Biochemical and Biophysical Research Communications 307 (2003) Cell shape provides global control of focal adhesion assembly
|
|
|
- Arron Perry
- 6 years ago
- Views:
Transcription
1 Biochemical and Biophysical Research Communications 307 (2003) BBRC Cell shape provides global control of focal adhesion assembly Christopher S. Chen, a Jose L. Alonso, b,1 Emanuele Ostuni, c,2 George M. Whitesides, c and Donald E. Ingber b, * a Departments of Biomedical Engineering and Oncology, Johns Hopkins University, Baltimore, MD 21205, USA b Departments of Surgery and Pathology (Ingber Laboratory), Vascular Biology Program, Children s Hospital, Harvard Medical School, Enders 1007, 300 Longwood Avenue, Boston, MA , USA c Department of Chemistry and Chemical Biology, Harvard University, Cambridge, MA 02138, USA Received 9 June 2003 Abstract Cell spreading was controlled independently of the amount and density of immobilized integrin ligand by culturing cells on single adhesive islands of different sizes ( lm 2 ) and shapes (squares, circles, and lines) or on many smaller (3 5 lm diameter) circular islands that were coated with a saturating density of fibronectin and separated by non-adhesive regions. The amount of focal adhesions (FAs) containing vinculin and phosphotyrosine increased in direct proportion to cell spreading under all conditions. FAs localized asymmetrically along the periphery of the small islands that experienced highest tensional stress, and FA staining increased when cytoskeletal tension was stimulated with thrombin, whereas inhibitors of contractility promoted FA disassembly. Thus, these findings demonstrate the existence of an inside-out mechanism whereby global cell distortion produces increases in cytoskeletal tension that feed back to drive local changes in FA assembly. This complex interplay between cell morphology, mechanics, and adhesion may be critical to how cells integrate from and function in living tissues. Ó 2003 Elsevier Inc. All rights reserved. Cell adhesion to extracellular matrix (ECM) plays a critical role in many cellular functions, ranging from migration and proliferation to apoptosis [1,2]. The primary subcellular structures that mediate the regulatory effects of ECM adhesion on cell behavior are the focal adhesions (FAs). These macromolecular complexes mediate cell anchorage to ECM by physically coupling integrins to the contractile actin cytoskeleton [3,4]; they also orient and concentrate numerous signaling proteins at sites of integrin binding and clustering [3,5]. Because FAs function not only as the mechanical linkages that anchor the intracellular cytoskeleton to bound integrins and the ECM, but also as biochemical signaling hubs for many regulatory pathways, regulation of their assembly and disassembly is a critical mechanism for controlling cell function [1,4,6]. * Corresponding author. Fax: address: donald.ingber@tch.harvard.edu (D.E. Ingber). 1 Current address: Drug Discovery Department, PharmaMar S. A., Avda. de los Reyes, 1, Colmenar Viejo (Madrid), Spain. 2 Current address: Surface Logix, Inc., 50 Soldiers Field Place, Brighton, MA 02135, USA. Early studies of cell adhesion on flat substrates described several distinct processes, including the initial clustering of integrins when they bind to ECM ligands, associated formation of FAs, and progressive maturation of these adhesion complexes as cells undergo active spreading and flattening. Because these processes proceed in parallel during initial attachment, it has been difficult to determine how each depends on the other. By varying the density of ECM ligand coated onto substrates, it was shown that FA formation and cell spreading both increase with increased integrin binding and clustering [7]. Studies have also suggested that cell shape depends on FA assembly, as knocking out FA proteins such as vinculin or focal adhesion kinase (FAK) results in cells that fail to spread normally [8,9]. Exposing suspended (round) cells to ECM-coated microbeads also induces FA formation within minutes [10,11], however, these structures appear to be more transient than the FAs formed on substrates. For example, ECM-coated beads only form mature, stable FAs if mechanical forces are applied to integrin-bound beads [12,13], and cells can generate such stabilizing forces at adhesions on planar X/03/$ - see front matter Ó 2003 Elsevier Inc. All rights reserved. doi: /s x(03)
2 356 C.S. Chen et al. / Biochemical and Biophysical Research Communications 307 (2003) substrates through the action of their own contractile cytoskeleton [14,15]. Interestingly, we recently found that changes in cell shape may directly alter the magnitude of contractile forces that cells can exert on their adhesions [16,17], suggesting the possibility that the global shape of the cell (i.e., the degree to which it distorts) may feed back to regulate local FA formation at the cell base through this mechanoregulatory pathway. We have previously developed a method to pattern ECM on surfaces to control cell shape while holding the ECM-coating density constant, as well as another strategy to control cell spreading independently of the total amount of ECM ligand that comes in contact with the cell [18,19]. In the present study, we use these approaches to vary endothelial cell shape and examine its effects on FA assembly. Our results show that the formation of stable FAs requires cell spreading and that this inside-out signaling is mediated by changes in mechanical forces exerted on integrin adhesion sites at the cell base. Materials and methods Cell culture. Bovine capillary endothelial (BCE) cells were cultured in standard growth media under 10% CO 2 on gelatin-coated tissue culture dishes in Dulbecco s modified Eagle s medium (DMEM) containing 10% calf serum, 2 mm glutamine, 100 lg/ml streptomycin, 100 lg/ml penicillin, and 1 ng/ml basic fibroblast growth factor (bfgf). Human endothelial cells (HMVEC) were cultured in endothelial basal medium (EBM; Clonetics) containing 10% fetal calf serum, 1 lg/ml hydrocortisone, 10 ng/ml epidermal growth factor (EGF), 10 lg/ml bovine brain extract, 50 lg/ml gentamycin, and 50 lg/ml amphotericin- B. Experiments with HMVECs were carried out in medium containing 2% serum; BCE cells were studied in defined medium containing DMEM supplemented with basic fibroblast growth factor (5 ng/ml), human high density lipoprotein (10 lg/ml), and transferrin (10 lg/ml). Microfabrication of patterned substrates. Culture substrates were patterned with FN in islands such that the regions between the islands were not adhesive to cells, as previously described [18,20,21]. Briefly, microfabricated elastomeric stamps were used to print hexadecanethiol onto gold-coated cover glass and remaining spaces were coated with tri(ethylene glycol)-terminated alkanethiol. Substrates were immersed in 50 lg/ml of FN in PBS, rinsed with PBS, and handled using standard cell culture techniques. Hexadecanethiol was purchased from Aldrich and purified by silica gel column chromatography; the tri(ethylene glycol)-terminated alkanethiol was synthesized as described previously. Immunofluorescence staining. Cultured cells were first permeabilized in a cytoskeletal stabilizing buffer (300 mm sucrose, 100 mm NaCl, 3 mm MgCl 2, 0.5% Triton X-100, and 10 mm Pipes, ph 6.8) [10], then fixed in 4% formaldehyde for 30 min, and washed in immunofluorescence buffer (IFB), containing 0.1% Triton X-100 and 0.1% BSA in PBS. The sample was incubated with primary antibody at 1 lg/ml in IFB for 1 h, washed, and incubated in fluorescent secondary antibody in IFB for 1 h and washed. Actin was stained with TRITC conjugated phalloidin (Sigma) in IFB for 1 h, and washed. Antibodies were obtained from commercial sources to recognize vinculin (Sigma), phosphotyrosine (UBI), talin (Sigma), paxillin (Sigma), FAK (Transduction Laboratories), avb3 integrin (Chemicon), and b1 integrin (Biosource). Secondary antibodies conjugated to Texas Red or FITC were obtained from Amersham and used at 1:40 dilution from stock. Quantitation of cell shape and cell-ecm contact areas. Image processing software (BDS Image, Oncor) was used to calculate projected cell areas from images grabbed from the microscope through a CCD camera. Projected cell areas were determined from interactive tracing of cell edges of phase images. ECM contact areas were calculated in square micrometers based on intersection of images of direct fluorescence staining of ECM islands with projected cell areas. Fluorescence quantitation of FA formation. The total amount of FAs formed per cell was measured using confocal microscopy to image cells stained by immunofluorescence for vinculin and phosphotyrosine. Images were taken at the plane of the cell substrate interface, and