arrier Isolation Technology
|
|
|
- Sherman Rose
- 6 years ago
- Views:
Transcription
1 B PharmaED s Register by November 15 th and receive a $300 Discount! arrier Isolation Technology New Technologies, Case Studies and Current Applications in the Usage of Isolator Systems JANUARY 12-13, 2009, RADISSON-PLAZA WARWICK, PHILADELPHIA, PA Featuring Case Studies and Lessons Learned from Industry Experts! Strategies for Assessing Isolator Performance and Containment Effectiveness Performing Sterility Testing and Environmental Monitoring within an Isolator System Case Studies and Design Considerations for Clinical Scale Aseptic Filling Containment Solutions for Closed Vial Technologies and Evaluating Isolator vs. RABS Technologies Decontamination of Isolators and Validating Glove Integrity Examining Blow/Fill/Seal Insert Technology Facility Design Considerations and Strategies for Process Equipment Integration Exploring Electron Beam Technologies Plus In-depth Session: Evaluating Contamination Concerns for Aseptic Manufacturing and Sterility Test Isolators Ken Muhvich, Ph.D., Principal Consultant, Micro-Reliance LLC Featuring Representation From: Advanced Electron Beams Applied Prime Technologies Rommelag USA, Inc Aseptic Barrier Systems LLC ASEPTIC Technologies Micro-Reliance LLC DSM Pharmaceuticals, Inc. IPS SKAN AG Isogen LLC. La Calhène, Inc. Walker Barrier SafeBridge Consultants, Inc. STERIS Corporation PharmaED R E S O U R C E S, I n c. P h a r m a E d Re s o u r c e s, I n c Ro b e s o n Pa r k D r iv e C h a m p a i g n, I L t e l fa x w e b. w w w. p h a r m a e d r e s o u r c e s. c o m
2 STRATEGIES FOR ASSESSING ISOLATOR PERFORMANCE AND CONTAINMENT EFFECTIVENESS Monday, January 12, :30 Chairperson s Welcome and Opening Remarks CONTROLLING MICROBIAL CONTAMINATION 8:45 Evaluating Contamination Concerns for Aseptic Manufacturing and Sterility Test Isolators Ken Muhvich, Ph.D., Principal Consultant, Micro-Reliance LLC Isolator environments are used for aseptic production and release testing of sterile pharmaceutical products. These isolators are barriers to the most significant source of microbial contamination human operators. If isolators are used properly, then sterile product contamination should not occur and there should be no sterility test false positive test results. However, it is often the case that, once qualified, the care and maintenance of both production and sterility test isolators is not the best. When this occurs, the result is often microbial contamination problems. This talk will provide case studies to illustrate what can happen when isolators of both types are not properly maintained. You will learn: 1) The best conditions/locations in which to operate the isolators to prevent microbial contamination. 2) How to properly disinfect sterility test samples before transfer into the sterility test isolator. 3) The optimal environmental monitoring sites and frequencies to prevent microbial contamination issues. 4) Environmental conditions that can lead to contamination of isolators once they have been decontaminated. 5) Proper cleaning and sanitization of isolators to prevent microbial contamination. 10:45 Refreshment Break STERILITY TESTING AND ENVIRONMENTAL MONITORING 11:00 Sterility Testing and Environmental Monitoring within an Isolator System Darcy Adee, Manager, Microbiology & Sample Management, DSM Pharmaceuticals, Inc. This session will address the advantages of sterility testing performed within an isolator system. The importance of an environmental monitoring program and how to handle breeches and excursions will be discussed. Other topics to be included are training, handling of personnel in regard to ergonomics and extrinsic contamination, and maintaining an excellent compliance record. 12:00 Luncheon ISOLATOR SUITE DESIGN 1:15 Case Studies and Design Considerations for Clinical Scale Aseptic Filling Patrice Cloué, Director, La Calhène, Inc. Within the last 10 years, isolator has been the technique of choice for aseptic filling suite, especially for small and medium production. Isolators offer containment solutions for aseptic and/or toxic product handling and are now common in the industry. This presentation will go over several clinical scale filling suites and will show the different possible approaches in the isolator suite design. The attendees will learn about isolator construction, component/product transfer, and classification inside the chamber for the different step of the process. CASE STUDY PRESENTATION 2:00 Closed Vial Technology: a Revolution in Aseptic Filling Benoit Verjans, Director, ASEPTIC Technologies The closed vial technology has been developed by GSK Biologicals to address the major issues of aseptic filling. s such as isolators and RABS will be illustrated. The first issue is the quality for the patient. First, many deadly accidents are recorded each year across the world due to injection of contaminated products. Second, counterfeiting is becoming a major issue for injectable drugs and the authorities such as FDA are expecting to have product traceability systems put in place. Finally, aseptic filling has reach such a complexity level due to WFI washing, sterilization, high speed stoppering, aluminum cap crimping that an easier solution is welcomed. The solution of the closed vial technology consists to provide a vial molded and assembled in class 100 and
3 CASE STUDIES AND DESIGN CONSIDERATIONS FOR CLINICAL SCALE ASEPTIC FILLING gamma-irradiated. This process generates a vial clean and sterile therefore ready-to-fill. The filling process is made of a needle passing through the stopper, dispensing the liquid and withdrawn. The piercing trace is re-sealed by laser and a plastic cap is placed by snap-fit. This process increases the quality by supplying a container which stays permanently closed and therefore behaves as an isolator by it-self, and eliminates the most complex steps of aseptic filling. As it is a new process with a new type of container, a specific containment system has been developed to address this process. This containment, called CVFS will be detailed and advantages over the classical barrier 2:45 Refreshment Break ASSESSING ISOLATOR PERFORMANCE 3:00 Measurement of Barrier Isolator and Containment Effectiveness Through the Use of Surrogate Powders and/or Active Pharmaceutical Ingredients Robert G. Sussman, Ph.D., DABT, Managing Principal Eastern Operations, SafeBridge Consultants, Inc. A proper approach to conducting a meaningful assessment of the performance of barrier isolators and other containment devices for the handling of powders and liquids including potent pharmaceutical materials will be discussed. The presentation will review the importance of conducting FAT and SAT testing for containment performance as well as worker exposure potential, the advantages and disadvantages of different surrogate materials, what to do when there is no monitoring method for the material of interest, and how to interpret data developed from such assessments. 3:45 Decontamination of Isolators: Past, Present, Future Claire Fritz, VHP Process Engineer, STERIS Corporation Isolation technology began in the 1980s. Liquid peracetic acid was one of the first decontamination methods available for isolators. Vaporized Hydrogen Peroxide (VHP ) was first introduced in 1991 by American Sterilizer Company. Many forms of the technology have been developed over the last 15 years as isolator applications have increased and expanded. Vapor phase hydrogen peroxide remains the dominant form of decontamination for isolators due to its excellent material compatibility, efficacy, and its innocuous byproducts of water vapor and oxygen. History of decontamination Process of vapor phase hydrogen peroxide Variables affecting efficacy Validation (biological indicators, real time sensors, residue analysis) How do you develop the fastest, most repeatable cycle? 4:30 Case Study for the Selection, Installation and Validation of an Aseptic Isolated Pre Sterilized Tub Syringe Filling Line Utilizing ebeam Technology for Tub Surface Decontamination at Entry to the Filling Isolator James E. Vogel P. E., Metall + Plastic The Objective is to provide attendees with an understanding of this approach to installing an aseptic pre sterilized tub syringe filling line. The case study will present the key factors in the selection, installation and validation of a nested syringe tub filling line utilizing isolation and ebeam technologies. The study will summarize available technologies and will cover in significant detail a description of how the low energy ebeam technology works, applicable health and safety requirements and the ebeam decontamination process validation requirements. This is the first aseptic syringe filling line utilizing the low energy ebeam decontamination technology installed in the U. S. The attendees will take home an understanding of the critical factors in the selection, installation and validation of an isolated tub syringe filling line utilizing ebeam technology to surface decontaminate tubs entering the filling isolator 5:15 Close of Day One Tuesday, January 13, 2009 CASE STUDY PRESENTATION 8:15 Validating Glove Integrity in Aseptic Barrier Isolators Dan Mohan, Ph.D, Director, Applied Prime Technologies Use of barrier isolator technology is becoming increasingly important in aseptic manufacturing of pharmaceuticals. The integrity of gloves in such isolator systems is crucial for successful maintenance of the manufacturing process as well as assurance of product sterility. A case study representing an aseptic media fill campaign for manufacturing a drug/device combination product will be presented. Development of the acceptance criteria for the glove
