DNA Structure Requirements for the Escherichia coli Complex Clamp Loader and DNA Polymerase III Holoenzyme*
|
|
|
- Abel Snow
- 6 years ago
- Views:
Transcription
1 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 275, No. 15, Issue of April 14, pp , by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. DNA Structure Requirements for the Escherichia coli Complex Clamp Loader and DNA Polymerase III Holoenzyme* (Received for publication, November 11, 1999) Nina Yao, Frank P. Leu, Jelena Anjelkovic, Jennifer Turner, and Mike O Donnell From the Joan and Sanford I. Weill Graduate School of Medical Sciences, Cornell University and the Laboratory of DNA Replication, Rockefeller University and the Howard Hughes Medical Institute, New York, New York The Escherichia coli chromosomal replicase, DNA polymerase III holoenzyme, is highly processive during DNA synthesis. Underlying high processivity is a ringshaped protein, the clamp, that encircles DNA and slides along it, thereby tethering the enzyme to the template. The clamp is assembled onto DNA by the multiprotein complex clamp loader that opens and closes the ring around DNA in an ATP-dependent manner. This study examines the DNA structure required for clamp loading action. We found that the complex assembles onto supercoiled DNA (replicative form I), but only at very low ionic strength, where regions of unwound DNA may exist in the duplex. Consistent with this, the complex does not assemble onto relaxed closed circular DNA even at low ionic strength. Hence, a 3 -end is not required for clamp loading, but a singlestranded DNA (ssdna)/double-stranded DNA (dsdna) junction can be utilized as a substrate, a result confirmed using synthetic oligonucleotides that form forked ssdna/dsdna junctions on M13 ssdna. On a flush primed template, the complex exhibits polarity; it acts specifically at the 3 -ssdna/dsdna junction to assemble onto the DNA. The complex can assemble onto a primed site as short as 10 nucleotides, corresponding to the width of the ring. However, a protein block placed closer than 14 base pairs (bp) upstream from the primer 3 terminus prevents the clamp loading reaction, indicating that the complex and its associated clamp interact with bp at a ssdna/dsdna junction during the clamp loading operation. A protein block positioned closer than bp from the 3 terminus prevents use of the clamp by the polymerase in chain elongation, indicating that the polymerase has an even greater spatial requirement than the complex on the duplex portion of the primed site for function with. Interestingly, DNA secondary structure elements placed near the 3 terminus impose similar steric limits on the complex and polymerase action with. The possible biological significance of these structural constraints is discussed. Escherichia coli DNA polymerase III holoenzyme (pol III 1 * This work was supported by Grant GM38839 from the National Institutes of Health. The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. To whom correspondence should be addressed: Laboratory of DNA Replication, Rockefeller University, P. O. Box 228, 1230 York Ave., New York, NY The abbreviations used are: pol III, DNA polymerase III; ssdna, single-stranded DNA; dsdna, double-stranded DNA; bp, base pair(s); SSB, E. coli single-stranded DNA-binding protein; ATP S, adenosine 5 -O-(thiotriphosphate); PCNA, proliferating cell nuclear antigen holoenzyme) is a highly processive multisubunit replicase (1). Processivity is conferred to the polymerase by the subunit (2, 3). The subunit is a ring-shaped protein that completely encircles DNA and slides along the duplex (4, 5). The ring endows the polymerase with high processivity by binding directly to it, continuously tethering it to the template during synthesis (4). The ring does not assemble onto DNA by itself; for this, it requires the clamp loading action of the complex. The complex is composed of five different proteins (,,,, and ) (1). Upon binding ATP, the complex opens the ring and positions it around the primed template (7, 8). Hydrolysis of two molecules of ATP results in closing the ring around DNA and dissociation of the complex from the clamp (7, 49). Following the release of the clamp loader from the clamp, the catalytic core polymerase subassembly (pol III core) within pol III holoenzyme couples with the clamp at the 3 terminus to form a highly processive polymerase (9, 10). The pol III core is composed of three subunits:,, and (11). The subunit is the DNA polymerase (6), and the subunit is the proofreading 3 5 exonuclease (12). Chromosome replication is performed by the large pol III holoenzyme assembly, which contains two catalytic cores, one clamp loader, and two clamps (1, 13). At the center of the holoenzyme structure is a dimer of. The dimer organizes the structure, as it holds together two molecules of pol III core and associates with one molecule of the complex. This assembly is referred to as pol III* and has the composition ( ) (13). The and subunits are related, as they are encoded by the same gene, dnax (14). A translational frameshift at amino acid 430 results in one unique C-terminal residue before termination, thus forming the 47-kDa subunit; translation of the entire dnax gene results in the 71-kDa subunit (15 17). The subunit is the only protein in the complex that binds and hydrolyzes ATP; and therefore, is the motor of the clamp loader machine (15, 18). Multiple copies of the subunit are present in the isolated complex; estimates range from two to four (19 21). The remaining subunits of the complex (,,, and ) are each present as a single copy (19 21). By itself, the subunit binds the clamp and opens the ring without ATP (7, 22), but cannot assemble onto DNA. Hence, other subunits and ATP are needed for the organizational task of positioning DNA inside the open ring and reclosing the clamp around DNA. In the complex, the subunit is sequestered from interaction with (22). Upon binding ATP, the complex undergoes a conformational change that exposes for interaction with, whereupon the clamp opens, and the ATP complex composite binds DNA (8, 22). Hydrolysis of ATP is required for the clamp to close around DNA and to effect the dissociation of the complex from (7, 8). Two molecules of ATP are hydrolyzed in a sequential fashion during the clamp loading process (49). This study examines the complex for ability to assemble This paper is available on line at
2 Clamp Loader Template Requirements onto different DNA structures. Overall, the complex can act on a surprisingly wide array of DNA templates. We found that on a flush primed site, the complex exhibits polarity, utilizing the primer/template junction at the 3 -end of the primer during the clamp assembly process. However, the 3 terminus of the primed site does not need to be annealed to the template strand. Indeed, no ends are required for the complex to assemble onto DNA; a ssdna/dsdna junction appears to be a sufficient substrate upon which the complex may load. Although the complex can assemble onto a 10-mer duplex, it has spatial requirements that extend beyond this small site. A protein positioned 12 bp from the 3 terminus of a primed site blocks clamp loading action; at least 14 bp 3 to the bound protein are required for assembly of onto DNA. Likewise, a large DNA secondary structure positioned within 16 nucleotides of the 3 terminus blocks complex action. Use of by pol III* in chain elongation requires even more space on the primed site; a protein or DNA secondary structure placed closer than bp from the 3 terminus prevents chain extension. The biological significance of these structural constraints for DNA polymerase III holoenzyme action is discussed. MATERIALS AND METHODS Reagents Unlabeled ribonucleoside and deoxyribonucleoside triphosphates were obtained from Amersham Pharmacia Biotech. Radioactive nucleoside triphosphates were from NEN Life Science Products. Bio-Gel A-15m agarose was from Bio-Rad. Restriction enzymes were from New England Biolabs Inc. DNA oligonucleotides were synthesized by Oligos etc. and Life Technologies, Inc. All oligonucleotides of 30 nucleotides were purified by SDS-polyacrylamide gel electrophoresis. Proteins were purified as described:,,, and (23); (5); and (24); and (25); (26); and SSB (27). pol III* and the complex were reconstituted from pure subunits and purified as described (13, 20). Replacements of Asp 12 and Glu 14 with Ala eliminated the 3 5 exonuclease activity of (28). with these two replacements ( mut ) was constructed by polymerase chain reaction. The gene was placed into pet11, and mut was prepared from induced cells as described (12). mut was used with the other subunits of pol III* to reconstitute pol III* mut lacking exonuclease activity (13). PK is containing a 6-residue C-terminal kinase recognition site (22). EBNA1 PK is the 200-residue C-terminal DNA-binding domain of EBNA1 to which a 6-residue kinase recognition site has been attached; it was isolated and purified as described (29). PK and EBNA1 PK were labeled with [ - 32 P[ATP using the recombinant catalytic subunit of camp-dependent protein kinase produced in E. coli (a generous gift of Dr. Susan Taylor, University of California at San Diego) as described (27). [ 3 H] (520 cpm/fmol) was prepared by reductive methylation using formaldehyde and [ 3 H]NaBH 4 (30). Buffers Buffer A contained 10 mm Tris-HCl, 300 mm NaCl, and 30 mm sodium citrate (final ph 8.0). Buffer B contained 20 mm Tris-HCl (ph 7.5), 4% (v/v) glycerol, 0.1 mm EDTA, 40 g/ml bovine serum albumin, 5 mm dithiothreitol, 1 mm ATP, and 8 mm MgCl 2. Buffer C contained 20 mm Tris-HCl (ph 7.5), 8 mm MgCl 2, 4% (v/v) glycerol, 0.5 mm EDTA, 100 g/ml bovine serum albumin, 2 mm dithiothreitol, and 100 mm NaCl. Buffer D contained 90 mm Tris base and 90 mm boric acid. DNA Templates Supercoiled pbluescript SK Supercoiled pbluescript SK (2.96 kilobases; Stratagene) was propagated in the DH5 strain of E. coli, and plasmid was extracted by alkaline lysis and then purified through QIAGEN columns and further purified by ultracentrifugation in cesium chloride as described (31). Relaxed Closed Circular DNA Relaxed closed circular DNA was prepared upon adding 1.2 g of vaccinia topoisomerase (a generous gift of Drs. Stewart Shuman and JoAnn Sekiguchi) to 20 g of psb01 EP3ori DNA (a generous gift of Dr. Jerard Hurwitz) (32) in 400 l of buffer containing 40 mm Tris 7.5, 150 mm NaCl, and 10 mm MgCl 2 at 37 C for 30 min. Relaxation was judged to be complete within 30 min as indicated by analysis of time points on a neutral agarose gel (ethidium bromide added after electrophoresis). M13mp18 and M13EBNA1 Circular ssdna M13mp18 phage was prepared by two consecutive bandings in cesium chloride as described (33). M13EBNA1 ssdna was prepared as described (27). Tailed Primed Templates The oligonucleotides used in the series of experiments of Fig. 3 contain 41 nucleotides complementary to M13EBNA1 ssdna (map positions ). The 3 -tail 56-mer oligonucleotide has 41 nucleotides complementary to M13EBNA1 ssdna at the 5 -end, followed by a stretch of 15 T residues at the 3 -end. The 5 -tail 56-mer oligonucleotide has 15 T residues at the 5 -end, followed by 41 nucleotides complementary to M13EBNA1 ssdna. The 3 - and 5 -tail 71-mer oligonucleotide has 41 nucleotides complementary to M13EBNA1 flanked by 15 T residues on both the 3 - and 5 -ends. The control flush 41-mer oligonucleotide was annealed to M13EBNA1 ssdna. EBNA1-blocked Primed Templates Two types of oligonucleotide primers were implemented to determine the polarity of complex clamp loading and to define the minimal length of DNA complex required for clamp loading. For the polarity experiments, two 34-mer oligonucleotides were synthesized having an 18-nucleotide EBNA1-binding sequence at either the 3 or 5 terminus. For the length determination, a set of nine primers was synthesized that had the EBNA1-binding sequence located at the 5 end. In this latter set of 5 EBNA1-block primers, the distance between the 3 end and the EBNA1 block ranged from 10 to 45 nucleotides (i.e. counting the 18 nucleotide EBNA1 site and one 5 nucleotide beyond it, these primers ranged in size from 29 to 45 nucleotides). To verify that each template contained a primer with an EBNA1-binding site, [ 32 P]EBNA1 PK (dimer) of known specific activity was added to a defined molar amount of M13EBNA1 ssdna hybridized with each EBNA1 block oligonucleotide. Results showed approximately one molecule of [ 32 P]EBNA1 PK bound to each EBNA1-blocked template as expected (for 5 -EBNA1 block, 420 fmol of EBNA 2 /417 fmol of template; and for 3 -EBNA1 block, 391 fmol of EBNA 2 /417 fmol of template). 10-mer and 15-mer Primed Templates A 10-mer DNA oligonucleotide with the sequence 5 -GGGCGCGAGC-3 was annealed to M13EBNA1 ssdna (map positions 40 49) in a 200:1 primer/template ratio. Another singly primed template containing a 15-mer oligonucleotide with the sequence 5 -ATTTGGGGCGCGAGC-3 (map positions on M13EBNA1 ssdna) was also constructed and annealed in an identical fashion. Bubble Primed Template Eight oligonucleotides ranging in length from 56 to 70 nucleotides (5 -map position 6239) were designed such that each contains a central stretch of 20 residues identical to the template (map positions ), thereby forming a 20-mer singlestranded DNA bubble when hybridized to M13EBNA1 ssdna. The bubble has equal amounts of ssdna on each strand. The bubble is flanked by 24 bp on the 5 -side and by bp on the 3 -side of the primer. Stem-Loop Primed Template Eight oligonucleotides ranging in length from 65 to 79 nucleotides (5 -map position 6239) were synthesized such that each contains a 29-nucleotide central region composed of two inverted sequences (12 nucleotides long) separated by 5 T residues that form a 12-bp stem-loop (this stem-loop sequence is 5 -GGAAGGC- CTTGGTTTTTCCAAGGCCTTCC-3 ). Upon hybridization of the oligonucleotides to M13EBNA1 ssdna, the stem-loop is flanked by bp on the 3 -side and by 24 bp on the 5 -side. Hybridization of Oligonucleotides to ssdna Annealing of oligonucleotides to ssdna templates was performed in reactions containing 12.5 pmol of ssdna template and 250 pmol of primer(s) in 20 l of buffer A in a 1.5-ml microcentrifuge tube. The reactions were heated in a mm test tube containing 100 C water and then allowed to slowly cool to room temperature ( 22 C, 30 min). For templates hybridized with short primers ( 15 nucleotides), 2500 pmol of primer were added to 12.4 pmol of ssdna circles. Analysis of Clamps Assembled onto DNA by Agarose Gel Electrophoresis Reactions were performed in buffer B in a volume of 100 l. Exact amounts of DNA and proteins and the concentration of NaCl are indicated in the figure legends. Mixtures were incubated for 10 min at 37 C and then analyzed (upon adding 20 l of glycerol) by electrophoresis on a 1.5% native agarose gel prepared with buffer D (containing 1 g/ml ethidium bromide) at 100 V for 150 min at 4 C. A photograph of the UV-induced ethidium bromide fluorescence was obtained, and the gel was dried and exposed to a PhosphorImager screen.
