REVIEW OF FACTORS THAT AFFECT THE EARTH'S OZONE DEPLETION. Dr. J. J. Hurtak, Ph.D AFFS Corporation P.O. Box FE Los Gatos, California USA.
|
|
|
- Stewart Ross
- 6 years ago
- Views:
Transcription
1 REVIEW OF FACTORS THAT AFFECT THE EARTH'S OZONE DEPLETION Dr. J. J. Hurtak, Ph.D AFFS Corporation P.O. Box FE Los Gatos, California USA and Patrick Bailey, Ph.D Lockheed Corporation Sunnyvale, CA Abstract Mapping ozone depletion over the Arctic and Antarctic to study the chemically perturbed regions has used airborne and satellite imaging systems onboard the specialized ER- 2 plane (at - 19 km) and the modified DC-8-72 aircraft (at km), the Nimbus-7 TOMS system, the UARS, as well as Dobson ground stations. Measurements are designed to gauge not only the extent of ozone depletion over the Antarctic and Arctic, but other chemical changes in the stratosphere. Their chemical findings have resulted in the Montreal Protocol and the Copenhagen revisions which will ban the production of CFCs by A Model For Ozone Destruction Ozone measurements have been underway since the 1920s using ground-based ultraviolet spectrometers known as Dobson instruments. The British Antarctic Survey team stationed at Halley Bay (Antarctica 76oS) first noted ozone changes as early as the late 1970s. Significant ozone loss in the Antarctic lower stratosphere during September and October (springtime) were first reported in 1985 by J.C. Farman, et al.1 This was confirmed in 1986 during the NOZE I expedition that noted evidence of high chlorine chemistry and low nitrogen compounds with ozone levels depleted in the 12 to 20 km region. Since 1987, ozone measurements have been globally monitored and an extensive network of ozone observations stations have been established. The systems include a world-wide network of Dobson ground stations, observations from the solar backscattered ultraviolet (SBUV) instrument, the Stratospheric Aerosol Measurement (SAM) II and the Total Ozone Mapping Spectrometer (TOMS) aboard the Nimbus 7 satellite which has been mapping ozone levels since November Ozonesonde stations have also been established with balloon-launched electrochemical sondes that measure ozone from the ground to 30km altitude.3 The ER-2 and DC-8 planes were employed for the 1987 Airborne Antarctic Ozone Experiment (AAOE) and the winter Airborne Arctic Stratospheric Expedition (AASE I) using both in situ and remote sensing equipment on board. During the winter of (October-March) similar equipment was used for the AASE II. The DC-8 carries differential absorption lidar (DIAL) and the ER-2 flies in situ carrying a wide variety of samplers and spectrometers for aerosol and cloud particles studies, as well as to measure the chemistry of the stratosphere.4 One recent addition on the ER-2 instrumentation was the ALIAS (aircraft laser infrared absorption spectrometer) to measure HCl.5 The most notable ozone destruction occurs in the Antarctic in springtime and the destruction is greatest in the lower stratosphere at an altitude of 20 to 25km, extending to 12 km, also the dominant regions for Polar Stratospheric Clouds (PSCs). Losses initially were concentrated in a 1-to-2 kilometer-thick region of the stratosphere above an altitude of 16 kilometers, but as the depletion has increased ozone destruction extends to larger regions at lower altitudes. In 1991 losses at 10 kilometer altitudes were recorded.6 Scientists at the National Oceanic and Atmospheric Administration (NOAA), the National Science Foundation, NASA, and other institutions after careful study now believe catalytic reactions of nitrogen, hydrogen, chlorine, and bromine oxides are the chemistry creating the destruction in the stratosphere. The long Antarctic polar winter night produces atmospheric temperatures as low as -90oC at 15 to 20km which produces PSC type II where HCl and ClONO2 that are usually present as nonreactive phase are photolyzed to radicals. Ice particles seeded by nitric acid trihydrate are created:7 HCl + ClONO2 HNO3 + Cl2 H2O + ClONO2 HNO3 + HOCL where HNO3 (nitric acid) remains on PSCs and Cl2 as well as HOCl (hypochlorous acid) continues in the gas phase. In some cases, water vapor and nitrogen compounds are removed to lower altitudes and Cl remains in the vortex. Chlorine as a reservoir species is
2 transformed on aerosols and cloud particles to form active chlorine (ClO) where the reaction of ClO + ClO yields Cl2O2 which photolyses to atomic chlorine and ClOO and ends with a catalytic destruction of ozone, where atomic chlorine (Cl) reacts with O3 to form ClO + O2.8 The effect of sunlight appears to accelerate the reaction, focusing on ice crystals with attached heterogeneous (surface) chlorine molecules. The Cl2 rapidly separates to atomic chlorine in the presence of ultraviolet light. When spring arrives the atomic chlorine is released, triggering ClOx chain reactions. The important factor in the ClOx chain is that after the chlorine reactant initiates the process of ozone destruction (or the destruction of any odd oxygen, i.e. O1 or O3), the Cl is not removed in a denitrified environment, but is able to once again repeat the process. Each chlorine atom could destroy approximately 100,000 molecules of ozone.9 If nitrogen radicals were in abundance, ClO would rapidly react to form chlorine nitrate, ClONO2, which like HCl is a reservoir molecule, nonreactive to ozone. Since little NOx exists, there is no stopping the reaction for 5-6 weeks, causing ozone depletion in early spring of up to 95% in the stratosphere and 60% in all latitudes surrounding the PSCs.10 In the Arctic less ozone depletion occurs than at the Antarctic since the area of the Northern Hemisphere is not as cold due in part to the greater land mass, for PSCs type II to form causing significantly less ozone destruction. Similar chemistry is present where levels of ClONO2 and HCl are high prior to the polar night. When the atmosphere cools to -77oC and PSCs type I, a significant depletion of both ClONO2 and HCl occurs and an increase of ClO (up to 1000 pptv from a previous < 150 pptv at 20 km) takes place. There is a clear reversal between the levels of ClO and HCl within the polar vortex, again HCl + ClONO2 Cl2 + HNO3. Nitrogen is driven out of the winter vortex and N2O levels reached 65 ppbv during mid-february recordings of the AASE II, a third of their usual level. By the end of February and March as the vortex breaks down, the ClO levels again decrease and ClONO2 increases where ClO + NO2 ClONO2. HCl levels require more time to recover.11 During the AASE II expedition, scientists calculated Arctic ozone loss of 15-25% in January and February 1992 which confirmed earlier models by Browell et al. To date, although ozone levels at mid- latitudes in the Northern Hemisphere are deceasing from 1-8% at certain times of the year. Only in approximately five of the last 30 winters have January temperatures in the Northern polar region been cold enough for preliminary PSCs type I to form.12 The polar vortex is not as constricted and there is little sunlight due to the polar night. However, by April 1991 the wintertime ozone layer in the Arctic showed ozone depletion as high as 5.6% with some depletion as far south as Florida.13 Origin of Ozone Depletion Scientists believe that the conditions of wind, light, and temperature over the Antarctic and the Arctic regions provide the proper environment for atomic Cl to cause ozone depletion. In 1974, Dr. F. Sherwood Rowland of the University of California, Irvine, and Mario Molina now at Jet Propulsion Laboratory, California put forth the theory that chlorofluorocarbons (CFCs) may add chlorine radicals to the stratosphere.14 Their concern was that compounds of CFCs are chemically inert and could remain in the atmosphere for years eventually photolyzing to reactant Cl. CFCs are stable, nonflammable, nontoxic and noncorrosive. CFCs vaporize at low temperatures and therefore act as high efficient coolants for refrigerators and air conditioners. As insulators they are used in rigid and flexible plastic-foams. Their nonreactive properties make them simple to use as solvents for microchips and telecommunications equipment. Atmospheric lifetimes of some CFCs are as follows: Gas Atmospheric Lifetimes for CFCs and Halons Years CFC-11 aerosol propellent 59 (trichlorofluoromethane - CCl3F) CFC-12 refrigerant122 (dichlorodiflurormethane - CCl2F2) CFC (trichlorotrifluoroethane - CCl2FCClF2) CFC (dichlorotetrafluoroethane - C2F4Cl2) CFC (monochloropentafluoroethane - C2F5Cl2) Halon 1211 fire retardant 25 (bromochlorordifluoromethane - CF2ClBr) Halon (bromotrifluoromethane - CF3Br) HCFC-22 (CHF2Cl) 15 (monochlorodifluoromethane - CHF2Cl) As CFCs are released at ground level, they are rapidly distributed in the troposphere until a vertical transport slowly carries the chemicals into the stratosphere. Normally chemicals are removed from the environment via photodissociation, rainout, and oxidation. However, CFC-11 and most other CFCs are only removed by photodissociation in the mid-stratosphere by solar UV radiation whereby:15 CCl3F + h ( <230nm) Cl + CCl2F Page 2 / 7
