WHO Global Task Force on Cholera Control Target Product Profile (TPP) for the development of improved Cholera rapid diagnostic tests
|
|
|
- Valerie Douglas
- 6 years ago
- Views:
Transcription
1 WHO Global Task Force on Cholera Control Target Product Profile (TPP) for the development of improved Cholera rapid diagnostic tests June
2 Table of content Part I Target Product Profile (TPP) for Cholera Rapid Diagnostic Tests Part II Supplementary Information Abbreviations I. Objectives and methodology II. Introduction III. Attributes of Cholera rapid diagnostic tests as established by the consultation a. Preliminary considerations b. Priority features c. Test procedure d. Test characteristics e. Related equipment f. Manufacturing process IV. Members of Cholera RDTs Expert Group V. References 2
3 Part I: Target Product Profile for Cholera rapid diagnostics tests (RDTs) The product specifications tabled below are called the target product profile (TPP). This table must be read in conjunction with Part II of this document (Supplementary information) that provides the rationale for the selection of the criteria and proposes refined information on the product attributes and the distinction between desired and acceptable attributes. DIAGNOSTIC PRODUCT SPECIFICATIONS TPP CHOLERA TARGET POPULATION GROUP/PATIENT: Patients clinically suspect of cholera HEALTH FACILITY WHERE THE TEST WILL BE USED: Primary health care level with no access to standard laboratory facility or settings and no access to electricity. Community settings outside health facilities where cholera outbreak is suspected ATTRIBUTES definitions DESIRED ACCEPTABLE Priority features Intended use of the test Target molecule classic approach is based on LipoPolySaccharide (LPS) (O antigen), additional markers can be considered provided they show adequate performance Specimen type Analytical Sensitivity/Limit of Detection (Identification of positive reference material) Clinical/Diagnostic Sensitivity (identification of clinical cases with toxigenic Vibrio cholerae only) Analytical Specificity (assessment of cross reactivity with other pathogens ) Clinical /Diagnostic Specificity (identification of the cases not due to toxigenic vibrio cases) Early detection, declaration, and monitoring of outbreak without need for cholera confirmation First intention test to be used on a predefined number of cholera suspect cases Biomarker for toxigenic Vibrio cholerae O1: LPS (O antigen) and cholera toxin marker (CT Monoclonal antibody) Stool/Rectal swab Or Samples easier to collect (capillary blood if new markers are used) 100% 95% 95% 95%CI (90-100) 100% 100% 98% 95%CI (95-100) Test to declare a cholera alert, to be confirmed by culture and/or PCR Biomarker for Vibrio cholerae O1 and O139 (test will distinguish O1 from O139): LPS (O antigen) Stool/Rectal swab 90% 95%CI (85 95) 95% 95%CI (93 98) Result output Qualitative result Qualitative result Time to result < 15 minutes < 30 minutes 3
4 Throughput: number of tests to be performed in an hour Intended users Ease of use Health worker from outbreak investigation team with dedicated training, present at community level And Health Community Worker at primary or secondary care levels for early detection of cholera transmission in patients presenting with compatible symptoms One-signal test (+ control) No need of interpretation, result should be selfexplanatory IFU available in all UN languages Invalid rate < 0,1% < 1% Non-laboratory trained health personnel Two-signal test (+ control) IFU available in English and French Test procedure Number of steps (use of different reagents/incubation step) Sample preparation Need for operator to transfer a precise volume of sample or reagents Biosafety requirements Internal control demonstrating proper migration of sample positive and negative controls Not needed/ Fully integrated in use of test device No None in addition to standard precautions Included in test device Provided with the kit, ideally embedded in each single test One step conducted before use of test device Acceptable if robust transfer device is provided with the test device and if variation do not affect test results None in addition to standard precautions Included in test device To be purchased separately Result recording Permanent via a reader Manual transfer Connectivity Tests Characteristics Adaptable to generic reader and digital data transfer Same Operating conditions 10 C 50 C 10 C - 40 C Format of test Volume of sample required Cassette Sufficient space to write patient ID Biodegradable plastic Easy and safe to mail (after inactivation) and used for further molecular testing No need to measure volume < 0,1 ml (rectal swab) Dipstick Sufficient space to write patient ID < 1ml 4
5 In use stability End-point stability (time window during which signal remain valid) 1 hour after opening of individual pouch 1 hour 30mn Shelf-life 24 months 12 months Storage conditions for test device (stability) Lot to lot variation - Sensitivity - Specificity Reader to reader variation Reagents reconstitution (need to prepare the reagents prior to utilization) Disposal requirements Training needs (time dedicated to training session for end users) Kit presentation Related equipment Equipment needed 2 C - 40 C, relative humidity up to 98%, no cold chain required Should be able to tolerate stress during transport (cycles of temperature of 30 to 50 C) without affecting the labelled expiry date - SE: up to 50% in end-point sensitivities with all lots meeting the sensitivity specification - SP: no variation 90% of readers should detect a positive result near the limit of detection Not needed None, device and accessories should be disposed in standard biological waste containers no sharps or glassware Or be biodegradable or combustible Less than half a day Job aid provided in test kit 5 test kit allowing use in exploratory mission with minimal wastage Test components individually packed Accessories not too small to be used with regular gloves Include all required components and accessories to perform the test Abbreviated IFU None (optional: use of a separate generic reader) 5 30mn after opening of individual pouch Up to 35 C, no cold chain required Should be able to tolerate stress during transport cycles of temperature of 30 to 50 C without affecting the labelled expiry date Same Same Not needed Device and accessories should be disposed in standard biological waste containers Less than one day of any type of end-user. 10 test kit Accessories not too small to be used with regular gloves Include all required components and accessories to perform the test Abbreviated IFU Small dedicated device, portable Power source None Battery operated Need for maintenance None Once a year, on site Manufacturing process Cost of test device < 1.00$ < 5.00$
6 Cost of reader (optional and if available) Expected scale of manufacture < 1 000$ < 1 500$ tests to be prepositioned in 25 at risk settings/countries Lead time for production 1 month maximum < 3 months Required QMS standard for production ISO ISO tests to be piloted in 5 priority countries 6
7 Part II: Target Product Profile (TPP) for Cholera rapid diagnostic tests Supplementary Information Abbreviations CDC Center for Disease Control CFU Colony Forming Unit CI Confidence Interval GFTCC Global Task Force on Cholera Control GHTF Global Harmonization Task Force ID Identification IFU Instructions For Use ISO International Organization for Standardization LPS Lipo Poly Saccharide MSF Médecins sans Frontières O1 Serogroup 1 O139 Serogroup 139 PCR Polymerase Chain Reaction PQ Pre-Qualification QMS Quality Management System R&D Research and Development SE Sensitivity SP Specificity TPP Target Product Profile VC Vibrio cholera WHO World Health Organization 7
8 I. Introduction Surveillance efforts to detect and react to the appearance of cholera in at-risk populations require the rapid identification of toxigenic V. cholerae in patients presenting with acute watery diarrhoea. There is an international consensus in the scientific community, supported by the recommendations issued by the WHO 1 that culture of the bacteria or its detection by PCR remains essential for the confirmation of the presence of the etiologic agent of cholera. Nevertheless, the timely and safe referral of specimens collected from cholera suspect cases to laboratory facilities where these techniques are available has been documented as challenging or just impossible due to the lack of capacity for classical microbiological and molecular testing in many areas at risk for cholera outbreaks. These barriers lead to loss of specimens, delayed results and late declaration of outbreaks. Therefore, reliable cholera rapid diagnostic tests (RDT) are critical to quickly alert medical authorities to the start of a potential outbreak. Due to its characteristics, such as ease of use, quick turnaround time for results, and robustness, the added value of a cholera RDT is expected to be important for resource-constrained settings. Unfortunately, while they are several cholera RDTs on the market with others under development, the most recent published evaluations (8-17) have shown their diagnostic accuracy to be less than optimal. Because of these limitations, the use of cholera RDTs has been erratic and insufficient in most of the settings at risk for cholera epidemics. The adoption of cholera RDTs will take place when good quality and performing diagnostic products are developed and made available to the teams facing suspicions of cholera outbreaks. New diagnostic products are needed to meet the specific requirements of health workers operating in harsh conditions. Several tests are probably required to answer all these needs but the priority was given to a test able to support an early detection of a cholera outbreak, this has been the scope of an consultation dedicated to cholera RDTs. All along the consultation process, the group of experts has tapped into its solid field experience and highly specialized knowledge and aligned their recommendations on the Common Technical Specifications usually considered by stringent regulatory authorities for the same category of diagnostic products namely a class A product according to the Global Harmonization Task Force (GHTF) classification (18)
