ANA and Antibody Series
|
|
|
- Mitchell Bennett
- 5 years ago
- Views:
Transcription
1 ANA and Antibody Series How to Do Scleroderma ANA and Antibody Testing Correctly (A Practical Guide for Clinicians) Background When a clinician has a reason to suspect that a patient may have an autoimmune disease, for example, systemic lupus erythematosus (SLE/lupus), rheumatoid arthritis, systemic sclerosis (scleroderma), or Sjogrens syndrome, s/he will usually order an antinuclear antibody (ANA) test as the first step. If the result of that test is positive, this can be a strong marker for the presence of an autoimmune disease. If the result is negative, an autoimmune disease is much less likely but still a possibility. Following a positive ANA result that has been correctly done, the next diagnostic step is to look at the patient s symptoms in combination with lab test results that look for the presence of individual autoantibodies, especially for autoimmune diseases that tend to be strongly associated with specific autoantibodies. If the ANA and antibody testing is done correctly, this can be a powerful tool for helping a clinician quickly and correctly diagnosis an autoimmune disease at an earlier stage, when it is often easier to treat. Unfortunately, for a variety of reasons, including recent changes in standard laboratory testing procedures that have been implemented to save money (often at the expense of diagnostic accuracy), it is increasingly likely that clinicians (who are not typically aware of these testing problems) will receive incorrect lab results, sometimes resulting in years of delay in making the correct clinical diagnosis. This article is a short, bullet point summary of the more detailed article titled Scleroderma ANA and Antibody Testing Basics one of the articles in the ANA and Antibody series that is part of this website. It is intended to educate clinicians as well as patients. Key references that document the issues raised in this article are included at the end. ANA Testing for Scleroderma The Problem Historically, all ANA testing was done by a method called indirect immunofluorescence (IFA or IIF). Now, however, most ANA testing is done (by default) using newer, less expensive and less "hands on" methods such as ELISA or Multiplex. ANA testing by IFA can detect up to 150 different antigens. In contrast, typical ANA testing by ELISA detects 8 to 10 antigens, and testing by Multiplex detects 11 to 13 antigens, potentially missing key antigens critical for correct diagnosis. 1
2 ANA testing by ELISA or Multiplex is very accurate IF the patient has one of the antibodies included in the panel *. However, if the patient has an antibody that is not included in the testing panel, the ANA result itself will be falsely reported as negative, suggesting that the patient does not have an autoimmune disease. When a clinician suspects that a patient has an autoimmune disease, but the early symptoms are ambiguous enough so that s/he is unsure as to which autoimmune disease the patient may have (this is frequently the case since there are several symptoms/signs that overlap with certain autoimmune diseases), s/he will typically order a general ANA screening panel. This is sometimes referred to as an Extractable Nuclear Antigen (ENA) panel, an ANA profile, an Autoimmune Disorders Panel, or an Autoimmune Cascade. In other cases, if s/he suspects a specific disease, e.g., systemic scleroderma, and the reference lab that will do the testing offers it, s/he may instead order a specific screening panel, for example, a scleroderma screening panel or a lupus screening panel. Unfortunately, there is absolutely no standardization of what is included in any of these disease-specific panels. To illustrate the problem for scleroderma patients in particular, consider the following: While all scleroderma-specific screening panels will include the two main antibodies that have been linked to scleroderma for decades [anti-scl70 (associated with one of the diffuse scleroderma variants) and anti-centromere (associated with one of the limited scleroderma variants)], these panels commonly leave out a number of other antibodies that have been linked to scleroderma based on recent research. In one recent study 1, a typical autoimmune screening panel done by Multiplex resulted in an almost 43% false negative rate for scleroderma patients. Notably, one of these typically omitted antibodies anti-rna polymerase III - has an incidence rate of about 20%, comparable to the general rates for anti-scl70 and anti-centromere antibodies. The problem is even worse for general autoimmune disease screening panels. It is very common for these panels to only include one scleroderma-related antibody anti-scl70 - and leave out the next most frequent, anti-centromere antibody. Moreover, some of these panels refer to the anti-scl70 antibody as the scleroderma antibody. So if you are a primary care clinician with little experience in diagnosing autoimmune disorders (no less a patient reading his/her lab results), when you get a (potentially false) negative ANA result from a general autoimmune screening panel that includes a negative result for the "scleroderma antibody, it is not at all surprising that an accurate scleroderma diagnosis can be delayed for years. Because of these issues, in a 2011 Position Statement, the American College of Rheumatology strongly recommends that ANA testing by indirect immunofluorescence (IFA) should remain the gold standard for ANA testing. 2 This is especially important when doing initial ANA and antibody screening for patients who may have some form of scleroderma, but also applies to other autoimmune diseases, including lupus. * There is a known problem with false-positive Scl-70 antibody results when done by ELISA or Multiplex assay. These will usually be low positive results. See the separate article in this series titled False-positive Scl-70 (Topoisomerase) Antibody Testing: A Major Problem in Systemic Sclerosis Diagnosis for more information about this important issue. 2
3 ANA Testing for Scleroderma The Solution The best way to do initial testing for scleroderma is to order an ANA done by IFA using human HEp-2 substrate. To the best of our knowledge, all major labs in the US now use HEp-2 substrate for ANA/IFA testing. However, this may not be true in other parts of the world. If you are unsure about this, check with the lab before ordering the test. If the lab is using a different substrate, e.g., rodent substrate, centromere antibodies are not reliably detected, so it is best to order a separate anticentromere antibody test in this situation. It is perfectly reasonable for a clinician to order instead an ANA test and reflex scleroderma screening panel that is done using ELISA or Multiplex, as long as s/he orders a confirming reflex ANA by IFA if the panel yields a negative result. If the ANA result turns out positive, then it is most likely correct (however, see note above about false-positive Scl-70 results), and since very few scleroderma patients have more than one scleroderma-related antibody, the test results will give the best information that the clinician needs to help make a correct diagnosis. Note that labs never do a reflex ANA by IFA test automatically upon getting a negative result this is something that the clinician needs to be aware of and to order, either as part of the original test order or in a subsequent testing round. (Ironically, with many reference labs, if the ANA result done by ELISA or Multiplex is positive, then the lab will often automatically rerun the ANA testing using IFA since this procedure yields additional information that may be useful to the clinician.) Scleroderma Antibody Testing The Final Piece of the Puzzle Assuming that the clinician has ordered an ANA test that has been done by IFA using human HEp-2 cell substrate and has received a positive result, the next step is to try to determine which specific scleroderma-related antibody the patient might have, since each scleroderma antibody may have a different clinical presentation and prognosis. Since ANA by IFA can potentially detect up to 150 different antibodies, determining the specific antibody is the next critical step. Recent research has identified at least ten different antibodies that are associated with specific variants of scleroderma. Some of these fall into a category called "overlap syndromes," where the patient can have symptoms of more than one autoimmune disease. (See the Antibody section of the Scleroderma FAQ on this website for a list of these ten antibodies and disease associations.) Unfortunately, only a very small number of reference labs even test for the majority of these scleroderma antibodies. If the clinician is not aware of this and just orders a scleroderma antibody screening panel, the number of antibodies included in this panel can be only the two originally identified antibodies, anti- Scl70 and anti-centromere, entirely missing other important antibodies. At a bare minimum, if the clinician suspects scleroderma, the following three antibodies should be included in the panel or ordered separately if not included: Scl70, centromere, and RNA Polymerase III. These three antibodies are present in between 60% and 80% of patients with diagnosed systemic sclerosis. 3
