Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency
|
|
|
- Audrey Page
- 6 years ago
- Views:
Transcription
1 Resource Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency Graphical Abstract Authors Yannick Simoni, Michael Fehlings, Henrik N. Kløverpris,..., Iain Beehuat Tan, Florent Ginhoux, Evan W. Newell Correspondence (Y.S.), (E.W.N.) In Brief Animal models have highlighted the importance of innate lymphoid cells (ILCs) in multiple immune responses. Simoni et al. (2016) profile human ILCs using mass cytometry across tissues. The results provide a global, comprehensive, and detailed description of ILC populations and their heterogeneity across individuals and tissues. Highlights d Comprehensive profiling of human ILCs across tissues d d d Detailed description of previously defined ILC subsets except helper-type ILC1 ieilc1-like cells are present in several tissues and functionally similar to NK cells Identification of markers expressed on ILCs, including functional IL-18R Simoni et al., 2017, Immunity 46, January 17, 2017 ª 2017 Elsevier Inc.
2 Immunity Resource Human Innate Lymphoid Cell Subsets Possess Tissue-Type Based Heterogeneity in Phenotype and Frequency Yannick Simoni, 1, * Michael Fehlings, 1 Henrik N. Kløverpris, 2,3 Naomi McGovern, 1 Si-Lin Koo, 4 Chiew Yee Loh, 1 Shawn Lim, 1 Ayako Kurioka, 5 Joannah R. Fergusson, 5 Choong-Leong Tang, 6 Ming Hian Kam, 6 Koh Dennis, 6,7 Tony Kiat Hon Lim, 8 Alexander Chung Yaw Fui, 9 Chan Weng Hoong, 10 Jerry Kok Yen Chan, 11,12 Maria Curotto de Lafaille, 1,15 Sriram Narayanan, 13 Sonia Baig, 14 Muhammad Shabeer, 14 Sue-Anne Ee Shiow Toh, 12,14,16,17 Henry Kun Kiaang Tan, 18 Rosslyn Anicete, 18 Eng-Huat Tan, 4 Angela Takano, 8 Paul Klenerman, 5 Alasdair Leslie, 2 Daniel S.W. Tan, 4,19 Iain Beehuat Tan, 4,12,19 Florent Ginhoux, 1 and Evan W. Newell 1,20, * 1 Agency for Science, Technology and Research (A*STAR), Singapore Immunology Network (SIgN), Singapore 2 Africa Health Research Institute (AHRI), 4001 Durban, South Africa 3 Department of Immunology and Microbiology, University of Copenhagen, 1165 Copenhagen, Denmark 4 Division of Medical Oncology, National Cancer Centre Singapore (NCCS), Singapore 5 Peter Medawar Building for Pathogen Research, University of Oxford, OX1 3SY Oxford, UK 6 Department of Colorectal surgery, Singapore General Hospital, Singapore 7 Colorectal Practice, Mount Elizabeth Medical Centre, Singapore 8 Department of Anatomical Pathology, Singapore General Hospital, Singapore 9 Department of Hepatopancreatobiliary/Transplant Surgery, Singapore General Hospital, Singapore 10 Department of Upper GI/Bariatric Surgery, Singapore General Hospital, Singapore 11 KK Women s and Children s Hospital, Department of Reproductive Medicine, Singapore 12 Duke-NUS Graduate Medical School, Singapore 13 Agency for Science, Technology and Research (A*STAR), Institute of Molecular and Cell Biology (IMCB), Singapore 14 Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore 15 New York University School of Medicine, New york, USA 16 Department of Medicine, National University Health System, Singapore 17 Perelman School of Medicine, University of Pennsylvania, USA 18 KK Women s and Children s Hospital, Department of Otolaryngology, Singapore 19 Agency for Science, Technology and Research (A*STAR), Genome Institute of Singapore (GIS), Singapore 20 Lead Contact *Correspondence: yannick_simoni@immunol.a-star.edu.sg (Y.S.), evan_newell@immunol.a-star.edu.sg (E.W.N.) SUMMARY Animal models have highlighted the importance of innate lymphoid cells (ILCs) in multiple immune responses. However, technical limitations have hampered adequate characterization of ILCs in humans. Here, we used mass cytometry including a broad range of surface markers and transcription factors to accurately identify and profile ILCs across healthy and inflamed tissue types. High dimensional analysis allowed for clear phenotypic delineation of ILC2 and ILC3 subsets. We were not able to detect ILC1 cells in any of the tissues assessed, however, we identified intra-epithelial (ie)ilc1-like cells that represent a broader category of NK cells in mucosal and non-mucosal pathological tissues. In addition, we have revealed the expression of phenotypic molecules that have not been previously described for ILCs. Our analysis shows that human ILCs are highly heterogeneous cell types between individuals and tissues. It also provides a global, comprehensive, and detailed description of ILC heterogeneity in humans across patients and tissues. INTRODUCTION Innate lymphoid cells (ILCs) represent a heterogeneous population of innate immune cells in mice and humans that include classically defined natural killer (NK) cells as well as more recently described helper-type ILCs (ILC1, ILC2, and ILC3) (Artis and Spits, 2015; Cella et al., 2009; Satoh-Takayama et al., 2008). These cells can be involved in infection, chronic inflammation, and in metabolic disease (Artis and Spits, 2015; Diefenbach et al., 2014; McKenzie et al., 2014). In contrast to T- and B-lymphocytes, ILCs lack somatic rearrangement of antigen receptors (e.g., TCR, BCR), and can be activated by cytokines and/or through natural cytotoxicity receptors (e.g., NKp44) (Glatzer et al., 2013). In contrast to NK cells, helper-type ILCs do not possess efficient cytotoxic capacity (Hazenberg and Spits, 2014). In mice and humans, helper-type ILCs can be subdivided into three main groups analogous to the main subsets of T helper lymphocytes. ILC1 cells can produce T helper-1 (Th1) cell signature cytokines (i.e., IFN-g) and express the transcription factor T-bet (Bernink et al., 2013), whereas ILC2 cells are capable of secreting Th2 cell-type 148 Immunity 46, , January 17, 2017 ª 2017 Elsevier Inc.
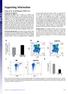
3 A B C D E Figure 1. t-sne Analysis Objectively Delineates ILCs Populations with the Exception of ILC1 Cells (A) Visualized t-sne map of cells isolated from cord blood, tonsil, and colon tissue. t-sne was performed after depleting B and T cells and gating on live CD45+ Lin (FcεR1a CD14 CD19 CD123, see Figure S1A for gating strategy). Clusters (cells populations) were identified by manual gating. Data shown are representatives of one donor per tissue from 3 to 10 independent experiments. (legend continued on next page) Immunity 46, , January 17,
4 cytokines and display a GATA3 + phenotype. ILC3 cells have been shown to be associated with interleukin-17 (IL-17) and IL-22 production, as well as RORgt transcription factor expression, characteristic of Th17 helper T cells (Hazenberg and Spits, 2014). Moreover, similar to ILC2 cells, ILC3 cells express CD127 (IL-7R), which is crucial for their development and survival (Vonarbourg and Diefenbach, 2012). Murine models have demonstrated that ILC subset diversity, assessed using single analyses including mass cytometry, can be heavily influenced by pathogens exposure (Gury-BenAri et al., 2016). ILC1 cells have been controversially discussed in the literature due to their similarity with NK cells (Serafini et al., 2015). In mice and humans, two non-nk cell ILC1 cell subsets have been described. One subset expresses CD127 but no cytotoxic granules and plays a role in intestinal pathologies (Bernink et al., 2013; McKenzie et al., 2014). The other subset has been described as intraepithelial ILC1 cells (ieilc1) that do not express CD127, but are capable of producing cytotoxic granules (Fuchs et al., 2013). Recently, a transcriptomic in-depth analysis of murine ILC subsets has demonstrated that gene-expression patterns of ILC1 cells overlap with NK cells. This raises the question of whether NK cells and ILC1 cells can truly be classified as distinct cell types (Robinette et al., 2015). In humans, single-cell mrna analysis has shown that ILC1 cells express transcripts that encode variable regions of the TCR, as well as various other specific T cell markers (Björklund et al., 2016). Consistent with this, another recent paper has shown that the majority of ILC1 cells also express CD5, which is expressed by T cells but not ILCs (Vely et al., 2016). Murine models have helped to effectively classify ILCs, especially since similarities have been observed between ILCs identified in mice and humans. However, differences in murine and human immunology pose a challenge. The characterization of human ILCs is mainly based on ex vivo analysis and on in vitro-derived cell lines (Bernink et al., 2013) and although human specimens have been used to analyze helper-type ILCs in different tissues (Hazenberg and Spits, 2014), studies analyzing all known ILC subsets for individual tissues in parallel are lacking. In addition, Hazenberg and Spits have demonstrated that a clear definition of ILC2 and ILC3 populations in humans by multi-color flow cytometry requires at least eight fluorescence channels (Hazenberg and Spits, 2014). Consequently, technical limitations of flow cytometry devices such as spectral overlap hamper a further in-depth characterization of these cells. Moreover, reported discrepancies in defining ILC subsets between studies have complicated an accurate comparison of these cells. Here, we use mass cytometry (cytometry by time of flight, CyTOF), to analyze NK, ILC1, ieilc1, ILC2, and ILC3 cells in parallel across nine different healthy tissues and three pathological tissues. To these ends, we simultaneously assessed a wide range of surface markers, cytotoxic granule components, transcription factors, and activation and proliferation markers on a single cell basis. Our data provide a clear global picture of ILC diversity and heterogeneity in humans. RESULTS Mass Cytometry Allows the Identification of Human ILC Subsets To resolve inconsistencies in ILC subset definitions, we developed a mass cytometry panel consisting of 38 heavy metallabeled antibodies to identify and characterize ILC populations in humans. This panel included a broad range of phenotypic markers that allow segregation of previously described ILC subsets. Additional markers allowed for simultaneous quantification of lineage defining transcription factors as well as other phenotypic and functional cellular proteins. To demonstrate this approach, we used cord blood, tonsils, and colon specimen because these tissues are known to contain all ILC subsets (NK, ieilc1, ILC1, ILC2, and ILC3 cells). Prior to CyTOF acquisition, T cells (CD3 + ) and B cells (CD20 + ) were depleted. Using standard biaxial gating, we further excluded monocytes (CD14 + ), plasmacytoid dendritic cells (pdc) (CD123 + ), mast and/or basophils (FcεR1a + ), as well as contaminating B cells (CD19 + ) from live immune cells (Cisplatin DNA + CD45 + )(Figure S1A). To assess ILC profiles, we also performed t-distribution Stochastic Neighbor Embedding (t-sne) analysis. t-sne projects high dimensional data into low dimensional space by making a pairwise comparison of cellular phenotypes to optimally plot similar cells close to each other (see Supplemental Experimental Procedure). For our analysis, we used t-sne to reduce 29 parameters (dimensions) into two dimensions (t-sne1 and t-sne2). Based on this projection, we identified up to 13 distinct cell clusters in each tissue (Figure 1A). For each cluster, the median intensity of each single marker molecules assessed was quantified and summarized by using a heat-map illustration (Figure 1B). We identified a group of clusters corresponding to contaminating CD4 + T cells (CD5 + CD4 + ), CD8 + T cells (CD5 + CD8 + ), hematopoietic stem cells (HSC) (CD34 + ), and dendritic cells (DC) (HLA-DR + CD11c + ), as well as unidentified cell populations (clusters 13 in tonsils and 9 in colon tissue, which did not express any known ILC marker. This analysis also segregated ILC2 cells (green cluster, CD127 + CRTH2 + KLRG1 + CD25 + GATA3 high ) and ILC3 cells (blue clusters, CD127 + CRTH2 NKp44 + or ), as well as various NK cell subsets (orange clusters, CD56 + NKp46 + T-bet + CD16 + Granzyme + Perforin + ). Notably, in tonsils, we observed a cluster that shared all characteristics with previously described mucosal associated ieilc1 cells, which was consistent across different (B) Based on the clusters identified in (A), median intensities for each of the marker were calculated and plotted as a heatmap to identify the respective ILC populations. Data shown are from donor represented in (A). (C) Identification of putative ILC1 cells using standard bi-axial gating (CD45 + Lin CD94 CD127 CRTH2 c-kit NKp44 ) from tonsil. Data shown are from donor represented in (A). (D) Histogram of T-bet expression by putative ILC1 cells in tonsils. Cell clusters corresponding to ILC2, ILC3, and NK cells were used as controls. Data shown are from donor represented in (A). (E) Visualization of all different ILC subsets identified by standard bi-axial gating by t-sne. Data shown are from donor represented in (A). Please also see Figures S1 and S Immunity 46, , January 17, 2017
