The Comet Assay How to recognise Good Data
|
|
|
- Chad Carter
- 6 years ago
- Views:
Transcription
1 The Comet Assay How to recognise Good Data William Barfield 4 th September 2015 ICAW
2 Content Regulatory Genetic Toxicology JaCVAM trial overview and results Protocols Historical control data Statistics Power curves Control charts
3 Regulatory Genotoxicity Testing Revision, designated S2(R1), November 2011 Replaces and combines the previous S2A and S2B guidelines, issued in 1995 and 1997 respectively. Strategy to reduce delays due to high frequency of non relevant in vitro positive results thus reducing animal use
4 ICH S2(R1) 2 Options Option 1 Ames Chromosome aberration assay or Mouse lymphoma assay or In vitro micronucleus assay In vivo chromosome aberration assay or In vivo micronucleus assay (Acute or repeat dose)
5 ICH S2(R1) 2 Options Option 1 2 nd In vivo test? Comet assay Negative results in two appropriate in vivo assays with adequate exposure are sufficient to demonstrate lack of genotoxicity
6 ICH S2(R1) 2 Options Option 2 Ames In vivo micronucleus assay (Acute or repeat dose) In vivo Comet assay
7 ICH S2(R1) - Comet ICH S2(R1) released in 2011 Comet OECD guideline released in 2014 Although there was no OECD guideline for the comet assay at the time of release of ICH S2(R1) it was considered a suitable 2 nd in vivo assay. UDS assay was losing popularity due to poor sensitivity
8 JaCVAM validation trial Within and or between-lab variability of assay results examined in phases 1-3 Variability disappeared with protocol refinement, confirmed in phase 4.1 Criteria for negative and positive controls confirmed % tail intensity 1 8% Liver, 1-20% Glandular stomach In validation study no clear false positives
9 Phase 4.2 Results Final judgement All results discussed under a blind condition with coded chemicals Statistics and histopathology Predictive capability determined Carcinogens Positive sensitivity Non carcinogens Negative specificity
10 Phase 4.2 Results Genotoxic carcinogens Expect positive judgement 13/19 = Positive sensitivity 68% Non genotoxic non carcinogens Expect negative judgement 7/8 = Negative specificity 88%
11 Phase 4.2 Results Genotoxic non carcinogens Prefer negative judgement 5/6 = Negative specificity 83% Non genotoxic carcinogens Prefer negative judgement 7/7 = Negative specificity 100%
12 Phase 4.2 Results CONCORDANCE 32/40 = 80% JaCVAM trial concluded comet assay could be at least equal or more sensitive to detect genotoxic carcinogens which could be detected in the UDS assay.
13 Life before the guideline What does a positive look like? What does it mean? What measurement to use? What does it measure? What tissues are they the same? Image analysis or eye? What statistics? What protocol to use? Cell or nucleus? Not sensitive enough! It s too sensitive! What ph? What about Apoptosis, Necrosis It doesn t detect mutagens!, hyperplasia etc? What end point? What on earth are hedgehogs and ghost cells!
14 Protocol - JaCVAM Design Rat: Crl:CD (SD), 7-9 weeks at dosing, 5 per group Vehicle control and 3 dose levels Dosing 0hrs, 24hrs, 45hrs sampling at 48hrs Positive control EMS at 200 mg/kg/day Tissues liver and stomach Electrophoresis 0.7 V/cm, voltage 0.30A, 2-8 C Measured % tail DNA
15 Experimental variability Cell death (apoptosis, necrosis) and endonuclease activity are associated with increased DNA fragmentation This could be caused by a test item or inappropriate assay conditions (e.g., time and temperature during tissue processing), electrophoresis conditions misleading data interpretation
16 Protocols Dose Dose Sample Micronucleus 0hr 24hr 48hr Comet Dose Dose Sample 0hr 24hr 27hr Combined Dose Dose Dose Sample 0hr 24hr 45hr 48hr
17 What does good data look like? How many animals per group? >=5 How many slides per animal? 3 How many cells per slide? 50 Which parameter to measure? % tail intensity What does vehicle or positive control data look like? Is it the same for all tissues? Dose (mg/kg/day) Animal number Slide Number Number of cells scored Single slide mean tail intensity (%) Animal mean tail intensity (%) Group mean tail intensity (%) Single slide median tail intensity (%) Animal mean of median tail intensity (%) Group mean of median tail intensity (%)
18 What does good data look like? Four-way hierarchical nested design: Treatment Animal Gel (slide) Cell Fixed effect Random effects Treatments tested against the animal variability
19 What does good data look like? Group Vehicle Low Test substance Medium High Positive Control Animal a b c a b c a b c a b c
20 Historical control As more experimental data are added to the historical control distribution, concurrent negative controls should ideally be within the 95% control limits of that distribution. The laboratory s historical negative control database should be statistically robust to ensure the ability of the laboratory to assess the distribution of their negative control data. The literature suggests that a minimum of 10 experiments may be necessary but would preferably consist of at least 20 experiments conducted under comparable experimental conditions.
21 Historical control Animal reference ranges Mean % tail intensity Species/strain Control Tissue Mean SD Max Med Min Rats Crl:CD(SD) Vehicle Liver Positive Liver Animal reference ranges Median % tail intensity Species/strain Control Tissue Mean SD Max Med Min Rats Crl:CD(SD) Vehicle Liver Positive Liver
22 Historical control Group reference ranges Mean % tail intensity Species/strain Control Tissue Max Med Min Rats Crl:CD(SD) Vehicle Liver Positive Liver Group reference ranges Median % tail intensity Species/strain Control Tissue Max Med Min Rats Crl:CD(SD) Vehicle Liver Positive Liver
23 Statistical analyses The median value from each slide is obtained for tail intensity The average of the three medians is calculated and used in the statistical analysis For tail intensity from each organ, separate analyses are performed for comparisons of Control vs. Increasing dose levels of Test Compound Control vs. Positive Control If any variable contains any zero values, will be added to all responses to enable a logarithmic transformation where necessary
24 Methodology For the comparison of test compound groups to control, the following pathway is followed: pass Test of equal variability (Bartlett s test) fail Transform data Test for trend (F1 test) Test for trend (H1 test) pass fail pass fail Williams test Dunnett s test Shirley s test Steel s test
25 Methodology For the comparison of positive control to control, the following pathway is followed: pass Test of equal variability (Bartlett s test) fail Transform data t-test Wilcoxon rank sum test
26 Power curves The power has been calculated based on the power of a two sided t-test (which is equivalent to the comparison of the lowest dose group with the control using the statistical methods currently used for comet). Power has been calculated for group size of 6, which is standard for non positive control groups. Power has been calculated for the three main parameters of tail intensity, length and moment. Of these, tail intensity is the key parameter.
27 Power Power Power Curves, HLS Data 100% Liver - Tail intensity - n=6 Males Females 80% X of Control 100% Liver - Tail moment - n=6 We could reduce the number of animals used per group 80% 60% 40% Males Females 20% 0% X of Control
28 Control charts Laboratories should use quality control methods, such as control charts (e.g. C-charts or X-bar charts) to identify how variable their data are, and to show that the methodology is 'under control' in their laboratory.
