Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways
|
|
|
- Kelly Chambers
- 6 years ago
- Views:
Transcription
1 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 Substrate rigidity and force define form through tyrosine phosphatase and kinase pathways Grégory Giannone 1,2 and Michael. Sheetz 1 1 Department of Biological Sciences, Columbia University, New York, NY10027, USA 2 CNRS, UMR 5091, Universite Bordeaux 2, Bordeaux, France Cell forces define cell morphology, alterations in which are caused by tyrosine kinase and phosphatase mutations, which implies a causal linkage. Recent studies have shown that phosphotyrosine signaling is involved in force sensing for cells on flat surfaces. Early force-dependent activation of Src family kinases by phosphatases or cytoskeleton stretch leads to the activation of downstream signaling. In addition, force generation by cells depends on a feedback mechanism between matrix rigidity or force generation and myosin contractility. Components of the force-sensing pathway are linked to the integrin cytoskeleton complex at sites of force application and serve as scaffolds for signaling processes. Thus, early events in force detection are mechanically induced cytoskeletal changes that result in biochemical signals to mechanoresponsive pathways that then regulate cell form. Introduction Architectural remodeling and changes in tissue tension are common in living tissues (Box 1). There are local tension changes during the addition or removal of cells, cell movements linked to morphogenesis, muscle contraction and relaxation, as well as during bone compression and decompression. Therefore, cell extracellular matrix (ECM) and cell cell contacts are subjected to force fluctuations and adjust to changes in tension. Studies demonstrate that mechanical factors affect cellular functions. At the level of cell growth and viability, normal cells require a rigid substrate or internal cytoskeletal tension for growth, whereas transformed cells have lost this requirement [1,2]. ECM rigidity and shear flow can alter migration [3,4], tyrosine kinase activities [5 7], gene expression [8,9] and cellular differentiation [10,11] in various cell types. transducers involved in mechanosensation are ion channels that convert mechanical force into an electrical or chemical signal [12]. However, the detailed mechanisms of force transduction in responses to the ECM during adhesion, migration or cell differentiation have yet to be identified. Corresponding author: Sheetz, M.. (ms2001@columbia.edu). Available online 10 March 2006 Integrins are important plasma membrane proteins that bind to the ECM, and antibodies directed against them can block cellular adhesion and migration. Integrin binding to the ECM induces the clustering and recruitment of scaffolding proteins that connect integrins to the actin cytoskeleton [13,14], activation of tyrosine kinase and phosphatase signaling, and the coupling of cell-generated forces with the ECM (reviewed in [15]). As transmembrane linkers that are involved in adhesion and motility, integrins are involved in the transduction of ECM rigidity to modifications of cellular morphology. s applied to ECM integrin cytoskeleton connections, which can be generated by internal actin or external ECM motion, induce maturation of adhesion sites to focal adhesions, which are coupled to bundles of actin called stress fibers [16,17] (Box 2). Conversely, loss of force triggers the disassembly of stress fibers and adhesion sites [16]. These observations indicate that structural and signaling functions of ECM integrin cytoskeleton molecular complexes are modified depending on the magnitude of the forces. Early evidence that forces exerted by the ECM proteins and rigidity are sensed through integrin adhesions was acquired using magnetic and optical tweezers in endothelial cells [18] and fibroblasts [14]. Beads coated with integrin ligands were twisted or restrained at the cell surface, mimicking the forces that are generated during adhesion-site formation. Local generation of forces caused cytoskeletal stiffening or the mechanical reinforcement of integrin cytoskeleton linkages, which increased in direct proportion to the applied stress. Although general changes in the cytoskeletal rigidity have been described as a result of external forces [19], adhesion site initiation and maturation occur locally where the forces are applied in fibroblasts [14,16,17,20 22] or in the direction of shear flow in endothelial cells [4]. Additional studies showed that forces affect integrin-dependent adhesion properties [23], linkage to the actin cytoskeleton [14] and recruitment and activation of signaling proteins [6]. Rigidity responses are difficult to understand because local, transient mechanical perturbations can be converted to distinct biochemical signals that have either limited or global effects on cellular functions. These temporally and spatially restricted signals depend on tyrosine kinase and/or phosphatase activities in the adhesion sites at the junction between integrins and the /$ - see front matter Q 2006 Elsevier Ltd. All rights reserved. doi: /j.tcb
2 214 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 Box 1. 2D versus 3D matrices Although most studies of cell adhesion, migration and rigidity responses have been performed in a two-dimensional (2D) environment, several studies performed in three-dimensional (3D) environments have demonstrated that adhesion sites, actin cytoskeleton structures, integrins signaling and cell morphology are altered compared with 2D [69,70]. Furthermore, the rigidity of the 2D environment, usually glass or plastic, used in most studies is higher than the rigidity of 3D matrices encountered in normal tissue [24]. Therefore, one should be careful extrapolating from 2D to 3D matrices. For example, mature focal adhesions and flat lamellipodia, which are a hallmark of cells migrating on 2D matrices, do not have an equivalent in 3D matrices [69,70]. However, some cell behavior is seen in both. Cells migrate towards a more rigid substrate [3,69,71] and this migration involves cycles of contraction and relaxation, and of extension and retraction [49,50]. cytoskeleton, that is, at the first intracellular molecular complex that will be subjected to the application of force. Local deformation can be either mechanically propagated to remote parts of the cell by the connecting cytoskeleton or converted to a global contraction or relaxation signal and therefore dictate cell shape. However, recent studies have found that confined rigidity response events that are dependent on phosphotyrosine signaling control locally and temporally the formation, stabilization and disassembly of adhesion sites and associated cytoskeletal structures. Consequently, we suggest that the integration of local changes in contractile state and motility behavior results in major effects on cell morphology. Alteration of tyrosine kinase activity affects rigiditydependent growth, migration and cell morphology Early observations linked transformation to uncontrolled cellular growth and to profound alterations in cell shape and migration. Transformation is often characterized by deregulation of tyrosine kinase and phosphatase activity. The first defined oncogene, vsrc, encodes an early recognized tyrosine kinase. In most studies on tumor cells, changes in morphology, but not cytoskeletal dynamics, have been reported. However, changes in environmental factors (i.e. changes in ECM rigidity) and internal force generation (i.e. inappropriate rigidity responses) might be key factors in determining transformed cell morphology and the malignant phenotype [24] (Box 3). Certain transformed cells in culture have an altered morphology (they are typically rounder) and are more refractive than are normal cells (Figure 1a). In transformed cells, the macromolecular structures that are responsible for cell morphology and migration are affected. Focal adhesions can be replaced by podosomes and stress fibers can be absent [25,26] (Figure 1b,c). Transformed cells acquire anchorage independence, that is, they can grow without attachment to a substrate, suggesting rigidity response deregulation [1,2]. For example, transformed cells generate weak, poorly coordinated traction forces [27]. However, transformation is also associated with increased contractility (Box 3). Cell motility depends on substrate density and rigidity and, therefore, also on the processes that respond to rigidity (Box 4) [28]. Many of the proteins involved in the rigidity response have been linked to motility disorders, including cancer, malformations in development and neuronal connectivity. Src family kinases (SFKs) [29,30], focal adhesion kinase (FAK) [31,32], the SH2 domaincontaining phosphatase SH-2 [32] and receptor-like protein tyrosine phosphatases (RTs) [22] are important components of the force-dependent signal transduction pathways that lead to the assembly of adhesion sites. The force-dependent initiation of adhesion sites, named reinforcement, occurs in protruding portions of cells, where adhesion sites can transmit cell propulsive forces [20,27]. In extending regions of the cell, forces are generated on integrins by actin rearward flow rather than stress fibers. At the trailing end of the cell, mature focal adhesions create passive resistance during cell migration. To overcome this resistance, high forces must be generated by nascent adhesion sites [20]. However, in some static cells, higher forces are correlated with mature Box 2. From nascent ECM integrin cytoskeleton connections to fibrillar adhesion sites Recruitment of integrin-associated proteins to nascent adhesion sites is hierarchical. Integrin aggregation or occupancy can be controlled using micrometer-sized beads coated with ECM proteins or antibodies, possibly in combination with soluble integrin ligand [13,14]. s can be applied to these coated-beads by optical or magnetic tweezers. Binding of specific cytoplasmic proteins to integrin adhesions can be triggered by integrin aggregation, a combination of aggregation and matrix ligand binding, and aggregation and/or occupancy in addition to force generation [13,14,21]. After integrins interact with the clustered ECM, scaffolding proteins connect integrins to the actin cytoskeleton in motion. Nascent ECM integrin cytoskeleton connections develop to focal complexes, which are defined as early adhesion sites and are localized at the cell periphery [15]. generated by myosin II is responsible for the maturation of focal complexes to focal adhesions [17,46], which require sustained forces for their stabilization [16] and are connected to stress fibers. Further maturation leads to the transition from mature focal adhesions to fibrillar adhesion sites [62]. The nomenclature of adhesion sites is mainly derived from studies of Rho GTases [72]. Excessive Rac activity is associated with the presence of focal complexes and the absence of stress fibers. However, elevated Rho activity is characterized by the presence of focal adhesions linked to stress fibers [72]. Antagonistic activities between Rac and Rho are responsible for the transition from focal complexes to focal adhesions [72,73]. It is not clear whether transition from nascent ECM integrin cytoskeleton connections to focal complexes requires the generation of forces on those linkages. Focal complexes are less tension dependent than focal adhesions and accumulate along the cell edge following treatment with inhibitors of actomyosin contractility [42]. s applied to these nascent ECM integrin cytoskeleton connections induce strengthening of the integrin cytoskeleton interactions [14], a phenomenon that might mimic focal complex initiation and stabilization [21]. Focal complexes are dissociated by inhibitors of myosin II-dependent contractility, but not by an inhibitor of Rho kinase [73]. Accumulation of adhesion site proteins, paxillin and vinculin, around ECM-coated beads is dependent on Rac but not Rho activity and is inhibited by a myosin light chain kinase inhibitor, indicating that physical forces are involved in the initiation of focal complexes [21].
