In situ investigation of electrochemical lithium intercalation into graphite powder
|
|
|
- Benedict Carpenter
- 6 years ago
- Views:
Transcription
1 Journal of Electroanalytical Chemistry 489 (2000) In situ investigation of electrochemical lithium intercalation into graphite powder Chunsheng Wang a, *, Imran Kakwan a, A. John Appleby a, Frank E. Little b a Center for Electrochemical Systems and Hydrogen Research, Texas Engineering Experiment Station, Texas A&M Uni ersity, College Station, TX , USA b Center for Space Power, Texas Engineering Experiment Station, Texas A&M Uni ersity, College Station, TX , USA Received 22 November 1999; received in revised form 17 April 2000; accepted 9 May 2000 Abstract The reaction kinetics for the electrochemical insertion extraction of lithium into and out of graphite and the electrode intrinsic resistance have been studied using galvanostatic intermittent titration with applied microcurrent pulses (GIT), electrochemical impedance spectroscopy (EIS) and in situ intrinsic (i.e. physical) resistance measurements. The formation of a so-called solid electrolyte interphase (SEI) or passivation layer on the graphite surface at about +0.8 V versus Li + /Li increases the total intrinsic resistance within the electrode on the first cycle. The gradual growth (i.e. change in volume) of the SEI film during repeated lithium insertion extraction results in a continued increase in intrinsic resistance. The good agreement between EIS and in situ intrinsic resistance measurements indicates that the latter is a simple and powerful method for investigating the change in this parameter for graphite electrodes. GIT showed a gradual increase in kinetic rate in an initial single-phase region, followed by a decrease in a two-phase transformation region, which was in turn followed by an increase in kinetic rate in a second single-phase region. This is explained by a shrinking unreacted core model. The controlling step of lithium insertion extraction during the two-phase transformation region was investigated by EIS and GIT Elsevier Science S.A. All rights reserved. Keywords: Electrochemical reaction kinetics; Intrinsic resistance; Electrochemical impedance spectroscopy 1. Introduction Graphites are extensively used as secondary lithiumion battery anodes because of their high practical energy density, charge discharge reversibility, non-toxicity, safety, and cost. Their reversible and irreversible capacity, rate capability and cycling capability characteristics determine their utility. It is generally accepted that reversible lithium charging capacities of graphite depends on their structural characteristics. Their irreversible capacities and longterm cycling capability largely depend on the amount of particle disintegration resulting from expansion/contraction during lithium insertion and extraction and on the relative stability of passivating surface films, which are commonly referred to as a solid electrolyte interphase (SEI) [1]. The SEI forms from solvent molecules * Corresponding author. Fax: address: cswang@pop.tamu.edu (C. Wang). when the double layer potential difference reaches a critical value, and it covers all the electrode surface exposed to the electrolyte. It must be permeable to Li + cations to permit reaction, but must also be an electronic insulator to prevent further electrolyte decomposition. Hence, both the formation of SEI films on individual graphite particles, and the gradual disintegration of these particles, will influence the overall conductivity of the graphite electrode. In situ monitoring of electrode conductivity is important for understanding the long-term cycling capability of graphite electrodes. Nishizawa et al. [2] have studied the in situ change in conductivity of a single mesocarbon microbead (MCMB) particles and SEI films on MCMBs using cyclic voltammetry (CV) and microelectrodebased methods. They found that the conductivity and redox activity of MCMB SEI films decreased on continued CV cycling, while these parameters remain stable on individual MCMB particles. The resistance (or conductivity) measured by dc methods is the total intrinsic /00/$ - see front matter 2000 Elsevier Science S.A. All rights reserved. PII: S (00)
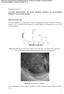
2 56 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) Table 1 Nominal particle sizes and BET surface areas of graphite powders Sample Nominal particle size/ m BET surface area/m 2 g 1 Distributor Timrex KS Timcal A.G. MCMB Osaka Chemical Co. JM Johnson Matthey or physical resistance resulting from the ionic conduction of SEI films and the electronic interconnection resistance between carbon particles themselves and their current collector contacts. As the authors state, the results obtained by CV cannot be quantitatively compared with those obtained by constant-current potentiometry, which has been frequently used for lithium-ion battery characterization. Finally, the faradaic reaction current may also interfere with the intrinsic conductivity measured. Microperturbation techniques such as electrochemical impedance spectroscopy (EIS) and galvanostatic intermittent titration with applied microcurrent pulses (GIT, i.e. galvanostatic microdischarge as a function of state-of-charge, SOC), are effective for the determination of impedances or rates of individual electrode kinetic processes [3]. EIS can give the individual resistances for each reaction step if their time constants are resolvable [4]. GIT can give the equilibrium potential and total reaction resistance of lithium insertion extraction as a function of lithium content if the pulsed current is small and the relaxation time is sufficiently long for the equilibrium potential to be attained [5]. In this work, the kinetics and cycling stability of graphite electrodes have been studied by using in situ intrinsic (i.e. physical) resistance measurements, EIS, and GIT. The reaction resistances and intrinsic resistances measured using EIS and GIT as a function of lithium content are compared. 2. Experimental 2.1. Electrode and cell preparation Three graphite powder samples (MCMB 10-28, KS 15, and JM 287) were used as anode materials. Their nominal particle size, BET surface area, and source are shown in Table 1. Electrodes consisted of 92 wt.% graphite powder and 8 wt.% polyvinylidene fluoride (Kynar, Elf-Atochem) in 1-methyl-2-pyrrolidinone solvent. After drying overnight at 120 C, they were pressed between two Ni mesh current collectors into a sandwich structure with a geometric area of 2.0 cm 2 containing ca. 50 mg of active graphite (Fig. 1). Electrochemical measurements were performed in a three-compartment PTFE cell of conventional design, with lithium foil counter and reference electrodes. Potentials are given versus this reference electrode in the electrolyte used, 1.0 M LiPF 6 in a 1:1:3 by volume ethylene carbonate (EC) propylene carbonate (PC) dimethylcarbonate (DMC) mixture (High Purity Lithium Battery Grade, Mitsubishi Chemical Company). Cells were assembled in an argon-filled glove box. Charge (lithium intercalation) and discharge (lithium extraction) characteristics were measured between +0.0 and +1.5 V at a constant current using an Arbin Corporation (College Station, TX) automatic battery cycler In situ intrinsic resistance measurement The in situ intrinsic resistance measurement is shown in Fig. 1. Two potentiostats were used, one for electrochemical lithium insertion extraction to and from the electrode, the other for measuring intrinsic resistance by application of constant current (10 ma for JM 287, 5 ma for KS 15, and 1 ma for the low bulk density MCMB electrodes, chosen to give less than 10 mv between the two outer current collectors). To avoid confusion over the various electrode resistances discussed here, the expression intrinsic resistance is used below to refer to those physical elements of resistance that are present within the thickness of the electrode, excluding any electrochemical (faradaic) elements. These include the electronic resistance of the series-parallel array of graphite particles themselves, the transmission line of contact resistances between the particles, ionic (electrolytic) contacts, and the electronic resistances of the current collectors and the contact resistances between them and the neighboring graphite particles. This intrinsic resistance may be evaluated by an in situ direct current method. Fig. 1. Schematic diagram of graphite electrode configuration.