29 Control charts X bar chart to monitor control group means Control limits are set at 3 sigma. Means probability of data falling out the limit is (370 points plotted before outside limits). Stage 1 20 studies advised to set limits (10 minimum) Stage 2 All studies included unless extreme outlier No clear guidance on limit recalculation Practical advice is to recalculate limits after every 3 to 4 studies.
30 Control charts Rules Nelson rules are a method in process control of determining if some measured variable is out of control (unpredictable versus consistent) One point is more than 3 sd from the mean. Nine (or more) points in a row are on the same side of the mean. Six (or more) points in a row are continually increasing (or decreasing) Fourteen (or more) points in a row alternate in direction, increasing then decreasing.
31 Control charts Rules - continued Two (or three) out of three points in a row are more than 2 sd from the mean in the same direction. Four (or five) out of five points in a row are more than 1 sd from the mean in the same direction. Fifteen points in a row are all within 1 sd of the mean on either side of the mean. Eight points in a row exist with none within 1 sd of the mean and the points are in both directions from the mean.
32 Liver % tail intensity X bar chart obs Group mean UCL LCL Mean of group means X bar control limits UCL 4.58 LCL 2.77
33 HLS data various tissues Means and SDs
34 Thank you for listening- Any Questions?
Three recently approved in vivo genotoxicity test guidelines
 Three recently approved in vivo genotoxicity test guidelines (Revised in February 2018) a. Title of the test guideline (year of original adoption, year of update) In Vitro Mammalian Cell Gene Mutation
Three recently approved in vivo genotoxicity test guidelines (Revised in February 2018) a. Title of the test guideline (year of original adoption, year of update) In Vitro Mammalian Cell Gene Mutation
EFSA Guidance on the Submission of a. Evaluation: Toxicological data
 Committed since 2002 to ensuring that Europe s food is safe EFSA Guidance on the Submission of a Dossier on Food Enzymes for Safety Evaluation: Toxicological data Prof. Karl-Heinz Engel CEF Panel member
Committed since 2002 to ensuring that Europe s food is safe EFSA Guidance on the Submission of a Dossier on Food Enzymes for Safety Evaluation: Toxicological data Prof. Karl-Heinz Engel CEF Panel member
S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use
 S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use Peter Kasper BfArM and S2(R1)EWP International Conference on Harmonisation of
S2(R1) Revision of the Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use Peter Kasper BfArM and S2(R1)EWP International Conference on Harmonisation of
Our Business. in vitro / in vivo. in vitro / in vivo ADME. in vitro / in vivo. Toxicology. Cell Battery V79. Analytical Services. Service.
 GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
REQUEST FOR A RE-EVALUATION OF HAIR DYES LISTED IN ANNEX III TO DIRECTIVE 76/768/EEC ON COSMETIC PRODUCTS
 OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING REQUEST FOR A RE-EVALUATION OF HAIR DYES LISTED IN ANNEX III TO DIRECTIVE 76/768/EEC ON
OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING REQUEST FOR A RE-EVALUATION OF HAIR DYES LISTED IN ANNEX III TO DIRECTIVE 76/768/EEC ON
SERVA DNA Stain Clear G Cat. No
 1 SERVA DNA Stain Clear G Cat. No. 39804 1. Introduction Ethidium bromide (EtBr) is most commonly used nucleic acid stain in molecular biology laboratories. It has been proved to be strong carcinogen and
1 SERVA DNA Stain Clear G Cat. No. 39804 1. Introduction Ethidium bromide (EtBr) is most commonly used nucleic acid stain in molecular biology laboratories. It has been proved to be strong carcinogen and
OECD/OCDE OECD GUIDELINE FOR THE TESTING OF CHEMICALS. In Vivo Mammalian Alkaline Comet Assay
 Adopted: 29 July 2016 OECD GUIDELINE FOR THE TESTING OF CHEMICALS In Vivo Mammalian Alkaline Comet Assay INTRODUCTION 1. The in vivo alkaline comet (single cell gel electrophoresis) assay (hereafter called
Adopted: 29 July 2016 OECD GUIDELINE FOR THE TESTING OF CHEMICALS In Vivo Mammalian Alkaline Comet Assay INTRODUCTION 1. The in vivo alkaline comet (single cell gel electrophoresis) assay (hereafter called
LAWSONE OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS. SCCNFP/0561/02, final.
 SCCNFP/0561/02, final OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING LAWSONE Colipa n C146 adopted by the SCCNFP during the 19 th plenary
SCCNFP/0561/02, final OPINION OF THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS CONCERNING LAWSONE Colipa n C146 adopted by the SCCNFP during the 19 th plenary
Ames Data Submissions and Other Qualification Data for Impurities in Drug Substances
 Ames Data Submissions and Other Qualification Data for Impurities in Drug Substances Mark W. Powley, Ph.D. Pharmacologist FDA/CDER/Office of New Drugs Outline Regulatory Background Ames Assay ICH M7 Submission
Ames Data Submissions and Other Qualification Data for Impurities in Drug Substances Mark W. Powley, Ph.D. Pharmacologist FDA/CDER/Office of New Drugs Outline Regulatory Background Ames Assay ICH M7 Submission
Glyphosate Research Scoping
 Glyphosate Research Scoping Stephanie L. Smith-Roe, Ph.D. Biomolecular Screening Branch National Institute of Environmental Health Sciences NTP Board of Scientific Counselors Meeting June 15 16, 2016 Glyphosate
Glyphosate Research Scoping Stephanie L. Smith-Roe, Ph.D. Biomolecular Screening Branch National Institute of Environmental Health Sciences NTP Board of Scientific Counselors Meeting June 15 16, 2016 Glyphosate
International Council for Harmonisation (ICH) Safety Guideline Updates
 International Council for Harmonisation (ICH) Safety Guideline Updates ICH Regional Public Meeting Canada-U.S. Regulatory Cooperation Council November 12, 2015 Alisa Vespa, Ph.D. Health Canada Active ICH
International Council for Harmonisation (ICH) Safety Guideline Updates ICH Regional Public Meeting Canada-U.S. Regulatory Cooperation Council November 12, 2015 Alisa Vespa, Ph.D. Health Canada Active ICH
