ASQ Madison, WI Section Using your ISO 9001:2008 QMS to Plan for the 2015 Changes
|
|
|
- Stella Dorsey
- 6 years ago
- Views:
Transcription
1 ASQ Madison, WI Section 1217 Using your ISO 9001:2008 QMS to Plan for the 2015 Changes
2 SESSION AGENDA High Level Review of Requirements Historical Perspective Basic Requirements Change It s a Process Inputs, Processes, Outputs Plan, Do, Check, Act Key Components of Your QMS Next steps Information Sources
3 Annex SL - High Level Structure 1 - Scope 2 - Normative References 3 - Terms and definitions 4 - Context of the Organization 5 Leadership 6 Planning 7 Support 8 Operation 9 Performance Evaluation 10 Improvement
4 ISO 9001 Common Clauses 4 Context of the Organization Understanding the organization and its context Understanding the needs and expectation of interested parties Determining the scope of the QMS 4.4 Quality management system and its processes
5 ISO 9000 Common Clauses 5 Leadership Leadership and commitment 5.2 Quality policy Organizational roles, responsibilities and authorities
6 ISO 9000 Common Clauses 6 Planning for the QMS Actions to address risk 6.2 Quality objectives and planning to achieve Planning of changes
7 ISO 9000 Common Clauses 7 Support 7.1 Resources Competence Awareness 7.4 Communication Documented information
8 ISO 9000 Common Clauses 8 Operation 8.1 Operational Planning/Control 8.2 Requirements for Products and Services 8.3 Design and Development 8.4 Control of Externally Provided Process/Products/Services 8.5 Production and Service Provision 8.6 Release of Products/Services 8.7 Control of Nonconforming Outputs
9 ISO 9000 Common Clauses 9 Performance Evaluation Monitoring, measurement, analysis and evaluation Internal audit Management review
10 ISO 9000 Common Clauses 10 Improvement 10.1 General Nonconformity and corrective action Continual improvement
11 A HISTORICAL PERSPECTIVE
12 AVOIDING THE CLIFF ISO 9001:2008 ISO 9001:2015
13 Customers Customers BASIC PRINCIPLES OF QMS Improvement of QMS Management Responsibility Resource Management Measurement, analysis & improvement Requirements Satisfaction Product Realization
14 BASICS OF YOUR QMS Quality management system 4 The organization shall establish, document, implement and maintain a quality management system in accordance with the requirements of this International Standard
15 BASICS OF YOUR QMS Quality management system 4 These processes shall be managed by the organization in accordance with the requirements of this International Standard
16 CHANGE IT S A PROCESS INPUTS ACTIVITIES OUTPUTS
17 IT S THERE FOR A REASON! Document Control Control of documents of external origin determined by the organization to be necessary for the planning and operation of the quality management system
18 PLANNING Quality management system planning Top management shall ensure that The planning of the quality management system is carried out in order to meet the requirements given in 4.1 and,
19 PLANNING The integrity of the quality management system is maintained when changes to the quality management system are planned and implemented
20 PLANNING Management Representative a) ensuring that processes needed for the quality management system are established, implemented and maintained
21 PLANNING Management review General This review shall include assessing the need for changes to the quality management system
22 PLANNING Review input The input to management review shall include information on f) changes that could affect the quality management system
23 PLANNING Review output The output of from the management review shall include any decisions and actions relating to a) Improvement of the effectiveness of the quality management system and its processes c) Resource needs
24 PLANNING RESOURCE NEEDS Competency, awareness and training a) determine the necessary competence of personnel performing work affecting quality
25 CHECKING Internal audit The organization shall conduct internal audits at planned intervals to determine whether the quality management system a) conforms to the planned arrangements (see 7.1) to the requirements of this International Standard and to the quality management requirements established by the organization
26 CHECKING Internal audit cont d An audit programme shall be planned, taking into consideration the status and importance of the processes
27 CHECKING Internal audit 2015 wording An audit programme shall be planned, taking into consideration the importance of the processes, customer feedback and changes
28 HOW ABOUT YOUR ORGANIZATION? What (likely) key changes in ISO 9001:2015 are going to require decisions and actions from your Organization s management review of the QMS?
29 KEEPING INFORMED Keep informed NQA website NQA blog NQA Linkedin NQA Newsletter
30
31 NQA USA BLOG
32 LinkedIn & Face Book
33 QUESTIONS? CONTACT INFO: NAME: ANDY NICHOLS PHONE: (248)
ISO 9001: 2015 Quality Management System Certification. Awareness Training
 ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
Correlation matrices between ISO 9001:2008 and ISO 9001:2015
 Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
Continual Improvement
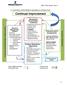 15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
Correlation Matrix & Change Summary
 The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT
 INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
ISO 9001:2015. Presented By: ASEAN Eng. DEXTER T. CHUA, PIE. Conference Room, University of Mindanao March 17, 2017
 ISO 9001:2015 Presented By: ASEAN Eng. DEXTER T. CHUA, PIE Conference Room, University of Mindanao March 17, 2017 @CQL Business Systems Consulting (11-2015) ISO The International Organization for Standardization
ISO 9001:2015 Presented By: ASEAN Eng. DEXTER T. CHUA, PIE Conference Room, University of Mindanao March 17, 2017 @CQL Business Systems Consulting (11-2015) ISO The International Organization for Standardization
Clause Map IATF 16949:2016 to ISO/TS 16949:2009
 Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
Performance Evaluation Standard as per ISO 9001:2015
 Performance Evaluation Standard as per ISO 9001:2015 PMI, PMP, PMBOK and the PMI Registered Education Provider logo are registered marks of the Project Management Institute, Inc. At the end of the class,
Performance Evaluation Standard as per ISO 9001:2015 PMI, PMP, PMBOK and the PMI Registered Education Provider logo are registered marks of the Project Management Institute, Inc. At the end of the class,
ISO 9001:2015 Quality Management System. New/Revised Requirements
 ISO 9001:2015 New/Revised The Quality System Checklist is intended to only identify the new/revised requirements. ISO 9001:2015 requires the adoption of the process approach which extends to internal quality
ISO 9001:2015 New/Revised The Quality System Checklist is intended to only identify the new/revised requirements. ISO 9001:2015 requires the adoption of the process approach which extends to internal quality
ISO 9001:2015. October 5 th, Brad Fischer.
