AB. OUR ISO CONFORMANCE AUDIT QUESTIONNAIRES 8. ASSESS HOW WELL YOU CONFORM TO ISO S REMEDIAL REQUIREMENTS
|
|
|
- Brianne Paulina Parker
- 6 years ago
- Views:
Transcription
1 8.1 PLANNING REQUIREMENTS 1 Do you plan monitoring, measurement, and analytical processes? 2 Do you plan how monitoring will be used to ensure conformity and effectiveness? 3 Do you plan how it will be used to ensure that requirements are being met? 4 Do you plan how it will be used to ensure products meet requirements? 5 Do you plan how it will be used to ensure QMS meets requirements? 6 Do you plan how it will be used to maintain the effectiveness of your QMS? 7 Do you plan how measurement will be used to ensure conformity and effectiveness? 8 Do you plan how it will be used to ensure that requirements are being met? 9 Do you plan how it will be used to ensure that products meet requirements? 10 Do you plan how measurement will be used to ensure product conformance? 11 Do you plan how statistical measurement techniques will be used? 12 Do you plan how it will be used to ensure that QMS meets requirements? 13 Do you plan how measurement will be used to ensure QMS conformance? 14 Do you plan how statistical measurements will be used to study QMS? 15 Do you plan how it will be used to maintain the effectiveness of your QMS? 16 Do you plan how statistical measurements will be used to maintain QMS? 17 Do you plan how analytics will be used to ensure conformity and effectiveness? 18 Do you plan how analytics will be used to ensure that requirements are met? 19 Do you plan how they will be used to ensure that products meet requirements? 20 Do you plan how analytics will be used to ensure product conformance? PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 61
2 21 Do you plan how analytical statistical techniques will be used? 22 Do you plan how analytics will be used to ensure QMS meets requirements? 23 Do you plan how analytics will be used to ensure QMS conformance? 24 Do you plan how analytical statistics will be used to study QMS? 25 Do you plan how analytics will be used to maintain the effectiveness of QMS? 26 Do you plan how analytical statistical methods will be used to maintain QMS? 27 Do you use monitoring, measurement, and analytical processes? 28 Do you apply your organization's monitoring methods? 29 Do you monitor your organization's conformance? 30 Do you monitor product conformance? 31 Do you monitor QMS conformance? 32 Do you monitor the effectiveness of your QMS? 33 Do you apply your organization's measurement methods? 34 Do you measure your organization's conformance? 35 Do you measure product conformance? 36 Do you measure QMS conformance? 37 Do you measure the effectiveness of your QMS? 38 Do you apply your organization's analytical methods? 39 Do you analyze your organization's conformance? 40 Do you analyze product conformance? 41 Do you analyze QMS conformance? 42 Do you analyze the effectiveness of your QMS? PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 62
3 8.2 RESEARCH REQUIREMENTS IMPLEMENT SUITABLE FEEDBACK METHODS AND PROCEDURES 43 Have you established feedback methods and procedures? 44 Did you establish customer feedback methods and procedures? 45 Did you establish production feedback methods and procedures? 46 Did you establish post-production feedback methods and procedures? 47 Did you document your feedback methods and procedures? 48 Did you implement your feedback methods and procedures? 49 Do you gather information about your customers? 50 Do you monitor customer perception and satisfaction? 51 Do you find out if customer requirements are being met? 52 Do you gather information about your production activities? 53 Do you gather information about your post-production activities? 54 Do you review your experience if regulations expect you to do so? 55 Do you maintain your feedback methods and procedures? 56 Do you examine the information you have gathered? 57 Do you use your feedback to measure QMS effectiveness? 58 Do you use your feedback to facilitate risk management? 59 Do you use it to monitor and maintain product requirements? 60 Do you use your feedback to support improvement processes? PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 63
4 61 Do you use your feedback to enhance product realization? DEVELOP AND DOCUMENT COMPLAINT HANDLING PROCEDURES 62 Have you established complaint handling procedures? 63 Do you expect procedures to comply with relevant regulations? 64 Do you expect complaints to be handled in a timely manner? 65 Do you expect uninvestigated complaints to be documented? 66 Do you expect people to justify why each complaint is ignored? 67 Do you document your complaint handling procedures? 68 Do you specify related responsibilities and requirements? 69 Do you specify how feedback should be managed? 70 Do you specify how feedback should be received? 71 Do you specify how feedback should be recorded? 72 Do you specify how feedback should be evaluated? 73 Do you explain when feedback is treated as a complaint? 74 Do you specify how complaints should be investigated? 75 Do you specify how complaints are discussed with external parties? 76 Do you explain when information may be exchanged with others? 77 Do you specify how information should be reported? 78 Do you specify when reports are sent to regulatory authorities? 79 Do you specify how remedial action should be initiated? 80 Do you specify when corrections need to be initiated? 81 Do you specify when corrective actions need to be initiated? PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 64
5 82 Do you specify how related products should be handled? 83 Do you specify how related records should be maintained? 84 Do you specify how complaints should be documented? 85 Do you explain how to document uninvestigated complaints? 86 Do you specify how remedial actions should be documented? 87 Do you explain how corrections should be documented? 88 Do you explain how corrective actions should be documented? 89 Did you implement your complaint handling procedures? 90 Do you maintain your complaint handling procedures? ESTABLISH AND MAINTAIN REGULATORY REPORTING PROCEDURES 91 Do you establish reporting procedures when regulators expect you to report to them? 92 Do you create procedures if they expect you to notify them about adverse events? 93 Do you create procedures if they expect you to notify them about advisory notices? 94 Do you document reporting procedures if regulators expect you to report to them? 95 Do you implement reporting procedures if regulators expect you to report to them? 96 Do you notify regulators when complaints meet adverse event reporting criteria? 97 Do you notify regulators when your organization issues official advisory notices? 98 Do you maintain reporting procedures if regulators expect you to report to them? 99 Do you keep a record of your organization's regulatory reports and notifications? PLAN AND PERFORM INTERNAL AUDITS AT PLANNED INTERVALS 100 Have you established an internal audit procedure? 101 Did you document your internal audit procedure? PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 65
