Chemistry Exam #2 Answer Key -- November 4, 2008 There are 6 pages
|
|
|
- Shona Lane
- 6 years ago
- Views:
Transcription
1 ame: Answer Key 1 Chemistry Exam #2 Answer Key -- ovember 4, 2008 There are 6 pages 1. (10 pts) The proteins found in silk fibers are formed from stacked antiparallel β-sheets in which long stretches are composed of alternating glycine and alanine residues. This results in all the alanine sidechains being on the same side of the sheet. Draw a detailed structure of a portion of a silk antiparallel β-sheet containing at least 6 amino acids in each strand and 3 strands wide. Be sure to indicate hydrogen bonds with dotted lines. The stereochemistry at the alpha carbon should also be clearly shown. 2. (10 pts) What are the two major experimental methods used for determining the detailed three dimensional structure of proteins? Compare and contrast these two methods, clearly indicating their strengths and weaknesses. Both MR and Crystallography require relatively large amounts (mg's) of protein. In both techniques, some parts of the structure may not be well defined. Both techniques may have difficulty with integral membrane proteins. MR Determined in solution, the "normal" condition of proteins But must be very soluble Protons are observed Can be used for following dynamic processes (e.g., folding) Can be used to determine pka of groups on the protein Limited to relatively small proteins (< 50 kd) Proteins may need to be labeled by growing on 15 and C13 enriched media if > 15 kd Typical RMSD of backbone atoms ~ 1 Å Reported as an ensemble of structures that fit the constraints X-ray Crystallography Must be able to grow well-ordered crystals Protons are normally not seen, -bonding patterns must be inferred. Crystal contacts may perturb orientation of flexible surface features Can be used for large or small proteins xygen, nitrogen, and carbon atoms can't normally be distinguished, must be inferred eed to solve "phase problem", e.g., by making heavy metal derivative Typical RMSD of backbone atoms ~0.1 Å Reported as a single "average" structure
2 ame: Answer Key 2 3. (25 pts) Assume that you are an experienced student in a lab and that you are demonstrating a purification for citrate synthase (CS) to a new student. Briefly explain to the new student what is happening in the various steps and answer the questions at the end of each section. a) After some initial steps to isolate the mitochondria (which contain the citrate synthase), you lyse the mitochondria. To this lysate you add ammonium sulfate. You centrifuge the solution, remove the supernatant, and discard the pellet. To the supernatant you add more ammonium sulfate. nce again you centrifuge the sample, but this time you keep the pellet. Why is ammonium sulfate often added during the early stages of protein purifications? What is the rationale for the two-step addition of ammonium sulfate? As the concentration of ammonium sulfate increases the solubility of proteins decreases and they start to precipitate out of solution. Different proteins will precipitate at different ammonium sulfate concentrations. So we can purify our protein by first adding ammonium sulfate to a concentration below the concentration needed to precipitate out our protein, but high enough that other contaminant proteins precipitate. We can then remove the supernatant, which still contains our protein, and then add more ammonium sulfate to precipitate it out. This time we take the precipitate (since it contains our protein), but leave behind the supernatant which now contains other contaminant proteins. b) You solubilize the ammonium sulfate pellet and dialyze it overnight against large volumes of a buffer solution at p 7.2. What does the dialysis do? Dialysis is used to remove small molecules from the protein solution. (Small molecules can go through the pores of the dialysis membrane, but proteins cannot.) In this case the dialysis removes the ammonium sulfate and replaces it with the desired buffeer. c) You run the dialyzed solution over a gel filtration column. Following the protocol, you collect the first protein fraction that exits from the column, and discard the rest of the fractions that elute from the column later. What property of a molecule determines how it elutes in gel filtration chromatography? What does the fact that you collect the first protein fraction tell you about the protein? What would be a simple method to monitor for the presence of protein in the eluted fractions? Gel filtration separates molecules on the basis of size (and to some extent on shape). Small molecules must travel through the pores of the gel, and thus take longer to elute than large molecules. The fact that the CS is in the first protein fraction indicates that it is one of the largest protein molecules in the sample. The simplest method for monitoring for the presence of protein in the eluted fractions would be to measure the Absorption at 280 nm. d) The pi of CS is 5.6. The next step in the purification involves ion exchange chromatography. Would you use an anion exchange resin or a cation exchange resin? Briefly explain. As part of your explanation indicate what functional group is on the ion-exchange residue you chose. ow would you elute your protein from the column? At p 7.2 a protein with a pi of 5.6 will be negatively charged. Therefore you would want to use an anion exchange resin. A typical anion exchange resin contains positively charged ammonium groups. These positively charged groups will attract the negative charge on the protein and cause it to be retained on the column. The typical method for eluting the protein is to increase the ionic strength (for example by increasing acl concentration). Another method would be to lower the p so that the protein becomes positively charged.
3 ame: Answer Key 3 e) The final, purified protein is analyzed by SDS-PAGE. The SDS-PAGE shows a single band with a MW of 42,000±2000. owever from the gel filtration column you estimate a MW of 240,000±16,000. Describe briefly how SDS-PAGE works (start by describing what SDS-PAGE stands for). Then explain the difference in MW determined by SDS-PAGE and gel filtration. What can you infer about the structure of the CS from this data? SDS-PAGE -- Sodium Dodecyl Sulfate - PolyAcrylamide Gel Electrophoresis In this technique, proteins are exposed to high concentrations of the detergent sodium dodecyl sulfate. This detergent causes the proteins to denature and assume a rodlike shape. The large number of detergent molecules bound to the protein result in a net negative charge. The protein sample is applied to a polyacrylamide gel containing an electrolyte and an electric potential is applied to the gel. The protein molecules (coated with SDS) move through the gel toward the positive end of the electric field at a rate that is related to the logarithm of their molecular masses. The important distinction between SDS-PAGE and regular gel filtration is that the proteins are denatured in SDS-PAGE and in normal gel filtration they are in their native conformation. This means that if a protein is composed of multiple polypeptide chains, these chains will show up as separate bands on SDS-PAGE (assuming they are not cross-linked by disulfide bonds). In normal gel-filtration a protein composed of several chains will run together. Thus, in the case of CS, we can infer that the protein exists as a hexamer of 40,000 D subunits. f) You later decide to make a mutant form of CS in which several Ser residues on the surface are changed to Lys. Will the pi of the mutant protein be higher, lower, or about the same as the native enzyme? igher g) What experimental procedure(s) could be used to confirm that the mutant CS protein that you isolated has the desired amino acid sequence. Digest with trypsin and use mass spectrometry to determine the sequence of the resulting peptides. 4. (5 pts) ow many amino acids are there in each turn of an alpha helix? 3.6 The following sequence is an alpha helix from flavodoxin. Circle the residue whose side-chain is oriented in the most similar direction to the isoleucine sidechain. Asp-Asp-Arg-Ile-Lys-Ser-Trp-Val-Ala-Glu-Leu-Lys-Ser-Glu
