INSTRUCTIONS FOR USE INFANT/CHILD REDUCED ENERGY DEFIBRILLATION ELECTRODES
|
|
|
- Natalie Wells
- 6 years ago
- Views:
Transcription
1 INSTRUCTIONS FOR USE INFANT/CHILD REDUCED ENERGY DEFIBRILLATION ELECTRODES
2 IMPORTANT INFORMATION!USA Rx only Infant/Child Reduced Energy Defibrillation Electrodes are to be used by authorized personnel only. Responsibility for Information It is the responsibility of our customers to ensure that the appropriate person(s) have access to the information in these instructions for use. Operators should be thoroughly familiar with their defibrillator operating instructions before using therapy electrodes. These instructions are specific to Infant/Child disposable products and are intended to complement local protocol and your defibrillator operating instructions. Medtronic Emergency Response Systems Willows Road Northeast Redmond, WA USA Telephone: Toll Free (USA only): Fax: Internet: Medtronic Europe S.A. Medtronic Emergency Response Systems Rte. du Molliau 31 Case postale Tolochenaz Switzerland Telephone: Fax: LIFEPAK, LIFEPAK CR, and LIFEPAK EXPRESS are registered trademarks of Medtronic Emergency Response Systems, Inc. ADAPTIV, PARTSLINE, QUIK-COMBO, and Shock Advisory System are trademarks of Medtronic Emergency Response Systems, Inc. Medtronic is a registered trademark of Medtronic, Inc. Specifications are subject to change without notice Medtronic Emergency Response Systems, Inc. All rights reserved. Publication Date: 11/2005 MIN / CAT ii Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use
3 TABLE OF CONTENTS General Warnings... 1 Symbols... 2 Indications for Use... 3 About The Electrodes... 4 Procedure for Use... 4 Cleaning and Sterilizing... 6 Product Recycling Information... 6 Recycling Assistance... 6 Preparation... 6 Recycling of Disposable Electrodes... 6 Packaging... 6 Ordering Information... 6 Specifications... 6 Defibrillator Compatibility... 7 Waveform Parameters for Pediatric Defibrillation... 7 Energy Parameters for Pediatric Defibrillation... 8 Electrode Pads... 8 Environmental... 9 Clinical Studies... 9 Non-Clinical Studies... 9 Declaration of Conformity Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use Medtronic Emergency Response Systems, Inc. iii
4
5 General Warnings English GENERAL WARNINGS The following are general warning statements. Warning: Hazards or unsafe practices that may result in serious personal injury or death. WARNINGS! Shock hazard. When discharged, a defibrillator delivers up to 360 joules of electrical energy. Do not touch the paddle electrode surface or the Infant/Child electrodes when discharging the defibrillator. Shock hazard. If a person is touching the patient, bed, or any conductive material in contact with the patient during defibrillation, the delivered energy may be partially discharged through that person. Make sure that everyone stands away from the patient, bed, and other conductive material before discharging the defibrillator. Ineffective energy delivery. Do not use Infant/Child electrodes on adults or large children. Delivering defibrillation energy (<100J) with these electrodes will reduce effectiveness. Possible skin burns. During defibrillation air pockets between the skin and Infant/Child electrodes can cause patient skin burns. Apply Infant/Child electrodes so that entire electrode adheres to skin. Do not reposition the electrodes once applied. If the position must be changed, remove and replace with new electrodes. Possible skin burns and ineffective energy delivery. Infant/Child electrodes that are dried out or damaged may cause electrical arcing and patient skin burns during defibrillation. Do not use electrodes that have been removed from foil package for more than 24 hours. Do not use electrodes beyond Use By date. Check that electrode adhesive is intact and undamaged. Possible fire or explosion. Do not use a defibrillator in the presence of flammable gases or anesthetics. Use care when operating this device close to oxygen sources (such as bag-valve-mask devices or ventilator tubing). Turn off gas source or move source away from patient during defibrillation. Possible fire, burns, and ineffective energy delivery. Do not discharge standard paddles on top of Infant/Child electrodes or ECG electrodes. Do not allow Infant/Child electrodes to touch each other, ECG electrodes, lead wires, dressings, transdermal patches, and the like. Such contact can cause electrical arcing and patient skin burns during defibrillation and may divert defibrillating energy away from the heart muscle. Possible interference with implanted electrical device. Defibrillation may cause implanted electrical devices to malfunction. Place Infant/Child electrodes away from implanted devices if possible. Check implanted device function after defibrillation. Possible electrical interference with device performance. Equipment operating in close proximity may emit strong electromagnetic or radio frequency interference (RFI) which could affect the performance of this device. RFI may result in improper device operation, distorted ECG, and failure to detect a shockable rhythm. Avoid operating the device near cauterizers, diathermy equipment, cellular phones, or other portable and mobile RF communications equipment. Maintain equipment separation of at least 1.2 m (4 ft) and do not rapidly key EMS radios on and off. Contact a technical support representative if assistance is required. Shock or fire hazard. Do not immerse any portion of the therapy cables or electrodes in water or other fluids. Avoid spilling any fluids on the adapters, cables, connectors, or Infant/Child electrodes. Do not autoclave, steam, or gas sterilize unless otherwise specified. Do not clean the Infant/Child electrodes or their permanently attached electrode cables with isopropyl alcohol. Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use Medtronic Emergency Response Systems, Inc.
6 Symbols WARNINGS! Safety risk and possible equipment damage. Monitors, defibrillators, and their accessories (including electrodes and cables) contain ferromagnetic materials. As with all ferromagnetic equipment, these products must not be used in the presence of the high magnetic field created by a magnetic resonance imaging (MRI) device. The high magnetic field created by an MRI device will attract the equipment with a force sufficient to cause death or serious personal injury to persons between the equipment and the MRI device. Skin burns will also occur due to heating of electrically conductive materials such as patient leads and pulse oximeter sensors. This magnetic attraction may also damage the equipment. Consult the MRI manufacturer for more information. SYMBOLS The following symbols may appear on the Infant/Child Reduced Energy Defibrillation Electrodes or on the packaging: LOT Lot code Use by date shown: yyyy-mm-dd or yyyy-mm REF CAT # MIN Reorder number Catalogue number Manufacturer s Item Number Single use only Attention, consult only accompanying documents BF patient connection Warning, high voltage Infant Child Reduced Energy Electrodes are not compatible with QUIK-COMBO defibrillation and therapy cables. To use Infant/Child Electrodes, connect Infant/Child electrodes directly to the AED. Mark of conformity according to the European Medical Device Directive 93/42/EEC Canadian Standards Association certification for Canada and the United States!USA Rx only For USA audiences only Federal (USA) law restricts this product to sale by or on the order of a physician. Latex-free 2 Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use
7 Indications for Use English 35 C 50 C 15 C 0 C 122 F 95 F 59 F 32 F Store in a cool, dry location (0 to 50 C or 32 to 122 F) Two electrodes in one package One package in one shelf-pak Twenty-five shelf-paks in one case Open package Remove electrodes Plug connector into the LIFEPAK CR Plus or LIFEPAK EXPRESS defibrillator Plug connector into LIFEPAK 500 AED Remove release liner Apply pads and follow voice prompts INDICATIONS FOR USE Use Infant/Child Reduced Energy Defibrillation Electrodes on an infant or child patient who is up to 8 years old or weighs up to 25 kg (55 lbs). Do not delay therapy to determine precise age or weight. These electrodes are to be used only with LIFEPAK CR Plus and LIFEPAK EXPRESS defibrillators and biphasic LIFEPAK 500 AEDs configured with a pink cable connector. Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use Medtronic Emergency Response Systems, Inc.