 ISO 9001:2015 October 5 th, 2017 Brad Fischer www.sdmanufacturing.com Purpose of presentation Provide a summary of notable changes from ISO 9001:2008 to ISO 9001:2015 Key perspectives ISO 9001 needs to
ISO 9001:2015 October 5 th, 2017 Brad Fischer www.sdmanufacturing.com Purpose of presentation Provide a summary of notable changes from ISO 9001:2008 to ISO 9001:2015 Key perspectives ISO 9001 needs to
ISO 9001:2015 Readiness Review
 ISO 9001:2015 Readiness Review Company Name Address Certification No. Contact Name Job Title Telephone Email BSI is committed to ensuring a smooth assessment for all clients wishing to certify to ISO 9001:2015,
ISO 9001:2015 Readiness Review Company Name Address Certification No. Contact Name Job Title Telephone Email BSI is committed to ensuring a smooth assessment for all clients wishing to certify to ISO 9001:2015,
ISO/DIS 9001: 2014 comparison with ISO 9001:2008. ISO 9001:2015 Updates. (Based on Draft International Standard, DIS) ISO/DIS 9001 ISO 9001:2008
 ISO 9001:2015 Updates (Based ondraft International Standard, DIS) August 2014 Page 1 ISO 9001:2015 Updates (Based on Draft International Standard, DIS) ISO/DIS 9001: 2014 comparison with ISO 9001:2008
ISO 9001:2015 Updates (Based ondraft International Standard, DIS) August 2014 Page 1 ISO 9001:2015 Updates (Based on Draft International Standard, DIS) ISO/DIS 9001: 2014 comparison with ISO 9001:2008
Sections of the Standard. Evidence / Comments. (Y) / Nonconforming (NC)
 Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
QMS Team: MR and all HODs (Internal Auditors) MR March 10. Quality policy Define quality policy The Steering committee Objectives and targets
 QMS Roles, Responsibility and Authority Process Clause Activities Records Required Responsibility Authority Deadline Clause 4: Process Development 4.1 Develop processes and sequence, operation controls
QMS Roles, Responsibility and Authority Process Clause Activities Records Required Responsibility Authority Deadline Clause 4: Process Development 4.1 Develop processes and sequence, operation controls
Summary of ISO 9001:2015 New and Changed Requirements
 This is a summary of the new and changed ISO 9001:2015 requirements compared to ISO 9001:2008. 4. Context of the Organization 4.1 Changes Understanding the Organization and its Context New requirement
This is a summary of the new and changed ISO 9001:2015 requirements compared to ISO 9001:2008. 4. Context of the Organization 4.1 Changes Understanding the Organization and its Context New requirement
We are a global classification, certification, technical assurance and advisory company Ungraded
 We are a global classification, certification, technical assurance and advisory company 1 Global reach local competence 150 300 100 15,000 years offices countries employees 2 DNV GL :: Focused on your
We are a global classification, certification, technical assurance and advisory company 1 Global reach local competence 150 300 100 15,000 years offices countries employees 2 DNV GL :: Focused on your
Fitting ISO 9001:2000 into a 20 Element Quality System
 Fitting ISO 9001:2000 into a 20 Element Quality System ASQ Washington DC Section 0509 Rockville Maryland Presented by Norman P. Moreau, P.E., CSQE, CQA President Theseus Professional Services 410-857-0023
Fitting ISO 9001:2000 into a 20 Element Quality System ASQ Washington DC Section 0509 Rockville Maryland Presented by Norman P. Moreau, P.E., CSQE, CQA President Theseus Professional Services 410-857-0023
ISO 9001:2015 Transition Presentation. Presented by Fredric Leung
 ISO 9001:2015 Transition Presentation Presented by Fredric Leung 1 2 ISO Technical Committees TC 176 ISO = International Organization for Standardization Standards development work is done by Technical
ISO 9001:2015 Transition Presentation Presented by Fredric Leung 1 2 ISO Technical Committees TC 176 ISO = International Organization for Standardization Standards development work is done by Technical
AB. OUR ISO CONFORMANCE AUDIT QUESTIONNAIRES 8. ASSESS HOW WELL YOU CONFORM TO ISO S REMEDIAL REQUIREMENTS
 8.1 PLANNING REQUIREMENTS 1 Do you plan monitoring, measurement, and analytical processes? 2 Do you plan how monitoring will be used to ensure conformity and effectiveness? 3 Do you plan how it will be
8.1 PLANNING REQUIREMENTS 1 Do you plan monitoring, measurement, and analytical processes? 2 Do you plan how monitoring will be used to ensure conformity and effectiveness? 3 Do you plan how it will be
Transition to ISO 9001:2015
 Transition to ISO 9001:2015 Whittington & Associates, LLC 6175 Hickory Flat Highway, Suite 110-303, Canton, GA 30115 www.whittingtonassociates.com 770-862-1766 Version 1.6: 02/01/16 2015-2016 Whittington
Transition to ISO 9001:2015 Whittington & Associates, LLC 6175 Hickory Flat Highway, Suite 110-303, Canton, GA 30115 www.whittingtonassociates.com 770-862-1766 Version 1.6: 02/01/16 2015-2016 Whittington
ISO 9001 Auditing Practices Group. Aligning the QMS with the achievement of organizational and business success
 Slide 1 ISO 9001 Auditing Practices Group Guidance on: Effectiveness Aligning the QMS with the achievement of organizational and business success Slide 2 Business, Quality and Excellence Models and Tools
