Lab 2: Determination of the empirical formula of the product of magnesium heating
|
|
|
- Melanie Rice
- 6 years ago
- Views:
Transcription
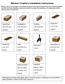
1 Chemistry 140 Please have parts 1, 2, 3 and 4 (tables only) ready before class on Wednesday, October 11. Write an abstract and paper-clip it to the front of your individual writeup. The abstract and the copied pages of the notebook are due in class on Wednesday, October 18. This is the standard lab manual format for a lab. It will be your job to translate it into the familiar of the report expected in Chem 140 (some guidelines are given at the end of the lab protocol below). Lab 2: Determination of the empirical formula of the product of magnesium heating In this experiment, you will determine with which component of air (nitrogen or oxygen) magnesium will react when heated, by identifying the percent composition of the product of that reaction. Air is composed of 78% nitrogen (in the form of N 2 ) and 21% oxygen (in the form of O 2 ), with a trace of other gases. When a strip (or ribbon) of magnesium is heated in a porcelain crucible, it emits an intense white light for a few minutes and a gray-white powdery residue remains. The experiment will help you determine the identity of that powdery residue. One possibility is that magnesium metal simply oxidizes by heating it in air: 2 Mg (s) + O 2 (g) 2 MgO (s) The other possibility is that magnesium reacts with the nitrogen in the air to form magnesium nitride: 3 Mg (s) + N 2 (g) Mg 3 N 2 (s) By determining the % Mg in your product, you can identify the product as MgO or Mg 3 N 2. From the mass of magnesium before heating and the mass of the product, you can calculate the experimentally determined percent Mg in the product: experimental %Mg = mass Mg mass product " 100 Materials: Magnesium (Mg) Crucible (with cover) Bunsen burner
2 Matches Tongs Metal ring Clay triangle Wire gauze Digital balance Procedure: Work in groups of Figure out where the equipment for the lab is kept (some of it will be in the cabinet, some of it will be on the cart). 1. Inspect the crucible for cracks. If your crucible is cracked, exchange it for a new one. Clean the crucible and cover thoroughly. The crucible may be stained, so you will not be able to get it completely white. After cleaning be sure to handle it ONLY with metal tongs. 2. Place the clay triangle on an iron ring clamped to a ringstand. Using tongs, transfer the crucible and its cover to the clay triangle. See if you can identify the materials list above in the picture.
3 Use the striker to light the Bunsen burner. Begin heating them with the Bunsen burner. Adjust the burner to obtain a blue flame having an inner lighter blue cone. The tip of the cone is the hottest part of the flame; use this part of the flame to heat the crucible red hot.
4 3. After heating the crucible, cool it in place on the ringstand for about five minutes to room temperature. 4. Using the digital balance, weigh the crucible and cover to the nearest milligram (.001g) and record the mass in your notebook. Place the magnesium sample into the crucible and then weigh the crucible, cover and sample. Record this mass and the physical appearance of the magnesium metal in your notebook. Notice that you can't merely tare the crucible and cover. 5. Cover the crucible with its lid and place it inside the clay triangle supported by a ring and ringstand (see demo model). Heat GENTLY. After 10 minutes check to make sure that most of the metal has reacted by using tongs to lift the lid SLIGHTLY. If the metal is burning, do not look directly at it, but replace the lid and continue heating gently until flames are no longer visible. 6. Remove the lid and heat the crucible with the hottest part of the flame for 5 minutes. Record the physical appearance of the residue. 7. When the crucible and its contents have cooled (after five minutes), weigh them to the nearest milligram and record this mass in your notebook. Use the same digital balance you used for the previous massing. 8. Dispose of the magnesium product in the container designated for waste disposal. Clean the crucible and cover. 9. Repeat the experiment for a total of three trials.
5 Sample Data Sheet for magnesium experiment: Trial # Mass of empty crucible and cover Mass of crucible, cover and Mg Mass of Mg Mass of crucible, cover, and product Mass of product Results: theoretical % Mg in MgO theoretical % Mg in Mg 3 N 2 experimental % Mg in product To calculate the theoretical % Mg in a compound, calculate the mass of Mg in one formula weight of the compound, then divide by the formula weight (or molar mass) of the compound. % Mg = mass of Mg in 1 formula weight of compound formula weight of compound " 100 And now for the hints on how to set up your lab book: Your name, your partner s name, date of experiment Lab 2: Determination of the empirical formula of the product of magnesium heating Part 1. Purpose Please write a sentence or two stating the goal of this experiment, based on the information above. Part 2. Materials and methods Chemicals needed: magnesium metal strip Examine the setup shown above, draw the equipment shown and label the various pieces of equipment. Part 3. Procedure There should be no major modifications to the procedure above.
6 Dispose of any magnesium-containing materials in the container designated for magnesium waste disposal in the hood. Part 4. Original data Perhaps the sample data sheet shown above could be converted into a table for the three trials you will doing. Part 5. Calculated results Note that you should include your calculations for both of the theoretical magnesium percent compositions as well as your experimental magnesium percent composition (one trial s worth will do; I trust your other two trials; calculations will be done in an identical manner!). Organize your calculated results into a table. Part 6. Group results Write all three of your experimental magnesium percent compositions on the board and record all groups' experimental magnesium percent compositions. Calculate the mean and standard deviation, and comment about where your results fit in (for instance, are your results outliers?). Part 7. Questions 1. Why can't you simply tare the crucible and cover when recording masses in step 4? 2. From your results, is your product MgO or Mg 3 N 2? Looking over the results of your three trials, did you have a systematic error? In any case, identify the sources of error in your experiment and how you would expect the error to affect your results. 3. Compare the appearance of magnesium metal with the product after heating. In what way could you tell that a chemical reaction occurred? 4. After looking at the group results from part 6, are you more or less confident of your answer to question 2? Mention how the standard deviation can be used in this context. Did the class as a whole suffer from a systematic error? Were there any outlier values which, if thrown out, improve the standard deviation dramatically? Part 8. Conclusion The conclusion should be a brief recap of your results and sources of error (confidence you have in your results). First sentence: We determined that the magnesium percent composition of our material was,, and in three trials, which resulted in a mean value of ± %.
7 Second sentence: From these results, we concluded the empirical formula of the product of heating magnesium in air is because. Next couple of sentences: Mention any significant random and/or systematic errors and how they could be fixed or not fixed next time. Next sentence: The class mean was ± %, which is (consistent/inconsistent) with our result. Last sentences: Did you accomplish the goal of the experiment? How confident are you of your results? Abstract Again, a short (less than 100 word) summary of the major result(s) of your experiment, and the method by which you achieved this result. Name, school affiliation, title, text as usual. Keep in mind: What was the major result (and uncertainty)? By what method did you achieve the result?