6 102 Do you specify how internal audits should be planned? 103 Do you define internal audit planning responsibilities? 104 Do you define internal audit planning requirements? 105 Do you specify how internal audits should be performed? 106 Do you define internal audit performance responsibilities? 107 Do you define internal audit performance requirements? 108 Do you specify how internal audit records should be kept? 109 Do you define internal audit record keeping responsibilities? 110 Do you define internal audit record keeping requirements? 111 Do you specify how internal audit results should be reported? 112 Do you define internal audit reporting responsibilities? 113 Do you define internal audit reporting requirements? 114 Did you implement your organization's internal audit procedure? 115 Do you maintain your organization's internal audit procedure? 116 Do you plan your organization's internal audit program? 117 Do you use your audit procedure to plan your audits? 118 Do you clarify the scope of your internal audits? 119 Do you establish your internal audit criteria? 120 Do you examine the results of previous audits? 121 Do you define and record your audit methods? 122 Do you consider the status and importance of audit areas? 123 Do you give more attention to important audit areas? 124 Do you give more attention to important processes? PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 66
7 125 Do you select impartial and objective auditors? 126 Do you make sure they don't audit their own work? 127 Do you specify how often audits should be performed? 128 Do you schedule internal audits at planned intervals? 129 Do you carry out your internal audits at planned intervals? 130 Do you determine if your organization's QMS meets requirements? 131 Do you find out whether QMS meets ISO requirements? 132 Do you find out whether QMS meets your organization's own requirements? 133 Do you find out whether QMS meets the requirements imposed by regulators? 134 Do you determine if your QMS conforms to planned arrangements? 135 Do you find out whether your QMS conforms to documented arrangements? 136 Do you determine if your organization's QMS is effectively implemented? 137 Do you determine if your QMS is being maintained effectively? 138 Do you maintain a record of audit plans and performance? 139 Do you establish a record of your planned audit program? 140 Do you establish a record of your audit scopes and activities? 141 Do you identify the areas and processes being audited? 142 Do you document your audit conclusions and results? 143 Do you eliminate all detected nonconformities and causes? 144 Do you make corrections and take corrective action without undue delay? 145 Do you expect managers to act whenever problems are found in their areas? 146 Do you follow-up on steps taken to resolve nonconformities? 147 Do you verify the corrections and the corrective actions that are taken? PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 67
8 148 Do you report corrections, corrective actions, and verification results? FIND OUT WHETHER PROCESSES ACHIEVE PLANNED RESULTS 149 Do you establish suitable methods to monitor and measure each QMS process? 150 Do you develop methods that can find out if planned results are being achieved? 151 Do you apply suitable methods to monitor and measure each QMS process? 152 Do you determine whether or not each QMS process is achieving planned results? 153 Do you monitor each QMS process to see if planned results are being achieved? 154 Do you measure each QMS process to see if planned results are being achieved? 155 Do you take remedial action whenever processes fail to achieve planned results? 156 Do you make corrections whenever a process fails to achieve planned results? 157 Do you take corrective action whenever a process fails to achieve planned results? MONITOR AND MEASURE MEDICAL DEVICE CHARACTERISTICS 158 Do you monitor and measure your organization's product characteristics? 159 Do you verify that relevant medical device requirements are being met? 160 Do you verify products at applicable stages during product realization? 161 Do you verify products in accordance with planned arrangements? 162 Do you verify products in accordance with documented arrangements? 163 Etcetera... Now that you've seen a sample of our approach, please consider purchasing our complete ISO Internal Audit Program (Title 47). If you purchase our Internal Audit Program, you'll find that it's integrated, detailed, exhaustive, and easy to understand. We guarantee it. Title 47 is 172 pages long and comes in both pdf and MS Word file formats. PART 8 COPYRIGHT 2016 BY PRAXIOM RESEARCH GROUP LIMITED. ALL RIGHTS RESERVED. PAGE 68
Correlation Matrix & Change Summary
 The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
ISO 9001: 2000 (December 13, 2000) QUALITY MANAGEMENT SYSTEM DOCUMENTATION OVERVIEW MATRIX
 In completing your Documented Quality Management System Review, it is important that the following matrix be completed and returned to us as soon as possible. This will save time during the review and
In completing your Documented Quality Management System Review, it is important that the following matrix be completed and returned to us as soon as possible. This will save time during the review and
A S D T R A N S L A T E D I N T O P L A I N E N G L I S H 8. O P E R A T I O N S
 8.1 DEVELOP, IMPLEMENT, AND CONTROL YOUR OPERATIOL PROCESSES 1 Plan the implementation and control of your operational processes. Black identifies ISO 9001 task. 2 Consider how you're going to implement
8.1 DEVELOP, IMPLEMENT, AND CONTROL YOUR OPERATIOL PROCESSES 1 Plan the implementation and control of your operational processes. Black identifies ISO 9001 task. 2 Consider how you're going to implement
QUALITY SYSTEM MANUAL
 QUALITY SYSTEM MANUAL This Manual is a Proprietary Document and any Unauthorized Reproduction is prohibited. ISSUE DATE July 26, 2012 AUTHORIZED BY: Quality Management Representative Eric Hoff Managing
QUALITY SYSTEM MANUAL This Manual is a Proprietary Document and any Unauthorized Reproduction is prohibited. ISSUE DATE July 26, 2012 AUTHORIZED BY: Quality Management Representative Eric Hoff Managing
Quality and Medical Devices: ISO 13485:2003
 Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
QUALITY MANUAL. Number: M-001 Revision: C Page 1 of 18 THIS DOCUMENT IS CONSIDERED UNCONTROLLED UNLESS ISSUED IDENTIFIED AS CONTROLLED