quantified by integrating the pixel intensities across the area of a cell, defined by manually tracing the cell boundary. The signals were normalized to a standard sample that was used to calibrate the laser between each sample. Results To first examine whether cell shape might affect the extent of FA formation, square islands of different sizes were created on planar glass substrates (Fig. 1A) by microcontact printing self-assembled monolayers of the protein-adhesive alkanethiol, HS(CH 2 ) 15 CH 3 in square forms; the areas between the islands were then filled in with a self-assembling, non-adhesive, and ethylene glycol-terminated alkanethiol. When the engineered substrates were incubated in a solution of FN, this ECM protein rapidly adsorbed only to the surface of the square islands and not to the surrounding regions (Fig. 1B). BCE cells plated on these substrates in defined medium containing saturating concentrations of growth factor selectively attached to the islands, and spread to take on the size and shape of the squares, ranging from 10 to 50 lm in side length (Fig. 1C). Thus, the degree of cell spreading and flattening was directly controlled by the size of the island, without varying the local density of FN in contact with cells. To examine FA formation in cells spread to different degrees, samples were fixed, immunofluorescently probed for vinculin and phosphorylated tyrosine [10], and imaged using confocal microscopy. Qualitatively, vinculin staining appeared to be most prominent along cell borders regardless of island size (Fig. 1D). However, the punctate focal adhesions typically found underneath the central region of adherent cells only became prominent in cells that were spread on squares with sides of 40 lm or larger. When the amount of vinculin staining was quantified, we found that the amount of FA formation in cells correlated with the degree of cell spreading regardless of island size (Fig. 1E). Quantitation of phosphotyrosine within FAs indicated the existence of a similar correlation between the level of tyrosine kinase signaling within FAs and the extent of cell spreading (Fig. 1E). Cells plated on larger islands were not only more spread, but they also came in direct contact with more immobilized FN due to the increased surface area covered. Thus, it remained unclear whether the increase in the total amount of FA per cell was a result of contact with more FN causing increased binding of integrins, or
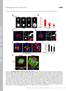
3 C.S. Chen et al. / Biochemical and Biophysical Research Communications 307 (2003) Fig. 1. Cell shape-dependent control of FA formation on micropatterned adhesive islands of different size coated with FN. (A) Diagram of an assortment of square adhesive islands with sides ranging from 10 to 50 lm in length that were micropatterned onto substrates using microcontact printing. (B) An immunofluorescence micrograph showing staining for adsorbed FN selectively limited to the square islands. (C) A differential interference contrast micrograph of BCE cells cultured on different sized, square FN islands. (D) Fluorescent confocal micrographs of individual, vinculin-labeled cells cultured on square islands of different sizes (lengths of sides are indicated). (E) Quantitation of total vinculin and total phosphotyrosine labeling per cell, for cells cultured on different sized squares. Over 30 cells per condition were averaged; error bars indicate standard error of the mean. a result of changes in cell shape and intracellular structure. To distinguish these two possibilities, cells were seeded onto closely spaced subcellular-scale islands (again separated by non-adhesive regions) such that single cells could spread across multiple islands. Using this approach to change the size and spacing of the islands, we could vary cell spreading while maintaining the total area of cell-fn contact constant (Fig. 2A). In all experiments, the local molecular ECM coating density also remained constant, and at a level that would optimally promote cell spreading on unpatterned substrates (Fig. 2A, bottom). When BCE cells were plated on substrates containing 20 lm or larger spaces between islands, they remained on single islands, whereas cells on islands spaced 10 lm or less consistently spread across multiple islands. When the island spacing was decreased further, cell spreading continued to increase. Immunofluorescence studies revealed that vinculin-containing FAs preferentially localized along the circumference of the subcellular-sized islands and coalesced to form larger, more intensely fluorescent FAs as compared to those observed within control cells cultured on unpatterned substrates (Fig. 2A, right). Furthermore, the circumferential localization of vinculin around these islands was not symmetric. Most notably, the FAs that formed on 3 lm diameter islands formed horseshoe-like structures where the brightest staining faced away from the centroid of the cell. Using this method, three island coating patterns (3, 5, and 10 lm circular islands) were chosen such that cell spreading could be varied more than ten fold, even though the cells came in contact with the same total amount of FN per cell (Fig. 2B). The amount of FAs formed in each cell under different spreading conditions was quantified using confocal microscopy to measure the total area of vinculin and phosphotyrosine staining per cell. The total FA area formed per cell increased directly as cell spreading was promoted, though the amount of ECM per cell was held constant (Fig. 2B). To confirm this finding, a second series of patterns containing parallel lines of similar widths and spacings (3 10 lm line widths and 5 20 lm spacings) was used to promote cell spreading without varying cell-ecm contact area. Again, FAs formed in proportion to the degree at which cells spread across the substrate. When all of the data for vinculin and phosphotyrosine per cell obtained from cells cultured on the different shaped islands used in this study (squares, circles, and lines) were pooled and plotted as a function of ECM-cell contact area or cell spreading area, no correlation between total ECM amount and FA area could be demonstrated (not shown). In contrast, the projected cell area correlated directly with the total area of FA formed per cell as well as the total amount of phosphotyrosine staining (Fig. 2C). The FAs that formed when cells were plated on 3 and 5 lm circles, and contained both vinculin and phosphotyrosine, appeared significantly brighter and larger than FAs formed in cells cultured on control, unpatterned substrates. To confirm that these structures were FAs, cells were stained for many other proteins found within FAs, including paxillin, talin, FAK, and integrins a5b1 and avb3. In all cases, like vinculin, these FA proteins were found in the ring-like structures oriented
4 358 C.S. Chen et al. / Biochemical and Biophysical Research Communications 307 (2003) Fig. 2. FA formation increases as cell spreading is promoted. (A) Diagrams of adhesive patterns (left), phase contrast images of cells (middle), and immunofluorescence images of vinculin in a series of micropatterned substrates where single cells can spread across multiple, small adhesive islands. The bottom row shows a cell on an unpatterned FN substrate. Scale bars indicate 10 lm in length. (B) Quantitation of total contact area of FN per cell (ECM per cell), projected cell area (cell spreading), and amount of vinculin per cell on three different micropatterned substrates containing 3, 5, or 10 lm circular islands. (C) Quantitation of total vinculin and phosphotyrosine per cell plotted against projected area of cells for cells cultured on many different micropatterned geometries, including squares, circles, lines, and unpatterned substrates. along the periphery of the FN islands (Fig. 3). Doublelabeling experiments also confirmed that the different FA proteins co-localized in similar locations (Fig. 3). Again, these ring-like structures were asymmetric and appeared to stain most intensely on the distal side of the islands (i.e., relative to the cell center). FA assembly can be modulated by the level of tensional forces that are transmitted across integrin recep- Fig. 3. Distribution of focal adhesion components in cells cultured on substrates micropatterned with small (3 and 5 lm) FN islands. Cells were immunostained for vinculin, paxillin, talin, phosphotyrosine, FAK, and integrins a5b1 and avb3. Both single (vinculin and paxillin) and double (talin vs. phosphotyrosine, integrin a5b1 vs. phosphotyrosine, and integrin avb3 vs. FAK) immunofluorescence images demonstrated localization of FA components to the periphery of these FN islands. Scale bars indicate 10 lm in length.
