Created by: Karl Ekwall. 4. Chemicals, Cytostatics and Prescription Drugs Cell media (GMM/GMO see below)... 6.
|
|
|
- Rudolf Gallagher
- 5 years ago
- Views:
Transcription
1 Dept. of Biosciences and Nutrition Dept. of Medicine (MedH/HERM/ LipidLab) Dept. of Neurobiology, Care Sciences and Society (NVS) Dept. of Laboratory Medicine (LabMed) Document Waste handling Dnr: Created by: Version: 1.3 Work Environment Group Approved by: Karl Ekwall Page 1 of 10. Date (prod./rev.): / Håkan Ottosson Content Waste handling Recycling and waste rooms Recycling Syringes, needles and scalpels Chemicals, Cytostatics and Prescription Drugs Liquid Chemical Waste Solid Chemical Waste Radioactivity Infectious/biological material Cell media (GMM/GMO see below) GMM/GMO Instruments and computer equipment Compounds that in small amounts can be poured out into the drain Sorting and Labelling of Hazardous Waste Recycling and waste rooms Waste handling 1 On floor 6, 7 and 8 recycling rooms (Miljörum 8360, 7360 and 6360) are located close to the transport elevator. On floor 3 the waste rooms (Avfall 3313, Avfall-Kylrum 3314, and Kemikalieavfall 3315) are located by the loading dock. All laboratory waste are transported down to the waste rooms at floor 3 by each research group. The sharps and cutting/infectious waste, in yellow bins, is stored in the refrigerated room 3314 and biological waste, in black bins, in the freezer in room Chemical waste is stored in room 3315 (Kemikalieavfall). Flammable liquid waste has to be stored in the fire proof cabinets, non-flammable waste that has hazardous vapours need to be stored in the ventilated cabinets. Other chemical waste may be stored at the shelves. In the waste depositing area at floor 3 (room 3313) there are containers for ordinary garbage (combustible waste), see below. Access to the waste rooms at floor 3 is limited to dedicated staff. The chemical and biological waste is picked up from the waste rooms by contracted companies. They will not pick up hazardous waste if the container is missing proper labels. 1 For an overview, see the Waste Handling poster
2 Page 2 of Recycling Ordinary copy- and printer paper (not glossy brochures, folder, etc.) should be deposited in the plastic containers for recovered paper that are placed at specific locations in the corridors and in the recycling rooms at each floor, 6360, 7360 and These containers are transported down to the main waste room by cleaning- or service staff. Cardboard boxes (corrugated cardboard) should be unfolded and preferentially discarded in the designated larger green plastic containers in the recycling rooms at each floor. Empty glass containers, should be deposited in one of the containers for recycled glass (separate container for coloured and uncoloured glass) with exception of those that have contained chemicals that are toxic (fig 1), carcinogenic/mutagenic (fig 2) or toxic to the environment (fig 3). Before depositing, rinse out residual content, if only a few ml it may be evaporated in a fume hood. When bottle is clean place it, without cap, in the container. This is valid for all volatile organic solvents, unless they are marked with hazardous labels. Empty inorganic acid bottles, e.g. hydrochloric acid, may be rinsed with plenty of water and then recycled. The glass containers used for chemicals, that cannot be recycled 2 should not be rinsed out, send them as chemical waste, see Ch. 6, Solid Chemical Waste. Discarded laboratory glass made of borosilicate glass (beakers, etc. made of Jena-, Pyrex- or Duran glass) should be deposited in the containers for this in the recycling room 3. Empty plastic containers, solvent bottles for ethanol, acetone, and inorganic acids are cleaned using the same procedure as for the glass containers, above, recycled as plastic (unless they are labelled with the hazardous symbols in fig 1, 2 or 3). Each research group should have a bag for recycling of plastics in the lab (hard and soft plastics can go together). If containers used for chemicals are included they have to be rinsed before deposited, see description above. The full bags should be closed and put in the container for plastics in the recycling room (Miljörum 6360, 7360, or 8360). Don't throw plastic pipettes or tips for automatic pipettes directly in the garbage bags in the lab. Contaminated plastic pipettes or pipette tips should be discarded together with the waste in question (contagious, chemical or radioactive) or if considered clean in any cardboard box which when full is sealed with tape and discarded as normal garbage (use a smaller empty plastic container or any other suitable container or a plastic bag that is not penetrable for the pipettes or the tips) Smaller metal objects should be deposited in recycling room in the box for metals. Metal objects larger than the recycling box should also be deposited as metal, contact janitor to handle this. Mercury and other toxic metals should be disposed of as chemical waste, with a proper label. Batteries should be saved and deposited in a box in the recycling room. Figure 1 Highly Toxic Figure 2 Health hazard Figure 3 Environmental hazard Used fluorescent lamps, other mercury containing lamps and normal light bulbs should be deposited in the designated box in the recycling room. Discarded X-ray films and other films should be deposited in the designated box in the recycling room. 2 3 Containers used for toxic or environmental hazardous chemicals must not be rinsed (GHS Pictogram, fig 1, 2 or 3) Due to an agreement with the waste contractors, small amounts of cleaned lab glass were can be disposed of in the stained glass container
3 Page 3 of Syringes, needles and scalpels Used disposable glass, syringes, injection needles and scalpels from work with human samples, micro-organisms should be deposited in the yellow bin for Sharps & Infectious waste. They may temporary be deposited in smaller yellow containers for sharps and infections waste. Noncollect in any contaminated (no toxic or infectious agents) sharps and pipette tips, may be suitable container, e.g. an empty ethanol bottle, for temporary storage properly marked as Sharps. When full, put them in the yellow bin for Sharps & Infectious waste. 4. Chemicals, Cytostatics and Prescription Drugs Almost all chemical waste is classified as hazardous waste ( farligt avfall ). See KI staff portal 4 for KIs rules and how to handle different types of chemical waste. Disposal of cytostatics and prescription drugs are regulated by different provisions and should be handledd slightly different than normal chemical waste, see the document for this and KI web pages. Organic solvent waste should be collected in "UN"-labelled 5 litre plastic jerricans 5. Other plastic containers should not be used. If large volumes are produced a largerr metal container would be preferred. Make sure that all caps are properly tightened and do not fill more than to the mark for 5 litres. Solid waste should be collected in 10 litre plastic containers (with larger cap) or in a designated box for hazardous waste (containing an inner plastic bag), but other plastic containers could also be used. Both type of plastic containers as well as designated cardboard boxes of a single size (and thicker plastic bags) are available in the storage rooms. Every type of waste has to be labelled separately with enough information to decide how the waste can be transported and incinerated. The current fractions and thus the information on how to label the waste are given in Ch. 13 Sorting and Labelling of Hazardous Waste. Completely or partly pre-printed labels are available in the storage rooms 6638, 7638, 8638 and You fill the content, if that is not pre-printed, your name, department, KI and your phone number. If you have some questions regarding waste handling or if you want to get rid of some old chemicals contact the work environment coordinator. All chemical and hazardous waste is transported down to the storage room on floor 3 by dedicated staff from each research group. 5. Liquid Chemical Waste Waste containing chemicals or mixtures containing chemicals labeled with pictograms for highly toxic, health hazard or environment hazards (see below) as are treated as chemicals waste. Organic Solvent waste is collected in 5 L jerrican (thicker plastic UN-labelled, black cap). Use an appropriate label, Ch. 13, below. Halogenated solvents (> 1% halogenated) are preferably collected separately and in the same type of containers as non-halogenated. Affix label, fill in requested information and deposit the container in the storage for chemical waste, 3315, on floor 3. Ether, dioxane and tetrahydrofuran and other ethers free from peroxides can, after a negative result from peroxidee testing be sent as organic solvent waste (with peroxide test label attached). For information on destruction of peroxides, contact the work environment group if needed and check the information on the KI web 6. Other non-halogenated solventss (not ether and other solvents that might contain peroxides, see above), are collected in approved containers, see Ch. 13 below. Do not use metal containers for solvents that might contain acid or base. Affix label, fill in requested information and deposit the container in the storage for chemical waste on floor Laboratory waste especially KIs rules for laboratory waste management, See the Waste Handling poster and Chemical, Infectious and Biological Waste Containers (Ch.11)