 Page 1 of 18 THIS DOCUMENT IS CONSIDERED UNCONTROLLED UNLESS ISSUED IDENTIFIED AS CONTROLLED Page 2 of 18 REVISION HISTORY DATE CHANGE DESCRIPTION 10/11/06 Original release 10/21/09 Revised to ISO9001:2008
Page 1 of 18 THIS DOCUMENT IS CONSIDERED UNCONTROLLED UNLESS ISSUED IDENTIFIED AS CONTROLLED Page 2 of 18 REVISION HISTORY DATE CHANGE DESCRIPTION 10/11/06 Original release 10/21/09 Revised to ISO9001:2008
Stanley Industries, Inc. ISO 9001:2008 Quality Policy Manual
 Stanley ISO 9001:2008 Table of Contents and STANLEY Document Reference Related STANLEY Section Page Procedure(s) 1. Introduction 1 None 2. Scope 1 None 3. Organizational Structure & 1 STANLEY Company History
Stanley ISO 9001:2008 Table of Contents and STANLEY Document Reference Related STANLEY Section Page Procedure(s) 1. Introduction 1 None 2. Scope 1 None 3. Organizational Structure & 1 STANLEY Company History
Sections of the Standard. Evidence / Comments. (Y) / Nonconforming (NC)
 Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
ISO 9001:2015 Quality Management System. New/Revised Requirements
 ISO 9001:2015 New/Revised The Quality System Checklist is intended to only identify the new/revised requirements. ISO 9001:2015 requires the adoption of the process approach which extends to internal quality
ISO 9001:2015 New/Revised The Quality System Checklist is intended to only identify the new/revised requirements. ISO 9001:2015 requires the adoption of the process approach which extends to internal quality
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 9001 Quality management systems Requirements Systèmes de management de la qualité Exigences Fourth edition 2008-11-15 Reference number ISO 9001:2008(E) ISO 2008 PDF disclaimer
INTERNATIONAL STANDARD ISO 9001 Quality management systems Requirements Systèmes de management de la qualité Exigences Fourth edition 2008-11-15 Reference number ISO 9001:2008(E) ISO 2008 PDF disclaimer
Clause Map IATF 16949:2016 to ISO/TS 16949:2009
 Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
Correlation matrices between ISO 9001:2008 and ISO 9001:2015
 Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
25 D.L. Martin Drive Mercersburg, PA (717)
 QUALITY MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 14 August 2012 Michael A. White Manager, QA & Engineering D.L. Martin Co. Quality Manual UNCONTROLLED
QUALITY MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 14 August 2012 Michael A. White Manager, QA & Engineering D.L. Martin Co. Quality Manual UNCONTROLLED
Quality Management Evaluation & Audit Policy
 Title Quality Management Evaluation & Audit policy Document ID Director Mark Reynolds Status Final Owner Neil McCrirrick Version 1.1 Author Mark Reynolds Version Date 07/11/2011 Quality Management Evaluation
Title Quality Management Evaluation & Audit policy Document ID Director Mark Reynolds Status Final Owner Neil McCrirrick Version 1.1 Author Mark Reynolds Version Date 07/11/2011 Quality Management Evaluation
QUALITY MANAGEMENT SYSTEM MANUAL ISO 9001:2008
 QUALITY MANAGEMENT SYSTEM MANUAL ISO 9001:2008 Revision: 9 Issue Date: 28 April 2014 CONTROLLED COPY Number: Issued to: UNCONTROLLED COPY A.M.S. Electronics, Inc. 113 Pillow Street, Butler, PA 16001 (724)
QUALITY MANAGEMENT SYSTEM MANUAL ISO 9001:2008 Revision: 9 Issue Date: 28 April 2014 CONTROLLED COPY Number: Issued to: UNCONTROLLED COPY A.M.S. Electronics, Inc. 113 Pillow Street, Butler, PA 16001 (724)
Reliance Aerospace Solutions
 Reliance Aerospace Solutions Quality Manual The information contained in this document is the property of Reliance Aerospace Solutions, a division of Reliance Steel & Aluminum Company This manual is a
Reliance Aerospace Solutions Quality Manual The information contained in this document is the property of Reliance Aerospace Solutions, a division of Reliance Steel & Aluminum Company This manual is a
Summary of ISO 9001:2015 New and Changed Requirements
 This is a summary of the new and changed ISO 9001:2015 requirements compared to ISO 9001:2008. 4. Context of the Organization 4.1 Changes Understanding the Organization and its Context New requirement
This is a summary of the new and changed ISO 9001:2015 requirements compared to ISO 9001:2008. 4. Context of the Organization 4.1 Changes Understanding the Organization and its Context New requirement
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru
 FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
ISO 9001: 2015 Quality Management System Certification. Awareness Training
 ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT
 INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
MDSAP Purchasing Process
 MDSAP Purchasing Process The Medical Device Single Audit Program, MDSAP, evaluates companies on their compliance to requirements based on ISO 13485:2016 and national regulations. An MDSAP audit looks at
MDSAP Purchasing Process The Medical Device Single Audit Program, MDSAP, evaluates companies on their compliance to requirements based on ISO 13485:2016 and national regulations. An MDSAP audit looks at
HACCP audit checklist
 Requirement HACCP audit checklist Prerequisite Program Management Commitment 1. Senior management ensures that the responsibilities and authorities are defined and communicated within the company Internal
Requirement HACCP audit checklist Prerequisite Program Management Commitment 1. Senior management ensures that the responsibilities and authorities are defined and communicated within the company Internal
Beaver Machine. Quality Manual
 Beaver Machine Quality Manual This manual has been written to the ISO 9001:2000 International Quality Standard Beaver Machine Inc. 5273 Hanson Court Minneapolis, MN 55429 763-535-2204 www.beavermachine.com
Beaver Machine Quality Manual This manual has been written to the ISO 9001:2000 International Quality Standard Beaver Machine Inc. 5273 Hanson Court Minneapolis, MN 55429 763-535-2204 www.beavermachine.com
APS Cleaning Quality Management System Scope of Certification The provision of commercial and industrial cleaning services throughout Queensland.
 Quality Management System Scope of Certification The provision of commercial and industrial cleaning services throughout Queensland. Table of Contents Contents 1. Introduction... 3 1.1. Process Approach...
Quality Management System Scope of Certification The provision of commercial and industrial cleaning services throughout Queensland. Table of Contents Contents 1. Introduction... 3 1.1. Process Approach...
ISO 22000:2005 Standard INTERNATIONAL STANDARDS REGISTRATIONS
 ISO 22000:2005 Standard Food Safety Management System INTERNATIONAL STANDARDS REGISTRATIONS 3.1 FOOD SAFETY concept that food will not cause harm to the consumer when it is prepared and/or eaten according
