Novel Approaches to Monitor and Manipulate Single Neurons In Vivo
|
|
|
- Bonnie Short
- 6 years ago
- Views:
Transcription
1 The Journal of Neuroscience, October 20, (42): Annual Meeting Mini-Symposium Novel Approaches to Monitor and Manipulate Single Neurons In Vivo Michael Brecht, 1 Michale S. Fee, 2 Olga Garaschuk, 3 Fritjof Helmchen, 4 Troy W. Margrie, 6 Karel Svoboda, 7 and Pavel Osten 5 1 Department of Neuroscience, Erasmus Medical Center, University Medical Center Rotterdam, 3015 DR Rotterdam, The Netherlands, 2 Department of Brain and Cognitive Sciences, McGovern Institute, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, 3 Institute of Physiology, Ludwig- Maximilians University Munich, Munich, Germany, Departments of 4 Cell Physiology and 5 Molecular Neurobiology, Max Planck Institute for Medical Research, Heidelberg, Germany, 6 Department of Physiology, The Wolfson Institute for Biomedical Research, University College London, London WC1E 6BT, United Kingdom, and 7 Howard Hughes Medical Institute, Cold Spring Harbor Laboratory, Cold Spring Harbor, New York Key words: cortex; patch clamp; in vivo; extracellular recording; two-photon imaging; lentivirus The complexity of the vertebrate brain poses an enormous challenge to experimental neuroscience. One way of dealing with this complexity has been to investigate different aspects of brain function in widely different preparations, each best suited to address a particular question. Accordingly, cellular questions are typically addressed with intracellular recordings in in vitro preparations such as brain slices or neuronal cultures, whereas network behavior and sensory or motor response properties are analyzed in vivo, often with extracellular recordings. This division of labor has proved to be an experimentally effective strategy. However, although there seems to be no limit to the wealth of data that can be generated in this way, integrating results derived in different preparations comes with its own set of challenges. The enormous difficulties encountered when one attempts to link cellular phenomena such as synaptic plasticity to systems properties such as spatial memory (Martin et al., 2000) have shown us that close collaboration between molecular cellular and systems neuroscience is required (Tonegawa et al., 2003) and that we need more convergence of experimental techniques to analyze the cellular basis of neural function under more natural conditions. Studying neurons under naturalistic conditions is, however, easier said than done. A return to in vivo preparations will only be successful if we are able to solve the technical problems that led previous researchers to abandon the study of intact brains in the first place. Thus, studying neurons at the cellular level in vertebrate brains is today first and foremost a technological challenge. Here we highlight recent efforts to improve our ability to analyze functions of single neurons in vivo. Given the mini-review format, we cannot aim for completeness but must focus on the techniques featured in the accompanying mini-symposium without meaning to imply that other novel developments are less significant. Received Aug. 15, 2004; revised Sept. 1, 2004; accepted Sept. 2, Correspondence should be addressed to Michael Brecht, Department of Neuroscience, Erasmus Medical Center, University Medical Center Rotterdam, Dr. Molewaterplein 50, 3015 DR Rotterdam, The Netherlands. m.brecht@erasmusmc.nl. DOI: /JNEUROSCI Copyright 2004 Society for Neuroscience /04/ $15.00/0 Monitoring neurons with improved cellular resolution Structural plasticity and synaptic function Synapses are the smallest units of organization in neural networks, and they are thought to encode memories. What happens at synapses when we learn? To understand synaptic dynamics in intact animals, it will be necessary to monitor the structure and function of individual synapses in vivo over times of milliseconds to months. This goal has been made attainable by the invention of two-photon laser scanning microscopy (2PLSM) (Denk et al., 1990), which allows imaging in the scattering environment of the intact brain (Denk and Svoboda, 1997). In addition, fluorescent proteins [with their large extinction ratios, quantum efficiencies, and resistance to photobleaching (Tsien, 1998)] are ideal for in vivo imaging and can be genetically targeted to neurons of interest (see below) (Feng et al., 2000). Long-term 2PLSM imaging of green fluorescent protein (GFP)-expressing neurons is used to track structural plasticity in vivo (Grutzendler et al., 2002; Trachtenberg et al., 2002). Whereas dendritic arbors in the adult brain are essentially stable, a fraction of dendritic spines appear and disappear over time periods of days (Trachtenberg et al., 2002). The turnover of dendritic spines is increased after novel sensory experience, suggesting that spine addition and subtraction, together with synapse formation and elimination, is a substrate of experience-dependent plasticity (Trachtenberg et al., 2002) (Fig. 1A). Fluorescent proteins have been engineered to indicate aspects of cellular activity, including ph changes (Miesenbock et al., 1998), Ca 2 concentration (Miyawaki et al., 1997), membrane potential (Siegel and Isacoff, 1997), and others (Miyawaki, 2003). Thus, aspects of synaptic function such as glutamate release (Oertner et al., 2002) will become accessible in vivo. Two-photon imaging can be easily combined with fluorescence lifetime measurements for quantitative fluorescence resonance energy transfer imaging (So et al., 2000). These techniques may help to decipher the cascades of signal transduction underlying synaptic plasticity in vivo. Network analysis Understanding neuronal computations requires a multilevel approach, and, hence, tools for analysis of network activity need to complement the synaptic and cellular analysis methods. Network
2 9224 J. Neurosci., October 20, (42): Brecht et al. Monitoring and Manipulating Neurons In Vivo activity has microscopic (individual neurons) and macroscopic (neuronal ensembles) aspects, which should preferably be monitored simultaneously. Current techniques [extracellular multielectrode recordings, functional magnetic resonance imaging, imaging of intrinsic optical signals, and voltage-sensitive dye-based imaging (Orbach et al., 1985; Grinvald et al., 1988; Raichle, 1998; Shoham et al., 1999; Nicolelis and Ribeiro, 2002)] are well suited for monitoring activity at the macroscopic level but are restricted in their ability to read out the activity of individual neurons. The divide between the macroscopic and the microscopic brain activity can be bridged by the recently developed multi-cell bolus loading (MCBL) technique, which provides a targeted staining of neuronal populations with calciumsensitive dyes (Stosiek et al., 2003). The cells are stained by a brief bolus injection of a membrane-permeant calcium indicator dye (e.g., Calcium Green-1 AM) into extracellular space and imaged by means of two-photon laser scanning microscopy. This technique allows simultaneous functional analyses of many individual neurons, i.e., a monitoring of brain activity at both the microscopic and macroscopic level (Fig. 1B). To clarify the identity of the imaged cells, mixtures of a Ca 2 -sensitive dye and different cell-specific markers (e.g., an astrocyte marker sulforhodamine 101) (Nimmerjahn et al., 2004) may be used for bolus injections. Identified cells can be further subjected to two-photon targeted patching (TPTP) (Margrie et al., 2003) to measure electrophysiological signals underlying their activity. MCBL is also very useful for monitoring activity in awake, behaving animals (Helmchen et al., 2001; Adelsberger et al., 2004), thus providing a versatile tool for analysis of intact neuronal circuits. Monitoring neural activity under natural conditions Extracellular recordings Although recent advances in electrode fabrication and microdrive techniques have led to the ability to record from many neurons in freely behaving animals (Bragin et al., 2000; Serruya et al., 2002; Nicolelis et al., 2003), many challenges remain in the effort to develop chronic recording systems with the flexibility and selectivity of acute recording systems. For mechanical stability, implanted multielectrode arrays are usually constructed from electrodes (e.g., microwires) that are optimal for multiunit (multi-cell) rather than single-unit (single-cell) signals (Gray et al., 1995). These signals can be decomposed into putative single-unit signals using spike-sorting techniques, but this method is relatively insensitive to smaller neurons and neurons with low firing rates. To avoid these difficulties, chronic recordings could be made with large numbers of electrodes optimized for single-unit recording that are individually moved in the brain to achieve high-quality single-unit signals on every electrode. We and others have recently made advances toward this goal by developing a motorized microdrive in which electrodes can be individually and remotely manipulated to isolate single neurons (Fee and Leonardo, 2001; Cham et al., 2004). Figure 1. Monitoring neurons with improved cellular resolution. A, Long-term in vivo imaging of GFP-expressing axons in the adult mouse barrel cortex. Note gained (e.g., green arrowhead), lost (blue arrowhead), and stable (yellow arrowheads) putative synaptic terminals (A. Holtmaat, personal communication). B,Ca 2 transients evoked by whisker deflection. Left, A highmagnification image of layer 2/3 neurons in vivo (depth, 130 m) in the barrel cortex of a 13-d-old mouse. Right, Line-scan recordings of Ca 2 transients evoked in two neurons by a deflection of the majority of whiskers on the contralateral side of the mouse s snout. The position of the scanned line and the cells analyzed are indicated (left) (modified from Stosiek et al., 2003). Another limitation of extracellular recording techniques is that neurons are usually found during recording process by searching for spontaneous spikes as the recording electrode is slowly advanced through the brain. Unfortunately, in some cases, Figure 2. Monitoring neural activity under natural conditions. A, Top, Overview of motorized microdrive. Electrodes are driven independently by small brushless DC motors. Bottom, Sample recording from a single neuron in the nucleus robustus archistriatalis of a zebra finchduringsinging(adaptedfromfeeandleonardo, 2001). B, Top, Principaldesignoftheminiaturizedtwo-photonmicroscope. Excitation light and fluorescence emission light are guided through optical fibers. The two-photon fiberscope is mounted above a small cranial windowandcanbecarriedbyfreelymovingrats. Bottom, Two-photonfiberscope-acquiredimageofalayer2neuronfilledwithCalcium Green-1 (left) and small blood vessels labeled via tail-vein injection of FITC dextran (right) (adapted from Helmchen et al., 2001).