4 ame: Answer Key 4 5. (18 pts) In an article in the European Journal of Biochemistry entitled "Ligand-binding properties and structural characterization of a novel rat odorant-binding protein variant" (Eur. J. Biochem. 2000, 267, 3079) the binding of 1-aminoanthracene (1-AMA) to the odorant-binding protein (BP) isolated from rat nasal mucus was studied. (ote: I am snot making this up!) A shorthand for the binding of AMA to BP is shown below, along with the fluorescence as a function of 1-AMA concentration. AMA + BP AMA BP a) Using the definition of, derive an expression for the fluorescence as a function of 1-AMA concentration. (int: This is similar to the derivation for the binding of 2 to myoglobin.) In your expression use to represent the dissociation constant and F max to represent the maximum fluorescence (i.e., the fluorescence when protein is completely saturated with 1-AMA.) ote that the observed fluorescence will be equal to the fraction of protein with 1-AMA bound times the maximum fluorescence. BP AMA [BP][AMA] = [BP AMA] BP + AMA [BP AMA] = [BP][AMA] (Eqn. 2) Fraction bound = [BP AMA] = [BP] + [BP AMA] Substituting in Eqn. 2 [BP][AMA] [BP] + [BP][AMA] = [BP] [BP] [AMA] ( + [AMA]) Fraction bound = [AMA] + [AMA] Fluorescence = F max [AMA] + [AMA] b) From the data shown in the figure estimate. (Briefly explain how you obtained this estimate.) = 1 µm. When [AMA] =, Fluorescence = 0.5 F max By inspection of the plot, it looks like F max is approximately 120. When Fluorescence = 60, [AMA] ~ 1 µm. c) Assume you find another molecule and determine that it has a of 3 mm for binding to the rat odorant-binding protein. Does this new molecule bind more tightly or less tightly than 1-AMA? Less Tightly
5 ame: Answer Key 5 6. (6 pts) Briefly compare and contrast myoglobin and hemoglobin in terms of their structure, oxygen binding characteristics, and physiological function. Myoglobin is a monomer (1 polypeptide chain), while hemoglobin is a tetramer (α 2 β 2 ). Although only 18% of the residues in the polypeptide chain in myoglobin are identical to those in the α or β chains of hemoglobin, the secondary and tertiary structures of these polypeptides are nearly identical, consisting of mostly α helices. The polypeptide chain of myoglobin and each of the polypeptide chains of hemoglobin contain one heme group that binds oxygen. The binding of oxygen to hemoglobin results in a conformational change that changes the relative orientations of the α and β subunits. Myoglobin binds oxygen with a "normal" hyperbolic binding curve and a p50 of 2.8 torr. Each tetrameric emoglobin can bind 4 oxygen atoms. The binding is not hyperbolic, but rather sigmoidal, reflecting cooperative interaction between the binding sites in a hemoglobin tetramer. That is, binding of oxygen at one site increases the affinity for binding at the other sites. The p50 for hemoglobin binding to oxygen in vivo is around 26 torr. The binding affinity in hemoglobin is affected by p (Bohr effect), the binding of BPG, and the binding to C 2. Myoglobin is an intracellular protein in vertebrate muscle. Its physiological role is to facilitate oxygen diffusion in muscle. emoglobin is the major protein in red blood cells and its role is to transport oxygen from the lungs (or gills) to other tissues in the body (e.g., the muscles). Since hemoglobin binds oxygen more weakly than myoglobin, oxygen is transferred from hemoglobin to myoglobin.
6 ame: Answer Key 6 7. (12 pts) Draw as precisely as possible a plot showing the oxygen binding curve (Y 2 versus p 2 ) for hemoglobin under normal conditions. When you go to higher altitudes your body rapidly adapts by increasing the concentration of BPG in red blood cells. Use a dotted line to show the oxygen binding curve in the presence of the increased BPG and show graphically how this helps compensate for the lower external oxygen pressure. (Assume the venous p 2 is 30 Torr, normal arterial p 2 is 100 torr, and at high altitude the arterial 2 drops to 60 torr.) Briefly explain the mechanism by which BPG changes hemoglobin's affinity for oxygen. n the graph above you can see that the decreased 2 affinity seen in the +BPG curve above leads to a larger fraction of 2 being delivered than would occur if the BPG concentration were not increased. BPG binds tightly to the T state hemoglobin but not the R state. Thus, increasing the amount of BPG shifts the equilibrium from the R state (high oxygen affinity) to the T state (low oxygen affinity) form, resulting in lower oxygen affinity.
7 ame: Answer Key 7 8. (7 pts) Describe a general pathway by which proteins fold into their 3-dimensional structure. (Include general time frames for these steps.) From pages in your text. 1. < 5 ms: Rapid formation of local segments of secondary structure (α helices and β sheets). This forms a "molten globule" containing a lot of the secondary structure, but little of its tertiary structure ms: The secondary structure becomes stabilized and tertiary structure begins to form. During this stage, the nativelike elements are thought to take the form of subdomains that are not yet properly docked to form domains s: The protein undergoes a series of complex motions in which it attains its relatively stable internal side chain packing and hydrogen bonding while it expels the remaining water molecules from its hydrophobic core. 4. In multidomain and multisubunit proteins, the respective units then assemble in a similar manner, with a few slight conformational adjustments required to produce the protein's native tertiary or quaternary structure. 9. (7 pts) What are prions, why were they controversial when first proposed, list two diseases that are caused by prions, and describe how prions cause disease? See pages in your text. Prions are infections protein particles that lack nucleic acids and cause disease. They were controversial when first proposed since it was thought that all infectious diseases required nucleic acids in order to be propagated. Diseases caused by prions include: Scrapie, Mad-cow disease (aka bovine spongiform encephalopathy, BSE), kuru, Creutzfeldt-Jakob disease (CJD). Prions are proteins that induce abnormal folding of a normal cellular protein (normally in the brain). The abnormally folded proteins catalyze the formation of more abnormally folded proteins (i.e., the process is autocatalytic). The abnormally folded proteins aggregate into amyloid fibrils that then disrupt brain function.
Protein Structure/Function Relationships
 Protein Structure/Function Relationships W. M. Grogan, Ph.D. OBJECTIVES 1. Describe and cite examples of fibrous and globular proteins. 2. Describe typical tertiary structural motifs found in proteins.
Protein Structure/Function Relationships W. M. Grogan, Ph.D. OBJECTIVES 1. Describe and cite examples of fibrous and globular proteins. 2. Describe typical tertiary structural motifs found in proteins.
Protein analysis. Dr. Mamoun Ahram Summer semester, Resources This lecture Campbell and Farrell s Biochemistry, Chapters 5
 Protein analysis Dr. Mamoun Ahram Summer semester, 2015-2016 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 5 Bases of protein separation Proteins can be purified on the basis Solubility
Protein analysis Dr. Mamoun Ahram Summer semester, 2015-2016 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 5 Bases of protein separation Proteins can be purified on the basis Solubility
Solutions to 7.02 Quiz II 10/27/05
 Solutions to 7.02 Quiz II 10/27/05 Class Average = 83 Standard Deviation = 9 Range Grade % 87-100 A 43 74-86 B 39 55-73 C 17 > 54 D 1 Question 1 (56 points) While studying deep sea bacteria, you discover
Solutions to 7.02 Quiz II 10/27/05 Class Average = 83 Standard Deviation = 9 Range Grade % 87-100 A 43 74-86 B 39 55-73 C 17 > 54 D 1 Question 1 (56 points) While studying deep sea bacteria, you discover
Purification: Step 1. Lecture 11 Protein and Peptide Chemistry. Cells: Break them open! Crude Extract
 Purification: Step 1 Lecture 11 Protein and Peptide Chemistry Cells: Break them open! Crude Extract Total contents of cell Margaret A. Daugherty Fall 2003 Big Problem: Crude extract is not the natural
Purification: Step 1 Lecture 11 Protein and Peptide Chemistry Cells: Break them open! Crude Extract Total contents of cell Margaret A. Daugherty Fall 2003 Big Problem: Crude extract is not the natural
Purification: Step 1. Protein and Peptide Chemistry. Lecture 11. Big Problem: Crude extract is not the natural environment. Cells: Break them open!
