The processing and cleaning of instruments provided by the company Weber Instrumente GmbH & Co. KG
|
|
|
- Gwendolyn Garrison
- 6 years ago
- Views:
Transcription
1 The processing and cleaning of instruments provided by the company Weber Instrumente GmbH & Co. KG SOP Contents 1 Preface Further applicable norms, directives and additional information General requirements for the processing of medical devices Manual pre-cleaning Pre-cleaning of instruments with a through hole Ultra sonic cleaning Mechanical cleaning Drying before maintenance and sterilization Maintenance of instruments Application with Forcipes and similar instruments Application with couplings and ratchets Sterilization Storage Marking of products Preface Please read this instruction on processing and cleaning of instruments provided by the company Weber Instrumente GmbH & Co. KG thoroughly before you start with the processing/sterilization of instruments. In the event that questions arise referring these instructions, you may contact Weber GmbH & Co. KG or the respective manufacturer of cleaning/disinfection equipment, sterilizer and ultrasonic bath or the manufacturer of cleaning, neutralization and disinfection agents. All instruments must undergo a visual inspection between each operational treatment. Here, instruments should be checked with regard to damages of surfaces or indication of corrosion (rust formation). Besides, the functioning of movable parts should be inspected upon cleaning. Damaged, corroded or inoperable instruments must not be reused. You may contact Weber Instrumente GmbH & Co. KG if you have further questions. 2 Further applicable norms, directives and additional information In this chapter, a short overview of further applicable norms, directives and additional information will be given which are needful for the processing and disinfection of instruments. No responsibility is taken for the completeness of this information. For general technical as well as regulatory information and hints about processing/sterilization of instruments you may consider applicable legislation, literature and recommendations by the Robert Koch Institute. \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 1/8
2 Please consider instructions of the disinfection/cleaning agent manufacturer for instruments with regard to concentration, dwell time and temperature. Disinfection agents without chlorine, ammonia and aldehyde with proven efficacy against HBV, HCV und HIV must be used only. They must correspond to the applicable national law for disinfection agents (e.g. FDA-admission, DDGHM (2002)/VAH (2011)-Listing, CE-marking etc.). Due to the protein-fixing effect of disinfection agents containing aldehyde, a disinfection should be carried out with agents free of aldehyde. Always consider the specification of the manufacturer of cleaning and disinfection equipments, ultrasonic bath and sterilizers. With regard to sterile packages (e.g. sterilization container) you should consider manufacturer instruction s. Please consider the specification DIN EN ISO sterilization of medical devices - information to be provided by the manufacturer for the processing of resterilizable medical devices (ISO 17664:2004); German version EN ISO 17664:2004 Please consider the specification of the RKI directive 2001: Anforderungen an die Hygiene bei der Aufbereitung von Medizinprodukten (Bundesgesundheitsblatt 44: ) Please consider the specification of the directive by the DGKH, DGSV and AKI for validation and routine monitoring of mechanical cleaning and thermic disinfection processes for medical devices and for principles of equipment selection (3rd edition 2008). Please consider the specifications of the Draft Guidance for Industry and FDA Staff / Processing/ Reprocessing Medical Devices in Health Care Settings: Validation Methods and Labeling 2May 2011 Please consider the specifications of ANSI/AAMI TIR12 September 2010 Designing, testing, and labeling reusable medical devices for reprocessing in health care facilities: A guide for medical device manufacturers Please consider the specifications of DIN EN ISO ( ) Reinigungs-Desinfektionsgeräte - Teil 1: Allgemeine Anforderungen, Begriffe und Prüfverfahren (ISO :2006); Deutsche Fassung EN ISO : General requirements for the processing of medical devices To execute an adequate processing of instruments, you should consider the national applicable legislation and hygiene regulations of medical practice and/or hospitals. This applies foremost for specifications regarding to an effective inactivation of prions (e.g. Creutzfeldt-Jakob-disease). Targeted preventive measures during treatment minimize permanent existing risks of contamination and infection. Including: The assessment of dangers connected to medical activities and definition of appropriate preventive measures Systematic proceeding of processing and work sequences with the primary target of prevention of contamination and injuries Thorough anamnesis, which registers all infection risks based on a patient Instruments are contaminated when they have been used or opened (even unused!). They must then be hygienically processed without exception. The personnel has to wear an appropriate protective clothing, foremost gloves, for its own safety. Instruments should not be kept longer than necessary in physiological saline solution as the contact can cause corrosion (rust formation). The moistening of instruments should be effected thoroughly in an adequate container. After disinfection, subsequent flushing should be carried out only with demineralized water. This proceeding minimizes the generation of water spots and other deposits which may influence the following sterilization process negatively. In order to achieve the necessary safety upon sterilization of instruments, the staff member in charge for processing of instruments must apply validated procedures for cleaning, disinfection and sterilization only. Regular maintenance, monitoring and compliance with pre-defined parameters for each cycle of processing are also mandatory. Processing is accomplished upon release for application by the staff member in charge. The respective SOP of the equipment lists modalities of the sterile-package (e.g. sterile-container). A sterilization indicator and indication of the date of sterilization must be marked in general. Basically, it is distinguished between manual and mechanical procedures for processing. Mechanical procedures for processing should be preferably deployed, as the process parameter and procedures can be reproduced. The following chapters describe the instruction for manual and mechanical processing and sterilization. \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 2/8
3 4 Manual pre-cleaning Touch dry contamination can make the processing of instruments difficult. Removable parts must be disassembled. Hence, pre-cleaning should be carried out promptly after deployment of instruments. For prevention of touch -dry contamination and for the protection of the personnel, all in struments should be put into an adequate disinfection solution with bactericide, fungicide, sporocide und antiviral effect. This must be thoroughly swayed. Rough contaminations, as blood, and residues from tissues and bones must be removed subsequently. For this purpose, the instruments must be taken from the bowl and all visible residues and contaminations must be washed up with fluent cold water. Alternatively, instruments may be cleaned in a disinfection solution with a soft nylon brush or a clean soft cloth which will be used for the purpose of this cleaning only. The instruments must not be treated with metal brushes, steel wool or other similar abrasive tools. Mechanical cleaning should be followed within the subsequent 2 hours. Attention: Danger of protein coagulation exists upon temperatures from 40 C, connected with an impeded removal of organic residues. The optimal working temperature for a disinfection bath is around room temperature. The initial disinfection is not a replacement for the subsequent sterilization of instruments! 4.1 Pre-cleaning of instruments with a through hole Due to the small diameter of through holes, for e.g. guide wires, requirements for re-processing are high. To assure a reliable processing of such instruments, these relatively small lumens must be manually pre-cleaned as well. Thereby, the following listing is decisive: The instrument must be put into cold water for soaking of the contaminations promptly after its deployment. Thereby, the instrument has to be fully wetted with water. The through holes must be released by rough contaminations by using a water-cleaning gun (at least 15 seconds, pressure 4.2 bar). Passability has to be checked. Instruments can be cleaned mechanically afterwards. 4.2 Ultra sonic cleaning Ultra sonic cleaning should be effected if instruments are strongly contaminated or if rough contaminations cannot be removed easily through manual pre-cleaning (see chapter 4.1). Application time and concentration of the cleaning agents must be considered and complied with manufacturer information. Beside, manufacturer information referring to the amount of water in the ultra sonic bath and compatibility of cleaning agent and instruments must be considered. In case of doubt, the manufacturer of the cleaning agent should be contacted. 5 Mechanical cleaning Adequate and qualified cleaning and disinfection equipment should be used for mechanical cleaning. They must allow the pre-defined and validated cleaning processes and inspections by the user. Manufacturer information of cleaning and disinfection equipment must be complied. This also applies for manufacturer information of cleaning and neutralization agents, especially dosing. The preferable water quality for cleaning, notably for the subsequent flush -phase, is fully desalinated water or water which meets the required purity degree. Cleaning programs are regarded as optimal if they achieve thermic disinfection and a best possible removing of blood. In case of doubt, the manufacturer of cleaning and disinfection equipment as well as cleaning and neutralization agents should be contacted. The company Weber Instrumente recommends the following cleaning method (or an equivalent validated procedure): Machine/program: Vario TD Program by the company Miele Professional with cleaning agent: neodischer MediClean, disinfection agent: Getinge 88. Pre-cleaning with cold water for 4 min. Cleaning with alkaline cleaner, max 55 C 10 min. Neutralization 6 min. Inbetween-cleaning 3 min. Disinfection 5 min. Drying 30 min. \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 3/8
