STUDY OF THE DENATURATION OF HUMAN SERUM ALBUMIN BY SODIUM DODECYL SULFATE USING THE INTRINSIC FLUORESCENCE OF ALBUMIN
|
|
|
- Dwain Knight
- 5 years ago
- Views:
Transcription
1 Journal of Applied Spectroscopy, Vol. 76, No. 4, 2009 STUDY OF THE DENATURATION OF HUMAN SERUM ALBUMIN BY SODIUM DODECYL SULFATE USING THE INTRINSIC FLUORESCENCE OF ALBUMIN I. M. Vlasova * and A. M. Saletsky UDC ; An analysis of the intrinsic tryptophan fluorescence of human serum albumin (HSA) confirms that the denaturation of HSA by sodium dodecyl sulfate takes place in two stages for different ph levels: the first is the disintegration of globules and the second is the complete unfolding of the amino acid chain of HSA. At ph levels below the isoelectric point (pi 4.7) of HSA, denaturation proceeds through both stages, but when the ph is above pi, denaturation ceases in the first stage. Key words: fluorescence, tryptophan, human serum albumin, denaturation, sodium dodecyl sulfate Introduction. Human serum albumin (HSA, 66.4 kdalton, isoelectric point pi 4.7) is the globular protein in human blood plasma [1]. The unique ability of HSA to bind a wide range of organic and inorganic ligands determines one of the basic functions of this protein the transport of physiological metabolites. Research on the denaturation of this protein is extremely important in connection with its physiological functions in blood, since this can provide information on the native conformation of the protein and, therefore, on the extent to which its physiological properties are maintained when various external factors act on it. Denaturation refers to a significant change in the secondary and tertiary structure of the protein, i.e., a breakup or disordering of the system of noncovalent interactions, which do not affect its covalent structure. Effective denaturing agents include the ionic detergents, of which the detergent sodium dodecyl sulfate (SDS) is widely used in biochemistry: H 3 C (CH 2 ) 11 OSO 3 Na + [2 7]. The denaturation of HSA by SDS has been studied [3 5] using Raman scattering spectroscopy, laser correlation spectroscopy, and fluorescence analysis using eosine as a probe. In this paper we investigate the mechanism by which HSA molecules are denatured by different concentrations of SDS at different levels of ph by studying the intrinsic tryptophan fluorescence of HSA, a method widely used to evaluate the conformation state of protein molecules [8 13]. The only amino acid residual of tryptophan (Trp-214) in HSA lies in domain II. The fluorescence properties of tryptophan in the HSA molecule are extremely sensitive to realignments of the protein globule. Changes in the spectral characteristics of the fluorescence of HSA tryptophan (intensity and position of the peak in the fluorescence spectrum and the polarization of the fluorescence) can be used to establish the conformational state of the protein globules in solutions with denaturing agents, such as SDS. Materials and Methods. Solutions of HSA were prepared by dilution of the protein to a concentration of 5 μm in two different buffer systems: 0.1 M CH 3 COOH KOH (ph ) and 0.1 M KH 2 PO M NaOH (ph ). Different concentrations of SDS ( mm) were added to solutions of HSA at different ph ( ). The fluorescence studies were made with a Perkin Elmer LS55 spectrofluorimeter and the spectral-fluorescence characteristics of the prepared samples were studied at room temperature. The tryptophan fluorescence of the HSA was recorded over nm with excitation by λ = 295 nm light. The degree r of anisotropy in the tryptophan fluorescence of the HSA was calculated from the values of I and I at the peak of the protein fluorescence spectrum, where I and I are the fluorescence intensities measured through a polarizer with its electric field axis directed parallel to or perpendicular to the polarization of the exciting light. The fluorescence spectra were processed using the Perkin Elmer FL Winlab program. To whom correspondence should be addressed. M. V. Lomonosov Moscow State University, Leninskie Gory, Moscow, , Russia; Translated from Zhurnal Prikladnoi Spektroskopii, Vol. 76, No. 4, pp , July August Original article submitted March 26, /09/ Springer Science+Business Media, Inc.
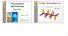
2 Results and Discussion. The protein macromolecules are capable of undergoing structural readjustments when acted on by various factors and agents. Two types of dynamic readjustments of this sort are possible: a transition of the protein from its native state to a denatured functionally inactive state (a denaturation transition) and a transition between two functionally active forms (a functional transition). We first examine the functional transition of HSA in the absence of SDS. The modifications in the intramolecular dynamics of HSA in solutions as the ph of the solutions vary within the range studied here ( ) when SDS is not present are functional transitions, i.e., they take place within the confines of the native structure of the protein. The dependence of the peak intensity (I fl max ) in the spectrum of the tryptophan fluorescence of HSA was determined as a function of the ph of solutions without SDS (Fig. 1a, inset). As the ph is raised from 3.5 to 6.0, I fl max rises; this indicates a change in the surroundings of the amino acid group of the tryptophan in the HSA molecules owing to small conformational readjustments. The dependence of the wavelength of the peak in the spectrum of the tryptophan fluorescence (λ fl max ) as a function of the ph of solutions without SDS was also obtained (Fig. 1b, inset). It is evident that besides an increase in the intensity of the tryptophan fluorescence of HSA with increasing ph there is a red shift in the fluorescence spectrum. A change in the ph (reduction from 6.0 to 3.5) leads to changes in the ionization state of the protein groups which trigger conformational changes in the protein within the native structure; this is the so-called N F transition. This conformational functional transition in HSA changes the surroundings of the protein chromophore the amino acid group of the tryptophan and, therefore, modifies the tryptophan fluorescence of the HSA. This N F transition of HSA as the ph is reduced from 6.0 to 3.5 is related to protonation of the carboxyl groups of the protein and is typical of all serum albumins [1]. The F-form of HSA, which exists for low ph solutions, is characterized by a looser structure with disordered hydrophobic regions. We now examine the denaturation transitions of HSA produced by different concentrations of SDS at different ph levels. For characterizing the conformational changes in the protein during denaturation, it is possible to use the parameters of the intrinsic protein (tryptophan) fluorescence: the intensity and position of the spectral peak of the steady state fluorescence, as well as the degree of anisotropy in the polarized fluorescence. Spectra of the tryptophan steady-state fluorescence of HSA in solutions containing different concentrations of SDS for different ph levels were obtained. Figure 1 shows the dependence of I fl max on the SDS concentration for different values of the ph. In the solutions with SDS the tryptophan fluorescence of HSA is quenched; this explained by denaturation of the protein, so that as the protein globule unfolds the chromophore tryptophan group becomes more accessible to water molecules which quench its fluorescence emission. Figure 1a shows that the stronger quenching of the intrinsic tryptophan fluorescence of HSA observed in solutions with SDS takes place at lower ph levels in solutions with equal concentrations of SDS. This behavior is indicative of an electrostatic mechanism for the interaction of the HSA with the SDS. SDS molecules in solution dissociate into positively charged sodium ions and dodecyl sulfate anions. It is these which interact with the HSA. On the whole, HSA molecules are positively charged for ph < pi of HSA. Thus, at low ph, the overall positively charged HSA macromolecules bond rapidly to the dodecyl sulfate anions, so that quenching of the tryptophan fluorescence of the HSA in solutions with SDS is strong compared to solutions which do not contain SDS. As the ph is raised, the overall positive charge of the HSA macromolecules decreases, and when ph > pi, the HSA molecules acquire an overall negative charge. Thus, for high ph (>pi), there is weaker bonding of the dodecyl sulfate anions to the overall negatively charged HSA molecules, which still do retain some positively charged segments, so that weak quenching of the tryptophan fluorescence of HSA is observed in solutions with SDS. The dependences of I fl max on [SDS] for different ph levels (Fig. 1a) obtained from the experimental data can explain the two-stage mechanism for denaturation of HSA in the presence of SDS. Here the behavior of the variation in I fl max with [SDS] differs, depending on whether the ph of the solution is higher or lower than pi for HSA. It is clear (Fig. 1a) that for ph < pi of HSA (ph ), denaturation of HSA in the presence of SDS is a stepwise, two-stage process. For concentrations [SDS] < 1 mm, the tryptophan fluorescence of the HSA is quenched, while for [SDS] = 1 2 mm I fl max is essentially constant. The first transition stage of denaturation of the protein takes place at these concentrations of SDS (up to 2 mm): the protein globules of the HSA are loosened, but complete unfolding has not yet taken place. As [SDS] is increased to 3 mm, further quenching of the tryptophan fluorescence of the HSA is observed. This indicates that the protein molecules change from a transitional, loose state to the second denaturation 537
