Monitoring the Effects of Acid Rain on Freshwater Metal Chemistry. Tyler Ueltschi University of Puget Sound Tacoma, WA
|
|
|
- Clara Nichols
- 6 years ago
- Views:
Transcription
1 Monitoring the Effects of Acid Rain on Freshwater Metal Chemistry Tyler Ueltschi University of Puget Sound Tacoma, WA Advisor: Anne Giblin Marine Biological Laboratory 7 MBL Street, Woods Hole, MA 2543 December 17, 213 Since the Industrial Revolution, circa 19, pollutants have been released into the atmosphere progressively lowering the ph of rain. This acidic deposition has resulted in a decline of the ph of lakes as well. Fortunately due to legislation the ph of rain is now recovering to normal levels, but it is yet to be determine whether lakes will also recover. To check in on the status of 9 lakes around Cape Cod (Duck, Dyer, Gull, Hamblin, Johns, Mares, Miles, Spectacle, and Wakeby) a lake chemistry survey was performed measuring alkalinity, ph, and concentrations of chromium, copper, iron, lead, manganese, and zinc; sediment cores were also taken from 6 of the lakes and analyzed for metal concentrations. In addition, a set of sediment cores from Johns Pond were incubated under 3 conditions, a control, sulfuric acid added, and nitric acid added, to determine the possible affects of acidic deposition on the storage of metals in lake sediments. From the general survey it was found that metal concentrations did not correlate as much with lake boat traffic as was predicted; instead the metal concentrations in the sediments correlated strongly with percent organic matter in the sediments. As for the incubations, the sediments were able to buffer the ph change more quickly than expected resulting in similar conditions between all the cores as time progressed. This buffering resulted in initial metal release from the sediments up to day 4, then very minimal metal release and some metal storage in the sediments through the end of the incubations. These results yielded insight on possible future water-sediment interactions to investigate. Introduction From the start of the Industrial Revolution pollutants have been released in higher and higher quantities into the atmosphere. This has caused a decrease in the ph of rain and an increase in dry deposition of pollutants, resulting in a significant decrease in the ph and/or alkalinity of lakes (Charles 1991). As a result of this and the general smog associated with air pollution, the Clean Air Act was passed. This legislation, which has been successful, has substantially decreased the amount of dry deposition and increased the ph of rain (NADP). 1
2 Sulfate deposited into lakes by acid rain in the form of H 2 SO 4 can be reduced in the sediment to form iron sulfides (e.g., FeS or FeS 2 ) and decrease the levels of acidity and sulfur in the water (Silver et. al. 22). This reduction of available iron oxides in the sediments reduces the lakes ability to store phosphates in the sediment, leading to more primary production in phosphorus limited lake systems (Silver et. al. 22). Now with a decrease in acid rain, we should see a decrease in iron sulfide storage in the sediments and a possible increase of iron in the water column or more iron oxides in the sediments compared to historical data. We also expect to a see an increase in alkalinity in all of the lakes due to an increase in the ph of rain over the last 2 years (NADP). With less sulfur in the water column to be reduced and form complexes with other metals that are more soluble under oxic conditions, such as Zn, Pb and Cu, but near constant rates of metal pollution we may see a decrease in metal storage in the sediments as depth decreases (our proxy for time) (Brick and Moore 1996). These results will be interesting to observe because a decrease in acidic deposition is generally believed to be positively influencing the environment, but it could actually decrease the storage of metals in the sediments and increase their concentrations in the water column. The affects of acid deposition on lake chemistry will be further investigated in a sediment core incubations experiment. In this portion of the research acid will be added to sets of cores in the form of sulfuric acid and nitric acid to explore the effects of added reducing power on metal storage/release along with the effects of added sulfate on metal storage. The sediments, being under anoxic conditions, should exhibit release of iron and manganese in all of the cores; however, if sulfate in the sulfuric acid incubation is reduced along with the iron oxides in the water column there may be storage of iron and 2
3 sulfur as pyrite. In the nitric acid incubation the lack of sulfates to be reduced may result in consistent release of iron and other metals throughout the incubation. The metal release in both of the acid treated cores should be greater than that of the control core due to the added reducing power of the acidic waters. Methods Lake Survey Water samples, 2 L each, were taken from 9 lakes in three regions on Cape Cod. The three regions were a coastal region including Duck, Dyer, and Gull Pond, an inland region including Spectacle, Hamblin, and Wakeby Pond, and an intermediate/falmouth region including Miles, Mares, and Johns Pond (Figure 1.). These water samples were analyzed for nutrients, sulfur isotopes, and alkalinity by Becky Leone, while I measured chromium, copper, iron, manganese, and zinc using an atomic adsorption spectrometer. In addition to the water samples sediment cores were taken from 6 of the lakes, Duck, Dyer, Gull, Spectacle, Miles, and Johns. These cores were taken using a pole corer with a 4 inch diameter core at about 5 meters depth in all but Johns Pond where a 3 inch diameter gravity corer was used to obtain a core at a depth of about 13 meters. The cores were then sectioned approximately every 1 cm throughout the entire depth of the core. These sections were dried and weighed to obtain a bulk density profile. The dried sediments were ground with a mortar and pestle to be used for further analyses. The portion of these analyses performed by Becky Leone included percent sulfur and percent organic matter. To estimate sedimentation rates, I measured the anthropogenically deposited lead throughout the core using a 1% nitric acid digestion. A complete 3
4 digestion of the sediment for measuring total metal in all of the sections was performed using concentrated nitric acid and concentrated hydrochloric acid (described in Forestner and Salomons 198). The metal concentrations were then determined using an Atomic Adsorption Spectrometer. Incubations For the incubation experiment an additional 9 cores were collected from Johns Pond at a depth of 13 meters using a 3 inch diameter gravity corer. All the cores collected contained approximately 2 cm of sediment. In addition to the cores, approximately 2 liters of water was collected. In the lab 5 liters of water was prepared for each of the 3 incubation conditions. For the control condition N2 gas was simply bubbled in the water to promote oxygen release and create anoxic conditions. For the nitric acid treatment 2 µeq of HNO3 was added to generate 2 µeq of acidity and then the water was bubbled with N2 gas. For the sulfuric acid treatment 1 µeq of H2SO4 was added to generate the same 2 µeq of acidity and then the water was bubbled with N2 gas. For each treatment 3 cores were prepared by removing the headwater and replacing it with the appropriate treated water. Initial samples and an initial ph were then taken from the 9 cores and the removed water was replaced with treated water before sealing the cores and placing them in a water bath incubation at approximately 19 C. These initial samples were analyzed for phosphate, sulfate, ammonium, alkalinity, and the metals measured in the lake survey samples. The three cores for each of the treatments were pulled consecutively at 4, 8, and 13 days. The ph of the headwater was measure and then it was analyzed for phosphate, sulfate, ammonium, alkalinity, and the metals 4
5 measured in the lake survey samples. The top 5 cm of the sediment was removed in 1 cm sections, dried, and analyzed for percent organic, percent sulfur, and the metals measured in the lake survey samples. Results and Discussion Lake Survey The dissolved metal concentrations did not vary significantly between the nine lakes except with regard to iron where Gull and Duck had higher levels than the others, and with zinc where Mares had higher levels with respect to the other lakes (Figure 2). Sediment cores were obtained from five lakes and analyzed for metals including lead, copper, chromium, iron, manganese, and zinc. The concentrations of metals in the top layer of sediment varied greatly (Figure 3.). Miles Pond and Johns Pond had the highest concentrations of all metals while Gull and Dyer were much lower than all of the other lakes except with regards to Chromium. Also obtained from analysis of the Gull, Johns, Miles, and Spectacle cores were metal concentrations, percent sulfur, and percent organic matter measured every 1 cm to create a complete profile of percent sulfur, percent organic, and naturally occurring metals (Figures 4-7) as well as anthropogenically deposited metals (Figures 8-11). These profiles, in general, showed a large correlation between percent organic matter and metal concentrations in the sediment. For this reason, the historically accurate lead profiles were only obtained in lakes with high percent organic matter, which included Johns and Miles Pond (Figure 9 and 1). For the Miles Pond profile the lead concentrations dropped to preindustrial revolution levels at a depth of about 12 cm. 5
