Investigation of an In Vitro Method for Protein Hazard Characterization
|
|
|
- Alice Anderson
- 6 years ago
- Views:
Transcription
1 Investigation of an In Vitro Method for Protein Hazard Characterization Bryan Delaney, PhD, DABT, Fellow, ATS DuPont Pioneer Johnston, Iowa
2 Conflict of Interest Statement I have no conflict of interest. Funding for all of the studies presented here was provided by DuPont Pioneer. Studies at MGH were conducted as contract research funded by DuPont Pioneer.
3 Agricultural Biotechnology Many crops produced using biotechnology express proteins from a nonnative source Insect resistance Cry proteins from Bacillus thuringiensis Herbicide tolerance CP4 EPSPS from Agrobacterium Disease resistance Viral coat proteins
4 Agricultural Biotechnology Proteins are tested for safety before commercialization Weight of evidence approach Tier I Hazard identification Tier II Hazard Characterization History of safe use - Acute toxicity Bioinformatics - Repeated dose toxicity Mode of action/specificity - Hypothesis-based studies Resistance to digestion in vitro Expression level and dietary intake Tier II Hazard characterization Delaney et al., Food Chem Toxicol 46 (Suppl 2):s71-s97
5 Agricultural Biotechnology Some crops (will) express proteins that are difficult or impossible to isolate in quantities necessary to conduct animal trials Characterized as Intractable proteins Includes: Membrane proteins Signaling proteins Transcription factors N-glycosylated proteins Resistance proteins (R-proteins)
6 Agricultural Biotechnology Tier I Hazard identification Requires little or no protein History of safe use Bioinformatics Mode of action/specificity Resistance to digestion in vitro Expression level and dietary intake Tier II Hazard characterizatio Acute toxicity Requires gram quantities Repeated dose toxicity Hypothesis-based studies
7 What Do We Know about Hazardous Proteins? Many proteins exist in nature that are hazardous but most need to be administered parenterally Stinging, biting, injecting Some proteins exist in nature that cause adverse effects from oral exposure Undercooked kidney beans (Phytohaemagglutinin-E) Adverse effects include: Damage the intestinal epithelium Absorbed intact and produce a systemic effect
8 Consideration of an In Vitro Testing Method Goal At least as good as an animal study Much smaller quantity of protein Reduce use of laboratory animals Inexpensive reagents and equipment
9 Consideration of an In Vitro Testing Method Human intestinal epithelial cell line monolayers Examples: T84, Caco-2, and HCT-8 Derived from colon cancer Develop into differentiated monolayer when grown on Transwell insert Have been utilized in investigation of drug bioavailability
10 Consideration of an In Vitro Testing Method Addition of known protein toxins to apical side: Cytotoxicity LDH MTT Monolayer integrity Transepithelial Electrical Resistance(TEER) [ 3 H]-Inulin or FITC-Inulin HRP
11 Consideration of an In Vitro Testing Method Outline Proof of concept investigation Effect of digestive enzymes Primary human polarized small intestinal epithelial barriers Intractable proteins
12 Consideration of an In Vitro Testing Method Outline Proof of concept investigation Effect of digestive enzymes Primary human polarized small intestinal epithelial barriers Intractable proteins
13 Proof of Concept Investigation Comparison of effects following addition of innocuous or known hazardous proteins Hazardous proteins: Streptolysin O (SLO) Clostridium difficile toxin A (ToxA) Clostridium difficile toxin B (ToxB) Lymphotoxin (LT) Lysteriolysin O (LLO) Mastoparan (Mast) Melittin (Mel) Innocuous proteins: Bovine serum albumin (BSA) Porcine serum albumin (PSA) Fibronectin (Fib) Rubisco (Rub)
14 24 hr Cytotoxicity Monolayer Integrity LDH MTT [ 3 H]-Inulin HRP TEER T84/Caco2/HCT-8 T84/Caco2/HCT-8 T84/Caco2/HCT-8 T84/Caco2/HCT-8 T84/Caco2/HCT-8 Toxin SLO N/N/N N/N/N N/N/N N/N/N N/N/N ToxA N/N/N N/N/N Y/Y/Y Y/N/N Y/Y/Y ToxB N/N/N N/N/N Y/Y/Y Y/Y/Y Y/Y/Y LT N/N/N N/N/N N/N/N N/N/N Y/N/N LLO N/Y/Y N/N/N N/Y/N N/N/N N/N/N Mast Y/Y/Y Y/Y/N Y/Y/Y Y/Y/N Y/Y/Y Mel Y/Y/Y Y/Y/Y Y/Y/Y Y/Y/Y Y/Y/Y Dietary BSA N/N/N N/N/N N/N/N N/N/N N/N/N PSA N/N/N N/N/N N/N/N N/N/N N/N/N Fib N/N/N N/N/N N/N/N N/N/N N/N/N Rub N/N/N N/N/N N/N/N N/N/N N/N/N
15 Proof of Concept Investigation Summary Known hazardous proteins damaged monolayers TEER was the most sensitive indicator None of the tested innocuous proteins damaged monolayers
16 Consideration of an In Vitro Testing Method Outline Proof of concept investigation Effect of digestive enzymes Primary human polarized small intestinal epithelial barriers Intractable proteins
17 Effect of Digestive Enzymes Design Hazardous proteins: Phytohaemagglutinin E (PHA-E) Concanavalin A (Con A) Wheat germ agglutinin (WGA) Melittin (Mel) Innocuous proteins: Bovine serum albumin (BSA) β-lactoglobulin (β-lg) Fibronectin (Fib) Rubisco (Rub)
18 Effect of Digestive Enzymes Design Symbol Treatment Enzymes Exposure Stop I None None G SGF Pepsin 37 C, 1 hr NaOH to ph 7.5 GL SGF Lyophilized/Suspended Pepsin 37 C, 1 hr NaOH to ph 7.5 S Sequential Pancreatin 37 C, 2 hr 100 C, 10 min SL Sequential Lyophilized/Suspended Pancreatin 37 C, 2 hr 100 C, 10 min
19 Effect of Digestive Enzymes Design Measurement at 24 and 48 hr: Cytotoxicity Neutral red uptake Monolayer integrity FITC dextran (70 kda) Tight junction integrity TEER TRITC-dextran (4.4 kda) Light microscopy
20 Effect of Digestive Enzymes Effects on monolayers (selected)
21 Effect of Digestive Enzymes Light microscopy
22 Effect of Digestive Enzymes Light microscopy
23 Effect of Digestive Enzymes Summary First level bullet No effects from innocuous proteins +/- digestive enzymes Hazardous proteins that were completely degraded in the presence of digestive enzymes did NOT alter monolayer integrity Hazardous proteins that resisted degradation in the presence of digestive enzymes DID alter monolayer integrity
24 Consideration of an In Vitro Testing Method Outline Proof of concept investigation Effect of digestive enzymes Primary human polarized small intestinal epithelial barriers Intractable proteins
25 Primary Human Polarized Small Intestinal Epithelial Barriers Design Primary cells are not transformed = $$$ Heterogeneous cell population (not just epithelial cells) Comparison of BSA and C. difficile toxin A TEER FITC-Inulin flux HRP flux MTT conversion LDH release
26 Primary Human Polarized Small Intestinal Epithelial Barriers TEER
27 Primary Human Polarized Small Intestinal Epithelial Barriers FITC-Inulin flux
28 Primary Human Polarized Small Intestinal Epithelial Barriers HRP flux