4 5.1 Drying before maintenance and sterilization Usually the drying of instruments will be effected automatically in the drying cycle of the clean ing and disinfection equipment. Residue-free compressed air can be used for the drying of lumens. Afterwards, all cleaned and disinfected instruments must be checked once again with regard to damages of surfaces and corrosion. 6 Maintenance of instruments Disassembled components of instruments can be reassembled before maintenance. To ensure efficiency of removable parts, notably the coupling and ratchets, they must always be lubricated after mechanical cleaning and before steam pressure sterilization. The components of the instruments should never dry run, i.e. deployed without lubricants as this may cause increasing wear-out failure and rapid failure of the entire instrument. The company Weber Instrumente recommends the care spray for instruments by the manufacturer Dr. Schuhmacher GmbH (D Malsfeld). For lubrication, equivalent care spray-lubricants for medical devices may also be used. Before each sterilization, a function check of removable parts must be carried out. If instruments are damaged, they must not be redeployed. 6.1 Application with Forcipes and similar instruments Forcipes and similar instruments with swivel joints or prismatic joints and threads have to be lubricated at the bearing surfaces. The user has to take care that the instrument is half opened and a thin oil film is sprayed on (Figure 1 - example of forcipes). Generally a distance of the spray to the instrument of approximately 25 cm should be kept and approx. 1 second should be sprayed. Excessive oil should be removed with a clean lint free cloth (please follow the instructions of the manufacturer). The instrument is ready for sterilization. Figure 1: Example of forcipes (compressor) with bearing surfaces that have to be lubricated (circuited areas) 6.2 Application with couplings and ratchets Important for the maintenance of couplings and ratchets is the lubrication of the inner functional parts. The following points have to be followed to achieve a sufficient lubrication : The spray has to be moved with the nozzle pipe to the limit stop into the drilling which is counterpart to the push button (if this is present). Spray for approx. 1 sec (Figure 2). Spray inside the drill which is counterpart to the push button Figure 2: Spray into the drill \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 4/8
5 Spray with nozzle pipe to the limit stop inside the main through hole of the coupling and the ratchet (that is valid for all designs). Spray for approx. 1 sec. Spray inside the gap between push button (if this is present) for one second (Figure 3). Push the push button (if this is present) 3 times (Figure 3). Lubrication of the gap at the push button and the through hole (spray approx. 1 sec each) Push the push button (3x) Figure 3: Lubricate the gap and through hole, push the push button 3 times The gap between the coupling body and the housing has to be lubricated (spray for approx. 1 sec). Move the sliding sleeve (if this is present) 3 times (Figure 4). Lubrication of the gap between coupling body and housing (spray for approx. 1 sec) Move sliding sleeve (3x) Figure 4: Lubrication of gap between coupling body and housing, move sliding sleeve 3 times To achieve a homogeneous lubrication of the inner functional parts the regulation sleeve has to be placed at a limit stop (right or left). Spray between the gap of the regulation sleeve and housing at the surface that is free. Take care that the nozzle pipe is at the level of the screw / labeling. Spray for approx. 1 sec (Figure 5). Lubricate gap between regulation sleeve and housing at the level of the screw (spray approx. 1 sec) Move regulation sleeve to the limit stop Figure 5: Lubricate the gap between the regulation sleeve and housing \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 5/8
6 Move the regulation sleeve to the opposite limit stop. Spray between the gap of the regulation sleeve and housing at the surface that is free. Take care that the nozzle pipe is at the level of the screw / labeling. Spray for approx. 1 sec (Figure 6). Lubricate gap between regulation sleeve and housing at the level of the screw (spray approx. 1 sec) Move regulation sleeve to the limit stop (opposite side) Figure 6: Lubricate the gap between the regulation sleeve and housing (opposite side) Move the ratchet with your fingers at least one complete turn in the free direction of rotation. Move the regulation sleeve in the opposite direction. Move the ratchet with your fingers at least one complete turn in the free direction of rotation (Figure 7). Move regulation sleeve for left- and right-rotation of ratchet Rotation of the ratchet (at least one complete turn left- and right) Figure 7: Left and right rotation of the ratchet, movement of regulation sleeve Move regulation sleeve to ratchet fixed position (centric position). Remove excessive oil with a clean and lint free cloth (follow the instructions of the manufacturer). The instrument is ready for sterilization. 7 Sterilization The instruments can be sterilized individually. However, it is recommended to sort them to the designated sterilizable tray. Afterwards, the instruments or trays must be packed into single-use-sterilization packages suitable for steam pressure sterilization (single or double packing) and /or must be packed into a sterilization -container. All packages for steam pressure sterilization must adhere to the requirements according to DIN EN ISO / ANSI/AAMI ST79/ AAMI TIR12:2010, e.g. single-use-sterilization package (single or double package) with a temperature resistance until at least 137 C (279 F) and a sufficient vapor permeability. It must have sufficient protection against mechanical damages. Sterilization container must be maintained according to manufacturer information. Sterilization must be effected in autoclaves with the following parameters: Fractional pre-vacuum (threefold) Sterilization temperature at least 132 C Dwell time for at least 6 min. Drying time at least for 10 min. Release for anew deployment of instruments must be effected by the staff member in charge upon evaluation of the recorded sterilization parameters. \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 6/8
7 8 Storage Store sterile packaged instruments dry and dust-free at room temperature. Further notes may be listed in the manufacturer information of the respective packages. 9 Marking of products This SOP applies only for products provided by Weber Instrumente GmbH & Co. KG, which are marked by the company Weber Instrumente GmbH & Co. KG. Identically constructed products, which are marked user-specifically by customers of the company Weber Instrumente GmbH & Co. KG, require a separate user-specific modified instruction for cleaning and processing. \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 7/8
8 Revision status Revision Modification Issued by / Date 00 First version Arne Jansen-Troy / 03/04/ Translation of Revision 00 (English Version) Irene Troy / 03/09/2015 Inspected by / Date Arian Mingo / 03/04/2015 Arne Jansen-Troy / 03/09/2015 Approved by / Date Armin Studer / 03/04/2015 Armin Studer / 03/09/2015 \\server\dokumente\qm\dqm0000\word\dqm r00.dotx Page 8/8
Instruction Guide Re-Usable Surgical Instruments. Instruction Guide Re-usable surgical instruments (GA 30.1)
 Instruction Guide Re-usable surgical instruments (GA 30.1) Part A: General Information 1. Field of application 2. Basics 3. General 4. Construction and Material 5. Compatibility 6. Labelling label symbols
Instruction Guide Re-usable surgical instruments (GA 30.1) Part A: General Information 1. Field of application 2. Basics 3. General 4. Construction and Material 5. Compatibility 6. Labelling label symbols
Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants
 Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA
Guide for Cleaning, Sterilization and Storage of Introducers for Caldera Medical Implants M Manufactured by: Caldera Medical, Inc. 5171 Clareton Drive Agoura Hills, CA 91301 U.S. Toll Free: 866-4-CALDERA
ATTENTION: READ THESE INSTRUCTIONS VERY CAREFULLY BEFORE YOU PREPARE AND USE THE PRODUCT FOR THE FIRST TIME.
 INSTRUCTION MANUAL Field of application All reusable surgical instruments, which consist of only one piece may contain simple joints or simple movable parts may be composed of several exchangeable parts
INSTRUCTION MANUAL Field of application All reusable surgical instruments, which consist of only one piece may contain simple joints or simple movable parts may be composed of several exchangeable parts
CAUTION: FEDERAL LAW (USA) RESTRICTS THESE DEVICES TO SALE BY OR ON THE ORDER OF A PHYSICIAN.
