Totally Integrated Solutions for Pharmaceutical Industry
|
|
|
- Christina Peters
- 6 years ago
- Views:
Transcription
1 Totally Integrated Solutions for Pharmaceutical Industry
2 ABOUT AUSTAR As a global leading solution provider for the pharmaceutical industry, AUSTAR provides pharmaceutical enterprises with comprehensive products and services. Headquartered in Beijing, AUSTAR has opened factories, offices and branch companies in Asia and Europe. AUSTAR has globally constituted a gigantic development and research, marketing and service network. AUSTAR products and solutions have been applied to more than 40 countries and regions in the world. Besides our traditional role as a manufacturer and supplier of technological equipment, AUSTAR supports customer from inception to completion of project. AUSTAR is committed to promoting pharmaceutical industry advancement internationally, and becoming your partner in improving human health. AUSTAR HEADQUARTERS Tel: Fax: info@austarunion.com 01 / ABOUT AUSTAR ABOUT AUSTAR / 02
3 40 COUNTRIES AND REGIONS 24 YEARS OF EXPERIENCE AUSTAR IN THE WORLD 03 / AUSTAR IN THE WORLD AUSTAR IN THE WORLD / 04
4 CONTENTS Design Conceptual Design (CD) Basic Design (BD) Detail Design (DD) Production Line Vial Liquid Filling Line Wet Granulation Line for Oral Solid Dosage Soft Bag Production Line GMP Compliance Services Validation Consultancy Pharmaceutical Quality System Consultancy Drug Technology Transfer Engineering Clean Room System Liquid Process System Pharmaceutical Automation Engineering Package Line Blister Packing Line Bottle Counting Line Consumables Personal Protection Cleaning Sterile Packaging 05 / CONTENTS CONTENTS / 06
5 Drug Technology Transfer Customized drug technology transfer services Design THE DESIGN PROCESS Facility URS Project definition, Basis of Design, Concept Study Conceptual Design (CD) Design basis and conceptual design Large Volume Parenteral Oral Solid Dosage Detailed Design (DD) Planning for construction, preparation of tender documents Basic Design (BD) Layout development, utility definition, detailed requirements for DD APIs Small Volume Injectables AUSTAR ADVANTAGES Standard Professional Flexible Efficient Request for Quotation (RFQ) Others Biological Products Process core is standard Process support functions are adaptable for local conditions Equipment manufactured to standard GMP compliance Quality by design Risk-based approach Highly educated, experienced and trained engineers and consultants Flexibility and adaptability to requirements response within 2 weeks on standard equipment of service Total design (CD/BD/DD) 3 mouths for complex process Project completion within budget, quality and predetermined schedule 07 / DRUG TECHNOLOGY TRANSFER DESIGN / 08
6 CLEAN ROOM SYSTEM Engineering AUSTAR provides complete project management services including designs, manufacturing and installation. Our clean room system combines European technology and customized solutions with guaranteed quality and reliable services. AUSTAR clean room system is flexible and easy to install that accepts changes to design in the field and can be reconfigured for use in manufacturing area. Our clean room system meets the requirement of US FDA cgmp, EU GMP, PIC/S and WHO. Business Scope Access Control Clean Room Design Project Management Clean Room Partition, Doors, Windows PVC/ Epoxy Floor Clean Room Equipment HVAC System 09 / ENGINEERING HAVC Automation Control Electrical System 01 Air Flow 02 Wall Panel 03 Door 04 Air Shower 05 HEPA Box 06 Window 07 Return Air Grille 08 Ceiling Panel 09 Light 10 Pass Box 11 PVC/Epoxy Floor ENGINEERING /10
7 LIQUID PROCESS SYSTEM CIP SKID Preparation Skid WFI Generator Pure Steam Generator Pretreatment PW Generator PW Tank Distribution Skid WFI Tank WFI Distribution Skid AUSTAR provides pharmaceutical liquid process technology, international level of AUSTAR will take experience-gained advantages to provide worldwide pharmaceutical design, project management services, manufacturing, installation and validation, as well enterprises with world-class pharmaceutical water system solutions. AUSTAR water as comprehensive integrated service. system meets US FDA cgmp, EU GMP, WHO and other national requirements. 11 / ENGINEERING ENGINEERING / 12
8 PHARMACEUTICAL AUTOMATION ENGINEERING ONE STOP AUTOMATION SOLUTION INTEGRATOR AUSTAR automation engineering provides integrated process automation lifecycle solutions for pharmaceutical companies. With pharmaceutical industry know-how and excellent abilities of automation application, AUSTAR can provide customized solutions to meet client's requirements. AUSTAR also provides information system such as BMS, EMS, SCADA, MES, warehouse management system and LIMS. 20th More than 20 years experience in pharmaceutical industry 100 More than 100 worldwide clients Process Automation Utility Automation Information System GAMP5 GAMP5 compliant design and Advantages construction procedures 20+ years experience (Focusing on pharmaceutical industry) Highly experienced and qualified engineering team (100+ professional technical experts) World-class system platform (SIEMENS, Rockwell, Emerson) Compliance with FDA, WHO, EMA regulations (System validated to current version of GAMP) Reliable after-sale service (20+ worldwide after-sales service engineers) Process Automation API Process Control System Preparations Process Control System Biopharmaceutical Process Control System Factory Information System Supervisory Control And Data Acquisition (SCADA) Manufacturing Execution System (MES) Laboratory Information Management System (LIMS) Utility Engineering Automation HVAC Control System Environment Monitoring System (EMS) PW / WFI System Light Current System Integration(IT, Access control, telephone, CCTV) 13 / ENGINEERING ENGINEERING / 14
9 VIAL LIQUID FILLING LINE Vial liquid filling line is composed of rotary or linear washing machine, sterilizing & depyrogenation tunnel, filling & stoppering machine, and capping machine. Production Line This production line is integrated in accordance with process requirements. The vial liquid filling line capacities extend to filling volumes of 2ml-500ml. 15 / PRODUCTION LINE PRODUCTION LINE / 16
10 ORAL SOLID DOSAGE SOLUTION AUSTAR offers complete solutions for solid processing plant. With the process of granulation, drying, WET GRANULATION LINE FOR ORAL SOLID DOSAGE tableting, capsule filling, coating, handling, weight checking, inspection and washing. AUSTAR process engineers can provide complete production line to customers' requirements. Bin Blender Drum Movable Drum Lifter Tablet Press Movable Drum Lifter Coating Pan IBC Bins High Shear Mixer Fluid Bed Dryer/Granulator FBD Bowl Pharma Lifting Mill Bin Blender Bin Bin Tablet Press Movable Drum Lifter Coating Pan Pharma Bin Bin Blender Bin Blender Drum Movable Drum Lifter Capsule Filler Automatic Bin Washing Station IBC Bins High Shear Mixer Fluid Bed Dryer/Granulator Vacuum Lifting Mill Bin Blender Bin Bin Capsule Filler Semi-automatic Bin Washing Station HANDLING Dispensing and Transfer System, Bins, Drums, Lifting Columns, Blenders COATING Perforated Coating Pan for Film and Sugar Coating TABLETING Force Feed Tablet Press Machines and Peripheral Machines INSPECTION Semi Automatic and Automatic Inspection Machine for Tablets and Capsules GRANULATION & DRYING WEIGHT CHECKING CAPSULE FILLING WASHING High Shear Mixer, Fluid Machine for 100% Weight Intermittent and Continuous Wash-In-Place Station, Bed Dryer/Processor Checking of Capsules/Tablets Motion Capsule Filling Machine Semi-Automatic and Fully and Peripheral Machine Automatic Bin Washing Station 17 / PRODUCTION LINE PRODUCTION LINE / 18