4 Page 4 of 10. Larger volumes of water-based waste are collected in 5 L jerrican (thinner plastic NOT UNlabelled). Use an Appropriate label, see Ch. 13, below. Small amounts of working solution of some chemicals can be poured into drains. See Ch. 12, below, and read the document on the KI web 7 before doing this. 6. Solid Chemical Waste Empty chemical containers with original label can be placed in any suitable cardboard box or in a transparent plastic and placed in the chemicals waste room Mark the box with your name, department and phone number. If the chemical is not toxic, hazardous to health or hazardous to the environment the container should be recycled as glass (or plastic).u Chemical waste which is mainly solid, primarily those that are containing phenol/ chloroform residues and Ethidium Bromide 8 stained gels, is preferably deposited in the 10 litre plastic container with a large cap available in the storage rooms. Affix label, fill in requested information and deposit the container in the storage for chemical waste on floor 3. Remaining alkali metals (e.g. sodium metal) are dissolved in small portions in 2-propanol, which can be flushed down the drain after dissolving. Acids and bases free from heavy metals (non of the hazardous labels in fig 1, 2 or 3) can after dilution and neutralization (if needed, ph should be within 5 to 11.5) be flushed down the drain together with plenty of water. Scintillation vials, see Ch. 7 Developing- and fixation solutions must not be poured out into drains, but collected in designated waste container. For the dark room the containers are transported down to the storage room for chemical waste on the 3 rd floor by the persons responsible for the dark room. The containers should be labelled "fix" or "developer", respectively, and your department/group. If you produce that kind of waste in the lab you can pour it into one of the containers in the dark room. Staining solutions should be handled in different ways. Silver nitrate solutions are collected in a plastic container with a white cap (or other non-un labelled plastic container) on which you write "silver nitrate" and your department/group. For methanol solutions containing Coomassie Blue use you should use UN labelled plastic container. Affix label, fill in requested information and deposit the container in the storage for chemical waste on floor 3 Ethidium Bromide, working with ethidium bromide is not allowed unless special permit, see and instruction on the web of how to decontaminate 9 Mercury from broken thermometers should be alloyed with zinc or other material and place the waste in a tight plastic container, label it "Mercury alloy and your department/group and place it in the storage room for chemical waste on floor 3. Residuals of other toxic heavy metals and their salts are packed in a suitable container. Affix label, fill in requested information and deposit the container in the storage for chemical waste on floor 3. Waste oil from pumps and other instruments is poured into plastic containers and marked with "Used oil" and your name and department/group. Those containers should be brought down to the storage room for chemical waste at the 3 rd floor. All cell media and other solutions containing antibiotics have to be collected in P5 containers and labelled Cytostatics and Drug Contaminated Waste. Disposable pipets, without hazardous contamination (e.g. used for pipetting buffers) may be collected in a suitable, non-penetrable, container. This may later be disposed in ordinary trash (combustibles or recycling plastics) if properly sealed Working with ethidium bromide is not allowed unless special permit Ethidium bromide: Destruction and decontamination of solutions, doc
5 Page 5 of Radioactivity 10 Radioactive waste is disposed in the hospital. Special training is needed before any handling of radioactive isotopes. See separate document for radioactive waste. Radioactive material, including samples in scintillation liquids, should be put in green plastic boxes that are exclusively used for this purpose. Radioactive materials must be properly contained to minimize the risk of leakage from the boxes. Scintillation vials are preferably collected in plastic 5L jerricans, black or white cap depending of scintillation liquid being organic solution or not, before placed in the green box. When stored at the lab, each box should be marked with: i. a warning or warning symbol that informs of its radioactive content. ii. information about the radioactive isotope and activity at a certain date To facilitate further handling of the waste, it is appropriate to place only one kind of radionuclide per box. There are limits for how much activity that each box may contain (SSMFS 2010:2). For 14 C the limit is 10 MBq (0,27 mci) per box and for 3 H the limit is 1000 MBq (27 mci) per box. It is therefore important to know approximately how much each box contains. For the more short-lived radionuclides 32 P, 33 P, 35 S, 51 Cr and 125 I, there are no activity limits for the boxes submitted to the waste room. The boxes will be stored until the activity is low enough before leaving the hospital. However, it is still important to keep an estimate of the contained activity in these boxes. The green boxes should be submitted to the room dedicated for radioactive waste in the Karolinska University Hospital, room F2:3505. In this room, each box should be registered in the waste management system RadWaste with the following information: i. the radioactive isotope, ii. the contained activity at a certain date and iii. the laboratory it comes from. When successfully registered, an adhesive label is printed and put on the box. Medical physicists from the department of nuclear medicine supervises the further handling of the radioactive waste according to the rules set by the Swedish Radiation Safety Authority. They also give access to the waste room and login to the waste management system. Sealed sources, e.g. calibration sources, must not be placed in a green box. Contact nuclear medicine for disposal of these. Even though the law allows for some activity to be poured into the drain, the first choice for disposal of radioactive waste should be in green boxes. The disposal of radioactive waste is also described in the Local safety directions at KI. 8. Infectious/biological material Summary Yellow waste bins, with a white absorption cloth in bottom are used for infectious or sharp waste and labeled with Skärande/stickande/smittförande avfall (Infectious waste, fig. 5). Contagious material is collected in yellow bins (fig. 4) in the lab. When filled it is transported to waste room All potentially contaminated waste such as tubes with blood or cells must be placed in the yellow bin. Fit the yellow lid properly (even if not full!) but don t lock/seal it. Sealed (locked) waste boxes are not allowed to weigh more than 12 kg. Full and sealed boxes are placed in the cold room 3314 at floor 3. Figure 4 Yellow plastic containers 10 Lokala strålskyddsföreskrifter för arbete med Radioaktiva ämnen vid Karolinska Institutet, f Local safety directions for working with radioactive substances at Karolinska Institutet, safety_directions_at_ki.pdf
6 Page 6 of 10. Animal carcasses should be left at the animal department, packed and marked according to the rules of that department. Biological waste (body parts, organs, etc,) should be put into plastic bags in black bins (fig. 6). It may be stored a short time at +4 C in plastic bags in your fridge and/or in the black bin in cold room 7353 until moved to the locked -20 C freezer in room A key to the locked freezer will be found in the cold room Figure 5 Sharps and Infectious Waste Figure 6 Black plastic containers and label All solid waste from work with mammalian cells or micro-organisms (including GMM, see below), and those containing small amounts of animal or human material, is classified as infectious waste. Always use the yellow plastic containers for contagious waste (available at the purchase unit at floor 8, room 8621). If your waste contains liquid you should place an absorption pad (also available in the purchase) in the bottom. When the container is full close it and attach the label for Stickande/skärande/smittförande avfall on the side of the container, at the top. Labels are available at storage room. Transport the full container to the room for chemical and contagious waste, floor 3. All filled and closed contagious waste containers should be placed inside the cold room Cell media (GMM/GMO see below) Be sure that you have labelled the box properly, see Ch. 13. Sharp things, needles and scalpels should go into the small yellow bucket (or in a small sealable plastic container of any kind, properly labelled). This container is then put in the yellow bin. Other general noninfectious waste such as closed empty tubes and other plastics, paper towels and packaging goes into ordinary waste bins or recycled as glass or plastics, if possible. Don't forget that all plastic bags inside the boxes for hazardous waste have to be sealed with tie-straps 11. Make sure the yellow bin is decontaminated on the outside before moving it out of the lab. Cell media without drugs or antibiotics Containers with culture media/broth from work with mammalian cells or micro- (e.g organisms should always be inactivated in accordance with an approved method treated 12 with DesiDos or sodium hypochlorite 13 if applicable). After this treatment the cell media may be poured out in the drain (unless it contains hazardous chemicals according to Ch. 5, then label it and dispose it as chemical waste). Figure 7 Chemical Waste in Water Cell media with drugs or antibiotics If the media contain antibiotics, cytostatics or other drugs it should be labelled with the label for cytostatics and drugs (fig. 8). Collect cell media in 5 litre plastic containers (P5, Ch.13). Use the containers which have white caps and which can be found in the storage room (these containers cost just a fraction of those we use for solvents). Do not fill the containers all the way up. Affix the designated label (fig. 7 and Ch.13), transport the container to the storage room for chemical waste, Figure 8 Cytostatics and Drugs Waste Available in the storage rooms If the media is deactivated with Virkon it should be disposed of as chemical waste. See for more information about inactivation of cells and microorganisms