 Manufacturer Silony Medical GmbH Leinfelder Strasse 60 70771 Leinfelden-Echterdingen Germany US Representative: Silony Medical Corp. 8200 NW 27TH STR, STE#104, DORAL, FL 33122 USA Phone: +1 305 916 0016
Manufacturer Silony Medical GmbH Leinfelder Strasse 60 70771 Leinfelden-Echterdingen Germany US Representative: Silony Medical Corp. 8200 NW 27TH STR, STE#104, DORAL, FL 33122 USA Phone: +1 305 916 0016
Sterilization of Schweickhardt Instruments
 Sterilization of Schweickhardt Instruments INTRODUCTION l Most dental instruments are made of corrosion-resistant highgrade steels. The requirements for grades of steel are defined in national and international
Sterilization of Schweickhardt Instruments INTRODUCTION l Most dental instruments are made of corrosion-resistant highgrade steels. The requirements for grades of steel are defined in national and international
RIGID AUTOCLAVABLE ENDOSCOPES
 IMPORTANT ADVICES PLEASE READ CAREFULLY AND KEEP THE INSTRUCTIONS FOR FUTURE REFERENCE RIGID AUTOCLAVABLE ENDOSCOPES Applications RUDOLF endoscopes serve the purpose of illumination and visualisation during
IMPORTANT ADVICES PLEASE READ CAREFULLY AND KEEP THE INSTRUCTIONS FOR FUTURE REFERENCE RIGID AUTOCLAVABLE ENDOSCOPES Applications RUDOLF endoscopes serve the purpose of illumination and visualisation during
HemoCheck TM Sample Policy. SUBJECT: Detection of blood residue on various surfaces
 HemoCheck TM Sample Policy SUBJECT: Detection of blood residue on various surfaces DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue on
HemoCheck TM Sample Policy SUBJECT: Detection of blood residue on various surfaces DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue on
Entellus Medical Sinuscope INSTRUCTIONS FOR USE
 Entellus Medical Sinuscope INSTRUCTIONS FOR USE Table of contents Entellus Medical Sinuscope Instructions for Use 1. About this document... 1 1.1 Purpose... 1 1.2 Symbols used... 1 2. Intended use... 2
Entellus Medical Sinuscope INSTRUCTIONS FOR USE Table of contents Entellus Medical Sinuscope Instructions for Use 1. About this document... 1 1.1 Purpose... 1 1.2 Symbols used... 1 2. Intended use... 2
Cranio-caudal Fixation Unit CC-GE MR10063-CC-GE
 Cranio-caudal Fixation Unit CC-GE MR10063-CC-GE Cranio caudal Fixation Unit CC-GE for Immobilization with the GE 8 channel breast coil Operator s Manual Revision 04 Issued Date: August 2016 Technichal
Cranio-caudal Fixation Unit CC-GE MR10063-CC-GE Cranio caudal Fixation Unit CC-GE for Immobilization with the GE 8 channel breast coil Operator s Manual Revision 04 Issued Date: August 2016 Technichal
One such problem is blood residual inside the channels of a robotic arm.
 SUBJECT: Robotic Arm Testing for residue blood DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue inside and outside of a robotic arm and
SUBJECT: Robotic Arm Testing for residue blood DEPARTMENT: Central Service APPROVED BY: EFFECTIVE: REVISED: 07/2016 PURPOSE: To test for detection of blood residue inside and outside of a robotic arm and
Best Practices for Reprocessing Robotic Instruments
 Best Practices for Reprocessing Robotic Instruments Nupur Jain Manager, Validation Engineering Services Intuitive Surgical, US Agenda 1. Background on robotic instruments 2. Reprocessing procedure for
Best Practices for Reprocessing Robotic Instruments Nupur Jain Manager, Validation Engineering Services Intuitive Surgical, US Agenda 1. Background on robotic instruments 2. Reprocessing procedure for
Best Practices for Reprocessing Endoscopes
 Best Practices for Reprocessing Endoscopes Reprocessing of Endoscopes Reprocessing Room Standards The resources referenced include: Canadian Standards Association Provincial Infectious Disease Advisory
Best Practices for Reprocessing Endoscopes Reprocessing of Endoscopes Reprocessing Room Standards The resources referenced include: Canadian Standards Association Provincial Infectious Disease Advisory
Recommendations for Care, Cleaning, Maintenance and Sterilization. Manual Orthopaedic Surgical Instruments
 Recommendations for Care, Cleaning, Maintenance and Sterilization Manual Orthopaedic Surgical Instruments Manual Orthopaedic Surgical Instruments Manual Orthopaedic Surgical Instruments Table of Contents
Recommendations for Care, Cleaning, Maintenance and Sterilization Manual Orthopaedic Surgical Instruments Manual Orthopaedic Surgical Instruments Manual Orthopaedic Surgical Instruments Table of Contents
Guidelines for processing and sterilising instruments
 Guidelines for processing and sterilising instruments Table of contents Underlying concept of the document 1 5 Purpose, scope, glossary, symbols General information concerning reprocessing 6 13 Preliminary
Guidelines for processing and sterilising instruments Table of contents Underlying concept of the document 1 5 Purpose, scope, glossary, symbols General information concerning reprocessing 6 13 Preliminary
Instructions for use. Reusable surgical instruments. Hersteller:
 Hersteller: ROLAN Instruments GmbH Ernst-Thälmann-Str. 50 15859 Storkow / Germany Tel.: 0049 33678 449433 E-Mail: info@rolan-instruments.de Item codes 01-02- 03-04- 05-06- 07-08- 09-10- 11-12- 13-14- 15-16-
Hersteller: ROLAN Instruments GmbH Ernst-Thälmann-Str. 50 15859 Storkow / Germany Tel.: 0049 33678 449433 E-Mail: info@rolan-instruments.de Item codes 01-02- 03-04- 05-06- 07-08- 09-10- 11-12- 13-14- 15-16-
New Requirements of ISO 17664/AAMI ST 81
 New Requirements of ISO 17664/AAMI ST 81 How the instrument manufacturer validate the cleaning process Klaus Roth SMP GmbH Tübingen Germany 21.09.2017 SMP GmbH 2017 1 Operation, which have been cancelled
New Requirements of ISO 17664/AAMI ST 81 How the instrument manufacturer validate the cleaning process Klaus Roth SMP GmbH Tübingen Germany 21.09.2017 SMP GmbH 2017 1 Operation, which have been cancelled
SUBJECT: Detection of Blood residue inside the biopsy channel of a scope
 SUBJECT: Detection of Blood residue inside the biopsy channel of a scope DEPARTMENT: Central Service/ Endoscopy APPROVED BY: EFFECTIVE: REVISED: 2/2012 PURPOSE: To test for detection of blood residue inside
SUBJECT: Detection of Blood residue inside the biopsy channel of a scope DEPARTMENT: Central Service/ Endoscopy APPROVED BY: EFFECTIVE: REVISED: 2/2012 PURPOSE: To test for detection of blood residue inside
GEISTER. I.F.U-Re/Processing Reusable Medical Devices. Medical articulated arm with quick fixation + Components
 GEISTER Page 1 of 12 Table of Contents 1 Introduction... 3 1.1 General Information... 3 1.2 Symbols used... 3 1.3 Definitions (according to the norm EN ISO 17664)... 4 2 Intended uses... 4 3 Product description...
GEISTER Page 1 of 12 Table of Contents 1 Introduction... 3 1.1 General Information... 3 1.2 Symbols used... 3 1.3 Definitions (according to the norm EN ISO 17664)... 4 2 Intended uses... 4 3 Product description...
RITTER Pickling Fluids finished blending VA 10 concentrate 1:1 VA 15 concentrate 1:2 VA 20
 How to use RITTER Pickling Products RITTER Pickling Fluids finished blending VA 10 concentrate 1:1 VA 15 concentrate 1:2 VA 20 30 kg 200 kg 1000 kg RITTER Pickling Fluids for diving, spraying and revolving
How to use RITTER Pickling Products RITTER Pickling Fluids finished blending VA 10 concentrate 1:1 VA 15 concentrate 1:2 VA 20 30 kg 200 kg 1000 kg RITTER Pickling Fluids for diving, spraying and revolving
LIQUID PENETRANTS USER S MANUAL in accordance to specifications: EN 571, ASTME 1417, AMS 2644, MIL, ASME, DIN, UNI, BS, AFNOR, etc..
