Mutational analysis of the antigenomic trans-acting delta ribozyme: the alterations of the middle nucleotides located on the P1 stem
|
|
|
- Berniece Wilkerson
- 6 years ago
- Views:
Transcription
1 1999 Oxford University Press Nucleic Acids Research, 1999, Vol. 27, No Mutational analysis of the antigenomic trans-acting delta ribozyme: the alterations of the middle nucleotides located on the P1 stem Sirinart Ananvoranich, Daniel A. Lafontaine and Jean-Pierre Perreault* Département de Biochimie, Faculté de Médecine, Université de Sherbrooke, Sherbrooke, Québec J1H 5N4, Canada Received November 12, 1998; Revised and Accepted January 20, 1999 ABSTRACT Our previous report on delta ribozyme cleavage using a trans-acting antigenomic delta ribozyme and a collection of short substrates showed that the middle nucleotides of the P1 stem, the substrate binding site, are essential for the cleavage activity. Here we have further investigated the effect of alterations in the P1 stem on the kinetic and thermodynamic parameters of delta ribozyme cleavage using various ribozyme variants carrying single base mutations at putative positions reported. The kinetic and thermodynamic values obtained in mutational studies of the two middle nucleotides of the P1 stem suggest that the binding and active sites of the delta ribozyme are uniquely formed. Firstly, the substrate and the ribozyme are engaged in the formation of a helix, known as the P1 stem, which may contain a weak hydrogen bond(s) or a bulge. Secondly, a tertiary interaction involving the base moieties in the middle of the P1 stem likely plays a role in defining the chemical environment. As a consequence, the active site might form simultaneously or subsequently to the binding site during later steps of the pathway. INTRODUCTION Delta ribozymes, derived from the genome of hepatitis delta virus (HDV), are metalloenzymes. Like other catalytically active ribozymes, namely hammerhead and hairpin ribozymes, the delta ribozymes cleave a phosphodiester bond of their RNA substrates and give rise to reaction products containing a 5 -hydroxyl and a 2,3 -cyclic phosphate termini. Two forms of delta ribozymes, namely genomic and antigenomic, were derived and referred to by the polarity of HDV genome from which the ribozyme was generated. Both delta ribozyme forms exhibit self-cleavage activity and it has been suggested that they are involved in the process of viral replication (1). This type of activity has been described as cis-acting delta ribozymes (2). Previously we have described the kinetics and the substrate specificity of a trans-acting antigenomic delta ribozyme, which is composed of 57 nt derived from the antigenomic self-cleaving motif, and its substrate is 11 nt long (Fig. 1; 3,4). Despite the same Figure 1. Secondary structure of the engineered trans-acting antigenomic delta ribozymes and their complementary substrates. The base paired regions of the pseudoknot-like structure are numbered according to Perrotta and Been (5). The secondary structure of the complex formed between δrzp1.1 and its substrate, SP1.1, is shown. The arrow indicates the cleavage site. The nucleotide numbering of both the substrate and ribozyme is indicated and referred to throughout the text. The mutational analyses were carried on positions 7 and 8 of the substrate and positions 23 and 24 of the ribozyme. substrate recognition sequence, trans-acting antigenomic delta ribozyme systems, generated by a similar approach in the elimination of the J1/2 portion, are of different lengths, ranging from 52 to 80 nt long, and exhibit various kinetic values (5 8). It has been previously discussed that the differences were results of the variation in the non-ribozyme flanking sequences (4,5). In our previous report on the substrate specificity, we studied the interactions between the delta ribozyme and the substrate, which are generally accepted as the formation of a helix referred to as the P1 stem (Fig. 1). We introduced a single mutation into each individual nucleotide of the substrate (positions 5 11 of the substrate) and showed that the base pairs in the middle of the P1 stem (corresponding to A23 and C24 of the delta ribozyme) are important not only for substrate binding, but also for subsequent steps in the cleavage pathway, including chemical cleavage (3). Recently, the crystal structure of a self-cleaving genomic delta ribozyme has been reported to have a putative active site buried in a unique nested double pseudoknot without any indication of *To whom correspondence should be addressed. Tel: ; Fax: ; jperre01@courrier.usherb.ca
2 Nucleic Nucleic Acids Acids Research, 1999, 1994, Vol. Vol. 27, 22, No. 61 the involvement of the nucleotides located on the P1 stem (9). Regardless of the similar predicted secondary structures of genomic and antigenomic delta ribozymes (2), it is possible that the X-ray structure of both delta ribozymes are different. More studies are required to achieve a better understanding of the native structure of antigenomic and genomic delta ribozymes, especially for their trans-active versions. In order to elaborate the effect of the P1 stem sequence on the cleavage pathway, we performed a detailed kinetic analysis of the wild-type delta ribozyme (δrzp1.1) and six of its variants (three δrzp1-a23n and three δrzp1-c24n ribozymes). Comparative analysis using both kinetic and thermodynamic parameters in this report further confirms the presence of tertiary interactions involving the positions A23 and C24 in defining the chemical environment of the trans-acting antigenomic delta ribozyme and its substrate complex. MATERIALS AND METHODS Preparation of RNAs Ribozymes and substrates were synthesized using T7 RNA polymerase as described previously (3). Uncleavable substrate analogs having a deoxyribonucleotide substitution at position 4 were chemically synthesized by the Keck Oligonucleotide Synthesis Facility (Yale University), deprotected as described by Perreault and Altman (10) and purified on 20% denaturing PAGE gels. Substrates were end-labeled by T4 polynucleotide kinase as described previously (3). The presence of alternative forms of ribozymes and substrates were examined using a non-denaturing gel electrophoresis system. Various concentrations of ribozymes and/or substrates within the range used in these experiments were electrophoresed on 12% non-denaturing polyacrylamide gels using 29:1 acrylamide:bis-acrylamide in 45 mm Tris borate, ph 7.5, and 10 mm MgCl 2 buffer and the relative amounts of the various forms quantified using a phosphorimaging screen and a Molecular Dynamics radioanalytic scanner. Kinetic analysis Pseudo first-order cleavage rate constants (k 2 and K m ) were measured with an excess of ribozyme (5 600 nm) and trace amounts of 5-32 P-end-labeled substrate (<0.1 nm). Unless otherwise stated, the reactions were carried out under standard conditions in 20 µl mixtures containing 50 mm Tris HCl, ph 7.5, and 10 mm MgCl 2 and were incubated at 37 C. At least two independent experiments were performed for each measurement. Reaction rates (k obs ) were obtained from fitting the experimental data to the equation A t = A α (1 e kt ) where A t is the percentage of cleavage at time t, A α is the maximum cleavage (or the end point of cleavage) and k is the reaction rate. Rates of substrate dissociation (k 1 ) were measured by pulse chase experiments. Partitioning experiments as described by Hertel et al. (11) and Fedor and Uhlenbeck (12) were performed with a slight modification. An inactive version of the ribozyme, which contains the substitution of A for C at position 47 in the wild-type ribozyme, was assumed to have similar substrate binding and dissociation properties as an active ribozyme. Trace amounts of end-labeled substrate were mixed with an inactive ribozyme (250 nm 1 µm) to form an enzyme substrate complex. After an initial binding period (10 s 5 min), the initial mixture (3 µl) was added to 27 µl of the active ribozyme. The concentration of active ribozyme during the chase period ranged from 2 to 20 µm, or 8 80 times the concentration of the inactive ribozyme. The observed rate of cleavage was obtained from fitting the experimental data describing the substrate left during the chase period to a pseudo first-order equation as mentioned earlier. The observed cleavage rate (k obs ) in the pulse chase reactions corresponds to the rate of cleavage (k 2 ) and the rate of substrate dissociation (k 1 ), i.e. k obs = k 2 + k 1. Control experiments were carried out by adding trace amounts of end-labeled substrate to a solution containing both inactive and active ribozymes at the same concentrations as in the test experiments. Observed cleavage rates were similar to those obtained from parallel reactions containing only active ribozyme. This observation indicated that the concentrations of active ribozyme were high enough to prevent the reassociation of the substrate to the inactive ribozyme during the chase period, reflecting the rate of cleavage (k 2 ). Rates of substrate association (k 1 ) were calculated from the equation described for the equilibrium state of the substrate dissociation (K d S = k 1 /k 1 ). Equilibrium dissociation constants of both the substrate and the product (K d S and K d P ) were measured by non-denaturing PAGE. Substrate analogs and reaction products were chemically synthesized so as to have sequences corresponding to the P1 stem of the cleavable complementary substrates. For the uncleavable substrate analog, the ribose was substituted by the 2 -deoxyribose moiety at position 4, resulting in an uncleavable substrate. For example, for the wild-type substrate SP1.1, SdC4 was synthesized as GGGdC 4 GGGUCGG. Various ribozyme concentrations ( nm) were individually mixed with trace amounts of end-labeled substrate analog (<0.1 nm) under similar cleavage assay conditions. To achieve an equilibrium