Installation Qualification/Operational Qualification Protocols and Instructions
|
|
|
- Bethanie Gordon
- 6 years ago
- Views:
Transcription
1 Installation Qualification/Operational Qualification Protocols and Instructions AquaLab PRE Decagon Devices, Inc July 20, :05 PM 1
2 Table of Contents Section 1 Section 2 Section 2.1 Section 2.2 Section 2.3 Section 2.4 Section 2.5 Section 3 Section 3.1 Section 3.2 Section Section Appendix 1 Appendix 2 Introduction Installation Qualification (IQ) Equipment Identification Receiving and Unpacking Visual Inspection Environmental Conditions Power Up Test Operational Qualification (OQ) Hardware testing System information Sensor Verification Equipment Familiarization Training Record Deficiencies and Corrective Actions Pg. 3 Pg. 5 Pg. 5 Pg. 5 Pg. 7 Pg. 8 Pg. 8 Pg. 10 Pg. 10 Pg. 10 Pg. 10 Pg. 12 Pg. 13 Pg. 14 Decagon Devices, Inc July 21, :51 AM 2
3 Section 1 Introduction This Qualification protocol is solely intended to be used with new or relocated AquaLab Water Activity instruments. It is written to assist the end-user in validation of predetermined specifications. The use of this document does not replace the need for the AquaLab PRE User s Manual. Information within the User s Manual is required to complete this IQ/OQ Protocol. If the manual has been misplaced, copies can be obtained from the manufacturer or downloaded from their website, Qualification of instrumentation is a formal process of documenting that an instrument is fit for its intended use and that it is kept maintained and calibrated. Responsibilities The instrument qualification carried out onsite is the sole responsibility of the instrument owner/user. However, Decagon Devices supports their customers in performing the qualification by providing the instrument qualification dedicated documentation and offering a qualification service. In this regard, the following responsibilities are defined: Performance of Qualification Execution of the instrument qualification and entire qualification of the installed system covered in this document is performed by a Decagon Devices trained and authorized service personnel when ordered from a customer. Review and final qualification approval Final approval for the qualification has to be completed after review of the qualification documentation filled out during performance of the qualification procedures (IQ/OQ protocols). The customer representative then signs the approved form. Installation Qualification (IQ) Installation qualification is documented proof that the instrument was received as designed and specified by the manufacturer, that it is properly installed in the selected environment, and that this environment is suitable for the operation and use of the instrument. The IQ section therefore describes and documents the instrument installation in the pre-determined environment. Further, the IQ verifies and ensures that all ordered parts and documentation are in place and that all supplied items are in working order and condition. Operational Qualification (OQ) The operational qualification serves as proof that the equipment operates as designed and intended, as well as fulfills acceptance criteria defined and stated in the Operational Qualification documentation. These criteria are defined and are based on the equipment technical specifications of the manufacturer. Performance Qualification (PQ) Performance qualification is documented proof that an instrument consistently performs according to the specifications appropriate for its routine use. Monitoring of equipment during routine operation is essential for ensuring that the ongoing performance is within specifications. The performance qualification, execution Decagon Devices, Inc July 21, :51 AM 3
4 and frequency are solely under responsibility of the user. Performance validation should be designed to meet the specifications and accuracy for a given application. Equipment familiarization and operator training records All equipment users are to be instructed in basic operation, functionality, instrument parameters, as well as on basic hardware features of the installed system including routine maintenance and cleaning procedures. Please contact Decagon Devices to learn about available training and seminars. Authorized support specialists perform the qualification services offered by Decagon Devices. Decagon Devices, Inc July 21, :51 AM 4
5 Section 2 Installation Qualification (IQ) Initial Qualification and Requalification The IQ protocols described below are dedicated to initial qualification and/or to requalification. Installation Qualification tests should be performed, 1) when the system is installed, 2) when the system is moved to a new location, 3) prior to running OQ tests. This section describes the procedure for receiving, unpacking, and installing a PRE AquaLab Water Activity instrument. The purchased AquaLab Water Activity instrument undergoing qualification is located at: Company Department: Address: City: Country: Zip code/postal Code: Phone Number: State: 2.1 Equipment identification Fill out this section after unpacking the AquaLab instrument and corresponding accessories. Dewpoint Volatiles Manufacturer: DECAGON DEVICES Model Number: Serial Number: Decagon Devices Authorized Representative 2.2 Receiving and Unpacking Verify that the external packaging was not damaged during shipment in a way that the internal package content might be damaged. External package condition Satisfactory Not Satisfactory Decagon Devices, Inc July 21, :51 AM 5
6 Compare shipment list with supplied items to ensure completeness of order. Water Activity Instrument Complete Not Complete Quick Start Guide Complete Not Complete User s Manual Complete Not Complete Certificate of Calibration Complete Not Complete Trial Verification Standards Complete Not Complete SDS Documents Complete Not Complete Power Cable Complete Not Complete USB/Serial Cable Complete Not Complete Filters (Volatiles only) Complete Not Complete Cleaning Kit Complete Not Complete LDPE Sample Cups and Lids Complete Not Complete All parts were received as ordered and the delivery is complete. Yes No Any parts that were missing at the time of this supply verification and reported as Not Complete must be delivered to complete the shipment. Any parts marked as Not Complete must be indicated below and reviewed with the customer. Minor parts or accessories that do not impact the installation or qualification procedure or the functionality of the instrument can be accepted, if agreed upon by the customer in order to complete the remainder of the IQ/OQ process. Completed can be marked once the item has been received. Item: Accepted Completed Date Initials Accepted Accepted Completed Completed Decagon Devices, Inc July 21, :51 AM 6
7 Date Initials Accepted Accepted Accepted Completed Completed Completed Qualified by: 2.3 Visual Inspection After unpacking, verify that there is no physical damage to the instrument, cables, and accessories. Note all observed damage in the Remarks section. Minor defects that do not affect functionality can be marked as accepted, if approved by the customer. AquaLab Instrument Satisfactory Not Satisfactory Accepted Documentation Satisfactory Not Satisfactory Accepted Other Satisfactory Not Satisfactory Accepted Qualified by: Severe damage to any of the delivered parts interrupts the installation qualification until the part is replaced. Completion of the installation qualification after replacement is documented below. AquaLab Instrument Satisfactory Not Satisfactory Documentation Satisfactory Not Satisfactory Other Satisfactory Not Satisfactory Decagon Devices, Inc July 21, :51 AM 7
8 Qualified by: 2.4 Environmental Conditions Installation of the AquaLab Water Activity instrument includes placing the instrument on a level surface in a location where the temperature remains fairly stable. This location should be well away from air conditioner and heater vents, open windows, outside doors, or other items that may cause rapid temperature fluctuation. Location Satisfactory Not Satisfactory Adequate Power Satisfactory Not Satisfactory Stable Surface Satisfactory Not Satisfactory Temperature Satisfactory Not Satisfactory Qualified by: 2.5 Power Up Test After finding a good location for the AquaLab Water Activity meter, plug the power cord into the back of the unit and a standard AC outlet. The ON/OFF switch is located on the back panel. Instrument is powered upon switching on Yes No Decagon Devices, Inc July 21, :51 AM 8
9 Completeness of Installation Qualification (IQ) Installation Qualification was completed and documented according to manufacturer s guidelines. Initial Qualification Requalification Decagon Devices Authorized Representative Function: Company: Installation Qualification was reviewed by the representative of the system owner. Reviewed and approved by: Function: Company: Decagon Devices, Inc July 21, :51 AM 9