 LIQUID PENETRANTS USER S MANUAL in accordance to specifications: EN 571, ASTME 1417, AMS 2644, MIL, ASME, DIN, UNI, BS, AFNOR, etc.. The liquid penetrant examination method is an effective means for detecting
LIQUID PENETRANTS USER S MANUAL in accordance to specifications: EN 571, ASTME 1417, AMS 2644, MIL, ASME, DIN, UNI, BS, AFNOR, etc.. The liquid penetrant examination method is an effective means for detecting
Sirona competency in hygiene DAC PROFESSIONAL. Hygiene systems. Vacuum autoclave class B to satisfy the most demanding requirements
 Sirona competency in hygiene Hygiene systems Vacuum autoclave class B to satisfy the most demanding requirements Professional and fast Sirona s is an autoclave which satisfies the most demanding medical
Sirona competency in hygiene Hygiene systems Vacuum autoclave class B to satisfy the most demanding requirements Professional and fast Sirona s is an autoclave which satisfies the most demanding medical
2-finger angular gripper SGB 32-50
 www.comoso.com Translation of the original manual 2-finger angular gripper SGB 32-50 Assembly and Operating Manual Superior Clamping and Gripping Imprint www.comoso.com Imprint Copyright: This manual remains
www.comoso.com Translation of the original manual 2-finger angular gripper SGB 32-50 Assembly and Operating Manual Superior Clamping and Gripping Imprint www.comoso.com Imprint Copyright: This manual remains
PRODUCT DATA SHEET Sika Unitherm Platinum
 PRODUCT DATA SHEET Solvent free, ultra high build, two pack, modified epoxy resin based, intumescent fire protection coating for internally or externally exposed structural steel. Ideal for in-shop application.
PRODUCT DATA SHEET Solvent free, ultra high build, two pack, modified epoxy resin based, intumescent fire protection coating for internally or externally exposed structural steel. Ideal for in-shop application.
Cranio-caudal Fixation Unit CC-320-Plus
 Cranio-caudal Fixation Unit CC-320-Plus 118341 for immobilization combined with the NORAS Breast Biopsy 4-Ch Coil, the Siemens Breast Biopsy 4-Ch Coil, the Breast Biopsy 4-Ch Coil for GE or the NORAS Patient
Cranio-caudal Fixation Unit CC-320-Plus 118341 for immobilization combined with the NORAS Breast Biopsy 4-Ch Coil, the Siemens Breast Biopsy 4-Ch Coil, the Breast Biopsy 4-Ch Coil for GE or the NORAS Patient
The next generation in ultrasound probe disinfection _Trophon_EPR_MM0001-DE-BR-V03_cc5.indd 1
 The next generation in ultrasound probe disinfection 19695_Trophon_EPR_MM0001-DE-BR-V03_cc5.indd 1 Automated disinfection for enhanced patient safety The Robert-Koch-Institutes processing guidelines stipulate
The next generation in ultrasound probe disinfection 19695_Trophon_EPR_MM0001-DE-BR-V03_cc5.indd 1 Automated disinfection for enhanced patient safety The Robert-Koch-Institutes processing guidelines stipulate
Sterilization Policy. Georgia Regents Medical Center Policy Library. Policy Owner: Epidemiology POLICY STATEMENT
 POLICY STATEMENT The ability to sterilize instruments and equipment for use during operative or other invasive procedures is critical to promoting successful patient outcomes and preventing infections.
POLICY STATEMENT The ability to sterilize instruments and equipment for use during operative or other invasive procedures is critical to promoting successful patient outcomes and preventing infections.
ARABIC CANADIAN MEDICAL CENTER CSSD POLICIES AND PROCEDURES
 CONTENTS Section Title 2 Disinfection, Packaging and Sterilization 3 Validation of Sterilization (Physical, Chemical and Biological) 4 Sterilizer Requalification 5 Sterile Package Shelf Life 6 Acquisition
CONTENTS Section Title 2 Disinfection, Packaging and Sterilization 3 Validation of Sterilization (Physical, Chemical and Biological) 4 Sterilizer Requalification 5 Sterile Package Shelf Life 6 Acquisition
pump pressure conveying quantity chamber geometry flow conditions Washing arms trays water quantity dosing temperature foam performance
 Validation of cleaning and disinfecting processes in WDs: Implementation of the ÖGSV- Guideline M. Gehrer, T. Miorini, V. Buchrieser, A. Gruber, G. Maierl Processing of instruments preferably automated
Validation of cleaning and disinfecting processes in WDs: Implementation of the ÖGSV- Guideline M. Gehrer, T. Miorini, V. Buchrieser, A. Gruber, G. Maierl Processing of instruments preferably automated
2-Finger-Parallel-Gripper RH
 Translation of the original manual 2-Finger-Parallel-Gripper RH 901-940 Assembly and Operating Manual Superior Clamping and Gripping Imprint Imprint Copyright: This manual remains the copyrighted property
Translation of the original manual 2-Finger-Parallel-Gripper RH 901-940 Assembly and Operating Manual Superior Clamping and Gripping Imprint Imprint Copyright: This manual remains the copyrighted property
Translation authorised by DIBt. to: 1 December January 2017 I /16
 Date: Reference: 24 January 2017 I 29-1.21.1-23/16 Approval number: Z-21.1-1711 Validity from: 1 December 2016 to: 1 December 2021 Applicant: fischerwerke GmbH & Co. KG Klaus-Fischer-Straße 1 72178 Waldachtal
Date: Reference: 24 January 2017 I 29-1.21.1-23/16 Approval number: Z-21.1-1711 Validity from: 1 December 2016 to: 1 December 2021 Applicant: fischerwerke GmbH & Co. KG Klaus-Fischer-Straße 1 72178 Waldachtal
Instruction of use. Annastr.25/1 D Fridingen. Reusable surgical Instruments (Steel, Titanium, TITANIT and antimagnetic Titanium Instruments)
 You get with the purchase this instrument and it is a hi quality product, its proper handling and use are described below. To keep hazards for patients and users as possible, we ask you to follow the instructions
You get with the purchase this instrument and it is a hi quality product, its proper handling and use are described below. To keep hazards for patients and users as possible, we ask you to follow the instructions
National technical approval Coupler
 National technical approval Coupler Z-1.5-100 12.02.2015 english Mechanical connection of B500B reinforcing steel bars with screw couplers Nominal diameter: 12 to 32 mm Tested by: DIBt, Berlin Max Frank
National technical approval Coupler Z-1.5-100 12.02.2015 english Mechanical connection of B500B reinforcing steel bars with screw couplers Nominal diameter: 12 to 32 mm Tested by: DIBt, Berlin Max Frank
Validation of cleaning and sterilization of reusable medical devices
 Synergy makes sence Validation of cleaning and sterilization of reusable medical devices MG-FSI72-119 Last revision: June 2012 Aurélien BIGNON 5, Chemin du Catupolan - 69120 Vaulx en Velin - France - Tel.
Synergy makes sence Validation of cleaning and sterilization of reusable medical devices MG-FSI72-119 Last revision: June 2012 Aurélien BIGNON 5, Chemin du Catupolan - 69120 Vaulx en Velin - France - Tel.
