If you have an extra electron where do you put it?
|
|
|
- Stephen Stone
- 6 years ago
- Views:
Transcription
1 If you have an extra electron where do you put it? Jouni Lehtoranta Finnish Environment Institute Marine Research Center Systems Ecological Perspectives on Sustainability Conference
2 Spatial scale Redox reactions - Significance in large scales - Description of main reactions APERITIF TALK TIME Linking reactions to sediment phosphorus - Microscale processes From micro- to macroscale again Human impact on redox-reactions LUNCH
3 Sun e - Energy goes through the system (open system) Producers Consumers Life is based on electron transfer i.e. redox - reactions Inorganic nutrient pool Decomposers Nasa Nutrients cycle in the system (closed system) J. Lehtoranta
4 Transfer of electrons Transfer of electrons from water to organic carbon Oxidation i.e. transfer of electrons from organic carbon to????? CO 2 + H 2 O [CH 2 O] + O 2 [CH 2 O] + O 2 CO 2 + H 2 O J. Lehtoranta
5 Pathways of organic matter oxidation i.e. transfer of electrons Reduction reaction Formula Depth in sediment oxic Aerobic respiration CH 2 O + O 2 CO 2 +H 2 O mm anoxic Denitrification 5CH 2 O + 4NO H + 5CO 2 + 2N 2 + 7H 2 O mm anoxic Manganese reduction CH 2 O + MnO 2 + 4H + CO 2 + 2Mn H 2 O cm anoxic Iron reduction CH 2 O + 4FeOOH + 8H + CO 2 + 4Fe H 2 O cm anoxic Sulfate reduction 2CH 2 O + SO H + 2CO 2 + H 2 S + 2H 2 O m anoxic Methanogenesis CH 3 COO - + CH 4 + CO 2 HCO H 2 + H + CH 4 + 3H 2 O Anaerobic mineralization causes indirectly O 2 consumption (reoxidation of NH 4, Mn(II), Fe(II), H 2 S, CH 4 ) O 2 is the ultimate acceptor of electrons released during mineralization In long run oxygen production = oxygen consumption (1:1) Not true in geological timescales: Long term burial of organic carbon and formation of FeS 2 (iron oxidizes J. Lehtoranta sulphur) we have free O 2 in athmosphere m
6 Biogeochemical cycles (closed system) Reservoir chemical is held for long periods of time in one place (coal and apatite deposits) Exchange pool chemical is held for only short period of time. Generally reservoirs are abiotic factors and exchange pools are biotic Residence the amount of time that a chemical is held in one place Abiotic compartments Water = hydrosphere Land = lithosphere Air = atmosphere
7 Terrestrial and aquatic systems Differences in reservoirs and exchange pools Nutrient pools a) Reactive sites b) Sites of nutrient storage Biota a) Lifespan of primary producers b) C:N, C:P of primary producers c) N-fixers d) Ratio consumers:producers Terrestrial Minerals, org. horizons, rhizosphere Soils, vegetation Long High Symbiotic with long-lived organisms Lower Lake Particles, sedim.-water Sediment, fish Short Low Free living Higher Prevalence of anoxia Rare, microsites only Sediments, hypolimnion
8 P-pools in marine and terrestrial living organisms P-pool (50 to 70 x g) in marine plankton is only 2 % of that in terrestrial living biomass However, the marine primary production incorporates 1200 x g P yr -1 is 3 to 4 times higher than the terrestrial incorporation rate
9 Turnover times ( residence ) The turnover time of living oceanic biomass is short: few days for prokaryotes week for phytoplankton few months for zooplankton In terrestrial systems the P is mainly bound to long-lived forests average turnover time for terrestrial living biomass is at least 10 years
10 What does this mean? Phosphorus cycles much faster in aquatic than terrestrial systems With a same amount of phosphorus we get more organic carbon (i.e. electron packages) into aquatic than terrestrial system
11 O 2 NO 3, Mn(IV), Fe(III), SO 4 CO 2 Seija Hällfors Photo: Ilkka Heikkinen, Inkoo
12 Energy flow goes through the system (open system) Proportion of background phosphorus Anthropogenic loading from Finland to the Baltic Sea is 30% phosphorus loading 70% Eutrophication increases amount of organic matter - Human actions have a large and growing importance on mineralization processes (redox-reactions) in aquatic systems
13 Pathways of organic matter oxidation Reduction reaction Formula Depth in sediment oxic Aerobic respiration CH 2 O + O 2 CO 2 +H 2 O mm anoxic Denitrification - 5CH 2 O + 4NO 3 + 4H + 5CO 2 + 2N 2 + 7H 2 O mm anoxic Manganese reduction CH 2 O + MnO 2 + 4H + CO 2 + 2Mn H 2 O cm anoxic Iron reduction CH 2 O + 4FeOOH + 8H + CO 2 + 4Fe H 2 O cm anoxic Sulfate reduction 2-2CH 2 O + SO 4 + 2H + 2CO 2 + H 2 S + 2H 2 O m anoxic Methanogenesis CH 3 COO - + CH 4 + CO 2 HCO H 2 + H + CH 4 + 3H 2 O Canfield and Thamdrup 2009, Geobiology 7: m One element is missing Phosphorus J. Lehtoranta
14 Phosphorus: What kind of sediment we would like to have considering mineralization pathways? If you have an extra electron (organic matter) where do you put it (which electron acceptor you prefer) J. Lehtoranta
15 Pathways of organic matter oxidation Reduction reaction Formula Depth in sediment oxic Aerobic respiration Geobacter CH 2 O + O 2 CO 2 +H 2 O mm anoxic Denitrification metallireducens - 5CH 2 O + 4NO 3 + 4H + 5CO 2 + 2N 2 + 7H 2 O mm anoxic Manganese reduction CH 2 O + MnO 2 + 4H + CO 2 + 2Mn H 2 O cm anoxic Iron reduction CH 2 O + 4FeOOH + 8H + CO 2 + 4Fe H 2 O cm anoxic Sulfate reduction 2-2CH 2 O + SO 4 + 2H + 2CO 2 + H 2 S + 2H 2 O m anoxic Methanogenesis CH 3 COO - + CH 4 + CO 2 Desulfovibrio vulgaris HCO H 2 + H + CH 4 + 3H 2 O Fe(III) is sensitive towards mineralization processes Phosphorus starts react on redox-reactions m J. Lehtoranta
16 Significance of sulphate Lehtoranta, Ekholm, Pitkänen Ambio 38: Lehtoranta & Ekholm 2013 Vesitalous 2: 40-42
17 Sulphate removed i.e. change in electron acceptor SO 4 2- (mg L -1 ) cores open cores closed cores open 100 A Fe(II) (mg L -1 ) cores open B Low sulphate High sulphate cores closed cores open cores open cores closed cores open DP (µg L -1 ) C Day Ekholm et al The effect of gypsum on phosphorus losses at the catchment scale. THE FINNISH ENVIRONMENT
18 Addition of organic carbon (i.e. change in electron donor) How microbial iron and sulphate reduction can be noticed after organic matter addition? Fresh sample Anoxia, No carbon addition Anoxia, Carbon addition 300 Cores open Cores closed Fe:P < 2 (C+) Cores open 0.02 Concentration (µmol l -1 ) Fe(II) C+ Fe(II) C- DP C+ DP (C-) Fe:P < 2 (C-) Days 1.6 2B J. Lehtoranta Lehtoranta, Ekholm and Pitkänen 2009 Ambio 38:
19 Up-stream thinking: Soil erosion and anerobic microbial processes in brackish sediment Standard field soil Sandy clay ( mg ) 80 ml filtered Gulf of Finland water Incubation: At dark (a) +10 C, (b) +8 C (a) 308 d, (b) 745 d Natrium acetate ( mg C) Pasi Valkama 10 µl sediment Lehtoranta, J., Ekholm, P., Wahlström, S. Tallberg, P. and Uusitalo, R. Under revision
20 Mn 2+ Fe 2+ H 2 S, HS- FeS High dose of C Small amount of C
21 Laakso Johanna, Kahma Tuomas, Ekholm Petri, Lehtoranta Jouni, Uusitalo Risto, Yli-Halla Markku Constructed wetland of Ojainen in Jokioinen Black sediment indicating presence of Fe sulphides
22 Consequences of eutrophication linked to electron transfer in sediments J. Lehtoranta
23 Eutrophication associated hypoxic areas
24 Shift in microbial processes in the Baltic Sea? Shift in microbial processes 2 Anoxic sediment surfa ce SO reduction dominates 4 S. Knuuttila State indicator 1 Oxic sediment surface Microbial Fe reduction domina tes Driver Lehtoranta, Ekholm and Pitkänen 2009 Ambio 38: Threshold Flux of labile organic C J. Lehtoranta Could change in microscale processes driven by micro-organisms cause macroscale consequences?
