Ozonesonde. Ozonesonde
|
|
|
- Georgia Benson
- 6 years ago
- Views:
Transcription
1 Ozonesonde Introduction In this exercise you will participate in a group activity which involves measuring and then interpreting the profile of ozone in the atmosphere from the ground to the stratosphere. This activity has two distinct aims: 1. To give you experience on how to plan a field experiment to make the best use of limited resources to investigate worthwhile scientific questions. 2. To understand the properties of ozone in the troposphere and stratosphere and to understand and interpret data from an ozonesonde profile. Format of activity Ozonesondes are much like normal meterological sondes as they are carried on a balloon and measure some aspect of the atmosphere. They are moderately expensive ( 500) pieces of atmospheric monitoring equipment used to produce profiles of atmospheric ozone in the vertical. On Arran we have available a single ozonesonde. It is therefore important that we choose to launch the ozonesonde in conditions where it will produce the most scientifically interesting and valuable data. The ozonesonde activity has three parts:! On the first afternoon each group will have one hour to design a short project proposal for when to launch the ozonesonde. You will have been informed in the introductory lecture about any restrictions on when the ozonesonde can be launched. Each group will produce a proposal of 1 side of A4 which should be submitted to the shared drive with your groups name by 1600 Local Time. The proposal should contain the following information: - When you would like to launch the ozonesonde? - What scientific questions you hope to answer? - The reasons behind your launch choice When designing you experiment, a critical part of your choice will be the meteorological conditions predicted for the day in question. You can make use of the forecast charts provided for the weather forecasting activity and the other data sources described later.! Once the hour is over each group will nominate a spokesperson to present their case to the scientific steering group made up of three members of staff. You should present your groups case using no overheads and talk for not longer than five minutes outlining the case as written in your document. Following all the presentations the steering group will meet to decide which of the proposals to take forward and will announce the chosen proposal during dinner. You will not be assessed on the project proposal that you write but on your reflective discussion of the comparison of your group s proposal with the proposal which is successful (see below for more details). The criteria for assessing the project proposals will be as follows: i. Maximum chance of observing interesting atmospheric phenomena. ii. Best scientific arguments for launching at that particular time. iii. Best technical arguments for launching at that particular time. 56 of 92
2 ! The ozonesonde will be launched following the instructions in the chosen proposal. Once the data is collected it will be available to you on the shared drive. Your group should produce a plot of the ozone profile from the ozonesonde and then follow the instructions in the assessment section on how to complete your analysis.! Some scientific projects that you might like to consider for your launch proposal are as follows, see below for more details of these phenomena: i. The identification of different tropospheric air masses and their origin above Arran. ii. An investigation of the influence of the passage of a front on atmospheric chemical structure. iii. The investigation of high ozone smog in the boundary layer. iv. The analysis of the structure within a tropopause fold. v. The identification of an ozone mini-hole vi. The validation of an ECMWF/GEMS ozone forecast There are however many other uses for an ozonesonde and you should attempt to be as creative as possible. Background information on atmospheric ozone Ozone as an atmospheric gas Ozone (O 3 ) plays a central role in both the chemistry and physics of the atmosphere. It is a molecule made up of 3 oxygen atoms. It is found throughout the atmosphere but its chemistry and physics is very different in the troposphere and in the stratosphere. Ozone plays a significant role in climate. Figure 1 is taken from the latest report by the Intergovernmental Panel of Climate Change and shows the radiative impact of different gases. You will notice that the increase in O 3 due to anthropogenic emission in the troposphere has led to a net warming of the planet, whereas the loss of O 3 in the stratosphere again by anthropogenic emissions has lead to a net cooling. Figure 1. Taken from: Contribution of Working Group I to the Fourth Assessment Report of the IPCC, Cambridge University Press, ISBN of 92
3 Ozone in the stratosphere Most of the ozone in the atmosphere resides in the stratosphere between about 70 and 1hPa. An example zonal (averaged along a latitude band) climatology of ozone (derived from a combination of models, ozonesondes and satellite data, is shown in Figure 2). There is a discussion of units for measuring the concentration of O 3 later.. Figure 2. Annual mean ozone climatology taken from Fortuin and Helder climatology. Units are parts per million by volume. Ozone in the stratosphere is formed through the interaction of ultra-violet radiation (short-wave radiation from the sun) and standard double bonded oxygen molecules (O 2 ) which exist throughout the atmosphere. The high ozone concentrations in the tropical middle stratosphere are related to the large amounts of ultra-violet radiation observed there. Ozone is transported through the stratosphere by the planetary scale Brewer-Dobson circulation. This circulation involves transport of air upwards in the tropical stratosphere, toward the pole and then downward into polar regions. Due to the Brewer-Dobson circulation, total column amounts of ozone (the total amount of ozone summed from the bottom of the atmosphere to the top) are generally largest in the polar regions. The amount of ozone in a column of air (add up all the ozone molecules from the ground to the top of the atmosphere at a single point) is measured using Dobson Units (DU) (Units are described in more detail later in this section). A typical daily maps of total column ozone, derived from two satellite instruments is shown in Fig of 92
4 Figure 3: Combined SBUV/2 and TOVS observations of total column ozone in Dobson Units. Taken from Stratospheric ozone has received much attention over the past thirty years because of the influence of anthropogenically produced chlorofluorocarbons or CFCs on ozone concentrations. CFCs are long-lived atmospheric gases used for refrigeration and aerosol sprays. Although these gases are normally unreactive, through a sequence of complex chemical and physical transformations in the stratosphere they are able to destroy stratospheric ozone. The ozone depleting reactions require the presence of sunlight and are much faster in the presence of polar stratospheric clouds. Polar stratospheric clouds form at very low temperatures in the stratosphere (< 195K). Some of the, normally slow, gas phase reactions involved in ozone depletion can proceed much faster on the surfaces of polar stratospheric cloud droplets. Although chemical ozone depletion has been observed in both the Arctic and Antarctic, it is generally much more intense and persistent in the Antarctic. In the Antarctic, stratospheric temperatures are colder than the Arctic, and allow polar stratospheric clouds to form more frequently and over a larger area leading to the more intense ozone depletion. The destruction of stratospheric ozone is important to understand because ozone forms the primary shield against the dangerous effects of incoming stratospheric UV-B radiation. When stratospheric ozone amounts are low, large amounts of UV-B can reach the surface. Exposure of biological life, including humans, to UV-B radiation increases the risks of cataracts, skin cancer and suppression of the immune system. 59 of 92
5 Ozone in the troposphere Ozone in the troposphere is usually a destructive force. It is an oxidizing agent and thus causes human health impact by attacking people s lungs, it attacks plants reducing crop yields and damages plastics and other materials increasing wear etc. Not enough high-energy radiation from the sun enters the troposphere for O 3 to be made by the photolysis of O 2, instead tropospheric O 3 is made by a complex set of chemical catalytic reactions involving oxides of nitrogen (NO and NO 2 collectively knows as NO x ) and oxides of hydrogen (OH and HO 2 collectively known as HO x ) NO x is naturally emitted into the atmosphere by microbes in the soils and from lightning. However the dominant source is the high temperature combustion associated with internal combustion engines, jets etc. Eventually the NO x emitted into the atmosphere reacts to form nitric acid. This is stable and soluble and is eventually removed to the surface by rain. HO x is mainly made in the atmosphere by the photolysis of O 3 and the subsequent reaction of the high energy oxygen atom with water. This produces an OH radical. The OH radical can be converted into an HO 2 radical through its reaction with CO. The HO 2 radical can be converted back to an OH radical through its reaction with NO. It is the interaction between HO x and NO x that leads to the production of an O 3 molecule. NO can react with HO 2 to produce NO 2 and OH. The NO 2 can rapidly be photolysed by radiation from the sun (indicated by h below) which returns an NO molecule and an oxygen atom which rapidly reacts with an oxygen molecule to make an O 3 molecule. This can all be represented as NO + HO 2! NO 2 + OH NO 2 +h!! NO + O O + O 2! O 3 OH +CO + O 2! CO 2 + HO 2 Overall 2O 2 + CO! CO 2 +O 3 This cycle is known as being catalytic as neither the NO x or the HO x are used up in making the O 3. Each NO x molecule can make many O 3 molecules. On the other hand the CO molecule is used up. Other species can act to convert OH into HO 2 and these are dominated by hydrocarbons. Overall the oxidation of gases such as CO and hydrocarbons in the presence of NO x leads to the production of O 3. Ozone is removed from the atmosphere through the reaction of sun light and water vapour Overall O 3 +h!! O 2 + O( 1 D) O( 1 D)+H 2 O! 2 OH O 3 +h!+h 2 O! O OH Thus air which has been recently subject to NO x emissions and sunshine are likely to have high O 3 concentrations, whereas those which been away from recent emission of NO x and have been in wet sunny conditions are likely to be low in O 3. Remember that airmasses can be lifted in the atmosphere by convection and frontal activity. These processes lead to layers being formed which are different O 3 concentrations. Tropopause dynamics and ozone mini-holes 60 of 92