8 About The Electrodes LIFEPAK CR Plus and LIFEPAK EXPRESS defibrillators and biphasic LIFEPAK 500 AEDs are indicated for use on patients in cardiopulmonary arrest. The patient must be unconscious, not breathing normally, and showing no signs of circulation (for example, no pulse, and/or no coughing, no movement) before the device is used to analyze the patient s ECG rhythm. LIFEPAK CR Plus and LIFEPAK EXPRESS defibrillators and biphasic LIFEPAK 500 AEDs are intended to be used by personnel who are trained on the device operation and in basic life support or other physician-authorized emergency medical response system. ABOUT THE ELECTRODES Infant/Child Reduced Energy Defibrillation Electrodes are pre-gelled, self-adhesive, therapy electrodes that allow hands-free defibrillation. These electrodes reduce the energy delivered to the patient by a factor of 4:1. Infant/Child electrodes are not compatible with any other AED or manual LIFEPAK defibrillator/monitor, such as the LIFEPAK 12 and 20 defibrillators, because of safety considerations. (There is a risk that the defibrillator operator may not take into account the 4:1 energy reduction of the Infant/Child electrodes and inadvertently deliver less energy than expected when manually selecting a pediatric dose.) Use adult QUIK-COMBO electrodes for larger children and if the adult electrodes fit completely on the torso as pictured in the placement figure on page 5. To help prevent damage to Infant/Child electrodes: Do not trim the electrodes. Do not crush, fold, or store the electrodes under heavy objects. Store electrodes in a cool, dry location. These electrodes are designed to withstand environmental temperature fluctuations between -40 to 50 C (-40 to 122 F). Continuous exposure to temperatures above 23 C (73 F) will reduce the shelf life of electrodes.. Medtronic Emergency Response Systems electrodes are latex-free. WARNING! Delay of therapy The QUIK-COMBO defibrillation cable is not compatible with Infant/Child Reduced Energy Defibrillation Electrodes. To use Infant/Child electrodes, remove the defibrillation cable and connect the Infant/Child electrodes to the AED. PROCEDURE FOR USE Remove all clothing from the patient s chest. Clean and dry the skin, if necessary. Do not use alcohol or tincture of benzoin. 4 Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use
9 Procedure for Use English Open package and remove the electodes. LIFEPAK CR Plus and LIFEPAK EXPRESS or LIFEPAK 500 AED Disconnect and remove the adult defibrillation electrodes from the defibrillator. Insert the pink Infant/Child electrode connector into the defibrillator. Slowly remove the release liner from each electrode. If the infant has not experienced obvious trauma, place the electrodes on the front and back of the infant s bare chest as shown on each electrode. If the child s chest is large enough or if the child has experienced any type of trauma, this placement is encouraged (approximately 1 inch is required between electrodes for safe use). If the child s chest is small and the child has not experienced obvious trauma, this alternate placement may be used. Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use Medtronic Emergency Response Systems, Inc.
10 Cleaning and Sterilizing Follow the defibrillator voice prompts. CLEANING AND STERILIZING Infant/Child electrodes are not sterile or sterilizable. They are disposable and intended only for single use. Do not autoclave, gas sterilize, immerse in fluids, or clean electrodes with alcohol or solvents. PRODUCT RECYCLING INFORMATION Recycle this product at the end of its useful life. Recycling Assistance The product should be recycled according to national and local regulations. Contact your local Medtronic representative for assistance. Preparation This product should be clean and contaminant-free prior to being recycled. Recycling of Disposable Electrodes After disposable electrodes are used, follow your local clinical procedures for recycling. Packaging Packaging should be recycled according to national and local regulations. ORDERING INFORMATION To order Infant/Child electrodes (Catalog Number ), contact your local Medtronic sales or service representative. In the USA, call the Medtronic PARTSLINE at Infant/Child Reduced Energy Defibrillation Electrodes: Catalog Number (outside USA) SPECIFICATIONS The following performance specifications are for a specially configured biphasic LIFEPAK 500 AED or a LIFEPAK CR Plus or LIFEPAK EXPRESS defibrillator connected directly to the Infant/Child Reduced Energy Defibrillation Electrodes. All specifications are at 20 C (68 F) unless otherwise stated. 6 Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use
11 Specifications English Defibrillator Compatibility Defibrillator Compatibility: Medtronic biphasic LIFEPAK 500 AEDs with pink patient connectors and LIFEPAK CR Plus and LIFEPAK EXPRESS defibrillators only Waveform Parameters for Pediatric Defibrillation Waveform: Energy Delivered (25 125Ω): Energy Accuracy (50Ω): Biphasic Truncated Exponential AED device energy 4, 86 J Max AED DEVICE Energy (Joules) Energy Delivered to Pediatric Patient (Joules) 150 * 38 ±15% 175 * 44 ±15% ±15% ±15% ±15% ±15% ±15% ±15% ±15% Phase 2 Duration: * Energy settings available outside the USA only. Consult the specifications section of the defibrillator operating instructions for energy selection information. 2/3 Phase 1 duration ±200µs Tilt: 50 75% Phase 1 Duration: Patient Impedance LIFEPAK CR Plus and LIFEPAK EXPRESS Duration (ms) LIFEPAK 500 Duration (ms) ± ± ± ± ± ± ± ±1.2 Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use Medtronic Emergency Response Systems, Inc.
12 Specifications Phase 1 Phase 2 Biphasic Waveform Figure Energy Parameters for Pediatric Defibrillation Using Infant/Child Reduced Energy Defibrillation Electrodes: Sample nominal doses delivered to a 50-ohm load Age 50 th Percentile Weight * (Kg) Energy Dose in Joules Per Kilogram for Adult Energy Settings 200 J 300 J 360 J Newborn year years years years years years years years *Doses indicated are based on CDC growth charts for the 50 th percentile weights for boys. National Center for Statistics in collaboration with the National Center for Chronic Disease Prevention and Health Promotion (2000). Electrode Pads Conductive/Gel Surface Area: Overall Size: Standard Pad Placement: 52 cm 2 (104 cm 2 combined) 8.9 x 10.2 cm (3.5 x 4 in) Anterior-Lateral or Anterior-Posterior 8 Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use
13 Specifications English Environmental Note: All performance specifications defined assume that the unit has been stored (two hours minimum) at the operating temperature prior to operation. Shelf Life: Operating Temperature: Storage Temperature: Relative Humidity: Atmospheric Pressure: 30 months 0 to 50 C (32 to 122 F) -30 to 65 C (-22 to 149 F), maximum exposure time limited to one week 5 to 95% (non-condensing) 760 to 439 mmhg, 0 to 15,000 feet above sea level Clinical Studies The Shock Advisory System in the biphasic LIFEPAK 500 AED and the LIFEPAK CR Plus and LIFEPAK EXPRESS defibrillators was tested using an ECG database of shockable and non-shockable rhythms from a broad range of pediatric patients. The results indicate the Medtronic Shock Advisory System can be used safely and effectively for both adults and children. Refer to defibrillator operating instructions for results. Non-Clinical Studies The safety and effectiveness of the Medtronic ADAPTIV Biphasic waveform was studied thoroughly using a pediatric animal cardiac arrest model across the weight range of the intended population and across the range of energy settings available on the compatible AEDs. This study demonstrated that these attenuated biphasic shocks successfully resuscitated a significantly higher percentage of animals than did monophasic shocks of 2 4 J/kg. 1, 2 1 RA Berg, RW Hilwig, FW Chapman, RG Walker, RC Nova, MD Berg, KB Kern, and GA Ewy. Comparison of weight-based monophasic and fixed-sequence biphasic defibrillation dosing for resuscitation in a model of pediatric prolonged cardiac arrest. Journal of the American College of Cardiology 2003 (in press), abstract. 2 RA Berg, FW Chapman, RW Hilwig, KB Kern, MD Berg, and GA Ewy. Piglet biphasic defibrillation with the same dosage over a wide weight range is as safe as monophasic weight-based dosing. Critical Care Medicine 2003 (in press), abstract. Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use Medtronic Emergency Response Systems, Inc.
14
15 Declaration of Conformity English EC DECLARATION OF CONFORMITY Declaration of Conformity Manufacturer s Name: Manufacturer s Address: Medtronic Emergency Response Systems, Inc Willows Road NE Redmond, WA USA declares that the CE-marked product Product Name: Infant/Child Reduced Energy Defibrillation Electrodes Part Number(s): Complies with: 93/42/EEC (Medical Device Directive) class IIb. Conformity assessed per Annex II. Safety: EN :1996/ IEC :1995 Type BF IEC :1983 Supplementary Information: For use only with LIFEPAK CR Plus defibrillators, LIFEPAK EXPRESS defibrillators, and specially configured biphasic LIFEPAK 500 AEDs. Redmond, November 17, 2005 James W. Dennison Vice President, Quality and Regulatory Affairs This declaration applies to CE marked devices produced after the date of issuance of this declaration and before it is either superseded by another declaration or withdrawn. Authorized EC Representative: Medtronic B.V., Earl Bakkenstraat 10, 6422 PJ Heerlen, The Netherlands Infant/Child Reduced Energy Defibrillation Electrodes Instructions for Use Medtronic Emergency Response Systems, Inc.