 Lecture 11 Protein and Peptide Chemistry Margaret A. Daugherty Fall 2003 Purification: Step 1 Cells: Break them open! Crude Extract Total contents of cell Big Problem: Crude extract is not the natural
Lecture 11 Protein and Peptide Chemistry Margaret A. Daugherty Fall 2003 Purification: Step 1 Cells: Break them open! Crude Extract Total contents of cell Big Problem: Crude extract is not the natural
Protein Structure/Function
 Protein Structure/Function C483 Spring 2013 1. Proteins segments which fold first can promote the folding of other sections of the protein into the native conformation by a process known as A) renaturation.
Protein Structure/Function C483 Spring 2013 1. Proteins segments which fold first can promote the folding of other sections of the protein into the native conformation by a process known as A) renaturation.
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor
 Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Focus concept Purification of a novel seed storage protein allows sequence analysis and determination of the protein
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Focus concept Purification of a novel seed storage protein allows sequence analysis and determination of the protein
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Last modified 29 September 2005
 Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Last modified 9 September 005 Focus concept Purification of a novel seed storage protein allows sequence analysis and
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Last modified 9 September 005 Focus concept Purification of a novel seed storage protein allows sequence analysis and
Globins. The Backbone structure of Myoglobin 2. The Heme complex in myoglobin. Lecture 10/01/2009. Role of the Globin.
 Globins Lecture 10/01/009 The Backbone structure of Myoglobin Myoglobin: 44 x 44 x 5 Å single subunit 153 amino acid residues 11 residues are in an a helix. Helices are named A, B, C, F. The heme pocket
Globins Lecture 10/01/009 The Backbone structure of Myoglobin Myoglobin: 44 x 44 x 5 Å single subunit 153 amino acid residues 11 residues are in an a helix. Helices are named A, B, C, F. The heme pocket
METHODS IN CELL BIOLOGY EXAM II, MARCH 26, 2008
 NAME KEY METHODS IN CELL BIOLOGY EXAM II, MARCH 26, 2008 1. DEFINITIONS (30 points). Briefly (1-3 sentences, phrases, word, etc.) define the following terms or answer question. A. depot effect refers to
NAME KEY METHODS IN CELL BIOLOGY EXAM II, MARCH 26, 2008 1. DEFINITIONS (30 points). Briefly (1-3 sentences, phrases, word, etc.) define the following terms or answer question. A. depot effect refers to
Protein Structure. Protein Structure Tertiary & Quaternary
 Lecture 4 Protein Structure Protein Structure Tertiary & Quaternary Dr. Sameh Sarray Hlaoui Primary structure: The linear sequence of amino acids held together by peptide bonds. Secondary structure: The
Lecture 4 Protein Structure Protein Structure Tertiary & Quaternary Dr. Sameh Sarray Hlaoui Primary structure: The linear sequence of amino acids held together by peptide bonds. Secondary structure: The
MOLEBIO LAB #3: Electrophoretic Separation of Proteins
 MOLEBIO LAB #3: Electrophoretic Separation of Proteins Introduction: Proteins occupy a central position in the structure and function of all living organisms. Some proteins serve as structural components
MOLEBIO LAB #3: Electrophoretic Separation of Proteins Introduction: Proteins occupy a central position in the structure and function of all living organisms. Some proteins serve as structural components
Extracting Pure Proteins from Cells
 Extracting Pure Proteins from Cells 0 Purification techniques focus mainly on size & charge 0 The first step is homogenization (grinding, Potter Elvejhem homogenizer, sonication, freezing and thawing,
Extracting Pure Proteins from Cells 0 Purification techniques focus mainly on size & charge 0 The first step is homogenization (grinding, Potter Elvejhem homogenizer, sonication, freezing and thawing,
BC 367, Exam 2 November 13, Part I. Multiple Choice (3 pts each)- Please circle the single best answer.
 Name BC 367, Exam 2 November 13, 2008 Part I. Multiple Choice (3 pts each)- Please circle the single best answer. 1. The enzyme pyruvate dehydrogenase catalyzes the following reaction. What kind of enzyme
Name BC 367, Exam 2 November 13, 2008 Part I. Multiple Choice (3 pts each)- Please circle the single best answer. 1. The enzyme pyruvate dehydrogenase catalyzes the following reaction. What kind of enzyme
Chapter 10. Oxygen Transporting Proteins
 Chapter 10 Oxygen Transporting Proteins Oxygen-transport proteins Vertebrates Myoglobin (Muscle) Hemoglobin (Blood) Invertebrates Hemerythrin Hemocyanin Heme Cu Cu O 22 Cu O 2 -binding site of hemocyanin
Chapter 10 Oxygen Transporting Proteins Oxygen-transport proteins Vertebrates Myoglobin (Muscle) Hemoglobin (Blood) Invertebrates Hemerythrin Hemocyanin Heme Cu Cu O 22 Cu O 2 -binding site of hemocyanin
PROTEINS. *Adapted from Biotechnology: Science for the New Millennium by Ellyn Daugherty.
 PROTEINS Most biotech products have something to do with proteins either containing amino acids or entire proteins. To manufacture protein products, researchers must understand protein structure and function.
PROTEINS Most biotech products have something to do with proteins either containing amino acids or entire proteins. To manufacture protein products, researchers must understand protein structure and function.