3 Brecht et al. Monitoring and Manipulating Neurons In Vivo J. Neurosci., October 20, (42): Figure 3. Targeting and manipulating single neurons. A, Top, Targeted whole-cell recording from a calretinin-positive interneuronintheglomeruluslayerofthemouseolfactorybulb(postnatalday21). Imageisanoverlayoftwochannels(redandgreen) showing the illuminated patch pipette filled with Alexa 594 attached to the GFP-labeled interneuron. Bottom, Trace shows membrane potential in response to odor presentation (2 sec). B, Enhanced GFP-expressing lentivirus was injected into the parenchymaofratlayer2/3somatosensorycortex(postnatalday28). Aftera2weekexpressionperiod, theratwaskilled, andenhanced GFP fluorescence was visualized from100 m paraformaldehyde-fixed brain sections. The image is a maximal projection of 40 confocal sections, separated by 2 nm. C, Intracellular stimulation of the rat primary whisker motor cortex. Top trace, Position of whisker E1 during an intracellular stimulation trial. Bottom trace, Membrane potential recordings during current injection (10 action potentials at 50 Hz); the stimulation current is not shown. neurons exhibit little or no spontaneous activity and cannot be found using this approach. We recently used the classic technique of antidromic activation (Swadlow, 1998) to find premotor neurons in the songbird brain that are completely silent in the awake bird, except when the bird sings. Furthermore, during the song, these neurons generate a single brief burst of spikes (Hahnloser et al., 2002). The discovery of extremely sparse activity in a critical population of premotor neurons in the songbird, as well as other studies (Beloozerova et al., 2003), suggest that a combination of remote manipulation of selective electrodes, and antidromic identification techniques, should be useful to study brain circuitry in freely behaving animals. Microscope miniaturization The development of miniaturized fiber-optic devices for imaging neurons at microscopic resolution in brains of awake, freely moving animals is both an important goal and a major technological challenge. Two types of fiber-optic fluorescence microscopes have been developed. The first type delivers excitation light through a single-core fiber, in which case two-dimensional images are obtained by a scanning mechanism in the microscope headpiece (Delaney and Harris, 1995; Helmchen et al., 2002). Using such a fiberscope, we could resolve neurons and dendrites in cortical layer 2 of anesthetized rats and small blood vessels in freely moving animals (Helmchen et al., 2001) (Fig. 2B). A particular challenge in this case has been the use of two-photon excitation through optical fiber to gain imaging depth (Helmchen et al., 2002). In standard glass fibers, nonlinear effects cause broadening of the ultrafast light pulses and thus reduce two-photon excitation efficiency (Helmchen et al., 2002). Fortunately, radically new types of optical fibers (with hollow air-filled cores) now permit distortion-free propagation of femtosecond pulses (Göbel et al., 2004a). A second type of fiber-optic microscope is based on so-called coherent optical fiber bundles, which contain many thousands of fiber cores and transmit entire images, albeit with low spatial resolution (Hirano et al., 1996; Knittel et al., 2001; Göbel et al., 2004b). Fiber bundles are now available in small diameters and can be attached to small cylindrical lenses suitable for endoscopic imaging. These ultrathin ( 1 mm diameter) microscope probes will extend imaging to deeper brain structures (Jung and Schnitzer, 2003; Levene et al., 2004). Microscope miniaturization together with the improvements in methods for fluorescence labeling in vivo (Stosiek et al., 2003) promises to shed light on cellular activity in behaving animals. Targeting and manipulating single neurons Targeted recordings An increasing refinement of recording specificity has been a leading theme in the development of the whole-cell patch-clamp recording technique (Hamill et al., 1981). When combined with differential interference contrast or fluorescence microscopy, the readily visible tip of the patch pipette could be targeted not only to a specific cell genetically manipulated to express a particular fluorophore but also to multiple locations on the same neuron (Stuart et al., 1993). The stability of whole-cell recordings combined with the possibility of rapidly loading fluorescent indicators (Helmchen and Waters, 2002) has therefore made blind whole-cell recordings attractive to in vivo experimentalists (Pei et al., 1991; Jagadeesh et al., 1993). Whole-cell recordings can be obtained from the anesthetized and awake preparation, superficial and deep structures (Margrie et al., 2002), extremely small cells (Chadderton et al., 2004), and even from the dendrites of large cortical neurons (Larkum and Zhu, 2002). One major drawback of blind whole-cell recordings is the random but not necessarily represen-
4 9226 J. Neurosci., October 20, (42): Brecht et al. Monitoring and Manipulating Neurons In Vivo tative sampling of neurons, which severely limits the interpretation and the rapidity of data acquisition in neuronally heterogeneous brain regions. Recently, it has become possible to target electrophysiological recordings to genetically labeled neurons in vivo. To this end, two-photon microscopy is used to guide a whole-cell patch pipette filled with a fluorescent dye onto a GFPexpressing neuron in the intact brain (Margrie et al., 2003) (Fig. 3A). This TPTP technique was initially used to record from the sparsely distributed parvalbumin-positive interneurons in the somatosensory cortex. TPTP experiments on GFP-expressing parvalbumin-positive interneurons provided the first piece of direct evidence in support of a role of electrical coupling in these cells during sensory processing in vivo (Margrie et al., 2003). More recently, TPTP has been used to target cortical cells that have been altered in their intrinsic and synaptic conductances (Komai et al., 2004) by lentivirus-based genetic means (see Single-cell genetics). Single-cell genetics The establishment of novel recording and stimulation techniques makes it highly desirable to develop methods that would combine the optical and electrophysiological single-cell analyses with the genetic means to manipulate individual neurons. Our approach for providing such a methodology, termed single-cell genetics, is based on stereotactic delivery of lentiviruses (Naldini and Verma, 2000). Lentiviral infection can be titrated to affect small numbers of neurons (on the order of tens to few hundreds) within intact brain networks (Fig. 3B), providing an excellent spatiotemporal control of the onset of the genetic manipulations. Importantly, the lentiviral vector-encompassed genetic information is stably integrated into the host cell genome, allowing for subsequent functional analysis as early as several days and as late as weeks or months after the viral delivery. We optimized the use of the self-inactivating lentiviral vectors (Lois et al., 2002) specifically for combination with cortical physiology, primarily by developing pyramidal neuron-specific expression from synapsin I or -CaMKII (Ca 2 /calmodulin-dependent protein kinase II) promoters (Dittgen et al., 2004). Coexpression of GFP and Cre recombinase from these vectors can now be used in mice with floxed genes for creating gene knock-out, whereas coexpression of GFP and double-stranded short-interfering RNAs is an efficient method for gene knock-down in the infected population of pyramidal neurons in the otherwise intact brain (Elbashir et al., 2001; Pfeifer et al., 2001; Licznerski et al., 2003; Qin et al., 2003). The advantages of this approach lie in the ease of its application, as well as in the fact that modification of a small neuronal population avoids lethality of phenotype or activation of compensatory mechanisms that are often associated with standard genetics approaches affecting the whole brain or particular brain regions. Reverse physiology It is unlikely that, if one had access to all the spike trains in all brain cells of an animal of choice, it would be possible to derive mechanistic explanations for the animal s mental events and behavioral performance. Thus, in addition to the correlative analysis of single-neuron activity, we need to study the brain by altering singleneuron activity. On long timescales, such manipulations might be done very elegantly by genetic approaches. On short