11 SOFT BAG PRODUCTION LINE Non-PVC Soft Bag Complete Production Line Form - Fill - Seal (FFS) Automatic Conveying System Packaging System Sterilizer Pharmaceutical Non-PVC Soft Bag Production Line A high level of flexibility and reliability is reflected in soft bag production line. Different types of multi-coextruded polypropylene (PP) films and different types of port can be processed. This line can produce up to 5000 bags per hour. The standard bag sizes vary between 50 ml and 3000 ml. Air Knife Dryer Visual Inspection Highlight Capacity: 1000 bags/hr-5000 bags/hr Package material: PP bags Bag size: 50ml-3,000ml Bag tube system: versatile Complete production system Customized Design 19 / PRODUCTION LINE PRODUCTION LINE / 20
12 Package Line BLISTER PACKING LINE Blister Packing Machine Cartoner Machine Bundling Machine Semi-automatic Case Packing Machine Application Application Application Application Blister packing machine can be widelyused for talbets, Cartoner machine is suitable for blisters, ampoules All kinds of Pharmaceutical cartons, or similar boxes. Applicable to packs and various kinds of quadrate capsules, pills, vials and ampoules with PVC feeding, heating, forming, sealing batch numbering and automatically cutting functions. Features Wide range for solid dosage products Processing of all film type: PVC, PVCD, Aluminum Camera Inspection blisters, vials, bottles, syringes, cartridges, and similar packaging. Features Multi-purpose use Continuous processing Cartoning packaging in process quality control Features Film feed protection Safeguard door protection Friendly design & Space saving Servo motor segment accuracy guarantee boxes. Features Widely used in pharmaceuticals & food industry Auto & semi-automatic option Sensors control in key segment Cylinder as execution component Continuous flow process: Forming, feeding, identifying mark, bar coding, slitting/cutting 21 / PACKAGE LINE PACKAGE LINE / 22
13 BOTTLE COUNTING LINE Unscrambler Counter Desiccant Inserter Screw Capper Sealer Labeler Complete Tablet / Capsule Counting Line Solution Maximum speed up to 200bpm CCD scanning sensor with high accurate counting Modularized design sensor for different types of solid Product detecting sensors to avoid incorrect counting Dipping nozzles to increase speed and prevent jams 12 channel can work separately when small count required Pre-count function to automatically count after buffer stop 23 / PACKAGE LINE PACKAGE LINE / 24
14 GMP Compliance Services AUSTAR provides integrated solutions for validation compliance covering the whole lifecycle of a pharmaceutical product and provides complete commissioning and validation services for the successful completion of the project to satisfy the customer, s requirements for such services as consultancy, commissioning, qualification, validation, re-qualification and re-validation at different stages, and help the project to meet the requirements of US FDA, EMA certification or WHO pre-qualification. CONTENT OF INTEGRATED SOLUTION FOR RISK BASED VALIDATION COMPLIANCE Premises,systems,equipment Utilities HVAC system Clean gases Revalidation (Continuous verification) PW and WFI Pure steam API equipment Transportation validation Sterile product equipment Equipment OSD equipment Biological product equipment Blood product equipment Process validation Quality Project Plan (QPP) Validation Master Plan (VMP) Computerized Systems TCM extraction equipment EMS BMS WMS DCS Cleaning validation SCADA Risk Management Plan(QRMP) Analytical instrument QC Lab. Microbial analytical method Physical and chemical analytical method 25 / GMP COMPLIANCE SERVICES GMP COMPLIANCE SERVICES / 26
15 CONTENT OF INTEGRATED SOLUTION FOR Pharmaceutical Quality System AUSTAR is devoted to providing pharmaceutical enterprises with integrated quality system solutions throughout the whole lifecycle of a pharmaceutical product, including quality system establishment, maintenance and training in compliance with EU GMP, WHO GMP, FDA cgmp and ICH Q10 requirements. NEW PQS STRATEGIES (ICH Q8/Q9/Q10) Transfer of personnel training Process Based QM Regulatory Compliance Based On 6 Major Systems documentation in conformity with EU GMP, FDA cgmp and ICH Q10 standards Science Based Approaches Risk Based Approaches 27 / GMP COMPLIANCE SERVICES GMP COMPLIANCE SERVICES / 28
16 Consumables AUSTAR provides high quality products such as cleanroom cleaning tools, personal protection, sterile packaging and other supplies to meet the stringent requirements of the pharmaceutical and life science industries. We offer products suitable for ISO class 5 (Federal class 100) of cleanrooms. The quality-approved products we offer in the market are widely appreciated by our global clients for their immense quality standards such as durability, longer functional life, reliability and their higher performance. Area 1 The Cleanroom Production area: validated and contamination controlled (air, surfaces and personnel) Only cleanroom products allowed. Area 2 The Personnel Entrance/ Gowning room Gowning area reduces the risk of potential source of contamination entering the clean rooms. 1 Area 3 The Controlled Environment 2 Controlled but not classified (CNC) areas include non GMP and packaging areas. These areas may have impact on clean rooms, suitable cleaning regime can prevent up to 80% of contaminants entering the clean room. 3 Personal Protection Clean room Garments Clean room Shoes & Boots Clean room Gloves Clean room Goggles Clean room Face Masks Cleaning Polyester Wiper Non-woven Wiper Microfiber Wiper Cleaning Mop Cleaning System Sterile Packaging Ultraclean PE Bags Cleansteam Bags/Coils Ultraclean Sterile LDPE Freeze-dry Film Bioprocess Vessels(BPV) Powder Transfer Vessels (PTV) 29 / CONSUMABLES CONSUMABLES / 30
17 Offices and Factories AUSTAR Headquaters 18F, Tower B, Chaowai MEN Office Building, No.26 Chaowai Street, Chaoyang District, Beijing, P. R. China Tel: Fax: Beijing Manufacturing Center No.15 Yanqi Road, Yanqi Economic Develpment Zone, Huairou District, Beijing, P. R. China Tel: Fax: Shanghai Manufacturing Center No.799, Yuyang Road, Cangqiao Industry Zone, Songjiang District, Shanghai, P. R. China Tel: Fax: Shijiazhuang Manufacturing Center No.389, Taihang Street, Gaoxin District, Shijiazhuang, Hebei Province, P. R. China Tel: Fax:
Page 1
 Engineers to Healthcare Industry INTERTECH EQUIPTECHNOLOGIES PVT. LTD. Company Presentation 2014 www.intertechequip.com Page 1 INTERTECH Production facility Total Fabrication Area 75,000 ft2 & Clean room