3 Fig. 1. The intensity (a) and wavelength (b) of the peak in the tryptophan fluorescence spectrum (λ exc = 295 nm) of HSA (5 μm) as functions of the concentration of sodium dodecyl sulfate for ph 3.5 (1), 4.0 (2), 4.5 (3), 5.0 (4), 5.5 (5), and 6.0 (6). The insets show I fl (a) and λ fl (b) at the spectral maximum of the tryptophan fluorescence (λ exc = 295 nm) of HSA (5 μm) as functions of the ph of solutions without SDS. stage a completely unfolded state. When the SDS concentration is raised beyond 3 mm (to 7 mm), stronger quenching of the tryptophan fluorescence of HSA does not occur; this indicates complete denaturation of the HSA molecules. Further increases in [SDS] change nothing in this system, as is confirmed by data from [3 5]. The two-stage quenching of the intrinsic fluorescence of the HSA as [SDS] is increased for ph < pi of HSA is explained by a two-stage mechanism for its denaturation and subsequent conformational readjustments of the protein globule which lead to exposure of the tryptophan and increased access to the tryptophan by water molecules which quench its fluorescence. When ph > pi of HSA (ph ), denaturation of HSA by SDS proceeds slowly and (Fig. 2) the twostage process for denaturation of HSA in the presence of SDS only reaches the first stage within the range of [SDS] that was studied. When [SDS] is raised to 1 2 mm, the tryptophan fluorescence of HSA is quenched, i.e., denaturation loosening of the protein globules (the first state of denaturation) is observed, which leads to increased accessibility of the tryptophan of the HSA to water molecules which quench it. Higher concentrations of SDS (>2 mm) at these ph levels do not lead to the next, second stage of denaturation. The mechanism for denaturation of HSA by SDS was also studied by analyzing the position of λ fl max. Figure 1b shows the variation in λ fl max with [SDS] for different ph levels. When an initial concentration of SDS (
4 Fig. 2. The degree r of anisotropy in the tryptophan fluorescence (λ exc = 295 nm) of HSA (5 μm) as a function of [SDS] for ph 3.5 (1), 4.0 (2), 4.5 (3), 5.0 (4), 5.5 (5), 6.0 (6). mm) is added to the solution, there is a sharp blue shift in λ fl max (by 8 12 nm, depending on ph). Further addition of SDS (to mm) still leads to a small blue shift in the tryptophan fluorescence spectra of HSA. The blue shift in λ fl max in solutions with SDS is explained by a change in the nearest surroundings of the tryptophan chromophore group of the HSA owing to the denaturation readjustment of the protein globules and to the influence of the dodecyl sulfate anions that bond to the protein. The polarized tryptophan fluorescence of HSA was also studied. The degree (r) of anisotropy of the tryptophan fluorescence of HSA was determined as a function of [SDS] for different values of the ph (Fig. 2). r was calculated from the intensities I and I at the peak of the HSA fluorescence spectra. Changes in the polarization of the fluorescence are known to have two causes: first, rotational diffusion of fluorophores and second, nonradiative energy transfer between fluorophores. Given the choice of experimental conditions (highly dilute solutions of the protein [5 μm] were studied and the tryptophan fluorescence was excited at the far long wavelength edge [295 nm]), there is no contribution from the second (nonradiative energy transfer). Thus, for the given experimental conditions only rotational diffusion of the fluorophore (the tryptophan group of the HSA molecule) has a significant effect on the polarization of the fluorescence of the HSA tryptophan. This polarization of the fluorescence of the tryptophan group of the HSA molecule is, in general, caused both by rotation of the entire protein molecule (Brownian diffusional motion) and by rotation of domain II of the HSA, which contains the tryptophan group, and the rotation of the chromophore (tryptophan) itself relative to its nearest surroundings owing to dipole-orientational relaxation of the chromophore after excitation. The measurements of the polarized steady state fluorescence of HSA make it possible to analyze the rotation of the entire protein molecule, while the contribution from the rotation of the domain containing the tryptophan and the rotation of the tryptophan group with respect to the nearby surroundings are considered to be negligible. Figure 2 shows that r increases in the region up to 1 mm SDS for all values of the ph; this is indicative of the first stage of denaturation of the HSA loosening of the globules, which reduces the rotational diffusion coefficient for the HSA molecules and leads to an increase in r. Subsequent increases (above 1 mm) in [SDS] for ph > pi of HSA do not change r. This shows that denaturation has come to a halt in the first stage. The way r varies with [SDS] is different for ph < pi of HSA (Fig. 2): for [SDS] = 1 2 mm, r is essentially constant and for [SDS] = 2 4 mm r increases further, which indicates a decrease in the rotational diffusion coefficient of the HSA molecules, which in turn is explained by the increase, reported in [4], in the linear-longitudinal dimensions of the HSA molecules as the amino acid chain unfolds. Thus, the HSA molecules enter the second stage of denaturation the stage of complete unfolding. As [SDS] is raised above 4 mm (to 7 mm), r is essentially constant, which indicates that the HSA molecules have been completely denatured. Based on the two stage increase in r as 539
5 [SDS] is raised, we may conclude that for ph < pi of HSA, denaturation of HSA in the presence of SDS is a stepwise, two-stage process. As opposed to the behavior of I fl max and r, which reflects the multistage denaturation of HSA by SDS, λfl max gives evidence only of a denaturation process with no signs of its occurring in stages. This is explained by the fact that the variation in λ fl max is sensitive only to the change in the nearest surroundings of the tryptophan, while the variation in I fl max and r bears traces both of local readjustments in the nearest surroundings of the tryptophan surroundings and of more distant peripheral changes in the HSA globules. Tryptophan-214 (Trp(214)) is known to lie in the hydrophobic pocket of HSA, which consists of Lys(212) Ala(213) Trp(214) Ala(215) Val(216) Ala(217) Arg(218), and is located on the surface of subdomain 4 in domain II. This hydrophobic pocket, which is responsible for the bonding of organic anions, contains five nonpolar amino acid groups which form a region bounded by polar cation (for the ph levels used in the present work) groups of lateral chains of lysine-212 and arginine-218, positioned at the entrance to the hydrophobic pocket. These two amino acid groups (Lys(212) and Arg(218)), located at the entrance to the hydrophobic pocket, within which Trp(214) is found, are the first targets for the dodecyl sulfate anions. From this point of view, because of the interaction of Lys(212) and Arg(218) with dodecyl sulfate anions, when SDS is added in concentrations up to 1 mm major changes occur in the nearest surroundings of the Trp(214), which lead, on one hand, to a blue shift in λ fl max and, on the other, to partial opening of the hydrophobic pocket with Trp(214) and partial exposure of the Trp(214) (because of the loosening of the globule) to water molecules which quench its fluorescence; this produces the first large reduction in I fl max (the first stage of denaturation) and an increase in r. As [SDS] is raised (>1 2 mm), depending on the ph, the bonding of new dodecyl sulfate anions leads to: (1) complete denaturation (the second stage of denaturation) of the HSA for ph < pi of the protein, involving complete exposure of the Trp(214) to water molecules that quench it, which causes a second large increase in I fl max, i.e., the unfolding of the globules leading to reduced rotational diffusion of the HSA molecules and, therefore, to an increase in r (here the nearest surroundings of Trp(214) change little, so that the blue shift in λ fl max is very small); (2) the absence of a second HSA denaturation stage for ph > pi of the protein here only a slight change in the surroundings of the Trp(214) takes place which shows up as a small blue shift in λ fl max. Conclusion. The intrinsic tryptophan fluorescence of human serum albumin (both the native protein and denatured by sodium dodecyl sulfate) has been used to study the functional transitions of HSA within the confines of the native structure as the ph is varied in the absence of SDS and the denaturing transitions of HSA produced by SDS at different ph levels. The tryptophan fluorescence of HSA gives an indication of a functional transition (N F transition) of HSA within the confines of its native structure (in the absence of SDS) when the ph is varied from 6.0 to 3.5 that leads to a reduction in I fl max and a blue shift in λfl max. An analysis of the intrinsic tryptophan fluorescence of HSA (λ fl max, Ifl max, and r) has been used to study the denaturing transitions of HSA acted on by SDS at different ph levels. The two-stage changes in I fl max and r as the concentration of SDS is increased indicate that the denaturation of the protein occurs in two stages: the first stage involves loosening of the protein globules and the second, complete unfolding of the amino acid chain of the HSA. When ph > pi of HSA, denaturation passes through two stages, and when ph < pi of HSA, denaturation is slow and ceases in the first stage. The variation in the tryptophan fluorescence of HSA shows that denaturation by SDS is more efficient for ph of the HSA, and that as the ph is increased, the efficiency of denaturation of HSA by SDS falls off. Acknowledgement. This work was supported by the Russian Foundation for Basic Research (grant No ). REFERENCES 1. Yu. A. Gryzunov and G. E. Dobretsov, Human Serum Albumin in Clinical Medicine [in Russian], IRIUS, Moscow (1994). 2. L. A. Osterman, Protein and Nucleic Acid Research Techniques [in Russian], MTsNMO, Moscow (2002). 3. I. M. Vlasova, D. V. Polyansky, and A. M. Saletsky, Laser Phys. Lett., 4, No. 12, (2007). 4. A. N. Baranov, I. M. Vlasova, V. E. Mikrin, and A. M. Saletskii, Zh. Prikl. Spektr., 71, (2004). 5. I. M. Vlasova, A. Yu. Zemlyanskii, and A. M. Saletskii, Zh. Prikl. Spektr., 73, (2004). 540
