Anergy and Exhaustion Are Independent Mechanisms of Peripheral T Cell Tolerance By Benedita Rocha,* Alf Grandien,~ and Antonio A.
|
|
|
- Jessica Day
- 6 years ago
- Views:
Transcription
1 Anergy and Exhaustion Are Independent Mechanisms of Peripheral T Cell Tolerance By Benedita Rocha,* Alf Grandien,~ and Antonio A. Freitas~ From *Institut Nationale de la Sant/ et de la Recherche M(dicale, U345, CHU Necker, and ~Unite d'lmmunobiologie, Centre National de la Recherche Scientifique URA 359, lnstitut Pasteur, Paris, France Summary We studied the interactions of male-specific T cell receptor (TCR)-ot/B-transgenic (TG) cells with different concentrations of male antigen in vivo. We constructed mouse chimeras expressing different amounts of male antigen by injecting thymectomized, lethally irradiated mice with various ratios of male (immunoglobulin [Ig] H a) and female (IgH b) bone marrow. These chimeras were injected with male-specific TCR-ot/~-transgenic ceils. These experiments allowed us to monitor antigen persistence and characterize antigen-specific T cells in terms of their frequency, reactivity, and effector functions (as tested by elimination of male B cells in vivo). In the absence of antigen, virgin TG cells persisted but did not expand. Transient exposure to antigen resulted in cell expansion, followed by the persistence of increased numbers of antigen-reactive T cells. In contrast, antigen persistence was followed by two independent mechanisms of tolerance induction: anergy (at high antigen concentrations), where T cells did not differentiate into effector functions but persisted in vivo as unresponsive T cells, and exhaustion (at lower antigen concentrations), where differentiation into effector functions (B cell elimination) occurred but was followed by the disappearance of antigen-specific T cells. he mechanisms underlying the generation and main- T tenance of secondary ("memory") T cell responses are unclear. T cell memory may result from the presence of an increased frequency of antigen-specific cells (1). It is also possible that antigen stimulation may induce changes in "virgin" T cells and generate a memory T lymphocyte, of poorly characterized properties (2-4). Classically, in vivo antigen immunization should induce cell division, but eventually a fraction of antigen-specific T cells would revert to the resting state and persist by increased cell survival. Thus, virgin T cells would be short-lived and memory cells long-lived, the latter forming the basis of long-term memory responses (5). These concepts were challenged by recent in vivo studies. First, it was shown that virgin T lymphocytes can persist as nondividing cells (6-9), whereas immunization occasionally led to the rapid disappearance of antigen-reactive T cells (10--12). It has also been suggested that activated T cells cannot survive in the absence of antigen. In these circumstances, longterm memory, rather than resulting from the generation of long-lived cells, might be due to antigen persistence and continued cell division (13-15). Other experimental results suggested, however, that survival of memory cells might take place in the absence of antigen (16, 17). In experimental systems where accurate follow-up of antigen-specific cells is possible, confrontation with antigen usually leads to tolerance rather than to secondary responses. Studies of TCR-transgenic (TG) 1 mice (6, 7, 18) and immune reactivity to superantigens (19, 20) have shown that immunization, besides inducing T cell activation and expansion, can render antigen-specific cells unresponsive (anergic) to further antigen stimulation (6, 7, 19), and/or lead to the disappearance of antigen-specific cells (18). Anergy and the disappearance of antigen-specific cells were both interpreted as being part of the same process and a consequence of extensive cell division (18). Tolerance induction in these and other systems was attributed to inadequate helper activity (10, 21) and to differences in antigen dose (18) or presentation (22). It is difficult to extrapolate most of these results, since not all the parameters of the immune response were evaluated simultaneously. We have constructed thymectomized male/female mouse bone marrow (BM) chimeras (repopulated with different numbers of male BM cells) and used them to study interactions of TG TCR-ot//3 male-specific cells with different amounts of male antigen in vivo. Using this approach, we were able to induce either tolerance or immune responses, study antigen persistence, and follow the frequency, func- 1 Abbreviations used in this paper: BM, bone marrow; FSC, forward scatter; MoTG, T cell populations monoclonal for the TCR-TG receptor; Slg, serum Ig; TG, transgenic; TX, thymectomized. 993 J. Exp. Med. 9 The Rockefeller University Press /95/03/0993/11 $2.00 Volume 181 March
2 tional reactivity, and effector functions of antigen-specific T lymphocytes. Materials and Methods Mice. C57B1/6 Thyl.1 or Thyl.2 mice TG for a TCR-oJ/~/ specific for the male antigen have been described elsewhere (23, 24). In some experiments, TG RAG2-/- mutant mice were also used. These and syngeneic C57BL/6-IgH-C b mice were obtained from Centre de Service des Animaux de Laboratoire (CSAL) (Orl6ans, France) C57BL/6-IgH-C ~ mice congenic for the IgM heavy chain locus were obtained from the breeding colonies of the Institut Pasteur. Mouse Chimeras. 8-wk-old thymectomized (TX) B6 mice were irradiated (850 fads) and reconstituted with 5 x 106 T celldepleted BM cells containing different percentages of male B6.IgH- C ~ and female B6.IgH-C b cells. Where noted, these mice were injected with Ig- lymph node cells from normal, TG, or TG RAG2-/- mice 24 h after BM transfer. Cell Labeling. We used the following mabs for cell surface staining (6): F23.2 (anti-tcr-b TG chain ~T) (23) and T3.70 (anti-tcr-ce TG chain cet) (24); H (anti-cd8-~ chain) (25); 19XE5 (anti-thyl.1) (26); 30H12 (anti-thyl.2); anti-b220 (Phar- Mingen, San Diego, CA) (27); RS3.1 (anti-~t ~) (28); MB8.6 (anti- #b) (29); 331 (anti-igm); 1M781 (anti-cd44) (10). These antibodies were used directly coupled to PE, FITC, or biotin (revealed with streptavidin-tricolor) or -PE [CALTAG Labs, South San Francisco, CA]). Cells were analyzed on a FACScan by use of the Lysis II (Becton Dickinson & Co., Mountain View, CA) program. Identification of T~ Populations in Recipient Mice. In these studies, we identified CD8 + populations from female TG mice by use of an anti-cd8 mab that recognizes the ~ chain of the CD8 molecule (25). This antibody was used (rather than an anti-cd8c~ chain antibody) since its expression is restricted to CD8 populations generated in the thymus. Expression of CD8ce can be induced in multiple cell types by activation events at the periphery. The absolute number of male-specific TG T cells was evaluated as described elsewhere (6). Briefly, the total number of TG CD8 + cells was calculated from the percentage of CD8 + TG lymphocytes and the total number of cells recovered from the spleen. This number, plus the percentage of CD8-expressing C~T, was used to calculate numbers of male-specific lymphocytes in the spleen. To determine the percentage of CD8 cells expressing C~T, cell suspensions were first depleted of B and CD4 + lymphocytes by magnetic sorting with coated Dynabeads (Dynal, A.S., Oslo, Norway), and stained with anti-tcr-cet mab T3.70 and anti-cd8b mab. Since these were kinetic experiments (in which the same injected cell suspension was studied at different time points after transfer), all recipient mice could not be analyzed simultaneously, and slight fluctuations of labeling intensity between different experiments were unavoidable. To overcome this handicap, T cell populations recovered after transfer were compared in each experiment with those from one female TG mouse analyzed simultaneously. CD8c~T + can be readily visualized in female TG CD8 ~ cells (24). CD8ceT cells in recipient mouse chimeras were identified as those expressing similar levels of TCR-c~T. PCR Amplification of Y Chromosome-specific Genes. Spleen and BM cells (5 x 106) from individual mouse chimeras were pelleted and incubated in lysis buffer (0.5% Tween 20, 50 mm KC1, 2.5 mm MgC12, 600/~g/ml proteinase K in 10 mm Tris-HCL, ph 7.5) for 1 h at 55~ followed by 10 rain at 100~ The lysate was spun for 5 min at 10,000 rpm, and the DNA in the supernatant was extracted with phenol-chloroform and ethanol precipi- tated. For PCR analysis, 10/zl of DNA dilutions corresponding to different cell numbers was added to a total 50-/zl reaction mixture containing 200/zM each dntp, 10 mm Tris-HCL, ph 8.8, 1.5 mm MgC12, 50 mm KC1, 0.1% Triton X-100, 2 U of Taq polymerase (Bioprobe International, Inc., Tustin, CA) and 500 ng of each nucleotide primer. Amplification consisted of 35 cycles at 94~ for 10 s, 65~ for 30 s, and 72~ for 30 s in a thermocycler (model 9600; Cetus Corp., Berkeley, CA). A 15-20/~1 aliquot was eleetrophoresed on a 4% Nusieve agarose (FMC BioProducts, Rockland, ME) TBE gel. Primers for the Y chromosome Zfy-1 gene were (5'-3'): CCTATTGCATGGACTGCAGCTTATG and GAC- TAGACATGTCTTAACATCTGTCC (30). DNA from male cells was titrated and, in the conditions used, we were able to amplify the Zfy-I gene from the DNA corresponding to a single male cell. In Vitro Proliferation. 5 x 104 CD4- SIg- spleen cells were cultured with 5 x 10 s irradiated (1,800 rad) spleen cells from male or female nude mice. 3 d later, cultures were supplemented with 10 U/ml of rib2 and expanded for a further 2 d. At the end of this period, cell growth was evaluated by [3H]thymidine incorporation (I mci/culture) (7). Results Experimental Protocol In these experiments, we produced mice expressing different amounts of male antigen. These mice were injected with TG T ceils expressing a male-specific (cevbt) TG receptor and studied at different time points after cell transfer. Host Mice Expressing Different Amounts of Male Antigen. TX lethally irradiated C57BL/6 (B6) mice were injected with a total of 5 x 106 T ceu-depleted BM cells containing female (B6.IgH-C b) and 10, 50, or 90% male (B6.IgH-C a) congenic BM cells. These BM donor mice differ in their IgM allotype, a property used to identify B cells and secreted IgM of male (/~a+) or female (/~b+) origin in the host mice. 1-2 mo after BM injection, we studied the distribution of female and male B cells in the BM, spleen, and lymph nodes, as well as the presence of the female or male IgM allotype in the serum of host mice. The peripheral B call compartment was fully reconstituted. The percentage of #a+ male B cells in the B cell pools and that of the #~+ allotype in the serum of these mice was identical to the percentage of male BM cells injected in the BM inoculum, that is, these mice contained 0, 10, 50, or 90% of male B cells. Although the precise quantitation of any antigen is not possible in vivo (because of unknown variables related to the rates of antigen processing and presentation, etc.), the 10-90% range of male BM cells injected ensured significant variations in the total amount of HY antigen present in the different mouse chimeras. T Cell Populations Expressing a TG TCR-oz/fl Specific for themale Antigen. T lymphocytes injected into host chimeras were TG for an TCR-c~/~ receptor (CeT~/T) specific for the male antigen. Recognition of the male antigen by TG cells requires coexpression of high surface levels of TCR-OLT~T and CD8. In male TG mice, male-reactive T cells are eliminated in the thymus. Peripheral T cells that escape deletion are tolerant of the male antigen and express little or no CD8 (Os176 Female TG mice contain a population of 994 Antigen Load and CD8 T Cell Persistence
3 male-reactive T cells (OIT~TCD8 hi) that is positively selected TX ~-~ in the thymus and represents 25-50% of the CD8 + periph- RECIPIENTS eral T cell pool. Both female and male mice also have T cells TG T ceils (CD4 or CD8 expressing endogenous TCR-cr chains injected: (OCE~T) that are not specific for the male antigen (23, 24). ~o~ In some experiments, T cell populations injected were from 10~ female TG KAG2-/- mutant mice. These mice do not rear- range endogenous T cell receptors, and all T cells injected 0 % exclusively expressed the TG TCR-aTBT. In Vivo Elimination of Male Cells by TG-TCR Male-specific T Cells Injection of tolerant T cells from male TG mice had no effect on the number of male B lymphocytes recovered from chimeric mice (Fig. I A). In contrast, injection of T cells from female TG mice (that contained a male-reactive OCTBTCD8 hi population) was able to induce the disappearance of #~+ male B cells (Fig. 1 B). Elimination of B cells was dependent on their expression of the male antigen, as the female TG population never affected female B cells: the loss of the #a+ male population was readily compensated for by the increase in/~b+ female B cells, and both the total number of B cells and serum IgM levels were similar in all chimeras studied. T cells from female TG mice injected into male/female chimeras contain male-reactive OtT~TCD8 hi as well as cells expressing endogenous T cell receptors. To verify if elimination of male B cells in these chimeras was due to the effect of the male-reactive OtT~TCD8 hi cells, we performed two types of experiments. First, male/female chimeras were injected with similar numbers of T lymphoeytes from female B6 mice, but these T cells were found to have a minimal effect on male B cells (Fig. 1 D). Second, the same chimeras were injected with a monoclonal OtTBTCD8 hi population, obtained from female TG RAG2-/- mice. As shown in Fig. 1 E, these monoclonal T cells eliminated male B cells in vivo and, on a per cell basis, they were actually far more efficient than T populations from TG polyclonal mice. These findings show that male-reactive c~vbtcd8 hi T cells can reject target hematopoiesis-derived cells expressing the male antigen in vivo. We cannot determine the precise mechanism by which male B cells are eliminated in vivo. As OtTBTCD8 hi T cells are unable to induce polyclonal B cell activation and thus to induce terminal B cell differentiation (von Boehmer, H., personal communication), it is likely that B cell elimination in vivo results from direct cytotoxicity of this CD8 + TG population for male B cells and/or their immature BM precursors. We conclude that OtTBTCD8 hi T cells are able to mediate effector functions and reject hematopoiesis-derived cells expressing the male antigen in vivo. These eliminated male cells may be either BM precursors or mature B cells. The present protocol thus mimics the confrontation of T cells with replicating antigen during infection or BM transplantation. Depending on how the immune system deals with the initial cohort of antigen, the antigen load may gradually increase or diminish. O t- O o e- # % 90 % B ~ X 1066 ` 3 X X 1069 (4 X losott~cd8 ~~ (4 X 10Scc, I~TCD8hl) (4 X 10SCZT~I-CD8 h~) 10"! ~ 10" ~r-r-~,~r~ 103~ 102 I 101 I ' 0 2 ~ o ~ 1(2o ~ 10 o IO3 104 Ig a T \ RECIPIENTS s B6 $ B6 Tcellsinjected: 3X1065 B6 2.5X RAG TG c 50 lo~ "o %.~. 101 U -- 10' "r" 90 lo= _~ % 04 10t I Ig a Figure 1. Frequency of male (#0 B cells in the spleen of chimeric mice 1 mo after BM reconstitution. Female (A, B, D, and E) and male (C) TX irradiated mice, reconstituted with 0, 10, 50, and 90% IgH a male BM cells, were injected i.v. with (A) 2.5 x 106 T cells from male TG mice (containing 4 x 105 OtT/SaCD8 Io T cells), (B and C) 3 x 106 T cells from female TG mice (containing 4 x 10 s at~tcd8 hi T cells); (D) 3 x 106 T cells from female B6 mice, or (E) 2.5 x 10 s OeT/STCD8 hi monoclonal T cells from female TG RAG2-deficient mice and studied 1 mo (A-C) and 6 wk (D and E) later. Data are for one of three mice studied at these time points with similar results. 995 Rocha et al.
