The Pennsylvania State University. The Graduate School. Department of Chemical Engineering ENGINEERING AND ANALYSIS OF COFACTOR PARTITIONING FOR
|
|
|
- Herbert Cole
- 6 years ago
- Views:
Transcription
1 The Pennsylvania State University The Graduate School Department of Chemical Engineering ENGINEERING AND ANALYSIS OF COFACTOR PARTITIONING FOR NADPH-DEPENDENT XYLITOL PRODUCTION IN ESCHERICHIA COLI A Dissertation in Chemical Engineering by Jonathan W. Chin 2010 Jonathan W. Chin Submitted in Partial Fulfillment of the Requirements for the Degree of Doctor of Philosophy August 2010
2 The dissertation of Jonathan W. Chin was reviewed and approved* by the following: Patrick C. Cirino Assistant Professor of Chemical Engineering Dissertation Advisor Chair of Committee Costas Maranas Donald B. Broughton Professor of Chemical Engineering Wayne Curtis Professor of Chemical Engineering Ali Demirci Professor of Agricultural and Biological Engineering Andrew Zydney Walter L. Robb Chair, and Professor of Chemical Engineering Head of the Department of Chemical Engineering *Signatures are on file in the Graduate School
3 iii ABSTRACT This research is focused on whole-cell biocatalysis as a way to regenerate reduced cofactors that are used to drive heterologous redox reactions of interest. Specifically, the focus is on engineering and understanding cofactor partitioning for heterologous production of xylitol in Escherichia coli. By replacing E. coli s native cyclic AMP receptor protein (CRP) with a cyclic AMP-independent mutant (CRP*), xylose uptake and xylitol production from mixtures of glucose and xylose was facilitated, with glucose serving as the growth substrate and source of reducing equivalents. Numerous xylose reductases (XRs) and xylulose dehydrogenases (XDHs) with varying nicotinamide cofactor specificities were screened in a crp*, ΔxylB double mutant strain (PC09). It was found that a NADPH-dependent xylose reductase from Candida boidinii (CbXR) consistently produced the highest concentration of xylitol in shake flask cultures (~275 mm in LB cultures, ~180 mm in minimal cultures). Use of non-growing, metabolically-active resting cells was next examined as a means of improving xylitol yield (Y RPG, mols of xylitol produced per mol of glucose consumed). An increase of Y RPG in resting cells compared to batch cultures (~3.4 and ~1.8 respectively) was observed. By altering various conditions and parameters of resting cells, xylitol Y RPG was further increased to ~4.0, while limiting fermentation product secretion (e.g. lactate, acetate, and ethanol). It was then sought to understand the role of NADPH supply in xylitol yield and the contribution of key central carbon metabolism enzymes toward xylitol production. Studies in which the expression of CbXR or a xylose transporter was increased suggested that enzyme activity and xylose transport are not limiting xylitol production in PC09. A constraints-based stoichiometric metabolic network model was used to understand the roles of central carbon metabolism reactions and xylose transport energetics on the theoretical maximum molar xylitol yield (xylitol produced per glucose consumed). These results were then compared to
4 iv experimentally determined xylitol yields (Y RPG ), which were measured from resting cell biotransformations with various PC09 derivative strains. For the case of xylose-proton symport, omitting the Zwf (glucose-6-phosphate dehydrogenase), PntAB (membrane-bound transhydrogenase) reactions, or TCA cycle activity from the model reduces the theoretical maximum yield from 9.2 to 8.8, 3.6, and 8.0 mol of xylitol per mol of glucose, respectively. Experimentally, deleting pgi (encoding phosphoglucose isomerase) from strain PC09 improves the yield from 3.4 to 4.0, while deleting either or both E. coli transhydrogenases (stha and pnta) has no significant effect on the measured yield. Deleting either zwf or succ (TCA cycle) significantly reduces the yield from 3.4 to 2.0 and 2.3 mol of xylitol per mol of glucose, respectively. Although the metabolic role of transhydrogenases during E. coli biocatalysis has remained largely unspecified, these results demonstrate the importance of direct NADPH supply by NADP + -utilizing enzymes in central metabolism for driving heterologous NADPH-dependent reactions, and suggest that the pool of reduced cofactors available for biotransformation is not readily interchangeable via transhydrogenase. Finally, two fundamentally different strategies were studied to improve the coupling between glucose oxidation and xylose reduction based on the result that the pool of reduced cofactors is not readily interchangeable. It was first examined the effects of deleting the phosphofructokinase (pfk) gene(s) on growth-uncoupled xylitol production and found that deleting both pfka and stha (encoding the E. coli soluble transhydrogenase) improved the xylitol Y RPG from 3.4 to 5.4. The second strategy focuses on coupling growth with xylose reduction. Deleting the pgi and stha genes resulted in a strain that was severely growth inhibited; however the growth was able to be partially restored upon expressing the NADPH-dependent CbXR to the double mutant strain (µ max = 0.12 from 0.06 hr -1 ) with concomitant xylitol production, which is a potential suitable strain for adaptive evolution. Intracellular nicotinamide cofactor levels were also quantified, and the magnitude of the change in the NADPH/NADP + ratio measured from
5 cells consuming glucose in the absence versus presence of xylose showed a strong correlation to the resulting Y RPG. v
6 vi TABLE OF CONTENTS LIST OF FIGURES... x LIST OF TABLES... xiv ACKNOWLEDGEMENTS... xviii Chapter 1 Background... 1 Introduction... 2 Literature review... 3 Xylitol... 3 Escherichia coli... 6 Nicotinamide cofactors... 7 Cofactor manipulation... 9 Stoichiometric model Proposed research References Chapter 2 Engineering Escherichia coli for Xylitol Production from Glucose-Xylose Mixtures Abstract Introduction Materials and Methods General Genetic Methods Shake-Flask Cultures Results Strain Construction and Characterization Xylitol Production in Strain PC09 Expressing XR s and XDH s Cofactor Utilization Analysis Discussion Conclusion References Chapter 3 Optimization of Resting Cell Parameters for Growth-Uncoupled Product Formation Abstract Introduction Materials and Methods General Resting Cell Cultures Glucose Limited Cultures Results... 51
7 vii Batch vs. Resting Cells Working OD Growth Inhibition Oxygen-Limited Cultures Glucose Fed Cultures Effect of Growth Medium on Resting Cell Yield Conclusion References Chapter 4 Analysis of NADPH Supply During Xylitol Production by Engineered Escherichia coli Abstract Introduction Materials and Methods General Gene Deletions Transhydrogenase Expression Plasmids Shake-Flask Cultures Resting Cell Cultures Transhydrogenase Activity Assay CbXR Activity Assay Simulations Results Role of Xylose Transport and CbXR Expression Simulation Studies Resting Cell Assays Discussion Conclusion References Chapter 5 Development of a Protocol to Determine the Internal Concentration of Nicotinamide Cofactors in Escherichia coli Abstract Introduction Spectrophotometric/Fluorometric Cycling Assay Materials and Methods Spectrophotometric Cycling Assay Resting Cell Cultures Results Spectrophotometric Cycling Assay Discussion References
8 Chapter 6 Improved NADPH Supply for Xylitol Production by Engineered Escherichia coli with Glycolytic Mutations Abstract Introduction Materials and Methods General Gene Deletions Resting Cell Cultures Cultures for Growth-Coupled Strain Studies Cofactor Analysis Phosphofructokinase Assay Description of Genome-scale E. coli Stoichiometric Model Results Analysis of pfk Mutant Resting Cells Growth-Coupled Strain Cofactor Analysis Discussion References Chapter 7 Conclusions and Future Works Conclusions Future Works Resting Cell Test Overexpression of Enzymes Adaptive Evolution Flux Balance Analysis Refernces Appendix A Strains, Plasmids, and Primers A.1 Strains A.2 Plasmids A.3 Primers Appendix B Methods and Protocols B.1 HPLC Start Up B.2 Cofactor Analysis B.3 Resting Cell Protocol B.4 CbXR Activity Assay B.5 Transhydrogenase Activity Assay B.6 Glucose-6-Phosphate Activity Assay B.7 Phosphofructokinase Assay B.8 References Appendix C Assays C.1 Construction of Plasmid ppcc C.2 Xylose Reductase Activity of Plasmid ppcc C.3 Construction of Plasmids ppcc118, and ppcc C.4 Glucose-6-Phosphate Dehydrogenase Activity Assay C.5 References Appendix D Fermentation viii
9 D.1 Methods D.2 Results D.3 References Appendix E OptKnock and Growth Coupled Strains E.1 Simulation Formulation E.2 Strain Construction E.3 Growth Shake-Flask Culture Method E.4 Simulation Results E.5 Growth Shake-Flask Cultures Results E.6 OptKnock Conclusion E.7 CtXR-Based Growth-Coupled Strains E.8 References Appendix F Batch Cultures F.1 Overexpression of Transhydrogenases Plasmids ppcc106 (CbXR & pntab) and ppcc500 (CbXR & stha) in Strain JC F.2 Overexpression of Glucose-6-Phosphate Dehydrogenase (Zwf) in Strain PC09, JC72, and JC F.3 Overexpression of Glucose-6-Phosphate Dehydrogenase and Proton- Symport Xylose Transporter in Strain JC F.4 References Appendix G Resting Cell G.1 Resting Cell Comparison of Strains W3110, PC07, and PC G.2 Strain JC79 Harboring Plasmids ploi3815 (CbXR), ppcc106 (CbXR, & pntab), or ppcc500 (CbXR & stha) Under Glucose Limitation and Excess Conditions G.3 Overexpression of Glucose-6-Phosphate Dehydrogenase (Zwf) in Strain PC09, JC72, and JC G.4 Table of all Resting Cell Studies Appendix H Supplemental Information Cofactor Determination H.1 Spectrophotometric Results H.2 Cycling Assay Results H.3 References ix
10 x LIST OF FIGURES Figure 1-1: Central carbon metabolism of glucose and xylose. Also shown are major sources and sinks for the reduced cofactors NADH and NADPH Figure 2-1: Xylose uptake and metabolism into the pentose phosphate pathway (PPP) in E. coli, and options for xylitol production via heterologous XDH or XR Figure 2-2: Effects of crp* and xylb on growth. Results are for 50-ml shake flask cultures grown at 37 o C and containing LB medium supplemented with 100 mm each of glucose, xylose and MOPS buffer. PC05 and PC09 express the mutant CRP* protein; the xylb gene is deleted in PC07 and PC09. Glucose and xylose consumption after 8 hours of growth Figure 2-3: Effects of crp* and xylb on growth. Results are for 50-ml shake flask cultures grown at 37 o C and containing LB medium supplemented with 100 mm each of glucose, xylose and MOPS buffer. PC05 and PC09 express the mutant CRP* protein; the xylb gene is deleted in PC07 and PC09. Growth curves (monitoring turbidity (OD 550 ) over time) Figure 2-4: Xylitol production in 50-mL shake-flask cultures of strain PC09 expressing the enzymes tested in this study. Refer to Table I for the corresponding plasmid used to express each enzyme. Cultures contained xylose (300 mm), glucose (100 mm), MOPS (50 mm), kanamycin (50 g ml -1 ), IPTG (100 M). Average final culture OD s are given in parentheses. Data points represent the average of at least two values Figure 2-5: Xylitol production by W3110 and PC09 expressing CbXR in shake-flask cultures containing LB plus glucose ( G, 100 mm) and/or xylose ( X, 300 mm). Average final culture OD s are given in parentheses Figure 3-1: Xylitol production (blue) and yield (pink) comparison between PC09 with plasmid ploi3815 cultured as a batch culture (solid lines) or resting cells (dashed lines). Figure used with permission from Wiley Sciences Figure 3-2: Xylitol per OD and uncorrected yield (mm xylitol produced per mm glucose consumed) comparison in resting cells with different working OD s. Cultures were grown in minimal medium and resuspended in minimal medium without nitrogen. Resuspended to an initial OD = 1.6, 3.6, 4.4 (as noted) Figure 3-3: Comparison between resting cell results using strain PC09 harboring plasmid ploi3815 (expressing CbXR) that was first cultured in rich medium (LB) versus minimal medium (NBS). The presented yield (Y RPG ), shown in parenthesis, is corrected for background production of xylitol (xylitol production in the absence of glucose) Figure 4-1: E. coli central carbon metabolism summarizing key reactions and pathways involved in NAD(P)H metabolism. Genes shown code for the following enzymes: pgi = phosphoglucose isomerase; zwf = glucose 6-phosphate-1-dehydrogenase; gnd
11 xi = 6-phosphogluconate dehydrogenase; edd = phosphogluconate dehydratase; eda = 2-keto-3-deoxy-6-phosphogluconate aldolase; nuoa-n = NADH dehydrogenase I; ndh = NADH dehydrogenase II; succ = succinyl-coa synthetase; stha = soluble pyridine nucleotide transhydrogenase; pntab = pyridine nucleotide transhydrogenase; pfk = phosphofrucokinase. Pathway abbreviations include the following: EMP = Embden-Meyerhof-Parnas, TCA = tricarboxylic acid Figure 4-2: Transhydrogenase activities measured from lysates of E. coli strain PC09 harboring plasmid ploi3815 (expressing CbXR), ppcc500 (expressing CbXR and SthA) or ppcc106 (expressing CbXR and PntAB). Activity reported as Units (mg total protein) -1 in the lysate Figure 5-1: Basic concept of the cycling assay used in this study. The amount of reduced MTT (MTT red ), which is readily measured at a wavelength of 570 nm, is proportional to the concentration of cofactor in the assay Figure 5-3: Chromatograms of a cofactor mixture standard (0.25 mm) of all four nicotinamide cofactors compared to a biological sample at two different absorbances. The grey chromatogram represents the absorbance of the cofactor standard at λ = 254 nm; the light grey chromatogram represents the absorbance of the cofactor standard at λ = 340 nm; the black chromatogram represents the absorbance of the biological sample (W3110) at λ = 254nm. Peak order is as follows: NADP +, NADPH, NAD +, NADH Figure 5-4: Chromatograms of the cofactor standards (1 mm each) using 0.1 M ammonium acetate as the running buffer at λ = 254. The green chromatogram represents a standard of NADH; the light blue chromatogram represents a standard of NAD + ; the pink chromatogram represents a standard of NADPH; the dark green chromatogram represents a standard of NADP Figure 5-5: Chromatograms of the cofactor standards (1 mm each) using 0.1 M ammonium acetate as the running buffer at λ = 340 nm. The green chromatogram represents a standard of NADH; the light blue chromatogram represents a standard of NAD + ; the pink chromatogram represents a standard of NADPH; the dark green chromatogram represents a standard of NADP Figure 6-1: Overview of E. coli central carbon metabolism highlighting key reactions and pathways involved in NAD(P)H metabolism. pgi = phosphoglucose isomerase; zwf = glucose-6-phosphate dehydrogenase; gnd = 6-phosphogluconate dehydrogenase; edd = phosphogluconate dehydrogenase; eda = 2-keto-3-deoxy-6- phosphogluconate aldolase; pfk = phosphofructokinase; nuoa-n = NADH dehydrogenase I; ndh = NADH dehydrogenase II; pntab = pyridine nucleotide transhydrogenase; stha = soluble pyridine nucleotide transhydrogenase. Metabolite abbreviations include the following: G6P = glucose-6-phosphate; F6P = fructose-6- phosphate; F1,6P = fructose-1,6-bisphosphate; G3P = glyceraldehyde-3-phosphate, 6-PG = 6-phospho-gluconate. Pathway abbreviations: EMP = Embden-Meyerhof- Parnas; TCA = tricarboxylic acid
12 Figure 6-2: OD vs time plot of pgi and pgi/stha deletion strains compared to their wildtype counterparts. All strains grown in minimal medium supplemented with 100 mm glucose Figure 6-3: Growth profile of JC11, JC128 (lacking pgi), and JC134 (lacking pgi and stha) grown in minimal medium supplemented with 100 mm xylose and/or 100 mm glucose. All cultures were induced with IPTG at time of inoculation Figure 6-4: Comparison of NADPH/NADP + ratio versus xylitol yield (Y RPG ). G represents the NADPH/NADP + ratio from the strain in the absence of xylose. Δ represents the difference in NADPH/NADP + in the absence of xylose and in the presence of xylose Figure D-1: Xylitol concentration as a function of time in fermenters with varying DO setpoints Figure D-2: OD 600 as a function of time in fermenters with varying DO setpoints Figure E-1: OD 600 vs time plot of JC11 and OptKnock predicted strains. Strains were grown in minimal medium supplemented with 100 mm xylose and/or 100 mm glucose, and induced with 100 µm IPTG. Final xylitol ( ) and calculated maximum growth rate [ ] are shown. Growth rate was calculated as described in the Shake Flask Culture Method Figure E-2: Growth profile of JC14, RP03 (lacking pgi), and RP05 (lacking pgi and stha) grown in minimal medium supplemented with 100 mm xylose and/or 100 mm glucose. All cultures were induced with IPTG at time of inoculation Figure F-1: OD 600 versus time plot of JC134 with the control plasmid (3809), overexpressed xyle (203) or overexpressed zwf (118). All strains were grown in minimal medium supplemented with 100 mm xylose and 100 mm glucose, and induced with 100 μm IPTG. Final xylitol production ( ) and growth rate [ ] are shown Figure G-1: Resting cell comparison between strain JC79 with plasmids ppcc106 (CbXR & pntab), ppcc500 (CbXR & stha), and ploi3815 (CbXR). Resting cell conditions were either with glucose excess or glucose limited condition Figure H-1: HPLC traces of nicotinamide cofactors using a 0.1 M KH 2 PO 4 solution to separate the four different forms. Light grey, contained 10% volume volume -1 methanol; grey, contained no methanol. Detection was performed with UV absorbance at λ = 254 nm; absorbance at λ = 340 nm was negligible (not shown) Figure H-2: Chromatograms of the cofactor standards (1 mm each) using ammonium acetate as the running buffer at λ = 254. The green chromatogram is a standard of NADH; the light blue chromatogram is a standard of NAD + ; the pink chromatogram is a standard of NADP + ; the dark green chromatogram is a standard of NADPH xii
13 Figure H-3: Chromatograms of the cofactor standards (1 mm each) using ammonium acetate as the running buffer at λ = 340 nm. The green chromatogram is a standard of NADH; the light blue chromatogram is a standard of NAD + ; the pink chromatogram is a standard of NADP + ; the dark green chromatogram is a standard of NADPH Figure H-4: Slope of the nicotinamide cofactor calibration curve versus the concentration of PES for NAD + and NADH. MTT concentration was either 2.4 mm or 4.2 mm MTT. Slopes were calculated from four averaged points Figure H-5: Slope of the nicotinamide cofactor calibration curve versus the concentration of PES for NADP + and NADPH. MTT concentration was either 2.4 mm or 4.2 mm. Slopes were calculated from four averaged points xiii
14 xiv LIST OF TABLES Table 1-1: Table comparing xylitol productivity and yield between different organisms and conditions Table 2-1: Strains and plasmids used in this study a Table 2-2: Xylose reductase (XR) and xylitol dehydrogenase (XDH) enzymes used Table 3-1: Glucose consumption and byproduct secretion levels in resting cells with different working OD at time point 96 hours are given in mm. Yield (Y RPG ) represents an uncorrected value (background xylitol was not subtracted). Cell cultures were grown in minimal medium and resuspended in minimal medium without nitrogen. The cultures were resuspended to an initial (working) OD = 1.6, 3.6, or 4.4. Ethanol and formate production was negligible Table 3-2: Glucose consumption and byproduct secretion levels (mm) in resting cells with different growth inhibitors at time point 24 hours. Cell cultures were grown in minimal medium and resuspended in minimal medium without nitrogen. The cultures were resuspended to an initial (working) OD = 2.0. Ethanol production was negligible. Note that the yield (Y RPG ) represents the uncorrected value Table 3-3:Glucose consumption and byproduct secretion levels (mm) in resting cells with different levels of aeration at time point 24 hours. Cell cultures were grown in LB medium and resuspended in minimal medium without nitrogen. The cultures were resuspended to an initial (working) OD = 2.0. Formate production was negligible. Yield (Y RPG ) is corrected for background production of xylitol (xylitol production in the absence of glucose) Table 3-4: Glucose consumption and byproduct secretion levels (mm) in resting cells with different glucose feeding strategies at time point 24 hours. Cell The cultures were grown in LB medium and resuspended in minimal media without nitrogen. Chloramphenicol was added prior to harvesting cells, and the culture was resuspended to an initial OD = 2.0. Formate and ethanol production was negligible. Yield (Y RPG ) is corrected for background production of xylitol (xylitol production in the absence of glucose) Table 4-1: Strains and plasmids used in this study. Other gene deletion strains described are derived from strain PC09. a Table 4-2: Predicted maximum theoretical yield and NADPH source(s) from simulated strains used in this study. Model constraints and parameters are described in the Materials and Methods. a Table 4-3: Experimental results from resting cell biotransformations for the various strains described. Y RPG is corrected for the background production of xylitol in the absence of glucose. Standard deviations were less than 10% of the average unless indicated
15 xv Table 4-4: Predicted maximum theoretical yields adjusted for experimentally determined glucose uptake and metabolite secretion profiles (reported in Table 4-3). Model parameters and constraints are described in the Materials and Methods. Y RPG is included for comparison between simulated and experimental results Table 6-1: Strains used in this study a Table 6-2: Phosphofructokinase activity in strains harboring plasmid ploi3815. Values represent the average and standard deviation of at least four points. Background activity was subtracted from the reported values Table 6-3: Experimental results from resting cell biotransformations at the 24 hour time point for the various strains described. Y RPG is corrected for the background production of xylitol in the absence of glucose (given as Bkgd Xylitol ). Standard deviations were less than 10% of the average unless indicated. *Results from PC09 and JC72 were reported in Chapter Table 6-4: Secretion profile of growth coupled strains and precursor strains. Culture supernatant was analyzed at the end of the culture as shown in Figure 6 3 and defined in the Materials and Methods section. Cultures were grown in minimal medium supplemented with 100 mm xylose and/or 100 mm glucose, and induced with 100 µm IPTG. Concentrations are given as mm; growth rate (hr -1 ) was estimated as described in the Materials and Methods section Table 6-5: Cofactor results of select resting cell conditions and strains. Cells were harvested 24 hours after resuspending the cells in nitrogen-limited medium. Xylitol is corrected for background production Table 6-6: Cofactor ratios measured during growth of strain JC134 and its precursors. All strains were grown in rich medium containing 100 mm glucose and in the presence or absence of 100 mm xylose, and induced with 100 μm IPTG Table 6-7: Cofactor results of select resting cell conditions and strains. Cells were harvested 24 hours after resuspending the cells in nitrogen-limited medium. Concentrations are given as μmol gdw Table 6-8: Cofactor results of JC134 and precursor strains under growing conditions. Cells were grown in rich medium supplemented with 100 mm xylose and/or 100 mm glucose and induced with 200 μm IPTG. Cells were harvested when the culture OD 600 was between 2 4. Concentrations are given as μmol gdw Table 6-9: Concentration and growth rate of JC134 with various plasmids. Cells were cultured in minimal medium supplemented with glucose (100 mm), xylose (100 mm), MOPS (50 mm), and IPTG (100 μm) Table A-1: Strains Table A-2: Plasmids
16 Table A-3: Primers Table C-1: Comparison of xylose reductase activity between ploi3815 (wild-type) and ppcc111 (single amino acid mutant) with NAD(P)H as the cosubstrate Table C-2: Glucose-6-phosphate activity measured from lysates of E. coli strains with a deletion in xylb harboring plasmid ploi3809 (control) and ppcc118 (zwf) with CbXR integrated into the chromosome, or plasimd ploi3815 (CbXR control) and ppcc119 (CbXR + zwf). Activity reported as units (mg total protein) -1 in the lysate. One Unit is defined as the activity that consumes 1 μmol of NADPH in 1 minute Table E-1: Select predicted optimal knockout strategies and corresponding yield results from the modified OptKnock formulation. Model constraints and parameters are described in the Simulation Formulation section. Knockouts code for the following enzymes: GapA = glyceraldehydes 3-phosphate dehydrogenase; Pgk = phosphoglycerate kinase; Eno = enolase; Pgm = phosphoglucomutase; Eda = 2-keto- 3-deoxy-6-phosphogluconate aldolase; TpiA = triose phosphate isomerase; Gnd = 6- phosphogluconate dehydrogenase; TktAB = transketolase I & II; SucB = α- ketoglutarate dehydrogenase; Pta = phosphate acetyltransferase Table E-2: Secretion profile of OptKnock predicted strains and precursor strain. Cultures were grown in minimal medium supplemented with 100 mm xylose and/or 100 mm glucose, and induced with 100 µm IPTG. Concentrations are given as mm; growth rate (hr -1 ) was estimated as described in the Growth Culture Method section. No significant amount of lactate was produced in any of the strains Table F-1: Batch culture data for strain JC79 containing plasmid ploi3815 (CbXR), ppcc106 (CbXR & pntab), or ppcc500 (CbXR & stha). Cultures were grown in LB medium, supplemented with xylose, glucose, and MOPS, at 30C with 250 shaking. Data is from the 96 hour time point. Fermentation data is JC79 containing ploi3815, taken at the 48 hour time point. Concentrations are given as mm Table F-2: Metabolic profile of strains PC09, JC72, and JC111 harboring either plasmid ploi3815 (CbXR) or ppcc119 (CbXR and zwf). Strains were grown in LB medium supplemented with 100 mm glucose, 300 mm xylose. Results are from 96 hours after inoculation. All concentrations are given in mm. Standard deviations were less than 10% of the average unless indicated Table F-3: Metabolic profile of JC11 mutant derived strains transformed with either the control plasmid (3809), XylE (203), or Zwf (118). Strains grown in minimal media supplemented with 100 mm xylose and 100 mm glucose. Concentrations are given as mm; growth rate (μ max ) was determined by calculating the slope of the ln(od 600 ) versus time plot during exponential growth (hr -1 ). Standard deviations were less than 10% of the average unless indicated Table G-1: Experimental results from resting cell biotransformations for strains W3110, PC07, and PC09 harboring plasmid ploi3815. Cultures were grown in minimal medium and resuspended with minimal medium without a nitrogen source. Y RPG is xvi
17 corrected for the background production of xylitol in the absence of glucose (given as Bkgd Xylitol ). Concentrations are given as mm Table G-2: Resting cell culture results of JC79 harboring different plasmids. Cultures were grown in rich medium and resuspended with minimal medium without a nitrogen source. Fed cultures were continuously fed glucose via a syringe pump. Yield is corrected for the background production. Concentrations are given in mm Table G-3: Experimental results from resting cell biotransformations for the various strains described. Y RPG is corrected for the background production of xylitol in the absence of glucose (given as Bkgd Xylitol ). Standard deviations were less than 10% of the average unless indicated. Concentrations are given as mm. Standard deviations were less than 10% of the average unless indicated Table G-4: Experimental results from resting cell biotransformations for strains harboring plasmid ploi3815. Plasmids other than ploi3815 were harbored in strain PC09. Cultures were grown in rich medium and resuspended with minimal medium lacking a nitrogen source. Xylitol concentration represents the uncorrected value; Y RPG is corrected for the background production of xylitol in the absence of glucose (given as Bkgd Xylitol ). Confidence intervals, where applicable, represent the standard deviation from the average. Concentrations are given as mm. IPTG concentration was varied with strain JC17, as described in the parenthesis (1x is equivalent to 100 μm IPTG) Table H-1: Resting cell cofactor results of W3110 using different quenching methods, as described. Cells were harvested 24 hours after resuspending the cells in nitrogenlimited medium. Concentration of cofactors is given in µmol gdw -1. Using a t- distribution, the probability that the quenching methods are equal to the no quench method is < 5% xvii
18 xviii ACKNOWLEDGEMENTS I would like to thank my advisor, Dr. Patrick Cirino, for his support, patience, and understanding during my graduate career at Penn State. I started in the Fall of 2004 with theoretical knowledge of molecular biology techniques but no real practical knowledge. Dr. Cirino gave me an opportunity to learn the experimental aspects of molecular biology and metabolic engineering in his lab and develop my technical, presentational, and writing skills over these past 5+ years. To reinforcing the knowledge that I gained, he gave me many opportunities to mentor and teach incoming undergraduate and graduate students in our lab. I would also like to thank him for all of the time and attention that he gave to both ensuring that I was prepared for the various national conferences which I presented and that the papers that I wrote were deserving of the journals in which they published. I would also like to thank Dr. Costas Maranas for giving me an opportunity to work on the computational aspect of metabolic engineering. I believe that the knowledge, education, and help that he has given me, during my first few years, expanded my education and skill set to include optimization strategies and simulations. Although my project was not the equal parts computational and experimental as originally planned, I know that I have sturdy foundation in the fundamentals of molecular biology modeling (e.g. protein engineering, flux balance analysis, stoichiometric metabolic networks). I would like to thank my thesis committee members, Dr. Wayne Curtis, and Dr. Ali Demirci, for their time, guidance, and valued opinions and help that they have given me. Dr. Wayne Curtis has also generously helped out our lab by supplying initial materials and lab equipment while we worked on getting our lab started. I would like to thank the staff of the Chemical Engineering Department at Penn State for making my life at Penn State that much easier. They were always very kind and patient when
19 xix answering the many questions that I had about taxes, classes, tuition, purchase orders, pay, and general building/equipment problems. I would like to thank all of my current and past labmates in not only the Dr. Cirino lab group, but neighboring lab groups as well. Specifically outside the lab group are Dave Latulippe, Kristin O Neil, and Jeff Larson for their help and feedback with fundamental knowledge problems and, Tony Burgard and Priti Pharkya for their help and patience as I was learning the stoichiometric metabolic modeling and OptKnock. Dr. Reza Khankal is a true friend and great labmate and classmate; we both started in Dr. Cirino s lab together and we have helped each other out with class work and experiments throughout our years together. I thank Dr. Hossein Fazelinia, for his patience and understanding, not only in teaching me basic laboratory skills but also taking the time to help me understand some of his work in computational protein engineering, specifically IPRO. Bolaji Akinterinwa deserves much praise for her dealing with the boys for so many years. She has been kind enough to help me edit various papers after I can no longer look at them, as well as take over lab management duties in our lab. Chris Frei has been a wonderful lab and officemate; without his presence, distractions, and food stash, the office would have been unbearable. Lois Eppihimer, Lexan Lhu, and the plethora of undergraduate helpers, dishwashers, and honors students also deserve to be acknowledged not only for the general help and work that they provided, but also for the entertainment value that they provided, making the lab a more enjoyable place. They have also given me the opportunity to not only pass on the knowledge that I have gained while working in the lab, but also reinforce that knowledge by asking those silly questions to help them understand either the specifics or bigger picture. I would also like to thank the various members of the Club Water Polo Team at Penn State for providing a wonderful supporting atmosphere to not only learn a new sport, but also provide a distraction from the everyday lab (and chemical engineering) life, and a means to burn off stress. Specifically, I would like to acknowledge the Radcliff brothers, Ben and Zach, for
20 xx being around to just hang out (Scrubs, The Office) and dragging me into the various shenanigans that they routinely found themselves in. I would like to thank my family and friends for all of their support during my time here at Penn State, as well as their understanding that I will graduate soon is not an empty promise. A special thanks goes out to my parents, Edmond and Susan Chin, who, with their love and support (especially financial support at the very end) helped me get over the trials and tribulations that is known as the Ph. D. dissertation. Lastly, but certainly not least, I would like to thank my housemates; Jason Binz, Chris Frei, Matt Marino, and Eric Moschetta; for putting up with me for the past several months. They were kind enough to share their housing arrangements with me for an undefined period of time while I finished up my research and dissertation, without which, I probably would have lived in Fenske, much to the janitorial staff s chagrin. I acknowledge Dr. T.W. Jeffries (University of Wisconsin) for his generous donation of the Pichia stipitis and Candida boidinii genes used in this work, Keio collection for providing single deletion mutant strains, and the NSF who provided funding for this work (grant number: BES ).
21 Chapter 1 Background
22 2 Introduction Biotransformations are increasingly being used as popular alternatives to traditional chemical catalysis. This is because of the relatively inexpensive feedstocks utilized, unmatched selectivity offered by enzymes, and the potential for clean, green processes. One important class of biotransformations are redox reactions requiring the reduced nicotinamide cofactors NAD(P)H to catalyze oxygen insertion (oxygenases) or reduction of carbonyl groups (reductases or dehydrogenases). Cost-effective and continuous delivery of reduced cofactors remains an important challenge that is associated with utilizing cofactor-dependent enzymes for biocatalysis. In vitro cofactor regeneration systems have been used where cosubstrates, such as formate or glucose, undergo a single (two electron) oxidation to yield stoichiometric amounts of NADH or NADPH (van der Donk and Zhao, 2003). However, in various scenarios, the use of whole-cell biocatalysis has been advantageous over in vitro systems (Duetz et al., 2001, Schmid et al., 2001). This is because multi-step transformations involving several enzymes in a biosynthetic pathway, as well as metabolite overproduction coupled to breakdown of a growth substrate both demand the use of whole-cell systems. Additionally, via sugar metabolism, the use of whole cells offers the opportunity to regenerate reduced cofactors which are utilized in driving heterologous redox reactions of interest. However, there is significant room for improvement in the efficiency with which glucose or other sugars are utilized for providing the reducing power for reactions of interest (i.e., the fraction of reducing equivalents resulting from cosubstrate oxidation that are actually used in the reaction of interest can be increased).
