Differentiation of Human Embryonic Stem Cell (hesc) Derived Pyramidal Neurons
|
|
|
- Randolf Copeland
- 5 years ago
- Views:
Transcription
1 University of Connecticut Honors Scholar Theses Honors Scholar Program Spring Differentiation of Human Embryonic Stem Cell (hesc) Derived Pyramidal Neurons Eagan Jacqueline University of Connecticut - Storrs, jacqueline_eagan@yahoo.com Follow this and additional works at: Part of the Neuroscience and Neurobiology Commons, and the Other Physiology Commons Recommended Citation Jacqueline, Eagan, "Differentiation of Human Embryonic Stem Cell (hesc) Derived Pyramidal Neurons" (2009). Honors Scholar Theses. Paper This Article is brought to you for free and open access by the Honors Scholar Program at DigitalCommons@UConn. It has been accepted for inclusion in Honors Scholar Theses by an authorized administrator of DigitalCommons@UConn. For more information, please contact digitalcommons@uconn.edu.
2 Differentiation of human embryonic stem cell (hesc) derived pyramidal neurons Jacqueline E. Eagan, 1 Radmila Filipovic, 1 Chris Fiondella, 1 Joseph LoTurco, 1 University of Connecticut, Storrs, Department of Physiology and Neurobiology Abstract The mammalian cerebral neocortex is a complex six-layered structure containing multiple types of neurons. Pyramidal neurons of the neocortex are formed during development in an inside-out manner, by which deep layer (DL) neurons are generated first, and upper layer (UL) neurons are generated last. Neurons within the six-layered neocortex express unique markers for their position, showing whether they are subplate, deep layer, upper layer, or Cajal-Retzius neurons. The sequential generation of cortical layers, which exists in vivo, has been partially recapitulated in vitro by differentiation of mouse embryonic stem cells (Gaspard et al., 2008) and human embryonic stem cells (hesc) (Eiraku et al., 2008). The timeline of generation of cortical neurons from hesc is still not well defined, and could be very important in the future of cell therapy. In this study we will define timeline for UL and DL neurons for our experimental paradigm as well as test the effects of fibroblast growth factors (FGF) 2 and 8 on this neuronal differentiation. Recent papers suggest that FGFs are critical for forebrain patterning (Storm et al., 2003). Neuronal differentiation after treatment with either FGF2 or FGF8 from hescs will be examined and the proportion of specific neuronal markers will be analyzed using immunocytochemistry. Our results show that the generated pyramidal neurons will express DL and UL laminar markers in vitro as they do in vivo and that the presence of FGF8 in induction media creates a proliferative effect, while FGF2 induces hesc to differentiate at a higher rate. Introduction The study of human embryonic stem cells (hecs) their pluripotent potential is leading to immense possibility in the future of medicine. hescs have the ability to differentiate into a variety of tissue cells when indicated to do so, including neurons. To be able to differentiate into neurons, hescs must be induced into a neuronal fate in vitro. This could be achieved by subjecting the cells to a serum-free induction media with growth factors that allow them to take on the potential for neuronal differentiation. To start neuronal induction and differentiation, hescs grown on irradiated mouse embryonic fibroblast (irrmef) feeder cells should be mechanically 1
3 scraped off and placed in a suspension of induction media, in which they create clusters called embryoid bodies (EBs). After proliferating as EBs, they should be plated on an adhesion surface to flatten and form neural tube shaped groups of cells termed rosettes that express early neuroectodermal markers such as Pax6 and Sox1 (Elkabetz et al., 2008). Once at rosette stage, the cells are at their highest potential for wide variety differentiation. It has been shown that by implanting rosette cells in vivo, they incorporate themselves into the brain and will test positive for β3-tubulin and nestin, two neurogenenic markers (Zhang et al., 2001) In vitro, these neuronal precursor cells have the ability to develop into different types of neurons when placed in environments with specific growth factors. It is important to isolate rosette cells and either freeze or differentiate them at this point (Elkabetz et al., 2008). We introduced fibroblast growth factor 2 (FGF2) to embryoid bodies and then divided them into two groups. The cultures are then plated as rosettes on poly-d-lysine and laminin substrates and treated with either FGF2 or FGF8. Fibroblast growth factors are proteins that control embryonic neurogenesis by binding receptor tyrosine kinases in high-affinity, which subsequently activates multiple signal transduction pathways (Storm et al., 2001). FGF2 has been shown to increase the dendritic growth, migration and survival of neural stem cells while developing (Vergaño-Vera et al., 2009), while also being crucial to the proliferation of neuroepithelial cells (Zhang et al., 2001.) At rosette stage, cells will be treated with either FGF2 or FGF8. Because of the inclusion of FGF2 or FGF8 in the medium, the majority of these progenitors are expected to produce forebrain pyramidal cells. FGF2 has been proven to allow an improvement of cell growth and colony quality (Zhang et al., 2001). It also directs developing neurons to be more forebrain specified. FGF8 is essential to the normal development of the forebrain; it is mainly expressed along the rostral-most point of the neural ridge, where most pre-anterior forebrain progenitors reside (Storm et al. 2003). Both FGF-2 and FGF-8 will be compared by observing the effect they have on the differentiation phase of the neuronal maturation. After rosette isolation, differentiation will be initiated. Addition of neuronal growth factors, neurotrophin 3 (NT3) and brain derived neurotrophic factor (BDNF) in the cell culture serum-free media induces differentiation. Neuronal progenitor cells have intrinsic signaling, allowing them to follow natural corticogenesis in vitro (Gaspard et al., 2008). Pyramidal neurons, anterior and posterior, are characterized by their monoaxonal shape, excitable behavior and specific markers (Gaspard et al., 2008). They develop from progenitors in the ventricular zone (VZ), and then migrate to deeper and upper cortical layers. When mature, neurons are found in six different cortical layers. DL neurons express signaling genes such as CTIP2 and Sox5, while upper layer neurons 2
4 express SATB2 and CUX1 (Fig. 1)(Gaspard et al., 2008, Leone et al., 2008)(Fig. 1). Using in vitro studies to observe presence of the layer-unique markers allows the possibility create and quantify a natural model of corticogenesis. This marking method gives the ability to identify a timeline of pyramidal neuron positioning. Our goal is to create layer specific pyramidal neurons from hesc and quantify them using immunocytochemistry with the important laminar markers (Fig. 1). Figure 1. Layer markers of the developing cortex. This diagram shows the markers characteristic of neurons in different cortical layers. Main deep layer markers include CTIP2, SOX5 and TRB1 where as upper layer neurons are marked for SATB2 and CUX1 (taken from Gaspard et al., 2008). Reproducing this natural timeline of hescs through pyramidal neurons includes the difficulties of cell culture as well as the precise timing by which the hescs and subsequent developing neurons must be in the presence of specific brain factors in order to mirror corticogenesis in vivo. The intrinsic neocorticogenesis patterning has been found, but mechanisms are still largely unknown. By following the induction and differentiation neuronal media process with these neuronal factors as well as FGF2 and FGF8, it will be possible to observe the fate of neurons derived from stem cells leading to mechanistic clues and methods for future therapy. In the future, this study will allow a better understanding of cortical layer mapping in vitro and the effects of fibroblasts growth factors 2 and 8 on the process of differentiation. Methods hesc culture and Induction of neuronal cell fate Human Embryonic Stem Cell line (H9, University of Connecticut Health Center Stem Cell Core) was grown on top of irrmef for approximately 1 week (n=3). The colonies were then scraped off and grown as a suspension in hesc media [(DMEM/F12, knockout serum replacer (KSR), ß-mercaptoethanol, 2mM L-glutamine, nonessential amino acids (NEAA)] to form free-floating aggregates. After 4 days, the aggregates were switched into high concentration FGF2 containing media (8ng/mL). After the total 3