4 The Amount of Male Antigen Determined the Fate of TG T Cells, As Well As Their Capacity to Eliminate Male B Cells Female TX Mice Reconstituted With 100% Female BM Cells. T cells from female TG mice transferred into these chimeras behaved in the same way as after adoptive transfer into female nude mice (6-8, 31). In the absence of the male antigen, male-specific OIT~TCD8 hi cells never expressed CD44 (Fig. 2 B) and did not expand (Fig. 3): the number of OtT~TCD8 hi cells recovered did not vary with time after injection and corresponded to the fraction of donor T cells expected to home to lymphoid organs after intravenous injection (32). Because TG cells expressing other TCR specificities expanded in these mice, r hi cells became a minority population after adoptive transfer. They could, however, be detected by FACS analysis (Fig. 2 B) as well as by their capacity to proliferate to the male antigen in vitro, a property that persisted for at least 4 mo after transfer (Table 1). These results suggest that T cells never exposed to nominal antigen (virgin T cells) can persist in vivo for long periods (6-9, 31). In the presence of male antigen, that is, in the male/female chimeras, all O~T~TCD8 hi cells expressed CD44 (Fig. 2 C) and their numbers increased. The kinetics of cell expansion differed from what we previously described after transfer into nude mice (6, 7), since it started by 2-3 wk after transfer and was maximal after 1 mo (Fig. 3). The fate of these cells, as well as their effect on male B cells, varied in the different experimental groups. Female TX Hosts Reconstituted With 10% Male BM. In these chimeras, injection of OtT~TCD8 hi TG cells resulted in the elimination of #a+ male B cells, which were rare up to 3 wk after BM injection and undetectable thereafter (Fig. 1 B). No #; Ig was then found in the serum of these mice. The OIT~TCD8 hi expanded population persisted (Figs. 3 and 4), maintaining the capacity to respond to the male antigen for up to 5 mo after transfer (Table 1). As we could not de- 106_.[ ~105- Figure 3. ";'\\... z~ 100 ~ S_10-9 ~ Months after transfer Months after transfer Fate of ~T/~TCD8 hi T cells after transfer into female TX recipients reconstituted with 0 (.~ -~), 10 (D-D), 50 (A-A), or 90% (0-0) male BM. Results are from one experiment in which mice were studied from 2 wk to 5 mo after BM transfer. In total, 97 mice were studied in three independent experiments, with 2-3 mice per experimental group and time point. (A) Number of CtTBTCD8 + T cells in the spleen (mean of 2 mice) evaluated as described in Materials and Methods. All mice had similar numbers of TG CD8 + lymphocytes in the spleen, but the percentage of OtT/Sr cells varied. In mice reconstituted with 50% male BM, we could not identify cer/$tcd8 + cells as distinct populations in the spleen 4 mo after BM injection: the proportion of t~t/3t within CD8 + cells was <0.8%. (B) Percentage of r + cells in the BM of the same mice. We did not evaluate absolute numbers of mate-specific cells in BM, since we found exhaustive BM suspensions difficult to obtain and quantification of cell yields inaccurate. We found variations in ~T/~T expression in mice reconstituted with 50% male BM. Thus, data for individual mice are shown rather than means. A A o ~ ii CD ~ 55.6 Figure 2. CD44 expression by TG CD8 + T lymphocytes recovered from a female TG mouse (A), TX irradiated mice reconstituted with 100% female BM cells (B), or 10% male BM cells (C) 5 mo after lethal irradiation and BM transfer. All mice were studied simultaneously. Spleen cell suspensions were depleted of SIg + and CD4 T cells and triple-labeled with the mabs T3.70, anti-cd8, and anti-cd44. The figure shows CD44 and T3.70 expression by gated CD8 + T cells. Male-specific cells expressing high levels of TG TCK-ot chain (T3.70 hi) can be readily visualized in female TG mice (.4). OtTBTCD8 hi T cells in chimeric mice were those expressing similar levels of the TG TCR-t~ chain. tect male B cells or serum #a later in these chimeras, whereas the male-reactive expanded population persisted, these results indicate that previously activated T cells might have survived in the absence of antigen. In Chimeras Reconstituted With 50% of Male BM, Elimination of Male B Cells Was Never Complete. Maximal depletion was seen 1-2 mo after BM reconstitution, and even then 1-3% of #a+ male B cells remained (Fig. 1 B). The initial expansion of the C~TC/TCD8 hl population was followed by a rapid decrease: 2 mo after transfer, male-specific cells were rare in the spleen and diminished in the BM; they were absent from both organs later on (Figs. 3 and 4). At these later times, T cells from these mice were unreactive to the male antigen (Table 1), confirming that OIT TCD8 hi ceils were absent. It should be noted that disappearance of male-specific T cells from these chimeras was not preceded by downregulation of the CD8 molecule at the cell surface (Fig. 4). In contrast, during anergy induction, downregulation of CD8 996 Antigen Load and CD8 T Cell Persistence
5 Table 1. In Vitro Response of Spleen Cells from Mouse Chimeras in the Presence of Male Antigen Time after transfer Percent male BM injected 1 mo 4 mo 5 mo 9 nu/nu CYnu/nu ~ nu/nu Cenu/nu 9 nu/nu Cenu/nu 0 3,688 20,645 5,840 23,520 ND ND 10 5,725 45,979 1,346 18,146 4,472 73, ,827 9,761 1,713 1,588 1, ,540 17,989 2,788 3,184 5,095 7,429 Female TX irradiated hosts were injected with 5 x 106 T cell-depleted BM containing 0, 10, 50, or 90% cells from IgH~ male donors; 24 h later, these chimeras received 4 x 10 s OtT/~TCD8 h~ TG T cells. At different times (mo) after transfer, 4 x 104 CD4-SIg- spleen cells from each individual chimera were cultured with 5 x 10 s irradiated (1,800 rad) spleen cells from either male or female (nu/nu) B6 mice, in the presence of rll-2 (7). Values represent the mean [3H]thymidine uptake of triplicate cultures. Similar results were obtained in all mice studied in two different experiments. expression preceded partial deletion of male-specific TG cells (6; see also Fig. 4 and below). The disappearance of OtT~TCD8 hi cells from these chimeras was not due to the absence of antigen: (a) male B cells were always detected (Fig. 1 B and below); (b) the amount of male antigen was sufficient to induce the expansion of a new set of virgin T cells (see below); and (c) in some mice, a substantial fraction of male hematopoietic precursors must have survived elimination by effector T cells since, after the disappearance of OITBTCD8 hi cells, the frequency of male B cells in some mice increased again (Fig. 5). These results demonstrated that the continuous presence of the male antigen was not sufficient for the survival of a male-reactive TCR TG population. Months after transfer t O! (3) "d E" a) E o3 T c4 t ~,..., ,1 CD8~ ![ 27.8 Figure 4. CD8 populations in male/female chimeras at different time points after transfer. The CD8 + T cells shown are from mice represented in Table 1, and not from the same kinetics experiment shown in Fig. 3. At each time point, mice from individual groups were studied simultaneously. This was a kinetics experiment (the same transferred population was studied at different time points after injection), and recipient mice at different time points were not studied on the same day. Slight variations of labeling intensity between individual assays were unavoidable. Therefore, in each experiment, a TG female mouse was simultaneously studied as a control. Male-specific cells expressing high levels of TG TCR-ct chain (T3.70 ~) can be readily visualized in female TG mice (Fig. 2 A). O~T~T CD8 hi T cells in recipient mice were those expressing similar levels of TG chain. This strategy was used in all experiments. 997 Rocha et al.
