STATE OF THE ART ON BIOBANKING INFRASTRUCTURE DEVELOPMENT AT IZSLER: BIOLOGICAL SPECIMEN COLLECTION, QUALITY CONTROL AND MANAGEMENT
|
|
|
- Randolf Tyler
- 5 years ago
- Views:
Transcription
1
2 ESBB Annual Meeting - Marseille, November th, 2011 STATE OF THE ART ON BIOBANKING INFRASTRUCTURE DEVELOPMENT AT IZSLER: BIOLOGICAL SPECIMEN COLLECTION, QUALITY CONTROL AND MANAGEMENT Maura Ferrari Istituto Zooprofilattico della Lombardia e dell Emilia Romagna Brescia (IZSLER), Italy
3 IZSLER Istituto Zooprofilattico Sperimentale della Lombardia e dell Emilia Romagna Veterinary Government Organization supported by Ministry of Health Guarantees Veterinary Service activities Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
4 Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
5 Where is IZSLER? LOMBARDIA: Brescia, Headquarter 8 Offsite Laboratories EMILIA ROMAGNA: 8 Offsite Laboratories Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
6 IZSLER Activities Animal health Food safety Zootechnic hygiene Animal welfare Several Reference Centres: Italian Ministry of Health, OIE, FAO Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
7 Livestock Population Lombardia Emilia Romagna % of italian population Bovine 1,526, , Equine 43,257 28, Swine 5,040,076 1,275, Ovine 211,825 86, Rabbit 611, , Avian Species 49,296,886 34,513, Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
8 Background Several laboratories involved in different branches Wide availability of biological resources and samples Development of a Biobank Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
9 Outline : Steps 1. Information processing / Records / Documentation 2. List of all the biological samples and resources at IZSLER 3. Selection of resources, materials to be collected, barcoding 4. Development of quality control criteria 5. Development of biobank infrastructure and backup system 6. Quality assurance system (ISO ), standard operating procedures (SOPs) Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
10 Step 1. BioBank Architecture The Architecture is an open system The system can communicate with other systems by applicative cooperation Legacy Image External system (monitoring, other biorepositories,.. ) Multichannel (desktop, internet, mobile,..) Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
11 Biobanking Architecture Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
12 Step 2. Census Algae: 294 Bacteria: 29,031 Chemical substrates: 26 Chlamydia: 18 Cell cultures: 602 Conjugated Ig: 40 Experimental immune sera: 380 Field sera: 382 Fungi: 25 Genomic sequences: 239 Monoclonal antibody: 64 Hybridomas: 6,427 Laboratory animals: 11 Mycoplasmas: 177 Metazoan parasites: 113 Nucleic acids: 24 Protozoa parasites: 63 Recombinant plasmids: 153 Prions: 58 Organs and tissues: 264 Viral pathological specimens: 426 Viruses: 5,271 Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
13 42,683 types of different samples Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
14 Step 3. Selection of the resources / specimens Preparation of forms specific for each biological sample Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
15 Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
16 Step 4. Development of quality control criteria and labelling Working groups for specimen preparation and quality control criteria: characterization typing absence of extraneous agents (viruses, mycoplasma, bacteria) Barcoding labelling Time consuming work with high labour costs Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
17 Barcode video Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
18 Currently Algae: 16 Bacteria: 196,000 Chemical substrates: 26 Chlamydia: 18 Cell cultures: 604 Conjugated Ig: 40 Experimental immune sera: 380 Field sera: 382 Fungi: 25 Genomic sequences: 239 Monoclonal antibody: 64 Hybridomas: 6,427 Laboratory animals: 11 Mycoplasmas: 177 Metazoan parasites: 113 Nucleic acids: 24 Protozoa parasites: 63 Recombinant plasmids: 153 Prions: 58 Organs and tissues: 264 Viral pathological specimens: 3 Viruses: 38 Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
19 Step 5. Identification of Biobank Infrastructures storage rooms + 4 C - 20 C Liquid Nitrogen Room temperature - 80 C Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
20 Step 6. Quality assurance system SOP preparation Biobank certification ISO Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
21 Field samples Reception Laboratory automation sample distribution and labelling Research Biobanking Sample processing: diagnostic services Datebase with traceability of all steps Veterinarians Clinical result transmission Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
22 Objective Construction of a new Italian Animal BioBanking Infrastructure Development of an European Animal BioBanking Infrastructure (ESFRI, BBMRI) Integration of international resources Improvement of scientific cooperation Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
23 Perspectives: Data and Samples Researcher Department Organization National International Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
24 Users Advantages Researchers Clinics involved in diagnostic laboratories Commercial companies Availability of biological resources, with well defined characteristics Biolological resource centres: reference material over time Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
25 Conclusions Bio-specimen resources Basic tool for Scientific Community and priceless Translational studies New perspectives in the future Improvement of Public Health Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
26 Thanks Researchers (our lab) Tina Lombardo Massimo Amadori Riccardo Villa Silvia Dotti IT Team Riccardo Possenti Giorgio Bontempi Stefano Vigliani Ricardo Kligman Roberta Pavesi All colleagues of IZSLER Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
27 Maura Ferrari. IZSLER biobanking ESBB Annual Meeting - Marseille, November 16-19th,
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2014 This report has been submitted : 2015-01-21 11:22:25 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Swine
OIE Reference Laboratory Reports Activities Activities in 2014 This report has been submitted : 2015-01-21 11:22:25 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Swine
OIE Collaborating Centres Reports Activities
 OIE Collaborating Centres Reports Activities Activities in 2016 This report has been submitted : 2017-01-16 17:19:29 Title of collaborating centre: Veterinary Biologicals Biobank Address of Collaborating
OIE Collaborating Centres Reports Activities Activities in 2016 This report has been submitted : 2017-01-16 17:19:29 Title of collaborating centre: Veterinary Biologicals Biobank Address of Collaborating
IZSLER IMPROVING SAFETY AND SECURITY
 IZSLER IMPROVING SAFETY AND SECURITY Biosafety and Biosecurity Compliance BIOSECURITY IN THE LABORATORY: EXPERIENCE OF THE OIE REFERENCE LABORATORY FOR FMD BIOSÉCURITÉ EN LABORATOIRE: EXPÉRIENCE DU LABORATOIRE
IZSLER IMPROVING SAFETY AND SECURITY Biosafety and Biosecurity Compliance BIOSECURITY IN THE LABORATORY: EXPERIENCE OF THE OIE REFERENCE LABORATORY FOR FMD BIOSÉCURITÉ EN LABORATOIRE: EXPÉRIENCE DU LABORATOIRE
Monoclonal Antibodies against FMDV type Asia 1: preliminary characterization and potential use in diagnostic assays
 Appendix 24 Monoclonal Antibodies against FMDV type Asia 1: preliminary characterization and potential use in diagnostic assays Grazioli S., Fallacara F. and Brocchi E. Istituto Zooprofilattico Sperimentale
Appendix 24 Monoclonal Antibodies against FMDV type Asia 1: preliminary characterization and potential use in diagnostic assays Grazioli S., Fallacara F. and Brocchi E. Istituto Zooprofilattico Sperimentale
OIE Reference Laboratory for Bovine Viral Diarrhoea Virus (BVDV).
