Gene therapies for spinocerebellar ataxia type 1
|
|
|
- Kristian Perkins
- 6 years ago
- Views:
Transcription
1 University of Iowa Iowa Research Online Theses and Dissertations Spring 2013 Gene therapies for spinocerebellar ataxia type 1 Megan Kathryn Keiser University of Iowa Copyright 2013 Megan Kathryn Keiser This dissertation is available at Iowa Research Online: Recommended Citation Keiser, Megan Kathryn. "Gene therapies for spinocerebellar ataxia type 1." PhD (Doctor of Philosophy) thesis, University of Iowa, Follow this and additional works at: Part of the Neuroscience and Neurobiology Commons
2 GENE THERAPIES FOR SPINOCEREBELLAR ATAXIA TYPE 1 by Megan Kathryn Keiser An Abstract Of a thesis submitted in partial fulfillment of the requirements for the Doctor of Philosophy degree in Neuroscience in the Graduate College of The University of Iowa May 2013 Thesis Supervisor: Professor Beverly L. Davidson
3 1 ABSTRACT Spinocerebellar ataxia type 1 (SCA1) is an adult onset, autosomal dominant neurodegenerative disease caused by a CAG repeat expansion in ataxin-1, which encodes the ataxin-1 protein. SCA1 is one of nine polyq-expansion gain-of-function diseases which includes Huntington s disease, spinal-bulbar muscular atrophy, dentatorubralpallidoluysian atrophy and other ataxias. Clinical symptoms of SCA1 include ataxia, dysarthria, ophthalmoparesis, muscle wasting, and extrapyramidal and bulbar dysfunction. Cerebellar Purkinje cells (PCs), neurons in the inferior olive and nuclei of the brainstem are affected. No disease-modifying therapy exists for SCA1. The goals of my thesis were to assess the safety and efficacy of AAV-delivered artificial mirnas targeting ataxin-1 to alleviate neuropathological and behavioral phenotypes in the knockin and transgenic SCA1 mouse models. In the knock-in SCA1 mouse model I delivered AAVs expressing an artificial mirna (misca1) targeting sequences conserved in mouse and human ataxin-1 directly to the deep cerebellar nuclei. This achieved long term silencing of ataxin-1 mrna and significantly improved rotarod performance, gait deficiencies, and neuropathology of the cerebellum. In the transgenic SCA1 mouse model I repeated this method of delivery with an artificial microrna (mir) (mis1) design that optimized potency, efficacy and safety to suppress Atxn1 expression. Additionally I examined the therapeutic potential of continuous overexpression of ataxin-1-like. Delivery of either ataxin-1-like or mis1 viral vectors to SCA1 mice cerebella resulted in widespread cerebellar Purkinje cell transduction. There was significant improvement to rotarod performance, gait deficiencies, coordination and balance, as well as the neuropathology of cerebellar Purkinje cells.
4 2 In summary, these data indicate the utility of these approaches as possible therapies for SCA1 patients. Abstract Approved: Thesis Supervisor Title and Department Date
5 GENE THERAPIES FOR SPINOCEREBELLAR ATAXIA TYPE 1 by Megan Kathryn Keiser A thesis submitted in partial fulfillment of the requirements for the Doctor of Philosophy degree in Neuroscience in the Graduate College of The University of Iowa May 2013 Thesis Supervisor: Professor Beverly L. Davidson
6 Graduate College The University of Iowa Iowa City, Iowa CERTIFICATE OF APPROVAL PH.D. THESIS This is to certify that the Ph.D. thesis of Megan Kathryn Keiser has been approved by the Examining Committee for the thesis requirement for the Doctor of Philosophy degree in Neuroscience at the May 2013 graduation. Thesis Committee: Beverly L. Davidson, Thesis Supervisor Alexander G. Bassuk Pedro Gonzalez-Alegre Paul B. McCray Peggy C. Nopoulos
7 To my loving parents, my supportive husband, and my dear, dear friends ii
8 ACKNOWLEDGMENTS First and foremost I would like to thank my mentor, Dr. Beverly Davidson for everything she has done to help me along my graduate journey. She has made me a better writer, a better speaker, and a better scientist. She pressed me to be better than I thought I could be. Dr. Davidson is an inspiration and I thank her for all of her encouragement and for believing in me. I would like to thank my thesis committee, Dr. Alex Bassuk, Dr. Pedro Gonzalez- Alegre, Dr. Paul McCray, and Dr. Peg Nopoulos for their support and insight on my thesis work. I am grateful to have had such a broad wealth of knowledge and in so many areas of expertise at my disposal for questions and advice. I would like to thank the members of the Davidson lab who have made my journey tolerable. A good work environment is crucial for good productivity. Thank you to Pat Staber, who was constantly there to help with technical difficulties and to help me locate any rogue reagents. Thank you to my bench mates and colleagues, always there to help me troubleshoot a difficult procedure or to instruct me when venturing into the new territories of cell culture and protein analysis. I would also like to thank Maria Scheel and the GTVC for providing my viruses and being so helpful. I would like to thank Dr. Daniel Tranel, director of the Neuroscience Graduate Program. He always puts his students first ensure that everyone makes it through the graduate program intact. He never questioned my abilities or my commitment when I stumbled in the beginning, and for this, I am forever grateful. Finally I wish to thank all of my friends and family for their unerring support. I would like to thank my mother and father who have stood by me since birth and thought I iii
9 could do anything I put my mind to; and my beloved husband Robby, who has been with me through the toughest times with love and support. Thank you.. iv
10 ABSTRACT Spinocerebellar ataxia type 1 (SCA1) is an adult onset, autosomal dominant neurodegenerative disease caused by a CAG repeat expansion in ataxin-1, which encodes the ataxin-1 protein. SCA1 is one of nine polyq-expansion gain-of-function diseases which includes Huntington s disease, spinal-bulbar muscular atrophy, dentatorubralpallidoluysian atrophy and other ataxias. Clinical symptoms of SCA1 include ataxia, dysarthria, ophthalmoparesis, muscle wasting, and extrapyramidal and bulbar dysfunction. Cerebellar Purkinje cells (PCs), neurons in the inferior olive and nuclei of the brainstem are affected. No disease-modifying therapy exists for SCA1. The goals of my thesis were to assess the safety and efficacy of AAV-delivered artificial mirnas targeting ataxin-1 to alleviate neuropathological and behavioral phenotypes in the knockin and transgenic SCA1 mouse models. In the knock-in SCA1 mouse model AAVs expressing an artificial mirna (misca1) targeting sequences conserved in mouse and human ataxin-1 were injected directly to the deep cerebellar nuclei. This achieved long term silencing of ataxin-1 mrna and significantly improved rotarod performance, gait deficiencies, and neuropathology of the cerebellum. In the transgenic SCA1 mouse model the same method of delivery was executed with an artificial microrna (mir) (mis1) designed to optimize potency, efficacy and safety to suppress Atxn1 expression. Additionally the therapeutic potential of continuous overexpression of ataxin-1-like was examined. Delivery of either ataxin-1-like or mis1 viral vectors to SCA1 mouse cerebellum resulted in widespread cerebellar Purkinje cell transduction. There was significant improvement to rotarod performance, gait deficiencies, coordination and balance, as well as the neuropathology of cerebellar Purkinje cells. In summary, these data indicate the utility of these approaches as possible therapies for SCA1 patients. v
11 TABLE OF CONTENTS LIST OF TABLES... viii LIST OF FIGURES... ix CHAPTER I. INTRODUCTION...3 Spinocerebellar Ataxia Type Mouse models...4 B05 transgenic SCA1 mouse model (82Q)...4 SCA1 knock-in mouse model (154Q)...5 Ataxin RNAi mechanism and function...9 RNAi as a tool for directed gene silencing...10 Viral delivery of RNAi sequences to the CNS...11 RNAi as a therapy for SCA Taking RNAi to the clinic...14 Allele-specific versus non-allele specific silencing...14 Dose, delivery, and distribution of RNAi in the human brain...15 Duration of silencing...16 II. DIRECT CEREBELLAR INJECTION OF AAV DELIVERED MIRNA TARGETING MOUSE ATAXIN-1 IN 154Q SCA1 KNOCK- IN MICE RESCUES BEHAVIORAL AND HISTOLOGICAL PHENOTYPES...19 Abstract...19 Introduction...19 Materials and Methods...21 Plasmids and viral vectors...21 Animals...22 AAV injections and brain tissue isolation...22 Immunohistochemistry analyses...23 In situ hybridization...24 Semi-quantitative PCR...24 Quantitative PCR...24 Behavioral analysis...25 Rotarod...25 Gait Analysis...26 Statistical analysis...26 Results...26 Expression of misca1 and reduction of ataxin-1 in vivo...26 AAV.miSCA1 protects against molecular layer thinning...27 AAV.miSCA1 rescues gait and coordination...28 Silencing ataxin-1 achieves cellular and synaptic rescue...29 Discussion...30 vi
12 III. ALTERNATIVE THERAPEUTIES ACHIEVE BEHAVIORAL AND HISTOLOGICAL PHENOTYPIC RESCUE IN B05 TRANSGENIC SCA1 MOUSE MODEL: RNAI AND OVEREXPRESSION OF HATXN1L...49 Abstract...49 Introduction...49 Materials and Methods...51 Cloning plasmids and viral vectors...51 Cell culture and transfection...53 Animals...53 AAV injections and brain tissue isolation...54 Immunohistochemistry analyses...54 In situ hybridization...54 Semi-quantitative PCR...54 Western blot analysis...55 Co-immunoprecipitation...55 Quantitative PCR...56 Behavioral analysis...56 Ledge test...57 Hindlimb clasping...57 Stride length measurements...58 Rotarod...58 Statistical analysis...59 Results...59 Optimization and validation of therapeutic delivery...59 Ataxin-1-like overexpression and knockdown of ataxin-1 rescues behavioral phenotypes...61 Analysis of in vivo protein interactions with overexpressed ataxin- 1-like...62 Treatment with HAtxn1L or mis1 is not toxic in vivo...63 Ataxin-1-like overexpression and human ataxin-1 knockdown improves neuropathology...63 Discussion...64 IV. CONCLUSIONS AND FUTURE DIRECTIONS...87 Known or implied ataxin-1 mechanisms and functions...87 Further elucidation into Capicua and Rbm17 complexes and the role of Ataxin-1-like...89 Cellular functions of ataxin Moving toward clinical applications...92 Summary...93 REFERENCES...95 vii
13 LIST OF TABLES Table 1. Proposed Co-IPs under specified conditions...94 viii
14 LIST OF FIGURES Figure 1. Cartoon of Ataxin RNAi Pathway and Viral Delivery Cartoon of AAV2/5 construct Viral spread shown by hrgfp fluorescence Semi-quantitative PCR of misca1 expression In situ hybridization of misca1 localization Quantitative PCR knockdown of matxn1 6 weeks post-injection IHC for Iba1 shows no toxicity Molecular layer widths improve in AAV.miSCA1 treated mice AAV.miSCA1 treated mice have longer strides than control mice AAV.miSCA1 treated mice have wider stances than control mice AAV.miSCA1 rescues rotarod phenotypes at 30- and 40-weeks of age Quantitative PCR shows matxn1 knockdown by AAV.miSCA1 at late time points mglur1 mrna expression levels are improved by AAV.miSCA mglur4 mrna expression levels are improved by AAV.miSCA Vegfa mrna expression levels are improved by AAV.miSCA Possible mechanisms underlying reduced Vegfa expression in SCA1 Purkinje cells Cartoon of viral constructs Quantitative PCR 3 weeks post-injection of AAV.miS1 confirms knockdown of human ataxin Western blot confirming expression of HAtxn1L in vivo Viral spread shown by egfp fluorescence Semi-quantitative PCR confirms mis1 expression in vivo In situ hybridization localizes mis1 to Purkinje cells...73 ix
15 24. Quantitative PCR confirms continual knockdown of human ataxin-1 by AAV.miS Quantitative PCR shows rescued Calbindin levels in AAV.miS1 injected mice AAV.miS1 and AAV.HAtxn1L rescues ledge test phenotype AAV.miS1 and AAV.HAtxn1L rescues hindlimb clasping phenotype AAV.miS1 and AAV.HAtxn1L improve stride length AAV.miS1 and AAV.HAtxn1L rescues rotarod deficit Immunoprecipitated Ataxin-1-like is detected in injected cerebellar lysates Co-immunoprecipitation confirms interaction between ataxin-1-like and ataxin-1 in vivo IHC Iba1 staining shows no toxicity in treated mice Quantitative PCR shows no increase in GFAP mrna Molecular layer widths improved by AAV.miS1 and AAV.HAtxn1L Purkinje cell number improved by AAV.miS1 and AAV.HAtxn1L % of ectopic Purkinje cells rescued by AAV.miS1 and AAV.HAtxn1L...86 x
16 3 CHAPTER I INTRODUCTION Spinocerebellar Ataxia Type 1 Spinocerebellar Ataxia Type 1 (SCA1) is among the most common inherited autosomal dominant neurodegenerative ataxias. SCA1 is characterized by an unstable CAG expansion in ataxin-1, which encodes the ataxin-1 protein (ATXN1) [1-3]. CAG encodes the amino acid glutamine, abbreviated as Q, and SCA1 is one of nine polyglutamine (polyq) diseases. Normally, there are 6-42 CAG repeats interspersed with 1-3 CAT units encoding histidine. Full penetrance occurs when alleles have pure CAG repeats [4-6]. Progressive neuronal dysfunction and cell death occur primarily in cerebellar Purkinje cells as well as in brain stem neurons. Symptoms present as problems with gait, balance and coordination. Additional symptoms include slurred speech, overactive reflexes, multiple ocular dysfunction, lack of movement coordination, an inability to perform rapidly alternative movements, a loss in muscle tone, and loss of executive function [7-10]. The average age of onset is in the fourth or fifth decade of life with death occurring within years [10, 11]. Currently there is no treatment for SCA1. Ataxin-1 is ubiquitously expressed, generally localized to neuronal nuclei neurons or in the cytoplasm for non-neuronal cells. However, ataxin-1 is found in both the nucleus and the cytoplasm of cerebellar Purkinje cells [6]. Post-necropsy analysis of patient cerebellar tissues identified ataxin-1 positive nuclear inclusions in affected areas, such as brainstem neurons, but also in unaffected neurons of the cerebrum [12, 13]. The
17 4 exact cellular and molecular mechanisms responsible for SCA1 remain unknown. Animal models have been generated to expand our basic knowledge of ataxin-1 function both in its normal and mutated form. Mouse Models B05 Transgenic SCA1 Mouse Model (82Q) In 1995, Burright et al. published a paper documenting the production of a SCA1 transgenic mouse model. This mouse expressed human SCA1 with an expanded polyglutamine tract encoded by 82 CAG repeats. The transgene expression was limited to cerebellar Purkinje cells [14] by the Purkinje cell-specific promoter Pcp2, also known as L7 [15]. This line, known as B05 line, had severe and aggressive behavioral and histological phenotypes that resembled SCA1 pathology. B05 mice exhibit normal cerebellar development. Morphological abnormalities become apparent at P25 when clear cytoplasmic vacuoles in Purkinje cells appear. Nuclear aggregates are evident in Purkinje cells at 6 weeks of age [16]. At 8 weeks of age, gliosis begins in the molecular layer, and progresses until 15 weeks of age, by which time the molecular layer has undergone significant shrinkage [17]. The original Burright et al. paper examined pathology at 16 weeks of age where there was significant Purkinje cell death and gliosis. Ectopic Purkinje cells were also present at 16 weeks of age [14]. The phenotype of ectopic Purkinje cells was specific to the 82Q transgenic SCA1 model. It was hypothesized that Purkinje cells translocate into the molecular layer to compensate for their reduction of dendrites in efforts to preserve their parallel fiber connections [14].