Proteins Higher Order Structures
 Proteins Higher Order Structures Dr. Mohammad Alsenaidy Department of Pharmaceutics College of Pharmacy King Saud University Office: AA 101 msenaidy@ksu.edu.sa Previously on PHT 426!! Protein Structures
Proteins Higher Order Structures Dr. Mohammad Alsenaidy Department of Pharmaceutics College of Pharmacy King Saud University Office: AA 101 msenaidy@ksu.edu.sa Previously on PHT 426!! Protein Structures
CHMI 2227E Biochemistry I
 CHMI 2227E Biochemistry I Proteins: - Quaternary structure CHMI 2227 - E.R. Gauthier, Ph.D. 1 CHMI 2227 - E.R. Gauthier, Ph.D. 2 hydrophobic Quaternary structure involves several polypeptides: Oligomers
CHMI 2227E Biochemistry I Proteins: - Quaternary structure CHMI 2227 - E.R. Gauthier, Ph.D. 1 CHMI 2227 - E.R. Gauthier, Ph.D. 2 hydrophobic Quaternary structure involves several polypeptides: Oligomers
Proteins and folding
 Proteins and folding October 8th, 2013. Kinga Futó Biopolymers Mitotic spindle of a dividing cell Actin filament network in the epidermal cell of Tobacco leaf. DNA strand released from bacteriophage 1
Proteins and folding October 8th, 2013. Kinga Futó Biopolymers Mitotic spindle of a dividing cell Actin filament network in the epidermal cell of Tobacco leaf. DNA strand released from bacteriophage 1
Amino Acids. Amino Acid Structure
 Amino Acids Pratt & Cornely Chapter 4 Alpha carbon Sidechain Proteins peptides Amino Acid Structure 1 L amino acids Glycine R/S vs D/L L isoleucine racemization Stereochemisty Common Amino Acids 2 Which
Amino Acids Pratt & Cornely Chapter 4 Alpha carbon Sidechain Proteins peptides Amino Acid Structure 1 L amino acids Glycine R/S vs D/L L isoleucine racemization Stereochemisty Common Amino Acids 2 Which
Biochem Fall Sample Exam I Protein Structure. Vasopressin: CYFQNCPRG Oxytocin: CYIQNCPLG
 Biochem Fall 2011 1. Primary Structure and amino acid chemistry Sample Exam I Protein Structure The peptide hormones vasopressin (ADH) and oxytocin each contain only nine amino acids. Vasopressin is an
Biochem Fall 2011 1. Primary Structure and amino acid chemistry Sample Exam I Protein Structure The peptide hormones vasopressin (ADH) and oxytocin each contain only nine amino acids. Vasopressin is an
Protein Purification and Characterization Techniques. Nafith Abu Tarboush, DDS, MSc, PhD
 Protein Purification and Characterization Techniques Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Extracting Pure Proteins from Cells Purification techniques focus
Protein Purification and Characterization Techniques Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Extracting Pure Proteins from Cells Purification techniques focus
Protein Techniques 1 APPENDIX TO CHAPTER 5
 Protein Techniques 1 APPENDIX T CHAPTER 5 Dialysis and Ultrafiltration If a solution of protein is separated from a bathing solution by a semipermeable membrane, small molecules and ions can pass through
Protein Techniques 1 APPENDIX T CHAPTER 5 Dialysis and Ultrafiltration If a solution of protein is separated from a bathing solution by a semipermeable membrane, small molecules and ions can pass through
Allosteric Effects & Cooperative Binding
 Allosteric Effects & Cooperative Binding The shape of the binding curve for oxygen to myoglobin is hyperbolic and follows the equation for non-cooperative binding: Y=[L]/(KD + [L]). The binding curve for
Allosteric Effects & Cooperative Binding The shape of the binding curve for oxygen to myoglobin is hyperbolic and follows the equation for non-cooperative binding: Y=[L]/(KD + [L]). The binding curve for
MBMB451A Section1 Fall 2008 KEY These questions may have more than one correct answer
 MBMB451A Section1 Fall 2008 KEY These questions may have more than one correct answer 1. In a double stranded molecule of DNA, the ratio of purines : pyrimidines is (a) variable (b) determined by the base
MBMB451A Section1 Fall 2008 KEY These questions may have more than one correct answer 1. In a double stranded molecule of DNA, the ratio of purines : pyrimidines is (a) variable (b) determined by the base
Isolation of Protein
 Isolation of Protein Ultra-centrifugation http://irfanchemist.wordpress.com/2009/04/19/isolation-of-protein / Protein solutions of various masses or densities may separated based on the time it takes to
Isolation of Protein Ultra-centrifugation http://irfanchemist.wordpress.com/2009/04/19/isolation-of-protein / Protein solutions of various masses or densities may separated based on the time it takes to
Lecture 5: 8/31. CHAPTER 5 Techniques in Protein Biochemistry
 Lecture 5: 8/31 CHAPTER 5 Techniques in Protein Biochemistry Chapter 5 Outline The proteome is the entire set of proteins expressed and modified by a cell under a particular set of biochemical conditions.
Lecture 5: 8/31 CHAPTER 5 Techniques in Protein Biochemistry Chapter 5 Outline The proteome is the entire set of proteins expressed and modified by a cell under a particular set of biochemical conditions.
Chapter 6. Techniques of Protein and Nucleic Acid Purification
 Chapter 6 Techniques of Protein and Nucleic Acid Purification Considerations in protein expression and purification Protein source Natural sources Recombinant sources Methods of lysis and solubilization
Chapter 6 Techniques of Protein and Nucleic Acid Purification Considerations in protein expression and purification Protein source Natural sources Recombinant sources Methods of lysis and solubilization
Globular proteins. Myoglobin and hemoglobin. Dr. Mamoun Ahram Summer semester,
 Globular proteins Myoglobin and hemoglobin Dr. Mamoun Ahram Summer semester, 2017-2018 Functions of myoglobin and hemoglobin Myoglobin is storage of O 2 in muscles. During periods of oxygen deprivation,
Globular proteins Myoglobin and hemoglobin Dr. Mamoun Ahram Summer semester, 2017-2018 Functions of myoglobin and hemoglobin Myoglobin is storage of O 2 in muscles. During periods of oxygen deprivation,
PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%)
 1 AFFINITY HIS-TAG PURIFICATION PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%) DESCRIPTION Nickel NTA Magnetic Agarose Beads are products that allow rapid and easy small-scale purification of
1 AFFINITY HIS-TAG PURIFICATION PROCEDURE FOR USE NICKEL NTA Magnetic Agarose Beads (5%) DESCRIPTION Nickel NTA Magnetic Agarose Beads are products that allow rapid and easy small-scale purification of
So.. Let us say you have an impure solution containing a protein of interest. Q: How do you (a) analyze what you have and (b) purify what you want?
 So.. Let us say you have an impure solution containing a protein of interest. Q: How do you (a) analyze what you have and (b) purify what you want? Polyacrylamide Gel Electrophoresis (PAGE) Note: proteins
So.. Let us say you have an impure solution containing a protein of interest. Q: How do you (a) analyze what you have and (b) purify what you want? Polyacrylamide Gel Electrophoresis (PAGE) Note: proteins
Protein Structure and Function! Lecture 4: ph, pka and pi!
 Protein Structure and Function! Lecture 4: ph, pka and pi! Definition of ph and pk a! ph is a measure of the concentration of H +.! + ph = log 10[H ] For a weak acid,! HA #!!"! H + + A!, K a = [H + ][A!
Protein Structure and Function! Lecture 4: ph, pka and pi! Definition of ph and pk a! ph is a measure of the concentration of H +.! + ph = log 10[H ] For a weak acid,! HA #!!"! H + + A!, K a = [H + ][A!
Solutions to Problem Set 1
 MIT Department of Biology 7.014 Introductory Biology, Spring 004 Question 1 Solutions to 7.014 Problem Set 1 a) Describe the conditions of the atmosphere on prebiotic earth and how these conditions differ
MIT Department of Biology 7.014 Introductory Biology, Spring 004 Question 1 Solutions to 7.014 Problem Set 1 a) Describe the conditions of the atmosphere on prebiotic earth and how these conditions differ
Chapter 5: Proteins: Primary Structure
 Instant download and all chapters Test Bank Fundamentals of Biochemistry Life at the Molecular Level 4th Edition Donald Voet https://testbanklab.com/download/test-bank-fundamentals-biochemistry-life-molecular-level-
Instant download and all chapters Test Bank Fundamentals of Biochemistry Life at the Molecular Level 4th Edition Donald Voet https://testbanklab.com/download/test-bank-fundamentals-biochemistry-life-molecular-level-
2 Liquid chromatography of biomolecules
 2 Liquid chromatography of biomolecules Proteins, peptides, DNA, RNA, lipids, and organic cofactors have various characteristics such as electric charge, molecular weight, hydrophobicity, and surface relief.
