Correlation Matrix EN 9100:2016 EN 9100:2009
|
|
|
- Asher Carpenter
- 6 years ago
- Views:
Transcription
1 Correlation Matrix EN 9100:2016 EN 9100:2009 EN 9100:2016 EN 9100: Context of the organization 4 Quality management system 4.2 Understanding the organization and its context Understanding the needs and expectations of interested parties 4.3 Determining the scope of the quality management system Quality management system and its processes 4 Quality management system Management review Application Quality manual Quality management system requirements 5 Leadership 5 Management responsibility 5.1 Leadership and commitment 5.1 Management commitment Management commitment Customer focus 5.2 Customer focus 5.2 Policy 5.3 Quality policy Developing the Quality Policy 5.3 Quality policy Communicating the Quality Policy 5.3 Quality policy 5.3 Organizational roles, responsibilities and authorities Responsibility and authority Management representative Quality management system planning 6 Planning Quality management system planning 6.1 Actions to address risks and opportunities Quality management system planning Preventive action 6.2 Quality objectives and planning to achieve them 5. Quality objectives 6.3 Planning of changes Quality management system planning 7 Support 6 Resource management 7.1 Resources 6 Resource management Provision of resources People 6.1 Provision of resources Infrastructure 6.3 Infrastructure Environment for the operation of processes 6.4 Work environment Monitoring and measuring resources 7.6 Control of monitoring and measuring equipment Control of monitoring and measuring equipment Measurement traceability 7.6 Control of monitoring and measuring equipment Organizational knowledge 7.2 Competence Competence, training and awareness 7.3 Awareness Competence, training and awareness 7.4 Communication Internal communication 7.5 Documented information 4.2 Documentation requirements TÜV SÜD Akademie GmbH
2 EN 9100:2016 EN 9100: Creating and updating Control of documented information Control of documents Control of records Control of documents Control of records 8 Operation 7 Product realization 8.1 Operational planning and control 7.1 Planning of product realization Operation risk management Risk management Configuration management Configuration management Product safety Design and development planning Prevention of counterfeit products 8.2 Requirements for products and 7.2 Customer-related processes Customer communication Customer communication Determining of requirements related to products and Determination of requirements related to the product Review of the requirements related to products and Review of requirements related to the product Changes to requirements for products and Review of requirements related to the product 8.3 Design and development of products and 7.3 Design and development Design and development planning Design and development planning Design and development planning Design and development inputs Design and development inputs Design and development controls Design and development review Design and development verification Design and development validation Design and development outputs Design and development outputs Design and development changes Control of design and development changes 8.4 Control of externally provided processes, products and 7. Purchasing process Type and extent of control requirements Purchasing process Purchasing process Verification of purchased product Verification of externally provided products and Verification of purchased product Information for external providers Purchasing information Verification of purchased product 8.5 Production and service provision 7.5 Production and service provision Control of production and service provision Control of production equipment, tools and software Control of production and service provision Validation of processes for production and service provision Control of production equipment, tools and software Validation and control of special processes Validation and control of special processes Production process verification Production process verification
3 EN 9100:2016 EN 9100: Identification and traceability Identification and traceability Property belonging to customers or external providers Customer property Preservation Preservation of product Post-delivery activities Control of production and service provision Post-delivery support Control of changes Control of design and development changes 8.6 Release of products and Verification of purchased product Monitoring and measurement of product 8.7 Control of nonconforming outputs 8.3 Control of nonconforming product 9 Performance evaluation 8 Measurement, analysis and improvement 9.1 Monitoring, measurement, analysis and evaluation 8 Measurement, analysis and improvement Monitoring and measurement processes Customer satisfaction Customer satisfaction Analysis and evaluation 8.4 Analysis of data 9.2 Internal audit Internal audit 9.3 Management review 5.6 Management review Management review input Review input Management review output Review output 10 Improvement 8.5 Improvement Continual improvement 10.2 Nonconformity and corrective action Continual improvement Control of nonconforming product Corrective action Continual improvement Preventive action
4 Correlation Matrix EN 9100:2009 EN 9100:2016 EN 9100:2009 EN 9100: Quality management system requirements Documentation requirements 7.5 Documented information Quality manual Control of documents Control of records Management responsibility 5 Leadership 5.1 Management commitment Context of the organization Understanding the organization and its context Understanding the needs and expectations of interested parties Quality management system and its processes Quality management system and its processes Control of externally provided processes, products and Determining the scope of the quality management system Quality management system and its processes Creating and updating Control of documented Information Creating and updating Control of documented Information Leadership and commitment 5.2 Customer focus Customer focus 5.3 Quality policy Policy Establishing the quality policy Communicating the quality policy 5.4 Planning 6 Planning 5. Quality objectives 6.2 Quality objectives and planning to achieve them Quality management system planning Organizational roles, responsibilities and authorities planning Actions to address risks and opportunities Planning of changes 5.5 Responsibility, authority and communication 5 Leadership Responsibility and authority 5.3 Organizational roles, responsibilities and authorities Management representative 5.3 Organizational roles, responsibilities and authorities Internal communication 7.4 Communication 5.6 Management review Context of the organization Understanding the organization and its context Understanding the needs and expectations of interested parties Management review Review input Management review input Review output Management review output
5 EN 9100:2009 EN 9100: Resource management Provision of resources Support Resources People 6.2 Human resources 7.2 Competence Competence Competence, training and awareness Competence Awareness 6.3 Infrastructure Infrastructure 6.4 Work environment Environment for the operation of processes 7 Product realization 8 Operation 7.1 Planning of product realization 8.1 Operational planning and control Project management 8.1 Operational planning and control Risk management Operational risk management Configuration management Configuration management Control of work transfers 8.1 Operational planning and control 7.2 Customer-related processes 8.2 Requirements to products and Determination of requirements related to the product Requirements related for products and Review of requirements related to the product Review of requirements related to products and Changes to requirements for products and Customer communication Customer communication 7.3 Design and development 8.3 Design and development of products and Design and development planning Design and development planning Product safety Design and development inputs Design and development inputs Design and development outputs Design and development outputs Design and development review Design and development controls Design and development verification Design and development controls Design and development validation Design and development controls Design and development verification and validation 8.3. (no title) testing Design and development verification and validation documentation 8.3. (no title) Control of design and development changes Design and development changes Control of changes 7.4 Purchasing 8.4 Control of externally provided processes, products and 7. Purchasing process Control of externally provided processes, products and Type and extent of control Purchasing information Information for external providers
