CDRH Scorecard Metric Determination. Presentation to the Balanced Scorecard Interest Group March 17, 2005
|
|
|
- Ralph Colin Wilson
- 6 years ago
- Views:
Transcription
1 CDRH Scorecard Metric Determination Presentation to the Balanced Scorecard Interest Group March 17, 2005
2 Organizational Structure DHHS FDA Food and Drug Administration Department of Health and Human Services ORA Office of Regulatory Affairs CBER Center for Biologics Evaluation and Research CDER Center for Drug Evaluation & Research CDRH Center for Devices and Radiological Health NCTR National Center for Toxiological Research CVM Center for Veterinary Medicine CFSAN Center for Food Safety and Applied Nutrition
3 CDRH Scorecard Development: 2000 CDRH Strategic Plan 2001 Determine Strategy 2002/2003 History Performance Scorecard Implementation 2004 Initiate process to Evaluate & Refine Scorecards
4 2002: Scorecard Development Process Collect Phase: Strategic Goals Determine obligations and commitments by reviewing Department and Agency documents
5 CDRH Strategic Goals Total Product Life Cycle Apply TPLC across Center activities to promote and protect the public health Public Health Impact Measure and communicate our impact on the public health Magnet for Excellence Attract and retain a diverse workforce to accomplish our public health mission Knowledge Management Manage knowledge to support TPLC in the information age
6 Obligations and Commitments President s Management Agenda One HHS Science Review Recommendations GPRA Goals Performance Plans (Commissioner & SES)
7 Create Phase: Develop Executive & Office Scorecards Each Office reviewed their core processes and proposed metrics which would be key indicators of success Philosophy: need an indicator for every process within our Office
8 2003 CDRH Executive Scorecard Key Result Areas (KRA s): 1. Public Health Promotion 2. Public Health Protection 3. Organizational Accountability 4. Stakeholder Collaboration 5. Workforce Excellence 6. Strategic Direction
9 FY-02/03 Metric Development CDRH Executive SC - Public Health Promotion (Expedited Approvals) 1. Average total elapsed days for approval 2. Average total elapsed time for final decisions 3. # of Expedited Applications 4. Average "Public Health Impact Score" based on population affected and risk reduction 5. # of agreement, determination, and other pre- submission meetings 6. % of Investigational Device Exemptions approved in the first 30-day cycle 7. % of submissions citing guidance or recognized standards
10 Office Scorecard Average total elapsed time for approval Median total elapsed time for device application approvals (1) expedited Pre-Market Approval (PMA) originals & expedited panel track PMA Supplements, (2) expedited 510(k)s, (3) Humanitarian Device Exemptions (HDEs( HDEs).
11 FY-02/03 Metric Development CDRH Executive SC Workforce Excellence 1. % CDRH employees receiving training 2. Average # of training hours per employee 3. % of CDRH managers completing core competency requirements 4. % of CDRH employees utilizing distance learning 5. # of CDRH employees participating in leadership programs 6. External Expertise Ratio (# of external engagements/ opportunities for engagements)
12 Office Level % CDRH employees receiving training 1. Staff College programs (in-house) 2. university/college courses 3. professional training programs 4. details 5. sabbaticals 6. fellowships 7. distance learning 8. mentoring 9. professional conferences..
13 2003 Executive & Office Scorecards Cultivate Phase: Gather, display, analyze historical data Did not have systems in place to capture all proposed metrics Reported what was available instead of developing systems to capture data
14 Performance Management System Vision, Mission, Core Values, And Core Passions Refinements Review, Evaluate, And Improve Business Performance Management System Rewards And Recognition Organization-Wide Strategic Planning Process Annual Deployment Cycle Workgroup And Individual Performance Management Process Develop Organization-Wide Performance Scorecard Management Cascade Operating Plans, Budget, And Scorecard Cascading Process Foundations For Achieving Performance Results Teaming System And Skills Process Improvement System And Skills Leadership Development System And Skills Project Management System And Skills Richard Chang Associates
15 Assessed Current Reality Disconnect between Scorecard Measures and Measures used to Manage Workload MDUFMA* Goals data had to be hand calculated from numerous data queries Data systems were not Centralized which impacted on data roll-up Medical Device User Fee and Modernization Act
16 2005 CDRH Executive Scorecard Key Result Areas (KRA s): 1. Public Health Promotion 2. Public Health Protection 3. Effective Management 4. Knowledge Management & Stakeholder Collaboration 5. Workforce Excellence
17 FY-04/05 Metric Development Collect Phase: Determine and prioritize obligations and commitments by reviewing Department and Agency documents Highest priority: MDUFMA requirements GPRA Goals and other Budget Document Obligations
18 Prioritization Goal Statement $ & Strategic Plans Ag. $ FDA CDRH Ctr. Perf. Plans Com MDUFMA 1. Utilize Risk management to target inspection coverage for Class II and Class III domestic medical device manufacturers at 20% or an estimate 5,500 firms. (Also FY 05 Goal-Only the Performance Plan specifies the target percentage) #11 2. Conduct 295 domestic and foreign BIMO inspections with an emphasis on scientific misconduct, data integrity, innovative products, and vulnerable populations. (Also FY 05 Goal) # Complete Review and Decision on premarket FY 2004 actions defined in the MDUFMA Legislation (MDUFMA) 4. Work with Stakeholders to implement MDUFMA. This will include providing MDUFMA training by December (MDUFMA)
19 FY-04/05 Metric Development CDRH Executive SC - Public Health Promotion 1. % on-time decisions and actions 2. % of Investigational Device Exemptions approved in the first 30-day cycle 3. % on-time consults
20 FY-04/05 Metric Development CDRH Executive SC Workforce Excellence 1. Average # of training hours per employee (limited to formal training & conferences) 2. # employees meeting training target 3. % of CDRH managers completing core competency requirements 4. % of operational dollars used for external expertise
21 Utilized the Performance Management System Concept to Assign Resources and Track Progress Project Management Developed Milestones Automate: target funds toward modifying or developing systems to capture high priority metrics Centralize Data Systems: internal benchmarking to develop efficient and productive systems
22 Lessons Learned Assess what is important to Stakeholders & Customers Periodic & Systematic Review Prioritize Systematic Implementation
