Application of a Differential Fuel-Cell Analyzer for Measuring Atmospheric Oxygen Variations
|
|
|
- Hugh Perry
- 6 years ago
- Views:
Transcription
1 82 J O U R N A L O F A T M O S P H E R I C A N D O C E A N I C T E C H N O L O G Y VOLUME 24 Application of a Differential Fuel-Cell Analyzer for Measuring Atmospheric Oxygen Variations BRITTON B. STEPHENS Earth Observing Laboratory, National Center for Atmospheric Research,* Boulder, Colorado PETER S. BAKWIN AND PIETER P. TANS NOAA/Climate Monitoring and Diagnostics Laboratory, Boulder, Colorado RON M. TECLAW AND DANIEL D. BAUMANN Forest Sciences Laboratory, USDA Forest Service, Rhinelander, Wisconsin (Manuscript received 19 December 2005, in final form 10 May 2006) ABSTRACT A commercially available differential fuel-cell analyzer has been adapted to make field-based ppm-level measurements of atmospheric O 2 variations. With the implementation of rapid calibrations and active pressure and flow control, the analysis system described here has a 1 precision of 2.5 per meg ( 0.5 ppm) for a 2-min measurement. Allowing for system stabilization after switching inlet lines, a 6-min measurement with a precision of 1.4 per meg ( 0.3 ppm) every 20 min is obtained. The elimination of biases in any atmospheric O 2 measurement depends critically on careful gas-handling procedures, and after screening for known sources of bias a comparability of 10 per meg ( 2 ppm) with the present setup is estimated. In comparison to existing techniques, the relatively small size, low cost, fast response, motion insensitivity, and ease of implementation of the fuel-cell analyzer make it particularly useful for a wide range of unattended field applications. This system has been used to measure atmospheric O 2 concentrations at the WLEF tall-tower research site in northern Wisconsin semicontinuously from June 2000 to December These measurements represent the first extended O 2 record in and above a forest ecosystem, and are being used to investigate global carbon budgeting, plant physiology, continental boundary layer mixing and synoptic transport, and potential means of industrial emission verification. In this paper, the measurement technique is described in detail and several weeks of data are presented to illustrate its performance. 1. Introduction Marine boundary layer atmospheric O 2 measurements have proven to be valuable constraints on the global partitioning of terrestrial and oceanic CO 2 sources, seasonal net production by the marine biosphere, hemispheric gas exchange rates, and interhemispheric oceanic transport (Keeling and Shertz 1992; * The National Center for Atmospheric Research is sponsored by the National Science Foundation. Corresponding author address: Dr. Britton Stephens, National Center for Atmospheric Research, Earth Observing Laboratory, 1850 Table Mesa Dr., Boulder, CO stephens@ucar.edu Bender et al. 1996; Keeling et al. 1996, 1998b; Stephens et al. 1998; Battle et al. 2000; Manning and Keeling 2005). The precision requirements for these measurements are challenging, with background variations of interest on the order of per meg (Keeling et al. 1993), where O 2 N 2 per meg O 2 N 2 sample O 2 N 2 reference In this context, the addition of 1 mol of O 2 to 1 mol of dry air results in a change of 4.8 per meg (Keeling et al. 1998a). Existing techniques for measuring parts per million (ppm)-level background O 2 variations now include interferometric (Keeling 1988), mass spectro- 1 DOI: /JTECH American Meteorological Society
2 JANUARY 2007 S T E P HENS ET AL. 83 metric (Bender et al. 1994), paramagnetic (Manning et al. 1999), vacuum ultraviolet absorption (Stephens 1999; Stephens et al. 2003), and gas chromatography (Tohjima 2000). However, for autonomous in situ measurements, these methods all have specific disadvantages; the instruments are either too large, too expensive, or too motion sensitive, or require either extensive construction, frequent user intervention, or too much time to obtain a precise measurement. We present here a new technique, based on a commercially available differential fuel-cell analyzer, that addresses these limitations and significantly advances our atmospheric O 2 measurement capabilities. The advanced capabilities of the fuel-cell analyzer can enable a wide range of new studies. For example, the fuel-cell O 2 measurement system can be used to improve the temporal resolution of marine boundary layer records typically based on flask sampling, thereby significantly advancing our understanding of seasonal marine productivity and episodic oceanic O 2 fluxes (Manning et al. 1999; Lueker et al. 2003). In addition, this system can be used to improve upon the spatial and temporal resolution of shipboard flask measurements, which will help to address uncertainties in meridional atmospheric O 2 gradients and their implications for large-scale ocean circulation (Stephens 1999; Stephens et al. 2003; Thompson 2005; Tohjima et al. 2005; Battle et al. 2006). The development of this robust in situ technique has also allowed us to extend atmospheric O 2 measurements from the marine boundary layer to the interior of continents, and to apply these continental measurements to study global CO 2 budgeting, terrestrial biospheric processes, atmospheric transport, and industrial emissions. Presently, observed trends in atmospheric O 2 provide our best constraint on the long-term global partitioning of terrestrial and oceanic sinks for anthropogenic CO 2 (Houghton et al. 2001). A key value in calculating this partitioning is the assumed O 2 :CO 2 ratio for the terrestrial uptake of anthropogenic CO 2. The relationship between O 2 and CO 2 during terrestrial exchange is not exactly 1 because of the incorporation of nitrogen and other species during photosynthesis, and their oxidation during respiration. Keeling (1988) estimated an oxidation ratio (OR O 2 :CO 2 ) for wood of 1.05 based on a survey of elemental abundance studies. Severinghaus (1995) measured oxidation ratios of around 1.2 for forest soil samples, but estimated that the net ecosystem exchange occurs at a ratio closer to 1.1. The OR for respiration in forest soils and roots depends on the form of nitrogen (NO 3 or NH 4 ) produced by soil microbes and utilized by trees (Bloom et al. 1989, 1992). However, this fundamental distinction is not widely characterized in nature and is ignored in most terrestrial ecosystem models. Furthermore, the photosynthetic quotient (PQ O 2 : CO 2 ) for trees also depends on the allocation of new carbon to either wood or nitrogen-rich leaves and fine roots. The ability to measure this partitioning would be very useful in identifying the growth stage of a forest and locating net carbon sinks. Existing flask-based studies of terrestrial O 2 :CO 2 ratios are limited by the number and scatter of the available data points (Seibt et al. 2004; Marca 2004; Sturm et al. 2005a). Thus, continuous measurements of atmospheric O 2 and CO 2 in a forest environment can improve our constraints on the terrestrial O 2 :CO 2 value used to partition global CO 2 sinks, and they can have direct impacts on our understanding of the physiological behavior of forest plants. By combining O 2 and concurrent CO 2 measurements, we can derive the tracer atmospheric potential oxygen (APO O CO 2 ; Stephens et al. 1998). The definition of APO includes the simplifying assumption of a constant and known O 2 :CO 2 ratio, but to the extent that photosynthesis and respiration do not change this scaled sum of O 2 and CO 2, APO is conservative with respect to terrestrial processes. In contrast, because air sea O 2 and CO 2 fluxes are largely decoupled, APO provides a sensitive tracer for oceanic gas exchange. Continental interior observations of this primarily oceanic tracer can then provide valuable information on aspects of continental boundary layer mixing and synoptic-scale transport that can be used to validate atmospheric models. For example, measurements of the APO gradient between the North Pacific and the central United States can help to constrain the seasonal variation in continental boundary layer mixing, which is a dominant source of uncertainty in global CO 2 inversions (Denning et al. 1995; Gurney et al. 2002). The O 2 :CO 2 ratios for fossil fuel combustion vary from 1.95 for natural gas, to 1.44 for liquid fuels, to 1.17 for coal (Keeling 1988). Because of this, atmospheric O 2 measurements can help to distinguish between industrial and terrestrial influences on observed CO 2 variations. Furthermore, it may be possible to use atmospheric O 2 measurements in future international emission verification efforts. For example, if we knew how much natural gas and liquid fuel were being combusted in a particular country, we could measure the O 2 :CO 2 ratio in downwind pollution signals to estimate how much coal was also being burned. We can test this idea by measuring O 2 :CO 2 ratios in continental U.S. pollution events and comparing them to those expected from reported distributions of fuel types for the source regions.
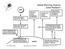
3 84 J O U R N A L O F A T M O S P H E R I C A N D O C E A N I C T E C H N O L O G Y VOLUME 24 To investigate these various processes and applications, in June of 2000 we installed the differential fuelcell analyzer and associated equipment described here for ongoing O 2 measurements at the WLEF tall-tower research site (Bakwin et al. 1998; Davis et al. 2003) in northern Wisconsin. More recently, similar systems have been installed at a tall-tower site in Ochsenkopf, Germany (A. Manning 2003, personal communication), on research ships in the Southern Ocean (Thompson 2005), and at Harvard Forest, Massachusetts (M. Battle 2005, personal communication); and installation has been planned at Mace Head, Ireland (R. Keeling, 2005 personal communication) and Syowa, Antarctica (T. Nakazawa 2005, personal communication). To assist in this growing use of fuel-cell O 2 analyzers, we provide a detailed description of our WLEF implementation in section 2. Also, as expected with the exploratory nature of this project, we have encountered a number of unique challenges to making accurate continuous O 2 measurements, and we share what we have learned about potential sources of measurement bias associated with fractionation in reference cylinders, at tubing tee junctions, and at sample inlets. We will present the major results from the first three-and-a-half years of measurements in a subsequent publication, but present several weeks of data here in section 3 to demonstrate the performance and potential applications of this measurement approach. 2. Instrument description and performance Figure 1 shows a schematic of the instrumentation and gas-handling hardware we have implemented at the WLEF tall-tower site for background atmospheric O 2 measurements. a. O 2 analyzer The Sable Systems Oxzilla I differential O 2 analyzer is based on a comparison of potentials from two lead oxygen fuel cells. These cells contain a weak acid electrolyte, which is isolated from air by a gas-permeable membrane, and within which the net reaction O 2 4H 2Pb 2H 2 O 2Pb 2 takes place. The voltage across the cell is thus proportional to the rate at which O 2 diffuses across the membrane, which in turn is proportional to the partial pressure of O 2 in the analyzed airstreams. By differencing the voltages from the two cells, most sources of drift in the signals can be eliminated, and a very precise measurement is produced. For differential measurements, the Oxzilla I came with a quoted short-term noise of 2 ppm ( 10 per meg) and a long-term drift of 20 ppm ( 100 per meg). For our unit we found a differential 1 precision at the serial data output rate of 1.16 Hz of 25 per meg, and drift rates in the differential signal of 100 per meg over several hours. The analyzer also does not appear to be adversely affected by acceleration or vibration. To improve the instrument for background atmospheric O 2 measurements we made several modest changes to the off-the-shelf unit. Because differential permeation of O 2 and N 2 through plastic tubing can have significant effects on O 2 mole fractions, we removed the internal plastic tubing and filters and replaced them with 2-mm inner-diameter (ID) stainless steel tubing. Also, because gas temperatures equilibrate very rapidly in metal tubing, we removed the original heat exchanger tubing coil and fan. To avoid the potential for small downstream pressure differences, we plumbed the two sample-cell outlets and the internal pressure sensor into a common volume immediately downstream of the cells. Finally, to minimize absolute and differential pressure fluctuations in the air flowing past the fuel cells, we actively control mass flow and pressure at various points, as shown in Fig. 1. To improve upon the precision and stability characteristics, we rapidly switch sample and reference gases between the two cells, using an upstream four-way valve (see Fig. 1) composed of two miniature solenoid valves and a manifold (Numatics, TM series). The diffusivity of the fuel-cell membranes leads to a characteristic response time for the sensors of 12 s to reach 90% of a step change. Therefore, we switch every 64.5 s (75 serial data values) and ignore the first 21.5 s (25 serial data values) of data after each switch. An example of the resulting signal when switching between two known calibration gases is shown in Fig. 2. Because the Oxzilla differential signal represents alternately gas A gas B and then gas B gas A, the measured amplitude of this square wave ( 1-min ) represents 2 gas A 2 gas B. By switching in this manner we eliminate most sources of drift with time scales longer than 1 min, and we increase the sensitivity of the measurement by a factor of 2. The 1 precision on an average of the differential signal over one 43-s period is 3.5 per meg, which is consistent with a standard error from averaging 50 values having white noise of 25 per meg. The precision on 1-min is 2.5 per meg in the actual concentration difference between the two switched gases, which is consistent with summing two 3.5 per meg errors in quadrature then dividing by a factor of 2. The drift rate on 1-min is typically 2.5 per meg over 6 h, which ultimately determines the required calibration frequency. To convert the Oxzilla measurements of apparent