29 Primary human polarized small intestinal epithelial barriers Viability
30 Primary Human Polarized Small Intestinal Epithelial Barriers Summary C. difficile toxin A altered monolayer integrity at comparable doses with cell line monolayers Innocuous protein (BSA) did not damage monolayers at any concentration
31 Consideration of an In Vitro Testing Method Outline Proof of concept investigation Effect of digestive enzymes Primary human polarized small intestinal epithelial barriers Intractable proteins
32 Intractable Proteins
33 Intractable Proteins
34 Intractable Proteins Summary Various types of intractable proteins were tested in human intestinal epithelial cell monolayers None of the tested proteins altered membrane integrity
35 Conclusions In vitro testing for protein hazard characterization: Human intestinal epithelial cell line monolayers appear to respond differently to hazardous and non-hazardous proteins Effect of digestive enzymes can be incorporated Results in cell lines correlate with primary cell monolayers May be useful for intractable proteins
36 References (all available open access) Bushey DF, Bannon GA, Delaney B, Graser G, Hefford M, Jiang X, Lee TC, Madduri KM, Pariza M, Privalle LS, Ranjan R, Saab-Rincon G, Schafer BW, Thelen JJ, Zhang JXQ, and Harper MS Characteristics and safety assessment of intractable proteins in genetically modified crops. Regulatory Toxicology and Pharmacology 69: Hurley BP, Pirzai W, Eaton AD, Harper M, Ladics G, Roper J,, Zimmermann C, Layton RJ, and Delaney B An experimental platform using human intestinal epithelial cell lines to differentiate between hazardous and non-hazardous proteins. Food and Chemical Toxicology 92: Hurley BP, Eaton AD, Zimmermann C, and Delaney B Polarized monolayer cultures of human intestinal epithelial cell lines exposed to intractable proteins - In vitro hazard identification studies. Food and Chemical Toxicology 98: Eaton AD, Hurley BP, Zimmermann C, and Delaney B Primary human intestinal epithelial barriers respond differently to a hazardous and an innocuous protein. Food and Chemical Toxicology 106 (Part A): Markell LK, Wezalis SM, Roper JM, Zimmermann C, and Delaney B Incorporation of in vitro digestive enzymes in an intestinal epithelial cell line model for protein hazard identification. Toxicology In Vitro 44:85-93.
37 Acknowledgements DuPont Pioneer Cindi Zimmermann Marc Harper Ray Layton MGH/Harvard Medical School Bryan Hurley Waheed Pirzai Alex Eaton DuPont Haskell Global Centers for Health and Environmental Sciences Jason Roper Stephanie Wezalis Lauren Markell Greg Ladics
Bringing True Novelty to the Anti-Infective Space
 Bringing True Novelty to the Anti-Infective Space New Class of Antibacterials Based on a Unique Mechanism of Action Dr Dawn Firmin SMi s 17 th Annual Conference on Superbugs & Superdrugs March 2015 1 Contents
Bringing True Novelty to the Anti-Infective Space New Class of Antibacterials Based on a Unique Mechanism of Action Dr Dawn Firmin SMi s 17 th Annual Conference on Superbugs & Superdrugs March 2015 1 Contents
PREPUBLICATION CODEX PRINCIPLES AND GUIDELINES ON FOODS DERIVED FROM BIOTECHNOLOGY CONTENTS
 PREPUBLICATION CODEX PRINCIPLES AND GUIDELINES ON FOODS DERIVED FROM BIOTECHNOLOGY CONTENTS PRINCIPLES FOR THE RISK ANALYSIS OF FOODS DERIVED FROM MODERN BIOTECHNOLOGY... 1 GUIDELINE FOR THE CONDUCT OF
PREPUBLICATION CODEX PRINCIPLES AND GUIDELINES ON FOODS DERIVED FROM BIOTECHNOLOGY CONTENTS PRINCIPLES FOR THE RISK ANALYSIS OF FOODS DERIVED FROM MODERN BIOTECHNOLOGY... 1 GUIDELINE FOR THE CONDUCT OF
State of the Art in the Cramer Classification Scheme and Threshold of Toxicological Concern. March 29, 2016
 State of the Art in the Cramer Classification Scheme and Threshold of Toxicological Concern March 29, 2016 Welcome Mary E. Torrence, DVM, PhD Director, Office of Applied Research and Safety Assessment
State of the Art in the Cramer Classification Scheme and Threshold of Toxicological Concern March 29, 2016 Welcome Mary E. Torrence, DVM, PhD Director, Office of Applied Research and Safety Assessment
Diffusional Contributions and Electrostatic Interactions during Ultrafiltration
 Diffusional Contributions and Electrostatic Interactions during Ultrafiltration Andrew L. Zydney Department Head and Walter L. Robb Family Chair Department of Chemical Engineering The Pennsylvania State
Diffusional Contributions and Electrostatic Interactions during Ultrafiltration Andrew L. Zydney Department Head and Walter L. Robb Family Chair Department of Chemical Engineering The Pennsylvania State
Genetically Modified Organisms II. How are transgenic plants generated? The components of T DNA transfer. Plants
 Genetically Modified Organisms II Plants How are transgenic plants generated? The bacterium Agrobacterium tumefaciens is a pathogen of plants that causes crown gall tumors. Crown gall tumor Agrobacterium
Genetically Modified Organisms II Plants How are transgenic plants generated? The bacterium Agrobacterium tumefaciens is a pathogen of plants that causes crown gall tumors. Crown gall tumor Agrobacterium
Pharmacology. Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University.
 Pharmacology Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University. 1 PHARMACODYNAMIC STUDIES A. Primary pharmacodynamics primary action in target
Pharmacology Chatchai Chinpaisal, Ph.D. Department of Pharmacology and Toxicology, Faculty of Pharmacy, Silpakorn University. 1 PHARMACODYNAMIC STUDIES A. Primary pharmacodynamics primary action in target
Workshop: Applying Exposure Science to Increase the Utility of Non-Animal Data in Efficacy and Safety Testing, 16 Feb. 2017
 Workshop: Applying Exposure Science to Increase the Utility of Non-Animal Data in Efficacy and Safety Testing, 16 Feb. 2017 Presentation Outline Should we account for kinetics in vitro and in vivo for
Workshop: Applying Exposure Science to Increase the Utility of Non-Animal Data in Efficacy and Safety Testing, 16 Feb. 2017 Presentation Outline Should we account for kinetics in vitro and in vivo for
CompoZr Transporter Knockout Cell Lines
 biotransport CompoZr Transporter Knockout Cell Lines Assessment of Substrates with Functionally Knocked Out Transporters MDR1, BCRP and MRP2 biotransport CompoZr Transporter Knockout Cell Lines The Increasing
biotransport CompoZr Transporter Knockout Cell Lines Assessment of Substrates with Functionally Knocked Out Transporters MDR1, BCRP and MRP2 biotransport CompoZr Transporter Knockout Cell Lines The Increasing
Our Business. in vitro / in vivo. in vitro / in vivo ADME. in vitro / in vivo. Toxicology. Cell Battery V79. Analytical Services. Service.
 GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
GenPharmTox is one of the few CROs who can guarantee the success of our project within the given time framework and on the terms agreed. Dr. Michael Runkel, Apogepha GmbH 3 3 In vitro 3.1. Phototoxicity
Material Safety Data Sheet
 Material Safety Data Sheet Date of issue: 14.02.2014 Supersedes edition of: 31.01.2014 1. Chemical Product and Company Identification Trade name: Common name: Kcentra Prothrombin Complex Concentrate (Human)
Material Safety Data Sheet Date of issue: 14.02.2014 Supersedes edition of: 31.01.2014 1. Chemical Product and Company Identification Trade name: Common name: Kcentra Prothrombin Complex Concentrate (Human)
Proteomics. Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Limitations: Examples
 Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
GM (Genetically Modified) Plants. Background
 1 GM (Genetically Modified) Plants Background Genetically modified crops (GM) have been used since 1996 in the U.S. GM crops contain foreign genetic material The DNA may be from another plant or from a
1 GM (Genetically Modified) Plants Background Genetically modified crops (GM) have been used since 1996 in the U.S. GM crops contain foreign genetic material The DNA may be from another plant or from a
TECHNICAL BULLETIN. CompoZr ADME/Tox Cell Lines Caco-2 BCRP Knockout Cell Line 24 Well Assay Ready Plate
 CompoZr ADME/Tox Cell Lines Caco-2 BCRP Knockout Cell Line 24 Well Assay Ready Plate Catalog Number MTOX1002P24 Store at Room Temperature TECHNICAL BULLETIN Product Description CompoZr zinc finger nuclease
CompoZr ADME/Tox Cell Lines Caco-2 BCRP Knockout Cell Line 24 Well Assay Ready Plate Catalog Number MTOX1002P24 Store at Room Temperature TECHNICAL BULLETIN Product Description CompoZr zinc finger nuclease
Regulatory and GM Food Safety Assessment Perspectives: Kenya Prof. Theophilus M. Mutui, PhD Chief Biosafety Officer
 Regulatory and GM Food Safety Assessment Perspectives: Kenya Prof. Theophilus M. Mutui, PhD Chief Biosafety Officer HESI Food Allergy and Safety Workshop KICC Nairobi, Kenya, 12 th August 2014 MISSION
Regulatory and GM Food Safety Assessment Perspectives: Kenya Prof. Theophilus M. Mutui, PhD Chief Biosafety Officer HESI Food Allergy and Safety Workshop KICC Nairobi, Kenya, 12 th August 2014 MISSION
Supporting Information
 Supporting Information Groschwitz et al. 10.1073/pnas.0906372106 SI Methods In vitro permeability. Caco2-bbe human intestinal adenocarcinoma cells (ATCC) were maintained in DMEM supplemented with 10% FCS,
Supporting Information Groschwitz et al. 10.1073/pnas.0906372106 SI Methods In vitro permeability. Caco2-bbe human intestinal adenocarcinoma cells (ATCC) were maintained in DMEM supplemented with 10% FCS,
Clostridium difficile (toxin B)
 Techne qpcr test Clostridium difficile (toxin B) Toxin B (toxb) gene 150 tests For general laboratory and research use only 1 Introduction to Clostridium difficile (toxin B) Clostridium difficile is a
Techne qpcr test Clostridium difficile (toxin B) Toxin B (toxb) gene 150 tests For general laboratory and research use only 1 Introduction to Clostridium difficile (toxin B) Clostridium difficile is a
IgG Purity/Heterogeneity and SDS-MW Assays with High- Speed Separation Method and High Throughput Tray Setup
 IgG Purity/Heterogeneity and SDS-MW Assays with High- Speed Separation Method and High Throughput Tray Setup High Throughput Methods to Maximize the Use of the PA 800 Plus system Jose-Luis Gallegos-Perez
IgG Purity/Heterogeneity and SDS-MW Assays with High- Speed Separation Method and High Throughput Tray Setup High Throughput Methods to Maximize the Use of the PA 800 Plus system Jose-Luis Gallegos-Perez
Biotechnological approaches in Pest Management
 Biotechnological approaches in Pest Management Biotechnology in Agriculture? Any technique that uses living organisms or substances from these organisms, to make or modify a product, to improve plants
Biotechnological approaches in Pest Management Biotechnology in Agriculture? Any technique that uses living organisms or substances from these organisms, to make or modify a product, to improve plants
Supplementary Figure Legend
 Supplementary Figure Legend Supplementary Figure S1. Effects of MMP-1 silencing on HEp3-hi/diss cell proliferation in 2D and 3D culture conditions. (A) Downregulation of MMP-1 expression in HEp3-hi/diss
Supplementary Figure Legend Supplementary Figure S1. Effects of MMP-1 silencing on HEp3-hi/diss cell proliferation in 2D and 3D culture conditions. (A) Downregulation of MMP-1 expression in HEp3-hi/diss
**MATERIAL SAFETY DATA SHEET**
 Date Issued: August 19, 2009 Version: 5.0 Supersedes: August 8, 2006 Page 1 of 5 Section 1 Product and Company Identification Product Name: Herceptin Chemical Name: Anti-p185 HER2 Chemical Family: High
Date Issued: August 19, 2009 Version: 5.0 Supersedes: August 8, 2006 Page 1 of 5 Section 1 Product and Company Identification Product Name: Herceptin Chemical Name: Anti-p185 HER2 Chemical Family: High
Chapter 8 Recombinant DNA Technology. 10/1/ MDufilho
 Chapter 8 Recombinant DNA Technology 10/1/2017 1 MDufilho The Role of Recombinant DNA Technology in Biotechnology Biotechnology? Recombinant deoxyribonucleic acid (DNA) technology Intentionally modifying
Chapter 8 Recombinant DNA Technology 10/1/2017 1 MDufilho The Role of Recombinant DNA Technology in Biotechnology Biotechnology? Recombinant deoxyribonucleic acid (DNA) technology Intentionally modifying
Toxicological Assessment of (silver) Nanomaterials: Challenges and Pitfalls
 Dr Helinor Johnston, Nano Safety Research Group Toxicological Assessment of (silver) Nanomaterials: Challenges and Pitfalls BfR Conference: Nanosilver Feb 2012 h.johnston@hw.ac.uk Nanotoxicology Nanotechnology
Dr Helinor Johnston, Nano Safety Research Group Toxicological Assessment of (silver) Nanomaterials: Challenges and Pitfalls BfR Conference: Nanosilver Feb 2012 h.johnston@hw.ac.uk Nanotoxicology Nanotechnology
Clostridium difficile (toxin B) genesig Easy Kit for use on the genesig q reaction. Primerdesign Ltd
 TM Primerdesign Ltd Clostridium difficile (toxin B) genesig Easy Kit for use on the genesig q16 50 reaction For general laboratory and research use only 1 genesig Easy: at a glance guide For each DNA test
TM Primerdesign Ltd Clostridium difficile (toxin B) genesig Easy Kit for use on the genesig q16 50 reaction For general laboratory and research use only 1 genesig Easy: at a glance guide For each DNA test
Elemental Speciation as an Essential Part of Formulating Exposure Assessments that Support Risk Estimates
 Elemental Speciation as an Essential Part of Formulating Exposure Assessments that Support Risk Estimates John T. Creed 1, Patricia A. Creed 1, Tatyana Pinyayev 1, Madhavi Mantha 2, John Trent 3, Edward
Elemental Speciation as an Essential Part of Formulating Exposure Assessments that Support Risk Estimates John T. Creed 1, Patricia A. Creed 1, Tatyana Pinyayev 1, Madhavi Mantha 2, John Trent 3, Edward