2 Liquid chromatography of biomolecules Proteins, peptides, DNA, RNA, lipids, and organic cofactors have various characteristics such as electric charge, molecular weight, hydrophobicity, and surface relief.
Introduction to Protein Purification
 Introduction to Protein Purification 1 Day 1) Introduction to Protein Purification. Input for Purification Protocol Development - Guidelines for Protein Purification Day 2) Sample Preparation before Chromatography
Introduction to Protein Purification 1 Day 1) Introduction to Protein Purification. Input for Purification Protocol Development - Guidelines for Protein Purification Day 2) Sample Preparation before Chromatography
Preparative Protein Chemistry
 Biochemistry 412 Preparative Protein Chemistry 19 February 2008 The Three Eras of Protein Purification 1. The Classical (Pre-Recombinant DNA) Era (pre-1978) - Proteins purified from natural sources only
Biochemistry 412 Preparative Protein Chemistry 19 February 2008 The Three Eras of Protein Purification 1. The Classical (Pre-Recombinant DNA) Era (pre-1978) - Proteins purified from natural sources only
Appendix IV Version
 APPENDIX IV. Gel Electrophoresis. Migration of biological molecules in the presence of an electric field through a gel matrix is the heart of many biochemistry experiments. The variety of electrophoresis
APPENDIX IV. Gel Electrophoresis. Migration of biological molecules in the presence of an electric field through a gel matrix is the heart of many biochemistry experiments. The variety of electrophoresis
Kinetics Review. Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets)
 Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Basic concepts of molecular biology
 Basic concepts of molecular biology Gabriella Trucco Email: gabriella.trucco@unimi.it Life The main actors in the chemistry of life are molecules called proteins nucleic acids Proteins: many different
Basic concepts of molecular biology Gabriella Trucco Email: gabriella.trucco@unimi.it Life The main actors in the chemistry of life are molecules called proteins nucleic acids Proteins: many different
Zool 3200: Cell Biology Exam 3 3/6/15
 Name: Trask Zool 3200: Cell Biology Exam 3 3/6/15 Answer each of the following questions in the space provided; circle the correct answer or answers for each multiple choice question and circle either
Name: Trask Zool 3200: Cell Biology Exam 3 3/6/15 Answer each of the following questions in the space provided; circle the correct answer or answers for each multiple choice question and circle either
Sheet #7 Dr. Mamoun Ahram 10/07/2014
 1 Recap: Globular Proteins - There are two types of proteins according to their structure: a. Fibrous proteins. b. Globular proteins: are globe-like (spherical) with three-dimensional compact structures
1 Recap: Globular Proteins - There are two types of proteins according to their structure: a. Fibrous proteins. b. Globular proteins: are globe-like (spherical) with three-dimensional compact structures
Zymogram: Study of an Active Enzyme with Electrophoresis
 PR106 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Zymogram: Study of an Active Enzyme with Electrophoresis Teachers Handbook (Cat. #
PR106 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Zymogram: Study of an Active Enzyme with Electrophoresis Teachers Handbook (Cat. #
蛋白質體學. Proteomics Amino acids, Peptides and Proteins 陳威戎 & 21
 蛋白質體學 Proteomics 2015 Amino acids, Peptides and Proteins 陳威戎 2015. 09. 14 & 21 Outline 1. Amino Acids 2. Peptides and Proteins 3. Covalent Structure of Proteins Amino Acids Proteins are polymers of amino
蛋白質體學 Proteomics 2015 Amino acids, Peptides and Proteins 陳威戎 2015. 09. 14 & 21 Outline 1. Amino Acids 2. Peptides and Proteins 3. Covalent Structure of Proteins Amino Acids Proteins are polymers of amino
MBP Excellose handbook - Purification of MBP fusion proteins -
 Introduction MBP Excellose handbook - Purification of MBP fusion proteins - MBP Excellose is a affinity chromatography medium used for simple and rapid purification of MBP (maltose binding protein) fusion
Introduction MBP Excellose handbook - Purification of MBP fusion proteins - MBP Excellose is a affinity chromatography medium used for simple and rapid purification of MBP (maltose binding protein) fusion
Amino Acids and Proteins
 Various Functions of Proteins SB203 Amino Acids and Proteins Jirundon Yuvaniyama, Ph.D. Department of Biochemistry Faculty of Science Mahidol University Enzymes Transport proteins utrient and storage proteins
Various Functions of Proteins SB203 Amino Acids and Proteins Jirundon Yuvaniyama, Ph.D. Department of Biochemistry Faculty of Science Mahidol University Enzymes Transport proteins utrient and storage proteins
Lecture 7: Affinity Chromatography-II
 Lecture 7: Affinity Chromatography-II We have studied basics of affinity purification during last lecture. The current lecture is continuation of last lecture and we will cover following: 1. Few specific
Lecture 7: Affinity Chromatography-II We have studied basics of affinity purification during last lecture. The current lecture is continuation of last lecture and we will cover following: 1. Few specific
Biochemistry Proteins
 MIT Department of Biology 7.014 Introductory Biology, Spring 2005 Recitation Section 3 February 9-10, 2005 A. CBS proteins, 3D representation Biochemistry Proteins The protein we will consider today and
MIT Department of Biology 7.014 Introductory Biology, Spring 2005 Recitation Section 3 February 9-10, 2005 A. CBS proteins, 3D representation Biochemistry Proteins The protein we will consider today and
1997 Nobel Prize in Physiology or Medicine Dr. Stanley Prusiner
 1997 Nobel Prize in Physiology or Medicine Dr. Stanley Prusiner for the discovery of prions* - a new biological principal of infection I. PRIONS - Definition Prions are simple proteins that are much smaller
1997 Nobel Prize in Physiology or Medicine Dr. Stanley Prusiner for the discovery of prions* - a new biological principal of infection I. PRIONS - Definition Prions are simple proteins that are much smaller
6-Foot Mini Toober Activity
 Big Idea The interaction between the substrate and enzyme is highly specific. Even a slight change in shape of either the substrate or the enzyme may alter the efficient and selective ability of the enzyme
Big Idea The interaction between the substrate and enzyme is highly specific. Even a slight change in shape of either the substrate or the enzyme may alter the efficient and selective ability of the enzyme
BIL 256 Cell and Molecular Biology Lab Spring, 2007 BACKGROUND INFORMATION I. PROTEIN COMPOSITION AND STRUCTURE: A REVIEW OF THE BASICS
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 BACKGROUND INFORMATION I. PROTEIN COMPOSITION AND STRUCTURE: A REVIEW OF THE BASICS Proteins occupy a central position in the structure and function
BIL 256 Cell and Molecular Biology Lab Spring, 2007 BACKGROUND INFORMATION I. PROTEIN COMPOSITION AND STRUCTURE: A REVIEW OF THE BASICS Proteins occupy a central position in the structure and function
Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 29, 2015
 Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 29, 2015 PHRM 836 Exam I - 1 Name: Instructions 1. Check your exam to make certain that it has 9 pages including this cover
Examination I PHRM 836 Biochemistry for Pharmaceutical Sciences II September 29, 2015 PHRM 836 Exam I - 1 Name: Instructions 1. Check your exam to make certain that it has 9 pages including this cover
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Purification and biochemical characterization of acid phosphatase-i from seeds of Nelumbo nucifera Sanaullah Khan a*, Shahnaz Asmat c, Sajida Batool a, Mushtaq Ahmed b a Department
SUPPLEMENTARY MATERIAL Purification and biochemical characterization of acid phosphatase-i from seeds of Nelumbo nucifera Sanaullah Khan a*, Shahnaz Asmat c, Sajida Batool a, Mushtaq Ahmed b a Department
Specificity: Induced Fit
 Specificity: Induced Fit Conformational changes may occur upon ligand binding (Daniel Koshland in 1958) This adaptation is called the induced fit Induced fit allows for tighter binding of the ligand Induced
Specificity: Induced Fit Conformational changes may occur upon ligand binding (Daniel Koshland in 1958) This adaptation is called the induced fit Induced fit allows for tighter binding of the ligand Induced
Plasmids & Transposable Elements. Dr. Wiaam Ahmed Al-Amili
 Plasmids & Transposable Elements BY Dr. Wiaam Ahmed Al-Amili Plasmid DNA preparation In order to use a vector for cloning, sequencing, etc., it is necessary to isolate the vector in a highly purified form.