3 CFCs diffuse when they reach altitudes of 25 to 40 km in the stratosphere (the greatest ozone region lying between 15 to 35 km). They yield atomic chlorine which later returns to the troposphere and is deposited as HCl. In 1989, during AASE I to analyze the chemical composition of the Arctic polar stratosphere, chlorine monoxide (ClO) levels were elevated to a factor of 50 in the Arctic stratosphere. Toon also reported that levels of ClO in the Arctic region were as high as 8 pptv which is capable of destroying about 1/2-1% of stratospheric ozone per day.16 The observation added up to a consistent picture with that of the Antarctic data in 1987,17 wherein the chemically perturbed region (CPR) of the Antarctic, ClO levels were found to be more than 100 times those expected from mid-latitude measurements. Concentrations where found to be as high as 1.3 ppbv18 and had ny 1991 increased to 3 ppbv19 as opposed to the total ppbv chlorine available in the atmosphere in The CPR areas of the vortex were defined at the abrupt rise in concentration of ClO. The high levels of chlorine monoxide (ClO), however, were not consistent throughout the year. The levels were very low in late winter when there was little sun which has led to the theory that there is a correspondence between the increase in sunlight, the growth of chlorine monoxide, and the decrease in ozone. Although chlorine continues to show increased levels in the stratosphere, observations show that ozone is decreasing in the 12 to 25km region of the Antarctic stratosphere, as well as the Arctic at a faster rate than expected for only chlorine-related processes.20 Bromide (Br) may also play the same role as reactive chlorine when exposed to ozone, and recent studies are revealing that they account for up to 25% of ozone loss from chemicals in the stratosphere.21 It is also now believed that the existence of OClO could be the result of a reaction that started with ClO + BrO.22 Recently methane has also been taken as a serious target in both the north and south hemispheres. Findings have shown the increase yearly of about ppmv.23 The incremental greenhouse effect of methane is about 20 times that for CO2. Flurocarbons (FCs) such as CF4 also undergo UV-driven photolysis. However, fluorine atoms have a high kinetic and thermodynamic affinity for hydrogen. Thus, most stratospheric fluorine is bound in inactive forms like HF before returning to the troposphere.24 There is some direct effect of loss of ozone due to meteorological influences where sunlight, wind and temperature appear to be the controlling factor. One event occurred over the Palmer Peninsula on September 5, 1986 where over a period of 24 hours total ozone decreased by about 10% over an area of approximately 3 million square miles.25 Such a rapid decrease is difficult to explain chemically. There is also a growing correlation between ozone loss and the aerosol clouds from volcanic eruptions as measured by the SAGE-II satellite. Where SO2 clouds such as those from Mount Pinatubo at 10o N in June 1991 and Mount Hudson (Chile) at 46o S in August 1991 added to the aerosol chemistry at the 15 to 22km region. After the eruptions total Antarctic ozone was 15-20% lower than observed in the previous five years.26 The depletion did not occur in the presence of extensive PSCs therefore it may be interpreted that 25% of the deficit may be caused by gas-phased chemistry in the Antarctic27 and Arctic.28 This could also mean that surfaces other than PSCs may be able to change the chemistry, whereby the global stratospheric aerosol layer enhanced by these eruptions, composed of H2SO4/H2O, could be a surface for ozone depleting chemistry. The conclusion is that chemical and meteorological data cannot be separated. Since 1986, most data appears to correlate with the appearance and formation of PSCs between 15 to 24 km with calculations taken from 1 µm SAM II.29 However, ozone depletion does occur in regions where PSCs are episodic and ClO levels have been found elevated outside the chemically perturbed regions (CPR) polar regions of the vortex.30 Ozone Depletion and Greenhouse Gases The "greenhouse effect" refers to an incremental increase in infrared absorption within the atmosphere because of the increase of various trace gases. Carbon dioxide has largely been targeted as our greatest problem. CO2 levels are currently 25% greater than in preindustrial times and are increasing exponentially. Atmospherical concentrations have increased from 315 ppmv in 1957 to 350 ppmv in 1988, which is approximately 1,500 ppbv yearly. This increase does account for half or more of the greenhouse effect. However, increases have also been noted due to CFCs with CFC 11 increasing yearly by ppbv and CFC 12 increasing ppbv. The important factor is that each molecule of CFC is 10,000 times more efficient in absorbing infrared radiation than that of CO2.31 Relative Infrared Global forcing per Warming molecule Potential* CO2 1 1 CFC-11 12,400 1,360 CFC-12 15,800 3,700 CFC ,800 1,900 *Calculated with Estimated Atmospheric Lifetimes and relative Infrared forcing per molecule where atmospheric lifetime for CO2 is 230 years. It is clear that CFCs do not have the same impact on global warming at CO2 gases. CFCs may account for as much as 15-20% of the global warming. Other scientists have argued that any global warming due to CFCs may be Page 3 / 7
4 canceled by the cooling from ozone depletion they induce. Regardless of the final analysis, CFCs and ozone depletion are part of the greenhouse effect dilemma since stratospheric climate is controlled by the balance between heating through absorption of UV by ozone and the cooling through CO2 through the emission of IR radiation. Increases in CO2 anticipated over the next 50 years are expected to produce a cooling in the lower stratosphere leading to the possible occurrence of PSCs including type II in the Northern Hemisphere. John Austin et al. at England's Meteorological Office created computer models using the Cray-YMP to show possible ozone destruction in the Northern Hemisphere if the level of CO2 increased from the current 330 ppmv to a future 660 ppmv. The doubling of CO2 could lead to an Arctic ozone hole comparable to that observed over Antarctica.32 Since CO2 is increasing in the atmosphere, the stratosphere will become cooler; methane, an important source of water in the stratosphere will also increase the probability of PSCs and lead to higher levels of ozone depletion.33 Findings have shown the increase yearly of about ppmv.34 The incremental greenhouse effect of methane is about 20 times that for CO2. In addition, aerosol particles are known to function as antigreenhouse materials, reflecting incoming visible solar radiation and decreasing surface temperatures. Sulfur aerosol gases in particular from volcanoes are known to cool the earth's surface. Therefore, reducing chlorine levels may not be sufficient without a serious reduction in CO2 levels. Changing the Ozone Molecule Even if we immediately stop the production of all CFCs, we will still suffer for the next 50 years from the chemical effects in our atmosphere. In both the North and South Hemispheres there is a clear decline of ozone even in midlatitude tracking stations. The chlorine molecule clearly plays a major role in ozone depletion and many scientists and world leaders agree that restrictions must be enforced. Major uses of CFCs in the United States and the world can be placed into five major categories: 24% as solvents in cleaning of electronics and dry-cleaning (CFC 113 and Halon 1301); 26% for rigid and flexible urethane foams (CFC 11 and CFC 12), phenolic foams (CFC 113), polyolefin foams and nonurethane forms (CFC 12 and CFC 114); 25% for air conditioners and refrigeration devices (CFC 11, CFC 12, CFC 113, 114, 115); 10% for fire extinguishers (Halon 1301); 15% is divided between aerosols, fluid technologies, and other uses.35 Two companies have been monitoring the sales and production of CFCs in the United States, the Chemical Manufacturers Association (CMA) receives annual information from about 85% of all companies who globally use CFCs and the Grant Thornton Company. Their reports of three of the major CFCs are as follows: Annual Major CFC production summary (in metric ktons) Year CFC 113 CFC 114CFC *Years: Reported CMA (1991) 36 Years: Grant Thornton (1992)37 This summary only accounts for three major CFCs out of well over 15 chlorine combinations and our yearly emissions of CFCs is more on the order of one million tons per year, which translates into