6 In addition to the long-term trends in stratospheric ozone caused by chemical ozone destruction there is also a great deal of variability in the amount of ozone in the total column above a particular location (see Fig. 2). Most of this variability occurs on the time and spatial scales associated with typical synoptic processes in the middle and high latitudes. In the Northern Hemisphere (NH) ozone mini-holes are regions of around 1000km with total column ozone values around 100DU less than the seasonal mean ozone column at their centre. Ozone mini-holes are formed when there is divergence in the atmospheric column at lower and middle-stratospheric levels, causing ozone rich air to flow out of the column. Most studies (see for example James and Peters, Annales Geophysicae, 2002) of the meteorological state associated with ozone mini-holes suggest that this divergence is caused by a combination of a tropospheric anti-cyclonic anomaly and a stratospheric cyclonic anomaly. The coincidence of these two meteorological structures causes strong lifting of the tropopause and horizontal transport of ozone-rich air out of the column. Over the UK, there are typically 2-3 events of this size each year, lasting for a few days. In some cases, particularly at high northern latitudes (e.g. Scandinavia) ozone mini-holes can occur in regions where some chemical ozone loss is also taking place, thereby causing very large ozone deficits. However, for latitudes close to the UK this is very uncommon. The characteristic signature of an ozone mini-hole is the reduction of ozone content below typical climatological values in the lower and middle stratosphere. Measuring ozone using an ozone-sonde Ozone-sondes measure O 3 using a chemical reaction in the liquid phase between potassium iodide (KI) and Ozone (O 3 ) which produced iodine molecules (I 2 ). 2 KI + O 3 +H 2!"# 2 +O KOH We can measure the concentration of I 2 using an electro-chemical cell so that the concentration of I 2 is proportional to the the current that flows through the system. By measuring the current within the cell, the I 2 concentration can be found and so the O 3 concentration can be deduced. Figure 4. Makeup of a ozone sonde cell. The pump draws air through the KI solution. The ozone sensor then measures the current within the electro chemical cell (ECC) and reports this to the radio system which relays the information to the ground. Units 61 of 92
7 We can think about O 3 in many different ways. Some times we are interested in the fraction of ozone molecules in a sample of air compared to all the other molecules in the air. This is known as a volume mixing ratio. In the troposphere there are usually 40 O 3 molecules for every billion air molecules (mainly nitrogen and oxygen) so we refer to this as 40 part per billion air molecules (ppbv). In the stratosphere there are usually 5 O 3 molecules for every million air molecules (ppmv). Sometime we not interested in the relative concentration of the gas to the air but its number density how many molecules of gas are there per cubic cm of air (# cm -3 ). People who make ozone-sonde observations often use the partial pressure of ozone as their unit. This is the pressure that the ozone molecules would exert if all the other molecules in the air were removed. If you look at Figure 5 the O 3 is presented as a pressure in milli-pascals (mpa). You can convert these numbers to a mixing ratio by dividing by the pressure of the air at that point. So for example at the surface the O 3 partial pressure is 5 mpa. The pressure at that point is ~1013 hpa so the mixing ratio of O3 is 5x10-3 /1013x10 2 = 5x10-8 molecules of O 3 per molecule of air or 50 parts per billion. Sometimes we are interested in the total amount of O 3 above our heads. In this case we use Dobson units. If you image you took all the O 3 from above your head and brought it down to the surface at 0ºC and a pressure of 1013 hpa how thick would it be? For historical reasons this thickness is given in units of 0.01 mm. So if you brought all the O 3 above your head down to the ground at 0ºC and it formed a column 3 mm thick the O 3 column would be 300 DU You may like to attempt to estimate the total ozone column from your sounding of temperature and ozone. To do this, you will need to calculate the number of molecules of ozone present in different parts of the profile if the air were at standard temperature and pressure. The ozone number density (in molecules cm -3 can be estimated using the formula: You will then need to calculate the absolute number of molecules in that layer using the thickness relation and sum the total number of molecules. One Dobson Unit is equivalent to 2.69 x molecules of ozone per square centimeter. 62 of 92
8 Typical Ozone soundings Here is a typical ozone-sounding: Figure 5: Typical Ozone sonding. Taken from the Scientific Assessment of Ozone Depletion: 1994, Executive Summary, World Meteorological Organization Global Ozone Research and Monitoring Project - Report No. 37 You can see the tropopause at around 15 km with high concentrations of O 3 above in the stratospheric ozone layer and lower concentrations in the troposphere. The O 3 concentrations peaks at around 22km and then drops off above that. In the troposphere the O 3 concentration is fairly constant and then increases towards the surface due to recent pollution (this isn t a feature of all soundings). A small peak in O 3 at 13km is probably due to convection lifting polluted airmasses with higher O 3 into the upper troposphere (again this isn t a feature of all soundings). Data sources The observations of O 3 made during our sonde launch will be placed on the shared drive and you will be told where to find it. The file will contain the observations made by the sonde as a function of time from the launch. They contain the pressure (hpa) and altitude (m) of the sonde, the temperature (deg C), O 3 concentration (parts per billion by volume ppbv) and the relative humidity (%). We will also give you a tephigram of the sonde ascent. The European Centre for Medium Range Weather Forecasting (ECMWF) makes forecasts for the O 3 concentration at various pressures over the next few days. You can find their forecasts at: You may have to ask a member of staff to use a staff computer to see this page. 63 of 92
9 We may (depending upon the speed of the internet connection) provide you with forecast back trajectories for Arran, and we will provide trajectories for the ozone-sonde itself. You can use met charts to investigate your O 3 profile. Assessment Your analysis of the ozonesonde sounding will be expected to contain the following (each section is worth 50% of the final grade): a. A comparison of your proposal and the proposal chosen for the launch (you will need to stick a copy of each proposal in your lab book). You should discuss the strengths and weaknesses of each proposal and comment on why you think the successful proposal was chosen. For the group that submitted the successful proposal you should compare your winning proposal with another unsuccessful proposal. b. A discussion of the resulting ozonesonde profile. You should produce a plot of the ozonesonde profile as a minimum. You also might like to compare the ozone data with data obtained from other experiments. Your final report should consider some of the following questions (depending on the properties of the profile which you should discuss with course staff): i. What are the main features of the profile? ii. Can you see the presence of different air masses based on the concentration of ozone? Are these properties confirmed by the temperature structure from a standard tephigram? Where might the air masses have come from given the synoptic picture and the available trajectories? iii. Can you see the position of the tropopause? Is it high or low? Does this fit with the synoptic picture? iv. How do the concentrations of ozone in the troposphere and stratosphere compare to climatological amounts? Is there evidence of active chemistry in any of the air masses in the column? Is there evidence of significant advection of ozone rich air out of the column? v. Does the profile answer the scientific questions posed in the successful proposal? If it does, what are the answers and how does this improve our knowledge of the chemistry and dynamics of the atmosphere? If it doesn t, what went wrong and how could you change the experimental design to avoid these problems in the future? This activity is very open-ended. We want you to explore the data and the background material as much as possible. Marks will be awarded for innovative use of the data and discussion of the results. 64 of 92
Lecture 29 Air Pollution. Air Pollution. Clean Boundary Layer. Clean Boundary Layer
 Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
Lecture 29 Air Pollution Air Pollution Conditions that promote air pollution episodes Ozone Hole Air Pollution Elevated levels of aerosols and harmful gases Most pollution enters atmosphere near the surface.
Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica?
 Ozone Depletion and Climate Change Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica? In a recent (last August 2016) BBC documentary on the Antarctic weather changes, it has
Ozone Depletion and Climate Change Why are there large quantities of the un-natural (Man Made) CFCs in Antarctica? In a recent (last August 2016) BBC documentary on the Antarctic weather changes, it has
ATM S 211 Final Examination June 4, 2007
 ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
ATM S 211 Final Examination June 4, 2007 Name This examination consists of a total of 100 points. In each of the first two sections, you have a choice of which questions to answer. Please note that you
1 Characteristics of the Atmosphere
 CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
CHAPTER 22 1 Characteristics of the Atmosphere SECTION The Atmosphere KEY IDEAS As you read this section, keep these questions in mind: What are the layers of Earth s atmosphere? How has Earth s atmosphere
4. Stratospheric ozone depletion
 112 4. Stratospheric ozone depletion The thickness of the ozone layer above Europe has decreased significantly since the beginning of the 198s, and is declining at a rate of 4 5 % per decade. The gradual
112 4. Stratospheric ozone depletion The thickness of the ozone layer above Europe has decreased significantly since the beginning of the 198s, and is declining at a rate of 4 5 % per decade. The gradual
Casterlin Environmental Systems pg. 1
 s of the Earth's Atmosphere The atmosphere is divided into five layers. It is thickest near the surface and thins out with height until it eventually merges with space. 1. The troposphere is the first
s of the Earth's Atmosphere The atmosphere is divided into five layers. It is thickest near the surface and thins out with height until it eventually merges with space. 1. The troposphere is the first
Chapter 11: Atmosphere
 To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
To get you thinking This is our atmosphere. All life on Earth exists within this tiny protective blanket. Why is the atmosphere important to us? What do you think it does for us? Chapter 11: Atmosphere
Lecture 14 Stratospheric Ozone Loss ATOC/CHEM 5151
 Lecture 14 Stratospheric Ozone Loss ATOC/CHEM 5151 1 Setting the Stage: An Historical Perspective To begin, we need to go back to the 1970s Concern about the environment is high and people are starting
Lecture 14 Stratospheric Ozone Loss ATOC/CHEM 5151 1 Setting the Stage: An Historical Perspective To begin, we need to go back to the 1970s Concern about the environment is high and people are starting
History of significant air pollution events
 Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
Ch17 Air Pollution A thick layer of smoke and haze covers Santiago, Chile. History of significant air pollution events Many of the worst air pollution episodes occurred in the last two centuries in London
HUMAN IMPACT on the BIOSPHERE part 4
 HUMAN IMPACT on the BIOSPHERE part 4 Charting a course for the Future http://www.claybennett.com/pages2/mistletoe.html ENVIRONMENTAL PROBLEMS DEAD ZONES OZONE DEPLETION ACID RAIN GLOBAL WARMING WASTE http://www.acmecompany.com/stock_thumbnails/13808.greenhouse_effect_2.jpg
HUMAN IMPACT on the BIOSPHERE part 4 Charting a course for the Future http://www.claybennett.com/pages2/mistletoe.html ENVIRONMENTAL PROBLEMS DEAD ZONES OZONE DEPLETION ACID RAIN GLOBAL WARMING WASTE http://www.acmecompany.com/stock_thumbnails/13808.greenhouse_effect_2.jpg
Composition and Energy AOSC 200 Tim Canty
 Composition and Energy AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Atmospheric composition cont. Energy transfer Lecture 03 Sept 5 2017 1 Today s Weather
Composition and Energy AOSC 200 Tim Canty Class Web Site: http://www.atmos.umd.edu/~tcanty/aosc200 Topics for today: Atmospheric composition cont. Energy transfer Lecture 03 Sept 5 2017 1 Today s Weather
The Earth s Global Energy Balance
 The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
The Earth s Global Energy Balance Electromagnetic Radiation Insolation over the Globe World Latitude Zones Composition of the Atmosphere Sensible Heat and Latent Heat Transfer The Global Energy System
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32)
 Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Lecture 2: Greenhouse Gases - Basic Background on Atmosphere - GHG Emission and Concentration Rise - California Regulation (AB32) METR 113/ENVS 113 Spring Semester 2011 February 15, 2011 Suggested Reading
Directed Reading. Section: Global Change. than in the rest of the United States. b. In the United States and Canada, many lakes are dying as their ph
 Section: Global Change In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Scientists have discovered that acid rain is caused
Section: Global Change In the space provided, write the letter of the term or phrase that best completes each statement or best answers each question. 1. Scientists have discovered that acid rain is caused
Environmental Impacts of. Energy Production
 CH2356 Energy Engineering Environmental Impacts of Energy Production Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
CH2356 Energy Engineering Environmental Impacts of Energy Production Dr. M. Subramanian Associate Professor Department of Chemical Engineering Sri Sivasubramaniya Nadar College of Engineering Kalavakkam
The atmosphere. The atmosphere is layered. Inversions affect air quality 3/2/2015. The sun influences weather and climate
 The atmosphere Chapter 13 Atmosphere Absorbs radiation and moderates climate Transports and recycles water and nutrients Human activity is now changing the amount of some gases CO 2, methane (CH 4 ), ozone
The atmosphere Chapter 13 Atmosphere Absorbs radiation and moderates climate Transports and recycles water and nutrients Human activity is now changing the amount of some gases CO 2, methane (CH 4 ), ozone
RECENT MAJOR FINDINGS AND CURRENT SCIENTIFIC UNDERSTANDING
 The provisions of the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer include the requirement that the Parties to the Protocol base their future decisions on the current scientific, environmental,
The provisions of the 1987 Montreal Protocol on Substances that Deplete the Ozone Layer include the requirement that the Parties to the Protocol base their future decisions on the current scientific, environmental,
Air Pollution. Asian Brown Cloud. Developed Countries have reduced emissions recently
 Study Questions 1. Compare and contrast primary vs. secondary pollutants, giving examples of each. 2. Compare and contrast indoor vs. outdoor pollution, listing specific examples and sources of each. 3.