4 DECONTAMINATION OF ISOLATORS AND VALIDATING GLOVE INTEGRITY leak rate and root cause failure analysis of the isolator glove assembly will be reviewed including recommendations for appropriate corrective actions. Glove leak testing methodologies Principle of SKAN glove tester Developing leak rate acceptance criteria Glove Testing scheme in an aseptic isolator campaign Leak rate failure modes analysis 9:15 Glovebox Glove Training - How to Choose the Correct Gloves for your Application Lynn Aurelius, Manager, PIERCAN USA Learn about the newest polymers and thermoplastics used today for the manufacturing of glove box gloves and how these new materials and hybrid gloves can help prevent punctures and loss of product and production time while protecting the operator. Understandvthe limits of glove materials and learn what is new in gamma irradiation of isolator gloves. 9:45 Refreshment Break BLOW/FILL/SEAL TECHNOLOGIES 10:00 Examining Blow/Fill/Seal Insert Technology Tim Kram, General Manager, Rommelag USA, Inc. Blow/Fill/Seal is a 40+ year old advanced aseptic technology that has been applied to the aseptic pharmaceutical industry since the early 1970's. Conventional Blow/Fill/Seal technology is characterized by mono-layer plastic resin containers. The typically mono-layer container has a unit-dose function. Blow/Fill/Seal insert technology was developed to enable sterile filled multidose container format manufacturing. Insert technology utilizes an isolator and feeding systems to supply sterile parts to the Blow/Fill/Seal machine where they are placed into the Blow/Fill/Seal container and then sealed over. The entire process is performed within a controlled environment. This technology can be used to make sterile filled multi-dose vials, ophthalmic bottles, and other unique devices. This presentation examines Blow/Fill/Seal insert technology and studies the various applications the process makes possible. 10:45 Barrier Isolator integration with the Blow/Fill/Seal Process Andrew Goll, Technical Sales Manager, Weiler Engineering Inc. Blow/Fill/Seal is increasingly being recognized as an advanced form of aseptic processing by the global regulatory community. Blow/Fill/Seal in various formats enables the end user high sterility assurance with the versatility in container design and multiple product configurations. Through the integration of a barrier isolator the range of products is ever expanding to reach broader markets and product diversity. The integration of a barrier isolator with a BFS machine utilizes presterilized components and aseptically inserts these components into the Blow/Fill/Seal container. After the insertion step, the vials are hermetically sealed in one continuous operation without human intervention. This presentation will cover the integration of a barrier isolator with a Blow/Fill/Seal machine and the various product configurations that can be applied using this advanced aseptic technology. The attendees will learn about the dynamics of insertion technology; including pressure cascades, the decontamination cycle and parts containment. They will also be introduced to an end-users perspective for Environmental Monitoring, Observations and Lessons Learned. FACILITY DESIGN CONSIDERATIONS 11:15 Building a Process Focused Clinical Aseptic Filling Facility for Potent Compounds Les Edwards, CEO, Isogen LLC. Many clinical manufacturing facilities are designed around a specific piece of process equipment or the constraints of current building layouts, which often do not lend themselves to potent compound processing. Isogen has recently converted a pharmaceutical development laboratory building into a GMP Clinical Manufacturing facility. This facility is equipped with QC Chem, QC Micro, Analytical Chemistry and Material Sciences laboratories to support the special process and product development needs of clinical manufacturing and scale-up with an eye toward facilitating tech transfer and cgmp compliance. Using a process-based model, the presenter will discuss: Selecting baseline vial and syringe filling capacities Defining component preparation and support processes Sizing critical utility systems to support process equipment and constraints Integration of isolation technology, including a multiuse isolator platform Material, personnel, and process flows for containment and compliance 12:15 Luncheon
5 EXAMINING BLOW/FILL/SEAL INSERT TECHNOLOGY 1:15 Facility Design for Isolator Technology Based Aseptic Processing Sterling Kline, Director of Project Development, IPS This presentation will cover the historical context, design philosophies, layout options, and architectural considerations for Isolator Technology Based Aseptic Facilities. It will include the practical applications of regulatory guidance including the new Annex 1. Design options will be reviewed in detail using six recent isolator projects. Examples are taken from large pharma, generic, contract and small bio manufacturers. They include liquid and lyo vials nested syringe, and BFS technologies. Learn practical applications of the current regulatory guidance on facility design Insight into isolator verses RABS technologies Review recent approved isolator facility designs Learn latest approaches to functional room classifications Review Annex 1 impact on capping process design PROCESS EQUIPMENT INTEGRATION 2:00 Integrating Isolator Technology with Process Equipment Peter Schofield,Technical Engineer, Walker Barrier The presentation will review basic isolator technology including considering what types of products are to be produced. The size of Isolator System. The Configuration of System. Materials of Construction. Method of Decontamination or Cleaning. Material Compatibility. Laminar or Turbulent Air Flow. Manipulation-Range of Motion. Transfer of Equipment and Material. Illumination of Enclosure. The discussion will then turn to examples of isolator projects showing isolators integrated with various pieces of production, process or test equipment. Key learning points: Isolator Design Considerations Isolator Material of Construction Airflow Schemes - Containment and Aseptic Material Handling Integrated Systems - fill machines, mills, freeze dryers, autoclaves etc. 2:45 Refreshment Break ELECTRON BEAM TECHNOLOGIES 3:00 Technology for Nested Syringe Filling with Focus on E-Beam for Tub Introduction James J. Spolyar, Aseptic Barrier Systems LLC, SKAN AG Agent for the Americas This presentation will highlight recent aseptic processing lines that have been installed for pharmaceutical syringe filling around the world, including sites at GlaxoSmithKline, Janssen (Belgium), Sanofi-Aventis (France), Novartis(Switzerland). Review the number of installations in Europe and the US Review reasons for choosing E-beam entry for tubs and other choices for entry Review the isolator and E-Beam design features and latest developments Review customer experiences in choosing isolators over a traditional clean room or Restricted Access Barrier System (RABS) 3:45 Low Energy Electron Beams for In Line Sterilization of Pharmaceutical and Medical Device Packaging Josh Epstein, Product Manager, Advanced Electron Beams The trend toward barrier isolator filling brings new requirements and opportunities for inline sterilization technologies. Electron beam disinfection tunnels have become the defacto standard for the transfer of nested syringe tubs into barrier isolator filling lines. The availability of compact, modular electron beam technologies opens up new opportunities beyond nested syringe tub transfer including: Sterile transfer of other pre-sterilized packaging components Direct inline sterilization of primary packaging components Sterile assembly of medical devices In line electron beam sterilization offers high sterility assurance and high throughput rates while simplifying monitoring and potentially reducing the size of barrier isolator systems. What attendees will learn: Overview of ionizing radiation sterilization and how it relates to other in line sterilization technologies How low energy electron beams are currently deployed in aseptic drug manufacturing New opportunities for integrating electron beams into barrier isolator filling lines Process for evaluating electron beams for new application Validation considerations for deploying in line electron beams 4:30 Close of Conference
6 PharmaED R E S O U R C E S, I n c. P h a r m a E d R e s o u r c e s, I n c R o b e s o n P a r k D r i v e C h a m p a i g n, I L About your conference destination: The Radisson-Plaza Warwick is located in the heart of downtown Philadelphia, and adjacent to beautiful Rittenhouse Square. From the conference venue, you can access many points of interest in Philadelphia including Independence Hall, the Kimmel Center and the Avenue of the Arts and numerous shops, hotels and excellent restaurants! REGISTRATION INFORMATION Register for the conference using one of four options: Online: Phone: (217) Fax: (847) Mail: 2810 Robeson Park Drive, Champaign, IL PLEASE COMPLETE THE FOLLOWING: FIRST NAME: LAST NAME: TITLE: COMPANY: ADDRESS: Please register me for: BARRIER ISOLATION TECHNOLOGIES: Technologies, Case Studies and Current Applications in the Usage of Isolator Systems January 12-13, 2009, Radisson-Plaza Warwick, Philadelphia, PA $1,895 USD REGISTER BY NOVEMBER 15 th AND TAKE $300 OFF ADDRESS: CITY: ZIP: OFFICE PHONE: MOBILE PHONE: FAX: STATE: COUNTRY CODE: PAYMENT METHOD CREDIT CARD REGISTRATION: CREDIT CARD VISA MASTERCARD AMEX NAME: CARD #: EXPIRATION: / SIGNATURE: BILLING ADDRESS: VENUE INFORMATION: Dates: January 12-13, 2009 Hotel: Radisson Warwick Plaza Hotel Hotel Address: 1701 Locust Street Philadelphia, PA Reservations: (888) US Hotel Telephone: (215) Fax: (215) rhi_plph@radisson.com CHECK REGISTRATION: To pay by check, please provide a purchase order below. Please note that all payments must be received five (5) days prior to the conference to ensure space. Attendees will not be admitted to the conference without full payment. PURCHASE ORDER #: PLEASE NOTE: PharmaEd Resources does not offer refunds. However, if you cannot attend after registering, we are happy to apply your registration fee to another PharmaEd Resources event, or transfer your registration to a colleague. Notice of cancellation must be received at least 5 days prior to the event.