6 When examining the lake chemistry survey results it was difficult to distinguish between lakes based simply on the metal concentrations in the water column (Figure 1.). It was expected that lakes with higher amounts of boat traffic and larger amounts of recreational use would have higher amounts of metal pollution in the water column. These metals associated with boat use, mostly chromium, copper, and zinc, were not significantly higher in Johns Pond or Gull Pond where recreational use is the highest. This indicates that there either isn t a significant amount of pollution input to the lakes or this pollution is being stored in the sediment. For this reason sediment cores, from the lakes at which they could be obtained, were examined. The sediments, sectioned and acid digested, showed much more variation in metal concentrations near the surface of the sediment (Figure 2.). However, the high traffic lakes, Johns and Gull, did not necessarily have the highest metal concentrations at the surface of their sediments. Miles Pond actually had higher concentrations of zinc than Johns Pond, with similar concentrations of copper in both and higher chromium in Johns Pond than Miles. This originally raised concern because it was believed that Miles was a rather pristine lake with little to no recreation use so it should not have high concentrations of anthropogenically deposited metals. But after conversation with John Hobbie, it was noted that the lake had an ice manufacture along its shore historically and was most likely the source of the zinc and copper pollution. It was also unexpected that Gull Pond would have relatively low concentrations of anthropogenic metals because it has been used for recreation heavily in the past. 6
7 To determine how metal content may have been influenced by other sediment parameters, we plotted the percent organic matter in the top section of the cores against the total concentration of the anthropogenic metals measured (zinc, copper, and chromium) (Figure 12.). By plotting these two against each other and using a linear fit, a correlation of.998 was found indicating that the amount of metal held within the sediment is directly related to the amount of organic matter in the sediment. So to accurately compare metal concentrations in these lakes, cores from deeper parts (presumably higher in organic content) of Dyer, Gull, and Spectacle would need to be collected. Cores with higher organic content throughout the depth of the core would also allow us to develop better depth profiles for the metals which were for the most part inconclusive in terms of dating based on lead deposition in all but Miles Pond. Incubations For the incubation experiment the goal was to determine how acid rain or ph drop in a lake affected metal release or storage in the sediments. Johns Pond cores were used for the experiment because of the high concentrations of metals and the relatively uniform core. The initial spike of acidity to the two sets of treated cores resulted in a drop of the ph from 6.7 to 5. (Figure 13). While this drop in ph is substantial it is possible that a similar drop could occur in nature due to acid rain over time. The water in the incubations, previously bubbled with N2 gas, was presumably anoxic producing good reducing conditions in the cores. After 4 days the first core was pulled from the incubation and analyzed. The ph of all of the cores had converged around at this point so the only difference in the incubations 7
8 after this time point would be the added sulfate or nitrate. This convergence of ph was somewhat unexpected and if the experiment was repeated I would either add more acid at the initial time point to keep the ph lower longer or add smaller amounts throughout the incubations to maintain a more consistent ph. The sulfate concentrations in all of the cores were consistent through day 4 and indicated that there weren t any considerable amounts of sulfur storage in the sediment. At day 4 the sulfuric acid treatment had the largest amount of metal release for all but chromium, which decreased in concentration for all of the treatments (Figure 14 and 15). This was the expected result for the sulfuric acid treatment especially since there was no sulfur storage that could promote iron or other metal storage. For the last two time points the ph and sulfate concentration stayed consistent in all of the cores, while the phosphate concentration increased overall in all of the cores (Figure 13.). It is tough to draw solid conclusions based on the behavior of individual cores, and it was odd that the phosphate showed the highest release in the control core where the least amount of phosphorous should have been released. The metal concentration for these last two time points was also interesting with the sulfuric acid treatment showing a spike in iron at 8 days but a large drop at 13 days without a large corresponding drop in the concentration of sulfate. As for the iron concentrations in the rest of the cores, the control showed the same pattern as the sulfuric treatment (Figure 14.). The nitric acid treatment followed the expected behavior of slow continual release, but it is hard to determine if this is because of discrepancies in the individual cores or actually attributed to continual iron release from the sediments. In all of the cores the manganese concentrations remained 8
9 consistent through day 13 with a slight spike at day 8 in the sulfuric treatment and a slight depression at day 8 in the nitric treatment (Figure 14.). For the rest of the metals over the last two time points, copper remained fairly consistent in all of the cores while zinc had a depression at day 8 in the treated cores then increased at day 13 with the control consistent throughout and chromium increasing slightly over all of the cores at day 13 (Figure 15.). The results for copper and chromium show such low variation that the fluxes are inconclusive. The results for zinc, which show larger amounts of change, are odd because they drop considerably at day 8 but none of the other measure concentrations showed similar behavior that would suggest zinc storage in the sediments so it is hard to attribute this to a result of the treatments. Overall it was difficult to draw conclusions from the incubation experiment with only one core per treatment and time point. In the future I would like to repeat this experiment in a similar fashion with replicate cores, more time points, and longer incubations. Acknowledgements I would like to acknowledge Anne Giblin for her help as my advisor on the project, providing considerable background knowledge to the project as well as supplies and equipment. Also Becky Leone as my co-research assisting with all of the sample collection and taking care of the non-metal analyses. And the course assistants, Fiona Jevon, Alice Carter, Sarah Nalven along with Rich McHorney and the rest of the SES MBL staff/researchers. References Brick, C. M., Moore, J. N Diel Variation of Trace Metals in the Upper Clark Fork River. Montana Environmental Science & Technology, pp
10 Charles D.F. (ed.) Acidic Deposition and Aquatic Ecosystems. Springer- Verlag, NY, pp Forstner, U. and W, Salomons Trace metal analysis on polluted sediments. Environmental Technology Letters 1 pp Gibbs, M. M, Hickey, C. W, Özkundakci, D Sustainability Assessment And Comparison Of Efficacy Of Four P-Inactivation Agents For Managing Internal Phosphorus Loads In Lakes: Sediment Incubations, Hydrobiologia pp Macleod, C. K. et. al Measuring Hypoxia Induced Metal Release From Highly Contaminated Estuarine Sediments During A 4 Day Laboratory Incubation. Experiment Science Of The Total Environment pp National Atmospheric Deposition Program. October 3, Website. Siver, P. A, R, Ricard, R, Goodwin, A. E, Giblin. 22 Estimating Historical Inlake Alkalinity Generation from Sulfate Reduction and its Relationship to Lake Chemistry as Inferred from Algal Microfossils, Journal of Paleolimnology pp
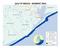
11 Inland: Spectacle, Hamblin, Wakeby Falmouth: Johns, Mares, Miles Coastal: Duck, Gull, Dyer Figure 1. A map of Cape Cod with the lakes surveyed labeled and listed by region.
12 Concentration (mg/l) Copper Zinc Chromium Manganese Iron John Wakeby Spectacle Hamblin Dyer Duck Gull Miles Mares Figure 2. A bar graph of the metal concentrations in the water column from the nine lakes surveyed on Cape Cod.
13 Concentration (µg/g) 1 Zinc Manganese 1 Copper Chromium Iron (mg/g) 1 1 Miles Johns Spectacle Gull Dyer Figure 3. A bar graph of the metal concentrations in the top layer of sediment from the five lakes cored on Cape Cod.
14 Depth (cm) Depth (cm) 2 4 % Organic % Sulfur Manganese (µg/g) Iron (mg/g) Figure 4. The depth profiles of Gull Pond for % organic matter, % sulfur, manganese, and iron.
15 Depth (cm) Depth (cm) 2 4 % Organic % Sulfur Manganese (µg/g) Iron (mg/g) Figure 5. The depth profiles of Johns Pond for % organic matter, % sulfur, manganese, and iron.
16 Depth (cm) Depth (cm) % Organic % Sulfur Manganese (µg/g) Iron (mg/g) Figure 6. The depth profiles of Miles Pond for % organic matter, % sulfur, manganese, and iron.
17 Depth (cm) Depth (cm) % Organic Sulfur % Manganese (µg/g) Iron (mg/g) Figure 7. The depth profiles of Spectacle Pond for % organic matter, % sulfur, manganese, and iron.
18 Depth (cm) Depth (cm) 2 4 Lead (µg/g) Chromium (µg/g) Zinc (µg/g) Copper (µg/g) Figure 8. The depth profiles of Gull Pond for lead, chromium, zinc, and copper.