25 phosphorus Winter surface concentration of phosphate in 2003 (µg l -1 ) Microbial iron reduction dominates? Iron oxides Tot-Fe to DIP ratio (mol:mol) ,1 0,01 State 1 State Station Eutrophy Microscale redox-processes driven by micro-organisms cause macroscale consequences phosphorus Microbial sulphate reduction dominates? Iron sulphides Lehtoranta J. Lehtoranta, Ekholm & Pitkänen (J. Mar. Syst. 74: ) J. Lehtoranta Data: PAPA Partners Figure: Mikko Kiirikki/SYKE
26 C:N:P 12/2/2014 Petri Ekholm Photos: Ekholm 26
27 Summary Eutrophication increases mineralization All terminal electron acceptors used produce CO 2 Reduced substances formed in the mineralization participate to further redox-reactions (Mn(II), Fe(II), H 2 S, CH 4 ) They have different consequences on the element cycles in the system So if you have an extra electron where do you put it, depends what you are trying to get
28 Thank you 28
What to do with extra electrons how combating eutrophication may affect mineralization pathways
 What to do with extra electrons how combating eutrophication may affect mineralization pathways Jouni Lehtoranta Finnish Environment Institute Marine Research Centre Sun e - Energy goes through the system
What to do with extra electrons how combating eutrophication may affect mineralization pathways Jouni Lehtoranta Finnish Environment Institute Marine Research Centre Sun e - Energy goes through the system
Regional variation in N vs. P limitation in the Baltic Sea the role of sediment mineralisation processes
 Regional variation in N vs. P limitation in the Baltic Sea the role of sediment mineralisation processes Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) COST869 Mitigation options
Regional variation in N vs. P limitation in the Baltic Sea the role of sediment mineralisation processes Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) COST869 Mitigation options
Should we focus only on P load when aiming to reduce eutrophication in a P-limited aquatic system?
 Should we focus only on P load when aiming to reduce eutrophication in a P-limited aquatic system? Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) International Phosphorus Workshop
Should we focus only on P load when aiming to reduce eutrophication in a P-limited aquatic system? Petri Ekholm & Jouni Lehtoranta Finnish Environment Institute (SYKE) International Phosphorus Workshop
The Phosphorus Cycle
 Lecture Outline Introduction Global Cycle Overview Major Forms Anthropogenic Influences Terrestrial Processes Freshwater Processes Ocean Processes The Phosphorus Cycle No important gaseous form, present
Lecture Outline Introduction Global Cycle Overview Major Forms Anthropogenic Influences Terrestrial Processes Freshwater Processes Ocean Processes The Phosphorus Cycle No important gaseous form, present
Reducing agricultural phosphorus load by gypsum: results from the first year after amendment
 Reducing agricultural phosphorus load by gypsum: results from the first year after amendment Photo: Janne Artell Petri Ekholm Finnish Environment Institute SYKE Contents What is gypsum and how it works
Reducing agricultural phosphorus load by gypsum: results from the first year after amendment Photo: Janne Artell Petri Ekholm Finnish Environment Institute SYKE Contents What is gypsum and how it works
CO 2 (g) + H 2 O = H 2 CO 3 log K H = HCO 3 log K 1 = HCO - 3 = H CO 3 log K 2 = -9.0
 Ocean 400 Chemical Oceanography Winter 2006 Your Name Final Exam Read all questions carefully before you begin to answer. Use the back of the pages if necessary. Points are assigned to each question in
Ocean 400 Chemical Oceanography Winter 2006 Your Name Final Exam Read all questions carefully before you begin to answer. Use the back of the pages if necessary. Points are assigned to each question in
BIOGEOCHEMICAL CYCLES
 BIOGEOCHEMICAL CYCLES BIOGEOCHEMICAL CYCLES A biogeochemical cycle or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth.
BIOGEOCHEMICAL CYCLES BIOGEOCHEMICAL CYCLES A biogeochemical cycle or cycling of substances is a pathway by which a chemical element or molecule moves through both biotic and abiotic compartments of Earth.
Nutrient Cycling & Soils
 Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
Nutrient Cycling & Soils tutorial by Paul Rich Outline 1. Nutrient Cycles What are nutrient cycles? major cycles 2. Water Cycle 3. Carbon Cycle 4. Nitrogen Cycle 5. Phosphorus Cycle 6. Sulfur Cycle 7.
CHEMICAL COMPOSITION OF NATURAL WATERS
 CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
CHEMICAL COMPOSITION OF NATURAL WATERS DISSOVLED GASES Oxygen (and E h ) Why important? product of photosynthesis needed for aerobic respiration - Much of an aquatic organisms energy budget is devoted
Nutrients elements required for the development, maintenance, and reproduction of organisms.