ISO 22000:2005 Standard Food Safety Management System INTERNATIONAL STANDARDS REGISTRATIONS 3.1 FOOD SAFETY concept that food will not cause harm to the consumer when it is prepared and/or eaten according
Quality Manual Revision: C Effective: 03/01/10
 TABLE OF CONTENTS DESCRIPTION SECTION PAGE INTRODUCTION 1.0 1 APPROVAL SIGNATURE PAGE 1.1 1 AMENDMENT RECORD 1.2 2 SCOPE 2.0 3 EXCLUSIONS 2.1 3 CORPORATE POLICY 3.0 3 QUALITY MANAGEMENT SYSTEM 4.0 4 GENERAL
TABLE OF CONTENTS DESCRIPTION SECTION PAGE INTRODUCTION 1.0 1 APPROVAL SIGNATURE PAGE 1.1 1 AMENDMENT RECORD 1.2 2 SCOPE 2.0 3 EXCLUSIONS 2.1 3 CORPORATE POLICY 3.0 3 QUALITY MANAGEMENT SYSTEM 4.0 4 GENERAL
14620 Henry Road Houston, Texas PH: FX: WEB: QUALITY MANUAL
 14620 Henry Road Houston, Texas 77060 PH: 281-447-3980 FX: 281-447-3988 WEB: www.texasinternational.com QUALITY MANUAL ISO 9001:2008 API Spec Q1, 9th Edition API Spec 8C 5 Th Edition MANUAL NUMBER: Electronic
14620 Henry Road Houston, Texas 77060 PH: 281-447-3980 FX: 281-447-3988 WEB: www.texasinternational.com QUALITY MANUAL ISO 9001:2008 API Spec Q1, 9th Edition API Spec 8C 5 Th Edition MANUAL NUMBER: Electronic
Quality Manual ISSUED JANUARY Approved By: January 12, 2004 (President & Chief Executive Officer)
 Quality Manual ISSUED JANUARY 2004 Approved By: January 12, 2004 (President & Chief Executive Officer) (Date) Quality Policy To be the industrial control industry's most preferred supplier of sensor integration
Quality Manual ISSUED JANUARY 2004 Approved By: January 12, 2004 (President & Chief Executive Officer) (Date) Quality Policy To be the industrial control industry's most preferred supplier of sensor integration
ISO 9001:2008 Quality Management System QMS Manual
 2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
Revision. Quality Manual. Multilayer Prototypes. Compliant to ISO / AS9100 Rev C
 1 of 29 Quality Manual Multilayer Prototypes Compliant to ISO 9001-2008 / AS9100 Rev C This Quality Manual sets forth the quality system policies and Defines compliance with the ISO 9001-2008 SAE AS 9100
1 of 29 Quality Manual Multilayer Prototypes Compliant to ISO 9001-2008 / AS9100 Rev C This Quality Manual sets forth the quality system policies and Defines compliance with the ISO 9001-2008 SAE AS 9100
Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry
 Addendum 1 June 2010 Effective Date: December 1, 2010 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry ANSI/API SPECIFICATION Q1 EIGHTH EDITION, DECEMBER 2007
Addendum 1 June 2010 Effective Date: December 1, 2010 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry ANSI/API SPECIFICATION Q1 EIGHTH EDITION, DECEMBER 2007
QUALITY MANAGEMENT SYSTEM QUALITY MANUAL ISO 9001:2008
 QUALITY MANAGEMENT SYSTEM QUALITY MANUAL ISO 9001:2008 EXPRESS CONTRACTING SERVICES PTY LTD TRADING AS GOLDEN BROWN CLEANING SERVICES Unit 8/217 Mickleham Road Tullamarine VIC 3043 www.goldenbrown.com.au
QUALITY MANAGEMENT SYSTEM QUALITY MANUAL ISO 9001:2008 EXPRESS CONTRACTING SERVICES PTY LTD TRADING AS GOLDEN BROWN CLEANING SERVICES Unit 8/217 Mickleham Road Tullamarine VIC 3043 www.goldenbrown.com.au
P. 1. Identify the Differences between ISO9001:2000 與 ISO9001:2008 ISO9001:2008 ISO9001:2000 版本的異同. 5 January 2009 ISO 9000 SERIES
 Identify the Differences between ISO9001:2000 and ISO 9001:2008 審視 ISO9001:2000 與 ISO9001:2008 版本的異同 ISO 9000 SERIES ISO 19011 ISO9000 5 January 2009 ISO9001 ISO9004 2 ISO 9000 SERIES ISO 9001 ISO 9000
Identify the Differences between ISO9001:2000 and ISO 9001:2008 審視 ISO9001:2000 與 ISO9001:2008 版本的異同 ISO 9000 SERIES ISO 19011 ISO9000 5 January 2009 ISO9001 ISO9004 2 ISO 9000 SERIES ISO 9001 ISO 9000
Objective Evidence Record (OER)
 This material is derived from SAE AS9101D, which is copyrighted intellectual property of SAE international. SAE is not responsible for outcomes resulting from use of this material. APPENDIX A OBJECTIVE
This material is derived from SAE AS9101D, which is copyrighted intellectual property of SAE international. SAE is not responsible for outcomes resulting from use of this material. APPENDIX A OBJECTIVE
AEROSPACE STANDARD. Quality Systems - Aerospace - Model for Quality Assurance in Design, Development, Production, Installation and Servicing
 AEROSPACE STANDARD AS9100 Technically equivalent to AECMA pren 9100 Issued 1999-11 Revised 2001-08 Superseding AS9100 REV. A Quality Systems - Aerospace - Model for Quality Assurance in Design, Development,
AEROSPACE STANDARD AS9100 Technically equivalent to AECMA pren 9100 Issued 1999-11 Revised 2001-08 Superseding AS9100 REV. A Quality Systems - Aerospace - Model for Quality Assurance in Design, Development,
QUALITY SYSTEM MANUAL
 TITLE: QUALITY SYSTEM MANUAL Page 1 of 15 QUALITY SYSTEM MANUAL TITLE: QUALITY SYSTEM MANUAL Page 2 of 15 Index PARAGRAPH TITLE... PAGE 1.0 GENERAL INFORMATION... 3 2.0 DEFINITIONS... 5 3.0 RELATED DOCUMENTS...
TITLE: QUALITY SYSTEM MANUAL Page 1 of 15 QUALITY SYSTEM MANUAL TITLE: QUALITY SYSTEM MANUAL Page 2 of 15 Index PARAGRAPH TITLE... PAGE 1.0 GENERAL INFORMATION... 3 2.0 DEFINITIONS... 5 3.0 RELATED DOCUMENTS...
FINAL DOCUMENT. International Medical Device Regulators Forum. Medical Device Regulatory Audit Reports
 FINAL DOCUMENT International Medical Device Regulators Forum Title: Authoring Group: Medical Device Regulatory Audit Reports IMDRF MDSAP Working Group Date: 2 October 2015 Toshiyoshi Tominaga, IMDRF Chair
FINAL DOCUMENT International Medical Device Regulators Forum Title: Authoring Group: Medical Device Regulatory Audit Reports IMDRF MDSAP Working Group Date: 2 October 2015 Toshiyoshi Tominaga, IMDRF Chair
Webster - Hoff Corporation. Quality Manual
 Registered to ISO 9001:2008 ANSI/ISO/ASQ Q9001-2008 QM-1 Page 2 of 35 TABLE OF CONTENTS Section Name Page Management Approval Of / List of Exclusions 3 Corporate Quality Policy 4 Webster-Hoff Corporation,
Registered to ISO 9001:2008 ANSI/ISO/ASQ Q9001-2008 QM-1 Page 2 of 35 TABLE OF CONTENTS Section Name Page Management Approval Of / List of Exclusions 3 Corporate Quality Policy 4 Webster-Hoff Corporation,
Quality Management System Guidance. ISO 9001:2015 Clause-by-clause Interpretation
 Quality Management System Guidance ISO 9001:2015 Clause-by-clause Interpretation Table of Contents 1 INTRODUCTION... 4 1.1 IMPLEMENTATION & DEVELOPMENT... 5 1.2 MANAGING THE CHANGE... 5 1.3 TOP MANAGEMENT
Quality Management System Guidance ISO 9001:2015 Clause-by-clause Interpretation Table of Contents 1 INTRODUCTION... 4 1.1 IMPLEMENTATION & DEVELOPMENT... 5 1.2 MANAGING THE CHANGE... 5 1.3 TOP MANAGEMENT
Document ID: Revision: Date. Approved:
 Document ID: Q&EMSM Standard: ISO 9001 / ISO 14001 Title: Quality and Environmental Management System Manual Approved By: Revision: ED170616 Date Approved: 6/26/17 Quality and Environmental Management