6 O Ckl OJ rn (12, 10+" 104 % male B cells i 9.3 I031 ~ ~, ~ t 10: 10: Ig a % C~T{3TCO8 cells 0 CD8~ Figure S. Frequency of male (/~') B cells (right) and C~T~TCD8 hi T cells (left) in the spleen of two chimeric mice injected with 50% male BM and TG T cells 4 mo after BM reconstitution. We tested 25 such chimeras. In the 15 chimeras studied I or 2 mo after BM transfer, ~TBTCD8 hi cells were present, and male B cells represented up to 3% of the B cell pool. In the 10 chimeric mice studied from 3 to 6 mo after BM injection, ~T~TCD8 hi cells were absent. Half these mice maintained a low percentage (1-3%) of male B cells. In the other mice, the percentage of male B ceils increased to 8-25% of the B cell pool. Results for two of these latter mice (not studied on the same day) are shown. In TX Female Hosts Reconstituted With 90% of Male BM, OIT~TCD8 hi TC Cells Induced a Small and Transient Decrease in the Number of Male B Cells 1 mo After Transfer (Fig. 1 B). The fraction of male B cells increased thereafter, to represent,,o90% of the peripheral B cell pool 2-5 mo after reconstitution (not shown). In male TX hosts, OtTC/TCD8 hi TG cells had no substantial effect on male B cells, even when they only constituted a small fraction of the B cell pool (Fig. 1 C). In all these chimeras, OtTBTCD8 hi cells expanded for 1 too, slowly decreasing thereafter; they were still present in substantial numbers 4-5 mo after transfer (Figs. 3 and 4). Persisting male-specific cells downregulated surface levels of CD8 (Fig. 4) and were unresponsive to the male antigen (Table 1), that is, they became "anergic" We have described similar phenomena when ~T~TCD8 hi TG lymphocytes were transferred to male nude recipients (6, 7). These anergic T cells were devoid of suppressor activity: the fraction of /z,- secreting cells and #~ serum levels were comparable to those found in similar chimeras that had not received male-specific cells (not shown). It should be noted that CD8 downregulation and anergy induction were also observed when O~T~TCD8 hi TG lymphocytes were transferred to TX male mice reconstituted with 100% female B cells (not shown). Thus, male B cells appeared unnecessary for anergy induction. Persistence of Virgin T Cells in the Absence of Antigen Our previous results suggested that long-term survival of virgin mature T lymphocytes did not require antigen recognition: when we transferred O~T~TCD8 hi cells to female mice a oo o 0 t~ ~ lo % IgHa male BM injected 50% 0% O 20.1,0, , I, i,,.,,,,,,,.,,,.,,,...,,,,vrm ~ 10 ~ CD3 O 97.3,,,,,,,,,,,,,rrm,.,,,,,.,,,,., ~ CD8 2, Antigen Load and CD8 T Cell Persistence Figure 6. Persistence of virgin T cells in the absence of antigen. TX irradiated mice reconstituted with 50 (left) or 0% male BM (100% female, right) were injected 24 h later with 2.5 x 10 s monoclonal OtTBTCD8 hi cells from female TG RAG2-/- mice and studied 6 wk after transfer. ('/bp) CD8 expression in total spleen cells. (Bottom) T3.70 expression by CD8 + T cells. Spleens were depleted of SIg cells before this study. Data are for one of three mice studied at this time point with similar results.
7 reconstituted with 100% female BM cells, these T cells did not expand but persisted for long periods (Figs. 2 and 3 and Table 1). In these experiments, however, we could not exclude the possibility that persisting O~TflTCD8 hi cells coexpressed low levels of endogenous TCR-oe chains at their surface, and thus had multiple specificities. In such circumstances, cell survival could be due to the recognition of other antigens. To verify this hypothesis, we injected female TX irradiated mice reconstituted with 100% female BM with mature T cells from female TG RAG2-/- mice. These donor mice cannot rearrange endogenous TCR, and all T cell populations are monoclonal for the TCR-TG receptor (MoTG). TG RAG2-/- mice have very few mature T cells in the peripheral pools (Rocha, B., manuscript in preparation), and so we injected our chimeras with a few T cells (2.5 x 10 s i.v.). In these experiments, and in the absence of TG cell expansion or cell death, about 5 x 104 TG cells should be recovered from the spleen of host mice (only 20% of a T cell cohort settles in the spleen after i.v. injection; 32). The results of one of these experiments are shown in Fig. 6. MoTG cells expanded after transfer into male/female chimeras, and numerous CD8 + T cells were seen in the spleen (Fig. 6, upper left) of these mice. These CD8 + T cells were virtually all MoTG (Fig. 6, lower left). In 100% female BM chimeras injected with MoTG cells, )>90% of spleen cells were B lymphocytes, and few T cells were present (Fig. 6, upper right). These minority CD8 + populations could be analyzed after depletion of SIg + cells. Most of these CD8 + cells were non-tg (probably residual host cells surviving irradiation), but an MoTG population was clearly identified (Fig. 6, lower left). These results demonstrate that virgin mature T cells can survive in vivo in the absence of their nominal antigen. The number of MoTG cells present in this mouse spleen can be calculated. Since spleen cell recovery was 74 x 106, the number of CD8 + T cells (3.1% of spleen cells) was 2.3 x 106, and the number of MoTG (2.2% of CD8 +) was 4.82 x 104. This number was very similar to the cohort of MoTG cells expected to home to the spleen after i.v. injection (5 X 104 TG cells). We obtained similar results in three other experiments. These results indicate that CCT3TCD8 hi virgin cells persisted but did not expand after transfer. Presence of the Male Antigen in Different Chimeric Mice 5-6 Mo After T Cell Transfer In the above experiments, we identified the male antigen in male/female chimeras by scoring ~a+ male B cells or #a+ SIg. It should be noted, however, that the absence of both these parameters does not rule out the presence of the HY antigen in chimeric mice. First, male BM-derived cells other than B cells may be present in these mice. Second, we cannot exclude presentation by female APC (although the HY antigen is presented by class I MHC molecules). Since the HY antigen, like most minor transplantation antigens, has not yet been isolated, and as elution and identification of small amounts of specific peptides present in vivo may not be possible, we tested for the presence of male antigen indirectly in two independent sets of experiments. 999 Rocha et al. First we investigated if the quantity of antigen present in these chimeras was sufficient to induce the expansion of a new cohort of virgin TG ceus. For this purpose, chimeric mice were reconstituted with 0, 10, 50, or 90% male BM and injected with Thyl.2 + ~T~TCD8 hi T cells; they were then transferred, 5 mo later, with a new set of virgin Thyl.1 + O/T~TCD8 hi T cells and studied 1 mo later. As shown in Fig. 7 (top), most of the CD8 + T lymphocytes present in these chimeric mice were Thyl.2 +, that is, they belonged to the first cohort of TG lymphocytes injected. These results were expected, since Thyl.2 + TG cells were injected into lethally irradiated (and thus T cell-deficient) mice, whereas Thyl.1 + TG cells were injected into T cellreconstituted mice. We have previously observed that T lymphocytes expand readily after transfer into T cell-deficient hosts, whereas little expansion is observed when T cells are injected into T ceu-reconstituted mice (26). As described above, in Thyl.2 + CD8 + cells, a male-specific O~T~TCD8 hi TG population was readily detected in chimeras reconstituted with 10 or 90% male BM, but was absent from mice reconstituted with 50% male BM. In contrast, Thyl.2- O~T~TCD8 hi TG cells (probably Thyl.1 +) were more abundant in chimeras reconstituted with 50 and 90% male BM than in mice reconstituted with 10% BM. To study this second set of virgin Thyl.1 + TG cells, CD8 + populations from recipient mice were further depleted of Thyl.2 cells. As shown in Fig. 7 (middle and bottom), OeTfTCD8 hi Thyl.1 cells parked for 1 mo in 0 or 10% male BM-reconstituted mice persisted as small resting cells and did not expand. After transfer into other chimeras (initially reconstituted with 50 or 90% male BM, in which we detected male B cells), this second set of virgin T cells was activated (as shown by increased size) and expanded. We also noted lower Thyl expression in these latter populations, as well as in "anergic" Thyl.2 + cells (recovered in mice reconstituted with 90% male BM). These results demonstrated that, 5-6 mo after BM transfer, the amount of male antigen present in 10% of male BM chimeras was not sufficient to induce the expansion of virgin TG populations. In contrast, in 50 or 90% of male BM chimeras, male antigen persisted in sufficient amounts to stimulate a newly transferred set of virgin TG cells. In a second set of experiments, we tested for male DNA by PCR amplification of the Y chromosome--specific Zfy-1 gene (30). Fig. 8 A shows the sensitivity of the method, that is, amplification of DNA in two male cells, but bands of similar intensity were observed when one cell, rather than two cells, was studied. As expected in the limiting conditions of one cell per well per PCR, negative and positive results were obtained. This method thus identified BM-derived male cells other than B cells and also increased the threshold of detection of male cells 10,000-fold relative to FACS analysis, since we were able to amplify this gene in the DNA from one or two male cells. We tested for the presence of the Zfy-1 gene in 20 mice (5 mice from each group) 5-6 mo after BM injection. In the 10 chimeras reconstituted with either 90 or 50% male BM, the Zfy-1 gene was identified even when only 103 spleen cells were studied. As expected, the 180-bp-specific band was ab-
8 % IgH a <5 BM injected 10% 50 % go % g 0,7 ~,,o Thy ,6 Total CD8 + cells % IgH a 6" BM injected O% 10% 50 % 90 % (I O o5 I-'- Thy 1+CD8 + ceils Thy 1.1 f \ FSC 9 t 0tT~ T CD8 + Thy 1 + cells Figure 7. Expansion of a new cohort of male-specific virgin T cells in chimeric mice 5-6 me after BM reconstitution and T cell injection. Female TX irradiated mice were injected with 0, 10, 50, or 90% male BM cells and 4 10 s ~TBTCD8hiThyl.2 + male-specific T cells. 5 me later, all mice received 4 10 s OCTflTCD86h ~ Thy-L1 + male-specific cells, and they were studied 1 me later. Data are for one of three mice tested in each experimental group, with similar results. (Tbp) Spleen cells depleted of Ig + and CD4 ceils were triple-labeled with anti-thyl.2, anti-cds, and T3.70 mabs. T3.70 and Thyl.2 expression in CD8 + gated populations is shown. (Middle) Spleen cells depleted of Ig +, CD4 +, and Thyl.2 + T cells were triple-labeled with anti-thyl.1, anti-cds, and T3.70 mabs. T3.70 and Thy-l.1 expression in CD8 gated populations is shown. (Bottom) Forward-scatter (FSC) profiles of CD8 + O~TflT Thyl.1 cells in the same chimeric mice. The FSC profile of otrflr virgin Thy1.1 + cells, parked for 1 me in chimeras reconstituted with 100% female BM cells, is reproduced in all graphs (solid lines) and compared with the FSC profile of ~vflr T cells in other chimeric mice (dotted lines). DNA from the spleen of the mouse reconstituted with 10% male BM shown in this figure was tested for the presence of the Zfy.1 Y-specific gene and was found positive. The proportions of #' male B cells within B220 IgM + cells in these mice were (from left to right) 0.3, 0.2, 2, and 75%, respectively. sent from mice that had received 100% female BM. Results varied in individual mice reconstituted with 10% male BM. In 3 out of 5 chimeras studied, we failed to detect the Zfy-1 gene in either spleen or BM, indicating that male cells, if present, had a frequency <10-6. In the remaining two mice, we could not detect this gene among BM cells, whereas in the spleen we only identified a specific band when the DNA from 10 ~ cells was amplified, suggesting that the frequency of male cells in this organ was <10-5 (Fig. 8). To summarize, in 90 or 50% male BM chimeras studied 5-6 me after reconstitution, male DNA was always present. In mice reconstituted with 10% male BM, male cells, if present, were at a frequency <10-5 or This amount of male antigen was not sufficient to induce the expansion of newly transferred male-specific T cells (Fig. 7). Discussion We found that injection of T cells from female TG mice or monoclonal c~rfla-cd8 hi T cells (MoTG) from female TG RAG2-/- mice into male/female BM chimeric mice induced the rejection of male-derived hematopoietic cells. This demon Antigen Load and CD8 T Cell Persistence
9 Figure 8. PCK amplification of the Y chromosome-specific Zfy-1 gene (30) in the spleens of different mice. (A) Titration of male spleen cells. We tested DNA from different numbers of male spleen cells diluted in female spleen DNA (see Materials and Methods). We show the results of amplification of the DNA of two male cells, rather than one cell, since bands of similar intensity were seen in the two cases. M, marker bands (1,353, 1,078, 872, 603, 310, 281, 271, 234, and 194 base pairs). (B) DNA from different numbers of spleen cells from chimeric mice. TX irradiated mice were injected with 0, 10, 50, or 90% male BM and 4 x 105 OtT~TCD8 hi Thyl.2 + T cells. A group of these mice was tested 5 mo after BM transfer. A second group was injected with CD8+OtTBT Thyl.1 + virgin cells and studied 1 mo later. In both cases, the 180-bpspecific band, corresponding to the amplification product of the Zfy gene, was present in all mice reconstituted with 90 or 50% BM and absent from all mice reconstituted with 100% female BM. This band was present in two out of five tested mice reconstituted with 10% male BM. Results for a positive and a negative mouse are shown. PCR amplifications shown in A and B were done simultaneously. strates that male-specific transgenic T cells (OtT/~TCD8 hi) are able to mediate effector functions in vivo. It has previously been shown that anti-hy female TG mice, although containing large numbers of male-specific cells, show slow rejection of male skin grafts (32a). The anti-hy clone from which the TG TCK was isolated was selected after in vivo boosting with hematopoietic ceils and long-term in vitro stimulation with male nude spleen cells (23). TG T cells may thus show some tissue specificity, being more prompt to reject male hematopoiesis-derived cells in vivo than, for example, male skin grafts. Rejection of male B ceils was dependent on the amount of male antigen, confirming that differentiation into effector functions and elimination of target B cells by CD8 + lymphocytes is dependent on the antigen load (18, 33). Similarly, the rate of elimination of infected or transformed ceils by effector T cells in vivo may depend on the concentration of antigen. In TX mice injected with different quantities of male BM, the fate of male-specific peripheral T cells, in the absence of thymic output, also appeared to be critically dependent on antigen load. In the absence of the nominal antigen, virgin functionally reactive T cells persisted in vivo but did not expand, and their frequencies were low. Low antigen concentrations induced expansion, effector functions (shown by the elimination of male B cells), and persistence of increased numbers of functionally reactive OtT3TCD8 hi lymphocytes. Effector functions and partial elimination of male B cells were observed when the amount of antigen increased, but antigenspecific TG cells disappeared at a later stage. Very high antigen concentrations induced anergy, after which antigenspecific cells did not mediate effector functions (i.e., did not reject male B cells) but persisted in vivo as CD8 l~ tolerant T ceils. These results may be particularly relevant to the understanding of the conditions in which antigen-specific T ceils persist in vivo, long-term T cell responses are maintained, and peripheral T cell tolerance is induced. Our results confirm that virgin T ceils (including MoTG populations) can survive in the absence of nominal antigen in a resting state (6, 9). It is possible that previously activated T cells survive in vivo, like virgin T cells, in the absence of cell division (8): as the expansion potential of T lymphocytes is limited (26), long-term T cell survival is unlikely in the presence of continuous division. The maximal number of divisions a eukaryotic cell can undergo in vitro was estimated by Hayflick (34) to average 45. Similarly, the number of divisions that mature T cells are able to undergo in vivo is equivalent to the Hayflick number (26). Considering that the in vivo doubling time of OtT~TCD8 hi cells is 48 h (Rocha, B., unpublished observations), when cell division is continuous, the Hayflick number should be reached within 3 mo, and exhaustion of immune responses should then occur. Long-term T cell survival must require at least a temporary quiescent state. This may only be possible when antigen encounters are rare or absent. Indeed, in mice injected with 10% male BM, male cells rapidly disappeared, and high frequencies, of male-reactive T cells were maintained in the apparent absence of antigen. The amount of male antigen present was not sufficient to induce activation or expansion of a second cohort of virgin T cells parked for 1 mo in these chimeras. Moreover, using a PCR technique that permitted identification of male cells at frequencies of 10-6, we could not always detect the presence of male DNA in these chimeras. Although previous antigen exposure 1001 Rocha et al.