an ozone loss 105 times larger. Continued destruction of ozone has global ramifications which would start with the disruption of the food chain of marine bacteria. Raymond Smith and Barbara Prezelin of the University of California, Santa Barbara recorded observations in the Bellingshausen Sea and measured the photosynthetic rate of marine phytoplankton at various UV conditions. Phytoplankton are microscopic free-floating plants which are the basis of the oceanic food chain. They manufacture organic materials that sustains most organisms in the marine ecosystem. Phytoplankton utilize shorter wavelengths of solar radiation for photosynthesis. Smith and Prezelin studies in 1991 showed that when compared to phytoplankton raised under ambient light, those exposed to the enhanced UV-B ( nm) reduced their production by 6-12% during the Antarctic springtime. And recorded that phytoplankton are currently being exposed to twice the amount of normal UV radiation in the Antarctic springtime under the influence of the ozone hole which can reach depths of 70 meters under water.38 It is already well established that in all life forms DNA can be damaged by UV, but organisms differ in the amount of damage they can received and their ability to repair the damage. The initial findings set forth by the EPA suggests that each 1% depletion of ozone leads to a 2-3% increase in skin cancer among fair-skinned people.39 There is already a large data base indicating Page 4 / 7
5 that UV-B is stressful to biological life even at current levels. Suppression of the immune system due to UV-B may also be a factor in the development of skin cancers.40 Atmospheric abundances of CFCs have also been reported by the World Meteorological Organization where abundances in the troposphere for CFC 113 in 1980 was 22.3 in 1990 they were recorded to be 78.2 pptv. CFC 114 in 1980 were reported to be 10.1 pptv and in 1991 they jumped to 14.7 pptv. CFC 115 in 1980 were 1.5 pptv and in 5.7 pptv.41 If, as Toohey, et al. predicts that elevated levels of ClO are found in the lower stratosphere and at mid-latitudes, ozone could be destroyed on the order of.5% per ozone for 100 pptv. ClO in an area where ozone replenishing would occur less rapidly.42 Recent EPA and NASA estimates suggest that the ozone layer has been depleted by 4-5% over the United States since 1978 increasing chances of skin cancer as stated in a report released to the Associated Press (April 5, 1991). As early as 1987, the Montreal Protocol Conference called for the elimination of CFC production by the end of the century with limits of 50% of production by After additional expeditions were sent to the North Pole in 1989, 81 nations met at Helsinki in May of 1989 and adopted a declaration calling for a complete phase-out of CFC's by the year 2000, and a ban on the use of halons beyond the Montreal Protocol.43 In July 1992, the United States initiated its own Clean Air Act Amendment calling for a updated schedule to accelerate the phaseout of CFCs by January 1, The 1990 Clean Air Act has already made venting HCFCs and CFCs illegal as of July 1, The EPA sent out warnings to encourage owners of HVAC and refrigeration equipment to begin preparing for the deadline to eliminate production of CFCs by After international meetings in London (1990) and Geneva (June, 1992) 80 countries have followed the United States with final amendments, revised in Copenhagen in November 1992, which will ban CFCs by January 1, Levels of consumption of CFCs have already been mandated placing limits of production as of January 1994 at 25% of the levels recorded in In addition, carbon tetrachloride will be prohibited by 1995, the use of methyl chloroform in dry cleaning by 1996 and the complete phaseout of halons by January 1, The Copenhagen provisions also provide a phaseout schedule for HCFCs beginning January 1, 1996 consumption will be limited in a progressive schedule until complete phaseout by It is hoped that this will meet the goal of reducing chlorine content in the atmosphere below 2 ppbv by the year 2075 with the atmospheric lifetimes for HCFCs from 2 to 20 years. Less developed nations (approx. 16% of world production) have fewer restrictions and are not currently making steps to stop production. These less developed nations such as China and India have yet to make commitments because of their own concerns about the cost of discarding equipment which uses CFCs. To overcome this dilemma, the Helsinki declaration called for provisions to assist developing countries through funding and transfer of technology. The current option chosen by most companies is to substitute CFCs for HFCs (hydrogluorocarbons) and HCFCs (hydrochloro-fluorocarbons) intended to replace major industrial applications. HCFCs do have some ozone depletion potential is considered 90% less harmful than CFCs. Some substitutes are as follows: CFC 11 and CFC 12 used in centrifugal chillers will be replaced with HCFC-123 and HFC- 134a respectively. Ford Motor's Atlanta, Georgia was one of the first assembly plants which begun in 1992 building Taurus cars with air conditioners that used HFC-134a instead of CFCs. According to the Congressional Research Service report, there are approximately 130 million auto air conditioners currently in use in the U.S., housing 200 million pounds of CFCs (CFC 12) having a capital cost of $20 billion and a CFC leak rate of 15-25% which will require million pounds to replenish CFC loss.45 Electronics companies that cleaned PC-boards with CFC- 113 have turned to aqueous cleaning systems which use de-ionized water and non-chlorine solvents. No-clean fluxes are also being used. IBM Rochester's plant used 902,800 pounds of CFCs in 1987, by 1989 it reduced its usage to 757,000 pounds and by 1991 it became CFC free implementing seven large aqueous cleaning systems in a three sift, 7-day week production schedule.46 Overall, in 1992 U.S. PC-board manufacturers were using 50% less CFCs than they did in Refrigeration devices are mainly industrial but include mobile air conditioners and retail food and domestic refrigerators (CFC 12) with domestic refrigeration counting for only about 1%. CFC-12 has been the most widely used chlorofluorocarbon primarily for refrigerant coolants. Several alternatives now used are: HFC 32, HFC 125 and HFC 143. However, mixtures are also possible such as a zeotrope mixture of HFC 32 and HFC 134a called KLEA 32. Chemists at Du Pont replaced their common coolant in refrigerators and air conditioners with a new compound called HCFC The air conditioner and refrigerant industry faces one of the most difficult challenges because most equipment has a useful life of years. There are about 70,000 commercial chillers which contain about 80 million pounds of CFCs (usually CFC 11). Their leakage rate is 15%-25% requiring 12 to 20 pounds of refrigerant make-up requirements annually. The EPA recently asked Du Pont to agree to continue manufacturing CFCs beyond 1994 Page 5 / 7
6 and until the federal ban December 31, 1995 in hopes of heading off a projected refrigerant shortage. Du Pont had announced it would cease production at the end of The ARI (Air Conditioning and Refrigeration Institute) estimates that there will be 67,000 unconverted large tonnage chillers in use in 1996 requiring 5-8 million pounds of CFCs. Another million pounds of CFCs will be needed to service commercial refrigeration equipment in Conclusions Concentrations of CFCs and halocarbons and such products as Halon 1301 and 1211 (fire retardants) both contribute to stratospheric ozone destruction. According to findings, each year has brought a worsening ozone picture, not only over the Southern which has areas of up to 95% depletion, but even in the Northern hemisphere and at mid-latitudes. Each year scientists seem to confirm their worst fears. Now in 1994 the Airborne Southern Hemisphere Experiment (ASHOE) has been underway. However, as monitoring continues over the next few decades we are also simultaneously cutting back on many of the chemical elements that are causing this depletion and scientists are optimistic that a recovery will come about by reducing future emissions of chlorine and Bromide species. References 1. Farman, J.C., et. al. "Large losses of total ozone in Antarctica reveal seasonal ClOx/Nox interaction, Nature, Vol , pp Caroille,D. et al. "Mountain Waves, Polar Stratospheric Clouds, and the Ozone Depletion Over Antarctica" J. of Geophysical Res. August 30, 1989, vol. 94. No. D9, pp 11,233-11, Komhyr, W.D., NOAA Tech Mem ERL ARL-149. NOAA/GMCC, Boulder, CO, and Komhyr, W.D. and Grass, R.D. "Dobson Spectrophotometer 83: A Standard for Total Ozone Measurements, " Journal of Geophysical Research, Vol 94, No. D7 July 20, l989, pp Tuck, A.F. et al. "The Planning and Execution of ER-2 and DC-8 Aircraft Flights Over Antarctica, August