FLEXIBLE CONTAINMENT
|
|
|
- Duane Mathews
- 5 years ago
- Views:
Transcription
1 FLEXIBLE CONTAINMENT
2 Collaboration Partners Bectochem produces a comprehensive range of pharmaceutical process equipment & turnkey solids dosage and liquid manufacturing systems. Introducing barrier isolator technology to India in 2004, Bectochem is the acknowledged expert in the specialised and technically demanding field of design & manufacture of high specification barrier containment systems. The Industries We Serve Pharmaceutical, API Manufacturing, Solids Formulations, Liquid Formulations, Ointment Formulations, Research & Development, Food & Cosmetics Bectochem s product range is to be found in facilities across 5 continents. We strive to provide continuous improvement in our quality and customer satisfaction, supplying right first time and on time with fast manufacturing lead times. Containment Service Providers Co. Ltd (CS) is an engineering company specializing in the application of flexible containment An international leader in the design and supply of flexible containment and integrated systems for all aspects of the containment of potent materials for the Pharmaceutical, Chemical, Medical and Food industries. Extensive experience of the Pharmaceutical Industry Unrivalled practical knowledge of flexible containment technology and the application of flexible containment to new and existing equipment anywhere in the world. A hands on-can do approach to all problems
3 CONTAINMENT AROUND PROCESS, NOT THE PEOPLE!!
4 Flexible Containment A Step Ahead Segregation of equipment or plant Remote charging stations Easy Decontamination and product change overs Safe storage areas Added Benefits of Flexible Containment Any size achievable Can be designed and fitted around existing plant, equipment and pipe work Segregated room/areas within the suite Pressure cascades and HEPA filtration Fully disposable Faster Replacement of PU structure. Canopy around only critical area - A custom built application Easily installed
5 Customer Delight Wide range of flexible isolators / Glove bags to enable customers to safely handle potent compounds. Custom designed and fit flexible isolators to existing and new process systems. Isolators in Flexible film (upvc, PU, PE, etc.),clear acrylic, Stainless steel (grade 316 & 316L) for Aseptic Processing. Sterility Testing. Controlled Atmosphere, including dry air, N 2, low oxygen, etc. Sterile Containment Isolators. Laboratory isolators. Stretcher Transit isolators. Bespoke isolators. Gassed isolators using H 2 O 2 & Ozone. Multi chamber isolators. Transfer hatches (all versions) Rapid transfer ports. Continuous liner transfer systems.
6 Flexible Isolators Features Dedicated airflow systems specified to meet the process application Materials of construction specified in relation to the process requirements Product transfer systems, Waste transfer systems Pressure gauges, Alarm systems, Service connections / ports, Controlled atmosphere systems Equipment interface arrangements Quickly detachable and easily replaced flexible enclosures Flexible materials offering good visibility
7 Flexible Isolators Equipment interface arrangements Multi chamber isolator systems Active air sampler connections Particle counter connections Drum connection systems Advice on glove specification and type Ergonomic test facilities Features Replacement liner units Quickly detachable and easily replaced flexible enclosures Elliptical ports (sizes specified against requirements) Variable height facility (available on request) Easy access transfer systems
8 Rapid transfer port connections Active air sampler connections Replacement liner units Filter Replacing Glove Bags Flexible Isolators Features
9 Flexible Isolator System Advantages The Flexible Units serve 3-fold purposes : Protect operator from getting exposed to the hazardous product. Protect product from getting contaminated with the outer surroundings. Protect process from outside environment. The cost of setting up a central HVAC unit is drastically reduced The are significantly abridged decontamination costs. Negligible chances of a false positive resulting during testing Can be moved and set up anywhere with marginal utility requirements. Offer a high degree of protection and design flexibility. Affordable prices Faster deliveries. Can be used as ventilated/un-ventilated or inert atmosphere. Easily integrated to ozonizers and VHP sterilizers for maintaining sterile conditions while testing. Reduced Operating electricity and utility costs.
10 Flexible Containment
11 Services We Provide Consultancy to the Chemical and Pharmaceutical Industry on chemical containment. Design and supply generic glove bags for for non-routine maintenance operations and process requirements. for valve removal, filter change and any other operations and areas required for wet/dry product sampling. Design and installation of mobile containment units. Generate structured cleaning / decontamination schedules which are area specific. Consultancy on control access procedures for personal working. Provide consultancy for powder handling (packaging, reactor charging, sampling etc.) Provide plant specific procedures for materials, personnel and equipment movement Provide wet/dry sampling, area access control, gowning, de-gowning, housekeeping & maintenance procedures, training for all engineering solutions.. Training in any/all of the above methods and procedures. Design and installation of rigid & flexible decontamination showers and de-gowning areas. Procedures and Provide solutions and procedures for planned breaks in containment. Planning containment for all processes. Front-end containment design for new facilities
12 THANK YOU
Production and Test Facilities Via Romagnoli, Fiorenzuola d Arda (PC) - Italy - Tel Fax Containment Systems
 Containment Systems our approach FPS containment approach FPS is specialized in containment systems and fine size reduction equipment for many industrial applications. Our approach to a containment system
Containment Systems our approach FPS containment approach FPS is specialized in containment systems and fine size reduction equipment for many industrial applications. Our approach to a containment system
GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR
 GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR 2 GETINGE ISOFLEX THE ANSWER TO YOUR DAILY NEEDS FOR STERILE HANDLING In any sterile operation, maintaining the sterility of goods -- whether you have purchased them
GETINGE ISOFLEX MULTI-PURPOSE ISOLATOR 2 GETINGE ISOFLEX THE ANSWER TO YOUR DAILY NEEDS FOR STERILE HANDLING In any sterile operation, maintaining the sterility of goods -- whether you have purchased them
CONTAINED ASEPTIC TRANSFER VALVES
 CONTAINED ASEPTIC TRANSFER VALVES Applications Contained filling and dispensing for all production processes. aseptic transfer valves can be integrated into the production of both powder and liquid formulations
CONTAINED ASEPTIC TRANSFER VALVES Applications Contained filling and dispensing for all production processes. aseptic transfer valves can be integrated into the production of both powder and liquid formulations
Containment Why and How to evaluate
