Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedures (SOPs) MDD-013 Supplier Business Reviews
|
|
|
- Maurice Rice
- 6 years ago
- Views:
Transcription
1 Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedures (SOPs) MDD-013 Supplier Business Reviews
2 Purpose of the Supplier Business Review SOP Establishes a consistent supplier Business Review process across the Johnson & Johnson Family of Medical Device & Diagnostics Companies (MD&D) The Business Review process provides for periodic structured conversations with suppliers about their relationship with J&J MD&D, for: Identifying improvement opportunities Communicating expectations Monitoring projects, metrics and overall performance The Business Review will result in a defined set of actionable follow-ups The common approach will allow multiple J&J MD&D Operating Companies to participate in a single business review 2
3 Scope of the Supplier Business Review SOP The SOP identifies both a standard process and metrics for Business Reviews The process includes both the topics and the general process for running the Business Review 3
4 Responsibilities At a minimum, the supplier s Account Representation and Quality Manager should be present for the Business Review The supplier should involve additional functions in the Business Review, as needed The supplier is responsible for preparing for the Business Review The supplier is responsible for any supplier-owned action items resulting from the Business Review 4
5 Business Review Structure The Business Review Structure covers four primary areas: Organizational Quality Business General 5
6 Organizational Topics Organizational changes: Staffing/structure changes impacting J&J MD&D Certifications: Review latest copy of applicable certificates or registrations Training: Review supplier s training program, including cross training and replacement of key positions 6
7 Quality Topics Review of Quality Agreements: Review applicable quality agreements and/or other agreements between the supplier and J&J MD&D Audit Observations: Any observations or findings derived from J&J MD&D audits, past Business Reviews or other audits or inspections Product Quality Review: Review general issues related to product quality Nonconformance report review: Review trends CAPA: Review applicable corrective and preventive action plans Compliance Update: Review progress on relative to applicable 2 nd /3 rd party quality system assessments 7
8 Quality Topics (continued) Change Control: Discuss planned component or related changes; consider if control plan or other updates are needed. Sterilization Requirements and Monitoring: Review periodic activities for EMs who provide sterilized, or ready to be sterilized product Sub-tier Supplier Performance: Review the supplier s own supplier management program and performance of sub tier suppliers Preventive Maintenance: Review PM program, spare parts, etc. Additional Quality Metrics: Review Field Actions and Complaints 8
9 Business Topics General Business Discussion: Review important issues not covered in other parts of the meeting Continuous Improvement: Review supplier scorecard (if applicable) and performance/actions relative to J&J MD&D metrics Business Continuity Plan: Review organizational health and business continuity planning J&D Owned Equipment: Discuss/review J&J MD&D owned assets Capacity Forecast: Discuss projected demand changes vs. capacity 9
10 Business Topics (continued) Inventory Levels: Discuss inventory levels/targets (for select products) Production Schedules: Discuss impact of items such as shutdowns J&J MD&D Sustainability Standards: Discuss supplier sustainability efforts Financials: Review financials including outstanding items and aging inventory Contract Review: Review supply agreement (if applicable) Conflict Minerals: Review Conflict Minerals Compliance, if applicable 10
11 General Discussion Topics Open Action Items: Review open items from previous Business Review Records Review: Review records retention, where applicable, and alignment between J&J and supplier revisions for key documents J&J MD&D Projects: Review active J&J MD&D projects J&J MD&D SOPs: Review supplier s awareness of applicable SOPs Supplier Feedback: Capture feedback from the supplier 11
Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedure (SOP)
 Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedure (SOP) Component Qualification Process for External Manufacturers and Suppliers 1 Purpose The purpose of the Component
Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedure (SOP) Component Qualification Process for External Manufacturers and Suppliers 1 Purpose The purpose of the Component
ISO 13485: :2015 CLIENT TRANSITION CHECKLIST
 - 9001:2015 CLIENT TRANSITION CHECKLIST Audit Conclusions: All requirements have been addressed. The organization is recommended for ISO 13485:2016 certification. Recommendation for registration is dependent
- 9001:2015 CLIENT TRANSITION CHECKLIST Audit Conclusions: All requirements have been addressed. The organization is recommended for ISO 13485:2016 certification. Recommendation for registration is dependent
Sections of the Standard. Evidence / Comments. (Y) / Nonconforming (NC)
 Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedures (SOPs)
 Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedures (SOPs) Supplier Responsibilities for Failure Investigations and Problem Solving Purpose of this SOP Establishes
Supplier Overview of Johnson & Johnson MD&D Supplier Quality Standard Operating Procedures (SOPs) Supplier Responsibilities for Failure Investigations and Problem Solving Purpose of this SOP Establishes
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT
 INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
Clause Map IATF 16949:2016 to ISO/TS 16949:2009
 Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru
 FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
SUPPLIER SURVEY FORM Instructions
 SUPPLIER SURVEY FORM Instructions 1. The following Supplier Survey was developed by Vishay Measurements Group, Inc. to assess and document the capability of its supplier base. 2. The Supplier Survey is
SUPPLIER SURVEY FORM Instructions 1. The following Supplier Survey was developed by Vishay Measurements Group, Inc. to assess and document the capability of its supplier base. 2. The Supplier Survey is
AESOP 15604; ISSUE 2; STATUS PENDING APPROVAL; AUTHORITY CARL BLAZIK This document is the property of NSF ISR. Page 1 of 9
 This document is the property of NSF ISR. Page 1 of 9 ISO 9001 Quality Management Systems registration provides a set of uniform requirements for a quality management system. A number of quality management
This document is the property of NSF ISR. Page 1 of 9 ISO 9001 Quality Management Systems registration provides a set of uniform requirements for a quality management system. A number of quality management
ISO and UAV Audits Preparation and Client Rights
 1 ISO 13485 and UAV Audits Preparation and Client Rights Medical Devices Quality Management Systems Requirements for Regulatory Purposes 24301 Woodsage Dr. Bonita Springs, FL 34134 (239)-307-6060 www.promedic.cc
1 ISO 13485 and UAV Audits Preparation and Client Rights Medical Devices Quality Management Systems Requirements for Regulatory Purposes 24301 Woodsage Dr. Bonita Springs, FL 34134 (239)-307-6060 www.promedic.cc