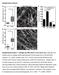
3 Review TRENDS in Cell Biology Vol.16 No.4 April Box 3. Transformation: increase or decrease contractility? Transformed cells have an altered rigidity homeostasis. It is not yet clear whether transformation is associated with enhanced contractility or impaired contractility. The current model is that contractility is impaired in malignant cells because malignant cells are associated with migration, which is enhanced when focal adhesions and stress fibers are destabilized. Transformed cells are less able to sense and respond to different surface rigidities compared with normal cells [2] and are less efficient at generating forces [27]. However, other studies demonstrate that rigid tumors are formed by transformed cells and uncontrolled growth is promoted by increased contractility [24]. Nevertheless, both unregulated growth and migration, which characterize transformed cells, could be explained by a decoupling of the cell response to the rigidity of its environment. focal adhesions [16]. At the cell rear, traction stresses induce the disassembly instead of the reinforcement of focal adhesions and linked stress fibers; this is dependent on mechanosensitive ion channels and calcium signaling in keratocytes and astrocytoma cells [33 35]. SFKs, FAK and EST domain-enriched tyrosine phosphatase (T- EST) are also crucial factors in adhesion site disassembly [26,29,34,36]. This suggests that different modalities of force generation and rigidity response at the cell front and rear correlate with position-dependent regulation of phosphotyrosine signaling, and that different mechanisms of rigidity responses based on phosphotyrosine signaling can independently direct cell morphology. At the subcellular level, the actin cytoskeleton appears to be the major site for force transduction. Cytoskeletal stretching correlates with the recruitment of adhesioncomplex proteins, especially tyrosine kinases and their substrates, both in intact [17,21] and triton-treated fibroblasts, which are devoid of membrane [37,38]. Figure 1. Cell transformation: a tight link between change in morphology and tyrosine kinase activity. (a) The distinct morphology of normal (left) and transformed fibroblasts (right). (b) Fluorescent staining of the actin cytoskeleton showing its disorganization in transformed (right) compared with normal (left) fibroblasts. (c) Fluorescent staining of adhesion sites illustrates the replacement of mature focal adhesions in normal fibroblasts (left) by podosomes in transformed fibroblasts (right); green fluorescence corresponds to paxillin GF. Adapted, with permission, from [77] (a) and [26] (b). Furthermore, cytoskeleton stretching triggers specific biochemical signals, particularly tyrosine kinase and phosphatase pathways [38]. Thus, mechanical alterations of cytoskeletal proteins probably underlie rigidity sensing. All of these observations point to tight links between morphology, migration, rigidity responses and tyrosine kinase activity. sensor elements at adhesion site cytoskeleton junctions The rigidity of an object is defined by the amount of force that is required for a given deformation. Therefore, rigidity responses require an element that will be deformed by the force (force sensor) and an element that will convert this into a biochemical signal (signal generator) (Figure 2a). Cells bind to specific ECM molecules through specific integrins and forces are focused on these sites by macromolecular complexes that connect the integrin cytoplasmic domains to the actin cytoskeleton (reviewed in [15]). For complexes to sense rigidity, they must contain proteins that: (i) mechanically link ECM ligand integrins and actin; and (ii) signal to the rest of the cell in response to mechanical stimuli while bound to actin and/or integrin (including tyrosine signaling) (Table 1). The mechanical link is a force sensor that can be deformed by changes in force that are generated by the ECM and the cell cytoskeleton; these changes are then translated into a biochemical signal by a signal generator. Therefore, the force sensor and signal generator should be co-localized at integrin cytoskeleton junctions. Although components of the cytoskeleton are also involved in rigidity responses, the most extensively studied rigidity response mechanisms involve elements at adhesion sites. s applied to integrin ECM bonds only cause a deformation in the intracellular and ECM elements when the ECM is connected to a rigid support. Cellular force moves the ECM in proportion to its rigidity as well as modifying the force-sensitive elements in the integrin cytoskeleton linkage (Figure 2a). The ECM is modified by force (reviewed in [39]). Thus, forces generated from outside and inside the cell will lead to deformation of forcesensitive linkages, which are probably localized at the intracellular side of adhesion sites. Application of external forces using fluid flow [4], ECM stretching [31,37], laser and magnetic tweezers [14,18] and pipette deformation [17] lead to the initiation and expansion of adhesion cytoskeleton complexes. The site of the greatest response correlates with the site of greatest applied force, indicating that the rigidity response process is locally activated by force. This might explain why cells move towards a more rigid substrate, which induces
4 216 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 Box 4. Definition of mechanotransduction and rigidity response pathways The broad definition of mechanotransduction is the conversion of changes in mechanical factors into changes in protein activity. s applied to a protein can cause conformational modifications ranging from distortion to domain unfolding. Work done by the force could lower or increase the energy barrier between the bound and free states, for slip bonds [52] or catch bonds [74], respectively. Deformation induced by force could mask or expose binding sites [39,75]. In addition, the application of force to an enzyme could change its activity [6,76] or the opening behavior of an ionic channel [12]. Rigidity response is a case of mechanotransduction in which the variations in the compliance of the ECM are converted into changes in protein activity. Greater rigidity results in less integrin movement for a given cell-generated force. Thus, not only are the forces and their site of application crucial, but the time and/or position-dependent changes in force are also important for cellular function. Therefore, here we have focused on mechanisms of force transduction, which can be time and position dependent. greater formation of adhesion sites and force generation [3,31]. The assembly of components at adhesion sites has been linked to early phosphotyrosine signals. dependent activation of SFKs by phosphatases [22] or by cytoskeleton stretch [38,40] is an upstream event in the signaling pathways that leads to activation of small G proteins [38] and MA kinases [41]. Small GTases can be activated by force [9] (and in the case of Rap1 downstream of the tyrosine kinases) through the activation of guanine exchange factors (GEFs) at appropriate sites [38]. Thus, we suggest that tyrosine kinases and phosphatases are directly involved in the force sensing mechanism. Rapid activation of the SFKs occurs in response to local forces at the cell edge [6]. In all of these cases, the details of the (a) (b) (i) Tyr TyrK Integrin actin linkage Integrins Fibronectin (ii) Tyr TyrK (iii) Tyr TyrK (iv) TRENDS in Cell Biology Figure 2. Rigidity detection and phosphotyrosine signaling. (a) The basic elements of the rigidity response machinery can be represented by a force sensor element (green spring) and a signal generator element (blue shapes). Extracellularly, the interaction between the transmembrane integrins (yellow) and proteins of the ECM (fibronectin, black triangle) provide one anchor point. The black spring represents the rigidity of the ECM. The green spring represents the actin-associated proteins that are regrouped into an integrin cytoskeleton complex (composedof scaffolding, signalingand structural proteins), which couplesthe actin filaments to the adhesion protein. Intracellularly, the actin filaments are pulled away by motors (generally myosin), generating forces on the integrin cytoskeleton complex and therefore on the ECM (red arrow). The signal generator (blue triangles), which is not directly experiencing force, can bind to a deformed force-sensor component (green spring) of the integrin cytoskeleton complex and be activated or inhibited (left). Or, the signal generator (H, inactivated; A, activated) might be incorporated into the force-sensor element and be directly activated or inhibited by force generation (right). (b) Stretching of the integrin cytoskeleton complex is the primary transducer of force into biochemical changes that involve changes in phosphotyrosine signaling (tyrosine phosphorylation and dephosphorylation). (i) Without force, the signal-generator element (blue shapes) is inactive. (ii) Some structural and/or scaffolding proteins (brown triangle) connect integrins and the cytoskeleton through phosphotyrosine-independent processes that enable the accumulation of force. generated by the cytoskeleton activates tyrosine kinases and/or phosphatases (transition of the blue H to the blue A). Balance of phosphotyrosine signaling (double arrows) defines the level of substrate phosphorylation. Substrates can also be tyrosine kinases and/or phosphatases. Structural (green solid) and signaling (blue solid) proteins, which are substrates of phosphotyrosine signaling, are either cytosolic or part of the force-sensor elements. (iii) The binding of structural and signaling proteins to phosphotyrosine and/or phosphotyrosine signaling induces changes in protein binding and/or catalytic activity. (iv) Binding and/or changes in activity induce modification of the strength of integrin cytoskeleton linkage (shown by thickening of the green spring) and downstream signaling (blue arrow).
5 Review TRENDS in Cell Biology Vol.16 No.4 April Table 1. Tyrosine kinases and phosphatases and their function in rigidity sensing rotein Function Target in rigidity response Localization Rigidity response FAK Tyr K, IB a-actinin? AS Y SS, SF RT-a Tyr, IB Src, Fyn activation SH-2 Tyr FAK inhibition Src Tyr K axillin? Vinculin? Assay Reinforcement Cytoskeleton stretch Y inhibition? Recruitment Assembly Disassembly Effects on AS Cell type Refs Fibroblast Astrocytoma Endothelial AS?, CM Y OT Y activation Assembly Fibroblast [22] [31,32,37]- [34] [4] AS Y OT Y activation Assembly Fibroblast [32,47] AS Y FRET, OT, SS Y inhibition Recruitment Disassembly Fibroblast Endothelial [21,22,29, 30,37] [6,26] Fyn Tyr K axillin? AS Y OT Y activation Assembly Fibroblast [22] Vinculin? T- Tyr Disassembly Fibroblast [36] EST ECAM-1 Scaffolding Cadherin Flow SF Assembly Endothelial [7] Tyr Signal VEGFR2 sensing Integrin MT p130cas Scaffolding AS, CM Y SS Y? Recruitment Fibroblast [38] Tyr Signal axillin Scaffolding AS (early) Y OT, Y recruitment Recruitment Fibroblast [17,22,37] SS, S Vinculin AB AS (early) Y? OT, SS, Y? recruitment No effect Fibroblast [16,17,21,- 22,37] S Zyxin AS (late) Fibroblast [20] a-actinin AB, IB AS (late) Y OT Y recruitment Fibroblast [32] Tensin AB, IB AS Fibroblast [62] Talin-1 AB, IB, scaffolding axillin, Vinculin, Integrin AS (early) Y OT Y recruitment Fibroblast [52,53] Filamin AB, IB AS, CM Y OT N Fibroblast [53] Integrin a v b 3 Adhesion protein RTa, Talin-1 AS, CM Y OT, SF Y Fibroblast Endothelial [22,52,53] [7,9,54] AB: actin-binding protein; AS: adhesion site; CM: cell margin; IB: integrin-binding protein; MT: magnetic tweezers; N, No; OT: optical tweezers; S: pipette stretch; SF: shear flow; SS: substrate stretch; Tyr K: tyrosine kinase; Tyr : tyrosine phosphatase; Y, Yes. A question mark indicates that the hypothesis is not yet confirmed. assembly by force and disassembly by relaxation have yet to be clarified but there is clearly a mechanical regulation of kinase and phosphatase activities. sensing has been linked to ion channel opening and cytoskeleton stretch. Although there is strong evidence for ionic movements in some force responses [12,33,35], a completely cytoskeleton-dependent mechanism has been observed in several cases [37]. Thus, the force sensor might be incorporated into the cytoskeleton (i.e. as a force-sensitive element). For example, forces applied to the cytoskeleton can cause protein deformation (unfolding or distortion) and induce a direct change in protein activity (indicating that the signal generator directly senses force). Alternatively, the signal generator could bind to a deformed component or elastic element in