7 Page 7 of GMM/GMO Laboratory work with gene modified micro-organisms (GMM) is regulated by AFS provision 2011:02 and refers to any experiment with unicellular organisms (including cell cultures) that contain genetic material which have been introduced into the cell in a way that would not happen in nature. Depending upon the risk the activity is divided into three classes; Negligible risk (Factivity, mainly E. coli and cell cultures), low risk (L-activity, such as work with adeno- and lenti-virus), and risky activities (R-activity, not carried out in ). Before work with GMM is started applications have been submitted to the Work Environment Authority (Arbetsmiljöverket) and a risk assessment should be carried out on a form. The waste handling rules for Infectious/biological material (see Ch.0) are also valid for GMM at level F. All solid waste from work with GMM is classified as infectious waste. Always use the yellow plastic containers for contagious waste and when the container is full close it and attach the label for Stickande/skärande/smittförande avfall on the side of the container, at the top. Liquid waste from work with GMM should be treated 14 with sodium hypochlorite and collect the media in 5 litre plastic containers. For waste generated from work at L-activity the local instructions for the BSL2 laboratories (virus rooms) should be followed and the cell media container should be placed inside the yellow container. 11. Instruments and computer equipment For electronic equipment (all instrument having a plug or a battery) is discarded in the waste room at floor 3, Discarded computer equipment should be handed to the IT unit. For heavier equipment and refrigerator/freezer (emptied, defrosted and reasonably cleaned) contact the service unit for deposition. 12. Compounds that in small amounts can be poured out into the drain. Small amounts of water solutions of some solvents/chemicals 15 can be poured into the drain - if it can be done without risk. These substances are decomposed very quickly or have such a low toxicity that small amounts in the common drain do not involve any environmental risk. Please read further on Laboratory waste, Ch 2.5 Discharge to drains 16 before pouring chemicals into the drain. Some cell cultures may also be poured out, if deactivated, see Cell media without drugs or antibiotics, Ch. 9. Solution containing chemicals with hazardous classifications highly toxic, health hazard or environmental hazard must not be poured out (see Ch. 5) Make sure the treatment is effective against the organism. E.g. buffer solutions of common salts, very diluted solution containing acetic acid, acetone, acetonitrile, ethanol, propanol, and some other alcohols (no hazardous chemicals according to Ch. 5)
8 Page 8 of Sorting and Labelling of Hazardous Waste. See Waste Handling poster for an overview and KI intraweb 17 and Ch 0 for more details Chemical, Infectious and Biological Waste Containers YB Yellow bin Yellow plastic container For Sharps & infectious waste, e.g. cells and blood, and all sharp objects, such as canullas/needles, syringes, scalpels, lancets, suture needles and glass slides. See Ch. 8 BB Black bin Black plastic container For human tissue, body parts, organs, anatomical preparations, and animals - but only if they do not contain sharp objects, See Ch. 8 GB Green bin Green plastic container. For radioactive waste. See Ch. 7 and KI safety directions 18 PHD5 Plastic Heavy Duty container 5 litres jerrican with black cap, sturdy container (UN marking 19 ). This container can withstand higher internal pressure than the P5 container. Suitable for hazardous chemicals and organic solvents. See Ch. 5 P5 P10 Plastic container Plastic container 5 litres jerrican with white cap made of thinner plastic, not suitable for organic solvents For waste in water solution. See Ch litres plastic container with black cap For solid chemical waste. See Ch. 6 CB Cardboard box Any suitable cardboard box For original chemical bottles (with hazardous labels, empty or with content) and other properly marked containers used for chemicals A transparent plastic bag may also be used for empty chemical containers. See Ch Laboratory waste: Lokala strålskyddsföreskrifter för arbete med Radioaktiva ämnen vid Karolinska Institutet, oaktiva_amnen_ki_0.pdf Local safety directions for working with radioactive substances at Karolinska Institutet, UN type marking Y or X e.g. 5L container w black cap: 3H1/Y1.9/150/15/N/NET0109A1.9/150/15/N/NET0109A 3: Jerri can; H: Plastic; 1: Closed head (non-remova removable lid); Y: for Packaging Group II (medium hazard level) and III (low hazard level) (when marked with X, it can be used for Group I: Great Danger - high hazard level ); 1.9: Hydraulic pressure (vapour pressure, kpa or weight of solid material); 15: Year of production (2015); N: Country where container was manufactured, NET=Code for manufacturing plant
9 Page 9 of 10. Labels All containers transferred to the waste storage room at floor 3 must be labelled or they will not be transported away. Pre-printed labels for the waste categories below are available in the waste rooms or at the goods reception unit at floor 8, room Remember to add your name, dept./group/lab and phone no. Specify the content as accurately as possible. Generic label for Chemical Waste May be used for all type of chemical waste if properly filled in. Solutions Containing Non-Halogenated Solvents Container: PHD5 Mixtures of solvents with less than 1% halogenated solvents (< 1%). See Ch. 5 Solutions Containing Halogenated Solvents Container: PHD5 Mixtures of solvents with more than 1% halogenated solvents (> 1%). E.g. Chloroform, dichloromethane See Ch. 5 Solid Non-Halogenated Chemical Waste Scintillation Fluids with 3 H, 14 C 35 S or 32 P < 1% halogenated. All radioactive waste is E.g. Ethidium transported to the hospital Bromide, Phenol and/or temporary stored in Residues, etc. the isotope lab Container: P10 See Ch. 7 See Ch. 6 Water Solution with Chemical Waste Container: P5 Including cell media treated with Virkon (See Ch. 5, except GMM, see Ch. 10) Coomassie Blue in Methanol Container: PHD5 See Ch. 5 Trichloroacetic Acid in Methanol Container: PHD5 See Ch. 5 Used chemical containers Container: Cardboard Original containers Box with the following labels should always Exemption: Solvent be handled as bottles with all solvent chemical waste: removed. Bottles of inorganic acid and bases, properly rinsed. These bottles may be placed in normal recycling.
10 Page 10 of 10. Cytostatics and Drug Contaminated Waste including Antibiotics Container: P5 Pharmaceuticals including vaccines, antibiotics, cytostatics and narcotic drugs. Packaging that has contained antibiotics. If waste is both infectious and pharmaceutical use the pharmaceutical and pollution labels. Handling drug containing waste, see Documents Routines handling narcotics and Routines handling prescription drugs. Handling cytostatics drugs, see KI web 1.2 Pharmaceuticals, including cytostatic waste ( and Document Routines handling cytostatics. See Ch. 4 Sharps and Infectious waste Container: Yellow Bin From work with human material, mammalian cells, micro-organisms or genetically modified microorganisms. Human blood and blood products, micro-organisms, cell cultures and materials that has come in contact with these items (e.g. gloves, tubes, pipette tips, paper towels etc.) Exemption: Deactivated waste if an approved method for deactivation has been used and there are no hazardous chemicals or antibiotics in addition to the infectious waste. (see Water Solution with Chemical Waste, above) Sharps/infectious waste labels must NOT be used for inactivated/sterilised waste. Sharps waste is defined as all sharp objects, such as canullas/needles, syringes with fixed needles, scalpels, lancets, suture needles and glass slides. This applies even if there is no suspicion of any infectious agent being present. Minor sharps waste may be placed straight in yellow bins or in small jars/cans/boxes, which are then placed in yellow bins. The box should be properly sealed and clean on the outside. The personnel that collect the waste are not wearing protective gloves See Ch. 3 and Ch. 8 Do not overfill the box! Boxes are handled manually and should not be too heavy to carry. Maximum weight is 12 kg. Name, telephone number, date, group and department are necessary information Boxes without a completed label are not collected. Human tissue and organs 20 Container: Black Bin For human tissue, body parts, organs, anatomical preparations, and animals - but only if they do not contain sharp objects. Temporary storage in the lab at +4 C for maximum one week. Then stored in the designated freezer in the waste storage at floor 3 until pickup for incineration. See Ch SOSFS 2001:8: pdf (SOSFS 2005:26 replaces SOSFS 1999:27. Changed by SOSFS 2009:19) SOSFS 2005:26: SOSFS 2009:19:
KI rules for management of laboratory waste
 Ref. number: Ref. no. 1-313/2017 Ref. no. of previous version: Ref. no. 3584/2012-204 Decision: Review of existing rules for management of laboratory waste and discharge of chemicals to drains Processed
Ref. number: Ref. no. 1-313/2017 Ref. no. of previous version: Ref. no. 3584/2012-204 Decision: Review of existing rules for management of laboratory waste and discharge of chemicals to drains Processed
KI rules for management of laboratory waste
 Ref. number: Ref. no. 1-313/2017 Ref. no. of previous version: Ref. no. 3584/2012-204 Decision: Review of existing rules for management of laboratory waste and discharge of chemicals to drains Processed
Ref. number: Ref. no. 1-313/2017 Ref. no. of previous version: Ref. no. 3584/2012-204 Decision: Review of existing rules for management of laboratory waste and discharge of chemicals to drains Processed
LABORATORY WASTE DISPOSAL GUIDE
 LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
LABORATORY WASTE DISPOSAL GUIDE Purpose This guide will help lab members properly identify and manage hazardous waste in their laboratory. Identifying the types of waste generated is crucial to handling
WASTE MANAGEMENT GUIDE. Creighton University Environmental Health and Safety
 WASTE MANAGEMENT GUIDE Creighton University Environmental Health and Safety www.creighton.edu/ehs 402 546 6400 Overview With over 150 teaching and research labs, as well as other areas such as fine arts
WASTE MANAGEMENT GUIDE Creighton University Environmental Health and Safety www.creighton.edu/ehs 402 546 6400 Overview With over 150 teaching and research labs, as well as other areas such as fine arts
Laboratory Waste Disposal
 Laboratory Waste Disposal 1. Purpose This guideline details the procedures to follow in disposing of hazardous waste that is generated in the laboratory in order to minimise risks associated with the disposal
Laboratory Waste Disposal 1. Purpose This guideline details the procedures to follow in disposing of hazardous waste that is generated in the laboratory in order to minimise risks associated with the disposal
LABORATORY WASTE MANAGEMENT PROGRAMMES
 LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
LABORATORY WASTE MANAGEMENT PROGRAMMES Sylvia Wanjiru Kamau INTERNATIONAL LIVESTOCK RESEARCH INSTITUTE LABORATORY MANAGEMENT AND EQUIPMENT OPERATIONS WORKSHOP. 17 TH -21 ST JUNE 2012 TRAINING OBJECTIVES
Laboratory Close-Out Guidelines
 Office of Laboratory Safety 2300 I Street, NW Ross Hall, Suite B- 05 Washington, DC 20037 t. 202-994- 8258 I labsafety@gwu.edu Guidelines The Principal Investigator (PI) is responsible for leaving laboratories
Office of Laboratory Safety 2300 I Street, NW Ross Hall, Suite B- 05 Washington, DC 20037 t. 202-994- 8258 I labsafety@gwu.edu Guidelines The Principal Investigator (PI) is responsible for leaving laboratories
THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY
 THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY STANDARD OPERATING PROCEDURE PROCEDURE NO: GDP 020 MOD: 1st Issue Page: 1 of 5 Procedure Type: General Discipline Procedure Title: Handling
THE UNIVERSITY OF NEWCASTLE- DISCIPLINE OF MEDICAL BIOCHEMISTRY STANDARD OPERATING PROCEDURE PROCEDURE NO: GDP 020 MOD: 1st Issue Page: 1 of 5 Procedure Type: General Discipline Procedure Title: Handling
2/07. PROCEDURE for DISPOSAL of RADIOACTIVE MATERIALS
 2/07 CORNELL UNIVERSITY PROCEDURE for DISPOSAL of RADIOACTIVE MATERIALS This procedure has been developed to ensure the safety of those individuals who handle radioactive waste and to comply with University,
2/07 CORNELL UNIVERSITY PROCEDURE for DISPOSAL of RADIOACTIVE MATERIALS This procedure has been developed to ensure the safety of those individuals who handle radioactive waste and to comply with University,
Biosafety Training. WVU Shared Research Facilities 2012
 Biosafety Training WVU Shared Research Facilities 2012 Training Overview DEFINE Biosafety, Biohazard, Biosafety Levels Protection (PPE, biosafety cabinets) CELL CULTURE Hazards (Blood Borne Pathogens)
Biosafety Training WVU Shared Research Facilities 2012 Training Overview DEFINE Biosafety, Biohazard, Biosafety Levels Protection (PPE, biosafety cabinets) CELL CULTURE Hazards (Blood Borne Pathogens)
Delaware State University
 Delaware State University University Area Responsible: Risk and Safety Management Policy Number and Name: 7-22: Laboratories Closeout Approval Date: 7/28/11 Reviewed: 7/26/2013 Revisions: 8/13/2013 Related
Delaware State University University Area Responsible: Risk and Safety Management Policy Number and Name: 7-22: Laboratories Closeout Approval Date: 7/28/11 Reviewed: 7/26/2013 Revisions: 8/13/2013 Related
Guidance Note H1402 Packaging and Transport of waste from suspect
 uid Guidance Note H1402 Packaging and Transport of waste from suspect and confirmed cases of the Ebola Virus Revision: 1.0 Date: 21/10/2014 Contents 1 Purpose... 3 2 Introduction... 3 3 Regulations...
uid Guidance Note H1402 Packaging and Transport of waste from suspect and confirmed cases of the Ebola Virus Revision: 1.0 Date: 21/10/2014 Contents 1 Purpose... 3 2 Introduction... 3 3 Regulations...
PROGRAM FOR THE MANAGEMENT AND DISPOSAL OF RADIOACTIVE WASTE
 RADIATION SAFETY DEPARTMENT West Virginia University Health Sciences Center WVU Hospitals Jefferson Memorial Hospital G-139 Health Sciences Center PO Box 9006 Morgantown, WV 26506-9006 Phone: 304-293-3413
RADIATION SAFETY DEPARTMENT West Virginia University Health Sciences Center WVU Hospitals Jefferson Memorial Hospital G-139 Health Sciences Center PO Box 9006 Morgantown, WV 26506-9006 Phone: 304-293-3413
Biological and Regulated Medical Waste Plan
 Biological and Regulated Medical Waste Plan TUFTS ENVIRONMENTAL HEALTH AND SAFETY OCTOBER 2016 Table of Contents I. Purpose... 3 II. Responsibilities... 3 III. Sink and sewer discharge of specific medical
Biological and Regulated Medical Waste Plan TUFTS ENVIRONMENTAL HEALTH AND SAFETY OCTOBER 2016 Table of Contents I. Purpose... 3 II. Responsibilities... 3 III. Sink and sewer discharge of specific medical
WHO NEEDS TO KNOW THIS PROGRAM All NYU employees that generate, handle or transport RMW should be familiar with this written program.
 Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Title: NYU Regulated Medical Waste Safety Written Program Effective Date: October 2002 Revision Date: April 14, 2017 Issuing Authority: Responsible Officer: VP, Facilities and Construction Management Director
Welcome to Courses facilities in building 301 at Department of Systems Biology
 Welcome to Courses facilities in building 301 at Department of Systems Biology The laboratories consists of Lab 039, Lab 049, Elektroforese/noise room 048 (lab 048), Photo room 052, Seminar room 040 and
Welcome to Courses facilities in building 301 at Department of Systems Biology The laboratories consists of Lab 039, Lab 049, Elektroforese/noise room 048 (lab 048), Photo room 052, Seminar room 040 and
Radioactive Waste Radiation Safety Orientation Open Source Booklet 7 (June 1, 2018)
 Radioactive Waste Radiation Safety Orientation Open Source Booklet 7 (June 1, 2018) For more information, refer to the Radiation Safety Manual, 2017 RSP-3 and the Quick Step for waste in the radiation
Radioactive Waste Radiation Safety Orientation Open Source Booklet 7 (June 1, 2018) For more information, refer to the Radiation Safety Manual, 2017 RSP-3 and the Quick Step for waste in the radiation
Central Bio-Medical Waste Treatment Facility
 Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Central Bio-Medical Waste Treatment Facility Central Biomedical Waste Treatment Facility, Swami Ram Cancer Institute and Research Centre Campus, Government Medical College, Rampur Road, Haldwani is the
Laboratory Biosafety Plan
 DUKE NEUROBIOLOGY SCHOOL OF MEDICINE Laboratory Biosafety Plan Laboratory of Boris Kantor Laboratory Biohazards: Biosafety Level 1 Recombinant DNA cloning in E. coli K-12 and derivatives Adeno-Associated
DUKE NEUROBIOLOGY SCHOOL OF MEDICINE Laboratory Biosafety Plan Laboratory of Boris Kantor Laboratory Biohazards: Biosafety Level 1 Recombinant DNA cloning in E. coli K-12 and derivatives Adeno-Associated
Biomedical Waste Management Guide
 Biomedical Waste Management Guide Yale University Office of Environmental Health & Safety 135 College Street, Suite 100, New Haven, CT 06510 Updated April 2017 Telephone: 203-785-3550 / Fax: 203-785-7588
Biomedical Waste Management Guide Yale University Office of Environmental Health & Safety 135 College Street, Suite 100, New Haven, CT 06510 Updated April 2017 Telephone: 203-785-3550 / Fax: 203-785-7588
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS
 UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
UNIVERSITY OF TOLEDO HEALTH SCIENCE CAMPUS SUBJECT: SPECIMEN TRANSPORT IN Procedure No: S-08-011 COMPUTERIZED TUBE SYSTEM PROCEDURE STATEMENT All laboratory specimens will be transported in a manner consistent
Hazardous Materials Management Policies and Procedures
 Hazardous Materials Management Policies and Procedures Table of Contents I. Purpose... 2 II. Scope... 2 III. Policy... 2 III.A. Methods for Managing Hazardous Materials... 2 III.A.1. Ordering... 2 III.A.2.