 Nutrient Cycles Energy flows through ecosystems (one way trip). Unlike energy, however, nutrients (P, N, C, K, S ) cycle within ecosystems. Nutrients are important in controlling NPP in ecosystems. Bottom-up
Nutrient Cycles Energy flows through ecosystems (one way trip). Unlike energy, however, nutrients (P, N, C, K, S ) cycle within ecosystems. Nutrients are important in controlling NPP in ecosystems. Bottom-up
7.014 Lecture 20: Biogeochemical Cycles April 1, 2007
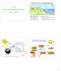 Global Nutrient Cycling - Biogeochemical Cycles 7.14 Lecture 2: Biogeochemical Cycles April 1, 27 Uptake Bioelements in Solution Weathering Precipitation Terrestrial Biomass Decomposition Volatile Elements
Global Nutrient Cycling - Biogeochemical Cycles 7.14 Lecture 2: Biogeochemical Cycles April 1, 27 Uptake Bioelements in Solution Weathering Precipitation Terrestrial Biomass Decomposition Volatile Elements
Biogeochemical cycles
 Biogeochemical cycles Microbial Ecology SS2010 www.icbm.de/pmbio Biogeochemistry The study of the exchange of material between the living and nonliving components of the biosphere. The biogeochemical cycling
Biogeochemical cycles Microbial Ecology SS2010 www.icbm.de/pmbio Biogeochemistry The study of the exchange of material between the living and nonliving components of the biosphere. The biogeochemical cycling
Sediment Diagenesis. 1/61
 Sediment Diagenesis http://eps.mcgill.ca/~courses/c542/ 1/61 Sequence of catabolic reactions In the presence of free O 2 : G (kj/mol) (1) Aerobic respiration: (CH 2 O) 106 (NH 3 ) 16 H 3 PO 4 + 138O 2
Sediment Diagenesis http://eps.mcgill.ca/~courses/c542/ 1/61 Sequence of catabolic reactions In the presence of free O 2 : G (kj/mol) (1) Aerobic respiration: (CH 2 O) 106 (NH 3 ) 16 H 3 PO 4 + 138O 2
Biogeochemistry of Wetlands
 Institute of Food and Agricultural Sciences (IFAS) Biogeochemistry of Wetlands Si Science and da Applications Biogeochemical Properties of Wetlands Wetland Biogeochemistry Laboratory Soil and Water Science
Institute of Food and Agricultural Sciences (IFAS) Biogeochemistry of Wetlands Si Science and da Applications Biogeochemical Properties of Wetlands Wetland Biogeochemistry Laboratory Soil and Water Science
Lakes, Primary Production, Budgets and Cycling
 OCN 401-Biogeochemical Systems Lecture #10 (9.22.11) Lakes, Primary Production, Budgets and Cycling (Schlesinger: Chapter 7) 1. Primary Production and Nutrient Cycling in Lakes Physical aspects and nomenclature
OCN 401-Biogeochemical Systems Lecture #10 (9.22.11) Lakes, Primary Production, Budgets and Cycling (Schlesinger: Chapter 7) 1. Primary Production and Nutrient Cycling in Lakes Physical aspects and nomenclature
Microorganisms & Biogeochemical Cycles
 Microorganisms & Biogeochemical Cycles Biogeochemical Cycles: Movement & transformation of chemical elements (nutrien) in the environment caused by biological and chemical processes in the atmosphere,
Microorganisms & Biogeochemical Cycles Biogeochemical Cycles: Movement & transformation of chemical elements (nutrien) in the environment caused by biological and chemical processes in the atmosphere,
Another cause of diversity may be the creation of different habitats within a region by periodic disturbance A community that forms if the land is
 Another cause of diversity may be the creation of different habitats within a region by periodic disturbance A community that forms if the land is undisturbed and that perpetuates itself for as long as
Another cause of diversity may be the creation of different habitats within a region by periodic disturbance A community that forms if the land is undisturbed and that perpetuates itself for as long as
Appendix F. SMAST Report Sediment Nutrient Flux and Oxygen Demand
 Appendix F SMAST Report Sediment Nutrient Flux and Oxygen Demand Technical Report Merrimack River Sediment Nutrient Regeneration 2009 Sampling Season By: Dr. David R. Schlezinger Dr. Brian L. Howes Roland
Appendix F SMAST Report Sediment Nutrient Flux and Oxygen Demand Technical Report Merrimack River Sediment Nutrient Regeneration 2009 Sampling Season By: Dr. David R. Schlezinger Dr. Brian L. Howes Roland
Nutrient Cycles. Nutrient cycles involve flow of high quality energy from the sun through the environment & of elements.
 Nutrient Cycles Nutrient cycles (= biogeochemical cycles): natural processes that involve the flow of nutrients from the environment (air, water, soil, rock) to living organisms ( ) & back again. Nutrient
Nutrient Cycles Nutrient cycles (= biogeochemical cycles): natural processes that involve the flow of nutrients from the environment (air, water, soil, rock) to living organisms ( ) & back again. Nutrient
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger,, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from
Agricultural phosphorus losses and eutrophication in Nordic-Baltic countries
 Agricultural phosphorus losses and eutrophication in Nordic-Baltic countries Petri Ekholm Finnish Environment Institute (SYKE) NJF 401: Phosphorus management in Nordic-Baltic agriculture - reconciling
Agricultural phosphorus losses and eutrophication in Nordic-Baltic countries Petri Ekholm Finnish Environment Institute (SYKE) NJF 401: Phosphorus management in Nordic-Baltic agriculture - reconciling
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 18 September 2012 Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 18 September 2012 Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 24 September 2013 Reading: Schlesinger & Bernhardt, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification
Why Water Quality? FOR IMMEDIATE RELEASE March 26, 2013
 Nutrients Nutrients Microbes Rulers of the World Microbes and Redox Potential Dissolved Oxygen Nitrogen Cycle From Waste to Gas Phosphorus Cycle or Recycle Algae The Miracle of Life Stormwater - Loadings
Nutrients Nutrients Microbes Rulers of the World Microbes and Redox Potential Dissolved Oxygen Nitrogen Cycle From Waste to Gas Phosphorus Cycle or Recycle Algae The Miracle of Life Stormwater - Loadings
A Robust Model for Biotic and Abiotic Degradation of Chlorinated Aliphatic Hydrocarbons in an In Situ Bioreactor
 A Robust Model for Biotic and Abiotic Degradation of Chlorinated Aliphatic Hydrocarbons in an In Situ Bioreactor Robert Edwards- Noblis Gabriel Moreno-Ferguson Camp Stanley Storage Activity Chris Beal
A Robust Model for Biotic and Abiotic Degradation of Chlorinated Aliphatic Hydrocarbons in an In Situ Bioreactor Robert Edwards- Noblis Gabriel Moreno-Ferguson Camp Stanley Storage Activity Chris Beal
CHEMICAL: NITROGEN AND PHOSPHORUS (read pp in Dodson)