 Containment Why and How to evaluate IDMA- APA PAC 2018 May 18 & 19 At The Club, Andheri - Mumbai Presentation by: Sasidharan S Menon Subject Matter Expert (SME) Design and Evaluation Controlled environments
Containment Why and How to evaluate IDMA- APA PAC 2018 May 18 & 19 At The Club, Andheri - Mumbai Presentation by: Sasidharan S Menon Subject Matter Expert (SME) Design and Evaluation Controlled environments
Creating A Transfer Solution. Applying the Doverpac Drum Transfer System
 Creating A Transfer Solution Applying the Doverpac Drum Transfer System One of the simplest, most convenient and most cost-effective ways to ship, receive, and store bulk powder ingredients, including
Creating A Transfer Solution Applying the Doverpac Drum Transfer System One of the simplest, most convenient and most cost-effective ways to ship, receive, and store bulk powder ingredients, including
CONTAINED ASEPTIC TRANSFER VALVES
 1 CONTAINED ASEPTIC TRANSFER VALVES Perform aseptic transfers that maintain critical area integrity. Reduce risk of cross contamination with closed transfers that limit manual intervention. Meet GMP and
1 CONTAINED ASEPTIC TRANSFER VALVES Perform aseptic transfers that maintain critical area integrity. Reduce risk of cross contamination with closed transfers that limit manual intervention. Meet GMP and
Section 6.6 BSL3 & ABSL3 Biocontainment
 NIH Design Requirements Manual 8-22-12 Section 6.6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 Section 6.6
NIH Design Requirements Manual 8-22-12 Section 6.6 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 37 38 39 40 41 42 43 44 45 46 47 48 49 Section 6.6
Compounding Aseptic Isolators (CAI) James T Wagner
 Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
STERIS Continuous Effluent Decontamination (CED) System
 LABORATORY RESEARCH STERIS Continuous Effluent Decontamination (CED) System The Safety and Efficacy of Continuous Effluent Decontamination A Breakthrough in Decontamination Technology Continuous Effluent
LABORATORY RESEARCH STERIS Continuous Effluent Decontamination (CED) System The Safety and Efficacy of Continuous Effluent Decontamination A Breakthrough in Decontamination Technology Continuous Effluent
Containment Barrier Systems
 Containment Barrier Systems As an international leader, focused on Quality, Telstar partners with clients to provide high value on a global basis through innovation, advanced technology, absolute reliability
Containment Barrier Systems As an international leader, focused on Quality, Telstar partners with clients to provide high value on a global basis through innovation, advanced technology, absolute reliability
Overview and Introduction Annex 1 Revision
 Overview and Introduction Annex 1 Revision Paul Moody, Inspector Pharmig Current Hot Topics in Pharmaceutical Microbiology, Dublin May 30 th, 2018 Scope History Process of Revision Rationale for Revision
Overview and Introduction Annex 1 Revision Paul Moody, Inspector Pharmig Current Hot Topics in Pharmaceutical Microbiology, Dublin May 30 th, 2018 Scope History Process of Revision Rationale for Revision
filtration technology pharmaceutical, Nutraceutical, & Cosmetic industries
 filtration technology pharmaceutical, Nutraceutical, & Cosmetic industries cleaner air with confidence Donaldson Torit delivers the confidence you need to process powders in the pharmaceutical, nutraceutical
filtration technology pharmaceutical, Nutraceutical, & Cosmetic industries cleaner air with confidence Donaldson Torit delivers the confidence you need to process powders in the pharmaceutical, nutraceutical
Process Containment Solutions
 Process Containment Solutions www.dec-group.net Powder Handling Excellence Process Containment Technology Containment is a major concern for GMP industries. With a proven track record in managing highly
Process Containment Solutions www.dec-group.net Powder Handling Excellence Process Containment Technology Containment is a major concern for GMP industries. With a proven track record in managing highly
How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs
 WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
WHITE PAPER 7004 How single-use connections advance aseptic processing: Increased process flexibility and reliability, reduced costs By John Boehm Business Unit Manager Colder Products Company Today s
HVAC and Risk Management RACI Meeting 7 th December Agenda. Design. Project Management. Context. Qualification. Construction
 HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
HVAC and Risk Management RACI Meeting 7 th December 2011 Dr Stephen Firmer Asia Pacific Consultants Pty Ltd Agenda Context Project Management Design Construction Qualification Routine Control /Potential
Current Trends in Sterile Manufacturing. by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS)
 Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY
 INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY John Klostermyer, Ph.D. ISPE Product Show Track 3 Session 3 September 26, 2018 AGENDA 1. BACKGROUND INFORMATION 2. BENEFITS 3. PROCESS 4. BEST
INTEGRATED VPHP DECONTAMINATION SYSTEMS THE EMERGING UTILITY John Klostermyer, Ph.D. ISPE Product Show Track 3 Session 3 September 26, 2018 AGENDA 1. BACKGROUND INFORMATION 2. BENEFITS 3. PROCESS 4. BEST
CLASS I MICROBIOLOGICAL SAFETY CABINET - BIO 1
 CLASS I MICROBIOLOGICAL SAFETY CABINET - BIO 1 PRODUCT SHEET NO. 101 VERSION 2.0 COMPACT HIGHLY SPECIFIED, HIGH PERFORMANCE CLASS I MICROBIOLOGICAL SAFETY CABINETS Applications Handling of biological agents
CLASS I MICROBIOLOGICAL SAFETY CABINET - BIO 1 PRODUCT SHEET NO. 101 VERSION 2.0 COMPACT HIGHLY SPECIFIED, HIGH PERFORMANCE CLASS I MICROBIOLOGICAL SAFETY CABINETS Applications Handling of biological agents
ROLL COMPACTION THE FITZPATRICK COMPANY. associates
 ROLL COMPACTION THE FITZPATRICK COMPANY associates The Fitzpatrick Company is a manufacturer of stainless steel, sanitary process equipment, used by the Food, Chemical and Pharmaceutical Industries. The
ROLL COMPACTION THE FITZPATRICK COMPANY associates The Fitzpatrick Company is a manufacturer of stainless steel, sanitary process equipment, used by the Food, Chemical and Pharmaceutical Industries. The
Containment Booths. FOR MORE INFORMATION ON CONTAINMENT BOOTHS CALL +44 (0) OR
 www.extract-technology.com ENGINEERED CONTAINMENT SOLUTIONS Containment Booths AT Extract Technology, we provide complete containment solutions based around an innovative range of Downflow Containment
www.extract-technology.com ENGINEERED CONTAINMENT SOLUTIONS Containment Booths AT Extract Technology, we provide complete containment solutions based around an innovative range of Downflow Containment
Design and Manufacture of HOT CELLS
 Aquila Nuclear Engineering Ltd Design and Manufacture of HOT CELLS Process is King Prepared for 51 st Annual Meeting HOTLAB-2014 at Paul Scherrer Institute Baden, Switzerland 21st -25th September 2014
Aquila Nuclear Engineering Ltd Design and Manufacture of HOT CELLS Process is King Prepared for 51 st Annual Meeting HOTLAB-2014 at Paul Scherrer Institute Baden, Switzerland 21st -25th September 2014
Flexible Isolators MULTIPLE CONFIGURATIONS. MODULAR DESIGN. QUICK DELIVERY.
