Towards More Successful Gene Therapy Clinical Trials for -Thalassemia
|
|
|
- Vanessa Stanley
- 6 years ago
- Views:
Transcription
1 Send Orders for Reprints to Current Molecular Medicine 2013, 13, Towards More Successful Gene Therapy Clinical Trials for -Thalassemia E. Drakopoulou 1,2, E. Papanikolaou 1,2, M. Georgomanoli 1,2 and N.P. Anagnou *,1,2 1 Laboratory of Cell and Gene Therapy, Centre for Basic Research, Biomedical Research Foundation of the Academy of Athens (BRFAA), Athens, Greece 2 Laboratory of Biology, University of Athens School of Medicine, Athens, Greece Abstract: -thalassemias constitute hereditary blood disorders characterized by reduced or absence of -globin synthesis resulting in mild to severe anemia, depending on the genotype. More than 200 mutations in the -globin gene are responsible for their specific features leading to a very heterogeneous phenotype. Current therapies for -thalassemia include blood transfusions, usually along with iron chelation and in selected cases with bone marrow transplantation (BMT) of HLA-matched hematopoietic stem cells (HSCs). However, these approaches are limited by factors, such as iron overload and donor availability, respectively. Since 2000, when globin lentiviral vectors (LVs) were first successfully tested for transfer efficiency of the therapeutic transgene, which led to disease amelioration in murine models, attention was drawn towards the improvement of such vectors for -thalassemia gene therapy. Constantly improving vector design and efficient HSC manipulation led recently to the first successful clinical trial in France, which further proved that this genetic approach can be curative. Furthermore, improved new efficient vectors and methods to safely monitor integration sites and therapeutic transgene position effects, promise a new era for -thalassemia gene therapy, with more and safer clinical trials yet to come. Keywords: -thalassemia, clinical trial, CD34 + cells, gene therapy, -globin, hematopoietic stem cells (HSCs), lentiviral vectors (LVs). 1. INTRODUCTION -Thalassemia syndromes represent a group of hereditary, monogenic, blood disorders inherited in an autosomal recessive manner. There are more than 200 different mutations affecting the human -globin gene, which are responsible for the significant heterogeneity of the thalassemic phenotype [1], leading either to reduction or absence of -chain synthesis. As a result, -globin chain molecules are produced in excess and precipitate in red blood precursors, leading to impaired erythrocyte maturation, mechanical damage and ultimately to apoptosis [2]. Current therapies for -thalassemia include mainly blood transfusions, usually together with life-long iron chelation and in specific cases combined with hydroxyurea treatment, which induces fetal hemoglobin (HbF) production leading to decrease of the unpaired -globin chains. The only available curative therapy so far, is the allogeneic hematopoietic stem cell (HSC) transplantation of human leukocyte antigen (HLA)- matched sibling donors [3]; however, this is restricted due to limited matched-related donors and the need for long-term immunosuppression to prevent or delay Address correspondence to this author at the Laboratory of Cell and Gene Therapy, Biomedical Research Foundation of the Academy of Athens (BRFAA), Athens , Greece; Tel: ; , ; Fax: ; anagnou@med.uoa.gr graft-versus-host disease (GVHD). Therefore, a molecular approach, such as gene therapy, seems quite promising, as it overcomes a significant number of the afore-mentioned limitations and exploits the beneficial effects that originate from the re-infusion of the corrected cells. For a successful -thalassemia gene therapy, the need for improved vector design exhibiting safety, lacking genotoxicity and leading to higher numbers of corrected HSC yields, is imperative. This paper summarizes the current state of the field, including all the in vitro studies using globin lentiviral vectors (LVs) and human thalassemic CD34 + cells, the first successful clinical trial in France by the team of Philippe Leboulch, as well the strategies for improved gene transfer and effective HSC manipulation, mainly ex vivo, which will eventually lead to more successful clinical trials for -thalassemia gene therapy. Although, a lot of pioneering experiments, which significantly contributed to our current knowledge and success in gene therapy for -thalassemia, were carried out in mice, they are not described in full here. Lastly, this review highlights the newly developed methods for vector safety and integration site analysis, which point towards a safe and controlled gene therapy outcome /13 $ Bentham Science Publishers
2 2 Current Molecular Medicine, 2013, Vol. 13, No. 8 Drakopoulou et al. 2. LENTIVIRAL VECTOR DESIGN FOR GLOBIN GENE TRANSFER 2.1. Leaving Oncoretroviruses Behind A critical step for a successful -thalassemia gene therapy is the identification and development of the most effective vector system for globin gene transfer. More than two decades ago, investigators utilized -retroviral vectors to transfer -globin genes into murine HSCs [4-6]; however the results of these studies were not satisfactory since the gene transfer efficiency was poor and expression of the transgene reached only up to 2% of the endogenous RNA levels. Even when Novak et al. [7] incorporated the newly identified DNA-enhancer elements from the -globin locus control region (LCR), shown to drive high-level expression of globin genes [8, 9], they failed to improve expression, as again poor vector production and genetic instability of the viral vector genome were observed. Pioneering experiments on retroviral vectors from Leboulch et al. [10] and Sadelain et al. [11] indicated that vector instability might be caused by splicing of the retroviral RNA genome, a consequence of the presence of cryptic splice sites within the incorporated human genomic sequences. Apparently, lentiviral vectors were less prone to splicing due to the expression of the Rev gene that allows the production of full-length unspliced viral RNA genomes [12]. Therefore, the use of a lentiviral backbone in globin vectors and Rev expression in a lentiviral packaging cell line, could minimize splicing of globin vectors containing large genomic fragments, thus leading to vector stability. The significant breakthrough in the field of globin gene therapy took place when May et al. [13] and Pawliuk et al. [14] constructed a human immunodeficiency virus (HIV)-based vector combining the -globin gene along with its LCR elements. This vector was able to transmit the significantly larger LCR and -globin configuration without rearrangement, and be produced in high titers, sufficient to ameliorate the disease in -thalassemic mice. Following this approach, several groups working also on hemoglobinopathies employed -globin LVs in their studies, leading to correction of murine -thalassemia [15]. Furthermore, the observation that compound thalassemic patients with the syndrome of hereditary persistence of fetal hemoglobin (HPFH) typically have less anemia, milder clinical symptoms and are often transfusion-independent, drew the attention towards constructing -globin vectors [16-18]. Derek Persons and colleagues were the first to design and test such vectors; the latter contained the extended -globin LCR and succeeded in demonstrating significant correction of the thalassemic phenotype [18]. However, the inconsistent globin expression by these vectors due to chromosomal position effects, eventually led to the use of chromatin insulators [19], which represent DNA elements capable to shield the therapeutic gene from the negative and/or positive effects of the surrounding DNA Self-Inactivating (SIN) Vectors, Insulators and Insertional Mutagenesis Integration of viral DNA constitutes the most basic step in the retroviral life cycle, since the viral genomes are permanently fixed as pro-viruses into the DNA of the host. During this process, the retroviral DNA is present in a large complex with a subset of retroviral and cellular proteins referred to as the pre integration complex (PIC). For -retroviruses, such as Murine Leukemia Virus (MLV), uncoating, DNA synthesis, and formation of PIC occur at the same rate, both in dividing and non-dividing cells, but integration fails to occur. During mitosis, however, due to disassembly of the nuclear membrane, the chromosomes are rendered accessible to the virus and integration occurs, suggesting that infection by oncoretroviruses, such as MLV, requires cell division [20]. This does not seem to be the case for lentiviruses, as it has been extensively documented that they are able to infect both dividing and non-dividing cells, due to their capacity to cross the nuclear membrane of interphasic cells. This represents a crucial asset for genetically modifying tissues, especially those considered as the main potential targets of gene therapy, such as the brain, muscle, liver and the hematopoietic system [21]. As with any new therapeutic approach, gene transfer using retroviral vectors may also induce novel kinds of side effects due to integration of the viral DNA within or near a proto-oncogene, a phenomenon called insertional mutagenesis. The report of lymphoproliferation due to insertional activation of the LMO2 gene following gene therapy for X1-linked severe combined immunodeficiency (SCID-X1) [22], as well as the detection of unexpected increase in the proportion of gene-corrected cells carrying retroviral insertion into the MDS1-EVI1 region in both nonhuman primates [23] and in patients in the German X-linked chronic granulomatous disease (X-CGD) clinical trial [24], has led to a re-evaluation of the mechanisms of insertional mutagenesis. While insertional mutagenesis using replicationdefective vectors is possible [25], such risks were originally underestimated [26], primarily based on the assumption that proviral integration into the genome is random. However, with the new and readily accessible human genome sequence data, mapping studies of retroviral integration sites in cell lines have uncovered semi-random integration patterns, using wild-type HIV, HIV-derived, or MLV-derived vectors [27-30]. Moreover, the integration patterns have only been recently investigated in HSCs [31-33] and have shown that while MLV integrants were located predominantly around transcription start sites, HIV integrants strongly favoured transcription units and gene-dense regions of the genome. These integration patterns suggest different mechanisms for integration, as well as distinct safety implications for oncoretroviral versus lentiviral vectors and imply a correlation between the stage of chromatin condensation and transcription [34]. The basis for these preferences is unknown, but they may reflect interaction of the PIC with specific proteins or
3 Thalassemia Gene Therapy Clinical Trials Current Molecular Medicine, 2013, Vol. 13, No. 8 3 with specific DNA sequences or structures that are associated with transcription. While copy number can be largely controlled with retroviral technology, the choice of the insertion site within the genome cannot be easily achieved with the current technology. This establishes a considerable risk for insertional up-regulation, silencing or disruption of cellular genes, a subset of which (>200) may act as proto-oncogenes or belong to the considerably smaller group of tumour suppressor genes. However, oncogene de-regulation requires a specific epigenetic and systematic context to foster tumour development. Therefore, mutagenesis is not necessarily predicting oncogenesis. However, the onset of leukemia in the SCID-X1 trial has been considered as the most severe side-effect of gene transfer, urging the gene therapy society and the monitoring organisms to create new methods in order to overcome the respective obstacle. The first strategy adopted was the construction of selfinactivating (SIN) vectors. This strategy essentially includes deletion of the enhancer sequences located within the U3 segment of the long terminal repeats (LTRs) of retroviruses. By employing the SIN configuration combined with the use of tissue-specific regulatory elements to drive expression of the transgene, activation of adjacent cellular genes becomes less likely. The second strategy involved the use of insulators. Transcriptional insulators are DNA elements that set boundaries on the actions of enhancer and silencer elements and thereby organize the eukaryotic genome into regulatory domains [35]. Thus, when an insulator is incorporated in the U3 region of the LTR, thereby flanking the transgene at both sides, the expression of the transgene is greatly protected, regardless of the integration site (position effect). At the same time, it provides extra safety, concerning the activation of adjacent proto-oncogenes. Arumugam et al. [19] improved globin gene expression by incorporating into the globin vector the 1.2 kb DNA element from the chicken hypersensitive site 4 (chs4) -globin locus; however, the vector s practical use was hampered, as the incorporation of the above element, significantly reduced the titer. The latter was rescued when Hanawa et al. [36] included only the 0.25 kb core element of chs4 and managed to obtain significantly increased transgene expression from an internal promoter, due to improved transcriptional termination. This element, in an orientation-dependent manner, demonstrated also some barrier activity, reducing variability of expression due to position effects, indicating that some insulation can be achieved with this