10 Section 3 Operational Qualification (OQ) This section describes tests that are to be executed for Operational Qualification of the AquaLab Water Activity instrument in order to prove proper operation of the installed instrument. 3.1 Hardware testing Display is functional Yes No Keypad is functional Yes No Qualified by: 3.2 System Information AquaLab Water Activity Instrument Information Manufacturer: Model Number: Serial Number: Firmware Version: Sensor Verification The AquaLab PRE line of instruments contain a chilled mirror dewpoint sensor for determining water activity. The Volatiles option contains a capacitance relative humidity sensor. The performance of the sensors is verified by measuring specially prepared calibration standards that have a specific molality and water activity. Performance Verification Standards in four water activity levels are used for qualification: 0.250, 0.500, 0.760, a w. The AquaLab dew point sensor will read each standard within ±0.001 a w of the stated value. The capacitance (volatiles) sensor will read each standard within ±0.015 a w of the stated value. To measure the water activity of the standards, follow the instructions in the User s Manual for taking a reading. Decagon Devices, Inc July 21, :51 AM 10
11 Dew Point Sensor Volatiles Sensor 25 C Lot # a w ±0.01 C a w ±0.015 C 13.41m LiCl m LiCl m NaCl Steam Distilled H 2 O Water Activity Verification Standards Within Specification Yes No If verification standards are out of specification, clean the instrument and follow the procedure in the User s Manual to perform a linear offset. Repeat the verification process with fresh standards. Dew Point Sensor Volatiles Sensor 25 C Lot # a w ±0.01 C a w ±0.015 C 13.41m LiCl m LiCl m NaCl Steam Distilled H Water Activity Verification Standards Within Specification Yes No Qualified by: Decagon Devices, Inc July 21, :51 AM 11
12 3.2.2 Equipment Familiarization This section ensures that the instrument operators receive appropriate equipment training to ensure proper operation, maintenance, and generation of results with the AquaLab instrument. Product familiarization covers instruction on basic operations, functionality and features of the instrument, and routine maintenance including cleaning procedures. Equipment familiarization and training completed for the AquaLab. Yes No Qualified by: Completeness of Operational Qualification (OQ) Operational Qualification was completed and documented according to manufacturers guidelines. Initial Qualification Requalification Qualifications met Vendor Acceptance Criteria Yes No If any deficiencies are found, fill out the instructions for a corrective action on Pg. 14 of this document. Decagon Devices Authorized Representative Function: Company: Operational Qualification was reviewed by the representative of the system owner. Reviewed and approved by: Function: Company: Decagon Devices, Inc July 21, :51 AM 12
13 Appendix 1 Training Record This training record is for instruction in basic operation, functionality, instrument parameters, as well as on basic hardware features of the installed system including routine maintenance and cleaning procedures. Please contact Decagon Devices to learn about available training and seminars. Authorized support specialists perform the qualification services offered by Decagon Devices. Decagon Devices Authorized Representative Function: Company: Reviewed and approved by: Function: Company: Decagon Devices, Inc July 21, :51 AM 13
14 Appendix 2 Deficiencies and Corrective Actions If any deficiencies were found, they are to be followed with an instruction for Corrective Action. Once acceptable results are obtained, the deficiency is accepted by checking the accepted box under the deficiency. Deficiency: Corrective Action: Accepted Initial Deficiency: Corrective Action: Accepted Initial Decagon Devices Authorized Representative Function: Company: Decagon Devices, Inc July 21, :51 AM 14
AQUALAB 4TE, 4TEV, 4TE DUO, 4TEV DUO BENCHTOP WATER ACTIVITY
 AQUALAB 4TE, 4TEV, 4TE DUO, 4TEV DUO BENCHTOP WATER ACTIVITY Installation Qualification + Operational Qualification Protocols and Instructions TABLE OF CONTENTS 1. Introduction.. 3 2. Installation Qualification
AQUALAB 4TE, 4TEV, 4TE DUO, 4TEV DUO BENCHTOP WATER ACTIVITY Installation Qualification + Operational Qualification Protocols and Instructions TABLE OF CONTENTS 1. Introduction.. 3 2. Installation Qualification
Installation Qualification/Operational Qualification Protocols and Instructions
 Installation Qualification/Operational Qualification Protocols and Instructions AquaLab VSA Vapor Sorption Analyzer Decagon Devices, Inc June 28, 2016 11:03 AM 1 Table of Contents Section 1 Section 2 Section
Installation Qualification/Operational Qualification Protocols and Instructions AquaLab VSA Vapor Sorption Analyzer Decagon Devices, Inc June 28, 2016 11:03 AM 1 Table of Contents Section 1 Section 2 Section
Compliance and qualification services. Trust your validation needs to the people who design, build, test, and support your systems
 Compliance and qualification services Trust your validation needs to the people who design, build, test, and support your systems Validation can be challenging If your laboratory is required to qualify
Compliance and qualification services Trust your validation needs to the people who design, build, test, and support your systems Validation can be challenging If your laboratory is required to qualify
QIAGEN Service Solutions Professional Services
 QIAGEN Service Solutions Professional Services Sample to Insight Installation Services Facilitate the quick integration of QIAGEN automated systems and instruments into your lab workflow by minimizing
QIAGEN Service Solutions Professional Services Sample to Insight Installation Services Facilitate the quick integration of QIAGEN automated systems and instruments into your lab workflow by minimizing
á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION
 USP 41 General Information / á1058ñ 1 á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION INTRODUCTION A large variety of analytical instruments, ranging from a simple apparatus to complex computerized systems,
USP 41 General Information / á1058ñ 1 á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION INTRODUCTION A large variety of analytical instruments, ranging from a simple apparatus to complex computerized systems,
The procurement, qualification and calibration of lab instruments: An Overview
 The procurement, qualification and calibration of lab instruments: An Overview The reliability of analytical data generated from chemical and physical analyses is critically dependent on three factors:
The procurement, qualification and calibration of lab instruments: An Overview The reliability of analytical data generated from chemical and physical analyses is critically dependent on three factors:
+ IQ/OQ Protocol Installation Qualification/ Operation Qualification
 + IQ/OQ Protocol Installation Qualification/ Operation Qualification PuriCare Vertical Flow Animal Transfer Station Labconco o: 1059601 Rev.- Purpose and Scope This Qualification Protocol is intended
+ IQ/OQ Protocol Installation Qualification/ Operation Qualification PuriCare Vertical Flow Animal Transfer Station Labconco o: 1059601 Rev.- Purpose and Scope This Qualification Protocol is intended
Tank Scale Service Checklist
 Tank Scale Service Checklist Specifying Service for Optimized Weighing Processes Selecting the right weighing equipment is an important first step to ensuring that your weighing processes are able to meet
Tank Scale Service Checklist Specifying Service for Optimized Weighing Processes Selecting the right weighing equipment is an important first step to ensuring that your weighing processes are able to meet
Managing Validation. Paperless Recorders
 Managing Validation Paperless Recorders Multitrend Plus Validation Background The past five years has seen an increase in the use of computerized systems and products in the pharmaceutical and bio-pharmaceutical
Managing Validation Paperless Recorders Multitrend Plus Validation Background The past five years has seen an increase in the use of computerized systems and products in the pharmaceutical and bio-pharmaceutical
DETAILED PROCEDURE AND GUIDELINES TEMPERATURE MAPPING STUDY & QUALIFICATION OF COLD ROOMS, WAREHOUSES, VANS, TRUCKS, REEFERS, REFRIGERATORS & BOXES
 DETAILED PROCEDURE AND GUIDELINES ON TEMPERATURE MAPPING STUDY & QUALIFICATION OF COLD ROOMS, WAREHOUSES, VANS, TRUCKS, REEFERS, REFRIGERATORS & BOXES FOR PHARMACEUTICAL INDUSTRY Revision History: Revision
DETAILED PROCEDURE AND GUIDELINES ON TEMPERATURE MAPPING STUDY & QUALIFICATION OF COLD ROOMS, WAREHOUSES, VANS, TRUCKS, REEFERS, REFRIGERATORS & BOXES FOR PHARMACEUTICAL INDUSTRY Revision History: Revision
+ IQ/OQ Protocol Installation Qualification/ Operation Qualification
 + IQ/OQ Protocol Installation Qualification/ Operation Qualification Purifier Logic Series Biological Safety Cabinets Labconco o: 1058602 Rev. - Available at www.labconco.com or by e-mail in Word 2000
+ IQ/OQ Protocol Installation Qualification/ Operation Qualification Purifier Logic Series Biological Safety Cabinets Labconco o: 1058602 Rev. - Available at www.labconco.com or by e-mail in Word 2000
Optimizing Performance Bench Scale Service Checklist
 Optimizing Performance Bench Scale Specifying Service For optimized weighing processes Selecting the right weighing equipment is an important first step to ensuring that your weighing processes are able
Optimizing Performance Bench Scale Specifying Service For optimized weighing processes Selecting the right weighing equipment is an important first step to ensuring that your weighing processes are able
Is monitoring water activity part of your risk management strategy? AquaLab Water activity meters.
