The germ line lineage in ukigori, Gymnogobius species (Teleostei: Gobiidae) during embryonic development
|
|
|
- Ethan Daniels
- 5 years ago
- Views:
Transcription
1 Int. J. ev. Biol. 48: (2004) doi: /ijdb ts Original Article The germ line lineage in ukigori, Gymnogobius species (Teleostei: Gobiidae) during embryonic development TAIJU SAITO*,1, SATOSHI OTANI 2, TAKAFUMI FUJIMOTO 1, TOHRU SUZUKI 3, TAKAKO NAKATSUJI 4, KATSUTOSHI ARAI 1 and ETSURO YAMAHA 5 1 Laboratory of Breeding Science, Graduate School of Fisheries Science, Hokkaido University, 2 Laboratory of Aquatic Biology, Faculty of Agriculture, Kinki University, 3 Laboratory of Bioindustrial Informatics, Graduate School of Agriculture Science, Tohoku University, 4 epartment of Marine Biology, Graduate School of Marine Science and Technology, Tokai University and 5 Nanae Fresh-Water Laboratory, Field Science Center for Northern Biosphere, Hokkaido University, Japan. ABSTRACT In order to determine the origin and migration of ukigori primordial germ cells (PGCs), we observed the aggregation of vasa mrna by whole mount in situ hybridization. To observe PGC migration in the germ layers, we analyzed HE-stained paraffin sections. The germ line lineages were derived from the edge of the first, second and third cleavage furrows. uring subsequent cleavages, vasa mrna aggregations were respectively taken into four to eight cells in each embryo and vasa expressing cells proliferated from the sphere stage. At the bud to early somitogenesis period, PGCs aligned from head to tail bud regions on both sides of the embryonic body. uring the late somitogenesis period, PGCs mainly aggregated just underneath the body axis. After gut formation, PGCs aligned along both sides of the gut at the 4th- to 8th- somite regions. Finally, PGCs reached the genital ridge via the inside of the lateral plate mesoderm and dorsal peritoneum. These results suggest that localized patterns of vasa transcripts and the migration routes of PGCs are different among fish (Teleost) species, perhaps depending on the amount of germinal cytoplasm derived maternally and the timing of endoderm differentiation. KEY WORS: primordial germ cell, PGC, vasa, cell lineage, ukigori Introduction All sexually reproducing organisms arise from gametes. In turn, all gametes arise from primordial germ cells (PGCs), a small population of cells set aside from other cell lineages early in development. In many animals, PGCs have to migrate from the position where they are specified toward the genital ridge during the embryonic development (Reviewed by Wiley, 1999). uring migration, PGC properties continuously change, although they essentially maintain developmental totipotency. These characteristics of PGCs make them an attractive system for studying cell fate specification, differentiation and migration (Reviewed by Raz, 2002). In addition, it is possible that PGCs could serve as tools for advanced technologies and reproductive biology, including genetic modification, cryopreservation and surrogate propagation (Reviewed by Yoshizaki et al., 2004; Saito and Yamaha., 2004). Studies of rosophila and Xenopus laevis PGCs revealed that they were specified by maternally provided cytoplasmic determinants (the germ plasm) that were partially localized in the eggs (Reviewed by Wiley, 1999). The germ plasm is characterized by polar granules and an electron dense structure and contains the mitochondria, mrna and proteins. In those components, in particular, vasa revealed the origin and migration of PGCs as molecular makers in many species, including chick (Tsunekawa et al., 2000) and zebrafish (Yoon et al., 1997). vasa is an ATPdependent RNA helicase belonging to the EA-box family (Hay et al., 1988; Lasko and Ashburner, 1988). It was originally identified in rosophila as a maternal effector gene required for abdominal segmentation and germ cell specification (Schüpbach and Wieshaus, 1986). In zebrafish, whole mount in situ hybridization using vasa during embryonic development revealed that the germ cell lineage is determined by maternally provided factors and separates from somatic cells early in development, as in rosophila and Xenopus (reviewed by Howard, 1998; Rongo et al., 1997; Wylie, 1999). In detail, vasa mrna aggregates at both edges of the cleavage furrow(s) at the 2- to 4-cell stage. Occa- Abbreviations used in this paper: HE, Hematoxylin & Eosin; PGC, primordial germ cell; WISH, whole mount in situ hybridization; YSL, yolk syncytial layer. *Address correspondence to: r. Taiju Saito. Nanae Fresh Water Laboratory, Field Science Center for Northern Biosphere, Hokkaido University, Nanae, , Japan. Fax: taiju@fish.hokudai.ac.jp /2004/$25.00 UBC Press Printed in Spain
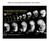
2 1080 T. Saito et al. sionally, vasa mrna localize at the edge of the third cleavage furrows, but this aggregation is degraded after the blastula stage. The PGCs proliferate after the blastula period and cluster around the first somites at the early segmentation period. Finally, clusters of PGCs migrate posterior to the junction of the yolk ball and yolk extension, the prospective genital ridge, keeping two clusters at both sides of the embryonic body (Yoon et al., 1997; Weidinger et al., 1999). PGC migration during embryonic development has also been investigated in several fishes using vasa gene homologs. In goldfish, vasa transcripts are localized at both edges of the third cleavage furrows in addition to the first and second cleavage furrows (Otani et al., 2002). Moreover, it has been shown that the cells taking vasa transcripts from the third cleavage furrows proliferate after the blastula stage, unlike zebrafish. At the early segmentation period, PGCs align from head to tail on both sides of the body axis. Thereafter, the PGCs aggregate to the yolk extension (prospective genital ridge region) and form the two clusters on both sides of the body axis. On the other hand, in medaka, vasa positive cells were not detected until the late gastrula period (Shinomiya et al., 2000; Tanaka et al., 2001). vasa positive cells were apparent at the dorsal marginal region randomly at the 70% epiboly stage. At the early segmentation period, PGCs form two clusters beside the somites. Thereafter, PGCs migrate to the gut, remaining in two clusters. These results suggest that the determination mechanisms of PGCs vary between fish species, although it is necessary that additional PGC specific molecular markers, such as nanos homologue gene mrna (Kobayashi et al., 1996; Köprunner et al., 2001), should be examined in medaka. However, the PGC migration patterns in these fishes correspond to the following points: 1) PGC origins are not related to the region of genital ridge formation, 2) PGCs localize on both sides of the body axis at the early segmentation period and 3) PGCs aggregate at the prospective genital ridge region while remaining in two clusters. The migration routes of PGCs, especially during the stages from the mesendodermal region to the genital ridge, have also been investigated histologically in several fishes. PGCs in medaka appear around the lateral endoderm at somitogenesis and move passively to the genital ridge via the outer layer of the lateral plate mesoderm at the side of gut (Gamo, 1961; Hamaguchi 1982). However, in other fishes, e.g. Fundulus heterclitus and black bass (Micropterus salmonides salmonides), PGCs migrate to the genital ridge via the inner layer of the lateral plate mesoderm (Richards and Thompson, 1921; Johnston, 1951). On the other hand, in zebrafish, during the course to genital ridge, PGCs form the pseudopodium, suggesting active movement (Braat et al., 1999). Results of these studies suggest that the final step of the PGC migration mechanism to the genital ridge varies between fish species. The origin and migration of PGCs in teleost fishes has not been explained by common concepts, as mentioned above. This might be caused by the remarkable diversity of teleost fishes. For comprehensive understanding of teleost PGCs, genetic analysis such as large-scale mutant screening as in zebrafish is one strategy, another workable strategy is comparison between model fishes and other fish species. However, studies of PGCs using molecular markers are restricted to a few fishes, namely, zebrafish, goldfish and medaka. In Perciformes, the most evolved group of teleost fish, the origin and migration of PGCs have not been investigated in detail. In this study, we investigate these aspects of PGCs in ukigori, Chaenogobius species (Family Gobiidae), by whole mount in situ hybridization and histological observation in HEstained paraffin sections. Results vasa RNA expression in the early embryo To reveal the origin of PGCs, localization of vasa transcripts was investigated from the 2-cell stage to 5 day post-fertilization (dpf) by WISH, using shiro-uo vasa probes. We did not observe localization of vasa transcripts in unfertilized eggs or 1-cell stage embryos, on account of difficulties in sampling and fixing embryos of those stages. At the 2-cell and 4-cell stage, vasa transcripts were enriched at the marginal positions of the first (Fig. 1 A,B) and the second cleavage plane (Fig. 1 C,). At the third cleavage plane, new vasa transcripts accumulation(s) were observed in some embryos. In the case that the third cleavage furrows divide horizontally, vasa mrna was enriched between the upper and lower tiers of the blastomeres at the 8- cell stage (Fig. 1 E,F). At the 16-cell stage, 4 to 8 signals of vasa mrna were observed in the cleavage planes of each embryo (Table 1). At the 512-cell stage, the number of vasa dots was 4 to 7 in each embryo (Table 1). Accumulations of vasa tran- A C E Fig. 1. istribution of vasa-positive dots from the 2-cell to 8-cell stage. (A,B) 2-cell, (C,) 4-cell and (E,F) 8-cell stage embryos. (A, C, E) are lateral views, while (B,, F) are animal pole views. Arrowheads indicate the accumulation of vasa transcripts. The scale bar indicates 50 µm. B F