4 JANUARY 2007 S T E P HENS ET AL. 85 FIG. 1. Schematic and legend showing the gas-handling arrangement as implemented at the WLEF tall tower. Components are included for air sampling, air drying, calibration, regulator and sample line purging, active flow and pressure control, and O 2 and CO 2 analysis. mole fraction into relative per-meg units requires correcting for the diluting effect of changing CO 2 concentrations (Keeling et al. 1998a). We first convert our reference gas (O 2 /N 2 ) values into apparent mole fraction differences ( X O2 ) using their known CO 2 concentrations. Then, after linearly interpolating the Oxzilla differential signals to get apparent sample mole fraction differences we convert these values back into relative per-meg units using simultaneously measured CO 2 concentrations. All of these conversions are captured in the following equation [from Keeling et al. 1998a, their Eq. (4)]: O 2 N 2 X O 2 X O2 CO , 3 X O2 1 X O2 where the O 2 mole fraction (X O2 ) is , [CO 2 ]isthe measured CO 2 concentration ( mol mol 1 ) in dry air for the sample gas, and is the average CO 2 concentration of the reference cylinders defining zero on the Scripps O 2 scale. This conversion assumes that N 2 and Ar are constant, so it is important to only apply it in situations where the references gases are composed of natural air. In addition to the modifications just described, a number of other conditions must be met to make comparable background atmospheric O 2 measurements. These include 1) using reference gases tied to established calibration scales and introducing them to the instrument at the appropriate frequency, pressure, and flow; 2) providing suitable flows through sample inlet
5 86 J O U R N A L O F A T M O S P H E R I C A N D O C E A N I C T E C H N O L O G Y VOLUME 24 FIG. 2. Example of the Oxzilla differential O 2 signal over several 1-min switching cycles, while comparing two reference gases. The vertical gray lines indicate the time at which the changeover valve is switched and open circles show data that are ignored after each switch. In this case the gases have an apparent O 2 mole fraction difference of %, which results in a signal amplitude of % because of the crossover switching arrangement. lines and the instrument while avoiding fractionation effects at inlets and tubing junctions; 3) drying the sample and calibration gases to below several ppm of H 2 O or a dewpoint of 80 C; 4) maintaining constant flows and pressures throughout the system, and allowing time for equilibration of diffusive concentration gradients after any perturbation; 5) eliminating dead volumes; and 6) making simultaneous high-accuracy measurements of CO 2 concentrations for use in applying the dilution correction and in scientific analyses. The equipment we used to achieve these critical tasks is shown in Fig. 1 and described below. b. Reference gases We employed five high pressure gas cylinders to calibrate our O 2 and CO 2 measurements. These are or 46.4-L aluminum cylinders (Luxfer) with brass Ceodeux valves, fitted with brass two-stage pressure regulators (Scott Specialty Gases, model 14). The high-span (HS), low-span (LS), and long-term (LT) reference tanks were prepared and assigned O 2 and CO 2 values in the Atmospheric Oxygen Laboratory at the Scripps Institution of Oceanography (SIO; Keeling et al. 1998a). These cylinders were also analyzed for CO 2 concentration at the National Oceanic and Atmospheric Administration (NOAA) Climate Monitoring and Diagnostics Laboratory (CMDL; Zhao et al. 1997). The HS, LS, and LT reference gases were adjusted to have nominal (O 2 /N 2 ) values of 100, 600, and 300 per meg on the SIO oxygen scale and nominal CO 2 concentrations of 340, 450, and 390 ppm, respectively. Using reference gases with inverted O 2 and CO 2 ranges leads to smaller apparent mole fraction differences in the fuel-cell instrument [Eq. (3)], but more closely matches natural variations. These ranges were intended to span the full range of observations, but at low heights at night during summer they were occasionally exceeded. An additional CO 2 reference tank (CT) was prepared and assigned a CO 2 value at CMDL, with a nominal concentration of 370 ppm. The working tank (WT) reference gas was ordered (Scott Marrin) as natural air with a nominal CO 2 concentration of 380 ppm and ambient O 2 levels, but we treat these values as unknowns in our calibration scheme. The HS, LS, LT, and CT cylinders are connected to two miniature solenoid valve manifolds (Numatics, TM series). Miniature solenoid valves such as these can develop significant leaks, so caution and frequent automated leak checks should be applied in their use. One of these solenoid manifolds selects a reference gas or sample air to be purged, while the other selects a reference gas or sample air to be analyzed. The gas selected to be analyzed passes through a mass-flow controller (MKS Instruments, M100B) set to either 100 or 50 ml min 1 (see section 2c). Every 6 h the HS and LS gases are analyzed for 20 or 30 min each (see section 2d). To sweep out and condition the regulators and stagnant lines, we purge each of these gases for 15 min at 300 ml min 1 prior to introducing them to the analyzer. To avoid known cylinder drift near depletion (Keeling et al. 1998a, 2004, 2005; Langenfelds et al. 2005), we normally stop using the HS and LS cylinders when their pressure reaches 30 atm, and return them for reanalysis. The 46.4-L HS and LS cylinders last 6 months at a 100 ml min 1 measurement flow and our analysis frequency. Once every 3 days, we analyze the LT gas, after purging for 20 min at 300 ml min 1, while once every 6 days we also analyze the CT gas after purging for 10 min at 300 ml min 1. The 46.4-L LT and 29.5-L CT cylinders should have useful lifetimes at 100 ml min 1 measurement flow of 6 and 8 yr, respectively, although they should be returned to a laboratory for recalibration sooner than that. The WT gas flows to the analyzer continuously, and using 29.5-L cylinders they must be replaced every month with a 100 ml min 1 flow. We determine (O 2 /N 2 ) values by referencing 1-min obtained by comparing WT and sample gases to those obtained comparing WT and other calibration gases.
6 JANUARY 2007 S T E P HENS ET AL. 87 We calculate the WT value and span using a linear calibration between the HS and LS values, interpolated in time between the 6-hourly calibrations. Analyses of the LT gas provide independent checks on the stability and accuracy of our O 2 calibrations. For our CO 2 measurements we use a LiCor 6251 dual-channel infrared gas analyzer (IRGA), and calculate 1-min means of the IRGA signal corresponding to the downstream switching cycle. To obtain CO 2 concentrations, we use the approximately weekly calibrations that include all four reference gases to calculate second-order polynomial calibration curves. We then apply linear adjustments to these second-order curves based on the 6-hourly HS and LS runs. For an independent check on our CO 2 measurements, we compare to the existing highaccuracy CO 2 measurement system in place at the WLEF tall tower (Bakwin et al. 1998). c. Air sampling and drying Another critical challenge of all atmospheric O 2 measurement systems is the delivery of air from the inlet location to the instrument without fractionating O 2 relative to N 2. For the first year of the WLEF deployment, we maintained sample flows of 100 ml min 1. However, in an attempt to minimize the demands of replacing WT cylinders every month and HS and LS cylinders every 6 months, in June 2001 we reduced the flow rate to 50 ml min 1. To avoid unreasonably long gas residence times in the inlet tubes at these flow rates and to take advantage of existing L min 1 sample inlet lines, for three of our lines we chose to pick off a small measurement flow from the larger sample flow at a tee junction. Because tee junctions can fractionate O 2 relative to N 2, as an additional check on the effect of these tees we installed a dedicated line at 30 m with no tee (see Fig. 1). Comparisons between measurements from the 30-m lines with and without a tee then provide a means of assessing fractionation effects in the field. To further minimize pressure fluctuations at these tees, we installed 5 m of 4.3-mm ID tubing downstream of the tees and upstream of the pickoff for the Bakwin et al. (1998) CO 2 concentration system (not shown), and 15 m of 9.6-mm ID tubing downstream of the flux LiCor and upstream of the main diaphragm pumps for these lines. As illustrated in Fig. 1, our inlets consist of a 9-cmdiameter inverted plastic funnel acting as a rain shield connected to a 5-cm-diameter, 3- m-pore-sized filter in a plastic housing (Cole Parmer). Inside the instrument trailer, the sample air passes through 7- m filters, then mass-flow controllers (MKS Instruments, M100B), and then to a miniature solenoid valve manifold (Numatics, TM series). For the first year and a half of the WLEF deployment, we used manual needle valves instead of mass-flow controllers on the inlet lines, but because variable pump efficiency and needle valve impedance required frequent adjustments to maintain constant flows, we switched to the illustrated configuration in February The inlet solenoid valves select one of the four inlet lines to be sampled, and direct the other three to a diaphragm pump (KNF, N010), which continuously flushes the unused lines. Gas from the selected inlet line is pressurized by a second diaphragm pump (KNF, N05) to approximately 160 kpa before entering a custom glass refrigerator trap held between 0 and 5 C. This sample pump has a Teflon valve plate and Teflon-coated neoprene diaphragm based on early success with these materials at SIO (R. Keeling 2006, personal communication). However, we must also use four Viton o-rings in custom-cut grooves in the pump heads to prevent leakage along the Teflon valve plate. It is necessary to position the refrigerator trap lower than the pump so that any compression-induced condensation will flow downstream into the trap and not collect at the pump head. Water collected in this trap is removed by a peristaltic pump (Cole Parmer) running at 0.06 ml min 1, which prevents liquid from accumulating in the trap without the potential for fractionation that would exist with an open vent (Manning 2001). The refrigerator trap removes the bulk of water vapor present in the airstream. However, because the dilution associated with only a 1 ppm change in water vapor would appear as a 1.3 per meg change in O 2, we must dry the air much further. The sample air next passes through a 2.5-cm-diameter glass trap in a cryogenic bath (FTS VT490) held below 90 C. This trap would normally last 2 weeks before clogging, but we swap it once per week with a second trap. The trap that is not in use is dried by the intake line and calibration gas purge flows (Fig. 1). After this second stage of drying, the sample gas stream joins the four calibration gases at the purge and analysis solenoid valve manifolds. As a result of thermal diffusion, large gradients in O 2 and N 2 form going into and out of cold traps (Keeling et al. 1998a). At steady flows, conservation of mass ensures that the sample is not altered passing through the traps, but any disturbance to the pressure or flow through the traps can result in large transient responses in the instrument while these gradients reestablish. Consequently, when we are analyzing a calibration gas, we must continue to purge the traps with sample air at the same flow and pressure. The flow rate of the calibration gas purge is set by a downstream manual needle valve, while the flow rate of the sample gas is fixed by the upstream mass-flow controllers. A manual needle valve on the sample purge line then allows us to match