Genesis Corporate Strategy Diagnostic
 Genesis Corporate Strategy Diagnostic Objectives 1. To stimulate strategic thinking 2. To explore possible new strategic directions 3. To explore possible improvements in strategic alignment and readiness
Genesis Corporate Strategy Diagnostic Objectives 1. To stimulate strategic thinking 2. To explore possible new strategic directions 3. To explore possible improvements in strategic alignment and readiness
ISO 14001:2015 Updates and Key Themes
 ISO 14001:2015 Updates and Key Themes November 10, 2016 Alex Lowry Agenda Overview of changes in ISO 14001:2015 standard Discussion of key ISO 14001:2015 themes Context of the organization Internal and
ISO 14001:2015 Updates and Key Themes November 10, 2016 Alex Lowry Agenda Overview of changes in ISO 14001:2015 standard Discussion of key ISO 14001:2015 themes Context of the organization Internal and
QUALITY ASSURANCE MANUAL
 Earle M. Jorgensen Company QUALITY ASSURANCE MANUAL Revision 7, February 19 th, 2018 If Printed - Uncontrolled Copy This manual was re-written for Transition to the ISO9001:2015 Standard. All manuals generated
Earle M. Jorgensen Company QUALITY ASSURANCE MANUAL Revision 7, February 19 th, 2018 If Printed - Uncontrolled Copy This manual was re-written for Transition to the ISO9001:2015 Standard. All manuals generated
Sales and Operations Planning (S&OP) WHAT S STOPPING YOU? Kaushik Dutta
 Sales and Operations Planning (S&OP) WHAT S STOPPING YOU? by Kaushik Dutta Agenda Sales and Operations planning 10-step methodology Key considerations Summary 2 S&OP it s all about ALIGNMENT Vision Goals
Sales and Operations Planning (S&OP) WHAT S STOPPING YOU? by Kaushik Dutta Agenda Sales and Operations planning 10-step methodology Key considerations Summary 2 S&OP it s all about ALIGNMENT Vision Goals
Supplier Quality Expectations for 2011
 Supplier Quality Expectations for 2011 May 2, 2011 FXI Quality Policy The FXI Team is committed to do our jobs right the first time every time in order to obtain the highest level of customer delight.
Supplier Quality Expectations for 2011 May 2, 2011 FXI Quality Policy The FXI Team is committed to do our jobs right the first time every time in order to obtain the highest level of customer delight.
Document Type: Main Process: Revision Level: Page: POLICY QUALITY ASSURANCE 3 1 of 6 Process Owner Title:
 POLICY QUALITY ASSURANCE 3 1 of 6 Process Owner A. SCOPE This document contains requirements for conducting business with Tri Star Metals, LLC and its customers. It is applicable to suppliers that provide
POLICY QUALITY ASSURANCE 3 1 of 6 Process Owner A. SCOPE This document contains requirements for conducting business with Tri Star Metals, LLC and its customers. It is applicable to suppliers that provide
Prevent Quality System Deficiencies by Conducting Effective Internal Audits. Whitepaper
 Prevent Quality System Deficiencies by Conducting Effective Internal Audits Whitepaper An internal audit system is one of the most effective ways to monitor, analyze, control, and improve quality management
Prevent Quality System Deficiencies by Conducting Effective Internal Audits Whitepaper An internal audit system is one of the most effective ways to monitor, analyze, control, and improve quality management
MDSAP AUDIT PROCESS. A Manufacturer s Perspective. Connie Hoy EVP Regulatory Affairs Cynosure, Inc.
 MDSAP AUDIT PROCESS A Manufacturer s Perspective Connie Hoy EVP Regulatory Affairs Cynosure, Inc. Cynosure Located in Westford, MA Largest manufacturer of Medical Lasers Second location in Hicksville,
MDSAP AUDIT PROCESS A Manufacturer s Perspective Connie Hoy EVP Regulatory Affairs Cynosure, Inc. Cynosure Located in Westford, MA Largest manufacturer of Medical Lasers Second location in Hicksville,
ADVANCED MAINTENANCE PLANNING SCHEDULING AND WORK CONTROL NON-TECHNICAL & CERTIFIED TRAINING COURSE SECTOR / OIL&GAS
 ADVANCED MAINTENANCE PLANNING SCHEDULING AND WORK CONTROL SECTOR / OIL&GAS NON-TECHNICAL & CERTIFIED TRAINING COURSE In this course various techniques will be discussed that will assist you in due course
ADVANCED MAINTENANCE PLANNING SCHEDULING AND WORK CONTROL SECTOR / OIL&GAS NON-TECHNICAL & CERTIFIED TRAINING COURSE In this course various techniques will be discussed that will assist you in due course
VERSION 6.0 EXAM CONTENT MANUAL PREVIEW
 VERSION 6.0 EXAM CONTENT MANUAL PREVIEW APICS Certified in Production and Inventory Management CPIM Part 2 Preview of CPIM Exam Content Manual Version 6.0 Please be aware, this is not the full APICS Certified
VERSION 6.0 EXAM CONTENT MANUAL PREVIEW APICS Certified in Production and Inventory Management CPIM Part 2 Preview of CPIM Exam Content Manual Version 6.0 Please be aware, this is not the full APICS Certified
QUALITY and ENVIRONMENTAL, HEALTH & SAFETY MANAGEMENT SYSTEM MANUAL
 00 Revision E February 009 ECOM OUTSIDE PLANT & QUALITY and ENVIRONMENTAL, HEALTH & SAFETY MANAGEMENT SYSTEM MANUAL 00 www.tycoelectronics.com www.telecomosp.com www.ampnetconnect.com 00 DCR TQ09/005 Revision
00 Revision E February 009 ECOM OUTSIDE PLANT & QUALITY and ENVIRONMENTAL, HEALTH & SAFETY MANAGEMENT SYSTEM MANUAL 00 www.tycoelectronics.com www.telecomosp.com www.ampnetconnect.com 00 DCR TQ09/005 Revision
Wilson-Hurd ATF Quality Manual
 Wilson-Hurd ATF Quality Manual Wilson-Hurd Manufacturing Company Advanced Technical Facility (ATF) 311 Winton Street Wausau, WI 54403 Rev. Date: 04 May 2017 Pete Dehne Vice President, Operations Table
Wilson-Hurd ATF Quality Manual Wilson-Hurd Manufacturing Company Advanced Technical Facility (ATF) 311 Winton Street Wausau, WI 54403 Rev. Date: 04 May 2017 Pete Dehne Vice President, Operations Table
SUPPLIER QUALITY SYSTEM SURVEY
 AERO BENDING USE ONLY APPROVED DISAPPROVED CONDITIONAL APPROVAL (SEE NOTES AND COMMENTS) BY: DATE: Assigned Vendor # Supplier : Facility Address: Phone: Email: Fax: Please check all that apply: Manufacturer
AERO BENDING USE ONLY APPROVED DISAPPROVED CONDITIONAL APPROVAL (SEE NOTES AND COMMENTS) BY: DATE: Assigned Vendor # Supplier : Facility Address: Phone: Email: Fax: Please check all that apply: Manufacturer
Advanced Maintenance Management. Contents are subject to change. For the latest updates visit
 Advanced Maintenance Management Page 1 of 7 Why Attend To survive in today s world of 'lean and mean' operations, we cannot wait for breakdowns. As a matter of fact, we should make responding to breakdowns
Advanced Maintenance Management Page 1 of 7 Why Attend To survive in today s world of 'lean and mean' operations, we cannot wait for breakdowns. As a matter of fact, we should make responding to breakdowns
Strategies to Revise Quality Policy and Quality Objectives. Eileen Cortes February 22, 2017
 Strategies to Revise Quality Policy and Quality Objectives Eileen Cortes February 22, 2017 Agenda Elements of Compliance Quality Policy Outline Quality Strategy and Objectives Case Study Open Discussion
Strategies to Revise Quality Policy and Quality Objectives Eileen Cortes February 22, 2017 Agenda Elements of Compliance Quality Policy Outline Quality Strategy and Objectives Case Study Open Discussion
CORRECTIVE AND PREVENTIVE ACTION
 1 of 7 uncontrolled. This copy is only valid only at the time of printing. Unauthorized reproduction of this document is prohibited. 2 of 7 CONTENTS 1.0 PURPOSE... 3 2.0 SCOPE... 3 3.0 RESPONSIBILITY...