3 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) ensure a low overpotential ( 10 mv). This and the 1.0 h charge discharge time ensured a uniform state of charge. Equally, the long open-circuit relaxation time ensured an equilibrium open-circuit potential. Hence, the lithium insertion extraction reaction resistance at different lithium contents could be calculated directly from the ratio of overpotential to the charge discharge current, and the electrode intrinsic resistance could be obtained with no interference from the charge discharge current. An intrinsic resistance of 1.0 could be measured quite accurately by application of 10 ma across the electrode. The experimental reaction resistance determined from the overpotential charge discharge current slope should be the resistance of the electrolyte, the SEI film, the charge transfer reaction, lithium diffusion into graphite, together with any resistance associated with transfer between phases in the phase transformation region. Fig. 2. Potential and intrinsic resistance of the KS 15 electrode during the first lithium insertion extraction and second lithium insertion cycles. Charge discharge current: 2.8 ma g 1. Current for measuring intrinsic resistance: 5 ma EIS measurement This was measured over a frequency range of 65 khz to 1.0 mhz at a potentiostatic signal amplitude of 5 mv using a Solartron PRA 1250 frequency response analyzer with a Solartron Model 1286 electrochemical interface after electrodes were left at open circuit for 90 min for potential stabilization. For electrochemical reaction rate measurements, the signal was applied to the current collectors on both sides of the electrode. For intrinsic resistance measurement at high frequency, the EIS was measured between the two sides GIT measurements Lithium was inserted and extracted to or from the electrode by the use of a series of current pulses of equal duration (1.0 h), such that the overall time required to pass 372 ma h g 1 was 72 h (C/72 rate). Following each pulse, the cell was left on open circuit for a time long enough to ensure equilibrium, yet short enough to prevent loss of lithium by self-discharge. The time chosen was 1.5 h. Meanwhile, the potential was recorded by computerized data acquisition. Depending on the electrode (see above), a constant 1 ma or 10 ma dc current was passed between the external current collectors to obtain the intrinsic resistance via the resulting voltage difference. The GIT charge discharge current was lower than that used in Ref. [3], and the relaxation time was much longer. A low charge discharge current of 0.25 ma (5.1 ma g 1 ) was used to 3. Results and discussion 3.1. In situ intrinsic resistance during initial cycling Fig. 2 shows the potential and intrinsic resistance profile of KS 15 during the first charge discharge and second charge cycles after allowing 3.0 h of immersion in the electrolyte for equilibration. The first charge curve has been subdivided into five sectors between initial state A and the five sharp discontinuities B-F. In the A-B zone, the electrode was at open-circuit for 3.0 h when the intrinsic resistance was about 2.2. SEI film formation is indicated by the increase in both the potential and the intrinsic resistance around 0.8 V (Fig. 3). The changes in these quantities at a lower charge current (1.25 ma g 1 ) are shown for comparison. On charge, the potential and intrinsic resistance both fell rapidly to V and 1.3, respectively, in the B-C zone. Here lithium insertion occurs to form a dilute Stage 1 phase in which lithium is intercalated uniformly within the host, decreasing the potential [6], but increasing the conductivity of the host graphite [7] and reducing the intrinsic resistance. Simultaneously, formation of a small amount of SEI film on the surface occurs above +0.8 V [8,9]. The SEI film is permeable to Li + cations, but is electronically insulating, increasing the intrinsic resistance. The combined effect of the two opposing processes is first to reduce the rate of decrease of the intrinsic resistance, then to increase it starting at C (Fig. 3). When KS 15 was charged further, the potential fell from +0.8 to +0.2 V (zones C-D-E). Here, the principal reaction has been considered to be SEI formation (zone C-D) and solvated lithium intercalation (zone D-E) [10]. The plateau potentials at ca V in D-E may be explained as stages in the formation of solvated graphite intercalation com-
4 58 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) Fig. 3. Potential and intrinsic resistance of a KS 15 electrode during SEI film formation on the graphite surface. (1) Charge current: 2.8 ma g 1. (2) Charge current: 1.25 ma g 1. Current for intrinsic resistance measurement: 5 ma. pounds [10]. Fig. 3 shows that the rapid increase in intrinsic resistance in the C-D plateau is due to the formation of SEI film. The resistance reaches a maximum after D because the film continues to grow at the high charge current density used (2.8 ma g 1 ). On decreasing the charge current density to 1.25 ma g 1 (Fig. 3, dashed line), the intrinsic resistance reached a maximum value at point D, and the potential plateau in D-E disappeared. Comparison of the capacities in the C-D and D-E zones shows that the irreversible faradaic capacity of the SEI film in C-D is ca. 14 ma h g 1 for 2.8 and 1.25 ma g 1 charge currents. However, a large faradaic capacity difference (8.4 ma h g 1 ) appears in D-E. This result indicates that a high lithium intercalation current has little effect on the SEI film in the C-D zone, but results in solvated lithium intercalation in the D-E zone. At high charge currents, the higher intrinsic resistance at E may be associated with solvated lithium intercalation. In zone E-F in Fig. 2, the principal reaction is the reversible formation of the next step of the binary (per C 6 unit) lithium graphite insertion compound (GIC), Li x C 6. These steps are represented by potential plateaus; those at ca. +0.2, +0.1, and V have been assigned to phase transitions between dilute Stage 1 and Stage 4, Stage 2L and Stage 2, and Stage 2 and Stage 1, respectively [6]. In this zone the changes in intrinsic resistance and potential go in parallel, i.e. in successive discharge/charge cycles, both the intrinsic resistance and the potential changed in a similar manner, although the resistance showed an independent increase in Cycle 2 (Fig. 2). The intrinsic resistance and the potential of JM 287 and MCMB behaved similarly to those of KS 15, although the increases with charge discharge cycle number varied with each type of graphite. Since the intrinsic resistance is calculated from the voltage difference across the electrode, the potential of the side of the electrode receiving the charge discharge current might influence measurements. This was eliminated by superposing GIT on the charge discharge cycles when the intrinsic resistance was calculated from the voltage between the two sides of the electrode with a small current imposed at open circuit (see below) GIT Fig. 4. Potential and intrinsic resistance as a function of time for the sixth cycle of a JM 287 electrode from galvanostatic intermittent titration via applied microcurrent pulses (GIT) technique. Charge discharge current: 5.1 ma g 1. The current pulse was imposed for 1.0 h then switched off for 1.5 h. Current for intrinsic resistance measurement: 10 ma. Fig. 4 shows the potential and intrinsic resistance measured by applying a current signal across the electrode for JM 287 Cycle 6 obtained under GIT conditions as a function of time. The charge discharge current pulse of 5.1 ma g 1 (0.255 ma for a 50 mg electrode) had little influence on the intrinsic resistance measurement, with an error not exceeding 6%. The overpotential gradually increased in the phase transformation region, but rapidly decreased in the single phase
5 Fig. 5. Dependence of the open-circuit potential, intrinsic resistance and reaction resistance of JM 287 on lithium content. Before measurement, the electrode was cycled six times. Current for intrinsic resistance measurement: 10 ma. C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) lithium diffusion currents, respectively. The reaction resistance measured via dc pulses (Fig. 5) should therefore be the total resistances of the four steps. Resistance changes in the electrolyte, and SEI film, and in charge transfer are small during charge/discharge process [13,14], so the changes observed must correspond to changes in lithium diffusion and phase transformation resistances in graphite. The change in diffusion resistance during phase transformation may be explained by the shrinking unreacted core model [15] (to be discussed later), which has satisfactorily explained potential-step chronoamperometry results [16]. The measured resistance parallels the potential change for lithium charge discharge, with a small variation, then a rapid change in the phase transformation and single phase regions, respectively. A more rapid increase was observed during discharge with JM 287 and MCMB The latter also showed a reaction resistance maximum at the end of the potential plateaus, with a gradual decrease in measured intrinsic resistance with decreasing potential (Fig. 6). Intrinsic resistances of JM 287 and MCMB at different open circuit potentials during Cycles 5 and 6 are shown in Fig. 7. Both graphites show similar behavior as a function of potential and cycle number, i.e. there is a slow decrease in intrinsic resistance on charge region, while the intrinsic resistance slowly decreased during lithium insertion but rapidly increased during lithium extraction. The open circuit potential, reaction resistance and intrinsic resistance at different lithium insertion extraction contents are shown in Fig. 5. About 20 ma h g 1 (0.054 Li/C 6 ) of irreversible faradaic capacity with 10 mv of hysteresis in the potential had appeared by Cycle 6. This irreversible loss may result from lithium self-discharge over the long charge discharge and relaxation times, or from continued SEI film growth, since the intrinsic resistance on charge was higher than on discharge. Hysteresis in the potential between charge and discharge may be related to expansion of graphite spacing on phase transformation [11]. The electrochemical reaction resistance on both charge and discharge gradually increased in the threephase transformation region (the potential plateau), but fell to its original value in the single phase region. Lithium charging appears first to involve partially-solvated ion diffusion through the SEI film, electron transfer on the graphite surface, then lithium diffusion into graphite, followed by phase transformation. At small overpotentials, the charge transfer and lithium diffusion resistances may be linearized as RT/nFI o and RT/nFI L, respectively [12], where I o and I L are the exchange and Fig. 6. Dependence of open-circuit potential, intrinsic resistance and reaction resistance of MCMB on lithium content. Before measurement, the electrode was cycled for six times. Current for intrinsic resistance measurement: 1 ma.
6 60 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) below +0.2 V and a rapid increase during discharge above 0.1 V. Nishizawa et al. explain this by volume changes on charge and discharge [2], so a further increase should occur during phase transformation, where a large volume change takes place. However, a stable intrinsic resistance in this region is shown in the present results. Since the intrinsic resistance measured galvanostatically gives the total resistance, a more discriminatory technique such as EIS is required to investigate the contact resistance, which is part of the intrinsic resistance EIS measurement Fig. 7. Dependence of intrinsic resistance on open-circuit potential of graphite. (a) JM 287; (b) MCMB JM 287 anodes were investigated to determine their reaction and intrinsic resistances as a function of lithium content. The latter were measured between the two sides of the electrode. Fig. 8 shows the open-circuit potential and instrinsic resistance during Cycle 9 (which were almost identical on charge and discharge) measured using 5.1 ma g 1 current pulses as for Cycle 6 in Fig. 5. The points indicate where the reaction resistance and intrinsic resistance were measured by EIS in the single phase region (dilute Stage 1, Stage 4, 2L) and in the phase transformation region (between Stage 1 and Stage 2). By Cycle 9s, the capacity and intrinsic resistance during charge and discharge were almost identical. Since the total charge discharge times vary greatly with lithium content, and self-discharge may occur at long times, accurate reaction resistances at high lithium content were difficult to obtain. However, reaction resistances for JM 287 in the charge cycles 5 7 were very similar (Fig. 9), despite the capacity loss on cycling, i.e. reaction kinetics on this graphite are relatively invariant and capacity-independent, although the intrinsic resistance showed an increase in these cycles (Fig. 7). Fig. 8. Variation of open-circuit potential, intrinsic resistance of JM 287 during the ninth charge discharge cycle. Circles: charge; Squares: discharge. EIS was used for intrinsic and reaction resistance measurement at each point. Fig. 9. Reaction resistance profile of JM 287 anode during Cycles 5 7. Applied current pulse: 5.1 ma g 1 for 1.0 h, followed by relaxation for 1.5 h.
7 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) Fig. 10. Nyquist plots for (a) charge and (b) discharge kinetics into JM 287 in the potential range +1.5 to V EIS in the single-phase region (dilute stage 1, and stages 2L, 4) Fig. 10(a, b) show the differences of the electrochemical reaction impedance spectra between charge discharge for JM 287 between +1.5 and V. At high potentials a depressed high-frequency semicircle occurs along with a low-frequency positive slope, which becomes very steep at the lowest frequencies (Fig. 10(a)). The former may be attributed to the SEI film impedance and the latter to lithium diffusion [13]. On charge a further charge transfer semicircle is overlapped by the inclined lithium diffusion line at high potentials, but starts to appear at potentials below +0.2 V, and becomes clearer below 0.1 V (Fig. 13). Increase in the real part of the high-frequency semicircle between charge and discharge indicate some SEI film growth during Cycle 9. At high potentials (+1.38 V), the EIS for intrinsic impedance measurement shows two semicircles whose real resistances decrease with potential and become almost zero below +0.2 V (Fig. 11). The intersection of the high-frequency line with the real axis decreases rapidly with decreasing potential. The two semicircles (see above) gradually become an inductive-resistive reactance as the potential is reduced from +2.3 to V. On JM 287 and MCMB graphites, the appearance of the inductive reactance depends on the intrinsic resistance, not on the potential. For example, it appeared in Cycle 1 for MCMB when the intrinsic resistance increased from 1.6 to 0.2, but disappeared in Cycle 6 when the intrinsic resistance became greater than 10 (Fig. 6). That the inductive reactance is an instrumental artifact is shown by EIS measured on the Solatron electrochemical interface terminals CE plus RE 1 and WE plus RE 2 connected together, or on an EIS of nickel wire, where the intercept to the real axis is the wire resistance. Hence, the intersection of the high-frequency line with the real axis in Fig. 11 must be the electronic resistance of the graphite electrode. Such artifacts are rather frequent, and may be avoided (when so identified) by omitting high frequency data. The two semicircles in Fig. 11 may be due to the contact impedance between the current collector to graphite powder, and graphite to graphite powder, respectively, which result from the SEI film giving incomplete particle contact after the volume change on cycling. For example, the electronic resistance of the graphite electrode decreased with increasing lithium content in dilute Stage 1 and Stage 2L (Fig. 11). Similar resistance changes have been noted in other dilute alkali metal graphite compounds (for example, C x K, Rb, Cs) [7].
