PHARMACEUTICAL ENGINEERING
|
|
|
- Charlotte Boyd
- 6 years ago
- Views:
Transcription
1 PHARACEUICAL ENGINEERING Crystallization Dr. Jasmina Khanam Reader of Pharmaceutical Engineering Division Department of Pharmaceutical echnology Jadavpur University Kolkatta ( ) CONENS Introduction Crystal geometry Crystal size and shape factors Uniformity in size or crystals distribution Yield Purity of product Nucleation and Crystal growth iers super saturation theory Super saturation method Crystallization Equipments ass balance in crystallization Energy balances Factors controlling crystallization Concentration gradient and emperature gradient Effect of emperature on solubility Caking of crystals Keywords Crystal characteristics, yield, crystallizing equipment, factors controlling crystallization, caking
2 Introduction here has been an increasing effort in designing pharmaceutical particles with controllable qualities in terms of purity, size, shape, morphology, size distribution and surface characteristics and pharmacological properties. Polymorphism and particle size distribution of pharmaceutical particles are two very important criteria as per the guidelines of regulatory bodies and requirements of pharmaceutical process and the uses of drugs. So, undergraduate Pharmacy students must understand the process involved in production and purification of medicinal compounds in the laboratory / bulk drug manufacturing industry, characterization of these compounds and stepwise handling of drug materials in formulation industry. A formulation pharmacist has to solve many problems related to undesirable growth of crystalline active ingredients in liquid formulations. As for eample, crystal growth in acetaminophen suspension can be prevented by the use of some additives like, polyvinyl pyrolidone, bovine serum albumin, benzalkonium chloride, sucrose etc. herefore, pharmacy students must acquire basic knowledge of crystals and crystallization to encounter and solve various problems in the laboratory and pharmaceutical industry. Crystallization is a method of formation of solid particles within a homogeneous phase (vapor phase, liquid melt). ost of the crystalline solid substances are prepared either by solidification from liquid melt or by precipitation in chemical reaction. 'Crystallization' is one of the important unit operations that are taught in undergraduate curriculum of Chemical Engineering and Pharmaceutical Engineering. his unit operation involves knowledge of mass transfer mechanism, diffusion theory, heat transfer mechanism, hydrodynamics, solubility curves, design of equipments and selection of equipments on the basis of principles of crystallization. Each manufacturing plant requires many unique features and these must be evaluated on an individual basis in order to achieve optimum result. It is true that mechanical design of the crystallizer has a significant influence on the nucleation rate due to contact nucleation with the parts of pump, impellor etc. when suspended in a supersaturated solution. his phenomenon yields varying rates of nucleation in scale up and differences in nucleation rate when the same piece of equipment is used to crystallize different materials. All these must be taken care of in order to achieve super saturation of liquid. Crystallization of a material from solution is important industrially because of the markets demand due to some advantages of its crystalline solid forms. One of the important advantages of crystallization compared with other means of separation technique is that it produces highly pure products with good appearance from impure solutions. Energy input is low and products with high bulk density are obtained. Drying requirement is minimum due to low moisture content. Commercial crystallization processes manufacture crystalline products from the standpoint of special demands based on definite crystal shape, size and size range of particles (crystal size distribution, CSD). Generally, narrow size range is desired. Of course, requirements of scientific works are solely restricted to yield of crystal as high as possible and its purity. Crystalline products have high demand in the market due to some reasons and some advantages over liquid form products.
3 Handling of solid form products with convenience than solution. ore economical than liquid form. Less degradation, less contamination, more stability than solution. ore sales appeal than liquid. Purity of crystalline product is more than that of a solution. Crystallization process may be analyzed and judged from the standpoint of following aspects in order to produce good products and avoid problems encountered both within the industry and outside the industry. 1. Yield of a given product 2. Quality of product in terms of size, shape and purity of crystals 3. Uniformity in size and CSD 4. Rate of production of the desired crystals. 5. Energy requirements for cooling and evaporation, etc. High yield and rate of production of a crystalline product at acceptable cost leads to its economical viability. But, purity of a crystalline material as medicinal compound is mandatory requirement in Pharmaceutical industry. So, purity is the first and foremost demand of the purchaser in Pharmaceutical sector. Size, uniformity in size, crystal size distribution and crystal geometry are characteristics of particles that control many physicochemical factors in making of formulation drugs, stability and pharmacological factors. Crystal geometry A crystal is the most highly organized type of non-living matter. olecules, atoms and ions are arranged in a crystal in orderly repetitive arrays called crystal lattices and it appears as polyhedrons having sharp corners and flat sides or faces. Although relative sizes of the faces and edge of various crystals of the same materials may vary, the angle between adjacent faces is constant for all crystals of the same materials and that is the characteristics of the material causing difference in physical properties among chemicals. herefore it is not unepected that the formation of the crystalline material from its solution or mother liquor is accompanied by unique growth and nucleation characteristics. By limiting the degree of super saturation crystal growth rate and crystal sizes can be controlled. he crystal systems are a grouping of crystal structures according to the aial systems used to describe their lattice. Each crystal system consists of a set of three aes in a particular geometric arrangement. here are seven unique crystal systems. he cubic / isometric system has the symmetry of a cube, that is the three aes are mutually perpendicular and of equal length. he other si systems in order to decreasing symmetry are as follows: Heagonal: hree equal aes are in one plane at 60 o to each other, one is at right angles to this plane but not necessarily of the same length as the others. Cross section is heagonal. etragonal: hree aes are at right angles, one is longer than the other two.
4 Orthorhombic: It is like tetragonal crystals ecept not square in cross section (when viewing the crystal on end), forming rhombic prisms or dipyramids (two pyramids stuck together). rigonal: here is single 3 fold ais of rotation instead of the 6 fold ais of heagonal division. onoclinic: wo aes are at right angles in one plane and a third ais at some odd angle to this plane like skewed tetragonal crystals often forming prisms and double pyramids. riclinic: hree aes are at odd angles to each other; usually it is not symmetrical from one side to the other, which can lead to some fairly strange shapes. A crystalline substance may have the ability to form more than one form, called polymorphism. Different polymorphs possess different physicochemical properties causing difference in solubility and melting point that affect dissolution, adsorption, absorption and stability. Nowadays, atomic force microscopy (AF) is used to map the crystal structure by imaging the molecular lattice space in all three directions. Crystal size and shape factors Shape factor or sphericity, φ s is used to calculate the ratio of the total surface (S P ) of a crystal to the crystal volume where S P = πd 2 p and V p = 1/6 πd 3 p where D p is the diameter of crystal. Sp 6 6 Vp = and L = φ s D p = V φ D S p s p p L is the length of crystal; when φ s =1, L = D p. For cubes and spheres, φ s = 1. For granular materials range of φ s is from 0.6 to he ratio S p /V p is very important parameter in any surface related phenomenon. Product containing smaller crystals is more soluble than that of bigger crystals since surface area per unit volume (S p /V p ) is greater is smaller particles. hree dimensional surface morphology, surface area and crystal structure of many therapeutic agents have effects on the manufacturing of crystal, ease of delivery of drug to the system, bioavailability, dissolution rate, absorption and adsorption of drugs. Uniformity in size or crystals distribution Particles of uniform size in a product are desirable for the convenience of selection of filter, washing, uniform time of dissolution and good appearance of product. Besides these, caking tendency of crystals during its storage period can be prevented since number of points of contact between crystalline particles is significantly less in uniform crystals. Actually a product batch contains various fractions of particles of different particle sizes. Wide range of particle sizes creates problem in many practical situations. So, crystal size range is made narrow by controlling some process parameters like adding seed crystals with desired size range. avoiding contact nucleation as far as possible removing a fraction of small size /finer particles dissolving smaller crystals, nuclei either by heating or adding some solvent increasing residence time for larger particles.
5 he objective is to reduce sharply the number of nuclei present so that bigger crystals formed earlier can grow properly under super saturated condition. he presence of too many nuclei leads to a large surface area and makes the supersaturation small, so the growth rate of individual crystal is low since there is decrease in potential (or concentration) between the bulk of the liquor and saturated liquid film around particle. If the process were carried out without formation of additional nuclei, it would be possible to predict eactly the size distribution of the product from the size distribution of seed crystals, because the rate of growth of a linear dimension of crystal is constant for all crystals in the batch. he size distribution is generally epressed by the mass fractions in a screen analysis rather than number of particles present in particular fraction of sizes. Yield In crystallization processes, it is often needed to calculate the theoretical yield so that actual yield can be compared with it. Crystals are allowed to grow for sufficient period to reach targeted growth. Finally, mother liquor sheds its super saturation and attains equilibrium at final temperature. Now, solid crystals are separated from mother liquor and further processed. Computation of yield of crystal involves a material balance. he yield can be predicted from the difference of concentration of feed solution and solubility of mother liquor (saturated). Predicted yield may not match actual yield due to slow growth longer time required to reach equilibrium, insufficient eposure of surface area when heavier particles settle at the bottom, less growth in the location with poor super saturation, viscous nature of solution etc. In such a situation, the final mother liquor may retain appreciable super saturation and actual yield is always less unless mode of nucleation is changed and above-mentioned causes are solved. he solubility is epressed commonly in terms of anhydrous salt per 100 mass of pure solvent or mass fraction. he calculation of yield of a nonhydrated salt (crystal without water of crystallization) from solution is simple since the amount of solvent in the liquid remains unchanged. 1 2 Yield = total amount of solvent, 1, 2 are the concentration of anhydrous material and solubility of crystal in feed solution and in mother liquor respectively. If evaporation loss (z) is accountable and y be the amount of initial solvent Yield = 1 y 2 (y z) If the product is hydrated with water of crystallization, quantity of solvent decreases during the operation, but the amount of water (free water) in ecess of that required hydrating total anhydrous material (salt (P) in solid product + salt (S) left in saturated mother liquor) is constant. A material balance can be carried out on the basis of ecess (free) solvent. Solubility is easily epressed in terms of mass of hydrate per unit mass of ecess water. Let w 1, w 2 be the respective amount of water associated with P lb and S lb of anhydrous material as water of crystallization. Let w 3 be the amount of free water. Considering no loss due to evaporation, we may write mass balance on the basis of 1000 lb of total water = w 1 + w 2 + w 3
6 ass of product salt (hydrated) = P + w 1 and ass of salt (hydrated) = S + w 2 in mother liquor. (P+S) is the total amount of anhydrous substance. Concentration of total hydrate per unit amount of free water in the solution is (P + w 1 + S + w 2 )/w 3 and solubility of hydrate in mother liquor is (S + w 2 ) / w 3 P + S + w1 + w 2 S + w 2 yield = w 3 w 3 w = P + w 1 3 Amount of water of hydrate is calculated as follows: (number of water of crystallization) (w 1 + w 2 ) = (P + S) molecular weight of water olecular weight of anhydrous salt Purity of product Purity of a crystalline product is judged by many ways, for eample determination of melting point of crystalline substance. Generally it is thought that crystal form is the purest form of a substance, but it may retain impurities on its surface due to adherence of impure mother liquor on its surface while separating it from final magma. Smaller crystals will retain relativity more impurities than large crystals due to large specific surface area of the smaller crystals. Washing of crystal s surface is done with the same solvent to remove impurities. Sometimes mother liquor is occluded within the empty space of an agglomerate / lump that leads to poor quality product. Proper washing of agglomerated crystal is difficult. Formation of agglomerated crystal during crystallization can be minimized by proper agitation. Even after thorough washing, the product cannot reach the limit of purity, then it is further processed to produce more pure product by the recrystallization method by utilizing the difference in solubility of crystal and unwanted impurities. Recrystallization is often practiced both in laboratory research work and industry to achieve high-grade purity in pharmaceutical ingredient. Recrystallization is done by repeated crystallization of the same material by dissolving in solvent to remove the residual soluble impurities that has been adhered to the surface of crystal from mother liquor. It is possible to produce crystals of varying particle shape and of specific polymorph by selection of proper operating variables and design of equipment. Recrystallization can be eplained by the following eample: Purity of benzoic acid (antimicrobial agent) is assessed by assay method and melting point (122 o C). If impurities like pthalic acid, benzyl benzoate are present with benzoic acid, dissolving it in hot water and filtering it can remove these. Upon cooling benzoic acid crystals are formed and then crystals are separated by filtration. his step may be repeated unless satisfactory product is obtained. After purification, the amount of final product will have been reduced due to removal of impurities and loss of material in attaining equilibrium during repeated recrystallization. Calculation of percentage yield is done as follows. ass of final product % Yield = 100 ass of initial sample his indicates etent of impurity in the original sample.
