Real-time Evaluation of Research and Development (R&D) Response to Zika. Second Review Mission: 28 th to 30 th June 2016
|
|
|
- Myron Atkinson
- 6 years ago
- Views:
Transcription
1 Introduction Real-time Evaluation of Research and Development (R&D) Response to Zika Second Review Mission: 28 th to 30 th June 2016 Background details to this mission are Acronyms found in the concept note related to ANVISA National Health Surveillance Authority (Brazil) real-time evaluation of the R&D ELISA Enzyme Linked Immunosorbent Assay EUAL Emergency Use Assessment and Listing response to Zika. A preliminary scoping FDA Food and Drug Administration mission was conducted in Geneva from Fiocruz Fundação Oswaldo Cruz GLOPID-R Global Research Collaboration for Infectious Disease Preparedness April This second mission IVM Integrated Vector Management sought to collect evidence on progress MPTF Multi-Partner Trust Fund NAT Nucleic Acid Testing towards agreed milestones and NTD Department of Control of Neglected Tropical Diseases indicators and also to answer a number OHE Outbreaks and Health Emergencies PAHO Pan-American Health Organization of generic and specific questions. Details PCR Polymerase Chain Reaction R&D Research and Development of these are presented in Annex 1 (p11). RDT Rapid Diagnostic Test During the mission, a number of TDR Special Programme for Research and Training in Tropical Diseases TPP Target Product Profile documents were reviewed (see Annex 2, UN United Nations p12) and interviews were held with a UNDP United Nations Development Programme UNFPA United Nations Population Fund number of key informants (see Annex 3, UNICEF United Nations Children s Fund p14). This report is organised as follows. US Unites States VCAG Vector Control Advisory Group First, it identifies and highlights a VLP Virus-like Particles WHO World Health Organization number of issues relating to the R&D response to Zika, in general, before considering more detailed issues relating to scientific evidence and progress on R&D related to diagnostics, vaccines, vector control and treatment. It ends with suggested conclusions and recommendations. The extent to which progress towards specific milestones has been assessed and particular questions answered is covered in Annex 4 (p15). General issues Role and scope of the Blueprint In the work done for planning for the Blueprint to identify and prioritise pathogens that were likely to cause severe outbreaks in the near future and for which few or no medical countermeasures exist, Zika was identified as a serious threat requiring action by WHO to promote R&D as soon as possible. The prioritisation meeting also noted that if the link to microcephaly was confirmed, this would mean Zika would need to be moved to an even higher priority. In addition, work on regulatory environments conducted under work-stream 2 of the Blueprint has proved relevant and useful for the R&D response to Zika. Similarly, work carried out under the Blueprint s work-stream 4 meant that this real-time evaluation was able to be rapidly established. One external respondent commented that the Blueprint has resulted in R&D being brought to the forefront much more quickly than previously. They noted that had the Blueprint been in place prior to the Ebola outbreak, less time might have been spent in pursuing conventional but ineffective approaches. One recurring issue is the scope of the Blueprint. While it is agreed that the focus is on research and development, there does not seem to be consensus as to precisely what this means and this may need to be clarified. 1
2 WHO has also been developing separately an overall research agenda for Zika. The research agenda has three main pillars characterisation; prevention and control; and women, communities and health systems (see Figure 1). Figure 1: WHO Zika Virus Research Agenda Implementation Framework (from Zika research agenda) Clearly, elements of product research and development that are included in the Blueprint are reflected in the Zika research agenda, particularly the second pillar focused on prevention and control. Elements relating to research and development of diagnostic tests (including landscape analysis, target product profiles and emergency assessment procedures) are included under the first pillar focused on characterisation. Implementing the full research agenda would be expected to cost around $12m over two years. Work already conducted under the research agenda includes harmonisation of research protocols. Challenges identified include: Ensuring someone is responsible within the emergency response for developing a research agenda for the specific pathogen causing the outbreak. Quickly identifying people with the appropriate research skills and experience to conduct necessary research identified in the research agenda. Building up (and establishing framework contracts with) a network of outbreak investigators could be helpful in this regard. Coordination In general, respondents were positive about coordination of the R&D response to Zika drawing, in particular, positive comparisons to what had been the case for the R&D response to Ebola. One respondent highlighted that there was better coordination across WHO, including headquarters and 2
3 regional/country offices. There had been strong leadership from the management team centrally and from PAHO. One external respondent commented that WHO had used their convening authority well and pointed to the meeting that was held in March This meeting brought together more than 130 experts from 30 countries to focus on issues relating to R&D of vaccines, treatment and vector control for Zika. One external respondent commented that this meeting went very well and brought people together to seek consensus. They noted that it would have been good to be clearer as to how WHO would communicate and coordinate after the meeting. Concerning coordination within WHO among those working on ZIka R&D, there have been regular meetings of the Blueprint team in relation to Zika with the next meeting scheduled for 7 th July. These meetings bring together those working on the R&D of diagnostics, vaccines, treatment and vector control. In addition, there has been coordination between those working on Zika R&D and those working on the Zika response more broadly. These two elements are managed by different departments within WHO. The coordination arrangements are reported to be the same as they were at the beginning of the response to Ebola, i.e. R&D is considered part of the overall Zika response. In terms of WHO communications with other actors and partners, external respondents appreciated the regular donor calls but commented that these could be documented more systematically. In general, WHO could communicate more clearly and regularly about what it is doing in relation to Zika R&D. For example, some of the external respondents reported that they had not seen the WHO Zika research agenda. A regular update with clickable links to access more detailed information might be the best way of sharing such information. One respondent commented that meetings convened by WHO had been a good way of sharing up-to-date information. Another commented that it was important for WHO and its partners to understand each other s structures and communication channels to ensure that communications are both effective and efficient. Respondents did highlight a number of issues related to coordination: - There are some areas where efforts appear to be duplicated, e.g. different companies developing similar vaccines. - Substantial efforts have been made to coordinate work on diagnostic R&D between UNICEF and WHO, including at a meeting organised by UNICEF in Copenhagen. - External respondents expressed some uncertainty about potential overlaps and duplications between the work of the WHO Blueprint and the Global Research Collaboration for Infectious Disease Preparedness (GLOPID-R) and the desire that these two bodies work more closely together. This may involve delineating more clearly the boundaries between the two bodies, i.e. who does what, to avoid unnecessary duplications and overlaps. Landscape analyses WHO conducted an initial landscape analysis in March 2016 related to Zika diagnostics, vaccines, vector control and treatment (see Box 1). Since that time, these analyses have been kept regularly updated. 3
4 Box 1: More detail of initial landscape analysis conducted by WHO on Zika diagnostics, vaccines, vector control and therapeutics 1 For diagnostics, WHO has mapped what diagnostics are being developed by whom. This includes nucleic acid test kits (for both Zika alone and in combination with other viruses); integrated nucleic acid testing (NAT); immunoassays (enzyme-linked immunosorbent assays [ELISA]); and rapid diagnostic tests (RDTs). This mapping includes those products submitted to WHO for EUAL but it also includes others. The landscape analysis notes that information may be incomplete because companies may choose not to disclose the current state of their development. WHO updates this information on a weekly basis with a focus on those products submitted for EUAL. For vaccines, WHO conducted a landscape analysis based on feedback from 25 developers related to at least 15 active programmes. A range of different technologies were identified including inactivated purified virus; live vectored; live attenuated/recombinant; subunit/peptides; virus-like particles (VLP); and nucleic acid based vaccines. The landscape analysis considered potential advantages and disadvantages of the different technologies. All programmes were at an early preclinical stage with phase 1 clinical trials expected towards the end of Following the landscape analysis, WHO began a consultative process to develop a target product profile (TPP) for a Zika vaccine. For vector control, the landscape analysis focused on identifying new tools including mechanical, chemical, biological and genetic approaches. It also examined how vector control methods could be made more efficient, effective and ecologically sound and referred to the work of the Vector Control Advisory Group. For therapeutics, given the lack of understanding of Zika virus infection, clinical progression and pathogenesis, the role for therapeutic products is uncertain. Nevertheless, the landscape analysis did include a product pipeline for a number of therapeutic agents including Amodiaquine, Chloroquine, Ribavirin, Interferon A, BCX4430, GS-5734, NITD008 and monoclonal antibodies. 