Engineers to Healthcare Industry INTERTECH EQUIPTECHNOLOGIES PVT. LTD. Company Presentation 2014 www.intertechequip.com Page 1 INTERTECH Production facility Total Fabrication Area 75,000 ft2 & Clean room
HISTORY AND MILESTONES
 HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
Conducting Supplier Audits: Ensure Validation Compliance
 Conducting Supplier Audits: Ensure Validation Compliance Presented by: Gamal Amer, Ph. D. Principal Premier Compliance Services, Inc. All rights reserved. Do not copy without permission. 1 Outline Why
Conducting Supplier Audits: Ensure Validation Compliance Presented by: Gamal Amer, Ph. D. Principal Premier Compliance Services, Inc. All rights reserved. Do not copy without permission. 1 Outline Why
ABOUT HAMELN PHARMA About us
 Welcome ABOUT HAMELN PHARMA About us With over 60 years of experience hameln pharma is the specialist for contract manufacturing of sterile solutions and suspensions filled in ampoules and vials. 2 ABOUT
Welcome ABOUT HAMELN PHARMA About us With over 60 years of experience hameln pharma is the specialist for contract manufacturing of sterile solutions and suspensions filled in ampoules and vials. 2 ABOUT
Actavis Italy. Nerviano Plant
 Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
Isolator Technology TRUKING TECHNOLOGY LIMITED
 Isolator TRUKING TECHNOLOGY LIMITED Add No.1 xinkang Road,Yutan Town,Ningxiang,Changsha,China P.C 410600 Tel 86 731-87938222 87938255 Fax 86 731-879338201 Web www.truking.cn Any technical renewal will
Isolator TRUKING TECHNOLOGY LIMITED Add No.1 xinkang Road,Yutan Town,Ningxiang,Changsha,China P.C 410600 Tel 86 731-87938222 87938255 Fax 86 731-879338201 Web www.truking.cn Any technical renewal will
WHO PUBLIC INSPECTION REPORT (WHOPIR) Finished Product Manufacturer. Mylan Nashik ( Sinnar in CRM) AND
 SOP 408.4 Annex D 20, avenue Appia CH-1211 Geneva 27 Switzerland Tel central +41 22 791 2111 Fax central +41 22 791 3111 www.who.int WHO PUBLIC INSPECTION REPORT (WHOPIR) Finished Product Manufacturer
SOP 408.4 Annex D 20, avenue Appia CH-1211 Geneva 27 Switzerland Tel central +41 22 791 2111 Fax central +41 22 791 3111 www.who.int WHO PUBLIC INSPECTION REPORT (WHOPIR) Finished Product Manufacturer
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE
 XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
XTREMA HIGH SPEED FILLING & STOPPERING MACHINE XTREMA HIGH SPEED FILLING & STOPPERING MACHINE FOR TIME HAS COME TO TAKE CONTROL This is the refrain that has driven IMA Life to further innovate. State-of-the-art
BEACONS. Exceeding. Expectations. Pharmaceuticals. Quality Pharmaceutical Manufacturing Services
 BEACONS Pharmaceuticals Exceeding Expectations Quality Pharmaceutical Manufacturing Services BEACONS Pharmaceuticals Quality with Trust, Service with Excellence Founded in 1970, with headquarters in, Beacons
BEACONS Pharmaceuticals Exceeding Expectations Quality Pharmaceutical Manufacturing Services BEACONS Pharmaceuticals Quality with Trust, Service with Excellence Founded in 1970, with headquarters in, Beacons
Polestar Power Industries (Pharma Division) Pioneer Manufacturer Of Beta-Lactum & Cephalosporin
 Polestar Power Industries (Pharma Division) Pioneer Manufacturer Of Beta-Lactum & Cephalosporin R Polestar Power Industries (Pharma Division) Vill. Damuwala, Haripur Road, Barotiwala, Tehsi l Baddi, Distt.
Polestar Power Industries (Pharma Division) Pioneer Manufacturer Of Beta-Lactum & Cephalosporin R Polestar Power Industries (Pharma Division) Vill. Damuwala, Haripur Road, Barotiwala, Tehsi l Baddi, Distt.
Aseptic Powder Dosing Solutions
 Aseptic Powder Dosing Solutions Beyond Technology The new business model from Romaco sets valuable incentives for meeting customer requirements. For the design of innovative solutions, Romaco relies on
Aseptic Powder Dosing Solutions Beyond Technology The new business model from Romaco sets valuable incentives for meeting customer requirements. For the design of innovative solutions, Romaco relies on
STERIS FINN-AQUA Pure Steam Generator for Pharmaceutical Manufacturing
 cgmp MANUFACTURING STERIS FINN-AQUA Pure Steam Generator for Pharmaceutical Manufacturing Life Sciences STERIS Corporation has achieved worldwide leadership in the pharmaceutical and biotech industries
cgmp MANUFACTURING STERIS FINN-AQUA Pure Steam Generator for Pharmaceutical Manufacturing Life Sciences STERIS Corporation has achieved worldwide leadership in the pharmaceutical and biotech industries
An Introduction to Double Dragon Consulting
 An Introduction to Double Dragon Consulting www.doubledragonconsulting.com 1 June 2012 About DDC Our Experience: All dosage forms, aseptic processes, OTC Remediation of 483 observations, warning letters,
An Introduction to Double Dragon Consulting www.doubledragonconsulting.com 1 June 2012 About DDC Our Experience: All dosage forms, aseptic processes, OTC Remediation of 483 observations, warning letters,
Prequalification Team WHO PUBLIC INSPECTION REPORT Vaccine Manufacturer
 Part 1: General information Name of Manufacturer Production Block Physical address Contact address Prequalification Team WHO PUBLIC INSPECTION REPORT Vaccine Manufacturer. Clean Utilities in the Basement.
Part 1: General information Name of Manufacturer Production Block Physical address Contact address Prequalification Team WHO PUBLIC INSPECTION REPORT Vaccine Manufacturer. Clean Utilities in the Basement.
SMART PWD ASEPTIC POWDER FILLING AND STOPPERING MACHINE
 SMART PWD ASEPTIC POWDER FILLING AND STOPPERING MACHINE SMART PWD ASEPTIC POWDER FILLING & STOPPERING MACHINE THANKS TO INTELLIGENT SERVO TECHNOLOGY, THE MACHINE IS EXTREMELY USER-FRIENDLY AND FLEXIBLE
SMART PWD ASEPTIC POWDER FILLING AND STOPPERING MACHINE SMART PWD ASEPTIC POWDER FILLING & STOPPERING MACHINE THANKS TO INTELLIGENT SERVO TECHNOLOGY, THE MACHINE IS EXTREMELY USER-FRIENDLY AND FLEXIBLE
Aseptic Liquid Filling Solutions
 Aseptic Liquid Filling Solutions Beyond Technology The new business model from Romaco sets valuable incentives for meeting customer requirements. For the design of innovative solutions, Romaco relies
Aseptic Liquid Filling Solutions Beyond Technology The new business model from Romaco sets valuable incentives for meeting customer requirements. For the design of innovative solutions, Romaco relies
Second Edition. New York London
 Second Edition Second Edition New York London Published in 2006 by CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 2006 by Taylor & Francis Group, LLC
Second Edition Second Edition New York London Published in 2006 by CRC Press Taylor & Francis Group 6000 Broken Sound Parkway NW, Suite 300 Boca Raton, FL 33487-2742 2006 by Taylor & Francis Group, LLC
PLANKSTADT EXPERTS TAKING CARE
 CordenPharma PLANKSTADT EXPERTS TAKING CARE Our History 1977 - Start of Packaging activities (ICI Pharma) 1980 - Start of Formulation activities (ICI Pharma) 1995 - First Pre-approval Inspection by FDA
CordenPharma PLANKSTADT EXPERTS TAKING CARE Our History 1977 - Start of Packaging activities (ICI Pharma) 1980 - Start of Formulation activities (ICI Pharma) 1995 - First Pre-approval Inspection by FDA
ISPE Aseptic Conference February 2014 Washington, D.C.