4 If these antibodies are negative, then the next group of antibodies that should be tested for include: Th/To, PM-Scl, U3-RNP, U1-RNP (Mixed Connective Tissue Disease), and Ku. Unfortunately, to the best of our knowledge, no commercial testing is yet available for U11/U12-RNP or RuvBL1/2 antibodies. At least two reference labs in the US offer comprehensive scleroderma screening panels that include almost all of the above-mentioned antibodies: RDL and ARUP. Note that the Ku antibody is not included in the standard scleroderma screening panel but it can be added by request if the initial seven antibodies are all negative. Unfortunately, we currently have no information on the availability of comprehensive scleroderma screening panels in other countries. It is important to note that antibodies are currently used diagnostically to identify specific disease subsets in order to better monitor and manage potential disease complications or progression. There is no currently identified clinical need to do antibody level trending as antibody levels are generally stable over time and are not thought to be directly correlated with symptom severity in systemic scleroderma. If ANA testing by IFA yields a centromere staining pattern, many labs may not do additional testing for Scl70, RNA Polymerase III, or other scleroderma related antibodies. While in most cases the IFA centromere pattern interpretation is correct, reading ANA staining patterns is subjective and has been shown to be insufficient clinically and in some cases incorrect. 4 Because of this concern, it is recommended that centromere staining patterns be confirmed with separate anti-centromere antibody testing. If there are cost constraints, stepwise antibody testing tailored to the patient s presenting symptom profile may be appropriate rather than expensive comprehensive antibody screening. Here is a chart that suggests sequential antibody testing. Note that the first step is always an ANA/IFA. If that is negative, additional antibody testing should not be done. Symptom Profile Antibody Testing Level 1 Antibody Testing Level 2 Potential diffuse SSc: severe pain and fatigue, Raynaud s, skin thickening, GI symptoms Potential limited SSc: Raynaud s, GI symptoms, swollen fingers, telangiectasia Overlap syndrome: myositis, SLE symptoms Scl-70, RNA Polymerase III Centromere Centromere, U1-RNP U3-RNP, Centromere, Th/To Th/To, U1-RNP PM/Scl, Ku 4
5 References 1. Shanmugam VK, Swistowski DR, Saddic N, Wang H, Steen VD. Comparison of indirect immunofluorescence and multiplex antinuclear antibody screening in systemic sclerosis. Clinical Rheumatology Oct (10): American College of Rheumatology Position Statement. Methodology of testing for antinuclear antibodies. Approved by Board of Directors: Aug Beutner EH, Krasny SA, Chorzelski TP, Rodnan G, Jablonska S, Kumar V. Evaluation of methods for detection of anticentromere antibodies and other antinuclear antibodies. J Am Acad Dermatol 1985; 12: Pham, BN, Albarede S, Guyard A, Burg E, Maisonneuve P. "Impact of external quality assessment on antinuclear antibody detection performance." Lupus 14.2 (2005): Acknowledgement The author wishes to thank Allan L. Metzger, MD (RDL Reference Laboratory) for his invaluable assistance in helping to make the information in this article as accurate and up-todate as possible. Copyright Scleroderma Education Project Ltd All Rights Reserved 5
ANA and Antibody Series The Puzzle of ANA-Negative Systemic Scleroderma
 ANA and Antibody Series The Puzzle of ANA-Negative Systemic Scleroderma Author s Note I decided to write this article when I first read the new Salazar et al. (2015) paper Antinuclear antibody-negative
ANA and Antibody Series The Puzzle of ANA-Negative Systemic Scleroderma Author s Note I decided to write this article when I first read the new Salazar et al. (2015) paper Antinuclear antibody-negative
Right and wrong in antinuclear antibody tests. Timo Walle HUSLAB, Department of Immunology
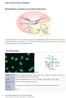
 Right and wrong in antinuclear antibody tests Timo Walle HUSLAB, Department of Immunology Antinuclear antibodies (ANA) Autoantibodies reacting with nuclear antigens Nucleoplasm Nuclear membrane Nucleoli
Right and wrong in antinuclear antibody tests Timo Walle HUSLAB, Department of Immunology Antinuclear antibodies (ANA) Autoantibodies reacting with nuclear antigens Nucleoplasm Nuclear membrane Nucleoli
INTERPRETIVE INFORMATION: Jo-1 Antibody, IgG 29 AU/mL or less...negative AU/mL...Equivocal 41 AU/mL or greater...positive
 Client: Example Client ABC123 123 Test Drive Salt Lake City, UT 84108 UNITED STATES Physician: Doctor, Example DOB Unknown Gender: Unknown Collection Date: 00/00/0000 00:00 Myositis Extended Panel ARUP
Client: Example Client ABC123 123 Test Drive Salt Lake City, UT 84108 UNITED STATES Physician: Doctor, Example DOB Unknown Gender: Unknown Collection Date: 00/00/0000 00:00 Myositis Extended Panel ARUP
immuno concepts Immuno Concepts is proud to announce that when present the characteristic SS-A/Ro pattern detected by the HEp-2000 substrate
 immuno concepts Immuno Concepts is proud to announce that when present the characteristic SS-A/Ro pattern detected by the HEp-2000 substrate is now considered confirmatory for the presence of antibodies
immuno concepts Immuno Concepts is proud to announce that when present the characteristic SS-A/Ro pattern detected by the HEp-2000 substrate is now considered confirmatory for the presence of antibodies
RNA polymerase II precursor mrna (hnrna) introns introns U1RNP. splicing
 DNA RNA polymerase II precursor mrna (hnrna) introns introns U2RNP splicing mrna U1RNP U5RNP U4/U6RNP splicing The genetic information of DNA is transcribed to precursor mrna (heterogeneous nuclear RNA,
DNA RNA polymerase II precursor mrna (hnrna) introns introns U2RNP splicing mrna U1RNP U5RNP U4/U6RNP splicing The genetic information of DNA is transcribed to precursor mrna (heterogeneous nuclear RNA,
Ed Harris - Background (Revised 1/25/16)
 Ed Harris - Background (Revised 1/25/16) Contact: eharris@sclerodermainfo.org Personal, Professional, and Academic Background Age: 68, currently retired Academic background: o BS Mathematics 1967 o MS
Ed Harris - Background (Revised 1/25/16) Contact: eharris@sclerodermainfo.org Personal, Professional, and Academic Background Age: 68, currently retired Academic background: o BS Mathematics 1967 o MS
Bio-Rad Laboratories BIOPLEX 2200 SYSTEM. BioPlex 2200 ANA Screen with MDSS. The first and only fully-automated, multiplexed ANA Screen
 Bio-Rad Laboratories BIOPLEX 2200 SYSTEM BioPlex 2200 ANA Screen with MDSS The first and only fully-automated, multiplexed ANA Screen Bio-Rad Laboratories BIOPLEX 2200 SYSTEM Like no other The BioPlex
Bio-Rad Laboratories BIOPLEX 2200 SYSTEM BioPlex 2200 ANA Screen with MDSS The first and only fully-automated, multiplexed ANA Screen Bio-Rad Laboratories BIOPLEX 2200 SYSTEM Like no other The BioPlex
Evaluation of Multiplexed Fluorescent Microsphere Immunoassay for Detection of Autoantibodies to Nuclear Antigens
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Nov. 2004, p. 1054 1059 Vol. 11, No. 6 1071-412X/04/$08.00 0 DOI: 10.1128/CDLI.11.6.1054 1059.2004 Copyright 2004, American Society for Microbiology. All
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Nov. 2004, p. 1054 1059 Vol. 11, No. 6 1071-412X/04/$08.00 0 DOI: 10.1128/CDLI.11.6.1054 1059.2004 Copyright 2004, American Society for Microbiology. All
SAMPLE I/LA02-A2. Archived Document
 Archived Document This archived document is no longer being reviewed through the CLSI Consensus Document Development Process. However, this document is technically valid as of September 2016. Because of
Archived Document This archived document is no longer being reviewed through the CLSI Consensus Document Development Process. However, this document is technically valid as of September 2016. Because of
Prevalence and Clinical Significance of anti-dfs70 in ANA Positive Patients Undergoing Routine ANA Testing in a New Zealand Public Hospital.