23 Questions? Questions Comments? Frances A. Benedict
FDA Nanotechnology Regulatory Science Program Science Board Presentation August 2010
 FDA Nanotechnology Regulatory Science Program Science Board Presentation August 2010 FDA Nanotechnology Task Force Carlos Peña, PhD Office of the Commissioner & Subhas Malghan, PhD Center for Devices and
FDA Nanotechnology Regulatory Science Program Science Board Presentation August 2010 FDA Nanotechnology Task Force Carlos Peña, PhD Office of the Commissioner & Subhas Malghan, PhD Center for Devices and
Navigating the FDA, and understanding Medical Device Preemption. Special Counsel Foulston Siefkin
 Navigating the FDA, and understanding Medical Device Preemption Presented by Cydney Boler Special Counsel Foulston Siefkin cboler@foulston.com 913-253-2158 March 9, 2010 A bit of Legalese upfront: The
Navigating the FDA, and understanding Medical Device Preemption Presented by Cydney Boler Special Counsel Foulston Siefkin cboler@foulston.com 913-253-2158 March 9, 2010 A bit of Legalese upfront: The
Medical Device Labeling HealthPack 2004 Program
 Medical Device Labeling HealthPack 2004 Program Elizabeth Kempen Overview Regulatory Agencies and Pathways Labeling Regulations General Medical Device Labeling Requirements Electronic Labeling FDA s Current
Medical Device Labeling HealthPack 2004 Program Elizabeth Kempen Overview Regulatory Agencies and Pathways Labeling Regulations General Medical Device Labeling Requirements Electronic Labeling FDA s Current
Regulatory Approach to Nanotechnology-Based Drugs For Animals
 Regulatory Approach to Nanotechnology-Based Drugs For Animals Steven Fleischer, DVM CSL/JIFSAN Joint Symposium on Food Safety and Nutrition: Nanotechnology in Foods and Cosmetics June 28, 2007 Introduction
Regulatory Approach to Nanotechnology-Based Drugs For Animals Steven Fleischer, DVM CSL/JIFSAN Joint Symposium on Food Safety and Nutrition: Nanotechnology in Foods and Cosmetics June 28, 2007 Introduction
Tina Morrison, Ph.D. Chair, Committee on Modeling and Simulation Office of the Chief Scientist U.S. Food and Drug Administration
 Tina Morrison, Ph.D. Chair, Committee on Modeling and Simulation Office of the Chief Scientist U.S. Food and Drug Administration Tina.Morrison@fda.hhs.gov Joseph Pellettiere Overview FDA s Strategic Plan
Tina Morrison, Ph.D. Chair, Committee on Modeling and Simulation Office of the Chief Scientist U.S. Food and Drug Administration Tina.Morrison@fda.hhs.gov Joseph Pellettiere Overview FDA s Strategic Plan
The Role of Chemists in the FDA Drug Approval Process
 The Role of Chemists in the FDA Drug Approval Process 231 st ACS National Meeting Atlanta, GA M. Scott Furness, Ph.D. March 26, 2006 Introduction Presentation Outline FDA Organization CDER Organization
The Role of Chemists in the FDA Drug Approval Process 231 st ACS National Meeting Atlanta, GA M. Scott Furness, Ph.D. March 26, 2006 Introduction Presentation Outline FDA Organization CDER Organization
FDA s Center for Devices and Radiological Health: Strategic Priorities for 2017 and Beyond
 FDA s Center for Devices and Radiological Health: Strategic Priorities for 2017 and Beyond Jeff Shuren, MD, JD Center for Devices and Radiological Health U.S. Food and Drug Administration May 4, 2017 1
FDA s Center for Devices and Radiological Health: Strategic Priorities for 2017 and Beyond Jeff Shuren, MD, JD Center for Devices and Radiological Health U.S. Food and Drug Administration May 4, 2017 1
Preparing for a United States Food and Drug Administration (FDA) Inspection: VOICE
 Preparing for a United States Food and Drug Administration (FDA) Inspection: VOICE This project has been funded in whole or in part with Federal funds from the Division of AIDS (DAIDS), National Institute
Preparing for a United States Food and Drug Administration (FDA) Inspection: VOICE This project has been funded in whole or in part with Federal funds from the Division of AIDS (DAIDS), National Institute
FDA Drug Compliance. Pepe Rodriguez-Perez, PhD Business Excellence Consulting Inc / BEC Spain SL.
 FDA Drug Compliance Presented by Pepe Rodriguez-Perez, PhD Business Excellence Consulting Inc / BEC Spain SL www.calidadpr.com email pepe@calidadpr.com Agenda 1. FDA s Strategic Priorities 2014-18 2. CDER
FDA Drug Compliance Presented by Pepe Rodriguez-Perez, PhD Business Excellence Consulting Inc / BEC Spain SL www.calidadpr.com email pepe@calidadpr.com Agenda 1. FDA s Strategic Priorities 2014-18 2. CDER
WORKGROUP-LEVEL OVERVIEW. What You Will Learn. What You Will Apply To Your Workgroup
 INTRODUCTION TO PERFORMANCE SCORECARDS WORKGROUP-LEVEL OVERVIEW What You Will Learn 1. By implementing Performance Scorecards, you are adopting an organized, proven method of defining key business outcomes
INTRODUCTION TO PERFORMANCE SCORECARDS WORKGROUP-LEVEL OVERVIEW What You Will Learn 1. By implementing Performance Scorecards, you are adopting an organized, proven method of defining key business outcomes
Enhancing Staff Training for New Medical Device Reviewers
 Enhancing Staff Training for New Medical Device Reviewers Carole C. Carey, BSEE, MEng. carole.carey@fda.hhs.gov Center for Devices and Radiological Health U.S. Food and Drug Administration TOPICS Addressing
Enhancing Staff Training for New Medical Device Reviewers Carole C. Carey, BSEE, MEng. carole.carey@fda.hhs.gov Center for Devices and Radiological Health U.S. Food and Drug Administration TOPICS Addressing
The FDA/CDRH Perspective in the Regulation of Surgical Mesh
 The FDA/CDRH Perspective in the Regulation of Surgical Mesh David Krause, Ph.D., Branch Chief Plastic & Reconstructive Surgery Branch Division of Surgical, Orthopedic & Restorative Devices Office of Device
The FDA/CDRH Perspective in the Regulation of Surgical Mesh David Krause, Ph.D., Branch Chief Plastic & Reconstructive Surgery Branch Division of Surgical, Orthopedic & Restorative Devices Office of Device
Statistical Computing Challenges at FDA. Paul Schuette, Ph.D. Scientific Computing Coordinator Office of Biostatistics FDA/CDER/OTS
 Statistical Computing Challenges at FDA Paul Schuette, Ph.D. Scientific Computing Coordinator Office of Biostatistics FDA/CDER/OTS What does the FDA do? FDA is responsible for protecting the public health
Statistical Computing Challenges at FDA Paul Schuette, Ph.D. Scientific Computing Coordinator Office of Biostatistics FDA/CDER/OTS What does the FDA do? FDA is responsible for protecting the public health
STRATEGIC PLAN. Tier 2 Strategic Planning
 STRATEGIC PLAN Tier 2 Strategic Planning December 2018 Tier 2 Strategic Planning All Tier 2 plans replicate the balanced scorecard framework and involve a strategy map. Two formats may be used for a Tier