and September 1987" J. of Geophysical Res. Aug 30, 1989, vol 94, pp. 11, Stolarski, R. et al. "Measured Trends in Stratospheric Ozone" Science. Ap 17, 1992, vol Deshler, T. and Adriani, A. "Volcanic aerosol and ozone depletion within the antarctic polar vortex during the austral spring of 1991." Antarctic Jounral vol. 27, no. 5, 1992 pp Schoeberl, M.; Hartmann, D. "The Dynamics of the Stratospheric Polar Vortex and Its Relation to Springtime Ozone Depletions," Science Jan. 4, 1991, vol 251 pp Rowland, F. Sherwood et al. "Variations Since 1978 in Tropospheric Concentrations of Halocarbons" Symposium Proceedings of WMO Technical Conference on Observation and Measurement of Atmospheric Contaminants Vienna, October Gandrud, B.W. "Filter Measurement Results From the Airborne Antarctic Ozone Experiment" J. of Geophysical Res, Aug. 30, 1989, vol 94, p. 11, Rowland, F. S., "Chlorofluorocarbons and the Depletion of Stratospheric Ozone" American Scientist, Jan-Feb 1989, vol. 77, p Webster, C.R. et. al. "Chlorine Chemistry on Polar Stratospheric Cloud Particles in the Arctic Winter." Science. Aug 27, 1993, vol. 261, pp Zurer, P.S. "Arctic Ozone Loss: Fact-Finding Mission Concludes Outlook is Bleak," C&EN March 6, l989, p Miro, C. and Cox, J.E. "Montreal Protocol Revised in Copenhagen." ASHRAE Journal Feb p Molina, Mario J. and F.S. Rowland, "Stratospheric Sink for Chlorofluoro-methanes:" Nature June 18, 1974, vol. 249, pp Rowland, F.S. "The Stratospheric Photochemistry of Chlorine Compounds and Its Influence on the Ozone Layer" (Dept of Chemistry, Un. of California, Irvine, Toon, O.B. et al. "Condensation of HNO3 and HCl in the winter polar stratospheres," Geophysical Res. Letters, 1986, 13: Watson, R. Film documentation, NASA Headquarters chief on the Upper Atmospheric and Research and Tropospheric Chemistry, Washington DC, Proffitt, M.H. "A Chemical Definition of the Boundary of the Antarctic Ozone Hole" J. of Geophysical Res., Aug 30, l989, vol. 94, pp. 11, Chafee, J. "Stratospheric Ozone: The Problem." EPA Journal January/February Hofmann, D.J. et al. "Observation and possible causes of new ozone depletion in Antarctica in 1991" Nature Sept 1992, vol Cicerone, R. "Fires, Atmospheric Chemical, and the Ozone Layer." Science, 4 Mar 1994, vol 263,p Wahner,A.R. Jakoubek, et. al.,"remote Sensing Obervations of Nightime OClO Column During the Airborne Antarctic Ozone Experiment, September 8, 1987" J. of Geophysical Res., Aug 30, l989, vol. 94, pp. 11, Blake, D.; Rowland, F.S. "Continuing World-wide Increase in Tropospheric Methane," , Science, 1987, vol 239,pp Mocella, M. "Plasma Etching and the Ozone Issue: Historical Perspecive, Status, and Implications for the Future. Private paper Du Pont de Nemours and Co, Wilmington, DEL. 25. McKenna, D.S. et al. "Diagnostic Studies of the Page 6 / 7
7 Antarctic Vortex During the 1987 Airborne Antarctic Ozone Experiment: Ozone Miniholes" J. of Geophysical Res., Aug 30, l989, vol. 94, pp. 11, Hofmann, D.J. et al. "Observation and possible causes of new ozone depletion in Antarctica in 1991" Nature Sept 1992, vol Ibid. 28. Rodriguez, J. "Probing Straospheric Ozone." Science. Aug 27, 1993, vol. 261, pp Poole, L.R. and M. McCorwick, "Polar stratospheric clouds and the Antarctic Ozone Hole," J. of Geophys. Res. 1988, vol. 93, pp Kelly, K.K. et al., "Dehydration in the Lower Antarctic Stratosphere During Late Winter and Early Spring, 1987," J. of Geophysical Res. Aug. 30, 1989, vol. 94, pp. 11,317-11, Rowland, F. S., "Chlorofluorocarbons and the Depletion of Stratospheric Ozone" American Scientist, Jan-Feb 1989, vol. 77, p Austin, J., et al. "Possibility of an Arctic ozone hole in a doubled-co2 climate" Nature November 19, 1992 vol. 360, pp Schoeberl, M.; Hartmann, D. Science Jan. 4, 1991, vol 251 pp Blake, D.; Rowland, F.S. Science, 1987, vol 239,pp EPA Estimates for 1988 CFC Use in the United States. 36. Chemical Manufacturers Association, "Production, sales and calculated of CFC 113 CFC 114 CFC 115 for the years " CMA, 2501 M Street NW, Washington DC 20037, Grant Thornton, Chlorofluorocarbons (CFC's) 113, CFC 114 CFC 115 Annual production and sales for the years " Grant Thornton, 1850 M Street NW, Washington, DC Smith, R.C. et. al. "Ozone depletion: Ultraviolet radiation and phytoplankton biology in antarctic waters." Science. Ap 10, 1992, vol. 255, pp Zurer, P.S. C&EN March 6, l989, p Scott, E.L. "Epidemiology of skin cancer under increasing UV radiation" Proceedings 1983 Indo-US Workshop on Global Ozone Problem, National Phys. Lab, New Delhi, World Meteorological Organization, Geneve, Switzerland, Toohey, D.W. Geophysical Research Letters. January Maussan, Jaime, "El Piel de la Tierra" documentary, Televisa, 1989, Mexico, D.F. 44. Miro, C. and Cox, J.E. ASHRAE Journal Feb p Seventy-Seventh American Assembly (Final Report) on Preserving the Global Environment: The Challenge of Shared Leadership. Arden House, Harriman, NY, April and Benedick, Richard E., Ozone Diplomacy: New Directions in Safeguarding the Planet. Cambridge, Mass: Harvard Un. Press, Rochester IBM report Du Pont Position Statement, on CFCs-Ozone- Greenhouse Issues," Environ Conser 13:4, Randazzo, M. "DuPont Will Comply with EPA Request to Produce CFC's Through 1995," Energy User News February 1994 p. 8. Page 7 / 7
Stratospheric Chemistry HS 2017 Solution to Homework Problem Set 3
 Stratospheric Chemistry HS 2017 Solution to Homework Problem Set 3 For questions: andrea.stenke@env.ethz.ch (CHN P14) Problem 1: The Montreal Protocol and Climate (a) Chemical Formulas CFC-11 : CFCl 3
Stratospheric Chemistry HS 2017 Solution to Homework Problem Set 3 For questions: andrea.stenke@env.ethz.ch (CHN P14) Problem 1: The Montreal Protocol and Climate (a) Chemical Formulas CFC-11 : CFCl 3
4. Stratospheric ozone depletion
 112 4. Stratospheric ozone depletion The thickness of the ozone layer above Europe has decreased significantly since the beginning of the 198s, and is declining at a rate of 4 5 % per decade. The gradual
112 4. Stratospheric ozone depletion The thickness of the ozone layer above Europe has decreased significantly since the beginning of the 198s, and is declining at a rate of 4 5 % per decade. The gradual
Lecture 14 Stratospheric Ozone Loss ATOC/CHEM 5151
 Lecture 14 Stratospheric Ozone Loss ATOC/CHEM 5151 1 Setting the Stage: An Historical Perspective To begin, we need to go back to the 1970s Concern about the environment is high and people are starting
Lecture 14 Stratospheric Ozone Loss ATOC/CHEM 5151 1 Setting the Stage: An Historical Perspective To begin, we need to go back to the 1970s Concern about the environment is high and people are starting
TROPICS: insolation high year round, high sun angle and ~ constant duration
 GE 101, February 6, 14 Finish insolation variation Global environmental issues associated with insolation TRPICS: insolation high year round, high sun angle and ~ constant duration MID-LATITUDES: insolation
GE 101, February 6, 14 Finish insolation variation Global environmental issues associated with insolation TRPICS: insolation high year round, high sun angle and ~ constant duration MID-LATITUDES: insolation
September 16 th 2010 International Day for the Preservation of the Ozone Layer
 September 16 th 2010 International Day for the Preservation of the Ozone Layer Next 16 th of September it will be celebrated the 2010 edition of the International Day for the Preservation of the Ozone
September 16 th 2010 International Day for the Preservation of the Ozone Layer Next 16 th of September it will be celebrated the 2010 edition of the International Day for the Preservation of the Ozone
Lecture 29 Air Pollution. Air Pollution. Clean Boundary Layer. Clean Boundary Layer
 Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
RECENT MAJOR FINDINGS AND CURRENT SCIENTIFIC UNDERSTANDING
 The provisions of the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer include the requirement that the Parties to the Protocol base their future decisions on the current scientific, environmental,
The provisions of the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer include the requirement that the Parties to the Protocol base their future decisions on the current scientific, environmental,
History of significant air pollution events
 Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica?
 Ozone Depletion and Climate Change Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica? In a recent (last August 2016) BBC documentary on the Antarctic weather changes, it has
Ozone Depletion and Climate Change Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica? In a recent (last August 2016) BBC documentary on the Antarctic weather changes, it has
Chapter 11: Atmosphere
 To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
Module 3, Investigation 2: Briefing The loss of stratospheric ozone: Where are lives at risk?