1 of 7 uncontrolled. This copy is only valid only at the time of printing. Unauthorized reproduction of this document is prohibited. 2 of 7 CONTENTS 1.0 PURPOSE... 3 2.0 SCOPE... 3 3.0 RESPONSIBILITY...
Good Distribution Practices Toolkit. Quality Management System 08 March 2016
 Good Distribution Practices Toolkit Quality Management System 08 March 2016 1 Quality Policy GDP, Legal, Environmental, H&S Processes & Systems Resources Risk Assessments 2 Quality Policy & Objectives
Good Distribution Practices Toolkit Quality Management System 08 March 2016 1 Quality Policy GDP, Legal, Environmental, H&S Processes & Systems Resources Risk Assessments 2 Quality Policy & Objectives
D107: DEMO OF ISO: FOOD SAFETY DOCUMENT KIT Price 360 USD
 Chapter-1.0 CONTENTS OF ISO 22000 DOCUMENT KIT (More than 100 document files) A. The entire Document kit has 5 main directories as below. Sr. No. List of Directory Document of Details 1. Food Safety Manual
Chapter-1.0 CONTENTS OF ISO 22000 DOCUMENT KIT (More than 100 document files) A. The entire Document kit has 5 main directories as below. Sr. No. List of Directory Document of Details 1. Food Safety Manual
The Logistics Enablers ISO 9001:2000 POLICIES AND PROCEDURES ORIENTATION
 The Logistics Enablers ISO 9001:2000 POLICIES AND PROCEDURES ORIENTATION 2 INTRODUCTION JLMI is committed to quality and achieving registration to ISO 9001:2000 - the International Standard for a Quality
The Logistics Enablers ISO 9001:2000 POLICIES AND PROCEDURES ORIENTATION 2 INTRODUCTION JLMI is committed to quality and achieving registration to ISO 9001:2000 - the International Standard for a Quality
PMO In A Box. Prepared for UBS
 PMO In A Box Prepared for UBS Roadmap Why PMO In A Box? Establish PMO Governance Standardize Methodology Create a Stakeholder Partnership Plan 2 PMOs Are In Transition 3 CEB PMO Executive Council pmo in
PMO In A Box Prepared for UBS Roadmap Why PMO In A Box? Establish PMO Governance Standardize Methodology Create a Stakeholder Partnership Plan 2 PMOs Are In Transition 3 CEB PMO Executive Council pmo in
THE CERTIFIED SUPPLIER
 THE CERTIFIED SUPPLIER QUALITY PROFESSIONAL HANDBOOK Mark Allen Durivage, editor ASQ Quality Press Milwaukee, Wisconsin Table of Contents List offigures and Tables Preface Acknowledgments xi xv xvii Part
THE CERTIFIED SUPPLIER QUALITY PROFESSIONAL HANDBOOK Mark Allen Durivage, editor ASQ Quality Press Milwaukee, Wisconsin Table of Contents List offigures and Tables Preface Acknowledgments xi xv xvii Part
PMO Services Checklist
 PMO Services Checklist by IMPACTbyLaura.com Services Checklist This resource is a list of possible services and categories that you can consider when determining how you will drive IMPACT with your PMO.
PMO Services Checklist by IMPACTbyLaura.com Services Checklist This resource is a list of possible services and categories that you can consider when determining how you will drive IMPACT with your PMO.