5 C.S. Chen et al. / Biochemical and Biophysical Research Communications 307 (2003) tors [15,22] and tractional forces exerted by the cytoskeleton are directed from the periphery towards the cell center [12,14]. Thus, one possibility for this asymmetric localization of FAs in the present study is that intracellular mechanics may alter the architecture of the adhesions: tension may be greater at the distal side of the FAs compared to its proximal side. While immunofluorescence studies revealed that neither microtubules nor intermediate filaments localized to these FAs, the distribution of actin stress fibers (as visualized by rhodaminated-phalloidin labeling) was dramatically altered by the artificial geometry of the surface (Fig. 4). Stress fibers were anchored at the periphery of islands where FAs formed and stretched from island to island across non-adhesive regions as if they mapped out tension field lines within the cytoskeleton. Confocal microscopy of FAs double stained for vinculin and F-actin confirmed that these cytoskeletal filaments inserted in a crescent only on the distal side of the 3 lm circles (Fig. 4). Thus, the site of greatest tensional stress application, as implied by the position of the actin stress fibers, correlated closely with the asymmetric distribution of FAs around these islands (Fig. 3). These results suggested that cell shape may modulate FA formation by controlling the amount of cytoskeletal tension that is exerted on integrin receptors bound to immobilized FN at the cell base. To test directly whether the assembly and asymmetric shape of these FAs were sensitive to changes in cytoskeletal tension, BCE cells spread across 5 lm diameter islands were exposed to inducers and inhibitors of actomyosin-based tension generation. Control (untreated) cells again formed FAs that were more intensely stained distally on the islands (Fig. 5A). The addition of thrombin, a potent vasoconstrictor, caused a dramatic increase in total FA staining on the islands (Fig. 5B) as more of the island became mechanically engaged, and the asymmetry of the staining pattern intensified. To examine this effect more closely, we examined this effect of cytoskeletal tension on FAs using the much larger HMVECs. Again, FA staining was restricted to the circumference in control cells (Fig. 5E) and intensified with the addition of thrombin (Fig. 5F). In these cells, the tension-dependent intensification clearly illustrates that the degree of asymmetry and circumferential localization of the FAs is driven by cytoskeletal tension. In contrast, 2,3-butanedione monoxime (BDM) and KT5926, inhibitors of myosin-activated contractility that act via distinct mechanisms, induced almost Fig. 4. Structural asymmetry within FAs on micropatterned substrates. (A) Immunofluorescence image of F-actin (red) and vinculin (green) in a BCE cell cultured on 3 lm diameter FN islands. Scale bar indicates 10 lm in length. (B D) High magnification confocal images of a single FA formed on one FN island, showing F-actin (B), vinculin (C), and combined staining (D). Scale bars indicate 3 lm in length. Fig. 5. Mechanical tension in the cytoskeleton modulates FA amount and structure. Immunofluorescence images of vinculin in BCE (A D) and HMVEC (E H) cells on 5 lm islands that were untreated (A,E) or exposed to thrombin (B,F), BDM (C,G), or KT5926 (D,H). Scale bars indicate 10 lm in length.
6 360 C.S. Chen et al. / Biochemical and Biophysical Research Communications 307 (2003) complete FA disassembly in both BCEs (Figs. 5C and D) and HMVECs (Figs. 5G and H). Interestingly, these inhibitors also reduced the asymmetry previously observed in untreated cells, although the FA staining still remained limited to the periphery of the islands. These results show that the size and position of FAs are modulated by both the level and direction of intracellular forces. Discussion By using a microprinting approach to vary the geometry of cell substrate interactions and thereby control cell shape, we have identified a previously unrecognized causal relationship between ECM, cell shape, and FA regulation. In general, it is assumed that FA assembly is controlled by clustering of integrin receptors induced by binding to immobilized ECM ligands, and that FA formation is then necessary for cell spreading. In contrast, in the present study we show that FA assembly can be varied independently of the total amounts of ECM binding, and instead, that it scales directly with cell spreading. Importantly, when cells spread to the same degree had different amount of ECM beneath them (e.g., cells on single islands vs. on multiple dots), the FA quantity per cell was not significantly different. Thus, although ECM binding is required for FA formation, neither binding to a high density of immobilized ECM ligand nor to a large amount of ECM is sufficient to promote FA assembly. Taken together, these results demonstrate that FA formation is also governed by changes in internal cytoskeletal structure and mechanics that result from large-scale changes in cell shape (i.e., cell distortion). The mechanism by which cell shape regulates FA formation was suggested by previous studies which showed that cytoskeletal tension and mechanical stress directly affect FA assembly [12,14,15,23]. However, while past studies implied that mechanical stresses applied directly to integrins modulate associated FA assembly, our results demonstrate that the global shape of cells and their internal cytoskeletal structure play an important role in constraining the magnitude, distribution, and direction of such stresses across the cell surface, and thereby control local FA assembly and organization. In fact, the asymmetric structure of the FAs on small islands correlated directly with the tension field patterns and distribution of actin filament staining. FA formation also could be increased or decreased by raising or lowering cytoskeletal tension, respectively, using pharmacological agents. Moreover, the horseshoelike distribution of FAs on the subcellular-sized, circular islands appeared to be polarized in the axis of the overlying stress fibers, and exaggerated when tension was increased. Conversely, inhibiting actomyosin tension decreased FAs significantly, and abolished the horseshoe polarity. But even in these non-contractile cells, FAs appeared as faint symmetric hollow rings, rather than be evenly distributed across the entire island, again confirming that integrin contact with ECM alone is not sufficient to explain FA formation. Taken together with recent work demonstrating that cell shape can regulate the magnitude of contractile force generated in cells [16,17], these findings suggest that mechanical tension in the cytoskeleton, rather than ECM presentation, molds the shape and distribution of FAs. Past studies have demonstrated that many fundamental biological processes, such as growth, differentiation, migration, and apoptosis, are mediated by changes in cell shape and cytoskeletal integrity [1,2]. Here, we identify the existence of a control mechanism whereby cell shape also regulates the quantity, size, and organization of