6 EN 9100:2009 EN 9100: Verification of purchased product Type and extent of control Information for external providers Release of products and 7.5 Production and service provision 8.5 Production and service provision Control of production and service provision Control of production and service provision Post-delivery activities Production process verification Production process verification Control of production process changes 8.1 Operational planning and control Control of production equipment, tools and software Control of production equipment, tools and software Post-delivery support Post-delivery activities Validation of processes for production and service Control of production and service provision provision Identification and traceability Identification and traceability Customer property Property belonging to customers or external providers Preservation of product Preservation 7.6 Control of monitoring and measuring equipment Measurement, analysis and improvement Monitoring and measuring resources Measurement traceability Performance evaluation Monitoring, measurement, analysis and evaluation Monitoring and measurement 9.1 Monitoring, measurement, analysis and evaluation Customer satisfaction Customer satisfaction Internal audit 9.2 Internal audit Monitoring and measurement of processes Monitoring and measurement of product 8.6 Release of products and 8.3 Control of nonconforming product Control of nonconforming outputs Nonconformity and corrective action 8.4 Analysis of data Analysis and evaluation 8.5 Improvement 10 Improvement Continual improvement Continual Improvement Corrective action 10.2 Nonconformity and corrective action Preventive action Actions to address risks and opportunities (see and 6.1.2) Continual improvement TÜV SÜD Akademie GmbH Westendstrasse Munich Telephone +49 (0) akd.managementsysteme@tuev-sued.de AC110-ggüDINEN9100LuftE-pb-210x
Comparison: ISO 45001:2018 versus BS OHSAS 18001:2007
 Comparison: ISO 45001:2018 versus BS OHSAS 18001:2007 ISO 45001:2018 BS OHSAS 18001:2007 1 Scope 1 Scope 2 Normative references 2 Reference publications 3 Terms and definitions 3 Terms and definitions
Comparison: ISO 45001:2018 versus BS OHSAS 18001:2007 ISO 45001:2018 BS OHSAS 18001:2007 1 Scope 1 Scope 2 Normative references 2 Reference publications 3 Terms and definitions 3 Terms and definitions
Correlation matrices between ISO 9001:2008 and ISO 9001:2015
 Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
Correlation matrices between ISO 9001:2008 and ISO 9001:2015 ISO 9001:2015 ISO 9001:2008 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system 4.1 Understanding the organization
4. Quality management system 4. Context of the organization. 5 Management responsibility 5 Leadership
 AS 9100:2009 (Rev.C) AS 9100:2016 (Rev.D) 4. Quality management system 4. Context of the organization 4.1 General requirements 4.4 Quality management system and its processes products and 4.2 Documentation
AS 9100:2009 (Rev.C) AS 9100:2016 (Rev.D) 4. Quality management system 4. Context of the organization 4.1 General requirements 4.4 Quality management system and its processes products and 4.2 Documentation
9110 Correlation matrices
 9110 Correlation matrices 9110:2016 to 9110:2012 9110:2012 to 9110:2016 This document provides correlation matrices from 9110:2016 to 9110:2012 and 9110:2012 to 9110:2016. This document can be used to
9110 Correlation matrices 9110:2016 to 9110:2012 9110:2012 to 9110:2016 This document provides correlation matrices from 9110:2016 to 9110:2012 and 9110:2012 to 9110:2016. This document can be used to
ISO/TC 176/SC 2 Document N1224, July 2014
 ISO/TC 176/SC 2 Document N1224, July 2014 Correlation matrices between ISO 9001:2008 and ISO/DIS 9001 This document gives correlation matrices from ISO 9001:2008 to the current Draft International Standard
ISO/TC 176/SC 2 Document N1224, July 2014 Correlation matrices between ISO 9001:2008 and ISO/DIS 9001 This document gives correlation matrices from ISO 9001:2008 to the current Draft International Standard
ISO 13485: :2015 CLIENT TRANSITION CHECKLIST
 - 9001:2015 CLIENT TRANSITION CHECKLIST Audit Conclusions: All requirements have been addressed. The organization is recommended for ISO 13485:2016 certification. Recommendation for registration is dependent
- 9001:2015 CLIENT TRANSITION CHECKLIST Audit Conclusions: All requirements have been addressed. The organization is recommended for ISO 13485:2016 certification. Recommendation for registration is dependent
Quality Systems Compliance LLC is your Compliance Partner
 Mark Durivage, Managing Principal Consultant and Member Quality Systems Compliance LLC 3118 Chanson Valley Road Lambertville, MI 48144 mark.durivage@qscompliance.com www.qscompliance.com 419-265-2862 This
Mark Durivage, Managing Principal Consultant and Member Quality Systems Compliance LLC 3118 Chanson Valley Road Lambertville, MI 48144 mark.durivage@qscompliance.com www.qscompliance.com 419-265-2862 This
Correlation Matrix & Change Summary
 The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
The correlation matrix compares the new requirements of ISO 9001:2015 to the requirements of ISO 9001:2008, and provides a summary of the changes. Correlation Matrix & Change Summary Introduction Correlation
Business Management System: Requirements 00-BMS-002
 Business Management System: Requirements 00-BMS-002 Preface This document defines the applicability of International s requirements to the Business Management System (BMS) processes implemented at GM Nameplate
Business Management System: Requirements 00-BMS-002 Preface This document defines the applicability of International s requirements to the Business Management System (BMS) processes implemented at GM Nameplate
Sections of the Standard. Evidence / Comments. (Y) / Nonconforming (NC)
 Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
Date: Sections of the Standard 4 Context of the Organization 4.1 Understanding the organization and its context 4.2 Understanding the needs and expectations of interested parties 4.3 Determining the scope
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT
 INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
INTEGRATING ISO 9000 METHODOLOGIES WITH PROJECT QUALITY MANAGEMENT M a r ch 2015 OBJECTIVE ISO and Project Quality Management Process Are they different or the same? ISO 9000 QMS FAMILY ISO 9000:2005 Vocabulary
UNIVERSITY OF NAIROBI
 Q# UNIVERSITY OF NAIROBI 4 CONTEXTS OF THE ORGANIZATION Yes No Remarks 4.1 UNDERSTANDING THE ORGANIZATION AND ITS CONTEXT 1 Have we determined the external and internal issues that are relevant to our
Q# UNIVERSITY OF NAIROBI 4 CONTEXTS OF THE ORGANIZATION Yes No Remarks 4.1 UNDERSTANDING THE ORGANIZATION AND ITS CONTEXT 1 Have we determined the external and internal issues that are relevant to our
QUALITY POLICIES HANDBOOK
 QUALITY POLICIES HANDBOOK Origination Date: (mo/yr) Document Identifier: Date: Document Status: Document Link: Latest Revision Date Draft, Redline, Released, Obsolete Location on Server (if used) Abstract:
QUALITY POLICIES HANDBOOK Origination Date: (mo/yr) Document Identifier: Date: Document Status: Document Link: Latest Revision Date Draft, Redline, Released, Obsolete Location on Server (if used) Abstract:
ISO 9001:2015. Presented By: ASEAN Eng. DEXTER T. CHUA, PIE. Conference Room, University of Mindanao March 17, 2017
 ISO 9001:2015 Presented By: ASEAN Eng. DEXTER T. CHUA, PIE Conference Room, University of Mindanao March 17, 2017 @CQL Business Systems Consulting (11-2015) ISO The International Organization for Standardization
ISO 9001:2015 Presented By: ASEAN Eng. DEXTER T. CHUA, PIE Conference Room, University of Mindanao March 17, 2017 @CQL Business Systems Consulting (11-2015) ISO The International Organization for Standardization
Correlation Matrix. ISO 9001:2008 and ISO 9001: INTRODUCTION CORRELATION MATRIX ISO 9001:2008 AND ISO 9001:2015
 Correlation Matrix ISO 9001:2008 and ISO 9001:2015 INTRODUCTION This document provides a correlation between the requirements of ISO 9001:2008 and the ISO 9001:2015 standard. The document will provide
Correlation Matrix ISO 9001:2008 and ISO 9001:2015 INTRODUCTION This document provides a correlation between the requirements of ISO 9001:2008 and the ISO 9001:2015 standard. The document will provide
QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES
 QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Policies and Procedures Latest Revision Date Abstract: This handbook documents
QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Policies and Procedures Latest Revision Date Abstract: This handbook documents
QM-1 Supplement QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT. Revision 7. Quality Director / Management Representative n. B.
 QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT Revision 7 APPROVED BY: TITLE DATE B. Olevson Quality Director / Management Representative 07 3-0 n DOCUMENT REVISION HISTORY Paragraph Revisions: or Rev: Date:
QUALITY MANAGEMENT SYSTEMS MANUAL SUPPLEMENT Revision 7 APPROVED BY: TITLE DATE B. Olevson Quality Director / Management Representative 07 3-0 n DOCUMENT REVISION HISTORY Paragraph Revisions: or Rev: Date:
ISO 9001:2015. Presented By: ASEAN Eng. DEXTER T. CHUA, PIE. November 25, Business Systems Consulting ( )
 ISO 9001:2015 Presented By: ASEAN Eng. DEXTER T. CHUA, PIE November 25, 2016 @CQL Business Systems Consulting (11-2015) ISO The International Organization for Standardization based in Geneva, Switzerland
ISO 9001:2015 Presented By: ASEAN Eng. DEXTER T. CHUA, PIE November 25, 2016 @CQL Business Systems Consulting (11-2015) ISO The International Organization for Standardization based in Geneva, Switzerland
Clause Map IATF 16949:2016 to ISO/TS 16949:2009
 Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
Table of Contents Table of Contents Foreword Foreword + Foreword Automotive QMS Standard + History + Goal + 0.5 Goal of this Technical Specification + Remarks for Certification + Remarks for Certification
9100 Team July, IAQG is a trademark the International Aerospace Quality Group. Copyright 2014 IAQG. All rights reserved.
 9100 Series 2016 Revision Overview 9100 Team July, 2014 1 9100 Revision The Plan 9100 Series Revision High Level Plan The 9100 is based on ISO 9001 and is thus affected by the ISO TC176 revision activity
9100 Series 2016 Revision Overview 9100 Team July, 2014 1 9100 Revision The Plan 9100 Series Revision High Level Plan The 9100 is based on ISO 9001 and is thus affected by the ISO TC176 revision activity
Continual Improvement
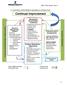 15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
15. CONTINUAL IMPROVEMENT SEQUENCE & INTERACTION Continual Customer and Stakeholders Requirements Resource / Support Finance Document Control Information System Human Resources Competence Training Performance
AESOP 15604; ISSUE 2; STATUS PENDING APPROVAL; AUTHORITY CARL BLAZIK This document is the property of NSF ISR. Page 1 of 9
 This document is the property of NSF ISR. Page 1 of 9 ISO 9001 Quality Management Systems registration provides a set of uniform requirements for a quality management system. A number of quality management
This document is the property of NSF ISR. Page 1 of 9 ISO 9001 Quality Management Systems registration provides a set of uniform requirements for a quality management system. A number of quality management
ISO 9001: 2015 Quality Management System Certification. Awareness Training
 ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
ISO 9001: 2015 Quality Management System Certification Awareness Training ISO 9001: 2015 STRUCTURE The new standard is modeled around the ISO Directive Annex SL, a high level structure (HSL) based on the
CO-ORDINATION OF NOTIFIED BODIES PPE Regulation 2016/425 RECOMMENDATION FOR USE
 CO-ORDINATION OF NOTIFIED BODIES PPE Regulation 2016/425 PPE-R/00.018 Version 1 RECOMMENDATION FOR USE Number of pages: 5 Approval stage : Approved on : Origin : Horizontal Committee, C2D Ad hoc group
CO-ORDINATION OF NOTIFIED BODIES PPE Regulation 2016/425 PPE-R/00.018 Version 1 RECOMMENDATION FOR USE Number of pages: 5 Approval stage : Approved on : Origin : Horizontal Committee, C2D Ad hoc group
This document is a preview generated by EVS
 2015 by SNV / Foqus Quality AG / MSB Bartels & Partner Consulting page: 1 von 77 0. Introduction On the occasion of the complete revision of the Standard ISO 9001 the Audit-Checklist (edition 2008) has
2015 by SNV / Foqus Quality AG / MSB Bartels & Partner Consulting page: 1 von 77 0. Introduction On the occasion of the complete revision of the Standard ISO 9001 the Audit-Checklist (edition 2008) has
ISO 9001:2015. October 5 th, Brad Fischer.
 ISO 9001:2015 October 5 th, 2017 Brad Fischer www.sdmanufacturing.com Purpose of presentation Provide a summary of notable changes from ISO 9001:2008 to ISO 9001:2015 Key perspectives ISO 9001 needs to
ISO 9001:2015 October 5 th, 2017 Brad Fischer www.sdmanufacturing.com Purpose of presentation Provide a summary of notable changes from ISO 9001:2008 to ISO 9001:2015 Key perspectives ISO 9001 needs to
ISO 9001:2000 Making the Transition
 ISO 9001:2000 Making the Transition ISO 9001:2000 Making the Transition Annette Dennis McCully Debra L. Reese Excerpts from interviews conducted by the author with Garnett Davis and William Poliseo published
ISO 9001:2000 Making the Transition ISO 9001:2000 Making the Transition Annette Dennis McCully Debra L. Reese Excerpts from interviews conducted by the author with Garnett Davis and William Poliseo published
Executive Overview. Transitioning to ISO 9001:2015 Quality Management System. Biafore Associates Inc. Overview Objectives
 Executive Transitioning to ISO 9001:2015 Quality Management System Biafore Associates Inc. This guideline is for training purposes only; Not ISO controlled Objectives The overview objectives are as follows:
Executive Transitioning to ISO 9001:2015 Quality Management System Biafore Associates Inc. This guideline is for training purposes only; Not ISO controlled Objectives The overview objectives are as follows:
ISO 9001:2015 Gap Analysis Check Sheet
 CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context Organization shall determine external and internal issues that are relevant to its purpose and strategic direction and that
CONTEXT OF THE ORGANIZATION 4.1 Understanding the organization and its context Organization shall determine external and internal issues that are relevant to its purpose and strategic direction and that
SUPPLIER SURVEY FORM Instructions
 SUPPLIER SURVEY FORM Instructions 1. The following Supplier Survey was developed by Vishay Measurements Group, Inc. to assess and document the capability of its supplier base. 2. The Supplier Survey is
SUPPLIER SURVEY FORM Instructions 1. The following Supplier Survey was developed by Vishay Measurements Group, Inc. to assess and document the capability of its supplier base. 2. The Supplier Survey is
ISO 9001: Understanding the changes
 ISO 9001: 2015 Understanding the changes Tel: 01952 288325 Email: info@interface-nrm.co.uk Why has ISO 9001 been Revised? ISO 9001, the world's leading Quality Management System has been revised to a new
ISO 9001: 2015 Understanding the changes Tel: 01952 288325 Email: info@interface-nrm.co.uk Why has ISO 9001 been Revised? ISO 9001, the world's leading Quality Management System has been revised to a new
Summary of ISO 9001:2015 New and Changed Requirements
 This is a summary of the new and changed ISO 9001:2015 requirements compared to ISO 9001:2008. 4. Context of the Organization 4.1 Changes Understanding the Organization and its Context New requirement
This is a summary of the new and changed ISO 9001:2015 requirements compared to ISO 9001:2008. 4. Context of the Organization 4.1 Changes Understanding the Organization and its Context New requirement
ISO 9001 PMA and 14 CFR 21 QUALITY POLICIES HANDBOOK