the cytoskeleton (the force sensor) and be activated or inhibited (indicating that the signal generator senses force indirectly) (Figure 2a) (Box 4). In the case of the rigidity response mechanism, no definitive force sensor or signal generator has been identified; however, a tyrosine-kinase dependent phosphorylation of p130cas has been found in stretched cytoskeletons [38]. Strikingly, adhesion sites under tension [42] and the lamellipodium [43], which are structures that are involved in rigidity responses at the cell edge, contain proteins that have high concentrations of phosphotyrosine. Thus, we favor cytoskeleton stretch rather than ion movements as the primary transducer of force from the environment into biochemical changes that involve changes in tyrosine phosphorylation levels by substrate, kinase and/or phosphatase modification (Figure 2b). Spatio-temporal aspects of force sensing A migrating cell on a soft substrate that encounters a border with a rigid substrate will cross towards the rigid substrate [3]. This process, known as durotaxis, indicates that rigidity responses are based on localized and dynamic processes, which can locally and temporally change cell contractility, motility and shape [31]. In these dynamic processes, adhesion sites and the actin cytoskeleton are assembled, stabilized, moved and disassembled on a time frame of seconds to minutes [44,45]. In addition, the molecular components of these complexes are rapidly exchanging on a timescale of seconds, even if the macromolecular structures are relatively immobile in space. Cycles of tyrosine phosphorylation and dephosphorylation are implied in both of these dynamic processes. Adhesion-site dynamics Treatments that increase or decrease tyrosine phosphorylation promote enhancement or inhibition of adhesion-site formation, respectively [46]. For example, the non-specific tyrosine phosphatase inhibitor AO induces the formation
6 218 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 (a) SH-2 FAK RTα SFKs (c) Fibronectin (b) FAK SH-2 N α-actinin Talin-1 Integrins RT-α C Fyn c-src (d) FAK N c-src C axillin Vinculin Fyn RT-α FAK SH-2 N C c-src Fyn N C Rap1 p38 Crk/C3G TRENDS in Cell Biology Figure 3. Two particular cases of rigidity responses involving phosphotyrosine signaling, SH-2 and FAK (left) and RT-a and SFKs (right). The left side illustrates the reinforcement of integrin cytoskeleton linkage after the force-dependent activation of SH-2. (a) Without force, active FAK phosphorylates a-actinin (green triangle) decreasing its interaction with the integrin cytoskeleton complex. (b) induces activation of SH-2, which dephosphorylates and inhibits FAK. (c) FAK inhibition decreases a-actinin tyrosine phosphorylation, increasing its interaction with the integrin cytoskeleton complex. (d) Increased association of a-actinin induces reinforcement of the integrin cytoskeleton complex. The right side illustrates the reinforcement of integrin cytoskeleton linkage after the force-dependent activation of the tyrosine phosphatase, RT-a, and the phosphotyrosine signaling cascade activated by SFKs after cytoskeleton stretch. (a) Without force, RT-a and SFKs are inactive. Integrins are linked to the actin cytoskeleton through talin-1. (b) induces RT-a activation, which dephosphorylates and activates SFKs. (c) SFKs activity induces association (through the activity of Fyn) or dissociation (through the activity of c-src) of structural proteins (green triangle) to the integrin cytoskeleton complex (through scaffolding proteins, e.g. talin-1). The activity of SFKs in stretched cytoskeleton induces tyrosine phosphorylation of integrin cytoskeleton complex proteins (e.g. p-130cas). (d) Recruitment of structural proteins (such as paxillin and vinculin) correlates with reinforcement of the integrin cytoskeleton complex. Recruitment of signaling proteins (such as Crk and C3G) on phosphotyrosine site (perhaps on p-130cas) induces activation of downstream signaling cascades. of stable adhesion sites on soft substrates, where such sites are normally irregularly shaped and highly dynamic, inducing an inappropriate response to rigidity [5]. However, FAK-deficient fibroblasts exhibit a reduced rate of cell motility that is associated with long-lasting adhesion sites, suggesting that FAK-mediated tyrosine phosphorylation events are involved in adhesion-site turnover. SH-2 and T-EST maintain FAK dephosphorylation and inactivation but they are involved in maturation and disassembly of adhesion sites, respectively [36,47]. Furthermore, tyrosine phosphatases are involved in the reinforcement of integrin cytoskeleton linkages [14] and the membrane tyrosine phosphatase RTa, which is required to dephosphorylate the inhibitory phosphotyrosine residue of SFKs, is involved in the activation of Fyn that is required for early reinforcement [22] (Figure 3). To reconcile these observations, it might be necessary to view the phosphotyrosine signaling of integrin-associated proteins within the context of a dynamic process that involves repeated cycles of phosphorylation and dephosphorylation of specific substrates at defined times and subcellular locations in the life cycle of an adhesion site. For example, tyrosine dephosphorylation of FAK by SH- 2 induces the stabilization and maturation of the early focal complexes to focal adhesions [32] (Figure 3), although the formation of paxillin-positive immature adhesion sites is not affected by SH-2. Fyn and c-src have opposite effects in the force-dependent reinforcement of integrin cytoskeleton linkages. Similar to FAK, c-src is involved in the turnover of adhesion sites [26] and, accordingly, force-dependent reinforcement is increased by the loss of c-src [21,29], whereas loss of the closely related kinase Fyn inhibits reinforcement [22]. We suggest that time- and positiondependent processes account for the specificity of SFKs in reinforcement and rigidity responses (Figure 4). For example, Fyn and c-src could have a different threshold of force-dependent activation, leading to a temporal shift in their activity. Alternatively, Fyn activity could be higher close to the cell edge, whereas c-src activity could be predominant further back, leading to a spatial difference in the activities of these proteins. Most components of the integrin cytoskeleton complex have fast exchange rates with half-lives ranging from a few seconds to a few minutes [32,34,48]. Only integrins have molecular dynamics sufficiently slow to support the half-life of adhesion sites [44]. Of particular interest, tyrosine dephosphorylation of FAK by SH-2 controls FAK-dependent tyrosine phosphorylation of a-actinin and modulates the association of a-actinin with the integrin cytoskeleton complex. A SH-2-dependent decrease in FAK activity increases the association of a-actinin and force-dependent strengthening of integrin cytoskeleton linkages [32] (Figure 3). At the cellular level, this stable association enables the maturation of focal complexes. Therefore, adhesion-site molecular dynamics are regulated during adhesion formation. The disassembly of focal adhesions is also regulated by the residency time of FAK
7 Review TRENDS in Cell Biology Vol.16 No.4 April Rigid substrate Soft substrate (d) (a) 30 s x=0 t=0 2 µm Stiff (b) x1 >x1 Intermediary x2 >x2 (c) Soft t1 t1 t2 t2 t3 t3 TRENDS in Cell Biology Figure 4. Spatial and temporal aspect of rigidity responses. ossible mechanisms are shown for the transduction of motile and force-dependent events that are important in defining cell morphology and are restricted in space and time. (a) On a rigid substrate (black spring, left) force rises after a small displacement (grey shading represents displacement) and time period, leading to the initiation and maturation of adhesion sites. The reinforcement of the integrin cytoskeleton linkage is represented by thickening of the green spring. However, on a soft substrate (right), a larger displacement and time period are required to produce the same force. This inappropriate force generation prevents the formation of adhesion sites because dissociation of integrin cytoskeleton linkage (known as a slip bond) occurs before stabilization of the connection. (b) osition-dependent rigidity responses. For a rigid substrate, the position of the unfolded or distorted force-sensor element (green spring) is co-localized with the signal generator (blue triangles), which leads to rigidity responses. The conformation of the force-sensor element after unfolding and/or distortion corresponds to the conformation that binds to the signal generator. For a soft substrate, a larger displacement of the ECM is required to attain the same force-sensor distortion (force) so the force sensor will not be co-localized with the signal generator and no rigidity responses will occur. (x1 and x2 refer to the position of the force-sensor element) (c) Time-dependent rigidity responses. The signal generator (blue triangles) is activated for a limited amount of time (shown in t2, which refers to the time when the signal generator is active). For a rigid substrate, the time necessary to unfold or deform the force-sensor element (green spring) corresponds to this activation window leading to rigidity responses. For a soft substrate, because a longer time is required to attain the same force-sensor distortion (and therefore the same force), the signal generator will be no longer activated and no rigidity response will occur. (d) Motile cells are characterized by the generation of periodic contractions (period of 25 s here). The three panels are kymographs that are used to visualize movements (y axis) of the cell edge (black) as function of time (x axis). The arrows indicate the direction of cell edge protrusion. These kymographs are generated from cells spreading on polyacrylamide gels of different rigidity. This phenomenon of periodic contractions occurs mostly on rigid substrates (top panel) and only poorly on intermediate to soft substrates (middle and lower panels). This process illustrates important characteristics of the rigidity responses mechanism. It is a positive feedback mechanism between forces that are generated by the cell and substrate rigidity. Initiation and sustained cycling requires myosin II activity and a rigid substrate. It is a timedependent process, every 25 s the cell is probing the substrate rigidity by a contraction of the cell edge, and is position dependent, the contraction and probing occur on a limited portion of the cell edge. Scale bars: verticalz2 mm; horizontalz30 s. Adapted, with permission, from [49]. at focal adhesions. rolonged association of FAK within focal adhesions is correlated with increased FAK activity and focal adhesions disassembly [34]. Rigidity responses involve force sensing and distance or time sensing The biological rigidity-sensing mechanism must have means of transforming the force and displacement (i.e. distance and time information) into a biochemical signal (Figure 4b,c). Cells display complex patterns of successive extensions and retractions that seem to be part of an active rigidity-response mechanism. In one case, the cell periodically probes the rigidity of its surroundings during spreading and migration [49] (Figure 4d). Also, the forces that are applied on a collagen filament are oscillating [50], implying that a rigidity response might involve a threshold force that is reached after a defined distance and during a limited time. From the study of rigidity responses using laser tweezers during fibroblast migration, the movements involved are calculated to be over distances of w100 nm and durations of w1 s [51]. Consequently, time and distance are plausible parameters