 OIE Reference Laboratory for Bovine Viral Diarrhoea Virus (BVDV). P.D. Kirkland, Virology Laboratory, Elizabeth Macarthur Agricultural Institute (EMAI) OIE Reference Laboratory for Bovine Viral Diarrhoea
OIE Reference Laboratory for Bovine Viral Diarrhoea Virus (BVDV). P.D. Kirkland, Virology Laboratory, Elizabeth Macarthur Agricultural Institute (EMAI) OIE Reference Laboratory for Bovine Viral Diarrhoea
Evaluation of three rapid diagnostic tests used in bovine spongiform encephalopathy monitoring in Italy
 830 Brief Research Reports J Vet Diagn Invest 21:830 836 (2009) Evaluation of three rapid diagnostic tests used in bovine spongiform encephalopathy monitoring in Italy Elena Carra, 1 Roberta Taddei, Ilaria
830 Brief Research Reports J Vet Diagn Invest 21:830 836 (2009) Evaluation of three rapid diagnostic tests used in bovine spongiform encephalopathy monitoring in Italy Elena Carra, 1 Roberta Taddei, Ilaria
Research in rabbit science and production in Italy Monday, September 13, 2010
 Research in rabbit science and production in Italy Monday, September 13, 2010 University of Milano: research in rabbit science PROF. FABIO LUZI Sezione di Zootecnica Veterinaria DIPAV Dipartimento di Patologia
Research in rabbit science and production in Italy Monday, September 13, 2010 University of Milano: research in rabbit science PROF. FABIO LUZI Sezione di Zootecnica Veterinaria DIPAV Dipartimento di Patologia
Can be integrated with any immunodetection technology (e.g. ELISA, Western Blot, fluorescence, SPR )
 The ANANAS nanoparticles are a unique kind of poly-avidin nanoassemblies that override the limits of current technology for diagnostic amplification. ü HIGHER SENSITIVITY: Reduce detection limits ü VERSATILITY:
The ANANAS nanoparticles are a unique kind of poly-avidin nanoassemblies that override the limits of current technology for diagnostic amplification. ü HIGHER SENSITIVITY: Reduce detection limits ü VERSATILITY:
Chapter 17: Immunization & Immune Testing. 1. Immunization 2. Diagnostic Immunology
 Chapter 17: Immunization & Immune Testing 1. Immunization 2. Diagnostic Immunology 1. Immunization Chapter Reading pp. 505-511 What is Immunization? A method of inducing artificial immunity by exposing
Chapter 17: Immunization & Immune Testing 1. Immunization 2. Diagnostic Immunology 1. Immunization Chapter Reading pp. 505-511 What is Immunization? A method of inducing artificial immunity by exposing
1. Immunization. What is Immunization? 12/9/2016. Chapter 17: Immunization & Immune Testing. 1. Immunization 2. Diagnostic Immunology
 Chapter 17: Immunization & Immune Testing 1. Immunization 2. Diagnostic Immunology 1. Immunization Chapter Reading pp. 505-511 What is Immunization? A method of inducing artificial immunity by exposing
Chapter 17: Immunization & Immune Testing 1. Immunization 2. Diagnostic Immunology 1. Immunization Chapter Reading pp. 505-511 What is Immunization? A method of inducing artificial immunity by exposing
AD HOC GROUP ON HIGH THROUGHPUT SEQUENCING, BIOINFORMATICS AND COMPUTATIONAL GENOMICS 1 Paris, June 2017
 Original: English June 2017 AD HOC GROUP ON HIGH THROUGHPUT SEQUENCING, BIOINFORMATICS AND COMPUTATIONAL GENOMICS 1 Paris, 27 29 June 2017 The Fourth meeting of the OIE ad hoc Group on High Throughput
Original: English June 2017 AD HOC GROUP ON HIGH THROUGHPUT SEQUENCING, BIOINFORMATICS AND COMPUTATIONAL GENOMICS 1 Paris, 27 29 June 2017 The Fourth meeting of the OIE ad hoc Group on High Throughput
Handling and Shipping of Infectious Substances for Research/Clinical Trials
 OFFICE FOR RESEARCH MELBOURNE HEALTH STANDARD OPERATING PROCEDURE: SOP012 Handling and Shipping of Infectious Substances for Research/Clinical Trials Prepared by Sarah Rickard Position Manager of Research
OFFICE FOR RESEARCH MELBOURNE HEALTH STANDARD OPERATING PROCEDURE: SOP012 Handling and Shipping of Infectious Substances for Research/Clinical Trials Prepared by Sarah Rickard Position Manager of Research
Application for Research Involving Biological Materials and Recombinant DNA
 BATES COLLEGE Institutional Biosafety Committee Application for Research Involving Biological Materials and Recombinant DNA INSTRUCTIONS: All submissions must be typed. E-mail completed applications to
BATES COLLEGE Institutional Biosafety Committee Application for Research Involving Biological Materials and Recombinant DNA INSTRUCTIONS: All submissions must be typed. E-mail completed applications to
Fabrizio Agnoletti. Istituto Zooprofilattico Sperimentale delle Venezie.
 Fabrizio Agnoletti Istituto Zooprofilattico Sperimentale delle Venezie Mission: prevention, control and research activities in 3 main areas: animal health and welfare food safety environmental protection
Fabrizio Agnoletti Istituto Zooprofilattico Sperimentale delle Venezie Mission: prevention, control and research activities in 3 main areas: animal health and welfare food safety environmental protection
HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) POINTS TO CONSIDER ON SAFETY OF HOMEOPATHIC MEDICINAL PRODUCTS FROM BIOLOGICAL ORIGIN
 HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) POINTS TO CONSIDER ON SAFETY OF HOMEOPATHIC MEDICINAL PRODUCTS FROM BIOLOGICAL ORIGIN DISCUSSION IN THE HMPWG January 2001-April 2005 RELEASE FOR CONSULTATION
HOMEOPATHIC MEDICINAL PRODUCT WORKING GROUP (HMPWG) POINTS TO CONSIDER ON SAFETY OF HOMEOPATHIC MEDICINAL PRODUCTS FROM BIOLOGICAL ORIGIN DISCUSSION IN THE HMPWG January 2001-April 2005 RELEASE FOR CONSULTATION
In ensuring the safety of veterinary vaccines demonstration of freedom from EA is one of high importance.
