LATEST RESEARCH IN ALZHEIMER S DISEASE THERAPIES
|
|
|
- Laurence Perkins
- 6 years ago
- Views:
Transcription
1 LATEST RESEARCH IN ALZHEIMER S DISEASE THERAPIES Associate Professor Michael Woodward Austin Health Alzheimer s Australia national conference Brisbane May
2 Third generation immunotherapy Beyond active immunization and current mabs Agadjanyan s group have developed epitope vaccines that add a foreign T-cell interacting sequence to the Aβ fragmentcontaining antigen so there is no induction of the unwanted human T cell response that causes meningoencephalitis His group prefers DNA rather than protein vaccines Requires additional efforts need to ensure adequate immunogenicity include placing an adjuvant patch, using an electrical field over the vaccination site, gene gun therapy and following the DNA vaccine by a protein vaccine His third generation immunotherapy has been taken up by Lundbeck and is being trialled in monkeys Aβ 1-24 is the fragment that is being utilized- the vaccine is called VAR 24. 2
3 DUALITY OF INTEREST Member of Alzheimer s Advisory Boards for Novartis, Pfizer, Janssen-Cilag, Lundbeck Paid honorarium for these Financial support from several companies to attend and report on international conferences on dementia Honorarium from several companies for speaking Dementia research Hospital paid for Principal Investigator duties for several trials 3
4 Relative Prevalence of Dementia Australia Rate per 100,000 population PREVALENCE Multiple Sclerosis Stroke Dementia Parkinson's Disease 4 [AIHW & Other Sources]
5 OUTLINE Latest research impacting on therapies New symptomatic therapies Disease-modifying approaches What the future holds 5
6 LATEST RESEARCH Impact on therapies Improved understanding of the amyloid cascade Better biomarkers and understanding of their significance AD as a continuum- a risk state 6
7 Potential Molecular Targets for AD Therapies Amyloid Plaque Inflammation Oxidative stress Neuronal cell death Ab Fibrils Ab Oligomers Acutely Neurotoxic Ab40 Accumulates Amyloid Precursor Protein (APP) Beta-Secretase Mitochondrial Dysfunction Soluble Ab40 Gamma-Secretase Complex Nucleus 7
8 The amyloid cascade The peptide Aβ is the initiator of AD Why it is produced in pathogenic amounts in some older individuals remains unclear Probably has direct effects on neurones and on memory Esp the oligomers (small aggregates of Aβ) Also increases tau production/pathology Hyperphosphorylated/misfolded/truncated tau recruits normal tau to create toxic fibrils and then tangles This tau is also toxic to mitochondria Most new disease-modifying approaches target the amyloid cascade If they fail, it will place the importance of the cascade under severe stress 8
9 Biomarkers Increasingly essential to diagnosis of early stages of AD The earliest changes reflect Aβ pathology CSF and plasma Aβ Amyloid imaging Later biomarkers can detect neurodegeneration MRI atrophy CSF (and possibly plasma) tau Newer ones being developed Proteomics, genetic, other 9
10 CSF Aβ42 Amyloid imaging CSF tau MRI hipp FDG-PET Cog Function P. Aisen, UCSD
11 IS MCI EARLY AD? Almost all with specific episodic memory disorder and one other phenotypic feature progress to AD So (this subtype of) MCI is really early AD We need to see AD as a continuum, not a discrete sudden- onset illness Aβ deposits years before subject fulfils current clinical criteria for AD Dubois et al 1 criteria for AD include much of what we currently call MCI Cued recall deficit One or more of Hippocampal atrophy FDG or PIB PET abnormality Biomarker positive (eg raised CSF p-tau) Known AD gene or strong family history Note- not prodromal/early AD if no biomarker Problematic if poorer country Dubois et al. Lancet Neurology 2007; 6:
12 Presymptomatic AD Stage one- asymptomatic cerebral amyloidosis PIB-PET positive CSF Aβ reduced Stage two- amyloidosis plus evidence of neurodegeneration MRI atrophy or elevated tau Stage three- the above plus subjective memory complaint May have abnormality on sophisticated neuropsychological testing- eg Cog State 12
13 Disease modifying trials- endpoints Most early MCI trials failed Too few conversions to AD New criteria for prodromal AD as a continuum to AD allow new measures of disease modification eg biomarker modification Likely that new criteria will select those that have more conversion annually to AD Around 20-25%/year 13
14 Rx OF amci/ PRODROMAL AD Huge effort Target of many current/planned trials BMS gamma-secretase inhibitor trial Roche mab trial both here in Australia No drug demonstrated beneficial as yet in earlier trials Trial of donepezil showed no effect at 3 years; partial benefit at 18 months Cognitive training may be useful Prevention even more relevant (diet, depression, diabetes and control of vascular risk factors) Recent BMJ 1 and NIH reviews AD disease modifying treatment may be used here 1.BMJ 2010; 341:c
15 New symptomatic therapies 5-HT 6 receptor antagonists Improved cognition in preclinical studies Two under trial here Histamine-3 receptor agonists Several under trial, here Partial nicotine α4b2 agonist, ABT-089 Phase II trial terminated due to futility Probably the end to nicotinic receptor approach New selective M 1 and M 5 agonists/allosteric activators being developed should avoid SLUDGE Newer disease- modifying agents will likely also be symptomatic therapies 15
16 Disease Modification Targeting amyloid Targeting tau Combined approaches Others Mitochondria 16
17 STATUS OF INVESTIGATIONAL DISEASE-MODIFYING TREATMENTS FOR AD IMMUNOTHERAPY Passive immunisation Active immunisation SECRETASE INHIBITORS Gamma secretase inhibitors Beta secretase inhibitors Solanezumab (Lilly) Bapineuzumab (Elan Wyeth) Ponezumab (Pfizer) Ganteneruzumab (Roche) Intravenous immunoglobulin (Baxter) ACC-001 (Elan Wyeth) CAD-106 (Novartis) Semagacestat (Lilly) MK-0752 (Merck) BMS KMI-429 Phase II Phase III Phase III Phase II (pro AD) Phase III Phase I Phase II Phase III (negative) Phase II Phase II (pro/early AD) early Phase ANTI-AGGREGATION AGENTS Tramiprosate (Neurochem) Curcumin (John Douglas) PBT-2 (Prana) Phase II (negative) Phase II Phase III (planned) 17
18 OTHER DISEASE-MODIFYING APPROACHES (Mostly targeting amyloid) SELECTIVE AMYLOID- LOWERING AGENTS Tarenflurbil (Myriad) Posiphen Phase III (negative) Phase I PPAR - AGONISTS Rosiglitazone (GSK) Phase II (negative) RAGE LIGAND INHIBITORS Several (eg Pfizer) Phase I PHOPHODIESTERASE (PDE9A) INHIBITORS STATINS (several mechanisms) PF (Pfizer) Atorvastatin (Pfizer) Phase I Phase II (negative) DUAL AChEI/AMYLOID PRODUCTION REDUCER Phenserine (Axonyx) Phase II (negative) 18
19 OTHER DISEASE-MODIFYING THERAPIES NEURORESTORATIVE NGF & RELATED THERAPIES Cerebrolysin Gene therapy TRANSPLANTATION / INFUSION Genetically engineered cells expressing NGF Stem cells NEUROTROPHIC FACTOR ENHANCER Xaliproden (negative) 19
20 Newer gamma secretase inhibitors Semagacestat failed Greater decline in group receiving active drug Also toxicity- skin cancer, rash, gastrointestinal BMS Phase II trial CSF Aβ 42 decreased Safe, well tolerated so far Soon to be trialled here Both in AD and MCI/prodromal AD 20
21 Advances in secretase therapies The 3 APP secretases have been the target of a huge drug development program But the recent failure of semagacestat, a γ secretase inhibitor is very concerning It is surprising that these have become a target as in sporadic AD there is only a very slight increase in Aβ production the main problem is oligomeric toxicity, and reduced Aβ clearance. 21
22 Alpha secreatase therapies The good secretase that prevents Aβ production When ADAM-10, an α secretase, is overexpressed in APP Tg mice, they no longer deposit excess amyloid Retinoic acid, and various related retinoids, activate ADAM- 10 provides a therapeutic option using a drug that is already marketed Acitretin, one retinoid, has reached Phase II trial stage in Germany In Osaka tamibarotene, a retinoic acid receptor activator, is also being evaluated Intriguingly, caloric restriction, which prevents amyloid toxicity in animal models, induces ADAM-10 activity, possibly by an epigenetic mechanism. 22
23 Β Secretase inhibition For many years it was thought that it was impossible to make an inhibitor of this very large protein complex any drug able to block the catalytic sites would be too large to cross the Blood Brain Barrier (BBB) Encouragingly, BACE knock out (KO) mice have a normal phenotype Amyloidogenic Tg mice with BACE KO also do not form excess amyloid, showing the benefit of reducing β-secretase activity. LY , a non-peptide BACE inhibitor developed by Lily and the Clinical Research Organization Parexel, has undergone preclinical trials and certainly looked promising binds to several catalytic pockets of the BACE-1 enzyme, and has good BBB and cellular penetration in mice and beagles it reduces Aβ deposition Phase I trials showed a reduction in plasma Aβ42 and good pharmacokinetics Continuous (36hr) CSF catheterization in healthy volunteers also showed reduced central Aβ42 levels Preliminary safety efficacy was also good.but changes in animal retinal epithelium were seen an off mechanism effect results with the drug provide a proof of concept but due to this retinal effect, development has been halted. 23
24 SECOND GENERATION IMMUNOTHERAPY Several approaches Aβ fragments Monoclonal antibodies Over 10 companies developing these Should avoid the T-cell response that led to the unwanted inflammation Aβ fragments could be delivered mucosally Effective antibody response in mice when given weekly this way Also an oral vaccine developed Anti- Aβ DNA vaccination also promising Olgomeric targetting Human IgG 24