6 6. P. Dutta, P. Sen, A. Halder, S. Mukherjee, S. Sen, and K. Bhattacharyya, Chem. Phys. Lett., 377, (2003). 7. E. L. Gelamo, C. H. T. P. Silva, H. Imasato, and M. Tabak, Biochim. Biophys. Acta, 1594, (2002). 8. L. V. Levshin and A. M. Saletskii, Optical Methods for Studying Molecular Systems. Part 1. Molecular Spectroscopy [in Russian], Izd-vo MGU, Moscow (1994). 9. Yu. A. Vladimirov, Protein Photochemistry and Luminescence [in Russian], Nauka, Moscow (1965). 10. E. A. Permyakov, The Technique of Intrinsic Protein Luminescence [in Russian], Nauka, Moscow (2003). 11. A. P. Demchenko, Luminescence and Dynamics of Protein Structure [in Russian], Naukova dumka, Kiev (1988) 12. X. Diaz, E. Abuin, and E. Lissi, J. Photochem. Photobiol. A: Chem., 155, (2003). 13. A. Brahma, C. Mandal, and D. Bhattacharyya, Biochim. Biophys. Acta, 1751, (2005). 541
UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change
 A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
A p p l i c a t i o n N o t e UV Fluorescence Polarization as a Means to Investigate Protein Conformational and Mass Change Using Intrinsic Tryptophan Fluorescence in Conjunction with UV-capable Polarizers
Fluorescence Quenching of Human Serum Albumin by Caffeine
 CHEM 411L Instrumental Analysis Laboratory Revision 2.1 Fluorescence Quenching of Human Serum Albumin by Caffeine In this laboratory exercise we will examine the fluorescence of Human Serum Albumin (HSA)
CHEM 411L Instrumental Analysis Laboratory Revision 2.1 Fluorescence Quenching of Human Serum Albumin by Caffeine In this laboratory exercise we will examine the fluorescence of Human Serum Albumin (HSA)
Fluorescense. Aromatic molecules often fluoresce (but DNA bases don't) Excitation ~ absorbance (Differences due to: ν h ' Fluorescence
 Fluorescense Two types of spectra: Excitation: Detection λ fixed and scan excitation λ. Emission: Excitation λ fixed (usually at A max ) & scan detection λ. What goes up must come down Radiationless decay
Fluorescense Two types of spectra: Excitation: Detection λ fixed and scan excitation λ. Emission: Excitation λ fixed (usually at A max ) & scan detection λ. What goes up must come down Radiationless decay
DENATURATION OF HEMOGLOBIN IN THE PRESENCE OF TANNIC ACID
 PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 2014, 1, p. 23 27 C h emistr y DENATURATION OF HEMOGLOBIN IN THE PRESENCE OF TANNIC ACID K. R. GRIGORYAN, L. S. SARGSYAN
PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 2014, 1, p. 23 27 C h emistr y DENATURATION OF HEMOGLOBIN IN THE PRESENCE OF TANNIC ACID K. R. GRIGORYAN, L. S. SARGSYAN
MOLEBIO LAB #3: Electrophoretic Separation of Proteins
 MOLEBIO LAB #3: Electrophoretic Separation of Proteins Introduction: Proteins occupy a central position in the structure and function of all living organisms. Some proteins serve as structural components
MOLEBIO LAB #3: Electrophoretic Separation of Proteins Introduction: Proteins occupy a central position in the structure and function of all living organisms. Some proteins serve as structural components
The mechanism(s) of protein folding. What is meant by mechanism. Experimental approaches
 The mechanism(s) of protein folding What is meant by mechanism Computational approaches Experimental approaches Questions: What events occur and in what time sequence when a protein folds Is there a specified
The mechanism(s) of protein folding What is meant by mechanism Computational approaches Experimental approaches Questions: What events occur and in what time sequence when a protein folds Is there a specified
Binding of bioactive coomassie brilliant blue G with protein:
 Binding of bioactive coomassie brilliant blue G with protein: Insights from spectroscopic studies A B S T R A C T The binding of CBB to BSA was investigated under imitated physiological conditions employing
Binding of bioactive coomassie brilliant blue G with protein: Insights from spectroscopic studies A B S T R A C T The binding of CBB to BSA was investigated under imitated physiological conditions employing
Extracting Pure Proteins from Cells
 Extracting Pure Proteins from Cells 0 Purification techniques focus mainly on size & charge 0 The first step is homogenization (grinding, Potter Elvejhem homogenizer, sonication, freezing and thawing,
Extracting Pure Proteins from Cells 0 Purification techniques focus mainly on size & charge 0 The first step is homogenization (grinding, Potter Elvejhem homogenizer, sonication, freezing and thawing,
Contact Details. Dr Alexander Galkin. Office: MBC Room 186. Tel: (028) Frequency and wavelength.
 Contact Details The electromagnetic spectrum Biological Spectroscopy Dr Alexander Galkin Email: a.galkin@qub.ac.uk Dr Alexander Galkin MSc Biomolecular Function - BBC8045 Office: MBC Room 186 Tel: (028)
Contact Details The electromagnetic spectrum Biological Spectroscopy Dr Alexander Galkin Email: a.galkin@qub.ac.uk Dr Alexander Galkin MSc Biomolecular Function - BBC8045 Office: MBC Room 186 Tel: (028)
LED Light Sources for Stopped- Flow Spectroscopy
 SX SERIES APPLICATION NOTE LED Light Sources for Stopped- Flow Spectroscopy Abstract: Applied Photophysics Ltd is offering a range of light emitting diode (LED) light sources for use in stopped-flow spectroscopy
SX SERIES APPLICATION NOTE LED Light Sources for Stopped- Flow Spectroscopy Abstract: Applied Photophysics Ltd is offering a range of light emitting diode (LED) light sources for use in stopped-flow spectroscopy
Origin of Tryptophan Fluorescence Lifetimes in Solution and in Proteins. Jihad René Albani Université de Lille 1. Laboratoire Biophysique Moléculaire
 Origin of Tryptophan Fluorescence Lifetimes in Solution and in Proteins Jihad René Albani Université de Lille 1. Laboratoire Biophysique Moléculaire Fluorescence instruments Fluorescence lifetimes were
Origin of Tryptophan Fluorescence Lifetimes in Solution and in Proteins Jihad René Albani Université de Lille 1. Laboratoire Biophysique Moléculaire Fluorescence instruments Fluorescence lifetimes were
Protein analysis. Dr. Mamoun Ahram Summer semester, Resources This lecture Campbell and Farrell s Biochemistry, Chapters 5
 Protein analysis Dr. Mamoun Ahram Summer semester, 2015-2016 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 5 Bases of protein separation Proteins can be purified on the basis Solubility
Protein analysis Dr. Mamoun Ahram Summer semester, 2015-2016 Resources This lecture Campbell and Farrell s Biochemistry, Chapters 5 Bases of protein separation Proteins can be purified on the basis Solubility
Interaction of 4-Nitroaniline with Serum Albumin
 Applied Mechanics and Materials Online: 214-2-6 ISSN: 1662-7482, Vols. 522-524, pp 337-34 doi:1.428/www.scientific.net/amm.522-524.337 214 Trans Tech Publications, Switzerland Interaction of 4-Nitroaniline
Applied Mechanics and Materials Online: 214-2-6 ISSN: 1662-7482, Vols. 522-524, pp 337-34 doi:1.428/www.scientific.net/amm.522-524.337 214 Trans Tech Publications, Switzerland Interaction of 4-Nitroaniline
INVESTIGATION OF THE INTERACTION BETWEEN NAPROXEN AND HUMAN SERUM ALBUMIN
 INVESTIGATION OF THE INTERACTION BETWEEN NAPROXEN AND HUMAN SERUM ALBUMIN A. PÎRNĂU, M. BOGDAN National R&D Institute for Isotopic and Molecular Technologies, 71 103 Donath str., 400293Cluj-Napoca, Romania
INVESTIGATION OF THE INTERACTION BETWEEN NAPROXEN AND HUMAN SERUM ALBUMIN A. PÎRNĂU, M. BOGDAN National R&D Institute for Isotopic and Molecular Technologies, 71 103 Donath str., 400293Cluj-Napoca, Romania
Self-Assembled Nanostructures Based on Activatable Red. Fluorescent Dye for Site-Specific Protein Probing and. Conformational Transition Detection
 Supporting Information Self-Assembled Nanostructures Based on Activatable Red Fluorescent Dye for Site-Specific Protein Probing and Conformational Transition Detection Yang Yu,, Yanyan Huang,*,, Fang Hu,,
Supporting Information Self-Assembled Nanostructures Based on Activatable Red Fluorescent Dye for Site-Specific Protein Probing and Conformational Transition Detection Yang Yu,, Yanyan Huang,*,, Fang Hu,,
Protein Structure and Function! Lecture 4: ph, pka and pi!