 BIOE 155, Fall 010 BACKGROUND CHEMICAL: NITROGEN AND PHOSPHORUS (read pp39-50 in Dodson) Lakes are often classified according to trophic status, specifically how much energy or food is available for the
BIOE 155, Fall 010 BACKGROUND CHEMICAL: NITROGEN AND PHOSPHORUS (read pp39-50 in Dodson) Lakes are often classified according to trophic status, specifically how much energy or food is available for the
Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p
 OCN 401-Biogeochemical Systems Lecture #12 (10.8.13) Angelos Hannides, hannides@hawaii.edu Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p. 288-308 1. Physical
OCN 401-Biogeochemical Systems Lecture #12 (10.8.13) Angelos Hannides, hannides@hawaii.edu Lakes, Primary Production, Budgets and Cycling Schlesinger and Bernhardt (2013): Chapter 8, p. 288-308 1. Physical
Ecosystems and Nutrient Cycles Chapters 3
 Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
Ecosystems and Nutrient Cycles Chapters 3 Prokaryotic and Eukaryotic cells Figure 3-2 Prokaryotic cells: Have organelles. Bacteria and Archaea are composed of prokaryotic cells. Eukaryotic cells: cells,
Nitrogen Cycling in the Sea
 Nitrogen Cycling in the Sea Matt Church (MSB 612 / 9568779/ mjchurch@hawaii.edu) Marine Microplankton Ecology / OCN 626 NH 4 N0 2 N0 2 NH 4 Outline Nitrogen species in marine watersdistributions and concentrations
Nitrogen Cycling in the Sea Matt Church (MSB 612 / 9568779/ mjchurch@hawaii.edu) Marine Microplankton Ecology / OCN 626 NH 4 N0 2 N0 2 NH 4 Outline Nitrogen species in marine watersdistributions and concentrations
Lecture 9. Nutrient and BOD Overloading in Fresh Waters
 Lecture 9 Nutrient and BOD Overloading in Fresh Waters Reading: chapter 4 (this week and next) Today 1. Nutrient cycles and Nutrient Overloading Next Time 2. OM (organic matter) and BOD (biological oxygen
Lecture 9 Nutrient and BOD Overloading in Fresh Waters Reading: chapter 4 (this week and next) Today 1. Nutrient cycles and Nutrient Overloading Next Time 2. OM (organic matter) and BOD (biological oxygen
GLOBAL BIOGEOCHEMICAL CYCLES. GEOG/ENST 3331 Lecture 10 Turco: Chapter 10; Dearden and Mitchell: Chapter 4
 GLOBAL BIOGEOCHEMICAL CYCLES GEOG/ENST 3331 Lecture 10 Turco: Chapter 10; Dearden and Mitchell: Chapter 4 Assignment 4 1. Suppose that a layer of air 1000 m thick has conditional stability. A rising parcel
GLOBAL BIOGEOCHEMICAL CYCLES GEOG/ENST 3331 Lecture 10 Turco: Chapter 10; Dearden and Mitchell: Chapter 4 Assignment 4 1. Suppose that a layer of air 1000 m thick has conditional stability. A rising parcel
Chapter 46 Ecosystems and Global Ecology
 Chapter 46 Ecosystems and Global Ecology Section 46.1 Climate and Nutrients Affect Ecosystem Function 1. How does the definition of ecosystem expand on the concept of the community? 2. Which ecosystems
Chapter 46 Ecosystems and Global Ecology Section 46.1 Climate and Nutrients Affect Ecosystem Function 1. How does the definition of ecosystem expand on the concept of the community? 2. Which ecosystems
Ocean Production and CO 2 uptake
 Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Ocean Production and CO 2 uptake Fig. 6.6 Recall: Current ocean is gaining Carbon.. OCEAN Reservoir size: 38000 Flux in: 90 Flux out: 88+0.2=88.2 90-88.2 = 1.8 Pg/yr OCEAN is gaining 1.8 Pg/yr Sum of the
Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical, & biological processes
 OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
OPEN Wetland Ecology Lectures 14-15-16 Wetland Biogeochemistry What is biogeochemical cycling? Transport & Transformation of chemicals in an ecosystem, involving numerous interrelated physical, chemical,
Estuarine and Coastal Biogeochemistry
 Estuarine and Coastal Biogeochemistry OCN 623 Chemical Oceanography 9 April 2013 Reading: Seitzinger & Mayorga (2008) 2013 Frank Sansone 1. Global coastal zone Outline 2. Nutrient loading in estuaries
Estuarine and Coastal Biogeochemistry OCN 623 Chemical Oceanography 9 April 2013 Reading: Seitzinger & Mayorga (2008) 2013 Frank Sansone 1. Global coastal zone Outline 2. Nutrient loading in estuaries
Geochemical Nutrient Scavenging. High mountain lakes as model systems. Research Questions
 Research Questions Geochemical Nutrient Scavenging High mountain lakes as model systems Kurt Hanselmann, Microbiology: Ecology: Biogeochemistry: Geobiology: How do microorganisms cope with low nutrient
Research Questions Geochemical Nutrient Scavenging High mountain lakes as model systems Kurt Hanselmann, Microbiology: Ecology: Biogeochemistry: Geobiology: How do microorganisms cope with low nutrient
Cycling and Biogeochemical Transformations of N, P and S
 Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Cycling and Biogeochemical Transformations of N, P and S OCN 401 - Biogeochemical Systems Reading: Schlesinger, Chapter 6 1. Nitrogen cycle Soil nitrogen cycle Nitrification Emissions of N gases from soils
Eutrophication test questions
 Eutrophication test questions From the course materials find answers to all questions. There may be (and often is) more than one correct choice. You have to mark down the right combination of choices.
Eutrophication test questions From the course materials find answers to all questions. There may be (and often is) more than one correct choice. You have to mark down the right combination of choices.
Biogeochemical Cycles. Nutrient cycling at its finest!
 Biogeochemical Cycles Nutrient cycling at its finest! Four Criteria for Sustainability Sustainable Ecosystems Need: Reliance on Solar Energy High Biodiversity Population Control Nutrient Cycling This note
Biogeochemical Cycles Nutrient cycling at its finest! Four Criteria for Sustainability Sustainable Ecosystems Need: Reliance on Solar Energy High Biodiversity Population Control Nutrient Cycling This note
Ecosystems: Nutrient Cycles
 Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Ecosystems: Nutrient Cycles Greeks, Native Peoples, Buddhism, Hinduism use(d) Earth, Air, Fire, and Water as the main elements of their faith/culture Cycling in Ecosystems the Hydrologic Cycle What are
Particulate Soil Phosphorus and Eutrophication in Lakes and Streams
 Particulate Soil Phosphorus and Eutrophication in Lakes and Streams Paul R. Bloom Soil, Water, & Climate Department University of Minnesota With contributions by John Moncrief, Carl Rosen and David Mulla
Particulate Soil Phosphorus and Eutrophication in Lakes and Streams Paul R. Bloom Soil, Water, & Climate Department University of Minnesota With contributions by John Moncrief, Carl Rosen and David Mulla
Chapter 34 Nature of Ecosystems. Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display.