 VICH/16/070 22 July 2016 Final Concept Paper for two VICH Guidelines: (1) general principles for detection of extraneous viruses in veterinary vaccines and defining the testing of seeds and materials of
VICH/16/070 22 July 2016 Final Concept Paper for two VICH Guidelines: (1) general principles for detection of extraneous viruses in veterinary vaccines and defining the testing of seeds and materials of
ROUND TABLE ARISLA Milano 26 marzo 2014 Le BioBanche Nodo Italiano di Biobanking and Biomolecular Resources Research Infrastructure
 ROUND TABLE ARISLA Milano 26 marzo 2014 Le BioBanche Nodo Italiano di Biobanking and Biomolecular Resources Research Infrastructure Marialuisa Lavitrano Università Milano-Bicocca Coordinatore BBMRI-Italia
ROUND TABLE ARISLA Milano 26 marzo 2014 Le BioBanche Nodo Italiano di Biobanking and Biomolecular Resources Research Infrastructure Marialuisa Lavitrano Università Milano-Bicocca Coordinatore BBMRI-Italia
HANDLING AND SHIPPING OF INFECTIOUS SUBSTANCES FOR CLINICAL TRIALS
 STANDARD OPERATING PROCEDURE HANDLING AND SHIPPING OF INFECTIOUS SUBSTANCES FOR CLINICAL TRIALS Standard Operating Procedure Western Health SOP reference 012 Version: 2.0 dated December 2015 Effective
STANDARD OPERATING PROCEDURE HANDLING AND SHIPPING OF INFECTIOUS SUBSTANCES FOR CLINICAL TRIALS Standard Operating Procedure Western Health SOP reference 012 Version: 2.0 dated December 2015 Effective
TOPICS GENERAL BACTERIOLOGY
 II year (2st semester) Scientific Field MICROBIOLOGY TUTOR ECTS PICA F. COORDINATOR MED/07 Microbiology Ceccherini Silberstein 1 Francesca MED/07 Microbiology Pica Francesca 5 MED/07 Microbiology Svicher
II year (2st semester) Scientific Field MICROBIOLOGY TUTOR ECTS PICA F. COORDINATOR MED/07 Microbiology Ceccherini Silberstein 1 Francesca MED/07 Microbiology Pica Francesca 5 MED/07 Microbiology Svicher
Arto Mannermaa. Research Manager, KUH Professor of personalized medicine and Biobanking, UEF
 Doctoral Programme of Clinical Research Introduction to Clinical Research, 2017 Arto Mannermaa Research Manager, KUH Professor of personalized medicine and Biobanking, UEF 040-3552752 arto.mannermaa@uef.fi
Doctoral Programme of Clinical Research Introduction to Clinical Research, 2017 Arto Mannermaa Research Manager, KUH Professor of personalized medicine and Biobanking, UEF 040-3552752 arto.mannermaa@uef.fi
GUIDELINES TO BLOCKING ENZYME IMMUNOSORBENT ASSAY FOR THE DETECTION OF AFRICAN HORSE SICKNESS VIRUS ANTIBODIES
 GUIDELINES TO BLOCKING ENZYME IMMUNOSORBENT ASSAY FOR THE DETECTION OF AFRICAN HORSE SICKNESS VIRUS ANTIBODIES Content 1. PURPOSE... 2 2. SCOPE... 2 3. REFERENCES... 2 4. TEST PRINCIPLE... 3 5. SAFETY
GUIDELINES TO BLOCKING ENZYME IMMUNOSORBENT ASSAY FOR THE DETECTION OF AFRICAN HORSE SICKNESS VIRUS ANTIBODIES Content 1. PURPOSE... 2 2. SCOPE... 2 3. REFERENCES... 2 4. TEST PRINCIPLE... 3 5. SAFETY
MVD-Enfer Chlamydia abortus-specific ELISA
 MVD-Enfer Chlamydia abortus-specific ELISA 01H14 (90Tests) Test for the in vitro detection of Chlamydia abortus antibodies in sheep For in vitro veterinary diagnostic use only Manufactured & Distributed
MVD-Enfer Chlamydia abortus-specific ELISA 01H14 (90Tests) Test for the in vitro detection of Chlamydia abortus antibodies in sheep For in vitro veterinary diagnostic use only Manufactured & Distributed
Destruction of Rinderpest Virus Containing Materials
 Destruction of Rinderpest Virus Containing Materials Date: May 2016 Supersedes: RP12.0 Contacts: FAO Secretariat Email: Rinderpest-Secretariat@fao.org OIE Secretariat Email: Rinderpest@oie.int Changes
Destruction of Rinderpest Virus Containing Materials Date: May 2016 Supersedes: RP12.0 Contacts: FAO Secretariat Email: Rinderpest-Secretariat@fao.org OIE Secretariat Email: Rinderpest@oie.int Changes
Research Compliance Requirements. Office of Research Compliance, The Texas A&M University System
 Research Compliance Requirements Office of Research Compliance, The Texas A&M University System http://www.tamus.edu/research/research-compliance/ Principal Investigator Responsibility It is the responsibility
Research Compliance Requirements Office of Research Compliance, The Texas A&M University System http://www.tamus.edu/research/research-compliance/ Principal Investigator Responsibility It is the responsibility
Introduction, by Tortora, Funke and Case, 11th Ed. TENTATIVE LECTURE OUTLINE DATE TOPIC CHAPTER
 MICROBIOLOGY 220 Spring 2014 TTh Section Professor: Scott Rose Text: Microbiology; An Introduction, by Tortora, Funke and Case, 11th Ed. TENTATIVE LECTURE OUTLINE DATE TOPIC CHAPTER Jan. 23 Introduction
MICROBIOLOGY 220 Spring 2014 TTh Section Professor: Scott Rose Text: Microbiology; An Introduction, by Tortora, Funke and Case, 11th Ed. TENTATIVE LECTURE OUTLINE DATE TOPIC CHAPTER Jan. 23 Introduction
The Global control of FMD - Tools, ideas and ideals Erice, Italy October 2008
 The Global control of FMD - Tools, ideas and ideals Erice, Italy 14-17 October 2008 Appendix 66 EVALUATION OF A LATERAL FLOW DEVICE FOR THE PEN-SIDE DIAGNOSIS OF FOOT- AND-MOUTH DISEASE N. P Ferris* 1,
The Global control of FMD - Tools, ideas and ideals Erice, Italy 14-17 October 2008 Appendix 66 EVALUATION OF A LATERAL FLOW DEVICE FOR THE PEN-SIDE DIAGNOSIS OF FOOT- AND-MOUTH DISEASE N. P Ferris* 1,
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2016 This report has been submitted : 2017-05-18 07:24:55 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Infection
OIE Reference Laboratory Reports Activities Activities in 2016 This report has been submitted : 2017-05-18 07:24:55 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Infection
Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Study Update / Change Request
 Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Study Update / Change Request 1 Submit the renewal electronically via email attachment to: IBCOFFICE@swmail.sw.org 2 Fax a Signed copy
Scott & White Healthcare Institutional Biosafety Committee (IBC) IBC Study Update / Change Request 1 Submit the renewal electronically via email attachment to: IBCOFFICE@swmail.sw.org 2 Fax a Signed copy
Adventitious Agents in Pharmaceutical Manufacturing. Deborah Gessell-Lee, Ph.D.