18 5 While ataxia was visually discernible by home cage behavior in B05 mice at 12 weeks of age [14], a more detailed analysis of behavioral deficits in this model was later characterized by Clark et al. [17]. Gait analysis was performed at 6-, 12-, and 52-week old animals. B05 mice first showed abnormalities in gait at 12-weeks of age. They had significantly shorter stride lengths than wild type littermates. At 1 year of age B05 mice presented a significantly wider hindlimb stance than wild type littermates. Accelerating rotarod was performed on naïve mice at 5-, 12-, 19-, and 52-weeks of age. Clark et al. reported that B05 mice performed significantly worse than their wild type littermates by as early as 5 weeks of age [17]. At later time points, B05 mice performed progressively worse on the accelerated rotarod until at 1 year of age they were unable to perform the task. These results have replicated since the original characterization in publications by the same research group [18-20] The B05 transgenic mouse model has proven beneficial in expanding our knowledge of ataxin-1 function and protein interactions, discussed below, in Purkinje cells. However, B05 mice have restricted neuronal degradation, occurring predominantly in Purkinje cells, and showed no decrease in life span [14]. This was not representative of the natural progression of SCA1. A mouse model that presumably would more accurately portray SCA1 disease progression spatially and temporally was important to advance the field. SCA1 Knock-In Mouse Model (154Q) In 2002, Watase et al. published a paper detailing a new mouse model for SCA1 that ubiquitously expressed a 154 expanded pure CAG tract in the endogenous ataxin-1
19 6 locus of C57Bl/6J mice [21]. The ubiquitous expression of polyglutamine expanded Atxn1 showed pathological differences from the B05 model. The characteristic nuclear inclusions were visible and present predominantly in the cerebral cortex at 20 weeks of age, with few inclusions in Purkinje cells. By 40 weeks of age there were significantly higher numbers of aggregates in all areas of the cerebrum and cerebella, but most notably in the hippocampus. At 19 weeks of age, a reduction of Purkinje cell dendrites was first reported. Progressive loss of dendrites and finally Purkinje cell death occurred at 34 weeks of age. Interestingly, there was limited reactive astrocytosis seen in the cerebellum and brain stem, by GFAP staining, whereas there was significantly enhanced immunoreactivity in the spinal cord [21]. 154Q mice showed growth retardation by 11 weeks of age, weighing ~20% less than wild type litter mates. Kyphosis presented at 30 weeks of age with premature death reported between 35 and 45 weeks [21]. This conflicts with personal work done in 154Q mice, which live more than 60 weeks before they began to perish by natural causes. Accelerated rotarod analysis performance deficits were noted by 5 weeks in 154Q mice [21]. This again conflicts with personal work, in which 154Q mice do not show any significant deficit in rotarod performance before 15 weeks of age when compared to wild type littermates. Hindlimb clasping in this model presented at 9 weeks of age, with obvious ataxia and abnormal gait seen by home cage behavior at 20 weeks of age. Learning and memory in 154Q was impaired by ~8 weeks of age as measured by Morris water maze analysis, both with visual and hidden platform tasks, and by a context fear assay [21].
20 7 Ataxin-1 While there are limitations to the B05 model, much has been learned about how mutant Atxn1 exerts its function. For example, Klement et al., found that nuclear localization fo the protein was paramount to this phenotype [22]. They originally believed nuclear inclusions drove toxicity in Purkinje cells [22]. More recently there has been data suggesting that the inclusions are of a protective nature, sequestering mutant Atxn1 [23, 24]. Important work identified that obstructing Atxn1 from translocating to the nucleus rescues all disease progression and no inclusions form, regardless of an expanded polyglutamine tract [25, 26]. The most recent belief, gleaned from experimental data, is that SCA1 pathology is not dependent on the formation on mutant atxn1 inclusion [22, 24-27]. To further understand function of Atxn1 and mechanisms of pathology in SCA1, studies have examined ATXN1 protein interactions. In addition to the expanded polyglutamine tract several important structural elements are critical to atxn1 function and disease progression: the AXH (ataxin-1 and HMG-box protein 1(HBP1)) domain [28] allows RNA binding and dimerization with other AXH containing proteins [29] and the nuclear localization sequence under the control of the phosphorylation site at serine 776 (S776) [22] (Figure 1). Under normal conditions S776 undergoes phosphorylation in the cytoplasm becoming ps776 [30]. Once phosphorylated binds Atxn1 near ps776 obscuring its nuclear localization site. This protects Atxn1 from dephosphorylation while inhibiting Atxn1 translocation to the nucleus [31]. Dissociation from allows Atxn1 to enter
21 8 the nucleus whereupon, in its phosphorylated state, it binds to RNA-Binding Motif 17 (Rbm17) or is dephosphorylated and binds to Capicua (Cic) [31]. Cic, a transcriptional repressor, contains an AXH domain [32] allowing it to bind with Atxn1 or Ataxin-1-like (Atxn1l). Work done in HeLa cells established that Atxn1 competes with Atxn1l for interaction with Cic [23]. This finding led to the creation of a double knock-in SCA1 mouse model expressing a 154Q expansion in the endogenous atxn1 locus and two copies of atxn1l. Results showed that duplication of endogenous atxn1l suppressed neuropathology in SCA1 double knock-in mice [23]. From this work, they hypothesized that elevated levels of Atxn1l displaced mutant Atxn1 from complexing with Cic to partially rescue SCA1 phenotypes [23]. Further work to elucidate the role of Cic in Atxn1 function found that complete loss of Atxn1 led to a decrease in Cic expression and normal Cic repressor activity was modulated when bound to mutant ataxin-1 [32]. Recently it was demonstrated that Cic when bound to polyglutamine expanded Atxn1 caused concomitant gain- and loss-offunction mechanisms on specific transcriptional targets [18]. SCA1 knock-in mouse data suggested that polyglutamine expanded Atxn1:Cic complexes enhanced binding of Cic to transcriptional targets and caused hyper-repression. Concomitantly, polyglutamine expanded Atxn1:Cic complexes reduced binding of Cic to transcriptional targets, causing de-repression [18]. Splicing factor Rbm17 interacts with phosphorylated Atxn1. Its binding affinity correlates directly to the length of the atxn1 polyglutamine expansion [33]. A study in 2008 hypothesized that healthy mice maintain a balance of Atxn1 complexing with Cic versus Rbm17, a balance that is dependent on the phosphorylation status of S776.
22 9 Polyglutamine expanded Atxn1 disrupts this balance because Rbm17 prefers atxn1 with expanded polyglutamine tracts [33]. This study concluded that elevated levels of Atxn1- Rbm17 complexes caused more toxicity than of Atxn1- Cic complexes. This study did not take Atxn1l into consideration. RNAi mechanism and function RNAi is an innate gene regulatory mechanism that is essential to many cellular processes such as proliferation, differentiation, cell death, and remodeling [34, 35]. RNAi also plays an important role in host defense by protecting against viral infection and transposable elements [36]. In RNAi, the cell makes use of double stranded RNA molecules to silence the expression of an mrna molecule by complementary base pairing. One form of naturally occurring double stranded RNA molecules are micrornas (mirnas), which are transcribed in the nucleus as stem loop structured primary mirnas (pri-mirnas) from pol II or pol III promoters [37]. Pri-miRNAs are cleaved by the Drosha-DGCR8 microprocessor complex in the nucleus to form ~60-70 nucleotide hairpin-like structures called precursor-mirnas (pre-mirnas) [38, 39]. The premirnas are then exported to the cytoplasm by Exportin-5, and further processed by the Dicer (an RNase III endonuclease)-containing complex which cleaves the loop structure of the pre-mirna to release short, ~21 nucleotide, mature mirna sequences [40, 41]. Dicer also processes exogenous long dsrnas (such as viral RNAs) into smaller 21 nucleotide small interfering RNAs (sirnas) [42]. One strand of the mirna or sirna duplex, known as the antisense or guide strand, is selectively loaded into the Agocontaining RNA-induced silencing complex (RISC). This process is known as strand
23 10 biasing; the non-loaded strand is referred to as the passenger strand [43]. This activated RISC carries out gene silencing, either by Ago2 mediated cleavage of the complementary target mrna (in the case of sirnas) or by target destabilization or translational repression (in the case of mirnas) after imperfect base pairing to the 3 UTR (Figure 2) [44-46]. RNAi as a tool for directed gene silencing RNAi has rapidly evolved as a tool for directed gene silencing. The RNAi machinery can be co-opted in many ways to achieve gene expression knockdown of a select target (Figure 2). Synthetic sirnas (~21nt) can be introduced into cells, which are directly loaded into RISC or, in the case of longer dsrnas (25-27nt), first processed by Dicer and then loaded into the RISC to achieve gene silencing [47]. Gene targeting sirna duplexes can also be embedded in hairpin-based structures made to mimic the primirna (called artificial mirnas) or the pre-mirna (called short hairpin or shrnas); when placed into expression vectors, they are transcribed in the nucleus and processed by the endogenous RNAi pathway to achieve gene silencing. shrnas are typically expressed from strong Pol III promoters (such as U6 or H1), while artificial mirnas can be expressed from pol II or pol III promoters. While shrnas may have more potent silencing capability, they are often expressed at very high levels and can saturate the RNAi machinery, which disrupts endogenous mirna processing and can induce toxicity [48, 49]. Artificial mirnas on the other hand are generally safer and less toxic and they do not appear to disrupt endogenous mirna processing [48-53]. Although artificial
24 11 mirnas are less toxic, their safety profile is also dictated by the design of the RNAi sequence. Viral delivery of RNAi sequences to the CNS RNAi delivery to the CNS faces unique challenges. For effective RNAi delivery via the blood, the presence of the blood-brain barrier (BBB) is an obstacle that must be overcome, while for direct injection into the brain, steps must be taken to avoid toxic or inflammatory reactions. An ideal delivery system for RNAi to the CNS should be: minimally immunogenic, non-toxic, target specific cells of the CNS, knockdown the specific target mrna efficiently and be easy to manufacture [54]. There are a number of viral vectors that can be used for gene delivery to the CNS [54]. The two main viral vector systems that are used to transduce the CNS are lentiviruses (LV) and adeno-associated viruses (AAV). Both viruses are minimally immunogenic and can transduce a number of CNS cell types. Recombinant lentiviruses are pseudotyped with various glycoproteins which can impart different tropisms after directed delivery into brain [55, 56] and they have been used successfully in gain of function studies [57] and loss of function studies [58-61]. One difference between lentivirus and AAV or Ad-based systems is the level of expression. This is due in part because lentivirus-mediated transduction often results in low copy numbers of transgene/cell. Also, the placement of the expression cassette in the lentivirus genome can affect expression levels [62]. Another difference is that most lentivirus vectors integrate, while AAVs do not.
25 12 AAV belongs to the genus Dependovirus and in its wild type state requires a helper virus, such as Adenovirus, to replicate. A number of factors make AAV suitable for gene delivery in vivo. AAV can be easily manufactured and it is scalable for human use [63, 64]; particularly for the relatively low volumes needed for brain-expressed targets. As stated above, AAVs rarely integrates. In general, AAVs are non-pathogenic and have low immunogenic properties, which make them ideal for gene delivery in vivo [54]. AAVs confer robust expression, efficiently transduce neurons and other cell types, and in the absence of an immune response to what is being expressed, can afford longterm expression [65-67]. Tissue tropism of AAV is dictated by the capsid serotype. AAV capsids with different cell/tissue tropisms have been identified and depending on the capsid serotype, AAV can transduce neurons, astrocytes, glia and ependymal cells with high transduction efficiency [68-72]. The AAV capsid can be modified to alter its tropism by several ways including directed evolution, capsid shuffling and incorporation of targeting peptides. Directed evolution involves mutagenesis of the capsid, which may alter tropism [73, 74]. Capsid shuffling involves the assembly of variant capsid sequences to give rise to recombinant capsids with tropisms to different cell types [75-77]. AAV tropism can also be altered by the incorporation of targeting ligand into the capsid, to mediate ligand specific receptor binding [78-80]. Gene transfer after direct delivery of AAV vectors by intraparenchymal, intraventricular or intrathecal injections to target cells of the brain is used for RNAi delivery. Direct delivery by intra-parenchymal injections has proven effective for targeting neurons in various neurodegenerative diseases, and it limits transduction to
26 13 those tissues most relevant to disease. Widespread exposure of a transgene product occurs after intraventricular or intrathecal delivery of AAVs, when the transgene product is a secreted molecule [81]. Recently, intrathecal injection of AAV9 or AAV2.5 showed robust transduction of the brain and spinal cord in non-human primates [82]. Peripheral delivery of AAVs for brain targeting has also been used [78-80, 83-85]. Concerns with this approach for clinical application are the high dose needed, the transduction of peripheral organs (which may not be desirable) and the induction of a robust anti-aav and likely anti-transgene response. Nonetheless, using vectors that can cross the BBB may be beneficial for some applications. Intravenous delivery of AAV9, a recently identified serotype, can cross the BBB after and transduce neurons in neonatal mice and astrocytes and scattered neurons in adult mice and rhesus macaques [83, 84]. Also, variants of AAV9 transduce motor neurons and astrocytes after systemic delivery by intravenous injection to adult mice [84, 85]. Personal pilot injections using AAV9 systemically resulted in sporadic transduction of predominantly glia. Although AAVs have a small packaging capacity (~4.7kb), they are ideally suited to deliver the small RNAi expression cassette. RNAi sequences delivered to the brain after AAV injection have shown therapeutic promise in mouse models of dominantly inherited polyglutamine (polyq) diseases and other neurological disorders. RNAi as a therapy for SCA1 In 2004, RNAi was established as a potential therapy for SCA1 after successful rescue of disease phenotypes in B05 SCA1 transgenic mice [86]. AAVs expressing shrnas against human Atxn1 were injected to SCA1 mice cerebellar cortices. Using
27 14 this injection technique, Xia et al. transduced ~10% of medially located cerebellar Purkinje cells. This amount of transduction resulted in significantly improved rotarod performance at 21 weeks of age compared to control treated B05 littermates. shrna delivery also rescued molecular layer width compared to control treated B05 littermates. They also saw a reduction of phenotypic nuclear inclusions in transduced cells relative to control treated B05 littermates. This was the premier publication to demonstrate the possibility of RNAi as a therapeutic for SCA1 specifically, and polyq diseases generally, in vivo. Since then, alternate injection coordinates targeting the deep cerebellar nuclei have achieved a higher level of Purkinje cell transduction. Also, safe and efficient artificial mirnas have been developed to target either conserved regions of atxn1 or alternatively human and rhesus-specific sequences in atxn1, to be described in later chapters. Taking RNAi to the clinic As RNAi therapies for CNS diseases approach the clinic, there are obvious considerations to moving each potential drug forward. Allele-specific versus non-allele specific silencing While targeting the mutant allele is always desirable, it may not be necessary for some diseases, as RNAi reduces but does not fully remove the targeted gene product. Moreover, wild type levels of the gene being targeted may not be required for maintenance of cell viability. For example, SCA1, SCA2 and SCA3 knockout mice are viable and fertile, indicating that knockdown of the wild type allele function may be
28 15 tolerable [87-89]. Non-allele specific silencing of HTT in HD mice resulted in a significant rescue of the HD phenotype and two studies have shown that reducing levels of wild type HTT in the adult rhesus macaque striatum is safe and well tolerated for at least 6 months [90-92]. However, as the HD null mice are embryonic lethal, and the levels of HTT required for cell viability of adult neurons is unknown, researchers are also investigating allele-specific silencing options [93]. For every disease being tested by nonallele specific silencing, it is important to consider whether partial loss of function of the wild type allele is sufficient to retain function long-term. Dose, delivery, and distribution of RNAi in the human brain Ultimately, the goal of developing RNAi therapies and testing them in animal models is for treatment in humans. Thus, it is important to understand what kind of dose may be appropriate and how long the RNAi efficacy is retained in the human brain. A number of studies have focused on determining an appropriate dose of RNAi that is efficacious and safe in the primate brain using viral and non-viral methods [91, 92, 94, 95]. Non-viral delivery systems used cannulas implanted in the brain or convection enhanced delivery (CED) systems that use flow pressure to increase the volume of distribution. For long-term effects, repeated dosing would be required. For AAVs, directed delivery can be done, or if the targeted structure is larger, CED is effective [94]. Various methods are used to inform investigators about the distribution of the drug after delivery. To detect sirnas after delivery, Stiles et.al. used radiolalebeled sirnas, which allowed comparison of the volume of brain for which there was target suppression as a function of dose and spread [94]. For viral vectors, a common strategy to determine the
29 16 distribution of transduced cells takes advantage of reporter genes, such as egfp [90, 92]. In the future, targeting the brain after systemic delivery may be possible. Alternatively, researchers may be able to take advantage of the impaired BBB in some neurological diseases, allowing for diffusion of viruses, drugs and other small molecules into the brain that are delivered systemically [73, 96]. Duration of silencing Expression of transgenes after AAV delivery has been observed to last many years (> 8 years) in the primate brain [66]. Whether these same platforms can provide for lasting expression of RNAi triggers sufficient to last the life of the patient, or at the least, many years, is not yet known. The longevity of expression from viral vectors may vary depending on the vector type, the promoter, the cell types transduced, and the pathology of the particular disease. While longevity of the transgene expression by viral vectors is important, it is also important to consider regulating its expression in the case of adverse effects from off-targeting. In this regard, regulating expression of the transgene by exogenous factors such as the erythromycin based on-off system may be an important parameter to consider [97]. In summary, cumulative and ongoing studies with RNAi delivery for CNS therapies are encouraging. RNAi activity in neurons continues to show efficacy in animal models for treatment of gain-of-function neurodegenerative diseases, and with careful choice of sirna to avoid off-target effects, holds much promise for translation to the clinic.