25 Aβ Fragment Immunotherapy Could be last shot at active immunotherapy Novartis Phase I CAD 106 vaccination. Aβ 1-6 fragment inserted into a biosphere to increase immunogenicity doesn t stimulate full Aβ-reactive T cells. 2 small cohorts tested, using 50 and 150 microgram doses, with MMSE Each of the subjects received 3 injections followed for 12 months clinical, MRI and CSF endpoints. Eighteen of 22 actively treated patients developed detectable antibodies No serious adverse events. Cognitive endpoints negative Other endpoints not yet reported 25
26 Aβ Monoclonal Antibody Programs Solanezumab Targets AA 16-24; IgG 1 Bapineuzumab Targets AA 1-5; IgG 1 Ponezumab Targets AA 33-40; IgG 2 Da N-Terminus C-Terminus Ganteneruzumab targets Aβ aggregates Sources: Cowen and Company Dec 17, 2007; Siemers et al, International Conference on Alzheimer s disease; Madrid, Spain 2006; Natixis Bleichroeder; 26
27 Solanezumab Mid-terminus mab does cross the BBB Aβ fragments, which are normally only found in the brain, appear in the plasma by day 80, in a dose-dependent manner. Phase II trial in progress, including here 27
28 Ponezumab 2 Phase I single dose studies presented at ICAD 2010 (one 10 min infusion, one 2hr) Pharmacokinetics favourable CSF Aβ increased No effects on CSF tau No safety issues No Vasogenic Oedema reported yet (including ongoing Phase II study) 28
29 Bapineuzumab Wyeth / Elan N-terminus monoclonal Ab Phase II results N = 234 Mean baseline MMSE = 20 Mean age endpoints (ADAS-Cog & DAD) did not significantly improve But moved in expected direction Did achieve significance in Apo E 4 non-carriers, especially for completers ADAS-Cog = 7.3 In CSF, only p-tau affected (not A 42 ) Less brain volume loss 29
30 Bapineuzumab- results, all patients 30
31 Bapineuzumab- Apo E 4 non-carrier results 31
32 Bapineuzumab- MRI results 32
33 Bapineuzumab - Safety Vasogenic oedema in 9.7% (N = 12) Mostly in higher dose cohort Usually just MRI finding One had lethargy and confusion ICAD 2010 presented theory that may be due to blockage of perivascular brain interstitial fluid drainage 3 deaths (all on active treatment) Not considered drug-related Largest ever AD Phase III trial program in progress (N=4,000) Stratified for Apo E 4 (actually as 2 separate trials) Using lower doses than in the Phase II, to reduce adverse events 33
34 Bapineuzumab-results from 3D6 3D6 is the murine equivalent of bapineuzumab Not only does the N-terminus binding 3D6 act against the amyloid plaques, it also is active against Aβ oligomers these are the entities that are most toxic in AD a C-terminus equivalent mab had no such effect on oligomers interesting as the same company that has an interest in bapineuzumab, Pfizer, is also is developing a C-terminus mab, ponuzemab D6 also blocks oligomeric Aβ-induced tau phosphorylation In Tg mice, 3D6 increased synapse formation and reversed memory/behavioural deficits If bapineuzumab proves to be effective, it may well be due to this effect on oligomeric Aβ rather than on the monomeric or plaque form.
35 Monoclonal Ab s from Reverse Translational Medicine From sera of a cohort of individuals with mild cognitive impairment (MCI) that had improved on cognitive testing over time Hock s Geneva team isolated anti Aβ antibodies selected ones that bound to aggregated Aβ using RTM created monoclonal Abs in transgenic (Tg) mice (genetically changed to overexpress Aβ) these human mabs reduced Aβ levels by 50% through activation of microglia seem to be acting against a conformational epitope of these Aβ aggregates rather than a linear sequence on the monomers most encouragingly, there was increased dendritic branching and neuronal connectivity, along with neurogenesis. memory also improved Human trials with these unique human mabs planned.
36 Aβ oligomeric immunotherapy Arctic APP mutation is not associated with amyloid plaque formation This mid- APP mutation is, instead, associated with increased Aβ protofibril formation and it does cause AD Lars Lannfelt, from Uppsala, has developed a humanized mab against these Arctic mutation protofibrils Lisensed to Eisai in 2007 after demonstrated efficacy in mouse models Phase I study of BAN 2401 completed A Phase II study is planned Biomarker endpoints will include MRI volumetrics and CSF Gives us a fall-back option if current mab trials, directed largely against Aβ monomers and amyloid plaques, fail.
37 Human IgG Contains anti-a Abs Given IV Original trial positive Nine month data (poster at ICAD 2008) Results: Improvement in ADAS-Cog Only significant at 9 months Phase III trial in progress 37
38 Scylloinositol Binds to the c-terminus of Aβ This induces phagocytosis Numerous beneficial effects in Tg mice reduced cortical Aβ reduced CSF Aβ Improved memory Seems to induce many genes related to Aβ degradation An amyloid imaging ligand is also being developed, based on this product 38
39 Attacking tangles tau protein..but- tau is downstream from amyloid 39
40 Tau- directed therapies Recent discoveries have moved the tau-ists more towards centre-field eg the finding that endogenous tau is necessary for Aβ- induced neuronal, synaptic and cognitive impairment reducing tau production reduces learning deficits in APP Tg mice (overexpressing amyloid), without affecting amyloid deposition- just making the amyloid much less toxic. This neuroprotective effect of tau depletion seems to be Aβ-dependent as tau reduction is not neuroprotective in other models of neurotoxicity Aβ may be causing a toxic gain of function of tau, or at least permitting tau to become toxic the truncated and hyperphosphorylated forms of tau seem to be the toxic entity Therapeutic approaches suggested by this understanding of the interdependence of Aβ and tau include: directly inhibiting tau reducing tau expression inhibiting tau hyperphosphorylation Increased tau acetylation seems to be associated with tau hyperphosphorylation, so another therapeutic approach could be to use acetylation inhibitors inhibiting tau aggregation stabilizing microtubules in a tau-independent way. 40
41 Rember Leuko-methylthiominium chloride (methylene blue) Inhibitor of tau aggregation N = 321 T = 84 weeks Primary ADAS-Cog 5 significant CDR also significant 41
42 Rember Neuroimaging studies SPECT in 125 No decline in 60mg group Placebo did decline FDG PET in 19 Impressive results 42
43 Rember Safety diarrhoea (poorly absorbed) UTI / urgency / dysuria Phase III to commence By 2050, 600 million will be BRAAK II ( tau staging) or above Succor for the tau-ists But baptists not yet in retreat We may need both pathologies targeted 43
44 OTHER ANTI-TAU THERAPIES Selenium Promotes tau dephosphorylation Phosphorylation inhibitors Anti-tau immunotherapy Microtubule-binding region of tau Phospho-tau itself ie active tau immunotherapy Truncated tau seems most toxic need to develop drugs targeting this only in animal models so far, but promising Aβ immunotherapy also lowers CSF tau May be useful for other tau-relayed dementias, such as frontotemporal lobar degeneration 44
45 Other disease-modifying Dimebon letredipine targets mitochondria Metal binding agents PBT-2 Bacterial phages Nerve growth factors Stem cell therapies Anti- TNF therapy approaches 45
46 Dimebon (letredipine) N = 183, RCT (2 arms) Russian sites Not on AChEI T = 26 weeks 1 0 outcome result (one only) ADAS-Cog positive 4.0 (p=<0.0001) Well tolerated 46
47 ADAS-Cog 47
48 Dimebon Two large phase III trials One ongoing (add-on to AChEI) CONCERT trial Worldwide, including here Recent monotherapy trial (CONNECT) negative No decline in placebo group Probably has implications for add-on trial But only 6 month New mechanisms of dimebon effects on mitochondria presented at ICAD
49 PBT-2 ( son of chlioquinol ) Binds zinc and copper Required for Aβ aggregation and synaptic function Phase IIa trial Sweden, Australia N = 78 T = 12 weeks 3 arms (placebo, 50mg, 250mg) Mean age = 72 Mean MMSE = 23 49
50 PBT-2 Results Non significant for cognition significant for 2 executive subscales for 250mg dose Reduced CSF A 42 (250mg, p=0.006) but? significance Phase IIb/III to begin soon will have PIB (amyloid) PET as primary endpoint 50
51 PDE9A Inhibition Modulate cgmp and camp signalling affect gene expression that impact on memory First In Humans study reported at ICAD 2010 Only safety and PK presented Safe, well tolerated Half life hr 51
52 Other therapies- Human Nerve Growth Factor Phase I trial completed 8 patients with mild AD Intracerebral injections of their own primary fibroblasts genetically modified to make NGF into the region of basal forebrain cholinergic neurons Mean MMSE decline = 3.0 ± 1.0 points per yr ( 51%); no AEs Tuszynski et al. (2005). Nature Medicine; 11:
53 Other NGF approaches Direct CSF infusion caused axial pain and neuraesthenia/ weight loss New approach uses capsule containing human retinal epithelial cells transfected with human NGF gene Phase Ib study implanting into both medial basal forebrains (N= 6 only) Safe (no pain or wt loss) Cog function improved in 2 of the 6 53
54 Other therapies-continued Anti-TNF monoclonal antibodies Unblinded single-centre study with 12 subjects 1 6 month endpoint Etanercept infused perispinally weekly Need to invert subject Marked improvement in all cognitive endpoints esp letter fluency (in FAS) 2 aphasic subjects began talking within minutes lots of publicity RCT said to begin soon Trial of a different new generation antiinflammatory agent planned for here soon 1. Tobinick et al. BMC Neurology 2008; 8: 27 54
