STRESSED AND STRUNG OUT: THE DEVELOPMENT AND TESTING OF AN IN UNDER SHEAR STRESS. Thesis. Submitted to. The School of Engineering of the
|
|
|
- Shavonne Meagan Taylor
- 6 years ago
- Views:
Transcription
1 STRESSED AND STRUNG OUT: THE DEVELOPMENT AND TESTING OF AN IN VIVO LIKE BENCH-TOP BIOREACTOR FOR THE OBSERVATION OF CELLS UNDER SHEAR STRESS Thesis Submitted to The School of Engineering of the UNIVERSITY OF DAYTON In Partial Fulfillment of the Requirements for The Degree of Master of Science in Bioengineering By Andrea Marie Chambers UNIVERSITY OF DAYTON Dayton, Ohio August, 2015
2 STRESSED AND STRUNG OUT: THE DEVELOPMENT AND TESTING OF AN IN VIVO LIKE BENCH-TOP BIOREACTOR FOR THE OBSERVATION OF CELLS UNDER SHEAR STRESS Name: Chambers, Andrea Marie APPROVED BY: Robert J. Wilkens, Ph.D., P.E. Advisory Committee Chair Member Professor, Director of Bioengineering Department of Chemical and Materials Engineering Carissa M. Krane, Ph.D. Advisory Committee Chair Member Associate Professor Department of Biology Kristen Comfort, Ph.D. Committee Chair Member Assistant Professor Department of Chemical and Materials Engineering John G. Weber, Ph.D. Associate Dean School of Engineering Eddy M. Rojas, Ph.D., M.A., P.E. Dean School of Engineering ii
3 ABSTRACT STRESSED AND STRUNG OUT: THE DEVELOPMENT AND TESTING OF AN IN VIVO LIKE BENCH-TOP BIOREACTOR FOR THE OBSERVATION OF CELLS UNDER SHEAR STRESS Name: Chambers, Andrea Marie University of Dayton Advisors: Dr. Robert Wilkens and Dr. Carissa Krane Bioreactor systems used for tissue engineering applications are an essential component of understanding the development of new tissues and studying the biochemical interactions between cells and their environment. A bioreactor is typically designed to mimic physiological, environmental, and mechanical stimuli that occur in vivo, and bioreactors are generally created for a specific application, such as for studying 3-dimensional tissues or dynamic fluid flow in 1-dimensional cell monolayers. The leading cause of death in the United States is coronary artery disease, which is treated with bypass graft surgery using a left internal mammary artery or human saphenous vein as the graft. Since human saphenous vein grafts often fail, investigating vascular function as a whole will help to understand more about the method of graft failure. A bioreactor system to study vascular function was successfully developed using the application of endothelial cells under shear stress in a microfluidic slide. The iii
4 temperature control and diffusion rate of CO 2 were recorded inside the bioreactor to confirm the system could stay within a temperature range of 37ºC +/- 0.5ºC and a CO 2 concentration between 56,000 ppm and 45,000 ppm. Also, a physiological level of shear stress was determined to be feasible with the peristaltic pump. The performance characteristics of the bioreactor were analyzed, and the apparatus was determined to be successful in generating physiological relevant conditions. Then, human umbilical vein endothelial cells were exposed to both static conditions and venous shear stress conditions for up to four days in an IBIDI microfluidic chamber. The cell morphology, alignment, and elongation were also evaluated. The cells stayed viable during the duration of all of the dynamic flow experiments, and the cells showed evidence of cell division. The cells were also more aligned and elongated towards the direction of flow for the 48 a nd 72 hour flow experiments compared to the 48 and 72 hour static experiments (P-value < 0.05). The 96 hour flow experiment cells were also more aligned than the cells exposed to static conditions (P-value < 0.05). The 48 hour, 72 hour, and 96 hour dynamic flow experiments had a statistically significant difference in cell alignment compared to the 24 hour flow test, and the 72 hour dynamic flow experiment also had a statistically significant difference in cell alignment compared to the 48 and 96 hour flow experiments (P-value < 0.05). The 72 hour flow experiment was more elongated than the 24, 48, and 96 hour flow experiments (P-value < 0.05). Overall, the lab setup and bioreactor system yielded desirable results and provided a system that was fully capable of studying endothelial cells under venous shear stress conditions for studies up to 4 days. iv
5 Dedicated to my family and friends v
6 ACKNOWLEDGEMENTS First, I would like to thank my advisors, Dr. Robert Wilkens and Dr. Carissa Krane for providing me the opportunity to work on such a challenging and stimulating project. I would also like to thank my other committee member, Dr. Kristen Comfort for her guidance and support, not only with my thesis, but also as a professor for many of my graduate school classes. I am grateful to Mike Green who helped with the beginning stages of assembling my bioreactor and for the equipment I was able to use, and Justin DelMar for using his tools during the bioreactor construction. I also would like to thank Dr. John Sternick for his valuable troubleshooting advice and support. I also owe a debt of gratitude to both Kyle McGrail and Emily Breitner who provided assistance throughout my thesis. I am indebted to Street Barnett for his tremendous support and assistance through almost the entire process of completing my thesis. Lastly, I am thankful for the continuous encouragement from both of my parents and my brother, and for them listening to me whenever I needed support. vi
7 TABLE OF CONTENTS ABSTRACT... iii DEDICATION.v ACKNOWLEDGEMENTS... vi LIST OF FIGURES... ix LIST OF TABLES... xiii LIST OF ABBREVIATIONS AND NOTATIONS... xiv CHAPTER 1: INTRODUCTION... 1 CHAPTER 2: BACKGROUND Assessment of Fluid Flow Dynamics In Vitro Bioreactors Fluid Flow Systems Applications for Bioreactors for Vascular Tissues Coronary Artery Disease, Bypass Graft Treatment, and Graft Failure Coronary Artery Disease and Bypass Graft Treatment Role of the Endothelium in Vascular Graft Failure CHAPTER 3: EXPERIMENTAL APPARATUS Test Apparatus Materials and Hardware vii
8 3.2 Procedure Testing CO 2 Diffusion Testing Cell Morphology CHAPTER 4: RESULTS CO 2 Diffusion Bioreactor Control and Cell Morphology CHAPTER 5: CONCLUSIONS AND FUTURE RECOMMENDATIONS REFERENCES APPENDIX A: Bioreactor Drawings APPENDIX B: Bioreactor Calculations APPENDIX C: Laboratory Protocols viii
9 LIST OF FIGURES Figure 1: Typical advanced bioreactor illustration (reproduced from Massai et al., 2013) 6 Figure 2: Spinner-flask bioreactor (reproduced from Plunkett and O'Brien, 2011)... 8 Figure 3: Parallel-plate flow chamber constructed from polycarbonate (reproduced from Lawrence, et al., 1987) Figure 4: Harvard Apparatus ORCA Bioreactor System ("Harvard Apparatus", 2015) Figure 5: Schematic of a cone-and-plate flow system (reproduced from Cheresh, 2008) 16 Figure 6: Circular parallel-plate flow chamber (reproduced from Kaur, et al., 2011) Figure 7: Microfluidic chamber with three culture units (reproduced from Hattori, et al., 2014) Figure 8: Novel hemodynamically-equivalent pulsatile bioreactor system (reproduced from Iwasaki et al., 2008) Figure 9: Schematic representation of the pulsatile flow bioreactor (reproduced from Engbers-Buijtenhuijs et al., 2006) Figure 10: Top and side view of all-in-one bioreactor (reproduced from Schulte et al., 2014) Figure 11: Rotating wall bioreactor (reproduced from Dermenoudis and Missirlis, 2010) Figure 12: Layers of a blood vessel (reproduced from LaMorte, 2013) Figure 13: 3-dimensional model of bioreactor with hardware inside Figure 14: Schematic of the benchtop bioreactor with all hardware ix
10 Figure 15: System set up with insulation around the bioreactor Figure 16: Setup of the fan inside of the bioreactor with the fan facing upwards Figure 17: Temperature logged from the CO 2 sensor with apparatus upright Figure 18: Temperature logged with the CO 2 sensor with apparatus on side Figure 19: Temperature logged with CO 2 sensor with fan added into apparatus Figure 20: Warm-up time for the heater to reach 37 C Figure 21: Correlation of shear stress to the setting on the peristaltic pump Figure 22: CO 2 diffusion through the bioreactor system with no dh 2 O or cell culture media Figure 23: CO 2 diffusion through the bioreactor system with dh 2 O Figure 24: CO 2 diffusion through the bioreactor system with RPMI cell culture media. 69 Figure 25: CO 2 concentration for the 24 hour flow experiment Figure 26: Temperature in the bioreactor system for the 24 hour flow experiment Figure 27: CO 2 concentration for the 48 hour flow experiment Figure 28: Temperature in the bioreactor system for the 48 hour flow experiment Figure 29: CO 2 concentration for the 72 hour flow experiment Figure 30: Temperature in the bioreactor system for the 72 hour flow experiment Figure 31: CO 2 concentration for the 96 hour flow experiment Figure 32: Temperature in the bioreactor system for the 96 hour flow experiment Figure 33: Static and dynamic fluid flow across HUVECs for 24, 48, 72, and 96 hours in the bioreactor system (scale bar = 150µm) Figure 34: Average angle of the orientation of the HUVECs under static and dynamic flow conditions (Bars represent the SEM, *p < 0.05 for dynamic vs. static control and dynamic vs. dynamic, Numbers at the bottom of each graph represent number of cells analyzed) x
11 Figure 35: Cell Elongation (Long Axis/Short Axis Ratio) of the HUVECs under static and dynamic flow conditions (Bars represent the SEM, *p < 0.05 for dynamic vs. static control, Numbers at the bottom of each graph represent number of cells analyzed) Figure 36: Sealed benchtop bioreactor Figure 37: Bioreactor with the components inside view Figure 38: Bioreactor with the components inside view Figure 39: Three-dimensional depiction and right side of the bioreactor Figure 40: Dimensions for the left and front sides of the bioreactor Figure 41: Dimensions for the top view of the bioreactor Figure 42: Dimensions for the inside and outside of the top door on the bioreactor Figure 43: Dimensions for the front door of the bioreactor Figure 44: Dimensions for the left side steel tubes and stopper in the bioreactor Figure 45: Dimensions for the right side steel tubes for the bioreactor Figure 46: Gas permeability correlation with different types of polymers (Krevelen and Nijenhuis, 2009) Figure 47: Schematic for laminar flow through a narrow slit (Bird, et al., 2007) Figure 48: Image of the side door cut out on the bench top bioreactor Figure 49: Image of the silicone rubber on the door of the bioreactor Figure 50: Image of the side door for the bioreactor Figure 51: Image of the top and top door of the bioreactor with the screws in place Figure 52: Image of the extreme temperature silicone rubber gasket for the bioreactor lid Figure 53: Image of the wires for the fan attachment Figure 54: Cords sealed into the ½ diameter hole on the right side of the bioreactor. 124 Figure 55: Seal around the lid of the bench top bioreactor xi
12 Figure 56: 33 mm filter connected to the stopcock and bioreactor Figure 57: 50 mm PTFE in-line filter connected to the CO 2 tank Figure 58: Three way valve adaptor set up Figure 59: Steel tubes inside of the rubber stopper and 125 ml flask Figure 60: Setup of the bioreactor in the hood for sterilization Figure 61: Setup of the UV lights for sterilization of the bioreactor holes Figure 62: Bioreactor setup with fan, media, and CO 2 sensor Figure 63: Direction of the top door cover of the bioreactor when sealed Figure 64: Left side of the bioreactor with the insulation foam Figure 65: Right side of the bioreactor with the insulation foam Figure 66: Foam insulation around the bioreactor Figure 67: Tubing wrapped inside the peristaltic pump Figure 68: Setting on the CO 2 regulator for the addition of CO 2 into the bioreactor Figure 69: 60 cc syringe connection to the 3 way valve connector Figure 70: Setting on the CO 2 regulator for the addition of CO 2 into the bioreactor xii
13 LIST OF TABLES Table 1: Summary of temperature inside of the bioreactor during flow experiments xiii
14 LIST OF ABBREVIATIONS AND NOTATIONS ACL - Anterior cruciate ligament BSA Bovine Serum Albumin CABG Coronary artery bypass graft CABS Coronary artery bypass surgery CAD Coronary artery disease CRP C-reactive protein dh 2 O Deionized water EtO - Ethylene oxide FBS Fetal bovine serum HUVECS Human umbilical vein endothelial cells HSV Human saphenous vein LIMA Left internal mammary artery PPM Parts per million xiv
15 CHAPTER 1 INTRODUCTION Heart disease has been the leading cause of mortality in the United States for more than 80 years, with a cardiovascular related death occurring about once every 30 seconds. The cost of cardiovascular disease and stroke is more expensive than any other malady, and in 2010, cardiovascular disease was estimated to cost about $315.4 billion (Murphy, et al., 2013; Go, et al., 2013). Coronary artery disease (CAD) is the most common type of cardiovascular disease in the United States, and without treatment, atherosclerosis arises, blood flow is restricted, blood clots form, and myocardial infarction occurs (Go, et al., 2013; NIH, 2012). Once CAD becomes increasingly severe, intervention is needed through coronary artery bypass surgery (CABS). In 2010, about 395,000 CABS procedures were performed in the United States (CDC, 2010). Bypass grafting involves rerouting the blood flow around the blockage, typically using either the left internal mammary artery (LIMA) or human saphenous vein (HSV) as a graft. The LIMA grafts are normally used for the left anterior descending artery, while the HSV grafts are used for the other two coronary arteries. The LIMA grafts have a high longterm patency rate with a 10% failure rate over 10 years, but HSV grafts often fail within 5 1
16 years causing the surgery to be repeated (Madaric, J., et al., 2008; Kim, et al., 2013). The HSV graft failure can be caused by thrombosis, intimal hyperplasia, or atherosclerosis, and studies have hypothesized that the vessel wall thickening and graft failure is a response from the vein to the increased arterial pressure and stress. However, the mechanistic reasons for the development of failure are not completely understood (Kim, et al., 2013; Parang and Arora, 2009). To study vascular function and understand more about graft failure, systems to culture the cells and keep them at a physiological relevant environment are needed. Often, the cultured cells are exposed to different mechanical stimuli to recreate a mechanical environment similar to what is found in vivo. A blood vessel is exposed to fluid pressure, fluid shear stress, blood gas tension, circumferential stress, and longitudinal stress inside of the body (Ohashi and Sato, 2005). Recreating even one type of these stresses allows for a better understanding of vascular function. One of the most common types of stresses that vascular cells are exposed to is shear stress, which is caused from the blood flow. Depending upon the location inside of the body, a human arterial shear stress is around 16 dynes/cm 2, while a human venous shear stress is around 6 dynes/cm 2 (Ando and Yamamoto, 2011). Many different types of flow chambers are used to study shear stress, with the most common being cone-and-plate flow chambers, oscillating orbital shaker flow chambers, parallel plate flow chambers, or microfluidic chambers. Shear stress has also been studied in 3-dimensional constructs that replicate the structure of a blood vessel (Zhou, et al., 2010; Brown, 2000; Prabhakarpandian, et al., 2011). 2
17 In order to recreate a physiological environment similar to in vivo, bioreactor systems are used. A bioreactor is a s ystem with a cl osed culture environment that is specifically created to simulate different physiological, environment, and mechanical factors found in nature. Many different types of bioreactor systems have been developed to support both 3-dimensional and 1-dimensional tissues, and each type of bioreactor system developed depends on its specific application. When studying vascular function, a bioreactor may mimic one or all of the mechanical stimuli found in a blood vessel, and they may also incorporate physiological relevant temperature, carbon dioxide, humidity, and ph conditions (Chaudhuru and Al-Rubeai, 2005). Given the important role to better understand vascular function and how and why HSV grafts fail, designing a system to study vascular function is needed. Designing, building, and testing a bioreactor apparatus will be achieved to successfully study vascular function using endothelial cells under shear stress in a microfluidic chamber. The bioreactor will be able to sustain a temperature of 37ºC and a CO 2 concentration between 56,000 ppm and 45,000 t o maintain the ph of the cell culture media. The apparatus will also allow for the use of a peristaltic pump to create a shear stress across the endothelial cells. Biocompatibility of materials, portability, cost, and steriliziability will also be taken into account when designing the system. Experimental data, such as cell morphology, temperature, and CO 2 concentration, will be analyzed to evaluate the bioreactor system. 3
18 CHAPTER 2 BACKGROUND 2.1 Assessment of Fluid Flow Dynamics In Vitro Bioreactors A bioreactor is defined as a system with a closed culture environment that simulates different physiological, environmental, and mechanical factors (Chaudhuru and Al-Rubeai, 2005). The environment inside of a bioreactor allows for the creation, conditioning, testing, and production of cells, metabolic products, tissues, support structures, and organs in vitro (Barron et al., 2003; Chaudhuru and Al-Rubeai, 2005). There are two main types of bioreactors, those used in industrial biotechnology and those used for tissue engineering applications. Industrial biotechnology bioreactors are mostly for growing high densities of prokaryotic or eukaryotic cells to produce large volumes of metabolic products, proteins, enzymes, or genes. These types of bioreactors are not normally utilized in tissue engineering applications, but are vessels where chemical processes are continuously carried out. Industrial bioreactors make it possible to apply batch, fed-batch, perfusion, and continuous cultivation to the cells cultured inside. Although the most common type of industrial bioreactor is the batch stirred tank reactor, 4
19 the continuous stirred tank reactor, packed bed, and membrane bioreactor are other commonly used types (Chaudhuru and Al-Rubeai, 2005). One company, Applikon Biotechnology, developed single use bioreactors, anaerobic and aerobic bioreactors, and autoclavable fermenters for cultivation. These bioreactors give the possibility of introducing gasses, controlling temperature, ph, and foam, and allowing for the removal of media for sample collection ("Applikon Biotechnology", 2015). Other companies that have produced similar bioreactors include New Brunswick ("Eppendorf ", 2015), Sartorius ("Sartorius", 2015), and Solaris ("Solaris Group, 2015). Bioreactors for tissue engineering are made to be closed systems that provide an in vitro environment that mimic in vivo conditions. These systems should be optimized to allow for controllable, repeatable, and sustainable conditions. Culture media, cells, and tissues are cultivated in environmental conditions that mimic in vivo and include a temperature of 37 C, humidity around 100%, an arterial ph of , venous ph of , or media ph of , an atmospheric concentration of CO 2 at 50,000 parts per million (5%). Keeping the system at an atmospheric concentration of 5% CO 2 helps to control the ph of the media the cells are placed in. Bioreactors are typically designed to perform at least one of the following functions: (1) to establish spatially uniform cell distributions on 3D (3-dimensional) scaffolds; (2) to control the conditions in culture medium (such as temperature, gas, ph, nutrients, and regulatory molecules); (3) to provide mass transfer between the cells and culture environment; (4) to expose the developing tissue or cells to physiologically relevant stimuli (such as shear stress, pressure, fluid flow, and stretch); (5) to provide information regarding the developing tissues or cells such as cell differentiation, tissue maturation, drug screening, disease 5
20 model investigation, and cell expansion (Massai et al., 2013; Ma and Elisseeff, 2006; Barron et al., 2003; Chaudhuru and Al-Rubeai, 2005; Lanza et al., 2000). An example of a typical advanced bioreactor system as described previously is shown in Figure 1. The bioreactor shown in Figure 1 consists of an area for the placement of a 3-dimensional scaffold, a control and sensor system to regulate the culture medium, media to provide mass transfer between the cells, pulsatile flow, mechanical, and electrical stimulation for physiologically relevant stimuli, and a control system to provide information about the tissue in real time. Figure 1: Typical advanced bioreactor illustration (reproduced from Massai et al., 2013) Bioreactors can be designed for a specific application, and their design depends on the appropriate conditions and environment desired. Various applications for bioreactors include bone tissue engineering, cardiovascular tissue engineering, and bladder regeneration to name a few. Today, a number of different types of bioreactors have been created, such as static systems, dynamic systems, 1-dimenisonal systems, 3- dimensional systems, and microfluidic chambers. 6
21 Static Systems vs. Dynamic Systems A static flask system is the simplest type of bioreactor. S tatic flask systems generally consist of a flask with cells or tissue constructs seeded on the bottom. These systems are similar to standard tissue culture flasks; however, the cells can be grown in either a static environment or a mixed environment with a magnetic stirrer. In the static environment, mass transfer occurs by molecular diffusion, while the mixed environment uses turbulent convection for mass transfer. The turbulent convection that occurs within the flask can negatively affect the cells by creating non-uniform tissue constructs (Lanza et al., 2000; Barron et al., 2003). A dynamic system is used to create a chamber with physical stimuli interacting on the cellular environment. The main types of dynamic tissue engineering systems include the spinner flask, rotating wall, compression, strain, hydrostatic pressure, flow perfusion, and a combination of the types (Chaudhuru and Al-Rubeai, 2005; Barron, et al., 2003; Plunkett and O'Brien, 2011). One of the first systems created was the rotating wall bioreactor, which was introduced by NASA. Originally made for experiments to interact with high forces during space shuttle take off, the wall of the vessel rotates horizontally for a low shear stress environment (Schwarz, et al., 1992). Spinner flask bioreactors are similar to those used in industrial biotechnology. An example of a spinner flask bioreactor is shown in Figure 2. In spinner flasks, scaffolds are suspended into the culture media, and a magnetic stirrer mixes the media to create turbulent flow (Plunkett and O'Brien, 2011). Spinner flasks also allow for sampling of the media through the port on the top of the flask. 7
22 Figure 2: Spinner-flask bioreactor (reproduced from Plunkett and O'Brien, 2011) Both a compression and strain bioreactor are similar in design, but differ in the way the force is transferred. Compression bioreactors use a flat platen to distribute the force across the tissue construct, while a strain bioreactor uses tensile strength. Hydrostatic pressure bioreactors are normally used for cartilage tissue engineering by applying a high pressure to the construct using a piston and actuator system. Flow perfusion bioreactors have been shown to be the best type of bioreactor for fluid transport because they can continuously recirculate the media (Chaudhuru and Al-Rubeai, 2005; Barron, et al., 2003; Plunkett and O'Brien, 2011). Perfusion bioreactors are also the most common type used for studying vascular function (Barron, et al., 2003). Comparison of static and dynamic tissue engineering systems for bone, cartilage, and vascular smooth muscle cells showed differences in cell morphology, expression of proteins, and oxygen content inside of the constructs. When seeding chondrocytes onto scaffolds in a static culture versus a dynamic perfusion bioreactor, change in ph was slower, and cells aligned in the direction of media flow in the dynamic system (Pazzano, et al., 2000). Similar results occurred when studying vascular smooth muscle cells under shear stress. The smooth muscle cell morphology changed under the shear stress with alignment towards the direction of flow (Henry, 2011). Perfusion bioreactors also were 8
23 able to prevent cell death and eliminate part of the oxygen gradient that can occur in a 3- dimensional culture system for bone tissue engineering (Volkmer et al., 2008). Although a static system is beneficial for certain types of applications, its lack of a r ealistic physiological environment make dynamic systems more favorable when designing a bioreactor D Systems vs. 3D Systems Bioreactors are used to study 1-dimensional, or monolayers of cells, as well as 3- dimensional tissues. Typically, bioreactors can study one system type or the other, and the decision on which type to choose is based on the specific study to be performed. Onedimensional systems are often chosen for protein expression, mechanical stress, drug delivery, or gene expression studies. These one-dimensional systems are frequently referred to as fluid flow systems since a fluid is flowing through or around the cell monolayer. Three-dimensional systems are often used to replicate a particular organ and to support tissue growth for the organ. One of the first types of 1-dimensional systems created to study fluid flow was in 1987, when human umbilical vein endothelial cells (HUVECs) were seeded onto glass slides and placed inside of a parallel plate flow chamber, Figure 3. One side of the flow chamber consisted of a cover slip that enabled the adherence and culturing of cells, while the other side of the flow chamber was used to hold the cover slip and was manufactured from polycarbonate. A Silastic rubber gasket was placed in between the two sides of the flow chamber to create a small channel using a vacuum. The flow chamber was then attached to an inverted-stage microscope, while a syringe pump controlled the flow. All 9
24 of the equipment was set inside of an air-curtain incubator to maintain the environment at 37 C (Lawrence, et al., 1987). Although this system was one of the first created, it allowed for the visual observation of cells under shear stress using a microscope. Since this system did not have circulating media, the experiments performed under flow used a large amount of fluid and lasted approximately 10 minutes, which is not long enough to study the effect of shear stress. Also, this system did not allow for the perfusion of gasses into the environment. Figure 3: Parallel-plate flow chamber constructed from polycarbonate (reproduced from Lawrence, et al., 1987) Since then, more flow chambers have been developed similar to the design described above, but with variable heights and widths or with possibilities of multiple shear rates (Cozens-Roberts, et al., 1990; Xiao and Truskey, 1996; Prabhakarpandian, et al., 2011). Currently, there is a commercially available flow chamber made by Glyotech that uses a circular or rectangular parallel plate flow chamber ("Glycotech", 2015). Many of the larger flow chambers, however, are not desirable because of the difficulty in fabrication, need for extensive cleaning, and use of an extensive amount of reagents. Therefore, microfluidic devices have been developed to solve these problems. Many companies have been developing microfluidic devices, including Ibidi, Cellix, 10
25 and Fluxion. Each of these systems allow for a wide range of assays, and the systems are easy to fabricate (Prabhakarpandian, et al., 2011). Since most three-dimensional bioreactor systems have been created to replicate or study specific organs, they have been designed for optimizing cell sources, scaffold materials, and fluid dynamics. Each tissue or cell source in the body is slightly different from each other, so a single bioreactor design is unlikely to be used for all types of cell and tissue operations. However, one company, Harvard Apparatus, has produced a bioreactor system capable of culturing either hollow or solid organs of different sizes and shapes called the ORCA Bioreactor. This bioreactor created by Harvard Apparatus has mostly been used for organ decellularization and recellularization. The ORCA bioreactor can be purchased with different chambers to accommodate large to small organs, such as hearts or lungs. Another type of bioreactor has been created by Harvard Apparatus specifically for trachea replacements. These are the first systems that have been developed for 3-dimensional tissues that are versatile for all types of tissues, and that contain a control system designed especially for this process. Figure 4 depicts the ORCA bioreactor system used for organ decellularization and recellularization sold by Harvard Apparatus. The ORCA bioreactor system, as shown in Figure 4, has a temperature control reservoir, a peristaltic pump to stimulate the organ, such as the stimulation of the ventricular action in the heart, a bioreactor chamber for growing the organ, and a controller for sensor monitoring, pump control, and other key functions. A variety of pumps can be incorporated into the system as needed, and the chamber where the organ is placed can be autoclaved ("Harvard Apparatus, 2015"). 11
26 Figure 4: Harvard Apparatus ORCA Bioreactor System ("Harvard Apparatus", 2015) Other whole tissue or 3-dimensional systems that have been developed were for a specific application or specific organ. Faraday Technology, along with the University of Dayton Research Institute and Grandview Medical Center, created an ex vivo bioprocess fluid flow system to further understand the environment that could affect bone implants or other materials implanted into the body. Preliminary studies showed that orthopedic fixation constructs made of Ti6A17NB used screws that could seize inside of the plates over time. Using physiological conditions to study this process made it possible reproduce a realistic in vivo condition ex vivo (Hansen, et al., 2012). Bioreactors have also been created that incorporate mechanical forces to apply cyclic strains or translational and rotational deformations for the promotion of 3- dimensional tissue formation. A piezoelectric-driven mechanism to create cyclic deformations into tissue constructs made of collagen for bone and cartilage engineering was created by Meyer et al. (2006). This mechanism allowed for the biaxial elongation of cells and for increased synthesis of extracellular matrix proteins for a more realistic cellular environment (Meyer et al., 2006). Another bioreactor system was made for 12