Purchasing Department Finance Group INVITATION TO BID
 Purchasing Department Finance Group March 6, 2014 INVITATION TO BID The City of Norwalk, Norwalk Public Schools, is soliciting bids for the supply of twenty-three (23) Medtronic Lifepak CR Plus devices.
Purchasing Department Finance Group March 6, 2014 INVITATION TO BID The City of Norwalk, Norwalk Public Schools, is soliciting bids for the supply of twenty-three (23) Medtronic Lifepak CR Plus devices.
LIFEPAK 1000 DEFIBRILLATOR PRODUCT BROCHURE
 DEFIBRILLATOR PRODUCT BROCHURE 1 Not every cardiac emergency is the same. Neither is every responder. Your world demands flexibility and that s exactly what the defibrillator from Physio-Control delivers.
DEFIBRILLATOR PRODUCT BROCHURE 1 Not every cardiac emergency is the same. Neither is every responder. Your world demands flexibility and that s exactly what the defibrillator from Physio-Control delivers.
LIFEPAK and HeartSine. AEDs. Which AED is right for you? Physio-Control has a solution for every need.
 LIFEPAK and HeartSine AEDs Which AED is right for you? Physio-Control has a solution for every need. Choose the AED solution you can rely on. Be ready for Sudden Cardiac Arrest. Sudden cardiac arrest
LIFEPAK and HeartSine AEDs Which AED is right for you? Physio-Control has a solution for every need. Choose the AED solution you can rely on. Be ready for Sudden Cardiac Arrest. Sudden cardiac arrest
LIFEPAK 1000 DEFIBRILLATOR PRODUCT BROCHURE
 LIFEPAK 1000 DEFIBRILLATOR PRODUCT BROCHURE Not every cardiac emergency is the same. Neither is every responder. Your world demands flexibility and that s exactly what the LIFEPAK 1000 defibrillator from
LIFEPAK 1000 DEFIBRILLATOR PRODUCT BROCHURE Not every cardiac emergency is the same. Neither is every responder. Your world demands flexibility and that s exactly what the LIFEPAK 1000 defibrillator from
LIFEPAK Defibrillator/Monitor Series
 LIFEPAK 12 Defibrillator/Monitor Series Setting the standard THE ONE LIFESAVING TOOL YOU NEED Across a Continuum of Care In life-threatening situations, you want a tool that s flexible, easy to use, and
LIFEPAK 12 Defibrillator/Monitor Series Setting the standard THE ONE LIFESAVING TOOL YOU NEED Across a Continuum of Care In life-threatening situations, you want a tool that s flexible, easy to use, and
Purchasing Department Finance Group INVITATION TO BID
 Purchasing Department Finance Group July 26, 2012 INVITATION TO BID The City of Norwalk is re-soliciting bids for the supply of six (6) Medtronic Lifepak 1000 Automatic External Defibrillator (AED) devices
Purchasing Department Finance Group July 26, 2012 INVITATION TO BID The City of Norwalk is re-soliciting bids for the supply of six (6) Medtronic Lifepak 1000 Automatic External Defibrillator (AED) devices
Defibtech DDU-2400 and DDU-2450 Automated External Defibrillator
 Defibtech DDU-2400 and DDU-2450 Automated External Defibrillator Operating Guide AHA/ERC 2010 ELECTRONIC DISTRIBUTION Notices Defibtech, LLC shall not be liable for errors contained herein or for incidental
Defibtech DDU-2400 and DDU-2450 Automated External Defibrillator Operating Guide AHA/ERC 2010 ELECTRONIC DISTRIBUTION Notices Defibtech, LLC shall not be liable for errors contained herein or for incidental
American Procurement Services: Distributor of Noctic Medical Equipment
 American Procurement Services: Distributor of Noctic Medical Equipment American Procurement Service: Medical Equipment & Furniture Division Please contact us for Pricing and product Information: Info@Americanprocurement.com
American Procurement Services: Distributor of Noctic Medical Equipment American Procurement Service: Medical Equipment & Furniture Division Please contact us for Pricing and product Information: Info@Americanprocurement.com
Intravascular Lithotripsy (IVL) Generator and Connector Cable LBL Rev E / Revision Date: March 2018
 OPERATOR S MANUAL Intravascular Lithotripsy (IVL) Generator and Connector Cable LBL 61876 Rev E / Revision Date: March 2018 Contents 1. Introduction... 2 1.1 The IVL Generator - How Supplied... 2 1.2 Required
OPERATOR S MANUAL Intravascular Lithotripsy (IVL) Generator and Connector Cable LBL 61876 Rev E / Revision Date: March 2018 Contents 1. Introduction... 2 1.1 The IVL Generator - How Supplied... 2 1.2 Required
Defibtech DDU-2000 Series Automated External Defibrillator DDU-2300 DDU-2400 DDU-2450
 Defibtech DDU-2000 Series Automated External Defibrillator DDU-2300 DDU-2400 DDU-2450 www.americanaed.com Operating Guide AHA/ERC 2010 ELECTRONIC DISTRIBUTION Notices Defibtech, L.L.C. shall not be liable
Defibtech DDU-2000 Series Automated External Defibrillator DDU-2300 DDU-2400 DDU-2450 www.americanaed.com Operating Guide AHA/ERC 2010 ELECTRONIC DISTRIBUTION Notices Defibtech, L.L.C. shall not be liable
AED Response Systems CONNECTED. READY.
 AED Response Systems CONNECTED. READY. LIFEPAK Express $1250 RRP:$1500 CODE: 80427-000136 Lifepak Express Semi Automatic Defibrillator Industry-leading 360 joules capability Step-by-step ClearVoice prompts
AED Response Systems CONNECTED. READY. LIFEPAK Express $1250 RRP:$1500 CODE: 80427-000136 Lifepak Express Semi Automatic Defibrillator Industry-leading 360 joules capability Step-by-step ClearVoice prompts
Choose both quality and value
 Key Content Supplies Choose both quality and value Match your Efficia devices with Philips supplies for pulse oximetry, ECG, and non-invasive blood pressure measurement Meeting your needs for measurement
Key Content Supplies Choose both quality and value Match your Efficia devices with Philips supplies for pulse oximetry, ECG, and non-invasive blood pressure measurement Meeting your needs for measurement
R Series. Confidence Comes with Knowing. You Are Code-Ready
 R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
Policy Document. The Management and Use of Defibrillators v1.3
 Policy Document The Management and Use of Defibrillators v1.3 Written: February 2013 Author: David Seymour, Director of Operations Co Author: Russell Wells SRODP, Clinical Team Leader Approved: Board of
Policy Document The Management and Use of Defibrillators v1.3 Written: February 2013 Author: David Seymour, Director of Operations Co Author: Russell Wells SRODP, Clinical Team Leader Approved: Board of
R Series. Confidence Comes with Knowing. You Are Code-Ready
 R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
R Series Confidence Comes with Knowing You Are Code-Ready The First and Only Code-Ready Defibrillator The worst time to find out a defibrillator isn t ready is at the code. Quick action is essential. Stress
PMA 31-G. English. Printed: Doc-Nr: PUB / / 000 / 00
 PMA 31-G English 1 Information about the documentation 1.1 About this documentation Read this documentation before initial operation or use. This is a prerequisite for safe, trouble-free handling and
PMA 31-G English 1 Information about the documentation 1.1 About this documentation Read this documentation before initial operation or use. This is a prerequisite for safe, trouble-free handling and
CODE-STAT 9.0 DATA REVIEW SOFTWARE PRODUCT BROCHURE
 CODE-STAT 9.0 DATA REVIEW SOFTWARE PRODUCT BROCHURE More powerful post-cardiac-event review. Improved performance. A higher level of care. 1 CODE-STAT 9.0 Improvement. Fueled by information. New CODE-STAT
