Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro
|
|
|
- Beryl Jordan
- 6 years ago
- Views:
Transcription
1 Experimental Neurology 207 (2007) Adipose-derived stem cells differentiate into a Schwann cell phenotype and promote neurite outgrowth in vitro Paul J. Kingham a,, Daniel F. Kalbermatten a,b, Daljeet Mahay a, Stephanie J. Armstrong a, Mikael Wiberg b, Giorgio Terenghi a a Blond McIndoe Research Laboratories, The University of Manchester, Room Stopford Building, Oxford Road, Manchester M13 9PT, UK b Departments of Surgical and Perioperative Science (Handsurgery) and Integrative Medical Biology (Anatomy), Umea University, Sweden Received 31 March 2007; revised 11 June 2007; accepted 29 June 2007 Available online 2 August 2007 Abstract Experimentally, peripheral nerve repair can be enhanced by Schwann cell transplantation but the clinical application is limited by donor site morbidity and the inability to generate a sufficient number of cells quickly. We have investigated whether adult stem cells, isolated from adipose tissue, can be differentiated into functional Schwann cells. Rat visceral fat was enzymatically digested to yield rapidly proliferating fibroblast-like cells, a proportion of which expressed the mesenchymal stem cell marker, stro-1, and nestin, a neural progenitor protein. Cells treated with a mixture of glial growth factors (GGF-2, bfgf, PDGF and forskolin) adopted a spindle-like morphology similar to Schwann cells. Immunocytochemical staining and western blotting indicated that the treated cells expressed the glial markers, GFAP, S100 and p75, indicative of differentiation. When co-cultured with NG motor neuron-like cells, the differentiated stem cells enhanced the number of NG cells expressing neurites, the number of neurites per cell and the mean length of the longest neurite extended. Schwann cells evoked a similar response whilst undifferentiated stem cells had no effect. These results indicate adipose tissue contains a pool of regenerative stem cells which can be differentiated to a Schwann cell phenotype and may be of benefit for treatment of peripheral nerve injuries Elsevier Inc. All rights reserved. Keywords: Adult stem cell; Axon; Glia; Peripheral nerve; Regeneration; Schwann cell Introduction Peripheral nerve injuries are a common occurrence and represent a major economic burden for society (Wiberg and Terenghi, 2003). Treatment usually involves direct end end surgical repair of the damaged nerves for minor defects whereas autologous nerve grafts are required for longer gaps (Lundborg, 2000). Despite good surgical advances, functional recovery is often poor. Tissue engineering techniques which enhance the beneficial endogenous responses to nerve injury could provide an alternative repair strategy. Schwann cells play a pivotal role in peripheral nerve regeneration (Ide, 1996) and are thus an attractive therapeutic target. Nerve injury disrupts the normal Schwann cell axon Corresponding author. Fax: address: paul.j.kingham@manchester.ac.uk (P.J. Kingham). interaction resulting in dedifferentiation of the Schwann cells and activation of a growth promoting phenotype (Hall, 2005). Proliferating Schwann cells release neurotrophic factors (Frostick et al., 1998) and form the bands of Büngner to direct regenerating axons across the lesion. When seeded in artificial nerve conduits, Schwann cells have been shown to enhance nerve regeneration (Li et al., 2006; Mosahebi et al., 2002; Rutkowski et al., 2004). However, cultured Schwann cells have limited clinical application. The requirement for nerve donor material evokes additional morbidity and the time required to culture and expand the cells would delay treatment. Instead, the ideal transplantable cell should be easily accessible, proliferate rapidly in culture and successfully integrate into host tissue with immunological tolerance (Tohill and Terenghi, 2004). Mesenchymal stem cells (MSC) are an attractive cell source for the regeneration of nerve tissue as they are able to self-renew with a high growth rate and possess multi-potent differentiation /$ - see front matter 2007 Elsevier Inc. All rights reserved. doi: /j.expneurol
2 268 P.J. Kingham et al. / Experimental Neurology 207 (2007) properties (Pittenger et al., 1999). There is also some evidence that MSC may be non-immunogenic or hypo-immunogenic (Barry and Murphy, 2004). MSC fibroblast-like cells can be isolated from the stromal cell population found in a number of tissues (Barrilleaux et al., 2006). Bone marrow has been used as the main source of MSC and under appropriate conditions we (Caddick et al., 2006; Tohill et al., 2004) and other groups (Dezawa et al., 2001; Keilhoff et al., 2006) have shown they can be selectively differentiated into Schwann cells. However, the harvest of bone marrow MSC is a highly invasive and painful procedure and alternative sources from which to isolate MSC should be investigated. In the last few years, adipose tissue has been identified as possessing a population of multi-potent stem cells (Gimble and Guilak, 2003; Strem et al., 2005) to which we assign the generic nomenclature, adipose-derived stem cells (ADSC). The phenotypic and gene expression profiles of ADSC are similar to MSC obtained from bone marrow (De Ugarte et al., 2003a,b; Strem et al., 2005) and these cells can be expanded in culture for extended periods (Zuk et al., 2002). Humans have abundant subcutaneous fat deposits and ADSC can easily be isolated by conventional liposuction procedures, thus overcoming the tissue morbidity associated with bone marrow aspiration. Furthermore the frequency of MSC in bone marrow is between 1 in 25,000 and 1 in 100,000 cells (Banfi et al., 2001; D'Ippolito et al., 1999; Muschler et al., 2001) whereas ADSC constitute approximately 2% of lipoaspirate cells (Strem et al., 2005). The apparent advantages of ADSC have led us to investigate whether they can be differentiated to a Schwann cell phenotype which could ultimately provide functional benefits for peripheral nerve repair. Animal models are critical to the development of applications utilising ADSC so in this study we have used stem cells isolated from rats. Since rats possess little subcutaneous fat, we used adipose tissue from the abdominal cavity. Previous studies have shown that stem cells isolated from rat visceral fat mimic the differentiation process of human ADSC and can adopt adipocyte, osteoblast, chondrocyte and neural phenotypes (Tholpady et al., 2003). Materials and methods Isolation and culture of adipose-derived stem cells (ADSC) ADSC were isolated from adult Sprague-Dawley rats euthanized by a schedule 1 method according to the UK Animal Scientific Procedures Act Visceral fat encasing the stomach and intestines was carefully dissected and minced using a sterile razor blade. Tissue was then enzymatically dissociated for 60 min at 37 C using 0.15% (w/v) collagenase type I (Invitrogen, UK). The solution was passed through a 70-μm filter to remove undissociated tissue, neutralized by the addition of Modified Eagle Medium (α- MEM; Invitrogen, UK) containing 10% (v/v) foetal bovine serum (FBS) and centrifuged at 800 g for 5 min. The stromal cell pellet was resuspended in MEM containing 10% (v/v) FBS and 1% (v/v) penicillin/streptomycin solution. Cultures were maintained at subconfluent levels in a 37 C incubator with 5% CO 2 and passaged with trypsin/edta (Invitrogen, UK) when required. Other cell culture Bone marrow-derived stem cells were harvested from adult rat femoral bones (Caddick et al., 2006) and maintained under the same conditions as ADSC. Schwann cells were isolated from the sciatic nerves of 1- to 2-day-old rat pups (Caddick et al., 2006) and cultured in Dulbecco's modified Eagle's medium (DMEM) containing 10% (v/v) FBS, 1% (v/v) penicillin/ streptomycin, 10 μm forskolin (Sigma, UK) and 63 ng/ml glial growth factor-2 (GGF-2; Acorda Therapeutics Inc., USA). The NG cell line was purchased from ECACC (Porton Down, UK) and was maintained in DMEM growth medium. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) cell proliferation assay At either passage 1 or passage 5, cells were trypsinised and plated at a density of 5000 cells/35 mm 2 dish. Cells were allowed to settle for 1 h after which time MTT was added (1 mg/ml final concentration) for a period of 2 h. The resulting formazan precipitate was then solubilised using 20% (v/v) Triton X-100 and the absorbance at 570 nm measured. This value was recorded as the baseline and further measurements were taken every 24 h. Characterisation of stem cell properties At passage 1, sub-confluent cultures were treated with agents to induce the phenotype of mesoderm-derived cells. For osteogenic induction, cultures were treated with 100 μg/ml ascorbate, 0.1 μm dexamethasone and 10 mm β-glycerophosphate for a period of 3 weeks. Cells were then fixed with 4% (w/v) paraformaldehyde for 30 min at room temperature, washed 3 times with phosphate-buffered saline (PBS) containing 1% (w/v) bovine serum albumin and then incubated with 1% (w/v) Alizarin Red solution to stain for calcium deposition. For induction of a chondrocyte phenotype, cells were treated with 0.1 μm dexamethasone, 50 μg/ml ascorbate, 10 ng/ml TGF-β1, 40 μg/ml proline and 1% (v/v) ITS + premix (BD Biosciences, UK) for 3 weeks. Cells were then fixed with 10% (v/v) formalin for 60 min, washed in H 2 O and stained for proteoglycan with 1% (w/v) toluidine blue. For immunocytochemical assessment of stem cell markers, ADSC were cultured on slide flasks (Nunc-Fisher Scientific, UK) for 24 h and then fixed in 4% (w/v) paraformaldehyde for 20 min. Fixative was removed and cells washed 1 10 min in PBS and permeabilised using 0.2% (v/v) Triton X-100 for 20 min. The cells were washed 2 10 min in PBS and 5% (v/v) normal goat serum blocking solution (Sigma, UK) was added for 1 h at room temperature. Monoclonal stro-1 (1:50; R&D Systems, UK) or nestin (1:500; Chemicon, USA) antibodies were added and incubated at 4 C overnight. Cells were washed 3 10 min in PBS and goat anti-mouse Cy3-labelled secondary antibody (1:200; Amersham Biosciences, UK) added for 1 h at room temperature. The cells were washed 3 10 min in PBS and slides mounted using an anti-fading Vectashield solution containing DAPI (Vector labs, UK). Slides were examined using an Olympus