FAs, and we show that this is mediated through changes in cell contractility. Because FAs contain signaling components from a large number of signaling cascades [1,24], shape-regulated FA formation could provide a molecular mechanism for how cell structure and physical distortion of cell shape can be transduced into biochemical signals inside the cell. Thus, this inside-out regulation of FA assembly may be critical in integrating many physical cues cell shape, ECM compliance, intracellular tension, and cytoskeletal architecture into the signals that drive cell function. Acknowledgments This work was made possible by funding from NIH grants CA45548 (D.E.I.) and GM30367 (G.M.W.). Microfabrication was carried out with the assistance of the Material Research Science and Engineering Center of Harvard University. We also would like to thank Bob Ezzell for his assistance with confocal microscopy. References [1] M.A. Schwartz, M.H. Ginsberg, Nat. Cell Biol. 4 (2002) E [2] N. Boudreau, M.J. Bissell, Curr. Opin. Cell Biol. 10 (1998) [3] S.K. Sastry, K. Burridge, Exp. Cell Res. 261 (2000) [4] N. Wang, J.P. Butler, D.E. Ingber, Science 260 (1993) [5] F.J. Alenghat, D.E. Ingber, Sci. STKE 2002 (2002) PE6. [6] B. Geiger, A. Bershadsky, Cell 110 (2002) [7] S.P. Massia, J.A. Hubbell, J. Cell Biol. 114 (1991) [8] R.M. Ezzell, W.H. Goldmann, N. Wang, N. Parasharama, D.E. Ingber, Exp. Cell Res. 231 (1997) [9] J.D. Owen, P.J. Ruest, D.W. Fry, S.K. Hanks, Mol. Cell. Biol. 19 (1999) [10] G. Plopper, D.E. Ingber, Biochem. Biophys. Res. Commun. 193 (1993) [11] S. Miyamoto, H. Teramoto, O.A. Coso, J.S. Gutkind, P.D. Burbelo, S.K. Akiyama, K.M. Yamada, J. Cell Biol. 131 (1995) [12] C.G. Galbraith, K.M. Yamada, M.P. Sheetz, J. Cell Biol. 159 (2002)
7 C.S. Chen et al. / Biochemical and Biophysical Research Communications 307 (2003) [13] C.J. Meyer, F.J. Alenghat, P. Rim, J.H. Fong, B. Fabry, D.E. Ingber, Nat. Cell Biol. 2 (2000) [14] N.Q. Balaban, U.S. Schwarz, D. Riveline, P. Goichberg, G. Tzur, I. Sabanay, D. Mahalu, S. Safran, A. Bershadsky, L. Addadi, B. Geiger, Nat. Cell Biol. 3 (2001) [15] D. Riveline, E. Zamir, N.Q. Balaban, U.S. Schwarz, T. Ishizaki, S. Narumiya, Z. Kam, B. Geiger, A.D. Bershadsky, J. Cell Biol. 153 (2001) [16] N. Wang, E. Ostuni, G.M. Whitesides, D.E. Ingber, Cell Motil. Cytoskel. 52 (2002) [17] J.L. Tan, J. Tien, D.M. Pirone, D.S. Gray, K. Bhadriraju, C.S. Chen, Proc. Natl. Acad. Sci. USA 100 (2003) [18] C.S. Chen, M. Mrksich, S. Huang, G.M. Whitesides, D.E. Ingber, Science 276 (1997) [19] K.K. Parker, A.L. Brock, C. Brangwynne, R.J. Mannix, N. Wang, E. Ostuni, N.A. Geisse, J.C. Adams, G.M. Whitesides, D.E. Ingber, FASEB J. 16 (2002) [20] R. Singhvi, A. Kumar, G.P. Lopez, G.N. Stephanopoulos, D.I. Wang, G.M. Whitesides, D.E. Ingber, Science 264 (1994) [21] C.S. Chen, E. Ostuni, G.M. Whitesides, D.E. Ingber, Methods Mol. Biol. 139 (2000) [22] D. Choquet, D.P. Felsenfeld, M.P. Sheetz, Cell 88 (1997) [23] M. Chrzanowska-Wodnicka, K. Burridge, J. Cell Biol. 133 (1996) [24] D.E. Ingber, J. Cell Sci. 116 (2003)
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Biochemical and Biophysical Research Communications 313 (2004)
 Biochemical and Biophysical Research Communications 313 (2004) 758 764 BBRC www.elsevier.com/locate/ybbrc Mechanical properties of individual focal adhesions probed with a magnetic microneedle Benjamin
Biochemical and Biophysical Research Communications 313 (2004) 758 764 BBRC www.elsevier.com/locate/ybbrc Mechanical properties of individual focal adhesions probed with a magnetic microneedle Benjamin
Geometric Determinants of Directional Cell Motility Revealed Using Microcontact Printing
 Langmuir 2003, 19, 1611-1617 1611 Geometric Determinants of Directional Cell Motility Revealed Using Microcontact Printing Amy Brock, Eric Chang, Chia-Chi Ho,,, Philip LeDuc, Xingyu Jiang, George M. Whitesides,
Langmuir 2003, 19, 1611-1617 1611 Geometric Determinants of Directional Cell Motility Revealed Using Microcontact Printing Amy Brock, Eric Chang, Chia-Chi Ho,,, Philip LeDuc, Xingyu Jiang, George M. Whitesides,
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization.
 Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
Micropatterning Tractional Forces in Living Cells
 Cell Motility and the Cytoskeleton 52:97 106 (2002) Micropatterning Tractional Forces in Living Cells Ning Wang, 1 * Emanuele Ostuni, 2 George M. Whitesides, 2 and Donald E. Ingber 3 1 Physiology Program,
Cell Motility and the Cytoskeleton 52:97 106 (2002) Micropatterning Tractional Forces in Living Cells Ning Wang, 1 * Emanuele Ostuni, 2 George M. Whitesides, 2 and Donald E. Ingber 3 1 Physiology Program,
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
5 HARNESSING THE PURSE STRING FOR
 107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Mechanical force plays a critical role in the interactions of
 Cells lying on a bed of microneedles: An approach to isolate mechanical force John L. Tan*, Joe Tien*, Dana M. Pirone*, Darren S. Gray*, Kiran Bhadriraju*, and Christopher S. Chen* Departments of *Biomedical
Cells lying on a bed of microneedles: An approach to isolate mechanical force John L. Tan*, Joe Tien*, Dana M. Pirone*, Darren S. Gray*, Kiran Bhadriraju*, and Christopher S. Chen* Departments of *Biomedical
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2007
 Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2007 Fibronectin isolation and fluorescent labeling Human plasma Fn was isolated from fresh human plasma (Swiss
Supplementary Material (ESI) for Lab on a Chip This journal is The Royal Society of Chemistry 2007 Fibronectin isolation and fluorescent labeling Human plasma Fn was isolated from fresh human plasma (Swiss
Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand
 SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
SUPPLEMENTAL FIGURES Beta3 integrin promotes long-lasting activation and polarization of Vascular Endothelial Growth Factor Receptor 2 by immobilized ligand C. Ravelli et al. FIGURE S. I Figure S. I: Gremlin
Promotion of HDF Cell Attachment and Proliferation
 Promotion of HDF Cell Attachment and Proliferation Objectives To qualitatively assess the effect of fibronectin (Fn) on HDF cell attachment Fn Attachment Assay To observe HDF cell proliferation and position
Promotion of HDF Cell Attachment and Proliferation Objectives To qualitatively assess the effect of fibronectin (Fn) on HDF cell attachment Fn Attachment Assay To observe HDF cell proliferation and position
Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways
 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways Grégory Giannone 1,2 and Michael. Sheetz 1 1 Department of
Review TRENDS in Cell Biology Vol.16 No.4 April 2006 Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways Grégory Giannone 1,2 and Michael. Sheetz 1 1 Department of
Control of the Direction of Lamellipodia Extension through Changes in the Balance between Rac and Rho Activities
 Copyright c 2005 Tech Science Press MCB, vol.2, no.3, pp.135-143, 2005 Control of the Direction of Lamellipodia Extension through Changes in the Balance between Rac and Rho Activities A.L. Brock and D.E.