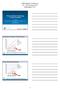 Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Aseptic Process Validation
 Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
GLOBAL QUALITY SOLUTIONS PACKAGE INTEGRITY SEAL INTEGRITY LEAK DETECTION
 GLOBAL QUALITY SOLUTIONS PACKAGE INTEGRITY SEAL INTEGRITY LEAK DETECTION Redefining the standards for accuracy and reliability. About PTI PTI Packaging Technologies & Inspection is headquartered in Tuckahoe,
GLOBAL QUALITY SOLUTIONS PACKAGE INTEGRITY SEAL INTEGRITY LEAK DETECTION Redefining the standards for accuracy and reliability. About PTI PTI Packaging Technologies & Inspection is headquartered in Tuckahoe,
TUNING UP YOUR PRODUCTION. A Member of Kiefel
 TUNING UP YOUR PRODUCTION A Member of Kiefel Tuning up your production Long-lasting experience with complex projects has turned us into one of the leading systems providers of turnkey projects using thermoforming,
TUNING UP YOUR PRODUCTION A Member of Kiefel Tuning up your production Long-lasting experience with complex projects has turned us into one of the leading systems providers of turnkey projects using thermoforming,
Aseptic vial filling line
 Pharma Forum - Schwäbisch Hall September 26, 2013 Aseptic vial filling line implementation of two separated filling paths including inline CIP/SIP Contact information Dr. Alexander Hohl Fresenius Kabi
Pharma Forum - Schwäbisch Hall September 26, 2013 Aseptic vial filling line implementation of two separated filling paths including inline CIP/SIP Contact information Dr. Alexander Hohl Fresenius Kabi
PHARMACEUTICAL PACKAGING
 GLOBAL SOLUTIONS FOR PHARMACEUTICAL PACKAGING High Barrier Bags Filling Vacuum Gas Flushing Sealing PACKAGING SOLUTIONS UNLOCKING VALUE FOR YOUR CHEMICAL PRODUCTS BERNHARDT is specialized in the production
GLOBAL SOLUTIONS FOR PHARMACEUTICAL PACKAGING High Barrier Bags Filling Vacuum Gas Flushing Sealing PACKAGING SOLUTIONS UNLOCKING VALUE FOR YOUR CHEMICAL PRODUCTS BERNHARDT is specialized in the production
Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite
 APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
ARIA FLUID BED PROCESSOR
 ARIA FLUID BED PROCESSOR ARIA Precise parameter control and premium quality engineering are the key features of IMA Active division s fluid bed processor. Simplified processing concepts based on smart
ARIA FLUID BED PROCESSOR ARIA Precise parameter control and premium quality engineering are the key features of IMA Active division s fluid bed processor. Simplified processing concepts based on smart
MASTER PLANNING FOR VALIDATION
 MASTER PLANNING FOR VALIDATION By: Gamal Amer, Ph.D. Principal, Premier Compliance Services, Inc. 1 Process Validation: General Principles and Practices Guidance to industry issued by the FDA in January
MASTER PLANNING FOR VALIDATION By: Gamal Amer, Ph.D. Principal, Premier Compliance Services, Inc. 1 Process Validation: General Principles and Practices Guidance to industry issued by the FDA in January
MEDIS INTERNATIONAL a.s Praha 1, Olivova 4/2096 MEDIS PHARMA Bolatice. Industrial zone Bolatice, region of Opava, Czech Republic
 MEDIS INTERNATIONAL a.s. 110 00 Praha 1, Olivova 4/2096 MEDIS PHARMA Bolatice Industrial zone Bolatice, region of Opava, Czech Republic In October 2008 MEDIS International a.s. started the building of
MEDIS INTERNATIONAL a.s. 110 00 Praha 1, Olivova 4/2096 MEDIS PHARMA Bolatice Industrial zone Bolatice, region of Opava, Czech Republic In October 2008 MEDIS International a.s. started the building of
GEA Pharma Systems. Tablet Compression - Courtoy R150 e With exchangeable turret for maximum flexibility. engineering for a better world
 GEA Pharma Systems Tablet Compression - Courtoy R150 e With exchangeable turret for maximum flexibility engineering for a better world GEA Pharma Systems supplies advanced technologies for the preparation
GEA Pharma Systems Tablet Compression - Courtoy R150 e With exchangeable turret for maximum flexibility engineering for a better world GEA Pharma Systems supplies advanced technologies for the preparation
PERFORMANCE QUALIFICATION PROTOCOL HVAC SYSTEM
 Page 1 of 24 PERFORMANCE QUALIFICATION PROTOCOL FOR HVAC SYSTEM Signing of this Performance Qualification Protocol indicates agreement with the Validation Master Plan approach of the equipment. Further
Page 1 of 24 PERFORMANCE QUALIFICATION PROTOCOL FOR HVAC SYSTEM Signing of this Performance Qualification Protocol indicates agreement with the Validation Master Plan approach of the equipment. Further
C40 BLISTER PACKAGING MACHINE
 C40 BLISTER PACKAGING MACHINE C40 A VALUE MACHINE Combined with the latest control systems and quality controls, the C40 machine is the answer for those customers who demand high production efficiency
C40 BLISTER PACKAGING MACHINE C40 A VALUE MACHINE Combined with the latest control systems and quality controls, the C40 machine is the answer for those customers who demand high production efficiency
Process Systems M+W Process Industries GmbH
 Process Systems M+W Process Industries GmbH Challenges in the Life Science Industry The manufacture of medicines in solid, semi-solid and liquid forms is a challenge for every pharmaceutical company. This
Process Systems M+W Process Industries GmbH Challenges in the Life Science Industry The manufacture of medicines in solid, semi-solid and liquid forms is a challenge for every pharmaceutical company. This
Biopharm Project Solutions Inc. Process Engineering Compliance
 Committed to providing reliable and expert solutions to Biologics and Pharmaceutical Projects BPS Inc, 119 Jaffrey Road, Malvern, PA, 19355 Phone: 484 614 0869 Fax: 610 296 1454 E-mail: surjs@biopharmprojects.com
Committed to providing reliable and expert solutions to Biologics and Pharmaceutical Projects BPS Inc, 119 Jaffrey Road, Malvern, PA, 19355 Phone: 484 614 0869 Fax: 610 296 1454 E-mail: surjs@biopharmprojects.com
TR135 BLISTER PACKAGING MACHINE
 TR135 BLISTER PACKAGING MACHINE TR135 MADE EASY FOR YOU TR135 is a medium speed blister packaging machine based on a established technology and ease of use, also for semi-skilled operators. With the best
TR135 BLISTER PACKAGING MACHINE TR135 MADE EASY FOR YOU TR135 is a medium speed blister packaging machine based on a established technology and ease of use, also for semi-skilled operators. With the best
Review Validation of aseptic processes for pharmaceuticals
 OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
HEADQUARTERS SPAIN SPAIN (LIFT)
 EPC & EPCM Services Telstar is a recognized global projects partner for technologybased clients in the Life Science and Healthcare industry, with specialist skills the provision of both conventional and
EPC & EPCM Services Telstar is a recognized global projects partner for technologybased clients in the Life Science and Healthcare industry, with specialist skills the provision of both conventional and
P RO V IDING S O L UT IO NS NO T JUST E Q UIPMENT
 P RO V IDING S O L UT IO NS NO T JUST E Q UIPMENT A E C P R O D U C T O V E R V I E W Auxiliary equipment for processors in the plastics, food, chemical, pharmaceutical, printing, and machine tool industries
P RO V IDING S O L UT IO NS NO T JUST E Q UIPMENT A E C P R O D U C T O V E R V I E W Auxiliary equipment for processors in the plastics, food, chemical, pharmaceutical, printing, and machine tool industries
Your benefit is our incentive
 MULTIVAC product overview Packaging solutions with method. 2 Your benefit is our incentive MULTIVAC stands for Better Packaging. But what lies behind these two words? As a benefactor of packaging solutions
MULTIVAC product overview Packaging solutions with method. 2 Your benefit is our incentive MULTIVAC stands for Better Packaging. But what lies behind these two words? As a benefactor of packaging solutions
TR200 BLISTER PACKAGING MACHINES
 TR200 BLISTER PACKAGING MACHINES TR200 TR200 is an extremely versatile, medium production speed blister packaging machine that was designed for production cycles that require frequent size changeovers.
