Guidance Note H1402 Packaging and Transport of waste from suspect
|
|
|
- Christopher Johns
- 6 years ago
- Views:
Transcription
1 uid Guidance Note H1402 Packaging and Transport of waste from suspect and confirmed cases of the Ebola Virus Revision: 1.0 Date: 21/10/2014
2 Contents 1 Purpose Introduction Regulations PROTEC620 packaging Alternative Packaging General Provisions: Packaging type Assembly of packaging Additional packaging and handling procedures Labelling of packaging Storage Collection Revision /10/2014 Page 2 of 16
3 1 Purpose This document is intended to provide hospitals and healthcare professionals with key information about the safe handling and disposal of medical waste generated from the care of persons with suspect and confirmed cases of the Ebola virus. 2 Introduction Packaging used for the transport of waste from patients with Ebola must meet the requirements of transport of dangerous goods by road regulations. There are two types of packaging available for waste from persons diagnosed with or suspected of having the Ebola virus: 1. PROTEC620 packaging This packaging is the Mauser WIVA PROTEC620 packaging. Guidance associated with this packaging is outlined in section 4 of the report. 2. Alternative packaging The Health and Safety Authority have granted an exemption whereby alternative packaging can be used for the packaging of waste from suspect and confirmed cases of the Ebola virus. Guidance associated with this packaging is outlined in section 5 of this report. Note: Either type of the above packaging can be used in healthcare facilities for waste from persons with suspect or confirmed cases of the Ebola virus. Appropriate general and personal protective clothing and equipment must be provided and used by staff involved in the handling of the waste and waste packaging. 3 Regulations Category A Ebola waste must be consigned nationally by road according to the following dangerous goods transport regulations: ADR European Agreement concerning the International Carriage of Dangerous Goods by Road European Communities (Carriage of Dangerous Goods by Road and Use of Transportable Pressure Equipment), 2011, S.I. No. 349 of European Communities (Carriage of Dangerous Goods by Road and Use of Transportable Pressure Equipment) (Amendment) Regulations 2013, S.I. No. 238 of To comply with the above regulations the waste from patients with the Ebola virus must be packaged in line with this guidance document. If you have any queries regarding this guidance document, please contact your Dangerous Goods Safety Advisor. Revision /10/2014 Page 3 of 16
4 4 PROTEC620 packaging This section outlines instructions for the use of the Mauser WIVA PROTEC620 packaging for waste from persons with suspect or confirmed cases of the Ebola Virus. Step 1 The outer box should be opened and the drum removed outside of the treatment room. Open the Box by folding the flaps outwards. Step 2 Remove the drum from the cardboard box and plastic packaging. Secure the cardboard box in a secure, safe, location outside the treatment room. Note: this cardboard box and outer plastic bag is not for disposal. This packaging is required for the safe transportation of the waste from the healthcare facility. Step 3 Line the drum with the 2 inner bags and fold back around the drum. Place granule pouch provided in the bottom of the inner plastic bag. The drum is now ready for use and can be brought to the treatment area. Step 4 Once the drum is ¾ full, secure the 1 st inner plastic bag with tie provided. Please note there are 3 cable ties provided on the lid of the drum. Secure the 2 nd inner plastic bag with the second tie provided. Revision /10/2014 Page 4 of 16
5 Step 5 Fold both bags inwards. Cover the bags with the polystyrene disc. Step 6 Place the lid on top and secure with the ring clamp. Step 7 Place the blue seal in opening on the clamp band. Please note this seal is provided on the back of the lid. Decontaminate external surface for the blue drum with 10,000ppm hypochlorite solution before it is removed from the treatment room. Step 8 Remove the drum from the treatment room. Retrieve the cardboard box from the secure location. Step 9 Place at the drum in the outer plastic bag and secure with the third cable tie provided. Revision /10/2014 Page 5 of 16
6 Step 10 Place the sealed drum in the cardboard box and close flaps in sequence 1 to 4 whilst removing adhesive strips. Affix the infectious substance label to the box. Remove the package to a secure facility pending collection. Revision /10/2014 Page 6 of 16
7 5 Alternative Packaging The Health and Safety Authority (HSA) has issued an exemption whereby alternative packaging can be used for waste from persons with suspect or confirmed cases of the Ebola virus. The guidance in this section must be strictly followed to ensure that the alternative packaging meets the requirements of the HSA exemption. 5.1 General Provisions: The following general provisions shall be complied with: 1. Medical or clinical wastes suspected of containing Ebola virus shall not be transported by road unless diagnosis of the patient/source has been confirmed. Pending diagnosis, the waste shall be packaged in accordance with section 5 of this document and stored in a secure location at the healthcare facility. 2. If the diagnosis is proven to be negative, the waste shall be treated as Category B waste and disposed of with standard clinical waste as appropriate. 3. If the diagnosis is proven to be positive, the waste shall be treated as Infectious Substances Category A waste. Additional packaging procedures in addition to those outlined in section 5 will be required prior to transport. The waste disposal company will advise you of these requirements. Revision /10/2014 Page 7 of 16
8 5.2 Packaging type The following packaging is permitted under this exemption from the Health and Safety Authority..Soft waste (blood/ bandages, tubing) Primary packaging Standard clinical waste bag. Secondary packaging Tertiary packaging Standard clinical waste bag (double bagging). 30/60 litre clinical waste rigid bin. Sufficient absorbent material must be placed in the bin to absorb the entire liquid contents. Sharps Waste (e.g. needles, syringes) Primary packaging Standard sharps bin. Secondary packaging Tertiary packaging Two layers of clinical waste bags 30/60 litre clinical waste rigid bin. Sufficient absorbent material must be placed in the bin to absorb the entire liquid contents. Liquid waste (e.g. urine bags) Primary packaging Standard clinical waste bag. Secondary packaging Tertiary packaging Standard clinical waste bag. (double bagging) 30 litre clinical waste rigid bin. Sufficient absorbent material must be placed in the bin to absorb twice the liquid content. The maximum quantity of liquids per bin should be 5 litres. Revision /10/2014 Page 8 of 16