3 11442 Clamp Loader Template Requirements Isolation of [ 32 P] Clamps on DNA by Gel Filtration Reactions were performed in 100 l of buffer B. Exact amounts of [ 32 P] or [ 3 H], the complex or pol III*, and the primed template used in each experiment are detailed in the figure legends. Reactions were incubated for 10 min at 37 C and applied to 5-ml columns of Bio-Gel A-15m equilibrated in buffer C at 4 C. Fractions of 180 l were collected. Aliquots of 150 l were analyzed for radioactivity by liquid scintillation counting. The molar amount of [ 32 P] in each fraction was calculated from the known specific activity of radioactive. The seven peak-excluded fractions (typically fractions 10 16) were summed to obtain the total amount of on DNA. All values presented are normalized for the full 180- l volume of each fraction. Recovery of total radioactive protein was typically 80 95%. Results were not corrected for recoveries. Replication of EBNA1-blocked Primed Templates Templates primed using 5 -EBNA1 block oligonucleotides (242 fmol as circles) were added to 308 fmol of, 3.6 pmol of EBNA1 (as dimer), and 55 pmol of SSB (as tetramer) in 22 l of buffer B containing 0.5 mm ATP. In a parallel control experiment, EBNA1 was omitted and replaced with storage buffer containing 20 mm Tris-HCl (ph 7.5), 50% (v/v) glycerol, 0.1 mm EDTA, 5 mm dithiothreitol, and 300 mm NaCl. Reactions were incubated at 30 C for 1 min to allow time for EBNA1 binding. Next, 100 fmol of pol III* were added to each reaction and incubated for 1.5 min at 30 C. Replication was initiated upon adding 2 l of 0.75 mm each datp, dctp, and dgtp and 0.25 mm [ - 32 P]dTTP and then allowed to proceed for 30 s at 30 C before being quenched with 25 l of stop solution (1% SDS and 40 mm EDTA). Reactions were spotted onto DE81 paper, and the filters were washed and counted as described (34). Replication of 20-mer Bubble- or 12-bp Stem-Loop-blocked Primed Templates Templates primed with either the 20-mer bubble oligonucleotides or the 12-bp stem-loop oligonucleotides were replicated with pol III* using the same protocol as with the 5 -EBNA1-blocked templates, except that EBNA1 protein was not included. RESULTS The Complex Assembles onto a Supercoiled Template Previous studies showed that the complex assembles onto the DNA in a supercoiled plasmid DNA preparation (i.e. by gel filtration analysis); however, it was not determined whether assembled onto the supercoiled plasmid or onto the small amount of nicked plasmid that contaminates supercoiled plasmid preparations (4). In the experiment of Fig. 1, we more thoroughly examined the ability of the complex to assemble onto a supercoiled plasmid. To detect the presence of on DNA, a modified form of was used in which a 6-residue kinase recognition motif was placed on the C terminus, allowing it to be radiolabeled with [ - 32 P]ATP using camp-dependent protein kinase (referred to herein as [ 32 P] ). [ 32 P] was incubated with a supercoiled plasmid, the complex, and ATP, and then the reaction was divided. One half was treated with BamHI to linearize the DNA, and the other half was treated with BamHI storage buffer. The reactions were then analyzed on a 1.5% native agarose gel to resolve the different forms of DNA, followed by autoradiography to detect the presence of [ 32 P] on the DNA. In Fig. 1A, the UV-induced ethidium bromide fluorescence shows a small amount of nicked DNA in the supercoiled DNA preparation (lane 1), and lane 2 shows that linearization of the supercoiled DNA was complete. The corresponding autoradiogram (right panel) shows that [ 32 P] was present on both the nicked plasmid and the supercoiled plasmid (lane 1), whereas no detectable [ 32 P] was observed on the DNA after linearization (lane 2). Therefore, the complex can assemble onto supercoiled DNA. The fact that [ 32 P] dissociates from the DNA upon linearization demonstrates that the clamp is topologically bound to these circular DNAs. The observation that the complex assembles onto supercoiled DNA is somewhat surprising, as this is not a template FIG. 1. Assembly of onto supercoiled plasmid DNA. A, the reaction contained 2.5 pmol of [ 32 P] (as dimer), 1.25 pmol of supercoiled pbluescript plasmid DNA, and 1.25 pmol of complex in 50 l of buffer B. After 10 min at 37 C, the reaction was divided: 100 units of BamHI were added to one half, and BamHI storage buffer was added to the other half. After a further 10 min at 37 C, 4 l of glycerol were added, and reactions were analyzed on a 1.5% native agarose gel. The photograph of the UV-induced ethidium bromide fluorescence is shown in the left panel, and the autoradiogram of the gel is shown in the right panel. In lane 1, the reaction lacked BamHI; in lane 2, the reaction contained BamHI; and in lane 3, the reaction lacked DNA. The positions of the nicked, linear, and supercoiled plasmid DNAs are indicated to the left of the gels. B, shown is the salt sensitivity of assembly onto plasmid DNA. Reactions contained 1500 fmol of [ 32 P], 750 fmol of supercoiled pbluescript plasmid DNA, and 650 fmol of complex in 100 l of buffer B. The buffer contained 0, 10, 20, or 30 mm NaCl. After 10 min at 37 C, reactions were analyzed on a native agarose gel. The photograph of the UV-induced ethidium bromide fluorescence is shown in the left panel, and the autoradiogram of the gel is shown in the right panel. The positions of the nicked and supercoiled plasmid DNAs are indicated to the left of the gels. with a 3 terminus for DNA synthesis. Perhaps superhelical tension produces unwound regions (i.e. bubbles), thereby providing ssdna/dsdna junctions upon which the complex may assemble. This predicts that the reaction will be highly sensitive to ionic strength, as salt should have the effect of stabilizing the duplex, thus replacing regions of unwound DNA in the supercoiled plasmid with a concomitant increase in the writhe (the number of times the helix axis crosses over itself). This prediction was tested by repeating the experiment in the presence of low amounts of NaCl, 0, 10, 20, or 30 mm (Fig. 1B). The result demonstrates that assembly of [ 32 P] onto supercoiled DNA is highly salt sensitive; at just 30 mm NaCl, practically no [ 32 P] was observed on supercoiled DNA, whereas the inverse held for assembly of onto the nicked circular DNA present in the plasmid preparation. Therefore, complex action on supercoiled DNA is significantly more sensitive to ionic strength than activity on nicked DNA, with the result that the
4 Clamp Loader Template Requirements complex directs its clamp loading activity to the nicked plasmid as the ionic strength is raised. The complex activity was not significantly affected over this narrow range of NaCl concentration, as the total amount of clamps assembled onto DNA remained constant. The oligomeric stability of the clamp is not affected by this difference in salt concentration ( remains a dimer even in 2 M NaCl). 2 The sensitivity to ionic strength of complex action on supercoiled DNA is consistent with the hypothesis that a supercoiled plasmid has regions of unwound DNA that act as a substrate for the complex. Alternatively, the complex may utilize fully duplex DNA as a substrate for at very low ionic strength, but not at slightly higher ionic strength. A test to distinguish between these possibilities is provided in the next experiment. The Complex Does Not Assemble onto a Closed Relaxed Plasmid Supercoiled plasmid DNA has inherent torsional strain that may unwind DNA (i.e. forming bubbles), providing a substrate for the complex. This torsional strain may be released upon treatment with topoisomerase to remove the supercoils. If the complex can assemble onto fully duplex DNA at low ionic strength, then the complex should assemble [ 32 P] onto a relaxed closed plasmid (form IV DNA) at low ionic strength. However, if the complex requires the plasmid to have torsional stress, possibly providing regions of unwound DNA, then little or no [ 32 P] should be assembled onto a relaxed plasmid even at low ionic strength. In Fig. 2, we examined whether the complex assembled onto plasmid DNA that had been relaxed by treatment with a topoisomerase. A control reaction containing a mixture of nicked and supercoiled DNAs was also performed. Reactions were analyzed by electrophoresis on a native agarose gel in the absence of ethidium bromide, allowing the DNA topoisomers to be resolved. Later, the gel was stained with ethidium bromide to visualize DNA (Fig. 2A). The autoradiogram in Fig. 2B shows that was assembled onto both supercoiled and nicked DNAs in the control (lane 2), but not onto the relaxed circular DNA (lane 3; individual topoisomers migrate between nicked and supercoiled DNAs). 3 This result suggests that the complex does not assemble onto duplex DNA and that assembly of onto supercoiled plasmid is mediated by a structure produced by superhelical tension, possibly a region of unwound DNA. The Complex Can Assemble at a ssdna/dsdna Junction The preceding experiments demonstrated that the complex can assemble onto DNA lacking an end, possibly at ssdna bubbles within the supercoiled plasmid. The complex does not utilize ssdna as template for assembly of onto DNA (Ref. 4 and see the control in the next experiment). Thus, it seems likely that the structural detail that the complex recognizes in a partially unwound plasmid is the forked ssdna/dsdna junction of the bubble. This structure is similar to a primed site, the major difference being the lack of an annealed end (indeed, a bubble in a closed circular duplex has no ends at all). In the next set of experiments, synthetic forked ssdna/dsdna junctions were constructed to test whether the complex can function on sites lacking an annealed terminus. Four different templates were prepared by annealing DNA oligonucleotides to the circular ssdna genome of an M13 derivative. The first template was a control in which a 41-mer DNA oligonucleotide was fully annealed to the circular ssdna (Fig. 3A). The second template was prepared by annealing a 56-mer oligonucleotide, containing a non-complementary 2 Z. Kelman and M. O Donnell, unpublished data. 3 The plasmid preparation used to make relaxed closed circular DNA contained very little nicked circular DNA, although a slight amount was present. FIG. 2.Assembly of onto closed relaxed plasmid DNA. Reactions contained 1500 fmol of [ 32 P], 650 fmol of complex, and 750 fmol (1.14 g) of closed relaxed pbluescript plasmid DNA in 100 l of buffer B. After 10 min at 37 C, 25 l of glycerol were added, and then reactions were analyzed on a 1.5% native agarose gel. A is a photograph of the UV-induced ethidium bromide fluorescence, and B is an autoradiogram of the gel. Lane 1, reaction lacking DNA; lane 2, reaction containing supercoiled and nicked plasmid DNAs; lane 3, reaction containing closed relaxed plasmid. The positions of the nicked plasmid DNA (slowest migrating band), supercoiled plasmid DNA (fastest migrating band), and topoisomers of the relaxed plasmid (series of bands migrating between nicked and supercoiled DNAs) are indicated to the right of the gels. stretch of 15 dt residues at the 5 -end, to the circular ssdna, forming a 41-bp primed site with a 15-residue 5 -tail (Fig. 3B). The third template was prepared by annealing a 56-mer oligonucleotide, containing a non-complementary stretch of 15 dt residues at the 3 -end, to the circular ssdna, forming a 41-bp primed site with a 15-residue 3 -dt tail (Fig. 3C). The fourth template had tails at both ends, prepared using a 71-mer oligonucleotide with 15-dT residue extensions flanking a 41-mer sequence complementary to the circular ssdna (Fig. 3D). The experiment was also performed using an unprimed M13EBNA1 ssdna circle to test whether the complex can assemble onto ssdna (Fig. 3E). Experiments were repeated at different concentrations of NaCl (0, 17.5, 50, and 76 mm) to assess whether the efficiency of clamp loading on a forked ssdna/dsdna junction is sensitive to ionic strength. Results using these different concentrations of NaCl did not differ significantly; and therefore, only results using 17.5 mm added NaCl are shown in Fig. 3. In the first experiment, the primed template containing a fully annealed oligonucleotide (Fig. 3A) was coated with SSB, and then [ 32 P] was assembled onto it using the complex and