5 EB propagation for 8 days, the cell aggregates were transferred onto poly-d lysine and laminin coated plates for adhesion and rosette formation. At this point, the cells were switched into neuronal induction media (DMEM/F12, N2 supplement, B27 supplement, 2mM L-glutamine, 4ng/mL FGF) to start priming the cells for neuronal fate. Half of the cells were placed in NIM FGF2 (4ng/mL) and the other half in NIM FGF8 (4ng/mL). Cells were proliferated until rosette-shaped colonies were formed (Fig 2). Differentiation of neurons from hesc After 7 days in NIM with FGF2 or FGF8, the rosettes were digested with trypsin and plated as monolayer cultures on laminin-coated plates in NIM with FGF2 or FGF8 with Y (4ng/mL), a ROCK inhibitor (Fig 2). After one day to adhere, the monolayer cultures were switched to NT3 differentiation medium (DMEM/F12, 2mM L- glutamine, N2 and B27 supplements, NT3 [20ng/mL], BDNF [20ng/mL]). These cells were then fixed and stained on day 7, 14 and 21 from the start of differentiation. Figure 2. Experimental plan to follow for hesc proliferation and differentiation. Floating cell aggregates are made at week 1, plated to form rosettes at week 2 and then trypsinized and plated in differentiation media at week 3.. Immunocytochemistry Cell cultures (n=3) were fixed with 4% paraformaldehyde every 7, 14 and 21 days. After being rinsed with PBS, a blocking solution containing 5% normal goat serum (NGS) and 0.5% Triton-100 was placed on the coverslips for 30 minutes followed by incubation with primary antibodies (overnight, RT) and secondary antibodies (2h, dark RT). The coverslips were then mounted using Vectasheild mounting serum. The primary antibodies are presented in Table 1: 4
6 Primary antibody β3-tubulin Ctip2 SATB2 Sox5 Labeling Immature neurons Deep layer neurons Upper layer neurons Deep layer neurons Type of antibody Antimouse IgG Anti-rabbit IgG Antimouse IgG Anti-rabbit IgG Oct3/4 Stem cells Anti-rabbit IgG Pax6 Nestin Neuroectodermal marker Anti mouse-igg Neural stem cells Antimouse IgG Dilution 1:200 1:200 1:200 1:200 1:200 1:200 1:200 Table 1. Primary antibodies for immunocytochemistry. Table includes: Abbreviated name of protein stained, where this protein is expressed during development, type of immunoglobulin antibody, and working dilution factor. After incubation with primary antibodies, cells were washed with PBS, and the secondary antibodies were added. The secondary antibodies consisted of either goat anti-rabbit IgG Alexa 488 conjugated with fluorescein (1:200, Invitrogen) or goat antimouse IgG Alexa 568. For nuclear staining cells were incubated with ToPro (1:1000, Invitrogen) or/and DAPI. Quantification Cells were viewed under a tabletop Nikon fluorescent microscope or Laica confocal microscope. Each set of cells from days 7,14 and 21 were quantified for the neuronal markers expressed at each time point. The number of β-iii tubulin, an immature neuronal marker, was expressed as a percentage of total cells, stained with DAPI. Five random fields were quantified under 20X magnification. Results Two full rounds of cell cultures were completed allowing us to compare and contrast the effects of FGF2 and FGF8. hecs (H9, University of Connecticut Health Center Stem Cell Core) were successfully grown on irrmef. They began as sharp edged cells with small colonies (Fig. 3A), grew into large, undifferentiated colonies (Fig. 3B) and were ready to be mechanically lifted and grown as undifferentiated cell aggregates, or embryoid bodies, for 8 days (Fig. 3C). Figure 3. Human embryonic stem cells in culture. Plated on irradiated mouse fibroblasts, hescs begin as single cells and then form small colonies (A). (B) is a larger colony of hescs ready to be transferred into a floating aggregate media. These floating undifferentiated aggregates are shown in (C) called neurospheres (NS). 5
7 The embryoid bodies (EB) were then plated on a poly-d lysine substrate to flatten as rosettes in neural induction media. Rosettes showed neural tube morphology (Fig. 4A,B), as well as neuronal precursor staining of nestin (Fig. 4C), Pax 6 (paired box gene), a gene regulating early progenitors to project to telencephalic neocortex (Molyneaux et al.,2007, Eiraku et al., 2008) (Fig. 4D), and Otx2 (orthodenticle homeobox 2)(Fig. 4E). Figure 4. Immunocytochemical representation of rosettes. (A) and (B) are contrast images showing rosette morphology and neural tube similarity. Nestin expression for staining for neuron precursors is present (C), (D) for Pax6 and(e) Otx2 protein staining. 6
8 Laminar marker expression visualized via immunocytochemistry allowed us to track the DL or UL position of the differentiated cells. After one week in neuronal differentiation media with NT3 (20ng/mL) and BDNF (20ng/mL), almost all cells expressed nestin (Fig. 5A,B). Nestin is expressed in neuronal precursor cells and cells that are dividing during early development of the CNS and down-regulates after differentiation. It is clear that these cells are still undifferentiated. β3-tubulin, an immature neuronal marker, which would show differentiation, is expressed very rarely (Fig. 5D). DL laminar markers Ctip2 and Sox5 have begun to express, as shown in Fig. 5 B-D. CTIP2, (COUP-TF) interacting protein 2, plays a dynamic role in the axonal extension and growth of the correct pathway during development of the cerebral cortex (Arlotta et al., 2008). During first week in differentiation media culture, only CTIP2+ neurons are detected (30±3.8%)(Fig. 7). The expression of this protein is strong at one week and Sox 5, another DL marker involved in the neuronal subtype specificity (Lai et al., 2008) is less prevalent and weaker when compared to the cells fixed at two weeks (Fig. 6B,C). Figure 5. Expression of neuronal precursor and laminar markers at week one in differentiation media. Nestin, expressed in neuronal precursors, is present in almost all cells from week 1 of differentiation showing lack of or premature differentiation (A). DL marker Ctip2 is expressed (B), as well as a weaker expression of DL marker Sox5 (C,D). A single differentiated cell, (D, top left, arrow), is shown by expression of β3-tubulin. In the second week of differentiation, the percentage increased (55±14.9%) and Satb2+ upper layer neurons appeared (2.2 ±1.9%)(Fig. 6B, 7) and Sox5, which was not expressed in every cell at week one, is now widespread, showing development (Fig 6C). It is clear that there is strong β3- tubulin in cells from week 2, shown in Figure 6 B-D. The Satb2+ cells along with strong DL neuronal expression 7
9 demonstrates temporal appearance of cortical neurons in vitro from hesc. Figure 6. Expression of laminar markers of differentiated neurons at week two. Early neuronal differentiation is expressed at two weeks by RFP staining of β3-tubulin in all cells (B,C). DL marker, Ctip2, has decreased in strength (B), while Sox5 is expressed in every cell at two weeks (C). Most importantly, UL neuron marker Satb2 is expressed in cells at two weeks (D, arrows). Fig.7. Graphical representation of percentages of upper and deep layer corti neurons in vitro. In a previous study, it was shown that FGF8 is critical in development of the anterior forebrain (Storm et al., 2003) and FGF2 is crucial to forebrain neuronal preparation (Zhang et al., 2001). By adding these separate fibroblast growth factors to the induction media, we came across interesting observations. We tested this as well as the fate of neurons based on their layer specifications. Under identical conditions there were general differences in the general health and behavior of the cultured cells. FGF2 cells aggregated much more with fewer cells in culture than FGF8. However, cells with FGF8 administration showed far less differentiated morphology and a smaller number of β3-tubulin+ immature neurons was noticed in these cultures (Fig. 8A-D). With induction media containing FGF2, at week 2 in differentiation media there was a average percentage of 7.5%±4.9 expression of β3-tubulin compared to the total number of cells shown by DAPI/ToPro expression (n=3). FGF8 inhibited this 8
10 much expression of β3-tubulin, only allowing 2.5%±.7 to express β3-tubulin (n= 3) (Fig. 8D). Furthermore, in week 3, there was an average of 23.3%±12 β3-tubulin expressing cells in FGF2 affected cells (n=4) and only a average of 1.2%±.8 from FGF8 cells (Fig. 8D). These data show that the FGF2 cells differentiated in a higher degree, while FGF8 cells proliferated instead; the cells would still retain the original β3tubulin expression if they had previously differentiated. FGF2 induced differentiating cells to cluster into small colonies, showing high expression of β3-tubulin (Fig. 8B). In contrast, FGF8 kept cells in a monolayer, singlized culture, with few differentiated cells that had long bipolar processes (Fig. 8A). Figure 8. Effects of FGF2 or FGF8 on hesc differentiation into pyramidal neurons. Cells affected with FGF8 show more proliferation, as with TOPRO cell body staining, and few cells differentiating into neurons stained with β3-tubulin (A). FGF2 (B,C) increases the amount of differentiated cells by showing clumps of neurons in which β3-tubulin is strongly expressed. From week 2 to week 3, the percentage of β3-tubulin expressing cells affected with FGF2 increased from 7.5%±4.9 to 23.3%±12, showing differentiation (D); that percentage increase was not found in FGF8 cells. Cells affected with FGF8 showed a decrease from 2.5%±.7 at week 2 to 1.2%±.8 at week 3, showing massive undifferentiated cell proliferation (D). Discussion Human embryonic stem cells are a popular focus of current research studies. Their ability to differentiate into brain cells, bone cells, muscle cells, and epithelial 9