Document ID: Q&EMSM Standard: ISO 9001 / ISO 14001 Title: Quality and Environmental Management System Manual Approved By: Revision: ED170616 Date Approved: 6/26/17 Quality and Environmental Management
PRODUCTS AND SERVICES:
 COMPANY INFORMATION: Company Name: Newcastle Aviation Partners, LLC Address: 3201 West County Road 42, Unit 104 Burnsville, MN 55306 Phone: 952-223-0317 Facsimile: 952-223-4470 AOG phone number: 952-223-0317,
COMPANY INFORMATION: Company Name: Newcastle Aviation Partners, LLC Address: 3201 West County Road 42, Unit 104 Burnsville, MN 55306 Phone: 952-223-0317 Facsimile: 952-223-4470 AOG phone number: 952-223-0317,
Fitting ISO 9001:2000 into a 20 Element Quality System
 Fitting ISO 9001:2000 into a 20 Element Quality System ASQ Washington DC Section 0509 Rockville Maryland Presented by Norman P. Moreau, P.E., CSQE, CQA President Theseus Professional Services 410-857-0023
Fitting ISO 9001:2000 into a 20 Element Quality System ASQ Washington DC Section 0509 Rockville Maryland Presented by Norman P. Moreau, P.E., CSQE, CQA President Theseus Professional Services 410-857-0023
Management System Policy and Procedure Manual. Based on the requirements of ISO17021, AS9104 and Associated ANAB Accreditation Rules
 1 Great Western Registrar LLC Management System Policy and Procedure Manual Based on the requirements of ISO17021, AS9104 and Associated ANAB Accreditation Rules 08/01/2017 Created and Approved by: Karey
1 Great Western Registrar LLC Management System Policy and Procedure Manual Based on the requirements of ISO17021, AS9104 and Associated ANAB Accreditation Rules 08/01/2017 Created and Approved by: Karey
ISO 14001: 2015 Environmental Gap Analysis
 Environmental Gap Analysis The revised ISO 14001 standard was published on 14 TH September 2015. How to use this document This document provides an overview of the changes between ISO 14001:2004 and ISO
Environmental Gap Analysis The revised ISO 14001 standard was published on 14 TH September 2015. How to use this document This document provides an overview of the changes between ISO 14001:2004 and ISO
CORRECTIVE AND PREVENTIVE ACTION. Co-X/QHS/SOP06
 CONVENTION & EXHIBITION (PUTRAJAYA) SDN. BHD. Co-X/QHS/SOP06 Revision No.: 03 Effective Date: 1 st November 2017 PREPARED BY REVIEWED BY APPROVED BY Name: Name: Name: Position: Position: Position: REFERENCE
CONVENTION & EXHIBITION (PUTRAJAYA) SDN. BHD. Co-X/QHS/SOP06 Revision No.: 03 Effective Date: 1 st November 2017 PREPARED BY REVIEWED BY APPROVED BY Name: Name: Name: Position: Position: Position: REFERENCE
QM-1 QUALITY MANAGEMENT SYSTEMS MANUAL. Revision 10
 QM-1 QUALITY MANAGEMENT SYSTEMS MANUAL Revision 10 Page: 1 of 54 TABLE OF CONTENTS 1. DOCUMENT REVISION HISTORY... 5 2. INTRODUCTION... 6 2.1 Purpose... 6 2.2 Scope... 7 Figure 1: Sequence and Interaction
QM-1 QUALITY MANAGEMENT SYSTEMS MANUAL Revision 10 Page: 1 of 54 TABLE OF CONTENTS 1. DOCUMENT REVISION HISTORY... 5 2. INTRODUCTION... 6 2.1 Purpose... 6 2.2 Scope... 7 Figure 1: Sequence and Interaction
SUPPLIER QUALITY SYSTEM SURVEY
 SUPPLIER QUALITY SYSTEM SURVEY DATE (M/d/yy): 55 Dragon Court Woburn, MA 01801 Tel: (781) 933-7300 Fax: (781) 935-4529 GENERAL INFORMATION Supplier Name: Physical Address: City: State: Zip: Sr. Company
SUPPLIER QUALITY SYSTEM SURVEY DATE (M/d/yy): 55 Dragon Court Woburn, MA 01801 Tel: (781) 933-7300 Fax: (781) 935-4529 GENERAL INFORMATION Supplier Name: Physical Address: City: State: Zip: Sr. Company
Analysis of ISO 9001:2015 against the ICoCA Certification Assessment Framework
 Analysis of ISO 9001:2015 against the ICoCA Certification Assessment Framework As detailed in the ICoCA Certification Procedure, the Board of Directors assesses and recognizes standards for potential recognition
Analysis of ISO 9001:2015 against the ICoCA Certification Assessment Framework As detailed in the ICoCA Certification Procedure, the Board of Directors assesses and recognizes standards for potential recognition
ISO 9001 QUALITY MANUAL
 ISO 9001 QUALITY MANUAL Origination Date: 10/01/14 Document Identifier: AIF quality control manual Date: 10/01/14 Project: Customer review Document Status: Released Document Link: www.aeroindfast.com Abstract:
ISO 9001 QUALITY MANUAL Origination Date: 10/01/14 Document Identifier: AIF quality control manual Date: 10/01/14 Project: Customer review Document Status: Released Document Link: www.aeroindfast.com Abstract:
ISO/DIS 9001: 2014 comparison with ISO 9001:2008. ISO 9001:2015 Updates. (Based on Draft International Standard, DIS) ISO/DIS 9001 ISO 9001:2008
 ISO 9001:2015 Updates (Based ondraft International Standard, DIS) August 2014 Page 1 ISO 9001:2015 Updates (Based on Draft International Standard, DIS) ISO/DIS 9001: 2014 comparison with ISO 9001:2008
ISO 9001:2015 Updates (Based ondraft International Standard, DIS) August 2014 Page 1 ISO 9001:2015 Updates (Based on Draft International Standard, DIS) ISO/DIS 9001: 2014 comparison with ISO 9001:2008
Desk Audit of. Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT
 Desk Audit of Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT-90-5001-02.1 Reviewed by: Element Requirements Applicable 1. Is a quality policy defined
Desk Audit of Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT-90-5001-02.1 Reviewed by: Element Requirements Applicable 1. Is a quality policy defined
Continual Improvement
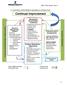 15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
Required Elements of a Quality Management System
 Quality Management System Review Procedures IAPMO Evaluation Service (IAPMO UES) No.: ES-010 Title: Review Procedures for Certified Manufacturer s Quality Management System (QMS) Documentation By: B. Berneche
Quality Management System Review Procedures IAPMO Evaluation Service (IAPMO UES) No.: ES-010 Title: Review Procedures for Certified Manufacturer s Quality Management System (QMS) Documentation By: B. Berneche
Quality Manual ISO 9001:2000
 Quality Manual ISO 9001:2000 Page 2 of 23 TABLE OF CONTENTS COVER PAGE...1 TABLE OF CONTENTS...2 SIGNATURES...3 QUALITY POLICY...4 INTRODUCTION...5 CORPORATE PROFILE...9 4.O QUALITY MANAGEMENT SYSTEM...10
Quality Manual ISO 9001:2000 Page 2 of 23 TABLE OF CONTENTS COVER PAGE...1 TABLE OF CONTENTS...2 SIGNATURES...3 QUALITY POLICY...4 INTRODUCTION...5 CORPORATE PROFILE...9 4.O QUALITY MANAGEMENT SYSTEM...10
YOUR CERTIFICATION PROCESS EXPLAINED
 ISO 22000 FOOD SAFETY MANAGEMENT SYSTEMS FSSC 22000 FOOD SAFETY SYSTEM CERTIFICATION This document outlines the audit process for the above referenced standard. It outlines the stages to audit and gives
ISO 22000 FOOD SAFETY MANAGEMENT SYSTEMS FSSC 22000 FOOD SAFETY SYSTEM CERTIFICATION This document outlines the audit process for the above referenced standard. It outlines the stages to audit and gives