7 Nucleation and Crystal growth In the formation of crystal two-steps is required (1) generation of a new particle (2) its growth to macroscopic size. he first step is called nucleation. Neither formation of a new crystal nor its growth is possible in saturated or unsaturated solution. Rate of nucleation is very important for controlling crystal size distribution (CSD). he rate of nucleation is the number of new particles formed per unit time per unit volume of magma or solid free mother liquor. he sequence of stages in the evolution of a crystal is as follows: Cluster embryo nucleus crystal Nuclei are in a state of unstable equilibrium if it looses units, it dissolves, if it gains units, it grows and becomes a crystal. Solubility of large crystal is less than that of smaller crystals in the micrometer range because of significant surface energy per unit mass on smaller crystals. Smaller crystals are in a state of unstable equilibrium in a supersaturated solution. As a result larger crystals grow until the small crystals disappear. his phenomenon is called Ostwald ripening. he effect of particle size on solubility is a key factor in nucleation. At first, a loose unstable aggregate called cluster is formed when several molecules/atoms of solute come into contact due to random collision. Formation of embryo initiates lattice arrangement and formation of a new and separate phase. hough embroys have short lives but it may grow to a size as that of nucleus, which is the smallest assemblage of particles. Formation of stable nucleus depends on number of units assemble together. Nucleation may be of three types: Spurious nucleation, primary nucleation and secondary nucleation. Spurious nucleation produces crystals of poor quality with abnormal needle like or whisker like growths from the ends of the crystals. It occurs at large super saturation ( >8 0 C). his is called needle breeding. Another growth related spurious nucleation called Veiled growth gives the crystal surface milky appearance due to rapid crystal growth and occlusion of mother liquor into the crystal faces. his problem can be avoided by growing crystals at low super saturation and by using well-designed and operated pumps and agitators. he first, step of nucleus formation is called nucleation or primary nucleation. On an industrial super saturation driving force is necessary to initiate primary nucleation. he second chief mechanism in crystallization is called secondary nucleation. here, crystal growth is initiated with contact. he formation of nuclei is attributable to fluid shear and to collisions between eisting crystals with each other or with the walls of the crystallizer and rotary impellers or agitator blades. On impact with these moving parts of crystallizer, soft or weak crystals can break into fragments and so give new crystals. Attrition is the only source of new crystals that is independent of super saturation. No complete theory is available to model secondary nucleation, so its behavior can be anticipated by eperimentation. It is the most common type of nucleation in industrial crystallizers and it is influenced by the intensity of agitation. Growth of crystals is optimum at low super saturation. he energy at which a crystal must be struck is very low and no visible effect is observable on the crystal surface.
8 Crystal growth is a diffusion process; solute molecules reach the growing surface by diffusion through the liquid phase and are organized into space lattice. Growth rate of most crystals is linear with super saturation. he rate of deposition is proportional to driving force between the bulk of the liquor and that wetting the surface of the crystal that is approimately saturated with respect to crystals of that size. he driving force will vary because of the increasing solubility for crystals with lower size range. Crystal growth takes place in metastable zone that lies between saturation and nucleation limits. In this region the solution is supersaturated and no nucleation occurs when crystals are growing. o tailor the growth process according to requirement, growth parameters such as temperature, ph, concentration and additive levels need to be optimized. Nowadays in situ visualization of growth by Atomic force microscopy (AF) help to optimize growth conditions in producing crystals with desired characteristics. iers super saturation theory In the year 1927, iers, SIR, H.A. postulated a theory on super saturation. iers theory eplains growth of nuclei with respect to super solubility and solubility curve under some limitations. Let us know first about solubility curve. When equilibrium is attained at final temperature, mother liquor becomes saturated in crystallization process and rate of formation of nucleus is balanced by the rate of dissolution of nucleus. he equilibrium relationship is the solubility curve. Solubility data for different solids had been epressed as function of temperature. Solubility chart displays various types of profiles like curves with positive and high slope (KNO 3 ), positive and very low slope (NaCl), negative slope (nso 4 H 2 O). For most of the materials, solubility curves follow firstly i.e. high and positive slope. Solubility curve represents the maimum concentration of solutions that can be obtained by bringing solute into equilibrium with solvent. his curve represents final concentration of mother liquor toward which supersaturated solution approaches. Super saturation is attained by decreasing temperature of highly concentrated solution or decreasing amount of solvent by evaporation or by both. iers super saturation theory is eplained with the help of following figure. Fig. 1: iers solubility curve AB super solubility curve, CD solubility curve, E Feed location, under saturation, F solution cools to saturation, G etastable zone, nucleation begins, H Concentration
9 of mother liquor decreases with crystal growth, I Crystal growth during cooling and decrease of concentration of solution, J Eit location, saturated solution Let us consider a concentrated solution that is free of any kind of solute either solute of crystalline material or any foreign substance. Point E represents certain composition and temperature. Now, this solution is cooled gradually in the direction as shown in the figure along EFG. It first crosses the solubility curve at F and it seems that crystallization would begin immediately. But it begins only when it is super cooled sufficiently past the curve CD, somewhere near the point G. When crystal formation begins the concentration of the substance in the solution falls and the curve follows roughly along GHIJ. he curve AB represents super solubility curve. Any short of this line, nuclei formation and its further growth cannot occur in pure solution. he theory eplains that formation of nuclei is possible under good etent of super saturation and for its subsequent growth necessary requirement is that concentration of solution should eceed normal solubility. he eact mechanism of arrangement of randomly moving molecules into a regular crystal lattice is not clearly understood. Both the molecules of solute and solvent move in their various molecular paths, when a group of molecules come in close proimity due to accidental collisions, form a loosely held cluster by mutual attraction. Stability of these clusters depends on high level of super saturation. As soon as crystal grows into appreciable size, it continues to grow due to continuous deposition of solute on the surface of crystal. hese crystals have lesser tendency to dissolve in the solution due to its bigger size. he super solubility line according to iers theory is not restricted to a definite curve like AB, but rather it is confined to an area or zone. So, formation of nucleus begins around the curve within the super solubility zone. iers theory is based on the postulation that the solution consists of molecules of pure solvent and solute, in which no seed particle or any foreign particle is present, so formation of nucleus depends solely on accidental combination of the solute molecules that form permanent crystal. Formation of nuclei in the large volume of supersaturated solution is very rapid than small volume liquor. In large volume liquid chance of collision is more. Nucleus formation is rather delayed in absence of any particulate matter. In commercial practice, solutions are eposed to the air and dust particles enter the solution and act as nuclei even if seed particles of same substance are not added. In order to justify the iers super solubility curve it is necessary to deal with pure solutions completely free of any type of particle of solid matter. he following are the limitations of iers theory: 1. ime be long enough 2. he volume of the solution be large enough 3. Presence of solute / foreign particle. If any one of the above three is maintained in crystallization then eistence of super solubility curve according to iers theory is no longer possible and solution does not need super cooling for crystal formation. Practically greater the degree of super saturation, the greater is the probability of nucleus formation and more rapid is the growth of nucleus, whether it is spontaneously formed nucleus or an accidental one.
10 Super saturation method 1. Super saturation by cooling 2. Super saturation by evaporation of solvent. 3. Super saturation by adiabatic evaporation (cooling plus evaporation) 4. Salting out by adding a substance that reduces the solubility of the substance of interest. In case of solubility with strong temperature dependencies the cooling method is more attractive whereas with low dependencies on temperature the evaporation method is adopted. Fig. 2: Solubility curves of salts When solubility curve decreases appreciably with temperature i.e., rate of decrease of solubility with the decrease of temperature is high, so curve is steeper. KCl, AgNO 3, CuSO 4, 5H 2 O and Na 2 HPO 4 H 2 O are the eample of crystals that follow ethod 1. Substances like NaCl, Na 2 SO 4, and CaCl 2 follow method 2. Solubility curves of these substances are flat type i.e. solubility does not decrease appreciably with the decrease of temperature. In this case, yield will be very poor if method 1 is followed. he third method, cooling adiabatically under a vacuum is the most important method for large-scale production. If a hot solution is evaporated under a vacuum, the solvent flashes because total pressure is less than the vapor pressure of the solvent at the temperature at which it is introduced. Now, the solution becomes supersaturated due to evaporation of solvent and adiabatic cooling. he last method is not very much used. In this method, a foreign substance is deliberately added to reduce the solubility to such an etent that the desired solute crystallizes. Crystallization Equipments here are different types of equipments used for making crystals. he design of these equipments is based on the method of super saturation.
11 Names of equipments: 1. Super saturation by cooling alone ank crystallizer, Agitated batch crystallizer. hese two are used in batch process, though tank crystallizer is obsolete one. Swenson Walker crystallizer falls under this category and it is used in continuous process. 2. Super saturation by adiabatic cooling: Different kinds of vacuum crystallizers are used to make crystals by this method. 3. Super saturation by evaporation: Krystal evaporator and salting evaporator are two eamples under this type of super saturation. All these equipments are made of stainless steel. Description of some crystallizers: (i)agitated batch crystallizer: he crystallizer body is equipped with a centrally located agitator, cooling pipes. he upper part of the vessel is cylindrical and closed at the top. he lower part of the vessel is conical and its bottommost part is used to drain out final magma. agma is defined as the slurry containing product crystal and saturated mother liquor. Hot concentrated solution of a substance is induced in the crystallizer and it is agitated with the impeller. Cold water flows though the cooling coils to transfer heat from the hot solution. Cold water transfers heat due to heat of crystallization too. As temperature drops, super saturation is achieved that initiates crystallization. Agitator facilities heat transfer operation throughout the solution and maintains uniformity of temperature in the solution and keeps the growing crystals in suspension so that these can grow uniformly. hus agitator prevents formation of aggregates. A Impellor, CC- cooling coil Fig. 3: Agitated batch crystallizer Rate of production of crystals is low due to batch process. Since temperature at the surface of cooling coil is least so rapidly formed crystals get deposited on the surface of cooling coil and cause hindrance in heat transfer rate. hese are the disadvantages of agitated batch crystallizer. After certain period of growth, magma is transferred to centrifuge where crystals are separated from mother liquor.