 Protein Structure and Function! Lecture 4: ph, pka and pi! Definition of ph and pk a! ph is a measure of the concentration of H +.! + ph = log 10[H ] For a weak acid,! HA #!!"! H + + A!, K a = [H + ][A!
Protein Structure and Function! Lecture 4: ph, pka and pi! Definition of ph and pk a! ph is a measure of the concentration of H +.! + ph = log 10[H ] For a weak acid,! HA #!!"! H + + A!, K a = [H + ][A!
Biochemistry. Biochemical Techniques. 18 Spectrofluorimetry
 Description of Module Subject Name Paper Name 12 Module Name/Title 1. Objectives 1.1 To understand technique of Spectrofluorimetry. 1.2 To explain instrumentation design 1.3 What are applications of Spectrofluorimetry?
Description of Module Subject Name Paper Name 12 Module Name/Title 1. Objectives 1.1 To understand technique of Spectrofluorimetry. 1.2 To explain instrumentation design 1.3 What are applications of Spectrofluorimetry?
Chemistry Exam #2 Answer Key -- November 4, 2008 There are 6 pages
 ame: Answer Key 1 Chemistry 460 -- Exam #2 Answer Key -- ovember 4, 2008 There are 6 pages 1. (10 pts) The proteins found in silk fibers are formed from stacked antiparallel β-sheets in which long stretches
ame: Answer Key 1 Chemistry 460 -- Exam #2 Answer Key -- ovember 4, 2008 There are 6 pages 1. (10 pts) The proteins found in silk fibers are formed from stacked antiparallel β-sheets in which long stretches
Fluorescence Measurements of Denatured Proteins within Electrospray Droplets
 Fluorescence Measurements of Denatured Proteins within Droplets Joel H. Parks, Sandra E. Rodriguez-Cruz and Joseph T. Khoury The Rowland Institute for Science, Cambridge, MA 02142 www.rowland.org OVERVIEW
Fluorescence Measurements of Denatured Proteins within Droplets Joel H. Parks, Sandra E. Rodriguez-Cruz and Joseph T. Khoury The Rowland Institute for Science, Cambridge, MA 02142 www.rowland.org OVERVIEW
Lecture 23: SDS Gel Electrophoresis and Stacking Gels
 Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 23: SDS Gel Electrophoresis and Stacking Gels 3 April 2018 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Biological Chemistry Laboratory Biology 3515/Chemistry 3515 Spring 2018 Lecture 23: SDS Gel Electrophoresis and Stacking Gels 3 April 2018 c David P. Goldenberg University of Utah goldenberg@biology.utah.edu
Fluorescence quenching, Fluorescence anisotropy, Fluorescence resonance energy transfer (FRET)
 Fluorescence quenching, Fluorescence anisotropy, Fluorescence resonance energy transfer (FRET) Timescale of fluorescence processes The excited electron decay possibilities k f k ph k q k t k ic Biophysics
Fluorescence quenching, Fluorescence anisotropy, Fluorescence resonance energy transfer (FRET) Timescale of fluorescence processes The excited electron decay possibilities k f k ph k q k t k ic Biophysics
and electron tunneling through proteins. Small-scale motions such as side-chain protein can take microseconds to seconds, depending on the protein.
 1 Chapter 1 1.1 Our Approach We are interested in dynamic processes in proteins that occur over a broad range of timescales: protein folding, intrachain diffusion in unfolded proteins, and electron tunneling
1 Chapter 1 1.1 Our Approach We are interested in dynamic processes in proteins that occur over a broad range of timescales: protein folding, intrachain diffusion in unfolded proteins, and electron tunneling
Fluorescence spectroscopy
 Fluorescence spectroscopy The light: electromagnetic wave Tamás Huber Biophysics seminar Dept. of Biophysics, University of Pécs 05-07. February 2013. Luminescence: light emission of an excited system.
Fluorescence spectroscopy The light: electromagnetic wave Tamás Huber Biophysics seminar Dept. of Biophysics, University of Pécs 05-07. February 2013. Luminescence: light emission of an excited system.
Types of chromatography
 Chromatography Physical separation method based on the differential migration of analytes in a mobile phase as they move along a stationary phase. Mechanisms of Separation: Partitioning Adsorption Exclusion
Chromatography Physical separation method based on the differential migration of analytes in a mobile phase as they move along a stationary phase. Mechanisms of Separation: Partitioning Adsorption Exclusion
Topics in Fluorescence Spectroscopy. Volume 3 Biochemical Applications
 Topics in Fluorescence Spectroscopy Volume 3 Biochemical Applications Topics in Fluorescence Spectroscopy Edited by JOSEPH R. LAKOWICZ Volume 1: Techniques Volume 2: Principles Volume 3: Biochemical Applications
Topics in Fluorescence Spectroscopy Volume 3 Biochemical Applications Topics in Fluorescence Spectroscopy Edited by JOSEPH R. LAKOWICZ Volume 1: Techniques Volume 2: Principles Volume 3: Biochemical Applications
Chapter 6. Techniques of Protein and Nucleic Acid Purification
 Chapter 6 Techniques of Protein and Nucleic Acid Purification Considerations in protein expression and purification Protein source Natural sources Recombinant sources Methods of lysis and solubilization
Chapter 6 Techniques of Protein and Nucleic Acid Purification Considerations in protein expression and purification Protein source Natural sources Recombinant sources Methods of lysis and solubilization
The Green Fluorescent Protein. w.chem.uwec.edu/chem412_s99/ppt/green.ppt
 The Green Fluorescent Protein w.chem.uwec.edu/chem412_s99/ppt/green.ppt www.chem.uwec.edu/chem412_s99/ppt/green.ppt Protein (gene) is from a jellyfish: Aequorea victoria www.chem.uwec.edu/chem412_s99/ppt/green.ppt
The Green Fluorescent Protein w.chem.uwec.edu/chem412_s99/ppt/green.ppt www.chem.uwec.edu/chem412_s99/ppt/green.ppt Protein (gene) is from a jellyfish: Aequorea victoria www.chem.uwec.edu/chem412_s99/ppt/green.ppt
Proteins Amides from Amino Acids
 Chapter 26 and Chapter 28 Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl group Joined as amides between the ¾NH 2 of one amino acid and the ¾CO 2 H to the
Chapter 26 and Chapter 28 Proteins Amides from Amino Acids Amino acids contain a basic amino group and an acidic carboxyl group Joined as amides between the ¾NH 2 of one amino acid and the ¾CO 2 H to the
QUENCHING MECHANISM OF HUMAN SERUM ALBUMIN FLUORESCENCE BY GANGLERON
 PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 03,, p. 6 0 C h emistr y QUENCHING MECHANISM OF HUMAN SERUM ALBUMIN FLUORESCENCE BY GANGLERON K. R. GRIGORYAN *, A. G.
PROCEEDINGS OF THE YEREVAN STATE UNIVERSITY C h e m i s t r y a n d B i o l o g y 03,, p. 6 0 C h emistr y QUENCHING MECHANISM OF HUMAN SERUM ALBUMIN FLUORESCENCE BY GANGLERON K. R. GRIGORYAN *, A. G.
Fluorescence spectroscopy
 Fluorescence spectroscopy The light: electromagnetic wave Tamás Huber Biophysics seminar Dept. of Biophysics, University of Pécs 05-06. February 2014. 1 Luminescence: light emission of an excited system.