 Chapter 34 Nature of Ecosystems 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 34.1 The Biotic Components of Ecosystems Ecosystems Abiotic components include
Chapter 34 Nature of Ecosystems 1 Copyright The McGraw-Hill Companies, Inc. Permission required for reproduction or display. 34.1 The Biotic Components of Ecosystems Ecosystems Abiotic components include
Ecosystems. Trophic relationships determine the routes of energy flow and chemical cycling in ecosystems.
 AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
AP BIOLOGY ECOLOGY ACTIVITY #5 Ecosystems NAME DATE HOUR An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 23 September 2014 Reading: Schlesinger & Bernhardt, Chapter 6 2014 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 23 September 2014 Reading: Schlesinger & Bernhardt, Chapter 6 2014 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Lakes: Primary Production, Budgets and Cycling
 OCN 401-Biogeochemical Systems (9.28.17) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
OCN 401-Biogeochemical Systems (9.28.17) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
11/9/2010. Stoichiometry of POM and DOM. DOC cycling via DO 14 C Williams, Oeschger, and Kinney; Nature v224 (1969)
 DOC cycling via DO 1 C Williams, Oeschger, and Kinney; Nature v22 (1969) UV photooxidation Radiocarbon in the Atlantic and Pacific Oceans Peter M. Williams and Ellen Druffel; Nature 1987, JGR 1992 DIC
DOC cycling via DO 1 C Williams, Oeschger, and Kinney; Nature v22 (1969) UV photooxidation Radiocarbon in the Atlantic and Pacific Oceans Peter M. Williams and Ellen Druffel; Nature 1987, JGR 1992 DIC
Oceans OUTLINE. Reading: White, Chapter 15 Today Finish estuaries and particles, then: 1. The oceans: currents, stratification and chemistry
 Oceans OUTLINE Reading: White, Chapter 15 Today Finish estuaries and particles, then: 1. The oceans: currents, stratification and chemistry Next Time Salinity Exercise bring something to calculate with
Oceans OUTLINE Reading: White, Chapter 15 Today Finish estuaries and particles, then: 1. The oceans: currents, stratification and chemistry Next Time Salinity Exercise bring something to calculate with
Chemistry that determines ph in Swedish waters Leif G. Anderson Department of Marine Sciences University of Gothenburg Sweden
 Chemistry that determines ph in Swedish waters Leif G. Anderson Department of Marine Sciences University of Gothenburg Sweden CO 2 The ocean takes up CO 2 when the surface water partial pressure (pco 2
Chemistry that determines ph in Swedish waters Leif G. Anderson Department of Marine Sciences University of Gothenburg Sweden CO 2 The ocean takes up CO 2 when the surface water partial pressure (pco 2
Reactions & Processes Influenced by Soil Wetness. Topics for Consideration. Reactions and Processes Influenced by Soil Wetness
 Reactions & Processes Influenced by Soil Wetness Topics for Consideration A. Organic matter (OM) accumulation B. Some reduction effects induced by soil saturation C. Redoximorphic features D. Calcium carbonate
Reactions & Processes Influenced by Soil Wetness Topics for Consideration A. Organic matter (OM) accumulation B. Some reduction effects induced by soil saturation C. Redoximorphic features D. Calcium carbonate
4/13/2015. The Biosphere
 The Biosphere Ecology- the scientific study of interactions among organisms and between organisms and their environment. The word ecology was first used in 1866 by Ernst Haeckel. Biosphere- contains the
The Biosphere Ecology- the scientific study of interactions among organisms and between organisms and their environment. The word ecology was first used in 1866 by Ernst Haeckel. Biosphere- contains the
Biogeochemistry of Wetlands
 Institute of Food and Agricultural Sciences (IFAS) Biogeochemistry of Wetlands Si Science and da Applications Biogeochemical Indicators Wetland Biogeochemistry Laboratory Soil and Water Science Department
Institute of Food and Agricultural Sciences (IFAS) Biogeochemistry of Wetlands Si Science and da Applications Biogeochemical Indicators Wetland Biogeochemistry Laboratory Soil and Water Science Department
Lakes: Primary Production, Budgets and Cycling. Lecture Outline
 OCN 401-Biogeochemical Systems (10.06.16) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
OCN 401-Biogeochemical Systems (10.06.16) Lakes: Primary Production, Budgets and Cycling Reading: Schlesinger, Chapter 8 Lecture Outline 1. Seasonal cycle of lake stratification Temperature / density relationship
Studying organisms in their environment
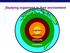 Studying organisms in their environment organism population community ecosystem biosphere Essential questions What limits the production in ecosystems? How do nutrients move in the ecosystem? How does
Studying organisms in their environment organism population community ecosystem biosphere Essential questions What limits the production in ecosystems? How do nutrients move in the ecosystem? How does
Nitrogen Cycling in the Sea
 Nitrogen Cycling in the Sea NH 4 + N0 2 N0 2 NH 4 + Outline Nitrogen species in marine watersdistributions and concentrations New, regenerated, and export production The processes: Assimilation, N 2 fixation,
Nitrogen Cycling in the Sea NH 4 + N0 2 N0 2 NH 4 + Outline Nitrogen species in marine watersdistributions and concentrations New, regenerated, and export production The processes: Assimilation, N 2 fixation,