2018 Pharma Analytics Summit:
 2018 Pharma Analytics Summit: Statistical Approaches for Improving Performance and Compliance March 26 27, 2018, Racquet Club of Philadelphia, PA Featured Speakers Include: Ajaz Hussain Former FDA Ying
2018 Pharma Analytics Summit: Statistical Approaches for Improving Performance and Compliance March 26 27, 2018, Racquet Club of Philadelphia, PA Featured Speakers Include: Ajaz Hussain Former FDA Ying
Current Trends in Sterile Manufacturing. by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS)
 Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
Current Trends in Sterile Manufacturing by Sterling Kline, RA Director of Project Development Integrated Project Services (IPS) Executive Summary Drug developers go to great lengths to assure that their
PharmaED s. Featuring Case Studies and Lessons Learned from Industry Experts! Including Special Coverage On: Featuring Representation From:
 P PharmaED s JULY 26-27, 2012, SHERATON LA JOLLA HOTEL, LA JOLLA, CA Register by June 1 st and receive a $300 Discount! re-filled Syringes Forum 2012 Strategic Development, Inspection, Safety & Regulatory
P PharmaED s JULY 26-27, 2012, SHERATON LA JOLLA HOTEL, LA JOLLA, CA Register by June 1 st and receive a $300 Discount! re-filled Syringes Forum 2012 Strategic Development, Inspection, Safety & Regulatory
Why change is inevitable in aseptic manufacturing?
 Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
Why change is inevitable in aseptic manufacturing? Chrissie Fuchs, Marketing & Communication at Fedegari Group Sergio Mauri, Manager, Integrated Projects at Fedegari Group Key words: Aseptic manufacturing
re-filled Syringes Forum 2012
 P PharmaED s Register by January 30 th and receive a $300 Discount! re-filled Syringes Forum 2012 Strategic Development, Inspection, Safety & Regulatory Compliance and Commercialization of Pre-Filled Syringes
P PharmaED s Register by January 30 th and receive a $300 Discount! re-filled Syringes Forum 2012 Strategic Development, Inspection, Safety & Regulatory Compliance and Commercialization of Pre-Filled Syringes
Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite
 APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
APV 2008 Basle Conference Basle, Switzerland: May 28 & 29 2008 Isolators v. RABS: Facility Design Considerations for a Fill-Finish Finish Suite John R. Chester Principal Engineer Global Pharmaceutical
Auditing Microbiology Media Suppliers
 Microrite, Inc. brings you this unique learning experience in Auditing Microbiology Media Suppliers; Part of Microrite s step-by-step webinar series. Auditing Microbiology Media Suppliers More often than
Microrite, Inc. brings you this unique learning experience in Auditing Microbiology Media Suppliers; Part of Microrite s step-by-step webinar series. Auditing Microbiology Media Suppliers More often than
Cleaning Validation Summit 2016
 May 23-24, 2016, Racquet Club of Philadelphia, PA Featured Speakers Include: Dushyant Varshney Dir. Manufacturing Assessment, Hospira Mariann Neverovitch Research Scientist at Bristol-Myers Squibb Michele
May 23-24, 2016, Racquet Club of Philadelphia, PA Featured Speakers Include: Dushyant Varshney Dir. Manufacturing Assessment, Hospira Mariann Neverovitch Research Scientist at Bristol-Myers Squibb Michele
ASEPTIC TECHNOLOGIES FILLING SOLUTIONS SAFER & EASIER
 ASEPTIC TECHNOLOGIES FILLING SOLUTIONS SAFER & EASIER 2013 CLOSED VIAL TECHNOLOGY FOR INJECTABLES 1. Is a ready-to-fill vial 2. Is an aseptic fill and finish equipment Closed Vial Technology 3. Offers
ASEPTIC TECHNOLOGIES FILLING SOLUTIONS SAFER & EASIER 2013 CLOSED VIAL TECHNOLOGY FOR INJECTABLES 1. Is a ready-to-fill vial 2. Is an aseptic fill and finish equipment Closed Vial Technology 3. Offers
Good Microbiology Laboratory Practices
 Microrite, Inc. brings you this unique learning experience in Good Microbiology Laboratory Practices ; Part of Microrite s step-by-step webinar series. Good Microbiology Laboratory Practices Good microbiology
Microrite, Inc. brings you this unique learning experience in Good Microbiology Laboratory Practices ; Part of Microrite s step-by-step webinar series. Good Microbiology Laboratory Practices Good microbiology
re-filled Syringes Forum 2008
 P PharmaED s Register by November 15 th and receive a $300 Discount! re-filled Syringes Forum 2008 Strategic Development, Inspection, Safety & Regulatory Compliance and Commercialization of Pre-Filled
P PharmaED s Register by November 15 th and receive a $300 Discount! re-filled Syringes Forum 2008 Strategic Development, Inspection, Safety & Regulatory Compliance and Commercialization of Pre-Filled
Process Validation 2018 Summit
 May 17 18, 2018, Racquet Club of Philadelphia, PA Featured Speakers Include: Kashappa Goud Dushyant B. Desai Varshney Investigator, Head of Manufacturing Biopharmaceutical Science & Technology, Product
May 17 18, 2018, Racquet Club of Philadelphia, PA Featured Speakers Include: Kashappa Goud Dushyant B. Desai Varshney Investigator, Head of Manufacturing Biopharmaceutical Science & Technology, Product
Environmental Monitoring Deficiencies
 Microrite, Inc. brings you this unique learning experience in Environmental Monitoring Deficiencies; Part of Microrite s learn from 483 s webinar series. Environmental Monitoring Deficiencies Learn from
Microrite, Inc. brings you this unique learning experience in Environmental Monitoring Deficiencies; Part of Microrite s learn from 483 s webinar series. Environmental Monitoring Deficiencies Learn from
Mold Contaminations-Prevention, Investigation, Remediation and Risk Assessment Durham, NC September 17 th & 18 th, 2018
 Mold Contaminations-Prevention, Investigation, Remediation and Risk Assessment Durham, NC September 17 th & 18 th, 2018 In recent years Mold Contaminations and Recalls have been a focus of attention for
Mold Contaminations-Prevention, Investigation, Remediation and Risk Assessment Durham, NC September 17 th & 18 th, 2018 In recent years Mold Contaminations and Recalls have been a focus of attention for
Strategy for Investigating Microbial Contaminations
 Microrite, Inc. brings you this unique learning experience in Strategy for Investigating Microbial Contaminations; Part of Microrite s step-by-step webinar series. Strategy for Investigating Microbial
Microrite, Inc. brings you this unique learning experience in Strategy for Investigating Microbial Contaminations; Part of Microrite s step-by-step webinar series. Strategy for Investigating Microbial
Microbial Detection Methods and Their Limitations
 Microrite, Inc. brings you this unique learning experience in Microbial Detection Methods and Their Limitations; Part of Microrite s step-by-step webinar series. Microbial Detection Methods and Their Limitations
Microrite, Inc. brings you this unique learning experience in Microbial Detection Methods and Their Limitations; Part of Microrite s step-by-step webinar series. Microbial Detection Methods and Their Limitations
Pharmaceutical Water System Sampling The Why s, Where s, and How s of Doing It Right
 Microrite, Inc. brings you this unique learning experience in Pharmaceutical Water System Sampling; Part of Microrite s step-by-step webinar series. Pharmaceutical Water System Sampling Problematic removal
Microrite, Inc. brings you this unique learning experience in Pharmaceutical Water System Sampling; Part of Microrite s step-by-step webinar series. Pharmaceutical Water System Sampling Problematic removal
Vetter Development Service
 A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
Compounding Aseptic Isolators (CAI) James T Wagner
 Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