27 developing an anterior cruciate ligament (ACL) using a multi-dimensional strain system. An autoclavable reactor vessel allowed for the development and growth of a 3- dimensional ACL, while a mechanical motion control subsystem created a tensional, rotational, and translational force onto the construct (Altman, et al., 2002; Altman, et al., 2004). U sing a rotating vessel to create and provide a 3 -dimensional tissue with appropriate nutrients and mechanical stimulation has also been used for the creation of a variety of cells and tissues. Including different sensors inside the rotating chamber to measure temperature, dissolved carbon dioxide, and nutrient concentration provided a system that could support a tissue with accurate environmental and physiological conditions (Asnaghi and Mantero, 2013). To create a system that allowed for shear stress, Seliktar et al. (1999) developed a bioreactor that could apply a shear flow stress from 1 to 100 dynes/cm 2 to a substrate composed of a monolayer of chondrocyte cells. The shear stress was applied to the substrate using a reservoir and pump, and a growth chamber was utilized to maintain the monolayer and provide an area for bathing the substrate with media (Seliktar, et al., 1999). Although many different bioreactor systems have been created, many of the apparatuses that have been designed can be cumbersome to assemble, which in turn may increase the risk of contamination. Takagi, et al. (2003) generated a bioreactor capable of decreasing this risk by employing the use of a detachable apparatus body made of a heat resistant material. The bioreactor also applied hydraulic pressure to the cultured medium as well as physical stimulation by flow. The bioreactor was made to be airtight to prevent contamination and allow for the desired gasses to flow through the system (Takagi, et al., 2003). Another type of bioreactor was also created to help sterilize and keep three- 13
28 dimensional tissue constructs contamination free. The bioreactor was made of a rigid material capable of being sterilized. A treatment chamber configured for a tendon or ligament construct was also inside the system. The bioreactor included a pump and pressure system for unidirectional or bidirectional flow and a magnetic field to create an axial load on the construct (Peterson, et al., 2000). Many bioreactors have been developed for a variety of applications, from studying a monolayer of cells to 3-dimensional tissues, and for a range of different mechanical and physiological conditions. Each bioreactor that has been created has been tailored for a specific application; therefore, there isn t one bioreactor that has been created that is versatile for all types of functions, tests, and tissues Limitations of Bioreactors Since many types of bioreactors have been developed for a variety of specific applications, the bioreactors are limited in mimicking all of the physiological conditions that occur inside of the body and/or they are not capable of sustaining the tissue construct or cell monolayer for long periods of time. Static bioreactor systems are beneficial because they allow for the opportunity to study the system under physiological environmental conditions, such as temperature and humidity. However, these systems do not necessarily mimic all of the mechanical stresses that can occur in vivo. Stresses such as tension, circumferential stress, and compression cannot always be simulated with a static system. A dynamic system is a m uch more accurate model in producing an environment similar to what occurs inside of the body. These systems are more versatile, as different mechanical stresses and environmental conditions can be studied inside one 14
29 system, and these systems can also be tailored for the desired application. Onedimensional systems are favorable when studying protein expression, gene expression, or drug delivery since these systems are capable of looking at a specific cell under one or two different environmental conditions. However, when studying the effects of an environmental condition on t he specific organ function as a whole, a one-dimensional system is not desired, while a three-dimensional system can be utilized Fluid Flow Systems A fluid flow system or perfusion system is a system that uses a fluid to flow within an apparatus to apply shear stress across many types of cells. Using a fluid flow system makes it possible to study the biochemical responses on cells, including vascular endothelial cells, under the influence of mechanical shear stress. There are two main types of fluid flow systems used, flow chambers, which include the cone-and-plate flow chamber system, oscillating orbital shaker flow chamber system, and parallel plate flow chamber, and microfluidic devices (Zhou, et al., 2010; Brown, 2000; Prabhakarpandian, et al., 2011). Flow chamber systems are larger than microfluidic systems, but both are capable of accurately reproducing in vivo conditions Flow Chambers Cone-and-plate systems consist of a flat tissue culture dish with a cone axis that can rotate around its center axis. The cone axis is typically oriented perpendicular to the surface of the flat plate and can rotate to produce a s table laminar flow with small Reynolds numbers. The separation between the cone and the plate in the system varies linearly with radial position, which allows for the spatially homogeneous fluid shear 15
30 stress on bot h of the surfaces. Depending on t he angle of the cone and the angular velocity, a wide range of fluid shear stresses, including turbulent flow, can be applied in the system (Brown, 2000). A diagram of a standard cone-and-plate system is shown in Figure 5. In this figure, the cone was designed to fit a 35-mm or 100-cm tissue culture dish, and unlike other common designs, this chamber included an upper lid on top of the dish to minimize evaporation. The cone also contained a 0.5 degree angle from its peak to its edge, and the cone was able to freely rotate by the steel shaft and ball bearing support system (Cheresh, 2008). Figure 5: Schematic of a cone-and-plate flow system (reproduced from Cheresh, 2008) Mun, et al. (2013), used a similar cone-and-plate system as previously described to study the effect of laminar shear stress in promoting wound healing in endothelial cells. Their system used a 100 mm culture dish with a 0.5 degree cone angle, and the system was able to apply a constant laminar shear stress across the plate up to 12 dynes/cm 2 (Mun, et al., 2012). There are many advantages of using a cone-and-plate system, including the production of a relatively stable flow through the whole system, the requirement of small 16
31 amounts of medium, and the large area for cell growth. One of the main disadvantages of the cone-and-plate system is that it can be difficult to place the chamber on a microscope for real-time observation. It is also difficult for continuous sampling of the media with this system (Cheresh, 2008). To improve on t he cone-and-plate system, Dreyer, et al., (2011) created an advanced system that could house the cone-and-plate chamber in an incubation chamber. The incubation chamber could control temperature, gas composition (CO 2, oxygen, and nitrogen) using a four-way gas mixer, and atmospheric pressure. This advanced system was also modified from the standard system described previously, by allowing for the possibility to mount the system on a confocal microscope for live-cell imaging. The main disadvantage of the advanced system created by Dreyer, et al., (2011) was that to exceed 10 dynes/cm 2 of shear stress in the system, the viscosity of the medium had to be increased (Dreyer, et al., 2011). To increase the viscosity of the medium, Kohn, et al., (2015) added dextran to the media. The addition of dextran made it possible to increase the fluid shear stress in their system to 12 dynes/cm 2. Although the system created by Kohn, et al., (2015) was not placed in an incubation chamber, the temperature of the media remained 37 +/- 2 C. Their system also used a 0.8 cone angle, 48 mm diameter plate and included a method to replace media during the experiment as necessary. The main disadvantage with this system was the lack of CO 2 in the environment (Kohn, et. al, 2015). Overall, when studying low levels of shear stress and requiring a l arge area for cell growth, the cone-and-plate flow chamber would be an appropriate choice. Another commonly used method to produce fluid flow and shear stress across cells has been to use an oscillating orbital shaking system. In this type of system, culture 17
32 dishes are positioned on an orbital shaker at various rotational frequencies, and the shaker is placed inside of a cell culture incubator. To determine the amount of maximal shear stress (ᴛ w ) across the cells in the culture dish, the standard equation as shown in Equation 1 is used, (Eq. 1) where is the radius of orbital rotation, is the density of the medium, is the viscosity of the medium, and is the frequency of rotations. Using the orbital shaker system, the majority of the cells in the culture dish are exposed to the near maximal shear stress (ᴛ w ); however, the entire monolayer is not exposed to a uniform application of shear stress (Pearce, et al., 1996; Yun, et al., 2002; Dardik, et al., 2005). To compare the effects of shear stress with the orbital shaker system and the parallel plate system, Dardik, et al. (2005), evaluated the differences of each system on e ndothelial cell morphology, proliferation, and apoptosis. The endothelial cells in both systems were exposed to similar levels of shear stress, 14 dynes/cm 2 for the parallel plate and 11.5 dynes/cm 2 for the orbital shaker. When observing the endothelial cell morphology in the orbital shaker system, the cells that were located in the center of the well had a morphology similar to a static culture, where the cells were randomly aligned and polygonal. However, the cells located in the peripheral area of the culture dish had a morphology similar to the parallel plate system, where the cells were aligned in the direction of flow. Both the cell proliferation and apoptosis in the orbital system were similar to the parallel plate system in the peripheral area of the culture dish, where there was low cell proliferation and apoptosis. The proliferation and cell death were opposite in the center of the well, but the 18
33 center of the well was similar to that of a static culture. Dardik, et al. (2005) also confirmed that the shear stress in the orbital shaker was not uniform, with a lower shear stress in the center of the well and a higher shear stress in the periphery. Although the orbital shaker system does not allow for uniform shear stress, it can be useful when comparing endothelial cells responses to different types of shear stress in the same system (Dardik, et al., 2005). Zhou, et al. (2010) quantified a similar system, where multiple culture dishes could be placed on a platform that rocked up and down like a see-saw. Depending on the angle of the culture dish and the media when the dish was rocking, the entire bottom of the dish may or may not have been covered. To keep the bottom of the dish covered with media and to keep relatively small variations of fluid shear stress in the apparatus, the rocking conditions had to be well controlled. The fluid shear stress also had to be kept at a low magnitude, on the order of 1 dyne/cm 2. This type of approach would be beneficial when trying to save on costs, using less culture medium, and studying tissues with low shear stress rates, such as cartilage or ligaments. However, this system did not have a spatially uniform fluid shear stress, and would not be practical when studying shear stress on endothelial cells or bone cells (Zhou, et al., 2010). A parallel plate flow chamber is one of the mostly commonly used fluid flow shear systems due to its simplicity, ease of operation, ease of medium sampling, ease of culture access, and small fluid requirements. Most parallel plate flow chambers are rectangular with two openings at each end for applying shear stress across the culture surface (Brown, 2000). However, some parallel plate flow chambers can be circular with a parallel flow chamber in the middle, such as the commercially available flow chamber made by Glycotech. The Glycotech flow chamber uses a 35 mm petri dish to seed 19
34 the cells and a gasket with a flow path placed on the top of the dish for applying shear stress. The setup is held under a vacuum during the experiment to create the fluid flow across the cells ( Glycotech, 2015). The fluid shear stress across a p arallel plate flow chamber is normally calculated by using Equation 2, where Q is the volumetric flow rate set by the pump, h is the height of the flow chamber, w is the width of the flow chamber, and µ is the fluid viscosity. (Eq. 2) Using a similar setup as Glycotech, Kaur, et al. (2011) created a circular parallel plate flow chamber using a peristaltic pump with a closed loop as shown in Figure 6. Figure 6: Circular parallel-plate flow chamber (reproduced from Kaur, et al., 2011) The parallel plate flow chamber was designed to use smaller solution volumes, be inexpensive, and have the ability to be mounted on a microscope. The chamber was also made to accept commercial gaskets such as the one made by Glycotech. To minimize flow effects from the inlet and outlet of the flow chamber, the ports were chamfered. The flow system by Kaur, et al. (2011) was able to successfully culture endothelial cells under low levels of shear stress at 4 and 8 dynes/cm 2 for 16 hou rs, and the cells were kept 20
35 contamination free during the duration of the experiment. Parallel plate flow chambers are able to have uniform shear stress across the flow chamber, but sometimes flow effects at the inlet and outlet of the chamber can occur, and the shear stress across the flow chamber is not constant. To combat this problem, a converging parallel plate flow chamber was created by Lu, et al. (2014). In the convergent fluid flow chamber, the width in the chamber was decreased in the flow direction to minimize entrance effects during flow. When the width of the chamber was narrowed, the velocity and shear stress in the chamber was increased. Although the convergent fluid flow chamber was able to successfully create a uniform distribution of shear stress across the center of the chamber, there was still some cross flow, especially near the side wall. To change the shear stress across the chamber, the chamber height and flow through the chamber needed to be adjusted. The ratio between the entrance and exit widths of the converging chamber could also be changed to alter the shear stress. However, when the ratios between the entrance and exit widths increased too much, the region of constant shear stress was reduced (Lu, et al., 2014). Although parallel plate flow chambers have been one of the most common types of flow chambers used to study the effects of shear stress, the mechanism of the mechanical stimulus experienced by the cells isn t completely understood. Vaughan, et al. (2013) used a fluid structure interaction computational approach to investigate the effects of pressure and shear stress to a single osteoblast cell in a parallel plate chamber. To drive the fluid through the channel, the parallel plate chamber used a syringe pump to apply a constant volumetric flow rate through the inlet. By changing the pressure and shear stress independently from each other in the parallel plate chamber, it was determined that the pressure dominated the deformation of the cell body and not the shear 21
36 stress. Vaughan, et al. (2013) also concluded that the mechanical stimulus applied across cells may not be the same in different parallel plate flow chamber systems since the pressure can vary greatly even if the shear stress does not. However, Vaughan, et al. (2013) looked at the deformation of only one single cell and didn t consider changes in cell morphology or biochemical behavior. Therefore, pressure and shear stress on a cell monolayer may affect the cells morphology and fluid flow response, and both types of stresses should be considered when designing a fluid flow system (Vaughan, et al., 2013). Fluid flow chambers are desirable when studying the biochemical and mechanical signals on specific cells, but sometimes these larger flow chambers need an extensive amount of reagents. Microfluidic devices have been created to help solve some of the problems with the flow chambers, including easier control over the height and width dimensions of the fluid channels for a more constant fluid flow Microfluidic Systems A microfluidic system is similar to a parallel plate flow chamber as previously described, except a m icrofluidic device can range from a f ew micrometers to 100s of micrometers. Instead of centimeter scale like the flow chambers, microfluidic devices can even have total channel dimensions less than 1 mm. Microfluidic systems don t have a great advantage over the macroscale flow chamber systems when studying the changes of endothelial cells or other cells under shear stress, but microfluidics do allow for an enhancement in throughput (Wong, et al., 2012). Microfluidic chambers also allow for a smaller flow rate to obtain the same level of shear stress as a larger flow chamber, and they have a larger ratio of surface area to volume than the macro sized flow chambers. 22
37 The microfluidic chambers help to reduce the resources and reagents needed, and they have a better ability of controlling a constant shear stress. Microfluidic chambers can be made out of a range of materials, such as metal, glass, or polymers, but polydimethylsiloxane (PDMS) is the most common type of material used. To create a microfluidic device, a process called soft lithography is used where the flow chamber is molded out from the chosen material (Prabhakarpandian, et al., 2011). One of the first known microfluidic chambers produced for flow studies was created by Brevig, et al. (2003). The microfluidic system design consisted of a microfluidic chip made from poly(methyl methacrylate) that had three influent streams and one effluent stream in each chamber. Two of the influent steams were for guiding the fluid, and the other was for the sample fluid. The microfluidic chamber designed by Brevig, et al. (2003) was developed mostly for studying drug delivery, drug response, or biochemical responses on different cells. One study done with the microfluidic chamber was using human umbilical vein endothelial cells (HUVECs) and leukocytes to study the recognition events that occur after the HUVECs are treated with cytokine tumor necrosis factor-alpha (TNF-α) (Brevig, et al., 2003). Another type of microfluidic chamber was developed by Ateya, et al., (2005) that was capable of measuring the volume of a small number of cells in real time using a volume sensor. The microfluidic chamber was fabricated on s ilicone and contained two sensing chambers. To measure the change in volume, the volume sensor depended on t he displacement of extracellular fluid that occurred when the cell volume increased. The microfluidic chip was tested with rat astrocytes that were exposed to different osmotic challenges. As expected, when the cell was in a hypertonic solution, rapid shrinking of the cell occurred and the regulatory 23
38 volume increased as shown by the volume sensor. The opposite occurred for a hypotonic solution. The microfluidic chip was also tested to be used as a rapid screen device for drugs or antibiotics (Ateya, et al., 2005). To create a system with a higher throughput, Hattori, et al., (2014) developed a microfluidic flow chamber with 3 chips and 10 culture channels per chip. Each chip had a different flow channel depth to create three different shear stresses ranging from 1 dyne/cm 2 to 10 dynes/cm 2 across the system. The chip was fabricated with PDMS using photolithography, and is shown in Figure 7. The microfluidic chip was tested with HUVECs under three different shear stress conditions. The chip was connected to a peristaltic pump and cell culture medium reservoir bottle and placed in a 5% CO 2 incubator during testing. As expected, the HUVECs survived in the microfluidic chamber and adhered to the culture channel surface. The more the shear stress increased between the three channels, the more the HUVECs were extended and aligned along the direction of flow, showing that the microfluidic chip successfully created a useful tool for studying endothelial cell biology (Hattori, et al., 2014). Figure 7: Microfluidic chamber with three culture units (reproduced from Hattori, et al., 2014) Li, et al., (2013), also created a microfluidic chamber for studying HUVECs or other cell monolayers, except the system was used for studying the cells under hydrostatic pressure instead of flow. Although the system was designed just for pressure, the cells could also 24
39 be tested under low levels of flow. The microfluidic design was made out of PDMS, and the system only contained one flow chamber connected to a syringe pump, syringe, and manometer. The system allowed for a pressure of up t o 18 k Pa, and when testing the HUVECs in the chamber, the higher the pressure, the more damage that was caused to the cells. Overall, the system created by Li, et al., (2013) was flexible and could be used for a variety of cells under different pressures (Li, et al., 2013). Several companies, which include IBIDI, Cellix, and Fluxion, have developed microfluidic devices for purchase. IBIDI makes all different types of microfluidic slides for various applications, such as for cell cultivation, live cell imaging, flow assays, wound healing assays, angiogenesis, and 3D cultures. Their slides are made with a special hydrophilic or hydrophobic plastic bottom that allows for numerous optical imaging techniques, such as for phase contrast or confocal fluorescence microscopy. The IBIDI slides are also compatible with most fluid flow pumps since they can connect to luer locks for easy assembly to the tubing during fluid flow studies ( Ibidi, 2015). Using the Ibidi microfluidic slides, Voyvodic, et al. (2012) seeded HUVECS on the slide and exposed them to a steady and pulsatile shear stress of 20 dynes/cm 2 with a multichannel peristaltic pump. Pulse dampeners were also used to reduce the pulsatility from the pump, and without the pulse dampeners, the cells would detach from the slide at the high shear stress. Overall, Voyvodic, et al. (2012) was able to show that the IBIDI system could study fluid flow across the chamber for high shear stress rates using a peristaltic pump and pulse dampener (Voyvodic, et al. 2012). Cellix has developed microfluidic chambers similar to the IBIDI slides, where the Cellix chambers can be used for analyzing cells under shear stress, for 25
40 rolling and adhesion assays on protein coatings, for chemotaxis studies, and for invasion assays. Unlike the IBIDI slides, Cellix biochips don t have any luer lock connections, and instead require a unique plug and play connection with their microfluidic pumps. Cellix also uses a high quality plastic that is compatible with brightfield, phase contrast, and fluorescence microscopy ( Cellix, 2015). Fluxion developed a high throughput microfluidic device that is able to run up to 96 experiments at once. The slides are made of a high quality glass bottom for imaging, and are very simple to use. Similar to the Cellix system, the Fluxion BioFlux system contains an accurate electropneumatic pump and controller which are compatible with their microfluidic slides ( Fluxion, 2015). Many different types of microfluidic chambers have been developed for various applications, such as for studying drug delivery or fluid flow. Although microfluidics are similar to parallel plate flow chambers, they use less reagents and have the potential for a higher throughput, which make them advantageous for fluid flow studies Limitations of fluid flow systems There are a v ariety of fluid flow systems that have been created to study individual cells or groups of cells in 2 dimensions, including cone-and-plate systems, oscillating orbital shaking systems, parallel plate flow chambers, and microfluidic chambers. Although fluid flow systems are one dimensional systems, they have been instrumental in understanding more about the individual roles of cells in the body, especially when studying drug delivery, biochemical signals and interactions, or shear and pressure forces. Cone-and-plate systems and oscillating orbital shaking systems are 26
41 beneficial when studying multiple ranges of shear stress at low levels, and when needing a large area for cell growth. However, oscillating orbital shaking systems are not capable of producing a constant uniform stress across the entire chamber, and would not be practical when studying cellular models that need a constant stress for the duration of the experiment. Both parallel plate flow chambers and microfluidic chambers are similar, except parallel plate flow chambers are larger in size. Both systems are valuable when studying flow conditions across different types of cells since a constant stress is typically applied through the entire chamber. The chambers are also able withstand high and low levels of shear stress during experimentation. The chambers are more realistic in mimicking in vivo conditions and often don t require large amounts of reagents. Overall, fluid flow systems are still not capable of creating an environment that can truly mimic in vivo since some of the critical components of the geometrical and cellular features of a 3- dimensional environment are lacking. However, fluid flow systems are still being developed, and have played an important role in understanding more about the cellular interactions in the body Applications for Bioreactors for Vascular Tissues Heart disease has been the leading cause of mortality in the United States for more than 80 years, with a cardiovascular related death occurring about once every 40 seconds (Murphy, et al., 2013; Go, et al., 2013). Bioreactors can play an important role in studying cardiovascular disease since they can be used to enhance the properties of tissue-engineered constructs, stimulate accurate physiological conditions, and study the causes of the disease (Barron et al., 2003; Massai et al., 2013). Depending upon the exact 27
42 specifications needed, bioreactors can be created to study vascular function as a whole, to study the exposure of all physiological mechanical stresses on individual layers a blood vessel, or to partially mimic the physiological environment on individual layers of a blood vessel Systems created for studying vascular function in 3-dimensions Typically, when creating a bioreactor to study vascular function, the physiological environment is mimicked as much as possible. Since a blood vessel is composed of three main layers, the intima, media, and adventitia, most systems try to incorporate at least one of these layers into their design (Bronzino, 2006; LaMorte, 2014). A blood vessel is also exposed to many different mechanical stresses, including fluid pressure, fluid shear stress, blood gas tension, circumferential stress, and axial stress (Ohashi and Sato, 2005). A dynamic mechanical environment that encompasses some or all of these stresses, as well as the appropriate temperature and gas conditions, is used to study vascular function. In order to accurately mimic the appropriate physiological flow rates and pressure, most of the types of bioreactors that have been created are pulsatile bioreactors. One type of bioreactor capable of integrating a tissue engineered vascular graft with all three layers of the blood vessel was created by Iwasaki et al. (2008) using polyglycolic acid sheets as templates to seed the cells. The bioreactor comprised of a left ventricular model with synthetic mitral and aortic valves to stimulate pulsatile circulation through the graft. The left ventricular model was pneumatically driven by positive and negative pressure. Using the left ventricular model and synthetic valves, the bioreactor was also capable of gradually increasing the pulsatile flow and pressure rates to adjust the 28
43 rates from venous to arterial pressure and to eventually reach realistic pulsatile aortic pressure. While the tissue engineered graft studied was in culture for 14 days, the media was exchanged from the system once, after 7 days. The bioreactor also consisted of a gas exchange unit with oxygen (O 2 ) and carbon dioxide (CO 2 ), a chamber to mount the vessel, a pulsatile pump, and a pressure transducer to measure the pressure and flow, as shown in Figure 8. Figure 8: Novel hemodynamically-equivalent pulsatile bioreactor system (reproduced from Iwasaki et al., 2008) Although this bioreactor system was capable of accurately regulating flow, pressure, heart rate, systolic fraction, ph, and carbon dioxide, similar to in vivo conditions, the authors did not mention any evidence that the tissue engineered graft was cultured at the appropriate physiological temperature (Iwasaki et al., 2008). Another type of bioreactor created by Shaikh, et al. (2010) was made to specifically look at one type of mechanical stress on the vascular graft, hypothesizing that applying only pulsatile cyclic wall pressure would be beneficial in the early stages of tissue growth. The major components of this bioreactor consisted of a pressure piston, filters, semi compliant tubes, and a sterilizable biochamber made of polyetherimide. For 29
44 the addition and removal of media, an inlet and outlet were placed above and below the bioreactor, with the outlet controlled by a valve. To create the pressure and mimic the normal physiological heart rate, positively pressurized air was introduced into the tubing system where the air exited the piston and entered the bioreactor through an air filter. This piston system made it possible to create pulsatile, unidirectional air-fluid pressure that could be controlled by an exit valve. The entire hydrostatic pressure bioreactor was then placed inside an incubator for the duration of the study. The hydrostatic pressure bioreactor created by Shaikh, et al. (2010) was useful because of its ability to be sterilizable, to accurately mimic physiological pressure, and to be small enough for working in sterile conditions. However, the bioreactor had some limitations, including its reliance on an incubator and its lack of all of the mechanical stresses present in an in vivo blood vessel environment (Shaikh, et al., 2010). Often, creating a bioreactor system where multiple samples can run at the same time is beneficial. Engbers-Buijtenhuijs et al., (2006) and Buttafoco et al., (2006) created a bioreactor system with four different flow chambers, a peristaltic roller-pump that could vary the pulsatile flow, and a Venturi valve to control pressure. A representation of the bioreactor system is shown in Figure 9. 30
45 Figure 9: Schematic representation of the pulsatile flow bioreactor (reproduced from Engbers-Buijtenhuijs et al., 2006) The bioreactor was also capable of simulating physiological shear stress rates and pressure wave forms by changing the roller speed of the pump and pressure values. Although this system was placed inside of an incubator, it was still possible to run experiments with this system up to 14 days, with a media change every 2 days (Engbers- Buijtenhuijs et al., 2006; Buttafoco et al., 2006). Xu, et al. (2008) created a similar system using a peristaltic pump and magnetic valve to control the flow rate and pressure. This bioreactor was successful in creating a s ystem with cyclic mechanical training for better vascular graft remodeling (Xu, et al., 2008). Although many different types of bioreactors have been developed, there is still an extensive need for an efficient all-in-one system. Schulte, et al., (2014) created a device that could fit neatly in an incubator and that allowed for the simultaneous seeding of a vascular graft while conditioning and perfusing the graft with shear stress. A completed apparatus is depicted in Figure 10. The completed bioreactor consisted of a seeding device, rotating mixer for the media, media reservoir, control unit, roller pump, and pulse generator. The seeding device contained a cylinder filled with culture medium and space for two tissue engineered grafts, and the media reservoir was manufactured to 31
