DNA Replication: Prokaryotes and Yeast
|
|
|
- Roy Dennis
- 6 years ago
- Views:
Transcription
1 DNA Replication: Prokaryotes and Yeast Derek J Hoelz, Indiana University School of Medicine, Indianapolis, IN, USA Robert J Hickey, Indiana University School of Medicine, Indianapolis, IN, USA Linda H Malkas, Indiana University School of Medicine, Indianapolis, IN, USA An organism s ability to pass on genetic information to further generations requires the accurate reproduction of its genomic DNA, a process termed DNA replication. Examination of the events that take place during DNA replication has revealed numerous well-orchestrated protein protein interactions and enzymatic reactions, which ultimately leads to synthesis of an essentially identical replica of the cell s complete genome. Advanced article Article Contents. Escherichia coli: The Archetype Model for Replication of a DNA Genome. Bacteriophage Models of DNA Replication. Yeast DNA Replication. Acknowledgments doi: /npg.els Escherichia coli: The Archetype Model for Replication of a DNA Genome General scheme for Escherichia coli (E. coli) DNA replication Over the last 30 years, an abundance of biochemical and genetic data has accumulated, identifying at least 30 proteins required to replicate the bacterial E. coli DNA genome (Marians, 1992). The E. coli genome consists of a relatively small circular chromosome comprising 4.7 million base pairs (Mbp) that, upon receiving the proper cellular signals, is replicated prior to cell division. Chromosomal DNA replication in E. coli can be divided into three discrete stages beginning with initiation. Once initiation occurs, a 42-min constant period (C period) is required for chromosomal replication. After initiation, the elongation stage takes place. The elongation stage is the most time-consuming phase of the replication process and is where the vast majority of the DNA is synthesized. The final stage of E. coli DNA replication, designated the termination phase, marks the end of the C period. It is accompanied by the generation of two independent daughter chromosomes. The cells then enter cell division (D period), which lasts approximately 20 min, yielding two genetically identical bacteria. Because of the min doubling time and the relatively small DNA content of the chromosome, E. coli was the first organism used to study the mechanisms of DNA replication. In this section the specific mechanisms involved in E. coli DNA replication will be presented. The initiation of E. coli DNA replication Chromosomal DNA replication in E. coli is initiated at a unique sequence, the chromosomal origin of replication or oric (Bramhill and Kornberg, 1988). oric is composed of a minimal region of 245 bp that contains three 13 bp AT-rich domains and four 9 bp sequences (dnaa boxes) that bind the initiation protein dnaa (Figure 1). Initiation at oric occurs through the binding of the dnaa protein to the 9 bp repeats in the presence of ATP and another protein, HU (Figure 2). Twenty to forty dnaa monomers organize with HU into a protein core to which the oric DNA is wrapped, forming the initial complex. Formation of the initial complex places the three 13 bp AT-rich domains in close proximity to the dnaa core and, in an ATP-dependent manner, dnaa melts the AT-rich domains sequentially, starting with the domain closest to the core. The melting of the ATrich domain results in the formation of a bubble of singlestranded DNA that quickly becomes coated with singlestranded DNA-binding protein (SSB). Binding of SSB to single-stranded DNA in effect stabilizes the single-stranded structure. A dimeric protein complex dnab dnac is then directed towards the single-stranded DNA through an interaction with dnaa. Here, dnab recognizes singlestranded DNA and, using its intrinsic 5!3 helicase activity, further unwinds the DNA in an ATP-dependent fashion. Extension of the unwound region of the DNA by dnab creates a prepriming complex. At this point, the DNA is ready for elongation. 13 bp AT-rich regions oric 9 bp dnaa boxes Figure 1 The E. coli chromosomal DNA replication origin, oric. The four boxes to the right, shaded blue, represent the 9 bp recognition sites for dnaa proteins, and the three red boxes to the left represent the 13 bp ATrich domains melted during the initiation of E. coli DNA replication. ENCYCLOPEDIA OF LIFE SCIENCES 2004, John Wiley & Sons, Ltd. 1
2 oric pol III HE AT-rich region +dnaa +HU +ATP dnag dnab E. coli chromosomal DNA Initial complex Singlestranded DNA +dnab dnac +ATP 38 5 mmol L 1 ATP (a) SSB Okazaki fragment Prepriming complex 38 Open complex Figure 2 Initiation of chromosomal DNA replication in E. coli. dnaa binds to the 9 bp sequences of oric in the presence of the protein HU and ATP, forming a complex of dnaa molecules around which the DNA of the origin is wrapped and is referred to as the initial complex. In the presence of 5 mmol L 2 1 ATP and at 388C, the initial complex is converted to an open complex via the ATP-dependent melting of the AT-rich domain by dnaa. The dnab dnac complex is then directed to the melted region of DNA through the interaction of dnac with dnaa. dnab is now able to recognize the single-stranded DNA, further unwinding the DNA and leading to the formation of the prepriming complex. E. coli DNA replication elongation events Once the prepriming complex has formed, single-stranded DNA is accessible, allowing for the synthesis of a new daughter strand of DNA on the parental template. However, in order for the replicative polymerase, DNA polymerase III holoenzyme (DNA pol III HE), to synthesize DNA, it must have a 3 -OH terminus from which it will elongate. This function is accomplished by a primosome. Although there is much confusion about what exactly constitutes a replicative primosome in E. coli, two proteins, the helicase dnab, and an RNA primase, dnag, are absolutely essential. However, for the purpose of this article, we will concentrate on replication initiated from oric, and thus the primosome to which we will refer simply consists of dnab, dnac, and dnag. dnag, an RNA primase, interacts with dnab, the helicase recruited to the prepriming complex, stimulating the activity of dnab. In turn, this interaction stimulates the primase activity of dnag, which then synthesizes a ribonucleotide (RNA) primer on both strands of the DNA duplex at the replication fork. The DNA pol III HE, which is the replicative DNA polymerase, is now directed to the replication fork by an interaction with the dnab dnag complex (Figure 3). At the fork, DNA pol III HE begins synthesizing nascent strands of DNA from the primers. DNA pol III HE is a protein complex composed of at least 10 different proteins and can carry out DNA synthesis Lagging strand movement Leading strand movement Daughter strand 5 3 DNA pol III core (b) 5 β dimer SSB dnab β dimer dnag χψ γ Complex τγτγδδ 3 5 Parental DNA Fork movement Figure 3 The E. coli replication fork. After initiation has occurred, two replication forks form at the points where single-stranded DNA meets double-stranded DNA. (a) Diagram of the proteins present at a replication fork in E. coli. The progression of the replication fork is directed through the helicase activity of dnab followed by the synthesis of RNA primers by dnag on both the leading strand (bottom strand) and lagging strand (top strand). The DNA polymerase III holoenzyme (DNA pol III HE) then synthesizes new strands of DNA, beginning with the RNA primers. The areas containing single-stranded DNA are stabilized through the binding of single-stranded DNA-binding proteins (SSB). A more accurate representation of a replication fork is shown in (b). Here, the DNA is unwound by dnab at the leading end of the replication fork, and a nascent strand of DNA is synthesized on the leading strand (top strand) by a DNA pol III HE. Tethered to the DNA by a b subunit dimer, the DNA pol III HE moves along the leading strand of DNA. Synthesis of the leading strand (top strand) is coordinated with synthesis of the lagging strand (bottom strand) through the dimerization of two DNA pol III HE complexes by the g subunit. The DNA of the lagging strand is looped around one DNA pol III complex and, following primer synthesis and loading of a b dimer at the primer terminus by the g complex, the DNA pol III HE synthesizes multiple short bp Okazaki fragments on to the lagging strand. These Okazaki fragments will become ligated to one another, forming a long, contiguous strand of DNA. The direction of fork movement and leading and lagging strand movement are denoted with arrows. 2
3 at a rate of nearly 1000 nucleotides per minute. As the replication fork progresses, DNA pol III HE must synthesize DNA on to two separate templates. As stated above, the DNA strand orientated in the 5!3 direction, the same direction as replication fork movement, is referred to as the leading strand, while the template orientated in the 3!5 direction is considered the lagging strand. Leading-strand synthesis is accomplished by DNA pol III HE synthesizing extensive lengths (>0.5 Mbp) of DNA. However, owing to the antiparallel nature of the DNA and the ability of DNA pol III to synthesize DNA exclusively in the 5!3 direction, a problem occurs when attempting to synthesize DNA on the lagging strand. How do you synthesize DNA in a 5!3 direction while moving in a 3!5 direction? Okazaki and co-workers first proposed the solution to this problem in the 1970s, and the answer lay in the discontinuous synthesis of small bp DNA fragments subsequently termed Okazaki fragments (Ogawa et al., 1977). Synthesis of the lagging strand is completed by looping small sections of the DNA strand and placing them in the 5!3 orientation. DNA pol III HE is then able to synthesize DNA on to these small looped sections of DNA, creating an Okazaki fragment. Once complete, the DNA pol III HE cycles to a new looped segment of the DNA strand 5 to the recently completed Okazaki fragment and again begins to synthesize DNA. The end result is a long stretch of Okazaki fragments on the lagging DNA strand that can be joined or ligated together to form one long contiguous stretch of DNA. DNA pol III HE can be resolved into its individual protein components. Isolation of these protein components has afforded valuable data about the enzymology and stoichiometry of DNA pol III HE. The core of DNA pol III HE is composed of three proteins, a, e, and y. The a subunit contains the catalytic activity and is able to synthesize DNA in the 5!3 direction, while the e subunit contains a 3!5 exonuclease and serves a structural function. The