state, the mixtures were incubated at 37 C for 1 h prior to fractionation on 12% acrylamide non-denaturing gels. The values of K d S were calculated by fitting experimental data to the simple binding equation (% bound substrate = [RZ]/K d + [RZ], where [RZ] is the concentration of ribozyme and K d is the equilibrium dissociation constant). K d P values were determined in the same manner using the labeled 3 -reaction products. RESULTS Delta ribozyme variants and their complementary substrates The trans-acting delta ribozyme variants were produced using plasmid pδrzp1.1 (3,4). The variants have either A23 or C24 mutated to one of the other three possible bases. The six resulting delta ribozyme variants are named for the altered nucleotide (δrzp1-a23c, -A23G, -A23U, -C24A, -C24G and -C24U; Table 1). Complementary or compensatory substrates (Table 1) were generated in which either position 7 or 8 of the wild-type substrate (SP1.1) was altered in order to restore the Watson Crick base pair formation of the P1 stem between the substrates and the ribozyme variants. Prior to performing a kinetic analysis, native gel electrophoresis was used to test for the possible presence of alternative forms of the transcripts as described in Materials and Methods. No alternative conformers were detected within the concentration range used ( nm) when individual transcripts were tested. Primarily, we assessed the cleavage activity of each variant and compared its extent of cleavage with that of the wild-type ribozyme, δrzp1.1 (Fig. 2). Despite the different cleavage rates
3 1475 Nucleic Acids Research, 1994, 1999, Vol. Vol. 22, 27, No. No Table 1. Sequences of transcripts used Subscript numbers represent nucleotide positions corresponding to the substrates, the reaction products and the ribozymes (as depicted in Fig. 1). observed, the extents of cleavage were observed to be between 0 and 80%, as compared with 60% for the wild-type ribozyme. Under single turnover conditions, δrzp1.1 is unable to cleave substrates with a mismatch at either position 7 or 8 reported previously (3). Ribozyme variants exhibited wider ranges of substrate specificity when G or U was introduced at either A23 or C24 of δrzp1.1. For example, δrzp1-a23g could cleave its complementary substrate (SU8C) as efficiently as the wild-type substrate (SP1.1) and cleaved SU8A with less efficiency. δrzp1-c24u cleaved single mismatched substrates (i.e. SP1.1, SG7U and SU7C). In contrast, δrzp1-c24a, having A substituted for C24, specifically cleaved only its complementary substrate, SG7U. Interestingly, δrzp1-a23c, which has C substituted for A23, did not efficiently cleave either its complementary substrate (SU8G) or any single mismatched substrates (i.e. SP1.1, SU8C and SU8A). In order to eliminate possible alternative forms of SU8G ribozyme occurring due to two repeated GGGC sequences in SU8G, the SU8G-9mer was synthesized with only 2 nt (GC) adjacent to the cleavage site instead of the usual 4 nt (Table 1). This shorter version of SU8G was weakly cleaved by δrzp1-a23c, with only a maximum cleavage extent of 7% after 4 h incubation. In order to determine what might cause this weak catalytic activity, various concentrations of δrzp1-a23c were pre-incubated with trace amounts of either end-labeled SU8G or SU8G-9mer and subjected to non-denaturing gel electrophoresis. Alternative conformers were detected when δrzp1-a23c was incubated with SU8G (11 nt), but not with SU8G-9mer. We also verified the effect of magnesium on the δrzp1-a23c cleavage of SU8G-9mer by increasing the magnesium concentration above Figure 2. Comparative analyses of the cleavage reactions catalyzed by delta ribozymes. The extents of cleavage of the δrzp1-a23n ribozyme variants were compared with that of the wild-type ribozyme, δrzp1.1 (A). The extents of cleavage of the δrzp1-c24n ribozyme variants were compared with that of the wild-type (B). The base pair formed between the ribozyme and the substrate is indicated by the capital and lower case letters, respectively, on each bar of the histogram. The values are an average calculated from at least two independent experiments. the 10 mm used under standard assay conditions (data not shown). Even after 3.5 h of incubation, a maximum cleavage extent of 15% was detected in the presence of 25 mm magnesium. However, at MgCl 2 concentrations >100 mm, we observed an inhibitory effect of the magnesium on δrzp1-a23c cleavage activity. Non-denaturing gel electrophoresis was also used for determining the binding affinity of δrzp1-a23c and -A23U for their uncleavable substrates (i.e. SP1.1 and SU8C; see Table 2), since δrzp1.1 was reported to bind its uncleavable substrates containing a single mutation at position 8 with the same affinity as the wild-type substrate (3). Similar phenomena were observed for the A23N ribozyme variants with their uncleavable substrates (Table 2). δrzp1-a23c could bind its complementary substrate analog with a K d S of 36 nm and the uncleavable substrates SP1.1 and SU8C with K d S of 33 ± 8 and 25 ± 8 nm, respectively. δrzp1-a23u could bind to its complementary substrate analog with K d S of 113 nm and SP1.1 as well as SU8C with K d S of 34 ± 3 and 140 nm, respectively. Since the δrzp1-c24n variants exhibited wider substrate specificities than those with the mutation at position A23, their substrate binding affinity was not determined. Kinetic studies of delta ribozyme variants The kinetic studies were employed to determine the effect of altering the P1 stem sequence on the cleavage pathway. The values of substrate association (k 1 ) and dissociation (k 1 ),
4 Nucleic Nucleic Acids Acids Research, 1999, 1994, Vol. Vol. 27, 22, No. 61 Table 2. Kinetic parameters for delta ribozymes Under single turnover conditions, the cleavage rate (k 2 ) and the ribozyme concentration at the half velocity (K m ) were determined. Calculated K P1 d values were based on the prediction of thermodynamic stability of the P1 stem duplex (13). K S d and K P d values were determined using end-labeled uncleavable substrate analogs and synthetic reaction products as described in Materials and Methods. a Kinetic parameters were determined using end-labeled SU8G-9mer. b The magnesium requirement could not be obtained by fitting the experimental data to the least squares equation. ND represents non-determined values. cleavage rate (k 2 ), as well as equilibrium substrate and product dissociation constants (K d S and K d P ) were measured for a trans-acting antigenomic delta ribozyme and its derived variants. Product formation or the chemical cleavage step (δrz S δrz P2 + P1) Wild-type delta ribozyme and its variants exhibited various cleavage extents and specificities against the collection of substrates examined. Therefore, we used the complementary pairs of substrates and ribozymes for all subsequent kinetic studies. The cleavage reactions were carried out under standard cleavage conditions described in Materials and Methods. The time courses of substrate cleavage by delta ribozyme variants were compared with that of the wild-type (Fig. 3). In these experiments trace amounts of substrate and 500 nm ribozyme were used. We observed both different extents of cleavage and different observed rates of cleavage. δrzp1.1 and δrz P1-A23U exhibited similar observed rates of cleavage (0.25 min 1 ), but δrzp1-a23u was found to have a higher extent of cleavage (Fig. 3A). Similar findings were observed for δrzp1-c24a and -C24G as compared with the wild-type ribozyme (Fig. 3B). δrzp1-a23g and -C24U cleaved their compensatory substrates 6 and 4 times less efficiently than δrzp1.1, respectively. Within 20 min, all ribozymes had reached their maximum cleavage extent. Various ribozyme concentrations were then used to obtain the experimental data required for the calculation of apparent K m (K m ) and apparent k 2 values (Table 2). In general, the mutations at either A23 or C24 reduced the rate, resulting in 2- to 6-fold lower k 2 values, but diversely affected the K m values. For example, the substitution of A or G at C24 resulted in ribozymes (δrzp1-c24a and -C24G) with similar k 2 ( 0.3 min 1 ), but different K m values (102 nm for δrzp1-c24a and 14 nm for δrzp1-a24g). As a consequence, the apparent second-order rate constants, which could be considered as a lower limit for substrate association, varied from 19 µm 1 min 1 for the wild-type to 4 µm 1 min 1 for δrzp1-a23g and -C24U, to 76 µm 1 min 1 for δrzp1-a23u. Under the standard assay conditions, we observed a decrease in k 2 values with most of the mutants. To verify whether higher magnesium concentrations could restore the lost activity, we Figure 3. Kinetics of cleavage reactions catalyzed by delta ribozymes. Time course cleavage experiments of the wild-type and variant ribozymes under the standard assay conditions described in Materials and Methods. The values for the δrzp1-a23n variants are presented in (A) and those for the δrzp1-c24n variants are presented in (B). Symbols indicating the plots for δrzp1.1 and the variants are listed in the square inset. The values were averages calculated from two independent experiments. determined the magnesium requirements of the wild-type and variant ribozymes as described previously (1; Table 2). We found the K Mg values, the concentration of magnesium at half-maximal velocity, to be 2 mm for most ribozymes. The mutations which replaced the purine (A) by the other purine (G) at position 23 (δrzp1-a23g) and the pyrimidine (C) by the other pyrimidine