Study Questions 1. Compare and contrast primary vs. secondary pollutants, giving examples of each. 2. Compare and contrast indoor vs. outdoor pollution, listing specific examples and sources of each. 3.
Air Transportation: Emissions and Effects
 Air Transportation: Emissions and Effects Joyce E. Penner University of Michigan Report Co-ordinator: IPCC Special Report on Aviation and the Global Atmosphere Presentation to the First Regional Symposium
Air Transportation: Emissions and Effects Joyce E. Penner University of Michigan Report Co-ordinator: IPCC Special Report on Aviation and the Global Atmosphere Presentation to the First Regional Symposium
September 16 th 2010 International Day for the Preservation of the Ozone Layer
 September 16 th 2010 International Day for the Preservation of the Ozone Layer Next 16 th of September it will be celebrated the 2010 edition of the International Day for the Preservation of the Ozone
September 16 th 2010 International Day for the Preservation of the Ozone Layer Next 16 th of September it will be celebrated the 2010 edition of the International Day for the Preservation of the Ozone
Planetary Energy Balance
 Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Planetary Energy Balance Overview of Planetary Energy Balance Energy coming into the Earth s atmosphere from the sun is always in balance with the energy leaving Earth s atmosphere going back out into
Announcements. Pollution week continues. Thinking about pollution. Why are polar bears so contaminated?
 Announcements Grades for exam 2 have been posted March 7 th - Last day to submit LEAD summary to TA, extra credit videos due next Tuesday (no late videos will be accepted) Next Thursday, Environmental
Announcements Grades for exam 2 have been posted March 7 th - Last day to submit LEAD summary to TA, extra credit videos due next Tuesday (no late videos will be accepted) Next Thursday, Environmental
The Chemistry of Climate Change. Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy
 The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
The Chemistry of Climate Change Reading: Chapter 8 Environmental Chemistry, G. W. vanloon. S. J. Duffy The Science of Global Climate There's a lot of differing data, but as far as I can gather, over the
SCIAMACHY book. Ozone Recovery? Michel Van Roozendael, BIRA- IASB. ATC14, October, Jülich, Germany
 SCIAMACHY book Ozone Recovery? Michel Van Roozendael, BIRA- IASB ATC14, 27-31 October, Jülich, Germany 1928: start of CFC production 1971: 1 st observation of CFC in the atmosphere (J. Lovelock) 1974:
SCIAMACHY book Ozone Recovery? Michel Van Roozendael, BIRA- IASB ATC14, 27-31 October, Jülich, Germany 1928: start of CFC production 1971: 1 st observation of CFC in the atmosphere (J. Lovelock) 1974:
Introduction to Environmental Physics
 Introduction to Environmental Physics Planet Earth, Life and Climate Nigel Mason Department of Physics and Astronomy University College, London, UK. Peter Hughes Kingsway College, London, UK. with Randall
Introduction to Environmental Physics Planet Earth, Life and Climate Nigel Mason Department of Physics and Astronomy University College, London, UK. Peter Hughes Kingsway College, London, UK. with Randall
Ozone and trace gases in India: Effects of transport and emissions. Shyam Lal Physical Research Laboratory, Ahmedabad
 Ozone and trace gases in India: Effects of transport and emissions Shyam Lal Physical Research Laboratory, Ahmedabad Second Workshop on Atmospheric Composition and the Asian Monsoon (ACAM), 8-10 June,
Ozone and trace gases in India: Effects of transport and emissions Shyam Lal Physical Research Laboratory, Ahmedabad Second Workshop on Atmospheric Composition and the Asian Monsoon (ACAM), 8-10 June,
Climate Change and Ozone Loss
 Climate Change and Ozone Loss During the past 900,000 years, the earth has undergone a series of cold glacial periods followed by warmer interglacial periods. The past 10,000 years has been an interglacial
Climate Change and Ozone Loss During the past 900,000 years, the earth has undergone a series of cold glacial periods followed by warmer interglacial periods. The past 10,000 years has been an interglacial
Section 4 The Air We Breathe
 Section 4 The Air We Breathe Key Concept Air is an important natural resource that is affected by human activities. What You Will Learn Air pollution is caused by human activities, such as burning fossil
Section 4 The Air We Breathe Key Concept Air is an important natural resource that is affected by human activities. What You Will Learn Air pollution is caused by human activities, such as burning fossil
Name: Class: Date: 6. Most air pollution is produced by a. thermal inversions. c. ozone layer depletion. b. fuel burning. d. volcanic eruptions.
 Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
Name: Class: Date: Air Test Multiple Choice Identify the choice that best completes the statement or answers the question. 1. Which of the following is often used to remove poisonous gases from industrial
The Good, the Bad, and the Ugly of Ozone
 Name: Date: Period: Background The Good, the Bad, and the Ugly of Ozone Ozone (O 3 ) is colorless and consists of three oxygen atoms (the third oxygen makes it highly reactive). There are two main types
Name: Date: Period: Background The Good, the Bad, and the Ugly of Ozone Ozone (O 3 ) is colorless and consists of three oxygen atoms (the third oxygen makes it highly reactive). There are two main types
Climate Change and Ozone Depletion Notes. Chapter 20
 Climate Change and Ozone Depletion Notes Chapter 20 PAST CLIMATE AND THE GREENHOUSE EFFECT Over the past 900,000 years, the troposphere has experienced prolonged periods of global cooling and global warming.
Climate Change and Ozone Depletion Notes Chapter 20 PAST CLIMATE AND THE GREENHOUSE EFFECT Over the past 900,000 years, the troposphere has experienced prolonged periods of global cooling and global warming.