The JaCVAM / OECD activities on the comet assay. Hajime Kojima NIHS, JaCVAM, Japan
 The JaCVAM / OECD activities on the comet assay Hajime Kojima NIHS, JaCVAM, Japan 1 Today s Topics 1.Summary of in vivo comet assay Validation Studies 2.Peer review by the OECD experts 3.General Introduction
The JaCVAM / OECD activities on the comet assay Hajime Kojima NIHS, JaCVAM, Japan 1 Today s Topics 1.Summary of in vivo comet assay Validation Studies 2.Peer review by the OECD experts 3.General Introduction
Combining the in vivo comet and micronucleus assays: a practical approach to genotoxicity testing and data interpretation
 Mutagenesis vol. 25 no. 2 pp. 187 199, 2010 doi:10.1093/mutage/gep060 Advance Access Publication 6 December 2009 Combining the in vivo comet and micronucleus assays: a practical approach to genotoxicity
Mutagenesis vol. 25 no. 2 pp. 187 199, 2010 doi:10.1093/mutage/gep060 Advance Access Publication 6 December 2009 Combining the in vivo comet and micronucleus assays: a practical approach to genotoxicity
APPENDIX IV OECD GUIDELINES FOR TESTING OF CHEMICALS. 1- The OECD Test Guidelines submission and adoption process
 APPENDIX IV OECD GUIDELINES FOR TESTING OF CHEMICALS 1- The OECD Test Guidelines submission and adoption process The OECD Test Guidelines Programme provides the mechanism for developing new Test Guidelines,
APPENDIX IV OECD GUIDELINES FOR TESTING OF CHEMICALS 1- The OECD Test Guidelines submission and adoption process The OECD Test Guidelines Programme provides the mechanism for developing new Test Guidelines,
Table 1: Current status of the test guidelines for genetic toxicology
 OECD TEST GUIDELINES FOR TESTING OF CHEMICALS: INTRODUCTION TO THE OECD GUIDELINES ON GENETIC TOXICOLOGY TESTING 1. GENERAL INTRODUCTION 1. This Introduction to the Test Guidelines (TG) on genetic toxicology
OECD TEST GUIDELINES FOR TESTING OF CHEMICALS: INTRODUCTION TO THE OECD GUIDELINES ON GENETIC TOXICOLOGY TESTING 1. GENERAL INTRODUCTION 1. This Introduction to the Test Guidelines (TG) on genetic toxicology
Midori Green Advance DNA Stain Safety Report
 Publishing date: 30.09.2011 Midori Green Advance DNA Stain Safety Report IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name Catalog number Supplier Information in case of emergency Midori Green
Publishing date: 30.09.2011 Midori Green Advance DNA Stain Safety Report IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name Catalog number Supplier Information in case of emergency Midori Green
HDGreen Safe DNA Dye Safety Test Reports
 HDGreen Safe DNA Dye Safety Test Reports IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name HDGreen Safe DNA Dye Catalog number ISII-HDGreen Supplier Information in case of emergency Intas Science
HDGreen Safe DNA Dye Safety Test Reports IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name HDGreen Safe DNA Dye Catalog number ISII-HDGreen Supplier Information in case of emergency Intas Science
Structure and content of an IMPD. What is required for first into man trial?
 What is required for first into man? The EU IMPD Thomas Sudhop, MD Scope Structure and content of an IMPD What is required for first into man trial? Only for IMPs that do not have a marketing authorisation
What is required for first into man? The EU IMPD Thomas Sudhop, MD Scope Structure and content of an IMPD What is required for first into man trial? Only for IMPs that do not have a marketing authorisation
Medical Device Testing. Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark
 Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
Training session: comet assay on plants
 Training session: comet assay on plants Dr Bertrand Pourrut, PhD Contact: bertrand.pourrut@isa-lille.fr Groupe ISA, Equipe Sols et Environnement, LGCgE Lille Nord de France, 59046 Lille Cedex ICAW 2015
Training session: comet assay on plants Dr Bertrand Pourrut, PhD Contact: bertrand.pourrut@isa-lille.fr Groupe ISA, Equipe Sols et Environnement, LGCgE Lille Nord de France, 59046 Lille Cedex ICAW 2015
Midori Green DNA Stain Safety Test Reports
 Midori Green DNA Stain Safety Test Reports IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name Catalog number Supplier Information in case of emergency Midori Green DNA Stain MG01 MG02 Nippon
Midori Green DNA Stain Safety Test Reports IDENTIFICATION OF THE PRODUCT AND OF THE COMPANY Product name Catalog number Supplier Information in case of emergency Midori Green DNA Stain MG01 MG02 Nippon
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) POSITION PAPER ON NON-CLINICAL SAFETY STUDIES TO SUPPORT CLINICAL TRIALS WITH A SINGLE MICRODOSE
 European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2004 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) POSITION PAPER ON NON-CLINICAL SAFETY STUDIES TO SUPPORT CLINICAL
European Medicines Agency Evaluation of Medicines for Human Use London, 23 June 2004 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) POSITION PAPER ON NON-CLINICAL SAFETY STUDIES TO SUPPORT CLINICAL
Supplementary Figure 1. Details of the component modelling and steps in generating and validating a PTGS. The steps (boxes) and the flow of
 Supplementary Figure 1. Details of the component modelling and steps in generating and validating a PTGS. The steps (boxes) and the flow of information (arrows) through the modelling process, including
Supplementary Figure 1. Details of the component modelling and steps in generating and validating a PTGS. The steps (boxes) and the flow of information (arrows) through the modelling process, including
Basic principles of the safety assessment of drugs
 Basic principles of the safety assessment of drugs SFT Annual meeting 2013 Björn Dahl, PhD Senior Project Director Global Safety Assessment AstraZeneca R&D Outline of presentation Safety assessment in
Basic principles of the safety assessment of drugs SFT Annual meeting 2013 Björn Dahl, PhD Senior Project Director Global Safety Assessment AstraZeneca R&D Outline of presentation Safety assessment in