 Flexible Isolators MULTIPLE CONFIGURATIONS. MODULAR DESIGN. QUICK DELIVERY. A TIMESAVING EDGE FOR PHARMACEUTICAL MANUFACTURERS Until now, the only way to get ILC Dover quality in a powder containment isolator
Flexible Isolators MULTIPLE CONFIGURATIONS. MODULAR DESIGN. QUICK DELIVERY. A TIMESAVING EDGE FOR PHARMACEUTICAL MANUFACTURERS Until now, the only way to get ILC Dover quality in a powder containment isolator
Discovering Dec Group an alliance of technology and expert knowledge. Powder Handling Excellence.
 Discovering Dec Group an alliance of technology and expert knowledge Powder Handling Excellence Dec Tailor Made Solutions Committed Innovative Successful Transferring Bulk Handling Micronizing Containment
Discovering Dec Group an alliance of technology and expert knowledge Powder Handling Excellence Dec Tailor Made Solutions Committed Innovative Successful Transferring Bulk Handling Micronizing Containment
Why change is inevitable in aseptic manufacturing?
 Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Sterility Test Isolators
 Latest developments Sterility Test Isolators with built-in H 2 O 2 vaporizer engineered and manufactured by Fedegari Innovation in a commodity market? When creativity is in your blood you don t settle
Latest developments Sterility Test Isolators with built-in H 2 O 2 vaporizer engineered and manufactured by Fedegari Innovation in a commodity market? When creativity is in your blood you don t settle
BQ-4 BQ-4. Introducing the Bioquell QUBE. Thinking Outside the Box. Performance - Process. Customers - Solutions. Thinking Outside the Box
 Introducing the Bioquell QUBE What is the Bioquell QUBE Modular, ISO 5, Grade A Aseptic Workstation with Integrated Rapid HPV Decontamination Technology How do the QUBE modules work QHPV Module QEXT Module
Introducing the Bioquell QUBE What is the Bioquell QUBE Modular, ISO 5, Grade A Aseptic Workstation with Integrated Rapid HPV Decontamination Technology How do the QUBE modules work QHPV Module QEXT Module
LIFE SCIENCE Isolation, Transfer, and Containment Solutions
 LIFE SCIENCE Isolation, Transfer, and Containment Solutions A Legacy of Innovative Experience In 1945, at the dawn of the Atomic Age, three scientists from the Massachusetts Institute of Technology founded
LIFE SCIENCE Isolation, Transfer, and Containment Solutions A Legacy of Innovative Experience In 1945, at the dawn of the Atomic Age, three scientists from the Massachusetts Institute of Technology founded
PDA: A Global. Association. Contamination Control: Particles, Bio-contamination, Aseptic manufacturing. PDA Parenteral 2014 Munich Conference
 PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
PDA Parenteral 2014 Munich Conference PDA: A Global Contamination Control: Particles, Bio-contamination, Bioburden Association and Endotoxins in Aseptic manufacturing. James Drinkwater F Ziel Head of Aseptic
GUIDE TO INSPECTIONS OF STERILE DRUG SUBSTANCE MANUFACTURERS
 GUIDE TO INSPECTIONS OF STERILE DRUG SUBSTANCE MANUFACTURERS Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA, and does no confer any
GUIDE TO INSPECTIONS OF STERILE DRUG SUBSTANCE MANUFACTURERS Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA, and does no confer any
MEDICINES CONTROL COUNCIL
 MEDICINES CONTROL COUNCIL PENICILLIN MANUFACTURING This document has been prepared to serve as a recommendation for penicillin manufacturing. It represents the Medicines Control Council s current thinking
MEDICINES CONTROL COUNCIL PENICILLIN MANUFACTURING This document has been prepared to serve as a recommendation for penicillin manufacturing. It represents the Medicines Control Council s current thinking
35.0 Sterile Supply Unit (SSU)
 35.0 Sterile Supply Unit (SSU) 35.1 Introduction 35.1.1 General A Hospital must provide adequate facilities for cleaning, sterilisation and storage of equipment and instruments to ensure the care and safety
35.0 Sterile Supply Unit (SSU) 35.1 Introduction 35.1.1 General A Hospital must provide adequate facilities for cleaning, sterilisation and storage of equipment and instruments to ensure the care and safety
PI s Name Date Bldg./Rm#
 PI s Name Date Bldg./Rm# Animal Biosafety Level 3 (ABSL-3) Yes No 1. Is access to the animal facility limited or restricted only to those persons authorized for program or support purposes? Yes No 2. Does
PI s Name Date Bldg./Rm# Animal Biosafety Level 3 (ABSL-3) Yes No 1. Is access to the animal facility limited or restricted only to those persons authorized for program or support purposes? Yes No 2. Does
filtration technology pharmaceutical Nutraceutical & Cosmetic industries
 filtration technology pharmaceutical Nutraceutical & Cosmetic industries cleaner air with confidence Donaldson Torit delivers the confidence you need to process powders in the pharmaceutical, nutraceutical
filtration technology pharmaceutical Nutraceutical & Cosmetic industries cleaner air with confidence Donaldson Torit delivers the confidence you need to process powders in the pharmaceutical, nutraceutical
AIR FLOW SYSTEM CATALOGUE. clean rooms
 AIR FLOW SYSTEM CATALOGUE clean rooms clean rooms clean rooms IGUÑA designs and manufactures air treatment equipment, with the highest effectiveness and efficiency, providing equipment solutions for
AIR FLOW SYSTEM CATALOGUE clean rooms clean rooms clean rooms IGUÑA designs and manufactures air treatment equipment, with the highest effectiveness and efficiency, providing equipment solutions for
Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite
 APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
Handling of high potency drugs: process and containment
 Environmental Exposure and Health 257 Handling of high potency drugs: process and containment G. Mari, A. Moccaldi & G. Ludovisi Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (Italian
Environmental Exposure and Health 257 Handling of high potency drugs: process and containment G. Mari, A. Moccaldi & G. Ludovisi Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (Italian
ISPE Aseptic Conference February 2014 Washington, D.C.