Lecture 22: Atmospheric Chemistry and Climate
 Lecture 22: Atmospheric Chemistry and Climate Required Reading: FP Chapter 14 (only sections that I cover) Suggested Introductory Reading: Jacob Chapter 7 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring
Lecture 22: Atmospheric Chemistry and Climate Required Reading: FP Chapter 14 (only sections that I cover) Suggested Introductory Reading: Jacob Chapter 7 Atmospheric Chemistry CHEM-5151 / ATOC-5151 Spring
Chapter 13. Atmospheric Science, Air Quality, and Pollution Control. Lecture Presentations prepared by Reggie Cobb Nash Community College
 Chapter 13 Atmospheric Science, Air Quality, and Pollution Control Lecture Presentations prepared by Reggie Cobb Nash Community College This lecture will help you understand: Earth s atmosphere Weather,
Chapter 13 Atmospheric Science, Air Quality, and Pollution Control Lecture Presentations prepared by Reggie Cobb Nash Community College This lecture will help you understand: Earth s atmosphere Weather,
K39: The Key Evidence That Current Global Warming is Human-Caused
 K39: The Key Evidence That Current Global Warming is Human-Caused Here are 13 lines of Evidence GW = AGW (Global Warming = Anthropogenic Global Warming) Evidence #1: A Warming Troposphere with a Cooling
K39: The Key Evidence That Current Global Warming is Human-Caused Here are 13 lines of Evidence GW = AGW (Global Warming = Anthropogenic Global Warming) Evidence #1: A Warming Troposphere with a Cooling
James R. Drummond Dalhousie University
 A Comparison of the Utility of 1st and 2nd-order Regularization Matrices for Controlling Non-Linear Effects in Optimally-Estimation Retrievals of Carbon Monoxide from Nadir Satellite Measurements James
A Comparison of the Utility of 1st and 2nd-order Regularization Matrices for Controlling Non-Linear Effects in Optimally-Estimation Retrievals of Carbon Monoxide from Nadir Satellite Measurements James
Earth Science Lesson Plan Quarter 2, Week 1, Day 1
 Earth Science Lesson Plan Quarter 2, Week 1, Day 1 1 Outcomes for Today Standard Focus: Earth Sciences 4.c Students know the different atmospheric gases that absorb the Earth s thermal radiation and the
Earth Science Lesson Plan Quarter 2, Week 1, Day 1 1 Outcomes for Today Standard Focus: Earth Sciences 4.c Students know the different atmospheric gases that absorb the Earth s thermal radiation and the
Climate Change. Some solar radiation is reflected by Earth and the atmosphere. Earth s Surface
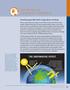 Q& A n The Basics of Greenhouse gases affect Earth s energy balance and climate The Sun serves as the primary energy source for Earth s climate. Some of the incoming sunlight is reflected directly back
Q& A n The Basics of Greenhouse gases affect Earth s energy balance and climate The Sun serves as the primary energy source for Earth s climate. Some of the incoming sunlight is reflected directly back
ECVS IN THE STRATOSPHERE
 METEOROLOGY ECVS IN THE STRATOSPHERE O 3, H 2 O, AEROSOL, N 2 O, CH 4, CFCS, HCFCS, HFCS, SF 6, PFCS, AND TEMPERATURE Michaela I. Hegglin, University of Reading UK GCOS COMPOSITION ECVs EXTEND INTO THE
METEOROLOGY ECVS IN THE STRATOSPHERE O 3, H 2 O, AEROSOL, N 2 O, CH 4, CFCS, HCFCS, HFCS, SF 6, PFCS, AND TEMPERATURE Michaela I. Hegglin, University of Reading UK GCOS COMPOSITION ECVs EXTEND INTO THE
Critique: The Signal and the Noise Nate Silver - his chapter on climate by Norman Rogers
 3 Oct 2016 Critique: The Signal and the Noise Nate Silver - his chapter on climate by Norman Rogers Silver s chapter 12 discusses global warming / climate. He makes massive mistakes and unsupported assumptions.
3 Oct 2016 Critique: The Signal and the Noise Nate Silver - his chapter on climate by Norman Rogers Silver s chapter 12 discusses global warming / climate. He makes massive mistakes and unsupported assumptions.
Convection Conduction
 L 18 Thermodynamics [3] Review Heat transfer processes convection conduction Greenhouse effect Climate change Ozone layer Review The temperature of a system is a measure of the average kinetic energy of
L 18 Thermodynamics [3] Review Heat transfer processes convection conduction Greenhouse effect Climate change Ozone layer Review The temperature of a system is a measure of the average kinetic energy of
Atmosphere Web quest
 Atmosphere Web quest 1. What are the four main layers of the atmosphere? Troposphere Stratosphere Mesosphere Thermosphere Ionosphere Exsosphere 2. Which layer is closest to space? Exosphere (upper layer
Atmosphere Web quest 1. What are the four main layers of the atmosphere? Troposphere Stratosphere Mesosphere Thermosphere Ionosphere Exsosphere 2. Which layer is closest to space? Exosphere (upper layer
Threats to Our Atmosphere
 Threats to Our Atmosphere A Reading A Z Level W Leveled Reader Word Count: 1,831 LEVELED READER W Written by Shaun Taylor Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
Threats to Our Atmosphere A Reading A Z Level W Leveled Reader Word Count: 1,831 LEVELED READER W Written by Shaun Taylor Visit www.readinga-z.com for thousands of books and materials. www.readinga-z.com
ENVIS- IITM NEWSLETTER The Air Quality: A Global Challenge
 ENVIS- IITM NEWSLETTER The Air Quality: A Global Challenge GLOBAL WARMING Editorial Prof. B.N. Goswami (Director, IITM, Pune) Dr. G. Beig (ENVIS Co-ordinetor) Ms. Neha S. Parkhi (Program Officer) Mr. Rajnikant
ENVIS- IITM NEWSLETTER The Air Quality: A Global Challenge GLOBAL WARMING Editorial Prof. B.N. Goswami (Director, IITM, Pune) Dr. G. Beig (ENVIS Co-ordinetor) Ms. Neha S. Parkhi (Program Officer) Mr. Rajnikant
TROPICS: insolation high year round, high sun angle and ~ constant duration
 GE 101, February 6, 14 Finish insolation variation Global environmental issues associated with insolation TRPICS: insolation high year round, high sun angle and ~ constant duration MID-LATITUDES: insolation
GE 101, February 6, 14 Finish insolation variation Global environmental issues associated with insolation TRPICS: insolation high year round, high sun angle and ~ constant duration MID-LATITUDES: insolation
TROPOSPHERIC AEROSOL PROGRAM - TAP
 THE DEPARTMENT OF ENERGY'S TROPOSPHERIC AEROSOL PROGRAM - TAP AN EXAMINATION OF AEROSOL PROCESSES AND PROPERTIES RG99060050.3 American Geophysical Union, Fall Meeting, San Francisco, December 12-17, 1999
THE DEPARTMENT OF ENERGY'S TROPOSPHERIC AEROSOL PROGRAM - TAP AN EXAMINATION OF AEROSOL PROCESSES AND PROPERTIES RG99060050.3 American Geophysical Union, Fall Meeting, San Francisco, December 12-17, 1999
Global warming. Models for global warming Sand analogy
 8.10 Global warming Assessment statements 8.6.1 Describe some possible models of global warming. 8.6. State what is meant by the enhanced greenhouse effect. 8.6.3 Identify the increased combustion of fossil
8.10 Global warming Assessment statements 8.6.1 Describe some possible models of global warming. 8.6. State what is meant by the enhanced greenhouse effect. 8.6.3 Identify the increased combustion of fossil
Introduction. Introduction. Introduction. Outline Last IPCC report : 2001 Last IPCC report :
 Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
Introduction Greenhouse Gases & Climate Change Laurent Bopp LSCE, Paris When did the story start? ¾1827 Fourier hypothesizes greenhouse effect ¾1860 Tyndal identifies CO2 and water vapor as heat trapping
Tuesday Dec 2nd TOPIC # 13 Global Warming Wrap Up TOPIC #14 IMPACTS & ISSUES
 Tuesday Dec 2nd TOPIC # 13 Global Warming Wrap Up TOPIC #14 IMPACTS & ISSUES SIT WITH YOUR GROUP TODAY ANNOUNCEMENTS: LINKING-TO-LIFE PROJECT PART A Your Ecological Footprint DUE in class TODAY! PART B
Tuesday Dec 2nd TOPIC # 13 Global Warming Wrap Up TOPIC #14 IMPACTS & ISSUES SIT WITH YOUR GROUP TODAY ANNOUNCEMENTS: LINKING-TO-LIFE PROJECT PART A Your Ecological Footprint DUE in class TODAY! PART B
What Exactly is a Greenhouse Gas?
