Mechanics of epithelial closure over non-adherent environments
|
|
|
- Nora Bell
- 6 years ago
- Views:
Transcription
1 Received 2 Oct 214 Accepted 15 Dec 214 Published 22 Jan 215 DOI: 1.138/ncomms7111 Mechanics of epithelial closure over non-adherent environments OPEN Sri Ram Krishna Vedula 1, *,w,grégoire Peyret 2, *, Ibrahim Cheddadi 3,4, Tianchi Chen 1, Agustí Brugués 5, Hiroaki Hirata 1, Horacio Lopez-Menendez 2, Yusuke Toyama 1,6,7, Luís Neves de Almeida 3,4, Xavier Trepat 5,8,9, Chwee Teck Lim 1,1,11 & Benoit Ladoux 1,2 The closure of gaps within epithelia is crucial to maintain its integrity during biological processes such as wound healing and gastrulation. Depending on the distribution of extracellular matrix, gap closure occurs through assembly of multicellular actin-based contractile cables or protrusive activity of border cells into the gap. Here we show that the supracellular actomyosin contractility of cells near the gap edge exerts sufficient tension on the surrounding tissue to promote closure of non-adherent gaps. Using traction force microscopy, we observe that cell-generated forces on the substrate at the gap edge first point away from the centre of the gap and then increase in the radial direction pointing into the gap as closure proceeds. Combining with numerical simulations, we show that the increase in force relies less on localized purse-string contractility and more on large-scale remodelling of the suspended tissue around the gap. Our results provide a framework for understanding the assembly and the mechanics of cellular contractility at the tissue level. 1 Mechanobiology Institute, National University of Singapore, Singapore , Singapore. 2 Institut Jacques Monod (IJM), CNRS UMR 7592 and Université Paris Diderot, 7513 Paris, France. 3 Sorbonne Universités, UPMC Univ Paris 6 and CNRS UMR 7598, Laboratoire Jacques-Louis Lions, F Paris, France. 4 INRIA-Paris-Rocquencourt, MAMBA Team, Domaine de Voluceau, BP15, Le Chesnay Cedex, France. 5 Institute for Bioengineering of Catalonia, C/ Baldiri Reixac 1-12, 828 Barcelona, Spain. 6 Department of Biological Sciences, National University of Singapore, 14 Science Drive 4, Singapore Singapore. 7 Temasek Life Sciences Laboratory, 1 Research Link, National University of Singapore, Singapore 11764, Singapore. 8 Institució Catalana de Recerca i Estudis Avanc ats (ICREA), Passeig Lluís Companys, Barcelona, Spain. 9 Unitat de Biofísica i Bioenginyeria, Facultat de Medicina, Universitat de Barcelona, and CIBERES, 836 Barcelona, Spain. 1 Department of Biomedical Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore , Singapore. 11 Department of Mechanical Engineering, National University of Singapore, 9 Engineering Drive 1, Singapore , Singapore. * These authors contributed equally to this work. w Present Address: L Oréal Research and Innovation, #6-6, Neuros, 8A Biomedical Grove, Singapore. Correspondence and requests for materials should be addressed to S.R.K.V. ( vkrishna@rd.loreal.com) or to C.T.L. ( ctlim@nus.edu.sg) or to B.L. ( benoit.ladoux@univ-paris-diderot.fr). NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
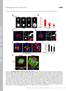
2 NATURE COMMUNICATIONS DOI: 1.138/ncomms7111 Studying the closure of gaps and discontinuities within multicellular sheets is of great interest because of the important role that it plays in various biological processes such as embryogenesis, tissue morphogenesis and wound healing. Typical examples include dorsal closure in drosophila 1,2, cell extrusion 3 and wound healing 4,5. When gaps or discontinuities appear in the epithelia, it is widely accepted that there are two major mechanisms that drive the closure of such gaps 6 8. The first mechanism, termed cell crawling, refers to the protrusive activity of filopodia or lamellipodia at the edge of the gap that propel them into the void The second mechanism, referred to as actin purse-string contraction, is mediated by the coordinated contraction of actin bundles running across multiple cells at the edge of the gap In many instances, epithelial gap closure occurs over regions where the extracellular matrix (ECM) proteins are either sparsely distributed or even non-existent. In order to close gaps under these environments, epithelial cells can switch from cell crawling mechanism to actin-based purse-string contraction 15. We have previously reported that keratinocytes migrating on micropatterned lines can form suspended epithelial bridges that rely on contractile actin bundles over regions devoid of ECM proteins to close gaps and maintain epithelial integrity 16. However, as both cell crawling and actin purse-string mechanisms co-exist during gap closure, this can induce discontinuities in the actin organization around the gap because of the presence of protrusive extensions as well as contractile actin bundles 7,1,17,18. Both mechanisms could thus influence each other during epithelial resealing. In addition to lamellipodia extensions towards the gap, the assembly of discontinuous supracellular contractile actin cables connected to the substrate through focal adhesions promotes efficient wound closure by compressing the underlying substrate 19. Because such mechanisms rely on cell substrate interactions, it is difficult to understand how gaps close in situations where the ECM is heterogeneous and/or poorly adherent. In such cases, the pursestring contraction of actin cables appears to be the crucial mechanism for gap closure but it remains poorly characterized. Here, by using micropatterned substrates 2, we study the closure of circular gaps devoid of ECM protein and functionalized h 9 h 15 h 16.5 h Y Early Z Intermediate Late Z X Figure 1 HaCaT cells close a 1 lm diameter non-adhesive gap using actin purse-string contraction. (a) Schematic and (b) phase contrast images showing closure of a circular non-adhesive gap with time. Cells are initially rounded and confined to the mcontact printed adhesive fibronectin (red) region but with time gradually spread and close the non-adhesive circular gap. (c) z-projection of confocal images and the corresponding xz and yz sections (along the white lines) of cells stained for F-actin during early, intermediate and late stages of closure of the circular non-adhesive gap (red circle). The top panels represent the approximate start of the phase and bottom panels mark the approximate end of each phase. Scale bars, (b) 5mm and (c) 2mm. 2 NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
3 NATURE COMMUNICATIONS DOI: 1.138/ncomms7111 ARTICLE with a cell non-adhesive polymer within sheets of keratinocytes. We find that closure of such non-adherent gaps is driven exclusively by contraction of multicellular actin-based cables. The ability to close these gaps is determined by geometrical cues such as size and curvature of the gap as well as intact intercellular junctions. Traction force microscopy (TFM) and numerical simulations suggest strong reinforcement of the contractile force driving the gap closure. Such reinforcement appears to be originating from large-scale remodelling of cells at the gap edge. Results Closure of non-adhesive gaps by purse-string contraction. Based on our previous experiments, we hypothesized that circular non-adhesive gaps within keratinocyte cell sheets would promote the formation of contractile purse-strings composed of actin filaments and thus help us to elucidate the mechanics of multicellular actin-based purse-string contraction within a well-defined environment. To test this hypothesis, HaCaT cells were seeded on micropatterns consisting of a 1 mm diameter circular nonadhesive gap (rendered non-adhesive with Pluronics, see methods) at the centre of a large (B8 mm) fibronectin-coated square. Surprisingly, we observed collective cellular movements towards the centre of the gap solely driven by actomyosin contraction ending in complete gap closure. Initially, cells confined themselves to the adhesive region leaving the non-adhesive gap empty (Fig. 1a,b and Supplementary Movie 1). With progression of time, they gradually moved in and closed the gap. Although no lamellipodia were observed at the advancing cell front, the edge showed a strong contrast in phase images suggesting that the closure was driven by contraction of actin cables (Fig. 1b). Staining for filamentous actin at different time points not only confirmed strong accumulation of F-actin at the edge but also allowed us to broadly divide the closure event into different stages (Fig. 1c). About 1 2 h after initial seeding (Fig. 1c, early stage, left panel), cells adhered to the fibronectin-coated region of the substrate. Gradually, cells started spreading and formed filopodia and lamellipodia over the fibronectin-coated pattern. After complete spreading, cells at the gap edge displayed strong accumulation of F-actin resulting in the formation of discontinuous actin cables around the gap. This was followed by an anisotropic closure of the gap with the formation of multicellular actin cables that partly extend over the non-adhesive region on one side and multiple contractile cables still connected to the substrate on another side (Fig. 1c, intermediate stage, middle panel). With further progression of time, the actin cables contracted rapidly resulting in complete closure and formation of a suspended epithelial sheet over the non-adhesive gap (Fig. 1c, late stage, right panel). To analyse whether these different stages reflected in the closure dynamics of the non-adhesive gap, the perimeter of the gap was tracked over time (Fig. 2a). The plot not only showed a large variation in the total time taken for closure from one gap to another (B4.5 to 17.5 h) but also suggested that the closure was highly non-linear and occasionally