Fluorescence spectroscopy The light: electromagnetic wave Tamás Huber Biophysics seminar Dept. of Biophysics, University of Pécs 05-06. February 2014. 1 Luminescence: light emission of an excited system.
Protein Purification and Characterization Techniques. Nafith Abu Tarboush, DDS, MSc, PhD
 Protein Purification and Characterization Techniques Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Extracting Pure Proteins from Cells Purification techniques focus
Protein Purification and Characterization Techniques Nafith Abu Tarboush, DDS, MSc, PhD natarboush@ju.edu.jo www.facebook.com/natarboush Extracting Pure Proteins from Cells Purification techniques focus
Crystal Growth, Optical and Thermal Studies of 4-Nitroaniline 4- Aminobenzoic Acid: A Fluorescent Material
 Research Article Crystal Growth, Optical and Thermal Studies of 4-Nitroaniline 4- Aminobenzoic Acid: A Fluorescent Material A. Silambarasan, P. Rajesh * and P. Ramasamy Centre for Crystal Growth, SSN College
Research Article Crystal Growth, Optical and Thermal Studies of 4-Nitroaniline 4- Aminobenzoic Acid: A Fluorescent Material A. Silambarasan, P. Rajesh * and P. Ramasamy Centre for Crystal Growth, SSN College
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor
 Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Focus concept Purification of a novel seed storage protein allows sequence analysis and determination of the protein
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Focus concept Purification of a novel seed storage protein allows sequence analysis and determination of the protein
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Last modified 29 September 2005
 Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Last modified 9 September 005 Focus concept Purification of a novel seed storage protein allows sequence analysis and
Case 7 A Storage Protein From Seeds of Brassica nigra is a Serine Protease Inhibitor Last modified 9 September 005 Focus concept Purification of a novel seed storage protein allows sequence analysis and
BIL 256 Cell and Molecular Biology Lab Spring, Molecular Weight Determination: SDS Electrophoresis
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 Molecular Weight Determination: SDS Electrophoresis Separation of Proteins by Electrophoresis A. Separation by Charge All polypeptide chains contain
BIL 256 Cell and Molecular Biology Lab Spring, 2007 Molecular Weight Determination: SDS Electrophoresis Separation of Proteins by Electrophoresis A. Separation by Charge All polypeptide chains contain
Chapter 4 Fluorescence Resonance Energy Transfer (FRET) by Minor Groove-Associated Cyanine-Polyamide Conjugates
 Chapter 4 Fluorescence Resonance Energy Transfer (FRET) by Minor Groove-Associated Cyanine-Polyamide Conjugates The work described in this chapter was accomplished in collaboration with V. Rucker (Dervan
Chapter 4 Fluorescence Resonance Energy Transfer (FRET) by Minor Groove-Associated Cyanine-Polyamide Conjugates The work described in this chapter was accomplished in collaboration with V. Rucker (Dervan
ESTIMATION OF THE BINDING ABILITY OF MAIN TRANSPORT PROTEINS OF BLOOD PLASMA WITH LIVER CIRRHOSIS BY THE FLUORESCENT PROBE METHOD
 Journal of Applied Spectroscopy, Vol. 74, No. 4, 2007 ESTIMATION OF THE BINDING ABILITY OF MAIN TRANSPORT PROTEINS OF BLOOD PLASMA WITH LIVER CIRRHOSIS BY THE FLUORESCENT PROBE METHOD E. A. Korolenko,
Journal of Applied Spectroscopy, Vol. 74, No. 4, 2007 ESTIMATION OF THE BINDING ABILITY OF MAIN TRANSPORT PROTEINS OF BLOOD PLASMA WITH LIVER CIRRHOSIS BY THE FLUORESCENT PROBE METHOD E. A. Korolenko,
Stability of sub-unit vaccines measured with the UNit label-free stability platform
 Application Note Stability of sub-unit vaccines measured with the UNit label-free stability platform Introduction Sub-unit vaccines and the need for adjuvants Of the many classes of vaccines that have
Application Note Stability of sub-unit vaccines measured with the UNit label-free stability platform Introduction Sub-unit vaccines and the need for adjuvants Of the many classes of vaccines that have
Toggling Between Blue and Red Emitting Fluorescent Silver Nanoclusters
 Supporting Information Toggling Between Blue and Red Emitting Fluorescent Silver Nanoclusters Uttam Anand, Subhadip Ghosh and Saptarshi Mukherjee * Department of Chemistry, Indian Institute of Science
Supporting Information Toggling Between Blue and Red Emitting Fluorescent Silver Nanoclusters Uttam Anand, Subhadip Ghosh and Saptarshi Mukherjee * Department of Chemistry, Indian Institute of Science
Imaging of protein crystals with two photon microscopy
 Supporting Information Imaging of protein crystals with two photon microscopy Pius Padayatti,*, Grazyna Palczewska,*, Wenyu Sun, Krzysztof Palczewski,# and David Salom Polgenix Inc., Cleveland, Ohio 44106,
Supporting Information Imaging of protein crystals with two photon microscopy Pius Padayatti,*, Grazyna Palczewska,*, Wenyu Sun, Krzysztof Palczewski,# and David Salom Polgenix Inc., Cleveland, Ohio 44106,
Lab 1: Ensemble Fluorescence Basics
 Lab 1: Ensemble Fluorescence Basics This laboratory module is divided into two sections. The first one is on organic fluorophores, and the second one is on ensemble measurement of FRET (Fluorescence Resonance
Lab 1: Ensemble Fluorescence Basics This laboratory module is divided into two sections. The first one is on organic fluorophores, and the second one is on ensemble measurement of FRET (Fluorescence Resonance
Physical Methods in Models of Cataract Disease. O. P. Srivastava Department of Vision Science University of Alabama at Birmingham
 Physical Methods in Models of Cataract Disease O. P. Srivastava Department of Vision Science University of Alabama at Birmingham Function of the Lens: Refraction Lens Specific Structural Proteins (α-,
Physical Methods in Models of Cataract Disease O. P. Srivastava Department of Vision Science University of Alabama at Birmingham Function of the Lens: Refraction Lens Specific Structural Proteins (α-,
Purification: Step 1. Lecture 11 Protein and Peptide Chemistry. Cells: Break them open! Crude Extract
 Purification: Step 1 Lecture 11 Protein and Peptide Chemistry Cells: Break them open! Crude Extract Total contents of cell Margaret A. Daugherty Fall 2003 Big Problem: Crude extract is not the natural
Purification: Step 1 Lecture 11 Protein and Peptide Chemistry Cells: Break them open! Crude Extract Total contents of cell Margaret A. Daugherty Fall 2003 Big Problem: Crude extract is not the natural
Purification: Step 1. Protein and Peptide Chemistry. Lecture 11. Big Problem: Crude extract is not the natural environment. Cells: Break them open!
 Lecture 11 Protein and Peptide Chemistry Margaret A. Daugherty Fall 2003 Purification: Step 1 Cells: Break them open! Crude Extract Total contents of cell Big Problem: Crude extract is not the natural
Lecture 11 Protein and Peptide Chemistry Margaret A. Daugherty Fall 2003 Purification: Step 1 Cells: Break them open! Crude Extract Total contents of cell Big Problem: Crude extract is not the natural
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA
 AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
AP Biology Book Notes Chapter 3 v Nucleic acids Ø Polymers specialized for the storage transmission and use of genetic information Ø Two types DNA Encodes hereditary information Used to specify the amino
DNA/Protein Binding, Molecular Docking and in Vitro Anti-cancer Activity of some Thioether-Dipyrrinato Complexes
 DNA/Protein Binding, Molecular Docking and in Vitro Anti-cancer Activity of some Thioether-Dipyrrinato Complexes Rakesh Kumar Gupta, Gunjan Sharma, ξ Rampal Pandey, Amit Kumar, Biplob Koch, ξ Pei- Zhou
DNA/Protein Binding, Molecular Docking and in Vitro Anti-cancer Activity of some Thioether-Dipyrrinato Complexes Rakesh Kumar Gupta, Gunjan Sharma, ξ Rampal Pandey, Amit Kumar, Biplob Koch, ξ Pei- Zhou
Drug/protein interactions studied by time-resolved fluorescence spectroscopy
 Drug/protein interactions studied by time-resolved fluorescence spectroscopy T. Gustavsson, D. Markovitsi, I. Vayá, P. Bonancía, M. C. Jiménez, M.L A. Miranda To cite this version: T. Gustavsson, D. Markovitsi,
Drug/protein interactions studied by time-resolved fluorescence spectroscopy T. Gustavsson, D. Markovitsi, I. Vayá, P. Bonancía, M. C. Jiménez, M.L A. Miranda To cite this version: T. Gustavsson, D. Markovitsi,
Protein Techniques 1 APPENDIX TO CHAPTER 5
 Protein Techniques 1 APPENDIX T CHAPTER 5 Dialysis and Ultrafiltration If a solution of protein is separated from a bathing solution by a semipermeable membrane, small molecules and ions can pass through
Protein Techniques 1 APPENDIX T CHAPTER 5 Dialysis and Ultrafiltration If a solution of protein is separated from a bathing solution by a semipermeable membrane, small molecules and ions can pass through
Fluorescence spectroscopy
 Fluorescence spectroscopy The light: electromagnetic wave Zoltán Ujfalusi Biophysics seminar Dept. of Biophysics, University of Pécs 14-16 February 2011 Luminescence: light is not generated by high temperatures!!!