3 Germ cell lineage in ukigori, Gymnogobius species 1081 A B C E F G Fig. 2. istribution of vasa-positive cells from the sphere to 30-somite stage. (A) Sphere, (B,C) 90% epiboly, () 10- somite, (E) 15-somite, (F) 20-somite and (G) 30-somite stage embryos. A and B are lateral views. C is a vegetal pole view., E, F and G are dorsal views. Arrows in (A) indicate the vasapositive cells that were located at the animal region. Arrows in B, F and G indicate ectopic vasa-positive cells. The arrowhead in (C) indicates dorsal position. od, oil droplet. The scale bar indicates 50 µm. scripts were respectively taken in single blastomeres until the blastula stage. After the late blastula stage (sphere), the number of vasa-positive cells increased and divided vasa-positive cells located adjacent to each other (Fig. 2A, Table 1). At the 30% epiboly stage, clusters of vasa positive cells were mainly observed at the marginal region of the blastodisc. The number of clusters was 4 to 6 at the 40% epiboly stage in each embryo (Table 2). At the 90% epiboly stage, vasa positive cells were located both at the germ ring region of the blastoderm and the dorsal anterior region along the embryonic body (Fig. 2 B,C). At the bud stage, vasa positive cells were aligned along both sides of the embryonic body from the head to the tail bud region. At the 10-somite stage, vasa positive cells were also found, similar to those at the bud stage (Fig. 2). At the 15-somite stage, vasa-positive cells migrated toward the axial region in a line (Fig. 2E). At the 20-somite stage, both sides of PGCs aggregated under the trunk region, although some vasa-positive cells were located at the head region (Fig. 2F). At the 30-somite stage, vasa positive-cells were aligned at both sides of the newly formed gut of the 4th- to 8th-somite region, however, some vasa-positive cells remained at the head region (Fig. 2G). These vasa-positive cells localized at the head region were detected until at least 5-dpf embryos. Observation of PGCs in HE-stained histological sections To identify the location of PGCs after the somitogenesis period and the migration of PGCs in the germ layer, we observed paraffin sections of ukigori embryos from the blastula to 12-dpf stages. When we observed the gonadal anlage of 8-dpf embryos, the cells located around the dorsal peritoneum and upper part of the body cavity were identified as PGCs by the characteristics as mentioned in Materials and Methods (Fig. 3 K,L). The PGCs could be traced back to the 10-somite stage. At the previous stages, PGCs could not be distinguished from somatic cells because eosinophilic yolk granules preserved in somatic cells prevent differentiation of PGCs and somatic cells. At the 10-somite stage, PGCs were located in the lateral to trunk region of the lateral plate mesoderm. In this stage, many PGCs came in contact with the yolk syncytial layer (YSL) (Fig. 3 A,B). At the 20-somite stage, PGCs were located at a more axial part of the mesendodermal layer than before (Fig. 3 C,). At the 20-somite stage, some PGCs were located on the dorsal midline of the endoderm region, but not observed in the region after the gut cavity was formed. At the 30-somite stage, the majority of PGCs were in contact with the lateral part of the gut (Fig. 3 E,F). In 4-dpf embryos, PGCs were more dorsally located in the lateral plate mesoderm lateral to the gut and separated from the YSL (Fig. 3 G,H). PGCs of this stage were in close contact with the gut. In 6-dpf embryos, the majority of PGCs were located on the dorsal part of the gut (Fig. 3 I,J). In TABLE 1 THE NUMBER OF VASA SIGNALS OR VASA-POSITIVE CELLS OB- SERVE BY WISH FROM THE 16-CELL TO 90% EPOBOLY STAGE Stage No. of vasa positive cells (dots) Average 16-cell 4, 4, 4, 4, 4, 4, 4, 4, 5, 5, 5, 5, 5, 6, 6, 6, 6, 6, 7, 7, 7, 7, 7, 8, 8, 8, 8, 8, cell 4, 5, 5, 5, 5, 5, 6, 6, 6, 7 shpere 4, 4, 4, 7, 7, 7, 8, 8, 8, 8, 8, 9, 9, 10, 10, 10, 11, 11, 12, 12, 13, 15, 16, % epiboly 8, 11, 11, 12, 12, 13, 14, 14, 14, 14, 15, 16, 18, 18, 19, 20, 20, 21, % epiboly 21, 22, 23, 25, 33, 34, 38, TABLE 2 THE NUMBER OF CLUSTERS OF VASA-POSITIVE CELLS OBSERVE BY WISH AT THE SHIEL STAGE Stage Total No. of No. of clusters (%) embryos % epiboly 40 1 (2.5) 16 (40.0) 16 (40.0) 7 (17.5)
4 1082 T. Saito et al. A C E B F G I K H J L Fig. 3. istribution of PGCs in transverse sections of ukigori embryos. (A,B) 10-somite, (C,) 20-somite, (E,F) 30-somite, (G,H) 4 dpf, (I,J) 6 dpf and (K,L) 8 dpf stage embryos. B,, F, H, J and L are higher magnifications of A, C, E, G, I and K, respectively. Scale bars indicate 50 µm. A C B 8-dpf embryos, most of them reached the dorsal peritoneum of the upper part of the body cavity (Fig. 3 K,L). Comparison of vasa positive cells and histological PGCs To elucidate whether vasa positive cells were identical to histologically detected PGCs or not, WISH samples hybridized with vasa probes were sectioned and analyzed. In latesomitogenesis period embryos, vasa positive cells were observed mainly in the trunk region (Fig. 4 A,B) and sometimes in the head region (Fig. 4 C,). As a result, vasa positive cells were located at the same positions as PGCs observed histologically at the 20-somite stage (Fig. 2F). Moreover, the Fig. 4. Light eosin-stained histological sections of WISH-stained embryos by vasa probe at the late-somitogenesis period. (A,B) Transverse section images of the somite region. (C,) Transverse section images of the head region. B and are higher magnifications of A and C, respectively. Arrows and arrowheads indicate vasapositive cells. ev, eye vesicle; fb, forebrain; g, gut; no, notochord; nt, neural tube; yc, yolk cell. Scale bars indicate 100 µm.
5 Germ cell lineage in ukigori, Gymnogobius species 1083 A B C E F G J K L M Fig. 5. A schematic illustration of PGC migration in Ukigori. (A-I) The origins and migration routes of PGCs. vasa transcripts localized at the cleavage furrows until third cleavage (A). vasa transcripts are taken into the single cells at the blastula stage (B). PGCs increase from the sphere stage and locate at the margin of the blastodisc (C). PGCs align at both sides of body axis from the bud to 10- somite stage (). PGCs locate under the body axis and gradually aggregate (E,F). PGCs align at both sides of the newly formed gut (G). PGCs migrate from the ventral side of the gut to the dorsal side, finally localizing in the genital ridge (H,I). (J-N) Transverse images of ukigori PGC migration. PGCs migrate toward the mid-line with close contact to the yolk H I syncytial layer (YSL) during the 10- and 30-somite stages (J-L). Thereafter, PGCs migrate to the genital ridge via the lateral plate mesoderm in close contact to the gut (L-N). The developmental stages of J, K, L, M and N correspond to, F, G, H and I, N respectively. Red circles indicate PGCs. Blue circles indicate PGCs located in the ectopic region. average number of vasa-positive cells in WISH embryos was approximately equal to those of PGCs in HE-stained histological sections, at 10-somite stage (Table. 