timescales, microstimulation techniques have been applied (Tehovnik, 1996; Cohen and Newsome, 2004) and have, for example, provided very direct evidence for the involvement of certain sets of cortical neurons in visual and tactile neural representations (Salzman et al., 1990; Romo and Salinas, 2001). The major drawback of microstimulation techniques is their lack of cellular specificity, i.e., the stimulated cellular elements are not identified and estimates of the number of stimulated neurons vary by more than an order of magnitude (Tehovnik, 1996). To overcome such limitations, intracellular stimulation has been applied recently in the vibrissa motor cortex (Woody and Black-Cleworth, 1973; Brecht et al., 2004). Quite unexpectedly, we found that single cells could drive noticeable movements. These evoked movements were spatiotemporally complex and varied as function of the lamina of the stimulated neuron (Fig. 3C). We hope to extend this work to a reverse physiology approach, in which one studies the perceptual and motor correlates of experimentally evoked spikes rather than (as is done conventionally) analyzing the occurrence of spikes as a correlate of external or internal events. Summary As yet, most in vivo records of cellular activity in the vertebrate brain come from unidentified cells, and most manipulations of neural activity affect the whole brain or large populations of neurons in particular brain regions. The combined application of the optical, electrophysiological, and genetic techniques outlined here will allow us to refine our focus to the structure and function of identified neurons in the intact brain. This is the only means by which we can determine with any precision the cellular basis of brain function. References Adelsberger H, Garaschuk OJM, Milos J, Enache A, Konnerth A (2004) In vivo monitoring of calcium waves in behaving mice. FENS Abstr 2:A Beloozerova IN, Sirota MG, Swadlow HA (2003) Activity of different classes of neurons of the motor cortex during locomotion. J Neurosci 23: Bragin A, Hetke J, Wilson CL, Anderson DJ, Engel Jr J, Buzsaki G (2000) Multiple site silicon-based probes for chronic recordings in freely moving rats: implantation, recording and histological verification. J Neurosci Methods 98: Brecht M, Schneider M, Sakmann B, Margrie TW (2004) Whisker movements evoked by stimulation of single pyramidal cells in rat motor cortex. Nature 427: Chadderton P, Margrie TW, Hausser M (2004) Integration of quanta in cerebellar granule cells during sensory processing. Nature 428: Cham JG, Branchaud EA, Nenadic Z, Greger B, Andersen RA, Burdick JW (2004) A semi-chronic motorized microdrive and control algorithm for autonomously isolating and maintaining optimal extracellular action potentials. J Neurophysiol, in press. Cohen MR, Newsome WT (2004) What electrical microstimulation has revealed about the neural basis of cognition. Curr Opin Neurobiol 14: Delaney PM, Harris MR (1995) Fiberoptics in confocal microscopy. In: Handbook of biological confocal microscopy (Pawley JB, ed), pp New York: Plenum. Denk W, Svoboda K (1997) Photon upmanship: why multiphoton imaging is more than a gimmick. Neuron 18: Denk W, Strickler JH, Webb WW (1990) Two-photon laser scanning fluorescence microscopy. Science 248: Dittgen T, Licznerski P, Grinevich V, Cetin A, Laudenklos S, Seeburg PH, Osten P (2004) Single cell genetics: a novel approach to study neuronal functions in vivo. FENS Abstr 2:A Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T (2001) Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 411: Fee MS, Leonardo A (2001) Miniature motorized microdrive and commutator system for chronic neural recording in small animals. J Neurosci Methods 112: Feng G, Mellor RH, Bernstein M, Keller-Peck C, Nguyen QT, Wallace M, Nerbonne JM, Lichtman JW, Sanes JR (2000) Imaging neuronal subsets in transgenic mice expressing multiple spectral variants of GFP. Neuron 28: Göbel W, Nimmerjahn A, Helmchen F (2004a) Distortion-free delivery of
5 Brecht et al. Monitoring and Manipulating Neurons In Vivo J. Neurosci., October 20, (42): femtosecond pulses from a Ti:sapphire laser through a hollow core photonic crystal fiber. Opt Lett 29: Göbel W, Kerr JND, Nimmerjahn A, Helmchen F (2004b) A miniaturized two-photon microscope using a flexible coherent fiber bundle and a gradient-index lens objective. Opt Lett, in press. Gray CM, Maldonado PE, Wilson M, McNaughton B (1995) Tetrodes markedly improve the reliability and yield of multiple single-unit isolation from multi-unit recordings in cat striate cortex. J Neurosci Methods 63: Grinvald A, Frostig RD, Lieke E, Hildesheim R (1988) Optical imaging of neuronal activity. Physiol Rev 68: Grutzendler J, Kasthuri N, Gan WB (2002) Long-term dendritic spine stability in the adult cortex. Nature 420: Hahnloser RH, Kozhevnikov AA, Fee MS (2002) An ultra-sparse code underlies the generation of neural sequences in a songbird. Nature 419: Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391: Helmchen F, Waters J (2002) Ca 2 imaging in the mammalian brain in vivo. Eur J Pharmacol 447: Helmchen F, Fee MS, Tank DW, Denk W (2001) A miniature headmounted two-photon microscope. High-resolution brain imaging in freely moving animals. Neuron 31: Helmchen F, Tank DW, Denk W (2002) Enhanced two-photon excitation through optical fiber by single-mode propagation in a large core. Appl Opt 41: Hirano M, Yamashita Y, Miyakawa A (1996) In vivo visualization of hippocampal cells and dynamics of Ca 2 concentration during anoxia: feasibility of a fiber-optic plate microscope system for in vivo experiments. Brain Res 732: Jagadeesh B, Wheat HS, Ferster D (1993) Linearity of summation of synaptic potentials underlying direction selectivity in simple cells of the cat visual cortex. Science 262: Jung JC, Schnitzer MJ (2003) Multiphoton endoscopy. Opt Lett 28: Knittel J, Schnieder L, Buess G, Messerschmidt B, Possner T (2001) Endoscope-compatible confocal microscope using a gradient index-lens system. Opt Commun 188: Komai S, Waters J, Licznerski P, Denk W, Brecht M, Osten P (2004) The role of active conductances in the development of Receptive Field properties in the rat barrel cortex studied with single-cell genetics and TPTP. Soc Neurosci Abstr 30:58.8. Larkum ME, Zhu JJ (2002) Signaling of layer 1 and whisker-evoked Ca 2 and Na action potentials in distal and terminal dendrites of rat neocortical pyramidal neurons in vitro and in vivo. J Neurosci 22: Levene MJ, Dombeck DA, Kasischke KA, Molloy RP, Webb WW (2004) In vivo multiphoton microscopy of deep brain tissue. J Neurophysiol 91: Licznerski P, Dittgen T, Seeburg PH, Osten P (2003) Lentivirus-based vectors for gene silencing in neurons. Soc Neurosci Abstr 29: Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D (2002) Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science 295: Margrie TW, Brecht M, Sakmann B (2002) In vivo, low-resistance, wholecell recordings from neurons in the anaesthetized and awake mammalian brain. Pflügers Arch 444: Margrie TW, Meyer AH, Caputi A, Monyer H, Hasan MT, Schaefer AT, Denk W, Brecht M (2003) Targeted whole-cell recordings in the mammalian brain in vivo. Neuron 39: Martin SJ, Grimwood PD, Morris RG (2000) Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci 23: Miesenbock G, De Angelis DA, Rothman JE (1998) Visualizing secretion and synaptic transmission with ph-sensitive green fluorescent proteins. Nature 394: Miyawaki A (2003) Visualization of the spatial and temporal dynamics of intracellular signaling. Dev Cell 4: Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY (1997) Fluorescent indicators for Ca 2 based on green fluorescent proteins and calmodulin. Nature 388: Naldini L, Verma IM (2000) Lentiviral vectors. Adv Virus Res 55: Nicolelis MA, Ribeiro S (2002) Multielectrode recordings: the next steps. Curr Opin Neurobiol 12: Nicolelis MA, Dimitrov D, Carmena JM, Crist R, Lehew G, Kralik JD, Wise SP (2003) Chronic, multisite, multielectrode recordings in macaque monkeys. Proc Natl Acad Sci USA 100: Nimmerjahn A, Kirchhoff F, Kerr JND, Helmchen F (2004) Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nature Methods, in press. Oertner TG, Sabatini BL, Nimchinsky EA, Svoboda K (2002) Facilitation at single synapses probed with optical quantal analysis. Nat Neurosci 5: Orbach HS, Cohen LB, Grinvald A (1985) Optical mapping of electrical activity in rat somatosensory and visual cortex. J Neurosci 5: Pei X, Volgushev M, Vidyasagar TR, Creutzfeldt OD (1991) Whole cell recording and conductance measurements in cat visual cortex in-vivo. NeuroReport 2: Pfeifer A, Brandon EP, Kootstra N, Gage FH, Verma IM (2001) Delivery of the Cre recombinase by a self-deleting lentiviral vector: efficient gene targeting in vivo. Proc Natl Acad Sci USA 98: Qin XF, An DS, Chen IS, Baltimore D (2003) Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA 100: Raichle ME (1998) Behind the scenes of functional brain imaging: a historical and physiological perspective. Proc Natl Acad Sci USA 95: Romo R, Salinas E (2001) Touch and go: decision-making mechanisms in somatosensation. Annu Rev Neurosci 24: Salzman CD, Britten KH, Newsome WT (1990) Cortical microstimulation influences perceptual judgements of motion direction. Nature 346: Serruya MD, Hatsopoulos NG, Paninski L, Fellows MR, Donoghue JP (2002) Instant neural control of a movement signal. Nature 416: Shoham D, Glaser DE, Arieli A, Kenet T, Wijnbergen C, Toledo Y, Hildesheim R, Grinvald A (1999) Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron 24: Siegel MS, Isacoff EY (1997) A genetically encoded optical probe of membrane voltage. Neuron 19: So PT, Dong CY, Masters BR, Berland KM (2000) Two-photon excitation fluorescence microscopy. Annu Rev Biomed Eng 2: Stosiek C, Garaschuk O, Holthoff K, Konnerth A (2003) In vivo two-photon calcium imaging of neuronal networks. Proc Natl Acad Sci USA 100: Stuart GJ, Dodt HU, Sakmann B (1993) Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Arch 423: Swadlow HA (1998) Neocortical efferent neurons with very slowly conducting axons: strategies for reliable antidromic identification. J Neurosci Methods 79: Tehovnik EJ (1996) Electrical stimulation of neural tissue to evoke behavioral responses. J Neurosci Methods 65:1 17. Tonegawa S, Nakazawa K, Wilson MA (2003) Genetic neuroscience of mammalian learning and memory. Philos Trans R Soc Lond B Biol Sci 358: Trachtenberg JT, Chen BE, Knott GW, Feng G, Sanes JR, Welker E, Svoboda K (2002) Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature 420: Tsien RY (1998) The green fluorescent protein. Annu Rev Biochem 67: Woody CD, Black-Cleworth P (1973) Differences in excitability of cortical neurons as a function of motor projection in conditioned cats. J Neurophysiol 36:
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope
 High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
High Throughput Whole Organ Imaging Based on Multifocal Multiphoton Microscope LBRC researchers: Peter So, Jae Won Cha, Elijah Yew, Vijay Singh External technology collaborators: Prof. Hanry Yu (University
Methods of Characterizing Neural Networks
 Methods of Characterizing Neural Networks Ashley Nord University of Minnesota Minneapolis, MN 55414 Advisors: Katsushi Arisaka, Adrian Cheng University of California Los Angeles Los Angeles, CA 90024 September
Methods of Characterizing Neural Networks Ashley Nord University of Minnesota Minneapolis, MN 55414 Advisors: Katsushi Arisaka, Adrian Cheng University of California Los Angeles Los Angeles, CA 90024 September
Multiphoton Microscopy: Seeing deeper and clearer
 Multiphoton Microscopy: Seeing deeper and clearer Since the invention of simple microscope by Leuwenhoek and Hooke in the 17th century, different types of light microscopy techniques (such as phase contrast,
Multiphoton Microscopy: Seeing deeper and clearer Since the invention of simple microscope by Leuwenhoek and Hooke in the 17th century, different types of light microscopy techniques (such as phase contrast,
Magneto: remote control over neuronal activity and behaviour. Yvette Zarb Division of Neurosurgery
 Magneto: remote control over neuronal activity and behaviour Yvette Zarb Division of Neurosurgery Gene expression Studying the actions specific cells and gene products is essential in understanding their
Magneto: remote control over neuronal activity and behaviour Yvette Zarb Division of Neurosurgery Gene expression Studying the actions specific cells and gene products is essential in understanding their
Simultaneously recording from four neurons using PatchStar & MicroStar manipulators
 SciMethods: Applications Simultaneously recording from four neurons using PatchStar & MicroStar manipulators Christian Wilms 1, Therese Abrahamsson 2, Per Jesper Sjöström 2 1: Scientifica Ltd. Uckfield,
SciMethods: Applications Simultaneously recording from four neurons using PatchStar & MicroStar manipulators Christian Wilms 1, Therese Abrahamsson 2, Per Jesper Sjöström 2 1: Scientifica Ltd. Uckfield,
Fundamentals of Central Nervous System Recording. Joseph E. O Doherty BME Neural Prosthetic Systems
 Fundamentals of Central Nervous System Recording Joseph E. O Doherty BME 265 - Neural Prosthetic Systems The Problem Spaghetti & Meatballs Rall 1962 (after Ramon y Cajal) Outline 1. Origin of Extracellular
Fundamentals of Central Nervous System Recording Joseph E. O Doherty BME 265 - Neural Prosthetic Systems The Problem Spaghetti & Meatballs Rall 1962 (after Ramon y Cajal) Outline 1. Origin of Extracellular
Genetically targeted all-optical electrophysiology with a transgenic Credependent
 Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
Genetically targeted all-optical electrophysiology with a transgenic Credependent Optopatch mouse Short title: Transgenic Optopatch mouse Shan Lou 1, Yoav Adam 1, Eli N. Weinstein 1,4, Erika Williams 2,
Nature Neuroscience: doi: /nn Supplementary Figure 1. Comprehensive opto-mechanical design of the dual-axis microscope.
 Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Supplementary Figure 1 Comprehensive opto-mechanical design of the dual-axis microscope. (a) The complete microscope is shown to scale. The laser beam is depicted in light red. (b) A close-up view of the
Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers
 Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Multiphoton Microscopy / Fiber Laser Simultaneous multi-color, multiphoton fluorophore excitation using dual-color fiber lasers Matthias Handloser, Tim Paasch-Colberg, Bernhard Wolfring TOPTICA Photonics
Improved in vivo two-photon imaging after blood replacement by perfluorocarbon
 J Physiol 587.13 (2009) pp 3153 3158 3153 Improved in vivo two-photon imaging after blood replacement by perfluorocarbon F. Haiss 1,R.Jolivet 1,M.T.Wyss 1,J.Reichold 2,N.B.Braham 3,F.Scheffold 3,M.P.Krafft
J Physiol 587.13 (2009) pp 3153 3158 3153 Improved in vivo two-photon imaging after blood replacement by perfluorocarbon F. Haiss 1,R.Jolivet 1,M.T.Wyss 1,J.Reichold 2,N.B.Braham 3,F.Scheffold 3,M.P.Krafft
In vivo fast imaging and optogenetic manipulation using genetically-encoded fluorescent indicators and actuators. Serena Bovetti
 In vivo fast imaging and optogenetic manipulation using genetically-encoded fluorescent indicators and actuators Serena Bovetti Istituto Italiano di Tecnologia Genova, Italy Bogliasco, June 6-8 2016 Analyzing
In vivo fast imaging and optogenetic manipulation using genetically-encoded fluorescent indicators and actuators Serena Bovetti Istituto Italiano di Tecnologia Genova, Italy Bogliasco, June 6-8 2016 Analyzing
Pioneering Applications of Two-Photon Microscopy to Mammalian Neurophysiology
 28 Pioneering Applications of Two-Photon Microscopy to Mammalian Neurophysiology Seven Case Studies Q.-T. Nguyen, G. O. Clay, N. Nishimura, C. B. Schaffer, L. F. Schroeder, P. S. Tsai, and D. Kleinfeld
28 Pioneering Applications of Two-Photon Microscopy to Mammalian Neurophysiology Seven Case Studies Q.-T. Nguyen, G. O. Clay, N. Nishimura, C. B. Schaffer, L. F. Schroeder, P. S. Tsai, and D. Kleinfeld
Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution
 SUPPLEMENTARY INFORMATION for Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution Yanjie Zhao 1,2, Tingting Yu 1,2, Chao Zhang 1,2, Zhao Li 1,2, Qingming Luo 1,2,
SUPPLEMENTARY INFORMATION for Skull optical clearing window for in vivo imaging of the mouse cortex at synaptic resolution Yanjie Zhao 1,2, Tingting Yu 1,2, Chao Zhang 1,2, Zhao Li 1,2, Qingming Luo 1,2,
Class 7: Methods in Research By: Ray
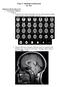 Class 7: Methods in Research By: Ray Method in Brain Research 1. Non-Invasive (Human) o Imaging Computerized (Axial) Tomography (X-rays). Static pictures and high spatial resolution. Horizontal plane only.
Class 7: Methods in Research By: Ray Method in Brain Research 1. Non-Invasive (Human) o Imaging Computerized (Axial) Tomography (X-rays). Static pictures and high spatial resolution. Horizontal plane only.