8 62 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) The contact resistance between graphite and the current collectors decreases progressively, and almost disappears at potentials below +0.2 V due to the almost continuous expansion of lithiated graphite in the single phase region between +1.5 and V [6]. The soft SEI film must thereby be compressed or pushed aside, as scanning electron microscope observations suggest [17], showing its apparent disappearance by compression onto graphite fiber at low potentials, reappearing again on discharge to +2.5 V [16]. The appearance of the two semicircles in the plot for intrinsic impedance (Fig. 11(b)) may therefore be associated with shrinkage Fig. 11. Nyquist plots for intrinsic resistance of JM 287 in the potential range +1.5 to V. (a) Charge; (b) discharge. of the discharging graphite and increasing SEI film thickness. If this is so, the SEI film must contain solvent under normal conditions. The EIS plots show further SEI film growth on cycling, since the SEI film resistance increases from 6 in the Cycle 1 to 10 in Cycle 6. Intrinsic resistances measured from in situ dc signals (Fig. 8) and from EIS are compared in Fig. 12, which shows good agreement, EIS giving somewhat lower values. Thus the simple in situ method developed is of considerable practical value EIS in the stage transformation region (stage 1 to stage 2) Fig. 13(a, b) shows the JM 287 electrochemical reaction impedance in the Stage 2 to 1 region and in the reverse transformation. Only a very small changes in SEI film and charge transfer resistance were observed during stage transformation. However, a large change in the low-frequency lithium diffusion line is observed. Theoretical EIS analysis for lithium diffusion into spherical particles with phase transformation shows that a low-frequency semicircle should occur [4]. The large low-frequency imaginary part indicates a high diffusion resistance. Diffusion becomes slower as phase transformation progresses, whether the phase transformation is from Stage 1 to 2 or the contrary (Fig. 13). These EIS results are in good agreement with the GIT reaction resistance changes (Fig. 5). EIS spectra in Fig. 13 are similar to EIS plots obtained in the stage transformation region [18]. In contrast to the diffusion resistance, the intrinsic resistance remains almost the same between charge and discharge between Stages 1 and 2 (Fig. 14). Its EIS had no obvious semicircle, so changes must reflect those of graphite. The continuous decrease from Stage 1 to 2 and the increase in the other direction were due to the phase change, since the conductivity of Stage 1 is twice as high as that of Stage 2 [7] Electrochemical kinetics during phase transformation Fig. 12. Dependence of intrinsic resistance on graphite lithium content using dc and EIS methods. The total electrolyte, SEI film, and charge transfer resistance was ca. 18 (Fig. 13), but the reaction resistance (without diffusion) from GIT was ca. 30 (Fig. 5). The 12 difference is evidently the phase transformation resistance, since GIT data should include it. To verify this, the GIT potential-time profile at the Stage 2 to 1 transformation (Fig. 4) was evaluated by examining the relaxation from the overpotential at each stage of charge and the corresponding equilibrium potential (Fig. 15). In all cases, a rapid relaxation of about 5 mv is initially observed, which is followed by a slow relaxation whose potential increases with the frac-
9 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) Fig. 13. Nyquist plots for charge discharge kinetics into JM 287 in the phase transformation region. (a) From Stage 2 to 1; (b) from Stage 1 to 2. tion of Stage 1 phase. The rapid change corresponds to the voltage drop for total electrolyte, SEI film and charge transfer reaction resistances, whereas the subsequent change may be attributed to phase transformation and lithium diffusion resistances. The former was ca. 20 (i.e. 5 mv/0.25 ma), close to the 18 determined by EIS (Fig. 13). The charge current pulse overpotential behaved correspondingly, as would be expected. The overpotential of the first charging pulse was stable after 0.5 h, but the region of stability shortened and finally disappeared in the following pulses as phase transformation progressed. A stable overpotential in the initial stage indicates that the phase transformation resistance is higher than that for lithium diffusion (discussed below). Phase transformation may be explained by a shrinking unreacted core model (Fig. 16). During the initial charging, the lithium concentration gradually increases at the graphite plane edges, giving a slow overpotential increase. When the edge concentration exceeds the maximum Stage 2 supersaturation (equivalent here to 0.25 ma charge for 0.5 h), the Stage 1 phase nucleates from supersaturated Stage 2 phase, rapidly forming a continuous sheet. The stable overpotential indicates that lithium diffusion showed no concentration polarization for 0.5 h because of the short lithium diffusion distance, which permitted the phase boundary to move towards the particle center. The initial overpotential largely results from the growth of the Stage 1 phase from Stage 2, but the lithium concentration polarization slowly increases as the phase boundary moves toward the particle center and the diffusion length increases. The shrinking unreacted core model assumes that the phase transformation resistance is constant and the lithium diffusion resistance is proportional to ln(1 X), where X is the fraction of the second phase (see Appendix). Thus: R 21 = RTr o 2 F 2 c 12 k 21 (c 12 c 21 ) 1 ln (1 X) (1) D i from Stage 2 to 1 R 12 = RTr o 2 F 2 c 21 k 12 (c 12 c 21 ) 1 ln 1 X (2) D 2 from Stage 1 to 2 where c 12 and c 21 are the lithium concentrations of the Stage 1 phase in equilibrium with Stage 2 and of the Stage 2 phase in equilibrium with Stage 1, D 1 and D 2
10 64 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) are the lithium diffusion coefficients in Stages 1 and 2, and k 12, k 21 are the rate constants for phase transformation from Stage 1 to 2 and from 2 to 1, respectively. r o is the average graphite particle radius. The constant resistance of stage transformation (first terms on the right side of Eqs. (1) and (2)) and gradual increasing resistance of lithium diffusion (second terms) are in agreement with the interpretation that increase of reaction resistance during phase transformation (Fig. 5) is due to lithium diffusion. Fig. 17 plots diffusion resistance in the between Stage 1 to 2 as a function of ln(1 X), showing an approximately linear relationship for the forward and backward transformation. The higher slope for the first compared with that for the second is because c 12 c 21 (Eqs. (1) and (2)). Even though it supposes cylindrical symmetry with the diffusion and phase transformations in a steady state, the experimental resistances are in satisfactory agreement with the model. A small negative experimental deviation in the high lithium content may result from the short relaxation time (1.5 h) used, which may not be enough to achieve equilibrium. Overall, the resistances are as follows (Fig. 5[13]): stage transformation, 12 SEI film, 10 charge transfer, 6 electrolyte 2. The diffusion resistance changes from 0 to 31 as the Stage 1 fraction increases. When it exceeds 0.52, the diffusion resistance becomes greater than 12, so the rate-determining step then changes from phase transformation to lithium diffusion. Similar behavior was also reported by Pyun et al. [11] and Barsoukov et al. [20] Electrochemical kinetics in the single-phase region Fig. 14. Nyquist plots for intrinsic resistance of JM 287 in the phase transformation region. (a) From Stage 2 to 1; (b) from Stage 1 to 2. Fig. 15. Overpotential relaxation-time profile for JM 287 at different stages of phase transformation from Stage 2 to 1 using GIT. The final overpotential at the end of each lithium insertion step is put equal to zero, and the relaxation potentials are measured from this value. R L, R ct, R SEI, R st, and R d are the electrolyte, charge transfer, SEI film, Stage transformation and lithium-in-graphite diffusion resistances, respectively. The high reaction resistance at the end of phase transformation becomes less in the single-phase region (Figs. 5 and 9). The reasons are not clear but the absence of a phase transformation resistance in this region, together with the decrease in the derivative of the open-circuit potential as a function of lithium content (dp oc /dx), may be significant. The latter indicates that the same lithium composition gradient between the surface and center of a graphite particle will result in a lower overpotential at low dp oc /dx. EIS modeling based on the shrinking unreacted core concept predicts that the diffusion impedance should have the properties of a Warburg impedance in series with a capacitance in the high frequency region. Published results [13,19] show good agreement with these predictions. The dp oc /dx value for the diffusion impedance Z w should be the slope of the potential versus lithium composition in a single phase outside of the phase transformation region, as Fig. 16 indicates. By regarding dp oc /dx as an unknown during fitting of experimental impedance spectra for MCMB graphite as a function of degree of intercalation, Barsoukov et al. [20] found that the best fit dp oc /dx value is higher than that measured from potential-composition curves in the phase transformation region, but is similar to it in the single phase region, which confirms the present predictions. In almost all diffusion coefficient measurements derived from EIS plots in the phase transformation
11 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) Fig. 16. (a) Equilibrium potential vs. lithium composition in the phase transformation region between Stage 2 and 1. (b) Schematic concentration profiles during stage transformation from Stage 2 to 1. region, a dp oc /dx value has been selected to calculate the lithium diffusion coefficient in the host material [13,19,21], which results in an apparent large change in lithium diffusion coefficient during phase transformation. Because the fitting accuracy in this region is low, Takami et al. have chosen to use an equation containing no dp oc /dx term to calculate the lithium diffusion coefficient [22]. 4. Conclusions In situ intrinsic resistance measurements and microperturbation techniques such as GIT via applied microcurrent pulses and EIS have been applied to investigate the charge discharge behavior over time and the kinetics of the lithium-insertion graphite anodes. The regions of rising intrinsic resistance observed in in situ resistance versus lithium composition curves at around + 0.8Vs.Li/Li + result from SEI film formation on the graphite host material. EIS of JM 287 graphite shows that the intrinsic resistance depends largely on the conductivity of lithiated graphite at potentials below +0.2 V, but the parts attributed to contact resistance between the current collector and graphite, and between graphite particles, appeared at potentials above +0.2 V. The increase in contact resistance above +0.2 V is presumably caused by the volume change during lithium insertion extraction and the increase in thickness of the SEI film. GIT with microcurrent pulsing shows that the reaction resistance increases in the three-stage transformation region, and then relaxes in the following single phase region. EIS measurement indicates that the increase in reaction resistance in the stage transformation region results from the gradual increase in lithium diffusion path length. Phase transformation and lithium diffusion in graphite may be satisfactorily described by the shrinking unreacted shrinking core model, which can satisfactorily explain charge discharge kinetics in this system. From the EIS and GIT measurements, we conclude that the electrochemical reaction for lithium insertion into JM 287 graphite in the phase transformation region is initially determined by the rate of phase change, and thereafter is controlled by the diffusion of lithium in the new phase. Fig. 17. The variation of diffusion resistance of JM 287 with ln(1 X) during stage transformation between Stages 1 and 2.