9 II. Objectives and Methodology Objectives In 2016, The World Health Organization (WHO) published its vision of the pathway leading to the development and the production of the most needed health products to fight epidemic outbreaks. In a document entitled An R&D Blueprint for Action to prevent Epidemics (1), WHO and partners describe a set of tools meant to foster the delivery of new products matching the unmet public health needs in the domain of epidemic prone diseases, one of them being the definition of Target Product Profiles (TPPs). For health products, such as diagnostic tests and vaccines (2), the publication of TPPs is meant to guide research and development (R&D) teams and foster the delivery of new products answering the priority unmet needs. In this context, a consultation with a group of experts was organised to support the definition of Target Product Profiles for Cholera rapid diagnostic tests. cholera rapid test specifications to inform a Target Product Profile, focussing on the detection target (VC O1 and/or VC O139) and expected targets for acceptable sensitivity and specificity. The objectives of this group of experts were to: Support transparent and pragmatic consultation of experts and stakeholders involved in the use and development of cholera rapid diagnostic tests Provide scientific and R&D community with key characteristics to guide the development of improved rapid diagnostic tests for cholera, allowing for early detection of cholera outbreaks Review the knowledge gaps and foster R&D efforts that facilitate the production and uptake of affordable quality cholera RDTs at country level Methodology The proposed TPPs presented in this document outline two sets of attributes for cholera rapid diagnostic tests, both desired and acceptable product criteria have been considered, in line with the approach used by WHO and partners for other diseases, such as diagnostic tests for Ebola disease, Zika, and to differentiate bacterial and non-bacterial infections (3, 4, 5). While acceptable attributes set the minimum requirements for a cholera rapid diagnostic test to answer the user needs and procurement obligations in terms of quality, the desired attributes are aspirational and intend to foster the competition among RDT manufacturers leading to the development of new products demonstrating improved performance. It is hoped that the use of new technological approaches together with a clear vision of what the test would ideally bring to the cholera programme can enhance the revision of the existing versions of cholera RDTs and result in improved test performance and alignment on enduser specific needs. These TPPs for cholera RDTs constitute the outcome of a joint-collaboration between WHO (6) and the Global Task Force on Cholera Control (GTFCC) (7). Together with a group of partners, the GFTCC Secretariat has set up group of experts, where individuals from different technical areas were invited to debate the profile of cholera RDTs answering the burning public health needs such as early detection of cholera outbreak in at risk settings. The group of experts is made of 14 highly specialized experts 2 in the domain of cholera disease, outbreak management, implementation and use of RDTs in underserved settings, development and production of antibodies and rapid tests, evaluation of RDTs and quality assessment of diagnostic products. WHO and the GTFCC paid special attention to reflect the opinion and the needs of the field end-users. For that, several representatives of cholera 2 See composition of the Expert Group in Section IV of the document 9
10 national programs from countries regularly facing cholera outbreaks were invited to join the group. The GFTCC Secretariat convened a series of consultations aiming at drafting the TPP for cholera RDTs. An initial set of characteristics was drafted to facilitate the discussion and the definition of TPPs for cholera RDT. Several rounds of mail exchanges took place between February and June 2017, resulting in a draft TPP document which was then shared with the members of the Epidemiology and Laboratory Working Group of the GFTCC in May All the suggestions but also the discordances were discussed until consensus was reached for each of the product attribute. The present TPP for cholera RDTs are also developed within a broader strategy aiming at improving the quality of the products made available to the stakeholders. WHO and its Pre- Qualification unit has developed a comprehensive assessment scheme 3 for the review of product dossiers. It is foreseen that this approach will convince the manufacturers of existing and new products to approach WHO for submission of data and product dossiers. A prequalification awarded to any cholera RDT will be considered as a critical asset by the teams in charge of procurement of these products. 3 WHO Pre-Qualification scheme for diagnostic products. 10
11 III. Attributes of improved cholera rapid diagnostic tests A. Preliminary considerations The procurement of cholera RDTs by WHO and the main organizations involved in the management of cholera epidemic outbreaks is based on the assessment of priority attributes as described below. To support the use and the selection of the cholera RDTs, the GTFCC has issued a temporary guidance document on the use of cholera RDTs 4. Of paramount importance are the populations to be tested and the health facilities where the tests will be deployed. If a cholera outbreak is suspected and for surveillance purposes, the cholera RDTs will be used on a small number of patients clinically suspect of cholera 5, typically patients presenting with acute watery diarrhoea. A positive result found in several patients in the same area will trigger a cholera alert, informing the health authorities and enhancing preparedness. It is only when the RDTs positive results are confirmed by positive culture or molecular testing that the outbreak is declared and relevant interventions initiated. To support this strategy, the selected cholera RDTs will be made available to health personnel and staff present in facilities where standard laboratory might not be present. The test must be suitable for end-users with limited knowledge on traditional laboratory testing. TARGET POPULATION GROUP/PATIENT: Patients clinically suspect of cholera HEALTH FACILITY WHERE THE TEST WILL BE USED: Primary health care level with no access to standard laboratory facility or settings and no access to electricity. Community settings outside health facilities where cholera outbreak is suspected B. Priority attributes While a cholera test may not meet all the attributes presented in the TPP, a restricted set of attributes are considered as critical for the selection of the best adapted cholera RDTs. This encompasses the intended-use for the test, its detection targets, its performance, and several additional characteristics. Basically, the cholera RDTs are intended to be used for early outbreak detection facilitating its management and reducing transmission. The diagnostic test presented in this document is not meant to be used for individual diagnostic of cholera among a general population. With improved technology and performance, the cholera RDT could be used within a test and act approach where positive cholera RDT results would allow a cholera outbreak to be declared without further confirmation. This context is referred to in the desired section. For the time being, the best cholera RDTs still need to be confirmed by culture or PCR for any positive result. Acceptable detection targets are biomarkers for detection of Vibrio cholerae O1 and O139 with the capacity of distinguishing between the two subgroups. The test must also distinguish these serogroups from the non-o1 and non-o139 ones. Currently, available cholera RDTs have been using lipopolysacharride (LPS) antigen O, unfortunately it is not sufficient to conclude that a VC strain is toxigenic. So, in addition to LPS biomarkers, it is highly desirable 4 Interim Technical Note: The Use of Cholera Rapid Diagnostic Tests. GTFCC Guidance on cholera surveillance. GTFCC
12 that the test detects toxigenic VC species using a cholera toxin marker to detect the strains with high potential for epidemic outbreaks. Because of the cost implications of having two markers attached to the same test and because the circulation of VC O139 has been limited in Africa so far, a test offering a single target molecule (VC O1) will be preferred. That being said, if the test is used for environmental surveillance as it is already the case in some countries and contexts, the presence of non-toxigenic VC O1 or non-toxigenic non-o1/non-o139 species will be interesting for background literature research. Regarding the expected minimal performance for a cholera RDT, the clinical sensitivity and specificity were intensively discussed in by the Expert Group. Sensitivity was felt as less important than specificity in the context of a test used to allow an outbreak alert to be declared. In the case of suspicion of an outbreak the current practice is to test a small number of patients presenting with cholera symptoms in the same area, therefore a test with a sensitivity of 90 95% will likely detect patients with high level of organisms. Unlike sensitivity, specificity will be the main parameter to consider as the end-user would not accept a high percentage of false positive results leading to the need of confirmatory testing or triggering unnecessary outbreak interventions or even distribution of cholera vaccines. In addition, prior to procurement, the manufacturer is expected to provide data demonstrating that the test does not cross react with a list of organisms for which differential diagnosis is crucial based on clinical evidence from historic records of outbreak and infections. As monoclonal antibodies can be very specific, we present here an indicative list of organisms to be tested for selection of the most specific ones: Vibrio parahaemolyticus, Vibrio alginolyticus, Vibrio mimicus, Vibrio fluvialis, Vibrio cholerae non-o1/non-o139, Plesiomonas shigelloides, Escherichia coli, Yersinia enterolitica, Shigella sonnei, Shigella flexneri, Salmonella typhi, Salmonella enteritidis, Salmonella typhimurium, Aeromonas hydrophila, Aeromonas sobria, Campylobacter coli, Campylobacter jejuni and additional Enterobacteriaceae responsible for diarrhoeal diseases. Should an ideally sensitive and specific cholera RDT be developed and validated, the recommendation on the use of cholera RDTs might be reviewed and amended accordingly. In addition, limits of the confidence intervals are indicated here for clinical sensitivity and specificity to guide the manufacturers in their validation studies and to suggest the number of tests to be conducted to reach statistically valid results. The limit of detection which is usually accepted is around a concentration of less than 10 6 CFU/mL and/or LPS at a concentration inferior to 100 ng/ml (referring to in-vitro cultured reference and clinical strains). The group of experts has also stressed the importance of the test attributes related to the ease of use for health workers with little or no knowledge of classical laboratory techniques. Cholera RDTs will be distributed in countries and settings affected with cholera outbreaks, most of them occurring in rural areas where trained health workers are often missing or not familiar with laboratory techniques. This deployment strategy will allow cholera alerts to be timely declared, this context of use has major consequences in terms of test attributes and kit presentation. The way the reading system of the test is built must prevent the end-user to interpret the result, this should be self-explanatory, thus a single or dual signal (one line or one spot) will be the most desired option. To facilitate the distribution of the cholera RDTs and its adoption, manufacturers must design clear Instructions for Use (IFU) with pictures and drawing rather than written explanations, available in the languages spoken in the countries where cholera outbreaks are likely to happen (English, French being the minimal requirement). 12