55 Other therapies Many presented at ICAD 2010 Phenolics (myricetin (red wine), curcumin, rosemary) All inhibit Aβ fibril formation and destabilize formed fibrils Also antioxidative and anti-inflammatory effects Tideglusib (GSK 3 inhibitor) Effects on both Aβ and tau in mice Phase I studies completed 7% had ALT elevations also seen in phase II studies Shen-Wu (ginseng+) Mitochondrial effects Lipoic acid Mitochondrial antioxidant Only animal studies to date 55
56 Epigenetics- another target for therapies Regulation of genes is through epigenetic mechanisms Gene expression necessary for the production of hippocampal synapses in memory formation Largely occurs through acetylation and deacetylation of histone proteins that condense the DNA Two opposing enzymes- histone acetyl transferase (HAT) and histone deacetylase (HDAC) Inhibiting the latter is beneficial for learning and memory in a mouse model of dementia and neurodegeneration (CK-p25), even after a year of neurodegeneration the use of a HDAC inhibitor restored learning- a remarkable observation. Suggests numerous new targets for AD therapies 56
57 Recently failed therapies Eight in a row presented at ICAD 2009 (Vienna) Tarenflurbil Initially developed as anti-inflammatory All anti-inflammatory therapies have failed May still have a role in prevention Also anti-amyloid (modulates γ secretase) Both placebo and active groups declined Alzhemed Large phase III trial showed insignificant benefits A glycosaminoglycan (GAG) mimetic Binds to Aβ, leading to disaggregation Atorvastatin Atorvastatin add-on to donepezil LEADe trial Insignificant ADAS-Cog effect Tamoxifen / Raloxifen ( Co Star ) 57
58 Reasons for failed trials wrong targets too simplistic an approach to AD therapies we may need to hit several targets simultaneously wrong trial designs wrong patients wrong endpoints we use those for symptomatic AD whereas many new trials are for disease modification
59 Trial endpoints Need better endpoints Group working with FDA developing a better CIBIC- like tool Probably will incorporate Goal Attainment Scaling- like dimension PROCOG developed for MCI/prodromal AD NTB also favoured by European Medicines Authority Fascinating presentation on the challenges of multinational trials at ICAD stage command has 42 syllables in Japanese cf 26 in English no ifs ands or buts can be very challenging. 59
60 Conclusions Both exciting and disappointing times 25 years after original tacrine report, we still only have 4 modestly effective symptomatic therapies not for want of trying! Likely that we will have a disease-modifying therapy within 5-10 years will be expensive probably most effective if used earlier (prodromal AD) add-on to symptomatic therapies possibly used sequentially, or in combination toxicity may be a limiting factor Most advanced are monoclonal antibodies, IgG and gammasecretase inhibitors Still very few advances in other dementias We must better recruit into trials for the next generation if not for the person with dementia clinicians should support research into trial methodology and recruitment, and create model centres of recruitment 60
AD/PD Conference, Nice, Fr, 2015
 AD/PD Conference, Nice, Fr, 2015 Overview, novelties and conclusions for domestic research NAP B MTA TTK MS Neuroproteomics Group Overlaping molecular mechanisms of miss-folded protein based neurodegenerative
AD/PD Conference, Nice, Fr, 2015 Overview, novelties and conclusions for domestic research NAP B MTA TTK MS Neuroproteomics Group Overlaping molecular mechanisms of miss-folded protein based neurodegenerative
Gunilla Osswald, PhD, CEO RedEye CNS Event, October 4, 2018
 BioArctic AB Company presentation Gunilla Osswald, PhD, CEO RedEye CNS Event, October 4, 2018 Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic ) solely for
BioArctic AB Company presentation Gunilla Osswald, PhD, CEO RedEye CNS Event, October 4, 2018 Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic ) solely for
Innovative therapies for Alzheimer s disease
 WHITE PAPER IMS DISEASE INSIGHTS Innovative therapies for Alzheimer s disease New treatments will drive significant market value growth over the next ten years Author: Peter Alfinito, Ph.D., lead AnAlyst,
WHITE PAPER IMS DISEASE INSIGHTS Innovative therapies for Alzheimer s disease New treatments will drive significant market value growth over the next ten years Author: Peter Alfinito, Ph.D., lead AnAlyst,
New family of Alzheimer s disease-modifying agents that hit multiple biological targets
 New family of Alzheimer s disease-modifying agents that hit multiple biological targets Barcelona, 4 de julio de 2013 Content 1. The Research Group 2. The Product a) Target Indications b) Innovative mechanisms
New family of Alzheimer s disease-modifying agents that hit multiple biological targets Barcelona, 4 de julio de 2013 Content 1. The Research Group 2. The Product a) Target Indications b) Innovative mechanisms
Bapineuzumab Phase 3 trials in mild to moderate Alzheimer s disease dementia in apolipoprotein E e4 carriers (Study 302) and non-carriers (Study 301)
 Phase 3 trials in mild to moderate Alzheimer s disease dementia in apolipoprotein E e4 carriers (Study 302) and non-carriers (Study 301) Safety and PiB PET Amyloid Imaging Sperling R, Salloway S, Raskind
Phase 3 trials in mild to moderate Alzheimer s disease dementia in apolipoprotein E e4 carriers (Study 302) and non-carriers (Study 301) Safety and PiB PET Amyloid Imaging Sperling R, Salloway S, Raskind
BioArctic AB Interim Report January - June Gunilla Osswald, CEO Jan Mattsson, CFO August 23, 2018
 BioArctic AB Interim Report January - June 2018 Gunilla Osswald, CEO Jan Mattsson, CFO August 23, 2018 Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic ) solely
BioArctic AB Interim Report January - June 2018 Gunilla Osswald, CEO Jan Mattsson, CFO August 23, 2018 Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic ) solely
BioArctic. Gunilla Osswald, PhD, CEO December 4, 2017 Lars Lannfelt, Professor, MD, Senior Advisor and Co-Founder
 BioArctic Gunilla Osswald, PhD, CEO December 4, 2017 Lars Lannfelt, Professor, MD, Senior Advisor and Co-Founder Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic
BioArctic Gunilla Osswald, PhD, CEO December 4, 2017 Lars Lannfelt, Professor, MD, Senior Advisor and Co-Founder Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic
Alzheimer's disease immunization strategies : a review of current research
 The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Alzheimer's disease immunization strategies : a review of current research Laura Ona Sirgedas The University
The University of Toledo The University of Toledo Digital Repository Master s and Doctoral Projects Alzheimer's disease immunization strategies : a review of current research Laura Ona Sirgedas The University
BioArctic Gunilla Osswald, CEO December 5, Carnegie Nordic Healthcare Day
 BioArctic Gunilla Osswald, CEO December 5, 2017 Carnegie Nordic Healthcare Day Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic ) solely for the benefit of
BioArctic Gunilla Osswald, CEO December 5, 2017 Carnegie Nordic Healthcare Day Disclaimer This presentation has been prepared and produced by BioArctic AB (publ) ( BioArctic ) solely for the benefit of
TARGETING ALZHEIMER S AND OTHER NEURODEGENERATIVE DISEASES WITH NOVEL THERAPEUTICS AND DIAGNOSTICS
 TARGETING ALZHEIMER S AND OTHER NEURODEGENERATIVE DISEASES WITH NOVEL THERAPEUTICS AND DIAGNOSTICS NASDAQ: ACIU Credit Suisse Healthcare Conference Prof. Andrea Pfeifer Nov 7, 2017 2017 AC Immune. Not
TARGETING ALZHEIMER S AND OTHER NEURODEGENERATIVE DISEASES WITH NOVEL THERAPEUTICS AND DIAGNOSTICS NASDAQ: ACIU Credit Suisse Healthcare Conference Prof. Andrea Pfeifer Nov 7, 2017 2017 AC Immune. Not
Engaging stakeholders for a holistic therapy in Alzheimer s disease
 Engaging stakeholders for a holistic therapy in Alzheimer s disease Brain Forum l Lausanne, May 26, 2016 l Prof. Andrea Pfeifer 2016 AC Immune. Not to be used or reproduced without permission. www.acimmune.com
Engaging stakeholders for a holistic therapy in Alzheimer s disease Brain Forum l Lausanne, May 26, 2016 l Prof. Andrea Pfeifer 2016 AC Immune. Not to be used or reproduced without permission. www.acimmune.com
BioArctic AB Interim Report Jan Sep Jan Mattsson, CFO November 8, 2018
 BioArctic AB Interim Report Jan Sep 2018 Gunilla Osswald, CEO Nasdaq Stockholm: BIOA B Jan Mattsson, CFO November 8, 2018 Disclaimer This presentation has been prepared and produced by BioArctic AB (publ)
BioArctic AB Interim Report Jan Sep 2018 Gunilla Osswald, CEO Nasdaq Stockholm: BIOA B Jan Mattsson, CFO November 8, 2018 Disclaimer This presentation has been prepared and produced by BioArctic AB (publ)
LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases Round 2
 LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases Round 2 DEADLINE: March 13, 2019, 2:00pm EDT Applicants will be notified of Proposal invitations in May 2019 This Letter of Intent
LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases Round 2 DEADLINE: March 13, 2019, 2:00pm EDT Applicants will be notified of Proposal invitations in May 2019 This Letter of Intent
LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases Round 2
 LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases Round 2 DEADLINE: May 15, 2019, 2:00pm EDT Applicants will be notified of Proposal invitations in August 2019 This Letter of
LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases Round 2 DEADLINE: May 15, 2019, 2:00pm EDT Applicants will be notified of Proposal invitations in August 2019 This Letter of
Capo Therapeutics, Inc.