12 66 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) This work shows that a high reaction resistance may decrease in the single phase region. From the kinetic viewpoint, an effective candidate anode material should undergo several phase transformations during charge and discharge. Acknowledgements We gratefully acknowledge NASA-Glenn Research Center, Cleveland, OH, for support of this work. Appendix A. Resistance lithium diffusion resistance in graphite with cylindrical symmetry Reaction resistance in phase transformation from Stage 2 to 1: It is generally accepted that lithium ions intercalate predominantly into graphite from the edge plane, and then diffuse inside in a direction parallel to the basal plane. JM 287 has been subjected to some mechanical milling. SEM images show that it has a circular cross section with a diameter of about 15 m. JM 287 particles may therefore be considered to be cylindrical in using the shrinking unreacted core model to describe the phase boundary moving toward to the particle center (Fig. 16). A further simplification assumes steady-state charge discharge. From Fick s first law, and lithium flux continuity in Stage 1, the following equation may be obtained: dc 1 2 rfd 1 dr =2 r dc 1 ofd 1 =2 r o I c (A1) dr r=r o where F is the Faraday constant, r o the average graphite particle radius, I c the cathodic current density, and D 1 is the diffusion coefficient for lithium in the Stage 1 phase, which is taken to be constant. Integrating Eq. (A1) with the boundary conditions r=r o, c 1 =c 1s, r=r 2, c 1 =c 1b : c 1s =c 1b I cr o ln r 2 (A2) FD 1 r o where r 2 is the radius of the unreacted Stage 2 material and c 1b is the lithium concentration in Stage 1 at the boundary with Stage 2. The fraction of Stage 1 transformed can be expressed as X=V stage1 /V=1 (r 2 /r o ) 2, therefore Eq. (A2) can be rewritten as: c 1s =c 1b I cr o ln 1 X (A3) FD 1 If the transformed Stage 1 is incoherent with Stage 2, the transformation rate of Stage 1 is [23]: dv stage 1 =k 21 (c 1b c 12 ) (A4) dt where c 12 is the lithium concentration of Stage 1 equilibrated with Stage 2, and k 21 is the rate constant for stage transformation. From mass conservation at the boundary between the Stage 1 and 2 phases: F(c 1b c 21 ) dv stage 1 dc =2 FD 1 dt 1 dr =2 r oi c (A5) where c 21 is the lithium concentration at the equilibrium Stage 2/Stage 1 boundary. Combining Eqs. (A4) and (A5), we obtain: c 1b =c r oi c (A6) Fk 21 (c 1b c 21 ) Substituting Eq. (A6) into Eq. (A3), then: c 1s =c r oi c Fk 21 (c 1b c 21 ) I cr o ln 1 X (A7) FD 1 Because the phase transformation resistance is small (12 ), c 1b c 12 c 21 hence Eq. (A7) may be rewritten as: c 1s =c r oi c Fk 21 (c 12 c 21 ) I cr o ln 1 X (A8) FD 1 The overpotential due to the phase transformation from Stage 2 to 1 and lithium diffusion is: 21 = RT F ln c 1s =25.6 ln 1 c 12 c 1s (A9) c 12 c 12 When the charge discharge current is low, the overpotential is small. At 10 mv, Eq. (A9) can be linearized: 21 = RT F ln c 1s = RT c 1s c 12 (A10) c 12 F c 12 Substituting Eq. (A8) into Eq. (A10), the resistance due to lithium diffusion into the host and phase transformation from Stage 2 to 1 can be obtained: R 21 = RTr o 2 F 2 c 12 k 21 (c 12 c 21 ) 1 ln 1 X (A11) D 1 Reaction resistance in phase transformation from stage 1 to 2: the overpotential in phase transformation from Stage 1 to 2 (cf. Eq. (A11)) can be expressed as: R 12 = RTr o 2 F 2 c 21 k 12 (c 12 c 21 ) 1 ln 1 X (A12) D 2 where I d is the lithium extraction current, D 2 is the lithium diffusion coefficient in Stage 2, and k 12 is the corresponding rate constant. The first term on the right side of Eqs. (A11) and (A12) indicates the resistances of the stage transformation are constant. The second terms, which reflect the lithium diffusion resistances, are proportional to ln(1 x).
13 C. Wang et al. / Journal of Electroanalytical Chemistry 489 (2000) References [1] E. Peled, J. Electrochem. Soc. 126 (1979) [2] M. Nishizawa, H. Koshika, I. Uchida, J. Phys. Chem. B 103 (1999) 192. [3] A.H. Whitehead, M. Perkins, J.R. Owen, J. Electrochem. Soc. 144 (1997) L93. [4] C.S. Wang, J. Electrochem. Soc. 145 (1801) [5] C.S. Wang, I. Kakwan, A.J. Appleby, F.E. Little, J. Electrochem. Soc., in press. [6] J.R. Dahn, Phys. Rev. B 44 (1991) [7] M.S. Dresselhaus, G. Dresselhaus, Adv. Phys. 30 (1981) 139. [8] K.A. Hirasawa, T. Sato, H. Asahina, S. Yamaguchi, S. Mori, J. Electrochem. Soc. 144 (1997) L81. [9] A.C. Chu, J.Y. Josefowicz, G.C. Farrington, J. Electrochem. Soc. 144 (1997) [10] M. Winter, P. Novak, A. Monnier, J. Electrochem. Soc. 145 (1998) 428. [11] S.-I. Pyun, Y.-G. Ryu, J. Power Sources 70 (1998) 34. [12] A.J. Bard, L.R. Faulkner, Electrochemical Methods: Fundamentals and Applications, Wiley, New York, [13] A. Funabiki, M. Inaba, Z. Ogumi, S.I. Yuasa, J. Ostuji, A. Tasaka, J. Electrochem. Soc. 145 (1998) 172. [14] T. Piao, S.-M. Park, C.-H. Doh, S.-I. Moon, J. Electrochem. Soc. 146 (1999) [15] C.S. Wang, X.H. Wang, Y.Q. Lei, C.P. Chen, Q.D. Lei, J. Hydrogen Energy 21 (1996) 471. [16] A. Funabiki, M. Inaba, T. Abe, Z. Ogumi, J. Electrochem. Soc. 146 (1999) [17] K. Zaghib, K. Tatsumi, Y. Sawda, S. Higuchi, H. Abe, T. Ohsaki, J. Electrochem. Soc. 146 (1999) [18] A. Metrot, A. Harrach, Electrochim. Acta 38 (1993) [19] M.D. Levi, D. Aurbach, J. Phys. Chem. B 101 (1997) [20] E. Barsoukov, J.H. Kim, J.H. Kim, C.O. Yoon, H. Lee, Solid State Ionics 116 (1999) 249. [21] E. Barsoukov, J.H. Kim, J.H. Kim, C.O. Yoon, H. Lee, J. Electrochem. Soc. 145 (1998) 27. [22] N. Takami, A. Satoh, M. Hara, T. Ohsaki, J. Electrochem. Soc. 142 (1995) 371. [23] D.A. Porter, Phase Transformation in Metals and Alloys, Van Nostrand Reinhold, New York,
Electrochemical impedance study of initial lithium ion intercalation into graphite powders
 Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
Electrochimica Acta 46 (2001) 1793 1813 www.elsevier.nl/locate/electacta Electrochemical impedance study of initial lithium ion intercalation into graphite powders Chunsheng Wang a, *, A. John Appleby
In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li 0.35 TiO 3 Ceramic at Different Li Insertion Levels
 A1196 Journal of The Electrochemical Society, 151 8 A1196-A1201 2004 0013-4651/2004/151 8 /A1196/6/$7.00 The Electrochemical Society, Inc. In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li
A1196 Journal of The Electrochemical Society, 151 8 A1196-A1201 2004 0013-4651/2004/151 8 /A1196/6/$7.00 The Electrochemical Society, Inc. In Situ IonicÕElectric Conductivity Measurement of La 0.55 Li
Supplemental Information for:
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplemental Information for: A Novel Lithium-sulfur Battery Cathode from Butadiene Rubber-caged
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 215 Supplemental Information for: A Novel Lithium-sulfur Battery Cathode from Butadiene Rubber-caged
Capacity fade study of lithium-ion batteries cycled at high discharge rates
 Journal of Power Sources 117 (2003) 160 169 Capacity fade study of lithium-ion batteries cycled at high discharge rates Gang Ning, Bala Haran, Branko N. Popov * Department of Chemical Engineering, University
Journal of Power Sources 117 (2003) 160 169 Capacity fade study of lithium-ion batteries cycled at high discharge rates Gang Ning, Bala Haran, Branko N. Popov * Department of Chemical Engineering, University
Supporting Information
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information High performance All-Solid-State Li-Se Batteries induced
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Supporting Information High performance All-Solid-State Li-Se Batteries induced
SUPPLEMENTARY INFORMATION
 Electrochemical stiffness in lithium-ion batteries Hadi Tavassol 1#, Elizabeth M. C. Jones 2,4#, Nancy R. Sottos 3,4*, and Andrew A. Gewirth 1* 1 Department of Chemistry, 2 Department of Mechanical Science
Electrochemical stiffness in lithium-ion batteries Hadi Tavassol 1#, Elizabeth M. C. Jones 2,4#, Nancy R. Sottos 3,4*, and Andrew A. Gewirth 1* 1 Department of Chemistry, 2 Department of Mechanical Science
PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES
 PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES A. Concheso, R. Santamaría, R. Menéndez, R. Alcántara #, P. Lavela #, J.L. Tirado # Instituto Nacional del Carbón (CSIC), Apdo.