Introduction. Figure 1. Oris Cell Migration Assay Principle
 Optimizing Performance of the Membrane-free, Oris Cell Migration Assay for High Throughput Screening using the BioTek Synergy HT Multi-Mode Microplate Reader Keren I. Hulkower, Renee L. Herber, and Scott
Optimizing Performance of the Membrane-free, Oris Cell Migration Assay for High Throughput Screening using the BioTek Synergy HT Multi-Mode Microplate Reader Keren I. Hulkower, Renee L. Herber, and Scott
Proteomics. Areas of Application for Proteomics. Most Commonly Used Proteomics Techniques: Limitations: Examples
 Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Proteomics Areas of Application for Proteomics Most Commonly Used Proteomics Techniques: Antibody arrays Protein activity arrays 2-D gels ICAT technology SELDI Limitations: protein sources surfaces and
Product Safety Assessment Herculex RW Rootworm Protection
 Product Safety Assessment Herculex RW Rootworm Protection Select a Topic: Names Product Overview Manufacture of Product: Plant Transformation Process Product Description Product Uses Exposure Potential
Product Safety Assessment Herculex RW Rootworm Protection Select a Topic: Names Product Overview Manufacture of Product: Plant Transformation Process Product Description Product Uses Exposure Potential
The Long Range Science Strategy (LRSS) of Cosmetics Europe
 The Long Range Science Strategy (LRSS) of Cosmetics Europe Repeated dose systemic toxicity 2016 Bas Blaauboer Aim of LRSS: Development of a strategy to determine safety of cosmetic ingredients and products
The Long Range Science Strategy (LRSS) of Cosmetics Europe Repeated dose systemic toxicity 2016 Bas Blaauboer Aim of LRSS: Development of a strategy to determine safety of cosmetic ingredients and products
Value Scale (1-5) 1 I. Final Product Profile Characteristics of the product required for patient care
 In this example, the majority of the compounds was active in the xenograft model and showed "drug-like" properties from in-silico and experimental data. However, the animal studies showed moderate toxicity
In this example, the majority of the compounds was active in the xenograft model and showed "drug-like" properties from in-silico and experimental data. However, the animal studies showed moderate toxicity
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations. Audrey Chang, PhD, Senior Director Development Services
 Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
Overview of Biologics Testing and Evaluation: Regulatory Requirements and Expectations Audrey Chang, PhD, Senior Director Development Services Definition of Biologics: PHS Act, section 351 Virus, therapeutic
Chapter - 9 IN VITRO CYTOTOXICITY ASSAY OF ZERUMBONE AND MDM3:1
 Chapter - 9 IN VITRO CYTOTOXICITY ASSAY OF ZERUMBONE AND MDM3:1 9.1 INTRODUCTION 9.2 CHAPTER OBJECTIVE 9.3 MATERIALS AND METHODS 9.4 RESULT 9.5 DISCUSSION In Vitro Cytotoxicity Assay of Zerumbone and MDM
Chapter - 9 IN VITRO CYTOTOXICITY ASSAY OF ZERUMBONE AND MDM3:1 9.1 INTRODUCTION 9.2 CHAPTER OBJECTIVE 9.3 MATERIALS AND METHODS 9.4 RESULT 9.5 DISCUSSION In Vitro Cytotoxicity Assay of Zerumbone and MDM
BIO1PS 2012 Plant Science Topic 4 Lectures 2 and 3 Introduction to Plant Biotechnology
 BIO1PS 2012 Plant Science Topic 4 Lectures 2 and 3 Introduction to Plant Biotechnology Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Some Key Words Agrobacterium Ti plasmid
BIO1PS 2012 Plant Science Topic 4 Lectures 2 and 3 Introduction to Plant Biotechnology Dr. Michael Emmerling Department of Botany Room 410 m.emmerling@latrobe.edu.au Some Key Words Agrobacterium Ti plasmid
Clostridium difficile
 TM Primerdesign Ltd Clostridium difficile Toxin A (tcda) gene genesig Advanced Kit 150 tests For general laboratory and research use only 1 Introduction to Clostridium difficile Clostridium difficile is
TM Primerdesign Ltd Clostridium difficile Toxin A (tcda) gene genesig Advanced Kit 150 tests For general laboratory and research use only 1 Introduction to Clostridium difficile Clostridium difficile is
Genetic engineering and the food we eat*
 Genetic engineering and the food we eat* Prof. Daniel Chamovitz Tel Aviv University *and the clothes we wear, and the medicines we take, and gasoline we burn Problem #1: World Population is exploding 2011
Genetic engineering and the food we eat* Prof. Daniel Chamovitz Tel Aviv University *and the clothes we wear, and the medicines we take, and gasoline we burn Problem #1: World Population is exploding 2011
Xfect Protein Transfection Reagent
 Xfect Protein Transfection Reagent Mammalian Expression Systems Rapid, high-efficiency, low-toxicity protein transfection Transfect a large amount of active protein Virtually no cytotoxicity, unlike lipofection
Xfect Protein Transfection Reagent Mammalian Expression Systems Rapid, high-efficiency, low-toxicity protein transfection Transfect a large amount of active protein Virtually no cytotoxicity, unlike lipofection
Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y. Report Price Publication date
 F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
F o r a c l e a r e r m a r k e t p e r s p e c t i v e Early Stage Drug Safety Strategies & Risk Management Maximizing opportunities towards achieving clinical success D R U G D I S C O V E R Y Report
Clostridium difficile
 TM Primerdesign Ltd Clostridium difficile Toxin A (tcda) gene genesig Standard Kit 150 tests For general laboratory and research use only 1 Introduction to Clostridium difficile Clostridium difficile is
TM Primerdesign Ltd Clostridium difficile Toxin A (tcda) gene genesig Standard Kit 150 tests For general laboratory and research use only 1 Introduction to Clostridium difficile Clostridium difficile is
Future Biotechnology Products and Opportunities to Enhance the Capabilities of the Biotechnology Regulatory System
 Future Biotechnology Products and Opportunities to Enhance the Capabilities of the Biotechnology Regulatory System June 2016 Synthetic Biology at Modular Genetics The Future of Chemical Manufacturing and
Future Biotechnology Products and Opportunities to Enhance the Capabilities of the Biotechnology Regulatory System June 2016 Synthetic Biology at Modular Genetics The Future of Chemical Manufacturing and
Tox21: Opportunities& Challenges. Richard A. Becker Ph.D., DABT American Chemistry Council
 Tox21: Opportunities& Challenges Richard A. Becker Ph.D., DABT American Chemistry Council October 21, 2010 Opportunities and Challenges The manifest challenge is to develop in vitro and in silico approaches
Tox21: Opportunities& Challenges Richard A. Becker Ph.D., DABT American Chemistry Council October 21, 2010 Opportunities and Challenges The manifest challenge is to develop in vitro and in silico approaches
Recombinant DNA Technology. The Role of Recombinant DNA Technology in Biotechnology. yeast. Biotechnology. Recombinant DNA technology.
 PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University C H A P T E R 8 Recombinant DNA Technology The Role of Recombinant DNA Technology in Biotechnology Biotechnology?
PowerPoint Lecture Presentations prepared by Mindy Miller-Kittrell, North Carolina State University C H A P T E R 8 Recombinant DNA Technology The Role of Recombinant DNA Technology in Biotechnology Biotechnology?
Genetically modified sugarcane and Eldana. Sandy Snyman Agronomist s Association Annual Symposium 27 October 2015
 Genetically modified sugarcane and Eldana Sandy Snyman Agronomist s Association Annual Symposium 27 October 2015 GM=Genetically modified What is GM? DNA/gene from one source is transferred to another organism
Genetically modified sugarcane and Eldana Sandy Snyman Agronomist s Association Annual Symposium 27 October 2015 GM=Genetically modified What is GM? DNA/gene from one source is transferred to another organism
BL-7040: Oligonucleotide for Inflammatory Bowel Disease
 BL-7040: Oligonucleotide for Inflammatory Bowel Disease December 2012 Forward Looking Statements This presentation contains "forward-looking statements." These statements include words like "may," "expects,"
BL-7040: Oligonucleotide for Inflammatory Bowel Disease December 2012 Forward Looking Statements This presentation contains "forward-looking statements." These statements include words like "may," "expects,"
Cell Migration, Chemotaxis and Invasion Assay Using Staining
 Cell Migration, Chemotaxis and Invasion Assay Using Staining Protocol Hillary Sherman and Mark Rothenberg Corning Life Sciences 836 North St. Bldg. 300, Suite 3401 Tewksbury, MA 01876 Introduction Cell
Cell Migration, Chemotaxis and Invasion Assay Using Staining Protocol Hillary Sherman and Mark Rothenberg Corning Life Sciences 836 North St. Bldg. 300, Suite 3401 Tewksbury, MA 01876 Introduction Cell
Abstract. Technical Aspects. Applying GastroPlus for Extensions of Biowaivers for BCS Class II Compounds 2
 Abstract GastroPlus is a mechanistically based simulation software package that predicts absorption, pharmacokinetics, and pharmacodynamics in humans and animals. GastroPlus modeling and simulation has
Abstract GastroPlus is a mechanistically based simulation software package that predicts absorption, pharmacokinetics, and pharmacodynamics in humans and animals. GastroPlus modeling and simulation has
Structure and content of an IMPD. What is required for first into man trial?
 What is required for first into man? The EU IMPD Thomas Sudhop, MD Scope Structure and content of an IMPD What is required for first into man trial? Only for IMPs that do not have a marketing authorisation
What is required for first into man? The EU IMPD Thomas Sudhop, MD Scope Structure and content of an IMPD What is required for first into man trial? Only for IMPs that do not have a marketing authorisation
How plants survive glyphosate
 IOWA STATE UNIVERSITY University Extension http://www.weeds.iastate.edu/mgmt/2007/glysurvival.pdf Weed Science Department of Agronomy How plants survive glyphosate Glyphosate is the most widely used herbicide
IOWA STATE UNIVERSITY University Extension http://www.weeds.iastate.edu/mgmt/2007/glysurvival.pdf Weed Science Department of Agronomy How plants survive glyphosate Glyphosate is the most widely used herbicide
Pocket K No. 42. Stacked Traits in Biotech Crops
 Pocket K No. 42 Stacked Traits in Biotech Crops The biotech method of gene stacking has brought us many notable products such as Golden Rice, Blue Rose, and SmartStax. What is gene stacking and why is
Pocket K No. 42 Stacked Traits in Biotech Crops The biotech method of gene stacking has brought us many notable products such as Golden Rice, Blue Rose, and SmartStax. What is gene stacking and why is
PKM2 Influences the Metabolic Fate of Butyrate in Colorectal Cancer Cells
 University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2018 PKM2 Influences the Metabolic
University of Tennessee, Knoxville Trace: Tennessee Research and Creative Exchange University of Tennessee Honors Thesis Projects University of Tennessee Honors Program 5-2018 PKM2 Influences the Metabolic
Real Time Cell Electronic Sensing (RT-CES) for Nanotoxicity Evaluation
 Real Time Cell Electronic Sensing (RT-CES) for Nanotoxicity Evaluation Reyes Sierra 1, Lila Otero 1, Scott Boitano 2 & Jim Field 1 1 Dept Chemical & Environmental Engineering 1 2 Dept Cell Physiology The
Real Time Cell Electronic Sensing (RT-CES) for Nanotoxicity Evaluation Reyes Sierra 1, Lila Otero 1, Scott Boitano 2 & Jim Field 1 1 Dept Chemical & Environmental Engineering 1 2 Dept Cell Physiology The
DEVELOPMENT OF NUCLEAR RECEPTOR TRANSFECTED CACO-2 CELL LINES. Timo Korjamo University of Kuopio Finland 27th October 2006
 DEVELOPMENT OF NUCLEAR RECEPTOR TRANSFECTED CACO-2 CELL LINES Timo Korjamo University of Kuopio Finland 27th October 2006 Background Cell lines Gene expression Functional experiments Conclusions Intestinal
DEVELOPMENT OF NUCLEAR RECEPTOR TRANSFECTED CACO-2 CELL LINES Timo Korjamo University of Kuopio Finland 27th October 2006 Background Cell lines Gene expression Functional experiments Conclusions Intestinal
Safety evaluation of technical enzyme products. legislation. 09 June 2009 Brussels
 Safety evaluation of technical enzyme products with regards to the REACH legislation 09 June 2009 Brussels ENZYMES What enzymes are Enzymes are proteins with highly specialized catalytic function Produced
Safety evaluation of technical enzyme products with regards to the REACH legislation 09 June 2009 Brussels ENZYMES What enzymes are Enzymes are proteins with highly specialized catalytic function Produced
SCIENTIFIC OPINION. Abstract
 SCIENTIFIC OPINION ADOPTED: 25 October 2017 doi: 10.2903/j.efsa.2017.5067 Assessment of genetically modified oilseed rape MS8, RF3 and MS83RF3 for renewal of authorisation under regulation (EC) No 1829/2003
SCIENTIFIC OPINION ADOPTED: 25 October 2017 doi: 10.2903/j.efsa.2017.5067 Assessment of genetically modified oilseed rape MS8, RF3 and MS83RF3 for renewal of authorisation under regulation (EC) No 1829/2003
FDA Public Hearing: Approval Pathway for Biosimilar. Products. November 2-3, 2010
 FDA Public Hearing: Approval Pathway for Biosimilar and Interchangeable Biological Products November 2-3, 2010 1 The Biotechnology Industry Organization Over 1,100 members, including biotechnology companies,
FDA Public Hearing: Approval Pathway for Biosimilar and Interchangeable Biological Products November 2-3, 2010 1 The Biotechnology Industry Organization Over 1,100 members, including biotechnology companies,
Molecular assessment of GMOs Ilona Kryspin Sørensen PhD DTU Food Division for Risk Assessment and Nutrition
 Molecular assessment of GMOs Ilona Kryspin Sørensen PhD DTU Food Division for Risk Assessment and Nutrition Objective : to assess the level of documentation necessary for the evaluation of the insertion
Molecular assessment of GMOs Ilona Kryspin Sørensen PhD DTU Food Division for Risk Assessment and Nutrition Objective : to assess the level of documentation necessary for the evaluation of the insertion
How do drugs work? Part 1. PK: Principles & Processes. PK vs. PD. Pharmacokinetics : Basic Principles Overview. What does the body do to a drug?