 Background Have you heard of the ozone hole? In the 1970s, scientists began noticing a hole developing in the stratospheric ozone layer high above Antarctica. Scientists attributed the ozone destruction
Background Have you heard of the ozone hole? In the 1970s, scientists began noticing a hole developing in the stratospheric ozone layer high above Antarctica. Scientists attributed the ozone destruction
HUMAN IMPACT on the BIOSPHERE part 4
 HUMAN IMPACT on the BIOSPHERE part 4 Charting a course for the Future http://www.claybennett.com/pages2/mistletoe.html ENVIRONMENTAL PROBLEMS DEAD ZONES OZONE DEPLETION ACID RAIN GLOBAL WARMING WASTE http://www.acmecompany.com/stock_thumbnails/13808.greenhouse_effect_2.jpg
HUMAN IMPACT on the BIOSPHERE part 4 Charting a course for the Future http://www.claybennett.com/pages2/mistletoe.html ENVIRONMENTAL PROBLEMS DEAD ZONES OZONE DEPLETION ACID RAIN GLOBAL WARMING WASTE http://www.acmecompany.com/stock_thumbnails/13808.greenhouse_effect_2.jpg
Threats to Our Atmosphere
 Threats to Our Atmosphere A Reading A Z Level W Leveled Reader Word Count: 1,831 LEVELED READER W Written by Shaun Taylor Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
Threats to Our Atmosphere A Reading A Z Level W Leveled Reader Word Count: 1,831 LEVELED READER W Written by Shaun Taylor Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32)
 Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Section 4 The Air We Breathe
 Section 4 The Air We Breathe Key Concept Air is an important natural resource that is affected by human activities. What You Will Learn Air pollution is caused by human activities, such as burning fossil
Section 4 The Air We Breathe Key Concept Air is an important natural resource that is affected by human activities. What You Will Learn Air pollution is caused by human activities, such as burning fossil
ACID RAIN. CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007
 ACID RAIN CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007 More accurate term may be acid deposition Occurs in two forms wet deposition (acidic rain, fog, and snow) dry deposition
ACID RAIN CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007 More accurate term may be acid deposition Occurs in two forms wet deposition (acidic rain, fog, and snow) dry deposition
Name: Class: Date: 6. Most air pollution is produced by a. thermal inversions. c. ozone layer depletion. b. fuel burning. d. volcanic eruptions.
 Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
Environmental Impacts of. Energy Production
 CH2356 Energy Engineering Environmental Impacts of Energy Production Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
CH2356 Energy Engineering Environmental Impacts of Energy Production Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
1 Characteristics of the Atmosphere
 CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
Announcements. Pollution week continues. Thinking about pollution. Why are polar bears so contaminated?
 Announcements Grades for exam 2 have been posted March 7 th - Last day to submit LEAD summary to TA, extra credit videos due next Tuesday (no late videos will be accepted) Next Thursday, Environmental
Announcements Grades for exam 2 have been posted March 7 th - Last day to submit LEAD summary to TA, extra credit videos due next Tuesday (no late videos will be accepted) Next Thursday, Environmental
Directed Reading. Section: Global Change. than in the rest of the United States. b. In the United States and Canada, many lakes are dying as their ph
 Section: Global Change In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Scientists have discovered that acid rain is caused
Section: Global Change In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Scientists have discovered that acid rain is caused
What Exactly is a Greenhouse Gas?
 1 What Exactly is a Greenhouse Gas? You may have stood in a greenhouse and felt the heat, but what do greenhouse gases have to do with greenhouses? A greenhouse gas is any gas that absorbs and re-emits
1 What Exactly is a Greenhouse Gas? You may have stood in a greenhouse and felt the heat, but what do greenhouse gases have to do with greenhouses? A greenhouse gas is any gas that absorbs and re-emits
Climate Change and Ozone Loss
 Climate Change and Ozone Loss During the past 900,000 years, the earth has undergone a series of cold glacial periods followed by warmer interglacial periods. The past 10,000 years has been an interglacial
Climate Change and Ozone Loss During the past 900,000 years, the earth has undergone a series of cold glacial periods followed by warmer interglacial periods. The past 10,000 years has been an interglacial
Ch Global Warming and Ozone Loss
 Ch. 19 - Global Warming and Ozone Loss The Greenhouse Effect and Global Warming About the Greenhouse Greenhouse effect - certain gases in the atmosphere trap heat in the lower atmosphere (troposphere).
Ch. 19 - Global Warming and Ozone Loss The Greenhouse Effect and Global Warming About the Greenhouse Greenhouse effect - certain gases in the atmosphere trap heat in the lower atmosphere (troposphere).
Air Pollution. Asian Brown Cloud. Developed Countries have reduced emissions recently
 Study Questions 1. Compare and contrast primary vs. secondary pollutants, giving examples of each. 2. Compare and contrast indoor vs. outdoor pollution, listing specific examples and sources of each. 3.
Study Questions 1. Compare and contrast primary vs. secondary pollutants, giving examples of each. 2. Compare and contrast indoor vs. outdoor pollution, listing specific examples and sources of each. 3.
Climate Change and Ozone Depletion Notes. Chapter 20
 Climate Change and Ozone Depletion Notes Chapter 20 PAST CLIMATE AND THE GREENHOUSE EFFECT Over the past 900,000 years, the troposphere has experienced prolonged periods of global cooling and global warming.
Climate Change and Ozone Depletion Notes Chapter 20 PAST CLIMATE AND THE GREENHOUSE EFFECT Over the past 900,000 years, the troposphere has experienced prolonged periods of global cooling and global warming.
Peace Independence Democracy Unity Prosperity. Decree on The Control of the Import, Export and Use of Ozone Depleting Substances
 The Prime Minister s LAO PEOPLE S DEMOCRATIC REPUBLIC Peace Independence Democracy Unity Prosperity ----------===000===---------- Office No.162/PM Vientiane, Date 13 October 2003 Decree on The Control
The Prime Minister s LAO PEOPLE S DEMOCRATIC REPUBLIC Peace Independence Democracy Unity Prosperity ----------===000===---------- Office No.162/PM Vientiane, Date 13 October 2003 Decree on The Control
SCIAMACHY book. Ozone Recovery? Michel Van Roozendael, BIRA- IASB. ATC14, October, Jülich, Germany
 SCIAMACHY book Ozone Recovery? Michel Van Roozendael, BIRA- IASB ATC14, 27-31 October, Jülich, Germany 1928: start of CFC production 1971: 1 st observation of CFC in the atmosphere (J. Lovelock) 1974:
SCIAMACHY book Ozone Recovery? Michel Van Roozendael, BIRA- IASB ATC14, 27-31 October, Jülich, Germany 1928: start of CFC production 1971: 1 st observation of CFC in the atmosphere (J. Lovelock) 1974:
The Antarctic Ozone Hole: A Unique Example of the Science and Policy Interface
 The Antarctic Ozone Hole: A Unique Example of the Science and Policy Interface Susan Solomon and Marie- Lise Chanin ABSTRACT. The discovery of an unexpected large depletion of the Antarctic ozone layer
The Antarctic Ozone Hole: A Unique Example of the Science and Policy Interface Susan Solomon and Marie- Lise Chanin ABSTRACT. The discovery of an unexpected large depletion of the Antarctic ozone layer
ATM S 211 Final Examination June 4, 2007
 ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
Ozone Layer Depletion and Its Effects: A Review
 Ozone Layer Depletion and Its Effects: A Review Sivasakthivel.T and K.K.Siva Kumar Reddy Abstract - There are many situations where human activities have significant effects on the environment. Ozone layer
Ozone Layer Depletion and Its Effects: A Review Sivasakthivel.T and K.K.Siva Kumar Reddy Abstract - There are many situations where human activities have significant effects on the environment. Ozone layer
Chapter 19: Global Change
 1 Summary Of the Case Study Polar Bear population in the Antarctic going down because temperatures are going up and melting the caps. Polar bears are losing their habitat, they also can t get their food
1 Summary Of the Case Study Polar Bear population in the Antarctic going down because temperatures are going up and melting the caps. Polar bears are losing their habitat, they also can t get their food