 ISO 9001 PMA and 14 CFR 21 QUALITY POLICIES HANDBOOK Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Quality Policies Handbook Latest Revision Date Orig Abstract: This quality
ISO 9001 PMA and 14 CFR 21 QUALITY POLICIES HANDBOOK Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Quality Policies Handbook Latest Revision Date Orig Abstract: This quality
ISO 9001:2015 QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES
 ISO 9001:2015 QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 Policies and Procedures Latest Revision Date Abstract: This handbook
ISO 9001:2015 QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 Policies and Procedures Latest Revision Date Abstract: This handbook
This is a preview - click here to buy the full publication. IEC Quality Assessment System for Electronic Components (IECQ System)
 QC 080000 Edition 4.0 2017-05 IECQ PUBLICATION IEC Quality Assessment System for Electronic Components (IECQ System) Hazardous Substance Process Management (HSPM) System Requirements INTERNATIONAL ELECTROTECHNICAL
QC 080000 Edition 4.0 2017-05 IECQ PUBLICATION IEC Quality Assessment System for Electronic Components (IECQ System) Hazardous Substance Process Management (HSPM) System Requirements INTERNATIONAL ELECTROTECHNICAL
ISO 9001:2015 GAP ANALYSIS CHECKLIST
 Context of the Organization 4.1 Understanding the organization and its context Organization shall determine external and internal issues that are relevant to its purpose and strategic direction and that
Context of the Organization 4.1 Understanding the organization and its context Organization shall determine external and internal issues that are relevant to its purpose and strategic direction and that
AP Quality Consulting works with you to provide a custom solution
 AP Quality Consulting works with you to provide a custom solution ISO 9001, ISO/IEC 17025, AS6081, AS5553, AS6171 (in development) Quality auditing and management of internal audit program Corporate training
AP Quality Consulting works with you to provide a custom solution ISO 9001, ISO/IEC 17025, AS6081, AS5553, AS6171 (in development) Quality auditing and management of internal audit program Corporate training
QMS Team: MR and all HODs (Internal Auditors) MR March 10. Quality policy Define quality policy The Steering committee Objectives and targets
 QMS Roles, Responsibility and Authority Process Clause Activities Records Required Responsibility Authority Deadline Clause 4: Process Development 4.1 Develop processes and sequence, operation controls
QMS Roles, Responsibility and Authority Process Clause Activities Records Required Responsibility Authority Deadline Clause 4: Process Development 4.1 Develop processes and sequence, operation controls
QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES
 Your Company Name QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Policies and Procedures Latest Revision Date Abstract:
Your Company Name QUALITY MANAGEMENT SYSTEM POLICIES AND PROCEDURES Origination Date: XXXX Document Identifier: Date: Document Revision: QMS-00 QMS Policies and Procedures Latest Revision Date Abstract:
AS9100 PMA and 14 CFR 21 QUALITY HANDBOOK
 AS9100 PMA and 14 CFR 21 QUALITY HANDBOOK Origination Date: XXXX Document Identifier: Date: Document Status: Document Link: Latest Revision Date Draft, Redline, Released, Obsolete Location on Server (if
AS9100 PMA and 14 CFR 21 QUALITY HANDBOOK Origination Date: XXXX Document Identifier: Date: Document Status: Document Link: Latest Revision Date Draft, Redline, Released, Obsolete Location on Server (if
NATO STANDARD AQAP-2110 NATO QUALITY ASSURANCE REQUIREMENTS FOR DESIGN, DEVELOPMENT AND PRODUCTION
 NATO STANDARD AQAP-2110 NATO QUALITY ASSURANCE REQUIREMENTS FOR DESIGN, DEVELOPMENT AND PRODUCTION Edition D Version 1 JUNE 2016 NORTH ATLANTIC TREATY ORGANIZATION ALLIED QUALITY ASSURANCE PUBLICATION
NATO STANDARD AQAP-2110 NATO QUALITY ASSURANCE REQUIREMENTS FOR DESIGN, DEVELOPMENT AND PRODUCTION Edition D Version 1 JUNE 2016 NORTH ATLANTIC TREATY ORGANIZATION ALLIED QUALITY ASSURANCE PUBLICATION
BUSINESS ASSURANCE GUIDANCE FOR THE TRANSITIONING FROM ISO 9001:2008 TO ISO 9001:2015 SAFER, SMARTER, GREENER
 BUSINESS ASSURANCE GUIDANCE FOR THE TRANSITIONING FROM ISO 9001:2008 TO ISO 9001:2015 SAFER, SMARTER, GREENER 2 ISO 9001 ABOUT THIS DOCUMENT The purpose of this document is to provide you with some useful
BUSINESS ASSURANCE GUIDANCE FOR THE TRANSITIONING FROM ISO 9001:2008 TO ISO 9001:2015 SAFER, SMARTER, GREENER 2 ISO 9001 ABOUT THIS DOCUMENT The purpose of this document is to provide you with some useful
NATO STANDARD AQAP-2310 NATO QUALITY ASSURANCE REQUIREMENTS FOR AVIATION, SPACE AND DEFENCE SUPPLIERS
 NATO STANDARD AQAP-2310 NATO QUALITY ASSURANCE REQUIREMENTS FOR AVIATION, SPACE AND DEFENCE SUPPLIERS Edition B Version 1 DECEMBER 2017 NORTH ATLANTIC TREATY ORGANIZATION ALLIED QUALITY ASSURANCE PUBLICATION
NATO STANDARD AQAP-2310 NATO QUALITY ASSURANCE REQUIREMENTS FOR AVIATION, SPACE AND DEFENCE SUPPLIERS Edition B Version 1 DECEMBER 2017 NORTH ATLANTIC TREATY ORGANIZATION ALLIED QUALITY ASSURANCE PUBLICATION
Main changes to ISO 9001 from the 2000 version to the 2008 version
 Introduction This document has been produced to outline the main changes to ISO 9001 from the 2000 version to the 2008 version, not all changes have been mentioned as most of them are typographical changes
Introduction This document has been produced to outline the main changes to ISO 9001 from the 2000 version to the 2008 version, not all changes have been mentioned as most of them are typographical changes
ISO/DIS 9001: 2014 comparison with ISO 9001:2008. ISO 9001:2015 Updates. (Based on Draft International Standard, DIS) ISO/DIS 9001 ISO 9001:2008
 ISO 9001:2015 Updates (Based ondraft International Standard, DIS) August 2014 Page 1 ISO 9001:2015 Updates (Based on Draft International Standard, DIS) ISO/DIS 9001: 2014 comparison with ISO 9001:2008
ISO 9001:2015 Updates (Based ondraft International Standard, DIS) August 2014 Page 1 ISO 9001:2015 Updates (Based on Draft International Standard, DIS) ISO/DIS 9001: 2014 comparison with ISO 9001:2008
ISO 9001: 2000 (December 13, 2000) QUALITY MANAGEMENT SYSTEM DOCUMENTATION OVERVIEW MATRIX
 In completing your Documented Quality Management System Review, it is important that the following matrix be completed and returned to us as soon as possible. This will save time during the review and
In completing your Documented Quality Management System Review, it is important that the following matrix be completed and returned to us as soon as possible. This will save time during the review and
PRI REGISTRAR AUDITOR ADVISORY #93
 PRI REGISTRAR AUDITOR ADVISORY #93 TO: PRI Registrar AS Auditors, Client Managers and AS Expert Reviewers FROM: Samantha Brock Production, Planning & Control Coordinator DATE: November 26, 2016 RE: AS
PRI REGISTRAR AUDITOR ADVISORY #93 TO: PRI Registrar AS Auditors, Client Managers and AS Expert Reviewers FROM: Samantha Brock Production, Planning & Control Coordinator DATE: November 26, 2016 RE: AS
ISO9001:2015 and ISO14001:2014 Transition Update March 2017
 ISO9001:2015 and ISO14001:2014 Transition Update March 2017 TIMESCALES The ISO 9001:2015 and ISO14001:2015 standards were issued on the 15 th September 2015 and they replace ISO 9001:2008 and ISO14001:2004.