3 P.J. Kingham et al. / Experimental Neurology 207 (2007) IX51 inverted fluorescence microscope and the number of immuno-positive cells counted from a minimum total of 100 cells per experiment. Differentiation to a Schwann cell phenotype Growth medium was removed from sub-confluent ADSC cultures at passage 2 and replaced with medium supplemented with 1 mm β-mercaptoethanol (Sigma-Aldrich, UK) for 24 h. Cells were then washed and fresh medium supplemented with 35 ng/ml all-trans-retinoic acid was added. A further 72 h later, cells were washed and medium replaced with differentiation medium; cell growth medium supplemented with 5 ng/ml platelet-derived growth factor (PDGF; PeproTech Ltd., UK), 10 ng/ml basic fibroblast growth factor (bfgf; PeproTech Ltd., UK), 14 μm forskolin and 252 ng/ml GGF-2. Cells were incubated for 2 weeks under these conditions with fresh medium added approximately every 72 h. Immunocytochemistry and western blotting Undifferentiated (uadsc) and differentiated (dadsc) cultures were trypsinised and replated on slide flasks for immunostaining as above. Cells were incubated with mouse anti-glial fibrillary acidic protein (GFAP; 1:200; Chemicon, USA), rabbit anti-s100 (1:500; Dako, Denmark) and rabbit antip75 (1:500; Promega, USA) overnight at 4 C. Washed slides were then treated with goat anti-rabbit FITC- (1:100; Vector Labs, UK) and goat anti-mouse CY3 (1:200; Amersham, UK)- conjugated secondary antibodies. Slides were examined using an Olympus IX51 inverted fluorescence microscope and the number of immuno-positive cells counted in a minimum total of 100 cells per experiment. For western blotting, individual lysates were prepared from one 75-cm 2 flask of confluent cultures. Cells were washed in PBS and then scraped into buffer containing 100 mm PIPES, 5 mm MgCl, 20% (v/v) glycerol, 0.5% (v/v) Triton X-100, 5 mm EGTA and protease inhibitors (Sigma, UK). Lysates wereincubatedfor15minoniceandthensubjectedto2 freeze thaw cycles prior to analysis of protein content using a commercially available protein assay kit (Bio-Rad, UK). 15 μg protein was prepared per sample, combined with Laemmli buffer and denatured at 95 C for 5 min. Proteins were resolved at 120 V on 15% (for S100) or 10% (for GFAP) sodium dodecyl sulphate polyacrylamide gels. Following electrophoretic transfer to nitrocellulose, membranes were blocked for 1 h in 5% (w/v) non-fat dry milk in TBS Tween (10 mm Tris ph 7.5, 100 mm NaCl, 0.1% (v/v) Tween), and then incubated overnight at 4 C with either monoclonal anti-gfap (1:200; LabVison, USA), monoclonal anti-s100 (1:750; Chemicon, UK) or polyclonal anti-p75 (1:250; Santa Cruz Biotechnology, USA) antibodies. Following 6 5 min washes in TBS Tween, membranes were incubated for 1 h with HRP-conjugated secondary antibodies [goat anti-mouse and goat anti-rabbit (1:1000; Cell Signalling Technology, USA)]. Membranes were washed as previously and treated with ECL chemiluminescent substrate (Amersham, UK) for 1 min and developed by exposure to Kodak X-OMAT light-sensitive film. Antibody was stripped from the membranes using 100 mm glycine ph 2.9 and the blots re-probed with β-tubulin antibody (1:1000; Abcam, UK) as a loading control. Stem cell and NG neuron co-culture uadsc and dadsc were plated at a density of 10,000 cells per slide flask and allowed to settle for 24 h. NG cells were then added to the ADSC monolayer at a density of 1000 cells and the co-cultures maintained for a further 24 h followed by fixation with 4% (w/v) paraformaldehyde for 20 min at room temperature. Fluorescent immunocytochemistry (as above) using mouse anti-neurofilament protein antibody (1:500; BioMol, UK) incubated at 4 C overnight followed by secondary labelling with goat anti-mouse Cy3 conjugate (1:200; Amersham Biosciences, UK) was used to visualise NG neurite outgrowth on the ADSC. Co-culture with NG cells did not alter the differentiation status of ADSC (as measured by expression of glial protein markers). Cultures were examined using an Olympus IX51 inverted fluorescence microscope and images captured using Image ProPlus software (MediaCybernetics, Marlow, UK). The trace function was used to determine the percentage of NG cells expressing Fig. 1. Proliferation of cells isolated from adipose tissue and bone marrow. An MTT assay was used to determine the growth rate of cells obtained from adipose tissue ( ) and bone ) plated at passage 1 (A) and passage 5 (B). Data are ( expressed as the mean % increase±s.e.m. in absorbance (570 nm). Pb0.05 significantly higher in adipose tissue derived cultures compared with those from bone.
4 270 P.J. Kingham et al. / Experimental Neurology 207 (2007) Results Characterisation of stem cell cultures Rat visceral adipose tissue was enzymatically digested and then centrifuged to isolate the stromal cell fraction from mature adipocytes. After approximately 1 week in culture, cells from the stromal fraction formed confluent fibroblast-like monolayers on 75-cm 2 flasks. Cells were then trypsinised and plated for MTT proliferation assays (Fig. 1). There was an apparent lag phase in growth of cells up to 48 h after which time the rate of proliferation expanded more rapidly. Cells isolated from Fig. 2. Adipose tissue-derived cells exhibit properties of stem cells. (A) Cultures were treated with agents to induce differentiation to cells of mesoderm origin. Alizarin red-stained and toluidine blue-stained cells indicate cells of osteogenic and chondrogenic lineages, respectively. Scale bar=40 μm. (B) Adipose tissuederived cells at passage 1 were stained with anti-stro-1 and anti-nestin antibodies and CY3-conjugated secondary antibody (red). DAPI staining (blue) indicates the total number of cells in the field and it was used to quantify the percentage of cells positive for each antigen. Scale bar=40 μm. (C) Data are expressed as the mean % positive±s.e.m. Pb0.01 significantly higher levels of nestinpositive cells in adipose tissue-derived cultures compared with those from bone. neurites and the number of neurites expressed per cell. For each experiment neurite data were ordered according to length (μm). The longest neurite in each experiment was thus identified and the mean of these calculated from 5 independent experiments. An average of one hundred NG cell bodies was analysed for each condition in each experiment. Statistical analysis Data are presented as mean ±S.E.M. from 4 to 5 independent cell cultures. Kruskal Wallis one way ANOVA with Dunn's comparison test was used to determine the statistical significance between data, p b0.05; pb0.01. Fig. 3. Adipose-derived stem cells differentiate to a Schwann cell phenotype. (A) Cultures of undifferentiated ADSC (uadsc) showed a flattened fibroblastlike morphology which adopt a spindle elongated shape characteristic of Schwann cells (dadsc) upon treatment with glial growth factors. Scale bar=40 μm. (B) Immunofluorescence staining indicated differentiated ADSC (dadsc) expressed GFAP, S100 and p75 proteins. Scale bar=30 μm. (C) Quantitative analysis of morphology and expression of GFAP, S100 and p75 proteins (data are mean % cells±s.e.m.).
5 P.J. Kingham et al. / Experimental Neurology 207 (2007) Fig. 4. Western blot analysis of Schwann cell proteins. Undifferentiated ADSC (uadsc), differentiated ADSC (dadsc) and Schwann cell (SC) lysates were blotted for GFAP, S100 and p75 proteins. β-tubulin was used as a loading control. expressed GFAP (Fig. 3C). Almost all of these GFAP-positive cells also stained for S100 protein (42.9 ± 3.3% positive). p75 expression was occasionally observed in uadsc but was readily apparent in the cultures treated with glial growth factors (36.38 ± 3.3% positive). A small fraction (16.8 ± 1.4%) of treated ADSC retained a fibroblast-like morphology, some of which expressed GFAP (5.9±1.3%), S100 (3.4±1.0%) and p75 (1.23±0.38%) proteins (Fig. 3C). A minority of cells (1.7±0.5%) displayed a rounded cell body with multiple processes. To confirm the results obtained by immunocytochemistry were not due to an artefact of cellular shrinkage, western blotting was performed (Fig. 4). Lysates of dadsc but not uadsc showed a GFAP-immunoreactive band corresponding to a molecular weight of 55 kda. This was present in Schwann cell lysates together with an additional lower band which is likely to represent a proteolytic fragment or alternate transcript. bone marrow exhibited a similar growth pattern; however, the overall proliferation rate of cells taken from adipose tissue was significantly faster in passage 1 cultures (Fig. 1A). Although the growth rate of passage 5 adipose cells in the lag phase was not different from bone-derived cells, they still proliferated significantly faster after 48 h (Fig. 1B). In order to determine whether the cells isolated from adipose tissue exhibited properties of mesenchymal stem cells, they were treated with agents known to induce differentiation to cells originating from the mesoderm. Osteogenic differentiation was confirmed by the production of calcium deposits detected with Alizarin Red (Fig. 2A) and chondrocyte differentiation by the presence of toluidine blue-positive proteoglycans (Fig. 2A). We also examined the passage 1 cultures for the presence of the stem cell marker, stro-1. A small proportion (11.38 ± 0.87%) of cells were positive for stro-1 in adipose cell cultures, a similar number to that found in bone marrow cultures (Figs. 2B and C). Since we were interested in deriving cells of a neural lineage we also determined whether the cells expressed nestin, a putative marker of neural progenitors (Dahlstrand et al., 1995). There were approximately three times more nestin-positive cells in cultures of cells taken from adipose tissue compared with those from bone (Fig. 2C; 14.6 ±0.8% vs. 5.1 ±1.6%, Pb0.01). Differentiation to a Schwann cell phenotype Having determined our cultures contained a population of stem-like cells, we assign the term adipose-derived stem cell (ADSC) to describe them. ADSC at passage 2 were treated with a mixture of glial growth factors for a period of 2 weeks after which time they were analysed morphologically and for the expression of the Schwann cell proteins, GFAP, S100 and p75. Cells cultured in the differentiation media changed from a fibroblast-like morphology to an elongated spindle shape (Fig. 3A), similar to that of Schwann cells. Neither GFAP nor S100 protein was detected in undifferentiated cultures (uadsc) (data not shown) but both were expressed in differentiated (dadsc) cells (Fig. 3B). Quantitative analysis indicated that 81.5±1.5% of the cells adopted a spindle-like morphology of which 44.5 ± 3.7% Fig. 5. Differentiated ADSC promote neurite outgrowth in NG cells. (A) NG cells were grown alone (con NG108-15) or on a monolayer of undifferentiated (NG uADSC) or differentiated (NG dADSC) stem cells. Cultures were stained with neurofilament antibody (red) to visualise neurites and DAPI (blue) to highlight individual cells. Scale bar=60 μm. (B) The percentage of NG cells expressing neurites, number of neurites per cell and length of longest neurite were determined in NG cells grown alone (con NG108-15) and co-cultures with stem cells (+uadsc, +dadsc) and Schwann cells (+SC). Data are mean±s.e.m. Pb0.05; Pb0.01 significantly different compared with NG grown alone.