 Adventitious Agents in Pharmaceutical Manufacturing Deborah Gessell-Lee, Ph.D. deborah_gessell_lee@baxter.com May 05, 2016 News from the microbiology bench Then and Now Source: CDC/ Barbara Jenkins, NIOSH
Adventitious Agents in Pharmaceutical Manufacturing Deborah Gessell-Lee, Ph.D. deborah_gessell_lee@baxter.com May 05, 2016 News from the microbiology bench Then and Now Source: CDC/ Barbara Jenkins, NIOSH
Office of Graduate and Professional Studies
 Reset Form Print Form Office of Graduate and Professional Studies PROPOSAL APPROVAL FORM FOR THESIS, DISSERTATION, OR RECORD OF STUDY Full proposal should be attached This form must be approved by OGAPS
Reset Form Print Form Office of Graduate and Professional Studies PROPOSAL APPROVAL FORM FOR THESIS, DISSERTATION, OR RECORD OF STUDY Full proposal should be attached This form must be approved by OGAPS
Atlanta Biologicals Fetal Bovine Serum. and Cell Culture Media & Reagents
 Atlanta Biologicals Fetal Bovine Serum and Cell Culture Media & Reagents Atlanta Biologicals by R&D Systems Bio-Techne, the parent company of R&D Systems, proudly acquired Atlanta Biologicals in 2018.
Atlanta Biologicals Fetal Bovine Serum and Cell Culture Media & Reagents Atlanta Biologicals by R&D Systems Bio-Techne, the parent company of R&D Systems, proudly acquired Atlanta Biologicals in 2018.
Global Biological Resource Leader
 Custom Solutions Partner with the Global Biological Resource Leader Since 1925, ATCC has set the standard for the manufacture, characterization, preservation, storage and distribution of biological materials.
Custom Solutions Partner with the Global Biological Resource Leader Since 1925, ATCC has set the standard for the manufacture, characterization, preservation, storage and distribution of biological materials.
Office of Graduate and Professional Studies
 Reset Form Print Form Office of Graduate and Professional Studies PROPOSAL APPROVAL FORM FOR THESIS, DISSERTATION, OR RECORD OF STUDY Full proposal should be attached This form must be approved by OGAPS
Reset Form Print Form Office of Graduate and Professional Studies PROPOSAL APPROVAL FORM FOR THESIS, DISSERTATION, OR RECORD OF STUDY Full proposal should be attached This form must be approved by OGAPS
Role of an OIE Reference Laboratory Trichinellosis. Edoardo Pozio Istituto Superiore di Sanità Rome, Italy
 Role of an OIE Reference Laboratory Trichinellosis Edoardo Pozio Istituto Superiore di Sanità Rome, Italy Historical background The OIE Reference Laboratory for Trichinellosis was appointed at the Istituto
Role of an OIE Reference Laboratory Trichinellosis Edoardo Pozio Istituto Superiore di Sanità Rome, Italy Historical background The OIE Reference Laboratory for Trichinellosis was appointed at the Istituto
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2015 This report has been submitted : 2016-02-02 02:33:29 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Epizootic
OIE Reference Laboratory Reports Activities Activities in 2015 This report has been submitted : 2016-02-02 02:33:29 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Epizootic
Specialty Lab Services. Deep science at scale
 Specialty Lab Services Deep science at scale Advancing biomarker research Our broad expertise and global laboratory footprint deliver deep science at scale Specialty assays drive insight into preclinical
Specialty Lab Services Deep science at scale Advancing biomarker research Our broad expertise and global laboratory footprint deliver deep science at scale Specialty assays drive insight into preclinical
International. Recruiting Briefing
 International Recruiting Briefing 1 1. Chia Tai Group Senior Swine Veterinary Positions Available Position Title: National Supervisor of Swine Veterinary Services Regional Supervisor Swine Veterinary Services
International Recruiting Briefing 1 1. Chia Tai Group Senior Swine Veterinary Positions Available Position Title: National Supervisor of Swine Veterinary Services Regional Supervisor Swine Veterinary Services
Cooperation initiative Italy/Tunisia
 Cooperation initiative Italy/Tunisia Hammamet, 13 December 2017 INNOVATIVE PROJECTS OF INTERNATIONAL COOPERATION OF THE UNIVERSITY OF BOLOGNA In February, 2017, the University of Bologna has launched a
Cooperation initiative Italy/Tunisia Hammamet, 13 December 2017 INNOVATIVE PROJECTS OF INTERNATIONAL COOPERATION OF THE UNIVERSITY OF BOLOGNA In February, 2017, the University of Bologna has launched a
Laboratory Perspective: The challenge of standardisation in the face of the revolution in biotechnology and data processing
 Laboratory Perspective: The challenge of standardisation in the face of the revolution in biotechnology and data processing Steven Edwards Consultant Editor, OIE Manual of Diagnostic Tests and Vaccines
Laboratory Perspective: The challenge of standardisation in the face of the revolution in biotechnology and data processing Steven Edwards Consultant Editor, OIE Manual of Diagnostic Tests and Vaccines
This training module covers the following animals housed in vivaria: rodents rabbits birds amphibians reptiles non-human primates other mammals
 www.ccac.ca This training module is relevant to all animal users working with animals housed in vivaria which are enclosed areas such as laboratories where animals are kept for research, teaching or testing
www.ccac.ca This training module is relevant to all animal users working with animals housed in vivaria which are enclosed areas such as laboratories where animals are kept for research, teaching or testing
Vision, aims and strategies. Department of Immunology, Genetics and Pathology Uppsala University
 Vision, aims and strategies Department of Immunology, Genetics and Pathology Uppsala University April19,2011 SCOPEOFACTIVITIESATIGP Research at the Department focuses on translational medicine through