13 ATTRIBUTES definitions Intended use of the test Target molecule classic approach is based on LipoPolySaccharide (LPS) (O antigen), additional markers can be considered provided they show adequate performance Specimen type Analytical Sensitivity/Limit of Detection (Identification of positive reference material) Clinical/Diagnostic Sensitivity (identification of clinical cases with toxigenic Vibrio cholerae only) Analytical Specificity (assessment of cross reactivity with other pathogens) Clinical /Diagnostic Specificity (identification of the cases not due to toxigenic vibrio cases) Early detection, declaration, and monitoring of outbreak without need for cholera confirmation First intention test to be used on a predefined number of cholera suspect cases Biomarker for toxigenic Vibrio cholerae O1: LPS (O antigen) and cholera toxin marker (CT Monoclonal antibody) Stool/Rectal swab Or Samples easier to collect (capillary blood if new markers are used) 100% 95% 95% 95%CI (90-100) 100% 100% 98% 95%CI (95-100) 13 Test to declare a cholera alert, to be confirmed by culture and/or PCR Biomarker for Vibrio cholerae O1 and O139 (test will distinguish O1 from O139): LPS (O antigen) Stool/Rectal swab 90% 95%CI (85 95) 95% 95%CI (93 98) Result output Qualitative result Qualitative result Time to result < 15 minutes < 30 minutes Throughput: number of tests to be performed in an hour Intended users DESIRED Health worker from outbreak investigation team with dedicated training, present at community level And Health Community Worker at primary or secondary care levels for early detection of cholera transmission in patients presenting with compatible symptoms ACCEPTABLE Non-laboratory trained health personnel
14 Ease of use One-signal test (+ control) No need of interpretation, result should be selfexplanatory IFU available in all UN languages Invalid rate < 0,1% < 1% Two-signal test (+ control) IFU available in English and French C. Test procedure Additional attributes related to the test procedure are important to consider for the selection of the best adapted cholera RDTs. As the cholera RDTs will be used in a context of emergency by health workers in basic facilities, a simplified procedure is highly desired with a limited number of steps which will decrease the risk of user errors. Here, we indicate a maximum of steps for each of the version of the TPP with a step being defined as a short preparation phase (a simple transfer, a couple of minutes) which is different from a 6-hour enrichment steps (not acceptable for the intended -users). Regarding the bio safety requirements, standard precautions assume the use of gloves together with the provisions for waste management, hand hygiene and splash protection. Anything more demanding will be considered as a non-appropriate option. The presence of internal controls must be carefully assessed for the selection of the best adapted diagnostic product. It is expected that a control demonstrating a proper sample flow is included in the test format, acknowledging that this might be a challenge for the test developers especially on stool samples. In addition, the availability of negative and positive controls within the test kit is a highly-appreciated attribute, alleviating the need for the enduser to store scarce cholera positive and negative samples. ATTRIBUTES definitions DESIRED ACCEPTABLE Number of steps (use of different reagents/incubation step) Sample preparation Need for operator to transfer a precise volume of sample or reagents Biosafety requirements Internal control demonstrating proper migration of sample positive and negative controls Not needed/ Fully integrated in use of test device No None in addition to standard precautions Included in test device Provided with the kit, ideally embedded in each single test One step conducted before use of test device Acceptable if robust transfer device is provided with the test device and if variation do not affect test results None in addition to standard precautions Included in test device To be purchased separately Result recording Permanent via a reader Manual transfer Connectivity Adaptable to generic reader and digital data transfer 14
15 D. Test characteristics In alignment with the recommendations issued for the improvement and harmonization of malaria RDTs (10), the Expert Group has highlighted several attributes for in-depth review. Because of the usual context of use in cholera prone areas, the cholera RDTs are expected to present specific characteristics in terms of robustness and composition of test kits. This section outlines the key attributes that would be reviewed by any stringent regulatory authority to which a cholera RDT would be submitted for product assessment. A cassette version is preferred to a strip one as it is considered as more user-friendly although the cost would probably be higher. To cope with the field operating conditions, the proposed range of temperature for stability is wide enough to accommodate cases where the test kits are accidently stored in a fridge although it is not required to do so ( desired column). Available products do not have sufficient space on the top of the cassette (even less on a strip version) allowing the identification of the patient to be recorded, this has been considered as a drawback by field users. Thus, it is recommended that the design of the new cholera RDTs presents a dedicated space for ID record. Given the conditions of use at the beginning of an outbreak it is important to avoid any mistake in the reporting of the test results. Regarding the requirements for disposal of the cholera RDTs, an acceptable approach would state that device and accessories should be able to be disposed in standard biological waste containers no sharps or glassware (note: glass cannot be incinerated). Accompanying non-infectious waste should be biodegradable or combustible. Stability and reproducibility are essential components for manufacturers to consider as specific data will be reviewed by the teams in charge of product selection leading to procurement. The expected stability during storage presents a range of temperatures covering the locations where air conditioning is not available especially in areas where ambient temperature is very high (over 37 C). The test must sustain these high temperatures, allowing the pre-positioning of the products at regional or district levels in the areas most at risk for cholera outbreaks. For stability attributes, the device must tolerate temperature stress without affecting the labelled expiry date. Testing on cycles of temperatures between 30 and 50 C is recommended as these modifications of temperature mimic transportation conditions more accurately and are more stressful. Reproducibility has been highlighted by the Expert Group as an important attribute, acknowledging that expressing reproducibility for a qualitative assay is somehow challenging. Lot to lot variation is a key component to investigate as it reflects a robust and regular quality of production. It is foreseen that no more than 50% difference in end-point sensitivities measured by logistic or probit analysis and all lots meeting the sensitivity specification would be acceptable. In any case, specificity should not change with all the different tested lots meeting the specification. The reader to reader reproducibility should reach 90% with typical, not expert, readers who should be able to detect a positive result near the limit of detection. The kit presentation is equally an important attribute, the ideal products would include clear bench aids (in addition to the IFU) describing the use of the individual test together with clear guidance on the interpretation of the test results which is known to be prone to errors for basically trained end-users. 15
16 ATTRIBUTES definitions DESIRED 16 ACCEPTABLE Operating conditions 10 C 50 C 10 C - 40 C Format of test Volume of sample required In use stability End-point stability (time window during which signal remain valid) Cassette Sufficient space to write patient ID Biodegradable plastic Easy and safe to mail (after inactivation) and used for further molecular testing No need to measure volume < 0,1 ml (rectal swab) 1 hour after opening of individual pouch Dipstick Sufficient space to write patient ID < 1ml 1 hour 30mn Shelf-life 24 months 12 months Storage conditions for test device (stability) Lot to lot variation - Sensitivity - Specificity Reader to reader variation Reagents reconstitution (need to prepare the reagents prior to utilization) Disposal requirements Training needs (time dedicated to training session for end users) Kit presentation 2 C - 40 C, relative humidity up to 98%, no cold chain required Should be able to tolerate stress during transport (cycles of temperature of 30 to 50 C) without affecting the labelled expiry date - SE: up to 50% in end-point sensitivities with all lots meeting the sensitivity specification - SP: no variation 90% of readers should detect a positive result near the limit of detection Not needed None, device and accessories should be disposed in standard biological waste containers no sharps or glassware Or be biodegradable or combustible Less than half a day Job aid provided in test kit 5 test kit allowing use in exploratory mission with minimal wastage Test components individually packed Accessories not too small to be used with regular gloves Include all required components and accessories to perform the test Abbreviated IFU 30mn after opening of individual pouch Up to 35 C, no cold chain required Should be able to tolerate stress during transport cycles of temperature of 30 to 50 C) without affecting the labelled expiry date - SE: up to 50% in end-point sensitivities with all lots meeting the sensitivity specification - SP: no variation 90% of readers should detect a positive result near the limit of detection Not needed Device and accessories should be disposed in standard biological waste containers Less than one day of any type of end-user. 10 test kit Accessories not too small to be used with regular gloves Include all required components and accessories to perform the test Abbreviated IFU