TR200 BLISTER PACKAGING MACHINES TR200 TR200 is an extremely versatile, medium production speed blister packaging machine that was designed for production cycles that require frequent size changeovers.
Novel Technologies in Plasma Fractionation. Dieter Fassnacht
 Novel Technologies in Plasma Fractionation Dieter Fassnacht Agenda Grifols Overview Grifols Engineering Innovations in - Pooling - Fractionation - Filling Developing new technologies to provide innovative
Novel Technologies in Plasma Fractionation Dieter Fassnacht Agenda Grifols Overview Grifols Engineering Innovations in - Pooling - Fractionation - Filling Developing new technologies to provide innovative
MEDIS International, a.s., Karlovo náměstí 3, Praha 2, tel :
 MEDIS INTERNATIONAL a.s. 110 00 Praha 1, Olivova 4/2096 MEDIS PHARMA Bolatice Industrial zone Bolatice, region of Opava, Czech Republic MEDIS International a.s. started to build a new facility in October
MEDIS INTERNATIONAL a.s. 110 00 Praha 1, Olivova 4/2096 MEDIS PHARMA Bolatice Industrial zone Bolatice, region of Opava, Czech Republic MEDIS International a.s. started to build a new facility in October
MIKRON G05 High volume automation solutions for the assembling of products
 MIKRON G05 High volume automation solutions for the assembling of products EN THE G05 PRODUCT FAMILY OFFERS CUSTOMIZED PRODUCTION SOLUTIONS BASED ON HIGH PERFORMANCE ASSEMBLY PLATFORMS COMBINED WITH FLEXIBLE
MIKRON G05 High volume automation solutions for the assembling of products EN THE G05 PRODUCT FAMILY OFFERS CUSTOMIZED PRODUCTION SOLUTIONS BASED ON HIGH PERFORMANCE ASSEMBLY PLATFORMS COMBINED WITH FLEXIBLE
We are located in, Yiyuan County,Shandong Province. Which between Beijing and Shanghai. It is about 600km from beijing,and 900km from Shanghai.
 Location We are located in, Yiyuan County,Shandong Province. Which between Beijing and Shanghai. It is about 600km from beijing,and 900km from Shanghai. Production Reyoung has involved in 8 categories,
Location We are located in, Yiyuan County,Shandong Province. Which between Beijing and Shanghai. It is about 600km from beijing,and 900km from Shanghai. Production Reyoung has involved in 8 categories,
Pharmaceutical Packaging
 Pharmaceutical Packaging 2017 2021 Section I: Introduction A. Pharmaceuticals defined B. Pharmaceutical packaging defined C. Study organization D. Methodology E. Geographic regions F. Conventions Section
Pharmaceutical Packaging 2017 2021 Section I: Introduction A. Pharmaceuticals defined B. Pharmaceutical packaging defined C. Study organization D. Methodology E. Geographic regions F. Conventions Section
ISPE Baseline Pharmaceutical Engineering Guide for New and Renovated Facilities Volume 2: Oral Solid Dosage Forms (Revision) Executive Summary
 Reprinted from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE September/October 2008, Vol. 28 No. 5 This executive summary provides an overview of the second edition of the ISPE Baseline Guide:
Reprinted from PHARMACEUTICAL ENGINEERING The Official Magazine of ISPE September/October 2008, Vol. 28 No. 5 This executive summary provides an overview of the second edition of the ISPE Baseline Guide:
Why change is inevitable in aseptic manufacturing?
 Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Anhydro Drying Systems FOR THE PHARMACEUTICAL INDUSTRY
 Anhydro Drying Systems FOR THE PHARMACEUTICAL INDUSTRY SPX Flow Technology Danmark A/S is an international engineering company with a consistent goal to provide our customers with the optimal processing
Anhydro Drying Systems FOR THE PHARMACEUTICAL INDUSTRY SPX Flow Technology Danmark A/S is an international engineering company with a consistent goal to provide our customers with the optimal processing
Int. J. Pharm. Sci. Rev. Res., 34(1), September October 2015; Article No. 23, Pages: Process Validation of Tablet Dosage Form in Industries
 Review Article Process Validation of Tablet Dosage Form in Industries Ram Mohan S.R, N. Vishal Gupta* Pharmaceutical Quality Assurance group, Dept of Pharmaceutics, JSS University, Sri ShivarathreeshwaraNagara,
Review Article Process Validation of Tablet Dosage Form in Industries Ram Mohan S.R, N. Vishal Gupta* Pharmaceutical Quality Assurance group, Dept of Pharmaceutics, JSS University, Sri ShivarathreeshwaraNagara,
MG2 at Interpack: new machines and new systems
 Newsletter 2 - April 2014 MG2 at Interpack: new machines and new systems MG2 will be attending Dusseldorf s kermis with a rich line-up of machines. At Interpack, the Italian company will introduce some
Newsletter 2 - April 2014 MG2 at Interpack: new machines and new systems MG2 will be attending Dusseldorf s kermis with a rich line-up of machines. At Interpack, the Italian company will introduce some
USP Chapter 823 USP 32 (old) vs. USP 35 (new)
 USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
Automatic inspection for vials, ampules and cartridges
 CS-series Automatic inspection for vials, ampules and cartridges CS-Series Purity and sterility of parenterals vital to patients health. In the pharmaceutical industry, quality standards are becoming more
CS-series Automatic inspection for vials, ampules and cartridges CS-Series Purity and sterility of parenterals vital to patients health. In the pharmaceutical industry, quality standards are becoming more
Packaging System for Liquids. Blow-Fill-Seal Technology
 Packaging System for Liquids Blow-Fill-Seal Technology Innovative packaging with bottelpack Blowing, Filling, Sealing and much more! bottelpack packaging systems are used worldwide for many decades for
Packaging System for Liquids Blow-Fill-Seal Technology Innovative packaging with bottelpack Blowing, Filling, Sealing and much more! bottelpack packaging systems are used worldwide for many decades for
CVT TM Slitec TM Series - inspection machines for vials, ampoules, cartridges and syringes
 CVT TM Slitec TM Series - inspection machines for vials, ampoules, cartridges and syringes The Highest Quality requires perfection inside and outside. CVT TM inspection machine series with the unique Slitec
CVT TM Slitec TM Series - inspection machines for vials, ampoules, cartridges and syringes The Highest Quality requires perfection inside and outside. CVT TM inspection machine series with the unique Slitec
AZO Benelux The expert partner in your neighbourhood
 AZO Benelux The expert partner in your neighbourhood AZO GmbH - Innovative, worldwide AZO-Osterburken with the new technology centre in the foreground. From the invention of the first cyclone screener
AZO Benelux The expert partner in your neighbourhood AZO GmbH - Innovative, worldwide AZO-Osterburken with the new technology centre in the foreground. From the invention of the first cyclone screener
GMP MANUAL Contents. Contents (1) 1 Pharmaceutical Quality System (PQS) 2 Personnel
 GMP MANUAL GMP MANUAL 1 Pharmaceutical Quality System (PQS) 1.A Preface 1.B The road to a Pharmaceutical Quality System 1.C Introduction to the PQS 1.C.1 General requirements 1.C (1) 1.C.2 Documentation
GMP MANUAL GMP MANUAL 1 Pharmaceutical Quality System (PQS) 1.A Preface 1.B The road to a Pharmaceutical Quality System 1.C Introduction to the PQS 1.C.1 General requirements 1.C (1) 1.C.2 Documentation
Preparing Your Aseptic Processing Facility for an FDA Inspection. Valerie Welter, Director Quality Bayer HealthCare March 10, 2014
 Preparing Your Aseptic Processing Facility for an FDA Inspection Valerie Welter, Director Quality Bayer HealthCare March 10, 2014 Agenda Regulatory Requirements Establishing your Approach Aseptic Controls
Preparing Your Aseptic Processing Facility for an FDA Inspection Valerie Welter, Director Quality Bayer HealthCare March 10, 2014 Agenda Regulatory Requirements Establishing your Approach Aseptic Controls
Our service offering: One stop accountability
 M+W Process Industries GmbH A Company of the M+W Group 28 January 2015 Our service offering: One stop accountability Consulting Engineering Construction & Commissioning Qualification & Validation Technical
M+W Process Industries GmbH A Company of the M+W Group 28 January 2015 Our service offering: One stop accountability Consulting Engineering Construction & Commissioning Qualification & Validation Technical
TECHNICAL SPECIFICATION
 TECHNICAL SPECIFICATION ABP SERIES OF AUTOMATIC BLISTER PACKAGING MACHINERY Ridat ABP series of machines are some of the most advanced models in the comprehensive range of Ridat blister packaging equipment.