Quality Management System MANUAL. SDIX, LLC Headquarters: 111 Pencader Drive Newark, Delaware 19702
 Quality Management System MANUAL SDIX, LLC Headquarters: 111 Pencader Drive Newark, Delaware 19702 Doc. No. G5500 Rev. 11 Status : APPROVED Effective: 09/07/2017 Page 2 of 32 Quality Manual Table of Contents
Quality Management System MANUAL SDIX, LLC Headquarters: 111 Pencader Drive Newark, Delaware 19702 Doc. No. G5500 Rev. 11 Status : APPROVED Effective: 09/07/2017 Page 2 of 32 Quality Manual Table of Contents
TITLE: TEKTRONIX COMPONENT SOLUTIONS SUPPLIER QUALITY MANUAL
 DATE OF RELEASE: 02-Jun-2017 Page 1 of 9 TITLE: TEKTRONIX COMPONENT SOLUTIONS SUPPLIER QUALITY MANUAL Change History: REV ECO ECO Completion/ Finished Date Summary of Change A01 S6-10550 29-JUL-15 Initial
DATE OF RELEASE: 02-Jun-2017 Page 1 of 9 TITLE: TEKTRONIX COMPONENT SOLUTIONS SUPPLIER QUALITY MANUAL Change History: REV ECO ECO Completion/ Finished Date Summary of Change A01 S6-10550 29-JUL-15 Initial
ASA-100 QUALITY MANUAL
 ASA-100 QUALITY MANUAL Origination Date: XXXX Document Identifier: Date: Document Revision: Latest Revision Date Abstract: This quality manual describes (your Company's) quality management system policies
ASA-100 QUALITY MANUAL Origination Date: XXXX Document Identifier: Date: Document Revision: Latest Revision Date Abstract: This quality manual describes (your Company's) quality management system policies
ISO 13485:2016 Mandatory documentation requirements & MyEasyISO
 ts & Mandatory documents All below mandatory documents can be created using Documented information module of. An organization can use their own procedures but if required can provide these procedures to
ts & Mandatory documents All below mandatory documents can be created using Documented information module of. An organization can use their own procedures but if required can provide these procedures to
Supplier Quality Terms & Conditions
 Rev 01. February 20, 2018 Page 1 of 5 Supplier Quality Terms & Conditions Century Spring Corporation (CSC) maintains a quality management system that adheres to the requirements of ISO 9001:2015 and AS9100
Rev 01. February 20, 2018 Page 1 of 5 Supplier Quality Terms & Conditions Century Spring Corporation (CSC) maintains a quality management system that adheres to the requirements of ISO 9001:2015 and AS9100
Railroad Friction Products Corporation
 Railroad Friction Products Corporation Our Quality Philosophy: "At Railroad Friction Products Corporation we will consistently provide products that meet and exceed customer and regulatory requirements
Railroad Friction Products Corporation Our Quality Philosophy: "At Railroad Friction Products Corporation we will consistently provide products that meet and exceed customer and regulatory requirements
Skyworks Solutions, Inc. SQ : Rev. 18 Sustainability Systems Manual Page 2 of 30
 Sustainability Systems Manual Page 2 of 30 Table of Contents 1 Introduction... 5 1.1 System structure... 5 1.2 Roles and responsibilities... 6 2 Sustainability policy... 7 2.1 Policy communication... 7
Sustainability Systems Manual Page 2 of 30 Table of Contents 1 Introduction... 5 1.1 System structure... 5 1.2 Roles and responsibilities... 6 2 Sustainability policy... 7 2.1 Policy communication... 7
ABBVIE PURCHASING AND SUPPLIER MANAGEMENT SUPPLIER PERFORMANCE PROGRAM
 ABBVIE PURCHASING AND SUPPLIER MANAGEMENT SUPPLIER PERFORMANCE PROGRAM SUPPLIER PERFORMANCE PROGRAM Without question, supplier relationships have a significant impact on AbbVie s corporate success. AbbVie
ABBVIE PURCHASING AND SUPPLIER MANAGEMENT SUPPLIER PERFORMANCE PROGRAM SUPPLIER PERFORMANCE PROGRAM Without question, supplier relationships have a significant impact on AbbVie s corporate success. AbbVie
Communicating an Evidence Based Asset Management Program By Daryush Esmaili
 Communicating an Evidence Based Asset Management Program By Daryush Esmaili December 1, 2017 Agenda Communicating Asset Management 2 Introduction Challenges Service-Supporting Assets Governance and Strategic
Communicating an Evidence Based Asset Management Program By Daryush Esmaili December 1, 2017 Agenda Communicating Asset Management 2 Introduction Challenges Service-Supporting Assets Governance and Strategic
Table of Contents. 1.0 Purpose. 2.0 Scope. 3.0 Quality System Requirements. 4.0 Approved Supplier List. 5.0 Supplier Assessments
 Issued 2/6/2009 Revision: 2/13/2017 Table of Contents 1.0 Purpose 2.0 Scope 3.0 Quality System Requirements 4.0 Approved Supplier List 5.0 Supplier Assessments 6.0 Advance Product Quality Planning (APQP)
Issued 2/6/2009 Revision: 2/13/2017 Table of Contents 1.0 Purpose 2.0 Scope 3.0 Quality System Requirements 4.0 Approved Supplier List 5.0 Supplier Assessments 6.0 Advance Product Quality Planning (APQP)
QUALITY MANUAL. This document defines the requirements, processes, structure and documentation for the Teledyne DGO Quality Management System.
 Page 1 of 13 1. PURPOSE This document defines the requirements, processes, structure and documentation for the Teledyne DGO Quality Management System. 2. SCOPE The Teledyne DGO Quality Management System
Page 1 of 13 1. PURPOSE This document defines the requirements, processes, structure and documentation for the Teledyne DGO Quality Management System. 2. SCOPE The Teledyne DGO Quality Management System
QMS CO-ORDINATOR & GENERAL PROCEDURES:-
 1. QMS CO-ORDINATOR & GENERAL PROCEDURES:- (1) How do you measure effectiveness of system for working of your company? How do you collect necessary information for the same? Are you getting information
1. QMS CO-ORDINATOR & GENERAL PROCEDURES:- (1) How do you measure effectiveness of system for working of your company? How do you collect necessary information for the same? Are you getting information
Global Trends in Audits: Demonstrating Training Effectiveness
 Global Trends in Audits: Demonstrating Training Effectiveness UL and the UL logo are trademarks of UL LLC 2013. Agenda Goals for this Discussion Our Panel Industry Audit Trends Our Benchmarking Responses
Global Trends in Audits: Demonstrating Training Effectiveness UL and the UL logo are trademarks of UL LLC 2013. Agenda Goals for this Discussion Our Panel Industry Audit Trends Our Benchmarking Responses
The Skyworks Quality Management System strives to:
 Skyworks has embraced a workplace where Quality is the number one differentiator to achieve Customer Loyalty. Skyworks has adopted a single Quality Management System which drives efficiency, consistency
Skyworks has embraced a workplace where Quality is the number one differentiator to achieve Customer Loyalty. Skyworks has adopted a single Quality Management System which drives efficiency, consistency