 Pharmacokinetics : Basic Principles verview 1. PK : Basic principles and processes 2. Important PK Parameters 3. Modulating PK Parameters 4. PK : Drugs vs. contrast agents Twan Lammers Dept. of Experimental
Pharmacokinetics : Basic Principles verview 1. PK : Basic principles and processes 2. Important PK Parameters 3. Modulating PK Parameters 4. PK : Drugs vs. contrast agents Twan Lammers Dept. of Experimental
MATERIAL SAFETY DATA SHEET
 Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
Page 1 of 6 1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND THE COMPANY/UNDERTAKING Pfizer Inc Pfizer Pharmaceuticals Group 235 East 42nd Street New York, New York 10017 1-212-573-2222 Emergency telephone
A Bridging Immunogenicity Assay Using SPARCL TM Technology
 A Bridging Immunogenicity Assay Using SPARCL TM Technology Wenhua F. Xie Lumigen Inc., a Beckman Coulter Company INTRODUCTION Evaluating the immune response in patients is an important aspect associated
A Bridging Immunogenicity Assay Using SPARCL TM Technology Wenhua F. Xie Lumigen Inc., a Beckman Coulter Company INTRODUCTION Evaluating the immune response in patients is an important aspect associated
PUTTING EXPOSURE BACK IN RISK ASSESSMENT OR: THE DOSE MAKES THE POISON
 PUTTING EXPOSURE BACK IN RISK ASSESSMENT OR: THE DOSE MAKES THE POISON Debra A. Kaden, PhD Presented to The International Society of Exposure Science and The Society of Toxicology WHAT I WILL DISCUSS TODAY
PUTTING EXPOSURE BACK IN RISK ASSESSMENT OR: THE DOSE MAKES THE POISON Debra A. Kaden, PhD Presented to The International Society of Exposure Science and The Society of Toxicology WHAT I WILL DISCUSS TODAY
A Level. A Level Biology. DNA Technology Questions. AQA, OCR, Edexcel. Name: Total Marks: Page 1
 AQA, OCR, Edexcel A Level A Level Biology DNA Technology Questions Name: Total Marks: Page 1 Q1.(a) (i) A mutation of a tumour suppressor gene can result in the formation of a tumour. Explain how.........(2)
AQA, OCR, Edexcel A Level A Level Biology DNA Technology Questions Name: Total Marks: Page 1 Q1.(a) (i) A mutation of a tumour suppressor gene can result in the formation of a tumour. Explain how.........(2)
Bovine Serum Albumin (BSA) Assay
 Bovine Serum Albumin (BSA) Assay Immunoenzymetric Assay for the Measurement of BSA Catalog # F030 Intended Use This kit is intended for use in quantitating bovine serum albumin (BSA). The kit is for Research
Bovine Serum Albumin (BSA) Assay Immunoenzymetric Assay for the Measurement of BSA Catalog # F030 Intended Use This kit is intended for use in quantitating bovine serum albumin (BSA). The kit is for Research
CytoSelect LDH Cytotoxicity Assay Kit
 Product Manual CytoSelect LDH Cytotoxicity Assay Kit Catalog Number CBA- 241 960 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The measurement and monitoring of cell cytotoxicity
Product Manual CytoSelect LDH Cytotoxicity Assay Kit Catalog Number CBA- 241 960 assays FOR RESEARCH USE ONLY Not for use in diagnostic procedures Introduction The measurement and monitoring of cell cytotoxicity
ENZYMES REACH PRE-CONSORTIUM
 ERpC/09/06 25MAR2009 Safety evaluation of technical enzyme products with regards to the REACH legislation 1 Introduction According to the REACH guidance on Substance Identification, the safety documentation
ERpC/09/06 25MAR2009 Safety evaluation of technical enzyme products with regards to the REACH legislation 1 Introduction According to the REACH guidance on Substance Identification, the safety documentation
Outline and learning objectives. From Proteomics to Systems Biology. Integration of omics - information
 From to Systems Biology Outline and learning objectives Omics science provides global analysis tools to study entire systems How to obtain omics - What can we learn Limitations Integration of omics - In-class
From to Systems Biology Outline and learning objectives Omics science provides global analysis tools to study entire systems How to obtain omics - What can we learn Limitations Integration of omics - In-class
ELISPOT and FLUOROSPOT kits
 ELISPOT and FLUOROSPOT kits Interleukins Interferons Granzymes and perforins TNF superfamily ligands and receptors Apoptosis markers And many more... ELISPOT and FLUOROSPOT: a cell-based assay to assess
ELISPOT and FLUOROSPOT kits Interleukins Interferons Granzymes and perforins TNF superfamily ligands and receptors Apoptosis markers And many more... ELISPOT and FLUOROSPOT: a cell-based assay to assess
Preclinical Development Drugs. Darrin Cowley PhD Executive Director Amgen BioBoot Camp 2015
 Preclinical Development Drugs Darrin Cowley PhD Executive Director Amgen BioBoot Camp 2015 Product Development: Development process: File Approval Drug Discovery Preclinical Phase 1 Phase 2 Phase 3 Lifecycle
Preclinical Development Drugs Darrin Cowley PhD Executive Director Amgen BioBoot Camp 2015 Product Development: Development process: File Approval Drug Discovery Preclinical Phase 1 Phase 2 Phase 3 Lifecycle
We are committed to translating ground-breaking science into genomic therapies that transform patients lives
 We are committed to translating ground-breaking science into genomic therapies that transform patients lives Liver-based expression of the human alpha-galactosidase A gene (GLA) in a murine Fabry model
We are committed to translating ground-breaking science into genomic therapies that transform patients lives Liver-based expression of the human alpha-galactosidase A gene (GLA) in a murine Fabry model
CHAPTER 08: RECOMBINANT DNA TECHNOLOGY Pearson Education, Inc.
 CHAPTER 08: RECOMBINANT DNA TECHNOLOGY The Role of Recombinant DNA Technology in Biotechnology Biotechnology the use of microorganisms to make practical products Recombinant DNA technology Intentionally
CHAPTER 08: RECOMBINANT DNA TECHNOLOGY The Role of Recombinant DNA Technology in Biotechnology Biotechnology the use of microorganisms to make practical products Recombinant DNA technology Intentionally
A) B) Ladder. Supplementary Figure 1. Recombinant calpain 14 purification analysis. A) The general domain structure of classical
 A) B) Ladder C) r4 r4 Nt- -Ct 78 kda Supplementary Figure 1. Recombinant calpain 14 purification analysis. A) The general domain structure of classical calpains is shown. The protease core consists of
A) B) Ladder C) r4 r4 Nt- -Ct 78 kda Supplementary Figure 1. Recombinant calpain 14 purification analysis. A) The general domain structure of classical calpains is shown. The protease core consists of
Genomics. Genomics. Understanding the human genome. The human genome. Genomics = study of an organism s entire genome or entire DNA sequence
 Genomics Genomics Genomics = study of an organism s entire genome or entire DNA sequence billion bases % of DNA shared Humans 3.2 99.5% Chimpanzee 2.8 98.5% Mouse 2.5 80% Chicken 1.0 So what s a genome?