fluctuated. Indeed, although the average time taken for complete closure of the gap was 11.7±3.7 h (n ¼ 17, mean±s.d.), the average time required for closure of the initial approximately one-third of the gap was 6.77±2.97 h, whereas the latter approximately two-third of the gap took 4.9±1.67 h to close. This strongly suggests that the slow first phase represents not only the time taken for the cells to spread but also the time required for cellular re-organization around the gap to build-up multiple contractile cables. In contrast, the rapid second phase represents a more coordinated and efficient contraction of the suspended cell front. The fluctuations in the perimeter suggest that the purse-string does not contract continuously but rather relaxes intermittently. As a control, we then compared this with closure of a 1 mm diameter adhesive gap induced by the removal of a micropillar stencil 1 (Fig. 2b). The gap closure was completed in 3.6±1.2 h (n ¼ 16), which is comparable to the speed of a migrating HaCaT monolayer 16. The closure process was mainly driven by cell crawling with lamellipodia extension with some reinforcement of actin at the edge of the gap (Fig. 2c and Supplementary Movie 2). This result shows that our experimental system is well-designed to exclusively study the influence of actomyosin purse-string contraction on epithelial gap closure h 1 h 2.5 h Figure 2 Closure dynamics of adhesive and non-adhesive gaps. Closure of (a) non-adhesive gaps (mediated by actin cable contraction) and (b) adhesive gaps (mediated by cell crawling mechanism). (c) Phase contrast images of HaCaT cells closing an adhesive epithelial gap created after the removal of a micropillar stencil. Inset shows cells forming lamellipodia. Scale bars, (c) 5 mm. NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
4 NATURE COMMUNICATIONS DOI: 1.138/ncomms7111 Curvature regulates purse-string contraction. To further investigate the effect of gap curvature on purse-string contraction, we varied the dimensions and the shapes of the non-adhesive regions. First, cells were seeded on non-adhesive gaps with diameters of 15 and 2 mm. In contrast to the 1 mm nonadhesive gaps that cells were always able to close, the 15 mm diameter non-adhesive gaps could be closed only in B2% of cases. However, in most instances (B8% of the cases), the cell front advanced to close the gap only partially (Fig. 3a and Supplementary Movie 3). On the 2 mm diameter gaps, the cell front barely advanced into the gap and no closure was observed (Fig. 3b and Supplementary Movie 4). These observations were further supported by the plot of the change in perimeter of the gap with time (Fig. 3c,d). This relationship between epithelial closure and gap curvature not only emphasizes the role of actinbased contractile forces at the edge of the gap in driving the closure process but also highlights the existence of a critical diameter of B15 mm, above which such contraction is ineffective. Also, as a larger gap can be interpreted as a geometrical feature with lower curvature, we hypothesized that the curvature of the ECM geometry influences the stability of actin cable formation as well as its efficient contraction. To test this hypothesis, we seeded cells on two different patterns. The first was an elliptical non-adhesive gap whose minor and major axes were B1 and B1, mm, respectively (Fig. 3e). As in the previous instances, cells were initially confined to the adhesive fibronectin region leaving the elliptical non-adhesive region empty. With time, cells started migrating into the gap from one end of the ellipse that displayed very high curvature (arrow, Fig. 3e and Supplementary Movie 5). Interestingly, the cell front reached an equilibrium-like state and stopped migrating after B24 h. The second pattern consisted of a non-adhesive gap with negative curvatures (convex projections into the gap, arrow in Fig. 3f) to the cell sheet. On this pattern, cell sheets initially migrated to close the corners of the gap that provided high curvature (Supplementary Movie 6). However, movement of the h 4 h h 48 h h 12 h 24 h h 18 h 31 h Figure 3 Closure of non-adhesive gaps is determined by the size and curvature of the gap. (a,b) Phase contrast images and (c,d) closure dynamics of 15 and 2 mm diameter non-adhesive gaps, respectively. (e) Elliptical non-adhesive gaps (minor/major axis ratio ¼ 1:1, mm) are partially closed in regions with high curvature (arrow) and (f) closure of non-adhesive gaps with negative curvature (arrow) is delayed. Scale bars, 5 mm. 4 NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
5 NATURE COMMUNICATIONS DOI: 1.138/ncomms7111 ARTICLE cell front was delayed across the sides of the gap with a strong negative curvature because of their convexity. Eventually, a circular actin-based purse-string was formed that resulted in gap closure. Together, these results suggest that geometrical cues such as curvature can strongly influence the formation, stability and contraction of actin cables. a-catenin is essential for closure of non-adhesive gaps. Epithelial reshaping processes involve a remodelling of adherens junctions We hypothesized that efficient force transmission through supracellular contractile actin cables would require intact intercellular adhesion during gap closure as described by previous studies 14,25. To test this, we generated HaCaT cells that were stably knocked down for a-catenin (a-hacat) and desmoplakin (dsp-hacat), which are considered as key adaptor molecules at the adherens junctions and desmosomes, respectively a-hacat cells did not form intercellular junctions and failed to close the non-adhesive gaps (Fig. 4a and Supplementary Movie 7). Instead, these cells migrated individually and single a-hacat cells occasionally stretched across the gap. In contrast, dsp-hacat cells could efficiently close the non-adhesive gap (Supplementary Movie 8) in a manner that was indistinguishable from wild-type HaCaT as well as HaCaT cells expressing nontargeting small hairpin RNA (shrna) (Supplementary Movie 9). This suggested that a-catenin but not desmoplakin was indispensable for coordinated contraction of the purse-string. This is consistent with recent studies that emphasize the role of adherens junctions in establishing tissue level tension 29. The importance of intercellular adhesion was also evident from the fact that simple epithelial cells such as MDCK that demonstrate a more viscous-like behaviour because of higher cell cell rearrangement (compared with HaCaT) 16 could not close a 1 mm non-adhesive gap even after B4 h despite reaching very high densities and eventually over confluence (Fig. 4b,c and Supplementary Movie 1). In fact, the partial closure of the gap that did occur was a result of cell overgrowth resulting in formation of three-dimensional-like tissue structures (Fig. 4d) as previously reported 3. Consequently, even though these findings demonstrate the importance of tensile stress through intercellular adhesions during epithelial gap closure, we could not exclude a possible role of proliferative pressure from the surrounding tissue. To test this, we inhibited cell proliferation in this process. When cell proliferation was inhibited, the 1 mm diameter non-adhesive gap could still be closed, although the closure was slightly delayed (Fig. 4e and Supplementary Movie 11). This delay h 4 h h 4 h 4 Y Z Z X h 14 h 18 h Figure 4 Strength and integrity of intercellular adhesion regulate closure of non-adhesive gaps. (a) Phase contrast images of a-catenin knockdown HaCaT cells on 1 mm diameter non-adhesive gaps. (b) Phase contrast images, (c) closure dynamics and (d) confocal images of actin staining of MDCK cells on 1 mm diameter non-adhesive gaps. (e) HaCaT cells close 1 mm non-adhesive gaps in the presence of 2 mm thymidine (proliferation inhibitor). Scale bars, (a,b,e) 5 mm and (d) 2 mm. NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
6 NATURE COMMUNICATIONS DOI: 1.138/ncomms7111 was not surprising as inhibition of proliferation would also increase the time taken for cells to completely occupy the adhesive region before migrating into the non-adhesive region. Thus, cell proliferation does not appear to be the major driving force in our model of epithelial gap closure. TFM of contracting purse-string. We thus considered that the closure of these epithelial gaps could result from a tug of war between the contracting actin-based purse-string at the edge of the gaps and the resistive forces offered by the cells adherent to the fibronectin-coated substrate. In such a model, the forces generated by the contracting actin-based purse-string should reflect in the traction forces exerted by cells in the vicinity of the edge of the non-adhesive gap. To test this hypothesis, we used TFM. Non-adhesive gaps of diameters 1 and 2 mm were patterned on soft silicone elastomeric substrates functionalized with fluorescent beads as described previously 16,31. Closure of the gap and deflection of beads (Fig. 5a) were tracked over time and unconstrained traction stresses were calculated from the bead displacement as previously described 32. Radial stresses pointing inward and tangential stresses directed counter-clockwise t= h t=17.5 h <T r > <T Pa t > < T r > < T t > Pa 1, t=22.5 h <T r > <T t > Pa Perimeter <T r > <T t > < T r > < T t > , 5 5 Stress (Pa) 1, < T r > < T t > Pa Perimeter <T r > <T t > 7 < T r > < T t > Stress (Pa) Figure 5 Traction force microscopy of HaCaT cells on 1 and 2 lm diameter non-adhesive gaps. (a) Evolution of traction stresses with time as the non-adhesive gap is closed. Arrows depict both the direction and magnitude of the stresses exerted by cells on the substrate. Kymographs showing (b,d) vectorial and (c,e) scalar average of radial (T r, left panel) and tangential (T t, right panel) stresses as a function of distance from the centre of 1 and 2 mm gaps, respectively. The dashed dotted line represents the gap edge. Average stresses along a 2 mm wide strip centred on the edge of the (f) 1 and (g) 2 mm diameter non-adhesive gap. 6 NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