core element. Furthermore, Lisowski and Sadelain [37] showed that addition of the HS1 element enhances the therapeutic efficacy of the globin gene transfer in murine -thalassemia, compared to the HS2-HS3-HS4 cassette, leading to even higher globin expression, along with lower vector copy numbers Inhibiting HbF Repressors The implication of the expression of -globin in thalassemia has long been recognized [1]. Firstly, the observation that thalassemic patients with HPFH syndromes show milder clinical symptoms of the disease and secondly, the great variability in -globin persistence in adults, led to efforts towards mapping modifier loci in these individuals, focusing on patients that do not have a mutation within the -globin locus on chromosome 11 [38]. These studies employed both murine and human cells and uncovered the role of BCL11A located on chromosome 2, on hemoglobin switching and inevitably on -globin expression [39]. More specifically, the pioneering work, of Sankaran et al. showed that low HbF expression in adult erythroid cells is associated with increased BCL11A expression. Moreover, knocking down BCL11A, by employing small interfering RNA (sirna) technology, led to the elevation of HbF levels, postulating that directed downregulation of BCL11A in thalassemic patients could ameliorate the severity of the disease through the re-activation of HbF in adult erythroid cells. Nearly a year later, two independent studies shed more light to HbF repression by BCL11A. Particularly, Borg et al. [40] with the aim to define the HPFH cause in a large Maltese family used a genome-wide singlenucleotide polymorphisms (SNPs) scan and found that KLF1 binds to human BCL11A in vivo and that its levels in affected individuals are directly proportional to BCL11A and inversely proportional to HbF levels. The above data indicated that BCL11A might regulate HbF expression via KLF1. A similar paper by Zhou et al. [41] published at the same time, verified the above, as it demonstrated that knockdown of KLF1 leads to reduced BCL11A expression in both murine and human adult erythroid cells, which in turn results in increased -globin expression with a concomitant decrease in -globin levels. Therefore, these data imply that KLF1, primarily in the adult life, is directly activating -globin and indirectly repressing -globin expression via the activation of BCL11A. The latter was shown to involve long-range interactions and cooperation with SOX6 [42]. The above results suggest that there is great therapeutic potential for interfering with KLF1 and/or BCL11A expression in the context of -thalassemia treatment. Construction of -globin LVs that combine the therapeutic gene, along with small hairpin RNA (shrna) that downregulate these HbF transcriptional repressors would, in theory, result in robust HbF expression. Our group is currently constructing such vectors. Recently, the Orkin group inactivated BCL11A in SCD transgenic mice and demonstrated correction of the hematologic and pathologic defects associated with SCD through a highlevel pancellular induction of HbF [43]. The above experiments verified that BCL11A can be a therapeutic target for -thalassemia gene therapy. Apart from the above -globin repressors, van Dijk et al. [44] showed that the chromatin factor Friend of
4 4 Current Molecular Medicine, 2013, Vol. 13, No. 8 Drakopoulou et al. Prmt1 (FOP) is also a critical modulator of HbF expression, since knockdown of the specific factor in adult erythroid precursors led to significantly elevated HbF levels. The above HbF induction was shown to occur independently of BCL11A, as BCL11A levels remained unaffected following FOP knockdown, but it involved modulation of SOX6, whose expression was reduced upon the specific experimental conditions [44]. Thus, utilization of shrnas against FOP in therapeutic LVs, alone or in combination with shrna against BCL11A and/or KLF1 could eventually lead to higher HbF expression levels. 3. IN VITRO STUDIES USING HUMAN THALAS- SEMIC CD34 + CELLS Both -globin and -globin vectors have been used in correcting thalassemic erythropoiesis in erythroid cultures. These studies are summarised in Fig. (1) and Table 1. The first study testing a -globin LV flanked by a chromatin insulator in human thalassemic CD34 + cells was carried out by Puthenveetil and co-workers [45] using transfusion-dependent human thalassemia major cells, where they demonstrated that the human -globin transgene was expressed at normal levels in erythroid cells produced in vitro. An in vivo significant outcome was also demonstrated, as these corrected progenitors were able to establish normal erythropoiesis when transplanted in immunodeficient mice. In addition, Roselli et al. [46] using the GLOBE vector, reported successful correction of thalassemia major in human cells, by achieving high transduction efficiency, restoration of hemoglobin A synthesis, rescue from apoptosis and correction of ineffective erythropoiesis. The same group, quite recently demonstrated that incorporation of the 200 bp erythroid-specific enhancer GATA1-HS2 into GLOBE, allows persistent and position-independent expression of -globin by reducing expression variegation and epigenetic silencing [47]. The improved globin vector, designated as G-GLOBE, managed to correct murine thalassemia with a lower vector copy number (VCV) than that of GLOBE (VCN = 1 vs VCN = 1.7). Initial experiments using human CD34 + cells from cord blood, suggest that the GATA1-HS2 element maintains high transgene expression in erythroid progeny of human HSCs, both in vitro and in vivo. Also, recently Breda et al. [48] developed a novel -globin lentiviral vector that carries the 200 bp erythroid-specific ankyrin 5 hypersensitive barrier insulator element and used it to transduce erythroid progenitor and CD34 + cells, originating from peripheral blood (PB) of thalassemic patients. AnkT9W was shown to contribute in increasing HbA synthesis from 0% to 62% in CD34 + derived cells (VCN = 0.92) or to 73% in erythroid progenitors-derived cells (VCN = 0.97). The group concluded that AnkT9W increased the synthesis of globin in most thalassemic specimens to levels comparable to carriers and control samples. However, 0 - thalassemic erythroid cells required higher VCN to reach therapeutic levels. Furthermore, the Persons group by employing the -globin vector V5m3-400 in an in vitro model of human erythropoiesis, showed recently that both LV-mediated -globin gene addition and genetic reactivation of endogenous -globin genes, either via a synthetic zinc finger transcription factor designed to interact with the -globin promoter, or via BCL11A inhibition, have the potential to provide therapeutic HbF levels to patients with -globin deficiency [49, 50]. Finally, our group, generated a novel insulated SIN LV, designated as GGHI (Gamma-globin HPFH insulated), containing the A -globin gene with the -117 HPFH point mutation and the HPFH-2 enhancer [51]. This vector managed to produce efficient amounts of HbF, resulting in phenotypic correction in erythroid cultures of CD34 + HSCs isolated from PB or bone marrow (BM) of thalassemic patients [51]. It is noteworthy that in this specific vector design, the incorporation of the full length 1.2 kb chs4 in the U3 region and the SIN configuration had no apparent effect on viral titer, as it reached 2x10 8 TU/ml. GGHI is the first LCR-free LV that has demonstrated efficient correction of thalassemia in vitro. 4. THE FIRST SUCCESSFUL CLINICAL TRIAL The first human clinical trial for -thalassemia commenced in France on June 7, 2007 and employed LentiGlobin, a -globin LV with a variant of the human -globin gene that carries a single base change at amino acid position 87. The specific variant, which was initially developed as an anti-sickling agent [14] was used in this setting to distinguish vector-encoded from endogenous -globin. Cavazzana-Calvo and colleagues selected two -thalassemia patients for BM transplantation (BMT) of LV-transduced HSCs [52, 53]. The initial patient, a 28-year old male, experienced a period of prolonged aplasia due to the low number of HSCs engrafted, and therefore the need for administration of his untransduced cells, which were kept as a back-up, was imperative. As a consequence, neither the LV-transduced cells reached a significant number in PB, nor did the therapeutic hemoglobin levels, thus leading to an inconclusive outcome for the specific patient, who was designated as patient 0. The next patient in the study (designated as patient 1) was a 18-year old male, a compound heterozygote for -thalassemia and HbE (HbE/ o ), requiring 160 ml of packed erythrocytes/kg/year, since the age of three. In this trial, he received 4x10 6 CD34 + cells/kg [53]. Within the first few months, the levels of the genetically modified cells increased to 2%, while they reached 11% at 33 months post-transplant, a rise that was also observed in the levels of the therapeutic -globin variant, which was expressed in 10 20% of genetically modified HSCs. As a result, improved production and quality of red blood cells was observed. The above patient was last transfused on June 6, 2008 and for more than four years post BMT he remains transfusion-
5 Thalassemia Gene Therapy Clinical Trials Current Molecular Medicine, 2013, Vol. 13, No. 8 5 Fig. (1). Schematic representation of the proviral elements of the / -globin lentiviral vectors currently used or planned for phase I/II clinical trials for -thalassemia gene therapy. All vectors have been constructed using a lentiviral backbone driven by a CMV or HIV promoter located at the 5' LTR. Following reverse transcription, the 398 bp enhancer U3 deletion (light grey triangle) of the 3' LTR is transferred to the 5' LTR, rendering the vector self-inactivating. All / -globin expression cassettes are in a reverse orientation compared to the orientation of the lentiviral vector reverse transcription. The proviral elements of each lentiviral vector used in preclinical trials, are depicted in detail. BG-I vector is insulated with the full length 1.2 kb chs4 insulator, carries the human -globin gene linked to a -globin 3' enhancer element, the 254 bp core element of the -globin promoter, and extended fragments of HS2, HS3 and HS4 hypersensitive sites of the LCR element [19]. LentiGlobin vector encodes a mutated -globin gene at codon 87, carries a double copy of the core 250 bp element of the chs4 insulator, a 266 bp -globin 3' enhancer element and different regions of HS2, HS3 and HS4 hypersensitive sites of the LCR [52, 53]. Both BG-I and LentiGlobin vectors have a 372 bp deletion in the intervening sequence (IVS) 2 of the -globin gene. GLOBE vector is the smallest in length lentiviral vector pre-clinically assessed, carries a minimal -globin gene with a 562 bp deletion in the IVS 2 (represented as a dark grey triangle) regulated by a 265 bp -globin promoter and contains only the HS2 and HS3 elements of the LCR, whereas it is not insulated [15]. G-GLOBE vector, which was recently constructed, has the same elements with GLOBE, but carries also the GATA1-HS2 element in both LTRs (shown between dotted lines), so as to allow persistent and position-independent expression of the -globin transgene [47]. TNS9 vector has similar control elements as the LentiGlobin, but it is not insulated and has control elements of different sizes, as depicted [13]. Its therapeutic efficiency has been tested only in mouse thalassemia models, and has not been clinically assessed yet. Notwithstanding, the novel version of TNS9, designated as TNS (not shown) is scheduled to be clinically assessed. AnkT9W vector represents a novel globin vector, which carries the 200 bp erythroid-specific ankyrin insulator element and was recently shown to increase HbA synthesis to therapeutic levels in human erythroid progenitors cells from thalassemic patients [48]. V5m3-400 and GGHI are -globin vectors, carrying the human A -globin coding sequence, with a 713 bp deletion in the IVS 2 region (black triangle), as well as the -globin or the -globin 3' UTR, respectively. V5m3-400 LV vector is flanked by the 400 bp core element, expresses the A -globin gene under the control of a minimal 130 bp -globin promoter and the HS2, HS3, HS4 fragments of the LCR [49, 50]. GGHI vector is a LCR-free, fulllength insulated -globin vector, which expresses A -globin coding sequences under the control of an A -globin promoter, carrying the -117 HPFH activating point mutation, and flanked by the HPFH-2 and HS-40 enhancer elements [51]. Triangles of different shades indicate deletions of different sizes as shown in figure; asterisks indicate point mutation; RRE, Rev Response Element; HS2, HS3, HS4, DNAase Hypersensitive sites 2, 3, 4 of the -globin LCR, the length of which is also indicated in each case; cppt, central Polypurine Tract; cppt/flap, DNA flap at the center of the viral cdna in between the central polypurine tract (cppt) and the Central Termination Sequence (CTS); LTR, Long Terminal Repeat; 3' Enh, -globin 3' enhancer element;, packaging signal; p, -globin gene promoter; A p, A -globin gene promoter; chs4, insulator from the chicken -like globin gene cluster; Ank, Ankyrin insulator; SD, Splice Donor site; SA, Splice Acceptor site; WPRE, Woodchuck Hepatitis Virus Posttranscriptional Regulatory Element; UTR, Untranslated Region; HPFH-2, Human Persistence of Fetal Hemoglobin-2 enhancer; HS-40, DNAase Hypersensitive Site-40 enhancer element of the human -like globin gene cluster; gag, 2 stop codons in gag; The length of the lentiviral vectors in kb is indicated adjacent to each vector.