Vision, aims and strategies Department of Immunology, Genetics and Pathology Uppsala University April19,2011 SCOPEOFACTIVITIESATIGP Research at the Department focuses on translational medicine through
Use of diagnostic kits and reagents
 Use of diagnostic kits and reagents in the European NRLs. Niels Jørgen Olesen, Nicole Nicolajsen 14 th Annual Meeting of the National Reference Laboratories for Fish Diseases Aarhus, Denmark, May 27-28,
Use of diagnostic kits and reagents in the European NRLs. Niels Jørgen Olesen, Nicole Nicolajsen 14 th Annual Meeting of the National Reference Laboratories for Fish Diseases Aarhus, Denmark, May 27-28,
Guideline on requirements for the production and control of immunological veterinary medicinal products
 14 June 2012 EMA/CVMP/IWP/206555/2010 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on requirements for the production and control of immunological veterinary Draft agreed by Immunologicals
14 June 2012 EMA/CVMP/IWP/206555/2010 Committee for Medicinal Products for Veterinary Use (CVMP) Guideline on requirements for the production and control of immunological veterinary Draft agreed by Immunologicals
Regional Disease Surveillance workshop for banana Rwanda 25 th -29 th January IITA-pathology,Uganda
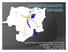 Regional Disease Surveillance workshop for banana Rwanda 25 th -29 th January 2010 Fen Beed Idd Ramathani IITA-pathology,Uganda Why lab-based diagnostic tools??? Field Diagnostic tools symptoms expression-bbtd
Regional Disease Surveillance workshop for banana Rwanda 25 th -29 th January 2010 Fen Beed Idd Ramathani IITA-pathology,Uganda Why lab-based diagnostic tools??? Field Diagnostic tools symptoms expression-bbtd
Need of strengthening the well defined in- vivo Systems for pre-clinical evaluation of vaccines in India
 8 th Indo Global summit and Expo on Vaccines, Therapeutics & Healthcare (VTH-2015) November 02-04, 2015 HICC, Hyderabad, India Chandrashekhar G Raut Sci-E & OIC National Institute of Virology, Bangalore
8 th Indo Global summit and Expo on Vaccines, Therapeutics & Healthcare (VTH-2015) November 02-04, 2015 HICC, Hyderabad, India Chandrashekhar G Raut Sci-E & OIC National Institute of Virology, Bangalore
Immunofluorescence Assays for Autoimmune and Infectious Disease
 life.science.discovery. Immunofluorescence Assays for Autoimmune and Infectious Disease PRODUCT CATALOG COMPANY MBL Bion, part of the MBL, has been manufacturing high-quality kits and components for autoimmune,
life.science.discovery. Immunofluorescence Assays for Autoimmune and Infectious Disease PRODUCT CATALOG COMPANY MBL Bion, part of the MBL, has been manufacturing high-quality kits and components for autoimmune,
Understand biotechnology in livestock animals. Objective 5.04
 Understand biotechnology in livestock animals. Objective 5.04 Biotechnology and Ethical Issues Biotechnology- technology concerning the application of biological and engineering techniques to microorganisms,
Understand biotechnology in livestock animals. Objective 5.04 Biotechnology and Ethical Issues Biotechnology- technology concerning the application of biological and engineering techniques to microorganisms,
Bachelor of Science (Hons) / Bachelor of Science Biomedical Science
 Bachelor of Science (Hons) / Bachelor of Science Biomedical Science Awarded by In collaboration with This degree course is designed to give you a broad understanding of the scientific investigation of
Bachelor of Science (Hons) / Bachelor of Science Biomedical Science Awarded by In collaboration with This degree course is designed to give you a broad understanding of the scientific investigation of
Annex to the Accreditation Certificate D PL according to DIN EN ISO/IEC 17025:2005
 Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D PL 13372 01 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 22.05.2014 to 25.03.2017
Deutsche Akkreditierungsstelle GmbH German Accreditation Body Annex to the Accreditation Certificate D PL 13372 01 00 according to DIN EN ISO/IEC 17025:2005 Period of validity: 22.05.2014 to 25.03.2017
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2016 This report has been submitted : 2017-01-18 10:31:01 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Contagious
OIE Reference Laboratory Reports Activities Activities in 2016 This report has been submitted : 2017-01-18 10:31:01 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Contagious
University Medical Microbiology
 Madison College 20806273 University Medical Microbiology Outline of Instruction Course Information Description Instructional Level Total Credits 5.00 Total Hours 116.00 University Medical Microbiology
Madison College 20806273 University Medical Microbiology Outline of Instruction Course Information Description Instructional Level Total Credits 5.00 Total Hours 116.00 University Medical Microbiology
MICROBIOLOGY (MICR) Microbiology (MICR) 1
 Microbiology (MICR) 1 MICROBIOLOGY (MICR) MICR 1513 Inquiry-Based Biology Description: Directed inquiry and hands-on study of biological principles. Restricted to elementary education majors or related
Microbiology (MICR) 1 MICROBIOLOGY (MICR) MICR 1513 Inquiry-Based Biology Description: Directed inquiry and hands-on study of biological principles. Restricted to elementary education majors or related
Short Title: CCI Biobank
 Patient Label Stamp or letterhead of the department Patient Information Donation, Storage and Use of Biological Materials and Collection, Processing and Use of Data of the CCI Biobank, Medical Center -
Patient Label Stamp or letterhead of the department Patient Information Donation, Storage and Use of Biological Materials and Collection, Processing and Use of Data of the CCI Biobank, Medical Center -
ICANtibodies TM. Has discovered more than 200 different antibodies for less than 2 years.