5 A B C D E F H G I (legend on next page) Immunity 46, , January 17,
6 donors (cluster 9 CD56 + T-bet + CD103 + NKp44 + CD94 +, Figures S1B and S1C) (Fuchs et al., 2013). Helper-Type ILC1 Cells Are Undetectable in Human Tissues None of the clusters determined by t-sne analysis was in line with previous ILC1 cell definitions. To reconcile our findings with previous observations, we used a standard bi-axial gating strategy (CD45 + Lin CD94 CD127 + CRTH2 c-kit NKp44 ) (Bernink et al., 2013) to delineate putative ILC1-like cells (Figure 1C). However, none of these cells expressed T-bet, a defining signature transcription factor that should be expressed by all cells in the ILC1 lineage (Bernink et al., 2013) (Figure 1D). When we plotted these putative ILC1 cells using the t-sne, no distinct cluster representing ILC1 cells was observed. Instead, these cells overlapped with T cells, dendritic cells, and ILC3 cell clusters (Figure 1E). In contrast, we observed that manual gating on ILC2 cells, NKp44 ILC3 or NKp44 + ILC3 cells resulted in the identification of independent individual clusters on the twodimensional t-sne projection map (Figure 1E). Similar findings were observed when analyzing cord blood, colon tissue, or fresh tissues (Figures S2A S2C). In all cases, we detected the expression of CD5 (contaminating CD4 + or CD8 + T cells), CD11c (contaminating DC) or CD34 (contaminating HSC) on putative ILC1 cells. It is plausible that contamination with T cells, DCs, ILC3 cells, HSCs, and NK cells might have resulted in an inaccurate definition of ILC1 cells in previous studies. This hypothesis was further supported by the observation of an NK cell population that expressed low amount of CD127 and shared other phenotypic characteristics of ILC1 cells (CD127 + T-bet + CRTH2 c-kit NKp44 ) (Figure S2D). However, these cells expressed key markers that characterize NK cells but should not be found on helper type ILC1 cells, such as CD94, CD56, NKp46, and perforin (Bernink et al., 2013). An ieilc1-like Population Can Be Found that Is Not Restricted to Mucosal Tissues and Displays Similarity to NK Cells Intraepithelial (ie)ilc1 cells have previously been described as cells present in mucosal tissues with an intraepithelial location, producing IFN-g and cytotoxic granules and having an unique integrin-expression profile and implicated in Crohn s disease (Fuchs et al., 2013). We also found a cell population displaying phenotypic characteristics of ieilc1 cells in non-mucosal pathological tissues (hereby referred to as ieilc1-like cells). Using t-sne, we identified a single cluster of these cells in pathological omentum adipose tissues (omentum AT) from obese patients (Ouchi et al., 2011), lung tumors, and colorectal tumors (Figure 2A). Compared to NK cells from the same tissue, these ieilc1-like cells expressed CD103, the transcription factors T-bet and EOMES, as well as surface NK cell markers CD56, NKp46, CD94, 2B4, CD161, CD160, CD122, CD69, and CD49a, but did not express CD16 or CD127 (Figures 2B, 2C, and S3A). On the basis of this analysis, we found that CD103 was consistently expressed on this population, implying that CD103 can be used to identify these cells by standard biaxial gating (Figure S3B). NKp44 is a marker that has been used to characterize ieilc1 cells (Bernink et al., 2015; Fuchs et al., 2013). However, we found that ieilc1-like cells heterogeneously expressed this marker across different individuals and tissues (Figure 2D). Unlike in tonsils where more than 75% of these cells expressed NKp44, frequencies of cells expressing this marker were lower and more variable in colon tissue, colorectal tumors, and lung tumors. Moreover, in omentum AT, NKp44 expression was almost absent on ieilc1-like cells (Figure 2D, lower panel). Using t-sne, we observed that the ieilc1-like cells did not entirely cluster together and were strongly associated with various NK cell populations (Figure 2A), thus suggesting a close similarity between these two populations. To explore this hypothesis, we investigated functional characteristics of these cells. Ex vivo ieilc1-like cells produced granzyme B and perforin but showed lower and more variable amounts of both cytotoxic granules as compared to CD103 NK cells of the same individual (Figure 2E). We found that both ieilc1-like and CD103 NK cells, expressed IL-18R (Figure 2F) and mrna coding for IL-15Ra (IL15RA) (Figures 2G and S3C). Consistent with previous ieilc1 studies (Fuchs et al., 2013), we also found that ieilc1-like cells derived from various tissues produced IFN-g in response to IL-15 or IL-18 stimulation at Figure 2. ieilc1-like Cells Are Present in Non-Mucosal Inflamed Tissues (A) Visualized t-sne map of cells from omentum adipose tissue (AT), lung tumor, and colorectal tumor. t-sne was performed after depleting B and T cells and gating on live CD45 + Lin (FcεR1a CD14 CD19 CD123 ). Cluster corresponding to NK cells is represented in orange. Data representative of one donor by tissue from 3 to 5 independent experiments. (B) Frequencies of cells positive for each marker molecule assessed. Based on the gates shown in (A), the frequencies of positive cells for each marker were calculated and represented as a heatmap. (C) Dot plots showing markers expressed by ieilc1-like cells. According to the clusters identified by t-sne (A) ieilc1-like cells (red) were displayed on a biaxial dot plot. CD45 + Lin (gray) cells were used as controls. Representative data from one donor (lung tumor) is shown from 3 to 5 independent experiments. (D) Top shows dot plots representing NKp44 expression by ieilc1 cells from three colorectal tumor samples (three donors). Data shown is gated on NK and ieilc1-like cells (see gating Figure S3B). Bottom shows expression of NKp44 by ieilc1 cells from tonsil, colon, omentum AT, lung, and colorectal tumor tissue (for gating see Figure S3B). Data shown are the mean values ± SD from at least 3 independent experiments. (E) Ex vivo expression of perforin and granzymeb by ieilc1 cells (red) and NK cells (orange) from omentum AT, lung, and colorectal tumor tissue. Helper type ILCs (gray) were used as controls (for gating see Figure S4A). Data shown are one representative from three different donors. (F) Dot plot representing expression of IL18Ra on ieilc1 (red) and CD103 NK cells (orange). Representative data from one individual from 3 independent experiments. (G) IL15RA mrna expression of ieilc1 and CD103 NK cells (see sorting gating strategy Figure S3C), means ± SD, n = 4. (H and I) Frequencies of IFN-g producing ieilc1-like or CD103 NK cells from PBMC, tonsil, colon, lung tumor, and colorectal tumor tissue following stimulation with IL-12 and IL-15 (H) or IL-12 and IL-18 (I). Data shown are the mean values ± SD from 3 independent experiments. n/a (data not available) (see Figure S3D for staining). Please also see Figure S Immunity 46, , January 17, 2017
7 A C B D E F G Figure 3. t-sne Analysis Objectively Delineates Helper-Type ILC Populations (A) Visualized t-sne map of cells from tonsil tissue. t-sne was performed after depleting B and T cells and gating on live CD45+ Lin (FcεR1a CD14 CD19 CD123 ). Manual gating identified three clusters corresponding to ILC2 (green), NKp44 ILC3 (light blue), and NKp44+ ILC3 (dark blue) cells. Data shown is from three donors. (B) A second round of t-sne analysis (t-sne #2) was applied to zoom-in exclusively on ILC2 and ILC3 cells cluster (black circle, A). Normalized expression intensities of each marker were calculated and overlayed on the t-sne plot (see Figure S4A for others markers). (legend continued on next page) Immunity 46, , January 17,
8 comparable quantities as detected for CD103 NK cells derived from the same tissues (Figures 2H and 2I, S3D). Human ILC2 and ILC3 cells Cannot Exclusively Be Defined by Transcription Factors GATA3 and RORgt To investigate the heterogeneity of helper type ILC subsets, we performed a second round of t-sne analysis (t-sne #2) to zoom-in exclusively on ILC2 and ILC3 cells (Figures 3A and 3B). This gave rise to the identification of a homogeneous and distinct cluster of cells that matched the previous definitions of ILC2 cells (CD127 + CD161 + CRTH2 + KLRG1 + )(Mjösberg et al., 2011) (Figure 3C). A higher degree of heterogeneity could be observed within the ILC3 cellular compartment (CD127 + CRTH2 )(Figures 3B and S4A). For instance, we observed a diverse expression of markers such as CD56, ICOS, 2B4, and natural cytotoxicity receptors (NCRs) such as NKp30, NKp46, and NKp44 across this plot (Figures 3B, S4A). Consistent with previous reports, we noted that cells could clearly be segregated based on their expression of NKp44 and this also correlated with other markers such as CD25 and ICOS (Figure 3A). It is clear, however, that these two cell subsets of ILC3 cells (NKp44 positive versus negative) were highly diverse in their expression of other NCRs that we tested (NKp30 and NKp46). This is in contrast to murine cells, where NCR expression in general has been used to segregate two major subsets of ILC3 cells. These findings suggest that human ILC3 cell subsets cannot be identified based on NCR expression alone. Nonetheless, on the basis of this analysis and the differences observed between tissues (e.g., absence of NKp44 + ILC3 cells in blood, or cord blood, Figure 1), we think that it is useful to segregate the two major subsets of ILC3 cells based on NKp44 expression. We also observed, although to a lesser degree, some diversity in terms of CD161 expression (Figure 3C). In particular, we found that NKp44 ILC3 cells displayed a broad distribution of CD161 and c-kit expression, which we think precludes the utility of these markers as defining features of this ILC3 population (Figure 3C). To assure that the t-sne analysis was reliable in the delineation of these helper type ILC subsets, we also investigated transcription factor expression in these cells. In comparison to NK and ILC3 cells, we found that ILC2 cells expressed significantly higher amount of transcription factor GATA3 (Figures 3D and 3E and S3A). This was further confirmed by measuring of the mrna amount of sorted cells and we found that both, NK and ILC3 cells expressed transcripts for GATA3 (Figure 3F). This observation is consistent with previous reports in mice (Zhong et al., 2015) and demonstrates that GATA3 is highly expressed by ILC2 cells, but cannot exclusively be used to distinguish ILC2 cells from other ILC types. We detected the expression of RORgt in both ILC3 subsets by using either antibody staining or quantitative gene-expression analysis of pre-sorted cells. Notably, NKp44 + ILC3 cells expressed higher amounts of RORgt