Surface Treatment of Titanium and its Alloys for Adhesion Promotion
|
|
|
- Karen Naomi Richardson
- 6 years ago
- Views:
Transcription
1 Surface Treatment of Titanium and its Alloys for Adhesion Promotion A Thesis Submitted to the University of Manchester for the Degree of Doctor of Philosophy in the Faculty of Engineering and Physical Sciences 2015 ZUOJIA LIU Corrosion and Protection Centre School of Materials
2 List of Contents List of Contents List of Contents... 2 List of Tables... 7 List of Figures... 8 List of Abbreviations Abstract Declaration Zuojia Liu Copyright Statement Acknowledgements Chapter 1 Introduction Background Research Objectives Layout of Thesis.28 Chapter 2 Literature Review Properties of Titanium and Its Alloys α Alloy α + β Alloy β Alloy Titanium Dioxide Anodizing of Titanium Barrier-type Anodic Film Porous-type Anodic Film Oxygen Evolution during Anodizing Overview of Adhesion Techniques Single-Lap Shear Test Tensile Test Wedge Test Cross-cut Test Chapter 3 Experimental Methods and Procedures Introduction Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Material and Pre-Treatments Anodizing Scanning & Transmission Electron Microscopes Focused-ion Beam X-ray Diffraction
3 List of Contents Glow Discharge Optical Emission Spectroscopy X-ray Photoelectron Spectroscopy Electrochemical Impedance Spectroscopy Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis Corrosion Behaviour of Anodic Oxide Film on CP -Titanium in NaCl environment Material and Anodizing Electrochemical Measurements Morphology Characterization Formation of Porous Anodic Oxide Film On CP-Titanium in Phosphoric Acid Electrolyte Material and Anodizing Scanning Electron Microscopy Raman Spectroscopy Electrochemical Impedance Spectroscopy Glow Discharge Optical Emission Spectroscopy Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Material and Anodizing Scanning & Transmission Electron Microscopes Raman Spectroscopy Glow Discharge Optical Emission Spectroscopy Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis Electrochemical Impedance Spectroscopy Degradation Test Single-lap Shear Test Figure 3.2. Schematic representation of the single-lap shear test of titanium materials Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Material and Anodizing Degradation Test Single-lap Shear Test Techniques for Characterization Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Material and Sputtering Anodizing Scanning & Transmission Electron Microscopes Glow Discharge Optical Emission Spectroscopy Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of Anodic Oxide Film Formed on Titanium Instrument
4 List of Contents Materials and Anodizing Ultramicrotomy Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Introduction Pre-Treated Surfaces of CP-Ti Anodizing and Film Characterizations Potentiodynamic Polarization and Current-Time Responses Interference Colours Microstructure of Anodic Films X-ray Diffraction Patterns Rutherford Backscattering Spectroscopy & Nuclear Reaction Analysis Glow Discharge Optical Emission Spectroscopy X-ray Photoelectron Spectroscopy Electrochemical Impedance Spectroscopy Discussion Summary.84 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl Environment Introduction Voltage-Time Responses Scanning Electron Microscopy Electrochemical Impedance Spectroscopy Potentiodynamic Polarization Measurements Discussion Summary Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte Introduction Current Time Responses Film Morphology Compositions of Anodic Films Crystallization Behaviour Dielectric Properties Discussion Summary Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Introduction Voltage Time Response.133 4
5 List of Contents 7.3. Scanning Electron Microscopy Raman Spectroscopy Transmission Electron Microscopy Glow Discharge Optical Emission Spectroscopy Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis Electrochemical Impedance Spectroscopy Degradation and Single-lap Shear Tests Surface Morphology after Degradation Load Displacement Responses Surface Morphology after Shear Bonding Discussion Summary Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Introduction Ti6Al4V Pre-Treated Alloy Voltage - Time Response Morphology of Anodic Oxide Films Crystalline Structures of Anodic Films Raman Spectra Transmission Electron Microscopy Film Compositions Glow Discharge Optical Emission Spectroscopy Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis Dielectric Properties of Anodic Films Degradation and Single-lap Shear Tests Surface Morphology after Degradation Load Displacement Responses Surface Morphology after Shear Bonding Discussion Summary Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Introduction Sputter-Deposited Titanium Layer Anodizing of ~100 nm Thick Sputter-Deposited Titanium Anodizing of ~290 nm Thick Sputter-Deposited Titanium Discussion Summary Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium
6 List of Contents Introduction Anodic Films formed on Sputter-Deposited Ti Other Use of STEM-in-SEM Discussion Summary Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Introduction Titanium before and after Etching Discussion Summary Chapter 12 Main Conclusions and Suggestions for Future Work Main Conclusions Suggestions for Future Work 233 References Appendices List of Publications The word count: 55,900 6
7 List of Tables List of Tables Table 2.1. Chemical compositions (wt%) of cp-titanium Grade 1 4 [22] Table 2.2. Chemical compositions of the as-received and heat-treated Ti-6Al-4V alloy analyzed by EDS (wt%) [37] Table 3.1. Chemical compositions (wt%) of Ti6Al4V alloy sheet used for anodizing, degradation and adhesive bonding tests Table 4.1. Current densities (A cm -2 ) of cp-titanium after anodizing at different constant voltages in H 2 SO 4 and H 3 PO 4 electrolytes for 900 s. 77 Table 5.1. The electrochemical impedance spectroscopy parameters of CP-Ti electrode in different experimental conditions.106 Table 5.2. The potentiodynamic polarization parameters of CP-Ti electrode in different experimental conditions Table 6.1. EIS fitted data for anodic films formed on CP-titanium at different voltages Table 7.1. RBS and NRA results of examinations of anodic oxide films on titanium in NaTESi electrolyte to different anodic voltages..136 Table 7.2. EIS fitting results of anodic films formed on titanium after anodizing to different anodic voltages in NaTESi electrolyte Table 7.3. EDS spectra of different regions on anodized titanium after adhesive bonding tests Table 8.1. EDS results of anodic films formed on Ti6Al4V alloy after anodizing to 10, 20, 30 and 40 V in NaTESi electrolyte Table 8.2. RBS and NRA results of anodized Ti6Al4V alloy to 10, 20, 30 and 40 V in NaTESi electrolyte Table 8.3. EIS fitting data obtained from the equivalent circuits of the titanium alloy anodized to 10, 20, 30 and 40 V respectively in the NaTESi electrolyte Table 8.4. EDS results of anodized Ti6Al4V alloy after degradation test in a continuous climatic chamber with a humidity of 90% at 50 o C for 1000 h Table 8.5. EDS results of anodized Ti6Al4V alloy after single-lap shear bonding tests immediately Table Pit depth and surface roughness of titanium after chemical etching in brominemethanol electrolyte for different times
8 List of Figures List of Figures Figure 1.1. Increase in application of titanium alloys in commercial Boeing aircraft [8] Figure 1.2. Percentage of aluminium, titanium, and steel alloys and carbon fibre reinforced polymer (CFRP) of the structural weight of modern large commercial aircraft and gas turbine engines [8] Figure 2.1. The two main crystal structures, hexagonal close packed (HCP) and body centred cubic (BCC), for the α and β phases of titanium [22, 24]...30 Figure 2.2. Scanning electron micrograph showing the typical microstructue of CP-Ti: grain boundary α (A), fine accicular α (B), Widmanstätten α (C) and serrated α (D) [27] Figure 2.3. A schematic illustration of microstructures transformation in Ti-6Al-4V after quenching at different temperatures [34] Figure 2.4. Optical micrograph of the microstructure of Ti6Al4V alloy showing a bi-modal microstructure consisting of α surrounded by transformed β (530X) [23, 33, 35] Figure 2.5. Scanning electron micrographs displaying the microstructures of Ti-6Al-4V alloy: (a) as-received and (b) solution annealed at 1066 o C/1h + furnace cooling [37]. 36 Figure 2.6. Scanning electron micrograph showing typical Ti6Al4V microstructure containing very fine acicular α (E), fine acicular α and β (F) and prior-β grain boundaries (G) [27] Figure 2.7. Optical micrograph showing the microstructure of the as-received Ti-15V-3Cr- 3Sn-3Al (Ti-15-3) alloy [40] Figure 2.8. Schematic diagrams showing the bulk structures of rutile and anatase. The stacking of the octahedral unit cells in both structures are shown on the right side [44] Figure 2.9. Interference between two waves reflected at both surfaces of an oxidised titanium metal substrate [51] Figure Transmission electron micrographs and electron diffraction patterns of the stripped anodic oxide of pure titanium obtained after anodized at 25V for 60 min [80] Figure Transmission electron micrograph of an ultramicrotomed section of the sputter deposited Ti 11.5 at.% Mo alloy anodized to 80 V at a constant current density of 50 ma in a 0.1 mol dm -3 ammonium pentaborate electrolyte at 293K [83] Figure Transmission electron micrographs of ultramicrotomed sections for the sputterdeposited (a) Ti 6 at.% Si, (b) Ti 12 at.% Si and (c) Ti 26 at.% Si alloys anodized at 50 A m 2 in 0.1 mol dm 3 ammonium pentaborate electrolyte at 293 K. The formation voltages are 75 V for Ti 6 at.% Si alloy and 100 V for the Ti 12 at.% Si and Ti 26 at.% Si alloys. The selected area electron diffraction patterns of the respective alloys are also shown [84] Figure Transmission electron micrographs showing regions of different porosity in anodic oxide films formed in H 2 SO 4 at 40 V on α-phase grains of Ti-6A1-4V (bright field) [85] Figure Scanning electron micrographs at top view (a), cross sectional (b), and bottom view images of titanium oxide nanotubes anodized in 0.5 wt% HF solution at 20 V for 20 min [93] Figure Scanning electron micrographs of anodic oxide films on CP-Ti after anodizing in 1.4 M H 3 PO 4 for 1 min: 200V (a) and 250V (b) [111] Figure Transmission electron micrograph of an ultramicrotomed section of the sputtering-deposited titanium anodized to 20 V at 50 A m -2 in 0.1 mol dm -3 ammonium pentaborate electrolyte at 293 K [120] Figure Schematic diagram of the specific single-lap joint experimental setup and its dimensions [125]
9 List of Figures Figure Schematic diagram of a butt tensile specimen. Metal rods are used for the adherends. The surfaces of the metal rods must be smooth and parallel when the bond is made [127] Figure ASTM D3762 wedge test for assessing bond durability [129] Figure Principle of classifying paint film adhesion in the cross-cut test [131] Figure 3.1. Schematic representation of the polarization and anodizing processes in a threecell electrochemical system. a) Application of potentiodynamic polarization at the range from 0 V vs. (Ref) to an anodic voltage vs. (Ref). b) Continued application of the constant anodic voltage to record the current-time response..58 Figure 3.2. Schematic representation of the single-lap shear test of titanium materials Figure 3.3. Schematic representation of thin film deposition using dc sputtering technique in Argon atmosphere Figure 3.4. Schematic showing the top view of parts (A), (B) and (C), represent for STEM detector, detector extension arm and carousel holder. Photo inside the SEM chamber corresponds to the schematics of the top view. The 6-sample carousel is aligned with the electron column, and the STEM detector is brought with the detector arm, and the imagining is ready after the working distance is set for both carousel and STEM detectors Figure 4.1. Scanning electron micrographs at different magnifications of as-received CP-Ti after GDOES sputter cleaning (a and b) and after etching in 48% HF + 70% HNO 3 (c and d) at ambient temperature.85 Figure 4.2. Electron backscatter diffraction (EBSD) of CP-Ti after etching in HF + HNO 3 ; a, the mapping area of titanium; b, mapping result; c, inverse pole figures (IPF) Figure 4.3. Potentiodynamic polarization scanning from 0 V (vs. SCE) to 60 V (vs. SCE) in 1 M sulphuric and 1 M phosphoric acids Figure 4.4. Time evolution of the current density at different anodic voltages in 1 M sulphuric acid, (a) in 1 M phosphoric acid (b) Figure 4.5. The colourations of anodic oxide formed on titanium following anodizing in sulphuric (up) and phosphoric acid (down) at different anodic voltages. The original photos were cropped to highlight the colours Figure 4.6. Scanning electron micrographs of the anodic films formed at 10 (a), 20 (b), 30 (c), 40 (d), 50 (e) and 60 (f) V for 900 s in 1 M sulphuric acid Figure 4.7. Scanning electron micrographs of the anodic films formed at 10 (a), 20 (b), 30 (c), 40 (d), 50 (e) and 60 (f) V for 900 s in 1 M phosphoric acid Figure 4.8. Transmission electron micrographs of the anodic films formed at 50 V for 900 s in 1 M sulphuric acid (a, b, c) and phosphoric acid (d, e, f) respectively at different magnifications Figure 4.9. X-ray diffraction patterns of anodic films formed at 10 V, 30 V and 50 V in sulphuric acid (a) and phosphoric acid (b) for 900 s Figure RBS spectra with fitted curves for the anodic film formed at 20 (a) and 50 (b) V respectively in 1 M H 2 SO 4 electrolyte Figure RBS spectra with fitted curves for the anodic film formed at 20 (a) and 50 (b) V respectively in 1 M H 3 PO 4 electrolyte Figure NRA spectra of anodic film formed at 20 (a) and 50 (b) V in 1 M H 2 SO 4 electrolyte Figure NRA spectra of anodic film formed at 20 (a) and 50 (b) V in 1 M H 3 PO 4 electrolyte Figure GDOES depth profiling analysis of anodic film formed in sulphuric acid and phosphoric acid at 10 V (a and c) and 50 V (b and d) Figure High resolution XPS spectra of anodic films formed at 50 V in sulphuric acid and phosphoric acid electrolytes respectively. (a) Ti2p peak; (b) O1s peak, (c) C1s peak, (d) S2p peak from sulphuric acid and (e) P2p peak from phosphoric acid Figure Bode plots as a function of voltages, and Nyquist diagrams in sulphuric acid (a, b and c) and phosphoric acid (d, e and f)
10 List of Figures Figure Relationship between film thicknesses and voltages in sulphuric and phosphoric acids Figure 5.1. Voltage-time responses during anodizing of CP-Ti in 1 M H 2 SO 4 (a) and 1 M H 3 PO 4 (b) electrolytes at 20 ma cm -2 respectively at ambient temperature.111 Figure 5.2. Scanning electron micrographs of titanium after etching in HF+HNO 3, and anodic films formed at 20 ma cm -2 to different voltages in 1 M H 2 SO 4 and 1 M H 3 PO 4 electrolytes respectively: (a) etched titanium; anodizing in H 2 SO 4 to (b) 20 V; (c) 40 V and (d) 60 V; anodizing in H 3 PO 4 to (e) 20 V; (f) 40 V and (g) 60 V Figure 5.3. Scanning electron micrographs of titanium after etching, and anodized at 20 ma cm -2 to different voltages in 1 M H 2 SO 4 and 1 M H 3 PO 4 electrolytes respectively followed by immersion for 60 days in naturally aerated 3.5% NaCl electrolyte; (a) etched titanium; anodized in H 2 SO 4 to (b) 20 V; (c) 40 V and (d) 60 V; anodized in H 3 PO 4 to (e) 20 V; (f) 40 V and (g) 60 V Figure 5.4. Bode plots of the etched titanium after immediate immersion, and after immersion for 60 days in naturally aerated 3.5% NaCl electrolyte Figure 5.5. Bode plots of titanium anodized in 1 M H 2 SO 4 to 20, 40 and 60 V respectively, and followed by immersion for 60 days in naturally aerated 3.5% NaCl electrolyte Figure 5.6. Bode plots of titanium anodized in 1 M H 3 PO 4 to 20, 40 and 60 V respectively, and followed by immersion for 60 days in naturally aerated 3.5% NaCl electrolyte Figure 5.7. Equivalent circuit for the monitoring of the impedance spectra. R s, C o and R o, as the electrolyte resistance, anodic film capacitance and film resistance respectively Figure 5.8. The relationship between film thickness and anodic voltage before and after immersion experiments in naturally aerated 3.5% NaCl electrolyte Figure 5.9. Potentiodynamic polarization plots of the etched titanium immediate immersed and immersed for 60 days in naturally aerated 3.5% NaCl electrolyte Figure Potentiodynamic polarization plots of the anodized titanium in 1 M H 2 SO 4 immediate immersion and after immersion for 60 days in naturally aerated 3.5% NaCl electrolyte Figure Potentiodynamic polarization plots of the anodized titanium in 1 M H 3 PO 4 immediate immersion and after immersion for 60 days in naturally aerated 3.5% NaCl electrolyte Figure 6.1. Current density-time responses of CP-Ti during anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid for 900 s at ambient temperature.126 Figure 6.2. Scanning electron micrographs of plan-views of the anodic films formed after anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid. The insets are higher magnification images of the respective anodic films Figure 6.3. Ultra-high magnification scanning electron micrographs of the anodic films formed after anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid Figure 6.4. Scanning electron micrographs of cross-sections of the anodic films formed after anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid. The inset images are higher magnification images of the respective anodic oxide film Figure 6.5. GDOES depth profiles of the anodic films formed on CP-Ti at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid; the intensities of phosphorus are multiplied by Figure 6.6. Raman spectra of anodic films formed on CP-Ti in 1 M phosphoric acid at 100, 150 and 200 V Figure 6.7. Electrochemical impedance spectroscopy results of anodic films formed after anodizing at 100, 150 and 200 V in 1 M phosphoric acid; as plotted in Bode diagrams; the left direction of arrow corresponds to the impedance modulus/dm.cm 2, and right direction corresponds to the impedance phase/degree Figure 6.8. Equivalent circuit model for anodic porous films formed at 100 V, 150 V and 200 V in 1 M phosphoric acid
11 List of Figures Figure 6.9. Schematic configuration of the growth state of the anodic film formed in phosphoric acid at different anodic voltages using a GDOES depth profile; the correlation for the crystallinity and electrolyte is included Figure 7.1. Voltage time curve during anodizing of titanium at 20 ma cm -2 in the NaTESi electrolyte at ambient temperature 144 Figure 7.2. Scanning electron micrographs of titanium surface anodized at 20 ma cm -2 in NaTESi electrolyte to 5 V (a), 10 V (b), 20 V (c) and 40 V (d) Figure 7.3. Raman spectra of titanium anodized in the NaTESi electrolyte at 20 ma cm -2 to 5, 10, 20 and 40 V respectively Figure 7.4. Transmission electron micrographs of cross-section of the anodic film formed on titanium in NaTESi electrolyte at 20 ma cm -2 to 10 V; (a) cross-section of the anodic oxide film at the Ti / Pt interface; (b) high magnification image of the titanium / anodic oxide interface with the diffraction pattern; (c) high magnification image of the Ti / TiO 2 interface at another region with the diffraction pattern Figure 7.5. GDOES elemental depth profiling analysis of the anodic films formed on titanium in NaTESi electrolyte at 20 ma cm -2 to 5 (a), 10 (b), 20 (c) and 40 V (d) in NaTESi electrolyte; the intensities of oxygen and silicon are multiplied by Figure 7.6. Experimental and simulated results of Rutherford backscattering spectroscopy for titanium anodized at 20 ma cm -2 to (a), 5 V; (b), 10 V; (c), 20 V and (d), 40 V in NaTESi electrolyte respectively Figure 7.7. Experimental results of nuclear reaction analysis for titanium anodized at 20 ma cm -2 to (a), 5 V; (b), 10 V; (c), 20 V and (d), 40 V in NaTESi electrolyte respectively Figure 7.8. Bode plots, measured in the NaTESi electrolyte, of the anodic films formed after anodizing to 5 (a) 10 (b) 20 (c) and 40 (d) V on titanium Figure 7.9. Schematic drawing of the equivalent circuits of anodized titanium in the NaTESi electrolyte after EIS measurements; anodic films formed after anodizing to 5, 10 and 20 V; b to 40 V Figure Scanning electron micrographs at different magnifications of titanium surfaces after anodizing to 10 V (a - b) and 20 (c - d) V followed by degradation treatment in a continuous climatic chamber with a humidity of 90% at 50 o C Figure Single-lap adhesive bonding tests of as-received titanium (1) and specimens anodized to 10 V (2) and 20 V (3) Figure Scanning electron micrographs at different magnifications of titanium surfaces anodized to 10 V (a, b) and 20 V (c, d) after adhesive bonding tests; EDS results are also presented at different regions Figure 8.1. Scanning electron micrographs at different magnifications of Ti6Al4V alloy after different surface treatments; a) after etching of the as-received alloy in 48% HF + 70% HNO 3, b) higher magnification; c) after rf-gd sputter cleaning for 10 s at 35 V, d) higher magnification.170 Figure 8.2. Transmission electron micrographs of cross-section of as-received Ti6Al4V alloy (a), and (b) higher magnification. The α and β phases are highlighted in the images respectively Figure 8.3. EBSD results of the Ti6Al4V alloy after mechanical polishing + etching treatment; a) scanning electron micrograph of an interested region; b) inverse pole figure map; c) phases map; d) phases showing 96% of hcp α structure and 4% of bcc β structure; e) pole figures Figure 8.4. Voltage time response of Ti6Al4V during anodizing in the NaTESi electrolyte at ambient temperature Figure 8.5. Scanning electron micrographs of the Ti6Al4V alloy etched in 48% HF + 70% HNO 3, and anodized to 10 V (b), 20 V (c), 30 V (d) and 40 V (e) in the NaTESi electrolyte; the regions of EDS analysis are highlighted by solid frames Figure 8.6. Raman spectra of the as-received Ti6Al4V alloy, and alloy anodizing in the NaTESi electrolyte at 20 ma cm -2 to 10, 20, 30 and 40 V
12 List of Figures Figure 8.7. EDS line scanning (a) by TECNAI F30 transmission electron microscopy to determine the locations of the anodic film formed on the Ti6Al4V alloy after anodizing to 10 V in the NaTESi electrolyte. After the location of anodic film is known, the higher magnification images are displayed in the Figs. 8.7 (b), (c) and (d); a diffraction pattern focused on a specific region of the anodic film which is highlighted in the image (d) Figure 8.8. GDOES depth profiles of the Ti6Al4V alloy anodized to 10, 20, 30 and 40 V in the NaTESi electrolyte. The intensities of aluminium, vanadium, oxygen and silicon are multiplied by Figure 8.9. Rutherford backscattering spectra of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 (d) V in the NaTESi electrolyte Figure Nuclear reaction analysis diagrams of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 (d) V in NaTESi electrolyte Figure Bode diagrams with fitted curves of anodic films formed on the Ti6Al4V alloy after anodizing to 10 (a), 20 (b), 30 (c) and 40 (d) V in the NaTESi electrolyte Figure Schematic drawing of the equivalent circuits of the Ti6Al4V alloy anodized in the NaTESi electrolyte after the EIS measurements; (a), anodic films formed after anodizing to 10 V; (b), for the films formed after anodizing to 20, 30 and 40 V Figure Scanning electron micrographs at different magnifications of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 (d) V in the NaTESi electrolyte after degradation treatment in climatic chamber with a humidity of 90% at 50 o C for 1000 h immediately Figure Adhesive bonding tests of the as-received Ti6Al4V alloy and the alloy after anodizing in the NaTESi electrolyte to 10, 20, 30 and 40 V Figure Scanning electron micrographs of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 V (d) V in the NaTESi electrolyte after single-lap shear bonding tests immediately. EDS spectra were detected in the regions shown by numbers; high magnification images are obtained in the regions highlighted by the squares Figure 9.1. (a) Scanning electron micrograph of sputter-deposited titanium surface on electropolished aluminium, (b) transmission electron micrograph of cross-section of sputter-deposited titanium layer Figure 9.2. Voltage time response of anodic oxide growth of sputter-deposited titanium on electropolished aluminium in 1 M H 3 PO 4 at 20 ma cm -2 at ambient temperature Figure 9.3. Transmission electron micrographs of the sputter-deposited titanium anodized in 1 M H 3 PO 4 to 10 (a), 30 (b) and 50 V (c) Figure 9.4. Transmission electron micrographs of the sputter-deposited titanium anodized in 1 M H 3 PO 4 to 80 V (a), 100 V (b), 130 V (c) and 150 V (d); EDS was scanned at a region of Al 2 O 3 /TiO 2 interface after anodizing to 80 V, highlighted by a circle solid line Figure 9.5. Transmission electron micrographs of the regions of the titanium layers where were ruptured completely after anodizing to 80 V (a) and 100 V (b) in 1 M H 3 PO Figure 9.6. Transmission electron micrographs of anodic film formed on sputter-deposited titanium in 1 M H 3 PO 4 after anodizing to 100 V; (a) highlighted different regions, (b) a region at TiO 2 / Al 2 O 3 interface, (c) a region at Ti / TiO 2 interface, (d) another region at TiO 2 / Al 2 O 3 interface, (e) a region containing an oxygen bubble Figure 9.7. Transmission electron micrographs of the 290 nm thick sputter-deposited titanium anodized at 20 ma cm -2 to 30 V in 1 M H 2 SO 4 (a) and H 3 PO 4 (b) Figure 9.8. GDOES depth profiles of ~100 nm thick sputter-deposited titanium after anodizing to 30 V in H 2 OS 4 (a) and H 3 PO 4 (b) Figure 9.9. GDOES depth profiles of ~290 nm thick sputter-deposited titanium after anodizing to 30 V in H 2 OS 4 (a) and H 3 PO 4 (b) Figure Schematic diagram of titanium and aluminium anodic films formed on the sputter-deposited titanium layer and the aluminium substrate; a) anodizing from 10 to 50 V, showing that the titanium anodic film is thickening with the formation of a thin aluminium anodic film at the Ti / Al interface; b) anodizing from 50 to 100 V, titanium 12
13 List of Figures anodic film becomes thicker with the thinning of sputtering titanium layer and the thickening of aluminium anodic film; c) anodizing from 100 to 150 V, revealing that the titanium anodic film becomes thinner with significant rupture of most of the sputtering titanium layer; d) anodizing over 80 V, showing a completely ruptured region of sputtering layer where the titanium anodic film could not form, and a thicker aluminium anodic film could be generated Figure Scanning electron micrographs in SE mode (a) and in STEM mode (b) at very low magnification located on the ultramicrotomed sections on a copper supported grid. SE mode was used to identify the location of the titanium sections.208 Figure Current-time responses of anodizing of titanium at 10 and 50 V in 1 M H 3 PO 4 for 900 s at ambient temperature Figure STEM-in-SEM micrographs at a low magnification (a) and a high magnification (b) of anodic film formed on titanium after anodizing at 10 V in 1 M H 3 PO 4 for 900 s Figure Scanning electron micrographs at different modes and different magnifications of anodic film formed on titanium after anodizing at 50 V in 1 M H 3 PO 4 for 900 s, a) Inlens mode; b) SE mode; c) STEM mode; d) low magnification in STEM mode; e) high magnification in STEM mode Figure An example of a specimen with a notched region in STEM-in-SEM a thin section of 15 nm thick was ultramicrotomed in cross-section and collected on a copper supported grid. (a) Low magnification of STEM-in-SEM image showing the depth of the notch. (b) High magnification of STEM-in-SEM image showing the effect of the propagation of notch morphology induced by diamond knife during ultramicrostomed processing Figure Transmission electron micrographs with diffraction patterns of different regions (a & b) of stripped anodic film from titanium after etching in bromine-methanol electrolyte for 300 s, the anodic film formed on titanium after anodizing at 20 V for 900 s in 1 M H 2 SO 4 at ambient temperature 219 Figure Scanning electron micrographs of as-received (a) and etched titanium in bromine-methanol electrolyte for 10 s (b), 30 s (c), 120 (d) and 300 (e) at ambient temperature Figure Electron backscatter diffraction mapping of etched titanium in brominemethanol electrolyte for 10 s Figure (a) Weights and weight losses obtained from the titanium specimens before and after etching in bromine-methanol electrolyte for different durations; (b) corrosion rates measured from weight loss records Figure Backscattering electron micrographs of etched titanium in bromine-methanol electrolyte for 10 (a), 30 (b), 120 (c) and 300 s (d); an EDS spectrum was scanned in a corroded region of titanium after etching for 300 s, highlighted by a solid square Figure Scanning electron micrographs of cross-section of titanium after etching for 30 (a) and 300 s (b); EDS spectra were scanned on corroded and non-corroded regions of etched titanium for 300 s, which are highlighted by solid squares in image (b) Figure White light interferometry profiles of etched titanium in bromine-methanol electrolyte for 10 s Figure X-ray diffraction patterns of as-received titanium (a) and titanium after etching for (b) 10 s, (c) 30 s, (d) 120 s and (e) 300 s
14 List of Abbreviations List of Abbreviations SE BSE SEM EDS TEM STEM EIS GDOES DC EDTA FFT XPS XRD FIB SCE wt% at% HCP BCC TiO 2 NaTESi Secondary electron Backscattered electron Scanning electron microscopy Energy-dispersive X-ray spectroscopy Transmission electron microscopy Scanning transmission electron microscopy Electrochemical impedance spectroscopy Glow discharge optical emission spectrometry Direct current Ethylenediaminetetraacetic acid Fast Fourier Transformation X-ray photoelectron spectroscopy X-ray diffraction pattern Focused-ion beam Saturated calomel electrode Weight percent Atomic percent Hexagonal close packed Body centred cubic Titanium dioxide NaOH + Na-tartrate + Na(SiO) 3 + EDTA 14
15 Abstract Abstract The anodic films formed on commercially pure titanium in sulphuric and phosphoric acids using a combination of potentiodynamic polarization and potentiostatic anodizing were investigated. Single-barrier anodic films were created in sulphuric and phosphoric acids from 10 to 60 V respectively. An amorphous-to-crystalline transition occurred within the films during the polarization and anodizing processes. Oxygen evolution was initiated within both stages, leading to the suppression of current efficiency for the growth of anodic films. The crystalline phases assisted gas bubbles to develop within the film, resulting in the formation of the blister textures. The rupture of the anodic film was found from anodizing at 20 V in the sulphuric acid but occurred at 50 V in the phosphoric acid. Further, the amorphous-tocrystalline transition could be impeded more by the phosphate incorporated into the anodic film from anodizing in the phosphoric acid compared with the sulphate incorporated from the sulphuric acid. The corrosion behaviour of the anodic oxide films formed on CP-Ti was studied in a near-neutral aerated 3.5% NaCl electrolyte. Ruptures and blisters of the films were found as a result of the release of a huge pressure by the bursting of oxygen bubbles. More ruptures were observed when anodizing to higher anodic voltages in the sulphuric and phosphoric acids. Further, the anodic films showed more ruptures after the anodized titanium specimens at higher anodic voltages were immersed for 60 days in the NaCl electrolyte compared with the immediate immersions. Additionally, the corrosion behaviours of the anodic films were examined by potentiodynamic polarization and electrochemical impedance spectroscopy. The corrosion resistance of the anodized titanium in the NaCl electrolyte increased with increased anodic voltage. For the same voltage, the film generated in the phosphoric acid showed a higher corrosion resistance compared with the sulphuric acid. It indicates that the corrosion protection by anodizing of titanium in the phosphoric acid should probably be considered preferential to the sulphuric acid. Porous anodic films were formed on CP-Ti after anodizing at 100, 150 and 200 V for 900 s respectively. The thickness of anodic porous film increased with increase of anodic voltage. Nano-particulates were found within the pores; the size and quantity of the pores increased due to the dissolution of the particulates. The amorphous-tocrystalline transition was initiated during anodizing. It was revealed that the degree of crystallinity was greater at a higher voltage. An increased content of phosphorus species was incorporated into the porous oxide film as the voltage increased, stabilizing the development of nanocrystals. The formation of anodic oxide films on CP-Ti in the NaTESi electrolyte at a constant current density of 20 ma cm -2 was investigated in the current thesis. Barrier-type titanium anodic films generated after anodizing to 5, 10 and 20 V were of thickness 15
16 Abstract 30, 37 and 67 nm respectively. Further, a porous anodic film of ~80.0 nm thickness was generated after anodizing to 40 V. Significant amounts of sodium species were found, which were incorporated into the anodic films, and the content increased with increased anodic voltage. The current efficiency for the film growth was reduced at higher anodic voltages due to the formation of crystalline phases and more oxygen generation. The degree of crystallinity of the anodic film increased at higher voltages. The dielectric permittivity of the anodic film was estimated as ~2.35 according to EIS and the TEM evidence. The degradation test was carried out in a continuous climatic chamber with a humidity of 90% at 50 o C. The anodic films formed on CP- Ti in the NaTESi electrolyte showed an excellent degradation resistance since no film damage was evident. Single-lap bonding tests were operated for the study of the adhesion joint performance, and the bonding strength increased with increase of the voltage associated with a thicker anodic TiO 2 coatings. The production of a thicker anodic film would increase the interfacial bonding strength. The formation of anodic oxide films on the Ti6Al4V alloy in the NaTESi electrolyte at a constant current density of 20 ma cm -2 was studied after the research investigations for the CP-Ti. An anodic film with shallow pores was formed after anodizing to 10 V. Porous anodic films were created after anodizing to 20, 30 and 40 V respectively. Significant amounts of sodium species were incorporated into the films, and the content increased with increase in voltage. The current efficiency for the anodic film growth increased from 10 to 30 V but decreased from 30 to 40 V due to oxygen evolution. The film thicknesses determined by Rutherford backscattering spectroscopy were ~15 nm, ~39 nm, ~1100 nm and 1800 nm for voltages of 10, 20, 30 and 40 V respectively. The film thickness at 10 V showed good agreement with 11 nm which was evident by TEM. The degree of crystallinity of the films was greater at a higher voltage. The dielectric permittivity of the film was estimated as ~118 according to the results of TEM and EIS. The degradation test was carried out in a continuous climatic chamber with a humidity of 90% at 50 o C. Without the evidence of damages, the anodic films formed on Ti6Al4V alloy in the NaTESi electrolyte showed an excellent degradation resistance. In addition, it was evident that the film formed after anodizing to 40 V was crystallized at the thermal temperature of 50 o C after the 1000 h degradation test. Single-lap bonding tests were employed to compare the strength of adhesively joined titanium alloy anodized with different film thicknesses, the results revealing a significant benefit from a thicker film. The ~100 nm thick 99.6% pure titanium layers were sputter-deposited on electropolished aluminium substrates by DC magnetron sputtering technique in order to investigate the anodic film growth behaviour of titanium in 1 M H 3 PO 4. The titanium anodic film and the sputter-deposited layer were ruptured by the bursting of oxygen bubbles. The phosphoric acid electrolyte penetrated into the ruptured regions of the sputter-deposited titanium layer, leading to the anodic oxide growth on aluminium. The thickness of the titanium anodic film increased from 10 to 100 V but 16
17 Abstract decreased from 100 to 150 V due to the rupture of the titanium layer. Above 80 V, some regions of the titanium layer where were completely ruptured did not generate the titanium anodic film. Consequently, a thin titanium layer would not provide sufficient current efficiency for the growth of the titanium anodic film when anodizing above 80 V due to the development of oxygen evolution. Further, due to the direct contact with the electrolyte, a thicker aluminium anodic film was formed on the regions of the titanium layer where had ruptured. Applications of STEM-in-SEM for morphology analysis of anodic oxide film on titanium were explored. Important structural details of anodic films with high quality images were obtained using the STEM-in-SEM technique, enabling the study of film morphologies, film thicknesses and oxygen bubble features. Combining the images with flexible magnification ranges in the STEM-in-SEM, it was possible to study a large-scale morphology of the anodic film. Additionally, a 6-specimen carousel holder would provide an increase in productivity by ~20% compared with a conventional single-specimen STEM or TEM. An air-formed oxide film was stripped from CP-Ti substrate by chemical etching in the bromine-methanol electrolyte, exposing the bare titanium substrate and grain boundaries with defects at specific regions. Without the protective air-formed TiO 2 film, pitting corrosion occurred on the bare titanium substrate due to the attack of bromine. The corrosion pits propagated with etching time from 10 to 300 s and were displayed using white light interferometry. Increased surface roughness was identified with etching time due to the occurrence of more pitting corrosion attacks. Bromine species and TiBr 4 compounds were detected by EDS and X-ray diffraction patterns, indicating that the dissolution of the titanium substrate was induced in each etching treatment. 17
18 Declaration Declaration I, Zuojia Liu, declare that no portion of the works relevant in this thesis has been submitted in support of an application for another degree or qualification of this or any other university or any other institution of learning. Zuojia Liu (2015) 18
19 Copyright Statement Copyright Statement i. The author of this thesis (including any appendices and/or schedules to this thesis) owns certain copyright or related rights in it (the Copyright ) and he has given The University of Manchester certain rights to use such Copyright, including for administrative purposes. ii. Copies of this thesis, either in full or in extracts and whether in hard or electronic copy, may be made only in accordance with the Copyright, Designs and Patents Act 1988 (as amended) and regulations issued under it or, where appropriate, in accordance with licensing agreements which the University has from time to time. This page must form part of any such copies made. iii. The ownership of certain Copyright, patents, designs, trademarks and other intellectual property (the Intellectual Property ) and any reproductions of copyright works in the thesis, for example graphs and tables ( Reproductions ), which may be described in this thesis, may not be owned by the author and may be owned by third parties. Such Intellectual Property and Reproductions cannot and must not be made available for use without the prior written permission of the owner(s) of the relevant Intellectual Property and/or Reproductions. iv. Further information on the conditions under which disclosure, publication and commercialisation of this thesis, the Copyright and any Intellectual Property and/or Reproductions described in it may take place is available in the University IP Policy (see in any relevant Thesis restriction declarations deposited in the University Library, The University Library s regulations (see and in The University s policy on Presentation of Theses. 19
20 To my parents Especially to my wife, I-Ling Tsai, I am grateful for her encouragement and love. 20
21 Acknowledgements Acknowledgements First, I would like to gratefully acknowledge my supervisor, Professor. George. Edward. Thompson, for his guidance and patience throughout my graduate study in The University of Manchester. I appreciate his time and valuable knowledge I learned from him. I would be impossible to finish this thesis without his inspiration, encouragement and advice. I also would like to thank my co-supervisor, Professor. Peter Skeldon, for his valuable comments and suggestions on my work. The technicians of the Corrosion and Protection Centre (Teruo Hashimoto, Shirley Zhong and Hong Liu) are also acknowledged, without their help and guidance, the researches cannot be completed. I would like to thank my friends in the office D10, The Mill (Uyime Donatus, Junjie Wang, Heike Krabs, Dawod Elabar and Aneta Nemcova), who always supported and encouraged me during my study until I finished my thesis. I would like to thank my parents for their supportive and patience with all of my problems. I appreciate their understanding and encouragement. Also, I would like to take this opportunity to thank LATEST2 Programme Grant, EPSRC for their financial support of my PhD in The University of Manchester. Finally, I would like to thank my wife, I-Ling Tsai, who took care of me during my most difficult time, and I appreciate for her love and encouragement. 21
22 Chapter 1 Introduction Chapter 1 Introduction 1.1. Background The process of corrosion influences various metals during their service in different applications. A study on the cost of corrosion in the worldwide economy was reported in Now is the Time, published recently by the World Corrosion Organization, which stated that the annual corrosion cost to the whole world was estimated as US $2.2/ UK 1.3 trillion or over 3% of the world s GDP [1]. Most corrosion issues exist in high-risk areas such as aircraft and oil pipelines. Simultaneously, some corrosion experts have concluded that a net of 20 to 25% of that annual cost can be saved by applying currently available corrosion control technologies [1]. Therefore, it is increasingly important to study the corrosion protection methods for the materials widely used in aircraft, oil and gas and chemical industries nowadays. One of my responsibilities in the present thesis is find out and understand the solutions for materials corrosion and surface protection. Nowadays, surface treatments of materials have been considered in the research fields of corrosion protection. The main surface treatment methods include applied coatings, reactive coatings, bio-film coatings and anodic oxidations. The appearance, surface quality and corrosion resistance of most metal profiles are improved by the application of such surface treatments. Even though some metals have an excellent corrosion resistant, surface treatments can be still utilized to modify other properties such as surface structure, colour, hardness, wear resistance, adhesive bonding performance, reflectivity and dielectric properties [1]. Further, in most cases, metals need to be adhesively bonded together to meet the demands from the current industrial process requirements. However, the failure effects such as cohesive fracture or adhesive fracture brought on materials adhesion have been reported that lead to the huge cost of construction [2]. It has been reported that cohesive fracture can be obtained from the crack propagation in the bulk polymer which constitutes the adhesive [3]. Further, the adhesive fracture is found that it occurs during debonding between the adhesive and the adherend [4]. Therefore, the research on surface treatments of relevant materials is required for the promotion of adhesion performance. 22
23 Chapter 1 Introduction Light alloys such as titanium alloys are widely used in aerospace applications. Saving the weight and increasing the corrosion resistance are the major reasons for choosing titanium alloys in the fuselage applications, thus making use of the high specific strength of those alloys. Therefore, investigations of surface treatments on titanium and its alloys have attracted attention. Titanium is named by Titans, the powerful sons of the earth in Greek mythology; it is the fourth most abundant metal on the Earth s crust (~0.86%) after aluminium, iron and magnesium [5]. Titanium shows a great immunity to environmental attack, regardless of pollutants. Where other metals exhibit limited life-spans, titanium endures. It withstands urban pollution, marine environments and the sulphur compounds of industrial areas, and it is failure-proof in even more aggressive environments. Because it is one of the most noble metals, the coupling of titanium with dissimilar metals does not accelerate galvanic corrosion of the titanium [6]. These properties make titanium perfect for use in aerospace applications nowadays. Titanium and its alloys are widely used for structural components and fasteners in aerospace applications [7]. The components may be adhesively bonded to composites and/or they may be painted [7]. Figure 1.1 charts the steady growth of titanium use in Boeing commercial aircraft since titanium has been introduced to fuselages in It shows that the Boeing aircraft series such as 707, 727, 737, 747, 747-SP (Special Performance), 757, 767 and 777 are all demanding titanium materials. Since 2000, it accounts for approximately 9% of the structural weight of the Boeing 777 manufacture [8]. 23
24 Chapter 1 Introduction Figure 1.1. Increase in application of titanium alloys in commercial Boeing aircraft [8]. Figure 1.2 shows the percentage of structural weight for different material classes in a modern large commercial aircraft, which are distinguished between airframe and engine materials. The fuselage of the Airbus A330/340 was manufactured using nearly two thirds of aluminium. At about 7%, titanium alloys have a similar share of the structural weight as steels. However, at over a third of the structural weight, titanium is the second most common material in the jet engine following Ni-based super-alloys. On the other hand, titanium alloys are the most abundant materials in the engine manufacture in the volume [8]. Figure 1.2. Percentage of aluminium, titanium, and steel alloys and carbon fibre reinforced polymer (CFRP) of the structural weight of modern large commercial aircraft and gas turbine engines [8]. 24
25 Chapter 1 Introduction Due to the excellent corrosion resistance, titanium and its alloys also attracted increasing attentions in the oil and gas industries for the manufacture of heat exchangers, automotive fields, biomedical prostheses or architectural constructions [9]. However, commercial production of titanium did not start until the 1950 s. At that time, titanium was only recognized for its strategic importance as a unique lightweight, high strength alloyed and structurally efficient metal for critical, highperformance aircraft, such as jet engine and airframe components. Today, titanium alloys are common, readily available engineering materials that compete directly with other metals such as aluminium alloys, stainless steels, copper alloys, nickelbased alloys and carbon fibre composites [10]. Extensive use of titanium for structural elements of spacecraft began with the early Mercury and Apollo programs, and titanium alloys continued to be widely used in space and nuclear plants. In addition to manned spacecrafts, titanium alloys are extensively employed in solid rocket booster cases, guidance control pressure vessels and a wide variety of applications demanding lightweight and reliability [11]. Even though titanium and its alloys have shown an excellent corrosion resistance, they still have weaknesses such as a poor wear resistance, and they can exhibit a poor corrosion resistance in some aggressive environments such as high-temperature reducing acids. A susceptibility to pitting/crevice corrosion in hot chloride or bromide solutions also restricts their use in certain offshore and chemical applications [12]. Adhesive connections are currently widely accepted as an extremely valuable tool in mechanical design, allowing the production of joints with a very good strength to weight ratio [13]. Aerospace, one of the largest industries cross the World, is among those industries that use adhesive techniques extensively, gaining benefits from the ability to join various different materials and the relatively simple manufacturing process, while reducing the weight [13]. As already stated, the aerospace industry is a large market for titanium products primarily due to the exceptional strength-toweight ratio, and high resistance to elevated temperatures and corrosion. Often in the fabrication processes, titanium sheets are joined by adhesives rather than by welding or riveting. The acceptance of adhesives as a high performance engineering material has grown steadily in the last few decades [14]. Adhesives contribute highly to 25
26 Chapter 1 Introduction structural integrity, ease of manufacturing, enhanced performance, improved safety, cost and time savings. The reasons for this are numerous and include the continuity of the adhesive bond. Therefore, upon loading, there is a more uniform distribution of stresses over the bonded area [14, 15]. However, the main problem with the application of adhesives for spacecraft and even aerospace is the thermal temperatures. This is caused by the aerodynamic friction heating of the structure as it moves through the air [16]. The commercial use of surface pre-treatments to improve the bondability of titanium and other metals was established [17]. The first step is to employ mechanical abrasion and solvents to remove contaminants such as millscale, rolling lubricants and adventitious surface impurities. This should create a clean, natural oxide surface. However, the oxide surface cannot afford optimum bond strength to structural adhesives, especially when the bond is exposed to moisture. Significant improvements in bond durability in aqueous environments have been achieved using a variety of chemical and electrochemical pre-treatments, of which the most useful have been listed in the literature [17]. Developments in titanium alloy environmental behaviour over the past few years have targeted cost reduction, corrosion resistance enhancement, and/or achievement of a synergistic combination of both higher mechanical performance and elevated corrosion resistance in titanium materials [18]. The usually excellent corrosion resistance of titanium under normal conditions results from the formation of very stable, continuous, highly adherent and protective oxide films on the surface. It has been considered that any surface treatment that can thicken and toughen the protective oxide film, such as anodizing treatments, will improve the corrosion resistance of titanium and its adhesive bonding behaviour [12]. Recently, research groups examining the protection of surfaces of titanium or titanium alloys, and more particularly in improving the anodizing methods for providing an anodic coating on such surfaces have reported their findings [19]. However, the growth mechanisms of anodic coatings on titanium and its alloys still need to be studied further to understand the useful knowledge for adhesive bonding promotion. Moreover, a particular anodizing process, called NaTESi, developed 26