23 3 Literature review Xylitol Xylitol (MW 152) was chosen to be the platform reduction product. Xylitol is considered by the United States Department of Energy s top twelve value-added products from biomass. Xylitol is a five-carbon sugar-alcohol with many useful properties as a sweetener; it has a similar sweetness to sucrose, and is also anticariogenic. The metabolism of xylitol within the body is not insulin-dependent, therefore it serves as a good sugar substitute for diabetics. Currently, xylitol is industrially produced by the catalytic hydrogenation of xylose at temperatures and pressures ranging from C and ~50 atm, respectively, with 50-60% conversion (Parajo et al., 1998). Production of xylitol using microorganisms is potentially a safer and more environmentally friendly process and could achieve higher product specificity. Some examples of xylitol bioproduction have been discussed previously (Akinterinwa et al., 2008, Parajo et al., 1998). Xylitol bioproduction, similar to many other biotransformations of interests, requires the use of reduced cofactors (NAD(P)H), and therefore a means of regenerating these cofactors. While these reactions can be performed in vitro using purified enzymes, it is often easier and less expensive to regenerate reduced cofactors by harnessing sugar metabolism in whole cells for in vivo bioproduction (Schmid et al., 2001). Other groups have examined the feasibility of bioproduction of xylitol in a variety of organisms (Akinterinwa et al., 2008, Parajo et al., 1998). Notable are the homologous and heterologous production in several yeast species and strains as well as bacterial (e.g. E. coli and Lactococcus lactis). Because bacterial strains do not naturally produce xylitol, expression of a heterologous gene is necessary. Mori s group has heterologously produced xylitol in E. coli, using a xylose reductase from Kluyveromyces lactis, with reported productivities ranging between 0.6 to 0.8 g l -1 hr -1 (Hibi et al., 2007). Expressing a xylose
24 4 reductase from C. tropicalis, Suzuki s group reported a productivity of 0.66 g l -1 hr -1 in E. coli (Suzuki et al., 1999). Nyyssola and coworkers have overexpressed the xylose reductase from Pichia stipitis in L. lactis to produce xylitol, with a production value of 2.7 g l -1 hr -1 (Nyyssola et al., 2005). Many groups have also heterologously produced xylitol in Saccharomyces cerevisiae. Overexpressing a xylose reductase from P. stipitis, Bae and coworkers, Kim and coworkers, and Lee and coworkers achieved batch productivities of 0.6, 0.8, and 1.9 g l -1 hr -1 respectively (Bae et al., 2004, Kim et al., 2002b, Lee et al., 2000). Kim also reported the overexpression of a nonspecific S. cerevisiae aldose reductase to produce xylitol, with a yield of 0.5 g l -1 hr -1 (Kim et al., 2002b). Homologous production of xylitol was achieved by using the strains C. guilliermondii, C. tropicalis, C. boidinii, P. stipitis, and Dabaryomyces hansenii (Converti and Dominguez, 2001, Jin et al., 2005, Kim et al., 2002a, Kim et al., 2004, Kim and Oh, 2003, Kwon et al., 2006, Rodrigues et al., 1998, Silva et al., 1999, Winkelhausen et al., 2004). Production values for the Candida species varied from 0.3 g l -1 hr -1 (C. boidinii) and 0.5 to 0.9 g l -1 hr -1 (C. guilliermondii) to 2.0 to 4.9 g l -1 hr -1 (C. tropicalis) (Kim et al., 2002a, Kim et al., 2004, Kim and Oh, 2003, Kwon et al., 2006, Rodrigues et al., 1998, Silva et al., 1999, Winkelhausen et al., 2004). With D. hansenii, production values ranged from 0.5 to 4.7 g l -1 hr -1 (Converti and Dominguez, 2001, Dominguez et al., 1999). Similar to the trend reported in S. cerevisiae, immobilizing D. hansenii cells for fermentation improved the productivity from batch-type fermentations (Dominguez et al., 1999, Silva et al., 1999). The productivity comparison can be more easily viewed in Table 1 1. Table 1-1: Table comparing xylitol productivity and yield between different organisms and conditions. Host organism Xylitol gene Media Productivity (g l -1 hr -1 ) ~ Yield Reference E. coli C. tropicalis Batch (Complex) mol (Suzuki et al., (mol glucose) ) E. coli K. lactis Batch (Defined) mol (Hibi et al., (mol glucose) )
25 Host organism Xylitol gene Media Productivity (g l -1 hr -1 ) 5 ~ Yield Reference (Nyyssola et (Bae et al., (Kim et al., (Lee et al., (Oh et al., (Kim et al., (Ko et al., (Kim et al., L. lactis P. stipitis Batch (Defined) mol (mol glucose) -1 al., 2005) S. cerevisiae P. stipitis Batch (Complex) mol (mol glucose) ) S. cerevisiae P. stipitis Batch (Complex) mol (mol glucose) b) S. cerevisiae P. stipitis Batch (Defined) mol (mol glucose) ) S. cerevisiae P. stipitis Batch (Complex) mol (mol glucose) ) S. cerevisiae S. Batch (Complex) mol cerevisiae (mol glucose) b) C. boidinii --- Batch (Complex) mol (Winkelhausen (mol xylose) -1 et al., 2004) C. --- Batch (Complex) mol (Dominguez et guilliermondii (mol xylose) -1 al., 1999) C. --- Semi continuous mol (Rodrigues et guilliermondii (Complex) (mol xylose) -1 al., 1998) C. --- Cell recycle mol (Silva et al., guilliermondii (Complex) (mol xylose) ) C. tropicalis --- Batch (Defined) mol (Kim and Oh, (mol xylose) ) C. tropicalis --- Batch (Complex) mol (mol xylose) ) C. tropicalis --- Fed batch mol (Complex) (mol xylose) a) C. tropicalis --- Submerged membrane mol (mol xylose) -1 (Kwon et al., 2006) (Complex) D. hansenii --- Batch (Complex) mol (Dominguez et D. hansenii --- Batch (immobilized cells Complex) (mol xylose) mol (mol xylose) -1 D. hansenii --- Batch (Complex) 1.9, mol (mol xylose) -1 P. stipitis --- Batch (Complex) mol al., 1999) (Dominguez et al., 1999) (Converti and Dominguez, 2001) (Jin et al., (mol xylose) ) More of interest to this research is the xylitol yield, or moles of xylitol produced per mole of substrate consumed. The groups using C. boidinii, C. guilliermondii, C. tropicalis, P. stipitis, and D. hansenii calculated yields in terms of moles of xylitol produced per mole of xylose consumed. Using P. stipitis, Jin and coworkers were able to obtain a yield of 0.30 mol xylitol per
26 6 mol of xylose (Jin et al., 2005), while Winkelhausen was able to achieve a xylitol yield of 0.44 mol xylitol per mol of xylose (Winkelhausen et al., 2004). Other groups were able to achieve higher xylitol yields using D. hansenii ( mol xylitol per mol xylose, (Converti and Dominguez, 2001, Dominguez et al., 1999)), C. guilliermondii ( mol xylitol per mol xylose, (Dominguez et al., 1999, Rodrigues et al., 1998, Silva et al., 1999)), and C. tropicalis ( mol xylitol per mol xylose, (Kim et al., 2002a, Kim and Oh, 2003, Ko et al., 2006, Kwon et al., 2006)). However, this research plans on using reducing equivalents derived from glucose metabolism for xylitol production which will allow for theoretical yields greater than 1. Using S. cerevisiae, Kim and coworkers were able to achieve a xylitol yield of 0.47 mol xylitol per mol glucose (Kim et al., 2002b). They were able to further increase this yield to 0.92 mol xylitol per mol glucose (Kim et al., 2002b). Other groups using a xylose reductase from P. stipitis in S. cerevisiae were able to achieve xylitol yields of mol xylitol per mol glucose (Bae et al., 2004, Lee et al., 2000, Oh et al., 2007). Expressing a xylose reductase from P. stipitis in L. lactis resulted in a xylitol yield of 2.5 mol xylitol per mol glucose (Nyyssola et al., 2005). Using E. coli, groups have been able to achieve xylitol yields of 1.0 and 3.15 mol xylitol per mol glucose, expressing either a xylose reductase from K. lactis (Hibi et al., 2007) or C. tropicalis (Suzuki et al., 1999). Yield comparisons can be more easily viewed in Table 1 1. Escherichia coli E. coli was chosen as the platform organism because it is a well understood, well studied, robust organism that has been demonstrated to be a viable platform production organism for both homologous and heterologous products (Akinterinwa et al., 2008, Boghigian and Pfeifer, 2008, Das et al., 2007, Jarboe et al., 2007, Lee et al., 2009, Neidhardt and Curtiss, 1996, Yan and Liao, 2009). It is a fast growing organism that is able to metabolize a variety of different sugars and
27 7 carbon sources. This makes it suitable for use under various environmental conditions and genetic alterations. E. coli is a proven platform production organism and has been reported to successfully produce many other value-added chemicals and metabolites such as 1,3-propanediol (Cervin et al., 2004, Tang et al., 2009, Nakamura and Whited, 2003), 1,2-propanediol (Altaras and Cameron, 2000), lactic acid (Zhou et al., 2006, Zhu and Shimizu, 2005, Zhu and Shimizu, 2004), succinate (Lin et al., 2005a, Lin et al., 2005b, Sanchez et al., 2006b), and pyruvate (Causey et al., 2003). Being a well understood organism, genetic modifications and manipulations in E. coli, such as gene deletion and overexpression are easy to implement. This allows E. coli to not only overproduce a specific metabolite, but also to overproduce non-native compounds (Cirino et al., 2006, Suzuki et al., 1999). Chromosomal modifications are easily transferred between strains with the use of phage transduction (Sambrook and Russell, 2001, Snyder and Champness, 2007) and a selectable marker. Site-directed mutations are implemented with the aid of recombinases, most notably λ-red recombinase (Datsenko and Wanner, 2000). Additionally, using a series of specific plasmids, site-directed chromosomal insertions can be used to integrate gene(s) into the bacterial chromosome at various insertion sites (Haldimann and Wanner, 2001). Finally, metabolic networks based on the stoichiometry of known enzymes and corresponding reactions in E. coli can be used to predict the stoichiometric boundaries of biochemical production. These are constantly being updated to better reflect experimental data (Edwards et al., 2001, Reed et al., 2003, Varma and Palsson, 1994). Nicotinamide cofactors There are two different nicotinamide reduced cofactors present in the cell: NAD(H) and NADP(H). Each cofactor serves a different biological purpose, and thus, has its own distinctive
28 regeneration cycle within normal cellular metabolism. This allows for strict regulation for the 8 oxidized and reduced states of the cofactors. NADH is generated by glycolysis and the tricarboxylic acid (TCA) cycle. Under aerobic conditions, NADH is primarily used to generate ATP via the electron transport chain and oxidative phosphorylation. Under anaerobic conditions, NADH is reoxidized by generating fermentation products (e.g. ethanol and lactate) in order to maintain a balance between its oxidized and reduced forms. NADPH is generated primarily through the pentose phosphate pathway, with minor contributions from the TCA cycle, and is used for anabolic cellular reactions (i.e. growth, gluconeogenesis, and biosynthesis). E. coli also contains two transhydrogenase enzymes, that have been reported to transfer electrons between NADH and NADPH. These transhydrogenases serve as another means to regulate the oxidized and reduced states of the cell (Boonstra et al., 1999, Sauer et al., 2004). The soluble transhydrogenase, encoded by the stha gene, was reported to predominantly transfer electrons from NADPH to NADH. The energy-dependent, membrane-bound transhydrogenase, encoded by the pntab genes, was reported to predominantly transfer electrons from NADH to NADPH (Sauer et al., 2004). A representation of the major sources and sinks of these cofactors is illustrated in Figure 1 1.
29 9 Figure 1-1: Central carbon metabolism of glucose and xylose. Also shown are major sources and sinks for the reduced cofactors NADH and NADPH. Cofactor manipulation There is significant effort and research to increase the availability of the reduced cofactors, via metabolic engineering, to improve production of a desired biochemical. Some examples of the manipulation techniques reported include: use of different sugars (San et al., 2002, Sanchez et al., 2005); overexpression of a gene that overproduces NAD(P)(H) (Berrios- Rivera et al., 2002c, Li et al., 2009, San et al., 2002), overexpression of genes that produce more
30 10 reduced cofactors (e.g. formate dehydrogenase from C. boidinii) (Berrios-Rivera et al., 2002b, Berrios-Rivera et al., 2002a, Sanchez et al., 2005), overexpression of the transhydrogenases (Sanchez et al., 2006a, Verho et al., 2003), and knock-out of certain genes or pathways (Lin et al., 2005a, Lin et al., 2005b, Yang et al., 1999, Yun et al., 2005). Carbon source San s groups reported that they could alter the internal concentrations of reduced cofactors by using carbon sources with different oxidation states, namely gluconate, glucose, and sorbitol, which have oxidation states of (+1), (0), and (-1) respectively (San et al., 2002, Sanchez et al., 2005). The theoretical maximum number of reducing equivalents per mole of substrate is dependent on the oxidation state of the carbon source. Assuming that the sugar was metabolized through EMP-glycolysis to generate pyruvate, gluconate will generate 1 mole of NADH per mole of gluconate consumed, glucose will generate 2 moles of NADH per mole of glucose consumed, and sorbitol will generate 3 moles of NADH per mole of sorbitol consumed. The study showed that using gluconate as the substrate resulted in the lowest ethanol/acetate ratio (which was used as an indicator of the oxidation state of the cell), while using sorbitol resulted in the highest ethanol/acetate ratio. In terms of the metabolite profile, gluconate had the highest amount of lactate and acetate as well as the lowest amount of ethanol. As the oxidation state decreased, an increase in the amount of ethanol produced and a subsequent decrease in the amount of lactate and acetate was observed. This suggested that higher amounts of NADH were available and was confirmed by measuring the cofactor concentrations and calculating the NADH to NAD + ratio.
31 11 Overexpression of nicotinic acid phosphoribosyltransferase (PncB) San s group has also tested the overproduction of NAD(H) in order to improve the availability of reduced cofactors for a reaction of interest. Specifically they overexpressed the nicotinic acid phosphoribosyltransferase (NAPRTase) enzyme, encoded by the pncb gene, which they concluded was the rate limiting step of the salvage pathway production of NAD(H) (Berrios- Rivera et al., 2002c). Assuming that the cell could maintain equilibrium between the oxidized and reduced states, they hypothesized that overexpressing NAPRTase would lead to an increase in production of NAD +, and hence, an increase both NAD + and NADH concentrations. Experimentally, using cells cultivated in anaerobic chemostat cultures, they were able to measure an increase in the overall amounts of NAD + & NADH using assays; however, neither a decrease in the NADH/NAD + ratio, nor a change in secreted metabolites occur when overexpressing pncb. With anaerobic tube cultures, they were able to increase the ratio of ethanol/lactate produced, which corresponded to an increase in NADH available to the cells. Therefore, they concluded that overexpressing pncb was beneficial for making reducing power available under anaerobic growing conditions but not anaerobic steady state (chemostat) conditions (Berrios-Rivera et al., 2002c, San et al., 2002). Use of an NAD + -dependent formate dehydrogenase (FDH) San s group has also replaced the native, cofactor-independent formate dehydrogenase (FDH) Replacing the native, cofactor-independent formate dehydrogenase with a NAD + - dependent formate dehydrogenase, cloned from Candida boidinii, San s group attempted to increase the availability of NADH in E. coli (Berrios-Rivera et al., 2002b, Berrios-Rivera et al., 2002a, Sanchez et al., 2005). They examined ethanol production of aerobic cultures in the
32 12 presence of formate (Berrios-Rivera et al., 2002b), and the metabolic secretion profiles of anaerobic cultures (chemostat, batch, and tube cultures) (Berrios-Rivera et al., 2002b, Berrios- Rivera et al., 2002a, Sanchez et al., 2005). Under aerobic conditions, formate is not normally produced and needed to be supplemented to the media to act as a substrate for the new NAD + -dependent FDH. Deleting the native FDH, they found that, aerobically, the mutant E. coli cells produced more ethanol than the wild-type, thus proving that the NAD + -dependent FDH increased the availability of reducing equivalents. Under anaerobic conditions, they observed that the ethanol/acetate ratio was higher when the expressing the FDH from C. boidinii, which they correlated to an increased NADH/NAD + ratio. These results were confirmed by measuring the internal concentrations of the cofactors NADH & NAD +. Use of an NADP + -dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) Verho and coworkers successfully utilized a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) which can utilize both NAD + and NADP + to improve the ability of S. cerevisiae to ferment xylose to ethanol (Verho et al., 2003, Verho et al., 2002). This enzyme, cloned from the organism Kluyveromyces lactis, corrected the cofactor imbalance associated with xylose metabolism in S. cerevisiae. This also resulted in a decrease in the amount of xylitol produced when grown in a xylose-containing medium. In a similar study, Martinez and coworkers cloned a NADP + -dependent glyceraldehyde-3-phosphate dehydrogenase (GAPDH) from Clostridium acetobutylicum (gapc) to improve lycopene and ε-caprolactone production and yield (Martinez et al., 2008). By knocking out the native GADPH (gapa), they ensured that NADPH was also produced through the reaction catalyzed by GAPDH. They found that the central metabolic flux was significantly changed upon overexpressing gapc, which they attributed to the exchange of
33 NAD + - with a NADP + -dependent GADPH, and reported an increase in yield of lycopene and ε- carpolactone when overexpressing gapc. 13 Overexpression of the native E. coli transhydrogenase E. coli is able to balance the internal levels of cofactors by regulating flux through both central metabolism and the transhydrogenase enzymes. The transhydrogenase enzymes were reported to transfer electrons from NADH to NADPH or vice versa (Boonstra et al., 1999, Boonstra et al., 2000, Sauer et al., 2004). The membrane-bound transhydrogenase, pntab, was reported to irreversibly catalyze the transfer of electrons from NADH to NADPH, at the expense of an external proton (Sauer et al., 2004). The soluble transhydrogenase, stha (udha), is energyindependent enzyme found in the cytosol. It has been reported to catalyze the reversible conversion of NADPH to NADH (Boonstra et al., 1999, Sauer et al., 2004). Based on the varying specificity of many enzymes (and products), several research groups have overexpressed the transhydrogenase enzymes in an effort to increase the availability of the required reduced cofactor. San s group overexpressed stha from E. coli to produce poly(3- hydroxybutyrate) (PHB), which is a biodegradable polymer produced by a NADPH-dependent reduction from acetoacetyl-coa (Sanchez et al., 2006a). By overexpressing the stha gene they were able to improve PHB formation compared to the control plasmid in aerobically grown cultures. In another study, Hummel s group aimed to produce chiral alcohols by an NADPHdependent reduction using whole-cell biotransformations, specifically (R)-phenylethanol (Weckbecker and Hummel, 2004). They report that overexpression of pntab improved (R)- phenylethanol production.
34 14 Elimination of competing pathways By eliminating reactions and/or pathways that naturally regenerate the cofactors, cofactor regeneration could only occur through the reaction of interest. Under aerobic conditions, NADH is most efficiently oxidized by using one of the two NADH dehydrogenases encoded by the genes nuoa-n and ndh (Leif et al., 1995, Tran et al., 1997, Weidner et al., 1993, Yun et al., 2005). During oxidative phosphorylation, these enzymes catalyze the transfer of electrons from NADH into the electron transport chain where the electrons are ultimately transferred to oxygen generating water and a proton gradient. The proton gradient is utilized in ATP synthesis via ATP synthase. Under anaerobic conditions, NADH is oxidized by producing fermentation products such as lactate, formate, and ethanol. NADPH is generally utilized in biosynthesis reactions which are necessary for cellular growth. The transhydrogenases, interconvert reduced cofactors to a form that is more easily oxidized to maintain internal cofactor balance. Several groups have reported their successes in eliminating these competing pathways to increase the bioproduction of a desired chemical (Lin et al., 2005a, Lin et al., 2005b, Yang et al., 1999, Yun et al., 2005). Resting cells In order to eliminate growth as both a variable between different strains and as a drain of carbons and cofactors, Walton and Stewart described a method in which cellular growth was inhibited, but metabolic activity was maintained (Walton and Stewart, 2002, Walton and Stewart, 2004). This was accomplished as follows: cells were grown in a fermenter containing Luria- Bertani (LB) medium supplemented with glucose. Upon entering the stationary phase, the cells were centrifuged and resuspended in a fermenter with a nitrogen-limited medium supplemented with glucose. Product formation was monitored over the course of 24 hours. Using this method,
35 15 they report a 20-fold increase in volumetric productivity of ε-caprolactone (Walton and Stewart, 2002). They then tested this method on other NADPH-dependent reactions, specifically they report success in increasing the productivity of 4-methylcyclohexanone and ethyl 3- hydroxybutyrate (Walton and Stewart, 2004). Cotter and coworkers have successfully utilized resting cell conditions to select for higher ethanol producing strains of Clostridium (Cotter et al., 2009). Forberg and coworkers demonstrated that tryptophan limitation in an auxotroph strain (growth inhibition by limiting tryptophan) could achieve phenylalanine overproduction from glucose. They also reported that the growth phase of the harvested cells affected the subsequent product yield; cells from the exponential phase had a higher yield than cells harvested from the stationary phase (Forberg et al., 1988). Emmerling and coworkers have used anaerobic resting cell conditions to simplify metabolism to better understand the effects of overexpressing various glycolytic enzymes (Emmerling et al., 1999, Emmerling et al., 2000). Stoichiometric model Using a stoichiometric metabolic model, the production boundaries of a desired chemical in various theoretical mutant strains can be predicted and explored with relative ease. The stoichiometric metabolic network model is a standard network that uses metabolites as nodes and reactions as the edges connecting these nodes. The network edges are directed, and reversibility of the reactions is accomplished by providing a separate backward reaction. Certain constraints, such as carbon source uptake, a minimum growth requirement, upper and lower bounds to all possible reaction fluxes, and pseudo steady state of the metabolites (e.g. no accumulation of any internal metabolites), are applied to this network, thereby decreasing the feasibility space. This underdetermined network is solved as a linear programming optimization problem by solving for the reaction fluxes that maximize or minimize a particular objective.
36 16 Objectives can be chosen to mimic cellular objectives, like maximizing growth or ATP generation; or to maximize a theoretical metabolite production, such as xylitol. An example outline of the linear program, with the associated equations, is shown below. Maximize: Biological objective/biochemical production (over fluxes) subject to fixed glucose uptake v 10 glc. up network stoichiometry j S ij v j 0, i regulatory constraints LB j v UB, j j j uptake/secretion restrictions blocked reactions v j 0, j (other carbon sources) v j 0, j (knockout reaction) where i represents the metabolites in the model; j represents the reaction pathways; v represents the flux through reaction j; LB is the lower bound; UB is the upper bound; S ij represents the stoichiometric matrix of the model Much of the preliminary work on E. coli metabolic networks has been performed by Palsson s group; they have verified the accuracy of their model by correctly predicting the secretion of metabolites under varying aerobic conditions (Varma and Palsson, 1994), and compared the predicted growth phenotype with the experimental phenotype under varying growth conditions (Covert et al., 2004, Covert and Palsson, 2002, Edwards et al., 2001). Their later models have begun implementing the idea of protein regulation in a dynamic model to more accurately predict experimental data (Covert et al., 2004, Covert and Palsson, 2002). Lastly, they, and other groups have developed and expanded upon models of other organisms, such as Helicobacter pylori (Forster et al., 2003, Schilling et al., 2002) Saccharomyces cerevisiae (Famili et al., 2003, Forster et al., 2003), Salmonella (Raghunathan et al., 2009, AbuOun et al., 2009),
37 17 Mycoplasma genitalium (Suthers et al., 2009), and Rhizobium etli (Resendis-Antonio et al., 2007). The Maranas group has taken the initial framework of the metabolic network and modified it for several different purposes. One purpose was to solve the true cellular objective (Burgard and Maranas, 2003), while another program computationally predicts the overproduction of a desired chemical in various theoretical knockout strains (Burgard and Maranas, 2001). They have also developed programs that can simultaneously maximize biomass (growth) and biochemical (desired metabolite) production. This program, OptKnock for E. coli (Burgard et al., 2003, Pharkya et al., 2004) and OptStrain for other microorganisms (Pharkya et al., 2004), essentially couples biochemical production with growth by selecting knockout strategies that forces the cell to grow with simultaneous production of the desired biochemical. By examining the existing framework under various conditions (e.g. gene deletion strategies, altered carbon source), the model can be used understand the importance of key central metabolic pathways for biochemical overproduction and increased yield. However, a caveat for using this framework is that enzyme activity for all reactions is never limiting. This allows all reactions that benefit the objective to be used exclusively and extensively, while competing reactions that hinder the objective have no flux. Also, the model assumes that there is minimal growth allowing for available reduced cofactors to be used to produce the desired chemical. Proposed research The focus of this research is to study and manipulate cellular metabolism of an organism to improve the availability of reducing equivalents for the production of a desired chemical. Specifically, this research aims to use E. coli as the platform organism for the heterologous bioproduction of xylitol. Metabolism of the cosubstrate, glucose, will serve as the means of
38 18 generating reduced cofactors for xylose reduction to xylitol. E. coli naturally metabolizes xylose, therefore it is desirable to eliminate xylose metabolism for two reasons. The first is to prevent cofactor generation from xylose metabolism, thereby requiring all reducing equivalents to come from glucose (or another sacrificial sugar) metabolism and allows for an easier yield (moles xylitol produced per mole sacrificial sugar consumed) calculations. The second is to ensure that xylose is solely used for xylitol production and not for biomass production/growth. This research aims to (a) determine a working strain and heterologous xylitol producing enzyme, (b) determine an optimized method for standardized comparisons between different strains and/or heterologous enzymes, (c) develop a method for increasing xylitol yield from glucose, (d) determine the source of reduced cofactors used for xylitol production, (e) manipulate the metabolism of E. coli in such a way as to improve the xylitol production for maximum yield, and (f) improve the metabolic network model by incorporating experimental data. For the purposes of this project, yield (Y RPG ) will be defined as the moles of xylitol produced per mole of glucose consumed. This research will test heterologous xylose reductases and xylulose dehydrogenases from various organisms with varying degrees of cofactor specificities. After determining a suitable protocol to equivalently compare different strains, this research will examine the effects of different gene deletion strategies that direct glucose metabolism towards or away from particular pathways and incorporate the data obtained from experiments to the stoichiometric model. References ABUOUN, M., SUTHERS, P. F., JONES, G. I., CARTER, B. R., SAUNDERS, M. P., MARANAS, C. D., WOODWARD, M. J. & ANJUM, M. F. (2009) Genome scale reconstruction of a Salmonella metabolic model: comparison of similarity and differences with a commensal Escherichia coli strain. J Biol Chem, 284,
39 AKINTERINWA, O., KHANKAL, R. & CIRINO, P. C. (2008) Metabolic engineering for bioproduction of sugar alcohols. Curr Opin Biotechnol, 19, ALTARAS, N. E. & CAMERON, D. C. (2000) Enhanced production of (R)-1,8-propanediol by metabolically engineered Escherichia coli. Biotechnology Progress, 16, BAE, S. M., PARK, Y. C., LEE, T. H., KWEON, D. H., CHOI, J. H., KIM, S. K., RYU, Y. W. & SEO, J. H. (2004) Production of xylitol by recombinant Saccharomyces cerevisiae containing xylose reductase gene in repeated fed-batch and cell-recycle fermentations. Enzyme and Microbial Technology, 35, BERRIOS-RIVERA, S. J., BENNETT, G. N. & SAN, K. Y. (2002a) The effect of increasing NADH availability on the redistribution of metabolic fluxes in Escherichia coli chemostat cultures. Metab Eng, 4, BERRIOS-RIVERA, S. J., BENNETT, G. N. & SAN, K. Y. (2002b) Metabolic engineering of Escherichia coli: increase of NADH availability by overexpressing an NAD(+)- dependent formate dehydrogenase. Metab Eng, 4, BERRIOS-RIVERA, S. J., SAN, K. Y. & BENNETT, G. N. (2002c) The effect of NAPRTase overexpression on the total levels of NAD, the NADH/NAD+ ratio, and the distribution of metabolites in Escherichia coli. Metab Eng, 4, BOGHIGIAN, B. A. & PFEIFER, B. A. (2008) Current status, strategies, and potential for the metabolic engineering of heterologous polyketides in Escherichia coli. Biotechnol Lett, 30, BOONSTRA, B., FRENCH, C. E., WAINWRIGHT, I. & BRUCE, N. C. (1999) The udha gene of Escherichia coli encodes a soluble pyridine nucleotide transhydrogenase. J Bacteriol, 181, BOONSTRA, B., RATHBONE, D. A., FRENCH, C. E., WALKER, E. H. & BRUCE, N. C. (2000) Cofactor regeneration by a soluble pyridine nucleotide transhydrogenase for biological production of hydromorphone. Appl Environ Microbiol, 66, BURGARD, A. P. & MARANAS, C. D. (2001) Probing the performance limits of the Escherichia coli metabolic network subject to gene additions or deletions. Biotechnology and Bioengineering, 74, BURGARD, A. P. & MARANAS, C. D. (2003) Optimization-based framework for inferring and testing hypothesized metabolic objective functions. Biotechnology and Bioengineering, 82, BURGARD, A. P., PHARKYA, P. & MARANAS, C. D. (2003) Optknock: a bilevel programming framework for identifying gene knockout strategies for microbial strain optimization. Biotechnol Bioeng, 84, CAUSEY, T. B., ZHOU, S., SHANMUGAM, K. T. & INGRAM, L. O. (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc Natl Acad Sci U S A, 100, CERVIN, M. A., SOUCAILLE, P. & VALLE, F. (2004) Process for the biological production of 1,3-propanediol with high yield. IN ORGANIZATION, W. I. P. (Ed.). CIRINO, P. C., CHIN, J. W. & INGRAM, L. O. (2006) Engineering Escherichia coli for xylitol production from glucose-xylose mixtures. Biotechnol Bioeng, 95, CONVERTI, A. & DOMINGUEZ, J. M. (2001) Influence of temperature and ph on xylitol production from xylose by Debaryomyces hansenii. Biotechnology and Bioengineering, 75, COTTER, J. L., CHINN, M. S. & GRUNDEN, A. M. (2009) Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst Eng, 32,
40 COVERT, M. W., KNIGHT, E. M., REED, J. L., HERRGARD, M. J. & PALSSON, B. O. (2004) Integrating high-throughput and computational data elucidates bacterial networks. Nature, 429, COVERT, M. W. & PALSSON, B. O. (2002) Transcriptional regulation in constraints-based metabolic models of Escherichia coli. J Biol Chem, 277, DAS, A., YOON, S. H., LEE, S. H., KIM, J. Y., OH, D. K. & KIM, S. W. (2007) An update on microbial carotenoid production: application of recent metabolic engineering tools. Appl Microbiol Biotechnol, 77, DATSENKO, K. A. & WANNER, B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A, 97, DOMINGUEZ, J. M., CRUZ, J. M., ROCA, E., DOMINGUEZ, H. & PARAJO, J. C. (1999) Xylitol production from wood hydrolyzates by entrapped Debaryomyces hansenii and Candida guilliermondii cells. Appl Biochem Biotechnol, 81, DUETZ, W. A., VAN BEILEN, J. B. & WITHOLT, B. (2001) Using proteins in their natural environment: potential and limitations of microbial whole-cell hydroxylations in applied biocatalysis. Curr Opin Biotechnol, 12, EDWARDS, J. S., IBARRA, R. U. & PALSSON, B. O. (2001) In silico predictions of Escherichia coli metabolic capabilities are consistent with experimental data. Nature Biotechnology, 19, EMMERLING, M., BAILEY, J. E. & SAUER, U. (1999) Glucose catabolism of Escherichia coli strains with increased activity and altered regulation of key glycolytic enzymes. Metab Eng, 1, EMMERLING, M., BAILEY, J. E. & SAUER, U. (2000) Altered regulation of pyruvate kinase or co-overexpression of phosphofructokinase increases glycolytic fluxes in resting Escherichia coli. Biotechnol Bioeng, 67, FAMILI, I., FORSTER, J., NIELSON, J. & PALSSON, B. O. (2003) Saccharomyces cerevisiae phenotypes can be predicted by using constraint-based analysis of a genome-scale reconstructed metabolic network. Proceedings of the National Academy of Sciences of the United States of America, 100, FORBERG, C., ELIAESON, T. & HAGGSTROM, L. (1988) Correlation of Theoretical and Experimental Yields of Phenylalanine from Non-Growing Cells of a Rec Escherichia- Coli Strain. Journal of Biotechnology, 7, FORSTER, J., FAMILI, I., FU, P., PALSSON, B. O. & NIELSEN, J. (2003) Genome-scale reconstruction of the Saccharomyces cerevisiae metabolic network. Genome Research, 13, HALDIMANN, A. & WANNER, B. L. (2001) Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol, 183, HIBI, M., YUKITOMO, H., ITO, M. & MORI, H. (2007) Improvement of NADPH-dependent bioconversion by transcriptome-based molecular breeding. Appl Environ Microbiol, 73, JARBOE, L. R., GRABAR, T. B., YOMANO, L. P., SHANMUGAN, K. T. & INGRAM, L. O. (2007) Development of ethanologenic bacteria. Adv Biochem Eng Biotechnol, 108, JIN, Y. S., CRUZ, J. & JEFFRIES, T. W. (2005) Xylitol production by a Pichia stipitis D- xylulokinase mutant. Applied Microbiology and Biotechnology, 68, KIM, J. H., HAN, K. C., KOH, Y. H., RYU, Y. W. & SEO, J. H. (2002a) Optimization of fedbatch fermentation for xylitol production by Candida tropicalis. J Ind Microbiol Biotechnol, 29,
41 KIM, M. D., JEUN, Y. S., KIM, S. G., RYU, Y. W. & SEO, J. H. (2002b) Comparison of xylitol production in recombinant Saccharomyces cerevisiae strains harboring XYL1 gene of Pichia stipitis and GRE3 gene of S-cerevisiae. Enzyme and Microbial Technology, 31, KIM, T. B., LEE, Y. J., KIM, P., KIM, C. S. & OH, D. K. (2004) Increased xylitol production rate during long-term cell recycle fermentation of Candida tropicalis. Biotechnol Lett, 26, KIM, T. B. & OH, D. K. (2003) Xylitol production by Candida tropicalis in a chemically defined medium. Biotechnol Lett, 25, KO, B. S., RHEE, C. H. & KIM, J. H. (2006) Enhancement of xylitol productivity and yield using a xylitol dehydrogenase gene-disrupted mutant of Candida tropicalis under fully aerobic conditions. Biotechnol Lett, 28, KWON, S. G., PARK, S. W. & OH, D. K. (2006) Increase of xylitol productivity by cell-recycle fermentation of Candida tropicalis using submerged membrane bioreactor. J Biosci Bioeng, 101, LEE, S. Y., KIM, H. U., PARK, J. H., PARK, J. M. & KIM, T. Y. (2009) Metabolic engineering of microorganisms: general strategies and drug production. Drug Discov Today, 14, LEE, W. J., RYU, Y. W. & SEO, J. H. (2000) Characterization of two-substrate fermentation processes for xylitol production using recombinant Saccharomyces cerevisiae containing xylose reductase gene. Process Biochemistry, 35, LEIF, H., SLED, V. D., OHNISHI, T., WEISS, H. & FRIEDRICH, T. (1995) Isolation and characterization of the proton-translocating NADH: ubiquinone oxidoreductase from Escherichia coli. Eur J Biochem, 230, LI, Z. J., CAI, L., WU, Q. & CHEN, G. Q. (2009) Overexpression of NAD kinase in recombinant Escherichia coli harboring the phbcab operon improves poly(3-hydroxybutyrate) production. Appl Microbiol Biotechnol, 83, LIN, H., BENNETT, G. N. & SAN, K. Y. (2005a) Chemostat culture characterization of Escherichia coli mutant strains metabolically engineered for aerobic succinate production: a study of the modified metabolic network based on metabolite profile, enzyme activity, and gene expression profile. Metab Eng, 7, LIN, H., BENNETT, G. N. & SAN, K. Y. (2005b) Metabolic engineering of aerobic succinate production systems in Escherichia coli to improve process productivity and achieve the maximum theoretical succinate yield. Metab Eng, 7, MARTINEZ, I., ZHU, J., LIN, H., BENNETT, G. N. & SAN, K. Y. (2008) Replacing Escherichia coli NAD-dependent glyceraldehyde 3-phosphate dehydrogenase (GAPDH) with a NADP-dependent enzyme from Clostridium acetobutylicum facilitates NADPH dependent pathways. Metab Eng, 10, NAKAMURA, C. E. & WHITED, G. M. (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotechnol, 14, NEIDHARDT, F. C. & CURTISS, R. (1996) Escherichia coli and Salmonella : cellular and molecular biology, Washington, D.C., ASM Press. NYYSSOLA, A., PIHLAJANIEMI, A., PALVA, A., VON WEYMARN, N. & LEISOLA, M. (2005) Production of xylitol from D-xylose by recombinant Lactococcus lactis. J Biotechnol, 118, OH, Y. J., LEE, T. H., LEE, S. H., OH, E. J., RYU, Y. W., KIM, M. D. & SEO, J. H. (2007) Dual modulation of glucose 6-phosphate metabolism to increase NADPH-dependent xylitol production in recombinant Saccharomyces cerevisiae. Journal of Molecular Catalysis B- Enzymatic, 47,
42 PARAJO, J. C., DOMINGUEZ, H. & DOMINGUEZ, J. M. (1998) Biotechnological production of xylitol. Part 1: Interest of xylitol and fundamentals of its biosynthesis. Bioresource Technology, 65, PHARKYA, P., BURGARD, A. P. & MARANAS, C. D. (2004) OptStrain: A computational framework for redesign of microbial production systems. Genome Research, 14, RAGHUNATHAN, A., REED, J., SHIN, S., PALSSON, B. & DAEFLER, S. (2009) Constraintbased analysis of metabolic capacity of Salmonella typhimurium during host-pathogen interaction. BMC Syst Biol, 3, 38. REED, J. L., VO, T. D., SCHILLING, C. H. & PALSSON, B. O. (2003) An expanded genomescale model of Escherichia coli K-12 (ijr904 GSM/GPR). Genome Biol, 4, R54. RESENDIS-ANTONIO, O., REED, J. L., ENCARNACION, S., COLLADO-VIDES, J. & PALSSON, B. O. (2007) Metabolic reconstruction and modeling of nitrogen fixation in Rhizobium etli. PLoS Comput Biol, 3, RODRIGUES, D. C., SILVA, S. S., PRATA, A. M. & FELIPE, M. D. G. (1998) Biotechnological production of xylitol from agroindustrial residues. Evaluation of bioprocesses. Appl Biochem Biotechnol, 70-72, SAMBROOK, J. & RUSSELL, D. W. (2001) Molecular cloning : a laboratory manual, Cold Spring Harbor, N.Y., Cold Spring Harbor Laboratory Press. SAN, K. Y., BENNETT, G. N., BERRIOS-RIVERA, S. J., VADALI, R. V., YANG, Y. T., HORTON, E., RUDOLPH, F. B., SARIYAR, B. & BLACKWOOD, K. (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng, 4, SANCHEZ, A. M., ANDREWS, J., HUSSEIN, I., BENNETT, G. N. & SAN, K. Y. (2006a) Effect of overexpression of a soluble pyridine nucleotide transhydrogenase (UdhA) on the production of poly(3-hydroxybutyrate) in Escherichia coli. Biotechnol Prog, 22, SANCHEZ, A. M., BENNETT, G. N. & SAN, K. Y. (2005) Effect of different levels of NADH availability on metabolic fluxes of Escherichia coli chemostat cultures in defined medium. J Biotechnol, 117, SANCHEZ, A. M., BENNETT, G. N. & SAN, K. Y. (2006b) Batch culture characterization and metabolic flux analysis of succinate-producing Escherichia coli strains. Metabolic Engineering, 8, SAUER, U., CANONACO, F., HERI, S., PERRENOUD, A. & FISCHER, E. (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem, 279, SCHILLING, C. H., COVERT, M. W., FAMILI, I., CHURCH, G. M., EDWARDS, J. S. & PALSSON, B. O. (2002) Genome-scale metabolic model of Helicobacter pylori Journal of Bacteriology, 184, SCHMID, A., DORDICK, J. S., HAUER, B., KIENER, A., WUBBOLTS, M. & WITHOLT, B. (2001) Industrial biocatalysis today and tomorrow. Nature, 409, SILVA, S. S., CHANTO, A. Q., VITOLO, M., FELIPE, M. G. & MANCILHA, I. M. (1999) A preliminary information about continuous fermentation using cell recycling for improving microbial xylitol production rates(scientific note). Appl Biochem Biotechnol, 77-79, SNYDER, L. & CHAMPNESS, W. (2007) Molecular genetics of bacteria, Washington, D.C., ASM Press. 22
43 SUTHERS, P. F., DASIKA, M. S., KUMAR, V. S., DENISOV, G., GLASS, J. I. & MARANAS, C. D. (2009) A genome-scale metabolic reconstruction of Mycoplasma genitalium, ips189. PLoS Comput Biol, 5, e SUZUKI, T., YOKOYAMA, S. I., KINOSHITA, Y., YAMADA, H., HATSU, M., TAKAMIZAWA, K. & KAWAI, K. (1999) Expression of xyra gene encoding for D- xylose reductase of Candida tropicalis and production of xylitol in Escherichia coli. Journal of Bioscience and Bioengineering, 87, TANG, X. M., TAN, Y. S., ZHU, H., ZHAO, K. & SHEN, W. (2009) Microbial Conversion of Glycerol to 1,3-Propanediol by an Engineered Strain of Escherichia coli. Applied and Environmental Microbiology, 75, TRAN, Q. H., BONGAERTS, J., VLAD, D. & UNDEN, G. (1997) Requirement for the protonpumping NADH dehydrogenase I of Escherichia coli in respiration of NADH to fumarate and its bioenergetic implications. Eur J Biochem, 244, VAN DER DONK, W. A. & ZHAO, H. (2003) Recent developments in pyridine nucleotide regeneration. Curr Opin Biotechnol, 14, VARMA, A. & PALSSON, B. O. (1994) Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl Environ Microbiol, 60, VERHO, R., LONDESBOROUGH, J., PENTTILA, M. & RICHARD, P. (2003) Engineering redox cofactor regeneration for improved pentose fermentation in Saccharomyces cerevisiae. Appl Environ Microbiol, 69, VERHO, R., RICHARD, P., JONSON, P. H., SUNDQVIST, L., LONDESBOROUGH, J. & PENTTILA, M. (2002) Identification of the first fungal NADP-GAPDH from Kluyveromyces lactis. Biochemistry, 41, WALTON, A. Z. & STEWART, J. D. (2002) An efficient enzymatic Baeyer-Villiger oxidation by engineered Escherichia coli cells under non-growing conditions. Biotechnol Prog, 18, WALTON, A. Z. & STEWART, J. D. (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog, 20, WECKBECKER, A. & HUMMEL, W. (2004) Improved synthesis of chiral alcohols with Escherichia coli cells co-expressing pyridine nucleotide transhydrogenase, NADP+dependent alcohol dehydrogenase and NAD+-dependent formate dehydrogenase. Biotechnol Lett, 26, WEIDNER, U., GEIER, S., PTOCK, A., FRIEDRICH, T., LEIF, H. & WEISS, H. (1993) The gene locus of the proton-translocating NADH: ubiquinone oxidoreductase in Escherichia coli. Organization of the 14 genes and relationship between the derived proteins and subunits of mitochondrial complex I. J Mol Biol, 233, WINKELHAUSEN, E., AMARTEY, S. A. & KUZMANOVA, S. (2004) Xylitol production from D-xylose at different oxygen transfer coefficients in a batch bioreactor. Engineering in Life Sciences, 4, YAN, Y. & LIAO, J. C. (2009) Engineering metabolic systems for production of advanced fuels. J Ind Microbiol Biotechnol, 36, YANG, Y. T., ARISTIDOU, A. A., SAN, K. Y. & BENNETT, G. N. (1999) Metabolic flux analysis of Escherichia coli deficient in the acetate production pathway and expressing the Bacillus subtilis acetolactate synthase. Metab Eng, 1, YUN, N. R., SAN, K. Y. & BENNETT, G. N. (2005) Enhancement of lactate and succinate formation in adhe or pta-acka mutants of NADH dehydrogenase-deficient Escherichia coli. J Appl Microbiol, 99,
44 ZHOU, S., SHANMUGAM, K. T., YOMANO, L. P., GRABAR, T. B. & INGRAM, L. O. (2006) Fermentation of 12% (w/v) glucose to 1.2 M lactate by Escherichia coli strain SZ194 using mineral salts medium. Biotechnology Letters, 28, ZHU, H. F. & SHIMIZU, K. (2005) Effect of a single-gene knockout on the metabolic regulation in Escherichia coli for D-lactate production under microaerobic condition. Metabolic Engineering, 7, ZHU, J. & SHIMIZU, K. (2004) The effect of pfl gene knockout on the metabolism for optically pure D-lactate production by Escherichia coli. Applied Microbiology and Biotechnology, 64,
45 Chapter 2 Engineering Escherichia coli for Xylitol Production from Glucose-Xylose Mixtures Portions of this chapter have been reprinted with permission from John Wiley and Sons (License number: ).