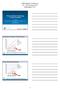 Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
John F. Koerner, MPH, CIH
 Critical Elements of Containment and Ventilation Practice for Healthcare Microbial Remediation John F. Koerner, MPH, CIH Vice President, Environmental Sciences Compliance Environmental International, Inc.
Critical Elements of Containment and Ventilation Practice for Healthcare Microbial Remediation John F. Koerner, MPH, CIH Vice President, Environmental Sciences Compliance Environmental International, Inc.
Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998)
 Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
INSPECTOR S OBSERVATIONS AND WARNING LETTER
 Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
Microrite, Inc. San Jose, CA, USA, http://www.microrite.com AIR VELOCITY MEASUREMENTS AND CORRELATION TO SMOKE STUDIES FOR ASEPTIC OPERATIONS KEYWORDS Air Velocity, Air Flows, Smoke Study, Air Pattern
Asbestos Abatement Standard Procedures Indoor Environment Group, Inc.
 The following work procedures are specified on all full containment asbestos abatement projects that we design. A. Preliminary Air Sampling: Many buildings contain background fiber levels prior to, or
The following work procedures are specified on all full containment asbestos abatement projects that we design. A. Preliminary Air Sampling: Many buildings contain background fiber levels prior to, or
As advanced therapies such as oncolytic viruses and gene
 Designing and Operating Flexible, High-Containment Vaccine Manufacturing Thomas Page A new facility type integrates next-generation mobile cleanroom systems. Thomas Page, PhD is vice-president, Engineering
Designing and Operating Flexible, High-Containment Vaccine Manufacturing Thomas Page A new facility type integrates next-generation mobile cleanroom systems. Thomas Page, PhD is vice-president, Engineering
Contract Manufacturing Services for Pre-filled Syringes
 Contract Manufacturing Services for Pre-filled Syringes From Standard Solutions to Complex Products Stefan Czvitkovich, PhD Director Product Partnering Sterile Pharmaceuticals Central Europe CPhI woldwide
Contract Manufacturing Services for Pre-filled Syringes From Standard Solutions to Complex Products Stefan Czvitkovich, PhD Director Product Partnering Sterile Pharmaceuticals Central Europe CPhI woldwide
ISOCell PRO Cell. therapy isolator
 ISOCell PRO Cell therapy isolator Everything you need for cell therapy products at your hand Producing artificial tissues and cell cultures for therapeutic purposes is a complex task, for which aseptic
ISOCell PRO Cell therapy isolator Everything you need for cell therapy products at your hand Producing artificial tissues and cell cultures for therapeutic purposes is a complex task, for which aseptic
Controlled dosimetry Fast and accurate process Versatile and easy to use Robust and reliable Meets the highest standards
 THE ART OF RADIOPHARMACEUTICALS: DEDICATED PET RADIOTRACER PREPARATION AND INJECTION Controlled dosimetry Fast and accurate process Versatile and easy to use Robust and reliable Meets the highest standards
THE ART OF RADIOPHARMACEUTICALS: DEDICATED PET RADIOTRACER PREPARATION AND INJECTION Controlled dosimetry Fast and accurate process Versatile and easy to use Robust and reliable Meets the highest standards
Aseptic Process Validation
 Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
NUCLEAR Isolation, Transfer, and Containment Solutions
 NUCLEAR Isolation, Transfer, and Containment Solutions A Legacy of Innovative Experience In 1945, at the dawn of the Atomic Age, three scientists from the Massachusetts Institute of Technology founded
NUCLEAR Isolation, Transfer, and Containment Solutions A Legacy of Innovative Experience In 1945, at the dawn of the Atomic Age, three scientists from the Massachusetts Institute of Technology founded
STANDARDS FOR COMPOUNDING PHARMACIES
 STANDARDS FOR COMPOUNDING PHARMACIES APPLICATION NOTE LC-139 (US) Introduction This publication provides excerpts from some of the many guidelines and standards that pertain to compounding pharmacies.
STANDARDS FOR COMPOUNDING PHARMACIES APPLICATION NOTE LC-139 (US) Introduction This publication provides excerpts from some of the many guidelines and standards that pertain to compounding pharmacies.
Amazon FILTRATION SOLUTIONS PHARMACEUTICAL MANUFACTURING
 Amazon FILTRATION SOLUTIONS PHARMACEUTICAL MANUFACTURING FILTRATION SOLUTIONS FOR PHARMACEUTICAL MANUFACTURING Delivering quality filtration products As one of Europe s leading manufacturers of process
Amazon FILTRATION SOLUTIONS PHARMACEUTICAL MANUFACTURING FILTRATION SOLUTIONS FOR PHARMACEUTICAL MANUFACTURING Delivering quality filtration products As one of Europe s leading manufacturers of process
O E L A B HAZ A RD NGINEERING S CONTAINMENT FLOW SCIENCES, INC.