6 272 P.J. Kingham et al. / Experimental Neurology 207 (2007) S100 and p75 proteins were also detected in dadsc but were absent in uadsc. Functional properties of differentiated cells The ability of ADSC to promote neurite outgrowth was determined by examining their interaction with NG cells, a motor neuron-like cell line (Jiang et al., 2003). uadsc and dadsc were plated on slide flasks to form monolayers and then NG cells were added (Fig. 5A). Computerised image analysis of co-cultures after 24 h was used to quantify three separate parameters: percentage of cells extending neurites, number of neurites per cell and length of longest neurite (Fig. 5B). Comparisons were made with control cultures of NG cells grown alone and NG cells seeded with Schwann cells. A small fraction (22.0 ± 2.5%) of control NG cells extended neurites which was significantly increased to 69.1±4.1% (Pb 0.05) and 57.6±5.3% (Pb0.05) in the presence of dadsc and Schwann cells respectively. uadsc had no significant effect. Likewise the number of neurites extended per cell was significantly (P b 0.05) increased in co-cultures of NG cells with dadsc or Schwann cells, when compared with NG cells grown alone. The mean longest neurite extended by control cultures of NG cells was 67.5±7.5 μm and in co-culture with uadsc it was 74.3±9.7 μm. In contrast, dadsc evoked a significant (Pb0.01) increase in neurite length to 205.2±2.7 μm and Schwann cells stimulated an increase to 309.8±31.5 μm (Pb 0.01). Discussion We have recently shown that MSC derived from bone marrow can undergo differentiation to a Schwann cell phenotype (Caddick et al., 2006; Tohill et al., 2004). Given the clinical advantages of adipose tissue as an alternative source of stem cells (Gimble and Guilak, 2003; Strem et al., 2005), we have now investigated whether it is also possible to derive Schwann cells from adipose tissue. We found that rat ADSC treated with a mixture of glial growth factors expressed GFAP, S100 and p75 proteins and enhanced neurite outgrowth in vitro, suggesting transition to a Schwann cell phenotype. As part of an initial characterisation of our cultures, we compared the growth rate of cells isolated from adipose tissue and bone marrow. We found that ADSC proliferated significantly faster than bone MSC. This is consistent with a recent study comparing various sources of rat MSC (Yoshimura et al., 2007) and if translated to human studies could mean a reduction in the time required to generate a therapeutically useful stock of cells. In order to determine the stem-ness of our cells we investigated the expression of stro-1. In contrast to the study by Ning et al. (2006) which identified all ADSC as stro-1 positive, only a small fraction of our cultures expressed this marker. This apparent discrepancy has also been reported by different groups examining human ADSC (De Ugarte et al., 2003a,b; Gronthos et al., 2001) and might reflect a difference in the region from which the tissue was obtained. Nestin is a protein commonly used to identify proliferating adult neural progenitor cells in the central nervous system (Dahlstrand et al., 1995). More recently nestin expression has been observed in bone marrow MSC (Caddick et al., 2006; Wislet-Gendebien et al., 2004) and its importance in controlling commitment to differentiation along the glial lineage has been demonstrated (Wislet-Gendebien et al., 2004). Our results showed that when compared with bone marrow MSC, a significantly greater proportion of ADSC expressed nestin protein. Murine ADSC have also been shown to express low levels of nestin which can be up-regulated upon neurogenic differentiation (Safford et al., 2002). These results suggest that ADSC are not restricted towards specific mesodermal cell lineages and rather they retain some ability for differentiation along a neuroglial lineage. To investigate this, we exposed the ADSC to a differentiation media we have previously used to induce Schwann cells from bone marrow MSC (Caddick et al., 2006). This produced a morphological change in the majority of the cells, toward an elongating spindle phenotype, characteristic of Schwann cells. Many of these cells also began to express the glial markers, GFAP, S100 and p75. The co-expression of these proteins taken with the morphological changes indicates we can produce high yields of Schwann-like cells from ADSC. In contrast, previous reports have shown that neural induction media converts ADSC to cells of a neuronal morphology with co-expression of GFAP, S100 and neuronal proteins including βiii-tubulin and neurofilament (Ning et al., 2006; Safford et al., 2004). Krampera et al. (2007) recently showed these changes were rapid and reversible, suggesting against a specific, full differentiation process. To induce a more selective Schwann cell differentiation, the authors co-cultured various MSC with Schwann cells and this produced a long lasting expression of PMP-20 and S100 proteins in the absence of other CNS glial and neuronal markers (Krampera et al., 2007). Interestingly, of all the MSC tested, those derived from adipose tissue produced the best response. These effects could not be induced by Schwann cell factors alone, indicating contact between the two cell types was necessary (Krampera et al., 2007). Whilst this suggests that some form of trans-differentiation might occur if ADSC were transplanted at a nerve injury site in vivo, the methodology does not provide a suitable approach for the generation of clinically useful cells. Instead, we have used a defined mixture of GGF-2, bfgf and PDGF, molecules which are known to play a role in the differentiation and proliferation of Schwann cells (Jessen and Mirsky, 1999; Li et al., 1998). These molecules together with forskolin are responsible for the induction of the glial protein expression we observed by immunocytochemistry. These results were confirmed by western blotting; arguing against the notion suggested by Lu et al. (2004) that increased staining is merely the result of an increase in antigen levels per unit area, due to disruption of the cytoskeleton. We also tested the function of our differentiated ADSC using a co-culture with the NG motor neuron-like cell line. We found that differentiated ADSC evoked a similar response to Schwann cells, in that they promoted neurite outgrowth and elongation. NG cells have been shown to express low levels of trka (Fu et al., 1997) and GFR-α1 (Lee et al., 2006), receptors for nerve growth factor (NGF) and glial-derived neurotrophic factor (GDNF), respectively, two proteins which
7 P.J. Kingham et al. / Experimental Neurology 207 (2007) are known to be released by Schwann cells (Frostick et al., 1998). To date, there have been no reports of the expression of neurotrophic factors produced by ADSC. However, a recent study has shown that rat bone marrow cells constitutively express NGF, GDNF and brain-derived neurotrophic factor (BDNF) (Chen et al., 2007). Human MSC also express NGF and BDNF although this is restricted to certain sub-populations (Crigler et al., 2006). These and other unidentified factors promoted neurite outgrowth and survival in a co-culture system with SH-SY5Y cells (Crigler et al., 2006). In our model, undifferentiated ADSC had no effect on neurite outgrowth suggesting that the process of differentiation led to the upregulation of nerve growth factors. In on-going experiments to identify the factors involved we have determined that conditioned medium from dadsc increases the number of NG cells producing neurites and neurite number per cell but does not enhance neurite length (data not shown). Thus direct contact (the model used in this study) between the cells is required for neurite elongation and this suggests that dadsc might also up-regulate extracellular matrix molecules such as laminin or fibronectin, which have also been shown to be expressed by rat bone marrow MSC (Chen et al., 2007). Our results suggest that ADSC differentiated to a Schwann cell phenotype might have a beneficial role for treatment of peripheral nerve injuries. We have previously shown that only differentiated bone marrow MSC, rather than untreated MSC, can stimulate nerve regeneration (Tohill et al., 2004), a result in agreement with other groups (Dezawa et al., 2001; Keilhoff et al., 2006). In contrast, other studies indicate that undifferentiated bone marrow MSC can also enhance regeneration and lead to improved motor function (Chen et al., 2007; Pereira Lopes et al., 2006). This suggests that some form of trans-differentiation might occur in vivo as the result of local signals from injured Schwann cells and axons. However, the long-term effect of undifferentiated cells remains to be determined and therefore we will continue to study the mechanisms of differentiation of ADSC, in the hope of generating clinically useful cells, for the treatment of peripheral nerve injuries. Acknowledgments This work was supported by grants from the UK Northwest Regional Development Agency, The Wellcome Trust, the UK and Swedish Medical Research Councils and the County of Vasterbotten and the Aners Foundation. We also gratefully acknowledge the continuing supply of GGF-2 from Acorda Therapeutics, USA. References Banfi, A., Bianchi, G., Galotto, M., Cancedda, R., Quarto, R., Bone marrow stromal damage after chemo/radiotherapy: occurrence, consequences and possibilities of treatment. Leuk. Lymphoma 42, Barrilleaux, B., Phinney, D.G., Prockop, D.J., O'Connor, K.C., Review: ex vivo engineering of living tissues with adult stem cells. Tissue Eng Barry, F.P., Murphy, J.M., Mesenchymal stem cells: clinical applications and biological characterization. Int. J. Biochem. Cell Biol. 36, Caddick, J., Kingham, P.J., Gardiner, N.J., Wiberg, M., Terenghi, G., Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia 54, Chen, C.J., Ou, Y.C., Liao, S.L., Chen, W.Y., Chen, S.Y., Wu, C.W., Wang, C.C., Wang, W.Y., Huang, Y.S., Hsu, S.H., Transplantation of bone marrow stromal cells for peripheral nerve repair. Exp. Neurol. 