46 26 Abstract The range of value-added chemicals produced by Escherichia coli from simple sugars has been expanded to include xylitol. This was accomplished by screening the in vivo activity of a number of heterologous xylitol-producing enzymes. Xylose reductases from Candida boidinii (CbXR), Candida tenuis (CtXR), Pichia stipitis (PsXR), and Saccharmoyces cerivisiae (ScXR), and xylitol dehydrogenases from Gluconobacter oxydans (GoXDH) and Pichia stipitis (PsXDH) were all functional in E. coli to varying extents. The nicotinamide cofactor requirements for the enzymes tested vary from being NADPH-dependent and preferring NADPH over NADH (XR s) to requiring NADH (XDH s). Replacement of E. coli s native cyclic AMP receptor protein (CRP) with a cyclic AMP-independent mutant ( CRP* ) facilitated xylose uptake and xylitol production from mixtures of glucose and xylose, with glucose serving as the growth substrate and source of reducing equivalents. Of the enzymes tested, overexpression CbXR produced the highest concentrations of xylitol in shake-flask cultures (~275 mm in LB cultures, ~180 mm using minimal medium). Introduction Xylitol is a pentahydroxy sugar alcohol found in fruits and vegetables and having sweetness similar to that of sucrose (Parajo et al., 1998a, Pepper and Olinger, 1988). Xylitol has many favorable properties as a natural, nutritive sweetener and food additive. Most notably, xylitol is noncariogenic and even inhibits the development of dental caries and therefore is used in toothpastes and sugarless confectionaries. In humans, metabolism of this polyol is not insulinmediated, so xylitol serves as a sugar substitute for diabetics (Parajo et al., 1998a). Additional auspicious qualities include its large negative heat of dissolution (greater than other sugar
47 27 substitutes), resulting in a clean, refreshing sensation in the mouth, and its inability to contribute to Maillard-based food browning and caramelization, in contrast to carbonyl-containing sugar substitutes. Finally, xylitol can serve as a valuable synthetic building block and was recently identified as one of the top twelve value-added materials to be produced from biomass, thereby serving as a key economic driver for biorefineries (Werpy et al., 2004). Commercial processes for xylitol production primarily use catalytic hydrogenation (reduction) of D-xylose derived from hemicellulose-xylan hydrolysates of biomass materials such as birchwood and corn, where about 50-60% of the initial xylose is converted to xylitol (Melaja and Hamalainen, 1977, Nigam and Singh, 1995, Parajo et al., 1998a). Alternate, biological approaches to xylitol production are also being developed. Most reports involve the whole-cell production of xylitol from xylose using various natural or genetically modified yeast strains (Carvalho et al., 2002, Kastner et al., 2003, Kim et al., 2004, Kim and Oh, 2003, Lee et al., 2000, Nigam and Singh, 1995, Oh et al., 1998, Parajo et al., 1998b, Rivas et al., 2003, Roca et al., 1996, Vandeska et al., 1996, Winkelhausen and Kuzmanova, 1998, Yahashi et al., 1996), although in vitro biocatalytic processes requiring cofactor regeneration systems are also being developed (Jang et al., 2003, Nidetzky et al., 1996, Suzuki et al., 2002). The first two steps of D-xylose assimilation in yeasts involve xylose reduction to xylitol via xylose reductase (XR, also called aldose or aldehyde reductase; alditol:nad(p) + 1-oxidoreductase, EC ) followed by xylitol oxidation to xylulose via xylitol dehydrogenase (XDH, also called D-xylulose reductase, xylitol:nad + 2-oxidoreductase, EC ). XR typically prefers NADPH while XDH utilizes NAD +, and it is this propensity for cofactor imbalance in many strains which ultimately leads to xylitol secretion rather than continued metabolism. Although some bacteria are naturally capable of synthesizing xylitol (Parajo et al., 1998a, Suzuki et al., 2002, Yoshitake et al., 1973), few research efforts have focused on using bacteria for xylitol production. E. coli is an ideal organism for industrial production of chemicals due to
48 28 its ability to assimilate both hexose and pentose sugars, rapid growth rates, ease of manipulation, and inexpensive growth medium requirements, as evidenced by its industrial implementation for production of 1,3-propanediol (Nakamura and Whited, 2003) and 3-hydroxy-propionic acid (Cameron, 2005). This research is interested in engineering E. coli strains which serve as biocatalysts for efficient conversion of biomass-derived sugars (e.g., glucose and xylose) into value-added products. One embodiment of this research involves the use of E. coli as host of heterologous, NAD(P)H-dependent transformations, whereby central metabolism serves as the cofactor regeneration system. Simple sugars can therefore serve as inexpensive energy sources ( cosubstrates ) to drive these transformations. The present report studies the reduction of xylose to xylitol as the cofactor-dependent, heterologous reaction of interest, and describes steps taken toward engineering an efficient xylitol-producing E. coli strain. Because mixtures of glucose and xylose are the primary products of biomass hydrolysis (Hayn et al., 1993, Ingram et al., 1999), it was considered important to engineer strains capable of simultaneous glucose metabolism and xylitol production. Results from these and future studies should lead to engineered strains which efficiently produce sugar alcohols or catalyze other heterologous NAD(P)H-dependent transformations utilizing glucose as the energy source for cofactor regeneration. Materials and Methods General Table 2 1 lists the strains and plasmids used in this study. E. coli K-12 wild-type W3110 (ATCC 27325) and derivative strains were maintained on plates containing either Luria-Bertani (LB) medium or minimal medium containing mineral salts (per liter: 3.5 g of KH 2 PO 4 ; 5.0 g of K 2 HPO 4 ; 3.5 of g (NH 4 ) 2 HPO 4, 0.25 g of MgSO 4 :7 H 2 O, 15 mg of CaCl 2 :2 H 2 O, 0.5 mg of
49 29 thiamine, and 1 ml of trace metal stock), 20 g of glucose, and 15 g of agar. The trace metal stock was prepared as described (Causey et al., 2003). 4-Morpholinopropanesulfonic acid (MOPS) was added to liquid media for ph control (50 mm, ph 7.4), but was not included in ph-controlled fermentations. Antibiotics were included as appropriate (kanamycin monosulfate, 50 g ml -1 ; ampicillin, 50 g ml -1 ; apramycin sulfate, 50 g ml -1 ; and tetracycline hydrochloride, 12.5 g ml - 1 ) and isopropyl -D-thiogalactopyranoside (IPTG) (100 M) was used to induce protein production under tac promoter control. Xylitol, xylose, xylulose, glucose and organic acid concentrations were determined using a Shimadzu LC-10AD HPLC equipped with a UV monitor (210 nm) and refractive index detector (RID). Products were separated using a Bio-Rad HPX- 87H column (10 μl injection) with 4 mm H 2 SO 4 as the mobile phase (0.4 ml min -1, 45 o C). Table 2-1: Strains and plasmids used in this study a. Strains/Plasmids Relevant Characteristics Reference Strains W3110 wild type ATCC ET25 E. coli K-12, crp*::tn10 (Tet R ) (Eppler and Boos, 1999) PC05 W3110, crp*::tn10 (Tet R ) This study PC06 W3110, ΔxylB::FRT-aac-FRT (Apr R ) This study PC07 W3110, ΔxylB::FRT This study PC08 PC05, ΔxylB::FRT-aac-FRT (Tet R, Apr R ) This study PC09 PC05, ΔxylB::FRT (Tet R ) This study Plasmids ploi3809 kan, pbr322-origin vector for expression of XR or This study XDH, under control of tac promoter ploi3815 ploi3809 carrying CbXR gene This study ppcc04 ploi3809 carrying ScXR gene This study ppcc05 ploi3809 carrying CtXR gene This study ppcc06 ploi3809 carrying PsXDH gene This study ppcc07 ploi3809 carrying PsXR gene This study ppcc12 ploi3809 carrying GoXDH gene This study a Tet = tetracycline, Apr = apramycin, Kan = kanamycin, Amp = ampicillin
50 30 Genetic Methods Standard methods were used for plasmid construction, phage P1 transduction, electroporation, and polymerase chain reaction (PCR) (Miller, 1992, Sambrook and Russell, 2001). Strain ET25, carrying the crp* gene next to a Tn::10 marker was obtained from W. Boos (Eppler and Boos, 1999). The crp* gene was introduced into W3110 via P1 phage transduction using a lysate from strain ET25 followed by selection on tetracycline plates. The resulting strain was named PC05. The crp* phenotype was verified in two ways. First, several Tet R transductants were grown in LB broth containing glucose (1%) and xylose (1%). Mid log-phase cells were harvested and washed twice in phosphate buffer containing kanamycin (50 g ml -1 ). After allowing time for residual sugars to be cleared, the cells were resuspended a final time in buffer containing xylose (1%), kanamycin, and 1% triphenyltetrazolium chloride (TTC). Reduction of TTC results in red color formation and indicates constitutive xylose utilization. The crp* phenotype was additionally confirmed using HPLC to verify simultaneous glucose and xylose consumption in batch cultures, as described in the text. Disruption of the xylulokinase gene (xylb) was accomplished using previously described methods (Causey et al., 2003, Datsenko and Wanner, 2000, Martinez-Morales et al., 1999, Posfai et al., 1997). Briefly, the xylb gene was amplified from W3110 genomic DNA using Taq DNA polymerase (New England Biolabs, Ipswich, MA) and Sigma-Genosys xylb ORFmers as primers. The resulting PCR fragment was TOPO-cloned into vector pcr2.1-topo (Invitrogen, Carlsbad, CA). A 240-bp fragment within the xylb gene was removed by digestion with BsiWI followed by Klenow fill-in. Next, a 1956-bp SmaI fragment containing an apramycin resistance gene (aac) flanked by FRT flipase recognition sequences (isolated from lab plasmid ploi3421) was ligated in place of the deleted xylb fragment. The corresponding ligation product (plasmid ploi3807) therefore contains the sequence xylb -FRT-aac-FRT-xylB. This sequence was
51 31 PCR-amplified using the xylb ORFmers as primers, and the PCR product was electroporated into E. coli W3110 expressing Red recombinase from plasmid pkd46 (Datsenko and Wanner, 2000). Apramycin-resistant colonies arising from homologous recombination of the xylb deletion construct were selected and verified by PCR. Strain PC06 (W3110, xylb::frt-aac-frt) was used for moving the xylb deletion into other strains (e.g., PC05) by P1 phage transduction. FRTflanked antibiotic resistance was deleted as described (Causey et al., 2003). All reductase and dehydrogenase genes listed in Table 2 2 were amplified using highfidelity polymerases (Pfu, Stratagene) and primers containing appropriate restriction sites for ligation into a multiple cloning site directly downstream of a tac promoter and upstream of a transcription termination sequence in plasmid ploi3809. All genes were cloned to contain the same sequence upstream of the start codon: AGGAGGACAGCTatg (Shine Delgarno sequence is underlined). Primer and plasmid sequences are available upon request. The source of DNA used to amplify each gene was as follows: GoXDH: genomic DNA prep of Gluconobacter oxydans (ATCC 621); GRE3 (ScXR): genomic prep of Baker s yeast; CtXR: genomic DNA prep of Candida tenuis (CBS 4435); PsXDH and PsXR: plasmids pcr2.1-topo- PSXYL2 and pcr2.1-topo-psxyl2, respectively, which were the gift of T.W. Jeffries (Jin and Jeffries, 2003); CbXR: plasmid prs424-cbar, another gift of T.W. Jeffries (Kang et al., 2003). Upon sequencing, one discrepancy was noted in the amino acid sequence of GoXDH compared to that reported (Sugiyama et al., 2003): Thr159 rather than Ala159 (this was verified by sequencing products from two separate PCR reactions). In addition, an error in primer synthesis resulted in a point mutation (Thr6Ala) in the ScXR (GRE3) amino acid sequence. Table 2-2: Xylose reductase (XR) and xylitol dehydrogenase (XDH) enzymes used. Enzyme Reductases: Xylose Xylitol Name used in this study Cofactor usage (K m, M) Reference
52 Enzyme Name used in this study Cofactor usage (K m, M) Reference (Bolen and Candida boidinii XYL1 CbXR NADPH (3-4) Mccracken, 1990, Kang et al., 2003) (Ford and Ellis, Saccharomyces cerevisiae GRE3 ScXR NADPH (7.6) 2001, Jeong et al., 2001) Candida tenuis XYL1 CtXR (Hacker et al., 1999, NADPH (4.8) > Neuhauser et al., NADH (25) 1997) Pichia stipitis XYL1 PsXR Dehydrogenases: Xylulose Xylitol NADPH (9) > NADH (21) 32 (Hallborn et al., 1991) Gluconobacter oxydans XDH GoXDH NADH a (Sugiyama et al., 2003) (Kotter et al., 1990, Pichia stipitis XYL2 PsXDH NADH (72) Rizzi et al., 1989) a Kinetic parameters have not been reported and were not determined. Shake-Flask Cultures All cultures were performed in duplicate or triplicate, and all data points reported are the average of at least two experiments. Shake flask cultures for xylitol production contained 50 ml medium in 250-ml baffled flasks and were grown at 30 o C and 250 rpm. All LB and minimal medium cultures were inoculated to an initial OD 550 of 0.1 from seed cultures of the same medium. Seed cultures were prepared by inoculating with a few colonies from a fresh plate (LB plates for LB cultures, minimal medium plates containing 2% glucose for minimal medium cultures) into 3 ml of medium (13 x 100 mm tube). Seeds were grown to an OD 550 of , and shake flask cultures were inoculated directly from the seed cultures by dilution to a final OD 550 of 0.1. Enzyme expression was induced with 100 M IPTG in shake flasks at the time of inoculation. This concentration of IPTG results in near-maximal induction.
53 33 Results Strain Construction and Characterization Figure 2 1 depicts the mechanisms for xylose uptake and metabolism in E. coli. Xylose uptake occurs primarily through either a high-affinity ABC transporter (XylFGH) or a lowaffinity proton symporter (XylE) (Neidhardt and Curtiss, 1996), although studies suggest that XylE has little activity even under high xylose concentrations (50 mm) (Hasona et al., 2004). As a result of cyclic AMP receptor protein (CRP)-dependent control of xyl genes (Song and Park, 1997), E. coli exhibits diauxic growth characteristics such that glucose is preferentially assimilated before xylose (Hasona et al., 2004, Hernandez-Montalvo et al., 2001, Song and Park, 1997, Stulke and Hillen, 1999). Most E. coli strains (including K-12) do not naturally synthesize or metabolize xylitol, although mutants have been isolated which are capable of converting xylitol to D-xylose using an oxidoreductase (Wu, 1976). Xylitol production should be possible either by expression of XR for direct reduction of xylose, or expression of the reversible XDH for reduction of xylulose to xylitol (see Figure 2 1). Figure 2-1: Xylose uptake and metabolism into the pentose phosphate pathway (PPP) in E. coli, and options for xylitol production via heterologous XDH or XR.
54 34 The initial studies involved expression of an XDH cloned from Gluconobacter oxydans (Sugiyama et al., 2003) in E. coli W3110 and its derivative PC07, containing a xylb (xylulokinase gene) deletion. Low concentrations of xylitol were produced in LB broth containing xylose alone, xylose plus sorbitol, and xylose plus glucose. For further studies strain PC09 was developed, constitutive in xylose metabolism due to the replacement of the native crp gene with a mutant gene (corresponding to three amino acid substitutions) encoding a camp-independent CRP variant (denoted CRP* or CRP-in ) (Eppler and Boos, 1999). The CRP* phenotype should promote xylose uptake in the presence of glucose by activating the native xylose transporters and/or by activating other CRP-controlled promiscuous transporters capable of xylose uptake (particularly when xylose concentrations are high (>50 mm)). PC09 additionally contains a deletion in xylb, preventing metabolism of xylose while still allowing conversion of xylose to xylulose for XDH-driven xylitol production. To verify elimination of diauxic growth by crp* strains, W3110, PC05, PC07 and PC09 (refer to Table 2 1) were grown in glucose-xylose mixtures and sugar concentrations were monitored during growth. Figure 2 2 shows the concentrations of xylose and glucose consumed after 10 hours of growth in batch cultures containing LB supplemented with 100 mm each of glucose and D-xylose and 100 mm MOPS buffer. Whereas xylose uptake in the presence of glucose is negligible for strains W3110 and PC07, strain PC05 (crp*) metabolizes both sugars simultaneously. Deletion of xylb from PC05 results in PC09, which is unable to metabolize xylose. Growth curves for these LB batch cultures are shown in Figure 2 3. Similar results were obtained when these strains were grown in minimal medium containing glucose and xylose, and when L-arabinose was used in place of xylose (although higher background levels of arabinose consumption occurred in wild-type crp strains (data not shown). PC05 grows slower than W3110, although growth is recovered to nearly that of W3110 upon deletion of xylb, indicating that xylose metabolism is partly responsible for reduced growth in PC05. Deletion of xylb in
55 Concentration (mm) W3110 (resulting in PC07) had no effect on growth, since xylose is not metabolized in the presence of glucose in the wild-type strain Glucose Consumed Xylose Consumed Xylulose Produced W3110 PC05 PC07 PC09 Figure 2-2: Effects of crp* and xylb on growth. Results are for 50-ml shake flask cultures grown at 37 o C and containing LB medium supplemented with 100 mm each of glucose, xylose and MOPS buffer. PC05 and PC09 express the mutant CRP* protein; the xylb gene is deleted in PC07 and PC09. Glucose and xylose consumption after 8 hours of growth OD W PC05 1 PC07 PC Time (h) (b) Figure 2-3: Effects of crp* and xylb on growth. Results are for 50-ml shake flask cultures grown at 37 o C and containing LB medium supplemented with 100 mm each of glucose, xylose and MOPS buffer. PC05 and PC09 express the mutant CRP* protein; the xylb gene is deleted in PC07 and PC09. Growth curves (monitoring turbidity (OD 550 ) over time).
56 36 Xylitol Production in Strain PC09 Expressing XR s and XDH s The enzymes listed in Table 2 2 were expressed in E. coli and tested for in vivo activity. These enzymes were selected because each has been previously characterized in some fashion (see references in Table 2 2) and it was felt that they comprise a representative group of enzymes with a spectrum of cofactor preferences. All genes tested were cloned in the same manner such that they are all maintained on a medium-copy vector (ploi3809) under the control of a tac promoter with identical Shine-Delgarno sequences (AGGAGGA). Plasmids ploi3815, ppcc04, ppcc05, ppcc06, ppcc07 and ppcc12 were each transformed into strain PC09 and the transformed strains were tested for xylitol production in LB or minimal medium containing glucose plus xylose under a variety of batch culture conditions. All xylitol-producing enzymes were functional at 30 o C, whereas only GoXDH, PsXDH and ScXR produced significant concentrations of xylitol at 37 o C. In agreement with these observations, Hacker et al. expressed CtXR in E. coli and noted significant inclusion body formation at 37 o C, whereas only 10-15% of the total expressed protein was present as insoluble material at 27 o C (Hacker et al., 1999). Differences in stability/activity at different temperatures also depended on whether the cells were grown in rich or minimal medium. All data reported below was collected at 30 o C. Xylitol production at 37ºC was significantly lower than that observed at 30ºC (data not reported). Figure 2 4 shows the xylitol production profiles from 50-ml batch shake-flask cultures in LB medium supplemented with 100 mm glucose plus 300 mm xylose and buffered with 50 mm MOPS. For simplification, the enzyme names are listed in the legend rather than the corresponding plasmids used. Also given in the legend next to each name is the average final density (OD 550 ) for each culture. Xylose consumption corresponded to xylitol production, with small differences between these values resulting from xylulose secretion. Cultures expressing the xylose reductase from Candida boidinii (CbXR) consistently produced the highest concentrations
57 37 of xylitol in shake flasks (~275 mm). Clones expressing ScXR and CtXR were generally not consistent: strain performance was clone-dependent, requiring multiple transformants to be screened to isolate an active clone. Strains which produced significant amounts of xylitol (CbXR, ScXR) also consumed all 100 mm glucose and secreted very little acid (less than 5 mm). In contrast, poor xylitol producers generally secreted high concentrations of acids (primarily acetic acid) resulting in lowered ph (~ ) in spite of the MOPS buffer and ultimately preventing complete glucose utilization. For example, the average final ph for the PsXR cultures was ~5.6, with 66 ± 17 mm acetic acid secreted from 84 ± 16 mm glucose consumed. Figure 2-4: Xylitol production in 50-mL shake-flask cultures of strain PC09 expressing the enzymes tested in this study. Refer to Table I for the corresponding plasmid used to express each enzyme. Cultures contained xylose (300 mm), glucose (100 mm), MOPS (50 mm), kanamycin (50 g ml -1 ), IPTG (100 M). Average final culture OD s are given in parentheses. Data points represent the average of at least two values.
58 38 All enzymes in strain PC09 were also conditioned to growth on minimal glucose plates and screened for xylitol production in shake flasks containing minimal medium with 100 mm glucose and 300 mm xylose. The relative activities of strains expressing these enzymes in minimal medium were similar to those in LB, with the exception of ScXR and CtXR. ScXR clones consistently produced very little xylitol in minimal medium, while the activity of CtXR was comparable to that of CbXR: PC09+pLOI3815 (CbXR) produced ~180 mm xylitol, with an average final culture OD 550 of 10.4, while PC09+pPCC05 (CtXR) produced ~170 mm xylitol, with a final culture OD 550 of Plasmid ploi3815 was also tested in W3110 and compared to PC09 for xylitol production. Shake flask cultures (50-ml) containing LB or minimal medium and including glucose (100 mm) plus xylose (300 mm) or xylose alone (300 mm) were studied. Results for the LB cultures are shown in Figure 2 5. In the presence of the sugar mixture, PC09 is clearly beneficial over W3110. W3110 expressing CbXR did produce low concentrations of xylitol (~58 mm) in the sugar mixture, implying low levels of xylose transport are possible even in the presence of glucose (however xylose is not metabolized, as shown in Figure 2 2). Similar to the results noted above for poor xylitol-producing strains, the W3110 culture with glucose secreted large amounts of acetic acid (~70 mm), ultimately dropping the ph and preventing further growth. Interestingly, CbXR expression in W3110 inhibits xylose metabolism, and very little xylose was consumed in the culture containing LB plus xylose. Likewise, using minimal medium with xylose as the only carbon source, the presence of CbXR in W3110 (as well as in PC05) severely inhibited xylose metabolism and cell growth and xylitol production was drastically reduced (~11 mm xylitol, final OD 550 ~4.4 after 96 hours of growth). In contrast, W3110 harboring ploi3815 in the absence of IPTG grew rapidly on xylose. A further study reports that the growth and xylitol production deficiency is due to the phosphorylation of xylitol by the xylulokinase (XylB) enzyme (Akinterinwa and Cirino, 2009).
59 Xylitol (mm) PC09, X+G (8.0) W3110, X (11.2) W3110, X+G (3.6) Time (h) Figure 2-5: Xylitol production by W3110 and PC09 expressing CbXR in shake-flask cultures containing LB plus glucose ( G, 100 mm) and/or xylose ( X, 300 mm). Average final culture OD s are given in parentheses. Cofactor Utilization Analysis In order to improve whole-cell production of sugar alcohols such as xylitol, it is important to know which metabolic pathways are responsible for reduced cofactor (NAD(P)H) regeneration and how these cofactors are partitioned for re-oxidation. One parameter of interest is the yield of (reduced) product per glucose consumed, which is defined as Y RPG. Note that this yield term is different from the typical fermentation yield because glucose carbon does not contribute to xylitol carbon, and xylose does not contribute to metabolic energization to fuel xylitol production. With zero growth, the theoretical maximum value of Y RPG is debatably between 10 and 12, depending on a number of factors including the relative participation of E. coli s two transhydrogenases to cofactor supply (Sauer et al., 2004) and whether the reaction of interest requires NADH or NADPH. CbXR not only produced the highest concentrations of xylitol, it also resulted in the highest Y RPG values in batch culture. In the minimal medium shake flask cultures, consumption of 100 mm glucose resulted in production of ~180 mm xylitol, a Y RPG of 1.8.