9 Notes: Clinical waste bags must be in-situ within the clinical waste rigid bin prior to adding the waste. Each clinical waste bag must be closed individually using the swan neck closure mechanism and plastic tie. A minimum of one 6 litre absorbent sheet or equivalent, should be placed in the 30/60 litre rigid bin. For liquids a minimum of two 6 litre absorbent sheets or equivalent, should be placed in the 30 litre rigid bin. The maximum quantity of liquids that can be disposed of in a 30 litre bin is 5 litres. 5.3 Assembly of packaging The following is the step by step guide for the assembly of the alternative packaging for waste contaminated or suspected of being contaminated with Ebola Virus. Soft waste i.e. bandages, gloves, ppe, tubing. Step 1 Insert absorption material into the bottom of the 30/60 litre rigid bin. (Recommended: 6 litre absorbent sheet or equivalent) Step 2 Line the 30/60 litre rigid bin with the 2 inner clinical waste bags and fold back around the bin. Step 3 The bin is now ready for the disposal of soft clinical waste. Revision /10/2014 Page 9 of 16
10 Step 4 When the bin is 2/3 full close the inner bag using the swan neck closure method with a plastic tie. Step 5: Close the outer bag using the swan neck closure method with a plastic tie. Step 6: Close the 30/60 litre rigid bin with a yellow lid. Ensure that the clips on the lid match up prior to closure. Step 7: When closed decontaminate external surface of the 30/60 litre rigid bin with 10,000ppm hypochlorite solution before it is removed from the treatment room. Remove the package to a secure storage room. Revision /10/2014 Page 10 of 16
11 Sharps waste i.e. needles, syringes, empty glass vials and bottles. Step 1 Insert absorbent material into the bottom of the 30/60 litre rigid bin. (Recommended: 6 litre absorbent sheet or equivalent) Step 2 Line the 30/60 litre rigid bin with the 2 inner clinical waste bags and fold back around the bin. Step 3 For sharps waste place the waste in the sharps bin. Ensure the sharps bin selected will fit comfortably into a 30/60 litre clinical waste rigid bin. Maintain sharps bins in the temporary closure position when being used by staff. When ¾ full or up to the manufacturer line lock the sharps bin. Sign and date upon closure. Step 4 Place the sharps bin into the 30/60 litre rigid bin. Step 5 Close the inner bag using the swan neck closure method with a plastic tie. Revision /10/2014 Page 11 of 16
12 Step 6 Close the outer bag using the swan neck closure method with a plastic tie. Step 7 Close the 30/60 litre rigid bin with a yellow lid. Ensure that the clips on the lid match up prior to closure. Step 8 When closed decontaminate the external surface of the 30/60 litre rigid bin with 10,000ppm hypochlorite solution before it is removed from the treatment room. Remove the package to a secure storage room. Note: Do not dispose of sharps bins in the same bin as soft waste. Revision /10/2014 Page 12 of 16
13 Liquid waste i.e. urine bags, blood bags. Step 1 Insert absorbent material into the bottom of a 30 litre rigid bin. (Recommended: two 6 litre absorbent sheets or equivalent) Note: The maximum quantity of liquid waste that should be disposed of in the bin is 5 litres. Step 2 Line the 30 litre rigid bin with the 2 inner clinical waste bags and fold back around the bin. Step 3 The bin is now ready for the disposal of liquid waste i.e. blood bags, urine bags. Step 4 When the bin is 2/3 full close the inner bag using the swan neck closure method with a plastic tie. Note: A maximum of 5 litres of liquid can be placed in the 30 litre bin. Step 5: Close the outer bag using the swan neck closure method with a plastic tie. Revision /10/2014 Page 13 of 16
14 Step 6: Close the 30 litre rigid bin with a yellow lid. Ensure that the clips on the lid match up prior to closure. Step 7: When closed decontaminate the external surface of the 30 litre rigid bin with 10,000ppm hypochlorite solution before it is removed from the treatment room. Remove the package to a secure storage room. Revision /10/2014 Page 14 of 16
15 5.4 Additional packaging and handling procedures Other dangerous substances shall not be packaged in the same packaging unless necessary for neutralising the hazardous nature of the infectious substances. 5.5 Labelling of packaging Each 30/60 litre rigid bin once removed from the treatment room must have a label with the following information: CATEGORY A EBOLA WASTE - QUERY CASE (Minimum dimensions 12 mm). Reference to the patient where the waste originated. Signature and date of responsible person. If the diagnosis is proven to be negative the 30/60 litre clinical waste rigid bin will have a label with the following: NEGATIVE DIAGNOSIS - Standard Clinical Waste (Minimum dimensions.12 mm). Reference to the patient where the waste originated. Signature and date of responsible person. If the diagnosis is proven to be positive the 30/60 litre clinical waste rigid bin will have a label with the following: CATEGORY A EBOLA WASTE - CONFIRMED CASE (Minimum dimensions.12 mm). Reference to the patient where the waste originated. Signature and date of responsible person. The 30 litre rigid bins containing liquid waste must have a label with the words Liquid waste (Minimum dimensions: 12 mm) 5.6 Storage The 30/60 litre clinical waste rigid bin must be stored in a locked and well ventilated covered facility on-site pending diagnosis. If the diagnosis is proven to be negative, the waste shall be removed from the storage facility and disposed of with standard clinical waste as appropriate. If the diagnosis is proven to be positive, the waste shall remain in the locked facility until the vehicle from the waste disposal company arrives for collection. Revision /10/2014 Page 15 of 16
16 6 Collection If the event of a suspect case of the Ebola virus the waste disposal company should be contacted immediately. The waste disposal company will advise on further packaging procedures to be adopted prior to transporting the waste by road. Revision /10/2014 Page 16 of 16
Procedure for Health Care Risk Waste Management. Procedure No. 403
 Procedure for Health Care Risk Waste Management Procedure No. 403 Print Name Title Date Prepared by J.G. MacNamara T.S.O. 01/11/04 Reviewed by J. Hoare CATSO 01/11/04 Corporate Authorisation J.G. MacNamara
Procedure for Health Care Risk Waste Management Procedure No. 403 Print Name Title Date Prepared by J.G. MacNamara T.S.O. 01/11/04 Reviewed by J. Hoare CATSO 01/11/04 Corporate Authorisation J.G. MacNamara
THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE
 Section V THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE The Trust is currently reviewing the requirements of the recent guidelines Health Technical Memorandum Safe Management of Healthcare Waste (HTML 07-01).