STRATEGIC PLAN Tier 2 Strategic Planning December 2018 Tier 2 Strategic Planning All Tier 2 plans replicate the balanced scorecard framework and involve a strategy map. Two formats may be used for a Tier
My Experiences as an FDA Statistician
 My Experiences as an FDA Statistician Yeh-Fong Chen, Ph.D. FDA/CDER/OB/DB3 CBA 2016-2017 Workshop series-3 Dec. 18, 2016 Disclaimer This presentation reflects the views of the author and should not be
My Experiences as an FDA Statistician Yeh-Fong Chen, Ph.D. FDA/CDER/OB/DB3 CBA 2016-2017 Workshop series-3 Dec. 18, 2016 Disclaimer This presentation reflects the views of the author and should not be
Inspection Prioritization
 FDA s Risk Toolbox: Inspection and Sampling Prioritization January 2011 Amy Barringer Division Director, Field Programs and Guidance FDA/CFSAN/Office of Compliance Inspection Prioritization 2 1 BACKGROUND
FDA s Risk Toolbox: Inspection and Sampling Prioritization January 2011 Amy Barringer Division Director, Field Programs and Guidance FDA/CFSAN/Office of Compliance Inspection Prioritization 2 1 BACKGROUND
FDA Regulation of In Vitro Diagnostics: Current Perspectives and Initiatives
 FDA Regulation of In Vitro Diagnostics: Current Perspectives and Initiatives Katherine Donigan, Ph.D. Office of In Vitro Diagnostics and Radiological Health, FDA Life Science Tennessee Annual Conference
FDA Regulation of In Vitro Diagnostics: Current Perspectives and Initiatives Katherine Donigan, Ph.D. Office of In Vitro Diagnostics and Radiological Health, FDA Life Science Tennessee Annual Conference
UNIVERSITY OF COLORADO DEPARTMENT OF INTERNAL AUDIT 2018 AUDIT PLAN As of June 1, 2017
 UNIVERSITY OF COLORADO DEPARTMENT OF INTERNAL AUDIT 2018 AUDIT PLAN As of June 1, 2017 Table of Contents I. Purpose 1 II. Internal Audit s Role, Objectives and Operational Strategy 1 III. Challenges and
UNIVERSITY OF COLORADO DEPARTMENT OF INTERNAL AUDIT 2018 AUDIT PLAN As of June 1, 2017 Table of Contents I. Purpose 1 II. Internal Audit s Role, Objectives and Operational Strategy 1 III. Challenges and
Cascading the BSC Using the Nine Steps to Success
 Cascading the BSC Using the Nine Steps to Success The Balanced Scorecard Institute uses a proven, disciplined framework, Nine Steps to Success, to systematically develop, implement, and sustain a strategic
Cascading the BSC Using the Nine Steps to Success The Balanced Scorecard Institute uses a proven, disciplined framework, Nine Steps to Success, to systematically develop, implement, and sustain a strategic
VISION, MISSION, VALUES
 VISION, MISSION, VALUES Vision Statement: Through a combination of best practices and well established strategic partnerships, the Department of Human Resources will recruit, develop, and support the diverse
VISION, MISSION, VALUES Vision Statement: Through a combination of best practices and well established strategic partnerships, the Department of Human Resources will recruit, develop, and support the diverse
FDA Regulatory Updates: Related to Cancer Immunotherapy
 FDA Regulatory Updates: Related to Cancer Immunotherapy Society for Immunotherapy of Cancer (SITC) Raj K. Puri, M.D., Ph.D. Director, DCGT Office of Cellular, Tissue and Gene Therapies, FDA, CBER Date:
FDA Regulatory Updates: Related to Cancer Immunotherapy Society for Immunotherapy of Cancer (SITC) Raj K. Puri, M.D., Ph.D. Director, DCGT Office of Cellular, Tissue and Gene Therapies, FDA, CBER Date:
FY2018 FY2021 Strategic Plan
 FY2018 FY2021 Strategic Plan It is the mission of the City of Virginia Beach Emergency Communications and Citizen Services operations and administration to provide efficient, accurate, professional processing
FY2018 FY2021 Strategic Plan It is the mission of the City of Virginia Beach Emergency Communications and Citizen Services operations and administration to provide efficient, accurate, professional processing
U.S. FDA CENTER FOR DEVICES AND RADIOLOGICAL HEALTH UPDATE. Jeff Shuren Director Center for Devices and Radiological Health
 U.S. FDA CENTER FOR DEVICES AND RADIOLOGICAL HEALTH UPDATE Jeff Shuren Director Center for Devices and Radiological Health 21 st Century Cures Implementation Establish Breakthrough Device Pathway Change
U.S. FDA CENTER FOR DEVICES AND RADIOLOGICAL HEALTH UPDATE Jeff Shuren Director Center for Devices and Radiological Health 21 st Century Cures Implementation Establish Breakthrough Device Pathway Change
CDER Keynote Address. Patrizia Cavazzoni M.D. Deputy Center Director for Operations CDER/FDA. AAM GRx+Biosims Conference September 5, 2018
 CDER Keynote Address Patrizia Cavazzoni M.D. Deputy Center Director for Operations CDER/FDA AAM GRx+Biosims Conference September 5, 2018 1 Outline CDER s modernization roadmap Initiatives to facilitate
CDER Keynote Address Patrizia Cavazzoni M.D. Deputy Center Director for Operations CDER/FDA AAM GRx+Biosims Conference September 5, 2018 1 Outline CDER s modernization roadmap Initiatives to facilitate
Guidance for Industry
 Guidance for Industry Formal Dispute Resolution: Scientific and Technical Issues Related to Pharmaceutical CGMP DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Guidance for Industry Formal Dispute Resolution: Scientific and Technical Issues Related to Pharmaceutical CGMP DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Guidance for Industry
 Guidance for Industry Formal Dispute Resolution: Scientific and Technical Issues Related to Pharmaceutical CGMP DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Guidance for Industry Formal Dispute Resolution: Scientific and Technical Issues Related to Pharmaceutical CGMP DRAFT GUIDANCE This guidance document is being distributed for comment purposes only. Comments
Achieving Performance Excellence
 Achieving Performance Excellence Baldrige 101 Workshop ASQ Professional Development Summit Today s Discussion Introduce the Baldrige framework Value and Benefits of Baldrige Regional Program MN, ND & SD
Achieving Performance Excellence Baldrige 101 Workshop ASQ Professional Development Summit Today s Discussion Introduce the Baldrige framework Value and Benefits of Baldrige Regional Program MN, ND & SD
Sec Short title; finding. Sec Authority to assess and use drug fees. Sec Reauthorization; reporting requirements.
 H.R. 2430, FDA Reauthorization Act of 2017 Section 1. Short Title. This Act may be cited as the FDA Reauthorization Act of 2017. Section 2. Table of Contents Table of Contents TITLE I: FEES RELATING TO
H.R. 2430, FDA Reauthorization Act of 2017 Section 1. Short Title. This Act may be cited as the FDA Reauthorization Act of 2017. Section 2. Table of Contents Table of Contents TITLE I: FEES RELATING TO
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program?
 Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
Voluntary Pilot Meeting Preview: How will CDRH apply assessments in the voluntary program? Cisco Vicenty Case for Quality Program Manager Center for Devices and Radiological Health U.S. Food and Drug Administration,
Association of Food & Drug Officials (AFDO) Drug and Device Session. June 5, 2012
 Association of Food & Drug Officials (AFDO) June 5, 2012 Drug and Device Session Presentation by: CAPT Domenic J. Veneziano Director, Division of Import Operations and Policy PRESENTATION OVERVIEW I. General
Association of Food & Drug Officials (AFDO) June 5, 2012 Drug and Device Session Presentation by: CAPT Domenic J. Veneziano Director, Division of Import Operations and Policy PRESENTATION OVERVIEW I. General
Food and Drug Administration (FDA) 101
 Food and Drug Administration (FDA) 101 What is the Food and Drug Administration (FDA)? The FDA is an agency within the U.S. Department of Health and Human Services that is responsible for protecting the
Food and Drug Administration (FDA) 101 What is the Food and Drug Administration (FDA)? The FDA is an agency within the U.S. Department of Health and Human Services that is responsible for protecting the
Paths to Market & FDA Product Lifecycle Regulation. Patricia Kaeding Design of Medical Devices University of Minnesota April 14, 2005
 Paths to Market & FDA Product Lifecycle Regulation Patricia Kaeding Design of Medical Devices University of Minnesota April 14, 2005 FDA Organization U.S. Food & Drug Administration Headed by a Commissioner
Paths to Market & FDA Product Lifecycle Regulation Patricia Kaeding Design of Medical Devices University of Minnesota April 14, 2005 FDA Organization U.S. Food & Drug Administration Headed by a Commissioner
Data Transparency and Quality Metrics
 Data Transparency and Quality Metrics Ann Ferriter Division of Analysis and Program Operations Office of Compliance Center for Devices and Radiological Health Why are we interested in transparency? What
Data Transparency and Quality Metrics Ann Ferriter Division of Analysis and Program Operations Office of Compliance Center for Devices and Radiological Health Why are we interested in transparency? What
Guidance for IRBs, Clinical Investigators and Sponsors
 Guidance for IRBs, Clinical Investigators and Sponsors IRB Responsibilities for Reviewing the Qualifications of Investigators, Adequacy of Research Sites, and the Determination of Whether an IND/IDE is
Guidance for IRBs, Clinical Investigators and Sponsors IRB Responsibilities for Reviewing the Qualifications of Investigators, Adequacy of Research Sites, and the Determination of Whether an IND/IDE is
Good Governance and Anti-Corruption: The Role of Supreme Audit Institutions (SAIs)
 Good Governance and Anti-Corruption: The Role of Supreme Audit Institutions (SAIs) Phillip Herr, Ph.D. Managing Director, Physical Infrastructure Issues U.S. Government Accountability Office The Vision
Good Governance and Anti-Corruption: The Role of Supreme Audit Institutions (SAIs) Phillip Herr, Ph.D. Managing Director, Physical Infrastructure Issues U.S. Government Accountability Office The Vision
Plans for Competency-Based Human Resources Management in KINS
 Plans for -Based Human Resources Management in KINS May 13, 2014 Young-Joon CHOI, Head Human Resources Development Department International Nuclear Safety School Contents I. Backgrounds II. What is a competency?
Plans for -Based Human Resources Management in KINS May 13, 2014 Young-Joon CHOI, Head Human Resources Development Department International Nuclear Safety School Contents I. Backgrounds II. What is a competency?
Excellence in Action: Division of Human Resources Summary Response to the Human Resources Assessment. Division of Human Resources
 Excellence in Action: Division of Human Resources Summary to the Human Resources Assessment Division of Human Resources March 26, 2010 Introduction The Human Resources Assessment was conducted in response
Excellence in Action: Division of Human Resources Summary to the Human Resources Assessment Division of Human Resources March 26, 2010 Introduction The Human Resources Assessment was conducted in response
IPMA-CANADA INTERNATIONAL CERTIFICATION PROGRAM IPMA-CP (IN TRAINING) IPMA-CP IPMA-ACP IPMA-EX IPMA-CE
 IPMA- Canada INTERNATIONAL PROGRAM IPMA-CP (IN TRAINING) IPMA-CP IPMA-ACP IPMA-EX IPMA-CE INTERNATIONAL PROGRAM is a national human resource management association whose mission is to promote excellence
IPMA- Canada INTERNATIONAL PROGRAM IPMA-CP (IN TRAINING) IPMA-CP IPMA-ACP IPMA-EX IPMA-CE INTERNATIONAL PROGRAM is a national human resource management association whose mission is to promote excellence
Rainforest Strategies
 Rainforest Strategies British Columbia s Innovation Ecosystem 03 October 2018 Webinar: TechDev 101 1 Rainforest Strategies Innovation Frontera JoeSterling@RFS-LLP.com +1-619-206-2403 www.rfs-llp.com 27
Rainforest Strategies British Columbia s Innovation Ecosystem 03 October 2018 Webinar: TechDev 101 1 Rainforest Strategies Innovation Frontera JoeSterling@RFS-LLP.com +1-619-206-2403 www.rfs-llp.com 27
The Future of Drug Safety
 The Future of Drug Safety IOM Committee on the Assessment of the US Drug Safety System R. Alta Charo Warren P. Knowles Professor of Law & Bioethics University of Wisconsin Charge to the Committee Examine
The Future of Drug Safety IOM Committee on the Assessment of the US Drug Safety System R. Alta Charo Warren P. Knowles Professor of Law & Bioethics University of Wisconsin Charge to the Committee Examine
CDRH s Inspection Strategy for 2018: How it Will Impact Your Company
 CDRH s Inspection Strategy for 2018: How it Will Impact Your Company CAPT Sean M. Boyd, MPH, USPHS Deputy Director for Regulatory Affairs CDRH Office of Compliance Patients are at the Heart of What We
CDRH s Inspection Strategy for 2018: How it Will Impact Your Company CAPT Sean M. Boyd, MPH, USPHS Deputy Director for Regulatory Affairs CDRH Office of Compliance Patients are at the Heart of What We
Disclosures 4/2/2011. No conflict of interest Not representing any organization Not a billing consultant
 San Francisco Radiation Oncology Conference April 2, 2011 Accelerated Partial Breast Irradiation: The Conundrum of Regulation and Reimbursement Disclosures No conflict of interest Not representing any
San Francisco Radiation Oncology Conference April 2, 2011 Accelerated Partial Breast Irradiation: The Conundrum of Regulation and Reimbursement Disclosures No conflict of interest Not representing any
Issues in Clinical Trial Designs for Devices