 Prevalence and Clinical Significance of anti-dfs70 in ANA Positive Patients Undergoing Routine ANA Testing in a New Zealand Public Hospital. Stacey Lucas A thesis submitted to Auckland University of Technology
Prevalence and Clinical Significance of anti-dfs70 in ANA Positive Patients Undergoing Routine ANA Testing in a New Zealand Public Hospital. Stacey Lucas A thesis submitted to Auckland University of Technology
Carmen Gelpí Elena Pérez Cristina Roldan
 Autoimmun Highlights (2014) 5:47 54 DOI 10.1007/s13317-014-0059-x REVIEW ARTICLE Efficiency of a solid-phase chemiluminescence immunoassay for detection of antinuclear and cytoplasmic autoantibodies compared
Autoimmun Highlights (2014) 5:47 54 DOI 10.1007/s13317-014-0059-x REVIEW ARTICLE Efficiency of a solid-phase chemiluminescence immunoassay for detection of antinuclear and cytoplasmic autoantibodies compared
Screening for IgG Antinuclear Autoantibodies by HEp-2 Indirect Fluorescent Antibody Assays and the Need for Standardization
 Immunopathology / HEp-2 Immunofluorescence Standardization Screening for IgG Antinuclear Autoantibodies by HEp-2 Indirect Fluorescent Antibody Assays and the Need for Standardization Susan S. Copple, MS,
Immunopathology / HEp-2 Immunofluorescence Standardization Screening for IgG Antinuclear Autoantibodies by HEp-2 Indirect Fluorescent Antibody Assays and the Need for Standardization Susan S. Copple, MS,
Immunofluorescence Assays for Autoimmune and Infectious Disease
 life.science.discovery. Immunofluorescence Assays for Autoimmune and Infectious Disease PRODUCT CATALOG COMPANY MBL Bion, part of the MBL, has been manufacturing high-quality kits and components for autoimmune,
life.science.discovery. Immunofluorescence Assays for Autoimmune and Infectious Disease PRODUCT CATALOG COMPANY MBL Bion, part of the MBL, has been manufacturing high-quality kits and components for autoimmune,
Fluoroenzymeimmunoassay to detect systemic sclerosis-associated antibodies: diagnostic performance and correlation with conventional techniques
 Fluoroenzymeimmunoassay to detect systemic sclerosis-associated antibodies: diagnostic performance and correlation with conventional techniques C. Bonroy 1, V. Smith 2, K. Van Steendam 3, J. Van Praet
Fluoroenzymeimmunoassay to detect systemic sclerosis-associated antibodies: diagnostic performance and correlation with conventional techniques C. Bonroy 1, V. Smith 2, K. Van Steendam 3, J. Van Praet
Interpath Laboratory, Inc. Test File Update
 As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
As an Interpath customer who receives electronic results or sends electronic orders you may need to be notified when we update our Service Manual. Although we try to keep these changes to a minimum, laboratory
ANA Screen 8 ELISA KIT
 ANA Screen 8 ELISA KIT Cat. No.:DEIA6289 Pkg.Size:96T Intended use ANA Screen 8 ELISA is for the qualitative screening of IgG antibodies against dsdna, RNP, Sm, SS-A/Ro, SS-B/La, Scl-70, CENP-B and Jo-1
ANA Screen 8 ELISA KIT Cat. No.:DEIA6289 Pkg.Size:96T Intended use ANA Screen 8 ELISA is for the qualitative screening of IgG antibodies against dsdna, RNP, Sm, SS-A/Ro, SS-B/La, Scl-70, CENP-B and Jo-1
Detection and identification of antinuclear antibodies (ANA) in a large and consecutive cohort of serum samples referred for ANA testing
 Ann Rheum Dis 2001;60:1131 1136 1131 Department of Rheumatology, Ghent University Hospital, Belgium I Peene EMVeys F De Keyser Innogenetics NV, Ghent, Belgium L Meheus Correspondence to: Dr F De Keyser,
Ann Rheum Dis 2001;60:1131 1136 1131 Department of Rheumatology, Ghent University Hospital, Belgium I Peene EMVeys F De Keyser Innogenetics NV, Ghent, Belgium L Meheus Correspondence to: Dr F De Keyser,
3. REAGENTS, MATERIALS AND INSTRUMENTATION
 DCM9-0 Ed. 03/202 ENA Profile for routine analysis Indirect immunoenzymatic determination of IgG class autoantibodies against ENA (Extractable Nuclear s) in human serum or plasma. IVD LOT See external
DCM9-0 Ed. 03/202 ENA Profile for routine analysis Indirect immunoenzymatic determination of IgG class autoantibodies against ENA (Extractable Nuclear s) in human serum or plasma. IVD LOT See external
Autoantibodies to RuvBL1 and RuvBL2: A Novel Systemic Sclerosis Related Antibody Associated With Diffuse Cutaneous and Skeletal Muscle Involvement
 Arthritis Care & Research Vol. 66, No. 4, April 2014, pp 575 584 DOI 10.1002/acr.22163 2014, American College of Rheumatology ORIGINAL ARTICLE Autoantibodies to RuvBL1 and RuvBL2: A Novel Systemic Sclerosis
Arthritis Care & Research Vol. 66, No. 4, April 2014, pp 575 584 DOI 10.1002/acr.22163 2014, American College of Rheumatology ORIGINAL ARTICLE Autoantibodies to RuvBL1 and RuvBL2: A Novel Systemic Sclerosis
NOVA Lite HEp-2 External EB Kits For In Vitro Diagnostic Use
 NOVA Lite HEp-2 External EB Kits For In Vitro Diagnostic Use Product Code: 704230, 704235 CLIA Complexity: High Intended Use This product (kit or substrate slides) is intended for use in the screening
NOVA Lite HEp-2 External EB Kits For In Vitro Diagnostic Use Product Code: 704230, 704235 CLIA Complexity: High Intended Use This product (kit or substrate slides) is intended for use in the screening
Enzyme-Linked Immunosorbent Assay Screening Then Indirect Immunofluorescence Confirmation of Antinuclear Antibodies A Statistical Analysis
 Immunopathology / Screening Antinuclear Antibodies Enzyme-Linked Immunosorbent Assay Screening Then Indirect Immunofluorescence Confirmation of Antinuclear Antibodies A Statistical Analysis Susan S. Copple,
Immunopathology / Screening Antinuclear Antibodies Enzyme-Linked Immunosorbent Assay Screening Then Indirect Immunofluorescence Confirmation of Antinuclear Antibodies A Statistical Analysis Susan S. Copple,
Human ANA IgG ELISA Kit
 Human ANA IgG ELISA Kit Cat.No:DEIA1695 Lot. No. (See product label) PRODUCT INFOMATION Storage specificity 1. Store the unopened kit at 2-8 C. 2. Coated microwell strips: Store between 2 and 8 C. Extra
Human ANA IgG ELISA Kit Cat.No:DEIA1695 Lot. No. (See product label) PRODUCT INFOMATION Storage specificity 1. Store the unopened kit at 2-8 C. 2. Coated microwell strips: Store between 2 and 8 C. Extra
Correlation of Line Immuno Assay with Indirect Immunofluoresence Assay for the Detection of Anti-Nuclear Antibodies in Various Autoimmune Disorders