SERIE 4. rapid, reliable, efficient
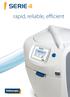 SERIE 4 rapid, reliable, efficient SERIE 4 Intuitive an machine 12/07/2014 15:23:00 1 35 C The power at your fingertips A NEW GENERATION OF INTERFACE The Serie 4 PA brings the benefit of a new intuitive
SERIE 4 rapid, reliable, efficient SERIE 4 Intuitive an machine 12/07/2014 15:23:00 1 35 C The power at your fingertips A NEW GENERATION OF INTERFACE The Serie 4 PA brings the benefit of a new intuitive
Performance, Beauty & Long Life STAINLESS STEEL CARE & MAINTENANCE. Stainless Steel Performance. Stain less Steel
 Stainless Steel Performance Stainless Steel Performs Best When Clean Cleanliness of stainless steel is essential for maximum resistance to corrosion. Properly maintained stainless steel provides excellent
Stainless Steel Performance Stainless Steel Performs Best When Clean Cleanliness of stainless steel is essential for maximum resistance to corrosion. Properly maintained stainless steel provides excellent
X-Cid 2 The automatic pre-sterilization cleaning device. Your Endo Specialist
 X-Cid 2 The automatic pre-sterilization cleaning device Your Endo Specialist X-Cid 2 The risk of infection in the dental office. Ensuring asepsis and controlling the risk of infection are of increasing
X-Cid 2 The automatic pre-sterilization cleaning device Your Endo Specialist X-Cid 2 The risk of infection in the dental office. Ensuring asepsis and controlling the risk of infection are of increasing
Disinfection and sterilisation
 Disinfection and sterilisation Mongolia 2011 Prof. Dr. Walter Popp Hospital Hygiene, University Clinics Essen, Germany 1 Term Definition Reduction factor of germs Cleaning Remove dirt including 10-100
Disinfection and sterilisation Mongolia 2011 Prof. Dr. Walter Popp Hospital Hygiene, University Clinics Essen, Germany 1 Term Definition Reduction factor of germs Cleaning Remove dirt including 10-100
Instruction Manual Rigid endoscopes with and without working / irrigation channel
 Instruction Manual Rigid endoscopes with and without working / irrigation channel 1. Content Chapter Page 1. Content 2 2. Legal advices 3 3. Signs and symbols 4 4. Introduction 5 5. Security advices/ warnings
Instruction Manual Rigid endoscopes with and without working / irrigation channel 1. Content Chapter Page 1. Content 2 2. Legal advices 3 3. Signs and symbols 4 4. Introduction 5 5. Security advices/ warnings
Best Practices for High Level Disinfection Part I
 Best Practices for High Level Disinfection Part I Nancy Chobin, RN, AAS, ACSP, CSPM, CFER 2017 Sterile Processing University, LLC. **This in-service has been Approved by the CBSPD for 1.5 CEUs. The Bible
Best Practices for High Level Disinfection Part I Nancy Chobin, RN, AAS, ACSP, CSPM, CFER 2017 Sterile Processing University, LLC. **This in-service has been Approved by the CBSPD for 1.5 CEUs. The Bible
Products made in Switzerland
 Products made in Switzerland HYGIENIC AND MEDICALLY CLEAR DIFFERENT PRODUCTS BUT THE SAME QUALITY LABEL HYGIENIC AND MEDICALLY CLEAR 2 MDD TECHNOLOGIES FOR: 1. Cleaning 2. Disinfection 3. High Level Disinfection
Products made in Switzerland HYGIENIC AND MEDICALLY CLEAR DIFFERENT PRODUCTS BUT THE SAME QUALITY LABEL HYGIENIC AND MEDICALLY CLEAR 2 MDD TECHNOLOGIES FOR: 1. Cleaning 2. Disinfection 3. High Level Disinfection
Administrative Policies and Procedures. Cross Reference: Date Issued: 2/14 Date Reviewed: 9/14 Date: Revised: 12/14 Attachment: None Page of 1 of 6
 Administrative Policies and Procedures Originating Venue: Infection Control Policy No.: IC 2306 Title: Cystoscope Reprocessing Policy & Procedure Cross Reference: Date Issued: 2/14 Date Reviewed: 9/14
Administrative Policies and Procedures Originating Venue: Infection Control Policy No.: IC 2306 Title: Cystoscope Reprocessing Policy & Procedure Cross Reference: Date Issued: 2/14 Date Reviewed: 9/14
Pipe Joints. Requirements and Test Methods. VdS-Guidelines for water extinguishing systems. VdS en. VdS en : (01)
 VdS-Guidelines for water extinguishing systems VdS 2100-6en Requirements and Test Methods Reproduction prohibited even for internal use VdS 2100-6en : 2004-01 (01) Publisher and Publishing house: VdS Schadenverhütung
VdS-Guidelines for water extinguishing systems VdS 2100-6en Requirements and Test Methods Reproduction prohibited even for internal use VdS 2100-6en : 2004-01 (01) Publisher and Publishing house: VdS Schadenverhütung
Industrial flue gas probes. Instruction manual
 Industrial flue gas probes Instruction manual 2 1 Contents 1 Contents 1 Contents... 3 2 Safety and the environment... 4 2.1. About this document... 4 2.2. Ensure safety... 4 2.3. Protecting the environment...
Industrial flue gas probes Instruction manual 2 1 Contents 1 Contents 1 Contents... 3 2 Safety and the environment... 4 2.1. About this document... 4 2.2. Ensure safety... 4 2.3. Protecting the environment...
geba fire damper WFK according to EN 15650
 Installation- and operation instruction geba fire damper WFK according to EN 15650 with a free cross-section for the usage in ventilation systems in buildings Abbildung 1 DN 250 Tested according to EN
Installation- and operation instruction geba fire damper WFK according to EN 15650 with a free cross-section for the usage in ventilation systems in buildings Abbildung 1 DN 250 Tested according to EN
Professional standard for Loaner instrumentation
 Sterilisatie Vereniging Nederland Professional standard for Loaner instrumentation Standard for loaning or renting of surgical instruments Version 04 November, 2010 Index Index... 2 Preface... 3 The Project
Sterilisatie Vereniging Nederland Professional standard for Loaner instrumentation Standard for loaning or renting of surgical instruments Version 04 November, 2010 Index Index... 2 Preface... 3 The Project
Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Webinar FAQ
 Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Webinar FAQ Disclaimer: The Society of Gastroenterology Nurses and Associates, Inc. assume no responsibility for the
Standards of Infection Prevention in Reprocessing Flexible Gastrointestinal Endoscopes Webinar FAQ Disclaimer: The Society of Gastroenterology Nurses and Associates, Inc. assume no responsibility for the
Water for Instrument Processing
 Water for Instrument Processing by Marcia Frieze, Case Medical Water, the universal solvent Water can dissolve more substances than any other liquid. It is essentially nonionic or neutral. While alkaline
Water for Instrument Processing by Marcia Frieze, Case Medical Water, the universal solvent Water can dissolve more substances than any other liquid. It is essentially nonionic or neutral. While alkaline
INSTRUMENT CLEANING & STERILISATION FOAMING BATH FORMULATION. Surgistain. Rapid Multi-Enzyme Cleaner. Kimguard Sterile Wrap. Instrument brushes $32.
 1Ltr Rapid Multi-Enzyme Cleaner Ÿ Proprietary combination enzyme Ÿ concentrate Ÿ Unparalleled combination of speed Ÿ and efficacy Ÿ Fully effective against proteins, Ÿ carbohydrates, lipids and Ÿ mucopolysaccharides
1Ltr Rapid Multi-Enzyme Cleaner Ÿ Proprietary combination enzyme Ÿ concentrate Ÿ Unparalleled combination of speed Ÿ and efficacy Ÿ Fully effective against proteins, Ÿ carbohydrates, lipids and Ÿ mucopolysaccharides
3034 Owen Drive Antioch, TN USA Fax: Maybachstraße 10. Revised 01/17
 CLN VersaPak Modular Instrument Tray Symmetry Surgical Inc. 3034 Owen Drive Antioch, TN 37013 USA 1-800-251-3000 Fax: 1-615-964-5566 www.symmetrysurgical.com EC REP Symmetry Surgical GmbH 78532 Tuttlingen,
CLN VersaPak Modular Instrument Tray Symmetry Surgical Inc. 3034 Owen Drive Antioch, TN 37013 USA 1-800-251-3000 Fax: 1-615-964-5566 www.symmetrysurgical.com EC REP Symmetry Surgical GmbH 78532 Tuttlingen,
2016. All rights reserved.
 DOCUMENT: Future AAMI/ISO 17664, 12 May 2017 Final Draft Standard Sterilization of health care products Information to be provided by the device manufacturer for the processing of medical devices Public
DOCUMENT: Future AAMI/ISO 17664, 12 May 2017 Final Draft Standard Sterilization of health care products Information to be provided by the device manufacturer for the processing of medical devices Public
AS Medizintechnik GmbH Sattlerstrasse 15, Tuttlingen, Germany Tel 07461/ Fax 07461/
 AS Medizintechnik GmbH Sattlerstrasse 15 78532 Tuttlingen, Germany Tel. (+49) 7461-966326 Fax: (+49) 7461-9663288 All rights reserved, any reproduction, adaption or translation, even in part, without the
AS Medizintechnik GmbH Sattlerstrasse 15 78532 Tuttlingen, Germany Tel. (+49) 7461-966326 Fax: (+49) 7461-9663288 All rights reserved, any reproduction, adaption or translation, even in part, without the
ENDOSCOPIC REPROCESSING ETD DOUBLE Advancing the Efficiency of Reprocessing.