Hazardous Materials Management Policies and Procedures Table of Contents I. Purpose... 2 II. Scope... 2 III. Policy... 2 III.A. Methods for Managing Hazardous Materials... 2 III.A.1. Ordering... 2 III.A.2.
Disposal of biological waste from containment level 2 laboratories in the WIMM
 Disposal of biological waste from containment level 2 laboratories in the WIMM This document describes how biological waste from containment level 2 laboratories in the WIMM is managed. Written by Malcolm
Disposal of biological waste from containment level 2 laboratories in the WIMM This document describes how biological waste from containment level 2 laboratories in the WIMM is managed. Written by Malcolm
For ethidium bromide waste that is generated at Harvard, each waste stream is to be managed or accumulated as described below.
 LABORATORY CHEMICAL WASTE FAQS ETHIDIUM BROMIDE 1. Q - How should I manage and dispose of ethidium bromide waste? Answer - Ethidium bromide is a mutagen that requires special storage, handling, and disposal
LABORATORY CHEMICAL WASTE FAQS ETHIDIUM BROMIDE 1. Q - How should I manage and dispose of ethidium bromide waste? Answer - Ethidium bromide is a mutagen that requires special storage, handling, and disposal
Appendix B: Decommissioning Checklist for Lab Move
 Laboratory Decommissioning/Commissioning Document - LS-003 12 Appendix B: Decommissioning Checklist for Lab Move This form is for lab moves only from one UTK location to another UTK location General Information
Laboratory Decommissioning/Commissioning Document - LS-003 12 Appendix B: Decommissioning Checklist for Lab Move This form is for lab moves only from one UTK location to another UTK location General Information
Hazard Waste Management Training. This training is a one-time requirement for personnel generating waste at WCU
 Hazard Waste Management Training This training is a one-time requirement for personnel generating waste at WCU Hazard Waste Compliance WCU is classified as a hazardous waste generator by the U.S. Environmental
Hazard Waste Management Training This training is a one-time requirement for personnel generating waste at WCU Hazard Waste Compliance WCU is classified as a hazardous waste generator by the U.S. Environmental
MANAGING HAZARDOUS WASTE AT WSU TRI-CITIES
 MANAGING HAZARDOUS WASTE AT WSU TRI-CITIES WHAT IS A HAZARDOUS WASTE? Most laboratory chemicals including mixtures and solutions Expired chemicals Pesticides, herbicides, fertilizers Paints, automotive
MANAGING HAZARDOUS WASTE AT WSU TRI-CITIES WHAT IS A HAZARDOUS WASTE? Most laboratory chemicals including mixtures and solutions Expired chemicals Pesticides, herbicides, fertilizers Paints, automotive
BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018
 1 BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018 BIOMEDICAL WASTE RULES APPLY TO:- All who generate, collect, receive, store, transport, treat, dispose, or handle bio medical waste :- Hospitals,
1 BIO-MEDICAL WASTE MANAGEMENT (AMENDMENT) RULES -2018 BIOMEDICAL WASTE RULES APPLY TO:- All who generate, collect, receive, store, transport, treat, dispose, or handle bio medical waste :- Hospitals,
Clinical Waste. Policy. Responsibilities
 Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
Clinical Waste Policy Clinical waste is defined as hazardous or offensive waste arising from: - any dental, medical, nursing or veterinary practice, or any other practice or establishment providing medical
Animal cell and tissue culture. Lab 1
 Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
Animal cell and tissue culture Lab 1 Tissue culture Laboratory Safety Outline Lab Safety Biohazards Biosafety Levels Biosafety Cabinets Decontamination Biological Waste Introduction A cell culture laboratory
THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE
 Section V THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE The Trust is currently reviewing the requirements of the recent guidelines Health Technical Memorandum Safe Management of Healthcare Waste (HTML 07-01).
Section V THE SAFE DISPOSAL OF CLINICAL/DOMESTIC WASTE The Trust is currently reviewing the requirements of the recent guidelines Health Technical Memorandum Safe Management of Healthcare Waste (HTML 07-01).
OREGON HEALTH & SCIENCE UNIVERSITY RADIATION SAFETY OPERATING PROCEDURE 1801 RADIOACTIVE WASTE HANDLING & STORAGE
 OREGON HEALTH & SCIENCE UNIVERSITY RADIATION SAFETY OPERATING PROCEDURE 1801 RADIOACTIVE WASTE HANDLING & STORAGE I. PURPOSE: To describe the manner in which radioactive waste shall be stored and/or disposed
OREGON HEALTH & SCIENCE UNIVERSITY RADIATION SAFETY OPERATING PROCEDURE 1801 RADIOACTIVE WASTE HANDLING & STORAGE I. PURPOSE: To describe the manner in which radioactive waste shall be stored and/or disposed
You are required to complete this course with a passing score of 85% or higher if you:
 OHS Biosafety BIO301L Medical Waste Management for Labs Training You are required to complete this course with a passing score of 85% or higher if you: Offer medical waste for transport from a UAB campus
OHS Biosafety BIO301L Medical Waste Management for Labs Training You are required to complete this course with a passing score of 85% or higher if you: Offer medical waste for transport from a UAB campus
Dartmouth College. Institutional Biosafety Committee. Biohazardous Waste Disposal Guide IBC Approved: 10/3/18
 Biohazardous Waste Disposal IBC Approved: 10/3/18 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical laboratories that may contain infectious
Biohazardous Waste Disposal IBC Approved: 10/3/18 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical laboratories that may contain infectious
Dartmouth College. Institutional Biosafety Committee. Biohazardous Waste Disposal Guide IBC Approved: 4/5/17
 Institutional Biosafety Committee Biohazardous Waste Disposal IBC Approved: 4/5/17 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical
Institutional Biosafety Committee Biohazardous Waste Disposal IBC Approved: 4/5/17 I. DEFINITION OF BIOHAZARDOUS WASTE: Biohazardous waste is any waste generated from working in biological or biomedical
CMU-Qatar Hazardous Wastes Disposal
 CMU-Qatar Hazardous Wastes Disposal Biological Wastes BSL1 from teaching/research labs Include Biological Strains, Petri dishes and Tissue culture flask. *Temporarily store the wastes on the following
CMU-Qatar Hazardous Wastes Disposal Biological Wastes BSL1 from teaching/research labs Include Biological Strains, Petri dishes and Tissue culture flask. *Temporarily store the wastes on the following
Waste Management. School Safety Advisor School of Chemistry UCD
 Waste Management School Safety Advisor School of Chemistry UCD Waste Who s responsibility is waste? School waste procedures School hazardous waste management plan School policy - enforced in all labs Clear
Waste Management School Safety Advisor School of Chemistry UCD Waste Who s responsibility is waste? School waste procedures School hazardous waste management plan School policy - enforced in all labs Clear
Radiation Safety Program Hunter College of the City University of New York
 Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
Radiation Safety Program Hunter College of the City University of New York 1. Guidelines for All Users of Radioactive Materials This document presents the guidelines for all persons using radioactive materials.
HAZARDOUS WASTE TRAINING
 Page 1 of 18 HAZARDOUS WASTE TRAINING The following guidelines are based on regulations enforced by the EPA and state Department of Environmental Regulation and must be implemented to avoid violations.
Page 1 of 18 HAZARDOUS WASTE TRAINING The following guidelines are based on regulations enforced by the EPA and state Department of Environmental Regulation and must be implemented to avoid violations.