1 Sources include Current Zika Product Pipeline produced March 2016 Comparisons to Ebola In general, WHO and others have learned many lessons from their experiences of responding to Ebola which have proved extremely useful in responding more effectively to Zika and other outbreaks that may occur. For example, lessons learned through Ebola concerning WHO s programme of prequalifying products led to the introduction of processes for EUAL which are being used in the response to Zika. While lessons have been learned for R&D from the response to Ebola that are relevant to the response to Zika, there are significant differences between the two diseases and their outbreaks which mean that the amount of cross-learning may be limited. For example, there were candidate vaccines available when the Ebola outbreak occurred. In addition, case mortality rate was very high in Ebola meaning there was a major focus on treatment. In terms of diagnostics, there were fewer problems of cross-reactivity because although there is potentially some cross-reactivity with other viruses, e.g. Marburg, this proved not to be a major issue in practice. Conversely, in relation to Zika, there was no candidate vaccine or treatment at the time of the outbreak. Zika also differed from Ebola in terms of being a relatively minor disease in most people but with potentially severe effects in fetuses. Consequently, potentially recommending vaccination for pregnant women has implications in terms of the risks and ethics of using a relatively untested vaccine in that group. In terms of Zika transmission, vector control has proven to date to be relatively ineffective. In addition, there are real practical problems for Zika diagnostics because of cross-reactivity with other viruses. In addition, the Zika virus is present in a person s blood for a relatively short period of time. 4
5 Funding and resources A key concern raised by respondents was that, to date, it has proved difficult to raise funding and resources needed for the response to Zika. The strategic response plan identifies that at least $122m is required by WHO and its partners from July 2016 to December However, the plan also notes that these figures are not exhaustive as some partners have not yet developed and finalised their plans. The budget is sub-divided into five objectives (see Figure 2). Although the plan is said to represent the work of more than 60 partners, almost all the budget (89%) is focused on the work of six UN agencies (see Figure 3). Of the total budget, around one third (33%, US$40m) is specified for research. Of this, almost all (95%) is allocated to either UNICEF (US$25.4m) or WHO ($12.3m). While it is unclear how much of the required budget is available, the plan is clear that, of the $25m that WHO and PAHO considered was needed for the first six months of 2016, only just over $4m (16%) was received. It is also reported that only $0.5m was made available for Zika R&D. Figure 2: Breakdown of Zika Strategic Response Plan Budget (US$122m) by Objective 33% 9% 7% 22% 29% Detection Prevention Care and Support Research Coordination Figure 3: Breakdown of Zika Strategic Response Plan Budget (US$122m) by Partner Partner Amount ($m) UNICEF 48.3 WHO 24.5 PAHO 15.1 UNFPA 10.0 UN Women 5.6 UNDP 5.2 Subtotal Save the Children 6.3 Other 7.2 Total In order to address this lack of funding, the UN established a Zika Multi-Partner Trust Fund (MPTF) in May The aim of this fund is to rapidly resource the UN system responses to Zika based on the needs and requirements of the Zika Response Framework. Although this framework is said to have three strategic objectives, i.e. surveillance, response and research, it is reported that the priorities of the MPTF needed to be people-centred so they focus on prevention, risk communication and care and support with no language specifically related to research. 1 See 5
6 Role of WHO One issue which continues to be discussed and debated is the role of WHO in effective R&D responses to Zika (and other pathogens). WHO is considered to have a comparative advantage in terms of coordinating and convening others and collating research done by others rather than in conducting research directly. One external respondent commented that it was not always clear from their documents what WHO is doing itself and what partners are doing. WHO structure, rules, procedures and culture WHO is currently reforming how it responds to emergencies 2. A new Executive Director has been appointed and is due to take up the post at the end of July. The Blueprint s manager has been representing R&D issues in that process. Emergence of other threats The response to Zika is not occurring in isolation. Some respondents noted that work related to Ebola is still ongoing and there is always a risk of other outbreaks occurring. Respondents expressed concern about the growing number of cases of Yellow Fever in Angola and the Democratic Republic of Congo. WHO is convening a research meeting at the end of September 2016 to seek to address the many things that are unknown about Yellow Fever. R&D issues in more detail Scientific knowledge There has been interest in why Zika appears to be associated with a higher rate of neonatal microcephaly in North East Brazil than elsewhere. Diagnostics Substantial work has been done in relation to R&D of Zika diagnostics including landscape analysis, work on target product profiles (TPPs) and work on Emergency Use Assessment and Listing (EUAL) procedures. EUAL procedures were introduced during the Ebola outbreak and were opened for Zika in February WHO is currently processing 20 EUAL applications relating to Zika diagnostics (see Box 2). The initial timeline for receiving applications has been extended and these can still be received. Box 2: Update on EUAL processes for Zika diagnostics (based on weekly update 1 st July 2016) Of the 20 diagnostic products submitted for EUAL, six had completed assessment of the manufacturer s quality management system. In five cases, additional information had been requested and in a further eight the process was ongoing. In two cases, the documentary evidence review had been completed. In five cases, additional information had been requested and in seven cases the process was ongoing. Three performance evaluations had been scheduled with a further seven in the planning phase. The EUAL process allows products to be assessed by WHO more quickly than the usual prequalification process which takes around 270 working days. The EUAL process has three main components (see also Box 2): - Assessment of a product dossier, i.e. documentary evidence. Manufacturers submit evidence and this is reviewed by technical experts using a standard checklist. 2 See 6
7 - Assessment of quality management system this component involves assessing the manufacturer. Unlike the standard prequalification process, the EUAL process does not require an inspection but relies on documentary evidence. - Review of product performance this requires independent assessment of the product in practice. This step is particularly important as manufacturers may not have full studies. In March 2016, WHO organised a consultative meeting which brought together experts to agree the level of documentary evidence needed in the first step and the study protocols to test product performance. One key challenge in relation to reviewing product performance of diagnostics is access to relevant clinical samples to build up a panel. It is particularly important to test diagnostics in Latin America because samples tested in other contexts, e.g. in Europe, might exhibit less cross reactivity because of other infections. It is reported that Brazil has passed a law which prevents samples being sent out of the country. This has made accessing and sharing samples more difficult. It means that product performance testing has to be done in country and WHO has been seeking to work with a laboratory in French Guiana and two laboratories operated by the Fundação Oswaldo Cruz (Fiocruz) in Brazil (in Recife and Rio). However, this is not straightforward because these laboratories, particularly the one in Recife, are overloaded and are targeted by others, e.g. manufacturers, researchers and national governments. It would be helpful if there were a network of laboratory centres which WHO and others could work with to gain access to specimens for product performance testing. Similar networks have been identified for specific diseases, e.g. influenza and dengue but it would be helpful to have such a network of laboratories that could be called on in different disease outbreaks. Such a network would allow WHO and others to identify laboratories to work with and could also allow sharing of specimens. Laboratories that are part of such a network would potentially benefit from increased visibility and recognition. Having such a network might also mean that requests, e.g. from companies, researchers and WHO, could be more coordinated. However, this might also involve more cooperation and coordination among those seeking to work with laboratories. It would also be helpful if WHO were able to support these laboratories with surge capacity, i.e. additional staff. It is important that these staff have relevant experience and language skills. WHO has been working closely with Brazil s National Health Surveillance Agency (ANVISA) who are reported to be very open on these matters. It would be ideal if sample banks could be established and shared with those who need access to them. However, this has proved to be very difficult to agree because of issues relating to ownership and sharing benefits and risks. WHO might consider establishing a unit to promote biobanking. Such a unit would need to address issues of ownership and benefits. They might also need to promote a mindset in which drug manufacturers are seen as suitable partners and not the enemy. Another key challenge is that while a number of steps have been speeded up under EUAL processes when compared with standard pre-qualification processes, there are a number of other elements, on which EUAL depends, which are reported to take as long as in non-emergency settings. These include ethical clearance and developing reference material. Vaccines WHO has developed a target product profile for a Zika vaccine and this has been posted and comments received. A first meeting of regulators has been held but there are many questions about 7