5 1477 Nucleic Acids Research, 1994, 1999, Vol. Vol. 22, 27, No. No (U) at position 24 (δrzp-c24u) increased the magnesium requirement 2-fold (Table 2). Under the standard assay conditions (10 mm Mg 2+ ), δrzp1-a23g has a k 2 value of 0.06 min 1. However, at a higher concentration of Mg 2+, we observed that its maximum observed cleavage rate was increased to 0.15 min 1. δrzp1-c24u, whose Mg 2+ requirement is 5.1 mm, was found to have a k 2 value at higher Mg 2+ concentrations of 0.11 min 1. The cleavage activity of δrzp1-a23c increased when the MgCl 2 concentration was <25 mm and decreased dramatically at MgCl 2 concentrations >100 mm (data not shown). Substrate association and dissociation step (δrz + S δrz S) Equilibrium substrate dissociation constants were measured in order to determine how the variants interact with their substrates. The six variants containing a single mutation at either A23 or C24 can form conventional base pairs with their respective substrates, resulting in the P1 stem of the pseudoknot-like structure (5). Most ribozyme variants exhibited similar K S d values of 30 nm, although the calculated K d values based on the P1 stem duplex were different (Table 3). Higher K S d values were observed for δrzp1-a23u (113 nm) and -C24A (164 nm). Table 3. Comparative analyses of the thermodynamic parameters affected by the P1 stem mutations A standard state of 1 M free substrate and ribozyme at K (37 C) is assumed to be the same for all delta ribozymes. Gibbs energy changes in units of kcal mol 1 are presented. a G P1 is the calculated value for the P1 stem duplex (13). b The free energy of the enzyme substrate complex ( G E.S ) and reaction product ribozyme complex ( G E.P ) were calculated from the K d S and K d P, respectively (as listed in Table 2), using the equation G E S = RTlnK d. c Transition energy ( G ) was calculated from k 2 using the equation G = RTlnk 2 h/k B T. R, molar gas constant; h, Planck constant; k B, Boltzman constant. The substrate association and dissociation constants (k 1 and k 1 ) for the wild-type ribozyme were determined using a combination of active and inactive ribozymes for determination of the k 1 values as described in Materials and Methods. The substrate molecules that dissociate from the inactive ribozyme (A47) substrate complex are cleaved by the excess active wild-type ribozyme (δrzp1.1) added during the chase period. We used non-denaturing gel electrophoresis to confirm that no alternative complex was present when the inactive and active ribozymes were mixed with the trace amount of substrate. The inactive ribozyme was shown to lack cleavage activity (unpublished data). Although the substrate binding affinity of the inactive ribozyme might be different from that of the active ribozyme, its use provided us with the lower limit for k 1 as determined by this method. Since we detected no alternative band in the non-denaturing gels, presumably due to their equivalent masses, we assumed that the observed value was reliable. We could measure the k 1 value of 0.13 ± 0.5 min 1 independent of either the substrate or the ribozyme concentration. Unfortunately, this method could not be used for the determination of k 1. Due to the limitation that the measurement method required sufficient cleavage activity, we could only determine the k 1 values for some variants, namely δrzp1-a23u, -C24A and -C24G (Table 2). We observed that δrzp1-c24g, having similar k 2, K m, K Mg and K d S values to those of δrzp1.1, gave k 1 values of a similar magnitude ( min 1 ). In contrast, δrzp1-a23u and -C24A exhibited k 1 of 0.02 min 1, although both have k 2 and K Mg similar to those of the wild-type ribozyme (Table 2). Product dissociation and association step (δrz P2 + P1 δrz + P1 + P2) Equilibrium product dissociation constants were measured using 5 -end-labeled P2 reaction products (7 nt long; see Table 1 for the sequences). The reaction products (P2) seem to have binding affinities for the ribozymes similar to those of the substrates. The K P d values range between 40 and 60 nm for most variants, similar to those measured for K S d (Table 2). Higher K P d values were measured for δrzp1-c24a, which also exhibited higher K S d values. We found that only 30% of the end-labeled products could bind to the ribozyme and examined whether the low binding capacity of the reaction products and the ribozymes was due to the presence of the phosphate group on the 5 -end of the reaction products. Under similar conditions, 3 -end-labeled (by 32 pcp and RNA ligase) reaction products were tested. We did not detect any increase in the binding capacity (data not shown). Unfortunately, due to the low product ribozyme binding capacity, we could not accurately measure the k 3 and k 3 values. Thermodynamic analysis of delta ribozyme variants The kinetic parameters obtained were used for the calculation of Gibbs free energy with the k 2 values listed in Table 2 being used to calculate the transition state energy ( G ) and the K S d and K P d values for the calculations of G E S and G E P, respectively (Table 3). In general, the mutations caused a decrease in substrate ribozyme binding affinity, even though the potential to form the P1 stem through conventional Watson Crick base pairs is identical to that in the wild-type situation. The destabilization of the substrate ribozyme complex was detected for δrzp1-a23u and -C24A complexes with their G E S values increasing 1 kcal mol 1. The mutations seemed to stabilize the reaction product ribozyme complex of δrzp1-a23u since the G E P value decreased 0.5 kcal mol 1, but destabilize the product ribozyme complex of δrzp1-c24a since the G E P value increased 0.6 kcal mol 1. We also noted changes in the transition state energy of kcal mol 1 among the ribozyme variants as compared with that of the wild-type. The calculated free energy therefore suggested that δrzp1-a23u catalyzed the cleavage of its substrate in the most favorable way as compared with all other ribozymes, including the wild-type. DISCUSSION Cleavage reactions of ribozyme variants Previously, we showed that the positions A23 and C24, which are in the middle of the P1 stem of δrzp1.1, might have significant
6 Nucleic Nucleic Acids Acids Research, 1999, 1994, Vol. Vol. 27, 22, No. 61 effect(s) on the cleavage pathway (3). In order to study their effects, we synthesized a collection of 11mer substrates containing a compensatory mutation at position G7 or U8 (Table 1). The SU8N substrates were used in the cleavage assays catalyzed by δrzp1-a23n ribozymes and the SG7N substrates in the assays catalyzed by δrzp1-c24n ribozymes. The mutations at either position SU8 or SG7 were designed to interfere with the formation of the P1 stem between the ribozyme and substrate and ranged in effect from a very mild one on complementary Watson Crick base pairing to the stronger interfering effect of non-cannonical base pairing. The base pairs between the ribozyme (A23) and the substrate (SU8) will be referred to as A23N SU8N and those between C24 and SG7 as C24N SG7N. The position A23 seems to be the more restricted of the two with respect to any mismatches introduced in the substrate. The conventional base pairing, for example A23 SU8 (A:u), A23U SU8A (U:a), C24 SG7 (C:g) and C24A SG7U (A:u), yielded the best P1 stem formation when the extent of cleavage was used to score the effect of these mutations (Fig. 2). However the A23C SU8G (C:g) pairing did not follow the pattern (see below). The wobble base pair between the two partners also resulted in an extent of cleavage similar to that of both A23G SU8 (G:u) and C24U SU8 (U:u). Some base pair preferences were detected for these two positions. For example, the wobble base pair (G-U) results in a higher extent of cleavage when the ribozyme possesses the G instead of the U. The introduction of any mutation might disturb optimal or proper interactions between the ribozyme and its substrate and therefore affect the minimum reaction steps in various ways depending upon how sensitive that position is to structural changes. Comparative analyses were undertaken to decipher the effect of a single mutation introduced into the middle of the delta ribozyme P1 stem. Only comparable kinetic parameters measured using the complementary substrate ribozyme pairs were analyzed (Table 2). In order to comprehend the effect of these mutations clearly, the mutation at each position will be discussed individually. δrzp1-a23n ribozymes and respective SU8N substrates The position A23 SU8 has previously been shown to have an important role in cleavage since any mismatches introduced into the substrate at this position resulted in a complete lack of cleavage with a ribozyme binding affinity similar to that of the wild-type substrate (3). One possible explanation might be that the initial step of the delta cleavage pathway involves an incomplete base pairing of the P1 stem. In this scenario, the P1 stem contains a weak hydrogen bond, presumably at position A23, which is flanked by three conventional base pairs on the top of the stem and two conventional base pairs plus an additional wobble base pair at the bottom of the P1 stem. This initial affinity with a weak hydrogen bond(s) is subsequently restored to a more stable form of a conventional base pair prior to the chemical cleavage. The formation of both the P1 stem and the proper substrate ribozyme complex will then result in product formation. We found that δrzp1-a23c could bind its substrate analog and uncleavable mismatched substrates with the same affinity, reminiscent of the situation observed with the wild-type ribozyme (3). The substrate binding affinity of the δrzp1-a23u variant was not compromised by mismatches in its uncleavable substrates (i.e. SP1.1 and SU8C), although it was surprising that it could bind SP1.1 better than its complementary substrate analog. When the adenine at position 23 was substituted by the other purine (G), the resulting δrzp1-a23g variant exhibited similar K m, K d S and K d P values, but showed a k 2 value 6.3 times lower than that of the wild-type ribozyme. We observed that this ribozyme required more magnesium to reach its maximum cleavage rate (0.14 ± 0.01 min 1 ; Table 2). The calculated free energy levels ( G E S, G E P and G ) were less stable than