17 E. Related equipment In the case an equipment is required to perform the cholera RDT (which is not considered as an advantage), it must be extremely simple to use without any constraint for the power source and the maintenance. This is most desired condition for the product to be used outside of classical laboratory premises and allow detection of cholera outbreaks. ATTRIBUTES definitions DESIRED ACCEPTABLE Equipment needed None (optional: use of a separate generic reader) Small dedicated device, portable Power source None Battery operated Need for maintenance None Once a year, on site F. Manufacturing process The indicated unit prices for the cholera RDTs are only indicative. In the absence of benchmark price for cholera tests, the Expert Group has used price information available for equivalent products implemented in similar contexts. Current versions are not expected to be procured in high volumes because of their limitations and the public health approach (testing of a small number of suspect cases), should the new cholera RDTs be massively improved they could be used for individual diagnostic therefore their unit price would be expected to decrease substantially. A rough estimate for production has been derived from historic procurement data from WHO and MSF, which are the main funders for this category of products in cholera endemic areas. Again, new cholera RDTs showing all the attributes listed in the desired section of this TPP document would likely trigger an increase demand as they would be able to support individual diagnosis outside of outbreak episodes. The lead time for production is critical attribute to know in advance as very few countries have stocks of tests available at national level even less at their periphery. This information is essential to ensure smooth procurement and delivery operations up to areas facing suspicions of cholera outbreaks. The requirement for ISO standard for Quality Management System is the benchmark for any diagnostic products to be procured by WHO and its affiliated partners. ATTRIBUTES definitions DESIRED ACCEPTABLE Cost of test device Cost of reader (optional and if available) Expected scale of manufacture < 1.00$ < 1 000$ tests to be prepositioned in 25 at risk settings/countries < 5.00$ < 1 500$ Lead time for production 1 month maximum < 3 months Required QMS standard for production ISO ISO
18 IV. Members of Cholera RDTs group of experts Members - Jerome Ateudjieu, Université de Dschang, Cameroon - Maurice Bangombe, Community Health Sciences Unit, MOH, Lilongwe, Malawi - Bhabatosh Das, Translational Health Science and Technology Institute, Haryana, India - Julian Duncan, diagnostic R&D expert, consultant - Jan Jacobs, Institute of Tropical Medicine, Antwerp, Belgium - Pierre Lafaye, Pasteur Institute, Paris, France - Jose Paulo Langa, Instituto Nacional de Saude, Maputo, Mozambique - Gisele Mbuy, DLM/MOH, Kinshasa, Democratic Republic of Congo - John Mwaba, University of Zambia, Lusaka, Zambia - Piero Olliaro, WHO/TDR, Geneva, Switzerland - Anne-Laure Page, Epicentre, MSF, Paris, France - Michele Parsons, CDC/OID, Atlanta, USA - Erwan Piriou, MSF, Amsterdam, The Netherlands - Anita Sands, Pre-Qualification Team Diagnostic, WHO, Geneva, Switzerland WHO/GTFCC Secretariat - Johanna Fihman, WHO/GTFCC Secretariat, Geneva, Switzerland - Dominique Legros, WHO/GTFCC Secretariat, Geneva, Switzerland - David Olson, WHO/GTFCC Secretariat, Geneva, Switzerland - Martine Guillerm, WHO/consultant, Geneva, Switzerland Acknowledgements Marie-Laure Quilici, National Reference Laboratory for Vibrio and Cholera, Pasteur Institute, Paris, France Contact : GTFCCsecretariat@who.int 18
19 V. References 1. WHO (2016) An R&D blueprint for action to prevent epidemics The process for developing WHO PPCs and TPPs for vaccines. WHO Ebola Diagnostics TPP. WHO. (2014) 4. Zika Diagnostics TPP; WHO (2016) Target Product Profile for a Diagnostic Assay to Differentiate between Bacterial and Non- Bacterial Infections and Reduce Antimicrobial Overuse in Resource-Limited Settings: An Expert Consensus. Dittrich S, et all. (2016). PLOS ONE 11(8): e WHO. Cholera website 7. The Global Task Force on Cholera Control 8. Field Evaluation of Crystal VC Rapid Dipstick test for cholera during a cholera outbreak in Guinea-Bissau (Trop Med Int Health. 2009) 9. Evaluation of a rapid dipstick test for identifying cholera cases during the outbreak (IJMR, 2012) 10. Performance and utility of a rapid diagnostic test for cholera: notes from Haiti (Diagn Microbiol Infect Dis, 2013) 11. Evaluation of a rapid immunochromatographic Dipstick Kit for Diagnosis of Cholera emphasizes its outbreak utility (Jpn. J. Infect. Dis. 2010) 12. Evaluation of a rapid dipstick (Crystal VC) for the Diagnosis of Cholera in Zanzibar and a comparison with previous studies (Plos ONE 2012) 13. Evaluation of a Rapid Test for the Diagnosis of Cholera in the Absence of Gold Standard (PLoS ONE 2012) 14. Rapid Detection of Vibrio Cholerae O1 and O139 in stool samples by one-step immunochromatographic dipstick test (Int J Biol Med Res. 2015) 15. Development and testing of monoclonal antibody-based rapid immunodiagnostic test kits for direct detection of vibrio cholerae O139 Synonym Bengal (J. Clin. Microbiol. 1995) 16. Evaluation of the monoclonal antibody -based kit Bengal SMART for rapid detection of vibrio cholerae O139 synonym Bengal in stool samples (J. Clin. Microbiol. 1995) 17. A novel kit for rapid detection of vibrio cholerae O1 (J. Clin. Microbiol. 1994) 18. A risk based approach for the assessment of in-vitro diagnostics. WHO. (2014). approach_buffet.pdf?ua=1 19. Jacobs, J. et al. (2014). Harmonization of malaria rapid diagnostic tests: best practices in labelling including instructions for use. Malaria Journal 13(1):
Interim Technical Note The Use of Cholera Rapid Diagnostic Tests November 2016
 Objective Interim Technical Note The Use of Cholera Rapid Diagnostic Tests November 2016 To provide interim guidance to Ministries of Health and other organizations on the potential use of commercially
Objective Interim Technical Note The Use of Cholera Rapid Diagnostic Tests November 2016 To provide interim guidance to Ministries of Health and other organizations on the potential use of commercially
Selection and use of Ebola in vitro diagnostic (IVD) assays
 EMERGENCY GUIDANCE Selection and use of Ebola in vitro diagnostic (IVD) assays June 2015 World Health Organization 2015. All rights reserved. The designations employed and the presentation of the material
EMERGENCY GUIDANCE Selection and use of Ebola in vitro diagnostic (IVD) assays June 2015 World Health Organization 2015. All rights reserved. The designations employed and the presentation of the material
Rapid Diagnostic Tests in malaria case management
 Rapid Diagnostic Tests in malaria case management Planning, Procuring and Implementing Suggestions for incorporation of malaria RDT-based diagnosis into proposals to the Global Fund Foundation for Innovative
Rapid Diagnostic Tests in malaria case management Planning, Procuring and Implementing Suggestions for incorporation of malaria RDT-based diagnosis into proposals to the Global Fund Foundation for Innovative
Global response plan for pfhrp2/3 deletions
 Global response plan for pfhrp2/3 deletions Jane Cunningham MPAC 17-19 October, 2017 The global response plan for pfhrp2/3 mutations that limit the effectiveness of HRP2- based RDTs comprises a global
Global response plan for pfhrp2/3 deletions Jane Cunningham MPAC 17-19 October, 2017 The global response plan for pfhrp2/3 mutations that limit the effectiveness of HRP2- based RDTs comprises a global
WHO Prequalification of In Vitro Diagnostics Programme Amended PUBLIC REPORT
 WHO Prequalification of In Vitro Diagnostics Programme Amended PUBLIC REPORT Product: SD BIOLINE Malaria Ag P.f/Pan and SD BIOLINE Malaria Ag P.f/Pan POCT PQDx Reference Number: PQDx 0030-012-01 Abstract
WHO Prequalification of In Vitro Diagnostics Programme Amended PUBLIC REPORT Product: SD BIOLINE Malaria Ag P.f/Pan and SD BIOLINE Malaria Ag P.f/Pan POCT PQDx Reference Number: PQDx 0030-012-01 Abstract
1. APPROVED MALARIA RDTS
 1. APPROVED MALARIA RDTS There are many different Rapid Diagnostic Tests available in the market. To assist partners of the Three Diseases Fund in the selection of the appropriate RDT, the Fund Manager
1. APPROVED MALARIA RDTS There are many different Rapid Diagnostic Tests available in the market. To assist partners of the Three Diseases Fund in the selection of the appropriate RDT, the Fund Manager
Noncommercial culture and drug-susceptibility testing methods for screening patients at risk for multidrugresistant.
 Noncommercial culture and drug-susceptibility testing methods for screening patients at risk for multidrugresistant tuberculosis Policy statement March 2010 1 Contents Abbreviations Executive summary 1.
Noncommercial culture and drug-susceptibility testing methods for screening patients at risk for multidrugresistant tuberculosis Policy statement March 2010 1 Contents Abbreviations Executive summary 1.
Surveillance strategy during Phase 3 of the Ebola response
 EMERGENCY GUIDANCE Surveillance strategy during Phase 3 of the Ebola response 5 November 2015 World Health Organization 2015. All rights reserved. The designations employed and the presentation of the
EMERGENCY GUIDANCE Surveillance strategy during Phase 3 of the Ebola response 5 November 2015 World Health Organization 2015. All rights reserved. The designations employed and the presentation of the
Point-Of-Care Tests -- Target Product Profiles and Research Questions Table of contents 1. Gonococcal Infection Chlamydial Infection
 Point-Of-Care Tests -- Target Product Profiles and Research Questions Table of contents 1. Gonococcal Infection... 3 2. Chlamydial Infection... 9 3. Syphilis Infection... 14 4. Human Papillomavirus...
Point-Of-Care Tests -- Target Product Profiles and Research Questions Table of contents 1. Gonococcal Infection... 3 2. Chlamydial Infection... 9 3. Syphilis Infection... 14 4. Human Papillomavirus...
Novel Diagnostic Methods of Tuberculosis
 Novel Diagnostic Methods of Tuberculosis Qimin YOU USTAR Biotechnologies I. The TB Diagnosis Challenge My presentation will focus on the new diagnostic methods of tuberculosis, developed by USTAR Biotechnologies.
Novel Diagnostic Methods of Tuberculosis Qimin YOU USTAR Biotechnologies I. The TB Diagnosis Challenge My presentation will focus on the new diagnostic methods of tuberculosis, developed by USTAR Biotechnologies.
Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS
 Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS Key Topics Definitions Essential Principles Classification Conformity Assessment Framework License to Manufacture, Import,
Medical Device Regulatory Framework 9 SEPTEMBER 2015 FUNDISA CONFERENCE JANE ROGERS Key Topics Definitions Essential Principles Classification Conformity Assessment Framework License to Manufacture, Import,
OIE Procedure for Validation and Certification of Diagnostic Assays
 OIE Procedure for Validation and Certification of Diagnostic Assays A FRAMEWORK FOR A HARMONISED APPROACH OF THE VALIDATION AND REGISTRATION OF VETERINARY DIAGNOSTIC ASSAYS ACROSS THE WORLD OIE Conference
OIE Procedure for Validation and Certification of Diagnostic Assays A FRAMEWORK FOR A HARMONISED APPROACH OF THE VALIDATION AND REGISTRATION OF VETERINARY DIAGNOSTIC ASSAYS ACROSS THE WORLD OIE Conference
Protocols for Laboratory Verification of Performance of the FilmArray Gastrointestinal (GI) Panel
 Protocols for Laboratory Verification of Performance of the FilmArray Gastrointestinal (GI) Panel Purpose The Clinical Laboratory Improvement Amendments (CLIA), passed in 1988, establishes quality standards
Protocols for Laboratory Verification of Performance of the FilmArray Gastrointestinal (GI) Panel Purpose The Clinical Laboratory Improvement Amendments (CLIA), passed in 1988, establishes quality standards
WHO Prequalification of In Vitro Diagnostics Programme
 P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s Information for Manufacturers on the Manufacturing Site(s) Inspection (Assessment of the Quality Management System) WHO Prequalification
P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s Information for Manufacturers on the Manufacturing Site(s) Inspection (Assessment of the Quality Management System) WHO Prequalification
Antisera QC and IQCP and Associated Challenges
 Antisera QC and IQCP and Associated Challenges Patti Fields Enteric Diseases Laboratory Branch (EDLB) CDC 2017 APHL Annual Meeting Providence, Rhode Island June 14, 2017 National Center for Emerging and
Antisera QC and IQCP and Associated Challenges Patti Fields Enteric Diseases Laboratory Branch (EDLB) CDC 2017 APHL Annual Meeting Providence, Rhode Island June 14, 2017 National Center for Emerging and
PRECISE AND POWERFUL MOLECULAR PATHOGEN DETECTION
 PRECISE AND POWERFUL MOLECULAR PATHOGEN DETECTION RAPID FOOD SAFETY YOU CAN RELY ON Food companies, service labs and government regulators around the world rely on the Hygiena BAX System, which uses the
PRECISE AND POWERFUL MOLECULAR PATHOGEN DETECTION RAPID FOOD SAFETY YOU CAN RELY ON Food companies, service labs and government regulators around the world rely on the Hygiena BAX System, which uses the
Molecular Diagnostics for Global Heath Problems. Proactive Investors Forum 5 October 2017
 Molecular Diagnostics for Global Heath Problems Proactive Investors Forum 5 October 2017 2 Document Information The information contained in this document and made verbally to you (together the Presentation
Molecular Diagnostics for Global Heath Problems Proactive Investors Forum 5 October 2017 2 Document Information The information contained in this document and made verbally to you (together the Presentation
A Multiplex Multi-Analyte Diagnostic Platform
 A Multiplex Multi-Analyte Diagnostic Platform Introduction Fever is one of the most common reasons for admission to hospitals in low-resource settings. 1,2 Among the millions of patients Médecins Sans
A Multiplex Multi-Analyte Diagnostic Platform Introduction Fever is one of the most common reasons for admission to hospitals in low-resource settings. 1,2 Among the millions of patients Médecins Sans
October 29 - November
 Les Pensières Veyrier-du-Lac (French Alps) October 29 - November 3 2017 Organized by The Mérieux Foundation and the London School of Hygiene & Tropical Medicine ACDx enhances the knowledge and professional
Les Pensières Veyrier-du-Lac (French Alps) October 29 - November 3 2017 Organized by The Mérieux Foundation and the London School of Hygiene & Tropical Medicine ACDx enhances the knowledge and professional
Malaria RDT Harmonization. Co chairs: Jan Jacobs, Institute of Tropical Medicine (ITM) Theodoor Visser, Clinton Health Access Initiative (CHAI)
 Malaria RDT Harmonization Co chairs: Jan Jacobs, Institute of Tropical Medicine (ITM) Theodoor Visser, Clinton Health Access Initiative (CHAI) WHO / UNICEF / UNFPA meeting with manufacturers and suppliers:
Malaria RDT Harmonization Co chairs: Jan Jacobs, Institute of Tropical Medicine (ITM) Theodoor Visser, Clinton Health Access Initiative (CHAI) WHO / UNICEF / UNFPA meeting with manufacturers and suppliers:
RIDA QUICK Clostridium difficile GDH
 RIDA QUICK Clostridium difficile GDH Product code: N0703 R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Tel.: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 C 1. Intended use
RIDA QUICK Clostridium difficile GDH Product code: N0703 R-Biopharm AG, An der neuen Bergstraße 17, D-64297 Darmstadt, Germany Tel.: +49 (0) 61 51 81 02-0 / Fax: +49 (0) 61 51 81 02-20 C 1. Intended use
OIE Standard on principles and methods of validation of diagnostic assays for infectious diseases
 OIE Standard on principles and methods of validation of diagnostic assays for infectious diseases OIE Regional Workshop for OIE National Focal Points for Veterinary Products Maputo, Republic of Mozambique
OIE Standard on principles and methods of validation of diagnostic assays for infectious diseases OIE Regional Workshop for OIE National Focal Points for Veterinary Products Maputo, Republic of Mozambique
THE INHERENT CHALLENGES OF TESTING C. DIFFICILE
 THE INHERENT CHALLENGES OF TESTING C. DIFFICILE Detecting Toxins Associated with C. difficile Infection Pritty Patel, MS, MBA, Global Director of Microbiology, Vaccines and Novel Immunotherapeutics, Covance
THE INHERENT CHALLENGES OF TESTING C. DIFFICILE Detecting Toxins Associated with C. difficile Infection Pritty Patel, MS, MBA, Global Director of Microbiology, Vaccines and Novel Immunotherapeutics, Covance
Stool GeneXpert MTB/Rif Assay
 Stool GeneXpert MTB/Rif Assay Standard Operating Procedure 1.0. Purpose The purpose of this standard operating procedure (SOP) is to detail the steps for correctly performing, interpreting, and documenting
Stool GeneXpert MTB/Rif Assay Standard Operating Procedure 1.0. Purpose The purpose of this standard operating procedure (SOP) is to detail the steps for correctly performing, interpreting, and documenting
Good morning ladies and gentlemen, Thank you for inviting me to talk about the IVD industry.
 Good morning ladies and gentlemen, Thank you for inviting me to talk about the IVD industry. We are entering a new era of much needed regulatory oversight which might seem a daunting task at first. I am
Good morning ladies and gentlemen, Thank you for inviting me to talk about the IVD industry. We are entering a new era of much needed regulatory oversight which might seem a daunting task at first. I am
Integrated human diagnostics and vector control towards OneHealth
 Integrated human diagnostics and vector control towards OneHealth Dr. Konstantinos Mitsakakis, et al. University of Freiburg & Hahn-Schickard, Germany 3 rd WHO Global Forum on Medical Devices, 12.05.2017,
Integrated human diagnostics and vector control towards OneHealth Dr. Konstantinos Mitsakakis, et al. University of Freiburg & Hahn-Schickard, Germany 3 rd WHO Global Forum on Medical Devices, 12.05.2017,
Transition from WHO Product Testing to WHO Prequalification as basis for procurement of malaria RDTs
 Transition from WHO Product Testing to WHO Prequalification as basis for procurement of malaria RDTs Malaria Policy Advisory Committee, Geneva, Switzerland, 6 March 206 Presentation outline WHO Product
Transition from WHO Product Testing to WHO Prequalification as basis for procurement of malaria RDTs Malaria Policy Advisory Committee, Geneva, Switzerland, 6 March 206 Presentation outline WHO Product
Quick Reference Instructions
 PROFILE -V Quick Reference Instructions Reader System Before performing the test, refer to the PROFILE -V MEDTOXScan Drugs of Abuse Test System Insert and MEDTOXScan User Manual for complete operating
PROFILE -V Quick Reference Instructions Reader System Before performing the test, refer to the PROFILE -V MEDTOXScan Drugs of Abuse Test System Insert and MEDTOXScan User Manual for complete operating
Technical Guidance Series (TGS) for WHO Prequalification Diagnostic Assessment
 Technical Guidance Series (TGS) for WHO Prequalification Diagnostic Assessment Designing Instructions for use for in vitro diagnostic medical devices Draft for comment, 19 May 2017 World Health Organization
Technical Guidance Series (TGS) for WHO Prequalification Diagnostic Assessment Designing Instructions for use for in vitro diagnostic medical devices Draft for comment, 19 May 2017 World Health Organization
Molecular Diagnostics at the Point of Need
 Molecular Diagnostics at the Point of Need 24 November 2016 David Budd CEO d.budd@genedrive.com 1 DOCUMENT INFORMATION The information contained in this document and made verbally to you (together the
Molecular Diagnostics at the Point of Need 24 November 2016 David Budd CEO d.budd@genedrive.com 1 DOCUMENT INFORMATION The information contained in this document and made verbally to you (together the
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness
 Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: (905) Fax: (905)
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com E.coli O157:H7 End-Point PCR Kit Product# EP41300 Product Insert
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com E.coli O157:H7 End-Point PCR Kit Product# EP41300 Product Insert
The need for mobile devices for rapid diagnosis of febrile or infectious diseases in resource-poor countries
 Dept. Medical Parasitology and Infection Biology Molecular Parasitology-Epidemiology Unit The need for mobile devices for rapid diagnosis of febrile or infectious diseases in resource-poor countries 2016
Dept. Medical Parasitology and Infection Biology Molecular Parasitology-Epidemiology Unit The need for mobile devices for rapid diagnosis of febrile or infectious diseases in resource-poor countries 2016