10 may reduce the threshold required for cell stimulation, encounters with antigen in these chimeras must be rare. Activated T cells (like virgin T cells) may thus survive in the absence of continuous antigen stimulation and in the absence of extensive terminal differentiation (see below). It is possible that interactions with the same ligands that induced intrathymic positive selection, although of insufficient affinity to induce cell proliferation (35), permit the survival of peripheral T cells in the absence of nominal antigen. Antigen persistence was clearly demonstrated in chimeras reconstituted with 90 or 50% male BM. We found that TG T cells became tolerant to the male antigen in these chimeras in two independent ways. In mice injected with 50% male BM, exhaustion was responsible for unresponsiveness: malespecific T cells were able to mediate effector functions and eliminate a large fraction of male cells, but eventually disappeared, allowing the late partial recovery of male B cells. In female mice reconstituted with 90% male BM, or after transfer into male hosts, male-specific cells initially proliferated, downregulated CD8 levels at the cell surface, became unable to mediate effector functions, and persisted in vivo as anergic T cells. As to the mechanisms inducing these two types of tolerance, it is unlikely that continuous division alone was responsible for clonal exhaustion of C~v3rCD8 h~ T cells in the 50% chimeras. Continuous division should generate a large clone (each cell may generate,,o3 x 10 t3 cells [26]), but this was never the case in these mice, in which ott3rcd8 hi T cell clones were smaller than those in other male/female chimeras. The gradual disappearance of antigen-specific cells was evident from 1 mo after transfer onwards, that is, well before expansion capacity should have been exhausted. It is likely that OtT3TCD8 hi T ceils disappeared by terminal differentiation: the continuous presence of antigen in mice reconstituted with 50% male BM may have induced continuous differentiation into effector functions, and cytotoxic effector T cells may have a short life span (36). Conversely, in chimeras reconstituted with 90% male BM after anergy induction, both cell expansion (7) and effector functions become paralyzed; it is thus not surprising that anergic T cells may persist in vivo in these conditions. Anergy was thought to emerge after extensive division of antigen-specific T cells and, therefore, to be similar to exhaustion (18). Our results show that anergy and exhaustion are different phenomena and independent mechanisms of tolerance. Induction of anergy required higher doses of antigen, which blocked proliferation (7) and effector functions, but antigen-specific T cells persisted. Exhaustion of the immune response was not preceded by CD8 downregulation or extensive division, permitted effector functions, and was probably caused by terminal differentiation of CD8 + T cells. It occurred at antigen concentrations insufficient to induce anergy. It should be noted that in mice reconstituted with 50% male BM, most/z a+ cells were eliminated 1 mo after transfer, but the remaining 1-3% of male B cells were sufficient to delete all antigen-specific T cells 2-3 mo later. These differences may also be important, as we have shown that anergy is reversible (7), whereas exhaustion is an irreversible mechanism of peripheral T cell tolerance. Exhaustion may be particularly efficient in removing effector CD8 T cells recognizing self-antigens in vivo: if antigen persistence rather than antigen load is responsible for the exhaustion of the immune response, then this process may occur in response to selfantigens continuously produced at low concentrations in vivo (low-dose tolerance). We found that persistence of antigen was not sufficient for the maintenance of functional reactive T cells. On the contrary, when antigen persistence could be demonstrated, that is, in chimeras reconstituted with 50 or 90% male BM, recipient mice became unresponsive to the male antigen (Table 1). If antigen persistence is associated with tolerance induction, it is likely that self-antigens, which are continuously present in vivo, induce tolerance rather than immune reactivity. We also found that the presence of male-specific T cells favored peripheral repopulation by female/z b+ B cells. The total number of B cells remained constant in all chimeras studied, that is, the loss of the/x a+ male population was readily compensated for by the increase in #b female B cells. Exploitation and mutualistic lymphocyte interactions may occur in an immune system in which the total number of cells is under strict control and each lymphocyte has to compete with other newly produced or resident lymphocytes for survival, proliferation, and/or differentiation (37). We are grateful to D. Guy-Grand, G. Marodon, M. Papiemick, and H. von Boehmer for reviewing this manuscript, K. McElreavey for suggestions, G. Bordenave for the gift of the B6.IgH-C a mice, F. Alt for the RAG2-/- mice, A.-M. Joret and M.-P. Lembezat for technical assistance, and D. Young for correcting our English. Dr. Matzinger was one of the referees of this article. Her criticisms definitely improved the quality of this manuscript. This work was supported by grants from DKET (#92/2023A) to B. Kocha and from ARC and the Institut Nationale de la Sant~ et de la Recherche M6dicale to A. A. Freitas. Address correspondence to Dr. Benedita Rocha, INSERM U345, CHU Necker, Paris, France. Received for publication I3 April 1994 and in revised form 18 October I Antigen Load and CD8 T Cell Persistence
11 ~efel~nces 1. Owen, J.A., M. Allouche, and P.C. Doherty Limiting dilution analysis of the specificity of influenza-immune cytotoxic T cells. Cell. Immunol. 67: Tabi, Z., F. Lynch, K. Ceredig, J.E. Allan, and P.C. Doherty Virus-specific memory T cells are Pgpl and can be selectively activated with phorbol ester and calcium ionophore. Cell, Immunol. 113: Hou, S., and PC. Doherty Partioning of responder CD8 + T cells in lymph node and lung of mice with Sendai virus pneumonia by LECAM-1 and CD45RB phenotype. J. Immunol. 150: Kos, F.J., and A. Mullbacher Ib2 independent activity of II,-7 in the generation of secondary antigen-specific cytotoxic T cell responses "in vitro." J. Immunol. 150: Beverley, PC Is T cell memory maintained by crossreactive stimulation? Immunol. Today. 11: Kocha, B., and H. yon Boehmer Peripheral selection of the T cell repertoire. Science (Wash. DC). 251: Rocha, B., C. Tanchot, and H. yon Boehmer Clonal anergy blocks in vivo growth of mature T cells and can be reversed in the absence of antigen.j. Extx Med. 177: von Boehmer, H., and K. Hafen The life span of naive el/b T cells in secondary lymphoid organs. J. Exp. Med. 177: Michie, C.A., A. McLean, C. Alcock, and P.C.L. Beverley The lifespan of human T lymphocyte subsets defined by CD45 isoforms. Nature (Lond.). 340: Kirberg, J., L. Bruno, and H. yon Boehmer CD4 - help prevents rapid deletion of CD8 cells after a transient response to antigen. Eur. J. Immunol. 1993: Carlow, D.A., S.J. Teh, N.S.C. van Oers, K.G. Miller, and H.-S. Teh Peripheral tolerance through clonal deletion of mature CD4-CD8 T cells. Int. Immunol. 4: Zhang, L., D.R. Martin, W.-F. Fung-Leung, H.-S. Teh, and R.G. Miller Peripheral deletion of mature CD8 antigen specific T cells after in vivo exposure to male antigen. J. Immunol. 148: Colic, J.-H., P. Truffa-Bachi, and A.A. Freitas Secondary antibody responses to thymus-independent antigens. Decline and life-span of memory. Eur. J. Immunol. 18: Gray, D., and P. Matzinger T cell memory is short-lived in the absence of antigen. J. Exp. Med. 174: Bell, E.B., and S.M. Sparshott Interconversion of CD45K subsets of CD4 T cells in vivo. Nature (Lond.). 348: Ahmed, R Immunological memory against viruses. Semin. Immunol. 4: Eichelberg, M.C., M. Wang, W. Allan, R.G. Webster, and PC. Doherty Influenza virus KNA in the lung and lymphoid tissue ofimmunologically intact and CD4-depleted mice. J. Gen. Virol. 72: Moskophidis, D., F. Lechner, H. Pitcher, and R..M. Zinkernagel Virus persistence in acutely infected immunocompetent mice by exhaustion of antiviral cytotoxic effector T cells. Nature (Lond.). 362: Webb, S., C. Morris, and J. Sprent Extrathymic tolerance of mature T cells: clonal elimination as a consequence of immunity. Cell. 63: MacDonald, H.K., S. Baschieri, and K.K. Lees Clonal expansion precedes anergy and death of Vfl 8 peripheral T ceils responding to staphylococcal enterotoxin B in vivo. Eur. j. Immunol. 21: Guerder, S., and P. Matzinger A fail safe mechanism for maintaining self-tolerance. J. Exlx Med. 176: Fuchs, E.J., and P. Matzinger B cells turn off virgin but not memory T cells. Science (Wash. DC). 258: Kisielow, P., H. Bluthman, U.D. Staerz, M. Steinmetz, and H. von Boehmer Tolerance in T cell receptor transgenic mice involves deletion of nonmature CD4 +CD8 thymocytes. Nature (Lond.). 333: Teh, H.S., P. Kisielow, B. Scott, H. Kishi, Y. Uematsu, H. Bluthman, and H. yon Boehmer Thymic MHC antigens and the c~ TCK determine the CD4/CD8 phenotype of mature T cells. Nature (Lond.). 335: Guy-Grand, D,, N. Cerf-Bensussan, B. Malissen, M. Malissen- Seris, C. Briottet, and P. Vassali Two gut intraepithelial CD8 lymphocyte populations with different T cell receptors: a role for the gut epithelium in T cell differentiation. J. Ex F Med. 173: Rocha, B., N. Dautigny, and P. Pereira Peripheral T lymphocytes: expansion potential and homeostatic regulation of pool sizes and CD4/CD8 ratios in vivo. Eur. J. Immunol. 19: Coffman, K.L., and I.L. Weissman A monoclonal antibody that recognizes B cells and B cell precursors in mice. J. Exp. Med. 153: Schiippel, R., J. Wilke, and E. WeiUer Monoclonal antiallotype antibody towards BALB/c IgM. Analysis of specificity and site of a V-C crossover in recombinant strain BALB-Igh- Va/Igh-Cb. Eur. J. Immunol. 17: Nishikawa, S.I., Y. Sasaki, T. Kina, T. Amagai, and Y. Katsura A monoclonal antibody against Igh6-4 determinant. Immunogenetics. 23: Koopman, P., J. Gubbay, N. Vivian, P. Goodfellow, and R. Lovell-Badge Male development of chromosomally female mice transgenic for Sty. Nature (Lond.). 351: von Boehmer, H., J. Kirberg, and B. Kocha An unusual lineage of c~/~ T cells that contains autoreactive cells.j. Ex F filed. 174: Freitas, A.A., and M. de Sousa Control mechanisms of lymphocyte traffic. Modification of the traffic of 51Cr labelled mouse lymph node cells by treatment with lectins in intact and splenectomized hosts. Eur. j. Immunol. 5: a.Sheng, B.D., K.T. Smith, andj. Odabralski FASEB (Fed. Am. Soa Exp. Biol.) J. 6:A Oehen, S., H. Waldner, T. Kundig, H. Hengartner, and K.M. Zinkemagel Antiviral protective cytotoxic T cell memory to lymphocytic choriomeningitis virus is governed by persisting antigen. J. Exp. Med. 176: Hayflick, L The limited "in vitro" lifetime of human diploid cell strains. Ex F Cell. Res. 37: Huesmann, M., B. Scott, P. Kisielow, and H. von Boehmer Kinetics and efficiency of positive selection in the thymus of normal and T cell receptor transgenic mice. Cell. 66: Walden, P.R., and H.N. Eisen Cognate peptides induce self-destruction of CD8 + cytolytic T lymphocytes. Proc. Natl. Acad. Sci. USA. 87: Freitas, A.A., and B. Kocha Lymphocyte lifespans: homeostasis, selection and competition. Immunol. Today. 14: R.ocha et al.