University Health Services Health and Safety
 ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
ADVISORY NO. 10.2: MANAGEMENT OF BIOLOGICAL AND INFECTIOUS WASTES The following recommendations are based on the Ohio Revised Code (ORC), Ohio Administrative Code (OAC) and EPA guidelines regarding biological
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES
 UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES October 2016 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
UNIVERSITY OF RICHMOND REGULATED MEDICAL WASTE MANAGEMENT GUIDELINES October 2016 Table of Contents Section Page I. Introduction.... 1 II. Characteristics of Regulated Medical Waste 1-2 III. Exclusions...2-3
10.0 DISPOSAL OF FARM WASTE
 DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
DISPOSAL OF FARM WASTE 10.1 Disposal of Dead Animals 10.1.1 Managing dead animal disposal 10.2 Disposal of Veterinary Waste 10.3 Pesticides 10.2.1 Sharps 10.2.2 Medicine disposal 10.3.1 Pesticide disposal
Handling of Specific Chemicals
 Handling of Specific Chemicals 1) ACETONITRILE 2) CARCINOGENS, MUTAGENS, TERATOGENS 3) COMBUSTIBLE LIQUIDS 4) CONTAMINATED CONTAINERS 5) CONTAMINATED EQUIPMENT 6) CORROSIVE ACIDS AND BASES 7) EXPLOSIVES
Handling of Specific Chemicals 1) ACETONITRILE 2) CARCINOGENS, MUTAGENS, TERATOGENS 3) COMBUSTIBLE LIQUIDS 4) CONTAMINATED CONTAINERS 5) CONTAMINATED EQUIPMENT 6) CORROSIVE ACIDS AND BASES 7) EXPLOSIVES
Policy & Procedure Manual
 Environmental Health & Safety Policy & Procedure Manual Title: 1. Purpose: To establish policies, work practices, and systematic procedures for the handling, packaging, collection, treatment, and disposal
Environmental Health & Safety Policy & Procedure Manual Title: 1. Purpose: To establish policies, work practices, and systematic procedures for the handling, packaging, collection, treatment, and disposal
Contents. Introduction
 Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
Introduction Contents Medi-Waste is a specialist company that evolved in 1987 providing the collection, transport and disposal of biomedical waste, (including off spec and out of date pharmaceuticals,
NJIT Biological Waste Management for Laboratories. NJIT Environmental Health and Safety Department Spring, 2017
 NJIT Biological Waste Management for Laboratories NJIT Environmental Health and Safety Department Spring, 2017 Biological Waste In broad terms, biological waste may be defined as: - liquid or solid waste
NJIT Biological Waste Management for Laboratories NJIT Environmental Health and Safety Department Spring, 2017 Biological Waste In broad terms, biological waste may be defined as: - liquid or solid waste
Standard Operating Procedure Title: Good laboratory practices (GLP) for microbiology and chemistry laboratories
 Standard Operating Procedure Title: Good laboratory practices (GLP) for microbiology and chemistry laboratories Department Micro Laboratory Document no MICLAB 155 Title Good laboratory practices (GLP)
Standard Operating Procedure Title: Good laboratory practices (GLP) for microbiology and chemistry laboratories Department Micro Laboratory Document no MICLAB 155 Title Good laboratory practices (GLP)
Standard Operating Procedure
 Section 1: Overview Page 1 of 5 Non-halogenated Organic Solvents Standard Operating Procedure Lab: 3724 Beckman Institute Department: Materials Science and Engineering PI: Paul V. Braun Written By: Eric
Section 1: Overview Page 1 of 5 Non-halogenated Organic Solvents Standard Operating Procedure Lab: 3724 Beckman Institute Department: Materials Science and Engineering PI: Paul V. Braun Written By: Eric
Consignment of Waste to the Disposal of Hazardous Waste Service
 Radioactive Waste LR 31 Who should read this document This document should be read by the Head of Department, Departmental Radiation Protection Supervisors, users of radioactive materials and their supervisors.
Radioactive Waste LR 31 Who should read this document This document should be read by the Head of Department, Departmental Radiation Protection Supervisors, users of radioactive materials and their supervisors.
Appendix J. University of Alabama at Birmingham Medical Waste Management Plan
 University of Alabama at Birmingham Medical Waste Management Plan I. Definitions per the Alabama Department of Environmental Management Land Division 13-Solid Waste Program, Chapter 335-13-1, Medical Waste
University of Alabama at Birmingham Medical Waste Management Plan I. Definitions per the Alabama Department of Environmental Management Land Division 13-Solid Waste Program, Chapter 335-13-1, Medical Waste
SESSION 4 AND HOSPITAL-GENERATED WASTE WASTE DISPOSAL AT THE MEDICAL CENTER OF THE UNIVERSITY OF ILLINOIS. Stephens. Raymond S.
 SESSION 4 RESEARCH- AND HOSPITAL-GENERATED WASTE WASTE DISPOSAL AT THE MEDICAL CENTER OF THE UNIVERSITY OF ILLINOIS Raymond S. Stephens This paper examines the main problems of hazardous waste disposal
SESSION 4 RESEARCH- AND HOSPITAL-GENERATED WASTE WASTE DISPOSAL AT THE MEDICAL CENTER OF THE UNIVERSITY OF ILLINOIS Raymond S. Stephens This paper examines the main problems of hazardous waste disposal
MEDICAL WASTE MANAGEMENT STUDY NOTES
 MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
MEDICAL WASTE MANAGEMENT STUDY NOTES UNIT 1 INTRODUCTION Hospital and other health care establishment has a duty of care for the environment and for public health and have particular responsibility in
INDEX. Laboratory Safety Guide
 INDEX A accident reporting, 1, 70, 125 acids, 154 neutralization, 164-168 strength table, 165 acrylamide, 154 acute toxicity, 31, 313, 314 acute toxicity classes, 32 administrative controls (fume hoods),
INDEX A accident reporting, 1, 70, 125 acids, 154 neutralization, 164-168 strength table, 165 acrylamide, 154 acute toxicity, 31, 313, 314 acute toxicity classes, 32 administrative controls (fume hoods),
OFFICE OF RESEARCH SAFETY RS104 Lab Closeout Policy
 OFFICE OF RESEARCH SAFETY RS104 Lab Closeout Policy Memphis Knoxville Chattanooga Nashville No./Title: RS104 Lab Closeout Policy Resp. Office: Research Safety Effective Date: 12/01/2017 Category: Research
OFFICE OF RESEARCH SAFETY RS104 Lab Closeout Policy Memphis Knoxville Chattanooga Nashville No./Title: RS104 Lab Closeout Policy Resp. Office: Research Safety Effective Date: 12/01/2017 Category: Research
Health Sciences Campus Biomedical Waste Management Standard Operating Procedure (SOP)
 Health Sciences Campus Biomedical Waste Management Standard Operating Procedure (SOP) NOTE: This SOP for biological waste management does not supersede the requirements for radioactive and/or hazardous
Health Sciences Campus Biomedical Waste Management Standard Operating Procedure (SOP) NOTE: This SOP for biological waste management does not supersede the requirements for radioactive and/or hazardous
University of Denver Environmental Health & Safety Department Laboratory Inspection Comment Sheet
 University of Denver Environmental Health & Safety Department Laboratory Inspection Comment Sheet The following briefly describes what EHS looks for during a typical laboratory inspection. Items in boxes
University of Denver Environmental Health & Safety Department Laboratory Inspection Comment Sheet The following briefly describes what EHS looks for during a typical laboratory inspection. Items in boxes
Hazardous Waste Management. Office of Environmental Health and Safety
 Hazardous Waste Management Office of Environmental Health and Safety 2016 Objectives To demonstrate the proper methods of laboratory hazardous waste management for compliance with state and federal regulations.
Hazardous Waste Management Office of Environmental Health and Safety 2016 Objectives To demonstrate the proper methods of laboratory hazardous waste management for compliance with state and federal regulations.