 Capo Therapeutics, Inc. Vaccine Therapies for Debilitating Diseases of the Brain San Diego, CA 1 SCIENTIFIC ADVISORY BOARD M. Flint Beal, M.D. Dr. Beal is an internationally recognized authority on neurodegenerative
Capo Therapeutics, Inc. Vaccine Therapies for Debilitating Diseases of the Brain San Diego, CA 1 SCIENTIFIC ADVISORY BOARD M. Flint Beal, M.D. Dr. Beal is an internationally recognized authority on neurodegenerative
LETTER OF INTENT Early Phase Clinical Trials 2018
 LETTER OF INTENT Early Phase Clinical Trials 2018 Applications are being accepted on a rolling basis. This Letter of Intent is an example only. Do not complete this paper application. Please submit the
LETTER OF INTENT Early Phase Clinical Trials 2018 Applications are being accepted on a rolling basis. This Letter of Intent is an example only. Do not complete this paper application. Please submit the
12-Month Interim Analysis of APOE4 Carriers for Fixed and Titration Dosing Regimens in PRIME, a Phase 1b study of Aducanumab
 12-Month Interim Analysis of APOE4 Carriers for Fixed and Titration Dosing Regimens in PRIME, a Phase 1b study of Aducanumab Vissia Viglietta, 1 John O Gorman, 1 Leslie Williams, 1 Tianle Chen, 1 Ahmed
12-Month Interim Analysis of APOE4 Carriers for Fixed and Titration Dosing Regimens in PRIME, a Phase 1b study of Aducanumab Vissia Viglietta, 1 John O Gorman, 1 Leslie Williams, 1 Tianle Chen, 1 Ahmed
The Importance of Early Identification of Alzheimer s Disease
 The Importance of Early Identification of Alzheimer s Disease Alessandro Padovani Professor of Neurology, Director of the Institute of Neurology University of Brescia X No, nothing to disclose Yes, please
The Importance of Early Identification of Alzheimer s Disease Alessandro Padovani Professor of Neurology, Director of the Institute of Neurology University of Brescia X No, nothing to disclose Yes, please
I «disease-modifying drugs» nello stato preclinico dell AD: quali evidenze?
 I «disease-modifying drugs» nello stato preclinico dell AD: quali evidenze? Daniela Galimberti Università di Milano, Fondazione Ca Granda, IRCCS Ospedale Maggiore Policlinico DEFICIT COLINERGICO Scarpini
I «disease-modifying drugs» nello stato preclinico dell AD: quali evidenze? Daniela Galimberti Università di Milano, Fondazione Ca Granda, IRCCS Ospedale Maggiore Policlinico DEFICIT COLINERGICO Scarpini
Tau-directed treatment approaches in Alzheimer s disease How far have we come?
 Tau-directed treatment approaches in Alzheimer s disease How far have we come? Lutz Frölich Department of Geriatric Psychiatry Central Institute of Mental Health University of Heidelberg, Germany EADC
Tau-directed treatment approaches in Alzheimer s disease How far have we come? Lutz Frölich Department of Geriatric Psychiatry Central Institute of Mental Health University of Heidelberg, Germany EADC
MY PERSONAL MEMORIES OF RITA LEVI-MONTALCINI G. NISTICÒ
 Vol. 2, no. 1, 1-3 (2013) MY PERSONAL MEMORIES OF RITA LEVI-MONTALCINI G. NISTICÒ General Director of the European Brain Research Institute (EBRI, Rita Levi-Montalcini Foundation) Vol. 2, no. 1, 5-17 (2013)
Vol. 2, no. 1, 1-3 (2013) MY PERSONAL MEMORIES OF RITA LEVI-MONTALCINI G. NISTICÒ General Director of the European Brain Research Institute (EBRI, Rita Levi-Montalcini Foundation) Vol. 2, no. 1, 5-17 (2013)
ADAPTIVE PHASE II STUDY OF BAN2401 IN EARLY ALZHEIMER S DISEASE CONTINUES TOWARD 18-MONTH ENDPOINT
 FOR IMMEDIATE RELEASE December 21, 2017 Eisai Co., Ltd. Biogen Inc. ADAPTIVE PHASE II STUDY OF BAN2401 IN EARLY ALZHEIMER S DISEASE CONTINUES TOWARD 18-MONTH ENDPOINT CRITERIA FOR SUCCESS AT 12-MONTH ANALYSIS
FOR IMMEDIATE RELEASE December 21, 2017 Eisai Co., Ltd. Biogen Inc. ADAPTIVE PHASE II STUDY OF BAN2401 IN EARLY ALZHEIMER S DISEASE CONTINUES TOWARD 18-MONTH ENDPOINT CRITERIA FOR SUCCESS AT 12-MONTH ANALYSIS
LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases
 LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases DEADLINE: Wednesday, August 1, 2018, 2:00pm EDT Applicants will be notified of Proposal invitations in September 2018. This Letter
LETTER OF INTENT Rapid Response: Canada 2019 Parkinson s & Related Diseases DEADLINE: Wednesday, August 1, 2018, 2:00pm EDT Applicants will be notified of Proposal invitations in September 2018. This Letter
Grifols demonstrates a significant reduction (61%) in the progression of moderate Alzheimer s disease using its AMBAR treatment protocol
 Grifols demonstrates a significant reduction (61%) in the progression of moderate Alzheimer s disease using its AMBAR treatment protocol Grifols presents AMBAR (Alzheimer Management by Albumin Replacement)
Grifols demonstrates a significant reduction (61%) in the progression of moderate Alzheimer s disease using its AMBAR treatment protocol Grifols presents AMBAR (Alzheimer Management by Albumin Replacement)
CNS Gene Regulation Platform
 CNS Gene Regulation Platform 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
CNS Gene Regulation Platform 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
Plenary Session: An update of brain circuits in Parkinson s and Deep Brain Stimulation
 Plenary Session: An update of brain circuits in Parkinson s and Deep Brain Stimulation Deep Brain Stimulation Andres M Lozano, University of Toronto Deep brain stimulation is the delivery of an electrical
Plenary Session: An update of brain circuits in Parkinson s and Deep Brain Stimulation Deep Brain Stimulation Andres M Lozano, University of Toronto Deep brain stimulation is the delivery of an electrical
Introducing MN-166 Multiple Sclerosis. July 9, 2008
 Introducing MN-166 A New Treatment Paradigm for Multiple Sclerosis July 9, 2008 MediciNova, Inc. 2008 Forward-Looking Statements Statements in this presentation that are not historical in nature constitute
Introducing MN-166 A New Treatment Paradigm for Multiple Sclerosis July 9, 2008 MediciNova, Inc. 2008 Forward-Looking Statements Statements in this presentation that are not historical in nature constitute
Clinical studies. BARBORA VLKOVÁ INSTITUTE OF MOLECULAR BIOMEDICINE
 Clinical studies BARBORA VLKOVÁ INSTITUTE OF MOLECULAR BIOMEDICINE www.imbm.sk barboravlk@gmail.com Biomedical research 1. Basic research a. In vitro = cell cultures b. In vivo = experimental animals c.
Clinical studies BARBORA VLKOVÁ INSTITUTE OF MOLECULAR BIOMEDICINE www.imbm.sk barboravlk@gmail.com Biomedical research 1. Basic research a. In vitro = cell cultures b. In vivo = experimental animals c.
