What to do with extra electrons how combating eutrophication may affect mineralization pathways
|
|
|
- Ella Lawrence
- 6 years ago
- Views:
Transcription
1 What to do with extra electrons how combating eutrophication may affect mineralization pathways Jouni Lehtoranta Finnish Environment Institute Marine Research Centre
2 Sun e - Energy goes through the system (open system) Producers Consumers Nutrients cycle in the system (closed system) Life is based on electron transfer i.e. redox - reactions Inorganic nutrient pool Decomposers Life on Earth just slows down the flow of solar energy Nasa
3 Oxygen cycle Transfer of electrons from water to organic carbon Oxidation i.e. transfer of electrons from organic carbon to????? CO 2 + H 2 O [CH 2 O] + O 2 [CH 2 O] + O 2 CO 2 + H 2 O Wikipedia
4 Pathways of organic matter oxidation i.e. transfer of electrons Reduction reaction Formula Depth in sediment oxic Aerobic respiration CH 2 O + O 2 CO 2 +H 2 O mm anoxic Denitrification 5CH 2 O + 4NO H + 5CO 2 + 2N 2 + 7H 2 O mm anoxic Manganese reduction CH 2 O + MnO 2 + 4H + CO 2 + 2Mn H 2 O cm anoxic Iron reduction CH 2 O + 4FeOOH + 8H + CO 2 + 4Fe H 2 O cm anoxic Sulfate reduction 2CH 2 O + SO H + 2CO 2 + H 2 S + 2H 2 O m anoxic Methanogenesis CH 3 COO - + Η + CH 4 + CO 2 HCO H 2 + H + CH 4 + 3H 2 O Anaerobic mineralization causes indirectly O 2 consumption (reoxidation of NH 4, Mn(II), Fe(II), H 2 S, CH 4 ) O 2 is the ultimate acceptor of electrons released during mineralization In long run oxygen production = oxygen consumption (1:1) m
5 Pathways of organic matter oxidation i.e. transfer of electrons Reduction reaction Formula Depth in sediment O 2 concentration in oxic Aerobic respiration atmosphere CH 2 O + is O21% 2 CO 2 +H 2 O mm and it is stable (annual anoxic - Denitrification fluctuations 5CH 2 O are + 4NO ±0.002%) 3 + 4H + 5CO 2 + 2N 2 + 7H 2 O mm anoxic Manganese reduction CH 2 O + MnO 2 + 4H + CO 2 + 2Mn H 2 O cm anoxic Iron reduction CH 2 O + 4FeOOH + 8H + CO 2 + 4Fe H 2 O cm 2- anoxic Sulfate reduction 2CH 2 O + SO 4 + 2H + 2CO 2 + H 2 S + 2H 2 O m anoxic Methanogenesis CH 3 COO - + Η + CH 4 + CO 2 m There HCO has to 4Hbe 2 + H + CH 4 + 3H 2 O a global balance between Anaerobic mineralization primary production causes vs. indirectly O 2 consumption (reoxidation of mineralization NH 4, Mn(II), and Fe(II), burial H 2 S, CH 4 ) O 2 is the ultimate acceptor of electrons released during mineralization In long run oxygen production = oxygen consumption (1:1) Not true in geological timescales: Long term burial of carbon and formation of FeS 2 (iron oxidizes sulphur) we have free O 2 in atmosphere
6 Is marine sediment a good sink for organic matter? Organic matter is continuously degraded in sediments and carbon fixed by phytoplankton in oceans is almost completely (99.9%) oxidized back to CO 2 (Middelburg & Meusmann, Science 316: ; Hedges & Keil 1995: Mar. Chem. 49: 81-86) Eventual preservation of organic matter is closely coupled to Flux of organic carbon to sediments (compared to amount and quality of electron acceptors) Oxygen exposure time before burial To surface area of clay particles in sediments (Rothman & Forney 2007: Science 316: )
7 What kind of bottom sediment we would like to have when combating eutrophication? What to do with extra electrons (organic matter) and which electron acceptors we prefer in mineralization
8 Aquatic Ecosystems and World Wide Eutrophication problem glossary/o_r/rain_splash.html Seija Hällfors Photo library on Soil erosion processes
9 Aquatic Ecosystems and World Wide Eutrophication problem glossary/o_r/rain_splash.html Seija Hällfors Photo library on Soil erosion processes
10 Aquatic Ecosystems and World Wide Eutrophication problem glossary/o_r/rain_splash.html Seija Hällfors Photo library on Soil erosion processes
11 Pathways of organic matter oxidation Reduction reaction Formula Depth in sediment oxic Aerobic respiration CH 2 O + O 2 CO 2 +H 2 O mm anoxic Denitrification - 5CH 2 O + 4NO 3 + 4H + 5CO 2 + 2N 2 + 7H 2 O mm anoxic Manganese reduction CH 2 O + MnO 2 + 4H + CO 2 + 2Mn H 2 O cm anoxic Iron reduction CH 2 O + 4FeOOH + 8H + CO 2 + 4Fe H 2 O cm anoxic Sulfate reduction 2CH 2 O + SO H + 2CO 2 + H 2 S + 2H 2 O m anoxic Methanogenesis CH 3 COO - + Η + CH 4 + CO 2 HCO H 2 + H + CH 4 + 3H 2 O m Mineralisation leads to formation of chemical energy in dissolved and gaseous form (NH 4, Mn(II), Fe(II), H 2 S, CH 4 ) Any favourites?
12 Utilization of chemical energy (chemolithoautotrophs) NH 4 (O 2, NO 2-, Mn) Fe 2+ (O 2, NO 3-, Mn) S, HS -, H 2 S, FeS (O 2, NO 3-, Mn, Fe) CH 4 (O 2, SO 4, Fe???NO 3???) Recent great discoveries in microbial processes Anaerobic oxidation of methane by sulphate (AOM, Hoehler et al. 1994, Hindrichs et al. 1999) Anaerobic oxidation of ammonium (ANAMMOX, van de Graaf et al. 1995, Jetten et al. 1999) Microbiologists are searching for microbes capable to oxidize methane with nitrate and Fe(III) oxides