Box 1a: Symbol key. Supplemental document Round 7: Annex (AID) - HarT checklist. Symbol Explanation Symbol Explanation
 Box 1a: Symbol key Symbol Explanation Symbol Explanation In vitro diagnostic medical device Content sufficient for < n > tests Lot number Date of manufacture YYYY-MM- (DD) Do not reuse Product code Consult
Box 1a: Symbol key Symbol Explanation Symbol Explanation In vitro diagnostic medical device Content sufficient for < n > tests Lot number Date of manufacture YYYY-MM- (DD) Do not reuse Product code Consult
Facilitating local production for improved access to in vitrodiagnostics. Tuberculosis. Ruth McNerney. Ruth McNerney & Rosanna Peeling
 Tuberculosis Facilitating local production for improved access to in vitrodiagnostics Ruth McNerney & Rosanna Peeling Ruth McNerney London School of Hygiene & Tropical Medicine Department of Pathogen Molecular
Tuberculosis Facilitating local production for improved access to in vitrodiagnostics Ruth McNerney & Rosanna Peeling Ruth McNerney London School of Hygiene & Tropical Medicine Department of Pathogen Molecular
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea
 How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
BIOPHARMACEUTICAL PROCESS EVALUATED FOR VIRAL CLEARANCE
 The purpose of Viral Clearance evaluation is to assess the capability of a manufacturing production process to inactivate and/or remove potential viral contaminants. Experience and knowledge in selecting
The purpose of Viral Clearance evaluation is to assess the capability of a manufacturing production process to inactivate and/or remove potential viral contaminants. Experience and knowledge in selecting
Preparation thin blood films and Giemsa staining
 TLM_PRO_026 Version No.001 Page 1 of 5 Department: Clinical sciences Unit: Tropical Laboratory Medicine Preparation thin blood films and Giemsa staining Project/study: Not applicable 1. Scope and application
TLM_PRO_026 Version No.001 Page 1 of 5 Department: Clinical sciences Unit: Tropical Laboratory Medicine Preparation thin blood films and Giemsa staining Project/study: Not applicable 1. Scope and application
Director Healthcare Biotech
 Director Healthcare Biotech EuropaBio, the European Association for Bioindustries, promotes an innovative and dynamic European biotechnology industry. EuropaBio and its members are committed to the socially
Director Healthcare Biotech EuropaBio, the European Association for Bioindustries, promotes an innovative and dynamic European biotechnology industry. EuropaBio and its members are committed to the socially
IGRAs for Serial Testing of Healthcare Workers
 IGRAs for Serial Testing of Healthcare Workers Jason Stout, MD, MHS Wake County TB Medical Consultant NC TB Medical Director Division of Infectious Diseases, Duke University Medical Center Disclosures-Funding
IGRAs for Serial Testing of Healthcare Workers Jason Stout, MD, MHS Wake County TB Medical Consultant NC TB Medical Director Division of Infectious Diseases, Duke University Medical Center Disclosures-Funding
i) Wild animals: Those animals that do not live under human supervision or control and do not have their phenotype selected by humans.
 The OIE Validation Recommendations provide detailed information and examples in support of the OIE Validation Standard that is published as Chapter 1.1.6 of the Terrestrial Manual, or Chapter 1.1.2 of
The OIE Validation Recommendations provide detailed information and examples in support of the OIE Validation Standard that is published as Chapter 1.1.6 of the Terrestrial Manual, or Chapter 1.1.2 of
CLIA Complexity: MODERATE
 CLIA Complexity: MODERATE INTENDED USE The is intended for the rapid detection of Group A Streptococcal antigen directly from throat swabs and beta-hemolytic colonies recovered from culture. This test
CLIA Complexity: MODERATE INTENDED USE The is intended for the rapid detection of Group A Streptococcal antigen directly from throat swabs and beta-hemolytic colonies recovered from culture. This test
WHO s IMS Organizational Structure Overview
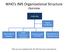 WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
Forecast diagnostics for antimicrobial resistance (AMR)
 Forecast diagnostics for antimicrobial resistance (AMR) Authors: Ann Van den Bruel, Philip Turner NIHR Diagnostic Evidence Cooperative Oxford, University of Oxford Context When asked to make forecasts
Forecast diagnostics for antimicrobial resistance (AMR) Authors: Ann Van den Bruel, Philip Turner NIHR Diagnostic Evidence Cooperative Oxford, University of Oxford Context When asked to make forecasts
Context and objectives of the workshop on platform technologies. 21 July 2016 David Wood and Virginia Benassi
 Context and objectives of the workshop on platform technologies 21 July 2016 David Wood and Virginia Benassi 2nd Technical workshop on platform technologies Geneva, Switzerland, 21 July 2016 Platform Technologies
Context and objectives of the workshop on platform technologies 21 July 2016 David Wood and Virginia Benassi 2nd Technical workshop on platform technologies Geneva, Switzerland, 21 July 2016 Platform Technologies
Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug development
 1 2 3 24 June 2010 EMA/CHMP/641298/2008 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug
1 2 3 24 June 2010 EMA/CHMP/641298/2008 Committee for Medicinal Products for Human Use (CHMP) 4 5 6 7 Reflection paper on co-development of pharmacogenomic biomarkers and Assays in the context of drug
FoodChek -E. coli O157 Kit for the Detection of E. coli O157. Product Information
 FoodChek -E. coli O157 Kit for the Detection of E. coli O157 Intended Use: Product Information The FoodChek -E. coli O157 kit is a rapid detection assay for Escherichia coli O157 in raw ground beef, beef
FoodChek -E. coli O157 Kit for the Detection of E. coli O157 Intended Use: Product Information The FoodChek -E. coli O157 kit is a rapid detection assay for Escherichia coli O157 in raw ground beef, beef
Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response. The WHO Blueprint Team
 Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response The WHO Blueprint Team Context of the Blueprint WHO Executive Board 1 (January 2015) WHO Summit on Ebola R&D
Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response The WHO Blueprint Team Context of the Blueprint WHO Executive Board 1 (January 2015) WHO Summit on Ebola R&D
Fast decisions instead of delays Quality Control through outstanding design
 Fast decisions instead of delays Quality Control through outstanding design We are CustomBiotech from Roche In your operations, behind your decisions, powering your products You are driving a paradigm
Fast decisions instead of delays Quality Control through outstanding design We are CustomBiotech from Roche In your operations, behind your decisions, powering your products You are driving a paradigm
Alere q. A platform to answer global health needs: TB and beyond. Duncan Blair Director, Public Health Initiatives
 Alere q A platform to answer global health needs: TB and beyond Duncan Blair Director, Public Health Initiatives 7 th FIND Symposium, Barcelona, October 2014 Alere q platform overview Comprising the Alere
Alere q A platform to answer global health needs: TB and beyond Duncan Blair Director, Public Health Initiatives 7 th FIND Symposium, Barcelona, October 2014 Alere q platform overview Comprising the Alere
GUIDELINES FOR ORGANIZING A QUALITY ASSURANCE PROGRAM FOR OV16 SEROLOGICAL RAPID TESTS
 GUIDELINES FOR ORGANIZING A QUALITY ASSURANCE PROGRAM FOR OV16 SEROLOGICAL RAPID TESTS About PATH PATH is the leader in global health innovation. An international nonprofit organization, we save lives
GUIDELINES FOR ORGANIZING A QUALITY ASSURANCE PROGRAM FOR OV16 SEROLOGICAL RAPID TESTS About PATH PATH is the leader in global health innovation. An international nonprofit organization, we save lives
Mycobacterium tuberculosis End-Point PCR Kit Product# EP42100
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Mycobacterium tuberculosis End-Point PCR Kit Product# EP42100
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Mycobacterium tuberculosis End-Point PCR Kit Product# EP42100
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
Diagnostics: Learning from successful experiences which made it to market. Mark Miller, MD Chief Medical Officer biomérieux France
 Diagnostics: Learning from successful experiences which made it to market Mark Miller, MD Chief Medical Officer biomérieux France Diagnostics: Learning from unsuccessful experiences which almost made it
Diagnostics: Learning from successful experiences which made it to market Mark Miller, MD Chief Medical Officer biomérieux France Diagnostics: Learning from unsuccessful experiences which almost made it
The response of research: from the field to the lab. K. Victoir PhD
 The response of research: from the field to the lab K. Victoir PhD Outline Responses to the Ebola outbreak: a nations response (France) and an institutional response (IP) Overview of the Field actions
The response of research: from the field to the lab K. Victoir PhD Outline Responses to the Ebola outbreak: a nations response (France) and an institutional response (IP) Overview of the Field actions
Update on Recast of IVD Directive
 Update on Recast of IVD Directive www.pei.de IVD Classification In-house Assays EU Reference Laboratories C M Nübling, PEI Concerns identified by the European Commission in the Medical Devices Roadmap
Update on Recast of IVD Directive www.pei.de IVD Classification In-house Assays EU Reference Laboratories C M Nübling, PEI Concerns identified by the European Commission in the Medical Devices Roadmap
Update on the IVDR. Sue Spencer
 Update on the IVDR Sue Spencer Caution The new regulations are draft the principles have now been agreed but the Annexes are subject to minor changes Further details will be added later pre and post application
Update on the IVDR Sue Spencer Caution The new regulations are draft the principles have now been agreed but the Annexes are subject to minor changes Further details will be added later pre and post application
What is the purpose of this website?