27 Chapter 1 Introduction by Matz for structural bonding of titanium, was studied by previous researchers on titanium alloys [20]. It has been reported that a nano-porous morphology with a cell diameter between 30 and 70 nm could be generated after titanium was anodized in the NaTESi electrolyte [21]. It has also been shown that the NaTESi anodized surface of a titanium alloy displays an inhomogeneous and open porous basket wave structure, but some areas of the surface are not nano-structured but dense, and the anodic oxide showed an improved long term bonding durability for titanium alloys [21]. Although the literature reported the anodizing trials of metals in the NaTESi electrolyte, the knowledge from them is very limited, and the anodizing mechanisms on commercially pure titanium and Ti6Al4V alloy processed in the NaTESi electrolyte remain the subject of discussion. Thus, the present thesis will also deal with the exploration of the anodizing behaviour of different titanium materials in the NaTESi electrolyte, with the characterizations of the anodic oxide films that are formed and the possible promotion of the adhesive bonding properties. To be familiar with the anodizing mechanism of titanium materials, the physical and chemical properties, formation mechanism of different types of anodic oxide films, oxygen evolution phenomenon and possible adhesion bonding techniques are introduced in the Literature Review Research Objectives The objectives of the thesis mainly aim to characterize the growth behaviours of anodic oxide film of CP-Ti in different electrolytes and Ti6AlV alloy in the NaTESi electrolyte using different techniques. Firstly, the growth behaviour of anodic oxide film on CP-Ti in sulphuric and phosphoric acids were characterized. Secondly, the corrosion behaviour of the anodic oxide films formed on CP-Ti in the 3.5% sodium chloride electrolyte was studied. Thirdly, characterizations of porous anodic oxide films formed on CP-Ti at breakdown anodic voltages were investigated. Fourthly, the growth behaviours of CP-Ti and Ti6Al4V alloy in the NaTESi electrolyte were investigated respectively. The anodic oxide films produced in the NaTESi electrolyte of the CP-Ti and the Ti6Al4V alloy were also investigated by the associated adhesive bonding experiments in order to examine the joint bonding surface performance before and after anodizing. Fifthly, the growth behaviour of anodic oxide films on sputter-deposited titanium substrate was evaluated. Sixthly, 27
28 Chapter 1 Introduction applications of scanning transmission electron microscopy technique in scanning electron microscopy on anodic film characterizations of pure titanium were explored. Finally, the corrosion behaviour of bare CP-Ti substrate in the bromine-methanol electrolyte was studied Layout of Thesis Chapter 2 presents the literature review that reports the mechanical, chemical and physical properties of titanium and its alloys. The growth mechanisms of the anodic oxide film on titanium materials are also documented and summarized in the literature review. Further, the oxygen evolution of anodizing processes and possible adhesion bonding joint techniques are introduced. Chapter 3 describes the experimental methods and procedures for all of the tests and the conditions of facilities for the researches in the respective chapter. Chapter 4 is the part of the results and discussion of the barrier-type anodic oxide film growth on CP-Ti in sulphuric and phosphoric acids. The anodizing mechanisms for barrier film growth of titanium in the two acid electrolytes are studied and discussed. Chapter 5 presents the results and discussion of the corrosion behaviour of CP-Ti in a near neutral-aerated 3.5% NaCl environment after anodizing galvanostatically in sulphuric and phosphoric acids to selected anodic voltages. Chapter 6 investigates the porous-type anodic oxide films formed on CP-Ti under dielectric breakdown conditions. The growth mechanism of porous anodic films on cp-ti is studied and discussed. Chapter 7 is devoted to the experimental study of anodic oxide film growth of CP-Ti in the NaTESi electrolyte with associated degradation and adhesive bonding behaviours. 28
29 Chapter 1 Introduction Chapter 8 studies and discusses the anodizing mechanism of Ti6Al4V alloy in the NaTESi electrolyte with associated degradation and adhesive bonding behaviours. Chapter 9 explores the anodizing behaviour of sputter-deposited pure titanium on electropolished aluminium in phosphoric acid electrolyte. Chapter 10 provides the results and discussion related to the applications of the STEM-in-SEM technique for morphology analysis of anodic film growth on titanium. Chapter 11 demonstrates the corrosion behaviour of bare CP-Ti substrate after the air-formed oxide film is stripped off in the bromine-methanol electrolyte and discusses the corrosion mechanism for the titanium substrate. Chapter 12 presents the main conclusions of the present work and suggestions for future work. 29
30 Chapter 2 Literature Review Chapter 2 Literature Review 2.2. Properties of Titanium and Its Alloys Pure titanium experiences an allotropic transformation from hexagonal close packed (α) to body centred cubic (β) (Figure 2.1) when the temperature is raised up to ~882 o C. Alloying elements that are dissolved in titanium can either i) stabilize the α phase by raising the α β transition temperature, ii) stabilize the β phase by lowering the α β transition temperature, or iii) act only as solid solution strengtheners and not affect the transition temperature. Of the interstitial elements, nitrogen, carbon and oxygen especially have a strong α stabilizing effect and, thereby, raise the transition temperature. Interestingly, hydrogen, which has a β stabilizing effect, reduces the transition temperature. Increasing the amount of interstitial elements leads to a dramatic increase in the strength of titanium alloys; however, a significant drop in ductility and the risk of embrittlement can be induced [22]. In the titanium alloys, the α phase and the β phase regions are not adjacent as they are in pure titanium. They are separated by a two-phase α + β region whose width increases as the solute concentration increases. Titanium materials are thus divided into three major groups, α alloys, α + β alloys and β alloys, depending on the type and amounts of alloying elements, which decide the dominating alloy phase at ambient temperature [23]. Figure 2.1. The two main crystal structures, hexagonal close packed (HCP) and body centred cubic (BCC), for the α and β phases of titanium [22, 24] 30
31 Chapter 2 Literature Review α Alloy Alpha titanium alloys are generally classified as unalloyed Ti (or known as commercially pure (CP) titanium), alpha alloys and near-alpha alloys. Within the first group, several grades of CP-Ti exist. The major difference between these grades is the concentrations of oxygen and iron. The oxygen is carefully controlled to obtain different strength levels in CP-titanium, ranging from 170 MPa (25 ksi) to 480 MPa (70 ksi) [22]. In addition, CP-Ti has an excellent corrosion resistance in applications where only modest strength is required [2]. Examples of such alloys corresponding to CP-Ti grade 1-4 are listed in Table 2.1 [22]: Table 2.1. Chemical compositions (wt%) of cp-titanium Grade 1 4 [22] Classification O N C H Fe Ti CP Ti Grade Bal. CP Ti Grade Bal. CP Ti Grade Bal. CP Ti Grade Bal. Alpha alloys contain stabilizing elements such as aluminium, tin, zirconium, and oxygen. These alloying elements give rise to significant solid solution hardening, thereby increasing tensile strengths by increments of MPa for each percent of the added element [25]. Alpha alloys are single phase materials. The hexagonal crystal structure possesses a high rate of work hardening that limits the formability [26]. Additionally, the alpha alloys also develop good welding behaviour. The general high aluminium content of this group of alloys ensures excellent strength characteristics and oxidation resistance at elevated temperatures. Further, alpha alloys cannot be heat-treated to develop higher strength since they are single phase alloys [22]. An example relating to pure titanium is reported in a literature [27]. The typical microstructure of commercially pure titanium can be observed using scanning electron microscopy, as shown in Figure 2.2. The typical grain boundary α, fine acicular α, Widmanstätten α and serrated α structures are observed and labelled by A, B, C and D respectively. 31
32 Chapter 2 Literature Review 250 µm Figure 2.2. Scanning electron micrograph showing the typical microstructue of CP-Ti: grain boundary α (A), fine accicular α (B), Widmanstätten α (C) and serrated α (D) [27] α + β Alloy The α + β alloy is composed of one or more α stabilizing or α soluble elements together with one or more β stabilizing phases. At ambient temperature, the equilibrium of the dual phase alloys depends on the amount and type of the β stabilizing elements [23]. The two-stabilizer titanium alloys can be strengthened significantly by heat treatment consisting of a quench from a temperature in the alpha-beta range, followed by an aging cycle at a reduced temperature. The beta-phase transformation normally occurs on slow cooling; however, it can be suppressed by the quenching. Further, the aging cycle induces the precipitation of some fine alpha particles from the metastable beta, imparting a structure that is stronger than the annealed alpha-beta structure. Even though heat-treated alpha-beta alloys are stronger than the alpha alloys, their ductility is proportionally lower [28]. Ti-6Al-4V is recognized as a powerful horse in the titanium industry because it is by far the most common Ti alloy, accounting for more than 50% of total titanium usage in the aerospace industries [29]. Ti-6Al-4V alloy is a duplex structured titanium alloy, which contains the hexagonal close packed (HCP) α phase and the body-centred cubic (BCC) β phase; it has been also widely used in the biomedical, pipeline, sporting goods and marine military fields. The Ti-6A-4V alloy exhibits a 32
33 Chapter 2 Literature Review wide range of microstructures and it is considered to be a heat treatable Ti alloy. Depending on heat treatment conditions, the α / β volume ratio and chemical compositions of the respective constituent phases can be adjusted to yield different properties [30, 31]. The β phase mainly transforms into a globular type of α for very slow cooling rates from high in the α + β region or above the β-transition temperature (99.5 o C ± 20 o C). Raising the cooling rate strengthens the α nucleation rate in the β grain boundaries, thereby enhancing the formation and growth of α platelets into the prior β grains. The length and width of these α platelets are determined by the cooling rate; an increased cooling rate enhances the nucleation rate and reduces the diffusion process (growth rate). For the age hardening purpose, the cooling rate determines whether the precipitation of coherent Ti 3 Al particles proceeds. At a certain point, the cooling rate is also sufficiently fast for nucleation of α to occur inside the prior β grains, leading to the formation of the basket weave structure. Finally, during the rapid cooling, the β phase will fully or partly transform into a martensitic type of α. Further, this martensite exists in two different forms of α having an hexagonal structure and α having an orthorhombic crystal structure [26, 32]. The type and amount of α and/or α formed during quenching are dependent upon the chemical composition of the β phase existing at the temperature prior to quenching. The β phase experiences a large compositional variation, which is reflected in significant mechanical property changes [23]. Vanadium enrichment of the β phase occurs in proportion to the reduction of the volume fraction of β phase. At vanadium contents 15wt%, the β phase is stabilized and retains its bcc crystal structure during cooling. When the β phase with 10 ± 2wt% vanadium is quenched (from a temperature range of approximately o C), it partly retains the bcc structure and partly transforms into the soft orthorhombic α martensite. The higher the solution heat treatment temperature, the smaller the vanadium enrichment in the β phase, which leads to transformation into the hexagonal α upon quenching (from temperatures above 900 o C) [23, 33]. Figure 2.3 is a schematic illustration of the microstructures resulting from quenching at different temperatures. The Figure attempts to illustrate the principle transformations upon quenching; in reality, mixtures of both α, α and metastable/stable β can be generated, depending on the variations of chemical compositions [34]. The left diagram of Figure 2.3 indicates that the allotriomorph α 33
34 Chapter 2 Literature Review phase starts to generate when the temperature reduces below the β transition temperature. It then continues to grow along the β phase, which is dependent upon the cooling rate. If the cooling rate is very slow, only the globular type of α phase can be observed. However, with increasing cooling rate, the transformation into the platelet type of α phase occurs, as highlighted in the right diagram of Figure 2.3 [34]. Figure 2.3. A schematic illustration of microstructures transformation in Ti-6Al-4V after quenching at different temperatures [34]. A typical example with regard to an as-received microstructure of Ti-6Al-4V is shown in Figure 2.4 [23, 33, 35]; the sample for optical microscopy was prepared using conventional metallographic techniques and was etched in the Kroll s reagent (1% HF (hydrofluoric acid), 2% HNO 3 (nitric acid) and 97% de-ionized water). The optical micrograph shows that the α stabilizers are located in the dark areas, and the bright areas are associated with the β stabilizers. 34
35 Chapter 2 Literature Review Figure 2.4. Optical micrograph of the microstructure of Ti6Al4V alloy showing a bi-modal microstructure consisting of α surrounded by transformed β (530X) [23, 33, 35]. Figure 2.5 shows a further example of the SEM micrographs of an as-received Ti- 6Al-4V alloy and a situation after the heat treatment. The dark area is the α phase and the bright area is the β phase within the as-received Ti-6Al-4V alloy, as shown in Figure 2.5(a); the major phase is the α phase while the β phase occupies about 10 vol. %. For the heat-treated alloy, although the size of β phase became coarser, as displayed in Figure 2.5(b), the volume ratio between the α and β phases did not change appreciably. According to the pseudo-binary phase diagram of Ti 6%Al with varying V content [36], the β phase is the only phase stable at 1066 o C for Ti- 6Al-4V. Cooling from 1066 o C to room temperature results in the precipitation of the α phase and gives rise to the formation of a dual phase microstructure. The pseudobinary phase diagram also reveals that the solubility of vanadium in the α phase varies in a small range while that in β phase varies considerably as the solid solution annealed alloy is cooled to room temperature [37]. 35
36 Chapter 2 Literature Review Figure 2.5. Scanning electron micrographs displaying the microstructures of Ti-6Al-4V alloy: (a) as-received and (b) solution annealed at 1066 o C/1h + furnace cooling [37]. It has been reported [27] that the unalloyed titanium materials and the materials containing only aluminium exhibited similar microstructures; however, the addition of vanadium could alter the microstructure. The microstructure consists predominantly of very fine acicular α and some of this acicular α is delineated by intergranular β, as shown in Figure
37 Chapter 2 Literature Review Figure 2.6. Scanning electron micrograph showing typical Ti6Al4V microstructure containing very fine acicular α (E), fine acicular α and β (F) and prior-β grain boundaries (G) [27]. In another study [37], the results of EDS (energy-dispersive X-ray spectroscopy) analysis for the two constituent phases of α and β are listed in Table 2.2. It is shown that the compositions of α and β phases in the as-received and the heat-treated alloys are relatively similar. In both cases, the Ti and Al elements are enriched in the α phase. Table 2.2. Chemical compositions of the as-received and heat-treated Ti-6Al-4V alloy analyzed by EDS (wt%) [37]. Element (wt%) Heat treatment As-received Heat-treated Phase α Phase β Phase α Phase β Ti Al V β Alloy The bcc phase is stabilized with the transition-metal (TM) solutes, so all β alloys generally comprise large amounts of one or more of the so-called β - isomorphous forming additions, e.g. vanadium, niobium, tantalum (transition metals group V) and molybdenum (group VI transition metal) [23]. Beta titanium alloys form one of 37
38 Chapter 2 Literature Review the most versatile classes of materials with respect to processing, microstructure and mechanical properties. These alloys include stable beta, metastable beta and betarich α / β alloys. They offer an attractive alternative to the α / β alloys due to the increased heat treatability, wide and unique range of strength-to-weight ratios, deep hardening potential, and inherent ductility which is the result of their body centred cubic structure. In addition, they possess superior fatigue resistance as compared with α / β alloys. These alloys are broadly used in aerospace, power plant, sporting goods, automotive, orthopaedic implants, and downhole service applications [38]. Ti-15V-3Cr-3Sn-3Al (Ti-15-3) alloy is one of the most broadly used beta titanium alloys which was developed in the 1980 s [39]. Due to its high strength-to-weight ratio, excellent corrosion resistance and good cold deformability, such alloy attracts attention in different industrial applications such as aerospace and automotive industries [39]. Tan [40] examined the microstructure of the Ti-15-3 alloy by optical microscopy, which is displayed in Figure 2.7. The β grain boundaries formed with different sizes of grains can be observed clearly in the optical micrograph. Figure 2.7. Optical micrograph showing the microstructure of the as-received Ti-15V-3Cr- 3Sn-3Al (Ti-15-3) alloy [40] Titanium Dioxide Titanium oxide is known to have varying stoichiometries, and the common compounds are Ti 3 O to Ti 2 O, Ti 3 O 2, TiO, Ti 2 O 3, Ti 3 O 5 and TiO 2 [41]. This is a consequence of the fact that titanium exists in many different stable oxidation states 38
39 Chapter 2 Literature Review and that oxygen is highly soluble in titanium. The most stable titanium oxide is TiO 2, with titanium in the preferred oxidation state of 4 +. The dioxide is normally less than 5 nm (1 nm = 10-9 m) thick and comprised mainly an amorphous phase [42]. However, the high affinity of oxygen within titanium results in several oxides of various crystalline structures. In a natural atmosphere, the thermodynamically stable oxide is primarily TiO 2, which can exist in three crystalline structures of anatase (tetragonal), rutile (tetragonal) and brookite (orthorhombic) [43]. In general, the anatase structure can be obtained by anodic oxidations and the rutile structure can be created by anodic oxidation followed by a thermal treatment [41]. The schematic diagrams of the rutile and anatase bulk structures are displayed in Figure 2.8. In both structures, the basic building block consists of a titanium atom surrounded by six oxygen atoms in a more or less distorted octahedral configuration. The two bonds between titanium and oxygen at the top of the octahedron are slightly longer. A sizable deviation from a 90 o bond angle is observed in anatase. In the rutile structure, the neighbouring octahedral unit cells share one corner along the <100> plane and they are stacked with their long axis alternating by 90 o [44]. On the other hand, natural anatase and rutile contain impurities of up to approximately 2% that include iron, chromium, vanadium, aluminium, niobium, tantalum, hafnium and zirconium [45] and, account for slight variations in density, colour and refractive indices. Since most commercial titanium dioxide is manufactured from natural material by dissolution of the parent mineral and re-precipitation as fine particles with the structure of anatase or rutile (referred to as titanium dioxide-anatase or titanium dioxide-rutile), most but not all of these chemical impurities are generally removed [45]. Additionally, this protective oxide film is very stable over a wide range of ph, potential and temperature and, is especially favoured as the oxidizing character of the environment raises. For this reason, titanium generally resists mildly reducing, neutral and highly oxidizing environments up to reasonably high temperatures. It is only under highly reducing conditions where breakdown of the oxide film and resultant corrosion may occur. For example, the useful resistance of titanium and its alloys is limited in strong, highly-reducing acid media, such as moderately or highly concentrated solutions of HCl, HBr, H 2 SO 4, and H 3 PO 4, and in HF solutions at all concentrations, particularly as the temperature increases [46]. This substantially inert 39
40 Chapter 2 Literature Review surface oxide shows a high integrity and tenacity. Further, the oxide will, if scratched or damaged, immediately restore itself in the presence of air or water. Figure 2.8. Schematic diagrams showing the bulk structures of rutile and anatase. The stacking of the octahedral unit cells in both structures are shown on the right side [44] Anodizing of Titanium An oxide film can be grown on certain metals such as aluminium, niobium, tantalum, titanium, tungsten and zirconium by using an electrochemical process termed anodizing in appropriate electrolytes. For each of these metals, there are process conditions which generate a thin, dense, barrier oxide of uniform thickness. The thickness of this layer and its properties vary greatly depending on the metals. Numerous patents about anodizing technology have been published since 1950, especially concerning the anodizing of aluminium for colouring [47]. In the early years, anodic oxidation processes using direct current (DC) or alternating current (AC), in either chromic, sulphuric or oxalic acid electrolytes, have been reported [48]. Consequently, it was observed that additives such as metal salts including copper, nickel, silver, arsenic, antimony, bismuth, tellurium, selenium or tin lead to a change of the physical and mechanical properties and the colours [48]. Between 40
41 Chapter 2 Literature Review 1970 and 1990, studies by the Manchester research group (led by Thompson and Wood) resulted in a deep insight for the growth mechanisms of the anodic oxide layer, especially for anodizing of aluminium. This was achieved by the use of advanced techniques such as transmission electron microscopy (TEM), marker methods and ultramicrotome sectioning [48]. As mentioned earlier, anodizing is also one of the greatest surface treatments for titanium and its alloys. Specifically, in an anodizing cell, the titanium workpiece is made as an anode by connecting it to a positive terminal DC power supply. The cathode is connected to the negative terminal of the supply. The cathode can be a plate or rod of carbon, lead, nickel, stainless steel, aluminium alloy or any electronic conductor that is unreactive (inert) in appropriate anodizing baths. When the circuit is closed, electrons are withdrawn from the metal at the positive terminal, allowing ions at the metal surface to react with water to form an oxide film on the metal. The anodizing colours of titanium are described as interference effects [49]. Due to light interference phenomena at the metal oxide air interfaces, the colour of these oxide layers changes with film thickness [50]. The oxide thickness is found to be directly proportional to the applied voltage [49]. The principal of the interference effect occurring on the anodic layer of titanium can be described as follows: Figure 2.9. Interference between two waves reflected at both surfaces of an oxidised titanium metal substrate [51]. 41
42 Chapter 2 Literature Review Some of the light is reflected from the top surface (reflected light ray 1) and some light will travel through the oxide film and will be reflected from the metal surface (reflected light ray 2) as shown in Figure 2.9. Both waves will interfere; depending on the phase difference, the addition of waves can increase or decrease the reflectance. In addition, this phenomenon is wavelength dependent and it will differ over the spectral region for a given thickness of the oxide and causes a specific wavelength or hue to be observed [51]. On the other hand, depending on several factors, two main types of anodic oxide films can be produced, which are defined as a barrier-type film and a porous-type film Barrier-type Anodic Film Titanium barrier oxide films can be produced by anodizing in acidic and basic solutions under potentiostatic or galvanostatic conditions. In view of its applications, it is possible to produce thick and thin anodic oxide films on the surface of titanium [52]. Anodic oxide films with different colours can be generated in H 2 SO 4 and H 3 PO 4 solutions. The coloration of the oxide produced through anodic oxidation can be indicative of the film thickness. This relationship between colour and thickness depends significantly on the anodizing process and the nature of the electrolyte [53]. It is reported that anodic films produced in H 3 PO 4 contain low percentages of phosphorus [53]. It is also found that anodic oxide films formed on titanium in H 3 PO 4 are compact and electrically insulating, and their thicknesses are determined to be higher than for films formed in H 2 SO 4 [54]. The properties of the anodic oxide layers on titanium and its alloys can be tailored to desired applications by changing the anodizing parameters [55, 56]. Certain anodizing treatments may shape the morphology, structure and chemical composition of oxide films to enhance the use of titanium materials in aerospace applications [57]. Since the anodic oxide films formed on titanium and its alloys have been studied by a few previous investigators; therefore, it has been known that the thickness of the film appears to be a linear function of applied voltage [53, 58-60]. Some researchers also reported that the oxide film formed on titanium is basically amorphous in crystal structure and morphologically homogeneous [61]. Moreover, 42
43 Chapter 2 Literature Review this thin oxide layer results in an excellent resistance to corrosion indicated by: (1) electrochemically a low level of electronic conductivity [62], (2) thermodynamically great stability [63], and (3) low ion-formation tendency in aqueous environments [64]. Growth of the oxide film thickness results in systemic changes of the surface topography, particularly in the surface pore configuration [65], whereas anion incorporation into oxide films will modify the chemical composition as well as the crystal structures. The last has been exemplified by anatase, rutile, and even the rare brukite structures [66-68]. However, results reported in the literature give a rather complex and somewhat contradictory picture on the solid state properties and the electrochemical growth behaviour of anodic oxide films [69-71]. It is generally known that titanium behaves as a typical valve metal for which oxide growth involves field-assisted migration of ions through the oxide films and for the thickness of the anodic oxide to follow Faraday s law [72-74]. Previous literature has demonstrated that the anodic films formed on titanium involve an amorphous-to-crystalline transition during anodizing at relatively low voltages of less than 10 V [75-77]. The crystallization results in the formation of oxygen during further anodizing and, hence, a uniform film cannot be attained after crystallization. Direct evidence of the formation of crystalline oxide on titanium has been obtained by in-situ and ex-situ Raman spectroscopy [78, 79] and transmission electron microscopy (TEM) [77]. It has been reported [80] that crystalline anodic oxide layer on pure titanium was found after a potentiostatic anodizing treatment, as shown in Figure Large dark areas associated with crystallinity were evident and spots were generally recorded in the TEM diffraction patterns, regardless of the location of the electron beam [80]. 43
44 Chapter 2 Literature Review Figure Transmission electron micrographs and electron diffraction patterns of the stripped anodic oxide of pure titanium obtained after anodized at 25V for 60 min [80]. Anodic oxide films formed on titanium and its alloys are usually contaminated with species derived from the electrolyte anions. Metallic foreign species can also be incorporated in the anodic films from the substrates by alloying of titanium. In this case, highly contaminated anodic films can be formed by increasing the concentration of alloying elements. It has been reported that alloying of titanium with silicon [81] and tungsten [82] suppresses effectively the amorphous-tocrystallization transition of anodic oxide films during anodizing. For example, the formation of amorphous anodic oxide films on the Ti Mo alloys has been confirmed by transmission electron microscopy [83]. As an example, a transmission electron micrograph of an ultramicrotomed section of Ti 11.5 at.% Mo alloy anodized to 80 V is shown in Figure 2.11 [83]. The anodic film and the alloy substrate are revealed in the micrograph. The anodic film of ~186.5 nm thickness, with relatively flat and parallel alloy / film and film / electrolyte interfaces, appears uniform and featureless, indicating the amorphous structure [83]. 44
45 Chapter 2 Literature Review Figure Transmission electron micrograph of an ultramicrotomed section of the sputter deposited Ti 11.5 at.% Mo alloy anodized to 80 V at a constant current density of 50 ma in a 0.1 mol dm -3 ammonium pentaborate electrolyte at 293K [83]. Tanvir et al [84] reported that the electron diffraction of the Ti 6 at.% Si alloy disclosed an hcp structure, while those of the alloys containing 12 and 26 at.% silicon reveal a diffuse ring, typical of amorphous material, as evident in Figure It is also observed that the anodic oxide film formed on the Ti 6 at.% Si alloy, where the rise of formation voltage is no longer linear with anodizing time [84], is mainly amorphous; however, crystalline regions are evident at a depth of 40% of the film thickness. At the region indicated by the arrow, relatively large areas of crystalline oxide are developed with oxygen bubbles generated around them. Consequently, the anodic oxide film at crystalline regions is slightly thinner than in the surrounding regions [84]. 45
46 Chapter 2 Literature Review a b c Figure Transmission electron micrographs of ultramicrotomed sections for the sputterdeposited (a) Ti 6 at.% Si, (b) Ti 12 at.% Si and (c) Ti 26 at.% Si alloys anodized at 50 A m 2 in 0.1 mol dm 3 ammonium pentaborate electrolyte at 293 K. The formation voltages are 75 V for Ti 6 at.% Si alloy and 100 V for the Ti 12 at.% Si and Ti 26 at.% Si alloys. The selected area electron diffraction patterns of the respective alloys are also shown [84]. For titanium alloy, anodizing of Ti6Al4V has attracted attention since it is often the preferred alloy in titanium groups for many practical applications. It is reported that a high degree of porosity could be produced on the anodic film of Ti6Al4V after anodizing in sulphuric acid [85] due to the addition of aluminium and vanadium alloying elements. Figure 2.13 shows an example that this type of porosity appears preferentially (or possibly exclusively) in an oxide film grown on the α-phase regions of the titanium alloy. 46
47 Chapter 2 Literature Review Figure Transmission electron micrographs showing regions of different porosity in anodic oxide films formed in H 2 SO 4 at 40 V on α-phase grains of Ti-6A1-4V (bright field) [85] Porous-type Anodic Film Porous film created in the presence of fluoride ions Porous anodic oxide films formed on titanium and other valve metals in fluoridecontaining electrolytes have attracted plenty of attention since their developments in a dilute hydrofluoric acid electrolyte was first reported [86, 87]. Several recent studies have shown that titania nanotubes have better properties compared to many other forms of titania for applications in photocatalysis [88, 89], gas sensors [86, 87], photoelectrolysis [86, 87], and photovoltaics [90]. Since Zwilling et al. [91] reported the anodizing of titanium in chromic acid and hydrofluoric acid for the first time in 1999, great achievements have been developed in the fabrication, characterization, application and formation mechanism of porous anodic oxide films as nanotubes on titanium materials [92]. So far, several different electrolytes have been used for producing the nanotubes. Gong et al. [93] reported that their anodic oxide film formed on titanium could be prepared in a 0.5 wt% HF aqueous solution at room temperature at different anodizing voltages, from 3 to 20 V. Figure 2.14 [93] shows scanning electron micrographs of a typical nanotube-type anodic oxide film generated on titanium. The appearance of separated nanotubes is obvious at high applied voltage. 47
48 Chapter 2 Literature Review Figure Scanning electron micrographs at top view (a), cross sectional (b), and bottom view images of titanium oxide nanotubes anodized in 0.5 wt% HF solution at 20 V for 20 min [93]. It was reported that the nanotube-film thickness could not be increased further from nm in HF-based electrolytes [93]. Fluoride-containing solution can help to dissolve TiO 2 by forming [TiF6] 2 anions [93]. However, too strong acidity of HFsolution results in a too fast dissolution of the formed TiO 2 nanotubes. Mixtures with other acids did not help very much, but the quality of the nanotube arrays could be modified. Mor et al. [94] reported that the addition of acetic acid to a 0.5 wt% HF electrolyte in a 1:7 ratio resulted in more mechanically robust nanotubes without changing their shapes and sizes. Ruan, et al. [95] found that the surface morphology of nanotube arrays anodized in an electrolyte composed of 2.5% HNO 3 and 1% HF at 20 V for 4 h showed a uniform, clean, regular nanotube structure with a length of ~400 nm. Consequently, an anodizing electrolyte of 0.5 M H 3 BO 3 2.5% HNO 3 1% HF at 20 V for 4 h led to a greater degree of pore irregularity, with a nanotube length about 560 nm was generated. For the use of a KF or NaF as an electrolyte, the thickness of nanotubes could be significantly increased [96]. The acidity of the electrolyte might be tuned by adding HF, H 2 SO 4 or Na 2 SO 4 in order to adjust the balance of dissociation of titania at the electrolyte/oxide interface and oxidation of titanium at the oxide/metal interface [97, 98]. A better electrolyte is probably a NH 4 F-based solution [99]. From a mixed solution of NH 4 SO 4 and NH 4 F, the anodic nanotube films on titanium can grow up to several micrometers in thickness [99]. Considering diffusion as the main effect on local acidification, which could lead to a temporarily increased dissolution rate, Macak et al. [99] used glycerol solutions as electrolytes with very low diffusion constant to suppress a ph burst at the pore tip, which they believed, led to the growth of ridges on the sidewall of anodic TiO 2 48
49 Chapter 2 Literature Review nanotubes. They demonstrated a sample containing an anodic film prepared in a glycerol electrolyte with 0.5 wt% NH 4 F with a length of 7 μm and a high degree of regularity and homogeneity [100]. Further, in combination with either HF, KF, or NaF to provide fluoride ions, Grimes and co-workers [101] obtained nanotube arrays up to ~220 μm in length using a variety of organic electrolytes including dimethyl sulfoxide (DMSO), formamide (FA), ethylene glycol, and N-methylformamide. It was suggested that the key to successfully achieving very long nanotube arrays was to minimize water content in the anodization bath to less than 5%. As with organic electrolytes, donation of oxygen is more difficult in comparison with water, thus, reducing the tendency to form oxide [102] and slowing down the process of the growth of nanotube. In the mean time, the reduction in the water content reduces the chemical dissolution of the oxide in the fluorine containing electrolytes and hence, assists the formation of longer-nanotubes. When individual nanotubes were examined by transmission electron microscopy, it was found that the wall of the nanotubes consists of two layers [103]. The inner layer was titania and the outer layer was titanium hydroxide, implying that OH anions can move from the electrolyte/oxide interface to the oxide/metal interface to form a titanium hydroxide layer, although the exact formula could not be determined. It was assumed that the hydroxide layer decomposed continuously into oxide during the anodization since its thickness at the nanotube bottom maintains constant. Consequently, the principal chemical reaction at the hydroxide/metal interface should be: Ti + xoh Ti(OH)x + 4e and titanium hydroxide decomposes to form TiO 2 at the oxide/hydroxide interface [103]. Porous film created by application of breakdown voltages Titanium materials that are widely used for implants have been mentioned; this has been attributed to the biocompatibility provided by the thin oxide layer that forms naturally on titanium metal. In order to understand the osseointegration process between implant and bone tissue, it is very important to know the surface properties of the film [104, 105]. For this reason, many researches of tissue reactions to implant surfaces have been directed at modifying the roughness of the thin film surface. The rougher surfaces were obtained by methods such as plasma spraying, blasting, etching and sintering [105]. Other techniques, such as sol-gel processing [106], 49
50 Chapter 2 Literature Review anodic plasma-chemical treatment [107], anodic oxidation under galvanostatic [108] and potentiostatic modes [109] and ion implantation [110], have been used recently to develop new surfaces. Accordingly, implants with porous oxide surface have been specially prepared to promote bone growth into the porous implant surface [111]. Additionally, this porous oxide film can also probably be used to improve adhesive bonding performance [112] and wear resistance [113, 114] in aerospace applications in the future. Examples have been reported by Neide et al. [111] of porous oxide films that were produced on commercially pure titanium through anodic oxidation with breakdown voltages (over 80 V) applied in the phosphoric acid, as shown in Figure As observed, the porous film formed at 200 V (Figure 2.15a) displayed substantial porous structures. The appearance of the anodic film produced at this voltage was that of pores and craters formed on a relatively flat ground oxide surface. At 250 V (Figure 2.15b), the film morphology changes and it shows smooth regions between the round-shaped pores. They reported that the film morphology and population porosity are strongly dependent upon the applied breakdown voltages. a b Figure Scanning electron micrographs of anodic oxide films on CP-Ti after anodizing in 1.4 M H 3 PO 4 for 1 min: 200V (a) and 250V (b) [111] Oxygen Evolution during Anodizing In the anodizing process, the growth of anodic oxide films on titanium is accompanied by the generation of oxygen bubbles [115]. Due to the small size of the bubbles, about several tens of nanometres, the pressure of the trapped gas is high and approaches to at least several hundred MPa, leading to the rupture of the film after 50
51 Chapter 2 Literature Review the bubble bursting. In addition, in films favouring the generation of oxygen, the number of bubbles can be extremely high, with bubbles occupying almost the same volume as the solid film [116]. Apart from loss of charge to generation of oxygen, the efficiency of film growth is reduced by the bursting of bubbles [117]. It is also reported that the oxygen bubbles in the oxide layer are approximately spherical and grow in diameter as the film thickens during anodizing [118], and the coalescence of bubbles can be induced. This remarkable behaviour of bubbles, and the accommodation of their growth by the film, is associated with the highly labile nature of the film during the processes of ionic migration [117]. The crystalline oxide in the film provides an electron-conducting path, which enables oxygen generation to occur on crystalline regions [119]. It has been reported that the crystalline oxide is formed in the inner part of the anodic film, where the film is developed at the metal/film interface [120]. Moreover, the crystalline oxide develops further during the thickening of the film, which can be evident from the increased size of crystalline regions with increasing distance from the metal/film interface. Actually, oxygen bubbles generated in the crystalline regions of the anodic oxide films can be directly observed by transmission electron microscopy, and increased gas pressure in the bubbles results in the breakdown of the film. In literature, observations of films in cross-section [121] and in plan-view [118] by transmission electron microscopy disclose the presence of bubbles as light regions or cavities. Oxygen bubbles can also form in anodic titania following the incorporation of foreign species such as aluminium, molybdenum, silicon and zirconium species, which result in the decrease in the permittivity of the anodic films. In the case of titania-based films, the incorporation of the transition metal species from the alloy or the electrolyte appears probably to modify locally the band structure, possibly reducing the band gap and introducing intermediate levels, such that electron transfer from oxygen ions to the conduction band is permitted [117]. The formation of crystalline oxide in the anodic film formed on titanium was observed by TEM in the Habazaki s literature [120]. He also revealed that the alloying elements often hinder the amorphous-to-crystallization transition occurred during the anodic film growth on titanium. An example showing the crystalline transition evidence of the film growth is displayed in Figure An anodic oxide 51
52 Chapter 2 Literature Review film of thickness nm, with irregular film/electrolyte and metal/film interfaces associated mainly with the rough surface of titanium is revealed. Lattice fringes, with spacing of 0.35 nm, are revealed in the middle of the anodic film, indicating nanocrystals of anatase. Further, nanoscale bubbles have developed around the nanocrystals due to the local generation of oxygen. The outer 30% of the film is composed of an amorphous oxide, with no lattice fringes evident. On the other hand, the distribution of the crystalline oxide can be correlated with the ionic transport during the film growth. The transport number of cations in amorphous anodic titania is [81, 122, 123]. Thus, the outer 35 38% of the film is formed at the film/electrolyte interface through migration of Ti 4+ ions outward, with the remaining film formed at the metal film interface by migration of O 2 /OH - ions inward. Electrolyte-derived species, are incorporated into the outer layer of anodic films and may stabilize the amorphous structure [124]. Figure Transmission electron micrograph of an ultramicrotomed section of the sputtering-deposited titanium anodized to 20 V at 50 A m -2 in 0.1 mol dm -3 ammonium pentaborate electrolyte at 293 K [120]. 52
53 Chapter 2 Literature Review 2.4. Overview of Adhesion Techniques There are a few adhesive joint techniques currently used to achieve adhesive connection purposes. Some of them are explained in detail as follows: Single-Lap Shear Test Shear joints impose uniform stresses across the bond area which results in the highest possible joint strength. ASTM D1002 is a common reference for measuring the shear strength of the adhesives that are used to bond metals [46] Shear lap joint tests were developed with different methods such as bevelled lap, joggle lap, single lap, double lap and double butt lap, etc [46]. The single lap shear joint is one of the major shear test techniques which are widely used nowadays for adhesive bond metals to determine the shear strength. An example of the experimental setup is outlined in Figure 2.17 [125], displaying an adhered/epoxy adhesive system where specimens were prepared individually, the adhesive was applied to the area across the end of one of the metal substrates. Two thick steel wires were used in this case, and located in the bond area across the overlap to control the bondline thickness. With the simple clamping arrangement, the joints were held rigidly during curing. After the specimen was securely joined, the test was accomplished by using a laboratory joint shear strength test instrument. Figure Schematic diagram of the specific single-lap joint experimental setup and its dimensions [125]. 53
54 Chapter 2 Literature Review Tensile Test The tensile test was the most common test which was used for the adhesion evaluation and, interestingly, there is no guarantee tensile loading in strict forms during the test designing. One of the advantages of the tensile test is to gain the most basic data, such as tensile strain, elastic modulus and tensile strength [126]. A typical specimen for evaluating the tensile properties of an adhesive is shown in Figure 2.20 [127]. This specimen is similar to that used in the ASTM Test Method D2095. Metal rods are generated to exacting specifications. The metal rods are butted up to an adhesive which joins them; hence, the term butt tensile test. After the adhesive cures or sets, the specimen is loaded in tension just as depicted in Figure 2.18 [127]. Although this test is useful in the development and quality control of adhesives, they are destructive and cannot offer failure prediction for in-service components [128]. Metal rod Adhesive Figure Schematic diagram of a butt tensile specimen. Metal rods are used for the adherends. The surfaces of the metal rods must be smooth and parallel when the bond is made [127] Wedge Test The Boeing wedge test is a commonly utilized method to test the durability of fractured and stressed adhesive joints when exposed to different environments. This fracture test is an ASTM standard (ASTM D 3762) and utilizes a mode I specimen configuration [2]. In the wedge test, two metal plates are bonded by the adhesive under consideration, following the recommended procedure, as shown in Figure 2.19 [129]. The wedge is retained in the specimen, and the assembly is placed into a test environment, typically an aqueous environment at elevated temperatures. Further 54
55 Chapter 2 Literature Review crack growth is measured by inspection following a prescribed time period. While the test is considered to be a useful method for investigating bond durability, the existing standard provides little guidance regarding specifics on test conditions and requirements that constitute an acceptable metal bonded joint. Of particular concern is the reduction in strength of the bonded metal joint over time due to moisture. Moisture absorption of the adhesive can lead to a reduction in bond strength through hydration [129]. When an adhesive-bonded joint is placed in an aggressive environment, its resistance to fracture will decrease [129]. Figure ASTM D3762 wedge test for assessing bond durability [129] Cross-cut Test This testing method is usually used to establish whether the adhesion of a coating to a substrate is at a generally adequate level. If this test is used on a multi-coated sample, assessment of the resistance to separation of individual layers of the coating from each other can be made [130]. The scope and procedure for practical and instructive methods have been established by the International Organization for Standardization (ISO). In order to obtain an idea of the adhesion of the coating, a lattice pattern is cut into it, penetrating through the film and into the substrate. Various cutting tools can be used either manually or mechanically for this purpose [131]. 55
56 Chapter 2 Literature Review The test results are evaluated according to the scheme indicated in Figure The classification is based on estimating the amount of paint flakes separated from the substrate. If in doubt about the real percentage of detachment, one may brush off the loose parts or remove them by means of an adhesive tape [131]. Figure Principle of classifying paint film adhesion in the cross-cut test [131]. 56
57 Chapter 3 Experimental Methods and Procedures Chapter 3 Experimental Methods and Procedures 3.1. Introduction This chapter introduces the materials studied in the present thesis and the experimental methods and procedures which have been employed. The different computer monitoring software used for the data analysis is also illustrated Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Material and Pre-Treatments Titanium specimens, of dimensions of cm, were obtained from grade II commercially pure titanium sheet of 1.0 mm thickness. The chemical compositions of the titanium are listed here (wt %): Fe, ; H, ; O, ; N, ; C, ; Ti, bal. The specimens were successively wet ground on silicon carbide (SiC) papers from grade 400 to 1200, and polished with an organic lubricant on a 2 µm diamond paste. After polishing, the specimens were cleaned in acetone, rinsed in de-ionized water and dried in a cool air stream. For removal of the contaminants and the observation of grain boundaries, the first pre-treatment was etching in 48% HF for 10 s, rinsing in 70% HNO 3 and cleaning in deionized water at ambient temperature, and finally drying in a cool air stream. The second surface treatment was carried out using the rf-gd (radio frequency-glow discharge) sputtering technique, employing a Jobin Yvon 5000 instrument in an argon atmosphere of 635 Pa, and a power of 35 W was applied. The sputtering treatment was processed for 10 s. The etched specimens from HF+HNO 3 were masked by lacquer 45 to expose a working area of 1.0 cm 2 for later anodizing tests Anodizing The anodizing processes were carried out in mildly stirred 1 M H 2 SO 4 and 1 M H 3 PO 4 electrolytes respectively at ambient temperature using a Solartron Modulab System. A classic electrochemical three-electrode cell was employed, using a platinum counter electrode, a saturated calomel reference electrode (SCE) and the 57
58 Voltage / V Voltage / V Chapter 3 Experimental Methods and Procedures titanium working electrode. Figure 3.1 presents a schematic diagram of the potentiodynamic polarization, which is performed at a scan rate of 100 mv s -1 until a selected anodic voltage is reached (Figure 3.1a). Followed by the polarization, a potentiostatic anodizing is continuously applied at the selected constant voltage for 900 s, and the current density-time curve is recorded (Figure 3.1b). The specimens were anodized at voltages of 10, 20, 30, 40, 50 and 60 (V) respectively. a b Current Density / A cm -2 Current Density / A cm -2 Time / s Figure 3.1. Schematic representation of the polarization and anodizing processes in a threecell electrochemical system. a) Application of potentiodynamic polarization at the range from 0 V vs. (Ref) to an anodic voltage vs. (Ref). b) Continued application of the constant anodic voltage to record the current-time response Scanning & Transmission Electron Microscopes The pre-treated and anodized surfaces of titanium were examined by scanning electron microscopy (SEM) using a Zeiss Ultra 55 instrument with an acceleration voltage of 5 kv. Electron backscatter diffraction (EBSD) mapping was employed in the same SEM at 20 kv, and the data was recorded using a Channel 5 EBSD system produced by Oxford Instruments HKL technology and analyzed using in-house software. The titanium specimen etched in HF+HNO 3 electrolyte was studied by EBSD. Selected anodic films were evaluated by transmission electron microscopy (TEM) using a Tecnai F30 instrument, operated at 300 kv Focused-ion Beam In this experiment, focused-ion beam was used for the TEM sample preparation. However, since the whole process of FIB requires a long time to accomplish; thus, the samples after anodizing in 1 M H 2 SO 4 and 1 M H 3 PO 4 at 50 V for 900 s were 58