 1 What Exactly is a Greenhouse Gas? You may have stood in a greenhouse and felt the heat, but what do greenhouse gases have to do with greenhouses? A greenhouse gas is any gas that absorbs and re-emits
1 What Exactly is a Greenhouse Gas? You may have stood in a greenhouse and felt the heat, but what do greenhouse gases have to do with greenhouses? A greenhouse gas is any gas that absorbs and re-emits
General Article. Abstract. Keywords: Pollutants, stratosphere, CFCS, colorimeter, skin cancer. Introduction Ozone, the O 3
 General Article Ozone:A Pollutant... Ozone: A Pollutant and a Protector Gas Thaneshwar Subedi Tribhuvan University Department of Chemistry P.N. Campus, Pokhara E-mail: thaneshworsubedi@gmail.com Abstract
General Article Ozone:A Pollutant... Ozone: A Pollutant and a Protector Gas Thaneshwar Subedi Tribhuvan University Department of Chemistry P.N. Campus, Pokhara E-mail: thaneshworsubedi@gmail.com Abstract
Name Date Class. This section describes Earth s atmosphere, or the layer of gases that surrounds the planet.
 The Atmosphere Name Date Class The Atmosphere Guided Reading and Study The Air Around You This section describes Earth s atmosphere, or the layer of gases that surrounds the planet. Use Target Reading
The Atmosphere Name Date Class The Atmosphere Guided Reading and Study The Air Around You This section describes Earth s atmosphere, or the layer of gases that surrounds the planet. Use Target Reading
In 2002, a group of university researchers joined together under the title of the Canadian Network for the Detection of Atmospheric Change (CANDAC)
 1 In 2002, a group of university researchers joined together under the title of the Canadian Network for the Detection of Atmospheric Change (CANDAC) with the objective of improving the state of observational
1 In 2002, a group of university researchers joined together under the title of the Canadian Network for the Detection of Atmospheric Change (CANDAC) with the objective of improving the state of observational
Energy, Greenhouse Gases and the Carbon Cycle
 Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Energy, Greenhouse Gases and the Carbon Cycle David Allen Gertz Regents Professor in Chemical Engineering, and Director, Center for Energy and Environmental Resources Concepts for today Greenhouse Effect
Stratospheric Chemistry HS 2017 Solution to Homework Problem Set 3
 Stratospheric Chemistry HS 2017 Solution to Homework Problem Set 3 For questions: andrea.stenke@env.ethz.ch (CHN P14) Problem 1: The Montreal Protocol and Climate (a) Chemical Formulas CFC-11 : CFCl 3
Stratospheric Chemistry HS 2017 Solution to Homework Problem Set 3 For questions: andrea.stenke@env.ethz.ch (CHN P14) Problem 1: The Montreal Protocol and Climate (a) Chemical Formulas CFC-11 : CFCl 3
Earth and Space Science (Earth's Atmosphere) Grade 7 Science Grade 7 Science Start Date: December 02, 2013 End Date : December 20, 2013
 Unit Overview Atmospheric properties Content Elaborations The atmosphere has different properties at different elevations and contains a mixture of gases that cycle through the lithosphere, biosphere,
Unit Overview Atmospheric properties Content Elaborations The atmosphere has different properties at different elevations and contains a mixture of gases that cycle through the lithosphere, biosphere,
CLIMATE CHANGE AND ACID RAIN. Mr. Banks 7 th Grade Science
 CLIMATE CHANGE AND ACID RAIN Mr. Banks 7 th Grade Science COMPOSITION OF AIR? COMPOSITION OF AIR? 78% Nitrogen 21% Oxygen 0.93% Argon and other noble gases 0.04% carbon dioxide Variable amounts of water
CLIMATE CHANGE AND ACID RAIN Mr. Banks 7 th Grade Science COMPOSITION OF AIR? COMPOSITION OF AIR? 78% Nitrogen 21% Oxygen 0.93% Argon and other noble gases 0.04% carbon dioxide Variable amounts of water
Downloaded from
 Question 14.1: Define environmental chemistry. Environmental chemistry is the study of chemical and biochemical processes occurring in nature. It deals with the study of origin, transport, reaction, effects,
Question 14.1: Define environmental chemistry. Environmental chemistry is the study of chemical and biochemical processes occurring in nature. It deals with the study of origin, transport, reaction, effects,
Grade 10 Academic Science Climate Change Unit Test
 Grade 10 Academic Science Climate Change Unit Test Part A - Multiple Choice: Circle the most correct answer. 1. What is the difference between weather and climate? a. Weather deals with wind and precipitation;
Grade 10 Academic Science Climate Change Unit Test Part A - Multiple Choice: Circle the most correct answer. 1. What is the difference between weather and climate? a. Weather deals with wind and precipitation;
Global Climate Change
 Global Climate Change Devizes & District U3A, 24 th November 2015 Prof. Richard Allan, Department of Meteorology University of Reading Why does Earth s climate change? Earth s Climate has always been changing
Global Climate Change Devizes & District U3A, 24 th November 2015 Prof. Richard Allan, Department of Meteorology University of Reading Why does Earth s climate change? Earth s Climate has always been changing
Convection. L 18 Thermodynamics [3] Conduction. heat conduction. radiation
![Convection. L 18 Thermodynamics [3] Conduction. heat conduction. radiation Convection. L 18 Thermodynamics [3] Conduction. heat conduction. radiation](/thumbs/72/66535645.jpg) L 18 Thermodynamics [3] Heat transfer processes convection conduction Thermodynamics of the atmosphere Greenhouse effect and climate change Effect of the ozone layer Convection heat is transferred from
L 18 Thermodynamics [3] Heat transfer processes convection conduction Thermodynamics of the atmosphere Greenhouse effect and climate change Effect of the ozone layer Convection heat is transferred from
Air Pollution in the Los Angeles Basin
 Air Pollution in the Los Angeles Basin Pollutants in L.A. Air Ozone Carbon Monoxide Nitrogen Oxides Heavy Metals Particulate Matter PAHs (polyaromatic hydrocarbons) Other things 1 Primary Pollutants The
Air Pollution in the Los Angeles Basin Pollutants in L.A. Air Ozone Carbon Monoxide Nitrogen Oxides Heavy Metals Particulate Matter PAHs (polyaromatic hydrocarbons) Other things 1 Primary Pollutants The
AST 105 Intro Astronomy The Solar System
 AST 105 Intro Astronomy The Solar System Next: How can we explain Earth s unique atmosphere. What kept Earth s climate stable? How did Earth's atmosphere end up so different? 1. Why did Earth retain most
AST 105 Intro Astronomy The Solar System Next: How can we explain Earth s unique atmosphere. What kept Earth s climate stable? How did Earth's atmosphere end up so different? 1. Why did Earth retain most
What is climate change? - BBC News
 What is climate change? - BBC News Media caption Why we should care about climate change? In December, of cials from across the world will gather in Paris, France, to try to hammer out a deal to tackle
What is climate change? - BBC News Media caption Why we should care about climate change? In December, of cials from across the world will gather in Paris, France, to try to hammer out a deal to tackle
At present rates of increase it would take about 360 years for atmospheric methane levels to double.