12 he Swenson Walker crystallizer: It consists of an open semi cylindrical trough, bottom part of which is welded with a jacket to run cold water through the jacket in counter current direction to that of solution. It is equipped with a slow speed long pitched spiral agitator moving at 7 r.p.m., that is fitted as close to the bottom of the trough as possible i.e. a narrow clearance is maintained. A single unit (trough) is 24 inch wide and 10 feet long. Length of crystallizer may be increased up to 40 feet by joining 4 units. If more units are required, these can be arranged one above another and solution fall from the upper row to the net lower one. Fig. 4: Swenson Walker Crystallizer: F-feed, J- Jacket, CI-coldwater inlet, CO-coldwater outlet, SA-spiral agitator, P- product. he hot concentrated solution is fed at one end of the trough and flows to the other end slowly. When solution is cooled to desired degree of super saturation, crystals start forming. At the end of the crystallizer, crystals along with mother liquor overflow to a draining table that separates mother liquor from crystals. other liquor is returned to the process and wet crystals are conveyed to a centrifuge. he spiral agitator functions by conveying crystals in the forward direction and it keeps particles in suspension and prevents accumulation of crystals at the bottom of trough. his agitator lifts the deposited crystals from the bottom and spreads throughout the solution and thus keeps these crystals in suspension facilitating uniform growth of crystals. Vacuum Crystallizer: his falls in the category of modern type crystallizers. It is closed vessel, at its top vacuum is maintained by a condenser with the help of a steam jet vacuum pump or booster. Large quantity of material can be processed in small floor space and labor cost is also saved in this type of crystallizer.
13 Vacuum crystallizer consists of a cone-bottomed vessel, which is fitted with a barometric condenser at the top and propellers in the conical part, elutriation leg at the bottom of the vessel. Hot saturated solution (feed) well above the boiling point at the pressure in the crystallizer is introduced into the main crystallizer body via a slow speed low head circulating pump and a heater, through a tangentially cut inlet pipe that causes a swirling motion within solution. Liquid level in the crystallizer body is maintained up to certain mark. At the top of liquid, vacuum is maintained that corresponds to be boiling point of the solution lower than the feed temperature. he solution so introduced into a vessel in which a vacuum is maintained, the solution flashes and solvent, evaporates due to adiabatic evaporation. As a result solution gets cooled spontaneously to equilibrium temperature; since it is adiabatically cooled i.e. both the enthalpy of cooling and the enthalpy of crystallization provide enthalpy of vaporization. Super saturation is generated due to both cooling and evaporation. emperature gradient and concentration gradient thus formed due cooling and evaporation are two driving potential to create nucleation. Vapors are formed in the empty space above the solution and discharged through the top outlet. It is evident that the vapor from the crystallizer cannot be condensed at low pressure with the usual cooling water temperatures available. his vapor is compressed and then condensed by ordinary cooling water. he crystals so formed in the body due to super saturation, fall through the conical bottom of vessel into elutriation leg that helps to Fig. 5: Vacuum Crystallizer: B- barometric condenser, D- Downpipe, F- feed S-steam inlet, SC- steam condensate, H-heater, P- Pump, C-centrifuge, - mother liquor, P-product crystal. classify and separate bigger size particles. Now, product magma (crystal along with mother liquor) is discharged through outlet and fed to centrifuge, part of mother liquor is re-circulated. Function of tangential inlet of the pipe is that it creates swirling motion and thus facilitates flashing of solvent and equilibrates magma within the vapor. he simple vacuum crystallizers are not equipped with propellers. So, the effect of static head due to solution creates problem in generating adequate nucleation. So, both the evaporation and
14 cooling are confined to the surface zone. As a result concentration and temperature gradients are formed near the surface. herefore, super saturation prevails near the surface and crystals tend to settles to the bottom region with little or no super saturation. his problem can be overcome if a stirrer is fitted the centrally. Function of propeller is to prevent the tendency of the feed to be short circuited to the discharge pipe without being flashed. It helps to maintain uniformity of temperature and concentration of solution. Draft tube baffle (DB) crystallizer: Draft tube baffle crystallizer is based on the same principle as that of vacuum crystallizer but it is more versatile and effective equipment. he main crystallizer body is equipped with a condenser at the top, elutriation leg at the conical bottom, a recycling loop at the side of the body, a centrally located propeller, a draft tube around the propeller and an annular space within the crystallizer body. A pump assembly is used to maintain circulation of fluid. Hot solution enters the main crystallizer body. he solution is super saturated adiabatically under the vacuum maintained at the top, due to evaporation of solvent and decrease of temperature of solution. Draft tube acts as battle. It controls circulation by promoting aial flow of fluid thus facilitating maintenance of temperature and concentration gradient throughout the solution and allowing fine particles to flow in the upstream. In this crystallizer, all the fine particles Fig.6 : Draft tube baffle crystallizer: BC- barometric condenser, CL-clear liquor, F-feed, CP- circulating pump, EP- elutriation pump, S-steam, SC-steam condensate, H-heater, E-elutriation leg, SZ- settling zone, D- draft tube, B-baffle, P-propeller. are not grown simultaneously. Some fraction of fine particles is removed in the upstream. When fluid enters the annular zone, bigger particles are settled here due to baffling effect and very finer particles float in the upstream and thus unwanted nuclei are removed through the recycle loop. In this way magma density is sharply increased within the body. A part of this recycled liquid is mied with the fresh feed and is pumped to the main crystallizer body via a heater and another part of liquid is introduced into the elutriation leg via an elutriation pump. Elutriation leg facilitates in classification of crystals according to size while flowing in the upward direction through it. Upward stream carries
15 away some smaller particles into the main crystallizer body for further growth. Product is discharged through the outlet and fed to the centrifuge to separate out crystals from mother liquor. his type of crystallizer produces crystals with narrow crystal size distribution. Draft ube Baffle Crystallizer is an elaborated ied Suspension ied Product Removal (SPR) design, which has proven to be well suited for vacuum cooling and for processes ehibiting a moderate evaporation rate. SPR is an idealized crystallizer model that follows some stringent requirements. he DB crystallizer has proven to be suitable for many products such as boric acid, Glauber salt, citric acid. he Krystal Crystallizer: he Krystal or Oslo crystallizer is etensively used where large quantities of crystals of controlled size are required. he basic principle is to flow a supersaturated solution to bed of crystals which are maintained in a fluidised state. he Krystal crystallizer consists of a vapor head (A) that is attached with a condenser at the top and connected with a long specially designed discharge tube (E) etended well to the bottom of crystal growth chamber B. Feed solution is usually introduced into the suction of pump C and then it is sent to vapor head through heater. Vapor formed at the top is released and discharged into condenser and vacuum pump. he hot solutions in the chamber A become supersaturated due to evaporation but crystals are not formed in this chamber. Super saturation produced in chamber A is discharged on the crystals suspended in an upward flowing stream of liquid at the bottom of Krystal growth chamber. When the liquid becomes saturated at equilibrium, it leaves the chamber through F for recirculation. Coarser particles remain at the bottom and finer particles remain at the top of crystal bed and re-circulated liquid carries away finest particles and enters the vapor chamber via the heater and pump. Coarse crystals are discharged through the bottom (G) at intervals. Fig.7: Krystal crystallizer: A- vapor head, B-crystal growth chamber, C- circulating pump, D- heater or cooler, E- discharge tube, F- overflow to circulating pump, G- product outlet. A typical Oslo crystallizer for the production of gso 4, 7H 2 O is fed with saturated solution at 393 o K and the temperature of the plant is 313 o K with a feed rate of 2000 kg/h and a circulation rate of kg/h, the circulating liquid becomes heated to 316 o K.
16 ass balance in crystallization ass balance in any operation acts as a monitor that facilitates quantification of converted products. In crystallization yield of product is calculated by mass balance let us consider an eample of hydrated magnesium sulphate (gso 4, 7H 2 O). Fig. 8: Solubility curve (ACF) of gso 4, X-mass fraction In solubility diagram (Fig.8) various hydrated forms of magnesium sulphate are displayed at various zones under the curve i.e. gso 4, 12H 2 O; gso 4, 7H 2 O; gso 4, 6H 2 O, gso 4, H 2 O. hese zones are in relation with concentration and temperature of magnesium sulphate. Let us consider a point A (0.3, 120 o ) that represents hot unsaturated solution. It is cooled to 60 o F (B) to achieve super saturation and kept at 60 o F for long period to form crystal. Now, magma is split to crystal and mother liquor (saturated liquor at 60 o F). Co ordinate of crystal or mass fraction ( c ) is obtained at D, where BD intersects vertical line. Co ordinate of mother liquor is obtained at C where CB intersects solubility curve. CD is isothermal line (60 o F). Crystal, C agma (slurry) other liquor, L = L + C Considering crystallization by cooling method without any loss due to evaporation, we may write material balance equation for anhydrous magnesium sulphate
17 = L L + C C Or, ( L + C ) = L L + C C Or, L C = C Now, adding 1 on both sides we get C = C L L L or, C = C L L (1) F, L, C are mass fractions of anhydrous magnesium sulphate in feed, mother liquor and crystal. c can be obtained by the following ratio or from solubility chart. olecular weight of gso4 c = = olecular weight of gso.7h O 4 2 Above equations help to calculate yield of crystal, when mass fraction are known. here is easy way to determine any ratio of masses ( L / C, L /, C / ) i.e. by the center of gravity principle. Let us consider now, crystallization by adiabatic evaporative or adiabatic cooling method where loss of solvent due to evaporation is accounted in mass balance. ass of feed ( F ) is not same as mass of magma or slurry ( ). able 1 Item ass, lb ass fraction Enthalpy, Bu/lb Feed, F F F h F Evaporated solvent, V V y H V Slurry, h other liquor,l L L h L Crystal, C C c h c Following are the mass balance equations for solution and anhydrous substance. V L F C F = V + ; = L + C and F F = V.y + ; = L L + C C
18 By algebraic method, above equations is solved and ratio of different masses can be epressed as before: V F = adding 1 on both sides, we get y F = F F y, y...(2) Ratio of masses can be epressed with the help of enthalpy of different items. and F V =.(3) h h F F H H h h V h H V F F = = (4) V h H V h h Fig.9: Enthalpy concentration diagram he above epressions (equation 2, 3 and 4) can be written easily with the help of center of gravity principle. In the Fig.10 (Enthalpy concentration diagram), V, F and are in equilibrium so these three points are on the same straight line. It is true for the L, and C. able 2 Items V,F, ass fraction and mass Numerator L.H.S. Denominator L.H.S. Ratio Numerator R.H.S. Denominator R.H.S. y, F, F F / y F y V, F,
