Lecture No. # 12 Assessment of biocompatibility of biomaterials
|
|
|
- Tracey Morrison
- 5 years ago
- Views:
Transcription
1 Introduction to Biomaterials Prof. Bikramjit Basu Prof. Kantesh Balani Department of Materials and Metallurgical Engineering Indian Institute of Technology, Kanpur Lecture No. # 12 Assessment of biocompatibility of biomaterials In the last lecture, I have discussed about the cell metal infraction, so I will continue with that and then I will discuss in this lecture on the in vitro testing. (Refer Slide Time: 00:26) So, different in vitro testing that is required to assess the biocompatibility property of biomaterials. Now, as you know that biocompatibility is the co-requirement or principle requirement for assessing the whether a material can be used in the in vivo applications or whether a material can be used for a given biomedical applications. So therefore, it is important to know more details that, how this biocompatibility property can be assessed. Now, first is that you need to know that biocompatibility is system property. System property means if your material is biocompatible for a particular applications, as I have mentioned couple of times during the last two lectures that if a material is biocompatible. For example, in the knee replacement that does not necessarily mean, that the same material is biocompatible for heart valve applications,
2 because your requirement is application specific; and therefore, your property requirement is also application specific. That is the number one point. Number two point for assessing the biocompatibility property you need to do in vitro testing and in vivo testing. In vitro testing essentially means that when you are doing the tests in the laboratory stimulated environments. In vivo essentially means when you do the experiments in the animal body itself or inside the animal body. Now, again if a material from the in vivo applications, if a material is found to be good biocompatible in the rat model or rabbit model that may not necessarily mean or they may, that may not necessarily tell you with guarantee that the same material will be successful in the human model experiments because, the in vivo conditions in the rat or rabbit is quite different from that inside the human being. Because human actually is a large animal model and in the large animal model your fluctuation in PH, your fluctuation in temperature or in that different type of collagen or different tissues and their composition should be different compare to that the same thing in the rat or rabbit. So therefore, in before one material can be applied in the human body, it is important that you do the test right from the small animal, intermediate animal right up to the human being before you can sell it into the market. So, this understanding is important and that is what I am saying here that biocompatibility depends on a variety of system parameters and it is essentially a system property. So if you change certain parameters in the system, then biocompatibility property also will be changing. Now, some of the things that I have mentioned here one is the toxicology, now toxicology essentially means that whether a biomaterial is toxic to the gene or the DNA or is capable of damaging the DNA, inside the nucleus. Now, this test is part of the genotoxicity testing which I will be showing later. Now biodegradation biodegradation essentially mean whether a material can degrade itself in the biological environment that means so, if if a material before putting the in vivo conditions or before putting in the animal model has a certain set of properties like hardness properties, strength properties etcetera etcetera.
3 Now, you put the same material inside a animal and then after let say 2 months or 3 months you take out the material do the test, now if if you see that the material loses its strength or the strength is decreased, that means there is a fate of the biological environment on the degradation of the material properties. So that is number 2. Number 3 design and construction now, it also depends on what is the shape of the biomaterial for example, if the shape is very much circular type of materials, then you do not have any stress concentration at any of the age of the biomaterial however, if you have a very sharp edge like a square of square type of implant that means, this corners are the potential sides where there will be stress rises or where the stress can be quiet high and accordingly the the implant when it is used for the load bearing applications those the presence of this square corners actually will effect on its biocompatibility. Then implant movement, now implant movement is that for example, your knee implant is an example of that implant which will experience I mean more number of movements compare to for example, any other implants inside the body or knee will take more load or more load r they will take more load during its applications compare to any other material. So, implant movement is also important and later all I will show you that how implant movement will cause the accepting loosening. Accepting loosening means whether how the bone or bone replacement materials or implants can be can can be detached from the natural bone or can lose may be can loosen out from the natural bone. Then surgery and sterility sterility is important and typically the sterilization is done either by gamma radiation for certain materials or by steam autoclave like you know steam treatment at typically at 121 degree Celsius in water vapor. Chemical reactions to implant chemical reactions, to implant means, like whether a biomaterial leaches out some of the elements from its components and thereby those leached out elements can cause certain toxic reactions in in vivo conditions. The last one is the biological in reactions to implant. Now, biological reactions to implant means for examples when you out the any material in the in vitro conditions many times that in vitro environment or in vitro biological fluid can cause, the hydroxyapatite or calcium phosphate rich layer formation on the material and if it does that is very good because,
4 the presence of hydroxyapatite calcium phosphate rich layer formation can induced more biocompatibility in the materials. So therefore, biocompatibility evaluation essentially will address or will focus some of the important properties that I have listed out here: cytotoxicity, cyto means cell and cytotoxicity means cell level toxicity, sensitization, genotoxicity I have already mentioned implantation means when you are putting the material as an implant in the animal body. So, that is the kind of in vivo experiments, Carcinogenicity whether the material can potentially cause the cancer inside the in vivo conditions. (Refer Slide Time: 07:26) So therefore, testing of the biomaterials are this biocompatibility assessment will try to address the following questions that how to characterize the material that will be processed into a medical device or implant. So this characterization is more from biological characterization remember, second one how biomaterials can be evaluated to determine if they are biocompatible. Now if they are biocompatible as I said biocompatible is a broad parameter. So, in the laboratory scale experiments people do only a set of particular experiments which are bad of the broad property which is known as the biocompatibility. So if you do the cell culture experiments, you cannot say on the basis of the cell culture experiments a given material is fully biocompatible, you can only say on the basis of the cell culture experiments whether a material is cytocompatible or cell compatible.
5 So, that is the cytocompatibility property is a much better description compared to biocompatibility if you use the word biocompatible right. Now, how biomaterials can be evaluated to determine whether they function appropriately in the in vivo environment. Now, again as I have mentioned that if a biomaterial passes through all the test of the in vitro conditions that even cannot guarantee that the same biomaterial can function very appropriately in the in vivo experiments unless, you carry out a particular set up in vivo experiments understand. So these things stand up here in mind all the time as long as you are dealing with the biomaterials. So therefore, the question is that how biomaterials can be evaluated, to determine whether the function appropriately in the in vivo experiment. So that question is a kind of peak time question and then you need to address it very carefully. (Refer Slide Time: 09:23) Now, testing a biomaterial four phases: first one is the raw material characterization in screening. So, raw material characterization means that what are the elements that you are using or what are the starting powders that you are using in the as a raw materials and they are chemical, physical and biological characteristics or biological properties. Chemical properties means, what are the elements that they contain like calcium, phosphorous, silicon etcetera. etcetera Physical properties means what is the size, what is the properties. Biological properties mean whether any of the starting material itself is biologically compatible or not.
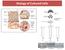
6 Then comes, the material biocompatibility and here this material biocompatibility again I have mentioned that all the set of in vitro and in vivo properties that is cytotoxicity irritation, systemic toxicity, hemocompatibility: hem means blood, hemocompatibility means blood compatibility. Carcinogenicity: carcinogenicity means cancer level toxicity or whether it can potentially cause cancer. Now, product and process validation: And product and process validation means like the way you prepare this biomaterial right from your raw material that is important and that depends on the manufacturing or sterility finished product etcetera and routine testing. Routine testing means all the ISO standard testing. ISO means, international standard organization so, tells you that certain set of test you need to do before you can release the product in the market. So, all those testing needs to be done before this product can be marketable. (Refer Slide Time: 11:04) Now, there are two types of materials interaction. It is important that you know how this implant will look like or how the implant will react, when it is put in the host. Host means here the animal or any human being. So here the implant is shown as the gray rectangular slab. Now this is the doctors are performing this surgery doctors are performing the surgery here, that means your doctors are putting this gray rectangular slab at the specific sides inside the human body. Now, when you put this implant inside the human body or the any animal then within t is equal to 1 minute.
7 So, you look at the timescale also like what is the time frame along which these reactions formed, whether t is equal to 1 minute the biomaterial absorbs a layer of proteins as I said in the beginning due to the cell material interaction, that protein absorption is the first reaction or the first (( )) before any further cell adhesion takes place. Now, within the first 1 minute or so that you biomaterial absorbs a layer of proteins then cells, cells means this is this is like fibroblast cells or macrophages. Now, these are your cells now these cells actually come and attracted get attracted towards the protein layer and you know from your basic in vitro study, that how this reaction takes place like cell surface protein they reacts with the absorb surface proteins on the biomaterial surface and therefore, these cells will get adhere to the protein layer there. Then fourth one is that cells fuse to form joint cells and secrete protein signaling agents like cytokinesis. Now, cells these joint cells means when number of cells they aggregate together and this aggregation can be done in a self organized fashion. Self organized means cells will attach to themselves like in a in their own fashion in a self organized manner and they form a joint cells and what they do? They secrete a protein signaling now this signaling process I have mentioned briefly in the last lecture, that signaling is important because, through this signaling one cell will transmit the cell signal to the other cell. So that cell movement, cell proliferation or cell differentiation or cell division can take place during the in vitro condition and this signaling can be transferred or this or or this signaling can go or this signals can go from one cell to another cell through E C M that is the Extra Cellular Matrix, now those things I have mentioned in the last lecture. Now, in response to the cytokines fibroblast arrive and began synthesizing collagen. Now what will happen once these joint cells formed, then they sends the signals to this fibroblast and because, of this proteins signaling fibroblast cells. Fibroblast means that is the cells of the connective tissue and once this fibroblast cells they are getting attracted towards this implant material and that takes place during t is equal to 5 days to 14 days then what will happen that within that time period this fibroblast cells they are they will arrive at the cell surface near to the cell surface and then they will secrete the collagen material.