6 6 Current Molecular Medicine, 2013, Vol. 13, No. 8 Drakopoulou et al. Table 1. Summary of the Key Findings of Preclinical and Clinical Assessment of Different and -Globin Vectors Used for -Thalassemia Gene Therapy LV Preclinical Assessment Using CD34 + Cells Clinical Trial MFI % Hb + Cells HPLC % Apoptosis CD34 + Hospital/Preliminary Source * % MTE VCN/Cell Results or Current Control Thal /LV Control Thal /LV Control Thal/LV Control Thal /LV Status BG-I LentiGlobin GLOBE AnkT9W V5m3-400 GGHI BM (n=4) CB (ND) BM (n=30) PB n=19 PB and BM (ND or Thal) (n=3) BM (n=20) ± ± ± 6 60 ± 5 7.3% 75% 49 ± ± 429 (ND) 2076 ± 647 (ND) 3170 ± ± ± 7.2 (ND) 44.6 ± (ND) (ND) (GFPtransduced) (V5m3-400 transduced) (ND) 0.79 (ND) 0% (ND) 39.76% (ND) 1.4 ± 0.4% (mock) 27.5% 25.9% % 74.63% 15.4 ± 4.9% 35.4% 34.9% 33.5 ± 5.4 GpA+/ AnnV 18.1 ± 3.1 PI 2 ± ± ± (mock) CHC Cincinatti Pending Necker Paris 21 months post BMT Hb: 9-10 g dl -1 HSR-TIGET Milano Pending Saint Jude Memphis Pending * CD34 + cell source for each lentiviral vector. For BG-I assessment, bone marrrow (BM) samples of o / o, + / + and o / + thalassemia patients were used [19]; for LentiGlobin preclinical assessment, cord blood (CB) samples from normal donors (ND) were used, while thalassemic BM were used in its clinical trial [52, 53]; For GLOBE vector, BM samples from o / o, o / + and + / + thalassemia patients were used [15, 47]. AnkT9W was tested on erythroid progenitor (data not shown) and CD34 + cells from peripheral blood (PB) of o / o, o / + and + / + thalassemic patients [48]. V5m3-400 was tested on mobilized PB or BM from ND or thalassemia major donors [49, 50]. Lastly, GGHI vector was tested on BM samples from o / o, + / o or + / + patients [51]. For GLOBE, AnkT9W and GGHI assessment, thalassemic patients with o / o genotypes are designated as o, while patients with + / o and/or + / + genotypes are included in one group, designated as +. The number of patients involved in each trial is provided. Mean transduction efficiency (MTE) is presented as percentage per vector-positive cells, calculated at clonal level by PCR and primers against vector-specific sequences. Average Vector Copy Number (VCN) per cell determined by quantitative PCR or Southern blot analysis, using genomic DNA isolated from LV-transduced CD34 + cells. For BG-I vector a corrected value for gene transfer efficiency is provided. Mean Fluorescence Intensity (MFI) is measured by FACS and intracellular staining of Thal cells from erythroid cultures transduced with either -globin or -globin LVs and compared to control samples. The latter are either untransduced Thal cells or cells from ND, as indicated in brackets. Percentage of HbA or HbF positive fraction of transduced cells from erythroid cultures determined by FACS analysis, with the exception of V5m3-400 vector, for which calculation of globin LV-transduced and GFP-transduced cells was determined by densitometry. For all other LVs, control is either untransduced Thal cells or cells from ND, as indicated in bracket. High Performance Liquid Chromatography (HPLC) for -globin or -globin chains, as estimated from transduced cell lysates from erythroid liquid cultures and compared to control (Thal, ND or mock), as indicated in brackets. For BG-I, the percentage of chains is estimated by reverse phase HPLC; for GLOBE vector the ratio of / chains is provided, as estimated with reverse phase HPLC analysis for radiolabelled neo-synthesized globin chains; for V5m3-400 the percentage of chains is estimated by HPLC and normalized per VCN and lastly for GGHI the percentage of -chains is estimated by HPLC. Percentage of apoptotic cells in erythroid liquid cultures assessed by FACS for apoptotic markers in Thal or mock-transduced CD34 + versus transduced Thal CD34 + cells, as indicated; for BG-1 and GGHI vectors, it was estimated by Annexin-V single staining; for GLOBE vector, it was estimated by both GpA+/Annexin-V double staining and propidium iodine (PI) staining, as indicated; for V5m3-400, the percentage of apoptotic cells was determined by TdT-mediated dutp nicked endlabelling (TUNEL) assay, which did not show a significant reduction, but just a trend. independent, while he is exhibiting sustained amelioration of the thalassemic phenotype for 64 months. Despite the above success, the group [53] reported clonal dominance of a single hematopoietic clone, harbouring a vector insertion into the HMGA2 gene. The specific clone, 20 months post transplant, accounted for 50% of the genetically modified HSCs, though with only 3% contribution to the circulating red blood cells. The potential clinical relevance of the aberrant HMGA2 expression alteration by the vector integration is highlighted by the fact that this gene may function as a potential oncogene in various types of cancer. However, as the above observation may simply reflect the consequences of engraftment from a small number of transduced HSCs, it remains unclear whether the insertion into the specific gene is responsible for the contribution of this clone to hematopoiesis [53].
7 Thalassemia Gene Therapy Clinical Trials Current Molecular Medicine, 2013, Vol. 13, No HEMATOPOIETIC STEM CELL (HSC) SOURCES FOR CLINICAL TRIALS IN -THALASSEMIA GENE THERAPY Before the French gene therapy thalassemia trial, allogeneic HSC transplantation was the only radical cure for -thalassemia major. Since gene therapy for thalassemia requires optimal numbers of vector transduced HSCs to be infused to the patient, the issue of supplying sufficient numbers of HSCs remains critical, while the originating source is a matter of debate. Until recently, there were two sources of procurement of HSCs, i.e. BM and mobilized PB. However, several research groups are now investigating the possibility of using alternative sources such as induced pluripotent stem (ips) cells Bone Marrow (BM) Bone marrow is undoubtedly the most well known source for CD34 + HSCs, as the majority of these cells under steady-state and normal conditions, reside in plethora within BM, organised in distinct niches [54]. Therefore, HSC transplantation has become synonymous to BMT. Until recently, BM was not considered a preferred source of HSCs in thalassemia gene therapy, primarily due to the invasive nature of the technique, requiring general anesthesia while providing insufficient yield of CD34 + cells. A recent study by Frittolli et al. [55] however, demonstrated for the first time that BM can indeed serve as a source of HSCs for gene therapy. Specifically, 20 BM harvests were carried out from 20 transfusion-dependent thalassemic patients undergoing allogeneic HSC transplantation, 29 BM harvests from thalassemia heterozygote donors and 20 from healthy donors. The group managed to collect sufficient amounts of CD34 + cells from the thalassemic patients, with concentrations being similar to that of healthy subjects, i.e. median 0.26 x 10 6 /ml in thalassemic patients, 0.36 x 10 6 /ml in thalassemia heterozygotes donors and 0.24 x 10 6 /ml in normal donors. All harvests were analyzed further for routine BM morphology. The group found an expected expansion in the erythroid compartment in thalassemic patients (70%) compared to heterozygotes (40%) and normal donors (35%). However, this expansion influenced neither the yield of CD45 + or CD34 + cells nor the proportion of myeloid and erythroid progenitors. Moreover, the HSC yield in the thalassemic patients was also not affected by disease-specific characteristics or iron overload. Lastly, none of the subjects suffered from any serious adverse effects following aspiration, suggesting that BM harvesting is safe and free from severe side effects. Based on the above clinical data, the Italian group has applied to the authorities for a phase I/II gene therapy clinical trial, using BM as a source of HSCs Mobilized Peripheral Blood Stem Cells (PBSCs) It represents the most widely used source of HSCs for autologous or allogeneic transplantation, primarily due to the higher yield of CD34 + cells, and to the noninvasive nature of the procedure compared to conventional BM harvest. The term PBSC mobilization refers to the forced migration of HSCs from the BM to the periphery. Granulocyte colony-stimulating factor (G- CSF) represents the major inducer of PBSC mobilization, and can be administered with or without chemotherapy [56, 57]. More specifically, Li et al. [58] administered G-CSF to mobilize stem and progenitor cells in thalassemia major pediatric patients and compared the kinetics of CD34 + cells and lymphocyte subsets with those of healthy PBSC donors. Results showed that G-CSF in concentrations of g/day/kg of body weight, succeeded in effectively mobilizing CD34 + cells of both thalassemic patients and healthy donors. Additionally, no significant difference was observed in the levels of daily stem cell counts between the two groups, indicating that efficient CD34 + cell levels in PB and collection of PBSC in -thalassemia patients are feasible [58]. In a different study, Yannaki et al. [59] used G-CSF to mobilize murine HSCs and concluded that thalassemic mice mobilized less efficiently than their control counterparts, possibly due to increased splenic trapping of HSCs and progenitor cells. The reduced mobilization efficiency was restored in splenectomized subjects. Recently, the same group published the results of a clinical trial using either G-CSF or plerixafor in splenectomized and nonsplenectomized individuals [60]. They concluded that for -thalassemia gene therapy, both G-CSF and plerixafor could be used as mobilization agents in nonsplenectomized subjects, whereas plerixafor is preferred in the case of splenectomized patients, both in terms of efficacy and safety. The French ongoing clinical trial, following authorization from the regulatory agency, could use either BM-derived or PB G-CSF mobilized CD34 + cells [52]. Also, Michel Sadelain in his strategy for phase I clinical trial using vector TNS9.3.55, is planning to mobilize PBSCs [61] Induced Pluripotent Stem (ips) Cells Induced pluripotent stem (ips) cells result from reprogramming of a differentiated somatic cell into a pluripotent embryonic-like stem cell (ESC) [62]. They exhibit many features of human ESCs and can theoretically generate any cell type in the human body. They can also serve as suitable cell types for the correction of mutant cells or tissues, mainly by homologous recombination. For ips cell generation, somatic cells from a patient are isolated and reprogrammed to a stem cell-like status, using the Oct3/4, Sox2 genes, with either Klf4 and c-myc or Nanog and Lin28. Following genetic manipulation and differentiation to the desired cell lineage, they can be administered back to the patient [62]. Several approaches have been described in detail for the generation of ips cells [62]. These mainly include direct reprogramming using viral vectors, primarily lentiviruses and adenoviruses and small molecules, such as plasmids and transposon elements. For -thalassemia gene therapy, ips cells can provide an alternative and patient-friendly strategy for