19 Fig.10: Enthalpy concentration diagram When numerator of L.H.S. involves mass of feed ( F ) then numerator of R.H.S. is equal to difference of mass fractions of slurry ( ) and vapor (y). Similarly, when denominator of L.H.S. involves mass of slurry ( ) then denominator of R.H.S. is equal to difference mass fractions of feed ( F ) and vapor (y), (see equation 2). By knowing required data from enthalpy concentration chart as for eample Fig.9, any unknown item can be calculated. here is another equation based on mass balance, for the estimation of yield of crystals produced by cooling method. y = RW 1 [C 1 C 2 (1 E)] / [1 C 2 (R 1)] y yield of crystal, R = (molecular weight of hydrate / molecular weight of anhydrous salt). E = (mass of solvent evaporated / mass of solvent in the initial solution) W 1 mass of initial solvent C 1,C 2 Initial and final concentration of solution in terms of mass of anhydrous salt per unit mass of solvent. Energy balances Energy balances are used to calculate the cooling requirements. In Swenson Walker crystallizer-cooling method is used and no evaporation occurs. Solution is split into solid crystal and mother liquor. If quantities and corresponding enthalpies are known then heat balance can be carried out. F h F = L h L + c h c + q F, L, C is the mass flow rate (lb/hr) of feed solution, mother liquor and crystal. h F, h L and h c are their respective enthalpies in BU/lb, q is the amount of heat withdrawn by cooling. q = ass of water (cooling agent) specific heat temperature difference + c h c q = c P t + c h c, here h c is called heat of crystallization, it is latent heat evolved when solid crystals are formed from a solution. It is eothermic and numerically it is
20 equal to heat of solution i.e. heat absorbed by crystals dissolving in a saturated solution. h c is negative of heat of solution for particular compound. q can be calculated from heat transfer equation q = U A t lm. U is overall heat transfer coefficient and t is the log means temperature difference. mc p t + c h c = UA t lm A is area of heat transfer. he amount of heat to be removed by cooling could be visualized as the heat required to cool the feed solution from the initial to final temperature without any solid phase precipitating out, plus the heat liberated due to heat of crystallization. Enthalpy data can be obtained from enthalpy concentration chart. Enthalpy concentration diagram illustrates various enthalpy concentration zones according to concentration, temperature of solution. It displays isothermal lines, isothermal triangles, unsaturated zone, and solubility curve. Problem: 1. A solution of 1000 kg of Na 2 SO 4 in 5000 kg of water is cooled from 333 to 283 o K in an agitated mild steel vessel of mass 1500 kg, the specific heat of steel being 0.5 KJ/kg deg k. At 283 o K the stable crystalline phase is Na 2 SO H 2 O and at 291 o K the heat of solution is 78.5 J/k mol. he mean heat capacity of the solution is 3.6 kj/kg deg K. If during cooling, 2% by mass of water is lost by evaporation, estimate the yield of crystals formed and the heat to be removed. he solubility of anhydrous salt at 283 o K is 8.9 kg/100 kg water. Solution: he heat of crystallization = heat of solution = kj/kmol = = 244 kj/kg 322 he latent heat of vaporization of water = 2395 kj/kg 1000 F = 6000 kg, F = % water is evaporated = 100 kg ( v ); herefore, 100 kg of water is evaporated. F = + V = 5900 Kg = weight of slurry X C = = ; XF = ; X = = ; y = 0; X L = C L = Q =, C L
21 herefore C = ( L ) / ( C L ) C = kg Heat of crystallization = = kj he heat to be removed from the solution = F mean heat capacity temperature drop = ( ) = kj Heat removed from the vessel = ( ) = kj he heat lost by evaporation = = kj and therefore, the heat to be removed = 10, = kj = J (appro.) Yield of crystal can also be calculated by using the following equation. Y = RW 1 [C 1 C 2 (1 E)] / [1 C 2 (R 1)] [R =, C1 = = 0.2 kg/kg of water, C2 = kg/kg of water at 283 o K, W 1 = 5000 kg water, E = 0.02 kg/kg water] 322 Y = 5000 [ (1 0.02)] / [ (2.27 1)] 142 = kg 2. An aqueous solution of NaNO 3 (.W.85) is fed to a continuous crystallizer at a rate of 5000 kg/hr. he solution is cooled from 90 to 40 o C. he cooling agent is water. Loss of water from feed due to evaporation in the crystallizer is 3% of the feed solution. Determine the quantity of heat (kilo watt) that must be withdrawn by the cooling agent. Data: ass fraction of NaNO 3 in feed ( F ), mother ( L ) liquor and crystal ( c ) are , and respectively. Heat capacity of feed solution (c p ) = J/kg o K Latent heat (λ) of vaporization of water 2345 kj/kg Heat of crystallization of NaNO 3 (h c ) = kj/k mole. Solution: Basis 1 hr. Amount of crystal produced = c By mass balance we get, = ( c ) L + c c.(1)
22 is the mass of slurry. = F V V is the mass of vapor evaporated. v = = 150 kg, therefore, = 4850 kg. Amount of NaNO 3 in feed = F F = = kg kg feed = kg (NaNO 3 ) kg (water) F F = = F V or, = Amount of NaNO From equation (1) we get, c = kg Heat withdrawn by cold water, q = F c p t + c h c V λ = (90 40) in solution = Joule / hr = J/S = kw. herefore, heat withdrawn from the crystallizer by the cooling is equal to kw (Appro). Factors controlling crystallisation (i)concentration gradient and emperature gradient: he rate of growth of a crystal in a solution is dependent on the temperature and concentration of the liquid at the crystal face. hese conditions are not generally the same as those in the bulk of the solution because concentration gradient and temperature gradient are necessary conditions for growth and dissipation of the heat of crystallization. Heat of crystallization is eothermic. For crystallization from a solution, very less super cooling of within 1 2 o K is possible in comparison to crystallisation from melt and so it involves much mass transfer rather than heat transfer since the resistance to heat and mass transfer lies predominantly in the laminar sub-layer close to the surface of the crystal, the rate of growth of the crystal is improved by increasing the relative velocity between the solid and the liquid. Rate of growth initially rises very rapidly in some cases with the increase of r.p.m. of impellor by overcoming the resistance in the solution but rate ceases after certain r.p.m. he temperature at the interface (i) is greater than that of the bulk of the liquid ( L ) otherwise there would not be any heat transfer. he higher the heat transfer coefficient, the smaller is the required temperature difference i L and so i approaches i when solution is agitated at high r.p.m. Noyes and Whiney [Noyes, A.A. and Whitney, W.R. J. Am. Chem. Soc. 19(1897) 930), the rate of solution of solid substances in their own solutions] assumed that the diffusion of material to the crystal face is controlled almost entirely by the resistance of the laminar
23 dm DA layer near the face, (C C ) dt = A B b dm is rate of growth where m is the mass of dt material deposited on the surface and t is time D is diffusivity, A is area of crystal surface, b is thickness of laminar layer, C A is the concentration in the bulk of liquid and C B is the concentration at equilibrium. In many instances, presence of soluble impurities affect both rate of nucleation and rate of crystal growth, substances like glue, tannin, detrin prevents nucleation and growth of calcium carbonate crystal in boiler in order to reduce scaling on the wall. (ii)effect of emperature on solubility: he rate of crystallization is a function of the degree of super saturation. here are different principles of super saturation. Accordingly methods as well as equipments have been designed and classified. Particular method of super saturation adopted for a specific substance depends on the effect of temperature on the solubility of that substance. In general, solubility increases with the increase of temperature. Fig.2 shows profiles of solubility of different substances at various temperatures KClO 3 has a large positive temperature coefficient, so spontaneous crystallization is possible by cooling saturated solution. In contrary, sodium chloride has a small coefficient; very little crystals are obtained by cooling method. Sodium hydrogen phosphate shows discontinuous profile due to changes in stable crystal form. At temperature less than 50 o C ferrous sulphate solution yields FeSO 4, 7H 2 O; whereas solution gives FeSO 4 4H 2 O in the range (65 50 o C). Anhydrous salt at temperature higher than 338 o K and temperature coefficient is negative in this case. Caking of crystals Increasingly there has been a trend towards improving quality of bulk solids in the industry in order to maintain a competitive advantage. It is often found that lumps/ aggregates are formed in the bulk products during its storage period or its transport from chemical / pharmaceutical / food industry tries due to effects of environmental conditions. his phenomenon is called caking. Caking of crystals caused by migration of moisture, create problems to such an etent that quality and handling properties are compromised. he caking tendency of crystalline materials arises due to liquid layer formation and evaporation of moisture from thin liquid layer (saturated with the crystal material) around the crystal surface when eposed to environmental condition that varies during storage and this fact leads to bonding together of crystals at points of contacts. If a crystal is brought into contact with air containing high amount of moisture (i.e. above the critical humidity) that would be in equilibrium with its saturated solution then the crystal will become damp. When these crystals are eposed to air with humidity less than critical humidity, then moisture present within saturated liquid layer in the interstices evaporates and it results locking of crystal particles with each other and formation of dry aggregates or cakes. For instance, vapor pressure of water at 70 o F is mm and vapor pressure of saturated sodium chloride solution at 70 o F is mm. Now, critical humidity of this sodium chloride solution is [(14.63/18.76) 100 = 78%] 78 percent, if NaCl crystal is eposed to air over 78% relative humidity for long period, salt will become damp and dissolve. So critical humidity of a solid salt is defined as the relative humidity above which it will always become damp and below which it will always stay
CRISTALIZATION UNIT YUSRON SUGIARTO
 CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
CRISTALIZATION UNIT YUSRON SUGIARTO COURSE OUTCOMES CO DESCRIBE the basic principles and applications of crystallization process. CALCULATE the yields, material and energy balance in crystallization. OUTLINES
PHEN 612 SPRING 2008 WEEK 13 LAURENT SIMON
 PHEN 612 SPRING 2008 WEEK 13 LAURENT SIMON Crystallization Crystallization is a common separation process in Commodity inorganic industry (e.g., salts) Food industry (e.g., sugars) Pharmaceutical manufacturing
PHEN 612 SPRING 2008 WEEK 13 LAURENT SIMON Crystallization Crystallization is a common separation process in Commodity inorganic industry (e.g., salts) Food industry (e.g., sugars) Pharmaceutical manufacturing
Chapter 4. Crystallization
 Chapter 4 Crystallization Topic Crystallization Principle of Crystallization Purity and Yields Solubility and Solubility Curves ass and nergy Balances in Crystallization earning utcome t is expected that
Chapter 4 Crystallization Topic Crystallization Principle of Crystallization Purity and Yields Solubility and Solubility Curves ass and nergy Balances in Crystallization earning utcome t is expected that
Physical pharmacy. dr basam al zayady
 Physical pharmacy Lec 5 dr basam al zayady Liquefaction of Gases: When a gas is cooled, it loses some of its kinetic energy in the form of heat, and the velocity of the molecules decreases. If pressure
Physical pharmacy Lec 5 dr basam al zayady Liquefaction of Gases: When a gas is cooled, it loses some of its kinetic energy in the form of heat, and the velocity of the molecules decreases. If pressure
Seader & Henley, Separation Process Principles f01_11
 Seader & Henley, Separation Process Principles f01_11 Crystallization (12.11, p.817) Solid-liquid separation where solid particles are formed from a homogenous liquid phase Ice crystals in freezing water
Seader & Henley, Separation Process Principles f01_11 Crystallization (12.11, p.817) Solid-liquid separation where solid particles are formed from a homogenous liquid phase Ice crystals in freezing water
A Study on Crystallization of Oxalic Acid in Batch Cooling Crystallizer
 C. SRINIVASAKANNAN et al., A Study on Crystallization of Oxalic Acid, Chem. Biochem. Eng. Q. 16 (3) 125 129 (2002) 125 A Study on Crystallization of Oxalic Acid in Batch Cooling Crystallizer C. Srinivasakannan*,
C. SRINIVASAKANNAN et al., A Study on Crystallization of Oxalic Acid, Chem. Biochem. Eng. Q. 16 (3) 125 129 (2002) 125 A Study on Crystallization of Oxalic Acid in Batch Cooling Crystallizer C. Srinivasakannan*,