CENTER FOR BRAIN EXPERIMENT
 CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
CENTER FOR BRAIN EXPERIMENT Section of Brain Structure Associate Professor: ARII, Tatsuo, PhD 1967 Graduated from Tohoku University, Faculty of Science. Completed the doctoral course in Engineering, Nagoya
Module BIO 628 Spring term 2015
 Module BIO 628 Spring term 2015 MD/PhD Neuroscience course This block course on Neuroscience is organized for students of the MD-PhD program and of the Master of Science in Medical Biology. It takes place
Module BIO 628 Spring term 2015 MD/PhD Neuroscience course This block course on Neuroscience is organized for students of the MD-PhD program and of the Master of Science in Medical Biology. It takes place
Detection of Action Potentials In Vitro by Changes in Refractive Index
 Detection of Action Potentials In Vitro by Changes in Refractive Index David Kleinfeld 1 and Arthur LaPorta 2 1 - Department of Physics, University of California, La Jolla, CA 92093-0319. 2- Laboratory
Detection of Action Potentials In Vitro by Changes in Refractive Index David Kleinfeld 1 and Arthur LaPorta 2 1 - Department of Physics, University of California, La Jolla, CA 92093-0319. 2- Laboratory
In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary
 Supplementary Methods In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary somatosensory cortex (S1), forepaw
Supplementary Methods In vivo recording, forepaw denervation, and isolation of slices: Methods for mapping the forepaw/lower jaw border in anesthetized adult rat primary somatosensory cortex (S1), forepaw
Dynamic Confocal Imaging of Living Brain. Miniaturization of fluorescence microscopes using fibre optics
 Dynamic Confocal Imaging of Living Brain Miniaturization of fluorescence microscopes using fibre optics Introduction High-resolution optical microscopy is the principal technique for characterizing biological
Dynamic Confocal Imaging of Living Brain Miniaturization of fluorescence microscopes using fibre optics Introduction High-resolution optical microscopy is the principal technique for characterizing biological
Biomimetic Computational Systems: Understanding Informational Processing in the Brain
 Institute of Medicine "Grand Challenges" 2008 Biomimetic Computational Systems: Understanding Informational Processing in the Brain Theodore W. Berger, Ph.D. David Packard Professor of Engineering Professor
Institute of Medicine "Grand Challenges" 2008 Biomimetic Computational Systems: Understanding Informational Processing in the Brain Theodore W. Berger, Ph.D. David Packard Professor of Engineering Professor
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/325/5941/756/dc1 Supporting Online Material for Synaptic Integration in Tuft Dendrites of Layer 5 Pyramidal Neurons: A New Unifying Principle Matthew E. Larkum,* Thomas
www.sciencemag.org/cgi/content/full/325/5941/756/dc1 Supporting Online Material for Synaptic Integration in Tuft Dendrites of Layer 5 Pyramidal Neurons: A New Unifying Principle Matthew E. Larkum,* Thomas
The question of how neuronal networks accomplish information
 In vivo two-photon calcium imaging of neuronal networks Christoph Stosiek*, Olga Garaschuk*, Knut Holthoff, and Arthur Konnerth Physiologisches Institut, Ludwig-Maximilians Universität München, Pettenkoferstrasse
In vivo two-photon calcium imaging of neuronal networks Christoph Stosiek*, Olga Garaschuk*, Knut Holthoff, and Arthur Konnerth Physiologisches Institut, Ludwig-Maximilians Universität München, Pettenkoferstrasse
Two-Photon Microscopy for Deep Tissue Imaging of Living Specimens
 for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
for Deep Tissue Imaging of Living Specimens Tilman Franke* and Sebastian Rhode TILL Photonics GmbH, an FEI company, Lochhamer Schlag 21, D-82166 Gräfelfing, Germany *tilman.franke@fei.com Introduction
Automated whole-cell patch-clamp electrophysiology of neurons in vivo
 Nature Methods Automated whole-cell patch-clamp electrophysiology of neurons in vivo Suhasa B Kodandaramaiah, Giovanni Talei Franzesi, Brian Y Chow, Edward S Boyden & Craig R Forest Supplementary File
Nature Methods Automated whole-cell patch-clamp electrophysiology of neurons in vivo Suhasa B Kodandaramaiah, Giovanni Talei Franzesi, Brian Y Chow, Edward S Boyden & Craig R Forest Supplementary File
Targeting neurons and photons for optogenetics
 f o c u s o n n e u r ot e c h n i q u e s r e v i e w Targeting neurons and photons for optogenetics Adam M Packer 1, Botond Roska 2 & Michael Häusser 1 Optogenetic approaches promise to revolutionize
f o c u s o n n e u r ot e c h n i q u e s r e v i e w Targeting neurons and photons for optogenetics Adam M Packer 1, Botond Roska 2 & Michael Häusser 1 Optogenetic approaches promise to revolutionize
Absorption of an electromagnetic wave
 In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
In vivo optical imaging?? Absorption of an electromagnetic wave Tissue absorption spectrum Extinction = Absorption + Scattering Absorption of an electromagnetic wave Scattering of an electromagnetic wave
* The Howard Hughes Medical Institute, The Salk Institute for
 In: J. M. Bower, (Ed.) CornpuPational Neuroscience: Trends In Research 1985, Sen DIsga. G cedernfc Press, 53-58 ( 1896). COMPUTATIONAL MODELS CONSTRAINED BY VOLTAGE-CLAMP DATA FOR INVESTIGATING DENDRITIC
In: J. M. Bower, (Ed.) CornpuPational Neuroscience: Trends In Research 1985, Sen DIsga. G cedernfc Press, 53-58 ( 1896). COMPUTATIONAL MODELS CONSTRAINED BY VOLTAGE-CLAMP DATA FOR INVESTIGATING DENDRITIC
Biophotonics?? Biophotonics. technology in biomedical engineering. Advantages of the lightwave
 Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Biophotonics - Imaging: X-ray, OCT, polarimetry, DOT, TIRF, photon migration, endoscopy, confocal microscopy, multiphoton microscopy, multispectral imaging - Biosensing: IR spectroscopy, fluorescence,
Controlling life with photons A new tool based on conjugated polymers
 Controlling life with photons A new tool based on conjugated polymers Maria Rosa Antognazza Center for Nanoscience and Technology @PoliMi The Italian Institute of Technology Controlling life with photons:
Controlling life with photons A new tool based on conjugated polymers Maria Rosa Antognazza Center for Nanoscience and Technology @PoliMi The Italian Institute of Technology Controlling life with photons:
Special Techniques 1. Mark Scott FILM Facility
 Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
Special Techniques 1 Mark Scott FILM Facility SPECIAL TECHNIQUES Multi-photon microscopy Second Harmonic Generation FRAP FRET FLIM In-vivo imaging TWO-PHOTON MICROSCOPY Alternative to confocal and deconvolution
t e c h n i c a l r e p o r t s
 t e c h n i c a l r e p o r t s Functional imaging of hippocampal place cells at cellular resolution during virtual navigation Daniel A Dombeck, Christopher D Harvey, Lin Tian, Loren L Looger & David W
t e c h n i c a l r e p o r t s Functional imaging of hippocampal place cells at cellular resolution during virtual navigation Daniel A Dombeck, Christopher D Harvey, Lin Tian, Loren L Looger & David W
University of Eastern Finland (UEF) Main Research Lines
 University of Eastern Finland (UEF) Main Research Lines Asla Pitkänen, MD, PhD Epilepsy Research Laboratory A.I.Virtanen Institute for Molecular Sciences University of Eastern Finland Kuopio, Finland E-mail:
University of Eastern Finland (UEF) Main Research Lines Asla Pitkänen, MD, PhD Epilepsy Research Laboratory A.I.Virtanen Institute for Molecular Sciences University of Eastern Finland Kuopio, Finland E-mail:
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 PCR-genotyping of the three mouse models used in this study and controls for behavioral experiments after semi-chronic Pten inhibition. a-c. DNA from App/Psen1 (a), Pten tg (b) and
Supplementary Figure 1 PCR-genotyping of the three mouse models used in this study and controls for behavioral experiments after semi-chronic Pten inhibition. a-c. DNA from App/Psen1 (a), Pten tg (b) and
Page 1 of 5. Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR Cy µg (~7.5 nmol) MIR 7901
 Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Page 1 of 5 Label IT RNAi Delivery Control Product Name Label Quantity Product No. Cy 3 10 µg (~0.75 nmol) MIR 7900 Label IT RNAi Delivery Control Cy 3 100 µg (~7.5 nmol) MIR 7901 Fluorescein 10 µg (~0.75
Light microscopy mapping of connections in the intact brain
 Light microscopy mapping of connections in the intact brain The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published
Light microscopy mapping of connections in the intact brain The MIT Faculty has made this article openly available. Please share how this access benefits you. Your story matters. Citation As Published
Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25
 Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25 9:30 9:45 Coffee and Gipfeli 9:45 10:00 Welcome address and introduction B. Hecht Uni Basel H.-J.
Symposium 20 years of nano-optics April 6th, 2004 Auditorium, Institute of Physics, St.Johanns-Ring 25 9:30 9:45 Coffee and Gipfeli 9:45 10:00 Welcome address and introduction B. Hecht Uni Basel H.-J.