8 this vaccine including what the end point is, that is based on clinical findings or laboratory tests. There is need for a further meeting to resolve these questions as human clinical trials are due to start. There are also issues because the first vaccines tested are likely to be DNA vaccines and although these have been shown to be effective in animals they have not yet been shown to be effective in humans. There are also issues about: Whether the vaccine can be given to pregnant women. The extent to which there is a commercial market for Zika vaccine. The extent to which vaccination with Zika might cause neurological problems and what the risks might be. There are also issues about how to assess these safety risks. Whether vaccination should be offered to men. External respondents were extremely positive about the meeting which had been held to discuss the vaccine TPP. The meeting was relatively small and was well-organised. Those who were there had something to contribute and the meeting was quite representative. This was considered to be much better than during Ebola when meetings were very large and there were people in the meetings who had no experience or little, if anything, invested in the response. During Ebola, there were concerns that some meetings organised by WHO had pre-determined outcomes but this was not so in this case. There was vigorous discussion on some topics, e.g. whether Zika vaccine should be available to pregnant women but the conversation was considered helpful and productive. Vector control Coordinating R&D on vector control in relation to Zika has involved liaising closely with two other groups with expertise in the more usual and established ways of vector control, including spraying and bed nets. These groups are the Department of Control of Neglected Tropical Diseases (NTD) and the Special Programme for Research and Training in Tropical Diseases (TDR). However, a number of new tools for vector control were identified during a landscape analysis of the research and development pipeline conducted under the WHO R&D Blueprint response to Zika. The subsequent WHO consultation in March 2016 on Zika R&D concluded that extreme rigour needs to be applied in evaluating novel tools, such as Wolbachia, recombinant and irradiated mosquitoes. The following week an emergency meeting of WHO s Vector Control Advisory Group (VCAG), jointly established by the Global Malaria Programme and NTD in 2013, recommended the carefully planned pilot deployment under operational conditions of two tools (Wolbachia-based biocontrol and OX513A transgenic mosquitoes) accompanied by rigorous independent monitoring and evaluation. The VCAG has commissioned a set of guidance on the evidence base needed to underpin vector control field trails. The guidance, which will cover both entomological and epidemiological end-points, is being developed by external experts. A preliminary report is expected to be available in September As part of the R&D Blueprint, a meeting will be organized in November, in close collaboration with NTD, to identify operational considerations when applying VCAG/WHO guidance in emergency settings The Zika strategic response framework emphasises that prevention should not focus only on vector control but on a process of integrated vector management (IVM) which also protects pregnant 8
9 women and women of reproductive age from infection and unintended pregnancy. Elements of IVM include advocacy, risk communication for behaviour change and community engagement and legislation; collaboration with the health sector and with other sectors; integrated approach to disease control; evidence-based decision-making; and capacity building. One external respondent expressed concern that WHO s messaging on this is confusing. On the one hand, strategic documents emphasise the need to move beyond conventional approaches to vector control while many programmes continue to emphasise these and work in this way. Clearer, evidence-based guidance is needed Treatment As noted above, there has not, as yet, been much focus on R&D of Zika treatment given that the illness is mild and self-limiting in most cases and there has been more focus on diagnostics, vaccines and vector control. Conclusions and recommendations This report concludes with a number of conclusions and recommendations for further discussion and potential action: 1. In relation to Zika, it would be useful to clarify the link between the research and development Blueprint and the broader research agenda. In particular, it would be helpful to have clear agreement on what falls within the scope of the R&D Blueprint and what falls beyond its scope. 2. It would be helpful if WHO could establish a network of outbreak investigators that could be available to provide research capacity in the face of emergencies. One option for doing this might be to establish a number of framework contracts with institutions with research capacity, e.g. universities and other academic institutions, that could be called on as needed. 3. There is need for someone within the OHE cluster to be responsible for developing a research agenda in an emergency situation. 4. It would be helpful if WHO were able to improve its communications about Zika R&D particularly with key external stakeholders. In the short-term, this should include sharing the Zika research agenda with them. In the medium-term, this should involve a regular update focused on issues of concern and interest to them. 5. It would also be helpful if WHO could clarify and explain how it is working and coordinating with other key partners working in this field, e.g. UNICEF and GLOPID-R. 6. There is need to recognise that while lessons can be learned from one disease outbreak that can inform responses to future outbreaks of different diseases, diseases and outbreaks differ from each other in many ways so may limit the transferability of lessons learned, e.g. from Ebola to Zika. 7. Given the importance of research (including R&D) to effective responses to Zika, it is crucial that efforts to raise resources for the response to Zika include resources for research. This is particularly important as the MPTF is established. 9
10 8. It is always important in documents to be clear as to what WHO s role is in the response to Zika. This will involve being as clear as possible as to what is done by WHO and what by partners. 9. A key priority for the new OHE Director should be to establish and communicate the cluster s structure. This should include articulating whether research (including R&D) fits within the OHE cluster and if so how. 10. It would be helpful if WHO could establish a network of laboratories that could be available to respond to disease outbreaks when they occur. 11. WHO might consider establishing a unit to promote biobanking of laboratory samples. 12. There are a number of areas where streamlined procedures might be needed in emergencies akin to the EUAL process in place of standard prequalification processes. These include ethical clearance, preparation of reference materials and WHO human resource procedures. 13. It would be helpful if WHO could review where additional staff might be needed to allow R&D for Zika and other emergencies to take place effectively. This could include dedicated staff for EUAL processes, for example. 14. It would be helpful for WHO and partners to have further discussions relating to Zika vaccine development including the appropriateness or otherwise of seeking to vaccinate pregnant women. 15. It would be helpful if WHO could produce clear and consistent guidance as to the role of vector control in relation to prevention of Zika and other related diseases. It may be particularly important to align policy statements and programme practices. 10
11 Annex 1: Proposed Zika review mission Introduction Background details to this mission are found in the concept note related to real-time evaluation of the R&D response to Zika. A preliminary scoping mission was conducted in Geneva from April. Based on this, it is proposed that a further mission be conducted in early June. It is proposed that the dates for face-to-face meetings in Geneva would be either the week of 30 th May or 6 th June. Remote interviews could be conducted during that week or prior to the trip to Geneva. Topics and questions It is proposed that evidence will be gathered concerning progress towards certain milestones and indicators, namely Global consultation on R&D in relation to Zika Landscape analysis table(s) completed. Number of conference calls held. Number of target product profiles developed. Products listed through Emergency Use Assessment and Listing (EUAL). It is also proposed that certain questions will be explored during each mission, namely: To what extent is progress being made on (1) diagnostics; (2) vector control; (3) vaccine development; and (possibly) (4) therapeutics? To what extent are supportive research activities being coordinated including the establishment and validation of appropriate animal models and sharing of information? To what extent have the conference calls held been valuable in terms of improving coordination? Has the regulatory support group been established? To what extent is this functioning well? It is also proposed that certain specific questions will be addressed during this particular mission, namely: What happened once Zika was identified as a priority pathogen and the cluster of microcephaly cases in Brazil was recognized as an emergency? In particular, was an operational plan developed and implemented? Were the appropriate stakeholders identified and informed? If so, what were the strengths and challenges of this process? If not, why not? Can any lessons be learned, e.g. for the development of a decision tree for new pathogens, from experiences of Zika, i.e. as a known pathogen developing new associations with diseases Stakeholders to consult Blueprint workstream leads PAHO Country offices Brazil, Colombia, Mexico Manica Balasegaram, MSF, Strategic Advisory Group Lucille Blumberg, National Institute for Communicable Diseases, South Africa Chris Lewis DFID 11
12 Annex 2: Documents Reviewed General for the Blueprint WHO (2016) An R&D Blueprint for Action to Prevent Epidemics: Monitoring and Evaluation Framework: Detailed Plans May 2016 WHO (2016) Questions for Team Leaders WHO (2016) Blueprint Project Management Plan WHO (2015/6) Notes of Blueprint leads meetings WHO (2015/6) Notes from SAG meetings WHO (2015) Notes of Blueprint Meeting WHO (2015) Short Concept Note on the Blueprint WHO (2015) Blueprint Proposal Other similar initiatives WHO (2016) Global Action Plan for Influenza Vaccines see WHO (2016) Pandemic Influenza Preparation Framework see Issues relating to Global Research Collaboration for Infectious Disease Preparedness (GLOPID-R) GLOPID-R (2016) Website see GLOPID-R (2016) Mind the Gap: Coordinating Funding in Public Health Emergencies letter to Lancet and details of authors Kieny, M-P, Rottingen, J-A. and Farrar, J. (2016) The Need for Global R&D Coordination for Infectious Diseases with Epidemic Potential letter to Lancet WHO and emergencies WHO (2016) Website see Specific to Zika UNDP (2016) UN Zika Response Multi Partner Trust Fund see WHO (2016) Zika: Strategic Response Framework and Joint Operations Plan WHO (2016) Zika: Organisational Structure WHO (2016) Zika: Research Agenda Matrix WHO (2016) Zika Virus Research Agenda WHO (2016) Zika: Finances WHO (2016) WHO Global Consultation on Research Related to Zika Virus Infection: List of Participants WHO (2016) Emergency Use Assessment and Listing (EUAL) Weekly Update: Update on Submission of Applications to the WHO EUAL for Zika Virus IVDs (in vitro diagnostics) and another table with details WHO (2016) Zika Vaccines see WHO (2016) DNA Vaccines see WHO (2016) Landscape Analysis on Zikavirus Vaccine Development Preliminary Findings WHO (2016) Current Zika Product Pipeline 12