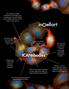 An innovative antibody development technology using breakthrough nanotaxis leading to the antigen expression by the chosen animal species. Has discovered more than 200 different antibodies for less than
An innovative antibody development technology using breakthrough nanotaxis leading to the antigen expression by the chosen animal species. Has discovered more than 200 different antibodies for less than
BIOSAFETY REGISTRATION FORM
 BRF 5/2015 Page 1 of 5 COMMITTEE USE ONLY 1. PERSONNEL TEMPLE UNIVERSITY Office of the Vice Provost for Research Division of Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax:
BRF 5/2015 Page 1 of 5 COMMITTEE USE ONLY 1. PERSONNEL TEMPLE UNIVERSITY Office of the Vice Provost for Research Division of Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax:
Biological and Select Agent Safety Policy
 4.20.1 POLICY PURPOSE All possession, use, and transportation of biological agents hazardous to human, animal, and plant health must conform to regulations and guidelines established by federal, state,
4.20.1 POLICY PURPOSE All possession, use, and transportation of biological agents hazardous to human, animal, and plant health must conform to regulations and guidelines established by federal, state,
Frequently Asked Questions (FAQs): FBS
 Frequently Asked Questions (FAQs): FBS What exactly is serum? Serum is the liquid fraction of clotted whole blood. Unlike plasma, no anti-coagulant will have been added to the blood following collection
Frequently Asked Questions (FAQs): FBS What exactly is serum? Serum is the liquid fraction of clotted whole blood. Unlike plasma, no anti-coagulant will have been added to the blood following collection
Departments of Biotechnology. Academic planner for (ODD semesters) Month I Semester III Semester V Semester
 Departments of Biotechnology Academic planner for 2014-15 (ODD semesters) Subject: Biotechnology Month I Semester III Semester V Semester JULY AUGUST Cell as a Basic unit of Living Systems, Discovery of
Departments of Biotechnology Academic planner for 2014-15 (ODD semesters) Subject: Biotechnology Month I Semester III Semester V Semester JULY AUGUST Cell as a Basic unit of Living Systems, Discovery of
Registration Document For Biohazards
 Protocol #: Registration Document For Biohazards All applicants are required to complete the following sections: Principal Investigator Information Location of Study Section A: General Administrative Information
Protocol #: Registration Document For Biohazards All applicants are required to complete the following sections: Principal Investigator Information Location of Study Section A: General Administrative Information
AuDit REPORt. Offi cial Control Audit Relating to On Farm Emergency Slaughtered Bovine Animals Department of Agriculture, Food and the Marine
 AuDit REPORt Offi cial Control Audit Relating to On Farm Emergency Slaughtered Bovine Animals Department of Agriculture, Food and the Marine Audit REPORT Official Control Audit Relating to On Farm Emergency
AuDit REPORt Offi cial Control Audit Relating to On Farm Emergency Slaughtered Bovine Animals Department of Agriculture, Food and the Marine Audit REPORT Official Control Audit Relating to On Farm Emergency
WORK PROGRAMME EUROPEAN UNION REFERENCE LABORATORY FOR PARASITES. European Union Reference Laboratory for Parasites. page 1 of 15
 EUROPEAN UNION REFERENCE LABORATORY FOR PARASITES WORK PROGRAMME 2015 page 1 of 15 INDEX 1. Providing national reference laboratories with details of analytical methods, including reference methods 4 1.1
EUROPEAN UNION REFERENCE LABORATORY FOR PARASITES WORK PROGRAMME 2015 page 1 of 15 INDEX 1. Providing national reference laboratories with details of analytical methods, including reference methods 4 1.1
VetBioNet. Veterinary Biocontained facility Network for excellence in animal infectiology research and experimentation. Newsletter Issue 1
 VetBioNet Veterinary Biocontained facility Network for excellence in animal infectiology research and experimentation Newsletter Issue 1 Summary VetBioNet Call for Proposals and Sample Requests... 2 VetBioNet
VetBioNet Veterinary Biocontained facility Network for excellence in animal infectiology research and experimentation Newsletter Issue 1 Summary VetBioNet Call for Proposals and Sample Requests... 2 VetBioNet
BIOSAFETY REGISTRATION FORM
 BRF 10/2015 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax: (215) 707-9100, Email: ibc@temple.edu research.temple.edu/research-compliance
BRF 10/2015 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # Research Compliance Institutional Biosafety Committee Tel: (215) 707-9741, Fax: (215) 707-9100, Email: ibc@temple.edu research.temple.edu/research-compliance
CHAPTER 24. Immunology
 CHAPTER 24 Diagnostic i Microbiology and Immunology Growth-Dependent Diagnostic Methods Isolation of Pathogens from Clinical Specimens Proper sampling and culture of a suspected pathogen is the most reliable
CHAPTER 24 Diagnostic i Microbiology and Immunology Growth-Dependent Diagnostic Methods Isolation of Pathogens from Clinical Specimens Proper sampling and culture of a suspected pathogen is the most reliable
BIOTECHNOLOGY. Course Syllabus. Section A: Engineering Mathematics. Subject Code: BT. Course Structure. Engineering Mathematics. General Biotechnology
 BIOTECHNOLOGY Subject Code: BT Course Structure Sections/Units Section A Section B Unit 1 Unit 2 Unit 3 Unit 4 Unit 5 Unit 6 Unit 7 Section C Section D Section E Topics Engineering Mathematics General
BIOTECHNOLOGY Subject Code: BT Course Structure Sections/Units Section A Section B Unit 1 Unit 2 Unit 3 Unit 4 Unit 5 Unit 6 Unit 7 Section C Section D Section E Topics Engineering Mathematics General
School of Allied Medical Sciences. Course Description Guide Associate in Medical Laboratory Sciences. Page 1 of 15
 School of Allied Medical Sciences Course Description Guide Associate in Medical Laboratory Sciences Page 1 of 15 Program Name & Definition: Associate in Medical Laboratory Sciences Laboratory sciences
School of Allied Medical Sciences Course Description Guide Associate in Medical Laboratory Sciences Page 1 of 15 Program Name & Definition: Associate in Medical Laboratory Sciences Laboratory sciences
Bachelor s Degree in Food Science and Technology. 3 rd YEAR Food Science II ECTS credits: 4,5 Semester: 1
 Bachelor s Degree in 3 rd YEAR 5168 Food Science II ECTS credits: 4,5 Semester: 1 1. Understand the basic aspects of the origin, identification, structure, composition and analysis of the foodstuffs covered
Bachelor s Degree in 3 rd YEAR 5168 Food Science II ECTS credits: 4,5 Semester: 1 1. Understand the basic aspects of the origin, identification, structure, composition and analysis of the foodstuffs covered
IBC Approval at TAMU What? Why? How?
 IBC Approval at TAMU What? Why? How? Biosafety Program Presented by Christine T. McFarland, Ph.D. Director, Biosafety, BSO, RO Why is IBC approval necessary? System Regulation 15.99.06: Use of Biohazards
IBC Approval at TAMU What? Why? How? Biosafety Program Presented by Christine T. McFarland, Ph.D. Director, Biosafety, BSO, RO Why is IBC approval necessary? System Regulation 15.99.06: Use of Biohazards
MICROBIO, IMMUN, PATHOLOGY-MIP (MIP)
 Microbio, Immun, Pathology-MIP (MIP) 1 MICROBIO, IMMUN, PATHOLOGY-MIP (MIP) Courses MIP 101 Introduction to Human Disease (GT-SC2) Credits: 3 (3-0-0) Survey of human systems and diseases. Additional Information:
Microbio, Immun, Pathology-MIP (MIP) 1 MICROBIO, IMMUN, PATHOLOGY-MIP (MIP) Courses MIP 101 Introduction to Human Disease (GT-SC2) Credits: 3 (3-0-0) Survey of human systems and diseases. Additional Information:
i) Wild animals: Those animals that do not live under human supervision or control and do not have their phenotype selected by humans.