 The Methane Misconceptions A paper by Dr Wilson Flood Summary A doubling of the amount of methane in the atmosphere with its present composition would produce a warming equal to only about one thirtieth
The Methane Misconceptions A paper by Dr Wilson Flood Summary A doubling of the amount of methane in the atmosphere with its present composition would produce a warming equal to only about one thirtieth
Chapter 17: Atmospheric Science and Air Pollution
 Name: Per. Due Dates: See the HW Guides for Week 1 and 2 of this unit EPA s 6 Criteria Pollutants (plus 2) Air Pollutant List Major Anthropogenic and Natural Sources (if applicable) Carbon monoxide (CO)
Name: Per. Due Dates: See the HW Guides for Week 1 and 2 of this unit EPA s 6 Criteria Pollutants (plus 2) Air Pollutant List Major Anthropogenic and Natural Sources (if applicable) Carbon monoxide (CO)
Answer Test Questions Finish Climate Discussion
 NREM 301 Forest Ecology & Soils Day 30 December 4, 2008 Answer Test Questions Finish Climate Discussion Take-Home Test Due Dec 11 5 pm No Final Exam Lab Today Finish & e-mail all materials to Dick Class
NREM 301 Forest Ecology & Soils Day 30 December 4, 2008 Answer Test Questions Finish Climate Discussion Take-Home Test Due Dec 11 5 pm No Final Exam Lab Today Finish & e-mail all materials to Dick Class
ACID RAIN. CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007
 ACID RAIN CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007 More accurate term may be acid deposition Occurs in two forms wet deposition (acidic rain, fog, and snow) dry deposition
ACID RAIN CE 326 Principles of Environmental Engineering Prof. Tim Ellis January 22, 2007 More accurate term may be acid deposition Occurs in two forms wet deposition (acidic rain, fog, and snow) dry deposition
CHANGE. Jean PLA, Frequency Management. Rapporteur ITU-D Question 24/2 ICT and Climate Change. CNES, Toulouse, FRANCE
 ITU climate change event, TURIN 6-7 May 2013 ICT AND CLIMATE CHANGE Jean PLA, Frequency Management Rapporteur ITU-D Question 24/2 ICT and Climate Change CNES, Toulouse, FRANCE jean.pla@cnes.fr Jean PLA
ITU climate change event, TURIN 6-7 May 2013 ICT AND CLIMATE CHANGE Jean PLA, Frequency Management Rapporteur ITU-D Question 24/2 ICT and Climate Change CNES, Toulouse, FRANCE jean.pla@cnes.fr Jean PLA
Lecture Outlines. Chapter 17. Environment: The Science behind the Stories 4th Edition Withgott/Brennan Pearson Education, Inc.
 Lecture Outlines Chapter 17 Environment: The Science behind the Stories 4th Edition Withgott/Brennan This lecture will help you understand: The Earth s atmosphere Weather, climate, and atmospheric conditions
Lecture Outlines Chapter 17 Environment: The Science behind the Stories 4th Edition Withgott/Brennan This lecture will help you understand: The Earth s atmosphere Weather, climate, and atmospheric conditions
Leif Backman HENVI Seminar February 19, 2009
 Methane Sources and Sinks Leif Backman HENVI Seminar February 19, 2009 Background Atmospheric methane Sources & Sinks Concentration variations & trends Objective & methods Objective & Goals Research plan
Methane Sources and Sinks Leif Backman HENVI Seminar February 19, 2009 Background Atmospheric methane Sources & Sinks Concentration variations & trends Objective & methods Objective & Goals Research plan
Air Pollution. GEOL 1350: Introduction To Meteorology
 Air Pollution GEOL 1350: Introduction To Meteorology 1 Overview Types and Sources of Air Pollutants Factors That Affect Air Pollution Air Pollution and the Urban Environment 2 Air pollutants are airborne
Air Pollution GEOL 1350: Introduction To Meteorology 1 Overview Types and Sources of Air Pollutants Factors That Affect Air Pollution Air Pollution and the Urban Environment 2 Air pollutants are airborne
Current Update on Climate Science
 Current Update on Climate Science Ben Santer Program for Climate Model Diagnosis and Intercomparison Lawrence Livermore National Laboratory, Livermore, CA 94550 Email: santer1@llnl.gov 3 rd Annual CAFE
Current Update on Climate Science Ben Santer Program for Climate Model Diagnosis and Intercomparison Lawrence Livermore National Laboratory, Livermore, CA 94550 Email: santer1@llnl.gov 3 rd Annual CAFE
Module 3, Investigation 2: Briefing The loss of stratospheric ozone: Where are lives at risk?
 Background Have you heard of the ozone hole? In the 1970s, scientists began noticing a hole developing in the stratospheric ozone layer high above Antarctica. Scientists attributed the ozone destruction
Background Have you heard of the ozone hole? In the 1970s, scientists began noticing a hole developing in the stratospheric ozone layer high above Antarctica. Scientists attributed the ozone destruction
Global Warming Science Solar Radiation
 SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
SUN Ozone and Oxygen absorb 190-290 nm. Latent heat from the surface (evaporation/ condensation) Global Warming Science Solar Radiation Turbulent heat from the surface (convection) Some infrared radiation
Teaching Time: 1 hour and 15 minutes
 Lesson Summary Students will discuss human output of greenhouse gasses and then calculate the amount of CO2 that their family cars produce per gallon. Prior Knowledge & Skills Data interpreting skills
Lesson Summary Students will discuss human output of greenhouse gasses and then calculate the amount of CO2 that their family cars produce per gallon. Prior Knowledge & Skills Data interpreting skills
CLIMATE MODELS: LARGE SCALE AND PREDICTABLE
 CCS 5-1 CLIMATE MODELS: LARGE SCALE AND PREDICTABLE 1. Print this file (and associated linked images if any). Also answer the "Concept of the Week" questions in the Weekly Climate News File. (Check for
CCS 5-1 CLIMATE MODELS: LARGE SCALE AND PREDICTABLE 1. Print this file (and associated linked images if any). Also answer the "Concept of the Week" questions in the Weekly Climate News File. (Check for
Human Activity and Climate Change
 Human Activity and Climate Change Textbook pages 482 501 Section 11.1 11.2 Summary Before You Read How might climate change affect the region where you live? Record your thoughts in the lines below. What
Human Activity and Climate Change Textbook pages 482 501 Section 11.1 11.2 Summary Before You Read How might climate change affect the region where you live? Record your thoughts in the lines below. What
Global Warming Potentials in AR4. V. Ramaswamy. NOAA/ Geophysical Fluid Dynamics Laboratory, Princeton University
 Global Warming Potentials in AR4 V. Ramaswamy NOAA/ Geophysical Fluid Dynamics Laboratory, Princeton University GWP Definition Defined as the ratio of the time-integrated radiative forcing from the instantaneous
Global Warming Potentials in AR4 V. Ramaswamy NOAA/ Geophysical Fluid Dynamics Laboratory, Princeton University GWP Definition Defined as the ratio of the time-integrated radiative forcing from the instantaneous
15.023J / J / ESD.128J Global Climate Change: Economics, Science, and Policy Spring 2008
 MIT OpenCourseWare http://ocw.mit.edu 15.023J / 12.848J / ESD.128J Global Climate Change: Economics, Science, and Policy Spring 2008 For information about citing these materials or our Terms of Use, visit:
MIT OpenCourseWare http://ocw.mit.edu 15.023J / 12.848J / ESD.128J Global Climate Change: Economics, Science, and Policy Spring 2008 For information about citing these materials or our Terms of Use, visit:
Aircraft Emissions. Ulrich Schumann. Edited by. Professor Ian Douglas. Encyclopedia of Global Environmental Change (ISBN )
 Aircraft Emissions Ulrich Schumann Volume 3, Causes and consequences of global environmental change, pp 178 186 Edited by Professor Ian Douglas in Encyclopedia of Global Environmental Change (ISBN 0-471-97796-9)
Aircraft Emissions Ulrich Schumann Volume 3, Causes and consequences of global environmental change, pp 178 186 Edited by Professor Ian Douglas in Encyclopedia of Global Environmental Change (ISBN 0-471-97796-9)
Unit 8. The atmosphere.