E24 PURIFICATION OF ORGANIC COMPOUNDS Distillation, recrystallisation, melting and boiling point determination
 E24 PURIFICATION OF ORGANIC COMPOUNDS Distillation, recrystallisation, melting and boiling point determination THE TASK To learn the main techniques of purifying organic compounds. THE SKILLS By the end
E24 PURIFICATION OF ORGANIC COMPOUNDS Distillation, recrystallisation, melting and boiling point determination THE TASK To learn the main techniques of purifying organic compounds. THE SKILLS By the end
CRYSTALLIZATION SOLUBILITY CURVE
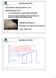 CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
CRYSTALLIZATION saturated solution cooled/evaporated forms crystal important industrially because: i) a crystal formed from an impure solution and itself pure ii) Practical method of obtaining pure chemical
Precipitation Crystallisation of Poorly Soluble Materials
 Precipitation Crystallisation of Poorly Soluble Materials Precipitation of Poorly Soluble Materials Kali Umwelttechnik GmbH (K-UTEC) Am Petersenschacht 7 99706 Sondershausen Germany Phone: ++ 49 3632 610-0
Precipitation Crystallisation of Poorly Soluble Materials Precipitation of Poorly Soluble Materials Kali Umwelttechnik GmbH (K-UTEC) Am Petersenschacht 7 99706 Sondershausen Germany Phone: ++ 49 3632 610-0
Developing Pharmaceutical Continuous Crystallization Processes - Knowledge & Gaps. Chris Price on behalf of the IMI team Product Development
 Developing Pharmaceutical Continuous Crystallization Processes - Knowledge & Gaps Chris Price on behalf of the IMI team Product Development Context - Innovative Manufacturing Initiative Move from Batch,
Developing Pharmaceutical Continuous Crystallization Processes - Knowledge & Gaps Chris Price on behalf of the IMI team Product Development Context - Innovative Manufacturing Initiative Move from Batch,
Eutectic freeze crystallization: Application to process streams and waste water purification
 Chemical Engineering and Processing 37 (1998) 207 213 Eutectic freeze crystallization: Application to process streams and waste water purification F. van der Ham *, G.J. Witkamp, J. de Graauw, G.M. van
Chemical Engineering and Processing 37 (1998) 207 213 Eutectic freeze crystallization: Application to process streams and waste water purification F. van der Ham *, G.J. Witkamp, J. de Graauw, G.M. van
Lecture 35. NPK Fertilizers Nitrophosphate Route
 Lecture 35 NPK Fertilizers Nitrophosphate Route Phosphate sources must be converted into a form which can be taken up by plants ( available ). This can be achieved by using the integrated Nitrophosphate
Lecture 35 NPK Fertilizers Nitrophosphate Route Phosphate sources must be converted into a form which can be taken up by plants ( available ). This can be achieved by using the integrated Nitrophosphate
R13 SET - 1 '' ''' '' ' '''' Code No: RT31035
 R13 SET - 1 III B. Tech I Semester Regular/Supplementary Examinations, October/November - 2016 THERMAL ENGINEERING II (Mechanical Engineering) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists
R13 SET - 1 III B. Tech I Semester Regular/Supplementary Examinations, October/November - 2016 THERMAL ENGINEERING II (Mechanical Engineering) Time: 3 hours Max. Marks: 70 Note: 1. Question Paper consists
Optimum Recirculation Rates in Phosphoric Acid Production
 Optimum Recirculation Rates in Phosphoric Acid Production Prepared by: Paul S. Waters, P.E. James Byrd Clearwater AIChE June 2013 INTRODUCTION Integral to modern phosphoric acid reaction circuits is the
Optimum Recirculation Rates in Phosphoric Acid Production Prepared by: Paul S. Waters, P.E. James Byrd Clearwater AIChE June 2013 INTRODUCTION Integral to modern phosphoric acid reaction circuits is the
AQA Chemistry A-level
 AQA Chemistry A-level Required Practical 10 Preparation of a pure organic solid, test of its purity, and preparation of a pure organic liquid Reflux Reflux: continuous boiling and condensing of a reaction
AQA Chemistry A-level Required Practical 10 Preparation of a pure organic solid, test of its purity, and preparation of a pure organic liquid Reflux Reflux: continuous boiling and condensing of a reaction
MIXING IMPACT ON ANTISOLVENT CRYSTALLIZATIONS. Dr. Wayne Genck Genck International
 MIXING IMPACT ON ANTISOLVENT CRYSTALLIZATIONS Dr. Wayne Genck Genck International Agitator Parameters Table 1. Agitator Parameters Agitator Type N P N Q (Q/P) R PBT Hydrofoil Rushton GL Retreat Blade*
MIXING IMPACT ON ANTISOLVENT CRYSTALLIZATIONS Dr. Wayne Genck Genck International Agitator Parameters Table 1. Agitator Parameters Agitator Type N P N Q (Q/P) R PBT Hydrofoil Rushton GL Retreat Blade*
Lecture 23. Nitrophosphate Fertilizers Part 1
 Lecture 23 Nitrophosphate Fertilizers Part 1 Introduction Nitrophosphate is the generally accepted term for any fertilizer that is produced by a process involving treatment of phosphate rock with nitric
Lecture 23 Nitrophosphate Fertilizers Part 1 Introduction Nitrophosphate is the generally accepted term for any fertilizer that is produced by a process involving treatment of phosphate rock with nitric
THE QUALITY OF SUGAR CRYSTALS AND THEIR STORAGE STABILITY
 Conference on Technical progress in the sugar industry organized by Polish Sugar Technicians Association on 12 and 13 May 2014 in Zakopane. THE QUALITY OF SUGAR CRYSTALS AND THEIR STORAGE STABILITY Mohamed
Conference on Technical progress in the sugar industry organized by Polish Sugar Technicians Association on 12 and 13 May 2014 in Zakopane. THE QUALITY OF SUGAR CRYSTALS AND THEIR STORAGE STABILITY Mohamed
SHRI RAMSWAROOP MEMORIAL COLLEGE OF ENGG. & MANAGEMENT B.Tech. [SEM IV (ME-41, 42,43 & 44)] QUIZ TEST-1 (Session: )
![SHRI RAMSWAROOP MEMORIAL COLLEGE OF ENGG. & MANAGEMENT B.Tech. [SEM IV (ME-41, 42,43 & 44)] QUIZ TEST-1 (Session: ) SHRI RAMSWAROOP MEMORIAL COLLEGE OF ENGG. & MANAGEMENT B.Tech. [SEM IV (ME-41, 42,43 & 44)] QUIZ TEST-1 (Session: )](/thumbs/72/67555697.jpg) QUIZ TEST-1 Q.1. In a stage of an impulse turbine provided with a single row wheel, the mean diameter of the blade ring is 80cm and the speed of the rotation is 3000rpm. The steam issues from the nozzle
QUIZ TEST-1 Q.1. In a stage of an impulse turbine provided with a single row wheel, the mean diameter of the blade ring is 80cm and the speed of the rotation is 3000rpm. The steam issues from the nozzle
SiC crystal growth from vapor
 SiC crystal growth from vapor Because SiC dissolves in Si and other metals can be grown from melt-solutions: Liquid phase epitaxy (LPE) Solubility of C in liquid Si is 0.029% at 1700oC high T process;
SiC crystal growth from vapor Because SiC dissolves in Si and other metals can be grown from melt-solutions: Liquid phase epitaxy (LPE) Solubility of C in liquid Si is 0.029% at 1700oC high T process;
SOLIDIFICATION, PHASE DIAGRAM & STEELS
 MODULE TWO SOLIDIFICATION, PHASE DIAGRAM & STEELS 4. SOLIDIFICATION Introduction Mechanism of solidification - crystallization and development of cast structure - nucleation and grain growth - dendritic
MODULE TWO SOLIDIFICATION, PHASE DIAGRAM & STEELS 4. SOLIDIFICATION Introduction Mechanism of solidification - crystallization and development of cast structure - nucleation and grain growth - dendritic
Fluid Mechanics, Heat Transfer, and Thermodynamics Fall Design Project. Production of Drying Oil
 Introduction Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2003 Design Project Production of Drying Oil Drying oils are additives to paints and varnishes to aid in the drying process when these
Introduction Fluid Mechanics, Heat Transfer, and Thermodynamics Fall 2003 Design Project Production of Drying Oil Drying oils are additives to paints and varnishes to aid in the drying process when these
Solids SECTION Critical Thinking
 SECTION 10.3 Solids A gas has neither a definite volume nor a definite shape. A liquid has a definite volume, but not a definite shape. A solid, the third state, has a definite volume and a definite shape.
SECTION 10.3 Solids A gas has neither a definite volume nor a definite shape. A liquid has a definite volume, but not a definite shape. A solid, the third state, has a definite volume and a definite shape.
Sanitary and Environmental Engineering I (4 th Year Civil)
 Sanitary and Environmental Engineering I (4 th Year Civil) Prepared by Dr.Khaled Zaher Assistant Professor, Public Works Engineering Department, Faculty of Engineering, Cairo University Wastewater Flow
Sanitary and Environmental Engineering I (4 th Year Civil) Prepared by Dr.Khaled Zaher Assistant Professor, Public Works Engineering Department, Faculty of Engineering, Cairo University Wastewater Flow
Valve Nest Filter with Time Clock Control Installation and Operation Manual
 Valve Nest Filter with Time Clock Control Installation and Operation Manual Effective 5/24/12 Effective 5/24/12 Table of Contents Operating Data. 1 Pre-Installation Data... 2 Filter Parameters Installation
Valve Nest Filter with Time Clock Control Installation and Operation Manual Effective 5/24/12 Effective 5/24/12 Table of Contents Operating Data. 1 Pre-Installation Data... 2 Filter Parameters Installation
The Recrystallization of Benzoic Acid and Determination of its Melting Point
 Doreen Foy Organic Chemistry 1 Laboratory The Recrystallization of Benzoic Acid and Determination of its Melting Point Hannah Loch 10/01/2012 Abstract The goal of the experiment was to obtain pure benzoic
Doreen Foy Organic Chemistry 1 Laboratory The Recrystallization of Benzoic Acid and Determination of its Melting Point Hannah Loch 10/01/2012 Abstract The goal of the experiment was to obtain pure benzoic
Effluent Treatment Using Evaporation and Crystallisation Technology
 Effluent Treatment Using Evaporation and Crystallisation Technology Brecknell C, Lilliott M, Pavlik A Wellman Process Engineering Newfield Road, Oldbury. B69 3ET. UK The authors will demonstrate with the
Effluent Treatment Using Evaporation and Crystallisation Technology Brecknell C, Lilliott M, Pavlik A Wellman Process Engineering Newfield Road, Oldbury. B69 3ET. UK The authors will demonstrate with the
USP s Perspective on Drug Product Performance Test
 USP s Perspective on Drug Product Performance Test Course Overview 1. The concept of in vitro dissolution Definition and application 2. Compendial dissolution/ drug release testing 3. Method development
USP s Perspective on Drug Product Performance Test Course Overview 1. The concept of in vitro dissolution Definition and application 2. Compendial dissolution/ drug release testing 3. Method development
Manufacture of Granulations Part 4. Industrial pharmacy 5 th class 1 st semester
 Manufacture of Granulations Part 4 Industrial pharmacy 5 th class 1 st semester Dry manufacturing methods The manufacture of granulations for tablet compression may follow one or a combination of three
Manufacture of Granulations Part 4 Industrial pharmacy 5 th class 1 st semester Dry manufacturing methods The manufacture of granulations for tablet compression may follow one or a combination of three
 TitleTHE SASAKI LABORATORY Author(s) Sasaki, Nobuji Citation The Commemoration volume for the si 117 Issue Date 1951-02-15 URL http://hdl.handle.net/2433/74792 Right Type Article Textversion publisher
TitleTHE SASAKI LABORATORY Author(s) Sasaki, Nobuji Citation The Commemoration volume for the si 117 Issue Date 1951-02-15 URL http://hdl.handle.net/2433/74792 Right Type Article Textversion publisher
Solubility Curve and Metastable zone Width using Lasentec FBRM & PVM
 Solubility Curve and Metastable zone Width using Lasentec FBRM & PVM Lasentec Users Forum, Charleston, 2002 Paul Barrett, Brian Glennon & Brian O Sullivan (paul.barrett@ucd.ie, brian.glennon@ucd.ie, brian.osullivan@ucd.ie)
Solubility Curve and Metastable zone Width using Lasentec FBRM & PVM Lasentec Users Forum, Charleston, 2002 Paul Barrett, Brian Glennon & Brian O Sullivan (paul.barrett@ucd.ie, brian.glennon@ucd.ie, brian.osullivan@ucd.ie)
 Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY LONG QUESTIONS Analytical Chemistry: The science of chemical characterization is called analytical chemistry. OR The branch of chemistry which deals with
Chapter No. 2 EXPERIMENTAL TECHNIQUES IN CHEMISTRY LONG QUESTIONS Analytical Chemistry: The science of chemical characterization is called analytical chemistry. OR The branch of chemistry which deals with
Keywords: Eutecic freeze crystallization, Solid-liquid equilibrium, Recovery of acetic acid, Heat of sublimation.
 IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY Removal of Organic Acids from Effluent via Freeze Crystallization Tarak C. Padhiyar *1, Prof. Suchen B. Thakore 2 *1,2 L.D. College
IJESRT INTERNATIONAL JOURNAL OF ENGINEERING SCIENCES & RESEARCH TECHNOLOGY Removal of Organic Acids from Effluent via Freeze Crystallization Tarak C. Padhiyar *1, Prof. Suchen B. Thakore 2 *1,2 L.D. College
ERT 318/4 UNIT OPERATIONS SEMESTER 1 (2013/2014)
 ERT 318/4 UNIT OPERATIONS SEMESTER 1 (2013/2014) WATER COOLING TOWER School of Bioprocess Engineering University Malaysia Perlis EXPERIMENT Water Cooling Tower 1.0 OBJECTIVES 1.1 To determine energy and
ERT 318/4 UNIT OPERATIONS SEMESTER 1 (2013/2014) WATER COOLING TOWER School of Bioprocess Engineering University Malaysia Perlis EXPERIMENT Water Cooling Tower 1.0 OBJECTIVES 1.1 To determine energy and
R13. II B. Tech I Semester Regular/Supplementary Examinations, Oct/Nov THERMODYNAMICS (Com. to ME, AE, AME) Time: 3 hours Max.
 SET - 1 1. a) Discuss about PMM I and PMM II b) Explain about Quasi static process. c) Show that the COP of a heat pump is greater than the COP of a refrigerator by unity. d) What is steam quality? What
SET - 1 1. a) Discuss about PMM I and PMM II b) Explain about Quasi static process. c) Show that the COP of a heat pump is greater than the COP of a refrigerator by unity. d) What is steam quality? What
Understanding Reverse Osmosis Mark Rowzee Mark Rowzee
 Understanding Reverse Osmosis Mark Rowzee Mark Rowzee RO produces some of the highest-purity water and is a workhorse technology for drinking water applications. Many people have heard of reverse osmosis
Understanding Reverse Osmosis Mark Rowzee Mark Rowzee RO produces some of the highest-purity water and is a workhorse technology for drinking water applications. Many people have heard of reverse osmosis
TESTS ON AGGREGATES 58
 TESTS ON AGGREGATES 58 59 3.1 DETERMINATION OF INDICES (FLAKINESS AND ELONGATION) STANDARD IS: 2386 (Part 1) 1963. DEFINITION The Flakiness Index of aggregates is the percentage by weight of particles
TESTS ON AGGREGATES 58 59 3.1 DETERMINATION OF INDICES (FLAKINESS AND ELONGATION) STANDARD IS: 2386 (Part 1) 1963. DEFINITION The Flakiness Index of aggregates is the percentage by weight of particles
PHARMACEUTICAL PRINCIPLES OF SOLID DOSAGE FORMS
 PHARMACEUTICAL PRINCIPLES OF SOLID DOSAGE FORMS J. T. Carstensen, Ph.D. Professor of Pharmacy University of Wisconsin Madison, Wisconsin TECHNOMIC ^PUBLISHING CO.. INC J 1.ANCASTER BASET, TABLE OF CONTENTS
PHARMACEUTICAL PRINCIPLES OF SOLID DOSAGE FORMS J. T. Carstensen, Ph.D. Professor of Pharmacy University of Wisconsin Madison, Wisconsin TECHNOMIC ^PUBLISHING CO.. INC J 1.ANCASTER BASET, TABLE OF CONTENTS
Efficient volume reduction and concentration of mining waste water with direct contact evaporation technology
 Efficient volume reduction and concentration of mining waste water with direct contact evaporation technology Steven Panz 1, Wesley Young 2 1. President, Inproheat Industries, Canada 2. Chief Technical
Efficient volume reduction and concentration of mining waste water with direct contact evaporation technology Steven Panz 1, Wesley Young 2 1. President, Inproheat Industries, Canada 2. Chief Technical
CHLOR-ALKALI INDUSTRY
 CHLOR-ALKALI INDUSTRY The chlor-alkali industry represents of three major industrial chemicals: Soda ash (sodium carbonate-na 2 CO 3 ) Caustic soda (sodium hydroxide-naoh) Chlorine (Cl 2 ) These chemicals
CHLOR-ALKALI INDUSTRY The chlor-alkali industry represents of three major industrial chemicals: Soda ash (sodium carbonate-na 2 CO 3 ) Caustic soda (sodium hydroxide-naoh) Chlorine (Cl 2 ) These chemicals
Slurry-Based Semi-Solid Die Casting
 Accepted for Publication in Advanced Materials and Processes, 159(10), October 2001 Slurry-Based Semi-Solid Die Casting Chris S. Rice and Patricio F. Mendez A new approach to semi-solid forming is described.
Accepted for Publication in Advanced Materials and Processes, 159(10), October 2001 Slurry-Based Semi-Solid Die Casting Chris S. Rice and Patricio F. Mendez A new approach to semi-solid forming is described.
1. To improve heat exchange between a gas & a liquid stream in a heat exchanger, it is decided to use fins. Correct the suitable option.
 1. To improve heat exchange between a gas & a liquid stream in a heat exchanger, it is decided to use fins. Correct the suitable option. a) Fins are generally attached on gas side. b) Fins are generally
1. To improve heat exchange between a gas & a liquid stream in a heat exchanger, it is decided to use fins. Correct the suitable option. a) Fins are generally attached on gas side. b) Fins are generally
Boiler Efficiency Testing. To understand the operation of a fire tube boiler To determine the operating efficiency of the boiler
 Boiler Efficiency Testing Objectives: To understand the operation of a fire tube boiler To determine the operating efficiency of the boiler Theory Most industrial processes require heating. This is usually
Boiler Efficiency Testing Objectives: To understand the operation of a fire tube boiler To determine the operating efficiency of the boiler Theory Most industrial processes require heating. This is usually
PRAYON PROCESSES FOR PHOSPHORIC ACID PRODUCTION
 PRAYON PROCESSES FOR PHOSPHORIC ACID PRODUCTION Our ideas make profitable plants www.prayon.com WWW.PRAYON.COM 2 PRAYON PROCESSES TABLE OF CONTENTS PRAYON TECHNOLOGIES: THE REFERENCE IN THE PHOSPHORIC
PRAYON PROCESSES FOR PHOSPHORIC ACID PRODUCTION Our ideas make profitable plants www.prayon.com WWW.PRAYON.COM 2 PRAYON PROCESSES TABLE OF CONTENTS PRAYON TECHNOLOGIES: THE REFERENCE IN THE PHOSPHORIC
Process Chem and Pharmaceutical Engineering 1. Solid Liquid Separation
 For updated version, please click on http://ocw.ump.edu.my Process Chem and Pharmaceutical Engineering 1 Solid Liquid Separation Wan Nurul Huda binti Wan Zainal Faculty of Engineering Technology wannurulhuda@ump.edu.my
For updated version, please click on http://ocw.ump.edu.my Process Chem and Pharmaceutical Engineering 1 Solid Liquid Separation Wan Nurul Huda binti Wan Zainal Faculty of Engineering Technology wannurulhuda@ump.edu.my
LAB II CRYSTAL STRUCTURE AND CRYSTAL GROWTH PART 1: CRYSTAL GROWTH. I. Introduction
 LAB II CRYSTAL STRUCTURE AND CRYSTAL GROWTH This lab will be divided into two parts. In the first part, you will be growing crystals from a seed crystal in a very visual demonstration of heterogeneous
LAB II CRYSTAL STRUCTURE AND CRYSTAL GROWTH This lab will be divided into two parts. In the first part, you will be growing crystals from a seed crystal in a very visual demonstration of heterogeneous
Fluid Mechanics, Heat Transfer, Thermodynamics. Design Project. Production of Ammonia
 Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Ammonia Your assignment is to continue evaluating the details of a process to produce 50,000 tonne/y of ammonia from a syngas
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Ammonia Your assignment is to continue evaluating the details of a process to produce 50,000 tonne/y of ammonia from a syngas
Heat Rejection using Cooling Towers
 MEBS6006 Environmental Services I http://www.hku.hk/bse/mebs6006 Heat Rejection using Cooling Towers Dr. Benjamin P.L. Ho (beplho@yahoo.com.hk) Part-time Lecturer Department of Mechanical Engineering The
MEBS6006 Environmental Services I http://www.hku.hk/bse/mebs6006 Heat Rejection using Cooling Towers Dr. Benjamin P.L. Ho (beplho@yahoo.com.hk) Part-time Lecturer Department of Mechanical Engineering The
Simulation of a Fluidized Bed Spray Granulation Pilot Plant GF/ProCell 25 with the Flowsheet-Simulation Software SolidSim
 Simulation of a Fluidized Bed Spray Granulation Pilot Plant GF/ProCell 25 with the Flowsheet-Simulation Software SolidSim ABSTRACT Fluidized bed spray granulation is commonly used for the production of
Simulation of a Fluidized Bed Spray Granulation Pilot Plant GF/ProCell 25 with the Flowsheet-Simulation Software SolidSim ABSTRACT Fluidized bed spray granulation is commonly used for the production of
COPYRIGHTED MATERIAL. Introduction to Crystallization Issues. Chapter 1
 Chapter 1 Introduction to Crystallization Issues Crystallization has been the most important separation and purification process in the pharmaceutical industry throughout its history. Many parallels exist
Chapter 1 Introduction to Crystallization Issues Crystallization has been the most important separation and purification process in the pharmaceutical industry throughout its history. Many parallels exist
Module: 9 Lecture: 40
 Module: 9 Lecture: 40 AMMONIUM SULFATE INTRODUCTION Ammonium sulfate containing 21% nitrogen is another important nitrogenous fertilizer. It occurs naturally as the mineral mascagnite and offers many advantages
Module: 9 Lecture: 40 AMMONIUM SULFATE INTRODUCTION Ammonium sulfate containing 21% nitrogen is another important nitrogenous fertilizer. It occurs naturally as the mineral mascagnite and offers many advantages
Fluid Mechanics, Heat Transfer, Fluid Mechanics Design Project. Production of Ethanol
 Fluid Mechanics, Heat Transfer, Fluid Mechanics Design Project Production of Ethanol Your assignment is to continue evaluating the details of a process to produce 30,000 tonne/y of ethanol from ethylene.
Fluid Mechanics, Heat Transfer, Fluid Mechanics Design Project Production of Ethanol Your assignment is to continue evaluating the details of a process to produce 30,000 tonne/y of ethanol from ethylene.