Section V THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE The Trust is currently reviewing the requirements of the recent guidelines Health Technical Memorandum Safe Management of Healthcare Waste (HTML 07-01).
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS
 UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
Standard Operating Procedures
 Document number: HSWM-0003 Standard Operating Procedure Title: Segregation & Disposal of from Theatres Written by: Martin Long Change Control Version: Date Author(s): Summary of Changes: 0.1 26.11.10 Martin
Document number: HSWM-0003 Standard Operating Procedure Title: Segregation & Disposal of from Theatres Written by: Martin Long Change Control Version: Date Author(s): Summary of Changes: 0.1 26.11.10 Martin
Collection, Transport & Disposal of Ebola Contaminated Wastes Reviewed: 30 October 2014
 THE COLLECTION, TRANSPORT AND DISPOSAL OF EBOLA CONTAMINATED WASTES 1. HAZARDS: Ebola is categorised as UN 2814 Infectious Substance - Category A: An infectious substance which is transported in a form
THE COLLECTION, TRANSPORT AND DISPOSAL OF EBOLA CONTAMINATED WASTES 1. HAZARDS: Ebola is categorised as UN 2814 Infectious Substance - Category A: An infectious substance which is transported in a form
Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date:
 STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
Clinical Waste. Policy. Responsibilities
 Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
In-Country shipment : How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea Step 1: Before handling the sample, prepare all shipping equipment (1) Manage
How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea
 INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
INTERIM GUIDELINE How to safely ship human blood samples from suspected Ebola cases within a country by road, rail and sea 2014 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
UN3373 Sample Transport Are You at Risk?
 UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
WHO NEEDS TO KNOW THIS PROGRAM All NYU employees that generate, handle or transport RMW should be familiar with this written program.
 Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
UN3373 Sample Transport Are You at Risk?
 UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
UN3373 Sample Transport Are You at Risk? Find out how to package and transport biological samples safely and in accordance with the UN3373 regulations. Supporting Category B sample transport compliance
Subject: Ebola Waste Management UPDATE Rev. 3
 !!!!!!!!!A"SAFE"AND"CLEAN"SOLUTION"FOR"YOUR"MEDICAL"WASTE"STREAM"" October 17, 2014 Subject: Ebola Waste Management UPDATE Rev. 3 Dear Valued Customer: Many of our customers have been asking about procedures
!!!!!!!!!A"SAFE"AND"CLEAN"SOLUTION"FOR"YOUR"MEDICAL"WASTE"STREAM"" October 17, 2014 Subject: Ebola Waste Management UPDATE Rev. 3 Dear Valued Customer: Many of our customers have been asking about procedures
Standard Operating Procedure Title: Good laboratory practices (GLP) for microbiology and chemistry laboratories
 Standard Operating Procedure Title: Good laboratory practices (GLP) for microbiology and chemistry laboratories Department Micro Laboratory Document no MICLAB 155 Title Good laboratory practices (GLP)
Standard Operating Procedure Title: Good laboratory practices (GLP) for microbiology and chemistry laboratories Department Micro Laboratory Document no MICLAB 155 Title Good laboratory practices (GLP)
Disposal of biological waste from containment level 2 laboratories in the WIMM
 Disposal of biological waste from containment level 2 laboratories in the WIMM This document describes how biological waste from containment level 2 laboratories in the WIMM is managed. Written by Malcolm
Disposal of biological waste from containment level 2 laboratories in the WIMM This document describes how biological waste from containment level 2 laboratories in the WIMM is managed. Written by Malcolm
UN 3291 Clinical and Related Wastes Services Specification
 SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
ENHANCED CONTAINMENT CL2 + (Level 3 practices in a Level 2 lab)
 ENHANCED CONTAINMENT CL2 + (Level 3 practices in a Level 2 lab) The use of Containment Level 3 (CL3) practices and procedures (as listed below) in a Containment Level 2 lab (often referred to as CL2+ ),
ENHANCED CONTAINMENT CL2 + (Level 3 practices in a Level 2 lab) The use of Containment Level 3 (CL3) practices and procedures (as listed below) in a Containment Level 2 lab (often referred to as CL2+ ),
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples
 Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Consignment of Waste to the Disposal of Hazardous Waste Service
 Radioactive Waste LR 31 Who should read this document This document should be read by the Head of Department, Departmental Radiation Protection Supervisors, users of radioactive materials and their supervisors.
Radioactive Waste LR 31 Who should read this document This document should be read by the Head of Department, Departmental Radiation Protection Supervisors, users of radioactive materials and their supervisors.