Beyond observation: Microscopy with ultrashort laser pulses to probe and manipulate cortical vasculature
 Prepared for SPIE's newsletter on Optics in Information Systems (Demetri Psaltis and Bahram Javidi, chairs); special issue on Information Processing Applications of Femtosecond Technology (Shaya Fainman,
Prepared for SPIE's newsletter on Optics in Information Systems (Demetri Psaltis and Bahram Javidi, chairs); special issue on Information Processing Applications of Femtosecond Technology (Shaya Fainman,
Confocal Microscopy Analyzes Cells
 Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Choosing Filters for Fluorescence A Laurin Publication Photonic Solutions for Biotechnology and Medicine November 2002 Confocal Microscopy Analyzes Cells Reprinted from the November 2002 issue of Biophotonics
Nonlinear optical endoscopy based on a doubleclad photonic crystal fiber and a MEMS mirror
 Nonlinear optical endoscopy based on a doubleclad photonic crystal fiber and a MEMS mirror Ling Fu a, Ankur Jain b, Huikai Xie b, Charles Cranfield a, and Min Gu a a Centre for Micro-Photonics, Faculty
Nonlinear optical endoscopy based on a doubleclad photonic crystal fiber and a MEMS mirror Ling Fu a, Ankur Jain b, Huikai Xie b, Charles Cranfield a, and Min Gu a a Centre for Micro-Photonics, Faculty
Preclinical models of negative symptoms and their implications for clinical trials. Martien Kas
 Preclinical models of negative symptoms and their implications for clinical trials Martien Kas From diagnose to biology ISCTM ~ ECNP Joint Conference 1 September 2017 Paris France The road to restoring
Preclinical models of negative symptoms and their implications for clinical trials Martien Kas From diagnose to biology ISCTM ~ ECNP Joint Conference 1 September 2017 Paris France The road to restoring
A Brief History of Light Microscopy And How It Transformed Biomedical Research
 A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
A Brief History of Light Microscopy And How It Transformed Biomedical Research Suewei Lin Office: Interdisciplinary Research Building 8A08 Email: sueweilin@gate.sinica.edu.tw TEL: 2789-9315 Microscope
Rice/TCU REU on Computational Neuroscience. Fundamentals of Molecular Imaging
 Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
Rice/TCU REU on Computational Neuroscience Fundamentals of Molecular Imaging June 2, 2009 Neal Waxham 713-500-5621 m.n.waxham@uth.tmc.edu Objectives Introduction to resolution in light microscopy Brief
P. J. Helm a),b) Department of Cell Physiology, The Max Planck Institute for Medical Research, P.O. Box , D Heidelberg, FRG
 A microscopic setup for combined, and time-coordinated electrophysiological and confocal fluorescence microscopic experiments on neurons in living brain slices P. J. Helm a),b) Department of Cell Physiology,
A microscopic setup for combined, and time-coordinated electrophysiological and confocal fluorescence microscopic experiments on neurons in living brain slices P. J. Helm a),b) Department of Cell Physiology,
Challenges to measuring intracellular Ca 2+ Calmodulin: nature s Ca 2+ sensor
 Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
A 0.4-mm-diameter probe for nonlinear optical imaging
 A 0.4-mm-diameter probe for nonlinear optical imaging Hongchun Bao and Min Gu* Centre for Micro-Photonics, Faculty of Engineering & Industrial Sciences, Swinburne University of Technology, Hawthorn, Victoria
A 0.4-mm-diameter probe for nonlinear optical imaging Hongchun Bao and Min Gu* Centre for Micro-Photonics, Faculty of Engineering & Industrial Sciences, Swinburne University of Technology, Hawthorn, Victoria
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING SMART IMAGING SYSTEM The NEWTON 7.0 system combines high sensitivity with advanced animal-handling features and userfriendly
Spontaneous network activity visualized by ultrasensitive Ca 2+ indicators, yellow Cameleon-Nano
 nature methods Spontaneous network activity visualized by ultrasensitive Ca 2+ indicators, yellow Cameleon-Nano Kazuki Horikawa, Yoshiyuki Yamada, Tomoki Matsuda, Kentarou Kobayashi, Mitsuhiro Hashimoto,
nature methods Spontaneous network activity visualized by ultrasensitive Ca 2+ indicators, yellow Cameleon-Nano Kazuki Horikawa, Yoshiyuki Yamada, Tomoki Matsuda, Kentarou Kobayashi, Mitsuhiro Hashimoto,
Measurement of ion channel functions under in vitro conditions. Dr. Norbert Nagy Research Associate Department of Pharmacology and Pharmacotherapy
 Measurement of ion channel functions under in vitro conditions Dr. Norbert Nagy Research Associate Department of Pharmacology and Pharmacotherapy Topics: -Electrophysiological techniques for basic research
Measurement of ion channel functions under in vitro conditions Dr. Norbert Nagy Research Associate Department of Pharmacology and Pharmacotherapy Topics: -Electrophysiological techniques for basic research
Implantable Microelectronic Devices
 ECE 8803/4803 Implantable Microelectronic Devices Fall - 2015 Maysam Ghovanloo (mgh@gatech.edu) School of Electrical and Computer Engineering Georgia Institute of Technology 2015 Maysam Ghovanloo 1 Outline
ECE 8803/4803 Implantable Microelectronic Devices Fall - 2015 Maysam Ghovanloo (mgh@gatech.edu) School of Electrical and Computer Engineering Georgia Institute of Technology 2015 Maysam Ghovanloo 1 Outline
Orflo Application Brief 3 /2017 GFP Transfection Efficiency Monitoring with Orflo s Moxi GO Next Generation Flow Cytometer. Introduction/Background
 Introduction/Background Cell transfection and transduction refer to an array of techniques used to introduce foreign genetic material, or cloning vectors, into cell genomes. The application of these methods
Introduction/Background Cell transfection and transduction refer to an array of techniques used to introduce foreign genetic material, or cloning vectors, into cell genomes. The application of these methods
Research area in the Strategic Objective Development of optical control technologies and elucidation of biological mechanisms
 Research area in the Strategic Objective Development of optical control technologies and elucidation of biological mechanisms 6.1.5 Development and application of optical technology for spatiotemporal
Research area in the Strategic Objective Development of optical control technologies and elucidation of biological mechanisms 6.1.5 Development and application of optical technology for spatiotemporal
Confocal Microscopes. Evolution of Imaging
 Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
Confocal Microscopes and Evolution of Imaging Judi Reilly Hans Richter Massachusetts Institute of Technology Environment, Health & Safety Office Radiation Protection What is Confocal? Pinhole diaphragm
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING
 NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
NEWTON 7.0 BIOLUMINESCENCE & FLUORESCENCE IMAGING IN VIVO - IN VITRO IMAGING The NEWTON s protocol driven image acquisition is as quick as it is intuitive: adjust your exposure, save, print or quantify.
Nature Neuroscience: doi: /nn Supplementary Figure 1
 Supplementary Figure 1 Nanoscale localization precision and relative quantification of CB 1 receptors by 3D-STORM imaging (a) Schematic representation of the experimental paradigm for combined confocal/storm
Supplementary Figure 1 Nanoscale localization precision and relative quantification of CB 1 receptors by 3D-STORM imaging (a) Schematic representation of the experimental paradigm for combined confocal/storm
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat
 Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Supplementary Figure 1: Expression of RNF8, HERC2 and NEURL4 in the cerebellum and knockdown of RNF8 by RNAi (a) Lysates of the cerebellum from rat pups at P6, P14, P22, P30 and adult (A) rats were subjected
Towards Retina Implants for Improvement of Vision in Humans with Retinitis Pigmentosa - Challenges and First Results 1
 Towards Retina Implants for Improvement of Vision in Humans with Retinitis Pigmentosa - Challenges and First Results 1 R. Eckmiller Division of Neuroinformatics, Department of Computer Science University
Towards Retina Implants for Improvement of Vision in Humans with Retinitis Pigmentosa - Challenges and First Results 1 R. Eckmiller Division of Neuroinformatics, Department of Computer Science University
Electrophysiology in the age of light Massimo Scanziani 1 & Michael Häusser 2
 INSIGHT REVIEW NATURE Vol 461 15 October doi:10.1038/nature08540 Electrophysiology in the age of light Massimo Scanziani 1 & Michael Häusser 2 Electrophysiology, the gold standard for investigating neuronal
INSIGHT REVIEW NATURE Vol 461 15 October doi:10.1038/nature08540 Electrophysiology in the age of light Massimo Scanziani 1 & Michael Häusser 2 Electrophysiology, the gold standard for investigating neuronal
CURRICULUM VITAE. David W. Tank