8 8 Current Molecular Medicine, 2013, Vol. 13, No. 8 Drakopoulou et al. obtaining higher number of HSCs for genetic manipulation and transplantation. Hanna et al. [63] were the first group to report gene correction in a murine SCD model, using ips cells. Cells from the skin of the SCD mouse were harvested and reprogrammed to ips cells, using a retrovirus to deliver Oct4, Sox2, Klf4 and c-myc genes. After removing the potential oncogenic c-myc gene, ips cells were cultured to produce hematopoietic stem cell precursors that carried the normal gene and were then transplanted back to SCD mice. The data documented disease amelioration in treated mice. ips cells were also used as an HSC source in human studies, with Ye et al. [64] being the first to show that skin fibroblasts from a thalassemic patient with o -thalassemia could be reprogrammed into ips cells. Specifically, fibroblasts from skin biopsies derived from a thalassemic patient homozygous for a CTTT deletion-mutation causing a frameshift that results in no -globin synthesis, were used for retroviral infections both in the presence and absence of c-myc gene, along with the Oct4, Sox2 and Klf4 transcription factors. The obtained colonies, expressed pluripotency markers, and were shown to carry the same 4-bp mutation found in the thalassemic patient, confirming that they derived from the patient s skin fibroblasts. Furthermore, these ips cells demonstrated the ability to differentiate towards hematopoietic lineage, upon cultivation in cytokine-containing differentiation medium and produced cells that stained positive for fetal hemoglobin. Taken together, the above findings suggest that reprogramming of skin fibroblasts from a thalassemic patient into ips cells is feasible [64]. Following this proof of principle, Zou et al. [65] recently reported a homologous recombination-based approach to correct SCD mutation in ips cells obtained from an adult patient carrying two mutated -globin alleles ( S ). Precise conversion of one mutated S gene to wild type A allele in the patient s ips cells was achieved, using a plasmid containing a drug-resistant gene cassette, flanked by loxp sites and S -specific zinc finger endonucleases. Up to 40% transcript expression upon differentiation to erythrocytes was documented, demonstrating that single nucleotide substitution using human ips cells is feasible in humans, representing an efficient strategy for gene correction [65]. Furthermore, in a recent study, besides the correction of the -thalassemia mutation, a successful transplantation of the corrected human ips cell-derived HSCs into an immune deficient mouse model was achieved [66]. More specifically, Wang et al. [66] reprogrammed skin fibroblasts into ips, derived from a 2-year old thalassemic patient homozygote for the CTTT deletion-mutation in codons 41-42, by introducing Oct3/4, Sox2 and Klf4 using retroviral vectors. Following successful correction of the mutation by specific gene targeting and homologous recombination, the ability of the specific cell lines to differentiate was assessed both in vitro and in vivo. It was shown that the gene-corrected cells showed improved hematopoietic differentiation capacity in vitro and could induce hematopoiesis in sub-lethally irradiated SCID mice. Furthermore, these cells could also produce adult human -globin after hematopoietic differentiation in vivo at about 9% of the endogenous mouse -globin. Furthermore, ips cells were also used as means of gene addition, leading to correction of -thalassemia in vitro. In a pioneering work by Papapetrou and colleagues [67], skin fibroblasts or BM mesenchymal cells from four patients with -thalassemia major were used to obtain -globin-expressing ips cell clones, in which the transgene was inserted in safe harbors and expressed in therapeutic levels. More specifically, somatic cells from thalassemia major patients were reprogrammed to patient-specific ips cells and following removal of the reprogramming vectors and transduction with the TNS9-derived -globin vector at safe harbors, they were differentiated towards erythroid lineage. Twelve out of the 13 ips cell clones used expressed detectable vector-encoded -globin, the average expression of which was 53% of the normal endogenous -globin allele. The above data proved that correction through addition of a therapeutic gene expressed in therapeutic levels into safe harbors in patient-specific ips cells is feasible and potentially could be used for the clinical treatment of -thalassemia. However, a major limitation to their use remains their inability for long-term engraftment, following generation and genetic modification. In 2009, Raya et al. [68] reported that disease-corrected ips cells originating from a Fanconi anemia patient, despite their successful differentiation into HSCs, they failed to engraft and reconstitute severe immunocompromised mice. It was only recently, that promising data arose, with Wang et al. [66] managing to produce patientspecific ips that could differentiate in HSCs and induce hematopoiesis in sub-lethally irradiated SCID mice, as seen above. In general however, ips cell use in human applications remains elusive. Despite extensive studies in mice, long-term engraftment of ES-originating HSCs in vivo has been reported only by ectopic expression of the transcription factor HoxB4 [69], however as the latter factor is implicated in various leukemias in both mice and primates [70, 71], this approach cannot be considered in human clinical applications. All the above strategies, which involved reprogramming using integrating viral vectors, require various genetic modifications of the target cells to stably express a transgene, and therefore the need for semi random and controlled integration of the transgene of interest is imperative to ensure safety. In their recent work, Papapetrou and Sadelain [67, 72, 73] demonstrated how the above issues could be addressed. Specifically, by employing bioinformatics and functional analysis, they screened patient-specific ips cell clones and retrieved safe integration sites, lowering thus the risk for semi random vector intergration, which could interfere with the expression of neighbouring genes, including oncogenes. The above safe harbors met the following five criteria: (i) distance of at least 50 kb from the 5 end of any gene, (ii) distance of at least 300 kb from any cancer-related
9 Thalassemia Gene Therapy Clinical Trials Current Molecular Medicine, 2013, Vol. 13, No. 8 9 gene, (iii) distance of at least 300 kb from any microrna (mirna) coding gene, (iv) location outside a transcription unit, and (v) location outside ultraconserved regions (UCRs) of the human genome [67]. Furthermore, they devised a 7-week protocol, which enables efficient derivation of genetically modified human ips cells, carrying the transgene at known genomic sites [72]. The group also proposed a 12 to 14-week protocol for generating transgene-free ips cells, employing the polycistronic LV pml-fsv2a, in which Oct4, Sox2, Klf4 and c-myc genes are flanked by loxp sites. Upon establishment of ips cell clones, in which vector integrations are again mapped to the human genome, expression of Cre recombinase, driven by an integrase-deficient vector, mediates excision of the above genes, leading thus to transgenefree ips cells [73]. Reprogramming of ips cells can be also achieved using methods that are devoid of integration, such as episomal plasmids, non-integrating vectors -such as adenoviruses- and transposons [74]. Use of such methods is promising for generation of ips cells, as they are free from issues, such as insertional mutagenesis and incomplete silencing of reprogramming factors. However, great concerns are also raised in certain occasions, as various genetic alterations, some affecting oncogenes during reprogramming phase under these conditions, have been reported. Specifically, in their recent report, Howden and colleagues [75], using an episomal reprogramming method, reported that comparison of ips cell lines before and after gene targeting revealed an accumulation of a number of mutations originating during reprogramming procedures. It is therefore worthwhile to extend our knowledge towards safe ips cell generation and genetic modification methods, before their use can be implemented in human applications. 6. ENHANCING GENE TRANSFER BY HSC MANIPULATION Human HSCs are more resistant to lentiviral gene transfer compared to murine ones, and so far the maximum number of genetically modified PB cells obtained is 15%, as demonstrated by various laboratories. Specifically, Kim et al. [76] working on rhesus macaques and using a GFP LV showed that only 7% of PB cells were transduced, an observation that was also made by Trobridge et al. [77] and Hanawa et al. [78], who worked on pigtail macaques. Lastly, in the French clinical trial [53], genetically modified HSCs did not exceed 15% on average, leading to the conclusion that higher percentages of genetically modified HSCs in the case of human and non-human primates might not be easily obtainable. Below, a description of the main methods for ex-vivo manipulation currently used for increasing HSC gene transfer suitable for clinical trials is presented Traditional vs Newly Developed Envelope Glycoproteins Significant efforts have been made towards improving the ability of globin LVs pseudotyped with different envelope glycoproteins to transduce human HSCs. Kim et al. [79] performed comparative transduction studies of human HSCs with GFP lentiviral vectors pseudotyped with either the vesicular stomatitis virus glycoprotein (VSV-G), the amphotropic (AMPHO) murine leukemia virus envelope protein, the endogenous feline leukemia viral envelope protein (RD114TR) or the feline leukemia virus type C envelope protein (FLVCTR). They concluded that VSV-G is more effective in transducing engrafting cells compared to other -retroviral glycoproteins, as it led from 2 to 10-fold higher transduction rates, with no indication of toxicity, despite the relatively high multiplicities of infection used, thus supporting its use in clinical-grade vectors [79]. Pioneering experiments by Verhoeyen et al. [80] demonstrated that HSC gene transfer is significantly increased when lentiviral particles are engineered to display early-acting cytokines on their surface, such as SCF and TPO, alone or combined. In this setting, the modified LVs would particularly stimulate the HSCs within the CD34 + pool, leading to increased gene transfer rates, with relatively low MOI and short incubation times. This procedure minimizes the risk for insertional mutagenesis resulting from high vector doses and also helps to preserve HSC stemness, which is usually lost, after cytokine cocktail stimulation, that is usually employed to sustain survival in vitro. Furthermore, Frecha et al. [81] from the same group, performed comparative analysis studies using novel LVs co-displaying SCF and TPO glycoproteins, fused to the N-terminus of the hemagglutinin protein of influenza virus (HA), and the mutant endogenous feline retroviral glycoprotein RDTR. The data disclosed that particularly RDTR/SCFHA can selectively target and transduce cord blood CD34 + cells, even in the absence of retronectin and with MOI as low as 1. Also, as RDTR and RDTR/SCFHA LVs, unlike VSV-G LVs, resist inactivation by complement factors present in human BM aspitates, they were further tested in unfractionated BM of healthy and Fanconi anemia donors, leading to selective transduction, again in the absence of retronectin and in low MOIs. Moreover, the RDTR/SCFHA lentiviral vector, when used to drive expression of a FANCA gene, resulted in 40% selective transduction of human CD34 + in the patients BM, suggesting that it can confer efficient gene targeting, allowing correction of CD34 + cells. Furthermore, Neff et al. [82], Ghani et al. [83] and Bell et al. [84], also proposed that the specific glycoprotein could potentially substitute the more cytotoxic VSV-G envelope protein and enhance viral transduction, as it demonstrates stability, its receptor is widely expressed on HSCs, and most importantly, it demonstrates no toxicity. More specifically, in a recent study by Bell and colleagues [84], the packaging efficiency of three envelope proteins, RD114, RDpro and VSV-G was compared. It was concluded that RD114-pseudotyped viruses, despite having one log
Pros and Cons of Gene Therapy in ß-Thalassemia
 Pros and Cons of Gene Therapy in ß-Thalassemia ß-Thalassemia: established and potential new therapeutic approaches Many ß-thalassemic patients are treated with blood transfusion and iron chelation. However,
Pros and Cons of Gene Therapy in ß-Thalassemia ß-Thalassemia: established and potential new therapeutic approaches Many ß-thalassemic patients are treated with blood transfusion and iron chelation. However,
Current SCID Gene Transfer Experience
 Current Perspectives on Gene Transfer for X-SCID Recombinant DNA Advisory Committee Safety Symposium March 15, 2005 Discussion Questions Current SCID Gene Transfer Experience 1. What data is available
Current Perspectives on Gene Transfer for X-SCID Recombinant DNA Advisory Committee Safety Symposium March 15, 2005 Discussion Questions Current SCID Gene Transfer Experience 1. What data is available
Peripheral Blood Stem Cell Collections in the Age of Gene Therapy
 Peripheral Blood Stem Cell Collections in the Age of Gene Therapy John P Manis MD Boston Children s Hospital Brigham and Women s Hospital Dana Farber Cancer Institute Boston MA No Disclosures Conflict
Peripheral Blood Stem Cell Collections in the Age of Gene Therapy John P Manis MD Boston Children s Hospital Brigham and Women s Hospital Dana Farber Cancer Institute Boston MA No Disclosures Conflict
Gene Therapy of FA: Premises and Promises
 Gene Therapy of FA: Premises and Promises Jakub Tolar tolar003@umn.edu Treat marrow failure Prevent leukemia Reduce endocrine problems Lower risk of head/neck cancer Improve quality of life A unified trial
Gene Therapy of FA: Premises and Promises Jakub Tolar tolar003@umn.edu Treat marrow failure Prevent leukemia Reduce endocrine problems Lower risk of head/neck cancer Improve quality of life A unified trial
John Gurdon was testing the hypothesis of genomic equivalence or that when cells divide they retain a full genomic compliment.