EMPIRICAL FORMULA OF MAGNESIUM OXIDE
 EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
EMPIRICAL FORMULA OF MAGNESIUM OXIDE
 EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
EXPERIMENT 7 Chemistry 110 EMPIRICAL FORMULA OF MAGNESIUM OXIDE PURPOSE: The purpose of this experiment is to determine the empirical formula of a compound. I. INTRODUCTION The object of this experiment
The empirical formula of a compound
 The empirical formula of a compound Reference: Chapter 1, Section 1.2, pages 21 24 Please note Aim A full risk assessment should be carried out prior to commencing this experiment. Personal safety equipment
The empirical formula of a compound Reference: Chapter 1, Section 1.2, pages 21 24 Please note Aim A full risk assessment should be carried out prior to commencing this experiment. Personal safety equipment
EMP I RICAL FORMULA OF MAGNESI U M OXIDE
 Experiment 6 Name: 53 EMP I RICAL FORMULA OF MAGNESI U M OXIDE In this experiment, you will synthesize oxide via the reaction pathways summarized in Figure 1. Note that [1] is the main reaction and [2]
Experiment 6 Name: 53 EMP I RICAL FORMULA OF MAGNESI U M OXIDE In this experiment, you will synthesize oxide via the reaction pathways summarized in Figure 1. Note that [1] is the main reaction and [2]
Determination of the Empirical Formula of Magnesium Oxide
 Determination of the Empirical Formula of Magnesium Oxide The quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. Magnesium
Determination of the Empirical Formula of Magnesium Oxide The quantitative stoichiometric relationships governing mass and amount will be studied using the combustion reaction of magnesium metal. Magnesium
EMPIRICAL FORMULA DETERMINATION
 Name Date Class Chapter 10 Chemical Quantities EXPERIMENT EMPIRICAL FORMULA DETERMINATION Text Reference Section 10.3 PURPOSE To determine the empirical formula of magnesium oxide. BACKGROUND Carbon dioxide
Name Date Class Chapter 10 Chemical Quantities EXPERIMENT EMPIRICAL FORMULA DETERMINATION Text Reference Section 10.3 PURPOSE To determine the empirical formula of magnesium oxide. BACKGROUND Carbon dioxide
Pre-Lab 5: Magnesium and Magnesium Oxide
 Name: Pre-Lab 5: Magnesium and Magnesium Oxide Section: Answer the following questions after reading the background information at the beginning of the lab. This should be completed before coming to lab.
Name: Pre-Lab 5: Magnesium and Magnesium Oxide Section: Answer the following questions after reading the background information at the beginning of the lab. This should be completed before coming to lab.
Experiment Twelve Empirical Formula of Magnesium Oxide
 Experiment Twelve Empirical Formula of Magnesium Oxide Objective The purpose of this experiment is to determine the stoichiometric ratio of magnesium and oxygen following the combustion of magnesium metal.
Experiment Twelve Empirical Formula of Magnesium Oxide Objective The purpose of this experiment is to determine the stoichiometric ratio of magnesium and oxygen following the combustion of magnesium metal.
Determination of the Empirical Formula of Magnesium Oxide
 Determination of the Empirical Formula of Magnesium Oxide 1 2 3 + magnesium (s) oxygen (g) magnesium oxide (s) [liquefied in picture] Background: During the latter half of the 18 th century, the French
Determination of the Empirical Formula of Magnesium Oxide 1 2 3 + magnesium (s) oxygen (g) magnesium oxide (s) [liquefied in picture] Background: During the latter half of the 18 th century, the French
Elemental Mass Percent and Empirical Formula From Decomposition of a Copper Oxide
 EXPERIMENT Elemental Mass Percent and Empirical Formula From Decomposition 6 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College The student will heat
EXPERIMENT Elemental Mass Percent and Empirical Formula From Decomposition 6 Prepared by Edward L. Brown, Lee University and Verrill M. Norwood, Cleveland State Community College The student will heat
DETERMINATION of the EMPIRICAL FORMULA
 DETERMINATION of the EMPIRICAL FORMULA One of the fundamental statements of the atomic theory is that elements combine in simple whole number ratios. This observation gives support to the theory of atoms,
DETERMINATION of the EMPIRICAL FORMULA One of the fundamental statements of the atomic theory is that elements combine in simple whole number ratios. This observation gives support to the theory of atoms,
Lab #3: Law of Definite Proportions
 Name Lab #3: Law of Definite Proportions Sept. 21, 2016 Purpose To find the percent composition and therefore definite ratio of the elements in magnesium oxide. Background When magnesium and oxygen are
Name Lab #3: Law of Definite Proportions Sept. 21, 2016 Purpose To find the percent composition and therefore definite ratio of the elements in magnesium oxide. Background When magnesium and oxygen are
Copper Smelting by an Ancient Method
 Copper Smelting by an Ancient Method EXPERIMENT ## Prepared by Paul C. Smithson, Berea College, based on Yee et al., 004 Using beads of a copper-containing mineral, students will produce beads of nearly
Copper Smelting by an Ancient Method EXPERIMENT ## Prepared by Paul C. Smithson, Berea College, based on Yee et al., 004 Using beads of a copper-containing mineral, students will produce beads of nearly
, to form the products magnesium oxide, MgO(s), and magnesium nitride, Mg 3
 Active Metals in the Periodic Table Periodic Trends and the Properties of the Elements SCIENTIFIC Introduction Like good experiments, good demonstrations often lead to other demonstrations. What follows
Active Metals in the Periodic Table Periodic Trends and the Properties of the Elements SCIENTIFIC Introduction Like good experiments, good demonstrations often lead to other demonstrations. What follows