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 9001 Third edition 2000-12-15 Quality management systems Requirements Systèmes de management de la qualité Exigences Reference number ISO 9001:2000(E) ISO 2000 Contents Page
INTERNATIONAL STANDARD ISO 9001 Third edition 2000-12-15 Quality management systems Requirements Systèmes de management de la qualité Exigences Reference number ISO 9001:2000(E) ISO 2000 Contents Page
AAMI Quality Systems White Paper: Comparison of 21 CFR Part 820 to ISO 13485:2016 1
 AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward, Owner, Ward Sciences
AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward, Owner, Ward Sciences
CMC Activities for Commercialization: PAI, QMS, and BLA/NDA. NEPDA Dinner Meeting Cambridge, MA 16 May 2018
 CMC Activities for Commercialization: PAI, QMS, and BLA/NDA Advancing from Clinical to Commercial The Commercialization Roadmap NEPDA Dinner Meeting Cambridge, MA 16 May 2018 Standards Certification Education
CMC Activities for Commercialization: PAI, QMS, and BLA/NDA Advancing from Clinical to Commercial The Commercialization Roadmap NEPDA Dinner Meeting Cambridge, MA 16 May 2018 Standards Certification Education
2 Day Seminar. Supplier Management for Medical Device Manufacturers 12 RAPS CREDITS. Jun Boston
 2 Day Seminar Supplier Management for Medical Device Manufacturers 12 RAPS CREDITS Jun 01 02 Boston Seminar One Registration $ 993 Special Group Discount Register for Four attendees $ 3495 COURSE SUMMARY
2 Day Seminar Supplier Management for Medical Device Manufacturers 12 RAPS CREDITS Jun 01 02 Boston Seminar One Registration $ 993 Special Group Discount Register for Four attendees $ 3495 COURSE SUMMARY
Auditing Within the Culture of the Medical Device Industry & ISO 13485:2016 Overview. ASQ Quality Conference 2017 for Region 5 October 25, 2017
 Auditing Within the Culture of the Medical Device Industry & ISO 13485:2016 Overview ASQ Quality Conference 2017 for Region 5 October 25, 2017 Introduction / Purpose Discuss the influences of regulatory
Auditing Within the Culture of the Medical Device Industry & ISO 13485:2016 Overview ASQ Quality Conference 2017 for Region 5 October 25, 2017 Introduction / Purpose Discuss the influences of regulatory
Supplier Relationship Management
 Microrite, Inc. brings you this unique learning experience in Supplier Relationship Management; Part of Microrite s step-by-step webinar series. Supplier Relationship Management Supplier relationship management
Microrite, Inc. brings you this unique learning experience in Supplier Relationship Management; Part of Microrite s step-by-step webinar series. Supplier Relationship Management Supplier relationship management
Risk-Based PMA. Process. Federal Aviation Administration. Presented to: AEA Technology Incubator By:
 Risk-Based PMA Process Presented to: AEA Technology Incubator By: Daniel J. Elgas, Certification Procedures Branch, AIR-6C0 Date: August 16, 2018 Project Background AIR evaluated the level of FAA resources
Risk-Based PMA Process Presented to: AEA Technology Incubator By: Daniel J. Elgas, Certification Procedures Branch, AIR-6C0 Date: August 16, 2018 Project Background AIR evaluated the level of FAA resources
ISO 13485:2016 Medical Devices-Quality Management Systems- Requirements for Regulatory Purposes. Theresa McCarthy
 ISO 13485:2016 Medical Devices-Quality Management Systems- Requirements for Regulatory Purposes Theresa McCarthy Welcome and Introductions Please interview and collect: Name Primary Quality Management
ISO 13485:2016 Medical Devices-Quality Management Systems- Requirements for Regulatory Purposes Theresa McCarthy Welcome and Introductions Please interview and collect: Name Primary Quality Management
DECISION TO ACCREDIT
 DECISION TO ACCREDIT Determine the requirements of the regulator, legislation and customer s needs prior to deciding on the standard to implement. Process is based on the following: Top management s decision
DECISION TO ACCREDIT Determine the requirements of the regulator, legislation and customer s needs prior to deciding on the standard to implement. Process is based on the following: Top management s decision
The Benefits and Challenges of Integrated Management Systems: A Registrar's Perspective AAC Conference. Presented by Steven Raduy April 8, 2014
 The Benefits and Challenges of Integrated Management Systems: A Registrar's Perspective Presented by Steven Raduy Presentation Outline 1. Trends, drivers and benefits of integrated management systems (MS)
The Benefits and Challenges of Integrated Management Systems: A Registrar's Perspective Presented by Steven Raduy Presentation Outline 1. Trends, drivers and benefits of integrated management systems (MS)
AAMI Quality Systems White Paper
 AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017, Updated February 2018 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward,
AAMI s White Paper Comparison of 21 CFR Part 820 to ISO 13485:2016 February 2017, Updated February 2018 AUTHORS Seb Clerkin, GMP Advisory Services Nicola Martin, Owner, Nicola Martin Consulting Jack Ward,
Sales & Operations Planning
 for Sales & / It is far better to foresee even without certainty than not to foresee at all. - Henri Poincare Trainer Profile Steve Allanson Managing Director of Veloc Ltd Steve Allanson is the Managing
for Sales & / It is far better to foresee even without certainty than not to foresee at all. - Henri Poincare Trainer Profile Steve Allanson Managing Director of Veloc Ltd Steve Allanson is the Managing
Continual Improvement
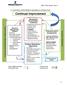 15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
Supplier Quality System Survey
 Supplier Division Address Date: Quality Contact: Reports to: Title: Title: Phone Fax e-mail Plant size (ft 2 ): Union: Contract expires: Total Employment: Direct Labor: Shifts: QA employees: This report
Supplier Division Address Date: Quality Contact: Reports to: Title: Title: Phone Fax e-mail Plant size (ft 2 ): Union: Contract expires: Total Employment: Direct Labor: Shifts: QA employees: This report
Conducting Quality Audit based on ISO 9001:2015
 Conducting Quality Audit based on ISO 9001:2015 FREE Professional Development Seminar Series At the end of the class, please fill the training feedback and referral forms! Return the form at the reception!
Conducting Quality Audit based on ISO 9001:2015 FREE Professional Development Seminar Series At the end of the class, please fill the training feedback and referral forms! Return the form at the reception!