7 88 J O U R N A L O F A T M O S P H E R I C A N D O C E A N I C T E C H N O L O G Y VOLUME 24 the trap pressures during purge and analysis. Nonetheless, even the smaller perturbations associated with switching between sample and calibration gas, or between two different sample lines, can cause noticeable transient instrument responses. In addition to these flow perturbation effects, it is necessary to sweep out the trap volumes and unavoidable dead spaces with the new gas after switching. At our low-flow rates, this can take a considerable amount of time. We have taken care to minimize the dead volumes at pressure sensors, and within the solenoid valves and valve manifolds. In practice, it takes approximately 5 min for the CO 2 and O 2 readings to stabilize after switching between calibration gases and min, because of the large-volume cold trap, after switching between sample lines (see Fig. 3). This sample line equilibration time could be reduced by minimizing the trap volumes further or increasing the flow rate, with the associated trade-offs being more frequent trap and reference cylinder replacement. d. O 2 and CO 2 measurements FIG. 3. Example of (a) the amplitude of the 1-min switching cycle for the Oxzilla differential O 2 signal ( 1-min ) and (b) the 1-min-averaged signal from the CO 2 analyzer on 9 Jun Symbols represent the averages of these values as determined from the last 6 min of each measurement period for 496 m (upwardpointing triangles), 122 m (squares), 30 m (diamonds), 30 m no tee (downward-pointing triangles), and calibration (circles) gas. Midnight (UTC) occurs at 1900 local time. The lower values of 1-min at night correspond to less O 2 in the sample air than in the reference gas. For the CO 2 analyzer, the higher voltages at night correspond to more CO 2 in the sample air than in the reference gas. The reference or sample gas selected for analysis passes through the mass-flow controller mentioned above and a final cold trap, consisting of a 3-mm ID stainless steel tubing loop, before entering the CO 2 analyzer at approximately 135 kpa. The WT reference gas passes through a similar cold trap at a pressure of 160 kpa. Its pressure is then reduced through a proportional solenoid valve (MKS, 248A) driven by a feedback controller (MKS, 250E) referenced to a 10-torr full-scale differential pressure gauge (MKS, 223B), in order to match the sample and reference pressures in the CO 2 analyzer. Downstream of the CO 2 analyzer, both gas streams pass through 0.5- m filters, mass-flow meters (Honeywell, AWM3100), and manual needle valves before reaching the four-way changeover valve described above. By adjusting these needle valves, we can balance the flows in the two lines. The four-way valve then switches once every minute to allow the rapid signal differencing and calculation of 1-min described above. After passing through the Oxzilla sample cells, the two gas streams join in a volume connected to the internal pressure sensor before exiting to the room. Changes in room pressure, and consequently the Oxzilla cell pressure, have a significant effect on the signals from the individual Oxzilla cells. However, this effect is much smaller on the difference signal between the two cells, and virtually insignificant on the 1-min amplitude of this difference signal that we use as the basis for our measurement. Figure 3 shows the 1-min O 2 signal and CO 2 analyzer voltage over 24 h of routine summertime measurements, covering a full O 2 and CO 2 diurnal cycle and including four calibration periods in which gas from the HS and LS cylinders were analyzed. Using the inlet and analysis solenoid valve manifolds, we switch the gas to be analyzed every 20 or 30 min. Initially, we allowed 30
8 JANUARY 2007 S T E P HENS ET AL. 89 min per line, but after installing the mass-flow controllers on each line in February 2002 we were able to reduce the switching time to 20 min. In either case, we use only the last 6 min of data from each gas for a measurement. This results in three independent 1-min values, which average to an expected 1 precision of 1.4 per meg. To apply the CO 2 dilution correction, a measurement accuracy of 1 ppm would be sufficient, but most scientific applications require significantly better resolution. We average the CO 2 analyzer voltage over the corresponding 6 min to achieve a 1 precision of 0.05 ppm. e. Potential sources of measurement bias High-precision, comparable atmospheric O 2 measurements are still very challenging to make. Our quoted precision of 1.4 per meg corresponds to the addition or removal of only one molecule of O 2 to every 3 million molecules of air, and there are numerous processes that can introduce biases that are times greater in magnitude. For example, it is known that thermal fractionation of O 2 relative to N 2 inside high pressure gas cylinders resulting from small ambient temperature changes can lead to drift in (O 2 /N 2 ) values for the delivered air on the order of per meg over the life of the cylinder (Keeling et al. 1998a, 2004, 2005; Langenfelds et al. 2005). Furthermore, if the cylinders are upright and exposed to room temperature variations of several degrees Celsius, significant shortterm oscillations in (O 2 /N 2 ) may be produced and the potential for long-term drift may be greater (W. A. Brand 2003, personal communication). Because of space constraints at the WLEF tall-tower site we were unable to place our reference cylinders horizontally or in an insulated enclosure. Temperature at specific points within the WLEF instrument trailer typically oscillates by 1 2 C on a 2-h air conditioner switching cycle, and at various times when the heaters or air conditioners failed or could not meet demands, even larger diurnal temperature oscillations occurred. We see evidence of short-term variations in our reference gases in our LT record (Fig. 4), with periods of peak-to-peak variations of per meg (approximately per meg RMS) corresponding to periods of room temperature oscillations of 2 12 C. It also appears from these records that (O 2 /N 2 ) in the air delivered from the HS and LS cylinders drifted up by approximately 20 per meg during the course of their use, resulting in apparent decreases in the LT value. Interestingly, comparing pre- and postuse calibrations at SIO and the National Center for Atmospheric Research (NCAR) for the eight HS and LS cylinders used (Fig. 4, lower panel), we only saw one case of a large FIG. 4. (top) Determinations of the LT cylinder over 3.5 yr. The dark line connects laboratory measurements on this cylinder before and after deployment. (middle) Large short-term variations in the field LT values are associated with large room temperature variations shown here as daily room temperature ranges. (bottom) Trends in LT values appear to be related to drifts in the HS and LS reference gases as their cylinder pressures decrease. The vertical gray lines indicate times when the HS and LS gases were replaced. change in (O 2 /N 2 ). The four HS cylinders showed changes of 5, 6, 3, and 5 per meg, the four LS cylinders showed changes of 3, 1, 17, and 2 per meg, and the LT cylinder showed a change of 6 per meg. It is possible that growing concentration gradients in the vertically oriented cylinders resulted in the observed field drift in their delivered air, but that these gradients were mixed out in the process of transporting the cylinders back to the laboratory and analyzing them in a horizontal position. Second, we know that temperature and pressure gradients at tee junctions can easily lead to (O 2 /N 2 ) changes on the order of 100 per meg in the smaller flow of air going to the instrument (Manning 2001; Stephens et al. 2003). In an attempt to overcome this obstacle, we experimented with various tee configurations in which the pick-off tube extended upstream of the tee, inside of the larger inlet tube. After trying various tee sizes a pick-off tube lengths, widths, and orientations, we found that running a 1.0-mm ID, 1.6-mm OD stainless steel tube 30 cm upstream of the tee inside 9.6-mm ID tubing had less than 10 per meg fractionation at a flow ratio of approximately 200:1. We note that many other pick-off configurations led to fractionation on the order
9 90 J O U R N A L O F A T M O S P H E R I C A N D O C E A N I C T E C H N O L O G Y VOLUME 24 of 100 per meg under these flow conditions. The amount of fractionation associated with a 2.1-mm ID, 3.2-mm OD pick-off tube was sensitive to the radial positioning of the tube end within the sample tube and to the orientation of the tube-end face relative to the sample flow. In contrast, the 1.0-mm ID pick-off tube that we used did not appear sensitive to these parameters. We used a high-precision vacuum ultraviolet O 2 analyzer (Stephens et al. 2003) in these tests but other limitations in the experimental design precluded tighter constraints. Our analyses have revealed that while this tee configuration may have minimized pressure-related fractionation effects, it was still susceptible to temperaturerelated effects. The integration of these tees with the existing plumbing at WLEF resulted in the pick-off tubes extending upstream into tubing that was outside of the instrument trailer, black in color, and only partially shaded by existing structures. Measurements from the three lines with tees show occasional isolated O 2 anomalies, both positive and negative, on the order of 20 per meg. These anomalous measurements occur within several hours of local noon and only when it is sunny. This suggests that the pick-off tubes may have been sampling within thermally induced O 2 gradients associated with differential solar heating of the exterior tubes. While the dedicated intake line at 30 m does provide an independent means of sampling for comparison, it is also vulnerable to potential fractionation effects, in this case resulting from thermal gradients at the inlet associated with the slower intake flows (Manning 2001; Blaine et al. 2005; Sturm et al. 2005b). The funnels we used as rain shields were white and the filter housings were light blue. For the first year of the WLEF deployment with sample flows of 100 ml min 1, we did not observe clear signs of inlet fractionation. However, after reducing the flow rate to 50 ml min 1, the dedicated 30-m line exhibited diurnal APO variations of up to 40 per meg in winter and up to 100 per meg in summer on sunny days with moderate winds. These variations were in the direction of low O 2 relative to N 2 during the daytime, which is consistent with sampling thermally induced gradients at the inlet associated with solar heating of the rain shield and or filter housing. Also, the magnitude of these variations is comparable to expectations from more recent inlet tests when measuring Ar/N 2 ratios (Blaine et al. 2005; Sturm et al. 2005b). Our improved understanding of these effects will naturally lead to different procedures in future deployments. High pressure gas cylinders should be horizontal and insulated from room temperature variations to prevent short-term oscillations and long-term drift in reference values. Also, it may be possible to use pick-off tees without significant fractionation, if pressure gradients can be minimized and all tubing can be maintained isothermal for sufficient distances upstream and downstream of the tee. However, even though installation and gas residence time concerns led us to use tees on three of the lines at WLEF, we do not recommend this approach. Finally, inlet flow rates and inlet geometry should be selected to minimize the potential for stagnant air and the development of thermal gradients. The most promising inlet approach based on Ar/N 2 tests appears to be that of using a fan to aspirate the inlet (Blaine et al. 2005). The presence of known artifacts in the WLEF O 2 data will require special care in their interpretation. For example, analyses of O 2 :CO 2 ratios will need to compare cloudy and sunny days, and different times of day, to quantify potential biases in the results. Fortunately some of the strongest signals, such as the predawn vertical gradients, should be less affected by the inlet and tee fractionation effects associated with solar heating. For O 2 comparisons between WLEF and other stations, the effect of cylinder drift will have to be assessed and possibly corrected for based on pre- and postdeployment laboratory determinations of the HS and LS values, and the operational LT measurements. It is important to point out that these potential effects are not unique to the fuel-cell instrument but are general to all in situ O 2 measurement techniques. The comparability of our measurements with respect to other sites and techniques clearly depends on a number of factors. However, assuming that we can adequately filter or correct for known biases and that we can eliminate them in future deployments, we conservatively estimate comparability (see definition in Worthy and Huang 2005) with respect to other O 2 measuring programs of 10 per meg for our fuel-cell system. A quantitative summation of the various components contributing to this comparability limit is difficult to make, but it is the potential effects that we cannot easily detect or correct for that contribute most to our subjective estimate. These are primarily short-term fractionation in vertically oriented and uninsulated cylinders, and incomplete stabilization of concentration gradients associated with cold traps after switching between inlet lines. We do not discuss our CO 2 measurements in detail here, but IRGAs are also known to have potential sources of bias that require careful attention to sample drying, pressure balancing, reference gas handling, and calibration schemes (Bakwin et al. 1998; Trivett and Köhler 1999). Our calibration frequency was optimized