30 Figure 1. Cartoon of Ataxin-1 Showing the four important regions of the Ataxin-1 protein. The polyglutamine tract length is responsible for disease manifestation. The conserved AXH domain allows interaction between Ataxin-1, Ataxin-1-like and Capicua. The nuclear localization sequence is adjacent to Serine 776. S776 is the site of and Rbm17 binding. The proximity of S776 to the NLS can interfere with correct NLS function particularly when is bound. 17
31 Figure 2. RNAi Pathway and Viral Delivery. Co-opting the mirna pathway for delivery of RNAi triggers to the CNS. Primary mirnas are transcribed in the nucleus and are processed by the Drosha- DGCR complex to give rise to pre-mirnas. Pre-miRNAs are exported out of the nucleus by Exportin-5 and undergo further processing by Dicer in the cytoplasm to give rise to mature mirnas. The mature mirna is then loaded into RISC to carry out silencing by binding to complementary mrna sequences. Artificial mirnas or shrnas can be delivered via an Adeno-associated virus (AAV) and enter the mirna pathway at different stages of the mirna pathway. sirnas that are complexed or delivered directly into cells, enter the pathway at the Dicer-to- RISC stage or can directly be incorporated into RISC to carry out gene silencing. 18
32 19 CHAPTER II DIRECT CEREBELLAR INJECTION OF AAV DELIVERED MIRNA TARGETING MOUSE ATAXIN-1 IN 154Q SCA1 KNOCK-IN MICE RESCUES BEHAVIORAL AND HISTOLOGICAL PHENOTYPES Abstract Spinocerebellar Ataxia Type 1 (SCA1) is an autosomal dominant late onset neurodegenerative disease caused by an expanded polyglutamine tract in ataxin-1 (Atxn1). Here we study the therapeutic effect using recombinant AAVs to deliver inhibitory RNAs to the knock-in mouse model of SCA1. Direct injection to the deep cerebellar nuclei of AAVs expressing an artificial mirna targeting sequences conserved in mouse and human ataxin-1 caused long term silencing of ataxin-1 mrna and significantly improved behavior and histological readouts. The phenotypic rescue observed in SCA1 knock-in mouse model demonstrates the utility of RNAi as a potential therapy for SCA1 patients. Introduction Spinocerebellar ataxia type 1 (SCA1) is an adult onset, autosomal dominant neurodegenerative disease caused by a CAG repeat expansion in ataxin-1, which encodes the ataxin-1 protein. SCA1 is one of nine polyq-expansion gain-of-function diseases which includes Huntington s disease, spinal-bulbar muscular atrophy, dentatorubralpallidoluysian atrophy and other ataxias [98]. Clinical symptoms of SCA1 include ataxia, dysarthria, ophthalmoparesis, muscle wasting, and extrapyramidal and bulbar dysfunction
33 20 [99, 100]. Although ubiquitously expressed, polyq expanded mutant ataxin-1 causes deleterious effects in specific neuronal populations [101]. Cerebellar Purkinje cells (PCs), neurons in the inferior olive and nuclei of the brainstem are affected [99, 100]. No disease-modifying therapy exists for SCA1. Therapies aimed at reducing expression of the mutant ataxin-1 would, by virtue of the fact that it is a gain of function disease, be beneficial. Methods to accomplish this include RNA interference (RNAi) [90, 92, 102, 103], antisense oligonucleotide therapy [104], and inhibitory antibodies [105]. RNAi is evolutionarily conserved, and results in post-transcriptional gene silencing [106, 107]. RNAi has been co-opted for therapeutic development for several gain-of-function CNS diseases [108, 109]. We recently showed that RNAi triggers released from first generation short hairpin RNAs (shrnas) or artificial microrna (mirna) platforms were therapeutic in a mouse model of SCA1 expressing an expanded human ataxin-1 allele specifically in Purkinje cells [102]. A more recent model of SCA1 was developed by knocking a expanded CAG repeat into the endogenous mouse ataxin-1 locus. This mouse shows behavioral deficits including impaired motor function and histological changes exemplified by Burright et al. [14]. The knock in (KI) model was also used to test the therapeutic benefits of lithium [110]. Finally, a cross of the KI model with a mouse transgenically over-expressing ataxin-1- like, an ataxin-1 interacting protein improved clinical phenotypes [23]. As the 154Q KI mouse model ubiquitously expresses an expanded polyq tract in the endogenous mouse locus of ataxin-1, it provides a more accurate model of SCA1 both spatially and temporally [14, 21]; the B05 transgenic expresses the mutant ataxin-1 transgene exclusively in Purkinje cells, and there is no affect on life span. Thus, the
34 21 154Q model gives us an opportunity to test if repression of mutant ataxin-1 specifically in Purkinje cells is therapeutic in a setting where the mutant gene exerts its effects also in the brain stem and inferior olive. To accomplish this, we used recombinant adeno-associated viruses (AAVs) expressing RNAi triggers targeting mouse ataxin-1. When injected into the deep cerebellar nuclei, we find efficient Purkinje cell transduction, reduction of ataxin-1, and robust improvements in disease readouts. These data show that exogenous delivery of agents designed to reduce mutant ataxin-1 expression in Purkinje cells, improves SCA1- like pathologies and motor deficits despite lack of targeting to other ataxin-1 expressing cells. Materials and Methods Plasmids and viral vectors The plasmid expressing mouse U6-driven artificial mirna, misca1 (5'- UGAUUGCUUGCUGCUGGCCGA -3'), was cloned as previously described [111]. Artificial mirna expression cassettes were cloned into paavmcscmvhrgfp plasmids which coexpressed CMV-driven hrgfp [48]. Recombinant AAV serotype 2/5 vectors (AAV.miC and AAV.miSCA1) were generated by the University of Iowa Vector Core facility as previously described [112]. AAV vectors were resuspended in buffer and titres (viral genomes/ml) were determined by QPCR.
35 22 Animals All animal protocols were approved by the University of Iowa Animal Care and Use Committee. Wild type C57Bl/6J mice were obtained from Jackson Laboratories (Bar Harbor, ME). Sca1 154Q/+ (154Q) mice were maintained on the C57Bl/6 background. Mice were genotyped using primers specific for the mutant mouse ataxin-1 [21], and heterozygous and age-matched wild-type littermates were used for the indicated experiments. In the therapeutic trials, the treatment groups comprised approximately equal numbers of male and female mice. Mice were housed in a controlled temperature environment on a 12-hour light/dark cycle. Food and water were provided ad libitum. AAV injections and brain tissue isolation 154Q mice were injected with AAV vectors as previously reported ([49]). For all studies mice were injected bilaterally into the deep cerebellar nuclei (coordinates -6.0 mm caudal to bregma, ±2.0 mm from midline, and -2.2 mm deep from cerebellar surface) with 4 μl of AAV5 virus (at 1 x viral genomes/ml) or saline (Formulation Buffer 18). Mice were anesthetized with a ketamine/xylazine mix and transcardially perfused with 20 ml of 0.9% cold saline. Mice were decapitated, and for histological analyses, brains were removed and post-fixed overnight in 4% paraformaldehyde. Brains were stored in a 30% sucrose/0.05% azide solution at 4 C until cut on a sliding knife microtome at 40 µm thickness and stored at -20 C in a cryoprotectant solution. For ISH, brains were put in OCT (Sakura Finetek USA) and frozen in slurry of dry ice and 70% ethanol, then kept at -80 C until cut on a cryostat at 10 µm thickness and stored at -80 C. For qpcr analyses, brains were removed sectioned into 1 mm thick coronal slices using
36 23 a brain matrix (Roboz, Gaithersburg, MD) and hrgfp expression was verified. Whole cerebellum was triturated in 100 µl of TRIzol (Life Technologies, Grand Island, NY) and flash frozen in liquid nitrogen and stored at -80 C until used. RNA was isolated from whole cerebellum using 1 ml of TRIzol. RNA quantity and quality was measured using a NanoDrop ND-1000 (Nanodrop, Wilmington, DE). Immunohistochemical analyses Free-floating sagittal cerebellar sections (40 µm thick) were washed in 1x PBS at room temperature and blocked for one hour in 10% serum, 0.03% Triton-100 in 1x PBS. Sections were incubated with primary antibody in 2% serum and 0.03% Triton-X in 1x PBS overnight at 4 C. Primary antibodies used were polyclonal anti-iba1 (1:1000; WAKO, Richmond, VA) and polyclonal rabbit anti-calbindin (1:2000; Cell Signaling Technology, Danvers, MA). For fluorescent IHC, sections were incubated with goat-antirabbit Alexa Fluor 568 (1:1000; Life Technologies, Grand Island, NY) in 2% serum and 0.03% Triton-100 in 1x PBS for 1 hour at room temperature. For DAB IHC, sections were incubated in goat anti-rabbit biotin-labeled secondary antibody (1:200; Jackson Immunoresearch) in 2% serum and 0.03% Triton-X at room temperature for 1 hour. Tissues were developed with VECTASTAIN ABC Elite Kit (Vector Laboratories, Burlingame, CA), according to manufacturer s instructions. All sections were mounted onto Superfrost Plus slides (Fischer Scientific, Pittsburgh, PA) and cover slipped with Fluoro-Gel (Electron Microscopy Sciences, Hatfield, PA). Images were captured on Leica Leitz DMR fluorescent microscope connected to a Olympus DP72 camera using the Olympus DP2-BSW software (Olympus, Melville, NY).
37 24 In situ hybridization A Locked-Nucleic-Acid (LNA) probe (Integrative DNA Technologies, Coralville, IA) was used to visually localize misca1 by in situ hybridization (ISH) designed to the reverse complement of the targeted misca1 mrna (5 TC+GGC+CAG+CAG+CAA+GCA+ATCA; + denotes LNA modification). The probe was labeled with 3 -end a DIG oligonucleotide tailing kit (Roche) according to the manufacturer s directions. AAV.miSCA1 injected samples were verified for expression by hrgfp fluorescence before treatment. Sections were treated by ISH methods previously described [113]. Semi-quantitative PCR Reverse transcription (High Capacity cdna Reverse Transcription Kit, Applied Biosystems, Foster City, CA) was performed on total RNA collected from cerebellum using a standard stem-loop PCR primer [114] designed to identify misca1 (5 GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACGGCCAG). cdna was subjected to RT-PCR with a standard reverse primer (5 GTGCAGGGTCCGAGGT) and a forward primer (5 CACAGATGGGTGATTGCTTGCTGC) to identify misca1 expression. Quantitative PCR Random-primer first-strand cdna synthesis was performed using 1 µg total RNA (High Capacity cdna Reverse Transcription Kit; Life Technologies, Grand Island, NY)
38 25 per manufacturer s instructions. Assays were performed on a sequence detection system using primers/probe sets specific for mouse ataxin-1, mouse Calbindin, mouse mglur1, mouse mglur4, mouse Vegfa or mouse β-actin (ABI Prism 7900 HT and TaqMan 2x Universal Master Mix; Life Technologies, Grand Island, NY). Behavioral analysis All assays were taken at 30 and 40 weeks of age and presented as means ± SEM unless otherwise specified. Uninjected wild type, n = 15; saline injected 154Q, n = 11; AAV.miC injected 154Q, n = 11; AAV.miSCA1 injected 154Q, n = 15 mice per group. Rotarod Mice were tested on an accelerated rotarod apparatus (model 47600; Ugo Basile, Comerio, Italy) at 4, 30 and 40 weeks of age. Baseline testing was conducted at 4 weeks of age to separate 154Q mice equally into treatment groups (data not shown). No difference between 154Q and wild type mice was seen at 4 weeks of age. Mice were first habituated on the rotarod for 4 minutes. Mice were then tested three trials per day (with at least 30 minutes of rest between trials) for four consecutive days. For each trial, acceleration was from 4 to 40 rpm over 5 minutes, and then speed maintained at 40 rpm. Latency to fall (or if mice hung on for two consecutive rotations without running) was recorded for each mouse per trial. The trials were stopped at 500 seconds, and mice remaining on the rotarod at that time were scored as 500 seconds.
39 26 Gait analysis Mice were allowed to walk across a paper lined chamber (100 cm X 10 cm with 10 cm walls) and into an enclosed recess. Mice were given one practice run. Non-toxic red and blue paint was applied to their fore- and hindpaws, respectively. Mice were then tested three times to produce three separate footprint tracings. Stride lengths were measured from the middle of each paw print between the same paws for steps taken during their gait. Steps were discarded in instances where a mouse stopped walking or turned around. Measurements from steps per mouse were averaged for all four paws and data were presented as box plots. Statistical analyses The results are expressed as the means ± SEM. For all studies unless indicated otherwise, statistical values were analyzed using ANOVA plus Bonferroni correction for multiple comparisons. Student s t-test was used for the pair-wise comparisons done for the baseline 4-week rotarod performance assay. In all statistical analysis, p < 0.05 was considered statistically significant. All photographs were formatted with Adobe Photoshop software, all graphs were made wish GraphPad Prism 5 software, and all figures were constructed with Adobe Illustrator software. Results Expression of misca1 and reduction of ataxin-1 in vivo We designed sequences which would inhibit mouse ataxin-1 using standard methods [107, ], and cloned them into AAV serotype 2/5 to generate
40 27 AAV.miSCA1 (Figure 3). These viruses also express GFP from a separate promoter (Methods). As a first test of their activity, we injected AAV.miSCA1, AAV.miC (control mirna sequence within AAV2/5) or saline into the deep cerebellar nuclei (DCN) of 5 week old 154Q mice. Six weeks later tissue was harvested for histology and quantification of knockdown. Extensive Purkinje cell transduction was evident by robust hrgfp expression from the rostral to caudal lobules of injected cerebella (Figure 4). Semi-quantitative PCR (sqpcr) analysis, used previously to examine endogenous mirnas [114, 118], demonstrated misca1 expression in cerebella of mice injected with AAV.miSCA1, but not AAV.miC injected mice (Figure 5). We found misca1 expression localized predominantly to Purkinje cells, as evidenced by in situ hybridization (Figure 6). Quantitative PCR (QPCR) analysis for endogenous ataxin-1 mrna levels in RNA harvested from whole cerebellar extracts showed ~20% knockdown compared to saline injected 154Q mice (Figure 7). As QPCR was done on whole cerebellar extracts, but misca1 expression is primarily in Purkinje cells, background levels of endogenous ataxin-1 in other cell types are likely obscuring the extent of silencing in Purkinje cells. Furthermore, Ib1 staining showed no overt neurotoxicity in animals injected with AAV.miSCA1 compared to saline injected 154Q littermates in cerebellar cortex or at the site of injection (Figure 8). AAV.miSCA1 protects against molecular layer thinning To test for possible beneficial effects of ataxin-1 silencing in the 154Q model, mice were injected as before and tissues harvested 65 weeks later. To determine molecular layer integrity, sagittal sections with evident transgene expression in
41 28 AAV.miSCA1 vs. control treated tissues were compared. Only molecular layers with Purkinje cells expressing misca1, verified by in situ hybridization, were utilized in the analysis (Figure 9). We measured 2 rostral locations and one caudal location from medial sagittal sections for all groups (Figure 9a). The data show that 154Q mice treated with AAV.miC or saline had significantly reduced molecular layer widths compared to wild type littermates at all locations (p < ) while mice treated with AAV.miSCA1 had significantly increased molecular layer widths (Figure 9b-d). In rostral sections, AAV.miSCA1 treated mice retained molecular layer widths equivalent to their wild type littermates (Figure 9c, d). AAV.miSCA1 rescues gait and coordination Gait analysis and accelerated rotarod assays were performed at 30 and 40 weeks of age on uninjected wild type mice and 154Q mice injected bilaterally with AAV.miSCA1, AAV.miC, or saline to the DCN. Saline or control treated 154Q mice have gait abnormalities as evidenced by significantly shorter stride lengths and a narrower hindlimb gate relative to wild type littermates at both time points tested (p < ; Figure 10, 11). 154Q mice treated with AAV.miSCA1, however, had strides 8 mm longer than control treated diseased mice, and this trend continued when assayed at 40 weeks of age (p < ; Figure 10). Moreover, AAV.miSCA1 treated mice had hindlimb stance widths similar to their wild type littermates (Figure 11). Prior work describing the generation of the KI model showed deficits on the accelerated rotarod by 5 weeks of age [21]. In our colony, there were no differences at this early time point, and disparity between diseased and normal littermates did not appear until 30
42 29 weeks. As such, we assessed misca1, mic and saline injected 154Q KI mice at 30 and 40 weeks, and compared them to wild type, age-matched littermates. At both the 30 and 40 week time points, 154Q mice injected with AAV.miSCA1 stayed on the rotarod apparatus more than 1 minute longer than saline or AAV.miC-treated 154Q mice (p < 0.05; Figure 12). Full rescue of the rotarod phenotype at extended time points supports the therapeutic potential of misca1. Silencing ataxin-1 causes cellular and synaptic rescue Whole cerebellar extracts were assayed for ataxin-1 knock down by QPCR 60 weeks after treatment. AAV.miSCA1 treated mice showed 30% reduction of ataxin-1 mrna levels relative to calbindin compared to all other treatment groups (p < 0.01; Figure 13). Granule cell parallel fibers synapse on Purkinje cell dendrites, and the integrity of this interaction is critical for coordinated motor function [119]. To test if the functional improvements noted reflect sustained synaptic integrity we examined pre- and postsynaptic glutamate receptor level expression. Metabotropic glutamate receptor 1 (mglur1) is a postsynaptic receptor localized to Purkinje cell dendritic spines. It is expressed robustly in healthy tissue [120, 121]. We found that 154Q littermates treated with saline or AAV.miC had 50% lower mglur1 mrna levels relative to wild type mice. 154Q mice treated with AAV.miSCA1 were indistinguishable from their normal littermates in their mglur1 mrna levels (p < ; Figure 14). AAV.miSCA1 mice had no significant difference compared to wild type littermates, indicating full rescue of this disease phenotype. The preservation of