 O E L A P O NGINEERING S A B HAZ A RD N P F CONTAINMENT P P T T DESIGN Y CONTRACT MANUFACTURING RESEARCH SOLUTIONS FLOW SCIENCES, INC. WWW.FLOWSCIENCES.COM 3 HIGH POTENCY API PREP SYSTEM 3 HPAPI GLOVEBOX
O E L A P O NGINEERING S A B HAZ A RD N P F CONTAINMENT P P T T DESIGN Y CONTRACT MANUFACTURING RESEARCH SOLUTIONS FLOW SCIENCES, INC. WWW.FLOWSCIENCES.COM 3 HIGH POTENCY API PREP SYSTEM 3 HPAPI GLOVEBOX
FILTRATION SOLUTIONS PhARmAceUTIcAL manufacturing FILTRATION SPecIALISTS
 FILTRATION SOLUTIONS Pharmaceutical Manufacturing filtration specialists Delivering quality filtration products As one of Europe s leading manufacturers of process filters, Amazon Filters is able to offer
FILTRATION SOLUTIONS Pharmaceutical Manufacturing filtration specialists Delivering quality filtration products As one of Europe s leading manufacturers of process filters, Amazon Filters is able to offer
COMPANY PROFILE. Dishman offers innovation, security of supply and value for money. Dishman Group. Research EARLY STAGE API. Preclinical.
 COMPANY PROFILE Dishman is a global outsourcing partner for the pharmaceutical industry offering a portfolio of development, scale-up and services. Dishman improves its customers businesses by providing
COMPANY PROFILE Dishman is a global outsourcing partner for the pharmaceutical industry offering a portfolio of development, scale-up and services. Dishman improves its customers businesses by providing
GUIDELINES FOR LABORATORY USE OF CHEMICAL CARCINOGENS
 GUIDELINES FOR LABORATORY USE OF CHEMICAL CARCINOGENS UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston SC 29425 843-792-3604 Revised: January 2015
GUIDELINES FOR LABORATORY USE OF CHEMICAL CARCINOGENS UNIVERSITY RISK MANAGEMENT Occupational Safety and Health Programs 19 Hagood Avenue, Suite 908 Charleston SC 29425 843-792-3604 Revised: January 2015
Biological contamination events in isolators: What lessons can be learned?
 WHITE PAPER SCIENCE DRIVEN BIO-DECONTAMINATION LS001-MKT-008 Rev 1US Biological contamination events in isolators: What lessons can be learned? James Drinkwater, Bioquell UK Process & Compliance Director.
WHITE PAPER SCIENCE DRIVEN BIO-DECONTAMINATION LS001-MKT-008 Rev 1US Biological contamination events in isolators: What lessons can be learned? James Drinkwater, Bioquell UK Process & Compliance Director.
Advancements on implementation of single use technology in vaccine manufacturing
 Advancements on implementation of single use technology in vaccine manufacturing October 25 th, 2013 DCVMN Meeting Rio de Janeiro, Brazil Outline Overview of single use products Applications of single
Advancements on implementation of single use technology in vaccine manufacturing October 25 th, 2013 DCVMN Meeting Rio de Janeiro, Brazil Outline Overview of single use products Applications of single
Commissioning the ABSL-3 Laboratory
 Commissioning the ABSL-3 Laboratory Michael C. Rosenberg P.E. Stanley Consultants Synopsis Airflow and pressurization relationships are adjusted and verified in most buildings with temperature / humidity
Commissioning the ABSL-3 Laboratory Michael C. Rosenberg P.E. Stanley Consultants Synopsis Airflow and pressurization relationships are adjusted and verified in most buildings with temperature / humidity
TEST REPORT. Purpose Personal Home Sterilizer in Each Use. January 2005
 TEST REPORT Measurement of Ozone (O 3 ) Leakage of Multi- Purpose Personal Home Sterilizer in Each Use January 2005 Air Resources Research Center Korea Institute of Science and Technology (KIST) KLENZ
TEST REPORT Measurement of Ozone (O 3 ) Leakage of Multi- Purpose Personal Home Sterilizer in Each Use January 2005 Air Resources Research Center Korea Institute of Science and Technology (KIST) KLENZ
Form 9: Infection Control Preventive Measures Level 4
 Form 9: Infection Control This form is filled out by the construction planning team or designated person(s) to identify the required preventive measures for the activity described in Form 1 Infection Control
Form 9: Infection Control This form is filled out by the construction planning team or designated person(s) to identify the required preventive measures for the activity described in Form 1 Infection Control
PHARMACY IV ADMIXTURE. Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor
 PHARMACY IV ADMIXTURE Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor Pharmacist is responsible for ensuring that compounded sterile preparations are properly prepared, labeled,
PHARMACY IV ADMIXTURE Pharmacy 483 January 17, 2006 Kim Donnelly Affiliate Associate Professor Pharmacist is responsible for ensuring that compounded sterile preparations are properly prepared, labeled,
DUQUESNE UNIVERSITY ASBESTOS OPERATIONS & MAINTENANCE PROGRAM
 DUQUESNE UNIVERSITY ASBESTOS OPERATIONS & MAINTENANCE PROGRAM Revised January 2017 Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Introduction 1 Scope 1 Policy Statement
DUQUESNE UNIVERSITY ASBESTOS OPERATIONS & MAINTENANCE PROGRAM Revised January 2017 Prepared by: Environmental Health and Safety Department TABLE OF CONTENTS Page Introduction 1 Scope 1 Policy Statement
Mobile processing systems for radioactive waste and disused sealed radioactive sources. Peter Ivanov
 Mobile processing systems for radioactive waste and disused sealed radioactive sources Peter Ivanov Radioactivity group National Physical Laboratory, UK ICTP/IAEA Workshop, 2 6 November 2015, ICTP - Trieste,
Mobile processing systems for radioactive waste and disused sealed radioactive sources Peter Ivanov Radioactivity group National Physical Laboratory, UK ICTP/IAEA Workshop, 2 6 November 2015, ICTP - Trieste,
FZ LABORATORY SERVICES