contribution of y to the core has yet to be determined. Although the pol III core has catalytic activity, its DNA synthetic processivity is extremely low. In other words, the pol III core is only able to synthesize very short stretches of DNA using a parental template. This processivity is increased by the addition of t, which causes two pol III core complexes to dimerize, forming a DNA pol III complex. Through an interaction with dnab, t is able to coordinate the helicase activity of the replication fork with leadingstrand synthesis. Addition of another group of proteins, the g complex, to the DNA pol III complex yields yet a more processive complex, DNA pol III. The g complex consists of six proteins, including t. A t dimer interacts with a g dimer, anchoring the g complex to DNA pol III. The t and g proteins are products of the same gene, dnax; however, g is truncated by a translational frame shift and is unable to dimerize the DNA pol III cores or interact with dnab. t and g are now able to associate with two other proteins d and d. High-affinity binding of dd to tg requires a preceding interaction with another two proteins, w and c (Olson et al., 1995; Leu et al., 2000). c,an insoluble protein by itself, tightly binds w and, although not much is known about the protein, appears to form a bridge between w and the g complex through its interaction with dd. w functions by an interaction with dnag primase and SSB, and it has been proposed that through this interaction the hand-off of the primer from dnag to the DNA polymerase is accomplished during Okazaki fragment synthesis of the lagging strand. wc is now bound to dd, which in turn is bound to tg, completing the functional g complex. Finally, the addition of a b protein dimer converts the DNA pol III complex to the highly processive DNA pol III HE (Kong et al., 1992; Kim and McHenry, 1996). The b subunit of DNA pol III HE is a member of a group of proteins called processivity factors. The b dimer encircles the DNA, forming a sliding clamp. The b sliding clamp then interacts with DNA pol III through an interaction with the a subunit and, in effect, tethers the polymerase to the DNA, greatly increasing its processivity. In order for b to act as a processivity factor, it must first be loaded on to the DNA and positioned in such a way as to promote DNA polymerization. As it turns out, b clamp loading on to DNA is accomplished through the action of the g complex of DNA pol III mentioned earlier. The g clamp-loading complex has been an area of intense study. This is because the complex is responsible not only for clamp loading but also for positioning b at the primer terminus. Clamp loading is an ATP-dependent reaction requiring the hydrolysis of two molecules of ATP per clamp-loading event. The reaction is initiated at the replication fork, where a b dimer is bound to the d subunit of the g complex. The interaction with d opens the dimer and loads the clamp on to the DNA in an ATP-independent manner. Recently, it has been suggested that d may also unload b from the DNA following completion of the synthesis of an Okazaki fragment. Once loaded on to the DNA, b must be positioned at or near the 3 -OH terminus of the RNA primer so as to allow for an interaction with the a catalytic subunit of the DNA polymerase. The translocation and positioning of b on the DNA is what requires the hydrolysis of ATP, an activity intrinsic to the tg subunits. With b now loaded and positioned at the primer termini of the leading and lagging strands, elongation commences. The replication forks now begin to move bidirectionally from the origin. The replication bubble formed at the end of initiation begins to expand, giving rise to a y structure consisting of the parental chromosome and two newly replicated half-circles of daughter chromosome. Elongation is completed approximately 40 min following the onset of initiation, and termination occurs at specific sequences on the E. coli chromosome approximately 1808 away from oric. 3
4 E. coli DNA replication termination Although the events involved in the termination of E. coli replication are poorly understood, some key features have been described that seem to be essential for the generation of two daughter chromosomes (Bussiere and Bastia, 1999). At the end of elongation, the replication forks eventually meet at a section of the E. coli chromosome approximately 1808 from oric (Figure 4). It is in this part of the genome that the replication forks will encounter specific DNA sequences called termination (ter) sequences. The ter sequences are arranged on the chromosome in two groups of three direct repeats, the groups having opposite orientations. The ter sequences function through the binding of a monomer of the protein Tus. The crystal structure of Tus complexed to a ter sequence has given insight into its binding and function in termination. Within the protein itself, Tus contains b sheets that contact the major groove of the DNA and give Tus its binding specificity. Projected over the DNA binding domain is the replication fork-arresting domain of Tus. Tus arrests the movement of the replication fork specifically by inhibiting the helicase activity of dnab and, although an interaction between dnab and Tus has been demonstrated in vitro, the significance of this interaction in vivo has yet to be determined. Another interesting aspect of Tus is that its helicase-arresting, or contra-helicase, activity is polar. Imagine a replication fork moving clockwise along the circular E. coli chromosome. Upon encountering the first group of three ter sequences complexed with Tus monomers, the replication fork moves through the sequences unabated. Owing to the orientation of the ter sequences, Tus is unable to block the helicase activity, and therefore is unable to arrest the replication fork. However, when the replication fork encounters the next group of three ter sequences with the opposite orientation, helicase activity is inhibited and replication fork movement is arrested. The arrest of replication fork movement, however, is only one of the steps involved in termination. Another terf terb terc τ 1 τ 2 tera τ 3 terd tere Figure 4 The chromosomal DNA replication terminus of E. coli. The replication terminus is located 1808 around the E. coli chromosome from oric and contains two sets of three directly repeated sequences called ter sites. At these ter sites, the Tus protein binds to the DNA and abrogates replication fork movement. However, the movement of one replication fork can only be stopped by a Tus protein binding to a single ter site that is in the proper orientation. This unidirectional or polar blockade of replication by Tus is represented by the sequences to the left and the leftward-moving replication fork (top arrow), and the sequences to the right and the rightward-moving replication fork (bottom arrow). When a replication fork encounters these sequences bound with Tus, an interaction between dnab and Tus occurs, most likely resulting in inhibition of its helicase activity and cessation of replication fork movement. step is the separation of the two daughter chromosomes. Through the action of topoisomerase IV, an enzyme that makes a cut in both strands of a DNA duplex, the daughter chromosomes are separated from one another. Bacteriophage Models of DNA Replication Bacteriophages (phages) have proved to be valuable model systems for the study of DNA replication. First, phage genomes are considerably smaller than bacterial genomes and are easier to manipulate. Second, their small genome size makes them rely on many bacterially encoded proteins for their replication. Finally, phage replication is not restricted by the temporal events of cell division. Several phage models have been developed and used to study the mechanisms of DNA replication in prokaryotes. Some of these models include single-stranded DNA phages represented by FX174, and others include double-stranded DNA phages represented by T4, T7 and l. Although the replication of the DNA of these phages shows many similarities from one to another, each of the phage mechanisms is unique. For the purpose of this article, only a few of these mechanisms (i.e. for l, T7, T4, and FX174) will be described. Each of these phage model systems has contributed tremendously to our understanding of the complex series of events involved in replicating DNA. k DNA replication The l phage is packaged with a double-stranded (ds) linear chromosome that, upon infection of a bacterium, becomes circular, or cyclizes, through the hybridization of two cohesive ends called cos sites (Figure 5). Formation of these circular molecules allows for replication to occur similar to that described for the circular E. coli chromosome (see above). Requiring only two phage-encoded proteins, protein O (lo) and protein P (lp), the l chromosomal replication initiates from a specific sequence, oril. Unlike the dnaa-dependent initiation of E. coli at oric, initiation at oril requires the action of a cellular RNA polymerase (RNAP). RNAP synthesizes an RNA transcript from one of two promoters present in the origin, through the oril region, which is composed of four direct 18 bp repeats and a bp AT-rich region. Synthesis of RNA through the oril, promotes the binding of lo to the 18 bp sequences much like the binding of dnaa to oric in E. coli, unwinding the AT-rich region. Priming and replication occur with the recruiting of dnab, the cellular helicase, through the interaction of lp with lo. dnab further unwinds the DNA at the oril and localizes dnag to the newly formed singlestranded DNA, enabling it to synthesize the RNA primers on the leading and lagging strands. As in cellular replication, DNA pol III HE is then recruited to the replication 4