 Issues in Clinical Trial Designs for Devices Soma Kalb, PhD Director, Investigational Device Exemption (IDE) Program Office of Device Evaluation Center for Devices and Radiological Health FDA Clinical
Issues in Clinical Trial Designs for Devices Soma Kalb, PhD Director, Investigational Device Exemption (IDE) Program Office of Device Evaluation Center for Devices and Radiological Health FDA Clinical
Strategic Plan The OSC: A 21 st Century Securities Regulator
 2012-2015 Strategic Plan The OSC: A 21 st Century Securities Regulator 2 The Ontario Securities Commission (OSC) is the regulatory body responsible for overseeing Ontario s capital markets, which include
2012-2015 Strategic Plan The OSC: A 21 st Century Securities Regulator 2 The Ontario Securities Commission (OSC) is the regulatory body responsible for overseeing Ontario s capital markets, which include
Bipartisan Policy Center, Top Medical Innovation Priorities
 Bipartisan Policy Center, Top Medical Priorities FDA: Advancing Medical is a Bipartisan Policy Center initiative led by former Senator Bill Frist, MD, former Congressman Bart Gordon, and BPC Health Initiative
Bipartisan Policy Center, Top Medical Priorities FDA: Advancing Medical is a Bipartisan Policy Center initiative led by former Senator Bill Frist, MD, former Congressman Bart Gordon, and BPC Health Initiative
Carrie McMahon, Ph.D. Center for Food Safety and Applied Nutrition Office of Food Additive Safety. October 14, 2015
 Carrie McMahon, Ph.D. Center for Food Safety and Applied Nutrition Office of Food Additive Safety October 14, 2015 FDA s Oversight of Food and Feed Safety food safety requirements how food safety requirements
Carrie McMahon, Ph.D. Center for Food Safety and Applied Nutrition Office of Food Additive Safety October 14, 2015 FDA s Oversight of Food and Feed Safety food safety requirements how food safety requirements
COMBINATION PRODUCTS Inspection Readiness and Outcomes
 WHITE PAPER COMBINATION PRODUCTS Inspection Readiness and Outcomes Many companies think that because they have a good relationship with the FDA Center they interacted with during their submission reviews
WHITE PAPER COMBINATION PRODUCTS Inspection Readiness and Outcomes Many companies think that because they have a good relationship with the FDA Center they interacted with during their submission reviews
HR Excellence. Confederation of Indian Industry
 HR Excellence HR Excellence Assessment Model 2010 Leadership Broad Parameters Human Resource Strategy HR Management & Processes People Knowledge & Competencies People well being & engagement Results Perception
HR Excellence HR Excellence Assessment Model 2010 Leadership Broad Parameters Human Resource Strategy HR Management & Processes People Knowledge & Competencies People well being & engagement Results Perception
Future of Pharmaceutical Quality and the Path to Get There
 Future of Pharmaceutical Quality and the Path to Get There Lawrence Yu, Ph.D. Deputy Director, Office of Pharmaceutical Quality FDA Center for Drug Evaluation and Research 3rd PQRI/FDA Conference on Advancing
Future of Pharmaceutical Quality and the Path to Get There Lawrence Yu, Ph.D. Deputy Director, Office of Pharmaceutical Quality FDA Center for Drug Evaluation and Research 3rd PQRI/FDA Conference on Advancing
Strategic Plan
 Strategic Plan 2017-2019 THE NATIONAL GRADUATE SCHOOL OF QUALITY MANAGEMENT Celebrating 20 YEARS of Quality Education This material is protected by United States copyright laws. You must treat this publication
Strategic Plan 2017-2019 THE NATIONAL GRADUATE SCHOOL OF QUALITY MANAGEMENT Celebrating 20 YEARS of Quality Education This material is protected by United States copyright laws. You must treat this publication
Guidance for Industry Part 11, Electronic Records; Electronic Signatures Scope and Application
 Guidance for Industry Part 11, Electronic Records; Electronic Signatures Scope and Application DRAFT GUIDANCE This guidance is being distributed for comment purposes only. Comments and suggestions regarding
Guidance for Industry Part 11, Electronic Records; Electronic Signatures Scope and Application DRAFT GUIDANCE This guidance is being distributed for comment purposes only. Comments and suggestions regarding
Building a Government Balanced Scorecard. Phase 1 - Planning
 Building a Government Balanced Scorecard Phase 1 - Planning Paul Arveson The Balanced Scorecard Institute March 2003 2003 Balanced Scorecard Institute 1 Example of a Government Balanced Scorecard Implementation
Building a Government Balanced Scorecard Phase 1 - Planning Paul Arveson The Balanced Scorecard Institute March 2003 2003 Balanced Scorecard Institute 1 Example of a Government Balanced Scorecard Implementation
Strategic & Operational Plan
 2015 2017 Strategic & Operational Plan 1 Strategic Plan Vision Statement: The elimination of occupational injury and illness in Saskatchewan. Mission Statement: To ensure the execution of an integrated
2015 2017 Strategic & Operational Plan 1 Strategic Plan Vision Statement: The elimination of occupational injury and illness in Saskatchewan. Mission Statement: To ensure the execution of an integrated
Update on Current FDA Policies and Priorities
 Update on Current FDA Policies and Priorities Vernessa Pollard MassMEDIC FDA Update December 12, 2017 Boston Brussels Chicago Dallas Düsseldorf Frankfurt Houston London Los Angeles Miami Milan Munich New
Update on Current FDA Policies and Priorities Vernessa Pollard MassMEDIC FDA Update December 12, 2017 Boston Brussels Chicago Dallas Düsseldorf Frankfurt Houston London Los Angeles Miami Milan Munich New
Expanding Leadership and Substantive Skills of Statistical Agency Personnel Panel Discussion
 Expanding Leadership and Substantive Skills of Statistical Agency Personnel Panel Discussion Lisa M. LaVange, PhD Director, Office of Biostatistics OTS/CDER/FDA FCSM Policy Seminar Washington, D.C. Dec.
Expanding Leadership and Substantive Skills of Statistical Agency Personnel Panel Discussion Lisa M. LaVange, PhD Director, Office of Biostatistics OTS/CDER/FDA FCSM Policy Seminar Washington, D.C. Dec.
Role of the FDA and PDUFA in Drug Development
 Role of the FDA and PDUFA in Drug Development Questions to be addressed 1. What is the Food and Drug Administration and why is it important? 2. Why was the Prescription Drug User Fee Act (PDUFA) created?
Role of the FDA and PDUFA in Drug Development Questions to be addressed 1. What is the Food and Drug Administration and why is it important? 2. Why was the Prescription Drug User Fee Act (PDUFA) created?