19 Depth (cm) Depth (cm) 2 4 Lead (µg/g) Chromium (µg/g) Zinc (µg/g) Copper (µg/g) Figure 9. The depth profiles of Johns Pond for lead, chromium, zinc, and copper.
20 Depth (cm) Depth (cm) Lead (µg/g) Chromium (µg/g) Zinc (µg/g) Copper (µg/g) Figure 1. The depth profiles of Miles Pond for lead, chromium, zinc, and copper.
21 Depth (cm) Depth (cm) Lead (µg/g) Chromium (µg/g) Zinc (µg/g) Copper (µg/g) Figure 11. The depth profiles of Spectacle Pond for lead, chromium, zinc, and copper.
22 Total Anthropogenic Metals (µg/g) y = x R² = % Organic Figure 12. A scatter plot of the total anthropogenic metal concentration from the top 1 cm of sediment in each of the lakes cored plotted against the percent organic matter in these sediments with a best fit line.
23 Sulfate (um) Phosphate (um) ph ph Control S N Phosphate Control S N Sulfate Time (Days) Control S N Figure 13. Scatter plots for the incubation experiment for the ph, concentration of phosphate, and concentration of sulfate in the water column at time, 4, 8, and 13 days.
24 Manganese (mg/l) Iron (mg/l) Iron Control Nitric Sulfuric Manganese Control Nitric Sulfuric Time (days) Figure 14. Scatter plots for the incubation experiment for the concentration of iron and manganese in the water column at time, 4, 8, and 13 days.
25 Zinc (mg/l) Chromium (mg/l) Copper (mg/l) Copper Control Nitric Sulfuric Chromium Control Nitric Sulfuric Zinc Time (days) Control Nitric Sulfuric Figure 15. Scatter plots for the incubation experiment for the concentration of copper, chromium, and zinc in the water column at time, 4, 8, and 13 days.
Base Metal and Iron Ore Mining
 Multilateral Investment Guarantee Agency Environmental Guidelines for Base Metal and Iron Ore Mining Industry Description and Practices This document addresses the mining of base metal ores (copper, lead
Multilateral Investment Guarantee Agency Environmental Guidelines for Base Metal and Iron Ore Mining Industry Description and Practices This document addresses the mining of base metal ores (copper, lead
Chapter Two: Cycles of Matter (pages 32-65)
 Biology 20 Chapter 2.1_keyed Chapter Two: Cycles of Matter (pages 32-65) 2.1 The Role of Water in the Cycles of Matter (pages 34 40) Due to its ability to form hydrogen bonds, water has several unique
Biology 20 Chapter 2.1_keyed Chapter Two: Cycles of Matter (pages 32-65) 2.1 The Role of Water in the Cycles of Matter (pages 34 40) Due to its ability to form hydrogen bonds, water has several unique
The Spatial Variability of Total and Bioavailable Metal Concentrations in the Sediments of Mitchell Lake
 The Spatial Variability of Total and Bioavailable Metal Concentrations in the Sediments of Mitchell Lake Summer J. Barber Department of Earth and Environmental Science Environmental Geochemistry Laboratory
The Spatial Variability of Total and Bioavailable Metal Concentrations in the Sediments of Mitchell Lake Summer J. Barber Department of Earth and Environmental Science Environmental Geochemistry Laboratory
Cycles of Ma,er. Lesson Overview. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
General Information on Nitrogen
 General Information on Nitrogen What is nitrogen? Nitrogen was discovered in 1772 by Daniel Rutherford in Scotland Nitrogen gas makes up nearly 80% of the air we breathe Nitrogen gas is not toxic Nitrogen
General Information on Nitrogen What is nitrogen? Nitrogen was discovered in 1772 by Daniel Rutherford in Scotland Nitrogen gas makes up nearly 80% of the air we breathe Nitrogen gas is not toxic Nitrogen
Chapter Two: Cycles of Matter (pages 32-65)
 Chapter Two: Cycles of Matter (pages 32-65) 2.2 Biogeochemical Cycles (pages 42 52) In order to survive and grow, organisms must obtain nutrients that serve as sources of energy or chemical building blocks,
Chapter Two: Cycles of Matter (pages 32-65) 2.2 Biogeochemical Cycles (pages 42 52) In order to survive and grow, organisms must obtain nutrients that serve as sources of energy or chemical building blocks,
TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY. Unit 2 Metals
 TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY Unit 2 Metals Part 2 Metals differ in their reactivity with other chemicals and this influences their uses. Describe observable changes when metals react
TWEED RIVER HIGH SCHOOL 2006 PRELIMINARY CHEMISTRY Unit 2 Metals Part 2 Metals differ in their reactivity with other chemicals and this influences their uses. Describe observable changes when metals react
ecostorm plus 400 Stormwater Filtration System For the removal of sediments, heavy metals and nutrients.
 ecostorm plus 400 Stormwater Filtration System For the removal of sediments, heavy metals and nutrients. Stormwater Filtration is vital to maintaining the quality of our finite water supply. Freytech presents
ecostorm plus 400 Stormwater Filtration System For the removal of sediments, heavy metals and nutrients. Stormwater Filtration is vital to maintaining the quality of our finite water supply. Freytech presents
REPORT NUMBER REPORT DATE SEND TO ISSUE DATE Apr 18, Apr 18, 2017 RECEIVED DATE Apr 05, 2017
 PAGE 1/7 Sample ID: EWA PELLETS Lab Number: 2653281 Date Sampled: 2017-04-04 0850 Carbon nitrogen ratio C/N 6 : 1 0.1 Calculation Auto-2017/04/12 Auto-2017/04/18 Carbon (total) 38.53 % 0.050 ASTM D 5373
PAGE 1/7 Sample ID: EWA PELLETS Lab Number: 2653281 Date Sampled: 2017-04-04 0850 Carbon nitrogen ratio C/N 6 : 1 0.1 Calculation Auto-2017/04/12 Auto-2017/04/18 Carbon (total) 38.53 % 0.050 ASTM D 5373
Water Chemistry. Water 101
 Water Chemistry Water 101 I. Introduction A. Water is not pure Many different kinds of chemicals dissolved in it Ions, organic chemicals, organic matter, particulate matter, and gases can all be in water
Water Chemistry Water 101 I. Introduction A. Water is not pure Many different kinds of chemicals dissolved in it Ions, organic chemicals, organic matter, particulate matter, and gases can all be in water
Ocean Production and CO 2 uptake
 Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Warm Mineral Springs Sampling by Sarasota County
 Warm Mineral Springs Sampling by Sarasota County John Ryan, Kathryn Meaux, Rene Janneman and Jon S. Perry Sarasota County Environmental Services Sarasota, Florida September 11 Warm Mineral Springs is a
Warm Mineral Springs Sampling by Sarasota County John Ryan, Kathryn Meaux, Rene Janneman and Jon S. Perry Sarasota County Environmental Services Sarasota, Florida September 11 Warm Mineral Springs is a
Strong Acid Leachable Metals (SALM) in Soil - Prescriptive
 Strong Acid Leachable Metals () in Soil - Prescriptive Metals Revision Date: Sept 15, 2017 Parameter Analytical Method Introduction Method Summary MDL(s) and EMS Analyte Code(s)* Metals in Soil and Sediment.
Strong Acid Leachable Metals () in Soil - Prescriptive Metals Revision Date: Sept 15, 2017 Parameter Analytical Method Introduction Method Summary MDL(s) and EMS Analyte Code(s)* Metals in Soil and Sediment.