Compounding Aseptic Isolators (CAI) James T Wagner jimwagner@cenvironment.com Disclaimer "Although I am a member of the USP Sterile Compounding Expert Committee, I am speaking today in my individual capacity
HISTORY AND MILESTONES
 HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
HISTORY AND MILESTONES HISTORY AND MILESTONES DOC S.r.l., Documentation Organization & Consultancy, was established in 1997 by MASCO group C.E.O. Eng. Alberto Borella and Eng. Paolo Curtò who became Managing
Stability Testing for Pre-Clinical and Early Phase Clinical Drug Products
 Microrite, Inc. brings you this unique learning experience in Stability Testing for Pre-Clinical and Early Phase Clinical Drug Products; Part of Microrite s step-by-step webinar series. Stability Testing
Microrite, Inc. brings you this unique learning experience in Stability Testing for Pre-Clinical and Early Phase Clinical Drug Products; Part of Microrite s step-by-step webinar series. Stability Testing
Auditing Cleanroom Gown Suppliers
 Microrite, Inc. brings you this unique learning experience in Auditing Cleanroom Gown Suppliers; Part of Microrite s step-by-step webinar series. Auditing Cleanroom Gown Suppliers Understanding product
Microrite, Inc. brings you this unique learning experience in Auditing Cleanroom Gown Suppliers; Part of Microrite s step-by-step webinar series. Auditing Cleanroom Gown Suppliers Understanding product
Contamination Risk Assessment in Aseptic, Non-Sterile and Terminally Sterilized Products March 7 th & 8 th Raleigh, NC
 Contamination Risk Assessment in Aseptic, Non-Sterile and Terminally Sterilized Products March 7 th & 8 th Raleigh, NC Contamination risk levels differ in aseptic, and non-sterile and terminally sterilized
Contamination Risk Assessment in Aseptic, Non-Sterile and Terminally Sterilized Products March 7 th & 8 th Raleigh, NC Contamination risk levels differ in aseptic, and non-sterile and terminally sterilized
Mold Contamination-Do's and Don'ts
 Microrite, Inc. brings you this unique learning experience in Mold Contamination-Do's and Don'ts; Part of Microrite s step-by-step webinar series. Mold Contamination-Do's and Don'ts Often the risk of mold
Microrite, Inc. brings you this unique learning experience in Mold Contamination-Do's and Don'ts; Part of Microrite s step-by-step webinar series. Mold Contamination-Do's and Don'ts Often the risk of mold
Vetter Development Service
 A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
A unique blend of expertise for clinical manufacturing Vetter Development Service Supporting your compound s success Answers that work 1 Your molecule s future starts here. By the time your new injectable
Isolator Technology Workshop Engineering - Validation - Operation
 Isolator Technology Workshop Engineering - Validation - Operation Participate in three workshops at AG SPEAKERS: Christian Doriath Philippe Jérôme Theresa Ladwig Katharina Schlereth Labor L+S Photo: Yves
Isolator Technology Workshop Engineering - Validation - Operation Participate in three workshops at AG SPEAKERS: Christian Doriath Philippe Jérôme Theresa Ladwig Katharina Schlereth Labor L+S Photo: Yves
Disinfectant Qualification Bacteria, Fungi, Viruses and Atypical Organisms A Step by Step Workshop
 Microrite, Inc. brings you this unique advanced course in ; part of Microrite s Step by Step Workshop Series Participate in the only workshop which will guide you through the process of choosing a cost
Microrite, Inc. brings you this unique advanced course in ; part of Microrite s Step by Step Workshop Series Participate in the only workshop which will guide you through the process of choosing a cost
Cleaning-Disinfection-Sterilization of Reusable Medical Devices
 Microrite, Inc. brings you this unique learning experience in Cleaning-Disinfection-Sterilization of Reusable Medical Devices; Part of Microrite s step-by-step webinar series. Cleaning-Disinfection-Sterilization
Microrite, Inc. brings you this unique learning experience in Cleaning-Disinfection-Sterilization of Reusable Medical Devices; Part of Microrite s step-by-step webinar series. Cleaning-Disinfection-Sterilization
Chemical Sterilization - Alternate Sterilization Methods
 Microrite, Inc. brings you this unique learning experience in Chemical Sterilization - Alternate Sterilization Methods. Part of Microrite s step-by-step webinar series. Chemical Sterilization - Alternate
Microrite, Inc. brings you this unique learning experience in Chemical Sterilization - Alternate Sterilization Methods. Part of Microrite s step-by-step webinar series. Chemical Sterilization - Alternate
NEW! Processing of cgmp Controlled Raw Materials
 NEW! Processing of cgmp Controlled Raw February 27, 2018 New Brunswick, NJ Directed by: Charity Ogunsanya, (Owner/CEO), Pharmabiodevice Consulting LLC Course Topics Include: FDA Regulations 21 CFR 110.80
NEW! Processing of cgmp Controlled Raw February 27, 2018 New Brunswick, NJ Directed by: Charity Ogunsanya, (Owner/CEO), Pharmabiodevice Consulting LLC Course Topics Include: FDA Regulations 21 CFR 110.80
USP Chapter 823 USP 32 (old) vs. USP 35 (new)
 USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
USP Chapter 823 USP 32 (old) vs. USP 35 (new) Sally W. Schwarz, MS, BCNP Research Associate Professor of Radiology Washington University School of Medicine St. Louis, MO Why USP Chapter ? FDA has
Auditing Cleanroom Gown Suppliers
 Microrite, Inc. brings you this unique learning experience in Auditing Cleanroom Gown Suppliers; Part of Microrite s step-by-step webinar series. Auditing Cleanroom Gown Suppliers Understanding product
Microrite, Inc. brings you this unique learning experience in Auditing Cleanroom Gown Suppliers; Part of Microrite s step-by-step webinar series. Auditing Cleanroom Gown Suppliers Understanding product
Key Elements of a Microbial Contamination Control Policy Why and how to write a policy for Microbial Contamination Control
 Microrite, Inc. brings you this unique learning experience Key Elements of a Microbial Contamination Control Policy; Part of Microrite s step-by-step webinar series. Key Elements of a Microbial Contamination
Microrite, Inc. brings you this unique learning experience Key Elements of a Microbial Contamination Control Policy; Part of Microrite s step-by-step webinar series. Key Elements of a Microbial Contamination
Cleaning-Sterilization-Disinfection Validation of Reusable Medical Devices
 Microrite, Inc. brings you this unique learning experience in Cleaning-Sterilization-Disinfection Validation of Reusable Medical Devices; Part of Microrite s step-by-step webinar series. Validation of
Microrite, Inc. brings you this unique learning experience in Cleaning-Sterilization-Disinfection Validation of Reusable Medical Devices; Part of Microrite s step-by-step webinar series. Validation of
Facility Water Management
 Microrite, Inc. brings you this unique learning experience in ; Part of Microrite s step-bystep webinar series. Water processing, use, validation, re-use, heating/cooling, storage, distribution, and disposal
Microrite, Inc. brings you this unique learning experience in ; Part of Microrite s step-bystep webinar series. Water processing, use, validation, re-use, heating/cooling, storage, distribution, and disposal
Handling of high potency drugs: process and containment
 Environmental Exposure and Health 257 Handling of high potency drugs: process and containment G. Mari, A. Moccaldi & G. Ludovisi Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (Italian
Environmental Exposure and Health 257 Handling of high potency drugs: process and containment G. Mari, A. Moccaldi & G. Ludovisi Istituto Superiore per la Prevenzione e la Sicurezza del Lavoro (Italian