10 JANUARY 2007 S T E P HENS ET AL. 91 FIG. 5. Seven-day time series (a) and correlation (b) plots for atmospheric O 2 and CO 2 variations measured at the WLEF tall-tower site in June 2001 for 496 (upward-pointing triangles), 122 (squares), 30 (diamonds), and 30 m no tee (downward-pointing triangles) gases. Midnight (UTC) occurs at 1900 local time. The y axis in (a) and both axes in (b) have been scaled to be equivalent on a mole O 2 to mole CO 2 basis. The slopes in (b) were determined from orthogonal distance regression with uncertainties on O 2 and CO 2 values set equal on a molar basis. for the requirements of the O 2 measurements and conservation of calibration gases, but it may not have been ideal for CO 2 measurements. The CO 2 analyzer has greater short-term drift than the O 2 analyzer because it is not calibrated by the 1-min switching cycle and has a greater thermal sensitivity. We account for short-term thermal drift in the IRGA by deriving an empirical temperature correction from our 6-hourly calibration data and apply this at the higher time resolution of the measurements. More frequent one-point calibrations or incorporating the CO 2 analyzer within the rapid switching loop would be more rigorous alternatives to this approach. 3. WLEF tall-tower observations The WLEF tower is a 447-m-tall communications tower located in a region of mixed forest in northern Wisconsin. It is part of the NOAA/Climate Modeling and Diagnostics Laboratory (CMDL) Cooperative Sampling Network and the Department of Energy (DOE) AmeriFlux network, and is the focal point of the Chequemegon Ecosystem Atmosphere Study (ChEAS) (Bakwin et al. 1998; Davis et al. 2003; Desai et al. 2006). Ongoing measurements at the tower include CO 2 concentration at six levels; eddy covariance measurements of CO 2 flux at three levels; gas chromatography measurements of CH 4, CO, CFCs, SF 6, and N 2 O concentrations at three levels; and weekly flask sampling for measurements of these and other gases. This suite of measurements has been valuable in elucidating the terrestrial ecosystem and boundary layer mixing processes affecting the atmospheric distribution of CO 2 and other species, and has encouraged the initiation of numerous collaborative studies at this site. We deployed the O 2 system described above at the WLEF site in June 2000 and operated it semicontinuously through December Our equipment was located inside the NOAA/CMDL instrument trailer at the site. Excluding the cryogenic chiller and reference gases, the O 2 equipment fit inside a 4-ft-tall standard instrument rack. We sampled air from intake lines at three heights 396, 122, and 30 m with a second dedicated sample line at 30 m. Figure 5 shows O 2 and CO 2 data from these four sample lines during a 1-week period in June During summer, CO 2 increases while O 2 decreases at night in response to net respiration, with the largest changes at the lowest levels. Conversely, CO 2 decreases while O 2 increases with the onset of photosynthesis and vertical mixing in the morning. The amplitude of this diurnal cycle varies with height, location, and weather, but at 30 m on a relatively calm summer day at WLEF it is typically 60 ppm in CO 2 and 300 per meg in O 2. The O 2 :CO 2 relationships are strongly influenced by local photosynthesis and respiration, and the statistical errors on linear fits (Fig. 5b) to the data are very small. During the period shown in Fig. 5, and throughout the four summers we sampled, the observed oxidation ratios ( O 2 :CO 2 ) were lower than soil incubation studies would suggest (Severinghaus 1995). Caution must be applied in interpreting O 2 :CO 2 slopes because it is possible for multiple processes operating at slightly different ratios, such as photosynthesis and respiration, to
11 92 J O U R N A L O F A T M O S P H E R I C A N D O C E A N I C T E C H N O L O G Y VOLUME 24 FIG. 6. Same as Fig. 5, but for a period in December The y axis in (a) and both axes in (b) have been expanded by a factor of 2. result in net atmospheric changes with very different relationships than either of the individual fluxes (Seibt et al. 2004). However, when one process dominates the observed gradients, as is the case for the influence of respiration on diurnal cycles at low levels and on predawn vertical gradients, it is possible to make meaningful links between observed ratios and fluxes. For example, the low-oxidation ratios we observe in the diurnal cycles at 30 m may reflect a reliance on ammonium rather than nitrate as a nitrogen substrate for the trees, such that net oxidation of nitrogen is not occurring in the soils (Bloom et al. 1989, 1992). Finally, it is important to point out that O 2 :CO 2 ratios determined from diurnal atmospheric variations cannot by themselves define the most appropriate biospheric O 2 :CO 2 value to use in the global partitioning of carbon sources (Houghton et al. 2001). This value must represent the O 2 :CO 2 ratio of the net change in the terrestrial carbon pool, and depends on whether this change is primarily in leaves, trunk wood, roots, soil carbon, or elsewhere. However, measurements such as these do provide valuable information on the potential variability of this value and its controlling influences. While synoptic variations in O 2 owing to latitudinal variations in airmass origin or industrial emission signals can be detected year-round, in summer they are generally too small relative to the diurnal variations to have much influence on the observed O 2 :CO 2 ratios. Figure 6 shows O 2 and CO 2 data from a week-long period in December In winter the diurnal cycle is not apparent, but the influence of synoptic pollution events can clearly be seen. During the large pollution event on 16 December, CO 2 increased by around 15 ppm and O 2 decreased by around 105 per meg. Backtrajectory analysis (not shown) indicates that this air had passed over or near both Green Bay, Wisconsin, and Chicago, Illinois. Two earlier events on 13 and 15 December, in which the lower two levels saw stronger pollution signals than the upper level, are suggestive of more local sources. Back trajectories on these days indicate westerly flows that may have been influenced by the small town of Park Falls, Wisconsin. The observed O 2 :CO 2 ratio (Fig. 6b) averaged over these three events of 1.46 is very close to that expected from the average mix of fossil fuel emissions in the United States in 2000 of 1.45 (Keeling 1988; Marland et al. 2005). With further work, atmospheric O 2 measurements may provide a tool for verifying fuel consumption mixtures reported for various countries or regions. Our data also reveal a seasonal cycle with O 2 lower and CO 2 higher during winter. Because atmospheric O 2 is strongly influenced by oceanic exchange, we can compare these seasonal cycles observed at WLEF to those observed at upwind marine boundary layer sites to investigate atmospheric transport and mixing over the continents. The seasonal amplitude in atmospheric O 2 is less at WLEF than at Cold Bay, Alaska (Keeling et al. 1998b), as expected from dilution of the oceanic O 2 signal through vertical mixing over the continent. This type of comparison is particularly sensitive to any systematic biases in the O 2 measurements, but preliminary analyses suggest that our measurements will be useful in testing atmospheric transport models. Information on the vertical gradients of O 2 over the oceans and continents would of course be useful in this context, but because the seasonal O 2 cycles at marine surface sites are already relatively well predicted by coupled ocean atmosphere models (Keeling et al. 1998b; Stephens et al. 1998) this information is not necessary for first-order tests.
12 JANUARY 2007 S T E P HENS ET AL Conclusions We have described a new technique for making ppmlevel atmospheric O 2 measurements based on the adaptation of a commercially available fuel-cell analyzer. This analyzer is well suited for unattended field-based O 2 observations because of its small size, low cost, motion insensitivity, ease of adaptation, robust operation, precision, and response time. After implementing the modifications for rapid calibrations and active pressure and flow control as described here, our analysis system achieves a precision of 1.4 per meg for a 6-minaveraged measurement made every 20 min. This high level of performance enables a wide range of new biogeochemical investigations and monitoring efforts. We have used the analysis system described here to measure atmospheric O 2 and CO 2 over a period of three-and-a-half years at the WLEF tall-tower research site in northern Wisconsin, and have presented several weeks of data here. The measurements define oxidative ratios for terrestrial respiration that have a bearing on plant physiology and on the application of O 2 data to constrain the global carbon cycle. By detecting the O 2 :CO 2 ratios in pollution events from U.S. cities, we can calculate corresponding mixes of fuel types and explore potential methods for future emission verification. Finally, these data provide information on aspects of continental atmospheric mixing important in atmospheric transport modeling and inverse calculations. While collecting one of the first extended records of in situ atmospheric O 2 measurements, we have encountered and characterized a number of potential sources of bias in such measurements. These include fractionation of O 2 with respect to N 2 in reference cylinders, at tubing tee junctions, and at sample inlets. The fuel-cell system has now been returned to our laboratory for refurbishment while we consider various redeployment options. In the future, we will minimize or eliminate known sources of bias by storing reference cylinders horizontally and in an insulated enclosure, by avoiding the use of tees, and by using high flow rates and air inlets designed to reduce thermal gradients. We note that our estimate of 10 per meg comparability after screening or correcting for known biases could be improved and better quantified with these modifications, and conversely that in situ O 2 observations without careful attention to gas-handling and robust performance diagnostics may be subject to even larger measurement biases. A number of fuel-cell O 2 analyzers have recently or will soon be put into service by other investigators at continental and marine boundary layer sites around the world, ensuring significant future contributions from this approach. Acknowledgments. We are indebted to Ralph Keeling, Bill Paplawsky, Adam Cox, and Laura Katz at the Scripps Institution of Oceanography for preparing and analyzing our reference cylinders. We thank Tara Schaefer, Frank Lenning, and staff at the Forest Sciences Laboratory in Rhinelander, Wisconsin, for assistance with field servicing and maintenance. The initial purchase of equipment and instrument deployment were supported by a Cooperative Institute for Research in the Environmental Sciences Innovative Research Program grant and later ongoing support was provided by the National Center for Atmospheric Research Atmospheric Technology Division. Reviews by Patrick Sturm and two anonymous reviewers were very helpful in improving this paper. REFERENCES Bakwin, P. S., P. P. Tans, D. F. Hurst, and C. Zhao, 1998: Measurements of carbon dioxide on very tall towers: Results of the NOAA/CMDL program. Tellus, 50B, Battle, M., M. L. Bender, P. P. Tans, J. W. C. White, J. T. Ellis, T. Conway, and R. J. Francey, 2000: Global carbon sinks and their variability inferred from atmospheric O 2 and 13 C. Science, 287, , and Coauthors, 2006: Atmospheric potential oxygen: New observations and their implications for some atmospheric and oceanic models. Global Biogeochem. Cycles, 20, GB1010, doi: /2005gb Bender, M. L., P. P. Tans, J. T. Ellis, J. Orchardo, and K. Habfast, 1994: A high precision isotope ratio mass spectrometry method for measuring the O 2 /N 2 ratio of air. Geochim. Cosmochim. Acta, 58, , J. T. Ellis, P. P. Tans, R. Francey, and D. Lowe, 1996: Variability in the O 2 /N 2 ratio of southern hemisphere air : Implications for the carbon cycle. Global Biogeochem. Cycles, 10, Blaine, T. W., R. F. Keeling, and W. J. Paplawsky, 2005: An improved inlet for precisely measuring the atmospheric Ar/N 2 ratio. Atmos. Chem. Phys. Discuss., 5, Bloom, A. J., R. M. Caldwell, J. Finazzo, R. L. Warner, and J. Weissbart, 1989: Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol., 91, , S. S. Sukrapanna, and R. L. Warner, 1992: Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol., 99, Davis, K. J., P. S. Bakwin, C. Yi, B. W. Berger, C. Zhao, R. M. Teclaw, and J. G. Isebrands, 2003: The annual cycles of CO 2 and H 2 O exchange over a northern mixed forest as observed from a very tall tower. Global Change Biol., 9, Denning, A. S., I. Y. Fung, and D. Randall, 1995: Latitudinal gradient of atmospheric CO 2 due to seasonal exchange with land biota. Nature, 376, Desai, A. R., and Coauthors, 2006: Influence of vegetation and surface forcing on carbon dioxide fluxes across the Upper Midwest, USA: Implications for regional scaling. Agric. For. Meteor., in press. Gurney, K. R., and Coauthors, 2002: Towards robust regional es-
Continuous measurements of atmospheric oxygen and carbon dioxide on a North Sea gas platform Ingrid Luijkx, R.E.M. Neubert, S. van der Laan, H.A.J.