8 And collagen material is important because, collagen also will constitute or collagen is the major component of the Extra Cellular Matrix. Then after that the biomaterial is encapsulated in an acellular and collagenous bag, now it is kind of you know that entire material is now contained in a inside the bag. Bag means it is like you know it is a big bag which will contain the collagenous material as well as some cells and then protein molecules also. So entire biomaterial is now is now contained in a biological bag kind of things. So, that is the way these two things that is the way this entire Foreign Body Reaction. Foreign Body Reaction means F B R that takes place towards the biomaterial. So, essentially its starts with t is equal to 0 and it it will go in the t is equal to 2 weeks so, entire process to take place it takes almost like a 2 weeks or 21 days and that is one of the reason that why many of the short coming plantation tends to see that whether a biomaterial is biocompatible in vivo, that people do up to the 12 weeks because, your initial thing takes place again 3 weeks then you see whether it causes inflammation to the neighboring tissues of the cells and that entire thing should be started up to a sufficient time period and that is the reason it takes 12 weeks time. Now, if I can describe this then you will understand it much better, there are two types of materials interaction one is to create an inert surface and not allowing the adsorption of proteins and adhesion of cells and thus preventing activation of the immune system, blood coagulation, thrombosis, extracellular matrix deposition and other interactions of the materials and the surrounding environments. So what I describe that to what happens from t is equal to 0 to t is equal to 3 weeks the entire process is not facilitated by certain material. So, what they call as bio-inert materials. Bio-inert means like it is biologically inert type of materials. Now, these kind of materials the examples are like for example, alumina or zirconia these kind of materials they cannot create and biologically active surface, they essentially creates an inert surface and they do not allow all these protein adsorption, cell adhesion and any activation of the immune system to take place. Now, examples are the heads and cups of the joint prostheses, intraocular lenses and blood-contacting devices. Blood-contacting devices means like heart valves so, these are like blood-contacting devices. Heads and cups of joint prostheses means, you have the
9 stem you have the acetabular cup and you have the you have that acetabular cup and you have the femoral ball. So femoral ball and acetabular cup they do not essentially require this kind of tissue formation because, there is essentially mechanical devices. So, those kinds of bones or tissues they essentially do not require any kind of the biologically active material so, within the bio active materials are safe to use that are perfectly. (Refer Slide Time: 17:42) Now, this is the other that is more general and advanced strategy aims at creation of materials promoting attachment, migration, proliferation, differentiation, long-term viability and cell functioning in a controllable manner. So that is the other biomaterials that is called bioactive materials and these bioactive materials essentially are the materials which belongs to the family of hydroxyapatite or calcium phosphate type of material and their uses bone implant or some other tissue replacement application. So, these materials in the in vivo environment they always create some surfaces which will promote the cell attachment or cell migration or cell proliferation. So, these are the examples of the cell-fate processes. Now these cell-fate processes are essentially promoted or essentially encouraged when this sort of materials like bioactive materials like hydroxyapatite, dicalcium phosphate they are implanted inside the animal body.
10 (Refer Slide Time: 18:45) Now cytotoxicity, now, in vitro cytotoxicity as say there essentially important to evaluate the toxicity of materials that can cause in close contact to the living tissue. So tissues mean these are aggregates or the self-organized. Self-organized aggregates of the biological cells and therefore, if the biological cells himself experience any toxic effect because, of the material then entire tissue also will experience similar toxic effect. Such materials may be toxic to the surrounding tissues under the action of body fluids or physical stresses. Now, one thing that I must mention here that typically cell culture experiments of cytotoxicity experiments, in the normal biology labs or molecular biology lab they do not involve any dynamic conditions. Dynamic conditions mean they do not involve any flow of the fluid, these are called static test. Static test means you simply put the material inside the solution and you absorb the cell proliferation, cell attachment, but under the dynamic condition this cell attachment or cell proliferation behavior can be quite different. So, on the basis of your simple static cell culture experiments you can again cannot comment how the cell attachment or proliferation will take place under the dynamic condition. So, these are the kind of things that you should remember so that you can understand that what happens in the entire process, the other thing is that physical stress. Physical stress means when you are putting that any bone implant materials so human body is not always in the rest condition or people do not sleep 24 hours right.
11 So, people sleep 8 hours and 16 hours in the working conditions, in 16 hours that entire things are moving right in the movement conditions,again under the physical stress of the movement when the bone is on the moving movement conditions, then that will also affect the way cell proliferate or cell differentiation. So, but however static test again cannot tell you how these two factors like action of the body fluids, like dynamic conditions or physical stress will affect the cell compatibility or the biocompatibility of the materials. (Refer Slide Time: 21:02) Now, in vitro testing now cell culture solution so these are like you know cells, so these are like cells with the disperse in the solution. So, let say you take any animal we said we dissect the tissue and you finally, chop. So, this dissection of the tissues and chopping of the tissues essentially require to extract the cells from that particular tissue. So if you are taking the connective tissue cells then you can chop it and you can extract biologically the fibroblast cells. But this can be done in the laboratory, in the advance laboratory and normally all the researches they get the cells as if commercially in the growing conditions on the living stem, then the another things is to that you have to free cells in the extracellular matrix via digestion.
12 So, cells also will be essentially dispersed in a E C M or extracellular matrix, but you want only cells not the cells with the extracellular matrix so, essentially you have to extract the cells from the extracellular matrix also place them I a dish with nutrients and keep them warm, now warm is it is like 37 degree Celsius. Now you have to always provide the cells with a environment which will be sufficient for it survival and sufficient for it survival means you have to give nutrients, like food like an human being cannot survive without foods similarly, cells also will not survive without nutrients and then second thing is that, you have to give them physiological environment which contains 37 degree Celsius, PH 7.4. (Refer Slide Time: 22:41) So, that environment must be given so that cells will survive. Now, this is the direct contact testing of the cytotoxicity and direct contact means this biomaterial you can directly mark and the surfaces that in direct contact with the cells and then cells are in the solution and this solution contacts D N A and phosphor buffer solution on all those things. Then you can have this monolayer and confluent so, monolayer means one layer the surface will contain one layer which is pool out in a particular cells and these cells can be L-929 fibroblast cells and again this culture conditions typically can be 24 hours depending on the cell lines and 37 degree Celsius. Now, cells may change morphology that is important, cells may die or cells may lose adherence to the dish. So, what I am saying that there are three scenarios: one scenario is
13 that cells die like, cells as soon as in coming contact with the material, cells will undergo apoptosis that means cell death will occur and that will essentially tell you thus this biomaterial is toxic. So, that biomaterial is toxic. Number 2 cells change their morphology, change their morphology means essentially cells which are in free condition in the solution they are like spherical shaped, now once it comes in contacts the biomaterials then adhered this spread and then cells change the morphology and that is very good as far as the cytocompatibility property is concerned. Third one cells will lose adherence to that is so, sometime what happens you know that these type of that lower surface of the cell culture solution, cells will not cells will be freely suspending in the solution, but cells will not adhere to the material as the at the same time cells which are suspended in the solution they are not dead. Do you understand what I am saying? What I am saying is that cells are surviving? Cells are cells will be dispersed in the solution, but cells will not adhere to the material itself. So that shows that the material is such that it the protein adhesion cannot take place or cell adhesion process cannot be trigger. Again those materials, you cannot call them as a bioactive material. Now, in most of the cell culture experiments in the laboratory scale people use L-929 that is the mouse fibroblast cell lines. Now, this mouse fibroblast cell lines why they are used? The question the answer is, they are easy to maintain this cell and easy to maintain. Second one, people have found good correlation with animal test. Animal test means if you use the mouse fibroblast cell lines and you say that these material is in vitro cytocompatible, many times people have to observed or people have reported that the same material is also in vivo biocompatibility, that means it is mold like one to one correlation if it is having the good cytocompatibility to L-929, it has good biocompatibility in vivo. The third point is that fibroblast present in the wound healing process. Wound healing means like essentially when you are talking some bones or joints in human body and put in the implants so, you are essentially you are essentially inducing some wound in the patient body and in the wound side the fibroblast cells are the first cells which will come and then they will form some tissue in contact with the implant. So, that is the reason that is why fibroblast cells are used primarily most of the cell culture experiments.
14 (Refer Slide Time: 26:15) Now, principles of cell culture, cells can grow either in suspension or as a monolayer culture on a special surface. Now monolayer cultures means most cells grow in monolayer with the exception of the hematopoietic cells that is the blood cells and third one is the suspension culture, cells can cells that can survive and proliferate without attachment this ability is restricted to hematopoietic cells or cells from malignant tumor. So, essentially what it means that is the other scenario that I have told the cells will not get themselves attached to the material, cells will still survive and they will suspend in the cell culture solution.