3). iscussion In this study, we carried out WISH by using shiro-uo vasa probes in ukigori embryos. vasa mrna accumulated at the cleavage furrows and thereafter, signals were continuously observed in the restricted cells. The locations of vasa mrna were similar to those observed in other fish species in which the origin and dynamics of PGCs were reported by vasa mrna (Yoon et al., 1997; Otani et al., 2002). When post-wish embryos at the 20- somite stage were sectioned and observed, the location of vasa positive cells was consistent with PGCs observed at the same stage histologically. Moreover, the average number of vasapositive cells in WISH embryos was approximately equal to those of PGCs in HE-stained histological sections, at 10-somite stage. Thus, we concluded that the vasa positive cells were PGCs. Furthermore, shiro-uo vasa probe have been shown to detect ukigori PGCs. The number of vasa signals varied (from 4 to 8) among embryos at the 16-cell stage. At the 40% epiboly stage, the number of PGC clusters observed was between 4 and 6. This suggests that vasa-positive cells originating from the third cleavage plane can divide and proliferate after the late blastula stage. In zebrafish, vasa signals from the third cleavage furrows did not proliferate (Yoon et al., 1997), but in goldfish, all signals from the third cleavage furrows proliferated (Otaninet al., 2002). This suggests that germ cells are determined by the amount of germinal factors and formation of PGCs are not restricted to specific edges of cleavage furrows. In the ukigori embryos, irregular dividing patterns were sometimes observed at the 8-cell stage (Saito et al., 2004). This irregular cleavage pattern may cause a distorted aggregation pattern of vasa mrna and result in unstable numbers. In ukigori embryos, vasa mrna aggregated at both edges of the first, second and third cleavage furrows. Knaut et al. (2002) discussed that the mechanisms of aggregation of vasa mrna at the cleavage plane are lost in the Euteleostei including medaka and trout, different from the Osteriophysans. However, in this study, vasa mrna aggregated at the edges of the cleavage furrows of ukigori embryos, which belong to the Euteleostei, at the cleavage period. Hence, there is room for reconsideration in terms of the relationship between the phylogenetic position of fish and the accumulation patterns of vasa transcripts. PGCs were observed at the head region during the somitogenesis period in high frequency (50%). Moreover, some head PGCs were histologically detected in the head ectoderm. In teleosts, TABLE 3 THE NUMBER OF VASA-POSITIVE CELLS AN PGCS IN WISH AN HE-STAINE HISTOLOGICAL SECTIONS Samples Stage Embryos The number of PGCs in respective embryos Average analyzed WISH 10-somite 11 15, 17, 18, 18, 24, 25, 25, 27, 35, 35, HE-stained histological 10-somite 12 18, 19, 22, 22, 22, 23, 23, 25, 27, 31, 33, sections
6 1084 T. Saito et al. PGCs have been observed in the trunk mesendoderm region during embryogenesis, but seldom around the head region, especially the head ectoderm. In goldfish, ectopic PGCs have been histologically detected around the head mesoderm and frequently in the head region (Kazama-Wakabayashi et al., 1999; Otani et al., 2002). These ectopic PGCs suggest that large scale mixing of blastomeres during the blastula stage carry some PGCs from the marginal zone to the animal pole region and consequently, that convergence movements produce ectopic head PGCs at the somitogenesis period (Otani et al., 2002). In ukigori embryos, vasa mrna aggregations are occasionally formed at the middle part of the blastodisc, by the third horizontal cleavage plane (present study) and large scale mixing of blastomeres is also observed from the blastula to the epiboly stage (Saito et al., 2004), suggesting localization of PGCs around the prospective head region of the blastoderm during gastrulation, as in goldfish. However, the actual movement of PGCs to the head region is still unknown. Examination of the route of visualized PGCs during gastrulation, as in zebrafish, is required (Köprunner et al., 2001). The migration route, shown by histological sections and WISH in vasa mrna, of PGCs in ukigori is summarized in Fig. 5. Germ plasm aggregating in early cleavage furrows is inherited by several blastomeres during the blastula stage, the resultant founder cells of PGCs proliferate during epiboly and PGCs are located at both sides of the body axis during the subsequent segmentation period. These processes of PGCs movement are similar to those of other teleost fish species reported. Thereafter, however, PGCs in ukigori immediately migrate in the dorsal direction to the body axis during the subsequent segmentation period, instead of in the posterior direction as reported in other species. In the center of the body axis, PGCs from both sides seem to be mingled on the endoderm and redistributed to both sides of the gut. This PGC migration pattern is similar to that of the shiro-uo, belonging to the same family gobiidae as the ukigori (Saito et al., 2002). The timing of PGC redistribution after 20-somite stage is corresponded to the definite gut formation. It seems probable that the newly formed gut affecting the PGCs migration. On the other hand, in other species of fishes, such as zebrafish, goldfish and medaka, PGCs at both sides migrate posterior-ward to the presumptive genital ridge region, then localize in sides of gut in the course of the posterior movement (Weidinger et al., 1999; Shinomiya et al., 2000; Tanaka et al., 2001; Otani et al., 2002). In zebrafish, it has been reported that migration of PGCs is guided by the chemokine signal SF-1a (oitsidou et al., 2002). Therefore, the expression patterns of guidance signals such as SF-1a remain to be examined. In final step of PGC migration to the genital ridge, ukigori PGCs migrate along the inner layer of the lateral plate mesoderm lateral to the gut. This route of PGC migration has been observed in Fundulus heteroclitus (Richards and Thompson, 1921) and black bass (Johnston, 1951). In this step, PGCs migrate actively with pseudopodia to the genital ridge in zebrafish (Braat et al., 1999). On the other hand, in medaka embryos, PGCs move to the outer layer of the lateral plate mesoderm and migrate passively dorsal-ward, from light- and electron-microscopic observations (Gamo, 1961; Hamaguchi, 1985). From this point, PGCs seem to adopt several mechanisms in the final step to the genital ridge, although it is unclear whether PGCs in ukigori migrate to genital ridge passively or actively. Since there is little knowledge of the migration of PGCs to the genital ridge, it is necessary to investigate the mechanism of migration of the later step to the genital ridge. In summary, we identified the migration route of PGCs from the origin to the genital ridge during embryonic development in ukigori, Perciformes gobiidae. These results suggest that the migration routes and localized patterns of PGCs are influenced by embryonic development in each fish, although are mainly conserved. Further research on the migration of PGCs in ukigori would clarify the behavior of fish PGCs. Materials and Methods Gametes and embryo culture Fertilized eggs of ukigori were sampled at the Moheji and Ryukei rivers, near Hakodate, in the southern part of Hokkaido, Japan, from April to June. Stones, on which eggs were spawned, were collected in the rivers and transferred to the Nanae Fresh-Water Laboratory. Eggs were removed from the stones and dechorionated by fine forceps. echorionated embryos were cultivated in Ringer s solution (120 mm NaCl, 2.8 mm KCl, 1.8 mm CaCl 2 ) containing 0.01% penicillin-streptomycin and 1.6% egg albumen at 20 C until the epiboly period. After the epiboly or segmentation period, the embryos were cultivated in culture medium (1.8 mm CaCl 2 and 1.8 mm MgCl 2 ) containing 0.01% penicillin-streptomycin at 20 C until the hatching period. The developmental stages were determined by external morphology according to Saito et al., (2004). Whole mount in situ hybridization using vasa probe Whole mount in situ hybridization (WISH) was carried out essentially as described by Thisse et al. (1994) with slight modification. Antisense vasa probe from shiro-uo, Leucopsarion petersii, (Accession No. AB098252) containing 0.38 Kb EA-box regions were prepared using a digoxigenin (IG) RNA labeling kit (Roche). Manually dechorionated embryos were fixed with 4% paraformaldehyde in PBS for 20- to 30 h. Fixed embryos were stored in 100% methanol at -20 C. Then embryos were rehydrated in PBT (1X PBS 0.1% Tween 20) 2 times for 5 min each. Embryos older than the beginning of somitogenesis were treated 10 to 60 min with proteinase K (10 µg/ml in PBT). Embryos were postfixed in 4% paraformaldehyde in PBS for 20 min and then rinsed in PBT 3 times for 5 min each. The embryos were prehybridized 3 hour at 62 C in hybridization buffer (50% formamide, 5X SSC, 50 µg/ml heparin, 500 µg/ ml trna, 0.1% Tween 20). The hybridization was done in the same buffer containing probe overnight at 62 C. Then the embryos were washed at 62 C for 10 min in (50% formamide, 50% 5X SSC, 0.1% Tween 20), 10 min in (50% formamide, 50% 2X SSC, 0.1% Tween 20), 10 min in (25% formamide, 75% 2X SSC, 0.1% Tween 20), 10 min in (2X SSC, 0.1% Tween 20), 4 times 15 min in (0.2X SSC, 0.1% Tween 20). Further washes were performed at room temperatures for 5 min in PBT and then 1~8 hour in Blocking solution (PBT with 10% blocking reagent). Then the embryos were incubate overnight at 4 C with anti-digoxigenin antiserum (Roche) at a 1/8000 dilution in a blocking solution and then embryos were washed 6 times for 20 min each in PBT at room temperature. Then embryos were washed 3 times for 5 min each in Staining buffer (100 mm Tris HCl ph 9.5, 50 mm MgCl 2, 100 mm NaCl, 0.1% Tween 20). etection was performed in Staining solution (3.375 mg/ml NBT, 1.75 mg/ml BCIP, Staining buffer) at 4 C. When the color was developed, the reaction was stopped in 1X PBS. Photographs were taken using an Olympus CAMEIA imaging system S3030U equipped with a SZX-12 stereomicroscope. Histology For histological observation, embryos were fixed with Bouin s fixative for 2 h, dehydrated in a butyl alcohol series and embedded in paraffin. Serial sections were cut 8 µm thick. PGCs were confirmed based on location and characteristics; such as round shape, large nuclei, relatively large size and clear nuclear membrane, similar to those observed in other fishes (Braat et al., 1999; Kazama-Wakabayashi et al., 1999; Nagai et al., 2001). The PGCs size varied between 10 and 20 µm. Using these