Alzheimer s disease: clinical trials and drug development
 Alzheimer s disease: clinical trials and drug development Francesca Mangialasche, Alina Solomon, Bengt Winblad, Patrizia Mecocci, Miia Kivipelto Lancet Neurol 2010; 9: 702 16 This online publication has
Alzheimer s disease: clinical trials and drug development Francesca Mangialasche, Alina Solomon, Bengt Winblad, Patrizia Mecocci, Miia Kivipelto Lancet Neurol 2010; 9: 702 16 This online publication has
Alzheimer s disease research in the 21 st century: Past and current failures and the way forward
 The European Commission s science and knowledge service Joint Research Centre Alzheimer s disease research in the 21 st century: Past and current failures and the way forward Francesca Pistollato francesca.pistollato@ec.europa.eu
The European Commission s science and knowledge service Joint Research Centre Alzheimer s disease research in the 21 st century: Past and current failures and the way forward Francesca Pistollato francesca.pistollato@ec.europa.eu
Alzheimer s Disease. Table of Contents. Differential processing of APP Hyperphosphorylation of Tau
 Alzheimer s Disease Alzheimer s disease (AD) is one of the most common neurodegenerative diseases worldwide. Clinically, it is characterized by the presence of extracellular amyloid plaques and intracellular
Alzheimer s Disease Alzheimer s disease (AD) is one of the most common neurodegenerative diseases worldwide. Clinically, it is characterized by the presence of extracellular amyloid plaques and intracellular
QPS Neuropharmacology Overview
 HISTOCHEMISTRY HISTOLOGY KO MODELS IN VIVO MODELS BLOOD BRAIN BARRIER IN VITRO MODELS NEUROPHARMACOLOGY OVERVIEW NEUROSCIENCES QPS Neuropharmacology Overview QPS is recognized all over the world as a leading
HISTOCHEMISTRY HISTOLOGY KO MODELS IN VIVO MODELS BLOOD BRAIN BARRIER IN VITRO MODELS NEUROPHARMACOLOGY OVERVIEW NEUROSCIENCES QPS Neuropharmacology Overview QPS is recognized all over the world as a leading
Course Agenda. Day One
 Course Agenda BioImmersion: Biotech for the Non-Scientist A three-day, in-depth course that provides the background required for understanding today s fast-paced biotech marketplace. Beginning with an
Course Agenda BioImmersion: Biotech for the Non-Scientist A three-day, in-depth course that provides the background required for understanding today s fast-paced biotech marketplace. Beginning with an
Alzheimer s Disease. Joachim Herz. STARS January 11, 2010
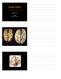 Alzheimer s Disease Joachim Herz STARS January 11, 2010 1 The Hallmarks of Alzheimer Disease Amyloid Plaques Neurofibrillary Tangles From: Medical Library of Utah 1906 First description of Alzheimer s
Alzheimer s Disease Joachim Herz STARS January 11, 2010 1 The Hallmarks of Alzheimer Disease Amyloid Plaques Neurofibrillary Tangles From: Medical Library of Utah 1906 First description of Alzheimer s
How Targets Are Chosen. Chris Wayman 12 th April 2012
 How Targets Are Chosen Chris Wayman 12 th April 2012 A few questions How many ideas does it take to make a medicine? 10 20 20-50 50-100 A few questions How long does it take to bring a product from bench
How Targets Are Chosen Chris Wayman 12 th April 2012 A few questions How many ideas does it take to make a medicine? 10 20 20-50 50-100 A few questions How long does it take to bring a product from bench
Nonclinical Data to Support FIH Clinical Trials for Cancer Immunotherapies. Whitney S. Helms, PhD IOM, February 29,2016
 Nonclinical Data to Support FIH Clinical Trials for Cancer Immunotherapies Whitney S. Helms, PhD IOM, February 29,2016 Disclaimer The views disseminated in this talk are my own and do not necessarily represent
Nonclinical Data to Support FIH Clinical Trials for Cancer Immunotherapies Whitney S. Helms, PhD IOM, February 29,2016 Disclaimer The views disseminated in this talk are my own and do not necessarily represent
Emerging Antibody Approaches
 Emerging Antibody Approaches Sudhir Paul University of Texas Houston Medical School With collaborations from Stephanie Planque, Yukie Mitsuda, Sari Sonoda, Sarah Murphy, Eric Brown, Yasuhiro Nishiyama
Emerging Antibody Approaches Sudhir Paul University of Texas Houston Medical School With collaborations from Stephanie Planque, Yukie Mitsuda, Sari Sonoda, Sarah Murphy, Eric Brown, Yasuhiro Nishiyama
Grifols has acquired 51% of the capital of Araclon Biotech to ensure the viability of the purchased company projects
 Alzheimer s disease acquires a growing role in Grifols R&D Grifols has acquired 51% of the capital of Araclon Biotech to ensure the viability of the purchased company projects Araclon Biotech, established
Alzheimer s disease acquires a growing role in Grifols R&D Grifols has acquired 51% of the capital of Araclon Biotech to ensure the viability of the purchased company projects Araclon Biotech, established
TARGETING ALZHEIMER S AND OTHER NEURODEGENERATIVE DISEASES WITH NOVEL THERAPEUTICS AND DIAGNOSTICS
 TARGETING ALZHEIMER S AND OTHER NEURODEGENERATIVE DISEASES WITH NOVEL THERAPEUTICS AND DIAGNOSTICS NASDAQ: ACIU KOL Event Tau Prof. Andrea Pfeifer Dec 1, 2017 2017 AC Immune. Not to be used or reproduced
TARGETING ALZHEIMER S AND OTHER NEURODEGENERATIVE DISEASES WITH NOVEL THERAPEUTICS AND DIAGNOSTICS NASDAQ: ACIU KOL Event Tau Prof. Andrea Pfeifer Dec 1, 2017 2017 AC Immune. Not to be used or reproduced
NEUROLOGY AND OPHTHALMOLOGY
 NEUROLOGY AND OPHTHALMOLOGY OPPORTUNITIES AT INSERM BD & L: Anne Cochi anne.cochi@inserm-transfert.fr September 2017 1 WE PROPOSE A SELECTION OF PROJECTS AT DIFFERENT STAGES 4 Neurodegeneration patents
NEUROLOGY AND OPHTHALMOLOGY OPPORTUNITIES AT INSERM BD & L: Anne Cochi anne.cochi@inserm-transfert.fr September 2017 1 WE PROPOSE A SELECTION OF PROJECTS AT DIFFERENT STAGES 4 Neurodegeneration patents
Engage with us on Twitter: #Molecule2Miracle
 Engage with us on Twitter: #Molecule2Miracle Kassy Perry President & CEO Perry Communications Group PhRMA Alliance Development Consultant.@kassyperry Emily Burke, Ph.D. Director of Curriculum BioTech
Engage with us on Twitter: #Molecule2Miracle Kassy Perry President & CEO Perry Communications Group PhRMA Alliance Development Consultant.@kassyperry Emily Burke, Ph.D. Director of Curriculum BioTech
Compound Re-Profiling. Dr Robert Scoffin CEO, Cresset
 Compound Re-Profiling Dr Robert Scoffin CEO, Cresset What is it? F F F N N H 2 N S O O Br > Compound Re-Profiling or Re-Purposing is the process of finding a new clinical use for an existing treatment
Compound Re-Profiling Dr Robert Scoffin CEO, Cresset What is it? F F F N N H 2 N S O O Br > Compound Re-Profiling or Re-Purposing is the process of finding a new clinical use for an existing treatment
LUPAS Luminescent Polymers for in vivo Imaging of Amyloid Signatures
 LUPAS Luminescent Polymers for in vivo Imaging of Amyloid Signatures A research project for innovative diagnostics for neurodegenerative disorders Funded by the European Union under the 7 th Framework
LUPAS Luminescent Polymers for in vivo Imaging of Amyloid Signatures A research project for innovative diagnostics for neurodegenerative disorders Funded by the European Union under the 7 th Framework
No new drugs for Alzheimer's disease in 15 years
 Published on ScienceNordic (http://sciencenordic.com) Home > Printer-friendly PDF > Printer-friendly PDF No new drugs for Alzheimer's disease in 15 years Health[1] Health[1]Alzheimer's disease [2]Health
Published on ScienceNordic (http://sciencenordic.com) Home > Printer-friendly PDF > Printer-friendly PDF No new drugs for Alzheimer's disease in 15 years Health[1] Health[1]Alzheimer's disease [2]Health
Credit Suisse 7 th Annual European Large Cap Pharmaceutical Conference. November 6, 2007
 Credit Suisse 7 th Annual European Large Cap Pharmaceutical Conference November 6, 2007 Safe Harbor Statement This presentation contains forward-looking statements about Elan s financial condition, results
Credit Suisse 7 th Annual European Large Cap Pharmaceutical Conference November 6, 2007 Safe Harbor Statement This presentation contains forward-looking statements about Elan s financial condition, results
A PHARMA MATTERS REPORT.