 Continuous measurements of atmospheric oxygen and carbon dioxide on a North Sea gas platform Ingrid Luijkx, R.E.M. Neubert, S. van der Laan, H.A.J. Meijer Centre for Isotope Research, University of Groningen,
Continuous measurements of atmospheric oxygen and carbon dioxide on a North Sea gas platform Ingrid Luijkx, R.E.M. Neubert, S. van der Laan, H.A.J. Meijer Centre for Isotope Research, University of Groningen,
Carbon Dioxide in Surface Seawaters of the Seto Inland Sea, Japan
 Journal of Oceanography Vol. 49, pp. 295 to 303. 1993 Carbon Dioxide in Surface Seawaters of the Seto Inland Sea, Japan EIJI OHTAKI 1, EIJI YAMASHITA 2 and FUKUICHI FUJIWARA 3 1 College of Liberal Arts
Journal of Oceanography Vol. 49, pp. 295 to 303. 1993 Carbon Dioxide in Surface Seawaters of the Seto Inland Sea, Japan EIJI OHTAKI 1, EIJI YAMASHITA 2 and FUKUICHI FUJIWARA 3 1 College of Liberal Arts
On the public release of carbon dioxide flux estimates based on the observational data by the Greenhouse gases Observing SATellite IBUKI (GOSAT)
 On the public release of carbon dioxide flux estimates based on the observational data by the Greenhouse gases Observing SATellite IBUKI (GOSAT) National Institute for Environmental Studies (NIES) Ministry
On the public release of carbon dioxide flux estimates based on the observational data by the Greenhouse gases Observing SATellite IBUKI (GOSAT) National Institute for Environmental Studies (NIES) Ministry
Estimation of CO 2 and CH 4 fluxes in Siberia using tower observation network (Abstract of the Interim Report)
 2 - i Global Environment Research Account for National Institutes Estimation of CO 2 and CH 4 fluxes in Siberia using tower observation network (Abstract of the Interim Report) Contact person MACHIDA,
2 - i Global Environment Research Account for National Institutes Estimation of CO 2 and CH 4 fluxes in Siberia using tower observation network (Abstract of the Interim Report) Contact person MACHIDA,
Chapter Two. Atmospheric Observations. 2.1 Overview
 ALarge-ScaleCO 2 Observing Plan 21 Chapter Two Atmospheric Observations 2.1 Overview C.D. Keeling of the Scripps Institution of Oceanography was the first to make regular measurements of atmospheric CO
ALarge-ScaleCO 2 Observing Plan 21 Chapter Two Atmospheric Observations 2.1 Overview C.D. Keeling of the Scripps Institution of Oceanography was the first to make regular measurements of atmospheric CO
2.4 CO 2 storage change
 Practical Handbook of Tower Flux Observation (Ver. 1.0) Chapter 2 Net Ecosystem CO 2 Exchange (NEE) is generally expressed as the sum of the CO 2 flux observed over the vegetation surface and the observed
Practical Handbook of Tower Flux Observation (Ver. 1.0) Chapter 2 Net Ecosystem CO 2 Exchange (NEE) is generally expressed as the sum of the CO 2 flux observed over the vegetation surface and the observed
Summer Campaign 2007 San Miguel Basin, Sonora. Luis Mendez-Barroso Soni Yatheendradas Whitney Lauren DeFoor Alexis Martinez
 Summer Campaign 2007 San Miguel Basin, Sonora Luis Mendez-Barroso Soni Yatheendradas Whitney Lauren DeFoor Alexis Martinez San Miguel Basin (map) Activities and experiments during Summer campaign 2007
Summer Campaign 2007 San Miguel Basin, Sonora Luis Mendez-Barroso Soni Yatheendradas Whitney Lauren DeFoor Alexis Martinez San Miguel Basin (map) Activities and experiments during Summer campaign 2007
Figure 1. Location of research sites in the Ameriflux network (from Ameriflux web site,
 CONTEXT - AMERIFLUX NETWORK Figure 1. Location of research sites in the Ameriflux network (from Ameriflux web site, http://public.ornl.gov/ameriflux/). AMERIFLUX OBJECTIVES: Quantify spatial and temporal
CONTEXT - AMERIFLUX NETWORK Figure 1. Location of research sites in the Ameriflux network (from Ameriflux web site, http://public.ornl.gov/ameriflux/). AMERIFLUX OBJECTIVES: Quantify spatial and temporal
Global Warming Science Solar Radiation
 SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
Science Questions. ) in the carbon cycle and for tracers used to diagnose the carbon cycle (SF 6
 HIAPER Pole-to to-pole Observations (HIPPO) of Carbon Cycle and Greenhouse Gases Will measure cross sections from the surface to the tropopause, at 5 times of year in a 3-year period, for a comprehensive
HIAPER Pole-to to-pole Observations (HIPPO) of Carbon Cycle and Greenhouse Gases Will measure cross sections from the surface to the tropopause, at 5 times of year in a 3-year period, for a comprehensive
Measurements of Carbon Dioxide in the Seto Inland Sea of Japan
 Journal of Oceanography Vol. 49, pp. 559 to 569. 1993 Measurements of Carbon Dioxide in the Seto Inland Sea of Japan EIJI YAMASHITA 1, FUKUICHI FUJIWARA 2, XIAOHU LIU 3 * and EIJI OHTAKI 4 1 Environmental
Journal of Oceanography Vol. 49, pp. 559 to 569. 1993 Measurements of Carbon Dioxide in the Seto Inland Sea of Japan EIJI YAMASHITA 1, FUKUICHI FUJIWARA 2, XIAOHU LIU 3 * and EIJI OHTAKI 4 1 Environmental
INFLUX (The Indianapolis Flux Experiment)
 INFLUX (The Indianapolis Flux Experiment) A top-down/bottom-up greenhouse gas quantification experiment in the city of Indianapolis Paul Shepson, Purdue University Kenneth Davis, Natasha Miles and Scott
INFLUX (The Indianapolis Flux Experiment) A top-down/bottom-up greenhouse gas quantification experiment in the city of Indianapolis Paul Shepson, Purdue University Kenneth Davis, Natasha Miles and Scott
Proceedings of the 43 rd Annual ISA Analysis Division Symposium
 Proceedings of the 43 rd Annual ISA Analysis Division Symposium Volume 31 Presented at: Sheraton Imperial Hotel research Triangle Park, NC April 26-29, 1998 Sponsored by ISA and ISA ANALYSIS DIVISION ISDN:
Proceedings of the 43 rd Annual ISA Analysis Division Symposium Volume 31 Presented at: Sheraton Imperial Hotel research Triangle Park, NC April 26-29, 1998 Sponsored by ISA and ISA ANALYSIS DIVISION ISDN:
CO and H 2 uptake and emission by soil
 CO and H 2 uptake and emission by soil variability of fluxes from long term soil chamber measurements M. E. Popa, M. Bolder, C. van der Veen, H. Snellen, T. Röckmann IMAU, Utrecht University, the Netherlands
CO and H 2 uptake and emission by soil variability of fluxes from long term soil chamber measurements M. E. Popa, M. Bolder, C. van der Veen, H. Snellen, T. Röckmann IMAU, Utrecht University, the Netherlands
NORTH AMERICAN CARBON PROGRAM. Continental carbon budgets, dynamics, processes, and management. Program Overview and Progress
 NORTH AMERICAN CARBON PROGRAM Continental carbon budgets, dynamics, processes, and management. Program Overview and Progress NACP Questions 1. What is the carbon balance of North America and adjacent oceans?
NORTH AMERICAN CARBON PROGRAM Continental carbon budgets, dynamics, processes, and management. Program Overview and Progress NACP Questions 1. What is the carbon balance of North America and adjacent oceans?
The amount of fixed nitrogen (N that has chemically combined with other
 Chapter 1: Introduction The cycle of N is unique in that it consists of a massive, well-mixed, and (to most organisms) wholly unavailable pool of nitrogen gas (N 2 ) in the atmosphere; a relatively small
Chapter 1: Introduction The cycle of N is unique in that it consists of a massive, well-mixed, and (to most organisms) wholly unavailable pool of nitrogen gas (N 2 ) in the atmosphere; a relatively small
ACE - The next-generation automatic long term unattended Soil CO Flux system.
 ACE - The next-generation automatic long term unattended Soil CO Flux system. World Class Design and Precision 40+ Years experience measuring CO U.S. warranty and service center Each station has its own
ACE - The next-generation automatic long term unattended Soil CO Flux system. World Class Design and Precision 40+ Years experience measuring CO U.S. warranty and service center Each station has its own
This presentation is on the value of reducing emissions and enhancing removals of greenhouse gases related to land use and land cover change in
 This presentation is on the value of reducing emissions and enhancing removals of greenhouse gases related to land use and land cover change in tropical wetland forests. 1 The objective of this presentation
This presentation is on the value of reducing emissions and enhancing removals of greenhouse gases related to land use and land cover change in tropical wetland forests. 1 The objective of this presentation
EC150, IRGASON, or EC155: Which CO 2 and H 2 O Eddy-Covariance System Is Best for My Application?
 WHITE PAPER EC150, IRGASON, or EC155: Which CO 2 and H 2 O Eddy-Covariance System Is Best for My Application? Introduction The eddy-covariance (EC) technique is widely used to quantify the exchange of
WHITE PAPER EC150, IRGASON, or EC155: Which CO 2 and H 2 O Eddy-Covariance System Is Best for My Application? Introduction The eddy-covariance (EC) technique is widely used to quantify the exchange of
Green-House-Gas budget of constructed wetlands: Understanding the sources to maximize benefits Principal Investigator: Gil Bohrer
 Green-House-Gas budget of constructed wetlands: Understanding the sources to maximize benefits Principal Investigator: Gil Bohrer Abstract Fixed nitrogen (N) is required for the growth for all biological
Green-House-Gas budget of constructed wetlands: Understanding the sources to maximize benefits Principal Investigator: Gil Bohrer Abstract Fixed nitrogen (N) is required for the growth for all biological
Many players have contributed to this John Miller, Arlyn Andrews, Pieter Tans, Oksansa Tarasova, and a host of partners.
 Many players have contributed to this John Miller, Arlyn Andrews, Pieter Tans, Oksansa Tarasova, and a host of partners. Whatever measurements are made supporting urban systems must be compatible with
Many players have contributed to this John Miller, Arlyn Andrews, Pieter Tans, Oksansa Tarasova, and a host of partners. Whatever measurements are made supporting urban systems must be compatible with
Environmental Science. Physics and Applications
 Environmental Science 1 Environmental Science. Physics and Applications. Carbon Cycle Picture from the IPCC report on the environment. 4. Carbon cycle 4.1 Carbon cycle, introduction 4.2 The oceans 4.3
Environmental Science 1 Environmental Science. Physics and Applications. Carbon Cycle Picture from the IPCC report on the environment. 4. Carbon cycle 4.1 Carbon cycle, introduction 4.2 The oceans 4.3
Science Mission Directorate Carbon Cycle & Ecosystems Roadmap NACP
 Science Mission Directorate Carbon Cycle & Ecosystems Roadmap NACP Bill Emanuel Program Scientist, Terrestrial Ecology Carbon Cycle & Ecosystems Focus Area Carbon Cycle & Ecosystems Focus Area Program
Science Mission Directorate Carbon Cycle & Ecosystems Roadmap NACP Bill Emanuel Program Scientist, Terrestrial Ecology Carbon Cycle & Ecosystems Focus Area Carbon Cycle & Ecosystems Focus Area Program
Conclusions and future directions
 Chapter 4 Conclusions and future directions 4.1 Summary of this work 4.1.1 Canopy-scale model A canopy model combining biochemical and inverse Lagrangian approaches has been presented. Source distributions
Chapter 4 Conclusions and future directions 4.1 Summary of this work 4.1.1 Canopy-scale model A canopy model combining biochemical and inverse Lagrangian approaches has been presented. Source distributions
The role of carbon dioxide in climate forcing from 1979 to 2004: introduction of the Annual Greenhouse Gas Index
 Tellus (2006), 58B, 614 619 Copyright C Blackwell Munksgaard, 2006 Printed in Singapore. All rights reserved TELLUS The role of carbon dioxide in climate forcing from 1979 to 2004: introduction of the
Tellus (2006), 58B, 614 619 Copyright C Blackwell Munksgaard, 2006 Printed in Singapore. All rights reserved TELLUS The role of carbon dioxide in climate forcing from 1979 to 2004: introduction of the
Collection of Samples for Analysis of Volatile Organic Compounds (VOCs) in Indoor Air Using Sorbent Tubes. BAA Guide October 1, 2007
 BERKELEY ANALYTICAL ASSOCIATES 815 Harbour Way South, Unit 6 Richmond, CA 94804-3612 Tel: 510-236-2325 Fax: 510-236-2335 Email: baalab@berkeleyanalytical.com www.berkeleyanalytical.com Collection of Samples
BERKELEY ANALYTICAL ASSOCIATES 815 Harbour Way South, Unit 6 Richmond, CA 94804-3612 Tel: 510-236-2325 Fax: 510-236-2335 Email: baalab@berkeleyanalytical.com www.berkeleyanalytical.com Collection of Samples
An emerging challenge for WMO
 WMO Congress Side Event 30 May 2011 at 13.30 to 14.30 Salle 6 International Conference Centre of Geneva (CICG) An emerging challenge for WMO: Supporting Greenhouse Gas Management Strategies with Observations,
WMO Congress Side Event 30 May 2011 at 13.30 to 14.30 Salle 6 International Conference Centre of Geneva (CICG) An emerging challenge for WMO: Supporting Greenhouse Gas Management Strategies with Observations,
ACE - The next-generation automatic long term unattended Soil CO Flux system.