Slide 1 ISO 9001 Auditing Practices Group Guidance on: Effectiveness Aligning the QMS with the achievement of organizational and business success Slide 2 Business, Quality and Excellence Models and Tools
ISO 9001:2015 READINESS CHECKLIST YOU RE CLOSER THAN YOU THINK EXECUTIVE SUMMARY CLAUSE 4 - CONTEXT OF THE ORGANISATION CLAUSE 5 - LEADERSHIP
 EXECUTIVE SUMMARY CLAUSE 4 - CONTEXT OF THE ORGANISATION CLAUSE 5 - LEADERSHIP CLAUSE 6 - PLANNING CLAUSE 7 - RESOURCES CLAUSE 8 - OPERATIONS CLAUSE 9 - PERFORMANCE EVALUATION CLAUSE 10 - IMPROVEMENTS
EXECUTIVE SUMMARY CLAUSE 4 - CONTEXT OF THE ORGANISATION CLAUSE 5 - LEADERSHIP CLAUSE 6 - PLANNING CLAUSE 7 - RESOURCES CLAUSE 8 - OPERATIONS CLAUSE 9 - PERFORMANCE EVALUATION CLAUSE 10 - IMPROVEMENTS
Industry Outreach Quality
 Mission and Installation Contracting Command Industry Outreach Quality Joe Merry Quality Assurance C-SPO Joe.merry@us.army.mil 10 August 2010 MICC Quality Program The MICC Quality Program is in the final
Mission and Installation Contracting Command Industry Outreach Quality Joe Merry Quality Assurance C-SPO Joe.merry@us.army.mil 10 August 2010 MICC Quality Program The MICC Quality Program is in the final
QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES
 Your Company Name QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Policies and Procedures Latest Revision Date Abstract:
Your Company Name QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Policies and Procedures Latest Revision Date Abstract:
ISO 9001:2015. Quality Management Systems Manual. [Preview] [Company Name] ADDRESS. Phone: Phone: Fax: Fax:
![ISO 9001:2015. Quality Management Systems Manual. [Preview] [Company Name] ADDRESS. Phone: Phone: Fax: Fax: ISO 9001:2015. Quality Management Systems Manual. [Preview] [Company Name] ADDRESS. Phone: Phone: Fax: Fax:](/thumbs/71/65619940.jpg) ISO 9001:2015 Quality Management Systems Manual [Preview] [Company Name] ADDRESS Phone: Phone: Fax: Fax: The holder of this manual is cautioned that the information contained herein must not be loaned
ISO 9001:2015 Quality Management Systems Manual [Preview] [Company Name] ADDRESS Phone: Phone: Fax: Fax: The holder of this manual is cautioned that the information contained herein must not be loaned
Pre Audit Transition Gap Analysis QMS (ISO 9001 Only)
 Pre Audit Transition Gap Analysis QMS (ISO 9001 Only) Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 standard and
Pre Audit Transition Gap Analysis QMS (ISO 9001 Only) Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 standard and
ISO 9001 REVISION INTRODUCTION TO ISO/FDIS 9001
 ISO 9001 REVISION INTRODUCTION TO ISO/FDIS 9001 AGENDA Introduction Structure and Terminology Changes to ISO 9001 Future Development How SGS can support you 2 INTRODUCTION ISO/DIS 9001 Issued May 2014
ISO 9001 REVISION INTRODUCTION TO ISO/FDIS 9001 AGENDA Introduction Structure and Terminology Changes to ISO 9001 Future Development How SGS can support you 2 INTRODUCTION ISO/DIS 9001 Issued May 2014
Moving from ISO/TS 16949:2009 to IATF 16949:2016. Transition Guide
 Moving from ISO/TS 16949:2009 to IATF 16949:2016 Transition Guide IATF 16949:2016 - Automotive Quality Management System - Transition Guide An effective Quality Management System is vital for organizations
Moving from ISO/TS 16949:2009 to IATF 16949:2016 Transition Guide IATF 16949:2016 - Automotive Quality Management System - Transition Guide An effective Quality Management System is vital for organizations
Clauses of the new ISO 9001:2015 standard
 Qudos Management Pty. Ltd. Quality Health & Safety Environmental management 320 Adelaide Street, Brisbane, QLD 4000 Tel: +61 (07) 3010 9259 3 Spring Street, Sydney, NSW 2000 Tel: +61 (02) 8249 4670 Email:
Qudos Management Pty. Ltd. Quality Health & Safety Environmental management 320 Adelaide Street, Brisbane, QLD 4000 Tel: +61 (07) 3010 9259 3 Spring Street, Sydney, NSW 2000 Tel: +61 (02) 8249 4670 Email:
P. 1. Identify the Differences between ISO9001:2000 與 ISO9001:2008 ISO9001:2008 ISO9001:2000 版本的異同. 5 January 2009 ISO 9000 SERIES
 Identify the Differences between ISO9001:2000 and ISO 9001:2008 審視 ISO9001:2000 與 ISO9001:2008 版本的異同 ISO 9000 SERIES ISO 19011 ISO9000 5 January 2009 ISO9001 ISO9004 2 ISO 9000 SERIES ISO 9001 ISO 9000
Identify the Differences between ISO9001:2000 and ISO 9001:2008 審視 ISO9001:2000 與 ISO9001:2008 版本的異同 ISO 9000 SERIES ISO 19011 ISO9000 5 January 2009 ISO9001 ISO9004 2 ISO 9000 SERIES ISO 9001 ISO 9000
What is ISO 9001 QMS? Business Beam
 1 What is ISO 9001 QMS? Business Beam Contents 2 What is Quality About Quality Management System About ISO 9001 ISO 9001 Certification Process 3 What is Quality? What is ISO 9001 QMS? What is Quality?
1 What is ISO 9001 QMS? Business Beam Contents 2 What is Quality About Quality Management System About ISO 9001 ISO 9001 Certification Process 3 What is Quality? What is ISO 9001 QMS? What is Quality?
ISO 14001:2015 Transition Presentation. Presented by Fredric Leung
 ISO 14001:2015 Transition Presentation Presented by Fredric Leung 1 2 ISO Technical Committees TC 207 ISO = International Organization for Standardization Standards development work is done by Technical
ISO 14001:2015 Transition Presentation Presented by Fredric Leung 1 2 ISO Technical Committees TC 207 ISO = International Organization for Standardization Standards development work is done by Technical