ICNIRP 7th International NIR Workshop
 ADVANTAGE, CHALLENGES AND LIMITS OF EXPERIMENTAL STUDIES CARMELA MARINO ICNIRP MEMBER Head of the Unit of Radiation Biology and Human Health, ENEA, Rome, Italy EXPERIMENTAL STUDIES ARE USED TO ASSESS RISK
ADVANTAGE, CHALLENGES AND LIMITS OF EXPERIMENTAL STUDIES CARMELA MARINO ICNIRP MEMBER Head of the Unit of Radiation Biology and Human Health, ENEA, Rome, Italy EXPERIMENTAL STUDIES ARE USED TO ASSESS RISK
Agreement of the Working Group of the National Coordinators of the Test Guidelines Program on the Follow-up to the Validation Peer Review Report
 DRAFT SUMMARY REPORT OF THE PEER REVIEW PANEL ASSESSING THE JACVAM INITIATIVE INTERNATIONAL VALIDATION STUDIES OF THE IN VIVO RODENT ALKALINE COMET ASSAY FOR THE DETECTION OF GENOTOXIC CARCINOGENS FORWORD
DRAFT SUMMARY REPORT OF THE PEER REVIEW PANEL ASSESSING THE JACVAM INITIATIVE INTERNATIONAL VALIDATION STUDIES OF THE IN VIVO RODENT ALKALINE COMET ASSAY FOR THE DETECTION OF GENOTOXIC CARCINOGENS FORWORD
11 th International Conference on Environmental Mutagens Foz du Iguaccu, Brazil; 7 November 2013
 Pig-a In Vivo Mutation Assay Workgroup Report 6 th International Workshop on Genotoxicity Testing Presented By: Bhaskar Gollapudi, Ph.D. Exponent, Inc. 11 th International Conference on Environmental Mutagens
Pig-a In Vivo Mutation Assay Workgroup Report 6 th International Workshop on Genotoxicity Testing Presented By: Bhaskar Gollapudi, Ph.D. Exponent, Inc. 11 th International Conference on Environmental Mutagens
Integrating Molecular Toxicology Earlier in the Drug Development Process
 Integrating Molecular Toxicology Earlier in the Drug Development Process Jonathan Hitchcock Molecular Toxicology, Drug Safety R&D Pfizer Global Research & Development Sandwich, Kent UK 4.5 4.0 3.5 3.0
Integrating Molecular Toxicology Earlier in the Drug Development Process Jonathan Hitchcock Molecular Toxicology, Drug Safety R&D Pfizer Global Research & Development Sandwich, Kent UK 4.5 4.0 3.5 3.0
Single cell resolution in vivo imaging of DNA damage following PARP inhibition. Supplementary Data
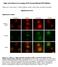 Single cell resolution in vivo imaging of DNA damage following PARP inhibition Katherine S. Yang, Rainer H. Kohler, Matthieu Landon, Randy Giedt, and Ralph Weissleder Supplementary Data Supplementary Figures
Single cell resolution in vivo imaging of DNA damage following PARP inhibition Katherine S. Yang, Rainer H. Kohler, Matthieu Landon, Randy Giedt, and Ralph Weissleder Supplementary Data Supplementary Figures
Test Report. The Report of Acute Oral Toxicity Test
 Test Report Sample No.: 2011KF0435 Test Item: Safe Nucleic Acid Stain Sponsor: Yekta Tajhiz Azma The Report of Acute Oral Toxicity Test Test item: Safe Nucleic Acid Stain Sample No.: 2011KF0435 Subject
Test Report Sample No.: 2011KF0435 Test Item: Safe Nucleic Acid Stain Sponsor: Yekta Tajhiz Azma The Report of Acute Oral Toxicity Test Test item: Safe Nucleic Acid Stain Sample No.: 2011KF0435 Subject
Statistical Analysis of the Comet-Assay
 Statistical Analysis of the Comet-Assay Bernd-Wolfgang Igl Principal Statistician Bayer AG Berlin PSI Toxicology SIG Webinar 17 th April 2018 Non-Clinical Drug Safety, Genetic Toxicology, Bayer AG Contents
Statistical Analysis of the Comet-Assay Bernd-Wolfgang Igl Principal Statistician Bayer AG Berlin PSI Toxicology SIG Webinar 17 th April 2018 Non-Clinical Drug Safety, Genetic Toxicology, Bayer AG Contents
Genotoxicity testing strategies: updated recommendations for the assessment of pesticides Riccardo Crebelli
 New Pesticide Regulation: Innovative Aspects and Emerging Problems October 5, 2010 Aula Pocchiari - Istituto Superiore di Sanità Viale Regina Elena, 299 ROMA Genotoxicity testing strategies: updated recommendations
New Pesticide Regulation: Innovative Aspects and Emerging Problems October 5, 2010 Aula Pocchiari - Istituto Superiore di Sanità Viale Regina Elena, 299 ROMA Genotoxicity testing strategies: updated recommendations
MB Expands Capabilities Adds Ames Test, MTD and Range Finding
 What's New @ MB? MB Expands Capabilities - Ames, PK, MTD, Range Finding New! - PorCORA - Non-Animal Ocular Irritation/Reversibility Test Under Development - PorFocal - Low-Level Ocular Irritation Screening
What's New @ MB? MB Expands Capabilities - Ames, PK, MTD, Range Finding New! - PorCORA - Non-Animal Ocular Irritation/Reversibility Test Under Development - PorFocal - Low-Level Ocular Irritation Screening
06/03/2009. Overview. Preclinical Support for Exploratory Phase I Clinical Trials. Micro-dosing IND. Pharmacological Active Single Dose IND
 Preclinical Support for Exploratory Phase I Clinical Trials Clive Joseph, DSRD Sandwich Overview Identify the most appropriate development paradigm - traditional vs alternative IND approach Confidence
Preclinical Support for Exploratory Phase I Clinical Trials Clive Joseph, DSRD Sandwich Overview Identify the most appropriate development paradigm - traditional vs alternative IND approach Confidence
A Strategy for Reducing Animal Use in the U.S. EPA's Endocrine Disruption Screening Program
 A Strategy for Reducing Animal Use in the U.S. EPA's Endocrine Disruption Screening Program Catherine Willett, PhD Science Policy Advisor Patricia Bishop, M.S. Research Associate Kristie Sullivan, MPH
A Strategy for Reducing Animal Use in the U.S. EPA's Endocrine Disruption Screening Program Catherine Willett, PhD Science Policy Advisor Patricia Bishop, M.S. Research Associate Kristie Sullivan, MPH
ICH S9 guideline on nonclinical evaluation for anticancer pharmaceuticals - questions and answers
 16 May 2018 EMA/CHMP/ICH/453684/2016 Committee for Human Medicinal Products ICH S9 guideline on nonclinical evaluation for anticancer pharmaceuticals - questions and answers Step 5 Transmission to CHMP
16 May 2018 EMA/CHMP/ICH/453684/2016 Committee for Human Medicinal Products ICH S9 guideline on nonclinical evaluation for anticancer pharmaceuticals - questions and answers Step 5 Transmission to CHMP
What can be done from regulatory side?