Fluorescence spectroscopy The light: electromagnetic wave Zoltán Ujfalusi Biophysics seminar Dept. of Biophysics, University of Pécs 14-16 February 2011 Luminescence: light is not generated by high temperatures!!!
SUPPLEMENTAL INFORMATION. Center Hamburg-Eppendorf, Center for Molecular Neurobiology, ZMNH, Hamburg, Germany
 SUPPLEMENTAL INFORMATION Title: Inter-motif communication induces hierarchical Ca 2+ -filling of Caldendrin Authors: Uday Kiran 1, Phanindranath Regur 1, Michael R. Kreutz 3,4*, Yogendra Sharma 1,2*, Asima
SUPPLEMENTAL INFORMATION Title: Inter-motif communication induces hierarchical Ca 2+ -filling of Caldendrin Authors: Uday Kiran 1, Phanindranath Regur 1, Michael R. Kreutz 3,4*, Yogendra Sharma 1,2*, Asima
Assembly and Photophysics of Ternary Luminescent Lanthanide Molecular Complex Systems
 Journal of the Chinese Chemical Society, 2004, 51, 697-702 697 Assembly and Photophysics of Ternary Luminescent Lanthanide Molecular Complex Systems B. Yan* ( ) and Q. Y. Xie ( ) Department of Chemistry,
Journal of the Chinese Chemical Society, 2004, 51, 697-702 697 Assembly and Photophysics of Ternary Luminescent Lanthanide Molecular Complex Systems B. Yan* ( ) and Q. Y. Xie ( ) Department of Chemistry,
Conformational Transitions of the Three Recombinant Domains of Human Serum Albumin Depending on ph*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 275, No. 5, Issue of February 4, pp. 3042 3050, 2000 2000 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Conformational
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 275, No. 5, Issue of February 4, pp. 3042 3050, 2000 2000 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Conformational
SECOND DERIVATIVE UV FOR RAPID CONFORMATIONAL ASSESSMENT OF PROTEINS
 SECOND DERIVATIVE UV FOR RAPID CONFORMATIONAL ASSESSMENT OF PROTEINS NOVEMBER 2016 KEVIN MCCOWEN SUPERVISOR ANALYTICAL DEVELOPMENT OVERVIEW Brief overview of UV absorbance Development of statistical analysis
SECOND DERIVATIVE UV FOR RAPID CONFORMATIONAL ASSESSMENT OF PROTEINS NOVEMBER 2016 KEVIN MCCOWEN SUPERVISOR ANALYTICAL DEVELOPMENT OVERVIEW Brief overview of UV absorbance Development of statistical analysis
Reminder: absorption. OD = A = - log (I / I 0 ) = ε (λ) c x. I = I ε(λ) c x. Definitions. Fluorescence quenching and FRET.
 Reminder: absorption Special fluorescence applications I 0 I Fluorescence quenching and FRET Miklós Nyitrai; 24 th of Februry 2011. substance OD = A = - log (I / I 0 ) = ε (λ) c x optical density I = I
Reminder: absorption Special fluorescence applications I 0 I Fluorescence quenching and FRET Miklós Nyitrai; 24 th of Februry 2011. substance OD = A = - log (I / I 0 ) = ε (λ) c x optical density I = I
Lecture 5: 8/31. CHAPTER 5 Techniques in Protein Biochemistry
 Lecture 5: 8/31 CHAPTER 5 Techniques in Protein Biochemistry Chapter 5 Outline The proteome is the entire set of proteins expressed and modified by a cell under a particular set of biochemical conditions.
Lecture 5: 8/31 CHAPTER 5 Techniques in Protein Biochemistry Chapter 5 Outline The proteome is the entire set of proteins expressed and modified by a cell under a particular set of biochemical conditions.
Different methods for the study of interactions of metal complexes with biological molecules
 Different methods for the study of interactions of metal complexes with biological molecules Dr. Santiago GómezG mez-ruiz Departamento de Química Inorgánica y Analítica, Escuela Superior de Ciencias Experimentales
Different methods for the study of interactions of metal complexes with biological molecules Dr. Santiago GómezG mez-ruiz Departamento de Química Inorgánica y Analítica, Escuela Superior de Ciencias Experimentales
Macromolecular environments influence proteins
 Research & Development Protein Dynamics Macromolecular environments influence proteins 6 www.q-more.com/en/ q&more 01.16 Studying proteins in the presence of high concentrations of macromolecules ( molecular
Research & Development Protein Dynamics Macromolecular environments influence proteins 6 www.q-more.com/en/ q&more 01.16 Studying proteins in the presence of high concentrations of macromolecules ( molecular
Multi-Volume Based Protein Quantification
 A p p l i c a t i o n G u i d e Multi-Volume Based Methods Why Quantify Proteins? Proteins are central to our understanding of biology. In cells, they are multipurpose: from actin providing structural
A p p l i c a t i o n G u i d e Multi-Volume Based Methods Why Quantify Proteins? Proteins are central to our understanding of biology. In cells, they are multipurpose: from actin providing structural
FLIM Fluorescence Lifetime IMaging
 FLIM Fluorescence Lifetime IMaging Fluorescence lifetime t I(t) = F0 exp( ) τ 1 τ = k f + k nr k nr = k IC + k ISC + k bl Batiaens et al, Trends in Cell Biology, 1999 τ τ = fluorescence lifetime (~ns to
FLIM Fluorescence Lifetime IMaging Fluorescence lifetime t I(t) = F0 exp( ) τ 1 τ = k f + k nr k nr = k IC + k ISC + k bl Batiaens et al, Trends in Cell Biology, 1999 τ τ = fluorescence lifetime (~ns to
Introduction to Fluorescence Jablonski Diagram
 ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
ntroduction to Fluorescence Jablonski Diagram Excited Singlet Manifold S1 internal conversion S2 k -isc k isc Excited riplet Manifold 1 S0 k nr k k' f nr fluorescence k p phosphorescence Singlet round
Acceleration of protein folding by four orders of magnitude through a single amino acid substitution
 Acceleration of protein folding by four orders of magnitude through a single amino acid substitution Daniel J. A. Roderer 1, Martin A. Schärer 1, Marina Rubini 2 * and Rudi Glockshuber 1 AUTHOR ADDRESS
Acceleration of protein folding by four orders of magnitude through a single amino acid substitution Daniel J. A. Roderer 1, Martin A. Schärer 1, Marina Rubini 2 * and Rudi Glockshuber 1 AUTHOR ADDRESS
Concept review: Fluorescence
 16 Concept review: Fluorescence Some definitions: Chromophore. The structural feature of a molecule responsible for the absorption of UV or visible light. Fluorophore. A chromophore that remits an absorbed
16 Concept review: Fluorescence Some definitions: Chromophore. The structural feature of a molecule responsible for the absorption of UV or visible light. Fluorophore. A chromophore that remits an absorbed
Directional Surface Plasmon Coupled Emission
 Journal of Fluorescence, Vol. 14, No. 1, January 2004 ( 2004) Fluorescence News Directional Surface Plasmon Coupled Emission KEY WORDS: Surface plasmon coupled emission; high sensitivity detection; reduced
Journal of Fluorescence, Vol. 14, No. 1, January 2004 ( 2004) Fluorescence News Directional Surface Plasmon Coupled Emission KEY WORDS: Surface plasmon coupled emission; high sensitivity detection; reduced
Ion exchange chromatography
 Ion exchange chromatography Objectives: 1- The objective of this experiment is to learn the principles of ion exchange chromatography by separating the charged molecules using buffer and salt. 2- A practical
Ion exchange chromatography Objectives: 1- The objective of this experiment is to learn the principles of ion exchange chromatography by separating the charged molecules using buffer and salt. 2- A practical
Microscale Thermophoresis
 AN INTRODUCTION Microscale Thermophoresis A sensitive method to detect and quantify molecular interactions OCTOBER 2012 CHAPTER 1 Overview Microscale thermophoresis (MST) is a new method that enables the
AN INTRODUCTION Microscale Thermophoresis A sensitive method to detect and quantify molecular interactions OCTOBER 2012 CHAPTER 1 Overview Microscale thermophoresis (MST) is a new method that enables the
Visualisation, Sizing and Counting of Fluorescent and Fluorescently-Labelled Nanoparticles
 Visualisation, Sizing and Counting of Fluorescent and Fluorescently-Labelled Nanoparticles Introduction Fluorescent molecules have long been used to specifically label particular structures and features
Visualisation, Sizing and Counting of Fluorescent and Fluorescently-Labelled Nanoparticles Introduction Fluorescent molecules have long been used to specifically label particular structures and features
Zetasizer ZS Helix (DLS/Raman) For Characterization of Pharmaceuticals and Biopharmaceuticals