 Presents - ACE - The next-generation automatic long term unattended Soil CO Flux system. World Class Precision 40+ Years experience measuring CO U.S. warranty and service center Each station has its own
Presents - ACE - The next-generation automatic long term unattended Soil CO Flux system. World Class Precision 40+ Years experience measuring CO U.S. warranty and service center Each station has its own
Technical Guidance Note (Monitoring) Medium Combustion Plant Directive and Generator Controls: monitoring point source emissions
 Technical Guidance Note (Monitoring) M5 Medium Combustion Plant Directive and Generator Controls: monitoring point source emissions Environment Agency XXXX 2018 Version X Draft for consultation Foreword
Technical Guidance Note (Monitoring) M5 Medium Combustion Plant Directive and Generator Controls: monitoring point source emissions Environment Agency XXXX 2018 Version X Draft for consultation Foreword
Tananyag fejlesztés idegen nyelven
 Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Calculation of greenhouse effect. The carbon cycle Lecture 11
Tananyag fejlesztés idegen nyelven Prevention of the atmosphere KÖRNYEZETGAZDÁLKODÁSI AGRÁRMÉRNÖKI MSC (MSc IN AGRO-ENVIRONMENTAL STUDIES) Calculation of greenhouse effect. The carbon cycle Lecture 11
Radiative forcing of climate change
 Radiative forcing of climate change Joanna D. Haigh Imperial College of Science, Technology and Medicine, London Radiative forcing concept, definition and applications On a global and annual average, and
Radiative forcing of climate change Joanna D. Haigh Imperial College of Science, Technology and Medicine, London Radiative forcing concept, definition and applications On a global and annual average, and
Figure 1 - Global Temperatures - A plot from the EarthScience Centre at
 GLOBAL WARMING Global warming is evidenced by a steady rise in average global temperatures, changing climate, the fact that snow cover has decreased 10% over the past half-century and that glaciers have
GLOBAL WARMING Global warming is evidenced by a steady rise in average global temperatures, changing climate, the fact that snow cover has decreased 10% over the past half-century and that glaciers have
(1) 4.1 DIURNAL CO 2 PROFILES FROM A ZEA MAYS L. CANOPY
 4.1 DIURNAL CO 2 PROFILES FROM A ZEA MAYS L. CANOPY Steven E. Hollinger*, Carl J. Bernacchi Illinois State Water Survey, Champaign, Illinois, and Tilden P. Meyers NOAA Atmospheric Turbulence Diffusion
4.1 DIURNAL CO 2 PROFILES FROM A ZEA MAYS L. CANOPY Steven E. Hollinger*, Carl J. Bernacchi Illinois State Water Survey, Champaign, Illinois, and Tilden P. Meyers NOAA Atmospheric Turbulence Diffusion
Solar Flat Plate Thermal Collector
 Solar Flat Plate Thermal Collector 1 OBJECTIVE: Performance Study of Solar Flat Plate Thermal Collector Operation with Variation in Mass Flow Rate and Level of Radiation INTRODUCTION: Solar water heater
Solar Flat Plate Thermal Collector 1 OBJECTIVE: Performance Study of Solar Flat Plate Thermal Collector Operation with Variation in Mass Flow Rate and Level of Radiation INTRODUCTION: Solar water heater
Introduction to Climate Science
 Introduction to Climate Science Vegetation and the Carbon Cycle Mike Unsworth Atmospheric Science Outline: Global carbon budget : role of vegetation How does weather and climate affect vegetation? How
Introduction to Climate Science Vegetation and the Carbon Cycle Mike Unsworth Atmospheric Science Outline: Global carbon budget : role of vegetation How does weather and climate affect vegetation? How
Chapter 19 Global Change. Wednesday, April 18, 18
 Chapter 19 Global Change Module 62 Global Climate Change and the Greenhouse Effect After reading this module you should be able to distinguish among global change, global climate change, and global warming.
Chapter 19 Global Change Module 62 Global Climate Change and the Greenhouse Effect After reading this module you should be able to distinguish among global change, global climate change, and global warming.
Stable and accurate measurements to quantify the causes of global climate change
 Stable and accurate measurements to quantify the causes of global climate change James Butler, Brad Hall, Ken Masarie, et al. Global Monitoring Division NOAA Earth System Research Laboratory Boulder, CO,
Stable and accurate measurements to quantify the causes of global climate change James Butler, Brad Hall, Ken Masarie, et al. Global Monitoring Division NOAA Earth System Research Laboratory Boulder, CO,
High School Climate Science Curriculum Course learning goals. October 2011
 1 High School Climate Science Curriculum Course learning goals October 2011 Current Climate 1. Earth climate is determined by a balance between absorbed sunlight and emitted infrared radiation. Because
1 High School Climate Science Curriculum Course learning goals October 2011 Current Climate 1. Earth climate is determined by a balance between absorbed sunlight and emitted infrared radiation. Because
On global estimates of monthly methane flux based on observational data acquired by the Greenhouse gases Observing SATellite IBUKI (GOSAT)
 On global estimates of monthly methane flux based on observational data acquired by the Greenhouse gases Observing SATellite IBUKI (GOSAT) March 27, 2014 National Institute for Environmental Studies Ministry
On global estimates of monthly methane flux based on observational data acquired by the Greenhouse gases Observing SATellite IBUKI (GOSAT) March 27, 2014 National Institute for Environmental Studies Ministry
Basin-scale aircraft measurements of oil and gas methane emissions
 Basin-scale aircraft measurements of oil and gas methane emissions Stefan Schwietzke Cooperative Institute for Research in Environmental Sciences (CIRES), University of Colorado, Boulder Earth System Research
Basin-scale aircraft measurements of oil and gas methane emissions Stefan Schwietzke Cooperative Institute for Research in Environmental Sciences (CIRES), University of Colorado, Boulder Earth System Research
OCO-3 Science and Status for CEOS
 OCO-3 Science and Status for CEOS John Worden presenting for Annmarie Eldering and the OCO-3 Team, October 2016 Copyright 2016. U.S. Government sponsorship acknowledged. Comparison of OCO-2 and OCO-3 Measurements
OCO-3 Science and Status for CEOS John Worden presenting for Annmarie Eldering and the OCO-3 Team, October 2016 Copyright 2016. U.S. Government sponsorship acknowledged. Comparison of OCO-2 and OCO-3 Measurements
The Ocean Carbon Cycle. Doug Wallace Dalhousie University, Halifax, Canada
 The Ocean Carbon Cycle Doug Wallace Dalhousie University, Halifax, Canada KISS Satellite to Seafloor Workshop, Pasadena, 6 October, 2013 Premise: There are Two Flavours (or Colours) of Carbon Natural carbon
The Ocean Carbon Cycle Doug Wallace Dalhousie University, Halifax, Canada KISS Satellite to Seafloor Workshop, Pasadena, 6 October, 2013 Premise: There are Two Flavours (or Colours) of Carbon Natural carbon
5.10 CLUSTER ANALYSIS OF METEOROLOGICAL STATES TO UNDERSTAND THE WEEKDAY-WEEKEND OZONE RESPONSE IN THE SAN FRANCISCO, CA BAY AREA
 5.10 CLUSTER ANALYSIS OF METEOROLOGICAL STATES TO UNDERSTAND THE WEEKDAY-WEEKEND OZONE RESPONSE IN THE SAN FRANCISCO, CA BAY AREA Scott Beaver and Ahmet Palazoglu University of California, Davis, California
5.10 CLUSTER ANALYSIS OF METEOROLOGICAL STATES TO UNDERSTAND THE WEEKDAY-WEEKEND OZONE RESPONSE IN THE SAN FRANCISCO, CA BAY AREA Scott Beaver and Ahmet Palazoglu University of California, Davis, California
TARGAS-1. Portable Photosynthesis System. Photosynthesis Soil Respiration Canopy Assimilation Environmental Monitoring
 TARGAS-1 Portable Photosynthesis System Photosynthesis Soil Respiration Canopy Assimilation Environmental Monitoring TARGAS-1 Main Console CO 2 & H 2 O Gas Analysis The TARGAS-1 console is compact, lightweight
TARGAS-1 Portable Photosynthesis System Photosynthesis Soil Respiration Canopy Assimilation Environmental Monitoring TARGAS-1 Main Console CO 2 & H 2 O Gas Analysis The TARGAS-1 console is compact, lightweight
Global Biogeochemical Cycles. Supporting Information for
 Global Biogeochemical Cycles Supporting Information for The annual cycle of gross primary production, net community production and export efficiency across the North Pacific Ocean Hilary I. Palevsky 1a,
Global Biogeochemical Cycles Supporting Information for The annual cycle of gross primary production, net community production and export efficiency across the North Pacific Ocean Hilary I. Palevsky 1a,
Objectives: New Science:
 Edge effects enhance carbon uptake and its vulnerability to climate change in temperate broadleaf forests Reinmann, A.B. and Hutyra, L.R., PNAS 114 (2017) 107-112 DOI: 10.1073/pnas.1612369114 Objectives:
Edge effects enhance carbon uptake and its vulnerability to climate change in temperate broadleaf forests Reinmann, A.B. and Hutyra, L.R., PNAS 114 (2017) 107-112 DOI: 10.1073/pnas.1612369114 Objectives:
In-situ measurements of CO2
 In-situ measurements of CO2 Calibration, error estimates, role of in-situ chemical measurements, quantification of fossil fuel emissions Pieter Tans NOAA Earth System Research Laboratory 3 March 2010 Keck
In-situ measurements of CO2 Calibration, error estimates, role of in-situ chemical measurements, quantification of fossil fuel emissions Pieter Tans NOAA Earth System Research Laboratory 3 March 2010 Keck
EuroFID Total Hydrocarbon Analyzer
 Product information EuroFID Total Hydrocarbon Analyzer Precise Determination of Total Hydrocarbons in Air for Corrosive as well as Condensing Gases Analyzers and Process Instrumentation Proven Analyzer
Product information EuroFID Total Hydrocarbon Analyzer Precise Determination of Total Hydrocarbons in Air for Corrosive as well as Condensing Gases Analyzers and Process Instrumentation Proven Analyzer
Planetary Energy Balance
 Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Energy, Greenhouse Gases and the Carbon Cycle
 Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
N-cycle: biogeochemistry. Biological flows of Nitrogen
 N-cycle: biogeochemistry SWES 410/510 April 4, 2014 I. N cycling A. simplest possible B. Global N budget C. Effects of N-cycling ( the Nitrogen Cascade ) II. Nitrous Oxide (N 2 O) budgets III. Data-Model
N-cycle: biogeochemistry SWES 410/510 April 4, 2014 I. N cycling A. simplest possible B. Global N budget C. Effects of N-cycling ( the Nitrogen Cascade ) II. Nitrous Oxide (N 2 O) budgets III. Data-Model
Boiler Efficiency Testing. To understand the operation of a fire tube boiler To determine the operating efficiency of the boiler
 Boiler Efficiency Testing Objectives: To understand the operation of a fire tube boiler To determine the operating efficiency of the boiler Theory Most industrial processes require heating. This is usually
Boiler Efficiency Testing Objectives: To understand the operation of a fire tube boiler To determine the operating efficiency of the boiler Theory Most industrial processes require heating. This is usually
Quantifying air-surface exchange of elemental mercury vapor using enclosure and micrometeorological methods
 Quantifying air-surface exchange of elemental mercury vapor using enclosure and micrometeorological methods C. Jerry Lin 1,2, W. Zhu 2, J. Sommar 2 & X. Feng 2 1 Lamar University and 2 CAS Institute of
Quantifying air-surface exchange of elemental mercury vapor using enclosure and micrometeorological methods C. Jerry Lin 1,2, W. Zhu 2, J. Sommar 2 & X. Feng 2 1 Lamar University and 2 CAS Institute of
Satellite data show that phytoplankton biomass and growth generally decline as the
 Oceanography Plankton in a warmer world Scott C. Doney Satellite data show that phytoplankton biomass and growth generally decline as the oceans surface waters warm up. Is this trend, seen over the past
Oceanography Plankton in a warmer world Scott C. Doney Satellite data show that phytoplankton biomass and growth generally decline as the oceans surface waters warm up. Is this trend, seen over the past
Battle, Bender et al (2000). Science.