15 (Refer Slide Time: 26:50) Now, cell cultures are normally handled under the sterile conditions and cell culture laboratories need to be equipped with incubators, sterile benches, autoclaves and all those things that you know, the other part is that various physical properties have to be taken into account to work with the cell culture, such as PH most cell lines grow well at the PH7.4 temperature, viscosity, surface tension and foaming. (Refer Slide Time: 27:12) Now, how this cell growth and proliferation can take place so, tissue can grow by increasing the number of cells, now when you have to increase in the number of cells
16 then that process is known as the hyperplasia. Increasing the size of the cell that is the hypertrophy, not atrophy, increasing the amount of intercellular substances so when a cell will increase a cell size, will increase in size that essentially means that increase in cell size must be accompanied by the increase in the amount of the intercellular substance, if the intercellular substance is the not increased then cells will not increase, will not be able to increase in size. And intercellular substance means like protein molecules if they are transported from outside the outside the cell body or outside the E C M or from other cells to the particular cells then cells will accumulate more and more number of protein molecules and then also it will increase in size. So, an increase in the cell number is by far the most important criteria in either normal or abnormal growth. So, what has been stated here that, when any process is accompanied by the increase in the number of cells and that increase in the number cells is important because, that will tell you the 12 cells that are in the growing stage or cells are proliferative pause. (Refer Slide Time: 28:30) Now, cell proliferation also or cell growth also takes place over the certain time duration. Now, this particular S E M micrograph tells you that what happens after 1 day and then right hand side micrograph tells you, that what happens after 7 day. So at the end of the 1 day L-929 fibroblast cells they are adhering, but the number is much less, but at the 7
17 days, the cells are adhering at certain point cells are forming, some cellular network or cellular bricks. (Refer Slide Time: 29:00) So, this you know that what is the meaning of the in vitro and what is the meaning of in vivo by now and why that both the of set of test are important, (Refer Slide Time: 29:10) and what is the necessity of the in vivo test as far as I mean per say
18 (Refer Slide Time: 29:15) Now, there are certain types of terminology that you should know what is known as systemic effect. Systemic effect means whether a biomaterial when implanted inside the animal body and that biomaterial, causes some effect in a in the system which is surrounded which surrounds the biomaterial and it can be acute like within 24 hours, it can be sub-acute within 14 to 28 days and it can be sub-chronic like up to 90 days or more than 10 percent of animal's life span and it can be chronic that is more than 90 days and more than 10 percent of the animal's life span. So, what is means by that suppose, it causes some toxic effect any biomaterial and this toxic effect is seen within the implantation of within the first 24 hours then we can call it as a very acute toxic effect. Now, if that toxic effect is observed in within 14 to 28 days then it is called sub-acute toxic effect. Now, it is called if it is a chronic, then it is typically it is observed after 90 days, months 3 months and within the 3 months if the animal or if the animal model is experienced that any toxic effect then is called subchronic type of effect.
19 (Refer Slide Time: 30:31) Now, host response as I mentioned earlier, host response means how the animal model itself will actually respond to the implant that you have put it inside that particular animal. So, biomaterials in contact with human tissues may have direct or indirect adverse toxic effect. Now, the various types of toxicity may be time limited and result so, this toxicity as you have seen in the earlier slide that it can be systemic means this toxicity effect may not be seen immediately after the implantation, like it cannot it it may not be very acute. Acute means what you see within 24 hours. But it can be chronic also, chronic means after long time lets a 90 days this toxic effect is seen, then that types of toxic effect is known as the chronic toxic effect and this in case of the chronic disease or chronic toxic effect, that initially the implant may perform well, but you know after 3 months the implant may not perform biologically well in the environment. So that is why it is saying it is said that this host response can be toxicity may be time limited and result in minimal and reversible effect minimal and reversibleeffect and may be longer lasting resulting in serious tissue damage. So, serious tissue damage means that within long term and were for a longer time interval and on your prolong basis, that material can cause some tissue damage. So this slide has been explained before,
20 (Refer Slide Time: 32:08) now the question is that, why a biomaterial or why certain types of biomaterial are shows or exhibit that kind of chronic disease or chronic toxicity after 3 months for example, now the answer is, is many times this biomaterials will experience some corrosion and wear in the in vivo environment and the wear takes place after long time and they generate some kind of particles, so that is what is mentioned here that wear particles may be released from the failed devices and are formed at the articulating surface of implanted devices. And these wear particles they are typically micron size particles or can be nano sized particles, finer the particles more is the toxicity effect. So if the particles are nano sized then it will have more toxicity compare to particles which are in the micron size and the particles may also be injected directly into the body for clinical purposes.
21 (Refer Slide Time: 33:02) Now, these are the standards in the biomaterials testing this is like ISO testing, I S O and which is approved by FDA. F D A is the U S food and drug administration and ISO means international standard organization. Now they have this set of documents and these documents there is like different subsets or set of documents like first one is the guidance on selection of tests, that tells you like suppose you are trying to develop a material x, for y application now for this material x, what are the kind of test a b c d that you need to perform for to satisfy or to say that this material x will be suitable or will be ideal for this biomaterial biomedical application y, I s will tell you that these you have to test from a to d or for example, a to g. Then second one is the animal welfare requirements like you know suppose if you put this material inside a particular animal or human being what is the animal welfare requirements that you must know those things will be mentioned in the Now that is a genotoxicity, carcinogenicity and reproductive toxicity, that is important, I mean those days guidelines are mentioned that is test for interaction with the blood that is the blood compatibility test, that is a test for cytotoxicity like in vitro method, so all these tests are essentially tells you that what are the guidelines that you need to follow when you want to do a particular set of tests.
22 (Refer Slide Time: 34:45) Ok We will start with the that is the set of guidelines which will tell you that what are tests that you need to carry out for a specific application. Now principles of the toxicity evaluation, that essentially characterized and identified the composition of the biomaterial and the potential impurities and extractables. Extractables means like when your material has a multiple composition x y z, now this you have to be judicious enough to be understand that whether x will be list out in the in vivo conditions or y will be list out in the in vivo conditions or the z will be list out in the in vivo conditions. And what are the impurities those will have this kind of effect, this second one is that account for toxic effect of the leachable chemicals and the degradation product so, leachable chemicals and the degradation product so remember that these two things may not be the same because, your element can be list out from the material, but then it can react with the biological fluid then it can cause some kind of a product. So that degradation product may have a different composition from what the element that is list out from the material. Third one is that testing perform by competent that informed person so that is the medical professionals. And fourth one is the reevaluate toxic effect if there is change in the composition manufacturing practice or intended use. Now, if you change your manufacturing process means the way you have the abrogated or you have sintered or you have compression
23 molded or you have injection molded particular material, if you change your process parameters,it is advisable that you need to revisit your all these toxicities studies again. Now, some generally recommended tests include cytotoxicity, sensitization or implantation test. Now, as I said that you know that in vitro cytotoxicity or that is the cell culture test that is under the guideline of and I will quickly go through that you know, what has been summarized to the last lecture? (Refer Slide Time: 36:45) So, these toxicity actually effects like when this particular material, this is the hatch is the material, that is the biomaterial it will release some kind of chemicals and these chemicals can enter the material, inside the material they can either transported through the nucleus then cause the DNA damage or it can cause some death of this intracellular elements like mitochondria, golgi body, golgi appearance it can cause some damage. So that, mitochondria cannot function like the way it performs in the normal healthy cells. Do you understand what I am saying? So your essentially culturing the normal healthy cells on the biomaterials. Now if the biomaterial leaches out some substances which can cause damage to the mitochondria which produces the energy in the inside the cell or which is the power house of the cell. Now, if the mitochondria cannot perform the way it should perform in the normal healthy cells and if it is damaged that means cells also will lose its normal functioning and essentially the cells will die over a time period.
24 So, that means that cells is causing a toxicity effect in vitro or if the material can be directly transported to the nucleus and then it has a toxicity effect, it can cause the genotoxicity or the DNA damage of the material. (Refer Slide Time: 38:16) Now, this is the structure of the connective tissue and the tissue underlying epithelium, so, epithelium is the top surface of the top tissue and here you can see that all the cells, eukaryotic cells are connected to each other and you know very dense manner. So, that is what I have mentioned earlier that in a tissue cells organize themselves and then tissue can be discuss self organized aggregates of the cells. Now, here you have a basal lamina and here you have the macrophages. The macrophages another cells also type of eukaryotic cells and you have the fibroblast cells which has a different type of shape, which does not have the same shape as the cells of the epithelium and this entire green stuff as you can see that matrix, it contains the E C M that is the extracellular matrix and extracellular matrix it has collagen and it has the collagen as well as the hydroxyapatite particles and so on. And you have that all other other type of cells like mast cells or capillary etcetera and this shows the collagen fiber, now this is called the connective tissue. So, the entire connective tissue contains a number of cells the other things that you should notice here that these cells they are kind of in direct contact with each other. However all the
25 connective tissues, these two connective tissues are not connected to each other, but they are separated by the extracellular matrix. So connected tissues therefore, have a different environment compare to the cells in the epithelium layer understood. (Refer Slide Time: 39:51) This is the cells how the cells which are surrounded by the E C M. So this is the part of your this white background or you know white matrix, this is the part of your E C M and you have the cell lines like you know connected tissues cells and so on. So this is the cell lines like fibroblast cells, so each all the fibroblast cells they are like separated from each other by the presence of the extracellular matrix. Now this shows how cell are surrounded by spaces which are filled by E C M.
26 (Refer Slide Time: 40:18) Now, this is the part of one cell and you have these fibers like structures you can see these are essentially like collagen fibers. Now this collagen fibers constitute your extracellular matrix and these fibers can be clearly seen and some fibers inside the cells do you notice these are the cytoskeleton that is the constitute of cytoskeleton. (Refer Slide Time: 40:46) Now this recent image clearly shows you that, what are the focal adhesions as the production sites of the intracellular signals? Now these focal adhesions are present at different sites in the extracellular matrix and these are the collagen fibers which are
27 constituting the extracellular matrix. So these appears as the green and this focal adhesions means this is the points of the focal adhesions where the signals are generated and signals can be transferred to other target cells, through the extracellular matrix collagen. (Refer Slide Time: 41:20) Now, this is the anchoring junctions and these anchoring junctions they join the cytoskeleton from two cells in the epithelium and these anchoring junctions are essentially these particular junctions are called anchoring junctions and these are the cytoskeleton filaments. Now what you see that cytoskeleton filaments from cell one, they are getting attached to the cell two and then they form this black level junctions and this black level junctions you can see here which are considering, which are known as the anchoring junctions. Now, the other thing is that you see that is that you have extracellular matrix E C M now, which is known as the labeled and this is the yellow junctions which is just outside the this epithelium cell lines.