 REUTERS / Todd Korol SPOTLIGHT ON ALZHEIMER S DISEASE A PHARMA MATTERS REPORT. A REVIEW OF JULY-SEPTEMBER 2012. PUBLISHED NOVEMBER 2012. Expert therapy area review of the key market players and deals highlights
REUTERS / Todd Korol SPOTLIGHT ON ALZHEIMER S DISEASE A PHARMA MATTERS REPORT. A REVIEW OF JULY-SEPTEMBER 2012. PUBLISHED NOVEMBER 2012. Expert therapy area review of the key market players and deals highlights
Developing innovative new medicines for acute and chronic neurological and psychiatric conditions Larry Glass, CEO
 Developing innovative new medicines for acute and chronic neurological and psychiatric conditions Larry Glass, CEO (lglass@neurenpharma.com) Neuren Pharmaceuticals Created to commercialize University of
Developing innovative new medicines for acute and chronic neurological and psychiatric conditions Larry Glass, CEO (lglass@neurenpharma.com) Neuren Pharmaceuticals Created to commercialize University of
Stealth BioTherapeutics Mission:
 The following is a summary of a live presentation offered through joint collaboration with UMDF, MitoAction and the Foundation for Mitochondrial Medicine to the mitochondrial disease patient and family
The following is a summary of a live presentation offered through joint collaboration with UMDF, MitoAction and the Foundation for Mitochondrial Medicine to the mitochondrial disease patient and family
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various
 Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Supplementary Figure 1. APP cleavage assay. HEK293 cells were transfected with various GST-tagged N-terminal truncated APP fragments including GST-APP full-length (FL), APP (123-695), APP (189-695), or
Considering a New Paradigm for Alzheimer s Disease Research
 The Humane Society Institute for Science and Policy Animal Studies Repository 8-2014 Considering a New Paradigm for Alzheimer s Disease Research Gillian R. Langley glangley@hsi.org Follow this and additional
The Humane Society Institute for Science and Policy Animal Studies Repository 8-2014 Considering a New Paradigm for Alzheimer s Disease Research Gillian R. Langley glangley@hsi.org Follow this and additional
TARGET VALIDATION. Maaike Everts, PhD (with slides from Dr. Suto)
 TARGET VALIDATION Maaike Everts, PhD (with slides from Dr. Suto) Drug Discovery & Development Source: http://dlab.cl/molecular-design/drug-discovery-phases/ How do you identify a target? Target: the naturally
TARGET VALIDATION Maaike Everts, PhD (with slides from Dr. Suto) Drug Discovery & Development Source: http://dlab.cl/molecular-design/drug-discovery-phases/ How do you identify a target? Target: the naturally
Investigations in Immune Suppression for Monoclonal Antibody Therapeutics
 Investigations in Immune Suppression for Monoclonal Antibody Therapeutics Vibha Jawa Principal Scientist AAPS NBC Meeting, May 2016 Clinical Immunology, Medical Sciences, Amgen Background Therapeutic proteins
Investigations in Immune Suppression for Monoclonal Antibody Therapeutics Vibha Jawa Principal Scientist AAPS NBC Meeting, May 2016 Clinical Immunology, Medical Sciences, Amgen Background Therapeutic proteins
Total urinary GAGs declined by 51%, dermatan sulfate by 32%, and heparan sulfate by 61% in Cohort 2 at 16 weeks
 September 5, 2018 Sangamo Announces 16 Week Clinical Results Including Reductions In Glycosaminoglycans In Phase 1/2 Trial Evaluating SB-913, A Zinc Finger Nuclease Genome Editing Treatment For MPS II
September 5, 2018 Sangamo Announces 16 Week Clinical Results Including Reductions In Glycosaminoglycans In Phase 1/2 Trial Evaluating SB-913, A Zinc Finger Nuclease Genome Editing Treatment For MPS II
Clinical Trials in the Age of Technology
 Clinical Trials in the Age of Technology Christopher G. Tarolli, M.D. Senior Instructor of Neurology Center for Health + Technology and Department of Neurology SCORE Seminar 6/5/2018 Disclosures I receive
Clinical Trials in the Age of Technology Christopher G. Tarolli, M.D. Senior Instructor of Neurology Center for Health + Technology and Department of Neurology SCORE Seminar 6/5/2018 Disclosures I receive
Department of Neurology David Geffen School of Medicine at UCLA Los Angeles, USA
 Supervisors: Lars Lannfelt, Professor Department of Public Health and Caring Sciences Uppsala University Uppsala, Sweden Frida Ekholm Pettersson, Assistant Professor Department of Public Health and Caring
Supervisors: Lars Lannfelt, Professor Department of Public Health and Caring Sciences Uppsala University Uppsala, Sweden Frida Ekholm Pettersson, Assistant Professor Department of Public Health and Caring
The Alzheimer s s Disease Neuroimaging Initiative (ADNI): A Public-Private. Private Partnership
 The Alzheimer s s Disease Neuroimaging Initiative (ADNI): A Public-Private Private Partnership Laurie M. Ryan, PhD Division of Neuroscience National Institute on Aging National Institutes of Health Department
The Alzheimer s s Disease Neuroimaging Initiative (ADNI): A Public-Private Private Partnership Laurie M. Ryan, PhD Division of Neuroscience National Institute on Aging National Institutes of Health Department
PARTNERING IN NEUROSCIENCE Sharing a Vision to Help Improve Patients Lives
 PARTNERING IN NEUROSCIENCE Sharing a Vision to Help Improve Patients Lives VISIT OUR WEBSITE FOR MORE INFORMATION: www.janssen.com/neuroscience MAY 2017 Johnson & Johnson Innovation LLC 2017. A Message
PARTNERING IN NEUROSCIENCE Sharing a Vision to Help Improve Patients Lives VISIT OUR WEBSITE FOR MORE INFORMATION: www.janssen.com/neuroscience MAY 2017 Johnson & Johnson Innovation LLC 2017. A Message
INTRODUCTORY GUIDE TO CNS DRUG DISCOVERY
 INTRODUCTORY GUIDE TO CNS DRUG DISCOVERY Table of Contents SUMMARY...3 OVERVIEW...4 1. Target Identification and Validation...4 2. Assay Development and Screening...4 2.1 High Throughput Screening (HTS)...4
INTRODUCTORY GUIDE TO CNS DRUG DISCOVERY Table of Contents SUMMARY...3 OVERVIEW...4 1. Target Identification and Validation...4 2. Assay Development and Screening...4 2.1 High Throughput Screening (HTS)...4
Priavoid. A life without dementia. Company Presentation, BIO, June, Prof. Dr. Dieter Willbold
 Priavoid A life without dementia Company Presentation, BIO, June, 2017 Prof. Dr. Dieter Willbold (co-founder and future Chairman of the Supervisory Board) The Company At a glance Established: probably
Priavoid A life without dementia Company Presentation, BIO, June, 2017 Prof. Dr. Dieter Willbold (co-founder and future Chairman of the Supervisory Board) The Company At a glance Established: probably
Monoclonal Antibodies for Treatment and Prevention of HIV-1 CROI Charles Boucher Erasmus MC
 Monoclonal Antibodies for Treatment and Prevention of HIV-1 CROI 2018 Charles Boucher Erasmus MC Introduction: Human monoclonal antibodies Monoclonal Antibodies: Are identical immunoglobulins made by clone
Monoclonal Antibodies for Treatment and Prevention of HIV-1 CROI 2018 Charles Boucher Erasmus MC Introduction: Human monoclonal antibodies Monoclonal Antibodies: Are identical immunoglobulins made by clone
COPYRIGHT 2006 SCIENTIFIC AMERICAN, INC.
 C R E D I T 72 S C I E N T I F I C A M E R I C A N M AY 2 0 0 6 AL ZHEIMER S DISE A SE gradually severs even one s oldest memories, but scientists are working on promising treatments. Some therapies could
C R E D I T 72 S C I E N T I F I C A M E R I C A N M AY 2 0 0 6 AL ZHEIMER S DISE A SE gradually severs even one s oldest memories, but scientists are working on promising treatments. Some therapies could
Biologic Drug Delivery Across the Blood-Brain Barrier with IgG Fusion Proteins. William M. Pardridge, M.D. University of California ArmaGen, Inc.