8 220 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 to be sensed in the rigidity response process. Changing the ECM rigidity affects these probing cycles (on softer substrates, none or fewer periodic probing events have been observed) suggesting that a feedback mechanism exists between ECM rigidity and the cellular architecture, and motile activities that are involved in the probing mechanism (Figure 4d). More studies are required to define the exact mechanism of force and displacement sensing involved in detecting rigidity and whether external forces can regularly activate the same signaling pathways. osition dependence and rigidity responses The position dependence of rigidity responses (Figure 4b) is exemplified by the fact that structural and signaling proteins that are necessary for rigidity responses are placed at strategic locations, for example, at the cell edge during protrusive events and at early adhesion sites. Many proteins involved in rigidity responses and/or phosphotyrosine signaling, including talin [52,53], integrins (avb3) [52,54], paxillin [22], a-actinin [32,49], RTa [22], Rap 1 [55] and p130cas [56], are localized at the leading edge of the cell, ready to respond to any contraction generated by the cell or by the ECM. There is a position-dependent binding-and-release cycle of fibronectin integrin cytoskeleton interactions, with preferential binding occurring at active edges of motile fibroblasts and release at mm back from the edge [57]. Interestingly, this position-dependent binding correlates with the efficiency of the reinforcement process in the rigidity response [51]. s generated at single ECM integrin cytoskeleton connections can lead to breaking or reinforcement of the integrin cytoskeleton linkage [51,52] (Figure 4a). With rigid optical tweezers holding fibronectin beads, contact reinforcement is favored over breaking of bead cytoskeleton contacts at the cell edge but not at 2 mm back, whereas no differences are observed using soft optical tweezers [51]. At the molecular level, the reinforcement of integrin cytoskeleton interactions are limited to linkages that have experienced force and not those nearby (!1 mm) [14]. s exerted on a macromolecular complex might affect the specific distances required to assemble that complex. The density and clustering of integrin ligand RGD affects cell adhesion [58], the establishment of integrin cytoskeleton linkages [52] and the formation of focal adhesions and stress fibers [59]. These processes might be dependent on a crucial minimum distance between occupied integrins. In agreement, the distance between RGD peptide sites must be!70 nm for the formation of stress fibers and focal adhesions [58,59]. This might be the length of the molecule that bridges and clusters integrins during the formation of integrin cytoskeleton complexes [52]. These data indicate that the distances between occupied integrins within an adhesion site can act as a rigidity response that responds to tension-dependent changes in integrin density [44]. At the cellular level, the periodic contractions of the lamellipodium involved in ECM probing are confined to a segment of the cell edge and involve local activation of a contractile signal that directs cell probing [49]. Therefore, at the cellular and molecular levels, rigidity response is a localized phenomenon. Time dependence and rigidity responses The time dependence of rigidity responses (Figure 4c) might involve the precise order in which components of the integrin cytoskeleton complex bind and detach during the life cycle of an adhesion site [45]. The formation of the elementary connection between integrin and the cytoskeleton, and its reinforcement, depend on talin, which is probably one of the first proteins that enter adhesion sites [52,53]. a-actinin and zyxin enter the adhesion site during its maturation [60,61]. The transition from mature focal adhesion to fibrillar adhesion is characterized by the segregation of tensin and specific integrins [62]. Because the ECM integrin cytoskeleton connection is a viscoelastic material (i.e. it is not purely elastic) [63], the time required to reach the threshold force for rigidity responses probably differs depending on the stiffness of the ECM. Accordingly, a soft optical trap could mimic the effects of a rigid trap on the stabilization of the integrin cytoskeleton linkages if externally applied forces rise rapidly [51]. In lamellipodia, the cytoskeletal-dependent radial transport of a contractile signal directs the timing of contraction and, probably, adhesion site initiation to stabilize protrusive events [49]. Consequently, formation of cell contacts with the ECM is not a continuous process, but involves cycles of contraction and relaxation. Furthermore, in a tissue, contractile activity and external forces should also produce a rigidity response. The limited time of contraction, during which the cell locally probes the ECM composition and physical properties, might reduce the time window for the activation of a rigidity response. Steady-state morphology of cells Cells in suspension usually adopt a spherical shape. After they contact a surface with suitable chemical (ECM proteins) and physical (rigidity) properties, cells spread and migrate through a series of motile events. Detailed analyses of cell movements on short timescales show that they move discontinuously, with a seemingly random mixture of brief extensions, retractions and quiet periods [49,64,65] (Figure 5a). However, motile events (extension and retraction) can be precisely defined in their geometry and dynamics by the structure and dynamics of the underlying actin cytoskeleton. Ena/VAS-capping activity, for example, determines the angle between actin filaments and the cell edge, thereby affecting the speed of protrusion [64]. The lamellipodial width is correlated with the periodicity of contractions that are generated by the cell edge during motility, indicating that the transport of a contractile signal by the rearward flow of actin controls the time between contractions [49]. Therefore, the periodicity, frequency and amplitude of extension and retraction events can be precisely defined by cell signaling pathways, and depend on external factors, for example, the nature of the ECM proteins and rigidity (Figure 4d). Many events are involved in defining cell shape because individual extension and
9 Review TRENDS in Cell Biology Vol.16 No.4 April (a) (i) 45 ο 0 ο hase 1 hase 2 hase 3 30 s 100 s 180 s 270 s 340 s 440 s 560 s 800 s 1240 s (ii) Angle ( o ) hase: (iii) hase: Time (min) Velocity (µm min 1 ) Area (µm 2 ) Time (min) (b) Key: rotrusion event Contraction event Adhesion site Cell ECM contact increase Cell ECM contact decrease TRENDS in Cell Biology Figure 5. sensing and generation control cell motility and cell morphology. (a) Total internal reflection fluorescence microscopy enables the measurement of the cell ECM contact during cell spreading (i). Detailed analysis of cell movements on short timescales (ii) shows that the cell moves discontinuously, with a mixture of brief extensions (yellow to red) and retractions (light blue to dark blue) (as seen in phase 3). The final surface contact of the cell with the substrate (iii) and its morphology are defined by the frequency, duration and direction of protrusion and contraction events. Cell movement is also characterized by transitions between different modes of protrusion and retraction dynamics. In this example, a fibroblast is spreading through initiation (hase 1), a fast spreading phase (hase 2, which has low activity of myosin II) and a contractile phase (hase 3, which has high activity of myosin II). Adapted, with permission, from [49]. (b) The final morphology of a cell is the integral of many individual rigid-substrate responses and the dynamics of adhesion sites and associated cytoskeletal structures. The black outlines of the cells show the evolution of the cell-surface contact with the ECM. Each increase in the contact area involves an individual and localized protrusion event followed by a contraction event (blue arrow) that leads to the formation of adhesion sites (green structures), which stabilize the protrusion on the ECM. Dissociation of stable interactions is represented as a gray area. rotrusion and contraction events are based on the dynamics of the actin cytoskeleton, protrusion events by actin polymerization and contraction events by myosin motor activities. s generated by the cytoskeleton stabilize its interaction with adhesion sites. Thus, if the adhesion is to a rigid substrate, a stable adhesion site will be formed. retraction events only last for s and involve mm of the cell edge. The precise timing and localization of the forces generated on different actin structures during motility will direct the amplitude and localization of forces that are applied on integrins and further adhesion-site initiation. Therefore, after the extension of the cell edge, the sensing of matrix rigidity is one of the earliest events in the formation of adhesion sites that leads to the stabilization of the extension on the ECM. Small movements can cause large changes in morphology. Therefore, the final morphology is the integral of many individual rigid substrate responses and the dynamics of the focal contacts and associated cytoskeletal structures (Figure 5b). Accordingly, cell spreading on soft substrates, which fails to stabilize cell protrusion [49], results in a greatly decreased cell area compared with cells on a rigid substrate [2,24,51]. In addition to this local effect of forces, the orientation of stress fibers (the axis where the internal forces are higher) also directs the
10 222 Review TRENDS in Cell Biology Vol.16 No.4 April 2006 protrusive activity of the cell [66]. Therefore, by sensing force and generating force in the appropriate areas, cells can control their motility as well as their morphology. The number of different motile activities is limited. For example, cellular spreading can be described by a few characteristic phases [67] (Figure 5a). Thus, only nine basic morphologies are produced by the expression of active small GTases [68]. For a complete description of the morphology, all factors must be included as each influences the overall dynamics. Changes in cell composition influence the duration and extent of individual processes, which, in turn, influences the overall behavior at the cellular level. Concluding remarks The morphology of a cell is the result of discrete rigidity response events that are integrated over time. These events are defined by the localized rigidity of the ECM that controls the local deformation of the force sensor at the integrin cytoskeleton junction and, therefore, the activation of a signal generator, which involves tyrosine signaling. Rigidity response events and motile events are based on the same molecular processes and are highly interconnected. The important task now is to define the distinct phases of motility and the dynamics of the cellular motile processes to obtain an overall working model of morphological changes that can then be tested by altering the activities and dynamics of contributing factors. References 1 Huang, S. and Ingber, D.E. (1999) The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1, E131 E138 2 Wang, H.B. et al. (2000) Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. hysiol. Cell hysiol. 279, C1345 C Lo, C.M. et al. (2000) Cell movement is guided by the rigidity of the substrate. Biophys. J. 79, Li, S. et al. (2002) The role of the dynamics of focal adhesion kinase in the mechanotaxis of endothelial cells. roc. Natl. Acad. Sci. U. S. A. 99, elham, R.J., Jr. and Wang, Y. (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. roc. Natl. Acad. Sci. U. S. A. 94, Wang, Y. et al. (2005) Visualizing the mechanical activation of Src. Nature 434, Tzima, E. et al. (2005) A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature 437, D Addario, M. et al. (2002) Interaction of p38 and Sp1 in a mechanical force-induced, b 1 integrin-mediated transcriptional circuit that regulates the actin-binding protein filamin-a. J. Biol. Chem. 277, Tzima, E. et al. (2002) Activation of Rac1 by shear stress in endothelial cells mediates both cytoskeletal reorganization and effects on gene expression. EMBO J. 21, Sikavitsas, V.I. et al. (2003) Mineralized matrix deposition by marrow stromal osteoblasts in 3D perfusion culture increases with increasing fluid shear forces. roc. Natl. Acad. Sci. U. S. A. 100, Engler, A.J. et al. (2004) Myotubes differentiate optimally on substrates with tissue-like stiffness: pathological implications for soft or stiff microenvironments. J. Cell Biol. 166, Sukharev, S. and Corey, D.. (2004) Mechanosensitive channels: multiplicity of families and gating paradigms. Sci. STKE 2004, re4 13 Miyamoto, S.et al. (1995) Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science 267, Choquet, D. et al. (1997) Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88, Geiger, B. et al. (2001) Transmembrane crosstalk between the extracellular matrix cytoskeleton crosstalk. Nat. Rev. Mol. Cell Biol. 2, Balaban, N.Q. et al. (2001) and focal adhesion assembly: a close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 3, Riveline, D. et al. (2001) Focal contacts as mechanosensors: externally applied local mechanical force induces growth of focal contacts by an mdia1-dependent and ROCK-independent mechanism. J. Cell Biol. 153, Wang, N. et al. (1993) Mechanotransduction across the cell surface and through the cytoskeleton. Science 260, Ingber, D.E. (2003) Tensegrity I. Cell structure and hierarchical systems biology. J. Cell Sci. 116, Beningo, K.A. et al. (2001) Nascent