Surface Water Samples: Containers, Preservation and Hold Times Table
 Surface Water Samples: Containers, Preservation and Hold Times Table North Carolina Division of Water Resources, Water Sciences Section Chemistry Laboratory Reference: 40 CFR Part 136.3 Table II Listed
Surface Water Samples: Containers, Preservation and Hold Times Table North Carolina Division of Water Resources, Water Sciences Section Chemistry Laboratory Reference: 40 CFR Part 136.3 Table II Listed
Water Resources on PEI: an overview and brief discussion of challenges
 Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
Water Resources on PEI: an overview and brief discussion of challenges Components: Components and links Atmospheric water Surface water (including glacial water) Groundwater Links: Precipitation (atm(
COOPERATIVE EXTENSION Bringing the University to You
 COOPERATIVE EXTENSION Bringing the University to You Fact Sheet-02-91 SAMPLING AND INTERPRETATION OF LANDSCAPE IRRIGATION WATER Robert Morris and Dr. Dale Devitt TAKING A WATER SAMPLE FOR ANALYSIS Contact
COOPERATIVE EXTENSION Bringing the University to You Fact Sheet-02-91 SAMPLING AND INTERPRETATION OF LANDSCAPE IRRIGATION WATER Robert Morris and Dr. Dale Devitt TAKING A WATER SAMPLE FOR ANALYSIS Contact
Particulate Soil Phosphorus and Eutrophication in Lakes and Streams
 Particulate Soil Phosphorus and Eutrophication in Lakes and Streams Paul R. Bloom Soil, Water, & Climate Department University of Minnesota With contributions by John Moncrief, Carl Rosen and David Mulla
Particulate Soil Phosphorus and Eutrophication in Lakes and Streams Paul R. Bloom Soil, Water, & Climate Department University of Minnesota With contributions by John Moncrief, Carl Rosen and David Mulla
2.2 Nutrient Cycles in Ecosystems. Review How energy flows What is the difference between a food chain, food web, and food pyramid?
 2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
2.2 Nutrient Cycles in Ecosystems Review How energy flows What is the difference between a food chain, food web, and food pyramid? https://www.youtube.com/watch?v=xhr1iebeops https://www.youtube.com/watch?v=alusi_6ol8m
BLACK STURGEON LAKES WATER QUALITY MONITORING
 BLACK STURGEON LAKES WATER QUALITY MONITORING 2017 REPORT Prepared by: Ryan Haines, B.Sc. Biologist and Project Management Kenora Resource Consultants Inc. Site 155, Compartment 14, RR #1 Kenora, ON P9N
BLACK STURGEON LAKES WATER QUALITY MONITORING 2017 REPORT Prepared by: Ryan Haines, B.Sc. Biologist and Project Management Kenora Resource Consultants Inc. Site 155, Compartment 14, RR #1 Kenora, ON P9N
Brian Hite. Research Assistant - CUW Environmental Science - UWT Civil and Environmental Engineering - UW
 Brian Hite Research Assistant - CUW Environmental Science - UWT Civil and Environmental Engineering - UW Acknowledgments I would like to thank: Joel Baker Andy James Kurt Marx Justin Miller-Schulze Sharon
Brian Hite Research Assistant - CUW Environmental Science - UWT Civil and Environmental Engineering - UW Acknowledgments I would like to thank: Joel Baker Andy James Kurt Marx Justin Miller-Schulze Sharon
IMPACT OF INCREASING OXYGEN IN STORMWATER PONDS. Astha Vashisht, WCI Environmental Solutions Inc.*
 IMPACT OF INCREASING OXYGEN IN STORMWATER PONDS Astha Vashisht, WCI Environmental Solutions Inc.* *WCI Environmental Solutions Inc., 1680 Woodward Drive, Suite 203, Ottawa, Ontario, K2C 3R7, avashisht@wcienvironmental.ca
IMPACT OF INCREASING OXYGEN IN STORMWATER PONDS Astha Vashisht, WCI Environmental Solutions Inc.* *WCI Environmental Solutions Inc., 1680 Woodward Drive, Suite 203, Ottawa, Ontario, K2C 3R7, avashisht@wcienvironmental.ca
Cadmium Testing and On Site Calibration for Water Testing Detection Range: ppm
 Document: AND016 04 2014 Cadmium Testing and On Site Calibration for Water Testing Detection Range: 0.1 1.0ppm April, 2014 Edition 3 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of
Document: AND016 04 2014 Cadmium Testing and On Site Calibration for Water Testing Detection Range: 0.1 1.0ppm April, 2014 Edition 3 ANDalyze, Inc., 2012. All rights reserved. Printed in USA. Table of
Hydrology and Water Quality. Water. Water 9/13/2016. Molecular Water a great solvent. Molecular Water
 Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Hydrology and Water Quality Water Molecular Water Exists as an equilibrium But equilibrium altered by what is dissolved in it Water Molecular Water a great solvent In reality, water in the environment
Forrest Bell, FB Environmental Mare Brook Stressor Analysis Methodology
 MEMORANDUM TO: Jared Woolston, Town of Brunswick FROM: SUBJECT: DATE: April 20, 2016 CC: Forrest Bell, FB Environmental Mare Brook Stressor Analysis Methodology Margaret Burns & Sabrina Vivian, FB Environmental;
MEMORANDUM TO: Jared Woolston, Town of Brunswick FROM: SUBJECT: DATE: April 20, 2016 CC: Forrest Bell, FB Environmental Mare Brook Stressor Analysis Methodology Margaret Burns & Sabrina Vivian, FB Environmental;
Chapter Seven: Factors Affecting the Impact of Nutrient Enrichment on the Lower Estuary
 Chapter Seven: Factors Affecting the Impact of Nutrient Enrichment on the Lower Estuary As presented in Chapter Six, the water quality data for the upper stations of the tidal freshwater Potomac Estuary
Chapter Seven: Factors Affecting the Impact of Nutrient Enrichment on the Lower Estuary As presented in Chapter Six, the water quality data for the upper stations of the tidal freshwater Potomac Estuary
Benefits of On-line Monitoring of Carbon, Nitrogen and Phosphorus
 Benefits of On-line Monitoring of Carbon, Nitrogen and Phosphorus New Jersey Water Environment Association Dan Davis Shimadzu Scientific Instruments Benefits of On-Line Monitoring Environment Consumers
Benefits of On-line Monitoring of Carbon, Nitrogen and Phosphorus New Jersey Water Environment Association Dan Davis Shimadzu Scientific Instruments Benefits of On-Line Monitoring Environment Consumers
Chapter 3 Ecosystem Ecology. Tuesday, September 19, 17
 Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
Protecting Our Water Keeping Our Water Healthy
 Protecting Our Water Keeping Our Water Healthy Draw a bubble map about "Why Water is Important in Our Daily Lives". Include at least 5 reasons. Compare answers with your neighbors, noting similarities
Protecting Our Water Keeping Our Water Healthy Draw a bubble map about "Why Water is Important in Our Daily Lives". Include at least 5 reasons. Compare answers with your neighbors, noting similarities
Bathing Water Quality Monitoring Programme
 Bathing Water Quality Monitoring Programme 2005 Report on monitoring of physico-chemical parameters Malta Environment and Planning Authority April 2006 Executive Summary As a Member State of the European
Bathing Water Quality Monitoring Programme 2005 Report on monitoring of physico-chemical parameters Malta Environment and Planning Authority April 2006 Executive Summary As a Member State of the European
Core Analysis with the Tracer
 BRUKER ELEMENTAL Core Analysis with the Tracer Prepared by: Lee Drake, Senior Application Scientist January 23, 2014 BRUKER ELEMENTAL Key Points OBJECTIVE 3 EXPLORATION 3 DECISION MAKING 3 RECLAMATION
BRUKER ELEMENTAL Core Analysis with the Tracer Prepared by: Lee Drake, Senior Application Scientist January 23, 2014 BRUKER ELEMENTAL Key Points OBJECTIVE 3 EXPLORATION 3 DECISION MAKING 3 RECLAMATION
Protecting Utah s Water Resources. Nutrient Issues
 Protecting Utah s Water Resources Nutrient Issues Nutrient Issues Questions What are they? Why are they important? Nationally Locally What are the impact on my community Costs Timing What are Nutrients?
Protecting Utah s Water Resources Nutrient Issues Nutrient Issues Questions What are they? Why are they important? Nationally Locally What are the impact on my community Costs Timing What are Nutrients?