 Biologic Drug Delivery Across the Blood-Brain Barrier with IgG Fusion Proteins William M. Pardridge, M.D. University of California ArmaGen, Inc. 1 The Brain and Biotechnology In 2017, there are no biologics
Biologic Drug Delivery Across the Blood-Brain Barrier with IgG Fusion Proteins William M. Pardridge, M.D. University of California ArmaGen, Inc. 1 The Brain and Biotechnology In 2017, there are no biologics
Target-mediated Clearance and Immunogenicity two sides, one coin. Daren Austin, PhD Senior Director and Head Biopharm Clinical Pharmacology
 Target-mediated Clearance and Immunogenicity two sides, one coin Daren Austin, PhD Senior Director and Head Biopharm Clinical Pharmacology Pharmacokinetic determinants of therapeutic proteins Pharmacokinetic
Target-mediated Clearance and Immunogenicity two sides, one coin Daren Austin, PhD Senior Director and Head Biopharm Clinical Pharmacology Pharmacokinetic determinants of therapeutic proteins Pharmacokinetic
University of Eastern Finland (UEF) Main Research Lines
 University of Eastern Finland (UEF) Main Research Lines Asla Pitkänen, MD, PhD Epilepsy Research Laboratory A.I.Virtanen Institute for Molecular Sciences University of Eastern Finland Kuopio, Finland E-mail:
University of Eastern Finland (UEF) Main Research Lines Asla Pitkänen, MD, PhD Epilepsy Research Laboratory A.I.Virtanen Institute for Molecular Sciences University of Eastern Finland Kuopio, Finland E-mail:
leading the way in research & development
 leading the way in research & development people. passion. possibilities. ABBVIE 2 immunology AbbVie Immunology has a demonstrated record of success in identifying and developing both small molecule and
leading the way in research & development people. passion. possibilities. ABBVIE 2 immunology AbbVie Immunology has a demonstrated record of success in identifying and developing both small molecule and
PHASE 1, MULTICENTER, OPEN-LABEL STUDY OF SINGLE-AGENT BISPECIFIC ANTIBODY T CELL ENGAGER GBR 1342 IN RELAPSED/REFRACTORY MULTIPLE MYELOMA
 PHASE 1, MULTICENTER, OPEN-LABEL STUDY OF SINGLE-AGENT BISPECIFIC ANTIBODY T CELL ENGAGER GBR 1342 IN RELAPSED/REFRACTORY MULTIPLE MYELOMA JOSHUA RICHTER 1 ; MARTIN WERMKE 2 ; JOHN KAUH 3 ; JONATHAN BACK
PHASE 1, MULTICENTER, OPEN-LABEL STUDY OF SINGLE-AGENT BISPECIFIC ANTIBODY T CELL ENGAGER GBR 1342 IN RELAPSED/REFRACTORY MULTIPLE MYELOMA JOSHUA RICHTER 1 ; MARTIN WERMKE 2 ; JOHN KAUH 3 ; JONATHAN BACK
Decision Process for Committing to a Therapeutic Development Approach: Target Validation Metrics and Biomarkers
 Decision Process for Committing to a Therapeutic Development Approach: Target Validation Metrics and Biomarkers Kalpana Merchant, PhD Chief Scientific Officer, Tailored Therapeutics - Neuroscience Eli
Decision Process for Committing to a Therapeutic Development Approach: Target Validation Metrics and Biomarkers Kalpana Merchant, PhD Chief Scientific Officer, Tailored Therapeutics - Neuroscience Eli
PHASE 1, MULTICENTER, OPEN-LABEL STUDY OF SINGLE-AGENT BISPECIFIC ANTIBODY T CELL ENGAGER GBR 1342 IN RELAPSED/REFRACTORY MULTIPLE MYELOMA
 PHASE 1, MULTICENTER, OPEN-LABEL STUDY OF SINGLE-AGENT BISPECIFIC ANTIBODY T CELL ENGAGER GBR 1342 IN RELAPSED/REFRACTORY MULTIPLE MYELOMA JOSHUA RICHTER 1 ; OLA LANDGREN 2 ; JOHN KAUH 3 ; JONATHAN BACK
PHASE 1, MULTICENTER, OPEN-LABEL STUDY OF SINGLE-AGENT BISPECIFIC ANTIBODY T CELL ENGAGER GBR 1342 IN RELAPSED/REFRACTORY MULTIPLE MYELOMA JOSHUA RICHTER 1 ; OLA LANDGREN 2 ; JOHN KAUH 3 ; JONATHAN BACK
Biomarkers in the differential diagnosis of Dementia: which role for Omics? Sergio Bernardini, Biochimica Clinica Tor Vergata
 Biomarkers in the differential diagnosis of Dementia: which role for Omics? Sergio Bernardini, Biochimica Clinica Tor Vergata FTD VaD AD a-syn Alzheimer s disease Slowly progressive memory loss and dementia
Biomarkers in the differential diagnosis of Dementia: which role for Omics? Sergio Bernardini, Biochimica Clinica Tor Vergata FTD VaD AD a-syn Alzheimer s disease Slowly progressive memory loss and dementia
Klaus Romero MD MS FCP. (Supported by the CAMD AD Modeling & Simulation Team)
 Baseline ICV-adjusted Hippocampal Volume as a Biomarker for Enrichment in Alzheimer s Disease Trials Co-Chairs: Patricia Cole (Takeda) & Derek Hill (IXICO) A Modeling Approach to Demonstrate Trial Enrichment
Baseline ICV-adjusted Hippocampal Volume as a Biomarker for Enrichment in Alzheimer s Disease Trials Co-Chairs: Patricia Cole (Takeda) & Derek Hill (IXICO) A Modeling Approach to Demonstrate Trial Enrichment
From Biomarkers to Diagnostics: Applications from Target Engagement to Patient Stratification
 Session: Wednesday February 22, 2017 at 08.40-09:10 am From Biomarkers to Diagnostics: Applications from Target Engagement to Patient Stratification Andrew Satlin, M.D. EVP Eisai Neurology Business Group
Session: Wednesday February 22, 2017 at 08.40-09:10 am From Biomarkers to Diagnostics: Applications from Target Engagement to Patient Stratification Andrew Satlin, M.D. EVP Eisai Neurology Business Group
1 Name. 1. (3 pts) What is apoptosis and how does it differ from necrosis? Which is more likely to trigger inflammation?
 1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
Immunogenicity of Therapeutic Proteins. Steven J Swanson, Ph.D. Executive Director, Clinical Immunology
 Immunogenicity of Therapeutic Proteins Steven J Swanson, Ph.D. Executive Director, Clinical Immunology swanson@amgen.com Causes of Immunogenicity Sequence differences between therapeutic protein and endogenous
Immunogenicity of Therapeutic Proteins Steven J Swanson, Ph.D. Executive Director, Clinical Immunology swanson@amgen.com Causes of Immunogenicity Sequence differences between therapeutic protein and endogenous
About OMICS Group Conferences
 About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
About OMICS Group OMICS Group International is an amalgamation of Open Access publications and worldwide international science conferences and events. Established in the year 2007 with the sole aim of
Application of Modeling and Simulation to Support Clinical Drug Development Decisions in Alzheimer's Disease
 Application of Modeling and Simulation to Support Clinical Drug Development Decisions in Alzheimer's Disease Marc R. Gastonguay, Ph.D. Scientific Director, Metrum Institute marcg@metruminstitute.org President
Application of Modeling and Simulation to Support Clinical Drug Development Decisions in Alzheimer's Disease Marc R. Gastonguay, Ph.D. Scientific Director, Metrum Institute marcg@metruminstitute.org President
Andrew Nesbitt, PhD. Disclaimer 10/10/2014. Determining the Immunogenicity of Biologics: a Tricky Problem. Employee of UCB ן
 Determining the Immunogenicity of Biologics: a Tricky Problem Andrew Nesbitt, PhD UCB Director Cimzia PST Disclaimer 2 Employee of UCB The theories expressed in this presentation are the views of the speaker
Determining the Immunogenicity of Biologics: a Tricky Problem Andrew Nesbitt, PhD UCB Director Cimzia PST Disclaimer 2 Employee of UCB The theories expressed in this presentation are the views of the speaker
Immunotherapy in myeloma
 Immunotherapy in myeloma Horizons Infosheet Clinical trials and novel drugs This Horizons Infosheet provides information on immunotherapy, a type of treatment being investigated in myeloma. The Horizons
Immunotherapy in myeloma Horizons Infosheet Clinical trials and novel drugs This Horizons Infosheet provides information on immunotherapy, a type of treatment being investigated in myeloma. The Horizons
Programa Cooperación Farma-Biotech Neurociencias G79
 G79 A Neuroprotective Therapy Barcelona, 15 de febrero 2011 Content 1. The Company 2. The Product a) Therapeutic focus b) Innovative mechanisms of action c) Differential features facing the market d) Current
G79 A Neuroprotective Therapy Barcelona, 15 de febrero 2011 Content 1. The Company 2. The Product a) Therapeutic focus b) Innovative mechanisms of action c) Differential features facing the market d) Current
Biogen Idec Neurology Pipeline. Alfred Sandrock, MD, PhD SVP, Neurology Research & Development
 Biogen Idec Neurology Pipeline Alfred Sandrock, MD, PhD SVP, Neurology Research & Development March 25, 2009 Robust Neurology Pipeline Neurology Multiple Sclerosis Discovery Pre-Clinical Phase 1 Phase
Biogen Idec Neurology Pipeline Alfred Sandrock, MD, PhD SVP, Neurology Research & Development March 25, 2009 Robust Neurology Pipeline Neurology Multiple Sclerosis Discovery Pre-Clinical Phase 1 Phase
HARNESSING THE POWER OF PRECISION MEDICINE TO TREAT ALZHEIMER S DISEASE AND ALS (LOU GEHRIG S DISEASE)
 HARNESSING THE POWER OF PRECISION MEDICINE TO TREAT ALZHEIMER S DISEASE AND ALS (LOU GEHRIG S DISEASE) Toronto Stock Exchange (TSX) ticker: PMN.TO June 2017 1 Forward Looking Statement: Safe Harbor This
HARNESSING THE POWER OF PRECISION MEDICINE TO TREAT ALZHEIMER S DISEASE AND ALS (LOU GEHRIG S DISEASE) Toronto Stock Exchange (TSX) ticker: PMN.TO June 2017 1 Forward Looking Statement: Safe Harbor This
We are committed to translating ground-breaking science into genomic therapies that transform patients lives