59 Chapter 3 Experimental Methods and Procedures only selected for the TEM evaluations. A FIB instrument of FEI QUANTA 3D FEG was used. In order to improve the electronic conductivity, the anodized titanium was coated with a thin gold layer in an Argon atmosphere of 10-1 Pa for 30 s, and a Platinum layer was coated above the gold layer for the protection of the surface. In addition, the last FIB milling step was attaching the sectioned specimens to a particular TEM sample holder X-ray Diffraction The crystalline structures of anodic films were examined by X-ray diffraction (XRD), using a Philips X pert Modular Powder Diffractometer and a long fine focus copper anode X-ray source. The scanning range (2θ) from 10 o to 85 o was employed. The compositions of the films were qualitatively compared using the height of diffraction peaks at crystal structures and semi-quantitatively calculated by summing up the integrated intensity of the main peaks of anatase. The resulting peaks were fitted by X Pert HighScore Plus software Glow Discharge Optical Emission Spectroscopy Depth profiling analysis of anodic films was carried out by glow discharge optical emission spectroscopy (GDOES), employing a Jobin Yvon 5000 instrument in an argon atmosphere of 635 Pa by applying a power of 40 W. Light emissions at 365.3, 130.2, and nm for Ti, O, S and P respectively were monitored during the analysis with a sampling time of 0.1 s. The area of analysis is ~4 mm diameter X-ray Photoelectron Spectroscopy The valence states of titanium, oxygen, carbon, sulphur and phosphorus were determined by X-ray photoelectron spectroscopy (XPS), using a Kratos Axis Ultra spectrometer with a monochromatic Al Kα source (15 ma, 14 kv). High resolution spectra were charge-compensated by setting the binding energy of the C1s peak to ev. Spectra were analyzed by the CasaXPS software Electrochemical Impedance Spectroscopy The dielectric properties of anodic oxide films were determined by electrochemical impedance spectroscopy, which was carried out in a potentiostatic mode and vs. open circuit. A voltage perturbation amplitude of 10 mv with 10 points per decade 59
60 Chapter 3 Experimental Methods and Procedures was employed. The frequency was scanned from 100 khz to 1 Hz, where the capacitive effect associated with the anodic oxide dominates [80, 132] Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis The compositions, thicknesses of anodic oxide films and current efficiencies for the films growth on CP-Ti in the sulphuric and phosphoric acids were determined by Rutherford backscattering spectroscopy (RBS) and nuclear reaction analysis (NRA). RBS employed 2.0 MeV 4He + ions produced by the Van de Graaff accelerator of the Institute of Reference Materials and Measurements (IRMM), Belgium. The analysed region was of 1 mm diameter with the ion beam at normal incidence to the specimen surface and with a scattering angle of 165 o. NRA employed the 16 O(d, p1) 17 O reactions, using 0.87 MeV protons and deuterons respectively, at normal incidence, with the detector positioned at 150 o to the direction of the incident beam. The analysed area was ~1 mm diameter. A 13 µm-thick Mylar film was placed in front of the detector to stop elastically-scattered particles, thereby providing almost a background-free detection. For quantitative analyses, the reaction yields were compared with tantalum oxide references containing 690 ± O atoms cm -2. The accuracies of the 16 O analysis were ~3%. The data were interpreted by RUMP software Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment Material and Anodizing Titanium specimens, with dimension of cm, were obtained from grade II commercially pure titanium (CP-Ti) sheet of 1.0 mm thickness and masked by beeswax to expose an area of 1.0 cm 2 for the anodizing and electrochemical tests. The composition of titanium in percent weight contains Fe, ; H, ; O, ; N, ; C, ; Ti, bal. In order to remove the contaminants, the titanium materials were etched in 48% HF for 15 s at ambient temperature, rinsed in 70% HNO 3, cleaned in deionized water and dried in cool air. The anodizing treatments employed a two-electrode cell, employing an aluminium sheet of a dimension of 8 10 cm as the counter electrode. The anodizing tests were carried out at a constant current of 20 ma cm -2 in 1 M phosphoric acid and 1 M 60
61 Chapter 3 Experimental Methods and Procedures sulphuric acid electrolytes to 20, 40 and 60 V respectively at ambient temperature. The voltage variation was recorded by a computer interfaced with the laboratory DC power supply. Following anodizing, the specimens were removed from the electrolyte, cleaned in deionized water, dried in a cool air stream and stored in a desiccator until required for analysis. Selected specimens after anodizing were immediate immersed or immersed for 60 days in a near-neutral aerated 3.5% NaCl electrolyte for corrosion tests Electrochemical Measurements Electrochemical experiments were carried out in an aqueous 3.5% NaCl electrolyte at ambient temperature using a Solartron Analytical Modulab System. A classic three-electrode system with the titanium as working electrode, a Platinum auxiliary electrode and a saturated calomel reference electrode (SCE), was employed. Potentiodynamic polarization measurements were investigated over the potential range from -1.0 V (SCE) to 6.0 V (SCE) at a scan rate of 1.0 mv s -1 in order to probe the respective anodic and cathodic behaviours. Electrochemical impedance spectroscopy measurements were carried out in a potentiostatic mode vs. the open circuit modulus. A voltage perturbation amplitude of 10 mv with 10 points per decade without start delay was employed. The frequency range from 100 khz to 0.01 Hz was selected. The impedance results were analyzed by the ZSim software. All electrochemical curves were plotted by origin 9.0 software. Further, the potentiodynamic polarization and electrochemical impedance spectroscopy measurements were conducted on the etched specimen before and after the anodizing processes. The electrochemical measurements were also carried out on the etched and anodized specimens after immediate immersion or immersion for 60 days in the 3.5% NaCl electrolyte. Prior to the polarization and EIS experiments, the working electrode was immersed in the NaCl electrolyte to approach a stable potential by applying an open circuit test for 30 min Morphology Characterization The etched surface of titanium, the anodic films formed to 20, 40 and 60 V in 1 M sulphuric and 1 M phosphoric acids respectively and the etched or anodized surface of titanium specimens after immersion in the 3.5% NaCl for 60 days were examined 61
62 Chapter 3 Experimental Methods and Procedures by scanning electron microscopy (SEM) using the Zeiss Ultra 55 instrument at an acceleration voltage of 5 kv Formation of Porous Anodic Oxide Film On CP-Titanium in Phosphoric Acid Electrolyte Material and Anodizing Titanium specimens, of dimensions of cm, were cut from 99.6% grade II pure titanium sheet of thickness of 1.0 mm. The chemical compositions of titanium are given here (wt %): Fe, ; H, ; O, ; N, ; C, ; Ti, bal. Specimens were cleaned in acetone, rinsed in deionized water and, finally dried in a cool air stream. The anodizing treatments were carried out in 1 M phosphoric acid electrolyte at constant voltages of 100, 150 and 200 V for 900 s respectively at ambient temperature. A two-electrode cell was employed, and an aluminium sheet of a dimension of 8 10 cm as the counter electrode was used. The current-time variation was recorded by a computer interfaced using a laboratory DC power supply. After anodizing, specimens were removed from the electrolyte, cleaned in deionized water, dried in a cool air stream and masked by lacquer 45 to define a working area of 1.0 cm 2, finally, stored in a desiccator until required for analysis Scanning Electron Microscopy Selected specimens were examined by scanning electron microscopy in plan-view and cross-section, using an INCA PentaFET 3, Ultra 55 at an acceleration voltage of 5 kv. Prior to observations, the cross-section of specimens were mounted in nonconductive epoxy resins, mixed with hardeners based on the ratio of 10:1 and dried for 24 h in air. Then, the surfaces of finished specimens were coated by gold in an Argon atmosphere of vacuum condition of 10-1 Pa for 30 s in order to strengthen the electrical conductivity Raman Spectroscopy The crystalline structure of titanium anodic oxide films was examined by Raman spectroscopy, using a HORIBA Jobin Yvon LabRAM Aramis instrument with a He- Ni laser power and an excitation wavelength of 633 nm. Spectroscopic ellipsometry 62
63 Chapter 3 Experimental Methods and Procedures was carried out at an incidence angle of 70 o with a wavelength range of nm. The wave number was scanned from 800 to 100 cm Electrochemical Impedance Spectroscopy The testing procedure is the same with the description in the part Glow Discharge Optical Emission Spectroscopy Depth profiles of the anodic oxide films were determined by glow discharge optical emission spectrometry (GDOES), employing a Jobin Yvon 5000 instrument in an argon atmosphere of 635 Pa with MHz and a power of 40 W. Light emissions of sputtered species were monitored with a sampling time of 0.01 s to obtain the depth profiles. The wavelengths of the spectral lines used were / Ti, / P and / O nm. The signals were detected from a circular area of approximately 4 mm diameter Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Material and Anodizing Titanium specimens, with dimensions of cm were obtained from 99.6% grade II titanium sheet of 1.0 mm thickness. The chemical compositions of the titanium can be seen (%): Fe, ; H, ; O, ; N, ; C, ; Ti, bal. Before anodic treatments, the titanium specimens were cleaned in acetone, rinsed in de-ionized water and dried in a cool air stream. The specimens were masked by lacquer 45 to define a working area of 1.0 cm 2. The anodizing electrolyte is composed of 7.5 mol l -1 NaOH, 0.33 mol l -1 Na-tartrate, 0.1 mol l -1 ethylenediaminetetraacetic acid (EDTA) and 0.05 mol l -1 Na 2 SO 3 [20, 133], and the ph value of the electrolyte is ~ Anodizing was performed at a constant current of 20 ma cm -2 to 5, 10, 20 or 40 V, using a Solartron Modulab System with a high voltage modulus in order to reach the voltages targeted in a three-electrode electrochemical cell, with a Platinum counter electrode and a saturated calomel reference electrode (SCE) at ambient temperature under continuous mild electrolyte 63
64 Chapter 3 Experimental Methods and Procedures stirring. Following anodizing, the specimens were cleaned by deionized water, dried in a cool air stream and stored in a desiccator Scanning & Transmission Electron Microscopes The anodic films were examined by scanning electron microscopy (SEM) using a Zeiss Ultra 55 instrument with an acceleration voltage of 5 kv, equipped with an energy dispersive X-ray spectroscopy (EDS) analysis facility (Oxford Instruments). The cross-section of titanium anodized to 10 V in the NaTESi electrolyte was milled by focused-ion beam (FIB) under the same procedure described in the part 3.2.4, and examined by transmission electron microscopy using the Tecnai F30 instrument with an acceleration voltage of 300 kv Raman Spectroscopy The crystalline structure of the anodic oxide films was examined by a Renishaw 2000 Raman spectrometer system using a He-Ne laser beam (633 nm excitation). The Raman testing duration for each specimen was monitored within 20 s, and scanned from 100 to 900 cm -1 where the titanium crystalline oxide dominates [78, 134, 135] Glow Discharge Optical Emission Spectroscopy Depth profiling analysis of oxides was carried out by glow discharge optical emission spectroscopy (GDOES), employing a Jobin Yvon 5000 instrument in an argon atmosphere of 635 Pa by applying a power of 40 W. Light emissions of sputtered species were monitored with a sampling time of 0.01 s to generate the depth profiles. The wavelengths of the spectral lines used were nm / Ti, nm / O, nm / Si and nm / Na respectively. The signals were detected from an area of ~4 mm diameter Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis The compositions and thicknesses of anodic oxide films prepared on CP-Ti in the NaTESi electrolyte were determined by Rutherford backscattering spectroscopy (RBS) and nuclear reaction analysis (NRA). RBS employed 2.0 MeV 4He + ions produced by the Van de Graaff accelerator of the Institute of Reference Materials and Measurements (IRMM), Belgium. The analysed region was of 1 mm diameter 64
65 Chapter 3 Experimental Methods and Procedures with the ion beam at normal incidence to the specimen surface and with a scattering angle of 165 o. NRA employed the 16 O(d, p1) 17 O reactions, using 0.87 MeV protons and deuterons respectively, at normal incidence, with the detector positioned at 150 o to the direction of the incident beam. The analysed area was ~1 mm diameter. A 13 µm-thick Mylar film was placed in front of the detector to stop elastically-scattered particles, thereby providing almost a background-free detection. For quantitative analyses, the reaction yields were compared with tantalum oxide references containing 690 ± O atoms cm -2. The accuracies of the 16 O analysis were ~3%. The data were interpreted by RUMP software Electrochemical Impedance Spectroscopy Dielectric properties of the anodic films were evaluated by electrochemical impedance spectroscopy. The electrochemical impedance spectroscopy measurements were carried out in a potentiostatic mode vs. the open circuit modulus. A voltage perturbation amplitude of 10 mv with 10 points per decade without start delay was employed. The frequency range from 100 khz to 0.01 Hz was scanned Degradation Test After anodizing, selected titanium specimens were exposed to a demanding environment with humidity of 90% at 50 o C for 1000 h, which was conducted in a constant climatic chamber (BINDER) Single-lap Shear Test Epoxy resin mixed with hardener in a 10:1 ratio was carefully spread on the anodized specimens without introducing air bubbles. After cleaning away the excess epoxy resin, the specimens were cured at 60 o C for 24 h in an oven. A schematic diagram is presented in Figure 3.2, showing the dimensions of the adhesive bonding region. The bond length was 10 mm, and the length of each specimen was 55 mm. The width of bonded area was 10 mm, and the area of test grip region was 100 mm 2. The thicknesses of the titanium specimens were 1 mm. The specimens were tested at ambient temperature using a Hounsfield H20-W lab instrument at a constant velocity of 2 mm min
66 Chapter 3 Experimental Methods and Procedures Figure 3.2. Schematic representation of the single-lap shear test of titanium materials Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Material and Anodizing Ti6Al4V alloy specimens, of dimensions of cm, were obtained from grade 5 titanium sheet of 2.0 mm thickness, supplied by TIMET Ltd, UK. The chemical composition of Ti6Al4V is given in Table 1. Prior to anodizing, the specimens were etched in 48% HF + 70% HNO 3 electrolyte for 10 s in order to remove the naturally air-formed oxide layer, and finally rinsed in deionized water and dried in a cool air stream. The specimens were masked in lacquer 45 to expose a working area of 1.0 cm 2. The anodizing treatment was carried out at a constant current density of 20 ma cm -2, using a Solartron Modulab workstation at a high voltage modulus and a classic three-electrode cell, employing a Platinum counter electrode and a saturated calomel reference electrode (SCE). The NaTESi was prepared with ph of ~12.3 by dissolving NaOH (7.5 M), Na-tartrate (0.33 M), ethylenediaminetetraacetic acid (EDTA) (0.1 M) and Na 2 SO 3 (0.05 M) in deionized water. The voltage-time response was recorded electronically during anodizing, employing Labview software with a sampling time of 20 ms. The anodizing process was terminated after a significant reduction of voltage rise in the curve was observed. 66
67 Chapter 3 Experimental Methods and Procedures Table 3.1. Chemical compositions (wt%) of Ti6Al4V alloy sheet used for anodizing, degradation and adhesive bonding tests. Al% V% Fe% H% C% O% N% Ti% Degradation Test After anodizing, selected titanium alloy specimens were placed in a demanding environment with humidity of 90% at 50 o C for 1000 h, which was conducted in a constant climatic chamber (BINDER) Single-lap Shear Test The same procedure as previously shown in Figure 3.2 was used for the single-lap shear bonding tests. The epoxy resin mixed with hardener in a 10:1 ratio was carefully spread on the anodized specimens without introducing air bubbles. After cleaning away the excess epoxy resin, the specimens were cured for 24 h in air. The bond length was 10 mm, and the length of each specimen was 55 mm. The width of bonded area was 10 mm, and the area of test grip region was 100 mm 2. The thicknesses of the titanium specimens were 2 mm. The specimens were tested at ambient temperature using a Hounsfield H20-W lab instrument at a constant velocity of 2 mm min Techniques for Characterization Titanium specimens were examined using a Zeiss Ultra 55 scanning electron microscopy (SEM) instrument at an acceleration voltage of 8 kv, equipped with an energy dispersive X-ray (EDS) analysis facility (Oxford Instruments). Electron backscatter diffraction (EBSD) mapping was employed in the same SEM at 20 kv; the data were recorded using a Channel 5 EBSD system produced by Oxford Instruments HKL technology and analyzed by in-house software. An ~100 nm thick cross-section for transmission electron microscopy (TEM) was fabricated by a FEI Nova Lab dual beam focused-ion beam (FIB) facility. The section was examined using a TECNAI F30 G2 instrument, operated at 300 kv with a Gatan imaging filter (GIF2001). The oxide crystalline structure was investigated by a Renishaw 2000 Raman spectrometer using a He-Ne laser beam (633 nm excitation). 67
68 Chapter 3 Experimental Methods and Procedures Depth profiling analysis of anodic oxide films was carried out by glow discharge optical emission spectroscopy (GDOES), employing a Jobin Yvon 5000 instrument in an argon atmosphere of 635 Pa by applying a power of 40 W. Light emissions at 365.3, 396.2, 311.1, 130.2, and nm for Ti, Al, V, O, Si and Na respectively were monitored during the analysis with a sampling time of 0.1 s. The area of analysis is ~4 mm diameter. The compositions and thicknesses of the anodic oxide films were determined by Rutherford backscattering spectroscopy (RBS) using a 2.0 MeV beam of He + particles. The scattered particles were detected at 165 o to the direction of the incident beam, which was normal to the surface of the specimen. The data were analysed using RUMP software. The dielectric properties of the anodic films were evaluated by electrochemical impedance spectroscopy, and the procedure is the same with the description in the part Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Material and Sputtering 99.9% pure aluminium foil, of dimensions of cm, was used as the substrate. Prior to the DC magnetron sputtering treatment, aluminium specimens were electropolished at 20 V for 3 min in a 4:1 by vol. mixture of ethanol/perchloric acid, and the temperature was maintained at 278 K, followed by rinsing in ethanol and deionized water and drying in cold air. After the electropolishing procedure, the specimens were stored in a desiccator. DC magnetron sputtering was carried out using an Oxford Applied Research system, with a 99.6% titanium target of 50 mm diameter. The system was first evacuated to Pa, with subsequent deposition of titanium layers at 300 ma in 99.99% argon at 0.5 Pa for 30 min, and the ~100 nm thick layers was produced. On the other hand, the ~290 nm thick titanium layer was generated by extending the sputtering time to 150 min. Figure 3.3 presents a schematic diagram of the working mechanism of a DC magnetron sputtering instrument, consisting of mainly four steps to operate: (1). A plasma is ignited in the vacuum chamber by applying a selected and admitting argon gas. 68
69 Chapter 3 Experimental Methods and Procedures (2). An electric field accelerates the positively charged argon ions towards the negatively charged cathode (the target) which is the electropolished aluminium substrate. (3). The argon particles dislodge atoms from the surface of the sputtering target. (4). The titanium atoms that are released from the target travel through the vacuum chamber toward the substrate opposite where they are deposited as a thin layer. Figure 3.3. Schematic representation of thin film deposition using dc sputtering technique in Argon atmosphere Anodizing After masking with lacquer 45 to expose one side area, specimens were anodized to voltages in the range V at 20 ma cm -2 in 1 M phosphoric acid with continuous mild stirring at ambient temperature. A sheet of pure aluminium, of size 8 10 cm, was used as the cathode. The voltage time response was recorded electronically, employing Labview data acquisition system (National Instruments) with data plotted by Origin 9.0. After anodizing, specimens were rinsed in the deionized water and dried in a cool air stream Scanning & Transmission Electron Microscopes The top-view of the sputtering titanium layer was observed by scanning electron microscopy (SEM) using a ZEISS Ultra 55 instrument with an acceleration voltage of 5 kv. The cross-sections of anodized specimens, ~25 nm thickness were fabricated by ultramicrotomy and examined by transmission electron microscopy (TEM) in a JEOL FX 2000 II instrument operated at 120 kv. The composition at a selected region of the section was qualitatively determined by energy dispersive X- 69
70 Chapter 3 Experimental Methods and Procedures ray spectroscopy (EDS). High resolution imaging of a selected section was studied by TEM, using a TECNAI F30 instrument at an acceleration voltage of 300 kv Glow Discharge Optical Emission Spectroscopy Depth profiling analyses of anodic films formed on 100 nm and 290 nm thick sputter-deposited titanium layers respectively were carried out by glow discharge optical emission spectroscopy (GDOES), employing a Jobin Yvon 5000 instrument in an argon atmosphere of 635 Pa by applying a power of 40 W. Light emissions of sputtered species were monitored with a sampling time of 0.01 s to generate the depth profiles. The wavelengths of the spectral lines used were nm / Ti, nm / O, nm / Al, nm / P and nm / S. The signals were detected from an area of ~4 mm diameter Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of Anodic Oxide Film Formed on Titanium Instrument A Zeiss Ultra 55 scanning electron microscopy equipped with a scanning transmission electron microscopy detector was used throughout the present study. The schematic diagrams showing the specimen holder and SEM chamber inside are displayed in Figure 3.4. Inside the SEM chamber, the STEM detector unit contains the detector itself and an extension arm. The extension arm carries the detector back and forth between a rest position and an active position when imaging. In the active imaging mode, the 6-specimen carousel holder used and the STEM detector were perfectly aligned. In the rest mode, the detector is parked away by simply retracting the detector by a safe distance. Also, if the detector is not parked away, the joystick function was used to freeze it during the active mode in order to protect the STEM detector, and when in the rest mode, joystick was released and rotated to change the position of grid holder. STEM-in-SEM evaluations were conducted at a typical operating acceleration voltage of 30 kv in order to improve the electron penetration and the brightness of the source with an optimized working distance of 4 mm. 70
71 Chapter 3 Experimental Methods and Procedures Inside SEM (C) (A) (B) Figure 3.4. Schematic showing the top view of parts (A), (B) and (C), represent for STEM detector, detector extension arm and carousel holder. Photo inside the SEM chamber corresponds to the schematics of the top view. The 6-sample carousel is aligned with the electron column, and the STEM detector is brought with the detector arm, and the imagining is ready after the working distance is set for both carousel and STEM detectors. 71
72 Chapter 3 Experimental Methods and Procedures Materials and Anodizing First, 99.9% pure aluminium foils, of dimensions of cm, were electropolished at 20 V for 3 min in a 4 to 1 by vol. mixture of ethanol/perchloric acid, and the temperature was maintained at 278 K, followed by rinsing in ethanol, deionized water and drying in cold air. Second, DC magnetron sputtering was carried out using an Oxford Applied Research system, with a 99.6% titanium target of 50 mm diameter on the electropolished aluminium substrates. The chamber was first evacuated to a vacuum condition of Pa; subsequently, the titanium was deposited on the aluminium at 300 ma in 99.99% argon atmosphere at 0.5 Pa for 60 min, and an ~120 nm thick titanium sputtering layer was generated. Third, the finished specimens were covered by lacquer 45 to expose a region with dimensions of 1.0 cm 2 for the anodizing treatments. The specimen was anodized at 10 and 50 V in 1 M phosphoric acid for 900 s respectively with continuous mild stirring at ambient temperature. A sheet of pure aluminium, of size 8 10 cm, was used as the cathode. The voltage - time response was recorded electronically, employing Labview data acquisition system (National Instruments). After anodizing, the specimen was cleaned in deionized water and dried in a cool air stream Ultramicrotomy Ultramicrotomed sections, nominally 15 nm thickness of the specimen was prepared using a Leica EM UC7 instrument with a cutting speed of 0.15 mm s -1. The slices were floated on water in the groove of diamond knife, and collected by standard TEM mesh copper grids Chemical Etching Behaviour of CP-Titanium in Bromine- Methanol Electrolyte Material and Etching Commercial pure titanium (CP-Ti) of 99.6% grade II titanium sheet (Fe wt.%, H wt.%, O, wt.%, N wt.%, C wt.%, Ti balance), with dimensions of cm and 2.0 mm thickness, was obtained from TIMET Ltd, UK. Prior to etching, the specimens were cleaned ultrasonically in acetone and deionized water baths for 5 min respectively and, finally, dried in a cool 72
73 Chapter 3 Experimental Methods and Procedures air stream. The etching treatment was carried out in a 1 to 9 by vol. mixture of liquid bromine and methanol electrolyte for 10, 30, 120 and 300 s at ambient temperature Characterization of Stripped Anodic Oxide Film In order to support the assumption that the anodic oxide film can be stripped off the titanium substrate even though the metal would not be dissolved completely after etching in the bromine-methanol electrolyte, anodizing was carried out to form an anodic film on titanium. The anodizing was operated potentiostatically in 1 M H 2 SO 4 acid at 20 V for 900 s at ambient temperature. A two-electrode cell was employed, using an aluminium sheet of dimension of 8 10 cm as the counter electrode. After the anodizing, the specimen was rinsing in deionized water, drying in cool air, and then, etching in the bromine-methanol electrolyte for 300 s. After the etching, the transparent anodic oxide film stripped from the titanium substrate was collected using standard nickel mesh grids, and they were examined immediately by transmission electron microscopy using a JEOL 2000 FX instrument at an acceleration voltage of 120 kv Characterization of Titanium The titanium specimens, before and after etching, were examined using a Carl Zeiss Ultra 55 scanning electron microscopy (SEM), equipped with energy dispersive X- ray spectroscopy (EDS). SEM was operated at an acceleration voltage of 8 kv, using secondary (SE) and backscattered electron (BSE) detectors respectively. Electron backscatter diffraction (EBSD) scan analysis was employed in the same SEM instrument at 20 kv; the data were recorded using a Channel 5 EBSD system produced by Oxford Instruments HKL technology and analyzed by in-house software. White light interferometry was employed for measurements of the surface roughness and the depths of corrosion pits, using a contourgt optical surface profiler configuration (Veeco Instruments). The interferometry was mapped for 15 µm with a back scan of 5 µm, using the vertical scanning interferometry (VSI) mode with an optical microscope at 50 x magnification. The chemical compositions and structures of etched titanium were analyzed by energy-dispersive X-ray spectroscopy (EDS) and X-ray diffraction (XRD). XRD measurements were carried out using a Philips X pert Modular Powder Diffractometer (CuKα). The scanning range (2 Theta) 73
74 Chapter 3 Experimental Methods and Procedures of 20 o to 85 o was employed. The patterns were qualitatively compared using the height of chemical substances and semi-quantitatively calculated by summing up the integrated intensity of the main peaks of Ti and TiBr 4. The resulting peaks were fitted by the X Pert HighScore software. 74
75 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Chapter 4 Barrier Anodic Oxide Film Growth of CP- Titanium in Sulphuric and Phosphoric Acids 4.1. Introduction CP-Ti is used in the present study to investigate the growth behaviour of barrier anodic oxide films in sulphuric and phosphoric acids using potentiodynamic polarization and potentiostatic anodizing techniques. The properties of the anodic oxide films are dependent upon the nature of the electrolytes and the applied voltages. In this chapter, a comprehensive understanding of the barrier film growth mechanisms is gained from the detailed structure of film morphologies, film thicknesses, crystalline structure and compositions Pre-Treated Surfaces of CP-Ti Scanning electron micrographs of the as-received CP-Ti after rf-gd (radio frequency-glow discharge) sputter cleaning and after etching in HF + HNO 3 are displayed in Figures 4.1a - d. Figure 4.1a shows the microstructure of the grains on the titanium substrate after the rf-gd sputter cleaning. The rough surface is displayed due to the sputtering energy from the rf-gd sputtering. The grain boundaries can be observed more clearly in the higher magnification image (Figure. 4.1b). Hydrofluoric acid that readily attacks TiO 2 reacts with Ti to form soluble titanium fluorides and hydrogen. Incorporation of hydrogen in titanium can cause embrittlement of the surface layer, but the nitric acid can minimize the formation of free hydrogen [136]. Thus, Figure 4.2c shows the micro-pores developed within the substrate, which were induced by the HF attack where some of the local titanium substrate regions were dissolved [137]. These pores are appeared within the grain boundaries. At a higher magnification image, the grain boundaries are observed more clearly (Figure 4.1d). Therefore, both surface treatments are suitable for cleaning the air-formed TiO 2 on titanium substrate and thus, an excellent observation of grain boundaries can be provided. Electron backscatter diffraction (EBSD) was carried out to investigate the grain orientations of titanium after etching in HF + HNO 3. The mapping is displayed in Figure 4.2b based on the image of Figure 4.2a, showing a 99.6% hexagonal close packed (HCP) crystal structure. The grain 75
76 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids orientations of [0001] and [11-20] and [10-10] are identified by the inverse pole figures (Figure 4.2c) Anodizing and Film Characterizations Potentiodynamic Polarization and Current-Time Responses Figure 4.3 shows potentiodynamic polarization curves from 0 V (vs. SCE) to 60 V (vs. SCE) in 1 M H 2 SO 4 and 1 M H 3 PO 4 respectively. Both polarization plots showed that the oxide films started growing from the voltage of ~ 1 to ~ 6 V. The current densities increased significantly due to the occurrence of oxygen evolution which was evident by the visible oxygen bubbles between ~ 6 and ~ 13 V. From ~ 13 to ~ 60 V, the currents were spent for the growth of re-passive oxide films and oxygen evolution; thus, the rising tendency of current density was reduced as a result of the film thickening. The current density in the phosphoric acid related to the repassive oxide film growth region was slightly decreased. In contrast, the current density in the re-passive region for the polarization occurred in the sulphuric acid still increased gradually at a small extent. Further, the current densities measured in the phosphoric acid were lower compared with sulphuric acid during the repassivation of the films. The potentiostatic anodizing responses in 1 M H 2 SO 4 are displayed in Figure 4.4a. The current densities were rapidly decreased at the commencement of 50 s, and subsequently, decreased gradually within the next 850 s. Table 4.1 shows that the current density measured after 900 s increases with increase of anodic voltage. Figure 4.4b shows that the current density is similarly decreased sharply for the first 50 s in 1 M H 3 PO 4, and dropped slowly until the tests were completed. The relative current densities are listed in Table 4.1, showing that the current density increases with increase in anodic voltage. The oxygen evolution was confirmed by the observation of gas bubbles originating from the titanium surface during anodizing. 76
77 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Table 4.1. Current densities (A cm -2 ) of cp-titanium after anodizing at different constant voltages in H 2 SO 4 and H 3 PO 4 electrolytes for 900 s. Current Density (A cm -2 ) 10 V 20 V 30 V 40 V 50 V 60 V H 2 SO H 3 PO Interference Colours Figure 4.5 shows the colour scales of the titanium surfaces after anodizing due to the interference effect [50, 138]. Depending on the voltage, the colour scale varies from golden, dark blue, pale blue, paler blue, golden yellow and dark yellow violet between 10 and 60 V in the sulphuric acid. For the phosphoric acid, dark golden yellow, brown, dark blue, pale blue, pale yellow and paler yellow are associated with the voltages (10 60 V) Microstructure of Anodic Films Figure 4.6a shows that a compact oxide film was formed at 10 V in H 2 SO 4. The anodic film ruptures at some of the local regions at 20 V (Figure 4.6b). The oxide blisters were formed due to the bursting of oxygen bubbles within these regions at 30 V (Figure 4.6c). The film cracks were initiated by the film rupture, which are observed at 40 and 50 V (Figure 4.6d - e). Further, similar cracking textures are observed after anodizing at 60 V (Figure 4.6f). For H 3 PO 4, small blisters were found within the anodic films after anodizing at 10, 20 and 30 V (Figure 4.7a, b and c). Blisters were formed with larger quantities at 40 V (Figure 4.7d). For 50 V, the film rupture was initiated within the blisters (Figure 4.7e), which is similar to 60 V (Figure 4.7f). According to the scanning electron micrographs, the rupture of the film was probably developed at lower voltages in the H 2 SO 4 compared with the H 3 PO 4. Transmission electron micrographs of anodic films formed at 50 V in the two acid electrolytes are shown in Figure 4.8. In terms of sulphuric acid (Figure 4.8a), the film thickness is ~75 nm which is consistent with a growth ratio of ~1.5 nm V -1. The amorphous-to-crystalline transition proceeded near the Ti/oxide interface is revealed 77
78 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids in Figure 4.8b, indicating that the crystalline phases were formed during the polarization stage. Further, more nanocrystals were nucleated during the following potentiostatic anodizing. Lattice fringes at the various regions of the film, with spacings of 3.5 Å and 1.7 Å are evident in Figure 4.8c, showing a good agreement with (101) and (004) crystal planes of anatase [120]. Nano-scale bubbles were developed around the nanocrystals, introducing the oxygen-filled voids. For the phosphoric acid, the film thickness is ~85 nm (Figure 4.8d), which is consistent with a growth ratio of ~1.7 nm V -1. The amorphous-to-crystalline transition is evident due to the observation of lattice fringe structures, indicating that the crystallization is induced and developed from the Ti/oxide interface (Figure 4.8e). In the high magnification image, lattice fringes are observed more clearly, with spacings of 3.5 Å and 1.6 Å, showing a good agreement with (101) and (002) crystal planes of anatase [120] (Figure 4.8f) X-ray Diffraction Patterns The X-ray diffraction patterns of the anodic film formed at 50 V in the sulphuric acid show different anatase peaks, representing the anatase crystal planes of (101), (200), (105) and (224) through the angles from 10 o to 85 o (Figure 4.9a). In contrast, the anodic films formed at 30 and 10 V show mostly titanium peaks, indicating a poorly crystalline nature of the anodic oxide films. In the phosphoric acid, the crystalline anatase peak was poorly detected after anodizing at 10, 30 and 50 V respectively, and only an anatase crystal plane of (224) was found (Figure 4.9b) Rutherford Backscattering Spectroscopy & Nuclear Reaction Analysis Figure 4.10 shows the RBS spectra for the specimen after anodizing in 1 M H 2 SO 4 electrolyte at 20 and 50 V respectively. Figures 4.10a and b reveal that the anodic film thicknesses generated after anodizing at 20 and 50 V are ~46 nm and ~66 nm respectively. They both disclose yields from oxygen and titanium, with a peak signalling the presence of oxygen. It is also measured that the anodic film formed at 20 V contains Ti and O atoms cm -2, and Ti and O atoms cm -2 at 50 V. The current efficiency of anodic oxide growth at 20 V is estimated as ~ 62.4%, and ~ 68.2% for 50 V. 78
79 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids The RBS spectra for the specimen after anodizing in 1 M H 3 PO 4 electrolyte are shown in Figure Figures 4.11a and b show that the film formed at 20 and 50 V are of thickness ~51 nm and ~72 nm respectively. The spectra of the anodic films formed at 20 and 50 V also disclose yields from oxygen and titanium, with a peak signalling the presence of the oxygen. It is measured that the anodic film formed at 20 V contains Ti and O atoms cm -2, Ti and O atoms cm -2 at 50 V. The current efficiency for the anodic oxide film growth at 20 V is ~73.7%, and ~76.9% for 50 V. It is also revealed that the current efficiency in the phosphoric acid is higher than in sulphuric acid when anodizing at 20 or 50 V. Figure 4.12a displays the NRA spectrum for the specimen following anodizing at 20 V in 1 M H 2 SO 4 electrolyte, revealing peaks due to the (d, p) reactions with oxygen and carbon. Peaks are evident which contribute to 16 O(d, p 1 ) 17 O and 16 O(d, p o ) 17 O reactions for oxygen in the single barrier anodic film on the titanium substrate near channel 40 and 90, and for the 12 C(d, p o ) 13 C reaction near channel 123. The low yield to the right of the main peak originates from oxygen of the anodic oxide and indicates an upper limit of about atoms cm -2 which is similar to the RBS analysis. Figure 4.12b shows the similar peaks for 16 O(d, p 1 ) 17 O and 16 O(d, p o ) 17 O reactions near channels 110 and 240 for oxygen in the anodic film after anodizing at 50 V. For the 12 C(d, p o ) 13 C reaction, it is located near channel 310. The main peak originating from oxygen of the anodic oxide shows a content of atoms cm -2 which is similar to the oxygen content measured in the RBS result. The yields of the 12 C(d, p o ) 13 C reactions for the anodized specimen arise mainly from adventitious carbon contamination of the titanium substrate during the specimen handling. Figure 4.13a shows the NRA spectrum of the titanium anodized at 20 V in 1 M H 3 PO 4 electrolyte. Peaks displayed are attributed to 16 O(d, p 1 ) 17 O and 16 O(d, p o ) 17 O reactions for oxygen in the single barrier anodic film on the titanium substrate, which are located around the channels 40 and 90 respectively. In terms of the 12 C(d, p o ) 13 C reaction, it is detected at the channel of 143. The low yield to the right of the main peak originates from oxygen of the anodic oxide shows an upper limit of
80 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids atoms cm -2 which is close to the oxygen content measured in the RBS analysis. Figure 4.13b reveals similar peaks for 16 O(d, p 1 ) 17 O and 16 O(d, p o ) 17 O reactions near channels 110 and 240, and for the 12 C(d, p o ) 13 C reaction near channel 310. The main peak originating from oxygen of the anodic oxide shows a content of atoms cm -2 which is close to the oxygen content measured in the RBS estimation. The similar yields for the 12 C(d, p o ) 13 C reactions result from carbon contamination of the titanium substrate Glow Discharge Optical Emission Spectroscopy GDOES analyzes for the anodic films formed at 10 and 50 V in the sulphuric and phosphoric acids are displayed in Figure Anodic films formed in an aqueous electrolyte at ambient temperature are usually contaminated with species derived from electrolyte anions. As observed in Figures 4.14a and b, titanium, oxygen, and sulphur species are present throughout the film. The wavy profiles of titanium originate from an interference effect, and the sputtering time (dash line) to reach the titanium substrate is increased from 10 to 50 V due to the increased film thickness. It is also shown that phosphors and oxygen species are incorporated into the films after anodizing in the phosphoric acid (Figures 4.14c and d). The amount of oxygen increased from 20 to 50 V, and a low concentration of phosphors species was found at both voltages X-ray Photoelectron Spectroscopy Figure 4.15a shows the high resolution Ti2p peaks for films formed in both acids. A characteristic Ti2p doublet structure, consistent with peaks at and ev, is identified. These peaks are attributed to Ti 4+ [139]. The high resolution O1s spectra, shown in Figure 4.15b, are disclosed at 530.2, and ev. The peaks could be deconvoluted into peaks at 530.1, and ev for Ti-O bonds, Ti-OH bonds and adsorbed water (H 2 O) respectively [140]. The O1s spectra indicate the production of hydroxide, hydroxyl groups, bound water and hygroscopic water; thus, oxygen could also be induced during the decomposition of hydroxides. The C1s spectra are presented in Figure 4.15c; the carbon species are detected at 282.8, and ev. The peaks can be assigned to C-C/C-H (284.8 ev), C-O (286.7 ev) and O-C=O (288.9 ev) groups [141]. The high resolution spectrum of the C1s peaks 80
81 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids features a small amount of carbonaceous species within the oxide. The presence of various carbon species is probably attributable to surface contaminations by absorbing carbon-containing molecules during sample handling or material machining. The XPS resolution spectrum in terms of S2p from the sulphuric acid electrolyte indicates the presence of sulphated titanium oxide such as TiSO 4 at a peak line position of ~169.2 ev (Figure 4.15d). For the phosphoric acid, XPS spectra disclose the presence of phosphate titanium oxide such as TiPO 4 (Figure 4.15e). The phosphate film layer formed on the oxide surface may inhibit certain degrees for the amorphous-to-crystalline transition Electrochemical Impedance Spectroscopy The Bode diagrams of the anodic oxide films formed in the sulphuric and phosphoric acids at the six different anodic voltages are displayed respectively in Figure The appearance of the impedance spectra as a function of the voltage is presented in Figure 4.16a and Figure 4.16d. Over the frequency range, the impedance increases slightly with increase of anodic voltage, suggesting a reduced capacitance of the anodic oxide film. Further, for the frequency window inspected, one time constant is observed since the impedance changes approximately linearly with frequency, with a slope of ~ -1. A predominantly capacitive behaviour is evident, indicating from medium to low frequencies by phase shift approaching -90 o (Figure 4.16b and Figure 4.16e). It is suggested that a highly stable oxide film is formed on titanium in the both acids. The Nyquist diagrams are shown in Figure 4.16c and Figure 4.16f respectively, also indicating that one time constant was resolved. Thus, it is confirmed that a single barrier-layer oxide film was grown during anodizing. The results were fitted by an equivalent circuit consisting of a capacitance, accounting for the capacitive behaviour of the anodic film, in parallel with a resistance for the dissipative resistance to current flow across the oxide, both in series with a solution resistance. The data from the capacitance were also used to gain a qualitative estimation of the permittivity of the anodic oxide film in the two electrolytes. According to the calculation by T = εε 0 /C [59], where T is the oxide thickness (nm), ε is the dielectric permittivity of TiO 2 formed in the acid electrolyte, ε 0 is dielectric permittivity of free space ( F m -1 ) and C is the oxide 81
82 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids capacitance (F cm -2 ). The dielectric permittivity of the anodic oxide maintained at 50 V in sulphuric and phosphoric acids are measured as ~77.6 and ~62.4 respectively. Figure 4.17 shows that the linear relationship between the oxide thicknesses and the voltages is identified in both acids. The thicknesses of anodic oxide films formed in the phosphoric acid from 10 to 60 V are all higher compared with the sulphuric acid Discussion The potentiodynamic polarization mainly accounts for the current density associated with an oxide film growth and oxygen evolution [80]. An oxide film is formed at the potentiodynamic polarization stage, and the film continuously grows during the following potentiostatic anodizing. The suppression of current efficiency for the oxide growth is triggered by the amorphous-to-crystalline transition which induces electron-conducting passages [142]. During the potentiostatic anodizing, the oxide thickness progressively increases from 10 to 60 V to maintain the order of ~1.5 nm V -1 and ~1.7 nm V -1 in the sulphuric acid and phosphoric acids respectively. From the X-ray diffraction patterns, the formation of anatase is evident within the anodic film formed at 50 V in the sulphuric acid, showing an abundant degree of crystalline phases of anatase. In contrary, the amorphous-to-crystalline transitions during the oxide growth may be impeded slightly by the incorporation of phosphate anions for anodizing at 50 V in the phosphoric acid. The distribution of the crystalline oxide can be correlated with the ionic transport. In general, the crystalline phase has a higher ionic resistivity, and hence a higher electric field than the amorphous phase [142]. Thus, the probability of excitation of electrons in the valence band, formed by overlapping of O2p orbitals in the crystalline titania, to the conduction band is enhanced, leading to the oxidation of O 2- ions to form O 2 molecules and bubbles. Finally, an oxide containing oxygen-filled cavities is developed. The increased gas pressure in the bubbles within the film results in the formation of blisters and eventual rupture of the film. Additionally, the potentiodynamic polarization is scanned first, ranging from 0 V (vs. SCE) to a selected anodic voltage (vs. SCE), and oxygen evolution occurs between specific regimes. Oxygen bubbles are developed within the oxide film formed before the potentiostatic anodizing. The quantities of oxygen-filled cavity and oxide blisters 82
83 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids could be induced significantly under such condition, as indicated from SEM comparisons. Thus, the amounts of oxygen are probably higher after anodizing compared with the traditional anodizing method without an application of potentiodynamic polarization. Also, nanocrystals are nucleated within the oxide, and an increase in electronic conduction occurs. More crystalline phases are probably generated by an increase in the anodic current density with increase of anodic voltage, resulting in the increase of the electron conduction. Consequently, during the anodizing, the localized bursting of the oxygen bubbles creates the cracking textures of the film. Further, the dielectric permittivity estimated by EIS measurements confirms that it is dependent upon the nature of electrolyte. The value of oxide film permittivity from the sulphuric acid anodizing is higher than the film formed from the phosphoric acid anodizing. It is inferred that the electron conductivity of the titanium oxide generated in sulphuric acid is higher than phosphoric acid with the same concentration, introducing probably more conducting passages for the development of oxygen bubbles [143]. During the polarization and anodizing stages, titanium is rapidly oxidized by this reaction: Ti + O 2- = Ti e -, and an TiO 2 film with ruptures due to oxygen evolution is formed. The polarization stage may assist the ruptured film to re-grow from the oxidative re-passivation; however, this is still not known until future studies. Additionally, the XPS results show that for the anodizing in the phosphoric acid, the reduction of current efficiency for the film growth may be inhibited in somewhat by the phosphate film layer formed within the oxide. Moreover, a small amount of carbon species detected by XPS comprise hydrocarbon species with small amounts of singly and doubly bound oxygen. Therefore, the carbon species could be of interest as an inhibitor that hinders oxygen evolution to a certain extent by adsorbing a certain amount of oxygen through carbon species, which optimizes the current efficiency for anodic oxide growth. However, this inhibition mechanism is not clear until further studies. 83
84 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids 4.5. Summary 1). The grain boundaries of CP-Ti can be observed after etching in HF+HNO 3 or rf- GD sputter cleaning. The grain orientations of titanium are distinguished by EBSD, and three main orientations of [0001] and [11-20] and [10-10] are detected. 2). A stable single-barrier anodic film can be formed using an electrochemical technique with a combination of potentiodynamic polarization and potentiostatic anodizing in the sulphuric and phosphoric acid electrolytes respectively. 3). An amorphous-to-crystalline transition is generated with the nucleation of nanocrystals during the potentiodynamic polarization stage from 10 to 60 V. Oxygen evolution is developed in the both potentiodynamic polarization and potentiostatic anodizing processes, resulting in the formation of blister structures of the films. The rupture of the anodic oxide film is initiated with the bursting of bubbles. 4). The anodic film is ruptured after anodizing from 20 V for the sulphuric acid electrolyte but induced from 50 V in the phosphoric acid electrolyte since the amorphous-to-crystalline transition within the oxide growth is impeded by the incorporation of phosphate anions to a certain degree. The higher dielectric permittivity of the film in the sulphuric acid indicates higher electron conductivity compared with the phosphoric acid. The thicknesses of anodic oxide films formed from 10 to 60 V in the sulphuric acid are lower compared with the phosphoric acid. 84
85 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a b c d Figure 4.1. Scanning electron micrographs at different magnifications of as-received CP-Ti after GDOES sputter cleaning (a and b) and after etching in 48% HF + 70% HNO 3 (c and d) at ambient temperature. 85
86 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a b c Figure 4.2. Electron backscatter diffraction (EBSD) of CP-Ti after etching in HF + HNO 3 ; a, the mapping area of titanium; b, mapping result; c, inverse pole figures (IPF). 86
87 Potential / V. vs (SCE) Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids H 2 SO 4 H 3 PO 4 Current spent for oxide film growth and oxygen evolution Current spent for oxygen evolution Current spent for oxide film growth -10 1E-6 1E-5 1E-4 1E Current Density / A. cm -2 Figure 4.3. Potentiodynamic polarization scanning from 0 V (vs. SCE) to 60 V (vs. SCE) in 1 M sulphuric and 1 M phosphoric acids. 87