those of the wild-type and the increased amount of magnesium seemed to stabilize the transition state conformation, lowering the G value by 0.6 kcal mol 1. The substitution of A23 with pyrimidine bases resulted in two ribozyme variants with quite astonishing characteristics. While the δrzp1-a23c variant was almost inactive, δrzp1-a23u cleaved efficiently. δrzp1-a23c was shown to form an alternative conformation with its 11mer complementary substrate, SU8G. However, SU8G-9mer, for which only one form of substrate ribozyme complex was detected, could not be efficiently cleaved by δrzp1-a23c. When we measured the cleavage rate (reflecting k 2 ), we detected that the magnesium requirement of this ribozyme was very different from that of the wild-type and other variants. The optimal MgCl 2 concentration was estimated to be 25 mm. The δrzp1-a23u variant cleaved its substrate with a rate similar to that of the wild-type ribozyme, but has 4-times less affinity for its complementary substrate as compared with the wild-type substrate ribozyme complex (Table 2). A higher affinity of δrzp1-a23u for its reaction product, with the K d P value being 2 times lower than the K d P value of the δrzp1.1 and its product, was detected (Table 2). These kinetic results suggest that δrzp1-a23u alone, among all variants tested, used the most favorable catalytic pathway. Due to the lower G E P ( 11 kcal mol 1 ) as compared with G E S ( 9.8 kcal mol 1 ), product formation was favorable. Also, this variant kinetically exhibited the highest apparent second-order rate constant (76 µm 1 min 1 ; Table 2) and its calculated k 1 value was 0.17 µm 1 min 1. Therefore, the product formation step is unlikely to be a rate determining step, rather the substrate association step is more likely to be the rate determining step. δrzp1-c24n ribozymes and respective SG7N substrates The position C24 has been shown to be important for both the substrate binding and cleavage steps. The substitution of C24 by U gave rise to the variant δrzp1-c24u that could recognize the complementary substrate with the same affinity as the wild-type ribozyme, but cleaved it at a much slower rate. The resulting transition energy was 1 kcal mol 1 higher than that of wild-type, indicating that the uridine ring could not stabilize the transition conformation as efficiently as the cytosine ring. However, an increase in the Mg 2+ concentration restored the cleavage rate to approximately one-third (0.1 min 1 ) of that of the wild-type ribozyme (Table 2). A similar effect was described earlier for δrzp1-a23g, suggesting that there is some change in the chemical environment. The variants δrzp1-c24a and -C24G could bind their complementary substrates producing less stable substrate ribozyme complexes as evidenced by the fact that their Gibbs free energy of binding was 1 kcal mol 1 greater than that of the wild-type ribozyme (Table 3). Interestingly, the destabilization of the substrate ribozyme complex did not affect the cleavage rate,
7 1479 Nucleic Acids Research, 1994, 1999, Vol. Vol. 22, 27, No. No although the transition energy increased by 1.1 kcal mol 1. These findings suggest that the transition state conformations formed by δrzp1-c24a and -C24G and their substrates differed from that formed by wild-type ribozyme with its substrate. The magnesium requirement remained similar to that of the wild-type ribozyme, indicating that the structures of the complexes are different. In summary, these mutational studies suggest that the binding and active sites of the delta ribozyme are formed in a unique way. Firstly, the substrate and the ribozyme are engaged in the formation of a helix, known as the P1 stem, which may contain a weak hydrogen bond(s). Secondly, a tertiary interaction involving the base moieties in the middle of the P1 stem likely plays a role in defining the chemical environment. As a consequence, the active site might form simultaneously or subsequently to the binding site during later steps of the pathway, possibly including the product formation step. These conclusions are based on the findings that not only the substrate affinity of the ribozyme changed upon point mutation, but so did the cleavage rate. Furthermore, a single mutation in the P1 stem seemed to affect the transition conformation, thereby directing the pathway. In order to confirm this hypothesis regarding the trans-acting delta ribozyme, more structural studies are required, including the identification of nucleotides involved in this putative tertiary interaction. Similarly, single functional group mutation on hammerhead and hairpin ribozymes was shown to affect their cleavage pathway (14,15). Clearly, more studies are required for a better understanding of the structure and activity requirements of ribozymes. ACKNOWLEDGEMENTS This work was supported by a grant from the Medical Research Council (MRC) of Canada to J.P.P. S.A. is the recipient of a post-doctoral fellowship from Natural Sciences and Engineering Research Council (NSERC) of Canada. D.A.L. is the recipient of a pre-doctoral fellowship program from the Fonds de la Recherche en Santé du Québec (FRSQ). J.P.P. is an MRC scholar. REFERENCES 1 Lazinski,D.W. and Taylor,J.M. (1995) RNA, 1, Perrotta,A.T. and Been,M.D. (1991) Nature, 350, Ananvoranich,S. and Perreault,J.P. (1998) J. Biol. Chem., 273, Mercure,S., Lafontaine,A.D., Ananvoranich,S. and Perreault J.P. (1998) Biochemistry, 37, Perrotta,A.T. and Been,M.D. (1992) Biochemistry, 31, Kawakami,J., Yuda,K., Suh,Y.A., Kumar,P.K.R., Nishikawa,F., Maeda,H., Taira,K., Ohtsuka,E. and Nishikawa,S. (1996) FEBS Lett., 394, Nishikawa,F., Fauzi,H. and Nishikawa,S. (1997) Nucleic Acids Res., 25, Fauzi,H., Kawakami,J., Nishikawa,F. and Nishikawa,S. (1997) Nucleic Acids Res., 25, Ferre-D Amare,A.R., Zhou,K. and Doudna,J.A. (1998) Nature, 395, Perreault,J.P. and Altman,S. (1992) J. Mol. Biol., 226, Hertel,K.J., Herschlag,D. and Uhlenbeck,O.C. (1994) Biochemistry, 33, Fedor,M.J. and Uhlenbeck,O.C. (1992) Biochemistry, 31, Serra,M.J. and Turner,D.H. (1995) Methods Enzymol., 259, Baidya,N. and Uhlenbeck,O.C. (1997) Biochemistry, 36, Shippy,R., Siwkowski,A. and Hampel,A. (1998) Biochemistry, 37,
BASIC MOLECULAR GENETIC MECHANISMS Introduction:
 BASIC MOLECULAR GENETIC MECHANISMS Introduction: nucleic acids. (1) contain the information for determining the amino acid sequence & the structure and function of proteins (1) part of the cellular structures:
BASIC MOLECULAR GENETIC MECHANISMS Introduction: nucleic acids. (1) contain the information for determining the amino acid sequence & the structure and function of proteins (1) part of the cellular structures:
Characterization of the Trans Watson-Crick GU Base Pair Located in the Catalytic Core of the Antigenomic HDV Ribozyme
 Characterization of the Trans Watson-Crick GU Base Pair Located in the Catalytic Core of the Antigenomic HDV Ribozyme Dominique Lévesque, Cédric Reymond, Jean-Pierre Perreault* Département de Biochimie,
Characterization of the Trans Watson-Crick GU Base Pair Located in the Catalytic Core of the Antigenomic HDV Ribozyme Dominique Lévesque, Cédric Reymond, Jean-Pierre Perreault* Département de Biochimie,
RNA is a single strand molecule composed of subunits called nucleotides joined by phosphodiester bonds.
 The Versatility of RNA Primary structure of RNA RNA is a single strand molecule composed of subunits called nucleotides joined by phosphodiester bonds. Each nucleotide subunit is composed of a ribose sugar,
The Versatility of RNA Primary structure of RNA RNA is a single strand molecule composed of subunits called nucleotides joined by phosphodiester bonds. Each nucleotide subunit is composed of a ribose sugar,
Monitoring of an RNA Multistep Folding Pathway by Isothermal Titration Calorimetry
 132 Biophysical Journal Volume 96 January 2009 132 140 Monitoring of an RNA Multistep Folding Pathway by Isothermal Titration Calorimetry Cédric Reymond, Martin Bisaillon, and Jean-Pierre Perreault* RNA
132 Biophysical Journal Volume 96 January 2009 132 140 Monitoring of an RNA Multistep Folding Pathway by Isothermal Titration Calorimetry Cédric Reymond, Martin Bisaillon, and Jean-Pierre Perreault* RNA
Supplementary Information. Single-molecule analysis reveals multi-state folding of a guanine. riboswitch
 Supplementary Information Single-molecule analysis reveals multi-state folding of a guanine riboswitch Vishnu Chandra 1,4,#, Zain Hannan 1,5,#, Huizhong Xu 2,# and Maumita Mandal 1,2,3,6* Department of
Supplementary Information Single-molecule analysis reveals multi-state folding of a guanine riboswitch Vishnu Chandra 1,4,#, Zain Hannan 1,5,#, Huizhong Xu 2,# and Maumita Mandal 1,2,3,6* Department of
Feedback D. Incorrect! No, although this is a correct characteristic of RNA, this is not the best response to the questions.
 Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
Supplemental Figure 1. Ribose-614 cleavage is unaffected by the addition of 10%
 Supplemental Figure 1. Ribose-614 cleavage is unaffected by the addition of 1% complementary strand. Addition of equimolar amounts of the complement greatly lowers cleavage rate and plateau. (A) Cleavage
Supplemental Figure 1. Ribose-614 cleavage is unaffected by the addition of 1% complementary strand. Addition of equimolar amounts of the complement greatly lowers cleavage rate and plateau. (A) Cleavage
Molecular biology WID Masters of Science in Tropical and Infectious Diseases-Transcription Lecture Series RNA I. Introduction and Background:
 Molecular biology WID 602 - Masters of Science in Tropical and Infectious Diseases-Transcription Lecture Series RNA I. Introduction and Background: DNA and RNA each consists of only four different nucleotides.
Molecular biology WID 602 - Masters of Science in Tropical and Infectious Diseases-Transcription Lecture Series RNA I. Introduction and Background: DNA and RNA each consists of only four different nucleotides.