9100 Team July, IAQG is a trademark the International Aerospace Quality Group. Copyright 2014 IAQG. All rights reserved.
 9100 Series 2016 Revision Overview 9100 Team July, 2014 1 9100 Revision The Plan 9100 Series Revision High Level Plan The 9100 is based on ISO 9001 and is thus affected by the ISO TC176 revision activity
9100 Series 2016 Revision Overview 9100 Team July, 2014 1 9100 Revision The Plan 9100 Series Revision High Level Plan The 9100 is based on ISO 9001 and is thus affected by the ISO TC176 revision activity
ISO Current status of development
 ISO 45001 Current status of development July 2015 1 Disclaimers Verbal statements made by the presenter may represent personal opinions and/or interpretations The presentation includes information related
ISO 45001 Current status of development July 2015 1 Disclaimers Verbal statements made by the presenter may represent personal opinions and/or interpretations The presentation includes information related
TL 9000 R6.0 REQUIREMENTS
 Logo or heading here TL 9000 R6.0 REQUIREMENTS Nancy Patterson Nokia Tom Yohe - Consultant TL 9000 CONTAINS ALL OF THE AUDITABLE CLAUSES OF ISO 9001:2008 http://tl9000.org/alerts/alerts.html#10 QUEST FORUM
Logo or heading here TL 9000 R6.0 REQUIREMENTS Nancy Patterson Nokia Tom Yohe - Consultant TL 9000 CONTAINS ALL OF THE AUDITABLE CLAUSES OF ISO 9001:2008 http://tl9000.org/alerts/alerts.html#10 QUEST FORUM
ISO 9001:2015 Key Changes in the University QMS
 ISO 9001:2015 Key Changes in the University QMS QMS Review Committee August 2016 University of Nairobi ISO 9001:2008 1 Certified http://www.uonbi.ac.ke Intro The ISO 9001:2015 standard was published in
ISO 9001:2015 Key Changes in the University QMS QMS Review Committee August 2016 University of Nairobi ISO 9001:2008 1 Certified http://www.uonbi.ac.ke Intro The ISO 9001:2015 standard was published in
Pre Audit Transition Gap Analysis QMS and EMS
 Pre Audit Transition Gap Analysis QMS and EMS Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 and ISO 14001:2015 standards
Pre Audit Transition Gap Analysis QMS and EMS Company: Contact Name: Certification Number: Email: Contact Number: This document should be used in conjunction with the ISO 9001:2015 and ISO 14001:2015 standards
IMPORTANT NOTE: My remarks are my personal remarks and not that of any organization that I belong.
 JP Russell Author of several books about quality, auditing & standards Editor of The ASQ Auditing Handbook, 1st-4th editions Columnist for Quality Progress Magazine for 15 years Member of US TAG TC 176
JP Russell Author of several books about quality, auditing & standards Editor of The ASQ Auditing Handbook, 1st-4th editions Columnist for Quality Progress Magazine for 15 years Member of US TAG TC 176
AS9100:2016 SERIES TRANSITION UPDATES
 AS9100:2016 SERIES TRANSITION UPDATES BUDDY CRESSIONNIE Introductions Buddy Cressionnie Americas Aerospace Quality System Committee (AAQSC) Chair AAQSC Americas Leader of the IAQG Requirements Strategy
AS9100:2016 SERIES TRANSITION UPDATES BUDDY CRESSIONNIE Introductions Buddy Cressionnie Americas Aerospace Quality System Committee (AAQSC) Chair AAQSC Americas Leader of the IAQG Requirements Strategy
Moving to the AS9100:2016 series. Transition Guide
 Moving to the AS9100:2016 series Transition Guide AS9100-series - Quality Management Systems for Aviation, Space and Defense - Transition Guide Successful aviation, space and defense businesses understand
Moving to the AS9100:2016 series Transition Guide AS9100-series - Quality Management Systems for Aviation, Space and Defense - Transition Guide Successful aviation, space and defense businesses understand
Quality and Medical Devices: ISO 13485:2003
 Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
con cen tric (ken-sĕn trĭk) adjective - Having a common center. con cen tric (ken-sĕn trĭk) adjective - Having a common center. acommoncenter.
 Changes to ISO 9001:2015 Presented by Jim Thompson 266 Meeting Street - Suite B Charleston, SC 29401 843.469.8279 acommoncenter.com January 26 th, 2016 ASQ Charleston con cen tric (ken-sĕn trĭk) adjective
Changes to ISO 9001:2015 Presented by Jim Thompson 266 Meeting Street - Suite B Charleston, SC 29401 843.469.8279 acommoncenter.com January 26 th, 2016 ASQ Charleston con cen tric (ken-sĕn trĭk) adjective
QM-1 Supplement QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT. Revision 7. Quality Director / Management Representative n. B.
 QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT Revision 7 APPROVED BY: TITLE DATE B. Olevson Quality Director / Management Representative 07 3-0 n DOCUMENT REVISION HISTORY Paragraph Revisions: or Rev: Date:
QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT Revision 7 APPROVED BY: TITLE DATE B. Olevson Quality Director / Management Representative 07 3-0 n DOCUMENT REVISION HISTORY Paragraph Revisions: or Rev: Date:
Moving from ISO 14001:2004 to ISO 14001:2015 Transition Guide
 ISO Revisions Final Standard Moving from ISO 14001:2004 to ISO 14001:2015 Transition Guide ISO 14001 - Environmental Management System - Transition Guide Successful businesses understand that it is the
ISO Revisions Final Standard Moving from ISO 14001:2004 to ISO 14001:2015 Transition Guide ISO 14001 - Environmental Management System - Transition Guide Successful businesses understand that it is the
ISO/DIS9001:2008 VS ISO9001:2000. Speaker: Ir. Dr. Aaron TONG Date:
 ISO/DIS9001:2008 VS ISO9001:2000 Speaker: Ir. Dr. Aaron TONG Date:2008-08-01 1 Outline Background Information of ISO/DIS 9001 : 2008 Main Changes of 2008 Edition What we should do? Q & A Section 2 Ir.
ISO/DIS9001:2008 VS ISO9001:2000 Speaker: Ir. Dr. Aaron TONG Date:2008-08-01 1 Outline Background Information of ISO/DIS 9001 : 2008 Main Changes of 2008 Edition What we should do? Q & A Section 2 Ir.
ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015. Joan Richter ASQ Baltimore (Section 502) June 2016
 ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015 Joan Richter ASQ Baltimore (Section 502) June 2016 HISTORY OF ISO 9001 Standard (28 Years of ISO) 1987 Procedures
ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015 Joan Richter ASQ Baltimore (Section 502) June 2016 HISTORY OF ISO 9001 Standard (28 Years of ISO) 1987 Procedures
ISO 9001: 2000 (December 13, 2000) QUALITY MANAGEMENT SYSTEM DOCUMENTATION OVERVIEW MATRIX
 In completing your Documented Quality Management System Review, it is important that the following matrix be completed and returned to us as soon as possible. This will save time during the review and
In completing your Documented Quality Management System Review, it is important that the following matrix be completed and returned to us as soon as possible. This will save time during the review and
QUALITY MANUAL. Number: M-001 Revision: C Page 1 of 18 THIS DOCUMENT IS CONSIDERED UNCONTROLLED UNLESS ISSUED IDENTIFIED AS CONTROLLED
 Page 1 of 18 THIS DOCUMENT IS CONSIDERED UNCONTROLLED UNLESS ISSUED IDENTIFIED AS CONTROLLED Page 2 of 18 REVISION HISTORY DATE CHANGE DESCRIPTION 10/11/06 Original release 10/21/09 Revised to ISO9001:2008
Page 1 of 18 THIS DOCUMENT IS CONSIDERED UNCONTROLLED UNLESS ISSUED IDENTIFIED AS CONTROLLED Page 2 of 18 REVISION HISTORY DATE CHANGE DESCRIPTION 10/11/06 Original release 10/21/09 Revised to ISO9001:2008
Risk Based Thinking & QMS Risk Management as per ISO
 Risk Based Thinking & QMS Risk Management as per ISO 9001-2015 PMI, PMP, PMBOK and the PMI Registered Education Provider logo are registered marks of the Project Management Institute, Inc. At the end of
Risk Based Thinking & QMS Risk Management as per ISO 9001-2015 PMI, PMP, PMBOK and the PMI Registered Education Provider logo are registered marks of the Project Management Institute, Inc. At the end of
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru
 FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
Integrating ISO 9001:2015 and ISO 14001:2015
 Integrating ISO 9001:2015 and ISO 14001:2015 Seize the opportunity and make efficiencies Whitepaper Integrating ISO 9001 and ISO 14001: there s no better time Why now? ISO standards have changed. The introduction
Integrating ISO 9001:2015 and ISO 14001:2015 Seize the opportunity and make efficiencies Whitepaper Integrating ISO 9001 and ISO 14001: there s no better time Why now? ISO standards have changed. The introduction
Type Your Company Name Here. Quality Manual. AS9100 Rev C
 Blue text throughout the manual highlight areas for customization Type Your Company Name Here AS9100 Rev C Documents are in Microsoft Word for ease of editing Provides P general purpose and description
Blue text throughout the manual highlight areas for customization Type Your Company Name Here AS9100 Rev C Documents are in Microsoft Word for ease of editing Provides P general purpose and description
ISO 9001:2015 How your ISO 9001 audit will be different. Whitepaper
 ISO 9001:2015 How your ISO 9001 audit will be different Whitepaper Introduction The new ISO 9001 introduces some key changes to the way a quality management system (QMS) is incorporated into your organization
ISO 9001:2015 How your ISO 9001 audit will be different Whitepaper Introduction The new ISO 9001 introduces some key changes to the way a quality management system (QMS) is incorporated into your organization
Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry
 Addendum 1 June 2010 Effective Date: December 1, 2010 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry ANSI/API SPECIFICATION Q1 EIGHTH EDITION, DECEMBER 2007
Addendum 1 June 2010 Effective Date: December 1, 2010 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry ANSI/API SPECIFICATION Q1 EIGHTH EDITION, DECEMBER 2007
Lessons Learned from an ISO 9001:2015 Transition. Tamara Hueston 12 October 2017
 Lessons Learned from an ISO 9001:2015 Transition Tamara Hueston 12 October 2017 Agenda: What initial steps were taken What plans were created How associates were prepared What were the certification audit
Lessons Learned from an ISO 9001:2015 Transition Tamara Hueston 12 October 2017 Agenda: What initial steps were taken What plans were created How associates were prepared What were the certification audit
Welcome ISO9001:2015 /ISO14001:2015
 Welcome ISO9001:2015 /ISO14001:2015 DQS 2017 All rights reserved. Unless otherwise specified, no part of this publication may be reproduced or utilized otherwise in any form or by any means, electronic
Welcome ISO9001:2015 /ISO14001:2015 DQS 2017 All rights reserved. Unless otherwise specified, no part of this publication may be reproduced or utilized otherwise in any form or by any means, electronic
TECHNICAL GUIDE. How to manage the transition successfully AUTOMOTIVE MANAGEMENT SYSTEM TRANSITION FROM ISO/TS TO IATF EDITION OCT 2017
 How to manage the transition successfully AUTOMOTIVE MANAGEMENT SYSTEM TRANSITION FROM ISO/TS 16949 TO IATF 16949 TECHNICAL GUIDE EDITION OCT 2017 Move Forward with Confidence IATF HAS PUBLISHED THE NEW
How to manage the transition successfully AUTOMOTIVE MANAGEMENT SYSTEM TRANSITION FROM ISO/TS 16949 TO IATF 16949 TECHNICAL GUIDE EDITION OCT 2017 Move Forward with Confidence IATF HAS PUBLISHED THE NEW
PRODUCTS AND SERVICES:
 COMPANY INFORMATION: Company Name: Newcastle Aviation Partners, LLC Address: 3201 West County Road 42, Unit 104 Burnsville, MN 55306 Phone: 952-223-0317 Facsimile: 952-223-4470 AOG phone number: 952-223-0317,
COMPANY INFORMATION: Company Name: Newcastle Aviation Partners, LLC Address: 3201 West County Road 42, Unit 104 Burnsville, MN 55306 Phone: 952-223-0317 Facsimile: 952-223-4470 AOG phone number: 952-223-0317,
Stanley Industries, Inc. ISO 9001:2008 Quality Policy Manual
 Stanley ISO 9001:2008 Table of Contents and STANLEY Document Reference Related STANLEY Section Page Procedure(s) 1. Introduction 1 None 2. Scope 1 None 3. Organizational Structure & 1 STANLEY Company History
Stanley ISO 9001:2008 Table of Contents and STANLEY Document Reference Related STANLEY Section Page Procedure(s) 1. Introduction 1 None 2. Scope 1 None 3. Organizational Structure & 1 STANLEY Company History
ISO 14001:2015 Gap Analysis Check Sheet
 ? CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context The organization shall determine external and internal issues that are relevant to its purpose and that affect its ability
? CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context The organization shall determine external and internal issues that are relevant to its purpose and that affect its ability
Document Number: QM001 Page 1 of 19. Rev Date: 10/16/2009 Rev Num: 1. Quality Manual. Quality Manual. Controlled Copy
 QM001 Page 1 of 19 Quality Manual QM001 Page 2 of 19 Table of Contents Page Company Profile 4 Approval 4 Revision History 4 Distribution List 4 1.0 Scope 5 Section 2: Normative Reference 6 2.0 Quality
QM001 Page 1 of 19 Quality Manual QM001 Page 2 of 19 Table of Contents Page Company Profile 4 Approval 4 Revision History 4 Distribution List 4 1.0 Scope 5 Section 2: Normative Reference 6 2.0 Quality
US survey of user experiences
 Implementing ISO 9001:2000 US survey of user experiences Results are in from a survey developed by the US Technical Advisory Group to ISO/TC 176 quantifying the experiences of 227 US organizations implementing
Implementing ISO 9001:2000 US survey of user experiences Results are in from a survey developed by the US Technical Advisory Group to ISO/TC 176 quantifying the experiences of 227 US organizations implementing
ISO 14001:2015 PREPARING FOR A SUCCESSFUL TRANSITION
 ISO 14001:2015 PREPARING FOR A SUCCESSFUL TRANSITION Scott Jones EHS Program Manager Welcome From PJR Headquarters: 755 W. Big Beaver Rd, Suite 1340 Troy, MI 48084 Phone: 1-800-800-7910 Email: PJR@PJR.com
ISO 14001:2015 PREPARING FOR A SUCCESSFUL TRANSITION Scott Jones EHS Program Manager Welcome From PJR Headquarters: 755 W. Big Beaver Rd, Suite 1340 Troy, MI 48084 Phone: 1-800-800-7910 Email: PJR@PJR.com
9 1.0 Step 1 Overview of what should be considered Step 2 ISO 9001:2015 Context of an organisation
 INDEX Page Section Description 1 Index 2 0.0 Introduction and Summary 9 1.0 Step 1 Overview of what should be considered 11 2.0 Step 2 ISO 9001:2015 Context of an organisation 16 3.0 Annex SL (New ISO
INDEX Page Section Description 1 Index 2 0.0 Introduction and Summary 9 1.0 Step 1 Overview of what should be considered 11 2.0 Step 2 ISO 9001:2015 Context of an organisation 16 3.0 Annex SL (New ISO
Harmonization day 2015 FSSC audit reporting
 Harmonization day 2015 FSSC 22000 audit reporting Today s topics 1. Basics audit reporting 2. Definition audit report 3. Audit report requirements 4. Conclusions 1. Basics audit reporting Starting point
Harmonization day 2015 FSSC 22000 audit reporting Today s topics 1. Basics audit reporting 2. Definition audit report 3. Audit report requirements 4. Conclusions 1. Basics audit reporting Starting point
SPECIAL AUDITS WHAT, WHY AND HOW?