28 (Refer Slide Time: 42:07) Now, this is the conditions that when the two cells are connected to each other as an epithelium, but when the two cells are separated from each other like the way it is separated here,this is cell 1 and this is the cell 2 and this you have this plasma membrane which is the cell membrane. And now you have the cytoskeleton filaments here this green one, red one and you have this intercellular actin pro, actin intercellular anchor proteins and you have also transmembrane protein. Now there are different type of proteins as you say as you noticed in the earlier lectures like you have the proteins the number of proteins can be as has 10 to the power 19, 10 to the 9 to 10 to the power 13 numbers. So this large number of proteins can be of different types, now different types is protein is called trans one is the transmembrane adhesion proteins. Transmembrane means they are essentially present between the two membranes and they are essentially participate in the adhesion process between the two cells. Now this is mediated by this adhesion and this is your actually intracellular anchor proteins. intracellular Intracellular means from one cell to another cell it is helping in the anchor anchoring anchoring process. So these cells are kind of called anchorage dependent cells and the examples of the anchorage dependent cells are fibroblast cell. Anchorage dependent cells means like essentially two cells will be attached to each other by the intracellular anchoring proteins as well as the transmembrane proteins as you can
29 see that this is like a forming a bridge, between the two cells and therefore, the two cells will be attached to each other. (Refer Slide Time: 44:02) Now, other a way that two cells can be attached to each other is the joining of the actin filaments from cell to cell by transmembrane adhesion proteins that is called Cadherins. Now this is cell 1 again part of the cell 1 and this is part of the cell 2 and you have this actin filaments here. Now, this actin filament and you have these anchor proteins also, this anchor proteins is the blue one and you have this cadherin proteins that is the green one. So, what happens that cell to cell junction is formed here which is established by these anchor proteins which are getting connected to the cadherin dimers. And you know that how protein complexes takes place, because protein has certain anchoring sites and through this sites the other proteins can be coming and can come together and two individual proteins can come together and form a protein complex. So similar protein to protein a junction is essentially develop between the cell 1 and cell 2.
30 (Refer Slide Time: 45:09) So, this is the mechanism to illustrate that how cell surface molecules they mediate, now this is there are three types of binding it has been shown that one is the homophilic binding. Homophilic means this is the two same type of cells and then from this one cell to other cell this similar type of protein molecules or cell adhesion molecules they come out and they make a bonding together the other is called heterophilic binding. Heterophilic means like this is again two different cell lines or it can be same cell lines and then this is the one type of protein molecules coming so if it is the protein molecule type x, from other protein molecule type y, they form and they form a bond here. The third one is the binding through an extracellular linker molecule like two surface proteins which are coming together, but they are not capable of binding to each other at site, this binding is essentially mediated by this positive this plus molecule and this plus essentially molecule from the extracellular matrix. So this binding here is mediated by the extracellular matrix molecule. So these are like homophilic binding, heterophilic binding and then whether this binding is actual when its extracellular linker molecule. The statement, that has been written here that mechanism 3 is less common in case of mammalian cells like whatever biological cells or mammalian cells that you are using in the laboratory or it is of relevance to the various biomedical applications, it is mostly the mechanism 1 or mechanism 2 which are mostly common or which are kind of hominine.
31 (Refer Slide Time: 46:49) Standards in biomaterials testing that ISO that is the materials characterization, now here you have to find out that what is the chemical, physical, electrical, morphological and mechanical properties of the materials that you are using. So, most of these things some of this things I have already mentioned that what is really mean, (Refer Slide Time: 47:10) now mechanical tests essentially means you can do the test as a tension, compression, bending depending on whether you have a brittle material like ceramic or whether you have a ductile material like metal. If it is a ductile material you can do that in tension
32 test, if it is a brittle material you can you have to do compression tests or bending tests. And accordingly you calculate what is elastic modulus? What is the string? What is ultimate denses? Etcetera. You can measure the fracture toughness, now this toughness can be measured from the simple tensile test from the area under the curve or this fracture toughness can be measured for the ceramic by single (( )) or put in the indentation measuring the crack length and so on and other things are equal to. Now, fatigue test is also important, but I thought metallic materials like you know you have the (( )) curve. so fatigue testing means when a material is under repeated stress cycle what is the maximum stress level, that the material can which stand before fracture so when you do simple tension tests, simple tension test means you have a material you are pulling in tension from two ends. Now, you are you are giving the uniform stress throughout the destiny, but fatigue testing means you are not giving uniform stress but, your stress cycles are going like sinusoidal or cosine or whatever typical repeated stress cycle or reverse stress cycle etcetera and then you were trying to assess that what is the property is of this material, under that repeated stress cycle and typically if your material is under uniform stress then the material will have higher load bearing capability compare to the material which is under the repeated stress cycle or reverse stress cycle. So, fatigue strength is much lower compare to your typical tensile strength of material. So that is the message that you should remember.
33 (Refer Slide Time: 48:56) Now, as I this has been mentioned early that these are the set of biocompatibility testing that one is to evaluate here. (Refer Slide Time: 49:04) Now, genotoxicity that is the next five slides I will teach and before I finish today, now genotoxicity tests are performed as tests in vitro to evaluate the risk of leachable substances form materials damaging D N A or causing mutations or chromosome damage. So, that is the core idea of the genotoxicity test and these are very important for pharmaceuticals as well as medical devices.
34 (Refer Slide Time: 49:28) Now, what is genotoxicity this is repeated here again that is the toxicity effect in the chromosome gene D N A and gene level and genotoxicity compares as either mutagenic or toxinogenic processes and up these tests essentially screen medical implants and materials for its toxic effects at nucleotides for the safe and long term implantation. So, what it means that is the long term level this material should not wear out and produce some wear particles which will cause directed DNA damage. So, that is what is essentially investigated here. (Refer Slide Time: 50:03)
35 Now, why genotoxicity to assess the effect of biomaterial eluates? To evaluate the size, dependent wear debris particles and third one analysis of the physical and chemical basis of nanobiocomposite. So size dependent wear debris particle effects as I mentioned to you earlier that finer the particles, more is the potential for the particles to cause genotoxic effect. So, if you are comparing the effect of 10 micron persists 10 nanometer. 10 nanometer particles definitely will have more genotoxic effect, compared to 10 micro meter particles. (Refer Slide Time: 50:39) Now this slide essentially tells you that you know the basically the concept of this genotoxicity effect, now you have this half of the cells here and in the half of the cells, this is the nucleus and nucleus contains this DNA. Now, DNA has a typical double helix type of patterns and this double helix patterns when it is exposed to biomaterial eluate. Eluate means you have this wear debris particles which is in solution like d a e m a and phosphate buffer solution and so on. So this entire solution is called eluates. Now, when this D NA is directly exposed to biomaterial eluates what will happen some particles may damage this hydrogen bonds which are essentially linking between two helix and if the hydrogen bond is broken that means you are essentially causing some damage sides on the D N A and what it will have what this will lead to this fragmented DNA, that means part of the DNA that will be immediately disperse in neighborhood of
36 the D N A. So, this is essentially cause by the debris particles and as I repeat finite the debris particles more is the chances of dies genotoxicity effect (Refer Slide Time: 51:49) Now, what is the motivation for genotoxicity evaluation number 1 is the aseptic loosening of implants and periprosthetic osteolysis. Aseptic loosening means when implants are put it inside the animal of and then after long time then implants can be loose by can be detached from the neighboring tissues or the bones. Periprosthetic osteolysis also is caused by the aseptic loosening third one is a apoptotic cell death at the implant site. So, that is also another thing which will happen
37 (Refer Slide Time: 52:17) now this individual particles you can see these particles are coated by DMM that is the dimeth (( )) medium (( )) medium and these DMM solution is statistically codes preferentially all the particles and if you see this micron bar here these particles is roughly around 100 to little bit higher than the 100 nanometer. So, essentially you can call it as ultra fine size particles are all most like nano sized particles. (Refer Slide Time: 52:46) Now when you put these materials
38 (Refer Slide Time: 52:49) in the solution and then there is the amount of these eluates and then see that what is the D N A damage of the L-929 cells now this is the case for the control material and this is the condition of L-929 cells Now you do not see any damage here, but if you increase this concentration of these eluates here now if you go to A and E for example, in both the cases what you notice here that this is the fragmented DNA region which shows these concentrations have the genotoxic effect and is the positive control, positive control means it is a reference sample which will definitely have the genotoxic effect and that is what has been illustrated here that it has a long tail. Now, if you take multiple images by the fluorescent microscopy and then what you can do you can measure the length of this tail and this tail length is first proposed by scientist called Olive and accordingly this is known as Olive Tail Movement, this is O T M.
39 (Refer Slide Time: 53:58) Now, if you measure this OTM for different conditions and put it in a graph, what you can see here, that if this particular concentration more than 25 percent, they have a statistically significant genotoxic effect. And the positive control has a much more genotoxic effect. So, this is the O T M that Olive Tail Movement for this particular biomaterial eluates. So, I think I will stop here, and in the next class I will start with the blood compatibility.