5 11444 Clamp Loader Template Requirements FIG. 3. Assembly of onto ssdna/dsdna junctions. Radiolabeled was assembled onto four different templates to which oligonucleotides were annealed to circular M13EBNA1 ssdna with flush ends (A),a5 -tail (B),a3 -tail (C), ora5 -tail and a 3 -tail (D). E is circular M13EBNA1 ssdna lacking an annealed oligonucleotide. Reactions contained 2300 fmol of [ 32 P], 850 fmol of primed M13EBNA1 ssdna, 210 pmol of SSB, and 700 fmol of complex in 100 l of buffer B containing 17.5 mm NaCl. Reactions were incubated at 37 C for 5 min before analysis by gel filtration as described under Materials and Methods. ATP in the presence of 17.5 mm NaCl. After 5 min at 37 C, the reaction was analyzed by gel filtration through a large-pore Bio-Gel A-15m column to quantitate [ 32 P] on DNA. Large DNA molecules and [ 32 P] clamps bound to them eluted in the void volume (fractions 10 16) and were resolved from the relatively small unbound [ 32 P] that eluted later in the included fractions of this large pore resin (fractions 18 30). The amount of [ 32 P] in each fraction was quantitated by liquid scintillation, and the specific activity of [ 32 P] was determined. The result shows that most of the is assembled onto the template containing a fully annealed primer (974 fmol of was loaded onto 846 fmol of DNA template). In Fig. 3B, complex action was examined using the 5 -tailed template. The results demonstrate that the complex assembles onto the 5 -tailed primed template as efficiently as onto the control template with a fully annealed primer (1082 fmol of was assembled onto 846 fmol of template). The results using the 3 -tailed template are shown in Fig. 3C. Approximately FIG. 4. Polarity of the complex in assembly of onto a primed template. clamps were assembled onto either of two primed templates that contained an EBNA1-binding site at either the 5 -terminal (A) or3 -terminal (B) 18 nucleotides. 18 pmol of EBNA1 dimer, when present (depicted as gray ovals), were preincubated with 850 fmol of primed template and with 210 pmol of SSB in 100 l of buffer B. Reactions were incubated at 37 C for 5 min, and then 1500 fmol of [ 3 H] and 500 fmol of complex were added. After a further 5 min at 37 C, reactions were analyzed by gel filtration as described under Materials and Methods., reactions containing EBNA1;, reactions lacking EBNA1. 76% the amount of was assembled onto this template relative to the control template with a fully annealed oligonucleotide (742 fmol of was loaded onto 846 fmol of template). Similar results were obtained at different concentrations of NaCl. The results using the template with both 3 - and 5 -tails show a comparable amount of clamp assembled onto this template as with the 3 -tailed primed template (574 fmol of were loaded onto 846 fmol template). The somewhat diminished efficiency of complex action on 3 -tailed primed templates is probably explained by the polarity of the complex in assembly of onto DNA (see experiment below in Fig. 4). In summary, these experiments indicate that the complex can assemble clamps onto a forked ssdna/dsdna junction and that annealed DNA ends are not essential to the clamp loading reaction. However, the complex cannot assemble onto DNA that is completely duplex (i.e. relaxed circular duplex in Fig. 2), nor can it assemble onto unprimed circular ssdna (Fig. 3E). Polarity in Complex Assembly of onto Primed DNA The head-to-tail arrangement of protomers in the dimer generates a ring with sidedness; the structure of one face is distinct
6 Clamp Loader Template Requirements from that of the other (e.g. as illustrated in the diagram in Fig. 4). A previous study has shown that the complex and pol III core both interact with the same face of the ring (10). Therefore, it seems likely that the clamp loader functions on the ssdna/dsdna junction at the 3 -end of the primer so that the clamp would be oriented properly for interaction with the polymerase (see diagram in Fig. 4). In this experiment, we asked whether the complex assembles at the 3 terminus of a primed template or whether it is perhaps capable of using the 5 -end, or either end, to assemble onto DNA. To address this issue of directionality, we designed a primed template that could be blocked at either the 3 or 5 terminus of the oligonucleotide primer with a protein, EBNA1. EBNA1 is a viral protein that binds and activates the latent replication origin (orip) of the Epstein-Barr virus. To use this protein as a block, we constructed an M13mp18 ssdna template containing one 18-bp EBNA1-binding site. Then we hybridized either of two 35-mer oligonucleotide primers to this M13EBNA1 ssdna circle such that the EBNA1 site was present at either the extreme 3 -or5 -end. Addition of EBNA1 to these constructs resulted in approximately one dimer bound to the DNA. If the complex acts upon one ssdna/dsdna junction at a primed site, EBNA1 should block clamp loading action on only one of these DNA constructs. In Fig. 4A, EBNA1 was added to the primed template with the EBNA1 site at the 5 -end, followed by addition of [ 32 P], the complex, and ATP. After 5 min at 37 C, the reaction was analyzed by gel filtration to quantitate [ 32 P] that assembled onto DNA. The results show that the complex is capable of loading onto this 5 -EBNA1-blocked primed template (1066 fmol of loaded per 833 fmol of 5 -blocked template). As a control, the experiment was repeated in the absence of EBNA1. The result shows that 50% more assembled onto the DNA in the absence of EBNA1 (1632 fmol of loaded per 833 fmol of 5 -unblocked template). A plausible explanation for this increased level of is the extra 18 bp available in the absence of EBNA1, which may accommodate another clamp, consistent with previous studies showing that multiple clamps accumulate on duplex DNA molecules (4, 35). In summary, the result suggests that the complex does not require interaction with the ssdna/dsdna junction at the 5 -end of the primer to assemble onto DNA. In Fig. 4B, the experiment was repeated using the primed template with the EBNA1 site at the 3 terminus. The results show that the complex is incapable of assembling onto the primed site in the presence of EBNA1; 3% the amount of was loaded onto the template relative to the unblocked control (54 and 1708 fmol of assembled, respectively, onto 833 fmol of template). This result suggests that the complex requires interaction with the ssdna/dsdna junction at the 3 -end of a primed template for clamp loader function. A 10-Base Pair Primed Site Is Sufficient for RNA primers in vivo are nucleotides long in E. coli (36). This length is consistent with the crystal structure of, which shows it to have a depth of 34 Å, suggesting that it is capable of encircling 10 bp of DNA (5). The ability of the complex to function on short primed sites was tested in the experiment of Fig. 5 using circular ssdna templates to which short DNA oligonucleotides (either 10 or 15 nucleotides long) were hybridized. The results demonstrate that the complex assembles onto 10-mer and 15-mer oligonucleotide-primed templates. Assembly of onto these templates was inefficient, probably due to the underlying inefficiency of hybridization of these short primers to DNA (396 and 494 fmol of /833 fmol of 10-mer and 15-mer primed DNAs, respectively). The concentration of primer was elevated in these experiments to increase the priming efficiency of the FIG. 5.Assembly of onto primed templates. The clamp was assembled onto templates that were primed with either a 10-mer or 15-mer DNA oligonucleotide. Reactions contained 850 fmol of primed template, 210 pmol of SSB, 1232 fmol of [ 3 H], and 500 fmol of complex in 100 l of buffer B. Reactions were incubated at 37 C for 5 min and then analyzed by gel filtration as described under Materials and Methods., M13mp18 ssdna primed with 10-mer DNA;, M13mp18 ssdna primed with 15-mer DNA. template (200-fold molar excess of primer to template). However, the increased concentration of free primer was found to be inhibitory to the complex in assembly of onto DNA (the concentration of primer used in the experiment of Fig. 4 inhibited the complex by 50% (data not shown)). Spatial Requirements for Clamp Loader Function The preceding experiment demonstrated that the complex can assemble onto a primed site as small as 10 bp. Primers shorter than this length could not be tested due to instability of these short hybrids. To test even smaller lengths of duplex for loading, we hybridized oligonucleotides over the binding site for EBNA1 such that the 3 terminus extended out 10 bp or more from the EBNA1 site. The crystal structure of the EBNA1 DNA-binding domain indicates that it does not protrude beyond either side of this 18-bp sequence (37). Thus, a 28-mer with an EBNA1 site at the 5 -end is sufficiently long to hybridize stably to the ssdna circle at low concentration. Upon addition of EBNA1, the 5 18 bp will be bound to protein, but the 3 10 bp should be available for the complex to assemble onto DNA. This experimental strategy was employed in the experiment of Fig. 6 using primers of different length (see diagrams). After annealing oligonucleotides of nucleotides, primed templates were coated with SSB, followed by addition of EBNA1 (binding was confirmed as described under Materials and Methods ), and then [ 32 P], the complex, and ATP were added. The reaction was analyzed by gel filtration to quantitate the amount of [ 32 P] assembled onto the DNA (Fig. 6, left panels). Use of the two shortest oligonucleotides showed essentially no on the DNA in the presence of EBNA1 (Fig. 6, A and
7 11446 Clamp Loader Template Requirements FIG. 6. Assembly of onto DNA is inhibited by EBNA1 positioned closer than 14 base pairs from the 3 terminus. clamps were assembled onto primed templates that contained an EBNA1- binding site near the 5 terminus. Assembly reactions were performed using either the complex (left panels) or pol III* (right panels). The distance from the 3 -end of the primer to the EBNA1 site ranges from 10 to 18 nucleotides in steps of 2 (A E). 2.3 pmol of EBNA1 dimer, when present (gray ovals), were preincubated with 850 fmol of primed DNA template and 210 pmol of SSB in 100 l of buffer B. Reactions were incubated at 37 C for 1 min, and then 2300 fmol of [ 32 P] and either 700 fmol of complex or 2000 fmol of pol III* were added. After a further 5 min at 37 C, reactions were analyzed by gel filtration as described under Materials and Methods., reactions containing EBNA1; E, reactions lacking EBNA1. The length of duplex between the EBNA1 site and the 3 terminus is noted in the upper right-hand corner of each panel. Results are summarized in F by bar graphs showing the ratio of clamps assembled onto EBNA1-blocked templates versus unblocked templates as a function of the distance between the 3 terminus of the primer and the EBNA1-binding sequence. B, squares), demonstrating that 3 -extensions of 10 and 12 bp beyond the EBNA1 site were insufficient length for assembly of. This result indicates that some space beyond the 12 bp length is needed for complex action. Control experiments lacking EBNA1 confirmed that these templates were suitable as substrates for the complex (Fig. 6, A and B, circles). This result would appear to contradict the experiment of Fig. 5 showing that the complex can assemble onto a 10-bp primed site. However, the two results can be rationalized if there is a spatial requirement for the complex such that a distance of 10 bp is needed to load onto DNA. A protein positioned too close to the 3 terminus blocks this assembly, but the presence of ssdna 5 to a 10-bp primed site satisfies the spatial requirement, allowing the complex to assemble onto a short primed site. Of course, it remains possible that EBNA1 itself interferes with by occupying more DNA than expected or by altering the DNA structure. Experiments that address this concern, by using DNA structures as blocks instead of EBNA1, are presented below. To further delineate the spatial requirement of complex action on a primed site, the experiment using EBNA1 was repeated using longer primers that provided 3 -extensions of 14, 16, or 18 bp beyond the EBNA1 site (Fig. 6, C E, respectively). The results demonstrate that a 14-bp extension is sufficient for assembly of onto DNA in the presence of EBNA1. These results are summarized in the bar graphs of Fig. 6F, which show the ratio of assembled onto each 5 -EBNA1- blocked template compared with the corresponding unblocked control. Fig. 6 (right panels) also shows the results of these assembly experiments using pol III* as the clamp loader. To prevent the 3 5 exonuclease activity of from digesting the 3 terminus of these primed templates, a mutant of that inactivates the 3 5 exonuclease activity was constructed and used in the reconstitution of pol III* (pol III* mut ). 