Energy, Greenhouse Gases and the Carbon Cycle
 Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
The Chemistry of Climate Change. Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy
 The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
Introduction. Introduction. Introduction. Outline Last IPCC report : 2001 Last IPCC report :
 Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
greenhouse effect 1 of 5
 This website would like to remind you: Your browser (Apple Safari 4) is out of date. Update your browser for more security, comfort and the best experience on this site. Encyclopedic Entry greenhouse effect
This website would like to remind you: Your browser (Apple Safari 4) is out of date. Update your browser for more security, comfort and the best experience on this site. Encyclopedic Entry greenhouse effect
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore
 Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore GREENHO U SE EFFECT For the complete encyclopedic entry with media
Your web browser (Safari 7) is out of date. For more security, comfort and the best experience on this site: Update your browser Ignore GREENHO U SE EFFECT For the complete encyclopedic entry with media
Air Transportation: Emissions and Effects
 Air Transportation: Emissions and Effects Joyce E. Penner University of Michigan Report Co-ordinator: IPCC Special Report on Aviation and the Global Atmosphere Presentation to the First Regional Symposium
Air Transportation: Emissions and Effects Joyce E. Penner University of Michigan Report Co-ordinator: IPCC Special Report on Aviation and the Global Atmosphere Presentation to the First Regional Symposium
ENVIS- IITM NEWSLETTER The Air Quality: A Global Challenge
 ENVIS- IITM NEWSLETTER The Air Quality: A Global Challenge GLOBAL WARMING Editorial Prof. B.N. Goswami (Director, IITM, Pune) Dr. G. Beig (ENVIS Co-ordinetor) Ms. Neha S. Parkhi (Program Officer) Mr. Rajnikant
ENVIS- IITM NEWSLETTER The Air Quality: A Global Challenge GLOBAL WARMING Editorial Prof. B.N. Goswami (Director, IITM, Pune) Dr. G. Beig (ENVIS Co-ordinetor) Ms. Neha S. Parkhi (Program Officer) Mr. Rajnikant
Air Pollution. GEOL 1350: Introduction To Meteorology
 Air Pollution GEOL 1350: Introduction To Meteorology 1 Overview Types and Sources of Air Pollutants Factors That Affect Air Pollution Air Pollution and the Urban Environment 2 Air pollutants are airborne
Air Pollution GEOL 1350: Introduction To Meteorology 1 Overview Types and Sources of Air Pollutants Factors That Affect Air Pollution Air Pollution and the Urban Environment 2 Air pollutants are airborne
Global Warming Science Solar Radiation
 SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
GLOBAL CLIMATE CHANGE
 1 GLOBAL CLIMATE CHANGE From About Transportation and Climate Change (Source; Volpe center for Climate Change and Environmental forecasting, http://climate.volpe.dot.gov/trans.html Greenhouse effect has
1 GLOBAL CLIMATE CHANGE From About Transportation and Climate Change (Source; Volpe center for Climate Change and Environmental forecasting, http://climate.volpe.dot.gov/trans.html Greenhouse effect has
The atmosphere. The atmosphere is layered. Inversions affect air quality 3/2/2015. The sun influences weather and climate
 The atmosphere Chapter 13 Atmosphere Absorbs radiation and moderates climate Transports and recycles water and nutrients Human activity is now changing the amount of some gases CO 2, methane (CH 4 ), ozone
The atmosphere Chapter 13 Atmosphere Absorbs radiation and moderates climate Transports and recycles water and nutrients Human activity is now changing the amount of some gases CO 2, methane (CH 4 ), ozone
Grade 10 Academic Science Climate Change Unit Test
 Grade 10 Academic Science Climate Change Unit Test Part A - Multiple Choice: Circle the most correct answer. 1. What is the difference between weather and climate? a. Weather deals with wind and precipitation;
Grade 10 Academic Science Climate Change Unit Test Part A - Multiple Choice: Circle the most correct answer. 1. What is the difference between weather and climate? a. Weather deals with wind and precipitation;
Criteria Pollutants. Sulfur Dioxide (SO 2 ) Nitrogen Oxides (NOx)
 1) Sulfur dioxide 2) Nitrogen oxides 3) Carbon monoxide 4) Ozone 5) Particulates 6) Lead Criteria Pollutants Sulfur Dioxide (SO 2 ) SO 2 is a colorless gas that is formed from the combustion of sulfur-containing
1) Sulfur dioxide 2) Nitrogen oxides 3) Carbon monoxide 4) Ozone 5) Particulates 6) Lead Criteria Pollutants Sulfur Dioxide (SO 2 ) SO 2 is a colorless gas that is formed from the combustion of sulfur-containing
Composition and Energy AOSC 200 Tim Canty
 Composition and Energy AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Atmospheric composition cont. Energy transfer Lecture 03 Sept 5 2017 1 Today s Weather
Composition and Energy AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Atmospheric composition cont. Energy transfer Lecture 03 Sept 5 2017 1 Today s Weather
Unit 3 Lesson 1 Earth s Support of Life. Copyright Houghton Mifflin Harcourt Publishing Company
 Living It Up What do living things need to survive? Earth is covered in living things. The basic necessities of life are air, water, a source of energy, and a habitat to live in. How do Earth and the sun
Living It Up What do living things need to survive? Earth is covered in living things. The basic necessities of life are air, water, a source of energy, and a habitat to live in. How do Earth and the sun
Leif Backman HENVI Seminar February 19, 2009
 Methane Sources and Sinks Leif Backman HENVI Seminar February 19, 2009 Background Atmospheric methane Sources & Sinks Concentration variations & trends Objective & methods Objective & Goals Research plan
Methane Sources and Sinks Leif Backman HENVI Seminar February 19, 2009 Background Atmospheric methane Sources & Sinks Concentration variations & trends Objective & methods Objective & Goals Research plan
Casterlin Environmental Systems pg. 1
 s of the Earth's Atmosphere The atmosphere is divided into five layers. It is thickest near the surface and thins out with height until it eventually merges with space. 1. The troposphere is the first
s of the Earth's Atmosphere The atmosphere is divided into five layers. It is thickest near the surface and thins out with height until it eventually merges with space. 1. The troposphere is the first
World avoided simulations with the Whole Atmosphere Community Climate Model
 JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 117,, doi:10.1029/2012jd018430, 2012 World avoided simulations with the Whole Atmosphere Community Climate Model Rolando R. Garcia, 1 Douglas E. Kinnison, 1 and Daniel
JOURNAL OF GEOPHYSICAL RESEARCH, VOL. 117,, doi:10.1029/2012jd018430, 2012 World avoided simulations with the Whole Atmosphere Community Climate Model Rolando R. Garcia, 1 Douglas E. Kinnison, 1 and Daniel
General Article. Abstract. Keywords: Pollutants, stratosphere, CFCS, colorimeter, skin cancer. Introduction Ozone, the O 3
 General Article Ozone:A Pollutant... Ozone: A Pollutant and a Protector Gas Thaneshwar Subedi Tribhuvan University Department of Chemistry P.N. Campus, Pokhara E-mail: thaneshworsubedi@gmail.com Abstract
General Article Ozone:A Pollutant... Ozone: A Pollutant and a Protector Gas Thaneshwar Subedi Tribhuvan University Department of Chemistry P.N. Campus, Pokhara E-mail: thaneshworsubedi@gmail.com Abstract
Climate Change. Some solar radiation is reflected by Earth and the atmosphere. Earth s Surface
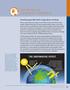 Q& A n The Basics of Greenhouse gases affect Earth s energy balance and climate The Sun serves as the primary energy source for Earth s climate. Some of the incoming sunlight is reflected directly back
Q& A n The Basics of Greenhouse gases affect Earth s energy balance and climate The Sun serves as the primary energy source for Earth s climate. Some of the incoming sunlight is reflected directly back
CEE 3510 Environmental Quality Engineering
 CEE 3510 Environmental Quality Engineering What Is Global Warming? An increasing trend in global temperature over the past century Presumably because of an increase of greenhouse gases in the atmosphere
CEE 3510 Environmental Quality Engineering What Is Global Warming? An increasing trend in global temperature over the past century Presumably because of an increase of greenhouse gases in the atmosphere
What is the carbon cycle?