 FZ LABORATORY SERVICES For those working in biological product manufacturing/aseptic process filling: you can greatly benefit from support by Franz Ziel GmbH (FZ) with many years experience in scientific
FZ LABORATORY SERVICES For those working in biological product manufacturing/aseptic process filling: you can greatly benefit from support by Franz Ziel GmbH (FZ) with many years experience in scientific
PHARMACY IV ADMIXTURE
 PHARMACY IV ADMIXTURE Pharmacy 483 January 18, 2007 Kim Donnelly Affiliate Assistant Professor kimdon@u.washington.edu Pharmacist is responsible for ensuring that compounded sterile preparations are properly
PHARMACY IV ADMIXTURE Pharmacy 483 January 18, 2007 Kim Donnelly Affiliate Assistant Professor kimdon@u.washington.edu Pharmacist is responsible for ensuring that compounded sterile preparations are properly
GUIDELINES FOR THE PACKAGING AND STORAGE OF PESTICIDES FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS
 GUIDELINES FOR THE PACKAGING AND STORAGE OF PESTICIDES FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS - ii - GUIDELINES FOR THE PACKAGING AND STORAGE OF PESTICIDES FOOD AND AGRICULTURE ORGANIZATION
GUIDELINES FOR THE PACKAGING AND STORAGE OF PESTICIDES FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS - ii - GUIDELINES FOR THE PACKAGING AND STORAGE OF PESTICIDES FOOD AND AGRICULTURE ORGANIZATION
Isolator Technology TRUKING TECHNOLOGY LIMITED
 Isolator TRUKING TECHNOLOGY LIMITED Add No.1 xinkang Road,Yutan Town,Ningxiang,Changsha,China P.C 410600 Tel 86 731-87938222 87938255 Fax 86 731-879338201 Web www.truking.cn Any technical renewal will
Isolator TRUKING TECHNOLOGY LIMITED Add No.1 xinkang Road,Yutan Town,Ningxiang,Changsha,China P.C 410600 Tel 86 731-87938222 87938255 Fax 86 731-879338201 Web www.truking.cn Any technical renewal will
While the global pharmaceutical industry tends to be conservative
 A new line at Handai Biken exemplifies the creative use of isolator technology found throughout Japan s pharmaceutical industry. By Jim Akers, Akers Kennedy and Associates, Kazuhito Tanimoto, Shibuya Kogyo
A new line at Handai Biken exemplifies the creative use of isolator technology found throughout Japan s pharmaceutical industry. By Jim Akers, Akers Kennedy and Associates, Kazuhito Tanimoto, Shibuya Kogyo
1972: Founded to manufacture laminar flow cabinets and design and build cleanrooms
 ENVAIR - History Envair History 1972: Founded to manufacture laminar flow cabinets and design and build cleanrooms 1979: Entered the field of microbiological safety cabinets 1986: Entered the field of
ENVAIR - History Envair History 1972: Founded to manufacture laminar flow cabinets and design and build cleanrooms 1979: Entered the field of microbiological safety cabinets 1986: Entered the field of
Media Fill A Process Simution. Presented By Shikha Chauhan
 Media Fill A Process Simution Presented By Shikha Chauhan 21 CFR 211.113 Validation of Aseptic Processing and Sterilization Process Simulation / Media Fill Filtration Efficacy Sterilization of Equipment,
Media Fill A Process Simution Presented By Shikha Chauhan 21 CFR 211.113 Validation of Aseptic Processing and Sterilization Process Simulation / Media Fill Filtration Efficacy Sterilization of Equipment,
CLEANER AIR for Pharmaceutical Processing
 CLEANER AIR for Pharmaceutical Processing Donaldson Torit systems installed for more than 1,000 Pharmaceutical Applications LESS EXPOSURE with Positive, Triple Sealed BIBO Cleaner Air With Confidence Donaldson
CLEANER AIR for Pharmaceutical Processing Donaldson Torit systems installed for more than 1,000 Pharmaceutical Applications LESS EXPOSURE with Positive, Triple Sealed BIBO Cleaner Air With Confidence Donaldson
Design Considerations for TGA Licensed Pharmacies. Presented by Ashley Isbel 11 th August 2015
 Design Considerations for TGA Licensed Pharmacies Presented by Ashley Isbel 11 th August 2015 Focus of the session Primarily of relevance to sterile compounding facilities, particularly those producing
Design Considerations for TGA Licensed Pharmacies Presented by Ashley Isbel 11 th August 2015 Focus of the session Primarily of relevance to sterile compounding facilities, particularly those producing
Hospital Pharmacy Isolator Solutions for USP <797> Compliance from Esco
 Hospital Pharmacy Isolator Solutions for USP Compliance from Esco Contents 1. Introduction 2. Clean Air Devices and Isolators 3. Cytotoxic Exposure 4. Esco Pharmacy Isolator (Positive Pressure Model)
Hospital Pharmacy Isolator Solutions for USP Compliance from Esco Contents 1. Introduction 2. Clean Air Devices and Isolators 3. Cytotoxic Exposure 4. Esco Pharmacy Isolator (Positive Pressure Model)
Flexible Pharma Containment Solutions
 IPT 23 2007 30/8/07 10:11 Page 78 Ingredients, Formulations & Finishing Flexible Pharma Containment Solutions Split valve technology has been the mainstay of pharmaceutical containment for many years;
IPT 23 2007 30/8/07 10:11 Page 78 Ingredients, Formulations & Finishing Flexible Pharma Containment Solutions Split valve technology has been the mainstay of pharmaceutical containment for many years;
Formulated Chemistries for Critical Environments
 STERIS CORPORATION Formulated Chemistries for Critical Environments Superior Cleaning Products Backed by Proven Expertise Unique in critical environments......................... AND/OR REGULATORY CLAIMS
STERIS CORPORATION Formulated Chemistries for Critical Environments Superior Cleaning Products Backed by Proven Expertise Unique in critical environments......................... AND/OR REGULATORY CLAIMS
Table of Contents. Welcome to Dishman. Our Mission. Our Focus: Client Satisfaction
 Welcome to Dishman Our Mission Dishman Group continually invests in the global pharmaceutical industry, ensuring Dishman s business can provide pharmaceutical customers with high-value, high-quality products