TECHNICAL SPECIFICATION ABP SERIES OF AUTOMATIC BLISTER PACKAGING MACHINERY Ridat ABP series of machines are some of the most advanced models in the comprehensive range of Ridat blister packaging equipment.
Bioprocess & Controlled Environments: Australia and South East Asia overview
 Environments: Australia and South East Asia overview AMEC is a focused supplier of consultancy, engineering and project management services to its customers in the world s oil and gas, mining, clean energy,
Environments: Australia and South East Asia overview AMEC is a focused supplier of consultancy, engineering and project management services to its customers in the world s oil and gas, mining, clean energy,
Partner with the Global Leader in Drug Delivery Systems
 3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems Northridge, CA, USA Manufacturing Facility Experts at Commercializing Innovation 3M: Transforming New Ideas into Thousands
3M DRUG DELIVERY SYSTEMS Partner with the Global Leader in Drug Delivery Systems Northridge, CA, USA Manufacturing Facility Experts at Commercializing Innovation 3M: Transforming New Ideas into Thousands
Robotized Integrated Blister Line INTEGRA 220. Liquids Solids Creams Packaging ENGLISH
 220 Robotized Integrated Blister Line Liquids Solids Creams Packaging ENGLISH 220 Robotized Integrated Blister Line A concentrate of innovation in less than 8 meters Robotized Blister Line, integrating
220 Robotized Integrated Blister Line Liquids Solids Creams Packaging ENGLISH 220 Robotized Integrated Blister Line A concentrate of innovation in less than 8 meters Robotized Blister Line, integrating
Water purification in the pharmaceutical industry
 ABB MEASUREMENT & ANALYTICS APPLICATION DESCRIPTION Water purification in the pharmaceutical industry Providing independent verification and validation of the water purification process for compliance
ABB MEASUREMENT & ANALYTICS APPLICATION DESCRIPTION Water purification in the pharmaceutical industry Providing independent verification and validation of the water purification process for compliance
MARIFARM LLC PRODUCTION ABOUT US
 MARIFARM LLC PRODUCTION ABOUT US 2018 KEY INFORMATION COMPANY NAME: HEADQUARTERS: OWNERSHIP: SHARE CAPITAL: SHARE CAPITAL: FOUNDED: Marifarm LLC Maribor, Slovenia (EU) 100% ARTERIUM International BV 4.539.731,46
MARIFARM LLC PRODUCTION ABOUT US 2018 KEY INFORMATION COMPANY NAME: HEADQUARTERS: OWNERSHIP: SHARE CAPITAL: SHARE CAPITAL: FOUNDED: Marifarm LLC Maribor, Slovenia (EU) 100% ARTERIUM International BV 4.539.731,46
AMERICAS Tel or Tel CHINA, SHENZHEN Tel
 www.uic.com email: universal@uic.com AMERICAS Tel. 1-800-432-2607 or Tel. +1-607-779-7522 CHINA, SHENZHEN Tel. +86-755-2685-9108 CHINA, SHANGHAI Tel. +86-21-6495-2100 EUROPE Tel. +36-23-445-500 2010 Universal
www.uic.com email: universal@uic.com AMERICAS Tel. 1-800-432-2607 or Tel. +1-607-779-7522 CHINA, SHENZHEN Tel. +86-755-2685-9108 CHINA, SHANGHAI Tel. +86-21-6495-2100 EUROPE Tel. +36-23-445-500 2010 Universal
Trends in Sterile Manufacturing Technologies
 Trends in Sterile Manufacturing Technologies ISPE Thailand Annual Meeting Charlotte Enghave Fruergaard 2013.07.18 Where we come from 1930s Danish Novo and Nordisk Gentofte (later Novo Nordisk) employed
Trends in Sterile Manufacturing Technologies ISPE Thailand Annual Meeting Charlotte Enghave Fruergaard 2013.07.18 Where we come from 1930s Danish Novo and Nordisk Gentofte (later Novo Nordisk) employed
Prequalification Team Inspection services WHO PUBLIC INSPECTION REPORT (WHOPIR) Finished Product Manufacturer. Tablets
 Prequalification Team Inspection services WHO PUBLIC INSPECTION REPORT (WHOPIR) Finished Product Manufacturer Part 1: information about the inspection Name of manufacturer Physical address production departments
Prequalification Team Inspection services WHO PUBLIC INSPECTION REPORT (WHOPIR) Finished Product Manufacturer Part 1: information about the inspection Name of manufacturer Physical address production departments
Inspections, Compliance, Enforcement, and Criminal Investigations
 Page 1 of 8 Inspections, Compliance, Enforcement, and Criminal Investigations Lupin Limited 5/7/09 Department of Health and Human Services Public Health Service Food and Drug Administration CENTER FOR
Page 1 of 8 Inspections, Compliance, Enforcement, and Criminal Investigations Lupin Limited 5/7/09 Department of Health and Human Services Public Health Service Food and Drug Administration CENTER FOR
User Requirement Specification Specyfikacja Wymagań Użytkownika
 \ Project / Projekt: Albumina dożylna nowej generacji oparta o wielostopniowy proces inaktywacji wirusów Plasma Fractionation Department BIOMED-LUBLIN Wytwórnia Surowic i Szczepionek S.A. ALBUMINA / ALBUMIN
\ Project / Projekt: Albumina dożylna nowej generacji oparta o wielostopniowy proces inaktywacji wirusów Plasma Fractionation Department BIOMED-LUBLIN Wytwórnia Surowic i Szczepionek S.A. ALBUMINA / ALBUMIN
Thermoforming packaging machine R 145 with MULTIVAC Clean Design. Maximum process reliability and cleanroom suitability
 Thermoforming packaging machine R 145 with MULTIVAC Clean Design Maximum process reliability and cleanroom suitability MULTIVAC R 145 Clean Design Thermoforming packaging machine R 145 with MULTIVAC Clean
Thermoforming packaging machine R 145 with MULTIVAC Clean Design Maximum process reliability and cleanroom suitability MULTIVAC R 145 Clean Design Thermoforming packaging machine R 145 with MULTIVAC Clean
G.F. S.p.A. Via T. Edison, Rubbiano fraz. Solignano (PR) - Italy Tel Fax Web:
 G.F. S.p.A. Via T. Edison, 3 43045 Rubbiano fraz. Solignano (PR) - Italy Tel. +39. 0525. 300311 Fax +39. 0525. 300350 Web: www.gfe.it E-mail: gfe@gfe.it Developed to process glass and plastic vials and
G.F. S.p.A. Via T. Edison, 3 43045 Rubbiano fraz. Solignano (PR) - Italy Tel. +39. 0525. 300311 Fax +39. 0525. 300350 Web: www.gfe.it E-mail: gfe@gfe.it Developed to process glass and plastic vials and