Nature Biotechnology: doi: /nbt.4086
 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3 p815 p815-hcd2 Ag (-) anti-cd3
Supplementary figures
 Mucida et al. Supplementary material Supplementary figures Supplementary Figure 1. Oral administration of OVA suppresses Th2 differentiation, Germinal Center (GC) formation and immunoglobulin class switching
Mucida et al. Supplementary material Supplementary figures Supplementary Figure 1. Oral administration of OVA suppresses Th2 differentiation, Germinal Center (GC) formation and immunoglobulin class switching
Brief Det~nitive Report
 Brief Det~nitive Report THYMIC RECONSTITUTION OF NUDE F MICE WITH ONE OR BOTH PARENTAL THYMUS GRAFTS* BY ROLF M. ZINKERNAGEL, A. ALTHAGE, AND G. CALLAHAN From the Departments of Immunopathology and of
Brief Det~nitive Report THYMIC RECONSTITUTION OF NUDE F MICE WITH ONE OR BOTH PARENTAL THYMUS GRAFTS* BY ROLF M. ZINKERNAGEL, A. ALTHAGE, AND G. CALLAHAN From the Departments of Immunopathology and of
First short refresh on tolerance
 First short refresh on tolerance Su et al. Immunity 2013 Number of Ag specific cells A Tet + cells (per 10 6 CD4) 100 No evidence for clonal deletion? 10 1 0.1 Antigen Specificity: HIV CMV HSV-naive
First short refresh on tolerance Su et al. Immunity 2013 Number of Ag specific cells A Tet + cells (per 10 6 CD4) 100 No evidence for clonal deletion? 10 1 0.1 Antigen Specificity: HIV CMV HSV-naive
MicroRNAs Modulate Hematopoietic Lineage Differentiation
 Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
Chen et al., page 1 MicroRNAs Modulate Hematopoietic Lineage Differentiation Chang-Zheng Chen, Ling Li, Harvey F. Lodish, David. Bartel Supplemental Online Material Methods Cell isolation Murine bone marrow
First short refresh on tolerance
 First short refresh on tolerance Death by neglect What is the diversity of the T cell repertoire? Parham cites estimate from Arstila [Science, 1999]: 2x10 6 Nowadays next generation sequencing (NGS):
First short refresh on tolerance Death by neglect What is the diversity of the T cell repertoire? Parham cites estimate from Arstila [Science, 1999]: 2x10 6 Nowadays next generation sequencing (NGS):
SUPPLEMENTARY INFORMATION
 doi:10.1038/nature10772 Supplementary Figures: Supplementary Figure 1. Location of CNS1 within of the Foxp3 locus highlighting CNS1 and indicating transcription factor binding motifs downstream of TCR,
doi:10.1038/nature10772 Supplementary Figures: Supplementary Figure 1. Location of CNS1 within of the Foxp3 locus highlighting CNS1 and indicating transcription factor binding motifs downstream of TCR,
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development
 Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
Strategies for Assessment of Immunotoxicology in Preclinical Drug Development Rebecca Brunette, PhD Scientist, Analytical Biology SNBL USA Preclinical Immunotoxicology The study of evaluating adverse effects
isolated from ctr and pictreated mice. Activation of effector CD4 +
 Supplementary Figure 1 Bystander inflammation conditioned T reg cells have normal functional suppressive activity and ex vivo phenotype. WT Balb/c mice were treated with polyi:c (pic) or PBS (ctr) via
Supplementary Figure 1 Bystander inflammation conditioned T reg cells have normal functional suppressive activity and ex vivo phenotype. WT Balb/c mice were treated with polyi:c (pic) or PBS (ctr) via
Receptor Revision Diminishes the Autoreactive B Cell Response after Antigen. PNA Tet. Day 8. Day 16
 Receptor Revision Diminishes the Autoreactive Cell Response after Antigen Activation Ying-Hua Wang and etty Diamond Supplemental data: PNA Tet 5 8 11 16 Supplemental Figure 1: Kinetic analysis of tetramer-binding
Receptor Revision Diminishes the Autoreactive Cell Response after Antigen Activation Ying-Hua Wang and etty Diamond Supplemental data: PNA Tet 5 8 11 16 Supplemental Figure 1: Kinetic analysis of tetramer-binding
Supplemental Information
 Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
Supplemental Information DLA-matched bone marrow transplantation reverses the immunodeficiency of SCID dogs. Bone marrow transplantation studies were initiated with the goal of reversing the immunodeficiency
In adult mice, the presence of a homeostatic control of B
 Published Online: 18 January, 1999 Supp Info: http://doi.org/10.1084/jem.189.2.319 Downloaded from jem.rupress.org on July 4, 2018 Transfer of Small Resting B Cells into Immunodeficient Hosts Results in
Published Online: 18 January, 1999 Supp Info: http://doi.org/10.1084/jem.189.2.319 Downloaded from jem.rupress.org on July 4, 2018 Transfer of Small Resting B Cells into Immunodeficient Hosts Results in
Supporting Information
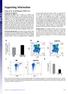 (xe number cells) LSK (% gated) Supporting Information Youm et al../pnas. SI Materials and Methods Quantification of sjtrecs. CD + T subsets were isolated from splenocytes using mouse CD + T cells positive
(xe number cells) LSK (% gated) Supporting Information Youm et al../pnas. SI Materials and Methods Quantification of sjtrecs. CD + T subsets were isolated from splenocytes using mouse CD + T cells positive
Recombination Lecture, Dr. Aguilera 2/17/2014
 Lymphocytes and Antigen Receptors Thymus T-Cells Lymph nodes Spleen } T+B-cells Paper Presentation: Bone Marrow Stem cells and B-cells Nat. Rev. Immunol. STEM CELL CLP Committed Lymphocyte Precursor T-cells
Lymphocytes and Antigen Receptors Thymus T-Cells Lymph nodes Spleen } T+B-cells Paper Presentation: Bone Marrow Stem cells and B-cells Nat. Rev. Immunol. STEM CELL CLP Committed Lymphocyte Precursor T-cells
1 Name. 1. (3 pts) What is apoptosis and how does it differ from necrosis? Which is more likely to trigger inflammation?
 1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
1 Name MCB 150 Midterm Eam #1 (100 points total) Please write your full name on each page of the eam!! The eam consists of 17 questions (6 pages). Each has a different point count as indicated. Please
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION
 CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
CHAPTER 7 CELLULAR BASIS OF ANTIBODY DIVERSITY: CLONAL SELECTION The specificity of humoral immune responses relies on the huge DIVERSITY of antigen combining sites present in antibodies, diversity which
Who pairs with whom? High-throughput sequencing of the human paired heavy and light chain repertoire
 Who pairs with whom? High-throughput sequencing of the human paired heavy and light chain repertoire Technical Journal Club September 15 th Christina Müller Background - antibody repertoire is the sum
Who pairs with whom? High-throughput sequencing of the human paired heavy and light chain repertoire Technical Journal Club September 15 th Christina Müller Background - antibody repertoire is the sum
SuperTCRExpress TM Human TCR Vβ Repertoire CDR3 Diversity Determination (Spectratyping) and Quantitative Analysis Kit
 SuperTCRExpress TM Human TCR Vβ Repertoire CDR3 Diversity Determination (Spectratyping) and Quantitative Analysis Kit Cat. No. H0521 Size: 2 sets (22 Vβ families/each, with enzymes) H0522 Size: 4 sets
SuperTCRExpress TM Human TCR Vβ Repertoire CDR3 Diversity Determination (Spectratyping) and Quantitative Analysis Kit Cat. No. H0521 Size: 2 sets (22 Vβ families/each, with enzymes) H0522 Size: 4 sets
In vitro cultures of bone marrow stromal cells and progenitor B cells can accurately recapitulate the normal steps of B cell development.
 Regular Office Hours: Tuesdays 11-12 Extra office hours: Wed, Feb 7 12-1pm Thurs, Feb 8 11am-12 Fri, Feb 9 2-4pm I WILL NOT BE HOLDING OFFICE HOURS ON TUESDAY Feb 13!! Dina, Tim, and I encourage all confused
Regular Office Hours: Tuesdays 11-12 Extra office hours: Wed, Feb 7 12-1pm Thurs, Feb 8 11am-12 Fri, Feb 9 2-4pm I WILL NOT BE HOLDING OFFICE HOURS ON TUESDAY Feb 13!! Dina, Tim, and I encourage all confused
B cell development The stages of B cell development
 Regular Office Hours: Tuesdays 11-12 Extra office hours: Wed, Feb 7 12-1pm Thurs, Feb 8 11am-12 Fri, Feb 9 2-4pm I WILL NOT BE HOLDING OFFICE HOURS ON TUESDAY Feb 13!! Dina, Tim, and I encourage all confused
Regular Office Hours: Tuesdays 11-12 Extra office hours: Wed, Feb 7 12-1pm Thurs, Feb 8 11am-12 Fri, Feb 9 2-4pm I WILL NOT BE HOLDING OFFICE HOURS ON TUESDAY Feb 13!! Dina, Tim, and I encourage all confused
OX40 MARKET LANDSCAPE
 OX40 Agonist OX40 MARKET LANDSCAPE OX40 (a.k.a. CD134) is a costimulatory molecule belonging to the TNF receptor family expressed primarily on activated effector T (T eff) cells and naive regulatory T
OX40 Agonist OX40 MARKET LANDSCAPE OX40 (a.k.a. CD134) is a costimulatory molecule belonging to the TNF receptor family expressed primarily on activated effector T (T eff) cells and naive regulatory T
SI Appendix. Tumor-specific CD8 + Tc9 cells are superior effector than Tc1 cells for. adoptive immunotherapy of cancers
 SI Appendix Tumor-specific CD8 + Tc9 cells are superior effector than Tc1 cells for adoptive immunotherapy of cancers Yong Lu, Bangxing Hong, Haiyan Li, Yuhuan Zheng, Mingjun Zhang, Siqing Wang, Jianfei
SI Appendix Tumor-specific CD8 + Tc9 cells are superior effector than Tc1 cells for adoptive immunotherapy of cancers Yong Lu, Bangxing Hong, Haiyan Li, Yuhuan Zheng, Mingjun Zhang, Siqing Wang, Jianfei
Adaptive Immunity: Specific Defenses of the Host
 PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 17 Adaptive Immunity: Specific Defenses of the Host The Adaptive Immune System Adaptive immunity:
PowerPoint Lecture Presentations prepared by Bradley W. Christian, McLennan Community College C H A P T E R 17 Adaptive Immunity: Specific Defenses of the Host The Adaptive Immune System Adaptive immunity:
Nature Immunology: doi: /ni.3694
 Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
Supplementary Figure 1 Expression of Bhlhe41 and Bhlhe40 in B cell development and mature B cell subsets. (a) Scatter plot showing differential expression of genes between splenic B-1a cells and follicular
ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development
 ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development University of Wisconsin-Madison, Production Assistance for Cellular Therapies (PACT) Over the
ISCT Telegraft Column: Mesenchymal Stromal Cell (MSC) Product Characterization and Potency Assay Development University of Wisconsin-Madison, Production Assistance for Cellular Therapies (PACT) Over the
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM
 Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
Supplementary Figure 1: Analysis of monocyte subsets and lineage relationships. (a) Gating strategy for definition of MDP and cmop populations in BM of Cx3cr1 GFP/+ mice related to Fig. 1a. MDP was defined
Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer
 SUPPLEMENTAL METHODS Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer For Celigo experiments, 0.1 ml containing 5 x 10 4 cells was seeded into 96 well plates for 30 min
SUPPLEMENTAL METHODS Measurement of peritoneal macrophage apoptosis by Celigo plate imaging cytometer For Celigo experiments, 0.1 ml containing 5 x 10 4 cells was seeded into 96 well plates for 30 min
Supplemental Information Inventory
 Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
Cell Stem Cell, Volume 6 Supplemental Information Distinct Hematopoietic Stem Cell Subtypes Are Differentially Regulated by TGF-β1 Grant A. Challen, Nathan C. Boles, Stuart M. Chambers, and Margaret A.