4 The results showed that the spatial requirement for the clamp loading activity within pol III* was the same as that for the complex alone. Spatial Requirements for DNA Synthesis by pol III Holoenzyme The experiments of Fig. 6 show that the complex can assemble onto duplex regions of 14 bp or more. Next we examined the spatial requirement for the replication activity of pol III holoenzyme. To address this, the series of EBNA1-block primed templates of Fig. 6 were tested in replication assays using, pol III*, and 32 P-labeled deoxyribonucleoside triphosphates (Fig. 7). pol III* mut was used in these experiments to ensure that the 3 terminus of the primed DNA was not modified (i.e. by exonuclease action) during assembly of the enzyme on DNA. We expected that the capacity of pol III* mut and to replicate these DNA templates would mirror the results of pol III* mut in assembly of onto DNA. Surprisingly, the two results differed substantially. Whereas pol III* mut was capable of assembling onto sites having only 14 bp 3 to the EBNA1 site, pol III* mut required at least 22 bp 3 to bound EBNA1 for chain extension (Fig. 7). Spatial Requirements for Clamp Loading and Synthesis on Primed Sites with Secondary Structure In the next experiment, the complex was examined for its ability to assemble onto primed sites with a bulky DNA secondary structure element instead of a protein positioned upstream from the primer 3 terminus. In addition, pol III* was tested for its ability to function with in DNA synthesis on these primed templates. Primed sites with two different secondary structures were studied: a 20-nucleotide ssdna bubble and a 12-bp stem connected by a 5-nucleotide loop. The results using the stem-loop positioned at various distances from the 3 terminus are shown in Fig. 8. The results were qualitatively similar to those in Figs. 6 and 7 using EBNA1 at various positions relative to the 3 terminus. For example, the length of duplex between the stemloop and the 3 terminus needed for the complex to assemble onto DNA was less than the length needed for pol III* to function with in chain elongation. Assembly of was inefficient on sites with 3 -extensions of 16 bp or less from the stem-loop. Therefore, the stem-loop appears to be a slightly more formidable barrier to the use of complex action compared with EBNA1 (where loading occurs at 14 bp). On the other hand, pol III* is active in synthesis with on templates in which the stem-loop is separated from the 3 terminus by 4 The pol III* mut is as active with in DNA synthesis as pol III* is with ; the speed and processivity of the two resulting holoenzymes are indistinguishable (N. Yao, F. P. Leu, J. Anjelkovic, J. Turner, and Mike O Donnell, unpublished data).
8 Clamp Loader Template Requirements FIG. 7. Replication competence of primed templates. pol III* mut and were used to replicate primed templates used in the experiments of Fig. 6 either in the presence or absence of EBNA1. The bar graph summarizes the results. The x axis shows the distance in base pairs from the 3 terminus to EBNA1. The y axis shows the ratio of DNA synthesis obtained for each template in the presence or absence of EBNA1. only 20 bp, whereas use of EBNA1 requires a 22-bp extension from the EBNA1 protein. The results of complex action and polymerase chain extension on primed sites with a ssdna bubble upstream from the 3 terminus are shown in Fig. 9. As with the stem-loop and EBNA1 blocks, the length of duplex between the bubble and 3 -end needed for the complex was less than the length required for polymerase function. The actual lengths for optimal function were 16 bp for the complex-catalyzed loading and 20 bp for pol III* function with, similar to that observed using the stem-loop. DISCUSSION Directionality of Clamp Assembly onto DNA The clamp is composed of two crescent-shaped protomers that form a protein ring with two structurally distinct faces (i.e. analogous to the different head and tail faces of a coin). A ring with distinct front and back faces initially suggested to us that one face is utilized for interaction with the complex and that the core polymerase may interact with the other face of. However, study of these interactions showed that the complex and core polymerase associate with the same face of the clamp (i.e. the face from which the two C termini protrude) (10). In fact, the core and complex compete for (10). This competition is important, as the complex can also remove clamps from DNA (10). This competitive arrangement between the core and complex for prevents the complex-catalyzed FIG. 8.Steric constraints on holoenzyme action imposed by a 12-bp stem-loop. The complex activity and pol III* function with were examined on primed templates with a stem-loop positioned at various distances from the 3 terminus as illustrated in the diagrams. A, the ability of the complex to assemble [ 32 P] onto each primed template was determined by gel filtration. Results are summarized in the bar graph (inset). B, the ability of pol III* mut to function with in DNA synthesis was tested for each primed template. removal of from DNA while the core polymerase functions with in processive synthesis (10). When replication is complete, the core polymerase dissociates from the clamp (and DNA), and then the complex is free to unload the clamp that is left behind on the completed DNA (10, 38). The fact that core polymerase interacts with the same face of as the complex suggests that the complex may act at the same 3 -terminal portion of the primer as the polymerase. This report shows that the complex does indeed require the 3 ssdna/dsdna junction to assemble onto DNA; it does not do so when this junction is blocked. A protein block at the 5 ssdna/dsdna junction of a primed template does not affect the clamp loading action of the complex (provided the primer is not too small). This polarity of complex action is consistent with a cross-linking study demonstrating that the subunit of the complex covalently reacts with a DNA photoreactive moiety positioned on the primed strand 3 bp upstream from the 3 terminus (39). The Complex Can Assemble onto a Forked ssdna/ dsdna Junction Previous studies have shown that the complex is functional in loading onto primed templates and onto nicked circular duplex DNA (4). This study expands these findings. This report documents the ability of the complex to assemble onto supercoiled plasmid under conditions of low ionic strength. The complex does not assemble onto a relaxed plasmid DNA, suggesting that superhelical tension may induce some single-stranded character in the supercoiled
9 11448 Clamp Loader Template Requirements FIG. 9.Steric constraints of a bubble on loading and replication. The action of the complex and pol III* with was examined on primed sites with a ssdna bubble positioned bp upstream from the 3 terminus as indicated in the diagram. A, the ability of the complex to assemble [ 32 P] onto primed templates was determined by gel filtration. The bar graph (inset) shows a summary of the results. B, the ability of pol III* mut to function with in DNA synthesis was examined for each primed template. plasmid, thus forming forked ssdna/dsdna junctions (i.e. bubbles). Indeed, this report demonstrates the ability of the complex to load onto a forked ssdna/dsdna junction lacking annealed ends. The fact that the complex can assemble onto superhelical plasmid DNA and a forked single-stranded/double-stranded structure lacking an annealed 3 terminus is consistent with the finding that the complex does not require the 3 -hydroxyl group of a primed site (i.e. it assembled onto a primed site that had been terminated with a dideoxynucleotide at the 3 terminus) (40). Spatial Requirement for the Complex in Loading and for pol III* in Chain Extension with In vivo, RNA primers are nucleotides in length (36). In keeping with this observation, we demonstrate here that the complex can assemble onto a primer as short as 10 nucleotides. pol III* is also able to extend the 10-mer oligonucleotide primer in a -dependent reaction (data not shown). The ring has a thickness of 34 Å, sufficient to span approximately one helical turn of DNA (i.e. 10 nucleotides) (5). Therefore, in vivo, primed sites are approximately the same length as is thick. These short RNA primers are probably held to DNA by primase, which remains bound to the primed site through contact with SSB (41). Primase is displaced by the complex during clamp loading via a competition between (a subunit of the complex) and primase for SSB (41, 42). The ability of the complex to assemble onto a 10-bp primed site and the fact that the complex acts from the 3 terminus of the primed site suggest that all clamp loading operations are confined to 10 bp of duplex DNA and perhaps some template ssdna at the 3 -dsdna/ssdna junction. This predicts that if a physical barrier such as EBNA1 were placed on a primed site 10 bp or more upstream from the 3 terminus, the complex would still be capable of assembling on the available 10 bp of duplex DNA. However, we performed this experiment and found that it could not; 14 bp or more must be provided. This result could be explained by protrusion of EBNA1 beyond its site, but crystal structure analysis indicated that this is not the case. Furthermore, repeating these experiments using primers with upstream secondary structure elements (i.e. a stem-loop or a bubble) showed that a DNA secondary structure placed 16 bp from the 3 terminus also blocks complex clamp loading activity. A plausible explanation for these observations is that the complex interacts with 4 6 bp at the 3 terminus in addition to the DNA occupied by the ring. On a very short primer only 10 bp in length, the complex may occupy some of the ssdna in back of the 5 -end of the primer in addition to the 10-bp primed site. This bp requirement for complex action with would appear to conflict with DNase I footprinting studies of and the complex that showed protection over the length of the entire 51-nucleotide primed site (43). However, this large footprint is likely explained by the ability of the complex to load multiple clamps onto DNA (4), thereby covering the duplex portion of the primed template with several rings. Studies such as this in the T4 system have demonstrated that a 660-bp stretch of duplex DNA can be protected from DNase I via packing the entire region with the T4 clamp, gp45 protein (50). pol III*, which has one molecule of the complex, has the same spatial requirement as the complex alone for assembly of onto DNA (i.e bp). However, use of the clamp by pol III* requires bp. Hence, the polymerase, coupled to, requires more space on DNA than the complex. These spatial requirements are summarized in Fig. 10. The spatial requirement of bp for pol III* function with fits nicely with protein/dna cross-linking studies of pol III holoenzyme that indicate that the holoenzyme makes contact with DNA as far as 22 nucleotides upstream from the 3 terminus (39). These results are also in agreement with DNase I footprinting studies of pol III holoenzyme on DNA that showed protection of bp at a primer terminus (43). DNase I is a rather large cleavage agent and generally overestimates binding sites by 3 5 nucleotides (44). Comparison with T4 and Eukaryotic Replicases The spatial requirements for assembly of E. coli DNA polymerase III holoenzyme onto a primed template can be compared with results from previous footprinting and cross-linking studies performed with T4 and eukaryotic replicases. Munn and Alberts (45, 46) utilized a hairpin helix with a single-stranded 5 -extension (a primer/template junction ) to footprint the T4 phage clamp (gp45) and clamp loader (gp44 gp62 complex). Analysis of the duplex portion of the primed template using DNase I in the presence of ATP S (which freezes the T4 clamp-clamp loader intermediate on DNA) showed that the clamp-clamp loader complex protects bp on one strand and bp on the other (45). Addition of the T4 DNA polymerase to the accessory proteins yields a somewhat expanded footprint encompassing 25 residues on one strand and 32 residues on the other (46). A DNase I footprinting study using a similar hairpin template performed with the eukaryotic clamp PCNA and the clamp loader replication factor C (in the presence of ATP S) showed a protected region of nucleotides on the double-stranded portion of the primed template; pol and PCNA yield a similarsized footprint of nucleotides (47). Taking into consideration the 4 5-nucleotide overestimate using DNase I, the re-