Guidance for Industry - Computerized Systems Used in Clinical Trials
 Page 1 of 14 Regulatory Information Computerized Systems Used in Clinical Trials Guidance for Industry - Computerized Systems Used in Clinical Trials
Page 1 of 14 Regulatory Information Computerized Systems Used in Clinical Trials Guidance for Industry - Computerized Systems Used in Clinical Trials
FAA PMIWDC LUNCHEON SERIES STRATEGIC PLANNING; WHAT, WHY, AND HOW
 FAA PMIWDC LUNCHEON SERIES STRATEGIC PLANNING; WHAT, WHY, AND HOW John Lever, Managing g Partner The Lever Group February 29 th, 2012 vision mission strategy performance INTRODUCTION AND SESSION OVERVIEW
FAA PMIWDC LUNCHEON SERIES STRATEGIC PLANNING; WHAT, WHY, AND HOW John Lever, Managing g Partner The Lever Group February 29 th, 2012 vision mission strategy performance INTRODUCTION AND SESSION OVERVIEW
The Institute of Medicine FDA s Role in Ensuring Safe Food January 28, 2011 PREDICT
 The Institute of Medicine FDA s Role in Ensuring Safe Food January 28, 2011 PREDICT Presentation by: CDR Domenic J. Veneziano, Director, Division of Import Operations Topics PREDICT (Predictive Risk-based
The Institute of Medicine FDA s Role in Ensuring Safe Food January 28, 2011 PREDICT Presentation by: CDR Domenic J. Veneziano, Director, Division of Import Operations Topics PREDICT (Predictive Risk-based
Metropolitan St. Louis Sewer District. Strategic Business and Operating Plan Fiscal Years
 Metropolitan St. Louis Sewer District Strategic Business and Operating Plan Fiscal Years 2013 2017 VISION STATEMENT Quality Service Always MISSION STATEMENT To protect the public s health, safety, and
Metropolitan St. Louis Sewer District Strategic Business and Operating Plan Fiscal Years 2013 2017 VISION STATEMENT Quality Service Always MISSION STATEMENT To protect the public s health, safety, and
State of Office of Pharmaceutical Quality (OPQ) Address
 State of Office of Pharmaceutical Quality (OPQ) Address Giuseppe Randazzo, M.S. Director, Office of Program and Regulatory Operations Office of Pharmaceutical Quality Center for Drug Evaluation and Research,
State of Office of Pharmaceutical Quality (OPQ) Address Giuseppe Randazzo, M.S. Director, Office of Program and Regulatory Operations Office of Pharmaceutical Quality Center for Drug Evaluation and Research,
BUSINESS ACCOMPLISHMENTS CONSUMER PROTECTION
 BUSINESS ACCOMPLISHMENTS 2016-2017 CONSUMER PROTECTION Goal: To regulate and enforce the legislation by which the Ontario travelling public can be confi dent in their travel purchases, including working
BUSINESS ACCOMPLISHMENTS 2016-2017 CONSUMER PROTECTION Goal: To regulate and enforce the legislation by which the Ontario travelling public can be confi dent in their travel purchases, including working
Government-Wide Diversity and Inclusion Strategic Plan 2011
 Government-Wide Diversity and Inclusion Strategic Plan 2011 Our Nation derives strength from the diversity of its population and from its commitment to equal opportunity for all. We are at our best when
Government-Wide Diversity and Inclusion Strategic Plan 2011 Our Nation derives strength from the diversity of its population and from its commitment to equal opportunity for all. We are at our best when
STRATEGIC PLAN. FY
 STRATEGIC PLAN FY 2017-2021 www.flynaples.com Adopted June 15, 2017 NAPLES AIRPORT AUTHORITY BOARD OF COMMISSIONERS Dick Evans Vice Chair Naples Airport began operation in 1943 as a military airfield.
STRATEGIC PLAN FY 2017-2021 www.flynaples.com Adopted June 15, 2017 NAPLES AIRPORT AUTHORITY BOARD OF COMMISSIONERS Dick Evans Vice Chair Naples Airport began operation in 1943 as a military airfield.
THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture
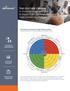 denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
denison TM THE CULTURE CANVAS A Working Guide and Checklist to Support the Development of a High-Performing Culture The Denison Model of High Performance A Systems Approach to Understanding and Managing
IPMA-Canada Certification Program
 Program IPMA-CP (In Training) IPMA-CP IPMA-ACP IPMA-EX IPMA-CE International Personnel Management Association Canada 3333 333 Program IPMA-Canada is a national human resource management association whose
Program IPMA-CP (In Training) IPMA-CP IPMA-ACP IPMA-EX IPMA-CE International Personnel Management Association Canada 3333 333 Program IPMA-Canada is a national human resource management association whose
November CFPB Diversity and Inclusion Strategic Plan
 November 2016 CFPB Diversity and Inclusion Strategic Plan 2016-2020 Message from Richard Cordray Director of the CFPB At the Consumer Financial Protection Bureau (CFPB), we are dedicated to making sure
November 2016 CFPB Diversity and Inclusion Strategic Plan 2016-2020 Message from Richard Cordray Director of the CFPB At the Consumer Financial Protection Bureau (CFPB), we are dedicated to making sure
The Brave New World of Medical Devices
 The Brave New World of Medical Devices December 17, 2014 BOSTON GERMANY ISRAEL www.bmtadvisors.com www.bmtcrogroup.com 1 It Is All About The Adoption More Than a Mantra David Barone December 17, 2014 www.bmtadvisors.com
The Brave New World of Medical Devices December 17, 2014 BOSTON GERMANY ISRAEL www.bmtadvisors.com www.bmtcrogroup.com 1 It Is All About The Adoption More Than a Mantra David Barone December 17, 2014 www.bmtadvisors.com
CERTIFICATIONS IN HUMAN RESOURCES. SPHRi TM Senior Professional in Human Resources - International TM SPHRi. Exam Content Outline
 CERTIFICATIONS IN HUMAN RESOURCES SPHRi TM Senior Professional in Human Resources - International TM 2018 SPHRi Exam Content Outline SPHRi Exam Content Outline At-a-Glance: SPHRi Exam Weighting by Functional
CERTIFICATIONS IN HUMAN RESOURCES SPHRi TM Senior Professional in Human Resources - International TM 2018 SPHRi Exam Content Outline SPHRi Exam Content Outline At-a-Glance: SPHRi Exam Weighting by Functional
Show value by learning to align initiatives, collecting data, and demonstrating the impact on business results. HUMAN CAPITAL. 30 T+D September 2013
 HUMAN CAPITAL Show value by learning to align initiatives, collecting data, and demonstrating the impact on business results. 30 T+D September 2013 PHOTO: JENNI SWEITZER podcast examining INTEGRATED TALENT
HUMAN CAPITAL Show value by learning to align initiatives, collecting data, and demonstrating the impact on business results. 30 T+D September 2013 PHOTO: JENNI SWEITZER podcast examining INTEGRATED TALENT
Organizational Change Through Metrics
 Organizational Change Through Metrics Speaker: Allen Hurst and Heather Rainey Company: Improving Enterprises Website: www.improvingenterprises.com Welcome to the PMI Houston Conference & Expo 2015 Please
Organizational Change Through Metrics Speaker: Allen Hurst and Heather Rainey Company: Improving Enterprises Website: www.improvingenterprises.com Welcome to the PMI Houston Conference & Expo 2015 Please
Center for Drug Evaluation and Research. CDER Small Business and Industry Assistance. (CDER SBIA) and New Drug Review.