protein and RORC mrna transcripts than NKp44 ILC3 cells (Figures 3D 3F). We further found that T-bet and TBX21 mrna expression were restricted to NK cells and was not detected for ILC2 and ILC3 cells. In summary, based on the high-dimensional perspective provided by these data and the t-sne analysis, we found that helper-type ILCs cannot be defined solely by the expression of different transcription factors. First, due to the weak RORgt antibody stainings (especially for NKp44 ILC3 cells, see Discussion), it is not possible to accurately discern ILC3 cells by using this marker in humans. Similarly, because GATA3 is expressed by other ILC subsets, the usage of this marker alone would not be an accurate approach to discriminate ILC2 cells from other ILC subsets. Thus, among helper-type ILCs (CD45 + Lin CD11c CD5 CD34 CD127 + CD94 ), ILC2 cells can be defined as CD161 + CRTH2 +, and ILC3 cells can be defined as CRTH2 which can further be divided into two major subpopulations based on the expression of NKp44 (Figure S4B). It is noteworthy, that the use of exclusion markers is important to remove contaminating cells, especially CD5 and CD11c. Based on these observations, we developed a panel and a gating strategy, which can be used by flow cytometry to identify helper-type ILC subsets in different tissues (Figure 3G, Experimental Procedures). Human ILC2 and ILC3 Cells Are Under-Represented in Non-mucosal and Lung Tissues Next, we analyzed the frequencies and phenotypic profiles of each of the ILC subsets across nine different non-pathological and three different pathological tissues by mass cytometry. Therefore, we used the gating strategies described above to simultaneously analyze all ILC subsets (Figure S4B). Due to the large sample size, we were unable to collect data for all different tissues simultaneously in a single batch of experiment, which is needed for an accurate t-sne analysis. We consequently used manual gating to quantify the frequencies of cells expressing marker molecules fitting the definitions of each ILC subset described for each of the cellular proteins measured across tissues and donors. In non-pathological tissues, we observed a clear distinction between mucosal and non-mucosal tissues (except for lung) with regard to the frequencies of different ILC subsets. In nonmucosal tissues (PBMCs, cord blood, bone marrow, spleen) and lung, ILC2, and ILC3 cells represented only a minority of the total ILC numbers (0.1% to 5%). In these tissues, NKp44 + ILC3 cells represented less than 0.5% of all ILCs. It is notable though, that the frequencies of ILC2 and NKp44 ILC3 cells (C) Representation of marker expression by standard bi-axial gating according to the gates shown in (A). (D) Expression of T-bet, GATA3, and RORgt by each ILC population. HSC (CD34 + ) were used as negative control. Representative data from one donor (tonsil) is shown. (E) Normalized mean signal intensities (MSI) of T-bet, GATA3, or RORgt of each ILC population. Data from >5 independent experiments, n = 17 tonsil tissue, paired t test, ns, non significant, *p % 0.05, **p % 0.01, ****p % (F) mrna expression of TBX21, GATA3, and RORC of each ILC population. Data from one experiment, n = 3 tonsil tissue, unpaired t test, ns, non significant, *p % 0.05, **p % (G) Flow cytometry based gating strategy for the identification of each helper-type ILC population from PBMCs and tonsil. Lineage markers: CD14, CD5, CD19, FcεR1a, CD123, CD11c. 154 Immunity 46, , January 17, 2017
9 A B C Figure 4. Frequencies of ILC Populations in Human Tissues (A) Pie charts displaying the frequencies of NK (orange), ieilc1-like (red), ILC2 (green), NKp44 ILC3 (light blue), and NKp44 + ILC3 (dark blue) cells in human tissues. Data shown are the mean values from at least 9 independent experiments, Nonpathological tissues included PBMC (n = 15), cord blood (n = 3), bone marrow (n = 4), spleen (n = 6), lung (n = 6), skin (n = 4), tonsil (n = 9), adenoid (n = 4), and colon (n = 9) tissue. Pathological tissues included omentum AT (n = 4), lung tumor (n = 6), and colorectal tumor (n = 7) (Table S1). (B) Heatmap displaying the frequencies of positive cells for markers expressed by ILC2 and NKp44 ILC3 cells. Data shown are PBMCs (n = 10) from 4 independent experiments. (C) Histograms displaying the percentages of ILC2 and NKp44 ILC3 cells expressing markers. Data shown are PBMCs from 3 different healthy donors from 4 independent experiments. Please also see Figure S4. were higher in cord blood as compared to adult blood (2.7% versus 0.63%, respectively, for ILC2 and 3.09% versus 1.05% for NKp44 ILC3 cells) (Figure 4A and Table S1). In lung, ILC2 and ILC3 cells represented less than 3% of total innate lymphoid cells (mostly composed of NK cells). In mucosal tissues (tonsil, adenoid, colon) and skin, helper-type ILC frequencies were higher than those of NK cells. As described above, among non-pathological tissues we found ieilc1-like cells only in tonsils (5% ± 4.1%) and colon tissue (25.1% ± 17%). NKp44 + ILC3 cells were highly represented (>14% of total ILCs) in these barrier tissues (tonsil, adenoid, colon, skin). In human skin, ILC2 cells represented the main population of ILCs, similar to what has been observed in murine dermis (Roediger et al., 2013). In pathological tissues, we observed ILC2 and ILC3 cell recruitment in all tissues studied (colorectal tumors, lung tumors, and omemtum AT). However, more than 95% of ILCs infiltrating these inflamed tissues were composed of NK and ieilc1-like cells (Figure 4A). As reflected by the standard deviation, we observed a remarkable heterogeneity in ILC frequencies between individuals that were higher in mucosal and skin tissues as compared to other tissues (Table S1). Despite this variation, the general ILC composition of each tissue was relatively constant (e.g., in all individuals helper-type ILCs outnumbered NK cells in colon, tonsil, adenoid, and skin). In addition to the overall ILC and NK cell subset composition, we observed phenotypic variations across different donors. Due to limitations in sample size and patient data from tissues, we highlight donor-to-donor variations by using ten PBMC samples from healthy donors without infection, chronic disease, or medical treatments (see Experimental Procedures). We observed a high degree of variation in the expression of c-kit, CD56, CCR4, CCR6, 2B4, NKp30, and NKp46. Expression of some of the markers assessed, such as CD160 (not previously described for ILC), were observed only in few patients (Figures 4B and 4C). As compared to murine studies, genetic variation and environmental exposure can greatly influence the immune cell composition of humans. The variability in expression of these markers in healthy donors might influence their response to stimuli and therefore should be considered in future human ILC studies. Variable Tissue-Specific Phenotypic Profiles of Helper- Type ILCs As observed for helper-type ILC frequencies (Figure 4A), we also observed a clear distinction between non-pathological, mucosal, and pathological tissues in terms of phenotypic profiles of ILCs (Figure 5). Under non-pathological condition, the majority of ILC2 cells expressed KLRG1, CD25, CCR6, and CCR4 and exhibited variable c-kit expression. We found that CD69 expression on ILC2 cells was restricted to skin, mucosal tissues, and a majority of cells found in the spleen (Figures 5 and 6A). ICOS expression is required for survival and efficient cytokine production by ILC2 cells (Maazi et al., 2015). Notably, ICOS expression was not ubiquitous and mainly limited to ILC2 cells derived from mucosal tissues (Figures 5 and 6A). In contrast to adult PBMCs, cord blood ILC2 cells expressed ICOS at a relatively high frequency (17.6% ± 5.7%). Some cord-blood-derived ILC2 cells also expressed NKp30 (49.5% ± 8.2%) and CD103 (5.2% ± 2.3%) (Figure 6B). On the basis of these findings, we investigated whether these phenotypic differences between adult PBMCs and cord blood ILC2 cells were indicative of functional differences. We found, however, that stimulation of sorted ILC2 cells with IL-33, a cytokine known to stimulate Th2 cell responses, elicited Immunity 46, , January 17,
10 Figure 5. Heterogeneity of Helper-Type ILC between Tissues and Donors Heatmap displaying the frequencies of ILC2 and ILC3 cells positive for 32 different marker molecules. Data shown are from at least 13 independent experiments, n/a (data not available). comparable quantities of IL-4, IL-5, IL-9, and IL-13 production by these cells (Figure 6C). Similar to ILC2, NKp44 ILC3 cells exhibited heterogeneous phenotypes across different tissues. ICOS and CD69 were only expressed on NKp44 ILC3 cells from skin and mucosal tissues (Figures 5 and 6A). The majority of these cells expressed CD25, NKp30, CCR6, and CCR4 and more than 40% expressed CD56, 2B4, and NKp46 across all tissues (Figure 5). We also observed differences in phenotypes of NKp44 ILC3 cells as compared to NKp44 + ILC3 cells. The average frequency of CD25 expression was lower on NKp44 + ILC3 cells as compared to NKp44 ILC3 cells in all tissues except for skin where both cell types displayed high frequencies of CD25 expression. In contrast, CD56, NKp46, and ICOS were highly expressed on NKp44 + ILC3 cells as compared to their NKp44 counterparts. In some patients, we also noticed expression of CD103 on various ILC2 and ILC3 cell subsets. Less than 5% of all of these ILC subsets were observed to be CD103 + (see Figure 6B for ILC2 cell staining). Within pathological tissues, we found that helper-type ILC displayed a phenotype similar to that observed in mucosal tissues. We observed an upregulation of CD69 and ICOS expression (Figures 5 and 6A), while expression of others markers remained similar to those observed in mucosal tissues (Figure 5). We were not able to detect significant differences in major histocompatibility complex (MHC) class II (HLA-DR) molecule expression between ILC subsets within the same tissues tested (Figure 6A). Nevertheless, since in some experiments we detected a slightly higher expression of MHCII expression in mucosal and pathological tissues as compared to non-mucosal tissue (e.g., Figure 6A). As previously described, ILCs express CD25, the non-signaling IL-2Ra subunit that increases the affinity of IL-2R (CD122 and gc) for IL-2. Under pathological conditions, we found that helper-type ILCs slightly upregulated CD122 expression in tissues. However, this expression remained very low as compared to NK cells (Figure 6D). One of the main roles of IL-2 signaling is the maintenance and proliferation of immune cells. In line with this, the frequency of proliferating helper-type ILCs (Ki67 + ) was relatively low in non-pathological, mucosal, or pathological tissues (< 5%) (Figure 6E). For instance, in colorectal tumors, we detected a high frequency of proliferating (Ki67 + ) ieilc1-like and/or NK cells but not helper-type ILCs (Figures 6E and S5A). We were also able to identify a number of previously unreported markers expressed by ILCs. For instance, we found that ILC3 cells can express 2B4 (CD244), a receptor having an activating or inhibitory function (Figures 5 and 3B). Moreover, we found that ILC2 cells can express integrin CD49a. However, unlike ieilc1-like cells, which also express CD49a, CD49a + ILC2 cells did not necessarily express integrin CD103 (data not shown). We further found that ILC3 cells can also express CD39, an ectoenzyme involved in suppression of inflammation (Figure 6F). Here, CD39 was mostly restricted to NKp44 + ILC3 cells from mucosal and inflamed tissues (Figure 5). We did not detect expression of any other receptors involved in immune suppression, such as PD-1, TIGIT, or CD38 by any of the ILC subsets across all tissues tested (Figure 5). Human ILC2 and ILC3 Cells Respond to IL-18 Stimulation by Secreting Th1, Th2, or Th17 Cytokines We observed the expression of IL-18Ra by ILC2 and ILC3 cells (Figure 6G), which has not been reported previously. By using quantitative-pcr, we confirmed that ILC2 and ILC3 cells express mrna coding for both chains of the IL-18 receptor (Figure S5B). Interaction between IL-18R and IL-18 is implicated in promoting production of Th1 cell-related cytokines. We found that ILC2 cells produced IL-4, IL-5, IL-9, IL-13, GM-CSF, IL-6, and IL-8 in response to IL-18 stimulation (Figure 6I). This cytokine profile produced by ILC2 cells was similar to that observed in response to IL-33 (Figure S5C) and demonstrates that IL-18 has a similar effect as IL-33 on ILC2 cell activation. ILC3 cells produced GM-CSF, TNF-a, and IL-8 in response to stimulation with IL-18, as well as in combination with IL-23 (Figure 6H) but only low amounts of IL-17. We did not detect IL-22 production by ILC3 cells in response to IL-18 stimulation. We also observed IFN-g secretion by ILC3 cells in the presence of IL-18 and IL-23. After in vitro culture with IL-18 and IL-23, these IFN-g producing ILC3 cells maintained their ILC3 cell phenotypes (i.e., expression of c-kit and upregulation of NKp44; Figure S5D). This confirms that ILC3 cells can be plastic in terms of cytokine production in vitro as described previously (Cella et al., 2009), but do not acquire an ILC1 cellular phenotype. DISCUSSION Our study aimed to clarify the composition and phenotypic profiles of ILCs in humans. In contrast to NK ILC2 and ILC3 cells, we did not detect any ILC1 population as previously described 156 Immunity 46, , January 17, 2017