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea
 How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
How to safely ship human blood samples from Lassa cases within a country by road, rail and sea Interim Guidance February 2018 Step 1: Before handling the sample, prepare all shipping equipment Step 1a:
Contents. Introduction
 Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
Copyright 2017 Aegis Sciences Corporation All Rights Reserved
 Oral Fluid Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control
Oral Fluid Collection Healthcare Collection Site Preparation 1. The collection site must have all the supplies needed to complete a specimen collection (e.g. collection kits, ink pens, Custody and Control
East Building, PHH-30 U.S. Department New Jersey Avenue S.E. of Transportation Washington, D.C DOT-SP 16279
 East Building, PHH-30 U.S. Department 1200 New Jersey Avenue S.E. of Transportation Washington, D.C. 20590 Pipeline and Hazardous Materials Safety Administration DOT-SP 16279 1. GRANTEE: (See individual
East Building, PHH-30 U.S. Department 1200 New Jersey Avenue S.E. of Transportation Washington, D.C. 20590 Pipeline and Hazardous Materials Safety Administration DOT-SP 16279 1. GRANTEE: (See individual
10.0 DISPOSAL OF FARM WASTE
 DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
/ Clinical Waste & Offensive Waste Disposal Procedures
 / Clinical Waste & Offensive Waste Disposal Procedures Document Control Document Created by Shane McAteer Ver. 1 26/01/2011 Gary Stratmann Ver. 3.1 08/01/2016 Last Updated by 1 Introduction This clinical
/ Clinical Waste & Offensive Waste Disposal Procedures Document Control Document Created by Shane McAteer Ver. 1 26/01/2011 Gary Stratmann Ver. 3.1 08/01/2016 Last Updated by 1 Introduction This clinical
Waste Management. School Safety Advisor School of Chemistry UCD
 Waste Management School Safety Advisor School of Chemistry UCD Waste Who s responsibility is waste? School waste procedures School hazardous waste management plan School policy - enforced in all labs Clear
Waste Management School Safety Advisor School of Chemistry UCD Waste Who s responsibility is waste? School waste procedures School hazardous waste management plan School policy - enforced in all labs Clear
Classification and packaging for infectious waste of Category A. Transmitted by the experts from Canada and the United Kingdom *
 United Nations Secretariat ST/SG/AC.10/C.3/2017/25 Distr.: General 13 April 2017 Original: English Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification
United Nations Secretariat ST/SG/AC.10/C.3/2017/25 Distr.: General 13 April 2017 Original: English Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification
Our Job: Prevent dis-ease as well as disease
 Our Job: Prevent dis-ease as well as disease 1 Kansas Ebola Virus Preparedness and Response Plan: Revised 15 October 2014 2 Background and Situation Update 3 West Africa (As of 12 October 2014) Total Cases
Our Job: Prevent dis-ease as well as disease 1 Kansas Ebola Virus Preparedness and Response Plan: Revised 15 October 2014 2 Background and Situation Update 3 West Africa (As of 12 October 2014) Total Cases
GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS
 GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS Table of Contents General Information Regulations Definitions Recommended packing procedure Universal precautions
GUIDELINES FOR SHIPPING NONPATHOGENIC BIOLOGICAL CULTURES AND NON-INFECTIOUS BIOLOGICAL MATERIALS Table of Contents General Information Regulations Definitions Recommended packing procedure Universal precautions
LABORATORY WASTE DISPOSAL GUIDE
 LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
Operational Policy. November 2009
 Operational Policy Waste Policy November 2009 Printed copies of this document may not be up to date. Always obtain the most recent version from GOSH Document Library. Document Control Information Lead
Operational Policy Waste Policy November 2009 Printed copies of this document may not be up to date. Always obtain the most recent version from GOSH Document Library. Document Control Information Lead
PROGRAM FOR THE MANAGEMENT AND DISPOSAL OF RADIOACTIVE WASTE
 RADIATION SAFETY DEPARTMENT West Virginia University Health Sciences Center WVU Hospitals Jefferson Memorial Hospital G-139 Health Sciences Center PO Box 9006 Morgantown, WV 26506-9006 Phone: 304-293-3413
RADIATION SAFETY DEPARTMENT West Virginia University Health Sciences Center WVU Hospitals Jefferson Memorial Hospital G-139 Health Sciences Center PO Box 9006 Morgantown, WV 26506-9006 Phone: 304-293-3413
Environmental Management System
 Environmental Management System Moreton Bay Research Station (MBRS) Clinical and Related Waste 1. Scope This policy applies to all clinical and related waste (refer to definitions in Section 6) from the
Environmental Management System Moreton Bay Research Station (MBRS) Clinical and Related Waste 1. Scope This policy applies to all clinical and related waste (refer to definitions in Section 6) from the
BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018
 1 BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018 BIOMEDICAL WASTE RULES APPLY TO:- All who generate, collect, receive, store, transport, treat, dispose, or handle bio medical waste :- Hospitals,
1 BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018 BIOMEDICAL WASTE RULES APPLY TO:- All who generate, collect, receive, store, transport, treat, dispose, or handle bio medical waste :- Hospitals,
COLLECTION AND TRANSPOSRT OF SPECIMENS
 COLLECTION AND TRANSPOSRT OF SPECIMENS Version 1 Abstract To provide accurate guidelines in the transport and collection of specimens for both internal and external users to ensure that the specimens are
COLLECTION AND TRANSPOSRT OF SPECIMENS Version 1 Abstract To provide accurate guidelines in the transport and collection of specimens for both internal and external users to ensure that the specimens are
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
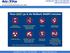 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
Last Revised: 1/2018 Prior Version: 12/2014 SOP NUMBER: SC-402 Page 1 of 9
 SOP NUMBER: SC-402 Page 1 of 9 1. PURPOSE: To standardize and describe the process for safely transporting human research participant specimens within, to or from University Hospitals (UH) facilities.
SOP NUMBER: SC-402 Page 1 of 9 1. PURPOSE: To standardize and describe the process for safely transporting human research participant specimens within, to or from University Hospitals (UH) facilities.
Cytotoxic Waste Sorting, Handling and Disposal
 Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
Approved by: Cytotoxic Waste Sorting, Handling and Disposal Corporate Director, Environmental Supports Environmental Services Operating Standards Manual Number: 3.1.3.14 Date Approved Next Review December
Next Review Date: Oct 2017 SWP Reference Number: Version: V3 Version Issue Date: Oct 2016
 Faculty/School: Faculty of Pharmacy Next Review Date: Oct 2017 SWP Reference Number: Version: V3 Version Issue Date: Oct 2016 SWP Title: Operation of the autoclaves Prepared by: Padmaja Dhanvate and Dr
Faculty/School: Faculty of Pharmacy Next Review Date: Oct 2017 SWP Reference Number: Version: V3 Version Issue Date: Oct 2016 SWP Title: Operation of the autoclaves Prepared by: Padmaja Dhanvate and Dr
LABORATORY WASTE MANAGEMENT PROGRAMMES
 LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
1.1 SUMMARY.1 Comply with requirements of this Section when performing following work:
 Page 1 of 6 Part 1 General 1.1 SUMMARY.1 Comply with requirements of this Section when performing following work:.1 Removing roofing materials that are suspected of containing asbestos using wet material
Page 1 of 6 Part 1 General 1.1 SUMMARY.1 Comply with requirements of this Section when performing following work:.1 Removing roofing materials that are suspected of containing asbestos using wet material
University Health Services Health and Safety
 ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
You are required to complete this course with a passing score of 85% or higher if you:
 OHS Biosafety BIO301L Medical Waste Management for Labs Training You are required to complete this course with a passing score of 85% or higher if you: Offer medical waste for transport from a UAB campus
OHS Biosafety BIO301L Medical Waste Management for Labs Training You are required to complete this course with a passing score of 85% or higher if you: Offer medical waste for transport from a UAB campus
BIOMEDICAL WASTE MANAGEMENT POLICY
 BIOMEDICAL WASTE MANAGEMENT POLICY Managing biomedical waste in a safe and environmentally responsible manner is a key focus of the Environmental Compliance and Sustainability office. 1.0 PURPOSE In an
BIOMEDICAL WASTE MANAGEMENT POLICY Managing biomedical waste in a safe and environmentally responsible manner is a key focus of the Environmental Compliance and Sustainability office. 1.0 PURPOSE In an
Dossier: What s the difference between P 650 (ADR) and PI 650 (IATA)?