88 Current Density (A cm -2 ) Current Density (A cm -2 ) Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a 10V 20V 30V 40V 50V 60V Time (s) b 10V 20V 30V 40V 50V 60V Time (s) Figure 4.4. Time evolution of the current density at different anodic voltages in 1 M sulphuric acid, (a) in 1 M phosphoric acid (b). 88
89 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Figure 4.5. The colourations of anodic oxide formed on titanium following anodizing in sulphuric (up) and phosphoric acid (down) at different anodic voltages. The original photos were cropped to highlight the colours. 89
90 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a b c d e f Figure 4.6. Scanning electron micrographs of the anodic films formed at 10 (a), 20 (b), 30 (c), 40 (d), 50 (e) and 60 (f) V for 900 s in 1 M sulphuric acid. 90
91 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a b c d e f Figure 4.7. Scanning electron micrographs of the anodic films formed at 10 (a), 20 (b), 30 (c), 40 (d), 50 (e) and 60 (f) V for 900 s in 1 M phosphoric acid. 91
92 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Figure 4.8. Transmission electron micrographs of the anodic films formed at 50 V for 900 s in 1 M sulphuric acid (a, b, c) and phosphoric acid (d, e, f) respectively at different magnifications. 92
93 Intensity (arb. unit) Intensity (arb. unit) Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a T T T: Titanium A: Anatase 50V 30V 10V (101) A T T T T T T T (200) A T T T 1 M H 2 SO 4 (105) A T T T T T T T (224) T A (224) TT A (224) TT A Position (2 o Theta) b 50V T T T T T T T T: Titanium A: Anatase 1 M H 3 PO 4 T T (224) T T A 30V T T T T T T (224) T T A 10V T T T T (224) TT A Position (2 o Theta) Figure 4.9. X-ray diffraction patterns of anodic films formed at 10 V, 30 V and 50 V in sulphuric acid (a) and phosphoric acid (b) for 900 s. 93
94 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Figure RBS spectra with fitted curves for the anodic film formed at 20 (a) and 50 (b) V respectively in 1 M H 2 SO 4 electrolyte. 94
95 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Figure RBS spectra with fitted curves for the anodic film formed at 20 (a) and 50 (b) V respectively in 1 M H 3 PO 4 electrolyte. 95
96 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids Figure NRA spectra of anodic film formed at 20 (a) and 50 (b) V in 1 M H 2 SO 4 electrolyte. Figure NRA spectra of anodic film formed at 20 (a) and 50 (b) V in 1 M H 3 PO 4 electrolyte. 96
97 Intensity / V Intensity / V Intensity / V Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a Ti Oxide Film O S Sputtering Time / s b Ti Oxide Film O S Sputtering Time / s c Ti Oxide Film O P Sputtering Time / s 97
98 Intensity / V Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids d Ti Oxide Film O P Sputtering Time / s Figure GDOES depth profiling analysis of anodic film formed in sulphuric acid and phosphoric acid at 10 V (a and c) and 50 V (b and d). 98
99 Intensity (arb. unit) Intensity (arb. unit) Intensity (arb. unit) Intensity (arb. unit) Intensity (arb. unit) Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a Ti 4+ H 2 SO 4 b Ti-O H 2 SO 4 H 3 PO 4 H 3 PO 4 Ti 4+ Ti-OH H 2 O Bind Energy (ev) Binding Energy (ev) c C-C/C-H H 2 SO 4 H 3 PO 4 d S 6+ S2p H 2 SO 4 C-O O-C=O Bind Energy (ev) Binding Energy (ev) e P 5+ P2p H 3 PO Binding Energy (ev) Figure High resolution XPS spectra of anodic films formed at 50 V in sulphuric acid and phosphoric acid electrolytes respectively. (a) Ti2p peak; (b) O1s peak, (c) C1s peak, (d) S2p peak from sulphuric acid and (e) P2p peak from phosphoric acid. 99
100 Impedance Phase / Degrees Phase Angle / Degree Impedance Modulus / ohm. cm 2 Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids a 10V 20V 30V 40V 50V 60V Impedance Modulus / ohm. cm d 10V 20V 30V 40V 50V 60V Frequency / Hz Frequency / Hz b 10V 20V 30V 40V 50V 60V e 10V 20V 30V 40V 50V 60V Frequency / Hz Frequency / Hz Zim / ohm.cm c 10 V 20 V 30 V 40 V 50 V 60 V Zim / ohm.cm f Zre / ohm.cm Zre / ohm.cm 2 Figure Bode plots as a function of voltages, and Nyquist diagrams in sulphuric acid (a, b and c) and phosphoric acid (d, e and f). 100
101 Anodic Oxide Thickness / nm Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids H 2 SO 4 H 3 PO Anodic Voltage / V Figure Relationship between film thicknesses and voltages in sulphuric and phosphoric acids. 101
102 Chapter 5 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl Environment 5.1. Introduction Commercially pure titanium exhibits significantly resistance to corrosion in neutral chloride environments even at relatively high temperatures [144]. However, titanium can still be probably attacked by the electrolytes containing chloride and oxygen ions [141]. The effects which might be caused by the integration between oxygen and chlorides for the destructive behaviour of the natural oxide film formed on titanium. Further, the corrosion resistance can probably be improved by anodizing to generate anodic films on titanium. Also, the comparison between the corrosion resistance of titanium anodic film formed from sulphuric acid and phosphoric acid respectively in NaCl environments has not been reported. Thus, in the present chapter, the corrosion behaviour of titanium anodic films in a near-neutral 3.5% NaCl electrolyte are studied Voltage-Time Responses The voltage-time response for the titanium specimen during anodizing in 1 M H 2 SO 4 resulted in an initial voltage rise to ~2 V due to the presence of the air-formed oxide film (Figure 5.1a). Then, the voltage rose to ~26 V at ~3.2 V s -1. The rise of voltage was slightly reduced from ~26 V to ~60 V followed by a significant reduction of voltage after ~60 V, and it was terminated at ~97 V. The colour scales vary from sky blue through pale blue to dark yellow associated with anodizing to 20, 40 and 60 V respectively due to interference effects [51]. On the other hand, anodizing of titanium in 1 M H 3 PO 4 showed a similar initial surge to ~3 V, followed by a linear rise at ~4 V s -1 (Figure 5.1b). The rise then was slightly increased to V s -1 for the time of ~7 s to 11 s. The rise of voltage was terminated at ~90 V at 18 s. Figure 5.1b also displays the surface colours of titanium after anodizing to different voltages. The colour varies from brown-pale through blue-pale to yellow for anodizing to 20, 40 and 60 V respectively. Further, the response time of anodic film growth in the phosphoric acid is shorter to reach 20, 40 and 60 V compared with the sulphuric acid. It is inferred that the current efficiency for the film growth in H 3 PO 4 102
103 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment is higher compared with H 2 SO 4. Further, oxygen bubbles were observed, which originated on the titanium surfaces during the anodizing tests; thus, the occurrence of oxygen evolution is confirmed for both anodizing conditions Scanning Electron Microscopy Figure 5.2a shows the as-received titanium after etching in the HF+HNO 3 electrolyte; grains and/or grain boundaries are observed due to the removal of the pre-existed airformed oxide film. Figure 5.2b shows the amorphous oxide, displaying a compact structure formed after anodizing to 20 V in the sulphuric acid. The oxide film ruptures from the initiation of a blister texture due to sufficient oxygen evolution occurred during anodizing to 40 V (Figure. 5.2c). The ruptures of the film within different regions are also observed after anodizing to 60 V (Figure 5.2d). For anodizing in the phosphoric acid, the oxide film without a blister structure is observed at 20 V (Figure 5.2e). However, the blisters are evident at 40 V (Figure 5.2f), and a similar oxide film texture is present after anodizing to 60 V (Figure 5.2g). Figure 5.3 shows the scanning electron micrographs of the etched and anodized titanium after immersion in naturally aerated 3.5% NaCl electrolyte for 60 days at ambient temperature. As it is known, an oxide film is formed on titanium in the presence of oxygen or water. Therefore, Figure. 5.3a shows that the titanium surface is covered by a thin air-formed oxide film during the immersion and no grains and/or grain boundaries are exposed. Figure 5.3b shows that the anodic film formed after anodizing to 20 V in the sulphuric acid followed by the immersion in the 3.5% NaCl electrolyte is less uniform compared with the anodic film prior to the immersion, and blisters initiated by the development of oxygen bubbles during anodizing are observed. It is revealed that the oxide film is similar compared with the morphology created immediately after the anodizing tests, except that the rupture of oxide film is more significant for anodizing to 40 and 60 V (Figure 5.3c and d). It is possible that the anodic films were attacked by the chloride ions to a certain extent, leading to more ruptures [145]. Figure 5.3e shows that the anodic film formed after anodizing to 20 V in the phosphoric acid followed by the immersion treatment in the NaCl electrolyte for 60 days comprises of micro-oxygen bubbles that the surface is less uniform compared with the original anodized surface. The oxide film morphology 103
104 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment formed after anodizing to 40 V and after the 60 days immersion displays a uniform region and localized film rupture; however, the bubble textures are not evident (Figure 5.3f). Additionally, Figure 5.3g shows that the anodic film generated after anodizing to 60 V and after the long-term immersion develops pores due probably to the dissolution of the film at certain regions Electrochemical Impedance Spectroscopy The as-received titanium after etching and immersion in the NaCl electrolyte was studied by electrochemical impedance techniques, with the results plotted in the form of Bode diagrams since they show all details for impedances and phase shifts, as shown in Figure 5.4. The impedance measured for the etched titanium is slightly higher compared with the etched specimen followed by the immersion in the NaCl for 60 days. The capacitive behaviour between medium and low frequency is more significant as the phase shift approaches -90 o for the specimen immersed for 60 days compared with the just etched specimen due to the removal of the protective airformed film previously. The relationship of the impedance spectra with the anodic voltage is presented in Figure 5.5 and Figure 5.6 associated with the anodic films formed in the sulphuric and phosphoric acids respectively. Over the frequency range inspected, the impedance for the anodic films generated in both acids increases slightly as the voltage increases. The impedance of the anodized titanium after immersion in the NaCl electrolyte for 60 days increases with increase of the voltage, and the impedances are lower than the anodized specimens without immersion. Moreover, according to the inspection of the frequency window, one time constant is evident since the impedances change linearly with frequency, and a slope of -1 is revealed. A capacitive behaviour is also evident by the phase shifts approaching -90 o. It is indicated that highly stable oxide films are formed after anodizing to different anodic voltages respectively. After the 60 days immersion in the NaCl, the oxide capacitance is reduced by lowering the phase shifts approaching -80 o from the medium to low frequency. 104
105 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment The results were fitted using an R(CR) equivalent circuit [80, 146], an oxide capacitance, for the capacitive behaviour of the anodic film, in parallel with an oxide resistance, responsible for the dissipative resistance to current density spent for the film growth, and both in series with an electrolyte resistance (Figure 5.7). The data are listed in Table 5.1, showing that the film impedance resistance is increased with anodic voltage for the just anodized titanium in both acids and after immersion in NaCl respectively. It is inferred that the corrosion resistance in the NaCl electrolyte is improved through anodizing at a higher anodic voltage. Further, the oxide film capacitance is reduced with increase of anodic voltage in either just anodized or anodized followed by the immersion for 60 days, and a high electric field could be developed across the anodic film due to the occurrence of amorphous-to-crystalline transition during the growth of the film [80]. The oxide capacitance can also characterize the function of the oxide film thickness to the voltage, as expressed as follows: 5.1 where the oxide film capacitance, C, is a function of the absolute permittivity in vacuum ɛ 0 ( F m -1 ), the relative permittivity of TiO 2 ɛ r (the value of 80 is assumed [147, 148], the exposed testing area, A (1.0 cm 2 ) and the oxide film thickness, d (nm). Figure 5.8 shows that the thickness of the anodic oxide film has a linear relationship with the anodic voltage for both just anodized and anodized followed by the immersion circumstances. A growth rate of 2.0 nm V -1 after anodizing in H 2 SO 4 and 2.3 nm V -1 in H 3 PO 4 electrolyte are revealed. The thicknesses of the anodic oxide films are reduced after the 60 days immersion in the NaCl. 105
106 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment Table 5.1. The electrochemical impedance spectroscopy parameters of CP-Ti electrode in different experimental conditions. Experimental Condition Anodic Voltage (V) R s (Ω.c m 2 ) R o (Ω.cm 2 ) C o (µf cm - 2 ) Etched in HF+HNO 3 / Immersed in NaCl / M H 2 SO 4 Immersed in NaCl after anodizing in H 2 SO 4 1 M H 3 PO 4 Immersed in NaCl after anodizing in H 3 PO Potentiodynamic Polarization Measurements The potentiodynamic polarization plot of the as-received titanium after etching in the HF+HNO 3 shows that a small passive region initiates from V (vs. SCE) to ~0 V (vs. SCE), followed by an rapid increase of the current due to the occurrence of oxygen evolution, until the potential reaches ~1.50 V (vs. SCE). Later, a secondary passive region, identified from the potential to 6 V (vs. SCE) is revealed (Figure 5.9). The potentiodynamic polarization plot obtained for the etched titanium after the immersion in the NaCl electrolyte for 60 days (Figure 5.9) shows a similar passive- O 2 evolution transition, starting from ~-0.35 V (vs. SCE) to ~1.80 V (vs. SCE), and a re-passive state is shown until the test is terminated at a potential of 6 V (vs. SCE). The corrosion potentials and corrosion current densities, measured from the polarization curves based on the Tafel method [149], are listed in Table 5.2. The Table shows that the corrosion potential and corrosion current density for the titanium after etching are both lower than the specimen after the 60 days immersion in the NaCl electrolyte. 106
107 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment Figure 5.10 shows the potentiodynamic polarization curves associated with titanium anodized to 20, 40 and 60 V in the sulphuric acid after immediate immersion and after immersion for 60 days in the NaCl electrolyte respectively. The evidence of a passive region in all of the conditions, from ~-0.60 V (vs. SCE), is displayed. Further, a decline in the current density from each relatively passive stage between ~-0.50 V (vs. SCE) and ~0.60 V (vs. SCE) is evident. The oxygen evolution occurs after the passive region, and it is maintained until the secondary-passive state with oxygen evolution is induced from ~3.10 V (vs. SCE), leading to a rise in the current density. The polarization curves of the titanium anodized to 20, 40 and 60 V respectively in the phosphoric acid associated with the immediate immersion and immersion for 60 days in the NaCl electrolyte respectively are presented in Figure A passive region in all the situations commences from ~-0.5 V (vs. SCE). The current density decreases slightly with increase of the corrosion potential within the passive region due to the formation of a protective passive oxide film. The current density increases rapidly, which is resulted from oxygen evolution after the passive state until a secondary-passive polarization dominates after ~ 3.21 V (vs. SCE). The corrosion potentials and corrosion current densities are listed in Table 5.2. Compared with the as-received etched titanium, the corrosion potentials for the anodized titanium are higher but the corrosion current densities are lower. Further, the corrosion potential for the anodized titanium shows a mild increasing trend with increase in the voltage while the current density decreases. Similar results are obtained for the titanium anodized in the H 2 SO 4 and H 3 PO 4 electrolytes respectively followed by immersion in the NaCl for 60 days. The increased anodic voltage results in a slight increase of corrosion potential but a reduction of the corrosion current density. 107
108 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment Table 5.2. The potentiodynamic polarization parameters of CP-Ti electrode in different experimental conditions. Experimental Condition Anodic Voltage (V) Corrosion Potential (V. vs SCE) Corrosion Current Density (A. cm -2 ) Etched in Kroll s reagent / Immersed in NaCl / M H 2 SO 4 Immersed in NaCl after anodizing in H 2 SO 4 1 M H 3 PO 4 Immersed in NaCl after anodizing in H 3 PO Discussion Comparison of anodic films formed on titanium in the sulphuric and phosphoric acid electrolytes was obtained from the SEM observations. Oxygen bubbles developed have a significant influence within the oxide film generated in the H 2 SO 4. In summary, the formation of blisters was initiated earlier than in the H 3 PO 4 electrolyte. The inhibition of oxygen evolution during the anodizing in the phosphoric acid is assumed to a greater extent compared with anodizing in sulphuric acid. Further, it has been reported that an amorphous-to-crystalline transition cannot be ruled out for the growth of anodic oxide films on titanium during anodizing [120]. The oxygen bubbles generated from the oxygen evolution would be developed within the crystalline phases due to their higher electron-conducting property compared with the adjacent amorphous phases [84], and ingress of the electrolyte in proximity to the metal-oxide interface, resulting in film rupture. Also, when the anodic voltage increased, more currents for the growth of the film were spent for oxygen evolution. Subsequently, the film textures containing oxygen bubbles, leading to the resultant blisters, are more significant. On the other hand, after the 60 days immersion in the 108
109 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment 3.5% NaCl electrolyte, the pore-like defects within the anodic oxide films were found, indicating that the dissolution of the film was induced by the development of oxygen bubbles and the attack of chloride ions. According to the point defect model [150], the oxide film contains oxygen vacancies which are generated at the TiO 2 / NaCl interface, and the chloride ions react with the vacancies. Thereby, more metal vacancies would be introduced. Further, it is also reported that the redundant metal vacancies located in the Ti / TiO 2 interface would result in the dissolution of the oxide film [151]. During the immersion experiments in the NaCl electrolyte for 60 days, the re-growth of anodic oxide film was probably triggered since titanium specimens were exposed to water and/or oxygen, maintaining the film thickness, which has been estimated by EIS. It was also evident that thicker anodic oxide films were formed by anodizing in the phosphoric acid compared with the sulphuric acid. A higher corrosion resistance in the NaCl environment can also be obtained from thicker anodic films compared with the films formed in the sulphuric acid. Thus, for certain harsh and crucial working or testing environments containing chloride ions, in order to provide surface protection of CP-Ti, the anodizing employed in phosphoric acid should be considered in the first place if compared with sulphuric acid. The corrosion rate can be determined from corrosion current density [152], and a higher current density is corresponding to a higher corrosion rate. Consequently, the dissolution rate of the anodic oxide films increases with increase of corrosion current density. Additionally, the oxygen bubbles developed within the anodic oxide film during anodizing in the sulphuric and phosphoric acids respectively resulted in the rupture of the film through the bursting of the bubbles. Thus, after the 60 days immersion in the NaCl electrolyte, the anodic films would be further ruptured in certain regions by the attack of chloride ions from the NaCl electrolyte [151]. Moreover, phosphate species what are incorporated into the anodic oxide films during anodizing in the phosphoric acid electrolyte may inhibit the amorphous-tocrystalline transition to a greater extent compared with the incorporation of the sulphate species into the anodic films generated from the sulphuric acid electrolyte [153]. Therefore, compared with the sulphate species, the existence of phosphate 109
110 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment species could increase the corrosion potential and reduce the current density during the polarization tests in the 3.5% NaCl electrolyte Summary 1). Prior to the corrosion tests, ruptures of the anodic films induced by the bursting of oxygen bubbles originated from oxygen evolution during anodizing are revealed. The bursting of oxygen bubbles releases a huge pressure within the film and a consequent blister surface texture is formed. 2). The rupture is more significant for the anodic films formed in the sulphuric acid compared with the phosphoric acid, indicating that oxygen evolution can be inhibited to a greater extent during anodizing in the phosphoric acid. Further, the anodic films with more ruptures are found after anodizing to higher anodic voltages. The films with more ruptures are also observed after the anodized titanium specimens are immersed for 60 days in the 3.5% NaCl compared with the immediate immersion condition. 3). The corrosion resistance for CP-Ti can be improved in the NaCl environment by anodizing treatments with increase of anodic voltage. 4). The anodic oxide films formed in the phosphoric acid provide a higher corrosion resistance compared with the anodic films formed in the sulphuric acid after anodizing to the same voltage. Consequently, the anodic oxide films formed on titanium in the phosphoric acid perform a greater corrosion resistance compared with the sulphuric acid for serving in chlorides-containing environments. Notification: some of the work in the Chapter 5 has been published in International Journal of ELECTROCHEMICAL SCIENCE, 9(2014) , please see Appendices. Thus, some of the published contents in this chapter have been rephrased to avoid the self-plagiarism issue. 110
111 Anodic Voltage (V) Anodic Voltage (V) Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment M H 2 SO 4 (a) Time (s) M H 3 PO 4 (b) Time (s) Figure 5.1. Voltage-time responses during anodizing of CP-Ti in 1 M H 2 SO 4 (a) and 1 M H 3 PO 4 (b) electrolytes at 20 ma cm -2 respectively at ambient temperature. 111
112 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment a b c d e f g Figure 5.2. Scanning electron micrographs of titanium after etching in HF+HNO 3, and anodic films formed at 20 ma cm -2 to different voltages in 1 M H 2 SO 4 and 1 M H 3 PO 4 electrolytes respectively: (a) etched titanium; anodizing in H 2 SO 4 to (b) 20 V; (c) 40 V and (d) 60 V; anodizing in H 3 PO 4 to (e) 20 V; (f) 40 V and (g) 60 V. 112
113 Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment a b c d e f g Figure 5.3. Scanning electron micrographs of titanium after etching, and anodized at 20 ma cm -2 to different voltages in 1 M H 2 SO 4 and 1 M H 3 PO 4 electrolytes respectively followed by immersion for 60 days in naturally aerated 3.5% NaCl electrolyte; (a) etched titanium; anodized in H 2 SO 4 to (b) 20 V; (c) 40 V and (d) 60 V; anodized in H 3 PO 4 to (e) 20 V; (f) 40 V and (g) 60 V. 113
114 Impedance Modulus / ohm. cm 2 Impedance Phase / degrees Impedance Modulus / ohm. cm 2 Impedance Phase / Degrees Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment Original Ti Original Ti, after immersion for 60 days Frequency / Hz Figure 5.4. Bode plots of the etched titanium after immediate immersion, and after immersion for 60 days in naturally aerated 3.5% NaCl electrolyte Anodizing to 20 V Anodizing to 40 V Anodizing to 60 V Immersion for 60 days after anodizing to 20 V Immersion for 60 days after anodizing to 40 V Immersion for 60 days after anodizing to 60 V Anodizing in 1 M H 2 SO Frequency / Hz Figure 5.5. Bode plots of titanium anodized in 1 M H 2 SO 4 to 20, 40 and 60 V respectively, and followed by immersion for 60 days in naturally aerated 3.5% NaCl electrolyte. 114
115 Impedance Modulus / ohm. cm 2 Impedance Phase / Degrees Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment 10 7 Anodizing to 20 V Anodizing to 40 V 10 6 Anodizing to 60 V Immersion for 60 days after anodizing to 20 V Immersion for 60 days after anodizing to 40 V 10 5 Immersion for 60 days after anodizing to 60 V Anodizing in 1 M H PO Frequency / Hz Figure 5.6. Bode plots of titanium anodized in 1 M H 3 PO 4 to 20, 40 and 60 V respectively, and followed by immersion for 60 days in naturally aerated 3.5% NaCl electrolyte. Figure 5.7. Equivalent circuit for the monitoring of the impedance spectra. R s, C o and R o, as the electrolyte resistance, anodic film capacitance and film resistance respectively. 115
116 Potential / V. vs SCE Anodic Oxide Thickness / nm Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment 180 Anodizing in 1 M H 2 SO Immersion in NaCl after anodizing in H 2 SO 4 Anodizing in 1 M H 3 PO Immersion in NaCl after anodizing in H 3 PO y = 2.0x y = 1.6x y = 2.3x y = 1.7x Anodic Voltage / V Figure 5.8. The relationship between film thickness and anodic voltage before and after immersion experiments in naturally aerated 3.5% NaCl electrolyte Original Ti Original Ti after immersion for 60 days 4 3 Secondary passivation O 2 evolution Passivation Current Density / A. cm -2 Figure 5.9. Potentiodynamic polarization plots of the etched titanium immediate immersed and immersed for 60 days in naturally aerated 3.5% NaCl electrolyte. 116
117 Poatential / V. vs SCE Potential / V. vs SCE Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment Anodizing to 20 V Anodizing to 40 V Anodizing to 60 V Immersion for 60 days after anodizing to 20 V Immersion for 60 days after anodizing to 40 V Immersion for 60 days after anodizing to 60 V Anodizing in H 2 SO E-9 1E-8 1E-7 1E-6 1E-5 1E-4 1E Current Density / A. cm -2 Figure Potentiodynamic polarization plots of the anodized titanium in 1 M H 2 SO 4 immediate immersion and after immersion for 60 days in naturally aerated 3.5% NaCl electrolyte Anodizing to 20 V Anodizing to 40 V Anodizing to 60 V Immersion for 60 days after anodizing to 20 V Immersion for 60 days after anodizing to 40 V Immersion for 60 days after anodizing to 60 V Anodizing in H 3 PO Current Density / A. cm -2 Figure Potentiodynamic polarization plots of the anodized titanium in 1 M H 3 PO 4 immediate immersion and after immersion for 60 days in naturally aerated 3.5% NaCl electrolyte. 117
118 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte 6.1. Introduction The use of titanium for medical implant materials has attracted attention on account of its good biocompatibility and ability to bind directly to bone tissues [154]. Such excellent characteristics have been attributed to the thin natural oxide layer, approximately nm in thickness that forms on the substrate of titanium on exposure to air or water at room temperature during manufacturing and machining. The investigations on performance of titanium biocompatibility were concerned with the development of titanium oxide layers, such as the reduction of the osseointegration time and thus, enhancing a patient s life. Accordingly, implants with porous surfaces have been particularly prepared to improve bone growth into the porous implant surface [111]. However, the naturally formed oxide layer contains many defects and it is bioinert. Therefore, studies have tried to explore various surface treatments to improve the oxide film as well as to endow bioactivity [154]. Although the structural and mechanical properties of anodic films on titanium have been studied, the morphological evolution and the electrochemical properties of the film at breakdown conditions are still rare. Particularly, the behaviour of crystallization within the porous oxide film formed on titanium is not clear. Therefore, this chapter investigates the formation mechanism porous anodic oxide films on CP-Ti in a phosphoric acid electrolyte at breakdown voltages. The film morphology, compositions and crystallization behaviour are evaluated Current Time Responses Figure 6.1 displays the current density time behaviour during anodizing at 100, 150 and 200 V in 1 M phosphoric acid electrolyte. At 100 V, the current density was initially held after a delay of ~1.0 s and decreased exponentially. A steady current density at ~ A cm -2 after ~900 s was maintained subsequently. According to the equation of Q = It, where Q is the electrical charge (C), I is the current density 118
119 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte (A cm -2 ) and t is the time (s), a charge of ~8.82 C was measured for the anodic film growth at 100 V after 900 s (Figure 6.1a). Further, sparking was not evident during the anodizing process at 100 V. For anodizing at 150 V, a similar qualitative transient of current density was evident compared with 100 V. A higher current density of ~ A cm -2 was measured after ~900 s, which results in a charge passed of ~22.41 C (Figure 6.1b). Sparking was observed during anodizing until the anodizing was terminated after 900 s. At 200 V, the sparks were significantly visible at the initiation of the anodizing, and continued for the remainder of the experiment. The current density time record shows a current density of ~ A cm -2 associated with a charge of ~ C that was passed after 900 s (Figure 6.1c). Further, oxygen bubbles that originated on the titanium surface were clearly observed for each anodizing condition Film Morphology Scanning electron micrographs in Figure 6.2 reveals that the morphology of the anodic oxide films formed on titanium is dependent upon the anodic voltage. At 100 V, initially, a uniform anodic oxide film was formed. Later, a few areas within the film dissolved locally, resulting in a porous film structure. Thereby, pores and craters were displayed in some regions of the oxide film (Figure 6.2a). At 150 V, the anodic oxide film broke down locally, and most of film regions were modified as the porosity increased. The film surface was occupied by pores in the size range of ~ nm or less (Figure 6.2b). At 200 V (Figure 6.2c), increased porosity, with an average pore size of ~ nm, is revealed. It was probably that the pores were interconnected with each other during anodizing, leading to the increase of porosity [65]. The number of pores in a unit surface region is significantly dependent on the anodic voltage. Further, it is shown that the pores in the anodic film are distributed more uniformly at 200 V compared with 150 and 100 V. In order to explore the surface morphologies of the porous anodic films in detail, the surface structures of the anodic films were examined at an ultra-high magnification in the SEM, as shown in Figure 6.3. The scanning electron micrographs reveal the surface structure around the pores developed at different voltages. The anodic film formed at 100 V comprised particulate-like structures in the nanometre range, and 119
120 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte they were connected with each other in different regions (Figure 6.3a). Some particulate-like structures were developed around pores within the film. The particulate-like structures were probably formed due to the breakdown dissolution of the film, and they were generated prior to the formation of the pores. Also, particulate-like structures appeared into the pores at the initiation of the pores. Additionally, the particulate-like oxides within the pores probably dissolved to increase the size of pores. Similar nanostructures are observed on the surface of the oxide film formed at 150 V, showing the film regions where pores were developed with the formation of nano-particulates (Figure 6.3b). In contrast, the nanoparticulates were not evident on the film formed at 200 V (Figure 6.3c). It is noticed that the size of pores is increased due probably to the complete dissolution of the particulates within these pores. Figure 6.4 shows cross-sections of the porous anodic films formed on titanium at different voltages. A porous anodic oxide film of ~4.7 µm thickness was generated on titanium at 100 V (Figure 6.4a). The thickness of anodic film formed at 150 and 200 V was ~9.8 µm and ~43.3 µm respectively (Figure 6.4b and c). According to the charges measured in the current density time curves and Faraday s law, the oxide film thicknesses related to the charges are estimated as ~5.3 µm, ~11.5 µm and ~52.9 µm for anodizing at 100, 150 and 200 V respectively. The current efficiencies are 89%, 85% and 81% for the film growth at 100, 150 and 200 V respectively. The cross-section of the anodic oxide film formed at 100 V comprises a porous texture which is developed uniformly towards the titanium substrate (Figure 6.4a). The inset micrograph of Figure 6.4a discloses the development of round pores that pass through almost the whole of the film region. Also, the sizes of the pores are ~170 nm or less. The inset image of Figure 6.4b shows that the anodic film formed at 150 V consists of a fine and round pore morphology developed within the cross-section. The pore size is estimated to be ~290 nm or less. At 200 V (Figure 6.4c), a porous anodic film with pores of a larger size of ~22.2 µm is evident in the inset graph Compositions of Anodic Films The presence of titanium, oxygen and phosphorus species throughout the film is identified by GDOES profiles for anodic films formed at different voltages (Figure 120
121 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte 6.5). The small wavy profile of titanium shown at 100 V is originated from an interference effect (Figure 6.5a) [153]. Anodic films formed in aqueous electrolytes are usually contaminated with species derived from the electrolyte anions. Thus, the film formed at 100 V shows the existence of phosphorus species. The amount of phosphorus species developed within the film is higher at 150 V (Figure 6.5b) in comparison with 100 V. At 200 V (Figure 6.5c), a relatively higher amount of phosphorus incorporation is displayed throughout the whole anodic film compared with 150 V. In addition, the amount of oxygen is similar at each voltage since GDOES is insensitive to oxygen species. For the porous film obtained at breakdown conditions, phosphorus species are incorporated into the anodic film from the electrolyte. During the film growth, phosphorus species migrate inwards under a high electric field as anionic species such as PO 3-4. In general, the migration of phosphorus species is slower than O 2- ions, and it is difficult for such species to approach the TiO 2 /Ti interface. However, the pores filled with the electrolyte may provide passages for phosphorus species developed in the film. The amount of phosphorus species could be increased if the porosity increased. Consequently, the amount of phosphorus increased with increased voltage Crystallization Behaviour Raman spectra disclose the crystalline structures of the titanium anodic films, with details of the related peaks, shown in Figure 6.6. The anatase crystalline peaks identified from Raman spectra mainly correspond to wavenumbers of , , , 516, cm -1 [134, 155, 156]. As detected, the anodic films formed at 100 and 150 V reveal similar peaks through the intensities of the spectra. However, the intensity for 100 V is lower than 150 V. The most intensive bands are found at 158 cm -1, which is assigned to the He-Ni laser energy instead of the samples. Peaks at 397, 516 and 632 cm -1 are characterized as crystalline anatase. At 200 V, three similar peaks, corresponding to 397, 516 and 633 cm -1, are disclosed. Further, the intensity is higher from 100 to 800 cm -1 compared with the other two voltages. 121
122 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte As observed, the intensity of anodic films formed in the phosphoric acid electrolyte is higher at higher voltage. Further, the amorphous-to-crystalline transition occurs with oxygen developed in the film, and the degree of the crystallization will be increased as the film thickness increases [120]. Thus, it is expected that the degree of crystallinity in the anodic oxide film generated at 200 V is more abundant than the other voltages Dielectric Properties The impedance spectra recorded as Bode diagrams for the porous anodic films formed on titanium are displayed in Figure 6.7. An outer porous oxide and an inner barrier oxide are evident from the two distinct capacitive behaviours from 100 k to 1 Hz. The phase shifts decreases since 10 0 Hz, and the degrees are lower than -70 o in the frequency range between 10 3 and 10 4 Hz, indicating that the anodic porous films are probably not fully capacitive. The impedance changes approximately linearly with frequency, with a slope of nearly -1. Over the frequency range inspected, the impedance is increased with increase of anodic voltage. On the other hand, the impedance has an inverse proportion with the capacitance [157]; therefore, a reduced capacitance of the anodic film is introduced at higher voltage. The electrochemical impedance systems are monitored using an electrical equivalent circuit. A circuit model of R s (Q p R p (Q b R b )) (Figure 6.8), where (R s ) represents the solution resistance, (Q p ) stands for the outer porous oxide capacitance and (Q b ) the inner barrier oxide capacitance, (R p ) and (R b ) are the charge transfer resistances of the outer porous layer and inner barrier layer. The fitted data of the circuit elements are listed in Table 6.1. It is shown that the R b values for 100, 150 and 200 V are nearly two orders larger than the R p values. Interestingly, the electrolyte may penetrate into the film during anodizing, and since the electrolyte has a lower resistance and higher dielectric constant than the air, the penetration may result in increase of the capacitance and decrease of resistance [158]. Conversely, R b and R p increase with increase of anodic voltage. Further, Q b and Q p both decrease with increase of anodic voltage. Thus, it is probabe that the phosphoric acid electrolyte penetrated into the porous film regions through pore channels to a small extent. The lower values of Q p at higher anodic voltages are related to thicker outer porous oxide 122
123 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte layers [159]. It is also shown that Q b for the inner oxide layer has a higher value compared with the outer oxide layer, indicating that the barrier layer might form at a steadier condition than the porous layer since pores are formed in the outer layer. Further, the crystallization results in a high electron conduction, enabling oxygen evolution to develop within crystalline regions [120, 160]. Compared with the lower voltages, the lowest values of the inner and outer oxide capacitances are evident at 200 V, indicating that the film has a higher electronic conductivity. Therefore, more oxygen bubbles could probably be induced within the both inner and outer layers formed at 200 V. Table 6.1. EIS fitted data for anodic films formed on CP-titanium at different voltages. Anodic Voltage (V) R s (Ω) R b (kω. cm 2 ) R p (kω. cm 2 ) Q b (μf cm -2 ) Q p (μf cm -2 ) Discussion The anodic oxide films generated by spark anodizing are typical of dielectric breakdown. The film forms due to the inward migration of O 2- ions to the metal/film interface and outward migration of the Ti 4+ ions from metallic Ti to the film/electrolyte interface. There are many reactions occurring during the process and the most relevant reactions that participate in the film growth are those which contribute to the formation of O 2 and TiO 2. Additionally, during the anodizing process, oxygen evolution occurred which reduces the current efficiency for anodic oxide growth with increase in anodic voltage. Thus, oxygen bubbles developed in the oxide produce a pressure that ruptures the film. The pores in the film are filled with the electrolyte favouring the passage of the current. These pores are also preferred sites for the development of oxygen evolution. Further, oxygen bubbles developed at the electrolyte/film interface are probably responsible for the formation of the circular shape of pores [111]. Figure 6.9 presents a schematic configuration of the porous anodic film growth when looking at each film formed in the phosphoric acid based on the GDOES profiles; a 123
124 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte double anodic oxide structure is proposed. Pores grow preferentially within the outer porous oxide layer. These pores are formed by the breakdown of the film and oxidation of O 2- ions either from the previously formed TiO 2 matrix or from migrating oxygen ions, to become molecular oxygen which accumulates as oxygen bubbles. In the porous layer, the oxygen bubbles are developed through pores, and the oxidation of O 2- ions is favoured by crystallites. Consequently, a high ionic resistivity is offered that facilitates the electron transfer [120]. Additionally, the anatase phase is known to have a better electron conducting behaviour than the amorphous phase, which would favour the transition of charges. With increasing the anodic voltage and thickening of oxide, the crystalline region nucleates, develops and propagates within the entire anodic film. The increased temperatures at high breakdown voltages may promote the growth of nanocrystals in regions of the barrier film adjacent to the breakdown sites as well as the nucleation of crystals in the amorphous regions. The electrolyte-derived contamination has a significant influence on the film growth. Phosphate ions diffuse and migrate through the oxide and generate chemical bonds, probably contributing to titanium phosphate compounds. The formation of titanium phosphates (e.g. TiPO 4 or Ti(HPO 4 ) 2-x ) during anodizing in phosphoric acid has been reported [108]; the XPS analyses allowed the determination of the chemical binding state of phosphorus species. This results in a high contamination level and a phosphorus concentration profile that slightly decreases towards the Ti/TiO 2 interface. Thus, the amorphous phase containing nanocrystals is stabilized almost throughout the entire double oxide layers. The crystallization initiates at the metal/inner oxide interface where the contamination level is lower, and develops further to the outer oxide layer. Additional reasons favouring the crystallization at the metal/oxide interface are assumed to be a template effect of the crystalline Ti substrate, leading to more ordered oxide regions and a higher stress field which is caused by the growth of oxide on Ti lattice regions Summary 1). The porous anodic film growth on CP-Ti by the breakdown anodizing in 1 M phosphoric acid electrolyte is significantly dependent upon the applied voltage. 124
125 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte 2). Anatase crystalline structures are developed within the porous anodic oxide films. The occurrence of oxygen evolution reduces the current efficiency for the growth of anodic oxide films with increase in anodic voltage. 3). The degree of crystallinity within the anodic film generated at a higher anodic voltage is more abundant. Particulate-like structures are developed within pores, and the increased size of pores is revealed due to the dissolution of the particulates. 4). The electronic conductivity of the oxide film formed at 200 V is probably higher compared with the film formed at lower voltages, leading to the development of more oxygen bubbles within the film. The phosphorus species are incorporated into the porous films formed at different voltages, and such species may stabilize the nanocrystals within the films. Notification: some of the works in the Chapter 6 have been published in Journal of Materials Engineering and Performance 24(2014)59-66, please see Appendices. Thus, some of the published contents in this chapter have been rephrased to avoid the self-plagiarism issue. 125
126 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte a 100 V Current Density / A.cm Time / s b 150 V Current Density / A cm Time / s c 200 V 3.0 Current Density / A.cm Time / s Figure 6.1. Current density-time responses of CP-Ti during anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid for 900 s at ambient temperature. 126
127 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte a b c Figure 6.2. Scanning electron micrographs of plan-views of the anodic films formed after anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid. The insets are higher magnification images of the respective anodic films. 127
128 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte a b c Figure 6.3. Ultra-high magnification scanning electron micrographs of the anodic films formed after anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid. 128
129 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte a Ti substrate Oxide Resin b Ti substrate Oxide c Resin Oxide Ti substrate Figure 6.4. Scanning electron micrographs of cross-sections of the anodic films formed after anodizing at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid. The inset images are higher magnification images of the respective anodic oxide film. 129
130 Intensity / a.u. Intensity / a.u Intensity / a.u. Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte a Ti O P Sputtering Time / s b Ti O P Sputtering Time / s c Ti O P Sputtering Time / s Figure 6.5. GDOES depth profiles of the anodic films formed on CP-Ti at 100 V (a), 150 V (b) and 200 V (c) in 1 M phosphoric acid; the intensities of phosphorus are multiplied by
131 Impedance Modulus / dm. cm 2 Impendance Phase / degree Intensity / a.u. Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte V V V Wavenumber / cm -1 Figure 6.6. Raman spectra of anodic films formed on CP-Ti in 1 M phosphoric acid at 100, 150 and 200 V V 150 V 200 V Frequency / Hz Figure 6.7. Electrochemical impedance spectroscopy results of anodic films formed after anodizing at 100, 150 and 200 V in 1 M phosphoric acid; as plotted in Bode diagrams; the left direction of arrow corresponds to the impedance modulus/dm.cm 2, and right direction corresponds to the impedance phase/degree. 131
132 Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte Figure 6.8. Equivalent circuit model for anodic porous films formed at 100 V, 150 V and 200 V in 1 M phosphoric acid. Figure 6.9. Schematic configuration of the growth state of the anodic film formed in phosphoric acid at different anodic voltages using a GDOES depth profile; the correlation for the crystallinity and electrolyte is included. 132
133 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours 7.1. Introduction The NaTESi electrolyte is composed of 7.5 mol l -1 NaOH, 0.33 mol l -1 Na-tartrate, 0.1 mol l -1 ethylenediaminetetraacetic acid (EDTA) and 0.05 mol l -1 Na 2 SO 3 [20, 133]. The anodizing treatment on commercially pure titanium in the NaTESi electrolyte has not yet been understood by any previous researchers. On the other hand, the literature has not reported that if the amorphous-to-crystalline transition would occur during the anodic oxide growth of titanium and its alloys in such an electrolyte. Thus, the present chapter tries to explore the anodizing behaviour of commercially pure titanium in the NaTESi electrolyte, with identifying the types of anodic oxide film that are formed, including the compositions and morphology; also understanding the behaviour of the amorphous-to-crystallization transition during the growth of the films is presented Voltage Time Response The voltage - time response for anodizing in the NaTESi electrolyte is shown in Figure 7.1. It reveals an initial voltage surge of 2-3 V due to the initiation of film growth, and a linear voltage increases at ~25.0 mv s -1 to 13 V. Then, a voltage rise of ~7.1 mv s -1 to ~35 V was evident with the occurrence of oxygen evolution. A real-time photograph for titanium anodized to 20 V is attached, showing the evidence of significant oxygen bubbles originating on the surface. The voltage rise is significantly weakened after the voltage of ~35 V. The surface of the specimen undergoes the following changes in appearance: pale grey to brown to blue to dark grey associated with anodizing voltage of 5, 10, 20 and 40 V respectively Scanning Electron Microscopy The nodular anodic films are revealed after anodizing to 5, 10 and 20 V in Figures 7.2a c. The non-uniform anodic films generated after anodizing to different voltages are shown. The titanium substrate was not pre-treated prior to anodizing and 133
134 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours thus, the rough substrate may introduce the non-uniform regions for the anodic film growth. Pore of ~36 nm diameter are created when the titanium is anodized to 40 V, and the pores are surrounded by nodules (Figure 7.2d) Raman Spectroscopy The crystalline structure of the anodic films was examined by Raman spectra, as shown in Figure 7.3. For anodizing to 5 V, no crystalline peaks are resolved; however, the spectrum for the film formed to 10 V reveals three anatase crystalline peaks (342, 480 and 617 cm -1 ) [161, 162]. Similar peaks for the anodic films formed to 20 and 40 V are observed with increased intensities compared with lower voltages. Thus, the higher energy intensity could be induced by a higher degree of crystallinity at higher anodic voltages Transmission Electron Microscopy The transmission electron micrograph of a cross-section of the titanium anodized to 10 V is shown in Figure 7.4. The anodic film, of ~37 nm thickness, is revealed between the titanium substrate and the Platinum layer (Figure 7.4a). The nonuniform anodic film is shown with oxygen bubbles evident within some of the film regions. The Ti / TiO 2 interface reveals lattice fringes which are highlighted by the circle areas, indicating the existence of nanocrystals in the oxide (Figure 7.4b). Crystal spots with prevailing orientations generated are evident in the diffraction pattern estimated by the fast Fourier transform (FFT) method [80]. The diffraction rings (1) and (2) associated with the crystal planes of (111) and (002) are observed. The crystal planes show a good agreement with the anatase crystalline structure, indicating that the planes identified here are probably prioritized to develop during the film growth. The crystalline phases are also highlighted in the anodic film at the Ti / TiO 2 interface, and the oxygen-filled voids found are indicated by the arrow (Figure 7.4c). The diffraction pattern in the inset image shows the crystalline characteristics, which also generates spots. These spots are corresponding to the anatase crystal planes of (002), (111) and (224) associated with rings (1), (2) and (3). 134