60 40 Discussion Lignocellulosic residues from plant biomass are largely composed of carbohydrate polymers which can be converted into sugar mixtures consisting primarily of glucose and xylose. This research therefore chose to engineer an E. coli strain which could process glucose-xylose mixtures into xylitol, where the reducing equivalents required to reduce xylose are derived from glucose. The in vivo activity of several different XR and XDH enzymes expressed in E. coli were compared in order to identify a suitable system for further engineering of xylitol production by this organism. This work demonstrates the biocatalytic potential of crp* E. coli strains for conversion of sugar mixtures containing glucose into value-added products. In addition to expressing the known xylose transporters (XylE and XylFGH), CRP* should also increase expression of other transporters capable of allowing xylose uptake (particularly in the presence of the high concentrations used in these studies). Carbohydrate transporters are known to exhibit promiscuous substrate specificity, and the mannose phosphotransferase (pts) systems of Lactobacillus sp have been shown to transport xylose by facilitated diffusion (concentrationdependent uptake) (Chaillou et al., 1999). Deletion of the phosphotransferase (pts) system has also proven useful for relieving glucose repression and eliminating diauxie (Gosset, 2005, Hernandez-Montalvo et al., 2001, Nichols et al., 2001), although increased expression of a secondary glucose transporter (either heterologously or by mutation) as well as glucokinase is required to recover growth deficits in the pts mutant strain (Gosset, 2005, Hernandez-Montalvo et al., 2001, Snoep et al., 1994). Microbial xylitol production using yeasts has been extensively studied (Nigam and Singh, 1995, Parajo et al., 1998b, Winkelhausen and Kuzmanova, 1998). Kim et al. (Kim et al., 2002) optimized xylitol production using Candida tropicalis for fed-batch fermentation of commercial sugars, reporting rates of 3.9 g liter -1 h -1, and 187 g liter -1 total xylitol, although complex medium
61 was used for cell growth. Kim and Oh used C. tropicalis in fed-batch fermentation and report production of up to 237 g liter -1 xylitol from xylose in chemically defined media containing urea 41 and several vitamin supplements (Kim and Oh, 2003). Numerous other reports of similar productivities are reviewed in the above references, and while it is difficult to compare economic feasibilities between these processes and yeast strains, a general limitation is the requirement for complex media. The initial results suggest that xylitol production and productivity (27 g liter -1, 0.28 g liter -1 h -1 ) from E. coli using a minimal medium containing xylose and glucose as cosubstrate is a viable competing technology, although significant improvements through metabolic engineering and process optimization are necessary. In shake-flask cultures, xylose reduction promoted further oxidation of glucose carbon and reduced acid secretion. This was attributed to increased flux through the TCA cycle as a result of higher rates of NAD(P) + regeneration and lower total NAD(P)H concentrations. Importantly, glucose-controlled catabolite repression of TCA cycle genes should also be alleviated due to the CRP* phenotype (Neidhardt and Curtiss, 1996). It is interesting to note that CbXR has the highest reported affinities for both xylose (K m ~13 mm (Bolen and Mccracken, 1990)) and cofactor (K m(nadph) ~3-4 M (Kang et al., 2003)), of the reductases tested. However, the intracellular concentrations of xylose (and xylulose) in these studies are not known, nor are the relative functional expression levels, or endogenous kinetics of the enzymes tested, making it difficult to speculate why expression of CbXR results in the most productive strain. Cost-effective, continuous delivery of reduced cofactors remains an important challenge in biocatalysis. While the use of whole-cells can have many advantages, the efficiency with which glucose or other sugars are utilized as cosubstrates can be improved considerably. Walton and Stewart expressed an NADPH-dependent short-chain dehydrogenase from S. cerevisiae in E. coli to reduce ethyl acetoacetate under glucose-fed, non-growing conditions, and they report the stoichiometry linking ketone reduction and glucose consumption (i.e., Y RPG ) to
62 42 be ~2.3 (Walton and Stewart, 2004). Walton s results led them to conclude that glucose is primarily channeled through the TCA cycle and therefore the majority of glucose consumed yields two NADPH per glucose. However, significant flux through the PPP as well as through the membrane-bound transhydrogenase (PntAB) in the direction of NADPH formation during growth on glucose has recently been demonstrated (Sauer et al., 2004). A strict NADPH requirement by an enzyme (e.g., reductase) therefore does not imply that its pool of available reducing equivalents is limited to NADPH produced directly through the PPP or TCA cycle (or other NADP + -dependent reactions). With the exception of possible energetic losses associated with proton translocation during NADPH synthesis by PntAB, there is no obvious benefit to employing an enzyme preferring one cofactor over another (NADH or NADPH) when using E. coli to host whole-cell transformations when glucose serves as co-substrate. In agreement with this line of reasoning, CtXR (which utilizes both NADH and NADPH) produced similar amounts of xylitol as CbXR in minimal medium (173 mm xylitol in 96 hours). Conclusion From the initial tests, it was observed that a double mutant strain, containing a crp* ΔxylB mutation (strain PC09) was suited well for simultaneous glucose and xylose consumption and subsequent xylitol production and will be used as the reference strain for this research project. Using strain PC09, it was found that overexpressing an NADPH-dependent xylose reductase from C. boidinii resulted in the highest production of xylitol compared to the other heterologous enzymes tested, and will be used as the working enzyme for further studies.
63 43 References AKINTERINWA, O. & CIRINO, P. C. (2009) Heterologous expression of D-xylulokinase from Pichia stipitis enables high levels of xylitol production by engineered Escherichia coli growing on xylose. Metab Eng, 11, BOLEN, P. L. & MCCRACKEN, D. A. (1990) Properties of Aldose Reductase from the Methanol Yeast Candida-Boidinii. Journal of Fermentation and Bioengineering, 69, CAMERON, D. C. (2005) 3-Hydroxypropionic acid: A new platform chemical from the biorefinery. Tenth Annual Meeting, Institute of Biological Engineering. Athens, Georgia. CARVALHO, W., SILVA, S. S., CONVERTI, A. & VITOLO, M. (2002) Metabolic behavior of immobilized Candida guilliermondii cells during batch xylitol production from sugarcane bagasse acid hydrolyzate. Biotechnol Bioeng, 79, CAUSEY, T. B., ZHOU, S., SHANMUGAM, K. T. & INGRAM, L. O. (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc Natl Acad Sci U S A, 100, CHAILLOU, S., POUWELS, P. H. & POSTMA, P. W. (1999) Transport of D-xylose in Lactobacillus pentosus, Lactobacillus casei, and Lactobacillus plantarum: evidence for a mechanism of facilitated diffusion via the phosphoenolpyruvate:mannose phosphotransferase system. J Bacteriol, 181, DATSENKO, K. A. & WANNER, B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A, 97, EPPLER, T. & BOOS, W. (1999) Glycerol-3-phosphate-mediated repression of malt in Escherichia coli does not require metabolism, depends on enzyme IIAGlc and is mediated by camp levels. Mol Microbiol, 33, FORD, G. & ELLIS, E. M. (2001) Three aldo-keto reductases of the yeast Saccharomyces cerevisiae. Chem Biol Interact, , GOSSET, G. (2005) Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate:sugar phosphotransferase system. Microb Cell Fact, 4, 14. HACKER, B., HABENICHT, A., KIESS, M. & MATTES, R. (1999) Xylose utilisation: cloning and characterisation of the Xylose reductase from Candida tenuis. Biol Chem, 380, HALLBORN, J., WALFRIDSSON, M., AIRAKSINEN, U., OJAMO, H., HAHN-HAGERDAL, B., PENTTILA, M. & KERASNEN, S. (1991) Xylitol production by recombinant Saccharomyces cerevisiae. Biotechnology (N Y), 9, HASONA, A., KIM, Y., HEALY, F. G., INGRAM, L. O. & SHANMUGAM, K. T. (2004) Pyruvate formate lyase and acetate kinase are essential for anaerobic growth of Escherichia coli on xylose. J Bacteriol, 186, HAYN, M., STEINER, W., KLINGER, R., STEINMULLER, H., SINNER, M. & ESTERBAUER, H. (1993) Basic research and pilot studies on the enzymatic conversion of lignocellulosics. IN SADDLER, J. (Ed.) Bioconcersion of forest and agricultural plant residues. Wallingford, CAB International. HERNANDEZ-MONTALVO, V., VALLE, F., BOLIVAR, F. & GOSSET, G. (2001) Characterization of sugar mixtures utilization by an Escherichia coli mutant devoid of the phosphotransferase system. Appl Microbiol Biotechnol, 57, INGRAM, L. O., ALDRICH, H. C., BORGES, A. C., CAUSEY, T. B., MARTINEZ, A., MORALES, F., SALEH, A., UNDERWOOD, S. A., YOMANO, L. P., YORK, S. W.,
64 ZALDIVAR, J. & ZHOU, S. (1999) Enteric bacterial catalysts for fuel ethanol production. Biotechnol Prog, 15, JANG, S. H., KANG, H. Y., KIM, G. J., SEO, J. H. & RYU, Y. W. (2003) Complete In vitro conversion of D-xylose to xylitol by coupling xylose reductase and formate dehydrogenase. Journal of Microbiology and Biotechnology, 13, JEONG, E. Y., SOPHER, C., KIM, I. S. & LEE, H. (2001) Mutational study of the role of tyrosine-49 in the Saccharomyces cerevisiae xylose reductase. Yeast, 18, JIN, Y. S. & JEFFRIES, T. W. (2003) Changing flux of xylose metabolites by altering expression of xylose reductase and xylitol dehydrogenase in recombinant Saccharomyces cerevisiae. Appl Biochem Biotechnol, , KANG, M. H., NI, H. & JEFFRIES, T. W. (2003) Molecular characterization of a gene for aldose reductase ( CbXYL1) from Candida boidinii and its expression in Saccharomyces cerevisiae. Appl Biochem Biotechnol, , KASTNER, J. R., EITEMAN, M. A. & LEE, S. A. (2003) Effect of redox potential on stationaryphase xylitol fermentations using Candida tropicalis. Appl Microbiol Biotechnol, 63, KIM, J. H., HAN, K. C., KOH, Y. H., RYU, Y. W. & SEO, J. H. (2002) Optimization of fedbatch fermentation for xylitol production by Candida tropicalis. J Ind Microbiol Biotechnol, 29, KIM, T. B., LEE, Y. J., KIM, P., KIM, C. S. & OH, D. K. (2004) Increased xylitol production rate during long-term cell recycle fermentation of Candida tropicalis. Biotechnol Lett, 26, KIM, T. B. & OH, D. K. (2003) Xylitol production by Candida tropicalis in a chemically defined medium. Biotechnol Lett, 25, KOTTER, P., AMORE, R., HOLLENBERG, C. P. & CIRIACY, M. (1990) Isolation and Characterization of the Pichia-Stipitis Xylitol Dehydrogenase Gene, Xyl2, and Construction of a Xylose-Utilizing Saccharomyces-Cerevisiae Transformant. Current Genetics, 18, LEE, W. J., RYU, Y. W. & SEO, J. H. (2000) Characterization of two-substrate fermentation processes for xylitol production using recombinant Saccharomyces cerevisiae containing xylose reductase gene. Process Biochemistry, 35, MARTINEZ-MORALES, F., BORGES, A. C., MARTINEZ, A., SHANMUGAM, K. T. & INGRAM, L. O. (1999) Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons used during construction. J Bacteriol, 181, MELAJA, A. & HAMALAINEN, L. (1977) Process for making xylitol. United States. MILLER, J. H. (1992) A short course in bacterial genetics : a laboratory manual and handbook for Escherichia coli and related bacteria, Plainview, N.Y., Cold Spring Harbor Laboratory Press. NAKAMURA, C. E. & WHITED, G. M. (2003) Metabolic engineering for the microbial production of 1,3-propanediol. Curr Opin Biotechnol, 14, NEIDHARDT, F. C. & CURTISS, R. (1996) Escherichia coli and Salmonella : cellular and molecular biology, Washington, D.C., ASM Press. NEUHAUSER, W., HALTRICH, D., KULBE, K. D. & NIDETZKY, B. (1997) NAD(P)Hdependent aldose reductase from the xylose-assimilating yeast Candida tenuis - Isolation, characterization and biochemical properties of the enzyme. Biochemical Journal, 326,
65 NICHOLS, N. N., DIEN, B. S. & BOTHAST, R. J. (2001) Use of catabolite repression mutants for fermentation of sugar mixtures to ethanol. Applied Microbiology and Biotechnology, 56, NIDETZKY, B., NEUHAUSER, W., HALTRICH, D. & KULBE, K. D. (1996) Continuous enzymatic production of xylitol with simultaneous coenzyme regeneration in a charged membrane reactor. Biotechnology and Bioengineering, 52, NIGAM, P. & SINGH, D. (1995) Processes for Fermentative Production of Xylitol - a Sugar Substitute. Process Biochemistry, 30, OH, D. K., KIM, S. Y. & KIM, J. H. (1998) Increase of xylitol production rate by controlling redox potential in Candida parapsilosis. Biotechnology and Bioengineering, 58, PARAJO, J. C., DOMINGUEZ, H. & DOMINGUEZ, J. M. (1998a) Biotechnological production of xylitol. Part 1: Interest of xylitol and fundamentals of its biosynthesis. Bioresource Technology, 65, PARAJO, J. C., DOMINGUEZ, H. & DOMINGUEZ, J. M. (1998b) Biotechnological production of xylitol. Part 2: Operation in culture media made with commercial sugars. Bioresource Technology, 65, PEPPER, T. & OLINGER, P. M. (1988) Xylitol in Sugar-Free Confections. Food Technology, 42, 98-&. POSFAI, G., KOOB, M. D., KIRKPATRICK, H. A. & BLATTNER, F. R. (1997) Versatile insertion plasmids for targeted genome manipulations in bacteria: Isolation, deletion, and rescue of the pathogenicity island LEE of the Escherichia coli O157:H7 genome. Journal of Bacteriology, 179, RIVAS, B., TORRE, P., DOMINGUEZ, J. M., PEREGO, P., CONVERTI, A. & PARAJO, J. C. (2003) Carbon material and bioenergetic balances of xylitol production from corncobs by Debaryomyces hansenii. Biotechnology Progress, 19, RIZZI, M., HARWART, K., BUITHANH, N. A. & DELLWEG, H. (1989) A Kinetic-Study of the Nad+-Xylitol-Dehydrogenase from the Yeast Pichia-Stipitis.6. Journal of Fermentation and Bioengineering, 67, ROCA, E., MEINANDER, N. & HAHNHAGERDAL, B. (1996) Xylitol production by immobilized recombinant Saccharomyces cerevisiae in a continuous packed-bed bioreactor. Biotechnology and Bioengineering, 51, SAMBROOK, J. & RUSSELL, D. W. (2001) Molecular cloning : a laboratory manual, Cold Spring Harbor, N.Y., Cold Spring Harbor Laboratory Press. SAUER, U., CANONACO, F., HERI, S., PERRENOUD, A. & FISCHER, E. (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem, 279, SNOEP, J. L., ARFMAN, N., YOMANO, L. P., FLIEGE, R. K., CONWAY, T. & INGRAM, L. O. (1994) Reconstruction of glucose uptake and phosphorylation in a glucose-negative mutant of Escherichia coli by using Zymomonas mobilis genes encoding the glucose facilitator protein and glucokinase. J Bacteriol, 176, SONG, S. G. & PARK, C. (1997) Organization and regulation of the D-xylose operons in Escherichia coli K-12: XylR acts as a transcriptional activator. Journal of Bacteriology, 179, STULKE, J. & HILLEN, W. (1999) Carbon catabolite repression in bacteria. Current Opinion in Microbiology, 2, SUGIYAMA, M., SUZUKI, S., TONOUCHI, N. & YOKOZEKI, K. (2003) Cloning of the xylitol dehydrogenase gene from Gluconobacter oxydans and improved production of xylitol from D-arabitol. Bioscience Biotechnology and Biochemistry, 67,
66 SUZUKI, S., SUGIYAMA, M., MIHARA, Y., HASHIGUCHI, K. & YOKOZEKI, K. (2002) Novel enzymatic method for the production of xylitol from D-arabitol by Gluconobacter oxydans. Biosci Biotechnol Biochem, 66, VANDESKA, E., AMARTEY, S., KUZMANOVA, S. & JEFFRIES, T. W. (1996) Fed-batch culture for xylitol production by Candida boidinii. Process Biochemistry, 31, WALTON, A. Z. & STEWART, J. D. (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog, 20, WERPY, T., PETERSON, G., ADEN, A., BOZELL, J., HOLLADAY, J., WHITE, J. & A., M. (2004) Top value added chemicals from biomass, Volume I: Results of screening for potential candidates from sugars and synthesis gas, U.S. Department of Energy. WINKELHAUSEN, E. & KUZMANOVA, S. (1998) Microbial conversion of D-xylose to xylitol. Journal of Fermentation and Bioengineering, 86, WU, T. T. (1976) Growth of a Mutant of Escherichia-Coli K-12 on Xylitol by Recruiting Enzymes for D-Xylose and L1,2-Propanediol Metabolism. Biochimica Et Biophysica Acta, 428, YAHASHI, Y., HORITSU, H., KAWAI, K., SUZUKI, T. & TAKAMIZAWA, K. (1996) Production of xylitol from D-xylose by Candida tropicalis: The effect of D-glucose feeding. Journal of Fermentation and Bioengineering, 81, YOSHITAKE, J., ISHIZAKI, H., SHIMAMURA, M. & IMAI, T. (1973) Xylitol Production by an Enterobacter Species. Agricultural and Biological Chemistry, 37,
67 Chapter 3 Optimization of Resting Cell Parameters for Growth-Uncoupled Product Formation Portions of this chapter have been reprinted with permission from John Wiley and Sons (License numbers: and ).
68 48 Abstract Use of whole cells offers the opportunity to regenerate reduced cofactors that are used to drive heterologous redox reactions of interest. In this scenario, metabolism of a sugar serves as a means of generating reducing power in the form of NAD(P)H, with a theoretical maximum of 12 per glucose. This theoretical maximum is never achieved, indicating that there is significant room for improvement in the efficiency with which glucose or other sugars are utilized as cosubstrates for providing the reducing power required to drive heterologous reactions of interest (i.e., the quantity of reducing equivalents resulting from cosubstrate oxidation that are actually used in the reaction of interest can be improved). In this study, the use of non-growing, metabolically-active resting cells is examined as a means of improving xylitol yield (Y RPG, moles xylitol produced per mole glucose consumed). An increase of Y RPG in resting cells compared to batch cultures (~4.2 and ~1.8 respectively) was observed. By altering various conditions and parameters of resting cells, a further increase the xylitol Y RPG while limiting fermentation product secretion (e.g. lactate, acetate, and ethanol) was able to be achieved. Introduction By eliminating growth as a drain for reducing equivalents, various groups have demonstrated an improvement in the bioproduction of a desired product (Cirino et al., 2006, Emmerling et al., 2000, Forberg et al., 1988, Walton and Stewart, 2002, Walton and Stewart, 2004, Cotter et al., 2009). Forberg and coworkers demonstrated that phenylalanine overproduction from glucose could be achieved by limiting tryptophan in an auxotrophic strain. They also reported that the growth phase of the harvested cells for biotransformations affected the subsequent product yield; cells from the exponential phase had a higher yield than cells harvested from the stationary phase
69 (Forberg et al., 1988). Walton and Stewart reported improved production through a heterologous 49 redox-dependent pathway with resting cells. Similar to Forberg s results, they reported a decrease in yield when the cells were harvested near or at stationary phase (Walton and Stewart, 2002, Walton and Stewart, 2004). Cotter and coworkers demonstrated an increase in ethanol production in two different Clostridium strains using resting cell conditions (Cotter et al., 2009). Emmerling and coworkers have used anaerobic resting cell conditions to simplify metabolism and better understand the effects of overexpressing various glycolytic enzymes (Emmerling et al., 1999, Emmerling et al., 2000). In this chapter, xylitol yield between batch cultures and resting cell cultures was compared and the effects of xylitol production and yield (Y RPG ) under various resting cell conditions (e.g. growing medium, working OD, glucose limitation, and oxygen availability) was examined. Materials and Methods General Strains (e.g. W3110, PC07, and PC09) and plasmid (ploi3815, containing a xylose reductase from Candida boidinii CbXR) used in this study are described in Chapter 2. Media components, antibiotic concentrations, and detection methods are also described in Chapter 2. The antibiotic chloramphenicol was included as required (50 μg ml -1 ). Standard methods were used for plasmid construction, phage P1 transduction, electroporation, and polymerase chain reaction (PCR) (Miller, 1992, Sambrook and Russell, 2001).
70 50 Resting Cell Cultures Resting cells were prepared using a protocol similar to that described by Walton (Walton and Stewart, 2002). Briefly, 25 ml seed cultures were grown in either minimal medium or LB medium at 37 C to a final OD 600 between and used to inoculate 200 ml cultures to an initial OD 600 of 0.05 in either minimal medium or LB medium supplemented with 50 mm xylose and 100 mm glucose in a one liter Erlenmeyer flask. This culture was induced with 100 M IPTG at inoculation, and shaken at 250 rpm and 30 C. When the cells reached an OD 600 between (exponential growth), chloramphenicol was added, unless otherwise specified, to the culture to inhibit further protein synthesis. Cells harvested in this growth stage were previously determined to behave reproducibly in resting culture and give stable yields for at least 48 hours (Cirino et al., 2006). The culture was then harvested by centrifugation, washed in minimal medium lacking a carbon and nitrogen source and resuspended to a final OD 600 of 2.0 (unless otherwise specified) in minimal medium lacking nitrogen but containing an appropriate amount of sugar (50 mm glucose and/or 300 mm xylose). Aerated resting cell experiments used 30 ml of resuspended cells in 250 ml baffled flasks shaking at 250 rpm and 30 C. Oxygen limited resting cell experiments (not shaken) used 30 ml of resuspended cells in 250 ml baffled flasks sitting in an incubator at a controlled temperature of 30 C. Confidence intervals represent the standard deviation. Molar yields of xylitol per glucose consumed (Y RPG ), were calculated by subtracting for the xylitol produced in the absence of glucose from the amount produced in the presence of glucose, before normalizing with respect to glucose consumption.
71 51 Glucose Limited Cultures Glucose limited cultures were performed by slowly adding a 90 mm glucose stock solution at a rate of 104 µl hr -1, via syringe pump (New Era Pump Systems) to a culture resuspended in resting cell medium supplemented with xylose only. Glucose was continuously fed in a manner to achieve a glucose consumption level equivalent to that observed in the case of excess glucose. From the 24 and 48 hour times points, HPLC analysis confirmed that the glucose concentration was negligible, verifying that glucose is not in excess (< 0.7 mm). Results Batch vs. Resting Cells The first test compared the results of resting cell cultures with batch cultures to determine if a higher Y RPG would be observed in the absence of growth. Both cultures were conditioned on minimal media plates prior to inoculation. For the resting cell cultures, growth was arrested by resuspending in a nitrogen-free medium (chloramphenicol was not used). What was found was that the Y RPG was significantly higher than that observed in batch cultures (Figure 3 1). Elimination of growth as a drain for available reduced cofactors increases the xylitol Y RPG, as expected. However, a lower xylitol production was observed in the resting cell cultures. The lower xylitol production was likely a result of a lower cell concentration in the resting cell cultures compared to the batch cultures; the OD 600 for the resting cell culture was ~ 2, whereas the final OD 600 for the batch culture was ~ 10.5.
72 Xylitol (mm) Yield (mm X'ol/mM Glucose) Batch Xylitol Resting Xylitol Batch Yield Resting Yield Time (hr) Figure 3-1: Xylitol production (blue) and yield (pink) comparison between PC09 with plasmid ploi3815 cultured as a batch culture (solid lines) or resting cells (dashed lines). Figure used with permission from Wiley Sciences. Working OD The effects of resting cell culture (working) OD on xylitol production and yield was next examined by testing resuspended the resting cell cultures to three different working OD s: 1.6, 3.6, and 4.4. It was hypothesized that an increased cell concentration would result in a higher xylitol concentration; however, because glucose consumption and xylitol production should be proportional to cell concentration, a constant xylitol Y RPG was expected. Growth was limited in these cultures by nitrogen limitation (chloramphenicol was not used). Increasing the working OD did increase the xylitol produced in the culture, and the xylitol per OD was relatively constant with all three working OD s (Figure 3 2). Interestingly, the xylitol Y RPG decreased slightly as the working OD increased, which was likely caused the biological
73 Xylitol/OD (mm) Yield (mm xylitol/mm glucose) 53 oxygen demand surpassing the oxygen availability in a baffled shake flask. This would lead to incomplete glucose oxidation, as evidenced by the secretion of acetate and lactate (Table 3 1). By secreting acetate, the cell would not be able to derive the potential reducing equivalents from the TCA cycle. Production of lactate not only lowered the potential maximum yield of reducing equivalents but also consumed a reducing equivalent (NADH) further decreasing the total pool of available reduced cofactors. It was determined that xylitol production could be increased with a higher working OD; however, a concurrent increase xylitol Y RPG could not be maintained. By resuspending the resting cell culture in 10 ml of medium in a 250 ml baffled flask, it was hypothesized that the oxygenation levels of the culture would be increased; however, an improvement upon the yield compared to a 30 ml culture was not observed (data not shown) Xylitol/OD, OD = 1.6 Xylitol/OD, OD = 3.6 Xylitol/OD, OD = 4.4 Yield, OD = 1.6 Yield, OD = 3.6 Yield, OD = Time (hour) Figure 3-2: Xylitol per OD and uncorrected yield (mm xylitol produced per mm glucose consumed) comparison in resting cells with different working OD s. Cultures were grown in minimal medium and resuspended in minimal medium without nitrogen. Resuspended to an initial OD = 1.6, 3.6, 4.4 (as noted). Table 3-1: Glucose consumption and byproduct secretion levels in resting cells with different working OD at time point 96 hours are given in mm. Yield (Y RPG ) represents an uncorrected value (background xylitol was not subtracted). Cell cultures were grown in minimal medium and resuspended in minimal medium without nitrogen. The cultures were resuspended to an initial (working) OD = 1.6, 3.6, or 4.4. Ethanol and formate production was negligible. Working OD Glucose consumed Xylitol Yield (Y RPG ) Lactate Acetate
74 Growth Inhibition In the previous tests, production of lactate and other fermentation products (e.g. pyruvate and ethanol) were detected, which were not observed in batch cultures. It was hypothesized that during the harvest, protein expression was altered to allow for fermentation to occur. In hopes to prevent the synthesis of fermentation enzymes and decrease the amount of secreted fermentation products, addition of a protein synthesis inhibitor (chloramphenicol) to the cultures prior to harvesting was examined. The effects of both nitrogen limitation and protein synthesis, as a means of inhibiting growth, on xylitol production and yield were tested. As shown in Table 3 2, using both nitrogen limitation and chloramphenicol resulted in the best xylitol yield (Y RPG ), but the worst xylitol production. No significant amount of byproducts (pyruvate, lactate, acetate, formate, or ethanol) was secreted in these cultures, indicating that glucose was completely oxidized to carbon dioxide. Lacking inhibition (+ N, C) resulted in the highest xylitol production (due to an increased cell concentration); however xylitol Y RPG was significantly lower than any of the cultures with growth inhibition. The lower Y RPG observed in the (+ N, C) cultures, was likely due to growth, which decreased the available reduced cofactors for xylitol production. Having nitrogen limitation without chloramphenicol addition ( N, C) resulted in the second best Y RPG. The lower Y RPG in ( N, C) was likely due to a slight production in lactate and acetate, which is indicative of incomplete glucose oxidation. The lower Y RPG in the (+ N, + C) cultures was likely due to an increased pyruvate and acetate production, indicative of incomplete glucose oxidation. Because this research project is focused on the improving the yield of reducing equivalents for a heterologous reaction of interest, the condition
75 of nitrogen limitation and chloramphenicol addition ( N, + C), was used for further resting cell studies. 55 Table 3-2: Glucose consumption and byproduct secretion levels (mm) in resting cells with different growth inhibitors at time point 24 hours. Cell cultures were grown in minimal medium and resuspended in minimal medium without nitrogen. The cultures were resuspended to an initial (working) OD = 2.0. Ethanol production was negligible. Note that the yield (Y RPG ) represents the uncorrected value. Growth inhibitors Glucose consumed Xylitol Yield (Y RPG ) Pyruvate Lactate Acetate Formate + N + C N - C N + C N C Oxygen-Limited Cultures The effects of aeration on xylitol Y RPG and production were examined by comparing resting cell cultures in an incubator with either shaking at 250 rpm or no shaking. Cultures shaking at 250 rpm were expected to be in a microaerobic or aerobic environment, while cultures without shaking (e.g. sitting ) should shift to more anaerobic conditions. Emmerling and coworkers have already demonstrated the effects of aerobic and anaerobic resting cell conditions cells harvested under aerobic conditions on the specific activity of ldha and adh (Emmerling et al., 1999, Emmerling et al., 2000). They have reported specific activity of both adh and ldha in aerobically grown cultures suggesting that lactate and ethanol production are possible. Although very little fermentation products were detected in the xylitol producing batch cultures (Chapter 2) or in the seed cultures for resting cells (data not shown), it was hypothesized that aerobic pathways (e.g. NADH dehydrogenases) and xylose reduction were effectively out-competing fermentation pathways for reduced cofactors and limiting the amount of lactate and ethanol detected in these cultures. In the cultures without agitation, lactate, acetate, pyruvate, and ethanol
76 56 production were all significantly increased (Table 3 3). Lactate was the major secretion product (~ 43 mm), which correlates well with the high specific activity of ldha (32 U/mg) that Emmerling reported (Emmerling et al., 2000). Because oxygen supply was limited, the electron transport chain was assumed to be inactive and leading to increased levels of NADH and ATP generation through substrate-level phosphorylation, which was supported by elevated production of acetate, and a significant increase in glucose consumption. Xylitol production was significantly reduced in the cultures without agitation. Under aerobic conditions, CbXR was effectively competing with the fermentation pathways for reducing equivalents, as evidenced by production of xylitol and insignificant amounts of fermentation products under aerobic conditions. However, under anaerobic conditions, xylitol production was significantly decreased, likely due to a lack of direct production of NADPH. The expected increase in NADH levels in anaerobic cultures may have allostericly inhibited the pentose phosphate pathway, specifically the glucose-6-phosphate dehydrogenase (zwf), as evidenced by Sanwal (Sanwal, 1970). Fischer also reported that relative flux through the pentose phosphate pathway was significantly decreased under anaerobic conditions compared to aerobic conditions (5% contribution under anaerobic conditions compared to 19% for aerobic conditions) (Fischer and Sauer, 2003). The TCA cycle, which is another source of NADPH, via isocitrate dehydrogenase (icd), is also allosterically controlled by NADH, as evidenced by Vemuri (Vemuri et al., 2006). Although there was a buildup of NADH under anaerobic conditions, the electrons may not be able to be effectively transferred from NADH to NADPH for xylitol production under aerobic conditions as evidenced in Chapter 4. Therefore aerobic conditions were necessary to promote efficient xylitol production (e.g. high xylitol Y RPG ) and complete oxidation of glucose and allow for greater flux through pathways that increase the availability of NADPH (e.g. pentose phosphate pathway and TCA cycle). Xylitol production under aerobic and anaerobic conditions was limited by the availability of NADPH. Therefore, pathway manipulation, via metabolic engineering, to increase either flux
77 through NADPH-generating pathways or NADPH availability should play an important role in host strain optimization. 57 Table 3-3:Glucose consumption and byproduct secretion levels (mm) in resting cells with different levels of aeration at time point 24 hours. Cell cultures were grown in LB medium and resuspended in minimal medium without nitrogen. The cultures were resuspended to an initial (working) OD = 2.0. Formate production was negligible. Yield (Y RPG ) is corrected for background production of xylitol (xylitol production in the absence of glucose). Condition Glucose consumed Xylitol (corrected) Yield (corrected) Pyruvate Lactate Acetate Ethanol Shaking Sitting Glucose Fed Cultures In order to minimize the amount of acetate, though negligible amounts were formed, in the LB grown cultures, a glucose-limitation strategy was tested. By minimizing the amount of excess glucose in the culture, it was hypothesized that overflow metabolism, which caused acetate production in the culture (Wolfe, 2005), could be prevented. Glucose limitation was performed by slowly adding glucose to the culture using a syringe pump at a rate similar to that observed in the case of excess glucose. It was observed that detectable glucose concentration was negligible at the 24 and 48 hour time points proving that glucose was not in excess (< 0.7 mm). Limiting glucose did decrease the amount of fermentation products observed in the cultures; however, because so little was produced under excess glucose conditions, there was not a significant change between the two different conditions. Although overall xylitol production was decreased, the xylitol Y RPG was increased, probably due to complete oxidation of glucose, as evidenced by the lack of fermentation products (Table 3 4). Table 3-4: Glucose consumption and byproduct secretion levels (mm) in resting cells with different glucose feeding strategies at time point 24 hours. Cell The cultures were grown in LB medium and resuspended in minimal media without nitrogen. Chloramphenicol was added prior to harvesting cells, and the culture was resuspended to an initial OD = 2.0. Formate and ethanol
78 production was negligible. Yield (Y RPG ) is corrected for background production of xylitol (xylitol production in the absence of glucose). Glucose source Glucose consumed Xylitol (corrected) Yield (corrected) Pyruvate Lactate Acetate Excess Fed Effect of Growth Medium on Resting Cell Yield Although the previous experiments grew the harvested cells in a minimal medium, other groups have grown the harvested cells in a complex (rich) medium (Cotter et al., 2009, Emmerling et al., 2000, Walton and Stewart, 2002, Walton and Stewart, 2004). It has also been reported that several of the gene deletion strains exhibit poor growth on minimal glucose medium (Hua et al., 2003, Sauer et al., 2004, Zhao et al., 2004). To avoid drastic growth differences between strains (e.g., culture time prior to harvesting) and selective pressures that may lead to compensatory mutations, the next test compared the resting cell performance (xylitol production and Y RPG ) of strain PC09 harboring plasmid ploi3815 when grown in rich (Luria-Bertani) medium and a minimal medium (both supplemented with 50 mm xylose and 100 mm glucose); the minimal resting cell medium remained unchanged and is free of a nitrogen source. As shown in Figure 3 3, the yield of strain PC09 was reduced as a result of growth in LB (3.4 versus 4.0 at 24 hours), while secretion of byproducts remained minimal. The increased Y RPG observed from cells grown in minimal medium (compared to LB-glucose) may be a result of higher flux through catabolic pathways producing NADPH for biosynthesis during growth, lower expression of genes involved in aerobic respiration (Tao et al., 1999), and/or higher total TCA cycle activity compared to cells grown in complex medium supplemented with glucose (Gray et al., 1966). Resting cells prepared without an energy source (glucose) produce low, background levels of xylitol, and when calculating Y RPG this background level of production was subtracted
79 59 from the amount produced in the presence of glucose. As shown in Figure 3 3, the background production of xylitol is 56% higher for cells grown in minimal medium compared to cells grown in LB (10.5 mm vs. 6.7 mm). This may be a result of increased production of carbohydrate reserves during growth in minimal glucose medium. Figure 3-3: Comparison between resting cell results using strain PC09 harboring plasmid ploi3815 (expressing CbXR) that was first cultured in rich medium (LB) versus minimal medium (NBS). The presented yield (Y RPG ), shown in parenthesis, is corrected for background production of xylitol (xylitol production in the absence of glucose). Conclusion Resting cells have previously been reported to be used both increase the production and yield of biochemicals (Cotter et al., 2009, Forberg et al., 1988, Walton and Stewart, 2002, Walton and Stewart, 2004) as well as simplify metabolism to get a better understanding of the effects of various metabolic engineering schemes (Emmerling et al., 2000, Emmerling et al., 1999). In this chapter, a higher xylitol Y RPG with resting cells compared to batch cultures was observed. This was attributed to a higher availability of NADPH due to eliminating the NADPH drain towards biomass production and being able to maintain a higher level of aeration in the culture (due to a lower cell density).