CODE-STAT 9.0 DATA REVIEW SOFTWARE PRODUCT BROCHURE More powerful post-cardiac-event review. Improved performance. A higher level of care. 1 CODE-STAT 9.0 Improvement. Fueled by information. New CODE-STAT
Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator
 Reprinted from FDA s website by Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator DRAFT GUIDANCE This guidance
Reprinted from FDA s website by Draft Guidance for Industry and Food and Drug Administration Staff Class II Special Controls Guidance Document: External Pacemaker Pulse Generator DRAFT GUIDANCE This guidance
Bedhead Service Systems. Instructions for Use. July 2016 ASF-18 (0)
 Bedhead Service Systems Instructions for Use July 2016 0197 ASF-18 (0) Contents 1. Instructions For Use 1.1. Introduction 1.2. Explanation of Symbols 1.3. General Safety Instructions Intended Use Operating
Bedhead Service Systems Instructions for Use July 2016 0197 ASF-18 (0) Contents 1. Instructions For Use 1.1. Introduction 1.2. Explanation of Symbols 1.3. General Safety Instructions Intended Use Operating
Implants for surgery Active implantable medical devices. Part 3: Implantable neurostimulators
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 14708-3 Second edition 2017-04 Implants for surgery Active implantable medical devices Part 3: Implantable neurostimulators Implants chirurgicaux
Instructions for Use Reusable Ablation Catheter Cables
 Processed by Instructions for Use Reusable Ablation Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized by Ethylene
Processed by Instructions for Use Reusable Ablation Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized by Ethylene
Trusted most often by those trained to save lives. Philips HeartStart FR2+ Defibrillator Product information
 Trusted most often by those trained to save lives Philips HeartStart FR2+ Defibrillator Product information Updated for Guidelines 2005 The Philips HeartStart FR2+ Defibrillator Each year sudden cardiac
Trusted most often by those trained to save lives Philips HeartStart FR2+ Defibrillator Product information Updated for Guidelines 2005 The Philips HeartStart FR2+ Defibrillator Each year sudden cardiac
QGO Oxygen Sensor. Building Technologies HVAC Products
 7 842 QGO20.000D17 QGO20.000D27 Oxygen Sensor QGO20.000... The QGO20... is an oxygen sensor designed for acquiring the residual oxygen content of flue gases in heat generation plant burning natural gas
7 842 QGO20.000D17 QGO20.000D27 Oxygen Sensor QGO20.000... The QGO20... is an oxygen sensor designed for acquiring the residual oxygen content of flue gases in heat generation plant burning natural gas
Trusted most often by those trained to save lives
 Trusted most often by those trained to save lives Philips HeartStart FR2+ Defibrillator Product information 340,000 deaths a year in the U.S. are attributed to Sudden Cardiac Arrest and hundreds of thousands
Trusted most often by those trained to save lives Philips HeartStart FR2+ Defibrillator Product information 340,000 deaths a year in the U.S. are attributed to Sudden Cardiac Arrest and hundreds of thousands
ZX70 & ZX80 SERIES PRECISION DECADE RESISTANCE BOX
 ZX70 & ZX80 SERIES PRECISION DECADE RESISTANCE BOX OPERATING INSTRUCTIONS SUBJECT TO CHANGE WITHOUT NOTICE This manual superseded all previous versions please keep for future reference TINSLEY, A DIVISION
ZX70 & ZX80 SERIES PRECISION DECADE RESISTANCE BOX OPERATING INSTRUCTIONS SUBJECT TO CHANGE WITHOUT NOTICE This manual superseded all previous versions please keep for future reference TINSLEY, A DIVISION
Protecting your people means being ready.
 Protecting your people means being ready. Heart Safe Solutions AED program implementation and management Kelly Kettler, RN-BSN, Cabela s Claims Manager Physio-Control Heart Safe Solution customer since
Protecting your people means being ready. Heart Safe Solutions AED program implementation and management Kelly Kettler, RN-BSN, Cabela s Claims Manager Physio-Control Heart Safe Solution customer since
LIFEPAK 15 Works like you work.
 LIFEPAK 15 MONITOR /DEfibrillator Works like you work. Lifesaving starts here. Building on a Proud Legacy The pioneer in portable defibrillation and monitoring technology, Physio-Control continues to
LIFEPAK 15 MONITOR /DEfibrillator Works like you work. Lifesaving starts here. Building on a Proud Legacy The pioneer in portable defibrillation and monitoring technology, Physio-Control continues to
MANUAL. ENGLISH Antenna Booster Ordercode: D1425. Highlite International B.V. Vestastraat EX Kerkrade the Netherlands
 MANUAL ENGLISH Antenna Booster V1 Highlite International B.V. Vestastraat 2 6468 EX Kerkrade the Netherlands Table of contents Warning... 2 Unpacking Instructions... 2 Safety Instructions... 2 Operating
MANUAL ENGLISH Antenna Booster V1 Highlite International B.V. Vestastraat 2 6468 EX Kerkrade the Netherlands Table of contents Warning... 2 Unpacking Instructions... 2 Safety Instructions... 2 Operating
MANUAL ENGLISH. Ordercode: D3423/D3423W. Highlite International B.V. Vestastraat EX Kerkrade the Netherlands
 MANUAL ENGLISH Xi-6 V1 Highlite International B.V. Vestastraat 2 6468 EX Kerkrade the Netherlands Table of contents Warning... 2 Safety Instructions... 2 Operating Determinations... 3 Return Procedure...
MANUAL ENGLISH Xi-6 V1 Highlite International B.V. Vestastraat 2 6468 EX Kerkrade the Netherlands Table of contents Warning... 2 Safety Instructions... 2 Operating Determinations... 3 Return Procedure...
CM-95 ORDERCODE D1355 Highlite International B.V.
 CM-95 ORDERCODE D1355 Highlite International B.V. Vestastraat 2 6468 EX Kerkrade The Netherlands Congratulations! You have bought a great, innovative product from DAP Audio. The DAP Audio CM-95 brings
CM-95 ORDERCODE D1355 Highlite International B.V. Vestastraat 2 6468 EX Kerkrade The Netherlands Congratulations! You have bought a great, innovative product from DAP Audio. The DAP Audio CM-95 brings
RESPIRATORY SENSING LEAD
 RESPIRATORY SENSING LEAD 4340 Technical Manual ONLY Explanation of symbols on product or package labeling Caution, consult accompanying documents eifu indicator Consult electronic instructions for use
RESPIRATORY SENSING LEAD 4340 Technical Manual ONLY Explanation of symbols on product or package labeling Caution, consult accompanying documents eifu indicator Consult electronic instructions for use
Essential Requirements Straumann Custom-made Dental Restorative Devices According to 93/42/EEC annex I as amended by 2007/47/EC
 Straumann's CAD (Computer Aided Design) software CARES Visual has built-in parameter checks to assure that a dental restorative device basically meets the generally applicable requirements. However, Institut
Straumann's CAD (Computer Aided Design) software CARES Visual has built-in parameter checks to assure that a dental restorative device basically meets the generally applicable requirements. However, Institut
PRODUCT BROCHURE LIFEPAK 15 MONITOR/DEFIBRILLATOR. For Hospitals
 PRODUCT BROCHURE LIFEPAK 15 MONITOR/DEFIBRILLATOR For Hospitals LIFEPAK 15 MONITOR/DEFIBRILLATOR In any area of the hospital, the most advanced emergency response monitor/defibrillator sets the standard
PRODUCT BROCHURE LIFEPAK 15 MONITOR/DEFIBRILLATOR For Hospitals LIFEPAK 15 MONITOR/DEFIBRILLATOR In any area of the hospital, the most advanced emergency response monitor/defibrillator sets the standard
Respond with confidence: Rely on service from Physio-Control