 DECEMBER 2015 Dossier: What s the difference between P 650 (ADR) and PI 650 (IATA)? Packing instruction 650 from both ADR and IATA imposes certain requirements on the packaging used for the transport of
DECEMBER 2015 Dossier: What s the difference between P 650 (ADR) and PI 650 (IATA)? Packing instruction 650 from both ADR and IATA imposes certain requirements on the packaging used for the transport of
DRAFT. Module 6. HEALTHCARE WASTE MANAGEMENT
 Module 6. HEALTHCARE WASTE MANAGEMENT Existing situation Learning Objectives To explain types of healthcare waste and associated risks To outline policy & guidelines on Healthcare Waste Management (HCWM)
Module 6. HEALTHCARE WASTE MANAGEMENT Existing situation Learning Objectives To explain types of healthcare waste and associated risks To outline policy & guidelines on Healthcare Waste Management (HCWM)
Transport of Infectious Substances. Best Practice Guidance for Microbiology Laboratories
 Transport of Infectious Substances Best Practice Guidance for Microbiology Laboratories Transport of Infectious Substances Best Practice Guidance for Microbiology Laboratories Prepared by: Inspector of
Transport of Infectious Substances Best Practice Guidance for Microbiology Laboratories Transport of Infectious Substances Best Practice Guidance for Microbiology Laboratories Prepared by: Inspector of
Emergency Medical Service General Order
 Date of Issue: October 15, 2014 Effective Date: October 15, 2014 Plan Classification: Field Operations Commissioner of : No. Pages: 1 of 12 Rescinds: All previous directives Purpose: The U.S. Department
Date of Issue: October 15, 2014 Effective Date: October 15, 2014 Plan Classification: Field Operations Commissioner of : No. Pages: 1 of 12 Rescinds: All previous directives Purpose: The U.S. Department
Trish Purfit Facilities Manager. Judy Carr - Lead Infection Prevention and Control Nurse. Policy and Procedures Committee Date: 17/12/15
 Facilities and Estates Waste Management: Standard Operating Procedure Document Control Summary Status: New Version: v1.0 Date: August 2015 Author/Title: Owner/Title: Trish Purfit Facilities Manager Judy
Facilities and Estates Waste Management: Standard Operating Procedure Document Control Summary Status: New Version: v1.0 Date: August 2015 Author/Title: Owner/Title: Trish Purfit Facilities Manager Judy
Table of Content. 1. Introduction. 2. Temperature Controlled Shipping. 3. Packaging, Labeling and Marking. 4. Shipment Documentation
 March 2017 Table of Content 1. Introduction 2. Temperature Controlled Shipping 3. Packaging, Labeling and Marking 3.1 Exempt Specimen 3.2 Category B 3.3 Category A 4. Shipment Documentation 5. Health
March 2017 Table of Content 1. Introduction 2. Temperature Controlled Shipping 3. Packaging, Labeling and Marking 3.1 Exempt Specimen 3.2 Category B 3.3 Category A 4. Shipment Documentation 5. Health
TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015
 TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015 The TJEMS Drug Box Program Best Practices relate to the use of the TJEMS Drug Box. These best practices sever to provide guidance
TJEMS Drug Box Program Best Practices Reviewed: Updated: November 2015 The TJEMS Drug Box Program Best Practices relate to the use of the TJEMS Drug Box. These best practices sever to provide guidance
THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY
 THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY STANDARD OPERATING PROCEDURE PROCEDURE NO: GDP 020 MOD: 1st Issue Page: 1 of 5 Procedure Type: General Discipline Procedure Title: Handling
THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY STANDARD OPERATING PROCEDURE PROCEDURE NO: GDP 020 MOD: 1st Issue Page: 1 of 5 Procedure Type: General Discipline Procedure Title: Handling
Laboratory Waste Disposal
 Laboratory Waste Disposal 1. Purpose This guideline details the procedures to follow in disposing of hazardous waste that is generated in the laboratory in order to minimise risks associated with the disposal
Laboratory Waste Disposal 1. Purpose This guideline details the procedures to follow in disposing of hazardous waste that is generated in the laboratory in order to minimise risks associated with the disposal
BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY
 BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY 2 Preamble This document describes the procedures to follow for safely
BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY 2 Preamble This document describes the procedures to follow for safely
Classification and packaging for infectious waste of Category A
 United Nations Secretariat ST/SG/AC.10/C.3/2018/20 Distr.: General 29 March 2018 Original: English Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification
United Nations Secretariat ST/SG/AC.10/C.3/2018/20 Distr.: General 29 March 2018 Original: English Committee of Experts on the Transport of Dangerous Goods and on the Globally Harmonized System of Classification
MODULE 14: Off-site Transport and Storage of Healthcare Waste
 MODULE 14: Off-site Transport and Storage of Healthcare Waste Module Overview Define external transport of healthcare waste Describe requirements for off-site transport of healthcare wastes including training
MODULE 14: Off-site Transport and Storage of Healthcare Waste Module Overview Define external transport of healthcare waste Describe requirements for off-site transport of healthcare wastes including training
Appendix J. University of Alabama at Birmingham Medical Waste Management Plan
 University of Alabama at Birmingham Medical Waste Management Plan I. Definitions per the Alabama Department of Environmental Management Land Division 13-Solid Waste Program, Chapter 335-13-1, Medical Waste
University of Alabama at Birmingham Medical Waste Management Plan I. Definitions per the Alabama Department of Environmental Management Land Division 13-Solid Waste Program, Chapter 335-13-1, Medical Waste
HackensackUMC Sustainability Program. Presented by Kyle Tafuri Sustainability Advisor
 HackensackUMC Sustainability Program Presented by Kyle Tafuri Sustainability Advisor About HackensackUHN HackensackUMC HackensackUMC Mountainside HackensackUMC at Pascack Valley Palisades Healthcare System
HackensackUMC Sustainability Program Presented by Kyle Tafuri Sustainability Advisor About HackensackUHN HackensackUMC HackensackUMC Mountainside HackensackUMC at Pascack Valley Palisades Healthcare System
Protocol for Cytotoxic Spills and Waste Management
 Occupational Health and Safety (OHS) Policy Document Number # QH-PTL-275-3-2:2012 Protocol for Cytotoxic Spills and Waste Management Custodian/Review O fficer: Graham Easterby, a/director, Safety and Wellbeing.