Copyright c 2005 Tech Science Press MCB, vol.2, no.3, pp.135-143, 2005 Control of the Direction of Lamellipodia Extension through Changes in the Balance between Rac and Rho Activities A.L. Brock and D.E.
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration
 /, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
/, Supplementary Advance Publications Materials 2016 CD93 and dystroglycan cooperation in human endothelial cell adhesion and migration Supplementary Materials Supplementary Figure S1: In ECs CD93 silencing
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues
 High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
System. Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells. Application Note No. 4 / March
 System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces
 Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces KEVIN KIT PARKER, AMY LEPRE BROCK, CLIFF BRANGWYNNE, ROBERT J. MANNIX, NING WANG,* EMANUELE
Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces KEVIN KIT PARKER, AMY LEPRE BROCK, CLIFF BRANGWYNNE, ROBERT J. MANNIX, NING WANG,* EMANUELE
Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces
 Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces KEVIN KIT PARKER, AMY LEPRE BROCK, CLIFF BRANGWYNNE, ROBERT J. MANNIX, NING WANG,* EMANUELE
Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces KEVIN KIT PARKER, AMY LEPRE BROCK, CLIFF BRANGWYNNE, ROBERT J. MANNIX, NING WANG,* EMANUELE
Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands).
 Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
Methods Materials Culture media, trypsin, penicillin and streptomycin were from Invitrogen (Breda, the Netherlands). Bovine fibroblast growth factor (BFGF), thrombin, forskolin, IBMX, H-89, BAPTA-AM and
CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
The Construction of Cell-Density Controlled Three- Dimensional Tissues by Coating Micrometer-Sized Collagen. Fiber Matrices on Single Cell Surfaces
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Page S1 Electronic Supplementary Information (ESI) for RSC Advances The Construction of Cell-Density
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Page S1 Electronic Supplementary Information (ESI) for RSC Advances The Construction of Cell-Density
Radius 24-Well Cell Migration Assay (Fibronectin Coated)
 Product Manual Radius 24-Well Cell Migration Assay (Fibronectin Coated) Catalog Number CBA-125-FN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Product Manual Radius 24-Well Cell Migration Assay (Fibronectin Coated) Catalog Number CBA-125-FN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
CytoSelect 48-Well Cell Adhesion Assay (ECM Array, Fluorometric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (ECM Array, Fluorometric Format) Catalog Number CBA-071 CBA-071-5 48 assays 5 x 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures
Product Manual CytoSelect 48-Well Cell Adhesion Assay (ECM Array, Fluorometric Format) Catalog Number CBA-071 CBA-071-5 48 assays 5 x 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures
Radius 24-Well Cell Migration Assay (Laminin Coated)
 Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Product Manual Radius 24-Well Cell Migration Assay (Laminin Coated) Catalog Number CBA-125-LN 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is a highly
Frequently Asked Questions (FAQ)
 Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
CytoSelect 48- Well Cell Adhesion Assay (Laminin- Coated, Colorimetric Format)
 Product Manual CytoSelect 48- Well Cell Adhesion Assay (Laminin- Coated, Colorimetric Format) Catalog Number CBA- 056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Product Manual CytoSelect 48- Well Cell Adhesion Assay (Laminin- Coated, Colorimetric Format) Catalog Number CBA- 056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Electronic Supplementary Information
 Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Electronic Supplementary Material (ESI) for Integrative Biology. This journal is The Royal Society of Chemistry 2015 Electronic Supplementary Information Table S1. Definition of quantitative cellular features
Characterization of cytoskeleton mechanical properties and 3D-actin structure in twisted adherent epithelial cells
 Biorheology 40 (2003) 241 245 241 IOS Press Characterization of cytoskeleton mechanical properties and 3D-actin structure in twisted adherent epithelial cells Redouane Fodil, Valérie Laurent, Emmanuelle
Biorheology 40 (2003) 241 245 241 IOS Press Characterization of cytoskeleton mechanical properties and 3D-actin structure in twisted adherent epithelial cells Redouane Fodil, Valérie Laurent, Emmanuelle
CytoSelect 24- Well Cell Contraction Assay Kit (Floating Matrix Model)
 Product Manual CytoSelect 24- Well Cell Contraction Assay Kit (Floating Matrix Model) Catalog Number CBA- 5020 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing
Product Manual CytoSelect 24- Well Cell Contraction Assay Kit (Floating Matrix Model) Catalog Number CBA- 5020 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation
 The FASEB Journal Research Communication Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation Nan Xia,* Charles K. Thodeti,* Tom P. Hunt, Qiaobing
The FASEB Journal Research Communication Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation Nan Xia,* Charles K. Thodeti,* Tom P. Hunt, Qiaobing
Protein patterning on hydrogels by direct microcontact. printing: application to cardiac differentiation SUPPLEMTARY INFORMATION
 Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Protein patterning on hydrogels by direct microcontact printing: application to cardiac differentiation
Electronic Supplementary Material (ESI) for RSC Advances. This journal is The Royal Society of Chemistry 2014 Protein patterning on hydrogels by direct microcontact printing: application to cardiac differentiation
T H E J O U R N A L O F C E L L B I O L O G Y
 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
CytoSelect 48-Well Cell Adhesion Assay (Laminin-Coated, Colorimetric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Laminin-Coated, Colorimetric Format) Catalog Number CBA-056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Laminin-Coated, Colorimetric Format) Catalog Number CBA-056 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell
Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing
 Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing Authors: Correa LG, Peter R, Clerici G, Ritter V. 2017
Negatively charged microspheres provide an additional surface for cell attachment leading to proliferation, tissue regeneration and wound healing Authors: Correa LG, Peter R, Clerici G, Ritter V. 2017
Cell Contraction Assay
 Product Manual Cell Contraction Assay Catalog Number CBA-201 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing comprises of three processes: epithelialization,
Product Manual Cell Contraction Assay Catalog Number CBA-201 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wound healing comprises of three processes: epithelialization,
CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Fluorometric Format) Catalog Number CBA-061 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Cellular Biomechanics. Linda Lowe-Krentz Bioscience in the 21 st Century October 17, 2011
 Cellular Biomechanics Linda Lowe-Krentz Bioscience in the 21 st Century October 17, 2011 Outline for today Tubes Vessel mechanics and disease Models Lung mechanics Technology integration Atherosclerosis
Cellular Biomechanics Linda Lowe-Krentz Bioscience in the 21 st Century October 17, 2011 Outline for today Tubes Vessel mechanics and disease Models Lung mechanics Technology integration Atherosclerosis
Effects of morphological patterning on endothelial cell migration
 Biorheology 38 (2001) 101 108 101 IOS Press Effects of morphological patterning on endothelial cell migration Song Li, Sangeeta Bhatia, Ying-Li Hu, Yan-Ting Shiu, Yi-Shuan Li, Shunichi Usami and Shu Chien
Biorheology 38 (2001) 101 108 101 IOS Press Effects of morphological patterning on endothelial cell migration Song Li, Sangeeta Bhatia, Ying-Li Hu, Yan-Ting Shiu, Yi-Shuan Li, Shunichi Usami and Shu Chien
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
Sélection Internationale Ens Ulm 2012, Cell Biology
 Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Radius 384-Well Cell Migration Assay
 Product Manual Radius 384-Well Cell Migration Assay Catalog Number CBA-127 CBA-127-5 384 assays 5 x 384 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is
Product Manual Radius 384-Well Cell Migration Assay Catalog Number CBA-127 CBA-127-5 384 assays 5 x 384 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration is
Fast, three-dimensional super-resolution imaging of live cells
 Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
Nature Methods Fast, three-dimensional super-resolution imaging of live cells Sara A Jones, Sang-Hee Shim, Jiang He & Xiaowei Zhuang Supplementary Figure 1 Supplementary Figure 2 Supplementary Figure 3
(B) Comparable expression of major integrin subunits and glycoproteins on the surface of resting WT and Lnk -/- platelets.