Syllabus for GUTS Lecture on DNA and Nucleotides
 Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
BIOLOGICAL SCIENCE. Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge. FIFTH EDITION Freeman Quillin Allison
 BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 4 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge In this chapter you will learn that Nucleic acids store the
BIOLOGICAL SCIENCE FIFTH EDITION Freeman Quillin Allison 4 Lecture Presentation by Cindy S. Malone, PhD, California State University Northridge In this chapter you will learn that Nucleic acids store the
Unit IX Problem 3 Genetics: Basic Concepts in Molecular Biology
 Unit IX Problem 3 Genetics: Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated to synthesize
Unit IX Problem 3 Genetics: Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated to synthesize
Module Overview. Lecture
 Module verview Day 1 2 3 4 5 6 7 8 Lecture Introduction SELEX I: Building a Library SELEX II: Selecting RNA with target functionality SELEX III: Technical advances & problem-solving Characterizing aptamers
Module verview Day 1 2 3 4 5 6 7 8 Lecture Introduction SELEX I: Building a Library SELEX II: Selecting RNA with target functionality SELEX III: Technical advances & problem-solving Characterizing aptamers
Gene and DNA structure. Dr Saeb Aliwaini
 Gene and DNA structure Dr Saeb Aliwaini 2016 DNA during cell cycle Cell cycle for different cell types Molecular Biology - "Study of the synthesis, structure, and function of macromolecules (DNA, RNA,
Gene and DNA structure Dr Saeb Aliwaini 2016 DNA during cell cycle Cell cycle for different cell types Molecular Biology - "Study of the synthesis, structure, and function of macromolecules (DNA, RNA,
Nucleotides: structure and functions. Prof. Dalė Vieželienė Biochemistry department Room No
 Nucleotides: structure and functions Prof. Dalė Vieželienė Biochemistry department Room No. 229 Email: daleveze@med.kmu.lt Composition of Nucleic Acids Nucleotide structure Two types of nucleic acids:
Nucleotides: structure and functions Prof. Dalė Vieželienė Biochemistry department Room No. 229 Email: daleveze@med.kmu.lt Composition of Nucleic Acids Nucleotide structure Two types of nucleic acids:
DNA Structures. Biochemistry 201 Molecular Biology January 5, 2000 Doug Brutlag. The Structural Conformations of DNA
 DNA Structures Biochemistry 201 Molecular Biology January 5, 2000 Doug Brutlag The Structural Conformations of DNA 1. The principle message of this lecture is that the structure of DNA is much more flexible
DNA Structures Biochemistry 201 Molecular Biology January 5, 2000 Doug Brutlag The Structural Conformations of DNA 1. The principle message of this lecture is that the structure of DNA is much more flexible
Nucleic Acids and the RNA World. Pages Chapter 4
 Nucleic Acids and the RNA World Pages 74-89 Chapter 4 RNA vs. Protein Chemical Evolution stated that life evolved from a polymer called a protein. HOWEVER, now many scientists question this. There is currently
Nucleic Acids and the RNA World Pages 74-89 Chapter 4 RNA vs. Protein Chemical Evolution stated that life evolved from a polymer called a protein. HOWEVER, now many scientists question this. There is currently
Chapter 3 Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA
 AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
Nucleic acids. How DNA works. DNA RNA Protein. DNA (deoxyribonucleic acid) RNA (ribonucleic acid) Central Dogma of Molecular Biology
 Nucleic acid chemistry and basic molecular theory Nucleic acids DNA (deoxyribonucleic acid) RNA (ribonucleic acid) Central Dogma of Molecular Biology Cell cycle DNA RNA Protein Transcription Translation
Nucleic acid chemistry and basic molecular theory Nucleic acids DNA (deoxyribonucleic acid) RNA (ribonucleic acid) Central Dogma of Molecular Biology Cell cycle DNA RNA Protein Transcription Translation
From Gene to Protein
 8.2 Structure of DNA From Gene to Protein deoxyribonucleic acid - (DNA) - the ultimate source of all information in a cell This information is used by the cell to produce the protein molecules which are
8.2 Structure of DNA From Gene to Protein deoxyribonucleic acid - (DNA) - the ultimate source of all information in a cell This information is used by the cell to produce the protein molecules which are
Multiple choice questions (numbers in brackets indicate the number of correct answers)
 1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
1 Multiple choice questions (numbers in brackets indicate the number of correct answers) February 1, 2013 1. Ribose is found in Nucleic acids Proteins Lipids RNA DNA (2) 2. Most RNA in cells is transfer
All This For Four Letters!?! DNA and Its Role in Heredity
 All This For Four Letters!?! DNA and Its Role in Heredity What Is the Evidence that the Gene Is DNA? By the 1920s, it was known that chromosomes consisted of DNA and proteins. A new dye stained DNA and
All This For Four Letters!?! DNA and Its Role in Heredity What Is the Evidence that the Gene Is DNA? By the 1920s, it was known that chromosomes consisted of DNA and proteins. A new dye stained DNA and
Genetics and Genomics in Medicine Chapter 3. Questions & Answers
 Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
N. GOOD NUCLEASE/BAD NUCLEASE
 N. GOOD NUCLEASE/BAD NUCLEASE Background Figure N.1. Simplified scheme for the general acid/base-catalyzed hydrolysis of RNA. A pentavalent intermediate is formed along the pathway. The hydrolysis of RNA
N. GOOD NUCLEASE/BAD NUCLEASE Background Figure N.1. Simplified scheme for the general acid/base-catalyzed hydrolysis of RNA. A pentavalent intermediate is formed along the pathway. The hydrolysis of RNA
GENETICS - CLUTCH CH.7 DNA AND CHROMOSOME STRUCTURE.
 !! www.clutchprep.com COCEPT: DA AS THE GEETIC MATERIAL DA wasn t always thought of as the genetic material To be genetic material, a molecule needs to have certain - Store information - Transmit information
!! www.clutchprep.com COCEPT: DA AS THE GEETIC MATERIAL DA wasn t always thought of as the genetic material To be genetic material, a molecule needs to have certain - Store information - Transmit information
Biochemistry 2000 Midterm 2 1 of 5. Student Name : KEY Student ID :
 Biochemistry 2000 Midterm 2 1 of 5 Student Name : KEY 2014-03-28 Student ID : Instructions: Write neatly and clearly. Cross out with a single line any material you do not wish to have marked. Marks will
Biochemistry 2000 Midterm 2 1 of 5 Student Name : KEY 2014-03-28 Student ID : Instructions: Write neatly and clearly. Cross out with a single line any material you do not wish to have marked. Marks will
Resources. How to Use This Presentation. Chapter 10. Objectives. Table of Contents. Griffith s Discovery of Transformation. Griffith s Experiments
 How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
How to Use This Presentation To View the presentation as a slideshow with effects select View on the menu bar and click on Slide Show. To advance through the presentation, click the right-arrow key or
Nucleic Acids: Structure and Function
 ucleic Acids: Structure and Function Components of ucleotides The building blocks (monomers) of the nucleic acids are called nucleotides. ucleotides are made up of: phosphoric acid, a pentose sugar, and
ucleic Acids: Structure and Function Components of ucleotides The building blocks (monomers) of the nucleic acids are called nucleotides. ucleotides are made up of: phosphoric acid, a pentose sugar, and
MOLECULAR STRUCTURE OF DNA
 MOLECULAR STRUCTURE OF DNA Characteristics of the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell (generation to generation) 2. Information Storage Biologically
MOLECULAR STRUCTURE OF DNA Characteristics of the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell (generation to generation) 2. Information Storage Biologically
Nucleic Acids: How Structure Conveys Information 1. What Is the Structure of DNA? 2. What Are the Levels of Structure in Nucleic Acids? 3.
 Fig. 9-CO, p.215 Nucleic Acids: How Structure Conveys Information 1. What Is the Structure of DNA? 2. What Are the Levels of Structure in Nucleic Acids? 3. What Is the Covalent Structure of Polynucleotides?
Fig. 9-CO, p.215 Nucleic Acids: How Structure Conveys Information 1. What Is the Structure of DNA? 2. What Are the Levels of Structure in Nucleic Acids? 3. What Is the Covalent Structure of Polynucleotides?
Ch 10 Molecular Biology of the Gene
 Ch 10 Molecular Biology of the Gene For Next Week Lab -Hand in questions from 4 and 5 by TUES in my mailbox (Biology Office) -Do questions for Lab 6 for next week -Lab practical next week Lecture Read
Ch 10 Molecular Biology of the Gene For Next Week Lab -Hand in questions from 4 and 5 by TUES in my mailbox (Biology Office) -Do questions for Lab 6 for next week -Lab practical next week Lecture Read
Road to the Double Helix
 Road to the Double Helix Watson and Crick Missing layer means alternating pattern (major & minor groove) Hydrogen bonding A pairs with T G pairs with C Double helix fits the data! Franklin and Wilkins
Road to the Double Helix Watson and Crick Missing layer means alternating pattern (major & minor groove) Hydrogen bonding A pairs with T G pairs with C Double helix fits the data! Franklin and Wilkins
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Supplementary Information for
 Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
How do we know what the structure and function of DNA is? - Double helix, base pairs, sugar, and phosphate - Stores genetic information
 DNA: CH 13 How do we know what the structure and function of DNA is? - Double helix, base pairs, sugar, and phosphate - Stores genetic information Discovering DNA s Function 1928: Frederick Griffith studied
DNA: CH 13 How do we know what the structure and function of DNA is? - Double helix, base pairs, sugar, and phosphate - Stores genetic information Discovering DNA s Function 1928: Frederick Griffith studied
Chapter 9. Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination
 Chapter 9 Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination 1 Genetics Genome Chromosome Gene Protein Genotype Phenotype 2 Terms and concepts gene Fundamental unit of heredity
Chapter 9 Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination 1 Genetics Genome Chromosome Gene Protein Genotype Phenotype 2 Terms and concepts gene Fundamental unit of heredity
Ch Molecular Biology of the Gene
 Ch. 12 - Molecular Biology of the Gene AP BIOLOGY CHAPTER GUIDE 1. In the middle of the unraveling the mysteries of DNA, researchers knew that genetic material must be able to. It must be stable so it
Ch. 12 - Molecular Biology of the Gene AP BIOLOGY CHAPTER GUIDE 1. In the middle of the unraveling the mysteries of DNA, researchers knew that genetic material must be able to. It must be stable so it
DNA and RNA Structure Guided Notes
 Nucleic acids, especially DNA, are considered as the key biomolecules that guarantee the continuity of life. DNA is the prime genetic molecule which carry all the hereditary information that's passed from
Nucleic acids, especially DNA, are considered as the key biomolecules that guarantee the continuity of life. DNA is the prime genetic molecule which carry all the hereditary information that's passed from
Lanthanide cofactors accelerate DNA-catalyzed synthesis of branched RNA
 Supporting Information for Javadi-Zarnaghi and Höbartner page S1 Lanthanide cofactors accelerate DNA-catalyzed synthesis of branched RNA Fatemeh Javadi-Zarnaghi and Claudia Höbartner* Research Group Nucleic
Supporting Information for Javadi-Zarnaghi and Höbartner page S1 Lanthanide cofactors accelerate DNA-catalyzed synthesis of branched RNA Fatemeh Javadi-Zarnaghi and Claudia Höbartner* Research Group Nucleic
What Are the Chemical Structures and Functions of Nucleic Acids?