 Respond with confidence: Rely on service from Physio-Control When lives are at stake, you need quality service for your lifesaving devices. Imagine having someone dedicated to managing your Physio-Control
Respond with confidence: Rely on service from Physio-Control When lives are at stake, you need quality service for your lifesaving devices. Imagine having someone dedicated to managing your Physio-Control
External Defibrillator Improvement Initiative
 External Defibrillator Improvement Initiative November 2010 Center for Devices and Radiological Health U.S. Food and Drug Administration External Defibrillator Improvement Initiative Table of Contents
External Defibrillator Improvement Initiative November 2010 Center for Devices and Radiological Health U.S. Food and Drug Administration External Defibrillator Improvement Initiative Table of Contents
Instructions for Use Reusable Electrophysiology Catheter Cables
 Processed by Instructions for Use Reusable Electrophysiology Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized
Processed by Instructions for Use Reusable Electrophysiology Catheter Cables STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by or on the order of a physician Sterilized
Turbocharger / TPL-C Original assembly instructions English
 Assembly Instructions Turbocharger / TPL-C Original assembly instructions English This document is valid for the TPL-C series: TPL79-C Purpose The assembly instructions explain how the ABB turbocharger
Assembly Instructions Turbocharger / TPL-C Original assembly instructions English This document is valid for the TPL-C series: TPL79-C Purpose The assembly instructions explain how the ABB turbocharger
Operation and Maintenance Manual
 M0088349-01 (en-us) February 2018 Operation and Maintenance Manual Product Link PL042 and PLE702 Systems PL7 1-UP (Machine Control & Guidance Products) SAFETY.CAT.COM Important Safety Information i06558969
M0088349-01 (en-us) February 2018 Operation and Maintenance Manual Product Link PL042 and PLE702 Systems PL7 1-UP (Machine Control & Guidance Products) SAFETY.CAT.COM Important Safety Information i06558969
Agilent Technologies 11752D/E Option K01 Connector Gage Kit. User s Guide
 Agilent Technologies 11752D/E Option K01 Connector Gage Kit User s Guide Manufacturing Part Number: 11752-90008 Print Date: September 2008 Copyright Agilent Technologies, Inc. 2000, 2008 i Warranty Statement
Agilent Technologies 11752D/E Option K01 Connector Gage Kit User s Guide Manufacturing Part Number: 11752-90008 Print Date: September 2008 Copyright Agilent Technologies, Inc. 2000, 2008 i Warranty Statement
INSTRUCTION MANUAL FOR WIRE WELDING MACHINE
 INSTRUCTION MANUAL FOR WIRE WELDING MACHINE IMPORTANT: BEFORE STARTING THE EQUIPMENT, READ THE CONTENTS OF THIS MANUAL, WHICH MUST BE STORED IN A PLACE FAMILIAR TO ALL USERS FOR THE ENTIRE OPERATIVE LIFE-SPAN
INSTRUCTION MANUAL FOR WIRE WELDING MACHINE IMPORTANT: BEFORE STARTING THE EQUIPMENT, READ THE CONTENTS OF THIS MANUAL, WHICH MUST BE STORED IN A PLACE FAMILIAR TO ALL USERS FOR THE ENTIRE OPERATIVE LIFE-SPAN
MEDICAL PRODUCTS. STERISETS CONVECTIVE WARMING BLANKETS Prevent & Treat Hypothermia
 MEDICAL PRODUCTS STERISETS CONVECTIVE WARMING BLANKETS Prevent & Treat Hypothermia History Since the start of Sterisets International B.V. in 1991 we have been growing our activities in Europe and beyond,
MEDICAL PRODUCTS STERISETS CONVECTIVE WARMING BLANKETS Prevent & Treat Hypothermia History Since the start of Sterisets International B.V. in 1991 we have been growing our activities in Europe and beyond,
HCP-7000 Opreator s Manual 1. Operator s Manual.
 -------------------------------------------------------------------- HCP-7000 Opreator s Manual 1 Operator s Manual Chart Projector 2 HCP-7000 Operator s Manual -----------------------------------------------------------------------
-------------------------------------------------------------------- HCP-7000 Opreator s Manual 1 Operator s Manual Chart Projector 2 HCP-7000 Operator s Manual -----------------------------------------------------------------------
ISO/TS TECHNICAL SPECIFICATION. Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device
 TECHNICAL SPECIFICATION ISO/TS 10974 First edition 2012-05-01 Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device Évaluation de la sécurité de
TECHNICAL SPECIFICATION ISO/TS 10974 First edition 2012-05-01 Assessment of the safety of magnetic resonance imaging for patients with an active implantable medical device Évaluation de la sécurité de
SelfCheck System R-Series. Site Planning Guide
 SelfCheck System R-Series Site Planning Guide Copyright 2006 2007, 3M. All rights reserved. SelfCheck System R-Series Site Planning Guide 78 8129 2082 1 Rev. C 3M, Tattle-Tape, and SelfCheck are trademarks
SelfCheck System R-Series Site Planning Guide Copyright 2006 2007, 3M. All rights reserved. SelfCheck System R-Series Site Planning Guide 78 8129 2082 1 Rev. C 3M, Tattle-Tape, and SelfCheck are trademarks
 EMPOWERMR INJECTOR SYSTEM SITE SURVEY AND SITE INSTALLATION INSTRUCTIONS ACIST Medical Systems, Inc., Eden Prairie, MN 55344 Phone: 952-941-3507 Fax: 952-941-4648 EmpowerMR Site Survey Document 900953-001
EMPOWERMR INJECTOR SYSTEM SITE SURVEY AND SITE INSTALLATION INSTRUCTIONS ACIST Medical Systems, Inc., Eden Prairie, MN 55344 Phone: 952-941-3507 Fax: 952-941-4648 EmpowerMR Site Survey Document 900953-001
Conversion Station Model 812
 Conversion Station Model 812 Owner s Manual 3M Library Systems 3M Center, Building 225-4N-14 St. Paul, Minnesota 55144-1000 www.3m.com/library Copyright 2006, 3M. All rights reserved. 78-8129-2472-4_A
Conversion Station Model 812 Owner s Manual 3M Library Systems 3M Center, Building 225-4N-14 St. Paul, Minnesota 55144-1000 www.3m.com/library Copyright 2006, 3M. All rights reserved. 78-8129-2472-4_A
THE MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION ORDER. January 19, Moscow
 Stamp: The Ministry of Justice of the Russian Federation REGISTERED Registration No.45896 dated March 10, 2017 THE MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION ORDER January 19, 2017 No.11n Moscow On approval
Stamp: The Ministry of Justice of the Russian Federation REGISTERED Registration No.45896 dated March 10, 2017 THE MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION ORDER January 19, 2017 No.11n Moscow On approval
Protecting your people means being ready.
 HEART SAFE SOLUTIONSM Protecting your people means being ready. AED program implementation and management Kelly Kettler, RN-BSN, Cabela s Claims Manager Physio-Control Heart Safe Solution customer since
HEART SAFE SOLUTIONSM Protecting your people means being ready. AED program implementation and management Kelly Kettler, RN-BSN, Cabela s Claims Manager Physio-Control Heart Safe Solution customer since
User Manual. Precision Balances XSR models
 User Manual XSR models Overview balances with S weighing platform 4 3 5 2 3 1 6 7 2 8 8 10 9 10 9 Legend balances with S weighing platform 1 MagicCube Draft Shield 6 MagicCube Draft Shield side door 2
User Manual XSR models Overview balances with S weighing platform 4 3 5 2 3 1 6 7 2 8 8 10 9 10 9 Legend balances with S weighing platform 1 MagicCube Draft Shield 6 MagicCube Draft Shield side door 2
INSTRUCTION MANUAL WARNING: BEFORE USING THE MACHINE READ THE INSTRUCTION MANUAL CAREFULLY!