 D E C A G O N Is monitoring water activity part of your risk management strategy? AquaLab Water activity meters. Your company invests valuable resources in product innovation. Product quality and business
D E C A G O N Is monitoring water activity part of your risk management strategy? AquaLab Water activity meters. Your company invests valuable resources in product innovation. Product quality and business
CRON HDI-920 Site Planning. Customer Responsibilities
 Customer Responsibilities CRON HDI-920 Site Planning You are responsible for providing the following items below to help ensure a trouble free installation. Adequate delivery access (i.e. Company Loading
Customer Responsibilities CRON HDI-920 Site Planning You are responsible for providing the following items below to help ensure a trouble free installation. Adequate delivery access (i.e. Company Loading
Your local gas generation partner. Installation Qualification/ Operational Qualification
 Your local gas generation partner Installation Qualification/ Operational Qualification IQ/OQ Certification Completed on-site by your local Peak Engineer at a time that suits you Page- 2 IQ/OQ Certification
Your local gas generation partner Installation Qualification/ Operational Qualification IQ/OQ Certification Completed on-site by your local Peak Engineer at a time that suits you Page- 2 IQ/OQ Certification
GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS
 PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS PH 1/97 (Rev. 3), 15 January 2002 ANNEX 15 QUALIFICATION AND
PHARMACEUTICAL INSPECTION CONVENTION PHARMACEUTICAL INSPECTION CO-OPERATION SCHEME GUIDE TO GOOD MANUFACTURING PRACTICE FOR MEDICINAL PRODUCTS PH 1/97 (Rev. 3), 15 January 2002 ANNEX 15 QUALIFICATION AND
Supplementary Training Modules on Good Manufacturing Practice. Validation. WHO Technical Report Series, No. 937, Annex 4.
 Supplementary Training Modules on Good Manufacturing Practice Validation WHO Technical Report Series, No. 937, 2006. Annex 4. Validation Slide 1 of 48 August 2006 Validation! Part 1. General overview on
Supplementary Training Modules on Good Manufacturing Practice Validation WHO Technical Report Series, No. 937, 2006. Annex 4. Validation Slide 1 of 48 August 2006 Validation! Part 1. General overview on
Quick Start Guide. AquaLab TDL Water Activity Meter
 Quick Start Guide AquaLab TDL Water Activity Meter The Setup Congratulations on your new AquaLab TDL instrument. This guide will help you properly setup and begin gathering data in just a few short steps.
Quick Start Guide AquaLab TDL Water Activity Meter The Setup Congratulations on your new AquaLab TDL instrument. This guide will help you properly setup and begin gathering data in just a few short steps.
LIGHT CONTROLLER LC 500
 LIGHT CONTROLLER LC 500 Manual and User Guide Please read this manual before operating this product PSI, spol. s r. o., Drásov 470, 664 24 Drásov, Czech Republic FAX: +420 511 440 901, TEL: +420 511 440
LIGHT CONTROLLER LC 500 Manual and User Guide Please read this manual before operating this product PSI, spol. s r. o., Drásov 470, 664 24 Drásov, Czech Republic FAX: +420 511 440 901, TEL: +420 511 440
Integrated IC Systems Installation Qualification Dionex Corporation
 Integrated IC Systems Installation Qualification 2009 Dionex Corporation Document. 031739 Revision 04 September 2009 2009, Dionex Corporation All rights reserved worldwide. Printed in the United States
Integrated IC Systems Installation Qualification 2009 Dionex Corporation Document. 031739 Revision 04 September 2009 2009, Dionex Corporation All rights reserved worldwide. Printed in the United States
Equipment Qualification Ensure Fit for Intended Use. IVT Amsterdam Conference Mary Sexton. 19 Mar 2015
 Equipment Qualification Ensure Fit for Intended Use IVT Amsterdam Conference Mary Sexton 19 Mar 2015 Equipment Qualification Ensure Fit for Intended Use 1. Types of Qualification 2. Validating Equipment
Equipment Qualification Ensure Fit for Intended Use IVT Amsterdam Conference Mary Sexton 19 Mar 2015 Equipment Qualification Ensure Fit for Intended Use 1. Types of Qualification 2. Validating Equipment
Reviewers, approvers and executers of this plan are captured in the approval routing tab of this document in IFS.
 DOCUMENT NUMBER: 1034722 LOCATION: FT COLLINS/LOVELAND PAGE 1 of 8 1.0 PURPOSE 1.1 The purpose of this Validation Master Plan (VMP) is to identify the validation and testing requirements necessary to qualify
DOCUMENT NUMBER: 1034722 LOCATION: FT COLLINS/LOVELAND PAGE 1 of 8 1.0 PURPOSE 1.1 The purpose of this Validation Master Plan (VMP) is to identify the validation and testing requirements necessary to qualify
Model FD 115 Drying and heating chambers Avantgarde.Line with forced convection
 Model FD 115 Drying and heating chambers Avantgarde.Line with forced convection A BINDER FD series Avantgarde.Line heating oven is always used when fast drying and sterilization is required. Thanks to
Model FD 115 Drying and heating chambers Avantgarde.Line with forced convection A BINDER FD series Avantgarde.Line heating oven is always used when fast drying and sterilization is required. Thanks to
Manual 069 The validation of facilities and system
 1. Purpose The purpose of this guideline is to provide requirements for the Validation of Facilities and Systems and to outline recommendations on how to achieve compliance. 2. Scope This guideline can
1. Purpose The purpose of this guideline is to provide requirements for the Validation of Facilities and Systems and to outline recommendations on how to achieve compliance. 2. Scope This guideline can
Model KMF 115 Constant climate chambers with expanded temperature / humidity range
 Model KMF 115 Constant climate chambers with expanded temperature / humidity range The BINDER KMF ensures absolutely constant test conditions throughout the testing area. A big advantage of this constant
Model KMF 115 Constant climate chambers with expanded temperature / humidity range The BINDER KMF ensures absolutely constant test conditions throughout the testing area. A big advantage of this constant
Benchtop Water Activity Meter
 Benchtop Water Activity Meter meyer.ca/product-shocase/aqualab/ Not a "quick mode" reading or an approximation, but a direct measure of ater activity accurate to 0.003 AQUALAB 4TE Speed and accuracy Measure
Benchtop Water Activity Meter meyer.ca/product-shocase/aqualab/ Not a "quick mode" reading or an approximation, but a direct measure of ater activity accurate to 0.003 AQUALAB 4TE Speed and accuracy Measure
Validation Strategies for Equipment from Multiple Vendors
 Welcome to our E-Seminar: Validation Strategies for Equipment from Multiple Vendors Page 1 Content FDA guidelines and inspectional observations Validation & Qualification Approaches Instrument Qualification
Welcome to our E-Seminar: Validation Strategies for Equipment from Multiple Vendors Page 1 Content FDA guidelines and inspectional observations Validation & Qualification Approaches Instrument Qualification
SESSION 14. Documentation Requirements and Management for Validation. 30 March Frits Vogt
 Validation Services SESSION 14 Documentation Requirements and Management for Validation 30 March 2017 Frits Vogt fritsvogt@valserv.nl Content Documentation Requirements and Management for Validation I.