 The OIE Validation Recommendations provide detailed information and examples in support of the OIE Validation Standard that is published as Chapter 1.1.6 of the Terrestrial Manual, or Chapter 1.1.2 of
The OIE Validation Recommendations provide detailed information and examples in support of the OIE Validation Standard that is published as Chapter 1.1.6 of the Terrestrial Manual, or Chapter 1.1.2 of
Syllabus for BIO 310 Microbiology Lecture 3.0 Credit Hours Fall 2012
 Syllabus for BIO 310 Microbiology Lecture 3.0 Credit Hours Fall 2012 I. COURSE DESCRIPTION A study of classification, cultivation, physiology, growth, morphology, pathogenicity, and economic importance
Syllabus for BIO 310 Microbiology Lecture 3.0 Credit Hours Fall 2012 I. COURSE DESCRIPTION A study of classification, cultivation, physiology, growth, morphology, pathogenicity, and economic importance
OIE Reference Laboratory Reports Activities
 OIE Reference Laboratory Reports Activities Activities in 2013 This report has been submitted : 2014-02-11 13:01:53 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Rabbit
OIE Reference Laboratory Reports Activities Activities in 2013 This report has been submitted : 2014-02-11 13:01:53 Name of disease (or topic) for which you are a designated OIE Reference Laboratory: Rabbit
AKESOgen, Inc, 3155 Northwoods Place, NW, Norcross, GA 30071, USA Tel: +1 (770) Page 1
 Page 1 HLA Sequencing Based Typing Workflow: A- Sample Collection: iswab-discovery (Mawi DNA Technologies) - Sample was transported by standard US postal services mail, no cold chain of any sort was involved
Page 1 HLA Sequencing Based Typing Workflow: A- Sample Collection: iswab-discovery (Mawi DNA Technologies) - Sample was transported by standard US postal services mail, no cold chain of any sort was involved
NIHR National Biosample Centre
 NIHR National Biosample Centre A key piece of national infrastructure Dr Kristian Spreckley Business Development Director Overview UK Biobank UK Biocentre NIHR National Biosample Centre UK Biobank: Overview
NIHR National Biosample Centre A key piece of national infrastructure Dr Kristian Spreckley Business Development Director Overview UK Biobank UK Biocentre NIHR National Biosample Centre UK Biobank: Overview
Microarray Industry Products
 Via Nicaragua, 12-14 00040 Pomezia (Roma) Phone: +39 06 91601628 Fax: +39 06 91612477 info@lifelinelab.com www.lifelinelab.com Microarray Industry Products Page 10 NBT / BCPIP Chromogenic phosphatase
Via Nicaragua, 12-14 00040 Pomezia (Roma) Phone: +39 06 91601628 Fax: +39 06 91612477 info@lifelinelab.com www.lifelinelab.com Microarray Industry Products Page 10 NBT / BCPIP Chromogenic phosphatase
Version 9/12/2016 Page 1 of 10
 Instructions for the Application for Teaching Activities Involving Biohazardous Agents (NIH Recombinant and Synthetic Nucleic Acid Molecules, Other Biohazardous Agents, and Human Materials) The Application
Instructions for the Application for Teaching Activities Involving Biohazardous Agents (NIH Recombinant and Synthetic Nucleic Acid Molecules, Other Biohazardous Agents, and Human Materials) The Application
COMMITTEE USE ONLY IBC REGISTRATION
 BRF September 2016 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # ASSOCIATED ACUP/IRB# Research Integrity and Compliance Institutional Biosafety Committee APPROVAL BIOSAFETY REGISTRATION FORM Tel: (215)
BRF September 2016 Page 1 of 6 COMMITTEE USE ONLY IBC REGISTRATION # ASSOCIATED ACUP/IRB# Research Integrity and Compliance Institutional Biosafety Committee APPROVAL BIOSAFETY REGISTRATION FORM Tel: (215)
ANTIBODIES A LABORATORY SECOND EDITION
 page 1 / 5 page 2 / 5 antibodies a laboratory second pdf Thyroid autoantibodies are antibodies that develop when a person's immune system mistakenly targets components of the thyroid gland or thyroid proteins,
page 1 / 5 page 2 / 5 antibodies a laboratory second pdf Thyroid autoantibodies are antibodies that develop when a person's immune system mistakenly targets components of the thyroid gland or thyroid proteins,
GENERAL PHARMACOPOEIA MONOGRAPH
 MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION GENERAL PHARMACOPOEIA MONOGRAPH Immunobiological GPM. 1.8.1.0002.15 medicinal products First Edition The present General Pharmacopoeia Monograph applies to
MINISTRY OF HEALTH OF THE RUSSIAN FEDERATION GENERAL PHARMACOPOEIA MONOGRAPH Immunobiological GPM. 1.8.1.0002.15 medicinal products First Edition The present General Pharmacopoeia Monograph applies to
SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS AND BIOLOGICAL WASTE
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
Tues 1/21. Today: Virus movie clip, ek paragraph for ch 20. Next class: collect Ch. 20 Guided Reading
 Tues 1/21 Today: Virus movie clip, ek paragraph for ch 20. Next class: collect Ch. 20 Guided Reading Pg. 104 Ch. 20 Guided Reading Pg. 105 EK Paragraph 3C3 Wed. 1/22 Collect-Ch 20 Guided Reading Today:
Tues 1/21 Today: Virus movie clip, ek paragraph for ch 20. Next class: collect Ch. 20 Guided Reading Pg. 104 Ch. 20 Guided Reading Pg. 105 EK Paragraph 3C3 Wed. 1/22 Collect-Ch 20 Guided Reading Today:
P a g e 1 Long Term Storage
 Page 1 Preface Revisions: This is a living document subject to ongoing improvement. Feedback or suggestions for improvement from registered Select Agent entities or the public are welcomed. Submit comments
Page 1 Preface Revisions: This is a living document subject to ongoing improvement. Feedback or suggestions for improvement from registered Select Agent entities or the public are welcomed. Submit comments
A Gateway for Health: BBMRI and Africa Prof. Jan-Eric Litton Director General BBMRI-ERIC
 A Gateway for Health: BBMRI and Africa Prof. Jan-Eric Litton Director General Most of our current knowledge on diseases as well as available diagnostic assays and drugs are based on investigation of biological
A Gateway for Health: BBMRI and Africa Prof. Jan-Eric Litton Director General Most of our current knowledge on diseases as well as available diagnostic assays and drugs are based on investigation of biological
Laboratory Procedures in Clinical Microbiology
 Laboratory Procedures in Clinical Microbiology Laboratory Procedures in Clinical Microbiology Edited by John A. Washington With Contributions by Members of the Section of Clinical Microbiology Department
Laboratory Procedures in Clinical Microbiology Laboratory Procedures in Clinical Microbiology Edited by John A. Washington With Contributions by Members of the Section of Clinical Microbiology Department
Animal Welfare Monitoring and Livestock Traceability During Transport
 Animal Welfare Monitoring and Livestock Traceability During Transport Adriano Di Pasquale (*), Enzo Isocrono (*), Luigi Possenti (*), Cesare Di Francesco (*), Walter Di Donato (*), Gianluca Fiore (^),
Animal Welfare Monitoring and Livestock Traceability During Transport Adriano Di Pasquale (*), Enzo Isocrono (*), Luigi Possenti (*), Cesare Di Francesco (*), Walter Di Donato (*), Gianluca Fiore (^),
Instructions for Biosafety Application Research and Sponsored Programs 201J University Hall Wright State University Dayton, OH (937)
 Instructions for Biosafety Application Research and Sponsored Programs 201J University Hall Wright State University Dayton, OH 45435 (937) 775-2425 Please use the attached application for requesting a
Instructions for Biosafety Application Research and Sponsored Programs 201J University Hall Wright State University Dayton, OH 45435 (937) 775-2425 Please use the attached application for requesting a
Regulatory Status of Veterinary Medicinal Products (VMPs)- An Indian Perspective. S.N.Singh
 Regulatory Status of Veterinary Medicinal Products (VMPs)- An Indian Perspective S.N.Singh snsingh.2006@gmail.com Regulatory Structure in India India is a federal form of government and the medical regulatory
Regulatory Status of Veterinary Medicinal Products (VMPs)- An Indian Perspective S.N.Singh snsingh.2006@gmail.com Regulatory Structure in India India is a federal form of government and the medical regulatory
Lecture Series 10 The Genetics of Viruses and Prokaryotes
 Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Lecture Series 10 The Genetics of Viruses and Prokaryotes The Genetics of Viruses and Prokaryotes A. Using Prokaryotes and Viruses for Genetic Experiments B. Viruses: Reproduction and Recombination C.