13 Electron transfer and combating eutrophication - any links to cycling of iron bound phosphorus?
14 Pathways of organic matter oxidation Reduction reaction Formula Depth in sediment oxic Aerobic respiration CH 2 O + O 2 CO 2 +H 2 O mm anoxic Denitrification - 5CH 2 O + 4NO 3 + 4H + 5CO 2 + 2N 2 + 7H 2 O mm anoxic Manganese reduction CH 2 O + MnO 2 + 4H + CO 2 + 2Mn H 2 O cm anoxic Iron reduction CH 2 O + 4FeOOH + 8H + CO 2 + 4Fe H 2 O cm anoxic Sulfate reduction 2CH 2 O + SO H + 2CO 2 + H 2 S + 2H 2 O m anoxic Methanogenesis CH 3 COO - + Η + CH 4 + CO 2 HCO H 2 + H + CH 4 + 3H 2 O m
15 Difference between microbial and chemical Fe reduction Microbial Fe(III) oxide reduction DissFe 2+ cells DissP Chemical Fe(III) oxide reduction Geobacter metallireducens DissP FeS/FeS 2 2 FeOOH + 3 H 2 S + 4 H + 2 FeS + S H 2 O, with possibly a further reaction to pyrite (Berner 1970): FeS + S 0 FeS 2
16 Fe and P cycling Oligotrophic marine system Microbial reduction dominates Fe reduction Eutrophic marine system Chemical reduction dominates Fe reduction Water O 2 Fe(III)oxides Fe(III) bound P org-p Fe(III) Oxic sediment binds PO Anoxic sediment Burial of Fe(III) bound P Fe(III)oxides Fe(III) bound P org P 3-4 O 2 Efficient Fe and P cycling Fe(III) reduction Fe(III) reduction by H S 2 Fe:P>2 Fe(II) Solid FeS FeS 2 State 1 Low efflux of P PO 4 3- Water O 2 Anoxic sedim Fe(III)oxides Fe(III) bound P org P Fe(III) binds PO 3- partly 4 Fe(III)oxides Fe(III) bound P org P Little Fe(III) bound P buried O Inefficient Fe and P cycling Fe(III) reduction by H S 2 2 Fe(III) reduction High efflux of P Fe:P<2 Fe(II) Solid FeS 2 FeS State 2 PO 4 3- A B
17 Fe and P cycling Oligotrophic marine system Microbial reduction dominates Fe reduction Eutrophic marine system Chemical reduction dominates Fe reduction Water O 2 Fe(III)oxides Fe(III) bound P org-p Fe(III) Oxic sediment binds PO Anoxic sediment Burial of Fe(III) bound P Fe(III)oxides Fe(III) bound P org P 3-4 O 2 Efficient Fe and P cycling Fe(III) reduction Fe(III) reduction by H S 2 Fe:P>2 Fe(II) Solid FeS FeS 2 State 1 Low efflux of P PO 4 3- Water O 2 Anoxic sedim Fe(III)oxides Fe(III) bound P org P Fe(III) binds PO 3- partly 4 Fe(III)oxides Fe(III) bound P org P Little Fe(III) bound P buried O Inefficient Fe and P cycling Fe(III) reduction by H S 2 2 Fe(III) reduction High efflux of P Fe:P<2 Fe(II) Solid FeS 2 FeS State 2 PO 4 3- A B
18
19 Regional pattern in Fe to P ratio can be explained with microbial processes Intensive monitoring of water quality Monitoring of harmful substances in tissues of fish and evertebrates Zoobenthos monitoring Microbial iron reduction dominates 1000 State 1 Fe to P ratio 2:1 moles State State N km Tot-Fe to DIP ratio (mol:mol) ,1 0,01 State Station Microbial sulphate reduction dominates Lehtoranta, Ekholm & Pitkänen (J. Mar. Syst 2008)
20 In freshwater systems Oxygen exposure time before burial is short for org. C What is the importance of anaerobic pathways in mineralization? Flux of organic carbon compared to amount of electron acceptors in specific system can be high: inhibition of coupled nitrification/denitrification? large sulphide formation, blocking of Fe cycle resulting in high release of P? effective methane production?, efficient C burial? How we affect the flux of electron acceptors when combating eutrophication?
21 Amendment of electron acceptor Positive Negative Nitrate Inhibition of Fe, SO 4 reduction and CH 4 formation Acts as nutrient Iron oxides Coupled Fe and P cycling, inhibition of SO 4 reduction and CH 4 formation Increase of Fe causes problems for water purification Sulphate Inhibition of methane formation and oxidation of methane Formation of toxic H 2 S and blocking of Fe cycling
22 Summary Micro-organisms are main drivers in biogeochemical cycles and they control global redox state Consequences of amendments have to be understood in a wide perspective: they may have an effect on fundamental biogeochemical cycles in recipient aquatic systems (i.e. cycling of C, Mn, Fe, S, N, P)
23 Photo Petri Ekholm
24 Artificial oxygenation (PROPPEN-project) Delivery of oxygen to near-bottom water in order to trigger preferable mineralization pathway (Pitkänen, Lehtoranta) Shift in microbial processes State indicator 2 Anoxic sediment surface SO reduction dominates 4 Thresholds B 1 Oxic sediment surface Microbial Fe reduction dominates Flux of labile organic C
25 MYTVASII Coastal sediment manipulation with organic matter/electron acceptors to control the anaerobic mineralization processes (MYTVASII, Ekholm) Fresh sample Anoxia, No carbon addition Anoxia, Carbon addition 300 Cores open Cores closed Fe:P < 2 (C+) Cores open 0.02 Concentration (µmol l -1 ) Fe(II) C+ Fe(II) C- DP C+ DP (C-) Fe:P < 2 (C-) Days 1.6 2B Lehtoranta, Ekholm and Pitkänen. Thresholds in coastal regions a matter of sediment microbial processes, AMBIO 2009
26 Application Up-stream thinking: Soil erosion from a point of view of processes in marine sediment (MYTVAS, Ekholm, Lehtoranta) Day 16: Clay+ labile C + sulphate + Baltic sediment microbes Day 23: Clay+ labile C + sulphate + Baltic sediment microbes Photo: Pasi Valkama
27 Wauer et al Wat. Res.39:
28
If you have an extra electron where do you put it?
 If you have an extra electron where do you put it? Jouni Lehtoranta Finnish Environment Institute Marine Research Center Systems Ecological Perspectives on Sustainability Conference 25.09.2014 Spatial
If you have an extra electron where do you put it? Jouni Lehtoranta Finnish Environment Institute Marine Research Center Systems Ecological Perspectives on Sustainability Conference 25.09.2014 Spatial
Regional variation in N vs. P limitation in the Baltic Sea the role of sediment mineralisation processes
 Regional variation in N vs. P limitation in the Baltic Sea the role of sediment mineralisation processes Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) COST869 Mitigation options
Regional variation in N vs. P limitation in the Baltic Sea the role of sediment mineralisation processes Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) COST869 Mitigation options
Should we focus only on P load when aiming to reduce eutrophication in a P-limited aquatic system?