 SPECIAL AUDITS WHAT, WHY AND HOW? Mike McRandall and Don McFarland (NSF-ISR) Introductions: Mike McRandall NSF-ISR Business Unit Manager, Aerospace mmcrandall@nsf.org Don McFarland NSF-ISR Technical Scheme
SPECIAL AUDITS WHAT, WHY AND HOW? Mike McRandall and Don McFarland (NSF-ISR) Introductions: Mike McRandall NSF-ISR Business Unit Manager, Aerospace mmcrandall@nsf.org Don McFarland NSF-ISR Technical Scheme
9100 revision Changes presentation clause-by-clause. IAQG 9100 Team November 2016
 Changes presentation clause-by-clause IAQG 9100 Team November 2016 INTRODUCTION In September 2016, a revision of the 9100 standard has been published by the IAQG (International Aerospace Quality Group)
Changes presentation clause-by-clause IAQG 9100 Team November 2016 INTRODUCTION In September 2016, a revision of the 9100 standard has been published by the IAQG (International Aerospace Quality Group)
QUALITY MANUAL Revision H
 QUALITY MANUAL Revision H JADE PRECISION MEDICAL COMPONENTS, LLC 3063 B Philmont Avenue Huntingdon Valley, PA 19006 Page: 1 of 15 Table of Contents 1. Purpose & Scope... 2 2. Applicable Standards... 2
QUALITY MANUAL Revision H JADE PRECISION MEDICAL COMPONENTS, LLC 3063 B Philmont Avenue Huntingdon Valley, PA 19006 Page: 1 of 15 Table of Contents 1. Purpose & Scope... 2 2. Applicable Standards... 2
0. 0 TABLE OF CONTENTS
 QUALITY MANUAL Conforming to ISO 9001:2008 0. 0 TABLE OF CONTENTS Section Description ISO 9001 Clause Page 0 TABLE OF CONTENTS n/a 2 1 PIMA VALVE, INC. DESCRIPTION n/a 3 2 QUALITY MANUAL DESCRIPTION 4.2.2
QUALITY MANUAL Conforming to ISO 9001:2008 0. 0 TABLE OF CONTENTS Section Description ISO 9001 Clause Page 0 TABLE OF CONTENTS n/a 2 1 PIMA VALVE, INC. DESCRIPTION n/a 3 2 QUALITY MANUAL DESCRIPTION 4.2.2
Document: ISO/TC 176/SC 2/N 1147
 ISO 2013 All rights reserved Document: ISO/TC 176/SC 2/N 1147 Secretariat of ISO/TC 176/SC 2 Date: 3 June 2013 To the Members of ISO/TC 176/SC 2 - Quality Management and Quality Assurance/ Quality Systems
ISO 2013 All rights reserved Document: ISO/TC 176/SC 2/N 1147 Secretariat of ISO/TC 176/SC 2 Date: 3 June 2013 To the Members of ISO/TC 176/SC 2 - Quality Management and Quality Assurance/ Quality Systems
ISO 9001:2015 TRANSITION INFORMATION
 ISO 9001:2015 TRANSITION INFORMATION ISO 9001:2015 Transition It Doesn t Need to be Difficult Whether your organization is transitioning from ISO 9001:2008 to the new ISO 9001:2015, or implementing an
ISO 9001:2015 TRANSITION INFORMATION ISO 9001:2015 Transition It Doesn t Need to be Difficult Whether your organization is transitioning from ISO 9001:2008 to the new ISO 9001:2015, or implementing an
INTERNATIONAL STANDARD
 INTERNATIONAL STANDARD ISO 9001 Quality management systems Requirements Systèmes de management de la qualité Exigences Fourth edition 2008-11-15 Reference number ISO 9001:2008(E) ISO 2008 PDF disclaimer
INTERNATIONAL STANDARD ISO 9001 Quality management systems Requirements Systèmes de management de la qualité Exigences Fourth edition 2008-11-15 Reference number ISO 9001:2008(E) ISO 2008 PDF disclaimer
List of Documented Information (Document & Records)
 As per IATF 16949:2016 Clause Type Clause Reference 4.3 Determining the scope of the quality management system 5.1.1.1 Corporate responsibility The only permitted exclusion for this Automotive QMS Standard
As per IATF 16949:2016 Clause Type Clause Reference 4.3 Determining the scope of the quality management system 5.1.1.1 Corporate responsibility The only permitted exclusion for this Automotive QMS Standard
ISO In 2014 Asset Management System. Benny Mok March 2013
 ISO 55000 In 2014 Asset Management System Benny Mok March 2013 WELCOME TO THE SEMINAR! Overview Introduction What is ISO 55000? Development of ISO 55000 Principles of Asset Management Definition ISO 55000
ISO 55000 In 2014 Asset Management System Benny Mok March 2013 WELCOME TO THE SEMINAR! Overview Introduction What is ISO 55000? Development of ISO 55000 Principles of Asset Management Definition ISO 55000
9001:2015. Quality Manual 9001:2015. Engineering Value through Quality and Innovation.