Filling and Packaging Validation for FDA Regulated Industries
 Microrite, Inc. brings you this unique learning experience in Filling and Packaging Validation for FDA Regulated Industries; Part of Microrite s step-by-step webinar series. Filling and Packaging Validation
Microrite, Inc. brings you this unique learning experience in Filling and Packaging Validation for FDA Regulated Industries; Part of Microrite s step-by-step webinar series. Filling and Packaging Validation
Single-Use Final Fill: Benefits and Considerations
 Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
Single-Use Final Fill: Benefits and Considerations TENDÊNCIAS DE TECNOLOGIAS DE FABRICAÇÃO E ASSÉPTICA DE MEDICAMENTOS Ana Luísa Lampert Cadore Sales Specialist SU & Aseptic Process Solutions ana.cadore@merckgroup.com
Environmental Monitoring Requirements and Best Practices for Medical Devices and Drugs New AAMI and ISO EM Reference Documents and Standards
 Microrite, Inc. brings you this unique learning experience in Environmental Monitoring Requirements and Best Practices for Medical Devices and Drugs; Part of Microrite s step-by-step webinar series. Environmental
Microrite, Inc. brings you this unique learning experience in Environmental Monitoring Requirements and Best Practices for Medical Devices and Drugs; Part of Microrite s step-by-step webinar series. Environmental
Exhibitor Prospectus. NYSPA s 81st Annual Convention. New York Marriott East Side June 1-3, 2018
 Exhibitor Prospectus NYSPA s 81st Annual Convention New York Marriott East Side June 1-3, 2018 New York State Psychological Association With years of experience and credibility in the profession of psychology,
Exhibitor Prospectus NYSPA s 81st Annual Convention New York Marriott East Side June 1-3, 2018 New York State Psychological Association With years of experience and credibility in the profession of psychology,
NO 2 Gas Sterilization and Decontamination: Keeping Pace, from Start to Finish
 NO 2 Gas Sterilization and Decontamination: Keeping Pace, from Start to Finish Evan Goulet, Ph.D., VP of Microbiology Applications Laboratory PDA Midwest: Sterility Assurance and Risk Mitigation October
NO 2 Gas Sterilization and Decontamination: Keeping Pace, from Start to Finish Evan Goulet, Ph.D., VP of Microbiology Applications Laboratory PDA Midwest: Sterility Assurance and Risk Mitigation October
Chuck Reed at Weiler Engineering, Inc compares aseptic blow-fill-seal technology with traditional aseptic processing of pharmaceutical liquids
 Manufacturing Aseptic Advances Chuck Reed at Weiler Engineering, Inc compares aseptic blow-fill-seal technology with traditional aseptic processing of pharmaceutical liquids Acknowledged by the FDA as
Manufacturing Aseptic Advances Chuck Reed at Weiler Engineering, Inc compares aseptic blow-fill-seal technology with traditional aseptic processing of pharmaceutical liquids Acknowledged by the FDA as
EXHIBITOR PROSPECTUS
 Texas Pediatric Society 2017 Annual Meeting EXHIBITOR PROSPECTUS October 5-6, 2017 Renaissance Dallas at Plano Legacy West Meeting Exhibit i e t i ng a l Me o n a l P 2017 A nnu West y c a g e L Exhibit
Texas Pediatric Society 2017 Annual Meeting EXHIBITOR PROSPECTUS October 5-6, 2017 Renaissance Dallas at Plano Legacy West Meeting Exhibit i e t i ng a l Me o n a l P 2017 A nnu West y c a g e L Exhibit
Trends in Sterile Manufacturing Technologies
 Trends in Sterile Manufacturing Technologies ISPE Thailand Annual Meeting Charlotte Enghave Fruergaard 2013.07.18 Where we come from 1930s Danish Novo and Nordisk Gentofte (later Novo Nordisk) employed
Trends in Sterile Manufacturing Technologies ISPE Thailand Annual Meeting Charlotte Enghave Fruergaard 2013.07.18 Where we come from 1930s Danish Novo and Nordisk Gentofte (later Novo Nordisk) employed
Actavis Italy. Nerviano Plant
 Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
Actavis Italy Nerviano Plant Starting out in 1901 in Jerusalem The company known today as Teva was established as a small wholesale drug business by Chaim Salomon, Moshe Levin and Yitschak Elstein. (They
Adaptive Clinical Trials
 MARCH 22 23, 2018 WYNDHAM PHILADELPHIA HISTORIC DISTRICT PHILADELPHIA, PA Adaptive Clinical Trials SYMPOSIUM Analyze Statistics, Data Management, and Operations to Improve Clinical Performance FEATURED
MARCH 22 23, 2018 WYNDHAM PHILADELPHIA HISTORIC DISTRICT PHILADELPHIA, PA Adaptive Clinical Trials SYMPOSIUM Analyze Statistics, Data Management, and Operations to Improve Clinical Performance FEATURED
DELIVERING SOLUTIONS FOR PATIENTS
 a clear vision for eyecare Co-Development DELIVERING SOLUTIONS FOR PATIENTS Patients suffer because of preservatives in eye drops. Scientific research has shown that preservatives cause irritations, allergies
a clear vision for eyecare Co-Development DELIVERING SOLUTIONS FOR PATIENTS Patients suffer because of preservatives in eye drops. Scientific research has shown that preservatives cause irritations, allergies
Microbial Contamination Testing and Validation Challenges Non-Sterile Products and Raw Materials
 Microrite, Inc. brings you this unique learning experience in Microbial Contamination Testing and Validation ; Part of Microrite s step-by-step webinar series. Microbial Contamination Testing and Validation
Microrite, Inc. brings you this unique learning experience in Microbial Contamination Testing and Validation ; Part of Microrite s step-by-step webinar series. Microbial Contamination Testing and Validation
NFPA CONFERENCE & EXPO MARKETING OPPORTUNITIES
 NFPA & MARKETING OPPORTUNITIES and LOW Cost Marketing Opportunities Marketing your participation is crucial to helping you have a successful NFPA Conference & Expo experience. We encourage you to take
NFPA & MARKETING OPPORTUNITIES and LOW Cost Marketing Opportunities Marketing your participation is crucial to helping you have a successful NFPA Conference & Expo experience. We encourage you to take
Medical Device Complaint Management
 An Interactive Workshop Featuring 11 Hands on Exercises Presented by Ombu Enterprises and FDAnews March 11 12, 2014 Westin Waltham-Boston Hotel Waltham, MA (Boston) Medical Device Complaint Management
An Interactive Workshop Featuring 11 Hands on Exercises Presented by Ombu Enterprises and FDAnews March 11 12, 2014 Westin Waltham-Boston Hotel Waltham, MA (Boston) Medical Device Complaint Management
Direct Marketing Metrics and Analysis
 Accurately measure your direct marketing success and create viable marketing plans with the mathematical and analytical tools you ll learn to use at this interactive two-day workshop. Direct Marketing
Accurately measure your direct marketing success and create viable marketing plans with the mathematical and analytical tools you ll learn to use at this interactive two-day workshop. Direct Marketing
Critical Environment Products and Services
 Critical Environment Products and Services For over two decades, EP Scientific products and services have defined clean for environmental sampling containers and services. Our proprietary cleaning methods
Critical Environment Products and Services For over two decades, EP Scientific products and services have defined clean for environmental sampling containers and services. Our proprietary cleaning methods
Computer Validation - Introduction to Risk Management - The GAMP 5 Approach
 Including implications of EU GMP Annex 11 computerised systems Computer Validation - Introduction to Risk Management - The GAMP 5 Approach Learn How to Plan, Implement and Document Effectively Computer