7 NATURE COMMUNICATIONS DOI: 1.138/ncomms7111 ARTICLE were considered negative. Kymographs of vectorial and scalar average of radial (ot r 4, o T r 4) and tangential (ot t 4, o T t 4) stresses as a function of distance from centre of the gap showed that the maximal stresses were localized to B1 mm on either side of the edge of the gap (Fig. 5b e). Accordingly, average radial and tangential stresses in this strip or region of interest were plotted as a function of time for further analysis. For the 1 mm diameter gap, ot r 4 was initially positive (directed outwards) consistent with the idea of cells that were spreading or crawling to reach the edge of the gap (Fig. 5f). With the formation of a contractile purse-string and movement of cells into the gap, ot r 4 became increasingly negative (pointing inwards) and reached a nadir (approximately 6 Pa) just about the time the gap closed (Fig. 5f). After the closure of the gap, ot r 4 showed a slight increase but remained negative suggesting that cells bridging the gap exhibited residual tension despite the dissolution of the actin-based purse-string. The average tangential stress ot t 4, on the other hand, fluctuated but remained close to zero throughout the observation period (Fig. 5f). However, the scalar average of the radial (o T r 4) and tangential (o T t 4) stresses increased and reached a peak just around the time the gap closed after which they showed a sudden fall. The force increase in the radial direction suggests a reinforcement of the contracting actin cable as gap closure progresses. Further support for such a force balance between cell substrate adhesion and contracting actin cables came from estimation of the critical length scale based on estimated cell adhesion energy density g (J m 2 ). The total work done to move a suspended cell sheet of radius R over the non-adhesive region by a further distance dr is given by g(2pr)dr. The radial force within the actin cable should be large enough to overcome this energy barrier, that is, Fdr ¼ g(2pr)dr. The maximal radial force within the cable estimated by TFM is B4. mn (B65 Pa over B6,3 mm 2 area of the strip of interest). Although data for g for keratinocytes are not available in the literature, the estimated value for fibroblasts on fibronectin is B15 mj m 2 (ref. 33). This gives a critical length scale R equal to B4 mm for closure that correlates well with our experimental observations. For the 2 mm diameter gap, minimal stresses were detected at the edge of the hole that corroborated with the fact that the cell front had barely migrated into the gap (Fig. 5g). To directly test the influence of such contractile tension on gap closure, we laser ablated actin bundles at the edge (Fig. 6a and Supplementary Movie 12) and inhibited actomyosin contractility with blebbistatin (Fig. 6b,c and Supplementary Movie 13). Both experiments induced a relaxation of a partially closed nonadhesive gap and thus supported the idea of force transmission from the contracting actin cable to cells adherent to fibronectin at the edge of the gap. Modelling the closure of a non-adhesive circular gap. To further test our hypothesis of a force balance between contractile forces of the actin cable and resistive viscoelastic responses of the remaining adherent cells, we developed a mechanical model that captures our experimental findings. The tissue was modelled as a solid viscoelastic Kelvin-Voigt material, and the interaction with the adhesive part of the substrate was modelled so that the velocity of the tissue is zero if the force exerted on the tissue is less than a threshold f y, and linearly increases with the force above this threshold (Fig. 7a, Supplementary Note 1 and Supplementary Fig. 1). Assuming that the actomyosin structure on the border of the gap acts as a cable under tension T, the resulting force per unit length exerted on the tissue is Tk~n, where k is the local curvature and ~n is the normal vector to the border directed towards the interior of the gap 34,35. Numerical s 5.5 s * h 4.5 h Figure 6 Closure of non-adhesive gap requires actomyosin contractility. (a) Laser ablation of the actin cables at the edge results in immediate relaxation of the cell front. The asterisk represents the point of laser ablation, red circle represents the edge of the 15 mm diameter non-adhesive gap and blue dotted line is the edge of the cell front before laser ablation. (b) Phase contrast images and (c) relaxation dynamics of a partially closed 1 mm diameter non-adhesive gap after addition of 5 mm blebbistatin. Scale bars, (a) 2mm and (b) 5mm. NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
8 NATURE COMMUNICATIONS DOI: 1.138/ncomms X Y Z X T fy Stress (Pa) 2 4 κ 2 model 6 κ model Experimental Model 35 Experimental Model Experimental Distance from centre of the gap (µm) (a.u.) Perimeter Intensity Intensity (a.u.) Figure 7 Numerical simulation of the gap closure. (a) Schematic of the model showing a balance between the tension in the actin cable (green) T and the frictional force f y arising from tissue adhesion to substrate on the fibronectin (red) and the tissue itself represented as a viscoelastic material. (b) Average radial stress at the edge of the gap predicted by the model with (k 2 model) or without (k model) reinforcement of tension in the actin pursestring. Change in the perimeter of the gap over time predicted by the tension reinforcement model for (c) 1 mm and (d) 2 mm diameter gaps. Simulation values have been scaled to fit the experimental data. (e) Kymograph of actin intensity from the centre of the gap. Black line is a guide to show an B12 mm wide strip along the edge of the cell front. (f) Change in average intensity of actin with time in the strip of interest bounded by the black line in e. (g) Actin network visualized using GFP-lifeact-expressing HaCaT cells and (h) basal confocal section of cells fixed and stained for actin showing strong re-organization within cells localized to the edge of the gap (insets). Scale bars 2 mm. simulations showed that a constant tension (k model) could not account for the increasing stress at the edge of the gap; however, a simple model of cable reinforcement (tension increasing linearly with curvature, k 2 model) could predict this experimental observation (Fig. 7b). Such a tension reinforcement model (k 2 model) together with a threshold friction force could not only account for the fact that 1 mm but not 2 mm diameter gaps close but could also explain the experimentally observed increase in closure velocity for the 1 mm diameter gaps with time (Fig. 7c,d). To understand the origin of the reinforcement, we performed live-cell imaging of HaCaT cells stably expressing GFP-lifeact (Supplementary Movie 14). Although there was some increase in the intensity of actin at the edge of the suspended cell front (Fig. 7e,f), a more prominent observation was the large-scale 8 NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
9 NATURE COMMUNICATIONS DOI: 1.138/ncomms7111 ARTICLE re-organization of the actin cytoskeleton within the cells around the gap directed tangentially to the gap (Fig. 7g, inset). Such a reorganization was also consistently observed in cells fixed and stained for actin immediately before gap closure (Fig. 4h, inset). We believe that this re-organization of actin cytoskeleton at tissue level probably plays a significant role in reinforcing overall contractility of the suspended cell front. Discussion In view of this relationship, our assay determines that fine tuning between cell substrate interactions and strengthening of multicellular actin cables through intercellular adhesions remains crucial to understand epithelial gap closure. Indeed, the mechanistic characterization of actin-based purse-stringmediated closure of epithelial gaps has remained elusive because of the lack of an assay that could faithfully and consistently reproduce it in vitro. Most of our understanding about this mechanism of gap closure comes from experiments involving laser ablation of cell clusters in vitro 13,19 or in vivo 34,36. However, in both experimental systems it is almost impossible to segregate the individual contributions of cell crawling and purse-string contraction mechanisms to the overall closure process. Furthermore, in the absence of a direct readout, quantification of forces generated by the actin cable is indirectly inferred from the retraction velocity of tissues following laser ablation. In contrast, our experimental setup provides a novel platform to isolate and characterize the mechanics of purse-string contraction during epithelial gap closure. Using a similar experimental approach, Kim et al. recently studied traction forces in cell monolayers that surrounded but never invaded non-adherent islands 37. They found that cells enveloping the islands exerted radial forces pointing uniformly away from the island. This intriguing behaviour, which they called kenotaxis, was independent of the orientation of the cell velocity vector and principal stress directions. Using cells that are able to invade non-adherent islands, here we found traction force patterns that are similar in orientation but opposite in sign. Indeed, we found that forces are mostly radial but point towards the island rather than away from it (note that Kim et al. reportedforces exerted by the gel on the cells rather than by the cells on the gel as we did