Genomics Genomics Genomics = study of an organism s entire genome or entire DNA sequence billion bases % of DNA shared Humans 3.2 99.5% Chimpanzee 2.8 98.5% Mouse 2.5 80% Chicken 1.0 So what s a genome?
Oncology Biopharmaceuticals and Preclinical Development: Evolving Regulatory Challenges
 The content of this presentation reflects the opinion of the speaker and does not necessarily represent the official position of CDER Oncology Biopharmaceuticals and Preclinical Development: Evolving Regulatory
The content of this presentation reflects the opinion of the speaker and does not necessarily represent the official position of CDER Oncology Biopharmaceuticals and Preclinical Development: Evolving Regulatory
Material Safety Data Sheet
 Material Safety Data Sheet Kit for the preparation of Technetium Tc99m Medronate for Injection ISSUE DATE: 20-Jun-08 ORIGINATOR: P.E. Buck REVIEWER: Lars Waldmann CHEMICAL PRODUCT/COMPANY IDENTIFICATION
Material Safety Data Sheet Kit for the preparation of Technetium Tc99m Medronate for Injection ISSUE DATE: 20-Jun-08 ORIGINATOR: P.E. Buck REVIEWER: Lars Waldmann CHEMICAL PRODUCT/COMPANY IDENTIFICATION
Managing Corn Pests with Bt Corn: Some Questions and Answers March 2003
 Managing Corn Pests with Bt Corn: Some Questions and Answers March 2003 Frank B. Peairs Department of Bioagricultural Sciences and Pest Management Colorado State University Introduction New technology
Managing Corn Pests with Bt Corn: Some Questions and Answers March 2003 Frank B. Peairs Department of Bioagricultural Sciences and Pest Management Colorado State University Introduction New technology
Staining Techniques. Staining Techniques. There are many dyes. Histochemical Stains: chemical reactions. Feulgen reaction -DNA
 Staining Techniques There are many dyes. http://medinfo.ufl.edu/~dental/denhisto/stains.html Examples: Sudan black -Lipids Myelinated axons- blue ihcworld.com/imagegallery/displayimage.php?al... Weigert
Staining Techniques There are many dyes. http://medinfo.ufl.edu/~dental/denhisto/stains.html Examples: Sudan black -Lipids Myelinated axons- blue ihcworld.com/imagegallery/displayimage.php?al... Weigert
TECHNICAL BULLETIN. In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits
 In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits Catalog Numbers APPA001 In Vitro Bacterial Split GFP "Fold 'n' Glow" Solubility Assay Kit (Green) APPA008 In Vitro Bacterial
In Vitro Bacterial Split Fluorescent Protein Fold n Glow Solubility Assay Kits Catalog Numbers APPA001 In Vitro Bacterial Split GFP "Fold 'n' Glow" Solubility Assay Kit (Green) APPA008 In Vitro Bacterial
Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research
 Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research Drug Development Process by which new chemical entities
Drug Development: Why Does it Cost so Much? Lewis J. Smith, MD Professor of Medicine Director, Center for Clinical Research Associate VP for Research Drug Development Process by which new chemical entities
Diphoterine, An Amphoteric, Polyvalent, Hypertonic Eye/Skin Chemical Splash Decontamination Solution: 14 Years Updated Data
 Diphoterine, An Amphoteric, Polyvalent, Hypertonic Eye/Skin Chemical Splash Decontamination Solution: 14 Years Updated Data Hall AH 1, Mathieu L 2, Blomet J 2 1Toxicology Consulting and Medical Translating
Diphoterine, An Amphoteric, Polyvalent, Hypertonic Eye/Skin Chemical Splash Decontamination Solution: 14 Years Updated Data Hall AH 1, Mathieu L 2, Blomet J 2 1Toxicology Consulting and Medical Translating
Supplementary Information
 Supplementary Information Extended Loop Region of Hcp1 is Critical for the Assembly and Function of Type VI Secretion System in Burkholderia pseudomallei Yan Ting Lim, Chacko Jobichen, Jocelyn Wong, Direk
Supplementary Information Extended Loop Region of Hcp1 is Critical for the Assembly and Function of Type VI Secretion System in Burkholderia pseudomallei Yan Ting Lim, Chacko Jobichen, Jocelyn Wong, Direk
RapidFACT: Accelerated Formulation Development for Poorly Soluble Drugs and Modified Release Products
 RapidFACT: Accelerated Formulation Development for Poorly Soluble Drugs and Modified Release Products Kevin Kane, Scientific Director, BCP 7 th Annual Global Drug Delivery & Formulation Summit 28 th August
RapidFACT: Accelerated Formulation Development for Poorly Soluble Drugs and Modified Release Products Kevin Kane, Scientific Director, BCP 7 th Annual Global Drug Delivery & Formulation Summit 28 th August
Genetic Modification in Our World. By: Paisley, Michael, Blake, and Elyse
 Genetic Modification in Our World By: Paisley, Michael, Blake, and Elyse Biopharming Genetically Modified Foods BIoremediation process of genetically modifying plants and animals to produce substances
Genetic Modification in Our World By: Paisley, Michael, Blake, and Elyse Biopharming Genetically Modified Foods BIoremediation process of genetically modifying plants and animals to produce substances
Supplementary Figure 1. Isolation of GFPHigh cells.
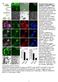 Supplementary Figure 1. Isolation of GFP High cells. (A) Schematic diagram of cell isolation based on Wnt signaling activity. Colorectal cancer (CRC) cell lines were stably transduced with lentivirus encoding
Supplementary Figure 1. Isolation of GFP High cells. (A) Schematic diagram of cell isolation based on Wnt signaling activity. Colorectal cancer (CRC) cell lines were stably transduced with lentivirus encoding
PUBLIC CONSULTATION AT STEP 4 OF THE VICH PROCEDURE OVERVIEW OF COMMENTS RECEIVED
 VICH/12/056 FINAL PUBLIC CONSULTATION AT STEP 4 OF THE VICH PROCEDURE OVERVIEW OF COMMENTS RECEIVED VICH draft Guideline 54 - Studies to Evaluate the Safety of Residues of Veterinary Drugs in Human Food:
VICH/12/056 FINAL PUBLIC CONSULTATION AT STEP 4 OF THE VICH PROCEDURE OVERVIEW OF COMMENTS RECEIVED VICH draft Guideline 54 - Studies to Evaluate the Safety of Residues of Veterinary Drugs in Human Food:
Intestinal Epithelial Cell-Specific Deletion of PLD2 Alleviates DSS-Induced Colitis by. Regulating Occludin
 Intestinal Epithelial Cell-Specific Deletion of PLD2 Alleviates DSS-Induced Colitis by Regulating Occludin Chaithanya Chelakkot 1,ǂ, Jaewang Ghim 2,3,ǂ, Nirmal Rajasekaran 4, Jong-Sun Choi 5, Jung-Hwan
Intestinal Epithelial Cell-Specific Deletion of PLD2 Alleviates DSS-Induced Colitis by Regulating Occludin Chaithanya Chelakkot 1,ǂ, Jaewang Ghim 2,3,ǂ, Nirmal Rajasekaran 4, Jong-Sun Choi 5, Jung-Hwan
Antibody-Drug Conjugate Bioanalytical Assay Development:
 Antibody-Drug Conjugate Bioanalytical Assay Development: Immunogenicity Challenges November 16, 2016 Presented by Corinna Fiorotti, Ph.D. Presentation Overview ADC Overview ADC Assays ADC Immunogenicity
Antibody-Drug Conjugate Bioanalytical Assay Development: Immunogenicity Challenges November 16, 2016 Presented by Corinna Fiorotti, Ph.D. Presentation Overview ADC Overview ADC Assays ADC Immunogenicity
Laboratory Reagents. for life science research.