INJECTING LIQUID HOG MANURE FOR IMPROVING CROP YIELDS
 INJECTING LIQUID HOG MANURE FOR IMPROVING CROP YIELDS IMPROVING SOIL QUALITY BY SOIL INJECTING LIQUID HOG MANURE AND ELEMENTAL SULPHUR Mike Grevers and Jeff Schoenau SUMMARY The application of hog manure
INJECTING LIQUID HOG MANURE FOR IMPROVING CROP YIELDS IMPROVING SOIL QUALITY BY SOIL INJECTING LIQUID HOG MANURE AND ELEMENTAL SULPHUR Mike Grevers and Jeff Schoenau SUMMARY The application of hog manure
ELEMENTS OF URBAN ECOLOGY: WATER QUALITY ASSESSMENT OF THE LAKES BUILD UP ON COLENTINA RIVER IN BUCHAREST AREA
 INCD ECOIND INTERNATIONAL SYPOSIU SII 2015 THE ENVIRONENT AND THE INDUSTRY ELEENTS OF URBAN ECOLOGY: WATER QUALITY ASSESSENT OF THE LAKES BUILD UP ON COLENTINA RIVER IN BUCHAREST AREA Bogdan Stanescu,
INCD ECOIND INTERNATIONAL SYPOSIU SII 2015 THE ENVIRONENT AND THE INDUSTRY ELEENTS OF URBAN ECOLOGY: WATER QUALITY ASSESSENT OF THE LAKES BUILD UP ON COLENTINA RIVER IN BUCHAREST AREA Bogdan Stanescu,
LABORATORY TURNAROUND TIMES AND CHARGES
 LABORATORY TURNAROUND TIMES AND CHARGES The enclosed prices reflect a 5 business day turnaround time. There will be a $25.00 minimum charge per report. Same Day Rush 24 Hour Rush 48 Hour Rush 72 Hour Rush
LABORATORY TURNAROUND TIMES AND CHARGES The enclosed prices reflect a 5 business day turnaround time. There will be a $25.00 minimum charge per report. Same Day Rush 24 Hour Rush 48 Hour Rush 72 Hour Rush
EUTROPHICATION. Student Lab Workbook
 EUTROPHICATION Student Lab Workbook THE SCIENTIFIC METHOD 1. Research Background literature research about a topic of interest 2. Identification of a problem Determine a problem (with regards to the topic)
EUTROPHICATION Student Lab Workbook THE SCIENTIFIC METHOD 1. Research Background literature research about a topic of interest 2. Identification of a problem Determine a problem (with regards to the topic)
AUTOMATED WATER ANALYSIS
 APPLICATION NOTE - CFA AUTOMATED WATER ANALYSIS APPLICATIONS OF CONTINUOUS-FLOW ANALYZERS In laboratories around the world, the SEAL Analytical analyzers measure all types of water - quickly, accurately
APPLICATION NOTE - CFA AUTOMATED WATER ANALYSIS APPLICATIONS OF CONTINUOUS-FLOW ANALYZERS In laboratories around the world, the SEAL Analytical analyzers measure all types of water - quickly, accurately
Factsheet: Town of Deep River Water Quality and Stormwater Summary
 79 Elm Street Hartford, CT 06106-5127 www.ct.gov/deep Affirmative Action/Equal Opportunity Employer Factsheet: Town of Deep River Water Quality and Stormwater Summary This document was created for each
79 Elm Street Hartford, CT 06106-5127 www.ct.gov/deep Affirmative Action/Equal Opportunity Employer Factsheet: Town of Deep River Water Quality and Stormwater Summary This document was created for each
5-4 Chemical changes Trilogy
 5-4 Chemical changes Trilogy.0 A student investigated the reaction of sodium carbonate with dilute hydrochloric acid. The student used the apparatus shown in Figure. Figure Sodium carbonate This is the
5-4 Chemical changes Trilogy.0 A student investigated the reaction of sodium carbonate with dilute hydrochloric acid. The student used the apparatus shown in Figure. Figure Sodium carbonate This is the
CANADA BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT
 CANADA BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT WATER QUALITY ASSESSMENT OF KETTLE RIVER AT MIDWAY (1972 2) Prepared by: BWP Consulting Kamloops, B.C. January, 23 Environment Canada Environnement
CANADA BRITISH COLUMBIA WATER QUALITY MONITORING AGREEMENT WATER QUALITY ASSESSMENT OF KETTLE RIVER AT MIDWAY (1972 2) Prepared by: BWP Consulting Kamloops, B.C. January, 23 Environment Canada Environnement
The effects of salt marsh restoration on nutrient retention and nitrogen cycling
 The effects of salt marsh restoration on nutrient retention and nitrogen cycling Katherine Klammer Skidmore College 16 Advisor: Anne E. Giblin The Ecosystems Center at the Marine Biological Laboratory
The effects of salt marsh restoration on nutrient retention and nitrogen cycling Katherine Klammer Skidmore College 16 Advisor: Anne E. Giblin The Ecosystems Center at the Marine Biological Laboratory
Compounds & Reactions Week 1. Writing Formulas & Balancing Equations. Write the chemical formula for each molecular (covalent) compound.
 Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Compounds & Reactions Week 1 Name Writing Formulas & Balancing Equations Write the chemical formula for each ionic compound. 1. Lithium fluoride 2. Copper (II) chloride 3. Manganese (II) oxide 4. Potassium
Ecosystems: Nutrient Cycles
 Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Gravimetric Analysis: Determination of % Sulfur in Fertilizer
 Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
How to Collect Your Water Sample and Interpret the Results for the Poultry Analytical Package
 How to Collect Your Water Sample and Interpret the Results for the Poultry Analytical Package Bradley J. Austin, Josh B. Payne, Susan E. Watkins, Mike Daniels, and Brian E. Haggard Arkansas Water Resources
How to Collect Your Water Sample and Interpret the Results for the Poultry Analytical Package Bradley J. Austin, Josh B. Payne, Susan E. Watkins, Mike Daniels, and Brian E. Haggard Arkansas Water Resources
Scibond SL-23 Polymeric Lubrication System for Tube Drawing
 Scibond SL-23 Polymeric Lubrication System for Tube Drawing I. Introduction: Scibond SL-23 is a novel water-based a polymeric lubrication system. It was developed under National Science Foundation grant
Scibond SL-23 Polymeric Lubrication System for Tube Drawing I. Introduction: Scibond SL-23 is a novel water-based a polymeric lubrication system. It was developed under National Science Foundation grant
NUTRIENT LIMITATION AND TOP-DOWN, BOTTOM-UP CONTROLS ON PHYTOPLANKTON IN MIRROR LAKE
 NUTRIENT LIMITATION AND TOP-DOWN, BOTTOM-UP CONTROLS ON PHYTOPLANKTON IN MIRROR LAKE KRYSTLE BOUCHARD Wells College, Aurora, NY 1326 USA MENTOR SCIENTISTS: DRS. GENE E. LIKENS 1 AND DARREN BADE 2 1 Institute
NUTRIENT LIMITATION AND TOP-DOWN, BOTTOM-UP CONTROLS ON PHYTOPLANKTON IN MIRROR LAKE KRYSTLE BOUCHARD Wells College, Aurora, NY 1326 USA MENTOR SCIENTISTS: DRS. GENE E. LIKENS 1 AND DARREN BADE 2 1 Institute
Nitrate and Phosphorous Levels in Selected Surface Water Sites in Southern Ontario
 Nitrate and Phosphorous Levels in Selected Surface Water Sites in Southern Ontario 4-4 By: Ron Fleming P.Eng and Heather Fraser Ridgetown College-University of Guelph August,. Objectives Using existing
Nitrate and Phosphorous Levels in Selected Surface Water Sites in Southern Ontario 4-4 By: Ron Fleming P.Eng and Heather Fraser Ridgetown College-University of Guelph August,. Objectives Using existing
Threats to Forest Ecosystem Health Activities together influence ecosystem structure & function
 Threats to Forest Ecosystem Health Activities together influence ecosystem structure & function introduced species poor management air pollution global warming habitat fragmentation American Chestnut From
Threats to Forest Ecosystem Health Activities together influence ecosystem structure & function introduced species poor management air pollution global warming habitat fragmentation American Chestnut From
EU Water Analysis Using the Thermo Scientific icap 7400 ICP-OES Duo
 EU Water Analysis Using the Thermo Scientific icap 7400 ICP-OES Duo James Hannan, Applications Chemist, Thermo Fisher Scientific, Cambridge, UK Application Note 43171 Key Words Environmental, EU, waste,