 Research Article imedpub Journals http://www.imedpub.com/ Journal of Autoimmune Disorders Correlation of Line Immuno Assay with Indirect Immunofluoresence Assay for the Detection of Anti-Nuclear Antibodies
Research Article imedpub Journals http://www.imedpub.com/ Journal of Autoimmune Disorders Correlation of Line Immuno Assay with Indirect Immunofluoresence Assay for the Detection of Anti-Nuclear Antibodies
AccuDiag. SSB (La) ELISA
 AccuDiag SSB (La) ELISA Kit Cat# 2556-2Z See external Label 2-8 C = 96 Tests Test SSB (La) ELISA Method Enzyme Linked Immunosorbent Assay Principle Indirect; Antigen Coated Plate Detection Range Qualitative:
AccuDiag SSB (La) ELISA Kit Cat# 2556-2Z See external Label 2-8 C = 96 Tests Test SSB (La) ELISA Method Enzyme Linked Immunosorbent Assay Principle Indirect; Antigen Coated Plate Detection Range Qualitative:
Practical Evaluation of Methods for Detection and Specificity of Autoantibodies to Extractable Nuclear Antigens
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Mar. 2004, p. 297 301 Vol. 11, No. 2 1071-412X/04/$08.00 0 DOI: 10.1128/CDLI.11.2.297 301.2004 Copyright 2004, American Society for Microbiology. All Rights
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, Mar. 2004, p. 297 301 Vol. 11, No. 2 1071-412X/04/$08.00 0 DOI: 10.1128/CDLI.11.2.297 301.2004 Copyright 2004, American Society for Microbiology. All Rights
Quality Monitoring Approach for Optimizing Antinuclear Antibody Screening Cutoffs and Testing Work Flow
 ARTICLES Quality Monitoring Approach for Optimizing Antinuclear Antibody Screening Cutoffs and Testing Work Flow Danyel H. Tacker 1 * and Peter L. Perrotta 1 Background: An antinuclear antibody (ANA) testing
ARTICLES Quality Monitoring Approach for Optimizing Antinuclear Antibody Screening Cutoffs and Testing Work Flow Danyel H. Tacker 1 * and Peter L. Perrotta 1 Background: An antinuclear antibody (ANA) testing
ab Anti-ENA Screen Human ELISA Kit
 ab178619 Anti-ENA Screen Human ELISA Kit Instructions for Use For the semi-quantitative measurement of IgG class antibodies against extractable nuclear antigens (ENA) in Human serum and plasma. This product
ab178619 Anti-ENA Screen Human ELISA Kit Instructions for Use For the semi-quantitative measurement of IgG class antibodies against extractable nuclear antigens (ENA) in Human serum and plasma. This product
EL-ANAScr. An enzyme immunoassay for the screening of human serum to detect antinuclear antibodies (ANAs) Instruction Manual
 EL-ANAScr An enzyme immunoassay for the screening of human serum to detect antinuclear antibodies (ANAs) Instruction Manual Catalog Nos.: 100-002, 100-004 TheraTest Laboratories, Inc. 1111 North Main Street
EL-ANAScr An enzyme immunoassay for the screening of human serum to detect antinuclear antibodies (ANAs) Instruction Manual Catalog Nos.: 100-002, 100-004 TheraTest Laboratories, Inc. 1111 North Main Street
ENA-6 Screen ELISA KIT
 ENA-6 Screen ELISA KIT Cat. No.:DEIA05732 Pkg.Size:96T Intended use For the qualitative screening of human IgG antibodies to extractable nuclear antigens (ENA) in human serum by indirect enzyme immunoassay
ENA-6 Screen ELISA KIT Cat. No.:DEIA05732 Pkg.Size:96T Intended use For the qualitative screening of human IgG antibodies to extractable nuclear antigens (ENA) in human serum by indirect enzyme immunoassay
ENA Combined SCREEN (6 Antigen)
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
AccuDiag ENA Combined Screen (6 Antigen) ELISA Kit
 AccuDiag ENA Combined Screen (6 Antigen) ELISA Kit Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE Cat# 2552-2 See external Label 96 Tests ENA Combined Screen ELISA Enzyme
AccuDiag ENA Combined Screen (6 Antigen) ELISA Kit Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE Cat# 2552-2 See external Label 96 Tests ENA Combined Screen ELISA Enzyme
THE QUALITY ASSURANCE AND ORGANIZATION OF AUTOANTIBODY LABORATORY
 THE QUALITY ASSURANCE AND ORGANIZATION OF AUTOANTIBODY LABORATORY Tanja Kveder, Ph.D.; Assist. Prof. Borut Božič, Ph.D. Department of Rheumatology, University Medical Centre, 1000 Ljubljana, Slovenia University
THE QUALITY ASSURANCE AND ORGANIZATION OF AUTOANTIBODY LABORATORY Tanja Kveder, Ph.D.; Assist. Prof. Borut Božič, Ph.D. Department of Rheumatology, University Medical Centre, 1000 Ljubljana, Slovenia University
AccuDiag ENA Combined Screen (6 Antigen) ELISA Kit
 AccuDiag ENA Combined Screen (6 Antigen) ELISA Kit Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE Cat# 2552-2 See external Label 96 Tests ENA Combined Screen ELISA Enzyme
AccuDiag ENA Combined Screen (6 Antigen) ELISA Kit Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE Cat# 2552-2 See external Label 96 Tests ENA Combined Screen ELISA Enzyme
Performance of the BMD FIDIS Connective 10 ENA luminex method in external quality assurance proficiency programmes in a diagnostic laboratory setting
 Performance of the BMD FIDIS Connective 10 ENA luminex method in external quality assurance proficiency programmes in a diagnostic laboratory setting Paul M Austin, Helena T Thompson and Anthony R Brown
Performance of the BMD FIDIS Connective 10 ENA luminex method in external quality assurance proficiency programmes in a diagnostic laboratory setting Paul M Austin, Helena T Thompson and Anthony R Brown
Research Article Evaluation of a Multiplex ELISA for Autoantibody Profiling in Patients with Autoimmune Connective Tissue Diseases
 Hindawi Publishing Corporation Autoimmune Diseases Volume 2014, Article ID 896787, 6 pages http://dx.doi.org/10.1155/2014/896787 Research Article Evaluation of a Multiplex ELISA for Autoantibody Profiling
Hindawi Publishing Corporation Autoimmune Diseases Volume 2014, Article ID 896787, 6 pages http://dx.doi.org/10.1155/2014/896787 Research Article Evaluation of a Multiplex ELISA for Autoantibody Profiling
ANA ELISA Screen Enzyme Immunoassay Test Kit For In Vitro Diagnostic Use Catalog No Tests
 SUMMARY OF PROCEDURE 1. Prepare 1:101 dilutions of samples in Sample Diluent. Mix well. 2. Add 100 µl of diluted samples into the antigen wells. 3. Incubate at room temperature (18-30 o C) for 30 + 5 min.
SUMMARY OF PROCEDURE 1. Prepare 1:101 dilutions of samples in Sample Diluent. Mix well. 2. Add 100 µl of diluted samples into the antigen wells. 3. Incubate at room temperature (18-30 o C) for 30 + 5 min.