7 Germ cell lineage in ukigori, Gymnogobius species 1085 characteristics, the origin of the PGCs could be traced back to the 10- somite stage. WISH samples stained with vasa probes were dehydrated in a butyl alcohol series and embedded in paraffin. Serial sections were cut 12 µm thick. The location of vasa positive cells was compared with HE-stained PGCs. Acknowledgements We thank Mr. Shizuo Kimura, Ms. Chikako Nishida and the members of Nanae Fresh Water Laboratory (Field Science Center for Northern Biosphere, Hokkaido University. This study was supported by the Research Fellowships of the Japan Society for the Promotion of Science (JSPS) for Young Scientists, to T. Saito (No. 0446), a Grant-in Aid for Scientific Research (B) from JSPS to E.Y (No ) and COEprogram to Graduate School of Fisheries Science, Hokkaido University. References BRAAT, A.K., ZANBERGEN, T., VAN E WATER, S., GOOS H.J. and ZIVKOVIC,. (1999) Characterization of zebrafish primordial germ cells: morphology and early distribution of vasa RNA. ev. yn. 216: OITSIOU, M., REICHIMAN-FRIE, M., STEBLER, J., KÖPRUNNER, M., ORRIES, J., MEYER,., ESGUERRA, C.V., LEUNG, T. and RAZ, E. (2002) Guidance of primordial germ cell migration by the chemokine SF-1. Cell 111: GAMO, H. (1961) On the origin of germ cells and formation of gonad primordia in the medaka, Oryzias latipes. Japan J. Zool. 13: HAMAGUCHI, S. (1982) A light- and electron-microscopic study on the migration of primordial germ cells in the teleost, Oryzias latipes. Cell Tissue Res. 227: HAY, B., JAN, L.Y. and JAN, Y.N. (1988) A protein component of rosophila polar granules is encoded by vasa and has extensive sequence similarity to ATPdependent helicases. Cell 55: HOWAR, K. (1998) Organogenesis: rosophila goes gonadal. Curr. Biol. 8: R JOHNSTON, P.M. (1951) The embryonic histology of the germ cells of the largemouth black bass, Micropterus salmoides salmoides (Lacépède). J. Morphol. 88: KAZAMA-WAKABAYASHI, M., YAMAHA, E. and YAMAZAKI, F. (1999) The elimination and duplication of lower part of blastoderm effects on the number of primordial germ cells in goldfish. Fish. Sci. 65: KNAUT, H., STEINBEISSER, H., SCHWARZ, H. and NUSSLEIN-VOLHAR, C. (2002) An evolutionary conserved region in the vasa 3 UTR targets RNA translation to the germ cells in the zebrafish. Curr. Biol. 12: KOBAYASHI, S., YAMAA, M., ASAOKA, M. and KITAMURA, T. (1996) Essential role of the posterior morphogen nanos for germline development in rosophila. Nature 380: KÖPRUNNER, M., THISSE, C., THISSE, B. and RAZ, E. (2001) A zebrafish nanosrelated gene is essential for the development of primordial germ cells. Genes ev. 15: LASKO, P.F. and ASHBURNER, M. (1988) The product of the rosophila gene vasa is very similar to eukaryotic initiation factor-4a. Nature 335: NAGAI, T., YAMAHA, E. and ARAI, K. (2001) Histological differentiation of primordial germ cells in zebrafish. Zool. Sci. 18; OTANI. S., MAEGAWA, S., INOUE, K., ARAI, K. and YAMAHA, E. (2002) The germ cell lineage identified by vas-mrna during the embryogenesis in goldfish. Zool. Sci. 19: RAZ, E. (2002) Primordial germ cell development in zebrafish. Semin. Cell. ev. Biol : RICHARS, A. and THOMPSON, J.T. (1921) The migration of the primary sex-cells of Fundulus heteroclitus. Biol. Bull. 40: RONGO, C., BROIHIER, H.T., MOORE, L., VAN OREN, M., FORBES, A. and LEHMANN, R. (1997) Germ plasm assembly and germ cell migration in rosophila. Cold Spring Harb. Symp. Quant. Biol. 62: SAITO, T., OTANI, S., NAGAI, T., NAKATSUJI, T., Arai, K. and YAMAHA, E. (2002) Germ cell lineage from a single blastomere at 8-cell stage in shiro-uo (ice goby). Zool. Sci. 19: SAITO, T. and YAMAHA, E. (2004) Aspects and prospective of surrogate propagation in teleost fish. J. Anim. Genet. 31: (in Japanese). SAITO, T., ARAI, K. and YAMAHA, E. (2004) The embryonic development of shimaukigori, Gymnogobius opperiens. Suisanzoshoku 52: (in Japanese). SCHÜPBACH, T. and WIESCHAUS, E. (1986) Germline autonomy of maternaleffect mutations altering the embryonic body pattern of rosophila. ev. Biol. 113: SHINOMIYA, A., TANAKA, M., KOBAYASHI, T., NAGAHAMA, Y. and HAMAGUCHI, S. (2000) The vasa-like gene, olvas, identifies the migration path of primordial germ cells during embryonic body formation stage in the medaka, Oryzias latipes. ev. Growth. iffer. 42: TANAKA, M., KINOSHITA, M., KOBAYASHI,. and NAGAHAMA, Y. (2001) Establishment of medaka (Oryzias latipes) transgenic lines with the expression of green fluorescent protein fluorescence exclusively in germ cells: A useful model to monitor germ cells in a live vertebrate. Proc. Natl. Acad. Sci. USA. 98: THISSE, C., THISSE, B., SCHILLING, T.F. and POSTLETHWAIT, J.H. (1993) Structure of the zebrafish snail1 gene and its expression in wild-type, spadetail and no tail mutant embryos. evelopment 119: TSUNEKAWA, N., NAITO, M., SAKAI, Y., NISHIA, T. and NOCE, T. (2000) Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. evelopment 127: WEIINGER, G., WOLKE, U., KÖPRUNNER, M., KLINGER, M. and RAZ, E. (1999) Identification of tissues and patterning events required for distinct steps in early migration of zebrafish primordial germ cells. evelopment 126: WYLIE, C. (1999) Germ cells. Cell 96; YOON, C., KAWAKAMI, K. and HOPKINS, N. (1997) Zebrafish vasa homologue RNA is localized to the cleavage planes of 2- and 4-cell-stage embryos and is expressed in the primordial germ cells. evelopment 124: YOSHIZAKI, G., TAKEUCHI, Y., KOBAYASHI, T. and TAKEUCHI, T. (2004) Primordial germ cells: A novel tool for fish bioengineering. Fish. Physiol. Biochem. (in press). Received: June 2004 Reviewed by Referees: July 2004 Modified by Authors and Accepted for Publication: October 2004 Edited by: Makoto Asashima
Title. Author(s)Yamaha, Etsuro; Saito, Taiju; Goto-Kazeto, Rie; Arai. CitationJournal of Sea Research, 58(1): Issue Date Doc URL.
 Title Developmental biotechnology for aquaculture, with sp Author(s)Yamaha, Etsuro; Saito, Taiju; Goto-Kazeto, Rie; Arai CitationJournal of Sea Research, 58(1): 8-22 Issue Date 2007-07 Doc URL http://hdl.handle.net/2115/30181
Title Developmental biotechnology for aquaculture, with sp Author(s)Yamaha, Etsuro; Saito, Taiju; Goto-Kazeto, Rie; Arai CitationJournal of Sea Research, 58(1): 8-22 Issue Date 2007-07 Doc URL http://hdl.handle.net/2115/30181
+ + Development and Evolution Dorsoventral axis. Developmental Readout. Foundations. Stem cells. Organ formation.
 Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
Development and Evolution 7.72 9.11.06 Dorsoventral axis Human issues Organ formation Stem cells Developmental Readout Axes Growth control Axon guidance 3D structure Analysis Model + + organisms Foundations
BIOLOGY
 Int. J. Dev. Biol. 54: 1487-1492 (2010) doi: 10.1387/ijdb.092914rg THE INTERNATIONAL JOURNAL OF DEVELOPMENTAL BIOLOGY www.intjdevbiol.com Isolation of teleost primordial germ cells using flow cytometry
Int. J. Dev. Biol. 54: 1487-1492 (2010) doi: 10.1387/ijdb.092914rg THE INTERNATIONAL JOURNAL OF DEVELOPMENTAL BIOLOGY www.intjdevbiol.com Isolation of teleost primordial germ cells using flow cytometry
BIOLOGY
 Int. J. Dev. Biol. 54: 1481-1486 (2010) doi: 10.1387/ijdb.103111ts THE INTERNATIONAL JOURNAL OF DEVELOPMENTAL BIOLOGY www.intjdevbiol.com Inter-species transplantation and migration of primordial germ
Int. J. Dev. Biol. 54: 1481-1486 (2010) doi: 10.1387/ijdb.103111ts THE INTERNATIONAL JOURNAL OF DEVELOPMENTAL BIOLOGY www.intjdevbiol.com Inter-species transplantation and migration of primordial germ
Surrogate production technology in fish
 Surrogate production technology in fish Martin Pšenička, Taiju Saito www.frov.jcu.cz Content of presentation Introduction to a new biotechnological technique, surrogate production in fish. The surrogate
Surrogate production technology in fish Martin Pšenička, Taiju Saito www.frov.jcu.cz Content of presentation Introduction to a new biotechnological technique, surrogate production in fish. The surrogate
Double in situ Hybridization Lili Jing *
 Double in situ Hybridization Lili Jing * Department of Cell and Molecular Biology, University of Pennsylvania, Philadelphia, USA *For correspondence: lilijingcn@gmail.com [Abstract] Double in situ hybridization
Double in situ Hybridization Lili Jing * Department of Cell and Molecular Biology, University of Pennsylvania, Philadelphia, USA *For correspondence: lilijingcn@gmail.com [Abstract] Double in situ hybridization
Activity 47.1 What common events occur in the early development of animals? 1. What key events occur at each stage of development?
 Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
Notes to Instructors Chapter 47 Animal Development What is the focus of this activity? Chapter 21 provided a review of how genes act to control development. Chapter 47 reviews some of the major morphological
To achieve the ability to transmit genetic information via
 Establishment of medaka (Oryzias latipes) transgenic lines with the expression of green fluorescent protein fluorescence exclusively in germ cells: A useful model to monitor germ cells in a live vertebrate
Establishment of medaka (Oryzias latipes) transgenic lines with the expression of green fluorescent protein fluorescence exclusively in germ cells: A useful model to monitor germ cells in a live vertebrate
Later Development. Caenorhabditis elegans. Later Processes 10/06/12. Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development
 Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Later Development Cytoplasmic Determinants Fate Mapping & Cell Fate Limb Development Caenorhabditis elegans Nematoda 10,000 worms/petri dish in cultivation short life cycle (~ 3 days egg to egg) wild-type
Early Development and Axis Formation in Amphibians
 Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Biology 4361 Early Development and Axis Formation in Amphibians October 25, 2006 Overview Cortical rotation Cleavage Gastrulation Determination the Organizer mesoderm induction Setting up the axes: dorsal/ventral
Mass Isolation of Primordial Germ Cells from Transgenic Rainbow Trout Carrying the Green Fluorescent Protein Gene Driven by the vasa Gene Promoter
 BIOLOGY OF REPRODUCTION 67, 1087 1092 (2002) DOI 10.1095/biolreprod.102.005660 Mass Isolation of Primordial Germ Cells from Transgenic Rainbow Trout Carrying the Green Fluorescent Protein Gene Driven by
BIOLOGY OF REPRODUCTION 67, 1087 1092 (2002) DOI 10.1095/biolreprod.102.005660 Mass Isolation of Primordial Germ Cells from Transgenic Rainbow Trout Carrying the Green Fluorescent Protein Gene Driven by
7.22 Final Exam points
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 11 7.22 2004 FINAL FOR STUDY
Reading. Lecture III. Nervous System Embryology. Biology. Brain Diseases. September 5, Bio 3411 Lecture III. Nervous System Embryology
 Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 Bio 3411 Wednesday 2 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Lecture III. Nervous System Embryology
 Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
Bio 3411 Wednesday Reading NEUROSCIENCE: 5 th ed, pp. 477-506 NEUROSCIENCE: 4 th ed, pp. 545-575 2 1 Summary from Lecture II Biology Understanding the brain is THE major question in biology and science.
mrna IN SITU HYBRIDIZATION For Sectioned Zebrafish
 DAYS 1 2: Harvesting fish, tissue fixation, hybridization 1. Harvest and fix embryos in 4% paraformaldehyde overnight at 4C *Fix should be made fresh on the day it will be used. Do not store it for long
DAYS 1 2: Harvesting fish, tissue fixation, hybridization 1. Harvest and fix embryos in 4% paraformaldehyde overnight at 4C *Fix should be made fresh on the day it will be used. Do not store it for long
Blastula: An early staged embryo that is made up of sphere shaped cells that surround an inner fluid-filled cavity known as the blastocoel.
 1) Define the following terms: cleavage, blastomere, blastula, blastulation, microlecithal, mesolecithal, megalecithal, centrolecithal, isolecithal, telolecithal, holoblastic cleavage, meroblastic cleavage,
1) Define the following terms: cleavage, blastomere, blastula, blastulation, microlecithal, mesolecithal, megalecithal, centrolecithal, isolecithal, telolecithal, holoblastic cleavage, meroblastic cleavage,
Lecture 20: Drosophila embryogenesis
 Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
Lecture 20: Drosophila embryogenesis Mitotic recombination/clonal analyses Embrygenesis Four classes of genes: Maternal genes Gap genes Pair-rule genes Segment polarity genes Homeotic genes Read 140-141;
What s the most complex problem in biology?
 Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? 1 The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development
 Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
Chapter 47. Development What s the most complex problem in biology? The most complex problem How to get from here to there Development: cellular level Cell division Differentiation cells become specialized
ANAT2341 Embryology Introduction: Steve Palmer
 ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
ANAT2341 Embryology Introduction: Steve Palmer Course overview Course lecturers specializations and roles Steve Palmer 9385 2957 Course overview Summary, aims and expected outcomes Course overview Graduate
Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement
 Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement Hwei-Jan Hsu 1,2, Ming-Ren Liang 3, Chao-Tsen Chen 3, and Bon-Chu Chung 1 * Abstract Embryonic cell movement is essential
Pregnenolone Stabilizes Microtubules and Promotes Zebrafish Embryonic Cell Movement Hwei-Jan Hsu 1,2, Ming-Ren Liang 3, Chao-Tsen Chen 3, and Bon-Chu Chung 1 * Abstract Embryonic cell movement is essential
Effects of LiCl on Zebrafish Embryonic Development & Whole Mount in situ Hybridization of Goosecoid (gsc) Gene in Zebrafish
 Effects of LiCl on Zebrafish Embryonic Development & Whole Mount in situ Hybridization of Goosecoid (gsc) Gene in Zebrafish Introduction/background: [Instructors: this protocol was adapted from Stachel
Effects of LiCl on Zebrafish Embryonic Development & Whole Mount in situ Hybridization of Goosecoid (gsc) Gene in Zebrafish Introduction/background: [Instructors: this protocol was adapted from Stachel
Fertilization. Animal hemisphere. Sperm entry point
 Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Fertilization Animal hemisphere Sperm entry point Establishes the dorsal/ventral axis Ventral side sperm entry Dorsal side gray crescent Organized by sperm centriole Cleavage Unequal radial holoblastic
Whole Mount In Situ Hybridization Protocol
 Whole Mount In Situ Hybridization Protocol Purpose of in situs: To use an RNA probe to stain for the specific complementary mrna of an intact embryo, allowing visualization of mrna patterns. Note: When
Whole Mount In Situ Hybridization Protocol Purpose of in situs: To use an RNA probe to stain for the specific complementary mrna of an intact embryo, allowing visualization of mrna patterns. Note: When
Protocol for Whole-mount in situ Hybridization
 Protocol for Whole-mount in situ Hybridization 5/21/97 Kenji Shimamura I. Tissue Preparation 1. Dissect embryos in chilled PBS. 2. Fix embryos in an adequate amount (> 50 vol.) of fresh fixative (4% paraformaldehyde
Protocol for Whole-mount in situ Hybridization 5/21/97 Kenji Shimamura I. Tissue Preparation 1. Dissect embryos in chilled PBS. 2. Fix embryos in an adequate amount (> 50 vol.) of fresh fixative (4% paraformaldehyde
Note that steps 1-16 are identical to steps 1-16 in the immunostaining protocol, except 0.5X PBtween is used in this protocol rather than 0.5X PBT.
 Embryo In-situ Hybridization: This protocol is based closely on an immunostaining protocol for Hypsibius dujardini (Gabriel and Goldstein 2007) and available in-situ protocols for both insects (Tomoysu
Embryo In-situ Hybridization: This protocol is based closely on an immunostaining protocol for Hypsibius dujardini (Gabriel and Goldstein 2007) and available in-situ protocols for both insects (Tomoysu
Supporting Information
 Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
Supporting Information Lu et al. 10.1073/pnas.1106801108 Fig. S1. Analysis of spatial localization of mrnas coding for 23 zebrafish Wnt genes by whole mount in situ hybridization to identify those present
DIG-label In Situ Hybridizations (Jessell lab), from Scharen-Wiemers and Gerfin-Moser Histochemistry 100:
 DIG-label In Situ Hybridizations (Jessell lab), 2004 from Scharen-Wiemers and Gerfin-Moser Histochemistry 100:431-440. I. Tissue section: 1 Fix embryo overnight at 4 C in 4% paraformaldehyde, 0.1 M PB.
DIG-label In Situ Hybridizations (Jessell lab), 2004 from Scharen-Wiemers and Gerfin-Moser Histochemistry 100:431-440. I. Tissue section: 1 Fix embryo overnight at 4 C in 4% paraformaldehyde, 0.1 M PB.