Final pilot plant layout
 7.2.27 Final pilot plant layout Summary INTRODUCTION... 7.2-813 PILOT PLANT LAYOUT BASED ON THE RESULTS FROM LAB-SCALE TREATMENT RUNS... 7.2-813 CHANGES IN PILOT PLANT LAYOUT ON ACCOUNT OF THE AVAILABILITY
7.2.27 Final pilot plant layout Summary INTRODUCTION... 7.2-813 PILOT PLANT LAYOUT BASED ON THE RESULTS FROM LAB-SCALE TREATMENT RUNS... 7.2-813 CHANGES IN PILOT PLANT LAYOUT ON ACCOUNT OF THE AVAILABILITY
SIZE REDUCTION. Subject:Pharmaceutical Engineering Course:II B.Pharm I Sem Presented by Mr.C.Kishore M.Pharm(Pharmaceutics)
 SIZE REDUCTION Subject:Pharmaceutical Engineering Course:II B.Pharm I Sem Presented by Mr.C.Kishore M.Pharm(Pharmaceutics) CONTENTS INTRODUCTION CLASSIFICATION TECHNIQUES ROTARY CUTTER MILL MORTAR AND
SIZE REDUCTION Subject:Pharmaceutical Engineering Course:II B.Pharm I Sem Presented by Mr.C.Kishore M.Pharm(Pharmaceutics) CONTENTS INTRODUCTION CLASSIFICATION TECHNIQUES ROTARY CUTTER MILL MORTAR AND
Module: 8 Lecture: 36
 Module: 8 Lecture: 36 TRIPLE SUPERPHOSPHATE INTRODUCTION Triple superphosphate (TSP) is the more concentrated fertilizer than ordinary superphosphate, containing from 44 to 51% of available P2O5 or nearly
Module: 8 Lecture: 36 TRIPLE SUPERPHOSPHATE INTRODUCTION Triple superphosphate (TSP) is the more concentrated fertilizer than ordinary superphosphate, containing from 44 to 51% of available P2O5 or nearly
Electron Emission. The reader is familiar with the current conduction (i.e. flow of electrons) through a conductor. 28 Principles of Electronics
 28 Principles of Electronics 2 Electron Emission 2.1 Electron Emission 2.2 Types of Electron Emission 2.3 Thermionic Emission 2.4 Thermionic Emitter 2.5 Commonly Used Thermionic Emitters 2.6 Cathode Construction
28 Principles of Electronics 2 Electron Emission 2.1 Electron Emission 2.2 Types of Electron Emission 2.3 Thermionic Emission 2.4 Thermionic Emitter 2.5 Commonly Used Thermionic Emitters 2.6 Cathode Construction
Code No: RR Set No. 1
 Code No: RR310303 Set No. 1 III B.Tech I Semester Regular Examinations, November 2006 THERMAL ENGINEERING-II (Mechanical Engineering) Time: 3 hours Max Marks: 80 Answer any FIVE Questions All Questions
Code No: RR310303 Set No. 1 III B.Tech I Semester Regular Examinations, November 2006 THERMAL ENGINEERING-II (Mechanical Engineering) Time: 3 hours Max Marks: 80 Answer any FIVE Questions All Questions
PRAYON PROCESS FOR PHOSPHORIC ACID PRODUCTION
 PRAYON PROCESS FOR PHOSPHORIC ACID PRODUCTION Our ideas make profitable plants www.prayon.com WWW.PRAYON.COM 2 PRAYON PROCESS TABLE OF CONTENTS PRAYON TECHNOLOGIES: THE REFERENCE IN THE PHOSPHORIC ACID
PRAYON PROCESS FOR PHOSPHORIC ACID PRODUCTION Our ideas make profitable plants www.prayon.com WWW.PRAYON.COM 2 PRAYON PROCESS TABLE OF CONTENTS PRAYON TECHNOLOGIES: THE REFERENCE IN THE PHOSPHORIC ACID
Lab 4: Recrystallization
 Lab 4: Recrystallization Pre Lab Question: (Answer submitted in a separate piece of paper at the beginning of lab) 1. Calculate how much 95% ethanol will be required to dissolve 0.8 g of sulfanilamide
Lab 4: Recrystallization Pre Lab Question: (Answer submitted in a separate piece of paper at the beginning of lab) 1. Calculate how much 95% ethanol will be required to dissolve 0.8 g of sulfanilamide
CHAPTER 1 BASIC CONCEPTS
 GTU Paper Analysis CHAPTER 1 BASIC CONCEPTS Sr. No. Questions Jan 15 Jun 15 Dec 15 May 16 Jan 17 Jun 17 Nov 17 May 18 Differentiate between the followings; 1) Intensive properties and extensive properties,
GTU Paper Analysis CHAPTER 1 BASIC CONCEPTS Sr. No. Questions Jan 15 Jun 15 Dec 15 May 16 Jan 17 Jun 17 Nov 17 May 18 Differentiate between the followings; 1) Intensive properties and extensive properties,
PRESENTATION OF CONDENSATE TREATMENT
 Via Pietro Nenni, 15-27058 VOGHERA ITALY Tel. +39 0383 3371 Fax +39 0383 369052 E-mail: info@idreco.com PRESENTATION OF CONDENSATE TREATMENT THE CONDENSATE TREATMENT The absence of impurities in feed water
Via Pietro Nenni, 15-27058 VOGHERA ITALY Tel. +39 0383 3371 Fax +39 0383 369052 E-mail: info@idreco.com PRESENTATION OF CONDENSATE TREATMENT THE CONDENSATE TREATMENT The absence of impurities in feed water
White Paper. Crystallization in Process Chemistry Applying Simple PAT Tools. Author: Des O'Grady PhD, METTLER TOLEDO
 Crystallization in Process Chemistry Applying Simple PAT Tools Author: Des O'Grady PhD, Crystallization is a common step used during the synthesis of organic compounds to isolate and purify the desired
Crystallization in Process Chemistry Applying Simple PAT Tools Author: Des O'Grady PhD, Crystallization is a common step used during the synthesis of organic compounds to isolate and purify the desired
Diffusional Transformations in Solids
 Diffusional Transformations in Solids The majority of phase transformations that occur in the solid state take place by thermally activated atomic movements. The transformations that will be dealt with
Diffusional Transformations in Solids The majority of phase transformations that occur in the solid state take place by thermally activated atomic movements. The transformations that will be dealt with
WHITE PAPER: Amorphous Solid Dispersions-One approach to improving Bioavailability
 2017 WHITE PAPER: Amorphous Solid Dispersions-One approach to improving Bioavailability Eric C Buxton Clinical Associate Professor University of Wisconsin Madison School of Pharmacy, Division of Pharmacy
2017 WHITE PAPER: Amorphous Solid Dispersions-One approach to improving Bioavailability Eric C Buxton Clinical Associate Professor University of Wisconsin Madison School of Pharmacy, Division of Pharmacy
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project. Production of Ethylbenzene
 Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Ethylbenzene We continue to investigate the feasibility of constructing a new, grass-roots, 80,000 tonne/y, ethylbenzene facility.
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Ethylbenzene We continue to investigate the feasibility of constructing a new, grass-roots, 80,000 tonne/y, ethylbenzene facility.
SIMPACK - MODEL DEVELOPMENT PACKAGE FOR POWER PLANTS
 SIMPACK - MODEL DEVELOPMENT PACKAGE FOR POWER PLANTS 1.0 OVERVIEW SIMPACK is a totally integrated set of simulation software development modules for power plants. It is template based modeling tool and
SIMPACK - MODEL DEVELOPMENT PACKAGE FOR POWER PLANTS 1.0 OVERVIEW SIMPACK is a totally integrated set of simulation software development modules for power plants. It is template based modeling tool and
Bio5325 Fall Crystal Vocabulary
 Crystals and Crystallization Bio5325 Fall 2007 Crystal Vocabulary Mosaicity (mosaic spread) Protein crystals are imperfect, consisting of a mosaic of domains that are slightly misaligned. As a result,
Crystals and Crystallization Bio5325 Fall 2007 Crystal Vocabulary Mosaicity (mosaic spread) Protein crystals are imperfect, consisting of a mosaic of domains that are slightly misaligned. As a result,
1. Scaling. H.O.: H-5/21, KRISHNA NAGAR, DELHI Tel.: , Fax:
 Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Heat Treating Basics-Steels
 Heat Treating Basics-Steels Semih Genculu, P.E. Steel is the most important engineering material as it combines strength, ease of fabrication, and a wide range of properties along with relatively low cost.
Heat Treating Basics-Steels Semih Genculu, P.E. Steel is the most important engineering material as it combines strength, ease of fabrication, and a wide range of properties along with relatively low cost.
Sizing Methods Screening Classification
 Sizing Methods There are two methods of industrial sizing. i. Screening ii. Classification Screening is generally carried out on relatively coarse material, as the efficiency decreases rapidly with fineness.
Sizing Methods There are two methods of industrial sizing. i. Screening ii. Classification Screening is generally carried out on relatively coarse material, as the efficiency decreases rapidly with fineness.
Enthalpy Calculations. Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions.