 Should we focus only on P load when aiming to reduce eutrophication in a P-limited aquatic system? Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) International Phosphorus Workshop
Should we focus only on P load when aiming to reduce eutrophication in a P-limited aquatic system? Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) International Phosphorus Workshop
CHEMICAL COMPOSITION OF NATURAL WATERS
 CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
Why Water Quality? FOR IMMEDIATE RELEASE March 26, 2013
 Nutrients Nutrients Microbes Rulers of the World Microbes and Redox Potential Dissolved Oxygen Nitrogen Cycle From Waste to Gas Phosphorus Cycle or Recycle Algae The Miracle of Life Stormwater - Loadings
Nutrients Nutrients Microbes Rulers of the World Microbes and Redox Potential Dissolved Oxygen Nitrogen Cycle From Waste to Gas Phosphorus Cycle or Recycle Algae The Miracle of Life Stormwater - Loadings
CO 2 (g) + H 2 O = H 2 CO 3 log K H = HCO 3 log K 1 = HCO - 3 = H CO 3 log K 2 = -9.0
 Ocean 400 Chemical Oceanography Winter 2006 Your Name Final Exam Read all questions carefully before you begin to answer. Use the back of the pages if necessary. Points are assigned to each question in
Ocean 400 Chemical Oceanography Winter 2006 Your Name Final Exam Read all questions carefully before you begin to answer. Use the back of the pages if necessary. Points are assigned to each question in
Appendix F. SMAST Report Sediment Nutrient Flux and Oxygen Demand
 Appendix F SMAST Report Sediment Nutrient Flux and Oxygen Demand Technical Report Merrimack River Sediment Nutrient Regeneration 2009 Sampling Season By: Dr. David R. Schlezinger Dr. Brian L. Howes Roland
Appendix F SMAST Report Sediment Nutrient Flux and Oxygen Demand Technical Report Merrimack River Sediment Nutrient Regeneration 2009 Sampling Season By: Dr. David R. Schlezinger Dr. Brian L. Howes Roland
Biogeochemistry of Wetlands
 Institute of Food and Agricultural Sciences (IFAS) Biogeochemistry of Wetlands Si Science and da Applications Biogeochemical Properties of Wetlands Wetland Biogeochemistry Laboratory Soil and Water Science
Institute of Food and Agricultural Sciences (IFAS) Biogeochemistry of Wetlands Si Science and da Applications Biogeochemical Properties of Wetlands Wetland Biogeochemistry Laboratory Soil and Water Science
Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical, & biological processes
 OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
Geochemical Nutrient Scavenging. High mountain lakes as model systems. Research Questions
 Research Questions Geochemical Nutrient Scavenging High mountain lakes as model systems Kurt Hanselmann, Microbiology: Ecology: Biogeochemistry: Geobiology: How do microorganisms cope with low nutrient
Research Questions Geochemical Nutrient Scavenging High mountain lakes as model systems Kurt Hanselmann, Microbiology: Ecology: Biogeochemistry: Geobiology: How do microorganisms cope with low nutrient
Nitrogen Cycling in the Sea
 Nitrogen Cycling in the Sea Matt Church (MSB 612 / 9568779/ mjchurch@hawaii.edu) Marine Microplankton Ecology / OCN 626 NH 4 N0 2 N0 2 NH 4 Outline Nitrogen species in marine watersdistributions and concentrations
Nitrogen Cycling in the Sea Matt Church (MSB 612 / 9568779/ mjchurch@hawaii.edu) Marine Microplankton Ecology / OCN 626 NH 4 N0 2 N0 2 NH 4 Outline Nitrogen species in marine watersdistributions and concentrations
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 18 September 2012 Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 18 September 2012 Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions
Nitrogen Cycling in the Sea
 Nitrogen Cycling in the Sea NH 4 + N0 2 N0 2 NH 4 + Outline Nitrogen species in marine watersdistributions and concentrations New, regenerated, and export production The processes: Assimilation, N 2 fixation,
Nitrogen Cycling in the Sea NH 4 + N0 2 N0 2 NH 4 + Outline Nitrogen species in marine watersdistributions and concentrations New, regenerated, and export production The processes: Assimilation, N 2 fixation,
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Nutrient Cycling & Soils
 Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Microorganisms & Biogeochemical Cycles
 Microorganisms & Biogeochemical Cycles Biogeochemical Cycles: Movement & transformation of chemical elements (nutrien) in the environment caused by biological and chemical processes in the atmosphere,
Microorganisms & Biogeochemical Cycles Biogeochemical Cycles: Movement & transformation of chemical elements (nutrien) in the environment caused by biological and chemical processes in the atmosphere,
7.014 Lecture 20: Biogeochemical Cycles April 1, 2007
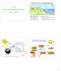 Global Nutrient Cycling - Biogeochemical Cycles 7.14 Lecture 2: Biogeochemical Cycles April 1, 27 Uptake Bioelements in Solution Weathering Precipitation Terrestrial Biomass Decomposition Volatile Elements
Global Nutrient Cycling - Biogeochemical Cycles 7.14 Lecture 2: Biogeochemical Cycles April 1, 27 Uptake Bioelements in Solution Weathering Precipitation Terrestrial Biomass Decomposition Volatile Elements
Lesson Overview. Cycles of Matter. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview 3.4 THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them up, so where do essential
Lesson Overview 3.4 THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them up, so where do essential
Sediment Diagenesis. 1/61
 Sediment Diagenesis http://eps.mcgill.ca/~courses/c542/ 1/61 Sequence of catabolic reactions In the presence of free O 2 : G (kj/mol) (1) Aerobic respiration: (CH 2 O) 106 (NH 3 ) 16 H 3 PO 4 + 138O 2
Sediment Diagenesis http://eps.mcgill.ca/~courses/c542/ 1/61 Sequence of catabolic reactions In the presence of free O 2 : G (kj/mol) (1) Aerobic respiration: (CH 2 O) 106 (NH 3 ) 16 H 3 PO 4 + 138O 2
Cycles of Ma,er. Lesson Overview. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
Lesson Overview Cycles of Ma,er Lesson Overview 3.4 Cycles of Matter THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these
Determining the f ratio 11/16/2010. Incubate seawater in the presence of trace 15
 Plankton production is supported by 2 types of nitrogen: 1) new production supported by external sources of N (e.g. NO 3 and N 2 ), 2) recycled or regenerated production, sustained by recycling of N. Assumptions:
Plankton production is supported by 2 types of nitrogen: 1) new production supported by external sources of N (e.g. NO 3 and N 2 ), 2) recycled or regenerated production, sustained by recycling of N. Assumptions:
3 3 Cycles of Matter