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Medical Device Testing. Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark
 Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
Medical Device Testing Andrew Makin, MSc, ERT, MRSB Scientific Director CiToxLAB Scantox, Denmark Medical device Risk evaluation Efficacy Biocompatibility Clinical testing Pharmaceutical product Preclinical
Cells Culture Techniques Marta Czernik
 Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Lecture - 03 To Grow the Cell Outside the Body
 Cell Culture Technologies Prof. Mainak Das Department of Biological Sciences & Bioengineering & Design Programme Indian Institute of Technology, Kanpur Lecture - 03 To Grow the Cell Outside the Body Welcome
Cell Culture Technologies Prof. Mainak Das Department of Biological Sciences & Bioengineering & Design Programme Indian Institute of Technology, Kanpur Lecture - 03 To Grow the Cell Outside the Body Welcome
Biocompatibility and War and Peace
 Professor David Williams, D.Sc.,F.R.Eng., Professor and Director of International Affairs, Wake Forest Institute of Regenerative Medicine, North Carolina, USA Editor-in-Chief, Biomaterials President-elect,
Professor David Williams, D.Sc.,F.R.Eng., Professor and Director of International Affairs, Wake Forest Institute of Regenerative Medicine, North Carolina, USA Editor-in-Chief, Biomaterials President-elect,
Cell and Tissue Culture
 Cell and Tissue Culture S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of IITs
Cell and Tissue Culture S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of IITs
Course Handbook MSc in Bioengineering Tissue Engineering Specialisation
 Course Handbook 2013-2014 MSc in Bioengineering Tissue Engineering Specialisation 1 Course Objectives & Learning Outcomes This programme aims to give a sound and broad basis in tissue engineering. In particular,
Course Handbook 2013-2014 MSc in Bioengineering Tissue Engineering Specialisation 1 Course Objectives & Learning Outcomes This programme aims to give a sound and broad basis in tissue engineering. In particular,
BNG 331 Cell-Tissue Material Interactions. Wound Healing I
 BNG 331 Cell-Tissue Material Interactions Wound Healing I Course update LBL 4 Friday; paper posted Need a volunteer to switch groups! Much better job on LBL 3 figure summaries BNG spring seminar 3 this
BNG 331 Cell-Tissue Material Interactions Wound Healing I Course update LBL 4 Friday; paper posted Need a volunteer to switch groups! Much better job on LBL 3 figure summaries BNG spring seminar 3 this
Biocompatibility: a risk based approach
 Biocompatibility: a risk based approach Legal framework The regulatory requirements include: Demonstration of safety Demonstration of efficacy Positive balance of risk and benefit The regulatory requirements
Biocompatibility: a risk based approach Legal framework The regulatory requirements include: Demonstration of safety Demonstration of efficacy Positive balance of risk and benefit The regulatory requirements
Concrete Technology Prof. B. Bhattacharjee Department of Civil Engineering Indian Institute of Technology, Delhi
 Concrete Technology Prof. B. Bhattacharjee Department of Civil Engineering Indian Institute of Technology, Delhi Lecture - 24 Strength of Concrete: Aggregate Contribution Welcome to module 6 lecture 2.
Concrete Technology Prof. B. Bhattacharjee Department of Civil Engineering Indian Institute of Technology, Delhi Lecture - 24 Strength of Concrete: Aggregate Contribution Welcome to module 6 lecture 2.
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
Ceramics, Glasses, and Glass-Ceramics
 Ceramics, Glasses, and Glass-Ceramics Ceramics, Glasses, and Glass-Ceramics include a broad range of inorganic/nonmetallic compositions. Eyeglasses Diagnostic instruments Thermometers Tissue culture flasks
Ceramics, Glasses, and Glass-Ceramics Ceramics, Glasses, and Glass-Ceramics include a broad range of inorganic/nonmetallic compositions. Eyeglasses Diagnostic instruments Thermometers Tissue culture flasks
Unit 5 Part B: Day 1 Cell Growth, Division & Reproduction
 Name Test Date Period Unit 5 Part B: Day 1 Cell Growth, Division & Reproduction CELL SIZE LIMITATIONS Cells that are will have diffusing materials through the cell. -,, and must enter cell at an efficient
Name Test Date Period Unit 5 Part B: Day 1 Cell Growth, Division & Reproduction CELL SIZE LIMITATIONS Cells that are will have diffusing materials through the cell. -,, and must enter cell at an efficient
Cell-Extracellular Matrix Interactions
 Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Endovascular Device Testing A Survey of Requirements for Product Validation
 MET A4 1p Endovascular Device Testing Info Sheet-02.qxp_MET A4 1p Endovascular Device Testing Info Sheet.qxp 05/04/2016 09:58 Page 1 Endovascular Device Testing A Survey of Requirements for Product Validation
MET A4 1p Endovascular Device Testing Info Sheet-02.qxp_MET A4 1p Endovascular Device Testing Info Sheet.qxp 05/04/2016 09:58 Page 1 Endovascular Device Testing A Survey of Requirements for Product Validation
For example: You are constantly loosing skin cells, in order to keep your skin healthy, your body needs to be constantly making new skin cells.
 Name: Cells and Reproduction A. The Cell Life Cycle The many cells in your body are constantly and. For example: You are constantly loosing skin cells, in order to keep your skin healthy, your body needs
Name: Cells and Reproduction A. The Cell Life Cycle The many cells in your body are constantly and. For example: You are constantly loosing skin cells, in order to keep your skin healthy, your body needs
CHAPTER 1. Survey of Clinical Cases of Biomaterials-Tissue Interactions: The Paradigm
 CHAPTER 1 Survey of Clinical Cases of Biomaterials-Tissue Interactions: The Paradigm 1.1 Introduction 1.2 Genotype/Phenotype (Fig. 1.1) 1.3 The Working Paradigm: The Unit Cell Process/The Control Volume
CHAPTER 1 Survey of Clinical Cases of Biomaterials-Tissue Interactions: The Paradigm 1.1 Introduction 1.2 Genotype/Phenotype (Fig. 1.1) 1.3 The Working Paradigm: The Unit Cell Process/The Control Volume
Biomaterials in Medical device design
 Biomaterials in Medical device design 1 Dr Joseph Buhagiar B. E n g ( H o n s. ) P h D ( B h a m ) D e p a r t m e n t o f M e t a l l u r g y a n d M a t e r i a l s E n g i n e e r i n g U n i v e r
Biomaterials in Medical device design 1 Dr Joseph Buhagiar B. E n g ( H o n s. ) P h D ( B h a m ) D e p a r t m e n t o f M e t a l l u r g y a n d M a t e r i a l s E n g i n e e r i n g U n i v e r
Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee
 Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 1 Engineering Materials and Processing Lecture -
Processing of Non-Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 1 Engineering Materials and Processing Lecture -
Required Biocompatibility Training and Toxicology Profiles for Evaluation of Medical Devices May 1, 1995 (G95-1)
 Required Biocompatibility Training and Toxicology Profiles for Evaluation of Medical Devices May 1, 1995 (G95-1) This guidance was written prior to the February 27, 1997 implementation of FDA s Good Guidance
Required Biocompatibility Training and Toxicology Profiles for Evaluation of Medical Devices May 1, 1995 (G95-1) This guidance was written prior to the February 27, 1997 implementation of FDA s Good Guidance
WHEATON DUAL. Vial. Perfecting the science of sample protection
 WHEATON Perfecting the science of sample protection WHEATON DualFusion vials combine the best properties of glass, with the mechanical strength and the precision molding of plastic. The vials are engineered
WHEATON Perfecting the science of sample protection WHEATON DualFusion vials combine the best properties of glass, with the mechanical strength and the precision molding of plastic. The vials are engineered
Joint Replacement Implants Hip Joint Prostheses
 Translated English of Chinese Standard: YY0118-2016 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.40 C 35 YY 0118-2016
Translated English of Chinese Standard: YY0118-2016 www.chinesestandard.net Sales@ChineseStandard.net PHARMACEUTICAL INDUSTRY STANDARD YY OF THE PEOPLE S REPUBLIC OF CHINA ICS 11.040.40 C 35 YY 0118-2016
THE EFFECTS OF POLYETHYLENE PARTICLE PHAGOCYTOSIS ON THE VIABILITY OF MATURE HUMAN MACROPHAGES BY PETER ASPINALL, KYRA BURNETT, JOE MAHER, CAT TJAN
 THE EFFECTS OF POLYETHYLENE PARTICLE PHAGOCYTOSIS ON THE VIABILITY OF MATURE HUMAN MACROPHAGES BY PETER ASPINALL, KYRA BURNETT, JOE MAHER, CAT TJAN THE AUTHORS S. Xing Institute of Biomaterials and Biomedical
THE EFFECTS OF POLYETHYLENE PARTICLE PHAGOCYTOSIS ON THE VIABILITY OF MATURE HUMAN MACROPHAGES BY PETER ASPINALL, KYRA BURNETT, JOE MAHER, CAT TJAN THE AUTHORS S. Xing Institute of Biomaterials and Biomedical
Testing and Evaluation Strategies for the Biological Evaluation of Medical Devices submitted for CE Mark and FDA Approval
 Testing and Evaluation Strategies for the Biological Evaluation of Medical Devices submitted for CE Mark and FDA Approval Today, millions of medical devices are used worldwide to treat or support patients.
Testing and Evaluation Strategies for the Biological Evaluation of Medical Devices submitted for CE Mark and FDA Approval Today, millions of medical devices are used worldwide to treat or support patients.
Growth factor delivery
 Growth factor delivery S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of IITs
Growth factor delivery S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of IITs
Colorants in Medical Devices:
 Colorants in Medical Devices: The Spectrum of Current Regulatory Expectations John Iannone Program Manager/ Technical Specialist Overview» Company Introduction» Why use Colorants in Devices?» Regulatory
Colorants in Medical Devices: The Spectrum of Current Regulatory Expectations John Iannone Program Manager/ Technical Specialist Overview» Company Introduction» Why use Colorants in Devices?» Regulatory
(Refer Slide Time: 04:02)
 Biomaterials for Bone Tissue Engineering Applications Professor Bikramjit Basu Materials Research Centre Indian Institute of Science Bangalore Lecture 1 Yeah! Welcome to the Popular Massive Open Online
Biomaterials for Bone Tissue Engineering Applications Professor Bikramjit Basu Materials Research Centre Indian Institute of Science Bangalore Lecture 1 Yeah! Welcome to the Popular Massive Open Online
ISO : A Risked Based Approach to Biological Safety
 ISO 10993 : A Risked Based Approach to Biological Safety Dr Arthur Brandwood Australian Delegation Leader to ISO TC 194 Biocompatibility and Clinical Trials Previous Director Devices Registration and Assessment
ISO 10993 : A Risked Based Approach to Biological Safety Dr Arthur Brandwood Australian Delegation Leader to ISO TC 194 Biocompatibility and Clinical Trials Previous Director Devices Registration and Assessment
Interview Questions. K: Marketing is not in the current plan. 6. Who will be using it? S: Undergrad students. 3rd-5 th years. Two professors.