Validation Services SESSION 14 Documentation Requirements and Management for Validation 30 March 2017 Frits Vogt fritsvogt@valserv.nl Content Documentation Requirements and Management for Validation I.
CALIBRATION AND QUALIFICATION OF EQUIPMENTS IN THE PHARMACEUTICAL INDUSTRY: EMPHASIS ON RADIOPHARMACEUTICALS PRODUCTION
 2011 International Nuclear Atlantic Conference - INAC 2011 Belo Horizonte, MG, Brazil, October 24-28, 2011 ASSOCIAÇÃO BRASILEIRA DE ENERGIA NUCLEAR - ABEN ISBN: 978-85-99141-04-5 CALIBRATION AND QUALIFICATION
2011 International Nuclear Atlantic Conference - INAC 2011 Belo Horizonte, MG, Brazil, October 24-28, 2011 ASSOCIAÇÃO BRASILEIRA DE ENERGIA NUCLEAR - ABEN ISBN: 978-85-99141-04-5 CALIBRATION AND QUALIFICATION
According to USP <1058>
 Analytical l Instrument t Qualification According to USP Scope, Approach, Requirements Dr. Ludwig Huber Chief Advisor, Global FDA Compliance ludwig_huber@labcompliance.com Overview FDA and International
Analytical l Instrument t Qualification According to USP Scope, Approach, Requirements Dr. Ludwig Huber Chief Advisor, Global FDA Compliance ludwig_huber@labcompliance.com Overview FDA and International
PERFORMANCE QUALIFICATION PROTOCOL HVAC SYSTEM
 Page 1 of 24 PERFORMANCE QUALIFICATION PROTOCOL FOR HVAC SYSTEM Signing of this Performance Qualification Protocol indicates agreement with the Validation Master Plan approach of the equipment. Further
Page 1 of 24 PERFORMANCE QUALIFICATION PROTOCOL FOR HVAC SYSTEM Signing of this Performance Qualification Protocol indicates agreement with the Validation Master Plan approach of the equipment. Further
AquaLab Water Activity Meter Operator s Manual For Series 4TE, 4TEV, DUO Version 8 Decagon Devices, Inc.
 Water Activity Meter Operator s Manual For Series 4TE, 4TEV, DUO Version 8 Decagon Devices, Inc. Decagon Devices, Inc 2365 NE Hopkins Court Pullman WA 99163 (509)332-5601 fax: (509)332-5158 www.aqualab.com
Water Activity Meter Operator s Manual For Series 4TE, 4TEV, DUO Version 8 Decagon Devices, Inc. Decagon Devices, Inc 2365 NE Hopkins Court Pullman WA 99163 (509)332-5601 fax: (509)332-5158 www.aqualab.com
TECHNICAL SERVICES MAXIMUM LIFE AND PERFORMANCE FROM YOUR MEASURING INSTRUMENT
 TECHNICAL SERVICES MAXIMUM LIFE AND PERFORMANCE FROM YOUR MEASURING INSTRUMENT The best results are achieved when man and technology work hand-in-hand. Thomas Zschockelt, Technical Support Engineer ENSURING
TECHNICAL SERVICES MAXIMUM LIFE AND PERFORMANCE FROM YOUR MEASURING INSTRUMENT The best results are achieved when man and technology work hand-in-hand. Thomas Zschockelt, Technical Support Engineer ENSURING
Services. Equipment, Software, Training and Validation Services.
 Life Sciences Nuclear Medicine / PET Services Equipment, Software, Training and Validation Services Radiation Safety Services Minimise downtime and maximise reliability At LabLogic, we understand the need
Life Sciences Nuclear Medicine / PET Services Equipment, Software, Training and Validation Services Radiation Safety Services Minimise downtime and maximise reliability At LabLogic, we understand the need
Operator s Manual Version 1
 Water Activity Meter Operator s Manual Version 1 for AquaLab Pre Decagon Devices, Inc. Decagon Devices, Inc. 2365 NE Hopkins Court Pullman WA 99163 (509) 332-5601 fax: (509) 332-5158 www.decagon.com support@aqualab.com
Water Activity Meter Operator s Manual Version 1 for AquaLab Pre Decagon Devices, Inc. Decagon Devices, Inc. 2365 NE Hopkins Court Pullman WA 99163 (509) 332-5601 fax: (509) 332-5158 www.decagon.com support@aqualab.com
Model BF 260 Incubators Avantgarde.Line with forced convection
 Model BF 260 Incubators Avantgarde.Line with forced convection The BINDER incubator of the BF Avantgarde.Line series with forced convection is suitable for all gentle incubation applications, particularly
Model BF 260 Incubators Avantgarde.Line with forced convection The BINDER incubator of the BF Avantgarde.Line series with forced convection is suitable for all gentle incubation applications, particularly
Standard Monitoring System
 Cleanroom-Requirements according to Annex 1, EU-GMP-Guidelines Strict requirements for compliance with the environmental conditions in the relative cleanroom classes Continuous data recording and archiving
Cleanroom-Requirements according to Annex 1, EU-GMP-Guidelines Strict requirements for compliance with the environmental conditions in the relative cleanroom classes Continuous data recording and archiving
Model BF 56 Standard-Incubators with forced convection
 Model BF 56 Standard-Incubators with forced convection The BINDER incubator of the BF Avantgarde.Line series with forced convection is suitable for all gentle incubation applications, particularly under
Model BF 56 Standard-Incubators with forced convection The BINDER incubator of the BF Avantgarde.Line series with forced convection is suitable for all gentle incubation applications, particularly under
WARNING LETTER AUG 3, 2016
 Ropack, Inc. 8/3/16 Department of Health and Human Services Public Health Service Food and Drug Administration 10903 New Hampshire Avenue White Oak Building 66 Silver Spring, MD 20993 WARNING LETTER AUG
Ropack, Inc. 8/3/16 Department of Health and Human Services Public Health Service Food and Drug Administration 10903 New Hampshire Avenue White Oak Building 66 Silver Spring, MD 20993 WARNING LETTER AUG
Kaye Product Line Calibration and Maintenance Service Solutions Rental
 Kaye Product Line Calibration and Maintenance Service Solutions Rental Amphenol Advanced Sensors EUROPE Value-added services that enhance your productivity Your Validation and Monitoring equipment is
Kaye Product Line Calibration and Maintenance Service Solutions Rental Amphenol Advanced Sensors EUROPE Value-added services that enhance your productivity Your Validation and Monitoring equipment is
2001 NBIC Interpretations
 2001 NBIC Interpretations INTERPRETATION 01-41 Subject: Appendix 2 and Appendix 5 with 2003 Addendum Question: In the event of an alteration to a boiler in which the boiler heating surface and steaming
2001 NBIC Interpretations INTERPRETATION 01-41 Subject: Appendix 2 and Appendix 5 with 2003 Addendum Question: In the event of an alteration to a boiler in which the boiler heating surface and steaming
Comprehensive. Flexible. Personalized. Illumina Product Support Services Customized service to meet your needs
 Comprehensive. Flexible. Personalized. Illumina Product Support Services Customized service to meet your needs Congratulations on choosing Illumina as your partner. We are here to help you maximize your
Comprehensive. Flexible. Personalized. Illumina Product Support Services Customized service to meet your needs Congratulations on choosing Illumina as your partner. We are here to help you maximize your
á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION
 First Supplement to USP 40 NF 35 General Information / á1058ñ Analytical Instrument Qualification 1 á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION INTRODUCTION A large variety of analytical instruments, ranging
First Supplement to USP 40 NF 35 General Information / á1058ñ Analytical Instrument Qualification 1 á1058ñ ANALYTICAL INSTRUMENT QUALIFICATION INTRODUCTION A large variety of analytical instruments, ranging
Spectrophotometer Service Solutions Comprehensive Service and Maintenance for Biochrom Spectrophotometers
 Spectrophotometer Service Solutions Comprehensive Service and Maintenance for Biochrom Spectrophotometers We understand the importance of your spectrophotometer within your lab and therefore the importance
Spectrophotometer Service Solutions Comprehensive Service and Maintenance for Biochrom Spectrophotometers We understand the importance of your spectrophotometer within your lab and therefore the importance
SUPPLIER QUALITY SYSTEM SURVEY
 SUPPLIER NAME DATE WEB ADDRESS ADDRESS CITY STATE ZIP PUBLICLY OR PRIVATELY HELD? TEL FAX CONTACT INFORMATION POSITION CONTACT NAME PHONE FAX E-MAIL PRESIDENT OPERATIONS MGR. QUALITY MGR. ENGINEERING MGR.