13 Lessons from Ebola High Level Panel on the Global Response to Health Crises (2016) Protecting Humanity from Future Health Crises Harvard-LSHTM Independent Panel on the Global Response to Ebola (2015) Will Ebola Change the Game? Ten Essential Reforms before the Next Pandemic Lancet article Issues relating to Yellow Fever PATH (2013) Yellow Fever Vaccination: The Potential of Dose-Sparing to Increase Vaccine Supply and Availability 13
14 Annex 3: People Interviewed WHO Virginia Benassi Nathalie Broutet Henry Dowlen Theo Grace Marie-Paule Kieny Robyn Meurant Bernadette Murgue Claudia Nannei Irena Prat David Wood External Lucille Blumberg, National Institute for Communicable Diseases South Africa Luciana Borio, Food and Drug Administration (FDA) Chris Lewis, Department for International Development (DFID) Hilary Marston, National Institutes of Health (NIH) 14
15 Annex 4: To what extent have milestones been achieved and questions answered? Milestone/question Comment Status Global consultation on R&D in relation to Zika Conducted in March 2016 Done Landscape analysis table(s) completed. These were done for diagnostics, vaccines, vector control and therapeutics. They continue to be updated, e.g. the diagnostics EUAL is updated Done weekly. Number of conference calls held There are a number of consultative calls and meetings being held. In general, it is better to assess the value and merit of these rather than the number of calls. Number of target product profiles developed Again, the absolute number of these may not be important but that they are being developed in areas where they are needed, e.g. diagnostics and vaccines. Products listed through EUAL diagnostics are going through the EUAL process diagnostics details of progress are in Box 2. in process To what extent is progress being made on (1) diagnostics; (2) vector control; (3) vaccine development; and (possibly) (4) therapeutics? To what extent are supportive research activities being coordinated including the establishment and validation of appropriate animal models and sharing of information? To what extent have the conference calls held been valuable in terms of improving coordination? Has the regulatory support group been established? To what extent is this functioning well? What happened once Zika was identified as a priority pathogen and the cluster of microcephaly cases in Brazil was recognized as an emergency? In particular, was an operational plan developed and implemented? Were the appropriate stakeholders identified and informed? If so, what were the strengths and challenges of this process? If not, why not? Can any lessons be learned, e.g. for the development of a decision tree for new pathogens, from experiences of Zika, i.e. as a known pathogen developing new associations with diseases Excellent progress on diagnostics and some progress on vaccines and vector control. Less focus to date on therapeutics. Good material on extent of coordination and sharing of information. Nothing specific on animal models. Is this still important? Wide range of coordinating mechanisms reviewed and considered. Broader than conference calls only. Not assessed. Good material presented on this although not conceptualised specifically as an operational plan. Good material on this from within WHO and small number of external stakeholders. Still need to consult more widely, e.g. PAHO and country offices. Not assessed. 15
Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response. The WHO Blueprint Team
 Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response The WHO Blueprint Team Context of the Blueprint WHO Executive Board 1 (January 2015) WHO Summit on Ebola R&D
Preparedness for the inevitable: overcoming obstacles to a timely and effective R&D response The WHO Blueprint Team Context of the Blueprint WHO Executive Board 1 (January 2015) WHO Summit on Ebola R&D
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness
 Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
Guidance on Request for Information on Rapid Response Platform Technologies for Epidemic Preparedness Purpose of this request for information The Bill & Melinda Gates Foundation (BMGF; also referred to
Vector control. REGIONAL COMMITTEE Provisional Agenda item 8.4. SEA/RC70/10 Maldives 6 10 September July Seventieth Session
 REGIONAL COMMITTEE Provisional Agenda item 8.4 Seventieth Session SEA/RC70/10 Maldives 6 10 September 2017 18 July 2017 Vector control Major vector-borne diseases account for an estimated 17% of the global
REGIONAL COMMITTEE Provisional Agenda item 8.4 Seventieth Session SEA/RC70/10 Maldives 6 10 September 2017 18 July 2017 Vector control Major vector-borne diseases account for an estimated 17% of the global
Technical Guidance and Specifications
 Technical Guidance and Specifications Deirde Healy and Robyn Meurant WHO PQ Team Diagnostics Assessment Joint UNICEF, UNFPA and WHO meeting with manufacturers and suppliers of in vitro diagnostics, vaccines,
Technical Guidance and Specifications Deirde Healy and Robyn Meurant WHO PQ Team Diagnostics Assessment Joint UNICEF, UNFPA and WHO meeting with manufacturers and suppliers of in vitro diagnostics, vaccines,
Innovation to Impact WHO change plan on evaluation of pesticides Malaria Policy Advisory Committee Geneva, Switzerland September 2015
 Innovation to Impact WHO change plan on evaluation of pesticides Malaria Policy Advisory Committee Geneva, Switzerland 16-18 September 2015 Raman Velayudhan and Abraham Mnzava 0 WHO leadership is strongly
Innovation to Impact WHO change plan on evaluation of pesticides Malaria Policy Advisory Committee Geneva, Switzerland 16-18 September 2015 Raman Velayudhan and Abraham Mnzava 0 WHO leadership is strongly
An R&D Blueprint for action to prevent epidemics. Report to the Scientific Advisory Group 8-9 February 2017
 An R&D Blueprint for action to prevent epidemics Report to the Scientific Advisory Group 8-9 February 2017 Why an R&D Blueprint? The Ebola epidemic has demonstrated that it is possible to accelerate R&D
An R&D Blueprint for action to prevent epidemics Report to the Scientific Advisory Group 8-9 February 2017 Why an R&D Blueprint? The Ebola epidemic has demonstrated that it is possible to accelerate R&D
EBOLA AND OTHER AFRICAN VIRAL HAEMORRHAGIC FEVERS
 EBOLA AND OTHER AFRICAN VIRAL HAEMORRHAGIC FEVERS In response to the 2014 West African Ebola epidemic, last year s G-FINDER survey tracked funding for Ebola R&D for the first time, capturing FY2014 investments.
EBOLA AND OTHER AFRICAN VIRAL HAEMORRHAGIC FEVERS In response to the 2014 West African Ebola epidemic, last year s G-FINDER survey tracked funding for Ebola R&D for the first time, capturing FY2014 investments.
CEPI Coalition for Epidemic Preparedness Innovations. CEPI a model for funding other initiatives and areas
 CEPI Coalition for Epidemic Preparedness Innovations CEPI a model for funding other initiatives and areas Foto: Daniel Berehulak, The New York Times Testing of an Ebola vaccine a successful but suboptimal
CEPI Coalition for Epidemic Preparedness Innovations CEPI a model for funding other initiatives and areas Foto: Daniel Berehulak, The New York Times Testing of an Ebola vaccine a successful but suboptimal
Exploring Good Clinical Practice guidance in clinical trials meeting summary
 Exploring Good Clinical Practice guidance in clinical trials meeting summary Summary The ICH Good Clinical Practice (GCP) guidelines are currently being revised, providing an opportunity to review the
Exploring Good Clinical Practice guidance in clinical trials meeting summary Summary The ICH Good Clinical Practice (GCP) guidelines are currently being revised, providing an opportunity to review the
Selection and use of Ebola in vitro diagnostic (IVD) assays
 EMERGENCY GUIDANCE Selection and use of Ebola in vitro diagnostic (IVD) assays June 2015 World Health Organization 2015. All rights reserved. The designations employed and the presentation of the material
EMERGENCY GUIDANCE Selection and use of Ebola in vitro diagnostic (IVD) assays June 2015 World Health Organization 2015. All rights reserved. The designations employed and the presentation of the material
Anticipating Epidemics Science and Research December Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation
 Anticipating Epidemics Science and Research December 2015 Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation Preparing for the inevitable With more frequent travel, globalized
Anticipating Epidemics Science and Research December 2015 Dr Marie-Paule Kieny Assistant Director-General Health Systems and Innovation Preparing for the inevitable With more frequent travel, globalized
Update on the ongoing development of a Global Vector Control Response
 Update on the ongoing development of a Global Vector Control Response Malaria Policy Advisory Committee 15th September 2016 Global Malaria Programme Department of Control of Neglected Tropical Diseases
Update on the ongoing development of a Global Vector Control Response Malaria Policy Advisory Committee 15th September 2016 Global Malaria Programme Department of Control of Neglected Tropical Diseases
Licenced Ebola Vaccine
 The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
The Market Shaping Goal Shape vaccine markets to ensure adequate supply of appropriate, quality vaccines at low and sustainable prices for developing countries. Supply and Procurement Roadmap Licenced
Health Impact of Ebola Crisis. Presentation for LSHTM / Options - Chris Lewis
 Health Impact of Ebola Crisis Presentation for LSHTM / Options - Chris Lewis Outline Introduction Ebola outbreak Impact of the crisis Timeline Early December 2013 - little boy in southern Guinea caught
Health Impact of Ebola Crisis Presentation for LSHTM / Options - Chris Lewis Outline Introduction Ebola outbreak Impact of the crisis Timeline Early December 2013 - little boy in southern Guinea caught
Lessons Learned: Bringing diagnostics to market in low and middle income countries