11 cells with the proper extrinsic signaling opens wide doors to possibilities of helping devastating diseases without present cures. In this study we described in vitro generation of neocortical neurons from human embryonic stem cells and the effect of two growth factors, FGF2 and FGF8 on their differentiation. Major findings of the study are as follows: 1. Human cortical neurons follow the same temporal pattern of generation in vitro, as in vivo; 2. Two members of the FGF family, FGF2 and FGF8 have differential effect on neuronal differentiation when added in early phase of cell culture, during neuronal induction. Acknowledging the fact that a human brain develops slower, this study shows in humans what Gaspard et al., 2008, discovered in rodents. Gaspard s previous study showed that nestin down-regulated in between day 14 and 21 in mouse. Our images show that the down regulation begins week 2, or day 29 including EB culture (8days) and rosette formation (7days), showing an initiation of differentiation 2 weeks behind rodent down-regulation. Human stem cells are important to research because this difference between mouse and human stem cell development is very different. Humans, because of an extended lifespan, have more delayed dividing of embryonic stem cells than of a mouse. Downstream of slower division means later differentiation and expression of laminar markers, perhaps withholding the expression of Sox5 in human ES cells. So far, are not studies except one (Eiraku et al., 2008) which focused on the laminar specification of neurons derived from hesc. In cortex deep layers 5 and 6, neurons express Ctip2 and Sox5 (Leone et al., 2008). After week one in differentiation media (NT3, BDNF 20ng/mL), Ctip2 was expressed in 30% of cells, showing that after week 1 of differentiation, 22 days in culture, there was axonal growth from forebrain DL neurons. In comparison, this is earlier than Eiraku et al.. Sox5 was weakly expressed at week one, or day 22 after EB formation, and widespread in week 2, day 29. The probable reason for the difference in expression is the difference between human and rodent neuronal development. The 2008 study of Eiraku et al., which shows DL staining of human neurogenic markers, did not describe UL markers of neurons developed from hesc. Our study shows Satb2 was shown to express after 2 weeks in differentiation media (29 days). Staining at week one for Satb2 (not shown) was negative, showing later expression and the presence of more mature neurons. Neurons that have migrated to the upper layers of the cortex express Satb2 and also Cux1 (not shown.) Satb2 is expressed in neurons in layers 2-4 that have projected into the cortical plate (Leone et al., 2008, Alcamo et al., 2008). This data and our experiment that shows Satb2 was expressed in a few cells after two weeks of differentiation media proves that these differentiated progenitor cells could now be layer 4 neurons. In sequence with Gaspard et al., it 10
12 follows that Satb2 would be expressed at 29 days in human as it was expressed in mouse at day 12, taking into account the delayed brain development in humans. This exemplifies the in vivo recapitulation of human ES cell differentiation in vitro. The growth factors FGF2 and FGF8 have been shown to regulate neocortical patterning in neuronal progenitors. In the study of Storm et al. from 2003, it was found that an elimination or high dosage of FGF8 produced an apoptotic effect, where as lowering the dosage increased cell survival in rodent neuronal progenitors. Here we used a low dosage (4ng/mL) and showed that only 1.2% of cells differentiated into β3-tubulin neurons, in high contrast with the 23.3% from cells treated with FGF2. A more recent study by Storm et al. in 2006 showed that FGF8 mutations led to abnormal telencephalon development due to decreased cell survival and increased cell death, proving that FGF8 does have significant proliferative effects. While FGF2 is expressed throughout the developing CNS, FGF8 is tightly localized to specific regions of the developing brain and is expressed only in the embryo during early phases of proliferation and neurogenesis. FGF8 is expressed first at the rostral most point of the neural plate and then following neural tube closure, in the rostral midline of the telencephalon. FGF8 has a role in initial patterning of mouse telenceplalon, but also in anterior-posterior patterning of the brain (Storm et al., 2003, Fukushi- Shigomori et al., 2001). Our future studies will test for this anterior-posterior patterning and progenitor projection when treated with FGF8 by focusing on markers such as BF-1, Otx2, Emx1 in rostral telencephelon, and COUP-TF1 in caudal telencephelon. By treating with varying dosages of FGF2 vs. FGF8, the underlying rostral or caudal fate of neuronal progenitors could be directed for future therapies. In summary, this study developed a reproducible method for the production of DL and UL neurons in vitro, and shown that FGF2 has a more potent effect for directing neuronal progenitors toward differentiation than FGF8. While not the only significant reason for this study, an example to recreate neurons of specified layers is to assist in the therapy of amyotrophic lateral sclerosis (ALS). UL neurons send projections to subcortical targets, spine, pons, thalamus, and DL neurons send their dendrites of sensory-motor cortex to corticospinal neurons. In ALS, these neurons degenerate. With the specificity of neuronal cortical layering shown, the necessary human stem cell differentiation for the degeneration characteristic of ALS is possible in the future, making this study very relevant to potential therapies for degenerative diseases like ALS. 11
13 References Alcamo EA., Chirivella L., et al. Satb2 Regulates Callosal Projection Neuron Identity in the Developing Cerebral Cortex. Neuron 2008;57: Arlotta, Paola et al. Ctip2 Controls the Differentiation of Medium Spiny Neurons and the Establishment of the Cellular Architecture or the Striatum. Journal of Neuroscience 2008;28(3): Du ZW, Zhang SC. Neural Differentiation from Embryonic Stem Cells: Which Way? Stem Cells and Development 2004; 13: Eiraku M., Watanabe K., et al. Self-Organized Formation of Polarized Cortical Tissues from ESCs and Its Active Manipulation by Extrinsic Signals. Cell Stem Cell 2008;3: Elkabetz Y., et al. Human ES cell-derived neural rosettes reveal a functionally distinct early neural stem cell stage. Genes & Development 2008:22;
14 Fukuchi-Shimogori, T., et al. Molecule Fgf8 Neocortex Patterning by the Sectreted Signaling. Science 2001:294; Gaspard N., et al. An intrisnsic mechanism of corticogenesis from embryonic stem cells. Nature 2008;455; Gu S, Huang H, Bi J, Yao Y, Wen T. Combined treatment of neurotrophin-3 gene and neural stem cells is ameliorative to behavior recovery of Parkinson's disease rat model. Lai T., Jabaudon D., et al. SOX5 Controls the Sequential Generation of Distinct Corticofugal Neuron Subtypes. Neuron 2008;57: Leone DP, Srinivasan K, et al. The determination of projection neuron identity in the developing cerebral cortex. Current Opinion in Neurobiology 2008;18: Li XJ, Zhang SC. Invitro differentiation of neural precursors from human embryonic stem cells. Methods of Mol. Biol. 2006, 331: Molyneaux B.J., Arlotta P., et al. Neuronal subtype specification in the cerebral cortex. Neuroscience 2007;8: Storm EE., et al. Dosage of Fgf8 determines whether cell survival is positively or negatively regulated in the developing forebrain. PNAS 2003:100-4; Storm EE., et al. Dose-dependent functions of Fgf8 in regulating telencephalic patterning centers. Development 2006:131; Zhang SC, Wernig M, Duncan ID, et al. In vitro differentiation of transplantable neural procursors from human embryonic stem cells. Nat Biotechnol 2001;19:
PluriQ TM Serum Replacement (PluriQ TM SR)
 PluriQ TM Serum Replacement (PluriQ TM SR) (PluriQ SR) is a defined, serum-free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated
PluriQ TM Serum Replacement (PluriQ TM SR) (PluriQ SR) is a defined, serum-free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated
PROTOCOL PluriQ Serum Replacement
 (PluriQ SR) is a serum free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated human embryonic stem cells (hesc) and induced
(PluriQ SR) is a serum free supplement formulated as a direct replacement for FBS (fetal bovine serum) used in the growth and maintenance of undifferentiated human embryonic stem cells (hesc) and induced
Culture and differentiation of embryonic stem cells. Hong-Lin Su Department of Life Sciences, National Chung-Hsing University
 Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
Culture and differentiation of embryonic stem cells Hong-Lin Su Department of Life Sciences, National Chung-Hsing University Topics-ES cell maintenance Establishment Culture condition, including the serum,
Therapeutic Cell Replacement. Steven McLoon Department of Neuroscience University of Minnesota
 Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Neuronal Death Neurons are lost due to four main causes: Trauma Toxin Hypoxia (typically loss of air or blood
Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Neuronal Death Neurons are lost due to four main causes: Trauma Toxin Hypoxia (typically loss of air or blood
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
doi:10.1038/nature22047 Supplementary discussion Overall developmental timeline and brain regionalization in human whole-brain organoids We first defined the timeline of generation of broadly-defined cell
Xeno-Free Systems for hesc & hipsc. Facilitating the shift from Stem Cell Research to Clinical Applications
 Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
Components Neural induction basal medium (Cat. # ) 100 ml x 5 Neural induction medium supplement 50x (Cat. # ) 2 ml x 5
 HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
All quality control test results are reported on a lot specific Certificate of Analysis which is available at or upon request.
 PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Application Note. Laminin/Entactin Complex: A Feeder-Free Surface for Culture of Human Embryonic Stem Cells. Introduction
 Laminin/Entactin Complex: A Feeder-Free Surface for Culture of Human Embryonic Stem Cells Deepa Saxena, Ph.D. and Susan Qian, Ph.D. Corning Incorporated, Tewksbury, MA, USA Application Note Contents 1
Laminin/Entactin Complex: A Feeder-Free Surface for Culture of Human Embryonic Stem Cells Deepa Saxena, Ph.D. and Susan Qian, Ph.D. Corning Incorporated, Tewksbury, MA, USA Application Note Contents 1
Protocol Reprogramming Human Fibriblasts using the Dox Inducible Reprogramming Polycistronic Lentivirus Set: Human 4F2A LoxP
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
Therapeutic Cell Replacement. Steven McLoon Department of Neuroscience University of Minnesota
 Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Dec 13) Thursday (Dec 14) Friday (Dec 15) 9:00-10:00am Surdyk s Café in
Therapeutic Cell Replacement Steven McLoon Department of Neuroscience University of Minnesota 1 Course News Coffee Hour Wednesday (Dec 13) Thursday (Dec 14) Friday (Dec 15) 9:00-10:00am Surdyk s Café in
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture. Catalog Number Guidelines for Use
 Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
hpsc Maintenance Media
 hpsc Maintenance Media Brigitte Arduini, version 2, 2013-Jun-12 Initially, it was found that pluripotency of human pluripotent stem cells (hpscs) can be maintained when plated in co-culture with mouse
hpsc Maintenance Media Brigitte Arduini, version 2, 2013-Jun-12 Initially, it was found that pluripotency of human pluripotent stem cells (hpscs) can be maintained when plated in co-culture with mouse
Protocol Using the Reprogramming Ecotropic Retrovirus Set: Mouse OSKM to Reprogram MEFs into ips Cells
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblast (MEF) cells into induced pluripotent stem (ips) cells in a 6-well format. Transduction
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblast (MEF) cells into induced pluripotent stem (ips) cells in a 6-well format. Transduction
Neural induction - Dual SMAD inhibition
 Neural induction - Dual SMAD inhibition Mark J. Tomishima, SKI Stem Cell Research Facility http://stemcells.mskcc.org, tomishim@mskcc.org This protocol is based on Chambers et al. 2009. [Plating pluripotent
Neural induction - Dual SMAD inhibition Mark J. Tomishima, SKI Stem Cell Research Facility http://stemcells.mskcc.org, tomishim@mskcc.org This protocol is based on Chambers et al. 2009. [Plating pluripotent
Protocol Reprogramming MEFs using the Dox Inducible Reprogramming Lentivirus Set: Mouse OKSM
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblasts (MEFs) into induced pluripotent stem (ips) cells in a 6-well format. Transduction
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of mouse embryonic fibroblasts (MEFs) into induced pluripotent stem (ips) cells in a 6-well format. Transduction
Formation of Embryonic Bodies to Produce Neural Stem Cells from ipscs Using the Corning Spheroid Microplate
 Formation of Embryonic Bodies to Produce Neural Stem Cells from ipscs Using the Corning Spheroid Microplate Application Note Wesley Hung and Joanne Hsu Corning Research Center Taiwan, Science & Technology
Formation of Embryonic Bodies to Produce Neural Stem Cells from ipscs Using the Corning Spheroid Microplate Application Note Wesley Hung and Joanne Hsu Corning Research Center Taiwan, Science & Technology
GENESDEV/2007/ Supplementary Figure 1 Elkabetz et al.,
 GENESDEV/2007/089581 Supplementary Figure 1 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 2 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 3 Elkabetz et al., GENESDEV/2007/089581
GENESDEV/2007/089581 Supplementary Figure 1 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 2 Elkabetz et al., GENESDEV/2007/089581 Supplementary Figure 3 Elkabetz et al., GENESDEV/2007/089581
STEMdiff Astrocyte Differentiation Kit and STEMdiff Astrocyte Maturation Kit
 Kit and Kit Catalog #08540 Catalog #08550 1 Kit 1 Kit Product Description Kit (Catalog #08540) is used to rapidly and efficiently generate astrocyte precursors from neural progenitor cells (NPCs) derived
Kit and Kit Catalog #08540 Catalog #08550 1 Kit 1 Kit Product Description Kit (Catalog #08540) is used to rapidly and efficiently generate astrocyte precursors from neural progenitor cells (NPCs) derived
Protocol Reprogramming Human Fibroblasts into ips Cells using the Stemgent Reprogramming Lentivirus Set: Human OKSM
 STEMGENT Page 1 Reprogramming Lentivirus Set: Human OKSM OVERVIEW The following procedure describes the reprogramming of BJ Human Fibroblasts (BJ cells) to induced pluripotent stem (ips) cells using the
STEMGENT Page 1 Reprogramming Lentivirus Set: Human OKSM OVERVIEW The following procedure describes the reprogramming of BJ Human Fibroblasts (BJ cells) to induced pluripotent stem (ips) cells using the
The Thermo Scientific Nunclon Sphera surface supports formation of embryoid bodies from pluripotent stem cells
 1 The Thermo Scientific surface supports formation of embryoid bodies from pluripotent stem cells Louise Gaarn, Amy Sinor-Anderson, Robert Scott, Cindy Neeley, Joseph Granchelli, and Tina Marwood Application
1 The Thermo Scientific surface supports formation of embryoid bodies from pluripotent stem cells Louise Gaarn, Amy Sinor-Anderson, Robert Scott, Cindy Neeley, Joseph Granchelli, and Tina Marwood Application
Induction of Neural Stem Cells from Human Pluripotent Stem Cells Using PSC Neural Induction Medium
 Induction of Neural Stem Cells from Human Pluripotent Stem Cells Using PSC Neural Induction Medium Publication Number MAN0008031 Revision A.0 Introduction Human pluripotent stem cells (PSCs), including
Induction of Neural Stem Cells from Human Pluripotent Stem Cells Using PSC Neural Induction Medium Publication Number MAN0008031 Revision A.0 Introduction Human pluripotent stem cells (PSCs), including
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Rat Cortical Stem Cells
 Rat Cortical Stem Cells Catalog Number NSC001 For cortical stem cell expansion and differentiation into neurons, astrocytes, and oligodendrocytes. This package insert must be read in its entirety before
Rat Cortical Stem Cells Catalog Number NSC001 For cortical stem cell expansion and differentiation into neurons, astrocytes, and oligodendrocytes. This package insert must be read in its entirety before
Matrices Sourcebook Life Technologies extracellular matrices products
 Matrices Sourcebook Life Technologies extracellular matrices products Introduction Our Gibco products, including extracellular matrices, 3D scaffolds, and attachment proteins, are essential tools for providing
Matrices Sourcebook Life Technologies extracellular matrices products Introduction Our Gibco products, including extracellular matrices, 3D scaffolds, and attachment proteins, are essential tools for providing
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex
 Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
Immunofluorescence Confocal Microscopy of 3D Cultures Grown on Alvetex 1.0. Introduction Immunofluorescence uses the recognition of cellular targets by fluorescent dyes or antigen-specific antibodies coupled
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE.
 NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
SUPPLEMENTARY INFORMATION
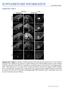 doi:10.1038/nature11094 Supplementary Figures Supplementary Figure 1: Analysis of GFP expression in P0 wild type and mutant ( E1-4) Fezf2-Gfp BAC transgenic mice. Epifluorescent images of sagittal forebrain
doi:10.1038/nature11094 Supplementary Figures Supplementary Figure 1: Analysis of GFP expression in P0 wild type and mutant ( E1-4) Fezf2-Gfp BAC transgenic mice. Epifluorescent images of sagittal forebrain
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW)
 Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
All quality control test results are reported on a lot specific Certificate of Analysis which is available at or upon request.
 PRIME-XV NPC Expansion XSFM PRIME-XV NPC Expansion XSFM is a xeno-and serum-free medium optimized for the cultivation and expansion of neural progenitor cells (NPC) that are able to maintain their ability
PRIME-XV NPC Expansion XSFM PRIME-XV NPC Expansion XSFM is a xeno-and serum-free medium optimized for the cultivation and expansion of neural progenitor cells (NPC) that are able to maintain their ability
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and
 Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Protocols for Neural Progenitor Cell Expansion and Dopaminergic Neuron Differentiation
 Protocols for Neural Progenitor Cell Expansion and Dopaminergic Neuron Differentiation In vitro neurological research presents many challenges due to the difficulty in establishing high-yield neuronal
Protocols for Neural Progenitor Cell Expansion and Dopaminergic Neuron Differentiation In vitro neurological research presents many challenges due to the difficulty in establishing high-yield neuronal
Table S1. Components for knockout serum replacer medium (KSR medium) (500 ml) Component Supplier Catalogue number
 Table S1. Components for knockout serum replacer medium (KSR medium) (500 ml) Component Supplier Catalogue number Stock Concentration Final concentration Volume used Advanced DMEM/F12 Invitrogen 12634010-78%
Table S1. Components for knockout serum replacer medium (KSR medium) (500 ml) Component Supplier Catalogue number Stock Concentration Final concentration Volume used Advanced DMEM/F12 Invitrogen 12634010-78%
Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration?
 Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
ADVANCED MEDIA TECHNOLOGY
 ADVANCED MEDIA TECHNOLOGY For Human ES and ips Cell Research The right medium makes all the difference in ensuring successful ES and ips cell culture. Stemgent offers high-quality, chemically-defined,
ADVANCED MEDIA TECHNOLOGY For Human ES and ips Cell Research The right medium makes all the difference in ensuring successful ES and ips cell culture. Stemgent offers high-quality, chemically-defined,
Sabrina Jedlicka. Interactions of Neurons and Materials
 Sabrina Jedlicka Interactions of Neurons and Materials Developmental Cell Biology Some Perspective Stem cells: Undifferentiated cells that are capable of proliferation, self-maintenance and differentiation
Sabrina Jedlicka Interactions of Neurons and Materials Developmental Cell Biology Some Perspective Stem cells: Undifferentiated cells that are capable of proliferation, self-maintenance and differentiation
Mouse/Rat Neural Stem Cell Functional Identification Kit
 Mouse/Rat Neural Stem Cell Functional Identification Kit Catalog Number SC013 Reagents for the identification of mouse/rat Neural Stem Cells (NSCs) by in vitro functional differentiation. This package
Mouse/Rat Neural Stem Cell Functional Identification Kit Catalog Number SC013 Reagents for the identification of mouse/rat Neural Stem Cells (NSCs) by in vitro functional differentiation. This package
Supplementary Figure 1. Retinogenesis during human embryonic development and in 3-D retinal cups (RCs) derived from hipsc. a, Main steps leading to
 Supplementary Figure 1. Retinogenesis during human embryonic development and in 3-D retinal cups (RCs) derived from hipsc. a, Main steps leading to the formation of the human retina in vivo. b, Diagram
Supplementary Figure 1. Retinogenesis during human embryonic development and in 3-D retinal cups (RCs) derived from hipsc. a, Main steps leading to the formation of the human retina in vivo. b, Diagram
Supporting Information for: Mucin-inspired thermoresponsive synthetic hydrogels induce stasis in human pluripotent stem cells and human embryos
 Supporting Information for: Mucin-inspired thermoresponsive synthetic hydrogels induce stasis in human pluripotent stem cells and human embryos Irene Canton*, Nicholas J. Warren, Aman Chahal, Katherine
Supporting Information for: Mucin-inspired thermoresponsive synthetic hydrogels induce stasis in human pluripotent stem cells and human embryos Irene Canton*, Nicholas J. Warren, Aman Chahal, Katherine
Protocol Using a Dox-Inducible Polycistronic m4f2a Lentivirus to Reprogram MEFs into ips Cells
 STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
STEMGENT Page 1 OVERVIEW The following protocol describes the transduction and reprogramming of one well of Oct4-GFP mouse embryonic fibroblasts (MEF) using the Dox Inducible Reprogramming Polycistronic
Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System
 Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System i Table of Contents 1.0 Introduction... 1 2.0 Materials, Reagents and Equipment... 2 2.1 Materials Required for Neural
Generation and Culture of Neural Progenitor Cells using the STEMdiff Neural System i Table of Contents 1.0 Introduction... 1 2.0 Materials, Reagents and Equipment... 2 2.1 Materials Required for Neural
HUMAN IPSC CULTURE PROTOCOLS
 HUMAN IPSC CULTURE PROTOCOLS FOR TRAINING COURSE IXCELLS BIOTECHNOLOGIES USA, LLC 10340 CAMINO SANTA FE, SUITE C, SAN DIEGO, CA 92121 TABLE OF CONTENTS CHAPTER 1 BEFORE STARTING 2-7 SECTION 1.1 COATING
HUMAN IPSC CULTURE PROTOCOLS FOR TRAINING COURSE IXCELLS BIOTECHNOLOGIES USA, LLC 10340 CAMINO SANTA FE, SUITE C, SAN DIEGO, CA 92121 TABLE OF CONTENTS CHAPTER 1 BEFORE STARTING 2-7 SECTION 1.1 COATING
Matrices sourcebook. Your guide to Gibco extracellular matrices products
 Matrices sourcebook Your guide to Gibco extracellular matrices products Introduction Gibco products, including extracellular matrices, 3D scaffolds, and attachment proteins, are essential tools for providing
Matrices sourcebook Your guide to Gibco extracellular matrices products Introduction Gibco products, including extracellular matrices, 3D scaffolds, and attachment proteins, are essential tools for providing
Cortical Neural Induction Kit. Protocol version 1.0
 Cortical Neural Induction Kit Protocol version 1.0 Protocol version 1.0 Table of Contents Product Information 2 Preparation of Reagents 3 Protocol Overview 4 Seeding ipscs 4 Cortical Neural Induction
Cortical Neural Induction Kit Protocol version 1.0 Protocol version 1.0 Table of Contents Product Information 2 Preparation of Reagents 3 Protocol Overview 4 Seeding ipscs 4 Cortical Neural Induction
Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP)
 Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP) in the cortex (area 1 as defined in Fig. 2a), and corpus
Supplementary Figure S1. Alterations in Fzr1( / );Nestin-Cre brains. (a) P10 Cdh1-deficient brains display low levels of Myelin basic protein (MBP) in the cortex (area 1 as defined in Fig. 2a), and corpus