here). These findings show that fundamentally distinct mechanical scenarios are at play in cells that invade gaps compared with cells that only envelop them. This is also reflected by the fact that, unlike cells enveloping non-adherent islands, cells that invade the islands show non-negligible tangential tractions (Fig. 5b,c) 19. Although closure mediated by contraction of actin cables appears to be slower and inefficient compared with cell crawling, it is probably important in situations where cell ECM contact is either poor or non-existent. Indeed, purse-string contraction appears to be the dominant mechanism of wound/gap closure in embryos, which exhibit low levels of ECM providing little cell substrate contact for migration 14. Furthermore, efficient formation and contraction of such actin-based purse-strings appears to be limited to cell types that are involved in wound healing (for example, skin keratinocytes and embryonic epidermal cells) as simple epithelial cells such as MDCK are unable to close similar non-adhesive gaps efficiently. Such specificity could arise from differences in the organization and strength of intercellular adhesion in these cell types. Our findings demonstrate the importance of contractile mechanical forces generated by large-scale re-organization of actin cytoskeleton in tissue remodelling during epithelial closure and thus provide new mechanistic insights into these processes. Methods Cell culture and reagents. HaCaT cells (Cell Lines Service) and MDCK cells were maintained in DMEM supplemented with 1% fetal bovine serum and antibiotics. Protein expression in HaCaT cells was depleted by retrovirus-mediated introduction of shrna into cells, as described previously 16. The target sequences used were 5 -GACTTAGGAATCCAGTATA-3 (for a-catenin) and 5 -GTGACC AACTTGTCCTCAA-3 (for desmoplakin). As a control, shrna with the nontargeting sequence (5 -ATAGTCACAGACATTAGGT-3 ) was used. For inhibition of proliferation, HaCaT cells were first maintained in 2 mm thymidine for 24 h following which cells were trypsinized, seeded on the patterns and imaged in medium containing 2 mm thymidine. Microcontact printing and microscopy. Patterned silicon wafers for soft lithography were prepared using SU-8 photoresist 2,38. For imaging the closure dynamics of the gaps, patterns were microcontact printed on non-culture treated Petri dishes (Greiner) and blocked with.2% Pluronics. Cells were seeded at high density and allowed to adhere for 1 2 h to completely fill the adhesive region of the pattern. Floating cells were washed off and the closure was imaged on a Biostation (Nikon). For closure of circular adhesive epithelial gaps by cell crawling, micropillar stencils removal assay was performed 1. The closure dynamics (contour of the non-adhesive gap) was obtained using the ABsnake plugin for Image J, fitted with an ellipse using MATLAB. For confocal imaging, patterning was done on glass bottom Petri dishes spin coated with a thin layer of polydimethylsiloxane (PDMS). Cells were imaged using a laser confocal microscope (Leica). Images were processed in Image J to enhance contrast. Laser ablation was done using ultraviolet laser (355 nm, Minilite II, Continuum) mounted on a confocal microscope (Nikon A1R) using a 4 water immersion objective 16. Traction force microscopy. Substrates for TFM were prepared from soft silicone gels 16,39. Briefly, soft silicone gels were mixed in a ratio of 1:1 and spin coated on a glass bottom Petri dish and cured. The gels were silanized by incubating in 5% (3-aminopropyl)triethoxysilane (APTES, Sigma) in ethanol for 5 min. The substrates were dried and incubated with 1 nm carboxylated fluorescent beads (Invitrogen) for 5 min, washed and dried. The beads were passivated with 1 mm Tris base (1st Base) in water and dried. Fibronectin pattern was stamped on the soft silicone gels using a water-soluble polyvinyl alcohol (PVA) membrane as an intermediate substrate 31. The gels were finally blocked with.2% Pluronics solution. Cells were seeded on the patterns and imaged on an Olympus inverted microscope. After imaging, cells were lysed and the stress free image of the beads was obtained. The unconstrained stresses were computed using custom written MATLAB codes 32. References 1. Jacinto, A., Woolner, S. & Martin, P. Dynamic analysis of dorsal closure in Drosophila: from genetics to cell biology. Dev. Cell 3, 9 19 (22). 2. Jacinto, A. et al. Dynamic analysis of actin cable function during Drosophila dorsal closure. Curr. Biol. 12, (22). 3. Marinari, E. et al. Live-cell delamination counterbalances epithelial growth to limit tissue overcrowding. Nature 484, (212). 4. Bement, W. M., Mandato, C. A. & Kirsch, M. N. Wound-induced assembly and closure of an actomyosin purse string in Xenopus oocytes. Curr. Biol. 9, (1999). 5. Martin, P. Wound healing--aiming for perfect skin regeneration. Science 276, (1997). 6. Jacinto, A., Martinez-Arias, A. & Martin, P. Mechanisms of epithelial fusion and repair. Nat. Cell. Biol. 3, E117 E123 (21). 7. Klarlund, J. K. Dual modes of motility at the leading edge of migrating epithelial cell sheets. Proc. Natl Acad. Sci. USA 19, (212). 8. Martin, P. & Lewis, J. Actin cables and epidermal movement in embryonic wound healing. Nature 36, (1992). 9. Fenteany, G., Janmey, P. A. & Stossel, T. P. Signaling pathways and cell mechanics involved in wound closure by epithelial cell sheets. Curr. Biol. 1, (2). 1. Anon, E. et al. Cell crawling mediates collective cell migration to close undamaged epithelial gaps. Proc. Natl Acad. Sci. USA 19, (212). 11. Jamora, C. & Fuchs, E. Intercellular adhesion, signalling and the cytoskeleton. Nat. Cell. Biol. 4, E11 E18 (22). 12. Bement, W. M., Forscher, P. & Mooseker, M. S. A novel cytoskeletal structure involved in purse string wound closure and cell polarity maintenance. J. Cell Biol. 121, (1993). 13. Tamada, M., Perez, T. D., Nelson, W. J. & Sheetz, M. P. Two distinct modes of myosin assembly and dynamics during epithelial wound closure. J. Cell Biol. 176, (27). 14. Brock, J., Midwinter, K., Lewis, J. & Martin, P. Healing of incisional wounds in the embryonic chick wing bud: characterization of the actin purse-string and demonstration of a requirement for Rho activation. J. Cell Biol. 135, (1996). 15. Grasso, S., Hernandez, J. A. & Chifflet, S. Roles of wound geometry, wound size, and extracellular matrix in the healing response of bovine corneal endothelial cells in culture. Am. J. Physiol. Cell Physiol. 293, C1327 C1337 (27). 16. Vedula, S. R. K. et al. Epithelial bridges maintain tissue integrity during collective cell migration. Nat. Mater. 13, (214). NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
10 NATURE COMMUNICATIONS DOI: 1.138/ncomms Garcia-Fernandez, B., Campos, I., Geiger, J., Santos, A. C. & Jacinto, A. Epithelial resealing. Int. J. Dev. Biol. 53, (29). 18. Cochet-Escartin, O., Ranft, J., Silberzan, P. & Marcq, P. Border forces and friction control epithelial closure dynamics. Biophys. J. 16, (214). 19. Brugues, A. et al. Forces driving epithelial wound healing. Nat. Phys. 1, (214). 2. Vedula, S. R. et al. Microfabricated environments to study collective cell behaviors. Methods Cell Biol. 12, (214). 21. Taguchi, K., Ishiuchi, T. & Takeichi, M. Mechanosensitive EPLIN-dependent remodeling of adherens junctions regulates epithelial reshaping. J. Cell Biol. 194, (211). 22. Huveneers, S. & de Rooij, J. Mechanosensitive systems at the cadherin-f-actin interface. J. Cell Sci. 126, (213). 23. Gomez, G. A., McLachlan, R. W. & Yap, A. S. Productive tension: force-sensing and homeostasis of cell-cell junctions. Trends Cell Biol. 21, (211). 24. Guillot, C. & Lecuit, T. Mechanics of epithelial tissue homeostasis and morphogenesis. Science 34, (213). 25. Danjo, Y. & Gipson, I. K. Actin purse string filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J. Cell Sci. 111(Pt 22): (1998). 26. Drees, F., Pokutta, S., Yamada, S., Nelson, W. J. & Weis, W. I. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actinfilament assembly. Cell 123, (25). 27. Yonemura, S., Wada, Y., Watanabe, T., Nagafuchi, A. & Shibata, M. alpha- Catenin as a tension transducer that induces adherens junction development. Nat. Cell. Biol. 12, 533 U535 (21). 28. Jefferson, J. J., Leung, C. L. & Liem, R. K. H. Plakins: Goliaths that link cell junctions and the cytoskeleton. Nat. Rev. Mol. Cell Biol. 5, (24). 29. Harris, A. R., Daeden, A. & Charras, G. T. Formation of adherens junctions leads to the emergence of a tissue-level tension in epithelial monolayers. J. Cell Sci. 127, (214). 3. Deforet, M., Hakim, V., Yevick, H. G., Duclos, G. & Silberzan, P. Emergence of collective modes and tri-dimensional structures from epithelial confinement. Nat. Commun. 5, 3747 (214). 31. Yu, H., Xiong, S., Tay, C. Y., Leong, W. S. & Tan, L. P. A novel and simple microcontact printing technique for tacky, soft substrates and/or complex surfaces in soft tissue engineering. Acta Biomater. 8, (212). 32. Butler, J. P., Tolic-Norrelykke, I. M., Fabry, B. & Fredberg, J. J. Traction fields, moments, and strain energy that cells exert on their surroundings. Am. J. Physiol. Cell Physiol. 282, C595 C65 (22). 33. Yamamoto, A., Mishima, S., Maruyama, N. & Sumita, M. Quantitative evaluation of cell attachment to glass, polystyrene, and fibronectin- or collagen-coated polystyrene by measurement of cell adhesive shear force and cell detachment energy. J. Biomed. Mater. Res. 5, (2). 34. Hutson, M. S. et al. Forces for morphogenesis investigated with laser microsurgery and quantitative modeling. Science 3, (23). 35. Almeida, L., Bagnerini, P. & Habbal, A. Modeling actin cable contraction. Comput. Math. Appl. 64, (212). 36. Fernandez-Gonzalez, R. & Zallen, J. A. Wounded cells drive rapid epidermal repair in the early Drosophila embryo. Mol. Biol. Cell 24, (213). 