 Federal Agency for Medicines and Health Products (FAMHP) What can be done from regulatory side? 9th Annual ecopa Workshop November 29-30, 2008, Brussels Dr. Sonja BEKEN Non-Clinical Assessor, Registration
Federal Agency for Medicines and Health Products (FAMHP) What can be done from regulatory side? 9th Annual ecopa Workshop November 29-30, 2008, Brussels Dr. Sonja BEKEN Non-Clinical Assessor, Registration
Inspections, Compliance, Enforcement, and Criminal Investigations
 Seite 1 von 6 Inspections, Compliance, Enforcement, and Criminal Investigations Sitek Research Laboratories 5/21/09 Department of Health and Human Services Public Health Service Food and Drug Administration
Seite 1 von 6 Inspections, Compliance, Enforcement, and Criminal Investigations Sitek Research Laboratories 5/21/09 Department of Health and Human Services Public Health Service Food and Drug Administration
Oncology Biopharmaceuticals and Preclinical Development: Evolving Regulatory Challenges
 The content of this presentation reflects the opinion of the speaker and does not necessarily represent the official position of CDER Oncology Biopharmaceuticals and Preclinical Development: Evolving Regulatory
The content of this presentation reflects the opinion of the speaker and does not necessarily represent the official position of CDER Oncology Biopharmaceuticals and Preclinical Development: Evolving Regulatory
Rapidly regulated genes are intron poor
 Supplementary material Rapidly regulated genes are intron poor Daniel C. Jeffares 1 *, Christopher J. Penkett 1,2 * and Jürg Bähler 1,3 1 Wellcome Trust Sanger Institute, Hinxton, Cambridge CB10 1SA, UK
Supplementary material Rapidly regulated genes are intron poor Daniel C. Jeffares 1 *, Christopher J. Penkett 1,2 * and Jürg Bähler 1,3 1 Wellcome Trust Sanger Institute, Hinxton, Cambridge CB10 1SA, UK
 Hazard identification Dose-response assessment Exposure assessment Risk characterization In vivo animal experiments Endpoint assays (mortality) Epidemiological studies Environmental monitoring Extrapolation
Hazard identification Dose-response assessment Exposure assessment Risk characterization In vivo animal experiments Endpoint assays (mortality) Epidemiological studies Environmental monitoring Extrapolation
486 Adopted: 21st July 1997
 486 Adopted: 21st July 1997 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells In Vivo INTRODUCTION 1. The purpose of the unscheduled DNA synthesis
486 Adopted: 21st July 1997 OECD GUIDELINE FOR THE TESTING OF CHEMICALS Unscheduled DNA Synthesis (UDS) Test with Mammalian Liver Cells In Vivo INTRODUCTION 1. The purpose of the unscheduled DNA synthesis
EUSAAT Linz (Austria) October The JaCVAM validation study of the ROS in vitro photoxicity assay
 EUSAAT2013 - Linz (Austria) 15-18 October 2012 The JaCVAM validation study of the ROS in vitro photoxicity assay Dr. med. Horst Spielmann Professor for Regulatory Toxicology, FU Berlin & Sate Animal Welfare
EUSAAT2013 - Linz (Austria) 15-18 October 2012 The JaCVAM validation study of the ROS in vitro photoxicity assay Dr. med. Horst Spielmann Professor for Regulatory Toxicology, FU Berlin & Sate Animal Welfare
S9 Implementation Working Group ICH S9 Guideline: Nonclinical Evaluation for Anticancer Pharmaceuticals Questions and Answers
 S9 Implementation Working Group ICH S9 Guideline: Nonclinical Evaluation for Anticancer Pharmaceuticals Questions and Answers Current Step 4 version International Council for Harmonisation of Technical
S9 Implementation Working Group ICH S9 Guideline: Nonclinical Evaluation for Anticancer Pharmaceuticals Questions and Answers Current Step 4 version International Council for Harmonisation of Technical
UPDATED RECOMMENDED STRATEGY TESTING HAIR DYES FOR THEIR POTENTIAL GENOTOXICITY/MUTAGENICITY/CARCINOGENICITY
 THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS UPDATED RECOMMENDED STRATEGY FOR TESTING HAIR DYES FOR THEIR POTENTIAL GENOTOXICITY/MUTAGENICITY/CARCINOGENICITY
THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS UPDATED RECOMMENDED STRATEGY FOR TESTING HAIR DYES FOR THEIR POTENTIAL GENOTOXICITY/MUTAGENICITY/CARCINOGENICITY
Correlation between the results of in vitro and in vivo chromosomal damage tests in consideration of exposure levels of test chemicals
 Yamamura et al. Genes and Environment (28) 4:6 https://doi.org/.86/s42-8-94-3 SHORT REPORT Correlation between the results of in vitro and in vivo chromosomal damage tests in consideration of exposure
Yamamura et al. Genes and Environment (28) 4:6 https://doi.org/.86/s42-8-94-3 SHORT REPORT Correlation between the results of in vitro and in vivo chromosomal damage tests in consideration of exposure
Testing Methods and Directives
 EUROPEAN COMMISSION DIRECTORATE GENERAL - JRC JOINT RESEARCH CENTRE Institute for Health and Consumer Protection Unit: Toxicology and Chemical Substances European Chemicals Bureau D:\My Documents\JR\Tables\OFFJOUR\Listofoj.doc
EUROPEAN COMMISSION DIRECTORATE GENERAL - JRC JOINT RESEARCH CENTRE Institute for Health and Consumer Protection Unit: Toxicology and Chemical Substances European Chemicals Bureau D:\My Documents\JR\Tables\OFFJOUR\Listofoj.doc
Overview. Presenter: Bill Cheney. Audience: Clinical Laboratory Professionals. Field Guide To Statistics for Blood Bankers
 Field Guide To Statistics for Blood Bankers A Basic Lesson in Understanding Data and P.A.C.E. Program: 605-022-09 Presenter: Bill Cheney Audience: Clinical Laboratory Professionals Overview Statistics
Field Guide To Statistics for Blood Bankers A Basic Lesson in Understanding Data and P.A.C.E. Program: 605-022-09 Presenter: Bill Cheney Audience: Clinical Laboratory Professionals Overview Statistics
Archived Content. This content was archived on February 4, 2017.
 This content was archived on February 4, 2017. Archived Content Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after
This content was archived on February 4, 2017. Archived Content Information identified as archived on the Web is for reference, research or recordkeeping purposes. It has not been altered or updated after
Guidance on Information Requirements and Chemical Safety Assessment. Chapter R.7a: Endpoint specific guidance
 1 GUIDANCE Guidance on Information Requirements and Chemical Safety Assessment Chapter R.7a: Endpoint specific guidance Version 3.0 August 2014 NOTE Please note that the present document is a proposed
1 GUIDANCE Guidance on Information Requirements and Chemical Safety Assessment Chapter R.7a: Endpoint specific guidance Version 3.0 August 2014 NOTE Please note that the present document is a proposed