11 A B C D E F G H I (legend on next page) Immunity 46, , January 17,
12 (Bernink et al., 2015; Bernink et al., 2013). Although we observed populations with shared phenotypic characteristics of previously defined ILC1 cells (CD127 + CRTH2 c-kit NKp44 ), other markers expressed by these cells were not compatible with the current definitions of ILC1 cells. Our analysis, including the t-sne analysis, showed that these cells derived from contaminating T cells, DCs, HSC, ILC3, and/or NK cells. Recently, as part of a single-cell transcriptomic analysis of ILC cells, it was reported that tonsil ILC1 cells express transcripts coding for rearranged TCR, as well as various other T cell-specific genes (Björklund et al., 2016). This together with another recent report using CD5 staining (Vely et al., 2016) supports our findings that T cells can heavily contaminate ILC1 cells. In vitro observations about ILC1 cell plasticity, differentiation into ILC3 cells, and the ability to produce both, Th1- and Th17 cell-related cytokines (Bernink et al., 2015) can also be explained by contaminating NK and ILC3 cells in ILC1 gating as we describe here. Furthermore, the first publication that describes ILC1 cells showed a bi-modal expression of T-bet by these cells (Bernink et al., 2013). This confirms that ILC1 cells are composed of at least two cell populations. Because a large number of markers was required for this detailed and accurate characterization of putative ILC1 cells, we think that it might be possible that previous identifications of human ILC1 cells comprised cellular contaminations, which could not be excluded due to technical limitations. Using t-sne analysis, we identified a unique subset of NK cells (ieilc1-like cells) that matches previous descriptions of ieilc1 cells (Fuchs et al., 2013), except for variable NKp44 expression between tissues and individuals. Remarkably, this population was also observed in non-mucosal pathological tissues. A similar population was previously described in uterine tissue from pregnant women and was termed decidual NK cells (Kopcow et al., 2005). As reported, we found that this population also expressed NKp44 and CD103 and had a low expression of cytotoxic granule components. In vitro experiments showed that decidual stromal medium (containing TGF-b) can convert blood peripheral NK cells into decidual NK cells sharing a similar phenotype. The profiles of these ieilc1-like cells also appeared to match those of a previously reported subset of intrahepatic NK cells and consistent with this, we found that this population expressed integrin CD49a. All of these observations strongly suggest that ieilc1-like cells do not represent an independent lineage of innate lymphoid immune cells specific to mucosal tissues, but a NK cell subset that acquired this specific phenotype under the influence of mucosal or inflamed tissue environments. Consistent with previous studies, our t-sne analysis confirmed that helper-type ILCs can be divided into three main populations: ILC2, NKp44 ILC3, and NKp44 + ILC3 cells. The use of t-sne was also useful for delineating ILC3 cells. By this analysis, we observe that all ILC3 cells coalesce into one amorphous population of cells. We observed diversity within these cells that results from the variable expression of numerous markers, such as CD56, ICOS, and the NCRs. As described for mice, this diversity might be related to how ILCs can integrate and differentiate in response to signals from the microenvironment (Gury-BenAri et al., 2016). Nonetheless, we found that NKp44 expression functions to divide this population, and this distinction is especially meaningful when using it to divide subsets of ILC3 cells and to compare the compositions of different tissue types. We also noted that RORgt antibody staining was more difficult to detect in the NKp44 subset of ILC3 cells. Because this antibody staining was weak, we also sorted these cells and showed that RORC expression could be detected. However, because the gene expression was not assessed at a single cell level, this does not rule out the possibility that some of the ILC3 cells (especially the NKp44 subset) do not express RORgt. Nonetheless, we argue that the t-sne analysis, which takes into account the composite of all markers (Newell and Cheng, 2016), should be an accurate mean to delineate this subset of cells. In contrast to published murine studies, we did not observe a coordinate segregation of ILC3 cells based on NKp46 or CCR6 expression in humans. Furthermore, we found that c-kit and CD161 were not sufficiently reliable for the identification of NKp44 ILC3 cells. We did not observe an ILC3 cell subset expressing T-bet as it has been reported in mice (Klose et al., 2013). However, we found that all human ILC subsets (NK and ILC3 cells included) expressed at least a low amount of GATA3. This is consistent with the role of GATA3 in promoting IL-22 expression in ILC3 cells as it has been observed in mice (Serafini et al., 2014; Zhong et al., 2015). On the basis of these Figure 6. Heterogeneity of Helper-Type ILCs (A) Expression of CD69, ICOS, and HLA-DR by ILC populations in non-mucosal (blood), mucosal (tonsil), and pathological tissue (lung tumor). Data shown is one representative from 3 different donors. (B) Comparison of NKp30, ICOS, and CD103 expression by ILC2 cells derived from adult PBMCs and cord blood. Data shown are the mean values ± SD from at least 6 independent experiments (n = 14 for PBMCs, and n = 3 for cord blood). n/a (data not available). (C) Expression of IL-4, IL-5, IL-9, and IL-13 by sorted ILC2 cells from blood (white) or cord blood (gray) after culture with or without IL-33. Data shown are the mean values ± SD from 2 independent experiments. n = 4, Mann-Whitney test, ns: non-significant, *p % 0.05, **p % (D) Expression of CD25 and CD122 by ILC population in non-pathological (PBMCs) and pathological tissue (lung tumor). Data shown is one representative from two different donors from at least 6 independent experiments. (E) Frequencies of proliferating ILCs (Ki67 + ) in all tissues analyzed. Data shown are the mean values ± SD from at least 9 independent experiments (see Figure S5A for staining). (F) Expression of CD49a and CD39 by ILC2 and ILC3 cell populations in tonsils. Data shown are the mean values ± SD from at least 6 independent experiments. (G) Expression of IL-18Ra by NK (orange), ILC2 (green), and ILC3 (blue) cells. Representative dot plot from one donor (PBMC) from at least 6 independent experiments. (H) Expression of GM-CSF, IL-8, IL-17A, TNFa, and IL-13 by sorted ILC3 cells from PBMCs after culture with or without IL-18 and IL-23. Data shown are the mean values ± SD from 2 independent experiments, n = 5, Mann-Whitney test, ns: non significant, *p % 0.05, **p % (I) Expression of IL-4, IL-5, IL-9, IL-13, GM-CSF, IL-8, and IL-6 by sorted ILC2 cells from PBMCs after culture with or without IL-18 and IL-23. Data shown are the mean values ± SD from 2 independent experiments, n = 4, Mann-Whitney test, ns: non significant, *p % 0.05, **p % 0.01 (see Figure S5D for sorting strategy). Please also see Figure S Immunity 46, , January 17, 2017
13 observations, we propose a panel and gating strategy to identify human helper-type ILCs using flow cytometry, which we further confirmed by qpcr and functional studies of sorted cells. Importantly, the expression of various markers on ILCs can be modulated in response to cytokine exposure (e.g., CD127, CD94, CRTH2) (Cella et al., 2009). Therefore, we think that this gating strategy should be adjusted depending on the tissue and/or inflammatory conditions being studied. Human tissues can be divided into two categories based on their overall ILC subsets composition. Under non-pathological conditions, non-mucosal and lung tissues displayed low frequencies of ILC2 and ILC3 cells (<5% of ILC), this questioning the importance of these cells in tissue homeostasis and immune surveillance in comparison to NK cells (>95%). NKp44 + ILC3 cells were nearly absent in these tissues (<0.5%). However, this observation does not undermine the role of these cells in infections and pathologies such as lung inflammation, where it is clear that rare cells can have profound effects (Huang et al., 2015). Our study demonstrates that ILC2 cells found in cord blood are functionally similar to ILC2 cells derived from adult blood when stimulated with IL-33. However, we cannot exclude the possibility that alternative stimulation conditions would be able to trigger functional differences between these cells. These cells displayed an increased frequency and distinct phenotypic profiles when compared with their counterparts derived from adult blood (expressing more ICOS, CD103 and NKp30 a receptor also involved in virus or parasite recognition), which suggests a possible role for ILC2 cells in normal neonatal immunity. Under inflammatory conditions, more than 95% of ILCs recruited were composed of NK or eilc1-like cells in all tissues analyzed. Despite a low frequency of helper-type ILCs, these cells represent a phenotype that is similar to mucosal ILCs with a remarkably high expression of CD69 and ICOS. In contrast to non-mucosal and lung tissues, oral and gastrointestinal mucosal and skin tissues contained high frequencies of helper-type ILCs, which is consistent with the role of these cells in human barrier surface immunity and which has already been partially confirmed in mice (Artis and Spits, 2015; Song et al., 2015). This distinction between mucosal and non-mucosal tissues also corresponds with an upregulation of CD69 and ICOS. Recently, it has been demonstrated that ICOS ICOS-L interaction between human ILC2 cells is required for efficient function and survival of these cells (Maazi et al., 2015). Expression of ICOS on ILC3 cells suggests a role for ICOS-L-mediated activation. However, this further raises the question about a potential ICOS ICOS-L interaction between ILC2 and ILC3 cells and the cytokines that might be produced by these cells (T H 2 or T H 17 cell-associated cytokines). Our analyses also identified a number of previously unreported