Ref : NTU/OHS/GUIDE/10 Date of issue : 23 September Updated by : Liew Ching Boon Next review date : 22 September 2012
 Office of Health and Safety Reg. No. 200604393R Ref : NTU/OHS/GUIDE/10 Date of issue : 23 September 2010 Updated by : Liew Ching Boon Next review date : 22 September 2012 Title : Guideline on Disposal
Office of Health and Safety Reg. No. 200604393R Ref : NTU/OHS/GUIDE/10 Date of issue : 23 September 2010 Updated by : Liew Ching Boon Next review date : 22 September 2012 Title : Guideline on Disposal
Next Review Date: Oct 2017 SWP Reference Number: Version: V3 Version Issue Date: Oct 2016
 Faculty/School: Faculty of Pharmacy Next Review Date: Oct 2017 SWP Reference Number: Version: V3 Version Issue Date: Oct 2016 SWP Title: Operation of the autoclaves Prepared by: Padmaja Dhanvate and Dr
Faculty/School: Faculty of Pharmacy Next Review Date: Oct 2017 SWP Reference Number: Version: V3 Version Issue Date: Oct 2016 SWP Title: Operation of the autoclaves Prepared by: Padmaja Dhanvate and Dr
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples
 Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Pointers on Shipping: Clinical Samples, Biological Substance Category B and Environmental Test Samples At FedEx, we understand the importance of ensuring the safe shipping of clinical samples such as human
Contact Information: Laboratory Supervisor: Denise Kind Laboratory Manager: Mat Ashby
 Biology and Wildlife STANDARD OPERATING PROCEDURE Autoclaving Location(s): Murie 215 Chemical(s): None Specific Hazards: o steam improper use of autoclave can expose user to dangerous steam burns o extremely
Biology and Wildlife STANDARD OPERATING PROCEDURE Autoclaving Location(s): Murie 215 Chemical(s): None Specific Hazards: o steam improper use of autoclave can expose user to dangerous steam burns o extremely
Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date:
 STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
STANDARD OPERATING PROCEDURE VETERINARY HEALTH COMPLEX Section: Administration Date of Issue: 05/09/2011 Issued By: Administration Part: Pneumatic Tube System Revision #: Revision Date: Pages: Board Approval
Management of Hazardous Wastes
 23 Management of Hazardous Wastes Dr. Mohammed Abu Kaff Environment & Public Health Expert Dubai Municipality Dubai. UAE Hazardous wastes have been defined by the United States Environmental Protection
23 Management of Hazardous Wastes Dr. Mohammed Abu Kaff Environment & Public Health Expert Dubai Municipality Dubai. UAE Hazardous wastes have been defined by the United States Environmental Protection
CONTACTS. Environmental Health and Safety. Regulated Materials Management Center
 Environmental Health & Safety Main Office, Wyoming Hall Room 102 Phone: (307) 766-3277 Fax: (307)766-6116 Regulated Materials Management Center Phone: (307)766-3696 Fax: (307)766-3699 Web: www.uwyo.edu/ehs
Environmental Health & Safety Main Office, Wyoming Hall Room 102 Phone: (307) 766-3277 Fax: (307)766-6116 Regulated Materials Management Center Phone: (307)766-3696 Fax: (307)766-3699 Web: www.uwyo.edu/ehs
Saves your life Protects your health Preserves your equipment Makes your customers happy Minimises risks Protects the environment
 Why handle dangerous cargo properly? Because it Saves your life Protects your health Preserves your equipment Makes your customers happy Minimises risks Protects the environment These are all very good
Why handle dangerous cargo properly? Because it Saves your life Protects your health Preserves your equipment Makes your customers happy Minimises risks Protects the environment These are all very good
Hazardous Waste Initial Training
 Hazardous Waste Initial Training Hazardous Waste Disposal Types of Waste Chemical Waste Radioactive Waste Biohazardous Waste/Medical Waste Pathological Waste Refer to the Waste Disposal Guide Typical Wastes
Hazardous Waste Initial Training Hazardous Waste Disposal Types of Waste Chemical Waste Radioactive Waste Biohazardous Waste/Medical Waste Pathological Waste Refer to the Waste Disposal Guide Typical Wastes
Development of a New Classification and Colour Code for Medical Waste Segregation
 Development of a New Classification and Colour Code for Medical Waste Segregation Ramani Bai V. 1*, Lokman Hakim S. 2, Ian Harrison 3, Vanitha G. 4, 1 Department of Civil Engineering, University of Nottingham
Development of a New Classification and Colour Code for Medical Waste Segregation Ramani Bai V. 1*, Lokman Hakim S. 2, Ian Harrison 3, Vanitha G. 4, 1 Department of Civil Engineering, University of Nottingham
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information New Jersey
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information New Jersey Identifying & Segregating Regulated Medical Waste RMW in New Jersey Six Categories of Regulated Medical
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information New Jersey Identifying & Segregating Regulated Medical Waste RMW in New Jersey Six Categories of Regulated Medical
Medical, Pathological and Low-level Radioactive Waste Management Plan for Incinerator Revised 3-08
 Background Medical, Pathological and Low-level Radioactive Waste Management Plan for Incinerator Revised 3-08 Many WSU laboratories and departments generate medical waste (contaminated solids), pathological
Background Medical, Pathological and Low-level Radioactive Waste Management Plan for Incinerator Revised 3-08 Many WSU laboratories and departments generate medical waste (contaminated solids), pathological
FACT SHEET : Using Autoclaves Safely
 CSULA Environmental Health and Safety Biosafety Office FACT SHEET : Using Autoclaves Safely Most science research laboratories on campus require the use of autoclaves. The primary purpose of the autoclave
CSULA Environmental Health and Safety Biosafety Office FACT SHEET : Using Autoclaves Safely Most science research laboratories on campus require the use of autoclaves. The primary purpose of the autoclave
Procedure for Health Care Risk Waste Management. Procedure No. 403
 Procedure for Health Care Risk Waste Management Procedure No. 403 Print Name Title Date Prepared by J.G. MacNamara T.S.O. 01/11/04 Reviewed by J. Hoare CATSO 01/11/04 Corporate Authorisation J.G. MacNamara
Procedure for Health Care Risk Waste Management Procedure No. 403 Print Name Title Date Prepared by J.G. MacNamara T.S.O. 01/11/04 Reviewed by J. Hoare CATSO 01/11/04 Corporate Authorisation J.G. MacNamara
Appendix C. Yes obtain containment compatible with chemical
 Appendix C Chemical Hazard Assessment Form Complete and attach a Chemical Hazard Assessment form for EACH hazardous chemical listed in the SOP. Chemical/ Reagent: Formaldehyde solutions/ Formalin CAS#:
Appendix C Chemical Hazard Assessment Form Complete and attach a Chemical Hazard Assessment form for EACH hazardous chemical listed in the SOP. Chemical/ Reagent: Formaldehyde solutions/ Formalin CAS#:
ADMINISTRATIVE PROCEDURE
 Page 1 of 6 1. Purpose: To provide safe and consistent procedures concerning the use, storage and disposal of hazardous materials/waste in compliance with the Environmental Protection Act (General- Waste
Page 1 of 6 1. Purpose: To provide safe and consistent procedures concerning the use, storage and disposal of hazardous materials/waste in compliance with the Environmental Protection Act (General- Waste
WARTBURG COLLEGE ENVIRONMENTAL AND OCCUPATIONAL SAFETY PROGRAM HAZARDOUS WASTE PLAN
 July 15, 2015 WARTBURG COLLEGE ENVIRONMENTAL AND OCCUPATIONAL SAFETY PROGRAM HAZARDOUS WASTE PLAN Table of Contents General.. 1 Responsibilities. 1 Program Development... 2 References... 2 Management Procedures
July 15, 2015 WARTBURG COLLEGE ENVIRONMENTAL AND OCCUPATIONAL SAFETY PROGRAM HAZARDOUS WASTE PLAN Table of Contents General.. 1 Responsibilities. 1 Program Development... 2 References... 2 Management Procedures
NOTE: Do NOT overfill sharps containers; there should be NO more than 50ml of residual liquid in the mailback kit when returned
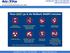 These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
These DO go in the Mailback SHARPS Containers Sharps Waste Such As: Needles & syringes Razor blades Orthodon?c wires Scalpel blades & lancets Glass pipebes, slides & tubes Broken, contaminated glass Staples
Biosafety Checklist. 07-BiosafetyChecklist-LTC-SOP-v2.0-17Feb of 6
 Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
Title: Biosafety Checklist Origination Date: 31 Jan 1997 Total Pages: 5 Effective Date: 17 Feb 2012 SOP Number LTC-SOP-07 v2.0 Supersedes SOP Written By: ACTG/IMPAACT Lab Tech Committee Dated: 01 Jun 2004
Ferris State University Academic Affairs Laboratory Safety Hazardous Waste Determination and Flowchart Master Flowchart. Yes
 Master Flowchart Is your material a SPECIAL WASTE? Refer to: Special Waste Flowchart - pg. 2 Your waste is a: HAZARDOUS WASTE Place with Hazardous Waste for proper pick-up disposal. Is your material a
Master Flowchart Is your material a SPECIAL WASTE? Refer to: Special Waste Flowchart - pg. 2 Your waste is a: HAZARDOUS WASTE Place with Hazardous Waste for proper pick-up disposal. Is your material a
Standard Operating Procedure (SOP) for loading AMS targets for Beryllium and Aluminum
 Standard Operating Procedure (SOP) for loading AMS targets for Beryllium and Aluminum #1 General Process Description This SOP describes the handling and loading of targets that will be sent to an AMS.
Standard Operating Procedure (SOP) for loading AMS targets for Beryllium and Aluminum #1 General Process Description This SOP describes the handling and loading of targets that will be sent to an AMS.