ISO9001:2015 and ISO14001:2014 Transition Update March 2017 TIMESCALES The ISO 9001:2015 and ISO14001:2015 standards were issued on the 15 th September 2015 and they replace ISO 9001:2008 and ISO14001:2004.
สร ปตารางเปร ยบเท ยบข อก าหนดระหว าง ISO 9001:2008 และ ISO 9001:2015/ ISO 14001:2004 และ ISO 14001:2015
 สร ปตารางเปร ยบเท ยบข อก าหนดระหว าง ISO 9001:2008 และ ISO 9001:2015/ ISO 14001:2004 และ ISO 14001:2015 ดร.ป ยะช ย จ นทรวงศ ไพศาล เปร ยบเท ยบข อก าหนดระหว าง ISO 9001:2008 และ ISO 9001:2015 ISO 9001:2008
สร ปตารางเปร ยบเท ยบข อก าหนดระหว าง ISO 9001:2008 และ ISO 9001:2015/ ISO 14001:2004 และ ISO 14001:2015 ดร.ป ยะช ย จ นทรวงศ ไพศาล เปร ยบเท ยบข อก าหนดระหว าง ISO 9001:2008 และ ISO 9001:2015 ISO 9001:2008
Summary of changes between ISO 9001:2008 and ISO/CD 9001
 Summary of changes between ISO 9001:2008 and ISO/CD 9001 Introduction This document is intended to summarise the proposed changes to ISO 9001:2008 as detailed in the Committee Draft (CD). It does not contain
Summary of changes between ISO 9001:2008 and ISO/CD 9001 Introduction This document is intended to summarise the proposed changes to ISO 9001:2008 as detailed in the Committee Draft (CD). It does not contain
ISO High Level Standards (HSL)
 ISO High Level Standards (HSL) 4. Context of the organization Understanding the organization and its context Understanding the needs and expectations of interested parties Determining the scope of the
ISO High Level Standards (HSL) 4. Context of the organization Understanding the organization and its context Understanding the needs and expectations of interested parties Determining the scope of the
Supplier Quality System Survey
 Supplier Division Address Date: Quality Contact: Reports to: Title: Title: Phone Fax e-mail Plant size (ft 2 ): Union: Contract expires: Total Employment: Direct Labor: Shifts: QA employees: This report
Supplier Division Address Date: Quality Contact: Reports to: Title: Title: Phone Fax e-mail Plant size (ft 2 ): Union: Contract expires: Total Employment: Direct Labor: Shifts: QA employees: This report
ISO 13485:2016 Mandatory documentation requirements & MyEasyISO
 ts & Mandatory documents All below mandatory documents can be created using Documented information module of. An organization can use their own procedures but if required can provide these procedures to
ts & Mandatory documents All below mandatory documents can be created using Documented information module of. An organization can use their own procedures but if required can provide these procedures to
ISO 9001:2015 Readiness Review
 ISO 9001:2015 Readiness Review Company Name Address Certification No. Contact Name Job Title Telephone Email BSI is committed to ensuring a smooth assessment for all clients wishing to certify to ISO 9001:2015,
ISO 9001:2015 Readiness Review Company Name Address Certification No. Contact Name Job Title Telephone Email BSI is committed to ensuring a smooth assessment for all clients wishing to certify to ISO 9001:2015,
NATO STANDARD AQAP-2110 NATO QUALITY ASSURANCE REQUIREMENTS FOR DESIGN, DEVELOPMENT AND PRODUCTION
 NATO STANDARD AQAP-2110 NATO QUALITY ASSURANCE REQUIREMENTS FOR DESIGN, DEVELOPMENT AND PRODUCTION Edition D Version 1 JUNE 2016 NORTH ATLANTIC TREATY ORGANIZATION ALLIED QUALITY ASSURANCE PUBLICATION
NATO STANDARD AQAP-2110 NATO QUALITY ASSURANCE REQUIREMENTS FOR DESIGN, DEVELOPMENT AND PRODUCTION Edition D Version 1 JUNE 2016 NORTH ATLANTIC TREATY ORGANIZATION ALLIED QUALITY ASSURANCE PUBLICATION
PRODUCTS AND SERVICES:
 COMPANY INFORMATION: Company Name: Newcastle Aviation Partners, LLC Address: 3201 West County Road 42, Unit 104 Burnsville, MN 55306 Phone: 952-223-0317 Facsimile: 952-223-4470 AOG phone number: 952-223-0317,
COMPANY INFORMATION: Company Name: Newcastle Aviation Partners, LLC Address: 3201 West County Road 42, Unit 104 Burnsville, MN 55306 Phone: 952-223-0317 Facsimile: 952-223-4470 AOG phone number: 952-223-0317,
Quality Manual ISSUED JANUARY Approved By: January 12, 2004 (President & Chief Executive Officer)
 Quality Manual ISSUED JANUARY 2004 Approved By: January 12, 2004 (President & Chief Executive Officer) (Date) Quality Policy To be the industrial control industry's most preferred supplier of sensor integration
Quality Manual ISSUED JANUARY 2004 Approved By: January 12, 2004 (President & Chief Executive Officer) (Date) Quality Policy To be the industrial control industry's most preferred supplier of sensor integration
ISO Food Safety Management System Compliance Summary
 ISO 22000 Clause ISO 9001 Clause FSMS Manual Reference Policy / Procedure Title 4. Quality Management System (ISO 9001) 4. Food Safety Quality Management System (ISO 22000) 4.1 General Requirements 4.1
ISO 22000 Clause ISO 9001 Clause FSMS Manual Reference Policy / Procedure Title 4. Quality Management System (ISO 9001) 4. Food Safety Quality Management System (ISO 22000) 4.1 General Requirements 4.1
How ISO 9001:2015 can improve your food safety management. Dr. David Rosenblatt Sher Consulting and Training
 How ISO 9001:2015 can improve your food safety management Dr. David Rosenblatt Sher Consulting and Training ISO management systems in the food industry A little bit of history 2000 ISO publishes the brand
How ISO 9001:2015 can improve your food safety management Dr. David Rosenblatt Sher Consulting and Training ISO management systems in the food industry A little bit of history 2000 ISO publishes the brand
Integrated Clause-byclause Guidance
 Integrated Clause-byclause Guidance ISO 9001:2015, ISO 14001:2015 & ISO 45001:2018 Table of Contents 1 INTRODUCTION... 4 2 IMPLEMENTATION & DEVELOPMENT... 5 2.1 MANAGING THE CHANGE... 6 2.2 TOP MANAGEMENT
Integrated Clause-byclause Guidance ISO 9001:2015, ISO 14001:2015 & ISO 45001:2018 Table of Contents 1 INTRODUCTION... 4 2 IMPLEMENTATION & DEVELOPMENT... 5 2.1 MANAGING THE CHANGE... 6 2.2 TOP MANAGEMENT
Contact information for the purchase of this quality manual. Paid version includes all the contents listed in the table of contents.