Plasmids & Transposable Elements BY Dr. Wiaam Ahmed Al-Amili Plasmid DNA preparation In order to use a vector for cloning, sequencing, etc., it is necessary to isolate the vector in a highly purified form.
BIOC 463A Expt. 4: Column Chromatographic Methods Column Chromatography
 Column Chromatography Chromatography is the process use to separate molecules based on SOME physical property of the molecule: Mass (i.e. size) Charge Affinity for ligands or substrates Hydrophobic interactions
Column Chromatography Chromatography is the process use to separate molecules based on SOME physical property of the molecule: Mass (i.e. size) Charge Affinity for ligands or substrates Hydrophobic interactions
Not The Real Exam Just for Fun! Chemistry 391 Fall 2018
 Not The Real Exam Just for Fun! Chemistry 391 Fall 2018 Do not open the exam until ready to begin! Rules of the Game: This is a take-home Exam. The exam is due on Thursday, October 11 th at 9 AM. Otherwise,
Not The Real Exam Just for Fun! Chemistry 391 Fall 2018 Do not open the exam until ready to begin! Rules of the Game: This is a take-home Exam. The exam is due on Thursday, October 11 th at 9 AM. Otherwise,
Basic concepts of molecular biology
 Basic concepts of molecular biology Gabriella Trucco Email: gabriella.trucco@unimi.it What is life made of? 1665: Robert Hooke discovered that organisms are composed of individual compartments called cells
Basic concepts of molecular biology Gabriella Trucco Email: gabriella.trucco@unimi.it What is life made of? 1665: Robert Hooke discovered that organisms are composed of individual compartments called cells
Strep-Spin Protein Miniprep Kit Catalog No. P2004, P2005
 INSTRUCTION MANUAL Strep-Spin Protein Miniprep Kit Catalog No. P2004, P2005 Highlights Fast protocol to purify Strep-tagged proteins from cell-free extracts Screen your recombinant colonies directly for
INSTRUCTION MANUAL Strep-Spin Protein Miniprep Kit Catalog No. P2004, P2005 Highlights Fast protocol to purify Strep-tagged proteins from cell-free extracts Screen your recombinant colonies directly for
Types of chromatography
 Chromatography Physical separation method based on the differential migration of analytes in a mobile phase as they move along a stationary phase. Mechanisms of Separation: Partitioning Adsorption Exclusion
Chromatography Physical separation method based on the differential migration of analytes in a mobile phase as they move along a stationary phase. Mechanisms of Separation: Partitioning Adsorption Exclusion
Strep-Spin Protein Miniprep Kit Catalog No. P2004 & P2005
 INSTRUCTION MANUAL Strep-Spin Protein Miniprep Kit Catalog No. P2004 & P2005 Highlights Fast & Simple: Purify Strep-tagged proteins from cell-free extracts using a spin-column in 7 minutes High-Quality:
INSTRUCTION MANUAL Strep-Spin Protein Miniprep Kit Catalog No. P2004 & P2005 Highlights Fast & Simple: Purify Strep-tagged proteins from cell-free extracts using a spin-column in 7 minutes High-Quality:
Ch Biophysical Chemistry
 Ch 247.53. Biophysical Chemistry Nina Rosario L. Rojas 2012-2013 sem 1 Review of Protein Structure Why structure? Primary, secondary, tertiary structure Disulfide bonds scheme 2 STRUCTURE- REGULAR STRUCTURE
Ch 247.53. Biophysical Chemistry Nina Rosario L. Rojas 2012-2013 sem 1 Review of Protein Structure Why structure? Primary, secondary, tertiary structure Disulfide bonds scheme 2 STRUCTURE- REGULAR STRUCTURE
SERVA Ni-NTA Magnetic Beads
 INSTRUCTION MANUAL SERVA Ni-NTA Magnetic Beads Magnetic beads for Affinity Purification of His-Tag Fusion Proteins (Cat. No. 42179) SERVA Electrophoresis GmbH - Carl-Benz-Str. 7-69115 Heidelberg Phone
INSTRUCTION MANUAL SERVA Ni-NTA Magnetic Beads Magnetic beads for Affinity Purification of His-Tag Fusion Proteins (Cat. No. 42179) SERVA Electrophoresis GmbH - Carl-Benz-Str. 7-69115 Heidelberg Phone
Conformational properties of enzymes
 Conformational properties of enzymes; Physics of enzyme substrate interactions; Electronic conformational interactions, cooperative properties of enzymes Mitesh Shrestha Conformational properties of enzymes
Conformational properties of enzymes; Physics of enzyme substrate interactions; Electronic conformational interactions, cooperative properties of enzymes Mitesh Shrestha Conformational properties of enzymes
Ali Yaghi. Tamara Wahbeh. Mamoun Ahram
 28 Ali Yaghi Tamara Wahbeh Mamoun Ahram This sheet is a continuation of protein purification methods. Isoelectric focusing Separation of proteins based on Isoelectric points(charge),and it is a horizontal
28 Ali Yaghi Tamara Wahbeh Mamoun Ahram This sheet is a continuation of protein purification methods. Isoelectric focusing Separation of proteins based on Isoelectric points(charge),and it is a horizontal
Proteins the primary biological macromolecules of living organisms
 Proteins the primary biological macromolecules of living organisms Protein structure and folding Primary Secondary Tertiary Quaternary structure of proteins Structure of Proteins Protein molecules adopt
Proteins the primary biological macromolecules of living organisms Protein structure and folding Primary Secondary Tertiary Quaternary structure of proteins Structure of Proteins Protein molecules adopt
2013 W. H. Freeman and Company. 5 Function of Globular Proteins
 2013 W. H. Freeman and Company 5 Function of Globular Proteins CHAPTER 5: Function of Globular Proteins Key topics in protein function: Reversible binding of ligands is essential Specificity of ligands
2013 W. H. Freeman and Company 5 Function of Globular Proteins CHAPTER 5: Function of Globular Proteins Key topics in protein function: Reversible binding of ligands is essential Specificity of ligands
1) The penicillin family of antibiotics, discovered by Alexander Fleming in 1928, has the following general structure: O O
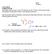 ame: TF ame: LS1a Fall 06 Problem Set #3 Due Friday 10/13 at noon in your TF s drop box on the 2 nd floor of the Science Center All questions including the (*extra*) ones should be turned in 1) The penicillin
ame: TF ame: LS1a Fall 06 Problem Set #3 Due Friday 10/13 at noon in your TF s drop box on the 2 nd floor of the Science Center All questions including the (*extra*) ones should be turned in 1) The penicillin
CFSSP: Chou and Fasman Secondary Structure Prediction server
 Wide Spectrum, Vol. 1, No. 9, (2013) pp 15-19 CFSSP: Chou and Fasman Secondary Structure Prediction server T. Ashok Kumar Department of Bioinformatics, Noorul Islam College of Arts and Science, Kumaracoil
Wide Spectrum, Vol. 1, No. 9, (2013) pp 15-19 CFSSP: Chou and Fasman Secondary Structure Prediction server T. Ashok Kumar Department of Bioinformatics, Noorul Islam College of Arts and Science, Kumaracoil
Chapter 3 Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
Unit 1. DNA and the Genome
 Unit 1 DNA and the Genome Gene Expression Key Area 3 Vocabulary 1: Transcription Translation Phenotype RNA (mrna, trna, rrna) Codon Anticodon Ribosome RNA polymerase RNA splicing Introns Extrons Gene Expression
Unit 1 DNA and the Genome Gene Expression Key Area 3 Vocabulary 1: Transcription Translation Phenotype RNA (mrna, trna, rrna) Codon Anticodon Ribosome RNA polymerase RNA splicing Introns Extrons Gene Expression
Introduction to Proteins
 Introduction to Proteins Lecture 4 Module I: Molecular Structure & Metabolism Molecular Cell Biology Core Course (GSND5200) Matthew Neiditch - Room E450U ICPH matthew.neiditch@umdnj.edu What is a protein?