 Lessons Learned: Bringing diagnostics to market in low and middle income countries Workshop on New & Innovative Approaches to Laboratory Diagnosis of Zika, Dengue & Other Arboviruses PDC, Fondation Merieux,
Lessons Learned: Bringing diagnostics to market in low and middle income countries Workshop on New & Innovative Approaches to Laboratory Diagnosis of Zika, Dengue & Other Arboviruses PDC, Fondation Merieux,
WHO Prequalification of in-vitro diagnostics, medicines and vaccines
 WHO Prequalification of in-vitro diagnostics, medicines and vaccines Overview of prequalification processes & supporting activities Deus Mubangizi PQT Coordinator 20 October 2016 2 WHO prequalification
WHO Prequalification of in-vitro diagnostics, medicines and vaccines Overview of prequalification processes & supporting activities Deus Mubangizi PQT Coordinator 20 October 2016 2 WHO prequalification
EBOLA IN DEMOCRATIC REPUBLIC OF CONGO (DRC) CRISIS INFO #2 06/06/2018
 EBOLA IN DEMOCRATIC REPUBLIC OF CONGO (DRC) CRISIS INFO #2 06/06/2018 1. Global overview 1.1. Context So far, the outbreak (officially declared on 8 th of May) has affected the Bikoro (Bikoro and Ikoko
EBOLA IN DEMOCRATIC REPUBLIC OF CONGO (DRC) CRISIS INFO #2 06/06/2018 1. Global overview 1.1. Context So far, the outbreak (officially declared on 8 th of May) has affected the Bikoro (Bikoro and Ikoko
Role of DIAGNOSTICS in BIOPREPAREDNESS Mark Miller, MD Chief Medical Officer biomerieux
 Role of DIAGNOSTICS in BIOPREPAREDNESS Mark Miller, MD Chief Medical Officer biomerieux 29.09.2016 IMI Stakeholder Forum 2016 Brussels, Belgium A distant history lesson Those who cannot remember the past
Role of DIAGNOSTICS in BIOPREPAREDNESS Mark Miller, MD Chief Medical Officer biomerieux 29.09.2016 IMI Stakeholder Forum 2016 Brussels, Belgium A distant history lesson Those who cannot remember the past
WHO Blood Regulators Network (BRN)
 Distribution: General English only WHO Blood Regulators Network (BRN) Position Paper on Use of Convalescent Plasma, Serum or Immune Globulin Concentrates as an Element in Response to an Emerging Virus*
Distribution: General English only WHO Blood Regulators Network (BRN) Position Paper on Use of Convalescent Plasma, Serum or Immune Globulin Concentrates as an Element in Response to an Emerging Virus*
Malaria Research Capability Strengthening in Africa
 July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
July 2005 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa INTRODUCTION
STATUS OF REVIEWS, AUTHORIZATIONS AND OVERSIGHT FOR CLINICAL TRIALS IN THE WHO AFRICAN REGION. Technical Document
 30 August 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS AND
30 August 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS AND
Evaluation: annual report
 EXECUTIVE BOARD EB137/7 137th session 8 May 2015 Provisional agenda item 8.2 Evaluation: annual report 1. The Executive Board at its 131st session approved the WHO evaluation policy. 1 The policy requires
EXECUTIVE BOARD EB137/7 137th session 8 May 2015 Provisional agenda item 8.2 Evaluation: annual report 1. The Executive Board at its 131st session approved the WHO evaluation policy. 1 The policy requires
STATUS OF REVIEWS, AUTHORIZATIONS AND OVERSIGHT FOR CLINICAL TRIALS IN THE WHO AFRICAN REGION. Technical Document
 15 June 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Provisional agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS
15 June 2017 REGIONAL COMMITTEE FOR AFRICA ORIGINAL: ENGLISH Sixty-seventh session Victoria Falls, Republic of Zimbabwe, 28 August 1 September 2017 Provisional agenda item 17 STATUS OF REVIEWS, AUTHORIZATIONS
Context and objectives of the workshop on platform technologies. 21 July 2016 David Wood and Virginia Benassi
 Context and objectives of the workshop on platform technologies 21 July 2016 David Wood and Virginia Benassi 2nd Technical workshop on platform technologies Geneva, Switzerland, 21 July 2016 Platform Technologies
Context and objectives of the workshop on platform technologies 21 July 2016 David Wood and Virginia Benassi 2nd Technical workshop on platform technologies Geneva, Switzerland, 21 July 2016 Platform Technologies
Malaria Research Capability Strengthening in Africa
 October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
October 2004 UNICEF/UNDP/World Bank/WHO Special Programme for Research & Training in Tropical Diseases (TDR) Multilateral Initiative on Malaria (MIM) Malaria Research Capability Strengthening in Africa
Introduction to Lassa fever
 Introduction to Lassa fever Managing infectious hazards Store food in containers with lids OpenWHO.org WHO2017 1 Learning objectives Describe signs, symptoms, and transmission of Lassa fever List 4 preventive
Introduction to Lassa fever Managing infectious hazards Store food in containers with lids OpenWHO.org WHO2017 1 Learning objectives Describe signs, symptoms, and transmission of Lassa fever List 4 preventive
Ebola virus disease outbreak in western Africa
 Action Plan Strategic Objectives 6. Promote innovation and research on new drugs International surveillance networks and information sharing on promising research Active role in research for governments
Action Plan Strategic Objectives 6. Promote innovation and research on new drugs International surveillance networks and information sharing on promising research Active role in research for governments
JOB DESCRIPTION. Job title: Senior Malaria Specialist Location: Abuja, Nigeria. Department: Technical Length of contract: Five years
 JOB DESCRIPTION Job title: Senior Malaria Specialist Location: Abuja, Nigeria Donor title: Senior Malaria Expert Department: Technical Length of contract: Five years Role type: Global Grade: 11 Travel
JOB DESCRIPTION Job title: Senior Malaria Specialist Location: Abuja, Nigeria Donor title: Senior Malaria Expert Department: Technical Length of contract: Five years Role type: Global Grade: 11 Travel
Regulatory system strengthening
 SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/32 Provisional agenda item 15.6 14 March 2014 Regulatory system strengthening Report by the Secretariat 1. The Executive Board at its 134th session noted an earlier
SIXTY-SEVENTH WORLD HEALTH ASSEMBLY A67/32 Provisional agenda item 15.6 14 March 2014 Regulatory system strengthening Report by the Secretariat 1. The Executive Board at its 134th session noted an earlier
JOB DESCRIPTION. Job title: LGA Field Officer Location: Jigawa 4, Katsina 2, Sokoto 2 and Yobe 2. Department: Technical Length of contract: 2-years
 JOB DESCRIPTION Job title: LGA Field Officer Location: Jigawa 4, Katsina 2, Sokoto 2 and Yobe 2 Department: Technical Length of contract: 2-years Role type: National Grade: 6 Travel involved: 40% within
JOB DESCRIPTION Job title: LGA Field Officer Location: Jigawa 4, Katsina 2, Sokoto 2 and Yobe 2 Department: Technical Length of contract: 2-years Role type: National Grade: 6 Travel involved: 40% within
Post Referendum Briefing: Business Continues. September 2016
 Post Referendum Briefing: Business Continues September 2016 The Agency 1. The Medicines and Healthcare products Regulatory Agency is an executive agency of the UK Department of Health (DH). The Agency
Post Referendum Briefing: Business Continues September 2016 The Agency 1. The Medicines and Healthcare products Regulatory Agency is an executive agency of the UK Department of Health (DH). The Agency
JOB DESCRIPTION. Job title: Regional Coordinator Location: Gulu, Lira. Department: Technical Length of contract: Fixed. Child safeguarding level:
 JOB DESCRIPTION Job title: Regional Coordinator Location: Gulu, Lira Department: Technical Length of contract: Fixed Role type: National Grade: 9 Travel involved: Up to 40% travel within region of operation
JOB DESCRIPTION Job title: Regional Coordinator Location: Gulu, Lira Department: Technical Length of contract: Fixed Role type: National Grade: 9 Travel involved: Up to 40% travel within region of operation
Democratic Republic of Congo: Ebola update May 2018
 Democratic Republic of Congo: Ebola update May 2018 30 May 2018 Summary Since the Ebola epidemic in Democratic Republic of Congo (DRC) was declared on 8 May 2018, 54 people who presented symptoms of haemorrhagic
Democratic Republic of Congo: Ebola update May 2018 30 May 2018 Summary Since the Ebola epidemic in Democratic Republic of Congo (DRC) was declared on 8 May 2018, 54 people who presented symptoms of haemorrhagic
PROMOTING ACCESS, QUALITY AND INNOVATION TO SAVE AND IMPROVE LIVES
 PROMOTING ACCESS, QUALITY AND INNOVATION TO SAVE AND IMPROVE LIVES Essential Medicines and Health Products 2 VISION: A WORLD WHERE EVERY CHILD, MAN AND WOMAN CAN AFFORD AND HAS ACCESS TO THE QUALITY ESSENTIAL
PROMOTING ACCESS, QUALITY AND INNOVATION TO SAVE AND IMPROVE LIVES Essential Medicines and Health Products 2 VISION: A WORLD WHERE EVERY CHILD, MAN AND WOMAN CAN AFFORD AND HAS ACCESS TO THE QUALITY ESSENTIAL
to protect human health throughout the life-cycle of medicinal products;
 ICMRA Communications Strategy: 2018-2020 Introduction The International Coalition of Medicines Regulatory Authorities (ICMRA) is a voluntary, highlevel, strategic coordinating, advocacy and leadership
ICMRA Communications Strategy: 2018-2020 Introduction The International Coalition of Medicines Regulatory Authorities (ICMRA) is a voluntary, highlevel, strategic coordinating, advocacy and leadership
WHO Prequalification of In Vitro Diagnostics
 WHO Prequalification of In Vitro Diagnostics 3 rd WHO Global Forum on Medical Devices Geneva, 10 May 2017 Helena Ardura & Deirdre Healy WHO PQ Team Diagnostics Assessment Essential Medicines and Health
WHO Prequalification of In Vitro Diagnostics 3 rd WHO Global Forum on Medical Devices Geneva, 10 May 2017 Helena Ardura & Deirdre Healy WHO PQ Team Diagnostics Assessment Essential Medicines and Health
Development of plasma therapies for emerging infectious diseases. Dr Glenn Smith Biological Science Section Scientific Evaluation Branch
 Development of plasma therapies for emerging infectious diseases Dr Glenn Smith Biological Science Section Scientific Evaluation Branch June 2017 Therapeutic Goods Administration (TGA) A part of the Australian
Development of plasma therapies for emerging infectious diseases Dr Glenn Smith Biological Science Section Scientific Evaluation Branch June 2017 Therapeutic Goods Administration (TGA) A part of the Australian
SUMMARY DOCUMENT FOR FROM PIPELINE TO PRODUCT: MALARIA R&D FUNDING NEEDS INTO THE NEXT DECADE
 SUMMARY DOCUMENT FOR FROM PIPELINE TO PRODUCT: MALARIA R&D FUNDING NEEDS INTO THE NEXT DECADE REPORT SUMMARY Introduction Malaria is a major public health challenge that threatens approximately half of