 1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
1. (15 pts) John Gurdon won the 2012 Nobel Prize in Physiology or Medicine for work he did in the 1960 s. What was the major developmental hypothesis he set out to test? What techniques did he development
Fundamental properties of Stem Cells
 Stem cells Learning Goals: Define what a stem cell is and describe its general properties, using hematopoietic stem cells as an example. Describe to a non-scientist the current progress of human stem cell
Stem cells Learning Goals: Define what a stem cell is and describe its general properties, using hematopoietic stem cells as an example. Describe to a non-scientist the current progress of human stem cell
Genetics and Genomics in Medicine Chapter 9 Questions
 Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
Genetics and Genomics in Medicine Chapter 9 Questions Multiple Choice Questions Question 9.1 Which, if any, of the following can be classified as a type of augmentation therapy? a) Treatment using a small
TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1
 AD Award Number: W81XWH-10-1-0181 TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1 PRINCIPAL INVESTIGATOR: Jonathan Chernoff, M.D., Ph.D. CONTRACTING ORGANIZATION: Institute
AD Award Number: W81XWH-10-1-0181 TITLE: Induced Pluripotent Stem Cells as Potential Therapeutic Agents in NF1 PRINCIPAL INVESTIGATOR: Jonathan Chernoff, M.D., Ph.D. CONTRACTING ORGANIZATION: Institute
A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo.
 Gus Frangou, Stephanie Palmer & Mark Groudine Fred Hutchinson Cancer Research Center, Seattle- USA A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo. During mammalian development and differentiation
Gus Frangou, Stephanie Palmer & Mark Groudine Fred Hutchinson Cancer Research Center, Seattle- USA A Genetic Screen to Identify Mammalian Chromatin Modifiers In Vivo. During mammalian development and differentiation
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms
 Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
Genetics - Problem Drill 19: Dissection of Gene Function: Mutational Analysis of Model Organisms No. 1 of 10 1. The mouse gene knockout is based on. (A) Homologous recombination (B) Site-specific recombination
ICH Considerations. Oncolytic Viruses September 17, 2009
 INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
INTERNATIONAL CONFERENCE ON HARMONISATION OF TECHNICAL REQUIREMENTS FOR REGISTRATION OF PHARMACEUTICALS FOR HUMAN USE ICH Considerations Oncolytic Viruses September 17, 2009 1. Introduction Oncolytic viruses
Test Bank for Molecular Cell Biology 7th Edition by Lodish
 Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
Test Bank for Molecular Cell Biology 7th Edition by Lodish Link download full: http://testbankair.com/download/test-bank-formolecular-cell-biology-7th-edition-by-lodish/ Chapter 5 Molecular Genetic Techniques
2111: ALL Post-HCT. Add/ Remove/ Modify. Manual Section. Date. Description. Comprehensive Disease- Specific Manuals
 2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
2111: ALL Post-HCT The Acute Lymphoblastic Leukemia Post-HCT Data Form is one of the Comprehensive Report Forms. This form captures ALL-specific post-hct data such as: planned treatments post-hct, the
Premade Lentiviral Particles for ips Stem Factors: Single vector system. For generation of induced pluripotent stem (ips) cells and other applications
 Premade Lentiviral Particles for ips Stem Factors: Single vector system For generation of induced pluripotent stem (ips) cells and other applications RESEARCH USE ONLY, not for diagnostics or therapeutics
Premade Lentiviral Particles for ips Stem Factors: Single vector system For generation of induced pluripotent stem (ips) cells and other applications RESEARCH USE ONLY, not for diagnostics or therapeutics
Phase I/II Gene therapy trial of Fanconi anemia patients with a new Orphan Drug consisting of a lentiviral vector carrying the FANCA
 Phase I/II Gene therapy trial of Fanconi anemia patients with a new Orphan Drug consisting of a lentiviral vector carrying the FANCA gene: A Coordinated International Action J. Bueren Hematopoietic Innovative
Phase I/II Gene therapy trial of Fanconi anemia patients with a new Orphan Drug consisting of a lentiviral vector carrying the FANCA gene: A Coordinated International Action J. Bueren Hematopoietic Innovative
TRANSGENIC ANIMALS. -transient transfection of cells -stable transfection of cells. - Two methods to produce transgenic animals:
 TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
TRANSGENIC ANIMALS -transient transfection of cells -stable transfection of cells - Two methods to produce transgenic animals: 1- DNA microinjection - random insertion 2- embryonic stem cell-mediated gene
Antibiotic Resistance: Ampicillin Bacterial Backbone: pbluescriptksii(+), Agilent Technologies
 G0656 pfiv3.2rsvmcs Coordinates Plasmid Features 1-682 CMV/5 LTR hybrid 958-1468 Partial Gag 1494-1653 Central Polypurine Tract 1695-2075 RSV Promoter 2104-2222 Multiple Cloning Sites 2244-2839 WPRE 2868-3009
G0656 pfiv3.2rsvmcs Coordinates Plasmid Features 1-682 CMV/5 LTR hybrid 958-1468 Partial Gag 1494-1653 Central Polypurine Tract 1695-2075 RSV Promoter 2104-2222 Multiple Cloning Sites 2244-2839 WPRE 2868-3009
G0411 pfiv3.2mcsegfp. Plasmid Features
 G0411 pfiv3.2mcsegfp Plasmid Features Coordinates Feature CMV/5 LTR hybrid 1:682 Major Splice Donor 926:957 Partial gag 958:1468 Central Polypurine Tract 1494:1653 Multiple cloning sites 1653:1714 egfp
G0411 pfiv3.2mcsegfp Plasmid Features Coordinates Feature CMV/5 LTR hybrid 1:682 Major Splice Donor 926:957 Partial gag 958:1468 Central Polypurine Tract 1494:1653 Multiple cloning sites 1653:1714 egfp
Solutions to Problem Set 6
 MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Name: Question 1 Solutions to 7.012 Problem Set 6 The
MIT Biology Department 7.012: Introductory Biology - Fall 2004 Instructors: Professor Eric Lander, Professor Robert A. Weinberg, Dr. Claudette Gardel Name: Question 1 Solutions to 7.012 Problem Set 6 The
TRANSGENIC ANIMALS. transient. stable. - Two methods to produce transgenic animals:
 Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
Only for teaching purposes - not for reproduction or sale CELL TRANSFECTION transient stable TRANSGENIC ANIMALS - Two methods to produce transgenic animals: 1- DNA microinjection 2- embryonic stem cell-mediated
ICH CONSIDERATIONS Oncolytic Viruses
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 ICH CONSIDERATIONS Oncolytic Viruses 20 November 2008 EMEA/CHMP/GTWP/607698/2008
Mid-term review. Recitation5 03/31/2014. Stem Cell Biology and Function W4193 1
 Mid-term review Recitation5 03/31/2014 Stem Cell Biology and Function W4193 1 What to expect in the midterm2 It will be all so far but mainly whatever was NOT covered by the previous mid-term- not a huge
Mid-term review Recitation5 03/31/2014 Stem Cell Biology and Function W4193 1 What to expect in the midterm2 It will be all so far but mainly whatever was NOT covered by the previous mid-term- not a huge
Gene Therapy for Fanconi Anaemia Hope or hype? Dr Phil Ancliff Great Ormond Street Hospital / UCL Institute of Child Health Twycross Zoo October 2017
 Gene Therapy for Fanconi Anaemia Hope or hype? Dr Phil Ancliff Great Ormond Street Hospital / UCL Institute of Child Health Twycross Zoo October 2017 Gene therapy in Seattle Grace Miranda Bailey had the
Gene Therapy for Fanconi Anaemia Hope or hype? Dr Phil Ancliff Great Ormond Street Hospital / UCL Institute of Child Health Twycross Zoo October 2017 Gene therapy in Seattle Grace Miranda Bailey had the
Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines)
 Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines) Jacqueline Corrigan-Curay, J.D. M.D. Acting Director Office of Biotechnology Activities National Institute
Updates to the NIH Guidelines for Research Involving Recombinant DNA Molecules (NIH Guidelines) Jacqueline Corrigan-Curay, J.D. M.D. Acting Director Office of Biotechnology Activities National Institute
ICH Considerations Oncolytic Viruses ONCOLYTIC VIRUSES (EMEA/CHMP/ICH/607698/2008) TRANSMISSION TO CHMP November 2008
 European Medicines Agency October 2009 EMEA/CHMP/ICH/607698/2008 ICH Considerations Oncolytic Viruses ONCOLYTIC VIRUSES (EMEA/CHMP/ICH/607698/2008) TRANSMISSION TO CHMP November 2008 TRANSMISSION TO INTERESTED
European Medicines Agency October 2009 EMEA/CHMP/ICH/607698/2008 ICH Considerations Oncolytic Viruses ONCOLYTIC VIRUSES (EMEA/CHMP/ICH/607698/2008) TRANSMISSION TO CHMP November 2008 TRANSMISSION TO INTERESTED
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Supplementary Figure 1 Schematic and results of screening the combinatorial antibody library for Sox2 replacement activity. A single batch of MEFs were plated and transduced with doxycycline inducible
Premade Lentiviral Particles for human ips Stem Factors
 Premade Lentiviral Particles for human ips Stem Factors For generating induced pluripotent stem (ips) cells or other applications RESEARCH USE ONLY, not for diagnostics or therapeutics Cat# Product Name
Premade Lentiviral Particles for human ips Stem Factors For generating induced pluripotent stem (ips) cells or other applications RESEARCH USE ONLY, not for diagnostics or therapeutics Cat# Product Name
From regulation to reality challenges in translation of gene therapy and cell-based medicinal products
 From regulation to reality challenges in translation of gene therapy and cell-based medicinal products Gene therapy case study: ADA-SCID Alessandro Aiuti Jonathan Appleby HSR-TIGET is focused on the implementation