 11157 ENDOSCOPIC REPROCESSING ETD DOUBLE Advancing the Efficiency of Reprocessing. ETD DOUBLE: INTRODUCING THE FIRST OLYMPUS PASS- THROUGH WASHER-DISINFECTOR With the ETD Double, Olympus Has Advanced Its
11157 ENDOSCOPIC REPROCESSING ETD DOUBLE Advancing the Efficiency of Reprocessing. ETD DOUBLE: INTRODUCING THE FIRST OLYMPUS PASS- THROUGH WASHER-DISINFECTOR With the ETD Double, Olympus Has Advanced Its
RIGID ENDOSCOPE OPERATING INSTRUCTIONS
 RIGID ENDOSCOPE OPERATING INSTRUCTIONS Note: Read this manual first. It contains important instructions and warnings concerning the proper assembly, use, and service of your new rigid endoscopy product(s).
RIGID ENDOSCOPE OPERATING INSTRUCTIONS Note: Read this manual first. It contains important instructions and warnings concerning the proper assembly, use, and service of your new rigid endoscopy product(s).
Getinge da Vinci Solution
 Getinge da Vinci Solution A comprehensive cleaning solution for da Vinci EndoWrist instruments This document is intended to provide information to an international audience outside of the US. Validated,
Getinge da Vinci Solution A comprehensive cleaning solution for da Vinci EndoWrist instruments This document is intended to provide information to an international audience outside of the US. Validated,
Standard Operating Procedure for performing a muscle biopsy
 Standard Operating Procedure for performing a muscle biopsy Effective date: 31.10.2016 Review due date: 30.08.2018 Original Author Name: Richard Metcalfe and Abdullah Alghannam Date: 14.12.2012 Position:
Standard Operating Procedure for performing a muscle biopsy Effective date: 31.10.2016 Review due date: 30.08.2018 Original Author Name: Richard Metcalfe and Abdullah Alghannam Date: 14.12.2012 Position:
PRODUCT DATA SHEET Sika Permacor -136 TW
 PRODUCT DATA SHEET Epoxy coating for use in water supply schemes, 100% volume solids PRODUCT DESCRIPTION Sika Permacor-136 TW is a 2-pack epoxy coating for steel and concrete. Solvent free according to
PRODUCT DATA SHEET Epoxy coating for use in water supply schemes, 100% volume solids PRODUCT DESCRIPTION Sika Permacor-136 TW is a 2-pack epoxy coating for steel and concrete. Solvent free according to
Technical Data Sheet EPOXY PRIMER 3:1 Anti-corrosion epoxy primer RELATED PRODUCTS. Epoxy primer hardener EPOXY PRIMER HARDENER PROPERTIES
 EPOXY PRIMER 3:1 Anti-corrosion epoxy primer RELATED PRODUCTS EPOXY PRIMER HARDENER Epoxy primer hardener Epoxy thinner PROPERTIES A product designed and dedicated for renovation of classic cars High solid
EPOXY PRIMER 3:1 Anti-corrosion epoxy primer RELATED PRODUCTS EPOXY PRIMER HARDENER Epoxy primer hardener Epoxy thinner PROPERTIES A product designed and dedicated for renovation of classic cars High solid
BakeMax Dough Mini Moulder BMMDM02
 BakeMax Dough Mini Moulder BMMDM02 2 Instruction Manual 1. Preface ------------------------------------------- P2 2. Machine Introduction -------------------------------- P2 3. Machine Specification and
BakeMax Dough Mini Moulder BMMDM02 2 Instruction Manual 1. Preface ------------------------------------------- P2 2. Machine Introduction -------------------------------- P2 3. Machine Specification and
IN-TANK TECHNOLOGY INSTRUCTIONS FOR USING FLO KING IN-TANK CARBON BAG
 Carbon Bag Instructions Page 1 INSTRUCTIONS FOR USING FLO KING IN-TANK CARBON BAG Do not attempt to carbon treat a solution before you have thoroughly filtered it! This almost certainly will result in
Carbon Bag Instructions Page 1 INSTRUCTIONS FOR USING FLO KING IN-TANK CARBON BAG Do not attempt to carbon treat a solution before you have thoroughly filtered it! This almost certainly will result in
Data Sheet 1806 Englisch
 Data Sheet 1806 Englisch Silicate Interior Paint ELF 1806 Silikat-Innenfarbe ELF 1806 low emission, solvent and plasticizer free, dull matt, wet abrasion resistance class 3, suitable for allergy sufferers,
Data Sheet 1806 Englisch Silicate Interior Paint ELF 1806 Silikat-Innenfarbe ELF 1806 low emission, solvent and plasticizer free, dull matt, wet abrasion resistance class 3, suitable for allergy sufferers,
STERILIZATION VALIDATION IN THIS SECTION REPROCESSING VALIDATIONS FOR REUSABLE MEDICAL DEVICES. Radiation Sterilization Validation
 STERILIZATION VALIDATION All sterilization processes require validation of the efficacy and reproducibility of the process. Depending on the type of sterilization, this may be accomplished by partial,
STERILIZATION VALIDATION All sterilization processes require validation of the efficacy and reproducibility of the process. Depending on the type of sterilization, this may be accomplished by partial,
Monitoring Flexible Endoscope Channels to Assure Cleaning
 Monitoring Flexible Endoscope Channels to Assure Cleaning Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers: Dr. M.
Monitoring Flexible Endoscope Channels to Assure Cleaning Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers: Dr. M.
Competency Guide: Care and Handling of Rigid Endoscopes
 Competency Guide: Care and Handling of Rigid Endoscopes This document details the basic steps to correctly process and maintain a rigid endoscope. This document can be used to perform a competency review
Competency Guide: Care and Handling of Rigid Endoscopes This document details the basic steps to correctly process and maintain a rigid endoscope. This document can be used to perform a competency review
Data Sheet 832 Englisch
 Englisch Primer LF 832 Floortec 2K-Epoxi-Grund LF 832 solvent-free, transparent primer resin as primer in Floortec PUR Coating System and under Floortec 2C Epoxy Thick Layer LF 834 Properties Solvent-free,
Englisch Primer LF 832 Floortec 2K-Epoxi-Grund LF 832 solvent-free, transparent primer resin as primer in Floortec PUR Coating System and under Floortec 2C Epoxy Thick Layer LF 834 Properties Solvent-free,
CLEANING AND PASSIVATING OF STAINLESS STEEL EQUIPMENT
 APPLICATIONS/CLEANING PROCEDURES C-1.30 October 2004 CLEANING AND PASSIVATING OF STAINLESS STEEL EQUIPMENT GENERAL CONSIDERATIONS Stainless steel derives its corrosion resistance from a very thin layer
APPLICATIONS/CLEANING PROCEDURES C-1.30 October 2004 CLEANING AND PASSIVATING OF STAINLESS STEEL EQUIPMENT GENERAL CONSIDERATIONS Stainless steel derives its corrosion resistance from a very thin layer
Sealing and blister punching machines
 Sealing and blister punching machines for MedTec, Dental, Watchmaking and Food industries ISO 9001:2008 EN ISO 13485:2012 Swiss Quality Products Hagmann Maschinenbau AG Bohnackerweg 6 CH- 2545 Selzach
Sealing and blister punching machines for MedTec, Dental, Watchmaking and Food industries ISO 9001:2008 EN ISO 13485:2012 Swiss Quality Products Hagmann Maschinenbau AG Bohnackerweg 6 CH- 2545 Selzach
Reprocessing of Single Use Devices (SUDs) MEDEC Regulatory Conference May 9, 2016
 Reprocessing of Single Use Devices (SUDs) MEDEC Regulatory Conference May 9, 2016 Objective and Outline Objective: Health Canada s requirements around licensing of Reprocessed Single Use Devices (RSUDS)
Reprocessing of Single Use Devices (SUDs) MEDEC Regulatory Conference May 9, 2016 Objective and Outline Objective: Health Canada s requirements around licensing of Reprocessed Single Use Devices (RSUDS)
Installation Guide ENAMELED CAST IRON APRON BATH. English page T01-A
 Installation Guide ENAMELED CAST IRON APRON BATH 368 201419 English page 1-9 10-17 Before You Begin A. Introduction Please read these instructions carefully to familiarize yourself with the required tools,
Installation Guide ENAMELED CAST IRON APRON BATH 368 201419 English page 1-9 10-17 Before You Begin A. Introduction Please read these instructions carefully to familiarize yourself with the required tools,