 3 3 Cycles of Matter Recycling in the Biosphere Energy - one way flow matter - recycled within and between ecosystems. biogeochemical cycles matter Elements, chemical compounds, and other forms passed
3 3 Cycles of Matter Recycling in the Biosphere Energy - one way flow matter - recycled within and between ecosystems. biogeochemical cycles matter Elements, chemical compounds, and other forms passed
Estuarine and Coastal Biogeochemistry
 Estuarine and Coastal Biogeochemistry OCN 623 Chemical Oceanography 9 April 2013 Reading: Seitzinger & Mayorga (2008) 2013 Frank Sansone 1. Global coastal zone Outline 2. Nutrient loading in estuaries
Estuarine and Coastal Biogeochemistry OCN 623 Chemical Oceanography 9 April 2013 Reading: Seitzinger & Mayorga (2008) 2013 Frank Sansone 1. Global coastal zone Outline 2. Nutrient loading in estuaries
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 23 September 2014 Reading: Schlesinger & Bernhardt, Chapter 6 2014 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 23 September 2014 Reading: Schlesinger & Bernhardt, Chapter 6 2014 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Nitrogen cycle Important steps
 Nitrogen cycle Nitrogen cycle Important steps Stage1 Entry and Accumulation Ammonia is introduced into the water via tropical fish waste, uneaten food, and decomposition. These will break down into ammonia
Nitrogen cycle Nitrogen cycle Important steps Stage1 Entry and Accumulation Ammonia is introduced into the water via tropical fish waste, uneaten food, and decomposition. These will break down into ammonia
Nutrient Cycles. Nutrient cycles involve flow of high quality energy from the sun through the environment & of elements.
 Nutrient Cycles Nutrient cycles (= biogeochemical cycles): natural processes that involve the flow of nutrients from the environment (air, water, soil, rock) to living organisms ( ) & back again. Nutrient
Nutrient Cycles Nutrient cycles (= biogeochemical cycles): natural processes that involve the flow of nutrients from the environment (air, water, soil, rock) to living organisms ( ) & back again. Nutrient
Biogeochemical cycles
 Biogeochemical cycles Microbial Ecology SS2010 www.icbm.de/pmbio Biogeochemistry The study of the exchange of material between the living and nonliving components of the biosphere. The biogeochemical cycling
Biogeochemical cycles Microbial Ecology SS2010 www.icbm.de/pmbio Biogeochemistry The study of the exchange of material between the living and nonliving components of the biosphere. The biogeochemical cycling
Lesson Overview. Cycles of Matter. Lesson Overview. 3.4 Cycles of Matter
 Lesson Overview 3.4 THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them up, so..where do essential
Lesson Overview 3.4 THINK ABOUT IT A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them up, so..where do essential
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 20 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2016 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 20 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2016 Frank Sansone 1. Nitrogen cycle Soil nitrogen
UNIT 1 SUSTAINING ECOSYSTEMS
 UNIT 1 SUSTAINING ECOSYSTEMS Chapter 2 Biogeochemical Cycles Science 10 Change & Recovery in Ecosystems (you do not need to copy) What happens to the materials that make up a truck when it begins to rust?
UNIT 1 SUSTAINING ECOSYSTEMS Chapter 2 Biogeochemical Cycles Science 10 Change & Recovery in Ecosystems (you do not need to copy) What happens to the materials that make up a truck when it begins to rust?
NUTRIENT CYCLES REVIEW
 52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
Chemistry that determines ph in Swedish waters Leif G. Anderson Department of Marine Sciences University of Gothenburg Sweden
 Chemistry that determines ph in Swedish waters Leif G. Anderson Department of Marine Sciences University of Gothenburg Sweden CO 2 The ocean takes up CO 2 when the surface water partial pressure (pco 2
Chemistry that determines ph in Swedish waters Leif G. Anderson Department of Marine Sciences University of Gothenburg Sweden CO 2 The ocean takes up CO 2 when the surface water partial pressure (pco 2
Ecosystems and Nutrient Cycles Chapters 3
 Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
Ecosystems. Trophic relationships determine the routes of energy flow and chemical cycling in ecosystems.
 AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 19 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2017 Frank Sansone Outline 1. Nitrogen cycle
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 19 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2017 Frank Sansone Outline 1. Nitrogen cycle
3 3 Cycles of Matter Slide 1 of 33
 1 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within and between ecosystems.
1 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within and between ecosystems.
Lecture 21: Soil Redox; Hydric Soils; Wetlands
 Lecture 21: Soil Redox; Hydric Soils; Wetlands Soil Oxidation-Reduction (Redox) Many Elements Exist in Multiple Oxidation States in Soils Oxygen: O 2 (0), H 2 O (-2) Carbon: CH 4 (-4), Organic C (-3 to
Lecture 21: Soil Redox; Hydric Soils; Wetlands Soil Oxidation-Reduction (Redox) Many Elements Exist in Multiple Oxidation States in Soils Oxygen: O 2 (0), H 2 O (-2) Carbon: CH 4 (-4), Organic C (-3 to
Lakes, Primary Production, Budgets and Cycling
 OCN 401-Biogeochemical Systems Lecture #10 (9.22.11) Lakes, Primary Production, Budgets and Cycling (Schlesinger: Chapter 7) 1. Primary Production and Nutrient Cycling in Lakes Physical aspects and nomenclature
OCN 401-Biogeochemical Systems Lecture #10 (9.22.11) Lakes, Primary Production, Budgets and Cycling (Schlesinger: Chapter 7) 1. Primary Production and Nutrient Cycling in Lakes Physical aspects and nomenclature
10/18/2010 THINK ABOUT IT CHAPTER 3 THE BIOSHPERE RECYCLING IN THE BIOSPHERE RECYCLING IN THE BIOSPHERE
 THINK ABOUT IT CHAPTER 3 THE BIOSHPERE 3.4 Mrs. Michaelsen A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them
THINK ABOUT IT CHAPTER 3 THE BIOSHPERE 3.4 Mrs. Michaelsen A handful of elements combine to form the building blocks of all known organisms. Organisms cannot manufacture these elements and do not use them
Dissociation of Orthophosphoric Acid
 { Phosphorus General Essential for all living things Component of DNA, RNA, ADP, ATP, and bone Usually the most limiting nutrient in phytoplankton productivity Many forms Soluble inorganic, soluble organic,
{ Phosphorus General Essential for all living things Component of DNA, RNA, ADP, ATP, and bone Usually the most limiting nutrient in phytoplankton productivity Many forms Soluble inorganic, soluble organic,
BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through living organisms and the physical environment.
 BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through living organisms and the physical environment. BIOCHEMIST: Scientists who study how LIFE WORKS at a CHEMICAL level. The work of biochemists has
BIOGEOCHEMICAL CYCLES: The RECYCLING of MATERIALS through living organisms and the physical environment. BIOCHEMIST: Scientists who study how LIFE WORKS at a CHEMICAL level. The work of biochemists has
Another cause of diversity may be the creation of different habitats within a region by periodic disturbance A community that forms if the land is
 Another cause of diversity may be the creation of different habitats within a region by periodic disturbance A community that forms if the land is undisturbed and that perpetuates itself for as long as
Another cause of diversity may be the creation of different habitats within a region by periodic disturbance A community that forms if the land is undisturbed and that perpetuates itself for as long as
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BIOGEOCHEMICAL CYCLES A biogeochemical cycle or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth.
BIOGEOCHEMICAL CYCLES BIOGEOCHEMICAL CYCLES A biogeochemical cycle or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth.
WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein!
 Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
Nitrogen Cycle 2.2 WHY DO WE NEED NITROGEN?? Nitrogen is needed to make up DNA and protein! In animals, proteins are vital for muscle function. In plants, nitrogen is important for growth. NITROGEN Nitrogen
The Phosphorus Cycle
 Lecture Outline Introduction Global Cycle Overview Major Forms Anthropogenic Influences Terrestrial Processes Freshwater Processes Ocean Processes The Phosphorus Cycle No important gaseous form, present
Lecture Outline Introduction Global Cycle Overview Major Forms Anthropogenic Influences Terrestrial Processes Freshwater Processes Ocean Processes The Phosphorus Cycle No important gaseous form, present
Chapter Two: Cycles of Matter (pages 32-65)
 Chapter Two: Cycles of Matter (pages 32-65) 2.2 Biogeochemical Cycles (pages 42 52) In order to survive and grow, organisms must obtain nutrients that serve as sources of energy or chemical building blocks,
Chapter Two: Cycles of Matter (pages 32-65) 2.2 Biogeochemical Cycles (pages 42 52) In order to survive and grow, organisms must obtain nutrients that serve as sources of energy or chemical building blocks,
Part 3. Oceanic Carbon and Nutrient Cycling
 OCN 401 Biogeochemical Systems (11.03.11) (Schlesinger: Chapter 9) Part 3. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Models of Carbon in the Ocean 2. Nutrient Cycling in the Ocean Atmospheric-Ocean
OCN 401 Biogeochemical Systems (11.03.11) (Schlesinger: Chapter 9) Part 3. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Models of Carbon in the Ocean 2. Nutrient Cycling in the Ocean Atmospheric-Ocean
10/17/ Cycles of Matter. Recycling in the Biosphere. How does matter move among the living and nonliving parts of an ecosystem?
 2 of 33 3-3 Cycles of Matter How does matter move among the living and nonliving parts of an ecosystem? 3 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the
2 of 33 3-3 Cycles of Matter How does matter move among the living and nonliving parts of an ecosystem? 3 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the
The Biosphere Chapter 3. What Is Ecology? Section 3-1
 The Biosphere Chapter 3 What Is Ecology? Section 3-1 Interactions and Interdependence Ecology is the scientific study of interactions among organisms and between organisms and their environment, or surroundings.
The Biosphere Chapter 3 What Is Ecology? Section 3-1 Interactions and Interdependence Ecology is the scientific study of interactions among organisms and between organisms and their environment, or surroundings.
Nitrogen & Bacteria. A biological journey through the environment
 Nitrogen & Bacteria A biological journey through the environment Sources of Nitrogen to the Environment Agricultural Natural Industrial Transportation Nitrogen as a pollutant Too much Nitrogen can cause
Nitrogen & Bacteria A biological journey through the environment Sources of Nitrogen to the Environment Agricultural Natural Industrial Transportation Nitrogen as a pollutant Too much Nitrogen can cause
Cycles of Matter. Slide 1 of 33. End Show. Copyright Pearson Prentice Hall
 Cycles of Matter 1 of 33 The purpose of this lesson is to learn the water, carbon, nitrogen, and phosphorus cycles. This PowerPoint will provide most of the required information you need to accomplish
Cycles of Matter 1 of 33 The purpose of this lesson is to learn the water, carbon, nitrogen, and phosphorus cycles. This PowerPoint will provide most of the required information you need to accomplish
A Robust Model for Biotic and Abiotic Degradation of Chlorinated Aliphatic Hydrocarbons in an In Situ Bioreactor
 A Robust Model for Biotic and Abiotic Degradation of Chlorinated Aliphatic Hydrocarbons in an In Situ Bioreactor Robert Edwards- Noblis Gabriel Moreno-Ferguson Camp Stanley Storage Activity Chris Beal
A Robust Model for Biotic and Abiotic Degradation of Chlorinated Aliphatic Hydrocarbons in an In Situ Bioreactor Robert Edwards- Noblis Gabriel Moreno-Ferguson Camp Stanley Storage Activity Chris Beal
Reducing agricultural phosphorus load by gypsum: results from the first year after amendment
 Reducing agricultural phosphorus load by gypsum: results from the first year after amendment Photo: Janne Artell Petri Ekholm Finnish Environment Institute SYKE Contents What is gypsum and how it works
Reducing agricultural phosphorus load by gypsum: results from the first year after amendment Photo: Janne Artell Petri Ekholm Finnish Environment Institute SYKE Contents What is gypsum and how it works
What s Happening in the Mud at the Bottom of the Bay?