Supplementary exam work (Number, form, scope) Courses (type of teaching) Contact time. Winter semester
 IEW-M1.1 Principles in Toxicology Number of credit points (CP): 12 Introduction to general toxicology Foundations of toxicokinetics (ADME: absorption, distribution, metabolism, and excretion) Foundations
IEW-M1.1 Principles in Toxicology Number of credit points (CP): 12 Introduction to general toxicology Foundations of toxicokinetics (ADME: absorption, distribution, metabolism, and excretion) Foundations
COMICS: developing high throughput comet assays for DNA damage and DNA repair
 COMICS: developing high throughput comet assays for DNA damage and DNA repair Amaya Azqueta, on behalf of COMICS partners Department of Nutrition University of Oslo is an EC strategic targeted project
COMICS: developing high throughput comet assays for DNA damage and DNA repair Amaya Azqueta, on behalf of COMICS partners Department of Nutrition University of Oslo is an EC strategic targeted project
Toxicology Testing. Genetic Toxicology Service List Comprehensive
 Toxicology Testing Genetic Toxicology Service List Comprehensive Table of Contents Preface Page Regulatory Guidelines 1 Assay Details Section Color Code Page GLP In Vitro Assays 2 GLP In Vivo Assays 7
Toxicology Testing Genetic Toxicology Service List Comprehensive Table of Contents Preface Page Regulatory Guidelines 1 Assay Details Section Color Code Page GLP In Vitro Assays 2 GLP In Vivo Assays 7
MAINTENANCE OF THE ICH GUIDELINE ON NON-CLINICAL SAFETY STUDIES FOR THE CONDUCT OF HUMAN CLINICAL TRIALS FOR PHARMACEUTICALS M3(R1)
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE MAINTENANCE OF THE ICH GUIDELINE ON NON-CLINICAL
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH HARMONISED TRIPARTITE GUIDELINE MAINTENANCE OF THE ICH GUIDELINE ON NON-CLINICAL
PROPOSAL THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS
 THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS PROPOSAL FOR A STRATEGY FOR TESTING HAIR DYE COSMETIC INGREDIENTS FOR THEIR POTENTIAL GENOTOXICITY/MUTAGENICITY
THE SCIENTIFIC COMMITTEE ON COSMETIC PRODUCTS AND NON-FOOD PRODUCTS INTENDED FOR CONSUMERS PROPOSAL FOR A STRATEGY FOR TESTING HAIR DYE COSMETIC INGREDIENTS FOR THEIR POTENTIAL GENOTOXICITY/MUTAGENICITY
NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS. Cutting-Edge Science and Constant Innovation: The Keys to Success
 NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge Science and Constant Innovation: The Keys to Success 2 NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge
NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge Science and Constant Innovation: The Keys to Success 2 NON-ANIMAL APPROACHES TO SAFETY ASSESSMENT OF COSMETIC PRODUCTS Cutting-Edge
Cosmetics Europe LRSS Programme
 Cosmetics Europe LRSS Programme 2016-20 Rob Taalman Objectives of CE Research To deliver tools & strategies for animal-free safety assessment Accurate Robust Efficient ultimately accepted by regulators
Cosmetics Europe LRSS Programme 2016-20 Rob Taalman Objectives of CE Research To deliver tools & strategies for animal-free safety assessment Accurate Robust Efficient ultimately accepted by regulators
Pre-Clinical Evaluation of ALN-AAT to Ameliorate Liver Disease Associated with Alpha-1 Antitrypsin Deficiency
 Pre-Clinical Evaluation of ALN-AAT to Ameliorate Liver Disease Associated with Alpha-1 Antitrypsin Deficiency A. Sehgal 1, K. Blomenkamp 2, K Qian 1, A. Simon 1, P. Haslett, S. Barros 1, J. Teckman 2 May
Pre-Clinical Evaluation of ALN-AAT to Ameliorate Liver Disease Associated with Alpha-1 Antitrypsin Deficiency A. Sehgal 1, K. Blomenkamp 2, K Qian 1, A. Simon 1, P. Haslett, S. Barros 1, J. Teckman 2 May
The comet assay for DNA damage and repair. Applications in genotoxicity testing and human biomonitoring
 The comet assay for DNA damage and repair Applications in genotoxicity testing and human biomonitoring Andrew Collins Department of Nutrition, University of Oslo Introduction measuring DNA damage Increasing
The comet assay for DNA damage and repair Applications in genotoxicity testing and human biomonitoring Andrew Collins Department of Nutrition, University of Oslo Introduction measuring DNA damage Increasing
Safety Qualification Process and Application of Thresholds. Jim Blanchard PQRI L&E Toxicology Subgroup Principal Scientist Aradigm
 Safety Qualification Process and Application of Thresholds Jim Blanchard PQRI L&E Toxicology Subgroup Principal Scientist Aradigm Outline Safety qualification process What to do when leachables exceed:
Safety Qualification Process and Application of Thresholds Jim Blanchard PQRI L&E Toxicology Subgroup Principal Scientist Aradigm Outline Safety qualification process What to do when leachables exceed:
Cost Reduction in REACH Alternatives to Testing ChemicalWatch EXPO Berlin, April 2017 Peter Jenkinson CEHTRA
 Consultancy for Environmental and Human Toxicology and Risk Assessment Science Beyond Regulatory Compliance Cost Reduction in REACH Alternatives to Testing ChemicalWatch EXPO Berlin, April 2017 Peter Jenkinson
Consultancy for Environmental and Human Toxicology and Risk Assessment Science Beyond Regulatory Compliance Cost Reduction in REACH Alternatives to Testing ChemicalWatch EXPO Berlin, April 2017 Peter Jenkinson
GLOBAL CHEMICAL REGISTRATIONS: TESTING STRATEGIES AND CMO COMMUNICATION
 GLOBAL CHEMICAL REGISTRATIONS: TESTING STRATEGIES AND CMO COMMUNICATION HPAPI Summit June 21, 2017 Jessica Graham, Ph.D., DABT Senior Research Investigator, Product Quality and Occupational Toxicology
GLOBAL CHEMICAL REGISTRATIONS: TESTING STRATEGIES AND CMO COMMUNICATION HPAPI Summit June 21, 2017 Jessica Graham, Ph.D., DABT Senior Research Investigator, Product Quality and Occupational Toxicology
Competency 1: The student will demonstrate knowledge of the safety regulations that must be implemented at the biotechnology laboratory by:
 Common Course Number: BSC-4420-L Course Title: Biotechnology Laboratory Catalog Course Description: This course provides students with practical, hands-on laboratory experiences to supplement the BSC-4330
Common Course Number: BSC-4420-L Course Title: Biotechnology Laboratory Catalog Course Description: This course provides students with practical, hands-on laboratory experiences to supplement the BSC-4330
Public Interest Incorporated Foundation BioSafety Research Center
 Public Interest Incorporated Foundation BioSafety Research Center http://www.anpyo.or.jp Ver. 201801 Building #1(Test and research annex) Building #2 (Administrat ion office) Building #3 (Toxicity test