Introduction to Proteins Lecture 4 Module I: Molecular Structure & Metabolism Molecular Cell Biology Core Course (GSND5200) Matthew Neiditch - Room E450U ICPH matthew.neiditch@umdnj.edu What is a protein?
Programme Good morning and summary of last week Levels of Protein Structure - I Levels of Protein Structure - II
 Programme 8.00-8.10 Good morning and summary of last week 8.10-8.30 Levels of Protein Structure - I 8.30-9.00 Levels of Protein Structure - II 9.00-9.15 Break 9.15-11.15 Exercise: Building a protein model
Programme 8.00-8.10 Good morning and summary of last week 8.10-8.30 Levels of Protein Structure - I 8.30-9.00 Levels of Protein Structure - II 9.00-9.15 Break 9.15-11.15 Exercise: Building a protein model
5.36 Biochemistry Laboratory Spring 2009
 MIT OpenCourseWare http://ocw.mit.edu 5.36 Biochemistry Laboratory Spring 2009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.36 Lecture Summary #3
MIT OpenCourseWare http://ocw.mit.edu 5.36 Biochemistry Laboratory Spring 2009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. 5.36 Lecture Summary #3
Proteins Amides from Amino Acids
 Chapter 26 and Chapter 28 Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl group Joined as amides between the ¾NH 2 of one amino acid and the ¾CO 2 H to the
Chapter 26 and Chapter 28 Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl group Joined as amides between the ¾NH 2 of one amino acid and the ¾CO 2 H to the
Protein Folding and Misfolding
 Folding of proteins into their native conformations occurs spontaneously under physiological conditions and is dictated by the primary structure of the protein. Learning Objective After interacting with
Folding of proteins into their native conformations occurs spontaneously under physiological conditions and is dictated by the primary structure of the protein. Learning Objective After interacting with
Bioinformatics. ONE Introduction to Biology. Sami Khuri Department of Computer Science San José State University Biology/CS 123A Fall 2012
 Bioinformatics ONE Introduction to Biology Sami Khuri Department of Computer Science San José State University Biology/CS 123A Fall 2012 Biology Review DNA RNA Proteins Central Dogma Transcription Translation
Bioinformatics ONE Introduction to Biology Sami Khuri Department of Computer Science San José State University Biology/CS 123A Fall 2012 Biology Review DNA RNA Proteins Central Dogma Transcription Translation
Refold SK Protein Refolding Kit
 Refold SK Protein Refolding Kit 2.5mg SK v5. 2 Table of Contents Introduction...4 Kit Content...5 Instruction for Use...6 Troubleshooting Guide...9 Application Notes...16 Introduction Overexpression of
Refold SK Protein Refolding Kit 2.5mg SK v5. 2 Table of Contents Introduction...4 Kit Content...5 Instruction for Use...6 Troubleshooting Guide...9 Application Notes...16 Introduction Overexpression of
Biochemistry 694:301 First Exam, Dr. Deis
 Biochemistry 694:301 First Exam, Dr. Deis Monday 26 July, 2004 Name Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth two points. Answer
Biochemistry 694:301 First Exam, Dr. Deis Monday 26 July, 2004 Name Row Letter Seat Number This exam consists of two parts. Part I is multiple choice. Each of these 25 questions is worth two points. Answer
Protein Function. chapter
 2608T_ch05sm_S54-S62 2/1/08 5:56PM Page S-54 ntt 102:WHQY028:Solutions Manual:Ch-05: chapter 5 Protein Function 1. Relationship between Affinity and Dissociation Constant Protein A has a binding site for
2608T_ch05sm_S54-S62 2/1/08 5:56PM Page S-54 ntt 102:WHQY028:Solutions Manual:Ch-05: chapter 5 Protein Function 1. Relationship between Affinity and Dissociation Constant Protein A has a binding site for
Bio 451 Examination I September 22, 1995
 Bio 451 Examination I September 22, 1995 PLEASE PRINT YOUR NAME CLEARLY ON EVERY PAGE OF THIS EXAMINATION; IF THIS IS NOT DONE, WE WILL SET YOUR EXAM ASIDE WHEN THE EXAMS ARE TAKEN APART FOR GRADING. Answer
Bio 451 Examination I September 22, 1995 PLEASE PRINT YOUR NAME CLEARLY ON EVERY PAGE OF THIS EXAMINATION; IF THIS IS NOT DONE, WE WILL SET YOUR EXAM ASIDE WHEN THE EXAMS ARE TAKEN APART FOR GRADING. Answer
BASIC MOLECULAR GENETIC MECHANISMS Introduction:
 BASIC MOLECULAR GENETIC MECHANISMS Introduction: nucleic acids. (1) contain the information for determining the amino acid sequence & the structure and function of proteins (1) part of the cellular structures:
BASIC MOLECULAR GENETIC MECHANISMS Introduction: nucleic acids. (1) contain the information for determining the amino acid sequence & the structure and function of proteins (1) part of the cellular structures:
Protein Methods. Second Edition. DANIEL M. BOLLAG Merck Research Laboratories West Point, Pennsylvania
 Protein Methods Second Edition DANIEL M. BOLLAG Merck Research Laboratories West Point, Pennsylvania MICHAEL D. ROZYCKI Department of Chemistry The Henry H. Hoyt Laboratory Princeton University Princeton,
Protein Methods Second Edition DANIEL M. BOLLAG Merck Research Laboratories West Point, Pennsylvania MICHAEL D. ROZYCKI Department of Chemistry The Henry H. Hoyt Laboratory Princeton University Princeton,
Lesson 2 Inoculation Growing a Cell Culture
 Lesson 2 Inoculation Growing a Cell Culture Isolation of Single Bacterial Colonies In this activity, students will pick one white colony from their LB/amp plates and one green colony from their LB/amp/ara
Lesson 2 Inoculation Growing a Cell Culture Isolation of Single Bacterial Colonies In this activity, students will pick one white colony from their LB/amp plates and one green colony from their LB/amp/ara
Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
Proteins. Amino Acids (APK) Peptides (APK) 5/23/2012. Peptide bond. Acid. Amino
 Proteins Amino Acids (APK) Acid Amino Image courtesy of Biotech (biotech.chem.indiana.edu/pages/ protein_intro.html) Peptides (APK) Peptide bond 1 Proteins (polypeptides) Segment of a protein Peptide bonds