 Laboratory Reagents Research, quality control, or routine analysis whatever the field of activity, where there is a need for laboratory reagents, Thermo Fisher Scientific has a suitable product. We offer
Laboratory Reagents Research, quality control, or routine analysis whatever the field of activity, where there is a need for laboratory reagents, Thermo Fisher Scientific has a suitable product. We offer
COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP)
 European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 22 February 2006 EMEA/CHMP/BMWP/42832/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON SIMILAR
European Medicines Agency Pre-authorisation Evaluation of Medicines for Human Use London, 22 February 2006 EMEA/CHMP/BMWP/42832/2005 COMMITTEE FOR MEDICINAL PRODUCTS FOR HUMAN USE (CHMP) GUIDELINE ON SIMILAR
The best and brightest
 Labeling and Detection The best and brightest Alexa Fluor 488 dye Labeling and Detection A superior alternative to FITC Brighter conjugate fluorescence Unequalled photostability Perfect spectral match
Labeling and Detection The best and brightest Alexa Fluor 488 dye Labeling and Detection A superior alternative to FITC Brighter conjugate fluorescence Unequalled photostability Perfect spectral match
Gunasekar Ramachandran a, Natesan Sundaramoorthy Karthikeyan a, b, Periyasamy Giridharan c, Kulathu Iyer Sathiyanarayanan a *
 Electronic Supplementary Information For Efficient iodine catalyzed three components domino reaction for the synthesis of 1- ((phenylthio)(phenyl)methyl)pyrrolidin-2-one derivatives possessing anticancer
Electronic Supplementary Information For Efficient iodine catalyzed three components domino reaction for the synthesis of 1- ((phenylthio)(phenyl)methyl)pyrrolidin-2-one derivatives possessing anticancer
Use of Antisense Oligonucleotides for the Treatment of Inheritable Rare Disorders. C. Frank Bennett Isis Pharmaceuticals
 Use of Antisense ligonucleotides for the Treatment of Inheritable Rare Disorders C. Frank Bennett Isis Pharmaceuticals Agenda Review different antisense strategies Delivery of oligonucleotides to the skin,
Use of Antisense ligonucleotides for the Treatment of Inheritable Rare Disorders C. Frank Bennett Isis Pharmaceuticals Agenda Review different antisense strategies Delivery of oligonucleotides to the skin,
Drug Tolerance in ADA Analysis
 Drug Tolerance in ADA Analysis Matthew Bentley www.eurofins.com DRUG TOLERANCE What is Drug Interference and Why an issue in ADA analysis? Methodologies to overcome Drug Interference Acid Dissociation
Drug Tolerance in ADA Analysis Matthew Bentley www.eurofins.com DRUG TOLERANCE What is Drug Interference and Why an issue in ADA analysis? Methodologies to overcome Drug Interference Acid Dissociation
MicroRNA Analysis Paired with Novel Cell Health Assays: A Complete Workflow
 MicroRNA Analysis Paired with Novel Cell Health Assays: A Complete Workflow Brad Hook, Ph.D Manager, NA Scientific Applications 2016 Presentation Outline From cells to RNA a seemingly easy, yet complex
MicroRNA Analysis Paired with Novel Cell Health Assays: A Complete Workflow Brad Hook, Ph.D Manager, NA Scientific Applications 2016 Presentation Outline From cells to RNA a seemingly easy, yet complex
PK and PK/PD Guided Starting Dose Selection for First-In-Human Trials. Sylvia Zhao ( 赵子微 ) Translational Clinical Oncology Novartis
 PK and PK/PD Guided Starting Dose Selection for First-In-Human Trials Sylvia Zhao ( 赵子微 ) Translational Clinical Oncology Novartis Disclaimer Contents are the opinion of the author and not that of Novartis
PK and PK/PD Guided Starting Dose Selection for First-In-Human Trials Sylvia Zhao ( 赵子微 ) Translational Clinical Oncology Novartis Disclaimer Contents are the opinion of the author and not that of Novartis
Cosmetics Europe LRSS Programme
 Cosmetics Europe LRSS Programme 2016-20 Rob Taalman Objectives of CE Research To deliver tools & strategies for animal-free safety assessment Accurate Robust Efficient ultimately accepted by regulators
Cosmetics Europe LRSS Programme 2016-20 Rob Taalman Objectives of CE Research To deliver tools & strategies for animal-free safety assessment Accurate Robust Efficient ultimately accepted by regulators
Immune System. Viruses vs. Bacteria
 Immune System Viruses vs. Bacteria Concept Map Section 19-1 Bacteria are classified into the kingdoms of Eubacteria Archaebacteria include a variety of lifestyles such as live in harsh environments such
Immune System Viruses vs. Bacteria Concept Map Section 19-1 Bacteria are classified into the kingdoms of Eubacteria Archaebacteria include a variety of lifestyles such as live in harsh environments such
The Role for Edible Vaccines
 The Role for Edible Vaccines JOHN A. HOWARD 1 ProdiGene College Station, TX For centuries, plants have been used as sources of pharmaceuticals. Since the advent of recombinant-dna technology, however,
The Role for Edible Vaccines JOHN A. HOWARD 1 ProdiGene College Station, TX For centuries, plants have been used as sources of pharmaceuticals. Since the advent of recombinant-dna technology, however,
Preclinical Drug Development
 Preclinical Drug Development A guidance prepared by From a 2004 NIH Summit Workshop: A major reason for the tremendous cost of drug development is the high rate of drug candidate failure during clinical
Preclinical Drug Development A guidance prepared by From a 2004 NIH Summit Workshop: A major reason for the tremendous cost of drug development is the high rate of drug candidate failure during clinical
A Review on Microdosing: Reduction in Cost & Time
 Human Journals Review Article June 2016 Vol.:6, Issue:3 All rights are reserved by Rupali Yevale et al. A Review on Microdosing: Reduction in Cost & Time Keywords: Microdosing, phase 0, LC-MS and AMS ABSTRACT
Human Journals Review Article June 2016 Vol.:6, Issue:3 All rights are reserved by Rupali Yevale et al. A Review on Microdosing: Reduction in Cost & Time Keywords: Microdosing, phase 0, LC-MS and AMS ABSTRACT
Material Safety Data Sheet
 Material Safety Data Sheet Cytochrome C, Bovine MSDS 0 He a lt h Fire Re a c t iv it y Pe rs o n a l Pro t e c t io n 0 E Section : Chemical Product and Company Identification Product Name: Cytochrome
Material Safety Data Sheet Cytochrome C, Bovine MSDS 0 He a lt h Fire Re a c t iv it y Pe rs o n a l Pro t e c t io n 0 E Section : Chemical Product and Company Identification Product Name: Cytochrome