43 30 mglur1 likely contributes to the maintenance of molecular layer width with AAV.miSCA1 treatment. Metabotropic glutamate receptor 4 (mglur4) is expressed at high levels presynaptically in the parallel fibers axons of granule cells [122, 123]. Mice deficient in mglur4 show impaired motor performance and decreased synaptic plasticity [124]. mglur4 mrna levels in all 154Q treatment groups were reduced relative to wild type littermates at 65 weeks of age, although AAV.miSCA1 treated animals had mglur4 levels significantly higher than control treated 154Q littermates (p < ; Figure 15). Vascular endothelial growth factor-a (Vegfa) is an angiogenic and trophic factor widely expressed in cerebellar neurons, glia, and endothelial cells, and its expression is decreased in SCA1 mice before behavioral or pathological signs emerge [125, 126]. Inhibition of ataxin-1 expression restored Vegfa levels to that of wild type littermates (Figure 16). These results support our hypothesis that AAV.miSCA1 provides therapeutic benefit in the setting of SCA1. Discussion We achieved behavioral and pathological rescue using RNAi to silence ataxin-1 in the SCA1 knock-in model. Previously, approaches using RNAi were demonstrated as feasible in SCA1 models using shrnas delivered to the cerebellar cortex of B05 transgenic mice [102]. Here, we used advanced platforms for expression of the RNAi trigger [49], and tested the efficacy of ataxin-1 silencing in the KI model of SCA1. Efficacy was evaluated by assessing the impact on both earlier described phenotypes and readouts that had not been previously described. Behavioral deficits previously
44 31 documented for the 154Q model include impaired rotarod activity between 5-7 weeks of age [21, 110], and abnormal gait at 20 weeks of age [21]. The general disturbances in gait were not quantified, however. In our studies we quantified these changes and found impaired stride length and hindlimb stance width at 30 weeks of age, both of which were improved by misca1 therapy. Notably, ataxin-1 silencing with misca1 rescued the rotarod performance to wild type levels, a therapeutic effect not previously demonstrated with small molecule therapy [110, 127]. Transcriptional changes are induced early in SCA1 and as disease progresses [18, 19, 110]. Ataxin-1 protein interacts with factors involved in transcriptional regulation including CIC, SMRTER, HDAC3, Gfi1, RBM17, and RORα-Tip60 [20, 32, 128]. Thus it is expected that expression of downstream targets of regulation factors change in the cells impacted in disease. One example of this is mglur1, a downstream target under RORα regulation[20], whose expression correlates directly with rotarod performance in a conditional SCA1 transgenic model [121]. mglur1 is expressed post-synaptically in Purkinje cell dendrites and receives excitatory synaptic input from parallel fibers. It is essential for synaptic plasticity in Purkinje cell-parallel fiber synapses as well as motor coordination [129, 130] As the Purkinje cell dendrites retract with SCA1 progression, mglur1 receptors are significantly down-regulated with loss of synapse function. AAV.miSCA1 treatment restored mglur1expression levels as well as their dendritic arborizations. The histological rescue and corresponding behavioral rescue implies improved synaptic functioning of Purkinje cell dendrites. Earlier work demonstrated the significance of mglur4, a downsteam target under Cic regulation [18], in the motor performance of mice. mglur4 knockout mice have reduced cerebellar plasticity and impaired rotarod performance [124], and mglur4
45 32 expression is reduced in 154Q mice [18]. mglur4 is an autoreceptor located on the presynaptic terminal functioning in a negative feedback loop to modulate signal transduction between Purkinje cells and parallel fibers [119]. The significant loss of mglur4 in the 154Q model at 65 weeks of age suggests a reduction in healthy parallel fiber axons. With the therapeutic application of misca1 mglur4 expression was partially restored. These data imply that synaptic parallel fiber axons remain healthy and active in a cerebellar environment with decreased levels of polyq-expanded ataxin-1. Although parallel fibers were not a direct target of our therapy, the relative improvement of Purkinje cells proved beneficial for multiple cell types in the cerebellum. Transcriptional changes in SCA1 models occur prior to behavioral symptoms. For instance, Vegfa mrna and protein levels are reduced very early in 154Q mice and in 82Q transgenic mice [125, 126]. Vegfa, an angiogenic and trophic factor, is implicated by its reduced expression in several motor neuron diseases including ALS, SMBA, and SCA1 [ ]. Delivery of exogenous Vegfa achieved behavioral and histological improvements in 154Q mice, suggesting Vegfa as a possible therapy for SCA1 patients [131]. misca1 treatment restored Vegfa to wild type levels. The significant increase of Vegfa in misca1-treated 154Q mice is in agreement with our previous results in a B05 model [126]. In those experiments reduction of the mutant allele, expressed solely in Purkinje cells, also rescued Vegfa levels. Figure 17 depicts possible mechanisms underlying reduced Vegfa expression in SCA1 Purkinje cells [126]. While our therapeutic AAV.miSCA1 transduced a large number of Purkinje cells, there not 100% coverage of the entire cerebellum. Regardless, we fully rescued the rotarod deficit, improved gait abnormalities, preserved molecular layer widths, and
46 33 maintained normal transcriptional levels of genes known to be reduced in SCA1. It is apparent that only a portion of Purkinje cells need to be affected to cause a significant improvement on phenotypes. Delivery of misca1 could potentially improve established SCA1 symptoms. A conditional transgenic SCA1 model demonstrated the recoverability of cerebellar dysfunction; a mutant transgene expressed in cerebellar Purkinje cells for 12 weeks caused disease symptoms that were significantly improved upon cessation of expression. Histologically there was recovery of Purkinje cell dendritic arborizations and transcription levels of Purkinje cell receptors [121]. In summary, delivery of AAV.miSCA1 to the DCN for Purkinje cell transduction provides sustained silencing of ataxin-1 with no adverse toxicity. SCA1 mice improved clinically and neuropathologically. AAV.miSCA1 is a promising therapeutic option for SCA1 patients.
47 Figure 3. Cartoon of recombinant AAV2/5 construct. Recombinant AAV2/5 vectors containing cassettes expressing misca1 driven by the mouse U6 promoter and hrgfp driven by CMV. 34
48 Figure 4. Viral spread shown by hrgfp fluorescence. 40 µm thick sagittal section cerebellum injected with AAV.miSCA1 to deep cerebellar nuclei. hrgfp fluorescence (green) defines AAV2/5 transduced cerebellar lobules. Scale bar = 500 µm. 35
49 Figure 5. Semi-quantitative PCR of misca1 expression. Samples from cerebellar injected with (+) AAV.miSCA1 or (-) AAV.miC were probed for misca1 expression. 36
50 Figure 6. In situ hybridization of misca1 localization. In situ hybridization localized misca1 expression to Purkinje cells in cerebella injected with AAV.miSCA1. Scale bar = 100 µm. 37
51 Figure 7. Quantitative PCR knowndown of matxn1 6 weeks post-injection. Quantitative PCR analysis 6 weeks post- injection shows ~20% knock down of mouse ataxin-1 from whole cerebellar mrna compared to saline injected cerebella. Data are expressed as mean ± SEM. (n = 4; samples assayed in triplicate, * indicates p < 0.05). 38
52 Figure 8. IHC for Iba1 shows no toxicity. Sagittal 8 µm-thick cerebellar sections at the location of injections (DCN) and in the target cerebellar cortex tissue. There is no adverse glial activation in AAV.miSCA1 treated tissues compared to saline injected controls or wild type littermates. Scale bar = 100 µm. 39
53 Figure 9. Molecular layer widths improve in AAV.miSCA1 treated mice. (A) 40 µm thick sagittal cerebellar section stained with α-calbindin (red) identifies location of sections that were measured. Scale bar = 500 µm. (B) Molecular layer between caudal lobules VIII and IX. Wild type mice had a significantly wider molecular layer than all other treatment groups at this position. 154Q mice treated with AAV.miSCA1 also had a significantly wider molecular layer than control treated 154Q littermates. (C) Molecular layer between rostral lobules IV/V and VI. No difference was seen in molecular layer width between wild type and 154Q mice treated with AAV.miSCA1. Both groups had significantly wider molecular layers than control treated 154Q littermates. (D) Molecular layer between rostral lobules III and IV/V. No difference was seen in the molecular layer widths between wild type and 154Q mice treated with AAV.miSCA1. Both groups had significantly wider molecular layers than control treated 154Q littermates. Results are shown as mean ± SEM (n 3; *p < 0.05, ***p < ). 40
54 Figure 10. AAV.miSCA1 treated mice have longer strides than control mice. Results shown by box plots display average stride lengths at 30 (left) and 40 (right) weeks of age. Measurements from steps per mouse were averaged for all four paws. AAV.miSCA1 treated mice have significantly longer strides than control treated 154Q littermates (***p < ). 41
55 Figure 11. AAV.miSCA1 treated mice have wider stances than control mice. Results shown by box plots display average hindlimb stance width at 30 (left) and 40 (right) weeks of age. AAV.miSCA1 treated mice have significantly wider stances than control treated 154Q littermates (***p < ). 42
56 Figure 12. AAV.miSCA1 rescues rotarod phenotypes at 30- and 40-weeks of age. Rotarod analysis was performed at 30 and 40 weeks of age. On day 4 at each age AAV.miSCA1 treated 154Q mice stay on the rotarod over 1 minute longer than control treated 154Q littermates. The performance of AAV.miSCA1 treated 154Q mice was no different from wild type littermates. Results are shown as mean ± SEM (*p < 0.05) 43
57 Figure 13. Quantitative PCR shows matxn1 knockdown by AAV.miSCA1 at late time points. Relative levels of ataxin-1 mrna. AAV.miSCA1 treated 154Q mice have ~30% reduction of ataxin-1 compared to saline treated 154Q littermates. Results are relative to saline injected 154Q littermates and displayed as mean ± SEM (n = 4; samples were run in triplicate;, ***p < ). 44
58 Figure 14. mglur1 mrna expression levels are improved by AAV.miSCA1. Relative levels of mglur1 mrna. AAV.miSCA1 treated 154Q mice maintain mglur1 mrna levels equivalent to wild type littermates, and both are significantly higher than control treated 154Q mice. Results are relative to saline injected 154Q littermates and displayed as mean ± SEM (n = 4; samples were run in triplicate; *p < 0.05, ***p < ). 45
59 Figure 15. mglur4 mrna expression levels are improved by AAV.miSCA1. Relative levels of mglur4 mrna. AAV.miSCA1 treated 154Q mice have significantly higher mrna levels of mglur4 than control treated 154Q mice. Wild type littermates have significantly higher mrna levels of mglur4 than all 154Q treated mice. Results are relative to saline injected 154Q littermates and displayed as mean ± SEM (n = 4; samples were run in triplicate; **p < 0.001, ***p < ). 46
60 Figure 16. Vegfa mrna expression levels are improved by AAV.miSCA1. Relative levels of Vegfa mrna. AAV.miSCA1 treated 154Q mice maintain Vegfa mrna levels equivalent to wild type littermates, and both are significantly higher than control treated 154Q mice. Results are relative to saline injected 154Q littermates and displayed as mean ± SEM (n = 4; samples were run in triplicate; ***p < ). 47
61 Figure 17. Possible mechanisms underlying reduced Vegfa expression in SCA1 Purkinje cells. In the nucleus mutant Ataxin-1 complexed with DNA binding proteins can occupy the Vegfa promoter and repress transcriptional activity. It is unknown if wild type ataxin-1 regulated Vegfa transcription in a similar manner. In the cytosol, under wild type coniditons, Vegfa expression is under posttranscriptional regulation of mirnas (left). Under SCA1 conditions, some mirnas have increased activity in Purkinje cells (right) and can act to further suppress Vegfa expression via post-transcriptional gene silencing [126]. 48
62 49 CHAPTER III ALTERNATIVE THERAPEUTIES ACHIEVE BEHAVIORAL AND HISTOLOGICAL PHENOTYPIC RESCUE IN B05 TRANSGENIC SCA1 MOUSE MODEL: RNAI AND OVEREXPRESSION OF HATXN1L Abstract Spinocerebellar Ataxia Type 1 (SCA1) is an autosomal dominant late onset neurodegenerative disease caused by an expanded polyglutamine tract in ataxin-1 (Atxn1). Here, we compared the protective effects of overexpressing ataxin-1-like using recombinant AAVs, or reducing expression of mutant ataxin-1 using virally delivered RNA interference (RNAi), in a transgenic mouse model of SCA1. For the latter, we used an artificial microrna (mir) design that optimizes potency, efficacy and safety to suppress Atxn1 expression (mis1). Delivery of either ataxin-1-like or mis1 viral vectors to SCA1 mice cerebella resulted in widespread cerebellar Purkinje cell transduction and improved behavioral and histological phenotypes. Our data indicate the utility of either approach as a possible therapy for SCA1 patients. Introduction Spinocerebellar Ataxia Type 1 (SCA1) is a late onset, autosomal dominant neurodegenerative disease caused by a polyglutamine (polyq) expansion in the ataxin-1 protein. The average age of onset is within the fourth decade of life, although juvenile cases have been documented [11]. Cell death in cerebellar Purkinje cells and brain stem neurons is characteristic of SCA1 [2, 100]. Although not fully understood, mechanisms
63 50 underlying neuropathy include an interplay between ataxin-1 and several proteins including [135], Rbm17 [33], Capicua [32] and Ataxin-1-like [28]. The B05 transgenic mouse model of SCA1 expresses a polyq expanded human ataxin-1 allele under control of the Purkinje cell specific promoter (Pcp2) [14]. Purkinje cell death occurs at approximately 24 weeks of age, with behavioral deficit onset at 5 weeks [17]. This implies that early symptoms reflect neuronal dysfunction but not overt cell loss, and raises the possibility that therapy can be initiated after disease onset. Work by Orr and colleagues using a doxycycline-inducible system investigated this possibility and found that if the disease gene was turned off after 6 weeks of expression, there was full reversibility. Notably, partial restoration of neuronal and behavioral deficits occurred if gene expression was turned off after 12 weeks [121]. Thus, there is a window of opportunity after disease onset to which therapies may have benefit. Therapeutic intervention for SCA1 may involve small molecule approaches, such as those which have been investigated for SCA2 [136], modulation of disease through overexpression of ataxin-1-like [23], or reducing expression of the disease allele through gene silencing [61, 137]. In SCA1 knock-in mice (154Q) overexpressing an ataxin-1- like-transgenic allele, disease phenotypes improved [33]. The presumed mechanism for therapy based on ataxin-1-like overexpression is that ataxin-1-like, ataxin-1 and mutant, polyq-expanded ataxin-1 all interact with Capicua through their AXH domain [29, 32, 33]. Interestingly, ataxin-1-like does not have a polyq region but if overexpressed in vitro it can effectively compete away the mutant ataxin-1:capicua interactions [23]. A separate study showed that Rbm17 competes with Capicua to bind ataxin-1, with Rbm17 favoring interactions with mutant, polyq-expanded ataxin-1, thus contributing to the
64 51 toxic gain-of-function phenotype [23]. To date, interactions between Rbm17 and ataxin- 1-like have not been reported. Modulating SCA1 pathogenesis through gene silencing takes advantage of the RNA interference (RNAi) pathway, a naturally occurring process that regulates expression through genomically encoded small RNAs, which include micrornas (mirs). RNAi has been utilized as a means to reduce target gene expression for potential treatment of various diseases [107], including the dominantly inherited gain of function mutations underlying SCA1 and Huntington s disease [90, 102, 138]. In early work, we established that sirnas processed from short hairpin RNAs (shrnas) expressed from viral vectors could reduce targets in brain [102, 103] and could improve disease phenotypes in SCA1 transgenic mice [102]. Here, we take advantage of recent improvements in expression systems and sirna design to deliver RNAi triggers that are appropriately expressed in vivo and possess low off targeting potential [48, 49, 115]. We test their therapeutic utility in the B05 mouse model, and compare this approach with ataxin-1-like overexpression via viral vectors. Materials and Methods Cloning plasmids and viral vectors The plasmid expressing mouse U6-driven artificial mirna, mis1, was cloned as previously described using DNA oligonucleotides [111]. Artificial mirna expression cassettes were cloned into paavmcscmvegfp plasmids which coexpressed CMVdriven egfp [48].