 Enthalpy Calculations Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions. Enthalpy Change as a Result of Temperature Sensible heat is the
Enthalpy Calculations Change in enthalpy can occur because of change in temperature, change in phase, or mixing of solutions and reactions. Enthalpy Change as a Result of Temperature Sensible heat is the
Crystallization Fouling Of The Aqueous Two-Component System CaSO 4 /CaCO 3
 Refereed Proceedings Heat Exchanger Fouling and Cleaning: Fundamentals and Applications Engineering Conferences International Year Crystallization Fouling Of The Aqueous Two-Component System CaSO /CaCO
Refereed Proceedings Heat Exchanger Fouling and Cleaning: Fundamentals and Applications Engineering Conferences International Year Crystallization Fouling Of The Aqueous Two-Component System CaSO /CaCO
THEORY AND PRACTICE OF FILTRATION IN THE ALUMINA INDUSTRY
 THEORY AND PRACTICE OF FILTRATION IN THE ALUMINA INDUTRY Abstract Jürgen Hahn; Reinhard Bott; Thomas Langeloh BOKELA GmbH, 76131 Karlsruhe, Germany Continuous rotary filters like disc, drum or pan filters
THEORY AND PRACTICE OF FILTRATION IN THE ALUMINA INDUTRY Abstract Jürgen Hahn; Reinhard Bott; Thomas Langeloh BOKELA GmbH, 76131 Karlsruhe, Germany Continuous rotary filters like disc, drum or pan filters
Crystallization Methods and Protein Crystal Properties. (adapted from )
 Crystallization Methods and Protein Crystal Properties (adapted from http://www.bioc.rice.edu/~bios482/xtal_ppt/ ) Four major steps in crystallization Obtain large amounts of pure protein samples Choose
Crystallization Methods and Protein Crystal Properties (adapted from http://www.bioc.rice.edu/~bios482/xtal_ppt/ ) Four major steps in crystallization Obtain large amounts of pure protein samples Choose
UNIT I: UNIFORM FLOW PART B
 UNIT I: UNIFORM FLOW PART-A 1 Define open channel flow with example BT-1-1 2 Distinguish between open channel flow and pipe flow. BT-4-1 3 Compute the hydraulic mean depth of a small channel 1m wide, 0.5m
UNIT I: UNIFORM FLOW PART-A 1 Define open channel flow with example BT-1-1 2 Distinguish between open channel flow and pipe flow. BT-4-1 3 Compute the hydraulic mean depth of a small channel 1m wide, 0.5m
Learning Objectives. Chapter Outline. Solidification of Metals. Solidification of Metals
 Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
Learning Objectives Study the principles of solidification as they apply to pure metals. Examine the mechanisms by which solidification occurs. - Chapter Outline Importance of Solidification Nucleation
3. STATE OF ART AND OBJECTIVES OF THESIS
 3. STATE OF ART AND OBJECTIVES OF THESIS 3.1. State of art 3.1.1. Crystallization time of drops From the simple model for droplet solidification [WAT92], the normalized crystallization time can be calculated
3. STATE OF ART AND OBJECTIVES OF THESIS 3.1. State of art 3.1.1. Crystallization time of drops From the simple model for droplet solidification [WAT92], the normalized crystallization time can be calculated
Metallurgy - Lecture (2) Solidification
 Metallurgy - Lecture (2) Solidification When molten metal enters a mold cavity, its heat is transferred through the mold wall. In the case of pure metals and eutectics, the solidification proceeds layer-bylayer
Metallurgy - Lecture (2) Solidification When molten metal enters a mold cavity, its heat is transferred through the mold wall. In the case of pure metals and eutectics, the solidification proceeds layer-bylayer
Lab 2 Guide: Recrystallization (Aug 31 Sept 4)
 Lab 2 Guide: Recrystallization (Aug 31 Sept 4) How Recrystallization Works- The Basic Idea Recrystallization is a purification technique; it allows us to remove impurities in a sample. The idea is you
Lab 2 Guide: Recrystallization (Aug 31 Sept 4) How Recrystallization Works- The Basic Idea Recrystallization is a purification technique; it allows us to remove impurities in a sample. The idea is you
Fundamentals of Casting
 Fundamentals of Casting Chapter 11 11.1 Introduction Products go through a series of processes before they are produced Design Material selection Process selection Manufacture Inspection and evaluation
Fundamentals of Casting Chapter 11 11.1 Introduction Products go through a series of processes before they are produced Design Material selection Process selection Manufacture Inspection and evaluation
UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEEING CHEMICAL PRECIPITATION REACOTRS
 UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEEING CHEMICAL PRECIPITATION REACOTRS PRECIPITATION PROCESS APPLICATIONS FACTORS AFFECTING THE REACTOR DESIGN MACROMIXING MESOMIXING FIGURES OF MESOMIXING
UNIVERSITY OF ROME LA SAPIENZA NANOTECHNOLOGIES ENGINEEING CHEMICAL PRECIPITATION REACOTRS PRECIPITATION PROCESS APPLICATIONS FACTORS AFFECTING THE REACTOR DESIGN MACROMIXING MESOMIXING FIGURES OF MESOMIXING
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project. Production of Styrene
 Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Styrene The feasibility of constructing a new, grass-roots, 100,000 tonne/y, styrene plant is being investigated. As part of
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Styrene The feasibility of constructing a new, grass-roots, 100,000 tonne/y, styrene plant is being investigated. As part of
ME ENGINEERING THERMODYNAMICS UNIT III QUESTION BANK SVCET
 1. A vessel of volume 0.04m 3 contains a mixture of saturated water and steam at a temperature of 250 0 C. The mass of the liquid present is 9 kg. Find the pressure, mass, specific volume, enthalpy, entropy
1. A vessel of volume 0.04m 3 contains a mixture of saturated water and steam at a temperature of 250 0 C. The mass of the liquid present is 9 kg. Find the pressure, mass, specific volume, enthalpy, entropy
Cooling Tower Operation
 Cooling Tower Operation Forced draught cooling towers use the evaporation of a liquid (often water) into air to achieve cooling. The tower often consists of a sprinkler system which wets a high-surface-area
Cooling Tower Operation Forced draught cooling towers use the evaporation of a liquid (often water) into air to achieve cooling. The tower often consists of a sprinkler system which wets a high-surface-area
CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES
 Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Ç engel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Objectives Develop the conservation
Thermodynamics: An Engineering Approach 8th Edition in SI Units Yunus A. Ç engel, Michael A. Boles McGraw-Hill, 2015 CHAPTER 5 MASS AND ENERGY ANALYSIS OF CONTROL VOLUMES Objectives Develop the conservation
GENERAL PROCESS CONSIDERATIONS IN THE SELECTION OF AGITATORS FOR SIMPLE APPLICATIONS AND IMPELLER DESIGN.
 GENERAL PROCESS CONSIDERATIONS IN THE SELECTION OF AGITATORS FOR SIMPLE APPLICATIONS AND IMPELLER DESIGN. The first thing to keep in mind is proper positioning of the agitator with respect to the vessel
GENERAL PROCESS CONSIDERATIONS IN THE SELECTION OF AGITATORS FOR SIMPLE APPLICATIONS AND IMPELLER DESIGN. The first thing to keep in mind is proper positioning of the agitator with respect to the vessel
S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering
![S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering](/thumbs/95/122495961.jpg) S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering Time: 3 Hrs. Prelim Question Paper Solution [Marks : 70 Q.1 Attempt any FIVE of the following. [10] Q.1(a) Explain difference between Thermodynamic
S.Y. Diploma : Sem. III [PG/PT/ME] Thermal Engineering Time: 3 Hrs. Prelim Question Paper Solution [Marks : 70 Q.1 Attempt any FIVE of the following. [10] Q.1(a) Explain difference between Thermodynamic
WASTEWATER TREATMENT (1)
 Wastewater Engineering (MSc program) WASTEWATER TREATMENT (1) Prepared by Dr.Khaled Zaher Assistant Professor, Public Works Engineering Department, Faculty of Engineering, Cairo University Wastewater Flow
Wastewater Engineering (MSc program) WASTEWATER TREATMENT (1) Prepared by Dr.Khaled Zaher Assistant Professor, Public Works Engineering Department, Faculty of Engineering, Cairo University Wastewater Flow
PESIT Bangalore South Campus Hosur road, 1km before Electronic City, Bengaluru -100 Department of Basic Science and Humanities
 USN 1 P E PESIT Bangalore South Campus Hosur road, 1km before Electronic City, Bengaluru -100 Department of Basic Science and Humanities INTERNAL ASSESSMENT TEST 1 Date : 28/08/2017 Marks: 40 Subject &
USN 1 P E PESIT Bangalore South Campus Hosur road, 1km before Electronic City, Bengaluru -100 Department of Basic Science and Humanities INTERNAL ASSESSMENT TEST 1 Date : 28/08/2017 Marks: 40 Subject &
Module: 9 Lecture: 39
 Module: 9 Lecture: 39 AMMONIUM CHLORIDE INTRODUCTION Ammonium chloride (NH4Cl) is white crystalline salt highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name
Module: 9 Lecture: 39 AMMONIUM CHLORIDE INTRODUCTION Ammonium chloride (NH4Cl) is white crystalline salt highly soluble in water. Solutions of ammonium chloride are mildly acidic. Sal ammoniac is a name
CHAPTER 4 LANGMUIR FILMS AT THE AIR/WATER INTERFACE
 CHAPTER 4 GROWTH OF POLY(ε-CAPROLACTONE) CRYSTALS IN LANGMUIR FILMS AT THE AIR/WATER INTERFACE Reproduced with permission from: Li, B.; Wu, Y.; Liu, M.; Esker, A. R. Brewster Angle Microscopy Study of
CHAPTER 4 GROWTH OF POLY(ε-CAPROLACTONE) CRYSTALS IN LANGMUIR FILMS AT THE AIR/WATER INTERFACE Reproduced with permission from: Li, B.; Wu, Y.; Liu, M.; Esker, A. R. Brewster Angle Microscopy Study of
Design Features of Combined Cycle Systems
 Design Features of Combined Cycle Systems 1.0 Introduction As we have discussed in class, one of the largest irreversibilities associated with simple gas turbine cycles is the high temperature exhaust.
Design Features of Combined Cycle Systems 1.0 Introduction As we have discussed in class, one of the largest irreversibilities associated with simple gas turbine cycles is the high temperature exhaust.
Three stages: Annealing Textures. 1. Recovery 2. Recrystallisation most significant texture changes 3. Grain Growth
 Three stages: Annealing Textures 1. Recovery 2. Recrystallisation most significant texture changes 3. Grain Growth Cold worked 85% Cold worked 85% + stress relieved at 300 C for 1 hr Cold worked 85% +
Three stages: Annealing Textures 1. Recovery 2. Recrystallisation most significant texture changes 3. Grain Growth Cold worked 85% Cold worked 85% + stress relieved at 300 C for 1 hr Cold worked 85% +
Chapter 3: Powders Production and Characterization
 Chapter 3: Powders Production and Characterization Course Objective... To introduce selective powder production processes and characterization methods. This course will help you : To understand properties
Chapter 3: Powders Production and Characterization Course Objective... To introduce selective powder production processes and characterization methods. This course will help you : To understand properties
COOLING TOWER DESIGN FOR CENTRAL GENERATORS OF CUET, BANGLADESH. Mohammad Sharif Khan, Golam Mainuddin, Abu Sadat Mohammad Sayem, Nadeem Nafis
 Proceedings of the 4 th BSME-ASME International Conference on Thermal Engineering 7-9 December, 008, Dhaka, Bangladesh COOLING TOWER DESIGN FOR CENTRAL GENERATORS OF CUET, BANGLADESH. Mohammad Sharif Khan,
Proceedings of the 4 th BSME-ASME International Conference on Thermal Engineering 7-9 December, 008, Dhaka, Bangladesh COOLING TOWER DESIGN FOR CENTRAL GENERATORS OF CUET, BANGLADESH. Mohammad Sharif Khan,
ENGINEERING INFORMATION Hot water and steam service
 ENGINEERING INFORMTION Hot water and steam service What is steam? Like other substances, water can exist in the form of a solid, when we call it ice; as a liquid when we call it water or as a gas when
ENGINEERING INFORMTION Hot water and steam service What is steam? Like other substances, water can exist in the form of a solid, when we call it ice; as a liquid when we call it water or as a gas when
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project. Production of Phthalic Anhydride
 Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Phthalic Anhydride Your assignment is to continue evaluating the details of a process to produce 75,000 tonne/y of phthalic anhydride
Fluid Mechanics, Heat Transfer, Thermodynamics Design Project Production of Phthalic Anhydride Your assignment is to continue evaluating the details of a process to produce 75,000 tonne/y of phthalic anhydride
ADVANTAGES OF MULTIPARTICULATES (PELLETS):
 INTRODUCTION: Multiparticulate Drug Delivery Systems (MDDS): The concept of multiple unit dosage form was initially introduced in the early 1950 s.these forms play a major role in the design of solid dosage
INTRODUCTION: Multiparticulate Drug Delivery Systems (MDDS): The concept of multiple unit dosage form was initially introduced in the early 1950 s.these forms play a major role in the design of solid dosage
Continuous Crystallization of Pharmaceuticals. Allan S. Myerson
 Continuous Crystallization of Pharmaceuticals Allan S. Myerson Dept. of Chemical Engineering Novartis-MIT Center for Continuous Manufacturing Massachusetts Institute of Technology Crystallization Process
Continuous Crystallization of Pharmaceuticals Allan S. Myerson Dept. of Chemical Engineering Novartis-MIT Center for Continuous Manufacturing Massachusetts Institute of Technology Crystallization Process
CHAPTER 2 CULTURE TYPE
 CHAPTER 2 CULTURE TYPE All types of micro-organisms are grown in suspension with the exception of cell culture, in which cells can also be anchorage dependent. Suspension the micro-organism can grow successfully
CHAPTER 2 CULTURE TYPE All types of micro-organisms are grown in suspension with the exception of cell culture, in which cells can also be anchorage dependent. Suspension the micro-organism can grow successfully