EU Water Analysis Using the Thermo Scientific icap 7400 ICP-OES Duo James Hannan, Applications Chemist, Thermo Fisher Scientific, Cambridge, UK Application Note 43171 Key Words Environmental, EU, waste,
Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical, & biological processes
 OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
KKB Micro Testing Labs Pvt. Ltd., , 2 nd Floor, Tarun Plaza, NFC Main Road, Krishna Nagar Colony, Moula Ali, Hyderabad, Telangana
 Last Amended on - Page 1 of 7 I. AIR, GASES & ATMOSPHERE 1. Ambient Air Monitoring RSPM ( PM 10) IS 5182 (Part 23): 2006 (RA ) 5 µg/m 3 to 750 µg/m 3 PM 2.5 SOP 11 dated 17.11.2014 12.5 µg/m 3 to 500 µg/m
Last Amended on - Page 1 of 7 I. AIR, GASES & ATMOSPHERE 1. Ambient Air Monitoring RSPM ( PM 10) IS 5182 (Part 23): 2006 (RA ) 5 µg/m 3 to 750 µg/m 3 PM 2.5 SOP 11 dated 17.11.2014 12.5 µg/m 3 to 500 µg/m
Partner: Cathy 22 March Separation and Qualitative Determination of Cations and Anions
 Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
Partner: Cathy 22 March 2012 Separation and Qualitative Determination of Cations and Anions Purpose: The purpose of this lab is to identify the cations and anions components in the unknown solution. This
A TEACHER-DIRECTED, GUIDED DISCOVERY ACTIVITY FOR ADVANCED PHYSICAL SCIENCE STUDENTS
 A TEACHER-DIRECTED, GUIDED DISCOVERY ACTIVITY FOR ADVANCED PHYSICAL SCIENCE STUDENTS 1 2 FEATURES OF THIS ACTIVITY Problem solving Cooperative learning Requires student reasoning Requires student initiative
A TEACHER-DIRECTED, GUIDED DISCOVERY ACTIVITY FOR ADVANCED PHYSICAL SCIENCE STUDENTS 1 2 FEATURES OF THIS ACTIVITY Problem solving Cooperative learning Requires student reasoning Requires student initiative
VI. WATER QUALITY MODELING
 VI. WATER QUALITY MODELING As was mentioned previously in Chapter V, the Hamblin Pond/Jehu Pond and Quashnet River sub-systems of Waquoit Bay were a part of the larger hydrodynamic model of the Waquoit
VI. WATER QUALITY MODELING As was mentioned previously in Chapter V, the Hamblin Pond/Jehu Pond and Quashnet River sub-systems of Waquoit Bay were a part of the larger hydrodynamic model of the Waquoit
Cultural accelerated by anthropogenic activities
 EUTROPHICATION IMPLICATIONS OF N & P Intent of this lecture? Link our discussions of terrestrial N & P dynamics with its influences on receiving water bodies How the relative amounts of N & P can influence
EUTROPHICATION IMPLICATIONS OF N & P Intent of this lecture? Link our discussions of terrestrial N & P dynamics with its influences on receiving water bodies How the relative amounts of N & P can influence
MARINE POLLUTION DEGRADATION MITIGATION MANAGEMENT IS ESSENTIAL FOR IMPROVING MARINE ENVIRONMENT
 MARINE POLLUTION DEGRADATION MITIGATION MANAGEMENT IS ESSENTIAL FOR IMPROVING MARINE ENVIRONMENT The health of the world s oceans and marine life is degrading rapidly as a result of excess human activities.
MARINE POLLUTION DEGRADATION MITIGATION MANAGEMENT IS ESSENTIAL FOR IMPROVING MARINE ENVIRONMENT The health of the world s oceans and marine life is degrading rapidly as a result of excess human activities.
How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package
 How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package Bradley J. Austin, Dirk Philipp, Mike Daniels, and Brian E. Haggard Arkansas Water Resources Center University
How to Collect Your Water Sample and Interpret the Results for the Livestock Analytical Package Bradley J. Austin, Dirk Philipp, Mike Daniels, and Brian E. Haggard Arkansas Water Resources Center University
Riffle Beetles to Riparian Buffers
 We care about WateR. it S What We do. Riffle Beetles to Riparian Buffers Exploring Methods of Assessing Surface Water Quality Grade level: 7-12 objective: Students will evaluate the quality of two water
We care about WateR. it S What We do. Riffle Beetles to Riparian Buffers Exploring Methods of Assessing Surface Water Quality Grade level: 7-12 objective: Students will evaluate the quality of two water
Climate: describes the average condition, including temperature and precipitation, over long periods in a given area
 Ch. 6 - Biomes Section 6.1: Defining Biomes Biome: a group of ecosystems that share similar biotic and abiotic conditions, large region characterized by a specific type of climate, plants, and animals
Ch. 6 - Biomes Section 6.1: Defining Biomes Biome: a group of ecosystems that share similar biotic and abiotic conditions, large region characterized by a specific type of climate, plants, and animals
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Loropetalum Screening for High Lime Induced Abnormalities
 Loropetalum Screening for High Lime Induced Abnormalities Nature of Work: Dr. James T. Midcap Department of Horticulture The University of Georgia Athens, GA 30602 Sizzling Pink Loropetalum has developed
Loropetalum Screening for High Lime Induced Abnormalities Nature of Work: Dr. James T. Midcap Department of Horticulture The University of Georgia Athens, GA 30602 Sizzling Pink Loropetalum has developed
Application of the AGF (Anoxic Gas Flotation) Process
 Application of the AGF (Anoxic Gas Flotation) Process Dennis A. Burke Environmental Energy Company, 6007 Hill Road NE, Olympia, WA 98516 USA (E-mail: dennis@makingenergy.com http//www.makingenergy.com)
Application of the AGF (Anoxic Gas Flotation) Process Dennis A. Burke Environmental Energy Company, 6007 Hill Road NE, Olympia, WA 98516 USA (E-mail: dennis@makingenergy.com http//www.makingenergy.com)
Consequences of Nitrogen Deposition to Rocky Mountain National Park
 Consequences of Nitrogen Deposition to Rocky Mountain National Park Jill S. Baron, US Geological Survey M.Hartman, D.S.Ojima, K. Nydick, H.M. Rueth B.Moraska Lafrancois, A.P. Wolfe, J. Botte, W.D. Bowman
Consequences of Nitrogen Deposition to Rocky Mountain National Park Jill S. Baron, US Geological Survey M.Hartman, D.S.Ojima, K. Nydick, H.M. Rueth B.Moraska Lafrancois, A.P. Wolfe, J. Botte, W.D. Bowman
SCOPE OF ACCREDITATION TO ISO GUIDE 34:2009
 SCOPE OF ACCREDITATION TO ISO GUIDE 34:2009 SCP SCIENCE 21800 Clark Graham Baie d'urfe, Quebec H9X 4B6 CANADA David Smith Phone: 514 457 0701 dsmith@scpscience.com REFERENCE MATERIAL PRODUCER Valid To:
SCOPE OF ACCREDITATION TO ISO GUIDE 34:2009 SCP SCIENCE 21800 Clark Graham Baie d'urfe, Quebec H9X 4B6 CANADA David Smith Phone: 514 457 0701 dsmith@scpscience.com REFERENCE MATERIAL PRODUCER Valid To:
Palm Beach County Board of County Commissioners Workshop October 29, Yongshan Wan, Ph.D. P.E. Section Leader Coastal Ecosystems Section
 S O U T H F L O R I D A W A T E R M A N A G E M E N T D I S T R I C T Palm Beach County Board of County Commissioners Workshop October 29, 2013 Yongshan Wan, Ph.D. P.E. Section Leader Coastal Ecosystems
S O U T H F L O R I D A W A T E R M A N A G E M E N T D I S T R I C T Palm Beach County Board of County Commissioners Workshop October 29, 2013 Yongshan Wan, Ph.D. P.E. Section Leader Coastal Ecosystems
ELECTRONIC GRADE SULFAMATE NICKEL Document ID: EFM1409
 ELECTRONIC GRADE SULFAMATE NICKEL Document ID: EFM1409 E-Form is an electronic grade nickel sulfamate electroforming concentrate designed and manufactured specifically for use with microlithography. DisChem
ELECTRONIC GRADE SULFAMATE NICKEL Document ID: EFM1409 E-Form is an electronic grade nickel sulfamate electroforming concentrate designed and manufactured specifically for use with microlithography. DisChem
GULF OF MEXICO - SEGMENT 2501