Syllabus for BIO 310 Microbiology Lecture 3.0 Credit Hours Spring 2015
 Syllabus for BIO 310 Microbiology Lecture 3.0 Credit Hours Spring 2015 I. COURSE DESCRIPTION This course is a study of the classification, cultivation, physiology, growth, morphology, pathogenicity, and
Syllabus for BIO 310 Microbiology Lecture 3.0 Credit Hours Spring 2015 I. COURSE DESCRIPTION This course is a study of the classification, cultivation, physiology, growth, morphology, pathogenicity, and
Microbiology An Introduction Tortora Funke Case Eleventh Edition
 Microbiology An Introduction Tortora Funke Case Eleventh Edition Pearson Education Limited Edinburgh Gate Harlow Essex CM20 2JE England and Associated Companies throughout the world Visit us on the World
Microbiology An Introduction Tortora Funke Case Eleventh Edition Pearson Education Limited Edinburgh Gate Harlow Essex CM20 2JE England and Associated Companies throughout the world Visit us on the World
Regional Biobank activity: getting started
 ESBB LIMS Workshop Regional Biobank activity: getting started Milan 13-14 March 2014 Maria Grazia Daidone Fondazione IRCCS Istituto Nazionale dei Tumori Milan Medical Treatment Clinical Biobanking Medical
ESBB LIMS Workshop Regional Biobank activity: getting started Milan 13-14 March 2014 Maria Grazia Daidone Fondazione IRCCS Istituto Nazionale dei Tumori Milan Medical Treatment Clinical Biobanking Medical
Touro University of California Mare Island, Vallejo
 Touro University of California Mare Island, Vallejo Institutional Biosafety Committee INITIAL REVIEW FORM Date Received: For IBC use only: Exempt Approved Approved with contingency(ies) Revisions required
Touro University of California Mare Island, Vallejo Institutional Biosafety Committee INITIAL REVIEW FORM Date Received: For IBC use only: Exempt Approved Approved with contingency(ies) Revisions required
ANIMAL EXPERIMENTATION FOR HEALTH RESEARCH. Prof. M. C. Sharma Director Indian Veterinary Research Institute IZATNAGAR (UP)
 ANIMAL EXPERIMENTATION FOR HEALTH RESEARCH Prof. M. C. Sharma Director Indian Veterinary Research Institute IZATNAGAR-243 122 (UP) E-mail:dirivri@ivri.res.in, directorivri@gmail.com Frontline Statement
ANIMAL EXPERIMENTATION FOR HEALTH RESEARCH Prof. M. C. Sharma Director Indian Veterinary Research Institute IZATNAGAR-243 122 (UP) E-mail:dirivri@ivri.res.in, directorivri@gmail.com Frontline Statement
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations. Audrey Chang, PhD, Senior Director Development Services
 Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
Can/How do we put data intensive biology in the picture?
 www.crs4.it Can/How do we put data intensive biology in the picture? 1 www.crs4.it Centro di Ricerca, Sviluppo e Studi Superiori in Sardegna Interdisciplinary research center focused on computational sciences
www.crs4.it Can/How do we put data intensive biology in the picture? 1 www.crs4.it Centro di Ricerca, Sviluppo e Studi Superiori in Sardegna Interdisciplinary research center focused on computational sciences
Chapter 5. Microbial Biotechnology. PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.
 PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 5 Microbial Biotechnology Lectures by Lara Dowland Chapter Contents 5.1 The Structure
PowerPoint Lectures for Introduction to Biotechnology, Second Edition William J.Thieman and Michael A.Palladino Chapter 5 Microbial Biotechnology Lectures by Lara Dowland Chapter Contents 5.1 The Structure
20/12/2013 HERACLES. KICK-OFF meeting Dec The EC launched a SPECIFIC CALL (First Time!!) on the neglected infectious diseases.
 HERACLES KICK-OFF meeting 17-18 Dec 2013 The EC launched a SPECIFIC CALL (First Time!!) on the neglected infectious diseases. 1 THEME: HEALTH.2013.2.3.4-1: Neglected infectious diseases of Central and
HERACLES KICK-OFF meeting 17-18 Dec 2013 The EC launched a SPECIFIC CALL (First Time!!) on the neglected infectious diseases. 1 THEME: HEALTH.2013.2.3.4-1: Neglected infectious diseases of Central and
Official Journal of the European Union L 55/11
 27.2.2009 Official Journal of the European Union L 55/11 COMMISSION REGULATION (EC) No 162/2009 of 26 February 2009 amending Annexes III and X to Regulation (EC) No 999/2001 of the European Parliament
27.2.2009 Official Journal of the European Union L 55/11 COMMISSION REGULATION (EC) No 162/2009 of 26 February 2009 amending Annexes III and X to Regulation (EC) No 999/2001 of the European Parliament
Making More Efficient Use of Biospecimens. ISBER Contributions and Initiatives
 Making More Efficient Use of Biospecimens. ISBER Contributions and Initiatives Members of the ISBER Education and Training Committee a,1, Members of the ISBER Science Policy a,1, Members of the ISBER Biospecimen
Making More Efficient Use of Biospecimens. ISBER Contributions and Initiatives Members of the ISBER Education and Training Committee a,1, Members of the ISBER Science Policy a,1, Members of the ISBER Biospecimen
Diagnostic Microbiology
 Diagnostic Microbiology Identification of Microbes Lecture: 1 Out lines What is expected out of this course??? At the end of this course, you will be able to apply Conventional/ Molecular diagnostic methods
Diagnostic Microbiology Identification of Microbes Lecture: 1 Out lines What is expected out of this course??? At the end of this course, you will be able to apply Conventional/ Molecular diagnostic methods