focal adhesions are responsible for the generation of strong propulsive forces in migrating fibroblasts. J. Cell Biol. 153, Galbraith, C.G. et al. (2002) The relationship between force and focal complex development. J. Cell Biol. 159, von Wichert, G. et al. (2003) RT-a acts as a transducer of mechanical force on av/b3-integrin-cytoskeleton linkages. J. Cell Biol. 161, Boettiger, D. et al. (2001) Activation of a(v)b3-vitronectin binding is a multistage process in which increases in bond strength are dependent on Y747 and Y759 in the cytoplasmic domain of b3. Mol. Biol. Cell 12, aszek, M.J. et al. (2005) Tensional homeostasis and the malignant phenotype. Cancer Cell 8, Linder, S. and Aepfelbacher, M. (2003) odosomes: adhesion hot-spots of invasive cells. Trends Cell Biol. 13, Frame, M.C. et al. (2002) v-src s hold over actin and cell adhesions. Nat. Rev. Mol. Cell Biol. 3, Munevar, S. et al. (2001) Traction force microscopy of migrating normal and H-ras transformed 3T3 fibroblasts. Biophys. J. 80, Janmey,.A. and Weitz, D.A. (2004) Dealing with mechanics: mechanisms of force transduction in cells. Trends Biochem. Sci. 29, Felsenfeld, D.. et al. (1999) Selective regulation of integrin cytoskeleton interactions by the tyrosine kinase Src. Nat. Cell Biol. 1, Volberg, T. et al. (2001) pp60(c-src) and related tyrosine kinases: a role in the assembly and reorganization of matrix adhesions. J. Cell Sci. 114, Wang, H.B. et al. (2001) Focal adhesion kinase is involved in mechanosensing during fibroblast migration. roc. Natl. Acad. Sci. U. S. A. 98, von Wichert, G. et al. (2003) -dependent integrin cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 22, Lee, J. et al. (1999) Regulation of cell movement is mediated by stretch-activated calcium channels. Nature 400, Giannone, G. et al. (2004) Calcium rises locally trigger focal adhesion disassembly and enhance residency of focal adhesion kinase at focal adhesions. J. Biol. Chem. 279, Doyle, A. et al. (2004) Calcium transients induce spatially coordinated increases in traction force during the movement of fish keratocytes. J. Cell Sci. 117, Angers-Loustau, A. et al. (1999) rotein tyrosine phosphatase-est regulates focal adhesion disassembly, migration, and cytokinesis in fibroblasts. J. Cell Biol. 144, Sawada, Y. and Sheetz, M.. (2002) transduction by Triton cytoskeletons. J. Cell Biol. 156, Tamada, M. et al. (2004) Activation of a signaling cascade by cytoskeleton stretch. Dev. Cell 7, Vogel, V. et al. (2001) Structural insights into the mechanical regulation of molecular recognition sites. Trends Biotechnol. 19, Han, B. et al. (2004) Conversion of mechanical force into biochemical signaling. J. Biol. Chem. 279, Sawada, Y. et al. (2001) Rap1 is involved in cell stretching modulation of p38 but not ERK or JNK MA kinase. J. Cell Sci. 114,
Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis. 3/9/17 ChE 575
 Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis 3/9/17 ChE 575 When, Where, Why do cells migrate? 1. Neutrophil Migration to Battle Infection 2. Development 3. Wound Healing 4. Disease 2 Wound
Cell Migration, the Cytoskeleton, Chemotaxis, and Haptotaxis 3/9/17 ChE 575 When, Where, Why do cells migrate? 1. Neutrophil Migration to Battle Infection 2. Development 3. Wound Healing 4. Disease 2 Wound
Sabrina Jedlicka. Interactions of Neurons and Materials
 Sabrina Jedlicka Interactions of Neurons and Materials Developmental Cell Biology Some Perspective Stem cells: Undifferentiated cells that are capable of proliferation, self-maintenance and differentiation
Sabrina Jedlicka Interactions of Neurons and Materials Developmental Cell Biology Some Perspective Stem cells: Undifferentiated cells that are capable of proliferation, self-maintenance and differentiation
Cell-Extracellular Matrix Interactions
 Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Cell-Extracellular Matrix Interactions Extracellular Matrix (ECM) Organised network outside of the cell s plasma membrane Between cells Composition varies throughout the ECM Continuous sheet of ECM = Basement
Sabrina Jedlicka. Interactions of Neurons and Materials
 Sabrina Jedlicka Interactions of Neurons and Materials Neurological Disorders Over 600 known neurological disorders ú Diseases of the CNS and PNS Epilepsy Alzheimer s Parkinson s Etc. ú Diseases that attack
Sabrina Jedlicka Interactions of Neurons and Materials Neurological Disorders Over 600 known neurological disorders ú Diseases of the CNS and PNS Epilepsy Alzheimer s Parkinson s Etc. ú Diseases that attack
Cellular Biomechanics. Linda Lowe-Krentz Bioscience in the 21 st Century October 17, 2011
 Cellular Biomechanics Linda Lowe-Krentz Bioscience in the 21 st Century October 17, 2011 Outline for today Tubes Vessel mechanics and disease Models Lung mechanics Technology integration Atherosclerosis
Cellular Biomechanics Linda Lowe-Krentz Bioscience in the 21 st Century October 17, 2011 Outline for today Tubes Vessel mechanics and disease Models Lung mechanics Technology integration Atherosclerosis
me239 mechanics of the cell me239 mechanics of the cell 3.1 biopolymers - motivation 3.2 biopolymers - polymerization
 4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
4. the cytoskeleton - fiber bundle models bathe, heussinger, claessens, bausch, frey [2008] me239 mechanics of the cell 1 me239 mechanics of the cell 2 biopolymers polymerization of actin and tubulin Figure
Lab Module 7: Cell Adhesion
 Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
Lab Module 7: Cell Adhesion Tissues are made of cells and materials secreted by cells that occupy the spaces between the individual cells. This material outside of cells is called the Extracellular Matrix
ANAT Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales. UNSW Copyright Notice
 ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
ANAT3231 - Cell Biology Lecture 11 School of Medical Sciences The University of New South Wales The actin cytoskeleton Prof Peter Gunning Oncology Research Unit Room 502A Wallace Wurth Building Email:
Quantitative analysis of Bidirectional Signaling (qbids)
 Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
Quantitative analysis of Bidirectional Signaling (qbids) EphB2 + cells were labeled independently with light (C 12 N 14 ) arginine and lysine or heavy (C 13 N 15 ) arginine and lysine ephrin-b1 + cells
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing
 Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
Actin cap associated focal adhesions and their distinct role in cellular mechanosensing Dong-Hwee Kim 1,2, Shyam B. Khatau 1,2, Yunfeng Feng 1,3, Sam Walcott 1,4, Sean X. Sun 1,2,4, Gregory D. Longmore
me239 mechanics of the cell homework biopolymers - motivation 3.1 biopolymers - motivation 3. biopolymers biopolymers biopolymers
 3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
3. biopolymers the inner life of a cell, viel & lue, harvard [2006] me239 mechanics of the cell 1 2 biopolymers Figure 3.1. Biopolymers. Characteristic length scales on the cellular and sucellular level..
BME Engineering Molecular Cell Biology. The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament
 BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
BME 42-620 Engineering Molecular Cell Biology Lecture 09: The Cytoskeleton (I): Actin The Cytoskeleton (II): Microtubule & Intermediate Filament BME42-620 Lecture 09, September 27, 2011 1 Outline Overviewofcytoskeletal
Cell-Environment Interactions. Chieh-Chun Chen
 Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Cell-Environment Interactions Chieh-Chun Chen Part 1: Soft Lithography in Biology and Biochemistry Chieh-Chun Chen Outlines Introduction Key features of soft lithography Applications In microscopic biochemical
Lecture 13. Motor Proteins I
 Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Lecture 13 Motor Proteins I Introduction: The study of motor proteins has become a major focus in cell and molecular biology. Motor proteins are very interesting because they do what no man-made engines
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization.
 Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
Printing of biochemically-patterned slides with the InnoStamp40 for deterministic cell immobilization. Jean christophe CAU 1 1 Innopsys, Parc activestre, Carbonne France contact@innopsys.fr www.innopsys.com
Acknowledgements. iii
 ŀ Abstract Tissue cells exhibit varying responses according to the stiffness of their extracellular matrix (ECM). The mechanism of this stiffness sensing is not fully understood; however, it is known that
ŀ Abstract Tissue cells exhibit varying responses according to the stiffness of their extracellular matrix (ECM). The mechanism of this stiffness sensing is not fully understood; however, it is known that
Sélection Internationale Ens Ulm 2012, Cell Biology
 Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Sélection Internationale Ens Ulm 2012, Cell Biology Please read the whole subject before starting. Questions 1-8 and 12-14 are general biology questions. In questions 9, 10, 11 and15, we ask you to interpret
Changes in E-cadherin rigidity sensing regulate cell adhesion
 Changes in E-cadherin rigidity sensing regulate cell adhesion Caitlin Collins a, Aleksandra K. Denisin b,c, Beth L. Pruitt b,c, and W. James Nelson a,1 a Department of Biology, Stanford University, Stanford,
Changes in E-cadherin rigidity sensing regulate cell adhesion Caitlin Collins a, Aleksandra K. Denisin b,c, Beth L. Pruitt b,c, and W. James Nelson a,1 a Department of Biology, Stanford University, Stanford,
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured
 Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
Supplementary Figure 1. Soft fibrin gels promote growth and organized mesodermal differentiation. Representative images of single OGTR1 ESCs cultured in 90-Pa 3D fibrin gels for 5 days in the presence
5 HARNESSING THE PURSE STRING FOR
 107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
107 5 HARNESSING THE PURSE STRING FOR ACCELERATED WOUND CLOSURE Abstract Wound healing is essential in maintaining tissue integrity. Wounds can close via lamellipodial crawling, which involves the rapid
Modeling Biochemical Interactions Controlling the Cytoskeleton of Spreading Cells
 Modeling Biochemical Interactions Controlling the Cytoskeleton of Spreading Cells By Mary Motz, Sophia Rick, and Dr. Magdalena Stolarska Abstract Biological cells respond to their environment. This is
Modeling Biochemical Interactions Controlling the Cytoskeleton of Spreading Cells By Mary Motz, Sophia Rick, and Dr. Magdalena Stolarska Abstract Biological cells respond to their environment. This is
Molecular Basis of Mechanotransduction in Endothelial Cells
 Molecular Basis of Mechanotransduction in Endothelial Cells Shu Chien, M.D., Ph.D. Departments of Bioengineering and Medicine, and The Whitaker Institute of Biomedical Engineering University of California
Molecular Basis of Mechanotransduction in Endothelial Cells Shu Chien, M.D., Ph.D. Departments of Bioengineering and Medicine, and The Whitaker Institute of Biomedical Engineering University of California
Single Cell Motility in Artificial Tissue
 Single Cell Motility in Artificial Tissue Rhoda J. Hawkins Lecturer in Physics, University of Sheffield Raphaël Voituriez, Jean-François Joanny, Jacques Prost Matthieu Piel, Ana-Maria Lennon-Dumenil, Philippe
Single Cell Motility in Artificial Tissue Rhoda J. Hawkins Lecturer in Physics, University of Sheffield Raphaël Voituriez, Jean-François Joanny, Jacques Prost Matthieu Piel, Ana-Maria Lennon-Dumenil, Philippe
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG
 Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Asymmetric endocytosis and remodeling of β1-integrin adhesions during growth cone chemorepulsion by MAG Jacob H. Hines, Mohammad Abu-Rub and John R. Henley Supplementary Figure 1. Validation of FM 5-95
Mechanism of action-1
 Mechanism of action-1 receptors: mediators of hormone action, membrane associated vs. intracellular receptors: measurements of receptor - ligand interaction, mechanism surface-receptors: kinases, phosphatases,
Mechanism of action-1 receptors: mediators of hormone action, membrane associated vs. intracellular receptors: measurements of receptor - ligand interaction, mechanism surface-receptors: kinases, phosphatases,
Ac(n cytoskeleton 12/7/11. The Cytoskeleton. Func0ons of the Cytoskeleton. B Visegrady. Elements of the Cytoskeleton
 The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
The Cytoskeleton Ac(n cytoskeleton B Visegrady The cytoskeleton is an intricate network of protein filaments that extends throughout the cytoplasm. A skin cell (fibroblast) stained with Coomassie blue.