 Zetasizer ZS Helix (DLS/Raman) For Characterization of Pharmaceuticals and Biopharmaceuticals Benefits of Zetasizer ZS Helix What it provides Develop mechanistic insight into oligomerization and aggregation
Zetasizer ZS Helix (DLS/Raman) For Characterization of Pharmaceuticals and Biopharmaceuticals Benefits of Zetasizer ZS Helix What it provides Develop mechanistic insight into oligomerization and aggregation
Addison Ault, Department of Chemistry, Cornell College, Mount Vernon, IA. The isoelectric point is a point on the ph scale. It is the ph at which the
 1 Isoelectric Points On the Back of the Envelope Addison Ault, Department of Chemistry, Cornell College, Mount Vernon, IA The isoelectric point is a point on the ph scale. It is the ph at which the average
1 Isoelectric Points On the Back of the Envelope Addison Ault, Department of Chemistry, Cornell College, Mount Vernon, IA The isoelectric point is a point on the ph scale. It is the ph at which the average
Proteomics 6/4/2009 WESTERN BLOT ANALYSIS
 SDS-PAGE (PolyAcrylamide Gel Electrophoresis) Proteomics WESTERN BLOT ANALYSIS Presented by: Nuvee Prapasarakul Veterinary Microbiology Chulalongkorn University Proteomics has been said to be the next
SDS-PAGE (PolyAcrylamide Gel Electrophoresis) Proteomics WESTERN BLOT ANALYSIS Presented by: Nuvee Prapasarakul Veterinary Microbiology Chulalongkorn University Proteomics has been said to be the next
Topics in Fluorescence Spectroscopy. Volume 2 Principles
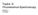 Topics in Fluorescence Spectroscopy Volume 2 Principles Topics in Fluorescence Spectroscopy Edited by JOSEPH R. LAKOWICZ Volume 1: Techniques Volume 2: Principles Volume 3: Biochemical Applications Topics
Topics in Fluorescence Spectroscopy Volume 2 Principles Topics in Fluorescence Spectroscopy Edited by JOSEPH R. LAKOWICZ Volume 1: Techniques Volume 2: Principles Volume 3: Biochemical Applications Topics
Time-resolved Measurements Using the Agilent Cary Eclipse Fluorescence Spectrophotometer A Versatile Instrument for Accurate Measurements
 Time-resolved Measurements Using the Agilent Cary Eclipse Fluorescence Spectrophotometer A Versatile Instrument for Accurate Measurements Technical Overview Authors Dr. Fabian Zieschang, Katherine MacNamara,
Time-resolved Measurements Using the Agilent Cary Eclipse Fluorescence Spectrophotometer A Versatile Instrument for Accurate Measurements Technical Overview Authors Dr. Fabian Zieschang, Katherine MacNamara,
Rapid Kinetics with IR Protein folding examples
 Rapid Kinetics with IR Protein folding examples Time dependent data with FTIR Stop-flow methods - msec limits so far Continuous, micro-flow methods - < 100 µsec Rapid scan FT-IR - msec Multichannel laser
Rapid Kinetics with IR Protein folding examples Time dependent data with FTIR Stop-flow methods - msec limits so far Continuous, micro-flow methods - < 100 µsec Rapid scan FT-IR - msec Multichannel laser
Fluorescence resonance energy transfer from tryptophan in human serum albumin to a bioactive indoloquinolizine system
 J. Chem. Sci., Vol. 119, No. 2, March 2007, pp. 77 82. Indian Academy of Sciences. Fluorescence resonance energy transfer from tryptophan in human serum albumin to a bioactive indoloquinolizine system
J. Chem. Sci., Vol. 119, No. 2, March 2007, pp. 77 82. Indian Academy of Sciences. Fluorescence resonance energy transfer from tryptophan in human serum albumin to a bioactive indoloquinolizine system
Chapter 5: Proteins: Primary Structure
 Instant download and all chapters Test Bank Fundamentals of Biochemistry Life at the Molecular Level 4th Edition Donald Voet https://testbanklab.com/download/test-bank-fundamentals-biochemistry-life-molecular-level-
Instant download and all chapters Test Bank Fundamentals of Biochemistry Life at the Molecular Level 4th Edition Donald Voet https://testbanklab.com/download/test-bank-fundamentals-biochemistry-life-molecular-level-
VARIATION OF SPECTRAL CHARACTERISTICS OF COELENTERAMIDE-CONTAINING FLUORESCENT PROTEIN FROM OBELIA LONGISSIMA EXPOSED TO DIMETHYL SULFOXIDE
 DOI 10.1007/s11182-016-0806-8 Russian Physics Journal, Vol. 59, No. 4, August, 2016 (Russian Original No. 4, April, 2016) VARIATION OF SPECTRAL CHARACTERISTICS OF COELENTERAMIDE-CONTAINING FLUORESCENT
DOI 10.1007/s11182-016-0806-8 Russian Physics Journal, Vol. 59, No. 4, August, 2016 (Russian Original No. 4, April, 2016) VARIATION OF SPECTRAL CHARACTERISTICS OF COELENTERAMIDE-CONTAINING FLUORESCENT
nanodsf 2bind: Your service provider for biophysical characterization of proteins Precisely revealing protein folding and stability
 nanodsf Precisely revealing protein folding and stability 2bind: Your service provider for biophysical characterization of proteins This booklet was written and designed by 2bind 08 2015 Any reproduction
nanodsf Precisely revealing protein folding and stability 2bind: Your service provider for biophysical characterization of proteins This booklet was written and designed by 2bind 08 2015 Any reproduction
Experiment 11. Protein Agarose Gel Electrophoresis. VY NGUYEN 8 April 2016
 Experiment 11 Protein Agarose Gel Electrophoresis VY NGUYEN 8 April 2016 ABSTRACT 1. Understand the concepts of protein structures, isoelectric point of amino acids and proteins. 2. Understand the principle
Experiment 11 Protein Agarose Gel Electrophoresis VY NGUYEN 8 April 2016 ABSTRACT 1. Understand the concepts of protein structures, isoelectric point of amino acids and proteins. 2. Understand the principle
BIL 256 Cell and Molecular Biology Lab Spring, 2007 BACKGROUND INFORMATION I. PROTEIN COMPOSITION AND STRUCTURE: A REVIEW OF THE BASICS
 BIL 256 Cell and Molecular Biology Lab Spring, 2007 BACKGROUND INFORMATION I. PROTEIN COMPOSITION AND STRUCTURE: A REVIEW OF THE BASICS Proteins occupy a central position in the structure and function
BIL 256 Cell and Molecular Biology Lab Spring, 2007 BACKGROUND INFORMATION I. PROTEIN COMPOSITION AND STRUCTURE: A REVIEW OF THE BASICS Proteins occupy a central position in the structure and function
Materials & Equipment 400 ml SDS running buffer Molecular weight markers (MWM) Chromatography samples (A476-1, A476 peak, A476+1)
 Texas A&M University-Corpus Christi CHEM4402 Biochemistry II Laboratory Laboratory 13: Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis In this final laboratory, we will use
Texas A&M University-Corpus Christi CHEM4402 Biochemistry II Laboratory Laboratory 13: Sodium Dodecyl Sulfate Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis In this final laboratory, we will use
F* techniques: FRAP, FLIP, FRET, FLIM,
 F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
F* techniques: FRAP, FLIP, FRET, FLIM, FCS Antonia Göhler March 2015 Fluorescence explained in the Bohr model Absorption of light (blue) causes an electron to move to a higher energy orbit. After a particular
Chapter 3 Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
Sheet #7 Dr. Mamoun Ahram 10/07/2014
 1 Recap: Globular Proteins - There are two types of proteins according to their structure: a. Fibrous proteins. b. Globular proteins: are globe-like (spherical) with three-dimensional compact structures
1 Recap: Globular Proteins - There are two types of proteins according to their structure: a. Fibrous proteins. b. Globular proteins: are globe-like (spherical) with three-dimensional compact structures
Regulatory Consideration for the Characterization of HOS in Biotechnology Products
 Regulatory Consideration for the Characterization of HOS in Biotechnology Products Maria Teresa Gutierrez Lugo, Ph.D. OBP/CDER/FDA 5 th International Symposium on Higher Order Structure of Protein Therapeutics
Regulatory Consideration for the Characterization of HOS in Biotechnology Products Maria Teresa Gutierrez Lugo, Ph.D. OBP/CDER/FDA 5 th International Symposium on Higher Order Structure of Protein Therapeutics
Stopped-Flow Fluorescence Polarisation/Anisotropy
 SX SERIES APPLICATION NOTE Stopped-Flow Fluorescence Polarisation/Anisotropy Abstract: Stopped-flow fluorescence polarisation/anisotropy is a highly useful technique for obtaining valuable kinetic information
SX SERIES APPLICATION NOTE Stopped-Flow Fluorescence Polarisation/Anisotropy Abstract: Stopped-flow fluorescence polarisation/anisotropy is a highly useful technique for obtaining valuable kinetic information
Supporting Information for. Electrical control of Förster energy transfer.