 Distribution number : Management classification c o n t r o l d o c u m e n t Document numbers QMS-A-01 Date of enactment 07/01/2016 Date of revision Revision numbers 1 Contact information for the purchase
Distribution number : Management classification c o n t r o l d o c u m e n t Document numbers QMS-A-01 Date of enactment 07/01/2016 Date of revision Revision numbers 1 Contact information for the purchase
84 Ways To Go Beyond ISO Rick Hill 13 October 2017
 84 Ways To Go Beyond ISO 9001 Rick Hill 13 October 2017 ISO 9001 is a great QMS standard but All the requirements of this International Standard are generic and are intended to be applicable to any organization,
84 Ways To Go Beyond ISO 9001 Rick Hill 13 October 2017 ISO 9001 is a great QMS standard but All the requirements of this International Standard are generic and are intended to be applicable to any organization,
ISO9001 QUALITY POLICY MANUAL
 1 OF 26 Metalife Industries, Inc. Serial No.: Master Revision: 1 Issue Date: October 14, 2011 Originator: Becky Wentling ISO9001 QUALITY POLICY MANUAL Prepared By (Document Controller): Becky Wentling
1 OF 26 Metalife Industries, Inc. Serial No.: Master Revision: 1 Issue Date: October 14, 2011 Originator: Becky Wentling ISO9001 QUALITY POLICY MANUAL Prepared By (Document Controller): Becky Wentling
ASQ Madison, WI Section Using your ISO 9001:2008 QMS to Plan for the 2015 Changes
 ASQ Madison, WI Section 1217 Using your ISO 9001:2008 QMS to Plan for the 2015 Changes SESSION AGENDA High Level Review of Requirements Historical Perspective Basic Requirements Change It s a Process Inputs,
ASQ Madison, WI Section 1217 Using your ISO 9001:2008 QMS to Plan for the 2015 Changes SESSION AGENDA High Level Review of Requirements Historical Perspective Basic Requirements Change It s a Process Inputs,
Summary of changes between ISO 9001:2008 and ISO/CD 9001
 Summary of changes between ISO 9001:2008 and ISO/CD 9001 Introduction This document is intended to summarise the proposed changes to ISO 9001:2008 as detailed in the Committee Draft (CD). It does not contain
Summary of changes between ISO 9001:2008 and ISO/CD 9001 Introduction This document is intended to summarise the proposed changes to ISO 9001:2008 as detailed in the Committee Draft (CD). It does not contain
PRECISE INDUSTRIES INC. Quality Manual
 PRECISE INDUSTRIES INC Revision N Issued July 5, 2017 Conforms to AS9100 Rev. D and ISO 9001:2015 Copyright Year2017 [PRECISE INDUSTRIES INC]; all rights reserved. This document may contain proprietary
PRECISE INDUSTRIES INC Revision N Issued July 5, 2017 Conforms to AS9100 Rev. D and ISO 9001:2015 Copyright Year2017 [PRECISE INDUSTRIES INC]; all rights reserved. This document may contain proprietary
Carbon of 7. IS-CMS-MUC/Mu Javier Castro. Telefax:
 TÜV SÜD Industrie Service GmbH 80684 Munich Germany CDM Executive Board DAP-PL-2885.99 DAP-IS-2886..00 DAP-PL-3089.00 DAP-PL-27222 DAP-IS-3516..01 DPT-ZE-3510.02 ZLS-ZE-219/99 ZLS-ZE-246/99 Your reference/letter
TÜV SÜD Industrie Service GmbH 80684 Munich Germany CDM Executive Board DAP-PL-2885.99 DAP-IS-2886..00 DAP-PL-3089.00 DAP-PL-27222 DAP-IS-3516..01 DPT-ZE-3510.02 ZLS-ZE-219/99 ZLS-ZE-246/99 Your reference/letter
ISO 9001:2008 Quality Management System QMS Manual
 2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
2501 Kutztown Road Reading, PA, 19605 Tel. 610-929-3330 Fax 610-921-6861 ISO 9001:2008 Quality Management System QMS Manual The information contained in this document is Fidelity Technologies Corporation
Electronics. Document: QUALITY MANUAL. Revision: W. Date: 08/22/2016
 Document: QUALITY MANUAL Revision: W Date: 08/22/2016 Electronics Gowanda Electronics One Magnetics Parkway Gowanda, NY 14070 Ph: 716-532-2234 Fax: 716-532-2702 www.gowanda.com Table of Contents Table
Document: QUALITY MANUAL Revision: W Date: 08/22/2016 Electronics Gowanda Electronics One Magnetics Parkway Gowanda, NY 14070 Ph: 716-532-2234 Fax: 716-532-2702 www.gowanda.com Table of Contents Table
Fitting ISO 9001:2000 into a 20 Element Quality System
 Fitting ISO 9001:2000 into a 20 Element Quality System ASQ Washington DC Section 0509 Rockville Maryland Presented by Norman P. Moreau, P.E., CSQE, CQA President Theseus Professional Services 410-857-0023
Fitting ISO 9001:2000 into a 20 Element Quality System ASQ Washington DC Section 0509 Rockville Maryland Presented by Norman P. Moreau, P.E., CSQE, CQA President Theseus Professional Services 410-857-0023
Quality Manual QM -07 Approval: D. Wheeler. AARD Spring & Stamping Quality Manual. Quality Manual. Page 1 of 24
 Quality Manual Page 1 of 24 ISO 9001:2015 Standard to Quality Manual Section Matrix ISO 9001:2015 Quality Manual Section 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system
Quality Manual Page 1 of 24 ISO 9001:2015 Standard to Quality Manual Section Matrix ISO 9001:2015 Quality Manual Section 1 Scope 1 Scope 1.1 General 4 Context of the organization 4 Quality management system
Monroe Engineering is committed to customer satisfaction; we strive for Continuous Improvement in our products and our people.