 Personal Birthdate: June 3, 1953 Citizenship : U.S. Address: CURRICULUM VITAE David W. Tank Dept. of Molecular Biology and Dept. of Physics Princeton University Princeton, NJ 05840 Phone: (609) 258-7371
Personal Birthdate: June 3, 1953 Citizenship : U.S. Address: CURRICULUM VITAE David W. Tank Dept. of Molecular Biology and Dept. of Physics Princeton University Princeton, NJ 05840 Phone: (609) 258-7371
Simultaneous Measurement of Neural Spike Recordings and Multi-Photon Calcium Imaging in Neuroblastoma Cells
 Sensors 2012, 12, 15281-15291; doi:10.3390/s121115281 Article OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Simultaneous Measurement of Neural Spike Recordings and Multi-Photon Calcium
Sensors 2012, 12, 15281-15291; doi:10.3390/s121115281 Article OPEN ACCESS sensors ISSN 1424-8220 www.mdpi.com/journal/sensors Simultaneous Measurement of Neural Spike Recordings and Multi-Photon Calcium
Fluorescence Light Microscopy for Cell Biology
 Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
Fluorescence Light Microscopy for Cell Biology Why use light microscopy? Traditional questions that light microscopy has addressed: Structure within a cell Locations of specific molecules within a cell
A 256-by-256 CMOS Microelectrode Array for Extracellular Neural Stimulation of Acute Brain Slices. Columbia University, New York, NY
 A 256-by-256 CMOS Microelectrode Array for Extracellular Neural Stimulation of Acute Brain Slices Na Lei 1, K L Shepard 1, Brendon O Watson 2, Jason N MacLean 2, Rafael Yuste 2 1 Department of Electrical
A 256-by-256 CMOS Microelectrode Array for Extracellular Neural Stimulation of Acute Brain Slices Na Lei 1, K L Shepard 1, Brendon O Watson 2, Jason N MacLean 2, Rafael Yuste 2 1 Department of Electrical
TOOLS sirna and mirna. User guide
 TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
TOOLS sirna and mirna User guide Introduction RNA interference (RNAi) is a powerful tool for suppression gene expression by causing the destruction of specific mrna molecules. Small Interfering RNAs (sirnas)
Supplementary Materials for
 advances.sciencemag.org/cgi/content/full/3/2/e1601966/dc1 Supplementary Materials for Ultraflexible nanoelectronic probes form reliable, glial scar free neural integration Lan Luan, Xiaoling Wei, Zhengtuo
advances.sciencemag.org/cgi/content/full/3/2/e1601966/dc1 Supplementary Materials for Ultraflexible nanoelectronic probes form reliable, glial scar free neural integration Lan Luan, Xiaoling Wei, Zhengtuo
SUMMER SCHOOL LABORATORY ACTIVITIES
 SUMMER SCHOOL LABORATORY ACTIVITIES ACTIVITIES Monday Tuesday Wednesday Thursday 18 th 19 th 20 th 21 st 1 and 2 A B C D 3 and 4 B C D A 5 and 6 C D A B 7 and 8 D A B C The students are divided into 4
SUMMER SCHOOL LABORATORY ACTIVITIES ACTIVITIES Monday Tuesday Wednesday Thursday 18 th 19 th 20 th 21 st 1 and 2 A B C D 3 and 4 B C D A 5 and 6 C D A B 7 and 8 D A B C The students are divided into 4
STRATEGY FOR FITTING NEURONAL MODELS TO DUAL PATCH DATA UNDER MULTIPLE STIMULATION PROTOCOLS
 STRATEGY FOR FITTING NEURONAL MODELS TO DUAL PATCH DATA UNDER MULTIPLE STIMULATION PROTOCOLS GongyuY.Shen 1,2, Shuming Ye 1, Wei R.Chen 2, Jens Midtgaard 3, Gordon.M.Shepherd 2, Michael L.Hines 2 1 Zhejiang
STRATEGY FOR FITTING NEURONAL MODELS TO DUAL PATCH DATA UNDER MULTIPLE STIMULATION PROTOCOLS GongyuY.Shen 1,2, Shuming Ye 1, Wei R.Chen 2, Jens Midtgaard 3, Gordon.M.Shepherd 2, Michael L.Hines 2 1 Zhejiang
OMICS Journals are welcoming Submissions
 OMICS Journals are welcoming Submissions OMICS International welcomes submissions that are original and technically so as to serve both the developing world and developed countries in the best possible
OMICS Journals are welcoming Submissions OMICS International welcomes submissions that are original and technically so as to serve both the developing world and developed countries in the best possible
SUPPLEMENTARY INFORMATION
 doi: 10.1038/nature06293 SUPPLEMENTARY INFORMATION a Testing the incompatibility of Lox2272 and LoxP Construct Expected Lox2272 promoter (CMV) c Testing the incompatibility of LoxN with Lox2272 and LoxP
doi: 10.1038/nature06293 SUPPLEMENTARY INFORMATION a Testing the incompatibility of Lox2272 and LoxP Construct Expected Lox2272 promoter (CMV) c Testing the incompatibility of LoxN with Lox2272 and LoxP
Channelrhodopsin-Assisted Patching: In Vivo Recording of Genetically and Morphologically Identified Neurons throughout the Brain
 Resource Channelrhodopsin-Assisted Patching: In Vivo Recording of Genetically and Morphologically Identified Neurons throughout the Brain Graphical Abstract Authors William Muñoz, Robin Tremblay, Bernardo
Resource Channelrhodopsin-Assisted Patching: In Vivo Recording of Genetically and Morphologically Identified Neurons throughout the Brain Graphical Abstract Authors William Muñoz, Robin Tremblay, Bernardo
λ N -GFP: an RNA reporter system for live-cell imaging
 λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
λ N -GFP: an RNA reporter system for live-cell imaging Nathalie Daigle & Jan Ellenberg Supplementary Figures and Text: Supplementary Figure 1 localization in the cytoplasm. 4 λ N22-3 megfp-m9 serves as
I t is essential for brain research to elucidate how a single neuron projects its dendrites and axon fiber into the
 SUBJECT AREAS: NEUROSCIENCE MULTIPHOTON MICROSCOPY DIODE LASERS FLUORESCENCE IMAGING Visualizing hippocampal neurons with in vivo two-photon microscopy using a 1030 nm picosecond pulse laser Ryosuke Kawakami
SUBJECT AREAS: NEUROSCIENCE MULTIPHOTON MICROSCOPY DIODE LASERS FLUORESCENCE IMAGING Visualizing hippocampal neurons with in vivo two-photon microscopy using a 1030 nm picosecond pulse laser Ryosuke Kawakami
Xiaohan Lai, Wen Li, Christopher Price, Liyun Wang. University of Delaware, Newark, DE, USA.
 Modeling and Testing Dual-Colored Fluorescence Recovery After Photobleaching: A Novel Approach in Quantifying the Osteocytic Pericellular Matrix In Situ Xiaohan Lai, Wen Li, Christopher Price, Liyun Wang.
Modeling and Testing Dual-Colored Fluorescence Recovery After Photobleaching: A Novel Approach in Quantifying the Osteocytic Pericellular Matrix In Situ Xiaohan Lai, Wen Li, Christopher Price, Liyun Wang.
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Background for Schizophrenia Research Article. Sekar A, et al., Schizophrenia risk from complex variation of complement component 4, Nature, 2016
 Background for Schizophrenia Research Article Sekar A, et al., Schizophrenia risk from complex variation of complement component 4, Nature, 2016 Topics The Major Histocompatibility Complex (MHC) region
Background for Schizophrenia Research Article Sekar A, et al., Schizophrenia risk from complex variation of complement component 4, Nature, 2016 Topics The Major Histocompatibility Complex (MHC) region
Reliability and Heterogeneity of Calcium Signaling at Single Presynaptic Boutons of Cerebellar Granule Cells
 7888 The Journal of Neuroscience, July 25, 2007 27(30):7888 7898 Cellular/Molecular Reliability and Heterogeneity of Calcium Signaling at Single Presynaptic Boutons of Cerebellar Granule Cells Stephan
7888 The Journal of Neuroscience, July 25, 2007 27(30):7888 7898 Cellular/Molecular Reliability and Heterogeneity of Calcium Signaling at Single Presynaptic Boutons of Cerebellar Granule Cells Stephan
Supplementary Figure 1
 Supplementary Figure 1 Line temporal focusing performance vs. widefield temporal focusing. (a) Imaging of 10μm fluorescent beads with the same laser power shows a much stronger fluorescence signal (x35)
Supplementary Figure 1 Line temporal focusing performance vs. widefield temporal focusing. (a) Imaging of 10μm fluorescent beads with the same laser power shows a much stronger fluorescence signal (x35)
Cell Processing Workstation. In Situ Laser-Mediated Cell Purification and Processing
 LEAP Cell Processing Workstation Cell Processing Workstation In Situ Laser-Mediated Cell Purification and Processing Expanding the Capabilities of Cell Processing Consistent, High-Yield, High-Purity Processing
LEAP Cell Processing Workstation Cell Processing Workstation In Situ Laser-Mediated Cell Purification and Processing Expanding the Capabilities of Cell Processing Consistent, High-Yield, High-Purity Processing
Dino-Lite knowledge & education. Fluorescence Microscopes
 Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
Dino-Lite knowledge & education Fluorescence Microscopes Dino-Lite Fluorescence models Smallest fluorescence microscope in the world Revolution to biomedical and educational applications Flexible Easy
Fluorescence Microscopy. Terms and concepts to know: 10/11/2011. Visible spectrum (of light) and energy
 Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Fluorescence Microscopy Louisiana Tech University Ruston, Louisiana Microscopy Workshop Dr. Mark DeCoster Associate Professor Biomedical Engineering 1 Terms and concepts to know: Signal to Noise Excitation
Generation and Application of Genetically Modified Mouse Models of Human Disease.