135 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours 7.6. Glow Discharge Optical Emission Spectroscopy Figure 7.5 shows the GDOES depth profiles for the titanium anodized to 5, 10, 20 and 40 V. It is observed that elements of titanium, aluminium, vanadium, sodium, oxygen and silicon are presented in each anodic film. The wavy profile of titanium at each voltage originates from an interference effect, and the sputtering time is expanded to reach the titanium substrate with increase of anodic voltage. Sodium species are also practically involved in the anodic film, and a significant sodium halo peak is revealed at 5, 10 and 20 V, indicating probably that the formation of a sodium-rich area exists at the TiO 2 /Ti interface. Two sodium-rich layers are probably formed by two sodium halo peaks derived at 40 V due to the fabrication of pores; thus, extra sodium species probably enter through the pore channels. Further, the intensity of sodium is increased with increase of anodic voltage. The amounts of oxygen are apparently low since the GODES test is insensitive to oxygen species. Silicon is also anticipated to be incorporated in the film since the ingredients of the electrolyte include sodium silicate; however, the amount of silicon concentration observed is relatively low Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis The RBS spectra for the anodic films formed after anodizing to 5, 10, 20 and 40 V were analyzed in Figure 7.6. The spectra presented in Figures 7.6a c indicate that a single barrier-type anodic film was formed after anodizing to 5, 10 and 20 V respectively. For 40 V, the anodic film is assumed to comprise an inner barrier layer and an outer porous layer according to the formation principle of a porous anodic film; however, only one porous layer is identified by the RBS fitting results (Figure 7.6d). Further, yields from oxygen and titanium in the anodic films are disclosed in the spectra; the peak and the width corresponding to oxygen increase with increase of anodic voltage due to the thickening of the anodic film, which is highlighted in the inset graphs. The data of RBS and NRA are listed in Table 7.1. The thickness of the anodic film generated increased from 5 to 40 V; TiO 2 with a density of 4.23 g cm -3 was employed for the estimations [163]. The anodic film thickness generated at 10 V in RBS shows a good agreement with the thickness derived from TEM. It is also shown that the content of titanium and the relative amount of oxygen in atoms 135
136 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours cm -2 within the film increase with increase of anodic voltage based on the NRA analysis, and Ta 2 O 5 was used as the reference (Figure 7.7). The cell charges responsible for the growth of film were estimated to increase from 5 to 40 V. The efficiencies of film growth, i.e. charge from NRA / charge from voltage-time response [164] are listed in Table 7.1, showing that a low efficiency for the film growth is evident at each voltage. Table 7.1. RBS and NRA results of examinations of anodic oxide films on titanium in NaTESi electrolyte to different anodic voltages. Anodic voltage (V) Ti ( atoms m -2 ) O ( atoms cm -2 by RBS) O ( atoms cm -2 by NRA) Oxide thickness (nm) Cell charge by NRA (C cm -2 ) Cell charge by V-t response (C cm -2 ) Efficiency (%) ± ± ± ± Electrochemical Impedance Spectroscopy The Bode plots of the anodic films formed to the four different anodic voltages are presented in Figure 7.8. The impedance increases slightly with increase of anodic voltage. Further, according to the frequency window, one time constant is evident for the anodic films formed to 5, 10 and 20 V (Figures 7.8a c), and the impedance changes approximately linearly with frequency, with a slope of nearly negative one. On the other hand, two-capacitive constants are observed for the film formed at 40 V, indicating that the film consists of an outer porous layer and an inner barrier oxide layer (Figure 7.8d). Furthermore, a predominantly capacitive behaviour is evident for each specimen, and performed by a phase shift approaching -85 o at the middle frequency region. The EIS curves can be fitted by an appropriate equivalent circuit, and one is adopted for the films layer formed to 5, 10 and 20 V which consists of the following elements: a solution resistance (R s ) of the electrolyte, the double layer capacitance (Q 1 ), accounting for the capacitive behaviour of the anodic film, in parallel with a 136
137 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours resistance (R 1 ) responsible for the dissipative resistance to current flow across the oxide (Figure 7.9a). For 40 V, the two-layer film including an inner barrier layer and an outer porous layer were formed, thus, an additional resistor (R 2 ) and capacitance (Q 2 ) were involved (Figure 7.9b). The corresponding parameters obtained from the circuit elements are listed in Table 7.2. It is shown that R 1 increased from 5 to 20 V, and the barrier film capacitance decreased due to the increase of the film thickness. Further, the film thickness after anodizing to 10 V has been examined by TEM, and thus, the dielectric permittivity of the anodic film formed in the NaTESi electrolyte can be clarified with the combination of the EIS results. The dielectric permittivity is estimated as ~2.35 followed by the calculation from T = εε 0 /C [59], where T is the film thickness (36.7 nm), ε is the dielectric permittivity of TiO 2 formed in the NaTESi electrolyte, ε 0 is dielectric permittivity of free space ( F m -1 ) and C is the oxide capacitance ( F, obtained from 5.66 F m m 2 ). In summary, other oxide thicknesses can be obtained using this equation, which are listed in Table 7.2. ~33 and ~71 nm thicknesses of the anodic films formed to 5 and 20 V are calculated, which are similar with the RBS estimations. Thus, the film thicknesses formed at 5 and 20 V are averagely read as ~30 and ~67 nm. Table 7.2. EIS fitting results of anodic films formed on titanium after anodizing to different anodic voltages in NaTESi electrolyte. Anodic voltage (V) R s (Ohm cm 2 ) R 1 (Ohm cm 2 ) R 2 (Ohm cm 2 ) Q 1 (F m -2 ) Q 2 (F m -2 ) Oxide thickness (nm) / 6.39 / / 5.66 / / 2.91 / / 7.9. Degradation and Single-lap Shear Tests Surface Morphology after Degradation Scanning electron micrographs at different magnifications of the anodized titanium after the specimens have endured a degradation treatment in a continuous climatic chamber with humidity of 90% at 50 o C for 1000 h are shown in Figure The degraded titanium after anodizing to 10 V presents a rough surface (Figure 7.10a). 137
138 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours The hole-like structure across the film is revealed at a high magnification image (Figure 7.10b). It is known that oxygen evolution occurred during the anodizing, which leads to defects of the film by the bursting of oxygen bubbles. However, the anodic films are not degraded or dissolved after the test, indicating that the film coatings show an excellent degradation resistance in such an atmospheric environment. Additionally, the anodic film formed after anodizing to 20 V is more uniform than the film formed to 10 V after the degradation test (Figure 7.10c). Moreover, non-uniform regions of the film are observed at a high magnification image (Figure 7.10d) Load Displacement Responses The load-displacement curves of the single-lap adhesive bonding tests for the asreceived and the titanium anodized to different anodic voltages are displayed in Figure The bonding behaviour of the as-received titanium reveals that the peak value of load is N at a displacement of 0.4 mm. The titanium joint anodized to 10 V is completely debonded when a peak load of N at a displacement of 0.5 mm is reached. A peak load of N is revealed for the titanium joint anodized to 20 V at a displacement of 0.7 mm. As the results show, the peak load with the related displacement performed at 20 V is the highest compared with the other conditions. It is inferred that the debonding resistance could be improved after anodizing, and increased with increased the anodic film thickness. The typical load-displacement curves for the single-lap joint specimens before and after anodizing to different voltages show that the behaviour can be divided into three stages: (I) the initial elastic region associated with elastic deformation of the epoxy and (II) a non-linear region associated with epoxy plastic deformation and interface debonding; all the specimens showed similar fracture behaviours. After the non-linear region, another elastic region (III) was observed due probably to the epoxy cohesive failure of the anodic film Surface Morphology after Shear Bonding Scanning electron micrographs of the titanium anodized to 10 V after the shear bonding test are shown in Figures 7.12a and b. It is observed that the epoxy resin is still retained on some of the film regions. Some parts of the epoxy resin were 138
139 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours detached to leave irregular areas. From EDS analysis (Table 7.3), the resin consists mainly of carbon species that are identified in the area 1, and only oxygen and titanium are found in area 2. A region of the anodic film is highlighted at a high magnification in Figure 7.12b, showing that some levels of oxide film were detached off due to the debonding force (dash circles). It is also probably attributed to the low concentration of oxygen identified in the film region. Scanning electron micrograph of the titanium anodized to 20 V after the shear bonding test is displayed in Figure 7.12c. A similar surface consisting of resin and the anodic film is observed. A region in the film characterized at increased magnification in Figure 7.12d shows that some areas of the anodic film were detached from the substrate due to the debonding force. As mentioned in Figure 7.11 previously, the peak load for 20 V is higher than the film formed to 10 V, which may result in more debonded areas in the film. However, the depth of the detached surface is difficult to be distinguished by the micrograph observations. The higher oxygen content found by the EDS result (area 3) is consistent with a thicker anodic film formed after anodizing to 20 V. Table 7.3. EDS spectra of different regions on anodized titanium after adhesive bonding tests. Area / Element (wt. %) C O Ti / 2 / / Discussion The amorphous-to-crystalline transition during the film growth of titanium has been reported in previous literature and it occurs at voltages of less than 10 V [84, 120] in specific electrolytes such as sulphuric acid and phosphoric acid [155, 165], ammonium pentaborate electrolyte [166], phosphate/glycerol electrolyte [153] and acetic acid [65]. The present study also reveals that the amorphous-to-crystalline transition of the anodic film formed on titanium was triggered during anodizing in the NaTESi electrolyte. The voltage-time transition disclosed that the voltage rose with the anodizing time, and oxygen evolution was observed clearly beyond 139
140 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours anodizing for 100 s. A linear voltage rise continued to the voltage of ~ 40 V, and the slope of voltage curve was reduced due to the development of significant oxygen evolution. The nodule film morphology, evident in Figure 7.2 may be influenced by the oxygen evolution. Raman spectra indicated that the crystalline oxide was initiated at voltages as low as ~ 10 V (Figure 7.3), in good agreement with previous study [120]. Further, the oxygen bubbles are evident in the TEM image (Figure 7.4a), and oxygen-filled voids are found within the lattice fringe regions associated with crystalline phases (Figures 7.4b - c). The reduced ionic resistivity of the crystalline oxide and the oxygen bubbles developed within improve the ionic transport in the anodic film at the high electric field [84]. The crystalline oxide is nucleated immediately above the titanium / oxide interface. It is also observed that a thinner oxide layer is formed where large areas of crystalline phases and oxygen bubbles are developed (Figure 7.4a). It is probably because the development of more oxygen bubbles can be induced by the crystalline phases. Consequently, the thickness of the film within the crystalline regions is reduced due to the film rupture induced by the bursting of oxygen bubbles. The amorphous anodic film is formed and grown simultaneously by cation egress and anion ingress through a pre-existing TiO 2. The crystalline oxide is probably developed at the commencement of anodizing, within the pre-existing TiO 2. Thus, it is probable that the pre-existing TiO 2 is a nucleation site for the crystalline oxide. The crystal planes of anatase were confirmed for the crystalline phases within the anodic film formed after anodizing to 10 V (Figures 7.4b - c). Anatase crystal planes of (002) and (111) are identified through the diffraction patterns, indicating that the crystalline oxide probably gives the preference for these two planes to develop at the initiation of the crystalline transition. The GDOES results in Figure 7.5 show clearly that sodium species are enriched in the anodic films, and the concentration increases with increase of anodic voltage. The significant amounts of sodium species may modify the structure of the anodic film, shaping the nodule structure of the surface and small pores within the cross-section. Moreover, the oxygen bubbles were probably induced within the pores and the crystalline regions. At the beginning of the anodizing, an amorphous film is generated and accompanied with oxygen evolution at the film / electrolyte interface. Afterwards, an amorphous- 140
141 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours to-crystalline transition occurs with the nucleation of nanocrystals within the oxide film. Initially, the transition results in an increase in the electron conductivity since the crystalline phases are more electron-conducting than the amorphous phases [80]. Oxygen evolution within the oxide is triggered when the crystalline phase nucleates. Then, an anodic film consisting of oxygen-filled voids is developed and, in such film, the effective space available for electron conduction is decreased, as the oxygenfilled bubbles inhibit the traverse of electrons and ions. The increased pressure in gas bubbles generated at the crystalline regions results in the rupture of the anodic films [84]. Conversely, during the anodizing, the localized rupture of the oxygen-filled bubbles within the anodic film formed is not evident (Figure 7.4a). According to the EIS results, it might be associated with the re-growth of oxide which took place to maintain the proportionality between the anodic voltage and the film thickness. The anatase type of the anodic film formed on titanium is determined from Raman spectra. The dielectric permittivity of anodic film of anatase, has been measured at ~77.6 in H 2 SO 4 and ~62.4 in H 3 PO 4 in Chapter 4. However, the dielectric permittivity of the anodic film in the NaTESi electrolyte is estimated at ~2.35 which is much lower than the permittivity estimated in sulphuric and phosphoric acids. The low dielectric permittivity is linearly functional to a low electrical conductivity [143], resulting in less charges spent for the growth of the anodic film. According to the charge measured by RBS fitted analysis (Table 7.1), the current efficiency is relatively low at each anodic voltage probably as a result of the low dielectric permittivity and oxygen evolution. The ejection of all of the outward migrating titanium ions to the electrolyte would also reduce the current efficiency. Dissolution of film species may also exist after titanium was immersed in the electrolyte, which might be attributable to the fabrication of nodules and pores. The nodules may be formed either by precipitation of Ti 4+ ions [167], which are probably dissolved at the pore base, or by selective dissolution of the outer regions of the film. Precipitation is possibly affected by the foreign species incorporated from the NaTESi electrolyte, which may be reduced due to the consumed current efficiencies by oxygen evolution. The single-lap shear bonding test results confirm the correlation between the morphology and the adhesive bond strength. It is found that by optimisation of the 141
142 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours morphology, the bonding strength can be increased. Additionally, the anodic films can be tailored based on the variation of applied voltages. Further, the loaddisplacement responses indicate that the shear bonding strength increases with increase of anodic voltage. It has been reported that [168] on metal oxides covalent bonds to the anodizing derived coating arise which lead to a good adhesion between the substrate and the coating. Therefore, it is likely that the good adhesion corresponds to a thicker TiO 2 film compared with a thinner film. According to the scanning electron micrographs of anodized CP-Ti before and after the single-lap shear bonding treatments, it is evident that a thicker anodic film can enhance the bonding friction since the degree of roughness of an anodic film is probably higher compared with a thinner film and the originate substrate. It was observed that after the bonding tests, epoxy resin is retained on some of the regions of the film, which are identified by EDS mappings. The region 1 shows the presence of mainly carbon species, indicating that it is the resin species. The regions of 2 and 3 show the existence of titanium and oxygen elements, confirming the presence of the anodic film. As has been observed, the thickness of the anodic film may play an important role for the resin, and also produce a composite interfacial region which will be difficult to be debonded completely [17]. Thus, it is suggested that a thicker anodic film formed at a higher anodic voltage provides a better mechanical key for the promotion of shear bonding strength Summary 1). A nodule-texture surface of anodic films is formed on commercially pure titanium in the NaTESi electrolyte after anodizing to 5, 10, 20 and 40 V respectively. 2). The thickness of the anodic film increases with increase of anodic voltage. Anodic films of ~ 30, 37 and 67 nm thicknesses are generated for anodizing to 5, 10 and 20 V respectively. Further, a porous anodic oxide film of ~ 80 nm thickness is formed when anodized to 40 V. 3). Amorphous-to-crystallization occurs during the growth of the anodic film at each voltage. Crystalline regions result in more oxygen bubbles, and the regions in the 142
143 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours film where are ruptured by the bursting of bubbles are thinner. The degree of crystallinity of the anodic film is increased with increased anodic voltage. 5). A dielectric permittivity of 2.35 for the anodic film formed to 10 V in the NaTESi electrolyte is estimated from the EIS and TEM results. 4). The low current efficiency responsible for the anodic film growth is probably induced by the low dielectric permittivity and oxygen evolution. 6). The anodic films formed after anodizing to 10 and 20 V in the NaTESi electrolyte show an excellent degradation resistance after the test for 1000 h. The shear bonding load of anodized titanium in the NaTESi electrolyte increases as the anodic voltage increases. EDS analysis indicates that the low oxygen contents within the film results from the debonding of the film region. 7). The single-lap shear bonding tests show that the shear bonding strength increases with increase of anodizing voltage. It is concluded that the good adhesion can be produced by a thicker anodic TiO 2 film compared with a thinner film. Additionally, a thicker anodic film would increase the interfacial bonding strength for the titanium materials. Notification: some of the works in the Chapter 7 have been published in Journal of Surface and Coatings Technology 258(2014) , please see Appendices. Thus, some of the published contents in this chapter have been rephrased to avoid the self-plagiarism issue. 143
144 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Figure 7.1. Voltage time curve during anodizing of titanium at 20 ma cm -2 in the NaTESi electrolyte at ambient temperature. 144
145 Intensity (a.u.) Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b c d Figure 7.2. Scanning electron micrographs of titanium surface anodized at 20 ma cm -2 in NaTESi electrolyte to 5 V (a), 10 V (b), 20 V (c) and 40 V (d) V 20 V V 5 V Wavelength (cm -1 ) Figure 7.3. Raman spectra of titanium anodized in the NaTESi electrolyte at 20 ma cm -2 to 5, 10, 20 and 40 V respectively. 145
146 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Figure 7.4. Transmission electron micrographs of cross-section of the anodic film formed on titanium in NaTESi electrolyte at 20 ma cm -2 to 10 V; (a) cross-section of the anodic oxide film at the Ti / Pt interface; (b) high magnification image of the titanium / anodic oxide interface with the diffraction pattern; (c) high magnification image of the Ti / TiO 2 interface at another region with the diffraction pattern. 146
147 Intensity / arb. unit Intensity / arb. unit Intensity / arb. unit Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours 40 a 30 Ti O Na Si Sputtering Time 40 b 30 Ti 20 Na 10 O Si Sputtering Time / s 40 c O Na Ti 10 Si Sputtering Time / s 147
148 Intensity / arb. unit Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours 40 d 30 Ti 20 O 10 Na Si Sputtering Time / s Figure 7.5. GDOES elemental depth profiling analysis of the anodic films formed on titanium in NaTESi electrolyte at 20 ma cm -2 to 5 (a), 10 (b), 20 (c) and 40 V (d) in NaTESi electrolyte; the intensities of oxygen and silicon are multiplied by
149 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Figure 7.6. Experimental and simulated results of Rutherford backscattering spectroscopy for titanium anodized at 20 ma cm -2 to (a), 5 V; (b), 10 V; (c), 20 V and (d), 40 V in NaTESi electrolyte respectively. 149
150 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Figure 7.7. Experimental results of nuclear reaction analysis for titanium anodized at 20 ma cm -2 to (a), 5 V; (b), 10 V; (c), 20 V and (d), 40 V in NaTESi electrolyte respectively. 150
151 Phase Shift / deg Phase Shift / deg Phase Shift / deg Phase Shift / deg Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Impedance / cm a Impedance Phase Shift Fitted curve Impedance / cm b Impedance Phase Shift Fitted curve Frequency / Hz Frequency / Hz Impedance / cm c Impedance Phase Shift Fitted curve Impedance / cm d Impedance Phase Shift Fitted curve Frequency / Hz Frequency / Hz Figure 7.8. Bode plots, measured in the NaTESi electrolyte, of the anodic films formed after anodizing to 5 (a) 10 (b) 20 (c) and 40 (d) V on titanium. 151
152 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b Figure 7.9. Schematic drawing of the equivalent circuits of anodized titanium in the NaTESi electrolyte after EIS measurements; anodic films formed after anodizing to 5, 10 and 20 V; b to 40 V. 152
153 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b c d Figure Scanning electron micrographs at different magnifications of titanium surfaces after anodizing to 10 V (a - b) and 20 (c - d) V followed by degradation treatment in a continuous climatic chamber with a humidity of 90% at 50 o C. 153
154 Load (N cm -2 ) Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours III II I As-received 2-10 V 3-20 V Displacement (mm) Figure Single-lap adhesive bonding tests of as-received titanium (1) and specimens anodized to 10 V (2) and 20 V (3). 154
155 Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b Resin 1 Oxide 2 Oxide c d Resin 3 Oxide C 1 Spectrum 3 2 O Full Scale 237 cts Cursor: (4 cts) kev 2 Spectrum 2 Ti O Ti Ti Full Scale 163 cts Cursor: (11 cts) kev Ti O Ti 3 3 Spectrum 2 Ti Full Scale 141 cts Cursor: (8 cts) kev Figure Scanning electron micrographs at different magnifications of titanium surfaces anodized to 10 V (a, b) and 20 V (c, d) after adhesive bonding tests; EDS results are also presented at different regions. 155
156 Chapter 8 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours 8.1. Introduction Within this chapter, the anodizing behaviour of Ti6Al4V alloy in the NaTESi electrolyte is explored. The types of anodic film that are formed on Ti6Al4V alloy, and the compositions and morphology are examined. Also, the degradation and adhesive bonding behaviours of the anodic films formed on Ti6Al4V alloy are investigated Ti6Al4V Pre-Treated Alloy Two different pre-treatments of Ti6Al4V alloy were carried out for the investigation of the metallurgical surface, as shown in Figure 8.1. It has been known that the Ti6Al4V alloy consists of two phases which are the α and β phases. The etching effect in 48% HF + 70% HNO 3 for 10 s in Figures 8.1a and b show that the α and β phases are observed and distinguished by the arrows in the image of higher magnification. The scanning electron micrographs show that the two phase alloy (α + β) is composed of hexagonal close-packed (hcp) α grains and body centred cubic β grains. The alloy surface after the rf-gd (radio frequency-glow discharge) sputtering shows sharp grain boundaries, as shown in Figure 8.1c. The depths of the grain boundaries are different due to the sputtering effect (Figures 8.1d). Additionally, a cross-section of Ti6Al4V alloy specimen without pretreatments was prepared by a focused-ion beam and examined by transmission electron microscopy. Figures 8.2a and b show that the grains and the boundaries are clearly observed since the airformed oxide film was removed by the focused-ion beam. Also, the α and β phases are distinguished clearly. EBSD mapping was carried out the specimen after etching in the HF+HNO 3 acid, as displayed in Figure 8.3. The EBSD grain and phase mappings and inverse pole figures show 96% of HCP (hexagonal close packed) structure from the α phase and 4.0% of BCC (body centred cubic) structure from the β phase. The basal crystal plane of (0001), two prismatic crystal planes of (11-20) and (10-10) were identified. 156
157 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours 8.3. Voltage - Time Response Figure 8.4 presents the voltage time response during anodizing of the Ti6Al4V alloy at a current density of 20 ma cm -2 in the NaTESi electrolyte. The voltage first rises linearly at a rate of ~3 V s -1 to ~34 V. It then increases at a reduced rate of ~2 V s -1 until ~40 V at ~75 s with fluctuations due to the occurrence of oxygen evolution. Thereafter, a significant reduction in gradient of voltage occurs until the anodizing was terminated. Oxygen bubbles that persisted on the surface of the titanium alloy were observed from the commencement of the anodizing process. Additionally, no sparks were evident during anodizing Morphology of Anodic Oxide Films Scanning electron micrographs of the specimen surfaces after anodizing to different voltages are displayed in Figure 8.5. The surface of the Ti6Al4V alloy anodized to 10 V presents an anodic film with shallow pores located mainly within the α phase. The anodic film formed to 20 V shows numerous pores of sizes up to ~1 µm diameters which are surrounded by honeycomb-like pore textures (Figure 8.5b). After anodizing to 30 V, the film contains pores of increased in size, namely ~ µm (Figure 8.5c). At 40 V, more large pores were found to develop through the coalescence of the small pores formed within the anodic film (Figure 8.5d). Interestingly, it is observed that the formation of shallow/small to deep/large pores is induced mainly within the α phase region at increased voltage. It is also evident that the pores of small sizes (less than 200 nm) coalesce to form large pores. Further, the quantity of the pores is promoted by the increase of anodic voltage. The EDS spectra (Table 8.1) show the presence of mainly titanium and oxygen, low amounts of aluminium and vanadium, and carbon from contamination. The amount of oxygen is increased with increase of anodic voltage since the thickness of the anodic film increases. 157
158 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Table 8.1. EDS results of anodic films formed on Ti6Al4V alloy after anodizing to 10, 20, 30 and 40 V in NaTESi electrolyte. Voltage (V)/Composition (wt. %) Ti Al V O C Crystalline Structures of Anodic Films Raman Spectra The Raman spectra of the as-received Ti6Al4V alloy and the anodic films formed on the alloy after anodizing in the NaTESi electrolyte are shown in Figure 8.6. No crystalline peaks were identified from the as-received Ti6Al4V specimen. For the anodic film formed to 10 V, the spectrum is relatively smooth, and a peak at 166 cm - 1 was resolved from the He-Ni laser beam. Three very weak anatase peaks (222, 447 and 599 cm -1 ) are resolved. It is reported that an amorphous-to-crystalline transition could be induced in anodic films formed on titanium when the voltage is less than 10 V [120]. For the anodic film formed after anodizing to 20, 30 and 40 V, three peaks are wider due to anatase [169]. It is observed that the degree of crystallinity of the films is more abundant at a higher voltage Transmission Electron Microscopy The Ti6Al4V alloy anodized to 10 V was selected for investigation by transmission electron microscopy, as shown in Figure 8.7. The cross-section was prepared by focused-ion beam; a gold layer and a Platinum layer above the oxide were prepared to improve the electronic conductivity and for surface protection. An EDS line analysis scanned from the Platinum layer to the titanium alloy substrate, and the location of the anodic film was identified by the oxygen rich region in the spectrum (Figure 8.7a). The EDS elemental map shows the presence of a region of wave profile highlighted by two dashed lines representing the anodic film area. After the film location is known, TEM images at high magnifications are taken to evaluate more detail, which are shown in Figures 8.7b - d. Figure 8.7b shows the anodic film 158
159 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours of ~ 11 nm thickness. Figure 8.7c shows that lattice fringes are observed within the film, indicating that the amorphous-to-crystalline transition and the formation of nanocrystals of anatase occurred during the anodizing. Further, oxygen-filled voids highlighted by an arrow are found. The diffraction pattern for a region of the anodic film by fast Frouier transform (FFT) analysis, displays the generation of spots with prevailing orientations that also confirm the existence of crystalline phases [146]. Further, oxygen-filled voids are developed within the crystalline phases (Figure. 8.7d). The observed diffraction rings of (1), (2), (3) and (4) show a good agreement with the (101), (004), (200) and (116) crystal planes of anatase [145] Film Compositions Glow Discharge Optical Emission Spectroscopy GDOES elemental analyses for Ti6Al4V alloy anodized to 10, 20, 30 and 40 V are displayed in Figure 8.8. The wavy profiles of titanium originate from an interference effect. The sputtering time to reach the titanium alloy substrate is increased with increase of anodic voltage and oxide thickness. Uniform distributions of aluminium species are evident in the anodic coatings. The vanadium species in the coatings are clearly seen in these depth profiles. Sodium halo peaks show that the anodic films formed to 10 and 20 V contain sodium species (Figures 8.8a - b). Further, more peaks and increased sodium intensities are revealed with increased anodic voltage to 30 and 40 V, indicating the formation of sodium-rich layers throughout the whole films (Figures 8.8c - d). The large quantity of sodium species probably penetrated within the film through the pore channels. The presence of silicon species here is apparently low. The oxygen content is increased slightly as the anodic voltage increases Rutherford Backscattering Spectroscopy / Nuclear Reaction Analysis The RBS spectra for the Ti6Al4V alloy specimens after anodizing in the NaTESi electrolyte to 10, 20, 30 and 40 V were fitted by the simulated spectrum to determine the anodic film compositions and thicknesses. Figure 8.9 shows the RBS spectra, displaying the experimental and simulated results. In Figures 8.9a and b, the spectra confirm that the anodic film thicknesses generated after anodizing of the Ti6Al4V alloy to 10 and 20 V are ~15 nm and 39 nm respectively; a density of 4.23 g cm -3 for 159
160 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours TiO 2 was used in the RBS analysis. As detected, the anodic oxide film thickness formed to 10 V shows a good agreement with the thickness identified from the previous TEM observations (~11 nm thickness). The spectra in Figures 8.9c and d indicate that the film formed to 30 and 40 V is ~1.11 µm and ~1.80 µm respectively. Further, all spectra disclose yields from oxygen, aluminium, titanium and vanadium in the anodic oxide films. However, the yields from titanium and vanadium become more significant with increase of anodic voltage, particularly for 30 and 40 V due to the formation of thicker porous oxide film compared with the films formed at 10 and 20 V. The yield from oxygen is more apparent due to the higher concentration of oxygen detected with increased voltage. This is also confirmed in Table 8.2, which shows the increasing trend of the oxygen content and the oxide thickness as the voltage rises. The NRA results are displayed in Figure 8.10, and the corresponding oxygen amounts are listed in Table 8.2. The Table shows that the oxygen amounts have a good agreement with the RBS results, and increase with increase of anodic voltage. The cell charges from RBS estimations responsible for the anodic film growth increase from 10 to 40 V. The current efficiencies for the anodic films growth based on the estimation of charges from the RBS data and the charges estimated by the voltage-time response are revealed in Table 8.2. The current efficiency increases from 44.8% to 77.9% associated with anodizing voltage of 10 and 30 V. However, it decreases to 30.9% for anodizing to 40 V due to the occurrence of significant oxygen evolution. Table 8.2. RBS and NRA results of anodized Ti6Al4V alloy to 10, 20, 30 and 40 V in NaTESi electrolyte. Anodic voltage (V) Ti ( atoms m -2 ) O ( atoms cm -2 by RBS) O ( atoms cm -2 by NRA) Oxide thickness (nm) Q by RBS (C cm -2 ) Q by V-t responses (C cm -2 ) Growth efficiency (%) ± ± ± ± Dielectric Properties of Anodic Films The dielectric properties of the anodic films formed on the Ti6Al4V alloy in the NaTESi electrolyte were examined by EIS. The Bode diagrams of the films formed 160
161 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours to the four different anodic voltages are revealed in Figure The impedance increases slightly from 10 to 20 V, but decreases from 20 to 40 V. Further, over the frequency window inspected, one time constant is observed for the film formed at 10 V (Figure 8.11a) since the impedance changes linearly with frequency, with a slope of ~ -1. However, two capacitive constants were shown after anodizing to 20, 30 and 40 V, suggesting that the oxide film formed at each of the voltage is composed of an outer porous oxide layer and an inner barrier oxide layer (Figure 8.11b - d). Moreover, a predominantly capacitive behaviour is evident for each specimen, indicated by a phase shift approaching -80 o at middle to low frequency associated with anodizing to 10, 20 and 30 V. However, for 40 V, the phase shift is lower than - 70 o in that region, indicating that the formation of the oxide film at 40 V is not fully capacitive. For a single barrier oxide film formed on the titanium alloy after anodizing to 10 V, the EIS data can be fitted using an equivalent circuit model shown in Figure 8.12a, which consists of the following elements: a solution resistance (R s ) of the electrolyte, the capacitance (Q 1 ) of the anodic film, in parallel with a resistance (R 1 ) which is responsible for the dissipative resistance to current flow across the oxide film. For the films formed at 20, 30 and 40 V respectively, two oxide layers consisting of an inner barrier layer and an outer porous layer were formed due to the observation of two time constant of phase shifts. Thus, an additional resistance (R 2 ) and capacitance (Q 2 ) are included (Figure 8.12b). The corresponding data of the circuit elements are listed in Table 8.3. It is indicated that R 1 increased from 10 to 20 V, but decreased from 20 to 40 V. According to the TEM results, the real oxide film thickness generated at 10 V has been confirmed as ~ 11 nm. Therefore, based on the equation of T = εε 0 A/C [59], where T is the oxide thickness (11 nm), ε is the dielectric permittivity of TiO 2 formed in the NaTESi electrolyte, ε 0 is the dielectric permittivity of free space ( F m -1 ) and C is the oxide capacitance ( F cm -2, obtained after multiplying by the exposed area A, 1 cm 2 ), the dielectric permittivity of the oxide film formed on Ti6Al4V to 10 V in the NaTESi is measured as ~118. Such a high value of dielectric permittivity of the anodic film is associated with the high 161
162 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours conductivity, which is mainly induced by the crystalline transition that provides electron passages [170]. Table 8.3. EIS fitting data obtained from the equivalent circuits of the titanium alloy anodized to 10, 20, 30 and 40 V respectively in the NaTESi electrolyte. Anodic voltage (V) R s (Ohm cm 2 ) R 1 (Ohm cm 2 ) R 2 (Ohm cm 2 ) Q 1 (F cm -2 ) Q 2 (F cm -2 ) / / Degradation and Single-lap Shear Tests Surface Morphology after Degradation Scanning electron micrographs of the anodized Ti6Al4V specimens after the degradation treatment in a continuous climatic chamber of 90% humidity at 50 o C for 1000 h are shown in Figure No film defects are evident after the degradation treatment (Figures 8.13a - d). The image of high magnification shows the similar shallow pores which were developed within the film formed at 10 V. Similar shallow pore textures are observed for 20 V and, interestingly, the film texture is different within different alloy phases, which results in the formation of different pattern shapes that can be distinguished in the graph of high magnification. With regard to 30 V, the high magnification image displays mainly honeycomb-like pore textures generated within the film; no large pores previously formed were found after the degradation. It is indicated that the anodic films formed on Ti6Al4V show an excellent degradation resistance in the atmospheric environment. Interestingly, the alloy surface anodized to 40 V shows that the film was crystallized during degradation at the thermal temperature of 50 o C (Figure 8.13d). The high magnification image reveals a nano-porous structure with the large pores formed previously during anodizing being interconnected with small pores. The EDS spectrum was mapped at each degraded anodic film, which is highlighted using a circle area in the images of low magnifications (Figures 8.13a - d). The EDS 162
163 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours analyses are listed in Table 8.4, and show that the oxygen content increases with increase of anodic voltage. Table 8.4. EDS results of anodized Ti6Al4V alloy after degradation test in a continuous climatic chamber with a humidity of 90% at 50 o C for 1000 h. Voltage (V)/Composition (wt %) Ti Al V O C Load Displacement Responses Figure 8.14 shows the single-lap shear adhesive bonding test results for the Ti6Al4V after anodizing in the NaTESi electrolyte to different voltages. The maximum load of the as-received Ti6Al4V alloy after the bonding was ~855 N at a displacement of 0.5 mm. With regard to anodic voltages of 10, 20, 30 and 40 V, the apparent maximum loads of the bonding tests were ~1034, 1151, 1192 and 1284 N at displacements of 0.51, 0.53, 0.57 and 0.67 mm respectively. It is observed that an increased anodic voltage increased the peak load. The load-displacement can be divided into two stages: (I) the initial elastic region associated with elastic deformation of the epoxy and (II) the non-linear region associated with plastic deformation of the epoxy and interface debonding. Compared with the as-received titanium alloy, the peak loads of anodized alloys are all higher. Further, all the specimens displayed a similar fracture behaviour. The fracture occurred during debonding was probably caused by the cohesive failure of the epoxy and/or oxide [112] Surface Morphology after Shear Bonding Scanning electron micrographs of the titanium surfaces anodized to 10, 20, 30 and 40 V after the shear bonding tests are shown in Figure It is observed that the epoxy resin is still retained on some of the regions within the surface. Some parts of epoxy resin were detached to expose the film areas. The EDS points analyzed (Table 8.5) show that the anodic oxide film is exposed near the resin since mainly carbon 163
164 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours species exist in regions 1 and 3; titanium, aluminium, vanadium and oxygen are largely identified in regions 2 and 4. The regions of the anodic films near the epoxy resins are highlighted to be observed at a higher magnification. Figures 8.15c and d display the scanning electron micrographs of the titanium surfaces anodized to 30 and 40 V after the shear bonding treatments. The EDS spectra distinguish the resin and the oxide film surface. A similar surface consisting of resin and anodic oxide film is observed. The higher magnification images show that some areas of the oxide films were detached from the substrate due to the debonding force. Further, the anodic oxide film with pores can still be observed. Moreover, the anodic oxide film formed to a higher voltage is thicker which probably increases the bonding strength or shear resistance. The increase of oxygen concentration with increased anodic voltage, evident by EDS is also consistent with increase of film thickness. Table 8.5. EDS results of anodized Ti6Al4V alloy after single-lap shear bonding tests immediately. Voltage (V)/Composition (wt. %) Ti Al V O C Area 1 / / / Area Area 3 / / / Area / Area 5 / / / Area Area 7 / / / Area Discussion With regard to the generation of oxygen, it is known that anodic films formed on titanium undergo an amorphous-to-crystalline transition at relatively low voltages, which is dependent upon the particular conditions of film growth [114]. Oxygen is then generated within the film at locations of crystals, forming oxygen bubbles within the film [114, 117]. The oxygen is eventually released when the film ruptures, leaving open pores. Further, the Al and V alloying elements are incorporated into the 164
165 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours films formed during anodizing. In such case, the main film material comprises a modified titanium anodic film containing units of both the alloying element oxides (Al 2 O 3 and V 2 O 5 ) and TiO 2. Consequently, the morphology of the film will probably be altered in responses to the changes in the field-assisted ejection and dissolution processes with the alloying elements included. If the film is more resistant to the dissolution, the steady-state cell dimensions achieved would require a balance between the film growth and the dissolution, which may lead the pores to form in finer sizes [171]. It is evident that the finer pore structures in the film preferably formed within the Ti α phase; then, alteration of both the ionic resistivity and the dissolution of the film will lead to different rates of oxidation of β phase. In previous literature [84, 166, ], the amorphous-to-crystalline transition within the anodic film formed on pure titanium could be impeded by the incorporation of alloying species such as W, Si, Mo and Zr. The anodic films formed on sputter-deposited Ti-20%W alloy consist of an outer amorphous layer of TiO 2 and an inner amorphous layer containing units of TiO 2 and WO 3. The latter units probably stabilize the film structure after anodizing in 0.01 M ammonium pentaborate electrolyte [172]. The amorphous films contrast with the crystalline oxide films grow on titanium [172]. It is evident that an amorphous-to-crystalline transition of anodic titanium oxide can be impeded by the incorporation of silicon species during anodizing of Ti-Si alloys containing 12 at.% or more silicon [84]. Uniform amorphous anodic films of low defect density can be developed at a high current efficiency to nearly 100 V on Ti-Mo alloys containing at.% Mo in ammonium pentaborate and phosphoric acid electrolytes[166]. The structures of anodic films formed on sputter-deposited Ti-Zr alloys are dependent upon alloy compositions. Nanocrystals of anatase could still develop in the amorphous anodic film on the Ti-10.5 at.% Zr alloy. Amorphous anodic films with no detectable oxide crystals grow on Ti-23 at.% Zr / 42.0 at.% Zr alloys [174]. For the Ti6Al4V alloy, it is known that the 6 wt.% Al and 4 wt.% V are incorporated in the pure titanium. The amorphous-to-crystalline transition within the anodic film should probably be impeded, stabilizing the amorphous oxide film. However, such an alloy has not been prepared by magnetron sputtering, and it has not been reported that the incorporation of Al and V could suppress the crystalline transition of anodic titania. Thus, the 165
166 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours anodic films formed on Ti6Al4V alloy can still probably undergo the amorphous-tocrystalline transition, enabling the development of oxygen bubbles to form oxygenfilled voids, and it is evident in the Raman spectra and TEM results (Figure 8.6 and Figure 8.7). The TEM evidence also reveals that the crystalline oxide is nucleated immediately above the Ti/TiO 2 interface. The crystalline anodic oxide formed and grew simultaneously by cation egress and anion ingress through a pre-existing airformed oxide film. Thus, it is probable that the pre-existing oxide acts as a nucleation site for crystalline oxide. The crystal planes were confirmed for the crystalline phases within the anodic oxide film when anodized to 10 V, which shows a good agreement with previous study [170]. The anatase crystal planes of (101), (004), (200) and (116) are determined by the diffraction patterns of TEM, indicating that the crystalline oxide probably gives preference for these planes to grow at the commencement of amorphous-to-crystalline transition. The GDOES profiles confirm that a larger amount of sodium species is present with increase of anodic voltage. The significant amounts of the sodium species may modify the structure of the anodic oxide film [170], enhancing the development of the porous structure. The Ti6Al4V alloy comprises duplex phases (α + β), which show different crystal structures such as HCP and BCC. Consequently, the two phases would generate an anodic film differently during anodizing. According to the SEM observations, the pores are formed preferentially within the α phase which is stabilized by the Al element. Further, it is reported that the incorporation of Al in Mg could introduce the formation of large pores on the anodic film after anodizing in NaOH solutions [175]. The pores formed within the anodic titania here are similar with the example for Mg- Al since the NaTESi electrolyte consists of 7.5 M NaOH. The pores are also similar compared to the anodizing of the Ti6Al4V alloy in the phosphoric acid with addition of various wt.% of phosphorus [176]. The micrographs show that the anodic film is dissolved more and deeper with increase of anodic voltage, and the dissolution mechanism is probably the main factor at a higher voltage. Further, oxygen bubbles are consumed more at a higher voltage, resulting in the formation of pores in larger sizes (less than 1.3 µm) and higher densities. It is studied that microporosity (pore diameter less than 10 µm) allows body fluid circulation whereas macroporosity (pore diameter greater than 100 µm) provides a scaffold for bone-cell colonization [177]. 166
167 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Thus, except for promoting the surface adhesion properties for aerospace, this anodic film may also act as scaffolding agent which allows circulation of body fluid [176]. However, it is not yet known until future investigations. The dielectric permittivity of the anodic oxide film formed on Ti6Al4V after anodizing to 10 V in the NaTESi electrolyte is estimated as ~118, showing a good agreement with the range between 32 and 173 identified in the literature [ ]. Such a high dielectric permittivity is probably linearly proportional to high electrical conductivity [143], leading to more charges spent for the film growth. RBS results confirmed the charge passed through the anodic oxide film during anodizing increased as the voltage increased, and the corresponding current efficiency was increased until 30 V. It is observed that the current efficiency is over 70% for anodizing to 30 V, and such a high efficiency is responsible for the oxide film growth with over 1 µm thickness. However, the current efficiency was reduced to ~30% during the anodizing from 30 to 40 V since the charge was consumed by the oxygen evolution, as observed during the anodizing process. Additionally, the ejection of all of the outward migrating titanium ions to the electrolyte would also reduce the efficiency for the film growth [170]. According to the degradation treatments for the anodic films formed on Ti6Al4V in an environment with a high degree of humidity, the anodic film is probably crystallized at a thermal temperature, 50 o C. The quantitative oxygen concentration identified in the respective EDS analysis is higher compared with the situation after anodizing immediately. However, no damages or defects were observed for the anodic films formed after anodizing to different voltages. Therefore, it is shown that the anodic films formed on Ti6Al4V alloy in the NaTESi electrolyte perform an excellent degradation resistance in such an atmospheric environment. The single-lap shear test results obtained confirm the correlation between morphology and adhesive bond strength, as already described by Venables [183]. It is established that by optimisation of the morphology, the bonding strength can be enhanced. On the other hand, the bonding strength is significantly dependent on the anodic voltage. The load-displacement responses indicate that the shear bonding 167
168 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours strength increases with increase of anodic voltage. Wapner and Grundmeier [168] demonstrated that on metal oxides covalent bonds with the anodizing derived coating arise, which lead to a good adhesion between the substrate and the coating. Therefore, it is likely that a good adhesion can be created by a thicker TiO 2 coating compared with a thinner TiO 2 coating. According to the scanning electron micrographs of anodized Ti6Al4V alloy in the NaTESi electrolyte before and after the single-lap shear bonding tests, it is possible that the anodic film with pores of larger size and population enhances the debonding friction since such a porous film may have a high degree of roughness. It is reported that the production of a porous oxide layer could significantly increase the interfacial strength [17]. It is observed that after the joint bonding tests, epoxy resin is retained on some of the regions of the oxide film. The porous oxide film may provide a mechanical key for the resin and produce a composite interfacial region which will be difficult to be debonded cleanly [17]. Thus, the evidence suggests that the anodic film formed on Ti6Al4V alloy with pores in larger sizes and higher population density provides a higher shear bonding strength Summary 1). α + β phases and grain boundaries of Ti6Al4V alloy can be observed after etching in HF + HNO 3 electrolyte or after the rf-gd sputter surface cleaning. The EBSD grain, phase mappings and inverse pole figures show 96% of HCP titanium structure from α phase and 4% BCC structure from β phase. The basal crystal plane of (0001), two prismatic crystal planes of (11-20) and (10-10) are identified. 2). The formation of pores within the anodic oxide film formed from 20 V, and the population density of the pores is increased with increase of the anodizing voltage. EDS spectra confirm that the oxygen concentration is increased with increased anodic voltage. 3). Three weak anatase crystalline peaks are found associated with the anodic film formed to 10 V. Similar anatase crystalline peaks are found when anodized to 20, 30 and 40 V respectively, and the degree of crystallinity of the film is more abundant at increased anodic voltage. 168