From regulation to reality challenges in translation of gene therapy and cell-based medicinal products Gene therapy case study: ADA-SCID Alessandro Aiuti Jonathan Appleby HSR-TIGET is focused on the implementation
Corporate Presentation. March 2018
 Corporate Presentation March 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
Corporate Presentation March 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation
Hemophilia and Gene Therapy
 Hemophilia and Gene Therapy Jackie Chu June 4, 2008 Overview Hemophilia, the disease Gene therapy Hemophilia as a target for gene therapy Gene delivery systems Clinical trials New methods Future of gene
Hemophilia and Gene Therapy Jackie Chu June 4, 2008 Overview Hemophilia, the disease Gene therapy Hemophilia as a target for gene therapy Gene delivery systems Clinical trials New methods Future of gene
Nature Biotechnology: doi: /nbt Supplementary Figure 1
 Supplementary Figure 1 Negative selection analysis of sgrnas targeting all Brd4 exons comparing day 2 to day 10 time points. Systematic evaluation of 64 Brd4 sgrnas in negative selection experiments, targeting
Supplementary Figure 1 Negative selection analysis of sgrnas targeting all Brd4 exons comparing day 2 to day 10 time points. Systematic evaluation of 64 Brd4 sgrnas in negative selection experiments, targeting
AQA Biology A-level Topic 8: The control of gene expression
 AQA Biology A-level Topic 8: The control of gene expression Notes Mutations Mutations are changes in the sequence of nucleotides in DNA molecules. Types of mutations include: Insertion/deletion mutations
AQA Biology A-level Topic 8: The control of gene expression Notes Mutations Mutations are changes in the sequence of nucleotides in DNA molecules. Types of mutations include: Insertion/deletion mutations
Regulation of metabolic pathways
 Regulation of metabolic pathways Bacterial control of gene expression Operon: cluster of related genes with on/off switch Three Parts: 1. Promoter where RNA polymerase attaches 2. Operator on/off, controls
Regulation of metabolic pathways Bacterial control of gene expression Operon: cluster of related genes with on/off switch Three Parts: 1. Promoter where RNA polymerase attaches 2. Operator on/off, controls
Stem Cel s Key Words:
 Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Stem Cells Key Words: Embryonic stem cells, Adult stem cells, ips cells, self-renewal, differentiation, pluripotent, multipotent, Inner cell mass, Nuclear transfer (Therapeutic cloning), Feeder cells,
Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression
 Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression LVP336 LVP336-PBS LVP339 LVP339-PBS LVP297 LVP297-PBS LVP013 LVP013-PBS LVP338 LVP338-PBS LVP027 LVP027-PBS LVP337 LVP337-PBS
Pre-made Lentiviral Particles for Nuclear Permeant CRE Recombinase Expression LVP336 LVP336-PBS LVP339 LVP339-PBS LVP297 LVP297-PBS LVP013 LVP013-PBS LVP338 LVP338-PBS LVP027 LVP027-PBS LVP337 LVP337-PBS
VECTOR SAFETY INFORMATION. AAV Vectors: Material Information
 VECTOR SAFETY INFORMATION AAV Vectors: Material Information AAV vectors contain recombinant transgene sequences (e.g. encoding reporter or therapeutic genes) flanked by the AAV inverted terminal repeats
VECTOR SAFETY INFORMATION AAV Vectors: Material Information AAV vectors contain recombinant transgene sequences (e.g. encoding reporter or therapeutic genes) flanked by the AAV inverted terminal repeats
The b-thalassemias constitute inherited anemias caused. Research Articles
 HUMAN GENE THERAPY 23:15 31 ( January 2012) ª Mary Ann Liebert, Inc. DOI: 10.1089/hum.2011.048 Research Articles The New Self-Inactivating Lentiviral Vector for Thalassemia Gene Therapy Combining Two HPFH
HUMAN GENE THERAPY 23:15 31 ( January 2012) ª Mary Ann Liebert, Inc. DOI: 10.1089/hum.2011.048 Research Articles The New Self-Inactivating Lentiviral Vector for Thalassemia Gene Therapy Combining Two HPFH
ANNOUNCEMENTS. HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134)
 ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
ANNOUNCEMENTS HW2 is due Thursday 2/4 by 12:00 pm. Office hours: Monday 12:50 1:20 (ECCH 134) Lectures 6 8 Outline Stem Cell Division - symmetric - asymmetric Stem cell lineage potential - pluripotent
Lac Operon contains three structural genes and is controlled by the lac repressor: (1) LacY protein transports lactose into the cell.
 Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
Regulation of gene expression a. Expression of most genes can be turned off and on, usually by controlling the initiation of transcription. b. Lactose degradation in E. coli (Negative Control) Lac Operon
DB3230 Midterm 1 11/15/2013 Name:
 1. (15 pts) Nuclear cloning by John Gurdon was rarely successful in producing fertile adults. Why not? Explain why serial transplantation improves the success rate. What else could you do to improve the
1. (15 pts) Nuclear cloning by John Gurdon was rarely successful in producing fertile adults. Why not? Explain why serial transplantation improves the success rate. What else could you do to improve the
Q4 and Full Year 2017 Conference Call. February 22, 2018
 Q4 and Full Year 2017 Conference Call February 22, 2018 Agenda Welcome McDavid Stilwell VP, Corporate Communications and Investor Relations Q4 2017 and Full Year Review and Recent Highlights Dr. Sandy
Q4 and Full Year 2017 Conference Call February 22, 2018 Agenda Welcome McDavid Stilwell VP, Corporate Communications and Investor Relations Q4 2017 and Full Year Review and Recent Highlights Dr. Sandy
What goes into my Biological Inventory?
 What goes into my Biological Inventory? What Information is Required for an Effective Risk Assessment? According to the Laboratory Biosafety Guidelines (2004), the risk group of an organism is determined
What goes into my Biological Inventory? What Information is Required for an Effective Risk Assessment? According to the Laboratory Biosafety Guidelines (2004), the risk group of an organism is determined
Nature Biotechnology doi: /nbt Supplementary Figure 1. Substrate-linked protein evolution components.
 Supplementary Figure 1 Substrate-linked protein evolution components. (a) loxbtr subsites used for the combinational directed-evolution process to generate Brec1. Sequence differences (compared to the
Supplementary Figure 1 Substrate-linked protein evolution components. (a) loxbtr subsites used for the combinational directed-evolution process to generate Brec1. Sequence differences (compared to the
Ectopic Gene Expression in Mammalian Cells
 Ectopic Gene Expression in Mammalian Cells Dr. Stefan Stein AG Grez Methodenseminar BCII Praktikum am Georg-Speyer-Haus 11.01. & 01.02.2012 1 Ectopic Gene Expression in Mammalian Cells Definition: ectopic
Ectopic Gene Expression in Mammalian Cells Dr. Stefan Stein AG Grez Methodenseminar BCII Praktikum am Georg-Speyer-Haus 11.01. & 01.02.2012 1 Ectopic Gene Expression in Mammalian Cells Definition: ectopic
Gene Expression. Chapters 11 & 12: Gene Conrtrol and DNA Technology. Cloning. Honors Biology Fig
 Chapters & : Conrtrol and Technology Honors Biology 0 Cloning Produced by asexual reproduction and so it is genetically identical to the parent st large cloned mammal: Dolly the sheep Animals that are
Chapters & : Conrtrol and Technology Honors Biology 0 Cloning Produced by asexual reproduction and so it is genetically identical to the parent st large cloned mammal: Dolly the sheep Animals that are
MLN8237 induces proliferation arrest, expression of differentiation markers and
 Supplementary Figure Legends Supplementary Figure 1 827 induces proliferation arrest, expression of differentiation markers and polyploidization of a human erythroleukemia cell line with the activating
Supplementary Figure Legends Supplementary Figure 1 827 induces proliferation arrest, expression of differentiation markers and polyploidization of a human erythroleukemia cell line with the activating
Experimental genetics - 2 Partha Roy
 Partha Roy Experimental genetics - 2 Making genetically altered animal 1) Gene knock-out k from: a) the entire animal b) selected cell-type/ tissue c) selected cell-type/tissue at certain time 2) Transgenic
Partha Roy Experimental genetics - 2 Making genetically altered animal 1) Gene knock-out k from: a) the entire animal b) selected cell-type/ tissue c) selected cell-type/tissue at certain time 2) Transgenic
Gene Therapy: The Basics. Mark A. Kay MD PhD Dennis Farrey Family Professor Stanford University
 Gene Therapy: The Basics Mark A. Kay MD PhD Dennis Farrey Family Professor Stanford University Definition of gene therapy Gene therapy is the introduction of nucleic acids (e.g. DNA/genes) into somatic
Gene Therapy: The Basics Mark A. Kay MD PhD Dennis Farrey Family Professor Stanford University Definition of gene therapy Gene therapy is the introduction of nucleic acids (e.g. DNA/genes) into somatic
Induced Pluripotent Stem Cell
 Induced Pluripotent Stem Cell When eventually mastered, the processes involved in the obtaining, cultivating and differencing ESC into cells of clinical interest, another practical limitation should be
Induced Pluripotent Stem Cell When eventually mastered, the processes involved in the obtaining, cultivating and differencing ESC into cells of clinical interest, another practical limitation should be
Applicazioni biotecnologiche
 Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Applicazioni biotecnologiche Analisi forense Sintesi di proteine ricombinanti Restriction Fragment Length Polymorphism (RFLP) Polymorphism (more fully genetic polymorphism) refers to the simultaneous occurrence
Supplemental Information
 Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
Exam 3 4/25/07. Total of 7 questions, 100 points.