 THE NUCLEIC ACIDS What Are the Chemical Structures and Functions of Nucleic Acids? Nucleic acids are polymers specialized for the storage, transmission, and use of genetic information. DNA = deoxyribonucleic
THE NUCLEIC ACIDS What Are the Chemical Structures and Functions of Nucleic Acids? Nucleic acids are polymers specialized for the storage, transmission, and use of genetic information. DNA = deoxyribonucleic
Chapter 9: DNA: The Molecule of Heredity
 Chapter 9: DNA: The Molecule of Heredity What is DNA? Answer: Molecule that carries the blueprint of life General Features: DNA is packages in chromosomes (DNA + Proteins) Gene = Functional segment of
Chapter 9: DNA: The Molecule of Heredity What is DNA? Answer: Molecule that carries the blueprint of life General Features: DNA is packages in chromosomes (DNA + Proteins) Gene = Functional segment of
2. True or False? The sequence of nucleotides in the human genome is 90.9% identical from one person to the next.
 1. True or False? A typical chromosome can contain several hundred to several thousand genes, arranged in linear order along the DNA molecule present in the chromosome. 2. True or False? The sequence of
1. True or False? A typical chromosome can contain several hundred to several thousand genes, arranged in linear order along the DNA molecule present in the chromosome. 2. True or False? The sequence of
Nucleic Acids. OpenStax College. 1 DNA and RNA
 OpenStax-CNX module: m44403 1 Nucleic Acids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be
OpenStax-CNX module: m44403 1 Nucleic Acids OpenStax College This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be
Small, highly-active DNAs that hydrolyze DNA
 Supplementary Information Small, highly-active DNAs that hydrolyze DNA Hongzhou Gu,, Kazuhiro Furukawa, Zasha Weinberg,, Daniel F. Berenson, and Ronald R. Breaker*,,, Department of Molecular, Cellular
Supplementary Information Small, highly-active DNAs that hydrolyze DNA Hongzhou Gu,, Kazuhiro Furukawa, Zasha Weinberg,, Daniel F. Berenson, and Ronald R. Breaker*,,, Department of Molecular, Cellular
DNA polymerase proofreading: active site switching catalyzed by the bacteriophage T4 DNA polymerase
 5452 5463 Nucleic Acids Research, 2007, Vol. 35, No. 16 Published online 15 August 2007 doi:10.1093/nar/gkm591 DNA polymerase proofreading: active site switching catalyzed by the bacteriophage T4 DNA polymerase
5452 5463 Nucleic Acids Research, 2007, Vol. 35, No. 16 Published online 15 August 2007 doi:10.1093/nar/gkm591 DNA polymerase proofreading: active site switching catalyzed by the bacteriophage T4 DNA polymerase
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA
 RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
Supplementary Figure 1 Telomerase RNA fragments used in single-molecule FRET experiments. A pseudoknot fragment (nts ) labeled at position U42
 Supplementary Figure 1 Telomerase RNA fragments used in single-molecule FRET experiments. A pseudoknot fragment (nts 32-195) labeled at position U42 with Cy3 (green circle) was constructed by a two piece
Supplementary Figure 1 Telomerase RNA fragments used in single-molecule FRET experiments. A pseudoknot fragment (nts 32-195) labeled at position U42 with Cy3 (green circle) was constructed by a two piece
specificity of a group I intron (ribozyme/self-splicing intron/exon-intron junction)
 Proc. Nadl. Acad. Sci. USA Vol. 86, pp. 7402-7406, October 1989 Biochemistry RNA structure, not sequence, determines the 5' splice-site specificity of a group I intron (ribozyme/self-splicing intron/exon-intron
Proc. Nadl. Acad. Sci. USA Vol. 86, pp. 7402-7406, October 1989 Biochemistry RNA structure, not sequence, determines the 5' splice-site specificity of a group I intron (ribozyme/self-splicing intron/exon-intron
3.A.1 DNA and RNA: Structure and Replication
 3.A.1 DNA and RNA: Structure and Replication Each DNA polymer is made of Nucleotides (monomer) which are made of: a) Phosphate group: Negatively charged and polar b) Sugar: deoxyribose- a 5 carbon sugar
3.A.1 DNA and RNA: Structure and Replication Each DNA polymer is made of Nucleotides (monomer) which are made of: a) Phosphate group: Negatively charged and polar b) Sugar: deoxyribose- a 5 carbon sugar
Structural Bioinformatics (C3210) DNA and RNA Structure
 Structural Bioinformatics (C3210) DNA and RNA Structure Importance of DNA/RNA 3D Structure Nucleic acids are essential materials found in all living organisms. Their main function is to maintain and transmit
Structural Bioinformatics (C3210) DNA and RNA Structure Importance of DNA/RNA 3D Structure Nucleic acids are essential materials found in all living organisms. Their main function is to maintain and transmit
Nucleic Acids. Information specifying protein structure
 Nucleic Acids Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats) Genome - the genetic information of an organism Information
Nucleic Acids Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats) Genome - the genetic information of an organism Information
Supplementary Table 1. Oligonucleotide sequences used in the study
 Supplementary Table 1. Oligonucleotide sequences used in the study Oligonucleotides Sequences (5 3 ) Substrate strand Lock -4 Lock -5 Lock -6 Lock -7 Free control DNAzyme DNAzyme strand linked to AuNP
Supplementary Table 1. Oligonucleotide sequences used in the study Oligonucleotides Sequences (5 3 ) Substrate strand Lock -4 Lock -5 Lock -6 Lock -7 Free control DNAzyme DNAzyme strand linked to AuNP
Information specifying protein structure. Chapter 19 Nucleic Acids Nucleotides Are the Building Blocks of Nucleic Acids
 Chapter 19 Nucleic Acids Information specifying protein structure Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats)
Chapter 19 Nucleic Acids Information specifying protein structure Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats)
translation The building blocks of proteins are? amino acids nitrogen containing bases like A, G, T, C, and U Complementary base pairing links
 The actual process of assembling the proteins on the ribosome is called? translation The building blocks of proteins are? Complementary base pairing links Define and name the Purines amino acids nitrogen
The actual process of assembling the proteins on the ribosome is called? translation The building blocks of proteins are? Complementary base pairing links Define and name the Purines amino acids nitrogen
}Nucleosides NUCLEIC ACIDS. Nucleic acids are polymers Monomer---nucleotides Nitrogenous bases Purines Pyrimidines Sugar Ribose Deoxyribose
 DNA STRUCTURE NUCLEIC ACIDS Nucleic acids are polymers Monomer---nucleotides Nitrogenous bases Purines Pyrimidines Sugar Ribose Deoxyribose Phosphates +nucleoside=nucleotide }Nucleosides The Sugars The
DNA STRUCTURE NUCLEIC ACIDS Nucleic acids are polymers Monomer---nucleotides Nitrogenous bases Purines Pyrimidines Sugar Ribose Deoxyribose Phosphates +nucleoside=nucleotide }Nucleosides The Sugars The
DNA and RNA Structure. Unit 7 Lesson 1
 Unit 7 Lesson 1 Students will be able to: Explain the structure and function of the DNA and RNA. Illustrate the structure of nucleotide. Summarize the differences between DNA and RNA. Identify the different
Unit 7 Lesson 1 Students will be able to: Explain the structure and function of the DNA and RNA. Illustrate the structure of nucleotide. Summarize the differences between DNA and RNA. Identify the different
1/4/18 NUCLEIC ACIDS. Nucleic Acids. Nucleic Acids. ECS129 Instructor: Patrice Koehl
 NUCLEIC ACIDS ECS129 Instructor: Patrice Koehl Nucleic Acids Nucleotides DNA Structure RNA Synthesis Function Secondary structure Tertiary interactions Wobble hypothesis DNA RNA Replication Transcription
NUCLEIC ACIDS ECS129 Instructor: Patrice Koehl Nucleic Acids Nucleotides DNA Structure RNA Synthesis Function Secondary structure Tertiary interactions Wobble hypothesis DNA RNA Replication Transcription
NUCLEIC ACIDS. ECS129 Instructor: Patrice Koehl
 NUCLEIC ACIDS ECS129 Instructor: Patrice Koehl Nucleic Acids Nucleotides DNA Structure RNA Synthesis Function Secondary structure Tertiary interactions Wobble hypothesis DNA RNA Replication Transcription
NUCLEIC ACIDS ECS129 Instructor: Patrice Koehl Nucleic Acids Nucleotides DNA Structure RNA Synthesis Function Secondary structure Tertiary interactions Wobble hypothesis DNA RNA Replication Transcription
C A T T A G C nitrogenous complimentary G T A A T C G to each other
 Name DNA RNA Review Worksheet Date 1. What does DNA stand for? Deoxyribonucleic acid 2. What is DNA s primary function? - Provides a pattern for protein manufacture - Provides a pattern for replication
Name DNA RNA Review Worksheet Date 1. What does DNA stand for? Deoxyribonucleic acid 2. What is DNA s primary function? - Provides a pattern for protein manufacture - Provides a pattern for replication
Molecular Biology (1)
 Molecular Biology (1) DNA structure and basic applications Mamoun Ahram, PhD Second semester, 2017-2018 Resources This lecture Cooper, pp. 49-52, 118-119, 130 What is molecular biology? Central dogma
Molecular Biology (1) DNA structure and basic applications Mamoun Ahram, PhD Second semester, 2017-2018 Resources This lecture Cooper, pp. 49-52, 118-119, 130 What is molecular biology? Central dogma
Chapter 5: Nucleic Acids, etc.