1. Where are nutrients accumulated or stored for short or long periods?
 Use with textbook pages 68 87. Nutrient cycles Answer the questions below. Comprehension 1. Where are nutrients accumulated or stored for short or long periods? 2. Name a biotic process and an abiotic
Use with textbook pages 68 87. Nutrient cycles Answer the questions below. Comprehension 1. Where are nutrients accumulated or stored for short or long periods? 2. Name a biotic process and an abiotic
Cycling and Biogeochemical Transformations of N, P, S, and K
 Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 20 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2016 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Cycling and Biogeochemical Transformations of N, P, S, and K OCN 401 - Biogeochemical Systems 20 September 2016 Reading: Schlesinger & Bernhardt, Chapter 6 2016 Frank Sansone 1. Nitrogen cycle Soil nitrogen
Aquatic respiration and ocean metabolism
 Aquatic respiration and ocean metabolism Remember what life is all about: Energy (ATP) Reducing power (NADPH) Nutrients (C, N, P, S, Fe, etc., etc.) Photosynthetic organisms use sunlight, H 2 O, and dissolved
Aquatic respiration and ocean metabolism Remember what life is all about: Energy (ATP) Reducing power (NADPH) Nutrients (C, N, P, S, Fe, etc., etc.) Photosynthetic organisms use sunlight, H 2 O, and dissolved
LIMNOLOGY. Inland Water Ecosystems. JACOB KALFF McGill University. Prentice Hall. Upper Saddle River, New Jersey 07458
 LIMNOLOGY Inland Water Ecosystems JACOB KALFF McGill University Prentice Hall Prentice Hall Upper Saddle River, New Jersey 07458 Contents CHAPTER 1 Inland Waters and Their Catchments: An Introduction and
LIMNOLOGY Inland Water Ecosystems JACOB KALFF McGill University Prentice Hall Prentice Hall Upper Saddle River, New Jersey 07458 Contents CHAPTER 1 Inland Waters and Their Catchments: An Introduction and
3 3 Cycles of Matter
 3 3 Cycles of Matter Recycling in the Biosphere Energy - one way flow matter - recycled within and between ecosystems. biogeochemical cycles matter Elements, chemical compounds, and other forms passed
3 3 Cycles of Matter Recycling in the Biosphere Energy - one way flow matter - recycled within and between ecosystems. biogeochemical cycles matter Elements, chemical compounds, and other forms passed
Lecture 5. Exchange Reactions. Cation exchange Salt/Sodium Affected Soils Acid Soils
 Lecture 5 Exchange Reactions Cation exchange Salt/Sodium Affected Soils Acid Soils General Classes (layer build-up) of Phyllosilicate Minerals: Layer Type Charge Trioctahedral Dioctahedral 1 octahedra
Lecture 5 Exchange Reactions Cation exchange Salt/Sodium Affected Soils Acid Soils General Classes (layer build-up) of Phyllosilicate Minerals: Layer Type Charge Trioctahedral Dioctahedral 1 octahedra
N-cycle: biogeochemistry. Biological flows of Nitrogen
 N-cycle: biogeochemistry SWES 410/510 April 4, 2014 I. N cycling A. simplest possible B. Global N budget C. Effects of N-cycling ( the Nitrogen Cascade ) II. Nitrous Oxide (N 2 O) budgets III. Data-Model
N-cycle: biogeochemistry SWES 410/510 April 4, 2014 I. N cycling A. simplest possible B. Global N budget C. Effects of N-cycling ( the Nitrogen Cascade ) II. Nitrous Oxide (N 2 O) budgets III. Data-Model
Carbon Cycling or. perspective. CE5508 Biogeochemistry
 Carbon Cycling or the importance of methods & perspective CE5508 Biogeochemistry Spring 2006 Topics to cover GPP vs. NPP vs. NEP (NCP) Carbon flow paths Allochthonous (exogenous) vs. autochthonous (endogenous)
Carbon Cycling or the importance of methods & perspective CE5508 Biogeochemistry Spring 2006 Topics to cover GPP vs. NPP vs. NEP (NCP) Carbon flow paths Allochthonous (exogenous) vs. autochthonous (endogenous)
River Water Quality. Section 2e: Hydrology & Ecology of Running Waters Prof. Maria Lazaridou School of Biology ARISTOTLE UNIVERSITY OF THESSALONIKI
 ARISTOTLE UNIVERSITY OF THESSALONIKI OPEN ACADEMIC COURSES Section 2e: Hydrology & Ecology of Running Waters Prof. Maria Lazaridou License The educational material subjects to Creative Commons licensing.
ARISTOTLE UNIVERSITY OF THESSALONIKI OPEN ACADEMIC COURSES Section 2e: Hydrology & Ecology of Running Waters Prof. Maria Lazaridou License The educational material subjects to Creative Commons licensing.
Studying organisms in their environment
 Ecosystems (Ch. 3) Studying organisms in their environment organism population community ecosystem biosphere Essential questions What limits the production in ecosystems? How does energy move through the
Ecosystems (Ch. 3) Studying organisms in their environment organism population community ecosystem biosphere Essential questions What limits the production in ecosystems? How does energy move through the
Impact of submarine groundwater discharge on biogeochemical processes and benthic fluxes in coastal sands
 Impact of submarine groundwater discharge on biogeochemical processes and benthic fluxes in coastal sands Daphne Donis 1,2, Felix Janssen 1,2, Frank Wenzhöfer 1,2, Olaf Dellwig 3, Peter Escher 3, Michael
Impact of submarine groundwater discharge on biogeochemical processes and benthic fluxes in coastal sands Daphne Donis 1,2, Felix Janssen 1,2, Frank Wenzhöfer 1,2, Olaf Dellwig 3, Peter Escher 3, Michael
Dissociation of Orthophosphoric Acid
 { Phosphorus General Essential for all living things Component of DNA, RNA, ADP, ATP, and bone Usually the most limiting nutrient in phytoplankton productivity Many forms Soluble inorganic, soluble organic,
{ Phosphorus General Essential for all living things Component of DNA, RNA, ADP, ATP, and bone Usually the most limiting nutrient in phytoplankton productivity Many forms Soluble inorganic, soluble organic,
Determining the f ratio 11/16/2010. Incubate seawater in the presence of trace 15
 Plankton production is supported by 2 types of nitrogen: 1) new production supported by external sources of N (e.g. NO 3 and N 2 ), 2) recycled or regenerated production, sustained by recycling of N. Assumptions:
Plankton production is supported by 2 types of nitrogen: 1) new production supported by external sources of N (e.g. NO 3 and N 2 ), 2) recycled or regenerated production, sustained by recycling of N. Assumptions:
Ecosystems. Physical Laws Law of Conservation of Energy - Energy can not be created or destroyed, only transformed. Chapter 55: Ecosystems. Fig. 55.
 Chapter 55: Ecosystems 1 Ecosystems consist of the living organisms in a community as well as the abiotic factors Microecosystem Two important considerations: Energy Flow Chemical cycling Fig. 55.1 2 Physical
Chapter 55: Ecosystems 1 Ecosystems consist of the living organisms in a community as well as the abiotic factors Microecosystem Two important considerations: Energy Flow Chemical cycling Fig. 55.1 2 Physical
Prepare for Learning. A 4000 year old corpse preserved in ice. Why hasn t it decomposed?