Including implications of EU GMP Annex 11 computerised systems Computer Validation - Introduction to Risk Management - The GAMP 5 Approach Learn How to Plan, Implement and Document Effectively Computer
Aseptic Process Validation
 Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
Aseptic Process Validation IMB GMP Information Seminar 27 th September 2012 Gerard Sheridan, Inspector Date Insert on Master Slide Slide 1 Overview Guidance Best Practices Common Deficiencies Slide 2 Aseptic
PDA: A Global. Association. Proposed Revisions to USP <1207> STERILE PRODUCT -
 Proposed Revisions to USP STERILE PRODUCT - PDA: A Global PACKAGE INTEGRITY EVALUATION Now in the Sep/Oct 2014 USP Pharmacopeial Forum For Public Comment Association Prepared by: Dana M. Guazzo
Proposed Revisions to USP STERILE PRODUCT - PDA: A Global PACKAGE INTEGRITY EVALUATION Now in the Sep/Oct 2014 USP Pharmacopeial Forum For Public Comment Association Prepared by: Dana M. Guazzo
Wednesday, June 13. 7:00 a.m. 5:30 p.m. Registration Open 7:00 a.m. 8:30 a.m. Continental Breakfast
 Wednesday, June 13 7:00 a.m. 5:30 p.m. Registration Open 7:00 a.m. 8:30 a.m. Continental Breakfast 2018 PDA Container Closure Performance and Integrity Conference Assuring Packaging Quality in Delivery
Wednesday, June 13 7:00 a.m. 5:30 p.m. Registration Open 7:00 a.m. 8:30 a.m. Continental Breakfast 2018 PDA Container Closure Performance and Integrity Conference Assuring Packaging Quality in Delivery
G.F. S.p.A. Via T. Edison, Rubbiano fraz. Solignano (PR) - Italy Tel Fax Web:
 G.F. S.p.A. ia T. Edison, 3 43045 Rubbiano fraz. Solignano (PR) - Italy Tel. +39. 0525. 300311 Fax +39. 0525. 300350 Web: www.gfe.it E-mail: gfe@gfe.it Developed to process ampoules, vials and cartridges
G.F. S.p.A. ia T. Edison, 3 43045 Rubbiano fraz. Solignano (PR) - Italy Tel. +39. 0525. 300311 Fax +39. 0525. 300350 Web: www.gfe.it E-mail: gfe@gfe.it Developed to process ampoules, vials and cartridges
Microbiological Consideration for Non-Sterile Pharmaceutical
 May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
May 1-3, 2012 Javits Center New York, NY Microbiological Consideration for Non-Sterile Pharmaceutical Dr. Leonard W. Mestrandrea Principal Consultant MESTRANDREA CONSULTING LLC Title Date Microbial Control
Technical Data Monograph. STERIS VHP LTS-V - Low Temperature Surfaces Terminal Sterilization
 Technical Data Monograph STERIS VHP LTS-V - Low Temperature Surfaces Terminal Sterilization STERIS VHP LTS-V Low Temperature Surfaces Terminal Sterilization Table of Contents 1. Abstract 3 2. VHP LTS-V
Technical Data Monograph STERIS VHP LTS-V - Low Temperature Surfaces Terminal Sterilization STERIS VHP LTS-V Low Temperature Surfaces Terminal Sterilization Table of Contents 1. Abstract 3 2. VHP LTS-V
PPC Best Practices Conference
 New! RISK ASSESSMENT REVISITED PPC Best Practices Conference Train with the experts! Cutting-edge content New Orleans, LA Sept 25 26 Las Vegas, NV Nov 6 7 from the Tax & Accounting business of Thomson
New! RISK ASSESSMENT REVISITED PPC Best Practices Conference Train with the experts! Cutting-edge content New Orleans, LA Sept 25 26 Las Vegas, NV Nov 6 7 from the Tax & Accounting business of Thomson
Coal Ash Landfill Management and Ash Pond Closure
 Coal Ash Landfill Management and Ash Pond Closure Rosen Centre Hotel COurse EUCI is authorized by IACET to offer 1.3 CEUs for the course. 1 Overview As coal generation continues, utilities are faced with
Coal Ash Landfill Management and Ash Pond Closure Rosen Centre Hotel COurse EUCI is authorized by IACET to offer 1.3 CEUs for the course. 1 Overview As coal generation continues, utilities are faced with
Aseptic vial filling line
 Pharma Forum - Schwäbisch Hall September 26, 2013 Aseptic vial filling line implementation of two separated filling paths including inline CIP/SIP Contact information Dr. Alexander Hohl Fresenius Kabi
Pharma Forum - Schwäbisch Hall September 26, 2013 Aseptic vial filling line implementation of two separated filling paths including inline CIP/SIP Contact information Dr. Alexander Hohl Fresenius Kabi
ABOUT HAMELN PHARMA About us
 Welcome ABOUT HAMELN PHARMA About us With over 60 years of experience hameln pharma is the specialist for contract manufacturing of sterile solutions and suspensions filled in ampoules and vials. 2 ABOUT
Welcome ABOUT HAMELN PHARMA About us With over 60 years of experience hameln pharma is the specialist for contract manufacturing of sterile solutions and suspensions filled in ampoules and vials. 2 ABOUT
ISPE Nordic, Denmark Yves Scholler SKAN AG
 Risk of contamination through pinholes in gloves and how to prove the integrity ISPE Nordic, Denmark Yves Scholler SKAN AG 07.10.2015 1 Agenda 1 Regulatory Requirements 2 Overview of Different Testing
Risk of contamination through pinholes in gloves and how to prove the integrity ISPE Nordic, Denmark Yves Scholler SKAN AG 07.10.2015 1 Agenda 1 Regulatory Requirements 2 Overview of Different Testing
Responses to Questions for SYSTEM 1E Process Monitoring and Validation Webinar
 Responses to Questions for SYSTEM 1E Process Monitoring and Validation Webinar MONITORING THE SYSTEM 1E PROCESSOR Q: I want to confirm that there is no need for a Biological Indicator (BI) and that use
Responses to Questions for SYSTEM 1E Process Monitoring and Validation Webinar MONITORING THE SYSTEM 1E PROCESSOR Q: I want to confirm that there is no need for a Biological Indicator (BI) and that use
Aseptic Processing Current Issues & Trends
 Aseptic Processing Current Issues & Trends 1 Introduction Richard M. Johnson Member, PDA for 20+ years President & CEO since 2009 Ladies and Gentlemen, I am happy to be here with you. Senhoras e Senhores,
Aseptic Processing Current Issues & Trends 1 Introduction Richard M. Johnson Member, PDA for 20+ years President & CEO since 2009 Ladies and Gentlemen, I am happy to be here with you. Senhoras e Senhores,
37th Annual Columbus Chapter ASIS Seminar and Exhibits
 May 12, 2016 37th Annual Columbus Chapter ASIS Seminar and Exhibits EDUCATION TECHNOLOGY EXHIBITS NETWORKING Hosted by the Columbus Chapter ASIS this one day event is the areas largest seminar with quality
May 12, 2016 37th Annual Columbus Chapter ASIS Seminar and Exhibits EDUCATION TECHNOLOGY EXHIBITS NETWORKING Hosted by the Columbus Chapter ASIS this one day event is the areas largest seminar with quality
MICROBIAL LIMIT TESTS FOR RAW MATERIALS AND NON-STERILE PRODUCTS REVIEW-DISCUSSIONS-INTERPRETATIONS
 Microrite, Inc. brings you this unique learning experience in microbial limit tests for raw materials and non-sterile products-review-discussions-interpretations Part of Microrite s step-by-step webinar
Microrite, Inc. brings you this unique learning experience in microbial limit tests for raw materials and non-sterile products-review-discussions-interpretations Part of Microrite s step-by-step webinar
MAKING PROPERTIES AND PROFITS
 AKING PROPERTIES AND PROFITS Twin-Screw Extrusion Seminar September 13-14, 2017 Seminar Leader: Adam Dreiblatt Akron Polymer Technology Services Akron, OH Program Overview This two-day seminar focuses
AKING PROPERTIES AND PROFITS Twin-Screw Extrusion Seminar September 13-14, 2017 Seminar Leader: Adam Dreiblatt Akron Polymer Technology Services Akron, OH Program Overview This two-day seminar focuses
Annex A2. Guidance on Process Validation Scheme for Aseptically Processed Products
 Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
Annex A2 Guidance on Process Validation Scheme for Aseptically Processed Products 1 Table of content 1 PURPOSE... 3 2 SCOPE... 3 3 GENERAL INFORMATION... 3 4 INFORMATION NEEDED FOR ASEPTIC PROCESSES VALIDATION...