10 Clamp Loader Template Requirements in this process indicate that the 3 terminus is masked by mismatch repair proteins. Presumably, pol III holoenzyme not only unlocks the 3 terminus, but clears away blocking proteins that are bound within 20 bp of the 3 terminus to generate sufficient room on the DNA for clamp loading action and polymerase extension. The space needed by pol III holoenzyme may also impose certain boundaries, or constraints, to initiating replication at D-loop recombination intermediates such as in double strand break repair. For example, a D-loop has ssdna/dsdna junctions directly in front and in back of the primed site, which may impose spatial requirements similar to the DNA secondary structures examined herein. This study indicates that pol IIImediated extension of a D-loop formed by homologous recombination would require that the invading strand form at least 20 bp of duplex DNA for the complex to load a clamp and for chain extension by the polymerase. Further spatial requirements for polymerase action may be imposed by the displaced ssdna strand of the loop, as sufficient room must be available between the primed site and the displaced ssdna to accommodate the radius of the ring. These spatial constraints may act to prevent recombination at sites of only limited homology. The coordination of pol III holoenzyme with other proteins in these several DNA metabolic activities may enable the holoenzyme to function in very restricted spaces. How these numerous proteins function together, especially with enzymes as large as pol III holoenzyme, remains an exciting area for future study. FIG. 10. Spatial requirements for pol III holoenzyme components at a primed site. A, the complex recognizes the 3 -ssdna/ dsdna junction to assemble the clamp onto the duplex portion of a primed site. Approximately bp of duplex are required in the clamp loading operation. B, once assembled onto DNA, the ring should occupy 10 bp, as the width of is 34 Å. C, the action of the polymerase with the clamp requires bp, implying that the DNA polymerase occupies bp in front of the clamp. sults of the T4 and human systems are quite similar to the requirement of and the complex for bp of duplex DNA and of bp for chain extension by pol III holoenzyme. Recent experiments on pol and PCNA using 5 -biotinylated primers demonstrated that streptavidin blocks PCNAdependent chain extension by pol on primers with lengths under 19 nucleotides, a length similar to the nucleotides observed in this study for pol III holoenzyme (48). Implications for Recombination and Repair pol III holoenzyme is utilized in a variety of recombination and repair processes. For example, pol III holoenzyme is required in replication restart after DNA damage. This process of restarting replication of a damaged chromosome involves participation of numerous proteins such as RecA, RecF, RecO, and RecR, which presumably function with pol III holoenzyme to circumvent lesions on the template (51, 52). This process may require recombination events to place a new 3 terminus across the damaged site, allowing pol III holoenzyme to restart chain extension past the lesion. The spatial requirements for pol III holoenzyme action outlined here suggest that at least 20 bp of the new primer terminus must be cleared of recombination factors for pol III holoenzyme to function. pol III holoenzyme is also required in mismatch repair to fill in the long ssdna gaps created by excision of DNA to and beyond the mismatched nucleotide (53, 54). The need for pol III holoenzyme and the inability of other polymerases to substitute Acknowledgments We are grateful to Maija Skangalis and Jeff Finkelstein for the reconstituted pol III*, Drs. Stewart Shuman and JoAnn Sekiguchi for the vaccinia topoisomerase, and Dr. Susan Taylor for the recombinant catalytic subunit of camp-dependent protein kinase. REFERENCES 1. Kornberg, A., and Baker, T. (1992) DNA Replication, 2nd Ed., pp , W. H. Freeman & Co., New York 2. Fay, P. J., Johanson, K. O., McHenry, C. S., and Bambara, R. A. (1981) J. Biol. Chem. 256, Burgers, P. M., and Kornberg, A. (1982) J. Biol. Chem. 257, Stukenberg, P. T., Studwell-Vaughan, P. S., and O Donnell, M. (1991) J. Biol. Chem. 266, Kong, X. P., Onrust, R., O Donnell, M., and Kuriyan, J. (1992) Cell 69, Maki, H., and Kornberg, A. (1985) J. Biol. Chem. 260, Turner, J., Hingorani, M. M., Kelman, Z., and O Donnell, M. (1999) EMBO J. 18, Hingorani, M. M., and O Donnell, M. (1998) J. Biol. Chem. 273, O Donnell, M. E. (1987) J. Biol. Chem. 262, Naktinis, V., Turner, J., and O Donnell, M. (1996) Cell 84, McHenry, C. S., and Crow, W. (1979) J. Biol. Chem. 254, Scheuermann, R. H., and Echols, H. (1984) Proc. Natl. Acad. Sci. U. S. A. 81, Onrust, R., Finkelstein, J., Turner, J., Naktinis, V., and O Donnell, M. (1995) J. Biol. Chem. 270, Kodaira, M., Biswas, S. B., and Kornberg, A. (1983) Mol. Gen. Genet. 192, Tsuchihashi, Z., and Kornberg, A. (1990) Proc. Natl. Acad. Sci. U. S. A. 87, Flower, A. M., and McHenry, C. S. (1990) Proc. Natl. Acad. Sci. U. S. A. 87, Blinkowa, A. L., and Walker, J. R. (1990) Nucleic Acids Res. 18, Xiao, H., Naktinis, V., and O Donnell, M. (1995) J. Biol. Chem. 270, Maki, S., and Kornberg, A. (1988) J. Biol. Chem. 263, Onrust, R., Finkelstein, J., Naktinis, V., Turner, J., Fang, L., and O Donnell, M. (1995) J. Biol. Chem. 270, Dallmann, H. G., and McHenry, C. S. (1995) J. Biol. Chem. 270, Naktinis, V., Onrust, R., Fang, L., and O Donnell, M. (1995) J. Biol. Chem. 270, Studwell, P. S., and O Donnell, M. (1990) J. Biol. Chem. 265, Dong, Z., Onrust, R., Skangalis, M., and O Donnell, M. (1993) J. Biol. Chem. 268, Xiao, H., Crombie, R., Dong, Z., Onrust, R., and O Donnell, M. (1993) J. Biol. Chem. 268, Studwell-Vaughan, P. S., and O Donnell, M. (1993) J. Biol. Chem. 268, Yao, N., Hurwitz, J., and O Donnell, M. (2000) J. Biol. Chem. 275, Fijalkowska, I. J., and Schaaper, R. M. (1996) Proc. Natl. Acad. Sci. U. S. A. 93, Zhang, D., Frappier, L., Gibbs, E., Hurwitz, J., and O Donnell, M. (1998)
Questions from chapters in the textbook that are relevant for the final exam
 Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
Questions from chapters in the textbook that are relevant for the final exam Chapter 9 Replication of DNA Question 1. Name the two substrates for DNA synthesis. Explain why each is necessary for DNA synthesis.
 The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
The replication of DNA Kornberg 1957 Meselson and Stahl 1958 Cairns 1963 Okazaki 1968 DNA Replication The driving force for DNA synthesis. The addition of a nucleotide to a growing polynucleotide
DNA Replication II Biochemistry 302. January 25, 2006
 DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 January 25, 2006 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA
 RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
RNA Expression of the information in a gene generally involves production of an RNA molecule transcribed from a DNA template. RNA differs from DNA that it has a hydroxyl group at the 2 position of the
III. Detailed Examination of the Mechanism of Replication A. Initiation B. Priming C. Elongation D. Proofreading and Termination
 Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
Outline for Replication I. General Features of Replication A. Semi-Conservative B. Starts at Origin C. Bidirectional D. Semi-Discontinuous II. Proteins and Enzymes of Replication III. Detailed Examination
DNA Replication II Biochemistry 302. Bob Kelm January 28, 2004
 DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
DNA Replication II Biochemistry 302 Bob Kelm January 28, 2004 Conceptual model for proofreading based on kinetic considerations Fig. 24.44 stalling transient melting exonuclease site occupancy Following
Chapter 11 DNA Replication and Recombination
 Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
Chapter 11 DNA Replication and Recombination Copyright Copyright 2009 Pearson 2009 Pearson Education, Education, Inc. Inc. 11.1 DNA is reproduced by Semiconservative Replication The complementarity of
The Size and Packaging of Genomes
 DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
DNA Replication The Size and Packaging of Genomes Vary greatly in size Ø Smallest viruses- 4 or 5 genes Ø Escherichia coli- 4,288 genes Ø Human cell- 20,000 to 25,000 genes E. coli 4 million base pairs
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory
 Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Molecular Biology: General Theory Author: Dr Darshana Morar Licensed under a Creative Commons Attribution license. DNA REPLICATION DNA replication is the process of duplicating the DNA sequence in the
Genetics and Genomics in Medicine Chapter 3. Questions & Answers
 Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Genetics and Genomics in Medicine Chapter 3 Multiple Choice Questions Questions & Answers Question 3.1 Which of the following statements, if any, is false? a) Amplifying DNA means making many identical
Molecular Biology (2)
 Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
Molecular Biology (2) DNA replication Mamoun Ahram, PhD Second semester, 2018-2019 Resources This lecture Cooper, pp. 191-207 2 Some basic information The entire DNA content of the cell is known as genome.
Proposed Models of DNA Replication. Conservative Model. Semi-Conservative Model. Dispersive model
 5.2 DNA Replication Cell Cycle Life cycle of a cell Cells can reproduce Daughter cells receive an exact copy of DNA from parent cell DNA replication happens during the S phase Proposed Models of DNA Replication
5.2 DNA Replication Cell Cycle Life cycle of a cell Cells can reproduce Daughter cells receive an exact copy of DNA from parent cell DNA replication happens during the S phase Proposed Models of DNA Replication
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature11988 Supplementary Figure 1. Digestion of model DNA substrates. a, Linearized plasmid DNA (pik31- PstI, lanes 1 and 2), supercoiled plasmid (pik31, lanes 3 and 4), singly nicked plasmid
doi:10.1038/nature11988 Supplementary Figure 1. Digestion of model DNA substrates. a, Linearized plasmid DNA (pik31- PstI, lanes 1 and 2), supercoiled plasmid (pik31, lanes 3 and 4), singly nicked plasmid
Genetic Information: DNA replication
 Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
Genetic Information: DNA replication Umut Fahrioglu, PhD MSc DNA Replication Replication of DNA is vital to the transmission of genomes and the genes they contain from one cell generation to the other.