5 P R1 P R2 oriλ P R2 P R1 cos cos (a) orit7 (a) (b) cos (b) + + (c) (c) (d) (d) 3 -OH (e) (f) pac + (e) (g) + Figure 5 The DNA replication of phage l. Upon infection, the l DNA is injected into the bacterium as a linear molecule. The linear l chromosome then cyclizes through the hybridization of cohesive termini (cos sites) at the ends of the molecule (a). Circle-to-circle DNA replication initiates at a single replication origin oril (b) by transcription of an RNA primer through the origin from one of two promoters (i.e. P R1 and P R2 ). Replication forks are formed, and circle-to-circle replication proceeds (c) producing two daughter molecules (d). After a few rounds of circle-to-circle replication, l switches to an alternative mode of DNA replication called rolling-circle replication (E G). Rolling-circle replication initiates by the nicking of one strand of the circular DNA, resulting in a free 3 -OH terminus that serves as a primer for DNA synthesis (e). Polymerization of the new strand, rolling round and round the circular molecule, causes displacement of the old strand, forming long tandemly repeated l chromosomes called concatemers (f). Single l chromosomes are generated from the sitespecific cleavage (lightning bolt) of the l concatemer at the cos sites (g). Figure 6 T7 DNA replication. DNA replication of the T7 phage initiates through the synthesis of an RNA transcript from one of two T7 promoters (P R1,P R2 ) located to the left of the T7 origin of replication (orit7), displacing the complementary DNA strand forming an R loop (a). The 3 -OH terminus of the RNA transcript then serves as a primer terminus for leading-strand synthesis, and two replication forks are assembled at the R loop (b). DNA replication then proceeds bidirectionally, copying the T7 genome. The T7 genome, unlike l, never cyclizes during its infection cycle and, because the DNA polymerases require a 3 -OH terminus from which to elongate, singlestranded DNA overhangs are generated after the removal of RNA primers from the 5 ends (c). To circumvent the loss of the DNA overhang at the ends of the T7 genome, the T7 phage forms long DNA concatemers by the hybridization of the terminal repeats () (d). Full-length T7 genomes are then generated through cleavage of the concatemers at specific sequences within the called pac sites (e). fork, leading to semiconservative DNA synthesis on the l parental DNA template. When the replication forks on the l DNA meet on the other side of the circular genome, replication is terminated and two daughter molecules are separated. After a few rounds of this circle-to-circle replication, l switches to a rolling-circle mechanism of replication. This requires the action of one additional phageencoded protein, Gam. Rolling-circle replication proceeds when one strand of the double-stranded circular l chromosome is cut, thus enabling the free 3 end to be used as a primer for DNA synthesis. Subsequent DNA synthesis produces a long stretch of double-stranded, tandemly repeated, linear l chromosomes, called concatemers. The concatemers are eventually cut at the cos sequences and packaged into the phage heads. T7 DNA replication The T7 bacteriophage, like l, contains a linear chromosome. However, in contrast to l, the T7 viral DNA encodes nearly all of the proteins needed for its own DNA replication, and does not cyclize after infection. Replication of the linear T7 DNA template presents a major problem (Figure 6). As mentioned above, the DNA polymerases are able to synthesize exclusively in the 5!3 direction and can only elongate from an existing 3 -OH primer terminus. As with E. coli, T7 also utilizes RNA primers, which presents a problem at the 5 ends of a linear template. After the RNA primer synthesized on the 5 end is degraded, the result is a single-stranded 3 DNA overhang (Figure 6c). Therefore, if these DNA overhangs are not corrected, the linear tem- 5
6 plate will get progressively smaller, eventually making the virus unviable. To correct 3 overhangs at the ends of the genome, phages T7 and T4 have developed clever DNA replication mechanisms, which will be discussed below. T7 DNA replication initiates with the action of a virally encoded RNA polymerase (vrnap). Synthesis of RNA by the vrnap from two promoters contained in the T7 origin, orit7, primes the initiation reactions, and is therefore considered R loop-dependent (Figure 6a). orit7 consists of a 200 bp region, which, in addition to the promoter elements, embodies a 61 bp AT-rich region that contains T7 gene 4 product (g4p)-binding sites. The resultant RNA/ DNA hybrid displaces the DNA strand at the AT-rich region, allowing for g4p to bind to the nontemplate strand. The helicase activity of g4p further unwinds orit7 in an ATP-dependent fashion. Translocating in the 5!3 direction, g4p unwinds the DNA until the first priming site is reached. At the priming site, g4p then synthesizes an RNA primer with its intrinsic primase activity. Exchange of the RNA primer from g4p to the T7 DNA polymerase, g5p, establishes the first Okazaki fragment, which will then serve as a 3 -OH primer terminus for leading-strand DNA synthesis and the progression of the replication fork. In the opposite direction, the RNA transcript initially synthesized in the initiation reaction serves as the primer for leading-strand synthesis, and another replication fork is assembled and DNA synthesis proceeds bidirectionally. Termination of DNA synthesis occurs when the replication forks reach the ends of the linear template. After termination, the T7 DNA now contains singlestranded 3 DNA overhangs. Resolution of the DNA loss associated with the single-stranded DNA overhangs is accomplished by hybridization of the complementary terminally repeated () ends present on each adjacent newly replicated chromosome (Figure 6d). This hybridization of the newly replicated DNA forms extensive lengths of endto-end chromosomes called concatemers. One T7 genome is subsequently packaged into one phage head by cutting the concatemer at specific sites called pac sites present between each genome (Figure 6e). After assembly of the phage particle is complete, the cell lyses and the viral particles are ready to infect another bacterium. T4 DNA replication Shortly after infection, the T4 phage takes over host DNA, RNA and protein synthesis, kidnapping the cell s RNA and protein synthetic machinery to produce its viral proteins. T4 then degrades the bacterial genome, increasing the free nucleotide pool to the advantage of its own DNA replication. This complete dominance over cellular activities makes the T4 phage an attractive model for the study of DNA replication, allowing researchers to monitor phage biosynthetic activities in the absence of any background activity of the host. In addition, the genome of T4 contains a double-stranded linear chromosome that, upon infection, does not cyclize. Encoding nearly all proteins needed to carry out DNA synthesis, the T4 phage has proved to be an extremely valuable model in the study of DNA replication. Interestingly, the T4 mechanism of replication occurs in two stages. In stage 1, initiation of replication occurs through an R loop-dependent mechanism (Figure 7). The host s RNAP synthesizes RNA primers from multiple promoters located throughout the T4 genome, and replication is initiated at seven or more origins. Later in infection, T4 no longer initiates by an R loop-dependent mechanism. Instead, in stage 2, T4 initiates replication using an alternative mechanism, which is referred to as D loop-dependent (Figure 7) (Krenzer, 2000). Initially discovered in T4, D loop-dependent replication requires the formation of recombination intermediates for initiation to occur. Study of T4 DNA replication initiated at D loops, referred to as recombination-dependent replication (RDR), proved the long-hypothesized involvement of the genetic recombination process in DNA replication. As with the replication of the linear T7 template, the replication of the T4 genome from defined origins in stage 1 leads to DNA molecules that contain 3 single-stranded DNA overhangs at their terminal sequences. However, these single-stranded 3 DNA overhangs do not hybridize end-to-end as in T7 but are then able to invade and displace a homologous strand of DNA on an adjacent T4 chromosome, forming recombination intermediates called D loops (Figure 7). The 3 -OH of the invading strand then serves as a primer for leading-strand DNA synthesis and an assembly site for the replication forks. D loops form because the T4 genome contains redundant sequences in combination with the packaging of slightly more DNA than that contained in a single chromosome (see below). Currently, there are three models for DNA synthesis occurring at D loops: unidirectional, bidirectional and tridirectional models (Figure 8). However, the actual mechanisms that take place in vivo have yet to be determined. Unidirectional replication is similar to bidirectional replication in that both models resolve the cross-strand structure of the recombination intermediate by cleavage of a DNA strand. The models differ only in the strand of DNA that is cleaved. The third, tridirectional, model, on the other hand, results from the total absence of DNA cleavage. After two replication forks are assembled at the D loop, similarly to the bidirectional model, the forks leave the area. The resultant Y-shaped structure could initiate a third replication fork, leading to another round of replication. Nevertheless, the replication fork(s) initiated from D loops will eventually reach the end of the DNA template, and 3 single-stranded DNA will be regenerated. The 3 single-stranded DNA will then be able to invade a homologous sequence, forming another D loop and beginning the process over again. This self-regenerating mechanism ultimately results in the formation of long concatemers of tandemly repeated T4 genomic DNA much 6