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2017-18 CELL BIOLOGY BIO-5005B Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section
System. Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells. Application Note No. 4 / March
 System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
System Application Note No. 4 / March 2008 Dynamic Monitoring of Receptor Tyrosine Kinase Activation in Living Cells www.roche-applied-science.com Dynamic Monitoring of Receptor Tyrosine Kinase Activation
LIFE Formation III. Morphogenesis: building 3D structures START FUTURE. How-to 2 PROBLEMS FORMATION SYSTEMS SYSTEMS.
 7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
7.013 4.6.07 Formation III Morphogenesis: building 3D structures VIRUSES CANCER HUMAN DISEASE START SYSTEMS BIOLOGY PROBLEMS FUTURE LIFE IMMUNE NERVOUS SYSTEMS How-to 1 FOUNDATIONS How-to 2 REC. DNA BIOCHEM
Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering.
 Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering. The journal welcomes manuscripts on the following topics: synthetic
Journal of Biological Engineering is an open access, peer-reviewed online journal that encompasses all aspects biological engineering. The journal welcomes manuscripts on the following topics: synthetic
Cell cycle oscillations
 Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
Positive and negative feedback produce a cell cycle Fast Slow Cell cycle oscillations Active Cdk1-Cyclin Inactive Cdk1-Cyclin Active APC 1 Ubiquitin mediated proteolysis Glycine Isopeptide bond Lysine
System of protein filaments in the cytoplasm of. eukaryotic cell that gives the cell shape and capacity for
 Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Cytoskeleton System of protein filaments in the cytoplasm of eukaryotic cell that gives the cell shape and capacity for directed movement. The protein filaments are responsible for the shaping, moving,
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking
 Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Regulation of Acetylcholine Receptor Clustering by ADF/Cofilin- Directed Vesicular Trafficking Chi Wai Lee, Jianzhong Han, James R. Bamburg, Liang Han, Rachel Lynn, and James Q. Zheng Supplementary Figures
Supplementary Figures
 Supplementary Figures Supplementary Figure 1: Collagen gel fibril sizes in vitro and in vivo. (a-b) Box and whisker plots for collagen fibril/bundle diameter (A) and length (B) for HR and FB16 matrices.*
Supplementary Figures Supplementary Figure 1: Collagen gel fibril sizes in vitro and in vivo. (a-b) Box and whisker plots for collagen fibril/bundle diameter (A) and length (B) for HR and FB16 matrices.*
Assembly of synapses by neuronal adhesion molecules: single molecule studies
 Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
Assembly of synapses by neuronal adhesion molecules: single molecule studies Olivier Thoumine Interdisciplinary Institute of Neurosciences CNRS - University of Bordeaux Connectivity in the brain 300 nm
Cell-Biomaterial Mechanical Interaction in the Framework of Tissue Engineering: Insights, Computational Modeling and Perspectives
 Int. J. Mol. Sci. 2011, 12, 8217-8244; doi:10.3390/ijms12118217 Review OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Cell-Biomaterial Mechanical Interaction
Int. J. Mol. Sci. 2011, 12, 8217-8244; doi:10.3390/ijms12118217 Review OPEN ACCESS International Journal of Molecular Sciences ISSN 1422-0067 www.mdpi.com/journal/ijms Cell-Biomaterial Mechanical Interaction
TRACTION and STRESS MICROSCOPY for CELLS:
 TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
TRACTION and STRESS MICROSCOPY for CELLS: the WOUND HEALING CASE Embryo Physics Course APRIL 2, 2014 Vito Conte, A. Brugués, E. Anon, J.H. Veldhuis, J. Colombelli, J.J. Muñoz, G.W. Brodland, B. Ladoux
CHAPTER 16 THE CYTOSKELETON
 CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
CHAPTER 16 THE CYTOSKELETON THE SELF-ASSEMBLY AND DYNAMIC STRUCTURE OF CYTOSKELETAL FILAMENTS HOW CELLS REGULATE THEIR CYTOSKELETAL FILAMENTS MOLECULAR MOTORS THE CYTOSKELETON AND CELL BEHAVIOR THE SELF-ASSEMBLY
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology
 Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Molecular Cell Biology - Problem Drill 01: Introduction to Molecular Cell Biology Question No. 1 of 10 1. Which statement describes how an organism is organized from most simple to most complex? Question
Cells Culture Techniques Marta Czernik
 Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Cells Culture Techniques 13.03.2018 Marta Czernik Why we need the cell/tissue culture Research To overcome problems in studying cellular behaviour such as: - confounding effects of the surrounding tissues
Rapid Chromatin Condensation Increases Stem Cell Nuclear Mechanics and Mechanosensitivity
 Rapid Chromatin Condensation Increases Stem Cell Nuclear Mechanics and Mechanosensitivity Su-Jin Heo, MS 1, Tristan P. Driscoll, BS 1, Stephen Thorpe, PhD 2, Woojin M. Han, MS 1, Dawn M. Elliott, PhD 3,
Rapid Chromatin Condensation Increases Stem Cell Nuclear Mechanics and Mechanosensitivity Su-Jin Heo, MS 1, Tristan P. Driscoll, BS 1, Stephen Thorpe, PhD 2, Woojin M. Han, MS 1, Dawn M. Elliott, PhD 3,
MICB688L/MOCB639 Advanced Cell Biology Exam II
 MICB688L/MOCB639 Advanced Cell Biology Exam II May 10, 2001 Name: 1. Briefly describe the four major classes of cell surface receptors and their modes of action (immediate downstream only) (8) 2. Please
MICB688L/MOCB639 Advanced Cell Biology Exam II May 10, 2001 Name: 1. Briefly describe the four major classes of cell surface receptors and their modes of action (immediate downstream only) (8) 2. Please
Quantification of Cell Adhesion Force with Atomic Force Microscope
 2nd International Conference on Materials Science, Machinery and Energy Engineering (MSMEE 2017) Quantification of Cell Adhesion Force with Atomic Force Microscope CHEN Sheng1,a,*, XIE Hui1,b 1 State Key
2nd International Conference on Materials Science, Machinery and Energy Engineering (MSMEE 2017) Quantification of Cell Adhesion Force with Atomic Force Microscope CHEN Sheng1,a,*, XIE Hui1,b 1 State Key
MEMS based sensors for cellular studies
 MEMS based sensors for cellular studies Taher Saif Mechanical Science and Engineering University of Illinois at Urbana-Champaign Part of GEM4 Summer School lectures on instruments for cell mechanics studies
MEMS based sensors for cellular studies Taher Saif Mechanical Science and Engineering University of Illinois at Urbana-Champaign Part of GEM4 Summer School lectures on instruments for cell mechanics studies
Sept 25 Biochemical Networks. Chemotaxis and Motility in E. coli Examples of Biochemical and Genetic Networks
 Sept 25 Biochemical Networks Chemotaxis and Motility in E. coli Examples of Biochemical and Genetic Networks Background Chemotaxis- signal transduction network Bacterial Chemotaxis Flagellated bacteria
Sept 25 Biochemical Networks Chemotaxis and Motility in E. coli Examples of Biochemical and Genetic Networks Background Chemotaxis- signal transduction network Bacterial Chemotaxis Flagellated bacteria
1 Scaffolds. 2 Extracellular matrix
 This lecture describes the components of the extracellular matrix and their influence on the cell properties and functions. 1 Scaffolds One of the key components of the tissue engineering triad is the
This lecture describes the components of the extracellular matrix and their influence on the cell properties and functions. 1 Scaffolds One of the key components of the tissue engineering triad is the
Extreme Mechanics Letters. Techniques to stimulate and interrogate cell cell adhesion mechanics
 Extreme Mechanics Letters 20 (2018) 125 139 Contents lists available at ScienceDirect Extreme Mechanics Letters journal homepage: www.elsevier.com/locate/eml Techniques to stimulate and interrogate cell
Extreme Mechanics Letters 20 (2018) 125 139 Contents lists available at ScienceDirect Extreme Mechanics Letters journal homepage: www.elsevier.com/locate/eml Techniques to stimulate and interrogate cell
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
DOI: 10.1038/ncb2386 Figure 1 Src-containing puncta are not focal adhesions, podosomes or endosomes. (a) FAK-/- were stained with anti-py416 Src (green) and either (in red) the focal adhesion protein paxillin,
SUPPLEMENTARY INFORMATION
 Transcription upregulation via force-induced direct stretching of chromatin Arash Tajik 1, 2#, Yuejin Zhang 1#, Fuxiang Wei 1#, Jian Sun 2#, Qiong Jia 1, Wenwen Zhou 1, Rishi Singh 2, Nimish Khanna 3,
Transcription upregulation via force-induced direct stretching of chromatin Arash Tajik 1, 2#, Yuejin Zhang 1#, Fuxiang Wei 1#, Jian Sun 2#, Qiong Jia 1, Wenwen Zhou 1, Rishi Singh 2, Nimish Khanna 3,
Kenneth Kwun Yin Ho. Doctoral Committee:
 Progressing Mechanobiology from a Simplified to a More Complex System: Development of Novel Platforms and Investigation of Actin Cytoskeletal Remodeling by Kenneth Kwun Yin Ho A dissertation submitted
Progressing Mechanobiology from a Simplified to a More Complex System: Development of Novel Platforms and Investigation of Actin Cytoskeletal Remodeling by Kenneth Kwun Yin Ho A dissertation submitted