F4/80, CD11b, Gr-1, NK1.1, CD3, CD4, CD8 and CD19. A-antigen was detected with FITCconjugated
 SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
SDC MATERIALS AND METHODS Flow Cytometric Detection of A-Antigen Expression Single cell suspensions were prepared from bone marrow, lymph node and spleen. Peripheral blood was obtained and erythrocytes
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A
 Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Discovery and Humanization of Novel High Affinity Neutralizing Monoclonal Antibodies to Human IL-17A Contacts: Marty Simonetti martysimonetti@gmail.com Kirby Alton kirby.alton@abeomecorp.com Rick Shimkets
Supplementary Figures
 Supplementary Figures Supplementary Figure 1: Phenotypically defined hematopoietic stem and progenitor cell populations show distinct mitochondrial activity and mass. (A) Isolation by FACS of commonly
Supplementary Figures Supplementary Figure 1: Phenotypically defined hematopoietic stem and progenitor cell populations show distinct mitochondrial activity and mass. (A) Isolation by FACS of commonly
Supplementary Figure 1. Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings.
 Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Supplementary Figure 1 Homozygous rag2 E450fs mutants are healthy and viable similar to wild-type and heterozygous siblings. (left) Representative bright-field images of wild type (wt), heterozygous (het)
Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help
 Washington University School of Medicine Digital Commons@Becker Open Access Publications 2006 Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help Laura
Washington University School of Medicine Digital Commons@Becker Open Access Publications 2006 Autoreactive marginal zone B cells are spontaneously activated but lymph node B cells require T cell help Laura
Supplementary Figure S1
 Supplementary Figure S1 Supplementary Figure S1. CD11b expression on different B cell subsets in anti-snrnp Ig Tg mice. (a) Highly purified follicular (FO) and marginal zone (MZ) B cells were sorted from
Supplementary Figure S1 Supplementary Figure S1. CD11b expression on different B cell subsets in anti-snrnp Ig Tg mice. (a) Highly purified follicular (FO) and marginal zone (MZ) B cells were sorted from
4 Themes in B cell development + Ig class switch and somatic mutation Tony DeFranco, 10/28/13 Checkpoints in B cell development: feedback from Ig
 4 Themes in B cell development + Ig class switch and somatic mutation Tony DeFranco, 10/28/13 Checkpoints in B cell development: feedback from Ig gene rearrangements Lineage commitment: transcription factors
4 Themes in B cell development + Ig class switch and somatic mutation Tony DeFranco, 10/28/13 Checkpoints in B cell development: feedback from Ig gene rearrangements Lineage commitment: transcription factors
Supplementary Figure 1. Characterization of the POP2 transcriptional and post-transcriptional regulatory elements. (A) POP2 nucleotide sequence
 1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
1 5 6 7 8 9 10 11 1 1 1 Supplementary Figure 1. Characterization of the POP transcriptional and post-transcriptional regulatory elements. (A) POP nucleotide sequence depicting the consensus sequence for
Supporting Information
 Supporting Information Tomura et al. 1.173/pnas.8227815 WT CFSE Con A stimulation Before After 2 3 1 CFSE Kaede- Tg No photoconversion Photoconversion Kaede Red Fig. S1. Dilution of photoconverted Kaede
Supporting Information Tomura et al. 1.173/pnas.8227815 WT CFSE Con A stimulation Before After 2 3 1 CFSE Kaede- Tg No photoconversion Photoconversion Kaede Red Fig. S1. Dilution of photoconverted Kaede
BD IMag. Streptavidin Particles Plus - DM. Technical Data Sheet. Product Information
 Technical Data Sheet Streptavidin Particles Plus - DM Product Information Material Number: Size: Storage Buffer: 557812 5 ml Aqueous buffered solution containing BSA and 0.09% sodium azide. Description
Technical Data Sheet Streptavidin Particles Plus - DM Product Information Material Number: Size: Storage Buffer: 557812 5 ml Aqueous buffered solution containing BSA and 0.09% sodium azide. Description
Nature Immunology: doi: /ni Supplementary Figure 1. Time course of OT-II T reg cell development in thymic slices.
 Supplementary Figure 1 Time course of OT-II T reg cell development in thymic slices. Time course of T reg cell development following addition of OT-II TCR transgenic Rag2 -/- mice (OT-II) thymocytes to
Supplementary Figure 1 Time course of OT-II T reg cell development in thymic slices. Time course of T reg cell development following addition of OT-II TCR transgenic Rag2 -/- mice (OT-II) thymocytes to
Application Note AN001
 Testing hybridoma supernatants with the Spots On Dots Antibody Screening Kit Application Note AN1 Table of Contents Overview... 2 Figure 1. Screening of hybridomas raised against peptide antigens... 3
Testing hybridoma supernatants with the Spots On Dots Antibody Screening Kit Application Note AN1 Table of Contents Overview... 2 Figure 1. Screening of hybridomas raised against peptide antigens... 3
ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity and percent identical sites.
 Identity Human Mouse Supplemental Figure 1. Amino acid sequence alignment of -MyHC. The alignment was created using the ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity
Identity Human Mouse Supplemental Figure 1. Amino acid sequence alignment of -MyHC. The alignment was created using the ClustalW algorithm. The alignment statistics are 96.9% for both pairwise identity
TIB6 B16F1 LLC EL4. n=2 n=7 n=9 n=6
 Shojaei et al., Supplemental Fig. 1 9 Mean Inhibition by anti-vegf (%) 7 5 3 1 TIB6 n=2 n=7 n=9 n=6 Supplemental Figure 1 Differential growth inhibition by anti-vegf treatment. The percent of tumor growth
Shojaei et al., Supplemental Fig. 1 9 Mean Inhibition by anti-vegf (%) 7 5 3 1 TIB6 n=2 n=7 n=9 n=6 Supplemental Figure 1 Differential growth inhibition by anti-vegf treatment. The percent of tumor growth
Multiple layers of B cell memory with different effector functions. Ismail Dogan, Barbara Bertocci, Valérie Vilmont, Frédéric Delbos,
 Multiple layers of B cell memory with different effector functions Ismail Dogan, Barbara Bertocci, Valérie Vilmont, Frédéric Delbos, Jérome Mégret, Sébastien Storck, Claude-Agnès Reynaud & Jean-Claude
Multiple layers of B cell memory with different effector functions Ismail Dogan, Barbara Bertocci, Valérie Vilmont, Frédéric Delbos, Jérome Mégret, Sébastien Storck, Claude-Agnès Reynaud & Jean-Claude
Supporting Information
 Supporting Information of Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for Treatment of Metastatic Tumor Lihua Luo, Chunqi Zhu, Hang Yin, Mengshi Jiang, Junlei Zhang, Bing Qin, Zhenyu
Supporting Information of Laser Immunotherapy in Combination with Perdurable PD-1 Blocking for Treatment of Metastatic Tumor Lihua Luo, Chunqi Zhu, Hang Yin, Mengshi Jiang, Junlei Zhang, Bing Qin, Zhenyu
Interplay of Cells involved in Therapeutic Agent Immunogenicity. Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology
 Interplay of Cells involved in Therapeutic Agent Immunogenicity Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology Disclosure The author works with Amicus on an immunogenicity project
Interplay of Cells involved in Therapeutic Agent Immunogenicity Robert G. Hamilton, Ph.D., D.ABMLI Professor of Medicine and Pathology Disclosure The author works with Amicus on an immunogenicity project
Exceptional Human Antibody Discovery. Corporate Overview
 Exceptional Human Antibody Discovery Corporate Overview Co 1 1 Our Mission Trianni is a biotech company with the scientific mission of creating optimized and highly versatile platforms for generation of
Exceptional Human Antibody Discovery Corporate Overview Co 1 1 Our Mission Trianni is a biotech company with the scientific mission of creating optimized and highly versatile platforms for generation of
Chapter 3. Clonal selection
 Chapter 3. Clonal selection I have called this principle, by which each slight variation, if useful, is preserved, by the term of Natural Selection -Charles Darwin, On the Origin of Species, 1859 4 The
Chapter 3. Clonal selection I have called this principle, by which each slight variation, if useful, is preserved, by the term of Natural Selection -Charles Darwin, On the Origin of Species, 1859 4 The
Jedi cells patrol the mouse
 Jedi cells patrol the mouse Technical Journal Club Josephin Wagner 19.01.2016 12 VOL.13 NO.1 JANUARY 2016 NATURE METHODS Introduction JEDI= Just EGFP Death Inducing t- cells Specific CD-8 T cells All EGFP
Jedi cells patrol the mouse Technical Journal Club Josephin Wagner 19.01.2016 12 VOL.13 NO.1 JANUARY 2016 NATURE METHODS Introduction JEDI= Just EGFP Death Inducing t- cells Specific CD-8 T cells All EGFP
Table S1. Antibodies and recombinant proteins used in this study
 Table S1. Antibodies and recombinant proteins used in this study Labeled Antibody Clone Cat. no. Streptavidin-PerCP BD Biosciences 554064 Biotin anti-mouse CD25 7D4 BD Biosciences 553070 PE anti-mouse
Table S1. Antibodies and recombinant proteins used in this study Labeled Antibody Clone Cat. no. Streptavidin-PerCP BD Biosciences 554064 Biotin anti-mouse CD25 7D4 BD Biosciences 553070 PE anti-mouse
THE BEHAVIOR OF SKIN GRAFTS INCOMPATIBLE WITH RESPECT TO SKIN ALLOANTIGENS ON MICE RENDERED TOLERANT AT BIRTH WITH LYMPHOID CELLS*
 Published Online: 1 June, 1976 Supp Info: http://doi.org/10.1084/jem.143.6.1317 Downloaded from jem.rupress.org on July 3, 2018 THE BEHAVIOR OF SKIN GRAFTS INCOMPATIBLE WITH RESPECT TO SKIN ALLOANTIGENS
Published Online: 1 June, 1976 Supp Info: http://doi.org/10.1084/jem.143.6.1317 Downloaded from jem.rupress.org on July 3, 2018 THE BEHAVIOR OF SKIN GRAFTS INCOMPATIBLE WITH RESPECT TO SKIN ALLOANTIGENS
Immunology 2011 Lecture 9 Immunoglobulin Biosynthesis 3 October
 Immunology 2011 Lecture 9 Immunoglobulin Biosynthesis 3 October APC Antigen processing (dendritic cells, MΦ et al.) Antigen "presentation" Ag/Ab complexes Antigenspecific triggering B T ANTIGEN Proliferation
Immunology 2011 Lecture 9 Immunoglobulin Biosynthesis 3 October APC Antigen processing (dendritic cells, MΦ et al.) Antigen "presentation" Ag/Ab complexes Antigenspecific triggering B T ANTIGEN Proliferation
Biological immune systems
 Immune Systems 1 Introduction 2 Biological immune systems Living organism must protect themselves from the attempt of other organisms to exploit their resources Some would-be exploiter (pathogen) is much
Immune Systems 1 Introduction 2 Biological immune systems Living organism must protect themselves from the attempt of other organisms to exploit their resources Some would-be exploiter (pathogen) is much
Supplemental Figure 1
 Supplemental Figure 1 A P1 NOD - SCID(X*X*)(0/0) NOD - SCID(X*Y)(A*0201/A*0201) B F1 (X*X)(A*0201/0) (X*X*)(0/0) F2 (X*X*)(0/0) (X*Y)(0/0) (X*X*)(A*0201/0) (X*X*)(A*0201/0) F3 (X*X*)(0/0) (X*X)(A*0201/0)
Supplemental Figure 1 A P1 NOD - SCID(X*X*)(0/0) NOD - SCID(X*Y)(A*0201/A*0201) B F1 (X*X)(A*0201/0) (X*X*)(0/0) F2 (X*X*)(0/0) (X*Y)(0/0) (X*X*)(A*0201/0) (X*X*)(A*0201/0) F3 (X*X*)(0/0) (X*X)(A*0201/0)
Supplementary Figure 1. Effect of FRC-specific ablation of Myd88 on PP and mln organization.