PERFORMANCE OF DIFFERENT COAL-TAR PITCH DERIVED CARBONS IN LI-ION BATTERIES A. Concheso, R. Santamaría, R. Menéndez, R. Alcántara #, P. Lavela #, J.L. Tirado # Instituto Nacional del Carbón (CSIC), Apdo.
The Initial Irreversible Capacity of the First Doping/Undoping of Lithium into Carbon
 Carbon Science Vol. 1, No. 3 & 4 January 2001 pp. 148-153 The Initial Irreversible Capacity of the First Doping/Undoping of Lithium into Carbon Chil-Hoon Doh, Hyun-Soo Kim and Seong-In Moon Battery Research
Carbon Science Vol. 1, No. 3 & 4 January 2001 pp. 148-153 The Initial Irreversible Capacity of the First Doping/Undoping of Lithium into Carbon Chil-Hoon Doh, Hyun-Soo Kim and Seong-In Moon Battery Research
EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY
 EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY Weiming Lu and D.D.L. Chung Composite Materials Research Laboratory University at Buffalo The State
EFFECT OF THE PITCH-BASED CARBON ANODE ON THE IRREVERSIBLE CAPACITY OF LITHIUM-ION SECONDARY BATTERY Weiming Lu and D.D.L. Chung Composite Materials Research Laboratory University at Buffalo The State
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral
 Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Supplementary Figure 1. Crystal structures of conventional layered and Li-rich layered manganese oxides. a, The crystal structure of rhombohedral LiMO 2 (M = Ni, Co, Mn) with the space group R3m. b, The
Supporting Information
 Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
Supporting Information In Situ-formed Li 2 S in Lithiated Graphite Electrodes for Lithium-Sulfur Batteries Yongzhu Fu, Chenxi Zu, Arumugam Manthiram Electrochemical Energy Laboratory & Materials Science
School of Materials Science and Engineering, South China University of Technology,
 Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
Supporting information Zn/MnO 2 Battery Chemistry With H + and Zn 2+ Co-Insertion Wei Sun, Fei Wang, Singyuk Hou, Chongyin Yang, Xiulin Fan, Zhaohui Ma, Tao Gao, Fudong Han, Renzong Hu, Min Zhu *, Chunsheng
Rechargeable Sodium-ion Batteries with a Cl-doped
 Supplementary Information: Room-Temperature All-solidstate Rechargeable Sodium-ion Batteries with a Cl-doped Na 3 PS 4 Superionic Conductor Iek-Heng Chu a, Christopher Kompella a, Han Nguyen a, Zhuoying
Supplementary Information: Room-Temperature All-solidstate Rechargeable Sodium-ion Batteries with a Cl-doped Na 3 PS 4 Superionic Conductor Iek-Heng Chu a, Christopher Kompella a, Han Nguyen a, Zhuoying
Cycle life performance of lithium-ion pouch cells
 Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Journal of Power Sources 158 (2006) 679 688 Cycle life performance of lithium-ion pouch cells Karthikeyan Kumaresan, Qingzhi Guo, Premanand Ramadass, Ralph E. White Department of Chemical Engineering,
Dynamic and Galvanic Stability of Stretchable Supercapacitors
 Supporting Information for Dynamic and Galvanic Stability of Stretchable Supercapacitors By Xin Li, Taoli Gu and Bingqing Wei* Department of Mechanical Engineering, University of Delaware, Newark, DE 19716
Supporting Information for Dynamic and Galvanic Stability of Stretchable Supercapacitors By Xin Li, Taoli Gu and Bingqing Wei* Department of Mechanical Engineering, University of Delaware, Newark, DE 19716
Electronic supplementary information. Efficient energy storage capabilities promoted by hierarchically MnCo 2 O 4
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Electronic supplementary information Efficient energy storage capabilities promoted by hierarchically
Journal of Power Sources
 Journal of Power Sources 196 (2011) 1442 1448 Contents lists available at ScienceDirect Journal of Power Sources journal homepage: www.elsevier.com/locate/jpowsour Strain accommodation and potential hysteresis
Journal of Power Sources 196 (2011) 1442 1448 Contents lists available at ScienceDirect Journal of Power Sources journal homepage: www.elsevier.com/locate/jpowsour Strain accommodation and potential hysteresis
A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 Supporting information A gel-ceramic multi-layer electrolyte for long-life lithium sulfur batteries
FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE POROSITY EVOLUTION MODEL
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supplementary data for : FAILURE MECHANISMS OF NANO SILICON ANODES: AN ELECTRODE
High-Performance All-Solid-State Lithium-Sulfur. Battery Enabled by a Mixed-Conductive Li 2 S
 Supporting information High-Performance All-Solid-State Lithium-Sulfur Battery Enabled by a Mixed-Conductive Li 2 S Nanocomposite Fudong Han, Jie Yue, Xiulin Fan, Tao Gao, Chao Luo, Zhaohui Ma, Liumin
Supporting information High-Performance All-Solid-State Lithium-Sulfur Battery Enabled by a Mixed-Conductive Li 2 S Nanocomposite Fudong Han, Jie Yue, Xiulin Fan, Tao Gao, Chao Luo, Zhaohui Ma, Liumin
Three-dimensional graphene-based hierarchically porous carbon. composites prepared by a dual-template strategy for capacitive
 Electronic Supplementary Information (ESI) Three-dimensional graphene-based hierarchically porous carbon composites prepared by a dual-template strategy for capacitive deionization Xiaoru Wen, a Dengsong
Electronic Supplementary Information (ESI) Three-dimensional graphene-based hierarchically porous carbon composites prepared by a dual-template strategy for capacitive deionization Xiaoru Wen, a Dengsong
De-ionized water. Nickel target. Supplementary Figure S1. A schematic illustration of the experimental setup.
 Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
Graphite Electrode Graphite Electrode De-ionized water Nickel target Supplementary Figure S1. A schematic illustration of the experimental setup. Intensity ( a.u.) Ni(OH) 2 deposited on the graphite blank
Supporting Information
 Supporting Information Earth Abundant Fe/Mn-Based Layered Oxide Interconnected Nanowires for Advanced K-Ion Full Batteries Xuanpeng Wang, Xiaoming Xu, Chaojiang Niu*, Jiashen Meng, Meng Huang, Xiong Liu,
Supporting Information Earth Abundant Fe/Mn-Based Layered Oxide Interconnected Nanowires for Advanced K-Ion Full Batteries Xuanpeng Wang, Xiaoming Xu, Chaojiang Niu*, Jiashen Meng, Meng Huang, Xiong Liu,
Corrosion. Lab. of Energy Conversion & Storage Materials. Produced by K. B. Kim
 Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
Corrosion 대기환경에의한금속소재 (organic film coated steel) 의퇴화현상평가연구 Lab. of Energy Conversion & Storage Materials Produced by K. B. Kim Introduction AC Impedance Spectroscopy Application of AC Impedance to Corrosion
The effect of pressure on the electroanalytical response of graphite anodes and LiCoO 2 cathodes for Li-ion batteries
 Journal of Electroanalytical Chemistry 516 (2001) 89 102 www.elsevier.com/locate/jelechem The effect of pressure on the electroanalytical response of graphite anodes and LiCoO 2 cathodes for Li-ion batteries
Journal of Electroanalytical Chemistry 516 (2001) 89 102 www.elsevier.com/locate/jelechem The effect of pressure on the electroanalytical response of graphite anodes and LiCoO 2 cathodes for Li-ion batteries
SUPPORTING INFORMATION. A Rechargeable Aluminum-Ion Battery Based on MoS 2. Microsphere Cathode
 SUPPORTING INFORMATION A Rechargeable Aluminum-Ion Battery Based on MoS 2 Microsphere Cathode Zhanyu Li a, Bangbang Niu a, Jian Liu a, Jianling Li a* Feiyu Kang b a School of Metallurgical and Ecological
SUPPORTING INFORMATION A Rechargeable Aluminum-Ion Battery Based on MoS 2 Microsphere Cathode Zhanyu Li a, Bangbang Niu a, Jian Liu a, Jianling Li a* Feiyu Kang b a School of Metallurgical and Ecological
Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries
 /8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
/8 SUPPORTING INFORMATION Single-crystalline LiFePO 4 Nanosheets for High-rate Li-ion Batteries Yu Zhao, Lele Peng, Borui Liu, Guihua Yu* Materials Science and Engineering Program and Department of Mechanical
Supporting Information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information In situ electrochemical activation of Ni-based colloids from NiCl 2 electrode
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2016 Supporting Information In situ electrochemical activation of Ni-based colloids from NiCl 2 electrode
Supporting Information
 Supporting Information Electrochemical reduction of CO 2 at Copper Nanofoams Sujat Sen a, Dan Liu a and G. Tayhas R. Palmore a, b, * a Department of Chemistry and b School of Engineering, Brown University,
Supporting Information Electrochemical reduction of CO 2 at Copper Nanofoams Sujat Sen a, Dan Liu a and G. Tayhas R. Palmore a, b, * a Department of Chemistry and b School of Engineering, Brown University,
Improving cyclic performance of Si anode for lithium-ion batteries by forming an intermetallic skin
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Improving cyclic performance of Si anode for lithium-ion batteries by
Effect of heat treatment on electrochemical characteristics of spinel lithium titanium oxide
 Korean J. Chem. Eng., 27(1), 91-95 (2010) DOI: 10.1007/s11814-009-0298-0 RAPID COMMUNICATION Effect of heat treatment on electrochemical characteristics of spinel lithium titanium oxide Sung-Chul Hong*,
Korean J. Chem. Eng., 27(1), 91-95 (2010) DOI: 10.1007/s11814-009-0298-0 RAPID COMMUNICATION Effect of heat treatment on electrochemical characteristics of spinel lithium titanium oxide Sung-Chul Hong*,
Chapter 7. Evaluation of Electrode Performance by. Electrochemical Impedance
 Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Chapter 7 Evaluation of Electrode Performance by Electrochemical Impedance Spectroscopy (EIS) 7.1 Introduction A significant fraction of internal resistance of a cell comes from the interfacial polarization
Supplementary Figure 1:
 b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
b a c Supplementary Figure 1: Calibration of the Cs + sputtering rate on composite LiNi 0.7 Mn 0.15 Co 0.15 O 2 electrodes (500 ev ion energy, ~40 na measured sample current): (a) Optical profilometry
In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries
 Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
Supporting Information: In situ generation of Li 2 FeSiO 4 coating on MWNT as a high rate cathode material for lithium ion batteries Yi Zhao, Jiaxin Li, Ning Wang, Chuxin Wu, Yunhai Ding, Lunhui Guan*
High energy all-solid-state lithium batteries with
 Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
Supporting Information High energy all-solid-state lithium batteries with ultralong cycle life Xiayin Yao, Deng Liu, Chunsheng Wang, Peng Long, Gang Peng, Yong-Sheng Hu, *, Hong Li, Liquan Chen, and Xiaoxiong
Topic: Electrochemical Application of Carbon Materials MILD-EXFOLIATED GRAPHITE AS AN ANODE MATERIAL FOR LITHIUM ION BATTERY.