HealthCaps. Quality our Priority
 HealthCaps I N D I A LTD. our Priority Introduction Healthcaps India Ltd is one the largest manufacturers of high quality Empty Capsules worldwide. We are a WHO-GMP, Halal & Kosher certified company in
HealthCaps I N D I A LTD. our Priority Introduction Healthcaps India Ltd is one the largest manufacturers of high quality Empty Capsules worldwide. We are a WHO-GMP, Halal & Kosher certified company in
Current Trends in Sterile Manufacturing. by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS)
 Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
Library Guide: Active Pharmaceutical
 Library Guide: Active Pharmaceutical Ingredients (API) Table of Contents Overview...3 Sample Curriculum...5 Course Descriptions: A Step-by-Step Approach to Process Validation (PHDV79)...7 A Tour of the
Library Guide: Active Pharmaceutical Ingredients (API) Table of Contents Overview...3 Sample Curriculum...5 Course Descriptions: A Step-by-Step Approach to Process Validation (PHDV79)...7 A Tour of the
1 of 17. Macpack Machineries Sdn. Bhd. Cone Mixer V Mixer. Shrink Tunnel. Liquid Filing Machine. Size can be custom
 Macpack Machineries Sdn. Bhd. Cone Mixer V Mixer Mixing Time Machine Material Mixing Volume Suitable Ingredient 10-30min Stainless Steel/ Mild Steel 50-10000ml Powder/ Small Granular Shrink Tunnel Speed
Macpack Machineries Sdn. Bhd. Cone Mixer V Mixer Mixing Time Machine Material Mixing Volume Suitable Ingredient 10-30min Stainless Steel/ Mild Steel 50-10000ml Powder/ Small Granular Shrink Tunnel Speed
PDA: A Global. Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG. Maturing Aseptic Technologies
 Maturing Aseptic Technologies PDA: A Global create more flexible Facilities Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG Content Changing Environment for Parenteral Manufacturing
Maturing Aseptic Technologies PDA: A Global create more flexible Facilities Association Dr. Friedrich Haefele Boehringer Ingelheim Pharma GmbH & Co. KG Content Changing Environment for Parenteral Manufacturing
SINTERED WIRE MESH.
 SINTERED WIRE MESH www.sinteredfilter.org "Total filtration solution provider" For over 15 years, Boegger has been providing filtration solutions to a wide range of filtering industries where air, liquid
SINTERED WIRE MESH www.sinteredfilter.org "Total filtration solution provider" For over 15 years, Boegger has been providing filtration solutions to a wide range of filtering industries where air, liquid
Extractables and leachables: An Introduction
 Extractables and leachables: An Introduction Tim Hulme Smithers Rapra THulme@smithers.com 44(0)1939 252 418 1 Extractables and leachables: An Introduction Tim Hulme Smithers Rapra thulme@smithers.com 2
Extractables and leachables: An Introduction Tim Hulme Smithers Rapra THulme@smithers.com 44(0)1939 252 418 1 Extractables and leachables: An Introduction Tim Hulme Smithers Rapra thulme@smithers.com 2
Sugar. g Liquefication (800) Reduce inbound syrup y p transportation. i costs by more than 32%.
 Reduce inbound syrup y p transportation i costs by more than 32%. Sugar g Liquefication n buying y g granular g sugar g on the open p market better control your sugar g supply problems and costs. Eliminate
Reduce inbound syrup y p transportation i costs by more than 32%. Sugar g Liquefication n buying y g granular g sugar g on the open p market better control your sugar g supply problems and costs. Eliminate
G.F. S.p.A. Via T. Edison, Rubbiano fraz. Solignano (PR) - Italy Tel Fax Web:
 G.F. S.p.A. ia T. Edison, 3 43045 Rubbiano fraz. Solignano (PR) - Italy Tel. +39. 0525. 300311 Fax +39. 0525. 300350 Web: www.gfe.it E-mail: gfe@gfe.it Developed to process ampoules, vials and cartridges
G.F. S.p.A. ia T. Edison, 3 43045 Rubbiano fraz. Solignano (PR) - Italy Tel. +39. 0525. 300311 Fax +39. 0525. 300350 Web: www.gfe.it E-mail: gfe@gfe.it Developed to process ampoules, vials and cartridges
Guiding Principles for the implementation of fluid management technologies for modern single use aseptic processing
 Guiding Principles for the implementation of fluid management technologies for modern single use aseptic processing Jean-Marc Cappia, Vice President Marketing Fluid Management Technologies Agenda 1. Aseptic
Guiding Principles for the implementation of fluid management technologies for modern single use aseptic processing Jean-Marc Cappia, Vice President Marketing Fluid Management Technologies Agenda 1. Aseptic
Your partner in the pharmaceutical industry
 English Micronisation- and milling service Micronised by GfM Your API Particle size (µm) Your partner in the pharmaceutical industry The... always that little bit finer. We are a family business in the
English Micronisation- and milling service Micronised by GfM Your API Particle size (µm) Your partner in the pharmaceutical industry The... always that little bit finer. We are a family business in the
 +91-8048763884 Siddhivinayak Engineering http://www.siddhivinayakengg.co.in/ We are the leading manufacturers, exporters, wholesalers and retailers of this impeccable range of Labeling & Capping s. The
+91-8048763884 Siddhivinayak Engineering http://www.siddhivinayakengg.co.in/ We are the leading manufacturers, exporters, wholesalers and retailers of this impeccable range of Labeling & Capping s. The
C360 DEEP DRAW THERMOFORMING MACHINE
 C360 DEEP DRAW THERMOFORMING MACHINE C360 DEEP DRAW THERMOFORMING MACHINE C360 The C360 is IMA SAFE s latest trend setting a thermoformer designed for deep draw packaging. This modular designed line has
C360 DEEP DRAW THERMOFORMING MACHINE C360 DEEP DRAW THERMOFORMING MACHINE C360 The C360 is IMA SAFE s latest trend setting a thermoformer designed for deep draw packaging. This modular designed line has
Our manufacturing facility is designed to comply in accordance to the stringent Schedule M GMP Guidelines.