Visualization of primordial germ cells in the fertilized pelagic eggs of the barfin flounder Verasper moseri
 Int. J. Dev. Biol. 59: 465-470 (2015) doi: 10.1387/ijdb.150008rg www.intjdevbiol.com Visualization of primordial germ cells in the fertilized pelagic eggs of the barfin flounder Verasper moseri RIE GOTO*,1,#,
Int. J. Dev. Biol. 59: 465-470 (2015) doi: 10.1387/ijdb.150008rg www.intjdevbiol.com Visualization of primordial germ cells in the fertilized pelagic eggs of the barfin flounder Verasper moseri RIE GOTO*,1,#,
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development
 Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Biology 4361 Developmental Biology Lecture 4. The Genetic Core of Development The only way to get from genotype to phenotype is through developmental processes. - Remember the analogy that the zygote contains
Lecture 20: Drosophila melanogaster
 Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Lecture 20: Drosophila melanogaster Model organisms Polytene chromosome Life cycle P elements and transformation Embryogenesis Read textbook: 732-744; Fig. 20.4; 20.10; 20.15-26 www.mhhe.com/hartwell3
Neural Development. How does a single cell make a brain??? How are different brain regions specified??? Neural Development
 Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Neural Development How does a single cell make a brain??? How are different brain regions specified??? 1 Neural Development How do cells become neurons? Environmental factors Positional cues Genetic factors
Mesoderm Formation. Fate map of early gastrula. Only two types of mesoderm are induced. Mesoderm induction by the vegetal hemisphere
 Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
Fate map of early gastrula Mesoderm Formation Animal hemisphere forms ectoderm (lacks ) Sperm Entry Point dbl dbl brachyury Vegetal hemisphere forms endoderm (requires ) dbl goosecoid Marginal zone forms
dead end, a Novel Vertebrate Germ Plasm Component, Is Required for Zebrafish Primordial Germ Cell Migration and Survival
 Current Biology, Vol. 13, 1429 1434, August 19, 2003, 2003 Elsevier Science Ltd. All rights reserved. DOI 10.1016/S0960-9822(03)00537-2 dead end, a Novel Vertebrate Germ Plasm Component, Is Required for
Current Biology, Vol. 13, 1429 1434, August 19, 2003, 2003 Elsevier Science Ltd. All rights reserved. DOI 10.1016/S0960-9822(03)00537-2 dead end, a Novel Vertebrate Germ Plasm Component, Is Required for
7.22 Example Problems for Exam 1 The exam will be of this format. It will consist of 2-3 sets scenarios.
 Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Massachusetts Institute of Technology Department of Biology 7.22, Fall 2005 - Developmental Biology Instructors: Professor Hazel Sive, Professor Martha Constantine-Paton 1 of 10 7.22 Fall 2005 sample exam
Xenopus gastrulation. Dorsal-Ventral Patterning - The Spemann Organizer. Two mesoderm inducing signals
 Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
Dorsal- atterning - The Spemann Organizer Two mesoderm inducing signals late-blastula early-gastrula nimal Blood Mesothelium Not Vegetal NC Endoderm Hans Spemann (1869-1941) Hilde Mangold (1898-1924) Dr
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
Lecture 3 MOLECULAR REGULATION OF DEVELOPMENT
 Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Lecture 3 E. M. De Robertis, M.D., Ph.D. August 16, 2016 MOLECULAR REGULATION OF DEVELOPMENT GROWTH FACTOR SIGNALING, HOX GENES, AND THE BODY PLAN Two questions: 1) How is dorsal-ventral (D-V) cell differentiation
Cell and Tissue Transplantation in Zebrafish Embryos
 Cell/Tissue Transplantation in Zebrafish 15 2 Cell and Tissue Transplantation in Zebrafish Embryos Toshiro Mizuno, Minori Shinya, and Hiroyuki Takeda 1. Introduction Zebrafish (Danio rerio) embryos have
Cell/Tissue Transplantation in Zebrafish 15 2 Cell and Tissue Transplantation in Zebrafish Embryos Toshiro Mizuno, Minori Shinya, and Hiroyuki Takeda 1. Introduction Zebrafish (Danio rerio) embryos have
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo.
 We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
We are walking and standing with parts of our bodies which could have been used for thinking had they developed in another part of the embryo. Hans Spemann, 1943 Reading from Chapter 3 - types of cell
WHOLE MOUNT IN SITU HYBRIDIZATION
 WHOLE MOUNT IN SITU HYBRIDIZATION Dissect embryos in cold PBS, wash in cold PBS in a 10 or 50 ml tube, and fixate o/n in cold 4% PFA/PBS at 4 C, while gently rocking. Young embryos or dissected tissues
WHOLE MOUNT IN SITU HYBRIDIZATION Dissect embryos in cold PBS, wash in cold PBS in a 10 or 50 ml tube, and fixate o/n in cold 4% PFA/PBS at 4 C, while gently rocking. Young embryos or dissected tissues
Code No. : 8421 Sub. Code : HZOM 13
 (6 pages) Reg. No. :... Sub. Code : HZOM 13 M.Sc. (CBCS) DEGREE EXAMINATION, APRIL 2014. First Semester Zoology DEVELOPMENTAL BIOLOGY (For those who joined in July 2012 onwards) Time : Three hours Maximum
(6 pages) Reg. No. :... Sub. Code : HZOM 13 M.Sc. (CBCS) DEGREE EXAMINATION, APRIL 2014. First Semester Zoology DEVELOPMENTAL BIOLOGY (For those who joined in July 2012 onwards) Time : Three hours Maximum
Readings. Lecture IV. Mechanisms of Neural. Neural Development. September 10, Bio 3411 Lecture IV. Mechanisms of Neural Development
 Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Readings Lecture IV. Mechanisms of Neural NEUROSCIENCE: References : 5 th ed, pp 477-506 (sorta) 4 th ed, pp 545-575 (sorta) Fainsod, A., Steinbeisser, H., & De Robertis, E. M. (1994). EMBO J, 13(21),
Assignment 11. Which of the following is true of the gray crescent region of frog eggs?
 Assignment 11 1. Multiple-choice (1 point) Which of the following is true of the gray crescent region of frog eggs? It contains mainly yolk. It may be seen in an unfertilized egg. Its content is usually
Assignment 11 1. Multiple-choice (1 point) Which of the following is true of the gray crescent region of frog eggs? It contains mainly yolk. It may be seen in an unfertilized egg. Its content is usually
In Situ Hybridization
 In Situ Hybridization Modified from C. Henry, M. Halpern and Thisse labs April 17, 2013 Table of Contents Reagents... 2 AP Buffer... 2 Developing Solution... 2 Hybridization buffer... 2 PBT... 2 PI Buffer
In Situ Hybridization Modified from C. Henry, M. Halpern and Thisse labs April 17, 2013 Table of Contents Reagents... 2 AP Buffer... 2 Developing Solution... 2 Hybridization buffer... 2 PBT... 2 PI Buffer
Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning.
 Supplemental Information Inventory of Supplemental Information Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning. Figure S2. Related to Figure 4, to validate Npn-1 signaling
Supplemental Information Inventory of Supplemental Information Figure S1. Related to Figure 1, to show 3-D images of nerve/vessel patterning. Figure S2. Related to Figure 4, to validate Npn-1 signaling
Developmental Zoology (ZOO ) Gatrulation
 Developmental Zoology (ZOO 228.1.0) Gatrulation 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis Aging
Developmental Zoology (ZOO 228.1.0) Gatrulation 1 Developmental Stages Ø Early Development Fertilization Cleavage Gastrulation Neurulation Ø Later Development Organogenesis Larval molts Metamorphosis Aging
Why Study Developmental Neurobiology? Terrific scientific challenge.
 Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
Developmental Neurobiology Textbook Readings: ( Neuroscience, 3rd Edition, Purves, et al.) Chapter 1 Studying Nervous Systems 7 Intracellular Signal Transduction 21 Early Brain Development 22 Construction
RESEARCH ARTICLE. Molecular Reproduction & Development 80: (2013) SUMMARY INTRODUCTION ß 2013 WILEY PERIODICALS, INC.
 RESEARCH ARTICLE Molecular Reproduction & Development 80:118 131 (2013) Identification and Migration of Primordial Germ Cells in Atlantic Salmon, Salmo salar: Characterization of Vasa, Dead End, and Lymphocyte
RESEARCH ARTICLE Molecular Reproduction & Development 80:118 131 (2013) Identification and Migration of Primordial Germ Cells in Atlantic Salmon, Salmo salar: Characterization of Vasa, Dead End, and Lymphocyte
Concepts and Methods in Developmental Biology
 Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
Biology 4361 Developmental Biology Concepts and Methods in Developmental Biology June 16, 2009 Conceptual and Methodological Tools Concepts Genomic equivalence Differential gene expression Differentiation/de-differentiation
5. Wash larvae briefly (<1min) 2mg/ml glycine in PTw (make fresh every time).
 In situ Hybridization Day 1 PRETREATMENT 1. Transfer embryos to small mesh filter cups in a tip box lid with 30ml 75% MeOH/25% PTw. Use ~50ul larvae per probe for each stage. Place in fume hood on top
In situ Hybridization Day 1 PRETREATMENT 1. Transfer embryos to small mesh filter cups in a tip box lid with 30ml 75% MeOH/25% PTw. Use ~50ul larvae per probe for each stage. Place in fume hood on top
Day 3 - Al incubation steps to be performed with gentile agitation on an orbital shaker, unless otherwise indicated.