DNA Replication II Biochemistry 302. Bob Kelm January 26, 2005
 DNA Replication II Biochemistry 302 Bob Kelm January 26, 2005 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
DNA Replication II Biochemistry 302 Bob Kelm January 26, 2005 Following in Dad s footsteps Original A. Kornberg E. coli DNA Pol I is a lousy replicative enzyme. 400 molecules/cell but ~2 replication forks/cell
Recitation CHAPTER 9 DNA Technologies
 Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
Recitation CHAPTER 9 DNA Technologies DNA Cloning: General Scheme A cloning vector and eukaryotic chromosomes are separately cleaved with the same restriction endonuclease. (A single chromosome is shown
MCB 110 SP 2005 Second Midterm
 MCB110 SP 2005 Midterm II Page 1 ANSWER KEY MCB 110 SP 2005 Second Midterm Question Maximum Points Your Points I 40 II 40 III 36 IV 34 150 NOTE: This is my answer key (KC). The graders were given disgression
MCB110 SP 2005 Midterm II Page 1 ANSWER KEY MCB 110 SP 2005 Second Midterm Question Maximum Points Your Points I 40 II 40 III 36 IV 34 150 NOTE: This is my answer key (KC). The graders were given disgression
Lecture Four. Molecular Approaches I: Nucleic Acids
 Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Lecture Four. Molecular Approaches I: Nucleic Acids I. Recombinant DNA and Gene Cloning Recombinant DNA is DNA that has been created artificially. DNA from two or more sources is incorporated into a single
Exam 2 Key - Spring 2008 A#: Please see us if you have any questions!
 Page 1 of 5 Exam 2 Key - Spring 2008 A#: Please see us if you have any questions! 1. A mutation in which parts of two nonhomologous chromosomes change places is called a(n) A. translocation. B. transition.
Page 1 of 5 Exam 2 Key - Spring 2008 A#: Please see us if you have any questions! 1. A mutation in which parts of two nonhomologous chromosomes change places is called a(n) A. translocation. B. transition.
Replication. Obaidur Rahman
 Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
Replication Obaidur Rahman DIRCTION OF DNA SYNTHESIS How many reactions can a DNA polymerase catalyze? So how many reactions can it catalyze? So 4 is one answer, right, 1 for each nucleotide. But what
The replication forks Summarising what we know:
 When does replication occur? MBLG1001 lecture 10 Replication the once in a lifetime event! Full blown replication only occurs once, just before cell division BUT the DNA template is constantly being repaired.
When does replication occur? MBLG1001 lecture 10 Replication the once in a lifetime event! Full blown replication only occurs once, just before cell division BUT the DNA template is constantly being repaired.
BIOCHEMISTRY REVIEW. Overview of Biomolecules. Chapter 11 DNA Replication
 BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 11 DNA Replication 2 3 4 5 6 7 8 9 Are You Getting It?? Which characteristics will be part of semi-conservative replication? (multiple answers) a) The
BIOCHEMISTRY REVIEW Overview of Biomolecules Chapter 11 DNA Replication 2 3 4 5 6 7 8 9 Are You Getting It?? Which characteristics will be part of semi-conservative replication? (multiple answers) a) The
Enzymes used in DNA Replication
 Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
Enzymes used in DNA Replication This document holds the enzymes used in DNA replication, their pictorial representation and functioning. DNA polymerase: DNA polymerase is the chief enzyme of DNA replication.
Fidelity of DNA polymerase
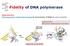 Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
Fidelity of DNA polymerase Shape selectivity: DNA polymerase's conformational change for determination of fidelity for each nucleotide Induced fit: Structure determines function Matched nucleotide Fidelity
Masayoshi Honda, Jeehae Park, Robert A. Pugh, Taekjip Ha, and Maria Spies
 Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Molecular Cell, Volume 35 Supplemental Data Single-Molecule Analysis Reveals Differential Effect of ssdna-binding Proteins on DNA Translocation by XPD Helicase Masayoshi Honda, Jeehae Park, Robert A. Pugh,
Nucleic Acid Structure:
 Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
Nucleic Acid Structure: Purine and Pyrimidine nucleotides can be combined to form nucleic acids: 1. Deoxyribonucliec acid (DNA) is composed of deoxyribonucleosides of! Adenine! Guanine! Cytosine! Thymine
Chapter Twelve: DNA Replication and Recombination
 This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
This is a document I found online that is based off of the fourth version of your book. Not everything will apply to the upcoming exam so you ll have to pick out what you thing is important and applicable.
Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function. Gary Peter
 Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function Gary Peter Learning Objectives 1. List and explain the mechanisms by which E. coli DNA is replicated 2. Describe
Plant Molecular and Cellular Biology Lecture 4: E. coli DNA Replicase Structure & Function Gary Peter Learning Objectives 1. List and explain the mechanisms by which E. coli DNA is replicated 2. Describe
CSIR UGC NET, GATE (ENGINEERING), GATE (Science), IIT-JAM, UGC NET, TIFR, IISc, NIMCET, JEST etc. JNU CEEB SAMPLE THEORY
 JNU CEEB SAMPLE THEORY REPLICATION TYPE OF DNA REPLICATION REPLICATION IN PROKARYOTES REPLICATION IN EUKARYOTES For IIT-JAM, JNU, GATE, NET, NIMCET and Other Entrance Exams 1-C-8, Sheela Chowdhary Road,
JNU CEEB SAMPLE THEORY REPLICATION TYPE OF DNA REPLICATION REPLICATION IN PROKARYOTES REPLICATION IN EUKARYOTES For IIT-JAM, JNU, GATE, NET, NIMCET and Other Entrance Exams 1-C-8, Sheela Chowdhary Road,
DNA Replication I Biochemistry 302. Bob Kelm January 24, 2005
 DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
DNA Replication I Biochemistry 302 Bob Kelm January 24, 2005 Watson Crick prediction: Each stand of parent DNA serves as a template for synthesis of a new complementary daughter strand Fig. 4.12 Proof
Storage and Expression of Genetic Information
 Storage and Expression of Genetic Information 29. DNA structure, Replication and Repair ->Ch 25. DNA metabolism 30. RNA Structure, Synthesis and Processing ->Ch 26. RNA metabolism 31. Protein Synthesis
Storage and Expression of Genetic Information 29. DNA structure, Replication and Repair ->Ch 25. DNA metabolism 30. RNA Structure, Synthesis and Processing ->Ch 26. RNA metabolism 31. Protein Synthesis
Gene Expression - Transcription
 DNA Gene Expression - Transcription Genes are expressed as encoded proteins in a 2 step process: transcription + translation Central dogma of biology: DNA RNA protein Transcription: copy DNA strand making
DNA Gene Expression - Transcription Genes are expressed as encoded proteins in a 2 step process: transcription + translation Central dogma of biology: DNA RNA protein Transcription: copy DNA strand making
Ligation Independent Cloning (LIC) Procedure
 Ligation Independent Cloning (LIC) Procedure Ligation Independent Cloning (LIC) LIC cloning allows insertion of DNA fragments without using restriction enzymes into specific vectors containing engineered
Ligation Independent Cloning (LIC) Procedure Ligation Independent Cloning (LIC) LIC cloning allows insertion of DNA fragments without using restriction enzymes into specific vectors containing engineered
CGTAAGAGTACGTCCAGCATCGGF ATCATTATCTACATCXXXXXTACCATTCATTCAGATCTCACTATCGCATTCTCATGCAGGTCGTAGCCXS
 5 CGTAAGAGTACGTCCAGCATCGGF ATCATTATCTACATCXXXXXTACCATTCATTCAGATCTCACTATCGCATTCTCATGCAGGTCGTAGCCXS 29 Supplementary Figure 1 Sequence of the DNA primer/template pair used in Figure 1bc. A 23nt primer is
5 CGTAAGAGTACGTCCAGCATCGGF ATCATTATCTACATCXXXXXTACCATTCATTCAGATCTCACTATCGCATTCTCATGCAGGTCGTAGCCXS 29 Supplementary Figure 1 Sequence of the DNA primer/template pair used in Figure 1bc. A 23nt primer is
DNA 5 End-Labeling System INSTRUCTIONS FOR USE OF PRODUCT U2010.
 Technical Bulletin DNA 5 End-Labeling System INSTRUCTIONS FOR USE OF PRODUCT U2010. PRINTED IN USA. Revised 12/12 DNA 5 End-Labeling System All technical literature is available on the Internet at: www.promega.com/protocols/
Technical Bulletin DNA 5 End-Labeling System INSTRUCTIONS FOR USE OF PRODUCT U2010. PRINTED IN USA. Revised 12/12 DNA 5 End-Labeling System All technical literature is available on the Internet at: www.promega.com/protocols/
Gene Expression- Protein Synthesis
 Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
Gene Expression- Protein Synthesis Protein synthesis is the key to expression of biological information - Structural Protein: bones, cartilage - Contractile Proteins: myosin, actin - Enzymes - Transport
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule.
 1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
1. True or False? At the DNA level, recombination is initiated by a single stranded break in a DNA molecule. 2. True or False? Dideoxy sequencing is a chain initiation method of DNA sequencing. 3. True
Polymerase Chain Reaction (PCR)
 Laboratory for Environmental Pathogens Research Department of Environmental Sciences University of Toledo Polymerase Chain Reaction (PCR) Background information The polymerase chain reaction (PCR) is an
Laboratory for Environmental Pathogens Research Department of Environmental Sciences University of Toledo Polymerase Chain Reaction (PCR) Background information The polymerase chain reaction (PCR) is an
Welcome to Class 18! Lecture 18: Outline and Objectives. Replication is semiconservative! Replication: DNA DNA! Introductory Biochemistry!
 Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
Lecture 18: Outline and Objectives Welcome to Class 18! Introductory Biochemistry! l DNA Replication! l DNA polymerase! l the enzymatic reaction! l proofreading and accuracy! l DNA synthesis! l origins
Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome
 SUPPLEMENTARY ONLINE MATERIAL For manuscript: Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome By Roxana E. Georgescu 1, Isabel Kurth 1 and Mike E. O Donnell 1 1 The Rockefeller
SUPPLEMENTARY ONLINE MATERIAL For manuscript: Single Molecule Studies Reveal the Function of a Third Polymerase in the Replisome By Roxana E. Georgescu 1, Isabel Kurth 1 and Mike E. O Donnell 1 1 The Rockefeller
Feedback D. Incorrect! No, although this is a correct characteristic of RNA, this is not the best response to the questions.
 Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
Biochemistry - Problem Drill 23: RNA No. 1 of 10 1. Which of the following statements best describes the structural highlights of RNA? (A) RNA can be single or double stranded. (B) G-C pairs have 3 hydrogen
XactEdit Cas9 Nuclease with NLS User Manual
 XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
XactEdit Cas9 Nuclease with NLS User Manual An RNA-guided recombinant endonuclease for efficient targeted DNA cleavage Catalog Numbers CE1000-50K, CE1000-50, CE1000-250, CE1001-250, CE1001-1000 Table of
Supplemental Materials and Methods
 Supplemental Materials and Methods Proteins and reagents Proteins were purified as described previously: RecA, RecQ, and SSB proteins (Harmon and Kowalczykowski 1998); RecF protein (Morimatsu and Kowalczykowski
Supplemental Materials and Methods Proteins and reagents Proteins were purified as described previously: RecA, RecQ, and SSB proteins (Harmon and Kowalczykowski 1998); RecF protein (Morimatsu and Kowalczykowski
DNA. Chapter 1. Molecular Diagnostics Fundamentals, Methods and Clinical Applications Second Edition 1/29/2013. Copyright 2012 F.A.