 What s Happening in the Mud at the Bottom of the Bay? Sarah Q. Foster PhD Candidate Earth and Environment Department, Boston University Research at the Reserve Coffee House Series / WBNERR, East Falmouth,
What s Happening in the Mud at the Bottom of the Bay? Sarah Q. Foster PhD Candidate Earth and Environment Department, Boston University Research at the Reserve Coffee House Series / WBNERR, East Falmouth,
Metabolism Lectures. Outline:! Part I: Fermentations! Part II: Respiration! Part III: Metabolic Diversity
 Outline:! Part I: Fermentations! Part II: Respiration! Part III: Metabolic Diversity Metabolism Lectures Learning objectives are:! Learn about anaerobic respiratory metabolisms.! How can an inorganic compound
Outline:! Part I: Fermentations! Part II: Respiration! Part III: Metabolic Diversity Metabolism Lectures Learning objectives are:! Learn about anaerobic respiratory metabolisms.! How can an inorganic compound
The Nitrogen Cycle. ) in the atmosphere is converted into ammonium ions ( NH 4 + ).
 The Nitrogen Cycle Nitrogen is essential for many processes; it is crucial for all life on Earth. It is in all amino acids, is incorporated into proteins, and is present in the bases that make up nucleic
The Nitrogen Cycle Nitrogen is essential for many processes; it is crucial for all life on Earth. It is in all amino acids, is incorporated into proteins, and is present in the bases that make up nucleic
Phytoplankton and bacterial biomass, production and growth in various ocean ecosystems
 Phytoplankton and bacterial biomass, production and growth in various ocean ecosystems Location Bact. Biomass (mg C m -2 ) Phyto. Biomass (mg C m -2 ) BactB: PhytoB BactP (mg C m -2 d -1 ) 1 o Pro (mg
Phytoplankton and bacterial biomass, production and growth in various ocean ecosystems Location Bact. Biomass (mg C m -2 ) Phyto. Biomass (mg C m -2 ) BactB: PhytoB BactP (mg C m -2 d -1 ) 1 o Pro (mg
Chapter 3 Ecosystem Ecology. Tuesday, September 19, 17
 Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
Denitrification 2/11/2011. Energy to be gained in oxidation. Oxidized N. Reduced N
 Oxidized N Energy to be gained in oxidation Reduced N (Sarmiento & Gruber, 2006) Denitrification The reduction of NO 3 and NO 2 to N 2 during heterotrophic respiration of organic matter. Occurs predominately
Oxidized N Energy to be gained in oxidation Reduced N (Sarmiento & Gruber, 2006) Denitrification The reduction of NO 3 and NO 2 to N 2 during heterotrophic respiration of organic matter. Occurs predominately
3 3 Cycles of Matter. EOC Review
 EOC Review A freshwater plant is placed in a salt marsh. Predict the direction in which water will move across the plant s cell wall, and the effect of that movement on the plant. a. Water would move out
EOC Review A freshwater plant is placed in a salt marsh. Predict the direction in which water will move across the plant s cell wall, and the effect of that movement on the plant. a. Water would move out
Biogeochemical Cycles. {Living World
 Biogeochemical Cycles {Living World What Sustains Life on Earth? Solar energy, the cycling of matter, and gravity sustain the earth s life. Earth's Spheres Atmosphere layer of air that surrounds the Earth
Biogeochemical Cycles {Living World What Sustains Life on Earth? Solar energy, the cycling of matter, and gravity sustain the earth s life. Earth's Spheres Atmosphere layer of air that surrounds the Earth
Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p
 OCN 401-Biogeochemical Systems Lecture #12 (10.8.13) Angelos Hannides, hannides@hawaii.edu Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p. 288-308 1. Physical
OCN 401-Biogeochemical Systems Lecture #12 (10.8.13) Angelos Hannides, hannides@hawaii.edu Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p. 288-308 1. Physical
Introduction. Wetland System. A Wetland Scene at Lorne C. Henderson Conservation Area near Petrolia
 Wetland Treatment of Wastewater This monograph, one in a series of single issue documents that deal with our local environment, has been prepared by the Sarnia-Lambton Environmental Association in co-operation
Wetland Treatment of Wastewater This monograph, one in a series of single issue documents that deal with our local environment, has been prepared by the Sarnia-Lambton Environmental Association in co-operation
Nutrients elements required for the development, maintenance, and reproduction of organisms.
 Nutrient Cycles Energy flows through ecosystems (one way trip). Unlike energy, however, nutrients (P, N, C, K, S ) cycle within ecosystems. Nutrients are important in controlling NPP in ecosystems. Bottom-up
Nutrient Cycles Energy flows through ecosystems (one way trip). Unlike energy, however, nutrients (P, N, C, K, S ) cycle within ecosystems. Nutrients are important in controlling NPP in ecosystems. Bottom-up
Lakes: Primary Production, Budgets and Cycling
 OCN 401-Biogeochemical Systems (9.28.17) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
OCN 401-Biogeochemical Systems (9.28.17) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
Estuarine and Coastal Biogeochemistry
 Estuarine and Coastal Biogeochemistry OCN 623 Chemical Oceanography 2 April 2015 Readings: Seitzinger& Mayorga(2008) Jeandelet al.(2011) 2015 Frank Sansone and S.V. Smith 1. Global coastal zone Outline
Estuarine and Coastal Biogeochemistry OCN 623 Chemical Oceanography 2 April 2015 Readings: Seitzinger& Mayorga(2008) Jeandelet al.(2011) 2015 Frank Sansone and S.V. Smith 1. Global coastal zone Outline
Understanding Nutrients and Their Affects on the Environment
 Understanding Nutrients and Their Affects on the Environment Humans & Ecosystems Humans are just like ecosystems, too much or too little of a nutrient is bad for the system. Nutrient management is a balancing
Understanding Nutrients and Their Affects on the Environment Humans & Ecosystems Humans are just like ecosystems, too much or too little of a nutrient is bad for the system. Nutrient management is a balancing
The Role of Alternative Respiration Pathways and the Effect of Nutrient Loading on Peat Decomposition in Plum Island Marsh Sediment
 1 The Role of Alternative Respiration Pathways and the Effect of Nutrient Loading on Peat Decomposition in Plum Island Marsh Sediment David A. Dodge 1, 2 Collaborator: Austin Ritter 1, 2 Advisor: Anne
1 The Role of Alternative Respiration Pathways and the Effect of Nutrient Loading on Peat Decomposition in Plum Island Marsh Sediment David A. Dodge 1, 2 Collaborator: Austin Ritter 1, 2 Advisor: Anne
Biogeochemical Cycles. Nutrient cycling at its finest!
 Biogeochemical Cycles Nutrient cycling at its finest! Four Criteria for Sustainability Sustainable Ecosystems Need: Reliance on Solar Energy High Biodiversity Population Control Nutrient Cycling This note
Biogeochemical Cycles Nutrient cycling at its finest! Four Criteria for Sustainability Sustainable Ecosystems Need: Reliance on Solar Energy High Biodiversity Population Control Nutrient Cycling This note
Elements essential for life also cycle through ecosystems.