 Fig. 1. The do 2 and CO 2 recordsmeasured(18) in air collectedat six sites (35). Axes have been scaled so that removals of 1 mol each of O 2 and CO 2 result in the same displacement.the secular trend in
Fig. 1. The do 2 and CO 2 recordsmeasured(18) in air collectedat six sites (35). Axes have been scaled so that removals of 1 mol each of O 2 and CO 2 result in the same displacement.the secular trend in
Preliminary Analysis of High Resolution Domestic Load Data
 Preliminary Analysis of High Resolution Domestic Load Data Zhang Ning Daniel S Kirschen December 2010 Equation Chapter 1 Section 1 1 Executive summary This project is a part of the Supergen Flexnet project
Preliminary Analysis of High Resolution Domestic Load Data Zhang Ning Daniel S Kirschen December 2010 Equation Chapter 1 Section 1 1 Executive summary This project is a part of the Supergen Flexnet project
USGCRP/CCSP Strategic Planning Building Block Global Carbon Cycle 1
 USGCRP/CCSP Strategic Planning Building Block Global Carbon Cycle 1 Summary The global carbon cycle is changing rapidly as a result of human actions, altering Earth s climate. Many research priorities
USGCRP/CCSP Strategic Planning Building Block Global Carbon Cycle 1 Summary The global carbon cycle is changing rapidly as a result of human actions, altering Earth s climate. Many research priorities
Digging Deeper SOLAR ENERGY. Forms of Solar Energy
 a) Is the wind speed the same in the morning; the afternoon; the evening? b) Move your anemometer to another location. Is it windier in other places? c) Do trees or buildings block the wind? 7. Back in
a) Is the wind speed the same in the morning; the afternoon; the evening? b) Move your anemometer to another location. Is it windier in other places? c) Do trees or buildings block the wind? 7. Back in
Measurement of Carbon Dioxide Concentration in the Outdoor Environment
 R32 Project: Measurement of Carbon Dioxide Concentration in the Outdoor Environment Woo Ka Ming Physics Department, from Chinese University of Hong Kong, CONTENTS: Introduction 1. Data Analysis: 1.1 Measurements
R32 Project: Measurement of Carbon Dioxide Concentration in the Outdoor Environment Woo Ka Ming Physics Department, from Chinese University of Hong Kong, CONTENTS: Introduction 1. Data Analysis: 1.1 Measurements
Lab 7 Measurement of Ozone
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 7 Measurement of Ozone Purpose of Lab 7: In this lab you will measure the ambient concentration of ozone
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2007 Lab 7 Measurement of Ozone Purpose of Lab 7: In this lab you will measure the ambient concentration of ozone
FGA 300 Panametrics Flue Gas Oxygen Analyzer. GE Sensing. Features. Applications
 Applications An ex situ zirconium oxide analyzer for excess oxygen measurement in dirty, rugged combustion applications such as: Boilers: all fuels and all types, including marine, recover, and utility
Applications An ex situ zirconium oxide analyzer for excess oxygen measurement in dirty, rugged combustion applications such as: Boilers: all fuels and all types, including marine, recover, and utility
PRODUCT INFORMATION. Model 140MX Flowmeter Functional Overview. PI 14-1 Rev: 1 March 2002
 PRODUCT INFORMATION Model 14MX Flowmeter Functional Overview PI 14-1 Rev: 1 March 22 DESCRIPTION The Model 14MX Flowmeter (Figure 1) works on the fluid phenomenon of momentum exchange (see page 6 for details),
PRODUCT INFORMATION Model 14MX Flowmeter Functional Overview PI 14-1 Rev: 1 March 22 DESCRIPTION The Model 14MX Flowmeter (Figure 1) works on the fluid phenomenon of momentum exchange (see page 6 for details),
Ocean Production and CO 2 uptake
 Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Sulfur Tail Gas Thermal Oxidizer Systems By Peter Pickard
 Sulfur Tail Gas Thermal Oxidizer Systems By Peter Pickard Introduction SRU s (Sulfur Recovery Units) are critical pieces of equipment in refineries and gas plants. SRUs remove sulfur compounds from certain
Sulfur Tail Gas Thermal Oxidizer Systems By Peter Pickard Introduction SRU s (Sulfur Recovery Units) are critical pieces of equipment in refineries and gas plants. SRUs remove sulfur compounds from certain
On-Line Gas Analysis In Air Separation Plants
 Application Data Sheet ADS 103-2815A.A01 March, 2008 Application Data On-Line Gas Analysis In Air Separation Plants APPLICATION Emerson provides several Rosemount Analytical gas analyzer technologies to
Application Data Sheet ADS 103-2815A.A01 March, 2008 Application Data On-Line Gas Analysis In Air Separation Plants APPLICATION Emerson provides several Rosemount Analytical gas analyzer technologies to
ATM S 211 Final Examination June 4, 2007
 ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1745 Isotopic ratios of nitrite as source and age tracers for oceanic nitrite Carolyn Buchwald 1 and Karen L. Casciotti 2 1 Massachusetts Institute of Technology/
SUPPLEMENTARY INFORMATION DOI: 10.1038/NGEO1745 Isotopic ratios of nitrite as source and age tracers for oceanic nitrite Carolyn Buchwald 1 and Karen L. Casciotti 2 1 Massachusetts Institute of Technology/
WBEA Standard Operating Procedure
 Page 1 WBEA Standard Operating Procedure SOP Title Procedures for operating continuous R&P TEOM PM 10 and PM 2.5 Analyzers Author Implementation date February 1 st 2013 Revision History Revision # Date
Page 1 WBEA Standard Operating Procedure SOP Title Procedures for operating continuous R&P TEOM PM 10 and PM 2.5 Analyzers Author Implementation date February 1 st 2013 Revision History Revision # Date
The Carbon Cycle: Global to local. Ruth Varner, PhD
 The Carbon Cycle: Global to local Ruth Varner, PhD Atmospheric CO 2 at Mauna Loa Keeling, C.D. and T.P. Whorf. 2004. Atmospheric CO 2 records from sites in the SIO air sampling An Impeccable Record of
The Carbon Cycle: Global to local Ruth Varner, PhD Atmospheric CO 2 at Mauna Loa Keeling, C.D. and T.P. Whorf. 2004. Atmospheric CO 2 records from sites in the SIO air sampling An Impeccable Record of
ACC Users Group 5 th Annual Meeting October 16 th, 2013 Performance Characteristics of a Novel Modular Air-Cooled Condenser
 The Development and Verification of a Novel Modular Air Cooled Condenser for Enhanced Concentrated Solar Power Generation ACC Users Group 5 th Annual Meeting October 16 th, 2013 Performance Characteristics
The Development and Verification of a Novel Modular Air Cooled Condenser for Enhanced Concentrated Solar Power Generation ACC Users Group 5 th Annual Meeting October 16 th, 2013 Performance Characteristics
Human Impact on the Environment: Part I
 Human Impact on the Environment: Part I The late Alan Gregg pointed out that human population growth within the ecosystem was closely analogous to the growth of malignant tumor cells, that man was acting
Human Impact on the Environment: Part I The late Alan Gregg pointed out that human population growth within the ecosystem was closely analogous to the growth of malignant tumor cells, that man was acting
Lecture 28: Radiative Forcing of Climate Change
 Lecture 28: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
Lecture 28: Radiative Forcing of Climate Change 1. Radiative Forcing In an unperturbed state, the net incoming solar radiation at the top of the atmosphere (Sn) must be balanced by the outgoing longwave
Songwut Nirunsin 1, *, and Yottana Khunatorn 1
 The 23 rd Conference of the Mechanical Engineering Network of Thailand November 4 7, 29, Chiang Mai Quantification of Liquid Water Saturation in a Transparent Single-Serpentine Cathode Flow Channels of
The 23 rd Conference of the Mechanical Engineering Network of Thailand November 4 7, 29, Chiang Mai Quantification of Liquid Water Saturation in a Transparent Single-Serpentine Cathode Flow Channels of
Research Question What ecological and other services do coastal wetlands provide?