 Unit 8. The atmosphere. Adapted from Natural Science. 1º ESO. Anaya Natural Science 1º ESO NAME 1 INDEX 1. VOCABULARY... 1 2. VOCABULARIO... 2 3. UNIT CHART...Cover and 3 4. UNIT ACTIVITIES AND NOTES...
Unit 8. The atmosphere. Adapted from Natural Science. 1º ESO. Anaya Natural Science 1º ESO NAME 1 INDEX 1. VOCABULARY... 1 2. VOCABULARIO... 2 3. UNIT CHART...Cover and 3 4. UNIT ACTIVITIES AND NOTES...
MODULE I. Learning Objectives
 MODULE I Learning Objectives To make the students aware of history of air pollution; definition of air pollution and various types of sources and classification of air pollutants. Lecture 1 Lecture 2 Lecture
MODULE I Learning Objectives To make the students aware of history of air pollution; definition of air pollution and various types of sources and classification of air pollutants. Lecture 1 Lecture 2 Lecture
Lightning and Atmospheric Chemistry
 Lightning and Atmospheric Chemistry 1785 Cavendish performed the first experiments with a spark discharge in glass tube. Discovered that oxidized nitrogen (NO x =NO + NO 2 ) compounds resulted from the
Lightning and Atmospheric Chemistry 1785 Cavendish performed the first experiments with a spark discharge in glass tube. Discovered that oxidized nitrogen (NO x =NO + NO 2 ) compounds resulted from the
From the exhaust to ozone production and methane destruction
 From the exhaust to ozone production and methane destruction Ivar S.A. Isaksen With contributions from Amund Søvde, Michael Gauss and Øyvind Hodnebrog Impact of NOx emission on ozone and methane Contribute
From the exhaust to ozone production and methane destruction Ivar S.A. Isaksen With contributions from Amund Søvde, Michael Gauss and Øyvind Hodnebrog Impact of NOx emission on ozone and methane Contribute
Earth as a System. Chapter 2. Table of Contents. Section 1 Earth: A Unique Planet. Section 2 Energy in the Earth System.
 Earth as a System Table of Contents Section 1 Earth: A Unique Planet Section 2 Energy in the Earth System Section 3 Ecology Section 1 Earth: A Unique Planet Objectives Describe the size and shape of Earth.
Earth as a System Table of Contents Section 1 Earth: A Unique Planet Section 2 Energy in the Earth System Section 3 Ecology Section 1 Earth: A Unique Planet Objectives Describe the size and shape of Earth.
GLOBAL CLIMATE CHANGE
 1 GLOBAL CLIMATE CHANGE From About Transportation and Climate Change (Source; Volpe center for Climate Change and Environmental forecasting, http://climate.volpe.dot.gov/trans.html Greenhouse effect has
1 GLOBAL CLIMATE CHANGE From About Transportation and Climate Change (Source; Volpe center for Climate Change and Environmental forecasting, http://climate.volpe.dot.gov/trans.html Greenhouse effect has
Overview of Climate Science
 1 Overview of Climate Science This overview of climate science is written to support the development of a K- 14 climate education plan for the Pacific Islands Climate Education Partnership (PCEP). It aims
1 Overview of Climate Science This overview of climate science is written to support the development of a K- 14 climate education plan for the Pacific Islands Climate Education Partnership (PCEP). It aims
Class IX Chapter 14 Natural Resources Science
 Question 1: How is our atmosphere different from the atmospheres on Venus and Mars? Earth s atmosphere is different from those of Venus and Mars. This difference lies essentially in their compositions.
Question 1: How is our atmosphere different from the atmospheres on Venus and Mars? Earth s atmosphere is different from those of Venus and Mars. This difference lies essentially in their compositions.
Chapter 2 9/15/2015. Chapter 2. Penny Boat. 2.1 The Role of Water in Cycles of Matter
 Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
Chapter 2 Chapter 2 Cycles of Matter 2.1 The Role of Water in Cycles of Matter 2.2 Biogeochemical Cycles 2.3 the Balance of the Matter and Energy Exchange 2.1 The Role of Water in Cycles of Matter In this
Air & Water Lesson 2. Chapter 6 Conserving Our Resources
 Air & Water Lesson 2 Chapter 6 Conserving Our Resources Objectives Summarize the importance of air. Describe the water cycle. Main Idea Living things use air and water to carry out their life processes.
Air & Water Lesson 2 Chapter 6 Conserving Our Resources Objectives Summarize the importance of air. Describe the water cycle. Main Idea Living things use air and water to carry out their life processes.
Atmosphere, the Water Cycle and Climate Change
 Atmosphere, the Water Cycle and Climate Change OCN 623 Chemical Oceanography 16 April 2013 (Based on previous lectures by Barry Huebert) 2013 F.J. Sansone 1. The water cycle Outline 2. Climate and climate-change
Atmosphere, the Water Cycle and Climate Change OCN 623 Chemical Oceanography 16 April 2013 (Based on previous lectures by Barry Huebert) 2013 F.J. Sansone 1. The water cycle Outline 2. Climate and climate-change
Renewable Energies and Low-Carbon Society: Application of CGE Model to Toyohashi City in Japan
 Renewable Energies and Low-Carbon Society: Application of CGE Model to Toyohashi City in Japan Yuzuru Miyata Department of Architecture and Civil Engineering, Toyohashi University of Technology and Shuai
Renewable Energies and Low-Carbon Society: Application of CGE Model to Toyohashi City in Japan Yuzuru Miyata Department of Architecture and Civil Engineering, Toyohashi University of Technology and Shuai
POLLUTION. Water Pollution Air Pollution
 POLLUTION Water Pollution Air Pollution Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The presence of a substance in the environment that prevents
POLLUTION Water Pollution Air Pollution Water Pollution Background Sources Types Eutrophication Sewage Management and Treatment Pollution = The presence of a substance in the environment that prevents