 Prepare for Learning A 4000 year old corpse preserved in ice. Why hasn t it decomposed? Why is carbon important? Carbon is the main constituent of all living cells (biochemistry, organic chemistry) Component
Prepare for Learning A 4000 year old corpse preserved in ice. Why hasn t it decomposed? Why is carbon important? Carbon is the main constituent of all living cells (biochemistry, organic chemistry) Component
Nutrients; Aerobic Carbon Production and Consumption
 Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography 5 February 2013 Reading: Libes, Chapters 8-10 Outline 1. Overview - photosynthesis & respiration 2. Nutrients - chemical
Nutrients; Aerobic Carbon Production and Consumption OCN 623 Chemical Oceanography 5 February 2013 Reading: Libes, Chapters 8-10 Outline 1. Overview - photosynthesis & respiration 2. Nutrients - chemical
NUTRIENT CYCLES REVIEW
 52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
52 Name A.P. Environmental Science Date Mr. Romano NUTRIENT CYCLES REVIEW 1. Which of the following chain of events would occur as a result of land clearing/deforestation? (vocabulary check: efflux means
Central Case: The Gulf of Mexico s Dead Zone
 Central Case: The Gulf of Mexico s Dead Zone The Gulf of Mexico brings in a billion pounds/year of shrimp, fish, and shellfish Gulf dead zone = a region of water so depleted of oxygen that marine organisms
Central Case: The Gulf of Mexico s Dead Zone The Gulf of Mexico brings in a billion pounds/year of shrimp, fish, and shellfish Gulf dead zone = a region of water so depleted of oxygen that marine organisms
14 th most abundant element in Earth s crust Sulfate is second most abundant anion in rivers (after bicarbonate) and in ocean after chloride
 Sulfur 14 th most abundant element in Earth s crust Sulfate is second most abundant anion in rivers (after bicarbonate) and in ocean after chloride Development of an oxygenated atmosphere facilitated this
Sulfur 14 th most abundant element in Earth s crust Sulfate is second most abundant anion in rivers (after bicarbonate) and in ocean after chloride Development of an oxygenated atmosphere facilitated this
Part 3. Oceanic Carbon and Nutrient Cycling
 OCN 401 Biogeochemical Systems (11.03.11) (Schlesinger: Chapter 9) Part 3. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Models of Carbon in the Ocean 2. Nutrient Cycling in the Ocean Atmospheric-Ocean
OCN 401 Biogeochemical Systems (11.03.11) (Schlesinger: Chapter 9) Part 3. Oceanic Carbon and Nutrient Cycling Lecture Outline 1. Models of Carbon in the Ocean 2. Nutrient Cycling in the Ocean Atmospheric-Ocean
Swedish experiences on the importance of N and P
 Danish Coastal Eutrophication Conference Copenhagen 213-6-19 Swedish experiences on the importance of N and P Ragnar Elmgren & Ulf Larsson Dept. Ecology, Environment & Plant Sciences Stockholm University
Danish Coastal Eutrophication Conference Copenhagen 213-6-19 Swedish experiences on the importance of N and P Ragnar Elmgren & Ulf Larsson Dept. Ecology, Environment & Plant Sciences Stockholm University
UNIT 1 SUSTAINING ECOSYSTEMS
 UNIT 1 SUSTAINING ECOSYSTEMS Chapter 2 Biogeochemical Cycles Science 10 Change & Recovery in Ecosystems (you do not need to copy) What happens to the materials that make up a truck when it begins to rust?
UNIT 1 SUSTAINING ECOSYSTEMS Chapter 2 Biogeochemical Cycles Science 10 Change & Recovery in Ecosystems (you do not need to copy) What happens to the materials that make up a truck when it begins to rust?
Oceanic CO 2 system - Significance
 OCN 401 Biogeochemical Systems (10.25.18) (10.30.18) (Schlesinger: Chapter 9) (11.27.18) Oceanic Carbon and Nutrient Cycling - Part 2 Lecture Outline 1. The Oceanic Carbon System 2. Nutrient Cycling in
OCN 401 Biogeochemical Systems (10.25.18) (10.30.18) (Schlesinger: Chapter 9) (11.27.18) Oceanic Carbon and Nutrient Cycling - Part 2 Lecture Outline 1. The Oceanic Carbon System 2. Nutrient Cycling in
Lecture 21: Soil Redox; Hydric Soils; Wetlands
 Lecture 21: Soil Redox; Hydric Soils; Wetlands Soil Oxidation-Reduction (Redox) Many Elements Exist in Multiple Oxidation States in Soils Oxygen: O 2 (0), H 2 O (-2) Carbon: CH 4 (-4), Organic C (-3 to
Lecture 21: Soil Redox; Hydric Soils; Wetlands Soil Oxidation-Reduction (Redox) Many Elements Exist in Multiple Oxidation States in Soils Oxygen: O 2 (0), H 2 O (-2) Carbon: CH 4 (-4), Organic C (-3 to
Chapter 3 Reading/Homework Quiz
 Name Chapter 3 Reading/Homework Quiz Date APES 1. Scientists estimate that tropical rain forests contain up to half of the earth s land plants and animal species. What percentage of the world s land surface
Name Chapter 3 Reading/Homework Quiz Date APES 1. Scientists estimate that tropical rain forests contain up to half of the earth s land plants and animal species. What percentage of the world s land surface
Introduction. Copyright 2002 Pearson Education, Inc., publishing as Benjamin Cummings
 Introduction An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an ecosystem involve two processes: energy flow
Introduction An ecosystem consists of all the organisms living in a community as well as all the abiotic factors with which they interact. The dynamics of an ecosystem involve two processes: energy flow
Chapter 24 Lecture Outline
 Chapter 24 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright 2016 McGraw-Hill Education. Permission required for reproduction
Chapter 24 Lecture Outline See separate PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright 2016 McGraw-Hill Education. Permission required for reproduction
Nitrogen Pollution and its Impacts
 Nitrogen Pollution and its Impacts OUTLINE: Background forms and cycling Sources Cycling, transport dynamics and loadings from watersheds Landuse and N exports Management Options to reduce N Effects and
Nitrogen Pollution and its Impacts OUTLINE: Background forms and cycling Sources Cycling, transport dynamics and loadings from watersheds Landuse and N exports Management Options to reduce N Effects and
Eutrophication processes in Alberta lakes
 Eutrophication processes in Alberta lakes Alexander P. Wolfe Department of Earth & Atmospheric Sciences University of Alberta, Edmonton Grand Beach Lake Winnipeg Eutrophication processes
Eutrophication processes in Alberta lakes Alexander P. Wolfe Department of Earth & Atmospheric Sciences University of Alberta, Edmonton Grand Beach Lake Winnipeg Eutrophication processes
Chemical Fluxes and Dynamics in River and Stream Ecosystems
 Chemical Fluxes and Dynamics in River and Stream Ecosystems W M Lewis, University of Colorado, Boulder, CO, USA ã 2009 Elsevier Inc. All rights reserved. Introduction Rivers and streams have in common
Chemical Fluxes and Dynamics in River and Stream Ecosystems W M Lewis, University of Colorado, Boulder, CO, USA ã 2009 Elsevier Inc. All rights reserved. Introduction Rivers and streams have in common
SPEEDING UP THE ECOLOGICAL RECOVERY OF THE BALTIC SEA
 SPEEDING UP THE ECOLOGICAL RECOVERY OF THE BALTIC SEA Esa Salminen, Ph.D. Vahanen Environment Oy Photograph: Janne Gröning SPEEDING UP THE ECOLOGICAL RECOVERY OF THE BALTIC SEA Assessment of: 1. the contribution
SPEEDING UP THE ECOLOGICAL RECOVERY OF THE BALTIC SEA Esa Salminen, Ph.D. Vahanen Environment Oy Photograph: Janne Gröning SPEEDING UP THE ECOLOGICAL RECOVERY OF THE BALTIC SEA Assessment of: 1. the contribution
3 3 Cycles of Matter Slide 1 of 33
 1 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within and between ecosystems.