Welcome to Dishman Our Mission Dishman Group continually invests in the global pharmaceutical industry, ensuring Dishman s business can provide pharmaceutical customers with high-value, high-quality products
Treatment of Radioactive Reactive Mixed Waste
 Treatment of Radioactive Reactive Mixed Waste S. Colby, Z. Turner, D. Utley Pacific EcoSolutions, Inc. 2025 Battelle Boulevard, Richland, Washington 99354 USA C. Duy Los Alamos National Laboratory LA-UR-05-8410
Treatment of Radioactive Reactive Mixed Waste S. Colby, Z. Turner, D. Utley Pacific EcoSolutions, Inc. 2025 Battelle Boulevard, Richland, Washington 99354 USA C. Duy Los Alamos National Laboratory LA-UR-05-8410
Webinar. The New EU-GMP Annex 1 draft Dupont. GOP-Innovations your Partner for Practical Training and e-learning. 8 June 2018
 Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
Webinar The New EU-GMP Annex 1 draft Dupont 8 June 2018 GOP-Innovations your Partner for Practical Training and e-learning Milenko Pavičić Pharmaceutical microbiologist, consultant, trainer, coach 10 years
WM2012 Conference, February 26 March 1, 2012, Phoenix, Arizona, USA
 Modular Design of Processing and Storage Facilities for Small Volumes of Low and Intermediate Level Radioactive Waste including Disused Sealed Sources - 12372 David R. Keene*, Susanta Kumar Samanta** and
Modular Design of Processing and Storage Facilities for Small Volumes of Low and Intermediate Level Radioactive Waste including Disused Sealed Sources - 12372 David R. Keene*, Susanta Kumar Samanta** and
AQUATIC HOUSING SOLUTIONS
 Because your research depends on it SUPERIOR WATER QUALITY PEACE OF MIND TIME TESTED FOR OVER 30 YEARS AQUATIC HOUSING SOLUTIONS ZEBRAFISH SYSTEMS FROM 12 TO 22,000 TANKS, WE HAVE YOUR RESEARCH NEEDS COVERED
Because your research depends on it SUPERIOR WATER QUALITY PEACE OF MIND TIME TESTED FOR OVER 30 YEARS AQUATIC HOUSING SOLUTIONS ZEBRAFISH SYSTEMS FROM 12 TO 22,000 TANKS, WE HAVE YOUR RESEARCH NEEDS COVERED
Overview of Inspection Issues with Legacy Products. Barbara Breithaupt Consumer Safety Officer
 Overview of Inspection Issues with Legacy Products Barbara Breithaupt Consumer Safety Officer 1 Overview of the presentation The role of the Quality Unit will be discussed in the context of the inspection
Overview of Inspection Issues with Legacy Products Barbara Breithaupt Consumer Safety Officer 1 Overview of the presentation The role of the Quality Unit will be discussed in the context of the inspection
Microbiological Consideration for Non-Sterile Pharmaceutical
 May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
MONAIR. Recirculating Fume Cabinet. Low cost, efficient and State-of-the-art design. Reliability and safety assured
 MONAIR Recirculating Fume Cabinet Low cost, efficient and State-of-the-art design Reliability and safety assured Fully compliant to BS 7989:2001 Why choose an ASTEC filtration fume cabinet? The principle
MONAIR Recirculating Fume Cabinet Low cost, efficient and State-of-the-art design Reliability and safety assured Fully compliant to BS 7989:2001 Why choose an ASTEC filtration fume cabinet? The principle
VHP M100 Biodecontamination Systems: Open Loop
 LOW TEMPERATURE BIODECONTAMINATION* VHP M100 Biodecontamination Systems: Open Loop The proven technology of vaporized hydrogen peroxide biodecontamination for a variety of applications Proven Technology
LOW TEMPERATURE BIODECONTAMINATION* VHP M100 Biodecontamination Systems: Open Loop The proven technology of vaporized hydrogen peroxide biodecontamination for a variety of applications Proven Technology
M A D E I N I T A L Y AIHP TECHNOLOGY DEVICES AND SERVICES FOR ENVIRONMENTAL BIODECONTAMINATION. Innovation for biodecontamination.
 M A D E I N I T A L Y AIHP TECHNOLOGY DEVICES AND SERVICES FOR ENVIRONMENTAL BIODECONTAMINATION Innovation for biodecontamination we defend quality AIHP TECHNOLOGY ACTIVATED IONIZED HYDROGEN PEROXIDE Achieve
M A D E I N I T A L Y AIHP TECHNOLOGY DEVICES AND SERVICES FOR ENVIRONMENTAL BIODECONTAMINATION Innovation for biodecontamination we defend quality AIHP TECHNOLOGY ACTIVATED IONIZED HYDROGEN PEROXIDE Achieve
MAX300-BIO PRODUCT NOTE. Bioreactor/Fermentation Control Process Efficiency Product Quality Analysis. Bioreactor/Fermentation Mass Spectrometer
 MAX300-BIO Bioreactor/Fermentation Mass Spectrometer www.extrel.com PRODUCT NOTE Bioreactor/Fermentation Control Process Efficiency Product Quality Analysis Introducing the MAX300-BIO Know Your Process
MAX300-BIO Bioreactor/Fermentation Mass Spectrometer www.extrel.com PRODUCT NOTE Bioreactor/Fermentation Control Process Efficiency Product Quality Analysis Introducing the MAX300-BIO Know Your Process
Single-Use Final Fill: Benefits and Considerations
 Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
INTEROX HYDROGEN PEROXIDE
 Product Leaflet FP 1.1.3 INTEROX HYDROGEN PEROXIDE 35% - 59.5% SOLUTIONS TECHNICAL & SPECIALTY GRADES UN 2014 H 0 0 H Molecular weight: 34.02 INTEROX Hydrogen Peroxide Description Hydrogen peroxide is
Product Leaflet FP 1.1.3 INTEROX HYDROGEN PEROXIDE 35% - 59.5% SOLUTIONS TECHNICAL & SPECIALTY GRADES UN 2014 H 0 0 H Molecular weight: 34.02 INTEROX Hydrogen Peroxide Description Hydrogen peroxide is
White Paper. Risk Management in Environmental Monitoring: Should you be spotting it sooner?
 White Paper Risk Management in Environmental Monitoring: Should you be spotting it sooner? Executive Overview Quality Risk Management is a comparatively new approach in the pharmaceutical manufacturing
White Paper Risk Management in Environmental Monitoring: Should you be spotting it sooner? Executive Overview Quality Risk Management is a comparatively new approach in the pharmaceutical manufacturing
Process Instruments (UK) Ltd.