169 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours 4). The oxide film thickness generated after anodizing to 10 V is ~11 nm, which is determined by TEM, and the amorphous-to-crystalline transition is also evident at higher magnifications of TEM. 5). GDOES profiles show the presence of titanium, aluminium, vanadium, oxygen, sodium and silicon species which are incorporated into the anodic films formed after anodizing to 10, 20, 30 and 40 V respectively. A significant amount of sodium is found in each film, and it is increased with increased the voltage. 6). RBS results confirm that the current efficiency for the anodic oxide growth is increased from 10 to 30 V; however, it is reduced when anodizing from 30 to 40 V due to oxygen evolution. The oxide film thicknesses estimated by RBS are ~15.0 nm, ~39.0 nm, ~1100 nm and 1800 nm associated with anodizing to 10, 20, 30 and 40 V respectively. 7). The dielectric permittivity of anodic TiO 2 formed on Ti6Al4V in NaTESi is measured as ~118 based on the EIS fitted data and the TEM evidence. 8). The degradation treatment evident that the anodic oxide films formed on Ti6Al4V alloy in the NaTESi perform an excellent degradation resistance in the 90% humidity environment at 50 o C for 1000 h. 9). The single-lap shear adhesive bonding test results confirm that the shear bonding strength increases with increase of the anodizing voltage. The good adhesion results from a thicker anodic TiO 2 coating compared with a thinner anodic TiO 2 is revealed. The production of the porous oxide film significantly increases the interfacial bonding strength between the titanium alloy materials since the porous film may have a high degree of roughness that increases the debonding friction. 169
170 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b β α c d Figure 8.1. Scanning electron micrographs at different magnifications of Ti6Al4V alloy after different surface treatments; a) after etching of the as-received alloy in 48% HF + 70% HNO 3, b) higher magnification; c) after rf-gd sputter cleaning for 10 s at 35 V, d) higher magnification. a b Pt Ti6Al4V β α α β Figure 8.2. Transmission electron micrographs of cross-section of as-received Ti6Al4V alloy (a), and (b) higher magnification. The α and β phases are highlighted in the images respectively. 170
171 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b c d e Figure 8.3. EBSD results of the Ti6Al4V alloy after mechanical polishing + etching treatment; a) scanning electron micrograph of an interested region; b) inverse pole figure map; c) phases map; d) phases showing 96% of hcp α structure and 4% of bcc β structure; e) pole figures. 171
172 Anodic Voltage / V Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Time / s Figure 8.4. Voltage time response of Ti6Al4V during anodizing in the NaTESi electrolyte at ambient temperature. 172
173 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b c d V Ti O Ti a Spectrum 2 C Al V Ti V Full Scale 206 cts Cursor: kev O V Ti Al Ti b Spectrum 1 C Ti V Full Scale 228 cts Cursor: kev O V V c Spectrum 1 Ti Al Ti C Ti V V Full Scale 100 cts Cursor: (2 cts) kev Spectrum 5 V d Ti O Al Ti C Ti V V Full Scale 100 cts Cursor: (3 cts) kev Figure 8.5. Scanning electron micrographs of the Ti6Al4V alloy etched in 48% HF + 70% HNO 3, and anodized to 10 V (b), 20 V (c), 30 V (d) and 40 V (e) in the NaTESi electrolyte; the regions of EDS analysis are highlighted by solid frames. 173
174 Intenstiy (a.u.) Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours V V 20 V 10 V As-received Wavelength (cm -1 ) Figure 8.6. Raman spectra of the as-received Ti6Al4V alloy, and alloy anodizing in the NaTESi electrolyte at 20 ma cm -2 to 10, 20, 30 and 40 V. 174
175 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a Oxide 175
176 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Figure 8.7. EDS line scanning (a) by TECNAI F30 transmission electron microscopy to determine the locations of the anodic film formed on the Ti6Al4V alloy after anodizing to 10 V in the NaTESi electrolyte. After the location of anodic film is known, the higher magnification images are displayed in the Figs. 8.7 (b), (c) and (d); a diffraction pattern focused on a specific region of the anodic film which is highlighted in the image (d). 176
177 Intensity / a.u. Intensity / a.u. Intensity / a.u. Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Ti Al V O Na Si a Sputtering Time / s Ti Al V O Na Si b Sputtering Time / s Ti Al V O Na Si c Sputtering Time / s 177
178 Intensity / a.u. Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Ti Al V O Na Si d Sputtering Time / s Figure 8.8. GDOES depth profiles of the Ti6Al4V alloy anodized to 10, 20, 30 and 40 V in the NaTESi electrolyte. The intensities of aluminium, vanadium, oxygen and silicon are multiplied by
179 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Figure 8.9. Rutherford backscattering spectra of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 (d) V in the NaTESi electrolyte. 179
180 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Figure Nuclear reaction analysis diagrams of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 (d) V in NaTESi electrolyte. 180
181 - Phase Angle - Phase Angle - Phase Angle - Phase Angle Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours Impedance / cm a Impedance Phase angle Fitted Impedance / cm b Impedance Phase angle Fitted Frequency / Hz Frequency / Hz Impedance / cm c Impedance Phase angle Fitted Impedance / cm d Impedance Phase angle Fitted Frequency / Hz Frequency / Hz Figure Bode diagrams with fitted curves of anodic films formed on the Ti6Al4V alloy after anodizing to 10 (a), 20 (b), 30 (c) and 40 (d) V in the NaTESi electrolyte. 181
182 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a b Figure Schematic drawing of the equivalent circuits of the Ti6Al4V alloy anodized in the NaTESi electrolyte after the EIS measurements; (a), anodic films formed after anodizing to 10 V; (b), for the films formed after anodizing to 20, 30 and 40 V. 182
183 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a β α b c d Figure Scanning electron micrographs at different magnifications of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 (d) V in the NaTESi electrolyte after degradation treatment in climatic chamber with a humidity of 90% at 50 o C for 1000 h immediately. 183
184 Load (N cm -2 ) Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours II I As-received 2-10 V 3-20 V 4-30 V 5-40 V Displacement (mm) Figure Adhesive bonding tests of the as-received Ti6Al4V alloy and the alloy after anodizing in the NaTESi electrolyte to 10, 20, 30 and 40 V. 184
185 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours a 1 2 b 3 4 c 6 5 d
186 Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi electrolyte with Associated Degradation and Adhesive Bonding Behaviours C Spectrum O 2 Spectrum 4 Ti V Ti Al O C V Ti V Full Scale 784 cts Cursor: (5 cts) Full Scale kev 191 cts Cursor: (0 cts) kev C Spectrum Ti 2 Spectrum 7 O V Al Ti O V Ti V Full Scale 396 cts Cursor: (0 cts) Full Scale 250 kev cts Cursor: (0 cts) kev C Spectrum V 2 Ti O Ti Al Spectrum O C Ti V V Full Scale 784 cts Cursor: (5 cts) Full Scale 513 kev cts Cursor: (0 cts) kev C Spectrum Ti 2 Spectrum 3 7 O 8 8 V Al Ti O C V Ti V Full Scale 396 cts Cursor: (0 cts) Full Scale kev 510 cts Cursor: (0 cts) kev Figure Scanning electron micrographs of the Ti6Al4V alloy anodized to 10 (a), 20 (b), 30 (c) and 40 V (d) V in the NaTESi electrolyte after single-lap shear bonding tests immediately. EDS spectra were detected in the regions shown by numbers; high magnification images are obtained in the regions highlighted by the squares. 186
187 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Chapter 9 Sputter-Deposited Titanium Anodic Oxide Film Growth on Magnetron 9.1. Introduction Magnetron sputtering is a popular and useful method that establishes the deposition of a wide range of industrially important coatings [184]. The hard, wear-resistant, corrosion resistant, friction or electrical properties can be improved by the magnetron sputtering method. This technique is also widely used for the simulation of metal surfaces for anodizing treatments in order to investigate the growth mechanism of anodic films [184]. The anodic films formed on the magnetron sputter-deposited titanium with the addition of various alloy elements has been studied in previous literature [84, ]. The plasma electrolytic oxidation on titanium was investigated by transmission electron microscopy, was referred to use magnetron sputtering titanium of 2000 nm thickness, and the anodizing treatment was proceeded to over 300 V on such titanium layer [188]. Crystallization analysis of anodic oxide on a sputtering titanium in sulphuric acid has been reported [189]. From the literature studying, it is known that the sputtering titanium layers over 200 nm thickness as the metal substrate for surface anodizing treatments have been used successfully. It is indicated that the DC magnetron sputtering technique offers a reliable titanium metal platform for the application of anodizing. Although anodizing treatments can be processed on a sputtering titanium layer, a thin titanium layer of ~100 nm thickness is still not certain if it can be successfully used for anodizing. Thus, the present chapter focuses on the anodic oxide growth mechanism on such a thin sputter-deposited titanium substrate Sputter-Deposited Titanium Layer The scanning electron micrograph in Figure 9.1a shows that the titanium layer is deposited and distributed uniformly; fine pores and minor defects are observed within the layer. The transmission electron micrograph in Figure 9.1b shows that the 187
188 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium cross-section of the titanium layer is of ~100 nm thickness which is sputtered homogeneously on the electropolished aluminium substrate (Figure 9.1b) Anodizing of ~100 nm Thick Sputter-Deposited Titanium The voltage - time response reveals an initial voltage surge of 2-3 V due to the presence of the air-formed oxide on the titanium layer (Figure 9.2). The voltage then increased rapidly at ~6.0 V s -1 to ~100 V. Over the voltage between ~100 and 110 V inspected, the response shows an unsteady signal as a slightly reduced slope of ~5.0 V s -1 to ~160 V is revealed. A further reduction occurred until the final voltage of ~155 V was achieved. Oxygen bubbles were visible to the naked eyes at the commencement of anodizing, confirming the occurrence of oxygen evolution. The transmission electron micrograph of a cross-section of the specimen anodized to 10 V and prepared by ultramicrotomy shows an anodic film of ~27.2 nm thickness, as shown in Figure 9.3a. After anodizing to 30 V, an anodic film of ~50.8 nm thickness is formed on the sputter-deposited titanium (Figure 9.3b). When anodized to 50 V, a titanium anodic film of ~58.5 nm thickness is generated, with the presence of oxygen bubbles which range from ~5 to ~20 nm (Figure 9.3c). Therefore, the film growth ratio for the sputtering titanium layer is ~1.7 nm V -1, which shows a good agreement with previous studies [ ]. Interestingly, due to probably the penetration of the phosphoric acid electrolyte into the defects of titanium layer, an aluminium anodic film is formed at the Ti / Al interface after the Al substrate is contacted with the electrolyte; consequently, the anodizing of aluminium is triggered. The titanium anodic film thicknesses of the specimens anodized to 80, 100, 130 and 150 V are ~111.9 nm (Figure 9.4a), ~133.0 nm (Figure 9.4b), ~108.5 nm (Figure 9.4c) and ~105.1 nm (Figure 9.4d). An EDS spectrum was scanned in the interface zone of TiO 2 / Al 2 O 3 to locate the sputter-deposited titanium layer when anodizing to 80 V. The spectrum displays the presence of peaks regarding the quantitative compositions of Ti, wt. 12.8%, O, wt. 66.8% and Cu, wt. 20.5%. It is shown that such a region mainly contains the TiO 2 film, and the existence of copper species is from the mesh grid used as the specimen holder. Relatively numerous bubble-like features are observed within the titanium anodic film; the size of bubbles is slightly increased in the range of ~44-60 nm from 80 to 100 V. However, the size is 188
189 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium decreased to ~20 or ~30 nm at 130 or 150 V. Interestingly, the thickness of the titanium anodic film decreased from 100 to 150 V since the layer was probably ruptured. An amorphous aluminium anodic film was also formed at the TiO 2 / Al interface. It is reported that the dielectric breakdown voltage for 99.6% pure titanium in 1 M H 3 PO 4 was estimated as ~80 V [193]; thus, a porous anodic film might probably form if anodizing is carried out above 80 V. However, such a porous titanium anodic film was not evident in the present cases. Further, it is observed that some regions of sputter-deposited titanium layer were ruptured since oxygen bubbles developed within such layer, resulting in the thickness reduction of the titanium layer. The aluminium substrate was connected with the electrolyte when the electrolyte penetrated through the ruptured titanium layer. Oxygen bubbles and an increased volume of bubbles were mainly developed within the titanium anodic film, which would reduce the current efficiency for the film growth. Additionally, a few bubbles of a small size of ~3 nm are also observed at the Al 2 O 3 / TiO 2 interface. The bursting of the bubbles would result in the rupture of the Al 2 O 3 and TiO 2 films at the interface region. Examples are shown for specimens anodized to 80 and 100 V respectively in Figure 9.5. The regions where the sputter-deposited titanium layer was completely ruptured are revealed. Amorphous aluminium anodic films of ~78.4 nm (Figure 9.5a) and ~93.4 nm (Figure 9.5b) thicknesses are formed on the aluminium metal. The region where the titanium layer was destroyed generated a thicker aluminium anodic film since the aluminium was directly connected with the electrolyte. High resolution TEM for studying the anodic film growth mechanism on the titanium layer and the aluminium substrate was employed. Different areas focused on the titanium anodic film formed after anodizing to 100 V and the TiO 2 / Al 2 O 3 interface are revealed in Figure 9.6a. The sputter-deposited titanium layer is not found at the TiO 2 / Al 2 O 3 interface due to the destruction of such a layer (Figure 9.6b). However, the Ti / TiO 2 interface can be observed in the area c ; O 2 -filled voids are developed and nanocrystals in the vicinity of the voids are evident within the film (Figure 9.6c). The sputtering titanium layer is negligible from the TiO 2 / Al 2 O 3 interface in the area d, as shown in Figure 9.6d, and the feature of O 2 -filled voids is also found within the anodic TiO 2. The crystal phases are evident through 189
190 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium the observation of lattice fringes highlighted by the dashed lines within the TiO 2. It is confirmed that the amorphous-to-crystallization transition occurred at localized regions of the titanium anodic film. Further, an area containing an oxygen bubble is highlighted; the high pressure is released by the bursting of the bubble and leads to the rupture of the film (Figure 9.6e). It is also displayed that the oxygen bubbles are mainly developed within the titanium anodic film due to the high electronconducting field induced by the crystalline transition occurred only in this film [80, 120] Anodizing of ~290 nm Thick Sputter-Deposited Titanium A titanium layer of ~290 nm thickness was deposited on the electropolished aluminium using the magnetron sputtering technique under the standard procedure except for a longer sputtering time. A transmission electron micrograph of an anodic oxide film of ~45 nm thickness formed on the sputter-deposited titanium after anodizing to 30 V in 1 M H 2 SO 4 is shown in Figure 9.7a, which is consistent with a growth ratio of ~1.5 nm V -1. Figure 9.7b presents an anodic film of ~75 nm thickness generated on the titanium in 1 M H 3 PO 4, which is corresponding to ~2.5 nm V -1. It is also observed that the sputter-deposited titanium layer is not ruptured by the development of oxygen evolution, and the anodic TiO 2 generates uniformly. Figure 9.8 shows the GDOES profiles of the anodic films formed on the titanium layer of ~100 nm thickness in the sulphuric and phosphoric acids when anodized to 30 V. The titanium anodic film and the titanium substrate can be distinguished within the titanium wave profile. The aluminium is displayed that it is located in the inner part of the oxide due to the growth of alumina. A low concentration of sulphur (Figure 9.8a) or phosphorus (Figure 9.8b) is shown, which is incorporated into the film derived from the sulphuric or phosphoric acid respectively. A low oxygen content can also be found that it is slightly higher in the anodic film formed in the sulphuric acid compared with the phosphoric acid. Figure 9.9 shows GDOES profiles of anodic films formed on the sputter-deposited titanium of ~290 nm thickness after anodizing to 30 V in the sulphuric and phosphoric acids. It is observed that the wave profile of titanium is wider for the part of anodic film and the titanium layer due to the larger thicknesses if compared with 190
191 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium the ~100 nm thick titanium layer and its anodic film. At the Al / Ti interface, the growth of alumina is not evident since the titanium layer is not ruptured for the penetration of the electrolytes. Similar low concentrations of oxygen, sulphur and phosphorus are also found compared with the anodizing case of the 100 nm thick titanium layer Discussion The present study shows that the anodic TiO 2 is formed on the sputter-deposited titanium layer of ~100 nm thickness, and the formation of an amorphous Al 2 O 3 at the Ti / Al interface is evident due to the penetration of electrolyte. The thickness of the anodic TiO 2 rises after anodizing from 10 to 100 V; however and interestingly, it is reduced from 100 to 150 V. The anodic Al 2 O 3 with thicknesses of ~10.5 nm (10 V), ~ 12.7 nm (30 V), ~17.3 nm (50 V), ~43.6 nm (80 V) and ~126.5 nm (100 V) are identified, indicating that it is increased with increased anodic voltage. For 130 and 150 V, the thickness of the Al 2 O 3 is decreased to ~112.1 and ~76.5 nm. Also, oxygen bubbles were developed at the TiO 2 / Al 2 O 3 interface. Thereby, a schematic illustration of the anodizing process for the ~100 nm thick sputter-deposited titanium layer is proposed, as shown in Figure First, when the anodic voltage is between 10 and 50 V, anodic TiO 2 is generated with the occurrence of oxygen evolution within the film and at the TiO 2 / Ti interface; a thin amorphous Al 2 O 3 is formed as a result of the penetration of the electrolyte through the defects of the titanium layer (Figure 9.10a). Second, for anodizing between 50 and 100 V, the anodic TiO 2 becomes thicker and oxygen bubbles are developed in larger sizes. Further, a thicker Al 2 O 3 also forms at the Al / Ti interface (Figure 9.10b). The development of oxygen bubbles is mainly induced within the crystalline phases of TiO 2, and the bubbles can probably be developed further into the sputter-deposited titanium. Then, the titanium layer would be ruptured due to the bursting of large volumes of oxygen bubbles. Therefore, the titanium layer will be ruptured, and the thickness of the ruptured regions is reduced. Third, for anodizing from 100 to 150 V, the sputtering layer can be ruptured to a great extent, and it will become very thin at the of TiO 2 / Al 2 O 3 interface; however, some oxygen bubbles of few nm dimensions can still be developed within the anodic Al 2 O 3 (Figure 9.10c). The thickness of the anodic TiO 2 is decreased since the current efficiency was suppressed for the film growth on such a thin titanium layer. Fourth, when anodizing above 80 V, a thicker anodic Al 2 O 3 is 191
192 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium formed within regions where the sputter-deposited titanium is ruptured totally, and these regions make the current efficiency mostly spend for the Al 2 O 3 growth after the aluminium metal directly connects with the electrolyte (Figure 9.10d). The sequence of the formation of the anodic TiO 2 or Al 2 O 3 is still an interesting research. It is not completely clear which anodic film is formed in the first place, or they are both generated contemporaneously. The anodic TiO 2 is possibly formed firstly due to the direct connection with the electrolyte in the initial stages, accompanied with oxygen evolution. Further, after oxygen bubbles are developed, a large pressure will be present in the anodic titania and the sputter-deposited titanium, and the bursting of bubble leads to the ruptures. Consequently, the electrolyte can penetrate into the defects and ruptures of the titanium anodic film and the layer, and the anodic Al 2 O 3 growth on aluminium is triggered. Further, the combined aluminium and titanium anodic films formed are probably useful for improving corrosion resistance of the pure aluminium metal in aggressive electrolytes; however, this is not yet understood and requiring future investigations Summary 1). Thin sputter-deposited titanium layers of ~100 nm thickness using a DC magnetron sputtering technique on electropolished aluminium substrates are used to study the growth of titanium anodic film growth in 1 M H 3 PO 4. The thickness of titanium anodic film increases from 10 to 100 V; however, it decreases from 100 to 150 V. 2). The titanium anodic film and the sputter-deposited layer are ruptured by the bursting of large volumes of oxygen bubbles. The thin titanium layer cannot provide the sufficient current efficiency for the film growth when anodizing above 80 V due to the occurrence of oxygen evolution. An amorphous aluminium anodic film is formed at the Ti / Al interface due to the penetration of electrolyte through the oxygen-filled voids of titanium anodic film and the ruptured regions of the sputtering layer. After the electrolyte connects with the aluminium substrate, the anodic Al 2 O 3 growth is triggered. 192
193 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium 3). The anodic film can be formed normally on a sputter-deposited titanium layer of ~290 nm thickness, which is evident from the anodizing examples to 30 V in the sulphuric and phosphoric acid electrolytes. 4). The results confirm that a thin sputtering titanium layer of ~100 nm thickness should not be used for the dielectric breakdown anodizing processes. Notification: some of the works in the Chapter 9 have been published in Journal of Materials Characterizations 98(2014) , please see Appendices. Thus, some of the published contents in this chapter have been rephrased to avoid the selfplagiarism issue. 193
194 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a b Ti Al Figure 9.1. (a) Scanning electron micrograph of sputter-deposited titanium surface on electropolished aluminium, (b) transmission electron micrograph of cross-section of sputterdeposited titanium layer. 194
195 Voltage / V Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Time / s Figure 9.2. Voltage time response of anodic oxide growth of sputter-deposited titanium on electropolished aluminium in 1 M H 3 PO 4 at 20 ma cm -2 at ambient temperature. 195
196 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a b TiO 2 TiO 2 Ti O 2 bubble Al 2 O 3 Al Ti O 2 bubble Al 2 O 3 Al c Al 2 O 3 Al TiO 2 Ti O 2 bubble Al 2 O 3 Al Figure 9.3. Transmission electron micrographs of the sputter-deposited titanium anodized in 1 M H 3 PO 4 to 10 (a), 30 (b) and 50 V (c). 196
197 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a b TiO 2 TiO 2 Al 2 O 3 Al O 2 bubble Al 2 O 3 Al O 2 bubble c d TiO 2 TiO 2 O 2 bubble O 2 bubble Al 2 O 3 Al 2 O 3 Al Al Figure 9.4. Transmission electron micrographs of the sputter-deposited titanium anodized in 1 M H 3 PO 4 to 80 V (a), 100 V (b), 130 V (c) and 150 V (d); EDS was scanned at a region of Al 2 O 3 /TiO 2 interface after anodizing to 80 V, highlighted by a circle solid line. 197
198 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a b Al 2 O 3 Al Ruptured Ti Ti Al Al 2 O 3 Ruptured Ti Figure 9.5. Transmission electron micrographs of the regions of the titanium layers where were ruptured completely after anodizing to 80 V (a) and 100 V (b) in 1 M H 3 PO
199 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Figure 9.6. Transmission electron micrographs of anodic film formed on sputter-deposited titanium in 1 M H 3 PO 4 after anodizing to 100 V; (a) highlighted different regions, (b) a region at TiO 2 / Al 2 O 3 interface, (c) a region at Ti / TiO 2 interface, (d) another region at TiO 2 / Al 2 O 3 interface, (e) a region containing an oxygen bubble. 199
200 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a Al b Al Ti TiO 2 Ti TiO 2 Figure 9.7. Transmission electron micrographs of the 290 nm thick sputter-deposited titanium anodized at 20 ma cm -2 to 30 V in 1 M H 2 SO 4 (a) and H 3 PO 4 (b). 200
201 Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a Ti Al O Ti oxide Ti S Sputtering Time (s) Sputtering Time (s) b Ti Al O 20 P Ti oxide Ti Sputtering Time (s) Sputtering Time (s) Figure 9.8. GDOES depth profiles of ~100 nm thick sputter-deposited titanium after anodizing to 30 V in H 2 OS 4 (a) and H 3 PO 4 (b). 201
202 Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Intensity (a.u.) Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a Ti Al O Ti oxide Ti S Sputtering Time (s) Sputtering Time (s) b Ti Al Ti oxide Ti Sputtering Time (s) Sputtering Time (s) O P Figure 9.9. GDOES depth profiles of ~290 nm thick sputter-deposited titanium after anodizing to 30 V in H 2 OS 4 (a) and H 3 PO 4 (b). 202
203 Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium a b c d Figure Schematic diagram of titanium and aluminium anodic films formed on the sputter-deposited titanium layer and the aluminium substrate; a) anodizing from 10 to 50 V, showing that the titanium anodic film is thickening with the formation of a thin aluminium anodic film at the Ti / Al interface; b) anodizing from 50 to 100 V, titanium anodic film becomes thicker with the thinning of sputtering titanium layer and the thickening of aluminium anodic film; c) anodizing from 100 to 150 V, revealing that the titanium anodic film becomes thinner with significant rupture of most of the sputtering titanium layer; d) anodizing over 80 V, showing a completely ruptured region of sputtering layer where the titanium anodic film could not form, and a thicker aluminium anodic film could be generated. 203
204 Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium Introduction Currently, transmission electron microscopy (TEM) has become the most often used technique for research on thin film coatings. However, such a technique still presents some disadvantages: the facility is expensive; the analysis is time-consuming and is skill-intensive to a certain extent. Recently, scanning transmission electron microscopy in scanning electron microscopy (STEM-in-SEM) has been reported in some publications related to the areas of mineralogy and petrology [194], semiconductors [195], nanomaterials [196, 197], polymers and catalysts [198]. The STEM-in-SEM has been recognized to be a rapid and easy method for the morphology characterization and the internal structure of mineral and rock specimens, and it was also shown to be particularly useful in microbiology research [199]. Since STEM-in-SEM has been explored in different applications, the application to anodic oxide films formed on titanium is of interest. In this chapter, some examples of anodic films formed on titanium are presented, with emphasis on the film morphology and oxygen bubble features Anodic Films formed on Sputter-Deposited Ti The scanning electron micrographs in Figure 10.1 compare the ultramicrotomed sections in the SE mode and STEM mode at a very low magnification. The contrast in the STEM mode is clearer than the SE mode. It is known that the SE mode detection is generated by secondary electrons emitted by atoms which are excited by the electron beam [200]. Then, by scanning the specimen and detecting the secondary electrons, an image displaying the topography of the surface is created. STEM is a type of TEM operation; as with any transmission illumination scheme, the electrons pass through a sufficiently thin specimen. STEM focuses the electron beam into a narrow spot, which is scanned over the specimen in a raster [201]. Thus, the image created in the SE mode (Figure 10.1a) is basically for identifying regions 204
205 Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium on the anodized titanium specimens; then, the STEM mode is employed for provision of further details (Figure 10.1b). The current time responses of anodizing at 10 and 50 V are shown in Figure 10.2 respectively. At 50 V, the current density decreased rapidly from an initial relatively high value. After ~15 s, the drop of current density was reduced until a minimal value was reached at ~30 s. Then, the current density increased slowly and maintained a stable value after ~200 s, which was corresponding to the growth of an oxide film on titanium in the phosphoric acid. At 10 V, a similar reduction of current density was recorded during the initial anodizing stage until ~15 s. The current density increased slightly until a steady value was maintained. The current density recorded for anodizing at 50 V after 900 s is higher compared with 10 V. Scanning transmission electron micrographs at different magnifications (Figure 10.3) show a 20 ± 5 nm thick anodic film formed on the sputtering titanium layer at 10 V. Figures 10.4a and b show the sputtering titanium after anodizing at 50 V in the Inlens and SE modes respectively, and the titanium layer and the film are not distinguished except for the aluminium substrate. The scanning transmission electron micrograph (Figure 10.4c) shows that an anodic film of 100 ± 10 nm thickness is formed on titanium after anodizing at 50 V, which corresponds to a growth rate of ~2 nm V -1. The round shape of oxygen bubbles within the anodic films can be observed in the range of ~5-30 nm diameters. It is confirmed that the film thicknesses and oxygen bubbles originating from oxygen evolution during anodizing can be characterized by STEMin-SEM. At a low magnification (~150 kx) or a high magnification (~500 kx) of the STEM mode, the morphology of anodic oxide film can be observed, and the image contrast for this mode is better than the other modes in SEM Other Use of STEM-in-SEM An important benefit which can be obtained from STEM-in-SEM is the large magnification range of the SEM from very low to very high combined with the high field of view compared with TEM. The large field of view can be relevant for failure analysis mechanisms such as crack propagation, which typically spans from a scale range of mm to µm. Figure 10.5 raises an example for such an application, where a thin ultramicrotomed section of a notched specimen was collected on a supported 205
206 Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium mesh grid, and imaged at low magnification (Figure 10.5a) to get a clear representation of the crack. The same specimen was then imaged at high magnification (Figure 10.5b), showing the crack grows as a notch within the aluminium. Thus, with the STEM-in-SEM, regions of interest from a large section can be quickly selected in order to generate further details at higher magnifications Discussion The main highlight of STEM-in-SEM over TEM is its suitability as the ideal equipment for environments that need rapid morphology analysis of specimens in large numbers at low cost. Such is the case in a typical production environment of anodic coatings on titanium, where morphological parameters can be monitored and critically controlled as quality control factors in manufacturing processes and research purposes. Practically, extensive morphology analysis using TEM sometimes is not carried out, even in the most advanced processing plants, since it is not ideal to carry out TEM analysis in large numbers and with a fast turnaround. The situation can be readily simplified with STEM-in-SEM. As found earlier, it is possible to conduct high throughput, TEM-like imaging using STEM-in-SEM in an industrial environment. In this work, the single-specimen STEM holder has been re-designed to handle up to 6 specimens at the same time. The throughput of the system is therefore considerably higher than a TEM with a single specimen holder. Most of the commercial SEM suppliers now offer a STEM detector as an attachment, with the capability to handle up to six specimens or more. The use of the multi-specimen carousel has enabled remarkably higher equipment productivity and better utilization of operation time. An estimation of the time saving through deployment of the STEM-in-SEM carousel, compared with a single-specimen system was derived from a careful analysis of the steps involved in the STEM-in-SEM process. Steps involved can be categorized into two main steps as follows: (1) Specimen preparation, involves the standard ultramicrotomy procedures to prepare cross-sections. (2) Imaging, involves loading the specimen holder into the microscope, STEM unit on the specimen holder, evacuating the chamber until the vacuum level is reached, finding the region of interest and acquiring images of relevant features, and finally removing the specimen. 206
207 Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium While both steps are common for TEM and STEM-in-SEM, but are improved in terms of speed and quality, major differences are identified in productivity which is estimated between the two techniques when a 6-specimen carousel system is designed for the STEM-in-SEM. It is estimated that the time saving per specimen resulted in a 20% increase in imaging productivity compared with TEM imaging. An added benefit of the carouse holder system is that it contributes to increasing the lifetime of the equipment by limiting the number of pumping and venting cycles and by limiting the filament warm-up of TEM Summary 1). A STEM-in-SEM system with a 6-specimen carousel allows straightforward analysis of the anodic oxide film coatings on titanium in terms of speed and quality. This makes the STEM-in-SEM a very practical and affordable alternative to TEM analysis, especially in industrial environments for the morphology characterization of anodic oxidation coatings. 2). Although TEM imaging at higher accelerating voltages is capable of providing more detailed images with higher resolutions, analysis of thin anodic film can be characterized by STEM-in-SEM. 3). STEM-in-SEM provides imaging at a large field of flexible magnification ranges from very low to very high level. Therefore, the whole anodic film thickness generated and oxygen bubbles originating from oxygen evolution during anodizing commonly required for analysis of most anodic films formed on titanium can be clearly identified by STEM-in-SEM. Notification: some of the work in the Chapter 10 has been published in Journal of Vacuum, 115(2015)19-22, please see Appendices. Thus, some of the published contents in this chapter have been rephrased to avoid the self-plagiarism issue. 207
208 Current Density (A cm -2 ) Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium a b Figure Scanning electron micrographs in SE mode (a) and in STEM mode (b) at very low magnification located on the ultramicrotomed sections on a copper supported grid. SE mode was used to identify the location of the titanium sections V 10 V Time (s) Figure Current-time responses of anodizing of titanium at 10 and 50 V in 1 M H 3 PO 4 for 900 s at ambient temperature. 208
209 Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium a b TiO 2 Ti Figure STEM-in-SEM micrographs at a low magnification (a) and a high magnification (b) of anodic film formed on titanium after anodizing at 10 V in 1 M H 3 PO 4 for 900 s. 209
210 Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium a d TiO 2? Ti? Al b e TiO 2? Ti? Al c TiO 2 Ti O 2 bubble Al Figure Scanning electron micrographs at different modes and different magnifications of anodic film formed on titanium after anodizing at 50 V in 1 M H 3 PO 4 for 900 s, a) Inlens mode; b) SE mode; c) STEM mode; d) low magnification in STEM mode; e) high magnification in STEM mode. 210
211 Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of the Anodic Oxide Film Formed on Titanium Figure An example of a specimen with a notched region in STEM-in-SEM a thin section of 15 nm thick was ultramicrotomed in cross-section and collected on a copper supported grid. (a) Low magnification of STEM-in-SEM image showing the depth of the notch. (b) High magnification of STEM-in-SEM image showing the effect of the propagation of notch morphology induced by diamond knife during ultramicrostomed processing. 211
212 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Introduction It is well known that an anodic oxide film can be generated on titanium surface using anodic oxidation techniques. Transmission electron microscopy has been widely used to study such air-formed or anodic oxide film layers. However, the specimen preparation is always a difficulty for titanium materials. Ultramicrotomy is a very useful tool for the preparation of TEM specimen, especially for aluminium and its alloys. However, it is not usually used for the preparation of titanium specimens due to damage incurred by the diamond knife. The focused-ion beam method is suitable for fabricating titanium specimens; nevertheless, the operation is complex, time consuming and somewhat skill-intensive. In some studies, methanol has been recommended as one of the most effective destabilising agents of titanium oxide films [202]. As reported in the literature, a bromine-methanol mixed electrolyte has been used to detach the anodic oxide films after the titanium substrate of 500 µm [80] or 50 µm [203] thickness was dissolved; the stripped film was less than 100 nm thick which can be observed by transmission electron microscopy (TEM). On the other hand, if a titanium specimen of ~ 2 mm thickness is used, although such a thick titanium metal is probably impossible to be dissolved in the bromine-methanol within a short time, the air-formed or anodic film could be stripped off to expose the metal substrate. If the substrate is exposed, it is assumed that the bare metal will be attacked by the bromine in the electrolyte. Further, it has been reported that titanium can suffer localized corrosion from a bromide-containing environment [46], and aqueous concentrated bromide solutions are commonly used as electrolytes in the electrochemical machining of titanium and, due to their high density, they are also used as fluids in the oil drilling process [204]. It has also been reported that titanium was used in concentrated LiBr solutions for absorbing air conditioning machines [205]. Moreover, the occurrence of pitting dissolution and a high surface roughness after the electrolytic polishing of titanium in NH 4 Br has been revealed [206]. 212
213 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte It is known that titanium is an important material which is widely used for the manufacture of facility units in oil and gas industries such as heat exchangers nowadays. During the oil refining process, crude oil was brought into a refinery as part of the feed slate. The crude oil contains some of the fluids at a level that filled with contaminants such as halogens. The halogen identified was bromine, and it was traced back to the fluids that had contaminated the crude shipment [207]. Thus, the facilities manufactured from titanium materials may be at risk from corrosion after the crude oil containing bromine is processed. In this chapter, the corrosion behaviour of bare titanium after the air-formed oxide film is stripped off from the etching in bromine-methanol electrolyte is explored Stripped Anodic Film Figure 11.1 shows that the anodic film, formed by anodizing at 20 V, is observed by TEM from different regions after being detached from the titanium substrate by immersion in the bromine-methanol electrolyte for 300 s. Particularly, the morphology of the anodic film was recorded in bright-field transmission electron micrographs, while the degree of crystallinity of the oxide was determined by electron diffraction patterns. In principle, amorphous oxide films generate diffraction patterns with relatively broad rings, whereas nanocrystalline oxides generate relatively sharp rings and crystalline oxides with a prevailing orientation producing diffraction spots. From both of the oxide regions (Figure 11.1a and Figure 11.1b), large dark areas associated with crystallinity were evident and spots were recorded in the diffraction patterns, also indicating the presence of crystal phases of anatase. Further, lighter areas in Figure 11.1b were detected within the oxide, associated with the presence of oxygen-filled bubbles occluded in the film Titanium before and after Etching Figure 11.2 shows scanning electron micrographs of the as-received and chemically etched titanium in the bromine-methanol electrolyte for 10, 30, 120 and 300 s. Figure 11.2a displays the micrograph of the surface of the as-received titanium with no visible grain boundaries since the surface is covered with a thin oxide film and the surface has not been exposed to the electrolyte. After etching for 10, 30, 120 and 300 s, the grain boundaries were revealed and the crystal grains were distinguishable due 213
214 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte to the removal of the air-formed TiO 2 film on the titanium substrate by the etching process. (Figure 11.2b - e). The grained surface showed micro-pits of less than 1 µm in diameter in certain regions. The depths of the grain boundary and micro-pits appear to increase with etching time. The pit-like defects or micro-pits were developed within the grains and/or grain boundaries. Further, the metal surface appears rougher with etching time compared with the as-received condition. The SEM micrograph and associated EBSD point scans examined from the surface of the chemically etched titanium, which was etched for 10 s, are shown in Figure The grains had mainly prism planes aligned toward the titanium surface. Some of the grains appeared brighter in the micrograph due to the different depths of attack on the individual grains. The prism planes revealed are located at different regions of the valley-like surface feature. The EBSD scans of the etched areas show the α phase titanium with hexagonal closed pack crystalline structures, as expected. The boundaries in the secondary electron image fit perfectly with the orientation observed, i.e., it shows the three same [ ] that represent for the prism grain orientations. Figure 11.4a displays the weights of specimens and their losses after etching for various times. The weight loss increased from to g cm -2 between 10 and 300 s of etching. The average corrosion rate is plotted in Figure 11.4b according to the weight loss, which was divided by the corresponding time of exposure. The corrosion rate increased from to g cm -2 s -1 corresponding to the etching times of 10 and 300 s respectively. The backscattered electron micrographs in Figure 11.5 show the specimen surfaces where pitting corrosion occurred after etching. This was induced after the air-formed oxide film was stripped off and the bare metal was attacked by the bromide ions. The pits were found to be ~ µm in radius within the surface after the etching time of 10 s (Figure 11.5a). The pits formed to sizes of µm after etching times of 30, 120 and 300 s, and appear larger compared with the pits after the etching time of 10 s (Figures 11.5b - d). The population density of pits increased with increase in etching time. Expectedly, the etching time has a significant influence on the growth of pits. The presence of titanium (97.76 at.%) and bromine (2.24 at.%) in the EDS 214
215 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte spectrum mapped on a corroded zone of the titanium after etching for 300 s, confirms the presence of significant quantity of bromide ions in such regions. It can be inferred from the corrosion attacks observed that titanium has a weak resistance to pitting corrosion in the environment containing bromine. Further, pits usually tend to initiate at preferred sites, such as grain boundaries, high dislocation density regions and micro-cracks [208]. It has also been reported that pits are preferably formed along the grain boundaries [209]. Thus, the large pits could be formed through the coalescence of several small pits around the same site. This is consistent with the evident that pits found on the titanium substrates after etchings in the brominemethanol electrolyte. Figure 11.6 displays the cross-sections of titanium after etching in the brominemethanol electrolyte for 30 and 300 s. It is revealed in the micrographs that the corroded area, with the occurrence of pitting, was enlarged with increase in the etching time (Figures 11.6a - b). EDS spectra show the presence of mainly titanium (98.02 at.%) and a small amount of bromine (1.98 at.%) in a corroded region of titanium after an etching time of 300 s, whilst only titanium (100 at.%) is present in the non-corroded area (Figure 11.6b). Having established that the bromine-methanol environment induces pitting corrosion on titanium, the severity of the attack in such environment was investigated by estimating the depths of the pits and the surface roughness using white light interferometry. A 3-D volumetric construction of a selected local region on the titanium, etched in the bromine-methanol electrolyte for 10 s, is presented in Figure It displays different colour scaling from blue, green to red that represents the map of the surface, to highlight the geometric arrangement of the depth level. The depth of the pits is measured through the dz range located between the bottom of the pits and the metal surface, which is schematically demonstrated in the inset graph. The interferometry profile reveals the depths of the micro-pits on the surface which were obtained by averaging the dz measurements of five pits. The results, listed in Table 11.1, revealed an increasing trend with increase in etching time. The surface roughnesses of the as-received and etched titanium specimens are also listed. The roughness increased with increasing etching time from 10 to 300 s due to the occurrence of more pitting corrosion as the exposure time increases. 215
216 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Table Pit depth and surface roughness of titanium after chemical etching in brominemethanol electrolyte for different times. Etching time / s Pit depth / µm / Roughness / µm The specific compositions of the etched titanium in the bromine-methanol electrolyte were identified by XRD pattern analysis, as shown in Figure After etching for 10 s, only one peak corresponding to TiBr 4 was revealed (Figure 11.8b). Two halo peaks associated with TiBr 4 compounds were detected in the XRD patterns after etching for 30, 120 and 300 s respectively (Figures 11.8c - e). The intensity of the peaks corresponding to TiBr 4 was slightly increased with increase in etching time due to the higher concentration of bromine on the titanium as etching time increases. The XRD results revealed that the Ti substrate was dissolved as Ti 4+ after the airformed oxide film was detached, and the dissolution reaction can be illustrated as follows: Ti + 2Br 2 TiBr 4 (1) Discussion It has been reported that aqueous solutions containing bromine act as effective etchants for semiconductors, etching the crystal (-1-1-1) and (100) faces in a diffusion-controlled way and the (111) face in a kinetically controlled way respectively [210]. The bromine-methanol mixtures have been considered hazardous at a certain concentration of Br 2 [211], and also methanol is toxic. Surprisingly, in practice, methanol is generally used as the solvent of Br 2 for the etching of materials. The air-formed TiO 2 on the titanium surface, which is known to be semi-conductive [212], may be stripped off effectively in such bromine-methanol etchant. Previous studies have shown that titanium exhibits a much higher susceptibility to localized corrosion in bromine-containing environments compared with chlorinecontaining environments [ ]. It is suggested that pit initiation of unalloyed titanium in bromide solutions is related to the adsorption of bromine into the defects 216
217 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte of bare titanium [216] or the formation of bromide nuclei at the impurity-rich sites containing mostly TiBr 4, which eventually reaches a critical concentration and results in the initiation of pits [217]. It is demonstrated that, in bromine media, a surface defect in the metal matrix encouraged localized dissolution of the substrate [9]. These regions of defects appear to be areas for the nucleation of pits, where halide ion mass transfer occurs and the formation of titanium halide is favoured. From the SEM evidences in Figure 11.4 and Figure 11.5, etching of titanium in the bromine-methanol electrolyte induces the dissolution of the metal since there is no protection from the air-formed oxide film. It is thought that bromide ions are adsorbed into the metal surface in an aqueous solution containing bromine: Br Br ad + e (2) Adsorbed bromines are related to the bromide ions by one of the following two steps: Br ad + Br - Br 2,aq + e - (3) Br ad + Br ad Br 2,aq (4) where Br 2,aq is bromine gas dissolving in the solution. Saturation of Br 2,aq enhances the initiation of corrosion pits. Further, as the etching time increases, more bromine react with the titanium substrate, forming TiBr 4. The main composition of the bromide nuclei is TiBr 4. Thus, the dissolution of titanium is predominant, and the thinning of the metal would be induced: Ti Ti e (5) The reaction in Eq. 5 is also evident from XRD measurements, and corresponding to the propagation of pitting corrosion. At the bare titanium surface, bromine penetrate more and more into the titanium surface as the etching time increases and larger depths of pits are formed. This was observed using the white light interferometry. The speculated manner of the dissolution of the titanium substrate followed by bromine-induced pitting and propagation seems to be similar to that of iron/steel system if without their protective passive oxide film in the environments containing chlorine, in which the iron/steel substrate dissolves as ferric ions and ferrous ions respectively [28, 218, 219]. The metal substrate also dissolves as metal ions as the corrosion pit propagates. 217
218 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Although it is well reported that bromide and chloride ions give rise to pitting of titanium, the present study shows and discusses the pitting corrosion of titanium in the bromine-methanol mixed environment under laboratory conditions for the first time. Further, understanding of titanium dissolution mechanism through corrosion attack by bromine could provide a useful reference for the material s applications in oil and gas industries Summary 1). The air-formed oxide film or anodic film can be stripped off from the titanium substrate in the bromine-methanol electrolyte after the etching of 300 s, and grain boundaries with defects at specific regions on the titanium substrate are exposed. 2). Pitting corrosion occurs on the bare titanium without the protective air-formed oxide film due to the attack of bromine. 3). The corrosion pit propagates with increase in etching time from 10 to 300 s, which is measured by white light interferometry. The surface roughness is increased as etching time increases. 4). The TiBr 4 compound identified by XRD indicates that titanium substrate is dissolved during the etchings from 10 to 300 s. 218