Public Interest Incorporated Foundation BioSafety Research Center http://www.anpyo.or.jp Ver. 201801 Building #1(Test and research annex) Building #2 (Administrat ion office) Building #3 (Toxicity test
Supplementary exam work (Number, form, scope) Courses (type of teaching) Contact time. Winter semester
 READING VERSION OF MODULE DESCRIPTIONS The module descriptions are not part of the Regulations; they are integrated into the First Amendment to the Module Catalog. IEW-M1.1 Principles in Toxicology Number
READING VERSION OF MODULE DESCRIPTIONS The module descriptions are not part of the Regulations; they are integrated into the First Amendment to the Module Catalog. IEW-M1.1 Principles in Toxicology Number
Reporting Checklist for Nature Neuroscience
 Corresponding Author: Manuscript Number: Manuscript Type: Chay T. Kuo NNA47008 Article Reporting Checklist for Nature Neuroscience # Main Figures: 7 # Supplementary Figures: 11 # Supplementary Tables:
Corresponding Author: Manuscript Number: Manuscript Type: Chay T. Kuo NNA47008 Article Reporting Checklist for Nature Neuroscience # Main Figures: 7 # Supplementary Figures: 11 # Supplementary Tables:
Bioanalytical Support to In Vitro Studies
 Bioanalytical Support to In Vitro Studies Presenter: Tim Sangster (on behalf of EBF (mini-workshop)) Open Symposium 2017 November 17 th 2017 Barcelona http://www.europeanbioanalysisforum.eu Agenda Ø Scope
Bioanalytical Support to In Vitro Studies Presenter: Tim Sangster (on behalf of EBF (mini-workshop)) Open Symposium 2017 November 17 th 2017 Barcelona http://www.europeanbioanalysisforum.eu Agenda Ø Scope
From Drug Discovery to IND Saving Time and Money Through Smart Study Design
 From Drug Discovery to IND Saving Time and Money Through Smart Study Design Stephen Madden BSc PhD CBiol FSB Head, Metabolism and Pharmacokinetics Charles River Preclinical Services, Edinburgh Outline
From Drug Discovery to IND Saving Time and Money Through Smart Study Design Stephen Madden BSc PhD CBiol FSB Head, Metabolism and Pharmacokinetics Charles River Preclinical Services, Edinburgh Outline
Accelerating Therapeutic Development through a look at current Regulatory Applications A Non-Clinical Perspective
 Accelerating Therapeutic Development through a look at current Regulatory Applications A Non-Clinical Perspective Imran M. Khan, Ph.D. Division of Psychiatry Center for Drug Evaluation and Research FDA
Accelerating Therapeutic Development through a look at current Regulatory Applications A Non-Clinical Perspective Imran M. Khan, Ph.D. Division of Psychiatry Center for Drug Evaluation and Research FDA
Super-marketing. A Data Investigation. A note to teachers:
 Super-marketing A Data Investigation A note to teachers: This is a simple data investigation requiring interpretation of data, completion of stem and leaf plots, generation of box plots and analysis of
Super-marketing A Data Investigation A note to teachers: This is a simple data investigation requiring interpretation of data, completion of stem and leaf plots, generation of box plots and analysis of
Overview Everything You Wanted to Know About GLPs. but were afraid to ask
 Overview Everything You Wanted to Know About GLPs. but were afraid to ask Janet Rose Christensen, M.S.P.H. Vice President, Regulatory Affairs and Quality AVI BioPharma, Inc. Portland, OR jrc@avibio.com/503.227.00554
Overview Everything You Wanted to Know About GLPs. but were afraid to ask Janet Rose Christensen, M.S.P.H. Vice President, Regulatory Affairs and Quality AVI BioPharma, Inc. Portland, OR jrc@avibio.com/503.227.00554
CYANOX 1790 Antioxidant 1,3,5-tris(4-t-butyl-3-hydroxy-2,6-dimethylbenzyl)-1,3,5-triazine-2,4,6-(1H,3H,5H)- trion
 CYTEC MSDS: 0001307 Date: 03/29/2006 Supersedes: 09/01/2005 MATERIAL SAFETY DATA SHEET 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Product Description: Intended/Recommended Use: Supplied
CYTEC MSDS: 0001307 Date: 03/29/2006 Supersedes: 09/01/2005 MATERIAL SAFETY DATA SHEET 1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Name: Product Description: Intended/Recommended Use: Supplied
ELECTRONIC SUPPLEMENTARY INFORMATION (ESI)
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 ELECTRONIC SUPPLEMENTARY INFORMATION (ESI) Naringin ameliorates radiation-induced hepatic damage
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2016 ELECTRONIC SUPPLEMENTARY INFORMATION (ESI) Naringin ameliorates radiation-induced hepatic damage
Trevigen s CometChip Platform (High Through-Put DNA Damage Analysis)
 Trevigen s CometChip Platform (High Through-Put DNA Damage Analysis) www.trevigen.com 301-216-2800 info@trevigen.com 8405 Helgerman Court, Gaithersburg MD 20817 Objectives Review Standard Comet Assay.
Trevigen s CometChip Platform (High Through-Put DNA Damage Analysis) www.trevigen.com 301-216-2800 info@trevigen.com 8405 Helgerman Court, Gaithersburg MD 20817 Objectives Review Standard Comet Assay.
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Standard Operating Procedure
 Standard Operating Procedure Title Subtitle NANoREG Work package/task: Owner and co-owner(s) HTS Comet Assay with and without FPG - 20 wells Comet assay with and without FPG using Trevigen 20-well slides
Standard Operating Procedure Title Subtitle NANoREG Work package/task: Owner and co-owner(s) HTS Comet Assay with and without FPG - 20 wells Comet assay with and without FPG using Trevigen 20-well slides
Living Environment. Directions: Use Aim # (Unit 4) to complete this study guide.
 Name: Date: Period: Living Environment Living Environment Unit 4 Genetics Study Guide Due Date: Test Date: Unit 5 Important Topics: I. Aim # 20 DNA Structure and Function II. Aim # 21 DNA Replication III.
Name: Date: Period: Living Environment Living Environment Unit 4 Genetics Study Guide Due Date: Test Date: Unit 5 Important Topics: I. Aim # 20 DNA Structure and Function II. Aim # 21 DNA Replication III.
ONAMER M. PRESERVATIVE and ANTIMICROBIAL ONAMER
 ONAMER M PRESERVATIVE and ANTIMICROBIAL ONAMER M Stepan Lipid Nutrition is a division of Stepan Company which manufactures lipid and polymer based ingredients. HO OH SUMMARY Our quaternary ammonium polymer
ONAMER M PRESERVATIVE and ANTIMICROBIAL ONAMER M Stepan Lipid Nutrition is a division of Stepan Company which manufactures lipid and polymer based ingredients. HO OH SUMMARY Our quaternary ammonium polymer
Carcinogenicity: No Carcinogenic substances as defined by IARC, NTP and/or OSHA.