SUPPLIER NAME DATE WEB ADDRESS ADDRESS CITY STATE ZIP PUBLICLY OR PRIVATELY HELD? TEL FAX CONTACT INFORMATION POSITION CONTACT NAME PHONE FAX E-MAIL PRESIDENT OPERATIONS MGR. QUALITY MGR. ENGINEERING MGR.
Q-CODE QV4 QUALITY REQUIREMENTS Supplier s Manufacturing Process Qualification (SMPQ)
 Rev Letter: E Page 1 of 5 REVISION HISTORY REV DATE ORIGIN REASON FOR CHANGE(S) A B 08Jan16 29Sep16 Eric Steif New Added clarification language to COC requirements C D E 09Dec16 27Jun17 5/04/18 Daniel
Rev Letter: E Page 1 of 5 REVISION HISTORY REV DATE ORIGIN REASON FOR CHANGE(S) A B 08Jan16 29Sep16 Eric Steif New Added clarification language to COC requirements C D E 09Dec16 27Jun17 5/04/18 Daniel
Model FD 23 Drying and heating chambers Classic.Line with forced convection
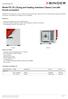 Model FD 23 Drying and heating chambers Classic.Line with forced convection A BINDER FD series heating oven is always used when fast drying and sterilization is required. Thanks to its fully homogeneous
Model FD 23 Drying and heating chambers Classic.Line with forced convection A BINDER FD series heating oven is always used when fast drying and sterilization is required. Thanks to its fully homogeneous
Model BF 720 Incubators Avantgarde.Line with forced convection
 Model BF 720 Incubators Avantgarde.Line with forced convection The BINDER incubator of the BF Avantgarde.Line series with forced convection is suitable for all gentle incubation applications, particularly
Model BF 720 Incubators Avantgarde.Line with forced convection The BINDER incubator of the BF Avantgarde.Line series with forced convection is suitable for all gentle incubation applications, particularly
SOP OPERATION OF PARTICULATE MATTER INSTRUMENTS CLARK COUNTY DEPARTMENT OF AIR QUALITY. Monitoring Division PROCEDURE NUMBER 400
 SOP OPERATION OF PARTICULATE MATTER INSTRUMENTS CLARK COUNTY DEPARTMENT OF AIR QUALITY Monitoring Division PROCEDURE NUMBER 400 STANDARD OPERATING PROCEDURE FOR Particulate Matter Instruments Revision
SOP OPERATION OF PARTICULATE MATTER INSTRUMENTS CLARK COUNTY DEPARTMENT OF AIR QUALITY Monitoring Division PROCEDURE NUMBER 400 STANDARD OPERATING PROCEDURE FOR Particulate Matter Instruments Revision
Istanbul May 31, 2001
 Pürifiye Su Sistemleri Validasyonu Istanbul, Turkey May 31, 2001 Peter Drechsler Aventis Pharm- Frankfurt, Germany Aventis Pharma Exubera Inhaled Insulin Table of content for Presentation: * Validation
Pürifiye Su Sistemleri Validasyonu Istanbul, Turkey May 31, 2001 Peter Drechsler Aventis Pharm- Frankfurt, Germany Aventis Pharma Exubera Inhaled Insulin Table of content for Presentation: * Validation
SUPPLEMENTARY GUIDELINES ON GOOD MANUFACTURING PRACTICES (GMP): VALIDATION
 Pharmapathway.com SUPPLEMENTARY GUIDELINES ON GOOD MANUFACTURING PRACTICES (GMP): VALIDATION Page 1 of 43 1. INTRODUCTION Validation is an essential and integral part of Good Manufacturing Practice (GMP).
Pharmapathway.com SUPPLEMENTARY GUIDELINES ON GOOD MANUFACTURING PRACTICES (GMP): VALIDATION Page 1 of 43 1. INTRODUCTION Validation is an essential and integral part of Good Manufacturing Practice (GMP).