 Supplementary Figure 1 Effect of FRC-specific ablation of Myd88 on PP and mln organization. (a) PP numbers in 8 10 week old Cre-negative littermate (Ctrl) and Myd88-cKO mice (n = 11 mice; each dot represents
Supplementary Figure 1 Effect of FRC-specific ablation of Myd88 on PP and mln organization. (a) PP numbers in 8 10 week old Cre-negative littermate (Ctrl) and Myd88-cKO mice (n = 11 mice; each dot represents
Corporate Tutorial IBA T-CATCH. Cell isolation in pipette tips. ISCT, Paris Lothar Germeroth
 Corporate Tutorial IBA T-CATCH Cell isolation in pipette tips ISCT, Paris 2014 Lothar Germeroth Workshop IBA Introduction of the Streptamer technology Staining with Streptamers off rate assay T-CATCH Cell
Corporate Tutorial IBA T-CATCH Cell isolation in pipette tips ISCT, Paris 2014 Lothar Germeroth Workshop IBA Introduction of the Streptamer technology Staining with Streptamers off rate assay T-CATCH Cell
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION doi:10.1038/nature11809 Supplementary Figure 1. Antibiotic treatment reduces intestinal bacterial load and allows access of non-pathogenic bacteria to the MLN, inducing intestinal
SUPPLEMENTARY INFORMATION doi:10.1038/nature11809 Supplementary Figure 1. Antibiotic treatment reduces intestinal bacterial load and allows access of non-pathogenic bacteria to the MLN, inducing intestinal
ANTIBODIES. Agents of Immunity
 ANTIBODIES Agents of Immunity - Antibodies are: The Organization What are they? Protective agents of the immune system Neutralize foreign agents called antigens Essential part of the Adaptive Immune System
ANTIBODIES Agents of Immunity - Antibodies are: The Organization What are they? Protective agents of the immune system Neutralize foreign agents called antigens Essential part of the Adaptive Immune System
BY POLLY MATZINGER AND GALADRIEL MIRKWOOD. (From the Department of Biology, University of California San Diego, La Jolla, California 92093)
 Published Online: 1 July, 1978 Supp nfo: http:doi.org10.1084jem.148.1.84 Downloaded from jem.rupress.org on May 18, 2018 N A FULLY H-2 NCOMPATBLE CHMERA, T CELLS OF DONOR ORGN CAN RESPOND TO MNOR HSTOCOMPATBLTY
Published Online: 1 July, 1978 Supp nfo: http:doi.org10.1084jem.148.1.84 Downloaded from jem.rupress.org on May 18, 2018 N A FULLY H-2 NCOMPATBLE CHMERA, T CELLS OF DONOR ORGN CAN RESPOND TO MNOR HSTOCOMPATBLTY
B cells Harry W Schroeder Jr MD PhD
 B cells Harry W Schroeder Jr MD PhD Division of Clinical Immunology and Rheumatology Departments of Medicine, Microbiology, and Genetics University of Alabama at Birmingham Director, UAB Program in Immunology
B cells Harry W Schroeder Jr MD PhD Division of Clinical Immunology and Rheumatology Departments of Medicine, Microbiology, and Genetics University of Alabama at Birmingham Director, UAB Program in Immunology
IMMUNOGLOBULINS ON THE SURFACE OF LYMPHOCYTES
 IMMUNOGLOBULINS ON THE SURFACE OF LYMPHOCYTES II. THE BONE MARROW AS THE MAIN SOURCE OF LYMPHOCYTES WITH DETECTABLE SURFACE-BouND IMMUNOGLOBULIN* BY EMIL R. UNANUE,{C M.D., HOWARD M. GREY, M.D., ENRIQUE
IMMUNOGLOBULINS ON THE SURFACE OF LYMPHOCYTES II. THE BONE MARROW AS THE MAIN SOURCE OF LYMPHOCYTES WITH DETECTABLE SURFACE-BouND IMMUNOGLOBULIN* BY EMIL R. UNANUE,{C M.D., HOWARD M. GREY, M.D., ENRIQUE
Mayumi Egawa, Kaori Mukai, Soichiro Yoshikawa, Misako Iki, Naofumi Mukaida, Yohei Kawano, Yoshiyuki Minegishi, and Hajime Karasuyama
 Immunity, Volume 38 Supplemental Information Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4 Mayumi Egawa, Kaori Mukai, Soichiro
Immunity, Volume 38 Supplemental Information Inflammatory Monocytes Recruited to Allergic Skin Acquire an Anti-inflammatory M2 Phenotype via Basophil-Derived Interleukin-4 Mayumi Egawa, Kaori Mukai, Soichiro
The Trianni Mouse: Best-In-Class Technology for Human Antibody Discovery
 The Trianni Mouse: Best-In-Class Technology for Human Antibody Discovery Corporate Overview Co David Meininger, PhD, MBA Chief Business Officer, Trianni 1 Our Mission Trianni is a biotech company with
The Trianni Mouse: Best-In-Class Technology for Human Antibody Discovery Corporate Overview Co David Meininger, PhD, MBA Chief Business Officer, Trianni 1 Our Mission Trianni is a biotech company with
Supporting Information for. Bongseo Choi, 1, Hyojin Moon, 1, Sung Joon Hong, 1 Changsik Shin, 1 Yoonkyung Do, 1 Seongho Ryu, 2,* Sebyung Kang 1,*
 Supporting Information for Effective Delivery of Antigen-Encapsulin Nanoparticle Fusions to Dendritic Cells Leads to Antigen-Specific Cytotoxic T Cell Activation and Tumor Rejection Bongseo Choi, 1, Hyojin
Supporting Information for Effective Delivery of Antigen-Encapsulin Nanoparticle Fusions to Dendritic Cells Leads to Antigen-Specific Cytotoxic T Cell Activation and Tumor Rejection Bongseo Choi, 1, Hyojin
Supplementary Figure 1
 Supplementary Figure 1 Ex2 promotor region Cre IRES cherry pa Ex4 Ex5 Ex1 untranslated Ex3 Ex5 untranslated EYFP pa Rosa26 STOP loxp loxp Cre recombinase EYFP pa Rosa26 loxp 1 kb Interleukin-9 fate reporter
Supplementary Figure 1 Ex2 promotor region Cre IRES cherry pa Ex4 Ex5 Ex1 untranslated Ex3 Ex5 untranslated EYFP pa Rosa26 STOP loxp loxp Cre recombinase EYFP pa Rosa26 loxp 1 kb Interleukin-9 fate reporter
Development of NOG mice
 Development of NOG mice General characteris5cs of NOG mice 1. T and B cell deficient 2. NK cell deficient 3. Reduced macrophage and dendri;c cell func;on 4. Complement ac;vity deficient 5. No incidence
Development of NOG mice General characteris5cs of NOG mice 1. T and B cell deficient 2. NK cell deficient 3. Reduced macrophage and dendri;c cell func;on 4. Complement ac;vity deficient 5. No incidence
Chapter 14. Second symmetry
 Chapter 14. Second symmetry Tyger Tyger, burning bright In the forests of the night What immortal hand or eye Could frame thy fearful symmetry? -William Blake Songs of Experience (1794) A good general
Chapter 14. Second symmetry Tyger Tyger, burning bright In the forests of the night What immortal hand or eye Could frame thy fearful symmetry? -William Blake Songs of Experience (1794) A good general
Mid-term review. Recitation5 03/31/2014. Stem Cell Biology and Function W4193 1
 Mid-term review Recitation5 03/31/2014 Stem Cell Biology and Function W4193 1 What to expect in the midterm2 It will be all so far but mainly whatever was NOT covered by the previous mid-term- not a huge
Mid-term review Recitation5 03/31/2014 Stem Cell Biology and Function W4193 1 What to expect in the midterm2 It will be all so far but mainly whatever was NOT covered by the previous mid-term- not a huge
Antibody Generation: challenges and solutions. Glen Marszalowicz, PHD May 10, AM
 Antibody Generation: challenges and solutions Glen Marszalowicz, PHD May 10, 2015 9AM GenScript the most cited biology CRO CRISPR Services Gene Services Peptide Services Protein Services Antibody Services
Antibody Generation: challenges and solutions Glen Marszalowicz, PHD May 10, 2015 9AM GenScript the most cited biology CRO CRISPR Services Gene Services Peptide Services Protein Services Antibody Services
Immunogenetics. Immunodeficiency
 4.05.009 Immune response represents a system of recognition of foreign molecules. Immunogenetics Foreign molecules (proteins, glycoproteins, carbohydrates, ssdna, viruses) or parts of foreign molecules
4.05.009 Immune response represents a system of recognition of foreign molecules. Immunogenetics Foreign molecules (proteins, glycoproteins, carbohydrates, ssdna, viruses) or parts of foreign molecules
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang *
 In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
In vivo BrdU Incorporation Assay for Murine Hematopioetic Stem Cells Ningfei An, Yubin Kang * Division of Hematology-Oncology, Department of Medicine, Medical University of South Carolina, Charleston,
A Modified Digestion-Circularization PCR (DC-PCR) Approach to Detect Hypermutation- Associated DNA Double-Strand Breaks
 A Modified Digestion-Circularization PCR (DC-PCR) Approach to Detect Hypermutation- Associated DNA Double-Strand Breaks SARAH K. DICKERSON AND F. NINA PAPAVASILIOU Laboratory of Lymphocyte Biology, The
A Modified Digestion-Circularization PCR (DC-PCR) Approach to Detect Hypermutation- Associated DNA Double-Strand Breaks SARAH K. DICKERSON AND F. NINA PAPAVASILIOU Laboratory of Lymphocyte Biology, The
Nature Immunology: doi: /ni Supplementary Figure 1. Zranb1 gene targeting.