SUMMARY DOCUMENT FOR FROM PIPELINE TO PRODUCT: MALARIA R&D FUNDING NEEDS INTO THE NEXT DECADE REPORT SUMMARY Introduction Malaria is a major public health challenge that threatens approximately half of
Preparations for Ebola Vaccine Deployment
 Preparations for Ebola Vaccine Deployment Ebola vaccine and vaccination - Session 8 Meeting of the Strategic Advisory Group of Experts on Immunization (SAGE) Geneva, 14 16 April 2015 Dr Marie-Pierre Preziosi,
Preparations for Ebola Vaccine Deployment Ebola vaccine and vaccination - Session 8 Meeting of the Strategic Advisory Group of Experts on Immunization (SAGE) Geneva, 14 16 April 2015 Dr Marie-Pierre Preziosi,
Data requirements and methods to support the evaluation of new vector control products
 Global Malaria Programme Data requirements and methods to support the evaluation of new vector control products DECEMBER 2017 RECOMMENDATIONS BACKGROUND WHO s process for the evaluation of vector control
Global Malaria Programme Data requirements and methods to support the evaluation of new vector control products DECEMBER 2017 RECOMMENDATIONS BACKGROUND WHO s process for the evaluation of vector control
MAIN OUTCOMES OF DISCUSSION FROM WHO CONSULTATION ON NUCLEIC ACID VACCINES. I. Knezevic, R. Sheets Feb, 2018 Geneva, Switzerland
 MAIN OUTCOMES OF DISCUSSION FROM WHO CONSULTATION ON NUCLEIC ACID VACCINES I. Knezevic, R. Sheets 21-23 Feb, 2018 Geneva, Switzerland CONTEXT OF DISCUSSION WHO Consultation held to determine whether the
MAIN OUTCOMES OF DISCUSSION FROM WHO CONSULTATION ON NUCLEIC ACID VACCINES I. Knezevic, R. Sheets 21-23 Feb, 2018 Geneva, Switzerland CONTEXT OF DISCUSSION WHO Consultation held to determine whether the
ACFID Code of Conduct PMEL Guidance Note. Prepared for ACFID by Learning4Development
 ACFID Code of Conduct PMEL Guidance Note Prepared for ACFID by Learning4Development September 2017 1 CONTENTS: 1. Introduction 2. What is PMEL? 3. Approaches to PMEL 3.1 At a strategic or policy level
ACFID Code of Conduct PMEL Guidance Note Prepared for ACFID by Learning4Development September 2017 1 CONTENTS: 1. Introduction 2. What is PMEL? 3. Approaches to PMEL 3.1 At a strategic or policy level
Learning from Past Emergency Use Authorizations
 Learning from Past Emergency Use Authorizations Elliot P. Cowan, Ph.D. Principal, Partners in Diagnostics, LLC PDC Workshop on New and Innovative Approaches to Laboratory Diagnosis of Zika, Dengue and
Learning from Past Emergency Use Authorizations Elliot P. Cowan, Ph.D. Principal, Partners in Diagnostics, LLC PDC Workshop on New and Innovative Approaches to Laboratory Diagnosis of Zika, Dengue and
Planning for Election Observation A Field Guide for Election Monitoring Groups
 Planning for Election Observation A Field Guide for Election Monitoring Groups Planning for Election Observation This field guide is designed as an easy- reference tool for domestic non- partisan election
Planning for Election Observation A Field Guide for Election Monitoring Groups Planning for Election Observation This field guide is designed as an easy- reference tool for domestic non- partisan election
A new Model of Vaccine R&D Global Health Public Private Partnership : The Global Health Vaccine Center of Innovation (GHVCI)
 A new Model of Vaccine R&D Global Health Public Private Partnership : The Global Health Vaccine Center of Innovation (GHVCI) Jean Lang avp R&D sanofi pasteur 9eme Rencontre Nationale des Directeurs de
A new Model of Vaccine R&D Global Health Public Private Partnership : The Global Health Vaccine Center of Innovation (GHVCI) Jean Lang avp R&D sanofi pasteur 9eme Rencontre Nationale des Directeurs de
Ebola outbreak in West Africa : Shift in paradigm
 Ebola outbreak in West Africa : Shift in paradigm Dr S.C Briand, Director Pandemic and Epidemic Diseases department, WHO Geneva EVD West Africa: many "first time" Enormous epidemic in the affected countries
Ebola outbreak in West Africa : Shift in paradigm Dr S.C Briand, Director Pandemic and Epidemic Diseases department, WHO Geneva EVD West Africa: many "first time" Enormous epidemic in the affected countries
JOB DESCRIPTION. Job title: Technical Officer Location: Gulu or Lira. Department: Technical Length of contract: Fixed. Role type: National Grade: 7
 JOB DESCRIPTION Job title: Technical Officer Location: Gulu or Lira Department: Technical Length of contract: Fixed Role type: National Grade: 7 Travel involved: Up to 40% travel to areas of operation
JOB DESCRIPTION Job title: Technical Officer Location: Gulu or Lira Department: Technical Length of contract: Fixed Role type: National Grade: 7 Travel involved: Up to 40% travel to areas of operation
WHO s IMS Organizational Structure Overview
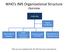 WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
WHO s IMS Organizational Structure Overview Leadership Partner Coordination Information & Planning Health Expertise & Operations Operations Support and Logistics Management & Administration *this structure
Certification in Humanitarian Logistics Level I HLC 2007
 Certification in Humanitarian Logistics Level I HLC 2007 1 How Certification came about.hlc 2003 HLC participant feedback Needs to be clear career map, not just specific training initiatives around warehousing
Certification in Humanitarian Logistics Level I HLC 2007 1 How Certification came about.hlc 2003 HLC participant feedback Needs to be clear career map, not just specific training initiatives around warehousing
Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1
 ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
ECONOMIC AND SOCIAL COUNCIL PARTNERSHIPS FORUM Dialogue 1: Partnerships in support of strengthening health systems: Building resilience to pandemics 1 Introduction 28 May 2015, 11:00 a.m. 1:00 p.m. UN
What Can I Do With My Data?
 What Can I Do With My Data? Utilizing Existing Data for Analysis and Hypothesis Development Falgunee Parekh, MPH, PhD Agenda My Research Background Background on Analysis of Surveillance (or Initial) Data
What Can I Do With My Data? Utilizing Existing Data for Analysis and Hypothesis Development Falgunee Parekh, MPH, PhD Agenda My Research Background Background on Analysis of Surveillance (or Initial) Data
JOB DESCRIPTION. Job title: Senior Malaria Specialist Location: Abuja, Nigeria. Department: Technical Length of contract: Five years
 JOB DESCRIPTION Job title: Senior Malaria Specialist Location: Abuja, Nigeria Donor title: Senior Malaria Expert Department: Technical Length of contract: Five years Role type: National Grade: 10 Travel
JOB DESCRIPTION Job title: Senior Malaria Specialist Location: Abuja, Nigeria Donor title: Senior Malaria Expert Department: Technical Length of contract: Five years Role type: National Grade: 10 Travel
WHO Prequalification Team Vector Control Products Assessment Group
 WHO Prequalification Team Vector Control Products Assessment Group Workshop on Dossier Requirements and Proposed Inspection Protocol 26-28 October 2016, Geneva, Switzerland 1 Recall: WHO leadership is
WHO Prequalification Team Vector Control Products Assessment Group Workshop on Dossier Requirements and Proposed Inspection Protocol 26-28 October 2016, Geneva, Switzerland 1 Recall: WHO leadership is
May 9, Meeting Summary. Facilitating Antibacterial Drug Development
 May 9, 2012 Meeting Summary Facilitating Antibacterial Drug Development Origins of the Current Public Health Crisis of Antibacterial Resistance Antibacterial drugs play a critical role in the ability to
May 9, 2012 Meeting Summary Facilitating Antibacterial Drug Development Origins of the Current Public Health Crisis of Antibacterial Resistance Antibacterial drugs play a critical role in the ability to
IDSA is pleased to offer the following comments on specific priority areas identified by FDA:
 October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
October 31, 2018 Shashi Malhotra Office of Acquisitions & Grants Services (OAGS) Food and Drug Administration Telephone: 240-402-7592 Email: Shashi.Malhotra@fda.hhs.gov SUBJECT: NOT-FD-18-16: Development
European Centre for Disease Prevention and Control. Call for expression of interest for Seconded National Experts (ECDC/2017/SNE)
 European Centre for Disease Prevention and Control Call for expression of interest for Seconded National Experts (ECDC/2017/SNE) Applications are invited for the secondment at the European Centre for Disease
European Centre for Disease Prevention and Control Call for expression of interest for Seconded National Experts (ECDC/2017/SNE) Applications are invited for the secondment at the European Centre for Disease
JOB DESCRIPTION. This position is contingent upon approved funding. Job title: Survey Coordinator Location: Phnom Penh, Cambodia
 JOB DESCRIPTION This position is contingent upon approved funding. Job title: Survey Coordinator Location: Phnom Penh, Cambodia Department: Technical Length of contract: 5 months Role type: Global Grade:
JOB DESCRIPTION This position is contingent upon approved funding. Job title: Survey Coordinator Location: Phnom Penh, Cambodia Department: Technical Length of contract: 5 months Role type: Global Grade:
was published. February 21, 2017
 701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
701 Pennsylvania Avenue, NW Suite 800 Washington, D.C. 20004 2654 Tel: 202 783 8700 Fax: 202 783 8750 www.advamed.org Division of Dockets Management (HFA-305) Food and Drug Administration 5630 Fishers
Simplifying and clarifying vector control product listing and policy guidance. May 14th, 2018
 Simplifying and clarifying vector control product listing and policy guidance May 14th, 2018 Context Last October, MPAC recognized recent progress, but requested that WHO continue to simplify the definitions
Simplifying and clarifying vector control product listing and policy guidance May 14th, 2018 Context Last October, MPAC recognized recent progress, but requested that WHO continue to simplify the definitions
Evaluation of the Contribution of UNDP to Environmental Management for Poverty Reduction ( :The Poverty and Environment Nexus)
 Evaluation of the Contribution of UNDP to Environmental Management for Poverty Reduction (2004-2009:The Poverty and Environment Nexus) Country-based Case Studies Terms of Reference I. Mandate The United
Evaluation of the Contribution of UNDP to Environmental Management for Poverty Reduction (2004-2009:The Poverty and Environment Nexus) Country-based Case Studies Terms of Reference I. Mandate The United
Diagnostic Preparedness Platform WHO R&D Blueprint for Priority Infectious Diseases with Epidemic Potential
 Diagnostic Preparedness Platform WHO R&D Blueprint for Priority Infectious Diseases with Epidemic Potential 2 nd round presentation Geneva July 21 st, 2016 Diagnostic Preparedness Platform Aim Surveillance