SUPPLIER QUALITY ASSESSMENT
 Supplier Organization Name: Supplier Number: Street Address: Date of This Audit: City, State, Zip Code: Date of Last Audit: Country: of Employees: Main Phone Number: of Buildings/Size: Fax Number: Principal
Supplier Organization Name: Supplier Number: Street Address: Date of This Audit: City, State, Zip Code: Date of Last Audit: Country: of Employees: Main Phone Number: of Buildings/Size: Fax Number: Principal
SAI Global Full Service Team
 General information regarding elements of the certification process is described below. A degree of flexibility and options in the certification process are available so please feel free to contact us
General information regarding elements of the certification process is described below. A degree of flexibility and options in the certification process are available so please feel free to contact us
List of Documented Information (Document & Records)
 As per IATF 16949:2016 Clause Type Clause Reference 4.3 Determining the scope of the quality management system 5.1.1.1 Corporate responsibility The only permitted exclusion for this Automotive QMS Standard
As per IATF 16949:2016 Clause Type Clause Reference 4.3 Determining the scope of the quality management system 5.1.1.1 Corporate responsibility The only permitted exclusion for this Automotive QMS Standard
ISCC 204 AUDIT REQUIREMENTS AND RISK MANAGEMENT. Version 3.0
 ISCC 204 AUDIT REQUIREMENTS AND RISK MANAGEMENT Version 3.0 II Copyright notice 2016 ISCC System GmbH This ISCC document is protected by copyright. It is freely available from the ISCC website or upon
ISCC 204 AUDIT REQUIREMENTS AND RISK MANAGEMENT Version 3.0 II Copyright notice 2016 ISCC System GmbH This ISCC document is protected by copyright. It is freely available from the ISCC website or upon
ISO /TS 29001:2010 SYSTEMKARAN ADVISER & INFORMATION CENTER SYSTEM KARAN ADVISER & INFORMATION CENTER
 SYSTEM KARAN ADVISER & INFORMATION CENTER PETROLEUM, PETROCHEMICAL AND NATURAL GAS INDUSTRIES -- SECTOR-SPECIFIC QUALITY MANAGEMENT SYSTEMS -- REQUIREMENTS FOR PRODUCT AND SERVICE SUPPLY ORGANIZATIONS
SYSTEM KARAN ADVISER & INFORMATION CENTER PETROLEUM, PETROCHEMICAL AND NATURAL GAS INDUSTRIES -- SECTOR-SPECIFIC QUALITY MANAGEMENT SYSTEMS -- REQUIREMENTS FOR PRODUCT AND SERVICE SUPPLY ORGANIZATIONS
ISO 9001:2015 READINESS CHECKLIST YOU RE CLOSER THAN YOU THINK EXECUTIVE SUMMARY CLAUSE 4 - CONTEXT OF THE ORGANISATION CLAUSE 5 - LEADERSHIP
 EXECUTIVE SUMMARY CLAUSE 4 - CONTEXT OF THE ORGANISATION CLAUSE 5 - LEADERSHIP CLAUSE 6 - PLANNING CLAUSE 7 - RESOURCES CLAUSE 8 - OPERATIONS CLAUSE 9 - PERFORMANCE EVALUATION CLAUSE 10 - IMPROVEMENTS
EXECUTIVE SUMMARY CLAUSE 4 - CONTEXT OF THE ORGANISATION CLAUSE 5 - LEADERSHIP CLAUSE 6 - PLANNING CLAUSE 7 - RESOURCES CLAUSE 8 - OPERATIONS CLAUSE 9 - PERFORMANCE EVALUATION CLAUSE 10 - IMPROVEMENTS
DEVELOPEMENT OF A PRODUCTION ORGANIZATION EXPOSITION SCHEME TAKING INTO ACCOUNT EN9100 REQUIREMENTS
 25 TH INTERNATIONAL CONGRESS OF THE AERONAUTICAL SCIENCES DEVELOPEMENT OF A PRODUCTION ORGANIZATION EXPOSITION SCHEME TAKING INTO ACCOUNT REQUIREMENTS Reyhaneh SAYFI*, Florence Leblond** * Civil Aviation
25 TH INTERNATIONAL CONGRESS OF THE AERONAUTICAL SCIENCES DEVELOPEMENT OF A PRODUCTION ORGANIZATION EXPOSITION SCHEME TAKING INTO ACCOUNT REQUIREMENTS Reyhaneh SAYFI*, Florence Leblond** * Civil Aviation
This procedure is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without prior written consent.
 www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This procedure is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This procedure is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
Harmonization day 2015 FSSC audit reporting
 Harmonization day 2015 FSSC 22000 audit reporting Today s topics 1. Basics audit reporting 2. Definition audit report 3. Audit report requirements 4. Conclusions 1. Basics audit reporting Starting point
Harmonization day 2015 FSSC 22000 audit reporting Today s topics 1. Basics audit reporting 2. Definition audit report 3. Audit report requirements 4. Conclusions 1. Basics audit reporting Starting point
Comparison ISO/TS (1999) to QS 9000, 3 rd edition (1998)
 1 SCOPE QS 9000: new: Introduction, applicability In addition to the applicability for supplier sites for production and services and their subcontractors for: - parts or materials, or - services like
1 SCOPE QS 9000: new: Introduction, applicability In addition to the applicability for supplier sites for production and services and their subcontractors for: - parts or materials, or - services like
ADVANCED INTERNAL AUDIT WORKSHOP
 ADVANCED INTERNAL AUDIT WORKSHOP By Paul J. Kunder RABQSA QMS Lead Auditor/Aerospace Industry Experienced Auditor RABQSA Approved Aerospace Auditor Transition Training (AATT) Trainer Vice Chair U.S. Z1A
ADVANCED INTERNAL AUDIT WORKSHOP By Paul J. Kunder RABQSA QMS Lead Auditor/Aerospace Industry Experienced Auditor RABQSA Approved Aerospace Auditor Transition Training (AATT) Trainer Vice Chair U.S. Z1A
How to be an Effective GMP Auditor
 The Daily Wrap-up (For multiple day audits) Classify Audit Observations The Close-Out Meeting Phase 3:Preparation, Approval and Distribution of the Audit Report Preparation of the audit report Approve
The Daily Wrap-up (For multiple day audits) Classify Audit Observations The Close-Out Meeting Phase 3:Preparation, Approval and Distribution of the Audit Report Preparation of the audit report Approve
KEVLIN CORPORATION DIVISION OF COBHAM DEFENSE ELECTRONIC SYSTEMS
 1.doc KEVLIN CORPORATION DIVISION OF COBHAM DEFENSE ELECTRONIC SYSTEMS QUALITY POLICY MANUAL QPM 4. 596 Lowell St Methuen, MA 01844 USA 2.doc TABLE OF CONTENTS Clauses Procedure Description of Contents
1.doc KEVLIN CORPORATION DIVISION OF COBHAM DEFENSE ELECTRONIC SYSTEMS QUALITY POLICY MANUAL QPM 4. 596 Lowell St Methuen, MA 01844 USA 2.doc TABLE OF CONTENTS Clauses Procedure Description of Contents
ISO 9001 Auditing Practices Group Guidance on:
 International Organization for Standardization International Accreditation Forum Date: 13 January 2016 ISO 9001 Auditing Practices Group Guidance on: What do we mean by adding value? Adding Value We hear
International Organization for Standardization International Accreditation Forum Date: 13 January 2016 ISO 9001 Auditing Practices Group Guidance on: What do we mean by adding value? Adding Value We hear
ISO/TS TECHNICAL SPECIFICATION
 TECHNICAL SPECIFICATION ISO/TS 29001 Third edition 2010-06-01 Petroleum, petrochemical and natural gas industries Sector-specific quality management systems Requirements for product and service supply