AccuDiag. SSA (Ro) ELISA INTENDED USE
 Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE AccuDiag SSA (Ro) Kit Cat# 2555-2 See external Label 96 Tests SSA (Ro) Enzyme Linked Immunosorbent Assay - Indirect; Antigen
Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE AccuDiag SSA (Ro) Kit Cat# 2555-2 See external Label 96 Tests SSA (Ro) Enzyme Linked Immunosorbent Assay - Indirect; Antigen
AccuDiag. SSB (La) ELISA TEST PRINCIPLE INTENDED USE SUMMARY AND EXPLANATION SPECIMEN COLLECTION AND PREPARATION See external Label
 Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE AccuDiag SSB (La) Kit See external Label 2556-2 96 Tests SSB (La) Enzyme Linked Immunosorbent Assay Indirect; Antigen Coated
Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE AccuDiag SSB (La) Kit See external Label 2556-2 96 Tests SSB (La) Enzyme Linked Immunosorbent Assay Indirect; Antigen Coated
ENA-6 Screen Enzyme Immunoassay Test Kit For In Vitro Diagnostic Use Catalog No Tests
 SUMMARY OF PROCEDURE ENA-6 Screen Enzyme Immunoassay Test Kit For In Vitro Diagnostic Use Catalog No. 720-230 96 Tests 1. Prepare a 1:101 dilution of samples in Sample Diluent. Mix well. 2. Add 100 µl
SUMMARY OF PROCEDURE ENA-6 Screen Enzyme Immunoassay Test Kit For In Vitro Diagnostic Use Catalog No. 720-230 96 Tests 1. Prepare a 1:101 dilution of samples in Sample Diluent. Mix well. 2. Add 100 µl
Detection of Anti-SSA Antibodies by Indirect Immunofluorescence
 Clinical Chemistry 50:12 2361 2369 (2004) Clinical Immunology Detection of Anti-SSA Antibodies by Indirect Immunofluorescence Xavier Bossuyt, 1* Johan Frans, 1 Ann Hendrickx, 1 Godelieve Godefridis, 1
Clinical Chemistry 50:12 2361 2369 (2004) Clinical Immunology Detection of Anti-SSA Antibodies by Indirect Immunofluorescence Xavier Bossuyt, 1* Johan Frans, 1 Ann Hendrickx, 1 Godelieve Godefridis, 1
ANA Screen ELISA Cat # Z
 DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
DIAGNOSTIC AUTOMATION, INC. 23961 Craftsman Road, Suite D/E/F, Calabasas, CA 91302 Tel: (818) 591-3030 Fax: (818) 591-8383 onestep@rapidtest.com technicalsupport@rapidtest.com www.rapidtest.com See external
AccuDiag ENA Profile-6 SM/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B ELISA Kit
 AccuDiag ENA Profile-6 SM/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B Kit with a homozygous C2 deficiency (13), and in a subset of patients who lack antidsdna antibodies (11). Scl-70 antibodies are highly specific
AccuDiag ENA Profile-6 SM/RNP, Sm, Jo-1, Scl-70, SS-A, SS-B Kit with a homozygous C2 deficiency (13), and in a subset of patients who lack antidsdna antibodies (11). Scl-70 antibodies are highly specific
AccuDiag. SSB (La) ELISA
 Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE AccuDiag SSB (La) ELISA Kit Cat# 2556-2 See external Label 96 Tests SSB (La) ELISA Enzyme Linked Immunosorbent Assay Indirect;
Test Method Principle Detection Range Sample Total Time Shelf Life INTENDED USE AccuDiag SSB (La) ELISA Kit Cat# 2556-2 See external Label 96 Tests SSB (La) ELISA Enzyme Linked Immunosorbent Assay Indirect;
Scl-86, A Marker Antigen for Diffuse Scleroderma
 Scl-86, A Marker Antigen for Diffuse Scleroderma W. J. van Venrooij, S. 0. Stapel, H. Houben, W. J. Habets, C. G. M. Kallenberg, E. Penner, and L. B. van de Putte Department of Biochemistry and Department
Scl-86, A Marker Antigen for Diffuse Scleroderma W. J. van Venrooij, S. 0. Stapel, H. Houben, W. J. Habets, C. G. M. Kallenberg, E. Penner, and L. B. van de Putte Department of Biochemistry and Department
Anti ENA Screen ELISA
 Anti ENA Screen ELISA GWB-521218 www.genwaybio.com FOR RESEARCH USE ONLY 1. INTRODUCTION Enzyme immunoassay for the quantitative determination of anti ENA in human serum or plasma Autoimmune diseases are
Anti ENA Screen ELISA GWB-521218 www.genwaybio.com FOR RESEARCH USE ONLY 1. INTRODUCTION Enzyme immunoassay for the quantitative determination of anti ENA in human serum or plasma Autoimmune diseases are
ANA Screening IgG. Enzyme Immunoassay (ELISA) for the qualitative determination of IgG antibodies to anti Nuclear Antigens in human serum and plasma
 Doc.: INS ANAS.CE/eng Page 1 of 7 Rev.: 0 Date: 0911 ANA Screening IgG Enzyme Immunoassay (ELISA) for the qualitative determination of IgG antibodies to anti Nuclear Antigens in human serum and plasma
Doc.: INS ANAS.CE/eng Page 1 of 7 Rev.: 0 Date: 0911 ANA Screening IgG Enzyme Immunoassay (ELISA) for the qualitative determination of IgG antibodies to anti Nuclear Antigens in human serum and plasma
(Enzyme immunoassay for the qualitative detection of auto-antibodies to extractable nuclear antigens) Codes: Well packs: Instructions for Use
 AUTOZYME TM ENA (Enzyme immunoassay for the qualitative detection of auto-antibodies to extractable nuclear antigens) Codes: Bulk pack: Well packs: Anti-Ro: Anti-La: Anti-Sm: Anti-Sm/RNP: Anti-Jo-1: Anti-Scl-70:
AUTOZYME TM ENA (Enzyme immunoassay for the qualitative detection of auto-antibodies to extractable nuclear antigens) Codes: Bulk pack: Well packs: Anti-Ro: Anti-La: Anti-Sm: Anti-Sm/RNP: Anti-Jo-1: Anti-Scl-70:
BIMM18 Dec 20 th - Flow cytometry in clinical diagnostics
 BIMM18 Dec 20 th - Flow cytometry in clinical diagnostics I. B cell leukemia and lymphomas Immunophenotyping as part of the diagnostic work- up of hematologic malignancies offers a rapid and effective
BIMM18 Dec 20 th - Flow cytometry in clinical diagnostics I. B cell leukemia and lymphomas Immunophenotyping as part of the diagnostic work- up of hematologic malignancies offers a rapid and effective
COMPARATIVE STUDY OF ANTI-DOUBLE STRANDED DNA DETECTION BY ELISA AND CRITHIDIA LUCILIAE IMMUNOFLUORESCENCE
 COMPARATIVE STUDY OF ANTI-DOUBLE STRANDED DNA DETECTION BY ELISA AND CRITHIDIA LUCILIAE IMMUNOFLUORESCENCE Kritsana Janyapoon, Pantakit Jivakanont, Kantima Choosang, Rungkarn Surbrsing, Vannapa Charoenying
COMPARATIVE STUDY OF ANTI-DOUBLE STRANDED DNA DETECTION BY ELISA AND CRITHIDIA LUCILIAE IMMUNOFLUORESCENCE Kritsana Janyapoon, Pantakit Jivakanont, Kantima Choosang, Rungkarn Surbrsing, Vannapa Charoenying
Prediction and Evaluation of Diagnostic Assays for Autoimmune Disorders
 International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 2, Number 3 (2011), pp. 215-224 International Research Publication House http://www.irphouse.com Prediction and Evaluation
International Journal of Genetic Engineering and Biotechnology. ISSN 0974-3073 Volume 2, Number 3 (2011), pp. 215-224 International Research Publication House http://www.irphouse.com Prediction and Evaluation
ENA-6-Profile ORG x 16 Tests. Immunometric Enzyme Immunoassay for the semiquantitative. of anti-ena-antibodies. Instruction for use
 ORGENTEC Diagnostika GmbH Carl-Zeiss-Straße 49 55129 Mainz Tel.: 06131-9258-0 Fax: 06131-9258-58 ENA-6-Profile ORG 546 6 x 16 Tests Immunometric Enzyme Immunoassay for the semiquantitative determination
ORGENTEC Diagnostika GmbH Carl-Zeiss-Straße 49 55129 Mainz Tel.: 06131-9258-0 Fax: 06131-9258-58 ENA-6-Profile ORG 546 6 x 16 Tests Immunometric Enzyme Immunoassay for the semiquantitative determination
SUMMARY AND EXPLANATION OF THE TEST
 RELISA ENA SINGLE WELL SCREENING TEST FOR ANTIBODIES TO EXTRACTABLE NUCLEAR ANTIGENS For in vitro Diagnostic Use For Professional Use Catalog numbers: 7096-10 (96 wells) and 7696-10 (576 wells) INTENDED
RELISA ENA SINGLE WELL SCREENING TEST FOR ANTIBODIES TO EXTRACTABLE NUCLEAR ANTIGENS For in vitro Diagnostic Use For Professional Use Catalog numbers: 7096-10 (96 wells) and 7696-10 (576 wells) INTENDED
AUTOIMMUNE DISEASE ASSAYS
 product range 1 Corgenix PRODUCT RANGE 04-05 Antiphospholipid Syndrome Assays 06-10 AUTOIMMUNE DISEASE ASSAYS 11 Liver Disease Assays 11 Liver Fibrosis Markers 12 Cardiovascular Markers 12-13 Platelet
product range 1 Corgenix PRODUCT RANGE 04-05 Antiphospholipid Syndrome Assays 06-10 AUTOIMMUNE DISEASE ASSAYS 11 Liver Disease Assays 11 Liver Fibrosis Markers 12 Cardiovascular Markers 12-13 Platelet
ANTINUCLEAR ANTIBODIES IN BULGARIAN PATIENTS WITH CHRONIC INFECTIONS
 PROCEEDINGS OF THE BALKAN SCIENTIFIC CONFERENCE OF BIOLOGY IN PLOVDIV (BULGARIA) FROM 19 TH TILL 21 ST OF MAY 2005 (EDS B. GRUEV, M. NIKOLOVA AND A. DONEV), 2005 (P. 246 252) ANTINUCLEAR ANTIBODIES IN
PROCEEDINGS OF THE BALKAN SCIENTIFIC CONFERENCE OF BIOLOGY IN PLOVDIV (BULGARIA) FROM 19 TH TILL 21 ST OF MAY 2005 (EDS B. GRUEV, M. NIKOLOVA AND A. DONEV), 2005 (P. 246 252) ANTINUCLEAR ANTIBODIES IN
Routine use of Zenit RA, a novel chemiluminescent immunoanalyzer in autoimmune disease diagnosis
 Autoimmun Highlights (2012) 3:27 31 DOI 10.1007/s13317-012-0032-5 ORIGINAL ARTICLE Routine use of Zenit RA, a novel chemiluminescent immunoanalyzer in autoimmune disease diagnosis P. Ghillani L. Dufat
Autoimmun Highlights (2012) 3:27 31 DOI 10.1007/s13317-012-0032-5 ORIGINAL ARTICLE Routine use of Zenit RA, a novel chemiluminescent immunoanalyzer in autoimmune disease diagnosis P. Ghillani L. Dufat
See AARDA Comments on CMS 1631 FC (Dec. 29, 2015), attached as an Appendix to these comments.