Proteins Amino Acids (APK) Acid Amino Image courtesy of Biotech (biotech.chem.indiana.edu/pages/ protein_intro.html) Peptides (APK) Peptide bond 1 Proteins (polypeptides) Segment of a protein Peptide bonds
His-Spin Protein Miniprep
 INSTRUCTIONS His-Spin Protein Miniprep Catalog No. P2001 (10 purifications) and P2002 (50 purifications). Highlights Fast 5 minute protocol to purify His-tagged proteins from cell-free extracts Screen
INSTRUCTIONS His-Spin Protein Miniprep Catalog No. P2001 (10 purifications) and P2002 (50 purifications). Highlights Fast 5 minute protocol to purify His-tagged proteins from cell-free extracts Screen
SUPPLEMENTAL INFORMATION. Center Hamburg-Eppendorf, Center for Molecular Neurobiology, ZMNH, Hamburg, Germany
 SUPPLEMENTAL INFORMATION Title: Inter-motif communication induces hierarchical Ca 2+ -filling of Caldendrin Authors: Uday Kiran 1, Phanindranath Regur 1, Michael R. Kreutz 3,4*, Yogendra Sharma 1,2*, Asima
SUPPLEMENTAL INFORMATION Title: Inter-motif communication induces hierarchical Ca 2+ -filling of Caldendrin Authors: Uday Kiran 1, Phanindranath Regur 1, Michael R. Kreutz 3,4*, Yogendra Sharma 1,2*, Asima
Suppl. Figure 1: RCC1 sequence and sequence alignments. (a) Amino acid
 Supplementary Figures Suppl. Figure 1: RCC1 sequence and sequence alignments. (a) Amino acid sequence of Drosophila RCC1. Same colors are for Figure 1 with sequence of β-wedge that interacts with Ran in
Supplementary Figures Suppl. Figure 1: RCC1 sequence and sequence alignments. (a) Amino acid sequence of Drosophila RCC1. Same colors are for Figure 1 with sequence of β-wedge that interacts with Ran in
Cobalt Chelating Resin
 078PR-05 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Cobalt Chelating Resin A Co-IDA IMAC resin for 6X-His Tagged Protein Purification
078PR-05 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Cobalt Chelating Resin A Co-IDA IMAC resin for 6X-His Tagged Protein Purification
SUPPLEMENTARY INFORMATION
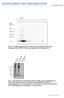 doi:10.1038/nature10324 Fig. S1: Two dimensional IEF/SDS-PAGE/Western blot analysis of RBC lysate crosslinked with 4 mm BS 3. The blot was probed with αsyn antibody C20. Fig. S2: SDS-PAGE/silver stain
doi:10.1038/nature10324 Fig. S1: Two dimensional IEF/SDS-PAGE/Western blot analysis of RBC lysate crosslinked with 4 mm BS 3. The blot was probed with αsyn antibody C20. Fig. S2: SDS-PAGE/silver stain
AFFINITY HIS-TAG PURIFICATION
 DESCRIPTION Resins are products that allow batch or column purifications. This product is supplied as a suspension in 50% aqueous suspension containing 30 vol % ethanol. INSTRUCTIONS The resins are adapted
DESCRIPTION Resins are products that allow batch or column purifications. This product is supplied as a suspension in 50% aqueous suspension containing 30 vol % ethanol. INSTRUCTIONS The resins are adapted
Nickel Chelating Resin Spin Columns
 326PR-02 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Nickel Chelating Resin Spin Columns A Ni-IDA IMAC resin for 6X-His Tagged Protein
326PR-02 G-Biosciences 1-800-628-7730 1-314-991-6034 technical@gbiosciences.com A Geno Technology, Inc. (USA) brand name Nickel Chelating Resin Spin Columns A Ni-IDA IMAC resin for 6X-His Tagged Protein
BIBC 103 Letter Grade Credit by Examination. Student Information Sheet
 BIBC 103 Letter Grade Credit by Examination Student Information Sheet This exam is a comprehensive test of the concepts, skills, competencies learned in the BIBC 103 (Biochemical Techniques) course. It
BIBC 103 Letter Grade Credit by Examination Student Information Sheet This exam is a comprehensive test of the concepts, skills, competencies learned in the BIBC 103 (Biochemical Techniques) course. It
Purification of (recombinant) proteins. Pekka Lappalainen, Institute of Biotechnology, University of Helsinki
 Purification of (recombinant) proteins Pekka Lappalainen, Institute of Biotechnology, University of Helsinki Physical properties of proteins that can be applied for purification -size -charge (isoelectric
Purification of (recombinant) proteins Pekka Lappalainen, Institute of Biotechnology, University of Helsinki Physical properties of proteins that can be applied for purification -size -charge (isoelectric
University of Guelph 3570 Analytical Biochemistry Fall 2004 In-class Quiz #1 Thursday, October 14, 2004 Instructor: Prof. David Josephy Solutions
 Student name: I.D. number: p. 1 University of Guelph 570 Analytical Biochemistry Fall 004 In-class Quiz #1 Thursday, October 14, 004 Instructor: Prof. David Josephy Solutions Instructions: Please write
Student name: I.D. number: p. 1 University of Guelph 570 Analytical Biochemistry Fall 004 In-class Quiz #1 Thursday, October 14, 004 Instructor: Prof. David Josephy Solutions Instructions: Please write
White Paper. Ion Exchange with PureSpeed Tips A Powerful Chromatography Tool
 Ion Exchange with PureSpeed Tips A Powerful Chromatography Tool Ion exchange chromatography separates molecules by exploiting differences in their overall charge characteristics. Its simplicity makes this
Ion Exchange with PureSpeed Tips A Powerful Chromatography Tool Ion exchange chromatography separates molecules by exploiting differences in their overall charge characteristics. Its simplicity makes this
Supplementary Note 1. Enzymatic properties of the purified Syn BVR
 Supplementary Note 1. Enzymatic properties of the purified Syn BVR The expression vector pet15b-syn bvr allowed us to routinely prepare 15 mg of electrophoretically homogenous Syn BVR from 2.5 L of TB-medium
Supplementary Note 1. Enzymatic properties of the purified Syn BVR The expression vector pet15b-syn bvr allowed us to routinely prepare 15 mg of electrophoretically homogenous Syn BVR from 2.5 L of TB-medium