TECHNICAL SPECIFICATION ISO/TS 29001 Third edition 2010-06-01 Petroleum, petrochemical and natural gas industries Sector-specific quality management systems Requirements for product and service supply
GENERAL QUALITY REQUIREMENTS FOR PURCHASED PARTS
 GENERAL QUALITY REQUIREMENTS FOR PURCHASED PARTS The following requirements shall apply to all the suppliers of the associated companies of SELCOM GROUP S.p.A., referred as SELCOM GROUP in the General
GENERAL QUALITY REQUIREMENTS FOR PURCHASED PARTS The following requirements shall apply to all the suppliers of the associated companies of SELCOM GROUP S.p.A., referred as SELCOM GROUP in the General
ISMS AUDIT CHECKLIST
 4.1 REQUIREMENT REFER TO BS ISO / IEC 27001 : 2005 Has the organisation developed a documented ISMS based on the PDCA model? Checked at Stage 1 for development and Stage 2/surveillance for implementation,
4.1 REQUIREMENT REFER TO BS ISO / IEC 27001 : 2005 Has the organisation developed a documented ISMS based on the PDCA model? Checked at Stage 1 for development and Stage 2/surveillance for implementation,
J. McCann & Co (Nottm) Ltd McCann House 110 Nottingham Road Chilwell Nottingham NG9 6DQ. Quality Manual
 McCann House 110 Nottingham Road Chilwell Nottingham NG9 6DQ Quality Manual Quality Manual Issue I Dated 8 th October 2012 Contents Page Section 1 - Introduction 2 Section 2 - Company Details 2 Section
McCann House 110 Nottingham Road Chilwell Nottingham NG9 6DQ Quality Manual Quality Manual Issue I Dated 8 th October 2012 Contents Page Section 1 - Introduction 2 Section 2 - Company Details 2 Section
TOOL ENGINEERING OLD GROVE RD. SAN DIEGO, CA
 Page 1 of 42 VERTECHS ENTERPRISES, INC. Dba LUCHNER TOOL ENGINEERING 10051 OLD GROVE RD. SAN DIEGO, CA 92131 Ph No. 1-858-578-3900. Fax No. 1-858-578-2910 Reviewed and Approved By: Geosef (Joey) Straza
Page 1 of 42 VERTECHS ENTERPRISES, INC. Dba LUCHNER TOOL ENGINEERING 10051 OLD GROVE RD. SAN DIEGO, CA 92131 Ph No. 1-858-578-3900. Fax No. 1-858-578-2910 Reviewed and Approved By: Geosef (Joey) Straza
ISO 14001:2015 Gap Analysis Check Sheet
 ? CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context The organization shall determine external and internal issues that are relevant to its purpose and that affect its ability
? CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context The organization shall determine external and internal issues that are relevant to its purpose and that affect its ability
We are a global classification, certification, technical assurance and advisory company Ungraded
 We are a global classification, certification, technical assurance and advisory company 1 Global reach local competence 150 300 100 15,000 years offices countries employees 2 DNV GL :: Focused on your
We are a global classification, certification, technical assurance and advisory company 1 Global reach local competence 150 300 100 15,000 years offices countries employees 2 DNV GL :: Focused on your
ISO GUIDE 34: 2009 WORKING DOCUMENT
 GUIDE 34: 2009 WORKING DOCUMENT NOTES: 1. This working document is intended as a checklist for the assessor when conducting Reference Material Producer (RMP) Accreditation Assessments according to ISO
GUIDE 34: 2009 WORKING DOCUMENT NOTES: 1. This working document is intended as a checklist for the assessor when conducting Reference Material Producer (RMP) Accreditation Assessments according to ISO
Quality Assurance Policy and Procedures
 650 Montana Ave, Suite A Las Cruces, NM 88001 (575) 522-0430 www.rmgovernmentservices.com Quality Assurance Policy and Procedures PURPOSE. The purpose of this policy is to outline the quality assurance
650 Montana Ave, Suite A Las Cruces, NM 88001 (575) 522-0430 www.rmgovernmentservices.com Quality Assurance Policy and Procedures PURPOSE. The purpose of this policy is to outline the quality assurance
Vendor Qualification Survey
 1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
QM-1 Supplement QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT. Revision 7. Quality Director / Management Representative n. B.
 QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT Revision 7 APPROVED BY: TITLE DATE B. Olevson Quality Director / Management Representative 07 3-0 n DOCUMENT REVISION HISTORY Paragraph Revisions: or Rev: Date:
QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT Revision 7 APPROVED BY: TITLE DATE B. Olevson Quality Director / Management Representative 07 3-0 n DOCUMENT REVISION HISTORY Paragraph Revisions: or Rev: Date:
ASQ Tappan Zee Section Medical Devices, New Regulations and Standards. 26 th September / V. Fischer / Rev. 01
 ASQ Tappan Zee Section Medical Devices, New Regulations and Standards 26 th September / V. Fischer / Rev. 01 Agenda Medical Device, General Aspects Examples Quality System(s) New Regulations & Standards
ASQ Tappan Zee Section Medical Devices, New Regulations and Standards 26 th September / V. Fischer / Rev. 01 Agenda Medical Device, General Aspects Examples Quality System(s) New Regulations & Standards
HP Standard Supplier Requirements for Safe and Legal Products
 HP Standard 014-2 Supplier Requirements for Safe and Legal Products Document Identifier Revision and Date Last Revalidation Date Abstract Applicability Status HX-00014-02 G, This standard describes HP
HP Standard 014-2 Supplier Requirements for Safe and Legal Products Document Identifier Revision and Date Last Revalidation Date Abstract Applicability Status HX-00014-02 G, This standard describes HP
FEC QUALITY MANUAL. Federal Equipment Company River Rd. Cincinnati, Ohio
 QMS-20 2016 FEC QUALITY MANUAL Federal Equipment Company 5298 River Rd Cincinnati, Ohio 45233 www.federalequipment.com www.fecheliports.com www.usdrillhead.com www.orionseals.com www.tkf.com REVISION:
QMS-20 2016 FEC QUALITY MANUAL Federal Equipment Company 5298 River Rd Cincinnati, Ohio 45233 www.federalequipment.com www.fecheliports.com www.usdrillhead.com www.orionseals.com www.tkf.com REVISION:
0. 0 TABLE OF CONTENTS
 QUALITY MANUAL Conforming to ISO 9001:2008 0. 0 TABLE OF CONTENTS Section Description ISO 9001 Clause Page 0 TABLE OF CONTENTS n/a 2 1 PIMA VALVE, INC. DESCRIPTION n/a 3 2 QUALITY MANUAL DESCRIPTION 4.2.2
QUALITY MANUAL Conforming to ISO 9001:2008 0. 0 TABLE OF CONTENTS Section Description ISO 9001 Clause Page 0 TABLE OF CONTENTS n/a 2 1 PIMA VALVE, INC. DESCRIPTION n/a 3 2 QUALITY MANUAL DESCRIPTION 4.2.2
Internal Audits Procedure
 Page: 1 of 8 Internal Audits Procedure Page: 2 of 8 1.0 PURPOSE AND SCOPE 1.1 Purpose 1.1.1 The purpose of this document is to provide the Quality Assurance staff with instructions for the planning, performance,
Page: 1 of 8 Internal Audits Procedure Page: 2 of 8 1.0 PURPOSE AND SCOPE 1.1 Purpose 1.1.1 The purpose of this document is to provide the Quality Assurance staff with instructions for the planning, performance,
Supplier Management. hago Automotive Corp.