80 60 The effect of resting cell working cell density on xylitol production and Y RPG was also examined. By increasing cell concentration, an increase in xylitol production was observed; however, the xylitol Y RPG decreases. This was attributed to a decrease in oxygen availability to the cells, which led to incomplete glucose oxidation and fermentation, as evidenced by the increase in lactate secreted by the cells. By reducing the oxygen availability to the cells, e.g. eliminating agitation, a corresponding increase in fermentation products secreted was observed, most notably lactate. The production of lactate in aerobically grown resting cells was expected as evidenced by a high specific activity of ldha (Emmerling et al., 1999, Emmerling et al., 2000) and indicated that the buildup of NADH was not effectively converted to NADPH, which could be reoxidized by the xylose reductase enzyme. This result correlates well with what the results reported in Chapter 4; the transhydrogenases are not effectively making more NADPH available for xylose reduction in resting cells. By using a glucose-limitation strategy, glucose oxidation was further improved and the amount of fermentation products detected in the culture was decreased. These results highlight the importance of aeration for the increased xylitol yield (Y RPG ) and also suggest that xylitol production may not be able to effectively compete for NADH. Different growth inhibition methods on resting cell xylitol Y RPG, including limiting nitrogen in the media and adding a protein synthesis inhibitor (chloramphenicol) to the culture was examined. In the presence or absence of nitrogen in the medium, chloramphenicol addition decreased the amount of glucose consumed and increased the xylitol Y RPG at a 24 hour time point. From this test it was concluded that to optimize Y RPG (uncorrected Y RPG of 1.5 with no growth inhibition or chloramphenicol addition to 5.5), a combination of nitrogen limitation and protein synthesis inhibition should be used. Lastly, use of rich (LB) medium to grow the cells to avoid drastic growth differences between strains (e.g., culture time prior to harvesting) and selective pressures that may lead to compensatory mutations was examined. Using a rich medium to grow the cells resulted in a decrease in the overall corrected Y RPG (3.4 compared to 4.0). These results
81 61 highlight the importance of the medium conditions, both harvesting and resuspending media, on xylitol production and yield (Y RPG ). Conditions that promote flux through the TCA cycle and promote complete glucose oxidation, while limiting growth and potential (over)expression of fermentation pathway enzymes were beneficial for enhanced xylitol production and yield. With a protocol that can be used for equivalent strain-by-strain comparisons, while eliminating the growth differences between different strains, this research sought to examine the study central metabolism to understand where the reducing equivalents were being generated. Although resting cells harvested from minimal medium did result in a higher xylitol Y RPG, further studies were harvested from cells grown in rich (LB) medium to eliminate any drastic growth differences and potential selective pressures. References CIRINO, P. C., CHIN, J. W. & INGRAM, L. O. (2006) Engineering Escherichia coli for xylitol production from glucose-xylose mixtures. Biotechnol Bioeng, 95, COTTER, J. L., CHINN, M. S. & GRUNDEN, A. M. (2009) Ethanol and acetate production by Clostridium ljungdahlii and Clostridium autoethanogenum using resting cells. Bioprocess Biosyst Eng, 32, EMMERLING, M., BAILEY, J. E. & SAUER, U. (1999) Glucose catabolism of Escherichia coli strains with increased activity and altered regulation of key glycolytic enzymes. Metab Eng, 1, EMMERLING, M., BAILEY, J. E. & SAUER, U. (2000) Altered regulation of pyruvate kinase or co-overexpression of phosphofructokinase increases glycolytic fluxes in resting Escherichia coli. Biotechnol Bioeng, 67, FISCHER, E. & SAUER, U. (2003) Metabolic flux profiling of Escherichia coli mutants in central carbon metabolism using GC-MS. Eur J Biochem, 270, FORBERG, C., ELIAESON, T. & HAGGSTROM, L. (1988) Correlation of Theoretical and Experimental Yields of Phenylalanine from Non-Growing Cells of a Rec Escherichia- Coli Strain. Journal of Biotechnology, 7, GRAY, C. T., WIMPENNY, J. W. & MOSSMAN, M. R. (1966) Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta, 117, HUA, Q., YANG, C., BABA, T., MORI, H. & SHIMIZU, K. (2003) Responses of the central metabolism in Escherichia coli to phosphoglucose isomerase and glucose-6-phosphate dehydrogenase knockouts. J Bacteriol, 185,
82 MILLER, J. H. (1992) A short course in bacterial genetics : a laboratory manual and handbook for Escherichia coli and related bacteria, Plainview, N.Y., Cold Spring Harbor Laboratory Press. SAMBROOK, J. & RUSSELL, D. W. (2001) Molecular cloning : a laboratory manual, Cold Spring Harbor, N.Y., Cold Spring Harbor Laboratory Press. SANWAL, B. D. (1970) Regulatory mechanisms involving nicotinamide adenine nucleotides as allosteric effectors. 3. Control of glucose 6-phosphate dehydrogenase. J Biol Chem, 245, SAUER, U., CANONACO, F., HERI, S., PERRENOUD, A. & FISCHER, E. (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem, 279, TAO, H., BAUSCH, C., RICHMOND, C., BLATTNER, F. R. & CONWAY, T. (1999) Functional genomics: expression analysis of Escherichia coli growing on minimal and rich media. J Bacteriol, 181, VEMURI, G. N., ALTMAN, E., SANGURDEKAR, D. P., KHODURSKY, A. B. & EITEMAN, M. A. (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol, 72, WALTON, A. Z. & STEWART, J. D. (2002) An efficient enzymatic Baeyer-Villiger oxidation by engineered Escherichia coli cells under non-growing conditions. Biotechnol Prog, 18, WALTON, A. Z. & STEWART, J. D. (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog, 20, WOLFE, A. J. (2005) The acetate switch. Microbiol Mol Biol Rev, 69, ZHAO, J., BABA, T., MORI, H. & SHIMIZU, K. (2004) Global metabolic response of Escherichia coli to gnd or zwf gene-knockout, based on 13C-labeling experiments and the measurement of enzyme activities. Appl Microbiol Biotechnol, 64,
83 Chapter 4 Analysis of NADPH Supply During Xylitol Production by Engineered Escherichia coli Portions of this chapter have been reprinted with permission from John Wiley and Sons (License numbers: ).
84 64 Abstract In Chapter 2, it was concluded that Escherichia coli strain PC09 (ΔxylB, camp-independent CRP (crp*) mutant) expressing an NADPH-dependent xylose reductase from Candida boidinii (CbXR) could produce xylitol from xylose while deriving reducing equivalents by metabolizing glucose. In Chapter 3, a protocol was developed that allowed for a more equivalent strain-bystrain comparison. This chapter aims to understand the role of NADPH supply in xylitol yield and the contribution of key central carbon metabolism enzymes toward xylitol production. Studies in which the expression of CbXR or a xylose transporter was increased suggest that enzyme activity and xylose transport are not limiting xylitol production in PC09. A constraintsbased stoichiometric metabolic network model was used to understand the roles of central carbon metabolism reactions and xylose transport energetics on the theoretical maximum molar xylitol yield (xylitol produced per glucose consumed), and xylitol yields (Y RPG ) were measured from resting cell biotransformations with various PC09 derivative strains. For the case of xylose-proton symport, omitting the Zwf (glucose-6-phosphate dehydrogenase) or PntAB (membrane-bound transhydrogenase) reactions or TCA cycle activity from the model reduces the theoretical maximum yield from 9.2 to 8.8, 3.6, and 8.0 mol xylitol (mol glucose) -1, respectively. Experimentally, deleting pgi (encoding phosphoglucose isomerase) from strain PC09 improves the yield from 3.4 to 4.0, while deleting either or both E. coli transhydrogenases (stha and pnta) has no significant effect on the measured yield. Deleting either zwf or succ (TCA cycle) significantly reduces the yield from 3.4 to 2.0 and 2.3 mol xylitol (mol glucose) -1, respectively. Expression of a xylose reductase with relaxed cofactor specificity increases the yield to 4.0. The large discrepancy between theoretical maximum and experimentally determined yield values suggests that biocatalysis is compromised by pathways competing for reducing equivalents and dissipating energy. The metabolic role of transhydrogenases during E. coli biocatalysis has
85 65 remained largely unspecified. The results presented in the chapter demonstrate the importance of direct NADPH supply by NADP + -utilizing enzymes in central metabolism for driving heterologous NADPH-dependent reactions, and suggest that the pool of reduced cofactors available for biotransformation is not readily interchangeable via transhydrogenase. Introduction Several strategies have been employed to improve the availability of NADPH in whole cells. Moreira dos Santos reported the use of NADP + -dependent malic enzymes to increase cytosolic or mitochondrial levels of NADPH within Saccharomyces cerevisiae (Moreira dos Santos et al., 2004). Weckbecker and Hummel reported the overexpression of the membrane-bound transhydrogenase PntAB in Escherichia coli to improve the NADPH-dependent conversion of acetophenone to (R)-phenylethanol (Weckbecker and Hummel, 2004). Sanchez reported the overexpression of the soluble transhydrogenase SthA in E. coli to improve the NADPHdependent production of poly(3-hydroxybutyrate) (Sanchez et al., 2006). Verho reported an increase in ethanol yield from pentose sugars in an S. cerevisiae strain by overexpressing an NADP + -dependent glyceraldehyde 3-phosphate dehydrogenase (GPD1) from Kluveromyces lactis (Verho et al., 2003). In Chapter 2 E. coli strain PC09 (crp*, ΔxylB) expressing NADPH-dependent xylose reductase from Candida boidinii (CbXR) was reported to be a viable xylitol-producing strain and plasmid combination. The research in Chapter 3 demonstrated that the availability of NADPH for xylose reduction (Y RPG ), was increased from 1.8 in batch cultures to > 3.7 in resting cells. This current study aims to understand which metabolic pathways are primarily responsible for supplying NADPH to the xylose reductase, as a step toward increasing the availability of NADPH for driving heterologous reactions in whole-cell biocatalysts.
86 66 The first step combined stoichiometric metabolic network modeling with knowledge of xylose transport and central metabolism to study how key enzymes influence the xylitol yield. Mutant strains were next created to investigate the contribution of particular pathways or reactions toward xylitol yield. Genes that were studied are shown in Figure 4 1 and include pgi (encoding phosphoglucose isomerase), zwf (glucose 6-phosphate-1-dehydrogenase), ndh (NADH dehydrogenase II), succ (succinyl-coa synthetase), edd-eda (phosphogluconate dehydrogenase and 2-keto-3-deoxy-6-phosphogluconate aldolase, respectively), and the transhydrogenase genes stha and pnta. Also, plasmid-based overexpression of the transhydrogenases was examined as a potential means of increasing NADPH supply. Resting cell assays were used to compare the strains in this study because xylitol production is growth-uncoupled and this assay permitted accurate comparisons between non-growing biocatalysts that display widely different growth characteristics.
87 Figure 4-1: E. coli central carbon metabolism summarizing key reactions and pathways involved in NAD(P)H metabolism. Genes shown code for the following enzymes: pgi = phosphoglucose isomerase; zwf = glucose 6-phosphate-1-dehydrogenase; gnd = 6-phosphogluconate dehydrogenase; edd = phosphogluconate dehydratase; eda = 2-keto-3-deoxy-6-phosphogluconate aldolase; nuoa-n = NADH dehydrogenase I; ndh = NADH dehydrogenase II; succ = succinyl- CoA synthetase; stha = soluble pyridine nucleotide transhydrogenase; pntab = pyridine nucleotide transhydrogenase; pfk = phosphofrucokinase. Pathway abbreviations include the following: EMP = Embden-Meyerhof-Parnas, TCA = tricarboxylic acid. 67
88 68 Materials and Methods General Strains and plasmids used in this study are listed in Table 4 1. E. coli W3110 (ATCC 27325) and derivative strains were maintained on plates containing either Luria-Bertani (LB) medium, or minimal medium containing mineral salts as described in Chapter 2. Antibiotic concentrations and detection methods are also described in Chapter 2 and 3. Table 4-1: Strains and plasmids used in this study. Other gene deletion strains described are derived from strain PC09. a Strain Relevant characteristic Reference W3110 Wild-type ATCC PC09 crp*, ΔxylB::FRT (Tet R ) Chapter 2 PAP01 W3110, ΔpntA:: FRT-kan-FRT (Kan R ) This study CF13 W3110, ΔsucC:: FRT-kan-FRT (Kan R ) This study JC16 PC09, CbXR integrated at HK022 (Tet R, Apr R ) This study JC17 PC09, CbXR integrated at HK022 (Tet R ) This study JC38 W3110, ΔsthA:: FRT-kan-FRT (Kan R ) This study JC53 W3110, Δpgi:: FRT-kan-FRT (Kan R ) This study JC54 W3110, Δzwf:: FRT-kan-FRT (Kan R ) This study JC62 BW25142 Δzwf-eda:: FRT-aac-FRT (Apr R ) This study JC63 BW25142 Δedd-eda:: FRT-aac-FRT (Apr R ) This study JC68 W3110, Δzwf-eda:: FRT-aac-FRT (Apr R ) This study JC69 W3110, Δedd-eda:: FRT-aac-FRT (Apr R ) This study Plasmid Relevant characteristic Reference ploi3809 kan, pbr322-origin vector for heterologous gene expression under control of tac promoter Chapter 2 ploi3815 ploi3809 carrying xylose reductase from Candida boidinii (CbXR) Chapter 2 ppcc05 ploi3809 carrying xylose reductase from Candida tenuis (CtXR) Chapter 2 ppcc09 ploi3809 carrying stha gene from W3110 This study ppcc102 ploi3809 carrying pntab gene from W3110 This study ppcc106 ploi3815 with pntab from ppcc102 This study ppcc107 ploi3815 with xyle from ppcc203 (Khankal et al., 2008) ppcc203 ploi3809 carrying xyle gene from W3110 (Khankal et al., 2008) ppcc205 ploi3809 carrying xylfgh gene from W3110 (Khankal et al., 2008)
89 69 Strain Relevant characteristic Reference ppcc207 ploi3815 with xylfgh from ppcc205 (Khankal et al., 2008) ppcc500 ploi3815 with stha gene from ppcc09 This study a Tet, tetracycline; Kan, kanamycin; Apr, apramycin Standard methods were used for plasmid construction, phage P1 transduction, electroporation, and polymerase chain reaction (PCR) (Miller, 1992, Sambrook, 2001). Strain JC17 was created by integrating a fragment of plasmid ploi3815 (containing the laci gene, tac promoter, CbXR gene, and terminator region) into the genome of PC09 using the CRIM method described by Haldimann and Wanner (Haldimann and Wanner, 2001). The FRT-flanked bla gene in plasmid pah68 (Haldimann and Wanner, 2001) was first replaced with the aac gene as follows. The aac gene was isolated by digesting lab plasmid ploi3821 with SmaI, and was ligated into plasmid pah68 which had been digested with MslI and phosphatase treated. The resulting plasmid is ppcc20. Plasmid ploi3815 was digested with FspI and a 4.4 kb fragment, containing the relevant genes, was isolated and ligated into ppcc20, which had been digested with SmaI and treated with CIP (calf intestinal alkaline phosphatase). The resulting plasmid is ppcc100. ppcc100 was subsequently integrated into the chromosome of PC09 at the HK022 site resulting in JC16. Apramycin resistant colonies arising from the successful integration of the ppcc100 region were selected and verified by PCR. Removal of the FRT-flanked apramycin resistance cassette was performed as described (Causey et al., 2003), resulting in strain JC17. Gene Deletions E. coli K-12 strains with single gene deletions carrying an FRT-flanked kanamycin resistance cassette in place of the selected gene (pgi, zwf, pnta, stha, succ, ndh) were obtained from the Keio collection (Baba et al., 2006). P1 phage transductions were performed to propagate the gene deletions. Combined deletions in the zwf-eda, and edd-eda genes were constructed using the one-
90 70 step inactivation method described by Datsenko and Wanner (Datsenko and Wanner, 2000). Briefly, apramycin resistance gene (aac) flanked by FRT flipase recognition sequences (in lab plasmid ploi3421) was PCR-amplified using the primer pairs: zwf for (ACG GGT GGA TAA GCG TTT ACA GTT TTC GCA AGC TCG TAA GGC GAT TAA GTT GGG TAA CG) and eda rev (GAC TTT TAC AGC TTA GCG CCT TCT ACA GCT TCA CGC GCC-TTC CGG TCT CCC TAT AGT GA) or edd for (CGT GCG GAT TCA CCC ACG AGG CTT TTT TTA TTA CAC TGA-GGC GAT TAA GTT GGG TAA CG) and eda rev. The underlined portions represent the region homologous to plasmid ploi3421. The non-underlined portions are homologous to the chromosomal location corresponding to ~120 bp upstream of the zwf start codon (zwf for), ~ 150 bp upstream of the edd start codon (eda for), and overlapping the stop codon of eda (eda rev). The PCR products were electroporated into strain BW25142 expressing the λ-red recombinase from plasmid pkd46 (Causey et al., 2003). Chromosomal deletions were verified by colony PCR. All FRT-flanked antibiotic resistance markers were removed using flipase recombinase (Causey et al., 2003). Transhydrogenase Expression Plasmids The pnta-pntb gene segment and stha gene were amplified from E. coli W3110 genomic DNA (performed in a Bio-Rad icycler thermocycler using iproof high fidelity polymerase (Bio- Rad)). Primers for pntab amplification were: pnta for HindIII, 5 -CGT AAG CTT CCG ATG GAA GGG AAT ATC ATG-3 (HindIII underlined); pntb rev KpnI 5 -ATC GGT ACC CAG GGT TAC AGA GCT TTC AG-3 (KpnI underlined). The resulting PCR product was digested with HindIII and KpnI and ligated into lab expression vector ploi3809 which had been digested with the same two restriction enzymes, creating plasmid ppcc102. Primers for the stha amplification were: sthafor1, 5 -CTA GCT CGA GCC GGC GGC GAA GGC GCT GC-3
91 71 (XhoI underlined); stharev1, 5 -CTA CGG TAC CCA AGA ATG GAT GGC CAT TTC G-3 (KpnI underlined). The resulting PCR product was digested with XhoI and KpnI then ligated to the 6.4 kb fragment isolated from lab plasmid ppcc02 which had been digested with the same two enzymes, creating plasmid ppcc09. Plasmids ppcc106 and ppcc500 were created as follows: the pntab gene segment from ppcc102 was prepared by digesting the plasmid with the HindIII, Klenow treating, digesting with BglII, and then isolating the 2.9 kb fragment via gel purification. The PntAB fragment was ligated into ploi3815, which had been digested with SmaI and BglII, resulting in plasmid ppcc106. The stha gene was isolated from ppcc09 after digesting the plasmid with KpnI and XhoI. The purified 1.4 kb fragment was ligated into ploi3815 vector, which had been previously digested with the same enzymes, resulting in ppcc500. Shake-Flask Cultures All cultures were performed in at least duplicates. Shake-flask cultures for xylitol production were performed as previously described in Chapter 2. Resting Cell Cultures Resting cells were grown in rich (LB) medium and prepared using a protocol as described in Chapter 3. Molar yields of xylitol per glucose consumed (Y RPG ), were calculated by subtracting for the xylitol produced in the absence of glucose from the amount produced in the presence of glucose, before normalizing with respect to glucose consumption.
92 72 Transhydrogenase Activity Assay Cells were grown in 50 ml of LB medium supplemented with 50 mm glucose, in a 250 ml baffled Erlenmeyer flask. The cultures were harvested at OD 600 between by pelleting the cells and washing twice with 0.9% (w/v) NaCl and 10 mm MgSO 4. Cell pellets were stored at -20 C until use. The cell pellet was resuspended to an OD 600 of 12.5 in lysis buffer (100 mm Tris- HCl ph 7.5, 5 mm MgCl 2, 3.33 μg ml -1 DNase I, Protease Arrest (G Biosciences, St. Louis, MO used at ½ manufacturer s recommended concentration), 0.2 mm dithiothrietol). The cell pellet was lysed by two passes through a French Pressure cell press and subsequently centrifuged at 4 C, 18,000 x g, for five minutes to remove cellular debris. The resulting supernatant contained both the soluble and membrane-bound transhydrogenases. To isolate the membrane, the lysates were further centrifuged at 50,000 x g for 60 min at 4 C (via ultracentrifugation) and the resulting pellets were resuspended in a volume equal to the initial sample of 50 mm sodium phosphate buffer ph 7.0. Transhydrogenase activity was measured according to the following procedure adapted from Sauer (Sauer et al., 2004) in a 96-well microtiter plate using a Spectra Max Plus 384 plate reader. Reactions contained different amounts of cell lysate in 200 μl total volume with 50 mm Tris-HCl ph 7.5, 2.0 mm MgCl 2, 2.0 mm β-nadph, 2.0 mm 3-acetyl pyridine adenine dinucleotide (APAD + ). APAD + is an analogue of NAD + ; the concentration of its reduced form (APADH) was readily determined by absorbance at λ = 400 nm (extinction coefficient ~2.9 (mm cm) -1 (Ciotti and Kaplan, 1956)) with minimal absorbance interference from β-nadph. Absorbance was monitored for one minute with readings taken every two seconds. One unit (U) is defined as the activity that produces one mol of APADH in one minute.
93 73 CbXR Activity Assay Seed cultures (10 ml in LB medium) were grown to an OD 600 of ~ 2.0, and were used to inoculate cultures by dilution to a final OD 600 of 0.1 in 125 ml of LB medium supplemented with 50 mm glucose, and 100 or 300 μm IPTG in a one-liter Erlenmeyer flask. When the cultures reached an OD 600 of , the cells were harvested by pelleting and washed twice with 25 ml of 50 mm potassium phosphate buffer ph 7.5. The cell pellets were stored at -20 C until use. The cell pellets were resuspended to a final OD 600 of 50 in lysis buffer (50 mm potassium phosphate buffer ph = 7.5, 4.0 mm MgCl 2, 3.3 ug ml -1 DNase I, Protease Arrest (G Biosciences, St. Louis, MO used at ½ manufacturer s recommended concentration). The cell pellet was lysed by two passes through a French Pressure cell press and subsequently centrifuged at 4 C, 13,000 x g, for 60 minutes to remove cellular debris. The resulting supernatant contained the xylose reductase. Xylose reductase activity was measured in a 96-well microtiter plate. Reactions contained 300 mm xylose, 300 μm β-nadph, 50 mm potassium phosphate buffer ph 7.5, and cell lysate supernatant in 200 μl total volume. Reduction in the β-nadph concentration was readily determined by absorbance at λ = 340 nm (extinction coefficient ~6.2 (mm cm) -1 ). One unit (U) is defined as the activity that consumes one mol of NADPH in one minute (background activity in the absence of xylose is subtracted). Simulations A genome-scale constraints-based metabolic model, as described by Reed (Reed et al., 2003), was adapted to examine xylitol production. Linear optimization was used to find a solution that maximized the production of xylitol. Simulation of gene deletion mutant strains was accomplished by setting the flux through the corresponding gene product(s) to zero, as described
94 74 by Covert and Palsson (Covert and Palsson, 2002). Sauer and Canonaco demonstrated that the energy-independent soluble transhydrogenase (SthA) converts NADPH to NADH (Canonaco et al., 2001, Sauer et al., 2004); therefore the SthA-catalyzed reaction was constrained to be irreversible in the direction of NADH production. The energy-dependent membrane-bound transhydrogenase (PntAB) was assumed to be irreversible in the direction of NADPH formation, as suggested by Sauer (Sauer et al., 2004). Though Pgi is known to be reversible (e.g., during gluconeogenesis) (Hua et al., 2003), metabolic flux analysis suggests that the net flux through Pgi during glucose metabolism is in the direction of glycolysis (Hua et al., 2003, Sauer et al., 2004) and Zhao and Shimizu have shown limited flux through Pgi in the reverse direction (fructose-6- phosphate to glucose-6-phosphate) when grown on acetate (Zhao and Shimizu, 2003). This led to an added model constraint that restricts flux through Pgi in the reverse direction to be, at most, equal to the flux necessary for biomass production. An ATP maintenance reaction with a flux of 7.6 mmol ATP (g cdw hr) -1 was also included in all simulations (Reed et al., 2003, Varma and Palsson, 1994). To allow for xylitol production, an irreversible NADPH-dependent xylose reduction reaction was added to the model, as well a xylitol diffusion pathway that allowed for xylitol secretion. An energy-independent diffusion pathway was chosen because no known xylitol-specific transporters exist in E. coli, though xylitol has been shown to be transported by glycerol facilitator (GlpF) (Heller et al., 1980). The added reactions are as follows: Xylitol production Xylose (int) + H + (int) + NADPH Xylitol (int) + NADP + Xylitol secretion Xylitol (int) Xylitol (ext)
95 75 Results Role of Xylose Transport and CbXR Expression To determine whether xylose transport and in vivo availability to the reductase could be limiting the yield, the effect of overexpressing the xylose-proton symporter XylE in PC09 was tested. The XylE gene (xyle) was cloned downstream of the CbXR gene in ploi3815 such that both genes were controlled by the tac promoter, yielding bicistronic plasmid ppcc107 (Khankal et al., 2008). This plasmid enabled xylose uptake and high levels of xylitol production in the presence of glucose in strain PC07 (W3110, ΔxylB, wild-type crp), verifying functional expression of XylE (xylitol production was low in PC07 carrying ploi3815) (Khankal et al., 2008). In contrast, resting cell Y RPG and xylitol production in batch cultures of PC09 (PC07, crp*) harboring ppcc107 were not improved relative to PC09 carrying ploi3815 (not shown), suggesting xylose transport does not impose a significant limitation to xylitol production in this strain (the CRP* phenotype already enables xylose transport in the presence of glucose, and added overexpression of XylE is of no benefit). The same observation was made when the ATPbinding cassette-type xylose transporter XylFGH (rather than XylE) was co-expressed with CbXR from plasmid ppcc207 (also verified to enable xylitol production in PC07 (Khankal et al., 2008)). Next, expression level of CbXR was tested to determine if this was limiting xylitol yield. Strain JC17 is similar to PC09 but contains a single, chromosomally-integrated copy of the CbXR gene (under p tac control). Resting cell yields and xylose reductase activities (measured from cell lysates) for strains JC17 and PC09 harboring ploi3815, after having been induced with IPTG during growth (300 M for JC17, 100 M for PC09), were compared. Although PC09 carrying ploi3815 had almost two-fold higher specific xylose reductase activity compared to JC17 (~400
96 76 mu (mg total protein) -1 compared to ~210 mu (mg total protein) -1 ), the yields were very similar (yield was actually slightly lower in PC09, which may reflect metabolic changes due to plasmid maintenance). Increasing the IPTG concentration to 300 M during growth of PC09 harboring ploi3815 resulted in a further ~70% increase in CbXR activity in the lysate without an increase in resting cell yield. These initial studies suggested that availability of xylose and enzyme do not significantly limit the molar xylitol yield in strain PC09. Nonetheless, the yield obtained from resting cells (~3.4) is significantly lower than the theoretical maximum yield (which depends on the metabolic network, as discussed below). NADPH availability therefore seems likely to be limiting the reduction of xylose to xylitol. Another strong indication that NADPH availability limits the xylitol yield is the fact that the yield obtained from minimal medium batch cultures (~1.8) is significantly lower than that obtained from resting cell cultures, where NADPH is no longer consumed in anabolic reactions, as shown in Chapter 3. Next a stoichiometric model of E. coli metabolism was examined to better understand energy- and pathway-related factors that contribute to NADPH availability and yield in this system. Simulation Studies Beginning from an E. coli genome-scale stoichiometric metabolic network (Covert and Palsson, 2002, Reed et al., 2003, Varma and Palsson, 1994), strain PC09 was simulated by eliminating the xylulokinase reaction (XylB) and allowing unlimited xylose uptake. Reactions for NADPH-dependent xylose reduction to xylitol and energy-free xylitol transport were also added to the stoichiometric network. Additional modeling details are described in Materials and Methods. With glucose uptake fixed at 10 mmol glucose (g cdw hr) -1, maximization of xylitol production was set as the optimization objective function. The maximum theoretical yield
97 77 corresponds to the resulting xylitol flux normalized with respect to glucose consumption. Table 4-2 summarizes the maximum xylitol yield results from simulations with PC09 and select mutant strains, and indicates the sources of NADPH leading to these yields. The maximum theoretical yield in PC09 is 9.2. As expected, the less energy-demanding xylose-proton symporter (XylE) was chosen over the ATP-dependent xylose transport system (XylFGH). Fixing all xylose uptake through XylFGH lowered the maximum theoretical yield to 6.9. Major contributors to NADPH supply are the pentose phosphate (PP) pathway (Zwf and Gnd enzymes), the tricarboxylic acid (TCA) cycle (Icd), and the proton-translocating transhydrogenase PntAB, which is consistent with the findings of Sauer (Sauer et al., 2004). Flux through Pgi and the soluble transhydrogenase (SthA) were zero when xylitol production was maximized in the model. Table 4-2: Predicted maximum theoretical yield and NADPH source(s) from simulated strains used in this study. Model constraints and parameters are described in the Materials and Methods. a Strain Xylitol Source of NADPH (Yield) PPP TCA PntAB PC Δpnt Δzwf Δpgi ΔsthA Δndh, ΔnuoA-N ΔxylE ΔTCA(sucC) Δpgi, ΔsthA ΔpntAB, Δzwf ΔpntAB, Δpgi ΔpntAB, ΔsthA ΔpntAB, ΔTCA(sucC)
98 a NADPH source may not match the xylitol yield due to rounding error and drain for minimal 78 biomass formation constraint ( g biomass (g cdw hr) -1 ). The primary physiological role of PntAB is believed to be the transfer of reducing equivalents from NADH to NADPH (Sauer et al., 2004), and PntAB is therefore a potential source of NADPH for xylose reduction. Upon deletion, the model predicted that the maximum theoretical yield decreased to 3.6, which is equivalent to the net supply of NADPH derived from the PP pathway and TCA cycle. In contrast, SthA is believed to primarily catalyze the conversion of NADPH to NADH (Canonaco et al., 2001, Sauer et al., 2004). A deletion in the stha gene could therefore potentially increase xylitol yield by eliminating a drain of NADPH. Note, however, that deleting SthA from the model did not impact the theoretical maximum yield. Deleting either pgi or zwf perturbs NADPH metabolism since these gene products determine the fate of glucose-6-phosphate (Hua et al., 2003, Sauer et al., 2004). A pgi mutation forces glucose- 6-phosphate oxidation through the PP and Entner-Doudoroff (ED) pathways and has been implicated in generating excess NADPH, as suggested by an observed slower growth rate (Hua et al., 2003, Sauer et al., 2004). A zwf mutation forces glucose metabolism through the Embden- Meyerhof-Parnas (EMP) pathway. This mutant strain has a lower maximum theoretical yield due to the added energy requirements for PntAB-dependent NADPH production from the reducing equivalents generated (NADH) through EMP. A mutant strain with a non-functional TCA cycle was modeled by removing the SucC reaction. As with the Zwf mutant, NADPH production is compensated by an increased flux through the PntAB reaction, resulting in a decreased maximum theoretical yield.