46 hold a capacity of 200 ml of liquid. To circulate and perfuse media through the bioreactor at a defined pulse frequency, a peristaltic roller pump and eccentric DC pulse device were used. Also, the bioreactor was designed for quick assembly in a laminar flow cabinet without the use of additional tools that could potentially introduce contamination. Figure 10: Top and side view of all-in-one bioreactor (reproduced from Schulte et al., 2014) The vascular grafts inside of the bioreactor created by Schulte, et al., (2014) could also be exposed to different shear stress values, including high values that might occur during thrombotic events. The bioreactor did have limitations in the sterilization of the equipment since, in this study, the bioreactor was sterilized with formaldehyde deposition. This technique of sterilization is difficult and not available in every laboratory. While the creation of this bioreactor allowed for ease of use, and the bioreactor was an all-in-one system, it still lacked the ability to be placed directly onto a benchtop and the ability to be easily sterilizable (Schulte, et al., 2014). Different types of mechanical stress tend to play a large role when creating a functional tissue engineered blood vessel, and Bilodeau, et al. (2005) decided to replicate all of the in vivo blood vessel mechanical stresses in their bioreactor design. Bilodeau, et al. (2005) first used finite element analysis to determine the accurate constraints that needed to be applied to the tissue engineered vessel. The design then focused on making 32
47 the stress parameters customizable so that the vessel could properly evolve from a fetal stage to an adult stage. The bioreactor included two different flow conditions, laminar pulsatile flow for the inside of the graft, and a continuous flow for the outside of the graft. The design also included the possibility of varying combinations of mechanical stresses, such as the internal pressure, axial stress and strain, and torsion. A temperature of 37 C was also maintained inside of the bioreactor for each experiment (Bilodeau, et al., 2005). There are also many different types of patents that have been submitted for vascular graft bioreactors. These bioreactors were mostly for creating, growing, processing, and conditioning the vascular grafts for re-implantation into the body. McAllister and L'Herureux (2010) created a system that could assemble a t issue engineered blood vessel inside of a bioreactor by using a rollable mandrel. The bioreactor consisted of an enclosed chamber, sheet growth module to grow the different layers of the blood vessel in sheets and a clamp for holding the mandrel in place. First, sheets of cells were cultured, and then the sheets were rolled into a multilayer vessel using the sheet growth module. Media and gas exchange could occur in the chamber using a value, and the environment inside of the chamber remained constant. The ph, CO 2, and sterility were also maintained inside of the bioreactor (McAllister and L'Herureux, 2010). Wolfinbarger (2002) developed a system similar to that previously described, but instead of rolling a vascular graft from sheets of cells, a re-cellularizing process from accellular vascular grafts was used. The developed graft could be attached to the bioreactor by inlet and outlet port connections or by sewing the graft to sewing rings. The entire bioreactor was a closed system to prevent contamination, and the bioreactor 33
48 allowed for solutions to be introduced into the graft from a syringe inlet port. The bioreactor also had an option, by using a peristaltic pump, to apply a slow controllable pulsatile flow of media into the graft to establish a cell population inside of it (Wolfinbarger, 2002). When creating vascular grafts for implantation into a patient, many bioreactors do not have the capability of processing and shipping the graft within a single, sterile apparatus. However, Elizondo, et al. (2001) created a s ystem able to process, evaluate, and store a vascular graft or heart valve material using a processing chamber, environmental control circuit, nutrient supply circuit, and pulse evaluation unit. The environmental control unit in the system was made using two heat exchangers that could alter the temperature of part or all of the entire system. The nutrient supply circuit allowed for the addition of nutrients to sustain the biological material, and the pulse evaluation circuit consisted of a pneumatic or peristaltic pump to test the vascular graft under different flow conditions (Elizondo, et al., 2001). Other patented work included systems that could integrate both engineering and biological principles with a realistic mechanical environment. A human blood vessel undergoes coupled pulsatile flow and pulsatile pressure which are also perpendicular to each other. These forces cause a stress phase angle whose value can change depending if the vessel is diseased or normal. Using this information, Dancu (2011) created a bioreactor system to condition a coronary bypass graft with endothelial cells or stem cells. Conditioning the graft gave the bioreactor the possibility of independent control over the flow and pressure, as well as a mounting system for introducing axial strain/torsion into the graft. Sensors could also be placed within the specimen unit to 34
49 measure different parameters of the graft, such as the wall thickness. With different options of conditioning the vascular graft with a realistic mechanical environment, the creation of a functional graft may be possible (Dancu, 2011). Overall, each of the bioreactors that have been developed for creating a functional vascular graft differ in some way. Some bioreactors have the possibility of mimicking all mechanical stresses that occur in vivo, while others just focus on one type of stress. Also, some of the designs include the appropriate physiological temperature, CO 2, and ph conditions inside of the system, while others do not. Since there are many different ways to create and maintain a vascular graft, there is not currently one bioreactor that addresses all of the physiological conditions and mechanical stresses that can occur in vivo. However, the likelihood of creating such a bioreactor is feasible using all of the different equipment discussed above Systems created for studying cell monolayers To study vascular function on one of the layers of a blood vessel, a monolayer of cells are typically used. The cells are normally seeded on constructs that are tubular or flat, but the cells can also be placed in a flow chamber or microfluidic slide. Similar to the bioreactor systems created for studying 3-dimensional tissue grafts and tissue engineered blood vessels, the cell monolayers are exposed to a d ynamic mechanical environment with at least one of the mechanical stresses and a suitable physiological environment as previously described. Previous evidence has shown that when studying vascular function, the cellular response to different mechanical stimuli depends on t he type of stimuli placed on t he 35
50 cells. To fully understand the effect of specific mechanical stimuli on one type of cell, systems have been made to exclusively study one particular stimulus at a time. One type of system, designed by Wang, et al. (2001), was created to understand how endothelial cells respond only to cyclic stretch. Human aortic endothelial cells were seeded on deformable silicone membranes and placed into an apparatus that could apply three different types of cyclic stretch. The apparatus consisted of four metal arms and four driving motors that attached to a square where the silicone membrane was placed. The type and magnitude of stretch was set and controlled by a control unit. One type of stretch created with the apparatus, called simple elongation, stretched the membrane in one direction while the other direction was compressed. The other type of stretch, called pure uniaxial stretching, controlled the orthogonal edges so the membrane was stretched, but not compressed. The last type of stretch was called equi-biaxial stretch, where the membrane was stretched equally in both directions. Using the apparatus, the cells were exposed to the different stretching conditions for 3 hou rs. In response to the different types of stretch applied to the membrane, the human aortic endothelial cells oriented to the direction with minimal substrate deformation. Thus, for the uniaxial stretching test, the cells aligned perpendicular to the stretch, while the cells under the simple elongation stretch aligned obliquely at about 70º. Using the type of apparatus built by Wang, et al. (2001), the endothelial cell response to a specific type of deformation could be studied. However, since the apparatus was only tested for experiments 3 hour s in length, long term experiments were not shown to be capable in this type of system. There was also no mention of the cells being cultured in a physiological environment after the cells were initially incubated before the experiment began (Wang, et al., 2001). 36
51 Williams and Wick (2005) developed another type of bioreactor to study just the individual mechanical stimulus of shear stress on m onolayers of cells co-cultured together. A biodegradable felt made of poly(glycolic acid) (PGA) and sutured into 4.5 mm inner diameter tubes was used to culture the cells. The PGA tubes and bioreactor system were created to study both endothelial cells and smooth muscle cells together in a physiological relevant environment with shear stress for long term. First, the PGA tubes were placed into a perfusion bioreactor and mounted onto hollow posts that penetrated the bioreactor wall. Then, the smooth muscle cells were seeded onto the constructs and perfused with cell culture media for 25 da ys at a shear stress of 4 ml/min. Endothelial cells were added into the lumen of the tube at 10 or 23 da ys into the 25 da ys of the experiment. The entire system was kept inside of an incubator at 37ºC with 5% CO 2 during the duration of the experiments. Williams and Wick (2005) not only wanted to create a system to grow tubular constructs of cells, but wanted to understand the interaction between the endothelial cells and smooth muscle cells. The bioreactor coculture system created was successful in performing long-term studies as well as incorporating pulsatile shear stress and tubular geometry. The main disadvantage of the system was its dependence on using a tubular 3-dimensional structure to study the interaction of monolayers of cells (William and Wick, 2005). Ballermann and Ott (1998) created a s imilar device, except the hollow tubular device was made of polypropylene instead of PGA. The system was developed to culture the cells under a shear stress between 0.4 dynes/cm 2 and 33 dynes/cm 2, and it was also made to allow for seeding a coculture of both endothelial cells and perivascular cells. Growing cells with the tubular device and using the method developed by Ballermann and Ott (1998), the co-culture of 37
52 cells could be seeded and transfected with genes to study vascular disease. The system was created to be versatile and the cells could also be grown to express specific proteins or fragments for isolation. Although the system did not create a physiological relevant environment, the system was small enough to be placed in an incubator for experiments (Ballermann and Ott, 1998). To construct a bioreactor system that could study most of the mechanical stresses that occur on the endothelial cells in a blood vessel, Dermenoudis and Missirlis (2010) designed a device that could apply shear stress, longitudinal stretch, and an altered gravitational field on a tubular construct of endothelial cells. A flow of growth medium across the construct created the shear stress, a stretch of the graft created the longitudinal stretch, and rotating the construct produced the gravitational field. Figure 11 shows what the fabricated bioreactor looks like, with A representing the plate delivering the longitudinal stretch, B exhibiting the tubular construct with endothelial cells, C showing the rotating mechanism, D displaying the medium inflow and outflow for the shear stress, and E showing the ends of stainless steel ducts that enter a hollow drum. The portion of the bioreactor that contacted the cells was made of stainless steel, and the specimens were mounted between two long ducts that penetrated an aluminum plate. The entire bioreactor assembly was placed inside of a cell culture incubator with a medium reservoir, while the peristaltic pump was placed outside of the incubator. When testing the device with endothelial cells, the cells elongated and formed swirls throughout the tubular construct. The cells also stayed viable throughout the 16 hour experiment, proving that the system created was capable of studying endothelial cells under a combination of mechanical factors (Dermenoudis and Missirlis, 2010). 38
53 Figure 11: Rotating wall bioreactor (reproduced from Dermenoudis and Missirlis, 2010) Overall, when developing a bioreactor for studying vascular function, the cells are typically exposed to different types of mechanical stimuli in a physiological relevant environment. Each type of bioreactor created is somewhat different, and the type of bioreactor designed depends on t he experiment. If a 3-dimensional vascular graft was going to be developed for implantation or for studying the interaction of cells in a blood vessel, a 3 -dimensional bioreactor would be advantageous. However, if studying the interaction of genes or proteins on specific cells or stimuli, a 1-dimensional bioreactor with microfluidic slides or tubular constructs would be beneficial. Although the type of bioreactor may be different, each system needs to be placed in a physiological relevant environment with suitable temperature, CO 2, humidity, and ph. 39
54 2.2 Coronary Artery Disease, Bypass Graft Treatment, and Graft Failure Coronary Artery Disease and Bypass Graft Treatment Heart Disease is the leading cause of death in the United States, and in the past ten years, the total number of inpatient cardiovascular operations has increased 28%. However, the number of those who have perished from cardiovascular disease has been decreasing each year. Coronary artery disease (CAD), the most common type of cardiovascular disease, causes about 1 i n every 6 de aths per year, and occurs approximately every minute (NIH, 2012). Atherosclerosis is the most frequent source of coronary artery disease, which happens when plaque buildup causes the arteries to become narrow, hardened, and blocked. This leads to restricted blood flow, blood clots, shortness of breath, and chest pain. Without treatment, the plaque can rupture, causing myocardial infarction and death to the heart muscle (Murphy, et al., 2013). Coronary artery bypass graft (CABG) surgery is commonly performed once CAD becomes serious, typically using either a left internal mammary artery (LIMA) or human saphenous vein (HSV). LIMA grafts usually have a high long-term patency rate, with a 10% failure rate over 10 years, while saphenous vein graft (SVG) patency at 1 year is 89% and 61% after 10 years (Madaric, J., et al., 2008; Kim, et al., 2013). Most people receive the LIMA grafts for the left anterior descending artery, and the SVG is given to all other diseased blood vessels. The high failure rate causes the bypass surgery to be frequently repeated. In the first month of having the CABG, thrombosis plays a major role in graft failure. Intimal hyperplasia generally causes failure between months 1 and 12, and atherosclerosis problems arise after one year (Kim, et al., 2013). Other bypass 40
55 graft treatment includes synthetic grafts and a mixture of synthetic and autologous grafts (Bronzino, 2006). However these bypass graft treatments are typically used for larger diameter blood vessels, such as the aorta, instead of for CABG surgery ( Johns Hopkins Medicine, 2013) Role of the Endothelium in Vascular Graft Failure A blood vessel is composed of three main layers, the intima, media and adventitia as shown in Figure 12. The intima is the inner most layer of the vessel, and is the only layer that directly contacts the blood. The intima is mostly composed of endothelial cells and is important in regulating vessel permeability. The middle layer, the media, is where the smooth muscle cells are located, and the outer most layer, the adventitia, functions as an anchor for the vessel. Although both arteries and veins have the same layers of tissues, the artery has a thicker media layer and experiences a h igher shear stress (Bronzino, 2006; Johns Hopkins Medicine, 2015; LaMorte, 2013). Figure 12: Layers of a blood vessel (reproduced from LaMorte, 2013) Disruption or damage to the vascular endothelium layer Disruption or damage to the intima or vascular endothelium layer can lead to graft thrombosis, intimal hyperplasia, and eventually graft failure. Many different biochemical signals have been shown to be involved in vascular graft failure, including nitric oxide, adenosine, and prostacyclin, since these signals are involved in inflammation and cell 41
56 proliferation (Somahlution, 2014; Loscalzo, 2000) O ne biochemical signal, C-reactive protein (CRP), has been shown to be a marker of inflammation in SVGs. Patients who tended to have a higher amount of CRP, had more endothelial dysfunction and graft failure, even when taking statin drugs (Momin, et al., 2007). There are also many biochemical signals shown to prevent vascular graft failure, such as 5-HT receptor antagonist, CBSS3830 (an inhibitor of p38 M ap Kinase), and SOCS1 (suppressor of cytokine signaling 1) (Ge, et al., 2013; Kodama, et al., 2014; Lingfeng, et al., 2014). When the SVG is placed into a patient for coronary artery bypass surgery (CABS), the thin-walled graft is exposed to an environment with much higher pressure and shear stress than normal. This mismatch in mechanical stress can cause the saphenous vein to bulge, and may be a major cause of failure. Many studies have shown that a disturbed flow can help mediate the process of intimal hyperplasia (Chiu and Chien, 2011). Also, the saphenous vein has a much larger diameter and a thinner wall than the coronary artery, which can cause a lower velocity of flow. When comparing the basal intimal thickness of human saphenous vein grafts obtained during CABS, the thicker the basal intima (greater than 120 µm), the more likely the graft will be predisposed to intimal hyperplasia. This basal intimal thickness is a predictor of endothelial dysfunction in the saphenous vein (Li, et al., 2013). Immediate graft thrombosis and neointimal thickness can also be prevented by treating the SVGs to an arterial shear stress of 25 dynes/cm 2 before implantation. Even after three months of implantation, a pre-treated graft with arterial shear stress still had a lower average thickness than one not pre-treated, showing that shear stress has an effect on SVG success (Dardik, et al., 1999). The results of these investigations demonstrate that 42
57 vascular function is very complex, with shear stress and biochemical signals playing a role in the success of a human saphenous vein graft. 43
58 CHAPTER 3 EXPERIMENTAL APPARATUS 3.1 Test Apparatus When studying monolayers of cells under shear stress, specifically to observe vascular function, an environment that mimics a physiological environment is needed. The appropriate materials and hardware were chosen to create this physiological environment, and the decision on t he type of hardware to purchase was based on biocompatibility, cost, and functionality. Since the bioreactor will need to provide steady temperature, CO 2, and shear stress over time, the bioreactor was designed to be an enclosed system. Once the apparatus was designed and developed, it was tested to determine if it could mimic physiological conditions and sustain the growth of endothelial cells. The bioreactor was developed to be a low cost, biocompatible, portable, enclosed system that could be adaptable for various applications, such as for studying both 1- dimensional monolayers and 3-dimensional tissue constructs. A 3-dimensional model of the bioreactor with its hardware components in their desired location is shown in Figure 13 and Appendix A-I. The inside of the bioreactor consists of a combined CO 2, humidity, and temperature sensor, heater and thermocouple system, fan to circulate air, flask to hold 44
59 cell culture media, valve to allow the addition and removal of CO 2, and a microfluidic IBIDI slide to maintain and culture the endothelial cells. Figure 13: 3-dimensional model of bioreactor with hardware inside A peristaltic pump, CO 2 tank, desktop computer, Variac, and power cords were placed outside of the device to allow for the control of shear stress and CO 2 addition to the system during experiments. A schematic of the completed system is shown in Figure 14, and an image of the system set up with the insulation around the bioreactor is shown in Figure 15. The peristaltic pump and Variac were used to control the flow rate and thus the shear stress across the endothelial cells. The CO 2 tank and valves were used to manually add CO 2 to the system, and the desktop computer was used to allow for monitoring and logging of the CO 2, temperature, and humidity over time. 45
60 Figure 14: Schematic of the benchtop bioreactor with all hardware Figure 15: System set up with insulation around the bioreactor Materials and Hardware The type of materials and hardware chosen to create the bioreactor system were based on what was already available in the laboratory, the cost, and the functionality in providing an environment to grow endothelial cells. The enclosure for the system, heater, CO 2 sensor, peristaltic pump, and culture system for the endothelial cells were selected for the complete bioreactor system The Bioreactor Enclosure The bioreactor enclosure was created using an acrylic radioactive waste container made by USA Scientific that was found in the laboratory. The bioreactor enclosure had outer dimensions of 8 in. X 8 in. X 13in., with a wall thickness of 0.5 i n. Holes were 46
61 drilled in the container to allow for the connection to a peristaltic pump, CO 2 supply and exhaust, power cords, and for the possible addition of future equipment, such as a humidity sensor. Although one door already existed in the top of the container, an additional door was created on the side of the bioreactor enclosure to allow for the easy addition of materials inside of the cell culture hood. A detailed protocol of how the bioreactor enclosure was built, as well as the complete dimensions in 2-dimensional drawings are located in Appendix A-II. Before deciding on the acrylic box, the biocompatibility, sterilization technique, heat transfer rate, and CO 2 diffusion rate were determined. Acrylic, which is also known as Poly(methyl methacrylate), is a strong polymer with high optical clarity. Acrylic has been shown to be biocompatible, and has been used in various medical applications, such as bone cements, adhesive resins, and ophthalmic applications (Meyers, 1995). The acrylic material is not recommended to be sterilized with steam sterilization or Wet Ethylene Oxide (EtO), but the acrylic material shows gamma radiation, UV radiation, E- beam radiation, and EtO gas resistance. Acrylic doesn t show any long term yellowing or discoloration after exposure to any of the recommended sterilization techniques; however, gamma radiation can show a temporary yellowing after exposure. Each of the recommended sterilization techniques also does not adversely affect the material s mechanical properties over time (Modjarrad and Ebnesajjad, 2013; Massey, 2005; Massay, 2003). To determine the heat transfer rate through the acrylic box, the heat transfer equations for a vertical plate were used. An internal bioreactor temperature of 37ºC and a room temperature of 20.8ºC were used to calculate the heat transfer through the acrylic 47
62 box. The final calculated value for the predicted heat transfer rate through the surface area of the acrylic box was Watts. The heat transfer rate was used to determine the power needed for the heater system to heat and maintain the inside of the bioreactor apparatus at 37ºC. The complete calculations are located in Appendix B-I. The CO 2 diffusion rate through the acrylic box was estimated using the Fick s law of rectangular diffusion and the permeability-diffusion equation. The CO 2 diffusivity was estimated to be 2.055*10-14 mol/second. The CO 2 diffusivity rate was used to determine how often the user would need to come in and add more CO 2 into the system. Although the CO 2 diffusivity rate is slow, the user would still need to periodically check the system to make sure it maintained the desired CO 2 concentration between 56,000 ppm and 45,000 ppm. The complete calculations are located in Appendix B-II. Overall, the properties of the acrylic make the acrylic box suitable as an application for a bench-top bioreactor Carbon Dioxide Sensor A carbon dioxide sensor was needed for the bench-top bioreactor that was capable of measuring a carbon dioxide concentration greater than 50,000 parts per million (ppm) or 5%. When choosing a carbon dioxide sensor, it was also required that the sensor was able to log data for the duration of the experiment. The K-33 BLG CO 2 Data Logger Sensor Development Kit was chosen from CO2Meter.com. Along with a carbon dioxide sensor, the development kit came with a thermistor temperature sensor and a humidity sensor. The operation range for the CO 2 sensor was 0-30% CO 2 with an accuracy of +/- 3% of the measured value. The temperature sensor had a measurement range of -40 to 60 C with an accuracy of +/- 0.4 C at 25 C. The relative humidity sensor had a measurement range of 0 to 100% relative non-condensing humidity with an accuracy of 48
63 +/- 3% ( Sensirion, 2015). The carbon dioxide sensor was placed on the top right-hand side of the bioreactor as shown in Appendix A-I and Figure 13. The location of the carbon dioxide sensor inside of the bioreactor was chosen to be near the top lid of the bioreactor for ease of removal, and the cords to plug into the carbon dioxide sensor were placed through a 0.5 in. hole in the Acrylic box Temperature Control To choose the appropriate temperature and heater control system for the acrylic bench-top bioreactor, a heater system was selected that could provide a power of at least Watts as previously calculated using the heat transfer rate. The Incukit Mini Thermostat, Fan, and Heater combination was purchased from Incubator Warehouse. The Incukit system had a 40 Watt heater, with a thermocouple that had an accuracy of +/- 1 C ( Incubator Warehouse, 2015). The heater was chosen to be placed on the top edge of the bioreactor as shown in Figure 13 and in Appendix A-I. The location for the heater was based off of ease of access and removal as well as to conserve as much space as possible. Insulation with a value of R-13 and R-5 was also added around the outside of the bioreactor to ensure better thermal insulation. Originally, the heater was placed with the fan facing upward, and the bioreactor was standing with its long edge upright. Since the fan attached to the heater blew toward the heater to remove hot air around the electronic components, the fan was situated facing upward to maximize air circulation within the apparatus as shown in Figure
64 Figure 16: Setup of the fan inside of the bioreactor with the fan facing upwards The temperature control was tested inside of the bioreactor for 12 h ours to determine if the temperature was stable. A graph of the temperature change over the 12 hours is in Figure 17. Although the temperature remained stable throughout the duration of the experiment, the temperature was about two degrees higher than the set target temperature of 37 C +/- 0.5 C. A temperature gradient was also found to be present inside of the chamber as measured by an additional mercury thermometer placed on the other end of the box in air and by a red alcohol thermometer placed in a flask of deionized water. The mercury thermometer measured 37 C while the alcohol thermometer measured 36 C. The warm-up temperature of the acrylic apparatus also overshot its target temperature by about 3 C. 50
65 Figure 17: Temperature logged from the CO 2 sensor with apparatus upright To decrease the temperature gradient inside of the bioreactor, the acrylic bioreactor setup was changed to place the apparatus on i ts side. A graph of the temperature change over 12 hour s is in Figure 18. The temperature remained stable throughout the duration of the entire experiment, and the temperature was recorded to stay in the set target temperature of 37 C +/- 0.5 C. The warm-up temperature of the apparatus also did not overshoot, and gradually increased until reaching the target temperature range. Although the temperature recorded by the temperature sensor was in the desired range, a temperature gradient was still observed in the chamber as shown by the mercury and red alcohol thermometers. The mercury thermometer read 35.5 C, while the red alcohol thermometer read 37.5 C. 51
66 Figure 18: Temperature logged with the CO 2 sensor with apparatus on side The apparatus was again changed, and an additional fan was added into the opposite end of where the Incukit was located, as shown in Figure 13 and Appendix A- 1. The additional 120 v olt cooling fan was purchased from RadioShack and had an airflow of 65 cubic feet per minute. The addition of a fan was proposed to circulate the air more efficiently, and to reduce the temperature gradient inside of the bioreactor. A graph of the temperature change over 12 hours with the additional fan is shown in Figure 19. Again, the temperature remained stable and the temperature stayed at its target set temperature throughout the duration of the experiment. The warm-up temperature of the bioreactor also did not overshoot, and the temperature increased slowly to reach its target set temperature. The temperature gradient inside of the apparatus disappeared, and the mercury thermometer and alcohol thermometer both measured 36.9 C. 52
67 Figure 19: Temperature logged with CO 2 sensor with fan added into apparatus When the apparatus is set up to run an experiment, all of its hardware and culture cells are inside before the heater is turned on. The CO 2 is also not added into the system until the bioreactor has heated to about 37ºC. Therefore, the warm-up time for the apparatus was also determined so the user would know when the bioreactor needed CO 2 to be added. A graph of the warm-up time for the apparatus is shown in Figure 20. The start temperature was the temperature of the room, which was at 20.7 C. As shown in Figure 20, it took between 40 and 50 minutes to reach the desired temperature set point of 37 C before the temperature stabilized. 53
68 Figure 20: Warm-up time for the heater to reach 37 C The apparatus lying on its side with two fans, one on each end, was used as the final set up for temperature control in the bioreactor. Insulation was also placed around the apparatus in the final set up as shown in Figure Cell Cultivation In order to properly culture endothelial cells under shear stress in the acrylic bench-top bioreactor, an Erlenmeyer flask to hold media, tubing, and an IBIDI slide were used. The Erlenmeyer flask needed to hold at least 100 ml of cell culture media to provide enough nutrients for the endothelial cells under flow for a 24 hour period. The tubing needed to be biocompatible, allow for CO 2 to diffuse through the tubing inside of the bioreactor, and prevent CO 2 and temperature loss from the tubing outside of the bioreactor. A tubing size of a 1/16 inner diameter and a 1/8 outer diameter was chosen, which is similar to what was previously used in the laboratory. The tubing for inside of the bioreactor was chosen to be silicone which is known to be very permeable to 54