 Supplier Management of hago Automotive Corp. Change 24/11/2016 L. Bendel 24/11/2016 J. Blumenstock 9999/ / 00 1 of 13 Table of Contents Table of Contents... 2 Table of Figures... 3 Abbreviations... 3 1
Supplier Management of hago Automotive Corp. Change 24/11/2016 L. Bendel 24/11/2016 J. Blumenstock 9999/ / 00 1 of 13 Table of Contents Table of Contents... 2 Table of Figures... 3 Abbreviations... 3 1
Expert Commentary on BS EN ISO 13485:2016, Medical devices Quality management systems Requirements for regulatory purposes
 Expert Commentary on BS EN ISO 13485:2016, Medical devices Quality management systems Requirements for regulatory purposes Author: Eamonn Hoxey, PhD, F.R.Pharm.S., Vice President, Medical Devices Quality
Expert Commentary on BS EN ISO 13485:2016, Medical devices Quality management systems Requirements for regulatory purposes Author: Eamonn Hoxey, PhD, F.R.Pharm.S., Vice President, Medical Devices Quality
SOLAR KEYMARK SPECIFIC SCHEME RULES ANNEX E: FACTORY PRODUCTION CONTROL INFORMATIVE BASED ON ISO 9001 STANDARD COVERING THE PRODUCTION LINE
 SOLAR KEYMARK SPECIFIC SCHEME RULES ANNEX E: FACTORY PRODUCTION CONTROL INFORMATIVE BASED ON ISO 9001 STANDARD COVERING THE PRODUCTION LINE 1. General This annex specifies the requirements for the Factory
SOLAR KEYMARK SPECIFIC SCHEME RULES ANNEX E: FACTORY PRODUCTION CONTROL INFORMATIVE BASED ON ISO 9001 STANDARD COVERING THE PRODUCTION LINE 1. General This annex specifies the requirements for the Factory
Procedures: QP 4 through QP 8, QP 16, QP 17, and QP 19
 SRI Quality System Registrar Procedures: QP 4 through QP 8, QP 16, QP 17, and QP 19 Booklet Version 171122 Revision Date QP 4.0 Pre-Audit Registration Procedures 15 11/07/15 QP 5.0 On-Site Audit Procedure
SRI Quality System Registrar Procedures: QP 4 through QP 8, QP 16, QP 17, and QP 19 Booklet Version 171122 Revision Date QP 4.0 Pre-Audit Registration Procedures 15 11/07/15 QP 5.0 On-Site Audit Procedure
QUALITY MANUAL RED CHECK DENOTES THIS IS A CONTROLLED COPY. APPROVED BY: (President) APPROVED BY: (Production Manager) APPROVED BY: (Quality Manager)
 PNC, Inc. QUALITY MANUAL, Rev. 6 Date: 11-10-08 Page 1 of 17 QUALITY MANUAL RED CHECK DENOTES THIS IS A CONTROLLED COPY APPROVED BY: (President) APPROVED BY: (Production Manager) APPROVED BY: (Quality
PNC, Inc. QUALITY MANUAL, Rev. 6 Date: 11-10-08 Page 1 of 17 QUALITY MANUAL RED CHECK DENOTES THIS IS A CONTROLLED COPY APPROVED BY: (President) APPROVED BY: (Production Manager) APPROVED BY: (Quality
WHO Prequalification of In Vitro Diagnostics Programme
 P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s Information for Manufacturers on the Manufacturing Site(s) Inspection (Assessment of the Quality Management System) WHO Prequalification
P r e q u a l i f i c a t i o n T e a m - D i a g n o s t i c s Information for Manufacturers on the Manufacturing Site(s) Inspection (Assessment of the Quality Management System) WHO Prequalification
ISO/TS TECHNICAL SPECIFICATION
 TECHNICAL SPECIFICATION ISO/TS 29001 Third edition 2010-06-01 Petroleum, petrochemical and natural gas industries Sector-specific quality management systems Requirements for product and service supply
TECHNICAL SPECIFICATION ISO/TS 29001 Third edition 2010-06-01 Petroleum, petrochemical and natural gas industries Sector-specific quality management systems Requirements for product and service supply
SAE AS9100 Revision C Requirements beyond ISO 9001:2008 UPDATING YOUR MANAGEMENT SYSTEM
 3 Terms and Definitions Ensure awareness and understanding of: Risk Special Requirements Critical Items Key Characteristic 4.1 Include process(es) that deploy(s) requirements from: Customers Applicable
3 Terms and Definitions Ensure awareness and understanding of: Risk Special Requirements Critical Items Key Characteristic 4.1 Include process(es) that deploy(s) requirements from: Customers Applicable
ISO 9001:2008 Clause PR017 Customer Satisfaction Procedure
 ISO 9001:2008 Clause 8.2.1 PR017 Customer Satisfaction Procedure Strode Park Foundation for People with Disabilities Page 1 of 7 Approvals The signatures below certify that this procedure has been reviewed
ISO 9001:2008 Clause 8.2.1 PR017 Customer Satisfaction Procedure Strode Park Foundation for People with Disabilities Page 1 of 7 Approvals The signatures below certify that this procedure has been reviewed
CORPORATE QUALITY MANUAL
 Corporate Quality Manual Preface The following Corporate Quality Manual is written within the framework of the ISO 9001:2008 Quality System by the employees of CyberOptics. CyberOptics recognizes the importance
Corporate Quality Manual Preface The following Corporate Quality Manual is written within the framework of the ISO 9001:2008 Quality System by the employees of CyberOptics. CyberOptics recognizes the importance
20 September 2017 Document No. QM-ISO revision T ASTRONAUTICS CORPORATION OF AMERICA S. AS 9100 and FAA QUALITY MANUAL. Proprietary Notice
 20 September 2017 Document No. QM-ISO revision T ASTRONAUTICS CORPORATION OF AMERICA S AS 9100 and FAA QUALITY MANUAL Proprietary Notice Information disclosed herein is the property of Astronautics Corporation
20 September 2017 Document No. QM-ISO revision T ASTRONAUTICS CORPORATION OF AMERICA S AS 9100 and FAA QUALITY MANUAL Proprietary Notice Information disclosed herein is the property of Astronautics Corporation
QUALITY MANUAL. IFS DOCUMENT CLASS: QLTY-100 IFS DOCUMENT NUMBER: QM4.2.2 PAGE 2 of 27 EFFECTIVE DATE: 30 MAY 2017 REV Q
 IFS DOCUMENT CLASS: QLTY-100 IFS DOCUMENT NUMBER: QM4.2.2 PAGE 2 of 27 Introduction Value Plastics, Inc., dba Nordson MEDICAL created a Quality Management System (QMS) to document the company s best business
IFS DOCUMENT CLASS: QLTY-100 IFS DOCUMENT NUMBER: QM4.2.2 PAGE 2 of 27 Introduction Value Plastics, Inc., dba Nordson MEDICAL created a Quality Management System (QMS) to document the company s best business
Moving to the AS9100:2016 series. Transition Guide
 Moving to the AS9100:2016 series Transition Guide AS9100-series - Quality Management Systems for Aviation, Space and Defense - Transition Guide Successful aviation, space and defense businesses understand
Moving to the AS9100:2016 series Transition Guide AS9100-series - Quality Management Systems for Aviation, Space and Defense - Transition Guide Successful aviation, space and defense businesses understand
Security Operations Manual
 2018-01-01 Security Operations Manual 1 INTRODUCTION 2 2 CONTEXT 2 3 RISK 3 4 SCOPE 3 5 REFERENCES 4 6 SECURITY OPERATIONS MANAGEMENT SYSTEM 4 7 MANAGEMENT RESPONSIBILITIES 5 7.1 Security policy 6 8 RESOURCE
2018-01-01 Security Operations Manual 1 INTRODUCTION 2 2 CONTEXT 2 3 RISK 3 4 SCOPE 3 5 REFERENCES 4 6 SECURITY OPERATIONS MANAGEMENT SYSTEM 4 7 MANAGEMENT RESPONSIBILITIES 5 7.1 Security policy 6 8 RESOURCE
4. RELATED DOCUMENTS AND FORMS 4.1. AS ISO Cambridge Valley Machining, Inc. QMS procedures, including, but not limited to:
 Approved By: Purchasing Manager Candice Lane (signature on file) Date: 5/26/17 Approved By: Process Owner James D. Moore (signature on file) Date: 5/26/17 1. PURPOSE 1.1. To define Cambridge Valley Machining,
Approved By: Purchasing Manager Candice Lane (signature on file) Date: 5/26/17 Approved By: Process Owner James D. Moore (signature on file) Date: 5/26/17 1. PURPOSE 1.1. To define Cambridge Valley Machining,