37. Kim, J. H. et al. Propulsion and navigation within the advancing monolayer sheet. Nat. Mater. 12, (213). 38. Fink, J. et al. Comparative study and improvement of current cell micropatterning techniques. Lab on a Chip 7, (27). 39. Mertz, A. F. et al. Scaling of traction forces with the size of cohesive cell colonies. Phys. Rev. Lett. 18, (212). Acknowledgements We thank A.J. Kabla, R.-M. Mège, W.J. Nelson and group members from the MBI and the IJM for helpful discussions. We also thank M. Ashraf and S. Vaishnavi for the microfabrication and F. Deshayes for the gift of actin-gfp cell line. Financial support from the European Research Council under the European Union s Seventh Framework Programme (FP7/27-213)/ERC grant agreement n , the Human Frontier Science Program (grant RGP4/212), Agence Nationale pour la Recherche (REGENR ANR-11-BSV5-21) and the Mechanobiology Institute are gratefully acknowledged. B.L. and H.L.-M. acknowledge the Institut Universitaire de France and the LABEX Who am I? for their support. X.T. acknowledges support by the European Research Council (StG ) and the Spanish Ministerio de Economía y Comptetitividad (BFU ). Author contributions S.R.K.V. and B.L. designed the research, S.R.K.V., G.P., T.C. and Y.T. performed the experiments, I.C., L.A., H.H., A.B., X.T., H.L.-M., B.L. and C.T.L. contributed new reagents, modelling and computational tools, S.R.K.V. and B.L. wrote the paper, C.T.L. and B.L. supervised the project. All authors read the manuscript and commented on it. Additional information Supplementary Information accompanies this paper at naturecommunications Competing financial interests: The authors declare no competing financial interests. Reprints and permission information is available online at reprintsandpermissions/ How to cite this article: Vedula, S. R. K. et al. Mechanics of epithelial closure over non-adherent environments. Nat. Commun. 6:6111 doi: 1.138/ncomms7111 (215). This work is licensed under a Creative Commons Attribution 4. International License. The images or other third party material in this article are included in the article s Creative Commons license, unless indicated otherwise in the credit line; if the material is not included under the Creative Commons license, users will need to obtain permission from the license holder to reproduce the material. To view a copy of this license, visit 1 NATURE COMMUNICATIONS 6:6111 DOI: 1.138/ncomms & 215 Macmillan Publishers Limited. All rights reserved.
5 HARNESSING THE PURSE STRING FOR
 107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
TRACTION and STRESS MICROSCOPY for CELLS:
 TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
SUPPLEMENTARY INFORMATION
 Epithelial bridges maintain tissue integrity during olletive ell migration Sri Ram Krishna Vedula 1, Hiroaki Hirata 1, Mui Hoon Nai 1, Agustí Brugés 2, Yusuke Toyama 1, 3, Xavier Trepat 2, Chwee Tek Lim
Epithelial bridges maintain tissue integrity during olletive ell migration Sri Ram Krishna Vedula 1, Hiroaki Hirata 1, Mui Hoon Nai 1, Agustí Brugés 2, Yusuke Toyama 1, 3, Xavier Trepat 2, Chwee Tek Lim
Forces driving epithelial wound healing
 Forces driving epithelial wound healing Agustí Brugués 1*, Ester Anon 1,2*, Vito Conte 1, Jim H. Veldhuis 3, Julien Colombelli 4, José J. Muñoz 5, G. Wayne Brodland 3, Benoit Ladoux 2,6, Xavier Trepat
Forces driving epithelial wound healing Agustí Brugués 1*, Ester Anon 1,2*, Vito Conte 1, Jim H. Veldhuis 3, Julien Colombelli 4, José J. Muñoz 5, G. Wayne Brodland 3, Benoit Ladoux 2,6, Xavier Trepat
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization.
 Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues
 High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
CytoSelect 24-Well Cell Migration Assay (12 µm, Colorimetric Format)
 Product Manual CytoSelect 24-Well Cell Migration Assay (12 µm, Colorimetric Format) Catalog Number CBA-107 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration
Product Manual CytoSelect 24-Well Cell Migration Assay (12 µm, Colorimetric Format) Catalog Number CBA-107 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration
Radius 24-Well Cell Migration Assay (Fibronectin Coated)
 Product Manual Radius 24-Well Cell Migration Assay (Fibronectin Coated) Catalog Number CBA-125-FN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Product Manual Radius 24-Well Cell Migration Assay (Fibronectin Coated) Catalog Number CBA-125-FN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Paul et al.,
 Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Supplemental material JCB Paul et al., http://www.jcb.org/cgi/content/full/jcb.201502040/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Mutant p53-expressing cells display limited retrograde actin flow at
Radius 24-Well Cell Migration Assay (Laminin Coated)
 Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
CytoSelect 24-Well Cell Migration Assay (5 µm, Fluorometric Format)
 Revised Protocol Product Manual CytoSelect 24-Well Cell Migration Assay (5 µm, Fluorometric Format) Catalog Number CBA-102 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Revised Protocol Product Manual CytoSelect 24-Well Cell Migration Assay (5 µm, Fluorometric Format) Catalog Number CBA-102 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Single Cell Motility in Artificial Tissue
 Single Cell Motility in Artificial Tissue Rhoda J. Hawkins Lecturer in Physics, University of Sheffield Raphaël Voituriez, Jean-François Joanny, Jacques Prost Matthieu Piel, Ana-Maria Lennon-Dumenil, Philippe
Single Cell Motility in Artificial Tissue Rhoda J. Hawkins Lecturer in Physics, University of Sheffield Raphaël Voituriez, Jean-François Joanny, Jacques Prost Matthieu Piel, Ana-Maria Lennon-Dumenil, Philippe
WOUND HEALING ON ARTIFICIAL EXTRACELLULAR MATRIX PROTEINS
 WOUND HEALING ON ARTIFICIAL EXTRACELLULAR MATRIX PROTEINS Thesis by Eileen Fong In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy California Institute of Technology Pasadena,
WOUND HEALING ON ARTIFICIAL EXTRACELLULAR MATRIX PROTEINS Thesis by Eileen Fong In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy California Institute of Technology Pasadena,
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
SUPPLEMENTAL INFORMATION: 1. Supplemental methods 2. Supplemental figure legends 3. Supplemental figures
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2008 A microfluidics-based turning assay reveals complex growth cone responses to integrated gradients of substrate-bound
Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues
 Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues Nikolce Gjorevski and Celeste M. Nelson Department of Chemical and Biological Engineering, and
Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues Nikolce Gjorevski and Celeste M. Nelson Department of Chemical and Biological Engineering, and
Journal of Cell Science Supplementary Material
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
CytoSelect 24-Well Cell Haptotaxis Assay (8 µm, Collagen I-Coated, Fluorometric Format)
 Product Manual CytoSelect 24-Well Cell Haptotaxis Assay (8 µm, Collagen I-Coated, Fluorometric Format) Catalog Number CBA-101-COL 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual CytoSelect 24-Well Cell Haptotaxis Assay (8 µm, Collagen I-Coated, Fluorometric Format) Catalog Number CBA-101-COL 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Changes in E-cadherin rigidity sensing regulate cell adhesion
 Changes in E-cadherin rigidity sensing regulate cell adhesion Caitlin Collins a, Aleksandra K. Denisin b,c, Beth L. Pruitt b,c, and W. James Nelson a,1 a Department of Biology, Stanford University, Stanford,
Changes in E-cadherin rigidity sensing regulate cell adhesion Caitlin Collins a, Aleksandra K. Denisin b,c, Beth L. Pruitt b,c, and W. James Nelson a,1 a Department of Biology, Stanford University, Stanford,
Radius 384-Well Cell Migration Assay
 Product Manual Radius 384-Well Cell Migration Assay Catalog Number CBA-127 CBA-127-5 384 assays 5 x 384 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is
Product Manual Radius 384-Well Cell Migration Assay Catalog Number CBA-127 CBA-127-5 384 assays 5 x 384 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is
CytoSelect 24- Well Cell Contraction Assay Kit (Floating Matrix Model)
 Product Manual CytoSelect 24- Well Cell Contraction Assay Kit (Floating Matrix Model) Catalog Number CBA- 5020 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing
Product Manual CytoSelect 24- Well Cell Contraction Assay Kit (Floating Matrix Model) Catalog Number CBA- 5020 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing
Sélection Internationale Ens Ulm 2012, Cell Biology
 Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplementary information
 Supplementary information Device Design The geometry of the microdevice channels were designed in autocad and modeled to simulate the microvasculature of the human body. In order to emulate physiologic
Supplementary information Device Design The geometry of the microdevice channels were designed in autocad and modeled to simulate the microvasculature of the human body. In order to emulate physiologic
supplementary information
 DOI: 10.1038/ncb1992 Figure S1 (a-b) Verification of actin flow patter using photobleaching of actin:gfp and Lifeact:RFP in migrating dendritic cells (DCs). (a) Actin:GFP and Lifeact:RFP double-trafected
DOI: 10.1038/ncb1992 Figure S1 (a-b) Verification of actin flow patter using photobleaching of actin:gfp and Lifeact:RFP in migrating dendritic cells (DCs). (a) Actin:GFP and Lifeact:RFP double-trafected
Supplemental material JCB. Pitaval et al., Cell shape and ciliogenesis Pitaval et al.
 Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Cell Contraction Assay
 Product Manual Cell Contraction Assay Catalog Number CBA-201 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing comprises of three processes: epithelialization,
Product Manual Cell Contraction Assay Catalog Number CBA-201 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing comprises of three processes: epithelialization,
Introduction. Figure 1. Oris Cell Migration Assay Principle
 Optimizing Performance of the Membrane-free, Oris Cell Migration Assay for High Throughput Screening using the BioTek Synergy HT Multi-Mode Microplate Reader Keren I. Hulkower, Renee L. Herber, and Scott
Optimizing Performance of the Membrane-free, Oris Cell Migration Assay for High Throughput Screening using the BioTek Synergy HT Multi-Mode Microplate Reader Keren I. Hulkower, Renee L. Herber, and Scott
me239 mechanics of the cell me239 mechanics of the cell 3.1 biopolymers - motivation 3.2 biopolymers - polymerization
 4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
Sabrina Jedlicka. Interactions of Neurons and Materials
 Sabrina Jedlicka Interactions of Neurons and Materials Developmental Cell Biology Some Perspective Stem cells: Undifferentiated cells that are capable of proliferation, self-maintenance and differentiation
Sabrina Jedlicka Interactions of Neurons and Materials Developmental Cell Biology Some Perspective Stem cells: Undifferentiated cells that are capable of proliferation, self-maintenance and differentiation
SUPPLEMENTARY INFORMATION
 SUPPLEMENTARY INFORMATION a b Supplementary Figure 1. Cellular viability in reformulated culture media cells were cultured in serum containing DMEM culture medium supplemented with 4%PEG and 5%DEX for
SUPPLEMENTARY INFORMATION a b Supplementary Figure 1. Cellular viability in reformulated culture media cells were cultured in serum containing DMEM culture medium supplemented with 4%PEG and 5%DEX for
INTRODUCTION Intrinsic cellular mechanical properties (e.g., instantaneous Young s modulus (E instantaneous ) and
 TUMOR CELL CLASSIFICATION BASED ON INSTANTANEOUS YOUNG S MODULUS USING CONSTRICTION CHANNEL BASED MICROFLUIDIC DEVICES Y. Luo 1, D. Chen 1, Y. Zhao 1, C. Wei 1, X. Zhao 2, W. Yue 2, R. Long 3*, J. Wang
TUMOR CELL CLASSIFICATION BASED ON INSTANTANEOUS YOUNG S MODULUS USING CONSTRICTION CHANNEL BASED MICROFLUIDIC DEVICES Y. Luo 1, D. Chen 1, Y. Zhao 1, C. Wei 1, X. Zhao 2, W. Yue 2, R. Long 3*, J. Wang
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table.
 Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
Supplementary Table 1. The Q-PCR primer sequence is summarized in the following table. Name Sequence (5-3 ) Application Flag-u ggactacaaggacgacgatgac Shared upstream primer for all the amplifications of
The Product and Experiment Guide
 The Product and Experiment Guide Providing Solutions for Your Research p. 3 p. 10 p. 4 CELL CULTURE & MICROSCOPY IMMUNO- FLUORESCENCE LIVE CELL IMAGING p. 8 p. 7 p. 11 ANGIOGENESIS ASSAYS CHEMOTAXIS ASSAYS
The Product and Experiment Guide Providing Solutions for Your Research p. 3 p. 10 p. 4 CELL CULTURE & MICROSCOPY IMMUNO- FLUORESCENCE LIVE CELL IMAGING p. 8 p. 7 p. 11 ANGIOGENESIS ASSAYS CHEMOTAXIS ASSAYS
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
3D Transfection System
 3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
3D Transfection System Why 3D Technology? Introduction to Three Dimensional (3D) Cell Culture The goal of three-dimensional (3D) cell culture is to eliminate the stress and artificial responses cells experience
CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
CytoSelect 48-Well Cell Adhesion Assay (Laminin-Coated, Colorimetric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Laminin-Coated, Colorimetric Format) Catalog Number CBA-056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Laminin-Coated, Colorimetric Format) Catalog Number CBA-056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force. Supporting Information
 Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force Benjamin M. Gaub and Daniel J. Müller Supporting Information Supporting Information Note
Mechanical stimulation of Piezo1 receptors depends on extracellular matrix proteins and directionality of force Benjamin M. Gaub and Daniel J. Müller Supporting Information Supporting Information Note
CytoSelect 48- Well Cell Adhesion Assay (Laminin- Coated, Colorimetric Format)
 Product Manual CytoSelect 48- Well Cell Adhesion Assay (Laminin- Coated, Colorimetric Format) Catalog Number CBA- 056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Product Manual CytoSelect 48- Well Cell Adhesion Assay (Laminin- Coated, Colorimetric Format) Catalog Number CBA- 056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figures
 1 Supplementary Figures Supplementary Figure 1 Dynamics of DCL cell division and its relation to cell dispersion during epiboly of A. nigripinnis. (a) Temporal changes in the cumulative number of DCL cell
1 Supplementary Figures Supplementary Figure 1 Dynamics of DCL cell division and its relation to cell dispersion during epiboly of A. nigripinnis. (a) Temporal changes in the cumulative number of DCL cell
SUPPLEMENTARY INFORMATION
 Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments Brendon M. Baker 1,2,*+, Britta Trappmann 1,2,*, William Y. Wang 1, Mahmut S. Sakar
Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments Brendon M. Baker 1,2,*+, Britta Trappmann 1,2,*, William Y. Wang 1, Mahmut S. Sakar
Arterial Tissue Mimics for Studying Cerebral Aneurysm Formation
 Arterial Tissue Mimics for Studying Cerebral Aneurysm Formation Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report Spring 2014 Project and
Arterial Tissue Mimics for Studying Cerebral Aneurysm Formation Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report Spring 2014 Project and
CytoSelect 24-Well Wound Healing Assay
 Product Manual CytoSelect 24-Well Wound Healing Assay Catalog Number CBA-120 CBA-120-5 24 assays 5 x 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wounded tissue initiates
Product Manual CytoSelect 24-Well Wound Healing Assay Catalog Number CBA-120 CBA-120-5 24 assays 5 x 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wounded tissue initiates
CytoSelect 48-Well Cell Adhesion Assay (ECM Array, Fluorometric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (ECM Array, Fluorometric Format) Catalog Number CBA-071 CBA-071-5 48 assays 5 x 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures
Product Manual CytoSelect 48-Well Cell Adhesion Assay (ECM Array, Fluorometric Format) Catalog Number CBA-071 CBA-071-5 48 assays 5 x 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures
MICROFILAMENTS IN EPITHELIAL MORPHOGENESIS
 Published Online: 1 January, 1972 Supp Info: http://doi.org/10.1083/jcb.52.1.206 Downloaded from jcb.rupress.org on November 15, 2018 MICROFILAMENTS IN EPITHELIAL MORPHOGENESIS NELLY AUERSPERG. From the
Published Online: 1 January, 1972 Supp Info: http://doi.org/10.1083/jcb.52.1.206 Downloaded from jcb.rupress.org on November 15, 2018 MICROFILAMENTS IN EPITHELIAL MORPHOGENESIS NELLY AUERSPERG. From the
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Nakajima and Tanoue, http://www.jcb.org/cgi/content/full/jcb.201104118/dc1 Figure S1. DLD-1 cells exhibit the characteristic morphology
Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing
 Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing Authors: Correa LG, Peter R, Clerici G, Ritter V. 2017
Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing Authors: Correa LG, Peter R, Clerici G, Ritter V. 2017
Nature Methods: doi: /nmeth Supplementary Figure 1. Real-time deformability cytometry: contour detection and theoretical modeling.