Indicon Gel Applications
 Indicon Gel Applications Trouble Finding Biofilm? Food processors know the challenges of sanitation and microbial trending. Recognizing trends and determining hot spots is one thing, but finding and resolving
Indicon Gel Applications Trouble Finding Biofilm? Food processors know the challenges of sanitation and microbial trending. Recognizing trends and determining hot spots is one thing, but finding and resolving
PRODUCT CATALOGUE. for gke Steri-Record and gke Clean-Record Chemical indicators, PCDs and accessories for cleaning and sterilization
 PRODUCT CATALOGUE for gke Steri-Record and gke Clean-Record Chemical indicators, PCDs and accessories for cleaning and sterilization Bowie-Dick Simulation Test Batch and Process Monitoring Systems, Documentation
PRODUCT CATALOGUE for gke Steri-Record and gke Clean-Record Chemical indicators, PCDs and accessories for cleaning and sterilization Bowie-Dick Simulation Test Batch and Process Monitoring Systems, Documentation
CONTROL OF LEGIONELLA FOR THE CAR WASH INDUSTRY
 CONTROL OF LEGIONELLA FOR THE CAR WASH INDUSTRY Legionella has become a focus for the car wash industry since the outbreak of Legionnaires disease at a car wash in Hoppers Crossing, Victoria. Legionnaires
CONTROL OF LEGIONELLA FOR THE CAR WASH INDUSTRY Legionella has become a focus for the car wash industry since the outbreak of Legionnaires disease at a car wash in Hoppers Crossing, Victoria. Legionnaires
Low temperature sterilizer Getinge STERIZONE VP4
 Low temperature sterilizer Getinge STERIZONE VP4 The new standard of low temperature sterilization This document is intended to provide information to an international audience outside of the US. Innovation
Low temperature sterilizer Getinge STERIZONE VP4 The new standard of low temperature sterilization This document is intended to provide information to an international audience outside of the US. Innovation
Construction. Pulastic Coating-221 W. 2 Component Polyurethane
 Product Data Sheet: Version: 08/11 ONLY TO BE APPLIED BY A SIKA APPROVED CONTRACTOR Pulastic Coating-221 W 2 Component Polyurethane Construction Positioning Description Water based, 2-component polyurethane
Product Data Sheet: Version: 08/11 ONLY TO BE APPLIED BY A SIKA APPROVED CONTRACTOR Pulastic Coating-221 W 2 Component Polyurethane Construction Positioning Description Water based, 2-component polyurethane
PRODUCT CATALOGUE. for gke Steri-Record and gke Clean-Record Chemical indicators, PCDs and accessories for cleaning and sterilization
 PRODUCT CATALOGUE for gke Steri-Record and gke Clean-Record Chemical indicators, PCDs and accessories for cleaning and sterilization Bowie-Dick Simulation Test Batch and Process Monitoring Systems, Documentation
PRODUCT CATALOGUE for gke Steri-Record and gke Clean-Record Chemical indicators, PCDs and accessories for cleaning and sterilization Bowie-Dick Simulation Test Batch and Process Monitoring Systems, Documentation
MODEL EXPOSURE CONTROL PLAN
 MODEL EXPOSURE CONTROL PLAN The Model Exposure Control Plan is intended to serve as an employer guide to the OSHA Bloodborne Pathogens standard. A central component of the requirements of the standard
MODEL EXPOSURE CONTROL PLAN The Model Exposure Control Plan is intended to serve as an employer guide to the OSHA Bloodborne Pathogens standard. A central component of the requirements of the standard
Scibond SL-23 Polymeric Lubrication System for Tube Drawing
 Scibond SL-23 Polymeric Lubrication System for Tube Drawing I. Introduction: Scibond SL-23 is a novel water-based a polymeric lubrication system. It was developed under National Science Foundation grant
Scibond SL-23 Polymeric Lubrication System for Tube Drawing I. Introduction: Scibond SL-23 is a novel water-based a polymeric lubrication system. It was developed under National Science Foundation grant
ISO/TC 198. Secretariat: ANSI
 DRAFT INTERNATIONAL STANDARD ISO/DIS 17664 ISO/TC 198 Secretariat: ANSI Voting begins on: Voting terminates on: 2016-04-28 2016-07-27 Processing of health care products Information to be provided by the
DRAFT INTERNATIONAL STANDARD ISO/DIS 17664 ISO/TC 198 Secretariat: ANSI Voting begins on: Voting terminates on: 2016-04-28 2016-07-27 Processing of health care products Information to be provided by the
SURFACE PREPARATION FOR SYNTHETIC ROPES
 Published date: June 30, 2005 SURFACE PREPARATION FOR SYNTHETIC ROPES Rust damaged chock typical of crude tankers. The following recommendations can be applied at the shipyard or during vessel operations.
Published date: June 30, 2005 SURFACE PREPARATION FOR SYNTHETIC ROPES Rust damaged chock typical of crude tankers. The following recommendations can be applied at the shipyard or during vessel operations.
iglidur A180 Very Appetising iglidur A180 Fax Phone
 Very Appetising The material complies with FOOD AND DRUG ADMINISTRATION (FDA) regulations For direct contact with food or pharmaceuticals For wet environments 7.1 Very Appetising The material complies
Very Appetising The material complies with FOOD AND DRUG ADMINISTRATION (FDA) regulations For direct contact with food or pharmaceuticals For wet environments 7.1 Very Appetising The material complies
S7 rhombos. expanded metal ceiling. Product data sheet. durlum.us. Your contact for North America
 S7 rhombos expanded metal ceiling Your contact for North America Product data sheet 2 4 1 3 1 2 U1045 U 1044 FK V 3 4 U 1042 U 1041 N 2 The open expanded metal ceiling S7-RHOMBOS offers a host of applications.
S7 rhombos expanded metal ceiling Your contact for North America Product data sheet 2 4 1 3 1 2 U1045 U 1044 FK V 3 4 U 1042 U 1041 N 2 The open expanded metal ceiling S7-RHOMBOS offers a host of applications.
Probe Manual EL Rev D. Handling, cleaning and sterilization of the Medistim EL Imaging Probe
 Handling, cleaning and sterilization of the Medistim EL 100015 Imaging Probe The purpose of this procedure is to guarantee that the probe performance is in accordance with the specifications during the
Handling, cleaning and sterilization of the Medistim EL 100015 Imaging Probe The purpose of this procedure is to guarantee that the probe performance is in accordance with the specifications during the
D E U T S C H E S I N S T I T U T F Ü R B A U T E C H N I K
 D E U T S C H E S I N S T I T U T F Ü R B A U T E C H N I K Corporation under Public Law 10829 Berlin, 17 July 2002 Kolonnenstraße 30 L Telefon: (0 30) 7 87 30-356 Telefax: (0 30) 7 87 30-320 GeschZ.:
D E U T S C H E S I N S T I T U T F Ü R B A U T E C H N I K Corporation under Public Law 10829 Berlin, 17 July 2002 Kolonnenstraße 30 L Telefon: (0 30) 7 87 30-356 Telefax: (0 30) 7 87 30-320 GeschZ.:
Operating instructions - Translation of the original -
 Operating instructions - Translation of the original - Duplex-Pigging-Systems KIESELMANN GmbH Paul-Kieselmann-Str.4-10 D - 75438 Knittlingen +49 (0) 7043 371-0 Fax: +49 (0) 7043 371-125 www.kieselmann.de
Operating instructions - Translation of the original - Duplex-Pigging-Systems KIESELMANN GmbH Paul-Kieselmann-Str.4-10 D - 75438 Knittlingen +49 (0) 7043 371-0 Fax: +49 (0) 7043 371-125 www.kieselmann.de
CAN STERILE REALLY BE STERILE?: EFFECTIVE AUTOCLAVING METHODS
 Vet Times The website for the veterinary profession https://www.vettimes.co.uk CAN STERILE REALLY BE STERILE?: EFFECTIVE AUTOCLAVING METHODS Author : James Gasson Categories : Vets Date : February 2, 2009
Vet Times The website for the veterinary profession https://www.vettimes.co.uk CAN STERILE REALLY BE STERILE?: EFFECTIVE AUTOCLAVING METHODS Author : James Gasson Categories : Vets Date : February 2, 2009
MODEL BE-100HT HEADSET TELEPHONE OWNER S MANUAL PLEASE READ THIS INSTRUCTION MANUAL CAREFULLY.