 Supplementary Figure 1 Zranb1 gene targeting. (a) Schematic picture of Zranb1 gene targeting using an FRT-LoxP vector, showing the first 6 exons of Zranb1 gene (exons 7-9 are not shown). Targeted mice
Supplementary Figure 1 Zranb1 gene targeting. (a) Schematic picture of Zranb1 gene targeting using an FRT-LoxP vector, showing the first 6 exons of Zranb1 gene (exons 7-9 are not shown). Targeted mice
Increased Hepatic Barr Body and Thymic Epithelial Cell Dysplasia Found in Chronic Immune Responses to Rat Male(H-Y)Antigen
 Acta Medica et Biologica Vol. 40, No.4, 145-150, 1992 Increased Hepatic Barr Body and Thymic Epithelial Cell Dysplasia Found in Chronic Immune Responses to Rat Male(H-Y)Antigen Tadako NAKATSUJI Department
Acta Medica et Biologica Vol. 40, No.4, 145-150, 1992 Increased Hepatic Barr Body and Thymic Epithelial Cell Dysplasia Found in Chronic Immune Responses to Rat Male(H-Y)Antigen Tadako NAKATSUJI Department
Creating a Model Antigen System to Study the Mechanisms of GCC-Specific Tolerance
 Creating a Model Antigen System to Study the Mechanisms of GCC-Specific Tolerance Patrick Ihejirika, Adam Snook, Laurence Eisenlohr. Abstract: Immunotherapeutics for colorectal cancer have been under investigation
Creating a Model Antigen System to Study the Mechanisms of GCC-Specific Tolerance Patrick Ihejirika, Adam Snook, Laurence Eisenlohr. Abstract: Immunotherapeutics for colorectal cancer have been under investigation
Hematopoietic stem cell transplantation without irradiation
 nature methods Hematopoietic stem cell transplantation without irradiation Claudia Waskow, Vikas Madan, Susanne Bartels, Céline Costa, Rosel Blasig & Hans-Reimer Rodewald Supplementary figures and text:
nature methods Hematopoietic stem cell transplantation without irradiation Claudia Waskow, Vikas Madan, Susanne Bartels, Céline Costa, Rosel Blasig & Hans-Reimer Rodewald Supplementary figures and text:
Supplemental Figure 1
 Supplemental Figure 1 A IL-12p7 (pg/ml) 7 6 4 3 2 1 Medium then TLR ligands MDP then TLR ligands Medium then TLR ligands + MDP MDP then TLR ligands + MDP B IL-12p4 (ng/ml) 1.2 1..8.6.4.2. Medium MDP Medium
Supplemental Figure 1 A IL-12p7 (pg/ml) 7 6 4 3 2 1 Medium then TLR ligands MDP then TLR ligands Medium then TLR ligands + MDP MDP then TLR ligands + MDP B IL-12p4 (ng/ml) 1.2 1..8.6.4.2. Medium MDP Medium
Immunogenicity Prediction Where are we?
 Pharma&Biotech Immunogenicity Prediction Where are we? European Immunogenicity Platform, 24th February 2016 Immunogenicity of Biopharmaceuticals Potential causes 2 Pre-clinical Immunogenicity Prediction
Pharma&Biotech Immunogenicity Prediction Where are we? European Immunogenicity Platform, 24th February 2016 Immunogenicity of Biopharmaceuticals Potential causes 2 Pre-clinical Immunogenicity Prediction
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam will have 40 multiple choice questions.
 MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
a. Hypoxanthine was present in the media. MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No.
 MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
MCB 4211, Fall 2018, Practice Exam 1 Last, First name Student ID # Seat No. ***NOTE: Exam 1 2018 will have 40 multiple choice questions. READ ALL THE CHOICES AND SELECT THE BEST 1. Which of the following
Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg
![Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg](/thumbs/84/90687567.jpg) Supplementary information Supplementary methods PCNA antibody and immunodepletion Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg extracts, one volume of protein
Supplementary information Supplementary methods PCNA antibody and immunodepletion Antibodies against PCNA were previously described [1]. To deplete PCNA from Xenopus egg extracts, one volume of protein
Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse
 Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse James W. Tung, Matthew D. Mrazek, Yang Yang, Leonard A. Herzenberg*, and Leonore A. Herzenberg Department
Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse James W. Tung, Matthew D. Mrazek, Yang Yang, Leonard A. Herzenberg*, and Leonore A. Herzenberg Department
Examination in Immunotechnology, 30 May 2011, 8-13
 Examination in Immunotechnology, 30 May 2011, 8-13 1 Each question can give 5p, with a total of 10 questions (i.e. 50 points in total). 2 Write name and personal number on ALL pages (including the cover).
Examination in Immunotechnology, 30 May 2011, 8-13 1 Each question can give 5p, with a total of 10 questions (i.e. 50 points in total). 2 Write name and personal number on ALL pages (including the cover).
Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP +
 Supplementary Figure 1 Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP + haematopoietic cells in the intestine. GFP + cells from E15.5 intestines were obtained after collagenase
Supplementary Figure 1 Supplementary Figure 1. Phenotype, morphology and distribution of embryonic GFP + haematopoietic cells in the intestine. GFP + cells from E15.5 intestines were obtained after collagenase
SOM 1 *** *** *** * * n.s GFP + (NK 10 4 ) Naive DNFB OXA. Donor sensitization: Naive OXA DNFB 1,200 6,000 4,000
 SOM 1 a n.s. 4. 3. GFP + (NK 1 4 ) 2. 1.. Naive DNFB OXA splenic NK hepatic NK b Donor sensitization: Naive OXA DNFB NK cells (% of CD45 + 1 5 ) 1,2 9 6 3 NK cells (% of CD45 + 1 5 ) 6, 4, 2, 24 48 72
SOM 1 a n.s. 4. 3. GFP + (NK 1 4 ) 2. 1.. Naive DNFB OXA splenic NK hepatic NK b Donor sensitization: Naive OXA DNFB NK cells (% of CD45 + 1 5 ) 1,2 9 6 3 NK cells (% of CD45 + 1 5 ) 6, 4, 2, 24 48 72
To determine the effect of N1IC in the susceptibility of T cells to the tolerogenic effect of tumorassociated
 Supplementary Methods Tolerogenic effect of MDSC To determine the effect of N1IC in the susceptibility of T cells to the tolerogenic effect of tumorassociated MDSC in vivo, we used a model described previously
Supplementary Methods Tolerogenic effect of MDSC To determine the effect of N1IC in the susceptibility of T cells to the tolerogenic effect of tumorassociated MDSC in vivo, we used a model described previously
Interferon Immunosuppression: Mediation by a Suppressor
 INFECTION AND IMMUNITY, Aug. 1980, p. 301-305 0019-9567/80/08-0301/05$02.00/0 Vol. 29, No. 2 Interferon Immunosuppression: Mediation by a Suppressor Factor HOWARD M. JOHNSON* AND J. EDWIN BLALOCK Department
INFECTION AND IMMUNITY, Aug. 1980, p. 301-305 0019-9567/80/08-0301/05$02.00/0 Vol. 29, No. 2 Interferon Immunosuppression: Mediation by a Suppressor Factor HOWARD M. JOHNSON* AND J. EDWIN BLALOCK Department
Antibody used for FC Figure S1. Multimodal characterization of NIR dyes in vitro Figure S2. Ex vivo analysis of HL60 cells homing
 Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Antibody used for FC The following antibodies were used following manufacturer s instructions: anti-human CD4 (clone HI3, IgG1, k - Becton Dickinson), anti-human CD33 (clone WM3, IgG1, k- Becton Dickinson),
Additional analysissu
 SSC-A FSC-A 65.6% FSC-H FSC-A 86.1% SSC-A 93.3% Viaprobe Additional analysissu Supplementary Figure S1. Gating strategy for flow cytometry Doublet cells were discriminated by FSC-A/SSC-A and FSC-A/FSC-H
SSC-A FSC-A 65.6% FSC-H FSC-A 86.1% SSC-A 93.3% Viaprobe Additional analysissu Supplementary Figure S1. Gating strategy for flow cytometry Doublet cells were discriminated by FSC-A/SSC-A and FSC-A/FSC-H
Exam 3 4/25/07. Total of 7 questions, 100 points.
 Exam 3 4/25/07 BISC 4A P. Sengupta Total of 7 questions, 100 points. QUESTION 1. Circle the correct answer. Total of 40 points 4 points each. 1. Which of the following is typically attacked by the antibody-mediated
Exam 3 4/25/07 BISC 4A P. Sengupta Total of 7 questions, 100 points. QUESTION 1. Circle the correct answer. Total of 40 points 4 points each. 1. Which of the following is typically attacked by the antibody-mediated
Antibody and Immunological Memory Responses. Rolf Zinkernagel University of Zurich, Switzerland
 Anti-Viral Antibody and Immunological Memory Responses Rolf Zinkernagel University of Zurich, Switzerland B cells react: Polymers (Polymeric selfantigens: collagen, DNA etc.) Monomers + LPS (CpG) 2 lymphoid
Anti-Viral Antibody and Immunological Memory Responses Rolf Zinkernagel University of Zurich, Switzerland B cells react: Polymers (Polymeric selfantigens: collagen, DNA etc.) Monomers + LPS (CpG) 2 lymphoid
T-cell response. Taken from NIAID: s.aspx
 T-cell receptor T-cell response 1. Macrophage or dendritic cell digest antigen bacteria, virus 2. Fragments of Ag bind to major histo-compatiblity (MHC) proteins in macrophage. 3. MHC I-Ag fragment expressed
T-cell receptor T-cell response 1. Macrophage or dendritic cell digest antigen bacteria, virus 2. Fragments of Ag bind to major histo-compatiblity (MHC) proteins in macrophage. 3. MHC I-Ag fragment expressed
Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning
 Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning Edward Seung, 1 John P. Mordes, 2 Aldo A. Rossini, 1,2,3 and Dale L. Greiner 1,2
Hematopoietic chimerism and central tolerance created by peripheral-tolerance induction without myeloablative conditioning Edward Seung, 1 John P. Mordes, 2 Aldo A. Rossini, 1,2,3 and Dale L. Greiner 1,2
The. Conception of. GCC- Specific. Chimeric Antigen Receptors
 The Conception of GCC- Specific Chimeric Antigen Receptors Raven Smith-Parris 8/6/2013 Abstract Colorectal Cancer is an aggressive disease that claims the lives of both men and women every year. It is
The Conception of GCC- Specific Chimeric Antigen Receptors Raven Smith-Parris 8/6/2013 Abstract Colorectal Cancer is an aggressive disease that claims the lives of both men and women every year. It is
Marker Antibody Supplier. CD7 CD7 PE-CY 7 anti-human CD7 ebioscience, San Diego, USA
 Supplementary Table 1: Flurochrome labelled antibody used Marker Antibody Supplier CD3 CD4 CD8 CD25 CD26 CD127 CCR4 CCR7 Ki67 Viability stain Alexa Fluor 700 anti-human CD3 Fluorescein isothiocyanate antihuman
Supplementary Table 1: Flurochrome labelled antibody used Marker Antibody Supplier CD3 CD4 CD8 CD25 CD26 CD127 CCR4 CCR7 Ki67 Viability stain Alexa Fluor 700 anti-human CD3 Fluorescein isothiocyanate antihuman
T Cell Memory Is Short-lived in the Absence of Antigen By David Gray and Polly Matzinger
 T Cell Memory Is Short-lived in the Absence of Antigen By David Gray and Polly Matzinger From the Basel Institute for Immunology, CH 4005, Basel, Switzerland Summary Immunological memory has generally
T Cell Memory Is Short-lived in the Absence of Antigen By David Gray and Polly Matzinger From the Basel Institute for Immunology, CH 4005, Basel, Switzerland Summary Immunological memory has generally
Antibody Structure. Antibodies
 Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibody Structure supports Function
 Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Antibodies Secreted by B lymphocytes Great diversity and specificity: >10 9 different antibodies; can distinguish between very similar molecules Tag particles for clearance/destruction Protect against
Phage Antibody Selection With Reichert SPR System
 Phage Antibody Selection With Reichert SPR System Reichert Technologies Webinar April 4, 2016 Mark A. Sullivan, Ph.D. Department of Microbiology and Immunology University of Rochester Presentation Outline
Phage Antibody Selection With Reichert SPR System Reichert Technologies Webinar April 4, 2016 Mark A. Sullivan, Ph.D. Department of Microbiology and Immunology University of Rochester Presentation Outline
Antibody-Mediated Immunity
 Color code: Important in red Extra in blue Antibody-Mediated Immunity For team error adjustments, click here Objectives To describe B-cells as the mediators of humoral immunity, (antibody-mediated immunity)
Color code: Important in red Extra in blue Antibody-Mediated Immunity For team error adjustments, click here Objectives To describe B-cells as the mediators of humoral immunity, (antibody-mediated immunity)
SUPPLEMENTARY INFORMATION
 doi: 1.138/nature875 a promoter firefly luciferase CNS b Supplementary Figure 1. Dual luciferase assays on enhancer activity of CNS1, 2, and 3. a. promoter sequence was inserted upstream of firefly luciferase
doi: 1.138/nature875 a promoter firefly luciferase CNS b Supplementary Figure 1. Dual luciferase assays on enhancer activity of CNS1, 2, and 3. a. promoter sequence was inserted upstream of firefly luciferase