 1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
1 Supporting Information for Electrical control of Förster energy transfer. Klaus Becker 1, John M. Lupton 1*, Josef Müller 1, Andrey. L. Rogach 1, Dmitri V. Talapin, Horst Weller & Jochen Feldmann 1 1
2012 HORIBA, Ltd. All rights reserved HORIBA, Ltd. All rights reserved.
 Fluorescence in Biomolecular Research HORIBA Jobin Yvon IBH Ltd A division of HORIBA Scientific Kulwinder Sagoo Product Specialist February 27, 2013 Outline History of IBH HORIBA Group What is Fluorescence?
Fluorescence in Biomolecular Research HORIBA Jobin Yvon IBH Ltd A division of HORIBA Scientific Kulwinder Sagoo Product Specialist February 27, 2013 Outline History of IBH HORIBA Group What is Fluorescence?
Kinetics Review. Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets)
 Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
Quiz 1 Kinetics Review Tonight at 7 PM Phys 204 We will do two problems on the board (additional ones than in the problem sets) I will post the problems with solutions on Toolkit for those that can t make
6-Foot Mini Toober Activity
 Big Idea The interaction between the substrate and enzyme is highly specific. Even a slight change in shape of either the substrate or the enzyme may alter the efficient and selective ability of the enzyme
Big Idea The interaction between the substrate and enzyme is highly specific. Even a slight change in shape of either the substrate or the enzyme may alter the efficient and selective ability of the enzyme
Quenching of the intrinsic fluorescence of bovine serum albumin by chlorpromazine and hemin
 Interaction Brazilian Journal of CPZ of and Medical hemin and with Biological bovine Research serum albumin (2004) 37: 963-968 ISSN 0100-879X Short Communication 963 Quenching of the intrinsic fluorescence
Interaction Brazilian Journal of CPZ of and Medical hemin and with Biological bovine Research serum albumin (2004) 37: 963-968 ISSN 0100-879X Short Communication 963 Quenching of the intrinsic fluorescence
Laser- and Light-Induced Autofluorescence Spectroscopy of Human Skin in Dependence on Excitation Wavelengths
 Vol. 112 (2007) ACTA PHYSICA POLONICA A No. 5 Proceedings of the International School and Conference on Optics and Optical Materials, ISCOM07, Belgrade, Serbia, September 3 7, 2007 Laser- and Light-Induced
Vol. 112 (2007) ACTA PHYSICA POLONICA A No. 5 Proceedings of the International School and Conference on Optics and Optical Materials, ISCOM07, Belgrade, Serbia, September 3 7, 2007 Laser- and Light-Induced
Protein Structure/Function
 Protein Structure/Function C483 Spring 2013 1. Proteins segments which fold first can promote the folding of other sections of the protein into the native conformation by a process known as A) renaturation.
Protein Structure/Function C483 Spring 2013 1. Proteins segments which fold first can promote the folding of other sections of the protein into the native conformation by a process known as A) renaturation.
single-molecule fluorescence spectroscopy
 single-molecule fluorescence spectroscopy 5 dynamics of a single molecule by FRET michael börsch 18/07/2003 topics theory of fluorescence resonance energy transfer solvent effects and fluorescence quenching
single-molecule fluorescence spectroscopy 5 dynamics of a single molecule by FRET michael börsch 18/07/2003 topics theory of fluorescence resonance energy transfer solvent effects and fluorescence quenching
Fluorescence Spectroscopy. Student: Marin Cristina Antonia Coordinator:S.l. Preda Liliana
 Fluorescence Spectroscopy Student: Marin Cristina Antonia Coordinator:S.l. Preda Liliana Fluorescence Electron in the ground state is excited to a higher energy state After loss of some energy in vibrational
Fluorescence Spectroscopy Student: Marin Cristina Antonia Coordinator:S.l. Preda Liliana Fluorescence Electron in the ground state is excited to a higher energy state After loss of some energy in vibrational
CONJUGATION BOVINE SERUM ALBUMIN WITH CDTE QUANTUM DOTS
 CONJUGATION BOVINE SERUM ALBUMIN WITH CDTE QUANTUM DOTS Jana CHOMOUCKA, Jana DRBOHLAVOVA, Petra Majzlikova, Jan Prasek, Jan Pekarek, Radim Hrdy, Jaromir Hubalek Brno University of Technology, Department
CONJUGATION BOVINE SERUM ALBUMIN WITH CDTE QUANTUM DOTS Jana CHOMOUCKA, Jana DRBOHLAVOVA, Petra Majzlikova, Jan Prasek, Jan Pekarek, Radim Hrdy, Jaromir Hubalek Brno University of Technology, Department
Investigation of dendrimers functionalized with eosin as macrophotoinitiators for polymerization-based signal amplification reactions
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Investigation of dendrimers functionalized with eosin as macrophotoinitiators
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Investigation of dendrimers functionalized with eosin as macrophotoinitiators
Quantitative Evaluation of the Ability of Ionic Liquids to Offset the Cold- Induced Unfolding of Proteins
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplimentary informations Quantitative Evaluation of the Ability of Ionic Liquids
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplimentary informations Quantitative Evaluation of the Ability of Ionic Liquids
Chapter 3-II Protein Structure and Function
 Chapter 3-II Protein Structure and Function GBME, SKKU Molecular & Cell Biology H.F.K. Active site of the enzyme trypsin. Enzymes (proteins or RNAs) catalyze making or breaking substrate covalent bonds.
Chapter 3-II Protein Structure and Function GBME, SKKU Molecular & Cell Biology H.F.K. Active site of the enzyme trypsin. Enzymes (proteins or RNAs) catalyze making or breaking substrate covalent bonds.
White Paper. Ion Exchange with PureSpeed Tips A Powerful Chromatography Tool
 Ion Exchange with PureSpeed Tips A Powerful Chromatography Tool Ion exchange chromatography separates molecules by exploiting differences in their overall charge characteristics. Its simplicity makes this
Ion Exchange with PureSpeed Tips A Powerful Chromatography Tool Ion exchange chromatography separates molecules by exploiting differences in their overall charge characteristics. Its simplicity makes this
The effects of N-acetyltryptophan and caprylic acid on protein aggregation
 J. Biol. Macromol., 16 (1), 3-7 (2016) Article The effects of N-acetyltryptophan and caprylic acid on protein aggregation Shogo Oki 1, Tsutomu Arakawa 2, Kentaro Shiraki 1,* 1 Institute of Applied Physics,
J. Biol. Macromol., 16 (1), 3-7 (2016) Article The effects of N-acetyltryptophan and caprylic acid on protein aggregation Shogo Oki 1, Tsutomu Arakawa 2, Kentaro Shiraki 1,* 1 Institute of Applied Physics,
SUPPLEMENTARY MATERIAL
 SUPPLEMENTARY MATERIAL Materials and Methods Circular dichroism (CD) spectroscopy. Far ultraviolet (UV) CD spectra of apo- and holo- CaM and the CaM mutants were recorded on a Jasco J-715 spectropolarimeter
SUPPLEMENTARY MATERIAL Materials and Methods Circular dichroism (CD) spectroscopy. Far ultraviolet (UV) CD spectra of apo- and holo- CaM and the CaM mutants were recorded on a Jasco J-715 spectropolarimeter