 We are committed to translating ground-breaking science into genomic therapies that transform patients lives Liver-based expression of the human alpha-galactosidase A gene (GLA) in a murine Fabry model
We are committed to translating ground-breaking science into genomic therapies that transform patients lives Liver-based expression of the human alpha-galactosidase A gene (GLA) in a murine Fabry model
Results to be Presented at LDN WORLD Symposium in February Initiation of Repeat-Dose Pompe Study Anticipated in 3Q13
 Amicus Therapeutics Announces Positive Results from All Four Cohorts in Phase 2 Chaperone-Enzyme Replacement Therapy (ERT) Co-Administration Study for Pompe Disease Strong Proof-of-Concept Data for Chaperone
Amicus Therapeutics Announces Positive Results from All Four Cohorts in Phase 2 Chaperone-Enzyme Replacement Therapy (ERT) Co-Administration Study for Pompe Disease Strong Proof-of-Concept Data for Chaperone
Peptide libraries: applications, design options and considerations. Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST
 Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Peptide libraries: applications, design options and considerations Laura Geuss, PhD May 5, 2015, 2:00-3:00 pm EST Overview 1 2 3 4 5 Introduction Peptide library basics Peptide library design considerations
Paving the way for Non-Clinical Bioanalytical Partnerships Louise Angell
 Paving the way for Non-Clinical Bioanalytical Partnerships Louise Angell Content Overview of non-clinical immunogenicity testing for biologics Regulatory guidance Bioanalytical considerations Risk based
Paving the way for Non-Clinical Bioanalytical Partnerships Louise Angell Content Overview of non-clinical immunogenicity testing for biologics Regulatory guidance Bioanalytical considerations Risk based
Immunotherapeutic Approaches for Alzheimer s Disease
 Immunotherapeutic Approaches for Alzheimer s Disease Thomas Wisniewski 1,2,3, * and Fernando Goñi 1 1 Department of Neurology, Center for Cognitive Neurology 2 Department of Pathology 3 Department of Psychiatry
Immunotherapeutic Approaches for Alzheimer s Disease Thomas Wisniewski 1,2,3, * and Fernando Goñi 1 1 Department of Neurology, Center for Cognitive Neurology 2 Department of Pathology 3 Department of Psychiatry
Partnering in Neuroscience Sharing a Vision to Help Improve Patients Lives. Janssen Research & Development, LLC
 Partnering in Neuroscience Sharing a Vision to Help Improve Patients Lives Janssen Research & Development, LLC 2 Yvonne Ford, Untitled Artwork Artwork from Reflections Art in Health, a user-led charity
Partnering in Neuroscience Sharing a Vision to Help Improve Patients Lives Janssen Research & Development, LLC 2 Yvonne Ford, Untitled Artwork Artwork from Reflections Art in Health, a user-led charity
Application of Deep Learning to Drug Discovery
 Application of Deep Learning to Drug Discovery Hase T, Tsuji S, Shimokawa K, Tanaka H Tohoku Medical Megabank Organization, Tohoku University Current Situation of Drug Discovery Rapid increase of R&D expenditure
Application of Deep Learning to Drug Discovery Hase T, Tsuji S, Shimokawa K, Tanaka H Tohoku Medical Megabank Organization, Tohoku University Current Situation of Drug Discovery Rapid increase of R&D expenditure
Roche to present new data at AAN reinforcing efficacy and safety of newly FDAapproved OCREVUS (ocrelizumab) in two types of multiple sclerosis
 Investor Update Basel, 19 April 2017 Roche to present new data at AAN reinforcing efficacy and safety of newly FDAapproved OCREVUS (ocrelizumab) in two types of multiple sclerosis Data presentations will
Investor Update Basel, 19 April 2017 Roche to present new data at AAN reinforcing efficacy and safety of newly FDAapproved OCREVUS (ocrelizumab) in two types of multiple sclerosis Data presentations will
National MS Society Information Sourcebook
 National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Interferons The interferons are a group of natural proteins that are produced by human cells in response to viral infection
National MS Society Information Sourcebook www.nationalmssociety.org/sourcebook Interferons The interferons are a group of natural proteins that are produced by human cells in response to viral infection
BEH.462/3.962J Molecular Principles of Biomaterials Spring 2003
 Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
Lecture 17: Drug targeting Last time: Today: Intracellular drug delivery Drug targeting Reading: T.J. Wickham, Ligand-directed targeting of genes to the site of disease, Nat. Med. 9(1) 135-139 (2003) Drug
The NYSCF Research Institute
 Regenerative Medicine Research Update Susan L. Solomon The NYSCF Research Institute New York Pharma Forum February 25, 2016 Regenerative Medicine Research Regenerative medicine uses laboratory grown human
Regenerative Medicine Research Update Susan L. Solomon The NYSCF Research Institute New York Pharma Forum February 25, 2016 Regenerative Medicine Research Regenerative medicine uses laboratory grown human
Phase 1 Clinical Studies First-In-Human (FIH) <Chapter 31> Pharmacologically-Guided Dose Escalation
 Phase 1 Clinical Studies First-In-Human (FIH) Pharmacologically-Guided Dose Escalation Jerry M. Collins, Ph.D. Developmental Therapeutics Program Division of Cancer Treatment and Diagnosis,
Phase 1 Clinical Studies First-In-Human (FIH) Pharmacologically-Guided Dose Escalation Jerry M. Collins, Ph.D. Developmental Therapeutics Program Division of Cancer Treatment and Diagnosis,
Immunotherapy in myeloma
 Immunotherapy in myeloma This Horizons Infosheet contains information on immunotherapy, a type of treatment being investigated in myeloma. The Horizons Infosheet series provides information relating to
Immunotherapy in myeloma This Horizons Infosheet contains information on immunotherapy, a type of treatment being investigated in myeloma. The Horizons Infosheet series provides information relating to
Evaluation of AMG 531 Efficacy in Splenectomized Patients with Chronic ITP in a Randomized Placebo- Controlled Phase 3 Study
 Evaluation of AMG 531 Efficacy in Splenectomized Patients with Chronic ITP in a Randomized Placebo- Controlled Phase 3 Study Plenary Session at ASH 2007 Presented by Terry B. Gernsheimer Madeleine Verhovsek
Evaluation of AMG 531 Efficacy in Splenectomized Patients with Chronic ITP in a Randomized Placebo- Controlled Phase 3 Study Plenary Session at ASH 2007 Presented by Terry B. Gernsheimer Madeleine Verhovsek
Introduction to Drug Development
 Introduction to Drug Development Yves Geysels, PhD Head Clinical Research Operations, Belgium Past President of the Associoation of Clinical Research Professionals (ACRP) Board Member of the European Forum
Introduction to Drug Development Yves Geysels, PhD Head Clinical Research Operations, Belgium Past President of the Associoation of Clinical Research Professionals (ACRP) Board Member of the European Forum
MultISyn: Multimodal Imaging of rare Synucleinopathies
 MultISyn: Multimodal Imaging of rare Synucleinopathies PUBLISHABLE EXECUTIVE SUMMARY FOR PERIODIC REPORT 1. Summary description of project context and main objectives Neurodegenerative diseases, such as
MultISyn: Multimodal Imaging of rare Synucleinopathies PUBLISHABLE EXECUTIVE SUMMARY FOR PERIODIC REPORT 1. Summary description of project context and main objectives Neurodegenerative diseases, such as
Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes
 Citation: Clin Transl Sci (2018) 11, 147 152; C 2017 ASCPT. All rights reserved doi:10.1111/cts.12491 REVIEW Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes Jeffrey INTRODUCTION
Citation: Clin Transl Sci (2018) 11, 147 152; C 2017 ASCPT. All rights reserved doi:10.1111/cts.12491 REVIEW Lessons Learned from Alzheimer Disease: Clinical Trials with Negative Outcomes Jeffrey INTRODUCTION
CBME/INSERM-Lille, France Mini-symposium
 CBME/INSERM-Lille, France Mini-symposium Contribution of Tau studies to Neuroregenerative Medicine & Development of Biomedical Engineering Tools March 6 th, 2018 8.45 am 14.00 pm Da An Campus, Room B204
CBME/INSERM-Lille, France Mini-symposium Contribution of Tau studies to Neuroregenerative Medicine & Development of Biomedical Engineering Tools March 6 th, 2018 8.45 am 14.00 pm Da An Campus, Room B204
Treating and Preventing Infectious Disease. November 2011 Nasdaq: INHX
 Treating and Preventing Infectious Disease November 2011 Nasdaq: INHX Safe Harbor This presentation contains forward looking statements about Inhibitex and its business, business prospects, strategy and
Treating and Preventing Infectious Disease November 2011 Nasdaq: INHX Safe Harbor This presentation contains forward looking statements about Inhibitex and its business, business prospects, strategy and
The Big Picture of FTD Research: Funding, Collaboration and the Scoop on Participation
 The Big Picture of FTD Research: Funding, Collaboration and the Scoop on Participation 1 st Annual FTD Caregiver Conference Raleigh, NC July 12, 2011 Our objectives today Snapshot of medical research process
The Big Picture of FTD Research: Funding, Collaboration and the Scoop on Participation 1 st Annual FTD Caregiver Conference Raleigh, NC July 12, 2011 Our objectives today Snapshot of medical research process