 www.aarda.org September 11, 2017 By Electronic Submission to www.regulations.gov Seema Verma, Administrator Centers for Medicare and Medicaid Services, Department of Health and Human Services Attention:
www.aarda.org September 11, 2017 By Electronic Submission to www.regulations.gov Seema Verma, Administrator Centers for Medicare and Medicaid Services, Department of Health and Human Services Attention:
Comparison of assay systems for detecting
 J Clin Pathol 1991;44:685-689 Department of Medicine, Divisions of Rheumatology and Clinical Immunology, Hospital for Joint Diseases Orthopaedic Institute, New York University Medical Center, 301 East
J Clin Pathol 1991;44:685-689 Department of Medicine, Divisions of Rheumatology and Clinical Immunology, Hospital for Joint Diseases Orthopaedic Institute, New York University Medical Center, 301 East
!""#$%&'(#)'%$*+!,-*
 ! How does methodology influence autoantibody test results? Johan Rönnelid MD, PhD Unit of Clinical Immunology and Transfusion medicine Uppsala University Hospital Department of Immunology, Genetics and
! How does methodology influence autoantibody test results? Johan Rönnelid MD, PhD Unit of Clinical Immunology and Transfusion medicine Uppsala University Hospital Department of Immunology, Genetics and
Diagnostic Automation, Inc Craftsman Road, Suite E/F Calabasas, CA Tel: , Fax:
 Diagnostic Automation, Inc. 23961 Craftsman Road, Suite E/F Calabasas, CA 91302 Tel:818-591-3030, Fax:818-591-8383 RNP IgG,A,M ELISA For in vitro diagnostic use. Catalog No. 2558-1 RNP IgG, A, M; Page
Diagnostic Automation, Inc. 23961 Craftsman Road, Suite E/F Calabasas, CA 91302 Tel:818-591-3030, Fax:818-591-8383 RNP IgG,A,M ELISA For in vitro diagnostic use. Catalog No. 2558-1 RNP IgG, A, M; Page
Anti-Sm ORG Tests. Immunometric Enzyme Immunoassay for the quantitative determination of anti-sm Antibodies. Instruction for use
 ORGENTEC Diagnostika GmbH Carl-Zeiss-Straße 49 55129 Mainz Tel.: 06131-9258-0 Fax: 06131-9258-58 Anti-Sm ORG 510 96 Tests Immunometric Enzyme Immunoassay for the quantitative determination of anti-sm Antibodies
ORGENTEC Diagnostika GmbH Carl-Zeiss-Straße 49 55129 Mainz Tel.: 06131-9258-0 Fax: 06131-9258-58 Anti-Sm ORG 510 96 Tests Immunometric Enzyme Immunoassay for the quantitative determination of anti-sm Antibodies
Epidermal nuclear immunoglobulin deposits in some connective tissue diseases: correlation with ENA antibodies
 Annals of the Rheumatic Diseases, 1983, 42, 163-167 Epidermal nuclear immunoglobulin deposits in some connective tissue diseases: correlation with ENA antibodies IRENE ANDERSEN,1 HANS GRAUDAL' PAUL ANDERSEN,2
Annals of the Rheumatic Diseases, 1983, 42, 163-167 Epidermal nuclear immunoglobulin deposits in some connective tissue diseases: correlation with ENA antibodies IRENE ANDERSEN,1 HANS GRAUDAL' PAUL ANDERSEN,2
Research Article A Comparison of Anti-Nuclear Antibody Quantification Using Automated Enzyme Immunoassays and Immunofluorescence Assays
 Hindawi Publishing Corporation Autoimmune Diseases Volume 2014, Article ID 534759, 8 pages http://dx.doi.org/10.1155/2014/534759 Research Article A Comparison of Anti-Nuclear Antibody Quantification Using
Hindawi Publishing Corporation Autoimmune Diseases Volume 2014, Article ID 534759, 8 pages http://dx.doi.org/10.1155/2014/534759 Research Article A Comparison of Anti-Nuclear Antibody Quantification Using
THE UTILITY OF AUTOANTIBODIES AS BIOMARKERS IN A WELL CHARACTERISED AUSTRALIAN SYSTEMIC SCLEROSIS (SCLERODERMA) COHORT
 THE UTILITY OF AUTOANTIBODIES AS BIOMARKERS IN A WELL CHARACTERISED AUSTRALIAN SYSTEMIC SCLEROSIS (SCLERODERMA) COHORT Karen Ann Patterson BMedSc BSc (Hons) Department of Immunology, Allergy and Arthritis
THE UTILITY OF AUTOANTIBODIES AS BIOMARKERS IN A WELL CHARACTERISED AUSTRALIAN SYSTEMIC SCLEROSIS (SCLERODERMA) COHORT Karen Ann Patterson BMedSc BSc (Hons) Department of Immunology, Allergy and Arthritis
QUANTA Lite SS-A ELISA For In Vitro Diagnostic Use CLIA Complexity: High
 Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 12/05/11 QUANTA Lite SS-A ELISA 708570 For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure Purified
Format to CLSI Standards GP2-A5 (Formerly NCCLS) Vol. 26 No. 12 Issue Date: 12/05/11 QUANTA Lite SS-A ELISA 708570 For In Vitro Diagnostic Use CLIA Complexity: High Principles of the Procedure Purified
Development of the Antinuclear and Anticytoplasmic Antibody Consensus Panel by the Association of Medical Laboratory Immunologists
 CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, May 2000, p. 436 443 Vol. 7, No. 3 1071-412X/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Development of the Antinuclear
CLINICAL AND DIAGNOSTIC LABORATORY IMMUNOLOGY, May 2000, p. 436 443 Vol. 7, No. 3 1071-412X/00/$04.00 0 Copyright 2000, American Society for Microbiology. All Rights Reserved. Development of the Antinuclear
Next-Generation Autoantibody Testing by Combination of Screening and Confirmation the CytoBead Technology
 Clinic Rev Allerg Immunol (2017) 53:87 104 DOI 10.1007/s12016-016-8574-3 Next-Generation Autoantibody Testing by Combination of Screening and Confirmation the CytoBead Technology Mandy Sowa 1 & Rico Hiemann
Clinic Rev Allerg Immunol (2017) 53:87 104 DOI 10.1007/s12016-016-8574-3 Next-Generation Autoantibody Testing by Combination of Screening and Confirmation the CytoBead Technology Mandy Sowa 1 & Rico Hiemann
PREPARED FOR: U.S. Army Medical Research and Materiel Command Fort Detrick, Maryland
 Award Number: W81XWH-12-1-0516 TITLE: Cadherin-11 Regulation of Fibrosis through Modulation of Epithelial-to- Mesenchymal Transition: Implications for Pulmonary Fibrosis in Scleroderma PRINCIPAL INVESTIGATOR:
Award Number: W81XWH-12-1-0516 TITLE: Cadherin-11 Regulation of Fibrosis through Modulation of Epithelial-to- Mesenchymal Transition: Implications for Pulmonary Fibrosis in Scleroderma PRINCIPAL INVESTIGATOR:
Antinuclear antibody determination in a routine
 Ann Rheum Dis 1996;55:723-727 723 NOW AND THEN Arthron, Research Centre for Rheumatic Diseases Amsterdam, Amsterdam, The Netherlands T E W Feltkamp Correspondence to: Professor Dr T E W Feltkamp, Arthron,
Ann Rheum Dis 1996;55:723-727 723 NOW AND THEN Arthron, Research Centre for Rheumatic Diseases Amsterdam, Amsterdam, The Netherlands T E W Feltkamp Correspondence to: Professor Dr T E W Feltkamp, Arthron,
Research Article A Comparison of Anti-Nuclear Antibody Quantification Using Automated Enzyme Immunoassays and Immunofluorescence Assays
 Autoimmune Diseases, Article ID 534759, 8 pages http://dx.doi.org/10.1155/2014/534759 Research Article A Comparison of Anti-Nuclear Antibody Quantification Using Automated Enzyme Immunoassays and Immunofluorescence
Autoimmune Diseases, Article ID 534759, 8 pages http://dx.doi.org/10.1155/2014/534759 Research Article A Comparison of Anti-Nuclear Antibody Quantification Using Automated Enzyme Immunoassays and Immunofluorescence
The following is an overview of diagnostic techniques for Ebola infection in humans.