VALIDATION AS A KEY TOOL IN SUCCESSFUL TRANSFER OR ESTABLISHMENT OF A MEDICAL DEVICE COMPANY IN A NEW FACILITY
 VALIDATION AS A KEY TOOL IN SUCCESSFUL TRANSFER OR ESTABLISHMENT OF A MEDICAL DEVICE COMPANY IN A NEW FACILITY Oren Levin, Diane Gordon VisionCare Ophthalmic Technologies, POB 3879, Petah Tikva 49130,
VALIDATION AS A KEY TOOL IN SUCCESSFUL TRANSFER OR ESTABLISHMENT OF A MEDICAL DEVICE COMPANY IN A NEW FACILITY Oren Levin, Diane Gordon VisionCare Ophthalmic Technologies, POB 3879, Petah Tikva 49130,
SECTION Inline ULTRASONIC FLOW METERS
 SECTION 40 71 66 Inline ULTRASONIC FLOW METERS PART 1- GENERAL 1.01 SUMMARY A. Ultrasonic flow meters for permanent in line installations. The meters shall utilize a transit time ultrasonic principle of
SECTION 40 71 66 Inline ULTRASONIC FLOW METERS PART 1- GENERAL 1.01 SUMMARY A. Ultrasonic flow meters for permanent in line installations. The meters shall utilize a transit time ultrasonic principle of
SECTION PRESSURE AND DIFFERENTIAL PRESSURE GAUGES
 SECTION 40 72 43 PRESSURE AND DIFFERENTIAL PRESSURE GAUGES PART 1- GENERAL A. The Waterpilot FMX21 is designed for hydrostatic level measurement. It has a robust ceramic sensor and integrated temperature
SECTION 40 72 43 PRESSURE AND DIFFERENTIAL PRESSURE GAUGES PART 1- GENERAL A. The Waterpilot FMX21 is designed for hydrostatic level measurement. It has a robust ceramic sensor and integrated temperature
Using Empower SystemsQT Qualification Tool
 Using Empower SystemsQT Qualification Tool for Waters Modular HPLC Systems A summary of Empower SystemsQT Qualification Tool features and benefits, with emphasis on the technical attributes of the qualification
Using Empower SystemsQT Qualification Tool for Waters Modular HPLC Systems A summary of Empower SystemsQT Qualification Tool features and benefits, with emphasis on the technical attributes of the qualification
Vaisala Life Science Services Integrated Service for Maximum Compliance, Safety and Performance LIFE SCIENCE SOLUTIONS FOR REAL LIFE
 Vaisala Life Science Services Integrated Service for Maximum Compliance, Safety and Performance LIFE SCIENCE SOLUTIONS FOR REAL LIFE Get Your Controlled Environment Under Control Simplified Compliance
Vaisala Life Science Services Integrated Service for Maximum Compliance, Safety and Performance LIFE SCIENCE SOLUTIONS FOR REAL LIFE Get Your Controlled Environment Under Control Simplified Compliance
Vaisala viewlinc Monitoring System Services
 Vaisala viewlinc Monitoring System Services Integrated Service for Maximum Compliance, Safety and Performance Vaisala viewlinc Life Cycle Maintenance Agreement Software Maintenance Technical Support Training
Vaisala viewlinc Monitoring System Services Integrated Service for Maximum Compliance, Safety and Performance Vaisala viewlinc Life Cycle Maintenance Agreement Software Maintenance Technical Support Training
Manufacturing Optimisation Inside the Pharmaceutical Supply Chain. Business System Compliance
 Manufacturing Optimisation Inside the Pharmaceutical Supply Chain. Business System Compliance Bob Mauger, Principal Validation Consultant Cork, April 3/4 2003 Agenda Business System Compliance Overview
Manufacturing Optimisation Inside the Pharmaceutical Supply Chain. Business System Compliance Bob Mauger, Principal Validation Consultant Cork, April 3/4 2003 Agenda Business System Compliance Overview
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY ISSN Review Article
 INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Review Article INTRODUCTION AND GENERAL OVERVIEW OF PHARMACEUTICAL PROCESS VALIDATION: A REVIEW Pandita Rachna* 1, Rana A C
INTERNATIONAL RESEARCH JOURNAL OF PHARMACY www.irjponline.com ISSN 2230 8407 Review Article INTRODUCTION AND GENERAL OVERVIEW OF PHARMACEUTICAL PROCESS VALIDATION: A REVIEW Pandita Rachna* 1, Rana A C
GG , , Appendix E (New), E101.1 (New)
 GG276-14 903.1, 903.1.2, Appendix E (New), E101.1 (New) Proponent: Brenda Thompson, Clark County Development Services, Las Vegas, NV, representing Chair, Sustainability, Energy & High Performance Building
GG276-14 903.1, 903.1.2, Appendix E (New), E101.1 (New) Proponent: Brenda Thompson, Clark County Development Services, Las Vegas, NV, representing Chair, Sustainability, Energy & High Performance Building
PROCESS VALIDATION. A Systematic Approach 2015 WHITE PAPER WHITE PAPER PRODUCED BY MAETRICS
 WHITE PAPER PROCESS VALIDATION A Systematic Approach 2015 WHITE PAPER PRODUCED BY MAETRICS For more information, please contact: USA Office: +1 317 706 1493 UK Office: +44 115 921 6200 globalsales@maetrics.com
WHITE PAPER PROCESS VALIDATION A Systematic Approach 2015 WHITE PAPER PRODUCED BY MAETRICS For more information, please contact: USA Office: +1 317 706 1493 UK Office: +44 115 921 6200 globalsales@maetrics.com
STAGING/STORAGE OF COMPONENTS CFR Part 50, Appendix B, Quality Assurance Criteria for Nuclear Power Plants and Fuel Reprocessing Plants
 Staging/Storage of Components Page 1 of 7 1.0 Objective STAGING/STORAGE OF COMPONENTS The objective of this surveillance is to ensure that before components or consumables are used in maintenance and repair
Staging/Storage of Components Page 1 of 7 1.0 Objective STAGING/STORAGE OF COMPONENTS The objective of this surveillance is to ensure that before components or consumables are used in maintenance and repair
TABLE OF CONTENTS. 1. Introduction Operation System... 21
 AQUALAB TABLE OF CONTENTS 18280-00 9.30.2018 1. Introduction... 1 2. Operation... 2 2.1 Installation... 2 2.2 Sample Preparation... 3 2.2.1 Sample Composition... 4 2.2.2 Sample Temperature... 6 2.3 Taking
AQUALAB TABLE OF CONTENTS 18280-00 9.30.2018 1. Introduction... 1 2. Operation... 2 2.1 Installation... 2 2.2 Sample Preparation... 3 2.2.1 Sample Composition... 4 2.2.2 Sample Temperature... 6 2.3 Taking
Quick Start Guide. Corporation Rev. 1
 Quick Start Guide Corporation 600269 Rev. 1 System Installation Note: Retain all packing materials. 1. Following the instructions provided in the SMART Turbo shipping carton, carefully remove the instrument
Quick Start Guide Corporation 600269 Rev. 1 System Installation Note: Retain all packing materials. 1. Following the instructions provided in the SMART Turbo shipping carton, carefully remove the instrument
SECTION CORIOLIS MASS FLOW MEASURING SYSTEM
 SECTION 40 71 73 CORIOLIS MASS FLOW MEASURING SYSTEM PART 1 - GENERAL 1.01 SUMMARY A. Coriolis flow meter for permanent installation above ground. The meter shall utilize a measuring principle based on
SECTION 40 71 73 CORIOLIS MASS FLOW MEASURING SYSTEM PART 1 - GENERAL 1.01 SUMMARY A. Coriolis flow meter for permanent installation above ground. The meter shall utilize a measuring principle based on
Model KB 23 Cooling incubators with compressor technology
 Model KB 23 Cooling incubators with compressor technology The most versatile among the cooled incubators for microorganisms: The BINDER KB series cooled incubator controls temperature ranges of -5 C to
Model KB 23 Cooling incubators with compressor technology The most versatile among the cooled incubators for microorganisms: The BINDER KB series cooled incubator controls temperature ranges of -5 C to
Facility and Equipment Maintenance
 Facility and Equipment Maintenance Deborah L. Griffin Manager, Quality Assurance for Cellular Therapies Hillman Cancer Center University of Pittsburgh Cancer Institute University of Pittsburgh Medical
Facility and Equipment Maintenance Deborah L. Griffin Manager, Quality Assurance for Cellular Therapies Hillman Cancer Center University of Pittsburgh Cancer Institute University of Pittsburgh Medical
RESERVE BANK OF INDIA
 RESERVE BANK OF INDIA www.rbi.org.in Empanelment of Vendors for supply of Computer Hardware, Software and Peripherals to RBI, Thiruvananthapuram and for the Maintenance of its Hardware, Software and Peripherals
RESERVE BANK OF INDIA www.rbi.org.in Empanelment of Vendors for supply of Computer Hardware, Software and Peripherals to RBI, Thiruvananthapuram and for the Maintenance of its Hardware, Software and Peripherals
TABLE OF CONTENTS. 1. Introduction Operation System... 21
 AQUALAB SERIES 4 TABLE OF CONTENTS 13484-14 6.15.2018 1. Introduction... 1 2. Operation... 2 2.1 Installation... 2 2.2 Sample Preparation... 3 2.2.1 Sample Composition... 4 2.2.2 Sample Temperature...
AQUALAB SERIES 4 TABLE OF CONTENTS 13484-14 6.15.2018 1. Introduction... 1 2. Operation... 2 2.1 Installation... 2 2.2 Sample Preparation... 3 2.2.1 Sample Composition... 4 2.2.2 Sample Temperature...