Diagnostic Preparedness Platform WHO R&D Blueprint for Priority Infectious Diseases with Epidemic Potential 2 nd round presentation Geneva July 21 st, 2016 Diagnostic Preparedness Platform Aim Surveillance
COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE REGIONS
 EUROPEAN COMMISSION Brussels, 2.10.2013 COM(2013) 686 final COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE
EUROPEAN COMMISSION Brussels, 2.10.2013 COM(2013) 686 final COMMUNICATION FROM THE COMMISSION TO THE EUROPEAN PARLIAMENT, THE COUNCIL, THE EUROPEAN ECONOMIC AND SOCIAL COMMITTEE AND THE COMMITTEE OF THE
Programme update WHO Prequalification of Diagnostics
 Prequalification of Medicines, Diagnostics and Vaccines h Consultative Stakeholders Meeting, Geneva, 4 April 2011 Programme update WHO Prequalification of Diagnostics Dr Gaby Vercauteren Diagnostics and
Prequalification of Medicines, Diagnostics and Vaccines h Consultative Stakeholders Meeting, Geneva, 4 April 2011 Programme update WHO Prequalification of Diagnostics Dr Gaby Vercauteren Diagnostics and
Overview of Nigeria s R & D Plan for Lassa fever
 Overview of Nigeria s R & D Plan for Lassa fever Presented by Bola Olayinka Preparing for Lassa Vaccine Clinical Trials with Targeted Epidemiology Studies Workshop 08 November 2018, Accra, Ghana Introduction
Overview of Nigeria s R & D Plan for Lassa fever Presented by Bola Olayinka Preparing for Lassa Vaccine Clinical Trials with Targeted Epidemiology Studies Workshop 08 November 2018, Accra, Ghana Introduction
Page 1 of 8. Better informed health care through better clinical guidelines
 Introduction The Guidelines International Network (G-I-N) Australian and New Zealand Regional Community was established to provide a forum to support the collaboration of organisations and individuals
Introduction The Guidelines International Network (G-I-N) Australian and New Zealand Regional Community was established to provide a forum to support the collaboration of organisations and individuals
ZIKAVAX PARTNERSHIP. Dr Odile LEROY DCVMN Seoul 26 th September 2017
 ZIKAVAX PARTNERSHIP Dr Odile LEROY DCVMN Seoul 26 th September 2017 1 Product Development Partnership THE EUROPEAN VACCINE INITIATIVE IS A PRODUC T DEVELOPMENT PARTNERSHIP WHICH AIMS TO ACCELERATE THE
ZIKAVAX PARTNERSHIP Dr Odile LEROY DCVMN Seoul 26 th September 2017 1 Product Development Partnership THE EUROPEAN VACCINE INITIATIVE IS A PRODUC T DEVELOPMENT PARTNERSHIP WHICH AIMS TO ACCELERATE THE
DIGITAL REACH INITIATIVE ROADMAP
 DIGITAL REACH INITIATIVE ROADMAP DIGITAL REGIONAL EAST AFRICAN COMMUNITY HEALTH INITIATIVE TOWARDS A REGIONAL COMMITMENT TO IMPROVE HEALTH OUTCOMES THROUGH DIGITAL TECHNOLOGY The Digital REACH Initiative
DIGITAL REACH INITIATIVE ROADMAP DIGITAL REGIONAL EAST AFRICAN COMMUNITY HEALTH INITIATIVE TOWARDS A REGIONAL COMMITMENT TO IMPROVE HEALTH OUTCOMES THROUGH DIGITAL TECHNOLOGY The Digital REACH Initiative
WHO-MSD Collaboration to Bring an Ebola Vaccine to the Populations in Need
 WHO-MSD Collaboration to Bring an Ebola Vaccine to the Populations in Need Jules Millogo, MD, MSc Director, Public Health Partnerships Merck & Co, Inc. North Wales, PA, USA V920: rvsvδg-zebov-gp Vaccine
WHO-MSD Collaboration to Bring an Ebola Vaccine to the Populations in Need Jules Millogo, MD, MSc Director, Public Health Partnerships Merck & Co, Inc. North Wales, PA, USA V920: rvsvδg-zebov-gp Vaccine
Joint Evaluation of Joint Programmes on Gender Equality in the United Nations System Management Response
 drafted by: Working Group (UN Women, UNDP, UNFPA, UNICEF, MDGF/UNDP) Date: 5/16/2014 Overall : The interagency group which commissioned the Joint Evaluation of Joint Programmes on Gender Equality in the
drafted by: Working Group (UN Women, UNDP, UNFPA, UNICEF, MDGF/UNDP) Date: 5/16/2014 Overall : The interagency group which commissioned the Joint Evaluation of Joint Programmes on Gender Equality in the
EDCTP perspective on biopreparedness and related capacity development in Africa
 EDCTP perspective on biopreparedness and related capacity development in Africa Consultation workshop on Preparedness for emerging diseases Dr. Michael Makanga EDCTP Executive Director. 29 September 2016
EDCTP perspective on biopreparedness and related capacity development in Africa Consultation workshop on Preparedness for emerging diseases Dr. Michael Makanga EDCTP Executive Director. 29 September 2016
Management Response to the Review of the Global Education Cluster Co-Leadership Arrangement UNICEF and Save the Children Draft 8 April 2011
 Management Response to the Review of the Global Cluster Co-Leadership Arrangement UNICEF and Save the Children Draft 8 April 2011 In November 2007 a Memorandum of Understanding (MoU) was signed between
Management Response to the Review of the Global Cluster Co-Leadership Arrangement UNICEF and Save the Children Draft 8 April 2011 In November 2007 a Memorandum of Understanding (MoU) was signed between
Evaluation: annual report
 EXECUTIVE BOARD EB141/7 141st session 16 May 2017 Provisional agenda item 7.3 Evaluation: annual report 1. The Executive Board approved the WHO evaluation policy at its 131st session in 2012. 1 The policy
EXECUTIVE BOARD EB141/7 141st session 16 May 2017 Provisional agenda item 7.3 Evaluation: annual report 1. The Executive Board approved the WHO evaluation policy at its 131st session in 2012. 1 The policy
JOB DESCRIPTION. Department: Global Length of contract: An initial period of 12 months with the possibility of renewal.
 JOB DESCRIPTION Job title: Regional Technical Specialist Location: Bangkok, Thailand Department: Global Length of contract: An initial period of 12 months with the possibility of renewal. Role type: International
JOB DESCRIPTION Job title: Regional Technical Specialist Location: Bangkok, Thailand Department: Global Length of contract: An initial period of 12 months with the possibility of renewal. Role type: International
Ebola Virus disease in West Africa : challenges and lessons learned
 Ebola Virus disease in West Africa : challenges and lessons learned Pr N. Shindo Dr S.C. Briand Pandemic and Epidemic Diseases Department WHO Geneva EVD outbreak in West Africa in Numbers From24 March
Ebola Virus disease in West Africa : challenges and lessons learned Pr N. Shindo Dr S.C. Briand Pandemic and Epidemic Diseases Department WHO Geneva EVD outbreak in West Africa in Numbers From24 March
Investing in Research, Development and Innovation for Global Health
 The Malaria Advocacy Working Group (MAWG) of the Roll Back Malaria Partnership s (RBM) contribution to the European Commission consultation on the Green Paper - From Challenges to Opportunities: Towards
The Malaria Advocacy Working Group (MAWG) of the Roll Back Malaria Partnership s (RBM) contribution to the European Commission consultation on the Green Paper - From Challenges to Opportunities: Towards
Terms of Reference (TOR)
 CONFÉRENCE DES NATIONS UNIES SUR LE COMMERCE ET LE DÉVELOPPEMENT UNITED NATIONS CONFERENCE ON TRADE AND DEVELOPMENT Terms of Reference (TOR) External Evaluation of Development Account Project 1415 AX Support
CONFÉRENCE DES NATIONS UNIES SUR LE COMMERCE ET LE DÉVELOPPEMENT UNITED NATIONS CONFERENCE ON TRADE AND DEVELOPMENT Terms of Reference (TOR) External Evaluation of Development Account Project 1415 AX Support
Accelerating Innovation in Global Health Crises The Fighting Ebola and Combating Zika and Future Threats Grand Challenge
 Accelerating Innovation in Global Health Crises The Fighting Ebola and Combating Zika and Future Threats Grand Challenge What will we do with what we ve learned? This was a test of the world s ability
Accelerating Innovation in Global Health Crises The Fighting Ebola and Combating Zika and Future Threats Grand Challenge What will we do with what we ve learned? This was a test of the world s ability
Emergencies preparedness, response Ebola virus disease Democratic Republic
 1 von 5 05.12.2018, 08:43 Emergencies preparedness, response Ebola virus disease Democratic Republic of the Congo Disease outbreak news: Update 29 November 2018 As the Ebola virus disease (EVD) outbreak
1 von 5 05.12.2018, 08:43 Emergencies preparedness, response Ebola virus disease Democratic Republic of the Congo Disease outbreak news: Update 29 November 2018 As the Ebola virus disease (EVD) outbreak
2015/2016 Year for Vaccine Development. Vasee Moorthy MD PhD Team Leader Vaccine Development
 2015/2016 Year for Vaccine Development Vasee Moorthy MD PhD Team Leader Vaccine Development Product Development for Vaccines Advisory Committee Established in 2014 to fill strategic gap in our advisory
2015/2016 Year for Vaccine Development Vasee Moorthy MD PhD Team Leader Vaccine Development Product Development for Vaccines Advisory Committee Established in 2014 to fill strategic gap in our advisory
7TH E-NEWSLETTER. 7 th EBOVAC2. e-newsletter
 7 th EBOVAC2 e-newsletter November 2018 1 Welcome to the EBOVAC2 e-newsletter! EBOVAC2: Getting up to date The EBOVAC2 project is one of 8 projects funded under the IMI Ebola+ program that was launched
7 th EBOVAC2 e-newsletter November 2018 1 Welcome to the EBOVAC2 e-newsletter! EBOVAC2: Getting up to date The EBOVAC2 project is one of 8 projects funded under the IMI Ebola+ program that was launched
Terms of Reference (TOR)
 CONFÉRENCE DES NATIONS UNIES SUR LE COMMERCE ET LE DÉVELOPPEMENT UNITED NATIONS CONFERENCE ON TRADE AND DEVELOPMENT Terms of Reference (TOR) External Evaluation of Development Account Project 1213 I Strengthening
CONFÉRENCE DES NATIONS UNIES SUR LE COMMERCE ET LE DÉVELOPPEMENT UNITED NATIONS CONFERENCE ON TRADE AND DEVELOPMENT Terms of Reference (TOR) External Evaluation of Development Account Project 1213 I Strengthening
Diagnostics for Epidemic Preparedness
 Diagnostics for Epidemic Preparedness Devy M. Emperador, MPH Scientific Officer Emerging Threats Foundation for Innovative New Diagnostics (FIND) ICREID 2018 12 March 2018 Addis Ababa, Ethiopia 1 Who is
Diagnostics for Epidemic Preparedness Devy M. Emperador, MPH Scientific Officer Emerging Threats Foundation for Innovative New Diagnostics (FIND) ICREID 2018 12 March 2018 Addis Ababa, Ethiopia 1 Who is
Stimulating introduction of new, innovative in vitro diagnostics Supporting product development
 Stimulating introduction of new, innovative in vitro diagnostics Supporting product development Gonzalo Domingo Diagnostics gdomingo@path.org 09/18/2017 PATH: Accelerating global health innovation E X
Stimulating introduction of new, innovative in vitro diagnostics Supporting product development Gonzalo Domingo Diagnostics gdomingo@path.org 09/18/2017 PATH: Accelerating global health innovation E X
G4 DEVELOPMENT. Document 2 of 12 Statistics: Quantitative Online Feedback. Second G4 Public Comment Period: Submissions.