Occupational Health and Safety (OHS) Policy Document Number # QH-PTL-275-3-2:2012 Protocol for Cytotoxic Spills and Waste Management Custodian/Review O fficer: Graham Easterby, a/director, Safety and Wellbeing.
Refer to the VCH Intranet to make sure you are using the most updated protocol
 Updated 0 April 05 Refer to the VCH Intranet to make sure you are using the most updated protocol General Guidelines:. Phlebotomy should be done by experienced personnel.. Specimen collection must not
Updated 0 April 05 Refer to the VCH Intranet to make sure you are using the most updated protocol General Guidelines:. Phlebotomy should be done by experienced personnel.. Specimen collection must not
USP800: A quick summary and disposal. Brenda Denson, Pharm.D.
 USP800: A quick summary and disposal Brenda Denson, Pharm.D. Objectives Review current USP800 guidelines on destruction of hazardous medications. Illustrate how a local pharmacy demonstrates compliance
USP800: A quick summary and disposal Brenda Denson, Pharm.D. Objectives Review current USP800 guidelines on destruction of hazardous medications. Illustrate how a local pharmacy demonstrates compliance
Infectious Waste Disposal Procedure 25 PA Code 284 Ursinus College Collegeville, PA 19426
 The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
Central Bio-Medical Waste Treatment Facility
 Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Special Specimen Collection Procedures for Coagulation Testing
 Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Dartmouth College. Institutional Biosafety Committee. Biohazardous Waste Disposal Guide IBC Approved: 10/3/18
 Biohazardous Waste Disposal IBC Approved: 10/3/18 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical laboratories that may contain infectious
Biohazardous Waste Disposal IBC Approved: 10/3/18 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical laboratories that may contain infectious
Dartmouth College. Institutional Biosafety Committee. Biohazardous Waste Disposal Guide IBC Approved: 4/5/17
 Institutional Biosafety Committee Biohazardous Waste Disposal IBC Approved: 4/5/17 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical
Institutional Biosafety Committee Biohazardous Waste Disposal IBC Approved: 4/5/17 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical
SESSION 4 AND HOSPITAL-GENERATED WASTE WASTE DISPOSAL AT THE MEDICAL CENTER OF THE UNIVERSITY OF ILLINOIS. Stephens. Raymond S.
 SESSION 4 RESEARCH- AND HOSPITAL-GENERATED WASTE WASTE DISPOSAL AT THE MEDICAL CENTER OF THE UNIVERSITY OF ILLINOIS Raymond S. Stephens This paper examines the main problems of hazardous waste disposal
SESSION 4 RESEARCH- AND HOSPITAL-GENERATED WASTE WASTE DISPOSAL AT THE MEDICAL CENTER OF THE UNIVERSITY OF ILLINOIS Raymond S. Stephens This paper examines the main problems of hazardous waste disposal
Location, Location, Location! Environmental Services
 Location, Location, Location! Environmental Services Kalpana Rengarajan, PhD, MPH, JM, RBP Director Research Safety / Biosafety Officer Environmental Health and Safety Office Office of Research Administration
Location, Location, Location! Environmental Services Kalpana Rengarajan, PhD, MPH, JM, RBP Director Research Safety / Biosafety Officer Environmental Health and Safety Office Office of Research Administration
Policy & Procedure Manual
 Environmental Health & Safety Policy & Procedure Manual Title: 1. Purpose: To establish policies, work practices, and systematic procedures for the handling, packaging, collection, treatment, and disposal
Environmental Health & Safety Policy & Procedure Manual Title: 1. Purpose: To establish policies, work practices, and systematic procedures for the handling, packaging, collection, treatment, and disposal
PACKAGING AND LABELLING OF HAZARDOUS WASTE
 PACKAGING AND LABELLING OF HAZARDOUS WASTE This guide has been produced to provide you with an overview of the basic steps to be taken when preparing waste for movement off your site. TABLE OF CONTENTS
PACKAGING AND LABELLING OF HAZARDOUS WASTE This guide has been produced to provide you with an overview of the basic steps to be taken when preparing waste for movement off your site. TABLE OF CONTENTS
Standard Operating Procedure for performing a muscle biopsy
 Standard Operating Procedure for performing a muscle biopsy Effective date: 31.10.2016 Review due date: 30.08.2018 Original Author Name: Richard Metcalfe and Abdullah Alghannam Date: 14.12.2012 Position:
Standard Operating Procedure for performing a muscle biopsy Effective date: 31.10.2016 Review due date: 30.08.2018 Original Author Name: Richard Metcalfe and Abdullah Alghannam Date: 14.12.2012 Position:
Eastman AdapT technical services sample kit procedure
 Eastman AdapT technical services sample kit procedure Introduction This sample kit is offered to customers by the Eastman AdapT Technical Service Department to facilitate the analysis of contaminated amine
Eastman AdapT technical services sample kit procedure Introduction This sample kit is offered to customers by the Eastman AdapT Technical Service Department to facilitate the analysis of contaminated amine
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information Washington
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Washington Biomedical Waste Regulation In the State of Washington biomedical waste, where regulated, is regulated
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Washington Biomedical Waste Regulation In the State of Washington biomedical waste, where regulated, is regulated
Purpose. Scope and restrictions. Responsibilities. Faculty of Health and Wellbeing Biosciences STANDARD OPERATING PROCEDURE.