204, Crigler, L., Robey, R.C., Asawachaicharn, A., Gaupp, D., Phinney, D.G., Human mesenchymal stem cell subpopulations express a variety of neuroregulatory molecules and promote neuronal cell survival and neuritogenesis. Exp. Neurol. 198, D'Ippolito, G., Schiller, P.C., Ricordi, C., Roos, B.A., Howard, G.A., Age-related osteogenic potential of mesenchymal stromal stem cells from human vertebral bone marrow. J. Bone Miner. Res. 14, Dahlstrand, J., Lardelli, M., Lendahl, U., Nestin mrna expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Brain Res. Dev. Brain Res. 84, De Ugarte, D.A., Alfonso, Z., Zuk, P.A., Elbarbary, A., Zhu, M., Ashjian, P., Benhaim, P., Hedrick, M.H., Fraser, J.K., 2003a. Differential expression of stem cell mobilization-associated molecules on multi-lineage cells from adipose tissue and bone marrow. Immunol. Lett. 89, De Ugarte, D.A., Morizono, K., Elbarbary, A., Alfonso, Z., Zuk, P.A., Zhu, M., Dragoo, J.L., Ashjian, P., Thomas, B., Benhaim, P., Chen, I., Fraser, J., Hedrick, M.H., 2003b. Comparison of multi-lineage cells from human adipose tissue and bone marrow. Cells Tissues Organs 174, Dezawa, M., Takahashi, I., Esaki, M., Takano, M., Sawada, H., Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur. J. Neurosci. 14, Frostick, S.P., Yin, Q., Kemp, G.J., Schwann cells, neurotrophic factors, and peripheral nerve regeneration. Microsurgery 18, Fu, A.K., Ip, F.C., Lai, K.O., Tsim, K.W., Ip, N.Y., Muscle-derived neurotrophin-3 increases the aggregation of acetylcholine receptors in neuron muscle co-cultures. Neuroreport 8, Gimble, J., Guilak, F., Adipose-derived adult stem cells: isolation, characterization, and differentiation potential. Cytotherapy 5, Gronthos, S., Franklin, D.M., Leddy, H.A., Robey, P.G., Storms, R.W., Gimble, J.M., Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 189, Hall, S., The response to injury in the peripheral nervous system. J. Bone Jt. Surg., Br. 87, Ide, C., Peripheral nerve regeneration. Neurosci. Res. 25, Jessen, K.R., Mirsky, R., Developmental regulation in the Schwann cell lineage. Adv. Exp. Med. Biol. 468, Jiang, J.X., Choi, R.C., Siow, N.L., Lee, H.H., Wan, D.C., Tsim, K.W., Muscle induces neuronal expression of acetylcholinesterase in neuron muscle co-culture: transcriptional regulation mediated by camp-dependent signaling. J. Biol. Chem. 278, Keilhoff, G., Goihl, A., Langnase, K., Fansa, H., Wolf, G., Transdifferentiation of mesenchymal stem cells into Schwann cell-like myelinating cells. Eur. J. Cell Biol. 85, Krampera, M., Marconi, S., Pasini, A., Galie, M., Rigotti, G., Mosna, F., Tinelli, M., Lovato, L., Anghileri, E., Andreini, A., Pizzolo, G., Sbarbati, A., Bonetti, B., Induction of neural-like differentiation in human mesenchymal stem cells derived from bone marrow, fat, spleen and thymus. Bone 40, Lee, R.H., Wong, W.L., Chan, C.H., Chan, S.Y., Differential effects of glial cell line-derived neurotrophic factor and neurturin in RET/GFRalpha1- expressing cells. J. Neurosci. Res. 83, Li, H., Wigley, C., Hall, S.M., Chronically denervated rat Schwann cells respond to GGF in vitro. Glia 24, Li, Q., Ping, P., Jiang, H., Liu, K., Nerve conduit filled with GDNF genemodified Schwann cells enhances regeneration of the peripheral nerve. Microsurgery 26, Lu, P., Blesch, A., Tuszynski, M.H., Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J. Neurosci. Res. 77,
8 274 P.J. Kingham et al. / Experimental Neurology 207 (2007) Lundborg, G., A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J. Hand Surg. [Am.] 25, Mosahebi, A., Fuller, P., Wiberg, M., Terenghi, G., Effect of allogeneic Schwann cell transplantation on peripheral nerve regeneration. Exp. Neurol. 173, Muschler, G.F., Nitto, H., Boehm, C.A., Easley, K.A., Age- and genderrelated changes in the cellularity of human bone marrow and the prevalence of osteoblastic progenitors. J. Orthop. Res. 19, Ning, H., Lin, G., Lue, T.F., Lin, C.S., Neuron-like differentiation of adipose tissue-derived stromal cells and vascular smooth muscle cells. Differentiation 74, Pereira Lopes, F.R., Camargo de Moura, C.L., Dias Jr., C.J., Balduino, A., Lora, S., Langone, F., Borojevic, R., Blanco Martinez, A.M., Bone marrow stromal cells and resorbable collagen guidance tubes enhance sciatic nerve regeneration in mice. Exp. Neurol. 198, Pittenger, M.F., Mackay, A.M., Beck, S.C., Jaiswal, R.K., Douglas, R., Mosca, J.D., Moorman, M.A., Simonetti, D.W., Craig, S., Marshak, D.R., Multilineage potential of adult human mesenchymal stem cells. Science 284, Rutkowski, G.E., Miller, C.A., Jeftinija, S., Mallapragada, S.K., Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration. J. Neural Eng. 1, Safford, K.M., Hicok, K.C., Safford, S.D., Halvorsen, Y.D., Wilkison, W.O., Gimble, J.M., Rice, H.E., Neurogenic differentiation of murine and human adipose-derived stromal cells. Biochem. Biophys. Res. Commun. 294, Safford, K.M., Safford, S.D., Gimble, J.M., Shetty, A.K., Rice, H.E., Characterization of neuronal/glial differentiation of murine adipose-derived adult stromal cells. Exp. Neurol. 187, Strem, B.M., Hicok, K.C., Zhu, M., Wulur, I., Alfonso, Z., Schreiber, R.E., Fraser, J.K., Hedrick, M.H., Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 54, Tholpady, S.S., Katz, A.J., Ogle, R.C., Mesenchymal stem cells from rat visceral fat exhibit multipotential differentiation in vitro. Anat. Rec., A Discov. Mol. Cell Evol. Biol. 272, Tohill, M., Terenghi, G., Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol. Appl. Biochem. 40, Tohill, M., Mantovani, C., Wiberg, M., Terenghi, G., Rat bone marrow mesenchymal stem cells express glial markers and stimulate nerve regeneration. Neurosci. Lett. 362, Wiberg, M., Terenghi, G., Will it be possible to produce peripheral nerves? Surg. Technol. Int. 11, Wislet-Gendebien, S., Bruyere, F., Hans, G., Leprince, P., Moonen, G., Rogister, B., Nestin-positive mesenchymal stem cells favour the astroglial lineage in neural progenitors and stem cells by releasing active BMP4. BMC Neurosci. 5, 33. Yoshimura, H., Muneta, T., Nimura, A., Yokoyama, A., Koga, H., Sekiya, I., Comparison of rat mesenchymal stem cells derived from bone marrow, synovium, periosteum, adipose tissue, and muscle. Cell Tissue Res. 327, Zuk, P.A., Zhu, M., Ashjian, P., De Ugarte, D.A., Huang, J.I., Mizuno, H., Alfonso, Z.C., Fraser, J.K., Benhaim, P., Hedrick, M.H., Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13,
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Cultrex Rat Mesenchymal Stem Cell Growth and Differentiation Products
 ITM-2011/06/07 Cultrex Rat Mesenchymal Stem Cell Growth and Differentiation Products Background: Mesenc ymal Stem Cells (MSC), also known as marrow stromal cells, are a self - renewing population of multipotent
ITM-2011/06/07 Cultrex Rat Mesenchymal Stem Cell Growth and Differentiation Products Background: Mesenc ymal Stem Cells (MSC), also known as marrow stromal cells, are a self - renewing population of multipotent
Comparison of Human Muscle-Derived Stem Cells and Human Adipose-Derived Stem Cells in Neurogenic Trans-Differentiation
 www.kjurology.org http://dx.doi.org/10.4111/kju.2011.52.12.852 Sexual Dysfunction Comparison of Human Muscle-Derived Stem Cells and Human Adipose-Derived Stem Cells in Neurogenic Trans-Differentiation
www.kjurology.org http://dx.doi.org/10.4111/kju.2011.52.12.852 Sexual Dysfunction Comparison of Human Muscle-Derived Stem Cells and Human Adipose-Derived Stem Cells in Neurogenic Trans-Differentiation
Human Bone Marrow Derived Mesenchymal Stem Cell (MSC) Care Manual
 Human Bone Marrow Derived Mesenchymal Stem Cell (MSC) Care Manual INSTRUCTION MANUAL ZBM0101.001 SHIPPING CONDITIONS Human Bone Marrow Derived MSC (HBMMSC-F) Orders are delivered via Federal Express courier.