CHEM 1215 LAB NOTES EXPT #2: PHYSICAL AND CHEMICAL CHANGES 1
 CHEM 1215 LAB NOTES EXPT #2: PHYSICAL AND CHEMICAL CHANGES 1 TECHNIQUES: chemical and physical changes, reactions, observations READING: PHYSICAL AND CHEMICAL CHANGES e.g. Tro chapter 1 SAFETY: Safety
CHEM 1215 LAB NOTES EXPT #2: PHYSICAL AND CHEMICAL CHANGES 1 TECHNIQUES: chemical and physical changes, reactions, observations READING: PHYSICAL AND CHEMICAL CHANGES e.g. Tro chapter 1 SAFETY: Safety
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION
 GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
GRAVIMETRIC DETERMINATION OF SULFATE IN AN UNKNOWN SOLUTION AIM The main objective of this experiment is to determine the concentration of sulfate ion in an unknown solution by using gravimetry. INTRODUCTION
EXPERIMENT 6. Determination of the Ideal Gas Law Constant - R. Magnesium metal reacts with hydrochloric acid according to the following reaction,
 EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
EXPERIMENT 6 Determination of the Ideal Gas Law Constant - R Magnesium metal reacts with hydrochloric acid according to the following reaction, Mg + 2 HCl MgCl 2 + H 2 (g) In this experiment you will use
Determination of the Empirical Formula of an Unknown Lead Oxide
 Determination of the Empirical Formula of an Unknown Lead Oxide by: Student Name Abstract Using a process known as smelting, 1 the empirical formula of an unknown lead oxide was determined 2 to be PbO
Determination of the Empirical Formula of an Unknown Lead Oxide by: Student Name Abstract Using a process known as smelting, 1 the empirical formula of an unknown lead oxide was determined 2 to be PbO
Chapter 8. Gravimetric Analysis
 Chapter 8 Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a :
Chapter 8 Gravimetric Analysis Gravimetric analysis is the use of weighing to determine the amount of a component in your sample. Gravimetric analysis, or gravimetry is normally performed either as a :
Equation Writing and Predicting Products Chemistry I Acc
 Introduction: Equation Writing and Predicting Products Chemistry I Acc If you examine your bicycle after it has been left out in the rain a number of times you will find that it has begun to rust. Rust
Introduction: Equation Writing and Predicting Products Chemistry I Acc If you examine your bicycle after it has been left out in the rain a number of times you will find that it has begun to rust. Rust
Experiment. Molar Mass of an Unknown Sulfate Salt by Gravimetric Techniques 1
 Experiment. Molar Mass of an Unknown Sulfate Salt by Gravimetric Techniques 1 This lab is to reacquaint you with some basic laboratory techniques and serves as a warm-up to the experiments in this course.
Experiment. Molar Mass of an Unknown Sulfate Salt by Gravimetric Techniques 1 This lab is to reacquaint you with some basic laboratory techniques and serves as a warm-up to the experiments in this course.
IDENTIFYING UNKNOWN SUBSTANCES
 IDENTIFYING UNKNOWN SUBSTANCES LAB 15 EXPERIMENT STUDENT BOOK Chapter 1, page 25 TOOLBOX Page 4 and 36 Goal Identify unknown substances with the help of different tests. 1. What is the independent variable
IDENTIFYING UNKNOWN SUBSTANCES LAB 15 EXPERIMENT STUDENT BOOK Chapter 1, page 25 TOOLBOX Page 4 and 36 Goal Identify unknown substances with the help of different tests. 1. What is the independent variable
INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES. Introduction. Electrochemistry Revised 4/28/14
 INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES Introduction Electrochemical Cells In this part of the experiment, four half cells are created by immersing metal strips of zinc, copper,
INTRODUCTION TO ELECTROCHEMISTRY: CURRENT, VOLTAGE, & BATTERIES Introduction Electrochemical Cells In this part of the experiment, four half cells are created by immersing metal strips of zinc, copper,
H N 2. Decolorizing carbon O. O Acetanilide
 Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
Experiment 1: Recrystallization of Acetanilide Reading Assignment Mohrig 2 4 (Glassware, Reagents, & Heating) & 14 15 (Melting Point & Recrystallization) The purification of organic compounds is a tedious,
The reduction of copper oxide
 The reduction of copper oxide Topic Formula determination, extraction of metals. Timing About 10 min. Level Pre-16. Description Copper(II) oxide can be reduced by hydrogen and its formula determined. Natural
The reduction of copper oxide Topic Formula determination, extraction of metals. Timing About 10 min. Level Pre-16. Description Copper(II) oxide can be reduced by hydrogen and its formula determined. Natural
EXPERIMENT 3: Identification of a Substance by Physical Properties
 EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
EXPERIMENT 3: Identification of a Substance by Physical Properties Materials: Hot plate Digital balance Capillary tubes (3) Thermometer Beakers (250 ml) Watch glass Graduated Cylinder (10 ml) Mel-Temp
Experiment 30A ENERGY CONTENT OF FUELS
 Experiment 30A ENERGY CONTENT OF FUELS FV 12/10/2012 MATERIALS: 12-oz. aluminum beverage can with top cut out and holes on side, thermometer, 100 ml graduated cylinder, 800 ml beaker, long-stem lighter,
Experiment 30A ENERGY CONTENT OF FUELS FV 12/10/2012 MATERIALS: 12-oz. aluminum beverage can with top cut out and holes on side, thermometer, 100 ml graduated cylinder, 800 ml beaker, long-stem lighter,
Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost
 Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost by Page 1/21 Contents What is the effect of compost on soil properties?... 3 Introduction:...
Porosity of Compost Water retention capacity of Compost Organic matter content of Compost Buffering capacity of Compost by Page 1/21 Contents What is the effect of compost on soil properties?... 3 Introduction:...