 MODEL BE-100HT HEADSET TELEPHONE OWNER S MANUAL PLEASE READ THIS INSTRUCTION - 1 - MANUAL CAREFULLY. - 2 - TABLE OF CONTENTS Introduction -------------------------------------------------------------------4
MODEL BE-100HT HEADSET TELEPHONE OWNER S MANUAL PLEASE READ THIS INSTRUCTION - 1 - MANUAL CAREFULLY. - 2 - TABLE OF CONTENTS Introduction -------------------------------------------------------------------4
AUTOMATED ENDOSCOPE REPROCESSOR. OER-Pro Easy. Fast. Reliable.
 AUTOMATED ENDOSCOPE REPROCESSOR OER-Pro Easy. Fast. Reliable. Olympus Endoscope Reprocessor The only reprocessor designed by an endoscope manufacturer. The OER-Pro provides high-level disinfection of Olympus
AUTOMATED ENDOSCOPE REPROCESSOR OER-Pro Easy. Fast. Reliable. Olympus Endoscope Reprocessor The only reprocessor designed by an endoscope manufacturer. The OER-Pro provides high-level disinfection of Olympus
INSTALLATION MANUAL. METAL INSULATED PANELS -ROXUL -Expanded Polystyrene
 INSTALLATION MANUAL METAL INSULATED PANELS -ROXUL -Expanded Polystyrene Insulated Metal Panels London Eco-Metal Manufacturing Inc. **Also see Eco-Metal s Handling Manual** These instructions are provided
INSTALLATION MANUAL METAL INSULATED PANELS -ROXUL -Expanded Polystyrene Insulated Metal Panels London Eco-Metal Manufacturing Inc. **Also see Eco-Metal s Handling Manual** These instructions are provided
Zinc Silicate (Inorganic)
 Product code: 477-041: part A 477-042: part B General description: Two-component, zinc-ethyl silicate-. Forms a continuous coat of metallic zinc that provides cathodic protection to metal (as in hot galvanization).
Product code: 477-041: part A 477-042: part B General description: Two-component, zinc-ethyl silicate-. Forms a continuous coat of metallic zinc that provides cathodic protection to metal (as in hot galvanization).
European Technical Approval ETA-12/0456
 European Technical Approval ETA-12/0456 - Original version in German language Handelsbezeichnung Trade name Zulassungsinhaber Holder of approval Zulassungsgegenstand und Verwendungszweck Generic type and
European Technical Approval ETA-12/0456 - Original version in German language Handelsbezeichnung Trade name Zulassungsinhaber Holder of approval Zulassungsgegenstand und Verwendungszweck Generic type and
Food Safety Audit Harvest Crew v07.04
 Food Safety Audit Harvest Crew v7.4 Auditor: Date Sent: Audit Start: Time/Date:: * Date Received: Audit End: Time/Date:: Foreman/Contact: Crew Name or Number: City/Location: State: Country: Scope G.P.S.:
Food Safety Audit Harvest Crew v7.4 Auditor: Date Sent: Audit Start: Time/Date:: * Date Received: Audit End: Time/Date:: Foreman/Contact: Crew Name or Number: City/Location: State: Country: Scope G.P.S.:
CASY Model TT E L E C T R I C A L C U R R E N T EXCLUSION ECE BASED CELL ANALYSIS
 CASY Model TT Cell Counter + AnalyZer E L E C T R I C A L C U R R E N T EXCLUSION ECE BASED CELL ANALYSIS ELECTRICAL CURRENT EXCLUSION CASY Model TT Cell Counter + Analyzer R&D Easy and fast adaptation
CASY Model TT Cell Counter + AnalyZer E L E C T R I C A L C U R R E N T EXCLUSION ECE BASED CELL ANALYSIS ELECTRICAL CURRENT EXCLUSION CASY Model TT Cell Counter + Analyzer R&D Easy and fast adaptation
Z5010 (Version 2) TV spigot with fixing plate Mounting instructions
 Z5010 (Version 2) TV spigot with fixing plate Mounting instructions Z5010 (Version 2) TV spigot with fixing plate References in the manual CAUTION! IMPORTANT! Symbols on the equipment This refers to a
Z5010 (Version 2) TV spigot with fixing plate Mounting instructions Z5010 (Version 2) TV spigot with fixing plate References in the manual CAUTION! IMPORTANT! Symbols on the equipment This refers to a
General Construction Supervisory Authority Approval
 General Construction Supervisory Authority Approval (Translation of the German original text which has not been checked by the DIBt.) Approval no.: Z-21.8-2018 Period of validity: April 1, 2014 until:
General Construction Supervisory Authority Approval (Translation of the German original text which has not been checked by the DIBt.) Approval no.: Z-21.8-2018 Period of validity: April 1, 2014 until:
AB-1020 EP Dust Free Vacuum Blast Machine
 AB-1020 EP Dust Free Vacuum Blast Machine This compact electric-pneumatic driven injection vacuum blasting machine with an average blasting pattern of 16 mm width, is excellent to apply for blast cleaning
AB-1020 EP Dust Free Vacuum Blast Machine This compact electric-pneumatic driven injection vacuum blasting machine with an average blasting pattern of 16 mm width, is excellent to apply for blast cleaning
Z5043 MAX Horizontal bracket Mounting instructions
 Z5043 MAX Horizontal bracket Mounting instructions Z5043 MAX Horizontal bracket References in the manual This refers to a potentially dangerous situation which may lead to personal injury. This refers
Z5043 MAX Horizontal bracket Mounting instructions Z5043 MAX Horizontal bracket References in the manual This refers to a potentially dangerous situation which may lead to personal injury. This refers
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy!
 Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers:
Flexible Endoscopes: The A,B,C s of Monitoring Manual Cleaning Efficacy! Dr. Michelle J. Alfa, Ph.D., FCCM Medical Director, Clinical Microbiology, Diagnostic Services of Manitoba, Winnipeg, Canada Disclaimers:
Technical Specification
 Technical Specification No.: Vacuum 005/2008 Version 1.6 / 22.09.2010 Guidelines for UHV-Components at DESY U. Hahn (FS-BT), K. Zapfe (MVS) Contents 1 General Information... 3 1.1 Introduction... 3 1.2
Technical Specification No.: Vacuum 005/2008 Version 1.6 / 22.09.2010 Guidelines for UHV-Components at DESY U. Hahn (FS-BT), K. Zapfe (MVS) Contents 1 General Information... 3 1.1 Introduction... 3 1.2
FLUXA Inspection Media for Magnetic Particle Testing
 FLUXA Inspection Media for Magnetic Particle Testing FLUXA Inspection Media for Magnetic Particle Testing UV-light fluorescent dry wet powder concentrates ready medium high powder DEUTROFLUX POWDER FLUXA
FLUXA Inspection Media for Magnetic Particle Testing FLUXA Inspection Media for Magnetic Particle Testing UV-light fluorescent dry wet powder concentrates ready medium high powder DEUTROFLUX POWDER FLUXA
Plastic Deep-Groove Ball Bearings
 Deutsche Star Plastic Deep-Groove Ball Bearings RE 82 93/1.98 RE 82 93/1.98 1 STAR Plastic Deep-Groove Ball Bearings Plastic Deep-Groove Ball Bearings Structural Design Inner/outer race polyacetal Cage
Deutsche Star Plastic Deep-Groove Ball Bearings RE 82 93/1.98 RE 82 93/1.98 1 STAR Plastic Deep-Groove Ball Bearings Plastic Deep-Groove Ball Bearings Structural Design Inner/outer race polyacetal Cage
DT 23 DT 24 DT 32 DT 33 DT 34 DT 44
 DT 23 DT 24 DT 32 DT 33 DT 34 DT 44 Operating Manual DURATRUSS B.V. Junostraat 2 6468 EW Kerkrade The Netherlands www.duratruss.com DURATRUSS - www.duratruss.com Operating Manual Page 1 Table of Contents
DT 23 DT 24 DT 32 DT 33 DT 34 DT 44 Operating Manual DURATRUSS B.V. Junostraat 2 6468 EW Kerkrade The Netherlands www.duratruss.com DURATRUSS - www.duratruss.com Operating Manual Page 1 Table of Contents