 Faculty of Health and Wellbeing Biosciences STANDARD OPERATING PROCEDURE Title: Disposal of Clinical waste bins SOP code: 017-SOP-01 Date: 26/11/2012 Author: Purpose Karen Bailey-Smith and Kay Simmonite
Faculty of Health and Wellbeing Biosciences STANDARD OPERATING PROCEDURE Title: Disposal of Clinical waste bins SOP code: 017-SOP-01 Date: 26/11/2012 Author: Purpose Karen Bailey-Smith and Kay Simmonite
Lisa Young David Jansen. Public Health Always Working for a Safer and Healthier Washington
 Lisa Young David Jansen Public Health Always Working for a Safer and Healthier Washington Radioactive waste, asbestos, execution chemicals, narcotics Straightforward, Yes? 2 Subdivides into two situations
Lisa Young David Jansen Public Health Always Working for a Safer and Healthier Washington Radioactive waste, asbestos, execution chemicals, narcotics Straightforward, Yes? 2 Subdivides into two situations
EXPIRATION DATE: November 30, 2014
 East Building, PHH-30 U.S. Department 1200 New Jersey Avenue S.E. of Transportation Washington, D.C. 20590 Pipeline and Hazardous Materials Safety Administration DOT-SP 16278 EXPIRATION DATE: November
East Building, PHH-30 U.S. Department 1200 New Jersey Avenue S.E. of Transportation Washington, D.C. 20590 Pipeline and Hazardous Materials Safety Administration DOT-SP 16278 EXPIRATION DATE: November
transport of biological materials
 UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO STANDARD OPERATING PROCEDURE transport of biological materials Prepared by Date Adopted Procedure # IBC Approved EHS B001 May 13, 2014 ENVIRONMENTAL
UNIVERSITY OF TEXAS HEALTH SCIENCE CENTER AT SAN ANTONIO STANDARD OPERATING PROCEDURE transport of biological materials Prepared by Date Adopted Procedure # IBC Approved EHS B001 May 13, 2014 ENVIRONMENTAL
3R approach towards bio-medical waste management
 3R approach towards bio-medical waste management 7 th IconSWM Conference 15-17 December 2017, Hyderabad, India Anupam Khajuria Researcher United Nations Centre for Regional Development (UNCRD), Japan 1
3R approach towards bio-medical waste management 7 th IconSWM Conference 15-17 December 2017, Hyderabad, India Anupam Khajuria Researcher United Nations Centre for Regional Development (UNCRD), Japan 1
Guidelines for the Use of Cytotoxic or Chemotherapeutic Drugs
 Guidelines for the Use of Cytotoxic or Chemotherapeutic Drugs Caltech Environment, Health and Safety 1200 E. California Blvd. M/C 25-6 Pasadena, CA 91125 626.395.6727 safety@caltech.edu www.safety.caltech.edu
Guidelines for the Use of Cytotoxic or Chemotherapeutic Drugs Caltech Environment, Health and Safety 1200 E. California Blvd. M/C 25-6 Pasadena, CA 91125 626.395.6727 safety@caltech.edu www.safety.caltech.edu
Biosafety Checklist. 07-BiosafetyChecklist-LTC-SOP-v2.0-17Feb of 6
 Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
WASTE MANAGEMENT POLICY
 TRUST-WIDE SERVICE BASED POLICY DOCUMENT WASTE MANAGEMENT POLICY Policy Number: SA22 Scope of this Document: All Staff Recommending Committee: Health & Safety Committee Approving Committee: Executive Committee
TRUST-WIDE SERVICE BASED POLICY DOCUMENT WASTE MANAGEMENT POLICY Policy Number: SA22 Scope of this Document: All Staff Recommending Committee: Health & Safety Committee Approving Committee: Executive Committee
Radiation Safety Program Hunter College of the City University of New York
 Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
Tamper Evident Anti-Doping Equipment from Versapak
 Phone: +44 (0)20 8333 5300 Email: info@versapak-anti-doping.com www.versapak-anti-doping.com Tamper Evident Anti-Doping Equipment from Versapak PRODUCT GUIDE Versapak Doping Control Limited has been manufacturing
Phone: +44 (0)20 8333 5300 Email: info@versapak-anti-doping.com www.versapak-anti-doping.com Tamper Evident Anti-Doping Equipment from Versapak PRODUCT GUIDE Versapak Doping Control Limited has been manufacturing
GUIDELINES FOR TRANSPORTING ANTHRAX AND ANTHRAX-CONTAMINATED OBJECTS AND MATERIALS
 RESEARCH AND SPECIAL PROGRAMS ADMINISTRATION GUIDELINES FOR TRANSPORTING ANTHRAX AND ANTHRAX-CONTAMINATED OBJECTS AND MATERIALS What regulations apply to the transportation of anthrax and other infectious
RESEARCH AND SPECIAL PROGRAMS ADMINISTRATION GUIDELINES FOR TRANSPORTING ANTHRAX AND ANTHRAX-CONTAMINATED OBJECTS AND MATERIALS What regulations apply to the transportation of anthrax and other infectious
Requirements for the construction and testing of packagings for class 6.2 infectious substances of category A
 Chapter 6.3 6.3.1 General Requirements for the construction and testing of packagings for class 6. infectious substances of category A NOTE: The requirements of this Chapter don't apply to packagings used
Chapter 6.3 6.3.1 General Requirements for the construction and testing of packagings for class 6. infectious substances of category A NOTE: The requirements of this Chapter don't apply to packagings used
The University of Texas Health Science Center at San Antonio Environmental Health & Safety Department Radiation Safety Division
 Page 1 of 6 The University of Texas Health Science Center at San Antonio Environmental Health & Safety Department Radiation Safety Division RADIOACTIVE MATERIAL AUTHORIZATION INITIAL APPLICATION AMENDENT
Page 1 of 6 The University of Texas Health Science Center at San Antonio Environmental Health & Safety Department Radiation Safety Division RADIOACTIVE MATERIAL AUTHORIZATION INITIAL APPLICATION AMENDENT