Rev Experiment 10
 Experiment 10 SPECTROPHOTOMETRIC DETERMINATION OF IRON IN DRINKING WATER 2 lab periods Reading: 1) Chapter 17, pg 393-403, Quantitative Chemical Analysis, 8 h Edition, Daniel C. Harris (7 th Edition: Chapter
Experiment 10 SPECTROPHOTOMETRIC DETERMINATION OF IRON IN DRINKING WATER 2 lab periods Reading: 1) Chapter 17, pg 393-403, Quantitative Chemical Analysis, 8 h Edition, Daniel C. Harris (7 th Edition: Chapter
BUREAU OF INDIAN STANDARDS DRAFT FOR COMMENTS ONLY. (Not to be reproduced without the permission of BIS or used as an Amendment)
 Doc No. CED 50 (7780) BUREAU OF INDIAN STANDARDS DRAFT FOR COMMENTS ONLY (Not to be reproduced without the permission of BIS or used as an Amendment) Draft AMENDMENT NO. 1 TO IS 12235 (Part 17):2004 THERMOPLASTICS
Doc No. CED 50 (7780) BUREAU OF INDIAN STANDARDS DRAFT FOR COMMENTS ONLY (Not to be reproduced without the permission of BIS or used as an Amendment) Draft AMENDMENT NO. 1 TO IS 12235 (Part 17):2004 THERMOPLASTICS
Analysis of Calcium Carbonate Tablets
 Experiment 9 Analysis of Calcium Carbonate Tablets Prepared by Ross S. Nord, Eastern Michigan University PURPOSE To perform a gravimetric exercise to determine weight percent of active ingredient in a
Experiment 9 Analysis of Calcium Carbonate Tablets Prepared by Ross S. Nord, Eastern Michigan University PURPOSE To perform a gravimetric exercise to determine weight percent of active ingredient in a
Worms and their environment
 s and their environment Objectives: The student will observe the growth of in different environments. The student will become familiar with biotechnology techniques. Specifically, how to manipulate organisms
s and their environment Objectives: The student will observe the growth of in different environments. The student will become familiar with biotechnology techniques. Specifically, how to manipulate organisms
ASEPTIC TRANSFER & PURE CULTURE TECHNIQUES
 ASEPTIC TRANSFER & PURE CULTURE TECHNIQUES GENERAL GUIDELINES & REMINDERS: SAFETY: NO EATING OR DRINKING IN THE LAB! Wash your hands with soap both BEFORE and AFTER lab, and, in addition, when you have
ASEPTIC TRANSFER & PURE CULTURE TECHNIQUES GENERAL GUIDELINES & REMINDERS: SAFETY: NO EATING OR DRINKING IN THE LAB! Wash your hands with soap both BEFORE and AFTER lab, and, in addition, when you have
Physical Behavior of Metals
 Activity 4 Physical Behavior of Metals GOALS In this activity you will: Discover what an alloy is. Make a brass-coated penny. Determine how the properties of a metal are affected by making it into an alloy.
Activity 4 Physical Behavior of Metals GOALS In this activity you will: Discover what an alloy is. Make a brass-coated penny. Determine how the properties of a metal are affected by making it into an alloy.
An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+
 An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+ LAB ADV COMP 8 From Advanced Chemistry with Vernier, Vernier Software & Technology, 2004 INTRODUCTION A titration, as you recall, is a
An Oxidation-Reduction Titration: The Reaction of Fe 2+ and Ce 4+ LAB ADV COMP 8 From Advanced Chemistry with Vernier, Vernier Software & Technology, 2004 INTRODUCTION A titration, as you recall, is a
The Goldschmidt Reaction
 CHEM 443L Inorganic Chemistry Laboratory Revision 2.0 The Goldschmidt Reaction In this laboratory we will carry-out several aluminothermic reactions, reactions involving the oxidation of Aluminum (Al)
CHEM 443L Inorganic Chemistry Laboratory Revision 2.0 The Goldschmidt Reaction In this laboratory we will carry-out several aluminothermic reactions, reactions involving the oxidation of Aluminum (Al)
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS
 EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
EXTRA CREDIT - EXPERIMENT G ELECTROCHEMISTRY ACTIVITY OF METALS INTRODUCTION The objective of this experiment is to develop an abbreviated activity series of metals using: 1. Displacement reactions 2.
1. Elemental mercury is a shiny, silver-colored, dense liquid that flows easily. Are these characteristics of mercury physical or chemical properties?
 Chemistry Dr. Saulmon 2014-15 School Year Unit 2: Basics of Chemistry Problem Set 3 Wednesday, September 3, 2014 1. Elemental mercury is a shiny, silver-colored, dense liquid that flows easily. Are these
Chemistry Dr. Saulmon 2014-15 School Year Unit 2: Basics of Chemistry Problem Set 3 Wednesday, September 3, 2014 1. Elemental mercury is a shiny, silver-colored, dense liquid that flows easily. Are these
Chem 355 Jasperse DISTILLATION
 Chem 355 Jasperse DISTILLATION 1 Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize.
Chem 355 Jasperse DISTILLATION 1 Background Distillation is a widely used technique for purifying liquids. The basic distillation process involves heating a liquid such that liquid molecules vaporize.
Pre- Lab Questions: Synthesis and Crystallization of Alum
 Name Date Grade Pre- Lab Questions: Synthesis and Crystallization of Alum MUST be completed before an experiment is started. Show all work and be sure to include units. Q1. Based on the general chemical
Name Date Grade Pre- Lab Questions: Synthesis and Crystallization of Alum MUST be completed before an experiment is started. Show all work and be sure to include units. Q1. Based on the general chemical
PLASTICS: A COMMON OCCURENCE
 PLASTICS: A COMMON OCCURENCE Introduction Plastics are very common in our society today. They are used extensively for food storage and in this area alone, generate tons of waste for landfills. If one
PLASTICS: A COMMON OCCURENCE Introduction Plastics are very common in our society today. They are used extensively for food storage and in this area alone, generate tons of waste for landfills. If one
CONSERVATION OF MATTER AND CHEMICAL PROPERTIES
 1 CONSERVATION OF MATTER AND CHEMICAL PROPERTIES I. OBJECTIVES AND BACKGROUND The object of this experiment is to demonstrate the conservation of matter- or more particularly, the conservation of "atoms"
1 CONSERVATION OF MATTER AND CHEMICAL PROPERTIES I. OBJECTIVES AND BACKGROUND The object of this experiment is to demonstrate the conservation of matter- or more particularly, the conservation of "atoms"
To identify and classify various types of chemical reactions.
 Cycle of Copper Reactions Minneapolis Community and Technical College v.11.17 Objectives: To observe and document copper s chemical changes in five different reactions and verify that copper is conserved
Cycle of Copper Reactions Minneapolis Community and Technical College v.11.17 Objectives: To observe and document copper s chemical changes in five different reactions and verify that copper is conserved
Experiment 13: Determination of Molecular Weight by Freezing Point Depression
 1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
1 Experiment 13: Determination of Molecular Weight by Freezing Point Depression Objective: In this experiment, you will determine the molecular weight of a compound by measuring the freezing point of a
Gas Chromatography Assignment Chem 543/443
 Gas Chromatography Assignment Chem 543/443 1. Introduction Capillary gas chromatography (GC) is one of the most popular analytical techniques used in today s research. Its popularity is mainly due to efficient
Gas Chromatography Assignment Chem 543/443 1. Introduction Capillary gas chromatography (GC) is one of the most popular analytical techniques used in today s research. Its popularity is mainly due to efficient
Skills in Science. Lab equipment. (Always draw 2D) Drawings below are NOT to scale. Beaker - A general purpose container with a pouring lip.
 Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
Skills in Science Safety: Do NOT enter or leave the lab without permission from a teacher. Keep the gaps between tables clear of stools and bags. Never run in the lab. Do not throw things around in the
Flares MULTIPOINT TOTALLY ENCLOSED GROUND FLARE STEAM ASSIST MULTIPOINT INTERNAL STEAM
 Flares Industry Leader / Compliance Driven / Certification Assured MULTIPOINT TOTALLY ENCLOSED GROUND FLARE INTERNAL STEAM MULTIPOINT STEAM ASSIST Internal Steam Flare Cast 310SS internal tube segments
Flares Industry Leader / Compliance Driven / Certification Assured MULTIPOINT TOTALLY ENCLOSED GROUND FLARE INTERNAL STEAM MULTIPOINT STEAM ASSIST Internal Steam Flare Cast 310SS internal tube segments
Application Notes for COD Analysis DETERMINATION OF CHEMICAL OXYGEN DEMAND (COD) IN WATER AND WASTE WATER.
 DETERMINATION OF CHEMICAL OXYGEN DEMAND (COD) IN WATER AND WASTE WATER. INTRODUCTION The chemical oxygen demand can be considered as an approximate measurement of the theoretical oxygen consumption, i.e.,
DETERMINATION OF CHEMICAL OXYGEN DEMAND (COD) IN WATER AND WASTE WATER. INTRODUCTION The chemical oxygen demand can be considered as an approximate measurement of the theoretical oxygen consumption, i.e.,
Soil Particle Density Protocol
 Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
Soil Particle Density Protocol Purpose To measure the soil particle density of each horizon in a soil profile Overview Students weigh a sample of dry, sieved soil from a horizon, mix it with distilled
NCEA Level 1 Chemistry (90933) 2012 page 1 of 5. Q Evidence Achievement Achievement with Merit Achievement with Excellence NØ N1 N2 A3 A4 M5 M6 E7 E8
 Assessment Schedule 2012 NCEA Level 1 Chemistry (90933) 2012 page 1 of 5 Chemistry: Demonstrate understanding of aspects of selected elements (90933) Evidence Statement Q Evidence with Merit with Excellence
Assessment Schedule 2012 NCEA Level 1 Chemistry (90933) 2012 page 1 of 5 Chemistry: Demonstrate understanding of aspects of selected elements (90933) Evidence Statement Q Evidence with Merit with Excellence
Evaluation copy. Total Dissolved Solids. Computer INTRODUCTION
 Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Total Dissolved Solids Computer 12 INTRODUCTION Solids are found in streams in two forms, suspended and dissolved. Suspended solids include silt, stirred-up bottom sediment, decaying plant matter, or sewage-treatment
Experiment 4 MELTING POINTS OF ORGANIC COMPOUNDS 1
 Experiment 4 MELTING POINTS OF ORGANIC COMPOUNDS 1 Background: The melting point of an organic solid is probably the most widely used physical constant. If you want a quick, easy, and cheap way to characterize
Experiment 4 MELTING POINTS OF ORGANIC COMPOUNDS 1 Background: The melting point of an organic solid is probably the most widely used physical constant. If you want a quick, easy, and cheap way to characterize
Workmanship Project STUDENT
 GMAW Workmanship Project STUDENT T N E D U T S E D I GU Lincoln Electric Workmanship Project 1 Guide for Safe Arc Welding STEP 1 Please refer to the E205 Safety Guide that was included in your kit and
GMAW Workmanship Project STUDENT T N E D U T S E D I GU Lincoln Electric Workmanship Project 1 Guide for Safe Arc Welding STEP 1 Please refer to the E205 Safety Guide that was included in your kit and
Choosing the Right Technologies for Reverb Furnaces. Russell Hewertson, Manager of Combustion Technology
 Choosing the Right Technologies for Reverb Furnaces Russell Hewertson, Manager of Combustion Technology Choosing the Right Technologies for Reverb Furnaces Russell Hewertson, Manager of Combustion Technology
Choosing the Right Technologies for Reverb Furnaces Russell Hewertson, Manager of Combustion Technology Choosing the Right Technologies for Reverb Furnaces Russell Hewertson, Manager of Combustion Technology
EXPERIMENT 5. Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline
 EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
EXPERIMENT 5 Molecular Absorption Spectroscopy: Determination of Iron with 1,10-Phenanthroline UNKNOWN Submit a clean, labeled 100-mL volumetric flask to the instructor so that your unknown iron solution
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley)
 Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
Experiment 1: The Densities of Liquids and Solids (from Masterson & Hurley) One of the fundamental properties of any sample of matter is its density, which is its mass per unit of volume. The density of
to the presentation Teaching Thermodynamics: Chemical Potential from the Beginning Regina Rüffler, Georg Job
 to the presentation Teaching Thermodynamics: Chemical Potential from the Beginning Regina Rüffler, Georg Job Thermo International 2006 Boulder, August 3, 2006 FOUNDATION Further informations on the homepage:
to the presentation Teaching Thermodynamics: Chemical Potential from the Beginning Regina Rüffler, Georg Job Thermo International 2006 Boulder, August 3, 2006 FOUNDATION Further informations on the homepage:
The UBI Hollow Core Fired (HCF) Biochar Oven
 The UBI Hollow Core Fired (HCF) Biochar Oven A number of people have requested information on low tech biochar production. This communication concerns the UBI Hollow Core Fired biochar oven design that
The UBI Hollow Core Fired (HCF) Biochar Oven A number of people have requested information on low tech biochar production. This communication concerns the UBI Hollow Core Fired biochar oven design that
Chem 2115 Experiment #9. Consumer Chemistry: Determining the Iron Content in Supplements
 Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
Chem 2115 Experiment #9 Consumer Chemistry: Determining the Iron Content in Supplements OBJECTIVE: The goal of this experiment is to use the quantitative technique of spectrophotometry to determine the
Best Practices for More Effective E-Newsletter Advertising:
 Best Practices for More Effective E-Newsletter Advertising: Practical tips for maximizing this marketing channel Contents 2 Executive Summary...3 Why E-Newsletter Advertising...4 E-Newsletter Trends in
Best Practices for More Effective E-Newsletter Advertising: Practical tips for maximizing this marketing channel Contents 2 Executive Summary...3 Why E-Newsletter Advertising...4 E-Newsletter Trends in
Calculating energy changes from burning fuels
 Calculating energy changes from burning fuels TEACHERS AND TECHNICIANS NOTES Specification reference: C3.3.1 Energy from reactions (a) The relative amounts of energy released when substances burn can be
Calculating energy changes from burning fuels TEACHERS AND TECHNICIANS NOTES Specification reference: C3.3.1 Energy from reactions (a) The relative amounts of energy released when substances burn can be
CARPETAMERICARECOVERYEFFORT SM
 CARPETAMERICARECOVERYEFFORT SM Developing market-based solutions for the recycling & reuse of post-consumer carpet CA CARPET STEWARDSHIP PROGRAM NOTICE TO PROCESSORS NOVEMBER 2015 1. New Ash & Moisture
CARPETAMERICARECOVERYEFFORT SM Developing market-based solutions for the recycling & reuse of post-consumer carpet CA CARPET STEWARDSHIP PROGRAM NOTICE TO PROCESSORS NOVEMBER 2015 1. New Ash & Moisture
Melting Point 1. Figure 2. A close-up of the "business end" of the Mel-Temp apparatus. Figure 1. The mel-temp device.