65 52 Human ataxin-1-like was originally cloned from HEK293 cells using forward primer 5 - AAACCTGTTCATGAAA and reverse primer 5 GGATCCTCATTTTCCCGCATTGGAAC containing a BamHI site and cloned into pcr4-topo plasmid (Life Technologies, Grand Island, NY). The sequence was expanded to contain a NheI site, a Flag tag and kozak sequence by consecutive PCR extensions using forward primers: a) 5 ATAAAGATCATGATATCGATTACAAGGATGACGATGACAAACCTGTTCAT; and b) GCTAGCGCCACCATGGACTACAAAGACCATGACGGTGATTATAAGATCAT. Sequential digests were conducted with BamHI and NheI followed by CIP treatment and electrophoresis of the digested product. The DNA construct band was purified using a QIAquick Gel Extraction Kit (Qiagen, Valencia, CA) per manufacturer s protocol. The purified DNA insert was ligated (T4 DNA Ligase; NEB, Ipswich, MA) into pfbaavmcsbghpa pre-digested with BamHI and NheI [113]. EF1α was cloned from pbud plasmid using forward primer 5 TTAATTAAGTGAGGCTCCGGTGCCCGTC containing a PacI site and reverse primer 5 GCTAGCGCCAGATCTCTCGAGTCCAC containing a NheI site into pcr 4-TOPO plasmid (Life Technologies, Grand Island, NY). EF1α was digested with PacI and NheI, gel purified and ligated into pfbaavmcsbghpa upstream of flag-tagged human ataxin-1-like. Recombinant AAV serotype 2/1 vectors (AAV.miC, AAV.miS1, and AAV.HAtxn1L) were generated by the University of Iowa Vector Core facility as previously described [112]. AAV vectors were resuspended in Formulation Buffer 18
66 53 (University of Iowa Gene Transfer Vector Core, Iowa City, IA) and titres (viral genomes/ml) were determined by quantitative PCR (qpcr). Cell culture and transfection HEK293 cells were transfected (Lipofectamine 2000, Life Technologies, Grand Island, NY) in triplicate in 24-well plates per manufacturer s instructions with 800 ng of pfbaavmcsbghpa plasmid containing the human ataxin-1-like construct, paavcmvegfp, paav, or no plasmid. Total RNA was harvested 48 hours later with TRIzol (Life Technologies, Grand Island, NY). Animals All animal protocols were approved by the University of Iowa Animal Care and Use Committee. Wild type FVB mice were obtained from Jackson Laboratories (Bar Harbor, ME). SCA1-1 TM (line B05) mice were generously provided by Dr. H.T. Orr [14] and the B05 line was maintained on the FVB background. Mice were genotyped using primers specific for the mutant human atxn1 transgene [14], and hemizygous and age-matched wild type littermates were used for the indicated experiments. In the therapeutic trials, the treatment groups comprised approximately equal numbers of male and female mice. Mice were housed in a controlled temperature environment on a 12- hour light/dark cycle. Food and water were provided ad libitum.
67 54 AAV injections and brain tissue isolation B05 mice were injected with AAV vectors as previously reported [49]. Brains were treated as described in Chapter II. Immunohistochemical analyses Tissues were processed as described in Chapter II. Primary antibodies used were polyclonal anti-iba1 (1:1000; WAKO, Richmond, VA) and polyclonal rabbit anti- Calbindin (1:2000; Cell Signaling Technology, Danvers, MA). In situ hybridization A 2 OMe ZEN probe (Integrated DNA Technologies, Coralville, IA) was used to visually localize mis1 by in situ hybridization (ISH) designed to the reverse complement of the targeted mis1 mrna (5 Dig-AzAGCAACGACCUGAAGAUCGzA-Dig 3 where AGCU = 2 OMe RNA, Dig = Digoxigenin, and z = ZEN modifier). AAV.miS1 injected samples were verified for expression by egfp fluorescence before treatment. Sections were treated by ISH methods previously described [113]. Semi-quantitative PCR Reverse transcription (High Capacity cdna Reverse Transcription Kit, Applied Biosystems, Foster City, CA) was performed on total RNA collected from cerebellum using a standard stem-loop PCR Primer [114] designed to identify mis1 (5 GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACAGCAAC). cdna was subjected to RT-PCR with a standard reverse primer (5
68 55 GTGCAGGGTCCGAGGT) and a forward primer (5 ACACTCCAGCTGGGTCGATCTTCAGGTC) to identify mis1 expression. Western blot analysis Protein was harvested from transfected HEK293 cells or whole cerebellar lysates using RIPA buffer (ThermoScientific Pierce, Rockford, IL) and 1X protease inhibitor using standard techniques and quantified using D C Protein Assay (Bio-Rad, Hercules, CA). Protein extracts were separated on a 7% acrylamide gel and transferred to Immobilon-P PDVF transfer membranes (Merck Millipore, Billerica, MA). Primary antibody to Flag (1:500; Sigma-Aldrich, St. Louis, MO) and β-actin (1:10,000; Sigma- Aldrich, St. Louis, MO) were used. Blots were developed using ECL Plus Western Blotting Detection System (GE Healthcare Life Sciences, Pittsburgh, PA). Co-immunoprecipitation Saline perfused cerebellum from either injected or control non-injected B05 mice were dounce homogenized in ice-cold TST Buffer (50 mm Tris ph 8.0, 75 mm NaCl, 0.5% Triton-X 100, 1 mm PMSF) containing complete mini protease inhibitors (Roche Applied Science, Indianapolis, IN). Lysates were solubilized on ice for one hour and then clarified by centrifugation at 16K x g for 10 minutes at 4 C. For anti-flag/ataxin-1-like immunoprecipitations, 2 mg of protein from the supernatant samples were combined with 20 μl of packed anti-flag M2 magnetic beads (Sigma-Aldrich) and allowed to incubate overnight on a rotator at 4 C. Using a magnet, the beads were washed three times with ice-cold Wash Buffer (50 mm Tris ph 7.4, 150 mm NaCl). Bound proteins were eluted
69 56 by heating in 2x LDS sample buffer (Life Technologies, Grand Island, NY) for 10 minutes at 70 C. Input and elution samples were analyzed by SDS-PAGE and western blotting. Flag tagged ataxin-1-like was detected with rabbit anti-flag primary antibody (1:1000; Cell Signaling Technology, Danvers, MA) and ataxin-1 was detected with rabbit anti-ataxin V primary antibody. HRP-conjugated goat anti-rabbit secondary antibody (1:2000; Cell Signaling Technology, Danvers, MA) was used for ECL detection. Quantitative PCR Random-primer first-strand cdna synthesis was performed using 1 μg total RNA (High Capacity cdna Reverse Transcription Kit; Life Technologies, Grand Island, NY) per manufacturer s instructions. Assays were performed on a sequence detection system using primers/probe sets specific for human ataxin-1, mouse GFAP, mouse Calbindin, or mouse β-actin (ABI Prism 7900 HT and TaqMan 2x Universal Master Mix; Life Technologies, Grand Island, NY). Behavioral analysis All assays were performed at 37 weeks of age and presented as means ± SEM unless otherwise specified. Uninjected wild type, n =14; saline injected B05, n = 8; AAV.miC injected B05, n = 11; AAV.miS1 injected B05, n = 13; AAV.HAtxn1L injected B05, n = 10 mice per group.
70 57 Ledge test Mice were placed on the ledge of a cage and scored from 0-3 on their ability, coordination, and use of hindlimb musculature to gracefully lower themselves onto the cage floor (~6 inches). A score of zero was given if a mouse could walk along the edge without losing its balance and gracefully lower itself onto the floor of the cage. A score of 1 was given if the mouse lost its footing and slipped while on the ledge, but otherwise lowered itself into the cage with coordination. A score of 2 was given if the mouse did not adequately use its hind limbs while lowering itself into the cage and landed on its head rather than its paws. A score of 3 was given if the mouse completely fell off the ledge while attempting to walk or lower itself into the cage, or alternatively the mouse shook and refused to move at all. The scorer s were blind to the mouse s genotype and/or treatment group. This assay was originally described by Geyenet et al. [139] Hindlimb clasping Mice were held suspended by the base of their tails for 10 seconds and scored 0-3 on the amount time spent clasping one or both hindlimbs. A score of zero was give if the mouse splayed their hindlimbs for the entire 10 seconds. A score of 1 was given if the mouse retracted one hindlimb toward its abdomen for > 50% of the time. A score of 2 was given if both hindlimbs were retracted toward its abdomen for > 50% of the time. A score of 3 was given if the mouse had both hindlimbs completely retracted and touching its abdomen for > 50% of the time held suspended. The scorer s were blind to the mouse s genotype and/or treatment group. This assay was originally described by Geyenet et al. [139]
71 58. Stride length measurements Mice were allowed to walk across a paper-lined chamber (100 cm x 10 cm with 10 cm walls) and into an enclosed recess. Mice were given one practice run. Non-toxic red and blue paint was applied to their fore- and hindpaws, respectively. Mice were then tested three times to produce three separate footprint tracings. Stride lengths were measured from the middle of each paw print between the same paws for steps taken during their gait. Steps were discarded in instances where a mouse stopped walking or turned around. Measurements were averaged for all four paws and data were presented as box plots. Rotarod Mice were tested on an accelerated rotarod apparatus (model 47600; Ugo Basile, Comerio, Italy) at 4, 27 and 37 weeks of age. Baseline testing was conducted at 4 weeks of age to separate B05 mice equally into treatment groups. No difference between B05 and wild type mice was seen at 4 weeks of age (data not shown). Mice were first habituated on the rotarod for 4 minutes. Mice were then tested three trials per day (with at least 30 minutes of rest between trials) for four consecutive days. For each trial, acceleration was from 4 to 40 rpm over 5 minutes, and then speed maintained at 40 rpm. Latency to fall (or if mice hung on for two consecutive rotations without running) was recorded for each mouse per trial. The trials were stopped at 500 seconds, and mice remaining on the rotarod at that time were scored as 500 seconds.
72 59 Statistical analyses For all studies, unless indicated otherwise, statistical values were obtained by using one-way analysis of variance followed by Bonferroni post-hoc analysis to assess for significant differences between individual groups. Student s t-test was used for the pair-wise comparisons for the baseline 4-week rotarod performance assay. In all statistical analysis, P < 0.05 was considered significant. Results Optimization and validation of therapeutic delivery Two vectors were generated to test alternative therapies; AAV expressing an sirna against human ataxin-1or one expressing human ataxin-1-like. While earlier work in our group demonstrated short term efficacy using an RNAi approach in the B05 model [102], we have substantially improved the safety of vector-based platforms by moving from shrna systems to those based on endogenous mirna backbones [49, 90, 111, 116, 140]. For this work, we also took advantage of recent progress in minimizing offsequence silencing to design an RNAi trigger that would target human ataxin-1 but have low off-target potential [49, 90, 111, 115]. Our sequence targeting human ataxin-1 (mis1) was cloned into shuttle plasmids also expressing egfp, and then subsequently packaged into AAV2/1 (AAV.miS1). Human ataxin-1-like (HAtxn1L) cdna was modified to contain an N-terminal 3X Flag tag and subsequently cloned downstream of the EF1α promoter for packaging in AAV2/1 (AAV.HAtxn1L) (Figure 18). AAV.miS1, AAV.HAtxn1L, AAV.miC (control mirna sequence within AAV2/1) or saline was injected bilaterally into the deep cerebellar nuclei (DCN) of 5 week old B05
73 60 mice to confirm either gene silencing activity or HAtxn1L overexpression, respectively. Tissue was harvested for RNA or protein extraction 3 weeks later. Q-RT-PCR showed 30% knockdown of human ataxin-1 mrna levels compared to control injected B05 mice (p < ; Figure 19). Ataxin-1-like expression was verified by western blot analysis of either transfected HEK293 cells or B05 mice injected previously with AAV.HAtxn1L into the DCN (Figure 20). At 40 weeks of age, harvested tissues showed robust egfp expression throughout rostral and caudal lobules of AAV.miS1 injected cerebella (Figure 21). Stem-loop PCR has been used previously to examine expression of endogenously expressed mirnas [114, 118]. We designed specific probes to similarly test for expression of mis1 in total RNA harvested from cerebellar extracts. Stem-loop PCR confirmed that mis1 was expressed in AAV.miS1 injected animals (Figure 22), but not those injected with AAV.miC. In situ hybridization using probes specific for the guide strand of the mirna liberated from the artificial mir platform demonstrated mis1 expression predominantly in Purkinje cells of AAV.miS1 treated mice (Figure 23). Q-RT-PCR on RNA harvested from whole cerebellar extracts showed a 70% reduction of human ataxin-1 mrna levels in AAV.miS1 treated animals relative to controls and AAV.HAtxn1L injected mice (Figure 24). By immunohistochemical analysis, calbindin, a Purkinje cell marker, is reduced in 6 week old B05 mice compared to wild type littermates [141, 142], and was therefore used as a surrogate marker for recovery of Purkinje cell viability. Here, we assessed calbindin transcript levels using Q-RT-PCR. Although there were not significant differences between control treated B05 mice and wild type littermates, AAV.miS1 animals had
74 61 significantly higher calbindin mrna levels relative to all other B05 treatment groups (p < 0.001; Figure 25). Ataxin-1-like over-expression and knockdown of ataxin1 rescues behavioral phenotypes The B05 mouse model displays many characteristics of human SCA1 patients including impaired gait, balance, and overall motor coordination as well as hindlimb muscle atrophy [14]. The ledge test measures coordination, which is compromised in the cerebellar ataxias [139]. As expected, control treated animals performed significantly worse than all other groups (p < ). Remarkably, there were no significant differences between AAV.miS1 or AAV.HAtxn1L-treated animals and their wild type littermates. Both ataxin-1 suppression and overexpression of HAtxn1L rescued the balance, agility, and hindlimb musculature needed to perform this task to the same level of coordination as wild type littermates (Figure 26). SCA1 mice display hindlimb clasping whereas wild type mice generally splay their hindlimbs [143]. At 37 weeks of age AAV.miS1 and AAV.HAtxn1L-treated animals treated mice showed significant improvements with reduced clasping of one or both hindlimbs relative to control-treated B05 mice (p < ). Again, there was no significant difference between AAV.miS1 or AAV.HAtxn1L- treated animals and their wild type littermates, who spent the vast majority of time with their hindlimbs splayed (Figure 27). Gait analysis has been used as a phenotypic measure of disease in SCA1 mice [17], and as a marker of rescue in HD mice subjected to RNAi therapy [138]. While wild type
75 62 mice had significantly longer stride lengths than all other groups at 37 weeks of age (p < ). AAV.miS1 and AAV.HAtxn1L treated animals had significantly longer strides (6-8mm) than control treated B05 mice (p < ; Figure 28), suggesting partial rescue of the gait phenotype with either therapy. There was no significant difference between AAV.miS1 or AAV.HAtxn1L treated animals. Mice were also tested on the accelerating rotarod at 27 and 37 weeks of age. At 27 weeks of age, AAV.miS1 or AAV.HAtxn1L treated animals stayed on the accelerated rotarod seconds longer than AAV.miC or saline treated B05 animals (p < 0.05). At 37 weeks of age AAV.miC and saline injected groups of B05 mice were indistinguishable from each other and were significantly worse than AAV.miS1 or AAV.HAtxn1L treated B05 mice or their wild type littermates (p < 0.05; Figure 29). Notably, mice in the AAV.miS1 or AAV.HAtxn1L treatment groups, and wild type mice, remained on the accelerated rotarod approximately two minutes longer than control treated B05 mice. Also, there was no significant difference between therapeutically treated B05 mice and wild type animals in their latency to fall. These results indicate that, even at extended time points, mice treated with mis1 or HAtxn1L perform with the same level of motor coordination as wild type animals, and demonstrated rescue of the rotarod phenotype. Analysis of in vivo protein interactions with overexpressed ataxin-1-like Polyglutamine expansion in ataxin-1 yields a toxic gain-of-function via enhanced complex formation with Rbm17 [33]. Given this, we were interested to know whether
76 63 overexpressed ataxin-1-like can also interact with Rbm17. Consistent with previous studies, we found that ataxin-1 and exogenously expressed ataxin-1-like coimmunoprecipitate using cerebellar extracts from B05 mice injected with AAV.HAtxn1L (Figure 30). However, unlike ataxin-1, Rbm17 did not co-immunoprecipitate with ataxin-1-like in either Rbm17 or ataxin-1-like immunoprecipitates (Figure 31). Treatment with HAtxn1L or mis1 is not toxic in vivo Iba1 is an antigen often used to assess microglia activation, as can occur in the setting of toxic shrnas [49]. Iba1 staining was not different among the groups, and there was no evident neurotoxicity in AAV.miS1 or AAV.HAtxn1L injected animals compared to wild type littermates, either at the injection site (DCN) or remotely in cerebellar lobules (Figure 32). Levels of GFAP, an astrocytic marker, were measured by Q-RT-PCR on RNA isolated from whole cerebellar extracts (Figure 33). AAV.miS1 and AAV.HAtxn1L treated animals had no significant increases in GFAP mrna levels compared to saline treated animals. AAV.miC injected animals had elevated GFAP levels relative to all other treatment groups (p < 0.05). Ataxin-1-like overexpression and human ataxin-1 knockdown improves neuropathology Dendritic pruning in Purkinje cells is an early phenotype in B05 mice [14]. Molecular layer width provides quantitative insight into dendritic integrity and overall Purkinje cell viability. Sagittal cerebellar sections were stained for the Purkinje cell marker Calbindin, and molecular layer width quantified in transduced cerebellar lobules IV/V and VI. B05
77 64 mice treated with AAV.miS1 or AAV.HAtxn1L showed partial rescue of the thinned molecular layer width compared to control treated B05 mice (p < ; Figure 34). Ectopic Purkinje cells are present by 16 weeks of age in B05 mice, with Purkinje cell death evident by 24 weeks [14]. We found partial but significant rescue in the numbers of Purkinje cells retained after AAV.miS1 or AAV.HAtxn1L treatment (p < ; Figure 35), and a marked reduction in ectopic Purkinje cell numbers (p < ; Figure 36). Indeed, for ectopic Purkinje cell counts among the B05 groups, there was no difference between AAV.miS1 or AAV.HAtxn1L treated mice and their wild type littermates. Thus, the partial histological rescue of molecular layer width and Purkinje cell number, but improved ectopic Purkinje cell phenotypes, suggests that behavioral recovery does not require full rescue of all neuropathological aspects. Discussion Here we demonstrate two different therapeutic approaches that improve disease phenotypes in B05 transgenic SCA1 mice. Silencing of mutant ataxin-1 using mirnas or, alternatively, overexpression of ataxin-1-like both rescued behavioral deficits and improved the well-documented neuropathology in B05 mice. Previous work from our lab using early generation shrnas showed that RNAi provided therapeutic benefit in this model in short-term experiments. Here, using improved methodology we designed a safer and more potent mirna, mis1, which achieved 70% knockdown of human ataxin-1 in vivo for extended time points (35 weeks post-injection), improved B05 neuropathy and rescued motor deficits while showing no adverse effects. Previous work using a transgenic approach demonstrated that doubling endogenous levels of mouse ataxin-1-like improved phenotypes and neuropathy in the
78 65 SCA1 knock-in model [23]. Here we showed that viral-mediated overexpression of ataxin-1-like delivered after cerebellar development improved motor and neuronal phenotypes in the B05 model. Overexpression of HAtxn1L was comparable to SCA1 phenotypic rescue by suppression of the mutant human ataxin-1 transgene. It is well established that degeneration of Purkinje cell dendrites occurs long before Purkinje cell death in SCA1 pathogenesis [14, 17, 22]. At later stages (24 weeks) in disease progression in B05 mice there is a significant reduction in the number of Purkinje cells and noticeable ectopic localization of remaining Purkinje cells [17]. B05 mouse cerebella develop normally, thus ectopic cells are not due to a developmental migratory deficit. Rather the Purkinje cells likely translocate from their normal position to maintain distal dendritic association with parallel fibers in the superficial molecular layer [17]. Both AAV.miS1 and AAV.HAtxn1L treatment rescued the ectopic Purkinje cell phenotype. This, in addition to the partial rescue of dendritic integrity indicated by the retention of molecular layer width suggests that both therapeutics improve multiple aspects of known cerebellar neuropathy in SCA1. Partial rescue of rotarod phenotypes, early in disease, occurred via duplication of ataxin-1-like in the knock-in model [23], and partial rescue using shrnas was seen at 21 weeks in B05 mice [102]. Here, we found full rotarod rescue at late time points, an important consideration for moving this platform into clinical application. We also made the original observation that SCA1 mice have a phenotype in the ledge test, a useful assay to further query balance, agility, coordination and hindlimb musculature. Moreover, we demonstrate full rescue in the ledge test phenotype with either treatment modality.