 Supplementary Figure 1 Real-time deformability cytometry: contour detection and theoretical modeling. (a) Image of cell deformed in constriction; contour (red) according to image analysis algorithm. Scale
Supplementary Figure 1 Real-time deformability cytometry: contour detection and theoretical modeling. (a) Image of cell deformed in constriction; contour (red) according to image analysis algorithm. Scale
SUPPLEMENTARY INFORMATION
 DOI: 0.038/ncb35 Supplementary Figure Effect of Merlin depletion on velocity correlation function and correlations lengths. (a) Representative images showing the sheet migration of MDCK monolayer at the
DOI: 0.038/ncb35 Supplementary Figure Effect of Merlin depletion on velocity correlation function and correlations lengths. (a) Representative images showing the sheet migration of MDCK monolayer at the
Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis. 3/9/17 ChE 575
 Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis 3/9/17 ChE 575 When, Where, Why do cells migrate? 1. Neutrophil Migration to Battle Infection 2. Development 3. Wound Healing 4. Disease 2 Wound
Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis 3/9/17 ChE 575 When, Where, Why do cells migrate? 1. Neutrophil Migration to Battle Infection 2. Development 3. Wound Healing 4. Disease 2 Wound
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Leaf-inspired Artificial Microvascular Networks (LIAMN) for Threedimensional
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Leaf-inspired Artificial Microvascular Networks (LIAMN) for Threedimensional
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2015 Supporting Information Leaf-inspired Artificial Microvascular Networks (LIAMN) for Threedimensional
Plutoni et al., http :// /cgi /content /full /jcb /DC1
 Supplemental material JCB Plutoni et al., http ://www.jcb.org /cgi /content /full /jcb.201505105 /DC1 THE JOU RNAL OF CELL BIO LOGY Figure S1. Cell morphology during migration. (a) Protein extracts (20
Supplemental material JCB Plutoni et al., http ://www.jcb.org /cgi /content /full /jcb.201505105 /DC1 THE JOU RNAL OF CELL BIO LOGY Figure S1. Cell morphology during migration. (a) Protein extracts (20
Cell-Extracellular Matrix Interactions
 Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
ELECTRONIC SUPPLEMENTARY INFORMATION. On-Skin Liquid Metal Inertial Sensor
 Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2017 ELECTRONIC SUPPLEMENTARY INFORMATION On-Skin Liquid Metal Inertial Sensor Matija Varga, a,b
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2017 ELECTRONIC SUPPLEMENTARY INFORMATION On-Skin Liquid Metal Inertial Sensor Matija Varga, a,b
Protein patterning on hydrogels by direct microcontact. printing: application to cardiac differentiation SUPPLEMTARY INFORMATION
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Protein patterning on hydrogels by direct microcontact printing: application to cardiac differentiation
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Protein patterning on hydrogels by direct microcontact printing: application to cardiac differentiation
CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Colorimetric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Colorimetric Format) Catalog Number CBA-060 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Colorimetric Format) Catalog Number CBA-060 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Methodology of the Microcontact Printing of Fibronectin and Blocking with Pluronic to Control Cell Adhesion
 Methodology of the Microcontact Printing of Fibronectin and Blocking with Pluronic to Control Cell Adhesion Jennifer M. Walz 1, Lidan You 2,3, Vikram Mukundan 2, Beth L. Pruitt 2, Christopher R. Jacobs
Methodology of the Microcontact Printing of Fibronectin and Blocking with Pluronic to Control Cell Adhesion Jennifer M. Walz 1, Lidan You 2,3, Vikram Mukundan 2, Beth L. Pruitt 2, Christopher R. Jacobs
SUPPLEMENTARY INFORMATION
 a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
a 14 12 Densitometry (AU) 1 8 6 4 2 t b 16 NMHC-IIA GAPDH NMHC-IIB Densitometry (AU) 14 12 1 8 6 4 2 1 nm 1 nm 1 nm 1 nm sirna 1 nm 1 nm Figure S1 S4 Quantification of protein levels. (a) The microtubule
cells (MLEC) that produce luciferase under the control of the PAI-1 promoter in response to
 Supplemental Materials and Methods TGF bioassay. To quantify the levels of active and total TGF, we used mink lung epithelial cells (MLEC) that produce luciferase under the control of the PAI-1 promoter
Supplemental Materials and Methods TGF bioassay. To quantify the levels of active and total TGF, we used mink lung epithelial cells (MLEC) that produce luciferase under the control of the PAI-1 promoter
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Monteiro et al., http://www.jcb.org/cgi/content/full/jcb.201306162/dc1 Figure S1. 3D deconvolution microscopy analysis of WASH and exocyst
µ Slide Membrane ibipore Flow
 The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
The ibidi product family is comprised of a variety of µ Slides and µ Dishes, which have all been designed for high end microscopic analysis of fixed or living cells. The high optical quality of the material
CytoSelect 96-Well Cell Migration Assay (8 µm, Fluorometric Format)
 Product Manual CytoSelect 96-Well Cell Migration Assay (8 µm, Fluorometric Format) Catalog Number CBA-106 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration
Product Manual CytoSelect 96-Well Cell Migration Assay (8 µm, Fluorometric Format) Catalog Number CBA-106 96 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration
TractionsForAll. v1.0. September 1 st, Created by Aleksandar Marinković, Sc.D. & Daniel Tschumperlin, Ph.D.