 Chapter 5: Nucleic Acids, etc. Voet & Voet: Sections 1 & 3 Pages 82-84 & 88-93 Any introductory Biochemistry textbook will have an introductory chapter on nucleic acids Slide 1 Nucleotides and Derivatives
Chapter 5: Nucleic Acids, etc. Voet & Voet: Sections 1 & 3 Pages 82-84 & 88-93 Any introductory Biochemistry textbook will have an introductory chapter on nucleic acids Slide 1 Nucleotides and Derivatives
Can have defects in any of the steps in the synthesis of arginine. With arginine in the medium, all arg mutants can grow on minimal medium.
 Molecular Biology I Biochemistry studying a single component in an organism Genetics studying an organism without that component Biochemical Genetics Look at the Arginine biosynthetic pathway: A B C Arginine
Molecular Biology I Biochemistry studying a single component in an organism Genetics studying an organism without that component Biochemical Genetics Look at the Arginine biosynthetic pathway: A B C Arginine
DNA: The Primary Source of Heritable Information. Genetic information is transmitted from one generation to the next through DNA or RNA
 DNA and Replication DNA: The Primary Source of Heritable Information Genetic information is transmitted from one generation to the next through DNA or RNA Chromosomes Non-eukaryotic (bacteria) organisms
DNA and Replication DNA: The Primary Source of Heritable Information Genetic information is transmitted from one generation to the next through DNA or RNA Chromosomes Non-eukaryotic (bacteria) organisms
Catalytically critical nucleotides in domain 5 of a group II intron
 Proc. Natl. Acad. Sci. USA Vol. 92, pp. 4422-4426, May 1995 Biochemistry Catalytically critical nucleotides in domain 5 of a group II intron (RNA splicing/active site/competitive inhibitor) CRAIG L. PEEBLES*t,
Proc. Natl. Acad. Sci. USA Vol. 92, pp. 4422-4426, May 1995 Biochemistry Catalytically critical nucleotides in domain 5 of a group II intron (RNA splicing/active site/competitive inhibitor) CRAIG L. PEEBLES*t,
NUCLEIC ACIDS Genetic material of all known organisms DNA: deoxyribonucleic acid RNA: ribonucleic acid (e.g., some viruses)
 NUCLEIC ACIDS Genetic material of all known organisms DNA: deoxyribonucleic acid RNA: ribonucleic acid (e.g., some viruses) Consist of chemically linked sequences of nucleotides Nitrogenous base Pentose-
NUCLEIC ACIDS Genetic material of all known organisms DNA: deoxyribonucleic acid RNA: ribonucleic acid (e.g., some viruses) Consist of chemically linked sequences of nucleotides Nitrogenous base Pentose-
Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
Fundamentals of Organic Chemistry. CHAPTER 10: Nucleic Acids
 Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 10: Nucleic Acids 2 o Nucleic
Fundamentals of Organic Chemistry CHEM 109 For Students of Health Colleges Credit hrs.: (2+1) King Saud University College of Science, Chemistry Department CHEM 109 CHAPTER 10: Nucleic Acids 2 o Nucleic
DNA: The Molecule Of Life
 DNA: The Molecule Of Life Introductory Concepts -One unique set of DNA in an organism is termed its genome (link to fig 1-3) -DNA is the main component of chromosomes -Humans are diploid organisms, with
DNA: The Molecule Of Life Introductory Concepts -One unique set of DNA in an organism is termed its genome (link to fig 1-3) -DNA is the main component of chromosomes -Humans are diploid organisms, with
Chapter 1 Structure of Nucleic Acids DNA The structure of part of a DNA double helix
 Chapter 1 Structure of Nucleic Acids DNA The structure of part of a DNA double helix Deoxyribonucleic acid ) (DNA) is a nucleic acid that contains the genetic instructions used in the development and functioning
Chapter 1 Structure of Nucleic Acids DNA The structure of part of a DNA double helix Deoxyribonucleic acid ) (DNA) is a nucleic acid that contains the genetic instructions used in the development and functioning
CHAPTER 8 Nucleotides and Nucleic Acids
 CHAPTER 8 Nucleotides and Nucleic Acids Key topics Biological function of nucleotides and nucleic acids Structures of common nucleotides Structure of double-stranded DNA Structures of ribonucleic acids
CHAPTER 8 Nucleotides and Nucleic Acids Key topics Biological function of nucleotides and nucleic acids Structures of common nucleotides Structure of double-stranded DNA Structures of ribonucleic acids
Chapter 12: Molecular Biology of the Gene
 Biology Textbook Notes Chapter 12: Molecular Biology of the Gene p. 214-219 The Genetic Material (12.1) - Genetic Material must: 1. Be able to store information that pertains to the development, structure,
Biology Textbook Notes Chapter 12: Molecular Biology of the Gene p. 214-219 The Genetic Material (12.1) - Genetic Material must: 1. Be able to store information that pertains to the development, structure,
Structure and Function of Nucleic Acids
 Structure and Function of Nucleic Acids E T Nyahangare Class Assignment 1. Write notes and outline the role of the following in protein biosynthesis a. DNA replication b. Transcription c. Genetic code
Structure and Function of Nucleic Acids E T Nyahangare Class Assignment 1. Write notes and outline the role of the following in protein biosynthesis a. DNA replication b. Transcription c. Genetic code
Molecular Genetics. The flow of genetic information from DNA. DNA Replication. Two kinds of nucleic acids in cells: DNA and RNA.
 Molecular Genetics DNA Replication Two kinds of nucleic acids in cells: DNA and RNA. DNA function 1: DNA transmits genetic information from parents to offspring. DNA function 2: DNA controls the functions
Molecular Genetics DNA Replication Two kinds of nucleic acids in cells: DNA and RNA. DNA function 1: DNA transmits genetic information from parents to offspring. DNA function 2: DNA controls the functions
THE CELLULAR AND MOLECULAR BASIS OF INHERITANCE
 Umm AL Qura University THE CELLULAR AND MOLECULAR BASIS OF INHERITANCE Dr. Neda Bogari www.bogari.net EMERY'S ELEMENTS OF MEDICAL GENETICS Peter Turnpenny and Sian Ellard 13 th edition 2008 COURSE SYLLABUS
Umm AL Qura University THE CELLULAR AND MOLECULAR BASIS OF INHERITANCE Dr. Neda Bogari www.bogari.net EMERY'S ELEMENTS OF MEDICAL GENETICS Peter Turnpenny and Sian Ellard 13 th edition 2008 COURSE SYLLABUS
Nucleic Acids. By Sarah, Zach, Joanne, and Dean
 Nucleic Acids By Sarah, Zach, Joanne, and Dean Basic Functions Carry genetic information (DNA storing it) Protein synthesis Helps in cell division (DNA replicates itself) RNA- numerous functions during
Nucleic Acids By Sarah, Zach, Joanne, and Dean Basic Functions Carry genetic information (DNA storing it) Protein synthesis Helps in cell division (DNA replicates itself) RNA- numerous functions during
STUDY GUIDE SECTION 10-1 Discovery of DNA
 STUDY GUIDE SECTION 10-1 Discovery of DNA Name Period Date Multiple Choice-Write the correct letter in the blank. 1. The virulent strain of the bacterium S. pneumoniae causes disease because it a. has
STUDY GUIDE SECTION 10-1 Discovery of DNA Name Period Date Multiple Choice-Write the correct letter in the blank. 1. The virulent strain of the bacterium S. pneumoniae causes disease because it a. has
CHAPTER 4, Part 1: LECTURE TOPICS: DNA and RNA - MOLECULES OF HEREDITY
 Chapter 4 Notes: Part 1 Biochemistry 461 Fall 2010 CHAPTER 4, Part 1: LECTURE TOPICS: DNA and RNA - MOLECULES OF HEREDITY 1) DNA/RNA structures, nomenclature, shorthand conventions 2) DNA and RNA as genetic
Chapter 4 Notes: Part 1 Biochemistry 461 Fall 2010 CHAPTER 4, Part 1: LECTURE TOPICS: DNA and RNA - MOLECULES OF HEREDITY 1) DNA/RNA structures, nomenclature, shorthand conventions 2) DNA and RNA as genetic
5-Formyl- and 5-carboxyl-cytosine reduce the rate and substrate specificity. of RNA polymerase II transcription
 SUPPLEMENTARY INFORMATION FOR 5-Formyl- and 5-carboxyl-cytosine reduce the rate and substrate specificity of RNA polymerase II transcription Matthew W. Kellinger 1, Chunxiao Song 2, Jenny Chong 1, Xingyu
SUPPLEMENTARY INFORMATION FOR 5-Formyl- and 5-carboxyl-cytosine reduce the rate and substrate specificity of RNA polymerase II transcription Matthew W. Kellinger 1, Chunxiao Song 2, Jenny Chong 1, Xingyu
Crystal Structure of a Self-Splicing Group I Intron with Both Exons
 Supplementary Data for: Crystal Structure of a Self-Splicing Group I Intron with Both Exons Peter L. Adams, Mary R. Stahley, Anne B. Kosek, Jimin Wang and Scott A. Strobel The supplementary material includes
Supplementary Data for: Crystal Structure of a Self-Splicing Group I Intron with Both Exons Peter L. Adams, Mary R. Stahley, Anne B. Kosek, Jimin Wang and Scott A. Strobel The supplementary material includes
DNA. translation. base pairing rules for DNA Replication. thymine. cytosine. amino acids. The building blocks of proteins are?