7 Stage 1: Initiation from origins Stage 2: No origins; recombinationdependent D-loop initiated DNA replication Figure 7 T4 DNA replication: initiation from R loops and D loops. T4 DNA replication occurs in two stages. In stage 1, T4 replication initiates from an RNA primer synthesized through one of multiple origins (R loop-dependent) scattered throughout the T4 genome. After a few rounds of origin-initiated DNA replication, the resultant T4 genomes contain free 3 single-stranded DNA overhangs at their ends. In stage 2, these 3 overhangs are then able to invade the DNA of an adjacent T4 chromosome, displacing a homologous region of DNA and forming a D-loop. The 3 -OH of the invading single-stranded DNA is then able to serve as a primer terminus for leading strand synthesis, enabling replication to proceed. like those developed by the l and T7 phages. The long concatemeric DNA is then packaged into T4 phage heads through a mechanism referred to as headful packaging. Headful packaging obtains its name because the T4 phage does not package a single genome into its head through cleavage at cos or pac sites as do l and T7, respectively. Instead, T4 packages as much DNA into its head as space will allow. Cleavage of the DNA takes place after the T4 phage head is full. This results in the packaging of slightly (a) (b) (c) Figure 8 The mechanisms of DNA replication initiation from D loops during T4 DNA replication. (a) Unidirectional DNA replication results from the cleavage of the hybridized (solid green circle) strand of DNA followed by the formation of a single replication fork. (b) Bidirectional DNA replication occurs through the cleavage of the displaced (solid blue circle) strand of DNA followed by formation of two replication forks. (c) Tridirectional DNA replication is the result of the formation of three replication forks on an uncleaved template. Alternatively, tridirectional DNA replication could also occur if two of the DNA strands were cleaved (not shown). more DNA than that contained in a single T4 genome. The excess DNA contains a section of the next adjacent T4 genome redundant in sequence to the first, and these redundant sequences at the ends of the packaged DNA will serve as the invading strands for D loop formation, producing the long concatemers of the next infection cycle. UX174 DNA replication Unlike the phages that have been discussed so far, the FX group represents a new group of E. coli phages that contain a small single-stranded circular DNA chromosome. These small phages are well suited as models for the study of DNA replication for many reasons. For example, the single-stranded chromosome of FX174 has proved easy to isolate and prepare intact; also, its small size limits the number of virally encoded proteins, and therefore the phage must rely on many of the host s cellular proteins to direct its DNA replication. In addition, the FX174 life cycle has attributes that make it attractive for study. After infection of a bacterium, the single-stranded circular FX174 genome is injected into the cell. Once inside the bacterium, the single-stranded chromosome is converted to double-stranded DNA, which then can be replicated using a mechanism similar to E. coli chromosomal replication. Much of the research done on the FX174 phage concentrates on the conversion of single-stranded circular DNA to double-stranded DNA, and we will therefore concentrate on the reactions that take place in this stage. 7
8 The conversion of FX174 single-stranded DNA to double-stranded occurs in two phases. In phase 1, a prepriming intermediate is formed, which will be elongated by the DNA pol III HE (see the section on E. coli DNA replication) in phase 2 (Marians, 2000). Phase 1 requires the action of a group of E. coli proteins that includes the dnab dnac complex that is involved in oric-directed chromosomal replication in E. coli. In addition to the dnab dnac complex, four additional proteins are involved in the formation of a prepriming complex. These proteins are PriA, a DNA-dependent ATPase with translocase and 3!5 helicase activities; PriB and PriC, whose functions remain elusive except for a redundant structural role; and dnat (Figure 9). This group of proteins assembles at a specific site on the FX174 single-stranded DNA template and is referred to as the primosome assembly site (PAS). Analysis of the PAS demonstrated that the sequence of the site is not directly responsible for protein binding; instead, primosome assembly is most likely dependent on the secondary DNA structure of the PAS site (Figure 9). In other words, primosome assembly results from topological recognition PAS dnac PriA PriB PriC + dnat dnab dnac complex Figure 9 The primosome assembly during covalently closed singlestranded RF DNA replication in the phage FX174. After infection, the single-stranded DNA of FX174 is converted to double-stranded replicative form (RF) DNA by the assembly of a primosome at a primosome assembly site (PAS). The cellular protein PriA first recognizes the secondary structure of the PAS and binds to the single-stranded DNA. PriA then recruits PriB and PriC, followed by dnat and dnab. dnab synthesizes an RNA primer on to the single-stranded DNA of FX174, which is elongated, forming a doublestranded RF DNA template. of structures formed by the interaction of the singlestranded DNA with itself. Preprimosome assembly initiates with recognition of the PAS site by PriA. PriA binding is followed by the recruitment of PriB, PriC, dnat and dnab, respectively; forming the preprimosome complex of phase 1. Formation of a functional primosome is completed with the association of dnag, and marks the beginning of phase 2. dnag of the primosome complex is then able to synthesize an RNA primer on the single-stranded DNA template followed by the initiation of nascent DNA synthesis by DNA polymerase I (pol I). After synthesis of about 300 bp, DNA pol III HE replaces pol I and the nascent DNA becomes the leading strand of a newly formed replication fork. The discovery of PriA has been instrumental in the study of recombination-dependent replication (RDR; see T4). Although it was initially thought not to be involved in E. coli chromosomal replication, because it is not necessary for primosome assembly at oric, recent research supports the view that PriA has a fundamental role in chromosomal DNA replication. Isolation of pria-null mutant bacteria reveals a phenotype that is constitutively induced for the SOS DNA damage response. These bacteria are also sensitive to ultraviolet damage and double-strand breaks. In addition, they are defective in homologous recombination. Cell survival of pria mutants is also less than 50% of the wild type. These phenotypes can now be explained through a recently realized activity of PriA, its ability to bind to D loops. As mentioned above, D loops are recombination intermediates generated from the invasion of a homologous strand of DNA, and, in addition to being involved in T4 DNA replication, are involved in double-strand break repair and homologous recombination. The first evidence for a connection between recombination and replication was provided by the analysis of pria-null bacteria, which are unable to carry out these basic cellular functions. Yeast DNA Replication As indicated in the preceding sections, the extensive study of E. coli and bacteriophage DNA replication has yielded an enormous wealth of information regarding the mechanisms involved in DNA synthesis. However, E. coli, being a prokaryotic organism, has a cellular architecture that is fundamentally different from that of even the simplest eukaryotic organism. Eukaryotic organisms contain a nucleus, whereas E. coli do not. The possession of a nucleus permits the physical separation of the processes of translation and transcription from that of DNA replication. Also, the DNA genome of most eukaryotic organisms consists of several distinct chromosomes; these chromosomes most often contain several different origins of replication. Therefore, studying only E. coli and bacteriophage DNA replication will not tell us how 8
9 eukaryotic cells regulate and organize their DNA replication process. With these concerns in mind, researchers needed to turn their attention to the specifics of eukaryotic DNA replication. Much of the study of eukaryotic DNA replication has been done on very simple eukaryotic microorganisms, such as yeasts. Yeast cells divide rapidly and contain only 3.5 times more DNA than E. coli cells. Yeast genetic systems have also been well defined and serve as an experimentally powerful arm in the study of yeast DNA replication. It has been found that many of the types of enzymes and proteins, as well as the molecular mechanisms, involved in the replication of the E. coli DNA genome are also used in yeast DNA synthesis. For more on the specifics of yeast DNA replication, readers are referred to several excellent reviews (Toyn et al., 1995; Wang and Li, 1995; Stillman, 1996). Acknowledgments This work is supported by the National Institutes of Health/National Cancer Institute through research awards to L.H.M. (CA83199, CA57350, CA73060, and CA65754) and to R.J.H. (CA74940), and the US Army Medical Research and Materiel Command through a predoctoral Breast Cancer Research Program Fellowship to D.H. We thank the Vera Bradley Foundation for their continued support of our work. References Bussiere DE and Bastia D (1999) Termination of DNA replication of bacterial and plasmid chromosomes. Molecular Microbiology 6: Kim DR and McHenry CS (1996) Identification of the b-binding domain of the a subunit of Escherichia coli polymerase III holoenzyme. Journal of Biological Chemistry 271: Kong XP, Onrust R, O Donnell M and Kuriyan J (1992) Three-dimensional structure of the b subunit of E. coli DNA polymerase III holoenzyme: a sliding clamp. Cell 69: Krenzer KN (2000) Recombination-dependent DNA replication in phage T4. Trends in Biochemical Sciences 25: Leu FP, Hingorami MM, Turner J and O Donnell M (2000) The d subunit of DNA polymerase III holoenzyme serves as a sliding clamp unloader in Escherichia coli. Journal of Biological Chemistry 275: Marians KJ (1992) Prokaryotic DNA replication. Annual Reviews of Biochemistry 61: Marians KJ (2000) PriA: at the crossroads of DNA replication and recombination. Progress in Nucleic Acids Research 63: Ogawa T, Hirose S, Okazaki T and Okazaki R (1977) Mechanism of DNA chain growth XVI. Analyses of RNA-linked DNA pieces in Escherichia coli with polynucleotide kinase. Journal of Molecular Biology 112: Olson MW, Dallmann HG and McHenry CS (1995) DnaX complex of Escherichia coli DNA polymerase III holoenzyme, the X. c complex functions by increasing the affinity of t and g for d. d to a physiologically relevant range. Journal of Biological Chemistry 270: Stillman B (1996) Cell cycle control of DNA replication. Science 274 (5293): Toyn JH, Toone WM, Morgan BA and Johnston LH (1995) The activation of DNA replication in yeast. Trends in Biochemical Sciences 20 (2): Wang TA and Li JJ (1995) Eukaryotic DNA replication. Current Opinion in Cell Biology 7 (3): Bramhill D and Kornberg A (1988) A model for initiation at origins of DNA replication. Cell 54:
The Size and Packaging of Genomes
 DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
Chapter 11 DNA Replication and Recombination
 Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
 The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
DNA Replication II Biochemistry 302. January 25, 2006
 DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
III. Detailed Examination of the Mechanism of Replication A. Initiation B. Priming C. Elongation D. Proofreading and Termination
 Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
The flow of Genetic information
 The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
The replication forks Summarising what we know:
 When does replication occur? MBLG1001 lecture 10 Replication the once in a lifetime event! Full blown replication only occurs once, just before cell division BUT the DNA template is constantly being repaired.