ELEMENTS OF MANUFACTURING BASICS
 ELEMENTS OF MANUFACTURING BASICS ELEMENTS OF MANUFACTURING BASICS Basic System Model Inputs Conversion Process RESULTS Resource Allocation for Improvement DATA Management Reviews MANUFACTURING BASICS CATEGORY
ELEMENTS OF MANUFACTURING BASICS ELEMENTS OF MANUFACTURING BASICS Basic System Model Inputs Conversion Process RESULTS Resource Allocation for Improvement DATA Management Reviews MANUFACTURING BASICS CATEGORY
Supplier Audit Procedure /20/17
 1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
Food Packaging Safety Management System
 Introduction The company has planned, established, documented and implemented a Food Packaging Safety Management System for the site in order to continually improve and ensure compliance with legislation,
Introduction The company has planned, established, documented and implemented a Food Packaging Safety Management System for the site in order to continually improve and ensure compliance with legislation,
Session W05 Measuring Quality Audit Effectiveness
 Session W05 Measuring Quality Audit Effectiveness Eric Richter, Ph.D. The Aerospace Corporation El Segundo, California May 26, 2010 ASQ World Conference on Quality and Improvement file W05 Richter.ppt
Session W05 Measuring Quality Audit Effectiveness Eric Richter, Ph.D. The Aerospace Corporation El Segundo, California May 26, 2010 ASQ World Conference on Quality and Improvement file W05 Richter.ppt
Osprey Technologies, LLC. Quality Manual ISO9001:2008 Rev -
 February 8, 2015 1 Osprey Technologies, LLC Quality Manual ISO9001:2008 Rev - February 8, 2015 Released by Dave Crockett President 6100 S. Maple Avenue, Suite 117 Tempe, AZ 85283 www.osprey-tech.com February
February 8, 2015 1 Osprey Technologies, LLC Quality Manual ISO9001:2008 Rev - February 8, 2015 Released by Dave Crockett President 6100 S. Maple Avenue, Suite 117 Tempe, AZ 85283 www.osprey-tech.com February
Boeing Enterprise Common Quality Surveillance An Introduction for Suppliers
 Boeing Enterprise Common Quality Surveillance An Introduction for Suppliers BOEING is a trademark of Boeing Management Company. Copyright 2008 Boeing. All rights reserved. 1 2 Boeing Enterprise Common
Boeing Enterprise Common Quality Surveillance An Introduction for Suppliers BOEING is a trademark of Boeing Management Company. Copyright 2008 Boeing. All rights reserved. 1 2 Boeing Enterprise Common
DRAFT. Contract Deliverables Requirement List. CI-3 Resource Plan for Contract Initiation For Authorization Contract Initiation
 Contract Deliverables Requirement List Requirement Contract Initiation DID Reference Deliverable Purpose Frequency CI-1 Service Delivery Regime For Acceptance Contract Initiation CI-2 Contract Transition
Contract Deliverables Requirement List Requirement Contract Initiation DID Reference Deliverable Purpose Frequency CI-1 Service Delivery Regime For Acceptance Contract Initiation CI-2 Contract Transition
OWNER USER INTEGRITY MANAGEMENT SYSTEM WRITTEN DESCRIPTION CHECKLIST AB-512(b)
 Company Name: Written Description of QMS Title and Rev. Status: Person who is responsible for preparing the owner s QMS written description: Name: Title: Telephone No.: ( ) Fax No.: ( ) Cell No.: ( ) E-Mail:
Company Name: Written Description of QMS Title and Rev. Status: Person who is responsible for preparing the owner s QMS written description: Name: Title: Telephone No.: ( ) Fax No.: ( ) Cell No.: ( ) E-Mail:
Quality Manual. This manual complies with the requirements of the ISO 9001:2015 International Standard. AW2 Logistics, Inc Ace Industrial Dr.
 Quality Manual This manual complies with the requirements of the ISO 9001:2015 International Standard. AW2 Logistics, Inc. 6001 Ace Industrial Dr. Cudahy, WI 53210 Quality Manual Rev 3 Page 1 of 30 Table
Quality Manual This manual complies with the requirements of the ISO 9001:2015 International Standard. AW2 Logistics, Inc. 6001 Ace Industrial Dr. Cudahy, WI 53210 Quality Manual Rev 3 Page 1 of 30 Table
PRINTED COPY IS NOT CONTROLLED
 Quality Manual We Deliver Quality on Time Form 1217 (01.16.2018) Rev. 12 SOR Inc. sorinc.com SOR QUALITY MANUAL Page 1 of 18 Table of Contents Quality Manual 8701-001 Revision 12 Introduction....4 1.0
Quality Manual We Deliver Quality on Time Form 1217 (01.16.2018) Rev. 12 SOR Inc. sorinc.com SOR QUALITY MANUAL Page 1 of 18 Table of Contents Quality Manual 8701-001 Revision 12 Introduction....4 1.0
EU MDR: Tips for Effectively Addressing the New Requirements
 Expert advisors. Exact practices. EU MDR: Tips for Effectively Addressing the New Requirements Mary Beth Henderson, Ph.D., MBA September 26, 2018 Thank you for joining us We will begin at 11:00 Central
Expert advisors. Exact practices. EU MDR: Tips for Effectively Addressing the New Requirements Mary Beth Henderson, Ph.D., MBA September 26, 2018 Thank you for joining us We will begin at 11:00 Central
The Examination Process...Comments
 Reading an Application Reviewing Key Factors Writing Comments Identifying OFIs 2 The Examination Process...Comments Read the Application Highlight/make notes n Key Factor integration n ADLI n LeTCI (Cat
Reading an Application Reviewing Key Factors Writing Comments Identifying OFIs 2 The Examination Process...Comments Read the Application Highlight/make notes n Key Factor integration n ADLI n LeTCI (Cat
Performing Multiple Regulatory Requirements within a Program Such as GMP, GLP, GCP, Medical Devices, and/or Controlled Substances
 Performing Multiple Regulatory Requirements within a Program Such as GMP, GLP, GCP, Medical Devices, and/or Controlled Substances Performing Multiple Regulatory Requirements within a Program Such as GMP,