 Melting Point 1 The temperature at which a solid melts is known as the melting point (MP) of that substance. The melting point is a physical property of a solid and can be used to help identify a substance.
Melting Point 1 The temperature at which a solid melts is known as the melting point (MP) of that substance. The melting point is a physical property of a solid and can be used to help identify a substance.
SAIOH Interpretation of Elemental Analyses Results. By: Jaco van Rensburg
 SAIOH 2013 Interpretation of Elemental Analyses Results By: Jaco van Rensburg WHY AEROSOL SAMPLING? Air sampling for aerosols, to determine the airborne concentrations of particulate matter, forms a major
SAIOH 2013 Interpretation of Elemental Analyses Results By: Jaco van Rensburg WHY AEROSOL SAMPLING? Air sampling for aerosols, to determine the airborne concentrations of particulate matter, forms a major
2. Crystallization. A. Background
 2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
2. Crystallization A. Background Crystallization is one of several available techniques available to purify organic compounds. Unlike other techniques, however, crystallization is specific to the purification
Aseptic Techniques. A. Objectives. B. Before coming to lab
 Aseptic Techniques A. Objectives Become familiar with 1. The ubiquity of microorganisms (see Note 1) 2. Aseptic techniques (see Note 2) 3. Standard methods for growing/observing microorganisms (see Note
Aseptic Techniques A. Objectives Become familiar with 1. The ubiquity of microorganisms (see Note 1) 2. Aseptic techniques (see Note 2) 3. Standard methods for growing/observing microorganisms (see Note
MICRO-SCALE COMBUSTION CALORIMETER
 FEATURES The Micro-scale Combustion Calorimeter was developed by the Federal Aviation Administration (FAA) to offer industry a research tool to assist the FAA in its mandate to dramatically improve the
FEATURES The Micro-scale Combustion Calorimeter was developed by the Federal Aviation Administration (FAA) to offer industry a research tool to assist the FAA in its mandate to dramatically improve the
Directions 1. Activate students' prior knowledge about secondary pollutants. 1 of 10. Activitydevelop
 Activitydevelop Pollutants Making More Pollutants How do pollutants interact with the environment to create more pollution, and what effects do secondary pollutants have on the environment and human health?
Activitydevelop Pollutants Making More Pollutants How do pollutants interact with the environment to create more pollution, and what effects do secondary pollutants have on the environment and human health?
BURNER FLAME TEMPERATURE DURING WARM UP AND HOT STANDBY. Alan D. Mosher KPS Technology & Engineering LLC
 BURNER FLAME TEMPERATURE DURING WARM UP AND HOT STANDBY Alan D. Mosher KPS Technology & Engineering LLC Presented at the Brimstone Sulfur Symposium Facilitated by Brimstone STS Limited Vail Colorado September
BURNER FLAME TEMPERATURE DURING WARM UP AND HOT STANDBY Alan D. Mosher KPS Technology & Engineering LLC Presented at the Brimstone Sulfur Symposium Facilitated by Brimstone STS Limited Vail Colorado September
EXPERIMENT 9 DEHYDRATION OF 2-METHYLCYCLOHEXANOL CH 3 H CH 3 OH H 3 PO 4 +
 EXPERIMENT 9 DEHYDRATION OF 2-METHYLCYCLOHEXANOL CH 3 CH 3 H CH 3 OH H 3 PO 4 + + H 2 O In this experiment, a microscale distillation apparatus will be used to perform an acid-catalyzed dehydration reaction
EXPERIMENT 9 DEHYDRATION OF 2-METHYLCYCLOHEXANOL CH 3 CH 3 H CH 3 OH H 3 PO 4 + + H 2 O In this experiment, a microscale distillation apparatus will be used to perform an acid-catalyzed dehydration reaction
TRANSFER OF BACTERIA USING ASEPTIC TECHNIQUE
 TRANSFER OF BACTERIA USING ASEPTIC TECHNIQUE GENERAL GUIDELINES: Safety Wear a lab coat and have your goggles on! ALWAYS disinfect the tables BEFORE and AFTER lab. Wash your hands with soap both BEFORE
TRANSFER OF BACTERIA USING ASEPTIC TECHNIQUE GENERAL GUIDELINES: Safety Wear a lab coat and have your goggles on! ALWAYS disinfect the tables BEFORE and AFTER lab. Wash your hands with soap both BEFORE
Experiment 3: The Chromatography of Organic Compounds
 Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
Experiment 3: The Chromatography of Organic Compounds INTRODUCTION Very often, in an organic synthesis, a reaction will proceed to produce multiple products or perhaps will only partially form the desired
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET
 INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
INDIAN SCHOOL MUSCAT SENIOR SECTION DEPARTMENT OF CHEMISTRY CLASS X- PRACTICAL WORKSHEET Different types of chemical reactions Experiment No: 1(a) Combination reaction Objectives: To study the Combination
Measuring Manganese Concentration Using Spectrophotometry
 Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
Measuring Manganese Concentration Using Spectrophotometry Objectives To use spectroscopy to determine the amount of Manganese is an unknown sample. Scenario Your have just joined a "Green Team" at the
SCHEDULE. Friday: Pet Investigations: Plate counts - how to know how many clones of your pet you have (pg. 9-10)
 SCHEDULE Wednesday: Pet Investigations: Phenol Red Broth with Durham tubes (pg. 3-4) Oxidation/Fermentation Agar (pg. 5-6) Anaerobic Growth (pg. 7) Growth in Liquid Culture (pg. 8-9) Friday: Pet Investigations:
SCHEDULE Wednesday: Pet Investigations: Phenol Red Broth with Durham tubes (pg. 3-4) Oxidation/Fermentation Agar (pg. 5-6) Anaerobic Growth (pg. 7) Growth in Liquid Culture (pg. 8-9) Friday: Pet Investigations:
RECENT COST REDUCTION DEVELOPMENTS IN THE HEATING OF STEEL