99 79 Resting Cell Assays To better understand the contributions of the central carbon metabolism reactions discussed above toward NADPH supply and xylitol yield in resting cells, variants of strain PC09 were next constructed and tested experimentally. Table 4 3 lists the Y RPG values and amounts of glucose consumed and metabolites secreted after 24 hours of resting cell biotransformation by these strains. Y RPG from the control strain PC09 (~3.4) is significantly lower than the predicted value of either 9.2 (assuming xylose transport through XylE) or 6.9 (assuming xylose transport through XylFGH). This is due, in small part, to the incomplete oxidation of glucose (indicated by the secretion of pyruvate, lactate, and acetate byproducts). Table 4 4 lists adjusted maximum theoretical yield values in which glucose consumption and metabolite secretion profiles were incorporated as constraints to the model. These adjusted theoretical values are listed both for the case of XylE- and XylFGH-mediated xylose transport. Note that the decrease in maximum yield for most strains tested is not significant, since the acid byproducts represent a small fraction of total glucose consumed. Thus, the experimentally achieved Y RPG s are still significantly lower than the maximum theoretical yields. Table 4-3: Experimental results from resting cell biotransformations for the various strains described. Y RPG is corrected for the background production of xylitol in the absence of glucose. Standard deviations were less than 10% of the average unless indicated. Strain Relevant genotype Y RPG Glucose (mm) Xylitol (mm) Acetate (mm) Pyruvate (mm) Lactate (mm) Bkgd Xylitol (mm) PC09 Reference 3.4± ± ± ± ± ± JC72 Δpgi 4.0± ± ± ± ± ± JC73 ΔpntA 3.6± ± ± ± ± ± ±1.2 JC74 ΔsthA 3.6± ± ± ± ± ±1.4 JC75 Δzwf 2.0± ± ± ± ± ±1.3 JC79 Δndh 0.6± ± ± ± ± ±1.9 MR02 ΔpntA, 5.4± ± ± ± ± ±0.1 ΔsthA JC87 ΔsucC 2.3± ± ± ± ±5.4
100 Strain Relevant genotype Y RPG Glucose (mm) Xylitol (mm) Acetate (mm) Pyruvate (mm) Lactate (mm) 80 Bkgd Xylitol (mm) JC88 Δpgi, 4.2± ± ± ±0.0 0 ΔsthA 11.4 ppcc107 a xyle + 2.0± ± ± ± ±1.3 ppcc207 a xylfgh + 1.9± ± ± ± ± ± ±1.7 ppcc106 a pntab + 1.2± ± ± ± ± ±1.2 ppcc500 a stha + 2.8± ± ± ± ± ±0.7 a Plasmid used to co-express the indicated gene(s) with CbXR in strain PC09. Table 4-4: Predicted maximum theoretical yields adjusted for experimentally determined glucose uptake and metabolite secretion profiles (reported in Table 4-3). Model parameters and constraints are described in the Materials and Methods. Y RPG is included for comparison between simulated and experimental results. Strain XylE simulated XylE adjusted simulated XylFGH simulated XylFGH adjusted simulated Y RPG experimental PC ±0.6 JC72 (Δpgi) ±0.4 JC73 (ΔpntA) ±0.4 JC74 (ΔsthA) ±0.4 JC75 (Δzwf) ±0.3 JC79 (Δndh) ±0.2 MR02 (ΔpntA, ΔsthA) ±0.6 JC87 (ΔsucC) ±0.3 JC88 (Δpgi, ΔsthA) ±0.7 EMP/PP/ED mutants Of the gene deletion strains tested, JC72 (Δpgi) had the highest Y RPG (~4.0). This suggests that some glucose-6-phosphate is directed through Pgi (EMP) in PC09 resting cells, and diverting flux away from Pgi and through Zwf (ED and/or PP) results in increased NADPH availability for xylitol production. A slower glucose uptake rate and slower growth rate due to a pgi mutation have been reported (Hua et al., 2003, Sauer et al., 2004, Blank, 2008), and are likely due to a decreased rate of oxidized cofactor regeneration. The slight reduction in glucose uptake rate for
101 81 JC72 compared to PC09 is in agreement with these observations. As with PC09, the amount of byproducts secreted is a small fraction of the glucose consumed. Zwf and Gnd play important roles in contributing NADPH for xylitol production in PC09. As shown in Table 4 3, Y RPG for JC75 (Δzwf) is reduced to 2.0, which is approximately 40% decreased compared to PC09 and 50% lower than JC72. The glucose consumption rate for JC75 was similar to that for PC09, but JC75 secreted more acetate and less xylitol. Because a higher fraction of glucose carbon was secreted as acid byproducts, the theoretical maximum yield decreased more significantly upon adjustment for byproducts (Table 4 4), although the adjusted yield (5.3 for xylose transport via XylFGH) was still much higher than the experimentally determined Y RPG. Whereas deletion of Zwf in the model causes only a mild reduction in the theoretical maximum xylitol yield (due to elevated flux through PntAB), the deletion has a much more significant impact on the experimental Y RPG. Though not generally used by E. coli growing on glucose (Flores et al., 2002, Hua et al., 2003, Sauer et al., 2004), the ED pathway was deleted in conjunction with the pgi and zwf gene deletions to ensure complete flux through either the PP or EMP pathway, respectively. Mutant strains JC94 (Δpgi, Δedd-eda) and JC76 (Δzwf, Δedd-eda) had Y RPG values comparable to JC72 and JC75, respectively (Appendix G). TCA Cycle and Respiration Mutants The nearly complete oxidation of glucose in resting cells suggests a functional TCA cycle. Inactivation of a complete TCA cycle by deleting succ (strain JC87) significantly lowers the experimental Y RPG (2.3) compared to PC09. As shown in Table 4 3, the glucose consumption rate in JC87 was similar to that in PC09, but JC87 produced more acetate and pyruvate. These results are expected since acetyl-coa can no longer be oxidized through the TCA cycle (Bock, 1996)
102 82 (aerated conditions prevent diversion of carbon to fermentation products) and indicate that a functional TCA cycle contributes to NADPH availability for xylitol production in PC09. Strain JC79 containing a deletion in ndh (NADH dehydrogenase II) was created to determine whether reducing NADH oxidation via respiration would feed back into increased NADPH availability. As shown in Table 4 3, the resulting Y RPG was only 0.6, due largely to incomplete oxidation of glucose. The observed high levels of pyruvate and lactate secretion are likely the result of an elevated NADH/NAD + ratio causing inhibition of pyruvate dehydrogenase (Schwartz et al., 1968) and TCA cycle activity (Vemuri et al., 2006), while pyruvate-formate lyase remains inactive given the aerobic conditions (Alexeeva et al., 2000). Transhydrogenase Deletion and Overexpression Weckbecker and Hummel showed that plasmid-based overexpression of PntAB in E. coli expressing NAD + -dependent formate dehydrogenase and NADP + -dependent alcohol dehydrogenase resulted in increased rates of whole-cell production of (R)-phenylethanol in the presence of formate as a reductant (Weckbecker and Hummel, 2004). In contrast, Sanchez showed that overexpression of SthA in E. coli improved the production of poly(3- hydroxybutyrate) (PHB) in an NADPH-dependent pathway (Sanchez et al., 2006). While not the physiological role of SthA, Sanchez speculated that with a sufficient NADPH drain from PHB synthesis (and therefore elevated concentrations of NADP + ), SthA would operate in the direction of NADPH production, and that overexpressing this enzyme would correspond to increased NADPH supply. In this study the two transhydrogenase systems PntAB and SthA were individually co-expressed with CbXR. Plasmids containing the CbXR gene and either the stha (ppcc500) or pntab (ppcc106) gene(s) were constructed. Transhydrogenase activity assays confirmed functional expression of the transhydrogenases from these plasmids (Figure 4 2). The
103 83 increase in transhydrogenase activity resulting from plasmid-based expression was additionally verified to be soluble for ppcc500 (~3-fold higher) and localized in membranes for ppcc106 (over 4-fold higher) (data not shown). Results for the resting cell cultures of ppcc106 and ppcc500 transformed into strain PC09 are shown in Table 4 3. Overexpression of either transhydrogenase did not improve the Y RPG in resting cells. Surprisingly, overexpression of PntAB (ppcc106) lowered the Y RPG when compared to the plasmids containing only CbXR (ploi3815) or CbXR plus SthA (ppcc500). Strain JC79 was also transformed plasmids ppcc106 and ppcc500 to determine the effects of an overexpressed transhydrogenase on NADPH availability in the context of an elevated NADH/NAD + ratio. As with PC09, an improvement in Y RPG was not obtained when overexpressing either plasmid (batch cultures: Appendix F; resting cell cultures: Appendix G). Figure 4-2: Transhydrogenase activities measured from lysates of E. coli strain PC09 harboring plasmid ploi3815 (expressing CbXR), ppcc500 (expressing CbXR and SthA) or ppcc106 (expressing CbXR and PntAB). Activity reported as Units (mg total protein) -1 in the lysate. Deleting pnta (strain JC73), stha (strain JC74), or both transhydrogenases (strain MR02) results in Y RPG values of ~3.6 for all three strains. Glucose uptake and xylitol production rates were similar to that of PC09, although JC74 and MR02 produced slightly elevated amounts of acetate. Transhydrogenase assays performed on lysate fractions verified the presence of
104 84 transhydrogenase activity in PC09 (Figure 4 2) and decreased activity from the deletion strains were similar to those reported by Sauer (Sauer et al., 2004) (not shown). While the result for JC74 may not be surprising, a reduction in Y RPG relative to PC09 was expected from the pnta deletions, and the modeling study demonstrated the large impact that PntAB has on the theoretical maximum yield. Interestingly, the Y RPG values of the pnta mutants are similar to the maximum theoretical yield predicted by the model (3.6). Furthermore, eliminating the PntAB reaction from other simulated strains ( zwf, pgi, succ) results in theoretical maximum yield values (given in Table 4 2) very similar to the experimentally determined values for the corresponding PC09 deletion strains given in Table 4 3 (these still carrying pntab). These results suggest that transhydrogenase activity does not play a significant role in the supply of NADPH to CbXR under these assay conditions. Metabolic fluxes are apparently adjusted in response to altered transhydrogenase expression such that the available NADPH for xylitol production is not affected. Alternately, in vivo transhydrogenase activity in these resting cells (in strains expressing transhydrogenase) may not be significant relative to the other pathways controlling cofactor metabolism. Strains with deletions in both pgi and stha are unable to grow on glucose minimal medium due to the inability to oxidize excess NADPH (Sauer et al., 2004). JC88 (Δpgi, ΔsthA) was created to determine if excess NADPH generated in this strain could be utilized for xylitol production (assuming SthA would otherwise convert the excess NADPH to NADH). Under resting cell conditions, the Y RPG was similar to that observed for JC72 (~4.0), supporting the notion that SthA does not contribute to xylitol production via interconversion of reducing equivalents between cofactors.
105 85 Expression of NADH-Utilizing Xylose Reductase Finally, the resting cell Y RPG obtained with NADPH-dependent CbXR expression in PC09 was compared to that achieved when expressing the xylose reductase from Candida tenuis (CtXR), which has dual cofactor specificity (i.e., it can use both NADPH (K m ~ 4.8μM) and NADH (K m ~ 25μM)) (Hacker et al., 1999, Neuhauser et al., 1997). The results from Chapter 2 suggest that CtXR allows for batch culture xylitol production levels comparable to those achieved with CbXR. The Y RPG for PC09 harboring plasmid ppcc05 was ~4.0, which is higher than the Y RPG of 3.4 obtained with plasmid ploi3815. Thus in PC09 resting cells the net availability of reduced cofactors is not limiting the yield to 3.4. This again suggests that excess reducing equivalents are not readily transferred from NADH to NADPH. Transforming ppcc05 into JC72 to express CtXR in the context of the pgi deletion (which increased yield with CbXR) did not further improve the yield. Discussion Xylitol yields from strains with deletions in key central metabolism genes were compared in resting cell conditions, and compared to the theoretical maximum values. Using a t-distribution, it was concluded that the probability of the mean Y RPG value of JC72, JC75, JC79, or JC87 being equal to the mean Y RPG value of PC09 is less than 0.2%. By comparing the adjusted Y RPG of PC09, JC75, and JC87, it was concluded that both the TCA cycle and pentose phosphate pathway are the primary contributors of NADPH for xylitol production (in PC09). The increased Y RPG with strain JC72 implies that flux at the glucose-6-phosphate node is split between the EMP and PP pathways in PC09 (as expected (Hua et al., 2003, Sauer et al., 2004, Zhao et al., 2004)). A lower
106 86 experimental Y RPG in strain JC87 implies that the TCA cycle is active under the employed resting cell conditions. While the TCA cycle is often considered to have very low activity under conditions of excess glucose or growth in LB medium (Cronan, 1996, Gray et al., 1966), it is possible that the added cofactor drain resulting from xylitol production and/or the crp* mutation in PC09 contribute to the observed role of the TCA cycle in this study. The metabolic role of transhydrogenases during E. coli biocatalysis has remained largely unspecified. Inferences about metabolic fluxes are readily made without considering the influence of transhydrogenases (e.g., (Walton and Stewart, 2004, Zhao et al., 2004)), and transhydrogenase functional roles inferred from flux analyses depend on the metabolic model and experimental conditions used (Hua et al., 2003, Sauer et al., 2004). The pnta and pnta/stha mutant strains in this study (JC73 and MR02) had Y RPG values similar to the maximum theoretical yields predicted by the metabolic model and similar to the Y RPG for PC09. In the knockout scenarios examined, removing PntAB from the model brought maximum theoretical yields to values close to those determined experimentally. Overexpressing either transhydrogenase did not increase Y RPG s. In the systems described by Weckbecker and Hummel (Weckbecker and Hummel, 2004), and Sanchez (Sanchez et al., 2006), a roughly calculated improved molar yield was less than one. The system described in this work has a reference yield of 3.4, which is significantly higher than the previously described systems. One possibility of why a molar yield improvement was not observed is that the transhydrogenases cannot increase the availability of NADPH in this system due to thermodynamic limitations; whereas the previously described systems have not encountered this limitation (e.g.
107 87 thermodynamically the yield can only be improved to 1.5 with an overexpressed transyhydrogenase). Use of a reductase with relaxed cofactor specificity (CtXR) only slightly improved the Y RPG to 4.0 (using the t-distribution, PC09 + CtXR has a 3.4% chance of having the same Y RPG value as PC09 + CbXR). Thus, it seems that reducing equivalents are not readily transferred from NADH to NADPH via PntAB in this system, and the net reducing equivalents available for xylose reduction does not exceed ~4 moles per mole glucose, regardless of the specific pathways involved. Results from this study raise important questions relating to cofactor trafficking and availability during biocatalysis in whole-cell systems. Whereas the theoretical maximum xylitol yield for strain PC09 is between 6.9 (for ATP-dependent xylose uptake) and 9.2 (for xylose-proton symport) (with growth rate and maintenance energy requirement set at g biomass (g cdw hr) -1 and 7.6 mmol ATP (g cdw hr) -1, respectively), the experimentally determined Y RPG for non-growing cells is only 3.4. The large discrepancy between theoretical maximum and experimentally determined yield values suggests that biocatalysis is compromised by pathways competing for reducing equivalents. More than 60% of the available energy in the form of NAD(P)H resulting from glucose oxidation is dissipated during biotransformation (note this energy consumption is beyond the maintenance energy constraint already included in the model). While scarce, similar cofactor yield analyses with other whole-cell biocatalyst systems reveal similar results. Walton and Stewart describe expressing an NADPH-dependent short-chain dehydrogenase in E. coli to reduce ethyl acetoacetate under glucose-fed, non-growing conditions (Walton and Stewart, 2004). They reported the stoichiometry linking ketone reduction and glucose consumption (i.e., Y RPG ) to be ~2.3. Schmid analyzed NADH
108 88 usage for styrene epoxidation in recombinant E. coli expressing styrene monooxygenase (Blank, 2008, Buhler et al., 2008). In aerobic continuous culture with glucose as the growth substrate, they observed net NAD(P)H consumption that was times higher than the amount of NADH required for styrene epoxidation, with a corresponding Y RPG near one (Buhler et al., 2008). In agreement with these results, switching to resting cells improved the styrene oxide (i.e., NADH) yield to nearly three, and this yield was not significantly altered when either transhydrogenase was deleted (Blank, 2008). In these examples it is unclear how the remaining reducing equivalents are dissipated. Schmid suggested that the deviations from predicted cofactor yields could be the result of increased maintenance energy requirements during biocatalysis, energy spilling, or uncoupling of NADH oxidation from styrene oxidation by the monooxygenase (Blank, 2008, Buhler et al., 2008). Assuming cofactor uncoupling by xylose reductase is not a significant drain of NADPH, the remaining reducing equivalents are most likely eliminated through respiration. This suggests that under physiological conditions the PntAB and reductase reactions do not effectively compete with respiration. Decreasing respiration by reducing oxygen availability or deleting ndh (NADH dehydrogenase II) lowered Y RPG values due to secretion of acids and fermentation products. Again, the conditions are such that transhydrogenase and reductase reactions apparently do not effectively compete for available NADH. It is not likely that true maintenance energy requirements are the primary drain on NAD(P)H, given that resting cells in this study are unable to synthesize protein. If there is not a demand for ATP, the question becomes at what point is energy lost? Many scenarios can be rationalized, such as ATP hydrolysis via futile cycles, uncoupling during
109 89 electron transport, dissipation of proton gradients, and significant inefficiencies in substrate transport. Addressing this issue by tracking the fate of all NAD(P)H in resting cells should provide insights toward the design of more efficient biocatalysts. Conclusion From the results presented in this chapter, it was observed that there was no xylitol yield (Y RPG ) change upon deletion of either or both transhydrogenase(s). These results imply that the transhydrogenases were not making more NADPH available for xylose reduction. The gene deletion strategy (pgi deletion) that directed metabolic flux towards reactions that generate NADPH increased xylitol yields (Y RPG ) found in resting cell cultures. Gene deletion strategies (zwf and succ) that directed metabolic flux away from reactions that generate NADPH decreased xylitol yields (Y RPG ). These results suggest that direct NADPH production is significant for increased xylitol yields. Therefore, this research should focus on determining methods and strategies that would improve the availability of NADPH to increase xylitol yields. This research should also try to determine an adequate method to measure the internal concentration of the nicotinamide cofactors to examine whether novel strategies are able to increase the availability of NADPH. References ALEXEEVA, S., DE KORT, B., SAWERS, G., HELLINGWERF, K. J. & DE MATTOS, M. J. (2000) Effects of limited aeration and of the ArcAB system on intermediary pyruvate catabolism in Escherichia coli. J Bacteriol, 182,
110 BABA, T., ARA, T., HASEGAWA, M., TAKAI, Y., OKUMURA, Y., BABA, M., DATSENKO, K. A., TOMITA, M., WANNER, B. L. & MORI, H. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol Syst Biol, 2, BLANK, L. M., EBERT,B.E., BÜHLER,B., SCHMID,A. (2008) Metabolic capacity estimation of Escherichia coli as platform for redox biocatalysis: Constraint based modeling and experimental verification. Biotechnology and Bioengineering, 100, BOCK, A., AND GARY SAWERS (1996) Fermentation. IN NEIDHARDT, F. C., CURTISS III,R., INGRAHAM,J.L, LIN,E.C.C, LOW,K.B., MAGASANIK,B., REZNIKOFF,W.S, RILEY,M., SCHAECHTER,M., AND UMBARGER,H.E. (Ed.) Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C., ASM Press. BUHLER, B., PARK, J. B., BLANK, L. M. & SCHMID, A. (2008) NADH availability limits asymmetric biocatalytic epoxidation in a growing recombinant Escherichia coli strain. Appl Environ Microbiol, 74, CANONACO, F., HESS, T. A., HERI, S., WANG, T., SZYPERSKI, T. & SAUER, U. (2001) Metabolic flux response to phosphoglucose isomerase knock-out in Escherichia coli and impact of overexpression of the soluble transhydrogenase UdhA. FEMS Microbiol Lett, 204, CAUSEY, T. B., ZHOU, S., SHANMUGAM, K. T. & INGRAM, L. O. (2003) Engineering the metabolism of Escherichia coli W3110 for the conversion of sugar to redox-neutral and oxidized products: homoacetate production. Proc Natl Acad Sci U S A, 100, CIOTTI, M. M. & KAPLAN, N. O. (1956) Chemistry and properties of the 3-acetylpyridine analogue of diphosphopyridine nucleotide. J Biol Chem, 221, COVERT, M. W. & PALSSON, B. O. (2002) Transcriptional regulation in constraints-based metabolic models of Escherichia coli. J Biol Chem, 277, CRONAN, J. E., AND C.O. ROCK (Ed.) (1996) Tricarboxylic acid cycle and glyoxylate bypass, Washington, D.C., ASM Press. DATSENKO, K. A. & WANNER, B. L. (2000) One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc Natl Acad Sci U S A, 97, FLORES, S., GOSSET, G., FLORES, N., DE GRAAF, A. A. & BOLIVAR, F. (2002) Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase system by (13)C labeling and NMR spectroscopy. Metab Eng, 4, GRAY, C. T., WIMPENNY, J. W. & MOSSMAN, M. R. (1966) Regulation of metabolism in facultative bacteria. II. Effects of aerobiosis, anaerobiosis and nutrition on the formation of Krebs cycle enzymes in Escherichia coli. Biochim Biophys Acta, 117, HACKER, B., HABENICHT, A., KIESS, M. & MATTES, R. (1999) Xylose utilisation: cloning and characterisation of the Xylose reductase from Candida tenuis. Biol Chem, 380, HALDIMANN, A. & WANNER, B. L. (2001) Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria. J Bacteriol, 183, HELLER, K. B., LIN, E. C. & WILSON, T. H. (1980) Substrate specificity and transport properties of the glycerol facilitator of Escherichia coli. J Bacteriol, 144, HUA, Q., YANG, C., BABA, T., MORI, H. & SHIMIZU, K. (2003) Responses of the central metabolism in Escherichia coli to phosphoglucose isomerase and glucose-6-phosphate dehydrogenase knockouts. J Bacteriol, 185, KHANKAL, R., CHIN, J. W. & CIRINO, P. C. (2008) Role of xylose transporters in xylitol production from engineered Escherichia coli. J Biotechnol, 134,
111 MILLER, J. H. (1992) A short course in bacterial genetics: A laboratory manual and handbook for Escherichia coli and related bacteria., Cold Spring Harbor, Cold Spring Harbor Press. MOREIRA DOS SANTOS, M., RAGHEVENDRAN, V., KOTTER, P., OLSSON, L. & NIELSEN, J. (2004) Manipulation of malic enzyme in Saccharomyces cerevisiae for increasing NADPH production capacity aerobically in different cellular compartments. Metab Eng, 6, NEUHAUSER, W., HALTRICH, D., KULBE, K. D. & NIDETZKY, B. (1997) NAD(P)Hdependent aldose reductase from the xylose-assimilating yeast Candida tenuis. Isolation, characterization and biochemical properties of the enzyme. Biochem J, 326 ( Pt 3), REED, J. L., VO, T. D., SCHILLING, C. H. & PALSSON, B. O. (2003) An expanded genomescale model of Escherichia coli K-12 (ijr904 GSM/GPR). Genome Biol, 4, R54. SAMBROOK, J., RUSSELL D.W. (2001) Molecular cloning: A laboratory manual., Cold Spring Harbor, Cold Spring Harbor Press. SANCHEZ, A. M., ANDREWS, J., HUSSEIN, I., BENNETT, G. N. & SAN, K. Y. (2006) Effect of overexpression of a soluble pyridine nucleotide transhydrogenase (UdhA) on the production of poly(3-hydroxybutyrate) in Escherichia coli. Biotechnol Prog, 22, SAUER, U., CANONACO, F., HERI, S., PERRENOUD, A. & FISCHER, E. (2004) The soluble and membrane-bound transhydrogenases UdhA and PntAB have divergent functions in NADPH metabolism of Escherichia coli. J Biol Chem, 279, SCHWARTZ, E. R., OLD, L. O. & REED, L. J. (1968) Regulatory properties of pyruvate dehydrogenase from Escherichia coli. Biochem Biophys Res Commun, 31, VARMA, A. & PALSSON, B. O. (1994) Stoichiometric flux balance models quantitatively predict growth and metabolic by-product secretion in wild-type Escherichia coli W3110. Appl Environ Microbiol, 60, VEMURI, G. N., ALTMAN, E., SANGURDEKAR, D. P., KHODURSKY, A. B. & EITEMAN, M. A. (2006) Overflow metabolism in Escherichia coli during steady-state growth: transcriptional regulation and effect of the redox ratio. Appl Environ Microbiol, 72, VERHO, R., LONDESBOROUGH, J., PENTTILA, M. & RICHARD, P. (2003) Engineering redox cofactor regeneration for improved pentose fermentation in Saccharomyces cerevisiae. Appl Environ Microbiol, 69, WALTON, A. Z. & STEWART, J. D. (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog, 20, WECKBECKER, A. & HUMMEL, W. (2004) Improved synthesis of chiral alcohols with Escherichia coli cells co-expressing pyridine nucleotide transhydrogenase, NADP+dependent alcohol dehydrogenase and NAD+-dependent formate dehydrogenase. Biotechnol Lett, 26, ZHAO, J., BABA, T., MORI, H. & SHIMIZU, K. (2004) Global metabolic response of Escherichia coli to gnd or zwf gene-knockout, based on 13C-labeling experiments and the measurement of enzyme activities. Appl Microbiol Biotechnol, 64, ZHAO, J. & SHIMIZU, K. (2003) Metabolic flux analysis of Escherichia coli K12 grown on 13C-labeled acetate and glucose using GC-MS and powerful flux calculation method. J Biotechnol, 101,
112 Chapter 5 Development of a Protocol to Determine the Internal Concentration of Nicotinamide Cofactors in Escherichia coli
113 93 Abstract Metabolic manipulations to increase the availability of reduced cofactors are often employed to improve the efficiency (titer and yield) of biotransformations of interest. Although flux analysis can provide some insight to the contributions of particular reactions and pathways (Zamboni and Sauer, 2009), relatively few groups have reported quantifying intracellular cofactor levels in efforts to establish relationships between cofactor ratios and availability for biocatalysis (Berrios-Rivera et al., 2002, Li et al., 2009, San et al., 2002, Sanchez et al., 2006, Walton and Stewart, 2004). Although there are several reported methods to quantify the internal concentration of the nicotinamide cofactors, this research seeks to develop a protocol that improves the time efficiency, while maintaining the accuracy of the existing methods. This chapter examines the use of spectrophotometric and cycling assay methods to accurately quantify the internal cofactor levels of Escherichia coli samples. Spectrophotometric results were often inconsistent, due to the instability of the cofactors, and lacked the sensitivity that was desired. The final modified protocol, a cycling assay in a 96-well format, improved the time efficiency, while retaining the sensitivity found in the cuvette format. Introduction Biotransformation reactions of importance often require the use of the reduced nicotinamide cofactors NAD(P)H. Many genetic modifications have been reported to improve the availability of reduced cofactors for biotransformation reactions of interest (Berrios-Rivera et al., 2002, Li et al., 2009, Lin et al., 2005a, Lin et al., 2005b, San et al., 2002, Sanchez et al., 2006, Weckbecker and Hummel, 2004, Yun et al., 2005). Several of these groups have reported concentrations of the nicotinamide cofactors to verify increased availability (Berrios-Rivera et al.,
114 , Li et al., 2009, San et al., 2002, Sanchez et al., 2006). However, there are many inherent difficulties associated with measuring the nicotinamide cofactors, which include lack of sensitivity, artificial oxidation of the reduced forms, and maintaining the stability of nicotinamide cofactors (Klaidman et al., 1995). Lowry and coworkers report that the oxidized forms of the cofactor are stable under acidic conditions, while the reduced forms of the reduced cofactors are stable under basic conditions (Lowry et al., 1961). Although longer term storage of the cofactors could be achieved, a separate procedure is necessary to isolate the oxidized and reduced forms for each sample. Artificial oxidation of the reduced forms may occur upon harvesting the sample without a suitable method to quench metabolism. A suitable quenching method should be able to immediately arrest metabolism, trap internal metabolites in their current state, and prevent metabolites from leaking out of the cell (Mashego et al., 2007). Although a low volume of cell culture can be quenched with immediate immersion into an acid, base, or cold methanol (Mashego et al., 2007), enough sample must be harvested to ensure that the total amount of desired the metabolite(s) is above the sensitivity of the detection method (Lilius et al., 1979). Detection of the nicotinamide cofactors can be accomplished by one of three ways: spectrophotometrically, fluorometrically, and enzymatic cycling (Matsumura and Miyachi, 1980). Spectrophotometric/Fluorometric Spectrophotometric detection methods are based on using high performance liquid chromatography (HPLC) to separate the different forms of the nicotinamide cofactors using a C- 18 reverse-phase chromatrography column and detecting the compounds with a UV detector. Absorbance at λ = 254 nm is used to detect the four different forms of the nicotinamide cofactors as well as nucleotides (i.e. AMP, ADP, ATP, etc); and absorbance at λ = 340 nm can be used to detect the reduced forms. Most groups isolated the nicotinamide cofactors using an acid/base
115 95 extraction method (Klaidman et al., 1995, Shalel-Levanon et al., 2005, Stocchi et al., 1985). Although the cofactors were relatively stable in the acidic or basic conditions, the oxidized and reduced samples were injected separately, and the samples needed to be kept chilled until injection to retard the rate of degradation. Specifically, Stocchi and coworkers developed a protocol to extract nicotinamide cofactors and nucleotides/nucleosides from red blood cells (Stocchi et al., 1985). They used perchloric acid and potassium hydroxide solutions to extract the oxidized and reduced forms of the cofactors, respectively. The solutions were neutralized (to ph 6.5) with either K 2 CO 3 (for acid treatment) or KH 2 PO 4 (for alkaline treatment) then analyzed using a LC-18 column with a mobile phase of 0.1 M KH 2 PO 4 (ph 6) with a gradient of methanol (up to 10% v v -1 ) (Stocchi et al., 1985). Shalel- Levanon and coworkers modified this method to measure the nicotinamide cofactors in bacterial cultures, adding in a concentration step (Shalel-Levanon et al., 2005). To concentrate the cells, they removed 20 ml of culture and immediately centrifuged for 10 minutes at 4 C. They used a cold alkaline treatment to lyse the cells and extract both the oxidized and reduced cofactors (NAD(H)), and neutralized the solution using KH 2 PO 4, ph 6.5. Although the running buffer was the same as described by Stocchi, the gradient protocol was changed, presumably to achieve a better separation (slower flow rate and a longer sample run time). This method detected both the oxidized and reduced cofactors simultaneously, though may be inaccurate due to potential oxidized cofactor (NAD + ) destruction under basic conditions, as evidenced by Lowry (Lowry et al., 1961). Klaidman and coworkers developed a method that eliminated the need for two separate extractions and analyses for the oxidized and reduced nicotinamide cofactors, while preserving the integrity of the different forms. Their method improved sensitivity by detecting fluorescence, which is inherently more sensitive than UV detection. By performing the extraction in a basic
116 96 solution supplemented with cyanide, the oxidized forms would react with the cyanide to produce NAD-CN and NADP-CN, which are stable in a basic solution (Klaidman et al., 1995). After extracting the nicotinamide cofactors from the lysate with chloroform, the cofactors were filtered, diluted with the mobile phase, and injected into the HPLC system. Separation was accomplished using a C-18 column with a running buffer of ammonium acetate and methanol. Detection was accomplished with a fluorescence detector exciting at a wavelength of 330 nm and emitting at a wavelength of 460 nm, which was able to detect the derivatized and reduced forms of the cofactors (Klaidman et al., 1995). Cycling Assay The basis of cycling assays is to transfer electrons from a sacrificial substance to the nicotinamide cofactors, which then transfers electrons to a terminal electron acceptor, which can be detected spectrophotometrically; an example can be seen in Figure 5 1. Increased sensitivity is achieved with cycling assays because the assays measure the rate of production, which is dependent on the concentration of the cofactor, rather than measuring the cofactor concentration directly. The specificity of the enzyme-catalyzed reaction allows the detection of either NAD(H) or NADP(H) with high accuracy. However, standardization of read times or inhibition of the terminal acceptor formation is necessary for an accurate comparison between samples and standards.
117 97 Figure 5-1: Basic concept of the cycling assay used in this study. The amount of reduced MTT (MTT red ), which is readily measured at a wavelength of 570 nm, is proportional to the concentration of cofactor in the assay. Bernofsky and Swan modified an existing cycling assay to use thiazolyl blue tetrazolium bromide (MTT) versus 2,6-dichlorophenolindophenol as the terminal electron acceptor to measure the concentration of NAD + and NADH. They reported that by using MTT, greater concentrations of the terminal acceptor and longer assay times can be used, which allowed for picomole levels of NAD(H) detection. By substituting PES (phenazine ethosulfate) for PMS (phenazine methosulfate), the assay conditions became more stable, which also allowed for longer assay times (Bernofsky and Swan, 1973). Gibon and Larher modified the protocol described by Bernofsky and Swan by adding an inhibitor that will terminate electron transfer to the final electron acceptor for both the alcohol dehydrogenase and glucose-6-phosphate dehydrogenase catalyzed reactions (Gibon and Larher, 1997). Although Bernofsky and Swan reported that iodoacetate could be used to terminate the reaction catalyzed by alcohol dehydrogenase, which would end electron transfer to MTT, they did not report any inhibitor of glucose-6-phosphate dehydrogenase, nor did they report measuring the concentrations of NADP + or NADPH (Bernofsky and Swan, 1973). Gibon and Larher demonstrated that by quenching both alcohol dehydrogenase and glucose-6-phosphate dehydrogenase reactions with a high concentration of NaCl, they could more accurately
118 98 determine the internal concentration of the cofactors by eliminating time as a variable between the standards and samples. The high NaCl concentration also effectively caused the reduced MTT to precipitate in the reaction solution, which could be easily separated via centrifugation, then resolubilized in a 96% ethanol solution (Gibon and Larher, 1997). Walton and Stewart provided a more recent account of performing the cycling assay to determine the internal concentrations of the four nicotinamide cofactors in bacterial cultures. Their method was based on that described by Gibon and Larher (Gibon and Larher, 1997), the difference coming in sample preparation. Quenching of cellular metabolism was accomplished by immediately adding 0.25 ml of a 0.3 M solution of either HCl (to extract the oxidized cofactors) or KOH supplemented with 10 mg CuCl (to extract the reduced forms) to 0.5 ml of cell culture (Walton and Stewart, 2004). The addition of CuCl was reported to protect the assay from nonprotein thiols (e.g. glutathione) (Lilius et al., 1979). After vortexing and heating at 60 C for 7 minutes, the samples were stored until the assay was ready to be performed. Materials and Methods Spectrophotometric Cofactor standards were initially prepared in 0.1 M KH 2 PO 4 and later in either 0.1 M HCl (for oxidized forms) or 0.1 M NaOH (for reduced forms) to help preserve the cofactors. The acidic and basic solutions were neutralized prior to injection in the HPLC. Biological concentrations of the cofactors were determined by comparing against a cofactor standard calibration curve. Cofactors were detected by using a Shimadzu LC-10AD HPLC equipped with a dual wavelength UV monitor measuring the absorbance at λ = 254 nm and λ = 340 nm, unless otherwise noted. The separation procedure was modified so that an isocratic pump running a
119 99 single buffer could be used instead of using a gradient method. The nicotinamide cofactors were initially separated using an Ascentis C18 column (Supelco, Bellefonte, PA); however, upon further testing, it was determined that a Discovery BIO Wide Pore C18 column (Supelco) was better suited for use with a completely aqueous running buffer, compared to the Ascentis column, which required an organic phase in the running buffer. The mobile phase was initially a 0.1 M KH 2 PO 4, ph 6 solution containing methanol (5-10% v v -1 ), but was changed to a 0.1 M KH 2 PO 4, ph 6 solution without methanol. Flow rates were varied in order to optimize separation, and are described in the results. Biological samples were prepared by modifying a protocol described by Shalel-Levanon et al. (Shalel-Levanon et al., 2005); modifications were added so that smaller volumes could be used. Quenching was initially attempted by aliquoting 4 ml of culture into 16 ml of 60% methanol prechilled to -20 C. The solution was then centrifuged at 0 C for 10 minutes at 3250 x g, and the supernatant was removed. Ice cold 0.5 M KOH (200 µl) was then added to the pellet and allowed to sit on ice for 10 minutes. Water at 4 C (200 µl) was then added to the cell lysate and allowed to sit on ice for 10 minutes. This mixture was then centrifuged at 0 C for 10 minutes at 3250 x g, and the supernatant was placed onto a filter plate to remove cellular debris. An equal amount of 1 M KH 2 PO 4 solution, ph 6.5 was then added to the filtrate to neutralize the solution. Quenching was finally accomplished using an acid/base extraction method described by Walton and Stewart (Walton and Stewart, 2004). Cycling Assay The procedure was based on the methods developed by Bernofsky and Swan (Bernofsky and Swan, 1973), and modified by Gibon and Larher (Gibon and Larher, 1997), and Walton and Stewart (Walton and Stewart, 2004). Biological samples were prepared by modifying a protocol
120 100 described by Walton and Stewart (Walton and Stewart, 2004). Cells were grown as described in the resting cell culture section (unless otherwise noted). Cells were harvested by centrifuging an appropriate amount of cells to achieve a final OD 600 of 30 in 1 ml (4 C, 10 min, 3250 x g). To isolate the oxidized forms, the pellet was resuspended in 0.5 ml of 0.3 M HCl supplemented with 50 mm Tricine-NaOH (ph 8.0). To isolate the reduced forms, the pellet was resuspended in 0.5 ml of 0.3 M NaOH. All samples were then heated to 60 C for 7 minutes followed by a neutralization step (0.5 ml 0.3 M NaOH for oxidized forms, 0.5 ml 0.3 M HCl, supplemented with 50 mm Tricine-NaOH (ph 8.0) for reduced forms). Tricine-NaOH (ph 8.0) was added to the cell lysates if neutral ph was not achieved (100 µl of 1 M stock). The neutralized solutions were then centrifuged (10 minutes, 18,000 x g) and the supernatants were transferred to a fresh microcentrifuge tube. Cofactor levels were measured in a 96-well microtiter plate. Either 40 μl of oxidized sample and 40 μl 0.1 M NaCl, or 80 μl of reduced sample was aliquoted to a single well. The 2X stock solution of the reaction mixture consisted of equal volumes of 1.0 M Tricine-NaOH (ph 8.0), 4.2 mm MTT, 40 mm EDTA, 1.67 mm PES, and substrate (either 5 M ethanol or 25 mm glucose-6-phosphate). After addition of the appropriate reaction mixture (ethanol for NAD(H), glucose-6-phosphate for NADP(H)), the plate was incubated at 37 C for 5 minutes. To start the reaction, either 10 units ml -1 alcohol dehydrogenase (from 100 units ml -1 stock) or 0.27 units ml -1 glucose-6-phosphate dehydrogenase (from 2.7 units ml -1 stock) was added. The formation of reduced thiazolyl blue tetrazolium bromide (MTT) was monitored using a SpectraMax 384 plate reader, taking readings every 15 seconds for 10 minutes using a wavelength of 570 nm while being incubated at 37 C. The cofactor concentration of the samples was calculated by subtracting the rate from the background of the sample (reaction without enzyme) and interpolating the corrected result with a standard calibration curve run on the same plate.