 Quality Manual 9001:2015 9001:2015 Suburban Manufacturing, Inc. encourages comments from customers, suppliers, employees and friends in order to improve our business and its performance. Information contained
Quality Manual 9001:2015 9001:2015 Suburban Manufacturing, Inc. encourages comments from customers, suppliers, employees and friends in order to improve our business and its performance. Information contained
Quality Manual ISSUED JANUARY Approved By: January 12, 2004 (President & Chief Executive Officer)
 Quality Manual ISSUED JANUARY 2004 Approved By: January 12, 2004 (President & Chief Executive Officer) (Date) Quality Policy To be the industrial control industry's most preferred supplier of sensor integration
Quality Manual ISSUED JANUARY 2004 Approved By: January 12, 2004 (President & Chief Executive Officer) (Date) Quality Policy To be the industrial control industry's most preferred supplier of sensor integration
MDSAP Purchasing Process
 MDSAP Purchasing Process The Medical Device Single Audit Program, MDSAP, evaluates companies on their compliance to requirements based on ISO 13485:2016 and national regulations. An MDSAP audit looks at
MDSAP Purchasing Process The Medical Device Single Audit Program, MDSAP, evaluates companies on their compliance to requirements based on ISO 13485:2016 and national regulations. An MDSAP audit looks at
AxiomLogics. Auditing Consulting Staffing Training. One Stop QUALITY
 AxiomLogics Auditing Consulting Staffing Training One Stop QUALITY Auditing INDEPENDENT ASSEMENT OF CONFORMNACE Business dictionary describes Quality Audits as Periodic verification by a competent authority
AxiomLogics Auditing Consulting Staffing Training One Stop QUALITY Auditing INDEPENDENT ASSEMENT OF CONFORMNACE Business dictionary describes Quality Audits as Periodic verification by a competent authority
ISO 9001:2008 Quality Management System QMS Manual
 2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
25 D.L. Martin Drive Mercersburg, PA (717)
 EMS MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 13 January 2017 Kip Heefner Environmental Management Representative Daniel J. Fisher President & CEO D.L.
EMS MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 13 January 2017 Kip Heefner Environmental Management Representative Daniel J. Fisher President & CEO D.L.
IAF Mandatory Document
 IAF-MD 11:2013 IAF Mandatory Document IAF MANDATORY DOCUMENT FOR THE APPLICATION OF ISO/IEC 17021 FOR AUDITS OF INTEGRATED MANAGEMENT SYSTEMS (IAF MD 11: 2013) Page 2 of 12 The (IAF) details criteria for
IAF-MD 11:2013 IAF Mandatory Document IAF MANDATORY DOCUMENT FOR THE APPLICATION OF ISO/IEC 17021 FOR AUDITS OF INTEGRATED MANAGEMENT SYSTEMS (IAF MD 11: 2013) Page 2 of 12 The (IAF) details criteria for
IAQG 9101:2014 (Rev. E)
 IAQG 9101:2014 (Rev. E) Changes Overview Conformity plus Performance Equals Effectiveness Prepared by the IAQG 9101 team 2014-04-08 Presentation Objectives Part One: Provide Overview of 9101:2014 (Rev.
IAQG 9101:2014 (Rev. E) Changes Overview Conformity plus Performance Equals Effectiveness Prepared by the IAQG 9101 team 2014-04-08 Presentation Objectives Part One: Provide Overview of 9101:2014 (Rev.
25 D.L. Martin Drive Mercersburg, PA (717)
 QUALITY MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 14 August 2012 Michael A. White Manager, QA & Engineering D.L. Martin Co. Quality Manual UNCONTROLLED
QUALITY MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 14 August 2012 Michael A. White Manager, QA & Engineering D.L. Martin Co. Quality Manual UNCONTROLLED
Correlation Matrix EN 9100:2016 EN 9100:2009
 Correlation Matrix EN 9100:2016 EN 9100:2009 EN 9100:2016 EN 9100:2009 4 Context of the organization 4 Quality management system 4.2 Understanding the organization and its context Understanding the needs
Correlation Matrix EN 9100:2016 EN 9100:2009 EN 9100:2016 EN 9100:2009 4 Context of the organization 4 Quality management system 4.2 Understanding the organization and its context Understanding the needs
Draft Sample ISO 9001:2015 Into the Future (KIS) October Annex SL (New ISO format for standards)
 INDEX Page Section Description 1 Index 2 0.0 Introduction and Summary 9 1.0 KIS Step 1 11 2.0 KIS Step 2 17 3.0 Annex SL (New ISO format for standards) 21 4.0 ISO Standards, structure and awareness 27
INDEX Page Section Description 1 Index 2 0.0 Introduction and Summary 9 1.0 KIS Step 1 11 2.0 KIS Step 2 17 3.0 Annex SL (New ISO format for standards) 21 4.0 ISO Standards, structure and awareness 27
ADVANCED INTERNAL AUDIT WORKSHOP
 ADVANCED INTERNAL AUDIT WORKSHOP By Paul J. Kunder RABQSA QMS Lead Auditor/Aerospace Industry Experienced Auditor RABQSA Approved Aerospace Auditor Transition Training (AATT) Trainer Vice Chair U.S. Z1A
ADVANCED INTERNAL AUDIT WORKSHOP By Paul J. Kunder RABQSA QMS Lead Auditor/Aerospace Industry Experienced Auditor RABQSA Approved Aerospace Auditor Transition Training (AATT) Trainer Vice Chair U.S. Z1A
Vendor Qualification Survey
 1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
ISO Food Safety Management System Compliance Summary
 ISO 22000 Clause ISO 9001 Clause FSMS Manual Reference Policy / Procedure Title 4. Quality Management System (ISO 9001) 4. Food Safety Quality Management System (ISO 22000) 4.1 General Requirements 4.2
ISO 22000 Clause ISO 9001 Clause FSMS Manual Reference Policy / Procedure Title 4. Quality Management System (ISO 9001) 4. Food Safety Quality Management System (ISO 22000) 4.1 General Requirements 4.2
MCBO B Jun 13. Subj: IMPLEMENTATION AND MANAGEMENT OF INTERNATIONAL ORGANIZATION OF STANDARDS QUALITY MANAGEMENT SYSTEM