 GULF OF MEXICO - SEGMENT 2501 GULF OF MEXICO - SEGMENT 2501 LAND COVER BACTERIA CHLOROPHYLL A Impairment Concern No Impairments or Concerns GULF OF MEXICO - SEGMENT 2501 OTHER IMPAIRMENTS Bays & Estuaries
GULF OF MEXICO - SEGMENT 2501 GULF OF MEXICO - SEGMENT 2501 LAND COVER BACTERIA CHLOROPHYLL A Impairment Concern No Impairments or Concerns GULF OF MEXICO - SEGMENT 2501 OTHER IMPAIRMENTS Bays & Estuaries
Read: Case Study: America s First River : A Success Story Summarize the story of the Hudson River and PCB s:
 Botkin & Keller: Environmental Science: Earth as a Living Planet- 8th Ed. APES- Chapter #19- Water Pollution and Treatment- Guided Reading Name: Brandon Tran Learning Objectives: Degradation of our surface-water
Botkin & Keller: Environmental Science: Earth as a Living Planet- 8th Ed. APES- Chapter #19- Water Pollution and Treatment- Guided Reading Name: Brandon Tran Learning Objectives: Degradation of our surface-water
What to do with extra electrons how combating eutrophication may affect mineralization pathways
 What to do with extra electrons how combating eutrophication may affect mineralization pathways Jouni Lehtoranta Finnish Environment Institute Marine Research Centre Sun e - Energy goes through the system
What to do with extra electrons how combating eutrophication may affect mineralization pathways Jouni Lehtoranta Finnish Environment Institute Marine Research Centre Sun e - Energy goes through the system
CHEMICAL MONITORING & MANAGEMENT LESSON 6: WATER QUALITY 1 SAMPLE RESOURCES
 YEAR 2 CHEM ISTRY CHEMICAL MONITORING & MANAGEMENT SAMPLE RESOURCES 300 008 008 www.matrix.edu.auu YEAR 2 CHEMISTRY. Water Quality Students perform first hand investigations to use qualitative and quantitative
YEAR 2 CHEM ISTRY CHEMICAL MONITORING & MANAGEMENT SAMPLE RESOURCES 300 008 008 www.matrix.edu.auu YEAR 2 CHEMISTRY. Water Quality Students perform first hand investigations to use qualitative and quantitative
Results of Water Quality Measurements in Messer Pond Bob Crane, Messer Pond Protective Association (MPPA) Board
 Results of Water Quality Measurements in Messer Pond Bob Crane, Messer Pond Protective Association (MPPA) Board The collection of water samples for the assessment of water quality in Messer Pond, New London,
Results of Water Quality Measurements in Messer Pond Bob Crane, Messer Pond Protective Association (MPPA) Board The collection of water samples for the assessment of water quality in Messer Pond, New London,
IBI Certified Biochar Production in California
 IBI Certified Biochar Production in California Fifth North American Biochar Symposium Presented on August 24, 2016 by Dr. Alan Propp, PE Syntech Bioenergy www.syntechbioenergy.com/www.gocpc.com SynTech
IBI Certified Biochar Production in California Fifth North American Biochar Symposium Presented on August 24, 2016 by Dr. Alan Propp, PE Syntech Bioenergy www.syntechbioenergy.com/www.gocpc.com SynTech
National Food Safety Standard
 National standard of People s Republic of China GB 5413.21 2010 National Food Safety Standard Determination of calcium, iron, zinc, sodium, potassium, magnesium, copper and manganese in foods for infants
National standard of People s Republic of China GB 5413.21 2010 National Food Safety Standard Determination of calcium, iron, zinc, sodium, potassium, magnesium, copper and manganese in foods for infants
Contaminated Extraction Pit treated to allow dewatering for continuation of mining works
 Contaminated Extraction Pit treated to allow dewatering for continuation of mining works Name Customer name withheld Site Location South East Queensland Site Problem ph, Acidity and Dissolved Metals Water
Contaminated Extraction Pit treated to allow dewatering for continuation of mining works Name Customer name withheld Site Location South East Queensland Site Problem ph, Acidity and Dissolved Metals Water
Discipline Chemical Testing Issue Date Certificate Number T-3553 Valid Until Last Amended on - Page 1 of 9
 Last Amended on - Page 1 of 9 I. ORES & MINERALS 1. Iron Ore Total Iron IS 1493: 1981 (RA 2006) 10 % to 70 % Silicon IS 1493: 1981 (RA 2006) 0.5 % to 25 % Aluminum IS 1493: 1981 (RA 2006) 0.5 % to 20 %
Last Amended on - Page 1 of 9 I. ORES & MINERALS 1. Iron Ore Total Iron IS 1493: 1981 (RA 2006) 10 % to 70 % Silicon IS 1493: 1981 (RA 2006) 0.5 % to 25 % Aluminum IS 1493: 1981 (RA 2006) 0.5 % to 20 %
WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein!
 Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
COPPER PRECIPITATION AND CYANIDE RECOVERY PILOT TESTING FOR THE NEWMONT YANACOCHA PROJECT
 COPPER PRECIPITATION AND CYANIDE RECOVERY PILOT TESTING FOR THE NEWMONT YANACOCHA PROJECT Michael Botz, Elbow Creek Engineering, Billings, MT Sevket Acar, Newmont Mining Corporation, Englewood, CO Introduction
COPPER PRECIPITATION AND CYANIDE RECOVERY PILOT TESTING FOR THE NEWMONT YANACOCHA PROJECT Michael Botz, Elbow Creek Engineering, Billings, MT Sevket Acar, Newmont Mining Corporation, Englewood, CO Introduction
Preparing for Nutrient Removal at Your Treatment Plant
 Summer Seminar Emerging Issues in the Water/Wastewater Industry Preparing for Nutrient Removal at Your Treatment Plant Rajendra P. Bhattarai, P.E., BCEE Austin Water Utility Ana J. Peña-Tijerina, Ph.D.,
Summer Seminar Emerging Issues in the Water/Wastewater Industry Preparing for Nutrient Removal at Your Treatment Plant Rajendra P. Bhattarai, P.E., BCEE Austin Water Utility Ana J. Peña-Tijerina, Ph.D.,
Study of Metals Distribution between Water and Sediment in the Smolnik Creek (Slovakia) Contaminated by Acid Mine Drainage
 A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 28, 2012 Guest Editor: Carlo Merli Copyright 2012, AIDIC Servizi S.r.l., ISBN 978-88-95608-19-8; ISSN 1974-9791 The Italian Association of Chemical
A publication of CHEMICAL ENGINEERING TRANSACTIONS VOL. 28, 2012 Guest Editor: Carlo Merli Copyright 2012, AIDIC Servizi S.r.l., ISBN 978-88-95608-19-8; ISSN 1974-9791 The Italian Association of Chemical
MURPHY DRAIN CATCHMENT
 The RVCA produces individual reports for 16 catchments in the Lower Rideau subwatershed. Using data collected and analysed by the RVCA through its watershed monitoring and land cover classification programs,
The RVCA produces individual reports for 16 catchments in the Lower Rideau subwatershed. Using data collected and analysed by the RVCA through its watershed monitoring and land cover classification programs,
Evaluation copy. Total Dissolved Solids. Computer INTRODUCTION
 Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Chapter 2 9/15/2015. Chapter 2. Penny Boat. 2.1 The Role of Water in Cycles of Matter
 Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
HYPOXIA Definition: ~63 µm; 2 mg l -1 ; 1.4 ml l -1 ; 30 %
 HYPOXIA Definition: ~63 µm; 2 mg l -1 ; 1.4 ml l -1 ; 30 % Consequences of hypoxia Reduce habitat for living resources Change biogeochemical processes P released from sediments Denitrification reduced
HYPOXIA Definition: ~63 µm; 2 mg l -1 ; 1.4 ml l -1 ; 30 % Consequences of hypoxia Reduce habitat for living resources Change biogeochemical processes P released from sediments Denitrification reduced
EUTROPHICATION. Teacher s Manual
 EUTROPHICATION Teacher s Manual Preface The following is a, hands on, and inquiry based lesson plan developed by COSEE Mid-Atlantic for teaching eutrophication. The National Education Science Standards
EUTROPHICATION Teacher s Manual Preface The following is a, hands on, and inquiry based lesson plan developed by COSEE Mid-Atlantic for teaching eutrophication. The National Education Science Standards