 Process Instruments (UK) Ltd. Coagulation Control; Past, Present and Future Mike Riding Process Instruments (UK) Ltd. What We Sell Biofilm ph Conductivity Dissolved Oxygen Free & Total Chlorine CRONOS
Process Instruments (UK) Ltd. Coagulation Control; Past, Present and Future Mike Riding Process Instruments (UK) Ltd. What We Sell Biofilm ph Conductivity Dissolved Oxygen Free & Total Chlorine CRONOS
Safe handling of pharmaceutical dust
 Safe handling of pharmaceutical dust Herding Filtertechnik offers the complete filtration package Herding GmbH Filtertechnik Amberg, Germany www.herding.de Stand: 10.08.10 The Herding Pharma-concept De-dusting
Safe handling of pharmaceutical dust Herding Filtertechnik offers the complete filtration package Herding GmbH Filtertechnik Amberg, Germany www.herding.de Stand: 10.08.10 The Herding Pharma-concept De-dusting
Cross Contamination Within a Pharmaceutical Manufacturing Plant. Belinda Whalan Operations QC Laboratory Manager, Pfizer
 Cross Contamination Within a Pharmaceutical Manufacturing Plant Belinda Whalan Operations QC Laboratory Manager, Pfizer Contamination The undesired introduction of impurities of a chemical or microbiological
Cross Contamination Within a Pharmaceutical Manufacturing Plant Belinda Whalan Operations QC Laboratory Manager, Pfizer Contamination The undesired introduction of impurities of a chemical or microbiological
Product Bulletin INTEROX HYDROGEN PEROXIDE
 Product Bulletin INTEROX HYDROGEN PEROXIDE UN 2014 35% - 59.5% SOLUTIONS TECHNICAL & SPECIALTY GRADES H 0 0 H Molecular weight: 34.02 FP 1.1.3 Version 06/16 Page 1 of 5 INTEROX Hydrogen Peroxide Description
Product Bulletin INTEROX HYDROGEN PEROXIDE UN 2014 35% - 59.5% SOLUTIONS TECHNICAL & SPECIALTY GRADES H 0 0 H Molecular weight: 34.02 FP 1.1.3 Version 06/16 Page 1 of 5 INTEROX Hydrogen Peroxide Description
Evolutionary Efficiency. Revolutionary Capacity.
 Central Sterile Services Evolutionary Efficiency. Revolutionary Capacity. The AMSCO Evolution Floorloader Steam Sterilizer One Integrated Approach to Healthcare The Amsco Evolution Floorloader Steam Sterilizer
Central Sterile Services Evolutionary Efficiency. Revolutionary Capacity. The AMSCO Evolution Floorloader Steam Sterilizer One Integrated Approach to Healthcare The Amsco Evolution Floorloader Steam Sterilizer
Blowing in Faster. Protection, safety, reliability. And more.
 Blowing in Faster. Protection, safety, reliability. And more. LAMINAR FLOW CABINETS AND SYSTEMS LAMINAR FLOW CABINETS AND SYSTEMS FASTER S.R.L., BASED IN ITALY SINCE 1984, HAVE BECOME IN 25 YEARS ONE OF
Blowing in Faster. Protection, safety, reliability. And more. LAMINAR FLOW CABINETS AND SYSTEMS LAMINAR FLOW CABINETS AND SYSTEMS FASTER S.R.L., BASED IN ITALY SINCE 1984, HAVE BECOME IN 25 YEARS ONE OF
Guidelines for QualiTru Line Sampling
 Guidelines for QualiTru Line Sampling QUALITRU GUIDELINES FOR LINE SAMPLING 1. Purpose: Following these Standard Operating Procedures, farms and processing plants may use the QualiTru aseptic sampling
Guidelines for QualiTru Line Sampling QUALITRU GUIDELINES FOR LINE SAMPLING 1. Purpose: Following these Standard Operating Procedures, farms and processing plants may use the QualiTru aseptic sampling
Section Asbestos Containment Procedures
 Section Cover Page Section 02 82 05 2017-10-01 Use this Section where full containment will be required, or if a Contractor option, may be required, depending on the removal method(s) selected by the Contractor.
Section Cover Page Section 02 82 05 2017-10-01 Use this Section where full containment will be required, or if a Contractor option, may be required, depending on the removal method(s) selected by the Contractor.
Improved conventional practices of the pharma industry serving Cost of Goods of Autologous Cell Therapy
 Tony Donolato Improved conventional practices of the pharma industry serving Cost of Goods of Autologous Cell Therapy Case Study: an Autonomous Tailor Made Isolator salamanderu: what we do? Regenerate
Tony Donolato Improved conventional practices of the pharma industry serving Cost of Goods of Autologous Cell Therapy Case Study: an Autonomous Tailor Made Isolator salamanderu: what we do? Regenerate
Asbestos in the Workplace: A Guide to Removal of Friable Asbestos Containing Materials
 Asbestos in the Workplace: A Guide to Removal of Friable Asbestos Containing Materials Revised November 21, 2013 Application Code of Practice Where asbestos is present or believed to be present in a workplace
Asbestos in the Workplace: A Guide to Removal of Friable Asbestos Containing Materials Revised November 21, 2013 Application Code of Practice Where asbestos is present or believed to be present in a workplace
NEW TECHNOLOGIES FOR SAFE AND CONTAINED POWDER HANDLING IN THE BULK PHARMACEUTICAL INDUSTRY
 NEW TECHNOLOGIES FOR SAFE AND CONTAINED POWDER HANDLING IN THE BULK PHARMACEUTICAL INDUSTRY Solutions to complex powder handling issues when designing a new factory for bulk pharmaceuticals by Frédéric
NEW TECHNOLOGIES FOR SAFE AND CONTAINED POWDER HANDLING IN THE BULK PHARMACEUTICAL INDUSTRY Solutions to complex powder handling issues when designing a new factory for bulk pharmaceuticals by Frédéric
USP <800> A practical approach to compliance. Ryan W. Naseman, PharmD, MS, BCPS Michael Storey, PharmD, MS, BCPS
 USP A practical approach to compliance Ryan W. Naseman, PharmD, MS, BCPS Michael Storey, PharmD, MS, BCPS Learning Objectives Explain the need for USP from an employee safety standpoint Describe
USP A practical approach to compliance Ryan W. Naseman, PharmD, MS, BCPS Michael Storey, PharmD, MS, BCPS Learning Objectives Explain the need for USP from an employee safety standpoint Describe