 Title: AS9100D Quality Manual Revision Date.: 05/04/2018 Page 1 of 22 The Quality Policy of Monroe Engineering is defined in the following statement: Monroe Engineering is committed to customer satisfaction;
Title: AS9100D Quality Manual Revision Date.: 05/04/2018 Page 1 of 22 The Quality Policy of Monroe Engineering is defined in the following statement: Monroe Engineering is committed to customer satisfaction;
ISO 9001:2015 TRANSITION INFORMATION
 ISO 9001:2015 TRANSITION INFORMATION ISO 9001:2015 Transition It Doesn t Need to be Difficult Whether your organization is transitioning from ISO 9001:2008 to the new ISO 9001:2015, or implementing an
ISO 9001:2015 TRANSITION INFORMATION ISO 9001:2015 Transition It Doesn t Need to be Difficult Whether your organization is transitioning from ISO 9001:2008 to the new ISO 9001:2015, or implementing an
IPD Quality Management System Supplier Questionnaire Instructions
 IPD Quality Management System Supplier Questionnaire Instructions 1. The following Supplier Questionnaire was developed by Industrial Parts Depot (IPD) to assess and document the capability of its supplier
IPD Quality Management System Supplier Questionnaire Instructions 1. The following Supplier Questionnaire was developed by Industrial Parts Depot (IPD) to assess and document the capability of its supplier
This document is a preview generated by EVS
 INTERNATIONAL STANDARD ISO 15378 Fourth edition 2017-09 Primary packaging materials for medicinal products Particular requirements for the application of ISO 9001:2015, with reference to good manufacturing
INTERNATIONAL STANDARD ISO 15378 Fourth edition 2017-09 Primary packaging materials for medicinal products Particular requirements for the application of ISO 9001:2015, with reference to good manufacturing
Patsy Brown Brown & Associates Quality Consulting, Inc. Pine Bluff, AR Documents. Standards ISO 9001:2008 ISO 22000:2005 PAS 220:2008
 Patsy Brown Brown & Associates Quality Consulting, Inc. Pine Bluff, AR www.brownqc.com Documents Standards ISO 9001:2008 PAS 220:2008 Supporting Documentation ISO 9000:2005 ISO 22004:2005 ISO 19011:2002
Patsy Brown Brown & Associates Quality Consulting, Inc. Pine Bluff, AR www.brownqc.com Documents Standards ISO 9001:2008 PAS 220:2008 Supporting Documentation ISO 9000:2005 ISO 22004:2005 ISO 19011:2002
Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry
 Addendum 1 June 2010 Effective Date: December 1, 2010 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry ANSI/API SPECIFICATION Q1 EIGHTH EDITION, DECEMBER 2007
Addendum 1 June 2010 Effective Date: December 1, 2010 Specification for Quality Programs for the Petroleum, Petrochemical and Natural Gas Industry ANSI/API SPECIFICATION Q1 EIGHTH EDITION, DECEMBER 2007
Desk Audit of. Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT
 Desk Audit of Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT-90-5001-02.1 Reviewed by: Element Requirements Applicable 1. Is a quality policy defined
Desk Audit of Based on Federal Transit Administration (FTA) Quality Assurance and Quality Control Guidelines FTA-IT-90-5001-02.1 Reviewed by: Element Requirements Applicable 1. Is a quality policy defined
ISO 9001:2015. Quality Management System. Manual
 ISO 9001:2015 Quality Management System Manual Introduction Company has made the Strategic Business Decision to develop and implement an effective Quality Management Systems (QMS) across all areas of the
ISO 9001:2015 Quality Management System Manual Introduction Company has made the Strategic Business Decision to develop and implement an effective Quality Management Systems (QMS) across all areas of the
25 D.L. Martin Drive Mercersburg, PA (717)
 QUALITY MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 14 August 2012 Michael A. White Manager, QA & Engineering D.L. Martin Co. Quality Manual UNCONTROLLED
QUALITY MANUAL D. L. MARTIN CO. 25 D.L. Martin Drive Mercersburg, PA 17236 (717) 328-2141 Revision 14 August 2012 Michael A. White Manager, QA & Engineering D.L. Martin Co. Quality Manual UNCONTROLLED
Quality Systems Manual Rev. NC Issued July 9 / 2018
 NMT Specialized Machining Inc 290 Shoemaker Street Kitchener, Ontario Canada N2E 3E1 Quality Systems Manual Rev. NC Issued July 9 / 2018 Conforms to AS9100 Rev D and ISO 9001:2015 Table of Contents Introduction
NMT Specialized Machining Inc 290 Shoemaker Street Kitchener, Ontario Canada N2E 3E1 Quality Systems Manual Rev. NC Issued July 9 / 2018 Conforms to AS9100 Rev D and ISO 9001:2015 Table of Contents Introduction
AS 9100 Rev C Quality Systems Manual AS-050C-QM
 AS 9100 Rev C Quality Systems Manual AS-050C-QM Innovative Control Systems, Inc. 10801 N. 24 th Ave. Suite 101-103 Phoenix, AZ 85029 U.S.A. www.icsaero.com +01-602-861-6984 VOICE +01-602-588-9440 FAX Table
AS 9100 Rev C Quality Systems Manual AS-050C-QM Innovative Control Systems, Inc. 10801 N. 24 th Ave. Suite 101-103 Phoenix, AZ 85029 U.S.A. www.icsaero.com +01-602-861-6984 VOICE +01-602-588-9440 FAX Table
ISO 14001:2004 Summary of significant changes
 Product information on certification to ISO 14001 Competence. Safety. Quality. A sustained economy is a law of common sense and our responsibility for the future. Circumspect, voluntary and systematic
Product information on certification to ISO 14001 Competence. Safety. Quality. A sustained economy is a law of common sense and our responsibility for the future. Circumspect, voluntary and systematic
Moving to the AS/EN 9100:2016 series. Transition Guide
 Moving to the AS/EN 9100:2016 series Transition Guide AS/EN 9100 series - Quality Management Systems for Aviation, Space and Defence - Transition Guide Successful aviation space and defence businesses
Moving to the AS/EN 9100:2016 series Transition Guide AS/EN 9100 series - Quality Management Systems for Aviation, Space and Defence - Transition Guide Successful aviation space and defence businesses
Quality Manual Revision: C Effective: 03/01/10
 TABLE OF CONTENTS DESCRIPTION SECTION PAGE INTRODUCTION 1.0 1 APPROVAL SIGNATURE PAGE 1.1 1 AMENDMENT RECORD 1.2 2 SCOPE 2.0 3 EXCLUSIONS 2.1 3 CORPORATE POLICY 3.0 3 QUALITY MANAGEMENT SYSTEM 4.0 4 GENERAL
TABLE OF CONTENTS DESCRIPTION SECTION PAGE INTRODUCTION 1.0 1 APPROVAL SIGNATURE PAGE 1.1 1 AMENDMENT RECORD 1.2 2 SCOPE 2.0 3 EXCLUSIONS 2.1 3 CORPORATE POLICY 3.0 3 QUALITY MANAGEMENT SYSTEM 4.0 4 GENERAL
Quality Manual Template ISO 9001:2015 Quality Management System
 Quality Manual Template Table of Contents 1 INTRODUCTION... 5 2 QUALITY MANAGEMENT PRINCIPLES... 6 3 REFERENCES & DEFINITIONS... 6 4 CONTEXT OF THE ORGANIZATION... 8 4.1 ORGANIZATIONAL CONTEXT... 8 4.2