Boy Lake CASS COUNTY
 Boy Lake 11-143- CASS COUNTY Summary Boy Lake is located near Remer, MN in Cass County. It covers 3,452 acres, which places it in the upper 1% of lakes in Minnesota in terms of size. Boy Lake has two main
Boy Lake 11-143- CASS COUNTY Summary Boy Lake is located near Remer, MN in Cass County. It covers 3,452 acres, which places it in the upper 1% of lakes in Minnesota in terms of size. Boy Lake has two main
Proposal. What is the issue? Why is it important? Key findings and draft recommendations
 Proposal Minnesota Pollution Control Agency, March 24, 2015 Protecting wild rice from excess sulfate What is the issue? Wild rice growth is negatively affected by excess sulfate, a naturally occurring
Proposal Minnesota Pollution Control Agency, March 24, 2015 Protecting wild rice from excess sulfate What is the issue? Wild rice growth is negatively affected by excess sulfate, a naturally occurring
Levels of zinc, copper, iron and manganese in soils of abandoned mine pits around the Tarkwa gold mining area of Ghana
 Available online at www.pelagiaresearchlibrary.com Advances in Applied Science Research, 2011, 2 (1): 280-288 ISSN: 0976-8610 CODEN (USA): AASRFC Levels of zinc, copper, iron and manganese in soils of
Available online at www.pelagiaresearchlibrary.com Advances in Applied Science Research, 2011, 2 (1): 280-288 ISSN: 0976-8610 CODEN (USA): AASRFC Levels of zinc, copper, iron and manganese in soils of
Modeling Surface Water Contamination
 Modeling Surface Water Contamination One of the resources required for an ecosystem to function is an available source of fresh water This is quite true for human settlements as well: If you examine the
Modeling Surface Water Contamination One of the resources required for an ecosystem to function is an available source of fresh water This is quite true for human settlements as well: If you examine the
Formula & Equation Writing
 Formula & Equation Writing Book 2 H H Al Al H Al(H) 3 H Ionic Equations Ionic Formulae Balanced Equations Formula Equations Word Equations Transition Metals Using Brackets Awkward Customers More than 2
Formula & Equation Writing Book 2 H H Al Al H Al(H) 3 H Ionic Equations Ionic Formulae Balanced Equations Formula Equations Word Equations Transition Metals Using Brackets Awkward Customers More than 2
Comparative Analysis of Minnesota Lakes Treated with Alum to Inform Spring Lake Treatment
 Comparative Analysis of Minnesota Lakes Treated with Alum to Inform Spring Lake Treatment Prepared for the Prior Lake Spring Lake Watershed District (PLSLWD) April 23, 2013 Comparative Analysis of Minnesota
Comparative Analysis of Minnesota Lakes Treated with Alum to Inform Spring Lake Treatment Prepared for the Prior Lake Spring Lake Watershed District (PLSLWD) April 23, 2013 Comparative Analysis of Minnesota
WATER QUALITY STATUS AND TREND MONITORING SYSTEM FOR THE CLARK FORK-PEND OREILLE WATERSHED
 WATER QUALITY STATUS AND TREND MONITORING SYSTEM FOR THE CLARK FORK-PEND OREILLE WATERSHED Summary Monitoring Report 3 Prepared for: TRI-STATE WATER QUALITY COUNCIL Diane Williams, Executive Director 37
WATER QUALITY STATUS AND TREND MONITORING SYSTEM FOR THE CLARK FORK-PEND OREILLE WATERSHED Summary Monitoring Report 3 Prepared for: TRI-STATE WATER QUALITY COUNCIL Diane Williams, Executive Director 37
Kapil Arora, Carl Pederson, Dr. Matt Helmers, and Dr. Ramesh Kanwar. DATE SUBMITTED: October 23, INDUSTRY SUMMARY
 TITLE: Evaluating Nutrient (nitrogen and ortho-phosphate) Export with Subsurface Drainage Water from Spring Applied Swine Manure to Soybean Planted Micro-watersheds - NPB #12-117 INVESTIGATORS: INSTITUTION:
TITLE: Evaluating Nutrient (nitrogen and ortho-phosphate) Export with Subsurface Drainage Water from Spring Applied Swine Manure to Soybean Planted Micro-watersheds - NPB #12-117 INVESTIGATORS: INSTITUTION:
Stabilising inorganic contaminants in soils: Considerations for the use of smart additives
 STARNET Conference 12. 13.4.25 Cambridge, England UK Stabilising inorganic contaminants in soils: Considerations for the use of smart additives H. Weigand, C. Gemeinhardt & C. Marb Funded by Background
STARNET Conference 12. 13.4.25 Cambridge, England UK Stabilising inorganic contaminants in soils: Considerations for the use of smart additives H. Weigand, C. Gemeinhardt & C. Marb Funded by Background
What s Happening in Lake Whatcom?
 What s Happening in Lake Whatcom? Dr. Robin A. Matthews, Director Institute for Watershed Studies Huxley College of the Environment Western Washington University June 6, 2011 Site 2 Basin 2 Lake Whatcom
What s Happening in Lake Whatcom? Dr. Robin A. Matthews, Director Institute for Watershed Studies Huxley College of the Environment Western Washington University June 6, 2011 Site 2 Basin 2 Lake Whatcom
85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO 3
 Metal and metal reactivity / Section 2 / Sect2pp.doc / S. W. Tse / P.1 85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO
Metal and metal reactivity / Section 2 / Sect2pp.doc / S. W. Tse / P.1 85 Q.51 Which of the following carbonates would give the metal when heated with carbon? (1) MgCO 3 (2) PbCO 3 (3) K 2 CO 3 (4) CuCO
Wetland Vegetation Effectiveness in Stormwater Treatment. Nazneen Hoque
 Wetland Vegetation Effectiveness in Stormwater Treatment Nazneen Hoque Abstract The use of natural or constructed wetlands for controlling stormwater contaminants is a relatively new and increasingly popular
Wetland Vegetation Effectiveness in Stormwater Treatment Nazneen Hoque Abstract The use of natural or constructed wetlands for controlling stormwater contaminants is a relatively new and increasingly popular
Nitrates are essential for plant growth
 THE NITROGEN CYCLE Nitrates are essential for plant growth Plant protein Root uptake Nitrate NO 3 Nitrates are recycled via microbes Animal protein Soil organic nitrogen Ammonification Ammonium NH 4 +
THE NITROGEN CYCLE Nitrates are essential for plant growth Plant protein Root uptake Nitrate NO 3 Nitrates are recycled via microbes Animal protein Soil organic nitrogen Ammonification Ammonium NH 4 +
1. Scaling. H.O.: H-5/21, KRISHNA NAGAR, DELHI Tel.: , Fax:
 Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Boiler Water Problems and Its Causes Water is the essential medium for steam generation. Conditioning it properly can increase the efficiency of boiler and as well as extend the boiler s life. Treating
Phosphorus (P) Lead (Pb) ~125 Years Remaining 2/19/2016
 /9/06 Taking the Next Step: Exploration of Naturally Produced, Organic Compounds to Alter the Mobility and Lability of Soil Elements Joseph (Jay) Weeks Jr. and Ganga M. Hettiarachchi (Akafia et al. 0)
/9/06 Taking the Next Step: Exploration of Naturally Produced, Organic Compounds to Alter the Mobility and Lability of Soil Elements Joseph (Jay) Weeks Jr. and Ganga M. Hettiarachchi (Akafia et al. 0)
Physicochemical Characteristics and Heavy Metal Levels in Water Samples from Five River Systems in Delta State, Nigeria
 JASEM ISSN 1119-8362 All rights reserved Full-text Available Online at www.bioline.org.br/ja J. Appl. Sci. Environ. Manage. March, 2010 Vol. 14(1) 83-87 Physicochemical Characteristics and Heavy Metal
JASEM ISSN 1119-8362 All rights reserved Full-text Available Online at www.bioline.org.br/ja J. Appl. Sci. Environ. Manage. March, 2010 Vol. 14(1) 83-87 Physicochemical Characteristics and Heavy Metal