121 101 The method described by Stewart was performed briefly as follows: in a centrifuge tube, take 100 μl cell lysate and 300 μl 0.1M NaCl (for NADH) or 50 μl cell lysate and 350 μl 0.1M NaCl (for NADP(H)) and add 500 μl of the reaction mixture. Incubate the sample and mixture at 37 C for 5 minutes. Add 100 μl of the enzyme, and incubate at 37 C for 40 minutes. After the reaction time, add 500 μl saturated NaCl then centrifuge for 10 minutes at 18,000 x g. Remove 1.4 ml of the supernatant and dissolve the pellet in 0.9 ml 100% ethanol and transfer 200 μl to a 96-well plate and measure the absorbance at λ = 570 nm (A 570 ) (Walton and Stewart, 2004). Resting Cell Cultures Resting cells were grown in rich (LB) medium and prepared using a protocol as described in Chapter 3. Molar yields of xylitol per glucose consumed (Y RPG ), were calculated by subtracting for the xylitol produced in the absence of glucose from the amount produced in the presence of glucose, before normalizing with respect to glucose consumption. Results Spectrophotometric Running buffer optimization The method developed by Stocchi et al (Stocchi et al., 1985) was initially tested to verify that the four different forms could be separated with the described gradient method with an Ascentis C18 column. Separation was achieved between the four forms of the nicotinamide cofactors using the gradient running buffer and this C18 column; however it was desired to
122 102 eliminate the need of a gradient method and use an isocratic method to achieve the desired separation. Therefore an optimal running buffer (e.g. methanol concentration) and flow rate needed to be determined to achieve adequate separation. Running buffers containing different concentrations of methanol were tested and it was determined to not adequately separate the cofactors (Appendix H), therefore a running buffer without methanol was tested. Standards of 1 M NAD + and NADPH were run using a method with a flow rate of 0.5 ml min -1, absorbance detection at λ = 254 and λ = 340 nm, a running time of 25 minutes, and a running buffer of 0.1 M KH 2 PO 4 solution, ph 6. The NAD + standard was detected by absorbance at λ = 254 nm with a retention time of 7.4 minutes. NADPH had a distinctive peak with a retention time of 5.5 minutes at both wavelengths (λ = 254 and 340 nm). A 50:50 mixture of the 1 M standards showed that the two cofactors could be adequately separated (Figure 5 2). Figure 5-2: HPLC trace of a 0.5 M mixture of NADPH and NAD +. The dark chromatogram is the absorbance at λ = 254 nm; the light chromatogram is the absorbance at λ = 340 nm. NADPH elutes with a retention time of ~ 5.5 minutes; NAD + elutes with a retention time of ~ 7.5 minutes.
123 103 Sample preparation Because adequate separation and detection of the nicotinamide cofactors was achieved, different extraction methods were tested to determine if cellular amounts of cofactors could be detected. The cofactors were extracted modifying the protocol described by Shalel-Levanon and coworkers (Shalel-Levanon et al., 2005), as described in the Materials and Methods section. Unfortunately, when the samples (2 different growing conditions with 2 different stopping OD 600 were run: LB OD 600 = 3.0 (Figure 5 3); LB OD 600 = 1.3; NBS OD 600 = 1.9; NBS OD 600 = 1.1 (data not shown)) were run, no cofactors were observed in any of the chromatograms. It was initially hypothesized that the quenching method degraded the extracted cofactors; however, preparing the cofactor standards using the quenching method did not affect peak height or retention time (data not shown). It was concluded that the cells were not concentrated enough, and a method needed to be developed to concentrate the cells to have a detectable amount of cofactor and quench metabolism so that metabolism was immediately arrested. Figure 5-3: Chromatograms of a cofactor mixture standard (0.25 mm) of all four nicotinamide cofactors compared to a biological sample at two different absorbances. The grey chromatogram represents the absorbance of the cofactor standard at λ = 254 nm; the light grey chromatogram
124 represents the absorbance of the cofactor standard at λ = 340 nm; the black chromatogram represents the absorbance of the biological sample (W3110) at λ = 254nm. Peak order is as follows: NADP +, NADPH, NAD +, NADH. 104 Mass spectrometry In order to achieve a lower detection limit without concentrating the cells, it was decided to incorporate the use of mass spectrometry (MS) after the chromatography separation step. However, because mass spectrometry involves vaporizing the compounds, the running buffer for the chromatography step needed to be volatile as well. It was suggested that ammonium acetate would be an adequate substitute to the potassium phosphate (KH 2 PO 4 ) because both would have the same ph with a 0.1 M concentration (ph ~ 6). Using ammonium acetate as the running buffer, the cofactor standards had a shorter retention time and adequate separation between NADP + and NADPH was no longer achieved (Figure 5 4, 5 5). Changing the concentration of ammonium acetate did not appear to help the separation: a lower concentration resulted in narrower peaks which were closer together; a higher concentration resulted in wider peaks (data not shown). However, because MS also determines the molecular weight of the compound, it should be able to differentiate between the two forms. The results of the MS showed that the nicotinamide cofactors were not detected at the expected time, which was confirmed when the samples were rerun using the lab HPLC setup. It was hypothesized that the stability of the cofactors was the issue and the resulting degradation caused the shift in retention time and lowered the peak heights. Further testing involving the mass spectrometer can be found in Appendix H.
125 105 Figure 5-4: Chromatograms of the cofactor standards (1 mm each) using 0.1 M ammonium acetate as the running buffer at λ = 254. The green chromatogram represents a standard of NADH; the light blue chromatogram represents a standard of NAD + ; the pink chromatogram represents a standard of NADPH; the dark green chromatogram represents a standard of NADP +. Figure 5-5: Chromatograms of the cofactor standards (1 mm each) using 0.1 M ammonium acetate as the running buffer at λ = 340 nm. The green chromatogram represents a standard of NADH; the light blue chromatogram represents a standard of NAD + ; the pink chromatogram represents a standard of NADPH; the dark green chromatogram represents a standard of NADP +.
126 106 Cyanide addition (sample preparation) A deeper literature search yielded an interesting prospect: by adding cyanide to the sample, the four forms of the nicotinamide cofactors could be prepared with a single method without degradation of any of the samples. It was reported that under basic conditions, the addition of cyanide would react with the oxidized forms to produce NAD-CN and NADP-CN which are stable under basic conditions (Klaidman et al., 1995). Fluorescence detection (excite at 330 nm, emit at 460 nm) could then be used to detect the cofactors with increased sensitivity compared to UV detection. However, due to the constraints of the lab setup (lack of a fluorescence detector), the UV absorbance was monitored. A wavelength of λ = 254 nm was monitored because that wavelength is able to detect the adenine portion of the cofactor. A wavelength of λ = 325 nm was monitored because it had been reported that a cyanide complex of the oxidized form will absorb at this wavelength (Matsumura and Miyachi, 1980). Unfortunately, when the cyanide treatment method was tested with the lab system, the conditions used were not able to differentiate NAD- CN from NADP-CN (data not shown). Although separation was achieved, due to the instability of the cofactors and the timing of the Proteomics and Mass Spectrometry Core Facility at Penn State, this research shifted focus from using spectrophotometric means of detection to cycling assays. Cycling Assay Pretest Several tests were conducted to verify the specificity of the enzyme, substrate, PES and MTT concentration, and length of the assay. Results for these tests can be found in Appendix H.
127 107 Concentrating the sample via centrifugation Because of the low levels of cofactors detected with the spectrophotometric tests (see Appendix H), it was decided to concentrate the cells in a similar method as Shalel-Levanon (Shalel-Levanon et al., 2005). The protocol for concentrating the cells is described in the Materials and Methods section. This method of concentration was found to be suitable for acquiring a detectable amount of nicotinamide cofactors. Supernatant and enzyme amounts were then varied to determine the optimal volumes of each, with respect to reducing background levels. It was observed that by increasing the cell lysate concentration, the background percentage levels appeared to have decreased. From this experiment, it was determined that complete neutralization of the lysate solution was necessary to keep the absorbance of the reduced sample in the linear range. Comparison between protocols Another test was performed to compare the adapted (96-well plate format) protocol and the protocol described by Stewart, with and without the use of CuCl in the reduced forms. This test was focused on reducing the background percentage and comparing the accuracy of the adapted method, but only tested the cofactors: NADP +, NADPH, and NADH. Strain W3110 was used as the biological sample to be compared. The first set of results with Stewart s method had significant amounts of precipitation at the bottom of the well, which affected the absorbance measurement. For the second set of results, the sample was centrifuged prior to measuring the supernatant in the 96-well plate. The values between the adapted method and Stewart s method do not seem significantly different; the exception being NADH for W3110 resuspended with CuCl (data not shown). It is unknown why that value was significantly different than the other
128 108 readings. Although the background levels were slightly lower with the method outlined by Stewart; it was concluded that the time to set up multiple reactions in separate tubes was significantly longer than the time necessary to set up the 96-well plate. Therefore it was more time efficient to utilize the modified method using a 96-well plate compared to the method described by Stewart. When the standards were prepared with CuCl, it was observed that CuCl did not affect the cofactor concentration in the biological sample or in the standards. Results comparing the standard curve between the different protocols can be found in Appendix H. Discussion Lilius and coworkers have previously demonstrated the use of CuCl as a means of improving the recovery of NAD(P)H from cellular extract (Lilius et al., 1979). However, from the results of the tests performed, it was observed that CuCl did not affect the cofactor results and was unnecessary with the modified protocol (Khankal et al., 2009). The use of CuCl may be necessary to protect the samples from interfering thiols if the samples were stored away for an extended time prior to testing, as described by others (Lilius et al., 1979, Walton and Stewart, 2004). However, because the assays were performed on the same day as the cofactor extraction, the thiols may not have had enough time to significantly reduce the recovery of the reduced nicotinamide cofactors. When testing the length of time for the cofactor assay, the cofactor standards steadily increased in measured absorbance over time then leveled off after approximately 60 minutes. The biological samples had a measured absorbance that continued to steadily increase over time and did not level off over the tested time. This led to an artificial increase in calculated concentration in the samples after 60 minutes, rather than a constant calculated concentration over time. Although it is unknown why the measured absorbance of the cofactor standards degraded
129 109 faster, it is hypothesized that the lysate may contain substances that either inhibits formation of the precipitating reduced MTT or allows the cycling reaction to continue. Regardless, the length of time used in the protocol was short enough that the measured absorbance of the cofactor standards remained in the linear region. Although concentration of the cell culture was not attempted in conjunction with the HPLC separation and spectrophotometric detection, it should be noted that a comparison of the peak amplitude from the cofactor standard run on the HPLC and cofactor concentration determined by the cycling assay that the UV detector would not be sensitive enough to distinguish the peaks from noise with only UV detection. With the use of the mass spectrometer, the detection limit was lowered; however, the time between lysing the cell and running the sample was significant (especially with a larger number of samples). Depending on the degradation rate of the cofactors, the time to inject the cofactor samples might be significant enough to impact the actual cofactor concentration. Using an HPLC/MS setup would eliminate the background issue observed with the cycling assay; however, optimization of the separation process remains to be accomplished. References BERNOFSKY, C. & SWAN, M. (1973) An improved cycling assay for nicotinamide adenine dinucleotide. Anal Biochem, 53, BERRIOS-RIVERA, S. J., SAN, K. Y. & BENNETT, G. N. (2002) The effect of NAPRTase overexpression on the total levels of NAD, the NADH/NAD+ ratio, and the distribution of metabolites in Escherichia coli. Metab Eng, 4, GIBON, Y. & LARHER, F. (1997) Cycling assay for nicotinamide adenine dinucleotides: NaCl precipitation and ethanol solubilization of the reduced tetrazolium. Anal Biochem, 251, KHANKAL, R., CHIN, J. W., GHOSH, D. & CIRINO, P. C. (2009) Transcriptional effects of CRP* expression in Escherichia coli. J Biol Eng, 3, 13. KLAIDMAN, L. K., LEUNG, A. C. & ADAMS, J. D., JR. (1995) High-performance liquid chromatography analysis of oxidized and reduced pyridine dinucleotides in specific brain regions. Anal Biochem, 228,
130 LI, Z. J., CAI, L., WU, Q. & CHEN, G. Q. (2009) Overexpression of NAD kinase in recombinant Escherichia coli harboring the phbcab operon improves poly(3-hydroxybutyrate) production. Appl Microbiol Biotechnol, 83, LILIUS, E. M., MULTANEN, V. M. & TOIVONEN, V. (1979) Quantitative extraction and estimation of intracellular nicotinamide nucleotides of Escherichia coli. Anal Biochem, 99, LIN, H., BENNETT, G. N. & SAN, K. Y. (2005a) Chemostat culture characterization of Escherichia coli mutant strains metabolically engineered for aerobic succinate production: a study of the modified metabolic network based on metabolite profile, enzyme activity, and gene expression profile. Metab Eng, 7, LIN, H., BENNETT, G. N. & SAN, K. Y. (2005b) Metabolic engineering of aerobic succinate production systems in Escherichia coli to improve process productivity and achieve the maximum theoretical succinate yield. Metab Eng, 7, LOWRY, O. H., PASSONNEAU, J. V. & ROCK, M. K. (1961) The stability of pyridine nucleotides. J Biol Chem, 236, MASHEGO, M. R., RUMBOLD, K., DE MEY, M., VANDAMME, E., SOETAERT, W. & HEIJNEN, J. J. (2007) Microbial metabolomics: past, present and future methodologies. Biotechnol Lett, 29, MATSUMURA, H. & MIYACHI, S. (1980) Cycling Assay for Nicotinamide Adenine Dinucleotides. Methods in Enzymology, 69, SAN, K. Y., BENNETT, G. N., BERRIOS-RIVERA, S. J., VADALI, R. V., YANG, Y. T., HORTON, E., RUDOLPH, F. B., SARIYAR, B. & BLACKWOOD, K. (2002) Metabolic engineering through cofactor manipulation and its effects on metabolic flux redistribution in Escherichia coli. Metab Eng, 4, SANCHEZ, A. M., ANDREWS, J., HUSSEIN, I., BENNETT, G. N. & SAN, K. Y. (2006) Effect of overexpression of a soluble pyridine nucleotide transhydrogenase (UdhA) on the production of poly(3-hydroxybutyrate) in Escherichia coli. Biotechnol Prog, 22, SHALEL-LEVANON, S., SAN, K. Y. & BENNETT, G. N. (2005) Effect of oxygen, and ArcA and FNR regulators on the expression of genes related to the electron transfer chain and the TCA cycle in Escherichia coli. Metab Eng, 7, STOCCHI, V., CUCCHIARINI, L., MAGNANI, M., CHIARANTINI, L., PALMA, P. & CRESCENTINI, G. (1985) Simultaneous Extraction and Reverse-Phase High- Performance Liquid-Chromatographic Determination of Adenine and Pyridine- Nucleotides in Human Red Blood-Cells. Analytical Biochemistry, 146, WALTON, A. Z. & STEWART, J. D. (2004) Understanding and improving NADPH-dependent reactions by nongrowing Escherichia coli cells. Biotechnol Prog, 20, WECKBECKER, A. & HUMMEL, W. (2004) Improved synthesis of chiral alcohols with Escherichia coli cells co-expressing pyridine nucleotide transhydrogenase, NADP+dependent alcohol dehydrogenase and NAD+-dependent formate dehydrogenase. Biotechnol Lett, 26, YUN, N. R., SAN, K. Y. & BENNETT, G. N. (2005) Enhancement of lactate and succinate formation in adhe or pta-acka mutants of NADH dehydrogenase-deficient Escherichia coli. J Appl Microbiol, 99, ZAMBONI, N. & SAUER, U. (2009) Novel biological insights through metabolomics and 13Cflux analysis. Curr Opin Microbiol, 12,
131 Chapter 6 Improved NADPH Supply for Xylitol Production by Engineered Escherichia coli with Glycolytic Mutations
132 112 Abstract Chapter 2 describes a strain of engineered Escherichia coli that is able to produce high levels of xylitol from a mixture of glucose and xylose when expressing NADPH-dependent xylose reductase from Candida boidinii (CbXR). Chapter 4 then describes the effects of deletions of key metabolic pathways (e.g., Embden-Meyerhof-Parnas and pentose phosphate pathway) and reactions (e.g., transhydrogenase and NADH dehydrogenase) on resting cell xylitol yield (Y RPG : moles xylitol produced/mole glucose consumed). These prior results demonstrated the importance of direct NADPH supply by NADP + -utilizing enzymes in central metabolism for driving heterologous NADPH-dependent reactions. The current study describes strain modifications that improve coupling between glucose catabolism (oxidation) and xylose reduction using two fundamentally different strategies. First, the effects of deleting the phosphofructokinase (pfk) gene(s) on growth-uncoupled xylitol production were examined and it was found that deleting both pfka and stha (encoding the E. coli soluble transhydrogenase) improved the xylitol Y RPG from 3.4 ± 0.6 to 5.4 ± 0.4. The second strategy focused on coupling aerobic growth on glucose to xylitol production by deleting pgi (encoding phosphoglucose isomerase) and stha. Impaired growth of this strain due to imbalanced NADPH metabolism (Sauer et al., 2004) was alleviated upon expressing the NADPH-dependent CbXR, resulting in xylitol production similar to that of the growth-uncoupled precursor strains but with much less acetate secretion and more efficient utilization of glucose. Intracellular nicotinamide cofactor levels were also quantified, and the magnitude of the change in the NADPH/NADP + ratio measured from cells consuming glucose in the absence versus presence of xylose showed a strong correlation to the resulting Y RPG.
133 113 Introduction Chapters 2, 3, and 4, as well as Akinterwina (Akinterinwa et al., 2008), describe multiple strategies to produce xylitol from mixtures of glucose and xylose by engineered strains of E. coli expressing NADPH-dependent xylose reductase from Candida boidinii (CbXR). Transport of xylose into the cells during glucose metabolism was accomplished by either expressing a relaxed catabolite repression mutant of camp receptor protein (CRP*), as described in Chapter 2, or by overexpressing a xylose-specific transporter, XylE or XylFGH (Khankal et al., 2008). Chapter 3 describes the use of resting cells, which was shown to improve the xylitol yield ( Y RPG, defined as moles xylitol produced per mole glucose consumed). In Chapter 4, an analysis of mutant strains lacking various enzymes in E. coli central metabolism (e.g., SthA, PntAB, Zwf, Pgi; refer to Figure 6 1) revealed the importance of direct NADPH supply from NADP + -reducing pathways to improve Y RPG in resting cell cultures, as compared to the transfer of reducing equivalents from NADH to NADP + via transhydrogenase. Reducing equivalents available from NADH were not readily accessible via transhydrogenase due to competition by respiration, while under nonrespiratory conditions glucose was incompletely oxidized.
134 114 Figure 6-1: Overview of E. coli central carbon metabolism highlighting key reactions and pathways involved in NAD(P)H metabolism. pgi = phosphoglucose isomerase; zwf = glucose-6- phosphate dehydrogenase; gnd = 6-phosphogluconate dehydrogenase; edd = phosphogluconate dehydrogenase; eda = 2-keto-3-deoxy-6-phosphogluconate aldolase; pfk = phosphofructokinase; nuoa-n = NADH dehydrogenase I; ndh = NADH dehydrogenase II; pntab = pyridine nucleotide transhydrogenase; stha = soluble pyridine nucleotide transhydrogenase. Metabolite abbreviations include the following: G6P = glucose-6-phosphate; F6P = fructose-6-phosphate; F1,6P = fructose-1,6-bisphosphate; G3P = glyceraldehyde-3-phosphate, 6-PG = 6-phospho-gluconate. Pathway abbreviations: EMP = Embden-Meyerhof-Parnas; TCA = tricarboxylic acid. This chapter describes two E. coli metabolic engineering strategies aimed at improving the coupling between glucose catabolism and xylitol production via NADPH-dependent xylose reductase. The first strategy involved deleting the phosphofructokinase gene pfka to increase flux through the (NADPH-producing) pentose phosphate pathway (PPP), resulting in improved growth-uncoupled xylitol production and yield. The roles of transhydrogenase and the Entner- Doudoroff (ED) pathway activities were also investigated in this context. In the second strategy,
Engineering E. coli for xylitol production during growth on xylose
 Engineering E. coli for xylitol production during growth on xylose Olubolaji Akinterinwa & Patrick C. Cirino IBE 2008 Annual Meeting, March 7th 008-17 Advances in Engineering Microbial Metabolism Xylitol
Engineering E. coli for xylitol production during growth on xylose Olubolaji Akinterinwa & Patrick C. Cirino IBE 2008 Annual Meeting, March 7th 008-17 Advances in Engineering Microbial Metabolism Xylitol
The Pennsylvania State University. The Graduate School. Department of Chemical Engineering IMPROVING THE EFFICIENCY OF NADPH-DEPENDENT XYLITOL
 The Pennsylvania State University The Graduate School Department of Chemical Engineering IMPROVING THE EFFICIENCY OF NADPH-DEPENDENT XYLITOL PRODUCTION IN ENGINEERED ESCHERICHIA COLI A Dissertation in
The Pennsylvania State University The Graduate School Department of Chemical Engineering IMPROVING THE EFFICIENCY OF NADPH-DEPENDENT XYLITOL PRODUCTION IN ENGINEERED ESCHERICHIA COLI A Dissertation in
Chapter 5: Microbial Metabolism (Part I)
 Chapter 5: Microbial Metabolism (Part I) Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living organism. These chemical reactions are generally of two types: Catabolic:
Chapter 5: Microbial Metabolism (Part I) Microbial Metabolism Metabolism refers to all chemical reactions that occur within a living organism. These chemical reactions are generally of two types: Catabolic:
Genetic Engineering for Biofuels Production
 Genetic Engineering for Biofuels Production WSE 573 Spring 2013 Greeley Beck INTRODUCTION Alternative transportation fuels are needed in the United States because of oil supply insecurity, oil price increases,
Genetic Engineering for Biofuels Production WSE 573 Spring 2013 Greeley Beck INTRODUCTION Alternative transportation fuels are needed in the United States because of oil supply insecurity, oil price increases,
Industrial microbiology
 Industrial microbiology pp. 166-173, 1032-1038, 1039-1045,1046-1050 Ed van Niel Ed.van_Niel@tmb.lth.se We are here Industrial microbiology biotechnology Why the increased interest Microbiological versus
Industrial microbiology pp. 166-173, 1032-1038, 1039-1045,1046-1050 Ed van Niel Ed.van_Niel@tmb.lth.se We are here Industrial microbiology biotechnology Why the increased interest Microbiological versus
Increase of Xylitol Production Rate by Controlling Redox Potential in Candida parapsilosis
 Increase of Xylitol Production Rate by Controlling Redox Potential in Candida parapsilosis Deok-Kun Oh, 1 Sang-Yong Kim, 2 Jung-Hoe Kim 3 1 Department of Food Science and Technology, Woosuk University,
Increase of Xylitol Production Rate by Controlling Redox Potential in Candida parapsilosis Deok-Kun Oh, 1 Sang-Yong Kim, 2 Jung-Hoe Kim 3 1 Department of Food Science and Technology, Woosuk University,
Cell Growth and DNA Extraction- Technion igem HS
 Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
Growing Cells and DNA Extraction Goals 1. Become familiar with the process of growing bacteria 2. Get to know the DNA extraction process 3. Perform miniprep in the lab Keywords 1. Growth stages 6. Techniques
while Bacilli is the class to which the order Lactobacillales belongs to.
 Interactive Questions Question 1: Lactic acid bacteria belong to which order? Lactobacillaceae Lactobacillales Lactobacillales is the correct order. Lactobacillaceae is one family within this order, while
Interactive Questions Question 1: Lactic acid bacteria belong to which order? Lactobacillaceae Lactobacillales Lactobacillales is the correct order. Lactobacillaceae is one family within this order, while
All commands and actions are indicated as highlighted yellow steps CellNetAnalyzer and MatLab commands are in Courier Font
 Steady State Metabolic Modeling Homework In this lab we will use the application CellNetAnalyzer (CNA) to model metabolic pathways in E. coli and M. tuberculosis. This lab is a revised version from a lab
Steady State Metabolic Modeling Homework In this lab we will use the application CellNetAnalyzer (CNA) to model metabolic pathways in E. coli and M. tuberculosis. This lab is a revised version from a lab
NADP + /NADPH Assay Kit (Colorimetric)
 Product Manual NADP + /NADPH Assay Kit (Colorimetric) Catalog Number MET-5018 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Nicotinamide adenine dinucleotide phosphate
Product Manual NADP + /NADPH Assay Kit (Colorimetric) Catalog Number MET-5018 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Nicotinamide adenine dinucleotide phosphate
DESIGN OF A SACCHAROMYCES CEREVISIAE STRAIN CAPABLE OF SIMULTANEOUSLY UTILIZING CELLOBIOSE AND XYLOSE SIJIN LI THESIS
 DESIGN OF A SACCHAROMYCES CEREVISIAE STRAIN CAPABLE OF SIMULTANEOUSLY UTILIZING CELLOBIOSE AND XYLOSE BY SIJIN LI THESIS Submitted in partial fulfillment of the requirements for the degree of Master of
DESIGN OF A SACCHAROMYCES CEREVISIAE STRAIN CAPABLE OF SIMULTANEOUSLY UTILIZING CELLOBIOSE AND XYLOSE BY SIJIN LI THESIS Submitted in partial fulfillment of the requirements for the degree of Master of
Photosynthetic production of biofuels from CO 2 by cyanobacteria using Algenol s Direct to Ethanol process Strain development aspects
 Photosynthetic production of biofuels from CO 2 by cyanobacteria using Algenol s Direct to Ethanol process Strain development aspects Paul Roessler - Oct 1, 2014 Algal Biomass Summit Algenol Overview Advanced
Photosynthetic production of biofuels from CO 2 by cyanobacteria using Algenol s Direct to Ethanol process Strain development aspects Paul Roessler - Oct 1, 2014 Algal Biomass Summit Algenol Overview Advanced
THE ROLE OF ALCOHOL DEHYDROGENASE IN THE FERMENTATION OF D-XYLOSE BY CANDIDA SHEHATAE ATCC 22984
 Biotechnology Letters Vol 10 No 1 37-42 (1988) Received December 3 THE ROLE OF ALCOHOL DEHYDROGENASE IN THE FERMENTATION OF D-XYLOSE BY CANDIDA SHEHATAE ATCC 22984 Bernard A. Prior 1,3 *, Michael A. Alexander
Biotechnology Letters Vol 10 No 1 37-42 (1988) Received December 3 THE ROLE OF ALCOHOL DEHYDROGENASE IN THE FERMENTATION OF D-XYLOSE BY CANDIDA SHEHATAE ATCC 22984 Bernard A. Prior 1,3 *, Michael A. Alexander
NIH Public Access Author Manuscript Nat Biotechnol. Author manuscript; available in PMC 2011 June 6.
 NIH Public Access Author Manuscript Published in final edited form as: Nat Biotechnol. 2010 March ; 28(3): 245 248. doi:10.1038/nbt.1614. What is flux balance analysis? Jeffrey D. Orth, University of California
NIH Public Access Author Manuscript Published in final edited form as: Nat Biotechnol. 2010 March ; 28(3): 245 248. doi:10.1038/nbt.1614. What is flux balance analysis? Jeffrey D. Orth, University of California
Efficient Cell-Free Hydrogen Production from Glucose A Feasibility Study Annual Report 5/1/09 to 4/30/10
 Efficient Cell-Free Hydrogen Production from Glucose A Feasibility Study Annual Report 5/1/09 to 4/30/10 Investigators James R. Swartz, Professor, Chemical Engineering and Bioengineering; Phil Smith, Jon
Efficient Cell-Free Hydrogen Production from Glucose A Feasibility Study Annual Report 5/1/09 to 4/30/10 Investigators James R. Swartz, Professor, Chemical Engineering and Bioengineering; Phil Smith, Jon
Biology (Microbiology): Exam #2
 NAME: ANSWER KEY PLEDGE: Biology 50-384 (Microbiology): Exam #2 1. You have isolated mutants in the following genes in the motile bacterium E. coli. These mutations result in the formation of a nonfunctional
NAME: ANSWER KEY PLEDGE: Biology 50-384 (Microbiology): Exam #2 1. You have isolated mutants in the following genes in the motile bacterium E. coli. These mutations result in the formation of a nonfunctional
Swammerdam Institute Klaas J. Hellingwerf. On the use and optimization of Synechocystis PCC6803 as a bio-solar cell factory in a bio-based economy
 Swammerdam Institute Klaas J. Hellingwerf On the use and optimization of Synechocystis PCC6803 as a bio-solar cell factory in a bio-based economy Acknowledgements: S.A. Angermayr, P. Savakis, Dr. A. de
Swammerdam Institute Klaas J. Hellingwerf On the use and optimization of Synechocystis PCC6803 as a bio-solar cell factory in a bio-based economy Acknowledgements: S.A. Angermayr, P. Savakis, Dr. A. de
TBT4170 v2014. Kapittel 4 White Biotechnology: Cells as Synthetic Factories. Part 1
 TBT4170 v2014 Kapittel 4 White Biotechnology: Cells as Synthetic Factories Part 1 1 The single definition, OECD Biotechnology The application of science and technology to living organisms, as well as parts,
TBT4170 v2014 Kapittel 4 White Biotechnology: Cells as Synthetic Factories Part 1 1 The single definition, OECD Biotechnology The application of science and technology to living organisms, as well as parts,
Abhishek Murarka, 1 James M. Clomburg 1 and Ramon Gonzalez 1,2 INTRODUCTION
 Microbiology (2010), 156, 1860 1872 DOI 10.1099/mic.0.036251-0 Metabolic flux analysis of wild-type Escherichia coli and mutants deficient in pyruvate-dissimilating enzymes during the fermentative metabolism
Microbiology (2010), 156, 1860 1872 DOI 10.1099/mic.0.036251-0 Metabolic flux analysis of wild-type Escherichia coli and mutants deficient in pyruvate-dissimilating enzymes during the fermentative metabolism
Metabolic Engineering for Fuels and Chemicals
 Metabolic Engineering for Fuels and Chemicals K.T. Shanmugam and Lonnie O. Ingram Dept. of Microbiology and Cell Science University of Florida Gainesville, Florida Florida Center for Renewable Chemicals
Metabolic Engineering for Fuels and Chemicals K.T. Shanmugam and Lonnie O. Ingram Dept. of Microbiology and Cell Science University of Florida Gainesville, Florida Florida Center for Renewable Chemicals
Volume: 2: Issue-3: July-Sept ISSN EFFECT OF NITROGEN SOURCES ON MICROBIAL PRODUCTION OF XYLITOL. K. Srivani 1 and Y.
 Volume: 2: Issue-3: July-Sept -2011 ISSN 0976-4550 EFFECT OF NITROGEN SOURCES ON MICROBIAL PRODUCTION OF XYLITOL K. Srivani 1 and Y. Pydi Setty 2 1 Department of Chemical Engineering, National Institute
Volume: 2: Issue-3: July-Sept -2011 ISSN 0976-4550 EFFECT OF NITROGEN SOURCES ON MICROBIAL PRODUCTION OF XYLITOL K. Srivani 1 and Y. Pydi Setty 2 1 Department of Chemical Engineering, National Institute
Alcoholic Fermentation in Yeast
 Lab 5. Alcoholic Fermentation in Yeast Prelab Assignment Before coming to lab, read carefully the introduction and the procedures of this experiment, and then answer the prelab questions at the end of
Lab 5. Alcoholic Fermentation in Yeast Prelab Assignment Before coming to lab, read carefully the introduction and the procedures of this experiment, and then answer the prelab questions at the end of
Influence of Oxygen Availability on Cell Growth and Xylitol Production by Candida guilliermondii
 Copyright Oxygen Availability 2002 by Humana on Press Cell Inc. Growth and Xylitol Production 449 All rights of any nature whatsoever reserved. 0273-2289/02/98-100/0449/$12.50 Influence of Oxygen Availability
Copyright Oxygen Availability 2002 by Humana on Press Cell Inc. Growth and Xylitol Production 449 All rights of any nature whatsoever reserved. 0273-2289/02/98-100/0449/$12.50 Influence of Oxygen Availability
Chapter 7 Outline. Microbial Physiology Introduction 5/22/2011
 Chapter 7 Outline Microbial Physiology Introduction Microbial Nutritional Requirements Categorizing Microorganisms According to Their Energy and Carbon Sources Metabolic Enzymes Biologic Catalysts Factors
Chapter 7 Outline Microbial Physiology Introduction Microbial Nutritional Requirements Categorizing Microorganisms According to Their Energy and Carbon Sources Metabolic Enzymes Biologic Catalysts Factors
MODEL 1. provides additional information about the performance of the CeSS algorithm.
 MODEL 1 Sections 1 to 4 describe Model 1, a metabolic model of E. coli s Central Carbon Metabolism. Section 5 provides additional information about the performance of the CeSS algorithm. 1 Model structure:
MODEL 1 Sections 1 to 4 describe Model 1, a metabolic model of E. coli s Central Carbon Metabolism. Section 5 provides additional information about the performance of the CeSS algorithm. 1 Model structure:
Boost in bioethanol production usin Title dehydrogenase. Citation Journal of biotechnology (2013), 16.
 Boost in bioethanol production usin Title Saccharomyces cerevisiae with mutat dependent xylose reductase and NADP dehydrogenase. Author(s) Khattab, Sadat Mohammad Rezq; Saimu Tsutomu Citation Journal of
Boost in bioethanol production usin Title Saccharomyces cerevisiae with mutat dependent xylose reductase and NADP dehydrogenase. Author(s) Khattab, Sadat Mohammad Rezq; Saimu Tsutomu Citation Journal of
Confirming the Phenotypes of E. coli Strains
 Confirming the Phenotypes of E. coli Strains INTRODUCTION Before undertaking any experiments, we need to confirm that the phenotypes of the E. coli strains we intend to use in the planned experiments correspond
Confirming the Phenotypes of E. coli Strains INTRODUCTION Before undertaking any experiments, we need to confirm that the phenotypes of the E. coli strains we intend to use in the planned experiments correspond
Pharma&Biotech. XS Microbial Expression Technologies Optimize Productivity, Speed and Process Robustness
 Pharma&Biotech XS Microbial Expression Technologies Optimize Productivity, Speed and Process Robustness Embracing Complexity Based on 30 years of innovation in microbial biotechnology, Lonza offers the
Pharma&Biotech XS Microbial Expression Technologies Optimize Productivity, Speed and Process Robustness Embracing Complexity Based on 30 years of innovation in microbial biotechnology, Lonza offers the
Glucose-6-Phosphate Dehydrogenase (G6PDH) Activity Assay Kit
 Product Manual Glucose-6-Phosphate Dehydrogenase (G6PDH) Activity Assay Kit Catalog Number MET-5081 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Glucose-6-phosphate
Product Manual Glucose-6-Phosphate Dehydrogenase (G6PDH) Activity Assay Kit Catalog Number MET-5081 100 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Glucose-6-phosphate
Aerobic and Anaerobic Biodegradation. Danny Clark ENSO Bottles LLC 06/29/2010
 2010 Aerobic and Anaerobic Biodegradation Danny Clark ENSO Bottles LLC 06/29/2010 Aerobic and Anaerobic Biodegradation A look into aerobic and anaerobic biodegradation By Danny Clark ENSO Bottles, LLC
2010 Aerobic and Anaerobic Biodegradation Danny Clark ENSO Bottles LLC 06/29/2010 Aerobic and Anaerobic Biodegradation A look into aerobic and anaerobic biodegradation By Danny Clark ENSO Bottles, LLC
Begin with the supplemental experiment handout and get all experiments set up first before beginning slide and model observations in Exercise 4.