Human Bone Marrow Derived Mesenchymal Stem Cell (MSC) Care Manual INSTRUCTION MANUAL ZBM0101.001 SHIPPING CONDITIONS Human Bone Marrow Derived MSC (HBMMSC-F) Orders are delivered via Federal Express courier.
Mouse/Rat Neural Stem Cell Functional Identification Kit
 Mouse/Rat Neural Stem Cell Functional Identification Kit Catalog Number SC013 Reagents for the identification of mouse/rat Neural Stem Cells (NSCs) by in vitro functional differentiation. This package
Mouse/Rat Neural Stem Cell Functional Identification Kit Catalog Number SC013 Reagents for the identification of mouse/rat Neural Stem Cells (NSCs) by in vitro functional differentiation. This package
Table of Contents. 2.1 NeuroCult NCFC Assay Kit (Rat) Components Additional Required Reagents Required Equipment...
 i Table of Contents 1.0 Overview of the NeuroCult NCFC Assay 2.0 Materials 2.1 NeuroCult NCFC Assay Kit (Rat) Components... 4 2.2 Additional Required Reagents... 4 2.3 Required Equipment... 4 3.0 Preparation
i Table of Contents 1.0 Overview of the NeuroCult NCFC Assay 2.0 Materials 2.1 NeuroCult NCFC Assay Kit (Rat) Components... 4 2.2 Additional Required Reagents... 4 2.3 Required Equipment... 4 3.0 Preparation
Osteogenic Differentiation and Analysis of MSC
 Osteogenic Differentiation and Analysis of MSC Application Note Background Mesenchymal Stem Cells (MSC) are fibroblastoid multipotent adult stem cells with a high capacity for self-renewal. So far, these
Osteogenic Differentiation and Analysis of MSC Application Note Background Mesenchymal Stem Cells (MSC) are fibroblastoid multipotent adult stem cells with a high capacity for self-renewal. So far, these
Supplemental Information. Materials and methods.
 Supplemental Information Materials and methods. Cell culture. hmscs were isolated from bone marrow of 3 male donors, undergoing orthopedic surgery (mean age 69.7). Cells were cultured in high glucose DMEM
Supplemental Information Materials and methods. Cell culture. hmscs were isolated from bone marrow of 3 male donors, undergoing orthopedic surgery (mean age 69.7). Cells were cultured in high glucose DMEM
UK +44 (0) CH +41 (0) DE +49 (0) US
 UK +44 (0) 1235 232100- CH +41 (0) 91 604 5522 - DE +49 (0) 69 779099 - US +1 855 267 2464 Featured Product Areas Stem Cell Fate Regulators and Synthetic Retinoid ec23 Recombinant Growth Factor Mimetics
UK +44 (0) 1235 232100- CH +41 (0) 91 604 5522 - DE +49 (0) 69 779099 - US +1 855 267 2464 Featured Product Areas Stem Cell Fate Regulators and Synthetic Retinoid ec23 Recombinant Growth Factor Mimetics
Cdc42 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Cdc42 Activation Assay Kit Catalog Number: 80701 20 assays 1 Table of Content Product Description 3 Assay
B. ADM: C. D. Apoptosis: 1.68% 2.99% 1.31% Figure.S1,Li et al. number. invaded cells. HuH7 BxPC-3 DLD-1.
 A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
A. - Figure.S1,Li et al. B. : - + - + - + E-cadherin CK19 α-sma vimentin β -actin C. D. Apoptosis: 1.68% 2.99% 1.31% - : - + - + - + Apoptosis: 48.33% 45.32% 44.59% E. invaded cells number 400 300 200
Culture of human mesenchymal stem cells in Nunc Cell Factory systems
 APPLICATION NOTE Cell Factory systems Culture of human mesenchymal stem cells in Nunc Cell Factory systems Key words: Cell culture, cell culture scale-up, Nunc Cell Factory system, hmsc, expansion and
APPLICATION NOTE Cell Factory systems Culture of human mesenchymal stem cells in Nunc Cell Factory systems Key words: Cell culture, cell culture scale-up, Nunc Cell Factory system, hmsc, expansion and
SUPPLEMENTARY NOTE 3. Supplememtary Note 3, Wehr et al., Monitoring Regulated Protein-Protein Interactions Using Split-TEV 1
 SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
SUPPLEMENTARY NOTE 3 Fluorescent Proteolysis-only TEV-Reporters We generated TEV reporter that allow visualizing TEV activity at the membrane and in the cytosol of living cells not relying on the addition
IgG TrueBlot Protocol for Mouse, Rabbit or Goatderived Antibodies - For Research Use Only
 IgG TrueBlot Protocol for Mouse, Rabbit or Goatderived Antibodies - For Research Use Only Introduction The IgG TrueBlot for mouse, rabbit, or goat-derived antibodies represents unique series of respective
IgG TrueBlot Protocol for Mouse, Rabbit or Goatderived Antibodies - For Research Use Only Introduction The IgG TrueBlot for mouse, rabbit, or goat-derived antibodies represents unique series of respective
RheB Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based RheB Activation Assay Kit Catalog Number: 81201 20 assays NewEast Biosciences 1 FAX: 610-945-2008 Table
Arf6 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Arf6 Activation Assay Kit Catalog Number: 82401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Arf6 Activation Assay Kit Catalog Number: 82401 20 assays NewEast Biosciences 1 Table of Content Product
Differentiation of Adipose-derived Stem Cells into Schwann Cell Phenotype in Comparison with Bone Marrow Stem Cells
 Iranian Journal of Basic Medical Sciences Vol. 13, No. 3, Summer 2010, 76-84 Received: Oct 21, 2009; Accepted: Feb 21, 2010 Original article Differentiation of Adipose-derived Stem Cells into Schwann Cell
Iranian Journal of Basic Medical Sciences Vol. 13, No. 3, Summer 2010, 76-84 Received: Oct 21, 2009; Accepted: Feb 21, 2010 Original article Differentiation of Adipose-derived Stem Cells into Schwann Cell
Marilyn G. Rimando, Hao-Hsiang Wu, Yu-An Liu, Chien-Wei Lee, Shu-Wen Kuo, Yin-
 Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
Supplementary Information Glucocorticoid receptor and Histone Deacetylase 6 mediate the differential effect of dexamethasone during osteogenesis of Mesenchymal stromal cells (MSCs) Marilyn G. Rimando,
Methods Western blot analysis of plg Quantification of plasminogen accumulation by ELISA Immunohistochemical analysis
 Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
Methods Western blot analysis of plg Wild-type mice first received a standardized burn wound and then were intravenously administered 2 mg of human plg (Omnio AB, Umeå, Sweden). 24 hours after wounding
Application Note. Introduction. Kerry Thompson, Jeff Partridge, Paula Flaherty, Susan Qian, and Deepa Saxena
 Page 1 BD PureCoat ECM Mimetic Cultureware Fibronectin Peptide: Novel Synthetic, Animalfree Surface for Culture of Human Bone Marrow-derived Mesenchymal Stem Cells Kerry Thompson, Jeff Partridge, Paula
Page 1 BD PureCoat ECM Mimetic Cultureware Fibronectin Peptide: Novel Synthetic, Animalfree Surface for Culture of Human Bone Marrow-derived Mesenchymal Stem Cells Kerry Thompson, Jeff Partridge, Paula
Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration?
 Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Kaleidoscope Volume 11 Article 30 July 2014 Do Sensory Neurons Secrete an Anti-Inhibitory Factor that Promotes Regeneration? Azita Bahrami Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Gα 13 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα 13 Activation Assay Kit Catalog Number: 80401 20 assays NewEast Biosciences 1 Table of Content Product
Anti-HB-EGF (Human) mab
 Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Page 1 For Research Use Only. Not for use in diagnostic procedures. CODE No. D308-3 Anti-HB-EGF (Human) mab CLONALITY CLONE ISOTYPE QUANTITY SOURCE IMMUNOGEN FORMURATION STORAGE Monoclonal 3H4 Mouse IgG1
Rab5 Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Rab5 Activation Assay Kit Catalog Number: 83701 20 assays 24 Whitewoods Lane 1 Table of Content Product
This Document Contains:
 This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
Effects Of Hypoxia-conditioned Medium On MSC Chondrogenesis
 Effects Of Hypoxia-conditioned Medium On MSC Chondrogenesis Marcel Betsch, M.D., Regina Wehrle, M.D., Brian Johnstone, Ph.D.. Oregon Health & Science University, Portland, OR, USA. Disclosures: M. Betsch:
Effects Of Hypoxia-conditioned Medium On MSC Chondrogenesis Marcel Betsch, M.D., Regina Wehrle, M.D., Brian Johnstone, Ph.D.. Oregon Health & Science University, Portland, OR, USA. Disclosures: M. Betsch:
Instructions For Research Use Only. Not For Use In Diagnostic Procedures. Rat Mesenchymal Stem Cell Starter Kit. Rat Mesenchymal Stem Cell Starter Kit
 Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cell Starter Kit Rat Mesenchymal Stem Cell Starter Kit Cat# 5000-001-K Primary Mesenchymal Stem Cells isolated
Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cell Starter Kit Rat Mesenchymal Stem Cell Starter Kit Cat# 5000-001-K Primary Mesenchymal Stem Cells isolated
Stem cells and tissue engineering
 Stem cells and tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
Stem cells and tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
APA105Hu01 100µg Active Nerve Growth Factor (NGF) Organism Species: Homo sapiens (Human) Instruction manual
 APA105Hu01 100µg Active Nerve Growth Factor (NGF) Organism Species: Homo sapiens (Human) Instruction manual FOR RESEARCH USE ONLY NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES [ PROPERTIES ] 1th Edition
APA105Hu01 100µg Active Nerve Growth Factor (NGF) Organism Species: Homo sapiens (Human) Instruction manual FOR RESEARCH USE ONLY NOT FOR USE IN CLINICAL DIAGNOSTIC PROCEDURES [ PROPERTIES ] 1th Edition
Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde
 Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Supplementary text Supplementary materials and methods Histopathological examination Segments of the obstructed intestinal loops were fixed in 4% paraformaldehyde (PFA) and embedded in paraffin wax with
Human ipsc-derived Sensory Neuron Progenitors. For the generation of ipsc-derived sensory neurons
 Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Product Information Catalog. No. Product Name Format Stock Conc. Storage on Arrival Thawing Instructions
Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Product Information Catalog. No. Product Name Format Stock Conc. Storage on Arrival Thawing Instructions
Human ipsc-derived Sensory Neuron Progenitors. For the generation of ipsc-derived sensory neurons
 Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Additional Reagents Product Name Supplier Product Code Mitomycin C Sigma-Aldrich M4287 Store at 4 o C for
Human ipsc-derived Sensory Neuron Progenitors For the generation of ipsc-derived sensory neurons Additional Reagents Product Name Supplier Product Code Mitomycin C Sigma-Aldrich M4287 Store at 4 o C for
Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW
 Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW Lyset, the human platelet derived supplement for cell culture Among the different alternatives to animal serum, platelet derived preparations
Lyset BOOST YOUR CELL CULTURE TODAY FOR THE EXPERIMENTS OF TOMORROW Lyset, the human platelet derived supplement for cell culture Among the different alternatives to animal serum, platelet derived preparations
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells
 Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
StemXVivo. Mesoderm Kit. Catalog Number SC030B. Reagents for the differentiation of human pluripotent stem cells into mesoderm.
 StemXVivo Mesoderm Kit Catalog Number SC030B Reagents for the differentiation of human pluripotent stem cells into mesoderm. This package insert must be read in its entirety before using this product.
StemXVivo Mesoderm Kit Catalog Number SC030B Reagents for the differentiation of human pluripotent stem cells into mesoderm. This package insert must be read in its entirety before using this product.
Instructions For Research Use Only. Not For Use In Diagnostic Procedures. Rat Mesenchymal Stem Cells. Rat Mesenchymal Stem Cells.
 Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cells Rat Mesenchymal Stem Cells Cat# 5000-001-01 Primary Mesenchymal Stem Cells Isolated from Rat Bone Marrow
Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cells Rat Mesenchymal Stem Cells Cat# 5000-001-01 Primary Mesenchymal Stem Cells Isolated from Rat Bone Marrow
Supplemental Materials and Methods
 Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Supplemental Materials and Methods In situ hybridization In situ hybridization analysis of HFE2 and genin mrna in rat liver tissues was performed as previously described (1). Briefly, the digoxigenin-labeled
Schwann cells differentiated from adipose derived stem cells for the treatment of brain contusion
 MOLECULAR MEDICINE REPORTS 9: 567-573, 2014 Schwann cells differentiated from adipose derived stem cells for the treatment of brain contusion LIANG YANG 1, JIASHENG FANG 1, DAGUANG LIAO 1, and WEI WANG
MOLECULAR MEDICINE REPORTS 9: 567-573, 2014 Schwann cells differentiated from adipose derived stem cells for the treatment of brain contusion LIANG YANG 1, JIASHENG FANG 1, DAGUANG LIAO 1, and WEI WANG
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1
 Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
Supplementary Information (Ha, et. al) Supplementary Figures Supplementary Fig. S1 a His-ORMDL3 ~ 17 His-ORMDL3 GST-ORMDL3 - + - + IPTG GST-ORMDL3 ~ b Integrated Density (ORMDL3/ -actin) 0.4 0.3 0.2 0.1
User Manual. OriCell TM Dog Adipose-Derived Mesenchymal. Stem Cells (ADSCs) Cat. No. CAXMD-01001
 User Manual OriCell TM Dog Adipose-Derived Mesenchymal Stem Cells (ADSCs) Cat. No. CAXMD-01001 Table of Contents Contents and Storage 3 Product Introduction... 3 Cell Characteristics and Identity. 3 Product
User Manual OriCell TM Dog Adipose-Derived Mesenchymal Stem Cells (ADSCs) Cat. No. CAXMD-01001 Table of Contents Contents and Storage 3 Product Introduction... 3 Cell Characteristics and Identity. 3 Product
Rat Cortical Stem Cells
 Rat Cortical Stem Cells Catalog Number NSC001 For cortical stem cell expansion and differentiation into neurons, astrocytes, and oligodendrocytes. This package insert must be read in its entirety before
Rat Cortical Stem Cells Catalog Number NSC001 For cortical stem cell expansion and differentiation into neurons, astrocytes, and oligodendrocytes. This package insert must be read in its entirety before
Gα i Activation Assay Kit
 A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
A helping hand for your research Product Manual Configuration-specific Monoclonal Antibody Based Gα i Activation Assay Kit Catalog Number 80301 20 assays NewEast Biosciences, Inc 1 Table of Content Product
Supplementary Information. Conversion of vascular endothelial cells into multipotent stem-like cells
 Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
Supplementary Information Conversion of vascular endothelial cells into multipotent stem-like cells Damian Medici 1, Eileen M. Shore 2,3,4, Vitali Y. Lounev 2,4, Frederick S. Kaplan 2,4,5, Raghu Kalluri
Quantum Dot Labeling of Stem Cells during Proliferation and Differentiation
 Copyright 2006 Tech Science Press MCB, vol.3, no.4, pp.153-155, 2006 Quantum Dot Labeling of Stem Cells during Proliferation and Differentiation E. K. Moioli 1, B. Shah 1, P. A. Clark 1, M. Stroscio 1
Copyright 2006 Tech Science Press MCB, vol.3, no.4, pp.153-155, 2006 Quantum Dot Labeling of Stem Cells during Proliferation and Differentiation E. K. Moioli 1, B. Shah 1, P. A. Clark 1, M. Stroscio 1
Effects of KAATSU Training on proliferation and differentiation of goat bone marrow mesenchymal stem cells
 Chinese Journal of Laboratory Diagnosis Issued on 25 Aug 2016 Vol. 20, No. 8 P.1240 Effects of KAATSU Training on proliferation and differentiation of goat bone marrow mesenchymal stem cells YANG Yu-hui,
Chinese Journal of Laboratory Diagnosis Issued on 25 Aug 2016 Vol. 20, No. 8 P.1240 Effects of KAATSU Training on proliferation and differentiation of goat bone marrow mesenchymal stem cells YANG Yu-hui,
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and
 Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Supplementary Figure 1: Derivation and characterization of RN ips cell lines. (a) RN ips cells maintain expression of pluripotency markers OCT4 and SSEA4 after 10 passages in mtesr 1 medium. (b) Schematic
Human ESC-derived Neural Progenitor Expansion Starter Panel. Reagents for the expansion of human neural progenitors.