 RECENT COST REDUCTION DEVELOPMENTS IN THE HEATING OF STEEL Steven J. O'Connor, Senior Applications Engineer, Bloom Engineering Company Anthony G. Fennell, Western Regional Manager, Bloom Engineering Company
RECENT COST REDUCTION DEVELOPMENTS IN THE HEATING OF STEEL Steven J. O'Connor, Senior Applications Engineer, Bloom Engineering Company Anthony G. Fennell, Western Regional Manager, Bloom Engineering Company
National Food Safety Standard
 National standard of People s Republic of China GB 5413.21 2010 National Food Safety Standard Determination of calcium, iron, zinc, sodium, potassium, magnesium, copper and manganese in foods for infants
National standard of People s Republic of China GB 5413.21 2010 National Food Safety Standard Determination of calcium, iron, zinc, sodium, potassium, magnesium, copper and manganese in foods for infants
Fixed (Inorganic Ash) and Volatile Solids by Gravimetric Determination
 SOP AMBL-105-E Page 1 of 7 Standard Operating Procedure AMBL-105-E Prepared: 12/28/2017 Revised: Prepared by: Terry E. Baxter Reviewed by: Fixed (Inorganic Ash) and Volatile Solids by Gravimetric Determination
SOP AMBL-105-E Page 1 of 7 Standard Operating Procedure AMBL-105-E Prepared: 12/28/2017 Revised: Prepared by: Terry E. Baxter Reviewed by: Fixed (Inorganic Ash) and Volatile Solids by Gravimetric Determination
15 (See Example E later in this lab)
 Tallahassee Community College APPLICATIONS OF ADDITION, SUBTRACTION, MULTIPLICATION AND DIVISION In an earlier chapter, you learned some of the basics of working with applications. Many of the prior applications
Tallahassee Community College APPLICATIONS OF ADDITION, SUBTRACTION, MULTIPLICATION AND DIVISION In an earlier chapter, you learned some of the basics of working with applications. Many of the prior applications
Gravimetric Analysis: Determination of % Sulfur in Fertilizer
 Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
Gravimetric Analysis: Determination % Sulfur in Fertilizer This is another "real world" sample experiment in this case we will analyze a fertilizer sample for the sulfate content and express the result
RFLP ANALYSIS OF DNA LABORATORY
 RFLP ANALYSIS OF DNA LABORATORY BIG PICTURE You will be working with an essential research method widely used in genetics, conservation biology, and forensics. The lab is divided into three sections. Part
RFLP ANALYSIS OF DNA LABORATORY BIG PICTURE You will be working with an essential research method widely used in genetics, conservation biology, and forensics. The lab is divided into three sections. Part
Windsor Fireplace Installation Instructions
 Windsor Fireplace Installation Instructions Thank you for your purchase of the Windsor Fireplace from Natural Concrete Products. Please familiarize yourself with these installation instructions before
Windsor Fireplace Installation Instructions Thank you for your purchase of the Windsor Fireplace from Natural Concrete Products. Please familiarize yourself with these installation instructions before
FLAMABILITY TESTS FOR ELECTRICAL CABLES
 Tests on electric and optical fibre cables under fire conditions Description E 60332-1-2 E 60332-2-2 acc. to IEC 60332-1-2 acc. to IEC 60332-2-2 Tests for flame Tests for flame propagation for a single
Tests on electric and optical fibre cables under fire conditions Description E 60332-1-2 E 60332-2-2 acc. to IEC 60332-1-2 acc. to IEC 60332-2-2 Tests for flame Tests for flame propagation for a single
SIGNIFICANT FIGURES WORKSHEET
 SIGNIFICANT FIGURES WORKSHEET PART 1 - Determine the number of significant figures in the following numbers. 1.) 0.02 2.) 0.020 3.) 501 4.) 501.0 5.) 5,000 6.) 5,000. 7.) 6,051.00 8.) 0.0005 9.) 0.1020
SIGNIFICANT FIGURES WORKSHEET PART 1 - Determine the number of significant figures in the following numbers. 1.) 0.02 2.) 0.020 3.) 501 4.) 501.0 5.) 5,000 6.) 5,000. 7.) 6,051.00 8.) 0.0005 9.) 0.1020
BEHAVIOUR OF EXPANDABLE GRAPHITE AS A FLAME RETARDANT IN FLEXIBLE POLYURETHANE FOAM
 BEHAVIOUR OF EXPANDABLE GRAPHITE AS A FLAME RETARDANT IN FLEXIBLE POLYURETHANE FOAM Vijay J. Bhagat Research & Development Center Cleanline Products Pvt. Ltd., Pune, India. Presented at Polyurethane Foam
BEHAVIOUR OF EXPANDABLE GRAPHITE AS A FLAME RETARDANT IN FLEXIBLE POLYURETHANE FOAM Vijay J. Bhagat Research & Development Center Cleanline Products Pvt. Ltd., Pune, India. Presented at Polyurethane Foam
As you saw in the last activity, a computer is made of many parts,
 23 Producing Circuit Boards R EA D I N G As you saw in the last activity, a computer is made of many parts, each manufactured from one or more materials. One essential part of a computer, and of many other
23 Producing Circuit Boards R EA D I N G As you saw in the last activity, a computer is made of many parts, each manufactured from one or more materials. One essential part of a computer, and of many other
Using Models to Make Predictions
 Activity APPLY For Educator 45 Minutes Grades 7-12+ Ages 12+ Using Models to Make Predictions How much do humans have to reduce greenhouse gas emissions to prevent major warming? For the complete activity
Activity APPLY For Educator 45 Minutes Grades 7-12+ Ages 12+ Using Models to Make Predictions How much do humans have to reduce greenhouse gas emissions to prevent major warming? For the complete activity
F321: Atoms, Bonds and Groups Group 2
 F321: Atoms, Bonds and Groups Group 2 87 Marks 1. Magnesium and strontium are in Group 2 of the Periodic Table. When reacted with oxygen, magnesium forms a white powder called magnesium oxide. Write the
F321: Atoms, Bonds and Groups Group 2 87 Marks 1. Magnesium and strontium are in Group 2 of the Periodic Table. When reacted with oxygen, magnesium forms a white powder called magnesium oxide. Write the