 Center for Drug Evaluation and Research Small Business and Industry Assistance (CDER SBIA) and New Drug Review LT Renu Lal, Pharm.D. CDER Small Business and Industry Assistance Division of Drug Information
Center for Drug Evaluation and Research Small Business and Industry Assistance (CDER SBIA) and New Drug Review LT Renu Lal, Pharm.D. CDER Small Business and Industry Assistance Division of Drug Information
PHARM 532 Regulation of Pharmaceuticals II
 FDA s Regulatory Structuret PHARM 532 Regulation of Pharmaceuticals II Spring 2009 Center(s) for Biologics Evaluation & Research (CBER) 1 Drug Evaluation & Research (CDER) 2 Device & Radiological Health
FDA s Regulatory Structuret PHARM 532 Regulation of Pharmaceuticals II Spring 2009 Center(s) for Biologics Evaluation & Research (CBER) 1 Drug Evaluation & Research (CDER) 2 Device & Radiological Health
Objective Standards Of Performance
 Objective Standards Of Performance Introduction...1 Section A - Laboratory Leadership...2 Section B Science And Technology...5 Section C - Performance Objectives, Criteria And Measures...7 1 Environment,
Objective Standards Of Performance Introduction...1 Section A - Laboratory Leadership...2 Section B Science And Technology...5 Section C - Performance Objectives, Criteria And Measures...7 1 Environment,
Guidance for Industry Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations
 Guidance for Industry Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations DRAFT GUIDANCE This draft guidance document is being distributed for comment purposes only.
Guidance for Industry Quality Systems Approach to Pharmaceutical Current Good Manufacturing Practice Regulations DRAFT GUIDANCE This draft guidance document is being distributed for comment purposes only.
Supply Management Three-Year Strategic Plan
 Supply Management Three-Year Strategic Plan 2010-2012 Message From the Vice President, Supply Management I am pleased to present our new three-year strategic plan for fiscal years 2010 2012. The plan
Supply Management Three-Year Strategic Plan 2010-2012 Message From the Vice President, Supply Management I am pleased to present our new three-year strategic plan for fiscal years 2010 2012. The plan
GW Human Resources Strategic Plan
 GW Human Resources Strategic Plan 2017-2021 OUR VISION We aspire to develop a diverse and engaged workforce to lead GW to excellence. OUR MISSION The mission of Human Resources is: to serve as an effective
GW Human Resources Strategic Plan 2017-2021 OUR VISION We aspire to develop a diverse and engaged workforce to lead GW to excellence. OUR MISSION The mission of Human Resources is: to serve as an effective
Dial-in Number: Access code: # Go to: Use the JOIN A SESSION button Enter the Access Code:
 Dial-in Number: 209 647-1075 Access code: 1013004# Go to: http://www.gatherplace.net Use the JOIN A SESSION button Enter the Access Code: 8285433 This presentation may appear different on your computer
Dial-in Number: 209 647-1075 Access code: 1013004# Go to: http://www.gatherplace.net Use the JOIN A SESSION button Enter the Access Code: 8285433 This presentation may appear different on your computer
Guidance for Industry and FDA Staff Procedures for Handling Post-Approval Studies Imposed by PMA Order
 Guidance for Industry and FDA Staff Procedures for Handling Post-Approval Studies Imposed by PMA Order Document issued on: [Level 2, June 15, 2009] This guidance supersedes the document issued under this
Guidance for Industry and FDA Staff Procedures for Handling Post-Approval Studies Imposed by PMA Order Document issued on: [Level 2, June 15, 2009] This guidance supersedes the document issued under this
Title: Review of Medical Devices Page: 1 of 5 Written by:
 THE NORTH SHORE MEDICAL CENTER Institutional Review Board (IRB) POLICIES AND PROCEDURES IRB Policy Number: 031.1 Title: Review of Medical Devices Page: 1 of 5 Written by: Approved by: Laura W. Knight,
THE NORTH SHORE MEDICAL CENTER Institutional Review Board (IRB) POLICIES AND PROCEDURES IRB Policy Number: 031.1 Title: Review of Medical Devices Page: 1 of 5 Written by: Approved by: Laura W. Knight,
FLORIDA DEPARTMENT OF HEALTH AGENCY WORKFORCE DEVELOPMENT IMPLEMENTATION PLAN
 FLORIDA DEPARTMENT OF HEALTH AGENCY WORKFORCE DEVELOPMENT IMPLEMENTATION PLAN Mission To protect, promote and improve the health of all people in Florida through integrated state, county, and community
FLORIDA DEPARTMENT OF HEALTH AGENCY WORKFORCE DEVELOPMENT IMPLEMENTATION PLAN Mission To protect, promote and improve the health of all people in Florida through integrated state, county, and community
LIFE SCIENCE BENCHMARKING STUDY. Trends and Best Practices Based on Our Work with the US FDA and Over 250 Companies
 LIFE SCIENCE 01 BENCHMARKING STUDY Trends and Best Practices Based on Our Work with the US FDA and Over 0 Companies SUMMARY In this report, UL EduNeering has identified the 01/01 compliance and business
LIFE SCIENCE 01 BENCHMARKING STUDY Trends and Best Practices Based on Our Work with the US FDA and Over 0 Companies SUMMARY In this report, UL EduNeering has identified the 01/01 compliance and business
Overview of USP Activities and How to Get Involved
 Overview of USP Activities and How to Get Involved Ravi Ravichandran, Ph.D. Principal Scientific Liaison, Chemical Medicines United States Pharmacopeia Outline Introduction to the USP USP Standard Setting
Overview of USP Activities and How to Get Involved Ravi Ravichandran, Ph.D. Principal Scientific Liaison, Chemical Medicines United States Pharmacopeia Outline Introduction to the USP USP Standard Setting
Recommendations for Strengthening the Investigator Site Community
 Recommendations for Strengthening the Investigator Site Community October 2017 CTTI MISSION: To develop and drive adoption of practices that will increase the quality and efficiency of clinical trials
Recommendations for Strengthening the Investigator Site Community October 2017 CTTI MISSION: To develop and drive adoption of practices that will increase the quality and efficiency of clinical trials
A conversation about the framework for performance excellence PERFORM LIKE A ROCK STAR!