 Supplemental Figure S1. Characteristics of Lnk -/- platelets. (A) Electron micrographs of resting platelets showing the normal intracellular structure of Lnk -/ platelets. Samples were fixed with 4% PFA
Supplemental Figure S1. Characteristics of Lnk -/- platelets. (A) Electron micrographs of resting platelets showing the normal intracellular structure of Lnk -/ platelets. Samples were fixed with 4% PFA
CytoSelect 24-Well Cell Migration Assay (12 µm, Colorimetric Format)
 Product Manual CytoSelect 24-Well Cell Migration Assay (12 µm, Colorimetric Format) Catalog Number CBA-107 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration
Product Manual CytoSelect 24-Well Cell Migration Assay (12 µm, Colorimetric Format) Catalog Number CBA-107 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Cell migration
WOUND HEALING ON ARTIFICIAL EXTRACELLULAR MATRIX PROTEINS
 WOUND HEALING ON ARTIFICIAL EXTRACELLULAR MATRIX PROTEINS Thesis by Eileen Fong In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy California Institute of Technology Pasadena,
WOUND HEALING ON ARTIFICIAL EXTRACELLULAR MATRIX PROTEINS Thesis by Eileen Fong In Partial Fulfillment of the Requirements For the Degree of Doctor of Philosophy California Institute of Technology Pasadena,
CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Colorimetric Format)
 Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Colorimetric Format) Catalog Number CBA-060 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Product Manual CytoSelect 48-Well Cell Adhesion Assay (Collagen IV-Coated, Colorimetric Format) Catalog Number CBA-060 48 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
This Document Contains:
 This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
This Document Contains: 1. In-Cell Western Protocol II. Cell Seeding and Stimulation Supplemental Protocol III. Complete Assay Example: Detailing the Seeding, Stimulation and Detection of the A431 Cellular
Nature Immunology: doi: /ni.1744
 Macrophage colony stimulating factor induces macrophage proliferation and survival through a pathway involving DAP12 and β-catenin Karel Otero, Isaiah R Turnbull, Pietro Luigi Poliani *, William Vermi
Macrophage colony stimulating factor induces macrophage proliferation and survival through a pathway involving DAP12 and β-catenin Karel Otero, Isaiah R Turnbull, Pietro Luigi Poliani *, William Vermi
CytoSelect 24-Well Wound Healing Assay
 Product Manual CytoSelect 24-Well Wound Healing Assay Catalog Number CBA-120 CBA-120-5 24 assays 5 x 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wounded tissue initiates
Product Manual CytoSelect 24-Well Wound Healing Assay Catalog Number CBA-120 CBA-120-5 24 assays 5 x 24 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction Wounded tissue initiates
Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering.
 Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering. The journal welcomes manuscripts on the following topics: synthetic
Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering. The journal welcomes manuscripts on the following topics: synthetic
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
CytoSelect 24-Well Cell Migration Assay (5 µm, Fluorometric Format)
 Revised Protocol Product Manual CytoSelect 24-Well Cell Migration Assay (5 µm, Fluorometric Format) Catalog Number CBA-102 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Revised Protocol Product Manual CytoSelect 24-Well Cell Migration Assay (5 µm, Fluorometric Format) Catalog Number CBA-102 12 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction
Visualizing mechanical tension across membrane receptors with a fluorescent sensor
 Nature Methods Visualizing mechanical tension across membrane receptors with a fluorescent sensor Daniel R. Stabley, Carol Jurchenko, Stephen S. Marshall, Khalid S. Salaita Supplementary Figure 1 Fabrication
Nature Methods Visualizing mechanical tension across membrane receptors with a fluorescent sensor Daniel R. Stabley, Carol Jurchenko, Stephen S. Marshall, Khalid S. Salaita Supplementary Figure 1 Fabrication
Focal adhesion kinase is involved in mechanosensing during fibroblast migration
 Focal adhesion kinase is involved in mechanosensing during fibroblast migration Hong-Bei Wang*, Micah Dembo, Steven K. Hanks, and Yu-li Wang* *Department of Physiology, University of Massachusetts Medical
Focal adhesion kinase is involved in mechanosensing during fibroblast migration Hong-Bei Wang*, Micah Dembo, Steven K. Hanks, and Yu-li Wang* *Department of Physiology, University of Massachusetts Medical
TRACTION and STRESS MICROSCOPY for CELLS:
 TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
TITLE: Development of New Approaches for Breast Cancer Therapy and Diagnosis Based on Angiogenesis
 AD AWARD NUMBER DAMD17-94-J-4283 TITLE: Development of New Approaches for Breast Cancer Therapy and Diagnosis Based on Angiogenesis PRINCIPAL INVESTIGATOR: Donald Ingber, M.D. CONTRACTING ORGANIZATION:
AD AWARD NUMBER DAMD17-94-J-4283 TITLE: Development of New Approaches for Breast Cancer Therapy and Diagnosis Based on Angiogenesis PRINCIPAL INVESTIGATOR: Donald Ingber, M.D. CONTRACTING ORGANIZATION:
Supporting Information
 Supporting Information Signal mingle: Micropatterns of BMP-2 and fibronectin on soft biopolymeric films regulate myoblast shape and SMAD signaling. Vincent Fitzpatrick, Laure Fourel, Olivier Destaing,
Supporting Information Signal mingle: Micropatterns of BMP-2 and fibronectin on soft biopolymeric films regulate myoblast shape and SMAD signaling. Vincent Fitzpatrick, Laure Fourel, Olivier Destaing,
Journal of Cell Science Supplementary Material
 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 SUPPLEMENTARY FIGURE LEGENDS Figure S1: Eps8 is localized at focal adhesions and binds directly to FAK (A) Focal
Arterial Tissue Mimics for Studying Cerebral Aneurysm Formation
 Arterial Tissue Mimics for Studying Cerebral Aneurysm Formation Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report Spring 2014 Project and
Arterial Tissue Mimics for Studying Cerebral Aneurysm Formation Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report Spring 2014 Project and
Rapid Chromatin Condensation Increases Stem Cell Nuclear Mechanics and Mechanosensitivity
 Rapid Chromatin Condensation Increases Stem Cell Nuclear Mechanics and Mechanosensitivity Su-Jin Heo, MS 1, Tristan P. Driscoll, BS 1, Stephen Thorpe, PhD 2, Woojin M. Han, MS 1, Dawn M. Elliott, PhD 3,
Rapid Chromatin Condensation Increases Stem Cell Nuclear Mechanics and Mechanosensitivity Su-Jin Heo, MS 1, Tristan P. Driscoll, BS 1, Stephen Thorpe, PhD 2, Woojin M. Han, MS 1, Dawn M. Elliott, PhD 3,
SUPPLEMENTARY INFORMATION
 Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments Brendon M. Baker 1,2,*+, Britta Trappmann 1,2,*, William Y. Wang 1, Mahmut S. Sakar
Cell-mediated fibre recruitment drives extracellular matrix mechanosensing in engineered fibrillar microenvironments Brendon M. Baker 1,2,*+, Britta Trappmann 1,2,*, William Y. Wang 1, Mahmut S. Sakar