 2 strands, has the 5-carbon sugar deoxyribose, and has the nitrogen base Thymine. The actual process of assembling the proteins on the ribosome is called? DNA translation Adenine pairs with Thymine, Thymine
2 strands, has the 5-carbon sugar deoxyribose, and has the nitrogen base Thymine. The actual process of assembling the proteins on the ribosome is called? DNA translation Adenine pairs with Thymine, Thymine
CH 4 - DNA. DNA = deoxyribonucleic acid. DNA is the hereditary substance that is found in the nucleus of cells
 CH 4 - DNA DNA is the hereditary substance that is found in the nucleus of cells DNA = deoxyribonucleic acid» its structure was determined in the 1950 s (not too long ago).» scientists were already investigating
CH 4 - DNA DNA is the hereditary substance that is found in the nucleus of cells DNA = deoxyribonucleic acid» its structure was determined in the 1950 s (not too long ago).» scientists were already investigating
A. Incorrect! A sugar residue is only part of a nucleotide. Go back and review the structure of nucleotides.
 Organic Chemistry - Problem Drill 24: ucleic Acids o. 1 of 10 1. What are the components of a nucleotide? (A) A sugar residue (B) A sugar residue + a nitrogenous base (C) A sugar residue + a nitrogenous
Organic Chemistry - Problem Drill 24: ucleic Acids o. 1 of 10 1. What are the components of a nucleotide? (A) A sugar residue (B) A sugar residue + a nitrogenous base (C) A sugar residue + a nitrogenous
7 Synthesizing the ykkcd Mutant Toxin Sensor RNA in vitro
 7 Synthesizing the ykkcd Mutant Toxin Sensor RNA in vitro 7.1 Learning Objective In the quest toward understanding how the ykkcd toxin sensor recognizes the antibiotic tetracycline you thus far designed
7 Synthesizing the ykkcd Mutant Toxin Sensor RNA in vitro 7.1 Learning Objective In the quest toward understanding how the ykkcd toxin sensor recognizes the antibiotic tetracycline you thus far designed
Chapter 6. DNA Structure, Replication, and Manipulation
 Chapter 6 DNA Structure, Replication, and Manipulation 1 Genome Size The genetic complement of a cell or virus constitutes its genome. In eukaryotes, this term is commonly used to refer to one complete
Chapter 6 DNA Structure, Replication, and Manipulation 1 Genome Size The genetic complement of a cell or virus constitutes its genome. In eukaryotes, this term is commonly used to refer to one complete
Chapter 13 - Concept Mapping
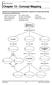 Chapter 13 - Concept Mapping Using the terms and phrases provided below, complete the concept map showing the discovery of DNA structure. amount of base pairs five-carbon sugar purine DNA polymerases Franklin
Chapter 13 - Concept Mapping Using the terms and phrases provided below, complete the concept map showing the discovery of DNA structure. amount of base pairs five-carbon sugar purine DNA polymerases Franklin
Appendix A DNA and PCR in detail DNA: A Detailed Look
 Appendix A DNA and PCR in detail DNA: A Detailed Look A DNA molecule is a long polymer consisting of four different components called nucleotides. It is the various combinations of these four bases or
Appendix A DNA and PCR in detail DNA: A Detailed Look A DNA molecule is a long polymer consisting of four different components called nucleotides. It is the various combinations of these four bases or
Genes to Proteins. Nucleic Acid Structure
 Genes to Proteins Pratt & Cornely Chapter 3 Nucleobase Nucleoside Nucleotide Nucleic acid Chromatin Chromosome Nucleic Acid Structure 1 Base Structure Purines and pyrimidines Aromatic Tautomers Nucleosides
Genes to Proteins Pratt & Cornely Chapter 3 Nucleobase Nucleoside Nucleotide Nucleic acid Chromatin Chromosome Nucleic Acid Structure 1 Base Structure Purines and pyrimidines Aromatic Tautomers Nucleosides
Chapter Fundamental Molecular Genetic Mechanisms
 Chapter 5-1 - Fundamental Molecular Genetic Mechanisms 5.1 Structure of Nucleic Acids 5.2 Transcription of Protein-Coding Genes and Formation of Functional mrna 5.3 The Decoding of mrna by trnas 5.4 Stepwise
Chapter 5-1 - Fundamental Molecular Genetic Mechanisms 5.1 Structure of Nucleic Acids 5.2 Transcription of Protein-Coding Genes and Formation of Functional mrna 5.3 The Decoding of mrna by trnas 5.4 Stepwise
Unit II Problem 3 Genetics: Summary of Basic Concepts in Molecular Biology
 Unit II Problem 3 Genetics: Summary of Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated
Unit II Problem 3 Genetics: Summary of Basic Concepts in Molecular Biology - The central dogma (principle) of molecular biology: Information from DNA are transcribed to mrna which will be further translated
DNA Replication. Packet #17 Chapter #16
 DNA Replication Packet #17 Chapter #16 1 HISTORICAL FACTS ABOUT DNA 2 Historical DNA Discoveries 1928 Frederick Griffith finds a substance in heat-killed bacteria that transforms living bacteria 1944 Oswald
DNA Replication Packet #17 Chapter #16 1 HISTORICAL FACTS ABOUT DNA 2 Historical DNA Discoveries 1928 Frederick Griffith finds a substance in heat-killed bacteria that transforms living bacteria 1944 Oswald
Nucleic Acids: DNA and RNA
 Nucleic Acids: DNA and RNA Living organisms are complex systems. Hundreds of thousands of proteins exist inside each one of us to help carry out our daily functions. These proteins are produced locally,
Nucleic Acids: DNA and RNA Living organisms are complex systems. Hundreds of thousands of proteins exist inside each one of us to help carry out our daily functions. These proteins are produced locally,
DNA Structure. DNA: The Genetic Material. Chapter 14
 DNA: The Genetic Material Chapter 14 DNA Structure DNA is a nucleic acid. The building blocks of DNA are nucleotides, each composed of: a 5-carbon sugar called deoxyribose a phosphate group (PO 4 ) a nitrogenous
DNA: The Genetic Material Chapter 14 DNA Structure DNA is a nucleic acid. The building blocks of DNA are nucleotides, each composed of: a 5-carbon sugar called deoxyribose a phosphate group (PO 4 ) a nitrogenous
Catalytic cleavage of cis- and trans-acting antigenomic delta ribozymes in the presence of various divalent metal ions
 4482 4492 Nucleic Acids Research, 2001, Vol. 29, No. 21 2001 Oxford University Press Catalytic cleavage of cis- and trans-acting antigenomic delta ribozymes in the presence of various divalent metal ions
4482 4492 Nucleic Acids Research, 2001, Vol. 29, No. 21 2001 Oxford University Press Catalytic cleavage of cis- and trans-acting antigenomic delta ribozymes in the presence of various divalent metal ions
MBioS 503: Section 1 Chromosome, Gene, Translation, & Transcription. Gene Organization. Genome. Objectives: Gene Organization
 Overview & Recap of Molecular Biology before the last two sections MBioS 503: Section 1 Chromosome, Gene, Translation, & Transcription Gene Organization Joy Winuthayanon, PhD School of Molecular Biosciences
Overview & Recap of Molecular Biology before the last two sections MBioS 503: Section 1 Chromosome, Gene, Translation, & Transcription Gene Organization Joy Winuthayanon, PhD School of Molecular Biosciences
Figure A summary of spontaneous alterations likely to require DNA repair.
 DNA Damage Figure 5-46. A summary of spontaneous alterations likely to require DNA repair. The sites on each nucleotide that are known to be modified by spontaneous oxidative damage (red arrows), hydrolytic
DNA Damage Figure 5-46. A summary of spontaneous alterations likely to require DNA repair. The sites on each nucleotide that are known to be modified by spontaneous oxidative damage (red arrows), hydrolytic
Drug DNA interaction. Modeling DNA ligand interaction of intercalating ligands
 Drug DNA interaction DNA as carrier of genetic information is a major target for drug interaction because of the ability to interfere with transcription (gene expression and protein synthesis) and DNA
Drug DNA interaction DNA as carrier of genetic information is a major target for drug interaction because of the ability to interfere with transcription (gene expression and protein synthesis) and DNA
Supporting Information
 Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany page S1 Modulation of DNA Constraints that Control Macromolecular Folding Chandrasekhar V. Miduturu and Scott K. Silverman* Department of Chemistry,
Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany page S1 Modulation of DNA Constraints that Control Macromolecular Folding Chandrasekhar V. Miduturu and Scott K. Silverman* Department of Chemistry,
What does DNA stand for?
 DNA and RNA What does DNA stand for? DNA = deoxribonucleic acid NOTE: the DNA from one cell would stretch 3 metre DNA are coiled and folded. DNA has two strands. What four bases are used in DNA? The four
DNA and RNA What does DNA stand for? DNA = deoxribonucleic acid NOTE: the DNA from one cell would stretch 3 metre DNA are coiled and folded. DNA has two strands. What four bases are used in DNA? The four
4) separates the DNA strands during replication a. A b. B c. C d. D e. E. 5) covalently connects segments of DNA a. A b. B c. C d. D e.
 1) Chargaff's analysis of the relative base composition of DNA was significant because he was able to show that a. the relative proportion of each of the four bases differs from species to species. b.
1) Chargaff's analysis of the relative base composition of DNA was significant because he was able to show that a. the relative proportion of each of the four bases differs from species to species. b.
Nucleic Acids. The Ribose Sugar
 Nucleic cids adenosine triphosphate (TP) a mononucleotide Nucleic acids consist of: a ribose (aldopentose) sugar; a heteroaromatic, glycosidic base; a phosphoester. ribonucleic acid (RN) a polynucleotide
Nucleic cids adenosine triphosphate (TP) a mononucleotide Nucleic acids consist of: a ribose (aldopentose) sugar; a heteroaromatic, glycosidic base; a phosphoester. ribonucleic acid (RN) a polynucleotide