219 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte a b Figure Transmission electron micrographs with diffraction patterns of different regions (a & b) of stripped anodic film from titanium after etching in bromine-methanol electrolyte for 300 s, the anodic film formed on titanium after anodizing at 20 V for 900 s in 1 M H 2 SO 4 at ambient temperature. a b c Pit Pit d e Pit Pit Figure Scanning electron micrographs of as-received (a) and etched titanium in bromine-methanol electrolyte for 10 s (b), 30 s (c), 120 (d) and 300 (e) at ambient temperature. 219
220 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Figure Electron backscatter diffraction mapping of etched titanium in brominemethanol electrolyte for 10 s. 220
221 Corrosion Rate (g. cm -2. s -1 ) Weight (g. cm -2 ) Weight Loss (g. cm -2 ) Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte 6.1x x10-1 a 2.0x x x x x x Time (s) 5.0x x10-4 b 3.0x x x Time (s) Figure (a) Weights and weight losses obtained from the titanium specimens before and after etching in bromine-methanol electrolyte for different durations; (b) corrosion rates measured from weight loss records. 221
222 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte a b c d Ti Ti Spectrum 7 Br Ti Full Scale 940 cts Cursor: (0 cts) kev Element Wt. % At. % Ti Br Figure Backscattering electron micrographs of etched titanium in bromine-methanol electrolyte for 10 (a), 30 (b), 120 (c) and 300 s (d); an EDS spectrum was scanned in a corroded region of titanium after etching for 300 s, highlighted by a solid square. 222
223 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte a b Corroded area Non-corroded Corroded area 1 Non-corroded 2 Ti Spectrum 2 Ti Br Ti Full Scale 409 cts Cursor: (0 cts) kev Ti Ti Spectrum 2 Ti Full Scale 1344 cts Cursor: (0 cts) kev Element 1 2 Ti Br Wt. % At. % Wt. % 3.26 / At. % 1.98 / Figure Scanning electron micrographs of cross-section of titanium after etching for 30 (a) and 300 s (b); EDS spectra were scanned on corroded and non-corroded regions of etched titanium for 300 s, which are highlighted by solid squares in image (b). 223
224 Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Pit d Z Dept h Figure White light interferometry profiles of etched titanium in bromine-methanol electrolyte for 10 s. 224
225 Intensity (arb. unit.) Intensity (arb. unit.) Intensity (arb. unit.) Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte a T T T 6000 T T T T T T Position (2 o Theta) b T T T TiBr T T T T T T Position (2 o Theta) c T T T TiBr 4 TiBr T T T T T T T Position (2 o Theta) 225
226 Intensity (arb. unit.) Intensity (arb. unit.) Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte d T T T T TiBr 4 TiBr T T T T T Position (2 o Theta) e T T T TiBr 4 TiBr T T T T T T T Position (2 o Theta) Figure X-ray diffraction patterns of as-received titanium (a) and titanium after etching for (b) 10 s, (c) 30 s, (d) 120 s and (e) 300 s. 226
227 Chapter 12 Main Conclusions and Suggestions for Future Work Chapter 12 Work Main Conclusions and Suggestions for Future Main Conclusions Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids The surface of pre-treated CP-Ti and barrier-type anodic films generated on the CP- Ti were investigated in this chapter. The growth mechanism of anodic oxide films formed were comprehensively understood and examined by scanning electron microscopy, transmission electron microscopy, X-ray diffraction pattern, Rutherford backscattering spectroscopy, nuclear reaction analysis, glow discharge optical emission spectroscopy, X-ray photoelectron spectroscopy and electrochemical impedance spectroscopy. The grain boundaries of CP-Ti can be observed after the two different pre-treatments involving etching in HF+HNO 3 acid and the rf-gd sputter surface cleaning. The grain orientations of the titanium are distinguished by EBSD, and three main orientations of [0001] and [11-20] and [10-10] are identified. A single-barrier anodic oxide film can be created using an electrochemical technique with a combination of a pre-scan of potentiodynamic polarization and continuous scan by a following potentiostatic anodizing in sulphuric and phosphoric acids respectively. An amorphous-to-crystalline transition of anodic titanium oxide is induced with the nucleation of nanocrystals during the potentiodynamic polarization stage. Oxygen evolution is developed in both the potentiodynamic polarization and potentiostatic anodizing processes, resulting in the formation of blister structures of the films. The rupture of the anodic film is initiated with the bursting of bubbles. The anodic oxide film formed in the sulphuric acid is thinner than in the phosphoric acid for the anodizing from 10 to 60 V. The oxide is ruptured from anodizing at 20 V in the sulphuric acid; however, such rupture is induced from anodizing at 50 V in the phosphoric acid since the amorphous-to-crystalline transition within the oxide growth is impeded by the incorporation of phosphate anions to a certain degree. The 227
228 Chapter 12 Main Conclusions and Suggestions for Future Work higher dielectric permittivity of the film in the sulphuric acid indicates a higher electron conductivity compared with the film formed in the phosphoric acid. Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment The anodic oxide films were generated on CP-Ti in 1 M H 2 SO 4 and 1 M H 3 PO 4 at a constant current of 20 ma cm -2 to selected voltages. The corrosion behaviour of anodic oxide films formed on CP-Ti was investigated in a near neutral 3.5% NaCl electrolyte. The comparison between the corrosion resistance of anodic films produced from sulphuric acid and phosphoric acid was studied. Prior to the corrosion tests, ruptures of the anodic films induced by the bursting of oxygen bubbles originated from the oxygen evolution during anodizing are revealed. The bursting of oxygen bubbles release a huge pressure within the film and a consequent blister surface texture is formed. The rupture is more significant for the anodic films formed in the sulphuric acid compared with the phosphoric acid, indicating that oxygen evolution can be inhibited to a greater extent during anodizing in the phosphoric acid. Further, the anodic films with more ruptures are found after the titanium specimens were anodized to higher anodic voltages. The films with more ruptures are also observed after the anodized titanium specimens were immersed for 60 days in the 3.5% NaCl compared with the immediate immersion conditions. The EIS and potentiodynamic polarization results show that the corrosion resistance of titanium can be improved in a NaCl environment by anodizing treatments and with increase of anodic voltage. The anodic films formed in the phosphoric acid provide a higher corrosion resistance compared with the anodic films formed in the sulphuric acid after anodizing to the same voltage. Consequently, the anodic oxide films formed on titanium in the phosphoric acid perform a greater corrosion resistance compared with the sulphuric acid for serving in chloridescontaining environments. 228
229 Chapter 12 Main Conclusions and Suggestions for Future Work Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte The anodizing behaviour involving structures, thicknesses and crystallization of anodic oxide films formed on titanium at breakdown voltages of 100, 150 and 200 V respectively are investigated in this chapter. The influences of the breakdown voltages on the film growth mechanism are evaluated. The porous anodic film growth on CP-Ti by the breakdown anodizing in 1 M phosphoric acid electrolyte is significantly dependent upon the applied voltages. Anatase crystalline structures are evident within the porous anodic films. Oxygen bubbles originating from oxygen evolution are developed around the nanocrystals. The oxygen bubbles are also the precursors of the breakdown sites as well as violent dissolution of the oxide, giving rise to the shape of the circular pores. The degree of crystallinity within the anodic film generated at a higher anodic voltage is more abundant than a lower anodic voltage. Particulate-like structures are found that developed within the pores and the increased size of pores is revealed due to the dissolution of the particulates. The electronic conductivity of the film formed at 200 V is probably higher compared with lower voltages, leading to the development of more oxygen bubbles within the film. The phosphorus species are incorporated into the porous films formed at different voltages, and such species may stabilize the nanocrystals within the films. Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi Electrolyte with Associated Degradation and Adhesive Bonding Behaviours The anodizing behaviour of CP-Ti in the NaTESi electrolyte with identification of the types of anodic film that are formed, including the compositions and morphology, were studied in the present chapter. The behaviour of the amorphous-tocrystallization transition during the growth of the films was also investigated. Additionally, the degradation and adhesive bonding behaviours of the anodic oxide films were investigated. 229
230 Chapter 12 Main Conclusions and Suggestions for Future Work A nodule-textured surface of the anodic films is formed on commercially pure titanium in the NaTESi electrolyte after anodizing to 5, 10, 20 and 40 V respectively. The thickness of the anodic film increases with increase of anodic voltage. Anodic films of thicknesses of ~ 30, 37 and 67 nm were generated after anodizing to 5, 10 and 20 V respectively. However, a porous anodic film of ~ 80 nm thickness was formed when anodized to 40 V. The amorphous-to-crystalline transition occurred within the film, and the reduced oxide thickness at some of the local regions resulted from crystallization is triggered by the development of oxygen evolution. The degree of crystallinity of the anodic film increased with increase of the voltage. The low current efficiency responsible for the anodic film growth is probably induced by oxygen evolution. A dielectric permittivity of 2.35 for the anodic film formed after anodizing to 10 V is estimated from the EIS and TEM results. The anodic films formed after anodizing to 10 and 20 V show an excellent degradation resistance after the degradation test for 1000 h. The shear bonding load of anodized titanium in the NaTESi electrolyte increases as the anodic voltage increases. EDS analysis indicates that the low oxygen contents within the film results from the debonding of the film region. It is observed from the single-lap shear bonding tests that the shear bonding strength increases with increase of anodic voltage associated with a thicker anodic TiO 2 coating. Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi Electrolyte with Associated Degradation and Adhesive Bonding Behaviours The anodizing behaviour of Ti6Al4V alloy in the NaTESi electrolyte was explored in this chapter. The identification of the types of anodic film formed on Ti6Al4V alloy, including the compositions and morphology, were examined. Also, the degradation and adhesive bonding behaviours of the anodic films formed on Ti6Al4V alloy in such an electrolyte were investigated. The α + β phases and grain boundaries within the Ti6Al4V alloy microstructure can be observed after etching in HF + HNO 3 electrolyte. The duplex phases can be also observed after the rf-gd sputter surface cleaning. The EBSD grain, phase mappings and inverse pole figures show 96% of the HCP titanium structure from the α phase and 4% of the BCC structure from the β phase. The basal crystal plane of (0001) and 230
231 Chapter 12 Main Conclusions and Suggestions for Future Work two prismatic crystal planes of (11-20) and (10-10) are identified. The formation of pores within the anodic film is observed after anodizing to 20 V. The quantity of the pores is increased with increased anodic voltage. EDS spectra confirm that the oxygen concentration is increased with increased anodic voltage. Three weak crystalline peaks can be found associated with the anodic film formed to 10 V. Similar anatase crystalline peaks are evident for anodizing to 20, 30 and 40 V respectively, and the degree of crystallinity of the film is more abundant at higher anodic voltages. The oxide film thickness generated after anodizing to 10 V is ~11 nm, which is determined by TEM. The amorphous-to-crystalline transition is also evident in higher magnification images of TEM. GDOES profiles show the presence of titanium, aluminium, vanadium, oxygen, sodium and silicon species, and the formation of sodium-rich layers throughout the films are detected. RBS results confirm that the current efficiency for the anodic film growth is increased from 10 to 30 V; however, it is reduced when anodizing from 30 to 40 V due to oxygen evolution. The oxide film thicknesses estimated by RBS are ~15.0 nm, ~39.0 nm, ~1100 nm and 1800 nm associated with anodizing to 10, 20, 30 and 40 V respectively. The dielectric permittivity of the anodic TiO 2 formed on Ti6Al4V in NaTESi is measured as ~118 from the EIS and the TEM evidence. The degradation treatment evident that the anodic films formed on Ti6Al4V alloy in the NaTESi electrolyte perform an excellent degradation resistance in the 90% humidity environment at 50 o C for 1000 h. The single-lap shear adhesive bonding test results confirm that the shear bonding strength increases with increase of anodic voltage associated with a thicker anodic TiO 2 coating compared with a thinner anodic TiO 2. The production of the porous oxide film significantly increases the interfacial bonding strength between the titanium alloy materials since the film may have a high degree of roughness that leads to the increase of debonding friction. Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Anodic oxide films formed on a magnetron sputter-deposited titanium layer of 100 nm thickness were investigated in the chapter by transmission electron microscopy. The anodic films formed on a sputter-deposited titanium layer of 290 nm thickness were also studied to compare with the film growth mechanism on the thinner titanium layer. 231
232 Chapter 12 Main Conclusions and Suggestions for Future Work Thin sputter-deposited titanium layers of ~100 nm thickness using a DC magnetron sputtering technique on electropolished aluminium substrates are used to study the titanium anodic film growth in 1 M H 3 PO 4. The thickness of the anodic film on titanium increases from 10 to 100 V but decreases from 100 to 150 V. The titanium anodic film and the sputter-deposited layer are ruptured by the bursting of large volumes of oxygen bubbles. The thin titanium layer cannot provide sufficient current efficiency for the titanium anodic film growth when anodizing above 80 V due to the occurrence of oxygen evolution. An amorphous aluminium anodic film is formed at the Ti / Al interface due to the penetration of electrolyte through the oxygen-filled voids of titanium anodic film and the ruptured regions of the sputtering layer. After the electrolyte connects with the aluminium substrate, the anodic Al 2 O 3 growth is triggered. The anodic film can be formed normally on a sputter-deposited titanium layer of ~290 nm thick, which is evident from anodizing examples to 30 V in the sulphuric and phosphoric acid electrolytes. The results confirm that a thin sputtering titanium layer of ~100 nm thickness should not be used for the dielectric breakdown anodizing processes. Chapter 10 Scanning Transmission Electron Microscopy Technique in Scanning Electron Microscopy for Morphology Analysis of Anodic Oxide Film Formed on Titanium The application using scanning transmission electron microscopy to anodic oxide films formed on titanium is explored. In this chapter, some examples of anodic films formed on titanium are presented, with emphasis on the film morphology and oxygen bubble features. A STEM-in-SEM system with a 6-specimen carousel allows straightforward analysis of the anodic oxide film coatings on titanium in terms of quality of information. The speed of analysis is also improved remarkably. This makes the STEM-in-SEM a very practical and affordable alternative to TEM analysis, especially in industrial environments for the morphological characterization of anodic oxidation coatings. Although TEM imaging at higher accelerating voltages is capable of providing more detailed images with higher resolution, analysis of thin anodic film can be realized by STEM-in-SEM. STEM-in-SEM provides imaging at a new field with flexible 232
233 Chapter 12 Main Conclusions and Suggestions for Future Work magnification ranges from very low to very high. Therefore, the whole anodic film thickness generated and the features of the oxygen bubbles originated from the oxygen evolution during anodizing that are commonly required for analysis of most thin barrier anodic films formed on titanium can be clearly identified in the STEMin-SEM. Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte This chapter studied and discussed the corrosion behaviour of titanium substrate in a bromine-methanol mixed electrolyte after the air-formed oxide film was stripped off by etching in the electrolyte. Chemical etching in the bromine-methanol electrolyte can strip off the air-formed or the anodic oxide film on titanium to expose the bare metal substrate. At specific regions of the bare titanium, grain boundaries can be observed. The results also provide direct evidences that pitting corrosion is induced on the bare titanium by etching in the bromine-methanol electrolyte. The depth of pits increases with increase in etching time due to the dissolution of the metal substrate. Further, the roughness of titanium substrate increases with the etching time due to the inducement of more pitting corrosion Suggestions for Future Work The suggestions for future work are separated based on the different relative chapters, and they are demonstrated in details as follows: Chapter 4 Barrier Anodic Oxide Film Growth of CP-Titanium in Sulphuric and Phosphoric Acids A comparison for using the combined techniques of potentiodynamic polarization and the potentiostatic method or the direct DC power supply to operate anodizing tests may be interesting. It is still not fully known that for anodizing, using potentiodynamic polarization + potentiostataic anodizing or using the traditional power supply for directly potentiostatic anodizing has any differences. Therefore, the 233
234 Chapter 12 Main Conclusions and Suggestions for Future Work future study could be raised for such comparison in order to further investigate possible new anodizing techniques. Chapter 5 Corrosion Behaviour of Anodic Oxide Film on CP-Titanium in NaCl environment The corrosion study of anodic oxide films formed on CP-Ti in the sulphuric and phosphoric acids will be carried out in other aggressive electrolytes containing halogen ions such as Br -, and hence, the corrosion resistance of the anodic films in such environments will be investigated. Chapter 6 Formation of Porous Anodic Oxide Film on CP-Titanium in Phosphoric Acid Electrolyte Further research will be required to obtain information about the bioactivity of the porous anodic films formed on CP-Ti through relevant tests in simulated body fluid to provide references for medical applications. Chapter 7 Characterization of Anodic Oxide Growth on CP-Titanium in NaTESi Electrolyte with Associated Degradation and Adhesive Bonding Behaviours Further work will explore the influence to the efficiency of film growth on CP-Ti in the NaTESi electrolyte and also the mechanism of the nodule-shape anodic oxide film development, including the role of foreign species in the electrolyte, which may contribute to influencing the structure and stability of the anodic film. Additionally, the consumption of titanium substrate is also required to be understood if it could be induced when immersed in such aggressive electrolyte and the effects for the anodizing behaviour. Chapter 8 Characterization of Anodic Oxide Growth on Ti6Al4V Alloy in NaTESi Electrolyte with Associated Degradation and Adhesive Bonding Behaviours Further study will explore the factors affecting the efficiency of film growth on Ti6Al4V in the NaTESi electrolyte and also the development mechanism of the 234
235 Chapter 12 Main Conclusions and Suggestions for Future Work porous anodic oxide film, including the role of foreign species in the electrolyte, which may contribute to influencing the structure and stability of the anodic oxide films. Chapter 9 Anodic Oxide Film Growth on Magnetron Sputter-Deposited Titanium Corrosion investigations of the anodic TiO 2 film and the anodic Al 2 O 3 film will be considered for the potential surface protection of pure aluminium in NaCl environments in the future. Chapter 10 Scanning Transmission Electron Microscopy Technique for Morphology Analysis of Anodic Oxide Film Formed on Titanium Future study will explore other uses of STEM-in-SEM, such as research on the deformation layer of aluminium materials and different types of anodic oxide film growth on titanium or aluminium materials. Chapter 11 Chemical Etching Behaviour of CP-Titanium in Bromine-Methanol Electrolyte Further study will explore the corrosion behaviour of the bare titanium in other aggressive environments to understand the corrosion knowledge in depth. Additionally, the understanding of corrosion behaviour of titanium substrate when the natural oxide film or anodic oxide film is dissolved can support the research study for failure analysis of titanium material. 235
236 References References [1] G.F. Hays, Now is the Time, in: Advanced Materials Research, [2] J.A. Marceau, Moji, Y., and McMillan, J. C, Bicentennial of Materials Progress, SAMPE, 21 (1976) [3] S. Cui, B.R.K. Blackman, A.J. Kinloch, A.C. Taylor, International Journal of Adhesion & Adhesives, 54 (2014) [4] H. Jin, G.M. Miller, S.J. Pety, A.S. Griffin, D.S. Stradley, D. Roach, N.R. Sottos, S.R. White, International Journal of Adhesion & Adhesives, 44 (2013) [5] D.Eylon, S.R. Seagle, Titanium '99, Science and Technology, CRISM "Prometey", St. Petersburg (2000). [6] J. John A. Mountford, Corrosion, (2002). [7] R.T. Cole, E.J. Bateh, J. Potter, Composites, 13 (1982) [8] M.P. Christoph Leyens, Titanium and Titanium Alloys, in, Die Deutsche Bibliothek, Germany, [9] Barbara. Del. Curto, Maria V. Diamanti, MariaPia Pedeferri, Color Research & Application, 33 (2008) [10] An RTI International Metals, Inc. Company, (2000). [11] H.W. Bonin, S. Bhowmik, V.T. Bui, R.D. Weir, International Journal of Adhesion & Adhesives, 26 (2006) [12] A. Bloyce, P.-Y. Qi, H. Dong, T. Bell, Surface and Coatings Technology, 107 (1998) [13] E.A.d.S. Marques, Analysis of adhesive joints for aerospace applications, in, [14] W. Leahy, V. Barron, M. Buggy, T. Young, A. Mas, F. Schue, T. McCabe and M. Bridge, Journal of Adhesion, 77 (2001) [15] J.J. Liming Liu, Materials Transactions, 50 (2009) [16] H.W. Bonin, S. Bhowmik, V.T. Bui, R.D. Weir, International Journal of Adhesion & Adhesives, 26 (2006) [17] M. Assefpour-Dezfuly, C. Vlachos, E.H. Andrews, J Mater Sci, 19 (1984) [18] R.W. Schutz, Proc. 8th World Conf. on Titanium, Birmingham, UK, (1995) [19] A. Zhecheva, W. Sha, S. Malinov, A. Long, Surface and Coatings Technology, 200 (2005) [20] C. Matz, International Journal of Adhesion and Adhesives, 8 (1988) [21] A. Kurtovic, E. Brandl, T. Mertens, H.J. Maier, International Journal of Adhesion & Adhesives, 45 (2013) [22] Titanium A Technical Guide, ASM International, Metals Park, USA, [23] R. Pederson, Licentiate Thesis, Lulea University of Technology, (2002). [24] M.R.Jackson, C.T. Sims, J. L. Walter, ASM International, Metals Park, USA, (1988). [25] I.J. Polmear, Light Alloys Metallurgy of the Light Metals, Edward Arnold, London, [26] S.L.S. I. Weiss, Materials Science and Engineering, A263 (1999) [27] S.D. McDonald, M.J. Bermingham, M.S. Dargusch, D.H. StJohn, Materials Forum, 31 (2007) [28] K. Fushimi, K. Azumi, M. Seo, Journal of the Electrochemical Society, 147 (2000) [29] - Titanium Alloy Ti 6Al-4V. [30] D.M. Garcia-Garcia, E. Blasco-Tamarit, J. García-Antón, A. Guenbour, Int. J. Electrochem. Sci, 6 (2011) [31] I.-H. Lo, W.-T. Tsai, Corrosion, 64 (2008) [32] G.R. Edwards, B.K. Damkroger, Metals & Materials Societ, (1990) [33] Materials Properties Handbook: Titanium Alloys, ASM International, [34] V. Seetharaman, S.L. Semiatin, I. Weiss, Metals and Materials Society, (1997). [35] G.R. Edwards, B.K. Damkroger, Metals & Materials Society, (1990)
237 References [36] A. Prince, P. Villars, H. Okamoto, Handbook of Ternary Alloy Phase Diagram, ASM International, OH, USA, [37] W.-T. Tsai. J.-R. Chen, Electrochimica Acta 56 (2011) [38] S.L. Seetharaman. I. Weiss, Materials Science and Engineering A243 (1998) [39] A.J. Huang, D. Hu, X. Wu, Journal of Alloy Compounds 413 (2006) [40] X.J. Zhu, M.J. Tan, S. Thiruvarudchelvan, K.M. Liew, Archives of Materials Science and Engineering, 28 (2007) [41] B.C. Yang, M. Uchida, H.M. Kim, X.D. Zhang, K. Tadashi, Biomaterials, 25 (2004) [42] D. Thierry, C. Leygraf, J. Pan, Electrochemica Acta, 41 (1996) [43] D.V. Velten, V. Biehl, F. Aubertin, B. Valeske, W. Possart, J. Breme, Journal of Biomedical Material Research 59 (2002) [44] P.K. Chu, X.Y. Liu, C.X. Ding, Materials Science and Engineering 47 (2004) [45] G.D.J.,Jr. Guthrie, B.T. Mossman, Health Effects of Mineral Dusts, Mineralogical Society of America, 28 (1993). [46] N. Casillas, S.J. Charlebois, W.H. Smyrl, Journal of the Electrochemical Society, 140 (1993) L142-L145. [47] B. Wielage, T. Lampke, M. Zacher, D. Nietrich, Key Engineering Materials, 384 (2008) [48] z.e.d.a. Grades, PhD Thesis of Fabrication of Monodomain Porous Alumina Using Nanoimprint Lithography and its Applications, Elektronisches ULB Sachsen-Anhalt, [49] D.C. Barbara, M.V. Diamanti, MP. Pedeferri, Color Research and Application, 33(3) (2008) [50] T. Mertens, H. Kollek, International Journal of Adhesion & Adhesives, 30 (2010) [51] S. Van Gils, P. Mast, E. Stijns, H. Terryn, Surface and Coatings Technology, 185 (2004) [52] J.L. Delplancke, A. Garnier, Y. Massiani, R. Winand, Electrochimica Acta, 39 (1994) [53] Y.-T. Sul, C.B. Johansson, Y. Jeong, T. Albrektsson, Medical Engineering & Physics, 23 (2001) [54] D.J. Blackwood, R. Greef, L.M. Peter, Electrochimica Acta, 34 (1989) [55] M. Ask, J. Lausmaa, B. Kasemo, Applied Surface Science, 35 (1989) [56] M. Ask, J. Lausmaa, B. Kasemo, Applied Surface Science, 35 (1989) [57] E. Krasicka-Cydzik, Anodic Layer Formation on Titanium and Its Alloys for Biomedical Applications, [58] J. Lausmaa, B. Kasemo, H. Mattsson, H. Odelius, Applied Surface Science, 45 (1990) [59] M. Schneider, S. Schroth, J. Schilm, A. Michaelis, Electrochimica Acta, 54 (2009) [60] A. Yildiz, N. Serin, M. Kasap, T. Serin, D. Mardare, Journal of Alloys and Compounds, 493 (2010) [61] G. Rådegran, J. Lausmaa, L. Mattsson, U. Rolander, B. Kasemo, Journal of Electron Microscopy Technique, 19 (1991) [62] H. Zitter, H. Plenk, Journal of Biomedical Materials Research, 21 (1987) [63] R.J. Solar, S.R. Pollack, E. Korostoff, Journal of Biomedical Materials Research, 13 (1979) [64] P. Tengvall, I. Lundström, Clinical Materials, 9 (1992) [65] Y.-T. Sul, C.B. Johansson, S. Petronis, A. Krozer, Y. Jeong, A. Wennerberg, T. Albrektsson, Biomaterials, 23 (2002) [66] L.D. Arsov, C. Kormann, W. Plieth, Journal of Raman Spectroscopy, 22 (1991) [67] G. Blondeau, M. Froelicher, M. Froment, A. Hugot-Le-Goff, Journal of the Less Common Metals, 56 (1977)
238 References [68] C. da Fonseca, A. Traverse, A. Tadjeddine, M.d.C. Belo, Journal of Electroanalytical Chemistry, 388 (1995) [69] B.J. Hwang, J.R. Hwang, Journal of Applied Electrochemistry, 23 (1993) [70] J.S.L. Leach, B.R. Pearson, Corrosion Science, 28 (1988) [71] N. Sato, Electrochimica Acta, 16 (1971) [72] J. Yahalom, J. Zahavi, Electrochimica Acta, 15 (1970) [73] I.A. Ammar, I. Kamal, Electrochimica Acta, 16 (1971) [74] I.A. Ammar, I. Kamal, Electrochimica Acta, 16 (1971) [75] A. Aladjem, Journal of Materials Science, 8 (1973) [76] F. Climent, R. Capellades, Materials Letters, 18 (1994) [77] T. Shibata, Y.C. Zhu, Corrosion Science, 37 (1995) [78] T. Ohtsuka, J. Guo, N. Sato, Journal of the Electrochemical Society, 133 (1986) [79] A. Felske, W.J. Plieth, Electrochimica Acta, 34 (1989) [80] A. Mazzarolo, M. Curioni, A. Vicenzo, P. Skeldon, G.E. Thompson, Electrochimica Acta, 75 (2012) [81] H. Habazaki, K. Shimizu, S. Nagata, P. Skeldon, G.E. Thompson, G.C. Wood, Corrosion Science, 44 (2002) [82] H. Habazaki, K. Takahiro, S. Yamaguchi, K. Shimizu, P. Skeldon, G.E. Thompson, G.C. Wood, Philosophical Magazine A: Physics of Condensed Matter, Structure, Defects and Mechanical Properties, 78 (1998) [83] H. Habazaki, M. Uozumi, H. Konno, S. Nagata, K. Shimizu, Surface and Coatings Technology, (2003) [84] M.T. Tanvir, K. Fushimi, K. Shimizu, S. Nagata, P. Skeldon, G.E. Thompson, H. Habazaki, Electrochimica Acta, 52 (2007) [85] M. Ask, U. Rolander, J. Lausmaa, and B. Kasemo, Materials Research Society, 5(8) (1990) [86] O.K. Varghese, D. Gong, M. Paulose, K.G. Ong, E.C. Dickey, C.A. Grimes, Advanced Material, 15(7-8) (2003) [87] G.K. Mor, M.A. Carvalho, O.K. Varghese, M.V. Pishko, C.A. Grimes, Journal of Materials Research, 19(2) (2004) [88] M. Adachi, Y. Harada M, S. Yoshikawa, Chemistry Letters, 29(8) (2000) [89] S.Z. Chu, S. Inoue, K. Wada, D. Li, H. Haneda, S. Awatsu, Journal of Physiccal Chemistry B, 107(27) (2003) [90] G.K. Mor, O.K. Varghese, C.A. Grimes, Journal of Materials Research, 19(10) (2004) [91] V. Zwilling, M. Aucouturier, E. Darque-Ceretti, Electrochimica Acta, 45(6) (1999) [92] G.K. Mor, O.K. Varghese, M. Paulose, K. Shankar, C.A. Grimes, Soler Energy Materials and Soler Cells, 90(14) (2006) [93] D. Gong, C.A. Grimes, O.K. Varghese, W. Hu, R.S. Singh, Z. Chen, E.C. Dickey, Journal of Materials Research, 16(12) (2001) [94] G.K. Mor, O.K. Varghese, M. Paulose, C.A. Grimes, Sensor Letters, 1(1) (2003) [95] C. Ruan, M. Paulose, O.K. Varghese, C.A. Grimes, Soler Energy Materials and Soler Cells, 90(9) (2006) [96] Q. Cai, M. Paulose, O.K. Varghese, C.A. Grimes, Journal of Materials Research, 20(1) (2005) [97] R. Beranek, H. Hildebrand, P. Schmuki, Electrochem Solid-State Letters, 6(3) (2003) B12-B14. [98] K. Shankar, G.K. Mor, H.E. Prakasam, S. Yoriya, M. Paulose, O.K. Varghese, C.A. Grimes, Nanotechnology, 18(1-11) (2007) [99] J.M. Macak, H. Tsuchiya, P. Schmuki, Angewandte Chemie International Edition, 44(14) (2005) [100] J.M. Macak, H. Tsuchiya, L. Taveira, S. Aldabergerova, P. Schmuki, Angewandte Chemie International Edition, 44(45) (2005)
239 References [101] M. Paulose, K. Shankar, S. Yoriya, H.E. Prakasam, O.K. Varghese, G.K. Mor, T.A. Latempa, A. Fitzgerald, C.A. Grimes, Journal of Physical Chemistry B, 110(33) (2006) [102] M. Christophersen, J. Carstensen, K. Voigt, H. Foll, Physica Status Solidi A, 197(1) (2003) [103] Z.X. Su, W.Z. Zhou, Advanced Materials, 20(19) (2008) [104] Y.T. Sul, C.B. Johansson, S. Petronis, A. Krozer, Y. Jeong, A. Wennerberg, T. Albrektsson, Biomaterials, 23 (2002) [105] Y.T. Sul, C.B. Johansson, Y. Kang, D.G. Jeon, T. Albrektsson, Clinical Implant Dentistry and Related Research, 4 (2002) [106] D. Velten, V. Biehl, F. Aubertin, B. Valeske, W. Possart, J. Breme, Journal of Biomedical Materials Research, 59 (2002) [107] V.M. Frauchiger, F. Schlottig, B. Gasser, M. Textor, Biomaterials, 25 (2004) [108] Y.T. Sul, Biomaterials, 24 (2003) [109] M. Kawashita, X.Y. Cui, H.M. Kim, T. Kokubo, T. Nakamura, Key Engineering Materials, 254 (2004) [110] Y.T. Sul, C.B. Johansson, T. Albrektsson, International Journal of Oral and Maxillofacial Implants, 17 (2002) [111] N.K. Kuromoto, R.A. Simão, G.A. Soares, Materials Characterization, 58 (2007) [112] P. He, K. Chen, B. Yu, C.Y. Yue, J. Yang, Composites Science and Technology, 82 (2013) [113] A.N. Minaev, S.V. Gnedenkov, S.L. Sinebryukhov, D.V. Mashtalyar, M.V. Sidorova, Y.V. Tsvetkov, A.V. Samokhin, Protection of Metals and Physical Chemistry of Surfaces, 47 (2011) [114] S. Aliasghari, P. Skeldon, G.E. Thompson, Applied Surface Science, 316 (2014) [115] P. Skeldon, G.E. Thompson, G.C. Wood, X. Zhou, H. Habazaki, K. Shimizu, Philosophical Magazine A, 76 (1997) [116] Y. Kihn, G.E. Thompson, G. Galaup, P. Skeldon, X. Zhou, K. Shimizu, H. Habazaki, Corrosion Science, 42 (2000) [117] E. Zhuravlyova, L. Iglesias-Rubianes, A. Pakes, P. Skeldon, G.E. Thompson, X. Zhou, T. Quance, M.J. Graham, H. Habazaki, K. Shimizu, Corrosion Science, 44 (2002) [118] X. Zhou, G.E. Thompson, H. Habazaki, M.A. Paez, K. Shimizu, P. Skeldon, G.C. Wood, Journal of the Electrochemical Society, 147 (2000) [119] A. Aladjem, D.G. Brandon, J. Yahalom, J. Zahavi, Electrochimica Acta, 15 (1970) [120] H. Habazaki, M. Uozumi, H. Konno, K. Shimizu, P. Skeldon, G.E. Thompson, Corrosion Science, 45 (2003) [121] H. Habazaki, P. Skeldon, G.E. Thompson, J. Wan, G.C. Wood, X. Zhou, J. De Laet, K. Shimizu, Journal of Electrochemical Society, 144 (1997) [122] H. Habazaki, K. Shimizu, S. Nagata, P. Skeldon, G.E. Thompson, G.C. Woodd, Journal of Electrochemical Society, 149 (2002) B70-B74. [123] N. Khalil, J.S.L. Leach, Electrochimica Acta, 31 (1986) [124] K. Shimizu, G.E. Thompson, G.C. Wood, Thin Solid Films, 77 (1981) [125] S.M. Tavakoli, E.J.C. Kellar, D. Nassiri, A.E. Joseph, SPE ANTEC 2001 Conference - Medical Plastics, (2001). [126] - Test methods for adhesives and bonding. [127] A.V. Pocius, Adhesion and Adhesives Technology, Hanser Gardner Publications, Inc, USA, [128] D.J. Dunn, Engineering and Structural Adhesives, Smithers Rapra Publishing, UK, [129] M. Chaudhury, A.V. Pocius, Adhesion Science and Engineering, Chapter 6 (20082)
240 References [130] - ASTM D [131] U. Zorll, Coating Technology Handbook, Chapter 6 - Adhesion Test, Taylor & Francis Group, LLC, [132] M. Curioni, E.V. Koroleva, P. Skeldon, G.E. Thompson, Electrochimica Acta, 55 (2010) [133] T. Mertens, F.J. Gammel, M. Kolb, O. Rohr, L. Kotte, S. Tschöcke, S. Kaskel, U. Krupp, International Journal of Adhesion & Adhesives, 34 (2012) [134] J. Xing, Z. Xia, J. Hu, Y. Zhang, L. Zhong, Corrosion Science, 75 (2013) [135] H.Y. Si, Z.H. Sun, X. Kang, W.W. Zi, H.L. Zhang, Microporous and Mesoporous Materials, 119 (2009) [136] American Society for Testing and Materials, ASTM standard B600, Annual Book of ASTM Standard, American Society for Testing and Materials, Philadelphia, PA, [137] X.Y. Liu, P.K. Chu, C.X. Ding, Materials Science and Engineering: R: Reports, 47 (2004) [138] M.V. Diamanti, B. Del Curto, M. Pedeferri, Color Research & Application, 33 (2008) [139] J.F. Moulder, W.M.F. Stickle, P.R.E. Sobol, K.H.D. Bomben, Handbook of X-ray photoelectron spectroscopy: a reference book of standard spectra for identification and interpretation of XPS data, Phys Electron, [140] E. McCafferty, J.P. Wightman, Surface and Interface Analysis, 26 (1998) [141] B.V. Crist, XPS International LLC, XPS Reports, 1 (2007) [142] K. Shimizu, G.E. Thompson, G.C. Wood, K. Kobayashi, Philosophical Magazine B: Physics of Condensed Matter; Electronic, Optical and Magnetic Properties, 63 (1991) [143] T. Ogawa, Journal of Applied Physics, 32 (1961) [144] R.W. Schutz, H.B. Watkins, Materials Science and Engineering: A, 243 (1998) [145] M. Atapour, A.L. Pilchak, M. Shamanian, M.H. Fathi, Materials & Design, 32 (2011) [146] A. Mazzarolo, M. Curioni, A. Vicenzo, P. Skeldon, G.E. Thompson, Electrochimica Acta, 75 (2012) [147] J. Li, S.J. Li, Y.L. Hao, R. Yang, International Journal of Hydrogen Energy, 39 (2014) [148] J. Robertson, EPJ Applied Physics, 28 (2004) [149] E. McCafferty, Corrosion Science, 47 (2005) [150] D.D. Macdonald, Electrochimica Acta, 56 (2011) [151] R. Narayanan, S.K. Seshadri, Corrosion Science, 50 (2008) [152] Rabab M. Abou Shahba, Waffa A. Ghannem, Azza El-Sayed El-Shenawy, Amal S.I. Ahmed, Safaa M. Tantawy, International Journal of Electrochemical Science, 6 (2011) [153] H. Habazaki, M. Teraoka, Y. Aoki, P. Skeldon, G.E. Thompson, Electrochimica Acta, 55 (2010) [154] I.S. Park, T.G. Woo, W.Y. Jeon, H.H. Park, M.H. Lee, T.S. Bae, K.W. Seol, Electrochimica Acta, 53 (2007) [155] G. Wu, M. Yu, J. Liu, S. Li, L. Wu, Y. Zhang, Surface and Interface Analysis, 45 (2013) [156] M. Pankuch, R. Bell, C.A. Melendres, Electrochimica Acta, 38 (1993) [157] [158] G.H. Lü, H. Chen, X.Q. Wang, H. Pang, G.L. Zhang, B. Zou, H.J. Lee, S.Z. Yang, Chinese Physics B, 19 (2010) 1-6. [159] S.A. Fadl-allah, Q. Mohsen, Applied Surface Science, 256 (2010) [160] C.K. Dyer, J.S.L. Leach, Journal of the Electrochemical Society, 125 (1978)
241 References [161] L.D. Arsov, C. Kormann, W. Plieth, Journal of Raman Spectroscopy, 22 (1991) [162] G.L. Wu, M. Yu, J.H. Liu, S.M. Li, L. Wu, Y. Zhang, Surface and Interface Analysis, 45 (2013) [163] S. Marinel, D.H. Choi, R. Heuguet, D. Agrawal, M. Lanagan, Ceramics International, 39 (2013) [164] C. Scheck, P. Evans, R. Schad, G. Zangari, J.R. Williams and T.F. Isaacs-Smith, Journal of Physics.: Condensed Matter, 14 (2002) [165] K. Asami, S.C. Chen, H. Habazaki, K. Hashimoto, Corrosion Science, 35 (1993) [166] H. Habazaki, M. Uozumi, H. Konno, K. Shimizu, S. Nagata, K. Asami, P. Skeldon, G.E. Thompson, Electrochimica Acta, 47 (2002) [167] H. Habazaki, Y. Oikawa, K. Fushimi, K. Shimizu, S. Nagata, P. Skeldon, G.E. Thompson, Electrochimica Acta, 53 (2007) [168] K. Wapner, G. Grundmeier, Surface and Coatings Technology, 200 (2005) [169] H.-J. Song, M.-K. Kim, G.-C. Jung, M.-S. Vang, Y.-J. Park, Surface and Coatings Technology, 201 (2007) [170] Z. Liu, H. Liu, X. Zhong, T. Hashimoto, G.E. Thompson, P. Skeldon, Surface and Coatings Technology, 258 (2014) [171] G.E. Thompson, H. Habazaki, K. Shimizu, M. Sakairi, P. Skeldon, X. Zhou, G.C. Wood, Aircraft Engineering and Aerospace Technology, 71 (1999) [172] H. Habazaki, K. Takahiro, S. Yamaguchi, K. Shimizu, P. Skeldon, G.E. Thompson, G.C. Wood, Philosophical Magazine A, 78 (1998) [173] Q. Lu, J. Alberch, T. Hashimoto, S.J. Garcia-Vergara, H. Habazaki, P. Skeldon, G.E. Thompson, Corrosion Science, 50 (2008) [174] H. Habazaki, M. Uozumi, H. Konno, K. Shimizu, S. Nagata, K. Asami, K. Matsumoto, K. Takayama, Y. Oda, P. Skeldon, G.E. Thompson, Electrochimica Acta, 48 (2003) [175] M.O. Seong-Jong Kim, Yoshihiro Mizutani, Ryoichi Ichino, Shoji Tanikawa and Saori Hasegawa, Materials Transactions, 44 (2003) [176] R. Narayanan, S.K. Seshadri, Corrosion Science, 49 (2007) [177] R.Z. Legeros, S. Lin, R. Rohanizadeh, D. Mijares, J.P. Legeros, Journal of Materials Science: Materials in Medicine, 14 (2003) [178] S. Kudelka, J.W. Schultze, Electrochimica Acta, 42 (1997) [179] T. Ohtsuka, T. Otsuki, Corrosion Science, 40 (1998) [180] F. Di Quarto, F. Di Franco, C. Monarca, M. Santamaria, H. Habazaki, Electrochimica Acta, 110 (2013) [181] S.K. Kim, W.-D. Kim, K.-M. Kim, C.S. Hwang, J. Jeong, Applied Physics Letters, 85 (2004) [182] H. Tang, K. Prasad, R. Sanjinès, P.E. Schmid, F. Lévy, Journal of Applied Physics, 75 (1994) [183] J.D. Venables, Journal of Materials Science, 19 (1984) [184] P.J. Kelly, R.D. Arnell, Vacuum, 56 (2000) [185] V.C. Nettikaden, A. Baron-Wiecheć, P. Bailey, T.C.Q. Noakes, P. Skeldon, G.E. Thompson, Corrosion Science, 52 (2010) [186] M. Santamaria, F. Di Quarto, H. Habazaki, Electrochimica Acta, 53 (2008) [187] F. Di Quarto, F. Di Franco, C. Monarca, M. Santamaria, H. Habazaki, Electrochimica Acta, 110 (2013) [188] E. Matykina, R. Arrabal, P. Skeldon, G.E. Thompson, Acta Biomaterialia, 5 (2009) [189] Z. Xia, H. Nanjo, H. Tetsuka, T. Ebina, M. Izumisawa, M. Fujimura, J. Onagawa, Electrochemistry Communications, 9 (2007) [190] D. Dunn, S. Raghavan, Surface and Coatings Technology, 50 (1992) [191] J.E. Sundgren, P. Bodö, I. Lundström, Journal of Colloid and Interface Science, 110 (1986)
242 References [192] H. Badekas, C. Panagopoulos, Surface and Coatings Technology, 31 (1987) [193] Y.-T. Sul, Biomaterials, 24 (2003) [194] C. Smith, M.R. Lee, M. MacKenzie, Microscopy and Analysis, 111 (2006) [195] M. Nakagawa, R. Dunne, H. Koike, M. Sato, J.J. Pérez-Camacho, B.J. Kennedy, Journal of Electron Microscopy, 51 (2002) [196] J. Russias, F. Frizon, C. Cau-Dit-Coumes, A. Malchère, T. Douillard, C. Joussot- Dubienz, Journal of the American Ceramic Society, 91 (2008) [197] D. Acevedo-Reyes, M. Perez, C. Verdu, A. Bogner, T. Epicier, Journal of Microscopy, 232 (2008) [198] G.M. Brown, A.D. Westwood, Microscopy and Microanalysis, 9 (2003) [199] M.R. Lee, C.L. Smith, Mineralogical Magazine, 70 (2006) [200] A.M. Paredes, MICROSCOPY Scanning Electron Microscopy, in: C.A.B.L. Tortorello (Ed.) Encyclopedia of Food Microbiology (Second Edition), Academic Press, Oxford, 2014, [201] R.J. Keyse, P. Goodhew, A.J. Garratt-Reed, G.W. Lorimer, Bios Scientific Publishers, (1998). [202] N.-S. Peighambardoust, F. Nasirpouri, Transactions of the IMF, 92 (2014) [203] E. Matykina, R. Arrabal, P. Skeldon, G.E. Thompson, H. Habazaki, Thin Solid Films, 516 (2008) [204] C. Madore, D. Landolt, Journal of Micromechanics and Microengineering, 7 (1997) 270. [205] E. Blasco-Tamarit, A. Igual-Muñoz, J. García Antón, D. García-García, Corrosion Science, 49 (2007) [206] E.A.a.D.I. R.Ittah, Int. J. Electrochem. Sci, 9 (2013) [207] G. Rittenhouse, The American Association of Petroleum Geologists Bulletin, 51 (1967) [208] Z. Jiang, X. Dai, T. Norby, H. Middleton, Corrosion Science, 53 (2011) [209] P. Marcus, V. Maurice, H.H. Strehblow, Corrosion Science, 50 (2008) [210] K.S. Ugent, W. Gomes, Journal of Electrochemical Society, 140 (1993) [211] P.T. Bowman, E.I. Ko, P.J. Sides, Journal of Electrochemical Society, 137 (1990) [212] A.M. Schmidt, D.S. Azambuja, E.M.A. Martini, Corrosion Science, 48 (2006) [213] D. Sazou, K. Saltidou, M. Pagitsas, Electrochimica Acta, 76 (2012) [214] J.L. Trompette, L. Massot, L. Arurault, S. Fontorbes, Corrosion Science, 53 (2011) [215] I. Dugdale, J.B. Cotton, Corrosion Science, 4 (1964) [216] T.R. Beck, Journal of Electrochemical Society, 120 (1973) [217] S. Huo, X. Meng, Corrosion Science, 31 (1990) [218] K.E. Heusler, L. Fischer, Werkstoffe und Korrosion, 27 (1976) [219] W. Tian, N. Du, S. Li, S. Chen, Q. Wu, Corrosion Science, 85 (2014)
243 Appendices Publications Appendices List of Publications Zuojia Liu, Xiaohui Liu, Uyime. Donatus, George. E. Thompson and Peter. Skeldon. Corrosion behaviour of the anodic oxide film on commercially pure titanium in NaCl Environment, International Journal of Electrochemical Science, 9 (2014) Z. Liu and G.E. Thompson. Formation of porous anodic oxide film on titanium in phosphoric acid electrolyte, Journal of Materials Engineering and Performance, (2015) 24: Z. Liu, H. Liu, X. Zhong, T. Hashimoto, G.E. Thompson and P. Skeldon. Characterization of anodic oxide growth on commercially pure titanium in NaTESi electrolyte, Surface & Coatings Technology, 258 (2014) Z. Liu, H. Liu, T. Hashimoto, G.E. Thompson and P. Skeldon. Anodic oxide film growth on thin magnetron sputter-deposited titanium layer, Materials Characterization, 98 (2014) Z. Liu, T. Hashimoto, I-Ling Tsai, G.E. Thompson, P. Skeldon and H. Liu. Scanning transmission electron microscopy technique for morphology analysis of anodic oxide film formed on titanium. Vacuum, 115 (2015) Publications are attached here in PDF image version, start from next page. 243
244 Appendices Publications 244
245 Appendices Publications 245
246 Appendices Publications 246
247 Appendices Publications 247
248 Appendices Publications 248
249 Appendices Publications 249
250 Appendices Publications 250
251 Appendices Publications 251
252 Appendices Publications 252
253 Appendices Publications 253
254 Appendices Publications 254
255 Appendices Publications 255
256 Appendices Publications 256
257 Appendices Publications 257
258 Appendices Publications 258
259 Appendices Publications 259
260 Appendices Publications 260
261 Appendices Publications 261
262 Appendices Publications 262
263 Appendices Publications 263
264 Appendices Publications 264
265 Appendices Publications 265
266 Appendices Publications 266
267 Appendices Publications 267
268 Appendices Publications 268
269 Appendices Publications 269
270 Appendices Publications 270
271 Appendices Publications 271
272 Appendices Publications 272
273 Appendices Publications 273
274 Appendices Publications 274
275 Appendices Publications 275
276 Appendices Publications 276
277 Appendices Publications 277
278 Appendices Publications 278
279 Appendices Publications 279
280 Appendices Publications 280
281 Appendices Publications 281
282 Appendices Publications 282
283 Appendices Publications 283
Microstructure and Corrosion Behaviour of Aerospace Aluminium Alloys
 Microstructure and Corrosion Behaviour of Aerospace Aluminium Alloys A thesis submitted to The University of Manchester for the Degree of Doctor of Philosophy in the Faculty of Engineering and Physical
Microstructure and Corrosion Behaviour of Aerospace Aluminium Alloys A thesis submitted to The University of Manchester for the Degree of Doctor of Philosophy in the Faculty of Engineering and Physical
Silver Diffusion Bonding and Layer Transfer of Lithium Niobate to Silicon
 Chapter 5 Silver Diffusion Bonding and Layer Transfer of Lithium Niobate to Silicon 5.1 Introduction In this chapter, we discuss a method of metallic bonding between two deposited silver layers. A diffusion
Chapter 5 Silver Diffusion Bonding and Layer Transfer of Lithium Niobate to Silicon 5.1 Introduction In this chapter, we discuss a method of metallic bonding between two deposited silver layers. A diffusion
De-ionized water. Nickel target. Supplementary Figure S1. A schematic illustration of the experimental setup.
 Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
CHAPTER 2 MATERIALS AND METHODS
 37 CHAPTER 2 MATERIALS AND METHODS This chapter discusses the methodology adopted for the development of zinc phosphate coating utilizing galvanic coupling and to evaluate the effect of cathode materials
37 CHAPTER 2 MATERIALS AND METHODS This chapter discusses the methodology adopted for the development of zinc phosphate coating utilizing galvanic coupling and to evaluate the effect of cathode materials
Galvanic corrosion evaluation of 6061 aluminum coupled to CVD coated stainless steel Elizabeth Sikora and Barbara Shaw 6/9/2016
 SHAW AND ASSOCIATES CONSULTING Galvanic corrosion evaluation of 6061 aluminum coupled to CVD coated stainless steel Elizabeth Sikora and Barbara Shaw 6/9/2016 Evaluation of galvanic corrosion of aluminum
SHAW AND ASSOCIATES CONSULTING Galvanic corrosion evaluation of 6061 aluminum coupled to CVD coated stainless steel Elizabeth Sikora and Barbara Shaw 6/9/2016 Evaluation of galvanic corrosion of aluminum
FORMATION OF TiO 2 THIN FILM BY ION-BEAM-MIXING METHOD AND ITS APPLICATION AS THE CORROSION PROTECTING FILM
 ORAL REFERENCE:ICF100266OR FORMATION OF TiO 2 THIN FILM BY ION-BEAM-MIXING METHOD AND ITS APPLICATION AS THE CORROSION PROTECTING FILM Yuji KIMURA 1 and Hirotsugu SAITO 1 1 Dept. of Materials Science and
ORAL REFERENCE:ICF100266OR FORMATION OF TiO 2 THIN FILM BY ION-BEAM-MIXING METHOD AND ITS APPLICATION AS THE CORROSION PROTECTING FILM Yuji KIMURA 1 and Hirotsugu SAITO 1 1 Dept. of Materials Science and
Influences of fluorine species on the anodizing behaviour of aluminium and AA 2024-T3 alloy
 Influences of fluorine species on the anodizing behaviour of aluminium and AA 2024-T3 alloy A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy in the Faculty of Science
Influences of fluorine species on the anodizing behaviour of aluminium and AA 2024-T3 alloy A thesis submitted to The University of Manchester for the degree of Doctor of Philosophy in the Faculty of Science
Characterization of Oxide Film Formed on Ck45 Steel by Plasma Electrolytic Oxidation Method
 Journal of Mechanical Research and Application ISSN: 2251-7383, eissn: 2251-7391 Vol. 4, No. 2, 2012, 57-61 Characterization of Oxide Film Formed on Ck45 Steel by Plasma Electrolytic Oxidation Method JMRA
Journal of Mechanical Research and Application ISSN: 2251-7383, eissn: 2251-7391 Vol. 4, No. 2, 2012, 57-61 Characterization of Oxide Film Formed on Ck45 Steel by Plasma Electrolytic Oxidation Method JMRA
Surface Engineering Challenges of Dissimilar Materials Joints
 Surface Engineering Challenges of Dissimilar Materials Joints Michele Curioni & LATEST2 team Corrosion And Protection Centre School of Materials The University of Manchester M13 9PL, Manchester, UK Microstructure
Surface Engineering Challenges of Dissimilar Materials Joints Michele Curioni & LATEST2 team Corrosion And Protection Centre School of Materials The University of Manchester M13 9PL, Manchester, UK Microstructure
Study of the Initial Stage and an Anisotropic Growth of Oxide Layers Formed on Zircaloy-4
 16 th International Symposium on Zirconium in the Nuclear Industry, Chengdu, P. R. China, May 10-13, 2010 Study of the Initial Stage and an Anisotropic Growth of Oxide Layers Formed on Zircaloy-4 B. X.
16 th International Symposium on Zirconium in the Nuclear Industry, Chengdu, P. R. China, May 10-13, 2010 Study of the Initial Stage and an Anisotropic Growth of Oxide Layers Formed on Zircaloy-4 B. X.
The effect of manganese and chromium on the selective oxidation of low carbon strip steels for tinplate applications
 University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2006 The effect of manganese and chromium on the selective oxidation
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2006 The effect of manganese and chromium on the selective oxidation
Crack Initiation and Crack Propagation of Pre-corroded Ni-16Cr Alloy in 4.5%NaCl Aqueous Solution
 IOSR Journal of Engineering (IOSRJEN) e-issn: 2250-3021, p-issn: 2278-8719 Vol. 3, Issue 8 (August. 2013), V2 PP 11-15 Crack Initiation and Crack Propagation of Pre-corroded Ni-16Cr Alloy in 4.5%NaCl Aqueous
IOSR Journal of Engineering (IOSRJEN) e-issn: 2250-3021, p-issn: 2278-8719 Vol. 3, Issue 8 (August. 2013), V2 PP 11-15 Crack Initiation and Crack Propagation of Pre-corroded Ni-16Cr Alloy in 4.5%NaCl Aqueous
The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
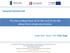 Honorata Kazimierczak The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes Supervisor: Assoc. Prof. Piotr Ozga The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
Honorata Kazimierczak The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes Supervisor: Assoc. Prof. Piotr Ozga The electrodeposition of Zn-Mo and Zn-Sn-Mo alloys from citrate electrolytes
Deformation and fracture of an alpha/beta titanium alloy
 ISSN 1517-7076 Revista Matéria, v. 15, n. 2, pp. 364-370, 2010. http://www.materia.coppe.ufrj.br/sarra/artigos/artigo11240 Deformation and fracture of an alpha/beta titanium alloy A. Andrade; A. Morcelli;
ISSN 1517-7076 Revista Matéria, v. 15, n. 2, pp. 364-370, 2010. http://www.materia.coppe.ufrj.br/sarra/artigos/artigo11240 Deformation and fracture of an alpha/beta titanium alloy A. Andrade; A. Morcelli;
CHEMICAL DEPTH PROFILING OF TOOL MATERIALS USING GLOW DISCHARGE OPTICAL EMISSION SPECTROSCOPY (GD-OES)
 CHEMICAL DEPTH PROFILING OF TOOL MATERIALS USING GLOW DISCHARGE OPTICAL EMISSION SPECTROSCOPY (GD-OES) T. Björk Swedish Institute for Metals Researc Drottning Kristinas väg 48 114 28 Stockholm Sweden Abstract
CHEMICAL DEPTH PROFILING OF TOOL MATERIALS USING GLOW DISCHARGE OPTICAL EMISSION SPECTROSCOPY (GD-OES) T. Björk Swedish Institute for Metals Researc Drottning Kristinas väg 48 114 28 Stockholm Sweden Abstract
on Electrochemical Etching of Pure Aluminium Foil for Aluminium Electrolytic Capacitor
 Volume 6 Paper C145 Effect of SO 4 2-, S 2 O 2-3 and HSO - 4 Ion Additives on Electrochemical Etching of Pure Aluminium Foil for Aluminium Electrolytic Capacitor S.-I. Pyun and K.-H. Na Dept. Mat. Sci.