Performing Multiple Regulatory Requirements within a Program Such as GMP, GLP, GCP, Medical Devices, and/or Controlled Substances Performing Multiple Regulatory Requirements within a Program Such as GMP,
Feed Quality Assurance and Certification Programs. Representing the Total Feed Industry
 Feed Quality Assurance and Certification Programs Quality Programs ISO 22000 Food Industry HAACP System Certification Program Comprehensive Quality Assurance Programs New US Food Safety Laws Animal Feed
Feed Quality Assurance and Certification Programs Quality Programs ISO 22000 Food Industry HAACP System Certification Program Comprehensive Quality Assurance Programs New US Food Safety Laws Animal Feed
PMP Exam Prep Coaching Program
 PMP Exam Prep Coaching Program Project Management 1 Project Management Develop Project Charter Develop Project Management Plan Monitor and Control Perform Integrated Change Control Close Project or Phase
PMP Exam Prep Coaching Program Project Management 1 Project Management Develop Project Charter Develop Project Management Plan Monitor and Control Perform Integrated Change Control Close Project or Phase
Meeting FDA Standards For Medical Device Quality: An Outof-the-box
 Meeting FDA Standards For Medical Device Quality: An Outof-the-box PLM Approach PTC s Medical Device Value-Ready Deployment TM Offering: Overview May 2016 Companies perceive that the regulatory framework
Meeting FDA Standards For Medical Device Quality: An Outof-the-box PLM Approach PTC s Medical Device Value-Ready Deployment TM Offering: Overview May 2016 Companies perceive that the regulatory framework
CORE. Integration. EXAM SPECIFICATIONS Certified Professional in Supply Management (CPSM ) Supply Management. Supply Management. CPSM Learning System
 CPSM EXAMS SPECIFICATIONS Leadership and Transformation in Supply Supply Integration EXAM SPECIFICATIONS Certified Professional in Supply (CPSM ) Supply CORE CPSM Learning System Institute for Supply 2018,
CPSM EXAMS SPECIFICATIONS Leadership and Transformation in Supply Supply Integration EXAM SPECIFICATIONS Certified Professional in Supply (CPSM ) Supply CORE CPSM Learning System Institute for Supply 2018,
PREDICTIVE METRICS FOR SUPPLY CHAINS
 Massachusetts Institute of Technology PREDICTIVE METRICS FOR SUPPLY CHAINS By: Linda Haydamous Advisor: Dr. Larry Lapide May 21, 2009 Outline 1. What are Predictive Metrics? 2. Research Question and Scope
Massachusetts Institute of Technology PREDICTIVE METRICS FOR SUPPLY CHAINS By: Linda Haydamous Advisor: Dr. Larry Lapide May 21, 2009 Outline 1. What are Predictive Metrics? 2. Research Question and Scope
Safety Management Systems. Presented by: Cathy Webb, MS, CQA, CQE
 Safety Management Systems Presented by: Cathy Webb, MS, CQA, CQE Health & Safety Management Systems What they are and why they matter Basic Elements Examples of Programs Benefits of ISO 45001 Challenges
Safety Management Systems Presented by: Cathy Webb, MS, CQA, CQE Health & Safety Management Systems What they are and why they matter Basic Elements Examples of Programs Benefits of ISO 45001 Challenges
SUPPLIER QUALITY MANUAL
 SUPPLIER QUALITY MANUAL LM-QU-PA-008 Revision Date:7/13/18 LM-QU-PA-008 Revision Date:7/13/18 Table of Contents 1.0 Purpose 2.0 Scope 3.0 Quality System Requirements 4.0 Approved Supplier List 5.0 Supplier
SUPPLIER QUALITY MANUAL LM-QU-PA-008 Revision Date:7/13/18 LM-QU-PA-008 Revision Date:7/13/18 Table of Contents 1.0 Purpose 2.0 Scope 3.0 Quality System Requirements 4.0 Approved Supplier List 5.0 Supplier
CAPA Management: Best Practices and FDA QSR Mandate WHITE PAPER. ComplianceQuest In-Depth Analysis and Review
 CAPA Management: Best Practices and FDA QSR Mandate WHITE PAPER ComplianceQuest In-Depth Analysis and Review CAPA Management: Best Practices and FDA QSR Mandate Establishing a corrective and preventive
CAPA Management: Best Practices and FDA QSR Mandate WHITE PAPER ComplianceQuest In-Depth Analysis and Review CAPA Management: Best Practices and FDA QSR Mandate Establishing a corrective and preventive
Conduct a More Effective Management Review. Rob Packard, Consultant
 Conduct a More Effective Management Review & Slide 1 of 31 Your Speaker Rob Packard Rob Packard is a regulatory consultant with 20 years experience in the medical device, pharmaceutical and biotechnology
Conduct a More Effective Management Review & Slide 1 of 31 Your Speaker Rob Packard Rob Packard is a regulatory consultant with 20 years experience in the medical device, pharmaceutical and biotechnology
How Can LuitBiz Help Your Company in ISO 9001:2008 Quality Certifications? Do What You Say. Improve It Say Whay You Do.
 How Can LuitBiz Help Your Company in ISO 9001:2008 Quality Certifications? www.luitinfotech.com Do What You Say Improve It Say Whay You Do Prove It In today's economy, ISO quality standards are the benchmark
How Can LuitBiz Help Your Company in ISO 9001:2008 Quality Certifications? www.luitinfotech.com Do What You Say Improve It Say Whay You Do Prove It In today's economy, ISO quality standards are the benchmark
CAPITAL AVIONICS, INC. Quality Manual
 CAPITAL AVIONICS, INC. Issued 31 July 2018 Conforms to ISO 9001:2015 2018 ; all rights reserved. This document may contain proprietary information and may only be released to third parties with approval
CAPITAL AVIONICS, INC. Issued 31 July 2018 Conforms to ISO 9001:2015 2018 ; all rights reserved. This document may contain proprietary information and may only be released to third parties with approval
3/17/ The FDA Group (508) Improving Efficiency and Quality of a CAPA Program. The FDA s Perspective Management's Perspective.