When does replication occur? MBLG1001 lecture 10 Replication the once in a lifetime event! Full blown replication only occurs once, just before cell division BUT the DNA template is constantly being repaired.
DNA Replication II Biochemistry 302. Bob Kelm January 28, 2004
 DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
DNA REPLICATION. Third Stage. Lec. 12 DNA Replication. Lecture No.: 12. A. Watson & Crick (1952) C. Cairns (1963) autoradiographic experiment
 Lec. 12 DNA Replication A. Watson & Crick (1952) Proposed a model where hydrogen bonds break, the two strands separate, and DNA synthesis occurs semi-conservatively in the same net direction. While a straightforward
Lec. 12 DNA Replication A. Watson & Crick (1952) Proposed a model where hydrogen bonds break, the two strands separate, and DNA synthesis occurs semi-conservatively in the same net direction. While a straightforward
Replication. Obaidur Rahman
 Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Gene Expression- Protein Synthesis
 Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
Genetic Information: DNA replication
 Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
Chapter Twelve: DNA Replication and Recombination
 This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
DNA Replication I Biochemistry 302. Bob Kelm January 24, 2005
 DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
DNA Replication II Biochemistry 302. Bob Kelm January 26, 2005
 DNA Replication II Biochemistry 302 Bob Kelm January 26, 2005 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 Bob Kelm January 26, 2005 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
Tutorial Week #9 Page 1 of 11
 Tutorial Week #9 Page 1 of 11 Tutorial Week #9 DNA Replication Before the tutorial: Read ECB Chapter 6 p195-207, and review your lecture notes Read this tutorial and create a table of definitions and functions
Tutorial Week #9 Page 1 of 11 Tutorial Week #9 DNA Replication Before the tutorial: Read ECB Chapter 6 p195-207, and review your lecture notes Read this tutorial and create a table of definitions and functions
Requirements for the Genetic Material
 Requirements for the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell-generation to generation. 2. Information Storage Biologically useful information in a stable
Requirements for the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell-generation to generation. 2. Information Storage Biologically useful information in a stable
Enzymes used in DNA Replication
 Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
Molecular Biology (2)
 Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
DNA Metabolism. I. DNA Replication. A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding
 DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
Chapter 3: Duplicating the DNA- Replication
 3. Basic Genetics Plant Molecular Biology Chapter 3: Duplicating the DNA- Replication Double helix separation New strand synthesis Plant Biotechnology Lecture 2 1 I've missed more than 9000 shots in my
3. Basic Genetics Plant Molecular Biology Chapter 3: Duplicating the DNA- Replication Double helix separation New strand synthesis Plant Biotechnology Lecture 2 1 I've missed more than 9000 shots in my
ARUNAI ACADEMY FOR PG TRB-BOTANY DHARMAPURI REPLICATION - ENZYMES.
 ARUNAI ACADEMY FOR PG TRB-BOTANY DHARMAPURI.9500244679 REPLICATION - ENZYMES DNA HELICASE Sparation of two strands- DNA helicase enzyme functions Unwinds DNA. DNA double helix by breaking the hydrogen
ARUNAI ACADEMY FOR PG TRB-BOTANY DHARMAPURI.9500244679 REPLICATION - ENZYMES DNA HELICASE Sparation of two strands- DNA helicase enzyme functions Unwinds DNA. DNA double helix by breaking the hydrogen
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA
 RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
CSIR UGC NET, GATE (ENGINEERING), GATE (Science), IIT-JAM, UGC NET, TIFR, IISc, NIMCET, JEST etc. JNU CEEB SAMPLE THEORY
 JNU CEEB SAMPLE THEORY REPLICATION TYPE OF DNA REPLICATION REPLICATION IN PROKARYOTES REPLICATION IN EUKARYOTES For IIT-JAM, JNU, GATE, NET, NIMCET and Other Entrance Exams 1-C-8, Sheela Chowdhary Road,
JNU CEEB SAMPLE THEORY REPLICATION TYPE OF DNA REPLICATION REPLICATION IN PROKARYOTES REPLICATION IN EUKARYOTES For IIT-JAM, JNU, GATE, NET, NIMCET and Other Entrance Exams 1-C-8, Sheela Chowdhary Road,
DNA replication. - proteins for initiation of replication; - proteins for polymerization of nucleotides.
 DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
Initiation of Replication. This section will begin with a brief discussion of chromosomal DNA sequences that sponsor the initiation of replication.
 Initiation of Replication This section will begin with a brief discussion of chromosomal DNA sequences that sponsor the initiation of replication. Replicons and origins of replication The unit of DNA replication
Initiation of Replication This section will begin with a brief discussion of chromosomal DNA sequences that sponsor the initiation of replication. Replicons and origins of replication The unit of DNA replication
Storage and Expression of Genetic Information
 Storage and Expression of Genetic Information 29. DNA structure, Replication and Repair ->Ch 25. DNA metabolism 30. RNA Structure, Synthesis and Processing ->Ch 26. RNA metabolism 31. Protein Synthesis
Storage and Expression of Genetic Information 29. DNA structure, Replication and Repair ->Ch 25. DNA metabolism 30. RNA Structure, Synthesis and Processing ->Ch 26. RNA metabolism 31. Protein Synthesis
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
DNA replication: Enzymes link the aligned nucleotides by phosphodiester bonds to form a continuous strand.
 DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
Replication of DNA and Chromosomes
 Chapter 9. Replication of DNA and Chromosomes 1. Semiconservative Replication 2. DNA Polymerases and DNA Synthesis In Vitro 3. The Complex Replication Apparatus 4. Unique Aspects of Eukaryotic Chromosome
Chapter 9. Replication of DNA and Chromosomes 1. Semiconservative Replication 2. DNA Polymerases and DNA Synthesis In Vitro 3. The Complex Replication Apparatus 4. Unique Aspects of Eukaryotic Chromosome
2/6/18. file:///users/jlc/ Downloads/ DNAi_replicatio n_vo2-lg.mov. 3x10 13 cells (Bianconi et al. Ann Hum Biol, 2013) DNA Replication in vivo
 file:///users/jlc/ Downloads/ DNAi_replicatio n_vo2-lg.mov 3x10 13 cells (Bianconi et al. Ann Hum Biol, 2013) 10 12 replicating cells (Cairns: Genetics (2006)174,1069). Lose about 1/3 of these per day
file:///users/jlc/ Downloads/ DNAi_replicatio n_vo2-lg.mov 3x10 13 cells (Bianconi et al. Ann Hum Biol, 2013) 10 12 replicating cells (Cairns: Genetics (2006)174,1069). Lose about 1/3 of these per day
Proposed Models of DNA Replication. Conservative Model. Semi-Conservative Model. Dispersive model
 5.2 DNA Replication Cell Cycle Life cycle of a cell Cells can reproduce Daughter cells receive an exact copy of DNA from parent cell DNA replication happens during the S phase Proposed Models of DNA Replication
5.2 DNA Replication Cell Cycle Life cycle of a cell Cells can reproduce Daughter cells receive an exact copy of DNA from parent cell DNA replication happens during the S phase Proposed Models of DNA Replication
BIOCHEMISTRY REVIEW. Overview of Biomolecules. Chapter 11 DNA Replication
 BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 11 DNA Replication 2 3 4 5 6 7 8 9 Are You Getting It?? Which characteristics will be part of semi-conservative replication? (multiple answers) a) The
BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 11 DNA Replication 2 3 4 5 6 7 8 9 Are You Getting It?? Which characteristics will be part of semi-conservative replication? (multiple answers) a) The
Zoo-342 Molecular biology Lecture 2. DNA replication
 Zoo-342 Molecular biology Lecture 2 DNA replication DNA replication DNA replication is the process in which one doubled-stranded DNA molecule is used to create two double-stranded molecules with identical
Zoo-342 Molecular biology Lecture 2 DNA replication DNA replication DNA replication is the process in which one doubled-stranded DNA molecule is used to create two double-stranded molecules with identical
Fidelity of DNA polymerase
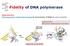 Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
Viral DNA replication
 Viral DNA replication Lecture 8 Biology 3310/4310 Virology Spring 2017 The more the merrier --ANONYMOUS Viral DNA genomes must be replicated to make new progeny Parvovirus Retrovirus Poliovirus VII Hepatitis
Viral DNA replication Lecture 8 Biology 3310/4310 Virology Spring 2017 The more the merrier --ANONYMOUS Viral DNA genomes must be replicated to make new progeny Parvovirus Retrovirus Poliovirus VII Hepatitis
Chapter 11 Part A: Metabolism: The synthesis of nucleic acids and proteins
 Chapter 11 Part A: Metabolism: The synthesis of nucleic acids and proteins I. Synthesis of DNA = REPLICATION A. Components of DNA (Fig. 11-1) 1. Composed of 4 different nucleotides that are joined by the
Chapter 11 Part A: Metabolism: The synthesis of nucleic acids and proteins I. Synthesis of DNA = REPLICATION A. Components of DNA (Fig. 11-1) 1. Composed of 4 different nucleotides that are joined by the
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule.
 1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
DNA Replication and Repair
 DN Replication and Repair http://hyperphysics.phy-astr.gsu.edu/hbase/organic/imgorg/cendog.gif DN Replication genetic information is passed on to the next generation semi-conservative Parent molecule with
DN Replication and Repair http://hyperphysics.phy-astr.gsu.edu/hbase/organic/imgorg/cendog.gif DN Replication genetic information is passed on to the next generation semi-conservative Parent molecule with
Nucleic Acid Structure:
 Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
Welcome to Class 18! Lecture 18: Outline and Objectives. Replication is semiconservative! Replication: DNA DNA! Introductory Biochemistry!
 Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
Replication of DNA Virus Genomes. Lecture 7 Virology W3310/4310 Spring 2013
 Replication of DNA Virus Genomes Lecture 7 Virology W3310/4310 Spring 2013 It s all about Initiation Problems faced by DNA replication machinery Viruses must replicate their genomes to make new progeny
Replication of DNA Virus Genomes Lecture 7 Virology W3310/4310 Spring 2013 It s all about Initiation Problems faced by DNA replication machinery Viruses must replicate their genomes to make new progeny
DNA REPLICATION & REPAIR
 DNA REPLICATION & REPAIR Table of contents 1. DNA Replication Model 2. DNA Replication Mechanism 3. DNA Repair: Proofreading 1. DNA Replication Model Replication in the cell cycle 3 models of DNA replication
DNA REPLICATION & REPAIR Table of contents 1. DNA Replication Model 2. DNA Replication Mechanism 3. DNA Repair: Proofreading 1. DNA Replication Model Replication in the cell cycle 3 models of DNA replication
DNA Replication semiconservative replication conservative replication dispersive replication DNA polymerase
 DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
Genetic material must be able to:
 Genetic material must be able to: Contain the information necessary to construct an entire organism Pass from parent to offspring and from cell to cell during cell division Be accurately copied Account
Genetic material must be able to: Contain the information necessary to construct an entire organism Pass from parent to offspring and from cell to cell during cell division Be accurately copied Account
DNA REPLICATION. DNA structure. Semiconservative replication. DNA structure. Origin of replication. Replication bubbles and forks.
 DNA REPLICATION 5 4 Phosphate 3 DNA structure Nitrogenous base 1 Deoxyribose 2 Nucleotide DNA strand = DNA polynucleotide 2004 Biology Olympiad Preparation Program 2 2004 Biology Olympiad Preparation Program
DNA REPLICATION 5 4 Phosphate 3 DNA structure Nitrogenous base 1 Deoxyribose 2 Nucleotide DNA strand = DNA polynucleotide 2004 Biology Olympiad Preparation Program 2 2004 Biology Olympiad Preparation Program
DNA metabolism. DNA Replication DNA Repair DNA Recombination
 DNA metabolism DNA Replication DNA Repair DNA Recombination Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology Central Dogma or Flow of genetic information
DNA metabolism DNA Replication DNA Repair DNA Recombination Chutima Talabnin Ph.D. School of Biochemistry,Institute of Science, Suranaree University of Technology Central Dogma or Flow of genetic information
Chapter 9. Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination
 Chapter 9 Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination 1 Genetics Genome Chromosome Gene Protein Genotype Phenotype 2 Terms and concepts gene Fundamental unit of heredity
Chapter 9 Topics - Genetics - Flow of Genetics - Regulation - Mutation - Recombination 1 Genetics Genome Chromosome Gene Protein Genotype Phenotype 2 Terms and concepts gene Fundamental unit of heredity
Molecular Genetics I DNA
 Molecular Genetics I DNA Deoxyribonucleic acid is the molecule that encodes the characteristics of living things. It is the molecule that is passed from a mother cell to daughter cells, and the molecule
Molecular Genetics I DNA Deoxyribonucleic acid is the molecule that encodes the characteristics of living things. It is the molecule that is passed from a mother cell to daughter cells, and the molecule
BIO 311C Spring Lecture 34 Friday 23 Apr.
 BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function. Gary Peter
 Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function Gary Peter Learning Objectives 1. List and explain the mechanisms by which E. coli DNA is replicated 2. Describe
Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function Gary Peter Learning Objectives 1. List and explain the mechanisms by which E. coli DNA is replicated 2. Describe
Fig. 16-7a. 5 end Hydrogen bond 3 end. 1 nm. 3.4 nm nm
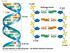 Fig. 16-7a end Hydrogen bond end 1 nm 3.4 nm 0.34 nm (a) Key features of DNA structure end (b) Partial chemical structure end Fig. 16-8 Adenine (A) Thymine (T) Guanine (G) Cytosine (C) Concept 16.2: Many
Fig. 16-7a end Hydrogen bond end 1 nm 3.4 nm 0.34 nm (a) Key features of DNA structure end (b) Partial chemical structure end Fig. 16-8 Adenine (A) Thymine (T) Guanine (G) Cytosine (C) Concept 16.2: Many
DNA Replication in Prokaryotes and Eukaryotes
 DNA Replication in Prokaryotes and Eukaryotes 1. Overall mechanism 2. Roles of Polymerases & other proteins 3. More mechanism: Initiation and Termination 4. Mitochondrial DNA replication DNA replication
DNA Replication in Prokaryotes and Eukaryotes 1. Overall mechanism 2. Roles of Polymerases & other proteins 3. More mechanism: Initiation and Termination 4. Mitochondrial DNA replication DNA replication
DNA is the genetic material. DNA structure. Chapter 7: DNA Replication, Transcription & Translation; Mutations & Ames test
 DNA is the genetic material Chapter 7: DNA Replication, Transcription & Translation; Mutations & Ames test Dr. Amy Rogers Bio 139 General Microbiology Hereditary information is carried by DNA Griffith/Avery
DNA is the genetic material Chapter 7: DNA Replication, Transcription & Translation; Mutations & Ames test Dr. Amy Rogers Bio 139 General Microbiology Hereditary information is carried by DNA Griffith/Avery
3.A.1 DNA and RNA: Structure and Replication
 3.A.1 DNA and RNA: Structure and Replication Each DNA polymer is made of Nucleotides (monomer) which are made of: a) Phosphate group: Negatively charged and polar b) Sugar: deoxyribose- a 5 carbon sugar
3.A.1 DNA and RNA: Structure and Replication Each DNA polymer is made of Nucleotides (monomer) which are made of: a) Phosphate group: Negatively charged and polar b) Sugar: deoxyribose- a 5 carbon sugar
Prokaryotic Physiology. March 3, 2017
 1. (10 pts) Explain the replication of both strands of DNA in prokaryotes. At a minimum explain the direction of synthesis, synthesis of the leading and lagging strand, separation of the strands and the
1. (10 pts) Explain the replication of both strands of DNA in prokaryotes. At a minimum explain the direction of synthesis, synthesis of the leading and lagging strand, separation of the strands and the
The Structure of DNA
 The Structure of DNA Questions to Ponder 1) How is the genetic info copied? 2) How does DNA store the genetic information? 3) How is the genetic info passed from generation to generation? The Structure
The Structure of DNA Questions to Ponder 1) How is the genetic info copied? 2) How does DNA store the genetic information? 3) How is the genetic info passed from generation to generation? The Structure
DNA REPLICATION. Anna Onofri Liceo «I.Versari»
 DNA REPLICATION Anna Onofri Liceo «I.Versari» Learning objectives 1. Understand the basic rules governing DNA replication 2. Understand the function of key proteins involved in a generalised replication
DNA REPLICATION Anna Onofri Liceo «I.Versari» Learning objectives 1. Understand the basic rules governing DNA replication 2. Understand the function of key proteins involved in a generalised replication
DNA Transcription. Dr Aliwaini
 DNA Transcription 1 DNA Transcription-Introduction The synthesis of an RNA molecule from DNA is called Transcription. All eukaryotic cells have five major classes of RNA: ribosomal RNA (rrna), messenger
DNA Transcription 1 DNA Transcription-Introduction The synthesis of an RNA molecule from DNA is called Transcription. All eukaryotic cells have five major classes of RNA: ribosomal RNA (rrna), messenger
Chapter 30. Replication. Meselson Stahl Experiment. BCH 4054 Chapter 30 Lecture Notes. Slide 1. Slide 2 Conceptual Mechanism of.