Volume 6 Paper C145 Effect of SO 4 2-, S 2 O 2-3 and HSO - 4 Ion Additives on Electrochemical Etching of Pure Aluminium Foil for Aluminium Electrolytic Capacitor S.-I. Pyun and K.-H. Na Dept. Mat. Sci.
EFFECTS OF CURRENT DENSITY ON SIZE AND SURFACE MORPHOLOGY OF HIGH SPEED DIRECT NANO-CRYSTALLINE NICKEL PLATING ON TITANIUM SURFACE
 EFFECTS OF CURRENT DENSITY ON SIZE AND SURFACE MORPHOLOGY OF HIGH SPEED DIRECT NANO-CRYSTALLINE NICKEL PLATING ON TITANIUM SURFACE Noor Zaimah 1, Azieyanti Nurain 1 and Sakhawat Hussain 2 1 Department
EFFECTS OF CURRENT DENSITY ON SIZE AND SURFACE MORPHOLOGY OF HIGH SPEED DIRECT NANO-CRYSTALLINE NICKEL PLATING ON TITANIUM SURFACE Noor Zaimah 1, Azieyanti Nurain 1 and Sakhawat Hussain 2 1 Department
CORROSION RESISTANCE OF PLASMA NITRIDED AND NITROCARBURIZED AISI 316L AUSTENITIC STAINLESS STEEL
 CORROSION RESISTANCE OF PLASMA NITRIDED AND NITROCARBURIZED AISI 316L AUSTENITIC STAINLESS STEEL F.A.P. Fernandes 1*, J. Gallego 2, G.E. Totten 3, C.A. Picon 2, L.C. Casteletti 1 1 Department of Materials
CORROSION RESISTANCE OF PLASMA NITRIDED AND NITROCARBURIZED AISI 316L AUSTENITIC STAINLESS STEEL F.A.P. Fernandes 1*, J. Gallego 2, G.E. Totten 3, C.A. Picon 2, L.C. Casteletti 1 1 Department of Materials
Corrosion Protect DLC Coating on Steel and Hastelloy
 Materials Transactions, Vol. 49, No. 6 (2008) pp. 1333 to 1337 #2008 The Japan Institute of Metals Corrosion Protect DLC Coating on Steel and Hastelloy Hironobu Miya and Jie Wang Semiconductor Equipment
Materials Transactions, Vol. 49, No. 6 (2008) pp. 1333 to 1337 #2008 The Japan Institute of Metals Corrosion Protect DLC Coating on Steel and Hastelloy Hironobu Miya and Jie Wang Semiconductor Equipment
Transmission Electron Microscopy (TEM) Prof.Dr.Figen KAYA
 Transmission Electron Microscopy (TEM) Prof.Dr.Figen KAYA Transmission Electron Microscope A transmission electron microscope, similar to a transmission light microscope, has the following components along
Transmission Electron Microscopy (TEM) Prof.Dr.Figen KAYA Transmission Electron Microscope A transmission electron microscope, similar to a transmission light microscope, has the following components along
SUPPLEMENTARY INFORMATION
 High Electrochemical Activity of the Oxide Phase in Model Ceria- and Ceria-Ni Composite Anodes William C. Chueh 1,, Yong Hao, WooChul Jung, Sossina M. Haile Materials Science, California Institute of Technology,
High Electrochemical Activity of the Oxide Phase in Model Ceria- and Ceria-Ni Composite Anodes William C. Chueh 1,, Yong Hao, WooChul Jung, Sossina M. Haile Materials Science, California Institute of Technology,
P. Møller Department of Mechanical Engineering, Technical University of Denmark, Lyngby, Denmark
 FIB-SEM investigation of trapped intermetallic particles in anodic oxide films on AA050 aluminium M. Jariyaboon Department of Mechanical Engineering, Technical University of Denmark, Lyngby, Denmark Department
FIB-SEM investigation of trapped intermetallic particles in anodic oxide films on AA050 aluminium M. Jariyaboon Department of Mechanical Engineering, Technical University of Denmark, Lyngby, Denmark Department
Specimen configuration
 APPLICATIONNOTE Model 1040 NanoMill TEM specimen preparation system Specimen configuration Preparing focused ion beam (FIB) milled specimens for submission to Fischione Instruments. The Model 1040 NanoMill
APPLICATIONNOTE Model 1040 NanoMill TEM specimen preparation system Specimen configuration Preparing focused ion beam (FIB) milled specimens for submission to Fischione Instruments. The Model 1040 NanoMill
CHARACTERIZATION OF SILICON CARBIDE AND PYROCARBON COATINGS FOR FUEL PARTICLES FOR HIGH TEMPERATURE REACTORS (HTR)
 CHARACTERIZATION OF SILICON CARBIDE AND PYROCARBON COATINGS FOR FUEL PARTICLES FOR HIGH TEMPERATURE REACTORS (HTR) D. Hélary 1,2, X. Bourrat 1, O. Dugne 2, G. Maveyraud 1,2, F. Charollais 3, M. Pérez 4,
CHARACTERIZATION OF SILICON CARBIDE AND PYROCARBON COATINGS FOR FUEL PARTICLES FOR HIGH TEMPERATURE REACTORS (HTR) D. Hélary 1,2, X. Bourrat 1, O. Dugne 2, G. Maveyraud 1,2, F. Charollais 3, M. Pérez 4,
Development of New Generation Of Coatings with Strength-Ductility Relationship, Wear, Corrosion and Hydrogen Embrittlement Resistance Beyond the
 Development of New Generation Of Coatings with Strength-Ductility Relationship, Wear, Corrosion and Hydrogen Embrittlement Resistance Beyond the Current Materials Accomplishments till date As the structural
Development of New Generation Of Coatings with Strength-Ductility Relationship, Wear, Corrosion and Hydrogen Embrittlement Resistance Beyond the Current Materials Accomplishments till date As the structural
Microstructural characterisation of as-deposited and reheated weld metal High Strength Steel Weld Metals
 Microstructural characterisation of as-deposited and reheated weld metal High Strength Steel Weld Metals Enda Keehan, Leif Karlsson, Mattias Thuvander, Eva-Lena Bergquist Abstract ESAB AB, Gothenburg,
Microstructural characterisation of as-deposited and reheated weld metal High Strength Steel Weld Metals Enda Keehan, Leif Karlsson, Mattias Thuvander, Eva-Lena Bergquist Abstract ESAB AB, Gothenburg,
Microstructural Characterization of Materials
 Microstructural Characterization of Materials 2nd Edition DAVID BRANDON AND WAYNE D. KAPLAN Technion, Israel Institute of Technology, Israel John Wiley & Sons, Ltd Contents Preface to the Second Edition
Microstructural Characterization of Materials 2nd Edition DAVID BRANDON AND WAYNE D. KAPLAN Technion, Israel Institute of Technology, Israel John Wiley & Sons, Ltd Contents Preface to the Second Edition
Corrosion Behaviour of the Anodic Oxide Film on Commercially Pure Titanium in NaCl Environment
 Int. J. Electrochem. Sci., 9 (214) 3558-3573 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Corrosion Behaviour of the Anodic Oxide Film on Commercially Pure Titanium in NaCl Environment
Int. J. Electrochem. Sci., 9 (214) 3558-3573 International Journal of ELECTROCHEMICAL SCIENCE www.electrochemsci.org Corrosion Behaviour of the Anodic Oxide Film on Commercially Pure Titanium in NaCl Environment
Enhancement of connectivity and flux pinning in MgB2 superconducting bulks and wires
 University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2009 Enhancement of connectivity and flux pinning in MgB2 superconducting
University of Wollongong Research Online University of Wollongong Thesis Collection 1954-2016 University of Wollongong Thesis Collections 2009 Enhancement of connectivity and flux pinning in MgB2 superconducting
Growth Of TiO 2 Films By RF Magnetron Sputtering Studies On The Structural And Optical Properties
 Journal of Multidisciplinary Engineering Science and Technology (JMEST) Growth Of TiO 2 Films By RF Magnetron Sputtering Studies On The Structural And Optical Properties Ahmed K. Abbas 1, Mohammed K. Khalaf
Journal of Multidisciplinary Engineering Science and Technology (JMEST) Growth Of TiO 2 Films By RF Magnetron Sputtering Studies On The Structural And Optical Properties Ahmed K. Abbas 1, Mohammed K. Khalaf
EFFECTS OF HAMMER PEENING AND AGING TREATMENT ON MICROSTRUCTURE, MECHANICAL PROPERTIES AND CORROSION RESISTANCE OF OIL-GRADE ALLOY 718
 EFFECTS OF HAMMER PEENING AND AGING TREATMENT ON MICROSTRUCTURE, MECHANICAL PROPERTIES AND CORROSION RESISTANCE OF OIL-GRADE ALLOY 718 Ting Chen 1, Hendrik John 2, Jing Xu 2, Jeffrey Hawk 3, Xingbo Liu
EFFECTS OF HAMMER PEENING AND AGING TREATMENT ON MICROSTRUCTURE, MECHANICAL PROPERTIES AND CORROSION RESISTANCE OF OIL-GRADE ALLOY 718 Ting Chen 1, Hendrik John 2, Jing Xu 2, Jeffrey Hawk 3, Xingbo Liu
CORROSION PROPERTIES OF CERMET COATINGS SPRAYED BY HIGH-VELOCITY OXYGEN-FUEL. Dragos UŢU, Iosif HULKA, Viorel-Aurel ŞERBAN, Hannelore FILIPESCU
 Abstract CORROSION PROPERTIES OF CERMET COATINGS SPRAYED BY HIGH-VELOCITY OXYGEN-FUEL Dragos UŢU, Iosif HULKA, Viorel-Aurel ŞERBAN, Hannelore FILIPESCU Politehnica University of Timisoara, Romania, dragosutu@yahoo.com,
Abstract CORROSION PROPERTIES OF CERMET COATINGS SPRAYED BY HIGH-VELOCITY OXYGEN-FUEL Dragos UŢU, Iosif HULKA, Viorel-Aurel ŞERBAN, Hannelore FILIPESCU Politehnica University of Timisoara, Romania, dragosutu@yahoo.com,
Corrosion resistance of Au/Ni thin films coated stainless steel used for a PEFC separator
 Materials Characterisation VI 217 Corrosion resistance of Au/Ni thin films coated stainless steel used for a PEFC separator Y. Kimura 1 & M. Hirano 2 1 Department of Environmental and Energy Chemistry,
Materials Characterisation VI 217 Corrosion resistance of Au/Ni thin films coated stainless steel used for a PEFC separator Y. Kimura 1 & M. Hirano 2 1 Department of Environmental and Energy Chemistry,
Remote Plasma Source Chamber Anodization
 Remote Plasma Source Chamber Anodization SUPERIOR ANODIC COATINGS IN THE XSTREAM RPS CHAMBER ENSURE RELIABLE, PARTICULATE-REE CHAMBER CLEANING Created by Advanced Energy Industries, Inc. Abstract Most
Remote Plasma Source Chamber Anodization SUPERIOR ANODIC COATINGS IN THE XSTREAM RPS CHAMBER ENSURE RELIABLE, PARTICULATE-REE CHAMBER CLEANING Created by Advanced Energy Industries, Inc. Abstract Most
Supplementary Materials for
 www.sciencemag.org/cgi/content/full/336/6084/1007/dc1 Supplementary Materials for Unidirectional Growth of Microbumps on (111)-Oriented and Nanotwinned Copper Hsiang-Yao Hsiao, Chien-Min Liu, Han-wen Lin,
www.sciencemag.org/cgi/content/full/336/6084/1007/dc1 Supplementary Materials for Unidirectional Growth of Microbumps on (111)-Oriented and Nanotwinned Copper Hsiang-Yao Hsiao, Chien-Min Liu, Han-wen Lin,
AP 5301/8301 LABORATORY MANUAL
 AP 5301/8301 LABORATORY MANUAL Department of Physics & Materials Science City University of Hong Kong Contents Table of Contents. 1 Project 1: Scanning Electron Microscopy (SEM). 2 Project 2: Microscopic
AP 5301/8301 LABORATORY MANUAL Department of Physics & Materials Science City University of Hong Kong Contents Table of Contents. 1 Project 1: Scanning Electron Microscopy (SEM). 2 Project 2: Microscopic
Improvement of corrosion resistance of HVOF thermal sprayed coatings by gas shroud
 Improvement of corrosion resistance of HVOF thermal sprayed coatings by gas shroud Jin Kawakita, Takeshi Fukushima, Seiji Kuroda, and Toshiaki Kodama National Research Institute for Materials Science Abstract
Improvement of corrosion resistance of HVOF thermal sprayed coatings by gas shroud Jin Kawakita, Takeshi Fukushima, Seiji Kuroda, and Toshiaki Kodama National Research Institute for Materials Science Abstract
Effect of Heat Treatment on the Corrosion Behaviour of GTM- SU-718 Superalloy in NaCl, HCl and H 2 SO 4 environments
 International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.10 No.6, pp 617-621, 2017 Effect of Heat Treatment on the Behaviour of GTM- SU- Superalloy in
International Journal of ChemTech Research CODEN (USA): IJCRGG, ISSN: 0974-4290, ISSN(Online):2455-9555 Vol.10 No.6, pp 617-621, 2017 Effect of Heat Treatment on the Behaviour of GTM- SU- Superalloy in
Laboratory Experiments in Corrosion Engineering II
 Lecture - 40 Laboratory Experiments in Corrosion Engineering II Keywords: Polarization Experiments, Pitting Potentials, Microbial Corrosion. A. Electrochemical tests in a given environment Polarization
Lecture - 40 Laboratory Experiments in Corrosion Engineering II Keywords: Polarization Experiments, Pitting Potentials, Microbial Corrosion. A. Electrochemical tests in a given environment Polarization
Sr and Pb additions. L. Affleck, C. Leach *
 Microstructures of BaTiO 3 based PTC thermistors with Ca, Sr and Pb additions Abstract L. Affleck, C. Leach * Manchester Materials Science Centre University of Manchester and UMIST Grosvenor Street, Manchester
Microstructures of BaTiO 3 based PTC thermistors with Ca, Sr and Pb additions Abstract L. Affleck, C. Leach * Manchester Materials Science Centre University of Manchester and UMIST Grosvenor Street, Manchester
Looking into the Past for Materials of the Future; Professor R. Balasubramaniam
 Seite 1 von 5 Looking into the Past for Materials of the Future Professor R. Balasubramaniam Department of Materials & Metallurgical Engineering email: bala@iitk.ac http://home.iitk.ac.in/~bala/ IITK -
Seite 1 von 5 Looking into the Past for Materials of the Future Professor R. Balasubramaniam Department of Materials & Metallurgical Engineering email: bala@iitk.ac http://home.iitk.ac.in/~bala/ IITK -
CORROSION BEHAVIOR OF LASER SURFACE MELTED 2014 ALUMINIUM ALLOY IN T6 AND T451 TEMPERS. P.H. Chong*, Z. Liu, P. Skeldon and G. E.
 CORROSION BEHAVIOR OF LASER SURFACE MELTED 2014 ALUMINIUM ALLOY IN T6 AND T451 TEMPERS P.H. Chong*, Z. Liu, P. Skeldon and G. E. Thompson Corrosion and Protection Centre, University of Manchester Institute
CORROSION BEHAVIOR OF LASER SURFACE MELTED 2014 ALUMINIUM ALLOY IN T6 AND T451 TEMPERS P.H. Chong*, Z. Liu, P. Skeldon and G. E. Thompson Corrosion and Protection Centre, University of Manchester Institute
High Performance Lithium Battery Anodes Using Silicon Nanowires
 Supporting Online Materials For High Performance Lithium Battery Anodes Using Silicon Nanowires Candace K. Chan, Hailin Peng, Gao Liu, Kevin McIlwrath, Xiao Feng Zhang, Robert A. Huggins and Yi Cui * *To
Supporting Online Materials For High Performance Lithium Battery Anodes Using Silicon Nanowires Candace K. Chan, Hailin Peng, Gao Liu, Kevin McIlwrath, Xiao Feng Zhang, Robert A. Huggins and Yi Cui * *To
A Study of Performance of Aluminium Anode by Addition of Zinc in Sea Water
 A Study of Performance of Aluminium Anode by Addition of Zinc in Sea Water G.M.Pradeep 1, R.M.Ravindran 2, A.Gokulram 3, R.Rajesh 4, B.YogeshEswaran 5, L. Samson Joshua 6 1 Assistant professor, Dept of
A Study of Performance of Aluminium Anode by Addition of Zinc in Sea Water G.M.Pradeep 1, R.M.Ravindran 2, A.Gokulram 3, R.Rajesh 4, B.YogeshEswaran 5, L. Samson Joshua 6 1 Assistant professor, Dept of
Carbon Nanotube-Based Supercapacitors with Excellent AC-Line
 SUPPORTING INFORMATION FOR: Carbon Nanotube-Based Supercapacitors with Excellent AC-Line Filtering and Rate Capability via Improved Interfacial Impedance Yverick Rangom, Xiaowu (Shirley) Tang*, and Linda
SUPPORTING INFORMATION FOR: Carbon Nanotube-Based Supercapacitors with Excellent AC-Line Filtering and Rate Capability via Improved Interfacial Impedance Yverick Rangom, Xiaowu (Shirley) Tang*, and Linda
THE INFLUENCE OF ANODISING PARAMETERS ON THE CORROSION PERFORMANCE OF ANODISED COATINGS ON MAGNESIUM ALLOY AZ91D
 THE INFLUENCE OF ANODISING PARAMETERS ON THE CORROSION PERFORMANCE OF ANODISED COATINGS ON MAGNESIUM ALLOY AZ91D Zhiming Shi, Guangling Song, and Andrej Atrens (CRC for Cast Metals Manufacturing (CAST),
THE INFLUENCE OF ANODISING PARAMETERS ON THE CORROSION PERFORMANCE OF ANODISED COATINGS ON MAGNESIUM ALLOY AZ91D Zhiming Shi, Guangling Song, and Andrej Atrens (CRC for Cast Metals Manufacturing (CAST),
Supporting Information. Hematite photoanode with gradient structure shows an unprecedentedly low onset
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information Hematite photoanode with gradient structure shows an unprecedentedly
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information Hematite photoanode with gradient structure shows an unprecedentedly
Sn Wears Super Skin: A New Design For Long Cycling Batteries
 Supporting Information Wears Super Skin: A New Design For Long Cycling Batteries Shuai Kang, Xi Chen, Junjie Niu* Department of Materials Science and Engineering, CEAS, University of Wisconsin-Milwaukee
Supporting Information Wears Super Skin: A New Design For Long Cycling Batteries Shuai Kang, Xi Chen, Junjie Niu* Department of Materials Science and Engineering, CEAS, University of Wisconsin-Milwaukee
Surface Rolled-in Defects of Titanium Hot Roll Band in HSM
 China Steel Technical Report, Wei-Lin No. 28, Wang, pp.29-34, Ming-Tao (2015) Wu, Chao-Chi Huang, Yeong-Tsuen Pan and Szu-Ning Lin 29 Surface Rolled-in Defects of Titanium Hot Roll Band in HSM WEI-LIN
China Steel Technical Report, Wei-Lin No. 28, Wang, pp.29-34, Ming-Tao (2015) Wu, Chao-Chi Huang, Yeong-Tsuen Pan and Szu-Ning Lin 29 Surface Rolled-in Defects of Titanium Hot Roll Band in HSM WEI-LIN
Effects of Surface Finishes on Corrosion Resistance of Welded Stainless Steels
 AIJSTPME (2) 3(3): 65-7 Effects of Surface Finishes on Corrosion Resistance of Welded Stainless Steels Daopiset S. Department of Production Engineering, Faculty of Engineering, KMUTNB, Bangkok, Thailand
AIJSTPME (2) 3(3): 65-7 Effects of Surface Finishes on Corrosion Resistance of Welded Stainless Steels Daopiset S. Department of Production Engineering, Faculty of Engineering, KMUTNB, Bangkok, Thailand
Carnegie Mellon MRSEC
 Carnegie Mellon MRSEC Texture, Microstructure & Anisotropy, Fall 2009 A.D. Rollett, P. Kalu 1 ELECTRONS SEM-based TEM-based Koseel ECP EBSD SADP Kikuchi Different types of microtexture techniques for obtaining
Carnegie Mellon MRSEC Texture, Microstructure & Anisotropy, Fall 2009 A.D. Rollett, P. Kalu 1 ELECTRONS SEM-based TEM-based Koseel ECP EBSD SADP Kikuchi Different types of microtexture techniques for obtaining
Institute for Diagnostic Imaging Research
 Institute for Diagnostic Imaging Research (Repair applications of the LPCS process) Roman Gr. Maev, Emil Strumban, Volf Leshchinskiy, and Dmitry Dzhurinskiy CSAT 2014, Worcester, MA Low Pressure Cold Spray
Institute for Diagnostic Imaging Research (Repair applications of the LPCS process) Roman Gr. Maev, Emil Strumban, Volf Leshchinskiy, and Dmitry Dzhurinskiy CSAT 2014, Worcester, MA Low Pressure Cold Spray
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral
 Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Titanium Surface Modification by Oxidation for Biomedical Application
 The University of New South Wales Faculty of Science School of Materials Science & Engineering Titanium Surface Modification by Oxidation for Biomedical Application A Thesis by Hasan Z. Abdullah Submitted
The University of New South Wales Faculty of Science School of Materials Science & Engineering Titanium Surface Modification by Oxidation for Biomedical Application A Thesis by Hasan Z. Abdullah Submitted
CHAPTER 3. Experimental Results of Magnesium oxide (MgO) Thin Films
 CHAPTER 3 Experimental Results of Magnesium oxide (MgO) Thin Films Chapter: III ---------------------------------------------------------------- Experimental Results of Magnesium oxide (MgO) Thin Films
CHAPTER 3 Experimental Results of Magnesium oxide (MgO) Thin Films Chapter: III ---------------------------------------------------------------- Experimental Results of Magnesium oxide (MgO) Thin Films
Formation of Sol-gel Coatings on Aluminium Alloys
 Formation of Sol-gel Coatings on Aluminium Alloys A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Sciences 2011 Zuwei
Formation of Sol-gel Coatings on Aluminium Alloys A thesis submitted to the University of Manchester for the degree of Doctor of Philosophy in the Faculty of Engineering and Physical Sciences 2011 Zuwei
Microstructural characterisation of interfaces in magnetic pulse welded aluminum/aluminum joints
 IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Microstructural characterisation of interfaces in magnetic pulse welded aluminum/aluminum joints To cite this article: S Sharafiev
IOP Conference Series: Materials Science and Engineering PAPER OPEN ACCESS Microstructural characterisation of interfaces in magnetic pulse welded aluminum/aluminum joints To cite this article: S Sharafiev
Development and characterization of novel metallic joint in power electronic device
 Development and characterization of novel metallic joint in power electronic device Guillaume LACOMBE 1-a, Jean-Marc HEINTZ 1-b, Akira KAWASAKI 2-c, Jean-François SILVAIN 1-d 1 CNRS, Université de Bordeaux,
Development and characterization of novel metallic joint in power electronic device Guillaume LACOMBE 1-a, Jean-Marc HEINTZ 1-b, Akira KAWASAKI 2-c, Jean-François SILVAIN 1-d 1 CNRS, Université de Bordeaux,
Characterization of Nano-Scale Fine Precipitates in Al-Mg-Si Alloys for Automotive Applications
 UDC 669. 715 721 782 : 629. 11. 011. 5 Characterization of Nano-Scale Fine Precipitates in Al-Mg-Si Alloys for Automotive Applications Makoto SAGA* 1 Naoki MARUYAMA* 1 Abstract Bake-hadenable Al-Mg-Si
UDC 669. 715 721 782 : 629. 11. 011. 5 Characterization of Nano-Scale Fine Precipitates in Al-Mg-Si Alloys for Automotive Applications Makoto SAGA* 1 Naoki MARUYAMA* 1 Abstract Bake-hadenable Al-Mg-Si
Corrosion Rate Measurement on C-Steel
 Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Measurements of corrosion rate on Carbon-steel using Electrochemical (potentiodynamic Polarization, EIS etc.) technique. Corrosion Rate Measurement on C-Steel Abdullah Al Ashraf 1. Introduction: The degradation
Low Thermal Budget NiSi Films on SiGe Alloys
 Mat. Res. Soc. Symp. Proc. Vol. 745 2003 Materials Research Society N6.6.1 Low Thermal Budget NiSi Films on SiGe Alloys S. K. Ray 1,T.N.Adam,G.S.Kar 1,C.P.SwannandJ.Kolodzey Department of Electrical and
Mat. Res. Soc. Symp. Proc. Vol. 745 2003 Materials Research Society N6.6.1 Low Thermal Budget NiSi Films on SiGe Alloys S. K. Ray 1,T.N.Adam,G.S.Kar 1,C.P.SwannandJ.Kolodzey Department of Electrical and
Specimen Preparation Technique for a Microstructure Analysis Using the Focused Ion Beam Process
 Specimen Preparation Technique for a Microstructure Analysis Using the Focused Ion Beam Process by Kozue Yabusaki * and Hirokazu Sasaki * In recent years the FIB technique has been widely used for specimen
Specimen Preparation Technique for a Microstructure Analysis Using the Focused Ion Beam Process by Kozue Yabusaki * and Hirokazu Sasaki * In recent years the FIB technique has been widely used for specimen
Direct nano-crystalline Ni plating on titanium surfaces
 Direct nano-crystalline Ni plating on titanium surfaces Dr Mohammad Sakhawat Hussain PhD (Aston) FIMF FIMMM CEng Department of Materials Engineering Faculty of Mechanical Engineering Universiti Teknologi
Direct nano-crystalline Ni plating on titanium surfaces Dr Mohammad Sakhawat Hussain PhD (Aston) FIMF FIMMM CEng Department of Materials Engineering Faculty of Mechanical Engineering Universiti Teknologi
Metals and alloys for biomedical applications
 http://pioneer.netserv.chula.ac.th/~pchedtha/ Metals and alloys for biomedical applications Fundamental of Materials Engineering 2189202 Chedtha Puncreobutr Department of Metallurgical Engineering Chulalongkorn
http://pioneer.netserv.chula.ac.th/~pchedtha/ Metals and alloys for biomedical applications Fundamental of Materials Engineering 2189202 Chedtha Puncreobutr Department of Metallurgical Engineering Chulalongkorn
Effect of Precorrosion and Temperature on the Formation Rate of Iron Carbonate Film
 7th Pipeline Technology Conference 2012 Effect of Precorrosion and Temperature on the Formation Rate of Iron Carbonate Film Winia Farida a,b, Tor Hemmingsen b, Tonje Berntsen b,c, Patrick Rabindran a a
7th Pipeline Technology Conference 2012 Effect of Precorrosion and Temperature on the Formation Rate of Iron Carbonate Film Winia Farida a,b, Tor Hemmingsen b, Tonje Berntsen b,c, Patrick Rabindran a a
CORROSION BEHAVIOUR OF TOMBAC USED IN CULT OBJECTS MANUFACTURING
 CORROSION BEHAVIOUR OF TOMBAC USED IN CULT OBJECTS MANUFACTURING P. HAGIOGLU 1, C. GHEORGHIES 3, A.M. CANTARAGIU 1, R. BOICIUC, N. TIGAU 3 1 Faculty of Mechanics, Dunarea de Jos University of Galati Arcelor
CORROSION BEHAVIOUR OF TOMBAC USED IN CULT OBJECTS MANUFACTURING P. HAGIOGLU 1, C. GHEORGHIES 3, A.M. CANTARAGIU 1, R. BOICIUC, N. TIGAU 3 1 Faculty of Mechanics, Dunarea de Jos University of Galati Arcelor
EFFECT OF POST WELD HEAT TREATMENTS ON THE ELEVATED TEMPERATURE MECHANICAL PROPERTIES OF Ti6Al4V FRICTION WELDS
 EFFECT OF POST WELD HEAT TREATMENTS ON THE ELEVATED TEMPERATURE MECHANICAL PROPERTIES OF Ti6Al4V FRICTION WELDS RAHUL 1#, K. V. RAJULAPATI 1, G. M. REDDY 2, T. MOHANDAS 3, K. B. S. RAO 4 1 School of Engineering
EFFECT OF POST WELD HEAT TREATMENTS ON THE ELEVATED TEMPERATURE MECHANICAL PROPERTIES OF Ti6Al4V FRICTION WELDS RAHUL 1#, K. V. RAJULAPATI 1, G. M. REDDY 2, T. MOHANDAS 3, K. B. S. RAO 4 1 School of Engineering
Combinatorial RF Magnetron Sputtering for Rapid Materials Discovery: Methodology and Applications
 Combinatorial RF Magnetron Sputtering for Rapid Materials Discovery: Methodology and Applications Philip D. Rack,, Jason D. Fowlkes, and Yuepeng Deng Department of Materials Science and Engineering University
Combinatorial RF Magnetron Sputtering for Rapid Materials Discovery: Methodology and Applications Philip D. Rack,, Jason D. Fowlkes, and Yuepeng Deng Department of Materials Science and Engineering University
TRIBOLOGICAL PROPERTIES OF SOLID LUBRICANT NANOCOMPOSITE COATINGS OBTAINED BY MAGNETRON SPUTTERED OF MOS 2 /METAL (TI, MO) NANOPARTICLES
 THE PUBLISHING HOUSE PROCEEDINGS OF THE ROMANIAN ACADEMY, Series A, OF THE ROMANIAN ACADEMY Volume 8, Number 3/2007, pp. 000-000 TRIBOLOGICAL PROPERTIES OF SOLID LUBRICANT NANOCOMPOSITE COATINGS OBTAINED
THE PUBLISHING HOUSE PROCEEDINGS OF THE ROMANIAN ACADEMY, Series A, OF THE ROMANIAN ACADEMY Volume 8, Number 3/2007, pp. 000-000 TRIBOLOGICAL PROPERTIES OF SOLID LUBRICANT NANOCOMPOSITE COATINGS OBTAINED
CHAPTER-4 EXPERIMENTAL DETAILS. 4.1 SELECTION OF MATERIAL FOR CC GTAW & PC GTAW OF 90/10 & 70/30 Cu-Ni ALLOY WELDS
 CHAPTER-4 EXPERIMENTAL DETAILS 4.1 SELECTION OF MATERIAL FOR CC GTAW & PC GTAW OF 90/10 & 70/30 Cu-Ni ALLOY WELDS Hot rolled plates of 90/10 and 70/30 Cu-Ni alloys of 5 mm thickness were selected as test
CHAPTER-4 EXPERIMENTAL DETAILS 4.1 SELECTION OF MATERIAL FOR CC GTAW & PC GTAW OF 90/10 & 70/30 Cu-Ni ALLOY WELDS Hot rolled plates of 90/10 and 70/30 Cu-Ni alloys of 5 mm thickness were selected as test
ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency
 ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency Elmira Memarzadeh Lotfabad a, Peter Kalisvaart a,*, Kai Cui b, Alireza Kohandehghan
ALD TiO 2 coated Silicon Nanowires for Lithium Ion Battery Anodes with enhanced Cycling Stability and Coulombic Efficiency Elmira Memarzadeh Lotfabad a, Peter Kalisvaart a,*, Kai Cui b, Alireza Kohandehghan
Effect of titanium additions to low carbon, low manganese steels on sulphide precipitation
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2008 Effect of titanium additions to low carbon, low manganese steels on sulphide precipitation
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2008 Effect of titanium additions to low carbon, low manganese steels on sulphide precipitation
Chapter 16 Corrosion and Degradation of Materials
 Chapter 16 Corrosion and Degradation of Materials Concept Check 16.1 Question: Would you expect iron to corrode in water of high purity? Why or why not? Answer: Iron would not corrode in water of high
Chapter 16 Corrosion and Degradation of Materials Concept Check 16.1 Question: Would you expect iron to corrode in water of high purity? Why or why not? Answer: Iron would not corrode in water of high
COMPATIBILITY OF THE ALTERNATIVE SEED LAYER (ASL) PROCESS WITH MONO- Si AND POLY-Si SUBSTRATES PATTERNED BY LASER OR WET ETCHING
 COMPATIBILITY OF THE ALTERNATIVE SEED LAYER (ASL) PROCESS WITH MONO- Si AND POLY-Si SUBSTRATES PATTERNED BY LASER OR WET ETCHING Lynne Michaelson 1, Anh Viet Nguyen 2, Krystal Munoz 1, Jonathan C. Wang
COMPATIBILITY OF THE ALTERNATIVE SEED LAYER (ASL) PROCESS WITH MONO- Si AND POLY-Si SUBSTRATES PATTERNED BY LASER OR WET ETCHING Lynne Michaelson 1, Anh Viet Nguyen 2, Krystal Munoz 1, Jonathan C. Wang
Chapter 7. Evaluation of Electrode Performance by. Electrochemical Impedance
 Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Ruthenium Oxide Films Prepared by Reactive Biased Target Sputtering
 Ruthenium Oxide Films Prepared by Reactive Biased Target Sputtering Hengda Zhang Anthony Githinji 1. Background RuO2 in both crystalline and amorphous forms is of crucial importance for theoretical as
Ruthenium Oxide Films Prepared by Reactive Biased Target Sputtering Hengda Zhang Anthony Githinji 1. Background RuO2 in both crystalline and amorphous forms is of crucial importance for theoretical as
ADVANCES in NATURAL and APPLIED SCIENCES
 ADVANCES in NATURAL and APPLIED SCIENCES ISSN: 1995-0772 Published BYAENSI Publication EISSN: 1998-1090 http://www.aensiweb.com/anas 2017 April 11(4): pages 551-556 Open Access Journal Study And Analysis
ADVANCES in NATURAL and APPLIED SCIENCES ISSN: 1995-0772 Published BYAENSI Publication EISSN: 1998-1090 http://www.aensiweb.com/anas 2017 April 11(4): pages 551-556 Open Access Journal Study And Analysis
The Study of SEM Examination of Crept Ceramic Samples Prepared by Cross Polishing Method
 The Study of SEM Examination of Crept Ceramic Samples Prepared by Cross Polishing Method Alper Uludag Faculty of Aeronautics and Astronautics Anadolu University Eskisehir, Turkey alperuludag@anadolu.edu.tr
The Study of SEM Examination of Crept Ceramic Samples Prepared by Cross Polishing Method Alper Uludag Faculty of Aeronautics and Astronautics Anadolu University Eskisehir, Turkey alperuludag@anadolu.edu.tr
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
High Rate and Durable, Binder Free Anode Based on Silicon Loaded MoO 3 Nanoplatelets
 Supplementary Information High Rate and Durable, Binder Free Anode Based on Silicon Loaded O 3 Nanoplatelets Alejandro Martinez-Garcia, Arjun Kumar Thapa,Ruvini Dharmadasa,, Tu Q. Nguyen, Jacek Jasinski,
Supplementary Information High Rate and Durable, Binder Free Anode Based on Silicon Loaded O 3 Nanoplatelets Alejandro Martinez-Garcia, Arjun Kumar Thapa,Ruvini Dharmadasa,, Tu Q. Nguyen, Jacek Jasinski,
A Parametric Study on the Electrodeposition of Copper Nanocrystals on a Gold Film Electrode. Andrea Harmer Co-op term #1 April 25, 2003
 A Parametric Study on the Electrodeposition of Copper Nanocrystals on a Gold Film Electrode Andrea Harmer Co-op term #1 April 25, 2003 Outline of Presentation: Introduction Purpose General method Parameters:
A Parametric Study on the Electrodeposition of Copper Nanocrystals on a Gold Film Electrode Andrea Harmer Co-op term #1 April 25, 2003 Outline of Presentation: Introduction Purpose General method Parameters:
Surface Layer Characterization of Atomized Magnesium for use in Powder Metallurgy Products Paul Burke and Georges J. Kipouros
 Surface Layer Characterization of Atomized Magnesium for use in Powder Metallurgy Products Paul Burke and Georges J. Kipouros Materials Engineering Program Process Engineering and Applied Science Dalhousie
Surface Layer Characterization of Atomized Magnesium for use in Powder Metallurgy Products Paul Burke and Georges J. Kipouros Materials Engineering Program Process Engineering and Applied Science Dalhousie
Investigation of Formation Process of the Chrome-free Passivation Film of Electrodeposited Zinc
 Chinese Journal of Aeronautics 20(2007) 129-133 Chinese Journal of Aeronautics www.elsevier.com/locate/cja Investigation of Formation Process of the Chrome-free Passivation Film of Electrodeposited Zinc
Chinese Journal of Aeronautics 20(2007) 129-133 Chinese Journal of Aeronautics www.elsevier.com/locate/cja Investigation of Formation Process of the Chrome-free Passivation Film of Electrodeposited Zinc
Supplementary Information
 Supplementary Information Atmospheric microplasma-functionalized 3D microfluidic strips within dense carbon nanotube arrays confine Au nanodots for SERS sensing Samuel Yick, Zhao Jun Han and Kostya (Ken)
Supplementary Information Atmospheric microplasma-functionalized 3D microfluidic strips within dense carbon nanotube arrays confine Au nanodots for SERS sensing Samuel Yick, Zhao Jun Han and Kostya (Ken)
LASER SURFACE MELTING OF 17-4 PH PRECIPITATION-HARDENABLE STAINLESS STEEL Paper 1203
 LASER SURFACE MELTING OF 7- PH PRECIPITATION-HARDENABLE STAINLESS STEEL Paper 0 Zhichao Cheng, Chi Tat Kwok, Kin Ho Lo, Department of Electromechanical Engineering, University of Macau, Taipa, Macau Abstract
LASER SURFACE MELTING OF 7- PH PRECIPITATION-HARDENABLE STAINLESS STEEL Paper 0 Zhichao Cheng, Chi Tat Kwok, Kin Ho Lo, Department of Electromechanical Engineering, University of Macau, Taipa, Macau Abstract
On the Long-Term Stability of 6013-T6 Aluminium Alloy Sheet
 Proceedings of the 9 th International Conference on Aluminium Alloys (2004) 986 Edited by J.F. Nie, A.J. Morton and B.C. Muddle Institute of Materials Engineering Australasia Ltd On the Long-Term Stability
Proceedings of the 9 th International Conference on Aluminium Alloys (2004) 986 Edited by J.F. Nie, A.J. Morton and B.C. Muddle Institute of Materials Engineering Australasia Ltd On the Long-Term Stability
Available online at ScienceDirect. Procedia Engineering 184 (2017 )
 Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 184 (2017 ) 418 422 Advances in Material & Processing Technologies Conference Development of Al-Zn-Cu Alloy for Low Voltage
Available online at www.sciencedirect.com ScienceDirect Procedia Engineering 184 (2017 ) 418 422 Advances in Material & Processing Technologies Conference Development of Al-Zn-Cu Alloy for Low Voltage
Global Journal of Engineering Science and Research Management
 DIFFUSION BONDING OF AL ALLOY USING DIFFERENT IINTERLAYERS Assist. Prof. Dr. Ahmed A. Akbar*, Samer K. Khaleel * Asst. Prof. Dr. at University of Technology, Production Engineering and Metallurgy, Iraq
DIFFUSION BONDING OF AL ALLOY USING DIFFERENT IINTERLAYERS Assist. Prof. Dr. Ahmed A. Akbar*, Samer K. Khaleel * Asst. Prof. Dr. at University of Technology, Production Engineering and Metallurgy, Iraq
A Case Study on Using Corrosion Analysis in Forensic Engineering. Reza Mirshams. Journal of Failure Analysis and Prevention
 A Case Study on Using Corrosion Analysis in Forensic Engineering Reza Mirshams Journal of Failure Analysis and Prevention ISSN 1547-7029 Volume 17 Number 4 J Fail. Anal. and Preven. (2017) 17:642-646 DOI
A Case Study on Using Corrosion Analysis in Forensic Engineering Reza Mirshams Journal of Failure Analysis and Prevention ISSN 1547-7029 Volume 17 Number 4 J Fail. Anal. and Preven. (2017) 17:642-646 DOI
Novel Fuel Cell MEA Based on Pt-C Deposited by Magnetron Sputtering
 10.1149/08008.0225ecst The Electrochemical Society Novel Fuel Cell MEA Based on Pt-C Deposited by Magnetron Sputtering A. Ostroverkh a, V. Johanek a, M. Dubau a, P. Kus a, K. Veltruska a, M. Vaclavu a,
10.1149/08008.0225ecst The Electrochemical Society Novel Fuel Cell MEA Based on Pt-C Deposited by Magnetron Sputtering A. Ostroverkh a, V. Johanek a, M. Dubau a, P. Kus a, K. Veltruska a, M. Vaclavu a,
Polycrystalline and microcrystalline silicon
 6 Polycrystalline and microcrystalline silicon In this chapter, the material properties of hot-wire deposited microcrystalline silicon are presented. Compared to polycrystalline silicon, microcrystalline
6 Polycrystalline and microcrystalline silicon In this chapter, the material properties of hot-wire deposited microcrystalline silicon are presented. Compared to polycrystalline silicon, microcrystalline
Characterization of Coatings on Grey Cast Iron Fabricated by Hot-dipping in Pure Al, AlSi11 and AlTi5 Alloys
 A R C H I V E S o f F O U N D R Y E N G I N E E R I N G Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences ISSN (1897-3310) Volume 14 Issue 1/2014 85 90 20/1 Characterization
A R C H I V E S o f F O U N D R Y E N G I N E E R I N G Published quarterly as the organ of the Foundry Commission of the Polish Academy of Sciences ISSN (1897-3310) Volume 14 Issue 1/2014 85 90 20/1 Characterization
In-situ Heating Characterisation Using EBSD
 Webinar In-situ Heating Characterisation Using EBSD Speakers Dr. Ali Gholinia Dr. Neil Othen Dr. Jenny Goulden Topics Introduction to EBSD Why do in-situ experiments? EBSD equipment requirements for in-situ
Webinar In-situ Heating Characterisation Using EBSD Speakers Dr. Ali Gholinia Dr. Neil Othen Dr. Jenny Goulden Topics Introduction to EBSD Why do in-situ experiments? EBSD equipment requirements for in-situ
SIMS Analysis of Hydride in Commercially Pure Titanium
 Memoirs of the Faculty of Engineering, Kyushu University, Vol.67, No.4, December 2007 SIMS Analysis of Hydride in Commercially Pure Titanium by Hideaki NISHIKAWA *, Shigeru HAMADA ** and Katsu OHNISHI
Memoirs of the Faculty of Engineering, Kyushu University, Vol.67, No.4, December 2007 SIMS Analysis of Hydride in Commercially Pure Titanium by Hideaki NISHIKAWA *, Shigeru HAMADA ** and Katsu OHNISHI
Research Article The Corrosion Behavior of Carburized Aluminum Using DC Plasma
 Metallurgy Volume 212, Article ID 25821, 4 pages doi:1.1155/212/25821 Research Article The Corrosion Behavior of Carburized Aluminum Using DC Plasma Somayeh Pirizadhejrandoost, Mehdi Bakhshzad Mahmoudi,
Metallurgy Volume 212, Article ID 25821, 4 pages doi:1.1155/212/25821 Research Article The Corrosion Behavior of Carburized Aluminum Using DC Plasma Somayeh Pirizadhejrandoost, Mehdi Bakhshzad Mahmoudi,
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION SUPPLEMENTARY DISCUSSION AND FIGURES 1. Chemical and Structural Characterization (a) Grazing-incidence small-angle X-ray scattering (GISAXS) The structural evolution of the mesoporous
SUPPLEMENTARY INFORMATION SUPPLEMENTARY DISCUSSION AND FIGURES 1. Chemical and Structural Characterization (a) Grazing-incidence small-angle X-ray scattering (GISAXS) The structural evolution of the mesoporous
Materials of Engineering ENGR 151 CORROSION ELECTRICAL PROPERTIES
 Materials of Engineering ENGR 151 CORROSION ELECTRICAL PROPERTIES more anodic (active) more cathodic (inert) GALVANIC SERIES Ranking of the reactivity of metals/alloys in seawater Platinum Gold Graphite
Materials of Engineering ENGR 151 CORROSION ELECTRICAL PROPERTIES more anodic (active) more cathodic (inert) GALVANIC SERIES Ranking of the reactivity of metals/alloys in seawater Platinum Gold Graphite
CORROSION PROTECTION OF MMCs BY DIAMOND-LIKE CARBON COATINGS
 CORROSION PROTECTION OF MMCs BY DIAMOND-LIKE CARBON COATINGS B. Wielage, A. Dorner Institute for Composites and Surface Technology, TU Chemnitz, D-09107 Chemnitz, Germany SUMMARY: Carbon fibre reinforced
CORROSION PROTECTION OF MMCs BY DIAMOND-LIKE CARBON COATINGS B. Wielage, A. Dorner Institute for Composites and Surface Technology, TU Chemnitz, D-09107 Chemnitz, Germany SUMMARY: Carbon fibre reinforced
THE RELATIONSHIP BETWEEN SURFACE TREATMENTS AND CORROSION RESISTANCE OF HOT-DIP GALVANIZED STEEL. Amirreza Bakhtiari
 Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC: 620.197.2:669.141; 620.193 THE RELATIONSHIP BETWEEN SURFACE TREATMENTS AND CORROSION RESISTANCE OF HOT-DIP GALVANIZED STEEL Amirreza
Association of Metallurgical Engineers of Serbia AMES Scientific paper UDC: 620.197.2:669.141; 620.193 THE RELATIONSHIP BETWEEN SURFACE TREATMENTS AND CORROSION RESISTANCE OF HOT-DIP GALVANIZED STEEL Amirreza