 Paper ID: 373 Topic: Electrochemical Application of Carbon Materials MILD-EXFOLIATED GRAPHITE AS AN ANODE MATERIAL FOR LITHIUM ION BATTERY Lin Zou, Yong-Ping Zheng, Feiyu Kang, Wanci Shen, Can Xu Laboratory
Paper ID: 373 Topic: Electrochemical Application of Carbon Materials MILD-EXFOLIATED GRAPHITE AS AN ANODE MATERIAL FOR LITHIUM ION BATTERY Lin Zou, Yong-Ping Zheng, Feiyu Kang, Wanci Shen, Can Xu Laboratory
Investigation of anode materials for lithium-ion batteries
 University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
University of Wollongong Thesis Collections University of Wollongong Thesis Collection University of Wollongong Year 2006 Investigation of anode materials for lithium-ion batteries Ling Yuan University
The Quantitative Evaluation of Anode Thickness Change for Lithium-ion Batteries
 The Quantitative Evaluation of node Thickness Change for Lithium-ion atteries Hiroko Takahashi* 1, Masanobu ragaki* 1, Toshiya Hikami* 2 The measurement technique of the electrode thickness to measure
The Quantitative Evaluation of node Thickness Change for Lithium-ion atteries Hiroko Takahashi* 1, Masanobu ragaki* 1, Toshiya Hikami* 2 The measurement technique of the electrode thickness to measure
Supporting Information
 Supporting Information Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO 3 Yifan Dong, Tyler Slade, Matthew J. Stolt, Linsen Li, Steven N. Girard, Liqiang
Supporting Information Low-Temperature Molten-Salt Production of Silicon Nanowires by the Electrochemical Reduction of CaSiO 3 Yifan Dong, Tyler Slade, Matthew J. Stolt, Linsen Li, Steven N. Girard, Liqiang
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes
 In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
In Situ Formation of Stable Interfacial Coating for High Performance Lithium Metal Anodes Haiping Wu 1, Yue Cao 1, Linxiao Geng 2 & Chao Wang 1* 1 Department of Chemistry, University of California Riverside,
Re-building Daniell Cell with a Li-Ion exchange Film
 Supplementary Information Re-building Daniell Cell with a Li-Ion exchange Film Xiaoli Dong, Yonggang Wang*, Yongyao Xia Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative
Supplementary Information Re-building Daniell Cell with a Li-Ion exchange Film Xiaoli Dong, Yonggang Wang*, Yongyao Xia Department of Chemistry and Shanghai Key Laboratory of Molecular Catalysis and Innovative
Intercalation of Bi nanoparticles into graphite enables ultrafast. and ultra-stable anode material for Sodium-ion
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Intercalation of Bi nanoparticles into
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2018 Electronic Supplementary Information Intercalation of Bi nanoparticles into
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED
 FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
FABRICATION AND ELECTROCHEMICAL CHARECTARIZATION OF THE CR2032 COIN CELLS USING THE DEVELOPED PURE AND CARBON COATED LiMPO 4 (M= Mn, Co & Ni) NANOPARTICLES CHAPTER VI 181 CHAPTER - VI FABRICATION AND ELECTROCHEMICAL
Supporting Information. Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries
 Supporting Information Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries Yu Lei,, Lei Qin,, Ruliang Liu,, Kah Chun Lau, Yiying Wu, Dengyun Zhai, *, Baohua Li, and Feiyu Kang *,
Supporting Information Exploring Stability of Nonaqueous Electrolytes for Potassium-Ion Batteries Yu Lei,, Lei Qin,, Ruliang Liu,, Kah Chun Lau, Yiying Wu, Dengyun Zhai, *, Baohua Li, and Feiyu Kang *,
Carbon Nanofiber Modified Graphite Electrode. Performance for Lithium Ion Secondary Battery
 Carbon Nanofiber Modified Graphite Electrode Performance for Lithium Ion Secondary Battery Seung-Hwan Moon, Myung-Soo Kim Department of Chemical Engineering, Myoungji University, Korea Corresponding author
Carbon Nanofiber Modified Graphite Electrode Performance for Lithium Ion Secondary Battery Seung-Hwan Moon, Myung-Soo Kim Department of Chemical Engineering, Myoungji University, Korea Corresponding author
Characterization of Nanocrystalline Si-MCMB Composite Anode Materials
 University of Wollongong Research Online Faculty of Engineering - Papers (Archive) Faculty of Engineering and Information Sciences 2004 Characterization of Nanocrystalline Si-MCMB Composite Anode Materials
University of Wollongong Research Online Faculty of Engineering - Papers (Archive) Faculty of Engineering and Information Sciences 2004 Characterization of Nanocrystalline Si-MCMB Composite Anode Materials
Li Ion Diffusivity and Improved Electrochemical Performances of the Carbon Coated LiFePO 4
 836 Bull. Korean Chem. Soc. 2011, Vol. 32, No. 3 ChangKyoo Park et al. DOI 10.5012/bkcs.2011.32.3.836 Li Ion Diffusivity and Improved Electrochemical Performances of the Carbon Coated LiFePO ChangKyoo
836 Bull. Korean Chem. Soc. 2011, Vol. 32, No. 3 ChangKyoo Park et al. DOI 10.5012/bkcs.2011.32.3.836 Li Ion Diffusivity and Improved Electrochemical Performances of the Carbon Coated LiFePO ChangKyoo
Supporting information
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting information An amorphous material with sponge-like structure as anode for Liion and
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Supporting information An amorphous material with sponge-like structure as anode for Liion and
Electrochemical properties of alkal. Citation Electrochimica Acta (2010), 55(3):
 KURENAI : Kyoto University Researc Title Electrochemical properties of alkal bis(trifluoromethylsulfonyl)amides Author(s) Kubota, Keigo; Tamaki, Kenichiro; N Takuya; Hagiwara, Rika Citation Electrochimica
KURENAI : Kyoto University Researc Title Electrochemical properties of alkal bis(trifluoromethylsulfonyl)amides Author(s) Kubota, Keigo; Tamaki, Kenichiro; N Takuya; Hagiwara, Rika Citation Electrochimica
Effect of Amorphous Transformation on Electrochemical Capacities of Rare Earth Mg Based Alloys
 Z. Phys. Chem. 220 (2006) 631 639 / DOI 10.1524/zpch.2006.220.5.631 by Oldenbourg Wissenschaftsverlag, München Effect of Amorphous Transformation on Electrochemical Capacities of Rare Earth Mg Based Alloys
Z. Phys. Chem. 220 (2006) 631 639 / DOI 10.1524/zpch.2006.220.5.631 by Oldenbourg Wissenschaftsverlag, München Effect of Amorphous Transformation on Electrochemical Capacities of Rare Earth Mg Based Alloys
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201704947 Bioinspired, Spine-Like, Flexible, Rechargeable Lithium-Ion
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2018. Supporting Information for Adv. Mater., DOI: 10.1002/adma.201704947 Bioinspired, Spine-Like, Flexible, Rechargeable Lithium-Ion
A novel rechargeable battery with magnesium anode, titanium dioxide cathode, and magnesim borohydride/tetraglyme electrolyte
 Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 A novel rechargeable battery with magnesium anode, titanium dioxide cathode, and magnesim borohydride/tetraglyme
Electronic Supplementary Material (ESI) for ChemComm. This journal is The Royal Society of Chemistry 2015 A novel rechargeable battery with magnesium anode, titanium dioxide cathode, and magnesim borohydride/tetraglyme
Galvanostatic charge discharge tests, 57 Fe and 119 Sn Mössbauer and XRD measurements on novel Sn-Ni-Fe electrodeposits
 Hyperfine Interact DOI 10.1007/s10751-012-0693-5 Galvanostatic charge discharge tests, 57 Fe and 119 Sn Mössbauer and XRD measurements on novel Sn-Ni-Fe electrodeposits G. B. Lak E. Kuzmann M. El-Sharif
Hyperfine Interact DOI 10.1007/s10751-012-0693-5 Galvanostatic charge discharge tests, 57 Fe and 119 Sn Mössbauer and XRD measurements on novel Sn-Ni-Fe electrodeposits G. B. Lak E. Kuzmann M. El-Sharif
Lithium Batteries with Nearly Maximum Metal. Storage Supporting Information
 Lithium Batteries with Nearly Maximum Metal Storage Supporting Information Abdul-Rahman O. Raji,, Rodrigo Villegas Salvatierra,, Nam Dong Kim, Xiujun Fan, Yilun Li, Gladys A. L. Silva, Junwei Sha and James
Lithium Batteries with Nearly Maximum Metal Storage Supporting Information Abdul-Rahman O. Raji,, Rodrigo Villegas Salvatierra,, Nam Dong Kim, Xiujun Fan, Yilun Li, Gladys A. L. Silva, Junwei Sha and James
Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries
 PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
PSI-SR-1261 Electroactive Polymer for Controlling Overcharge in Lithium-Ion Batteries A. Newman R. Pawle K. White J. Lennhoff A. Newman, R. Pawle, K. White, J. Lennhoff, "Electroactive Polymer for Controlling
Supplementary Information for
 Supplementary Information for An elastic and Li-ion-percolating hybrid membrane stabilizes Li metal plating Quan Pang, Laidong Zhou, Linda F. Nazar* Department of Chemistry and the Waterloo Institute for
Supplementary Information for An elastic and Li-ion-percolating hybrid membrane stabilizes Li metal plating Quan Pang, Laidong Zhou, Linda F. Nazar* Department of Chemistry and the Waterloo Institute for
THE USE OF GALVANOSTATIC PULSE MEASUREMENTS TO DETERMINE CORROSION PARAMETERS Galvanostatic Pulse Measurement of Corrosion
 THE USE OF GALVANOSTATIC PULSE MEASUREMENTS TO DETERMINE CORROSION PARAMETERS Galvanostatic Pulse Measurement of Corrosion D.W.LAW, S.G.MILLARD and J H BUNGEY Department of Civil Engineering, Liverpool
THE USE OF GALVANOSTATIC PULSE MEASUREMENTS TO DETERMINE CORROSION PARAMETERS Galvanostatic Pulse Measurement of Corrosion D.W.LAW, S.G.MILLARD and J H BUNGEY Department of Civil Engineering, Liverpool
Effect of Width of Gas/Liquid/Solid Three-Phase Boundary Zone of Discrete Water Film on Atmospheric Corrosion of Metals
 64 The Open Corrosion Journal, 2010, 3, 64-72 Open Access Effect of Width of Gas/Liquid/Solid Three-Phase Boundary Zone of Discrete Water Film on Atmospheric Corrosion of Metals Jia Wang *,1,2 and Jing
64 The Open Corrosion Journal, 2010, 3, 64-72 Open Access Effect of Width of Gas/Liquid/Solid Three-Phase Boundary Zone of Discrete Water Film on Atmospheric Corrosion of Metals Jia Wang *,1,2 and Jing
How initial nucleation influences discharge capacities of Li-O 2 cells
 How initial nucleation influences discharge capacities of Li-O 2 cells Ali Rinaldi 1, Olivia Wijaya 1, Denis Yu 2, Harry.E. Hoster 1 1TUM CREATE Centre for Electromobility #10-02 CREATE Tower, Singapore
How initial nucleation influences discharge capacities of Li-O 2 cells Ali Rinaldi 1, Olivia Wijaya 1, Denis Yu 2, Harry.E. Hoster 1 1TUM CREATE Centre for Electromobility #10-02 CREATE Tower, Singapore
The Li-O 2 Battery with a Dimethylformamide Electrolyte
 The Li-O 2 Battery with a Dimethylformamide Electrolyte Yuhui Chen, Stefan A. Freunberger, Zhangquan Peng, Fanny Bardé and Peter G. Bruce* School of Chemistry, University of St. Andrews, North Haugh, St.