Audit Validation Programs Like a Champ (When your time is limited!!) Cathelene Compton 12 October 2017
 Audit Validation Programs Like a Champ (When your time is limited!!) Cathelene Compton 12 October 2017 FDA 483 Observations 2 FDA 483 Observations 3 FDA 483 Observations 4 Auditing Validation How to cover
Audit Validation Programs Like a Champ (When your time is limited!!) Cathelene Compton 12 October 2017 FDA 483 Observations 2 FDA 483 Observations 3 FDA 483 Observations 4 Auditing Validation How to cover
Validation and Automated Validation
 TOP INDUSTRY QUESTIONS Validation and Automated Validation 1 Table of Contents 03 04 07 10 13 16 19 INTRODUCTION SECTION 1 - Validation Standards How is validation defined under Title 21 CFR Part 11? What
TOP INDUSTRY QUESTIONS Validation and Automated Validation 1 Table of Contents 03 04 07 10 13 16 19 INTRODUCTION SECTION 1 - Validation Standards How is validation defined under Title 21 CFR Part 11? What
18.H Questionnaire for preparing GMP-inspections
 Questionnaire for preparing -inspections Questionnaire for preparing -inspections Here you will find answers to the following questions: What questions are typically asked during inspections based on current
Questionnaire for preparing -inspections Questionnaire for preparing -inspections Here you will find answers to the following questions: What questions are typically asked during inspections based on current
R. Bulut & E.C. Leong. International Symposium Advanced Experimental Unsaturated Soil Mechanics Trento, Italy June 27-29, 2005
 State-of-the-Art Report on Indirect Measurement of Soil Suction R. Bulut & E.C. Leong International Symposium Advanced Experimental Unsaturated Soil Mechanics Trento, Italy June 27-29, 2005 Indirect Suction
State-of-the-Art Report on Indirect Measurement of Soil Suction R. Bulut & E.C. Leong International Symposium Advanced Experimental Unsaturated Soil Mechanics Trento, Italy June 27-29, 2005 Indirect Suction
Schedule of Tender. For. Nitrogen Parallel Seam Sealer
 Schedule of Tender For Nitrogen Parallel Seam Sealer Central Manufacturing Technology Institute Tumkur Road, Bangalore 560 022. INDIA Page 1 of 14 Central Manufacturing Technology Institute Tumkur Road,
Schedule of Tender For Nitrogen Parallel Seam Sealer Central Manufacturing Technology Institute Tumkur Road, Bangalore 560 022. INDIA Page 1 of 14 Central Manufacturing Technology Institute Tumkur Road,
Guidelines for Process Validation of Pharmaceutical Dosage Forms
 Guidelines for Process Validation of Pharmaceutical Dosage Forms Version 2.1 Date issued 21/02/2010 Date of implementation 21/05/2010 31 August 2010 Page 1 of 20 Guidelines for Process Validation of Pharmaceutical
Guidelines for Process Validation of Pharmaceutical Dosage Forms Version 2.1 Date issued 21/02/2010 Date of implementation 21/05/2010 31 August 2010 Page 1 of 20 Guidelines for Process Validation of Pharmaceutical
Gyrolab xp workstation
 Gyrolab xp workstation Product Information Sheet Nanoliter-scale immunoassays generating high quality data Automated assay workflows High throughput with unattended operation Fast time to result: up to
Gyrolab xp workstation Product Information Sheet Nanoliter-scale immunoassays generating high quality data Automated assay workflows High throughput with unattended operation Fast time to result: up to
QUALITY MANUAL. Revision K. JADE PRECISION MEDICAL COMPONENTS, LLC 3063 B Philmont Avenue Huntingdon Valley, PA 19006
 QUALITY MANUAL Revision K JADE PRECISION MEDICAL COMPONENTS, LLC 3063 B Philmont Avenue Huntingdon Valley, PA 19006 Page: 1 of 14 Table of Contents 1. Purpose & Scope... 2 2. Applicable Standards... 2
QUALITY MANUAL Revision K JADE PRECISION MEDICAL COMPONENTS, LLC 3063 B Philmont Avenue Huntingdon Valley, PA 19006 Page: 1 of 14 Table of Contents 1. Purpose & Scope... 2 2. Applicable Standards... 2
35 STATION DOUBLE ROTORY COMPRESSION MACHINE
 Page 1 of 16 User Requirement Specifications 35 STATION DOUBLE ROTORY Equipment ID: T-35COMP 01 Page 2 of 16 Table of Contents 1.0 APPROVAL SIGNATURES 2.0 OVERVIEW 3.0 PROCESS DESCRIPTION 4.0 PRODUCTIVITY
Page 1 of 16 User Requirement Specifications 35 STATION DOUBLE ROTORY Equipment ID: T-35COMP 01 Page 2 of 16 Table of Contents 1.0 APPROVAL SIGNATURES 2.0 OVERVIEW 3.0 PROCESS DESCRIPTION 4.0 PRODUCTIVITY
The Standard Illustrations ISO The International Standard for Quality Management Systems. Year 2000 Edition. Leland R.
 ISO 9001 The Standard Illustrations The International Standard for Quality Management Systems Year 2000 Edition Leland R. Beaumont ISO 9001, The Standard Illustrations Figures excerpted from ISO 9001,
ISO 9001 The Standard Illustrations The International Standard for Quality Management Systems Year 2000 Edition Leland R. Beaumont ISO 9001, The Standard Illustrations Figures excerpted from ISO 9001,
Installation Qualification Equipment (Reference SOP: )
 Installation Qualification Equipment (Reference SOP: ) Project: Equipment Description: Project No: Serial No: Manufacturer: Model No: Equipment No: Location: Protocol: PROGRAM INDEX 1. OBJECTIVE...3 2.
Installation Qualification Equipment (Reference SOP: ) Project: Equipment Description: Project No: Serial No: Manufacturer: Model No: Equipment No: Location: Protocol: PROGRAM INDEX 1. OBJECTIVE...3 2.
Service Description for IP Implementation. Issue 1.0. Date
 for IP Implementation Issue 1.0 Date 2012-11-01 for IP Implementation Contents 1 Overview... 3 1.1 Architecture... 3 2... 4 2.1 Engineering... 4 2.2 Supervision... 4 2.3 One-Off Support... 4 2.4 Details...
for IP Implementation Issue 1.0 Date 2012-11-01 for IP Implementation Contents 1 Overview... 3 1.1 Architecture... 3 2... 4 2.1 Engineering... 4 2.2 Supervision... 4 2.3 One-Off Support... 4 2.4 Details...