 What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,454 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,454 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
Global Warming Potentials in AR4. V. Ramaswamy. NOAA/ Geophysical Fluid Dynamics Laboratory, Princeton University
 Global Warming Potentials in AR4 V. Ramaswamy NOAA/ Geophysical Fluid Dynamics Laboratory, Princeton University GWP Definition Defined as the ratio of the time-integrated radiative forcing from the instantaneous
Global Warming Potentials in AR4 V. Ramaswamy NOAA/ Geophysical Fluid Dynamics Laboratory, Princeton University GWP Definition Defined as the ratio of the time-integrated radiative forcing from the instantaneous
Planetary Energy Balance
 Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
ECVS IN THE STRATOSPHERE
 METEOROLOGY ECVS IN THE STRATOSPHERE O 3, H 2 O, AEROSOL, N 2 O, CH 4, CFCS, HCFCS, HFCS, SF 6, PFCS, AND TEMPERATURE Michaela I. Hegglin, University of Reading UK GCOS COMPOSITION ECVs EXTEND INTO THE
METEOROLOGY ECVS IN THE STRATOSPHERE O 3, H 2 O, AEROSOL, N 2 O, CH 4, CFCS, HCFCS, HFCS, SF 6, PFCS, AND TEMPERATURE Michaela I. Hegglin, University of Reading UK GCOS COMPOSITION ECVs EXTEND INTO THE
20 Global Climate Change
 20 Global Climate Change Overview of Chapter 20 Introduction to Climate Change Causes of Global Climate Change Effects of Climate Change Melting Ice and Rising Sea Level Changes in Precipitation Patterns
20 Global Climate Change Overview of Chapter 20 Introduction to Climate Change Causes of Global Climate Change Effects of Climate Change Melting Ice and Rising Sea Level Changes in Precipitation Patterns
Overview of Climate Science
 1 Overview of Climate Science This overview of climate science is written to support the development of a K- 14 climate education plan for the Pacific Islands Climate Education Partnership (PCEP). It aims
1 Overview of Climate Science This overview of climate science is written to support the development of a K- 14 climate education plan for the Pacific Islands Climate Education Partnership (PCEP). It aims
Georgia IS HUMAN ACTIVITY A SUBSTANTIAL CAUSE OF GLOBAL CLIMATE CHANGE? ARGUMENTATIVE Task: Copyright 2014 by Write Score, LLC
 Georgia ARGUMENTATIVE Task: IS HUMAN ACTIVITY A SUBSTANTIAL CAUSE OF GLOBAL CLIMATE CHANGE? Copyright 2014 by, LLC Humans and Global Climate Change The yearly global temperature has been above normal
Georgia ARGUMENTATIVE Task: IS HUMAN ACTIVITY A SUBSTANTIAL CAUSE OF GLOBAL CLIMATE CHANGE? Copyright 2014 by, LLC Humans and Global Climate Change The yearly global temperature has been above normal
Carbon Dioxide and Global Warming Case Study
 Carbon Dioxide and Global Warming Case Study Key Concepts: Greenhouse Gas Carbon dioxide El Niño Global warming Greenhouse effect Greenhouse gas La Niña Land use Methane Nitrous oxide Radiative forcing
Carbon Dioxide and Global Warming Case Study Key Concepts: Greenhouse Gas Carbon dioxide El Niño Global warming Greenhouse effect Greenhouse gas La Niña Land use Methane Nitrous oxide Radiative forcing
In 2002, a group of university researchers joined together under the title of the Canadian Network for the Detection of Atmospheric Change (CANDAC)
 1 In 2002, a group of university researchers joined together under the title of the Canadian Network for the Detection of Atmospheric Change (CANDAC) with the objective of improving the state of observational
1 In 2002, a group of university researchers joined together under the title of the Canadian Network for the Detection of Atmospheric Change (CANDAC) with the objective of improving the state of observational
The Earth s Global Energy Balance
 The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
Refrigeration Cycle. Definitions , , 11-46, 11-49,
 Refrigeration Cycle Reading Problems - -7, -9 -, -46, -49, -03 Definitions the st law of thermodynamics tells us that heat flow occurs from a hot source to a cooler sink, therefore, energy in the form
Refrigeration Cycle Reading Problems - -7, -9 -, -46, -49, -03 Definitions the st law of thermodynamics tells us that heat flow occurs from a hot source to a cooler sink, therefore, energy in the form
Ozone and trace gases in India: Effects of transport and emissions. Shyam Lal Physical Research Laboratory, Ahmedabad
 Ozone and trace gases in India: Effects of transport and emissions Shyam Lal Physical Research Laboratory, Ahmedabad Second Workshop on Atmospheric Composition and the Asian Monsoon (ACAM), 8-10 June,
Ozone and trace gases in India: Effects of transport and emissions Shyam Lal Physical Research Laboratory, Ahmedabad Second Workshop on Atmospheric Composition and the Asian Monsoon (ACAM), 8-10 June,
Available online at Energy Procedia 18 (2012 )
 Available online at www.sciencedirect.com Energy Procedia 18 (2012 ) 807 816 Refrigerants and their environmental impact Substitution of hydro chlorofluorocarbon HCFC and HFC hydro fluorocarbon. Search
Available online at www.sciencedirect.com Energy Procedia 18 (2012 ) 807 816 Refrigerants and their environmental impact Substitution of hydro chlorofluorocarbon HCFC and HFC hydro fluorocarbon. Search
Take away concepts. Global Ozone Policy. Global Policy to Protect Stratospheric Ozone
 Global Policy to Protect Stratospheric Ozone Montreal Protocol on Substances That Deplete the Ozone Layer 20th Anniversary in 2007 Take away concepts Montreal Protocol in a nutshell: 1. Science shaped
Global Policy to Protect Stratospheric Ozone Montreal Protocol on Substances That Deplete the Ozone Layer 20th Anniversary in 2007 Take away concepts Montreal Protocol in a nutshell: 1. Science shaped
What is the carbon cycle?
 What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,160 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
What is the carbon cycle? By NASA Earth Observatory, adapted by Newsela staff on 03.29.17 Word Count 1,160 Carbon is both the foundation of all life on Earth and the source of the majority of energy consumed
Climate Change Frequently Asked Questions Scrambled Information Source: EPA Climate Change FAQ
 Climate Change Frequently Asked Questions Scrambled Information Source: EPA Climate Change FAQ Instructions: The questions and answers below have been scrambled. Cut the answers and questions apart. Separate
Climate Change Frequently Asked Questions Scrambled Information Source: EPA Climate Change FAQ Instructions: The questions and answers below have been scrambled. Cut the answers and questions apart. Separate
Atmosphere Web quest
 Atmosphere Web quest 1. What are the four main layers of the atmosphere? Troposphere Stratosphere Mesosphere Thermosphere Ionosphere Exsosphere 2. Which layer is closest to space? Exosphere (upper layer
Atmosphere Web quest 1. What are the four main layers of the atmosphere? Troposphere Stratosphere Mesosphere Thermosphere Ionosphere Exsosphere 2. Which layer is closest to space? Exosphere (upper layer
Measuring Ocean Color: The Basics
 Measuring Ocean Color: The Basics Radiation of energy from the Sun and the Earth s surface. Recall from previous lectures that the Sun (6000 K), radiates energy in three portions of the energy spectrum:
Measuring Ocean Color: The Basics Radiation of energy from the Sun and the Earth s surface. Recall from previous lectures that the Sun (6000 K), radiates energy in three portions of the energy spectrum:
K39: The Key Evidence That Current Global Warming is Human-Caused
 K39: The Key Evidence That Current Global Warming is Human-Caused Here are 13 lines of Evidence GW = AGW (Global Warming = Anthropogenic Global Warming) Evidence #1: A Warming Troposphere with a Cooling
K39: The Key Evidence That Current Global Warming is Human-Caused Here are 13 lines of Evidence GW = AGW (Global Warming = Anthropogenic Global Warming) Evidence #1: A Warming Troposphere with a Cooling
Earth Science Lesson Plan Quarter 2, Week 1, Day 1
 Earth Science Lesson Plan Quarter 2, Week 1, Day 1 1 Outcomes for Today Standard Focus: Earth Sciences 4.c Students know the different atmospheric gases that absorb the Earth s thermal radiation and the
Earth Science Lesson Plan Quarter 2, Week 1, Day 1 1 Outcomes for Today Standard Focus: Earth Sciences 4.c Students know the different atmospheric gases that absorb the Earth s thermal radiation and the
Basics of greenhouse gases and climate change
 Basics of greenhouse gases and climate change Facts and theories We need to distinguish between what we know (facts), and what we think will happen (theories). In the subject of greenhouse gases and global
Basics of greenhouse gases and climate change Facts and theories We need to distinguish between what we know (facts), and what we think will happen (theories). In the subject of greenhouse gases and global
Chapter 2 9/15/2015. Chapter 2. Penny Boat. 2.1 The Role of Water in Cycles of Matter
 Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
Earth and Space Science (Earth's Atmosphere) Grade 7 Science Grade 7 Science Start Date: December 02, 2013 End Date : December 20, 2013
 Unit Overview Atmospheric properties Content Elaborations The atmosphere has different properties at different elevations and contains a mixture of gases that cycle through the lithosphere, biosphere,
Unit Overview Atmospheric properties Content Elaborations The atmosphere has different properties at different elevations and contains a mixture of gases that cycle through the lithosphere, biosphere,
Dr David Karoly School of Meteorology
 Global warming: Is it real? Does it matter for a chemical engineer? Dr David Karoly School of Meteorology Email: dkaroly@ou.edu Recent global warming quotes Senator James Inhofe (R, Oklahoma), Chair, Senate
Global warming: Is it real? Does it matter for a chemical engineer? Dr David Karoly School of Meteorology Email: dkaroly@ou.edu Recent global warming quotes Senator James Inhofe (R, Oklahoma), Chair, Senate
Earth as a System. Chapter 2. Table of Contents. Section 1 Earth: A Unique Planet. Section 2 Energy in the Earth System.