The Li-O 2 Battery with a Dimethylformamide Electrolyte Yuhui Chen, Stefan A. Freunberger, Zhangquan Peng, Fanny Bardé and Peter G. Bruce* School of Chemistry, University of St. Andrews, North Haugh, St.
International Journal of Chemical Studies
 ISSN: 2321-4902 Volume 1 Issue 1 Online Available at www.chemijournal.com International Journal of Chemical Studies Calculation of Diffusion Coefficients and Layer Thickness for Oxidation the Ferrocene
ISSN: 2321-4902 Volume 1 Issue 1 Online Available at www.chemijournal.com International Journal of Chemical Studies Calculation of Diffusion Coefficients and Layer Thickness for Oxidation the Ferrocene
Supplementary Information
 Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2017 Supplementary Information Self-Standing Bi 2 O 3 Nanoparticles/Carbon Nanofiber
Electronic Supplementary Material (ESI) for Materials Chemistry Frontiers. This journal is the Partner Organisations 2017 Supplementary Information Self-Standing Bi 2 O 3 Nanoparticles/Carbon Nanofiber
A membrane-free ferrocene-based high-rate semiliquid
 Supporting Information A membrane-free ferrocene-based high-rate semiliquid battery Yu Ding,, Yu Zhao,, and Guihua Yu, * Materials Science and Engineering Program and Department of Mechanical Engineering,
Supporting Information A membrane-free ferrocene-based high-rate semiliquid battery Yu Ding,, Yu Zhao,, and Guihua Yu, * Materials Science and Engineering Program and Department of Mechanical Engineering,
Supporting Information
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Surface graphited carbon scaffold enables simple
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2017 Supporting Information Surface graphited carbon scaffold enables simple
Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface
 Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface Xiaobiao Wu a, Sihui Wang a, Xiaochen Lin a, Guiming Zhong a, Zhengliang Gong
Promoting long-term cycling performance of high-voltage Li 2 CoPO 4 F by the stabilization of electrode/electrolyte interface Xiaobiao Wu a, Sihui Wang a, Xiaochen Lin a, Guiming Zhong a, Zhengliang Gong
Preparation and Properties of Supercapacitor with Composite Electrodes
 Preparation and Properties of Supercapacitor with Composite Electrodes Chih-Ming Wang a,*, Chih-Yu Wen b, Ying-Chung Chen b, Jui-Yang Chang b, Jian-Zhen Huang b and Chien-Chung Hsu b a Department of Electrical
Preparation and Properties of Supercapacitor with Composite Electrodes Chih-Ming Wang a,*, Chih-Yu Wen b, Ying-Chung Chen b, Jui-Yang Chang b, Jian-Zhen Huang b and Chien-Chung Hsu b a Department of Electrical
Supporting Information
 Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Supporting Information Mg 2 B 2 O 5 Nanowires Enabled Multifunctional Solid-State Electrolyte with High Ionic Conductivity, Excellent Mechanical Properties and Flame-retardant Performance Ouwei Sheng,
Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from
 Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from polyimide used to seal Titanium (Ti) current collectors
Supplementary Figure 1: Sketch of XRD-EIS pouch cell design with Titanium current collectors serving as XRD windows, parafilm, kapton tape made from polyimide used to seal Titanium (Ti) current collectors
The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning
 2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
2016 International Conference on Intelligent Manufacturing and Materials (ICIMM 2016) ISBN: 978-1-60595-363-2 The Preparation of C/Ni Composite Nanofibers with Pores by Coaxial Electrospinning Yiqiang
Morphology controlled synthesis of monodispersed manganese. sulfide nanocrystals and their primary application for supercapacitor
 Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Electronic Supplementary Material (ESI) for Chemical Communications. This journal is The Royal Society of Chemistry 2015 Morphology controlled synthesis of monodispersed manganese sulfide nanocrystals
Supplementary Information
 Electronic Supplementary Material (ESI) for Sustainable Energy & Fuels. This journal is The Royal Society of Chemistry 2018 Supplementary Information for Chemical Science, DOI: 10.1039/ ((please add manuscript
Electronic Supplementary Material (ESI) for Sustainable Energy & Fuels. This journal is The Royal Society of Chemistry 2018 Supplementary Information for Chemical Science, DOI: 10.1039/ ((please add manuscript
Carbon-Silicon Core-Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries
 Carbon-Silicon Core-Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries NANO LETTERS XXXX Vol. xx, No. x - Li-Feng Cui, Yuan Yang, Ching-Mei Hsu, and Yi Cui* Department of Materials Science
Carbon-Silicon Core-Shell Nanowires as High Capacity Electrode for Lithium Ion Batteries NANO LETTERS XXXX Vol. xx, No. x - Li-Feng Cui, Yuan Yang, Ching-Mei Hsu, and Yi Cui* Department of Materials Science
Real-time XRD Studies of Li-O 2. Electrochemical Reaction in Nonaqueous. Lithium-Oxygen Battery
 Supporting Information Real-time XRD Studies of Li-O 2 Electrochemical Reaction in Nonaqueous Lithium-Oxygen Battery Hyunseob Lim, 1,2 Eda Yilmaz 1, and Hye Ryung Byon 1* 1 Byon Initiative Research Unit,
Supporting Information Real-time XRD Studies of Li-O 2 Electrochemical Reaction in Nonaqueous Lithium-Oxygen Battery Hyunseob Lim, 1,2 Eda Yilmaz 1, and Hye Ryung Byon 1* 1 Byon Initiative Research Unit,
Supporting Information. Hematite photoanode with gradient structure shows an unprecedentedly low onset
 Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information Hematite photoanode with gradient structure shows an unprecedentedly
Electronic Supplementary Material (ESI) for Physical Chemistry Chemical Physics. This journal is the Owner Societies 2014 Supporting Information Hematite photoanode with gradient structure shows an unprecedentedly
Access to the published version may require subscription. Published with permission from: Institute of Physics
 http://uu.diva-portal.org This is an author produced version of a paper published in Journal of Physics, Conference Series. This paper has been peer-reviewed but does not include the final publisher proof-corrections
http://uu.diva-portal.org This is an author produced version of a paper published in Journal of Physics, Conference Series. This paper has been peer-reviewed but does not include the final publisher proof-corrections
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications
 Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
Safe, Inexpensive, Long Life, High Power and Efficiency Batteries For Grid Scale Energy Storage Applications Investigators Yi Cui, Associate Professor; Robert Huggins, Professor; Mauro Pasta, Postdoctoral
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016)
 6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
6th International Conference on Advanced Design and Manufacturing Engineering (ICADME 2016) Porous Co3O4 irregular Micro-cubes with lithium storage performances Ting Wanga, Hao Zhengb, Jinsong Chengc,
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells
 Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Novel Mn 1.5 Co 1.5 O 4 spinel cathodes for intermediate temperature solid oxide fuel cells Huanying Liu, a, b Xuefeng Zhu, a * Mojie Cheng, c You Cong, a Weishen Yang a * a State Key Laboratory of Catalysis,
Final Report for AOARD Grant FA Lithium-air Battery Research. December 2009
 Final Report for AOARD Grant FA 4869-7-1-49 Lithium-air Battery Research December 29 Name of Principal Investigators: Prof. N. Munichandraiah - e-mail address : muni@ipc.iisc.ernet.in - Institution : Indian
Final Report for AOARD Grant FA 4869-7-1-49 Lithium-air Battery Research December 29 Name of Principal Investigators: Prof. N. Munichandraiah - e-mail address : muni@ipc.iisc.ernet.in - Institution : Indian
Formation and Growth of Surface Films on Graphitic Anode Materials for Li-Ion Batteries
 A128 1099-0062/2005/8 2 /A128/5/$7.00 The Electrochemical Society, Inc. Formation and Growth of Surface Films on Graphitic Anode Materials for Li-Ion Batteries J. S. Gnanaraj, a, *,z R. W. Thompson, a
A128 1099-0062/2005/8 2 /A128/5/$7.00 The Electrochemical Society, Inc. Formation and Growth of Surface Films on Graphitic Anode Materials for Li-Ion Batteries J. S. Gnanaraj, a, *,z R. W. Thompson, a
All-solid-state Batteries with Thick Electrode Configurations
 All-solid-state Batteries with Thick Electrode Configurations Yuki Kato, * Shinya Shiotani, Keisuke Morita, Kota Suzuki, Masaaki Hirayama, Ryoji Kanno Toyota Motor Europe NV/SA, Hoge Wei 33, 1930 Zaventem,
All-solid-state Batteries with Thick Electrode Configurations Yuki Kato, * Shinya Shiotani, Keisuke Morita, Kota Suzuki, Masaaki Hirayama, Ryoji Kanno Toyota Motor Europe NV/SA, Hoge Wei 33, 1930 Zaventem,
A design of Solid-State Li-S cell with evaporated Lithium anode to eliminate shuttle effects
 Supporting Information A design of Solid-State Li-S cell with evaporated Lithium anode to eliminate shuttle effects Yujie Hao, a Sheng Wang, a Feng Xu, a Yijie Liu, a Ningning Feng, a Ping He, *a Haoshen
Supporting Information A design of Solid-State Li-S cell with evaporated Lithium anode to eliminate shuttle effects Yujie Hao, a Sheng Wang, a Feng Xu, a Yijie Liu, a Ningning Feng, a Ping He, *a Haoshen
Metal Hydride Test Station (top) and alloy oxidation in bare metal hydrides (bottom) Bare LaNi Sn Cobalt coated LaNi 4.