79 66 Together with our histological studies, we find that rescue of motor deficits occurs in the absence of complete normalization of histological readouts. Capicua, a transcriptional repressor, interacts with ataxin-1. Capicua generally binds ataxin-1 when serine 776 (S776) is not phosphorylated regardless of polyq tract length. Complete loss of ataxin-1 decreases Capicua protein levels, and normal Capicua repressor activity is modulated when bound to mutant ataxin-1 [32]. Recently it was demonstrated that polyq-expanded ataxin-1 alters Capicua s activity at specific transcriptional targets [18]. SCA1 knock-in mouse data suggest that polyq-expanded ataxin-1:capicua interactions induce hyper-repression. Concomitantly, this reduces Capicua binding to other targets [18]. Important to this study, Capicua contains an AXH domain [32] and can bind ataxin-1 and ataxin-1-like. Early work tested the hypothesis that ataxin-1-like overexpression would compete with mutant, polyq expanded ataxin-1, displacing the mutant protein allowing it to aggregate into a form that is less toxic [23]. Ataxin-1-like lacks a polyq tract suggesting that binding with Capicua would mimic normal ataxin-1 binding thereby ablating the transcriptional modulation caused by polyq-expanded ataxin-1 binding. Whether phenotypic rescue in this study was the direct result of displaced mutant ataxin-1 or restoration of normal Capicua function is an interesting question and requires the development of tools to address their in vivo interactions. Earlier work showed that Rbm17 complexes with phosphorylated S776 ataxin-1 with a preference to bind polyq-expanded ataxin-1 over wild type ataxin-1 [33]. In our study exogenously expressed ataxin-1-like co-immunoprecipitated with ataxin-1 but not Rbm17. The lack of observed interaction between ataxin-1-like and Rbm17 suggests that
80 67 these two proteins either do not interact in vivo or that the interaction is too weak or transient to be detected in our co-ip assay. A lack of in vivo interaction between Rbm17 and ataxin-1-like is consistent with the Zoghbi model (Lam et al., 2006) wherein ataxin- 1-like overexpression restores normal Capicua activity. However, it is also possible that overexpressed ataxin-1-like sequesters mutant ataxin-1, thereby abrogating the toxic gain-of-function caused by the ataxin-1/rbm17 complex formation. Although the mechanism of rescue from overexpression of HAtxn1L is not fully understood, the results of our study indicate the ataxin-1-like is a promising candidate for further pre-clinical experiments in SCA1 therapies.
81 Figure 18. Cartoon of viral constructs. Top cartoon depicts AAV2/1 vector expressing mis1 driven by the mouse U6 promoter and egfp driven by CMV; bottom cartoon shows AAV2/1 with EF1α driving human 3x Flag-tagged ataxin-1-like. 68
82 Figure 19. Quantitative PCR 3 weeks post-injection of AAV.miS1 confirms knockdown of human ataxin-1. Mice were injected with either saline, AAV.miC.eGFP, or AAV.miS1.eGFP at 5 weeks of age and sacrificed 3 weeks later. Quantitative PCR was used to analyze the amount of human ataxin-1 mrna from whole cerebellum. Results are shown as mean ± SEM. AAV.miS1.eGFP treated mice have 30% less human ataxin-1 compared to control treated B05 littermates (*** indicated p < ). 69
83 Figure 20. Western blot confirming expression of HAtxn1L in vitro and in vivo. Western blotting for the Flag epitope verified AAV.HAtxn1L expression. Left panel shows HEK293 cells that were transfected with plasmids expressing indicated vectors. Protein was harvested 48-hours after transfection. Flag-tagged HAtxn1L expression (indicated by arrowhead) is seen only in cells transfected with AAV.HAtxn1L plasmid. Right panel shows mouse cerebellum injected with AAV.miC.eGFP, AAV.HAtxn1L or uninjected. Flag-tagged HAtxn1L expression is seen only in cerebellum injected with AAV.HAtxn1L. Beta Actin was used as a control (indicated by asterisk). 70
84 Figure 21. Viral spread shown by egfp fluorescence. Sagittal section of AAV.miS1.eGFP injected mouse cerebellum. egfp fluorescence defines AAV2/1 transduced lobules. Scale bar = 500 µm. 71
85 Figure 22. Semi-quantitative PCR confirms mis1 expression in vivo. Samples from cerebellar injected with AAV.miSCA1 or AAV.miC were probed for mis1 expression. 72
86 Figure 23. In situ hybridization localizes mis1 to Purkinje cells. Sagittal sections of injected B05 cerebella assayed by in situ hybridization showing mis1 expression localized predominantly to Purkinje cells (boxed) in cerebellar cortex. Scale bar = 100 µm. 73
87 Figure 24. Quantitative PCR confirms knockdown of human ataxin-1 by AAV.miS1. Quantitative PCR analysis of ataxin-1 expression in whole cerebellum showing 70% knockdown in AAV.miS1.eGFP treated mice relative to saline treated control B05 mice. Results are shown as mean ± SEM, normalized to saline injected B05 mice. (n = 4, * indicates p < 0.05, *** indicates p < ). 74
88 Figure 25. Quantitative PCR shows rescued Calbindin levels in AAV.miS1 injected mice. Quantitative PCR analysis of endogenous calbindin expression in whole cerebellum showing increased levels in AAV.miS1.eGFP treated mice compared to all other treated B05 mice. Results are shown as mean ± SEM, normalized to saline injected B05 mice. (n = 4, * indicates p < 0.05, *** indicates p < ). 75
89 Figure 26. AAV.miS1 and AAV.HAtxn1L rescues ledge test phenotype. Ledge Test assay showing AAV.miS1.eGFP injected, AAV.HAtxn1L injected and wild type mice perform significantly better than control treated B05 littermates.results are shown as mean ± SEM (*** indicates p < ). 76
90 Figure 27. AAV.miS1 and AAV.HAtxn1L rescues hindlimb clasping phenotype. Hindlimb Clasping assay showing AAV.miS1.eGFP-injected, AAV.HAtxn1Linjected and wild type mice perform significantly better than control treated B05 littermates. Results are shown as mean ± SEM (*** indicates p < ). 77
91 Figure 28. AAV.miS1 and AAV.HAtxn1L improve stride length. Box plots showing AAV.miS1.eGFP and AAV.HAtxn1L treated mice have significantly longer stride lengths than control injected B05 littermates. Measurements from steps per mouse were averaged for all four paws. (* indicates p < 0.05, *** indicates p < ). 78
92 Figure 29. AAV.miS1 and AAV.HAtxn1L rescues rotarod deficit. Rotarod performance at 27 and 37 weeks of age. At both time points, AAV.miC.eGFP and saline injected B05 littermates perform significantly worse than therapeutically treated and wild type mice (* indicates p < 0.05, the number of mice per group is indicated in parentheses). 79
93 Figure 30. Immunoprecipitated Ataxin-1-like is detected in injected cerebellar lysates. Arrowhead (~80 kda) denotes ataxin-1-like band. No band appeared in uninjected samples. I = 2% of total input; E = 30% of total elution. 80
94 Figure 31. Co-immunoprecipitation confirms interaction between ataxin-1-like and ataxin-1 in vivo. The same samples in Figure 30 were probed for ataxin-1 revealing that ataxin-1- like and ataxin-1 coimmunoprecipitate in the injected sample (arrowhead; ~85 kda) but not in the uninjected sample. I = 2% of total input; E = 30% of total elution. 81
95 Figure 32. IHC Iba1 staining shows no toxicity in treated mice. Sagittal 60 µm-thick cerebellar sections from all groups were stained for the microglia activation marker Iba1. Top panels show Iba1 levels in comparable sections of cerebellar cortex. Bottom panels show Iba1 levels at the injection site in the DCN. No gross difference in the amount of microglia activation was seen between wild type animals and individual treatment groups. Scale bar = 100 µm. 82
96 Figure 33. Quantitative PCR shows no increase in GFAP mrna. Quantitative PCR was used to quantify relative expression levels of GFAP, an astroglia marker. No significant statistical differences were seen between therapeutic treatment groups and saline treated animals. However, AAV.miC.eGFP injected B05 animals had higher levels of GFAP than all other groups. Results are shown as mean ± SEM (* indicates p < 0.05). 83
97 Figure 34. Molecular layer widths improved by AAV.miS1 and AAV.HAtxn1L. (Top) Sagittal 60 µm-thick cerebellar sections from all groups were stained for the Purkinje cell marker, calbindin. The molecular layer widths between lobules IV-V and VI were quantified in the region indicated by horizontal white lines. (Bottom) Molecular layer widths relative to WT mice. Mice treated with AAV.miS1.eGFP and AAV.HAtxn1L had significantly wider molecular layers than control treated B05 littermates. Scale bar = 75 µm. 84
98 Figure 35. Purkinje cell number improved by AAV.miS1 and AAV.HAtxn1L. Purkinje cell counts per unit area. AAV.miS1.eGFP and AAV.HAtxn1L treated mice had significantly more Purkinje cells than control treated B05 littermates. Results are shown as mean ± SEM (** indicated p < 0.001; *** indicated p < ). 85
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and
 This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
This article appeared in a journal published by Elsevier. The attached copy is furnished to the author for internal non-commercial research and education use, including for instruction at the authors institution
Investigating the regulation of mirna biogenesis and Argonaute2 by RNA binding proteins
 Investigating the regulation of mirna biogenesis and Argonaute2 by RNA binding proteins Patrick Peter Connerty Supervisor: Gyorgy Hutvagner Thesis Submitted for the Degree of Doctor of Philosophy (Science)
Investigating the regulation of mirna biogenesis and Argonaute2 by RNA binding proteins Patrick Peter Connerty Supervisor: Gyorgy Hutvagner Thesis Submitted for the Degree of Doctor of Philosophy (Science)
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
OmicsLink shrna Clones guaranteed knockdown even in difficult-to-transfect cells
 OmicsLink shrna Clones guaranteed knockdown even in difficult-to-transfect cells OmicsLink shrna clone collections consist of lentiviral, and other mammalian expression vector based small hairpin RNA (shrna)
OmicsLink shrna Clones guaranteed knockdown even in difficult-to-transfect cells OmicsLink shrna clone collections consist of lentiviral, and other mammalian expression vector based small hairpin RNA (shrna)
TOOLS sirna and mirna. User guide
 TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES
 SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
SUPPLEMENTARY INFORMATION SUPPLEMENTARY FIGURES Supplementary Figure 1. Generation of inducible BICD2 knock-out mice. A) The mouse BICD2 locus and gene targeting constructs. To generate an inducible Bicd2
Technology Overview. Figure 1. asirna structure
 BMT, Inc. Technology Overview Small interfering RNAs (sirnas) are short, double-stranded RNAs (dsrnas) that mediate efficient gene silencing in a sequence-specific manner. The specific cleavage of mrna
BMT, Inc. Technology Overview Small interfering RNAs (sirnas) are short, double-stranded RNAs (dsrnas) that mediate efficient gene silencing in a sequence-specific manner. The specific cleavage of mrna
Therapeutic & Prevention Application of Nucleic Acids
 Therapeutic & Prevention Application of Nucleic Acids Seyed Amir Hossein Jalali Institute of Biotechnology and Bioengineering, Isfahan University Of Technology (IUT). 30.7.2015 * Plasmids * DNA Aptamers
Therapeutic & Prevention Application of Nucleic Acids Seyed Amir Hossein Jalali Institute of Biotechnology and Bioengineering, Isfahan University Of Technology (IUT). 30.7.2015 * Plasmids * DNA Aptamers
Experimental genetics - 2 Partha Roy
 Partha Roy Experimental genetics - 2 Making genetically altered animal 1) Gene knock-out k from: a) the entire animal b) selected cell-type/ tissue c) selected cell-type/tissue at certain time 2) Transgenic
Partha Roy Experimental genetics - 2 Making genetically altered animal 1) Gene knock-out k from: a) the entire animal b) selected cell-type/ tissue c) selected cell-type/tissue at certain time 2) Transgenic
RNA interference therapy for the Spinocerebellar ataxias
 University of Iowa Iowa Research Online Theses and Dissertations Spring 2014 RNA interference therapy for the Spinocerebellar ataxias Pavitra Shyam Ramachandran University of Iowa Copyright 2014 Pavitra
University of Iowa Iowa Research Online Theses and Dissertations Spring 2014 RNA interference therapy for the Spinocerebellar ataxias Pavitra Shyam Ramachandran University of Iowa Copyright 2014 Pavitra
Technical tips Session 4
 Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
Technical tips Session 4 Biotinylation assay: Streptavidin is a small bacterial protein that binds with high affinity to the vitamin biotin. This streptavidin-biotin combination can be used to link molecules
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature12119 SUPPLEMENTARY FIGURES AND LEGENDS pre-let-7a- 1 +14U pre-let-7a- 1 Ddx3x Dhx30 Dis3l2 Elavl1 Ggt5 Hnrnph 2 Osbpl5 Puf60 Rnpc3 Rpl7 Sf3b3 Sf3b4 Tia1 Triobp U2af1 U2af2 1 6 2 4 3
doi:10.1038/nature12119 SUPPLEMENTARY FIGURES AND LEGENDS pre-let-7a- 1 +14U pre-let-7a- 1 Ddx3x Dhx30 Dis3l2 Elavl1 Ggt5 Hnrnph 2 Osbpl5 Puf60 Rnpc3 Rpl7 Sf3b3 Sf3b4 Tia1 Triobp U2af1 U2af2 1 6 2 4 3
Thermo Scientific Dharmacon SMARTvector 2.0 Lentiviral shrna Particles
 Thermo Scientific Dharmacon SMARTvector 2.0 Lentiviral shrna Particles Long-term gene silencing shrna-specific design algorithm High titer, purified particles Thermo Scientific Dharmacon SMARTvector shrna
Thermo Scientific Dharmacon SMARTvector 2.0 Lentiviral shrna Particles Long-term gene silencing shrna-specific design algorithm High titer, purified particles Thermo Scientific Dharmacon SMARTvector shrna
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various
 Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
CELL BIOLOGY - CLUTCH CH. 7 - GENE EXPRESSION.