Options for load release
 Approaches to Release of Sterile Goods from Steam Sterilization: Parametric Release and Routine Monitoring Craig Wallace Senior Technical Specialist 3M Infection Prevention Division Today s Topics 1. Options
Approaches to Release of Sterile Goods from Steam Sterilization: Parametric Release and Routine Monitoring Craig Wallace Senior Technical Specialist 3M Infection Prevention Division Today s Topics 1. Options
OAKDALE CHILLED WATER PLANT CAPACITY UPGRADES UI PROJECT INSTALL CHILLERS SECTION COMMISSIONING
 PART I GENERAL 1.01 SECTION INCLUDES A. Commissioning objectives. B. Roles and responsibilities. C. Test Equipment. D. Systems to be commissioned. E. Construction Checklist requirements. SECTION 01 91
PART I GENERAL 1.01 SECTION INCLUDES A. Commissioning objectives. B. Roles and responsibilities. C. Test Equipment. D. Systems to be commissioned. E. Construction Checklist requirements. SECTION 01 91
Vendor Qualification Survey
 1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
1200 West 96 th St Minneapolis, MN 55431 Ph: 952-888-7900 Fax: 952-888-2719 Vendor Qualification Survey Vendor Information Company Name: Date: Address: City: Phone Number: email address: Product or Service
User Requirement Specifications
 Page 1 of 15 User Requirement Specifications Tablet Inspection Belt Equipment ID: T-INSB01 Page 2 of 15 Table of Contents 1.0 APPROVAL SIGNATURES 2.0 OVERVIEW 3.0 PROCESS DESCRIPTION 4.0 PRODUCTIVITY REQUIREMENT
Page 1 of 15 User Requirement Specifications Tablet Inspection Belt Equipment ID: T-INSB01 Page 2 of 15 Table of Contents 1.0 APPROVAL SIGNATURES 2.0 OVERVIEW 3.0 PROCESS DESCRIPTION 4.0 PRODUCTIVITY REQUIREMENT
Validation and Control of Porous Load Sterilisation
 Validation and Control of Porous Load Sterilisation Crown Plaza Hotel, Santry; October 14 th 2010 Gerard Sheridan 14th October 2010 Slide 1 14th October 2010 Slide 2 14th October 2010 Slide 3 Overview
Validation and Control of Porous Load Sterilisation Crown Plaza Hotel, Santry; October 14 th 2010 Gerard Sheridan 14th October 2010 Slide 1 14th October 2010 Slide 2 14th October 2010 Slide 3 Overview
Water Activity Meter
 Water Activity Meter Operator s Manual METER Group, Inc. Version: July 19, 2017 06:45:45 AquaLab Pre METER Group, Inc. 2365 NE Hopkins Court Pullman WA 99163 Phone: 509-332-5601 Fax: 509-332-5158 Website:
Water Activity Meter Operator s Manual METER Group, Inc. Version: July 19, 2017 06:45:45 AquaLab Pre METER Group, Inc. 2365 NE Hopkins Court Pullman WA 99163 Phone: 509-332-5601 Fax: 509-332-5158 Website:
SUPPLIER QUALITY SYSTEM SURVEY
 SUPPLIER QUALITY SYSTEM SURVEY DATE (M/d/yy): 55 Dragon Court Woburn, MA 01801 Tel: (781) 933-7300 Fax: (781) 935-4529 GENERAL INFORMATION Supplier Name: Physical Address: City: State: Zip: Sr. Company
SUPPLIER QUALITY SYSTEM SURVEY DATE (M/d/yy): 55 Dragon Court Woburn, MA 01801 Tel: (781) 933-7300 Fax: (781) 935-4529 GENERAL INFORMATION Supplier Name: Physical Address: City: State: Zip: Sr. Company
Application Note. Baby Formula Water Activity Testing Guide
 Baby Formula Water Activity Testing Guide Introduction The research and development team for the AquaLab line of instruments is conducting water activity best testing practices research. This is an ongoing
Baby Formula Water Activity Testing Guide Introduction The research and development team for the AquaLab line of instruments is conducting water activity best testing practices research. This is an ongoing
Qualification of High-Performance Liquid Chromatography Systems
 Qualification of High-Performance Liquid Chromatography Systems L. Huber Hewlett- Packard GmbH, Waldbronn December 1998 Manuscript of an article published in BioPharm, Vol 11 Number 11, November 1998,
Qualification of High-Performance Liquid Chromatography Systems L. Huber Hewlett- Packard GmbH, Waldbronn December 1998 Manuscript of an article published in BioPharm, Vol 11 Number 11, November 1998,
Model KB 720 Cooling incubators with compressor technology
 Model KB 720 Cooling incubators with compressor technology The powerful virtuoso in cooling incubators for microorganisms: the KB masters temperature ranges from -5 C to 100 C. The new KB series consumes
Model KB 720 Cooling incubators with compressor technology The powerful virtuoso in cooling incubators for microorganisms: the KB masters temperature ranges from -5 C to 100 C. The new KB series consumes
Temperature mapping and autoclave validation specialists
 www.thermalcompliance.co.uk Temperature mapping and autoclave validation specialists Who are we? We are Thermal Compliance, specialists in temperature mapping and autoclave validation. Founded in 2007,
www.thermalcompliance.co.uk Temperature mapping and autoclave validation specialists Who are we? We are Thermal Compliance, specialists in temperature mapping and autoclave validation. Founded in 2007,
Procedure for Equipment Calibration and Maintenance
 Procedure for Equipment Calibration and Maintenance 1.0 Purpose This procedure specifies the schedule and requirements for calibration, performance verification, and maintenance of State Crime Laboratory
Procedure for Equipment Calibration and Maintenance 1.0 Purpose This procedure specifies the schedule and requirements for calibration, performance verification, and maintenance of State Crime Laboratory
Statement of Work. 1.0 Executive Summary Vendor Management Services. Table of Contents QBR. 1.0 Executive Summary. 2.0 Features & Benefits
 QBR Managed Services Statement of Work - 1 - Vendor Management Services Vendor Management Service 1.0 Executive Summary Table of Contents 1.0 Executive Summary 2.0 Features & Benefits 3.0 Details of Service
QBR Managed Services Statement of Work - 1 - Vendor Management Services Vendor Management Service 1.0 Executive Summary Table of Contents 1.0 Executive Summary 2.0 Features & Benefits 3.0 Details of Service
INSTALLATION QUALIFICATIONPROTOCOL FOR DYNAMIC PASS BOX
 Page 1 of 10 INSTALLATION QUALIFICATIONPROTOCOL FOR DYNAMIC PASS BOX CUSTOMER: M/s. NATCO PHARMA LIMITED. K O T H U R EQUIPMENT: DYNAMIC PASS BOX (600 W x 600 D x 1000 H mm) SUBMITTED BY: P H ARMA ENGINEERS
Page 1 of 10 INSTALLATION QUALIFICATIONPROTOCOL FOR DYNAMIC PASS BOX CUSTOMER: M/s. NATCO PHARMA LIMITED. K O T H U R EQUIPMENT: DYNAMIC PASS BOX (600 W x 600 D x 1000 H mm) SUBMITTED BY: P H ARMA ENGINEERS
WHITEPAPER: PROCESS VALIDATION A SYSTEMATIC APPROACH
 WHITEPAPER: PROCESS VALIDATION A SYSTEMATIC APPROACH White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 globalsales@maetrics.com With offices
WHITEPAPER: PROCESS VALIDATION A SYSTEMATIC APPROACH White paper produced by Maetrics For more information, please contact global sales +1 610 458 9312 +1 877 623 8742 globalsales@maetrics.com With offices
A guideline to good manufacturing practice (GMP) requirements
 A guideline to good manufacturing practice (GMP) requirements Part 2: Validation Get all guidelines on www.pharmaguideline.com Email- info@pharmaguideline.com Page 1 of 96 1. Abbreviations EP: GMP: MF:
A guideline to good manufacturing practice (GMP) requirements Part 2: Validation Get all guidelines on www.pharmaguideline.com Email- info@pharmaguideline.com Page 1 of 96 1. Abbreviations EP: GMP: MF:
WATER ACTIVITY AND SHALE STABILITY. Presented by: Dr. Martin Chenevert
 WATER ACTIVITY AND SHALE STABILITY Presented by: Dr. Martin Chenevert 1 Water Activity and Shale Stability Objectives: Know why shale react with water base muds Understand the development of shale swelling
WATER ACTIVITY AND SHALE STABILITY Presented by: Dr. Martin Chenevert 1 Water Activity and Shale Stability Objectives: Know why shale react with water base muds Understand the development of shale swelling
SUPPLIER QUALITY ASSESSMENT
 Supplier Organization Name: Supplier Number: Street Address: Date of This Audit: City, State, Zip Code: Date of Last Audit: Country: of Employees: Main Phone Number: of Buildings/Size: Fax Number: Principal
Supplier Organization Name: Supplier Number: Street Address: Date of This Audit: City, State, Zip Code: Date of Last Audit: Country: of Employees: Main Phone Number: of Buildings/Size: Fax Number: Principal