 Exam 3 4/25/07 BISC 4A P. Sengupta Total of 7 questions, 100 points. QUESTION 1. Circle the correct answer. Total of 40 points 4 points each. 1. Which of the following is typically attacked by the antibody-mediated
Exam 3 4/25/07 BISC 4A P. Sengupta Total of 7 questions, 100 points. QUESTION 1. Circle the correct answer. Total of 40 points 4 points each. 1. Which of the following is typically attacked by the antibody-mediated
Supplementary Methods
 Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
Supplementary Methods Reverse transcribed Quantitative PCR. Total RNA was isolated from bone marrow derived macrophages using RNeasy Mini Kit (Qiagen), DNase-treated (Promega RQ1), and reverse transcribed
A Survey of Genetic Methods
 IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
IBS 8102 Cell, Molecular, and Developmental Biology A Survey of Genetic Methods January 24, 2008 DNA RNA Hybridization ** * radioactive probe reverse transcriptase polymerase chain reaction RT PCR DNA
ICH Considerations on Viral/Vector Shedding; and Overview of Gene Therapy Activity in Canada
 ICH Considerations on Viral/Vector Shedding; and Overview of Gene Therapy Activity in Canada Anthony Ridgway, Ph.D. Senior Regulatory Scientist Biologics & Genetic Therapies Directorate Health Canada Open
ICH Considerations on Viral/Vector Shedding; and Overview of Gene Therapy Activity in Canada Anthony Ridgway, Ph.D. Senior Regulatory Scientist Biologics & Genetic Therapies Directorate Health Canada Open
Chapter 8 Healthcare Biotechnology
 Chapter 8 Healthcare Biotechnology Outline: 8.1 Introduction 8.2 Biopharming 8.3 Models of Human Disease 8.4 Detecting and Diagnosing Human Disease 8.5 Monoclonal Antibodies 8.6 Gene Therapy 8.7 Tissue
Chapter 8 Healthcare Biotechnology Outline: 8.1 Introduction 8.2 Biopharming 8.3 Models of Human Disease 8.4 Detecting and Diagnosing Human Disease 8.5 Monoclonal Antibodies 8.6 Gene Therapy 8.7 Tissue
Stem Cells Regenerative Medicine Congress September 15, 2014
 Stem Cells Regenerative Medicine Congress 2014 September 15, 2014 Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use
Stem Cells Regenerative Medicine Congress 2014 September 15, 2014 Forward Looking Statement These slides and the accompanying oral presentation contain forward-looking statements and information. The use
Supplementary Figure 1
 Supplementary Figure 1 Supplementary Figure 1: Vector maps of TRMPV and TRMPVIR variants. Many derivatives of TRMPV have been generated and tested. Unless otherwise noted, experiments in this paper use
Supplementary Figure 1 Supplementary Figure 1: Vector maps of TRMPV and TRMPVIR variants. Many derivatives of TRMPV have been generated and tested. Unless otherwise noted, experiments in this paper use
Stem Cells: Introduction and Prospects in Regenerative Medicine.
 Stem Cells: Introduction and Prospects in Regenerative Medicine www.gothamgazette.com/.../stemcell/stem_cell.jpg Ode to a Stem Cell, Part II by VCW There once was stem cell stuck in the hood Dividing endlessly,
Stem Cells: Introduction and Prospects in Regenerative Medicine www.gothamgazette.com/.../stemcell/stem_cell.jpg Ode to a Stem Cell, Part II by VCW There once was stem cell stuck in the hood Dividing endlessly,
Disease Clinical features Genotype
 Disease Clinical features Genotype Alpha 1 Antitrypsin deficiency (A1ATD) Patient 1 65 year old caucasian male, liver transplant recipient, explant biopsy confirmed A1ATD induced liver cirrhosis Patient
Disease Clinical features Genotype Alpha 1 Antitrypsin deficiency (A1ATD) Patient 1 65 year old caucasian male, liver transplant recipient, explant biopsy confirmed A1ATD induced liver cirrhosis Patient
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) <DRAFT>
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 17 November 2005 Doc. Ref. EMEA/273974/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 17 November 2005 Doc. Ref. EMEA/273974/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) NOTE
Immuno-Oncology Program
 Immuno-Oncology Program 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform
Immuno-Oncology Program 2018 Forward Looking Statements This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform
Student Learning Outcomes (SLOS) - Advanced Cell Biology
 Course objectives The main objective is to develop the ability to critically analyse and interpret the results of the scientific literature and to be able to apply this knowledge to afford new scientific
Course objectives The main objective is to develop the ability to critically analyse and interpret the results of the scientific literature and to be able to apply this knowledge to afford new scientific
Bone Marrow Failure Research Program
 Bone Marrow Failure Research Program Strategic Plan INTRODUCTION The Congressionally Directed Medical Research Programs (CDMRP) represents a unique partnership among the U.S. Congress, the military, and
Bone Marrow Failure Research Program Strategic Plan INTRODUCTION The Congressionally Directed Medical Research Programs (CDMRP) represents a unique partnership among the U.S. Congress, the military, and
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform
 This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements
This presentation contains forward-looking statements within the meaning of the "safe harbor" provisions of the Private Securities Litigation Reform Act of 1995, as amended. These forward-looking statements
Sangamo Therapeutics Announces Presentations at 2017 Annual meeting of the American Society of Gene & Cell Therapy
 April 24, 2017 Sangamo Therapeutics Announces Presentations at 2017 Annual meeting of the American Society of Gene & Cell Therapy RICHMOND, Calif., April 24, 2017 /PRNewswire/ -- Sangamo Therapeutics,
April 24, 2017 Sangamo Therapeutics Announces Presentations at 2017 Annual meeting of the American Society of Gene & Cell Therapy RICHMOND, Calif., April 24, 2017 /PRNewswire/ -- Sangamo Therapeutics,
Supplemental Table S1. RT-PCR primers used in this study
 Supplemental Table S1. RT-PCR primers used in this study -----------------------------------------------------------------------------------------------------------------------------------------------
Supplemental Table S1. RT-PCR primers used in this study -----------------------------------------------------------------------------------------------------------------------------------------------
(a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for
 Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
Supplementary Figure 1 Generation of Diaph3 ko mice. (a) Scheme depicting the strategy used to generate the ko and conditional alleles. (b) RT-PCR for different regions of Diaph3 mrna from WT, heterozygote
MicroRNAs Modulate Hematopoietic Lineage Differentiation
 Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
a previous report, Lee and colleagues showed that Oct4 and Sox2 bind to DXPas34 and Xite
 SUPPLEMENTARY INFORMATION doi:1.138/nature9496 a 1 Relative RNA levels D12 female ES cells 24h Control sirna 24h sirna Oct4 5 b,4 Oct4 Xist (wt) Xist (mut) Tsix 3' (wt) Tsix 5' (wt) Beads Oct4,2 c,2 Xist
SUPPLEMENTARY INFORMATION doi:1.138/nature9496 a 1 Relative RNA levels D12 female ES cells 24h Control sirna 24h sirna Oct4 5 b,4 Oct4 Xist (wt) Xist (mut) Tsix 3' (wt) Tsix 5' (wt) Beads Oct4,2 c,2 Xist
G0752 pfiv3.2mcswtiresegfp
 G0752 pfiv3.2mcswtiresegfp Plasmid Features Coordinates Feature 1-682 CMV/5 LTR Hybrid 958-1468 Partial Gag 1494-1653 Central Polypurine Tract 1653-1738 Multiple Cloning Sites 1739-2325 wtires 2326-3045
G0752 pfiv3.2mcswtiresegfp Plasmid Features Coordinates Feature 1-682 CMV/5 LTR Hybrid 958-1468 Partial Gag 1494-1653 Central Polypurine Tract 1653-1738 Multiple Cloning Sites 1739-2325 wtires 2326-3045
Schematic representation of the endogenous PALB2 locus and gene-disruption constructs
 Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Supplementary Figures Supplementary Figure 1. Generation of PALB2 -/- and BRCA2 -/- /PALB2 -/- DT40 cells. (A) Schematic representation of the endogenous PALB2 locus and gene-disruption constructs carrying
Supplementary Figures and Figure legends
 Supplementary Figures and Figure legends 3 Supplementary Figure 1. Conditional targeting construct for the murine Satb1 locus with a modified FLEX switch. Schematic of the wild type Satb1 locus; the conditional
Supplementary Figures and Figure legends 3 Supplementary Figure 1. Conditional targeting construct for the murine Satb1 locus with a modified FLEX switch. Schematic of the wild type Satb1 locus; the conditional
This study is currently recruiting participants. Verified July 2012 by Memorial Sloan-Kettering Cancer Center
 Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies Treatment of ß-Thalassemia Major With Autologous CD34+ Hematopoietic Progenitor Cells Transduced
Home Search Study Topics Glossary Search Full Text View Tabular View No Study Results Posted Related Studies Treatment of ß-Thalassemia Major With Autologous CD34+ Hematopoietic Progenitor Cells Transduced
Molecular Genetics Student Objectives
 Molecular Genetics Student Objectives Exam 1: Enduring understanding 3.A: Heritable information provides for continuity of life. Essential knowledge 3.A.1: DNA, and in some cases RNA, is the primary source
Molecular Genetics Student Objectives Exam 1: Enduring understanding 3.A: Heritable information provides for continuity of life. Essential knowledge 3.A.1: DNA, and in some cases RNA, is the primary source
Developing Targeted Stem Cell Therapeutics for Cancer. Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013
 Developing Targeted Stem Cell Therapeutics for Cancer Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013 The Challenge of Drug Delivery for Brain Cancer Stem Cells
Developing Targeted Stem Cell Therapeutics for Cancer Shawn Hingtgen, Ph.D. Assistant Professor UNC Eshelman School of Pharmacy May 22 nd, 2013 The Challenge of Drug Delivery for Brain Cancer Stem Cells
Pharmacogenetics: A SNPshot of the Future. Ani Khondkaryan Genomics, Bioinformatics, and Medicine Spring 2001
 Pharmacogenetics: A SNPshot of the Future Ani Khondkaryan Genomics, Bioinformatics, and Medicine Spring 2001 1 I. What is pharmacogenetics? It is the study of how genetic variation affects drug response
Pharmacogenetics: A SNPshot of the Future Ani Khondkaryan Genomics, Bioinformatics, and Medicine Spring 2001 1 I. What is pharmacogenetics? It is the study of how genetic variation affects drug response