Infectious Waste Disposal Procedure 25 PA Code 284 Ursinus College Collegeville, PA 19426
 The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
The purpose of the Infectious Waste Disposal procedure is to establish procedures for the safe handling and proper disposal of infectious waste generated at in accordance with regulation 25 PA Code Chapter
ANG 111 Summer 2009 Laboratory 0: Basic Laboratory Procedures. To provide a foundation in basic laboratory techniques and safety issues
 ANG 111 Summer 2009 Laboratory 0: Basic Laboratory Procedures Objective: To provide a foundation in basic laboratory techniques and safety issues Overview: Students enter this laboratory with a wide range
ANG 111 Summer 2009 Laboratory 0: Basic Laboratory Procedures Objective: To provide a foundation in basic laboratory techniques and safety issues Overview: Students enter this laboratory with a wide range
Healthcare Waste Regulatory Challenges. Selin Hoboy November 17, 2011
 Healthcare Waste Regulatory Challenges Selin Hoboy November 17, 2011 Overview What are healthcare wastes? What regulations and agencies affect healthcare waste? How to cope with it all! BMPs What are upcoming
Healthcare Waste Regulatory Challenges Selin Hoboy November 17, 2011 Overview What are healthcare wastes? What regulations and agencies affect healthcare waste? How to cope with it all! BMPs What are upcoming
CSULB Department of Biological Sciences Common Use Equipment Training. Autoclaves
 CSULB Department of Biological Sciences Common Use Equipment Training Autoclaves Outline/Learning Objectives Understand how gravity autoclaves work Learn how autoclaves are SAFELY operated What materials
CSULB Department of Biological Sciences Common Use Equipment Training Autoclaves Outline/Learning Objectives Understand how gravity autoclaves work Learn how autoclaves are SAFELY operated What materials
UN 3291 Clinical and Related Wastes Services Specification
 SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
SSS013 UN 3291 Clinical and Related Wastes Services Specification 1. Scope This specification describes the acceptable waste materials and packaging requirements for Daniels Health services for a subset
SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS AND BIOLOGICAL WASTE
 ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
ENVIRONMENTAL AND EMERGENCY MANAGEMENT Environmental Health and Safety University Crossing Suite 140 Lowell MA 01854 http://www.uml.edu/eem/ SOP BIO-006 USE OF AUTOCLAVE FOR STERILIZATION OF MATERIALS
Hazardous Waste Disposal Standard. Safety Resources
 Hazardous Waste Disposal Standard 2018 Safety Resources Table of Contents 1 Purpose... 1 2 Applicable To... 1 3 Definitions... 1 4 Scope... 1 5 Responsibilities... 2 5.1 College/Division/Department/Unit
Hazardous Waste Disposal Standard 2018 Safety Resources Table of Contents 1 Purpose... 1 2 Applicable To... 1 3 Definitions... 1 4 Scope... 1 5 Responsibilities... 2 5.1 College/Division/Department/Unit
Laboratory Decontamination and Decommissioning Policy and Procedures. Office of Environmental Health and Safety
 Laboratory Decontamination and Decommissioning Policy and Procedures Office of Environmental Health and Safety July 2018 1. Purpose and Scope 1.1 It is the policy of Cleveland State University that laboratory
Laboratory Decontamination and Decommissioning Policy and Procedures Office of Environmental Health and Safety July 2018 1. Purpose and Scope 1.1 It is the policy of Cleveland State University that laboratory
Lewis & Clark Policy and Procedure
 Lewis & Clark Policy and Procedure Subject: Hazardous Waste Program Policy No.: Division: Business and Finance Department: Facilities Services Original Effective Date: March 29, 1994 Date(s) Reviewed/Revised:
Lewis & Clark Policy and Procedure Subject: Hazardous Waste Program Policy No.: Division: Business and Finance Department: Facilities Services Original Effective Date: March 29, 1994 Date(s) Reviewed/Revised:
BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY
 BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY 2 Preamble This document describes the procedures to follow for safely
BIOSAFETY LEVEL 2 PROTOCOLS BIOPROCESS OPTIMIZATION LABORATORY - POULIOT BUILDING, ROOM 1541 ALAIN GARNIER JUNE 2007, LAVAL UNIVERSITY 2 Preamble This document describes the procedures to follow for safely
Biosafety Program. I. Policy. Authority. Definitions
 Biosafety Program I. Policy It is the policy of California State University, Fullerton to maintain, insofar as is reasonably possible, an environment that will not adversely affect the health, safety and
Biosafety Program I. Policy It is the policy of California State University, Fullerton to maintain, insofar as is reasonably possible, an environment that will not adversely affect the health, safety and
BioSpace Lab Policies and Practices. Corefacilties Unit
 BioSpace Lab Policies and Practices Corefacilties Unit corefacilities@ibecbarcelona.eu 1 The BioSpace Lab is part of IBEC Core Facilities which also consists of General Management of IBEC s Labs and the
BioSpace Lab Policies and Practices Corefacilties Unit corefacilities@ibecbarcelona.eu 1 The BioSpace Lab is part of IBEC Core Facilities which also consists of General Management of IBEC s Labs and the
LABORATORY SAFETY EVALUATION. S = Satisfactory N = Needs Improvement* N/A = Not Applicable
 LABORATORY SAFETY EVALUATION Principal Investigator: Department: PH# : Building: Date: Room(s) : S = Satisfactory N = Needs Improvement* N/A = Not Applicable *If an area is in need of improvement, please
LABORATORY SAFETY EVALUATION Principal Investigator: Department: PH# : Building: Date: Room(s) : S = Satisfactory N = Needs Improvement* N/A = Not Applicable *If an area is in need of improvement, please
Anseth Lab Orientation Checklist
 Anseth Lab Orientation Checklist In order for the lab to operate in the most efficient and safe manner possible, it is imperative that all lab members are familiar with lab policies and procedures. This
Anseth Lab Orientation Checklist In order for the lab to operate in the most efficient and safe manner possible, it is imperative that all lab members are familiar with lab policies and procedures. This
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa
 MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa ADMINISTRATIVE POLICY AND PROCEDURE Policy #: 163 Subject: Purpose: Policy: Pharmaceutical Waste Management Program Marshalltown Medical Surgical
MARSHALLTOWN MEDICAL & SURGICAL CENTER Marshalltown, Iowa ADMINISTRATIVE POLICY AND PROCEDURE Policy #: 163 Subject: Purpose: Policy: Pharmaceutical Waste Management Program Marshalltown Medical Surgical
Regulated Medical Waste Management and Proper Waste Segregation. State-Specific Information Washington
 Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Washington Biomedical Waste Regulation In the State of Washington biomedical waste, where regulated, is regulated
Regulated Medical Waste Management and Proper Waste Segregation State-Specific Information Washington Biomedical Waste Regulation In the State of Washington biomedical waste, where regulated, is regulated
Special Specimen Collection Procedures for Coagulation Testing
 Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
Special Specimen Collection Procedures for Coagulation Testing The accuracy of hemostasis testing depends upon the quality of the specimen submitted. Hemostasis specimens must be properly collected, labeled,
WARTBURG COLLEGE ENVIRONMENTAL AND OCCUPATIONAL SAFETY PROGRAM HAZARDOUS WASTE PLAN
 3 February, 2001 WARTBURG COLLEGE ENVIRONMENTAL AND OCCUPATIONAL SAFETY PROGRAM TABLE OF CONTENTS HAZARDOUS WASTE PLAN 1. General 2. Responsibilities 3. Program Development 4. References 5. Management
3 February, 2001 WARTBURG COLLEGE ENVIRONMENTAL AND OCCUPATIONAL SAFETY PROGRAM TABLE OF CONTENTS HAZARDOUS WASTE PLAN 1. General 2. Responsibilities 3. Program Development 4. References 5. Management
DEPARTMENT OF BIOLOGICAL SCIENCES MEDICAL/BIOHAZARDOUS WASTE DISPOSAL AUTOCLAVE TRAINING
 DEPARTMENT OF BIOLOGICAL SCIENCES MEDICAL/BIOHAZARDOUS WASTE DISPOSAL AUTOCLAVE TRAINING INTRODUCTION In accordance with the California Medical Waste Management Act, Health and Safety Code Stanford University
DEPARTMENT OF BIOLOGICAL SCIENCES MEDICAL/BIOHAZARDOUS WASTE DISPOSAL AUTOCLAVE TRAINING INTRODUCTION In accordance with the California Medical Waste Management Act, Health and Safety Code Stanford University
Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998)
 Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
Date: Location: Responsible: Project Title: Inspector: _ Animal Facility Biosafety Level 3 Checklist (date: April 16, 1998) These questions are based on the Biosafety Level 3 section of Biosafety in Microbiological
Biology and Wildlife STANDARD OPERATING PROCEDURE Ethidium Bromide
 Biology and Wildlife STANDARD OPERATING PROCEDURE Ethidium Bromide 4 0 0 This SOP addresses only ethidium bromide. Users must also follow the electrophoresis SOP and any other SOPs relevant to their particular
Biology and Wildlife STANDARD OPERATING PROCEDURE Ethidium Bromide 4 0 0 This SOP addresses only ethidium bromide. Users must also follow the electrophoresis SOP and any other SOPs relevant to their particular
96-well Genomic DNA Extraction Kit. Protocol Book. (Deep Well) Ver RBP96B-2
 96-well Genomic DNA Extraction Kit (Deep Well) Protocol Book RBP96B-2 Ver. 2013-1 Precautions I) Handling Requirements Do not use a kit after its expiration date has passed. Some reagents contain the
96-well Genomic DNA Extraction Kit (Deep Well) Protocol Book RBP96B-2 Ver. 2013-1 Precautions I) Handling Requirements Do not use a kit after its expiration date has passed. Some reagents contain the