 What is the purpose of this website? This website aims to explain: How it is possible for different methods to give different results for the same test on the same patient sample Why it is important to
What is the purpose of this website? This website aims to explain: How it is possible for different methods to give different results for the same test on the same patient sample Why it is important to
Evaluation of a Rapid Test for the Diagnosis of Cholera in the Absence of a Gold Standard
 Evaluation of a Rapid Test for the Diagnosis of Cholera in the Absence of a Gold Standard Anne-Laure Page, Kathryn P Alberti, Vital Mondonge, Jean Rauzier, Marie-Laure Quilici, Philippe J Guerin To cite
Evaluation of a Rapid Test for the Diagnosis of Cholera in the Absence of a Gold Standard Anne-Laure Page, Kathryn P Alberti, Vital Mondonge, Jean Rauzier, Marie-Laure Quilici, Philippe J Guerin To cite
Enteroinvasive Escherichia coli. genesig Easy Kit for use on the genesig q reaction. Primerdesign Ltd
 TM Primerdesign Ltd Enteroinvasive Escherichia coli genesig Easy Kit for use on the genesig q16 50 reaction For general laboratory and research use only 1 genesig Easy: at a glance guide For each DNA test
TM Primerdesign Ltd Enteroinvasive Escherichia coli genesig Easy Kit for use on the genesig q16 50 reaction For general laboratory and research use only 1 genesig Easy: at a glance guide For each DNA test
Molecular Diagnostics at the Point of Need Interim Results (to Dec-17) 20 March 2018
 Molecular Diagnostics at the Point of Need Interim Results (to Dec-17) 20 March 2018 2 Decentralising molecular diagnostics DOCUMENT INFORMATION The information contained in this document and made verbally
Molecular Diagnostics at the Point of Need Interim Results (to Dec-17) 20 March 2018 2 Decentralising molecular diagnostics DOCUMENT INFORMATION The information contained in this document and made verbally
Simplify your pathogen testing. Amplify your confidence.
 Simplify your pathogen testing. Amplify your confidence. 2 One protocol. Fewer steps. Better results. Be confident in the knowledge that your pathogen testing process is accurate and reliable with the
Simplify your pathogen testing. Amplify your confidence. 2 One protocol. Fewer steps. Better results. Be confident in the knowledge that your pathogen testing process is accurate and reliable with the
DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT
 November 2015 DRAFT DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface... iii 1 Introduction.
November 2015 DRAFT DRAFT MEDICAL DEVICE GUIDANCE DOCUMENT REQUIREMENTS FOR LABELLING OF MEDICAL DEVICES Medical Device Authority MINISTRY OF HEALTH MALAYSIA Contents Page Preface... iii 1 Introduction.
SAMPLE PROCEDURE , 11/07
 SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
SAMPLE PROCEDURE This Sample Procedure is not intended as a substitute for your facility s Procedure Manual or reagent labeling, but rather as a model for your use in customizing for your laboratory s
Public private partnerships
 THE INTERNATIONAL CONFERENCE ON (RE-)EMERGING INFECTIOUS DISEASES 14 March 2018 in Addis Ababa, Ethiopia Public private partnerships Engaging the private research sector to scale up infectious diseases
THE INTERNATIONAL CONFERENCE ON (RE-)EMERGING INFECTIOUS DISEASES 14 March 2018 in Addis Ababa, Ethiopia Public private partnerships Engaging the private research sector to scale up infectious diseases
Hepatitis A Virus genesig Easy Kit for use on the genesig q16
 TM Primerdesign Ltd genesig Easy Kit for use on the genesig q16 50 reaction For general laboratory and research use only 1 genesig Easy: at a glance guide For each RNA test Component Volume HAV primer/probe
TM Primerdesign Ltd genesig Easy Kit for use on the genesig q16 50 reaction For general laboratory and research use only 1 genesig Easy: at a glance guide For each RNA test Component Volume HAV primer/probe
Herpes Simplex 1 IgM (HSV1 IgM)
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
Malaria Research Capability Strengthening in Africa
 July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
SURVEILLANCE AND LABORATORY DIAGNOSTICS OF INFECTIOUS DISEASES IN THE RUSSIAN FEDERATION I. DYATLOV
 SURVEILLANCE AND LABORATORY DIAGNOSTICS OF INFECTIOUS DISEASES IN THE RUSSIAN FEDERATION FEDERAL SERVICE OF SURVAILLANCE IN THE FIELD OF CONSUMER RIGHT PROTECTION & HUMAN WELFARE, RUSSIA I. DYATLOV Russia
SURVEILLANCE AND LABORATORY DIAGNOSTICS OF INFECTIOUS DISEASES IN THE RUSSIAN FEDERATION FEDERAL SERVICE OF SURVAILLANCE IN THE FIELD OF CONSUMER RIGHT PROTECTION & HUMAN WELFARE, RUSSIA I. DYATLOV Russia
Draft Guidance for Industry Development and Use of Risk Minimization Action Plans
 Draft Guidance for Industry Development and Use of Risk Minimization Action Plans Docket Number [2004D-0188] Submitted to the U.S. Department of Health and Human Services Food and Drug Administration Center
Draft Guidance for Industry Development and Use of Risk Minimization Action Plans Docket Number [2004D-0188] Submitted to the U.S. Department of Health and Human Services Food and Drug Administration Center
Information for Manufacturers on the Inspection of Manufacturing Site(s) (Assessment of the Quality Management System)
 P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s Information for Manufacturers on the Inspection of Manufacturing Site(s) (Assessment of the Quality Management System) WHO Prequalification
P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s Information for Manufacturers on the Inspection of Manufacturing Site(s) (Assessment of the Quality Management System) WHO Prequalification
One Year Later, Where Does the U.S. Response to Ebola Stand?
 One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
PROCEDURE MANUAL. Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature
 Procedure: CLIA Complexity: Moderate Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
Procedure: CLIA Complexity: Moderate Procedure #: Prepared By Date Adopted Supersedes Procedure # Review Date Revision Date Signature Distributed to # of Copies Distributed to # of Copies This Procedural
FINAL DOCUMENT. Global Harmonization Task Force (revision of GHTF/SG1/N29:2005)
 GHTF/SG1/N071:2012 FINAL DOCUMENT Global Harmonization Task Force (revision of GHTF/SG1/N29:2005) Title: Definition of the Terms Medical Device and In Vitro Diagnostic (IVD) Medical Device Authoring Group:
GHTF/SG1/N071:2012 FINAL DOCUMENT Global Harmonization Task Force (revision of GHTF/SG1/N29:2005) Title: Definition of the Terms Medical Device and In Vitro Diagnostic (IVD) Medical Device Authoring Group:
Malaria Research Capability Strengthening in Africa
 October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
METHODS MANUAL FOR PRODUCT TESTING OF MALARIA RAPID DIAGNOSTIC TESTS
 METHODS MANUAL FOR PRODUCT TESTING OF MALARIA RAPID DIAGNOSTIC TESTS Manual of standard operating procedures for Assessment of Malaria Rapid Diagnostic Tests within the Product Testing Programme of the
METHODS MANUAL FOR PRODUCT TESTING OF MALARIA RAPID DIAGNOSTIC TESTS Manual of standard operating procedures for Assessment of Malaria Rapid Diagnostic Tests within the Product Testing Programme of the
Clarifying digital health and software regulation: FDA releases three new guidance documents
 Clarifying digital health and software regulation: FDA releases three new guidance documents December 15, 2017 On December 7, 2017, the Food and Drug Administration (FDA or the Agency) released three guidance
Clarifying digital health and software regulation: FDA releases three new guidance documents December 15, 2017 On December 7, 2017, the Food and Drug Administration (FDA or the Agency) released three guidance
Diagnostics gathering intelligence to fight antimicrobial resistance
 Diagnostics gathering intelligence to fight antimicrobial resistance Arjon van Hengel European Commission DG Research and Innovation, Health Directorate Unit "Fighting Infectious Diseases and Advancing
Diagnostics gathering intelligence to fight antimicrobial resistance Arjon van Hengel European Commission DG Research and Innovation, Health Directorate Unit "Fighting Infectious Diseases and Advancing
Role of the WHO IVD Prequalification Programme in Light of National Regulatory Authority Approval
 Role of the WHO IVD Prequalification Programme in Light of National Regulatory Authority Approval UN Prequalification of Medicines, Diagnostics and Vaccines 6 th Consultative Stakeholder Meeting 4 April
Role of the WHO IVD Prequalification Programme in Light of National Regulatory Authority Approval UN Prequalification of Medicines, Diagnostics and Vaccines 6 th Consultative Stakeholder Meeting 4 April
Classification under the IVD Regulation
 Classification under the IVD Regulation Nick Baker Head of IVD Notified Body LRQA Ltd Improving performance, reducing risk IVD regulation shift in regulatory scrutiny Under the current IVD directive 10-15%
Classification under the IVD Regulation Nick Baker Head of IVD Notified Body LRQA Ltd Improving performance, reducing risk IVD regulation shift in regulatory scrutiny Under the current IVD directive 10-15%
The BEI & CGF s Soft Commodities Compact: TECHNICAL GUIDANCE
 The BEI & CGF s Soft Commodities Compact: TECHNICAL GUIDANCE as of December 2015 Introducing this Technical Guidance This Technical Guidance has been developed by the BEI Compact Banks, convened and co-ordinated
The BEI & CGF s Soft Commodities Compact: TECHNICAL GUIDANCE as of December 2015 Introducing this Technical Guidance This Technical Guidance has been developed by the BEI Compact Banks, convened and co-ordinated
Product# TM Intended Use. use only and not for use in diagnostic procedures.