 Interview Questions 1. What issues are we currently encountering with cell culturing? S: It is a brand new device. Want under flow instead of 2D plastic surface. So far, developed advanced cell culture
Interview Questions 1. What issues are we currently encountering with cell culturing? S: It is a brand new device. Want under flow instead of 2D plastic surface. So far, developed advanced cell culture
Risk Management of Biological Evaluation What s the Future for ISO 10993?
 Risk Management of Biological Evaluation What s the Future for ISO 10993? ISO 10993 High level guidance on how to conduct a biological evaluation Detailed test methods for investigation of different aspects
Risk Management of Biological Evaluation What s the Future for ISO 10993? ISO 10993 High level guidance on how to conduct a biological evaluation Detailed test methods for investigation of different aspects
Cell Growth and Reproduction
 Cell Growth and Reproduction Robert Hooke was the first person to describe cells, in the year 1665. He was looking through his microscope at a piece of cork when he noticed a lot of repeating honeycomb
Cell Growth and Reproduction Robert Hooke was the first person to describe cells, in the year 1665. He was looking through his microscope at a piece of cork when he noticed a lot of repeating honeycomb
Lecture 42 Phase Diagram of Ceramic
 Phase Diagrams in Material Science Engineering Prof. Krishanu Biswas Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Lecture 42 Phase Diagram of Ceramic So, students
Phase Diagrams in Material Science Engineering Prof. Krishanu Biswas Department of Materials Science and Engineering Indian Institute of Technology, Kanpur Lecture 42 Phase Diagram of Ceramic So, students
Frequently Asked Questions (FAQ)
 Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
Sélection Internationale Ens Ulm 2012, Cell Biology
 Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing
 Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing Authors: Correa LG, Peter R, Clerici G, Ritter V. 2017
Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing Authors: Correa LG, Peter R, Clerici G, Ritter V. 2017
Selection criteria for Biomaterials
 Selection criteria for Biomaterials Biomaterials and biomedical devices are used throughout the human body. 2 important aspects: Functional performance Biocompatibility Hip joint prosthesis 1 Functional
Selection criteria for Biomaterials Biomaterials and biomedical devices are used throughout the human body. 2 important aspects: Functional performance Biocompatibility Hip joint prosthesis 1 Functional
(approved during the CWA Kick-off meeting on )
 2017.06.15 Project Plan for the CEN Workshop on NOVEL METHODS FOR ISOLATING WEAR PARTICLES FROM JOINT REPLACEMENTS AND RELATED DEVICES AND FOR EVALUATING THEIR BIOLOGICAL IMPACT IN VITRO (CEN/WS 87) (approved
2017.06.15 Project Plan for the CEN Workshop on NOVEL METHODS FOR ISOLATING WEAR PARTICLES FROM JOINT REPLACEMENTS AND RELATED DEVICES AND FOR EVALUATING THEIR BIOLOGICAL IMPACT IN VITRO (CEN/WS 87) (approved
Ceramic Biomaterials. Lecture #17
 Ceramic Biomaterials Lecture #17 Ceramics Refractory (maintain shape and composition at high temperatures) Polycrystalline Nonductile virtually zero creep at room temperatures Usually inorganic Silicates
Ceramic Biomaterials Lecture #17 Ceramics Refractory (maintain shape and composition at high temperatures) Polycrystalline Nonductile virtually zero creep at room temperatures Usually inorganic Silicates
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
Biology of Cultured Cells
 Biology of Cultured Cells Transfection of cells (particularly human cells) with RAS or RAF genes Transformation transfection or DNA transfer Transformation of cultured cells implies a spontaneous or induced
Biology of Cultured Cells Transfection of cells (particularly human cells) with RAS or RAF genes Transformation transfection or DNA transfer Transformation of cultured cells implies a spontaneous or induced
Required Biocompatibility Training and Toxicology Profiles for Evaluation of Medical Devices
 Use of International Standard ISO-10993, 'Biological Evaluation of Medical Devices Part 1: Evaluation and Testing' (Replaces #G87-1 #8294) (blue book memo)(text Only) This guidance was written prior to
Use of International Standard ISO-10993, 'Biological Evaluation of Medical Devices Part 1: Evaluation and Testing' (Replaces #G87-1 #8294) (blue book memo)(text Only) This guidance was written prior to
The advantages and use of ceramics in medicine
 The advantages and use of ceramics in medicine G. Carl Friedrich-Schiller-Universität Jena Otto-Schott-Institut (now with JSJ Jodeit GmbH Jena) ORTHOSONIC CERAMIC CONFERENCE Saturday 6 th July 2002 bioceramics
The advantages and use of ceramics in medicine G. Carl Friedrich-Schiller-Universität Jena Otto-Schott-Institut (now with JSJ Jodeit GmbH Jena) ORTHOSONIC CERAMIC CONFERENCE Saturday 6 th July 2002 bioceramics
Lecture - 01 Introduction: Manufacturing and Joining
 Joining Technologies of Commercial Importance Dr. D. K. Dwivedi Department of Mechanical and Industrial Engineering Indian Institute of Technology Roorkee Lecture - 01 Introduction: Manufacturing and Joining
Joining Technologies of Commercial Importance Dr. D. K. Dwivedi Department of Mechanical and Industrial Engineering Indian Institute of Technology Roorkee Lecture - 01 Introduction: Manufacturing and Joining
MEDICAL WASTE MANAGEMENT STUDY NOTES
 MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
Chapter 21. Toxicity Testing
 Chapter 21 Toxicity Testing Toxicity Testing There are two purposes of toxicity testing. There is a quantitative effort to elucidate a dose effect relationship There is a qualitative determination of the
Chapter 21 Toxicity Testing Toxicity Testing There are two purposes of toxicity testing. There is a quantitative effort to elucidate a dose effect relationship There is a qualitative determination of the
Practice Exam A. Briefly describe how IL-25 treatment might be able to help this responder subgroup of liver cancer patients.
 Practice Exam 2007 1. A special JAK-STAT signaling system (JAK5-STAT5) was recently identified in which a gene called TS5 becomes selectively transcribed and expressed in the liver upon induction by a
Practice Exam 2007 1. A special JAK-STAT signaling system (JAK5-STAT5) was recently identified in which a gene called TS5 becomes selectively transcribed and expressed in the liver upon induction by a
CELLULAR PROCESSES; REPRODUCTION. Unit 5
 CELLULAR PROCESSES; REPRODUCTION Unit 5 Cell Cycle Chromosomes and their make up Crossover Cytokines Diploid (haploid diploid and karyotypes) Mitosis Meiosis What is Cancer? Somatic Cells THE CELL CYCLE
CELLULAR PROCESSES; REPRODUCTION Unit 5 Cell Cycle Chromosomes and their make up Crossover Cytokines Diploid (haploid diploid and karyotypes) Mitosis Meiosis What is Cancer? Somatic Cells THE CELL CYCLE
Get the most out of Pure PRP
 Get the most out of Pure PRP The Basic Science of PRP Platelets are small non-nucleated bodies in peripheral blood known for role in hemostasis Platelets contain proteins, cytokines & other bioactive factors
Get the most out of Pure PRP The Basic Science of PRP Platelets are small non-nucleated bodies in peripheral blood known for role in hemostasis Platelets contain proteins, cytokines & other bioactive factors
Introduction to Cells
 Introduction to Cells Key terms: Cell Microscopy Nucleus Organelle Micrograph Cytoplasm Define: Cell Microscopy Cell structure Today, all of biology is based on an understanding of what is going on within
Introduction to Cells Key terms: Cell Microscopy Nucleus Organelle Micrograph Cytoplasm Define: Cell Microscopy Cell structure Today, all of biology is based on an understanding of what is going on within
Science 10 Unit 1 GENETICS
 Science 10 Unit 1 GENETICS 1.1 DNA Structure and Function Part I- The Nucleus: Control Centre of the Cell Every cell in your body has a specific JOB- but how do they become specialized? E.g. hair cells
Science 10 Unit 1 GENETICS 1.1 DNA Structure and Function Part I- The Nucleus: Control Centre of the Cell Every cell in your body has a specific JOB- but how do they become specialized? E.g. hair cells
Biological evaluation of medical devices --
 Translated English of Chinese Standard: GB/T16886.1-2011 Translated by: www.chinesestandard.net Wayne Zheng et al. Email: Sales@ChineseStandard.net ICS 11.040.01 C 30 NATIONAL STANDARD OF THE PEOPLE S
Translated English of Chinese Standard: GB/T16886.1-2011 Translated by: www.chinesestandard.net Wayne Zheng et al. Email: Sales@ChineseStandard.net ICS 11.040.01 C 30 NATIONAL STANDARD OF THE PEOPLE S
General Education Learning Outcomes
 BOROUGH OF MANHATTAN COMMUNITY COLLEGE City University of New York Department of Science Title of Course: Cell Biology Class hours 3 BIO Section: 260 Lab hours 3 Semester Spring 2018 Credits 4 Schedule:
BOROUGH OF MANHATTAN COMMUNITY COLLEGE City University of New York Department of Science Title of Course: Cell Biology Class hours 3 BIO Section: 260 Lab hours 3 Semester Spring 2018 Credits 4 Schedule:
Directives 90/385/EEC and 93/42/EEC MEDICAL DEVICES. Guidance document for the presentation of biological evaluation
 Directives 90/385/EEC and 93/42/EEC MEDICAL DEVICES Guidance document for the presentation of biological evaluation According to ISO 10993 1 standard (current version) This guidance document is applicable
Directives 90/385/EEC and 93/42/EEC MEDICAL DEVICES Guidance document for the presentation of biological evaluation According to ISO 10993 1 standard (current version) This guidance document is applicable
Introduction to Biomaterials
 Introduction to Biomaterials Objective To introduce the different biomaterials used in Biomedical Engineering To provide some fundamentals properties of these materials, And to indicate how they are used.