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay. Josephine MY Ko and Maria Li Lung *
 In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
In vitro Human Umbilical Vein Endothelial Cells (HUVEC) Tube-formation Assay Josephine MY Ko and Maria Li Lung * Clinical Oncology Department, The Univerisity of Hong Kong, Hong Kong, Hong Kong SAR *For
The role of the ninth and tenth type III domains of human fibronectin in cell adhesion
 ELSEVIER FEBS Letters 340 (1994) 197-201 FEBS 13711 LETTERS The role of the ninth and tenth type III domains of human fibronectin in cell adhesion Helen J. Mardon*, Kate E. Grant Nuffield Department of
ELSEVIER FEBS Letters 340 (1994) 197-201 FEBS 13711 LETTERS The role of the ninth and tenth type III domains of human fibronectin in cell adhesion Helen J. Mardon*, Kate E. Grant Nuffield Department of
Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues
 Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues Nikolce Gjorevski and Celeste M. Nelson Department of Chemical and Biological Engineering, and
Mapping of Mechanical Strains and Stresses around Quiescent Engineered Three-Dimensional Epithelial Tissues Nikolce Gjorevski and Celeste M. Nelson Department of Chemical and Biological Engineering, and
THE BASICS OF IMMUNOHISTOCHEMISTRY
 THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
THE BASICS OF IMMUNOHISTOCHEMISTRY Introduction Immunohistochemistry (IHC) identifies specific tissue components by means of a specific antigen/antibody reaction tagged with a visible label. IHC makes
Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna
 Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
Supplemental Materials and Methods Short hairpin RNA (shrna) against MMP14. Lentiviral plasmids containing shrna (Mission shrna, Sigma) against mouse MMP14 were transfected into HEK293 cells using FuGene6
Supplementary Figure 1
 Supplementary Figure a.5 fold of increase.5 plaa plaa with LY plaa /piaa plaa /piaa with LY b 5 5 5 plaa plaa /piaa No LY with LY No LY with LY c 6X fluorescence plaa plaa with LY plaa /piaa plaa /piaa
Supplementary Figure a.5 fold of increase.5 plaa plaa with LY plaa /piaa plaa /piaa with LY b 5 5 5 plaa plaa /piaa No LY with LY No LY with LY c 6X fluorescence plaa plaa with LY plaa /piaa plaa /piaa
Cell-Extracellular Matrix Interactions
 Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Supplementary Protocol. sirna transfection methodology and performance
 Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Supplementary Protocol sirna transfection methodology and performance sirna oligonucleotides, DNA construct and cell line. Chemically synthesized 21 nt RNA duplexes were obtained from Ambion Europe, Ltd.
Exploring Protein Phosphorylation with RayBio Antibody Arrays and ELISAs
 Exploring Protein Phosphorylation with RayBio Antibody Arrays and ELISAs Valerie Sloane Jones, Ph.D.* 1, Hao Tang, Ph.D. 1, Ruo-Pan Huang, M.D., Ph.D. 1,2 1 RayBiotech, Inc., 3607 Parkway Lane, Suite 100,
Exploring Protein Phosphorylation with RayBio Antibody Arrays and ELISAs Valerie Sloane Jones, Ph.D.* 1, Hao Tang, Ph.D. 1, Ruo-Pan Huang, M.D., Ph.D. 1,2 1 RayBiotech, Inc., 3607 Parkway Lane, Suite 100,
Supplemental material JCB. Pitaval et al., Cell shape and ciliogenesis Pitaval et al.
 Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental material THE JOURNAL OF CELL BIOLOGY Pitaval et al., http://www.jcb.org/cgi/content/full/jcb.201004003/dc1 S1 Figure S1. Cell cycle exit analysis. (A) Experimental procedure. RPE1 cells were
Supplemental Data Supplementary Figure Legends and Scheme Figure S1.
 Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
Supplemental Data Supplementary Figure Legends and Scheme Figure S1. UTK1 inhibits the second EGF-induced wave of lamellipodia formation in TT cells. A and B, EGF-induced lamellipodia formation in TT cells,
Use of Phase Contrast Imaging to Track Morphological Cellular Changes due to Apoptotic Activity
 A p p l i c a t i o n N o t e Use of Phase Contrast Imaging to Track Morphological Cellular Changes due to Apoptotic Activity Brad Larson and Peter Banks, Applications Department, BioTek Instruments, Inc.,
A p p l i c a t i o n N o t e Use of Phase Contrast Imaging to Track Morphological Cellular Changes due to Apoptotic Activity Brad Larson and Peter Banks, Applications Department, BioTek Instruments, Inc.,
Determination of Superior Culture Conditions for HDF Cells BIOE /28/08
 Determination of Superior Culture Conditions for HDF Cells BIOE 342 05/28/08 1 Objective Given a range of initial culture factors, determine the most optimal condition for maximum HDF tissue culture viable
Determination of Superior Culture Conditions for HDF Cells BIOE 342 05/28/08 1 Objective Given a range of initial culture factors, determine the most optimal condition for maximum HDF tissue culture viable
Introduction. Key words: adhesion-dependent signaling cell contractility GFP vinculin myosin II Rho
 Focal Contacts as Mechanosensors: Externally Applied Local Mechanical Force Induces Growth of Focal Contacts by an mdia1-dependent and ROCK-independent Mechanism Daniel Riveline,* Eli Zamir, Nathalie Q.
Focal Contacts as Mechanosensors: Externally Applied Local Mechanical Force Induces Growth of Focal Contacts by an mdia1-dependent and ROCK-independent Mechanism Daniel Riveline,* Eli Zamir, Nathalie Q.
This paper describes a method to pattern the attachment of
 Using electroactive substrates to pattern the attachment of two different cell populations Muhammad N. Yousaf, Benjamin T. Houseman, and Milan Mrksich* Department of Chemistry and the Institute for Biophysical
Using electroactive substrates to pattern the attachment of two different cell populations Muhammad N. Yousaf, Benjamin T. Houseman, and Milan Mrksich* Department of Chemistry and the Institute for Biophysical
Supporting Information
 Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information Cell culture. C2C12 cells (ATCC CRL 1772) were cultured in 75 cm 2
Electronic Supplementary Material (ESI) for Lab on a Chip. This journal is The Royal Society of Chemistry 2014 Supporting Information Cell culture. C2C12 cells (ATCC CRL 1772) were cultured in 75 cm 2
For labelling sub-cellular organelles in tissue sections, cell cultures and cell free experiments using our proprietary Red fluorescence probe
 ab112127 CytoPainter F-actin Staining Kit - Red Fluorescence Instructions for Use For labelling sub-cellular organelles in tissue sections, cell cultures and cell free experiments using our proprietary
ab112127 CytoPainter F-actin Staining Kit - Red Fluorescence Instructions for Use For labelling sub-cellular organelles in tissue sections, cell cultures and cell free experiments using our proprietary
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells
 Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Apoptosis And Anti-tumor Effect Induced By Mtor Inhibitor And Autophagy Inhibitor In Human Osteosarcoma Cells Ryosuke Horie. Kagawa University of medecine, Kita-gun, Japan. Disclosures: R. Horie: None.