B-27 Plus Neuronal Culture System
 USER GUIDE B-27 Plus Neuronal Culture System Catalog Number A3653401 Pub. No. MAN0017319 Rev. 1.0 WARNING! Read the Safety Data Sheets (SDSs) and follow the handling instructions. Wear appropriate protective
USER GUIDE B-27 Plus Neuronal Culture System Catalog Number A3653401 Pub. No. MAN0017319 Rev. 1.0 WARNING! Read the Safety Data Sheets (SDSs) and follow the handling instructions. Wear appropriate protective
STEMdiff Mesenchymal Progenitor Kit
 Defined culture kit for derivation and expansion of mesenchymal progenitor cells Catalog #05240 1 Kit Product Description STEMdiff Mesenchymal Progenitor Kit is a defined culture kit consisting of animal
Defined culture kit for derivation and expansion of mesenchymal progenitor cells Catalog #05240 1 Kit Product Description STEMdiff Mesenchymal Progenitor Kit is a defined culture kit consisting of animal
bfgf Supports Human ES Cell Self- Renewal
 APPLICATION NOTE Page 1 bfgf Supports Human ES Cell Self- Renewal Authors: Dongmei Wu, Wen Xiong, Yan Gao, Kristine Guerrero, Yi Chen, Liming Yang, Yang Liu, and Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle
APPLICATION NOTE Page 1 bfgf Supports Human ES Cell Self- Renewal Authors: Dongmei Wu, Wen Xiong, Yan Gao, Kristine Guerrero, Yi Chen, Liming Yang, Yang Liu, and Shuyuan Yao 1 Stemgent, Inc., 10575 Roselle
Human Pluripotent Stem Cell Functional Identification Kit
 Human Pluripotent Stem Cell Functional Identification Kit Catalog Number SC027B Reagents for the identification of human pluripotent stem cells by in vitro functional differentiation. This package insert
Human Pluripotent Stem Cell Functional Identification Kit Catalog Number SC027B Reagents for the identification of human pluripotent stem cells by in vitro functional differentiation. This package insert
Neural induction Dual SMAD inhibition
 Neural induction Dual SMAD inhibition Mark Tomishima, Sloan-Kettering Institute, Center For Stem Cell Biology, New York, NY 10065 US Introduction Dual SMAD inhibition takes a confluent, feeder free culture
Neural induction Dual SMAD inhibition Mark Tomishima, Sloan-Kettering Institute, Center For Stem Cell Biology, New York, NY 10065 US Introduction Dual SMAD inhibition takes a confluent, feeder free culture
StemXVivo. Mesoderm Kit. Catalog Number SC030B. Reagents for the differentiation of human pluripotent stem cells into mesoderm.
 StemXVivo Mesoderm Kit Catalog Number SC030B Reagents for the differentiation of human pluripotent stem cells into mesoderm. This package insert must be read in its entirety before using this product.
StemXVivo Mesoderm Kit Catalog Number SC030B Reagents for the differentiation of human pluripotent stem cells into mesoderm. This package insert must be read in its entirety before using this product.
MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE
 Neurodevelopment MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE UCSD Brain Development Proliferation Differentiation Migration Axon and dendrite development Synaptogenesis Synapse Pruning Apoptosis
Neurodevelopment MARY ET BOYLE, PH.D. DEPARTMENT OF COGNITIVE SCIENCE UCSD Brain Development Proliferation Differentiation Migration Axon and dendrite development Synaptogenesis Synapse Pruning Apoptosis
Sabrina Jedlicka. Interactions of Neurons and Materials
 Sabrina Jedlicka Interactions of Neurons and Materials Neurological Disorders Over 600 known neurological disorders ú Diseases of the CNS and PNS Epilepsy Alzheimer s Parkinson s Etc. ú Diseases that attack
Sabrina Jedlicka Interactions of Neurons and Materials Neurological Disorders Over 600 known neurological disorders ú Diseases of the CNS and PNS Epilepsy Alzheimer s Parkinson s Etc. ú Diseases that attack
StemXVivo. Mouse Oligodendrocyte Differentiation Kit. Catalog Number SC004
 StemXVivo Mouse Oligodendrocyte Differentiation Kit Catalog Number SC004 For the differentiation of mouse pluripotent stem cells to oligodendrocytes. This package insert must be read in its entirety before
StemXVivo Mouse Oligodendrocyte Differentiation Kit Catalog Number SC004 For the differentiation of mouse pluripotent stem cells to oligodendrocytes. This package insert must be read in its entirety before
Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent
 APPLICATION NOTE Page 1 Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent Authors: Amelia L. Cianci 1, Xun Cheng 1 and Kerry P. Mahon 1,2 1 Stemgent Inc.,
APPLICATION NOTE Page 1 Transfection of Mouse ES Cells and Mouse ips cells using the Stemfect 2.0 -mesc Transfection Reagent Authors: Amelia L. Cianci 1, Xun Cheng 1 and Kerry P. Mahon 1,2 1 Stemgent Inc.,
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit
 ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa)
 Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa) Cat. No. EK0474 96 Tests in 8 x 12 divisible strips Background Neurotrophin-3 (NT-3) is a new member of the nerve growth factor gene family which plays an important
Rat Neurotrophin-3 ELISA Kit (rnt-3-elisa) Cat. No. EK0474 96 Tests in 8 x 12 divisible strips Background Neurotrophin-3 (NT-3) is a new member of the nerve growth factor gene family which plays an important
StemXVivo TM. Endoderm Kit. Catalog Number SC019. Reagents for the differentiation of human pluripotent stem cells into definitive endoderm.
 StemXVivo TM Endoderm Kit Catalog Number SC019 Reagents for the differentiation of human pluripotent stem cells into definitive endoderm. This package insert must be read in its entirety before using this
StemXVivo TM Endoderm Kit Catalog Number SC019 Reagents for the differentiation of human pluripotent stem cells into definitive endoderm. This package insert must be read in its entirety before using this
ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming vector for generating induced pluripotent stem (ips)
 Kit for generating ips cells using ReproRNA -OKSGM, a non-integrating, self-replicating RNA reprogramming vector Product Description ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming
Kit for generating ips cells using ReproRNA -OKSGM, a non-integrating, self-replicating RNA reprogramming vector Product Description ReproRNA -OKSGM is a non-integrating, self-replicating RNA-based reprogramming
Xcelthera, Inc. San Diego Regenerative Medicine Institute
 Direct Conversion of Pluripotent Human Embryonic Stem Cells into Functional Human Neuronal or Cardiomyocyte Cell Therapy Derivatives for Regenerative Medicine Xuejun H Parsons, PhD Xcelthera, Inc. San
Direct Conversion of Pluripotent Human Embryonic Stem Cells into Functional Human Neuronal or Cardiomyocyte Cell Therapy Derivatives for Regenerative Medicine Xuejun H Parsons, PhD Xcelthera, Inc. San
ipsc Reprogramming from Human Peripheral Blood Using Sendai Virus Mediated Gene Transfer
 ipsc Reprogramming from Human Peripheral Blood Using Sendai Virus Mediated Gene Transfer Wenli Yang 1, Jason A. Mills 2, Spencer Sullivan 3, Ying Liu 1, Deborah L. French 2 and Paul Gadue 2, 1 Institute
ipsc Reprogramming from Human Peripheral Blood Using Sendai Virus Mediated Gene Transfer Wenli Yang 1, Jason A. Mills 2, Spencer Sullivan 3, Ying Liu 1, Deborah L. French 2 and Paul Gadue 2, 1 Institute
Human ESC-derived Neural Progenitor Expansion Starter Panel. Reagents for the expansion of human neural progenitors.