 IBM Deep Dive Topic 1/14/2015 Alex // operonlabs.com Diagnostics and Ebola: The following is an overview of diagnostic techniques for Ebola infection in humans. Current Status: The current status of Ebola
IBM Deep Dive Topic 1/14/2015 Alex // operonlabs.com Diagnostics and Ebola: The following is an overview of diagnostic techniques for Ebola infection in humans. Current Status: The current status of Ebola
dsdna Ab ELISA Kit Catalog Number KA assays Version: 01 Intended for research use only
 dsdna Ab ELISA Kit Catalog Number KA3171 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
dsdna Ab ELISA Kit Catalog Number KA3171 96 assays Version: 01 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
ab Anti-RNP70 IgG ELISA Kit
 ab178625 Anti-RNP70 IgG ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against RNP70 in Human serum and plasma. This product is for research use only and is not
ab178625 Anti-RNP70 IgG ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against RNP70 in Human serum and plasma. This product is for research use only and is not
Anti IFI16. for routine analysis Semi-quantitative determination of IgG class antibodies against IFI16 in human serum or plasma. DCM122-3 Ed.
 DCM122-3 Ed. 03/2012 Anti IFI16 for routine analysis Semi-quantitative determination of IgG class antibodies against IFI16 in human serum or plasma IVD LOT See external label = 96 test REF DKO122 INTENDED
DCM122-3 Ed. 03/2012 Anti IFI16 for routine analysis Semi-quantitative determination of IgG class antibodies against IFI16 in human serum or plasma IVD LOT See external label = 96 test REF DKO122 INTENDED
Anti-Centromer B ORG Tests. Immunometric Enzyme Immunoassay for the quantitative determination of Antibodies directed against Centromer B
 ORGENTEC Diagnostika GmbH Carl-Zeiss-Straße 49 55129 Mainz Tel.: 06131-9258-0 Fax: 06131-9258-58 Anti-Centromer B ORG 633 96 Tests Immunometric Enzyme Immunoassay for the quantitative determination of
ORGENTEC Diagnostika GmbH Carl-Zeiss-Straße 49 55129 Mainz Tel.: 06131-9258-0 Fax: 06131-9258-58 Anti-Centromer B ORG 633 96 Tests Immunometric Enzyme Immunoassay for the quantitative determination of
Catalog. Immunofluorescence assays (IFA) The IFA-Galaxy Instruments & Assays
 Catalog Immunofluorescence assays (IF) The IF-Galaxy Instruments & ssays Welcome to our galaxy of automated immunofluorescence assays (IF) Reducing manual routines is an effective step to save time and
Catalog Immunofluorescence assays (IF) The IF-Galaxy Instruments & ssays Welcome to our galaxy of automated immunofluorescence assays (IF) Reducing manual routines is an effective step to save time and
October Monthly Update, Quest Diagnostics Nichols Institute, Valencia
 October 2014 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated.
October 2014 - Monthly Update, NEW TESTS Please Note: Not all test codes assigned to each assay are listed in the table of contents. Please refer to the complete listing on the page numbers indicated.
May Immunohistochemistry. Immunology. Microbiology. Referral Testing. ROS1 screening
 May 2017 Immunohistochemistry ROS1 screening Immunology Immunofluorescence (IFA) assay platform change ANCA (Anti-Neutrophil Cytoplasmic Ab) Panel for Vasculitis Anti-Mitochondrial Antibody Anti-Native
May 2017 Immunohistochemistry ROS1 screening Immunology Immunofluorescence (IFA) assay platform change ANCA (Anti-Neutrophil Cytoplasmic Ab) Panel for Vasculitis Anti-Mitochondrial Antibody Anti-Native
Original papers. Introduction. Andrea Tešija Kuna* 1, Lovorka Đerek 2, Ana Kozmar 3, Vedrana Drvar 4
 Original papers Current practice in laboratory diagnostics of autoimmune diseases in Croatia. Survey of the Working group for laboratory diagnostics of autoimmune diseases of the Croatian Society of Medical
Original papers Current practice in laboratory diagnostics of autoimmune diseases in Croatia. Survey of the Working group for laboratory diagnostics of autoimmune diseases of the Croatian Society of Medical
anti-ssb Enzyme Immunoassay
 anti-ssb Enzyme Immunoassay For Individual Laboratory to Complete: Laboratory Name Adopted Reviewed Reviewed Revised Supercedes Method: Diamedix Corp., Immunosimplicity Manual or in conjunction with one
anti-ssb Enzyme Immunoassay For Individual Laboratory to Complete: Laboratory Name Adopted Reviewed Reviewed Revised Supercedes Method: Diamedix Corp., Immunosimplicity Manual or in conjunction with one
ZENIT RA ENA Screen REF ZENIT RA ENA Screen
 REF 41412 ZENIT RA ENA Screen Distributed by INSTRUCTIONS FOR USE 100 INTENDED USE The ZENIT RA ENA Screen test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for quantitative
REF 41412 ZENIT RA ENA Screen Distributed by INSTRUCTIONS FOR USE 100 INTENDED USE The ZENIT RA ENA Screen test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for quantitative
Proteomic approach to autoimmune disorders: A review
 Indian Journal of Biotechnology Vol 9, January 2010, pp 13-17 Proteomic approach to autoimmune disorders: A review Vandana D Pradhan, Neha R Deshpande and K Ghosh Department of Autoimmune Disorders, National
Indian Journal of Biotechnology Vol 9, January 2010, pp 13-17 Proteomic approach to autoimmune disorders: A review Vandana D Pradhan, Neha R Deshpande and K Ghosh Department of Autoimmune Disorders, National
immunofluorescence tests in routine laboratories Dr. Dirk Roggenbuck
 Standardization di ti of cellbased immunofluorescence tests in routine laboratories Dr. Dirk Roggenbuck Overview Automatic interpretation system AKLIDES - basis for standardization Cell-based immunofluorescence
Standardization di ti of cellbased immunofluorescence tests in routine laboratories Dr. Dirk Roggenbuck Overview Automatic interpretation system AKLIDES - basis for standardization Cell-based immunofluorescence
Antigen-antibody reactions with labeled reagents
 Antigen-antibody reactions with labeled reagents Department of Immunology Faculty of Medicine University of Belgrade ANTIGEN ANTIBODY REACTIONS WITH LABELED REAGENTS Enzyme immunoassay Radioimmunoassay
Antigen-antibody reactions with labeled reagents Department of Immunology Faculty of Medicine University of Belgrade ANTIGEN ANTIBODY REACTIONS WITH LABELED REAGENTS Enzyme immunoassay Radioimmunoassay
QUANTA Lite SS-A ELISA For In Vitro Diagnostic Use CLIA Complexity: High
 QUANTA Lite SS-A ELISA 708570 For In Vitro Diagnostic Use CLIA Complexity: High Intended Use QUANTA Lite SS-A is an enzyme-linked immunosorbent assay (ELISA) for the semi-quantitative detection of SS-A
QUANTA Lite SS-A ELISA 708570 For In Vitro Diagnostic Use CLIA Complexity: High Intended Use QUANTA Lite SS-A is an enzyme-linked immunosorbent assay (ELISA) for the semi-quantitative detection of SS-A