Introduction to Biomaterials Objective To introduce the different biomaterials used in Biomedical Engineering To provide some fundamentals properties of these materials, And to indicate how they are used.
Processing of Non- Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee
 Processing of Non- Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 4 Plastics: Properties and Processing Lecture -
Processing of Non- Metals Prof. Dr. Inderdeep Singh Department of Mechanical and Industrial Engineering Indian Institute of Technology, Roorkee Module - 4 Plastics: Properties and Processing Lecture -
Introduction to Materials Science & Engineering
 Introduction to Materials Science & Engineering Course Objective... Introduce fundamental concepts in Materials Science You will learn about: material structure how structure dictates properties how processing
Introduction to Materials Science & Engineering Course Objective... Introduce fundamental concepts in Materials Science You will learn about: material structure how structure dictates properties how processing
3D Transfection System
 3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
ISO INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 10993-9 Second edition 2009-12-15 Biological evaluation of medical devices Part 9: Framework for identification and quantification of potential degradation products Évaluation
INTERNATIONAL STANDARD ISO 10993-9 Second edition 2009-12-15 Biological evaluation of medical devices Part 9: Framework for identification and quantification of potential degradation products Évaluation
Greg LeBlanc Dir. Regulatory Affairs and Quality Systems Cook (Canada) Inc.
 Greg LeBlanc Dir. Regulatory Affairs and Quality Systems Cook (Canada) Inc. ISO/TC 194 OVERVIEW Biological and clinical evaluation of medical devices Secretariat: DIN Secretary: Mr. Karl Wenzelewski Chairperson:
Greg LeBlanc Dir. Regulatory Affairs and Quality Systems Cook (Canada) Inc. ISO/TC 194 OVERVIEW Biological and clinical evaluation of medical devices Secretariat: DIN Secretary: Mr. Karl Wenzelewski Chairperson:
ICH CONSIDERATIONS Oncolytic Viruses
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
Applications in Cardiology Hollow Fiber Membranes and Applications
 Applications in Cardiology Want is meant by the term silicones?; Describe in general terms a typical synthetic scheme for a silicone consisting of half PDMS and half polysiloxane; Describe three cross-linking
Applications in Cardiology Want is meant by the term silicones?; Describe in general terms a typical synthetic scheme for a silicone consisting of half PDMS and half polysiloxane; Describe three cross-linking
B i o m a t e r i a l s E n g i n e e r i n g
 B i o m a t e r i a l s E n g i n e e r i n g Collagen, a key factor for clinical success INTRODUCTION A REVOLUTIONARY INNOVATION Tecnoss exclusive manufacturing process is able to neutralize the antigenic
B i o m a t e r i a l s E n g i n e e r i n g Collagen, a key factor for clinical success INTRODUCTION A REVOLUTIONARY INNOVATION Tecnoss exclusive manufacturing process is able to neutralize the antigenic
Introduction [1] Introduction to the course; CEO and CLO
![Introduction [1] Introduction to the course; CEO and CLO Introduction [1] Introduction to the course; CEO and CLO](/thumbs/85/92432151.jpg) MME 297: Lecture 01 Introduction [1] Introduction to the course; CEO and CLO Dr. A. K. M. Bazlur Rashid Professor, Department of MME BUET, Dhaka Topics to discuss today. What are Biomaterials? Classes
MME 297: Lecture 01 Introduction [1] Introduction to the course; CEO and CLO Dr. A. K. M. Bazlur Rashid Professor, Department of MME BUET, Dhaka Topics to discuss today. What are Biomaterials? Classes
Experimental techniques: The Skin; Biocompatibility. Dr. Gábor Erős
 Experimental techniques: The Skin; Biocompatibility Dr. Gábor Erős Structure of the skin Possibilities - Human trials - Animal experiments - In vitro examinations Cosmetic products and their ingredients
Experimental techniques: The Skin; Biocompatibility Dr. Gábor Erős Structure of the skin Possibilities - Human trials - Animal experiments - In vitro examinations Cosmetic products and their ingredients
Affinity. A Paradigm Shift in Skeletal Reconstruction
 Affinity A Paradigm Shift in Skeletal Reconstruction INTRODUCING TRS AFFINITY SKELETAL RECONSTRUCTION BREAKTHROUGH Tissue Regeneration Systems (TRS ) is a start-up medical device company commercializing
Affinity A Paradigm Shift in Skeletal Reconstruction INTRODUCING TRS AFFINITY SKELETAL RECONSTRUCTION BREAKTHROUGH Tissue Regeneration Systems (TRS ) is a start-up medical device company commercializing
Biomaterials The Intersection of Biology and Materials Science
 Biomaterials The Intersection of Biology and Materials Science J. S. Temenoff Wallace H. Coulter Department of Biomedical Engineering Georgia Tech and Emory University, Atlanta, GA A. G. Mikos Departments
Biomaterials The Intersection of Biology and Materials Science J. S. Temenoff Wallace H. Coulter Department of Biomedical Engineering Georgia Tech and Emory University, Atlanta, GA A. G. Mikos Departments
The role of thermal spray in medical applications
 The role of thermal spray in medical applications Rajan Bamola Traditionally, thermal spray in the medical industry has been linked to the spraying of either titanium or hydroxyapatite on dental, spinal
The role of thermal spray in medical applications Rajan Bamola Traditionally, thermal spray in the medical industry has been linked to the spraying of either titanium or hydroxyapatite on dental, spinal
Lecture Outline. History. Purpose? Func:on of Bioscaffolds. Extracellular Matrix (ECM) 12/08/15
 Associate Professor Rod Dilley Dr Rob Marano Ear Sciences Centre School of Surgery Harry Perkins Research Building 4 th Floor Lecture Outline History Purpose Functions Properties Approaches to bioscaffold
Associate Professor Rod Dilley Dr Rob Marano Ear Sciences Centre School of Surgery Harry Perkins Research Building 4 th Floor Lecture Outline History Purpose Functions Properties Approaches to bioscaffold
1/24/2012. Cell. Plasma Membrane
 Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division Cell Basic unit of structure and function in body
Chapter 3 Outline Plasma Membrane Cytoplasm and Its Organelles Cell and Gene Expression Protein Synthesis and Secretion DNA Synthesis and Cell Division Cell Basic unit of structure and function in body
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. 1. (6 points) Distinguish between the innate and adaptive immune responses:
 BENG 100b: Frontiers in Biomedical Engineering Midterm Examination 28 February 2006 There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. SHORT ANSWER (Total=70 points) Read the questions
BENG 100b: Frontiers in Biomedical Engineering Midterm Examination 28 February 2006 There are 100 possible points on this exam. THIS EXAM IS CLOSED BOOK. SHORT ANSWER (Total=70 points) Read the questions
COMMISSION DIRECTIVE 2009/120/EC
 15.9.2009 Official Journal of the European Union L 242/3 DIRECTIVES COMMISSION DIRECTIVE 2009/120/EC of 14 September 2009 amending Directive 2001/83/EC of the European Parliament and of the Council on
15.9.2009 Official Journal of the European Union L 242/3 DIRECTIVES COMMISSION DIRECTIVE 2009/120/EC of 14 September 2009 amending Directive 2001/83/EC of the European Parliament and of the Council on
Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras. Lecture - 02 Sterilization
 Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras Lecture - 02 Sterilization Welcome, to this second lecture on Bioreactors. This is a mooc on Bioreactors.
Bioreactors Prof G. K. Suraishkumar Department of Biotechnology Indian Institute of Technology, Madras Lecture - 02 Sterilization Welcome, to this second lecture on Bioreactors. This is a mooc on Bioreactors.