 Human ESC-derived Neural Progenitor Expansion Starter Panel Catalog Number SC016 Reagents for the expansion of human neural progenitors. This package insert must be read in its entirety before using this
Human ESC-derived Neural Progenitor Expansion Starter Panel Catalog Number SC016 Reagents for the expansion of human neural progenitors. This package insert must be read in its entirety before using this
ApoTrack Cytochrome c Apoptosis ICC Antibody
 ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and nonapoptotic
ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and nonapoptotic
Suggested Method Reprogramming MEF Cells into pips Cells using the Stemgent Recombinant Human Protein Set: OSKM-11R
 Page 1 Reprogramming MEF Cells into pips Cells using the Cat. No. 00-0062 OVERVIEW The procedure outlined below describes the reprogramming of mouse embryonic fibroblasts (MEF) by the addition of four
Page 1 Reprogramming MEF Cells into pips Cells using the Cat. No. 00-0062 OVERVIEW The procedure outlined below describes the reprogramming of mouse embryonic fibroblasts (MEF) by the addition of four
Cells Culture Techniques Marta Czernik
 Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
hpsc Growth Medium DXF Dr. Lorna Whyte
 hpsc Growth Medium DXF Dr. Lorna Whyte 27.06.2014 Training from Heidelberg Overview Background: Stem Cells Introduction: Human Pluripotent Stem Cells (hpsc) vs. Adult Stem Cells Promise of PSC Research
hpsc Growth Medium DXF Dr. Lorna Whyte 27.06.2014 Training from Heidelberg Overview Background: Stem Cells Introduction: Human Pluripotent Stem Cells (hpsc) vs. Adult Stem Cells Promise of PSC Research
Transfection of pluripotent stem cells with Lipofectamine Stem reagent in mtesr1 Medium on Geltrex matrix
 TRANSFECTION PROTOCOL Lipofectamine Stem Transfection Reagent Transfection of pluripotent stem cells with Lipofectamine Stem reagent in mtesr1 Medium on Geltrex matrix PSC growth medium, passaging reagents,
TRANSFECTION PROTOCOL Lipofectamine Stem Transfection Reagent Transfection of pluripotent stem cells with Lipofectamine Stem reagent in mtesr1 Medium on Geltrex matrix PSC growth medium, passaging reagents,
The Ultimate Low Cell Binding Surface
 The Ultimate Low Cell Binding Surface Thermo Scientific Nunc HydroCell Surface SINGLE CELL CULTURES Grow cells that are sensitive to activation and differentiation signals arising from cell adhesion CELL
The Ultimate Low Cell Binding Surface Thermo Scientific Nunc HydroCell Surface SINGLE CELL CULTURES Grow cells that are sensitive to activation and differentiation signals arising from cell adhesion CELL
HUMAN NEURAL CELL-BASED BIOSENSOR FOR ENVIRONMENTAL TOXINS D. W. MACHACEK AND S. K. DHARA AND S. L. STICE ABSTRACT
 HUMAN NEURAL CELL-BASED BIOSENSOR FOR ENVIRONMENTAL TOXINS D. W. MACHACEK AND S. K. DHARA AND S. L. STICE ABSTRACT Environmental toxicants represent a growing concern for both military and civilian agencies.
HUMAN NEURAL CELL-BASED BIOSENSOR FOR ENVIRONMENTAL TOXINS D. W. MACHACEK AND S. K. DHARA AND S. L. STICE ABSTRACT Environmental toxicants represent a growing concern for both military and civilian agencies.
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry of cytochrome c and mitochondria.
 PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
PROTOCOL ApoTrack Cytochrome c Apoptosis ICC Antibody Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSA07 Rev.1 DESCRIPTION ApoTrack Cytochrome c Apoptosis ICC Antibody Kit: 2 color immunocytochemistry
Banking Human Neural Stem Cells for Clinical Applications. Lisa Fox Director, Cell Process Development & Operations
 Banking Human Neural Stem Cells for Clinical Applications Lisa Fox Director, Cell Process Development & Operations 7 th Annual Somatic Cell Therapy Symposium September 27, 2007 Stem Cells Inc. Approach
Banking Human Neural Stem Cells for Clinical Applications Lisa Fox Director, Cell Process Development & Operations 7 th Annual Somatic Cell Therapy Symposium September 27, 2007 Stem Cells Inc. Approach
Department of Clinical Application, Cebter for ips cell Research and Application, 53 Kawahara-cho Shogoin Sakyo-ku, Kyoto,
 Department of Clinical Application, Cebter for ips cell Research and Application, Kyoto University 53 Kawahara-cho Shogoin Sakyo-ku, Kyoto, 606-8507 JAPAN Phone: 81-75-366-7066, Fax: 81-75-366-7071 Daisuke
Department of Clinical Application, Cebter for ips cell Research and Application, Kyoto University 53 Kawahara-cho Shogoin Sakyo-ku, Kyoto, 606-8507 JAPAN Phone: 81-75-366-7066, Fax: 81-75-366-7071 Daisuke
Mouse Neurotrophin-3 ELISA Kit
 OriGene Technologies, Inc 9620 Medical Center Dr., Suite 200, Rockville, MD 20850 Phone: 1.888.267.4436 Fax: 301-340-9254 Email: techsupport@origene.com Web: Mouse Neurotrophin-3 ELISA Kit Catalog No.
OriGene Technologies, Inc 9620 Medical Center Dr., Suite 200, Rockville, MD 20850 Phone: 1.888.267.4436 Fax: 301-340-9254 Email: techsupport@origene.com Web: Mouse Neurotrophin-3 ELISA Kit Catalog No.
Nature Biotechnology: doi: /nbt Supplementary Figure 1. Generation of NSCs from hpscs in SDC medium.
 Supplementary Figure 1 Generation of NSCs from hpscs in SDC medium. (a) Q-PCR of pluripotent markers (OCT4, NANOG), neural markers (SOX1, PAX6, N-Cadherin), markers for the other germ layers (T, EOMES,
Supplementary Figure 1 Generation of NSCs from hpscs in SDC medium. (a) Q-PCR of pluripotent markers (OCT4, NANOG), neural markers (SOX1, PAX6, N-Cadherin), markers for the other germ layers (T, EOMES,
User Manual. OriCell TM Strain 129 Mouse Embryonic Stem Cells With GFP (ESCs/GFP) Cat. No. MUAES IMPI0066A3 MUAES Page 1 of 14
 User Manual OriCell TM Strain 129 Mouse Embryonic Stem Cells With GFP (ESCs/GFP) Cat. No. MUAES-01101 IMPI0066A3 MUAES-01101 Page 1 of 14 Table of Contents Contents and Storage 3 Product Introduction 3
User Manual OriCell TM Strain 129 Mouse Embryonic Stem Cells With GFP (ESCs/GFP) Cat. No. MUAES-01101 IMPI0066A3 MUAES-01101 Page 1 of 14 Table of Contents Contents and Storage 3 Product Introduction 3
Supplemental Information
 Cell Stem Cell, Volume 12 Supplemental Information Human ipsc-derived Oligodendrocyte Progenitor Cells Can Myelinate and Rescue a Mouse Model of Congenital Hypomyelination Su Wang, Janna Bates, Xiaojie
Cell Stem Cell, Volume 12 Supplemental Information Human ipsc-derived Oligodendrocyte Progenitor Cells Can Myelinate and Rescue a Mouse Model of Congenital Hypomyelination Su Wang, Janna Bates, Xiaojie
First xeno-free serum replacement for pluripotent stem cells
 Stem Cell Research First xeno-free serum replacement for pluripotent stem cells KnockOut SR XenoFree Stem Cell Research New, xeno-free growth and expansion of hescs and human ipscs KnockOut SR XenoFree
Stem Cell Research First xeno-free serum replacement for pluripotent stem cells KnockOut SR XenoFree Stem Cell Research New, xeno-free growth and expansion of hescs and human ipscs KnockOut SR XenoFree
CULTURE ENGINEERING DIFFERENTIATION CHARACTERIZATION. Stem Cell Research Solutions Promotional offers
 CULTURE ENGINEERING DIFFERENTIATION CHARACTERIZATION Stem Cell Research Solutions Promotional offers Stem Cell research products For more than a decade, we have provided you with key tools to address challenges
CULTURE ENGINEERING DIFFERENTIATION CHARACTERIZATION Stem Cell Research Solutions Promotional offers Stem Cell research products For more than a decade, we have provided you with key tools to address challenges
Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0
 Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0 Table of Contents General ICC Protocol 3 Synaptic Marker ICC Protocol 5 Recommended Markers 6 Technical Support 7 2 General
Axol Guide to Performing Immunocytochemistry (ICC) Application Protocol Version 2.0 Table of Contents General ICC Protocol 3 Synaptic Marker ICC Protocol 5 Recommended Markers 6 Technical Support 7 2 General
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
The Effects of Serum Starvation on Cell Cycle Synchronization
 OSR Journal of Student Research Volume 1 Article 4 2013 The Effects of Serum Starvation on Cell Cycle Synchronization Negin Baghdadchi California State University, San Bernardino Follow this and additional
OSR Journal of Student Research Volume 1 Article 4 2013 The Effects of Serum Starvation on Cell Cycle Synchronization Negin Baghdadchi California State University, San Bernardino Follow this and additional
BMP-2 and BMP-4 signalling in the developing spinal cord of human and rat embryos
 O R I G I N A L A R T I C L E Folia Morphol. Vol. 74, No. 3, pp. 359 364 DOI: 10.5603/FM.2015.0054 Copyright 2015 Via Medica ISSN 0015 5659 www.fm.viamedica.pl BMP-2 and BMP-4 signalling in the developing
O R I G I N A L A R T I C L E Folia Morphol. Vol. 74, No. 3, pp. 359 364 DOI: 10.5603/FM.2015.0054 Copyright 2015 Via Medica ISSN 0015 5659 www.fm.viamedica.pl BMP-2 and BMP-4 signalling in the developing
Table of Contents. 2.1 NeuroCult NCFC Assay Kit (Rat) Components Additional Required Reagents Required Equipment...