 G4 DEVELOPMENT Document 2 of 12 Statistics: Quantitative Online Feedback February 2013 INTRODUCTION ABOUT THE SUBMISSIONS DOCUMENTS In 2010 GRI began the development of the fourth generation of its Sustainability
G4 DEVELOPMENT Document 2 of 12 Statistics: Quantitative Online Feedback February 2013 INTRODUCTION ABOUT THE SUBMISSIONS DOCUMENTS In 2010 GRI began the development of the fourth generation of its Sustainability
One Year Later, Where Does the U.S. Response to Ebola Stand?
 One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
One Year Later, Where Does the U.S. Response to Ebola Stand? Kaiser Family Foundation, Washington, DC Jen Kates, PhD Vice President & Director, Global Health & HIV Policy Kaiser Family Foundation jkates@kff.org
Terms of Reference 1. BACKGROUND
 Terms of Reference Title: Capacity building, preventing and responding to Gender Based Violence Consultant Contract Type: Consultancy contract Duration: 5 months, starting immediately Duty Station: Geneva,
Terms of Reference Title: Capacity building, preventing and responding to Gender Based Violence Consultant Contract Type: Consultancy contract Duration: 5 months, starting immediately Duty Station: Geneva,
Cloning in human reproduction
 (^Ш World Health Organization Organisation mondiale de la Santé FIFTIETH WORLD HEALTH ASSEMBLY Supplementary agenda item A50/30 8 May 1997 Cloning in human reproduction Cloning,biomedical technology and
(^Ш World Health Organization Organisation mondiale de la Santé FIFTIETH WORLD HEALTH ASSEMBLY Supplementary agenda item A50/30 8 May 1997 Cloning in human reproduction Cloning,biomedical technology and
The Role of Research in Supporting Regulation of Biologicals: Building Bridges from Biomedical Discovery to Innovative Products
 The Role of Research in Supporting Regulation of Biologicals: Building Bridges from Biomedical Discovery to Innovative Products Jay S. Epstein, M.D. CBER, FDA Biologicals are Complex Products Critical
The Role of Research in Supporting Regulation of Biologicals: Building Bridges from Biomedical Discovery to Innovative Products Jay S. Epstein, M.D. CBER, FDA Biologicals are Complex Products Critical
NOT PROTECTIVELY MARKED. HM Inspectorate of Constabulary in Scotland. Inspection Framework. Version 1.0 (September 2014)
 HM Inspectorate of Constabulary in Scotland Inspection Framework Version 1.0 (September 2014) Improving Policing across Scotland Introduction I am pleased to introduce the new HMICS Inspection Framework.
HM Inspectorate of Constabulary in Scotland Inspection Framework Version 1.0 (September 2014) Improving Policing across Scotland Introduction I am pleased to introduce the new HMICS Inspection Framework.
Webinar IMI2 Call 13 CONCEPTION continuum of evidence from pregnancy exposures, reproductive toxicology and breastfeeding to improve outcomes now
 Webinar IMI2 Call 13 CONCEPTION continuum of evidence from pregnancy exposures, reproductive toxicology and breastfeeding to improve outcomes now 11 December 2017 15:00 CET Agenda How to use GoToWebinar
Webinar IMI2 Call 13 CONCEPTION continuum of evidence from pregnancy exposures, reproductive toxicology and breastfeeding to improve outcomes now 11 December 2017 15:00 CET Agenda How to use GoToWebinar
Report on meetings of expert committees and study groups 1
 EXECUTIVE BOARD EB137/9 137th session 27 March 2015 Provisional agenda item 10 Report on meetings of expert committees and study groups 1 Report by the Secretariat SPECIFICATIONS FOR PHARMACEUTICAL PREPARATIONS
EXECUTIVE BOARD EB137/9 137th session 27 March 2015 Provisional agenda item 10 Report on meetings of expert committees and study groups 1 Report by the Secretariat SPECIFICATIONS FOR PHARMACEUTICAL PREPARATIONS
The Future Landscape for Technical and Professional Education
 The Future Landscape for Technical and Professional Education ABOUT US Collab Group is a forward-thinking membership organisation which represents leading Colleges and College Groups who collaborate, at
The Future Landscape for Technical and Professional Education ABOUT US Collab Group is a forward-thinking membership organisation which represents leading Colleges and College Groups who collaborate, at
Organizing Framework for a Functional National HIV Monitoring & Evaluation System
 Regional Training on Strategic and Operational Planning in HIV & AIDS Organizing Framework for a Functional National HIV Monitoring & Evaluation System Final Draft for submission to the HIV Monitoring
Regional Training on Strategic and Operational Planning in HIV & AIDS Organizing Framework for a Functional National HIV Monitoring & Evaluation System Final Draft for submission to the HIV Monitoring
2. The first session concerned the scientific and technological aspects of infectious diseases and possible areas of activity.
 2(&'&21)(5(1&(21 %,27(&+12/2*
2(&'&21)(5(1&(21 %,27(&+12/2*
Global Health Cluster Interim Terms of Reference
 Global Health Cluster Interim Terms of Reference These interim Terms of Reference are effective as of January 2015 and will be reviewed in December 2015 in alignment with the new multi-year GHC strategy.
Global Health Cluster Interim Terms of Reference These interim Terms of Reference are effective as of January 2015 and will be reviewed in December 2015 in alignment with the new multi-year GHC strategy.
ANNEX I - TERMS OF REFERENCE
 ANNEX I - TERMS OF REFERENCE Review and Development of End User Monitoring (EUM) Standards and tools for Ready to Use Therapeutic Foods (RUTF) Supply Chain 1.0 Background The 2016 joint child malnutrition
ANNEX I - TERMS OF REFERENCE Review and Development of End User Monitoring (EUM) Standards and tools for Ready to Use Therapeutic Foods (RUTF) Supply Chain 1.0 Background The 2016 joint child malnutrition
MEDICAL DEVICES STUDIES
 AN OVERVIEW OF MEDICAL DEVICES STUDIES Outsourcing Trends & Regulatory Landscape in Europe and Latin America - whitepaper - MARKET & LANDSCAPE At Eurotrials, we work to deliver a best in class solution
AN OVERVIEW OF MEDICAL DEVICES STUDIES Outsourcing Trends & Regulatory Landscape in Europe and Latin America - whitepaper - MARKET & LANDSCAPE At Eurotrials, we work to deliver a best in class solution
WHO support and Technical Assistance _ In-Vitro Diagnostics
 WHO support and Technical Assistance _ In-Vitro Diagnostics Joint UNICEF, UNFPA & WHO Meeting with Manufacturers and suppliers UN City, Copenhagen, Denmark 24-27 September 2018 Dr Gaby Vercauteren Senior
WHO support and Technical Assistance _ In-Vitro Diagnostics Joint UNICEF, UNFPA & WHO Meeting with Manufacturers and suppliers UN City, Copenhagen, Denmark 24-27 September 2018 Dr Gaby Vercauteren Senior
SPC/CRGA 46 (16) Paper 3 ORIGINAL: ENGLISH
 SPC/CRGA 46 (16) Paper 3 ORIGINAL: ENGLISH FORTY-SIXTH MEETING OF THE COMMITTEE OF REPRESENTATIVES OF GOVERNMENTS AND ADMINISTRATIONS (Noumea, New Caledonia, 28 30 June 2016i) AGENDA ITEM 3: STRATEGIC
SPC/CRGA 46 (16) Paper 3 ORIGINAL: ENGLISH FORTY-SIXTH MEETING OF THE COMMITTEE OF REPRESENTATIVES OF GOVERNMENTS AND ADMINISTRATIONS (Noumea, New Caledonia, 28 30 June 2016i) AGENDA ITEM 3: STRATEGIC