 INSTRUCTION MANUAL WARNING: BEFORE USING THE MACHINE READ THE INSTRUCTION MANUAL CAREFULLY! SECTION 1 : PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME : SYNONYMS : PRODUCT CODES : Weldability ARC140i
INSTRUCTION MANUAL WARNING: BEFORE USING THE MACHINE READ THE INSTRUCTION MANUAL CAREFULLY! SECTION 1 : PRODUCT AND COMPANY IDENTIFICATION PRODUCT NAME : SYNONYMS : PRODUCT CODES : Weldability ARC140i
PRODUCT BROCHURE. LIFENET System PHYSIO-CONTROL IS A DIVISION OF MEDTRONIC
 PRODUCT BROCHURE LIFENET System PHYSIO-CONTROL IS A DIVISION OF MEDTRONIC LIFENET System In the vehicle or in the ED, more efficiency with your equipment, your information, and your decisions means working
PRODUCT BROCHURE LIFENET System PHYSIO-CONTROL IS A DIVISION OF MEDTRONIC LIFENET System In the vehicle or in the ED, more efficiency with your equipment, your information, and your decisions means working
ECG CONSUMABLES. NEW ZEALAND AUSTRALIA
 ECG CONSUMABLES PLG50 PLG30 Suitable for any in any ECG exam, where short and medium monitoring is needed. Dimension: 50mm diameter Gel quality: liquid Suitable for any pediatric ECG exam, for short and
ECG CONSUMABLES PLG50 PLG30 Suitable for any in any ECG exam, where short and medium monitoring is needed. Dimension: 50mm diameter Gel quality: liquid Suitable for any pediatric ECG exam, for short and
PRODUCT SHEET EL500 SERIES DISPOSABLE ELECTRODES
 EL500 SERIES DISPOSABLE ELECTRODES EL504 EL509 EL508 EL510 EL512 The EL500 Series disposable, Ag/AgCl snap electrodes provide the same signal transmission as BIOPAC s reusable electrodes, with added convenience
EL500 SERIES DISPOSABLE ELECTRODES EL504 EL509 EL508 EL510 EL512 The EL500 Series disposable, Ag/AgCl snap electrodes provide the same signal transmission as BIOPAC s reusable electrodes, with added convenience
User s Manual. Chart Projector GCP-7000
 User s Manual Chart Projector GCP-7000 ------------------------------------------------------------------------ Chart Projector GCP-7000 1 IMPORTANT NOTICE This product may malfunction due to electromagnetic
User s Manual Chart Projector GCP-7000 ------------------------------------------------------------------------ Chart Projector GCP-7000 1 IMPORTANT NOTICE This product may malfunction due to electromagnetic
RapID Raw Materials Verification System
 RapID Raw Materials Verification System Pre-Installation Guide NOTICE: This document contains references to Cobalt or Cobalt Light Systems. Please note that Cobalt Light Systems was purchased by Agilent
RapID Raw Materials Verification System Pre-Installation Guide NOTICE: This document contains references to Cobalt or Cobalt Light Systems. Please note that Cobalt Light Systems was purchased by Agilent
Preparation and Installation of the ENVIRO-SEAL I Rupture Disc
 Preparation and Installation of the ENVIRO-SEAL I Rupture Disc GEP-6019 Rev. AA 102335 Ref. I.D.: 10711 WARNING USER SHOULD READ AND THOROUGHLY UNDERSTAND THESE INSTRUCTIONS BEFORE INSTALLING RUPTURE DISC.
Preparation and Installation of the ENVIRO-SEAL I Rupture Disc GEP-6019 Rev. AA 102335 Ref. I.D.: 10711 WARNING USER SHOULD READ AND THOROUGHLY UNDERSTAND THESE INSTRUCTIONS BEFORE INSTALLING RUPTURE DISC.
Electrical Protection Methods and Devices SAMPLE. Learner Workbook Version 1. Training and Education Support Industry Skills Unit Meadowbank
 Electrical Protection Methods and Devices Learner Workbook Version 1 Training and Education Support Industry Skills Unit Meadowbank Product Code: 5635 Table of Contents Introduction... 5 1. Protection
Electrical Protection Methods and Devices Learner Workbook Version 1 Training and Education Support Industry Skills Unit Meadowbank Product Code: 5635 Table of Contents Introduction... 5 1. Protection
DJ Switch 10F V2 ORDERCODE 50365
 DJ Switch 10F V2 ORDERCODE 5035 Congratulations! You have bought a great, innovative product from Showtec. The Showtec DJ Switch brings excitement to any venue. Whether you want simple plug-&-play action
DJ Switch 10F V2 ORDERCODE 5035 Congratulations! You have bought a great, innovative product from Showtec. The Showtec DJ Switch brings excitement to any venue. Whether you want simple plug-&-play action
Whitening Lamp. Instructions for use. flaesh.com
 Whitening Lamp Instructions for use flaesh.com Whitening System Latest LED technology Guided treatment function Determination of whitening shades achieved Cosmetic and medical tooth whitening 2flaesh.com
Whitening Lamp Instructions for use flaesh.com Whitening System Latest LED technology Guided treatment function Determination of whitening shades achieved Cosmetic and medical tooth whitening 2flaesh.com
MNL, SEL DIMENSIONS, SITE AW PLANNING GUIDE ARTWORK SIZE A SHEET 1 OF 1
 Artwork and Signature File for: MAN-01120, MNL, SEL DIMENSIONS, SITE PLANNING GUIDE Artwork consists of: Thirty-Six (36) 8 ½ inch x 11 inch sheet(s) attached. REV AUTHORED BY DATE A.TAMBASCIO 01/20/10
Artwork and Signature File for: MAN-01120, MNL, SEL DIMENSIONS, SITE PLANNING GUIDE Artwork consists of: Thirty-Six (36) 8 ½ inch x 11 inch sheet(s) attached. REV AUTHORED BY DATE A.TAMBASCIO 01/20/10
CARE SERVICE USER GUIDE. Sonicision System
 CARE SERVICE USER GUIDE Sonicision System TABLE OF CONTENTS Sonicision device product return instructions for post-use cleaning and sterilization...3 How to contact the carrier...5 Frequently asked questions...6
CARE SERVICE USER GUIDE Sonicision System TABLE OF CONTENTS Sonicision device product return instructions for post-use cleaning and sterilization...3 How to contact the carrier...5 Frequently asked questions...6
FAST SPOTTER WT-FS-5.0
 . FAST SPOTTER WT-FS-5.0 Installation, Operating and Maintenance Manual WELDING TECHNOLOGIES www.weldingnet.com 1365 Horseshoe Dr., Blue Bell, PA 19422 Phone: 610-278-9325 Fax: 610-278-9325 Toll Free Number:
. FAST SPOTTER WT-FS-5.0 Installation, Operating and Maintenance Manual WELDING TECHNOLOGIES www.weldingnet.com 1365 Horseshoe Dr., Blue Bell, PA 19422 Phone: 610-278-9325 Fax: 610-278-9325 Toll Free Number:
INSTRUCTION MANUAL FOR WIRE WELDING MACHINE
 INSTRUCTION MANUAL FOR WIRE WELDING MACHINE IMPORTANT: BEFORE STARTING THE EQUIPMENT, READ THE CONTENTS OF THIS MANUAL, WHICH MUST BE STORED IN A PLACE FAMILIAR TO ALL USERS FOR THE ENTIRE OPERATIVE LIFE-SPAN
INSTRUCTION MANUAL FOR WIRE WELDING MACHINE IMPORTANT: BEFORE STARTING THE EQUIPMENT, READ THE CONTENTS OF THIS MANUAL, WHICH MUST BE STORED IN A PLACE FAMILIAR TO ALL USERS FOR THE ENTIRE OPERATIVE LIFE-SPAN
VBC Manual MManualManual. Installation and user manual for the VBC
 VBC400-600 Manual MManualManual Installation and user manual for the VBC400-600 Before You Begin Read these instructions before installing or operating this product. Note: This installation should be made
VBC400-600 Manual MManualManual Installation and user manual for the VBC400-600 Before You Begin Read these instructions before installing or operating this product. Note: This installation should be made
Appendix 1 Canadian Standards Association Standards for Equipment
 Can J Anesth/J Can Anesth (2018) Appendix 1 Canadian Standards Association Standards for Equipment Legend Example: C22.2 NO. 0.1-FM85 (C1999) F Indicates the standard is written in French. M Indicates
Can J Anesth/J Can Anesth (2018) Appendix 1 Canadian Standards Association Standards for Equipment Legend Example: C22.2 NO. 0.1-FM85 (C1999) F Indicates the standard is written in French. M Indicates
Imperial Series. Model IMP-425/525/625/825/1000AP IMD-425/525/625/825/1000AP IMP-1025/1200/1500/2000AP IMD-1025/1200/1500/2000AP
 Service Manual Imperial Series Model IMP-425/525/625/825/1000AP IMD-425/525/625/825/1000AP IMP-1025/1200/1500/2000AP IMD-1025/1200/1500/2000AP Contents of Service Manual 1. Safety Precautions ------------------------------------------------------------------
Service Manual Imperial Series Model IMP-425/525/625/825/1000AP IMD-425/525/625/825/1000AP IMP-1025/1200/1500/2000AP IMD-1025/1200/1500/2000AP Contents of Service Manual 1. Safety Precautions ------------------------------------------------------------------
MD Power Supply -48Vdc +Bias Tee
 215988-001MD Power Supply -48Vdc +Bias Tee Operation and Maintenance Manual Mitec Telecom Inc. Designers and manufacturers of telecom and wireless products 9000 Trans Canada, Pointe-Claire, Quebec, Canada
215988-001MD Power Supply -48Vdc +Bias Tee Operation and Maintenance Manual Mitec Telecom Inc. Designers and manufacturers of telecom and wireless products 9000 Trans Canada, Pointe-Claire, Quebec, Canada
Manual Supplement. This supplement contains information necessary to ensure the accuracy of the above manual.