1 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the biosphere very differently. Unlike the one-way flow of energy, matter is recycled within and between ecosystems.
Wetland Soils. Lecture outline: Importance of wetland soils
 Importance of wetland soils Wetland Soils Chemical transformations Chemical (nutrient) storage These affect plant growth and peat formation Lecture outline: What are soils? How does inundation change upland
Importance of wetland soils Wetland Soils Chemical transformations Chemical (nutrient) storage These affect plant growth and peat formation Lecture outline: What are soils? How does inundation change upland
Coastal Wetlands Formation, Functions, and Susceptibility. John Marton CWC Gulf Lagniappe November 23, 2013
 Coastal Wetlands Formation, Functions, and Susceptibility John Marton CWC Gulf Lagniappe November 23, 2013 What is a wetland? Wetlands are distinguished by the presence of water, either at the surface
Coastal Wetlands Formation, Functions, and Susceptibility John Marton CWC Gulf Lagniappe November 23, 2013 What is a wetland? Wetlands are distinguished by the presence of water, either at the surface
10/17/ Cycles of Matter. Recycling in the Biosphere. How does matter move among the living and nonliving parts of an ecosystem?
 2 of 33 3-3 Cycles of Matter How does matter move among the living and nonliving parts of an ecosystem? 3 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the
2 of 33 3-3 Cycles of Matter How does matter move among the living and nonliving parts of an ecosystem? 3 of 33 Recycling in the Biosphere Recycling in the Biosphere Energy and matter move through the
Metabolism Lectures. Outline:! Part I: Fermentations! Part II: Respiration! Part III: Metabolic Diversity
 Outline:! Part I: Fermentations! Part II: Respiration! Part III: Metabolic Diversity Metabolism Lectures Learning objectives are:! Learn about anaerobic respiratory metabolisms.! How can an inorganic compound
Outline:! Part I: Fermentations! Part II: Respiration! Part III: Metabolic Diversity Metabolism Lectures Learning objectives are:! Learn about anaerobic respiratory metabolisms.! How can an inorganic compound
PHOSPHORUS DYNAMICS & POLLUTION
 PHOSPHORUS DYNAMICS & POLLUTION (Source of some of the notes Zaimes & Shultz 2002 Phosphorus literature review Sharpley et al. 1999 Agricultural phosphorus & eutrophication) Introduction A major player
PHOSPHORUS DYNAMICS & POLLUTION (Source of some of the notes Zaimes & Shultz 2002 Phosphorus literature review Sharpley et al. 1999 Agricultural phosphorus & eutrophication) Introduction A major player
Nitrogen biogeochemistry. Lecture 1 Universidade do algarve
 Nitrogen biogeochemistry Lecture 1 Universidade do algarve Cycling of elements in the early stages of earth was slow, dependent on extreme conditions temperature, pressure, high energy radiations.. Purely
Nitrogen biogeochemistry Lecture 1 Universidade do algarve Cycling of elements in the early stages of earth was slow, dependent on extreme conditions temperature, pressure, high energy radiations.. Purely
Chapter 3 Ecosystem Ecology. Tuesday, September 19, 17
 Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
Chapter 3 Ecosystem Ecology Reversing Deforestation in Haiti Answers the following: Why is deforestation in Haiti so common? What the negative impacts of deforestation? Name three actions intended counteract
Chapter Two: Cycles of Matter (pages 32-65)
 Chapter Two: Cycles of Matter (pages 32-65) 2.2 Biogeochemical Cycles (pages 42 52) In order to survive and grow, organisms must obtain nutrients that serve as sources of energy or chemical building blocks,
Chapter Two: Cycles of Matter (pages 32-65) 2.2 Biogeochemical Cycles (pages 42 52) In order to survive and grow, organisms must obtain nutrients that serve as sources of energy or chemical building blocks,
Chapter 55: Ecosystems
 Ch. 55 Warm-Up 1. Draw an energy pyramid and label the following trophic levels: Primary producer Primary consumer Secondary consumer Tertiary consumer 2. What is an example of an organism at each level
Ch. 55 Warm-Up 1. Draw an energy pyramid and label the following trophic levels: Primary producer Primary consumer Secondary consumer Tertiary consumer 2. What is an example of an organism at each level
The Global Nitrogen Cycle
 OCN 401 The Global Nitrogen Cycle (11.30.10) Fig. 12.2. Units are 10 12 g N/yr (Tg) Role of N in Biogeochemistry Bioavailability of N (and/or P) can limit NPP on land/oceans; controls size of biomass N
OCN 401 The Global Nitrogen Cycle (11.30.10) Fig. 12.2. Units are 10 12 g N/yr (Tg) Role of N in Biogeochemistry Bioavailability of N (and/or P) can limit NPP on land/oceans; controls size of biomass N
CHEMICAL: CARBON and OXYGEN (read 44-45; in Dodson)
 BIOE 155, Fall BACKGROUND INFORMATION CHEMICAL: CARBON and OXYGEN (read -5; 3-39 in Dodson) Types of molecules Organic: compounds containing Carbon-Hydrogen bonds Inorganic: everything else. Photosynthesis
BIOE 155, Fall BACKGROUND INFORMATION CHEMICAL: CARBON and OXYGEN (read -5; 3-39 in Dodson) Types of molecules Organic: compounds containing Carbon-Hydrogen bonds Inorganic: everything else. Photosynthesis
an ecosystem is a community of different species interacting with one another and with their nonliving environment of matter and energy
 1 Ecocsystems: Energy Flow and Materials Cycling 2 EVPP 111 Lecture Dr. Largen Spring 2004 Energy Flow and Matter Cycling Energy flow s through ecosystems ecosystems global energy budget physical laws
1 Ecocsystems: Energy Flow and Materials Cycling 2 EVPP 111 Lecture Dr. Largen Spring 2004 Energy Flow and Matter Cycling Energy flow s through ecosystems ecosystems global energy budget physical laws
Trout Lake Big Lake Lake Balance NO 3
 1. You are a limnologist studying several lakes in northern Ontario. The lakes have little input of nutrients from streams. You measure the concentrations of nitrate and phosphate every two months, and
1. You are a limnologist studying several lakes in northern Ontario. The lakes have little input of nutrients from streams. You measure the concentrations of nitrate and phosphate every two months, and
Unit 3: Ecology II Section 1: Environmental Systems and Nutrient Cycling
 Unit 3: Ecology II Section 1: Environmental Systems and Nutrient Cycling Systems in the Environment are not Independent of one Another Central Case Study: The Vanishing Oysters of the Chesapeake Bay Chesapeake
Unit 3: Ecology II Section 1: Environmental Systems and Nutrient Cycling Systems in the Environment are not Independent of one Another Central Case Study: The Vanishing Oysters of the Chesapeake Bay Chesapeake