 UNITED STATES MARINE CORPS MARINE CORPS BASE 3250 CATLIN AVENUE QUANTICO VA 22134 5001 MCBO 5220.1 B 021 05 Jun 13 MARINE CORPS BASE ORDER 5220.1 From: Commander To: Distribution List Subj: IMPLEMENTATION
UNITED STATES MARINE CORPS MARINE CORPS BASE 3250 CATLIN AVENUE QUANTICO VA 22134 5001 MCBO 5220.1 B 021 05 Jun 13 MARINE CORPS BASE ORDER 5220.1 From: Commander To: Distribution List Subj: IMPLEMENTATION
Clause-byclause. Interpretation. Transitioning to ISO 9001:2015
 We re committed to helping you and your organization understand the updated requirements. This guidance document identifies the steps you should take to achieve compliance to ISO 9001:2015, and more importantly;
We re committed to helping you and your organization understand the updated requirements. This guidance document identifies the steps you should take to achieve compliance to ISO 9001:2015, and more importantly;
ISO Revisions. ISO 9001 Whitepaper. The importance of risk in quality management. Approaching change
 ISO Revisions ISO 9001 Whitepaper The importance of risk in quality management Approaching change Background and overview to the ISO 9001:2015 revision As an International Standard, ISO 9001 is subject
ISO Revisions ISO 9001 Whitepaper The importance of risk in quality management Approaching change Background and overview to the ISO 9001:2015 revision As an International Standard, ISO 9001 is subject
Atlantic Technical Systems, Inc. 415 Headquarters Drive # 2 Millersville, MD USA Office: (410) Fax: (410)
 ISO 9001 Page: 1 of 29 Quality Assurance Manual ISO 9001:2008 Atlantic Technical Systems, Inc. 415 Headquarters Drive # 2 Millersville, MD 21108 USA Office: (410) 507-2779 Fax: (410) 451-9609 APPROVALS
ISO 9001 Page: 1 of 29 Quality Assurance Manual ISO 9001:2008 Atlantic Technical Systems, Inc. 415 Headquarters Drive # 2 Millersville, MD 21108 USA Office: (410) 507-2779 Fax: (410) 451-9609 APPROVALS
ISO 9001:2015. Quality Manual Template.
 www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This quality manual is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This quality manual is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
Making the Transition to ISO 14001:2015 ISO EMS Support Tools
 Making the Transition to ISO 14001:2015 ISO EMS Support Tools Friday 15 th September 2017 12:30-13:30 BST (GMT +1) For more information, view the SC1 website: https://committee.iso.org/home/tc207sc1 Speakers
Making the Transition to ISO 14001:2015 ISO EMS Support Tools Friday 15 th September 2017 12:30-13:30 BST (GMT +1) For more information, view the SC1 website: https://committee.iso.org/home/tc207sc1 Speakers
Outsourcing and ISO 9001:2008
 Outsourcing and ISO 9001:2008 Dan O Leary CBA, CQA, CQE, CRE, SSBB, CIRM President Ombu Enterprises, LLC Dan@OmbuEnterprises.com www.ombuenterprises.com 603-209-0600 1 Speaker Biography Dan O Leary Dan
Outsourcing and ISO 9001:2008 Dan O Leary CBA, CQA, CQE, CRE, SSBB, CIRM President Ombu Enterprises, LLC Dan@OmbuEnterprises.com www.ombuenterprises.com 603-209-0600 1 Speaker Biography Dan O Leary Dan
Document ID: Revision: Date. Approved:
 Document ID: Q&EMSM Standard: ISO 9001 / ISO 14001 Title: Quality and Environmental Management System Manual Approved By: Revision: ED170616 Date Approved: 6/26/17 Quality and Environmental Management
Document ID: Q&EMSM Standard: ISO 9001 / ISO 14001 Title: Quality and Environmental Management System Manual Approved By: Revision: ED170616 Date Approved: 6/26/17 Quality and Environmental Management
IATF 16949:2016 TRANSITION INFORMATION
 IATF 16949:2016 TRANSITION INFORMATION ISO/TS 16949 Overview ISO/TS 16949 Technical Specification for Automotive Quality Management Systems, in conjunction with ISO 9001, defines the quality system requirements
IATF 16949:2016 TRANSITION INFORMATION ISO/TS 16949 Overview ISO/TS 16949 Technical Specification for Automotive Quality Management Systems, in conjunction with ISO 9001, defines the quality system requirements
To support organizations in making a successful transition to IATF Quality Partner has developed several documents to help:
 IATF 16949: 2016 has arrived! Hurry, time to make the transition is short Quality Partner Newsletter November 2016 For More Information Visit www.qualitypartner.co.uk Author: Paul Hardiman Welcome to the
IATF 16949: 2016 has arrived! Hurry, time to make the transition is short Quality Partner Newsletter November 2016 For More Information Visit www.qualitypartner.co.uk Author: Paul Hardiman Welcome to the
ISO /TS 29001:2010 SYSTEMKARAN ADVISER & INFORMATION CENTER SYSTEM KARAN ADVISER & INFORMATION CENTER
 SYSTEM KARAN ADVISER & INFORMATION CENTER PETROLEUM, PETROCHEMICAL AND NATURAL GAS INDUSTRIES -- SECTOR-SPECIFIC QUALITY MANAGEMENT SYSTEMS -- REQUIREMENTS FOR PRODUCT AND SERVICE SUPPLY ORGANIZATIONS
SYSTEM KARAN ADVISER & INFORMATION CENTER PETROLEUM, PETROCHEMICAL AND NATURAL GAS INDUSTRIES -- SECTOR-SPECIFIC QUALITY MANAGEMENT SYSTEMS -- REQUIREMENTS FOR PRODUCT AND SERVICE SUPPLY ORGANIZATIONS
ANCHOR ISO9001:2008 RPR-002 MARINE SERVICES REQUIRED PROCEDURE RECORDS CONTROL
 RECORD CONTROL (4.2.4) Document Control Revision History PAGE REASON FOR CHANGE REV. REVIEWER / AUTHORISED BY: ALL ALL RELEASE DATE: Revision Approval: J.BENTINK Signature: Date: 20/02/17 Revision: B UNCONTROLLED
RECORD CONTROL (4.2.4) Document Control Revision History PAGE REASON FOR CHANGE REV. REVIEWER / AUTHORISED BY: ALL ALL RELEASE DATE: Revision Approval: J.BENTINK Signature: Date: 20/02/17 Revision: B UNCONTROLLED