 Generation and Application of Genetically Modified Mouse Models of Human Disease. Nina Balthasar RCUK and BHF Research Fellow Department of Physiology and Pharmacology University of Bristol The Plan Techniques
Generation and Application of Genetically Modified Mouse Models of Human Disease. Nina Balthasar RCUK and BHF Research Fellow Department of Physiology and Pharmacology University of Bristol The Plan Techniques
Confocal Microscopy & Imaging Technology. Yan Wu
 Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Confocal Microscopy & Imaging Technology Yan Wu Dec. 05, 2014 Cells under the microscope What we use to see the details of the cell? Light and Electron Microscopy - Bright light / fluorescence microscopy
Ionscope SICM. About Ionscope. Scanning Ion Conductance Microscopy. Ionscope A brand of OpenIOLabs Limited
 SICM About is a brand of OpenIOLabs Ltd, headquartered in Cambridge UK, is the worldleader in (SICM), a rapidly emerging Scanning Probe Microscopy (SPM) technique which allows nanoscale topographical mapping
SICM About is a brand of OpenIOLabs Ltd, headquartered in Cambridge UK, is the worldleader in (SICM), a rapidly emerging Scanning Probe Microscopy (SPM) technique which allows nanoscale topographical mapping
Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression
 Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression LVP336 LVP336-PBS LVP339 LVP339-PBS LVP297 LVP297-PBS LVP013 LVP013-PBS LVP338 LVP338-PBS LVP027 LVP027-PBS LVP337 LVP337-PBS
Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression LVP336 LVP336-PBS LVP339 LVP339-PBS LVP297 LVP297-PBS LVP013 LVP013-PBS LVP338 LVP338-PBS LVP027 LVP027-PBS LVP337 LVP337-PBS
HELMUT J. KOESTER* AND BERT SAKMANN
 Proc. Natl. Acad. Sci. USA Vol. 95, pp. 9596 9601, August 1998 Neurobiology Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating
Proc. Natl. Acad. Sci. USA Vol. 95, pp. 9596 9601, August 1998 Neurobiology Calcium dynamics in single spines during coincident pre- and postsynaptic activity depend on relative timing of back-propagating
Voltage clamp and patch-clamp techniques
 Voltage clamp and patch-clamp techniques Dr. Nilofar Khan Objectives Historical background Voltage Clamp Theory Variations of voltage clamp Patch-clamp Principal Patch-clamp configurations Applications
Voltage clamp and patch-clamp techniques Dr. Nilofar Khan Objectives Historical background Voltage Clamp Theory Variations of voltage clamp Patch-clamp Principal Patch-clamp configurations Applications
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING. Marc Verhaegen
 HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
HYPERSPECTRAL MICROSCOPE PLATFORM FOR HIGHLY MULTIPLEX BIOLOGICAL IMAGING Marc Verhaegen CMCS, MONTREAL, MAY 11 th, 2017 OVERVIEW Hyperspectral Imaging Multiplex Biological Imaging Multiplex Single Particle
The Journal of Neuroscience. Primary Authors Jeremy N. Kay Harvard U., Department of MCB Research Associate in Sanes Lab University of San Francisco
 Retinal Ganglion Cells with Distinct Directional Preferences Differ in Molecular Identity, Structure, and Central Projections Jeremy N. Kay, Irina De la Huerta, In-Jung Kim, Yifeng Zhang, Masahito Yamagata,
Retinal Ganglion Cells with Distinct Directional Preferences Differ in Molecular Identity, Structure, and Central Projections Jeremy N. Kay, Irina De la Huerta, In-Jung Kim, Yifeng Zhang, Masahito Yamagata,
Optical Recording of Action Potentials with Second-Harmonic Generation Microscopy
 The Journal of Neuroscience, January 28, 2004 24(4):999 1003 999 Brief Communication Optical Recording of Action Potentials with Second-Harmonic Generation Microscopy Daniel A. Dombeck, 1 Mireille Blanchard-Desce,
The Journal of Neuroscience, January 28, 2004 24(4):999 1003 999 Brief Communication Optical Recording of Action Potentials with Second-Harmonic Generation Microscopy Daniel A. Dombeck, 1 Mireille Blanchard-Desce,
Transient and selective suppression of neural activity with infrared light
 Supplementary Material Transient and selective suppression of neural activity with infrared light Authors: Austin R. Duke 1, Michael W. Jenkins 2, Hui Lu 3, Jeffrey M. McManus 3, Hillel J. Chiel 3,2,4,
Supplementary Material Transient and selective suppression of neural activity with infrared light Authors: Austin R. Duke 1, Michael W. Jenkins 2, Hui Lu 3, Jeffrey M. McManus 3, Hillel J. Chiel 3,2,4,
Optogenetics and Multiphoton Excitation. June 2014
 Optogenetics and Multiphoton Excitation June 2014 Optogenetics and Multiphoton Excitation (MPE) MPE is used in Optogenetics for the usual advantages related to nonlinear excitation: Deeper penetration
Optogenetics and Multiphoton Excitation June 2014 Optogenetics and Multiphoton Excitation (MPE) MPE is used in Optogenetics for the usual advantages related to nonlinear excitation: Deeper penetration
Studying the Retina and Other Neurobiological Systems with a Physicist's Toolkit. What s a Physicist Doing in a Biology Lab?
 Studying the Retina and Other Neurobiological Systems with a Physicist's Toolkit or What s a Physicist Doing in a Biology Lab? Physics 10 A. A. Grillo SCIPP UCSC 1 Focus of Talk You may find this talk
Studying the Retina and Other Neurobiological Systems with a Physicist's Toolkit or What s a Physicist Doing in a Biology Lab? Physics 10 A. A. Grillo SCIPP UCSC 1 Focus of Talk You may find this talk
Supplementary Methods
 Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Supplementary Methods MARCM-based forward genetic screen We used ethylmethane sulfonate (EMS) to mutagenize flies carrying FRT 2A and FRT 82B transgenes, which are sites for the FLP-mediated recombination
Neural coding in a single sensory neuron controlling opposite seeking behaviors in Caenorhabditis elegans
 Neural coding in a single sensory neuron controlling opposite seeking behaviors in Caenorhabditis elegans Atsushi Kuhara, Noriyuki Ohnishi, Tomoyasu Shimowada & Ikue Mori Supplementary Information Supplementary
Neural coding in a single sensory neuron controlling opposite seeking behaviors in Caenorhabditis elegans Atsushi Kuhara, Noriyuki Ohnishi, Tomoyasu Shimowada & Ikue Mori Supplementary Information Supplementary
Dendritic targeting of mrna
 Dendritic targeting of mrna FMRP-dependent mglur-induced dendritic targeting of MAP1b and CaMKII mrna IMP-dependent NMDAR-induced dendritic targeting of β-action mrna Discussion (12/15) RNA and protein
Dendritic targeting of mrna FMRP-dependent mglur-induced dendritic targeting of MAP1b and CaMKII mrna IMP-dependent NMDAR-induced dendritic targeting of β-action mrna Discussion (12/15) RNA and protein
Supplementary Figure 1. Serial deletion mutants of BLITz.
 Supplementary Figure 1 Serial deletion mutants of BLITz. (a) Design of TEVseq insertion into J -helix. C-terminal end of J -helix was serially deleted and replaced by TEVseq. TEV cleavage site is labeled
Supplementary Figure 1 Serial deletion mutants of BLITz. (a) Design of TEVseq insertion into J -helix. C-terminal end of J -helix was serially deleted and replaced by TEVseq. TEV cleavage site is labeled
August 15, 2018 $ " % ' & OMe. NEt2 MeO
 Taking a deep look: a near infrared fluorescent dye for long term bioimaging ~ Promising photostable tool for single molecule tracking and multicolor imaging ~ August 15, 2018 A team of researchers at
Taking a deep look: a near infrared fluorescent dye for long term bioimaging ~ Promising photostable tool for single molecule tracking and multicolor imaging ~ August 15, 2018 A team of researchers at
Femtosecond micromachining in polymers
 Femtosecond micromachining in polymers Prof. Dr Cleber R. Mendonca Daniel S. Corrêa Prakriti Tayalia Dr. Tobias Voss Dr. Tommaso Baldacchini Prof. Dr. Eric Mazur fs-micromachining focus laser beam inside
Femtosecond micromachining in polymers Prof. Dr Cleber R. Mendonca Daniel S. Corrêa Prakriti Tayalia Dr. Tobias Voss Dr. Tommaso Baldacchini Prof. Dr. Eric Mazur fs-micromachining focus laser beam inside
LiveReceptor AMPAR <Endogenous AMPAR Labeling Reagent>
 9-7 Hongo 2-Chome, Bunkyo-Ku Tokyo 113-0033, Japan LiveReceptor AMPAR Product Background Neurotransmitter receptors including glutamate receptors and GABA receptors
9-7 Hongo 2-Chome, Bunkyo-Ku Tokyo 113-0033, Japan LiveReceptor AMPAR Product Background Neurotransmitter receptors including glutamate receptors and GABA receptors
The most extensively used technique for tissue analysis is light microscopy.
 Fluorescence Theory Quantum yield Wavelength shift Ligand interactions Membrane interactions Using quenchning effects Fluorescence in-vivo Localization Distance measurements FRET The most extensively used
Fluorescence Theory Quantum yield Wavelength shift Ligand interactions Membrane interactions Using quenchning effects Fluorescence in-vivo Localization Distance measurements FRET The most extensively used
Long-term dynamics of CA1 hippocampal place codes
 Long-term dynamics of CA1 hippocampal place codes Yaniv Ziv, Laurie D. Burns, Eric D. Cocker, Elizabeth O. Hamel, Kunal K. Ghosh, Lacey J. Kitch, Abbas El Gamal, and Mark J. Schnitzer Supplementary Fig.
Long-term dynamics of CA1 hippocampal place codes Yaniv Ziv, Laurie D. Burns, Eric D. Cocker, Elizabeth O. Hamel, Kunal K. Ghosh, Lacey J. Kitch, Abbas El Gamal, and Mark J. Schnitzer Supplementary Fig.
SUMMER SCHOOL LABORATORY ACTIVITIES
 SUMMER SCHOOL LABORATORY ACTIVITIES ACTIVITIES Monday Tuesday Wednesday Thursday 18 th 19 th 20 th 21 st 1 and 2 A B C D 3 and 4 B C D A 5 and 6 C D A B 7 and 8 D A B C The students are divided into 4
SUMMER SCHOOL LABORATORY ACTIVITIES ACTIVITIES Monday Tuesday Wednesday Thursday 18 th 19 th 20 th 21 st 1 and 2 A B C D 3 and 4 B C D A 5 and 6 C D A B 7 and 8 D A B C The students are divided into 4