markers expressed by helper-type ILCs in oral and gastrointestinal mucosal, as well as skin tissues. We found that ILC2 cells expressed CD49a, an integrin providing tissue retention and survival signals. We report that 2B4 was expressed on ILC3 cells. This might be relevant since, depending on the tissue location and inflammatory condition, 2B4 CD48 interactions can inhibit cytokine production by human NK cells (Morandi et al., 2005). We also found that CD39 was expressed by ILC3 cells, and particularly by NKp44 + ILC3 cells, which can play a role in immunosuppression through its ability to degrade extracellular ATP and the modulation of purinergic signaling. These observations about helper type ILC phenotypes could be considered as additional targets for therapeutics approaches. Across all tissues studied, we were unable to clearly detect MHC II expression by ILCs. As compared to mice, this observation can be explained by different molecular mechanisms regulating MHCII expression in humans (Robinette and Colonna, 2016). We can hypothesize that MHC II expression on ILCs is restricted to some specific inflammatory conditions. As reported, ILC2 and ILC3 cells proliferate in response to IL-2 stimulation (Cella et al., 2009; Moro et al., 2010). However, we observed that helper-type ILCs do not abundantly express CD122 (IL-2Rb). A weak expression of CD122 on ILC3 can be explained by the fact that IL-2 signaling constrains IL-17 production (Laurence et al., 2007). However, helper-type ILCs highly express CD25, the non-signaling subunit that increases the affinity of IL-2R. As observed for DCs, CD25 on helper-type ILCs could trans-present IL-2 to other immune cells as a way to provide survival signal (Boyman and Sprent, 2012). In this study, we show that both ILC2 and ILC3 cell subsets expressed a functional IL- 18R in all tissues analyzed. This receptor binds IL-18, a cytokine associated with Th1 cell cytokine production (Dinarello et al., 2013). We observed that IL-18 could elicit Th1 cell cytokine production by ILC3 cells. Despite this, we found that these cells maintained their ILC3 phenotype, excluding plasticity toward an ILC1 cellular phenotype. Importantly, we did not detect T-bet expression on ILC3 cells ex vivo. These observations confirm a functional plasticity of ILC3 cells in vitro, which needs to be further confirmed in vivo. We also found that both, ILC2 and ILC3 cells produce GM-CSF and IL-8 in response to IL-18, two cytokines promoting the recruitment and activation of myeloid cells, such as monocytes and neutrophils. Our data show that ILC2 cells can also produce Th2 cell-associated cytokines in response to this cytokine stimulation, at similar amounts as by IL-33 stimulation. In contrast, IL-18 in combination with IL-23 induced the secretion of IL-17 but not IL-22 by ILC3 cells. This might be relevant because human intestinal epithelial cells are capable of producing high amounts of IL-18 in Crohn s disease (Pizarro et al., 1999). Furthermore, macrophages that are present in inflamed tissues produce large amounts of IL-18 (Dinarello et al., 2013). Taken together, these observations reveal the potential for novel mechanisms of ILC2 and ILC3 cell activation under inflammatory conditions. In summary, this study provides a global, comprehensive, and detailed description of human heterogeneity in ILCs across patients, tissues, in non-pathological conditions, and within various pathological environments. Despite a homology with mice, our study highlights the uniqueness of human ILCs in terms of their composition, phenotypes, and heterogeneity. These differences between mice and humans should be considered as results from mouse ILC studies that are translated into humans. EXPERIMENTAL PROCEDURES Human Samples and Cells Isolation The use of human tissues was approved by the appropriate IRBs, A*STAR, the Singapore Immunology Network and the Health Sciences Authority of Singapore or the KwaZulu-Natal Research Institute for TB- HIV (K-RITH). Briefly, tissues were mechanically dissociated in small pieces and incubated at 37 C for 15 to 40 min in media with Collagenase IV and DNase. Dissociated Immunity 46, , January 17,
About ATCC. Established partner to global researchers and scientists
 Discovering ATCC Primary Immunology Cells - Model Systems to Study the Immune and Cardiovascular Systems James Clinton, Ph.D. Scientist, ATCC July 14, 2016 About ATCC Founded in 1925, ATCC is a non-profit
Discovering ATCC Primary Immunology Cells - Model Systems to Study the Immune and Cardiovascular Systems James Clinton, Ph.D. Scientist, ATCC July 14, 2016 About ATCC Founded in 1925, ATCC is a non-profit
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development
 Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development
 ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development University of Wisconsin-Madison, Production Assistance for Cellular Therapies (PACT) Over the
ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development University of Wisconsin-Madison, Production Assistance for Cellular Therapies (PACT) Over the
Nature Biotechnology: doi: /nbt.4086
 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Chapter 3 The Immune System
 Chapter 3 The Immune System Why is the Immune System Important? Why is the Immune System Relevant to HIV? T Lymphocyte Infected by HIV Brief History of Immunology Immunity- Observation reported in 430
Chapter 3 The Immune System Why is the Immune System Important? Why is the Immune System Relevant to HIV? T Lymphocyte Infected by HIV Brief History of Immunology Immunity- Observation reported in 430
initial single-cell analysis, with a pragmatic focus on surface markers with the highest potential for
 Supplementary Figure 1: Summary of the exclusionary approach to surface marker selection for initial single-cell analysis, with a pragmatic focus on surface markers with the highest potential for protein
Supplementary Figure 1: Summary of the exclusionary approach to surface marker selection for initial single-cell analysis, with a pragmatic focus on surface markers with the highest potential for protein
Mayumi Egawa, Kaori Mukai, Soichiro Yoshikawa, Misako Iki, Naofumi Mukaida, Yohei Kawano, Yoshiyuki Minegishi, and Hajime Karasuyama
 Immunity, Volume 38 Supplemental Information Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4 Mayumi Egawa, Kaori Mukai, Soichiro
Immunity, Volume 38 Supplemental Information Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4 Mayumi Egawa, Kaori Mukai, Soichiro
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11809 Supplementary Figure 1. Antibiotic treatment reduces intestinal bacterial load and allows access of non-pathogenic bacteria to the MLN, inducing intestinal
SUPPLEMENTARY INFORMATION doi:10.1038/nature11809 Supplementary Figure 1. Antibiotic treatment reduces intestinal bacterial load and allows access of non-pathogenic bacteria to the MLN, inducing intestinal
BEH.462/3.962J Molecular Principles of Biomaterials Spring 2003
 Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
1 Name. 1. (3 pts) What is apoptosis and how does it differ from necrosis? Which is more likely to trigger inflammation?
 1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
Best practices in panel design to optimize the isolation of cells of interest
 Sort Best practices in panel design to optimize the isolation of cells of interest For Research Use Only. Not for use in diagnostic or therapeutic procedures. Alexa Fluor is a registered trademark of Life
Sort Best practices in panel design to optimize the isolation of cells of interest For Research Use Only. Not for use in diagnostic or therapeutic procedures. Alexa Fluor is a registered trademark of Life
Supplementary Fig. 5
 Supplementary Fig. 5 Supplemental Figures legends Supplementary Figure 1 (A) Additional dot plots from CyTOF analysis from untreated group. (B) Gating strategy for assessment of CD11c + NK cells frequency
Supplementary Fig. 5 Supplemental Figures legends Supplementary Figure 1 (A) Additional dot plots from CyTOF analysis from untreated group. (B) Gating strategy for assessment of CD11c + NK cells frequency
Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages
 Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages Christoph S.N. Klose, 1,2,8,11 Melanie Flach, 1,2,11 Luisa Möhle, 3 Leif Rogell, 1,2,4 Thomas Hoyler,
Differentiation of Type 1 ILCs from a Common Progenitor to All Helper-like Innate Lymphoid Cell Lineages Christoph S.N. Klose, 1,2,8,11 Melanie Flach, 1,2,11 Luisa Möhle, 3 Leif Rogell, 1,2,4 Thomas Hoyler,
Supplemental Information
 Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
High-dimensional flow-cytometric analysis of human B-cell populations
 High-dimensional flow-cytometric analysis of human B-cell populations The BD FACSCelesta cell analyzer and FlowJo software together enable deep analysis of B-cell biology Features High-resolution analysis
High-dimensional flow-cytometric analysis of human B-cell populations The BD FACSCelesta cell analyzer and FlowJo software together enable deep analysis of B-cell biology Features High-resolution analysis
Supplementary Figure 1. Characterization of the POP2 transcriptional and post-transcriptional regulatory elements. (A) POP2 nucleotide sequence
 1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
Immune enhancement by increased number of B-cells
 Page 1 of 6 Immune enhancement by increased number of B-cells - A summary report of scientific studies with GlycaNova s beta-glucan, Lentinex Background Human immunity can be divided in two main parts,
Page 1 of 6 Immune enhancement by increased number of B-cells - A summary report of scientific studies with GlycaNova s beta-glucan, Lentinex Background Human immunity can be divided in two main parts,
Supplemental Information Inventory
 Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy
 In the format provided by the authors and unedited. DOI: 10.1038/NNANO.2017.69 Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy Hengfeng Yuan, Wen Jiang,* Christina A.
In the format provided by the authors and unedited. DOI: 10.1038/NNANO.2017.69 Multivalent bi-specific nanobioconjugate engager for targeted cancer immunotherapy Hengfeng Yuan, Wen Jiang,* Christina A.
Supplemental Information The Sensing of Environmental Stimuli by Follicular Dendritic Cells Promotes Immunoglobulin A Generation in the Gut
 Immunity, Volume 33 Supplemental Information The Sensing of Environmental Stimuli by Follicular Dendritic Cells Promotes Immunoglobulin A Generation in the Gut Keiichiro Suzuki, Mikako Maruya, Shimpei
Immunity, Volume 33 Supplemental Information The Sensing of Environmental Stimuli by Follicular Dendritic Cells Promotes Immunoglobulin A Generation in the Gut Keiichiro Suzuki, Mikako Maruya, Shimpei
ABOUT GLYCOSTEM. Company Overview
 ABOUT GLYCOSTEM The company is a clinical stage biotech company established in the Netherlands in 2007. The company s headquarters and new state-of-the-art lab and production facilities are based at Pivot
ABOUT GLYCOSTEM The company is a clinical stage biotech company established in the Netherlands in 2007. The company s headquarters and new state-of-the-art lab and production facilities are based at Pivot
Nature Immunology: doi: /ni.3694
 Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Examination in Immunotechnology, 30 May 2011, 8-13
 Examination in Immunotechnology, 30 May 2011, 8-13 1 Each question can give 5p, with a total of 10 questions (i.e. 50 points in total). 2 Write name and personal number on ALL pages (including the cover).
Examination in Immunotechnology, 30 May 2011, 8-13 1 Each question can give 5p, with a total of 10 questions (i.e. 50 points in total). 2 Write name and personal number on ALL pages (including the cover).