 13.5 Cycling of Matter KEY CONCEPT Matter cycles in and out of an ecosystem. MAIN IDEAS Water cycles through the environment. Elements essential for life also cycle through ecosystems. VOCABULARY hydrologic
13.5 Cycling of Matter KEY CONCEPT Matter cycles in and out of an ecosystem. MAIN IDEAS Water cycles through the environment. Elements essential for life also cycle through ecosystems. VOCABULARY hydrologic
5/6/2015. Matter is recycled within and between ecosystems.
 Biogeochemical Cycles/ Nutrient Cycles Biogeochemical Cycle Evaporation Water Cycle Transpiration Condensation Precipitation Runoff Vocabulary Seepage Root Uptake Carbon Cycle Phosphorus Cycle Nitrogen
Biogeochemical Cycles/ Nutrient Cycles Biogeochemical Cycle Evaporation Water Cycle Transpiration Condensation Precipitation Runoff Vocabulary Seepage Root Uptake Carbon Cycle Phosphorus Cycle Nitrogen
Ocean Production and CO 2 uptake
 Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Biology. Slide 1 of 33. End Show. Copyright Pearson Prentice Hall
 Biology 1 of 33 2 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within
Biology 1 of 33 2 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within
13.5. Cycling of Matter. Water cycles through the environment.
 13.5 Cycling of Matter VOCABULARY hydrologic cycle biogeochemical cycle nitrogen fixation KEY CONCEPT Matter cycles in and out of an ecosystem. Main Ideas Water cycles through the environment. Elements
13.5 Cycling of Matter VOCABULARY hydrologic cycle biogeochemical cycle nitrogen fixation KEY CONCEPT Matter cycles in and out of an ecosystem. Main Ideas Water cycles through the environment. Elements
Lakes: Primary Production, Budgets and Cycling. Lecture Outline
 OCN 401-Biogeochemical Systems (10.06.16) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
OCN 401-Biogeochemical Systems (10.06.16) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
Ecosystems: Nutrient Cycles
 Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
In Situ Remediation (ISR MT3DMS TM ) Features: Reactions
 In Situ Remediation (ISR MT3DMS TM ) Features: Reactions October 5, 2015 ISR MT3DMS TM Reactions 1 Introduction The core reaction framework in ISR MT3DMS TM was developed using the public domain reaction
In Situ Remediation (ISR MT3DMS TM ) Features: Reactions October 5, 2015 ISR MT3DMS TM Reactions 1 Introduction The core reaction framework in ISR MT3DMS TM was developed using the public domain reaction
CEE 370 Environmental Engineering Principles. Environmental Microbiology
 Updated: 1 October 015 Print version CEE 370 Environmental Engineering Principles Lecture #13 Environmental Biology II Metabolism Reading: Davis & Masten, Chapter 3 David Reckhow CEE 370 L#13 1 Environmental
Updated: 1 October 015 Print version CEE 370 Environmental Engineering Principles Lecture #13 Environmental Biology II Metabolism Reading: Davis & Masten, Chapter 3 David Reckhow CEE 370 L#13 1 Environmental
Eutrophication test questions
 Eutrophication test questions From the course materials find answers to all questions. There may be (and often is) more than one correct choice. You have to mark down the right combination of choices.
Eutrophication test questions From the course materials find answers to all questions. There may be (and often is) more than one correct choice. You have to mark down the right combination of choices.
THE CYCLING OF NUTRIENTS
 Unit 4 THE CYCLING OF NUTRIENTS LEARNING OBJECTIVES 1. Recognize the need for the recycling of the earth s chemicals and the consequences if this is not done. 2. Learn the difference between a global cycle
Unit 4 THE CYCLING OF NUTRIENTS LEARNING OBJECTIVES 1. Recognize the need for the recycling of the earth s chemicals and the consequences if this is not done. 2. Learn the difference between a global cycle
Impact of submarine groundwater discharge on biogeochemical processes and benthic fluxes in coastal sands
 Impact of submarine groundwater discharge on biogeochemical processes and benthic fluxes in coastal sands Daphne Donis 1,2, Felix Janssen 1,2, Frank Wenzhöfer 1,2, Olaf Dellwig 3, Peter Escher 3, Michael
Impact of submarine groundwater discharge on biogeochemical processes and benthic fluxes in coastal sands Daphne Donis 1,2, Felix Janssen 1,2, Frank Wenzhöfer 1,2, Olaf Dellwig 3, Peter Escher 3, Michael
CHEMICAL: NITROGEN AND PHOSPHORUS (read pp in Dodson)
 BIOE 155, Fall 010 BACKGROUND CHEMICAL: NITROGEN AND PHOSPHORUS (read pp39-50 in Dodson) Lakes are often classified according to trophic status, specifically how much energy or food is available for the
BIOE 155, Fall 010 BACKGROUND CHEMICAL: NITROGEN AND PHOSPHORUS (read pp39-50 in Dodson) Lakes are often classified according to trophic status, specifically how much energy or food is available for the
Coastal Wetlands Formation, Functions, and Susceptibility. John Marton CWC Gulf Lagniappe November 23, 2013
 Coastal Wetlands Formation, Functions, and Susceptibility John Marton CWC Gulf Lagniappe November 23, 2013 What is a wetland? Wetlands are distinguished by the presence of water, either at the surface
Coastal Wetlands Formation, Functions, and Susceptibility John Marton CWC Gulf Lagniappe November 23, 2013 What is a wetland? Wetlands are distinguished by the presence of water, either at the surface
CEE 370 Environmental Engineering Principles
 Updated: 12 October 2015 Print version CEE 370 Environmental Engineering Principles Lecture #13 Environmental Biology II Metabolism Reading: Davis & Masten, Chapter 3 David Reckhow CEE 370 L#13 1 Environmental
Updated: 12 October 2015 Print version CEE 370 Environmental Engineering Principles Lecture #13 Environmental Biology II Metabolism Reading: Davis & Masten, Chapter 3 David Reckhow CEE 370 L#13 1 Environmental
Nitrogen and phosphorus cycling in the ocean
 Nitrogen and phosphorus cycling in the ocean Deborah A. Bronk Department of Physical Sciences Outline: 1. The Redfield ratio 2. Liebig s Law of the Minimum 3. The nitrogen cycle 4. The phosphorus cycle
Nitrogen and phosphorus cycling in the ocean Deborah A. Bronk Department of Physical Sciences Outline: 1. The Redfield ratio 2. Liebig s Law of the Minimum 3. The nitrogen cycle 4. The phosphorus cycle