Quality Manual Template Table of Contents 1 INTRODUCTION... 5 2 QUALITY MANAGEMENT PRINCIPLES... 6 3 REFERENCES & DEFINITIONS... 6 4 CONTEXT OF THE ORGANIZATION... 8 4.1 ORGANIZATIONAL CONTEXT... 8 4.2
ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015. Joan Richter ASQ Baltimore (Section 502) June 2016
 ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015 Joan Richter ASQ Baltimore (Section 502) June 2016 HISTORY OF ISO 9001 Standard (28 Years of ISO) 1987 Procedures
ISO 9001:2015: Change or Not to Change or How to Transition from ISO9001:2008 and ISO9001:2015 Joan Richter ASQ Baltimore (Section 502) June 2016 HISTORY OF ISO 9001 Standard (28 Years of ISO) 1987 Procedures
Med-Info. Directive 98/79/EC on in-vitro diagnostic medical devices. TÜV SÜD Product Service GmbH
 Med-Info International expert information for the medical device industry Directive 98/79/EC on in-vitro diagnostic medical devices Practice-oriented summary of the most important aspects and requirements
Med-Info International expert information for the medical device industry Directive 98/79/EC on in-vitro diagnostic medical devices Practice-oriented summary of the most important aspects and requirements
MANAGE YOUR TRANSITION ISO 9001 TO ISO GAP GUIDE
 MANAGE YOUR TRANSITION ISO 9001 TO ISO 27001 GAP GUIDE ISO 27001:2013 the Information Security Management Standard is one of the fastest growing standards right now; partly due to the ever evolving digital
MANAGE YOUR TRANSITION ISO 9001 TO ISO 27001 GAP GUIDE ISO 27001:2013 the Information Security Management Standard is one of the fastest growing standards right now; partly due to the ever evolving digital
Overview of Draft API Standard 20M
 Overview of Draft API Standard 20M Qualification of Suppliers of Machining Services for Use in the Petroleum and Natural Gas Industries API STANDARD 20M Slide 1 of 9 Overview Standard 20M provides methods
Overview of Draft API Standard 20M Qualification of Suppliers of Machining Services for Use in the Petroleum and Natural Gas Industries API STANDARD 20M Slide 1 of 9 Overview Standard 20M provides methods
ISO 9001:2015. Quality Manual Template.
 www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This quality manual is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
www.iso-9001-checklist.co.uk Insert your company s name or logo, and address. This quality manual is the property of Your Company. It must not be reproduced in whole or in part or otherwise disclosed without
Description of the certification procedure ISO/TS 16949:2009
 Description of the certification procedure ISO/TS 16949:2009 Certific ation Table of contents 1 RUELS... 2 2 DESCRIPTION OF SERVICES... 6 If you should require any further information then please do not
Description of the certification procedure ISO/TS 16949:2009 Certific ation Table of contents 1 RUELS... 2 2 DESCRIPTION OF SERVICES... 6 If you should require any further information then please do not
Quality and Medical Devices: ISO 13485:2003
 Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
Quality and Medical Devices: ISO 13485:2003 Quality Management System requirements for Medical Devices manufacturers O3 Enterprise s.r.l. ISO 13485 Quality Management Systems Medical Device O3 Enterprise
Supplier Quality Survey. 1. Type of Business: g) Commodities supplied? Supplier Changes/comments: 2. Headcount breakdown by group: Purchasing
 Supplier: Phone: Prime Contact/Title: Sales Contact/Title: Address: Fax: e-mail address e-mail address Quality Contact/Title: e-mail address 1. Type of Business: a) Number of years in business? b) Company
Supplier: Phone: Prime Contact/Title: Sales Contact/Title: Address: Fax: e-mail address e-mail address Quality Contact/Title: e-mail address 1. Type of Business: a) Number of years in business? b) Company
P. 1. Identify the Differences between ISO9001:2000 與 ISO9001:2008 ISO9001:2008 ISO9001:2000 版本的異同. 5 January 2009 ISO 9000 SERIES
 Identify the Differences between ISO9001:2000 and ISO 9001:2008 審視 ISO9001:2000 與 ISO9001:2008 版本的異同 ISO 9000 SERIES ISO 19011 ISO9000 5 January 2009 ISO9001 ISO9004 2 ISO 9000 SERIES ISO 9001 ISO 9000
Identify the Differences between ISO9001:2000 and ISO 9001:2008 審視 ISO9001:2000 與 ISO9001:2008 版本的異同 ISO 9000 SERIES ISO 19011 ISO9000 5 January 2009 ISO9001 ISO9004 2 ISO 9000 SERIES ISO 9001 ISO 9000
9001:2015, ISO 14001:2015 & ISO
 Quality management input comprises the standard requirements from ISO 9001:2015 which are deployed by our organization to achieve customer satisfaction through process control. Environmental input comprises
Quality management input comprises the standard requirements from ISO 9001:2015 which are deployed by our organization to achieve customer satisfaction through process control. Environmental input comprises
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru
 FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
FDA 21 CFR Part 820 vs. ISO 13485:2016 Comparison Table created by greenlight.guru FDA QSR (21 CFR Part 820) ISO 13485:2016 820.1 Scope 1 Scope 2 Normative References 820.3 Definitions 3 Terms and Definitions
Brief Introduction to the ISO 9001:2000 and Quality Principles
 Vilmos Berényi H2509 H2509 Esztergom-Kertváros, Hőtáv Hőtávu. u. 59 59 Hungary Hungary +36703806768, fax: fax: +3633319117 berenyi@gmail.com Brief Introduction to the ISO 9001:2000 and Quality Principles
Vilmos Berényi H2509 H2509 Esztergom-Kertváros, Hőtáv Hőtávu. u. 59 59 Hungary Hungary +36703806768, fax: fax: +3633319117 berenyi@gmail.com Brief Introduction to the ISO 9001:2000 and Quality Principles
QUALITY MANUAL. Number: MAN Dept: Quality. Quality Manual. Rev: Page. 1 of 11
 QUALITY MANUAL 1 of 11 Contents 1 PURPOSE AND SCOPE... 3 2 TRIAD PROFILE... 3 3 APPLICALE DOCUMENTS... 3 4 DEFINITIONS / ACRONYMS... 3 5 QUALITY MANAGEMENT SYSTEM PROCESS... 3 6 MANAGEMENT PROCESSES...
QUALITY MANUAL 1 of 11 Contents 1 PURPOSE AND SCOPE... 3 2 TRIAD PROFILE... 3 3 APPLICALE DOCUMENTS... 3 4 DEFINITIONS / ACRONYMS... 3 5 QUALITY MANAGEMENT SYSTEM PROCESS... 3 6 MANAGEMENT PROCESSES...
Continental Steel & Tube Co. Quality Manual
 312 SE 22 nd Street Fort Lauderdale, FL 33316 Mailing address P.O. Box 030040 Fort Lauderdale, FL 33303-0040 954-332-2290 Fax 954-332-2296 info@continentalsteel.com www.continentalsteel.com Introduction
312 SE 22 nd Street Fort Lauderdale, FL 33316 Mailing address P.O. Box 030040 Fort Lauderdale, FL 33303-0040 954-332-2290 Fax 954-332-2296 info@continentalsteel.com www.continentalsteel.com Introduction