 TractionsForAll v1.0 September 1 st, 2013 TractionsForAll v1.0 is freely distributed program that calculates tractions exerted by an adherent cell on the soft hydrogel substrate underneath. It is created
TractionsForAll v1.0 September 1 st, 2013 TractionsForAll v1.0 is freely distributed program that calculates tractions exerted by an adherent cell on the soft hydrogel substrate underneath. It is created
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay. Josephine MY Ko and Maria Li Lung *
 In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
APPLICATION SPECIFIC PROTOCOL CELL MIGRATION FOR ADHERENT CELLS
 APPLICATION SPECIFIC PROTOCOL CELL MIGRATION FOR ADHERENT CELLS AIM 3D Cell Culture Chips are very useful for the study of 3D cell invasion and migration. The chips are not only suitable for endpoint measurement;
APPLICATION SPECIFIC PROTOCOL CELL MIGRATION FOR ADHERENT CELLS AIM 3D Cell Culture Chips are very useful for the study of 3D cell invasion and migration. The chips are not only suitable for endpoint measurement;
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
System. Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells. Application Note No. 4 / March
 System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
Cells Culture Techniques Marta Czernik
 Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Corning Microplates for Microscopy and High Content Imaging. Improve results with microplates for high resolution cell imaging
 Corning Microplates for Microscopy and High Content Imaging Improve results with microplates for high resolution cell imaging High Performance for Cell-based Assays Within the drug discovery process, high
Corning Microplates for Microscopy and High Content Imaging Improve results with microplates for high resolution cell imaging High Performance for Cell-based Assays Within the drug discovery process, high
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
DOI: 10.1038/ncb2271 Supplementary Figure a! WM266.4 mock WM266.4 #7 sirna WM266.4 #10 sirna SKMEL28 mock SKMEL28 #7 sirna SKMEL28 #10 sirna WM1361 mock WM1361 #7 sirna WM1361 #10 sirna 9 WM266. WM136
Supplementary Information. Quantifying cell-generated mechanical forces within living embryonic tissues
 Supplementary Information Quantifying cell-generated mechanical forces within living embryonic tissues Otger Campàs, Tadanori Mammoto, Sean Hasso, Ralph A. Sperling, Daniel O Connell, Ashley G. Bischof,
Supplementary Information Quantifying cell-generated mechanical forces within living embryonic tissues Otger Campàs, Tadanori Mammoto, Sean Hasso, Ralph A. Sperling, Daniel O Connell, Ashley G. Bischof,
SUPPLEMENTARY INFORMATION
 Nanomechanical mapping of first binding steps of a virus to animal cells David Alsteens, Richard Newton, Rajib Schubert, David Martinez-Martin, Martin Delguste, Botond Roska and Daniel J. Müller This PDF
Nanomechanical mapping of first binding steps of a virus to animal cells David Alsteens, Richard Newton, Rajib Schubert, David Martinez-Martin, Martin Delguste, Botond Roska and Daniel J. Müller This PDF
Frequently Asked Questions (FAQ)
 Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
Extreme Mechanics Letters. Techniques to stimulate and interrogate cell cell adhesion mechanics
 Extreme Mechanics Letters 20 (2018) 125 139 Contents lists available at ScienceDirect Extreme Mechanics Letters journal homepage: www.elsevier.com/locate/eml Techniques to stimulate and interrogate cell
Extreme Mechanics Letters 20 (2018) 125 139 Contents lists available at ScienceDirect Extreme Mechanics Letters journal homepage: www.elsevier.com/locate/eml Techniques to stimulate and interrogate cell
Pittsburgh Tissue Engineering Initiative Annual Progress Report: 2011 Formula Grant
 Pittsburgh Tissue Engineering Initiative Annual Progress Report: 2011 Formula Grant Reporting Period July 1, 2012 December 31, 2012 Formula Grant Overview The Pittsburgh Tissue Engineering Initiative received
Pittsburgh Tissue Engineering Initiative Annual Progress Report: 2011 Formula Grant Reporting Period July 1, 2012 December 31, 2012 Formula Grant Overview The Pittsburgh Tissue Engineering Initiative received
Monteiro, G., Sundararaghavan, H. Fernandes, A. and Shreiber, D. Rutgers, the State University of New Jersey, New Brunswick, NJ, USA
 Monteiro, G., Sundararaghavan, H. Fernandes, A. and Shreiber, D. Rutgers, the State University of New Jersey, New Brunswick, NJ, USA Introduction: Cell migration is a ubiquitous process that is of fundamental
Monteiro, G., Sundararaghavan, H. Fernandes, A. and Shreiber, D. Rutgers, the State University of New Jersey, New Brunswick, NJ, USA Introduction: Cell migration is a ubiquitous process that is of fundamental
CHAPTER 4 LANGMUIR FILMS AT THE AIR/WATER INTERFACE
 CHAPTER 4 GROWTH OF POLY(ε-CAPROLACTONE) CRYSTALS IN LANGMUIR FILMS AT THE AIR/WATER INTERFACE Reproduced with permission from: Li, B.; Wu, Y.; Liu, M.; Esker, A. R. Brewster Angle Microscopy Study of
CHAPTER 4 GROWTH OF POLY(ε-CAPROLACTONE) CRYSTALS IN LANGMUIR FILMS AT THE AIR/WATER INTERFACE Reproduced with permission from: Li, B.; Wu, Y.; Liu, M.; Esker, A. R. Brewster Angle Microscopy Study of
Supporting Information
 Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2014. Supporting Information for Adv. Funct. Mater., DOI: 10.1002/adfm.201402218 Dynamic Fluoroalkyl Polyethylene Glycol Co-Polymers:
Copyright WILEY-VCH Verlag GmbH & Co. KGaA, 69469 Weinheim, Germany, 2014. Supporting Information for Adv. Funct. Mater., DOI: 10.1002/adfm.201402218 Dynamic Fluoroalkyl Polyethylene Glycol Co-Polymers:
Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna
 Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
ANAT Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales. UNSW Copyright Notice
 ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
Promotion of HDF Cell Attachment and Proliferation
 Promotion of HDF Cell Attachment and Proliferation Objectives To qualitatively assess the effect of fibronectin (Fn) on HDF cell attachment Fn Attachment Assay To observe HDF cell proliferation and position
Promotion of HDF Cell Attachment and Proliferation Objectives To qualitatively assess the effect of fibronectin (Fn) on HDF cell attachment Fn Attachment Assay To observe HDF cell proliferation and position
Oris TM Pro 384 Cell Migration Assay Collagen I Coated
 Oris TM Pro 384 Cell Migration Assay Collagen I Coated Product No.: PRO384CMACC1 & PRO384CMACC5 384-well, 2-D Assay for Investigating Cell Migration of Adherent Cell Lines on Collagen I PROTOCOL & INSTRUCTIONS
Oris TM Pro 384 Cell Migration Assay Collagen I Coated Product No.: PRO384CMACC1 & PRO384CMACC5 384-well, 2-D Assay for Investigating Cell Migration of Adherent Cell Lines on Collagen I PROTOCOL & INSTRUCTIONS
Supporting Online Material for
 www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
www.sciencemag.org/cgi/content/full/323/5911/256/dc1 Supporting Online Material for HDAC4 Regulates Neuronal Survival in Normal and Diseased Retinas Bo Chen and Constance L. Cepko E-mail: bochen@genetics.med.harvard.edu,
Labware compatibility report. Directly order at:
 Labware compatibility report Directly order at: https://ibidi.com/12-labware Contents 1. Introduction 3 2. Compatible Labware 4 A. 35 mm dishes - high borders 4 µ-dish 35 mm, high 4 µ-dish 35mm, high Glass
Labware compatibility report Directly order at: https://ibidi.com/12-labware Contents 1. Introduction 3 2. Compatible Labware 4 A. 35 mm dishes - high borders 4 µ-dish 35 mm, high 4 µ-dish 35mm, high Glass
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rainero et al., http://www.jcb.org/cgi/content/full/jcb.201109112/dc1 Figure S1. The expression of DGK- is reduced upon transfection
Basal Cell-Extracellular Matrix Adhesion Regulates Force Transmission during Tissue Morphogenesis
 Article Basal Cell-Extracellular Matrix Adhesion Regulates Force Transmission during Tissue Morphogenesis Graphical Abstract Authors Katharine Goodwin, Stephanie J. Ellis, Emily Lostchuck, Teresa Zulueta-Coarasa,
Article Basal Cell-Extracellular Matrix Adhesion Regulates Force Transmission during Tissue Morphogenesis Graphical Abstract Authors Katharine Goodwin, Stephanie J. Ellis, Emily Lostchuck, Teresa Zulueta-Coarasa,
Epithelial Sheet Response to External Stimuli
 Old Dominion University ODU Digital Commons Mechanical & Aerospace Engineering Theses & Dissertations Mechanical & Aerospace Engineering Summer 2018 Epithelial Sheet Response to External Stimuli Yashar
Old Dominion University ODU Digital Commons Mechanical & Aerospace Engineering Theses & Dissertations Mechanical & Aerospace Engineering Summer 2018 Epithelial Sheet Response to External Stimuli Yashar
Epithelial Wound Healing Coordinates Distinct Actin Network Architectures to Conserve Mechanical Work and Balance Power
 Epithelial Wound Healing Coordinates Distinct Actin Network Architectures to Conserve Mechanical Work and Balance Power Visar Ajeti #,2,3, A. Pasha Tabatabai #,2,3, Andrew J. Fleszar +4, Michael F. Staddon
Epithelial Wound Healing Coordinates Distinct Actin Network Architectures to Conserve Mechanical Work and Balance Power Visar Ajeti #,2,3, A. Pasha Tabatabai #,2,3, Andrew J. Fleszar +4, Michael F. Staddon
Keller et al., 2008, Science. Guy Blanchard. Movie. Movie. Tomer et al., 2012 Nat. Methods. Stephen Young. Movie. Movie.
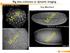 Big data solutions to dynamic imaging Keller et al., 2008, Science Guy Blanchard Stephen Young Tomer et al., 2012 Nat. Methods Zallen lab Mechanics Morphogenetic genotype Mechanics Morphogenetic phenotype
Big data solutions to dynamic imaging Keller et al., 2008, Science Guy Blanchard Stephen Young Tomer et al., 2012 Nat. Methods Zallen lab Mechanics Morphogenetic genotype Mechanics Morphogenetic phenotype
Application Note. BD PureCoat ECM Mimetic Cultureware Collagen I Peptide: Novel Synthetic, Animal-free Surface for Culture of Human Keratinocytes
 Page 1 BD PureCoat ECM Mimetic Cultureware Collagen I Peptide: Novel Synthetic, Animal-free Surface for Culture of Human Keratinocytes Kerry Thompson, Jeff Partridge, Elizabeth Abraham, Paula Flaherty,
Page 1 BD PureCoat ECM Mimetic Cultureware Collagen I Peptide: Novel Synthetic, Animal-free Surface for Culture of Human Keratinocytes Kerry Thompson, Jeff Partridge, Elizabeth Abraham, Paula Flaherty,