DNA Cloning with Cloning Vectors
 Cloning Vectors A M I R A A. T. A L - H O S A R Y L E C T U R E R O F I N F E C T I O U S D I S E A S E S F A C U L T Y O F V E T. M E D I C I N E A S S I U T U N I V E R S I T Y - E G Y P T DNA Cloning
Cloning Vectors A M I R A A. T. A L - H O S A R Y L E C T U R E R O F I N F E C T I O U S D I S E A S E S F A C U L T Y O F V E T. M E D I C I N E A S S I U T U N I V E R S I T Y - E G Y P T DNA Cloning
Pre-made Lentiviral Particles for nuclear permeant CRE recombinase expression
 Pre-made Lentiviral Particles for nuclear permeant CRE recombinase expression Cat# Product Name Amounts* LVP336 NLS-CRE (Bsd) LVP336-PBS NLS-CRE (Bsd), in vivo ready LVP339 NLS-CRE (Puro) LVP339-PBS NLS-CRE
Pre-made Lentiviral Particles for nuclear permeant CRE recombinase expression Cat# Product Name Amounts* LVP336 NLS-CRE (Bsd) LVP336-PBS NLS-CRE (Bsd), in vivo ready LVP339 NLS-CRE (Puro) LVP339-PBS NLS-CRE
Chapter 5 Genetic Analysis in Cell Biology. (textbook: Molecular Cell Biology 6 ed, Lodish section: )
 Chapter 5 Genetic Analysis in Cell Biology (textbook: Molecular Cell Biology 6 ed, Lodish section: 5.1+5.4-5.5) Understanding gene function: relating function, location, and structure of gene products
Chapter 5 Genetic Analysis in Cell Biology (textbook: Molecular Cell Biology 6 ed, Lodish section: 5.1+5.4-5.5) Understanding gene function: relating function, location, and structure of gene products
KNOW HOW & TECHNOLOGY LICENSING OPPORTUNITIES
 Vector platform developed at SAN RAFFAELE TELETHON INSTITUTE FOR GENE THERAPY SAN RAFFAELE HOSPITAL AND SCIENTIFIC INSTITUTE KNOW HOW & TECHNOLOGY LICENSING OPPORTUNITIES -regulated vectors and their uses
Vector platform developed at SAN RAFFAELE TELETHON INSTITUTE FOR GENE THERAPY SAN RAFFAELE HOSPITAL AND SCIENTIFIC INSTITUTE KNOW HOW & TECHNOLOGY LICENSING OPPORTUNITIES -regulated vectors and their uses
Lecture 8: Transgenic Model Systems and RNAi
 Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
Lecture 8: Transgenic Model Systems and RNAi I. Model systems 1. Caenorhabditis elegans Caenorhabditis elegans is a microscopic (~1 mm) nematode (roundworm) that normally lives in soil. It has become one
FDA Perspective on the Preclinical Development of Cancer Vaccines
 FDA Perspective on the Preclinical Development of Cancer Vaccines Richard D. McFarland Ph.D., M.D. Medical Officer CBER/OCTGT/DCEPT mcfarlandr@cber.fda.gov Cancer Vaccine Clinical Trials Workshop Alexandria,
FDA Perspective on the Preclinical Development of Cancer Vaccines Richard D. McFarland Ph.D., M.D. Medical Officer CBER/OCTGT/DCEPT mcfarlandr@cber.fda.gov Cancer Vaccine Clinical Trials Workshop Alexandria,
Orchard Therapeutics. Overcoming the complex challenges associated with ex vivo gene therapies. Adrien Lemoine VP Business Development & Operations
 Orchard Therapeutics Overcoming the complex challenges associated with ex vivo gene therapies Adrien Lemoine VP Business Development & Operations Orchard at a glance Who we are Our mission Team Academic
Orchard Therapeutics Overcoming the complex challenges associated with ex vivo gene therapies Adrien Lemoine VP Business Development & Operations Orchard at a glance Who we are Our mission Team Academic
Genome manipulation by homologous recombination in Drosophila Xiaolin Bi and Yikang S. Rong Date received (in revised form): 9th May 2003
 Xiaolin Bi is a post doctoral research fellow at the Laboratory of Molecular Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA. Yikang S. Rong is the principal
Xiaolin Bi is a post doctoral research fellow at the Laboratory of Molecular Cell Biology, National Cancer Institute, National Institutes of Health, Bethesda, Maryland, USA. Yikang S. Rong is the principal
Pre-made expression Adenovirus product manual
 Pre-made expression Adenovirus product manual Catalog# Product Name Amounts AVP001 RFP adenovirus AVP002 AVP004 AVP005 AVP011 AVP012 AVP017 AVP010 AVP013 AVP014 AVP015 AVP016 AVP-Null AVP001-PBS AVP002-PBS
Pre-made expression Adenovirus product manual Catalog# Product Name Amounts AVP001 RFP adenovirus AVP002 AVP004 AVP005 AVP011 AVP012 AVP017 AVP010 AVP013 AVP014 AVP015 AVP016 AVP-Null AVP001-PBS AVP002-PBS
Bi 8 Lecture 5. Ellen Rothenberg 19 January 2016
 Bi 8 Lecture 5 MORE ON HOW WE KNOW WHAT WE KNOW and intro to the protein code Ellen Rothenberg 19 January 2016 SIZE AND PURIFICATION BY SYNTHESIS: BASIS OF EARLY SEQUENCING complex mixture of aborted DNA
Bi 8 Lecture 5 MORE ON HOW WE KNOW WHAT WE KNOW and intro to the protein code Ellen Rothenberg 19 January 2016 SIZE AND PURIFICATION BY SYNTHESIS: BASIS OF EARLY SEQUENCING complex mixture of aborted DNA
Edexcel (B) Biology A-level
 Edexcel (B) Biology A-level Topic 7: Modern Genetics Notes Using Gene Sequencing Genome = all of an organism s DNA, including mitochondrial/chloroplast DNA. Polymerase chain reaction (PCR) is used to amplify
Edexcel (B) Biology A-level Topic 7: Modern Genetics Notes Using Gene Sequencing Genome = all of an organism s DNA, including mitochondrial/chloroplast DNA. Polymerase chain reaction (PCR) is used to amplify
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
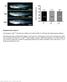 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Genetic Basis of Development & Biotechnologies
 Genetic Basis of Development & Biotechnologies 1. Steps of embryonic development: cell division, morphogenesis, differentiation Totipotency and pluripotency 2. Plant cloning 3. Animal cloning Reproductive
Genetic Basis of Development & Biotechnologies 1. Steps of embryonic development: cell division, morphogenesis, differentiation Totipotency and pluripotency 2. Plant cloning 3. Animal cloning Reproductive
Making Hope A Reality bluebird style. November, 2017 Nasdaq : BLUE
 Making Hope A Reality bluebird style November, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information. The
Making Hope A Reality bluebird style November, 2017 Nasdaq : BLUE 1 Forward Looking Statements These slides and the accompanying oral presentation contain forward-looking statements and information. The
Regulation of advanced blood cell therapies
 Regulation of advanced blood cell therapies www.pei.de Clinical trials using cell-based products Substantially manipulated cells and cells for non-homologous use Quality, safety and non-clinical aspects
Regulation of advanced blood cell therapies www.pei.de Clinical trials using cell-based products Substantially manipulated cells and cells for non-homologous use Quality, safety and non-clinical aspects
GENETICS - CLUTCH CH.15 GENOMES AND GENOMICS.
 !! www.clutchprep.com CONCEPT: OVERVIEW OF GENOMICS Genomics is the study of genomes in their entirety Bioinformatics is the analysis of the information content of genomes - Genes, regulatory sequences,
!! www.clutchprep.com CONCEPT: OVERVIEW OF GENOMICS Genomics is the study of genomes in their entirety Bioinformatics is the analysis of the information content of genomes - Genes, regulatory sequences,
Introducing new DNA into the genome requires cloning the donor sequence, delivery of the cloned DNA into the cell, and integration into the genome.
 Key Terms Chapter 32: Genetic Engineering Cloning describes propagation of a DNA sequence by incorporating it into a hybrid construct that can be replicated in a host cell. A cloning vector is a plasmid
Key Terms Chapter 32: Genetic Engineering Cloning describes propagation of a DNA sequence by incorporating it into a hybrid construct that can be replicated in a host cell. A cloning vector is a plasmid
Cell therapies to treat haematopoietic disorders
 Cell therapies to treat haematopoietic disorders Haematopoietic Stem Cells (CD34) RBCs Platelets Limitations A donor required for each treatment Transfusion-transmitted infection Immunological (HLA/AB0)
Cell therapies to treat haematopoietic disorders Haematopoietic Stem Cells (CD34) RBCs Platelets Limitations A donor required for each treatment Transfusion-transmitted infection Immunological (HLA/AB0)
Non-coding Function & Variation, MPRAs II. Mike White Bio /5/18
 Non-coding Function & Variation, MPRAs II Mike White Bio 5488 3/5/18 MPRA Review Problem 1: Where does your CRE DNA come from? DNA synthesis Genomic fragments Targeted regulome capture Problem 2: How do
Non-coding Function & Variation, MPRAs II Mike White Bio 5488 3/5/18 MPRA Review Problem 1: Where does your CRE DNA come from? DNA synthesis Genomic fragments Targeted regulome capture Problem 2: How do
Genetics Faculty of Agriculture and Veterinary Medicine. Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology
 Genetics 10201232 Faculty of Agriculture and Veterinary Medicine Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology 1 Biotechnology is defined as the technology that involves the use of living organisms
Genetics 10201232 Faculty of Agriculture and Veterinary Medicine Instructor: Dr. Jihad Abdallah Topic 16: Biotechnology 1 Biotechnology is defined as the technology that involves the use of living organisms
GC GC - AAV2/8-4E10
 SUPPLEMENTARY INFORMATION doi:.38/nature660 a Luciferase Expression (Photons/Sec) c f 12 11 11 GC - AAV2/8-Luciferase 9 GC - AAV2/8-Luciferase 0 8 16 24 32 40 48 56 64 Weeks Post-AAV Injection Human IgG
SUPPLEMENTARY INFORMATION doi:.38/nature660 a Luciferase Expression (Photons/Sec) c f 12 11 11 GC - AAV2/8-Luciferase 9 GC - AAV2/8-Luciferase 0 8 16 24 32 40 48 56 64 Weeks Post-AAV Injection Human IgG
Gene Technology classification of dealings involving self-inactivating, replication defective, retroviral vectors.
 Gene Technology classification of dealings involving self-inactivating, replication defective, retroviral vectors. The use of replication defective, retroviral vectors for research purposes has become
Gene Technology classification of dealings involving self-inactivating, replication defective, retroviral vectors. The use of replication defective, retroviral vectors for research purposes has become
PART 1 (COUNCIL DECISION 2002/813/EC)
 PART 1 (COUNCIL DECISION 2002/813/EC) SUMMARY NOTIFICATION INFORMATION FORMAT FOR THE RELEASE OF GENETICALLY MODIFIED ORGANISMS OTHER THAN HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 11 OF DIRECTIVE 2001/18/EC
PART 1 (COUNCIL DECISION 2002/813/EC) SUMMARY NOTIFICATION INFORMATION FORMAT FOR THE RELEASE OF GENETICALLY MODIFIED ORGANISMS OTHER THAN HIGHER PLANTS IN ACCORDANCE WITH ARTICLE 11 OF DIRECTIVE 2001/18/EC