 BCH 4054 Chapter 30 Lecture Notes 1 Chapter 30 DNA Replication and Repair 2 Conceptual Mechanism of Replication Strand separation, with copying of each strand by Watson-Crick base pairing Fig 30.2 Three
BCH 4054 Chapter 30 Lecture Notes 1 Chapter 30 DNA Replication and Repair 2 Conceptual Mechanism of Replication Strand separation, with copying of each strand by Watson-Crick base pairing Fig 30.2 Three
Answers to Module 1. An obligate aerobe is an organism that has an absolute requirement of oxygen for growth.
 Answers to Module 1 Short Answers 1) What is an obligate aerobe? An obligate aerobe is an organism that has an absolute requirement of oxygen for growth. What about facultative anaerobe? 2) Distinguish
Answers to Module 1 Short Answers 1) What is an obligate aerobe? An obligate aerobe is an organism that has an absolute requirement of oxygen for growth. What about facultative anaerobe? 2) Distinguish
GENETICS - CLUTCH CH.8 DNA REPLICATION.
 !! www.clutchprep.com CONCEPT: SEMICONSERVATIVE REPLICATION Before replication was understood, there were three of how DNA is replicated Conservative replication states that after replication, there is
!! www.clutchprep.com CONCEPT: SEMICONSERVATIVE REPLICATION Before replication was understood, there were three of how DNA is replicated Conservative replication states that after replication, there is
Prokaryotic cells divide by pinching in two. Fig. 10-CO, p.240
 Prokaryotic cells divide by pinching in two Fig. 10-CO, p.240 Learning Objectives 1. What Is the Flow of Genetic Information in the Cell? 2. What Are the General Considerations in the Replication of DNA?
Prokaryotic cells divide by pinching in two Fig. 10-CO, p.240 Learning Objectives 1. What Is the Flow of Genetic Information in the Cell? 2. What Are the General Considerations in the Replication of DNA?
BIOLOGY 101. CHAPTER 16: The Molecular Basis of Inheritance: Life s Operating Instructions
 BIOLOGY 101 CHAPTER 16: The Molecular Basis of Inheritance: Life s Operating Instructions Life s Operating Instructions CONCEPTS: 16.1 DNA is the genetic material 16.2 Many proteins work together in DNA
BIOLOGY 101 CHAPTER 16: The Molecular Basis of Inheritance: Life s Operating Instructions Life s Operating Instructions CONCEPTS: 16.1 DNA is the genetic material 16.2 Many proteins work together in DNA
The Genetic Material. The Genetic Material. The Genetic Material. DNA: The Genetic Material. Chapter 14
 DNA: Chapter 14 Frederick Griffith, 1928 studied Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia there are 2 strains of Streptococcus: - S strain is virulent - R strain is nonvirulent
DNA: Chapter 14 Frederick Griffith, 1928 studied Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia there are 2 strains of Streptococcus: - S strain is virulent - R strain is nonvirulent
DNA Structure. DNA: The Genetic Material. Chapter 14
 DNA: The Genetic Material Chapter 14 DNA Structure DNA is a nucleic acid. The building blocks of DNA are nucleotides, each composed of: a 5-carbon sugar called deoxyribose a phosphate group (PO 4 ) a nitrogenous
DNA: The Genetic Material Chapter 14 DNA Structure DNA is a nucleic acid. The building blocks of DNA are nucleotides, each composed of: a 5-carbon sugar called deoxyribose a phosphate group (PO 4 ) a nitrogenous
Questions from chapters in the textbook that are relevant for the final exam
 Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
DNA vs. RNA DNA: deoxyribonucleic acid (double stranded) RNA: ribonucleic acid (single stranded) Both found in most bacterial and eukaryotic cells RNA
 DNA Replication DNA vs. RNA DNA: deoxyribonucleic acid (double stranded) RNA: ribonucleic acid (single stranded) Both found in most bacterial and eukaryotic cells RNA molecule can assume different structures
DNA Replication DNA vs. RNA DNA: deoxyribonucleic acid (double stranded) RNA: ribonucleic acid (single stranded) Both found in most bacterial and eukaryotic cells RNA molecule can assume different structures
DNA Replication * OpenStax
 OpenStax-CNX module: m45475 1 DNA Replication * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able
OpenStax-CNX module: m45475 1 DNA Replication * OpenStax This work is produced by OpenStax-CNX and licensed under the Creative Commons Attribution License 4.0 By the end of this section, you will be able
Chapter 16 The Molecular Basis of Inheritance
 Chapter 16 The Molecular Basis of Inheritance Chromosomes and DNA Morgan s experiments with Drosophila were able to link hereditary factors to specific locations on chromosomes. The double-helical model
Chapter 16 The Molecular Basis of Inheritance Chromosomes and DNA Morgan s experiments with Drosophila were able to link hereditary factors to specific locations on chromosomes. The double-helical model
DNA, RNA, Replication and Transcription
 Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College DNA, RNA, Replication and Transcription The metabolic processes described earlier (glycolysis, cellular respiration, photophosphorylation,
Harriet Wilson, Lecture Notes Bio. Sci. 4 - Microbiology Sierra College DNA, RNA, Replication and Transcription The metabolic processes described earlier (glycolysis, cellular respiration, photophosphorylation,
Essential Question. What is the structure of DNA, and how does it function in genetic inheritance?
 DNA Dr. Bertolotti Essential Question What is the structure of DNA, and how does it function in genetic inheritance? What is the role of DNA in hereditary? Transformation Transformation is the process
DNA Dr. Bertolotti Essential Question What is the structure of DNA, and how does it function in genetic inheritance? What is the role of DNA in hereditary? Transformation Transformation is the process
Prokaryotic cells divide by pinching in two. Fig. 10-CO, p.240
 Prokaryotic cells divide by pinching in two Fig. 10-CO, p.240 Learning Objectives 1. What Is the Flow of Genetic Information in the Cell? 2. What Are the General Considerations in the Replication of DNA?
Prokaryotic cells divide by pinching in two Fig. 10-CO, p.240 Learning Objectives 1. What Is the Flow of Genetic Information in the Cell? 2. What Are the General Considerations in the Replication of DNA?