CLINICAL TRIAL BILLING COMPLIANCE BOOT CAMP
 CLINICAL TRIAL BILLING COMPLIANCE BOOT CAMP Become Clinical Trial Billing Proficient at the Only Hands-On Workshop to Guide You Through All of Your Billing Compliance Challenges ALL-NEW DATES AND LOCATIONS
CLINICAL TRIAL BILLING COMPLIANCE BOOT CAMP Become Clinical Trial Billing Proficient at the Only Hands-On Workshop to Guide You Through All of Your Billing Compliance Challenges ALL-NEW DATES AND LOCATIONS
Fluoroscopy Testing Workshop
 Hands-on Fluoroscopy Testing Workshop 2-day Workshop February 24-25, 2018 in Wichita, KS 4.5 SAMs offered A continuing education division of HERZING UNIVERSITY Workshop Schedule ~~~~ Day One ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Hands-on Fluoroscopy Testing Workshop 2-day Workshop February 24-25, 2018 in Wichita, KS 4.5 SAMs offered A continuing education division of HERZING UNIVERSITY Workshop Schedule ~~~~ Day One ~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~~
Achieving Greater Agility with BI and Analytics
 San Diego, CA // August 8 10 Achieving Greater Agility with BI and Analytics Learn how to accelerate time to value using agile BI development methods and models Discover insights from executive leaders
San Diego, CA // August 8 10 Achieving Greater Agility with BI and Analytics Learn how to accelerate time to value using agile BI development methods and models Discover insights from executive leaders
Vendor & Exhibitor Prospectus
 Vendor & Exhibitor Prospectus ATP Welcome Message to 2017 Sponsors/Exhibitors Dear Exhibitor: The Association for the Tutoring Profession (ATP) cordially invites you to exhibit at our 13th Annual Conference,
Vendor & Exhibitor Prospectus ATP Welcome Message to 2017 Sponsors/Exhibitors Dear Exhibitor: The Association for the Tutoring Profession (ATP) cordially invites you to exhibit at our 13th Annual Conference,
Partnering ACOs. with Summit. 4th. October 27-28, 2014 Hotel Palomar Los Angeles-Westwood, Los Angeles, CA
 4th Partnering ACOs October 27-28, 2014 Hotel Palomar Los Angeles-Westwood, Los Angeles, CA with Summit Improve Patient Outcomes and Reduce Costs While Building Valuable Strategic Cross Industry Collaborations
4th Partnering ACOs October 27-28, 2014 Hotel Palomar Los Angeles-Westwood, Los Angeles, CA with Summit Improve Patient Outcomes and Reduce Costs While Building Valuable Strategic Cross Industry Collaborations
HEALTHCARE AD-TECH & PROGRAMMATIC STRATEGY
 2ND HEALTHCARE AD-TECH & PROGRAMMATIC STRATEGY Leverage Programmatic and Advanced Ad-Tech Strategies to Effectively Target and Reach Patients and HCPs August 7-8, 2017 Hilton Philadelphia at Penn s Landing
2ND HEALTHCARE AD-TECH & PROGRAMMATIC STRATEGY Leverage Programmatic and Advanced Ad-Tech Strategies to Effectively Target and Reach Patients and HCPs August 7-8, 2017 Hilton Philadelphia at Penn s Landing
PPC NONPROFIT AND GOVERNMENT CONFERENCE
 New! PPC NONPROFIT AND GOVERNMENT CONFERENCE Train with the experts! Orlando, FL June 5 6 Beautiful resort location! from Thomson Tax & Accounting Information you need... at locations you ll love! trainingcpe.thomson.com
New! PPC NONPROFIT AND GOVERNMENT CONFERENCE Train with the experts! Orlando, FL June 5 6 Beautiful resort location! from Thomson Tax & Accounting Information you need... at locations you ll love! trainingcpe.thomson.com
On-Site GMP Training GMP COMPLIANCE TECHNICAL
 PharmaNet On-Site GMP Training GMP COMPLIANCE TECHNICAL 284 E Lake Mead Pkwy Suite C-278 Henderson, NV 89015 Phone: 702-558-0094 Fax: 702-558-0079 www.gmpseminars.com Key to Level of Program Level A: Program
PharmaNet On-Site GMP Training GMP COMPLIANCE TECHNICAL 284 E Lake Mead Pkwy Suite C-278 Henderson, NV 89015 Phone: 702-558-0094 Fax: 702-558-0079 www.gmpseminars.com Key to Level of Program Level A: Program
USP CHAPTER <797> INTRODUCTION RISK LEVELS
 USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
USP CHAPTER INTRODUCTION RISK LEVELS Introduction: The objective of this chapter is to describe conditions and practices to prevent harm, including death, to patients that could result from 1) microbial
DEVICES ARE KEY TO DRUG EFFICACY
 pump up your performance Co-Development DEVICES ARE KEY TO DRUG EFFICACY The intranasal route is widely used for both prescription and over-the-counter drugs. It is an attractive option for topical drugs
pump up your performance Co-Development DEVICES ARE KEY TO DRUG EFFICACY The intranasal route is widely used for both prescription and over-the-counter drugs. It is an attractive option for topical drugs
Executive Compensation Seminar
 The 2003 Executive Compensation Seminar The Metamorphosis of Executive Compensation June 24-25, 2003 Westin Chicago River North Chicago, IL The Metamorphosis of Executive Compensation: What s Happening
The 2003 Executive Compensation Seminar The Metamorphosis of Executive Compensation June 24-25, 2003 Westin Chicago River North Chicago, IL The Metamorphosis of Executive Compensation: What s Happening
Review Validation of aseptic processes for pharmaceuticals
 OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
OPEM www.opem.org Oriental Pharmacy and Experimental Medicine 2010 10(4), 231-238 DOI 10.3742/OPEM.2010.10.4.231 Review Validation of aseptic processes for pharmaceuticals Lincy Joseph*, Mathew George
Exhibitor/Sponsor Prospectus
 Exhibitor/Sponsor Prospectus GovTravels Expo Program: Lower Lobby Foyer, which is outside of the main meeting room, will serve as the GovTravels Resource Center. There and the right side of the Plaza Ballroom
Exhibitor/Sponsor Prospectus GovTravels Expo Program: Lower Lobby Foyer, which is outside of the main meeting room, will serve as the GovTravels Resource Center. There and the right side of the Plaza Ballroom
Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119
 Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119 February 28, 2018 Manufacture of sterile drugs (GUI-0119) Author: Health Canada Date issued: February 28, 2018 Implementation
Annex 1 to the Good manufacturing practices guide Manufacture of sterile drugs GUI-0119 February 28, 2018 Manufacture of sterile drugs (GUI-0119) Author: Health Canada Date issued: February 28, 2018 Implementation
ISPE Aseptic Conference February 2014 Washington, D.C.