 Dales Remedies Pvt Ltd was founded in 1994 as a pioneer in the manufacture of wide range of pharmaceutical formulations such as creams, gels, ointments, lotions and mouthwash. Our manufacturing facility
Dales Remedies Pvt Ltd was founded in 1994 as a pioneer in the manufacture of wide range of pharmaceutical formulations such as creams, gels, ointments, lotions and mouthwash. Our manufacturing facility
Critical Environment Products and Services
 Critical Environment Products and Services For over two decades, EP Scientific products and services have defined clean for environmental sampling containers and services. Our proprietary cleaning methods
Critical Environment Products and Services For over two decades, EP Scientific products and services have defined clean for environmental sampling containers and services. Our proprietary cleaning methods
AFB factory hybrid production
 AFB The modular concept factory for all hybrid production tasks The learning system for all processes associated with production automation and process automation: Production Handling of seals Packaging
AFB The modular concept factory for all hybrid production tasks The learning system for all processes associated with production automation and process automation: Production Handling of seals Packaging
Whitepaper. High temperature HEPA filtration. Dr.-Ing. Marc Schmidt, Dr.-Ing. Lothar Gail, Hugo Hemel MSc. Preview
 High temperature HEPA filtration Air filtration challenges and answers for dry heat sterilization tunnels Whitepaper Preview Dr.-Ing. Marc Schmidt, Dr.-Ing. Lothar Gail, Hugo Hemel MSc. Air filtration
High temperature HEPA filtration Air filtration challenges and answers for dry heat sterilization tunnels Whitepaper Preview Dr.-Ing. Marc Schmidt, Dr.-Ing. Lothar Gail, Hugo Hemel MSc. Air filtration
Regulatory perspectives on CQAs, CPPs, and Risk Analyses for Combination Products.
 Regulatory perspectives on CQAs, CPPs, and Risk Analyses for Combination Products. 3rd FDA/PQRI Conference on Advancing Product Quality March 22-24, 2017 TRACK #2 Achieving Drug Product Quality: Novel
Regulatory perspectives on CQAs, CPPs, and Risk Analyses for Combination Products. 3rd FDA/PQRI Conference on Advancing Product Quality March 22-24, 2017 TRACK #2 Achieving Drug Product Quality: Novel
HL Pharm Tech Capsule Filling Machine HLF Series
 EBSEOS GmbH Pharmaceutical Machinery & Services Industriestrasse 12 79664 Wehr Tel. ++49 (0)7762 80 68 966 Fax. ++49 (0)7762 80 68 967 HL Pharm Tech Capsule Filling Machine HLF Series The HL Pharm Tech
EBSEOS GmbH Pharmaceutical Machinery & Services Industriestrasse 12 79664 Wehr Tel. ++49 (0)7762 80 68 966 Fax. ++49 (0)7762 80 68 967 HL Pharm Tech Capsule Filling Machine HLF Series The HL Pharm Tech
GMP MANUAL Contents. 1 Pharmaceutical Quality System (PQS)
 GMP MANUAL GMP MANUAL 1 Pharmaceutical Quality System (PQS) 1.A Preface 1.B The road to a Pharmaceutical Quality System 1.C Introduction to the PQS 1.C.1 General requirements 1.C.2 Documentation 1.D Main
GMP MANUAL GMP MANUAL 1 Pharmaceutical Quality System (PQS) 1.A Preface 1.B The road to a Pharmaceutical Quality System 1.C Introduction to the PQS 1.C.1 General requirements 1.C.2 Documentation 1.D Main
Vetter Development Service
 A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
CIP Cleaning in the. The ASME Guide for Bioprocessing Equipment (BPE 2007) and CIP Design. Full TACCT functionality
 CIP Cleaning in the Life Science Industry The ASME Guide for Bioprocessing Equipment (BPE 2007) and CIP Design Decentralized CIP System Design with Full TACCT functionality Agenda Cleaning in the Life
CIP Cleaning in the Life Science Industry The ASME Guide for Bioprocessing Equipment (BPE 2007) and CIP Design Decentralized CIP System Design with Full TACCT functionality Agenda Cleaning in the Life
FS 1000/3000 SIP BIOREACTORS/FERMENTORS FOR INDUSTRIAL PROCESSES
 FS 1000/3000 SIP BIOREACTORS/FERMENTORS FOR INDUSTRIAL PROCESSES THE COMPANY Bionet is a specialist in bioprocesses engineering. We provide equipment (Bioreactors, Cross-Flow Filtration Systems and Cleaning-In-Place
FS 1000/3000 SIP BIOREACTORS/FERMENTORS FOR INDUSTRIAL PROCESSES THE COMPANY Bionet is a specialist in bioprocesses engineering. We provide equipment (Bioreactors, Cross-Flow Filtration Systems and Cleaning-In-Place
Microbiological Consideration for Non-Sterile Pharmaceutical
 May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
Inspection. Implementation of ICH Q8, Q9, Q10
 Implementation of ICH Q8, Q9, Q10 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Outline Aim of - as a key part of the regulatory
Implementation of ICH Q8, Q9, Q10 International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Outline Aim of - as a key part of the regulatory
Ampio Pharmaceuticals Production and Office Facility Englewood, Colorado
 Related Experience Ampio Pharmaceuticals Production and Office Facility Englewood, Colorado About the Project Design and construction of a 19,000-SF pharmaceutical production facility, which included cleanrooms,
Related Experience Ampio Pharmaceuticals Production and Office Facility Englewood, Colorado About the Project Design and construction of a 19,000-SF pharmaceutical production facility, which included cleanrooms,
A Case Study on the Application of Disposable Technologies in cgmp Manufacturing Processes for a Therapeutic Antibody.
 A Case Study on the Application of Disposable Technologies in cgmp Manufacturing Processes for a Therapeutic Antibody. Jeremy M Tong (presenting), Mark S Kettel, Edward M Perry, David J Pain, David M Valentine,
A Case Study on the Application of Disposable Technologies in cgmp Manufacturing Processes for a Therapeutic Antibody. Jeremy M Tong (presenting), Mark S Kettel, Edward M Perry, David J Pain, David M Valentine,
Guidelines for Process Validation of Pharmaceutical Dosage Forms
 Guidelines for Process Validation of Pharmaceutical Dosage Forms Version 2.1 Date issued 21/02/2010 Date of implementation 21/05/2010 31 August 2010 Page 1 of 20 Guidelines for Process Validation of Pharmaceutical
Guidelines for Process Validation of Pharmaceutical Dosage Forms Version 2.1 Date issued 21/02/2010 Date of implementation 21/05/2010 31 August 2010 Page 1 of 20 Guidelines for Process Validation of Pharmaceutical
NADERI ENGINEERING, INC.
 BIO-PHARMACEUTICAL ENGINEERING & VALIDATION CAPABILITIES "Cost Effective Solutions Through Innovation" 1240 Powell Street, Suite 2-B Emeryville, CA 94608 Tel: (510) 547-4040 Fax: (510) 547-4141 Website:
BIO-PHARMACEUTICAL ENGINEERING & VALIDATION CAPABILITIES "Cost Effective Solutions Through Innovation" 1240 Powell Street, Suite 2-B Emeryville, CA 94608 Tel: (510) 547-4040 Fax: (510) 547-4141 Website:
,1+#$# 1)0'+!0',+!) -&!.*!"$10'"!)/ ",*-!+4 !/ %.,3+ 0.,+% *!+!%$*$+0 0$!* )2,0$"&
 ALVOTECH 2017 ALVOGEN 9.5.2017 3 ALVOGEN WORLDWIDE 35 Alvogen worldwide offices BIOPHARMACEUTICALS ARE THE FUTURE Biologic molecules are the future of the pharmaceutical industry due to type of diseases
ALVOTECH 2017 ALVOGEN 9.5.2017 3 ALVOGEN WORLDWIDE 35 Alvogen worldwide offices BIOPHARMACEUTICALS ARE THE FUTURE Biologic molecules are the future of the pharmaceutical industry due to type of diseases