 Manual Supplement Manual Title: 4180, 4181 Users Supplement Issue: 4 Part Number: Web-Only Issue Date: 4/18 Print Date: March 2013 Page Count: 5 Revision/Date: This supplement contains information necessary
Manual Supplement Manual Title: 4180, 4181 Users Supplement Issue: 4 Part Number: Web-Only Issue Date: 4/18 Print Date: March 2013 Page Count: 5 Revision/Date: This supplement contains information necessary
Technical instructions. Mini Brio X15CA Underwater LED lighting. Table of Contents. Réf : PK10R303
 Technical instructions Mini Brio X15CA Underwater LED lighting Réf : PK10R303 Table of Contents 1. Packaging Contents... 2 2. Technical features... 3 3. Description... 4 3.1. Warnings... 4 3.2. Technical
Technical instructions Mini Brio X15CA Underwater LED lighting Réf : PK10R303 Table of Contents 1. Packaging Contents... 2 2. Technical features... 3 3. Description... 4 3.1. Warnings... 4 3.2. Technical
Date: 7 July 2009 Version: 1 Page 1 of Identification of the substance/mixture and company/undertaking
 Date: 7 July 2009 Version: 1 Page 1 of 6 1. Identification of the substance/mixture and company/undertaking Product name Use Supplier Clinell Instant Hand Sanitiser Hand sanitiser GAMA Healthcare Ltd.
Date: 7 July 2009 Version: 1 Page 1 of 6 1. Identification of the substance/mixture and company/undertaking Product name Use Supplier Clinell Instant Hand Sanitiser Hand sanitiser GAMA Healthcare Ltd.
NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use
 630 Komas Drive Suite 200 Salt Lake City UT 84108 USA P +1 801.582.5533 F +1 801.582.1509 www.blackrockmicro.com NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use Revision 2.00 / LB-0612
630 Komas Drive Suite 200 Salt Lake City UT 84108 USA P +1 801.582.5533 F +1 801.582.1509 www.blackrockmicro.com NeuroPort Array PN 4382, 4383, 6248, and 6249 Instructions for Use Revision 2.00 / LB-0612
Water Level Meter User Manual
 Man 054 Water Level Meter User Manual Soil Instruments Limited has an ongoing policy of design review and reserves the right to amend these specifications without notice. Man054 - Water Level Meter - MN1114
Man 054 Water Level Meter User Manual Soil Instruments Limited has an ongoing policy of design review and reserves the right to amend these specifications without notice. Man054 - Water Level Meter - MN1114
Resuscitation supplies
 Resuscitation supplies Changes to this edition NEW December 2012 New product added HeartStart MRx Accessories 989803176541 HeartStart MRx Quick Disconnect DC Power Cable 13 Cases and Pouches 989803180871
Resuscitation supplies Changes to this edition NEW December 2012 New product added HeartStart MRx Accessories 989803176541 HeartStart MRx Quick Disconnect DC Power Cable 13 Cases and Pouches 989803180871
Material Safety Data Sheet
 LI-ION BATTERY Page 1 of 5 1. Identification Of Substance Product Details Product Name: LI-ION BATTERY Product Model: 18650-2200mAh Manufacturer/Supplier By: SHENZHEN MING-BO SOURCE TECHNOLOGY CO., LTD
LI-ION BATTERY Page 1 of 5 1. Identification Of Substance Product Details Product Name: LI-ION BATTERY Product Model: 18650-2200mAh Manufacturer/Supplier By: SHENZHEN MING-BO SOURCE TECHNOLOGY CO., LTD
ISO INTERNATIONAL STANDARD. Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers
 INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
INTERNATIONAL STANDARD Provläsningsexemplar / Preview ISO 14708-2 First edition 2005-10-01 Implants for surgery Active implantable medical devices Part 2: Cardiac pacemakers Implants chirurgicaux Dispositifs
Saphyr System Safety Guide for Saphyr P/N 60325
 Saphyr System Safety Guide for Saphyr P/N 60325 Document Number: 30253 Document Revision: A For Research Use Only. Not for use in diagnostic procedures. Copyright 2019 Bionano Genomics Inc. All Rights
Saphyr System Safety Guide for Saphyr P/N 60325 Document Number: 30253 Document Revision: A For Research Use Only. Not for use in diagnostic procedures. Copyright 2019 Bionano Genomics Inc. All Rights
To Whom It May Concern:
 To Whom It May Concern: DeRoyal Foley Catheters with Temperature Sensors have been tested for safe use in magnetic resonance environments at 1.5 and 3.0 Tesla according to ASTM International F2052, Standard
To Whom It May Concern: DeRoyal Foley Catheters with Temperature Sensors have been tested for safe use in magnetic resonance environments at 1.5 and 3.0 Tesla according to ASTM International F2052, Standard
Government Management Committee. Chief and General Manager, Toronto EMS Director of Purchasing and Materials Management Division
 STAFF REPORT ACTION REQUIRED Sole source contract for proprietary consumable supplies, operating accessories and service with Zoll Medical Canada Inc. for monitor/defibrillator equipment Date: May 16,
STAFF REPORT ACTION REQUIRED Sole source contract for proprietary consumable supplies, operating accessories and service with Zoll Medical Canada Inc. for monitor/defibrillator equipment Date: May 16,
1501 Incubator for Ampoule Biological Indicators
 MesaLabs 1501 Incubator for Ampoule Biological Indicators MesaLabs Mesa Laboratories, Inc. Biological Indicator Division Omaha Manufacturing Facility 8607 Park Drive, Omaha, NE 68127 USA (402) 593-0781
MesaLabs 1501 Incubator for Ampoule Biological Indicators MesaLabs Mesa Laboratories, Inc. Biological Indicator Division Omaha Manufacturing Facility 8607 Park Drive, Omaha, NE 68127 USA (402) 593-0781
Agilent 8490G Coaxial Attenuators
 Agilent 849G Coaxial Attenuators Technical Overview Key specifications Maximize your operating frequency range for DC to 67 GHz application Minimize your measurement uncertainty with low SWR of 1.45 up
Agilent 849G Coaxial Attenuators Technical Overview Key specifications Maximize your operating frequency range for DC to 67 GHz application Minimize your measurement uncertainty with low SWR of 1.45 up
PRODUCT SPECIFICATION FOR APPROVAL
 Issue : 151XC14C08005 Date of Issue : October 27.2008 DIGI-KEY CORPORATION : New Changed PRODUCT SPECIFICATION FOR APPROVAL Product Description Product Part Number : Common Mode Noise Filter (RoHS) : EXC14CE900U
Issue : 151XC14C08005 Date of Issue : October 27.2008 DIGI-KEY CORPORATION : New Changed PRODUCT SPECIFICATION FOR APPROVAL Product Description Product Part Number : Common Mode Noise Filter (RoHS) : EXC14CE900U
Medi-Therm Precise. Easy. Versatile.