 i Table of Contents 1.0 Overview of the NeuroCult NCFC Assay 2.0 Materials 2.1 NeuroCult NCFC Assay Kit (Rat) Components... 4 2.2 Additional Required Reagents... 4 2.3 Required Equipment... 4 3.0 Preparation
i Table of Contents 1.0 Overview of the NeuroCult NCFC Assay 2.0 Materials 2.1 NeuroCult NCFC Assay Kit (Rat) Components... 4 2.2 Additional Required Reagents... 4 2.3 Required Equipment... 4 3.0 Preparation
Supplementary figures (Zhang)
 Supplementary figures (Zhang) Supplementary figure 1. Characterization of hesc-derived astroglia. (a) Treatment of astroglia with LIF and CNTF increases the proportion of GFAP+ cells, whereas the percentage
Supplementary figures (Zhang) Supplementary figure 1. Characterization of hesc-derived astroglia. (a) Treatment of astroglia with LIF and CNTF increases the proportion of GFAP+ cells, whereas the percentage
Human Neurotrophin-3 ELISA Kit
 Human Neurotrophin-3 ELISA Kit CATALOG NO: IRKTAH5200 LOT NO: SAMPLE INTENDED USE For quantitative detection of human Neurotrophin-3 in cell culture supernates and serum. BACKGROUND Neurotrophin-3 (NT-3)
Human Neurotrophin-3 ELISA Kit CATALOG NO: IRKTAH5200 LOT NO: SAMPLE INTENDED USE For quantitative detection of human Neurotrophin-3 in cell culture supernates and serum. BACKGROUND Neurotrophin-3 (NT-3)
UK +44 (0) CH +41 (0) DE +49 (0) US
 UK +44 (0) 1235 232100- CH +41 (0) 91 604 5522 - DE +49 (0) 69 779099 - US +1 855 267 2464 Featured Product Areas Stem Cell Fate Regulators and Synthetic Retinoid ec23 Recombinant Growth Factor Mimetics
UK +44 (0) 1235 232100- CH +41 (0) 91 604 5522 - DE +49 (0) 69 779099 - US +1 855 267 2464 Featured Product Areas Stem Cell Fate Regulators and Synthetic Retinoid ec23 Recombinant Growth Factor Mimetics
Application Note Use of a Defined, Low-Growth Factor Xeno-Free Culture Medium for the Long-Term Culture of Undifferentiated Human Embryonic Stem Cells
 Undifferentiated Human Authors: Dongmei Wu, M. D., Ph. D., Yan Gao, Wen Xiong, Ph. D., Shuyuan Yao, Ph. D. and Matthew A. Singer, Ph. D. 1 Stemgent, Inc., 10575 Roselle St., San Diego, CA 92121 USA 1 Corresponding
Undifferentiated Human Authors: Dongmei Wu, M. D., Ph. D., Yan Gao, Wen Xiong, Ph. D., Shuyuan Yao, Ph. D. and Matthew A. Singer, Ph. D. 1 Stemgent, Inc., 10575 Roselle St., San Diego, CA 92121 USA 1 Corresponding
Human Induced Pluripotent Stem Cells
 Applied StemCell, Inc. (866) 497-4180 www.appliedstemcell.com Datasheet Human Induced Pluripotent Stem Cells Product Information Catalog Number Description Tissue Age Sex Race Clinical information Quantity
Applied StemCell, Inc. (866) 497-4180 www.appliedstemcell.com Datasheet Human Induced Pluripotent Stem Cells Product Information Catalog Number Description Tissue Age Sex Race Clinical information Quantity
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
Rat Neurotrophin-3 ELISA Kit
 Rat Neurotrophin-3 ELISA Kit GenWay ID: GWB-ZZD026 Size 96T(8 12 divisible strips) For quantitative detection of rat Neurotrophin-3 in cell culture supernates and serum. Typical Data Obtained from Rat
Rat Neurotrophin-3 ELISA Kit GenWay ID: GWB-ZZD026 Size 96T(8 12 divisible strips) For quantitative detection of rat Neurotrophin-3 in cell culture supernates and serum. Typical Data Obtained from Rat
The NYSCF Research Institute
 Regenerative Medicine Research Update Susan L. Solomon The NYSCF Research Institute New York Pharma Forum February 25, 2016 Regenerative Medicine Research Regenerative medicine uses laboratory grown human
Regenerative Medicine Research Update Susan L. Solomon The NYSCF Research Institute New York Pharma Forum February 25, 2016 Regenerative Medicine Research Regenerative medicine uses laboratory grown human
Neural Precursor Cell-Based Screening & Bioassay Kit
 Neural Precursor Cell-Based Screening & Bioassay Kit Catalog Number SC014 Reagents for screening toxins or other bioactive agents for their effects on neural precursor cell proliferation and neuronal differentiation.
Neural Precursor Cell-Based Screening & Bioassay Kit Catalog Number SC014 Reagents for screening toxins or other bioactive agents for their effects on neural precursor cell proliferation and neuronal differentiation.
GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts
 Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Current Biology, Volume 24 Supplemental Information GM130 Is Required for Compartmental Organization of Dendritic Golgi Outposts Wei Zhou, Jin Chang, Xin Wang, Masha G. Savelieff, Yinyin Zhao, Shanshan
Stem Cells & Neurological Disorders. Said Ismail Faculty of Medicine University of Jordan
 Stem Cells & Neurological Disorders Said Ismail Faculty of Medicine University of Jordan Outline: - Introduction - Types & Potency of Stem Cells - Embryonic Stem Cells - Adult Stem Cells - ipscs -Tissue
Stem Cells & Neurological Disorders Said Ismail Faculty of Medicine University of Jordan Outline: - Introduction - Types & Potency of Stem Cells - Embryonic Stem Cells - Adult Stem Cells - ipscs -Tissue
Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays
 Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays Elizabeth J. Abraham, Ph.D. BD Biosciences Outline Overview of surfaces and culture
Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays Elizabeth J. Abraham, Ph.D. BD Biosciences Outline Overview of surfaces and culture
Human Neurotrophin-3 ELISA Kit
 OriGene Technologies, Inc 9620 Medical Center Dr., Suite 200, Rockville, MD 20850 Phone: 1.888.267.4436 Fax: 301-340-9254 Email: techsupport@origene.com Web: Human Neurotrophin-3 ELISA Kit Catalog No.
OriGene Technologies, Inc 9620 Medical Center Dr., Suite 200, Rockville, MD 20850 Phone: 1.888.267.4436 Fax: 301-340-9254 Email: techsupport@origene.com Web: Human Neurotrophin-3 ELISA Kit Catalog No.