 3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Norovirus TaqMan RT-PCR Kit Product# TM41400 Product Insert Intended
3430 Schmon Parkway Thorold, ON, Canada L2V 4Y6 Phone: 866-667-4362 (905) 227-8848 Fax: (905) 227-1061 Email: techsupport@norgenbiotek.com Norovirus TaqMan RT-PCR Kit Product# TM41400 Product Insert Intended
arxiv: v4 [q-bio.pe] 2 Sep 2014
![arxiv: v4 [q-bio.pe] 2 Sep 2014 arxiv: v4 [q-bio.pe] 2 Sep 2014](/thumbs/74/70358630.jpg) Pre-print version of manuscript published at PLOS Currents Outbreaks (2 September 2014) Estimating the reproduction number of Ebola virus (EBOV) during the 2014 outbreak in West Africa Christian L. Althaus
Pre-print version of manuscript published at PLOS Currents Outbreaks (2 September 2014) Estimating the reproduction number of Ebola virus (EBOV) during the 2014 outbreak in West Africa Christian L. Althaus
Improving the development and deployment of rapid diagnostic tests in LMICs. Workshop report 21 November 2016 London, United Kingdom
 Improving the development and deployment of rapid diagnostic tests in LMICs Workshop report 21 November 2016 London, United Kingdom The Academy of Medical Sciences is the independent body in the UK representing
Improving the development and deployment of rapid diagnostic tests in LMICs Workshop report 21 November 2016 London, United Kingdom The Academy of Medical Sciences is the independent body in the UK representing
OVERVIEW OF THE PREQUALIFICATION OF MALE CIRCUMCISION DEVICES ASSESSMENT PROCESS
 D i a g n o s t i c s a n d L a b o r a t o r y T e c h n o l o g y OVERVIEW OF THE PREQUALIFICATION OF MALE CIRCUMCISION DEVICES ASSESSMENT PROCESS Prequalification of Male Circumcision Devices PQMC_007
D i a g n o s t i c s a n d L a b o r a t o r y T e c h n o l o g y OVERVIEW OF THE PREQUALIFICATION OF MALE CIRCUMCISION DEVICES ASSESSMENT PROCESS Prequalification of Male Circumcision Devices PQMC_007
consent. With the exception, such as impossible to obtain informed consent objectively or there is no risk of the clinical trial for subjects, the res
 The Technical Guidelines for In Vitro Diagnostic Reagents Clinical Trial Article 1 In vitro diagnostic reagents clinical trial (include comparison experiment with marketed products) refers to the systematically
The Technical Guidelines for In Vitro Diagnostic Reagents Clinical Trial Article 1 In vitro diagnostic reagents clinical trial (include comparison experiment with marketed products) refers to the systematically
MicroSEQ Rapid Microbial Identification System
 MicroSEQ Rapid Microbial Identification System Giving you complete control over microbial identification using the gold-standard genotypic method The MicroSEQ ID microbial identification system, based
MicroSEQ Rapid Microbial Identification System Giving you complete control over microbial identification using the gold-standard genotypic method The MicroSEQ ID microbial identification system, based
ARN2016 Procurement Regulations Utility Sectors 2016
 ARN2016 Procurement Regulations Utility Sectors 2016 Preamble - Special sector companies are obliged to award assignments in accordance with the provisions of the Aanbestedingswet 2012 (Netherlands Procurement
ARN2016 Procurement Regulations Utility Sectors 2016 Preamble - Special sector companies are obliged to award assignments in accordance with the provisions of the Aanbestedingswet 2012 (Netherlands Procurement
Procedure 3. Statistical Requirements for CEC Test Methods
 Procedure 3 Issue 3 19th March 2014 Statistical Requirements for CEC Test Methods 1. Purpose...2 2. Overview...2 3. Processes...3 i) Repeatability/Discrimination...3 ii) Pilot inter-laboratory programme(s)...4
Procedure 3 Issue 3 19th March 2014 Statistical Requirements for CEC Test Methods 1. Purpose...2 2. Overview...2 3. Processes...3 i) Repeatability/Discrimination...3 ii) Pilot inter-laboratory programme(s)...4
E. coli O26 Latex Test Kit
 Importance of STEC Determination Shiga toxin-producing Escherichia coli strains (non-o157 STEC) have become an increasing public health concern. Some of the non-o157 STEC possess the same range of virulence
Importance of STEC Determination Shiga toxin-producing Escherichia coli strains (non-o157 STEC) have become an increasing public health concern. Some of the non-o157 STEC possess the same range of virulence
WHO EBOLA SCIENCE COMMITTEE
 WHO EBOLA SCIENCE COMMITTEE Prioritized Research Agenda World Health Organization 19 October 2015 The 2014-2015 epidemic of Ebola Virus Disease (EVD) in West Africa found the world unprepared, lacking
WHO EBOLA SCIENCE COMMITTEE Prioritized Research Agenda World Health Organization 19 October 2015 The 2014-2015 epidemic of Ebola Virus Disease (EVD) in West Africa found the world unprepared, lacking
OVERVIEW OF THE PREQUALIFICATION OF IN VITRO DIAGNOSTICS ASSESSMENT
 P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s OVERVIEW OF THE PREQUALIFICATION OF IN VITRO DIAGNOSTICS ASSESSMENT WHO Prequalification of In Vitro Diagnostics NOTE: Additional technical
P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s OVERVIEW OF THE PREQUALIFICATION OF IN VITRO DIAGNOSTICS ASSESSMENT WHO Prequalification of In Vitro Diagnostics NOTE: Additional technical
EBOLA SITUATION REPORT
 EBOLA SITUATION REPORT 30 MARCH 2016 SUMMARY The International Health Regulations (2005) Emergency Committee regarding Ebola virus disease (EVD) in West Africa met for a ninth time on 29 March. On the
EBOLA SITUATION REPORT 30 MARCH 2016 SUMMARY The International Health Regulations (2005) Emergency Committee regarding Ebola virus disease (EVD) in West Africa met for a ninth time on 29 March. On the
NDA Requirements: Surveillance, Clinical Trial Data, Breakpoints
 NDA Requirements: Surveillance, Clinical Trial Data, Breakpoints Kevin M. Krause Director Microbiology Achaogen, Inc. Presented at the ASM/ESCMID Conference on Drug Development to Meet the Challenge of
NDA Requirements: Surveillance, Clinical Trial Data, Breakpoints Kevin M. Krause Director Microbiology Achaogen, Inc. Presented at the ASM/ESCMID Conference on Drug Development to Meet the Challenge of
RealLine HPV 31 / 33 Str-Format
 Instructions for use A QUALITATIVE ASSAY KIT FOR THE DIFFERENTIAL DETERMINATION OF HUMAN PAPILLOMAVIRUS TYPE 31 AND 33 DNA BY REAL TIME PCR METHOD In vitro diagnosticum () VBD8471 96 Tests valid from May
Instructions for use A QUALITATIVE ASSAY KIT FOR THE DIFFERENTIAL DETERMINATION OF HUMAN PAPILLOMAVIRUS TYPE 31 AND 33 DNA BY REAL TIME PCR METHOD In vitro diagnosticum () VBD8471 96 Tests valid from May
Listeria Environmental Monitoring programs: Getting into the details
 Listeria Environmental Monitoring programs: Getting into the details Martin Wiedmann Department of Food Science Cornell University, Ithaca, NY E-mail: mw16@cornell.edu Phone: 607-254-2838 Thank you to
Listeria Environmental Monitoring programs: Getting into the details Martin Wiedmann Department of Food Science Cornell University, Ithaca, NY E-mail: mw16@cornell.edu Phone: 607-254-2838 Thank you to
Development of diagnostic kits for selected markers of resistance, virulence and zoonotic transmission among methicillinresistant
 Development of diagnostic kits for selected markers of resistance, virulence and zoonotic transmission among methicillinresistant Staphylococcus aureus strains Dr. Boris Oberheitmann, Constanze Seidel
Development of diagnostic kits for selected markers of resistance, virulence and zoonotic transmission among methicillinresistant Staphylococcus aureus strains Dr. Boris Oberheitmann, Constanze Seidel
Annex 1(1): Availability of panels of cultured P. falciparum parasites for preliminary testing of products by manufacturers (optional)
 Annex 1(1): Availability of panels of cultured P. falciparum parasites for preliminary testing of products by manufacturers (optional) A panel of samples derived from cultured P. falciparum, diluted and
Annex 1(1): Availability of panels of cultured P. falciparum parasites for preliminary testing of products by manufacturers (optional) A panel of samples derived from cultured P. falciparum, diluted and