69 CO 2. The CO 2 permeation rate for silicone at a temperature of 25 C was found to be 118,110 cm 3 * mm/m 2 * day * atm. The tubing for outside of the bioreactor was chosen to be clear PVC, and PVC is known to have low permeability to CO 2. The CO 2 permeation rate for PVC was found to be between 1.6 and 2.4 cm 3 * mm/m 2 * day * atm (Massey, 2003). Black norprene rubber tubing was also placed around the outside PVC tubing to help prevent heat loss. A detailed description of this setup is located in Appendix C-I and C-II. A n IBIDI slide was used to culture and adhere the endothelial cells for flow experiments. An IBIDI slide is a small microfluidic slide used to culture cells in a channel under flow or static experimental conditions. The IBIDI slide used was a µ- Slide I 0.4 Luer slide capable for use under a wide range of shear stresses for flow applications and for use in static cell culture studies. The µ-slide I 0.4 Luer IBIDI slide had a channel length of 50 mm, channel width of 5 mm, channel thickness of 400 µm, and a channel volume of 100 µl. The slides also allowed for easy connections to tubes and pumps using Luer adaptors and for easy use with a microscope ( Ibidi ). Using the IBIDI slide allowed for the exposure of the endothelial cells to shear stress conditions similar to a physiological environment Peristaltic Pump To create a fluid shear stress across the endothelial cells cultured in the IBIDI slide, a p eristaltic pump made by MittyFlex and a V ariac were utilized. Since the MittyFlex peristaltic pump did not have an available setting for a low venous shear stress, the Variac was used to decrease the amount of power going to the MittyFlex pump. The desired shear stresses for both arterial and venous shear stress conditions were determined across the IBIDI slide in ml/min using the laminar flow in a narrow slit 55
70 momentum-flux, shear stress, and velocity distribution equations. The equations for the narrow slit momentum-flux in directions x, y, and z relative to the z direction of flow as shown in Equations 3-7 were used. The normal force of gravity from the fluid was also included in the calculation as shown in Equation 6, a nd the fluid is not accumulating momentum and is at steady state. (Eq. 3) (Eq. 4) (Eq. 5) (Eq. 6) (Eq. 7) Using the narrow slit momentum-flux equations, the shear stress and velocity distribution equations were derived and are shown in Equations 8 and 9. In Equation 8, ( x) is the specific vertical position of the flow parabola in the slit. (Eq. 8) (Eq. 9) The ratio of average velocity to maximum velocity for the flow equation and mass flow rate using the Hagen-Poseuille equation were derived from Equations 8 and 9. Using the size of the IBIDI slide and the viscosity of standard DMEM/F-12 cell culture media, the shear stress in dynes/cm 2 was correlated with the volumetric flow rate in ml/min to determine the flow rate using the peristaltic pump. The final equation used to correlate the shear stress in dynes/cm 2 and the volumetric flow rate in ml/min is shown in Equation
71 (Eq. 10) The completed calculations are located in Appendix B-III. To obtain a venous shear stress of 6 dynes/cm 2 across the IBIDI slide, the peristaltic pump needed to deliver 6.15 ml/min, and to obtain an arterial shear stress of 16 dynes/cm 2 the peristaltic pump needed to deliver ml/min, as determined by Equation 10. Since the peristaltic pump needed to be set at a l ow value to obtain the appropriate shear stress rates, a V ariac was connected to the pump to reduce the AC voltage suppled to the pump. The Variac was set at 60%. Using deionized water, the volumetric flow rate in ml/min was determined by setting the peristaltic pump at different values and measuring the amount of water that would flow though the tubing connected to the pump over a time interval. A graph with shear stress values in ml/min correlated with the setting on the peristaltic pump is shown in Figure 21. A best fit line was created to determine the appropriate setting on the peristaltic pump. To measure a shear stress rate of 6 dynes/cm 2 across the IBIDI slide, the setting on the peristaltic pump needed to be set at 23.8, and to measure a shear stress rate of 16 dynes/cm 2, the peristaltic pump needed to be set at There is some uncertainty with the setting on the peristaltic pump accurately creating the desired shear stress. The graph in Figure 21 is not as linear as desired, which may be due to the age of the Variac and peristaltic pump. The Variac can fluctuate in its accuracy depending on the temperature of the room. Also, the peristaltic pump dial was easy to bump, and the settings on the pump were in intervals of 0.5, which could also cause some uncertainty in the set value. 57
72 Figure 21: Correlation of shear stress to the setting on the peristaltic pump Using all of the hardware and materials listed above, the acrylic bench-top bioreactor was created to optimize the physiological conditions for the endothelial cells under shear stress. After the development of the bioreactor, the system was tested to determine if it could accurately mimic physiological conditions and keep the endothelial cells viable over time. 3.2 Procedure The acrylic bench-top bioreactor system as previously described was developed to enhance and mimic physiological conditions for endothelial cells under shear stress. The inside of the system included an acrylic bench-top bioreactor with a 120 volt fan, CO 2 sensor and data logger, and heater/thermocouple system. An IBIDI slide and flask with tubing were also set up inside the bioreactor to allow for the cultivation of endothelial cells under shear stress. The outside of the bioreactor system consisted of a p eristaltic pump, Variac, CO 2 tank, desktop computer to log data, tubing, and power cords. Using the system, test methodology was developed to assess if the bioreactor system could 58
73 mimic physiological conditions and sustain the growth of endothelial cells over time. To create an environment for culturing media, cells, and tissues, a concentration of CO 2 at approximately 50,000 parts per million (5%) was needed to help control a media ph of for optimal endothelial cell growth. A temperature of 37ºC and humidity around 100% were also needed. During the first phase of testing, the CO 2 diffusion through the acrylic bioreactor was tested without any media, with dh 2 O, and then with RPMI media to determine how quickly the CO 2 left the system and how often additional CO 2 needed to be added. The CO 2 needed to have a concentration between 56,000 ppm and 45,000 ppm in the bioreactor system to maintain the desired media ph. Once the CO 2 diffusion rate was determined, the bioreactor was tested with human umbilical vein endothelial cells (HUVEC) for 24, 48, 72, and 96 hours under shear stress to determine if cell growth was maintained in the system. The temperature was also controlled by the IncuKit heater/thermocouple system, and the cells were bathed in media for 100% humidity. During the endothelial cell testing, the CO 2 data logger recorded the bioreactor system s temperature and CO 2 concentration. Finally, the endothelial cells were stained to observe the cell morphology under flow and static conditions, and to affirm endothelial cell growth and viability Testing CO 2 Diffusion A CO 2 tank was connected to the bioreactor system using 1/4 ID X 3/8 OD Clear Tygon PVC tubing that connected from the tank to a 50 mm PTFE in-line filter. The 1/4 ID X 3/8 OD Clear Tygon PVC tubing was then attached to a Male Luer lock, Harvard Apparatus Stopcock, and 33 mm syringe filter. To link the rest of the tubing to the bioreactor, the 33 mm syringe filter was connected to 3/32 ID X 5/32 OD black 59
74 PVC tubing, which was subsequently attached to the steel tubes on the bioreactor system. A similar setup was also constructed on another steel tube on the bioreactor system with a Harvard Apparatus stopcock, 33 m m syringe filter, and 3/32 ID X 5/32 OD black PVC tubing. This setup was used to release the pressure buildup when adding CO 2 into the bioreactor. A chemical resistant EPDM 3/8 X 1/4 stopper was placed inside the two holes on the left side of the bioreactor and connected to 1/16 ID X 1/8 OD clear Tygon PVC tubing that was inside of 3/16 X 3/8 OD Black Norprene Rubber tubing. The tubing was used to apply shear stress to the cells using the peristaltic pump during endothelial cell experiments, and the EPDM stoppers were placed through the holes to prevent CO 2 loss during testing. The EPDM stoppers were also removable for sterilization during endothelial cell experiments. Once the doors on the bioreactor were completely sealed and the bioreactor system was assembled, the valves on the CO 2 tank and the stopcock for relieving pressure was opened. Then, the CO 2 data logger was connected, and the CO 2 was added into the system in 5 second increments by opening the Harvard Apparatus stopcock connected to the CO 2 tank. The CO 2 was measured in the system using the CO 2 data logger, and once the CO 2 concentration reached the desired value, all of the stopcocks and CO 2 tank valves were closed. The optimal range for CO 2 concentration inside of the bioreactor when running an experiment was between 56,000 (5.6%) parts-per-million (ppm) and 45,000 ( 4.5%) parts-per-million. When testing the CO 2 diffusion rate, the CO 2 was added inside the system until the concentration was slightly above 56,000 ppm. The CO 2 data logger was then set to record the CO 2 concentration in 10 minute intervals until the concentration decreased below 45,000 ppm. 60
75 The rate of CO 2 diffusion was then calculated and compared to the estimated calculated value of CO 2 diffusion rate as shown in Appendix B-II. For the first step in testing the CO 2 diffusion through the bioreactor, the bioreactor was completely assembled without any media or dh 2 O and without the IBIDI slide and 125 ml flask. Media and dh 2 O were not used in the first test for the CO 2 diffusion since CO 2 can diffuse into the media and water, and the rate of diffusion through the acrylic bioreactor setup was needed. The Incukit heater/thermocouple system was also used to maintain the bioreactor at 37ºC during the testing. The CO 2 was added into the bioreactor system from the CO 2 tank until the concentration was slightly above 56,000 ppm. The CO 2 data logger was set to log CO 2 concentration data in percentage of CO 2 for every 10 minutes until the program was manually stopped. Every 24 hours, the CO 2 concentration inside of the bioreactor was checked, and once the CO 2 concentration decreased below 45,000 ppm, the data logger was stopped. A best fit line was correlated with the CO 2 concentration data, and the derivative of the best fit line was calculated to determine how quickly the CO 2 concentration was decreasing per minute. This value was compared with the estimated calculated value of CO 2 diffusion. To test the bioreactor with RPMI media or dh 2 O, the bioreactor was tested similarly to what was previously described when determining the CO 2 diffusion through the bioreactor with no media, except 100 ml of either dh 2 O or RPMI media was added to a 125 ml flask. The flask was then connected to an IBIDI slide with 1/16 ID 1/8 OD silicone rubber tubing. The outside of the bioreactor also had the 1/16 ID X 1/8 OD clear Tygon PVC tubing that was inside of 3/16 X 3/8 OD Black Norprene Rubber tubing connected to the EPDM stopper. The Black Norprene tubing was placed inside of 61
76 the peristaltic pump and the peristaltic pump was turned on at a 23.8 to mimic venous shear stress. Again, CO 2 was added to the system and the CO 2 concentration was logged at 10 minute intervals until the concentration fell below 45,000 ppm. These CO 2 values were compared with the previous value and the calculated value to determine how well the acrylic bioreactor could hold CO 2 over time Testing Cell Morphology To determine if the bioreactor system could maintain the growth of endothelial cells over time, the cell morphology of human umbilical vein endothelial cells (HUVECs) was tested for 24, 48, 72, and 96 hours inside of the bioreactor. The HUVEC cells were seeded on an IBIDI slide and exposed to a venous shear stress of 6 dynes/cm 2. During the testing, the bioreactor was to maintain a temperature of 37ºC and a CO 2 concentration between 56,000 ppm and 45,000 ppm. After testing, the HUVECs morphology was observed to affirm endothelial cell growth and viability Human Umbilical Vein Endothelial Cell (HUVEC) Cultures The human umbilical vein endothelial cells (HUVECs) were obtained and harvested from Coriell Cell Repository cell line AG Cell line AG14572 was established from a normal full-term umbilical cord vein, and the cell culture tested positive for the endothelial cell-specific von Willebrand factor and negative for alpha-smooth muscle actin. Once the cells arrived, they were cultured in a 25cm 2 cell culture treated flask on 1% gelatin. The cells were cultured in EGM -2 BulletKit media from Lonza. The EGM -2 BulletKit contained the Endothelial Basal Medium-2 (EBM -2 Medium), 0.5 ml human Epidermal Growth Factor (hegf), 0.5 ml Vascular Endothelial Growth Factor (VEGF), 0.5 ml R3-Insulin-like Growth Factor-1 (RE-IGF-1), 0.5 ml Ascorbic 62
77 Acid, 0.2 ml hydrocortisone, 2.0 ml human Fibroblast Growth Factor-Beta (hfgf-β), 0.5 ml Heparin, 10.0 ml Fetal Bovine Serum (FBS), and 0.5 m L Gentamicin/Amphotericin-B (GA). An additional 30 m l of Fetal Bovine Serum was added to the EGM -2 media for a final FBS concentration of 8%. The cells were grown to 80% confluence in the 25cm 2 cell culture treated flask, and were then subsequently split in a 1:2 ratio into two separate flasks. Before splitting, the cells were washed with Versene and were detached from the cell culture flask using 0.05% Trypsin for 5 minutes at room temperature. Prior to testing the cells in the bioreactor system, the cells were seeded on an IBIDI µ-slide I 0.4 Luer Family as per the manufacturer s instructions with 1% gelatin. The HUVEC cells were between a p assage of 8 and a p assage of 12. For the cells undergoing flow, they were seeded at a confluence of 1.2 * 10 6 cells/ml. The cells cultured for static conditions were seeded at a confluence of 7.0 * 10 5 cells/ml. The slides were incubated at 5% CO 2 and 37ºC with EGM -2 media. The cells undergoing flow were incubated for 24 hour s before exposing the cells to a venous shear stress of 6 dynes/cm 2. The cells under static conditions had media replenished in the slide every 24 hours with 200 µl of the EGM -2 media. The full protocols for the HUVECs are in Appendices C-V and C-VI Shear Stress in the Bioreactor System The IBIDI slides seeded with endothelial cells were placed inside of the bioreactor system to expose the cells to shear stress using a Mittyflex peristaltic pump and Variac. The Variac was set to 60% and the pump was set to 23.8 to expose the endothelial cells to a venous shear stress of 6 dynes/cm 2. Besides the peristaltic pump, the acrylic bioreactor 63
78 system included a 125 ml flask to hold 100 ml of EGM -2 media, a computer fan, Incukit heater/thermocouple system, CO 2 sensor and data logger, desktop computer, and tubing components. The tubing and the tubing components were all autoclavable and consisted of 1/16 ID 1/8 OD silicone rubber tubing for inside of the bioreactor. The outside of the bioreactor also had 1/16 ID X 1/8 OD clear Tygon PVC tubing that was inside of 3/16 X 3/8 OD Black Norprene Rubber tubing connected to the EPDM stopper inside of the holes on the bioreactor apparatus. The tubing on the outside of the bioreactor was also placed inside of the peristaltic pump. Once the slide and flask of media were placed inside of the bioreactor and the bioreactor was sealed, the Incukit heater/thermocouple system and fan were turned on. When the bioreactor system reached approximately 37ºC, CO 2 was added into the system through a CO 2 tank and valves on the bioreactor until the CO 2 concentration was approximately 55,000 ppm. The CO 2 data logger recorded data in 1 or 10 minute intervals for 24, 48, 72, or 96 h ours. Every 24 hours, 50% of the EGM -2 media was replaced in the 125 m l flask. Using an autoclavable three way valve and 28 m m syringe filter on t he tubing outside of the bioreactor, 50 m l of the EGM -2 media was removed from the 125 m l flask. Then, using a 60 cc syringe, 50 ml of fresh EGM -2 media was added back into the 125 flask through the three way valve. Every 24 hours, the CO 2 concentration was checked, and if the CO 2 concentration was nearing 45,000 ppm, more CO 2 was added to the system. After the cells were exposed to the venous shear stress, both the slide in the bioreactor and the static control slide in the incubator were fixed using 4% paraformaldehyde. The completed protocols for the bioreactor setup and media exchange are in Appendices C-II and C-III. 64
79 Fluorescent Cellular Staining Once the cells were fixed with 4% paraformaldehyde, a P BS-Triton X-100 solution was added to the IBIDI slides for 3-5 minutes to permeabilize the cell membrane. After the permeabilization step, 1% blocking solution using Bovine Serum Albumin was added to the IBIDI slides. The 1% blocking solution was left on the slide for one hour. Then, a phalloidin stain was diluted 1:40 with the 1% blocking buffer and was added to the IBIDI slides for 20 minutes. The phalloidin was used to visualize the F-actin in the endothelial cells. Next, the Topro-3 stain was diluted 1:500 with Vectashield Mounting Media and was added to the IBIDI slide. Topro-3 was used to visualize the nucleus inside of the endothelial cells. Between each step, except before the phalloidin was added, the cells were washed 3 times with 1X PBS. The slides were then imaged using an Olympus Fluoview Laser Scanning Confocal Microscope with the magnification set at 20X. The Texas Red-X Phalloidin F-actin stain was excited by a 591 nm light, and the emitted fluorescence was at 608 nm. The To-Pro-3 nuclear stain was excited by a 642 nm light, and the emitted fluorescence was at 661 nm. 65
80 CHAPTER 4 RESULTS 4.1 CO 2 Diffusion Before the HUVECs were tested inside of the bioreactor system, the CO 2 diffusion rate through the acrylic bioreactor was determined with no media or no dh 2 O, with dh 2 O, and with RPMI media. As described previously, CO 2 was added into the system until the concentration was above 56,000 ppm, and then the bioreactor was set up to run until the CO 2 concentration was below 45,000 ppm. The CO 2 concentration was logged using the data logger during the duration of the experiment, and the data for the first test with no media or dh 2 O is shown in Figure 22. A second order polynomial best fit line was fit with the data set as shown in Figure 22. The derivative of the second order polynomial was calculated to determine the slope and diffusion rate in parts-per-million (ppm)/minute. It was assumed that during the beginning of the diffusion tests, the bioreactor system was still acclimating to the temperature and CO 2 concentration inside. Therefore, the time of 500 minutes was used to access the diffusion rate through the bioreactor since at 500 minutes the system was assumed to be stable. Using the derivative of the second order polynomial and converting the ppm/minute to mol/s as described in Appendix B-II, the diffusion rate of CO 2 through the bioreactor was calculated to be 66
81 5.831*10-8 mol/s. Comparing this diffusion rate to the calculated rate of 2.055*10-14 mol/second, the diffusion rate of CO 2 through the bioreactor was much quicker in actuality. Since there were two doors created on the bioreactor as well as holes and tubing, the actual CO 2 diffusion rate should be higher than the calculated value. Figure 22: CO 2 diffusion through the bioreactor system with no dh 2 O or cell culture media The CO 2 diffusion test for the system with dh 2 O was executed similarly as the test with no media or dh 2 O, except a flask with 100 ml of dh 2 O was added into the bioreactor. Also, the peristaltic pump was turned on and the water was pumped through the tubing using the venous shear stress rate. A graph of the rate of CO 2 diffusion and a second order polynomial best fit line with the data are shown in Figure 23. The derivative was again calculated, and the rate of diffusion at 500 minutes was evaluated and converted to mol/second. The rate of CO 2 diffusion with dh 2 O was calculated to be 7.70*10-8 mol/second. This CO 2 diffusion rate was determined to be higher than the predicted diffusion rate. Additionally, the CO 2 diffusion rate for dh 2 O was higher than the diffusion rate with any additional fluid in the system. Since, CO 2 can diffuse into H 2 O, 67
82 some of the CO 2 may have diffused into the 100 ml of water pumped through the system. When CO 2 and water react together, carbonic acid is produced. The carbonic acid then disassociates into to a bicarbonate ion and a proton. Therefore, the observed CO 2 diffusion may have been quicker in the system with the dh 2 O than with no fluid since CO 2 diffusion occurs both out of the bioreactor system and into the 100 ml of dh 2 O inside of the flask. Figure 23: CO 2 diffusion through the bioreactor system with dh 2 O The CO 2 diffusion test for the system with 100 ml of RPMI cell culture media was tested similarly as the test with dh 2 O, and the peristaltic pump flowed fluid through the tubing at a venous shear stress during the test. A graph of the rate of CO 2 diffusion for the RPMI media, and a second order polynomial best fit line with the data are shown in Figure 24. The derivative was again calculated, and the rate of diffusion at 500 minutes was evaluated and converted to mol/second. The rate of CO 2 diffusion with RPMI media was calculated to be 1.006*10-8 mol/second. The diffusion rate for CO 2 in the bioreactor system with cell culture media was much slower than for the dh 2 O and the system with 68
83 no fluid. To control ph in the media, the cell culture media contained sodium bicarbonate to be used as a buffer along with the CO 2. This reaction caused the CO 2 to diffuse in and out of the media. Therefore, the CO 2 was able to be sustained inside of the bioreactor longer when using cell culture media. Also, the cell culture media contained a phenol red ph indicator, and if CO 2 diffused into the media, the cell culture media became red or pink in color. When testing the CO 2 diffusion rate through the system, the cell culture media was observed to stay red in color for the duration of the experiment, showing that the CO 2 was diffusing into the media. Figure 24: CO 2 diffusion through the bioreactor system with RPMI cell culture media Based off of the CO 2 diffusion rates for no f luid, dh 2 O, and RPMI media, it was determined that the CO 2 concentration in the bioreactor system needed to be checked between 20 and 24 hours during each experiment. By checking the CO 2 concentration in the system before 24 hours, the CO 2 concentration would be guaranteed not to decrease below the desired limiting value of 45,000 ppm. 69
84 4.2 Bioreactor Control and Cell Morphology To successfully mimic a physiological environment for culturing and studying endothelial cells under shear stress, an environmental temperature of 37ºC +/- 0.5ºC and an environmental CO 2 concentration between 56,000 ppm and 45,000 ppm were needed. Using a CO 2 sensor and data logger, CO 2 tank, and IncuKit heater system, in vivo conditions were simulated inside of the bioreactor system. The cell morphology was also accessed to determine if endothelial cells were sustained under a venous shear stress of 6 dynes/cm 2 inside of the bioreactor for up to 96 hours Bioreactor Control Four experiments at 24 hours, 48 hours, 72 hours, and 96 hours were run with the HUVEC cells under a v enous shear stress of 6 dynes/cm 2. The CO 2 concentration and temperature were recorded using the CO 2 Data Logger program for the duration of the experiment. The accuracy of the CO 2 sensor was +/- 3% of the measured value, and the accurary of the temperature sensor was +/- 0.4ºC. For the 24 hour experiment, the CO 2 concentration was added until the concentration was slightly below 56,000 ppm. After about 18 hour s, the CO 2 concentration was observed using the Data Logger, and additional CO 2 was added to the system as needed. As shown in Figure 25, the CO 2 concentration was added to the system when the CO 2 concentration was slightly above 48,000 ppm, and the CO 2 was added to about 54,000 ppm. The CO 2 was added into the system before the concentration decreased to 45,000 ppm to prevent the cells and cell culture media from being exposed to a low CO 2 concentration before the end of the experiment. During the 24 hour experiment, the CO 2 concentration successfully stayed between 45,000 ppm and 56,000 ppm. 70
85 Figure 25: CO 2 concentration for the 24 hour flow experiment Figure 26 shows the temperature recorded using the CO 2 Data Logger during the duration of the 24 hour experiment. The temperature stayed within the range of 37ºC +/- 0.5ºC. The small fluctuations seen in Figure 26 are from the heater turning on and off to keep the temperature within the set range. Figure 26: Temperature in the bioreactor system for the 24 hour flow experiment 71
86 For the 48 hou r flow experiment, the CO 2 concentration was added to about 56,000 ppm before starting the experiment. When checking the CO 2 concentration after the HUVECs were under venous shear stress for 24 hours, it was determined that more CO 2 did not need to be added into the system since the CO 2 concentration was still above 50,000 ppm. Figure 27 shows the CO 2 concentration for the 48 hour flow experiment. Although the 24 hour flow experiment needed to have more CO 2 added to the system, differences when sealing the door on t he top of the bioreactor caused the CO 2 concentration to decrease slower during the 48 hour flow experiment than the 24 hour experiment. The top door on t he bioreactor was screwed down into binding posts that were sealed through the holes in the bioreactor apparatus. Since the binding posts were not very strong, the screws couldn t be tightened too much otherwise the binding posts would break; therefore, the CO 2 diffusion out of the bioreactor could change from experiment to experiment. Figure 27: CO 2 concentration for the 48 hour flow experiment 72
87 Figure 28 shows the temperature recorded using the CO 2 Data Logger during the duration of the 48 hour experiment. The temperature stayed within the range of 37ºC +/- 0.5ºC for the duration of the experiment as shown in Figure 28. Figure 28: Temperature in the bioreactor system for the 48 hour flow experiment The 72 hour flow experiment started with an initial CO 2 concentration of about 55,000 ppm. After about 24 hour s the CO 2 level inside of the bioreactor system was increased to about 55,000 ppm to prevent the concentration from decreasing below 45,000 ppm during the rest of the experiment. Figure 29 shows the CO 2 concentration for the 72 hour flow experiment. During the initial edition of the CO 2 into the bioreactor system, the temperature and CO 2 were still stabilizing inside of the cell culture media. Therefore, a larger drop in CO 2 concentration was seen in the first 4 hour s of data collection. The drop in CO 2 concentration was not seen during the 24 hour and 48 hour experiments. 73
88 Figure 29: CO 2 concentration for the 72 hour flow experiment Figure 30 shows the temperature recorded using the CO 2 Data Logger for the 72 hour experiment, and the temperature stayed within the range of 37ºC +/- 0.5ºC for the duration of the experiment. Figure 30: Temperature in the bioreactor system for the 72 hour flow experiment The 96 hour flow experiment is shown in Figure 31, and the initial concentration of CO 2 was slightly below 56,000 ppm. More CO 2 was added into the system at about 30 74
89 hours and 75 hours to prevent the CO 2 from decreasing below 45,000 before the end of the experiment. At about 30 hour s, the CO 2 was added to around 56,000 ppm, and at about 75 hours the CO 2 was added to around 54,000 ppm. Similar to the 72 hour flow experiment, the temperature and CO 2 initialization inside of the bioreactor caused the CO 2 concentration to drop quicker in the initial stages of the experiment compared to the rest of the flow experiment. Figure 31: CO 2 concentration for the 96 hour flow experiment For the 96 hour experiment, the temperature stayed within the range of 37ºC +/- 0.5ºC during the duration of the experiment. Figure 32 shows the temperature recorded during the experiment using the CO 2 Data Logger. 75
90 Figure 32: Temperature in the bioreactor system for the 96 hour flow experiment For each flow experiment, the temperature inside of the bioreactor never exceeded its desired limits. Table 1 shows the maximum, minimum, average, and standard deviation for each flow experiment. The maximum temperature in each experiment never exceeded 37.5ºC, and the minimum temperature in each flow experiment was never below 36.5ºC. Table 1: Summary of temperature inside of the bioreactor during flow experiments Maximum Minumum Mean Standard Deviation 24 hours hours hours hours Cell Morphology The HUVECs were seeded onto IBIDI slides and were exposed to a venous shear stress of 6 dynes/cm 2 for 24 to 96 hours as previously explained. While the cells were exposed to the venous shear stress, 50 ml of EGM -2 was replaced into the system every 24 hours. During the exchange of media, the peristaltic pump was turned off, and 76
91 the media was statically left on the slides. The media exchange took approximately 10 minutes, and the cells were exposed to no fluid flow during that time. After exposure to shear stress, the endothelial cells were stained with Phalloidin to stain the F-actin, and Topro-3 to stain the nucleus. Then, the cells were imaged using the Olympus Flouoview Laser Scanning Confocal Microscope supplied by the University of Dayton. Figure 33 shows what the cells looked like during both exposure to the venous shear stress and the static conditions. As shown in Figure 33, the HUVECs survived during the duration of each experiment in the bioreactor system, and some cells also showed evidence of cell division. In the 24 hour flow experiment in Figure 33, the top middle of the image shows two cells in the process of cell divison in either anaphase or telephase, as there are two cell nuclei (shown by the Topro-3 stain) in one cell membrane. The 48 hour flow experiment also showed evidence of cell division as shown in the bottom middle of the image. Similar evidence of cell division was shown in the bottom middle of the 72 hour flow experiment image and in the top left of the 96 hour experiment image in Figure 33. The HUVECs also had intact cytoskeletons as shown by the Texas Red-X Phalloidin stain, which stains F-actin inside of the cells. F-actin supports the cell structurally by forming the cyotskeleton, and during flow conditions cellular remodeling can occur. As shown in Figure 33, the F-actin is intact in both static and flow conditions. Since the cells exposed to shear stress were not dying, the bio-enviornment was supportive of cell growth for up to 4 days. The HUVECs cultured in static conditions did show evidence of cell death since the cells became so confluent on the IBIDI slide, and since only a small amount of media was on the slide, the cells began to die. Evidence of cell death is shown when the F-actin and nucleus become very condensed, and the F-actin begins to collapse 77