Interferon- -producing immature myeloid cells confer protection. against severe invasive group A Streptococcus infections. Supplementary Information
 Supplementary Information Interferon- -producing immature myeloid cells confer protection against severe invasive group A Streptococcus infections Takayuki Matsumura, Manabu Ato, Tadayoshi Ikebe, Makoto
Supplementary Information Interferon- -producing immature myeloid cells confer protection against severe invasive group A Streptococcus infections Takayuki Matsumura, Manabu Ato, Tadayoshi Ikebe, Makoto
Rorα is essential for nuocyte development
 Rorα is essential for nuocyte development See Heng Wong,,, Jennifer A. Walker,, Helen E. Jolin,, Lesley F. Drynan, Emily Hams 3, Ana Camelo, Jillian L. Barlow, Daniel R. Neill,6, Veera Panova, Ute Koch,
Rorα is essential for nuocyte development See Heng Wong,,, Jennifer A. Walker,, Helen E. Jolin,, Lesley F. Drynan, Emily Hams 3, Ana Camelo, Jillian L. Barlow, Daniel R. Neill,6, Veera Panova, Ute Koch,
Supplemental figure 1: Phenotype of IMC and MDSC after purification. A. Gating
 Supplemental Figure Legend: Supplemental figure 1: Phenotype of IMC and MDSC after purification. A. Gating strategy for mouse MDSC. CD11b + Ly6C high Ly6G - cells are defined as M-MDSC. CD11b + Ly6C low
Supplemental Figure Legend: Supplemental figure 1: Phenotype of IMC and MDSC after purification. A. Gating strategy for mouse MDSC. CD11b + Ly6C high Ly6G - cells are defined as M-MDSC. CD11b + Ly6C low
Nature Immunology: doi: /ni Supplementary Figure 1
 Supplementary Figure 1 BALB/c LYVE1-deficient mice exhibited reduced lymphatic trafficking of all DC subsets after oxazolone-induced sensitization. (a) Schematic overview of the mouse skin oxazolone contact
Supplementary Figure 1 BALB/c LYVE1-deficient mice exhibited reduced lymphatic trafficking of all DC subsets after oxazolone-induced sensitization. (a) Schematic overview of the mouse skin oxazolone contact
Considerations in Product Development with Advanced Therapies and Cancer Vaccines
 Considerations in Product Development with Advanced Therapies and Cancer Vaccines Thomas Hinz Head of Section Therapeutic Vaccines Paul-Ehrlich-Institut hinth@pei.de Thomas Hinz, October 29, 2008, San
Considerations in Product Development with Advanced Therapies and Cancer Vaccines Thomas Hinz Head of Section Therapeutic Vaccines Paul-Ehrlich-Institut hinth@pei.de Thomas Hinz, October 29, 2008, San
Interplay of Cells involved in Therapeutic Agent Immunogenicity. Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology
 Interplay of Cells involved in Therapeutic Agent Immunogenicity Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology Disclosure The author works with Amicus on an immunogenicity project
Interplay of Cells involved in Therapeutic Agent Immunogenicity Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology Disclosure The author works with Amicus on an immunogenicity project
Biomarkers, Early Prediction of Vaccine Efficacy and Safety
 Biomarkers, Early Prediction of Vaccine Efficacy and Safety Giuseppe Pantaleo, M.D. Professor of Medicine Head, Division of Immunology and Allergy Executive Director, Swiss Vaccine Research Institute Lausanne
Biomarkers, Early Prediction of Vaccine Efficacy and Safety Giuseppe Pantaleo, M.D. Professor of Medicine Head, Division of Immunology and Allergy Executive Director, Swiss Vaccine Research Institute Lausanne
Jedi cells patrol the mouse
 Jedi cells patrol the mouse Technical Journal Club Josephin Wagner 19.01.2016 12 VOL.13 NO.1 JANUARY 2016 NATURE METHODS Introduction JEDI= Just EGFP Death Inducing t- cells Specific CD-8 T cells All EGFP
Jedi cells patrol the mouse Technical Journal Club Josephin Wagner 19.01.2016 12 VOL.13 NO.1 JANUARY 2016 NATURE METHODS Introduction JEDI= Just EGFP Death Inducing t- cells Specific CD-8 T cells All EGFP
Supplementary Figure 1
 Supplementary Figure 1 CITE-seq library preparation. (a) Illustration of the DNA-barcoded antibodies used in CITE-seq. (b) Antibody-oligonucleotide complexes appear as a high-molecular-weight smear when
Supplementary Figure 1 CITE-seq library preparation. (a) Illustration of the DNA-barcoded antibodies used in CITE-seq. (b) Antibody-oligonucleotide complexes appear as a high-molecular-weight smear when
Immunology 101: Implications for Medical Device Failure. Joshua B. Slee, PhD Assistant Professor of Biology
 Immunology 101: Implications for Medical Device Failure Joshua B. Slee, PhD Assistant Professor of Biology Learning Goals Understand innate and adaptive immunity Explain how innate and adaptive immunity
Immunology 101: Implications for Medical Device Failure Joshua B. Slee, PhD Assistant Professor of Biology Learning Goals Understand innate and adaptive immunity Explain how innate and adaptive immunity
MicroRNAs Modulate Hematopoietic Lineage Differentiation
 Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Hector R. Wong, MD Division of Critical Care Medicine Cincinnati Children s Hospital Medical Center Cincinnati Children s Research Foundation
 Mechanisms of Sepsis Exploiting Pathogenic Genes Hector R. Wong, MD Division of Critical Care Medicine Cincinnati Children s Hospital Medical Center Cincinnati Children s Research Foundation Critical Care
Mechanisms of Sepsis Exploiting Pathogenic Genes Hector R. Wong, MD Division of Critical Care Medicine Cincinnati Children s Hospital Medical Center Cincinnati Children s Research Foundation Critical Care
Fundamental properties of Stem Cells
 Stem cells Learning Goals: Define what a stem cell is and describe its general properties, using hematopoietic stem cells as an example. Describe to a non-scientist the current progress of human stem cell
Stem cells Learning Goals: Define what a stem cell is and describe its general properties, using hematopoietic stem cells as an example. Describe to a non-scientist the current progress of human stem cell
Keywords: mesenchymal, FGF2, MHC-classII, RANKL, osteoimmunology
 1 Supplementary data FGF2 induces RANKL gene expression as well as IL1 regulated MHC class II in bone marrow derived human mesenchymal progenitor stromal cells. 1,3 Chiara Bocelli-Tyndall, 1 Emanuele Trella,
1 Supplementary data FGF2 induces RANKL gene expression as well as IL1 regulated MHC class II in bone marrow derived human mesenchymal progenitor stromal cells. 1,3 Chiara Bocelli-Tyndall, 1 Emanuele Trella,
Discover more with FluoroSpot
 Discover more with FluoroSpot FluoroSpot combines the sensitivity of ELISpot with the capacity to analyze secretion of several analytes simultaneously. This highly sensitive cellular assay is robust, easy
Discover more with FluoroSpot FluoroSpot combines the sensitivity of ELISpot with the capacity to analyze secretion of several analytes simultaneously. This highly sensitive cellular assay is robust, easy
Supporting Information
 (xe number cells) LSK (% gated) Supporting Information Youm et al../pnas. SI Materials and Methods Quantification of sjtrecs. CD + T subsets were isolated from splenocytes using mouse CD + T cells positive
(xe number cells) LSK (% gated) Supporting Information Youm et al../pnas. SI Materials and Methods Quantification of sjtrecs. CD + T subsets were isolated from splenocytes using mouse CD + T cells positive
Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation
 Immunity Supplemental Information Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation Jernej Godec, Yan Tan, Arthur Liberzon, Pablo Tamayo, Sanchita
Immunity Supplemental Information Compendium of Immune Signatures Identifies Conserved and Species-Specific Biology in Response to Inflammation Jernej Godec, Yan Tan, Arthur Liberzon, Pablo Tamayo, Sanchita
Distribution of human ILCs during chronic lung disease.
 Supplementary Figure 1 Distribution of human ILCs during chronic lung disease. Quantification of flow cytometric analysis of lung tissue from patients with COPD or IPF identifying (a) frequencies of total
Supplementary Figure 1 Distribution of human ILCs during chronic lung disease. Quantification of flow cytometric analysis of lung tissue from patients with COPD or IPF identifying (a) frequencies of total
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/1/494/eaak972/dc1 Supplementary Materials for Blockade of surface-bound TGF-β on regulatory T cells abrogates suppression of effector T cell function in the tumor
www.sciencesignaling.org/cgi/content/full/1/494/eaak972/dc1 Supplementary Materials for Blockade of surface-bound TGF-β on regulatory T cells abrogates suppression of effector T cell function in the tumor
London, 11 October 2006 Doc. Ref. EMEA/CHMP/BWP/271475/2006 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency 1 2 London, 11 October 2006 Doc. Ref. 3 4 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) 5 DRAFT 6 7 GUIDELINE ON POTENCY TESTING OF CELL BASED IMMUNOTHERAPY MEDICINAL
European Medicines Agency 1 2 London, 11 October 2006 Doc. Ref. 3 4 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) 5 DRAFT 6 7 GUIDELINE ON POTENCY TESTING OF CELL BASED IMMUNOTHERAPY MEDICINAL
Cytomics in Action: Cytokine Network Cytometry
 Cytomics in Action: Cytokine Network Cytometry Jonni S. Moore, Ph.D. Director, Clinical and Research Flow Cytometry and PathBioResource Associate Professor of Pathology & Laboratory Medicine University
Cytomics in Action: Cytokine Network Cytometry Jonni S. Moore, Ph.D. Director, Clinical and Research Flow Cytometry and PathBioResource Associate Professor of Pathology & Laboratory Medicine University
IGF-1 promotes the development and cytotoxic activity of human NK cells regulated
 Supplementary Information for IGF-1 promotes the development and cytotoxic activity of human NK cells regulated by mir-483-3p Fang Ni 1, 2, Rui Sun 1, Binqing Fu 1, Fuyan Wang 1, Chuang Guo 1, Zhigang
Supplementary Information for IGF-1 promotes the development and cytotoxic activity of human NK cells regulated by mir-483-3p Fang Ni 1, 2, Rui Sun 1, Binqing Fu 1, Fuyan Wang 1, Chuang Guo 1, Zhigang
International Foundations of Medicine Basic Science Exam Blueprint
 International Foundations of Medicine Basic Science Exam Blueprint General Principles 28% 30% Biochemistry and molecular biology gene expression: DNA structure, replication, and exchange gene expression:
International Foundations of Medicine Basic Science Exam Blueprint General Principles 28% 30% Biochemistry and molecular biology gene expression: DNA structure, replication, and exchange gene expression:
Supporting Information
 Supporting Information of Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for Treatment of Metastatic Tumor Lihua Luo, Chunqi Zhu, Hang Yin, Mengshi Jiang, Junlei Zhang, Bing Qin, Zhenyu
Supporting Information of Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for Treatment of Metastatic Tumor Lihua Luo, Chunqi Zhu, Hang Yin, Mengshi Jiang, Junlei Zhang, Bing Qin, Zhenyu
To the Reviewers: POINT-BY-POINT REPLY
 To the Reviewers: We changed our manuscript according to the Reviewer s suggestions. We used a red character for the novel information added or for the sentences deleted from the original version of the
To the Reviewers: We changed our manuscript according to the Reviewer s suggestions. We used a red character for the novel information added or for the sentences deleted from the original version of the
Figure S1: GM-CSF upregulates CCL17 expression in in vitro-derived and ex vivo murine macrophage populations
 Figure S1 A B C Figure S1: GM-CSF upregulates CCL17 expression in in vitro-derived and ex vivo murine macrophage populations (A-B) Murine bone cells were cultured in either M-CSF (5,000 U/ml) or GM-CSF
Figure S1 A B C Figure S1: GM-CSF upregulates CCL17 expression in in vitro-derived and ex vivo murine macrophage populations (A-B) Murine bone cells were cultured in either M-CSF (5,000 U/ml) or GM-CSF
Outcomes in Mesenchymal Stem Cell Manufacturing. Athena Russell, MT(AAB) Human Cellular Therapy Laboratory Mayo Clinic Jacksonville, FL
 Outcomes in Mesenchymal Stem Cell Manufacturing Athena Russell, MT(AAB) Human Cellular Therapy Laboratory Mayo Clinic Jacksonville, FL Background HCTL established in 1992 to support BMT programs of Mayo
Outcomes in Mesenchymal Stem Cell Manufacturing Athena Russell, MT(AAB) Human Cellular Therapy Laboratory Mayo Clinic Jacksonville, FL Background HCTL established in 1992 to support BMT programs of Mayo
Resolving Stem Cell Heterogeneity Using Flow Cytometry
 Resolving Stem Cell Heterogeneity Using Flow Cytometry Mirko Corselli, PhD Senior Scientist We have not fully utilized the power of flow cytometry to address biological questions in other systems Flow
Resolving Stem Cell Heterogeneity Using Flow Cytometry Mirko Corselli, PhD Senior Scientist We have not fully utilized the power of flow cytometry to address biological questions in other systems Flow
Nature Immunology: doi: /ni Supplementary Figure 1
 Supplementary Figure 1 PPAR-γ is dispensable for the development of tissue macrophages in the heart, kidneys, lamina propria and white adipose tissue. Plots show the expression of F4/80 and CD11b (a) or
Supplementary Figure 1 PPAR-γ is dispensable for the development of tissue macrophages in the heart, kidneys, lamina propria and white adipose tissue. Plots show the expression of F4/80 and CD11b (a) or
Immunogenicity: Assessing the Clinical Relevance and Risk Minimization of Antibodies to Biopharmaceuticals
 Immunogenicity: Assessing the Clinical Relevance and Risk Minimization of Antibodies to Biopharmaceuticals Daniel Kramer/Kathleen Beach Need for public-private collaboration 1 All biopharmaceuticals are
Immunogenicity: Assessing the Clinical Relevance and Risk Minimization of Antibodies to Biopharmaceuticals Daniel Kramer/Kathleen Beach Need for public-private collaboration 1 All biopharmaceuticals are
Supplementary Figure 1
 Supplementary Figure 1 Ex2 promotor region Cre IRES cherry pa Ex4 Ex5 Ex1 untranslated Ex3 Ex5 untranslated EYFP pa Rosa26 STOP loxp loxp Cre recombinase EYFP pa Rosa26 loxp 1 kb Interleukin-9 fate reporter
Supplementary Figure 1 Ex2 promotor region Cre IRES cherry pa Ex4 Ex5 Ex1 untranslated Ex3 Ex5 untranslated EYFP pa Rosa26 STOP loxp loxp Cre recombinase EYFP pa Rosa26 loxp 1 kb Interleukin-9 fate reporter
Single-cell analysis reveals the continuum of human lympho-myeloid progenitor cells
 SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41590-017-0001-2 In the format provided by the authors and unedited. Single-cell analysis reveals the continuum of human lympho-myeloid progenitor
SUPPLEMENTARY INFORMATION Articles https://doi.org/10.1038/s41590-017-0001-2 In the format provided by the authors and unedited. Single-cell analysis reveals the continuum of human lympho-myeloid progenitor
Differentiation. Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU)
 Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
MegaClust. MegaClust. MegaClust automated and multidimensional processing of flow cytometry data enables the analysis of drugs mechanism of action
 Application Note automated and multidimensional processing of flow cytometry data enables the analysis of drugs mechanism of action Introduction Key Words - CyTof - analysis of large datasets - Identification
Application Note automated and multidimensional processing of flow cytometry data enables the analysis of drugs mechanism of action Introduction Key Words - CyTof - analysis of large datasets - Identification
Supplementary information
 Supplementary information Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer Je-In Youn, Vinit Kumar, Michelle Collazo, Yulia Nefedova, Thomas Condamine,
Supplementary information Epigenetic silencing of retinoblastoma gene regulates pathologic differentiation of myeloid cells in cancer Je-In Youn, Vinit Kumar, Michelle Collazo, Yulia Nefedova, Thomas Condamine,
leading the way in research & development
 leading the way in research & development people. passion. possibilities. ABBVIE 2 immunology AbbVie Immunology has a demonstrated record of success in identifying and developing both small molecule and
leading the way in research & development people. passion. possibilities. ABBVIE 2 immunology AbbVie Immunology has a demonstrated record of success in identifying and developing both small molecule and
At the conclusion of this lesson you should be able to:
 Learning Objectives At the conclusion of this lesson you should be able to: Understand the key terms and definitions regarding stem cells Differentiate between the adult and embryonic stem cells Differentiate
Learning Objectives At the conclusion of this lesson you should be able to: Understand the key terms and definitions regarding stem cells Differentiate between the adult and embryonic stem cells Differentiate
Structure of IgG and IgM
 Structure of IgG and IgM Fig. 5-1 A,B Crystal Structure of Secreted IgG Fig. 5-1 C Structure of an Ig Domain Fig. 5-2 Proteolytic Fragments of IgG (1) Fig. 5-3A Proteolytic Fragments of IgG (2) Fig. 5-3B
Structure of IgG and IgM Fig. 5-1 A,B Crystal Structure of Secreted IgG Fig. 5-1 C Structure of an Ig Domain Fig. 5-2 Proteolytic Fragments of IgG (1) Fig. 5-3A Proteolytic Fragments of IgG (2) Fig. 5-3B
Immunophenotyping of Peripheral Blood and Bone Marrow Cells by Flow Cytometry *Akanni EO and # Palini A.
 Immunophenotyping of Peripheral Blood and Bone Marrow Cells by Flow Cytometry *Akanni EO and # Palini A. * Department of Haematology & Blood Transfusion,College of Health Science, Ladoke Akintola University
Immunophenotyping of Peripheral Blood and Bone Marrow Cells by Flow Cytometry *Akanni EO and # Palini A. * Department of Haematology & Blood Transfusion,College of Health Science, Ladoke Akintola University
Session 3 Lecture 1 Dynamics of the GIT Microbiome: Microbial Darwinism
 Session 3 Lecture 1 Dynamics of the GIT Microbiome: Microbial Darwinism 10 14 bacteria representing ~1000 species and many phyla live in a crowded ecological space: The GIT Four major phyla dominate: By
Session 3 Lecture 1 Dynamics of the GIT Microbiome: Microbial Darwinism 10 14 bacteria representing ~1000 species and many phyla live in a crowded ecological space: The GIT Four major phyla dominate: By
Adaptive Immunity: Specific Defenses of the Host
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 17 Adaptive Immunity: Specific Defenses of the Host The Adaptive Immune System Adaptive immunity:
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 17 Adaptive Immunity: Specific Defenses of the Host The Adaptive Immune System Adaptive immunity:
CyTOF 2. Mass Cytometry System
 CyTOF 2 Mass Cytometry System Discover more. Imagine more. CyTOF Applications Mass Cytometry. The Future of Cytometry Today. Mass cytometry resolves multiple metal probes per cell with minimal signal overlap,
CyTOF 2 Mass Cytometry System Discover more. Imagine more. CyTOF Applications Mass Cytometry. The Future of Cytometry Today. Mass cytometry resolves multiple metal probes per cell with minimal signal overlap,
Supplementary Figure 1. Xbp1 deficiency does not alter hematopoietic cellularity.
 Supplementary Figure 1 Xbp1 deficiency does not alter hematopoietic cellularity. Absolute number of leukocytes obtained from bone marrow (BM, 2 tibias and 2 femurs per mouse) of Xbp1 f/f and Xbp1 Vav1
Supplementary Figure 1 Xbp1 deficiency does not alter hematopoietic cellularity. Absolute number of leukocytes obtained from bone marrow (BM, 2 tibias and 2 femurs per mouse) of Xbp1 f/f and Xbp1 Vav1
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM
 Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity and percent identical sites.
 Identity Human Mouse Supplemental Figure 1. Amino acid sequence alignment of -MyHC. The alignment was created using the ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity
Identity Human Mouse Supplemental Figure 1. Amino acid sequence alignment of -MyHC. The alignment was created using the ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity
Supplementary Materials for
 www.sciencesignaling.org/cgi/content/full/9/426/ra46/dc1 Supplementary Materials for Transforming growth factor and Notch ligands act as opposing environmental cues in regulating the plasticity of type
www.sciencesignaling.org/cgi/content/full/9/426/ra46/dc1 Supplementary Materials for Transforming growth factor and Notch ligands act as opposing environmental cues in regulating the plasticity of type
Immunological Genome Project:
 Immunological Genome Project: Does the big immunological/hematological database support good little science? Yasuyuki Saito, M.D., Ph.D. Exp. Hematology, USZ Overview 1. Immunological Genome Project 2.
Immunological Genome Project: Does the big immunological/hematological database support good little science? Yasuyuki Saito, M.D., Ph.D. Exp. Hematology, USZ Overview 1. Immunological Genome Project 2.