 Bringing Wetlands to Market Part 1 Introduction Blue, Green, and Bountiful: Wetlands and carbon Estuary Principle Principle 5: Humans, even those living far from the coast, rely on goods and services supplied
Bringing Wetlands to Market Part 1 Introduction Blue, Green, and Bountiful: Wetlands and carbon Estuary Principle Principle 5: Humans, even those living far from the coast, rely on goods and services supplied
Lab 6 Measurement of Ozone
 Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 6 Measurement of Ozone Purpose of Lab 6: In this lab you will measure the ambient concentration of ozone
Georgia Institute of Technology School of Earth and Atmospheric Sciences EAS 4641 Spring 2008 Lab 6 Measurement of Ozone Purpose of Lab 6: In this lab you will measure the ambient concentration of ozone
Model 140MX Flowmeter Functional Overview
 A higher level of performance Model 14MX Flowmeter Functional Overview DESCRIPTION The Model 14MX Flowmeter (Figure 1) works on the fluid phenomenon of momentum exchange (see page 6 for details), providing
A higher level of performance Model 14MX Flowmeter Functional Overview DESCRIPTION The Model 14MX Flowmeter (Figure 1) works on the fluid phenomenon of momentum exchange (see page 6 for details), providing
Use of Stable Oxygen Isotopes in Studies of Forest-Atmospheric CO 2 & H 2 O Exchange. Chun-Ta Lai San Diego State University
 Use of Stable Oxygen Isotopes in Studies of Forest-Atmospheric CO 2 & H 2 O Exchange Chun-Ta Lai San Diego State University June 2008 Atmospheric Composition Change Increasing CO 2 Increasing CH 4 Decreasing
Use of Stable Oxygen Isotopes in Studies of Forest-Atmospheric CO 2 & H 2 O Exchange Chun-Ta Lai San Diego State University June 2008 Atmospheric Composition Change Increasing CO 2 Increasing CH 4 Decreasing
Chapter Six{ TC "Chapter Six" \l 1 } System Simulation
 Chapter Six{ TC "Chapter Six" \l 1 } System Simulation In the previous chapters models of the components of the cooling cycle and of the power plant were introduced. The TRNSYS model of the power plant
Chapter Six{ TC "Chapter Six" \l 1 } System Simulation In the previous chapters models of the components of the cooling cycle and of the power plant were introduced. The TRNSYS model of the power plant
Soil Respiration and Carbon Sequestration of an Oakgrass Savanna in California: Roles of temperature, soil moisture, rain events and photosynthesis
 001-006 Mission Kearney Foundation of Soil Science: Soil Carbon and California's Terrestrial Ecosystems Final Report: 000, 1/1/003-1/31/00 Soil Respiration and Carbon Sequestration of an Oakgrass Savanna
001-006 Mission Kearney Foundation of Soil Science: Soil Carbon and California's Terrestrial Ecosystems Final Report: 000, 1/1/003-1/31/00 Soil Respiration and Carbon Sequestration of an Oakgrass Savanna
Session 14 Unit VI CLIMATIC CHANGE AND GLOBAL WARMING
 Session 14 Unit VI CLIMATIC CHANGE AND GLOBAL WARMING Dr. H.S. Ramesh Professor of Environmental Engineering S.J. College of Engineering, Mysore 570 006 Carbon di-oxide is a natural constituent of atmosphere,
Session 14 Unit VI CLIMATIC CHANGE AND GLOBAL WARMING Dr. H.S. Ramesh Professor of Environmental Engineering S.J. College of Engineering, Mysore 570 006 Carbon di-oxide is a natural constituent of atmosphere,
3.3 Air temperature. Types of instruments
 3.3 Air temperature 3.3 Air temperature Air temperature is measured with a. Thermometers are substantially affected by radiation. For accurate measurement they should be installed within a shelter to avoid
3.3 Air temperature 3.3 Air temperature Air temperature is measured with a. Thermometers are substantially affected by radiation. For accurate measurement they should be installed within a shelter to avoid
Variations of sound from wind turbines during different weather conditions
 Variations of sound from wind turbines during different weather conditions Conny Larsson 1 Olof Öhlund 2 Department of Earth Sciences, Uppsala University, Villavägen 16, SE-752 36 Uppsala, Sweden Long-term
Variations of sound from wind turbines during different weather conditions Conny Larsson 1 Olof Öhlund 2 Department of Earth Sciences, Uppsala University, Villavägen 16, SE-752 36 Uppsala, Sweden Long-term
University of Groningen
 University of Groningen Atmospheric oxygen and carbon dioxide observations from two European coastal stations 2000-2005 Sirignano, C.; Neubert, R.E.M.; Rödenbeck, C.; Meijer, Harro Published in: Atmospheric
University of Groningen Atmospheric oxygen and carbon dioxide observations from two European coastal stations 2000-2005 Sirignano, C.; Neubert, R.E.M.; Rödenbeck, C.; Meijer, Harro Published in: Atmospheric
Extensions of TerrSysMP-CO 2 to resolve dynamic processes induced by tall vegetation
 Extensions of TerrSysMP-CO 2 to resolve dynamic processes induced by tall vegetation Markus Übel and Andreas Bott Meteorological Institute, University of Bonn TerrSysMP-CO 2 model domain General Meeting,
Extensions of TerrSysMP-CO 2 to resolve dynamic processes induced by tall vegetation Markus Übel and Andreas Bott Meteorological Institute, University of Bonn TerrSysMP-CO 2 model domain General Meeting,
In situ methods to measure Primary Production and Net Community Production. What have we learned?
 In situ methods to measure Primary Production and Net Community Production What have we learned? Time Series Sites Time series sites provide: - test bed for new PP methods - evaluation of the annual carbon
In situ methods to measure Primary Production and Net Community Production What have we learned? Time Series Sites Time series sites provide: - test bed for new PP methods - evaluation of the annual carbon
Urban-Dome GHG Monitoring:
 Urban-Dome GHG Monitoring: The INFLUX Project Measurement Challenges and Perspectives J. Whetstone (1), P.B. Shepson (2), K.J. Davis (3), C. Sweeney (4), K.R. Gurney (5), N.L. Miles (3), S. Richardson
Urban-Dome GHG Monitoring: The INFLUX Project Measurement Challenges and Perspectives J. Whetstone (1), P.B. Shepson (2), K.J. Davis (3), C. Sweeney (4), K.R. Gurney (5), N.L. Miles (3), S. Richardson
GLOBAL CLIMATE CHANGE
 1 GLOBAL CLIMATE CHANGE From About Transportation and Climate Change (Source; Volpe center for Climate Change and Environmental forecasting, http://climate.volpe.dot.gov/trans.html Greenhouse effect has
1 GLOBAL CLIMATE CHANGE From About Transportation and Climate Change (Source; Volpe center for Climate Change and Environmental forecasting, http://climate.volpe.dot.gov/trans.html Greenhouse effect has
METHOD 3 - GAS ANALYSIS FOR THE DETERMINATION OF DRY MOLECULAR WEIGHT. NOTE: This method does not include all of the
 312 METHOD 3 - GAS ANALYSIS FOR THE DETERMINATION OF DRY MOLECULAR WEIGHT NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling) essential
312 METHOD 3 - GAS ANALYSIS FOR THE DETERMINATION OF DRY MOLECULAR WEIGHT NOTE: This method does not include all of the specifications (e.g., equipment and supplies) and procedures (e.g., sampling) essential
Chapter 8. Vapor Power Systems
 Chapter 8 Vapor Power Systems Introducing Power Generation To meet our national power needs there are challenges related to Declining economically recoverable supplies of nonrenewable energy resources.
Chapter 8 Vapor Power Systems Introducing Power Generation To meet our national power needs there are challenges related to Declining economically recoverable supplies of nonrenewable energy resources.
Context of the Program Element
 Context of the Program Element Scientific Rationale The need to understand how carbon cycles through the Earth system is critically important to our ability to predict future climate change. Carbon dioxide
Context of the Program Element Scientific Rationale The need to understand how carbon cycles through the Earth system is critically important to our ability to predict future climate change. Carbon dioxide
Atmospheric Chemistry and Physics
 Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Detection of regional scale sea-to-air oxygen emission related to spring bloom
Author(s) 2008. This work is distributed under the Creative Commons Attribution 3.0 License. Atmospheric Chemistry and Physics Detection of regional scale sea-to-air oxygen emission related to spring bloom
METHOD 4 - DETERMINATION OF MOISTURE CONTENT IN STACK GASES. NOTE: This method does not include all the
 347 METHOD 4 - DETERMINATION OF MOISTURE CONTENT IN STACK GASES NOTE: This method does not include all the specifications (e.g., equipment and supplies) and procedures (e.g., sampling) essential to its
347 METHOD 4 - DETERMINATION OF MOISTURE CONTENT IN STACK GASES NOTE: This method does not include all the specifications (e.g., equipment and supplies) and procedures (e.g., sampling) essential to its
Recent Scientific Applications of Underwater Mass Spectrometry
 World-changing solutions to make people safer, healthier, and more productive. Recent Scientific Applications of Underwater Mass Spectrometry R. T. Short 1, S. K. Toler 1, A. M. Cardenas-Valencia 1, R.
World-changing solutions to make people safer, healthier, and more productive. Recent Scientific Applications of Underwater Mass Spectrometry R. T. Short 1, S. K. Toler 1, A. M. Cardenas-Valencia 1, R.
NOTES AND CORRESPONDENCE. Toward a Robust Phenomenological Expression of Evaporation Efficiency for Unsaturated Soil Surfaces
 1330 JOURNAL OF APPLIED METEOROLOGY NOTES AND CORRESPONDENCE Toward a Robust Phenomenological Expression of Evaporation Efficiency for Unsaturated Soil Surfaces TERUHISA S. KOMATSU Department of Environmental
1330 JOURNAL OF APPLIED METEOROLOGY NOTES AND CORRESPONDENCE Toward a Robust Phenomenological Expression of Evaporation Efficiency for Unsaturated Soil Surfaces TERUHISA S. KOMATSU Department of Environmental
RC400G FC400G RESIDUAL CHLORINE ANALYZER RC400G NON-REAGENT FREE CHLORINE ANALYZER FC400G RESIDUAL CHLORINE ANALYZER
 RESIDUAL CHLORINE ANALYZER 400G NON-REAGENT FREE CHLORINE ANALYZER FC400G 400G RESIDUAL CHLORINE ANALYZER FC400G NON-REAGENT FREE CHLORINE ANALYZER Bulletin 12F01A01-01E www.yokogawa.com/an/ For Safe and
RESIDUAL CHLORINE ANALYZER 400G NON-REAGENT FREE CHLORINE ANALYZER FC400G 400G RESIDUAL CHLORINE ANALYZER FC400G NON-REAGENT FREE CHLORINE ANALYZER Bulletin 12F01A01-01E www.yokogawa.com/an/ For Safe and
Title: Standard Operating Procedure for Measurement of CO in Ambient Air by Gas Filter Correlation (GFC)
 Procedure No: SOP-024 Revision No: 0 (new document) Page No.: 1 of 7 1. INTRODUCTION AND SCOPE To obtain timely data for the purpose of air quality assessment, air quality trend reporting, air quality
Procedure No: SOP-024 Revision No: 0 (new document) Page No.: 1 of 7 1. INTRODUCTION AND SCOPE To obtain timely data for the purpose of air quality assessment, air quality trend reporting, air quality
Diffusion-based Soil Respiration Chamber Measurements
 Vaisala CARBOCAP Carbon Dioxide Probe GMP343: Diffusion-based Soil Respiration Chamber Measurements This application note contains general information about soil respiration and discusses some issues worth
Vaisala CARBOCAP Carbon Dioxide Probe GMP343: Diffusion-based Soil Respiration Chamber Measurements This application note contains general information about soil respiration and discusses some issues worth
Two Great Technologies Together At Last. The 700 Series NanoTrace Moisture And Oxygen Analyzers
 Two Great Technologies Together At Last The 700 Series NanoTrace Moisture And Oxygen Analyzers w w w. d e l t a - f. c o m THE DF-750 NANOTRACE MOISTURE ANALYZER: THE DF-760 NANOTRACE DUAL ANALYZER: Redefining
Two Great Technologies Together At Last The 700 Series NanoTrace Moisture And Oxygen Analyzers w w w. d e l t a - f. c o m THE DF-750 NANOTRACE MOISTURE ANALYZER: THE DF-760 NANOTRACE DUAL ANALYZER: Redefining
Title: Standard Operating Procedure for Measurement of Ozone in Ambient Air by Ultraviolet (UV) Photometry
 Procedure No: SOP-022 Revision No: 1.1 January 27, 2011 Page No.: 1 of 8 1. INTRODUCTION AND SCOPE To obtain timely data for the purpose of air quality assessment, air quality trend reporting, air quality
Procedure No: SOP-022 Revision No: 1.1 January 27, 2011 Page No.: 1 of 8 1. INTRODUCTION AND SCOPE To obtain timely data for the purpose of air quality assessment, air quality trend reporting, air quality
Evaluation of efficiency and collector time constant of a solar flat plate collector
 Evaluation of efficiency and collector time constant of a solar flat plate collector Abhijit Devaraj 1, Abhishek Hiremath 2, Akshay R Patil 3, Krushik B N 4 Department of Mechanical Engineering, BMS College
Evaluation of efficiency and collector time constant of a solar flat plate collector Abhijit Devaraj 1, Abhishek Hiremath 2, Akshay R Patil 3, Krushik B N 4 Department of Mechanical Engineering, BMS College