 DNA Chapter 1 1 Deoxyribonucleic acid (DNA) is a genetic information storage system. A T G C T A C G DNA is a polymer of nucleotides. Nucleotides are phosphorylated nucleosides. DNA Nucleosides comprise
DNA Chapter 1 1 Deoxyribonucleic acid (DNA) is a genetic information storage system. A T G C T A C G DNA is a polymer of nucleotides. Nucleotides are phosphorylated nucleosides. DNA Nucleosides comprise
Syllabus for GUTS Lecture on DNA and Nucleotides
 Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
Syllabus for GUTS Lecture on DNA and Nucleotides I. Introduction. DNA is the instruction manual for how to build a living organism here on earth. The instructions in DNA are propagated to future generations
* + * RecA * + + for RecA-dependent strand + + MIT Department of Biology 7.28, Spring Molecular Biology
 MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1) Question 1. 1A. You are studying the function of in vitro along with a lab partner. You run several reactions to examine the requirements
MIT Department of Biology 7.28, Spring 2005 - Molecular Biology 1) Question 1. 1A. You are studying the function of in vitro along with a lab partner. You run several reactions to examine the requirements
Expression of the genome. Books: 1. Molecular biology of the gene: Watson et al 2. Genetics: Peter J. Russell
 Expression of the genome Books: 1. Molecular biology of the gene: Watson et al 2. Genetics: Peter J. Russell 1 Transcription 1. Francis Crick (1956) named the flow of information from DNA RNA protein the
Expression of the genome Books: 1. Molecular biology of the gene: Watson et al 2. Genetics: Peter J. Russell 1 Transcription 1. Francis Crick (1956) named the flow of information from DNA RNA protein the
BIO 311C Spring Lecture 34 Friday 23 Apr.
 BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
BIO 311C Spring 2010 1 Lecture 34 Friday 23 Apr. Summary of DNA Replication in Prokaryotes origin of replication initial double helix origin of replication new growing polynucleotide chains Circular molecule
DNA Hybridization and Detection
 Chapter 6 DNA Hybridization and Detection Fluorescence Polarization Detection of DNA Hybridization........................................................ 6-2 Introduction.............................................................................................................
Chapter 6 DNA Hybridization and Detection Fluorescence Polarization Detection of DNA Hybridization........................................................ 6-2 Introduction.............................................................................................................
λ-terminase Cat. Nos. LT4450 and LT44200
 Cat. Nos. LT4450 and LT44200 Connect with Epicentre on our blog (epicentral.blogspot.com), Facebook (facebook.com/epicentrebio), and Twitter (@EpicentreBio). www.epicentre.com Lit. # 056 11/201 1 EPILIT056
Cat. Nos. LT4450 and LT44200 Connect with Epicentre on our blog (epicentral.blogspot.com), Facebook (facebook.com/epicentrebio), and Twitter (@EpicentreBio). www.epicentre.com Lit. # 056 11/201 1 EPILIT056
IDTutorial: DNA Replication
 IDTutorial: DNA Replication Introduction In their report announcing the structure of the DNA molecule, Watson and Crick (Nature, 171: 737-738, 1953) observe, It has not escaped our notice that the specific
IDTutorial: DNA Replication Introduction In their report announcing the structure of the DNA molecule, Watson and Crick (Nature, 171: 737-738, 1953) observe, It has not escaped our notice that the specific
DNA REPLICATION. Third Stage. Lec. 12 DNA Replication. Lecture No.: 12. A. Watson & Crick (1952) C. Cairns (1963) autoradiographic experiment
 Lec. 12 DNA Replication A. Watson & Crick (1952) Proposed a model where hydrogen bonds break, the two strands separate, and DNA synthesis occurs semi-conservatively in the same net direction. While a straightforward
Lec. 12 DNA Replication A. Watson & Crick (1952) Proposed a model where hydrogen bonds break, the two strands separate, and DNA synthesis occurs semi-conservatively in the same net direction. While a straightforward
Molecular Cell Biology - Problem Drill 11: Recombinant DNA
 Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
Molecular Cell Biology - Problem Drill 11: Recombinant DNA Question No. 1 of 10 1. Which of the following statements about the sources of DNA used for molecular cloning is correct? Question #1 (A) cdna
4) separates the DNA strands during replication a. A b. B c. C d. D e. E. 5) covalently connects segments of DNA a. A b. B c. C d. D e.
 1) Chargaff's analysis of the relative base composition of DNA was significant because he was able to show that a. the relative proportion of each of the four bases differs from species to species. b.
1) Chargaff's analysis of the relative base composition of DNA was significant because he was able to show that a. the relative proportion of each of the four bases differs from species to species. b.
Delve AP Biology Lecture 7: 10/30/11 Melissa Ko and Anne Huang
 Today s Agenda: I. DNA Structure II. DNA Replication III. DNA Proofreading and Repair IV. The Central Dogma V. Transcription VI. Post-transcriptional Modifications Delve AP Biology Lecture 7: 10/30/11
Today s Agenda: I. DNA Structure II. DNA Replication III. DNA Proofreading and Repair IV. The Central Dogma V. Transcription VI. Post-transcriptional Modifications Delve AP Biology Lecture 7: 10/30/11
Transcription. By : Lucia Dhiantika Witasari M.Biotech., Apt
 Transcription By : Lucia Dhiantika Witasari M.Biotech., Apt REGULATION OF GENE EXPRESSION 11/26/2010 2 RNA Messenger RNAs (mrnas) encode the amino acid sequence of one or more polypeptides specified by
Transcription By : Lucia Dhiantika Witasari M.Biotech., Apt REGULATION OF GENE EXPRESSION 11/26/2010 2 RNA Messenger RNAs (mrnas) encode the amino acid sequence of one or more polypeptides specified by
5. Which of the following enzymes catalyze the attachment of an amino acid to trna in the formation of aminoacyl trna?
 Sample Examination Questions for Exam 3 Material Biology 3300 / Dr. Jerald Hendrix Warning! These questions are posted solely to provide examples of past test questions. There is no guarantee that any
Sample Examination Questions for Exam 3 Material Biology 3300 / Dr. Jerald Hendrix Warning! These questions are posted solely to provide examples of past test questions. There is no guarantee that any
Kinetic Mechanism for Formation of the Active, Dimeric UvrD Helicase-DNA Complex*
 THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 278, No. 34, Issue of August 22, pp. 31930 31940, 2003 2003 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Kinetic Mechanism
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 278, No. 34, Issue of August 22, pp. 31930 31940, 2003 2003 by The American Society for Biochemistry and Molecular Biology, Inc. Printed in U.S.A. Kinetic Mechanism
DNA Transcription. Dr Aliwaini
 DNA Transcription 1 DNA Transcription-Introduction The synthesis of an RNA molecule from DNA is called Transcription. All eukaryotic cells have five major classes of RNA: ribosomal RNA (rrna), messenger
DNA Transcription 1 DNA Transcription-Introduction The synthesis of an RNA molecule from DNA is called Transcription. All eukaryotic cells have five major classes of RNA: ribosomal RNA (rrna), messenger
DNA 5 End-Labeling System
 DNA 5 End-Labeling System I. Description...1 II. Product Components...2 III. Dephosphorylation Reaction...2 IV. Phosphorylation Reaction...3 V. Determination of Percent Incorporation/Specific Activity...3
DNA 5 End-Labeling System I. Description...1 II. Product Components...2 III. Dephosphorylation Reaction...2 IV. Phosphorylation Reaction...3 V. Determination of Percent Incorporation/Specific Activity...3
Chapter 16 The Molecular Basis of Inheritance
 Chapter 16 The Molecular Basis of Inheritance Chromosomes and DNA Morgan s experiments with Drosophila were able to link hereditary factors to specific locations on chromosomes. The double-helical model
Chapter 16 The Molecular Basis of Inheritance Chromosomes and DNA Morgan s experiments with Drosophila were able to link hereditary factors to specific locations on chromosomes. The double-helical model
DNA: Structure & Replication
 DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
DNA Form & Function DNA: Structure & Replication Understanding DNA replication and the resulting transmission of genetic information from cell to cell, and generation to generation lays the groundwork
CircLigase ssdna Ligase
 Cat. Nos. CL4111K and CL4115K Connect with Epicentre on our blog (epicentral.blogspot.com), Facebook (facebook.com/epicentrebio), and Twitter (@EpicentreBio). www.epicentre.com Lit. # 222 10/2012 1 EPILIT222
Cat. Nos. CL4111K and CL4115K Connect with Epicentre on our blog (epicentral.blogspot.com), Facebook (facebook.com/epicentrebio), and Twitter (@EpicentreBio). www.epicentre.com Lit. # 222 10/2012 1 EPILIT222
Selected Techniques Part I
 1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
1 Selected Techniques Part I Gel Electrophoresis Can be both qualitative and quantitative Qualitative About what size is the fragment? How many fragments are present? Is there in insert or not? Quantitative
The flow of Genetic information
 The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
The flow of Genetic information http://highered.mcgrawhill.com/sites/0072507470/student_view0/chapter3/animation dna_replication quiz_1_.html 1 DNA Replication DNA is a double-helical molecule Watson and
DNA SEQUENCING BY SANGER METHOD
 DNA SEQUENCING BY SANGER METHOD First method described by Sanger and Coulson,1975 for DNA sequencing was called plus and minus. This method used E.coli DNA polymerase I and DNA ploymerase from bacteriophage
DNA SEQUENCING BY SANGER METHOD First method described by Sanger and Coulson,1975 for DNA sequencing was called plus and minus. This method used E.coli DNA polymerase I and DNA ploymerase from bacteriophage
Supporting Information
 Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany RNA ligands that distinguish metabolite-induced conformations in the TPP riboswitch Günter Mayer, Marie-Sophie L. Raddatz, Julia D. Grunwald,
Supporting Information Wiley-VCH 2006 69451 Weinheim, Germany RNA ligands that distinguish metabolite-induced conformations in the TPP riboswitch Günter Mayer, Marie-Sophie L. Raddatz, Julia D. Grunwald,
CircLigase II ssdna Ligase
 Cat. Nos. CL9021K and CL9025K www.lucigen.com MA298E-CircLigase II ssdna Ligase 2/2018 1 1. Introduction CircLigase II ssdna Ligase is a thermostable ligase that catalyzes iintramolecular ligation (i.e.,
Cat. Nos. CL9021K and CL9025K www.lucigen.com MA298E-CircLigase II ssdna Ligase 2/2018 1 1. Introduction CircLigase II ssdna Ligase is a thermostable ligase that catalyzes iintramolecular ligation (i.e.,
DNA replication: Enzymes link the aligned nucleotides by phosphodiester bonds to form a continuous strand.
 DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
DNA replication: Copying genetic information for transmission to the next generation Occurs in S phase of cell cycle Process of DNA duplicating itself Begins with the unwinding of the double helix to expose
DNA Replication in Prokaryotes and Eukaryotes
 DNA Replication in Prokaryotes and Eukaryotes 1. Overall mechanism 2. Roles of Polymerases & other proteins 3. More mechanism: Initiation and Termination 4. Mitochondrial DNA replication DNA replication
DNA Replication in Prokaryotes and Eukaryotes 1. Overall mechanism 2. Roles of Polymerases & other proteins 3. More mechanism: Initiation and Termination 4. Mitochondrial DNA replication DNA replication
Amplified segment of DNA can be purified from bacteria in sufficient quantity and quality for :
 Transformation Insertion of DNA of interest Amplification Amplified segment of DNA can be purified from bacteria in sufficient quantity and quality for : DNA Sequence. Understand relatedness of genes and
Transformation Insertion of DNA of interest Amplification Amplified segment of DNA can be purified from bacteria in sufficient quantity and quality for : DNA Sequence. Understand relatedness of genes and
Requirements for the Genetic Material
 Requirements for the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell-generation to generation. 2. Information Storage Biologically useful information in a stable
Requirements for the Genetic Material 1. Replication Reproduced and transmitted faithfully from cell to cell-generation to generation. 2. Information Storage Biologically useful information in a stable
Information specifying protein structure. Chapter 19 Nucleic Acids Nucleotides Are the Building Blocks of Nucleic Acids
 Chapter 19 Nucleic Acids Information specifying protein structure Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats)
Chapter 19 Nucleic Acids Information specifying protein structure Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats)
All This For Four Letters!?! DNA and Its Role in Heredity
 All This For Four Letters!?! DNA and Its Role in Heredity What Is the Evidence that the Gene Is DNA? By the 1920s, it was known that chromosomes consisted of DNA and proteins. A new dye stained DNA and
All This For Four Letters!?! DNA and Its Role in Heredity What Is the Evidence that the Gene Is DNA? By the 1920s, it was known that chromosomes consisted of DNA and proteins. A new dye stained DNA and
Replication of DNA Virus Genomes. Lecture 7 Virology W3310/4310 Spring 2013
 Replication of DNA Virus Genomes Lecture 7 Virology W3310/4310 Spring 2013 It s all about Initiation Problems faced by DNA replication machinery Viruses must replicate their genomes to make new progeny
Replication of DNA Virus Genomes Lecture 7 Virology W3310/4310 Spring 2013 It s all about Initiation Problems faced by DNA replication machinery Viruses must replicate their genomes to make new progeny
Bacterial DNA replication
 Bacterial DNA replication Summary: What problems do these proteins solve? Tyr OH attacks PO4 and forms a covalent intermediate Structural changes in the protein open the gap by 20 Å! 1 Summary: What problems
Bacterial DNA replication Summary: What problems do these proteins solve? Tyr OH attacks PO4 and forms a covalent intermediate Structural changes in the protein open the gap by 20 Å! 1 Summary: What problems
Bio 366: Biological Chemistry II Test #3, 100 points
 Bio 366: Biological Chemistry II Test #3, 100 points READ THIS: Take a numbered test and sit in the seat with that number on it. Remove the numbered sticker from the desk, and stick it on the back of the
Bio 366: Biological Chemistry II Test #3, 100 points READ THIS: Take a numbered test and sit in the seat with that number on it. Remove the numbered sticker from the desk, and stick it on the back of the
Supplementary Information for. Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment.
 Supplementary Information for Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation Thomas R. Beattie and Stephen D. Bell Sir William Dunn School of Pathology,
Supplementary Information for Coordination of multiple enzyme activities by a single PCNA in archaeal Okazaki fragment maturation Thomas R. Beattie and Stephen D. Bell Sir William Dunn School of Pathology,
Weaver 4th ed Ch 4+5 Methods etc
 Methods Weaver 4th ed Ch 4+5 Methods etc Chapter 4 Cloning pg 52 Restriction endonucleases pg 54 Vectors pg 56 Replica plating pg 63 Probes pg 64 cdnas pg 66 RACE (Rapid amplification of cdna ends) pg
Methods Weaver 4th ed Ch 4+5 Methods etc Chapter 4 Cloning pg 52 Restriction endonucleases pg 54 Vectors pg 56 Replica plating pg 63 Probes pg 64 cdnas pg 66 RACE (Rapid amplification of cdna ends) pg
The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity
 Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
Promega Notes Magazine Number 62, 1997, p. 02 The GeneEditor TM in vitro Mutagenesis System: Site- Directed Mutagenesis Using Altered Beta-Lactamase Specificity By Christine Andrews and Scott Lesley Promega
RNA synthesis/transcription I Biochemistry 302. February 6, 2004 Bob Kelm
 RNA synthesis/transcription I Biochemistry 302 February 6, 2004 Bob Kelm Overview of RNA classes Messenger RNA (mrna) Encodes protein Relatively short half-life ( 3 min in E. coli, 30 min in eukaryotic
RNA synthesis/transcription I Biochemistry 302 February 6, 2004 Bob Kelm Overview of RNA classes Messenger RNA (mrna) Encodes protein Relatively short half-life ( 3 min in E. coli, 30 min in eukaryotic
Lecture 1 Sunday, 4 March :24 pm
 Lecture 1 Sunday, 4 March 2018 10:24 pm Amino acid side chains can be Hydrophobic, hydrophilic Positive, negatively charged Movement of information OH removed from 2' carbon to make the end more stable
Lecture 1 Sunday, 4 March 2018 10:24 pm Amino acid side chains can be Hydrophobic, hydrophilic Positive, negatively charged Movement of information OH removed from 2' carbon to make the end more stable
DNA Metabolism. I. DNA Replication. A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding
 DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
DNA Metabolism I. DNA Replication A. Template concept: 1. How can you make a copy of a molecule? 2. Complementary Hydrogen bonding B. DNA replication follows a set of fundamental rules 1. Semiconservative
Plasmids & Transposable Elements. Dr. Wiaam Ahmed Al-Amili
 Plasmids & Transposable Elements BY Dr. Wiaam Ahmed Al-Amili Plasmid DNA preparation In order to use a vector for cloning, sequencing, etc., it is necessary to isolate the vector in a highly purified form.
Plasmids & Transposable Elements BY Dr. Wiaam Ahmed Al-Amili Plasmid DNA preparation In order to use a vector for cloning, sequencing, etc., it is necessary to isolate the vector in a highly purified form.
Nucleic Acids. Information specifying protein structure
 Nucleic Acids Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats) Genome - the genetic information of an organism Information
Nucleic Acids Nucleic acids represent the fourth major class of biomolecules (other major classes of biomolecules are proteins, carbohydrates, fats) Genome - the genetic information of an organism Information
DNA Replication semiconservative replication conservative replication dispersive replication DNA polymerase
 DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
DNA Replication DNA Strands are templates for DNA synthesis: Watson and Crick suggested that the existing strands of DNA served as a template for the producing of new strands, with bases being added to
DNA replication. - proteins for initiation of replication; - proteins for polymerization of nucleotides.
 DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
DNA replication Replication represents the duplication of the genetic information encoded in DNA that is the crucial step in the reproduction of living organisms and the growth of multicellular organisms.
MCB 110 Spring 2017 Exam 1 SIX PAGES
 MCB 110 Spring 2017 Exam 1 SIX PAGES NAME: SID Number: Question Maximum Points Your Points I 28 II 32 III 32 IV 30 V 28 150 PLEASE WRITE your NAME or SID number on each page. This exam must be written
MCB 110 Spring 2017 Exam 1 SIX PAGES NAME: SID Number: Question Maximum Points Your Points I 28 II 32 III 32 IV 30 V 28 150 PLEASE WRITE your NAME or SID number on each page. This exam must be written
User Manual. Topoisomerase I Assay Kit (plasmid based). Lot Number:
 Copyright TopoGEN, Inc., 2012. All rights reserved. User Manual Topoisomerase I Assay Kit (plasmid based). Catalog Number Catalog Number TG1015-1 100 Reaction Set TG1015-2 250 Reaction Set Lot Number:
Copyright TopoGEN, Inc., 2012. All rights reserved. User Manual Topoisomerase I Assay Kit (plasmid based). Catalog Number Catalog Number TG1015-1 100 Reaction Set TG1015-2 250 Reaction Set Lot Number:
Torsional Constraints of DNA Substrates Impact Cas9 Cleavage
 Supporting Information Torsional Constraints of DNA Substrates Impact Cas9 Cleavage Michael H. Räz, Kumi Hidaka, Shana J. Sturla, Hiroshi Sugiyama, *,, and Masayuki Endo *, Institute for Integrated Cell-Material
Supporting Information Torsional Constraints of DNA Substrates Impact Cas9 Cleavage Michael H. Räz, Kumi Hidaka, Shana J. Sturla, Hiroshi Sugiyama, *,, and Masayuki Endo *, Institute for Integrated Cell-Material
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB Chapters 34 & 35 DNA Replication and Repair
 BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
BCMB 3100 - Chapters 34 & 35 DNA Replication and Repair Semi-conservative DNA replication DNA polymerase DNA replication Replication fork; Okazaki fragments Sanger method for DNA sequencing DNA repair
The discovery of the role of RNA RNA structure, synthesis and function
 Central Dogma The discovery of the role of RNA RNA structure, synthesis and function! Fundamental observations in genetics!! Genes are located in nuclei (in eukaryotes)!! Polypeptides are synthesised in
Central Dogma The discovery of the role of RNA RNA structure, synthesis and function! Fundamental observations in genetics!! Genes are located in nuclei (in eukaryotes)!! Polypeptides are synthesised in
Chapters 31-32: Ribonucleic Acid (RNA)
 Chapters 31-32: Ribonucleic Acid (RNA) Short segments from the transcription, processing and translation sections of each chapter Slide 1 RNA In comparison with DNA RNA utilizes uracil in place of thymine
Chapters 31-32: Ribonucleic Acid (RNA) Short segments from the transcription, processing and translation sections of each chapter Slide 1 RNA In comparison with DNA RNA utilizes uracil in place of thymine
7.28 Spring 01 Problem Set #1 Answers
 1. You have a 2000 base linear, double-stranded DNA template, a 1000 base region of which you would like to amplify using polymerase chain reaction (PCR). In addition to the template, you add the appropriate
1. You have a 2000 base linear, double-stranded DNA template, a 1000 base region of which you would like to amplify using polymerase chain reaction (PCR). In addition to the template, you add the appropriate
Recombinant DNA Technology
 History of recombinant DNA technology Recombinant DNA Technology (DNA cloning) Majid Mojarrad Recombinant DNA technology is one of the recent advances in biotechnology, which was developed by two scientists
History of recombinant DNA technology Recombinant DNA Technology (DNA cloning) Majid Mojarrad Recombinant DNA technology is one of the recent advances in biotechnology, which was developed by two scientists
of the Triphosphate of ATP
 A Small Aptamer with Strong and Specific Recognition of the Triphosphate of Peter L. Sazani, Rosa Larralde and Jack W. Szostak Howard Hughes Medical Institute, and Department of Molecular Biology, Massachusetts
A Small Aptamer with Strong and Specific Recognition of the Triphosphate of Peter L. Sazani, Rosa Larralde and Jack W. Szostak Howard Hughes Medical Institute, and Department of Molecular Biology, Massachusetts
Supplementary Information for
 Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
Supplementary Information for Conformational landscapes of DNA polymerase I and mutator derivatives establish fidelity checkpoints for nucleotide insertion Hohlbein et al. 1 Supplementary Figure S1. Wt
M1 - Biochemistry. Nucleic Acid Structure II/Transcription I
 M1 - Biochemistry Nucleic Acid Structure II/Transcription I PH Ratz, PhD (Resources: Lehninger et al., 5th ed., Chapters 8, 24 & 26) 1 Nucleic Acid Structure II/Transcription I Learning Objectives: 1.
M1 - Biochemistry Nucleic Acid Structure II/Transcription I PH Ratz, PhD (Resources: Lehninger et al., 5th ed., Chapters 8, 24 & 26) 1 Nucleic Acid Structure II/Transcription I Learning Objectives: 1.