 Human ESC-derived Neural Progenitor Expansion Starter Panel Catalog Number SC016 Reagents for the expansion of human neural progenitors. This package insert must be read in its entirety before using this
Human ESC-derived Neural Progenitor Expansion Starter Panel Catalog Number SC016 Reagents for the expansion of human neural progenitors. This package insert must be read in its entirety before using this
Mouse Mesenchymal Stem Cell Functional Identification Kit
 Mouse Mesenchymal Stem Cell Functional Identification Kit Catalog Number SC010 Reagents for the identification of mouse bone marrow-derived stem cells (BMSC)/mesenchymal stem cells (MSC) by in vitro functional
Mouse Mesenchymal Stem Cell Functional Identification Kit Catalog Number SC010 Reagents for the identification of mouse bone marrow-derived stem cells (BMSC)/mesenchymal stem cells (MSC) by in vitro functional
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling
 Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Supplementary information to accompany: A novel role for the DNA repair gene Rad51 in Netrin-1 signalling Glendining KA 1, Markie D 2, Gardner RJM 4, Franz EA 3, Robertson SP 4, Jasoni CL 1 Supplementary
Description of supplementary material file
 Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
Description of supplementary material file In the supplementary results we show that the VHL-fibronectin interaction is indirect, mediated by fibronectin binding to COL4A2. This provides additional information
Instructions For Research Use Only. Not For Use In Diagnostic Procedures. Rat Mesenchymal Stem Cell Starter Kit. Rat Mesenchymal Stem Cell Starter Kit
 Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cell Starter Kit Rat Mesenchymal Stem Cell Starter Kit Cat# 5000-001-K Primary Mesenchymal Stem Cells isolated
Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cell Starter Kit Rat Mesenchymal Stem Cell Starter Kit Cat# 5000-001-K Primary Mesenchymal Stem Cells isolated
Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis
 Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis Diptiman Chanda 1*, Ashish Kurundkar 1, Sunad Rangarajan 1, Morgan Locy 1, Karen Bernard 1,
Developmental Reprogramming in Mesenchymal Stromal Cells of Human Subjects with Idiopathic Pulmonary Fibrosis Diptiman Chanda 1*, Ashish Kurundkar 1, Sunad Rangarajan 1, Morgan Locy 1, Karen Bernard 1,
Background. Background. Background. Background. Background 3/4/2013
 3/4/213 Human Very Small Embryonic-like Stem Cells (s) Capacity to Regenerate Bone Tissue: A Potential Cell-based Therapy for Autogenous Bone Grafting Aaron M. Havens, DMD Need to discover an improved
3/4/213 Human Very Small Embryonic-like Stem Cells (s) Capacity to Regenerate Bone Tissue: A Potential Cell-based Therapy for Autogenous Bone Grafting Aaron M. Havens, DMD Need to discover an improved
Rat Mesenchymal Stem Cell Starter Kit
 Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cell Starter Kit Cat # 5000-001-K Primary Mesenchymal Stem Cells isolated and purified from Rat Bone Marrow
Instructions For Research Use Only. Not For Use In Diagnostic Procedures Rat Mesenchymal Stem Cell Starter Kit Cat # 5000-001-K Primary Mesenchymal Stem Cells isolated and purified from Rat Bone Marrow
g: relative centrifugal force R: radius of the rotor S: speed of the centrifuge in revolutions per minute
 Propagation of Mesenchymal Stem Cells The density of the colonies is the primary consideration in determining when the cells should be passaged. Cells grow densely should be passaged, even though the cells
Propagation of Mesenchymal Stem Cells The density of the colonies is the primary consideration in determining when the cells should be passaged. Cells grow densely should be passaged, even though the cells
Protocol for induction of expression and cell lysate production
 Protocol for induction of expression and cell lysate production AV-04 Doxycyclin induction and cell lysate 1.0 Introduction / Description This method is intended for the treatment of the previously transfected
Protocol for induction of expression and cell lysate production AV-04 Doxycyclin induction and cell lysate 1.0 Introduction / Description This method is intended for the treatment of the previously transfected
Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor
 SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
SUPPLEMENTARY INFORMATION Single cell imaging of Bruton's Tyrosine Kinase using an irreversible inhibitor Anna Turetsky 1,a, Eunha Kim 1,a, Rainer H. Kohler 1, Miles A. Miller 1, Ralph Weissleder 1,2,
The Effects of Different Sources of Fetal Bovine Serum on Chondrocyte Growth
 The Effects of Different Sources of Fetal Bovine Serum on Chondrocyte Growth Stacey T. Chu, B.A., Nita Chen, B.A., Ruobin Wu, B.A., Alexis B.C. Dang, M.D.. University of California, San Francisco, San
The Effects of Different Sources of Fetal Bovine Serum on Chondrocyte Growth Stacey T. Chu, B.A., Nita Chen, B.A., Ruobin Wu, B.A., Alexis B.C. Dang, M.D.. University of California, San Francisco, San
ApoTrack Cytochrome c Apoptosis ICC Antibody Kit
 ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
ab110417 ApoTrack Cytochrome c Apoptosis ICC Antibody Kit Instructions for Use For the Immunocytochemistry analysis of cytochrome c and a mitochondrial marker (Complex Vα) in apoptotic cells and non-apoptotic
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE.
 NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
NEURONAL CELL CULTURE MATRIX FOR BETTER MAINTENANCE AND SURVIVAL OF NEURONAL CELL CULTURES IN TISSUE CULTURE. D. R. Aguirre, N. DiMassa, Chrystal Johnson, H. Eran, R. Perez, C.V.R. Sharma, M.V.R. Sharma,
Osteoblast Differentiation and Mineralization
 Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts (HOB) are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
Osteoblast Differentiation and Mineralization Application Note Background Osteoblasts (HOB) are specialized fibroblasts that secrete and mineralize the bone matrix. They develop from mesenchymal precursors.
All quality control test results are reported on a lot specific Certificate of Analysis which is available at or upon request.
 PRIME-XV NPC Expansion XSFM PRIME-XV NPC Expansion XSFM is a xeno-and serum-free medium optimized for the cultivation and expansion of neural progenitor cells (NPC) that are able to maintain their ability
PRIME-XV NPC Expansion XSFM PRIME-XV NPC Expansion XSFM is a xeno-and serum-free medium optimized for the cultivation and expansion of neural progenitor cells (NPC) that are able to maintain their ability
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplemental Information
 Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Supplemental Information Intrinsic protein-protein interaction mediated and chaperonin assisted sequential assembly of a stable Bardet Biedl syndome protein complex, the BBSome * Qihong Zhang 1#, Dahai
Apoptosis of Rat Adipose-Derived Stem Cells during Transdifferentiation to Schwann-Like Cell
 IJMS Vol 35, No 2, June 2010 Original Article Apoptosis of Rat Adipose-Derived Stem Cells during Transdifferentiation to Schwann-Like Cell Zolikha Golipoor 1, Iraj Ragerdi Kashani 1, Mohammad Akbari 1,
IJMS Vol 35, No 2, June 2010 Original Article Apoptosis of Rat Adipose-Derived Stem Cells during Transdifferentiation to Schwann-Like Cell Zolikha Golipoor 1, Iraj Ragerdi Kashani 1, Mohammad Akbari 1,
Expansion of Human Mesenchymal Stem Cells Using Corning HYPER Technology Cell Culture Vessels
 Expansion of Human Mesenchymal Stem Cells Using Corning HYPER Technology Cell Culture Vessels SnAPPshots A brief technical report from the Corning Development Group Ana Maria P. Pardo and Mark E. Rothenberg,
Expansion of Human Mesenchymal Stem Cells Using Corning HYPER Technology Cell Culture Vessels SnAPPshots A brief technical report from the Corning Development Group Ana Maria P. Pardo and Mark E. Rothenberg,
Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays
 Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays Elizabeth J. Abraham, Ph.D. BD Biosciences Outline Overview of surfaces and culture
Development of Novel Advanced Cell Culture Surfaces that Provide Better Cell Growth and Attachment for Cell-Based Assays Elizabeth J. Abraham, Ph.D. BD Biosciences Outline Overview of surfaces and culture
Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice
 Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice Supplementary Material Supplementary Methods Materials Spironolactone, aldosterone and β-glycerophosphate were
Spironolactone ameliorates PIT1-dependent vascular osteoinduction in klotho-hypomorphic mice Supplementary Material Supplementary Methods Materials Spironolactone, aldosterone and β-glycerophosphate were
Supporting Information
 Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information Cell culture. C2C12 cells (ATCC CRL 1772) were cultured in 75 cm 2
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information Cell culture. C2C12 cells (ATCC CRL 1772) were cultured in 75 cm 2
User Manual. OriCell TM C57BL/6 Mouse Adipose-derived Mesenchymal Stem Cells With GFP (ADSCs/GFP) Cat. No. MUBMD-01
 User Manual OriCell TM C57BL/6 Mouse Adipose-derived Mesenchymal Stem Cells With GFP (ADSCs/GFP) Cat. No. MUBMD-01 Table of Contents Contents and Storage. 3 Product Introduction. 3 Cell Characteristics
User Manual OriCell TM C57BL/6 Mouse Adipose-derived Mesenchymal Stem Cells With GFP (ADSCs/GFP) Cat. No. MUBMD-01 Table of Contents Contents and Storage. 3 Product Introduction. 3 Cell Characteristics
Impairment of Alveolar Macrophage Transcription in. Idiopathic Pulmonary Fibrosis. Online Data Supplement
 Impairment of Alveolar Macrophage Transcription in Idiopathic Pulmonary Fibrosis Online Data Supplement Ping Ren, Ivan O. Rosas, Sandra D. MacDonald, Hai-Ping Wu, Eric M. Billings, and Bernadette R. Gochuico
Impairment of Alveolar Macrophage Transcription in Idiopathic Pulmonary Fibrosis Online Data Supplement Ping Ren, Ivan O. Rosas, Sandra D. MacDonald, Hai-Ping Wu, Eric M. Billings, and Bernadette R. Gochuico
The Ultimate Low Cell Binding Surface
 The Ultimate Low Cell Binding Surface Thermo Scientific Nunc HydroCell Surface SINGLE CELL CULTURES Grow cells that are sensitive to activation and differentiation signals arising from cell adhesion CELL
The Ultimate Low Cell Binding Surface Thermo Scientific Nunc HydroCell Surface SINGLE CELL CULTURES Grow cells that are sensitive to activation and differentiation signals arising from cell adhesion CELL
Western Blot Tool Box
 Western Blot Tool Box BOX12/BOX12-03 V1.1 Store at 2-8 C For research use only Introduction The Western Blot Tool Box is designed to conveniently provide reagents/buffers needed for Western blotting, from
Western Blot Tool Box BOX12/BOX12-03 V1.1 Store at 2-8 C For research use only Introduction The Western Blot Tool Box is designed to conveniently provide reagents/buffers needed for Western blotting, from
Human Pluripotent Stem Cell Functional Identification Kit
 Human Pluripotent Stem Cell Functional Identification Kit Catalog Number SC027B Reagents for the identification of human pluripotent stem cells by in vitro functional differentiation. This package insert
Human Pluripotent Stem Cell Functional Identification Kit Catalog Number SC027B Reagents for the identification of human pluripotent stem cells by in vitro functional differentiation. This package insert
Immunoprecipitation Protocol
 Immunoprecipitation Protocol Immunoprecipitation is a general method to obtain the enrichment of a specific protein from tissue lysate and cell lysate. It can be used to purify a specific protein, to identify
Immunoprecipitation Protocol Immunoprecipitation is a general method to obtain the enrichment of a specific protein from tissue lysate and cell lysate. It can be used to purify a specific protein, to identify
User Manual. OriCell TM Rabbit Mesenchymal Stem Cells (MSCs) Cat. No. RBXMX-01001
 User Manual OriCell TM Rabbit Mesenchymal Stem Cells (MSCs) Cat. No. RBXMX-01001 Table of Contents Contents and Storage 3 Product Introduction 3 Cell Characteristics and Identity 3 Product Application
User Manual OriCell TM Rabbit Mesenchymal Stem Cells (MSCs) Cat. No. RBXMX-01001 Table of Contents Contents and Storage 3 Product Introduction 3 Cell Characteristics and Identity 3 Product Application
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Protocol Reprogramming Human Fibriblasts using the Dox Inducible Reprogramming Polycistronic Lentivirus Set: Human 4F2A LoxP
 STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
STEMGENT Page 1 OVERVIEW The following protocol describes the reprogramming of one well of BJ Human Fibroblasts (BJ cells) into induced pluripotent stem (ips) cells in a 6-well format. Transduction efficiency
PeproGrow hmsc Medium
 PeproGrow hmsc Medium Maintenance Media for Human Mesenchymal Stem Cells Complete Media Superior growth rates Xeno-free, phenol red-free Phenotypic integrity Maintained multipotency Affordable and competitive
PeproGrow hmsc Medium Maintenance Media for Human Mesenchymal Stem Cells Complete Media Superior growth rates Xeno-free, phenol red-free Phenotypic integrity Maintained multipotency Affordable and competitive
SUPPLEMENTAL MATERIAL. Supplemental Methods:
 SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
SUPPLEMENTAL MATERIAL Supplemental Methods: Immunoprecipitation- As we described but with some modifications [22]. As part of another ongoing project, lysate from human umbilical vein endothelial cells
All quality control test results are reported on a lot specific Certificate of Analysis which is available at or upon request.
 PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
PRIME-XV Neural Basal Medium PRIME-XV Neural Basal Medium is a chemically-defined basal medium optimized for the culture and maintenance of neuronal cells when supplemented with PRIME-XV IS21 Supplement
Day 7 Day 21 MNC CD14 + CD14- Depleted
 Day 7 Day 21 A B MNC C D CD14 + E F CD14- Depleted Supplemental Figure 1. DUOC-01 cell product develops from CD14 + monocytes in cord blood. Cultures were established with cord blood mononuclear cells
Day 7 Day 21 A B MNC C D CD14 + E F CD14- Depleted Supplemental Figure 1. DUOC-01 cell product develops from CD14 + monocytes in cord blood. Cultures were established with cord blood mononuclear cells
Components Neural induction basal medium (Cat. # ) 100 ml x 5 Neural induction medium supplement 50x (Cat. # ) 2 ml x 5
 HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
HPSC Neural Induction Medium (PSCNIM) Catalog #5931 Introduction Human embryonic stem cells (hesc) and induced pluripotent stem cells (hipsc) possess the capability of differentiating into all derivatives
Supplementary Material
 Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Material Supplementary Methods Cell synchronization. For synchronized cell growth, thymidine was added to 30% confluent U2OS cells to a final concentration of 2.5mM. Cells were incubated
Supplementary Figure 1 A green: cytokeratin 8
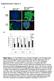 Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
Supplementary Figure 1 A green: cytokeratin 8 green: α-sma red: α-sma blue: DAPI blue: DAPI Panc-1 Panc-1 Panc-1+hPSC Panc-1+hPSC monoculture coculture B Suppl. Figure 1: A, Immunofluorescence staining
RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the
 Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Supplementary Methods RT-PCR and real-time PCR analysis RNA was isolated using NucleoSpin RNA II (Macherey-Nagel, Bethlehem, PA) according to the manufacturer s protocol and quantified by measuring the
Isolation, culture, and transfection of primary mammary epithelial organoids
 Supplementary Experimental Procedures Isolation, culture, and transfection of primary mammary epithelial organoids Primary mammary epithelial organoids were prepared from 8-week-old CD1 mice (Charles River)
Supplementary Experimental Procedures Isolation, culture, and transfection of primary mammary epithelial organoids Primary mammary epithelial organoids were prepared from 8-week-old CD1 mice (Charles River)
Zenon Goat IgG Labeling Kits
 Product Information Revised: 19 June 2007 Quick Facts Storage upon receipt: 2 6 C Protect from light Abs/Em: See Table 1 Unlabeled IgG antibody Zenon labeling reagent (labeled Fab fragment) Introduction
Product Information Revised: 19 June 2007 Quick Facts Storage upon receipt: 2 6 C Protect from light Abs/Em: See Table 1 Unlabeled IgG antibody Zenon labeling reagent (labeled Fab fragment) Introduction
Cells Culture Techniques Marta Czernik
 Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
ReliaBLOT TM. IP/Western Blot Reagents Cat. No. WB120, rabbit
 ReliaBLOT TM Introduction: IP/Western Blot Reagents Cat. No. WB120, rabbit ReliaBLOT TM IP/Western Blot Reagents and Procedures (patent pending) provide an improved method for the detection of immunoprecipitated
ReliaBLOT TM Introduction: IP/Western Blot Reagents Cat. No. WB120, rabbit ReliaBLOT TM IP/Western Blot Reagents and Procedures (patent pending) provide an improved method for the detection of immunoprecipitated
Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands).
 Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
Attenuation of MSC Hypertrophy in 3D Culture via Treatment with a Retinoic Acid Receptor Inverse Agonist
 Attenuation of MSC Hypertrophy in 3D Culture via Treatment with a Retinoic Acid Receptor Inverse Agonist Christian G. Pfeifer, M.D. 1,2, Minwook Kim 2, Megan J. Farrell, B.S. 2, Maurizio Pacifici, Ph.D.
Attenuation of MSC Hypertrophy in 3D Culture via Treatment with a Retinoic Acid Receptor Inverse Agonist Christian G. Pfeifer, M.D. 1,2, Minwook Kim 2, Megan J. Farrell, B.S. 2, Maurizio Pacifici, Ph.D.
Data Sheet. Hedgehog Signaling Pathway Gli Reporter NIH3T3 Cell Line Catalog #: 60409
 Data Sheet Hedgehog Signaling Pathway Gli Reporter NIH3T3 Cell Line Catalog #: 60409 Product Description The Gli Reporter NIH3T3 Cell Line is designed for monitoring the activity of the hedgehog signaling
Data Sheet Hedgehog Signaling Pathway Gli Reporter NIH3T3 Cell Line Catalog #: 60409 Product Description The Gli Reporter NIH3T3 Cell Line is designed for monitoring the activity of the hedgehog signaling
Protein Translation Study Label Protein with S35 Methionine in Cells Salma Hasan and Isabelle Plo *
 Protein Translation Study Label Protein with S35 Methionine in Cells Salma Hasan and Isabelle Plo * INSERM U1009, Gustave Roussy, Villejuif, France *For correspondence: isabelle.plo@gustaveroussy.fr [Abstract]
Protein Translation Study Label Protein with S35 Methionine in Cells Salma Hasan and Isabelle Plo * INSERM U1009, Gustave Roussy, Villejuif, France *For correspondence: isabelle.plo@gustaveroussy.fr [Abstract]
Xeno-Free Systems for hesc & hipsc. Facilitating the shift from Stem Cell Research to Clinical Applications
 Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
Xeno-Free Systems for hesc & hipsc Facilitating the shift from Stem Cell Research to Clinical Applications NutriStem Defined, xeno-free (XF), serum-free media (SFM) specially formulated for growth and
PRIMARY NEURONAL CULTURE
 PRIMARY NEURONAL CULTURE Products for Your Research Scientists Helping Scientists WWW.STEMCELL.COM TABLE OF CONTENTS 3 Primary Neuronal Culture NeuroCult SM1 and BrainPhys Neuronal Medium 4 Increased Neuronal
PRIMARY NEURONAL CULTURE Products for Your Research Scientists Helping Scientists WWW.STEMCELL.COM TABLE OF CONTENTS 3 Primary Neuronal Culture NeuroCult SM1 and BrainPhys Neuronal Medium 4 Increased Neuronal
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW)
 Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
Propagation of H7 hesc From: UW (John Stamatoyannopoulos) ENCODE group Date: 12/17/2009 Prepared By: S. Paige/S. Hansen (UW) Growth and Harvest Modifications Addendum to: Propagation of H7 hesc from UW
HEK293A cells were cultured in high glucose (4.500 mg/l) Dulbecco s modified
 Additional methods: HEK293A and C7 cell cultures HEK293A cells were cultured in high glucose (4.500 mg/l) Dulbecco s modified Eagle s medium (DMEM) with GlutaMAX I (Invitrogen, Cergy Pontoise, France),
Additional methods: HEK293A and C7 cell cultures HEK293A cells were cultured in high glucose (4.500 mg/l) Dulbecco s modified Eagle s medium (DMEM) with GlutaMAX I (Invitrogen, Cergy Pontoise, France),
Supplemental material
 Supplemental material THE JOURNAL OF CELL BIOLOGY Taylor et al., http://www.jcb.org/cgi/content/full/jcb.201403021/dc1 Figure S1. Representative images of Cav 1a -YFP mutants with and without LMB treatment.
Supplemental material THE JOURNAL OF CELL BIOLOGY Taylor et al., http://www.jcb.org/cgi/content/full/jcb.201403021/dc1 Figure S1. Representative images of Cav 1a -YFP mutants with and without LMB treatment.
Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis
 Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis Yan Liu 1,*, Zhipeng Han 1,*, Yingying Jing 1,*, Xue Yang 1, Shanshan Zhang 1, Chen
Contribution and Mobilization of Mesenchymal Stem Cells in a mouse model of carbon tetrachloride-induced liver fibrosis Yan Liu 1,*, Zhipeng Han 1,*, Yingying Jing 1,*, Xue Yang 1, Shanshan Zhang 1, Chen