 !! www.clutchprep.com CONCEPT: CONTROL OF GENE EXPRESSION BASICS Gene expression is the process through which cells selectively to express some genes and not others Every cell in an organism is a clone
!! www.clutchprep.com CONCEPT: CONTROL OF GENE EXPRESSION BASICS Gene expression is the process through which cells selectively to express some genes and not others Every cell in an organism is a clone
Learning Objectives. Define RNA interference. Define basic terminology. Describe molecular mechanism. Define VSP and relevance
 Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
Learning Objectives Define RNA interference Define basic terminology Describe molecular mechanism Define VSP and relevance Describe role of RNAi in antigenic variation A Nobel Way to Regulate Gene Expression
SureSilencing sirna Array Technology Overview
 SureSilencing sirna Array Technology Overview Pathway-Focused sirna-based RNA Interference Topics to be Covered Who is SuperArray? Brief Introduction to RNA Interference Challenges Facing RNA Interference
SureSilencing sirna Array Technology Overview Pathway-Focused sirna-based RNA Interference Topics to be Covered Who is SuperArray? Brief Introduction to RNA Interference Challenges Facing RNA Interference
pdsipher and pdsipher -GFP shrna Vector User s Guide
 pdsipher and pdsipher -GFP shrna Vector User s Guide NOTE: PLEASE READ THE ENTIRE PROTOCOL CAREFULLY BEFORE USE Page 1. Introduction... 1 2. Vector Overview... 1 3. Vector Maps 2 4. Materials Provided...
pdsipher and pdsipher -GFP shrna Vector User s Guide NOTE: PLEASE READ THE ENTIRE PROTOCOL CAREFULLY BEFORE USE Page 1. Introduction... 1 2. Vector Overview... 1 3. Vector Maps 2 4. Materials Provided...
mmu-mir-200a-3p Real-time RT-PCR Detection and U6 Calibration Kit User Manual MyBioSource.com Catalog # MBS826230
 mmu-mir-200a-3p Real-time RT-PCR Detection and U6 Calibration Kit User Manual Catalog # MBS826230 For the detection and quantification of mirnas mmu-mir-200a-3p normalized by U6 snrna using Real-time RT-PCR
mmu-mir-200a-3p Real-time RT-PCR Detection and U6 Calibration Kit User Manual Catalog # MBS826230 For the detection and quantification of mirnas mmu-mir-200a-3p normalized by U6 snrna using Real-time RT-PCR
sirna Overview and Technical Tips
 1 sirna Overview and Technical Tips 2 CONTENTS 3 4 5 7 8 10 11 13 14 18 19 20 21 Introduction Applications How Does It Work? Handy Tips Troubleshooting Conclusions Further References Contact Us 3 INTRODUCTION
1 sirna Overview and Technical Tips 2 CONTENTS 3 4 5 7 8 10 11 13 14 18 19 20 21 Introduction Applications How Does It Work? Handy Tips Troubleshooting Conclusions Further References Contact Us 3 INTRODUCTION
Concepts and Methods in Developmental Biology
 Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Sarker et al. Supplementary Material. Subcellular Fractionation
 Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Supplementary Material Subcellular Fractionation Transfected 293T cells were harvested with phosphate buffered saline (PBS) and centrifuged at 2000 rpm (500g) for 3 min. The pellet was washed, re-centrifuged
Robust and Durable Target Silencing in the CNS with sirna Conjugates
 Robust and Durable Target Silencing in the CNS with sirna Conjugates Stuart Milstein October 2, 2018 1 2018 Alnylam Pharmaceuticals, Inc. Alnylam Forward Looking Statements This presentation contains forward-looking
Robust and Durable Target Silencing in the CNS with sirna Conjugates Stuart Milstein October 2, 2018 1 2018 Alnylam Pharmaceuticals, Inc. Alnylam Forward Looking Statements This presentation contains forward-looking
NCode mirna profiling. Sensitive, reproducible mirna profiling
 Sensitive, reproducible mirna profiling Complete solutions for profiling mirna expression patterns Complete, optimized platform for mirna profiling Quick and efficient mirna expression analysis Superior
Sensitive, reproducible mirna profiling Complete solutions for profiling mirna expression patterns Complete, optimized platform for mirna profiling Quick and efficient mirna expression analysis Superior
Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides
 Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides Laboratory Sciences, MPI Research, A Charles River Company Amy Smith, BA, Senior Director, Bioanalytical/Analytical
Utility of Branched DNA Hybridization Methodology for the Quantitation of Oligonucleotides Laboratory Sciences, MPI Research, A Charles River Company Amy Smith, BA, Senior Director, Bioanalytical/Analytical
Nature Medicine doi: /nm.2558
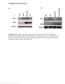 Supplementary. Fig. 1. (a) Sirt1 and mutant HTT (detected by HTT 81-90 antibody) protein levels were detected by Western blotting in cerebral cortex of N171-82Q mice. (b) Sirt1 and mutant HTT (detected
Supplementary. Fig. 1. (a) Sirt1 and mutant HTT (detected by HTT 81-90 antibody) protein levels were detected by Western blotting in cerebral cortex of N171-82Q mice. (b) Sirt1 and mutant HTT (detected
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency.
 Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
Supplementary Figure 1, related to Figure 1. GAS5 is highly expressed in the cytoplasm of hescs, and positively correlates with pluripotency. (a) Transfection of different concentration of GAS5-overexpressing
MISSION shrna Library: Next Generation RNA Interference
 Page 1 of 6 Page 1 of 6 Return to Web Version MISSION shrna Library: Next Generation RNA Interference By: Stephanie Uder, Henry George, Betsy Boedeker, LSI Volume 6 Article 2 Introduction The technology
Page 1 of 6 Page 1 of 6 Return to Web Version MISSION shrna Library: Next Generation RNA Interference By: Stephanie Uder, Henry George, Betsy Boedeker, LSI Volume 6 Article 2 Introduction The technology
UTR Reporter Vectors and Viruses
 UTR Reporter Vectors and Viruses 3 UTR, 5 UTR, Promoter Reporter (Version 1) Applied Biological Materials Inc. #1-3671 Viking Way Richmond, BC V6V 2J5 Canada Notice to Purchaser All abm products are for
UTR Reporter Vectors and Viruses 3 UTR, 5 UTR, Promoter Reporter (Version 1) Applied Biological Materials Inc. #1-3671 Viking Way Richmond, BC V6V 2J5 Canada Notice to Purchaser All abm products are for
Exam 3 4/25/07. Total of 7 questions, 100 points.
 Exam 3 4/25/07 BISC 4A P. Sengupta Total of 7 questions, 100 points. QUESTION 1. Circle the correct answer. Total of 40 points 4 points each. 1. Which of the following is typically attacked by the antibody-mediated
Exam 3 4/25/07 BISC 4A P. Sengupta Total of 7 questions, 100 points. QUESTION 1. Circle the correct answer. Total of 40 points 4 points each. 1. Which of the following is typically attacked by the antibody-mediated
sirna Transfection Into Primary Neurons Using Fuse-It-siRNA
 sirna Transfection Into Primary Neurons Using Fuse-It-siRNA This Application Note describes a protocol for sirna transfection into sensitive, primary cortical neurons using Fuse-It-siRNA. This innovative
sirna Transfection Into Primary Neurons Using Fuse-It-siRNA This Application Note describes a protocol for sirna transfection into sensitive, primary cortical neurons using Fuse-It-siRNA. This innovative
Input. Pulldown GST. GST-Ahi1
 A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
A kd 250 150 100 75 50 37 25 -GST-Ahi1 +GST-Ahi1 kd 148 98 64 50 37 22 GST GST-Ahi1 C kd 250 148 98 64 50 Control Input Hap1A Hap1 Pulldown GST GST-Ahi1 Hap1A Hap1 Hap1A Hap1 Figure S1. (A) Ahi1 western
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat
 Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Cell-penetrating peptides: news for dated cellular Trojan horses
 Technical Journal Club Cell-penetrating peptides: news for dated cellular Trojan horses Assunta Senatore August 5 th 2014 Overview Cell-penetrating peptides: classes and mechanisms of cell entry Overview
Technical Journal Club Cell-penetrating peptides: news for dated cellular Trojan horses Assunta Senatore August 5 th 2014 Overview Cell-penetrating peptides: classes and mechanisms of cell entry Overview
SANTA CRUZ BIOTECHNOLOGY, INC.
 TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
TECHNICAL SERVICE GUIDE: Western Blotting 2. What size bands were expected and what size bands were detected? 3. Was the blot blank or was a dark background or non-specific bands seen? 4. Did this same
What goes into my Biological Inventory?
 What goes into my Biological Inventory? What Information is Required for an Effective Risk Assessment? According to the Laboratory Biosafety Guidelines (2004), the risk group of an organism is determined
What goes into my Biological Inventory? What Information is Required for an Effective Risk Assessment? According to the Laboratory Biosafety Guidelines (2004), the risk group of an organism is determined
RNA Interference and the World of Small RNAs
 RNA Interference and the World of Small RNAs O, I die, Horatio; The potent poison quite o'er-crows my spirit: I cannot live to hear the news from England; But I do prophesy the election lights On Fortinbras:
RNA Interference and the World of Small RNAs O, I die, Horatio; The potent poison quite o'er-crows my spirit: I cannot live to hear the news from England; But I do prophesy the election lights On Fortinbras:
TRANSGENIC ANIMALS. transient. stable. - Two methods to produce transgenic animals:
 Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
Hairpin-it TM mirnas qpcr Quantitation Kit
 Hairpin-it TM mirnas qpcr Quantitation Kit For the detection and quantification of micrornas using real-time PCR detection instruments. Catalog No. QPM-010/ QPM-011/ QPM-012/ QPM-013 User Manual Table
Hairpin-it TM mirnas qpcr Quantitation Kit For the detection and quantification of micrornas using real-time PCR detection instruments. Catalog No. QPM-010/ QPM-011/ QPM-012/ QPM-013 User Manual Table
Test Bank for Molecular Cell Biology 7th Edition by Lodish
 Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
mmu-mir-34a Real-time RT-PCR Detection Kit User Manual
 mmu-mir-34a Real-time RT-PCR Detection Kit User Manual Catalog # CPK1272 For the detection and quantification of mirna mmu-mir-34a using Real-Time RT-PCR detection instruments. For research use only. Not
mmu-mir-34a Real-time RT-PCR Detection Kit User Manual Catalog # CPK1272 For the detection and quantification of mirna mmu-mir-34a using Real-Time RT-PCR detection instruments. For research use only. Not
CNS Gene Regulation Platform
 CNS Gene Regulation Platform 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
CNS Gene Regulation Platform 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
6th Form Open Day 15th July 2015
 6th Form Open Day 15 th July 2015 DNA is like a computer program but far, far more advanced than any software ever created. Bill Gates Welcome to the Open Day event at the Institute of Genetic Medicine
6th Form Open Day 15 th July 2015 DNA is like a computer program but far, far more advanced than any software ever created. Bill Gates Welcome to the Open Day event at the Institute of Genetic Medicine
RNAi HTS and Data Analysis
 Part I RNAi HTS and Data Analysis 1 Introduction to Genome-Scale RNAi Research 1.1 RNAi: An Effective Tool for Elucidating Gene Functions and a New Class of Drugs RNAi is a mechanism in living cells that
Part I RNAi HTS and Data Analysis 1 Introduction to Genome-Scale RNAi Research 1.1 RNAi: An Effective Tool for Elucidating Gene Functions and a New Class of Drugs RNAi is a mechanism in living cells that
sherwood - UltramiR shrna Collections
 sherwood - UltramiR shrna Collections Incorporating advances in shrna design and processing for superior potency and specificity sherwood - UltramiR shrna Collections Enabling Discovery Across the Genome
sherwood - UltramiR shrna Collections Incorporating advances in shrna design and processing for superior potency and specificity sherwood - UltramiR shrna Collections Enabling Discovery Across the Genome
Genetics Faculty of Agriculture and Veterinary Medicine. Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology
 Genetics 10201232 Faculty of Agriculture and Veterinary Medicine Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology 1 Biotechnology is defined as the technology that involves the use of living organisms
Genetics 10201232 Faculty of Agriculture and Veterinary Medicine Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology 1 Biotechnology is defined as the technology that involves the use of living organisms
GE Healthcare. DharmaconTM. Gene Editing, RNAi & Gene Expression. RNAi Application Guide 2016/17
 GE Healthcare DharmaconTM Gene Editing, RNAi & Gene Expression RNAi Application Guide 2016/17 Every discovery is a stepping stone toward clarity, firm conclusions, and ultimately an amazing breakthrough.
GE Healthcare DharmaconTM Gene Editing, RNAi & Gene Expression RNAi Application Guide 2016/17 Every discovery is a stepping stone toward clarity, firm conclusions, and ultimately an amazing breakthrough.
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
TITLE: Developing Gene Silencing for the Study and Treatment of Dystonia
 1 AWARD NUMBER: W81XWH-14-1-0282 TITLE: Developing Gene Silencing for the Study and Treatment of Dystonia PRINCIPAL INVESTIGATOR: Pedro Gonzalez-Alegre, MD, PhD CONTRACTING ORGANIZATION: Children s Hospital
1 AWARD NUMBER: W81XWH-14-1-0282 TITLE: Developing Gene Silencing for the Study and Treatment of Dystonia PRINCIPAL INVESTIGATOR: Pedro Gonzalez-Alegre, MD, PhD CONTRACTING ORGANIZATION: Children s Hospital
TRANSLATIONAL RESEARCH A possible approach for treating FSHD with RNAi therapeutics Perspectives and updates from the FSH Society
 FSH Society, Inc. Watertown, MA 02472 TRANSLATIONAL RESEARCH A possible approach for treating FSHD with RNAi therapeutics Perspectives and updates from the FSH Society Two exciting papers were recently
FSH Society, Inc. Watertown, MA 02472 TRANSLATIONAL RESEARCH A possible approach for treating FSHD with RNAi therapeutics Perspectives and updates from the FSH Society Two exciting papers were recently
Genetics and Genomics in Medicine Chapter 9 Questions
 Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
Quality Control Assays
 QUALITY CONTROL An integral part of the Penn Vector Core is its robust quality control program which is carried out by a separate quality control group. Quality control assays have been developed and optimized
QUALITY CONTROL An integral part of the Penn Vector Core is its robust quality control program which is carried out by a separate quality control group. Quality control assays have been developed and optimized
At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in
 Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supplementary Materials and Methods Barrier function assays At E17.5, the embryos were rinsed in phosphate-buffered saline (PBS) and immersed in acidic X-gal mix (100 mm phosphate buffer at ph4.3, 3 mm
Supporting Information
 Supporting Information Fujii et al. 10.1073/pnas.1217563110 A Human Cen chromosome 4q ART3 NUP54 SCARB2 FAM47E CCDC8 Tel B Bac clones RP11-54D17 RP11-628A4 Exon 1 2 34 5 6 7 8 9 10 11 12 5 3 PCR region
Supporting Information Fujii et al. 10.1073/pnas.1217563110 A Human Cen chromosome 4q ART3 NUP54 SCARB2 FAM47E CCDC8 Tel B Bac clones RP11-54D17 RP11-628A4 Exon 1 2 34 5 6 7 8 9 10 11 12 5 3 PCR region
Rapid Method for the Purification of Total RNA from Formalin- Fixed Paraffin-Embedded (FFPE) Tissue Samples
 Application Note 17 RNA Sample Preparation Rapid Method for the Purification of Total RNA from Formalin- Fixed Paraffin-Embedded (FFPE) Tissue Samples M. Melmogy 1, V. Misic 1, B. Lam, PhD 1, C. Dobbin,
Application Note 17 RNA Sample Preparation Rapid Method for the Purification of Total RNA from Formalin- Fixed Paraffin-Embedded (FFPE) Tissue Samples M. Melmogy 1, V. Misic 1, B. Lam, PhD 1, C. Dobbin,
CELL BIOLOGY - CLUTCH CH TECHNIQUES IN CELL BIOLOGY.