A Computational Model of Cell Movement Linked to Substrate Rigidity
 Lehigh University Lehigh Preserve Theses and Dissertations 2015 A Computational Model of Cell Movement Linked to Substrate Rigidity Jie Sheng Lehigh University Follow this and additional works at: http://preserve.lehigh.edu/etd
Lehigh University Lehigh Preserve Theses and Dissertations 2015 A Computational Model of Cell Movement Linked to Substrate Rigidity Jie Sheng Lehigh University Follow this and additional works at: http://preserve.lehigh.edu/etd
PAXILLIN S NUCLEAR TRANSPORT DEPENDS UPON FOCAL ADHESION DYNAMICS AND INTERACTIONS WITH THE LD4 BINDING REGION OF FAT DOMAINS
 PAXILLIN S NUCLEAR TRANSPORT DEPENDS UPON FOCAL ADHESION DYNAMICS AND INTERACTIONS WITH THE LD4 BINDING REGION OF FAT DOMAINS ANEESH RAJEEV SATHE (M.Sc. & M.Tech. Biotechnology, University of Pune) A THESIS
PAXILLIN S NUCLEAR TRANSPORT DEPENDS UPON FOCAL ADHESION DYNAMICS AND INTERACTIONS WITH THE LD4 BINDING REGION OF FAT DOMAINS ANEESH RAJEEV SATHE (M.Sc. & M.Tech. Biotechnology, University of Pune) A THESIS
Biochemical and Biophysical Research Communications 307 (2003) Cell shape provides global control of focal adhesion assembly
 Biochemical and Biophysical Research Communications 307 (2003) 355 361 BBRC www.elsevier.com/locate/ybbrc Cell shape provides global control of focal adhesion assembly Christopher S. Chen, a Jose L. Alonso,
Biochemical and Biophysical Research Communications 307 (2003) 355 361 BBRC www.elsevier.com/locate/ybbrc Cell shape provides global control of focal adhesion assembly Christopher S. Chen, a Jose L. Alonso,
cellular interactions
 cellular interactions chapter 20 tissues cellular interaction in some organisms, cells interact to form defined tissues extracellular matrix allows for cellular interaction extremely important in certain
cellular interactions chapter 20 tissues cellular interaction in some organisms, cells interact to form defined tissues extracellular matrix allows for cellular interaction extremely important in certain
Biophysics of contractile ring assembly
 Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
Biophysics of contractile ring assembly Dimitrios Vavylonis Department of Physics, Lehigh University October 1, 2007 Physical biology of the cell Physical processes in cell organization and function: Transport
T H E J O U R N A L O F C E L L B I O L O G Y
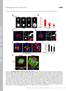 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Rodríguez-Fraticelli et al., http://www.jcb.org/cgi/content/full/jcb.201203075/dc1 Figure S1. Cell spreading and lumen formation in confined
J. Cell Sci. 128: doi: /jcs : Supplementary Material. Supplemental Figures. Journal of Cell Science Supplementary Material
 Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Supplemental Figures Figure S1. Trio controls endothelial barrier function. (A) TagRFP-shTrio constructs were expressed in ECs. Western blot shows efficient Trio knockdown in TagRFP-expressing ECs. (B)
Density-Enhanced Protein Tyrosine Phosphatase 1 (DEP-1) Regulation of Epithelial Cell Adhesions. Jennifer Leigh Sallee
 Density-Enhanced Protein Tyrosine Phosphatase 1 (DEP-1) Regulation of Epithelial Cell Adhesions Jennifer Leigh Sallee A dissertation submitted to the faculty of the University of North Carolina at Chapel
Density-Enhanced Protein Tyrosine Phosphatase 1 (DEP-1) Regulation of Epithelial Cell Adhesions Jennifer Leigh Sallee A dissertation submitted to the faculty of the University of North Carolina at Chapel
Article. Matrix Microarchitecture and Myosin II Determine Adhesion in 3D Matrices
 Current Biology 23, 1607 1619, September 9, 2013 ª2013 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.06.053 Matrix Microarchitecture and Myosin II Determine Adhesion in 3D Matrices
Current Biology 23, 1607 1619, September 9, 2013 ª2013 Elsevier Ltd All rights reserved http://dx.doi.org/10.1016/j.cub.2013.06.053 Matrix Microarchitecture and Myosin II Determine Adhesion in 3D Matrices
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues
 High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
High-Throughput Method for Microfluidic Placement of Cells in Micropatterned Tissues Emily N. Sevcik Faculty Mentor: Patrick W. Alford Undergraduate Research Opportunities Program Project Final Report
SUPPLEMENTARY INFORMATION
 DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
DOI: 10.1038/ncb2743 Figure S1 stabilizes cellular protein level, post-transcriptionally. (a, b) and DDR1 were RNAi-depleted from HEK.293.-CBG cells. Western blots with indicated antibodies (a). RT-PCRs
T H E J O U R N A L O F C E L L B I O L O G Y
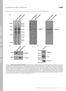 T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
T H E J O U R N A L O F C E L L B I O L O G Y Supplemental material Bays et al., http://www.jcb.org/cgi/content/full/jcb.201309092/dc1 Figure S1. Specificity of the phospho-y822 antibody. (A) Total cell
Challenges to measuring intracellular Ca 2+ Calmodulin: nature s Ca 2+ sensor
 Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
Calcium Signals in Biological Systems Lecture 3 (2/9/0) Measuring intracellular Ca 2+ signals II: Genetically encoded Ca 2+ sensors Henry M. Colecraft, Ph.D. Challenges to measuring intracellular Ca 2+
ANAT Cell Biology Lecture 12 School of Medical Sciences The University of New South Wales
 ANAT3231 - Cell Biology Lecture 12 School of Medical Sciences The University of New South Wales Prof Peter Gunning Microfilaments, 2010 Sllide 1 UNSW Copyright Notice COMMONWEALTH OF AUSTRALIA Copyright
ANAT3231 - Cell Biology Lecture 12 School of Medical Sciences The University of New South Wales Prof Peter Gunning Microfilaments, 2010 Sllide 1 UNSW Copyright Notice COMMONWEALTH OF AUSTRALIA Copyright
Monteiro, G., Sundararaghavan, H. Fernandes, A. and Shreiber, D. Rutgers, the State University of New Jersey, New Brunswick, NJ, USA
 Monteiro, G., Sundararaghavan, H. Fernandes, A. and Shreiber, D. Rutgers, the State University of New Jersey, New Brunswick, NJ, USA Introduction: Cell migration is a ubiquitous process that is of fundamental
Monteiro, G., Sundararaghavan, H. Fernandes, A. and Shreiber, D. Rutgers, the State University of New Jersey, New Brunswick, NJ, USA Introduction: Cell migration is a ubiquitous process that is of fundamental
The cytoskeleton. The cytoskeleton, the motor proteins, the muscle and its regulation. The cytoskeleton. The cytoskeleton.
 , the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
, the motor proteins, the muscle and its regulation Dept. of Biophysics, University of Pécs Zoltán Ujfalusi January-February 2012 Dynamic framework of the Eukaryotes Three main filament-class: 1. Intermedier
Prestress and adhesion site dynamics control cell sensitivity to extracellular stiffness
 Author manuscript, published in "Biophysical Journal 2009;96(5):2009-22" DOI : 10.1016/j.bpj.2008.10.072 1 Prestress and adhesion site dynamics control cell sensitivity to extracellular stiffness S. Féréol
Author manuscript, published in "Biophysical Journal 2009;96(5):2009-22" DOI : 10.1016/j.bpj.2008.10.072 1 Prestress and adhesion site dynamics control cell sensitivity to extracellular stiffness S. Féréol
Polypharmacology. Giulio Rastelli Molecular Modelling and Drug Design Lab
 Polypharmacology Giulio Rastelli Molecular Modelling and Drug Design Lab www.mmddlab.unimore.it Dipartimento di Scienze della Vita Università di Modena e Reggio Emilia giulio.rastelli@unimore.it Magic
Polypharmacology Giulio Rastelli Molecular Modelling and Drug Design Lab www.mmddlab.unimore.it Dipartimento di Scienze della Vita Università di Modena e Reggio Emilia giulio.rastelli@unimore.it Magic
Supplemental Information. A Computational Model of YAP/TAZ Mechanosensing. Meng Sun, Fabian Spill, and Muhammad H. Zaman
 Biophysical Journal, Volume 110 Supplemental Information A Computational Model of YAP/TAZ Mechanosensing Meng Sun, Fabian Spill, and Muhammad H. Zaman Supporting Methods Molecular intervention When latrunculin
Biophysical Journal, Volume 110 Supplemental Information A Computational Model of YAP/TAZ Mechanosensing Meng Sun, Fabian Spill, and Muhammad H. Zaman Supporting Methods Molecular intervention When latrunculin
Section 10. Junaid Malek, M.D.
 Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
Section 10 Junaid Malek, M.D. Cell Division Make sure you understand: How do cells know when to divide? (What drives the cell cycle? Why is it important to regulate this?) How is DNA replication regulated?
cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular processes (transcription, replication, microtubules)
 DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
DISCUSSION SECTIONS BY STUDENT NUMBER ENDING IN ODD NUMBERS 2-3, EVEN 3-4 Methods for Studying the Cell Cycle cell fusion live and fixed imaging genetics biochemistry in vitro systems inhibitors of cellular
TRANSMEMBRANE EXTRACELLULAR MATRIX CYTOSKELETON CROSSTALK. Benjamin Geiger*, Alexander Bershadsky*, Roumen Pankov and Kenneth M.