Experience of an university hospital on the implementation of I and II Brazilian Consensuses for Standardization of ANA in HEp-2 Cells
 Original Article Experience of an university hospital on the implementation of I and II Brazilian Consensuses for Standardization of in HEp-2 Cells Claudia Cilene Fernandes Correia Laurino (1), Priscila
Original Article Experience of an university hospital on the implementation of I and II Brazilian Consensuses for Standardization of in HEp-2 Cells Claudia Cilene Fernandes Correia Laurino (1), Priscila
Centromere B Ab ELISA Kit
 Centromere B Ab ELISA Kit Catalog Number KA1275 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
Centromere B Ab ELISA Kit Catalog Number KA1275 96 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the
Human Rheumatoid Factor IgM ELISA Kit
 Product Manual Human Rheumatoid Factor IgM ELISA Kit Catalog Number PRB- 5066 PRB- 5066 96 assays 5 x 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Rheumatoid arthritis
Product Manual Human Rheumatoid Factor IgM ELISA Kit Catalog Number PRB- 5066 PRB- 5066 96 assays 5 x 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Rheumatoid arthritis
ZENIT RA Scl-70 REF ZENIT RA Scl-70
 REF 41418 ZENIT RA Scl-70 Distributed by INSTRUCTIONS FOR USE 50 INTENDED USE The ZENIT RA Scl-70 test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for quantitative
REF 41418 ZENIT RA Scl-70 Distributed by INSTRUCTIONS FOR USE 50 INTENDED USE The ZENIT RA Scl-70 test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for quantitative
AccuDiag dsdna ELISA Kit
 AccuDiag dsdna ELISA Kit 2553-1 The first quantitative assay for DNA is the Farr assay.4 The original procedure used precipitation, radio-labeled DNA and separation techniques. The difficulties in obtaining
AccuDiag dsdna ELISA Kit 2553-1 The first quantitative assay for DNA is the Farr assay.4 The original procedure used precipitation, radio-labeled DNA and separation techniques. The difficulties in obtaining
ZENIT RA Centromere B
 REF 41431 ZENIT RA Centromere B Distributed by INSTRUCTIONS FOR USE 50 INTENDED USE The ZENIT RA Centromere B test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for
REF 41431 ZENIT RA Centromere B Distributed by INSTRUCTIONS FOR USE 50 INTENDED USE The ZENIT RA Centromere B test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for
Lecture 24. Autoimmunity. Origins of autoimmunity. Diseases. Immune Diseases - Chapter 16 - Allergies - Autoimmunity - Immunodeficiency
 Lecture 24 Immune Diseases - Chapter 16 - Allergies - Autoimmunity - Immunodeficiency Disease Diagnostics - Chapter 17 - Sample Collections - Phenotypic Method - Genotypic Method - Immunological Method
Lecture 24 Immune Diseases - Chapter 16 - Allergies - Autoimmunity - Immunodeficiency Disease Diagnostics - Chapter 17 - Sample Collections - Phenotypic Method - Genotypic Method - Immunological Method
Clinical relevance and frequency of cytoplasmic and nuclear dense fine speckled patterns observed in ANA-HEp-2
 ORIGINAL ARTICLE Clinical relevance and frequency of cytoplasmic and nuclear dense fine speckled patterns observed in ANA-HEp- Andréia Martini Pazini, Juliana Fleck, Rosane Souza dos Santos, Sandra Trevisan
ORIGINAL ARTICLE Clinical relevance and frequency of cytoplasmic and nuclear dense fine speckled patterns observed in ANA-HEp- Andréia Martini Pazini, Juliana Fleck, Rosane Souza dos Santos, Sandra Trevisan
AUTOIMMUNITY CATALOGUE
 AUTOIMMUNITY Autoimmune Rheumatic Disease Antiphospholipid Syndrome ANCA & Vasculitis Hashimoto Thyroiditis Celiac Disease Autoimmune Gastroenteritis Disease Inflammatory Bowel Disease Autoimmune Diabetes
AUTOIMMUNITY Autoimmune Rheumatic Disease Antiphospholipid Syndrome ANCA & Vasculitis Hashimoto Thyroiditis Celiac Disease Autoimmune Gastroenteritis Disease Inflammatory Bowel Disease Autoimmune Diabetes
ZENIT RA U1-snRNP REF ZENIT RA U1-snRNP
 REF 41417 ZENIT RA U1-snRNP Distributed by INSTRUCTIONS FOR USE 50 INTENDED USE The ZENIT RA U1-snRNP test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for quantitative
REF 41417 ZENIT RA U1-snRNP Distributed by INSTRUCTIONS FOR USE 50 INTENDED USE The ZENIT RA U1-snRNP test is a chemiluminescent immunoassay (CLIA) for use on the dedicated ZENIT RA Analyzer for quantitative
Research Article Automated Indirect Immunofluorescence Evaluation of Antinuclear Autoantibodies on HEp-2 Cells
 Clinical and Developmental Immunology Volume 2012, Article ID 651058, 7 pages doi:10.1155/2012/651058 Research Article Automated Indirect Immunofluorescence Evaluation of Antinuclear Autoantibodies on
Clinical and Developmental Immunology Volume 2012, Article ID 651058, 7 pages doi:10.1155/2012/651058 Research Article Automated Indirect Immunofluorescence Evaluation of Antinuclear Autoantibodies on
Molecular reclassification to find clinically useful biomarkers for systemic autoimmune diseases
 IMI Periodic Report Template Molecular reclassification to find clinically useful biomarkers for systemic autoimmune diseases PRECISESADS 115565 Prof. Chris Chamberlain UCB Biopharma SPRL Allée de la Recherche,
IMI Periodic Report Template Molecular reclassification to find clinically useful biomarkers for systemic autoimmune diseases PRECISESADS 115565 Prof. Chris Chamberlain UCB Biopharma SPRL Allée de la Recherche,
ab Anti-Sm IgG ELISA Kit
 ab178627 Anti-Sm IgG ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against Sm in Human serum and plasma. This product is for research use only and is not intended
ab178627 Anti-Sm IgG ELISA Kit Instructions for Use For the quantitative measurement of IgG class antibodies against Sm in Human serum and plasma. This product is for research use only and is not intended
Cellular RNA-Protein Particles
 Antibodies from Patients with Connective Tissue Diseases Bind Specific Subsets of Cellular RNA-Protein Particles JOHN A. HARDIN, DANIEL R. RAHN, CALVIN SHEN, MICHAEL R. LERNER, SANDRA L. WOLIN, MARGARET
Antibodies from Patients with Connective Tissue Diseases Bind Specific Subsets of Cellular RNA-Protein Particles JOHN A. HARDIN, DANIEL R. RAHN, CALVIN SHEN, MICHAEL R. LERNER, SANDRA L. WOLIN, MARGARET
SUMMARY AND EXPLANATION OF THE TEST
 HEp-2000 COLORZYME ANA-Ro TEST SYSTEM For in vitro Diagnostic Use For Professional Use INTENDED USE: This is an indirect enzyme antibody test for the semi-quantitative detection of antinuclear antibody
HEp-2000 COLORZYME ANA-Ro TEST SYSTEM For in vitro Diagnostic Use For Professional Use INTENDED USE: This is an indirect enzyme antibody test for the semi-quantitative detection of antinuclear antibody