 !! www.clutchprep.com CONCEPT: LIGHT MICROSCOPE A light microscope uses light waves and magnification to view specimens Can be used to visualize transparent, and some cellular components - Can visualize
!! www.clutchprep.com CONCEPT: LIGHT MICROSCOPE A light microscope uses light waves and magnification to view specimens Can be used to visualize transparent, and some cellular components - Can visualize
SUPPLEMENTARY INFORMATION FILE
 SUPPLEMENTARY INFORMATION FILE Existence of a microrna pathway in anucleate platelets Patricia Landry, Isabelle Plante, Dominique L. Ouellet, Marjorie P. Perron, Guy Rousseau & Patrick Provost 1. SUPPLEMENTARY
SUPPLEMENTARY INFORMATION FILE Existence of a microrna pathway in anucleate platelets Patricia Landry, Isabelle Plante, Dominique L. Ouellet, Marjorie P. Perron, Guy Rousseau & Patrick Provost 1. SUPPLEMENTARY
GENETICS - CLUTCH CH.15 GENOMES AND GENOMICS.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF GENOMICS Genomics is the study of genomes in their entirety Bioinformatics is the analysis of the information content of genomes - Genes, regulatory sequences,
!! www.clutchprep.com CONCEPT: OVERVIEW OF GENOMICS Genomics is the study of genomes in their entirety Bioinformatics is the analysis of the information content of genomes - Genes, regulatory sequences,
Lac Operon contains three structural genes and is controlled by the lac repressor: (1) LacY protein transports lactose into the cell.
 Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
Loss of Cul3 in Primary Fibroblasts
 PSU McNair Scholars Online Journal Volume 5 Issue 1 Humans Being: People, Places, Perspectives and Processes Article 15 2011 Loss of Cul3 in Primary Fibroblasts Paula Hanna Portland State University Let
PSU McNair Scholars Online Journal Volume 5 Issue 1 Humans Being: People, Places, Perspectives and Processes Article 15 2011 Loss of Cul3 in Primary Fibroblasts Paula Hanna Portland State University Let
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
SUPPLEMENTARY INFORMATION
 doi:.38/nature899 Supplementary Figure Suzuki et al. a c p7 -/- / WT ratio (+)/(-) p7 -/- / WT ratio Log X 3. Fold change by treatment ( (+)/(-)) Log X.5 3-3. -. b Fold change by treatment ( (+)/(-)) 8
doi:.38/nature899 Supplementary Figure Suzuki et al. a c p7 -/- / WT ratio (+)/(-) p7 -/- / WT ratio Log X 3. Fold change by treatment ( (+)/(-)) Log X.5 3-3. -. b Fold change by treatment ( (+)/(-)) 8
Page 1 of 5. Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR Cy µg (~7.5 nmol) MIR 7901
 Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
CRISPR/Cas9 Gene Editing Tools
 CRISPR/Cas9 Gene Editing Tools - Guide-it Products for Successful CRISPR/Cas9 Gene Editing - Why choose Guide-it products? Optimized methods designed for speed and ease of use Complete kits that don t
CRISPR/Cas9 Gene Editing Tools - Guide-it Products for Successful CRISPR/Cas9 Gene Editing - Why choose Guide-it products? Optimized methods designed for speed and ease of use Complete kits that don t
Applicazioni biotecnologiche
 Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
ksierzputowska.com Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster
 Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster Research plan: Specific aims: 1. To successfully engineer transgenic Drosophila expressing TALENs
Research Title: Using novel TALEN technology to engineer precise mutations in the genome of D. melanogaster Research plan: Specific aims: 1. To successfully engineer transgenic Drosophila expressing TALENs
A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo.
 Gus Frangou, Stephanie Palmer & Mark Groudine Fred Hutchinson Cancer Research Center, Seattle- USA A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo. During mammalian development and differentiation
Gus Frangou, Stephanie Palmer & Mark Groudine Fred Hutchinson Cancer Research Center, Seattle- USA A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo. During mammalian development and differentiation
MeCP2. MeCP2/α-tubulin. GFP mir1-1 mir132
 Conservation Figure S1. Schematic showing 3 UTR (top; thick black line), mir132 MRE (arrow) and nucleotide sequence conservation (vertical black lines; http://genome.ucsc.edu). a GFP mir1-1 mir132 b GFP
Conservation Figure S1. Schematic showing 3 UTR (top; thick black line), mir132 MRE (arrow) and nucleotide sequence conservation (vertical black lines; http://genome.ucsc.edu). a GFP mir1-1 mir132 b GFP
Enhancers mutations that make the original mutant phenotype more extreme. Suppressors mutations that make the original mutant phenotype less extreme
 Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Interactomics and Proteomics 1. Interactomics The field of interactomics is concerned with interactions between genes or proteins. They can be genetic interactions, in which two genes are involved in the
Motivation From Protein to Gene
 MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
MOLECULAR BIOLOGY 2003-4 Topic B Recombinant DNA -principles and tools Construct a library - what for, how Major techniques +principles Bioinformatics - in brief Chapter 7 (MCB) 1 Motivation From Protein
Clontech Product Selection Guide
 New New Clontech Product Selection Guide PCR THigh Yield - TITANIUM Taq High Yield & Fidelity - Advantage 2 polymerase High Fidelity CloneAmp HiFi Direct PCR- Terra PCR Direct polymearase Transfection
New New Clontech Product Selection Guide PCR THigh Yield - TITANIUM Taq High Yield & Fidelity - Advantage 2 polymerase High Fidelity CloneAmp HiFi Direct PCR- Terra PCR Direct polymearase Transfection
17.5 Eukaryotic Transcription Initiation Is Regulated by Transcription Factors That Bind to Cis-Acting Sites
 17.5 Eukaryotic Transcription Initiation Is Regulated by Transcription Factors That Bind to Cis-Acting Sites 1 Section 17.5 Transcription regulatory proteins, transcription factors, target cis-acting sites
17.5 Eukaryotic Transcription Initiation Is Regulated by Transcription Factors That Bind to Cis-Acting Sites 1 Section 17.5 Transcription regulatory proteins, transcription factors, target cis-acting sites
Design, Characterization, and Lead Selection of Therapeutic mirnas Targeting Huntingtin for Development of Gene Therapy for Huntington s Disease
 Citation: Molecular Therapy Nucleic Acids (16) 5, e297; doi:1.138/mtna.16.7 Official journal of the American Society of Gene & Cell Therapy All rights reserved 2162-2531/16 www.nature.com/mtna Design,
Citation: Molecular Therapy Nucleic Acids (16) 5, e297; doi:1.138/mtna.16.7 Official journal of the American Society of Gene & Cell Therapy All rights reserved 2162-2531/16 www.nature.com/mtna Design,
There are four major types of introns. Group I introns, found in some rrna genes, are self-splicing: they can catalyze their own removal.
 1 2 Continuous genes - Intron: Many eukaryotic genes contain coding regions called exons and noncoding regions called intervening sequences or introns. The average human gene contains from eight to nine
1 2 Continuous genes - Intron: Many eukaryotic genes contain coding regions called exons and noncoding regions called intervening sequences or introns. The average human gene contains from eight to nine
Cell proliferation was measured with Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan).
 1 2 3 4 5 6 7 8 Supplemental Materials and Methods Cell proliferation assay Cell proliferation was measured with Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). GCs were plated at 96-well
1 2 3 4 5 6 7 8 Supplemental Materials and Methods Cell proliferation assay Cell proliferation was measured with Cell Counting Kit-8 (Dojindo Laboratories, Kumamoto, Japan). GCs were plated at 96-well
Supplementary Fig.1. Over-expression of RNase L in stable polyclonal cell line
 Supplemental Data mrna Protein A kda 75 40 NEO/vector NEO/RNase L RNASE L β-actin RNASE L β-actin B % of control (neo vector), normalized 240 ** 200 160 120 80 40 0 Neo Neo/RNase L RNase L Protein Supplementary
Supplemental Data mrna Protein A kda 75 40 NEO/vector NEO/RNase L RNASE L β-actin RNASE L β-actin B % of control (neo vector), normalized 240 ** 200 160 120 80 40 0 Neo Neo/RNase L RNase L Protein Supplementary
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms
 Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Gene Therapy for MYO7A USH1B. Shannon Boye, Ph.D. Assistant Professor Department of Ophthalmology University of Florida
 Gene Therapy for MYO7A USH1B Shannon Boye, Ph.D. Assistant Professor Department of Ophthalmology University of Florida Hearing Vision Vestibular Function (balance) Type 1 Type 2 Type 3 Profound deafness
Gene Therapy for MYO7A USH1B Shannon Boye, Ph.D. Assistant Professor Department of Ophthalmology University of Florida Hearing Vision Vestibular Function (balance) Type 1 Type 2 Type 3 Profound deafness
Expressed genes profiling (Microarrays) Overview Of Gene Expression Control Profiling Of Expressed Genes
 Expressed genes profiling (Microarrays) Overview Of Gene Expression Control Profiling Of Expressed Genes Genes can be regulated at many levels Usually, gene regulation, are referring to transcriptional
Expressed genes profiling (Microarrays) Overview Of Gene Expression Control Profiling Of Expressed Genes Genes can be regulated at many levels Usually, gene regulation, are referring to transcriptional
CRISPR/Cas9 Gene Editing Tools
 CRISPR/Cas9 Gene Editing Tools - Separations Simply Spectacular INDELS Identify indels Determine if one or both copies of your gene have indels The Guide-it Genotype Confirmation Kit: Simple detection
CRISPR/Cas9 Gene Editing Tools - Separations Simply Spectacular INDELS Identify indels Determine if one or both copies of your gene have indels The Guide-it Genotype Confirmation Kit: Simple detection
BCH Graduate Survey of Biochemistry
 BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Glen Graham Lecture 30 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html
BCH 5045 Graduate Survey of Biochemistry Instructor: Charles Guy Producer: Ron Thomas Director: Glen Graham Lecture 30 Slide sets available at: http://hort.ifas.ufl.edu/teach/guyweb/bch5045/index.html
CHAPTERS 16 & 17: DNA Technology
 CHAPTERS 16 & 17: DNA Technology 1. What is the function of restriction enzymes in bacteria? 2. How do bacteria protect their DNA from the effects of the restriction enzymes? 3. How do biologists make
CHAPTERS 16 & 17: DNA Technology 1. What is the function of restriction enzymes in bacteria? 2. How do bacteria protect their DNA from the effects of the restriction enzymes? 3. How do biologists make
Rapid Method for the Purification of Total RNA from Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Samples
 Application Note 17 RNA Sample Preparation Rapid Method for the Purification of Total RNA from Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Samples M. Melmogy 1, V. Misic 1, B. Lam, PhD 1, C. Dobbin,
Application Note 17 RNA Sample Preparation Rapid Method for the Purification of Total RNA from Formalin-Fixed Paraffin-Embedded (FFPE) Tissue Samples M. Melmogy 1, V. Misic 1, B. Lam, PhD 1, C. Dobbin,
Site directed mutagenesis, Insertional and Deletion Mutagenesis. Mitesh Shrestha
 Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
Site directed mutagenesis, Insertional and Deletion Mutagenesis Mitesh Shrestha Mutagenesis Mutagenesis (the creation or formation of a mutation) can be used as a powerful genetic tool. By inducing mutations
Control of Eukaryotic Genes. AP Biology
 Control of Eukaryotic Genes The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different, specialized functions? Evolution
Control of Eukaryotic Genes The BIG Questions How are genes turned on & off in eukaryotes? How do cells with the same genes differentiate to perform completely different, specialized functions? Evolution
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
doi:10.1038/nature10810 Supplementary Fig. 1: Mutation of the loqs gene leads to shortened lifespan and adult-onset brain degeneration. a. Northern blot of control and loqs f00791 mutant flies. loqs f00791
SURVEY AND SUMMARY Oligonucleotide-based strategies to combat polyglutamine diseases
 Published online 21 May 2014 Nucleic Acids Research, 2014, Vol. 42, No. 11 6787 6810 doi: 10.1093/nar/gku385 SURVEY AND SUMMARY Oligonucleotide-based strategies to combat polyglutamine diseases Agnieszka
Published online 21 May 2014 Nucleic Acids Research, 2014, Vol. 42, No. 11 6787 6810 doi: 10.1093/nar/gku385 SURVEY AND SUMMARY Oligonucleotide-based strategies to combat polyglutamine diseases Agnieszka
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
New microribbon production
 New microribbon production In vitro transformation Excystation brief exposure to acidic ph (~2) flagellar activity within 5-10 min after return to neutral ph breakdown of cyst wall (proteases) trophozoite
New microribbon production In vitro transformation Excystation brief exposure to acidic ph (~2) flagellar activity within 5-10 min after return to neutral ph breakdown of cyst wall (proteases) trophozoite
A c t i v a t e R N A i w i t h P r e c i s i o n. Specifically elicit RNAi without dsrna. mrna binding region
 SM NEW TECHNOLOGY: Specifically elicit i without ds. attracting loop processing stem m binding region Novel Molecule Gene Knockouts Eliminate Off Target Suppression Use in-situ or in-vivo Avoid Interferon
SM NEW TECHNOLOGY: Specifically elicit i without ds. attracting loop processing stem m binding region Novel Molecule Gene Knockouts Eliminate Off Target Suppression Use in-situ or in-vivo Avoid Interferon
Practice Problems 5. Location of LSA-GFP fluorescence
 Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Life Sciences 1a Practice Problems 5 1. Soluble proteins that are normally secreted from the cell into the extracellular environment must go through a series of steps referred to as the secretory pathway.
Genetic Engineering & Recombinant DNA
 Genetic Engineering & Recombinant DNA Chapter 10 Copyright The McGraw-Hill Companies, Inc) Permission required for reproduction or display. Applications of Genetic Engineering Basic science vs. Applied
Genetic Engineering & Recombinant DNA Chapter 10 Copyright The McGraw-Hill Companies, Inc) Permission required for reproduction or display. Applications of Genetic Engineering Basic science vs. Applied
Lullaby sirna Transfection Reagent - Results
 sirna Transfection Reagent - Results OZ Biosciences is delighted to announce the launching of a new sirna transfection reagent: -sirna. This lipid based transfection reagent is specifically designed for
sirna Transfection Reagent - Results OZ Biosciences is delighted to announce the launching of a new sirna transfection reagent: -sirna. This lipid based transfection reagent is specifically designed for
Chapter 11: Regulation of Gene Expression
 Chapter Review 1. It has long been known that there is probably a genetic link for alcoholism. Researchers studying rats have begun to elucidate this link. Briefly describe the genetic mechanism found
Chapter Review 1. It has long been known that there is probably a genetic link for alcoholism. Researchers studying rats have begun to elucidate this link. Briefly describe the genetic mechanism found
CURRICULUM VITAE. Educational Qualification: DEGREE INSTITUTION YEAR PROGRESS. Bharathidasan University Tamil Nadu
 CURRICULUM VITAE M.BHARATH VIJAYARAGAVAN Masters in Biomedical sciences Nationality: Indian E-mail: bharathragavan@gmail.com Mobile: 91-9911738539 Sex: Male; Age: 23 Objective: Cell Signaling is an important
CURRICULUM VITAE M.BHARATH VIJAYARAGAVAN Masters in Biomedical sciences Nationality: Indian E-mail: bharathragavan@gmail.com Mobile: 91-9911738539 Sex: Male; Age: 23 Objective: Cell Signaling is an important
Value Correct Answer Feedback. Student Response. A. Dicer enzyme. complex. C. the Dicer-RISC complex D. none of the above
 1 RNA mediated interference is a post-transcriptional gene silencing mechanism Which component of the RNAi pathway have been implicated in cleavage of the target mrna? A Dicer enzyme B the RISC-siRNA complex
1 RNA mediated interference is a post-transcriptional gene silencing mechanism Which component of the RNAi pathway have been implicated in cleavage of the target mrna? A Dicer enzyme B the RISC-siRNA complex
Supporting Information
 Supporting Information Kolber et al. 10.1073/pnas.0803216105 SI Text Animal Husbandry. All mouse protocols were in accordance with National Institutes of Health guidelines and were approved by the Animal
Supporting Information Kolber et al. 10.1073/pnas.0803216105 SI Text Animal Husbandry. All mouse protocols were in accordance with National Institutes of Health guidelines and were approved by the Animal
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Generation of the AARE-Gene system construct. (a) Position and sequence alignment of AAREs extracted from human Trb3, Chop or Atf3 promoters. AARE core sequences are boxed in grey.
Supplementary Figure 1 Generation of the AARE-Gene system construct. (a) Position and sequence alignment of AAREs extracted from human Trb3, Chop or Atf3 promoters. AARE core sequences are boxed in grey.
Mutagenesis and Expression of Mammalian Clotting Factor IX. Mark McCleland The Children s Hospital of Philadelphia and Lycoming College
 Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Mutagenesis and Expression of Mammalian Clotting Factor IX Mark McCleland The Children s Hospital of Philadelphia and Lycoming College Hemophilia B X-linked blood clotting disorder characterized by a deficiency
Chapter 10 Genetic Engineering: A Revolution in Molecular Biology
 Chapter 10 Genetic Engineering: A Revolution in Molecular Biology Genetic Engineering Direct, deliberate modification of an organism s genome bioengineering Biotechnology use of an organism s biochemical
Chapter 10 Genetic Engineering: A Revolution in Molecular Biology Genetic Engineering Direct, deliberate modification of an organism s genome bioengineering Biotechnology use of an organism s biochemical