 Title: RNA in situ hybridization (from Dr. Eric Domyan) Rationale and background: To visualize gene expression by binding a tagged probe to target mrna Protocol: DAY 0 Dissect tissue, fix in 4% paraformaldehyde
Title: RNA in situ hybridization (from Dr. Eric Domyan) Rationale and background: To visualize gene expression by binding a tagged probe to target mrna Protocol: DAY 0 Dissect tissue, fix in 4% paraformaldehyde
High-Sensitivity and High-Resolution In Situ Hybridization of Coding and Long Noncoding RNAs in Vertebrate Ovaries and Testes
 Takei et al. Biological Procedures Online (2018) 20:6 https://doi.org/10.1186/s12575-018-0071-z METHODOLOGY Open Access High-Sensitivity and High-Resolution In Situ Hybridization of Coding and Long Noncoding
Takei et al. Biological Procedures Online (2018) 20:6 https://doi.org/10.1186/s12575-018-0071-z METHODOLOGY Open Access High-Sensitivity and High-Resolution In Situ Hybridization of Coding and Long Noncoding
Supplementary Data. Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells
 Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
Supplementary Data Supplementary Methods Three-step protocol for spontaneous differentiation of mouse induced pluripotent stem (embryonic stem) cells Mouse induced pluripotent stem cells (ipscs) were cultured
ProductInformation TECHNICAL BULLETIN MULTIPLE TISSUE NORTHERN BLOT, MOUSE. Product No. BLOT-2 Technical Bulletin No. MB-865 February 2000
 MULTIPLE TISSUE NORTHERN BLOT, MOUSE Product No. BLOT-2 Technical Bulletin No. MB-865 February 2000 ProductInformation TECHNICAL BULLETIN Product Description Sigma s Mouse Multiple Tissue Northern Blot
MULTIPLE TISSUE NORTHERN BLOT, MOUSE Product No. BLOT-2 Technical Bulletin No. MB-865 February 2000 ProductInformation TECHNICAL BULLETIN Product Description Sigma s Mouse Multiple Tissue Northern Blot
A zebrafish nanos-related gene is essential for the development of primordial germ cells
 A zebrafish nanos-related gene is essential for the development of primordial germ cells Marion Köprunner, 1 Christine Thisse, 2 Bernard Thisse, 2 and Erez Raz 1,3 1 Max Planck Institute for Biophysical
A zebrafish nanos-related gene is essential for the development of primordial germ cells Marion Köprunner, 1 Christine Thisse, 2 Bernard Thisse, 2 and Erez Raz 1,3 1 Max Planck Institute for Biophysical
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
www.sciencemag.org/cgi/content/full/331/6018/753/dc1 Supporting Online Material for Embryological Evidence Identifies Wing Digits in Birds as Digits 1, 2, and 3 Koji Tamura,* Naoki Nomura, Ryohei Seki,
Fluorescent In Situ Hybridization (FISH) Assay
 Fluorescent In Situ Hybridization (FISH) Assay 1 What is FISH 2 Probes 3 FISH Procedure 4 Application Definition, Principle and Sample Types The core of FISH technology A quick and simple FISH protocol
Fluorescent In Situ Hybridization (FISH) Assay 1 What is FISH 2 Probes 3 FISH Procedure 4 Application Definition, Principle and Sample Types The core of FISH technology A quick and simple FISH protocol
The Genetic Basis of Development
 Chapter 21 The Genetic Basis of Development Overview: From Single Cell to Multicellular Organism The application of genetic analysis and DNA technology Has revolutionized the study of development PowerPoint
Chapter 21 The Genetic Basis of Development Overview: From Single Cell to Multicellular Organism The application of genetic analysis and DNA technology Has revolutionized the study of development PowerPoint
Histological preparation of embryonic and adult zebrafish eyes
 Histological preparation of embryonic and adult zebrafish eyes Richard J. Nuckels 1 and Jeffrey M. Gross 1,2,3 1 Section of Molecular Cell and Developmental Biology 2 Institute of Cell and Molecular Biology
Histological preparation of embryonic and adult zebrafish eyes Richard J. Nuckels 1 and Jeffrey M. Gross 1,2,3 1 Section of Molecular Cell and Developmental Biology 2 Institute of Cell and Molecular Biology
An Evolutionary Conserved Region in the vasa 3 UTR Targets RNA Translation to the Germ Cells in the Zebrafish
 Current Biology, Vol. 12, 454 466, March 19, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0960-9822(02)00723-6 An Evolutionary Conserved Region in the vasa 3 UTR Targets RNA Translation to
Current Biology, Vol. 12, 454 466, March 19, 2002, 2002 Elsevier Science Ltd. All rights reserved. PII S0960-9822(02)00723-6 An Evolutionary Conserved Region in the vasa 3 UTR Targets RNA Translation to
Developmental Biology. Cell Fate, Potency, and Determination
 Developmental Biology Cell Fate, Potency, and Determination Definitions Fate The sum of all structures that the cell or its descendants will form at a later stage of normal development. Cell Fate & Fate
Developmental Biology Cell Fate, Potency, and Determination Definitions Fate The sum of all structures that the cell or its descendants will form at a later stage of normal development. Cell Fate & Fate
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Exam 1 ID#: October 1, 2006
 Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Biology 4361 Name: Exam 1 ID#: October 1, 2006 Multiple choice (one point each) 1. The formation of new structures in chick embryogenesis is an example of a. teratology. b. epigenesis. c. hybridization.
Differentiation. Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU)
 Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Differentiation Ahmed Ihab Abdelaziz MD, PhD Associate Prof. Of Molecular Medicine NewGiza University (NGU) 1 Developmental Genetics Objectives: Explain how a differentiated cell achieves and maintains
Neural Stem Cell Characterization Kit
 Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
Neural Stem Cell Characterization Kit Catalog No. SCR019 FOR RESEARCH USE ONLY Not for use in diagnostic procedures USA & Canada Phone: +1(800) 437-7500 Fax: +1 (951) 676-9209 Europe +44 (0) 23 8026 2233
KEY Reproductive cloning Therapeutic cloning
 1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
1. (20 pts) Define Reproductive and Therapeutic cloning. Make sure your descriptions clearly distinguish the critical differences between them. Describe an example of each. Reproductive cloning refers
Supplementary Information. Isl2b regulates anterior second heart field development in zebrafish
 Supplementary Information Isl2b regulates anterior second heart field development in zebrafish Hagen R. Witzel 1, Sirisha Cheedipudi 1, Rui Gao 1, Didier Y.R. Stainier 2 and Gergana D. Dobreva 1,3* 1 Origin
Supplementary Information Isl2b regulates anterior second heart field development in zebrafish Hagen R. Witzel 1, Sirisha Cheedipudi 1, Rui Gao 1, Didier Y.R. Stainier 2 and Gergana D. Dobreva 1,3* 1 Origin
Lecture 8: Transgenic Model Systems and RNAi
 Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
TUNEL Universal Apoptosis Detection Kit ( Biotin-labeled POD )
 TUNEL Universal Apoptosis Detection Kit ( Biotin-labeled POD ) Cat. No. L00290 Technical Manual No. 0267 Version 03112011 I Description. 1 II Key Features.... 1 III Kit Contents.. 1 IV Storage.. 2 V Procedure...
TUNEL Universal Apoptosis Detection Kit ( Biotin-labeled POD ) Cat. No. L00290 Technical Manual No. 0267 Version 03112011 I Description. 1 II Key Features.... 1 III Kit Contents.. 1 IV Storage.. 2 V Procedure...
Tracer Studies on Chick Embryos in vitro
 Tracer Studies on Chick Embryos in vitro by E. M. PANTELOURIS and L. MULHERKAR 1 From the Institute of Animal Genetics, Edinburgh WITH TWO PLATES INTRODUCTION IN the experiments to be reported here isotopic
Tracer Studies on Chick Embryos in vitro by E. M. PANTELOURIS and L. MULHERKAR 1 From the Institute of Animal Genetics, Edinburgh WITH TWO PLATES INTRODUCTION IN the experiments to be reported here isotopic
Aquatic Vertebrates Platform Services (See services description below, page 3)
 Aquatic Vertebrates Platform Services (See services description below, page 3) Services 1. Micro-injection of DNA, morpholino or mrna in zebrafish and medaka embryos. 2. Generation of stable zebrafish
Aquatic Vertebrates Platform Services (See services description below, page 3) Services 1. Micro-injection of DNA, morpholino or mrna in zebrafish and medaka embryos. 2. Generation of stable zebrafish
Hemidesmosome. Focal adhesion
 BIOLOGY 52 - - CELL AND DEVELOPMENTAL BIOLOGY - - FALL 2002 Third Examination - - November, 2002 -------------------------------------------------- Put your name at the top of each page. Please read each
BIOLOGY 52 - - CELL AND DEVELOPMENTAL BIOLOGY - - FALL 2002 Third Examination - - November, 2002 -------------------------------------------------- Put your name at the top of each page. Please read each
Nature Medicine doi: /nm.2548
 Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Supplementary Table 1: Genotypes of offspring and embryos from matings of Pmm2 WT/F118L mice with Pmm2 WT/R137H mice total events Pmm2 WT/WT Pmm2 WT/R137H Pmm2 WT/F118L Pmm2 R137H/F118L offspring 117 (100%)
Immuno-Labelling Cryosections
 Thin sections of biological material, mounted on nickel or gold grids, can be labelled by floating them, section-side down, on small, 10 µl, droplets of antibody. This process is conveniently carried out
Thin sections of biological material, mounted on nickel or gold grids, can be labelled by floating them, section-side down, on small, 10 µl, droplets of antibody. This process is conveniently carried out
Technical Advancement
 INVITED ARTICLE Acta Histochem. Cytochem. 35 (5): 361 365, 2002 Technical Advancement Using Antisense Morpholino Oligos to Knockdown Gene Expression in the Chicken Embryo Richard P. Tucker 1 1 Department
INVITED ARTICLE Acta Histochem. Cytochem. 35 (5): 361 365, 2002 Technical Advancement Using Antisense Morpholino Oligos to Knockdown Gene Expression in the Chicken Embryo Richard P. Tucker 1 1 Department
(From the Division of Biological and Medical Research, Argonne National Laboratory, Lemont, Illinois) Methods and Materials
 OBSERVATIONS ON FIBRILLOGENESIS IN THE CONNECTIVE TISSUE OF THE CHICK EMBRYO WITH THE AID OF SILVER IMPREGNATION* BY F. WASSERMANN, M.D., AND L. KUBOTA (From the Division of Biological and Medical Research,
OBSERVATIONS ON FIBRILLOGENESIS IN THE CONNECTIVE TISSUE OF THE CHICK EMBRYO WITH THE AID OF SILVER IMPREGNATION* BY F. WASSERMANN, M.D., AND L. KUBOTA (From the Division of Biological and Medical Research,
Methodology for Immunohistochemistry. Learning Objectives:
 Proteomics Methodology for Immunohistochemistry Methodology for Immunohistochemistry A staining process for identifying the proteins location in cells, tissues by using antigen-antibody property. Immuno
Proteomics Methodology for Immunohistochemistry Methodology for Immunohistochemistry A staining process for identifying the proteins location in cells, tissues by using antigen-antibody property. Immuno
Recipes for Groves Lab Stocks and Solutions
 Recipes for Groves Lab Stocks and Solutions 4% PARAFORMALDEHYDE... 2 5 X MABT... 3 10 X PBS... 4 10 X TBE... 5 20 X SSC... 6 50 X TAE... 7 AMPICILLIN... 8 CHLORAMPHENICOL... 9 HOWARD RINGERʼS SOLUTION...