MICROBES IN ECOLOGY INTRODUCTION
 MICROBES IN ECOLOGY INTRODUCTION - Microbes usually live in communities and rarely as individuals They are Present in every known ecosystem Over 99% of microbes contribute to the quality of human life
MICROBES IN ECOLOGY INTRODUCTION - Microbes usually live in communities and rarely as individuals They are Present in every known ecosystem Over 99% of microbes contribute to the quality of human life
Chapter 34 Nature of Ecosystems. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chapter 34 Nature of Ecosystems 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 34.1 The Biotic Components of Ecosystems Ecosystems Abiotic components include
Chapter 34 Nature of Ecosystems 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 34.1 The Biotic Components of Ecosystems Ecosystems Abiotic components include
Soil Quality: manifestations, mechanisms and measurement
 Soil Quality: manifestations, mechanisms and measurement John Witty and Lance Mytton Why is soil quality important? 54 The impact of biology on soil quality 54 Oxygen status in soils 55 Effect of oxygen
Soil Quality: manifestations, mechanisms and measurement John Witty and Lance Mytton Why is soil quality important? 54 The impact of biology on soil quality 54 Oxygen status in soils 55 Effect of oxygen
Reactions & Processes Influenced by Soil Wetness. Topics for Consideration. Reactions and Processes Influenced by Soil Wetness
 Reactions & Processes Influenced by Soil Wetness Topics for Consideration A. Organic matter (OM) accumulation B. Some reduction effects induced by soil saturation C. Redoximorphic features D. Calcium carbonate
Reactions & Processes Influenced by Soil Wetness Topics for Consideration A. Organic matter (OM) accumulation B. Some reduction effects induced by soil saturation C. Redoximorphic features D. Calcium carbonate
TABLE OF CONTENTS. 4, Environmental Chemistry 2, Biogeochemical cycle of carbon and nitrogen
 Subject Paper No and Title Module No and Title Module Tag CHE_P4_M2 TABLE OF CONTENTS 1. Learning outcomes 2. Introduction 2.1. Bio-distribution of elements 2.2. Biogeochemical cycles 3. Carbon cycle 3.1.
Subject Paper No and Title Module No and Title Module Tag CHE_P4_M2 TABLE OF CONTENTS 1. Learning outcomes 2. Introduction 2.1. Bio-distribution of elements 2.2. Biogeochemical cycles 3. Carbon cycle 3.1.
CHEMICAL: CARBON and OXYGEN (read 44-45; in Dodson)
 BIOE 155, Fall BACKGROUND INFORMATION CHEMICAL: CARBON and OXYGEN (read -5; 3-39 in Dodson) Types of molecules Organic: compounds containing Carbon-Hydrogen bonds Inorganic: everything else. Photosynthesis
BIOE 155, Fall BACKGROUND INFORMATION CHEMICAL: CARBON and OXYGEN (read -5; 3-39 in Dodson) Types of molecules Organic: compounds containing Carbon-Hydrogen bonds Inorganic: everything else. Photosynthesis
Streamside Management. How the area around your pond effects the water.
 Streamside Management Zones and Water Quality How the area around your pond effects the water. Stream(pond)side Management Zone A streamside management zone (SMZ) is a strip of land immediately adjacent
Streamside Management Zones and Water Quality How the area around your pond effects the water. Stream(pond)side Management Zone A streamside management zone (SMZ) is a strip of land immediately adjacent
Enhanced bioremediation of gasoline contaminated groundwater in Finland by injection of humic acids
 In situ workshop, Stockholm 31.5.2011, Pirjo Tuomi Enhanced bioremediation of gasoline contaminated groundwater in Finland by injection of humic acids Enhanced Natural Attenuation Most organic contaminants
In situ workshop, Stockholm 31.5.2011, Pirjo Tuomi Enhanced bioremediation of gasoline contaminated groundwater in Finland by injection of humic acids Enhanced Natural Attenuation Most organic contaminants
Biological Reductive Dechlorination of Chlorinated Compounds. Barry Molnaa WSW Remediation Practice Manager ARCADIS
 Biological Reductive Dechlorination of Chlorinated Compounds Barry Molnaa WSW Remediation Practice Manager ARCADIS 1 Presentation Outline What are we trying to do? How is it supposed to work? What are
Biological Reductive Dechlorination of Chlorinated Compounds Barry Molnaa WSW Remediation Practice Manager ARCADIS 1 Presentation Outline What are we trying to do? How is it supposed to work? What are
AP Biology. Ecosystems
 Ecosystems Studying organisms in their environment organism population community ecosystem biosphere Essential questions What limits the production in ecosystems? How do nutrients move in the ecosystem?
Ecosystems Studying organisms in their environment organism population community ecosystem biosphere Essential questions What limits the production in ecosystems? How do nutrients move in the ecosystem?
Nutrients, biology and elemental stoichiometry
 Nutrients, biology and elemental stoichiometry Subtropics and tropics: oligotrophic = low nutrient, low biomass. Equatorial upwelling regions: Elevated nutrients (1 10 MNO 3 ) and biomass (relative to
Nutrients, biology and elemental stoichiometry Subtropics and tropics: oligotrophic = low nutrient, low biomass. Equatorial upwelling regions: Elevated nutrients (1 10 MNO 3 ) and biomass (relative to
Evolution of Leachate Composition In a Calgary Landfill. Sean Buckles, M.Sc., P.Eng.
 Evolution of Leachate Composition In a Calgary Landfill Sean Buckles, M.Sc., P.Eng. Background Landfill Construction and Operation Leachate Generation Typical Characteristics 2 Landfill Construction and
Evolution of Leachate Composition In a Calgary Landfill Sean Buckles, M.Sc., P.Eng. Background Landfill Construction and Operation Leachate Generation Typical Characteristics 2 Landfill Construction and
1. Energy to do work 2. Raw material to build/repair things (nutrients)
 1. Energy to do work 2. Raw material to build/repair things (nutrients) Living things are built from water Nutrients: carbon, hydrogen, nitrogen, and oxygen 3. Essential nutrients are cycled through environment
1. Energy to do work 2. Raw material to build/repair things (nutrients) Living things are built from water Nutrients: carbon, hydrogen, nitrogen, and oxygen 3. Essential nutrients are cycled through environment