92 into the cell. All of the static flow conditions show evidence of cell death. In the 24 hour static experiment, the bottom middle of the slide shows many cells undergoing cell death. Cell death is also displayed in the static 48 hou r experiment in the bottom left of the image, in the 72 hour image in the middle left, and the 96 hour image in the top left in Figure 33. Figure 33: Static and dynamic fluid flow across HUVECs for 24, 48, 72, and 96 hours in the bioreactor system (scale bar = 150µm). (Direction of flow: ) 78
93 Then using Image J, the HUVECs were analyzed to determine the average orientation of the cells under static and dynamic flow conditions. The angle of the cells was calculated by determining the angle of the cell from the horizontal x-axis to a line drawn through the nucleus along the long axis of the cell. The angle orientation of the cell could be between 0º and 90º, with 0º meaning the cell was completely horizontal and towards the direction of the flow, and 90º meaning the cell was perpendicular to the direction of the flow. The average angle for both the static and dynamic flow conditions is shown in Figure 34. When the amount of time the endothelial cells were exposed to shear stress increased, the cells aligned more in the direction of the flow compared to the static conditions as shown by the F-actin in the cell. Using the F-test, the variances for the static compared to the dynamic flow conditions for each time point were equal, so a one-tailed t-test was used with a P-value of less than The one-tailed t-test was chosen because the static orientation was thought to be greater when compared to the dynamic orientation. The cell alignment for the 48 hour, 72 hour, and 96 hour flow experiments were statistically different than the static experiments. The 24 hour flow experiment was not statistically different from the static experiment. When comparing each static experiment between hours, the Bartlett Test showed the variances were equal. Therefore, a one way ANOVA with a P-value of less than 0.05 was used since the static time points were independent of each other. The one way ANOVA showed that the cell alignment was not statistically significant for the static experiments. Also, when comparing the dynamic flow conditions across the four experiments, the Bartlett Test showed that the variances between the groups were unequal, so a Kruskal-Wallis test with a P-value of less than 0.05 was used. The 24 hour experiment was shown to be statistically different than the 79
94 48, 72, and 96 hour experiments as shown in Figure 34. Also, the 48 hour experiment and 96 hour experiment were shown to be significantly different than the 72 hour experiment. Overall, the 72 hour flow experiment was more statistically aligned than the other flow experiments. The 96 hour flow experiment cells started to become not as aligned as the 72 hour experiment, and the difference may be due to some of the cells unattaching from the surface of the IBIDI slide from the high density of cells. When new cells took the place of the detached cells, the fluid flow across the slide could change, causing an effect in cell orientation. Also, new cells divided into cells that may have taken the place of the detached cells and may not have had enough time to orient in the direction of the flow for the 96 hour experiment. A two way ANOVA was also performed with all of the data, and the results were the same as previously described. Figure 34: Average angle of the orientation of the HUVECs under static and dynamic flow conditions (Bars represent the SEM, *p < 0.05 for dynamic vs. static control, for dynamic vs. dynamic flow, a represents a significant difference from 24 hour flow, b represents a significant difference from 48 hour flow, c represents a significant difference from 72 hour flow, and d represents a significant difference from 96 hour flow using a p < 0.05, Numbers at the bottom of each graph represent number of cells analyzed) 80
95 Using Image J, the HUVECs were then fit into the shape of an ellipse, and the longest and shortest axes of the cell were measured in µm. The longest and shortest axes ratio (LA/SA or cell elongation) was calculated and is shown in Figure 35. Except for the 96 hour experiment, when the cells were exposed to shear stress for a longer period of time, the cells began to become more elongated. The increase in elongation was shown by the increase in the long axis to short axis ratio. Using the F-test, the variances for the static conditions versus the dynamic conditions were unequal. Therefore, a one tailed Mann Whitney test was used with a P-value of less than As shown in Figure 35, the cells in the 48 hour and 72 hour flow experiments were statistically more elongated than the static experiments. Although the 96 hour flow experiment was not statistically different than the static flow experiment, the P-value was When comparing each of the static experiments at the different time points, the Bartlett test showed the variances were unequal, so the Kruskal-Wallis Test was used with a P-value of less than The static conditions were not statistically different for the cell elongation (LA/SA ratio) for each time point. When comparing each of the dynamic flow experiments at different time points, the Bartlett test showed the variances were unequal, so the Kruskal- Wallis Test with a P-value of less than 0.05 was used. For the dynamic flow conditions, the cell elongation was only statistically different for the 72 hour flow experiment compared to the 24 hou r, 48 hour, and 96 hour time points. Since the 72 hour flow experiment is more elongated than the other time points, the 72 hour flow experiment may be the ideal amount of time for the cells to elongate. More experiments may need to be performed to determine the differences in cell elongation between the two different 81
96 time points. A two way ANOVA was also performed with all of the data and the results were the same as previously described. Figure 35: Cell Elongation (Long Axis/Short Axis Ratio) of the HUVECs under static and dynamic flow conditions (Bars represent the SEM, *p < 0.05 for dynamic vs. static control and #p< 0.05 for dynamic vs. dynamic flow, Numbers at the bottom of each graph represent number of cells analyzed) Overall, the 72 hour flow experiment was more statistically aligned and elongated than the flow experiments for the other time points, showing that 72 hours may be the ideal amount of time for the cells to align and elongate towards the direction of flow. Throughout the flow experiments, the only condition changing between experiments was the amount of time the HUVECs were exposed to the venous shear stress. The same cells and the same cell density were on each IBIDI slide, and the temperature and CO 2 levels were also similar. Therefore, the bioreactor system was successful in maintaining and growing endothelial cells under venous shear stress for a period of up to 96 hours. 82
97 CHAPTER 5 CONCLUSIONS AND FUTURE RECOMMENDATIONS A laboratory apparatus was successfully constructed for evaluating monolayers of cells under shear stress, specifically for studying vascular function. The laboratory apparatus consisted of a bioreactor system with a CO 2 sensor, computer fan, IBIDI slide, 125 m l flask, tubing and an Incukit heater/thermocouple system inside of the apparatus. The CO 2 sensor, computer fan, and Incukit heater/thermocouple system were used to control and monitor the temperature and CO 2 inside of the bioreactor system, and the IBIDI slide, 125 m l flask, and tubing were used to culture the endothelial cells and apply a venous shear stress. The outside of the bioreactor system also included a CO 2 tank, computer desktop setup, data logger software, peristaltic pump, and tubing. The peristaltic pump and tubing were used to apply the venous shear stress, and the data logger software recorded the temperature and CO 2 concentration during experiments. Key performance characteristics to test the bioreactor for its capability in studying vascular function included testing the temperature control, diffusion rate of CO 2, and cell morphology, alignment and elongation of human umbilical vein endothelial cells in the bioreactor system. The temperature tests with the Incukit heater/thermocouple system originally showed a t emperature gradient in the bioreactor apparatus. After the addition of a 83
98 computer fan and placing the bioreactor apparatus on its side, the system was able to hold a temperature of 37ºC +/- 0.5ºC for the duration of each experiment. The CO 2 diffusion rate was also determined and compared to the estimated rate of CO 2 diffusion through the system. The calculated estimated rate of CO 2 diffusion was 2.055*10-14 mol/second, while the measured CO 2 diffusion rate for no media and no dh 2 O was 5.831*10-8 mol/second. The measured CO 2 diffusion rate for dh 2 O was 7.70*10-8 mol/second, and the measured CO 2 diffusion rate for RPMI media was 1.006*10-8 mol/second. The actual CO 2 diffusion rate was higher than the calculated rate due to the doors, tubing, and holes drilled into the apparatus affecting the CO 2 diffusion and causing the CO 2 to leave the system quicker. The CO 2 was also maintained between 56,000 ppm and 45,000 during each experiment, and CO 2 was added into the system when needed to prevent the CO 2 concentration from decreasing too low. To test the cell morphology of human umbilical vein endothelial cells in the bioreactor apparatus, the cells were exposed to a venous shear stress of 6 dynes/cm 2 for up to four days. The cells seeded under dynamic flow conditions were more statistically aligned toward the direction of the flow than the cells exposed to static conditions for the 48 hour, 72 hour, and 96 hour flow experiments (P-value < 0.05). Also, the 48, 72 hour, and 96 hour dynamic flow experiments had a statistically significant difference in cell alignment compared to the 24 hour test, and the 72 hour dynamic flow experiment also had a statistically significant difference in cell alignment compared to the 48 and 96 hour dynamic flow experiments (P-value < 0.05). The large concentration of cells on the IBIDI slide may have caused some cells to detach from the slide, which may also explain why the cells were not as aligned during the 96 hour test. The cells seeded under 84
99 dynamic flow conditions were more elongated toward the direction of the flow than the cells exposed to static conditions for the 48 and 72 hour tests, and the 72 dynamic flow experiment was statistically more elongated than the 24, 48, and 96 hour flow experiments ( P-value < 0.05). The endothelial cells stayed viable on the IBIDI slides, and showed low evidence of cell death during all of the dynamic flow experiments. The bioreactor system created was able to reproduce a p hysiologically relevant environment for culturing cells under dynamic shear stress conditions. However, the CO 2 would diffuse out of the system after a period of time and more would need to be added in. By purchasing a CO 2 controller, the carbon dioxide set point could accurately be held inside of the bioreactor, instead of continuously checking the CO 2 concentration and manually adding more CO 2 into the system as needed. It would also be desirable to change the orientation of the bioreactor so the system would be easier to set up. Since the door on t he bioreactor was on t op of the apparatus or was covered by the IncuKit heater system, it was difficult to arrange the IBIDI slide and flask inside. By changing the orientation of the door and placing the door on the front of the system instead of the top or the back, the experiment would be easier to set up in a cell culture hood. T he material of the bioreactor system should also be modified to make the system autoclavable for easier setup and to have a lower thermal conductivity to hold in heat without insulation. Materials such as polypropylene or polycarbonate may be suitable for an autoclavable application with a lower heat transfer rate compared to PMMA. Using the bioreactor, more experiments could also be performed, such as testing the human umbilical vein endothelial cells under an arterial shear stress or studying the expression of different proteins like aquaporin-1 when the cells are exposed to shear stress. 85
100 The bioreactor system created was fully capable of studying endothelial cells under shear stress conditions for studies up to 4 days. With some modifications to the bioreactor system as described, the system could become more efficient during setup and easier to use for long term studies. 86
101 REFERENCES Altman, G., et al., Advanced Bioreactor with Controlled Application of Multi- Dimensional Strain for Tissue Engineerng, Journal of Biomechanical Engineeering, 124.6, pp , Altman, G., G. Vunjak-Novakovic, and P. Stark, Multi-Dimensional Strain Bioreactor, Patent US 2004/ A1, Ando, J. and K. Yamamoto, Effects of Shear Stress and Stretch on Endothelial Function, Antioxidants and Redox Signaling, 15.5, pp , Applikon Biotechnology, last accessed January 20, Asnaghi, M.A., and S. Mantero, Rotating Bioreactor, Patent US 8,507,263 B2,2013 Ateya, D., et al., Volume Cytometry: Microfluidic Sensor for High-Throughput Screening in Real Time, Analytical Chemistry, 77.5, pp , Bacabac, R., et al., Chapter 2: Dynamic Shear Stress In Parallel-Plate Flow Chambers, Journal of Biomechanics, 38, pp , 2005 Ballermann, B. and M. Ott, Implantable Prosthetic Vascular Device Having an Adherent Cell Monolayer Produced Under Shear Stress, Patent US 5,843,781, Barron, V., et al., Bioreactors for Cardiovascular Cell and Tissue Growth: A Review, Annals of Biomedical Engineering, 31.9, pp , Bilodeau, K., et al., Design of a perfusion bioreactor specific to the regeneration of vascular tissues under mechanical stresses, Artificial Organs, 29.11, pp , 2005 Bird, B., W. Stewart, and E. Lightfoot, Transport Phenomena, 2nd edition, John Wiley and Sons, Inc.,
102 Brevig, T., et al., Hydrodynamic guiding for addressing subsets of immobilized cells and molecules in microfluidic systems. BMC Biotechnology, 3, pp 1-11, 2003 Bronzino, J (Eds.), Tissue Engineering and Artificial Organs, 3rd edition, Taylor and Francis Group, LLC., Boca Raton, Brown, T., Techniques for mechanical stimulation of cells in vitro: A review, Journal of Biomechanics, 33, pp 3-14, Buttafoco, L., et al., Physical characterization of vascular grafts cultured in a bioreactor, Biomaterials, 27.11, pp , CDC/NCHS, National Hospital Discharge Survey: 2010 table, Procedures by selected patient characteristics Number by procedure category and age, pp 1-2, Cellix, Biochips, last accessed May 30, 2015 Chaudhuru, J., and M. Al-Rubeai, Bioreactors for Tissue Engineering: Principles, Design, and Operation, Springer, The Netherlands, Cheresh, D. (Eds.), Methods in Enzymology: Angiogenesis: In Vitro Systems, Volume 443, Elsevier, San Diego, Chiu, J.J., and S. Chien, Effects of Disturbed Flow and Vascular Endothelium: Pathophysiological Basis and Clinical Perspectives, Physiological Reviews, 91.1, pp , Cozens-Roberts, C., J.A. Quinn, and D.A. Lauffenberger, Receptor-Mediated Adhesion Phenomena. Model Studies with the Radical-Flow Detachment Assay, Biophysical Journal, 58.1, pp , Dancu, M., Method of Conditioning a Hybrid Synthetic Tubular Structure to Yield a Functional Human Hybrid Coronary Bypass Graft, Patent US B2, Dardik, A., A. Liu, and B. Ballermann, Chronic in vitro shear stress stimulates endothelial cell retention on prosthetic vascular grafts and reduces subsequent in vivo neointimal thickness, Journal of Vascular Surgery, 29.1, pp , Dardik, A., et al., Differential effects of orbital and laminar shear stress on endothelial cells, Journal of Vascular Surgery, 41.5, pp , Dermenoudis, S., and Y. Missirlis, Design of a novel rotating wall bioreactor for the in vitro simulation of the mechanical environment of the endothelial function, Journal of Biomechanics, 43, pp ,
103 Dreyer, L., et al., An advanced cone-and-plate reactor for the in vitro-application of shear stress on adherent cells, Clinical Hemorheology and Microcirculation, 49, pp , Elizondo, D., T. Campbell, and R. Totten, Cardiovascular Bioreactor Apparatus and Method, Patent US B1, Engbers-Buijtenhujis, P., et al., Biological characterization of vascular grafts cultured in a bioreactor, Biomaterials, 27.11, pp , Engineering Toolbox, Air Properties, last accessed June 18, Eppendorf, New Brunswick Cell Culture Bioreactors, eppendorf.com/en/products/bioreactors/, last accessed January 20, Fluxion, Bioflux System: in-vivo Conditions for Live Cell Assays, com/bioflux/, last accessed May 30, Ge, J.J., et al., p38 MAPK inhibitor, CBS3830 Limits Vascular Remodeling in Arterialized Vein Grafts, Heart, Lung, and Circulation, 22.9, pp , GlycoTech, Parallel Plate Flow Chambers, /apparatus/parallel.html, last accessed January 15, Go, A., et al., Heart Disease and Stoke Statistics 2014 update, Circulation, 129, pp e28-e292, Hansen, D., et al., Investigation of the Seizing of Ti6A14V Orthopedic Constructs in Vitro: Further Results in Hanks Balanced Salt Solution, The 221 st Electrochemical Society Meeting, The Electrochemical Society, Seattle, pp 982, Harvard Apparatus, Research Products, regen.com/index.php/products.html, last accessed January 15, Hattori, K., et al., Microfluidic perfusion culture chip providing different strengths of shear stress for analysis of vascular endothelial function, Journal of Bioscience and Bioengineering,118.3, pp , Henry, A., Morphologic Examination of Isolated Vascular Smooth Muscle Cells Cultured Under Shear Stress Using a Novel Bioreactor System, B.S. Honors Thesis, University of Dayton, Dayton,
104 Ibidi, µ-slide I Luer Family, Chambers/m-Slide-I-Luer-Family, last accessed May 28, Incubator Warehouse, Incukit Mini for Desktop Incubators, last accessed May 28, Iwasaki, K., et al., Bioengineered Three-Layered Robust and Elastic Artery Using Hemodynamically-Equivalent Pulsatile Bioreactor, Circulation, 118.S1, pp S52- S57, Johns Hopkins Medicine, Coronary Artery Bypass Graft Surgery, coronary_artery_bypass_graft_surgery_cabg_92,p07967/, last accessed December 6, Kaur, H., R. Carriveau, and B. Mutus, A Simple Parallel Plate Flow Chamber to Study Effects of Shear Stress on Endothelial Cells, American Journal of Biomedical Sciences, 4.1, pp 70-79, Kim, F. et al., Saphenous Vein Graft Disease: Review of Pathophysiology, Prevention, and Treatment, Cardiology in Review, 21, pp , Kodama, A., T. Itoh, and K. Komori, Possible roles of 5-HT in vein graft failure due to intimal hyperplasia 5-HT, nitric oxide, and vein graft, Surgery Today, 44.2, pp , Kohn, J, et al., Cooperative Effects of Matrix Stiffness and Fluid Shear Stress on Endothelial Cell Behavior, Biophysical Journal, 108.3, pp , Krevelen, D.W. and K. Nijenhuis, Properties of Polymers, 4 th ed., Elsevier, Oxford, UK, LaMorte, W., Atherosclerosis, Modules/PH/PH709_Heart/index.html, last modified December 3, 2014, last accessed January 11, Lanza, R., R. Langer, and J. Vacanti (Eds.), Principles of Tissue Engineering, 2 nd ed., Academic Press, San Diego, Lawrence, M.B., L.V. McIntire, and S.G. Eskin, Effect of flow on polymorphonuclear leukocyte/endothelial cell adhesion, Blood, 70.5, pp , Li, F.D., et al., Intimal thickness associated with endothelial dysfunction in human 90
105 vein grafts, Journal of Surgical Research,180.1, pp e55-e62, Li, L., et al., A microfluidic system for the study of the response of endothelial cells under pressure, Microfluidics and Nanofluidics, 16, pp , Lingfeng, Q., et al., SOCS1 Prevents Graft Arteriosclerosis by Preserving Endothelial Cell Function, Journal of the American College of Cardiology, 63.1, pp 21-29, Loscalzo, J., Vascular Matrix and Vein Graft Failure, Circulation, 101, pp , Lu, Y., et al., Converging Parallel Plate Flow Chambers for Studies on the Effect of the Spatial Gradient of Wall Shear Stress on Endothelial Cells, Journal of Biosciences and Medicines, 2, pp 50-56, Ma, P., and J. Elisseeff (Eds.), Scaffolding in Tissue Engineering, Taylor and Francis Group LLC, Boca Raton, Madaric, J., et al., Left internal mammary artery bypass dysfunction after revascularization of moderately narrowed coronary lesions: Color duplex ultrasound versus angiography study, European Heart Journal of Cardiovascular Imaging, 9.2, pp , Massai, D., et al., Bioreactors as Engineering Support to Treat Cardiac Muscle and Vascular Disease, Journal of Healthcare Engineering, 4.3, pp , Massay, L., Peremability Properties of Plastics and Elastomers, 2nd Edition, William Andrew, Massay, L., The Effect of Sterilization Methods on Plastics and Elastomers, 2nd Edition, Elsevier Science, McAllister, T., and N. L Herureux, Bioreactor for the manufacture of tissue engineered blood vessels, Patent US B2, Meyer, U., et al., Design and performance of a bioreactor system for mechanically promoted three-dimensional tissue engineering, British Journal of Oral and Maxillofacial Surgery, 44.2, pp , Meyers, R. (Ed), Molecular Biology and Biotechnology: A Comprehensive Desk Reference, Wiley-WCH, Inc., Canada, Modjarrad, K, and S. Ebnesajjad, Handbook of Polymer Applications in Medicine and Medical Devices, Elsevier, Inc,
106 Momin, A., et al., The association between saphenous vein endothelial function, systemic inflammation, and statin therapy in patients undergoing coronary artery bypass surgery, Journal of Thoracic Cardiovascular Surgery, 134.2, pp , Mun, G.I., S.I. Jang, and Y.C. Boo, Laminar shear stress induces the expression of aquaporin-1 in endothelial cells involved in wound healing, Biochemical and Biophysical Research Communications, 430, pp , Murphy, S., J. Xu, and K. Kochanek, Deaths: Final Data for 2010, National Vital Statistics Reports, 61.4, pp 1-118, NIH: National Heart and Lung Institute, What is Coronary Artery Disease, last modified August 23, 2013, last accessed December 6, Ohashi, T. and M. Sato, Remodeling of vascular endothelial cells exposed to fluid shear stress: experimental and numerical approach, Fluid Dynamics Research, , pp 40-59, Parang, P. and R. Arora, Coronary vein and graft disease: pathogenesis and prevention, The Canadian Journal of Cardiology, 25.2, pp e57-e62, Pazzano, D., et al., Comparison of Chondrogensis in Static and Perfused Bioreactor Culture, Biotechnology Progress, 16.5, pp , Pearce, M., et al. Shear Stress Activates Cytosolic Phospholipase A 2 (cpla 2 ) and MAP Kinase in Human Endothelial Cells, Biochemical and Biophysical Research Communications, 218, pp , Peterson, A., et al., Apparatus and Method for Simulating In Vivo Conditions While Seeding and Culturing Three Dimensional Tissue Constructs, Patent US , Plunkett, N., and F. O Brien, Bioreactors in tissue engineering, Technology and Health Care, 19.1, pp , Prabhakarpandian, B., et al., Microfluidic Devices for Modeling Cell-Cell and Particle-Cell Interactions in the Microvasculature, Microvascular Research, 82.3, pp , Sartorius, Bioreactors/Fermentors, bioprocess/bioreactors-fermentors/?setcountry=220, last accessed January 20,
107 Schulte, J., et al., A Novel Seeding and Conditioning Bioreactor for Vascular Tissue Engineering, Processes, 2.3, pp , Schwarz, R., T. Goodwin, and D. Wolf, Cell culture for three-dimensional modeling in rotating-wall vessels: An application of simulated microgravity, Journal of Tissue Culture Methods, 14.2, pp 51-57, Seliktar, D., et al., Application of Shear Flow Stress to Chondrocytes or Chondrocyte Stem Cells to Produce Cartilage, Patent US A, Sensirion: The Sensor Company, SHT1x Digital Humidity and Temperature Sensor (RH/T) last accessed May 28, Shaikh, F., et al., New Pulsatile hydrostatic pressure bioreactor for vascular tissue engineered constructs, Artificial Organs,29.11, pp , Solaris Group, R&D Autoclavable & SIP Fermenters-Bioreactors, solarisgroup.org/autoclavable-sip-fermenters-bioreactors.html, last accessed January 15, Somahlution, Coronary Artery Bypass Surgery (CABG), medical-professionals/coronary-artery-bypass-surgery, last modified 2014, last accessed October 20, Takagi, T., et al., Method of and Apparatus for Cultivating a Cell or Tissue, Patent US B2, Vaughan, T., M. Haugh, and L. McNamara, A fluid-structure interaction model to characterize bone cell stimulation in parallel-plate flow chamber systems, Journal of The Royal Society Interface, 10.81, Volkmer, E., et al., Hypoxia in Static and Dynamic 3D Culture Systems for Tissue Engineering of Bone, Tissue Engineering Part A, 14.8, pp , Voyvodic, P., D. Min, and A. Baker, A multichannel dampened flow system for studies on shear stress-mediated mechanotransduction, Lab on a Chip, 12, pp , Wang, J., et al., Specificity of endothelial cell reorientation in response to cyclic mechanical stretching, Journal of Biomechanics, 34, pp , Williams, C. and T. Wick, Endothelial Cell-Smooth Muscle Cell Co-Culture in a Perfusion Bioreactor System, Annals of Biomedical Engineering, 33.7, pp
108 928, Wolfinbarger, L., Bioreactor Mediated Recellularization of Natural and Tissue Engineered Vascular Grafts, Patent US B1, Wong, K., et al., Microfluidic Models of Vascular Functions, The Annual Review of Biomedical Engineering, 14, pp , Xiao, Y. and G.A. Truskey, Effect of receptor-ligand affinity on the strength of endothelial cell adhesion, Biophysical Journal,71.5, pp , Xu, Z.C., et al., Engineering of an elastic large muscular vessel wall with pulsatile stimulation in bioreactor, Biomaterials, 29.10, pp , Yun, S., et al., Transcription Factor Sp1 Phosphorylation Induced by Shear stress Inhibits Membrane Type-1 Matrix Metalloproteinase Expression in Endothelium, The Journal of Biological Chemistry,277.38, pp , Zhou, X., et al., Quantifying fluid shear stress in a rocking culture dish, Journal of Biomechanics, 43, pp ,
109 APPENDIX A BIOREACTOR DRAWINGS A.1 Three-Dimensional Drawings of the Bioreactor in NX 7.5 Figure 36, Figure 37, and Figure 38 are three-dimensional images of the benchtop bioreactor using the computer aided design (CAD) software Siemens NX 7.5. Figure 36: Sealed benchtop bioreactor 95
110 Figure 37: Bioreactor with the components inside view 1 Figure 38: Bioreactor with the components inside view 2 96
111 A.2 Two-Dimensional Drawings of the Bioreactor in NX 7.5 Figure 39-Figure 45 are two dimensional images of the benchtop bioreactor using the computer aided design (CAD) software Siemens NX 7.5. The images include all of the dimensions needed to reproduce the bioreactor. Figure 39: Three-dimensional depiction and right side of the bioreactor 97
112 Figure 40: Dimensions for the left and front sides of the bioreactor 98
113 Figure 41: Dimensions for the top view of the bioreactor 99
114 Figure 42: Dimensions for the inside and outside of the top door on the bioreactor 100
115 Figure 43: Dimensions for the front door of the bioreactor 101
116 Figure 44: Dimensions for the left side steel tubes and stopper in the bioreactor 102
117 Figure 45: Dimensions for the right side steel tubes for the bioreactor 103
118 APPENDIX B BIOREACTOR CALCULATIONS B.1 Heat Transfer Across the Bioreactor Calculation To calculate the heat transfer rate across the bioreactor to determine the power needed for purchasing a heater system, the equation for the rate of heat transfer shown in Equation 11 w as used. Q is the heat transfer rate, U is the mean overall heat transfer coefficient, A is the surface area, and T is the change in temperature. Q = U* A* T (Eq. 11) The standard equation for the mean overall heat transfer coefficient for a rectangular system is shown in Equation U = 1 x 1 (Eq. 12) ( + + ) h k h fluida wall To determine the heat transfer coefficient ( h ), the Nusselt number is used as shown by Equation 13 where k is the thermal conductivity of air and L is the length of the wall. (Eq. 13) fluidb To solve for the heat transfer coefficient, the Nusselt number needs to be calculated by using the Grashof (Gr), Reynolds (Re), and Prandtl (Pr) numbers as shown in Equations 104
119 In the Grashof number shown in Equations 14 and 15, g is the gravity at 9.8 m/s 2, is the density of air, (T I T O ) is the change in temperature, L is the length of the rectangular wall, A is the surface area, p is the perimeter, and µ is the viscosity of air. β is the coefficient of thermal expansion and is found using the ideal gas law and is shown in Equation 16. (sides of bioreactor) (Eq. 14) (top of bioreactor) (Eq. 15) (Eq. 16) (Eq. 17) The Grashof number has to be calculated for both the density of the inside air and the density of the outside air, as well as the sides and top of the bioreactor. The calculations are shown in Equations = 3.3 * 10-3 (Eq. 18) (vertical inside sides) (Eq. 19) (vertical outside sides) Eq. 20) (horiz. inside top) (Eq. 21) (horiz. outside top) (Eq. 22) 105
120 Then, using Equation 17, and a Prandtl number of about for air at a temperature of about 30ºC (Engineering Toolbox, 2015), the Reynolds (Re) number is calculated as shown in Equations Re = 7 6 = (1.40 *10 )(0.712) 9.97 *10 (vertical inside side) (Eq. 23) Re = 7 7 = (1.60*10 )(0.712) 1.14 *10 (vertical outside side) (Eq. 24) Re = 5 5 = (4.14*10 )(0.712) 2.95*10 (horizontal inside top) (Eq. 25) Re = 5 5 = (4.72*10 )(0.712) 3.36 *10 (horizontal outside top) (Eq. 26) For the vertical side, since the Reynolds number was between 10 4 and 10 9, Equation 27 was used to calculate the Nussalt number. For the horizontal side, since the Reynolds number was between 10 5 and 10 11, Equation 28 was used to calculate the Nussalt number. Also, the thermal conductivity of air (k air ) is Nu h 4 vertical = 0.59Ra 1/ = (Eq. 27) k air * L A hhorizontal * ( ) 4 p Nu = 0.27Ra 1/ = (Eq. 28) k air Then, using Equations 27 and 28, the heat transfer coefficient numbers were calculated as shown in Equations h h 6 1/ *(9.97 *10 ) *0.026 W = = 4.24 (Eq. 29).2032 m K verticalinside 2 7 1/ *(1.14*10 ) *0.026 W = = 4.38 (Eq. 30).2032 m K verticaloutside 2 106
121 h h 5 1/ *(2.95*10 ) *0.026 W = = 2.60 (Eq. 31) m K ( ) horizontalinside 2 5 1/ *(3.36*10 ) *0.026 W = = 2.69 (Eq. 32) m K ( ) horizontaloutside 2 To calculate the overall heat transfer coefficient (U) for both the vertical sides and the horizontal side, Equation 12 is used. The thickness (x) of the bioreactor system is m and the thermal conductivity of acrylic (k acrylic ) is 0.2. The calculations for both the vertical and horizontal heat transfer coefficients are shown in Equations 33 and 34. U 1 1 W = = = 1.89 (Eq. 33) 1 x m K h k h vertical 2 U verticalinside acrylic verticaloutside 1 1 W = = = 1.21 (Eq. 34) 1 x m K h k h horiz 2 horizinside acrylic horizoutside There are four vertical sides and one horizontal side of the bioreactor since one of the horizontal sides is lying flat on the surface of the table. Using Equation 11, the power needed for the heater is calculated for both the horizontal side and the four vertical sides as shown in Equations 35 and 36. (Eq. 35) (Eq. 36) To determine the total amount of power needed for the heater, both Equation 35 and Equation 36 are added together as shown in Equation 37. (Eq. 37) 107