 Earth as a System Table of Contents Section 1 Earth: A Unique Planet Section 2 Energy in the Earth System Section 3 Ecology Section 1 Earth: A Unique Planet Objectives Describe the size and shape of Earth.
Earth as a System Table of Contents Section 1 Earth: A Unique Planet Section 2 Energy in the Earth System Section 3 Ecology Section 1 Earth: A Unique Planet Objectives Describe the size and shape of Earth.
Is the greenhouse effect good or bad?
 NAME 1. The diagram below represents energy being absorbed and reradiated by the Earth. Is the greenhouse effect good or bad? 5. Equal areas of which surface would most likely absorb the most insolation?
NAME 1. The diagram below represents energy being absorbed and reradiated by the Earth. Is the greenhouse effect good or bad? 5. Equal areas of which surface would most likely absorb the most insolation?
National Revision- Global Issues- Climate Change
 National Revision- Global Issues- Climate Change Our planet is encased in a blanket of gases, held in place by the force of gravity. This mixture gives us our life and makes our planet unique and distinctive.
National Revision- Global Issues- Climate Change Our planet is encased in a blanket of gases, held in place by the force of gravity. This mixture gives us our life and makes our planet unique and distinctive.
Convection Conduction
 L 18 Thermodynamics [3] Review Heat transfer processes convection conduction Greenhouse effect Climate change Ozone layer Review The temperature of a system is a measure of the average kinetic energy of
L 18 Thermodynamics [3] Review Heat transfer processes convection conduction Greenhouse effect Climate change Ozone layer Review The temperature of a system is a measure of the average kinetic energy of
THE SKY IS THE LIMIT: Strategies for Protecting the Ozone Layer
 THE SKY IS THE LIMIT: Strategies for Protecting the Ozone Layer Al.mS. Milli-r Irving \ l. Miiil/cr Research Report #3 \ ( )\l W l i l K VV ( ) R L f ) R E S O U R C E S IN STITUT E THE SKY IS THE LIMIT:
THE SKY IS THE LIMIT: Strategies for Protecting the Ozone Layer Al.mS. Milli-r Irving \ l. Miiil/cr Research Report #3 \ ( )\l W l i l K VV ( ) R L f ) R E S O U R C E S IN STITUT E THE SKY IS THE LIMIT:
Lecture 22: Atmospheric Chemistry and Climate
 Lecture 22: Atmospheric Chemistry and Climate Required Reading: FP Chapter 14 (only sections that I cover) Suggested Introductory Reading: Jacob Chapter 7 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring
Lecture 22: Atmospheric Chemistry and Climate Required Reading: FP Chapter 14 (only sections that I cover) Suggested Introductory Reading: Jacob Chapter 7 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring
Laws and Regulation for Fluorocarbons in Japan
 WS7-Th-2 Current global status of transition to lower GWP alternatives by laws and regulations Laws and Regulation for Fluorocarbons in Japan Atsuhiro MENO Ministry of Economy, Trade and Industry, Japan
WS7-Th-2 Current global status of transition to lower GWP alternatives by laws and regulations Laws and Regulation for Fluorocarbons in Japan Atsuhiro MENO Ministry of Economy, Trade and Industry, Japan
The ozone hole leaves a lasting impression on southern climate
 University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2014 The ozone hole leaves a lasting impression on southern climate Sharon
University of Wollongong Research Online Faculty of Science, Medicine and Health - Papers Faculty of Science, Medicine and Health 2014 The ozone hole leaves a lasting impression on southern climate Sharon
CLIMATE CHANGE AND ACID RAIN. Mr. Banks 7 th Grade Science
 CLIMATE CHANGE AND ACID RAIN Mr. Banks 7 th Grade Science COMPOSITION OF AIR? COMPOSITION OF AIR? 78% Nitrogen 21% Oxygen 0.93% Argon and other noble gases 0.04% carbon dioxide Variable amounts of water
CLIMATE CHANGE AND ACID RAIN Mr. Banks 7 th Grade Science COMPOSITION OF AIR? COMPOSITION OF AIR? 78% Nitrogen 21% Oxygen 0.93% Argon and other noble gases 0.04% carbon dioxide Variable amounts of water
Convection. L 18 Thermodynamics [3] Conduction. heat conduction. radiation
![Convection. L 18 Thermodynamics [3] Conduction. heat conduction. radiation Convection. L 18 Thermodynamics [3] Conduction. heat conduction. radiation](/thumbs/72/66535645.jpg) L 18 Thermodynamics [3] Heat transfer processes convection conduction Thermodynamics of the atmosphere Greenhouse effect and climate change Effect of the ozone layer Convection heat is transferred from
L 18 Thermodynamics [3] Heat transfer processes convection conduction Thermodynamics of the atmosphere Greenhouse effect and climate change Effect of the ozone layer Convection heat is transferred from
Global Warming and Climate Change
 Global Warming and Climate Change Weather vs. Climate Weather refers to short term conditions (e.g. 24 hrs.) in meteorological conditions such as temperature, pressure and rainfall Climate is average weather
Global Warming and Climate Change Weather vs. Climate Weather refers to short term conditions (e.g. 24 hrs.) in meteorological conditions such as temperature, pressure and rainfall Climate is average weather
Chapter 13. Atmospheric Science, Air Quality, and Pollution Control. Lecture Presentations prepared by Reggie Cobb Nash Community College
 Chapter 13 Atmospheric Science, Air Quality, and Pollution Control Lecture Presentations prepared by Reggie Cobb Nash Community College This lecture will help you understand: Earth s atmosphere Weather,
Chapter 13 Atmospheric Science, Air Quality, and Pollution Control Lecture Presentations prepared by Reggie Cobb Nash Community College This lecture will help you understand: Earth s atmosphere Weather,
Teaching Time: 1 hour and 15 minutes
 Lesson Summary Students will discuss human output of greenhouse gasses and then calculate the amount of CO2 that their family cars produce per gallon. Prior Knowledge & Skills Data interpreting skills
Lesson Summary Students will discuss human output of greenhouse gasses and then calculate the amount of CO2 that their family cars produce per gallon. Prior Knowledge & Skills Data interpreting skills
AST 105 Intro Astronomy The Solar System
 AST 105 Intro Astronomy The Solar System Next: How can we explain Earth s unique atmosphere. What kept Earth s climate stable? How did Earth's atmosphere end up so different? 1. Why did Earth retain most
AST 105 Intro Astronomy The Solar System Next: How can we explain Earth s unique atmosphere. What kept Earth s climate stable? How did Earth's atmosphere end up so different? 1. Why did Earth retain most
Global Warming: What is the role of aerosol?
 Global Warming: What is the role of aerosol? Barbara Wyslouzil, Sept. 10 2007 Outline Aerosols 101 The greenhouse effect Global temperature records The global warming problem How do aerosols play a role
Global Warming: What is the role of aerosol? Barbara Wyslouzil, Sept. 10 2007 Outline Aerosols 101 The greenhouse effect Global temperature records The global warming problem How do aerosols play a role
Closed Systems A closed system is a system in which energy, but not matter is exchanged with the surroundings.
 2.2 Notes Objectives Compare an open system with a closed system. List the characteristics of Earth s four major spheres. Identify the two main sources of energy in the Earth system. Identify four processes
2.2 Notes Objectives Compare an open system with a closed system. List the characteristics of Earth s four major spheres. Identify the two main sources of energy in the Earth system. Identify four processes
Twenty Questions and Answers About the Ozone Layer: 2014 Update
 Twenty Questions and Answers About the Ozone Layer: 2014 Update Scientific Assessment of Ozone Depletion: 2014 World Meteorological Organization United Nations Environment Programme National Oceanic and
Twenty Questions and Answers About the Ozone Layer: 2014 Update Scientific Assessment of Ozone Depletion: 2014 World Meteorological Organization United Nations Environment Programme National Oceanic and
Long Term Observations of Earth s Upper Atmosphere
 Long Term Observations of Earth s Upper Atmosphere SME UARS Aura EnviSat TIMED Nimbus VII Marty Mlynczak NASA Langley Research Center SORCE 11/20/2015 Sun-Climate Symposium 2015 1 Outline The golden age
Long Term Observations of Earth s Upper Atmosphere SME UARS Aura EnviSat TIMED Nimbus VII Marty Mlynczak NASA Langley Research Center SORCE 11/20/2015 Sun-Climate Symposium 2015 1 Outline The golden age