 Surface Modification to Improve Hydrogen Entry Efficiency and Storage Capabilities of Metal Hydride Alloys Branko N. Popov, Durairajan Anand, Bala S. Haran and Ralph E. White Center For Electrochemical
Surface Modification to Improve Hydrogen Entry Efficiency and Storage Capabilities of Metal Hydride Alloys Branko N. Popov, Durairajan Anand, Bala S. Haran and Ralph E. White Center For Electrochemical
Yuki Takeuchi, Koichi Matsuzawa, Yuji Kohno, and Shigenori Mitsushima
 .49/644.65ecst The Electrochemical Society Charge and Mass Transfer on a La O Added Li/Na or Li/K Molten Carbonate Meniscus Electrode of LaNiO Coated Au Ring for Oxygen Reduction Reaction. Yuki Takeuchi,
.49/644.65ecst The Electrochemical Society Charge and Mass Transfer on a La O Added Li/Na or Li/K Molten Carbonate Meniscus Electrode of LaNiO Coated Au Ring for Oxygen Reduction Reaction. Yuki Takeuchi,
Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes
 Manuscript# IMLB-070, LBNL-52142 Revised to Journal of Power Sources (Dec 13, 2002) Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes Seung-Wan Song, a Kathryn A. Striebel, a,z Xiangyun Song a
Manuscript# IMLB-070, LBNL-52142 Revised to Journal of Power Sources (Dec 13, 2002) Amorphous and Nanocrystalline Mg 2 Si Thin Film Electrodes Seung-Wan Song, a Kathryn A. Striebel, a,z Xiangyun Song a
Tutorial Corrosion II. Electrochemical characterization with EC-Lab techniques
 Tutorial Corrosion II Electrochemical characterization with EC-Lab techniques 1 OUTLINE 1. Introduction 2. Types of corrosion a) Uniform corrosion b) Localized corrosion 3. Corrosion experiment 4. EC-Lab
Tutorial Corrosion II Electrochemical characterization with EC-Lab techniques 1 OUTLINE 1. Introduction 2. Types of corrosion a) Uniform corrosion b) Localized corrosion 3. Corrosion experiment 4. EC-Lab
Supporting Information. Investigation of the Reversible Intercalation/Deintercalation of Al
 Supporting Information Investigation of the Reversible Intercalation/Deintercalation of Al into the Novel Li 3 VO 4 @C Microsphere Composite Cathode Material for Aluminum-Ion Batteries Jiali Jiang, He
Supporting Information Investigation of the Reversible Intercalation/Deintercalation of Al into the Novel Li 3 VO 4 @C Microsphere Composite Cathode Material for Aluminum-Ion Batteries Jiali Jiang, He
Nitrogen-Doped Graphdiyne Applied for Lithium-
 Supporting Information for Nitrogen-Doped Graphdiyne Applied for Lithium- Ion Storage Shengliang Zhang,, Huiping Du,, Jianjiang He,, Changshui Huang,*, Huibiao Liu, Guanglei Cui and Yuliang Li Qingdao
Supporting Information for Nitrogen-Doped Graphdiyne Applied for Lithium- Ion Storage Shengliang Zhang,, Huiping Du,, Jianjiang He,, Changshui Huang,*, Huibiao Liu, Guanglei Cui and Yuliang Li Qingdao
State Key Laboratory of Advanced Electromagnetic Engineering and Technology, School of
 Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Electrocatalysis of polysulfide conversion by conductive RuO 2 Nano Dots for lithium sulfur batteries
Electronic Supplementary Material (ESI) for Nanoscale. This journal is The Royal Society of Chemistry 2018 Electrocatalysis of polysulfide conversion by conductive RuO 2 Nano Dots for lithium sulfur batteries
Supplemental Information. A Low-Cost and High-Energy Hybrid. Iron-Aluminum Liquid Battery Achieved. by Deep Eutectic Solvents
 JOUL, Volume 1 Supplemental Information A Low-Cost and High-Energy Hybrid Iron-Aluminum Liquid Battery Achieved by Deep Eutectic Solvents Leyuan Zhang, Changkun Zhang, Yu Ding, Katrina Ramirez-Meyers,
JOUL, Volume 1 Supplemental Information A Low-Cost and High-Energy Hybrid Iron-Aluminum Liquid Battery Achieved by Deep Eutectic Solvents Leyuan Zhang, Changkun Zhang, Yu Ding, Katrina Ramirez-Meyers,
Supporting Information
 Supporting Information Silicon(lithiated)-sulfur full cells with porous silicon anode shielded by Nafion against polysulfides to achieve high capacity and energy density Chenfei Shen a,1, Mingyuan Ge a,1,2,
Supporting Information Silicon(lithiated)-sulfur full cells with porous silicon anode shielded by Nafion against polysulfides to achieve high capacity and energy density Chenfei Shen a,1, Mingyuan Ge a,1,2,
Novel Materials for Lithium-Ion Batteries
 Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
Novel Materials for Lithium-Ion Batteries John Bradley May 18th 2012 Project Supervisors: Prof. West & Chaou Tan Abstract The effect of carbon coating on two novel battery cathode materials LiMnP 2 O 7
Model Prediction and Experiments for the Electrode Design Optimization of LiFePO 4 /Graphite Electrodes in High Capacity Lithium-ion Batteries
 Model Prediction and Experiments for the Electrode Design Optimization Bull. Korean Chem. Soc. 2013, Vol. 34, No. 1 79 http://dx.doi.org/10.5012/bkcs.2013.34.1.79 Model Prediction and Experiments for the
Model Prediction and Experiments for the Electrode Design Optimization Bull. Korean Chem. Soc. 2013, Vol. 34, No. 1 79 http://dx.doi.org/10.5012/bkcs.2013.34.1.79 Model Prediction and Experiments for the
Electronic Supplementary Material (ESI) for Chemical Communications This journal is The Royal Society of Chemistry 2013
 Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
Sodium-ion battery based on ion exchange membranes as electrolyte and separator Chengying Cao, Weiwei Liu, Lei Tan, Xiaozhen Liao and Lei Li* School of Chemical and Chemistry Engineering, Shanghai Jiaotong
Effect of electrode structure on performance of Si anode in Li-ion batteries: Si particle size and conductive additive
 Journal of Power Sources 140 (2005) 139 144 Short communication Effect of electrode structure on performance of Si anode in Li-ion batteries: Si particle size and conductive additive Wei-Ren Liu a, Zheng-Zao
Journal of Power Sources 140 (2005) 139 144 Short communication Effect of electrode structure on performance of Si anode in Li-ion batteries: Si particle size and conductive additive Wei-Ren Liu a, Zheng-Zao
HIGH PRESSURE CO 2 CORROSION ELECTROCHEMISTRY AND THE EFFECT OF ACETIC ACID
 HIGH PRESSURE CO 2 CORROSION ELECTROCHEMISTRY AND THE EFFECT OF ACETIC ACID Shihuai Wang, Keith George and Srdjan Nesic Institute for Corrosion and Multiphase Technology Ohio University, Athens, OH 71
HIGH PRESSURE CO 2 CORROSION ELECTROCHEMISTRY AND THE EFFECT OF ACETIC ACID Shihuai Wang, Keith George and Srdjan Nesic Institute for Corrosion and Multiphase Technology Ohio University, Athens, OH 71
Unlocking the Potential of Amorphous Red Phosphorus Films as Long-term Stable. Negative Electrode for the Lithium Battery
 Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Unlocking the Potential of Amorphous Red Phosphorus
Electronic Supplementary Material (ESI) for Journal of Materials Chemistry A. This journal is The Royal Society of Chemistry 2016 Supporting Information Unlocking the Potential of Amorphous Red Phosphorus
Electronic Supporting Information. Synthesis of single crystalline hexagonal nanobricks of
 Electronic Supporting Information Synthesis of single crystalline hexagonal nanobricks of LiNi 1/3 Co 1/3 Mn 1/3 O 2 with high percentage of exposed {010} active facets as high rate performance cathode
Electronic Supporting Information Synthesis of single crystalline hexagonal nanobricks of LiNi 1/3 Co 1/3 Mn 1/3 O 2 with high percentage of exposed {010} active facets as high rate performance cathode
Soft silicon anodes for lithium ion batteries
 Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supplementary Information Soft silicon anodes for lithium ion batteries Qizhen
Electronic Supplementary Material (ESI) for Energy & Environmental Science. This journal is The Royal Society of Chemistry 2014 Supplementary Information Soft silicon anodes for lithium ion batteries Qizhen
Electrode and Molecular Architectures for Iron based Multivalent Systems
 Electrode and Molecular Architectures for Iron based Multivalent Systems Jagjit Nanda Materials Science and Technology Division 2 nd MRES, North Eastern University August 20 th 2014 Collaborators S. K.
Electrode and Molecular Architectures for Iron based Multivalent Systems Jagjit Nanda Materials Science and Technology Division 2 nd MRES, North Eastern University August 20 th 2014 Collaborators S. K.