 The Cell: Division (Mitosis & Cytokinesis) and Cellular Respiration Exercise 4 (begins page 30 in 8 th edition, page 39 in 9 th 10 th 11 th and 12 th editions) and Supplemental Experiment Handout Anaerobic
The Cell: Division (Mitosis & Cytokinesis) and Cellular Respiration Exercise 4 (begins page 30 in 8 th edition, page 39 in 9 th 10 th 11 th and 12 th editions) and Supplemental Experiment Handout Anaerobic
A Discovery Laboratory Investigating Bacterial Gene Regulation
 Chapter 8 A Discovery Laboratory Investigating Bacterial Gene Regulation Robert Moss Wofford College 429 N. Church Street Spartanburg, SC 29307 mosssre@wofford.edu Bob Moss is an Associate Professor of
Chapter 8 A Discovery Laboratory Investigating Bacterial Gene Regulation Robert Moss Wofford College 429 N. Church Street Spartanburg, SC 29307 mosssre@wofford.edu Bob Moss is an Associate Professor of
ab MitoTox Complete OXPHOS Activity Assay Kit (5 Assays)
 ab110419 MitoTox Complete OXPHOS Activity Assay Kit (5 Assays) Instructions for Use Assays measure the direct effects of drugs on the 5 OXPHOS complexes This product is for research use only and is not
ab110419 MitoTox Complete OXPHOS Activity Assay Kit (5 Assays) Instructions for Use Assays measure the direct effects of drugs on the 5 OXPHOS complexes This product is for research use only and is not
The non-oxidative pentose phosphate pathway controls the fermentation rate of xylulose but not of xylose in Saccharomyces cerevisiae TMB3001
 FEMS Yeast Research 2 (2002) 277^282 www.fems-microbiology.org The non-oxidative pentose phosphate pathway controls the fermentation rate of xylulose but not of xylose in Saccharomyces cerevisiae TMB3001
FEMS Yeast Research 2 (2002) 277^282 www.fems-microbiology.org The non-oxidative pentose phosphate pathway controls the fermentation rate of xylulose but not of xylose in Saccharomyces cerevisiae TMB3001
5.36 Biochemistry Laboratory Spring 2009
 MIT OpenCourseWare http://ocw.mit.edu 5.36 Biochemistry Laboratory Spring 2009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. SESSION 13 and SESSION
MIT OpenCourseWare http://ocw.mit.edu 5.36 Biochemistry Laboratory Spring 2009 For information about citing these materials or our Terms of Use, visit: http://ocw.mit.edu/terms. SESSION 13 and SESSION
Optimization of enzyme parameters for fermentative production of biorenewable fuels and chemicals
 Chemical and Biological Engineering Publications Chemical and Biological Engineering 10-2012 Optimization of enzyme parameters for fermentative production of biorenewable fuels and chemicals Laura R. Jarboe
Chemical and Biological Engineering Publications Chemical and Biological Engineering 10-2012 Optimization of enzyme parameters for fermentative production of biorenewable fuels and chemicals Laura R. Jarboe
Synthetic Biology for the Calvin-Cycle- Channeled (Photobiological) Synthesis of Butanol & Pentanol Utilizing Carbon Dioxide as the Sole Feedstock
 Synthetic Biology for the Calvin-Cycle- Channeled (Photobiological) Synthesis of Butanol & Pentanol Utilizing Carbon Dioxide as the Sole Feedstock 2012 Pacific Rim Summit on Industrial Biotechnology and
Synthetic Biology for the Calvin-Cycle- Channeled (Photobiological) Synthesis of Butanol & Pentanol Utilizing Carbon Dioxide as the Sole Feedstock 2012 Pacific Rim Summit on Industrial Biotechnology and
Production of xylitol from biomass using an inhibitor-tolerant fungus
 Production of xylitol from biomass using an inhibitor-tolerant fungus Nancy Nichols National Center USDA ARS National Center for Agricultural Utilization Research Peoria IL USA Peoria, IL Biomass conversion
Production of xylitol from biomass using an inhibitor-tolerant fungus Nancy Nichols National Center USDA ARS National Center for Agricultural Utilization Research Peoria IL USA Peoria, IL Biomass conversion
Biochemistry study of the molecular basis of life
 Biochemistry : An Introduction Biochemistry study of the molecular basis of life n Study of the chemistry of living organisms Studies organic molecules & organic reactions in living organisms n Living
Biochemistry : An Introduction Biochemistry study of the molecular basis of life n Study of the chemistry of living organisms Studies organic molecules & organic reactions in living organisms n Living
NADP+/NADPH Assay Kit
 NADP+/NADPH Assay Kit Catalog Number KA1663 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
NADP+/NADPH Assay Kit Catalog Number KA1663 100 assays Version: 03 Intended for research use only www.abnova.com Table of Contents Introduction... 3 Intended Use... 3 Background... 3 Principle of the Assay...
Genome-Scale Consequences of Cofactor Balancing in Engineered Pentose Utilization Pathways in Saccharomyces cerevisiae
 Genome-Scale Consequences of Cofactor Balancing in Engineered Pentose Utilization Pathways in Saccharomyces cerevisiae Amit Ghosh 1,4, Huimin Zhao 1,2,3 *, Nathan D. Price 1,3,4 * 1 Department of Chemical
Genome-Scale Consequences of Cofactor Balancing in Engineered Pentose Utilization Pathways in Saccharomyces cerevisiae Amit Ghosh 1,4, Huimin Zhao 1,2,3 *, Nathan D. Price 1,3,4 * 1 Department of Chemical
green B 1 ) into a single unit to model the substrate in this reaction. enzyme
 Teacher Key Objectives You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating enzymatic activity.
Teacher Key Objectives You will use the model pieces in the kit to: Simulate enzymatic actions. Explain enzymatic specificity. Investigate two types of enzyme inhibitors used in regulating enzymatic activity.
Lab Exercise 13: Growth Curve
 Lab Exercise 13: Growth Curve OBJECTIVES 1. Know the different phases of a standard growth curve. 2. Understand and perform direct measurement of bacterial growth through serial dilutions and standard
Lab Exercise 13: Growth Curve OBJECTIVES 1. Know the different phases of a standard growth curve. 2. Understand and perform direct measurement of bacterial growth through serial dilutions and standard
Butanol: : A Second Generation Biofuel. Hans P. Blaschek University of Illinois March 6, 2007
 Butanol: : A Second Generation Biofuel Hans P. Blaschek University of Illinois March 6, 2007 Outline Introduction History Rationale Microbe Development and Characterization Genetic and Post-genomic Characterization
Butanol: : A Second Generation Biofuel Hans P. Blaschek University of Illinois March 6, 2007 Outline Introduction History Rationale Microbe Development and Characterization Genetic and Post-genomic Characterization
Take-Home Quiz II. Summer 2005 Semester
 General Instructions and Information: Obtain an answer sheet from the instructor and legibly write your name in the appropriate space. After placing your name, you must enter your Patron ID Number (NOT
General Instructions and Information: Obtain an answer sheet from the instructor and legibly write your name in the appropriate space. After placing your name, you must enter your Patron ID Number (NOT
IDTox Lactate Dehydrogenase (LDH) Enzyme Cytotoxicity Kit For Cell Culture Supernatant Samples
 Rev Date: Mar 26 2015 Rev: 1 1 of 1 IDTox Lactate Dehydrogenase (LDH) Enzyme Cytotoxicity Kit For Cell Culture Supernatant Samples 192 Wells Enzyme Immunoassay for the determination of the Lactate Dehydrogenase
Rev Date: Mar 26 2015 Rev: 1 1 of 1 IDTox Lactate Dehydrogenase (LDH) Enzyme Cytotoxicity Kit For Cell Culture Supernatant Samples 192 Wells Enzyme Immunoassay for the determination of the Lactate Dehydrogenase
The Biochemistry of Vitreoscilla hemoglobin
 , http://dx.doi.org/10.5936/csbj.201210002 CSBJ The Biochemistry of Vitreoscilla hemoglobin Benjamin C. Stark a,*, Kanak L. Dikshit b, Krishna R. Pagilla c Abstract: The hemoglobin (VHb) from Vitreoscilla
, http://dx.doi.org/10.5936/csbj.201210002 CSBJ The Biochemistry of Vitreoscilla hemoglobin Benjamin C. Stark a,*, Kanak L. Dikshit b, Krishna R. Pagilla c Abstract: The hemoglobin (VHb) from Vitreoscilla
METABOLIC ENGINEERING OF ZYMOMONAS MOBILIS FOR IMPROVED PRODUCTION OF ETHANOL FROM LIGNOCELLULOSES
 METABOLIC ENGINEERING OF ZYMOMONAS MOBILIS FOR IMPROVED PRODUCTION OF ETHANOL FROM LIGNOCELLULOSES A Dissertation Presented to The Academic Faculty by Manoj Agrawal In Partial Fulfillment of the Requirements
METABOLIC ENGINEERING OF ZYMOMONAS MOBILIS FOR IMPROVED PRODUCTION OF ETHANOL FROM LIGNOCELLULOSES A Dissertation Presented to The Academic Faculty by Manoj Agrawal In Partial Fulfillment of the Requirements
Development of Acetic-acid Tolerant Zymomonas mobilis Strains. through Adaptation. A Thesis. Presented to. The Academic Faculty.
 Development of Acetic-acid Tolerant Zymomonas mobilis Strains through Adaptation A Thesis Presented to The Academic Faculty By Yun Wang In Partial Fulfillment of the Requirements for the Degree Master
Development of Acetic-acid Tolerant Zymomonas mobilis Strains through Adaptation A Thesis Presented to The Academic Faculty By Yun Wang In Partial Fulfillment of the Requirements for the Degree Master
Development & application of a molecular toolbox for the unconventional yeast Candida intermedia. Master s thesis in Biotechnology ADAM LARSSON
 Development & application of a molecular toolbox for the unconventional yeast Candida intermedia Master s thesis in Biotechnology ADAM LARSSON Department of Biology and Biological Engineering CHALMERS
Development & application of a molecular toolbox for the unconventional yeast Candida intermedia Master s thesis in Biotechnology ADAM LARSSON Department of Biology and Biological Engineering CHALMERS
NAD/NADH Cell-Based Assay Kit
 NAD/NADH Cell-Based Assay Kit Item No. 600480 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Safety Data
NAD/NADH Cell-Based Assay Kit Item No. 600480 Customer Service 800.364.9897 * Technical Support 888.526.5351 www.caymanchem.com TABLE OF CONTENTS GENERAL INFORMATION 3 Materials Supplied 3 Safety Data
Abstract Process Economics Program Report 188B BIOTECHNOLOGY SEPARATION PROCESSES (June 2002)
 Abstract Process Economics Program Report 188B BIOTECHNOLOGY SEPARATION PROCESSES (June 2002) The chemical industry has a renewed interest in developing processes for producing industrial chemicals from
Abstract Process Economics Program Report 188B BIOTECHNOLOGY SEPARATION PROCESSES (June 2002) The chemical industry has a renewed interest in developing processes for producing industrial chemicals from
 Special Additional Papers for B.Sc. (Hons.)Biotechnology Transcriptomics and Metabolomics (306) M.M.-100 Transcriptomics and Metabolomics Cloning and expression of heterologous genes: Redirecting metabolic
Special Additional Papers for B.Sc. (Hons.)Biotechnology Transcriptomics and Metabolomics (306) M.M.-100 Transcriptomics and Metabolomics Cloning and expression of heterologous genes: Redirecting metabolic
% Viability. isw2 ino isw2 ino isw2 ino isw2 ino mM HU 4-NQO CPT
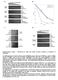 a Drug concentration b 1.3% MMS nhp1 nhp1 8 nhp1 mag1.5% MMS.3% MMS nhp1 nhp1 ino8 9 ino8 9 % Viability 4.5% MMS ino8 9 ino8 9 2.5.1.15 % MMS c d nhp1 nhp1 nhp1 nhp1 nhp1 nhp1 Control (YPD) γ IR (1 gy)
a Drug concentration b 1.3% MMS nhp1 nhp1 8 nhp1 mag1.5% MMS.3% MMS nhp1 nhp1 ino8 9 ino8 9 % Viability 4.5% MMS ino8 9 ino8 9 2.5.1.15 % MMS c d nhp1 nhp1 nhp1 nhp1 nhp1 nhp1 Control (YPD) γ IR (1 gy)
Optimizing Synthetic DNA for Metabolic Engineering Applications. Howard Salis Penn State University
 Optimizing Synthetic DNA for Metabolic Engineering Applications Howard Salis Penn State University Synthetic Biology Specify a function Build a genetic system (a DNA molecule) Genetic Pseudocode call producequorumsignal(luxi
Optimizing Synthetic DNA for Metabolic Engineering Applications Howard Salis Penn State University Synthetic Biology Specify a function Build a genetic system (a DNA molecule) Genetic Pseudocode call producequorumsignal(luxi
Fermentation monitoring. Dissolved oxygen ph Temperature Offgas monitoring Substrate (glucose)
 Bioreactor Monitoring & Control Bioreactor Monitoring & Control Basic principles of process control Fermentation monitoring Dissolved oxygen ph Temperature Offgas monitoring Substrate (glucose) Useful
Bioreactor Monitoring & Control Bioreactor Monitoring & Control Basic principles of process control Fermentation monitoring Dissolved oxygen ph Temperature Offgas monitoring Substrate (glucose) Useful
2.4 TYPES OF MICROBIAL CULTURE
 2.4 TYPES OF MICROBIAL CULTURE Microbial culture processes can be carried out in different ways. There are three models of fermentation used in industrial applications: batch, continuous and fed batch
2.4 TYPES OF MICROBIAL CULTURE Microbial culture processes can be carried out in different ways. There are three models of fermentation used in industrial applications: batch, continuous and fed batch
BIO 315 Lab Exam I. Section #: Name:
 Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
Section #: Name: Also provide this information on the computer grid sheet given to you. (Section # in special code box) BIO 315 Lab Exam I 1. In labeling the parts of a standard compound light microscope
High yield anaerobic succinate production by strategically regulating multiple metabolic pathways based on stoichiometric maximum in Escherichia coli
 DOI 10.1186/s12934-016-0536-1 Microbial Cell Factories RESEARCH Open Access High yield anaerobic succinate production by strategically regulating multiple metabolic pathways based on stoichiometric maximum
DOI 10.1186/s12934-016-0536-1 Microbial Cell Factories RESEARCH Open Access High yield anaerobic succinate production by strategically regulating multiple metabolic pathways based on stoichiometric maximum
STRUCTURAL BIOLOGY. α/β structures Closed barrels Open twisted sheets Horseshoe folds
 STRUCTURAL BIOLOGY α/β structures Closed barrels Open twisted sheets Horseshoe folds The α/β domains Most frequent domain structures are α/β domains: A central parallel or mixed β sheet Surrounded by α
STRUCTURAL BIOLOGY α/β structures Closed barrels Open twisted sheets Horseshoe folds The α/β domains Most frequent domain structures are α/β domains: A central parallel or mixed β sheet Surrounded by α
Industrial Microbiology Introduction and Overview. Dr. Gerard Fleming ext. 3562
 Industrial Microbiology Introduction and Overview Dr. Gerard Fleming ger.fleming@nuigalway.ie ext. 3562 The Scope: This course seeks to introduce students to those aspects of applied microbiology which
Industrial Microbiology Introduction and Overview Dr. Gerard Fleming ger.fleming@nuigalway.ie ext. 3562 The Scope: This course seeks to introduce students to those aspects of applied microbiology which
GCE A level 1074/01 BIOLOGY BY4
 Surname Other Names Centre Number 2 Candidate Number GCE A level 1074/01 BIOLOGY BY4 P.M. MONDAY, 13 June 2011 1¾ hours Question For s use Maximum Mark 1 10 Mark Awarded 2 10 1074 010001 3 12 4 14 5 9
Surname Other Names Centre Number 2 Candidate Number GCE A level 1074/01 BIOLOGY BY4 P.M. MONDAY, 13 June 2011 1¾ hours Question For s use Maximum Mark 1 10 Mark Awarded 2 10 1074 010001 3 12 4 14 5 9
DNA-PROTEIN CROSS-LINKS INDUCED BY BIS-ELECTROPHILES. Elisabeth M. Loecken. Dissertation. Submitted to the Faculty of the
 DNA-PROTEIN CROSS-LINKS INDUCED BY BIS-ELECTROPHILES By Elisabeth M. Loecken Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements
DNA-PROTEIN CROSS-LINKS INDUCED BY BIS-ELECTROPHILES By Elisabeth M. Loecken Dissertation Submitted to the Faculty of the Graduate School of Vanderbilt University in partial fulfillment of the requirements
Deposited research article Using Topology of the Metabolic Network to Predict Viability of Mutant Strains Zeba Wunderlich and Leonid Mirny*
 This information has not been peer-reviewed. Responsibility for the findings rests solely with the author(s). Deposited research article Using Topology of the Metabolic Network to Predict Viability of
This information has not been peer-reviewed. Responsibility for the findings rests solely with the author(s). Deposited research article Using Topology of the Metabolic Network to Predict Viability of
Cocultivation of Algae and Bacteria for Improved Productivity and Metabolic Versatility
 Cocultivation of Algae and Bacteria for Improved Productivity and Metabolic Versatility Pacific Rim Summit on Industrial Biotechnology and Bioenergy October 10-12, 2012 Vancouver, Canada Axenic Cultures
Cocultivation of Algae and Bacteria for Improved Productivity and Metabolic Versatility Pacific Rim Summit on Industrial Biotechnology and Bioenergy October 10-12, 2012 Vancouver, Canada Axenic Cultures
Soil and Environmental Biochemistry and Microbial Ecology (ENR 6610) Course Syllabus Winter/Spring Semester 2016
 Soil and Environmental Biochemistry and Microbial Ecology (ENR 6610) Course Syllabus Winter/Spring Semester 2016 Instructor: Office Hours: Warren A. Dick The Ohio State University / The Ohio Agricultural
Soil and Environmental Biochemistry and Microbial Ecology (ENR 6610) Course Syllabus Winter/Spring Semester 2016 Instructor: Office Hours: Warren A. Dick The Ohio State University / The Ohio Agricultural
OmniLog/PM Assay Technology. Leveraging Metabolism For Optimal Bioprocess Development
 OmniLog/PM Assay Technology Leveraging Metabolism For Optimal Bioprocess Development Importance of Metabolism and Operational Constraints Energy generation is an important driver of cell growth and bioproduction
OmniLog/PM Assay Technology Leveraging Metabolism For Optimal Bioprocess Development Importance of Metabolism and Operational Constraints Energy generation is an important driver of cell growth and bioproduction
Lab Exercise: Examining Water Quality: Most Probable Number & Colilert Test Kit Lab
 Lab Exercise: Examining Water Quality: Most Probable Number & Colilert Test Kit Lab OBJECTIVES 1. Understand the use of MPN to determine likely fecal water contamination. 2. Understand the use of MUG,
Lab Exercise: Examining Water Quality: Most Probable Number & Colilert Test Kit Lab OBJECTIVES 1. Understand the use of MPN to determine likely fecal water contamination. 2. Understand the use of MUG,
Module F06FB08. To gain knowledge about enzyme technology and production of enzymes and
 Module F06FB08 Enzyme technology Introduction and Production of enzymes This module would focus on enzyme technology which deals with the enzymes, the metabolic catalysts and their use in various Industries.
Module F06FB08 Enzyme technology Introduction and Production of enzymes This module would focus on enzyme technology which deals with the enzymes, the metabolic catalysts and their use in various Industries.
Summer Research Report to Wang Chu Chien Wen Summer Research Fund 1. Synthetic Microbial Consortium for Isobutanol Production August, 2012
 Summer Research Report to Wang Chu Chien Wen Summer Research Fund Synthetic Microbial Consortium for Isobutanol Production August, 212 Ning He Undergraduate student of Chemical Engineering Department University
Summer Research Report to Wang Chu Chien Wen Summer Research Fund Synthetic Microbial Consortium for Isobutanol Production August, 212 Ning He Undergraduate student of Chemical Engineering Department University
Aerobic and Anaerobic Biodegradation
 Polimernet Plastik San.Tic.Ltd.Şti. Tel:+90 216 393 77 46 / Email: info@polimernet.com www.polimernet.com 1 Aerobic and Anaerobic Biodegradation This document provides an in depth explanation, detailing
Polimernet Plastik San.Tic.Ltd.Şti. Tel:+90 216 393 77 46 / Email: info@polimernet.com www.polimernet.com 1 Aerobic and Anaerobic Biodegradation This document provides an in depth explanation, detailing
Contents... vii. List of Figures... xii. List of Tables... xiv. Abbreviatons... xv. Summary... xvii. 1. Introduction In vitro evolution...
 vii Contents Contents... vii List of Figures... xii List of Tables... xiv Abbreviatons... xv Summary... xvii 1. Introduction...1 1.1 In vitro evolution... 1 1.2 Phage Display Technology... 3 1.3 Cell surface
vii Contents Contents... vii List of Figures... xii List of Tables... xiv Abbreviatons... xv Summary... xvii 1. Introduction...1 1.1 In vitro evolution... 1 1.2 Phage Display Technology... 3 1.3 Cell surface
Now available to industry clients through Bioengineering Solutions. Bioprocess Engineering (TEA, Scale-Up / Scale Down, Tech Transfer)
 HO BDO OH Bioengineering Toolbox Now available to industry clients through Bioengineering Solutions Ideas Technology Platform Sector Independent Robust Commercial Processes Bioprocess Engineering (TEA,
HO BDO OH Bioengineering Toolbox Now available to industry clients through Bioengineering Solutions Ideas Technology Platform Sector Independent Robust Commercial Processes Bioprocess Engineering (TEA,
Link between iron homeostasis, oxidative mutagenesis and antibiotic resistance evolution
 Thesis of the PhD dissertation Link between iron homeostasis, oxidative mutagenesis and antibiotic resistance evolution Orsolya Katinka Méhi Supervisor: Dr. Csaba Pál, senior research associate PhD School
Thesis of the PhD dissertation Link between iron homeostasis, oxidative mutagenesis and antibiotic resistance evolution Orsolya Katinka Méhi Supervisor: Dr. Csaba Pál, senior research associate PhD School
Higher Human Biology Unit 1: Human Cells Pupils Learning Outcomes
 Higher Human Biology Unit 1: Human Cells Pupils Learning Outcomes 1.1 Division and Differentiation in Human Cells I can state that cellular differentiation is the process by which a cell develops more
Higher Human Biology Unit 1: Human Cells Pupils Learning Outcomes 1.1 Division and Differentiation in Human Cells I can state that cellular differentiation is the process by which a cell develops more
QUESTIONSHEET 1. The diagram shows a method of screening fungi for the production of an antibiotic. fungus A fungus B fungus C [2] ...
![QUESTIONSHEET 1. The diagram shows a method of screening fungi for the production of an antibiotic. fungus A fungus B fungus C [2] ... QUESTIONSHEET 1. The diagram shows a method of screening fungi for the production of an antibiotic. fungus A fungus B fungus C [2] ...](/thumbs/77/75444315.jpg) QUESTIONSHEET 1 The diagram shows a method of screening fungi for the production of an antibiotic. test fungus petri dish containing nutrient agar 1 2 3 4 5 6 streaks of different test bacteria The diagrams
QUESTIONSHEET 1 The diagram shows a method of screening fungi for the production of an antibiotic. test fungus petri dish containing nutrient agar 1 2 3 4 5 6 streaks of different test bacteria The diagrams
PROTOCOL 1850 Millrace Drive, Suite 3A Eugene, Oregon
 PROTOCOL Pyruvate Dehydrogenase (PDH) Enzyme Activity Dipstick Assay Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP30 Rev.4 DESCRIPTION Pyruvate Dehydrogenase (PDH) Enzyme Activity Dipstick
PROTOCOL Pyruvate Dehydrogenase (PDH) Enzyme Activity Dipstick Assay Kit 1850 Millrace Drive, Suite 3A Eugene, Oregon 97403 MSP30 Rev.4 DESCRIPTION Pyruvate Dehydrogenase (PDH) Enzyme Activity Dipstick
Metabolism and growth in selections of mutant libraries; modeling metabolic phenotypes and engineering sugar utilization in Escherichia coli
 University of Colorado, Boulder CU Scholar Chemical & Biological Engineering Graduate Theses & Dissertations Chemical & Biological Engineering Spring 1-1-2014 Metabolism and growth in selections of mutant
University of Colorado, Boulder CU Scholar Chemical & Biological Engineering Graduate Theses & Dissertations Chemical & Biological Engineering Spring 1-1-2014 Metabolism and growth in selections of mutant
STRAIN ISOLATION AND STUDY ON PROCESS PARAMETERS FOR XYLOSE- TO-XYLITOL BIOCONVERSION
 Article DOI: 1.2478/v1133-1-13-7 FB Food BIOTECHNOLOGY STRAIN ISOLATION AND STUDY ON PROCESS PARAMETERS FOR XYLOSE- TO-XYLITOL BIOCONVERSION K.-K. Cheng 1, H.-Z. Ling 2, J.-A. Zhang 1, W.-X. Ping 2, W.
Article DOI: 1.2478/v1133-1-13-7 FB Food BIOTECHNOLOGY STRAIN ISOLATION AND STUDY ON PROCESS PARAMETERS FOR XYLOSE- TO-XYLITOL BIOCONVERSION K.-K. Cheng 1, H.-Z. Ling 2, J.-A. Zhang 1, W.-X. Ping 2, W.
Characterization and Complementation of a Pichia stipitis Mutant Unable to Grow on D-Xylose or L-Arabinose
 Copyright 2000 by Humana Press Inc. All rights of any nature whatsoever reserved. 0273-2289/00/84-86/0201/$14.00 Characterization and Complementation of a Pichia stipitis Mutant Unable to Grow on D-Xylose
Copyright 2000 by Humana Press Inc. All rights of any nature whatsoever reserved. 0273-2289/00/84-86/0201/$14.00 Characterization and Complementation of a Pichia stipitis Mutant Unable to Grow on D-Xylose
Effect of the start-up length on the biological nutrient removal process
 Water Pollution IX 521 Effect of the start-up length on the biological nutrient removal process F. J. Fernández 1, J. Villaseñor 1 & L. Rodríguez 2 1 Department of Chemical Engineering, ITQUIMA, University
Water Pollution IX 521 Effect of the start-up length on the biological nutrient removal process F. J. Fernández 1, J. Villaseñor 1 & L. Rodríguez 2 1 Department of Chemical Engineering, ITQUIMA, University
Technical University of Denmark
 1 of 13 Technical University of Denmark Written exam, 15 December 2007 Course name: Introduction to Systems Biology Course no. 27041 Aids allowed: Open Book Exam Provide your answers and calculations on
1 of 13 Technical University of Denmark Written exam, 15 December 2007 Course name: Introduction to Systems Biology Course no. 27041 Aids allowed: Open Book Exam Provide your answers and calculations on
Chapter 12 Respiration
 2.2 Cell Metabolism Learning Objectives Chapter 12 Respiration 2.2.5 Respiration 1. Define, give the role and balanced equation for "aerobic respiration". 2. Explain the stages and molecules involved in
2.2 Cell Metabolism Learning Objectives Chapter 12 Respiration 2.2.5 Respiration 1. Define, give the role and balanced equation for "aerobic respiration". 2. Explain the stages and molecules involved in
Diffusional Contributions and Electrostatic Interactions during Ultrafiltration
 Diffusional Contributions and Electrostatic Interactions during Ultrafiltration Andrew L. Zydney Department Head and Walter L. Robb Family Chair Department of Chemical Engineering The Pennsylvania State
Diffusional Contributions and Electrostatic Interactions during Ultrafiltration Andrew L. Zydney Department Head and Walter L. Robb Family Chair Department of Chemical Engineering The Pennsylvania State
The Production of a Recombinant Biotechnology Product. Chapter 8
 The Production of a Recombinant Biotechnology Product Chapter 8 Objectives Give a basic overview of genetic engineering. Describe the processes involved in isolating a piece DNA of interest Mass producing
The Production of a Recombinant Biotechnology Product Chapter 8 Objectives Give a basic overview of genetic engineering. Describe the processes involved in isolating a piece DNA of interest Mass producing
Purification of Lactate Dehydrogenase
 Dominican University of California Dominican Scholar Scholarly & Creative Works Conference 2018 Scholarly and Creative Works Conference 2016 Apr 15th, 1:30 PM - 2:00 PM Purification of Lactate Dehydrogenase
Dominican University of California Dominican Scholar Scholarly & Creative Works Conference 2018 Scholarly and Creative Works Conference 2016 Apr 15th, 1:30 PM - 2:00 PM Purification of Lactate Dehydrogenase
HOUR EXAM II BIOLOGY 422 FALL, In the spirit of the honor code, I pledge that I have neither given nor received help on this exam.
 Name First Last (Please Print) PID Number - HOUR EXAM II BIOLOGY 422 FALL, 2010 In the spirit of the honor code, I pledge that I have neither given nor received help on this exam. 1 Signature 2 3 4 5 6
Name First Last (Please Print) PID Number - HOUR EXAM II BIOLOGY 422 FALL, 2010 In the spirit of the honor code, I pledge that I have neither given nor received help on this exam. 1 Signature 2 3 4 5 6
Cell Biology Homework
 Cell Biology Homework NAME: CLASS: 1 1. The diagram below shows two cells. Cell Structure a) Complete the table below to give the name and functions of the parts labelled A, B and C. (2) Letter Part Function
Cell Biology Homework NAME: CLASS: 1 1. The diagram below shows two cells. Cell Structure a) Complete the table below to give the name and functions of the parts labelled A, B and C. (2) Letter Part Function
Xylitol Production from Corn Cobs Hemicellulosic Hydrolysate by Candida tropicalis Immobilized Cells in Hydrogel Copolymer Carrier
 INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY 156 853/24/6 6 166 173 http://www.ijab.org Xylitol Production from Corn Cobs Hemicellulosic Hydrolysate by Candida tropicalis Immobilized Cells in Hydrogel
INTERNATIONAL JOURNAL OF AGRICULTURE & BIOLOGY 156 853/24/6 6 166 173 http://www.ijab.org Xylitol Production from Corn Cobs Hemicellulosic Hydrolysate by Candida tropicalis Immobilized Cells in Hydrogel
HAWAII. Renewable Bio-solar Hydrogen Production from Robust Oxygenic Phototrophs AFOSR MURI Progress Update: January 2007.
 PRINCETON PENNSTATE HAWAII Renewable Bio-solar Hydrogen Production from Robust Oxygenic Phototrophs AFOSR MURI Progress Update: January 2007 -BioSolarH 2 Team Charles Dismukes PU photosyn. metabolism/chemistry
PRINCETON PENNSTATE HAWAII Renewable Bio-solar Hydrogen Production from Robust Oxygenic Phototrophs AFOSR MURI Progress Update: January 2007 -BioSolarH 2 Team Charles Dismukes PU photosyn. metabolism/chemistry
Supporting Information
 Supporting Information Copper and zinc ions specifically promote non-amyloid aggregation of the highly stable human γ-d crystallin Liliana Quintanar, 1,* José A. Domínguez-Calva, 1 Eugene Serebryany, 2
Supporting Information Copper and zinc ions specifically promote non-amyloid aggregation of the highly stable human γ-d crystallin Liliana Quintanar, 1,* José A. Domínguez-Calva, 1 Eugene Serebryany, 2
SYSTEMATIC AND COMBINATORIAL APPROACHES FOR METABOLIC ENGINEERING OF OPTIMAL YEAST STRAINS TO PRODUCE FUELS AND CHEMICALS JOSHUA C.
 SYSTEMATIC AND COMBINATORIAL APPROACHES FOR METABOLIC ENGINEERING OF OPTIMAL YEAST STRAINS TO PRODUCE FUELS AND CHEMICALS BY JOSHUA C. QUARTERMAN DISSERTATION Submitted in partial fulfillment of the requirements
SYSTEMATIC AND COMBINATORIAL APPROACHES FOR METABOLIC ENGINEERING OF OPTIMAL YEAST STRAINS TO PRODUCE FUELS AND CHEMICALS BY JOSHUA C. QUARTERMAN DISSERTATION Submitted in partial fulfillment of the requirements
The microtubule-associated tau protein has intrinsic acetyltransferase activity. Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and
 SUPPLEMENTARY INFORMATION: The microtubule-associated tau protein has intrinsic acetyltransferase activity Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and Virginia M.Y. Lee Cohen
SUPPLEMENTARY INFORMATION: The microtubule-associated tau protein has intrinsic acetyltransferase activity Todd J. Cohen, Dave Friedmann, Andrew W. Hwang, Ronen Marmorstein and Virginia M.Y. Lee Cohen
Q1 (1 point): Explain why a lettuce leaf wilts when it is placed in a concentrated salt solution.
 Short questions 1 point per question. Q1 (1 point): Explain why a lettuce leaf wilts when it is placed in a concentrated salt solution. Answer: Water is sucked out of the cells by osmosis (this reduces
Short questions 1 point per question. Q1 (1 point): Explain why a lettuce leaf wilts when it is placed in a concentrated salt solution. Answer: Water is sucked out of the cells by osmosis (this reduces
PHEN 612 SPRING 2008 WEEK 4 LAURENT SIMON
 PHEN 612 SPRING 2008 WEEK 4 LAURENT SIMON Bioreactors Breads, yogurt, cheeses, etc Recombinant DNA techniques are used to make cheese. Fermentation is a microbial process that is used to produce food products
PHEN 612 SPRING 2008 WEEK 4 LAURENT SIMON Bioreactors Breads, yogurt, cheeses, etc Recombinant DNA techniques are used to make cheese. Fermentation is a microbial process that is used to produce food products
Stabilization of a virus-like particle and its application as a nanoreactor at physiological conditions
 Supporting Information Stabilization of a virus-like particle and its application as a nanoreactor at physiological conditions Lise Schoonen, b Sjors Maassen, b Roeland J. M. Nolte b and Jan C. M. van
Supporting Information Stabilization of a virus-like particle and its application as a nanoreactor at physiological conditions Lise Schoonen, b Sjors Maassen, b Roeland J. M. Nolte b and Jan C. M. van
ab Mitochondrial Viability Assay
 ab129732 Mitochondrial Viability Assay Instructions for Use For measuring mitochondrial/cellular viability in high throughput This product is for research use only and is not intended for diagnostic use.
ab129732 Mitochondrial Viability Assay Instructions for Use For measuring mitochondrial/cellular viability in high throughput This product is for research use only and is not intended for diagnostic use.
Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production
 Procedia Food Science 1 (2011) 908 913 11 th International Congress on Engineering and Food (ICEF11) Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production Siti M. Mustapa
Procedia Food Science 1 (2011) 908 913 11 th International Congress on Engineering and Food (ICEF11) Detoxification of sago trunk hydrolysate using activated charcoal for xylitol production Siti M. Mustapa
Continuous Xylose Fermentation by Candida shehatae in a Two-Stage Reactor
 In: Scott, Charles D., ed. Proceedings of the 9th symposium on biotechnology for fuels and chemicals; 1987 May 5-8; Boulder, CO. In: Applied Biochemistry and Biotechnology. Clifton, NJ: Humana Press; 1988:
In: Scott, Charles D., ed. Proceedings of the 9th symposium on biotechnology for fuels and chemicals; 1987 May 5-8; Boulder, CO. In: Applied Biochemistry and Biotechnology. Clifton, NJ: Humana Press; 1988:
