Comparison of the quality of grafts placed with implanter and forceps
|
|
|
- Nickolas Randall
- 6 years ago
- Views:
Transcription
1 Comparison of the quality of grafts placed with implanter and forceps Research Design Data Collection Procedures: The licensed hair restoration surgeon located at each of the participating sites will be responsible for collecting: (a) patient baseline and follow-up data and (b) performing all patient hair transplant procedures. Pre-Treatment: All patients will undergo a comprehensive history and physical prior to being enrolled in this study. After inclusionexclusion criteria have been applied, specific data regarding past medical treatments for hair loss will be sought and recorded. Baseline data: Pre-operative photographs using the Macrophotography from the recipient study area will document the presence of terminal hairs in the area so that final terminal counts can be reconciled at the end of the study. The hair in both of the 1cm x 2cm blocks within the test areas will be photographed for independent hair count analysis. Treatment: Follicular unit grafts will be harvested using a standard FUE technique using a 0.9mm ID (inside diameter) punch. The extractions will be performed and 140 completely intact grafts containing two terminal hairs will be collected for inclusion into the study. Only FUE grafts that contain follicles
2 that exhibit no transections at any level will be considered for the study. We divide grafts randomly to two groups of 70 grafts each. We collect the grafts in standard preserving solution and temperature before implanting them into the study areas. The total number of terminal hairs implanted into the study area in each group will be 140. The grafts will be handled with standard care and placed into saline or equivalent solution chilled to degrees Fahrenheit or 1-4 degrees Centigrade until planted. Two test areas; each approximately 1 cm by 2 cm, per patient will be identified for the study. The test sites for each method (Implanter versus Forceps) will be randomized per patient. The area should be completely devoid of hair and be non-contiguous. The study recipient sites will be created using a standard template, which has 2 test areas, each approximately 1cm X 2cm, with slots to create an even distribution of sites at 70 grafts/sq cm; alternatively, the marks could be made with the template and then incisions made accordingly. Each recipient site will be made with a 0.9mm, angled profile blade in the parallel orientation and the depth of the incision should be set as to reflect the average follicle length to minimize trauma. Each time the template is applied, it will allow the creation of a total of 70 recipient sites, evenly spaced, for the insertion of grafts implanted by a single implanting method. There should be at least 1 cm area between the two boxes so they could be distinguished in the future. The grafts will be placed within 2 hours of extraction from the donor area. The implantation on the Forceps group will be performed by the most accomplished placing staff and on the implanter
3 group by the surgeon. Proper sterilization techniques will be adhered to at all times. The post-operative care provided and those instructions routinely given to the patients will be administered. Post Treatment Data Collection: The patient will be instructed to return at 6 and 12 months following surgery and to allow the hair in the test areas to grow to at a length of at least 3 cm. The patient will then have the following evaluation performed: How to Evaluate: 1. HairCheck: Each of the test areas will have cross sectional trichometry (CST) performed using the HairCheck device (Divi Int l Co., Miami, Florida). The measurements will be performed three times and the average value of the three measurements recorded and the ratio of hair mass of two groups will be calculated. The ratio will provide the percentage of hair mass maintained. 2. Normal Photography: Standardized, non-magnified photographs will also be taken to compare the global appearance of each test site. 3. Manual count: The hair in each test region will be subjected to a manual count whereby the individual terminal hairs will be counted by a staff member blinded to the type of graft contained in the test area. The process will be repeated three times per test area and the average of the three values will be the terminal hair counts per test area. 4. The hair in the study areas will then be cut to a length of approximately 2-3 mm. 5. Macrophotography: a. The hair in each of the 1cm by 2cm study areas
4 will be photographed using the DermLite epiluminescence microscopy or similar device with photography capability for independent hair count analysis. b. The counting process will be repeated three times per test area and the average of the three values will be the terminal hair count per test area. The person performing the count will be blinded to the type of graft contained in each test region. c. Blinded observers will evaluate the global photographs to assess gross graft growth according to a predetermined scale. d. The process will be repeated for all test areas in all patients and the results recorded. All photography will be forwarded to the PI, who will have the hair counts done by an experienced hair restoration technician. The hair counts will be tracked by study ID number only, and whether they were baseline (BL) or final study (FS) photos, Data Analysis: Following data collection, the data will be entered into Excel and transferred into SPSS 20.0 for further analyses. The percentages of grown vs transplanted hair will be calculated to determine the survival rate of the hair grafts for each implanting method: (a) the surviving hair counts in each test area will be tallied and divided by the number of hairs implanted to give a hair survival rate for each implant method, (b) the data will then be combined for patients into a Center survival rate for each implant method, and (c) the data will then be combined again for the multi-center survival rate for each harvest method. Baseline data will first be analyzed using a two-tailed
5 independent t-test to determine if a statistically significant mean difference exists, in terms of hair count, between the FUE and strip method. If no statistically significant mean difference in baseline data is found to exist, then the research question will be answered using a two-tailed independent t-test. If a statistically significant mean difference is found to exist, then an Analysis of Covariance (ANCOVA) will be conducted controlling for baseline data. Effect size will be calculated using Cohen s d and coefficient of determination (r 2 ). A significance value of.05 will be used for this study. The questions that will be answered at conclusion of the study: 1. What is the survival rate of hair implanted by the implanter in comparison to hair implanted by the forceps? 2. Is there a statistically significant mean difference in the survival rate of hair grafts implanted by the implanter and the forceps method? 3. Is there a statistically significant mean difference in the cross-section surface area of hair grafts implanted by the implanter and the forceps method?
PEER-REVIEW DERMATOLOGY
 22 July 2014 prime-journal.com DERMATOLOGY PEER-REVIEW ROBOTICS, ARTIFICIAL INTELLIGENCE, AND THE FUTURE OF HAIR TRANSPLANTATION Chang Hun Huh reviews the latest technological advancement in hair restoration
22 July 2014 prime-journal.com DERMATOLOGY PEER-REVIEW ROBOTICS, ARTIFICIAL INTELLIGENCE, AND THE FUTURE OF HAIR TRANSPLANTATION Chang Hun Huh reviews the latest technological advancement in hair restoration
Technical Comparison 6-Feb-2015
 Technical Comparison 6-Feb-2015 Criteria Features FUETOR NEOGRAFT ARTAS Harvesting Implantation System Scoring Rotating Punch Rotating Punch Rotating Punch Harvesting of Follicle Vacuum Assisted Vacuum
Technical Comparison 6-Feb-2015 Criteria Features FUETOR NEOGRAFT ARTAS Harvesting Implantation System Scoring Rotating Punch Rotating Punch Rotating Punch Harvesting of Follicle Vacuum Assisted Vacuum
By Vision Medical, Inc.
 By Vision Medical, Inc. Long Term Successful Industry Experience; over 100 years of combined experience in bringing innovative technology to the medical market. Vision Medical History Founded in 2013 US
By Vision Medical, Inc. Long Term Successful Industry Experience; over 100 years of combined experience in bringing innovative technology to the medical market. Vision Medical History Founded in 2013 US
JOIN THE ARTAS GENERATION. A robotic revolution in speed, precision and accuracy.
 JOIN THE ARTAS GENERATION. A robotic revolution in speed, precision and accuracy. 2 A NEW GENERATION OF PERFORMANCE The ARTAS System enables physicians to harvest healthy follicular units in a minimally
JOIN THE ARTAS GENERATION. A robotic revolution in speed, precision and accuracy. 2 A NEW GENERATION OF PERFORMANCE The ARTAS System enables physicians to harvest healthy follicular units in a minimally
Quality Management. Carlos Bachier, MD
 Quality Management Carlos Bachier, MD Goals of Quality Management Ensure credibility of outcomes Improve patient safety and quality of processes Significantly reduce errors Quality Medical and Laboratory
Quality Management Carlos Bachier, MD Goals of Quality Management Ensure credibility of outcomes Improve patient safety and quality of processes Significantly reduce errors Quality Medical and Laboratory
Santen Acquisition of InnFocus, Developer of MicroShunt Glaucoma Implant Device
 Santen Acquisition of InnFocus, Developer of MicroShunt Glaucoma Implant Device Akira Kurokawa President & CEO August 2, 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. 1 External
Santen Acquisition of InnFocus, Developer of MicroShunt Glaucoma Implant Device Akira Kurokawa President & CEO August 2, 2016 Copyright 2016 Santen Pharmaceutical Co., Ltd. All rights reserved. 1 External
PATENT AGENT EXAMINATION, January 23, 2010 PAPER II TOTAL MARKS: 100. Total number of pages: 7
 PATENT AGENT EXAMINATION, 2010 (Under Section 126 of the Patents Act, 1970, as amended) January 23, 2010 PAPER II TOTAL MARKS: 100 Time : 3 PM to 6 PM (3 hours) Instructions: Total number of pages: 7 This
PATENT AGENT EXAMINATION, 2010 (Under Section 126 of the Patents Act, 1970, as amended) January 23, 2010 PAPER II TOTAL MARKS: 100 Time : 3 PM to 6 PM (3 hours) Instructions: Total number of pages: 7 This
Sponsor. Generic Drug Name. Trial Indication(s) Protocol Number. Protocol Title. Clinical Trial Phase. Study Start/End Dates
 Sponsor Sandoz GmbH Generic Drug Name Filgrastim Trial Indication(s) Neutropenia in breast cancer patients on myelosuppressive chemotherapy Protocol Number EP06-302 Protocol Title A randomized, double-blind,
Sponsor Sandoz GmbH Generic Drug Name Filgrastim Trial Indication(s) Neutropenia in breast cancer patients on myelosuppressive chemotherapy Protocol Number EP06-302 Protocol Title A randomized, double-blind,
Packaging, Labelling and Transport of Organs in Deceased and Living Donation and Transplantation. Summary of Significant Changes
 This National Operating Procedure replaces: NOP003 Summary of Significant Changes Effective: 6 th December 2016 Document updated to reflect changes in organ packaging and labelling including minor changes
This National Operating Procedure replaces: NOP003 Summary of Significant Changes Effective: 6 th December 2016 Document updated to reflect changes in organ packaging and labelling including minor changes
Designing Generalizable Trials: Why Inclusivity Matters. Estelle Russek-Cohen, PhD U.S. Food and Drug Administration Center for Biologics
 Designing Generalizable Trials: Why Inclusivity Matters Estelle Russek-Cohen, PhD U.S. Food and Drug Administration Center for Biologics 1 Disclaimer The thoughts expressed are my own and should not be
Designing Generalizable Trials: Why Inclusivity Matters Estelle Russek-Cohen, PhD U.S. Food and Drug Administration Center for Biologics 1 Disclaimer The thoughts expressed are my own and should not be
Trabecular Metal Revision Shell. Surgical Technique IMAGE TO COME. The Best Thing Next to Bone
 Trabecular Metal Revision Shell Surgical Technique IMAGE TO COME The Best Thing Next to Bone Trabecular Metal Revision Shell Surgical Technique Initial and Long-Term Stability Fully-interconnected trabecular
Trabecular Metal Revision Shell Surgical Technique IMAGE TO COME The Best Thing Next to Bone Trabecular Metal Revision Shell Surgical Technique Initial and Long-Term Stability Fully-interconnected trabecular
ROUTINE SPECIMEN COLLECTION BY SPECIMEN TYPE
 1. AUTOPSY EYE ROUTINE SPECIMEN COLLECTION BY SPECIMEN TYPE a. Arrangements: No special arrangements necessary. i. Immerse the specimen in a sufficient quantity of fixative so that the eye is covered completely
1. AUTOPSY EYE ROUTINE SPECIMEN COLLECTION BY SPECIMEN TYPE a. Arrangements: No special arrangements necessary. i. Immerse the specimen in a sufficient quantity of fixative so that the eye is covered completely
Clinical Commissioning Policy: Eculizumab for the treatment of refractory antibody mediated rejection post kidney transplant
 Clinical Commissioning Policy: Eculizumab for the treatment of refractory antibody mediated rejection post kidney transplant January 2014 Reference: NHS ENGLAND A07/P/c NHS England Clinical Commissioning
Clinical Commissioning Policy: Eculizumab for the treatment of refractory antibody mediated rejection post kidney transplant January 2014 Reference: NHS ENGLAND A07/P/c NHS England Clinical Commissioning
FDA Inspections: FDA Inspections: An Overview Overview
 FDA Inspections: An Overview Robert Lindblad, MD The EMMES Corporation August 11, 2011 researchcartoons.com Why Inspections? Why Inspections? To evaluate compliance with regulations To establish that an
FDA Inspections: An Overview Robert Lindblad, MD The EMMES Corporation August 11, 2011 researchcartoons.com Why Inspections? Why Inspections? To evaluate compliance with regulations To establish that an
How a diagnostic system operates in Ghana
 How a diagnostic system operates in Ghana Case finding: Community based volunteers Suspect cases are referred to treatment centres Clinical diagnosis established by local surgeons Eligible for laboratory
How a diagnostic system operates in Ghana Case finding: Community based volunteers Suspect cases are referred to treatment centres Clinical diagnosis established by local surgeons Eligible for laboratory
3.1. Overall Principal Investigator (PI), who holds the IDE and/or is the Sponsor
 POLICY #: RCO-101 Page: 1 of 11 1. POLICY STATEMENT: An Overall Principal Investigator (PI) who holds an Investigational Device Exemption (IDE) or who is the Sponsor of the research has additional responsibilities
POLICY #: RCO-101 Page: 1 of 11 1. POLICY STATEMENT: An Overall Principal Investigator (PI) who holds an Investigational Device Exemption (IDE) or who is the Sponsor of the research has additional responsibilities
Instructions for Use READY TO USE
 READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
READY TO USE Instructions for Use LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 1.908.947.1215 1.800.367.5737 Fax: 1.908.947.1089 E-mail: customerservicegroup@lifecell.com www.lifecell.com
Maurice Nahabedian, MD Plastic and Reconstructive Surgeon National Center for Plastic Surgery Mclean, VA, USA
 Regeneration of Full Thickness Hair-Bearing Skin in Chronic Refractory Wounds with an Autologous Homologous Skin Construct: Preclinical and Clinical Experience Nikolai Sopko, MD, PhD Chief Scientific Officer
Regeneration of Full Thickness Hair-Bearing Skin in Chronic Refractory Wounds with an Autologous Homologous Skin Construct: Preclinical and Clinical Experience Nikolai Sopko, MD, PhD Chief Scientific Officer
ALLODERM SELECT ALLODERM SELECT RESTORE
 ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
ALLODERM SELECT ALLODERM SELECT RESTORE Regenerative Tissue Matrix Instructions for Use Processed from donated human tissue by: LifeCell Corporation One Millennium Way Branchburg, NJ 08876-3876 DESCRIPTION
Matanuska-Susitna Borough Timber Inventory 2006
 Matanuska-Susitna Borough Timber Inventory 2006 1989 MSB Forest Management Plan 17 Forest Management Units (FMU) 138,000 acres classified forest land Approximately 84,700 acres Commercial Timber Land
Matanuska-Susitna Borough Timber Inventory 2006 1989 MSB Forest Management Plan 17 Forest Management Units (FMU) 138,000 acres classified forest land Approximately 84,700 acres Commercial Timber Land
HUMAN GENETICS INITIATIVE
 Clinical Protocol HUMAN GENETICS INITIATIVE Ancillary Study to A Comparison of Long-Acting Injectable Medications for Schizophrenia (ACLAIMS) National Institute of Mental Health Grant R01-MH081234 and
Clinical Protocol HUMAN GENETICS INITIATIVE Ancillary Study to A Comparison of Long-Acting Injectable Medications for Schizophrenia (ACLAIMS) National Institute of Mental Health Grant R01-MH081234 and
Anti- THrombosis with Enoxaparin in intubated Adolescents
 Anti- THrombosis with Enoxaparin in intubated Adolescents E. Vincent S. Faustino, MD, MHS October 2017 NHLBI submission S L I D E 0 Research question, central hypothesis and primary aim Research Question
Anti- THrombosis with Enoxaparin in intubated Adolescents E. Vincent S. Faustino, MD, MHS October 2017 NHLBI submission S L I D E 0 Research question, central hypothesis and primary aim Research Question
Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc.
 for wounds instructions for use Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc. marketed by KCI USA, Inc. San Antonio, TX 78219 USA 800.275.4524 www.kci1.com www.graftjacketbykci.com
for wounds instructions for use Processed from Donated Human Tissue by LifeCell Corporation for KCI USA, Inc. marketed by KCI USA, Inc. San Antonio, TX 78219 USA 800.275.4524 www.kci1.com www.graftjacketbykci.com
INVESTIGATIONAL DRUG MANAGEMENT OVERVIEW
 NORTHWELL HEALTH OFFICE OF RESEARCH COMPLIANCE STUDY FEASIBILITY AND APPROVAL Where can I learn about the investigational drug? Protocol Investigator s brochure Product insert or prescribing information
NORTHWELL HEALTH OFFICE OF RESEARCH COMPLIANCE STUDY FEASIBILITY AND APPROVAL Where can I learn about the investigational drug? Protocol Investigator s brochure Product insert or prescribing information
07/30/2013. Record of Revisions IRB Page 1 of 17
 Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
Current Protocol Version 7.1 (Continuing Review) Approved July 30, 2013 Version 7.1 (Amendment) September 6, 2012 Version 7.0 (Continuing Review) July 30, 2012 Version 6.0 (Continuing Review) July 30,
Matrix HD. wound covering. Sterile, room temperature human dermis graft
 Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD is an acellular human dermis allograft sterilized using
Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD wound covering Sterile, room temperature human dermis graft Matrix HD is an acellular human dermis allograft sterilized using
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength
 The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
The Biomechanics of ProLayer Acellular Dermal Matrix: suture retention strength Reginald Stilwell, B.S., C.T.B.S., Ryan Delaney, M.S. ProLayer, Centennial, CO Basic science volume 2 ProLayer Acellular
SYNOPSIS. Clinical Study Report for Study CV Individual Study Table Referring to the Dossier
 Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Individual Study Table Referring to the Dossier (For National Authority Use Only) Name of Active Ingredient: SYNOPSIS Clinical Study
Name of Sponsor/Company: Bristol-Myers Squibb Name of Finished Product: Individual Study Table Referring to the Dossier (For National Authority Use Only) Name of Active Ingredient: SYNOPSIS Clinical Study
Methods in Clinical Cancer Research Workshop Format for Protocol Concept Synopsis Sheet (blank) SYNOPSIS
 B Methods in Clinical Cancer Research Workshop Format for Protocol Concept Synopsis Sheet (blank) elow is the format to follow for the concept sheet for protocols. It is based on the International Committee
B Methods in Clinical Cancer Research Workshop Format for Protocol Concept Synopsis Sheet (blank) elow is the format to follow for the concept sheet for protocols. It is based on the International Committee
CRUISE DESIGN AND LAYOUT
 CRUISE DESIGN AND LAYOUT FOR 05: Forest Biometrics Lecture Notes Fall 015 Dr. Dean Coble Arthur Temple College of Forestry and Agriculture Stephen F. Austin State University CRUISE DESIGN AND LAYOUT Sample
CRUISE DESIGN AND LAYOUT FOR 05: Forest Biometrics Lecture Notes Fall 015 Dr. Dean Coble Arthur Temple College of Forestry and Agriculture Stephen F. Austin State University CRUISE DESIGN AND LAYOUT Sample
FirstDose Application Release Enhancements. FirstDose Application Release Enhancements. Complete list of Enhancements
 Release Notes FirstDose Application Release 3.1.4 Complete list of Enhancement of the system s barcode functionality. The system no longer requires every item in the MIL to have a Barcode in order to turn
Release Notes FirstDose Application Release 3.1.4 Complete list of Enhancement of the system s barcode functionality. The system no longer requires every item in the MIL to have a Barcode in order to turn
Tissue cleaning and sterilization provide an additional level of safety
 Managing biologics Understanding tissue processing Third in a series on managing bone allografts. In the October issue, articles included were Allografts: Overview of the process; and Donor screening:
Managing biologics Understanding tissue processing Third in a series on managing bone allografts. In the October issue, articles included were Allografts: Overview of the process; and Donor screening:
Vegetation Resources Inventory
 Vegetation Resources Inventory Ground Sampling Quality Assurance Procedures Prepared by Ministry of Forests Resources Inventory Branch for the Terrestrial Ecosystem Task Force Resources Inventory Committee
Vegetation Resources Inventory Ground Sampling Quality Assurance Procedures Prepared by Ministry of Forests Resources Inventory Branch for the Terrestrial Ecosystem Task Force Resources Inventory Committee
Device research sponsors, whether companies or investigators, are held responsible for meeting the same regulations.
 POLICY #: RCO-101 Page: 1 of 11 1. POLICY STATEMENT: A DF/HCC Investigator who holds an Investigational Device Exemption (IDE) or who is the Sponsor of the research has additional responsibilities that
POLICY #: RCO-101 Page: 1 of 11 1. POLICY STATEMENT: A DF/HCC Investigator who holds an Investigational Device Exemption (IDE) or who is the Sponsor of the research has additional responsibilities that
H E A L T H PROVIDER MANUAL
 H E A L T H PROVIDER MANUAL Table of Contents Welcome...1 Dimension Members...2 Verifying Eligibility and Benefits...2 Pre-Certification, Utilization Review and Case Management...2 Referral Authorizations...2
H E A L T H PROVIDER MANUAL Table of Contents Welcome...1 Dimension Members...2 Verifying Eligibility and Benefits...2 Pre-Certification, Utilization Review and Case Management...2 Referral Authorizations...2
Current Management of Empyema
 Current Management of Empyema George W. Holcomb, III, M.D., MBA Surgeon-in-Chief/Senior Vice-President Children s Mercy Hospital Kansas City, Missouri Thoracoscopic Operations Children s Mercy Experience
Current Management of Empyema George W. Holcomb, III, M.D., MBA Surgeon-in-Chief/Senior Vice-President Children s Mercy Hospital Kansas City, Missouri Thoracoscopic Operations Children s Mercy Experience
Policies Concerning Survival Surgery of Mice
 Policies Concerning Survival Surgery of Mice Date approved: October 25, 2016 Purpose This document details the minimum standards for survival (recovery) surgery in all laboratory rodent species. Responsibility
Policies Concerning Survival Surgery of Mice Date approved: October 25, 2016 Purpose This document details the minimum standards for survival (recovery) surgery in all laboratory rodent species. Responsibility
Thebiotutor.com A2 Biology OCR Unit F215: Control, genomes and environment Module 2.1 Cloning in plants and animals Notes & Questions
 Thebiotutor.com A2 Biology OCR Unit F215: Control, genomes and environment Module 2.1 Cloning in plants and animals Notes & Questions Andy Todd 1 Outline the differences between reproductive and non-reproductive
Thebiotutor.com A2 Biology OCR Unit F215: Control, genomes and environment Module 2.1 Cloning in plants and animals Notes & Questions Andy Todd 1 Outline the differences between reproductive and non-reproductive
Ask your surgeon for more information about Natrelle and visit GummyImplants.com
 The #1 surgeon-preferred gummy implant collection in the US* Two-stage breast reconstruction starts with Natrelle 133 Series Tissue Expanders Natrelle 133 Series Tissue Expanders are made to match Natrelle
The #1 surgeon-preferred gummy implant collection in the US* Two-stage breast reconstruction starts with Natrelle 133 Series Tissue Expanders Natrelle 133 Series Tissue Expanders are made to match Natrelle
Social and Ethical Issues in Systems Biology. HW: pg 120 #1-5, 9-11, 14
 Social and Ethical Issues in Systems Biology HW: pg 120 #1-5, 9-11, 14 Transplanting Organs Organ transplantation involves the removal of an organ from donor body and placement in a recipient body, wherein
Social and Ethical Issues in Systems Biology HW: pg 120 #1-5, 9-11, 14 Transplanting Organs Organ transplantation involves the removal of an organ from donor body and placement in a recipient body, wherein
SRTR Technical Advisory Committee (STAC) Report to UNOS/OPTN Board
 SRTR Technical Advisory Committee (STAC) Report to UNOS/OPTN Board November 16, 2009 Kim M. Olthoff, MD Chair, STAC Committee Slide 1 SRTR and OPTN: Complementary Roles SRTR Mission Research / Policy Evaluation
SRTR Technical Advisory Committee (STAC) Report to UNOS/OPTN Board November 16, 2009 Kim M. Olthoff, MD Chair, STAC Committee Slide 1 SRTR and OPTN: Complementary Roles SRTR Mission Research / Policy Evaluation
More than surface deep
 Tritanium PL Posterior Lumbar Cage More than surface deep Featuring Tritanium In-Growth Technology: 1 TM Built to fuse Tritanium In-Growth Technology1 Stryker s proprietary Tritanium In-Growth Technology,
Tritanium PL Posterior Lumbar Cage More than surface deep Featuring Tritanium In-Growth Technology: 1 TM Built to fuse Tritanium In-Growth Technology1 Stryker s proprietary Tritanium In-Growth Technology,
Specifically designed for abdominal wall repair STRUCTURAL
 Specifically designed for abdominal wall repair STRUCTURAL The Better Approach To a Better Allograft Better Standards: Board of Directors comprised primarily of surgeons 3-years run by surgeons, for surgeons
Specifically designed for abdominal wall repair STRUCTURAL The Better Approach To a Better Allograft Better Standards: Board of Directors comprised primarily of surgeons 3-years run by surgeons, for surgeons
10-CBA providing access to unlicensed cord blood units
 10-CBA providing access to unlicensed cord blood units Using the NMDP IND to access unlicensed cord blood units Amy Hays, Sr. Clinical Research Specialist, CIBMTR History of CBUs First CBU transplant done
10-CBA providing access to unlicensed cord blood units Using the NMDP IND to access unlicensed cord blood units Amy Hays, Sr. Clinical Research Specialist, CIBMTR History of CBUs First CBU transplant done
Controlled Document Number: Version Number: 2 Controlled Document Sponsor: Controlled Document Lead:
 University Hospitals Birmingham Policy on Human Tissue in Research NHS Foundation Trust CONTROLLED DOCUMENT CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 2 Controlled Document
University Hospitals Birmingham Policy on Human Tissue in Research NHS Foundation Trust CONTROLLED DOCUMENT CATEGORY: CLASSIFICATION: PURPOSE Controlled Document Number: Version Number: 2 Controlled Document
Clinical Research with Drugs/Biologics and Devices & Good Clinical Practices
 Clinical Research with Drugs/Biologics and Devices & Good Clinical Practices Jason Jobson, BLS, CCRP Research Compliance Officer Oklahoma City VA Medical Center October 2017 Goals Investigational New Drug
Clinical Research with Drugs/Biologics and Devices & Good Clinical Practices Jason Jobson, BLS, CCRP Research Compliance Officer Oklahoma City VA Medical Center October 2017 Goals Investigational New Drug
Clinical Trial Report Synopsis. Efficacy of twice daily applications of LEO ointment 30 mg/g for 12 weeks to subjects with alopecia areata
 Clinical Trial Report Synopsis Efficacy of twice daily applications of LEO 124249 ointment 30 mg/g for 12 weeks to subjects with alopecia areata Design of trial: A phase 2a, multi-centre, prospective,
Clinical Trial Report Synopsis Efficacy of twice daily applications of LEO 124249 ointment 30 mg/g for 12 weeks to subjects with alopecia areata Design of trial: A phase 2a, multi-centre, prospective,
Making Surgery Simpler
 Making Surgery Simpler The Lone Star Retractor System Making Surgery Simpler Lone Star Medical Products RECOMMENDED SURGICAL USES: Gynecology Urology General Colon/Rectal Otolaryngology Podiatry Hand Plastic
Making Surgery Simpler The Lone Star Retractor System Making Surgery Simpler Lone Star Medical Products RECOMMENDED SURGICAL USES: Gynecology Urology General Colon/Rectal Otolaryngology Podiatry Hand Plastic
ThromboLUX for determining platelet quality
 Updating information provided by the company October 2012 Horizon Scanning Centre October 2011 ThromboLUX for determining platelet quality SUMMARY Lay summary click here This briefing is based on information
Updating information provided by the company October 2012 Horizon Scanning Centre October 2011 ThromboLUX for determining platelet quality SUMMARY Lay summary click here This briefing is based on information
Clinical trial design issues and options for study of rare diseases
 Clinical trial design issues and options for study of rare diseases November 3, 2016 Jeffrey Krischer, PhD Rare Diseases Clinical Research Network Rare Diseases Clinical Research Network (RDCRN) is coordinated
Clinical trial design issues and options for study of rare diseases November 3, 2016 Jeffrey Krischer, PhD Rare Diseases Clinical Research Network Rare Diseases Clinical Research Network (RDCRN) is coordinated
2111: ALL Post-HCT. Add/ Remove/ Modify. Manual Section. Date. Description. Comprehensive Disease- Specific Manuals
 2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
Reduction Pruning. Community Forester Alabama Cooperative Extension System
 Reduction Pruning Restoring Storm Damaged dtrees W.J. Rowe II Community Forester Alabama Cooperative Extension System Looking for a path back from this To this Pruning: The Basics The goal of guiding tree
Reduction Pruning Restoring Storm Damaged dtrees W.J. Rowe II Community Forester Alabama Cooperative Extension System Looking for a path back from this To this Pruning: The Basics The goal of guiding tree
IEOR 130 Methods of Manufacturing Improvement Practice Examination Problems Part I of Course Prof. Leachman Fall, 2017
 IEOR 130 Methods of Manufacturing Improvement Practice Examination Problems Part I of Course Prof. Leachman Fall, 2017 1. The thickness of a film deposited on wafers at a particular process step is subject
IEOR 130 Methods of Manufacturing Improvement Practice Examination Problems Part I of Course Prof. Leachman Fall, 2017 1. The thickness of a film deposited on wafers at a particular process step is subject
Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation)
 Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation) Examination Guidelines for Patent and Utility Model Part II: REQUIREMENTS FOR PATENTABILITY Chapter 1 Industrially
Revised Examination Guidelines for Industrially Applicable Inventions (Provisional Translation) Examination Guidelines for Patent and Utility Model Part II: REQUIREMENTS FOR PATENTABILITY Chapter 1 Industrially
How to Survive an EBAA Site Inspection
 How to Survive an EBAA Site Inspection Woodford S. Van Meter,MD Lexington, KY EBAA Site Inspections: Scheduled by Accreditation Board Approximately 15-20 eyebanks inspected every six months Individual
How to Survive an EBAA Site Inspection Woodford S. Van Meter,MD Lexington, KY EBAA Site Inspections: Scheduled by Accreditation Board Approximately 15-20 eyebanks inspected every six months Individual
Development of a Finite Element Model to Analyse the Proximal Ring of an Endovascular Device
 Development of a Finite Element Model to Analyse the Proximal Ring of an Endovascular Device Emma McCummiskey Department of Mechanical Engineering University of Strathclyde Glasgow, UK. Overview Medical
Development of a Finite Element Model to Analyse the Proximal Ring of an Endovascular Device Emma McCummiskey Department of Mechanical Engineering University of Strathclyde Glasgow, UK. Overview Medical
3.1 Publishable summary
 3.1 Publishable summary This section must be of suitable quality to enable direct publication by the Commission. A summary description of project context and objectives, 4000 characters max The central
3.1 Publishable summary This section must be of suitable quality to enable direct publication by the Commission. A summary description of project context and objectives, 4000 characters max The central
Brain Tumour Australia Information FACT SHEET 22 Clinical Trials: Questions and Answers
 FACT SHEET 22 Clinical Trials: Questions and Answers What is a clinical trial? Clinical trials are research studies that answer scientific questions and try to find better ways to prevent, screen for,
FACT SHEET 22 Clinical Trials: Questions and Answers What is a clinical trial? Clinical trials are research studies that answer scientific questions and try to find better ways to prevent, screen for,
SOP# version e1.0 Material Handling and Documentation TMA's from Paraffin Embedded Tissue Blocks
 CTRNet Standard Operating Procedure SOP Number: 8.3.010 Version e1.0 Supersedes: SR 001.001 Effective Date 09 Jan 08 Subject: TMA's from Paraffin Embedded Tissue Blocks Category Material Handling and Documentation
CTRNet Standard Operating Procedure SOP Number: 8.3.010 Version e1.0 Supersedes: SR 001.001 Effective Date 09 Jan 08 Subject: TMA's from Paraffin Embedded Tissue Blocks Category Material Handling and Documentation
Genitope Corporation. Summary of MyVax Personalized Immunotherapy Phase 3 Clinical Trial Results
 Genitope Corporation Summary of MyVax Personalized Immunotherapy Phase 3 Clinical Trial Results Introduction In December 27, Genitope Corporation ( Genitope ) obtained data indicating that its pivotal
Genitope Corporation Summary of MyVax Personalized Immunotherapy Phase 3 Clinical Trial Results Introduction In December 27, Genitope Corporation ( Genitope ) obtained data indicating that its pivotal
RAPID Phase 1 Deliverables Use Cases, Flow Diagrams
 RAPID Phase 1 Deliverables Use Cases, Flow Diagrams James E. Tcheng, MD Duke Clinical Research Institute Durham, NC RAPID Think Tank 14 September 2016 1 Use Cases: Background & Rationale Why Use Cases?
RAPID Phase 1 Deliverables Use Cases, Flow Diagrams James E. Tcheng, MD Duke Clinical Research Institute Durham, NC RAPID Think Tank 14 September 2016 1 Use Cases: Background & Rationale Why Use Cases?
CHANGES TO AATB STANDARDS FOR TISSUE BANKING, 14 TH EDITION Effective May 31, 2018 SECTION A GENERAL INFORMATION
 CHANGES TO AATB STANDARDS FOR TISSUE BANKING, 14 TH EDITION Effective May 31, 2018 SECTION A GENERAL INFORMATION QUARANTINE The identification of tissue as not suitable for transplantation, including tissue
CHANGES TO AATB STANDARDS FOR TISSUE BANKING, 14 TH EDITION Effective May 31, 2018 SECTION A GENERAL INFORMATION QUARANTINE The identification of tissue as not suitable for transplantation, including tissue
China Biopsy Devices Market Outlook to 2020
 China Biopsy Devices Market Outlook to 2020 Reference Code: GDMECC0645DB Publication Date: July 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2 List of Figures... 5
China Biopsy Devices Market Outlook to 2020 Reference Code: GDMECC0645DB Publication Date: July 2014 Page 1 1 Table of Contents 1 Table of Contents... 2 1.1 List of Tables... 4 1.2 List of Figures... 5
Part 1- Donor and Transplant Number Alignment
 Part 1- Donor and Transplant Number Alignment Alisha Mussetter Clinical Research Coordinator II Tiffany Hunt, MS, CCRP Clinical Research Coordinator II Wednesday February 17 th, 2016 How to participate
Part 1- Donor and Transplant Number Alignment Alisha Mussetter Clinical Research Coordinator II Tiffany Hunt, MS, CCRP Clinical Research Coordinator II Wednesday February 17 th, 2016 How to participate
>> Rehabilitation and Return to Work. >> Skills Assessment. >> Perception. >> Motor Coordination. >> Personnel Selection and Development
 Manual Dexterity >> Rehabilitation and Return to Work >> Skills Assessment >> Perception >> Motor Coordination >> Personnel Selection and Development PO Box 5729 Lafayette, IN 47903 -- Phone: (765) 423-1505
Manual Dexterity >> Rehabilitation and Return to Work >> Skills Assessment >> Perception >> Motor Coordination >> Personnel Selection and Development PO Box 5729 Lafayette, IN 47903 -- Phone: (765) 423-1505
HCCA 13 TH ANNUAL COMPLIANCE INSTITUTE LAS VEGAS, NV APRIL 29, 2009
 HCCA 13 TH ANNUAL COMPLIANCE INSTITUTE LAS VEGAS, NV APRIL 29, 2009 MEDICARE COVERAGE ANALYSIS WORKSHOP: THE HOW TO OF MEDICARE COVERAGE IN RESEARCH Suzanne LivPage, J.D. Director, Clinical Research Initiation
HCCA 13 TH ANNUAL COMPLIANCE INSTITUTE LAS VEGAS, NV APRIL 29, 2009 MEDICARE COVERAGE ANALYSIS WORKSHOP: THE HOW TO OF MEDICARE COVERAGE IN RESEARCH Suzanne LivPage, J.D. Director, Clinical Research Initiation
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 AWARD NUMBER: W81XWH-13-2-0059 TITLE: The Johns Hopkins RTR Consortium: A Collaborative Approach to Advance Translational Science and Standardize Clinical Monitoring of Restorative Transplantation PRINCIPAL
AWARD NUMBER: W81XWH-13-2-0059 TITLE: The Johns Hopkins RTR Consortium: A Collaborative Approach to Advance Translational Science and Standardize Clinical Monitoring of Restorative Transplantation PRINCIPAL
liquids. 3) Mist/wipe the work surface wiped with disinfectant before conducting surgery.
 Institutional Animal Care Program Title: RODENT SURVIVAL SURGERY Policy#: IACP 004 Date in Effect: 12/14/07 Rev #: 01 Rev Date: 12/14/12 Rev #: 02 Rev Date: 03/08/13 Rev #: 03 Rev Date: 12/13/13 Rev #:
Institutional Animal Care Program Title: RODENT SURVIVAL SURGERY Policy#: IACP 004 Date in Effect: 12/14/07 Rev #: 01 Rev Date: 12/14/12 Rev #: 02 Rev Date: 03/08/13 Rev #: 03 Rev Date: 12/13/13 Rev #:
Glaucoma Sets and Implants
 238 Glaucoma Sets and Implants 39 Duckworth & Kent Moorfields Safe Surgery System designed by P.T. Khaw From Duckworth & Kent, the range of instruments designed by Prof. Khaw at Moorfields Eye Hospital
238 Glaucoma Sets and Implants 39 Duckworth & Kent Moorfields Safe Surgery System designed by P.T. Khaw From Duckworth & Kent, the range of instruments designed by Prof. Khaw at Moorfields Eye Hospital
Spray-on stem cells for rapid healing.
 Spray-on stem cells for rapid healing. Grafting is Painful, Expensive and Leaves Scarring Inadequate Options for Burn Patients Skin Grafting is Current Standard-of-Care Sheets of meshed skin for surgical
Spray-on stem cells for rapid healing. Grafting is Painful, Expensive and Leaves Scarring Inadequate Options for Burn Patients Skin Grafting is Current Standard-of-Care Sheets of meshed skin for surgical
TITLE: Potential Therapeutic Use of Relaxin in Healing Cranial Bone Defects
 AWARD NUMBER: W81XWH-15-1-0162 TITLE: Potential Therapeutic Use of Relaxin in Healing Cranial Bone Defects PRINCIPAL INVESTIGATOR: Kirk P. Conrad CONTRACTING ORGANIZATION: University of Florida Gainesville
AWARD NUMBER: W81XWH-15-1-0162 TITLE: Potential Therapeutic Use of Relaxin in Healing Cranial Bone Defects PRINCIPAL INVESTIGATOR: Kirk P. Conrad CONTRACTING ORGANIZATION: University of Florida Gainesville
Attachment B: A Guideline for Writing a Clinical Protocol for CPRN
 Attachment B: A Guideline for Writing a Clinical Protocol for CPRN This document provides guidelines for protocol submission. It is only guidance, and the format in which you choose to present the information
Attachment B: A Guideline for Writing a Clinical Protocol for CPRN This document provides guidelines for protocol submission. It is only guidance, and the format in which you choose to present the information
FREQUENTLY ASKED QUESTIONS
 FREQUENTLY ASKED QUESTIONS Table of Contents GORE ACUSEAL Vascular Graft Properties What is the GORE ACUSEAL Vascular Graft?...2 What is unique about the GORE ACUSEAL Vascular Graft?...3 What are the
FREQUENTLY ASKED QUESTIONS Table of Contents GORE ACUSEAL Vascular Graft Properties What is the GORE ACUSEAL Vascular Graft?...2 What is unique about the GORE ACUSEAL Vascular Graft?...3 What are the
3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description
 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing ( Securement System ) is designed
3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing Product Description 3M Tegaderm PICC/CVC Securement Device + I.V. Advanced Securement Dressing ( Securement System ) is designed
LITe LIF Less invasive posterior lumbar interbody fusion procedure
 LITe LIF Less invasive posterior lumbar interbody fusion procedure LITe LIF Access LITe Midline Retractor Fixation Xia CT Interbody Tritanium PL Cage Instruments LITe Decompression 2.0 Power CD3 and RemB
LITe LIF Less invasive posterior lumbar interbody fusion procedure LITe LIF Access LITe Midline Retractor Fixation Xia CT Interbody Tritanium PL Cage Instruments LITe Decompression 2.0 Power CD3 and RemB
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company ASCRS April 12, 2018 NASDAQ: EYPT
 psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ ASCRS April 12, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
psivida Transforms into Commercial Stage Specialty BioPharmaceutical Company OIS @ ASCRS April 12, 2018 NASDAQ: EYPT Forward Looking SAFE HARBOR STATEMENTS UNDER THE PRIVATE SECURITIES LITIGATION REFORM
FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies
 FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies Executive Summary, June 2015 Life Sciences Practice Group AUTHOR Areta L. Kupchyk Nixon Peabody LLP Washington,
FDA s Same Surgical Procedure Draft Guidance Could Stifle Use of Autologous Stem Cell Therapies Executive Summary, June 2015 Life Sciences Practice Group AUTHOR Areta L. Kupchyk Nixon Peabody LLP Washington,
Part 7: Clinical investigations
 Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11979-7 Third edition 2014-09-01 Ophthalmic implants Intraocular lenses Part 7: Clinical investigations Implants ophtalmiques Lentilles intraoculaires
Provläsningsexemplar / Preview INTERNATIONAL STANDARD ISO 11979-7 Third edition 2014-09-01 Ophthalmic implants Intraocular lenses Part 7: Clinical investigations Implants ophtalmiques Lentilles intraoculaires
Investor Presentation. Second Quarter 2017
 Investor Presentation Second Quarter 2017 1 Company Overview Imagin Medical... a medical imaging company with advanced optic and light sensor technology that will dramatically improve physicians ability
Investor Presentation Second Quarter 2017 1 Company Overview Imagin Medical... a medical imaging company with advanced optic and light sensor technology that will dramatically improve physicians ability
Kansas Pharmacy Act Amendments; Filling and Refilling Prescriptions; Biological Products; Senate Sub. for HB 2055
 Kansas Pharmacy Act Amendments; Filling and Refilling Prescriptions; Biological Products; Senate Sub. for HB 2055 (Act). Senate Sub. for HB 2055 makes several amendments to the Kansas Pharmacy Act The
Kansas Pharmacy Act Amendments; Filling and Refilling Prescriptions; Biological Products; Senate Sub. for HB 2055 (Act). Senate Sub. for HB 2055 makes several amendments to the Kansas Pharmacy Act The
DMC member experience: studies with adaptive designs. P.Bauer Medical University of Vienna December 2007
 DMC member experience: studies with adaptive designs P.Bauer Medical University of Vienna December 2007 A typical application: Dose selection and confirmative inference (the critical issue of combining
DMC member experience: studies with adaptive designs P.Bauer Medical University of Vienna December 2007 A typical application: Dose selection and confirmative inference (the critical issue of combining
SNC2D BIOLOGY 3/31/2013. TISSUES, ORGANS & SYSTEMS OF L Stem Cells & Meristematic Cells (P.40-41) Specialized Cells. Stem Cells
 SNC2D BIOLOGY TISSUES, ORGANS & SYSTEMS OF L & Meristematic Cells (P.40-41) Specialized Cells The cell theory states that all cells come from pre-existing cells. Every cell that makes up an animal s body
SNC2D BIOLOGY TISSUES, ORGANS & SYSTEMS OF L & Meristematic Cells (P.40-41) Specialized Cells The cell theory states that all cells come from pre-existing cells. Every cell that makes up an animal s body
Supplementary material
 Supplementary material Tracking mesenchymal stem cell contributions to regeneration in an immunocompetent cartilage regeneration model Daniela Zwolanek, María Satué, Verena Proell, Josè R. Godoy, Kathrin
Supplementary material Tracking mesenchymal stem cell contributions to regeneration in an immunocompetent cartilage regeneration model Daniela Zwolanek, María Satué, Verena Proell, Josè R. Godoy, Kathrin
Bacterial Adherence to Barbed Monofilament Suture in a Contaminated Wound Model
 Bacterial Adherence to Barbed Monofilament Suture in a Contaminated Wound Model John R Fowler, MD Tiffany A Perkins, BS Bettina A Buttaro, PhD Allan L Truant, PhD Joseph S Torg, MD Disclosures Funding
Bacterial Adherence to Barbed Monofilament Suture in a Contaminated Wound Model John R Fowler, MD Tiffany A Perkins, BS Bettina A Buttaro, PhD Allan L Truant, PhD Joseph S Torg, MD Disclosures Funding
Chapter XIII. USRDS Research Studies. Appreciation Of Renal Community Participation. Ongoing USRDS Special Studies
 Chapter XIII T he USRDS conducts two different kinds of studies: Existing Data Studies and Special Studies. Existing Data Studies analyze data that already have been collected; these studies will be discussed
Chapter XIII T he USRDS conducts two different kinds of studies: Existing Data Studies and Special Studies. Existing Data Studies analyze data that already have been collected; these studies will be discussed
January (San Francisco, CA) January 8, 2018
 January 2017 J.P. Morgan 36 th Annual Management Healthcare Presentation Conference (San Francisco, CA) January 8, 2018 DISCLAIMER Certain information contained in this presentation relates to or is based
January 2017 J.P. Morgan 36 th Annual Management Healthcare Presentation Conference (San Francisco, CA) January 8, 2018 DISCLAIMER Certain information contained in this presentation relates to or is based
EDWIN G. BUSS SECRETARY
 EDWIN G. BUSS SECRETARY PROCEDURE NUMBER: 604.606 PROCEDURE TITLE: IDENTIFICATION CARDS RESPONSIBLE AUTHORITY: OFFICE OF INSTITUTIONS ISSUE DATE: JULY 27, 2011 INITIAL ISSUE DATE: MAY 24, 2000 SUPERSEDES:
EDWIN G. BUSS SECRETARY PROCEDURE NUMBER: 604.606 PROCEDURE TITLE: IDENTIFICATION CARDS RESPONSIBLE AUTHORITY: OFFICE OF INSTITUTIONS ISSUE DATE: JULY 27, 2011 INITIAL ISSUE DATE: MAY 24, 2000 SUPERSEDES:
Agenzia Italiana del Farmaco
 Agenzia Italiana del Farmaco European Regulation on Advanced Therapies Cristina Pintus Head of European Relations Unit and Coordinator of the Advanced Therapy Project Italian Medicines Agency Proposal
Agenzia Italiana del Farmaco European Regulation on Advanced Therapies Cristina Pintus Head of European Relations Unit and Coordinator of the Advanced Therapy Project Italian Medicines Agency Proposal
John Gurdon was testing the hypothesis of genomic equivalence or that when cells divide they retain a full genomic compliment.
 1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
Life Sustaining Bone Marrow Stem Cells & Cancellous Bone Graft
 Life Sustaining Bone Marrow Stem Cells & Cancellous Bone Graft Bone Marrow Cells (BMC) reside deep inside bone cavities in the most protected part of the body and are redundant throughout the organism.
Life Sustaining Bone Marrow Stem Cells & Cancellous Bone Graft Bone Marrow Cells (BMC) reside deep inside bone cavities in the most protected part of the body and are redundant throughout the organism.
The purpose of these guidelines is to describe a standardized approach for the management of
 ASHP Guidelines for the Management of Investigational Drug Products Purpose The purpose of these guidelines is to describe a standardized approach for the management of investigational drug products by
ASHP Guidelines for the Management of Investigational Drug Products Purpose The purpose of these guidelines is to describe a standardized approach for the management of investigational drug products by
Chapter 2 Basic Equipment for Microsurgical Experiment
 Chapter 2 Basic Equipment for Microsurgical Experiment Peter Balaz Abstract Unlike of human organ transplantation, the most of the experimental procedures in small animal are performed under magnification
Chapter 2 Basic Equipment for Microsurgical Experiment Peter Balaz Abstract Unlike of human organ transplantation, the most of the experimental procedures in small animal are performed under magnification
The Journal of Thoracic and Cardiovascular Surgery
 Accepted Manuscript Commentary: Failing Grades Jacob A. Klapper, MD PII: S0022-5223(19)30711-1 DOI: https://doi.org/10.1016/j.jtcvs.2019.03.037 Reference: YMTC 14310 To appear in: The Journal of Thoracic
Accepted Manuscript Commentary: Failing Grades Jacob A. Klapper, MD PII: S0022-5223(19)30711-1 DOI: https://doi.org/10.1016/j.jtcvs.2019.03.037 Reference: YMTC 14310 To appear in: The Journal of Thoracic
2 Cruise Design. April 15, 2015 Amendment No
 Timber Pricing Branch Quality Assurance 2 Cruise Design April 15, 2015 Amendment No. 3 2-1 Cruising Manual Ministry of Forests, Lands and NRO 2.1 Cruise Objective The objective of the timber cruise is
Timber Pricing Branch Quality Assurance 2 Cruise Design April 15, 2015 Amendment No. 3 2-1 Cruising Manual Ministry of Forests, Lands and NRO 2.1 Cruise Objective The objective of the timber cruise is
Innovative Integration of Strategic Planning, Benchmarking, and Assessment
 Banking the Past--Investing the Future Innovative Integration of Strategic Planning, Benchmarking, and Assessment Robert L. Armacost Director, University Analysis and Planning Support Julia J. Pet-Armacost
Banking the Past--Investing the Future Innovative Integration of Strategic Planning, Benchmarking, and Assessment Robert L. Armacost Director, University Analysis and Planning Support Julia J. Pet-Armacost
ATTACHMENT 4: PROCEDURE FOR SOIL FERTILITY EXPERIMENT ON MICRO-DOSING
 ATTACHENT 4: PROCEDURE FOR SOIL FERTILITY EXPERIENT ON ICRO-DOSING DETERINING OPTIAL IXTURE OF ORGANIC AND INORGANIC FERTILIZERS FOR SOIL FERTILITY AND PLANT RESPONSE BACKGROUND Context: In south-central
ATTACHENT 4: PROCEDURE FOR SOIL FERTILITY EXPERIENT ON ICRO-DOSING DETERINING OPTIAL IXTURE OF ORGANIC AND INORGANIC FERTILIZERS FOR SOIL FERTILITY AND PLANT RESPONSE BACKGROUND Context: In south-central
Use of Quantitative Scanning Electron Microscopy of S. aureus Biofilm Formation in vitro to Identify Strain and Implant Material Specificities
 Use of Quantitative Scanning Electron Microscopy of S. aureus Biofilm Formation in vitro to Identify Strain and Implant Material Specificities Werasak Sutipornpalangkul, MD, PhD 1, Kohei Nishitani, MD,
Use of Quantitative Scanning Electron Microscopy of S. aureus Biofilm Formation in vitro to Identify Strain and Implant Material Specificities Werasak Sutipornpalangkul, MD, PhD 1, Kohei Nishitani, MD,
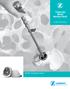
Marrow Cellution. Autologous Bone Marrow Aspiration & Cancellous Bone Graft Harvesting. aspire-medical.eu
 Marrow Cellution Autologous Bone Marrow Aspiration & Cancellous Bone Graft Harvesting aspire-medical.eu Aspire Medical Innovation Einsteinstr. 167 D-81677 München Traditional Trocar Design & Technique
Marrow Cellution Autologous Bone Marrow Aspiration & Cancellous Bone Graft Harvesting aspire-medical.eu Aspire Medical Innovation Einsteinstr. 167 D-81677 München Traditional Trocar Design & Technique