Introduction. Key words: adhesion-dependent signaling cell contractility GFP vinculin myosin II Rho
 Published Online: 4 June, 2001 Supp Info: http://doi.org/10.1083/jcb.153.6.1175 Downloaded from jcb.rupress.org on September 4, 2018 Focal Contacts as Mechanosensors: Externally Applied Local Mechanical
Published Online: 4 June, 2001 Supp Info: http://doi.org/10.1083/jcb.153.6.1175 Downloaded from jcb.rupress.org on September 4, 2018 Focal Contacts as Mechanosensors: Externally Applied Local Mechanical
Supporting Information
 Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
Supporting Information Stavru et al. 0.073/pnas.357840 SI Materials and Methods Immunofluorescence. For immunofluorescence, cells were fixed for 0 min in 4% (wt/vol) paraformaldehyde (Electron Microscopy
JCB. Supplemental material THE JOURNAL OF CELL BIOLOGY. Prospéri et al.,
 Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
Supplemental material JCB Prospéri et al., http://www.jcb.org/cgi/content/full/jcb.201501018/dc1 THE JOURNAL OF CELL BIOLOGY Figure S1. Myo1b Tail interacts with YFP-EphB2 coated beads and genistein inhibits
PARP-1 (cleaved) Human In-Cell ELISA Kit (IR)
 ab110215 PARP-1 (cleaved) Human In-Cell ELISA Kit (IR) Instructions for Use For the quantitative measurement of Human PARP-1 (cleaved) concentrations in cultured adherent and suspension cells. This product
ab110215 PARP-1 (cleaved) Human In-Cell ELISA Kit (IR) Instructions for Use For the quantitative measurement of Human PARP-1 (cleaved) concentrations in cultured adherent and suspension cells. This product
Supplemental data. Supplemental Materials and Methods
 Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
Supplemental data Supplemental Materials and Methods Transfection of plasmid. Transfection of plasmids into FRTL5 cells was performed using Lipofectamine LTX with Plus reagent (Invitrogen) according to
Please read manual carefully before starting experiment
 RayBio Cell-Based Human/Mouse/Rat EGFR (Multi-site) Phosphorylation ELISA Sampler Kit For the semi-quantitative detection of phosphorylated human, mouse, or rat EGFR (Tyr845), (Tyr992), (Tyr1068), (Activated)
RayBio Cell-Based Human/Mouse/Rat EGFR (Multi-site) Phosphorylation ELISA Sampler Kit For the semi-quantitative detection of phosphorylated human, mouse, or rat EGFR (Tyr845), (Tyr992), (Tyr1068), (Activated)
Supplementary Figures
 Supplementary Figures Supplementary Figure 1: Collagen gel fibril sizes in vitro and in vivo. (a-b) Box and whisker plots for collagen fibril/bundle diameter (A) and length (B) for HR and FB16 matrices.*
Supplementary Figures Supplementary Figure 1: Collagen gel fibril sizes in vitro and in vivo. (a-b) Box and whisker plots for collagen fibril/bundle diameter (A) and length (B) for HR and FB16 matrices.*
Introduction to N-STORM
 Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Introduction to N-STORM Dan Metcalf Advanced Imaging Manager Outline Introduction Principles of STORM Applications N-STORM overview Biological Scale Mitochondrion Microtubule Amino Acid 1Å Kinesin 1nm
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture. Catalog Number Guidelines for Use
 Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
Corning BioCoat Matrigel Matrix 6-well Plates for Embryonic Stem (ES) Cell Culture Catalog Number 354671 Guidelines for Use Discovery Labware, Inc., Two Oak Park, Bedford, MA 01730, Tel: 1.978.442.2200
Quantitative analysis of Bidirectional Signaling (qbids)
 Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
Supporting Information for. Bull s Eye Janus Particles as Artificial Antigen-Presenting Cells for T Cell Activation
 Supporting Information for Bull s Eye Janus Particles as Artificial Antigen-Presenting Cells for T Cell Activation Bo Chen, Yilong Jia, Yuan Gao, Lucero Sanchez, Stephen M. Anthony, and Yan Yu * Department
Supporting Information for Bull s Eye Janus Particles as Artificial Antigen-Presenting Cells for T Cell Activation Bo Chen, Yilong Jia, Yuan Gao, Lucero Sanchez, Stephen M. Anthony, and Yan Yu * Department
Repositioning of cells by mechanotaxis on surfaces with micropatterned Young s modulus
 Repositioning of cells by mechanotaxis on surfaces with micropatterned Young s modulus Darren S. Gray, Joe Tien, Christopher S. Chen Department of Biomedical Engineering, Johns Hopkins University School
Repositioning of cells by mechanotaxis on surfaces with micropatterned Young s modulus Darren S. Gray, Joe Tien, Christopher S. Chen Department of Biomedical Engineering, Johns Hopkins University School
Chimeric pan HLA I IgG1 was generated by fusion of the heavy and light chain variable regions from
 Materials and Methods HLA Antibodies Chimeric pan HLA I IgG1 was generated by fusion of the heavy and light chain variable regions from the murine monoclonal pan HLA class I antibody W6/32 with human constant
Materials and Methods HLA Antibodies Chimeric pan HLA I IgG1 was generated by fusion of the heavy and light chain variable regions from the murine monoclonal pan HLA class I antibody W6/32 with human constant
Brightfield and Fluorescence Imaging using 3D PrimeSurface Ultra-Low Attachment Microplates
 A p p l i c a t i o n N o t e Brightfield and Fluorescence Imaging using 3D PrimeSurface Ultra-Low Attachment Microplates Brad Larson, BioTek Instruments, Inc., Winooski, VT USA Anju Dang, S-BIO, Hudson,
A p p l i c a t i o n N o t e Brightfield and Fluorescence Imaging using 3D PrimeSurface Ultra-Low Attachment Microplates Brad Larson, BioTek Instruments, Inc., Winooski, VT USA Anju Dang, S-BIO, Hudson,
Received 23 November 2004/Returned for modification 15 February 2005/Accepted 24 May 2005
 MOLECULAR AND CELLULAR BIOLOGY, Aug. 2005, p. 6921 6936 Vol. 25, No. 16 0270-7306/05/$08.00 0 doi:10.1128/mcb.25.16.6921 6936.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
MOLECULAR AND CELLULAR BIOLOGY, Aug. 2005, p. 6921 6936 Vol. 25, No. 16 0270-7306/05/$08.00 0 doi:10.1128/mcb.25.16.6921 6936.2005 Copyright 2005, American Society for Microbiology. All Rights Reserved.
Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation 5
 Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation Application Note Authors Yoonseok Kam 1, Ned Jastromb 1, Joe Clayton, Paul Held, and Brian P. Dranka 1 1 Agilent
Normalization of Agilent Seahorse XF Data by In-situ Cell Counting Using a BioTek Cytation Application Note Authors Yoonseok Kam 1, Ned Jastromb 1, Joe Clayton, Paul Held, and Brian P. Dranka 1 1 Agilent