Recipes for Groves Lab Stocks and Solutions 4% PARAFORMALDEHYDE... 2 5 X MABT... 3 10 X PBS... 4 10 X TBE... 5 20 X SSC... 6 50 X TAE... 7 AMPICILLIN... 8 CHLORAMPHENICOL... 9 HOWARD RINGERʼS SOLUTION...
Nature Methods: doi: /nmeth Supplementary Figure 1. Retention of RNA with LabelX.
 Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Supplementary Figure 1 Retention of RNA with LabelX. (a) Epi-fluorescence image of single molecule FISH (smfish) against GAPDH on HeLa cells expanded without LabelX treatment. (b) Epi-fluorescence image
Developmental Biology 3230 Exam 1 (Feb. 6) NAME
 DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
DevelopmentalBiology3230Exam1(Feb.6)NAME 1. (10pts) What is a Fate Map? How would you experimentally acquire the data to draw a Fate Map? Explain what a Fate Map does and does not tell you about the mechanisms
The DIG System. Labeling and Detection of Nucleic Acids
 The DG System Labeling and Detection of Nucleic Acids The DG System Specifically Label and Detect Nucleic Acids Publishable results require high level specific detection and low background. Do your hybridizations
The DG System Labeling and Detection of Nucleic Acids The DG System Specifically Label and Detect Nucleic Acids Publishable results require high level specific detection and low background. Do your hybridizations
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Patterning of the Brain
 Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
Patterning of the Brain Clemens.Kiecker@kcl.ac.uk IoPPN 27 Oct 2015 Hundreds of billions of cells, hundreds of cell types Egg = single cell, some 20,000 genes Egg = single cell, some 20,000 genes How is
SUPPLEMENTARY INFORMATION
 Figure S1. lev-9 mutants are resistant to levamisole. The levamisole dose-response curves indicate that lev-9 mutants are partially resistant to levamisole similar to lev-10(kr26) mutants. unc-29(x29)
Figure S1. lev-9 mutants are resistant to levamisole. The levamisole dose-response curves indicate that lev-9 mutants are partially resistant to levamisole similar to lev-10(kr26) mutants. unc-29(x29)
Supplement Figure S1:
 Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Supplement Figure S1: A, Sequence of Xcadherin-11 Morpholino 1 (Xcad-11MO) and 2 (Xcad-11 MO2) and control morpholino in comparison to the Xcadherin-11 sequence. The Xcad-11MO binding sequence spans the
Sydney Brenner: Present
 Sydney Brenner: 1927 - Present Born in the small town of Germiston, South Africa Won a scholarship to the University of Witwatersrand at the age of 15 Received his PhD from Exeter College, Oxford Made
Sydney Brenner: 1927 - Present Born in the small town of Germiston, South Africa Won a scholarship to the University of Witwatersrand at the age of 15 Received his PhD from Exeter College, Oxford Made
Human CEACAM6 ELISA Pair Set
 Human CEACAM6 ELISA Pair Set ( CD66c ) Catalog Number : SEK10823 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Human CEACAM6 ELISA Pair Set ( CD66c ) Catalog Number : SEK10823 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Human Transferrin / TF ELISA Pair Set
 Human Transferrin / TF ELISA Pair Set Catalog Number : SEK11019 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Human Transferrin / TF ELISA Pair Set Catalog Number : SEK11019 To achieve the best assay results, this manual must be read carefully before using this product and the assay is run as summarized in the
Mos1 insertion. MosTIC protocol-11/2006. repair template - Mos1 transposase expression - Mos1 excision - DSB formation. homolog arm.
 MosTIC (Mos1 excision induced Transgene Instructed gene Conversion) Valérie Robert (vrobert@biologie.ens.fr) and Jean-Louis Bessereau (jlbesse@biologie.ens.fr) (November 2006) Introduction: MosTIC (Robert
MosTIC (Mos1 excision induced Transgene Instructed gene Conversion) Valérie Robert (vrobert@biologie.ens.fr) and Jean-Louis Bessereau (jlbesse@biologie.ens.fr) (November 2006) Introduction: MosTIC (Robert
A Supersandwich Fluorescence in Situ Hybridization (SFISH) Strategy. for Highly Sensitive and Selective mrna Imaging in Tumor Cells
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) A Supersandwich Fluorescence in Situ Hybridization (SFISH)
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information (ESI) A Supersandwich Fluorescence in Situ Hybridization (SFISH)
Technical Manual No Version
 TUNEL Apoptosis Detection Kit Cat. No. L00301 (For Cryopreserved Tissue Sections, FITC-labled POD) Technical Manual No. 0269 Version 01132011 I Description. 1 II Key Features.... 1 III Kit Contents.. 1
TUNEL Apoptosis Detection Kit Cat. No. L00301 (For Cryopreserved Tissue Sections, FITC-labled POD) Technical Manual No. 0269 Version 01132011 I Description. 1 II Key Features.... 1 III Kit Contents.. 1
Cell Lineage in the Development of the Leech Nervous System
 Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
Cell Lineage in the Development of the Leech Nervous System DAVID A. WEISBLAT Department of Molecular Biology, University of California, Berkeley, CA 94720 (U.S.A) INTRODUCTION Knowledge of the lines of
Combined Digoxigenin-labeled in situ hybridization/ Immunohistochemistry protocol (for fixed frozen cryostat sections)
 Combined Digoxigenin-labeled in situ hybridization/ Immunohistochemistry protocol (for fixed frozen cryostat sections) A. Digoxigenin-UTP labeling of crna antisense probe Refer to laboratory protocol and
Combined Digoxigenin-labeled in situ hybridization/ Immunohistochemistry protocol (for fixed frozen cryostat sections) A. Digoxigenin-UTP labeling of crna antisense probe Refer to laboratory protocol and
Muscle gene activation by induction and the nonrequirement for cell division
 /. Embryol. exp. Morph. 97 Supplement, 75-84 (1986) 75 Printed in Great Britain The Company of Biologists Limited 1986 Muscle gene activation by induction and the nonrequirement for cell division J. B.
/. Embryol. exp. Morph. 97 Supplement, 75-84 (1986) 75 Printed in Great Britain The Company of Biologists Limited 1986 Muscle gene activation by induction and the nonrequirement for cell division J. B.
sides of the aleurone (Al) but it is excluded from the basal endosperm transfer layer
 Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
Supplemental Data. Gómez et al. (2009). The maize transcription factor MRP-1 (Myb-Related-Protein-1) is a key regulator of the differentiation of transfer cells. Supplemental Figure 1. Expression analyses
Transgenic Medaka, Small Fresh Water Teleost (Oryzias latipes), is Now Available for Environmental Science
 Interdisciplinary Studies on Environmental Chemistry Environmental Pollution and Ecotoxicology, Eds., M. Kawaguchi, K. Misaki, H. Sato, T. Yokokawa, T. Itai, T. M. Nguyen, J. Ono and S. Tanabe, pp. 49
Interdisciplinary Studies on Environmental Chemistry Environmental Pollution and Ecotoxicology, Eds., M. Kawaguchi, K. Misaki, H. Sato, T. Yokokawa, T. Itai, T. M. Nguyen, J. Ono and S. Tanabe, pp. 49
An overview of the basics of generate transgenic hen bioreactors
 Available online at www.scholarsresearchlibrary.com Annals of Biological Research, 2011, 2 (6):213-220 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-1233 CODEN (USA): ABRNBW An overview of
Available online at www.scholarsresearchlibrary.com Annals of Biological Research, 2011, 2 (6):213-220 (http://scholarsresearchlibrary.com/archive.html) ISSN 0976-1233 CODEN (USA): ABRNBW An overview of
SUPPLEMENTARY INFORMATION
 a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
a before amputation regeneration regenerated limb DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS DERMIS SKELETON MUSCLE SCHWANN CELLS EPIDERMIS developmental origin: lateral plate mesoderm presomitic mesoderm