OpenStax-CNX module: m Antibodies * OpenStax. Abstract
 OpenStax-CNX module: m44823 1 Antibodies * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able to:
OpenStax-CNX module: m44823 1 Antibodies * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 3.0 By the end of this section, you will be able to:
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10772 Supplementary Figures: Supplementary Figure 1. Location of CNS1 within of the Foxp3 locus highlighting CNS1 and indicating transcription factor binding motifs downstream of TCR,
doi:10.1038/nature10772 Supplementary Figures: Supplementary Figure 1. Location of CNS1 within of the Foxp3 locus highlighting CNS1 and indicating transcription factor binding motifs downstream of TCR,
IMMUNOTOXICOLOGY: THE WHY, WHAT, WHEN, AND HOW
 IMMUNOTOXICOLOGY: THE WHY, WHAT, WHEN, AND HOW WJ Freebern May-2016 WHY Guidance for testing unintended immunosuppression or enhancement, not intended immunoenhancement caused by Immunomodulatory mabs
IMMUNOTOXICOLOGY: THE WHY, WHAT, WHEN, AND HOW WJ Freebern May-2016 WHY Guidance for testing unintended immunosuppression or enhancement, not intended immunoenhancement caused by Immunomodulatory mabs
Principles of Immunophenotyping
 Principles of Immunophenotyping % of Cell types? Immune activation? Changes based on health state? Moving from a heterogeneous population of blood cells to identifying the presence and proportion of different
Principles of Immunophenotyping % of Cell types? Immune activation? Changes based on health state? Moving from a heterogeneous population of blood cells to identifying the presence and proportion of different
Additional analysissu
 SSC-A FSC-A 65.6% FSC-H FSC-A 86.1% SSC-A 93.3% Viaprobe Additional analysissu Supplementary Figure S1. Gating strategy for flow cytometry Doublet cells were discriminated by FSC-A/SSC-A and FSC-A/FSC-H
SSC-A FSC-A 65.6% FSC-H FSC-A 86.1% SSC-A 93.3% Viaprobe Additional analysissu Supplementary Figure S1. Gating strategy for flow cytometry Doublet cells were discriminated by FSC-A/SSC-A and FSC-A/FSC-H
ANNOUNCEMENTS. HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134)
 ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells. in the injured sites
 Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites Yunyuan Li, Reza Baradar Jalili, Aziz Ghahary Department of Surgery, University of British Columbia,
Accelerating skin wound healing by M-CSF through generating SSEA-1 and -3 stem cells in the injured sites Yunyuan Li, Reza Baradar Jalili, Aziz Ghahary Department of Surgery, University of British Columbia,
Engage with us on Twitter: #Molecule2Miracle
 Engage with us on Twitter: #Molecule2Miracle Kassy Perry President & CEO Perry Communications Group PhRMA Alliance Development Consultant.@kassyperry Emily Burke, Ph.D. Director of Curriculum BioTech
Engage with us on Twitter: #Molecule2Miracle Kassy Perry President & CEO Perry Communications Group PhRMA Alliance Development Consultant.@kassyperry Emily Burke, Ph.D. Director of Curriculum BioTech
isolated from ctr and pictreated mice. Activation of effector CD4 +
 Supplementary Figure 1 Bystander inflammation conditioned T reg cells have normal functional suppressive activity and ex vivo phenotype. WT Balb/c mice were treated with polyi:c (pic) or PBS (ctr) via
Supplementary Figure 1 Bystander inflammation conditioned T reg cells have normal functional suppressive activity and ex vivo phenotype. WT Balb/c mice were treated with polyi:c (pic) or PBS (ctr) via
Immunological Techniques in Research and Clinical Medicine. Philip L. Cohen, M.D. Chief of Rheumatology, LKSOM 10 March 2016
 Immunological Techniques in Research and Clinical Medicine Philip L. Cohen, M.D. Chief of Rheumatology, LKSOM 10 March 2016 Antibodies Remarkable Tools for Research and Diagnosis You can make an antibody
Immunological Techniques in Research and Clinical Medicine Philip L. Cohen, M.D. Chief of Rheumatology, LKSOM 10 March 2016 Antibodies Remarkable Tools for Research and Diagnosis You can make an antibody
Designer Cells Finely Tuned for Therapy
 Designer Cells Finely Tuned for Therapy Gianluca Spaltro MDPhD student Prof. Manz group Technical Journal Club, 12.07.2016 Science Translational Medicine 16 Dec 2015 New trends in therapies Targeted therapy
Designer Cells Finely Tuned for Therapy Gianluca Spaltro MDPhD student Prof. Manz group Technical Journal Club, 12.07.2016 Science Translational Medicine 16 Dec 2015 New trends in therapies Targeted therapy
CyTOF 2 Mass Cytometry System
 CyTOF 2 Mass Cytometry System Discover more. Imagine more. Mass Cytometry. The Future of Cytometry Today. Mass cytometry resolves multiple metal probes per cell with minimal signal overlap, maximizing
CyTOF 2 Mass Cytometry System Discover more. Imagine more. Mass Cytometry. The Future of Cytometry Today. Mass cytometry resolves multiple metal probes per cell with minimal signal overlap, maximizing
Supplementary figures
 Mucida et al. Supplementary material Supplementary figures Supplementary Figure 1. Oral administration of OVA suppresses Th2 differentiation, Germinal Center (GC) formation and immunoglobulin class switching
Mucida et al. Supplementary material Supplementary figures Supplementary Figure 1. Oral administration of OVA suppresses Th2 differentiation, Germinal Center (GC) formation and immunoglobulin class switching
DC enriched CD103. CD11b. CD11c. Spleen. DC enriched. CD11c. DC enriched. CD11c MLN
 CD CD11 + CD + CD11 d g CD CD11 + CD + CD11 CD + CD11 + Events (% of max) Events (% of max) CD + CD11 Events (% of max) Spleen CLN MLN Irf fl/fl e Irf fl/fl h Irf fl/fl DC enriched CD CD11 Spleen DC enriched
CD CD11 + CD + CD11 d g CD CD11 + CD + CD11 CD + CD11 + Events (% of max) Events (% of max) CD + CD11 Events (% of max) Spleen CLN MLN Irf fl/fl e Irf fl/fl h Irf fl/fl DC enriched CD CD11 Spleen DC enriched
Humoral Immune Response. Dr. Iman Hussein Shehata Professor of Medical Microbiology and Immunology
 Humoral Immune Response Dr. Iman Hussein Shehata Professor of Medical Microbiology and Immunology Intended Learning Outcomes By the end of this lesson the student is expected to: 1-Decribe the sequence
Humoral Immune Response Dr. Iman Hussein Shehata Professor of Medical Microbiology and Immunology Intended Learning Outcomes By the end of this lesson the student is expected to: 1-Decribe the sequence
There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. 1. (6 points) Distinguish between the innate and adaptive immune responses:
 BENG 100b: Frontiers in Biomedical Engineering Midterm Examination 28 February 2006 There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. SHORT ANSWER (Total=70 points) Read the questions
BENG 100b: Frontiers in Biomedical Engineering Midterm Examination 28 February 2006 There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. SHORT ANSWER (Total=70 points) Read the questions
Nature Immunology: doi: /ni Supplementary Figure 1
 Supplementary Figure 1 Validation of the monoclonal antibody to mouse ACKR1 and expression of ACKR1 by BM hematopoietic cells. (a to d) Comparison of immunostaining of BM cells by anti-mouse ACKR1 antibodies:
Supplementary Figure 1 Validation of the monoclonal antibody to mouse ACKR1 and expression of ACKR1 by BM hematopoietic cells. (a to d) Comparison of immunostaining of BM cells by anti-mouse ACKR1 antibodies:
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb3206 Supplementary Figure 1 Autophagy-related gene expression in murine and human intestinal tumors. (a) Representative LC3 immunostaining in colonic adenoma and adjacent non tumoral tissue
DOI: 10.1038/ncb3206 Supplementary Figure 1 Autophagy-related gene expression in murine and human intestinal tumors. (a) Representative LC3 immunostaining in colonic adenoma and adjacent non tumoral tissue
The. Conception of. GCC- Specific. Chimeric Antigen Receptors
 The Conception of GCC- Specific Chimeric Antigen Receptors Raven Smith-Parris 8/6/2013 Abstract Colorectal Cancer is an aggressive disease that claims the lives of both men and women every year. It is
The Conception of GCC- Specific Chimeric Antigen Receptors Raven Smith-Parris 8/6/2013 Abstract Colorectal Cancer is an aggressive disease that claims the lives of both men and women every year. It is
Supplementary Figure 1. Effect of FRC-specific ablation of Myd88 on PP and mln organization.
 Supplementary Figure 1 Effect of FRC-specific ablation of Myd88 on PP and mln organization. (a) PP numbers in 8 10 week old Cre-negative littermate (Ctrl) and Myd88-cKO mice (n = 11 mice; each dot represents
Supplementary Figure 1 Effect of FRC-specific ablation of Myd88 on PP and mln organization. (a) PP numbers in 8 10 week old Cre-negative littermate (Ctrl) and Myd88-cKO mice (n = 11 mice; each dot represents
Nature Immunology: doi: /ni Supplementary Figure 1. Time course of OT-II T reg cell development in thymic slices.
 Supplementary Figure 1 Time course of OT-II T reg cell development in thymic slices. Time course of T reg cell development following addition of OT-II TCR transgenic Rag2 -/- mice (OT-II) thymocytes to
Supplementary Figure 1 Time course of OT-II T reg cell development in thymic slices. Time course of T reg cell development following addition of OT-II TCR transgenic Rag2 -/- mice (OT-II) thymocytes to
Specialty Lab Services. Deep science at scale
 Specialty Lab Services Deep science at scale Advancing biomarker research Our broad expertise and global laboratory footprint deliver deep science at scale Specialty assays drive insight into preclinical
Specialty Lab Services Deep science at scale Advancing biomarker research Our broad expertise and global laboratory footprint deliver deep science at scale Specialty assays drive insight into preclinical
Human skin punch biopsies were obtained under informed consent from normal healthy
 SUPPLEMENTAL METHODS Acquisition of human skin specimens. Human skin punch biopsies were obtained under informed consent from normal healthy volunteers (n = 30) and psoriasis patients (n = 45) under a
SUPPLEMENTAL METHODS Acquisition of human skin specimens. Human skin punch biopsies were obtained under informed consent from normal healthy volunteers (n = 30) and psoriasis patients (n = 45) under a
Find 1 cell in 100,000 with ELISpot
 Find 1 cell in 100,000 with ELISpot ELISpot is a sensitive assay used to quantify cytokine- or immunoglobulin-secreting cells at the single-cell level. ELISpot has been widely applied to investigate specific
Find 1 cell in 100,000 with ELISpot ELISpot is a sensitive assay used to quantify cytokine- or immunoglobulin-secreting cells at the single-cell level. ELISpot has been widely applied to investigate specific
BIOGRAPHICAL SKETCH. POSITION TITLE: Professor of Immunology; Director, Jill Roberts Institute for Research in Inflammatory Bowel Disease
 BIOGRAPHICAL SKETCH NAME: Artis, David era COMMONS USER NAME: dartis POSITION TITLE: Professor of Immunology; Director, Jill Roberts Institute for Research in Inflammatory Bowel Disease EDUCATION/TRAINING
BIOGRAPHICAL SKETCH NAME: Artis, David era COMMONS USER NAME: dartis POSITION TITLE: Professor of Immunology; Director, Jill Roberts Institute for Research in Inflammatory Bowel Disease EDUCATION/TRAINING
Managing Specimen Stability for Robust Flow Cytometric Clinical Biomarker Assays
 Managing Specimen Stability for Robust Flow Cytometric Clinical Biomarker Assays Dianna Wu, Richard Wnek Molecular Biomarkers & Diagnostics Merck Co., Inc 2014 AAPS Annual Meeting San Diego, CA (Fluorescent
Managing Specimen Stability for Robust Flow Cytometric Clinical Biomarker Assays Dianna Wu, Richard Wnek Molecular Biomarkers & Diagnostics Merck Co., Inc 2014 AAPS Annual Meeting San Diego, CA (Fluorescent
HSV-2 therapeutic vaccine program. Subunit vaccine candidates
 HSV-2 therapeutic vaccine program Subunit vaccine candidates Our HSV-2 vaccine program Therapeutic subunit vaccine T-cell-based Aim: Best-in-class antigens by engineering Excellent standing (Sept 2017):
HSV-2 therapeutic vaccine program Subunit vaccine candidates Our HSV-2 vaccine program Therapeutic subunit vaccine T-cell-based Aim: Best-in-class antigens by engineering Excellent standing (Sept 2017):
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
Supplementary Information
 1 2 Supplementary Information 3 4 5 6 7 8 Supplementary Figure 1. Loading of R837 into PLGA nanoparticles. The loading efficiency (a) and loading capacity (b) of R837 by PLGA nanoparticles obtained at
1 2 Supplementary Information 3 4 5 6 7 8 Supplementary Figure 1. Loading of R837 into PLGA nanoparticles. The loading efficiency (a) and loading capacity (b) of R837 by PLGA nanoparticles obtained at