 TRANSMEMBRANE EXTRACELLULAR MATRIX CYTOSKELETON CROSSTALK Benjamin Geiger*, Alexander Bershadsky*, Roumen Pankov and Kenneth M. Yamada Integrin-mediated cell adhesions provide dynamic, bidirectional links
TRANSMEMBRANE EXTRACELLULAR MATRIX CYTOSKELETON CROSSTALK Benjamin Geiger*, Alexander Bershadsky*, Roumen Pankov and Kenneth M. Yamada Integrin-mediated cell adhesions provide dynamic, bidirectional links
Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly
 Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly Colleen T. Skau a, Sergey V. Plotnikov b, Andrew. oyle c, and Clare
Inverted formin 2 in focal adhesions promotes dorsal stress fiber and fibrillar adhesion formation to drive extracellular matrix assembly Colleen T. Skau a, Sergey V. Plotnikov b, Andrew. oyle c, and Clare
Three major types of cytoskeleton
 The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
The Cytoskeleton Organizes and stabilizes cells Pulls chromosomes apart Drives intracellular traffic Supports plasma membrane and nuclear envelope Enables cellular movement Guides growth of the plant cell
Characterization of cytoskeleton mechanical properties and 3D-actin structure in twisted adherent epithelial cells
 Biorheology 40 (2003) 241 245 241 IOS Press Characterization of cytoskeleton mechanical properties and 3D-actin structure in twisted adherent epithelial cells Redouane Fodil, Valérie Laurent, Emmanuelle
Biorheology 40 (2003) 241 245 241 IOS Press Characterization of cytoskeleton mechanical properties and 3D-actin structure in twisted adherent epithelial cells Redouane Fodil, Valérie Laurent, Emmanuelle
Biochemistry 111. Carl Parker x A Braun
 Biochemistry 111 Carl Parker x6368 101A Braun csp@caltech.edu Central Dogma of Molecular Biology DNA-Dependent RNA Polymerase Requires a DNA Template Synthesizes RNA in a 5 to 3 direction Requires ribonucleoside
Biochemistry 111 Carl Parker x6368 101A Braun csp@caltech.edu Central Dogma of Molecular Biology DNA-Dependent RNA Polymerase Requires a DNA Template Synthesizes RNA in a 5 to 3 direction Requires ribonucleoside
Combined fluorescence and AFM imaging of cells
 Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Combined fluorescence and AFM imaging of cells Introduction Combining optical and AFM imaging of cells opens up many possibilities for correlating structural information about the cell surface with functional
Review Article Physicochemical Control of Adult Stem Cell Differentiation: Shedding Light on Potential Molecular Mechanisms
 Hindawi Publishing Corporation Journal of Biomedicine and Biotechnology Volume 2010, Article ID 743476, 14 pages doi:10.1155/2010/743476 Review Article Physicochemical Control of Adult Stem Cell Differentiation:
Hindawi Publishing Corporation Journal of Biomedicine and Biotechnology Volume 2010, Article ID 743476, 14 pages doi:10.1155/2010/743476 Review Article Physicochemical Control of Adult Stem Cell Differentiation:
(a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The
 1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
1 Supplementary Figure 1 Design of the DNA origami spring (nanospring). (a) Overview of the 2-helix bundle (2HB) nanospring design used in this study. The scheme was produced by cadnano software 1. Scaffold,
Frequently Asked Questions (FAQ)
 Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
Frequently Asked Questions (FAQ) Matrigen Softwell Hydrogels Version 1.0 Contents General Questions... 3 Cell Culture and Experimental Questions... 6 Quality Control Technical Questions... 8 SoftTrac Products...
Cell motility and proliferation in 2D and 3D substrata. Applications to chemotaxis, wound healing and collective migration.
 UNIVERSITÀ DEGLI STUDI DI NAPOLI FEDERICO II Dipartimento di Ingegneria Chimica, dei Materiali e della Produzione Industriale Dottorato di ricerca in Ingegneria Chimica XXVII CICLO Cell motility and proliferation
UNIVERSITÀ DEGLI STUDI DI NAPOLI FEDERICO II Dipartimento di Ingegneria Chimica, dei Materiali e della Produzione Industriale Dottorato di ricerca in Ingegneria Chimica XXVII CICLO Cell motility and proliferation
In vitro EGFP-CALI comprehensive analysis. Liang CAI. David Humphrey. Jessica Wilson. Ken Jacobson. Dept. of Cell and Developmental Biology
 In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
In vitro EGFP-CALI comprehensive analysis Liang CAI David Humphrey Jessica Wilson Ken Jacobson Dept. of Cell and Developmental Biology 1/15 Liang s Rotation in Ken s Lab, Sep-Nov, 2003 1. CALI background
Prestress and Adhesion Site Dynamics Control Cell Sensitivity to Extracellular Stiffness
 Biophysical Journal Volume 96 March 2009 2009 2022 2009 Prestress and Adhesion Site Dynamics Control Cell Sensitivity to Extracellular Stiffness S. Féréol, R. Fodil, V. M. Laurent, M. Balland, {k B. Louis,
Biophysical Journal Volume 96 March 2009 2009 2022 2009 Prestress and Adhesion Site Dynamics Control Cell Sensitivity to Extracellular Stiffness S. Féréol, R. Fodil, V. M. Laurent, M. Balland, {k B. Louis,
Supplementary Information
 Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
Supplementary Information STED nanoscopy combined with optical tweezers reveals protein dynamics on densely covered DNA Iddo Heller, Gerrit Sitters, Onno D. Broekmans, Géraldine Farge, Carolin Menges,
PLEASE MAKE SURE THAT ALL PAGES ARE NUMBERED and IDENTIFIED!!!!!.
 Instructions: The following pages contain twelve questions. You must answer ten out of the twelve questions. Any one question may ask for more than one response so please read each question carefully for
Instructions: The following pages contain twelve questions. You must answer ten out of the twelve questions. Any one question may ask for more than one response so please read each question carefully for
SUPPLEMENTARY INFORMATION
 Results Construct purification and coupling. Two A1-GP1bα ReaLiSM constructs, with and without cysteine residues near the N and C-termini (Fig. S2a), were expressed and purified by Ni affinity chromatography
Results Construct purification and coupling. Two A1-GP1bα ReaLiSM constructs, with and without cysteine residues near the N and C-termini (Fig. S2a), were expressed and purified by Ni affinity chromatography
FRET Biosensors and Live Cell Imaging
 08/3/2015 Summer Workshop FRET Imaging and Quantification FRET Biosensors and Live Cell Imaging Peter Yingxiao Wang Associate Professor Department of Bioengineering, Institute of Engineering in Medicine,
08/3/2015 Summer Workshop FRET Imaging and Quantification FRET Biosensors and Live Cell Imaging Peter Yingxiao Wang Associate Professor Department of Bioengineering, Institute of Engineering in Medicine,
The function and regulation of vinculin in cell-cell adhesions
 University of Iowa Iowa Research Online Theses and Dissertations 2011 The function and regulation of vinculin in cell-cell adhesions Xiao Peng University of Iowa Copyright 2011 Xiao Peng This dissertation
University of Iowa Iowa Research Online Theses and Dissertations 2011 The function and regulation of vinculin in cell-cell adhesions Xiao Peng University of Iowa Copyright 2011 Xiao Peng This dissertation
Chapter 3 Nucleic Acids, Proteins, and Enzymes
 3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
3 Nucleic Acids, Proteins, and Enzymes Chapter 3 Nucleic Acids, Proteins, and Enzymes Key Concepts 3.1 Nucleic Acids Are Informational Macromolecules 3.2 Proteins Are Polymers with Important Structural
Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in Section B and ONE question from Section C.
 UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 CELL BIOLOGY BIO-2B06 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in
UNIVERSITY OF EAST ANGLIA School of Biological Sciences Main Series UG Examination 2013-2014 CELL BIOLOGY BIO-2B06 Time allowed: 2 hours Answer ALL questions in Section A, ALL PARTS of the question in
A. Incorrect! Enzymes are not altered or consumed by the reactions they catalyze.
 CLEP Biology - Problem Drill 04: Enzymes and Cellular Metabolism No. 1 of 10 1. Which of the following statements about enzymes is correct? (A) Enzymes are consumed in a reaction. (B) Enzymes act by lowering
CLEP Biology - Problem Drill 04: Enzymes and Cellular Metabolism No. 1 of 10 1. Which of the following statements about enzymes is correct? (A) Enzymes are consumed in a reaction. (B) Enzymes act by lowering
Supplementary Materials for
 www.advances.sciencemag.org/cgi/content/full/1/1/e1400205/dc1 Supplementary Materials for Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling Michael
www.advances.sciencemag.org/cgi/content/full/1/1/e1400205/dc1 Supplementary Materials for Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling Michael
Post-translational modification
 Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
Protein expression Western blotting, is a widely used and accepted technique to detect levels of protein expression in a cell or tissue extract. This technique measures protein levels in a biological sample
October 24, BIOS 95: Cell Division and Chromosome Dynamics
 October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
October 24, 2008 BIOS 95: Cell Division and Chromosome Dynamics Cancer unregulated cell growth and migration Aneuploidy errors in chromosome segregation and genome maintenance living with an altered genome
Analysis of cytoskeletal features in dried and living cells and slow dynamic experiments using Atomic Force Microscopy
 Analysis of cytoskeletal features in dried and living cells and slow dynamic experiments using Atomic Force Microscopy Author Watson, Gregory, Watson, Jolanta Published 008 Journal Title Journal of Physics:
Analysis of cytoskeletal features in dried and living cells and slow dynamic experiments using Atomic Force Microscopy Author Watson, Gregory, Watson, Jolanta Published 008 Journal Title Journal of Physics:
supplementary information
 DOI: 10.1038/ncb2156 Figure S1 Depletion of p114rhogef with different sirnas. Caco-2 (a) and HCE (b) cells were transfected with individual sirnas, pools of the two sirnas or the On Target (OnT) sirna
DOI: 10.1038/ncb2156 Figure S1 Depletion of p114rhogef with different sirnas. Caco-2 (a) and HCE (b) cells were transfected with individual sirnas, pools of the two sirnas or the On Target (OnT) sirna
BNG-338: Lecture 11. Outlines due today Exam #1 on Friday Review Molecular Level Mechanobiology Cellular Level Mechanobiology. Monday, May 15, 17
 BNG-338: Lecture 11 Outlines due today Exam #1 on Friday Review Molecular Level Mechanobiology Cellular Level Mechanobiology Learning Objectives Review how cells sense strain on a molecular level Discover
BNG-338: Lecture 11 Outlines due today Exam #1 on Friday Review Molecular Level Mechanobiology Cellular Level Mechanobiology Learning Objectives Review how cells sense strain on a molecular level Discover
Gene Expression: Transcription
 Gene Expression: Transcription The majority of genes are expressed as the proteins they encode. The process occurs in two steps: Transcription = DNA RNA Translation = RNA protein Taken together, they make
Gene Expression: Transcription The majority of genes are expressed as the proteins they encode. The process occurs in two steps: Transcription = DNA RNA Translation = RNA protein Taken together, they make
From Molecular Signal Activation to Locomotion: An Integrated, Multiscale Analysis of Cell Motility on Defined Matrices
 From Molecular Signal Activation to Locomotion: An Integrated, Multiscale Analysis of Cell Motility on Defined Matrices Amit Pathak, Sanjay Kumar* Department of Bioengineering, University of California,
From Molecular Signal Activation to Locomotion: An Integrated, Multiscale Analysis of Cell Motility on Defined Matrices Amit Pathak, Sanjay Kumar* Department of Bioengineering, University of California,
Supplementary Information. Synergistic action of RNA polymerases in overcoming the nucleosomal barrier
 Supplementary Information Synergistic action of RNA polymerases in overcoming the nucleosomal barrier Jing Jin, Lu Bai, Daniel S. Johnson, Robert M. Fulbright, Maria L. Kireeva, Mikhail Kashlev, Michelle
Supplementary Information Synergistic action of RNA polymerases in overcoming the nucleosomal barrier Jing Jin, Lu Bai, Daniel S. Johnson, Robert M. Fulbright, Maria L. Kireeva, Mikhail Kashlev, Michelle