Module 2, Add on Lesson Turbidity Sensor. Student. 90 minutes
 Module 2, Add on Lesson Turbidity Sensor Student 90 minutes Purpose Construct a sensor to measure the turbidity of water Graph data and reason about curves and linear relationships Calibrate the turbidity
Module 2, Add on Lesson Turbidity Sensor Student 90 minutes Purpose Construct a sensor to measure the turbidity of water Graph data and reason about curves and linear relationships Calibrate the turbidity
Which of these is the formula for disulfur heptoxide? A. S 2 O 7 B. S 7 O 2 C. SO 2 D. N 2 O
 Which of these is the formula for disulfur heptoxide? A. S 2 O 7 B. S 7 O 2 C. SO 2 D. N 2 O Which of these is the correct chemical formula for a molecule of oxygen? A. O B. O -2 C. O +2 D. O 2 Which of
Which of these is the formula for disulfur heptoxide? A. S 2 O 7 B. S 7 O 2 C. SO 2 D. N 2 O Which of these is the correct chemical formula for a molecule of oxygen? A. O B. O -2 C. O +2 D. O 2 Which of
REPORT NUMBER REPORT DATE SEND TO ISSUE DATE Apr 18, Apr 18, 2017 RECEIVED DATE Apr 05, 2017
 PAGE 1/7 Sample ID: EWA PELLETS Lab Number: 2653281 Date Sampled: 2017-04-04 0850 Carbon nitrogen ratio C/N 6 : 1 0.1 Calculation Auto-2017/04/12 Auto-2017/04/18 Carbon (total) 38.53 % 0.050 ASTM D 5373
PAGE 1/7 Sample ID: EWA PELLETS Lab Number: 2653281 Date Sampled: 2017-04-04 0850 Carbon nitrogen ratio C/N 6 : 1 0.1 Calculation Auto-2017/04/12 Auto-2017/04/18 Carbon (total) 38.53 % 0.050 ASTM D 5373
Unit Automobile Body Repair Techniques 6006 Shrinking Sheet Metal Student Manual
 Auto Collision & Refinishing Programs Unit 6000- Automobile Body Repair Techniques 6006 Shrinking Sheet Metal Student Manual About This Publication This manual was designed with the materials contained
Auto Collision & Refinishing Programs Unit 6000- Automobile Body Repair Techniques 6006 Shrinking Sheet Metal Student Manual About This Publication This manual was designed with the materials contained
For ethidium bromide waste that is generated at Harvard, each waste stream is to be managed or accumulated as described below.
 LABORATORY CHEMICAL WASTE FAQS ETHIDIUM BROMIDE 1. Q - How should I manage and dispose of ethidium bromide waste? Answer - Ethidium bromide is a mutagen that requires special storage, handling, and disposal
LABORATORY CHEMICAL WASTE FAQS ETHIDIUM BROMIDE 1. Q - How should I manage and dispose of ethidium bromide waste? Answer - Ethidium bromide is a mutagen that requires special storage, handling, and disposal
Nomenclature. A systematic method of writing chemical formulas and naming compounds
 Nomenclature A systematic method of writing chemical formulas and naming compounds Chemical symbols Symbols are used to represent elements Either one capital letter, or a capital letter with a lower case
Nomenclature A systematic method of writing chemical formulas and naming compounds Chemical symbols Symbols are used to represent elements Either one capital letter, or a capital letter with a lower case
ENVR 1401 LAB EXERCISE Lab 11 Wastewater Treatment
 ENVR 1401 LAB EXERCISE Lab 11 Wastewater Treatment Name: SAFETY CONCERNS: Chemical splash goggles must be worn by everyone in the lab for the entire lab period. Goggles and a sterilizing cabinet have been
ENVR 1401 LAB EXERCISE Lab 11 Wastewater Treatment Name: SAFETY CONCERNS: Chemical splash goggles must be worn by everyone in the lab for the entire lab period. Goggles and a sterilizing cabinet have been
Chapter 6, Lesson 11: Chemical Reactions & Engineering Design
 Chapter 6, Lesson 11: Chemical Reactions & Engineering Design NGSS Standard: MS-PS1-6 Undertake a design project to construct, test, and modify a device that either releases or absorbs thermal energy by
Chapter 6, Lesson 11: Chemical Reactions & Engineering Design NGSS Standard: MS-PS1-6 Undertake a design project to construct, test, and modify a device that either releases or absorbs thermal energy by
XRF S ROLE IN THE PRODUCTION OF MAGNESIUM METAL BY THE MAGNETHERMIC METHOD
 Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 398 XRF S ROLE IN THE PRODUCTION OF MAGNESIUM METAL BY THE MAGNETHERMIC METHOD H. L. Baker Northwest Alloys,
Copyright(c)JCPDS-International Centre for Diffraction Data 2001,Advances in X-ray Analysis,Vol.44 398 XRF S ROLE IN THE PRODUCTION OF MAGNESIUM METAL BY THE MAGNETHERMIC METHOD H. L. Baker Northwest Alloys,
Iron filings (Fe) 56g IRON + SULPHUR IRON SULPHIDE
 W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
W.S.51. Chemical reactions. All of the different materials around us have been formed by chemical reactions from about one hundred simple elements. The diagram below shows a chemical reaction between the
An Inexpensive Corrosion Laboratory Experiment - Suitable for All Ages.
 An Inexpensive Corrosion Laboratory Experiment - Suitable for All Ages. John R. Williams Purdue University Statewide Technology Kokomo, IN 46904-9003 Abstract One of the most obvious effects of corrosion
An Inexpensive Corrosion Laboratory Experiment - Suitable for All Ages. John R. Williams Purdue University Statewide Technology Kokomo, IN 46904-9003 Abstract One of the most obvious effects of corrosion
Controlling NOx and other Engine Emissions
 Controlling NOx and other Engine Emissions Extensive Emissions Control Experience in Stationary Diesel and Natural Gas Engines Distributed Power Generation Cogeneration Plants (CHP) Gas Compression & Transmission
Controlling NOx and other Engine Emissions Extensive Emissions Control Experience in Stationary Diesel and Natural Gas Engines Distributed Power Generation Cogeneration Plants (CHP) Gas Compression & Transmission