 Acute Skin For Component: Chromium Oxide May cause mechanical irritation. Acute Eye For Component: Chromium Oxide May cause slight irritation. Carcinogenicity: No Carcinogenic substances as defined by
Acute Skin For Component: Chromium Oxide May cause mechanical irritation. Acute Eye For Component: Chromium Oxide May cause slight irritation. Carcinogenicity: No Carcinogenic substances as defined by
MATERIAL SAFETY DATA SHEET
 Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Animal Health Pfizer Inc 235 East 42nd Street New York, NY 10017 Poison Control Center Phone: 1-866-531-8896
Page 1 of 7 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Animal Health Pfizer Inc 235 East 42nd Street New York, NY 10017 Poison Control Center Phone: 1-866-531-8896
SCIENTIFIC OPINION. Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19 1
 The EFSA Journal (2008) 854, 1-5 SCIENTIFIC OPINION Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19 1 Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and
The EFSA Journal (2008) 854, 1-5 SCIENTIFIC OPINION Genotoxicity Test Strategy for Substances belonging to Subgroups of FGE.19 1 Statement of the Panel on Food Contact Materials, Enzymes, Flavourings and
STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: GENERAL APPROACH TO TESTING
 VICH GL33 (SAFETY: GENERAL APPROACH) October 2002 For implementation at Step 7 - Final STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: GENERAL APPROACH TO TESTING Recommended
VICH GL33 (SAFETY: GENERAL APPROACH) October 2002 For implementation at Step 7 - Final STUDIES TO EVALUATE THE SAFETY OF RESIDUES OF VETERINARY DRUGS IN HUMAN FOOD: GENERAL APPROACH TO TESTING Recommended
EURL ECVAM. A broader role for greater impact. Maurice Whelan. Head of Systems Toxicology Unit and EURL ECVAM
 EURL ECVAM A broader role for greater impact Maurice Whelan Head of Systems Toxicology Unit and EURL ECVAM Institute for Health and Consumer Protection DG Joint Research Centre Meeting of the EP Intergroup
EURL ECVAM A broader role for greater impact Maurice Whelan Head of Systems Toxicology Unit and EURL ECVAM Institute for Health and Consumer Protection DG Joint Research Centre Meeting of the EP Intergroup
Canadian Experience - Priority Setting and Screening of All Existing Substances under the Canadian Environmental Protection Act (CEPA)
 Canadian Experience - Priority Setting and Screening of All Existing Substances under the Canadian Environmental Protection Act (CEPA) ISRTP Workshop: Progress and Barriers to Incorporating Alternative
Canadian Experience - Priority Setting and Screening of All Existing Substances under the Canadian Environmental Protection Act (CEPA) ISRTP Workshop: Progress and Barriers to Incorporating Alternative
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Animal Health Pfizer Inc 235 East 42nd Street New York, NY 10017 Poison Control Center Phone: 1-866-531-8896
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Animal Health Pfizer Inc 235 East 42nd Street New York, NY 10017 Poison Control Center Phone: 1-866-531-8896
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Validation of the 21 st Century Toxicology Toolbox: Challenges, Opportunities, and the Way Forward
 Validation of the 21 st Century Toxicology Toolbox: Challenges, Opportunities, and the Way Forward William S. Stokes 1, Judy Strickland 2, and Warren Casey 1 1 National Toxicology Program Interagency Center
Validation of the 21 st Century Toxicology Toolbox: Challenges, Opportunities, and the Way Forward William S. Stokes 1, Judy Strickland 2, and Warren Casey 1 1 National Toxicology Program Interagency Center
Natural Products and Drug Discovery
 Natural Products and Drug Discovery Secondary metabolism Building blocks & Phytochemicals Drug discovery 26-Oct-18 2 Secondary metabolism: Biochemical reactions derived from primary metabolic pathways
Natural Products and Drug Discovery Secondary metabolism Building blocks & Phytochemicals Drug discovery 26-Oct-18 2 Secondary metabolism: Biochemical reactions derived from primary metabolic pathways
B2 Tuesday 12 th May (Additional Science)
 B2.1.1 Cells and cell structure State that all living things are made up of cells Identify, and describe the functions of, the following parts of human and animal cells: nucleus, cytoplasm, cell membrane,
B2.1.1 Cells and cell structure State that all living things are made up of cells Identify, and describe the functions of, the following parts of human and animal cells: nucleus, cytoplasm, cell membrane,
Biocompatibility: a risk based approach
 Biocompatibility: a risk based approach Legal framework The regulatory requirements include: Demonstration of safety Demonstration of efficacy Positive balance of risk and benefit The regulatory requirements
Biocompatibility: a risk based approach Legal framework The regulatory requirements include: Demonstration of safety Demonstration of efficacy Positive balance of risk and benefit The regulatory requirements
Accredited Laboratory
 Accredited Laboratory A2LA has accredited St. Paul, MN for technical competence in the field of Biological Testing This laboratory is accredited in accordance with the recognized International Standard
Accredited Laboratory A2LA has accredited St. Paul, MN for technical competence in the field of Biological Testing This laboratory is accredited in accordance with the recognized International Standard
Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system.
 Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system. Application for seeking approval of the Central Government for conduct of clinical trial in
Guidelines and criteria for seeking approval to conduct clinical trials in Ayurveda, Siddha and Unani system. Application for seeking approval of the Central Government for conduct of clinical trial in
OECD QSAR Toolbox Version 3.1
 OECD QSAR Toolbox Version 3.1 Strategies for grouping chemicals to fill data gaps to assess genetic toxicity and genotoxic carcinogenicity (where covalent DNA binding is the molecular initiating event)
OECD QSAR Toolbox Version 3.1 Strategies for grouping chemicals to fill data gaps to assess genetic toxicity and genotoxic carcinogenicity (where covalent DNA binding is the molecular initiating event)
MATERIAL SAFETY DATA SHEET Acetamiprid 20% SP
 SECTION 1 CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Use Insecticide Manufacturer s Name HERANBA INDUSTRIES LIMITED Street Address 101/102, Kanchanganga, Factory lane, Near
SECTION 1 CHEMICAL PRODUCT AND COMPANY IDENTIFICATION Product Identifier Product Use Insecticide Manufacturer s Name HERANBA INDUSTRIES LIMITED Street Address 101/102, Kanchanganga, Factory lane, Near
United States Environmental Protection Agency (EPA) Office of Pesticide Programs (OPP): The Vision for Global Pesticide Reviews
 2009/SOM2/SCSC/WKSP2/011 United States Environmental Protection Agency (EPA) Office of Pesticide Programs (OPP): The Vision for Global Pesticide Reviews Submitted by: United States Examination of Hot Issues
2009/SOM2/SCSC/WKSP2/011 United States Environmental Protection Agency (EPA) Office of Pesticide Programs (OPP): The Vision for Global Pesticide Reviews Submitted by: United States Examination of Hot Issues
Introduction to clinical trials
 Introduction to clinical trials Definition of a clinical trial A research activity that involves administration of a test treatment to some experimental unit in order to evaluate the treatment. Key words
Introduction to clinical trials Definition of a clinical trial A research activity that involves administration of a test treatment to some experimental unit in order to evaluate the treatment. Key words
, E C H A 1(25) Helsinki, 19 May 2017
 , E C H A 1(25) Helsinki, 19 May 2017 Registered substance name: reaction product: bisphenol-a-(epichlorhydrin); epoxy resin (number average molecular weight 700) EC No: 500-033-5 CAS No: 25068-38-6 Date
, E C H A 1(25) Helsinki, 19 May 2017 Registered substance name: reaction product: bisphenol-a-(epichlorhydrin); epoxy resin (number average molecular weight 700) EC No: 500-033-5 CAS No: 25068-38-6 Date
SAFETY DATA SHEET according to Regulation (EC) No. 1907/2006 Silsoft* ETS
 1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1 Product identifier Trade name : CAS : 17861-60-8 Chemical name : Heptamethyl ethyl trisiloxane ELINCS No. : 469-070-1 REACH
1. IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1 Product identifier Trade name : CAS : 17861-60-8 Chemical name : Heptamethyl ethyl trisiloxane ELINCS No. : 469-070-1 REACH
Non-clinical Assessment Requirements
 Non-clinical Assessment Requirements Presented by: Maria Nieto-Gutierrez Safety and Efficacy of Medicines/Human Medicines Development and Evaluation An agency of the European Union Contents: Relevance
Non-clinical Assessment Requirements Presented by: Maria Nieto-Gutierrez Safety and Efficacy of Medicines/Human Medicines Development and Evaluation An agency of the European Union Contents: Relevance
Combining Large Animal IND Enabling Toxicology and CV Sph Studies- PRO
 Safety Pharmacology Endpoints: Integration into Toxicology Studies Combining Large Animal IND Enabling Toxicology and CV Sph Studies- PRO Marc Bailie DVM, PhD Director In Vivo Facility Michigan State University,
Safety Pharmacology Endpoints: Integration into Toxicology Studies Combining Large Animal IND Enabling Toxicology and CV Sph Studies- PRO Marc Bailie DVM, PhD Director In Vivo Facility Michigan State University,