122 B.2 CO 2 Permeability Across the Bioreactor Calculation Since the bioreactor system is rectangular in shape, the standard molar flux equation for rectangular diffusion is used to determine the estimated CO 2 diffusion rate through the bioreactor. The standard molar flux equation is shown in Equation 38. J dt = C * DAB ( X Ax X A0 ) (Eq. 38) Dx Since the CO 2 diffusion rate is going to be calculated from the molar flux equation, the gas law as shown in Equation 39 is used to determine the concentration of moles/volume. PV= nrt (Eq. 39) P RT n = = C (Eq. 40) V Using the atmospheric pressure of P = 1 atm, the gas constant of (L *atm)/(mol * K), and a room temperature of 293 K, Equation 40 becomes: P 1 1 mol C = = = (Eq. 41) RT * L Since the CO 2 was diffusing through poly(methyl methacrylate) (PMMA), the diffusion rate of CO 2 through PMMA was determined. When searching for the CO 2 diffusion coefficient through PMMA, only the diffusion coefficient of O 2 through PMMA was found. However, since the diffusion coefficient for CO 2 and O 2 for similar glassy thermoplastics was known, the CO 2 diffusion coefficient for PMMA was estimated based off of the correlation between the other types of glassy thermoplastics and their diffusion coefficients for CO 2 and O 2 as shown in Figure 46 (Krevelen and Nijenhuis, 2009). A best fit line for 4 different types of glassy thermoplastics was created, and the best fit line 108
123 was calculated with the diffusion coefficient of O 2 through PMMA to determine an estimated diffusion coefficient of CO 2 through PMMA. Figure 46: Gas permeability correlation with different types of polymers (Krevelen and Nijenhuis, 2009) The CO 2 diffusion coefficient for PMMA was determined to be *10-10 m 2 /second when using the graph and best fit line in Figure 46. Then, the CO 2 concentration in parts per million (ppm) for both the air outside and air inside the bioreactor was converted into mole fractions as shown in Equation molofgas 1 ppm = (Eq. 42) 6 10 molofair Using an atmospheric CO 2 concentration of ppm and a starting CO 2 concentration of 56,000 ppm in the bioreactor system, the CO 2 concentrations were converted to Equations 43 and 44 as shown molofgas 401.3ppm = (Eq. 43) 6 10 molofair 56,000 molofgas 56,000 ppm = (Eq. 44) 6 10 molofair 109
124 Substituting the values above into Equation 38 and using a thickness of meters for the bioreactor thickness, Equation 38 becomes: J dt * , mol = * *( ) = 1.849*10 (Eq. 45) m * s To convert the molar flux from mol to 2 m * s mol, the molar flux is multiplied by the surface s area of the bioreactor system, where the surface area includes 5 out of 6 of the sides of the bioreactor. One side of the bioreactor is not included because one side of the system is touching the table below. The surface area was calculated to be m 2. Multiplying the molar flux with the surface area, the rate of diffusion becomes Equation 46. J * A = * dt mol s (Eq. 46) This value can be compared with the recorded values of CO 2 diffusion through the system. The values recorded with the CO 2 data logger were recorded in parts-per-million (ppm)/minute, and need to be converted into mol s to compare the values with the estimated value calculated above. First, the values are converted from ppm to mol of gas/mol of air using Equation 42. Then using Equation 41, multiplying the calculated concentration by Equation 42 converts the measured value into mol L * min below shows an example calculation for a measured rate of 8.73 ppm/min.. Equation 47 ppm 8.73 molofgas 1 1 mol 7 mol 8.73 = * * = 3.638*10 (Eq. 47) 6 min 10 molofair min L L * min 110
125 To convert the mol L*min to mol, Equation 47 is divided by 60 as shown in Equation 48. L * s 3.638*10 mol 1 mol * min = 6.06 *10 (Eq. 48) L * min 60 L * s 7 9 Then, to convert from mol L * s to mol, Equation 48 was multiplied by the total volume of s the inside of the bioreactor system, which was 9.63 Liters as shown in Equation *10 mol L * s * 9.63 L = * 10-8 mol s (Eq. 49) The general equation to covert from ppm to Equation 50. mol for this bioreactor system is shown in s ppm x 1 1 mol x = * * *9. 63 = x (Eq. 50) 6 min s 111
126 B.3 Shear Stress Across the IBIDI Slide Calculation The shell momentum balance equation is used to set up the calculations for determining the shear stress across the IBIDI slide similar to Figure 47 where the: {momentum in} {momentum out} = 0 (Eq. 51) Figure 47: Schematic for laminar flow through a narrow slit (Bird, et al., 2007). Assuming steady state through the system, the flow continuity equation shown in Equation 52 is used. (Eq.52) Since the fluid does not flow in the x and y directions, the x and y derivative cancel out and Equation 53 becomes: (Eq.53) Since the fluid through the slide is incompressible and the x direction has spatial changes because of the changes in the surface from the top and bottom of the slide, variation in fluid flow is only in the z direction. Using the momentum flux tensor and equations, a momentum balance in the z direction is set up as shown in Equations The normal force of gravity from the fluid was also included in the calculation as shown in Equation 57, and the fluid is not accumulating momentum and is at steady state. 112
127 (Eq. 54) (Eq. 55) (Eq. 56) (Eq. 57) In Cartesian coordinates, the shear stresses for the x, y and, z directions for a Newtonian fluid are directly proportional to the time rate of deformation of a fluid element and are shown in Equations (Eq. 58) (Eq. 59) (Eq. 60) Using equation 59, the shear stress in the y direction cancels out because the velocity in the z direction is not dependent on the y direction and there isn t fluid flow (velocity) in the y direction. Using equation 55, the flux in the y direction also cancels out because there isn t any shear stress or velocity in the y direction. Utilizing equation 58, there isn t a velocity in the x direction, but there is a velocity in the z direction that is dependent on the x direction. Using Equation 54, since there isn t a velocity in the x direction, but there is a shear stress in the x direction from the velocity in the z direction, the flux in the x direction only incorporates shear stress. The shear stress in the z direction also cancels out when using Equation 60 because from the continuity equation and because the velocity in the z direction is dependent on the x direction not the z direction. Also, since the fluid is an incompressible fluid, 113. Therefore, the flux equation in the
128 z direction only incorporates the velocity and pressure variables. The flux momentum equation is added together as shown in Equation 61. (Eq. 61) Since the flux in the y direction cancels out and the flux in the x and z directions only incorporate the variables described above, Equation 61 is rewritten as shown below: (Eq. 62) Since the velocity in the z direction is the same on both sides of the slide, the velocity in Equation 62 cancels out. The volume of the slide can also be divided to eliminate the width and length of the slide. Equation 62 then becomes: (Eq. 63) Taking the derivative of Equation 63 to obtain the shear stress, before integration, the equation then becomes: (Eq. 64) Integrating, Equation 64 then becomes Equation 65 for the shear stress across the parallel slide. (Eq. 65) Since the stresses are not specified at any boundary, C 1 cannot be solved until deriving the velocity equation. Substituting for ) and using Equation 65, a velocity distribution equation can be derived as shown in Equation 66. (Eq. 66) 114
129 Integrating the new equation and taking the boundary conditions where at x = B,, and x= -B,, C 1 = 0 and C 2 =. The velocity distribution equation then becomes Equation 67 as shown. (Eq. 67) Then, the equation for shear stress is also determined using C 1 = 0 a nd is shown in Equation 68. (Eq. 68) Then, the ratio of the average velocity to maximum velocity using Equation 67 is determined. Since at x = 0,, so. Replacing, Equation 67 becomes: (Eq. 69) Then the average velocity equation shown in Equation 70 is used, and the average velocity is obtained by dividing the volumetric flow rate by the cross-sectional area. (Eq. 70) Integrating Equation 70 using the cross-sectional area and substituting in and Equation 69, Equation 70 becomes: (Eq. 71) )dx (Eq. 72) 115
130 (Eq. 73) Then, using the mass flow rate from Equation 74, the mass flow rate was derived using the volumetric flow rate and Hagen Poseuille equation as shown. (Eq. 74) (Eq. 75) (Eq.76) The volumetric flow rate (Q) and density ( ) is substituted in for in Equation 76. Also, for, /x is substituted in for Equation 76. As the shear stress across the bottom of the slide is needed, B is substituted in for x since B is the distance from the coordinate plane to the edge of the parallel plate where the shear stress is located. The final equation for shear stress with the volumetric flow rate is shown in Equation 77. (Eq.77) Using the size of the IBIDI slide and the viscosity of standard DMEM/F-12 cell culture media, the shear stress in dynes/cm 2 was correlated with the volumetric flow rate in ml/min to determine the flow rate through IBIDI slide using the MittyFlex peristaltic pump. The IBIDI slide had a width (W) of m, and the DMEM/F-12 cell culture media had a viscosity (µ) of Pa-s (Bacabac, et al., 2005). Also, since the thickness of the total plate is 2B or meters, B would be meters ( Ibidi, 2015). The final equation to correlate the shear stress with the volumetric flow rate, while 116
131 using the desired units of dynes/cm 2 for the shear stress and ml/min for the volumetric flow rate, is shown in Equation 78. (Eq. 78) 117
132 APPENDIX C LABORATORY PROTOCOLS C.1 Building the Bioreactor Purpose: To build a portable bench top bioreactor for cell shear stress applications that mimics physiological and environmental conditions of temperature and CO 2. Materials: - Two 9 3/4" W x 8 1/2" D x 13 1/2" Acrylic Boxes (USA Scientific Part # ) - Silicone Rubber 3/16 thick Adhesive Back (McMaster Carr Part #8991K55) - JB Weld Epoxy 8265S (JB Weld Part #14956 from Walmart) - Silicone Sealant Model # GE500 3TG (GE Silicone II Part # from Home Depot) - #10-32 X 1-1/2, #8-32 X 1, and 1/4-20 X 1 1/2 Machine Screws (Lowes Part # 57855, Part # 57866, Part # 67796) and Washers (Lowes Part # 3834, Part # , McMaster Carr Part # 92141A029) - Threaded Binding Post 1/2 Aluminum Screw Extensions (Lowes Part # ) ID X 5/32 OD Stainless Steel Tubing (Grainger Part #4NUD7) ID X 1/8 OD Stainless Steel Tubing (Grainger Part #4NUC4) - Extreme Temperature Silicone Rubber Gasket Material 1/8 thick (McMaster Carr Part #5812T14) V Craftsman C3 3/8 Compact Power Drill (Sears Part # P) with Drill Bit diameters 5/32, 1/2, 3/16, 7/32, 5/16, 1/8 - Dewalt 7.5 Amp, 12,000 RPM Paddle Switch Small Angle Grinder ( Home Depot Part # DWE4012) - Dewalt General Purpose Metal Grinding Wheel (Lowes Part # ) - Inch Tap and Die Set (Home Depot Part # 7558) with Tap Thread #1/4-20 and Tap Thread # Grit 9. X 11. Sandpaper (3M Part # from Home Depot) AWG Blue Nylon Insulated Male Disconnect Wire Connector (Ace #34529) AWG Blue Nylon Insulated Female Disconnect Wire Connector (Grainger # 5X425) - Stranded Wire 300V AC for Fan ( McMaster Part # 8054T17) 118
133 - Hole Puncher#8-32 X 1/2 Binder Head Nylon Machine Screws (Lowes Part # )Chemical Resistant EPDM Rubber Stopper 3/8 large end and 1/4' small end (McMaster Carr Part # 6448K91) V AC 4 Fan (RadioShack Part # C) - X-Acto Knife (Michaels Item # ) - Heater/Thermocouple (Incubator Warehouse Part #1099) - CO 2 Sensor and Data Logger (GasLab Software) (CO 2 Meter.com Part # CM-0025) - Permanent Marker Building the Bioreactor: 1. Select side of the acrylic box, opposite of the side where the box top hinge is located, to cut out the door. 2. Measure a size hole 4 1/2 x 7 on the side of the bioreactor where the door will be cut, and draw an outline of the door with a permanent marker. The hole will be located 1 1/2 from the bottom of the bioreactor, and 1 1/4 from each side of the bioreactor. 3. Using the angle grinder, cut a hole in the side of the box where the outline is located. 4. Sand the edges of the inside of the hole with the 220 Grit 9 X 11 sandpaper down until smooth. Figure 48 shows the size and location of the side door cut out on the bioreactor system. Figure 48: Image of the side door cut out on the bench top bioreactor 5. Take the other acrylic box, and measure a 6 1/2 X 9 rectangle on any one side of the box. Draw an outline of the rectangle to create the door using a permeant marker. 6. Using the Dewalt angle grinder, cut a hole in the side of the box where the outline was drawn. 7. Sand down the edges of the outside of the door with the 200 Grit 9 X 11 sandpaper until smooth. 8. Take the 3/16 thick silicone rubber with the adhesive back, and cut out a gasket the size of 5 1/2 X
134 9. Measure a 4 1/ 2 X 7 1/ 4 square in the middle of the silicone rubber with the adhesive back, and cut out the square to make a hole. The silicone rubber should have a 1/2 thick border. Round out the edges of the silicone rubber as shown in Figure 49. Figure 49: Image of the silicone rubber on the door of the bioreactor 10. Remove the adhesive cover and place the silicone rubber with the adhesive back on the outside door that was cut with the Dewalt angle grinder. The silicone rubber should be placed 1/4 from the bottom of the door and ½ from each side of the door. Figure 50 shows the door with the adhesive silicone placed on top of it. Figure 50: Image of the side door for the bioreactor 11. Drill two 3/16 holes on the front of the bioreactor 3 1/ 4 from the top of the bioreactor and 1 ¼ from each side of the bioreactor. Drill two more 3/16 holes on the front of the bioreactor 1 1/4 from each side of the bioreactor and 1 from the bottom of the bioreactor. The drilled holes are shown in Figure 48, above. 12. Drill two 3/16 holes through the door of the bioreactor ½ from the top of the door and 3/8 from each side of the door. Drill two more 3/16 holes through the door of the bioreactor 3/8 from the bottom of the door and 3/8 from each side of the door. The drilled holes are shown in Figure 50, above. 13. Match the holes in the door with the holes in the front box cut out. Place a piece of tape on t he top right corner of the outside of the door to signify the correct orientation of the door when being placed on the box. 120
135 14. Prepare the JB Weld epoxy by mixing 1/2 of the steel mix with 1/2 of the hardener mix. 15. Take the 1/2 aluminum screw threaded posts, and epoxy the posts to the front box cutout with the threads facing upwards in the drilled holes. Epoxy takes 24 hours to dry. The 1/2 aluminum screw threaded posts with epoxy are shown in Figure To seal the front door with the adhesive rubber gasket on to the bioreactor, use four #8-32 X 1 screws with four #8 size washers. 17. Remove the hinge from the original lid on t he top of the bioreactor box using scissors or an X-Acto knife. 18. Drill 8 holes on the top of the box using the power drill. Two holes should be drilled using the 7/32 drill bit and tapped with a 1/4-20 thread tap. These two holes should be placed 1/8 from the bottom and 1/4 from the slides of the front of the bioreactor box. The other six holes should be drilled using the 5/32 drill bit and tapped with a #10-32 thread tap. One hole should be located 1/8 from the bottom and 4 from the sides in the front of the bioreactor box. Two holes should be located 4 from the bottom and 1/4 from the slides of the front of the bioreactor. Two holes should be located 7/8 from the back of the bioreactor and ¼ from the sides of the bioreactor. One hole should be located 4 from the slides of the bioreactor box and 1/4 from the back of the bioreactor box. 19. Take the top lid of the bioreactor that was removed and line up the lid with the top of the box and the drilled holes. Leave 1/2 of the door over the top front of the bioreactor. Mark where the holes in the bioreactor align with the lid using a permanent marker. The holes should all be located with the same dimensions as the previous step, except for the three front holes. Since the top of the door is ½ longer than the top of the bioreactor, two of the front holes should be located 5/8 from the front of the bioreactor and ¼ from the sides. The other hole should be located 5/8 from the bottom and 4 from the sides. Figure 51 shows where the holes and screws should be located on the top of the bioreactor and top lid. Figure 51: Image of the top and top door of the bioreactor with the screws in place 20. Drill holes in the bioreactor where the marks are located using 7/32 drill bit for the front two holes, and a 5/32 drill bit for the rest of the holes. The holes should be drilled through the lid. 121
136 21. Take the extreme temperature silicone rubber gasket material, and cut out a s quare piece 8 1/4 X 8 1/4 in size. Line up the silicone rubber gasket material with 1/8 on the right and left side over the lid of the bioreactor and the back lined up with the back of the lid of the bioreactor. Mark where the holes in the top of the bioreactor align with the holes drilled in the lid. 22. Using a hole puncher, punch out holes for the marks in the silicone rubber gasket material. 23. Using the permanent marker, draw a rectangle 2 1/2 from the back of the silicone rubber gasket and 5/8 from each side of the silicone rubber gasket. The rectangle should be 3 1/4 long and 7 wide. In the middle of the drawn rectangle, draw a 1 square flap so that 3 of the width is on each side. Draw this flap on each side of the rectangle. 24. Use an X-Acto knife and cut out the drawn rectangle. 25. Mark two holes to go on the square flap 3/8 from the top of each flap and 4 1/8 from the left and right side of the silicone rubber gasket. 26. Using the hole punch, punch out each hole. This is where the heater will be placed. Figure 52 shows what the silicone rubber gasket material looks like after it has been cut and the holes have been made. Figure 52: Image of the extreme temperature silicone rubber gasket for the bioreactor lid 27. Using the Binder Head Nylon Machine Screws, screw down two sides of the heater onto the rubber gasket. Place the heater with the heater plug facing the right side of the bioreactor and the fan of the heater facing upwards. The heater should look similar to Figure 51, above. 28. Plug the heater into the wall and set the temperature of the heater to 37 C as explained in the Testing the Temperature protocol. Unplug the heater. 29. Using 6 #10-32 X 1-1/2 screws and two 1/4-20 X 1-1/2 size screws with the #10 and ¼ washers, seal the silicone rubber gasket on to the top of the bioreactor using the original top lid and a screwdriver. 30. On the left side of the bioreactor, drill two 5/32 holes both located 1 1/4 from the top of the bioreactor side and 3 1/4 from each side of the bioreactor. 122
137 31. On the right side of the bioreactor, drill four 5/32 holes. Two holes are located 2 1/4 from each side of the bioreactor and 2 from the top of the bioreactor. The other two holes are located 4 from the top of the bioreactor and 2 1/4 from each side of the bioreactor. 32. Cut six 1 sizes of stainless steel tubing #4NUD7. Place the steel tubing inside each hole, with 1 1/ 2 on t he outside of the bioreactor and flush with the edge of the bioreactor on the inside. 33. Seal all six of the steel tubes on the box by placing epoxy on both sides of the steel tubes where the tubes touch the walls of the bioreactor. 34. Cut two pieces of the stranded wire 7 1/2 long. 35. In the two bottom steel tubes on the right side of the box, place the wires through the tubes with 3 of the wire inside of the bioreactor, and 4 of the wire outside of the bioreactor. 36. Attach two AWG blue nylon insulated male disconnect wire connectors to one end of each of the cut stranded wires. 37. Using the silicone sealant, seal the wires into the steel tubes where they touch the outside of the steel tubes. After 24 hours, seal the wires with the epoxy where the silicone sealant was used. The epoxy is used for a stronger seal, and it takes 24 hours to dry. Figure 53 shows the wires attached to the inside of the steel tubes. Figure 53: Image of the wires for the fan attachment 38. Attach the other two AWG blue nylon insulated male disconnect wire connectors to the other ends of the cut stranded wires that are connected to the bioreactor. 39. Attach two AWG blue nylon insulated female disconnect wire connectors to the two ends of the wires on the 120 V AC 4 Fan. Also, attach two AWG blue nylon insulated female disconnect wire connectors to two ends of the plug for the fan that will attach into the wall. 40. On the right side of the bioreactor, drill a 1/2 hole through the bioreactor and in between the top two steel tubes. The hole should be located 2 from the top of the box and 3 1/2 from the left edge of the right side of the box. 41. Place the cords for both the CO 2 sensor and the heater/thermocouple through the 1/2 diameter hole. Seal the cords down with the silicone sealant. Wait 24 hours and then seal the cords down with epoxy on top of the sealant for a stronger seal. Wait another 123
138 24 hours for the epoxy to dry. Figure 54 shows what the cords will look like after they are sealed on the bioreactor. Figure 54: Cords sealed into the ½ diameter hole on the right side of the bioreactor 42. Drill two 5/16 holes on the left side of the bioreactor. Both holes should be located 4 from each edge. One hole should be located 3 from the bottom of the bioreactor, and the other hole should be located 6 1/8 from the bottom of the bioreactor. 43. Cut two 1 3/4 size stainless steel tubing #4NUC Drill two 1/8 holes through the EPDM rubber stoppers. Place the steel tubing inside each hole with 1 on each side of the stopper, and epoxy the steel tubing on to both sides of the rubber stopper. Let the epoxy dry for 24 hours. 45. Place the stoppers with the steel tubes into the holes of the bioreactor with the 3/8 facing the inside of the bioreactor and the ¼ facing outside. These stoppers are removable for sterilization. 46. Before using the bioreactor, check the epoxy and silicone sealant has dried for at least 24 hours. 124
139 C.2 Assembling the Bioreactor Purpose: To assemble and prepare the bioreactor for experiments for cell monolayers exposed to shear stress. Materials: - Built Bioreactor from Building the Bioreactor Protocol - 3/32 ID X 5/32 OD Black PVC Tubing (McMaster Carr Part #5321K32) - 1/16 ID X 1/8 OD Clear Tygon PVC Tubing (McMaster Carr Part #6546T33) - 1/16 ID X 1/8 OD Silicone Rubber Tubing (McMaster Carr Part #51135K12) - 3/16 ID X 3/8 OD Black Norprene Rubber Tubing (McMaster Carr Part #51075K51) - 1/4 ID X 3/8 OD Clear Tygon PVC Tubing (McMaster Carr Part #6519T13) - IBIDI Slides u-slide I 0.4 Luer Family (Ibidi Part #80176) - Polystyrene Foam Insulation, R= 5.0 (Lowes Part # ) - 28 mm PES Syringe Filters, Pores Size 0.2 um (Fisher Scientific Part # ) - 50 mm PTFE In-line Filters Pore Size 0.2 um (Millex Part # SLFG05010) - 33 mm Fisher Scientific Syringe Filters Pore Size 0.45um (Fisher Scientific Part # ) - Fiber Glass Insulation R-13 (Lowes Part #31116) - Two 125 ml Erlenmeyer Flasks (Fisher Scientific Part # B) - dh2o or EGM-2 Cell Culture Media (Lonza Part # CC-3162) - Rubber Stopper #5 (Fisher Scientific Part #14-130G) - #10-32 X 1-1/2, #8-32 X 1, and 1/4-20 X 1 1/2 Machine Screws (Lowes Part # 57855, Part # 57866, Part # 67796) and Washers (Lowes Part # 3834, Part # , McMaster Carr Part # 92141A029) - 3/32 Male Polypropylene Luer Locks (Qosina Part # 11535) - 3/32 Female Polypropylene Luer Locks (Qosina Part # 11534) - Male X Male Luer Connectors (Cole Parmer Part # UX ) - Female luer lock to three-way valve adapter, kynar body and polypropylene insert (Cole Parmer Part # EW ) - Peristaltic Pump (MittyFlex Part # ) - Desktop Computer - Heater/Thermocouple System (Incubator Warehouse Part #1099) X 1/2 Binder Head Nylon Machine Screws (Lowes Part # ) V AC 4 Fan (RadioShack Part # C) - CO2 Sensor and Data Logger (CO2Meter.com Part # CM-0025) - Female X Female 1/4 CO 2 Needle Valve (McMaster Carr Part # 7833K76) - CO2 Tank - Gamma sterilized Petri Dishes (Fisher Scientific Part # FB ) - Two thermometers, one 6 and one 12 (alcohol or mercury thermometers work) - Miniature Cable Tie with a 0.36 thickness (Grainger Part # 36J127) - Three, 1/2 Round Rubber Bumper Feet (McMaster Carr Part # 9540K12) 125
140 - Harvard Apparatus 3-way Stopcocks (Harvard Apparatus Part # ) - EGM -2 BulletKit Cell Culture Media with additional 6% FBS (Fetal Bovine Serum) (Lonza Part # CC-3125 and Fisher Scientific Part # ) - JB Weld Epoxy 8265S (JB Weld Part #14956 from Walmart) - X-Acto Knife (Michaels Item # ) - 25 ml Disposable Polystyrene Serological Pipets (Fischer Scientific Part # K) - Drummond Scientific Pipet-Aid Pipet - 2 Compact Handheld UV Lamps 254 nm UV (UVP Model UVG-11 Part # ) - Fisherbrand 200 Series Spectacles (Fisher Scientific Part # ) - Variac (University of Dayton) - Covidien Luer Lock Sterile Syringe 140 cc (Grainger Part # 8YAE0) Assembling the Bioreactor: NOTE: The section below needs to be followed and checked before each experiment, but not each step needs to be fully repeated before each experiment. 1. Check the top lid of the bioreactor to confirm the heater fan is facing towards the lid, and the heater is attached to the heater connector inside of the bioreactor. 2. Check that the silicone rubber gasket is lined up with the holes in the top of the bioreactor, and that the silicone rubber gasket is completely sealed around the top of the bioreactor. There should be a dark black line around the edges of the door to indicate an appropriate seal. Figure 55 shows what the seal should look like around the lid of the bioreactor. Figure 55: Seal around the lid of the bench top bioreactor 3. Cut four, 2 pieces of the 3/32 ID X 5 32 OD black PVC tubing. On one end attach each piece of the tubing to a 3/32 male luer lock. Two of the black PVC tubing pieces/luer locks should also attach to a male X male luer connector. 4. Place the black PVC tubing into an autoclavable bag and autoclave the black PVC tubing in a dry gravity 2 autoclave cycle. 126
141 5. Place 4 Harvard Apparatus stopcocks into 70% ethanol for 30 minutes. 6. Add a 33 mm Fisher Scientific syringe filter to each end of the black PVC tubing. Two syringe filters should have the inlet facing the bioreactor, and two syringe filters should have the inlet facing away from the bioreactor. 7. Place each stopcock on the end of each syringe. Shut the valves on the stopcocks. Figure 56: 33 mm filter connected to the stopcock and bioreactor shows what the syringe filter, stopcocks, and luer locks should look like. Figure 56: 33 mm filter connected to the stopcock and bioreactor 8. Add four small cable ties around the black tubing where the black PVC tubing attaches to the steel tube in the bioreactor and tighten the cable ties until the tubing is snug. NOTE: The black PVC tubing pieces and attachments do not need to be autoclaved, sterilized, and put together before the start of each experiment. The PVC tubing needs to be replaced when the tubing starts to become loose or deteriorate. 9. Attach a CO 2 tank to the wall and secure the tank with wall bracket. 10. Attach the 1/4 CO 2 Needle Valve to the tank. 11. Cut between 4 and 5 of the 1/4 ID X 3/8 OD Clear Tygon PVC Tubing and connect one end of the tubing to the needle valve on the CO 2 tank and the other end to 50 mm PTFE in-line filters. The inlet of the filter should be facing the CO 2 tank. 12. Cut a 2 piece of the 1/4 ID X 3/8 OD Clear Tygon PVC Tubing and attach it to the other side of the PTFE in-line filter. Also attach a 3/32 female luer lock to the other end. Figure 57 shows what the PTFE and tubing connections should look like. Figure 57: 50 mm PTFE in-line filter connected to the CO 2 tank 127
142 13. Mix the epoxy together by mixing 1/2 of the steel tube with 1/2 of the white hardener. 14. Take the three round rubber bumper feet and epoxy the feet together. Let the round rubber bumper feet dry for at least 24 hours. Setting up the Bioreactor: NOTE: The section below needs to be repeated before the start of each experiment unless noted. 15. Cut two pieces of the 1/16 ID X 1/8 OD Clear Tygon PVC Tubing. One piece should be 1 and the other should be 6. Cut two pieces of the 3/16 ID X 3/8 OD Black Norprene Rubber Tubing. One piece should be 9 and the other should be 5. Place the clear Tygon PVC tubing inside of the black Norprene rubber tubing. 16. Take the female luer lock to three-way valve adapter and attach one male luer lock to one end, one female luer lock to one end, and a male X male connector to the middle of the three-way valve adaptor. Figure 58 shows what the three way valve adaptor will look like with the luer locks connected. Figure 58: Three way valve adaptor set up 17. Attach the clear Tygon PVC tubing to each end of the three-way valve adaptor, and leave the middle valve open. Turn the valve so the fluid will flow through the tubing, but not through the middle piece. 18. Place the setup inside of an autoclavable bag and autoclave the tubing in the dry gravity 2 cycle. 19. Cut two ID X 1/8 OD Stainless Steel Tubes 4 and 5 in length. 20. Drill two 1/8 holes into the Rubber Stopper #5 that are 1/4 apart. 21. Place the two steel tubes into the Rubber Stopper with the top of the steel tubes at the same level. Figure 59 shows what the steel tubes should look like inside of the rubber stopper and the 125 ml Erlenmeyer flask. NOTE: Steps do not need to be repeated after the steel tubes are cut and placed inside of the stopper unless the tubes become loose. 128
143 Figure 59: Steel tubes inside of the rubber stopper and 125 ml flask 22. Cut three pieces of the 1/16 ID X 1/8 OD silicone rubber tubing. Make two pieces 12 and one piece Attach two male luer locks on one side of one 8 piece and one side of one 12 piece of the silicone rubber tubing. Attach the other side of the 8 piece to the smaller of the steel tubes. Attach the 12 piece with the luer lock onto the steel tube of the small EPDM stopper and attach the other 12 piece without the luer lock to the other steel tube of the other small EPDM stopper. Attach the 12 pieces to the side with the larger diameter of the EPDM stopper. Attach the 12 with no luer lock to the longer of the steel tubes in the 125 ml Erlenmeyer flask. 24. Place both the 125 ml Erlenmeyer flask and the Stopper #5 with the steel tubes into an autoclavable bag and autoclave the setup in the dry gravity 2 cycle. Preparing the Bioreactor for an Experiment: NOTE: The section below needs to be repeated before the start of each experiment unless noted. 1. Once the parts in the above section are in the autoclave, place the bioreactor set up inside of the cell culture hood with the cords facing towards the back of the hood. 2. Put on the safety spectacles. 3. Turn on both Compact Handheld UV Lamps 254 nm and place them inside of the bioreactor with the both UV lights facing the heater of the bioreactor. 129
144 4. Place the fan, screws, CO 2 sensor, and top cover of the bioreactor inside of the hood with the top cover facing downward. Figure 60 shows what the bioreactor should look like in the hood with the UV lights on. Figure 60: Setup of the bioreactor in the hood for sterilization 5. Close the cell culture hood and put the UV light on inside of the hood. Sterilize the parts of the bioreactor for 30 minutes. 6. Turn off the UV hood light and move the two UV handheld lights to the outside of the bioreactor facing the two holes in the left side of the bioreactor. These holes need to be sterilized in case the holes get touched when putting in the EPDM stoppers. Figure 61 shows the setup for the UV lights when sterilizing the holes in the bioreactor. Figure 61: Setup of the UV lights for sterilization of the bioreactor holes 7. Turn the top cover of the bioreactor, fan and CO 2 sensor over to sterilize the other sides. 8. Close the cell culture hood and put the UV light on inside of the hood. Sterilize the parts of the bioreactor for 30 minutes. 9. Turn off the UV hood light and turn off the two UV handheld lights and put them on the bottom shelf of the rack next to the cell culture hood. 10. Put the sterilized parts of the bioreactor into the cell culture hood along with the EGM -2 media and IBIDI slide with the seeded endothelial cells. 11. Attach the fan to the connector cables inside of the bioreactor. Place the fan 1 from the back of the bioreactor with the back of the fan facing the wall. Connect the blue wire to the top wire in the bioreactor (front wire) and the red wire to the bottom wire (back wire) in the bioreactor. 12. Attach the CO 2 Sensor to the plugs inside of the bioreactor. 130