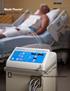 Medi-Therm Precise. Easy. Versatile. Medi-Therm Hyper/Hypothermia System Patient warming and cooling are often critical factors in achieving positive outcomes. The Stryker Medi-Therm system can be used
Medi-Therm Precise. Easy. Versatile. Medi-Therm Hyper/Hypothermia System Patient warming and cooling are often critical factors in achieving positive outcomes. The Stryker Medi-Therm system can be used
General Safety and Performance Requirements Regulation (EU) 2017/ 745 on medical devices (MDR) - Annex I I. GENERAL REQUIREMENTS
 I. GENERAL REQUIREMENTS No. General Safety and Performance Requirement Applies? (y/n/na) 1. Devices shall achieve the performance intended by their manufacturer and shall be designed and manufactured in
I. GENERAL REQUIREMENTS No. General Safety and Performance Requirement Applies? (y/n/na) 1. Devices shall achieve the performance intended by their manufacturer and shall be designed and manufactured in
Postmarket Safety Surveillance: A Medical Device Perspective
 Postmarket Safety Surveillance: A Medical Device Perspective Thomas P. Gross, MD, MPH Deputy Director, Postmarket Science Office of Surveillance and Biometrics Center for Devices and Radiological Health
Postmarket Safety Surveillance: A Medical Device Perspective Thomas P. Gross, MD, MPH Deputy Director, Postmarket Science Office of Surveillance and Biometrics Center for Devices and Radiological Health
Created by EBCCW 00:06. X65 Locating module. User Documentation
 Created by EBCCW 00:06 by EBCCW 96:05 00:06 X65 Locating module User Documentation Created by EBCCW 96:05 00:06 Created by EBCCW 00:06 by EBCCW 96:05 00:06 X65 Locating module Created by EBCCW 96:05 00:06
Created by EBCCW 00:06 by EBCCW 96:05 00:06 X65 Locating module User Documentation Created by EBCCW 96:05 00:06 Created by EBCCW 00:06 by EBCCW 96:05 00:06 X65 Locating module Created by EBCCW 96:05 00:06
Dual Port isolated USB Charger
 User & Installation Manual EN Dual Port isolated USB Charger 3A R-net cable P010-61 3A DX cable P010-62 Contact & Product mo-vis BVBA Biebuyckstraat 15 D 9850 Nevele - Belgium Website: www.mo-vis.com E-mail:
User & Installation Manual EN Dual Port isolated USB Charger 3A R-net cable P010-61 3A DX cable P010-62 Contact & Product mo-vis BVBA Biebuyckstraat 15 D 9850 Nevele - Belgium Website: www.mo-vis.com E-mail:
64C Amplification Block. Instruction Manual
 64C Amplification Block Instruction Manual Caution: All rights reserved. Quidel Corporation reserves the right to modify this manual at any time without notice. Any part of the manual shall not be duplicated,
64C Amplification Block Instruction Manual Caution: All rights reserved. Quidel Corporation reserves the right to modify this manual at any time without notice. Any part of the manual shall not be duplicated,
RePlay Hemostasis Clip
 RePlay Hemostasis Clip Instructions for use. Read carefully prior to use. Caution: Federal (U.S.A.) Law restricts this device to sale by or on the order of a physician. Diversatek Healthcare Corporate
RePlay Hemostasis Clip Instructions for use. Read carefully prior to use. Caution: Federal (U.S.A.) Law restricts this device to sale by or on the order of a physician. Diversatek Healthcare Corporate
3M Red Dot Cable and Leadwire Solutions for Patient Monitoring. Minimize risk. Standardize inventory. Utilize one source.
 3M Red Dot Cable and Leadwire Solutions for Patient Monitoring Minimize risk. Standardize inventory. Utilize one source. Rely on 3M for a wide selection of Disposable solutions. Disposable ECG leads reduce
3M Red Dot Cable and Leadwire Solutions for Patient Monitoring Minimize risk. Standardize inventory. Utilize one source. Rely on 3M for a wide selection of Disposable solutions. Disposable ECG leads reduce
LASER GUIDED PARKING SYSTEM
 LASER GUIDED PARKING SYSTEM Model 94318 ASSEMBLY AND OPERATING INSTRUCTIONS Due to continuing improvements, actual product may differ slightly from the product described herein. 3491 Mission Oaks Blvd.,
LASER GUIDED PARKING SYSTEM Model 94318 ASSEMBLY AND OPERATING INSTRUCTIONS Due to continuing improvements, actual product may differ slightly from the product described herein. 3491 Mission Oaks Blvd.,
CERTIFICATE OF ACCREDITATION
 CERTIFICATE OF ACCREDITATION In terms of section 22(2)(b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
CERTIFICATE OF ACCREDITATION In terms of section 22(2)(b) of the Accreditation for Conformity Assessment, Calibration and Good Laboratory Practice Act, 2006 (Act 19 of 2006), read with sections 23(1),
Technical Specification for Helical Fittings for Overhead Power Lines
 Ergon Energy Corporation Limited Technical Specification for Helical Fittings for Overhead Power Lines ETS01-09-03 Specification ETS01-09-03 Ver 3 Contents 1. Purpose and Scope... 4 2. References... 4
Ergon Energy Corporation Limited Technical Specification for Helical Fittings for Overhead Power Lines ETS01-09-03 Specification ETS01-09-03 Ver 3 Contents 1. Purpose and Scope... 4 2. References... 4
Instructions for Use Reprocessed Electrophysiology Catheter Cables
 Reprocessed by Instructions for Use Reprocessed Electrophysiology Catheter Cables Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by
Reprocessed by Instructions for Use Reprocessed Electrophysiology Catheter Cables Reprocessed Device for Single Use STERILE Explanation of Symbols Federal Law in the USA restricts this device to sale by
QuickNet-RK QUICKNET AND CERAPRO HEATING CABLE REPAIR KIT INSTALLATION INSTRUCTIONS
 QuickNet-RK QUICKNET AND CERAPRO HEATING CABLE REPAIR KIT INSTALLATION INSTRUCTIONS DESCRIPTION The QuickNet-RK repair kit is for repairing QuickNet or CeraPro heating cable that is damaged during installation.
QuickNet-RK QUICKNET AND CERAPRO HEATING CABLE REPAIR KIT INSTALLATION INSTRUCTIONS DESCRIPTION The QuickNet-RK repair kit is for repairing QuickNet or CeraPro heating cable that is damaged during installation.
Part 1: On-land pipelines
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 15589-1 Second edition 2015-03-01 Petroleum, petrochemical and natural gas industries Cathodic protection of pipeline systems Part 1: On-land pipelines
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 15589-1 Second edition 2015-03-01 Petroleum, petrochemical and natural gas industries Cathodic protection of pipeline systems Part 1: On-land pipelines
2200-Lb. Semi-Electric Stacker OWNER S MANUAL
 2200-Lb. Semi-Electric Stacker OWNER S MANUAL WARNING: Read carefully and understand all ASSEMBLY AND OPERATION INSTRUCTIONS before operating. Failure to follow the safety rules and other basic safety
2200-Lb. Semi-Electric Stacker OWNER S MANUAL WARNING: Read carefully and understand all ASSEMBLY AND OPERATION INSTRUCTIONS before operating. Failure to follow the safety rules and other basic safety
WARNING! Read all the information contained in this Manual, before you begin using the Equipment.
 INTRODUCTION: Congratulations! You have just purchased a product developed using the latest concepts of high technology on the market. This equipment has been designed following strict quality standards
INTRODUCTION: Congratulations! You have just purchased a product developed using the latest concepts of high technology on the market. This equipment has been designed following strict quality standards
U7242A USB3.0 Test Fixture
 U7242A USB3.0 Test Fixture User s Guide Agilent Technologies Notices Agilent Technologies, Inc. 2009 No part of this manual may be reproduced in any form or by any means (including electronic storage and
U7242A USB3.0 Test Fixture User s Guide Agilent Technologies Notices Agilent Technologies, Inc. 2009 No part of this manual may be reproduced in any form or by any means (including electronic storage and
KANE510. Portable Oxygen Analyser. Stock No: April Kane International Ltd
 KANE510 Portable Oxygen Analyser Stock No: 19673 April 2016 Kane International Ltd CONTENTS Page No: OWNERS MANUAL & MAINTENANCE 3 1. GETTING STARTED 5 1.1 SAFETY NOTES 5 1.2 PRE-TEST CHECKLIST 5 1.3 ANALYSER
KANE510 Portable Oxygen Analyser Stock No: 19673 April 2016 Kane International Ltd CONTENTS Page No: OWNERS MANUAL & MAINTENANCE 3 1. GETTING STARTED 5 1.1 SAFETY NOTES 5 1.2 PRE-TEST CHECKLIST 5 1.3 ANALYSER
MEGADYNE. MEGA 2000 Patient Return Electrode System. Frequently Asked Questions
 MEGADYNE MEGA 2000 Patient Return Electrode System Frequently Asked Questions Do any of MEGADYNE's products contain latex? Is the system FDA approved? How is the MEGA 2000 Patient Return Electrode system
MEGADYNE MEGA 2000 Patient Return Electrode System Frequently Asked Questions Do any of MEGADYNE's products contain latex? Is the system FDA approved? How is the MEGA 2000 Patient Return Electrode system