 A conversation about the framework for performance excellence PERFORM LIKE A ROCK STAR! Like Olympic athletes, pursing performance excellence in our work implies that we optimize every factor that goes
A conversation about the framework for performance excellence PERFORM LIKE A ROCK STAR! Like Olympic athletes, pursing performance excellence in our work implies that we optimize every factor that goes
Food and Drug Administration Guidance: Supervisory Responsibilities of Investigators
 Journal of Diabetes Science and Technology Volume 5, Issue 2, March 2011 Diabetes Technology Society REVIEW ARTICLE Food and Drug Administration Guidance: Supervisory Responsibilities of Investigators
Journal of Diabetes Science and Technology Volume 5, Issue 2, March 2011 Diabetes Technology Society REVIEW ARTICLE Food and Drug Administration Guidance: Supervisory Responsibilities of Investigators
Status of Food and Drug Administration Programs and Activities During the Current Government Shutdown
 Status of Food and Drug Administration Programs and Activities During the Current Government Shutdown Agency-Wide FDA can perform activities necessary to address imminent threats to the safety of human
Status of Food and Drug Administration Programs and Activities During the Current Government Shutdown Agency-Wide FDA can perform activities necessary to address imminent threats to the safety of human
Human Resources Strategic Update. Presented by Stephen Trncak October 28, 2011
 Human Resources Strategic Update Presented by Stephen Trncak October 28, 2011 Agenda Past and Future for the College The End Game (Elevating the Human and Organizational Capabilities and Capacity of the
Human Resources Strategic Update Presented by Stephen Trncak October 28, 2011 Agenda Past and Future for the College The End Game (Elevating the Human and Organizational Capabilities and Capacity of the
CSR / Sustainability Governance and Management Assessment By Coro Strandberg President, Strandberg Consulting
 Introduction CSR / Sustainability Governance and Management Assessment By Coro Strandberg President, Strandberg Consulting www.corostrandberg.com November 2015 Companies which adopt CSR or sustainability
Introduction CSR / Sustainability Governance and Management Assessment By Coro Strandberg President, Strandberg Consulting www.corostrandberg.com November 2015 Companies which adopt CSR or sustainability
FDA Requirements for Clinical Investigations of Medical Devices: A Review for European Manufacturers
 FDA Requirements for Clinical Investigations of Medical Devices: A Review for European Manufacturers Autumn Dawn Ediger, 1, * Birgit Limbach 2 and Dieter Dannhorn 3 1 Regulatory Affairs, Clinical QM and
FDA Requirements for Clinical Investigations of Medical Devices: A Review for European Manufacturers Autumn Dawn Ediger, 1, * Birgit Limbach 2 and Dieter Dannhorn 3 1 Regulatory Affairs, Clinical QM and
Re: Docket No. FDA 2015-D-1580:
 August 17, 2015 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2015-D-1580: Patient Preference Information Submission,
August 17, 2015 Dockets Management Branch (HFA-305) Food and Drug Administration 5630 Fishers Lane, Rm. 1061 Rockville, MD 20852 Re: Docket No. FDA 2015-D-1580: Patient Preference Information Submission,
Office of Pharmaceutical Quality Progress Update
 Office of Pharmaceutical Quality Progress Update Michael Kopcha, Ph.D., R.Ph. Director Office of Pharmaceutical Quality CDER/FDA 2017 3 rd PQRI/FDA Conference on Advancing Product Quality March 22-24,
Office of Pharmaceutical Quality Progress Update Michael Kopcha, Ph.D., R.Ph. Director Office of Pharmaceutical Quality CDER/FDA 2017 3 rd PQRI/FDA Conference on Advancing Product Quality March 22-24,
Milestones & Benchmarks
 2014 Center for Communities That Care, University of Washington Phase 1: Get Started 1.1 Organize the community to begin the Communities That Care Process. Designate a single point of contact to act as
2014 Center for Communities That Care, University of Washington Phase 1: Get Started 1.1 Organize the community to begin the Communities That Care Process. Designate a single point of contact to act as
DoD ENVIRONMENTAL MANAGEMENT SYSTEM (EMS) METRICS FISCAL YEARS
 DoD ENVIRONMENTAL MANAGEMENT SYSTEM (EMS) METRICS FISCAL YEARS 2009 2011 These metrics are identical to the Federal EMS Metrics and provide the mechanism by which DoD agencies, organizations and facilities
DoD ENVIRONMENTAL MANAGEMENT SYSTEM (EMS) METRICS FISCAL YEARS 2009 2011 These metrics are identical to the Federal EMS Metrics and provide the mechanism by which DoD agencies, organizations and facilities
New Approach to Drug Inspections
 New Approach to Drug Inspections Donald D. Ashley Director CDER Office of Compliance 2018 FDLI Annual Meeting May 4, 2018 Office of Compliance Structure Mission: The Office of Compliance shield s patients
New Approach to Drug Inspections Donald D. Ashley Director CDER Office of Compliance 2018 FDLI Annual Meeting May 4, 2018 Office of Compliance Structure Mission: The Office of Compliance shield s patients
Document issued on: March 19, The draft of this document was issued on May 20, 2010.
 Reprinted from FDA s website by Guidance for Industry, Third Parties and Food and Drug Administration Staff Medical Device ISO 13485:2003 Voluntary Audit Report Submission Pilot Program Document issued
Reprinted from FDA s website by Guidance for Industry, Third Parties and Food and Drug Administration Staff Medical Device ISO 13485:2003 Voluntary Audit Report Submission Pilot Program Document issued
Murray Sheldon, MD Associate Director for Technology and Innovation Center for Devices and Radiological Health (CDRH) Office of the Center Director
 Murray Sheldon, MD Associate Director for Technology and Innovation Center for Devices and Radiological Health (CDRH) Office of the Center Director The National Academies Innovation Policy Forum September
Murray Sheldon, MD Associate Director for Technology and Innovation Center for Devices and Radiological Health (CDRH) Office of the Center Director The National Academies Innovation Policy Forum September
Charter for Enterprise Risk Management
 for Enterprise Risk Management Prepared by: Shannon Sinclair Version: 1.2 Document Id: Date: Release Date TABLE OF CONTENTS TABLE OF CONTENTS... i 1. Background... 1 2. Objectives... 1 3. Scope... 2 3.1
for Enterprise Risk Management Prepared by: Shannon Sinclair Version: 1.2 Document Id: Date: Release Date TABLE OF CONTENTS TABLE OF CONTENTS... i 1. Background... 1 2. Objectives... 1 3. Scope... 2 3.1
Developing Enterprise-Wide Measures for Tracking Performance of Acquisition Organizations
 Pittsburgh, PA 15213-3890 Developing Enterprise-Wide Measures for Tracking Performance of Acquisition Organizations Wolfhart Goethert Carnegie Mellon University Pittsburgh, PA 15213-3890 Sponsored by the
Pittsburgh, PA 15213-3890 Developing Enterprise-Wide Measures for Tracking Performance of Acquisition Organizations Wolfhart Goethert Carnegie Mellon University Pittsburgh, PA 15213-3890 Sponsored by the
Evaluating and Building Portfolio Management Maturity
 Evaluating and Building Portfolio Management Maturity Hostetter and Norris, UMD PM Symposium 2016 Evaluating and Building Portfolio Management Maturity Susan Hostetter and Sherri Norris U.S. Census Bureau,
Evaluating and Building Portfolio Management Maturity Hostetter and Norris, UMD PM Symposium 2016 Evaluating and Building Portfolio Management Maturity Susan Hostetter and Sherri Norris U.S. Census Bureau,