ALTERNATIVE BEARING SURFACES: THE GOOD, BAD & UGLY
 ALTERNATIVE BEARING SURFACES: THE GOOD, BAD & UGLY AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 69th Annual Meeting February 13-17, 2002 Dallas, Texas COMMITTEE ON BIOMEDICAL ENGINEERING COMMITTEE ON HIP AND
ALTERNATIVE BEARING SURFACES: THE GOOD, BAD & UGLY AMERICAN ACADEMY OF ORTHOPAEDIC SURGEONS 69th Annual Meeting February 13-17, 2002 Dallas, Texas COMMITTEE ON BIOMEDICAL ENGINEERING COMMITTEE ON HIP AND
Artificial blood vessels
 Artificial blood vessels S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of
Artificial blood vessels S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of
me239 mechanics of the cell me239 mechanics of the cell 3.1 biopolymers - motivation 3.2 biopolymers - polymerization
 4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
A NEW CHALLENGE : ASSESSMENT of Metallic Toxicity. Professeur F. DESCHAMPS Dept of Occupational Health (IMTECA) Medicine Faculty REIMS- FRANCE
 3 rd International Summit on Toxicology CHICAGO Oct 2014 TRACK 4 : Industrial and Metallic Toxicology A NEW CHALLENGE : ASSESSMENT of Metallic Toxicity Professeur F. DESCHAMPS Dept of Occupational Health
3 rd International Summit on Toxicology CHICAGO Oct 2014 TRACK 4 : Industrial and Metallic Toxicology A NEW CHALLENGE : ASSESSMENT of Metallic Toxicity Professeur F. DESCHAMPS Dept of Occupational Health
BEH.462/3.962J Molecular Principles of Biomaterials Spring 2003
 Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
Cell Division. embryo: an early stage of development in organisms
 Over the past several years, a debate has been brewing over the use of stem cells. Stem cells can be used to treat certain diseases and conditions such as spinal cord injuries, diabetes, arthritis, and
Over the past several years, a debate has been brewing over the use of stem cells. Stem cells can be used to treat certain diseases and conditions such as spinal cord injuries, diabetes, arthritis, and
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength
 The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
10:10-10:22. YIA-1 A study of newly established human peripheral blood monocyte-derived ips cell line used in allergy research 10:22-10:32
 10:10-10:22 YIA-1 A study of newly established human peripheral blood monocyte-derived ips cell line used in allergy research 10:22-10:32 EPA-1 Integration of conventional cell viability assays- recruiting
10:10-10:22 YIA-1 A study of newly established human peripheral blood monocyte-derived ips cell line used in allergy research 10:22-10:32 EPA-1 Integration of conventional cell viability assays- recruiting
Standards for Safety Assessments of Food Additives produced Using Genetically Modified Microorganisms
 Standards for Safety Assessments of Food Additives produced Using Genetically Modified Microorganisms (Food Safety Commission Decision of March 25, 2004) Chapter 1 General Provisions No. 1 Background on
Standards for Safety Assessments of Food Additives produced Using Genetically Modified Microorganisms (Food Safety Commission Decision of March 25, 2004) Chapter 1 General Provisions No. 1 Background on
Composite Materials. Metal matrix composites
 Composite Materials Metal matrix composites Introduction The properties that make MMCs attractive are high strength and stiffness, good wear resistance, high service temperature, tailorable coefficient
Composite Materials Metal matrix composites Introduction The properties that make MMCs attractive are high strength and stiffness, good wear resistance, high service temperature, tailorable coefficient
High Speed Devices and Circuits Prof K. N. Bhat Department of Electrical Engineering Indian Institute of Technology, Madras
 High Speed Devices and Circuits Prof K. N. Bhat Department of Electrical Engineering Indian Institute of Technology, Madras Lecture 4 Ternary Compound Semiconductors and their Applications Last time we
High Speed Devices and Circuits Prof K. N. Bhat Department of Electrical Engineering Indian Institute of Technology, Madras Lecture 4 Ternary Compound Semiconductors and their Applications Last time we
Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay
 Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay Lecture - 31 Standard Costing - Material, Labor and Overhead Variances Dear students,
Managerial Accounting Prof. Dr. Varadraj Bapat Department of School of Management Indian Institute of Technology, Bombay Lecture - 31 Standard Costing - Material, Labor and Overhead Variances Dear students,
Tissue Engineering: The art of growing body parts. Robby Bowles, Ph.D Cornell University
 Tissue Engineering: The art of growing body parts Robby Bowles, Ph.D Cornell University What is Tissue Engineering? What is Tissue Engineering? TE is an interdisciplinary field that applies the principles
Tissue Engineering: The art of growing body parts Robby Bowles, Ph.D Cornell University What is Tissue Engineering? What is Tissue Engineering? TE is an interdisciplinary field that applies the principles
GENE EXPRESSSION. Promoter sequence where RNA polymerase binds. Operator sequence that acts as a switch (yellow) OPERON
 GENE EXPRESSSION 1 GENE REGULATION IN PROKARYOTES Bacteria can turn genes on or off depending on their environment Prokaryotes have operons clusters of related genes and regulatory sequences Promoter sequence
GENE EXPRESSSION 1 GENE REGULATION IN PROKARYOTES Bacteria can turn genes on or off depending on their environment Prokaryotes have operons clusters of related genes and regulatory sequences Promoter sequence
MECHANICAL PROPERTIES
 MECHANICAL PROPERTIES Dynamic S.C. BAYNE, 1 J.Y. Thompson 2 1 University of Michigan School of Dentistry, Ann Arbor, MI 48109-1078 sbayne@umich.edu 2 Nova Southeastern College of Dental Medicine, Ft. Lauderdale,
MECHANICAL PROPERTIES Dynamic S.C. BAYNE, 1 J.Y. Thompson 2 1 University of Michigan School of Dentistry, Ann Arbor, MI 48109-1078 sbayne@umich.edu 2 Nova Southeastern College of Dental Medicine, Ft. Lauderdale,
Chemical Process Instrumentation Prof. Debasis Sarkar Department of Chemical Engineering Indian Institute of Technology, Kharagpur
 Chemical Process Instrumentation Prof. Debasis Sarkar Department of Chemical Engineering Indian Institute of Technology, Kharagpur Lecture - 01 General Principles and Representation of Instruments Hello.
Chemical Process Instrumentation Prof. Debasis Sarkar Department of Chemical Engineering Indian Institute of Technology, Kharagpur Lecture - 01 General Principles and Representation of Instruments Hello.
Tissue Culture Sterilization and Contamination
 Tissue Culture lab #4 Tissue Culture Sterilization and Contamination Nowf Aldouweghri Introduction Successful cell culture depends heavily on keeping the cells free from contamination by microorganisms.
Tissue Culture lab #4 Tissue Culture Sterilization and Contamination Nowf Aldouweghri Introduction Successful cell culture depends heavily on keeping the cells free from contamination by microorganisms.
Short Aramid Fibre Reinforced Ultra-High Molecular Weight Polyethylene Composite. A new hip-prosthesis Hofsté, Joanna Maria
 University of Groningen Short Aramid Fibre Reinforced Ultra-High Molecular Weight Polyethylene Composite. A new hip-prosthesis Hofsté, Joanna Maria IMPORTANT NOTE: You are advised to consult the publisher's
University of Groningen Short Aramid Fibre Reinforced Ultra-High Molecular Weight Polyethylene Composite. A new hip-prosthesis Hofsté, Joanna Maria IMPORTANT NOTE: You are advised to consult the publisher's
Basic&Laboratory& Materials&Science&and&Engineering& Biocompatible&Tests&of& Materials&
 & Basic&Laboratory& Materials&Science&and&Engineering& Biocompatible&Tests&of& Materials& M111& Stand: 08.10.2013 Aim: This lab serves as an introduction to testing the biocompatibility of materials by
& Basic&Laboratory& Materials&Science&and&Engineering& Biocompatible&Tests&of& Materials& M111& Stand: 08.10.2013 Aim: This lab serves as an introduction to testing the biocompatibility of materials by
Mission (Im)possible: Plasmid Mapping Student Materials
 Mission (Im)possible: Plasmid Mapping Student Materials Introduction... 2 Pre-Lab Questions... 6 Lab Protocol... 7 Data Collection Worksheet... 11 Post-Lab Questions and Analysis... 12 Last updated: August
Mission (Im)possible: Plasmid Mapping Student Materials Introduction... 2 Pre-Lab Questions... 6 Lab Protocol... 7 Data Collection Worksheet... 11 Post-Lab Questions and Analysis... 12 Last updated: August
Our Business. in vitro / in vivo. in vitro / in vivo ADME. in vitro / in vivo. Toxicology. Cell Battery V79. Analytical Services. Service.
 GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
Ceramics in Orthopaedic and Neurosurgery. B. Sonny Bal, MD MBA JD PhD University of Missouri-Columbia Amedica Corporation
 Ceramics in Orthopaedic and Neurosurgery B. Sonny Bal, MD MBA JD PhD University of Missouri-Columbia Amedica Corporation Historic Concern: Wear Bearing Failures of the Past The Rationale for Alternatives
Ceramics in Orthopaedic and Neurosurgery B. Sonny Bal, MD MBA JD PhD University of Missouri-Columbia Amedica Corporation Historic Concern: Wear Bearing Failures of the Past The Rationale for Alternatives
Hydrogels: There is always room for Jello
 Hydrogels: There is always room for Jello Soft-solids and the evolution of medical device design Gavin Braithwaite, 56 Roland Street, Suite 310 Boston, MA 02129 Cambridge Polymer Group, Inc. Testing, Consultation,
Hydrogels: There is always room for Jello Soft-solids and the evolution of medical device design Gavin Braithwaite, 56 Roland Street, Suite 310 Boston, MA 02129 Cambridge Polymer Group, Inc. Testing, Consultation,
Photograph showing the components of an artificial total hip replacement
 Case Study 4 (CS4) Artificial Total Hip Replacement Learning Objectives After studying this case study, you should be able to do the following: 1.List and briefly explain six biocompatibility considerations
Case Study 4 (CS4) Artificial Total Hip Replacement Learning Objectives After studying this case study, you should be able to do the following: 1.List and briefly explain six biocompatibility considerations
COATING TECHNOLOGIES REVIEW:
 COATING TECHNOLOGIES REVIEW: How Orthopedic Implants Get Coated April, 2018 By: Tony Crivella Senior Global Commercial Manager, Coatings Parimal Bapat, Ph. D Research Engineer Shilesh Jani Engineering
COATING TECHNOLOGIES REVIEW: How Orthopedic Implants Get Coated April, 2018 By: Tony Crivella Senior Global Commercial Manager, Coatings Parimal Bapat, Ph. D Research Engineer Shilesh Jani Engineering
Chapter 27A: Bacteria and Archaea. 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes
 Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic Structures 1 µm 1 µm 3 µm
Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic Structures 1 µm 1 µm 3 µm
1. Extracellular Prokaryotic Structures
 1 µm 1 µm 3 µm 2/11/2015 Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic
1 µm 1 µm 3 µm 2/11/2015 Chapter 27A: Bacteria and Archaea 1. Extracellular Prokaryotic Structures 2. Intracellular Prokaryotic Structures 3. Genetic Diversity Prokaryotes 1. Extracellular Prokaryotic