Infectious Disease/EBOLA Guidelines for the School Clinic
 Infectious Disease/EBOLA Guidelines for the School Clinic Background: The procedures in the accompanying algorithm provide guidance on evaluation and management of individuals who present with possible
Infectious Disease/EBOLA Guidelines for the School Clinic Background: The procedures in the accompanying algorithm provide guidance on evaluation and management of individuals who present with possible
Packing, handling and storage information for Saft products
 PHSI 1.1 May 2015 Packing, handling and storage information for Saft products Saft AB, Box 709, SE-572 28 Oskarshamn, Sweden Visiting adress: Jugnergatan, Oskarshamn Phone: +46 (0)491 680 00 Fax: +46 (0)491
PHSI 1.1 May 2015 Packing, handling and storage information for Saft products Saft AB, Box 709, SE-572 28 Oskarshamn, Sweden Visiting adress: Jugnergatan, Oskarshamn Phone: +46 (0)491 680 00 Fax: +46 (0)491
SITE PROCEDURES NASAL SWAB
 SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
SITE PROCEDURES NASAL SWAB Nasal Swab Collection Nasal swab specimen labels EXAKT-PAK One Pak shipping system Cryogenic specimen vial with transport medium Outer transport tube with absorbent material
Biomedical Waste Management in Hospitals A Review
 May 2017 IRA-International Journal of Technology & Engineering ISSN 2455-4480; Vol.07, Issue 02 (2017) Pg. no. 10-16 Institute of Research Advances http://research-advances.org/index.php/irajte Biomedical
May 2017 IRA-International Journal of Technology & Engineering ISSN 2455-4480; Vol.07, Issue 02 (2017) Pg. no. 10-16 Institute of Research Advances http://research-advances.org/index.php/irajte Biomedical
UW LOCAL RISK ASSESSMENT WORKSHEET FOR WORK WITH BIOLOGICAL MATERIALS
 UW LOCAL RISK ASSESSMENT WORKSHEET FOR WORK WITH BIOLOGICAL MATERIALS Completed by: Date completed: Material description 1. Name or description of the material being handled: 2. Identify where the material
UW LOCAL RISK ASSESSMENT WORKSHEET FOR WORK WITH BIOLOGICAL MATERIALS Completed by: Date completed: Material description 1. Name or description of the material being handled: 2. Identify where the material
Customer Packaging Guidelines
 Customer Packaging Guidelines Customer Packaging Guidelines 05.02.16 v1 CONTENTS Introduction Packaging Tips: Parcels Envelopes Packaging Tips: Pallets Crates Drums Pipes Spools & Reels Packaging Tips:
Customer Packaging Guidelines Customer Packaging Guidelines 05.02.16 v1 CONTENTS Introduction Packaging Tips: Parcels Envelopes Packaging Tips: Pallets Crates Drums Pipes Spools & Reels Packaging Tips:
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information Florida
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Florida Biomedical Waste in Florida Regulated by Florida DOH Florida Administrative Code (FAC) 64E-16 Contains
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Florida Biomedical Waste in Florida Regulated by Florida DOH Florida Administrative Code (FAC) 64E-16 Contains
Zika Testing: Specimen Collection, Packaging and Shipping
 August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
August, 2017 New Jersey Public Health and Environmental Laboratories Technical Bulletin Supplement Update Zika Testing: Specimen Collection, Packaging and Shipping SAMPLE SPECIFICATIONS FOR ZIKA Trioplex
Management of Hazardous Wastes
 23 Management of Hazardous Wastes Dr. Mohammed Abu Kaff Environment & Public Health Expert Dubai Municipality Dubai. UAE Hazardous wastes have been defined by the United States Environmental Protection
23 Management of Hazardous Wastes Dr. Mohammed Abu Kaff Environment & Public Health Expert Dubai Municipality Dubai. UAE Hazardous wastes have been defined by the United States Environmental Protection
UNIVERSITY OF TOLEDO
 UNIVERSITY OF TOLEDO SUBJECT: SHIPPING, PACKAGING AND RECEIPT OF Procedure No: HM-08-031 HAZARDOUS MATERIALS PROCEDURE Employees of the University of Toledo attempting to transport hazardous materials,
UNIVERSITY OF TOLEDO SUBJECT: SHIPPING, PACKAGING AND RECEIPT OF Procedure No: HM-08-031 HAZARDOUS MATERIALS PROCEDURE Employees of the University of Toledo attempting to transport hazardous materials,
Standard Operating Procedure. Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples
 Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
Standard Operating Procedure Packing and Shipping of Ambient, Refrigerated, Frozen and Combined Biological Samples I. POLICY: The Puerto Rico Clinical and Translational Research Consortium (PRCTRC) ensure
WASTE MANAGEMENT GUIDE. Creighton University Environmental Health and Safety
 WASTE MANAGEMENT GUIDE Creighton University Environmental Health and Safety www.creighton.edu/ehs 402 546 6400 Overview With over 150 teaching and research labs, as well as other areas such as fine arts
WASTE MANAGEMENT GUIDE Creighton University Environmental Health and Safety www.creighton.edu/ehs 402 546 6400 Overview With over 150 teaching and research labs, as well as other areas such as fine arts
Regulated Medical Waste (RMW) Rules, Registration & Reports
 Regulated Medical Waste (RMW) Rules, Registration & Reports 1 Prepared by: Bret Reburn Envi. Specialist 3 Hazardous Waste/UST Compliance & Enforcement Central Regional Office - Trenton, NJ (609) 292-3949
Regulated Medical Waste (RMW) Rules, Registration & Reports 1 Prepared by: Bret Reburn Envi. Specialist 3 Hazardous Waste/UST Compliance & Enforcement Central Regional Office - Trenton, NJ (609) 292-3949