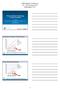 Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Trends of Barrier Technology in China and Japan Alex Cheng: Shanghai Tofflon Airex Science and Technology Co.,Ltd. Koji Kawaskai: Airex CoLtd. Japan Filling Barrier Isolators Global Deliveries 500 450
Porton Biopharma Limited 1/17/17
 Porton Biopharma Limited 1/17/17 10903 New Hampshire Avenue Silver Spring, MD 20993 Via UPS Warning Letter 320 17 19 Return Receipt Requested January 19, 2017 Dr. Roger J. Hinton Managing Director Porton
Porton Biopharma Limited 1/17/17 10903 New Hampshire Avenue Silver Spring, MD 20993 Via UPS Warning Letter 320 17 19 Return Receipt Requested January 19, 2017 Dr. Roger J. Hinton Managing Director Porton
Good Laboratory Practice Compliance
 Practice January 26-27, 2017 Hilton Crystal City at Reagan National Airport Arlington, Virginia Use GLP Standards to Remain Compliant and Successful in Preclinical Research Featured Speakers Amanda Ulrey
Practice January 26-27, 2017 Hilton Crystal City at Reagan National Airport Arlington, Virginia Use GLP Standards to Remain Compliant and Successful in Preclinical Research Featured Speakers Amanda Ulrey
Stronger Economies Through Licensing Is IP Policy Making or Breaking Your Deals?
 LES 2017 SPRING MEETING Stronger Economies Through Licensing Is IP Policy Making or Breaking Your Deals? Exhibitor & Sponsor Prospectus May 9-11 Willard InterContinental Hotel Washington, DC If your business
LES 2017 SPRING MEETING Stronger Economies Through Licensing Is IP Policy Making or Breaking Your Deals? Exhibitor & Sponsor Prospectus May 9-11 Willard InterContinental Hotel Washington, DC If your business
Reference Standards for Monoclonal Antibodies: Key Challenges Addressed
 CASSS WCBP 2012: 16th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products January 23-25, 2012 Reference Standards for Monoclonal Antibodies: Key Challenges
CASSS WCBP 2012: 16th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products January 23-25, 2012 Reference Standards for Monoclonal Antibodies: Key Challenges
Decon 2.0: Emerging Decontamination Technologies
 Decon 2.0: Emerging Decontamination Technologies Vaporized Hydrogen Peroxide (VHP) and Chlorine Dioxide Gas Decontamination Case Studies: Peter Harris Director of Operations B & V Testing, INC. 800.851.9081
Decon 2.0: Emerging Decontamination Technologies Vaporized Hydrogen Peroxide (VHP) and Chlorine Dioxide Gas Decontamination Case Studies: Peter Harris Director of Operations B & V Testing, INC. 800.851.9081
Your Marketing. Step-Up Marketing Opportunities. International Woodworking Fair, LLC is owned and sponsored by
 Step-Up Your Marketing 2013-2014 Marketing Opportunities International Woodworking Fair, LLC is owned and sponsored by Dear IWF 2014 Exhibitor, We always strive to bring the largest and most qualified
Step-Up Your Marketing 2013-2014 Marketing Opportunities International Woodworking Fair, LLC is owned and sponsored by Dear IWF 2014 Exhibitor, We always strive to bring the largest and most qualified
2018 IEDC LEADERSHIP SUMMIT January 28-30, 2018 Las Vegas, NV
 2018 IEDC LEADERSHIP SUMMIT January 28-30, 2018 Las Vegas, NV SPONSORSHIP INFORMATION Location: Golden Nugget Hotel and Casino 129 Fremont Street Experience Las Vegas, NV 89101 IEDC's Leadership Summit
2018 IEDC LEADERSHIP SUMMIT January 28-30, 2018 Las Vegas, NV SPONSORSHIP INFORMATION Location: Golden Nugget Hotel and Casino 129 Fremont Street Experience Las Vegas, NV 89101 IEDC's Leadership Summit
FDA s Guidance for Industry
 Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Sterile Drug Products Produced by Aseptic Processing - CGMPs Midwest FDC Conference November 4 2005 Susan Bruederle, Investigator FDA / ORA / Central Region Chicago District / Hinsdale IL FDA s Guidance
Sponsorship Opportunities. benefiting the
 Sponsorship Opportunities benefiting the WHO: WHAT: WHERE: WHEN: CONTACT: More than 1,000 attendees from metropolitan New York s real estate, business, social, and celebrity circles are expected to attend
Sponsorship Opportunities benefiting the WHO: WHAT: WHERE: WHEN: CONTACT: More than 1,000 attendees from metropolitan New York s real estate, business, social, and celebrity circles are expected to attend
INNOVATIVE PLASTIC CONTAINERS
 INNOVATIVE PLASTIC CONTAINERS Capitol Vial, Inc. is a vertically integrated ISO 9001-certified manufacturer of one-piece plastic containers. Patented molding technology enables Capitol Vial, Inc. to produce
INNOVATIVE PLASTIC CONTAINERS Capitol Vial, Inc. is a vertically integrated ISO 9001-certified manufacturer of one-piece plastic containers. Patented molding technology enables Capitol Vial, Inc. to produce
Quantos Solutions. Gravimetric Sample Preparation (GSP) Automated Weighing
 Quantos Solutions Gravimetric Sample Preparation (GSP) Automated Weighing Innovation Weighing Liquids and Solids to Reach Perfect Concentrations Do you spend a lot of time on sample preparation? Do you
Quantos Solutions Gravimetric Sample Preparation (GSP) Automated Weighing Innovation Weighing Liquids and Solids to Reach Perfect Concentrations Do you spend a lot of time on sample preparation? Do you
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective
 Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
Hot Topics in Drug Product Process Validation: A Reviewer s Perspective Colleen Thomas, Ph.D. Quality Assessment Lead (Acting) FDA/CDER/OPQ/OPF Division of Microbiology Assessment CASSS CMC Strategy Forum
EXHIBITOR PACKET Presented by Ogletree Deakins
 January 19, 2018 Denver Marriott Tech Center High SHRM 2018 HR Conference EXHIBITOR PACKET Presented by Ogletree Deakins Welcome High SHRM (MH-SHRM) is pleased to invite you to participate in Denver s
January 19, 2018 Denver Marriott Tech Center High SHRM 2018 HR Conference EXHIBITOR PACKET Presented by Ogletree Deakins Welcome High SHRM (MH-SHRM) is pleased to invite you to participate in Denver s
QUALIFICATION and VALIDATION Open Discussion Workshop QP Forum 21 st April 2016
 QUALIFICATION and VALIDATION Open Discussion Workshop QP Forum 21 st April 2016 Representatives from cross section of companies were at this workshop namely: Abbott, Stiefel, Abbvie, Pfizer, Helsinn, BMS,
QUALIFICATION and VALIDATION Open Discussion Workshop QP Forum 21 st April 2016 Representatives from cross section of companies were at this workshop namely: Abbott, Stiefel, Abbvie, Pfizer, Helsinn, BMS,