 Perspectives: CAPA and FDA Improving Efficiency and Quality of a CAPA Program The FDA s Perspective Management's Perspective. 33 rd Annual Meeting of the Society of Quality Assurance National Harbor, Maryland
Perspectives: CAPA and FDA Improving Efficiency and Quality of a CAPA Program The FDA s Perspective Management's Perspective. 33 rd Annual Meeting of the Society of Quality Assurance National Harbor, Maryland
Proposed Document. Global Harmonization Task Force GHTF/SG4(PD)/N83R5:2009
 1 2 3 4 5 GHTF/SG4(PD)/N83R5:2009 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Global Harmonization Task Force Proposed Document Title: Guidelines for Regulatory Auditing
1 2 3 4 5 GHTF/SG4(PD)/N83R5:2009 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 Global Harmonization Task Force Proposed Document Title: Guidelines for Regulatory Auditing
NQA-1 Graded Approach, 55-Gallon Drum Summary
 1, Organization 2, Quality Assurance program 3, Design 4, Procurement Document 5, Instructions, procedures, and Drawings 100 Basic 200 Structure and Responsibility 201 General 202 Delegation of Work 300
1, Organization 2, Quality Assurance program 3, Design 4, Procurement Document 5, Instructions, procedures, and Drawings 100 Basic 200 Structure and Responsibility 201 General 202 Delegation of Work 300
CO-ORDINATION OF NOTIFIED BODIES PPE Regulation 2016/425 RECOMMENDATION FOR USE
 CO-ORDINATION OF NOTIFIED BODIES PPE Regulation 2016/425 PPE-R/00.018 Version 1 RECOMMENDATION FOR USE Number of pages: 5 Approval stage : Approved on : Origin : Horizontal Committee, C2D Ad hoc group
CO-ORDINATION OF NOTIFIED BODIES PPE Regulation 2016/425 PPE-R/00.018 Version 1 RECOMMENDATION FOR USE Number of pages: 5 Approval stage : Approved on : Origin : Horizontal Committee, C2D Ad hoc group
IVD Inspections Technical Update Dr Dragana Milic Medical devices inspections
 IVD Inspections Technical Update 2018 Dr Dragana Milic Medical devices inspections 1 Objectives Harmonisation - internal and external (PQ Medicines/Vaccines/Vector Control and MDSAP) Transparency (clarify
IVD Inspections Technical Update 2018 Dr Dragana Milic Medical devices inspections 1 Objectives Harmonisation - internal and external (PQ Medicines/Vaccines/Vector Control and MDSAP) Transparency (clarify
The Impact of Quality Culture on Quality Risk Management. FDA Perspective on Quality Culture; how it Impacts Risk Management
 The Impact of Quality Culture on Quality Risk Management FDA Perspective on Quality Culture; how it Impacts Risk Management Teresa Gorecki Practice Lead Compliance Architects Agenda The WHAT Definitions
The Impact of Quality Culture on Quality Risk Management FDA Perspective on Quality Culture; how it Impacts Risk Management Teresa Gorecki Practice Lead Compliance Architects Agenda The WHAT Definitions
Supplier Audit Procedure /2/17
 1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
1.0 Purpose The purpose of this procedure is to establish a process for conducting a Supplier audit to determine if the Supplier has a quality system, if the Supplier follows an established quality system,
Integrity Management Program for
 Compliance Assurance Protocol Integrity Management Program for Pipelines April 2018 Version 1.9 Table of Contents BACKGROUND... 4 SAFETY CULTURE... 4 COMPLIANCE ASSURANCE PROCESS... 5 PHASE ONE... 5 PHASE
Compliance Assurance Protocol Integrity Management Program for Pipelines April 2018 Version 1.9 Table of Contents BACKGROUND... 4 SAFETY CULTURE... 4 COMPLIANCE ASSURANCE PROCESS... 5 PHASE ONE... 5 PHASE
Gaining Supply Chain Connectivity and Visibility with the Cloud
 Gaining Supply Chain Connectivity and Visibility with the Cloud At a Glance Your supply chain network needs to meet and exceed delivery targets for your business to remain competitive. The need for supply
Gaining Supply Chain Connectivity and Visibility with the Cloud At a Glance Your supply chain network needs to meet and exceed delivery targets for your business to remain competitive. The need for supply
Strategic Objectives. 3. Marketing Materials / Tools. 1. Training & Consulting for Human Capital Strategy
 Strategic Objectives We provide Professional Training & Consultation Services in the fields of: 1. Training & Consulting for Human Capital Strategy At ADHRT we believe that the development of Human capital
Strategic Objectives We provide Professional Training & Consultation Services in the fields of: 1. Training & Consulting for Human Capital Strategy At ADHRT we believe that the development of Human capital
Quality Manual. Manasota Optics, Inc & 1749 Northgate Boulevard Sarasota, FL Issue # 7 dated 05/10/2018
 Quality Manual Manasota Optics, Inc. 1743 & 1749 Northgate Boulevard Sarasota, FL 34234 Issue # 7 dated 05/10/2018 Schedule QM-01 Page:- 1 of 34 Issue Number:- 7 Effective Date:- 05/10/18 This document
Quality Manual Manasota Optics, Inc. 1743 & 1749 Northgate Boulevard Sarasota, FL 34234 Issue # 7 dated 05/10/2018 Schedule QM-01 Page:- 1 of 34 Issue Number:- 7 Effective Date:- 05/10/18 This document
ADVANCED INTERNAL AUDIT WORKSHOP
 ADVANCED INTERNAL AUDIT WORKSHOP By Paul J. Kunder RABQSA QMS Lead Auditor/Aerospace Industry Experienced Auditor RABQSA Approved Aerospace Auditor Transition Training (AATT) Trainer Vice Chair U.S. Z1A
ADVANCED INTERNAL AUDIT WORKSHOP By Paul J. Kunder RABQSA QMS Lead Auditor/Aerospace Industry Experienced Auditor RABQSA Approved Aerospace Auditor Transition Training (AATT) Trainer Vice Chair U.S. Z1A
How to Implement and Maintain a Modern CAPA System While Avoiding Common Pitfalls. Jon D. Speer, Founder & VP QA/RA Greenlight Guru
 How to Implement and Maintain a Modern CAPA System While Avoiding Common Pitfalls Jon D. Speer, Founder & VP QA/RA Greenlight Guru Jon Speer is the Founder & VP QA/RA of Greenlight Guru. 19+ years in medical
How to Implement and Maintain a Modern CAPA System While Avoiding Common Pitfalls Jon D. Speer, Founder & VP QA/RA Greenlight Guru Jon Speer is the Founder & VP QA/RA of Greenlight Guru. 19+ years in medical
Strategic and Operational Control
 Strategic and Operational Control Business Policy Please note that these slides are not intended as a substitute to reading the recommended text for this course. 0 Objectives Define strategic capability
Strategic and Operational Control Business Policy Please note that these slides are not intended as a substitute to reading the recommended text for this course. 0 Objectives Define strategic capability
DOCUMENT CONTROL INFORMATION
 DOCUMENT CONTROL INFORMATION System: Document Title: Document Status: Quality Manual Approved 5 11/5/2014 Current Document Revision Details Formal review. Added section 2, 3. Revised section 5.5.2, 6.2,
DOCUMENT CONTROL INFORMATION System: Document Title: Document Status: Quality Manual Approved 5 11/5/2014 Current Document Revision Details Formal review. Added section 2, 3. Revised section 5.5.2, 6.2,