IDTutorial: DNA Replication
 IDTutorial: DNA Replication Introduction In their report announcing the structure of the DNA molecule, Watson and Crick (Nature, 171: 737-738, 1953) observe, It has not escaped our notice that the specific
IDTutorial: DNA Replication Introduction In their report announcing the structure of the DNA molecule, Watson and Crick (Nature, 171: 737-738, 1953) observe, It has not escaped our notice that the specific
DNA: The Genetic Material. Chapter 14
 DNA: The Genetic Material Chapter 14 The Genetic Material Frederick Griffith, 1928 studied Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia there are 2 strains of Streptococcus: - S strain
DNA: The Genetic Material Chapter 14 The Genetic Material Frederick Griffith, 1928 studied Streptococcus pneumoniae, a pathogenic bacterium causing pneumonia there are 2 strains of Streptococcus: - S strain
Bacterial DNA replication
 Bacterial DNA replication Summary: What problems do these proteins solve? Tyr OH attacks PO4 and forms a covalent intermediate Structural changes in the protein open the gap by 20 Å! 1 Summary: What problems
Bacterial DNA replication Summary: What problems do these proteins solve? Tyr OH attacks PO4 and forms a covalent intermediate Structural changes in the protein open the gap by 20 Å! 1 Summary: What problems
Name: - Bio A.P. DNA Replication & Protein Synthesis
 Name: - Bio A.P. DNA Replication & Protein Synthesis 1 ESSENTIAL KNOWLEDGE Big Idea 3: Living Systems store, retrieve, transmit and respond to information critical to living systems Enduring Understanding:
Name: - Bio A.P. DNA Replication & Protein Synthesis 1 ESSENTIAL KNOWLEDGE Big Idea 3: Living Systems store, retrieve, transmit and respond to information critical to living systems Enduring Understanding:
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION
 M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
M I C R O B I O L O G Y WITH DISEASES BY TAXONOMY, THIRD EDITION Chapter 7 Microbial Genetics Lecture prepared by Mindy Miller-Kittrell, University of Tennessee, Knoxville The Structure and Replication
Molecular Biology, Lecture 3 DNA Replication
 Molecular Biology, Lecture 3 DNA Replication We will continue talking about DNA replication. We have previously t discussed the structure of DNA. DNA replication is the copying of the whole DNA content
Molecular Biology, Lecture 3 DNA Replication We will continue talking about DNA replication. We have previously t discussed the structure of DNA. DNA replication is the copying of the whole DNA content
BS GENOMES. DNA replication and repair
 BS2009 - GENOMES DNA replication and repair REPLICATION GENERAL PRINCIPLES START Must be ready Must know where to start FINISH Must all finish Must ensure that each piece of DNA is replicated only once
BS2009 - GENOMES DNA replication and repair REPLICATION GENERAL PRINCIPLES START Must be ready Must know where to start FINISH Must all finish Must ensure that each piece of DNA is replicated only once
Chromosomes. Chromosomes. Genes. Strands of DNA that contain all of the genes an organism needs to survive and reproduce
 Chromosomes Chromosomes Strands of DNA that contain all of the genes an organism needs to survive and reproduce Genes Segments of DNA that specify how to build a protein genes may specify more than one
Chromosomes Chromosomes Strands of DNA that contain all of the genes an organism needs to survive and reproduce Genes Segments of DNA that specify how to build a protein genes may specify more than one
DNApol then (4) adds deoxyribonucleotide triphosphates to the 3'-end of the primer until the molecules are completely replicated.
 Biochemistry II DNA Synthesis p-p-p-n-oh ssna is a nucleotide (of any particular base, as represented by the N) with a 5' triphosphate and a 3' hydroxide. This module is on DNA so the nucleotide is dntp
Biochemistry II DNA Synthesis p-p-p-n-oh ssna is a nucleotide (of any particular base, as represented by the N) with a 5' triphosphate and a 3' hydroxide. This module is on DNA so the nucleotide is dntp
DNA replication. DNA replication. replication model. replication fork. chapter 6
 DN chapter 6 DN two complementary s bases joined by hydrogen bonds separation of s each - template determines order of nucleotides in duplicate parent DN s separate two identical daughter s model dispersive
DN chapter 6 DN two complementary s bases joined by hydrogen bonds separation of s each - template determines order of nucleotides in duplicate parent DN s separate two identical daughter s model dispersive
Recitation CHAPTER 9 DNA Technologies
 Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
Tala Saleh. Tamer Barakat ... Anas Abu. Humaidan
 7 Tala Saleh Tamer Barakat... Anas Abu. Humaidan Some Information in this lecture may not be mentioned by the Dr. as thoroughly as this sheet. But they cannot be overlooked for a better understanding,
7 Tala Saleh Tamer Barakat... Anas Abu. Humaidan Some Information in this lecture may not be mentioned by the Dr. as thoroughly as this sheet. But they cannot be overlooked for a better understanding,
4) separates the DNA strands during replication a. A b. B c. C d. D e. E. 5) covalently connects segments of DNA a. A b. B c. C d. D e.
 1) Chargaff's analysis of the relative base composition of DNA was significant because he was able to show that a. the relative proportion of each of the four bases differs from species to species. b.
1) Chargaff's analysis of the relative base composition of DNA was significant because he was able to show that a. the relative proportion of each of the four bases differs from species to species. b.
Chapter 12. DNA Replication and Recombination
 Chapter 12 DNA Replication and Recombination I. DNA replication Three possible modes of replication A. Conservative entire original molecule maintained B. Semiconservative one strand is template for new
Chapter 12 DNA Replication and Recombination I. DNA replication Three possible modes of replication A. Conservative entire original molecule maintained B. Semiconservative one strand is template for new
CHAPTER 16 MOLECULAR BASIS OF INHERITANCE
 CHAPTER 16 MOLECULAR BASIS OF INHERITANCE DNA as genetic material? Deducted that DNA is the genetic material Initially worked by studying bacteria & the viruses that infected them 1928 Frederick Griffiths
CHAPTER 16 MOLECULAR BASIS OF INHERITANCE DNA as genetic material? Deducted that DNA is the genetic material Initially worked by studying bacteria & the viruses that infected them 1928 Frederick Griffiths
Name 10 Molecular Biology of the Gene Test Date Study Guide You must know: The structure of DNA. The major steps to replication.
 Name 10 Molecular Biology of the Gene Test Date Study Guide You must know: The structure of DNA. The major steps to replication. The difference between replication, transcription, and translation. How
Name 10 Molecular Biology of the Gene Test Date Study Guide You must know: The structure of DNA. The major steps to replication. The difference between replication, transcription, and translation. How
Please sign below if you wish to have your grades posted by the last five digits of your SSN
 BIO 226R EXAM II (Sample) PRINT YOUR NAME SSN Please sign below if you wish to have your grades posted by the last five digits of your SSN Signature BIO 226R Exam II has 6 pages, and 27 questions. There
BIO 226R EXAM II (Sample) PRINT YOUR NAME SSN Please sign below if you wish to have your grades posted by the last five digits of your SSN Signature BIO 226R Exam II has 6 pages, and 27 questions. There
Nucleic Acid Structure:
 Genetic Information In Microbes: The genetic material of bacteria and plasmids is DNA. Bacterial viruses (bacteriophages or phages) have DNA or RNA as genetic material. The two essential functions of genetic
Genetic Information In Microbes: The genetic material of bacteria and plasmids is DNA. Bacterial viruses (bacteriophages or phages) have DNA or RNA as genetic material. The two essential functions of genetic
Covalently bonded sugar-phosphate backbone with relatively strong bonds keeps the nucleotides in the backbone connected in the correct sequence.
 Unit 14: DNA Replication Study Guide U7.1.1: DNA structure suggested a mechanism for DNA replication (Oxford Biology Course Companion page 347). 1. Outline the features of DNA structure that suggested
Unit 14: DNA Replication Study Guide U7.1.1: DNA structure suggested a mechanism for DNA replication (Oxford Biology Course Companion page 347). 1. Outline the features of DNA structure that suggested
The Molecular Basis of Inheritance
 The Molecular Basis of Inheritance Chapter 16 Objectives Describe the contributions of the following people: Griffith; Avery, McCary, and MacLeod; Hershey and Chase; Chargaff; Watson and Crick; Franklin;
The Molecular Basis of Inheritance Chapter 16 Objectives Describe the contributions of the following people: Griffith; Avery, McCary, and MacLeod; Hershey and Chase; Chargaff; Watson and Crick; Franklin;
DNA stands for deoxyribose nucleic acid
 DNA DNA stands for deoxyribose nucleic acid This chemical substance is present in the nucleus of all cells in all living organisms DNA controls all the chemical changes which take place in cells DNA Structure
DNA DNA stands for deoxyribose nucleic acid This chemical substance is present in the nucleus of all cells in all living organisms DNA controls all the chemical changes which take place in cells DNA Structure
DNA: Structure & Replication
 DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
DNA replication. DNA replication. replication model. replication fork. chapter 6
 DN chapter 6 DN two complementary s bases joined by hydrogen bonds separation of s each - template determines order of nucleotides in duplicate parent DN s separate two identical daughter s model dispersive
DN chapter 6 DN two complementary s bases joined by hydrogen bonds separation of s each - template determines order of nucleotides in duplicate parent DN s separate two identical daughter s model dispersive
DNA Model Stations. For the following activity, you will use the following DNA sequence.
 Name: DNA Model Stations DNA Replication In this lesson, you will learn how a copy of DNA is replicated for each cell. You will model a 2D representation of DNA replication using the foam nucleotide pieces.
Name: DNA Model Stations DNA Replication In this lesson, you will learn how a copy of DNA is replicated for each cell. You will model a 2D representation of DNA replication using the foam nucleotide pieces.