145 13. Remove the Erlenmeyer flask from the autoclavable bag. Fill the flask with 100 ml of EGM -2. NOTE: If testing the temperature inside of the bioreactor, an additional flask with 100 ml of dh 2 0 will be added at this step. The small 6 thermometer will also be added into the flask. 14. Take the IBIDI slide and attach the two male luer locks with the tubing onto each side of the slide. 15. Put the Rubber Stopper #5 with the steel tubes into the flask with the 100 ml of EGM -2 media. 16. Put each EPDM stopper into the two holes in the left side of the bioreactor. The EPDM stopper with the tubing from the flask goes into the top hole (back hole) and the other EPDM stopper goes in the bottom hole (front hole). Take care to prevent the steel tubing from touching the hole in the bioreactor. 17. Push the EPDM stopper through the hole as much as possible so the stoppers are snug. 18. Put the IBIDI into a Petri dish for stabilization. 19. Put the flask with the EGM -2 media onto the round rubber bumper feet and put the flask inside of the bioreactor. Figure 62 shows what the bioreactor set up should look like after the fan, media, and CO 2 sensor are placed inside the bioreactor without the IBIDI slide. Figure 62: Bioreactor setup with fan, media, and CO 2 sensor 20. Attach the clear Tygon PVC tubing/black Norprene Rubber tubing to the outside steel tubes of the EPDM stoppers. Take care to hold the inside of the EPDM stopper when putting the outside PVC tubing on so the tubing does not slip. Also, don t touch the outer steel tube when putting the PVC tubing on. 21. Add a PES syringe filter to the middle of the three way valve adaptor on the end of the male X male luer connector. NOTE: If testing the temperature of the bioreactor, the second thermometer should be added here and placed in the bioreactor to read the temperature of the air. The thermometer may need to be taped to the side of the bioreactor is it can stay in place. 22. Put on the top cover of the bioreactor with the silicone rubber border, and face the border towards the inside of the bioreactor. Put the top door on so that all of the holes line up and the marker tape is on the top right corner. Hand screw the screws into the 131
146 holes. Figure 63 shows the direction the top door cover should face when placed on the top of the bioreactor. Figure 63: Direction of the top door cover of the bioreactor when sealed 23. Slowly remove the bioreactor from the cell culture hood so the flask and fan do not tip over. 24. Take the polystyrene foam insulation and cut out two long rectangular pieces 15 X 10, two long rectangular pieces 13 by 10 and two square pieces 10 X Take one of the 13 X 10 rectangular pieces and measure two holes with a permanent marker. One hole should measure 1 1/2 X 4 and should be located 2 from the bottom of the foam piece and in the middle of the foam insulation. The rectangular piece that was cut out can be placed back in the hole during the experiment to hold in heat more efficiently. The other hole should measure 2 1/2 X 1 and should be located in the middle of the insulation and 1 1/2 from the top. Figure 64 shows the left side of the bioreactor with the holes cut out in the insulation foam. Figure 64: Left side of the bioreactor with the insulation foam 26. Cut out the holes using an X-Acto knife. This rectangular piece is for the left side of the bioreactor. 27. Take the other 13 X 10 long rectangular pieces and measure three holes using a permanent marker. The first hole should be 5 1/2 X 1 1/2 and should be in the middle of the insulation and 2 from the top. The other two holes should be 1 X 1 1/2 and each hole should be located 2 from each side of the foam insulation and 4 1/2 from the top of the foam insulation. 132
147 28. Cut out the holes using an X-Acto knife. This rectangular side is for the right side of the bioreactor. Figure 65 shows the hole cutouts for the right side of the insulation foam around the bioreactor. Figure 65: Right side of the bioreactor with the insulation foam 29. Take the two square pieces and one 15 X 10 long rectangular piece and tape the ends together. NOTE: The foam insulation only needs to be cut out once unless the insulation becomes old or breaks. 30. Take the last 15 X 10 rectangular piece and put the bioreactor on top of it. Pull the cords and tubing through the holes of the two other rectangular pieces. Figure 66 shows what the insulation foam will look like around the bioreactor. Figure 66: Foam insulation around the bioreactor 31. Connect the heater and fan to the wall. Hook up the CO 2 sensor wires to the wall and desktop computer. 32. Check to make the fan and heater turn on. 33. Open the GasLab program on the desktop of the computer. Set the GasLab settings using the Port as Com3 and the Device as CM Connect the CO 2 sensor and confirm the CO 2 sensor can record data by pressing the CO 2, humidity, and temperature buttons 34. Change the CO 2 reading in GasLab from PPM (parts-per-million) to percent (%) 35. Close the top lid of the bioreactor by using a screwdriver. 36. Attach the Variac to the peristaltic pump and plug the Variac into the wall. Set the Variac at setting 60%. 133
148 37. Set the peristaltic pump at about 23.8 for a venous shear stress or 40.5 for an arterial shear stress. 38. Put the PVC tubing/black Norprene tubing in the peristaltic pump and seal the tubing down with the three screws and plate. Figure 67 shows how the tubing should fit around the peristaltic pump, and how the tubing should be sealed down with the plate. Figure 67: Tubing wrapped inside the peristaltic pump 39. Turn the peristaltic pump on by pressing the button downwards. Check the fluid starts flowing through the tubing, and the fluid flows counterclockwise or from the top steel tube out of the bioreactor to the bottom steel tube. 40. Use the fiber glass insulation, R-13 and place the insulation into the holes of the polystyrene foam insulation. 41. Put the polystyrene insulation cover on the top of the bioreactor. Adding the CO 2 : NOTE: The section below needs to be repeated before the start of each experiment. 1. Attach the female luer lock on the tubing for the CO 2 in line filter to the stopcock on the bioreactor. 2. In the GasLab Software, choose the amount of time to log the samples as 1 hour and the frequency of the samples at 1 second. Press Start. 3. Check the CO 2 reading in the GasLab software is at Percent (%) and not PPM. 4. Open the valve on the CO 2 tank one fourth of an entire turn. 5. Set the incoming pressure from the tank to be at the first notch on the left regulator. Figure 68 shows what the setting on the left regulator should look like when adding CO 2 into the bioreactor. Figure 68: Setting on the CO 2 regulator for the addition of CO 2 into the bioreactor 6. Open the needle valve with one eighth of a full twist so that the needle valve is barely open. 134
149 7. Open both stopcocks on the left side of the bioreactor to allow the CO 2 to enter the bioreactor and to prevent pressure buildup. 8. Add the CO 2 into the system for 5 seconds or less, and then close the stopcock valve on the stopcock connected to the tank. 9. Wait 30 seconds and then observe the percentage of CO 2 recorded by the CO 2 sensor in the GasLab program by clicking the CO 2 button. 10. Continue to slowly add the CO 2 until the CO 2 level is between 5% and 5.5% waiting 30 seconds in between each addition of CO Once the CO 2 percentage is at the desired value, turn off the valves to the CO 2 tank. Close the stopcock connected to the CO 2 tank. 12. If too much CO 2 was added into the system, open one of the stopcock valves on the right side of the bioreactor system that has the syringe filter inlet facing away from the bioreactor. 13. Using a 140 cc syringe, pull out air from the bioreactor system. Continue to do this step until the CO 2 percentage is at or right below 5.6%. 14. Close all of the stopcocks and ports. 15. Stop the CO 2 data logger. 16. Change the amount of time to log the samples by choosing a frequency of samples and amount of time to log samples. Typically, a frequency of samples would be chosen at either 1 minute or 10 minutes, and the amount of time to log the sample can range from 24 hours to forever. 17. Press Start and check that the data logger is recording samples. The CO 2, temperature, and humidity buttons can be pressed to check the CO 2 data logger is recording correctly. 18. If the experiment is running longer than 24 hours, CO 2 will need to be re-added into the system between 20 and 21 hours similarly to what was described above in steps 4 through
150 C.3 Changing the Media in Bioreactor Purpose: To change 50% of the media inside of the Erlenmeyer flask in the bioreactor when the cells are exposed to shear stress longer than 24 hours. Materials: - Male X Male Luer Connectors (Cole Parmer Part # UX ) - EGM -2 BulletKit Cell Culture Media with additional 6% FBS (Fetal Bovine Serum) (Lonza Part # CC-3125 and Fisher Scientific Part # ) - 28 mm PES Syringe Filters, Pores Size 0.2 um (Fisher Scientific Part # ) - Drummond Scientific Pipet-Aid Pipet - Covidien Luer Lock Sterile Syringe 60 cc (Grainger Part # 9VZF7) - 25 ml Disposable Polystyrene Serological Pipets (Fischer Scientific Part # K) - 1/16 ID X 1/8 OD Clear Tygon PVC Tubing (McMaster Carr Part #6546T33) or 1/16 ID X 1/8 OD Silicone Rubber Tubing (McMaster Carr Part #51135K12) - 3/32 Female Polypropylene Luer Locks (Qosina Part # 11534) - 50 ml BasiX Polypropylene Centrifuge Tubes (Fisher Scientific Part # ) ml Pyrex Glass Beaker (Fisher Scientific Part # ) Changing the Media: 1. Cut a 4-5 piece of either the clear Tygon PVC tubing or Silicone Rubber tubing. 2. Put a female luer lock on the end of the cut tubing and place the tubing in an autoclavable bag. 3. Place one male X male luer connector into an autoclavable bag. 4. Autoclave the male X male luer connector and the tubing/luer lock in the Gravity 2 cycle. NOTE: The tubing/luer lock doesn t need to be made before each experiment, and instead can be rinsed with dh 2 O. The tubing does need to be autoclaved before each media change. More than one set of tubing/luer lock and male X male luer connectors can be autoclaved before the start of each experiment, depending upon how many media changes are needed. 5. Remove the EGM -2 BulletKit media from the 4ºC and allow the media to warm to room temperature by either leaving the media on the counter or by putting the media in the incubator for about 20 minutes. 6. Place one 50 ml conical, the pre-warmed EGM -2 BulletKit media, a 25 ml pipet, the 60 cc syringe, and the autoclaved tubing/luer lock into the cell culture hood. 7. Using a 25 ml pipet and the Dummond Scientific Pipet-Aid Pipet, place 50 ml of EGM -2 media into the 50 ml conical. 8. Connect the tubing/luer lock onto the 60 cc syringe and pull up the 50 ml of media from the conical into the 60 cc syringe. 9. Remove the tubing/luer lock from the syringe, and place the syringe back into its bag. Remove the syringe from the cell culture hood. 136
151 10. Place the 100 ml Pyrex Glass Beaker underneath the 3 way valve and 28 mm PES syringe filter connected to the bioreactor. 11. Turn off the peristaltic pump. 12. Turn the 3-way valve so that the media inside of the bioreactor will flow from the inlet of the valve through the syringe filter, but not through outlet side (right side) of the 3-way valve. 13. Turn the peristaltic pump back on by pressing the button downwards and allow the media to flow through syringe filter and into the 100 ml Pyrex Glass Beaker. 14. Once the 100 ml Pyrex Glass Beaker fills up to 50 ml, turn off the peristaltic pump. 15. Turn the 3 way valve so that the media will flow through the tubing from the inlet of the valve through the outlet side (right side) of the 3 way valve. 16. Remove the 28 mm syringe filter and the male X male luer lock connector. 17. Connect the 60 cc syringe with EGM -2 media to the 3 way valve connector. 18. Turn the 3 way valve so that the media inside of the bioreactor will flow from the syringe filter through the inlet of the valve. Check that the outlet of the valve (right side) is closed. Figure 69 shows how the 60 cc syringe is connected to the 3 way valve and the direction the 3 way valve should be turned. Figure 69: 60 cc syringe connection to the 3 way valve connector 19. Turn on the peristaltic pump by pressing the button upwards so the media flows in the opposite direction as before. 137
Bioreactors in tissue engineering
 Bioreactors in tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
Bioreactors in tissue engineering S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative
Chapter 8. Comparison of static vs dynamic culture
 Chapter 8 Comparison of static vs dynamic culture 8.1. Literature Review Articular cartilage is a load-bearing connective tissue in which its functions not only transmitting the compressive joint loads
Chapter 8 Comparison of static vs dynamic culture 8.1. Literature Review Articular cartilage is a load-bearing connective tissue in which its functions not only transmitting the compressive joint loads
Reinnervate Perfusion Plate Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate
 Reinnervate Perfusion Plate Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate Application Note 9 Overview Much of our understanding of cell biology and the molecular mechanisms
Reinnervate Perfusion Plate Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate Application Note 9 Overview Much of our understanding of cell biology and the molecular mechanisms
Microfluidic for Testing Mechanical Properties of Cancer Cells
 Kaleidoscope Volume 11 Article 82 July 2014 Microfluidic for Testing Mechanical Properties of Cancer Cells Adrianne Shearer Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Kaleidoscope Volume 11 Article 82 July 2014 Microfluidic for Testing Mechanical Properties of Cancer Cells Adrianne Shearer Follow this and additional works at: https://uknowledge.uky.edu/kaleidoscope
Fabrication of Oxygenation Microfluidic Devices for Cell Cultures
 Fabrication of Oxygenation Microfluidic Devices for Cell Cultures Shauharda Khadka Department of Engineering Physics, Ramapo College of New Jersey, Mahwah, NJ Gerardo Mauleon and David T. Eddington Department
Fabrication of Oxygenation Microfluidic Devices for Cell Cultures Shauharda Khadka Department of Engineering Physics, Ramapo College of New Jersey, Mahwah, NJ Gerardo Mauleon and David T. Eddington Department
CHAPTER 2 CULTURE TYPE
 CHAPTER 2 CULTURE TYPE All types of micro-organisms are grown in suspension with the exception of cell culture, in which cells can also be anchorage dependent. Suspension the micro-organism can grow successfully
CHAPTER 2 CULTURE TYPE All types of micro-organisms are grown in suspension with the exception of cell culture, in which cells can also be anchorage dependent. Suspension the micro-organism can grow successfully
Perfusion Plate. Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate
 Perfusion Plate Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate Overview Much of our understanding of cell biology and the molecular mechanisms that control cell growth,
Perfusion Plate Dynamic Circulation and Perfusion of Culture Medium Within a Multi-welled Plate Overview Much of our understanding of cell biology and the molecular mechanisms that control cell growth,
Fabrication of Oxygenation Microfluidic Devices for Cell Cultures
 1 Fabrication of Oxygenation Microfluidic Devices for Cell Cultures Shauharda Khadka skhadka@ramapo.edu Department of Engineering Physics, Ramapo College of New Jersey Gerardo Mauleon mauleon2@uic.edu
1 Fabrication of Oxygenation Microfluidic Devices for Cell Cultures Shauharda Khadka skhadka@ramapo.edu Department of Engineering Physics, Ramapo College of New Jersey Gerardo Mauleon mauleon2@uic.edu
Leaf-inspired Artificial Microvascular Networks (LIAMN) for Threedimensional
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Leaf-inspired Artificial Microvascular Networks (LIAMN) for Threedimensional
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Leaf-inspired Artificial Microvascular Networks (LIAMN) for Threedimensional
BE.430/2.795//6.561/10.539/HST.544. Final Exam. Handed out: Monday, Dec. 6, 2004 Due: Thursday, Dec. 9 by 5pm
 BE.430/2.795//6.561/10.539/HST.544 Final Exam Handed out: Monday, Dec. 6, 2004 Due: Thursday, Dec. 9 by 5pm Problem 1 (30 %) ALG Notes Problem 2.6.3 Parts a f (not g) (Page 199) Problem 2 (35 %) When patients
BE.430/2.795//6.561/10.539/HST.544 Final Exam Handed out: Monday, Dec. 6, 2004 Due: Thursday, Dec. 9 by 5pm Problem 1 (30 %) ALG Notes Problem 2.6.3 Parts a f (not g) (Page 199) Problem 2 (35 %) When patients
Figure 1. Few examples of in-vitro models developed using our products
 Introduction IVTech offers products and know-how to improve the outcomes of your in-vitro research and refine your cell and tissue models. Using our systems, you can now implement and visualize dynamic
Introduction IVTech offers products and know-how to improve the outcomes of your in-vitro research and refine your cell and tissue models. Using our systems, you can now implement and visualize dynamic
1) Determining the best cell sources and scaffold materials for TEHV development.
 Broadly speaking, my primary research interests focus on the development and application of methodologies that can be employed in the basic understanding and optimization of tissue engineered heart valves
Broadly speaking, my primary research interests focus on the development and application of methodologies that can be employed in the basic understanding and optimization of tissue engineered heart valves
µ Slide Membrane ibipore Flow
 The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
Aggi-E Challenge The Perfusion Bioreactor Section Group 2a. Diego Garcia, Megan Maladecki, Nathanael Marshall, Ria Rao
 Aggi-E Challenge The Perfusion Bioreactor Section 511 - Group 2a Diego Garcia, Megan Maladecki, Nathanael Marshall, Ria Rao Types of Bioreactors Spinner Flask Scaffolds are fully submerged in a media-filled
Aggi-E Challenge The Perfusion Bioreactor Section 511 - Group 2a Diego Garcia, Megan Maladecki, Nathanael Marshall, Ria Rao Types of Bioreactors Spinner Flask Scaffolds are fully submerged in a media-filled
Perfusion culture and live cell imaging of Hepatitis C Virus Replicon cells in miniature bioreactor and analysis of shape of nuclei
 Perfusion culture and live cell imaging of Hepatitis C Virus Replicon cells in miniature bioreactor and analysis of shape of nuclei Anisha Prasad a, Prof. Saumitra Das b and Prof. G. K. Ananthasuesh c
Perfusion culture and live cell imaging of Hepatitis C Virus Replicon cells in miniature bioreactor and analysis of shape of nuclei Anisha Prasad a, Prof. Saumitra Das b and Prof. G. K. Ananthasuesh c
Revolutionising Stem Cell Research. Unique Biaxial Bioreactor Designed for TISSUE ENGINEERING & 3D CELL CULTURE. Regeneration System
 Regeneration System Unique Biaxial Bioreactor Designed for TISSUE ENGINEERING & 3D CELL CULTURE Revolutionising Stem Cell Research A Member of Quintech Life Sciences TisXell A Renaissance in Regenerative
Regeneration System Unique Biaxial Bioreactor Designed for TISSUE ENGINEERING & 3D CELL CULTURE Revolutionising Stem Cell Research A Member of Quintech Life Sciences TisXell A Renaissance in Regenerative
µ Slide Membrane ibipore Flow
 The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
The Product and Experiment Guide
 The Product and Experiment Guide Providing Solutions for Your Research p. 3 p. 10 p. 4 CELL CULTURE & MICROSCOPY IMMUNO- FLUORESCENCE LIVE CELL IMAGING p. 8 p. 7 p. 11 ANGIOGENESIS ASSAYS CHEMOTAXIS ASSAYS
The Product and Experiment Guide Providing Solutions for Your Research p. 3 p. 10 p. 4 CELL CULTURE & MICROSCOPY IMMUNO- FLUORESCENCE LIVE CELL IMAGING p. 8 p. 7 p. 11 ANGIOGENESIS ASSAYS CHEMOTAXIS ASSAYS
PimCell. Cell Culture Starter Kit ADVANCED CELL CULTURE PIMBIO CATALOGUE & APPLICATION NOTES
 2018 PimCell Cell Culture Starter Kit ADVANCED CELL CULTURE PIMBIO CATALOGUE & APPLICATION NOTES PimCell Microfluidic Cell Culture Starter Kit PimCell line is a group of related cell culture microfluidic
2018 PimCell Cell Culture Starter Kit ADVANCED CELL CULTURE PIMBIO CATALOGUE & APPLICATION NOTES PimCell Microfluidic Cell Culture Starter Kit PimCell line is a group of related cell culture microfluidic
Syringe Pumps. Please contact us for help in selecting the best pump for your application and a free quote.
 Syringe Pumps Microfluidic Pumps! We are proud to offer select Harvard Apparatus syringe pumps for use with SynVivo biochips. These high quality pumps have been extensively tested and offer a feature rich
Syringe Pumps Microfluidic Pumps! We are proud to offer select Harvard Apparatus syringe pumps for use with SynVivo biochips. These high quality pumps have been extensively tested and offer a feature rich
Tube Formation Assays in µ-slide Angiogenesis. 1. General Information. Application Note 19
 Tube Formation Assays in µ-slide Angiogenesis Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview... 3 4. Preparation of the Gel and the Slide... 4 4.1. Gel Application... 4 4.2.
Tube Formation Assays in µ-slide Angiogenesis Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview... 3 4. Preparation of the Gel and the Slide... 4 4.1. Gel Application... 4 4.2.
Supplementary information
 Supplementary information Device Design The geometry of the microdevice channels were designed in autocad and modeled to simulate the microvasculature of the human body. In order to emulate physiologic
Supplementary information Device Design The geometry of the microdevice channels were designed in autocad and modeled to simulate the microvasculature of the human body. In order to emulate physiologic
Artificial blood vessels
 Artificial blood vessels S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of
Artificial blood vessels S. Swaminathan Director Centre for Nanotechnology & Advanced Biomaterials School of Chemical & Biotechnology SASTRA University Thanjavur 613 401 Tamil Nadu Joint Initiative of
Mechanical Characterization and Stimulation Solutions for Biomaterials
 Mechanical Characterization and Stimulation Solutions for Biomaterials BioDynamic Instruments Biomaterials and Tissue Characterization Application Examples Bone Bending Creep Test Clinical Need: Understand
Mechanical Characterization and Stimulation Solutions for Biomaterials BioDynamic Instruments Biomaterials and Tissue Characterization Application Examples Bone Bending Creep Test Clinical Need: Understand
 3D Blood Brain Impedance Assay Using SynBBB Idealized Network (TEER configuration) Kits and Chips Technical Manual Catalog #s 402004, 402003, 102015-SB Schematic of the chip used for the SynBBB Model and
3D Blood Brain Impedance Assay Using SynBBB Idealized Network (TEER configuration) Kits and Chips Technical Manual Catalog #s 402004, 402003, 102015-SB Schematic of the chip used for the SynBBB Model and
ADVANCED BIOREACTOR SYSTEM FOR THE IMPLANTABLE BIOMATERIALS TESTING AND TISSUE ENGINEERING APPLICATIONS
 ADVANCED BIOREACTOR SYSTEM FOR THE IMPLANTABLE BIOMATERIALS TESTING AND TISSUE ENGINEERING APPLICATIONS Mohd Ramdan 1 and Irza Sukmana 2 1 MediTeg Research Group, Faculty of Biosciences and Biomedical
ADVANCED BIOREACTOR SYSTEM FOR THE IMPLANTABLE BIOMATERIALS TESTING AND TISSUE ENGINEERING APPLICATIONS Mohd Ramdan 1 and Irza Sukmana 2 1 MediTeg Research Group, Faculty of Biosciences and Biomedical
Rolling, Adhesion and Migration Assay Using SynRAM Idealized Network Kits and Chips Technical Manual Catalog #s , , SR
 Rolling, Adhesion and Migration Assay Using SynRAM Idealized Network Kits and Chips Technical Manual Catalog #s 401002, 401001, 102008-SR Schematic of the SynRAM model Chip. Vascular channels are for culture
Rolling, Adhesion and Migration Assay Using SynRAM Idealized Network Kits and Chips Technical Manual Catalog #s 401002, 401001, 102008-SR Schematic of the SynRAM model Chip. Vascular channels are for culture
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues
 High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
Labware compatibility report. Directly order at:
 Labware compatibility report Directly order at: https://ibidi.com/12-labware Contents 1. Introduction 3 2. Compatible Labware 4 A. 35 mm dishes - high borders 4 µ-dish 35 mm, high 4 µ-dish 35mm, high Glass
Labware compatibility report Directly order at: https://ibidi.com/12-labware Contents 1. Introduction 3 2. Compatible Labware 4 A. 35 mm dishes - high borders 4 µ-dish 35 mm, high 4 µ-dish 35mm, high Glass
Low-Cost Bioreactor to Enable Microbe Productivity Optimization
 Low-Cost Bioreactor to Enable Microbe Productivity Optimization Design Team David Christianson, Elizabeth Duffy Bryan Keen, Andrew Mazzotta Jameson Stark Design Advisor Prof. Jeffrey Ruberti Email: j.ruberti@neu.edu
Low-Cost Bioreactor to Enable Microbe Productivity Optimization Design Team David Christianson, Elizabeth Duffy Bryan Keen, Andrew Mazzotta Jameson Stark Design Advisor Prof. Jeffrey Ruberti Email: j.ruberti@neu.edu
3D Transfection System
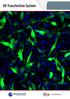 3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
EQUIPMENTS & MATERIALS COMMONLY USED IN A LABORATORY
 EQUIPMENTS & MATERIALS COMMONLY USED IN A LABORATORY a) Autoclave: An autoclave is a device used to sterilize equipment and supplies by subjecting them to high pressure saturated steam at 121 C for around
EQUIPMENTS & MATERIALS COMMONLY USED IN A LABORATORY a) Autoclave: An autoclave is a device used to sterilize equipment and supplies by subjecting them to high pressure saturated steam at 121 C for around
3D Endothelial Pericyte Coculturing Kit Product Description Kit Components
 3D Endothelial Pericyte Coculturing Kit (3D-EPC) Cat #8728 Product Description Blood vessels are responsible for transporting blood throughout the body as part of the circulatory system. Their main components
3D Endothelial Pericyte Coculturing Kit (3D-EPC) Cat #8728 Product Description Blood vessels are responsible for transporting blood throughout the body as part of the circulatory system. Their main components
Photoetched Regenerator for Use in a High Frequency Pulse Tube
 Photoetched Regenerator for Use in a High Frequency Pulse Tube W.F. Superczynski, G.F. Green Chesapeake Cryogenics, Inc. Arnold, MD 21012 ABSTRACT To produce refrigeration at 20 K in a single stage regenerative
Photoetched Regenerator for Use in a High Frequency Pulse Tube W.F. Superczynski, G.F. Green Chesapeake Cryogenics, Inc. Arnold, MD 21012 ABSTRACT To produce refrigeration at 20 K in a single stage regenerative
Tube Formation Assays in µ-slide Angiogenesis
 Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
Tube Formation Assays in µ-slide Angiogenesis Related topics: Application Note 27 Data Analysis of Tube Formation Assays. Contents 1. General Information... 1 2. Material... 2 3. Work Flow Overview...
A Physiological Cell based Microchip Platform. 1
 A Physiological Cell based Microchip Platform www.synvivobio.com 1 Drug Development Problem What passes here often fails here Discovery Pre clinical Clinical Trials in vitro in vivo Static Well plate Platforms
A Physiological Cell based Microchip Platform www.synvivobio.com 1 Drug Development Problem What passes here often fails here Discovery Pre clinical Clinical Trials in vitro in vivo Static Well plate Platforms
PimCell. microfluidic construction kit ADVANCED CELL CULTURE CATALOGUE & APPLICATION NOTES
 2018 PimCell microfluidic construction kit ADVANCED CELL CULTURE CATALOGUE & APPLICATION NOTES PimCell microfluidic construction kit PimCell line is a group of related cell culture microfluidic products
2018 PimCell microfluidic construction kit ADVANCED CELL CULTURE CATALOGUE & APPLICATION NOTES PimCell microfluidic construction kit PimCell line is a group of related cell culture microfluidic products
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Reimagine A New Instron Way
 Reimagine A New Instron Way Tissue Engineering and Regenerative Medicine 2014 Year in Review TABLE OF CONTENTS Welcome Since 1946, Instron has been dedicated to providing you with comprehensive solutions
Reimagine A New Instron Way Tissue Engineering and Regenerative Medicine 2014 Year in Review TABLE OF CONTENTS Welcome Since 1946, Instron has been dedicated to providing you with comprehensive solutions
Bio Reactor Systems. Contact for further information, or call +44 (0)
 Bio Reactor Systems The bench mark for R+D Bio-reactors Single and parallel fully automated, modular systems Aerobic, anaerobic plus microbial, cell cultures and bio-fuels Range of interchangeable size
Bio Reactor Systems The bench mark for R+D Bio-reactors Single and parallel fully automated, modular systems Aerobic, anaerobic plus microbial, cell cultures and bio-fuels Range of interchangeable size
The content is broken down into the following outline:
 Microfluidics has been around for a long time now, yet there are so many silos of information, and so many folks offering a variety of solutions, it s hard to ask the right questions to ensure you're choosing
Microfluidics has been around for a long time now, yet there are so many silos of information, and so many folks offering a variety of solutions, it s hard to ask the right questions to ensure you're choosing
A STUDY ON FLOW BEHAVIOR INSIDE A SIMPLE MODEL OF EJECTOR
 A STUDY ON FLOW BEHAVIOR INSIDE A SIMPLE MODEL OF EJECTOR Taketoshi Koita 1 and Junjiro Iwamoto ABSTRACT This paper is concerned with the experimental results on the internal flow of the ejector. To improve
A STUDY ON FLOW BEHAVIOR INSIDE A SIMPLE MODEL OF EJECTOR Taketoshi Koita 1 and Junjiro Iwamoto ABSTRACT This paper is concerned with the experimental results on the internal flow of the ejector. To improve
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Optimizing the HyPerforma Single-Use Bioreactor for adherent cell culture on microcarriers
 APPLICATION NOTE Harvestainer BPC for microcarrier bead separation Optimizing the HyPerforma Single-Use Bioreactor for adherent cell culture on microcarriers Introduction The scale-up of culture of adherent
APPLICATION NOTE Harvestainer BPC for microcarrier bead separation Optimizing the HyPerforma Single-Use Bioreactor for adherent cell culture on microcarriers Introduction The scale-up of culture of adherent
Optimizing the HyPerforma Single-Use Bioreactor for adherent cell culture on microcarriers
 APPLICATION NOTE Harvestainer BPC for microcarrier bead separation Optimizing the HyPerforma Single-Use Bioreactor for adherent cell culture on microcarriers Introduction The scale-up of culture of adherent
APPLICATION NOTE Harvestainer BPC for microcarrier bead separation Optimizing the HyPerforma Single-Use Bioreactor for adherent cell culture on microcarriers Introduction The scale-up of culture of adherent
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures. Xin Zhang Associate Professor.
 Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
Spying on Cells: Cellular and Subcellular Analysis using Novel Polymeric Micro- and Nanostructures Xin Zhang Associate Professor Boston University US-Korea Nano Forum April 2008 Road Map of Nanobio-sensors
Chemotaxis assay using µ-slide Chemotaxis
 Chemotaxis assay using µ-slide Chemotaxis 1. General information The µ-slide Chemotaxis is a tool for observing chemotactical responses of adherent migrating cells over extended periods of time. The linear
Chemotaxis assay using µ-slide Chemotaxis 1. General information The µ-slide Chemotaxis is a tool for observing chemotactical responses of adherent migrating cells over extended periods of time. The linear
Methods in Bioengineering: 3D Tissue Engineering
 Methods in Bioengineering: 3D Tissue Engineering Berthiaume, Francois ISBN-13: 9781596934580 Table of Contents Preface Chapter 1. Chemical Modification of Porous Scaffolds Using Plasma Polymers 1.1. Introduction
Methods in Bioengineering: 3D Tissue Engineering Berthiaume, Francois ISBN-13: 9781596934580 Table of Contents Preface Chapter 1. Chemical Modification of Porous Scaffolds Using Plasma Polymers 1.1. Introduction
Scaling Down Bioreactor Process Development: Comparison of Microbioreactor and Bench Scale Solutions.
 Scaling Down Bioreactor Process Development: Comparison of Microbioreactor and Bench Scale Solutions. Richard Lugg. Scientist I, MedImmune. European Laboratory Robotics Interest Group: High Throughput
Scaling Down Bioreactor Process Development: Comparison of Microbioreactor and Bench Scale Solutions. Richard Lugg. Scientist I, MedImmune. European Laboratory Robotics Interest Group: High Throughput
Design and Evaluation of the Flexible PDMS Microfluidic Diaphragm Pump with Check-Valve
 Design and Evaluation of the Flexible PDMS Microfluidic Diaphragm Pump with Check-Valve A Thesis Presented to Todd Sulchek, Ph.D. By: Soo Kim In Partial Fulfillment Of the Requirements for the Degree B.S.
Design and Evaluation of the Flexible PDMS Microfluidic Diaphragm Pump with Check-Valve A Thesis Presented to Todd Sulchek, Ph.D. By: Soo Kim In Partial Fulfillment Of the Requirements for the Degree B.S.
Scalability of the Mobius CellReady Single-use Bioreactor Systems
 Application Note Scalability of the Mobius CellReady Single-use Bioreactor Systems Abstract The Mobius CellReady single-use bioreactor systems are designed for mammalian cell growth and recombinant protein
Application Note Scalability of the Mobius CellReady Single-use Bioreactor Systems Abstract The Mobius CellReady single-use bioreactor systems are designed for mammalian cell growth and recombinant protein
Bioreactor System ERT 314. Sidang /2012
 Bioreactor System ERT 314 Sidang 1 2011/2012 Chapter 3:Types of Bioreactors Week 4-5 Handouts : Chapter 13 in Doran, Bioprocess Engineering Principles Background to Bioreactors The bioreactor is the heart
Bioreactor System ERT 314 Sidang 1 2011/2012 Chapter 3:Types of Bioreactors Week 4-5 Handouts : Chapter 13 in Doran, Bioprocess Engineering Principles Background to Bioreactors The bioreactor is the heart
The BioFlux 200 System Using Well Plate Microfluidics for Live Cell Assays Product Overview and Tutorial
 The BioFlux 200 System Using Well Plate Microfluidics for Live Cell Assays Product Overview and Tutorial Introduction to the BioFlux System Enables live-cell assays with precisely-controlled shear flow
The BioFlux 200 System Using Well Plate Microfluidics for Live Cell Assays Product Overview and Tutorial Introduction to the BioFlux System Enables live-cell assays with precisely-controlled shear flow
Preventive Maintenance and Calibration Program: IDM provides OEM services:
 FOAM / MATTRESS Industry Product Guide Preventive Maintenance and Calibration Program: The IDM Preventive Maintenance and Calibration (PM&C) program has been designed to make the maintenance and calibration
FOAM / MATTRESS Industry Product Guide Preventive Maintenance and Calibration Program: The IDM Preventive Maintenance and Calibration (PM&C) program has been designed to make the maintenance and calibration
Responses of Cells to Fluid Shear Stress in Vitro
 Responses of Cells to Fluid Shear Stress in Vitro Fumihiko SATO, Shigehiro HASHIMOTO Biomedical Engineering, Department of Mechanical Engineering, Kogakuin University, Tokyo, 163-8677, Japan shashimoto@cc.kogakuin.ac.jp
Responses of Cells to Fluid Shear Stress in Vitro Fumihiko SATO, Shigehiro HASHIMOTO Biomedical Engineering, Department of Mechanical Engineering, Kogakuin University, Tokyo, 163-8677, Japan shashimoto@cc.kogakuin.ac.jp
Contents. Preface XI Nomenclature XIII. Part I Basic Concepts and Principles 1
 V Preface XI Nomenclature XIII Part I Basic Concepts and Principles 1 1 Introduction 3 1.1 Background and Scope 3 1.2 Dimensions and Units 4 1.3 Intensive and Extensive Properties 6 1.4 Equilibria and
V Preface XI Nomenclature XIII Part I Basic Concepts and Principles 1 1 Introduction 3 1.1 Background and Scope 3 1.2 Dimensions and Units 4 1.3 Intensive and Extensive Properties 6 1.4 Equilibria and
Report on the application of BlueSens gas sensor in continuous bioh 2 process optimization
 Report on the application of BlueSens gas sensor in continuous bioh 2 process optimization Péter Bakonyi, Nándor Nemestóthy, Katalin Bélafi-Bakó Research Institute on Bioengineering, Membrane Technology
Report on the application of BlueSens gas sensor in continuous bioh 2 process optimization Péter Bakonyi, Nándor Nemestóthy, Katalin Bélafi-Bakó Research Institute on Bioengineering, Membrane Technology
Responses of Cells to Flow in Vitro
 Responses of Cells to in Vitro Shigehiro HASHIMOTO, Fumihiko SATO, Haruka HINO Biomedical Engineering, Department of Mechanical Engineering, Kogakuin University, Tokyo, 163-8677, Japan shashimoto@cc.kogakuin.ac.jp
Responses of Cells to in Vitro Shigehiro HASHIMOTO, Fumihiko SATO, Haruka HINO Biomedical Engineering, Department of Mechanical Engineering, Kogakuin University, Tokyo, 163-8677, Japan shashimoto@cc.kogakuin.ac.jp
miniperm The Bioreactor for the Production of Monoclonal Antibodies and Recombinant Proteins
 Cell/ 2 HTS 5 Tubes/Multi 0 Biochips/ Cryo The Bioreactor for the Production of Monoclonal Antibodies and Recombinant Proteins Labscale protein production is an easytouse bioreactor which was developed
Cell/ 2 HTS 5 Tubes/Multi 0 Biochips/ Cryo The Bioreactor for the Production of Monoclonal Antibodies and Recombinant Proteins Labscale protein production is an easytouse bioreactor which was developed
Kyle Anderson BSAC. Peter Guerin BWIG, BPEG. Rebecca Stoebe Communicator. Dan Thompson Team Leader. Professor Tracy Puccinelli Advisor
 Midsemester Presentation Kyle Anderson BSAC Peter Guerin BWIG, BPEG Rebecca Stoebe Communicator Dan Thompson Team Leader Professor Tracy Puccinelli Advisor Dr. Nathan Welham, Dr. Yutaka Yoya, Dr. Steve
Midsemester Presentation Kyle Anderson BSAC Peter Guerin BWIG, BPEG Rebecca Stoebe Communicator Dan Thompson Team Leader Professor Tracy Puccinelli Advisor Dr. Nathan Welham, Dr. Yutaka Yoya, Dr. Steve
Biomaterials. Third Edition
 Biomaterials Third Edition Joon Park R.S. Lakes Biomaterials An Introduction Third Edition Joon Park Biomedical and Mechanical Engineering University of Iowa Iowa City, IA 52242-1414 USA joon-park@uiowa.edu
Biomaterials Third Edition Joon Park R.S. Lakes Biomaterials An Introduction Third Edition Joon Park Biomedical and Mechanical Engineering University of Iowa Iowa City, IA 52242-1414 USA joon-park@uiowa.edu
In Vitro Angiogenesis Assay Kit
 In Vitro Angiogenesis Assay Kit Catalog Number KA1323 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
In Vitro Angiogenesis Assay Kit Catalog Number KA1323 100 assays Version: 02 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Background... 3 Principle of the Assay...
Basic design of laboratory bioreactor
 Basic design of laboratory bioreactor In terms of the construction, the following variants of the laboratory bioreactor can be made: 1. Glass bioreactor (without a jacket) with an upper stainless steel
Basic design of laboratory bioreactor In terms of the construction, the following variants of the laboratory bioreactor can be made: 1. Glass bioreactor (without a jacket) with an upper stainless steel
Affinity NT. Clearly the Choice for Consistent Performance. and Reliable Results
 Affinity NT Oxygenation System Clearly the Choice for Consistent Performance and Reliable Results Consistent performance starts with an oxygenator design that has set the standard for the industry. The
Affinity NT Oxygenation System Clearly the Choice for Consistent Performance and Reliable Results Consistent performance starts with an oxygenator design that has set the standard for the industry. The
Affinity NT. Clearly the Choice for Consistent Performance. and Reliable Results
 Affinity NT Oxygenation System Clearly the Choice for Consistent Performance and Reliable Results Consistent performance starts with an oxygenator design that has set the standard for the industry. The
Affinity NT Oxygenation System Clearly the Choice for Consistent Performance and Reliable Results Consistent performance starts with an oxygenator design that has set the standard for the industry. The
Synchronisation of P. falciparum v1.1. Procedure. In vitro Module WorldWide Antimalarial Resistance Network (WWARN)
 Synchronisation of P. falciparum v1.1 Procedure In vitro Module WorldWide Antimalarial Resistance Network (WWARN) Suggested citation: In vitro Module, WWARN. 2010. Synchronisation of P. falciparum. WWARN
Synchronisation of P. falciparum v1.1 Procedure In vitro Module WorldWide Antimalarial Resistance Network (WWARN) Suggested citation: In vitro Module, WWARN. 2010. Synchronisation of P. falciparum. WWARN
Cellab GmbH - Introduction. June 2014
 Cellab GmbH - Introduction June 2014 Content overview About Cellab GmbH Concept Introduction Applications Technology Summary 2 3 About Cellab GmbH Corporate structure Founded 1993 Plant engineering and
Cellab GmbH - Introduction June 2014 Content overview About Cellab GmbH Concept Introduction Applications Technology Summary 2 3 About Cellab GmbH Corporate structure Founded 1993 Plant engineering and
6-Well Bio-Assembler TM Kit Instruction Manual
 6-Well Bio-Assembler TM Kit Instruction Manual Introduction 1. Introduction... 2 2. Materials and Supplies... 3 3. Instructions a. Treating Cells with NanoShuttle TM -PL... 4 b. Cell Detachment... 5 c.
6-Well Bio-Assembler TM Kit Instruction Manual Introduction 1. Introduction... 2 2. Materials and Supplies... 3 3. Instructions a. Treating Cells with NanoShuttle TM -PL... 4 b. Cell Detachment... 5 c.
EXPERIMENTAL AERODYNAMICS FLOW TROUGH AN ORIFICE LAB SHEET
 EXPERIMENTAL AERODYNAMICS FLOW TROUGH AN ORIFICE LAB SHEET EMRE KOÇ ONUR GÜNEL 1- Introduction When a fluid passes through a constriction, such as through a sharp-edged hole or over a weir, the constriction
EXPERIMENTAL AERODYNAMICS FLOW TROUGH AN ORIFICE LAB SHEET EMRE KOÇ ONUR GÜNEL 1- Introduction When a fluid passes through a constriction, such as through a sharp-edged hole or over a weir, the constriction
Rolling processes. Fig. (5-1)
 Page1 Rolling processes 5-1 introduction: Rolling is the process of reducing the thickness or changing the cross section of a long workpiece by compressive forces applied through a set of rolls, as shown
Page1 Rolling processes 5-1 introduction: Rolling is the process of reducing the thickness or changing the cross section of a long workpiece by compressive forces applied through a set of rolls, as shown
National Tsing Hua University, Taiwan, R.O.C. Correspondence:
 A CONTINUOUS-FLOW CELL CULTURE ARRAY WITH CHAOTIC MIXERS FOR IDENTIFICATION OF THE OPTIMUM GROWTH FACTORS COMBINATIONS FOR MOUSE EMBRYONIC STEM CELLS DIFFERENTIATION Yun-Hua Hsiao 1, Kuang-Yuan Lee 1,
A CONTINUOUS-FLOW CELL CULTURE ARRAY WITH CHAOTIC MIXERS FOR IDENTIFICATION OF THE OPTIMUM GROWTH FACTORS COMBINATIONS FOR MOUSE EMBRYONIC STEM CELLS DIFFERENTIATION Yun-Hua Hsiao 1, Kuang-Yuan Lee 1,
Structural Performance of 8-inch NRG Concrete Masonry Units. Report Compiled for: Niagara Regional Group. Date: January 28, 2013
 Structural Performance of 8-inch NRG Concrete Masonry Units Report Compiled for: Niagara Regional Group Date: January 28, 2013 Report Prepared by: Dr. Shawn Gross, Associate Professor Dr. David Dinehart,
Structural Performance of 8-inch NRG Concrete Masonry Units Report Compiled for: Niagara Regional Group Date: January 28, 2013 Report Prepared by: Dr. Shawn Gross, Associate Professor Dr. David Dinehart,
Analysis of pressure-driven air bubble elimination in a. microfluidic device
 Analysis of pressure-driven air bubble elimination in a microfluidic device Joo H. Kang, a Yu Chang Kim a,b and Je-Kyun Park* a a Department of Bio and Brain Engineering, Korea Advanced Institute of Science
Analysis of pressure-driven air bubble elimination in a microfluidic device Joo H. Kang, a Yu Chang Kim a,b and Je-Kyun Park* a a Department of Bio and Brain Engineering, Korea Advanced Institute of Science
How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs
 WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
Single-Use Simplicity
 Single-Use Simplicity BioBLU c and BioBLU p Single-Use Vessels for cell culture »Proven stirred-tank design meets single-use technology.«reliable performance and ease-of-use Combine the benefits of single-use
Single-Use Simplicity BioBLU c and BioBLU p Single-Use Vessels for cell culture »Proven stirred-tank design meets single-use technology.«reliable performance and ease-of-use Combine the benefits of single-use
DESIGN OF A PERFUSION BIOREACTOR FOR SIMULATING SYNOVIAL JOINT CAVITY
 DESIGN OF A PERFUSION BIOREACTOR FOR SIMULATING SYNOVIAL JOINT CAVITY A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR THE DEGREE OF BACHELOR OF TECHNOLOGY IN BIOMEDICAL ENGINEERING By
DESIGN OF A PERFUSION BIOREACTOR FOR SIMULATING SYNOVIAL JOINT CAVITY A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENT FOR THE DEGREE OF BACHELOR OF TECHNOLOGY IN BIOMEDICAL ENGINEERING By
LARGE SCALE PRODUCTION OF LACCASE BY PLEUROTUS OSTREATUS IMI IN BIOREACTOR
 LARGE SCALE PRODUCTION OF LACCASE BY PLEUROTUS OSTREATUS IMI 395545 IN BIOREACTOR ABSTRACT In this chapter large scale laccase production from Pleurotus ostreatus IMI 395545 in a bench top bioreactor was
LARGE SCALE PRODUCTION OF LACCASE BY PLEUROTUS OSTREATUS IMI 395545 IN BIOREACTOR ABSTRACT In this chapter large scale laccase production from Pleurotus ostreatus IMI 395545 in a bench top bioreactor was
Scale-up & scale-down between the two. worlds of shaken and stirred bioreactors
 Scale-up & scale-down between the two worlds of shaken and stirred bioreactors Prof. Dr.-Ing. Jochen Büchs AVT - Biochemical Engineering, RWTH Aachen University Sammelbau Biologie, D - 52074 Aachen, Germany
Scale-up & scale-down between the two worlds of shaken and stirred bioreactors Prof. Dr.-Ing. Jochen Büchs AVT - Biochemical Engineering, RWTH Aachen University Sammelbau Biologie, D - 52074 Aachen, Germany
Single-Use Simplicity
 Single-Use Simplicity BioBLU c and BioBLU p Single-Use Vessels for cell culture »Proven stirred-tank design meets single-use technology.«reliable performance and ease-of-use Combine the benefits of single-use
Single-Use Simplicity BioBLU c and BioBLU p Single-Use Vessels for cell culture »Proven stirred-tank design meets single-use technology.«reliable performance and ease-of-use Combine the benefits of single-use
PROJECT STATEMENT AND SPECIFICATIONS THREE POINT BENDING DEVICE FOR FLEXURE TESTING OF SOFT TISSUES
 PROJECT STATEMENT AND SPECIFICATIONS THREE POINT BENDING DEVICE FOR FLEXURE TESTING OF SOFT TISSUES TEAM 4 Michael Harman Minh Xuan Nguyen Eric Sirois CLIENT CONTACT Wei Sun, Ph.D. Assistant Professor
PROJECT STATEMENT AND SPECIFICATIONS THREE POINT BENDING DEVICE FOR FLEXURE TESTING OF SOFT TISSUES TEAM 4 Michael Harman Minh Xuan Nguyen Eric Sirois CLIENT CONTACT Wei Sun, Ph.D. Assistant Professor
The Hollow Fiber Bioreactor and Cell Co-Cultivation
 The Hollow Fiber Bioreactor and Cell Co-Cultivation June 12, 2012 John J.S. Cadwell Historically, the scientific method had been based upon the reduction of complex systems to their simplest forms in order
The Hollow Fiber Bioreactor and Cell Co-Cultivation June 12, 2012 John J.S. Cadwell Historically, the scientific method had been based upon the reduction of complex systems to their simplest forms in order
Cells Culture Techniques Marta Czernik
 Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
CHAPTER 1. Survey of Clinical Cases of Biomaterials-Tissue Interactions: The Paradigm
 CHAPTER 1 Survey of Clinical Cases of Biomaterials-Tissue Interactions: The Paradigm 1.1 Introduction 1.2 Genotype/Phenotype (Fig. 1.1) 1.3 The Working Paradigm: The Unit Cell Process/The Control Volume
CHAPTER 1 Survey of Clinical Cases of Biomaterials-Tissue Interactions: The Paradigm 1.1 Introduction 1.2 Genotype/Phenotype (Fig. 1.1) 1.3 The Working Paradigm: The Unit Cell Process/The Control Volume
Mechano-dependent Biosynthetic Response of Micro-integrated Cells in Elastomeric Scaffolds
 Mechano-dependent Biosynthetic Response of Micro-integrated Cells in Elastomeric Scaffolds Lauren Anderson 1,2, John Stella 3, and Dr. Michael Sacks 3 1 Bioengineering and Bioinformatics Summer Institute,
Mechano-dependent Biosynthetic Response of Micro-integrated Cells in Elastomeric Scaffolds Lauren Anderson 1,2, John Stella 3, and Dr. Michael Sacks 3 1 Bioengineering and Bioinformatics Summer Institute,
2019 Award Nomination Title of Innovation: Microfluidic MIC model: Biocide studies
 2019 Award Nomination Title of Innovation: Microfluidic MIC model: Biocide studies Nominee(s) Susmitha Purnima Kotu, Texas A&M University Arul Jayaraman, Texas A&M University Sam Mannan, Texas A&M University
2019 Award Nomination Title of Innovation: Microfluidic MIC model: Biocide studies Nominee(s) Susmitha Purnima Kotu, Texas A&M University Arul Jayaraman, Texas A&M University Sam Mannan, Texas A&M University
Ready to use: Serum free culture in Insectomed SF express
 Ready to use: Serum free culture in Insectomed SF express Information from Biochrom AG, 19.05.2010 Serum free culture in Insectomed SF express is a proprietary formulation which has successfully been used
Ready to use: Serum free culture in Insectomed SF express Information from Biochrom AG, 19.05.2010 Serum free culture in Insectomed SF express is a proprietary formulation which has successfully been used
The effect of shear stress on circulating and vascular endothelial cells
 The effect of shear stress on circulating and vascular endothelial cells D.Bronneberg Report: BMT 02.11. Coaching: S.M. Kladakis Prof. R.M. Nerem Dr. C.V.C. Bouten Prof. F.T.P. Baaijens Department of Biomedical
The effect of shear stress on circulating and vascular endothelial cells D.Bronneberg Report: BMT 02.11. Coaching: S.M. Kladakis Prof. R.M. Nerem Dr. C.V.C. Bouten Prof. F.T.P. Baaijens Department of Biomedical
APPLICATION SPECIFIC PROTOCOL CELL MIGRATION FOR ADHERENT CELLS
 APPLICATION SPECIFIC PROTOCOL CELL MIGRATION FOR ADHERENT CELLS AIM 3D Cell Culture Chips are very useful for the study of 3D cell invasion and migration. The chips are not only suitable for endpoint measurement;
APPLICATION SPECIFIC PROTOCOL CELL MIGRATION FOR ADHERENT CELLS AIM 3D Cell Culture Chips are very useful for the study of 3D cell invasion and migration. The chips are not only suitable for endpoint measurement;
Single-Use Simplicity
 Single-Use Simplicity BioBLU Single-Use Vessels for cell culture »Proven stirred-tank design meets single-use technology.«reliable performance and ease-of-use Combine the benefits of single-use bioreactor
Single-Use Simplicity BioBLU Single-Use Vessels for cell culture »Proven stirred-tank design meets single-use technology.«reliable performance and ease-of-use Combine the benefits of single-use bioreactor
Custom Systems Built to Exacting Client Specification
 Custom Systems Built to Exacting Client Specification Laboratory Reactor Systems Parr Instrument Company is pleased to work with customers in the design and assembly of complete laboratory or pilot plant
Custom Systems Built to Exacting Client Specification Laboratory Reactor Systems Parr Instrument Company is pleased to work with customers in the design and assembly of complete laboratory or pilot plant
Artery-on-a-Chip: A Microfluidic Platform to Investigate Small Artery Function
 Artery-on-a-Chip: A Microfluidic Platform to Investigate Small Artery Function 1 C Lochovksy, 1 S Yasotharan, 1 A Vagaon, 1 D Lidington, 1,2 J Voigtlaender-Bolz, 1 S-S. Bolz, 1 A Günther 1 University of
Artery-on-a-Chip: A Microfluidic Platform to Investigate Small Artery Function 1 C Lochovksy, 1 S Yasotharan, 1 A Vagaon, 1 D Lidington, 1,2 J Voigtlaender-Bolz, 1 S-S. Bolz, 1 A Günther 1 University of
cardiovascular cell Solutions
 The Essentials of Life Science Research Globally Delivered cardiovascular cell Solutions We love what we do. And now we do it even better! As part of your portfolio for success, ATCC now provides a range
The Essentials of Life Science Research Globally Delivered cardiovascular cell Solutions We love what we do. And now we do it even better! As part of your portfolio for success, ATCC now provides a range
CHAPTER 4. Design of Innovative Creep Testing Facility under Flowing. Sodium
 CHAPTER 4 Design of Innovative Creep Testing Facility under Flowing Sodium 4.1 Introduction In this chapter, design of an innovative creep testing system comprising of a lever arm type creep machine with
CHAPTER 4 Design of Innovative Creep Testing Facility under Flowing Sodium 4.1 Introduction In this chapter, design of an innovative creep testing system comprising of a lever arm type creep machine with
Lehigh Preserve. Lehigh University. Sonam Srivastava Lehigh University. Theses and Dissertations
 Lehigh University Lehigh Preserve Theses and Dissertations 2013 Analytical Lateral Load Response of Unbonded Post-Tensioned Cast-in-Place Concrete Special Structural Walls with Bonded or Debonded Longitudinal
Lehigh University Lehigh Preserve Theses and Dissertations 2013 Analytical Lateral Load Response of Unbonded Post-Tensioned Cast-in-Place Concrete Special Structural Walls with Bonded or Debonded Longitudinal
14. SCREENS. in the Figure 8.1. BARS TROUGH
 1 14. SCREENS The primary treatment incorporates unit operations for removal of floating and suspended solids from the wastewater. They are also referred as the physical unit operations. The unit operations
1 14. SCREENS The primary treatment incorporates unit operations for removal of floating and suspended solids from the wastewater. They are also referred as the physical unit operations. The unit operations
Cellular Biomechanical Systems
 Cellular Biomechanical Systems 2D/3D Cell Culture Tension Compression Shear Stress Tissue Engineering Tension System Compression System Tension & Compression Systems: Computer regulated bioreactors that
Cellular Biomechanical Systems 2D/3D Cell Culture Tension Compression Shear Stress Tissue Engineering Tension System Compression System Tension & Compression Systems: Computer regulated bioreactors that
Sanitary and Environmental Engineering I (4 th Year Civil)
 Sanitary and Environmental Engineering I (4 th Year Civil) Prepared by Dr.Khaled Zaher Assistant Professor, Public Works Engineering Department, Faculty of Engineering, Cairo University Wastewater Flow
Sanitary and Environmental Engineering I (4 th Year Civil) Prepared by Dr.Khaled Zaher Assistant Professor, Public Works Engineering Department, Faculty of Engineering, Cairo University Wastewater Flow
Preventive Maintenance and Calibration Program: IDM provides OEM services:
 FOAM / MATTRESS Industry Product Guide Preventive Maintenance and Calibration Program: The IDM Preventive Maintenance and Calibration (PM&C) program has been designed to make the maintenance and calibration
FOAM / MATTRESS Industry Product Guide Preventive Maintenance and Calibration Program: The IDM Preventive Maintenance and Calibration (PM&C) program has been designed to make the maintenance and calibration
BioBundles. Real bioreactors, really small.
 BioBundles Real bioreactors, really small. Save Time and Money with mycontrol and Small-Scale Bioreactors Starting with smaller bioreactor cultures is the most cost effective way to develop a new process
BioBundles Real bioreactors, really small. Save Time and Money with mycontrol and Small-Scale Bioreactors Starting with smaller bioreactor cultures is the most cost effective way to develop a new process
DESIGN AND FABRICATION OF ARTIFICIAL SKIN USING EMBEDDED MICROCHANNELS AND LIQUID CONDUCTORS
 ONLY AND OF USING EMBEDDED MICROCHANNELS AND LIQUID CONDUCTORS ONLY Critical technology. Robots are expected to work more autonomously. Biomedical science has made a lot of progress. The robotics community
ONLY AND OF USING EMBEDDED MICROCHANNELS AND LIQUID CONDUCTORS ONLY Critical technology. Robots are expected to work more autonomously. Biomedical science has made a lot of progress. The robotics community
